Translate this page into:
Study of oxidative dehydrogenation of ethylbenzene with CO2 on supported CeO2-Fe2O3 binary oxides
⁎Corresponding author. kcsong@umd.edu (Deping Xu)
-
Received: ,
Accepted: ,
This article was originally published by Elsevier and was migrated to Scientific Scholar after the change of Publisher.
Peer review under responsibility of King Saud University.
Abstract
Nano-sheets Al2O3 supported CeO2-Fe2O3 binary oxides were prepared by the vacuum impregnation method. The structural and textural properties were characterized by pertinent techniques, and the materials were evaluated as catalysts for the oxidative dehydrogenation of ethylbenzene with carbon dioxide (CO2-ODEB). The characterization results show that all samples maintain the hierarchical structure, and CeO2-like and Fe2O3-like solid solutions were formed when changing the Ce-to-Fe molar ratio. The catalytic performances indicate that CeO2-Fe2O3 binary oxides were effective for CO2-ODEB, and the activity is determined by mobile oxygen, which can facilitate the dehydrogenation process. The DFT studies further identified the reaction pathway and rate-determining step. The inter-transmission of oxygen species and the presence of CO2 refill the oxygen vacancies and restore the redox cycle of CeO2-Fe2O3 binary oxides.
Keywords
CeO2-Fe2O3 binary oxides
Solid solution
Ethylbenzene dehydrogenation
Oxygen mobility
DFT
1 Introduction
Styrene, a very important raw material in the petrochemical industry, is used in the production of polymers, such as polystyrene and styrene-butadiene rubber, amongst many others (Lee, 1974; Baghalha and Ebrahimpour, 2007). Typically, styrene is produced by dehydrogenation of ethylbenzene, which requires an exorbitant supply of super-heated steam (Coulter et al., 1995; Wu et al., 1993). This process consumes a significant amount of energy and results in a challenging gas–liquid phase separation; the equilibrium efficiency is also quite low, with 79.7% ethylbenzene conversion occurring at the high temperature of 983 K. Due to these drawbacks, the replacement of steam with CO2 is believed to be energy-saving and theoretically favorable (Zhao et al., 2007; Kuśtrowski et al., 2006). It has been reported that the utilization of CO2 can reduce the required energy input from 6.27 × 106 to 7.9 × 105 kJ·ton−1. Additionally, an estimation of energy required for steam-assisted and oxidative dehydrogenation of ethylbenzene suggests that the energy required in case of CO2 utilization was very less (190 kcal/kg of styrene) compared to that of steam (1500 kcal/kg of styrene) (Mimura et al., 1998); second, the CO2 utilization offers several advantages, such as enhancement of the product selectivity, diminishing of the thermodynamic limitations, suppression of the over oxidation. Furthermore, the coupling of oxidative dehydrogenation of ethylbenzene with reverse water–gas shift reaction will improve the per-pass ethylbenzene conversion (Sun et al., 2004). In addition, the employment of CO2 is environmentally friendly and could establish a greenhouse gas conversion approach (Ansari and Park, 2012).
Various catalysts have been investigated for the CO2-ODEB (Brünig et al., 2018; Diao et al., 2017, 2016; Liu et al., 2014). Among different type of catalysts, CeO2-based materials have been extensively designed and used as catalyst due to the remarkable oxygen storage capacity (OSC) and enhanced redox properties (Wang et al., 2019a, 2019b; Fan et al., 2019; Kuntaiah et al., 2013; Kurnatowska et al., 2014; Zhou and Zhou, 2010). It has been shown that the substitution of Ce4+ by isovalent ions, like Zr4+ and Hf4+, shows superior activity, so Ce-Zr solid solution is the most widely investigated catalyst in CO2-ODEB (Su et al., 2018; Wang et al., 2017a). It is reported that introducing Zr4+ could lead to better lattice oxygen mobility and facile reduction of Ce4+, moreover, the reducible tetravalent ions like Sn4+ and Ti4+ exhibit better activity than a non-reducible Zr-substitution (Yao et al., 2013). However, isovalent ions dopants can increase the difficulty in creating oxygen vacancies (Ahn et al., 2012). Therefore, there is considerable scientific interest in introducing aliovalent cations into CeO2, such as Gd3+ and Sm3+, as they can generate extrinsic oxygen vacancies via charge compensation mechanism (Guo et al., 2011). One possibility is to introduce trivalent ions with similar radius, such as Eu3+, as a method of altering the characteristics. Another way is incorporating ions with an undersized radius, like Fe3+, and the resulting material was also studied in numerous reactions (Kano et al., 2013; Li et al., 2001; Pérez-Alonso et al., 2005; Wang et al., 2014). As a well-suited reducible dopant, Fe has attracted attention because of its unique advantages, easy handling, and low-cost features (Hedayati et al., 2012; Hong et al., 2010; Liang et al., 2009; Zuo et al., 2013). In particular, the redox potential of Fe3+ and Fe2+ is 0.77 V, very much lower than that of Ce4+ and Ce3+, 1.44 eV. (Hedayati et al., 2012). Zhang et al. proposed that the lattice oxygen in Fe2O3 participates in the reaction and reduces Fe3+ to Fe2+, which is then restored by CeO2 (Zhang et al., 2010). Singh reported the oxygen species in CeO2-Fe2O3 binary oxides were more labile and Fe dopants into ceria lead to strong structural distortion and lower oxygen vacancy formation energy, thus to the resulting catalyst is suited for CO2-ODEB (Kurnatowska et al., 2014).
There are two reported mechanisms for CO2-ODEB. One is a coupling mechanism, which involves the direct dehydrogenation of ethylbenzene, followed by the reverse water–gas shift reaction. The other is Mars-van-Krevelen redox mechanism involving the lattice oxygen (Liu et al., 2011; Mimura and Saito, 2000). Previous studies suggested CeO2-Fe2O3 binary oxides align best with the redox mechanism, where ethylbenzene reacts with mobile oxygen to produce styrene and water, simultaneously, a stoichiometric amount of oxygen vacancies is left over the oxide catalyst, which is then replenished by CO2 to complete the redox cycle. Thus, the amount and reversibility of mobile oxygen are crucial in determining the activity.
In this work, an Al2O3 assembled by nanosheets, which presents the hierarchical structure and reported to be beneficial to the dispersion and both chemical and thermal stability (Wang et al., 2017a, 2017b), was used as support to prepare the supported CeO2-Fe2O3 binary oxides, and evaluated as catalyst for the possible use in CO2-ODEB. The reaction results reflected that the activity of the catalysts was correlated with their structural and redox properties. The physicochemical properties were then investigated and discussed to reveal the determining factors in the reaction.
2 Experimental
2.1 Material
Aluminum nitrate nonahydrate (Al(NO3)3·9H2O), cerium nitrate hexahydrate (Ce(NO3)3·6H2O), and iron nitrate nonahydrate (Fe(NO3)3·9H2O) were purchased from Alfa Aesar (USA), urea (CH4N2O) was purchased from Aladdin (Shanghai, China). Catalytic reaction solution of benzene, toluene, styrene, and were purchased from Sigma-Aldrich (USA). All the reagents are analytical grade and used directly without further purification.
2.2 Catalyst preparation
Nano-sheets of γ-Al2O3 were synthesized via a controlled hydrothermal method according to literature (Abdollahifar et al., 2014), and labeled as NSA. The synthetic steps are as follows, 18.75 g of Al(NO3)3·9H2O and 6 g of urea, CH4N2O, were dissolved in 100 and 30 mL of distilled water, respectively, and magnetically stirred at room temperature to obtain homogeneous solutions A and B. Then solution B was added to solution A and stirred at room temperature for 15 min, then transferred the mixed solution into a 200 mL Teflon-lined stainless autoclave and heated at 473 K for 24 h under autogenous pressure. After being naturally cool down to room temperature, the precipitate was filtered, washed three times with distilled water, and finally dried in an oven at 333 K for 24 h, then calcined at 773 K in air for 5 h.
