Translate this page into:
Study on microwave selective treatment of mercury-containing acid mud
⁎Corresponding authors at: Faculty of Metallurgical and Energy Engineering, Kunming University of Science and Technology, Kunming, Yunnan 650093, China. truepsyche@sina.com (Kun Yang), zhanglibopaper@126.com (Libo Zhang)
-
Received: ,
Accepted: ,
This article was originally published by Elsevier and was migrated to Scientific Scholar after the change of Publisher.
Abstract
The subject of this paper is acid mud, a hazardous waste containing mercury. The current treatment process for acid mud involves high temperatures and extended roasting times. In order to address this issue, a new method utilizing microwave-assisted treatment was proposed. This method has demonstrated the effectiveness of microwave heating for acid mud treatment. The results of the study indicate that acid mud consists of PbSO4, HgS, HgSe, and HgCl2, with the main microwave absorbing material being the mercury-containing phase. A schematic diagram illustrating the microwave heating process is proposed in this study. After subjecting the acid mud to microwave treatment at a temperature of 600 ℃ for 30min, the volatilization rate of mercury was found to reach 99.94%. This rate is 6.55% higher compared to conventional roasting methods. Additionally, the microwave roasting slag exhibited increased pore size and specific surface area, indicating that microwave energy provided an improved channel for volatilization. These observations support the conclusion that microwave heating selectively targets the mercury-containing components in acid mud, thereby enhancing the volatilization rate of mercury.
Keywords
Acid mud
Microwave
Selective heating
Mercury
1 Introduction
The acid mud refers to a hazardous by-product generated during the treatment of ores with sulfuric acid. It is composed primarily of mercury and lead (Liu et al., 2017; Han et al., 2016; Bridges and Zalups, 2017). Environmental protection laws and regulations require proper management of waste containing lead and other heavy metals produced during the dilute acid washing process of flue gas. Specifically, mercury-containing acid mud must be promptly treated (UNEP, 2013; Seeley et al., 1984).
The acid mud treatment processes commonly reported in literature encompass the oxidation roasting method (Ni et al., 2014), the sulfate acid roasting method (Nong, 2004; Zhu and Li, 2017), and the calcium addition method for selenium fixation (Olli and Tian, 1991; Liang et al., 2008). The oxidation roasting method, being the earliest and simplest technique, involves the direct volatilization of mercury. However, this method has drawbacks, such as a lengthy treatment time and low efficiency in mercury volatilization (Bing et al., 2016; Raquel et al., 2013). The sulfat acid roasting method involves the use of sulfuric acid as an oxidizing agent to recover mercury. However, this method has drawbacks including the requirement of a large amount of sulfuric acid, lengthy oxidation time, and high cost consumption. Contrastingly, the calcium addition method for selenium fixation is currently the most widely employed acid mud treatment process. This technique demonstrates a high rate of mercury recovery but is also associated with disadvantages such as the consumption of significant quantities of lime, prolonged roasting time, and excessive cost consumption (Wang et al., 2013).Hence, the primary challenges faced by the current acid mud treatment processes are elevated processing temperature and prolonged roasting duration. A potential solution to these issues lies in the achievement of selective heating for the mercury-containing components within the acid mud. This approach presents a fundamental solution with the advantage of lower cost consumption.
Microwave heating is regarded as a green and efficient method, characterized by its non-contact nature. Unlike conventional heating methods, microwave heating does not require heat transmission through the interior of the material. Instead, it occurs through dipole transition and interface polarization within an electromagnetic field (Mingos et al., 1991; Ma et al., 2015; Lei et al., 2011). Extensive experimental evidence confirms that microwave heating offers several advantages including short reaction time, volumetric heating, high controllability, conduction of energy rather than heat, and selective heating (Menéndez et al., 2010; Haque, 1999). The volatilization removal process of mercury-containing compounds under microwave treatment has been investigated by Liu (Jian et al., 2018) and Xie (Huimin et al., 2019). Their studies revealed that mercury compounds have a high high-temperature dielectric loss factor, resulting in fast heating within a microwave field and leading to high efficiency in mercury removal, accompanied by relatively low energy consumption. Therefore, the microwave roasting method holds promising potential for application in acid mud treatment.
To date, there have been no reports on the treatment of acid mud using microwave technology. Therefore, our study aims to explore the reaction mechanism and the application of microwave treatment for acid mud. This investigation involves a comprehensive analysis of various factors, such as raw materials, thermogravimetric curves, high temperature dielectric properties, heat-up curves, and specific surface area analysis. Through these analyses, we aim to confirm the micro-mechanism of microwave selective heating of acid mud.
2 Material and experiment
2.1 Raw material analysis
The acid mud used in this experiment was obtained from Yunnan Chihong Zinc Germanium Company, a zinc smelting enterprise in Yunnan. The acid mud was dried and ground into powder, serving as the experimental raw material in this study. The content of main elements in the acid mud was analyzed using an AF-610A atomic fluorescence analyzer (Beijing Ruili Analyzer Co., Ltd.). The chemical composition of the acid mud is presented in Table 1. Notably, the acid mud contains a significant amount of mercury, with a content as high as 43.88 %. Therefore, it can be classified as a solid waste with a high mercury content.
