Translate this page into:
Study on thermal behavior of hazardous substances in the process of preparing corundum from black aluminum dross
⁎Corresponding authors. liufq@ustb.edu.cn (Fengqin Liu), zhaohl@ustb.edu.cn (Hongliang Zhao)
-
Received: ,
Accepted: ,
This article was originally published by Elsevier and was migrated to Scientific Scholar after the change of Publisher.
Peer review under responsibility of King Saud University.
Abstract
The black aluminum dross (BAD) from aluminum recycling industry is considered to be a hazardous material due to containing a large amount of AlN and fluoride salt. The potential environmental problems caused by BAD restrict the sustainable development of the aluminum recycling industry. A calcining-leaching process for preparing α-Al2O3 by using BAD is proposed in this paper. The transformation mechanism of toxic substances is discussed in detail based on the analysis of TG-DTG-DSC&MS. The results show that the reaction between AlN and O2 occurs in the range of 500–1000 °C. Most of the gaseous products are N2, and there is also a small amount of NO. The average conversion rate is 0.087 mg/min. The average volatilization rate of chloride salt in BAD under Ar atmosphere is 0.065 mg/min, and the average volatilization rate under Ar + O2 atmosphere is 0.089 mg/min, which is slightly higher than that of pure Ar atmosphere. The BAD processed by calcination-leaching process contain more than 95 % corundum and magnesia-aluminum spinel, which can be used as high-quality raw materials for the preparation of refractory materials.
Keywords
Aluminum recycling
Black aluminum dross
Aluminum nitride
α-Al2O3
TG-DTG-DSC&MS
1 Introduction
Aluminum is one of the most widely used non-ferrous metals in the world. Most aluminum products will not be corroded during their service due to the high corrosion resistance. Almost all aluminum can be recycled except for some aluminum chemical containers and devices. Recycling demands only 5 % of the energy required to produce primary aluminum (Brough and Jouhara, 2020).
The recovery and regeneration of aluminum is considered to be an effective measure of resource recycling. In the aluminum regeneration process, the waste aluminum products are heated and melted in the smelting furnace, and the flux is added to reduce the oxidation of the aluminum liquid and remove some impurities (Kvande and Drabløs, 2014). BAD is produced in this process (Liu and Chou, 2013; David and Kopac, 2012). Aluminum nitride is inevitably formed when molten aluminum is in contact with N2 in the air (Lv et al., 2020). The aluminum nitride in BAD has high reactivity and can release toxic ammonia gas in a humid environment, which can cause human health problems (Liu et al., 2021). A variety of salts in BAD can cause soil salinization (Mahinroosta and Allahverdi, 2018). Trace amounts of toxic elements such as fluorine will cause environmental pollution if they enter the soil and groundwater. Millions of tons of BAD are produced worldwide each year. Untreated storage and burial will cause serious pollution problems (Xie et al., 2020). At present, BAD has been included in China's National Hazardous Waste List due to its high reactivity (hazardous waste code: 321–224-48, 321–226-48, 321–34-48).
Although BAD contain harmful substances, aluminum nitride, it also contains rich alumina resources (Zuo et al., 2021). About 30–40 % of corundum (alpha alumina) in BAD is difficult to extract, and about 20 % of aluminum nitride with high reactivity is easy to hydrolyze, and harmful ammonia gas will be released. Some researches on the recycling and utilization of BAD have been carried out. Including the preparation of alumina (El-Katatny et al., 2003; Türk et al., 2020; Guo et al., 2018), aluminum chloride and aluminum sulfate water purifiers (Amer, 2002; Amer, 2010), steelmaking refining agents (Cheng et al., 2013), building and road materials (Uehara et al., 1998), refractory materials, (Li et al., 2014; Sooksaen and Puathawee, 2016) etc. Most of the researches remain in the laboratory-level stage due to economic infeasibility or technical problems (Abdulkadir et al., 2015; Dash et al., 2008).