A series of CeO2-Fe2O3 binary oxides with different molar ratios were loaded onto the supports. The synthesis of Ce50Fe50/NSA was explained as an example of the detailed synthesis. 0.43 g Ce(NO3)3·6H2O and 0.42 g Fe(NO3)3·9H2O were dissolved in 10 mL deionized water, respectively (Ce/Fe molar ratio = 1.0, Ce + Fe = 0.002 mol), then mixed above solutions and magnetic stirred for 30 min, then 5.0 g nano-sheets Al2O3 were dispersed into the solution under stirring, after 30 min ultrasonic processing, the resulting mixture was moved to a vacuum rotary evaporator, set to 343 K and 50 round per minute for 10 h. Finally, the obtained solids were calcined at 873 K in static air for 5 h at the heating rate of 1 K·min−1, leading to the 5 wt% Ce50Fe50/NSA. By simply changing the amount of Ce(NO3)3·6H2O and Fe(NO3)3·9H2O, the CexFe(100-x)/NSA (x = 0, 10, 30, 50, 70, 100) was synthesized, using the same procedure and parameters.
2.3 Characterization
Field emission scanning electron microscopy (FESEM) images were obtained on a Zeiss Supra 55 microscope. Transmission electrons were recorded on a FEI Tecnai G2-20 S-TWIN. The surface areas of the materials were determined by the BET method using an automatic Micromeritics ASAP 2020M. Before measurement, the samples were degassed at 473 K for 8 h, and the N2 adsorption–desorption was performed at 77 K. X-ray diffraction (XRD) patterns were recorded on a Rigaku X-ray diffractometer (D/Max 2550VB+/PC) equipped with Cu/Kα radiation Cu/Kα (40 kV, 30 mA). The samples were scanned from 2θ of 10-90° with a step size of 0.02◦ and a counting time of 2.5 s per step. The X-ray photoelectron spectroscopy (XPS) analysis was performed on a KRATOS Axis Ultra DLD spectrometer with an Mg Kα radiation from a double anode X-ray source. The binding energy correction was made using the adventitious C 1s at 284.5 eV as a reference for all the elements recorded. The reduction behavior of the composite oxides was investigated by hydrogen temperature-programmed reduction (TPR) in a Micromeritics Autochem 2920 instrument. Firstly, 50 mg of each sample was loaded and flushed with an Ar flow of 30 mL·min−1 at room temperature for 2 h and then increased from room temperature to 1273 K at a temperature ramp rate of 10 K·min−1 under 30 mL·min−1 flow of 10 vol% H2 in Ar. After condensing the water formed in a liquid N2 trap, the effluent was monitored with a TCD detector. The spent catalysts were characterized by the thermogravimetric analysis technique on a Netzsch STA 449 F5 thermo analyzer system (TA Instruments). The sample (ca. 10 mg) was heated at a rate of 10 K·min−1 under a constant flow of CO2 (50 mL·min−1)
2.4 Simulation details
The first-principles calculations have been performed by the VASP code (Kresse and Furthmüller, 1996; Kresse and Hafner, 1993). The generalized gradient approximation functional proposed by Perdew et al. was used (Perdew et al., 1996). The interactions between valence electrons and ion cores are represented by Blöchl’s all-electron-like projector augmented wave method (PAW), which regards the d7s1 states as the valence configuration for Fe, s2p4 for O, 4f15d16s2 for Ce and s2p2 for C (Blöchl, 1994). A plane wave energy cutoff of 400 eV was used in the present calculations. Geometries were relaxed using the conjugate gradient algorithm until the forces on all the unconstrained atoms are less than 0.03 eV/Å (Sheppard et al., 2008).
2.5 Catalytic reaction procedure
The oxidative dehydrogenation of ethylbenzene with CO2 was performed in a quartz fixed-bed reactor with an internal diameter of 6 mm, housed inside an electric furnace with three independently controlled temperatures zones. The temperature of the catalyst bed was measured by K-type thermocouple. A fritted quartz disk placed in the center of the reactor was used to hold the catalyst. Typically, 0.3 g catalyst (40–60 mesh) diluted with the same amount of quartz sands was loaded into the reactor, then heating up under N2 flow, when reached the reaction temperature, the catalyst was pre-treated at the reaction temperature for 30 min under flowing CO2 to remove the physically absorbed impurities and N2. The analytical grade ethylbenzene (99.999% purity) was then introduced into a gasifier using a HPLC pump at a flow rate of 0.006 mL·min−1, then the vaporized ethylbenzene carried by CO2 flow was fed into the reactor. The reaction temperature was 823 K, P = 0.1 MPa, total flow rate = 25 mL·min−1, CO2/EB molar ratio = 20, W/F = 4.747 g-cat·h·mol−1. The condensed products, including benzene (BE), toluene (TO), styrene (ST), and unreacted ethylbenzene (EB), were analyzed on a gas chromatograph equipped with a DB-1 capillary column (0.25 mm × 60 m) and an FID detector. The ethylbenzene conversion and styrene selectivity were calculated based on the calibrated GC peak areas of the components. The following expressions were used to determine the activity of different catalysts. The percent conversion for a reactant (Eq. (1)) and selectivity towards a product (Eq. (2)) were calculated as:
3 Results and discussion
3.1 Structure of supported CeO2-Fe2O3 binary oxides
To investigate the textural properties of the supported CeO2-Fe2O3 binary oxides, N2 adsorption–desorption isotherms were measured and shown in Fig. 1(A). All the isotherms exhibited IV type behavior with an H3 hysteresis loop, indicating the presence of slit-shaped pores associated with the unique structure of the support. Clearly, after the loading of CeO2-Fe2O3 binary oxides, the adsorption volume decreased when compared to the unloaded support, indicating that the CeO2-Fe2O3 binary oxides were located in the slit pores of the nanosheets support. Additionally, in the low relative pressure range, the uptake of supported samples was lower than pristine support, which suggested the occupation of the micropores.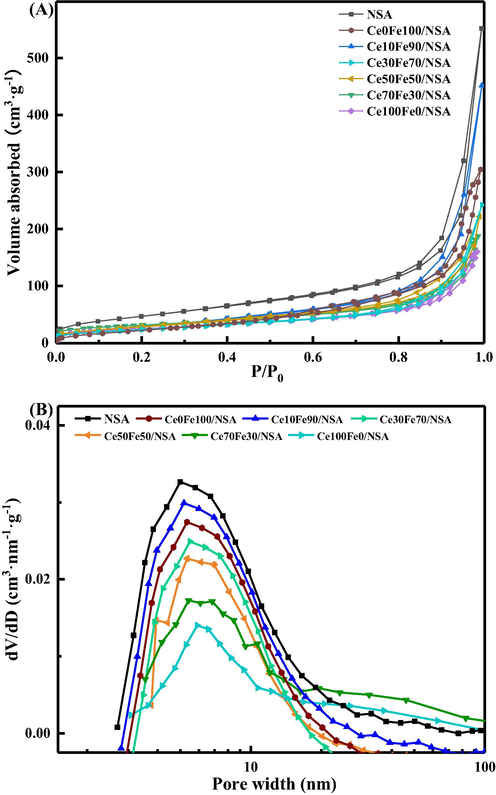
(A) N2 adsorption–desorption isotherms and (B) pore size distributions of NSA and supported CeO2-Fe2O3 binary oxides.
The pore size distributions (PSD) plot, as shown in Fig. 1(B), further underscored the textural properties. The pore size distribution curves were determined from the adsorption branch of N2 isotherms. All the samples showed a similar peak center at 3–5 nm, together with the presence of broad distribution consisting of wide mesopores and macropores, which means the hierarchical porous structure; no significant variation means the hierarchical structure was maintained after CeO2-Fe2O3 binary oxides loading.