Elements
Zn
Pb
Hg
Se
S
Cl
Impurity Elements
Total content (wt%)
0.37
22.04
43.88
6.89
8.71
1.68
16.43
To investigate the phase composition of mercury-containing components in the acid mud, X-ray diffraction (XRD) analysis was performed using the UltimaIV instrument. The results are depicted in Fig. 1(a). The acid mud primarily consists of PbSO4, HgSe, HgS, and HgCl2, indicating a complex phase structure in the raw material. To further determine the elemental distribution, scanning electron microscopy (SEM) analysis was conducted on the acid mud. The results are presented in Fig. 1 (b-f).The SEM mapping is clearly indicated within the red region shows that there are distinct areas of common occurrence between Hg and S, Hg and Cl, Hg and Se, as well as Pb, S, and O. This observation confirms the presence of HgS, HgSe, HgCl2 and PbSO4, which aligns with the findings from the XRD characterization. Thus, the SEM mapping results validate the XRD characterization results.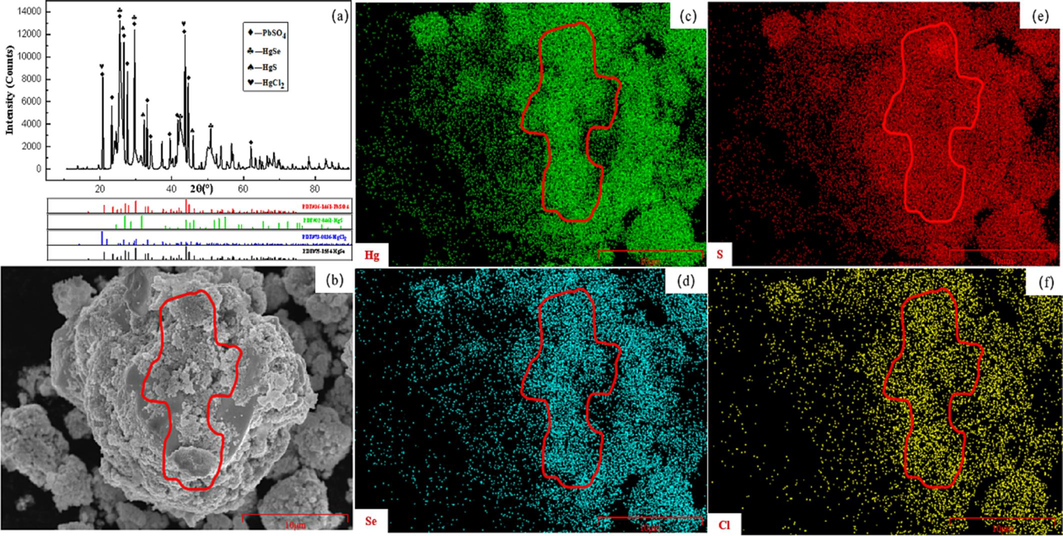
Acid mud characterization results: (a) XRD analysis of acid mud; (b-f) SEM mapping and EDS analysis of acid mud.
2.2 Instruments and measurement principles of dielectric properties
Various methods are employed to measure the dielectric performance, including the resonator method, free space method, short-circuit method, and coaxial deflection method. Among these, the cavity perturbation method offers distinct advantages, particularly in measuring small volumes and high losses in metallurgical materials. For the purpose of investigating the heating principles of the reduction process in this study, the authors utilized a medium broadband constant temperature test system (Agilent-E5071C, My Wave) to measure the complex dielectric constant of samples at different temperatures. This provided the foundation for determining the optimal microwave conditions. Fig. 2 illustrates the configuration of the test system, which primarily consists of a multi-mode cylindrical cavity (TM0n0), an air pump, a temperature controller, heating equipment, a water cooler, and a Vector Network Analyzer (Agilent-N5230C).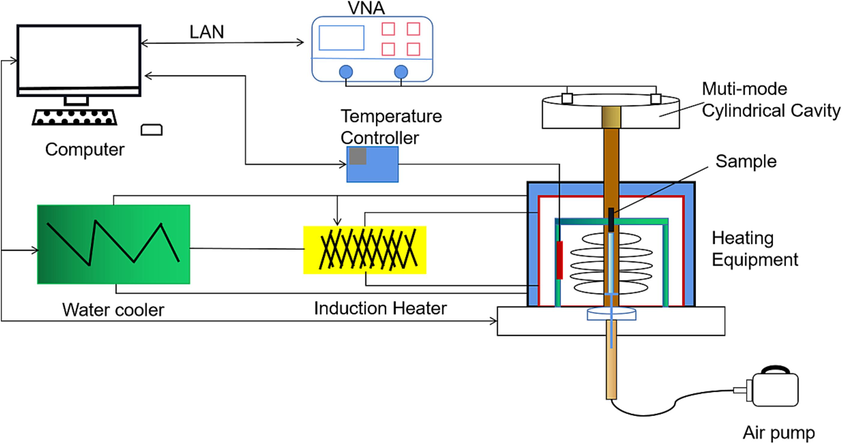
Dielectric experimental setup diagram.