Corundum is widely used in metallurgy, machinery, chemical and other industrial fields because of its excellent high-temperature properties and mechanical strength. Yang (Yang et al., 2014) used plasma as a high temperature source for BAD to produce corundum. The results show that pure white alumina powder can be obtained when the radio-frequency plasma power reaches 3.5 kW, and impurities enter the gas phase. The d50 of the powder is 8 μm and the purity is 99.95 %. Obviously, a high-purity alumina product is obtained in this way. The cost is expensive, and the reaction behavior of BAD at high temperature is still unclear. Das (Das et al., 2007) washed the soluble salts in BAD with water, and added sulfuric acid to form an aluminum sulfate solution. Then aqueous ammonia is added as a precipitant to generate Al(OH)3 precipitation. Finally, η-alumina with good surface activity was obtained after being calcined at 900 °C. Researchers have extensively studied the degradation of aluminum nitride in aqueous environments and found that formation of amorphous AlOOH takes place and after 16 h, the crystalline hydroxide Al(OH)3 forms (Meshram and Singh, 2018). The energy consumption of this method is low, but ammonia gas will be released violently when BAD is in a liquid environment. Ammonia gas is harmful when it occurs in uncontrolled landfills. However, ammonia is also a valuable product, and controlling hydrolysis may lead to the recovery of this compound (Jiménez et al., 2022).
In summary, the harmless treatment for BAD can be divided into hydrometallurgy and pyrometallurgy (Zuo et al., 2021). Hydrometallurgy is to convert aluminum nitride in BAD into ammonia, while pyrometallurgy is to convert aluminum nitride into nitrogen or nitrogen oxide. Considering the practical application difficulty and working environment, pyrometallurgy is considered to be a promising removal method of the hazardous material in BAD. Pyrometallurgical methods, like rotary salt furnaces and salt-free technologies, are industrially applied for recovery of aluminum. Many hydrometallurgical technologies have been patented that consume aluminum dross to produce aluminum alum and other industrially applicable products (Meshram and Singh, 2018).On the laboratory research front, hydrometallurgy and hydrothermal processes emerge as promising routes of dross recycling (Srivastava and Meshram, 2023). However, the mechanism of the phase transition of BAD in the high temperature process and the transformation mechanism of toxic substances has not been further studied. At present, the qualitative and quantitative analysis of thermal behavior such as the conversion and release characteristics of aluminum nitride and chloride salts has not been carried out, and the relevant basic data are rare. It is difficult for designers to determine operating conditions such as temperature and residence time if quantitative conversion data cannot be obtained. It is also difficult to choose a purification device such as a denitrification device if the composition and quantity of the flue gas, especially pollutant gases such as NH3 and NOx, are not clear. Therefore, it is of great significance to explore the conversion and release characteristics of aluminum nitride and chloride during BAD heat treatment, and to provide qualitative and quantitative data on the gas components released in the flue gas. The research on the preparation of corundum by BAD is carried out, and the analysis of TG-DTG-DSC&MS for the reaction of BAD at high temperature is discussed in detail in this paper.
2 Experimental section
2.1 Raw material preparation
The BAD used in this study comes from an aluminum alloy processing plant in southern China. First, the metal aluminum in BAD is pre-extracted away. Then it is finely ground to −100 mesh. Finally, it was dried at 95 °C for 2 h and put into a sealed bag for later use.
2.2 Experimental method
2.2.1 Preparation of corundum
Accurately weigh 150 g of BAD and divide them into five crucibles (each crucible contains about 30 g of sample). The BAD was calcined at 1200 °C for 2 h. The atmosphere of calcination is air. The calcined BAD were leached with water at 30 °C for 1 h, with a liquid–solid ratio of 5:1. After being leached by water, the BAD were continuously leached with 3 mol/L HCl at 30 °C for 1 h, with a liquid-to-solid ratio of 5:1. The phase and chemical composition of the products at different stages are tested, and the mass loss rate is also calculated.
2.2.2 TG-DTG-DSC&MS analysis
The measurement was performed on a Netzsch STA 449F3 thermal analyzer and a quadrupole mass spectrometer (QMS) 403C Aëolos. The measurement under an inert atmosphere is performed under high-purity analytical grade argon and a flow rate of 100 mL·min−1 at a heating rate of 10 K·min−1. The oxidation measurement is performed under 65 mL·min−1 Ar and 5 mL·min−1 oxygen. All samples were weighed approximately 15 or 30 mg in a corundum crucible and heated from room temperature to a maximum of 1100 °C, and a controlled temperature program was used. Each set of measurements was repeated twice to verify the results. The detailed conditions of TG-DTG-DSC&MS measurement are shown in Table 1. Explanation: -a is Ar atmosphere; -b is O2 atmosphere.
No.