The physical textural parameters were collected in Table 1. It was interesting to see that sample Ce0Fe100/NSA lost a lot of pore volume but kept a relatively high surface area, potentially due to agglomerated FeOx blocked the larger-sized mesopores which contribute little to the external surface area, while the smaller-sized mesopores were left intact (Oh et al., 2017), this is also supported by the PSD curve. For other samples, with the increase of Ce content, both surface area and pore volume decreased, which indicates that CeO2-Fe2O3 binary oxides were not only located on the outermost surface but also entered the slit-shaped pores. These changes may be ascribed to the unique porosity derived from the nano-sheets-like architecture, since the material can retain the accessibility to the internal pores and decrease the possibility of blocking, therefore providing the potential for high dispersion (Wang et al., 2017b).
Catalyst
Vtotal [cm3·g−1]
SBET [m2·g−1]
Mean pore diameter [nm]
NSA
0.85 ± 0.04
177.43 ± 8.87
4.32 ± 0.22
Ce0Fe100/NSA
0.42 ± 0.02
130.41 ± 6.62
3.81 ± 0.19
Ce10Fe90/NSA
0.51 ± 0.02
119.90 ± 5.99
3.64 ± 0.18
Ce30Fe70/NSA
0.38 ± 0.01
100.24 ± 5.01
3.62 ± 0.18
Ce50Fe50/NSA
0.35 ± 0.01
94.89 ± 4.74
3.38 ± 0.17
Ce70Fe30/NSA
0.33 ± 0.01
107.7 ± 5 0.38
3.41 ± 0.16
Ce100Fe0/NSA
0.27 ± 0.01
87.7 ± 4.38
3.43 ± 0.17
3.2 Crystal properties
Supported CeO2-Fe2O3 binary oxides and pure Fe2O3/CeO2 loaded crystalline structure after calcination at 873 K was investigated by XRD, and the spectra are presented in Fig. 2. The reflections can be assigned either to hematite, α-Fe2O3 (hexagonal, JCPD 33-0664), or cubic CeO2 (fluorite, JCPDS 65-5923) (Yin et al., 2012). In Ce0Fe100/NSA, typical patterns of hexagonal hematite structure were observed, however, peaks derived from NSA support were weak and occurred as a bump, this may shed some light on textural properties discussed above. For Ce10Fe90/NSA, the diffraction peaks assigned to Fe2O3 showed higher intensity than those in Ce0Fe100/NSA, and peaks of support occurred, meanwhile, the cubic CeO2 peaks were missing. With increasing of Ce-to-Fe molar ratio, the CeO2 peaks were identified and became stronger, in addition, sample Ce50Fe50/NSA still showed weak Fe2O3 peaks, while Ce70Fe10/NSA barely exhibited α-Fe2O3 peaks, with only CeO2 peaks appearing in the spectrum. It should be noted that the characteristic peak of CeO2 shifted towards lower 2θ position in some samples with the increase of Ce-to-Fe molar ratio, as shown in the left enlarged portion of Fig. 2, which, combined with absent Fe2O3 peaks, that may suggest the formation of solid solution or highly dispersed Fe2O3 (Zhu et al., 2014, 2013).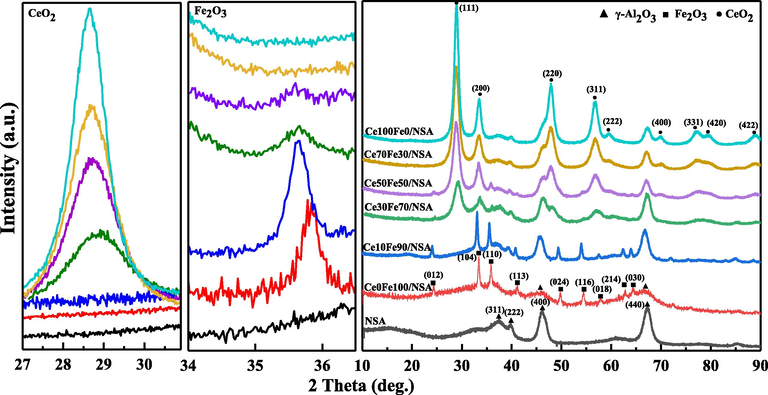
XRD patterns of NSA and supported CeO2-Fe2O3 binary oxides.
To further verify the formation of the solid solution, the unit cell parameters of each sample were calculated using Scherrer’s equation (Patterson, 1939) and collected in Table 2. The spectra from some samples were not suitable to accurately determine the unit cell parameters due to the low peak intensities. From Table 2, a shrinkage in CeO2 lattice constants was observed, indicating a CeO2-like solid solution was initially formed in Ce30Fe70/NSA, Ce50Fe50/NSA, and Ce70Fe30/NSA, as some Ce4+ in the CeO2 lattice were substituted by Fe3+, since the Fe3+ cation size is smaller than Ce4+ (0.0645 and 0.102 Å). On the other hand, increased peak intensity also reflected the increased concentration of CeO2-like solid solution (Pérez-Alonso et al., 2006). As for the hematite, the lattice constants also exhibited an increasing trend with the rising of the Ce-to-Fe molar ratio. This phenomenon can be explained by the incorporation of Ce cations into the hematite lattice, leading to the expansion, especially for the parameter c. Besides, this change can be verified by the shift of hematite characteristic peaks, as shown in the enlarged middle portion.
Sample
Crystallite size (nm)
Lattice constant (nm)
CeO2
Fe2O3
Ce0Fe100/NSA
15.57
–
a 0.6404
c 1.4059
Ce10Fe90/NSA
8.30
–
a 0.6563
c 1.4127
Ce30Fe70/NSA
8.60
a 0.5414
a 0.6780
c 1.4245
Ce50Fe50/NSA
8.84
a 0.5436
a 0.6815
c 1.4625
Ce70Fe30/NSA
9.35
a 0.5457
–
–
Ce100Fe0/NSA
9.40
a 0.5519
–
3.3 Morphologic characteristics
TEM and FESEM were performed to explore the morphology of NSA support and loaded samples. It is important to note that the calculation of the particle size from TEM images was rather difficult as there were no protective agents used, so no precise calculations were attempted in this study.
As shown in Fig. 3(a) and (b), the NSA supports consist of a great deal of nano-sheets-like architecture with an average size of about 476 × 226 × 42 nm (length × width × thickness). Fig. 3(c) and (d) were supported CeO2-Fe2O3 binary oxides, Ce10Fe90/NSA and Ce70Fe30/NSA, which appeared as a homogeneous agglomeration of particles. Sample Ce10Fe90/NSA showed a number of small particles (∼5–7 nm) with a clear grain boundary supported on a larger particle (>20 nm), whose lattice fringe with an interplanar spacing of ∼0.27 nm was associated with the (1 1 0) facet of Fe2O3. The Ce70Fe30/NSA sample displayed a notably different morphology from that of Ce10Fe90/NSA, and showed a lattice fringe of ∼0.31 nm, which can be attributed to the cubic CeO2 (1 1 1) at 28.6° crystal planes.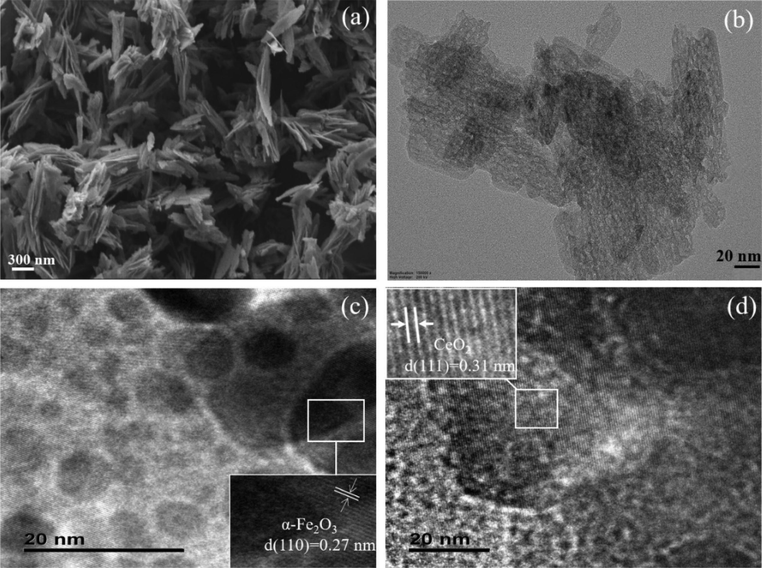
SEM image of (a) NSA, TEM images of (b) NSA, (c) Ce10Fe90/NSA, and (d) Ce70Fe30/NSA.