To measure the dielectric performance, the sample for the drying test is inserted into a small quartz tube and positioned in the resonator at the maximum magnetic field. The heating device is located on the side, and an air pump is used to connect the resonator with the heating device. This setup allows for the sample to be pushed into the resonator once it reaches the desired temperature. The heating device is primarily composed of a copper coil, graphite rod, and thermocouple. The experimental temperature is achieved through vorticity generated by each coil situated in the scaffold chamber. Quartz tubes are positioned within a graphite rod that connects to the coil's center, where an s-type thermocouple is placed. The measured temperature corresponds to the temperature of the graphite rods, albeit with a certain margin of error. To minimize these errors, we have conducted multiple corrections during the equipment manufacturing process, ensuring that the errors remain within 3 %. Measurements are taken within a temperature range of 25–600 °C, with a 2-minute holding time for each temperature point. The dielectric constant was measured at an ambient temperature of 50 °C using a microwave frequency of 2450 MHz. The dielectric parameters of the sample were obtained through cavity calibration, container calibration, sample testing, and subsequent computation of the complex dielectric constant. The cavity perturbation method is based on the theory that loading a material perturbs the field distribution, resulting in changes in resonance frequency and quality factor. The determination of the dielectric constant and dielectric losses relies on calculating the resonant frequencies and quality factors before and after material loading.
2.3 Characterization method
The phase compositions of the acid mud were determined using an UltimaIV X-ray diffraction instrument. The micromorphology and microcompositions of the samples were analyzed using an FEI QUANTA FEG 450 scanning electron microscope equipped with energy-dispersive spectroscopy (SEM-EDS). The thermal weight analysis (TGA) was conducted using a Netzsch TG 209 F1 instrument from Germany. The TGA test conditions involved a heating rate of 5 °C/min and an argon flow rate of 60 mL/min. The complex dielectric constant (εr'), complex dielectric loss (εr“), and dielectric loss angle tangent (tanδ) of the samples were measured using a self-made high-temperature dielectric testing platform with a frequency of 2450 MHz. The BET analysis was performed using a Quantachrome-EVO instrument for determining the specific surface area.
3 Results with discussion
3.1 Study on thermal decomposition properties
The heat map detection method was used to analyze the thermochemical properties and thermal decomposition behavior of the acid mud, with a heating rate of 5 °C/min. The experimental setup involved conducting the test in an inert gas atmosphere. The results of the analysis are depicted in Fig. 3.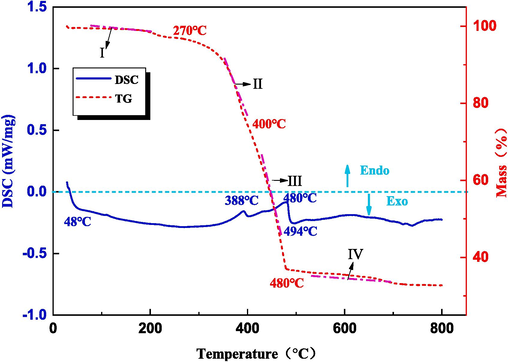
TG-DSC curves of acid mud.
The TG curve in Fig. 3 reveals that the weight loss of the acid mud can be divided into four stages. In the first stage, which occurs between 0 and 270 ℃, weight loss initiates at around 50 ℃. This can be attributed to the evaporation of moisture and crystal water present in the acid mud. The second stage, spanning from 270 to 400 ℃, exhibits an increased rate of weight loss.The weight loss observed during the third stage, which occurs between 400 and 480 ℃, reaches its peak value. This can be attributed to the steam-stripping and volatilization of HgS and HgSe present in the acid mud at high temperatures. Finally, in the fourth stage above 480 °C, the materials reach a balance as there are no significant changes in the composition. This is because the high-temperature easily decomposable substances in the acid mud have undergone complete decomposition. The TG curve presented in the figure provides a theoretical calcination temperature for future exploration of the volatilization of the mercury phase.
Based on the DSC curve depicted in the figure, four distinct thermal peaks can be identified. The first peak, occurring at 48 °C, displays an endothermic reaction associated with the volatilization of surface water and bound water in the material. The second peak, observed at 388 °C, exhibits an exothermic reaction attributed to the volatilization of HgCl2. The third peak, observed at 480 °C, corresponds to an endothermic reaction resulting from the recombination of mercury vapor and selenium, forming HgSe. Lastly, the fourth peak, occurring at 494 °C, represents an exothermic reaction due to the energy absorption required for the combustion of HgSe and mercury sulfide. These observations align with the weight loss patterns observed in the TG curve. It can be deduced from these findings that the volatilization of the mercury phase occurs at temperatures higher than 480 °C.