Sample
Mass (mg)
Argon flow
(ml/min)Oxygen flow
(ml/min)Heating rate
(K/min)
1-a
BAD
15.0
100
0
10
1-b
BAD
30.0
65
5
10
2-a
BAD + CaO
30.0
100
0
10
2-b
BAD + CaO
30.0
65
5
10
2.2.3 Tests and characterization
The phase component of sample is determined by X-ray diffraction analysis by using a PW 1710 diffractometer (XRD, Philips-Netherlands) and the morphology of the powders was tested using JSM-7500F scanning electron microscope (SEM, JOEL-Japan). The composition of the samples in the experiment was analyzed using X-ray fluorescence spectrometer (mAX, AXIOS, Netherlands).
3 Results and discussion
3.1 Thermodynamic analysis
The main aluminum-containing compounds in BAD are Al, Al2O3, AlN, and MgAl2O4. It also contains other impurities such as chloride (NaCl, KCl) and fluoride (CaF2). The possible reactions of these substances during the calcination process are as follows:
The Gibbs free energy of Eqs. (1)–(6) is shown in Fig. 1. As shown in Fig. 1a, the Gibbs free energy of NaCl, KCl, and CaF2 from solid phase to gas phase transition decreases with the increase of temperature. When the temperature reaches the boiling point of the substance (NaCl = 1465 °C, KCl = 1420 °C), the reaction Gibbs free energy △G < 0, the reaction can proceed spontaneously. The fluorite is difficult to transform into the gas phase during the calcination process because of its high boiling point (2500 °C).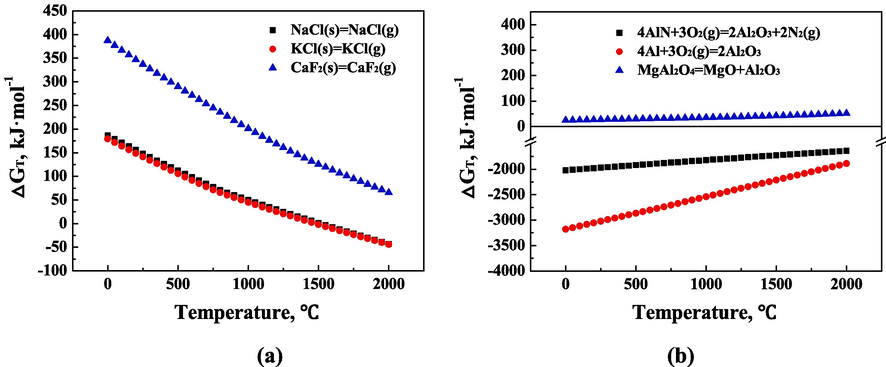
(a) Gibbs free energy of the transition of chloride, fluoride and (b) aluminum-containing compounds in BAD.
As shown in Fig. 1b, the Gibbs free energy of the oxidation reaction of metallic aluminum and aluminum nitride is less than zero, and the reactions can proceed spontaneously. However, the Gibbs free energy of the decomposition reaction of magnesium aluminum spinel is greater than zero, and the reaction is difficult to proceed spontaneously.
The thermodynamic analysis results show that the chloride salt in BAD can be volatilized and turned into gas phase, and metallic aluminum and aluminum nitride will decompose and turn into alumina and nitrogen under suitable calcination conditions. The fluorite and magnesium aluminum spinel will not be volatilized or decomposed, and will remain in BAD.
3.2 Thermal behavior of BAD under Ar and Ar + O2 atmospheres
The thermal behavior of aluminum nitride and chloride in BAD, such as the conversion and release characteristics, is still unclear. The TG-DTG-DSC&MS method is used in this section to qualitatively and quantitatively analyze the thermal behavior of harmful substances in BAD in high-temperature processes.
According to the results of thermogravimetric-mass spectrometry (TG-MS) in the Ar atmosphere in Fig. 2, BAD samples have been observed to have mass loss behavior in the temperature range of 180–500 °C and 800–1000 °C, which indicates that there is gas escape or salt volatilization. The mass spectrometry detection result in Fig. 2(b) shows that in the temperature range of 180–500 °C, three gases, H2O, NH3, and CO2, are generated. It is speculated that H2O mainly comes from the release of crystal water, and the generated H2O reacts with AlN in BAD to generate NH3 (Eq. (7)). For the generation of CO2 gas, it may come from the decomposition reaction of MgCO3 (Eq. (8)).