3.4 Reduction behavior
As a matter of fact, the OSC and its mobility were closely related to the reducibility and defects. To gain further insight into the composition and structure, supported CeO2-Fe2O3 binary oxides were investigated via H2-TPR, and the results were shown in Fig. 4. The hydrogen reduction of Fe2O3 is commonly reported to be stepwise, where Fe3+ converts to metallic Fe by going through Fe3O4 and FeO as intermediates. In the current study, peaks of the pure Fe2O3 loaded sample correspond to the above process at about 673, 923, and 1123 K. For pure CeO2, mainly two reduction steps, the reduction of surface-capping oxygen at about 793 K and the reduction of bulk phase oxygen reduction at about 1173 K, where, in the presently studied sample, Ce100Fe0/NSA only displayed the high-temperature reduction peak, surface oxygen barely existed.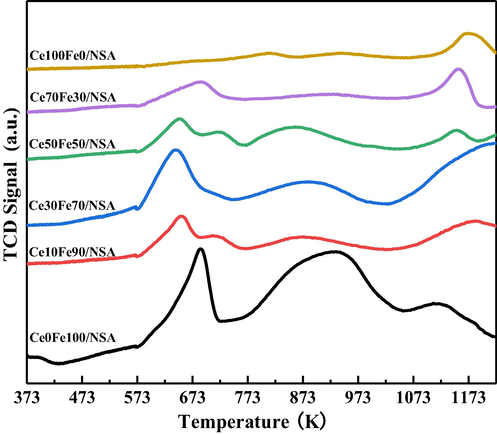
H2-TPR profiles of supported CeO2-Fe2O3 binary oxides.
For CeO2- Fe2O3 binary oxides, according to Lagun and Qiao (Laguna et al., 2011; Qiao et al., 2011), four well-defined reduction peaks at around 673, 873 and 1073 K corresponded to the Fe3+ and Ce4+ reduction in solid solution, Fe species intermediate reduction step, and bulk Ce4+ reduction, respectively. As shown in Fig. 4, sample Ce70Fe30/NSA, the XRD pattern was essentially the same as that of pure CeO2 loaded sample, but the reducibility was improved, as observed the significantly lowered first reduction temperature, likely due to the doping of Fe3+ into the CeO2 lattice and the strong metal-support interaction enhancing the reducibility (Ebiad et al., 2012; Figueiredo et al., 2019). For the sample Ce50Fe50/NSA, Ce30Fe70/NSA, and Ce10Fe90/NSA, the first peak could result from the reduction of segregated FeOx, and peak area was proportional to the Fe content. The shoulder peak appeared in Ce50Fe50/NSA and Ce10Fe90/NSA was due to the reduction of surface CeO2, which closely contacted with the surface segregated FeOx, and the peak area was increased with Ce-to-Fe molar ratio. The broad peak in the range of 723 to 973 K was ascribed to the Fe species intermediate reduction step, and the last peak at higher temperature was due to the reaction of bulk CeO2.
3.5 Surface composition
The XPS measurements were conducted to investigate the surface composition, and the results were plotted in Fig. 5. Fig. 5(A) depicted the Ce 3d spectrum, and it is known to be complex in CeO2-Fe2O3 binary oxides due to the hybridization of Ce 4f with ligand orbitals and fractional occupancy of the valence 4f orbitals, which assists the multiplet splitting of peaks into doublets showing further spectral activity due to final state effects. There were 8 peaks related to four different spin–orbit-split doublets. Among them, peaks denoted as U, U'' and U''' were attributed to Ce4+ 3d3/2, located at about 901.3, 907.3 and 916.9 ± 0.1 eV, and those denoted as V, V'' and V''' arisen from Ce4+ 3d5/2, located at about 882.7, 888.5 and 898.3 ± 0.1 eV, The pair of doublets, U' and V', were characterized by the lower binding energy intensities of Ce3+ 3d3/2 and Ce3+ 3d5/2, located at about 903.4 and 885.2 ± 0.1 eV (Bêche et al., 2008). As shown, CeO2-Fe2O3 binary oxides containing both Ce4+ and Ce3+, suggesting the rapid oxygen exchange and contributes to the enhancement of OSC. The Fe 2p spectrum, as shown in Fig. 5(B), two bands can be observed, which correspond to lower energy (Fe 2p3/2) and higher energy (Fe 2p1/2) asymmetric bands originated from the spin-orbital splitting. According to the literature, the standard Fe 2p3/2/Fe 2p1/2 signals corresponding to metallic iron, ferrous and ferric states are located at 706.7/719.8, 709.2/722.8 and 711.2/724.8 eV, respectively. Satellites peaks at 718 and 733 eV were also detected.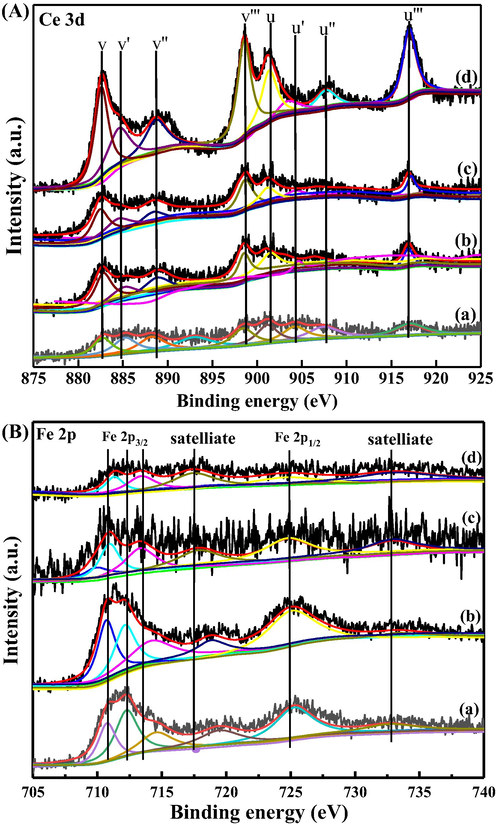
Ce 3d (A) and Fe 2p (B) XPS spectra of (a) Ce10Fe90/NSA, (b) Ce30Fe70/NSA, (c) Ce50Fe50/NSA and (d) Ce70Fe30/NSA.
The binding energy and concentration of each element on the surface of samples were calculated based on the peak area and shown in Table 3. The binding energy of Al 2p increased slightly compared with the pristine support, possibly due to the effects of different Fe/Ce molar ratios on the final material properties, which could account for the appearance of unsaturated Al species derived from the removal of hydroxyl groups on the surface of NSA (Wang et al., 2017b). Taking the loading of Fe and Ce species into consideration, the interaction between binary oxides and support could also contribute to these changes. Moreover, the Fe/Al and Ce/Fe value varied with different Ce-to-Fe molar ratios, which reflects the dispersion variations and different O species content. The (Ce + Fe)/Al ratio remained constant due to the sum of Ce and Fe were constant. On the other hand, the content of Ce3+ on the surface of CeO2-Fe2O3 binary oxides was strongly dependent on the Ce/Fe ratio. To make a quantitative comparison, the relative content of Ce3+ defined as the ratio of Ce3+/(Ce3++Ce4+) was calculated based on the peak area. By doping a small amount of Fe into CeO2, the content of Ce3+ was increased from 20.04 to 27.17. The oxygen vacancy is believed result from the reduction of Ce4+ to Ce3+, which will generate one oxygen vacancy to balance the charge, and thus the Ce3+/(Ce3++Ce4+) ratio could depict the concentration (Guo et al., 2011).