3.2 The micro-mechanism of microwave selective heating
In this study, the high-temperature dielectric properties of the acidic mud phase were analyzed to examine the microscopic mechanism of microwave selective heating. Fig. 4 presents the determined high-temperature dielectric properties, including the dielectric constant (εr'), dielectric loss coefficient (εr“), and loss tangent coefficient (tanδ), of the acid mud and its compositions.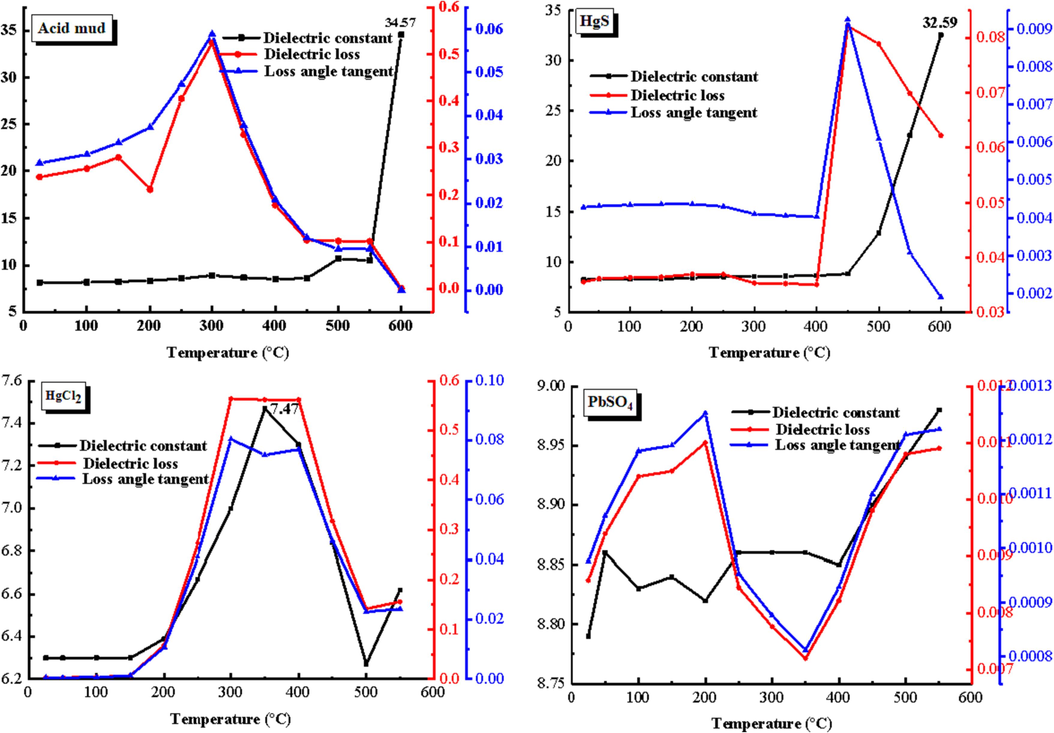
High temperature dielectric properties of acid mud and compositions.
One factor that contributes to the change in permittivity is the dipole moment, an inherent characteristic of polarized molecules. Increasing the temperature causes the reorientation of dipole moments in polar molecules, leading to a heightened directional polarization of these dipoles. Consequently, the orientation of polar molecules is favored, resulting in an increase in permittivity. In the case of the acidic mud, elevating the temperature will induce directional polarization in polar molecular dipole moments such as HgSe, HgCl2, and HgS. This phenomenon enhances the dielectric loss and subsequently enhances the wave absorbing performance.
The figure clearly illustrates the increase in dielectric constant of acid mud as temperature rises from 0 to 450 °C. This increase signifies the occurrence of noticeable dipole moment orientation polarization. In the case of HgCl2, its permittivity initially increases and then decreases, reaching its maximum dipole moment orientation polarization at 350 °C. On the other hand, the permittivity of PbSO4 remains relatively unchanged at approximately 8.9, indicating that temperature has minimal effect on the directional polarization of its dipole moment. Analyzing the changes in dielectric coefficients of acid mud and its components, it becomes evident that high temperature aids in the dipole moment directional polarization of molecules containing mercury. As a result, it enhances the overall polar moment directional polarization of the acid mud, highlighting the advantages of microwave roasting in acid mud treatment.