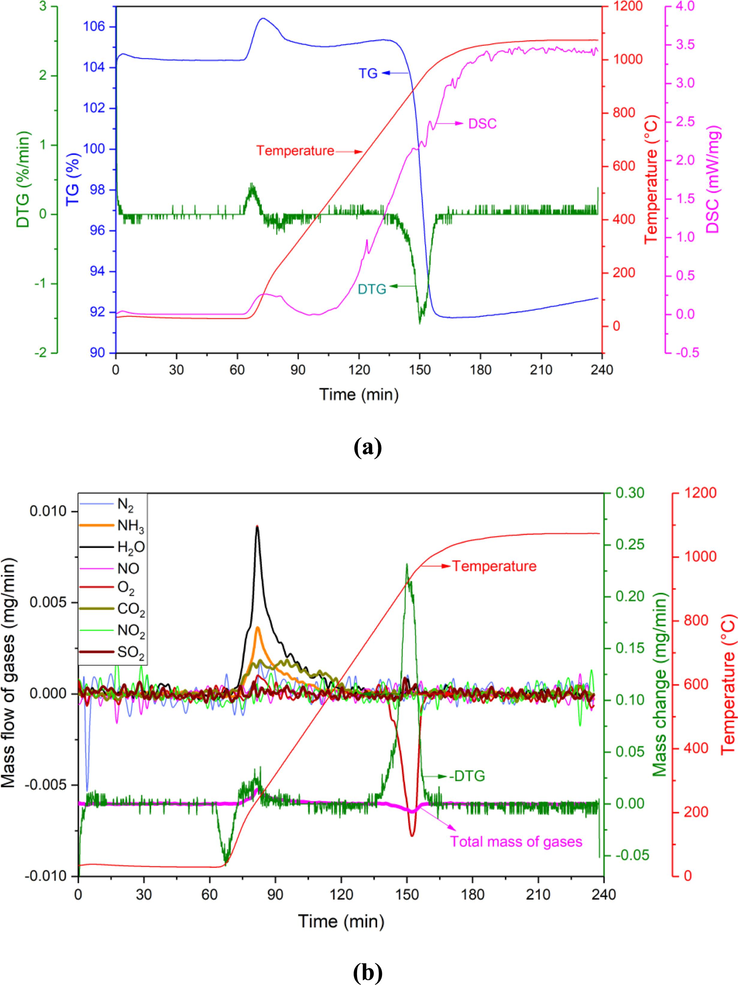
(a) TG-DTG-DSC and (b) MS of BAD in Ar atmosphere.
No gas escapes in the temperature range of 800–1000 °C, and its mass loss is mainly caused by the volatilization of three low-melting substances such as Al, NaCl, and KCl. Although the consumption of O2 is detected at this stage, the amount of Al2O3 produced by Al and O2 is very small, and the mass increase of oxidation is less than volatilization loss of Al, NaCl, and KCl. It can be calculated that the total mass loss by volatilization of Al, NaCl, and KCl in the range of 800–1000 °C is 1.96 mg, and the average volatilization rate is 0.065 mg/min, which accounts for 13.1 % of the total mass of BAD.
According to the results of thermogravimetric-mass spectrometry in the Ar + O2 atmosphere in Fig. 3, it can be seen that three gases, H2O, NH3, and CO2, are generated in the range of 180–500 °C, and their reaction behavior is similar to that in the Ar atmosphere. But in the range of 500–1000 °C, the mass loss behavior and gas volatilization of BAD have changed greatly compared with the Ar atmosphere. The mass of the BAD sample increases first and then decreases. The inflection point (TG peak) appears at 850 °C, a large amount of O2 is consumed, and a large amount of N2 and a small amount of NO and CO2 are also escaped. CO2 mainly comes from the decomposition of carbonates. N2 and NO are released during the reaction between AlN and O2. Therefore, the decomposition reaction of aluminum nitride during the calcination stage should also include the following reaction (Eq. (9)).