Sample
Surface atomic ratio (%)
Al 2p
Fe/Al
Ce/Fe
(Ce + Fe)/Al
Ce3+/(Ce3+ + Ce4+)
NSA
71.8
–
–
–
–
Ce10Fe90/NSA
74.1
0.029
0.10
0.12
20.04
Ce30Fe70/NSA
74.2
0.021
0.32
0.11
23.04
Ce50Fe50/NSA
74.3
0.018
0.92
0.13
26.04
Ce70Fe30/NSA
74.2
0.012
2.20
0.11
27.17
3.6 Catalytic performance
The catalytic performance of supported CeO2-Fe2O3 binary oxides and pure CeO2/Fe2O3 loaded samples were shown in Fig. 6. All samples displayed a high steady-state selectivity of styrene above 90% under the optimized reaction condition. When the initial activity was considered, except pure CeO2 loaded sample, all other samples showed around 55% ethylbenzene conversion. Ce30Fe70/NSA and Ce50Fe50/NSA displayed relatively higher and stable ethylbenzene conversion, while that of Ce70Fe30/NSA displayed a lower activity and stabilized gradually with TOS. For the sample Ce10Fe90/NSA and Ce0Fe100/NSA, suffer from the deactivation drastically, the EB conversion dropped more than 50% after 8 h TOS. Several related reported catalysts were listed in Table 4. Activity comparison of these cited catalysts such as Al2O3 supported Ce-Zr mixed oxides synthesized with impregnation and hydrothermal methods (Wang et al., 2019b, 2017b), vanadium-ceria-zirconia-alumina (Wang et al., 2019c), pure α-Fe2O3 (Wang et al., 2017c) and MnOx (Ren et al., 2017), respectively, indicate that the present studied nanosheets Al2O3 supported CeO2-Fe2O3 binary oxides show both higher activity and stability, could be a desired catalyst for CO2-ODEB.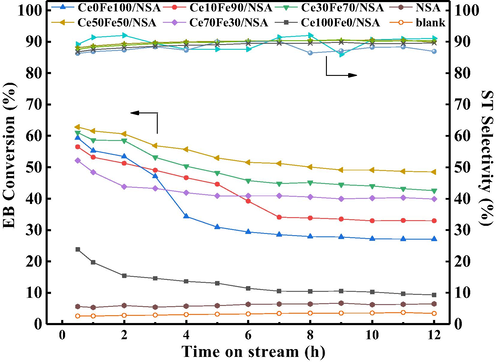
Catalytic performance of supported CeO2-Fe2O3 binary oxides for CO2-ODEB. (823 K, P = 0.1 MPa, CO2/EB molar ratio = 20, EB = 0.006 mL·min−1).
Catalyst
Reaction Conditions
EB Con. (%)
ST Sel. (%)
Ref.
Temperature (K)
Atmosphere
Ce-Zr/NFA
823
CO2
53
95
Wang et al. (2017a)
Fe/FA
823
CO2
38
>95
Wang et al. (2017b)
Ce1-xFexO2
823
CO2
35
92
Wang et al. (2014)
Ce1-xZrxO2
823
CO2
40
94
Wang et al. (2019b)
Ce1-xZrxO2
823
CO2
15
95
Wang et al. (2019a)
V-Ce-Zr-Al
823
CO2
62
92
Wang et al. (2019c)
α-Fe2O3
823
CO2
3
95
Wang et al. (2017c)
MnOx
823
CO2
35
76
Ren et al. (2017)
Ce-Fe/NSA
823
CO2
56
95
Present study
Additionally, the effect of feed gas was also studied. The catalytic test of sample Ce50Fe50/NSA was performed under N2 flow, and the results were displayed in Fig. 7. The results indicated that CO2 has a significant influence, as the styrene selectivity only dropped slightly, while the EB conversion under title reaction condition was much lower than under CO2 flow. The detailed discussion was made correlated to the reaction mechanism in the later section.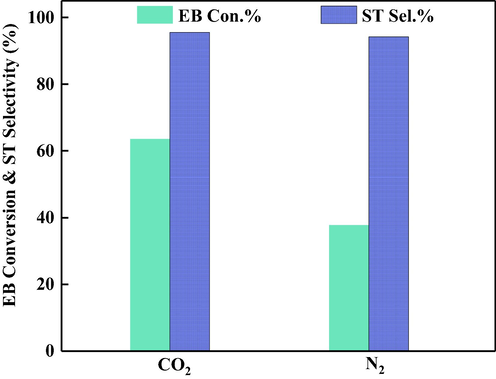
Catalytic performance of Ce50Fe50/NSA with different feed gases.
Taking the redox mechanism into consideration, higher activity was expected due to the mobile oxygen species (Zhang et al., 2010). And normally, the general mobile oxygen should be primarily related to both lattice and surface-absorbed oxygen (Li et al., 2014). Moreover, the available amount and reversibility of the mobile oxygen species are curial in determining the stability for CO2-ODEB. Thus, the oxygen species were then investigated. As shown in Fig. 8(A), the O 1s spectrum displayed 3 peaks after deconvolution. The lower binding energy at about 529 eV represents the surface lattice oxygen derived from binary oxides, labeled as OI, and the broad peak at about 531 eV, marked as OII, may be ascribed to monatomic oxygen (or surface-absorbed oxygen), such as O2−, O22− or O−, which considered as derived from surface oxygen vacancies/defects with unsaturated chemical bonds, can enhance the mobility of the lattice oxygen. The last peak at 532 eV was assigned to the absorbed oxygen, denoted as OIII, which could represent the hydroxyl-like groups or other weakly absorbed oxygen species (WEI et al., 2010). The calculated content of each oxygen species was listed in Table 5. The relationship between lattice oxygen (OI) and monatomic oxygen (OII) and EB conversion before and after the reaction was depicted in Fig. 9. From the results, samples Ce30Fe70/NSA and Ce50Fe50/NSA with a high proportion of OI and OII, were expected to exhibit higher catalytic activity in the CO2-ODEB reaction, as confirmed by the value of (OI + OII)/ OIII was higher than other samples, the remaining samples generally match well with the trend of EB conversion. Moreover, the changing pattern for the content of (OI + OII)/OIII also matched very well with that for the value of Ce3+/(Ce3+ + Ce4+) in Table 3, indicating that the content of OI + OII is equivalent to the proportion of Ce3+. On the other hand, the higher activity also can be ascribed to the well dispersed CeO2-Fe2O3 binary oxides in hierarchically structured support. As discussed, CeO2-Fe2O3 binary oxides could be located inside the slit-shaped pores, promoting the formation of two types of solid solutions, leading to a higher concentration of surface lattice oxygen (OI) and monatomic oxygen (OII) (Zhang and Lin, 2011).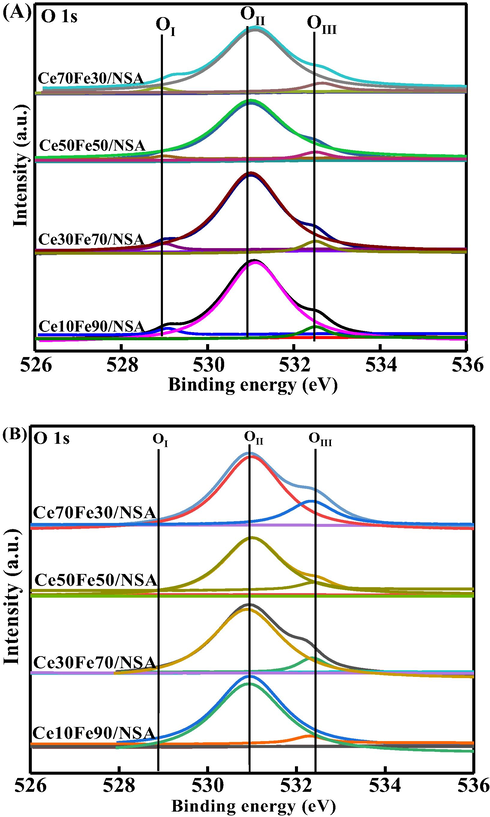
O 1s XPS spectra of fresh (A) and spent (B) supported CeO2-Fe2O3 binary oxides.