The determination of a polymer material's polarity relies on its dielectric constant. In general, substances with a relative permittivity greater than 3.6 are considered polar, those with a relative permittivity between 2.8 and 3.6 are classified as weakly polar, and those with a relative permittivity below 2.8 are categorized as non-polar (Kavanagh, 1975). With a dielectric constant of 8.17 (F/m), acid mud is determined to be a polar substance. When the temperature is in the range of 0 to 300℃, the dielectric loss coefficient of acid mud increases with rising temperature. At 300℃, the dielectric loss reaches 0.524 (F/m). This increase is attributed to the volatilization of certain HgCl2 components present in the acid mud. As the temperature range extends from 300 ℃ to 600 ℃, the dielectric loss coefficient of the acid mud decreases. This is because HgCl2 has completely evaporated, and phase transformations occur in HgSe and HgS within the acid mud. When the temperature reaches 600 ℃, the dielectric constant of the acid mud reaches 34.57 (F/m). This significant increase in dielectric constant is likely due to the temperature-induced transformation of red HgS into black HgS, resulting in a sudden change in the dielectric properties.
Based on the microwave electromagnetic characteristics observed in the acid mud, it is evident that the main absorbing material within the mud is the mercury-containing component. During microwave heating, the directional polarization of the molecular dipole moment of this component increases, which proves beneficial for the microwave treatment of the acid mud. At 300℃, some HgCl2 evaporates, leading to an increase in the dielectric loss of the acid mud. As the temperature further rises, HgSe and HgS undergo combustion, resulting in a reduction of the mercury-containing components and consequently a decrease in dielectric loss. Nonetheless, when the temperature surpasses 600 °C, the red HgS undergoes a transformation into black HgS, which triggers an abrupt change in the dielectric properties.Hence, when subjected to microwave heating, the mercury-containing components of the acid mud experience heating prior to other phases. This selective heating enables quicker roasting and volatilization of the mercury-containing components.
3.3 Analysis of microwave heating characteristics
The acid mud was subjected to microwave heating at power levels of 500 W, 700 W, and 900 W. The corresponding heating curves are presented in Fig. 5. The time required to heat the acid mud to a temperature of 500 ℃ was determined to be 700 s, 550 s, and 400 s for the respective power levels. The thermal behavior of the acid mud can be divided into three stages based on the upward trend observed. These stages include the temperature ranges of 25–200 ℃, 200–300 ℃, and 300–500 ℃. During the 25–200 ℃ stage, the heating rate was remarkably fast due to the strong wave-absorbing ability of the surface water and crystal water present in the acid mud. In the 200–300 ℃ stage, the heating rate decreased since the volatilization of mercuric chloride in the acid mud did not possess the same wave-absorbing capacity as water. However, during the 300–500 ℃ stage, the baking of mercury sulfide and HgSe in the acid mud led to the volatilization of mercury vapor, resulting in enhanced wave-absorbing ability and an increased heating rate. These findings demonstrate the excellent microwave-induced performance of the acid mud as observed in the microwave external field effect.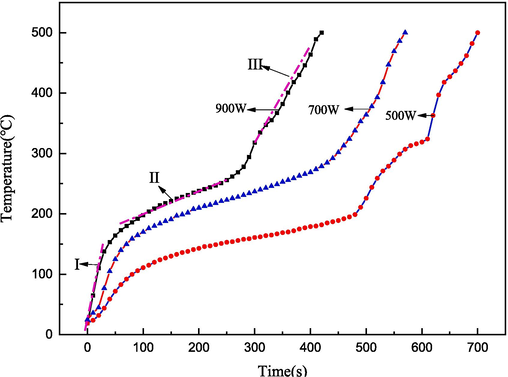
The heat-up curve of acid mud.
Fig. 6 presents a schematic diagram illustrating the microwave heating mechanism of acid mud. Initially, the microwave selectively heats the mercury-containing group elements, which exhibit a higher dielectric loss factor within the mud. The mercury-containing group constitutes a magnetoresistant material with significant conductivity in the acid mud, primarily controlled by conductive polarization within the microwave field. The absorption of microwave energy by the mercury-containing group element is primarily facilitated through the thermoconduction of the acid mud. This stage acts as the primary heat source for inducing metapolarization in the mercury-containing component. The dominance of the mercury-containing element and lead sulfate in the acid mud necessitates the dielectric constant and dielectric loss of the mercury-containing element to exceed that of lead sulfate. This property enables the mercury-containing element to absorb and heat the lead sulfide blocks more efficiently upon exposure to microwave radiation. As a result, the selective heating of mercury-containing components by microwave radiation reduces their absorption energy on the surface of the lead sulfide blocks, thereby enhancing the thermolysis process of these components.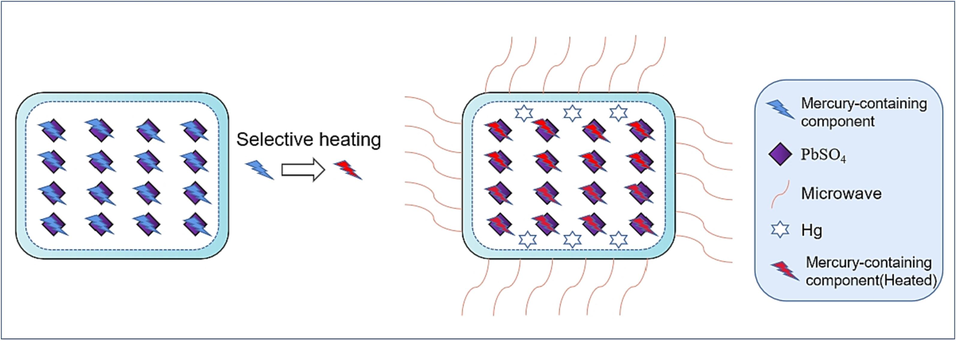
Schematic diagram of microwave heating process for acid mud.