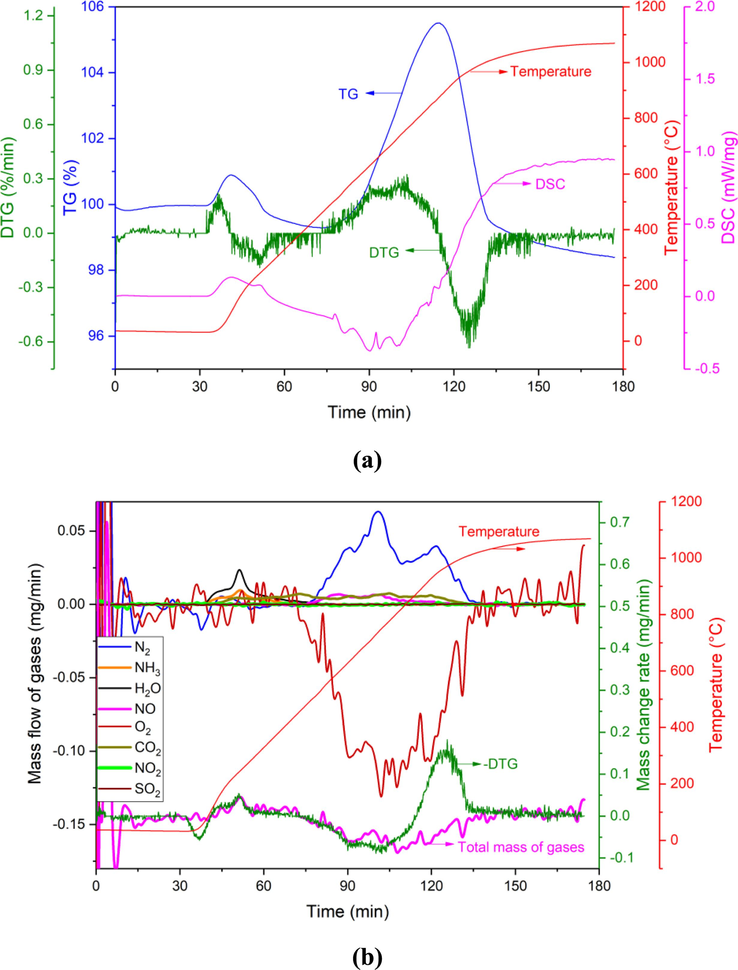
(a) TG-DTG-DSC and (b) MS of BAD in Ar + O2 atmosphere.
Furthermore, from the DTG and DSC curves in Fig. 3(a), it can be seen that in the range of 500 °C-800 °C, DTG gains mass and DSC releases heat. At this stage, the oxidation reaction of AlN and Al mainly occurs (Eqs. (4)–(5)). In addition, since the melting point of Al is 660 °C, a small amount of Al will volatilize as vapor at this stage. However, in an O2 atmosphere, the amount of oxidation will be much greater than the amount of volatilization.
From the analysis results, as well as the reaction Eq. (4) and reaction Eq. (9), the calculation shows that the mass of AlN in BAD is 5.53 mg, which accounts for 36.9 % of the total mass of BAD. The reaction between AlN and O2 is mainly to produce N2, and about 5 % of AlN reacts with O2 to produce NO gas. The reaction between AlN and O2 is very slow, the reaction is complete within 63 min, and the average conversion rate is 0.087 mg/min. In addition, combined with reaction Eq. (5), it can be inferred that the mass of oxidized metallic aluminum is 1.41 mg, accounting for 9.4 %. And comparing the DTG and “Total mass of gases” curves in the range of 500 °C–800 °C in Fig. 3(b), it can be seen that they are relatively consistent, indicating that the mass loss caused by the volatilization of NaCl and KCl is small in this temperature range.
After 800 °C, DSC absorbs heat and DTG loses mass, indicating that NaCl and KCl volatilize mainly occurred. However, MS detected that N2 and NO gas still escaped at this stage. It can be seen that the AlN reaction still exists, and the reaction is not complete until 1000 °C. After the temperature exceeds 800 °C, the DTG in Fig. 3(b) and the “Total mass of gases” curve show a large deviation, which also indicates that a large amount of NaCl and KCl volatilize in this temperature range. In fact, the area enclosed by the curve of DTG and “Total mass of gases” is the volatilization amount of NaCl and KCl. The total mass loss of NaCl and KCl is calculated to be 2.93 mg, accounting for 19.5 % of BAD. The volatilization rate above 800 °C is 0.089 mg/min, slightly higher than the volatilization rate under pure Ar atmosphere.