Sample
Relative content (%)
OI
OII
OIII
(OI + OII)/OIII
(a) Ce10Fe90/NSA
15.0
62.5
22.5
3.52
(b) Ce30Fe70/NSA
14.4
73.7
11.9
7.40
(c) Ce50Fe50/NSA
10.2
81.3
8.5
10.76
(d) Ce70Fe30/NSA
6.3
68.5
25.2
2.96
(a′) Ce10Fe90/NSA-spent
–
60.7
39.3
1.54
(b′) Ce30Fe70/NSA-spent
–
66.1
33.9
1.94
(c′) Ce50Fe50/NSA-spent
–
63.2
36.8
1.72
(d′) Ce70Fe30/NSA-spent
–
58.3
41.7
1.40
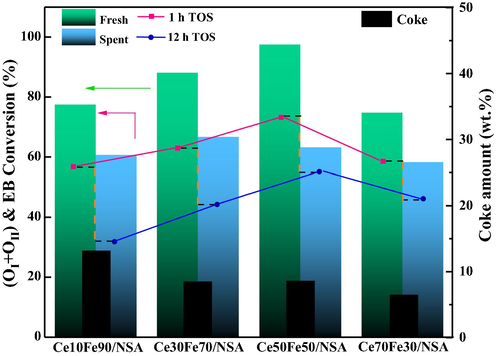
Mobile oxygen and catalytic performance of supported CeO2-Fe2O3 binary oxides.
Moreover, oxygen species of spent catalysts were also investigated and shown in Fig. 8(B). It can be seen that OI was almost exhausted during the reaction, as the peaks nearly vanished, and OII also slightly decreased. The comparison of OI and OII before and after reaction confirmed that the lattice oxygen (OI) and monatomic oxygen (OII) contributed to higher activity and both played an essential role in the reaction as discussed above. However, the amount of OIII was increased, and the (OI + OII)/ OIII value of spent catalysts kept the same order as before the reaction, but the gap between each other narrowed, combined the only slightly consumed OII which should have decreased more, and the presence of CO2 in the reaction, one deduction can be made that, the inter-transmission of oxygen species allowed the original OIII to replenish OII, and CO2 served as an oxidant can recover the OIII, however, OI cannot be restored completely.
Furthermore, as indicated by the reaction results, the redox cycle cannot be fully maintained throughout the reaction because of the coke. The deposited coke inhibited the reversibility of the mobile oxygen, leading to the increasing extent of deactivation. It is commonly known that the coke deposition is inevitable under typical conditions for CO2-ODEB, however, the participation of CO2 in the reaction may achieve dynamic equilibrium of coke forming and burning (Wang et al., 2010; Liu et al., 2011). The relative deactivation rate (R), defined as 100 × (X1 – X12)/X1, where X was the EB conversion, was then calculated. The deactivation rate of Ce70Fe30/NSA and Ce50Fe50/NSA were 21% and 27%, much lower than Ce10Fe90/NSA and Ce30Fe70/NSA, 42% and 30%, respectively, and pure Fe2O3 loaded sample suffered deactivation greatly. The deactivation rate indicated different coke resistance among samples. To get a deep understanding of the generated coke. Acquired spent supported CeO2-Fe2O3 binary oxides were evaluated by TG, as shown in Fig. 10. Clear weight loss can be observed in all samples, and the weight loss can be reasonably attributed to the gasification of the coke. The amount of coke in each sample was different, spent pure Fe2O3 loaded sample contained about 14 wt% coke, while spent CeO2-Fe2O3 binary oxides showed less. On the other hand, the gasification temperature varied. Form DTG data, it was obvious that the spent CeO2-Fe2O3 binary oxides exhibited a lower gasification temperature than the pure Fe2O3 loaded sample. In Fig. 10, the weight of spent CeO2-Fe2O3 binary oxides begin to drop at about 523 K, while the coke in Ce0Fe100/NSA began to gasification about 100 K higher. The varied coke amount and coke gasification temperatures indicate that the existence of proper amount CeO2 enhances the coke resistance and coke in CeO2-Fe2O3 binary oxides was easier to be eliminated than pure Fe2O3 loaded sample, which may due to the surface of the oxygen species, the relationship between deactivation rate and coke was depicted in Fig. 9.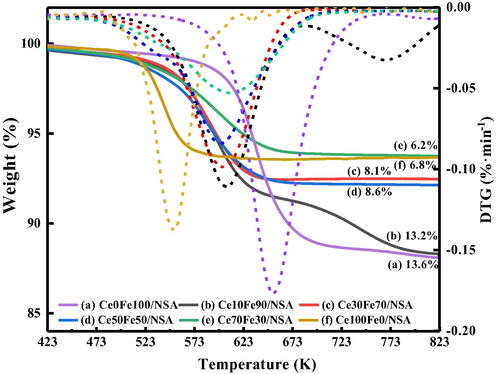
TG-DTG profiles of spent supported CeO2-Fe2O3 binary oxides.
3.7 Mechanism and simulation
A proposed reaction pathway was shown in Fig. 11. Firstly, ethylbenzene absorbed on the catalyst surface, dehydrogenated to styrene and H-H, mobile oxygen then reacted with H-H produced H2O (step 1 and 2). The high valence redox couple Fe3+/Ce4+ was reduced to Fe2+/Ce4+, as well as the oxygen vacancy was produced, because of the high energy barrier of CO2 decomposition and kinetically unfavored formation of CO on defective Fe surface, in this case, CeO2 then donated an oxygen atom to recover the oxygen vacancy, leading to Fe3+/Ce3+ (step 3) (He et al., 2011). Then, CO2 was absorbed and consequently replenished the oxygen vacancy on the CeO2 surface (step 4). Finally, the oxygen species spread and partially restored the redox couple to Fe3+/Ce4+. In the whole process, the presence of oxygen vacancies and the transmission of the oxygen species could accelerate the adsorption of CO2 and the diffusion speed of oxygen, thus prompting the entire reaction.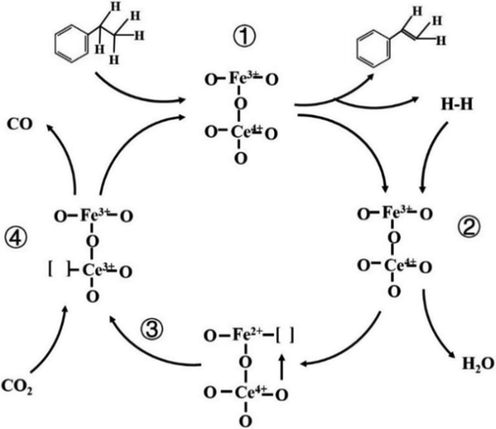
Proposed reaction scheme of CO2-ODEB.