3.4 Advantages of microwave roasting acid mud
3.4.1 Volatilization of mercury-containing components
Separately weigh 50g of acid mud in the same ark.The acid mud was subjected to roasting in both a microwave field and a conventional field separately. Following roasting at various temperatures for a duration of 30 min, the mercury content of the resulting roasting slag was measured to determine the efficiency of mercury volatilization. Fig. 7(a) presents the corresponding results.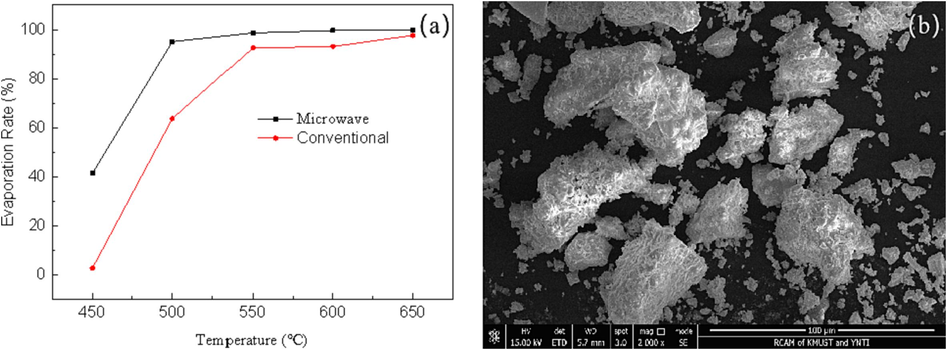
Mercury volatilization rate (a)and SEM analysis of roasting slag(b).
When baked at a temperature of 600℃, the volatilization rate of mercury was found to be 99.94 % under microwave conditions, whereas under conventional conditions, the volatilization rate of mercury was only 93.39 %. This significant difference demonstrates the superiority of microwave heating in facilitating the volatilization of mercury. Fig. 7(b) illustrates the remaining roasting slag following the process. It can be observed that the powder of the mercury component, such as the flocculent structure, has vanished, and the surface of the roasting slag has become smooth. Consequently, it can be concluded that microwave field heating offers distinct advantages for the selective heating of mercury-containing components.
3.4.2 Analysis of specific surface area of roasting slag
To examine the underlying reasons behind the improvement of the volatilization rate of mercury-containing components under microwave field, we conducted a BET analysis on the roasting slag.The results are shown in Fig. 8, where Ad denotes the adsorption curve and De denotes the desorption curve.
BET model of roasting slag.
The N2 adsorption/desorption curve was used to apply the BET formula in order to calculate the specific surface area of the roasting slag under normal conditions. The C constant was determined to be 29.518 and the correlation coefficient was observed to be 0.9976, indicating a high level of calculation reliability. The total pore volume was found to be 1.386e−03 cc/g, while the average pore diameter was measured at 29.93 nm.Similarly, the BET formula was employed to calculate the specific surface area of the roasting slag under microwave conditions. The C constant was determined to be 12.440, with the correlation coefficient indicating a high calculation reliability of 0.9947. The calculated specific surface area was determined to be 0.236 cm3/g, representing a 27.27 % increase in microwave-specific surface area. The total pore volume was measured to be 8.289e−04 cc/g, while the average pore diameter was determined to be 14.04 nm. These results clearly demonstrate that the application of a microwave field can effectively expand the reaction area, leading to a more thorough roasting of mercury-containing components and a faster and more efficient volatilization process.