In order to further study the thermal behavior of BAD in the high temperature process, calcium oxide was added to BAD for TG-DTG-DSC&MS analysis. As shown in Fig. 4, the mass loss of BAD after adding CaO under Ar atmosphere occurs in the temperature range of 80–220 °C, 220–500 °C, 500–700 °C and 800–1000 °C. From the mass spectrometry results in Fig. 4(b), it can be seen that there are three kinds of gases H2O, NH3 and O2 generated in the temperature range of 80–220 °C and 180–500 °C. It is speculated that H2O mainly comes from the release of crystal water, and the H2O reacts with AlN to form NH3 (Eq. (7)).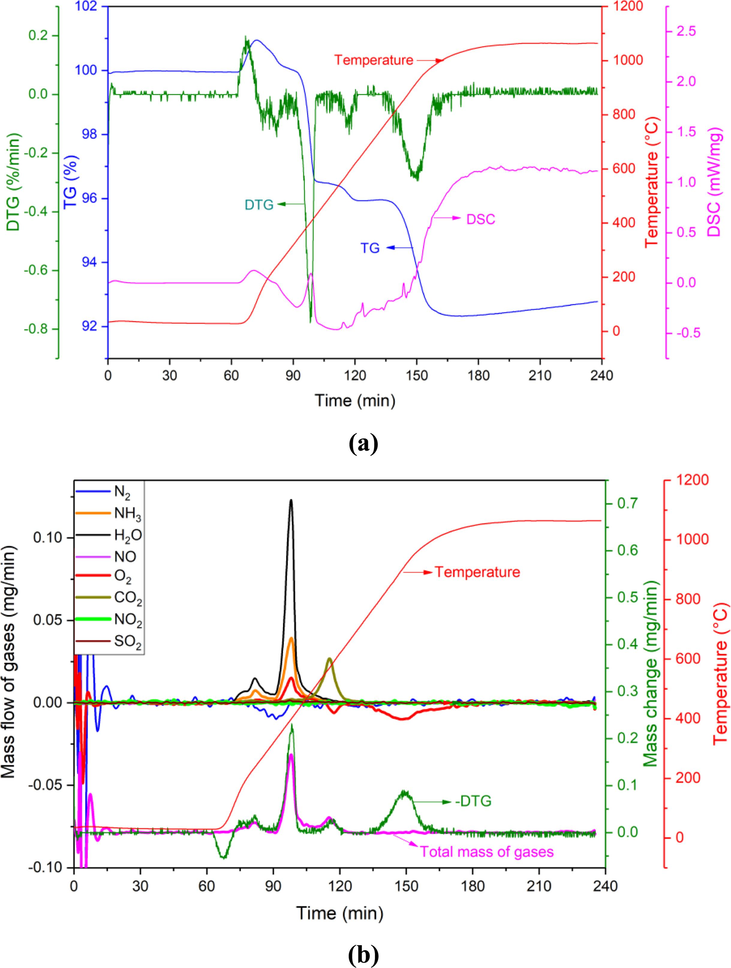
(a) TG-DTG-DSC and (b) MS of BAD + CaO in Ar atmosphere.
The CO2 gas generated at 500–700 °C is mainly from the decomposition of carbonate. The mass loss of 800–1000 °C is mainly caused by the volatilization of three low-melting substances such as Al, NaCl and KCl. According to the measurement results, it can be calculated that the total mass loss of Al, NaCl, and KCl in the range of 800–1000 °C is 0.87 mg, and the average volatilization rate is 0.029 mg/min, which accounts for 4.1 % of the total mass of raw materials.
Different from the pure Ar atmosphere, the mass of BAD + CaO in the Ar + O2 atmosphere increases first and then decreases within the temperature range of 500–1000 °C (Fig. 5a). The MS results in Fig. 5b show that a large amount of O2 is consumed, and a large amount of N2 escapes at the same time, and a small amount of NO also escapes within this temperature range. Therefore, the oxidation reaction of AlN and Al mainly occurs at this stage (Eq. (4), Eq. (5), Eq. (9)).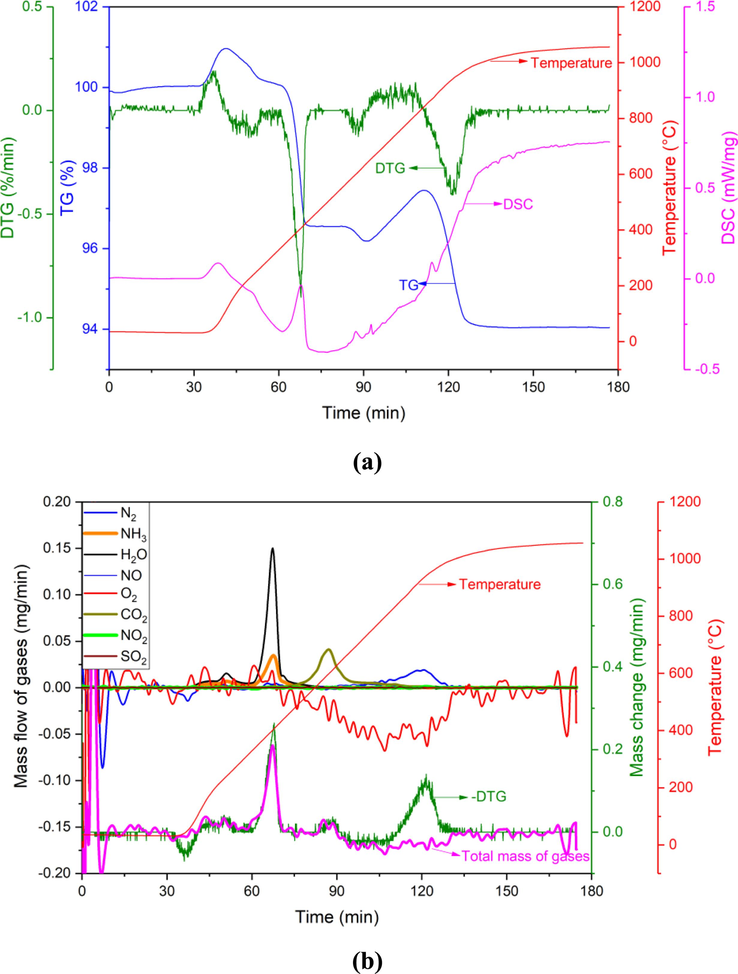
(a) TG-DTG-DSC and (b) MS of BAD + CaO in Ar + O2 atmosphere.