Density functional theory (DFT) calculations were performed to confirm the feasibility under typical conditions, and further understand the reaction pathway proposed in Fig. 11. All computational details and results were presented in Fig. 12. For step 1, ethylbenzene absorbed on the O-terminated Fe2O3 surface, then dehydrogenated to produce styrene and H—H, owing to the strong H—O binding, molecular H2 formation was difficult to form (He et al., 2011). The dehydrogenation reaction followed the Mars van Krevelen mechanism, the energy barrier was calculated to be 0.1 eV, and the whole process was exothermic. The following step 2, the dehydrogenated H atoms absorbed at the O site and reacted with an adjacent hydroxyl group to produce H2O, this elementary reaction was endothermic, and the energy barrier was 1.35 eV. Then the H2O was desorbed with an energy barrier of 1.16 eV, and the surface of the oxides was reduced. This step is the rate determining step due to the highest energy barrier. The step 4, oxygen vacancy replenishment process, a CO2 molecule contacted with an oxygen vacancy and provided an O atom to restore the defect and detached as a CO molecule.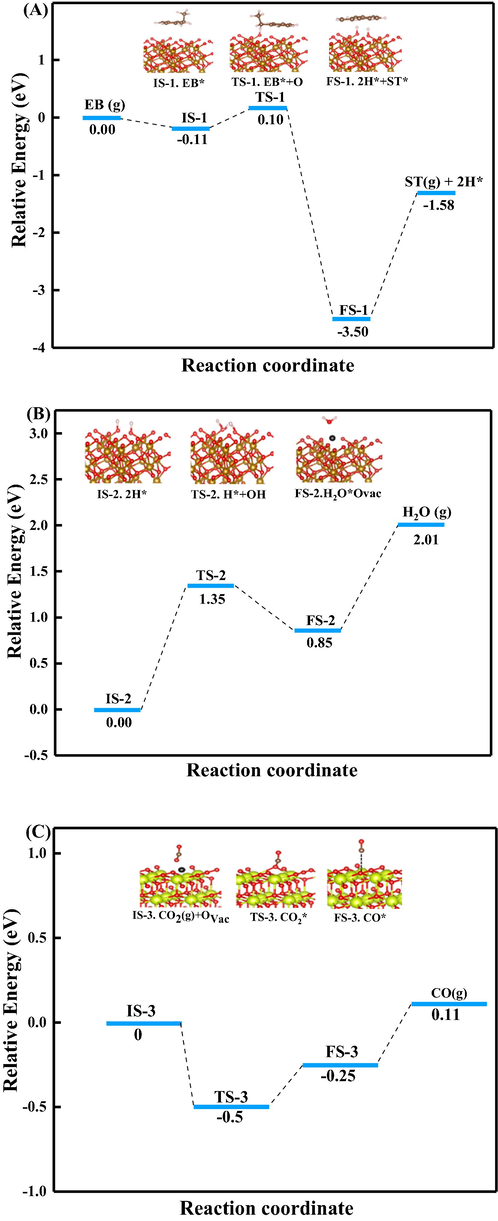
Potential energy diagrams and transition states of CO2-ODEB from DFT calculations. (A) Step ①, (B) Step ② and (C) step ④. (IS: Initial states; TS: transition state; FS: final state).
4 Conclusions
In summary, the hierarchical structure will be maintained after load CeO2-Fe2O3 binary oxides onto nano-sheet γ-Al2O3. The insertion of Ce or Fe species will cause the ion substitution and then form the solid solution. In the case of the catalytic activity, CeO2-Fe2O3 binary oxides show better ethylbenzene conversion and coke resistance than pure Fe2O3 loaded sample. Base on the redox mechanism of CO2-ODEB and characterization results of fresh and spent catalysts, surface lattice oxygen (OI) and monatomic oxygen (OII) were found to be the key factors in determining the activity for the title reaction. Moreover, the inter-transmission of oxygen species and the presence of CO2 can refill the oxygen vacancies and restore the redox cycle of CeO2-Fe2O3 binary oxides. The DFT studies verified the reaction pathway, and the oxygen species react with hydrogen act as the rate-determining step, and the oxygen vacancy replenishment process is favorable in studied conditions. With these understandings, the optimization of the synthetic parameters may develop not only a highly active but also a stable catalyst for the title reaction, which is worthy of being done.
Acknowledgments
We thank the writing assistance from Emily Schulman, University of Maryland, College Park.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
References
- Synthesis of micro-mesopores flowerlike γ-Al2O3 nano-architectures. J. Serbian Chem. Soc.. 2014;79:1007-1017.
- [CrossRef] [Google Scholar]
- Role of multivalent Pr in the formation and migration of oxygen vacancy in Pr-doped ceria: experimental and first-principles investigations. Chem. Mater.. 2012;24:4261-4267.
- [CrossRef] [Google Scholar]
- Carbon dioxide utilization as a soft oxidant and promoter in catalysis. Energy Environ. Sci.. 2012;5:9419-9437.
- [CrossRef] [Google Scholar]
- Structural changes and surface activities of ethylbenzene dehydrogenation catalysts during deactivation. Appl. Catal. A Gen.. 2007;326:143-151.
- [CrossRef] [Google Scholar]
- Ce 3d XPS investigation of cerium oxides and mixed cerium oxide (CexTiyOz) Surf. Interface Anal.. 2008;40:264-267.
- [CrossRef] [Google Scholar]
- Chemoselective supported ionic-liquid-phase (SILP) aldehyde hydrogenation catalyzed by an Fe(II) PNP pincer complex. ACS Catal.. 2018;8:1048-1051.
- [CrossRef] [Google Scholar]
- Kinetics of the dehydrogenation of ethylbenzene to styrene over unpromoted and K-promoted model iron oxide catalysts. Catal. Lett.. 1995;31:1-8.
- [CrossRef] [Google Scholar]
- Selective and stable ethylbenzene dehydrogenation to styrene over nanodiamonds under oxygen-lean conditions. ChemSusChem. 2016;9:662-666.
- [CrossRef] [Google Scholar]
- Fabrication of MgO–rGO hybrid catalysts with a sandwich structure for enhanced ethylbenzene dehydrogenation performance. Chem. Commun.. 2017;53:11322-11325.
- [CrossRef] [Google Scholar]
- Ni supported high surface area CeO2–ZrO2 catalysts for hydrogen production from ethanol steam reforming. RSC Adv.. 2012;2:8145-8156.
- [CrossRef] [Google Scholar]
- Promotional effect of oxygen storage capacity on oxy-dehydrogenation of ethylbenzene with CO2 over κ-Ce2Zr2O8(111) Appl. Surf. Sci.. 2019;486:411-419.
- [CrossRef] [Google Scholar]
- Understanding the strong metal-support interaction (SMSI) effect in CuxNi1–x/CeO2 (0 < x < 1) nanoparticles for enhanced catalysis. ACS Appl. Nano Mater.. 2019;2:2559-2573.
- [CrossRef] [Google Scholar]
- UV and visible Raman studies of oxygen vacancies in rare-earth-doped ceria. Langmuir. 2011;27:3872-3877.
- [CrossRef] [Google Scholar]
- Role of CO2 in ethylbenzene dehydrogenation over Fe2O3(0 0 0 1) from first principles. J. Mol. Catal. A Chem.. 2011;344:53-61.
- [CrossRef] [Google Scholar]
- Evaluation of novel ceria-supported metal oxides as oxygen carriers for chemical-looping combustion. Ind. Eng. Chem. Res.. 2012;51:12796-12806.
- [CrossRef] [Google Scholar]
- Direct decomposition of NO on Ba catalysts supported on Ce–Fe mixed oxides. Catal. Lett.. 2010;135:190-196.
- [CrossRef] [Google Scholar]
- Dehydrogenation of ethylbenzene over Fe–Ce–Rb and Fe–Ce–Cs mixed oxide catalysts. React. Kinet. Mech. Catal.. 2013;109:29-41.
- [CrossRef] [Google Scholar]
- Efficiency of ab-initio total energy calculations for metals and semiconductors using a plane-wave basis set. Comput. Mater. Sci.. 1996;6:15-50.
- [CrossRef] [Google Scholar]
- Ab initio molecular dynamics for open-shell transition metals. Phys. Rev. B. 1993;48:13115-13118.
- [CrossRef] [Google Scholar]
- Nanocrystalline Ce1−xSmxO2−δ (x = 0.4) solid solutions: structural characterization versus CO oxidation. RSC Adv.. 2013;3:7953-7962.
- [CrossRef] [Google Scholar]
- Nanocrystalline Ce1−xRuxO2 – Microstructure, stability and activity in CO and soot oxidation. Appl. Catal. B Environ.. 2014;148–149:123-135.
- [CrossRef] [Google Scholar]
- VOx supported SBA-15 catalysts for the oxidative dehydrogenation of ethylbenzene to styrene in the presence of N2O. Catal. Today. 2006;114:307-313.
- [CrossRef] [Google Scholar]
- Fe-doped ceria solids synthesized by the microemulsion method for CO oxidation reactions. Appl. Catal. B Environ.. 2011;106:621-629.