To simulate the pore size distribution, we employed a DFT model in Fig. 9(a,b). The results revealed that under both conventional and microwave calcination conditions, the pores were primarily distributed within the range of 10 to 50nm. Furthermore, all of the observed pore sizes were classified as mesopores.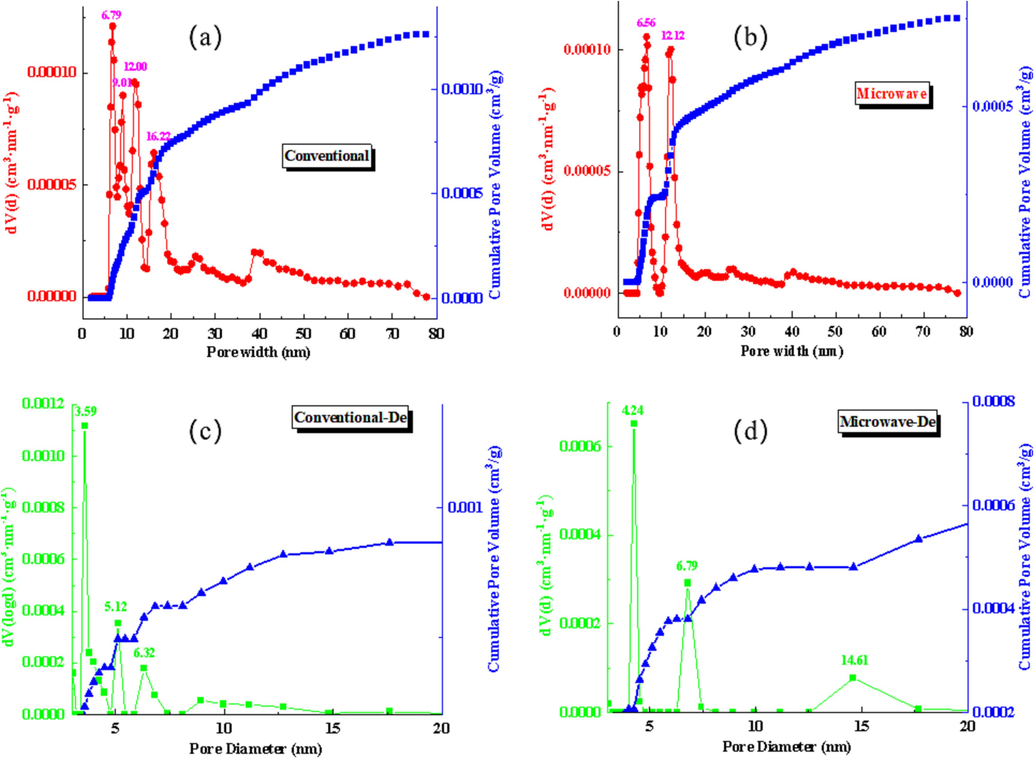
Roasting slag DFT model(a,b)and Roasting slag BJH model(c,d).
The pore size distribution of the lead slag was determined using the BJH model in Fig. 9(c,d). It was observed that the desorption curve exhibited a false peak at 0.38 nm, which was primarily influenced by the data from the adsorption curve. Under normal conditions, the pore size distribution of the roasting slag was primarily found to be concentrated at 3.59 nm, 5.12 nm, and 6.32 nm. However, under microwave conditions, the pore size distribution of the roasting slag shifted to larger sizes, with the main peaks observed at 4.24 nm, 6.79 nm, and 14.61 nm. This indicates that microwave conditions can result in the formation of larger pore sizes.The use of microwaves in the process provides larger volatilization channels, leading to higher rates of volatilization for mercury-containing components within the microwave field.
To provide a more intuitive comparison of the pore size between microwave-roasted slag and conventionally-roasted slag, separate SEM analyses were conducted for each (Fig. 10). The results clearly demonstrate that the pore size of microwave-roasted slag is larger than that of conventionally-roasted slag.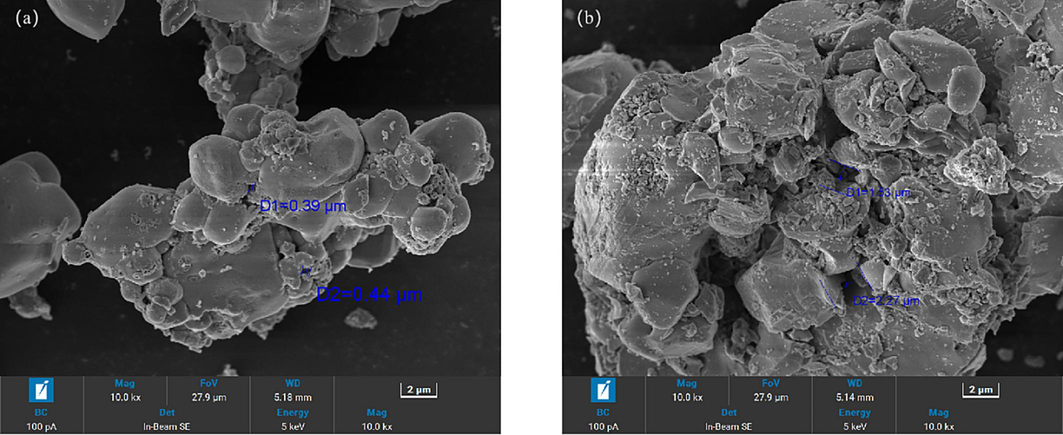
Conventional roasting slag(a) and microwave roasting slag(b).
In the process of microwave radiation, it has selective heating characteristics. Microwave heating can promote the opening and cross-linking of closed and blind pores in the medium and improve the hard-shell crusting of the material(Wang et al., 2018; Song et al., 2017).This process leads to an increase in the pore size of microwave-roasted slag, as well as an increase in the specific surface area. Additionally, the application of microwave energy provides improved pathways, resulting in a higher volatilization rate of mercury.