The calculation results show that the mass of AlN in the sample BAD + CaO is 3.08 mg, which accounts for 10.3 % of the total mass of the raw materials. The reaction between AlN and O2 is mainly to produce N2, about 12 % of AlN reacts with O2 to produce NO gas. Moreover, the reaction between AlN and O2 is quite slow, the reaction is complete within 63 min, and the average conversion rate is 0.049 mg/min. According to Eq. (5), it can be calculated that the mass of oxidized metallic aluminum is 1.69 mg, accounting for 5.6 %.
The area enclosed by the DTG and “Total mass of gases” curve in the range of 500–1000 °C is the volatile amount of NaCl and KCl. The total mass loss of NaCl and KCl is calculated to be 1.71 mg, accounting for 5.7 % of the total raw material, and the volatilization rate is 0.052 mg/min.
In summary, the reaction between AlN and O2 occurs in the range of 500–1000 °C, and the reaction rate is quite slow. The gaseous product of the reaction is mainly N2, and there is also a small amount of NO. The volatilization of salts such as NaCl and KCl mainly occurs under high temperature conditions higher than 800 °C, and the volatilization rate is also slow. The addition of calcium oxide to BAD does not significantly change its reaction behavior in the high temperature process. A schematic diagram of the thermal behavior of BAD during the calcination-leaching process is shown in Fig. 6.
Thermal behaviors of BAD in the calcination-leaching process.
3.3 Phase transition during the preparation of corundum
The phase composition of BAD in each stage of the calcination-leaching process is shown in Fig. 7. The phase analysis results show that the main phases in the untreated BAD are α-Al2O3, γ-Al2O3, AlN, Al, NaCl, KCl, NaAl11O17, MgAl2O4 and CaF2. After BAD are calcined at high temperature, the phases are mainly composed of α-Al2O3, MgAl2O4 and CaF2 phases. The results showed that γ-Al2O3 was transformed into α-Al2O3, AlN and NaAl11O17 were decomposed, NaCl and KCl were volatilized and removed during the calcination process. It is consistent with the thermodynamic calculation results in Section 3.1. There was no significant change in the phase of BAD after being leached by water and after being leached by acid.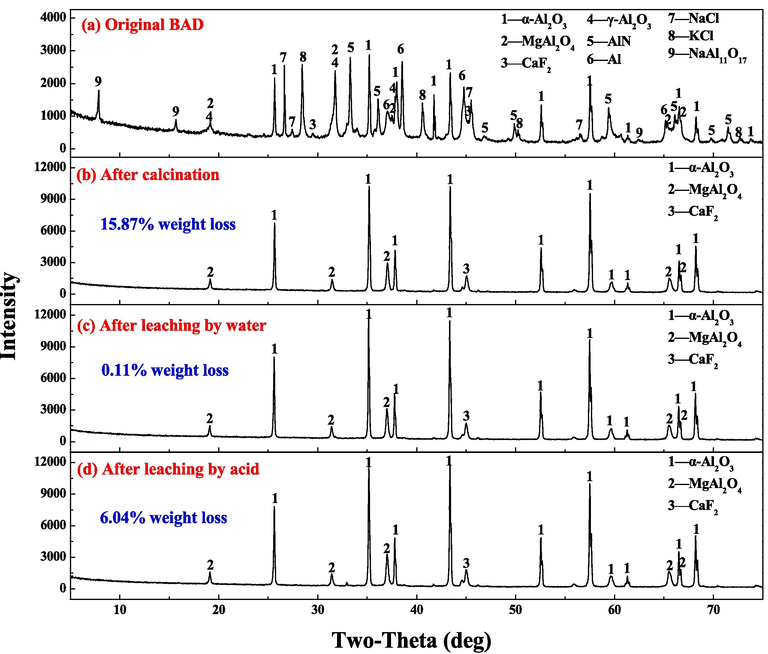
Phase transition in the preparation of corundum-based refractories.