- [CrossRef] [Google Scholar]
- Iron Oxide catalysts for dehydrogenation of ethylbenzene in the presence of steam. Catal. Rev.. 1974;8:285-305.
- [CrossRef] [Google Scholar]
- Synthesis of nanoscale Ce1-xFexO2 solid solutions via a low-temperature approach. J. Am. Chem. Soc.. 2001;123:11091-11092.
- [CrossRef] [Google Scholar]
- Microstructure and oxygen evolution of Fe–Ce mixed oxides by redox treatment. Appl. Surf. Sci.. 2014;289:378-383.
- [CrossRef] [Google Scholar]
- Template preparation of nanoscale CexFe1−xO2 solid solutions and their catalytic properties for ethanol steam reforming. J. Mater. Chem.. 2009;19:1417-1424.
- [CrossRef] [Google Scholar]
- A nanodiamond/CNT–SiC monolith as a novel metal free catalyst for ethylbenzene direct dehydrogenation to styrene. Chem. Commun.. 2014;50:7810-7812.
- [CrossRef] [Google Scholar]
- V2O5/Ce0.6Zr0.4O2-Al2O3 as an efficient catalyst for the oxidative dehydrogenation of ethylbenzene with carbon dioxide. ChemSusChem. 2011;4:341-345.
- [CrossRef] [Google Scholar]
- Dehydrogenation of ethylbenzene to styrene over Fe2O3/Al2O3 catalysts in the presence of carbon dioxide. Catal. Today. 2000;55:173-178.
- [CrossRef] [Google Scholar]
- Dehydrogenation of ethylbenzene over iron oxide-based catalyst in the presence of carbon dioxide. In: Inui T., Anpo M., Izui K., Yanagida S., eds. Advances in Chemical Conversions for Mitigating Carbon Dioxide. Elsevier; 1998. p. :415-418.
- [Google Scholar]
- External surface and pore mouth catalysis in hydrolysis of inulin over zeolites with different micropore topologies and mesoporosities. Catal. Sci. Technol.. 2017;7:1153-1166.
- [CrossRef] [Google Scholar]
- The Scherrer formula for X-ray particle size determination. Phys. Rev.. 1939;56:978-982.
- [CrossRef] [Google Scholar]
- Generalized gradient approximation made simple. Phys. Rev. Lett.. 1996;77:3865-3868.
- [CrossRef] [Google Scholar]
- Chemical structures of coprecipitated Fe−Ce mixed oxides. Chem. Mater.. 2005;17:2329-2339.
- [CrossRef] [Google Scholar]
- Synergy of FexCe1−xO2 mixed oxides for N2O decomposition. J. Catal.. 2006;239:340-346.
- [CrossRef] [Google Scholar]
- Preparation of Ce1−xFexO2 solid solution and its catalytic performance for oxidation of CH4 and CO. J. Mater. Sci.. 2011;46:3500-3506.
- [CrossRef] [Google Scholar]
- Catalytic behavior of manganese oxides for oxidative dehydrogenation of ethylbenzene with carbon dioxide. J. CO2 Util.. 2017;22:63-70.
- [Google Scholar]
- Optimization methods for finding minimum energy paths. J. Chem. Phys.. 2008;128:134106.
- [CrossRef] [Google Scholar]
- Highly active and stable CH4 oxidation by substitution of Ce4+ by two Pd2+ ions in CeO2(111) ACS Catal.. 2018;8:6552-6559.
- [CrossRef] [Google Scholar]
- Role of carbon dioxide in the ethylbenzene dehydrogenation coupled with reverse water–gas shift. J. Mol. Catal. A Chem.. 2004;210:189-195.
- [CrossRef] [Google Scholar]
- The dehydrogenation of ethylbenzene with CO2 over V2O5/CexZr1−xO2 prepared with different methods. J. Mol. Catal. A Chem.. 2010;329:64-70.
- [CrossRef] [Google Scholar]
- The role of CO2 in dehydrogenation of ethylbenzene over pure α-Fe2O3 catalysts with different facets. J. Catal.. 2017;345:104-112.
- [CrossRef] [Google Scholar]
- Two-step hydrothermally synthesized Ce1-xZrxO2 for oxidative dehydrogenation of ethylbenzene with carbon dioxide. J. CO2 Util.. 2019;34:99-107.
- [Google Scholar]
- Defect-rich Ce1-xZrxO2 solid solutions for oxidative dehydrogenation of ethylbenzene with CO2. Catal. Today. 2019;324:39-48.
- [CrossRef] [Google Scholar]
- Insights into the oxidative dehydrogenation of ethylbenzene with CO2 catalyzed by the ordered mesoporous V2O5–Ce0.5Zr0.5O2–Al2O3. Ind. Eng. Chem. Res.. 2019;58:21372-21381.
- [CrossRef] [Google Scholar]
- Promoting effect of Fe in oxidative dehydrogenation of ethylbenzene to styrene with CO2 (I) preparation and performance of Ce1−xFexO2 catalyst. Catal. Commun.. 2014;50:21-24.
- [CrossRef] [Google Scholar]
- Nanoflake-assembled Al2O3 -supported CeO2-ZrO 2 as an efficient catalyst for oxidative dehydrogenation of ethylbenzene with CO2. Appl. Surf. Sci.. 2017;398:1-8.
- [CrossRef] [Google Scholar]
- Flower-like Al2O3-supported iron oxides as an efficient catalyst for oxidative dehydrogenation of ethlybenzene with CO2. J. CO2 Util.. 2017;17:162-169.
- [CrossRef] [Google Scholar]
- Ce-Fe-O mixed oxide as oxygen carrier for the direct partial oxidation of methane to syngas. J. Rare Earths. 2010;28:560-565.
- [CrossRef] [Google Scholar]
- Dehydrogenation of ethylbenzene over TiO2-Fe2O3 and ZrO2-Fe2O3 mixed oxide catalysts. Catal. Letters. 1993;20:191-201.
- [CrossRef] [Google Scholar]
- Investigation of the physicochemical properties and catalytic activities of Ce0.67M0.33O2 (M = Zr4+, Ti4+, Sn4+) solid solutions for NO removal by CO. Catal. Sci. Technol.. 2013;3:688-698.
- [CrossRef] [Google Scholar]
- Solution synthesis of homogeneous plate-like multifunctional CeO2 particles. RSC Adv.. 2012;2:5976-5982.
- [CrossRef] [Google Scholar]
- Visible-light induced oxo-bridged ZrIV−O−CeIII redox centre in tetragonal ZrO2–CeO2 solid solution for degradation of organic pollutants. Phys. Chem. Chem. Phys.. 2011;13:3896-3905.
- [CrossRef] [Google Scholar]
- Determination of active site densities and mechanisms for soot combustion with O2 on Fe-doped CeO2 mixed oxides. J. Catal.. 2010;276:16-23.
- [CrossRef] [Google Scholar]
- Rational design of the carbon nanofiber catalysts for oxidative dehydrogenation of ethylbenzene. Appl. Catal. A Gen.. 2007;323:135-146.
- [CrossRef] [Google Scholar]
- Growth and surface structure of Ti-doped CeOx(111) thin films. J. Phys. Chem. Lett.. 2010;1:1714-1720.
- [CrossRef] [Google Scholar]
- CeO2 modified Fe2O3 for the chemical hydrogen storage and production via cyclic water splitting. Int. J. Hydrogen Energy. 2014;39:13381-13388.
- [CrossRef] [Google Scholar]
- Ce–Fe oxygen carriers for chemical-looping steam methane reforming. Int. J. Hydrogen Energy. 2013;38:4492-4501.
- [CrossRef] [Google Scholar]
- An ultra-stable nanosized Ce0.9Fe0.1O2 solid solution with an excellent catalytic performance towards CH4 oxidation. J. Mater. Chem. A. 2013;1:374-380.
- [CrossRef] [Google Scholar]