4 Conclusion
To address the limitations of high treatment temperature and long roasting time in the acid mud treatment process, microwave technology was employed for selective heating of the acid mud. Following roasting at 600℃, the volatilization rate of mercury under microwave conditions reached an impressive 99.94 %. In contrast, under normal conditions, the volatilization rate of mercury was only 93.39 %, highlighting a substantial increase of 6.55 % in the volatilization rate achieved through microwave roasting.Using our self-developed microwave dielectric test platform, we conducted high-temperature dielectric tests on acid mud and its components using the resonant cavity perturbation method. Our findings indicated that the primary microwave-absorbing substance in the acid mud was the mercury-containing phase. Additionally, it was observed that the dipole moment of mercury-containing molecules increased with rising temperatures, leading to an enhanced heating rate. Specifically, within the 300℃ range, the volatilization of HgCl2 contributed to an increase in the dielectric loss of the acid mud. Furthermore, the phase transition of HgSe and HgS resulted in an additional increase in the dielectric loss. Consequently, the selective heating of the acid slurry was successfully achieved.This observation was supported by BET and SEM analyses, which revealed an enlargement in the pore size and specific surface area of the slag following microwave roasting. It is evident that microwave energy creates a more efficient pathway for the roasting of acid mud, leading to an improvement in the volatilization rate of mercury.
Acknowledgements
This work was supported by National Natural Science Foundation of China [grant number 52204361] and Yunnan Science and Technology Talent and Platform Plan [grant number 2019HB081].
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
References
- The effect of selenite on mercury re-emission in smelting flue gas scrubbing system. Fuel. 2016;168:7-13.
- [Google Scholar]
- Mechanisms involved in the transport of mercuric ions in target tissues. Arch. Toxicol.. 2017;91(1):63-81.
- [Google Scholar]
- Study on the leaching of mercuric oxide with thiosulfate solutions. Metals. 2016;6(9):206.
- [Google Scholar]
- Microwave energy for mineral treatment processes—a brief review. Int. J. Miner. Process.. 1999;57(1):1-24.
- [Google Scholar]
- Microwave heating-assisted pyrolysis of mercury from sludge. Mater. Res. Express. 2019;6(1):015507
- [Google Scholar]
- Kinetics of the volatilization removal of mercury from mercury sludge by microwave heating. Mater. Res. Express. 2018;5(10):105503
- [Google Scholar]
- Effect of polymer adsorption on the properties of the electrical double layer. Faraday Discussions of the Chemical Society. 1975;59(1):242-249.
- [Google Scholar]
- Application of Microwave Selective Heating on Mining and Metallurgy Processing. Materials Review. 2011;25(15):119-122.
- [Google Scholar]
- Research progress on technology of dealing with copper anode slime. GOLD. 2008;29(12):32-38.
- [Google Scholar]
- Study on non-isothermal kinetics of the thermal desorption of mercury from spent mercuric chloride catalyst. J. Hazard. Mater.. 2017;322:325-333.
- [Google Scholar]
- Microwave dielectric properties and temperature increasing characteristics on zinc oxide dust with high content of Cl. Journal of Central South University(science and Technology). 2015;046(002):410-415.
- [Google Scholar]
- Microwave heating processes involving carbon materials. Fuel Process. Technol.. 2010;91(1):1-8.
- [Google Scholar]
- Application of microwave dielectric heating effects to synthetic problems in chemistry. Chem. Soc. Rev.. 1991;20:1-47.
- [Google Scholar]
- Speciation of mercury in sewage sludge and its dynamic changes in drying process. Acta Scientiae Circumstantiae. 2014;34(5):1262-1267.
- [Google Scholar]
- Nong, D., 2004. Process Improvement in Copper Anode Slime Treatment. China Nonferrous Metall. 33(6), 44–46, 49.
- Recovery of selenium and tellurium from copper refinery anode mud. China Nonferrous Metall.. 1991;1:19-24.
- [Google Scholar]
- Influence of Limestone Characteristics on Mercury Re-emission in WFGD Systems. Environmental Ence & Technology. 2013;47(6):2974-2981.
- [Google Scholar]
- Development of processes for the solubilization of uranium from waste leach residue. Oak Ridge National Lab. 1984
- [Google Scholar]
- Coal slime hot air/microwave combined drying characteristics and energy analysis. Fuel Process. Technol.. 2017;156:491-499.
- [Google Scholar]
- Research on Extraction of Selenium From Acid Mud Containing Selenium. Hydrometallurgy of China. 2013;5:316-318.
- [Google Scholar]
- Pore structure development in Xilingol lignite under microwave irradiation. J. Energy Inst.. 2018;91(1):75-86.
- [Google Scholar]
- Study on the recovery of selenium and mercury from acid mud containing selenium and mercury. In: Proceedings of the National Seminar on Smelting Technology of Tin, Antimony and Mercury and Comprehensive Recovery Technology and Equipment of Heavy Metal Smelting. 2017. p. :70-74.
- [Google Scholar]