The mass loss rate of BAD in each stage of the calcination-leaching process is calculated. The mass loss rate of BAD after calcination was 15.87 %, 0.11 % after being leached by water, and 6.04 % after being leached by acid. The maximum weight loss rate of BAD occurs in the calcination process, and the phase change at this stage is also obvious. Although the phase composition did not change after being leached by acid, the mass loss indicated that some acid-soluble substances were still dissolved. Therefore, the chemical composition of the product is analyzed, and the composition is shown in Table 2. The volatilization of fluoride salt caused the mass loss of BAD during the calcination process. The composition of the calcined BAD is almost unchanged before and after being leached by water. After being leached by acid, residual fluoride and chloride salts in BAD cannot be detected. After BAD is acid-leached, it mainly contains more than 95 % corundum and magnesia-aluminum spinel.
Materials
Al2O3
SiO2
Fe2O3
Na2O
MgO
CaO
K2O
Cl
F
Original BAD
70.87
2.01
0.42
4.72
5.50
0.71
3.99
5.76
5.24
Calcined BAD
84.66
2.41
0.71
0.46
5.22
0.68
0.19
0.018
0.29
Water leaching BAD
84.93
2.52
0.71
0.40
5.21
0.68
0.19
0.016
0.34
Acid leaching BAD
89.65
0.92
0.74
0.099
6.0
–
–
–
–
3.4 Characterization of the product
The particle size of the product obtained through the calcination-leaching process is shown in Fig. 8. The d50 of the raw material BAD is 77.46 μm, and the d90 is 298.52 μm. After being calcined, the particle size of BAD is slightly reduced, with d50 of 74.81 μm and d90 of 294.17 μm. After being leached by water, the particle size of BAD decreased slightly, with d50 of 67.04 μm and d90 of 297.28 μm. The particle size of BAD after acid leaching was significantly reduced, with d50 of 37.95 μm and d90 of 242.54 μm. It can be seen that the acid leaching stage has the most significant impact on the product particle size, while the calcination and water leaching processes have little effect on the product particle size.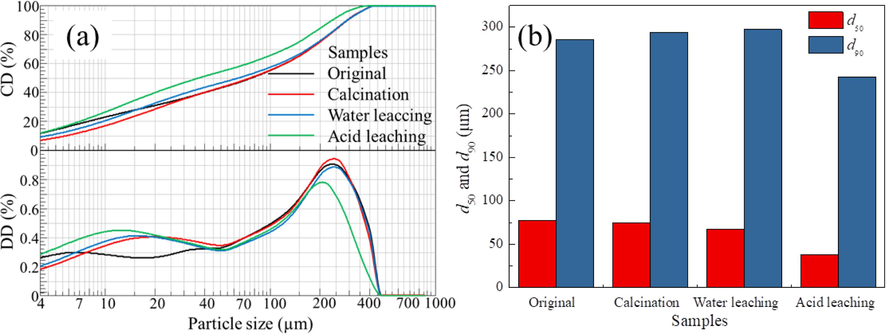
The particle size change during the preparation of corundum-based refractories.
After BAD are calcined, most of the aluminum nitride, β-alumina and metallic aluminum have been oxidized and converted to alpha alumina. Water-soluble chloride salts are also removed during the calcination process, which can be judged from the mass loss rate after water leaching and the phase of the calcined product. Acid-soluble impurities such as iron oxide are further removed in the acid leaching process. The morphology of the product obtained by the calcination-leaching process of BAD is shown in Fig. 9. The product is mainly composed of α-alumina and magnesia alumina spinel. The corundum phase presents different morphological characteristics, such as lamellar or rod-like, and cross-links with the magnesia-aluminum spinel. It can be used as a high-quality raw material for the preparation of refractory materials.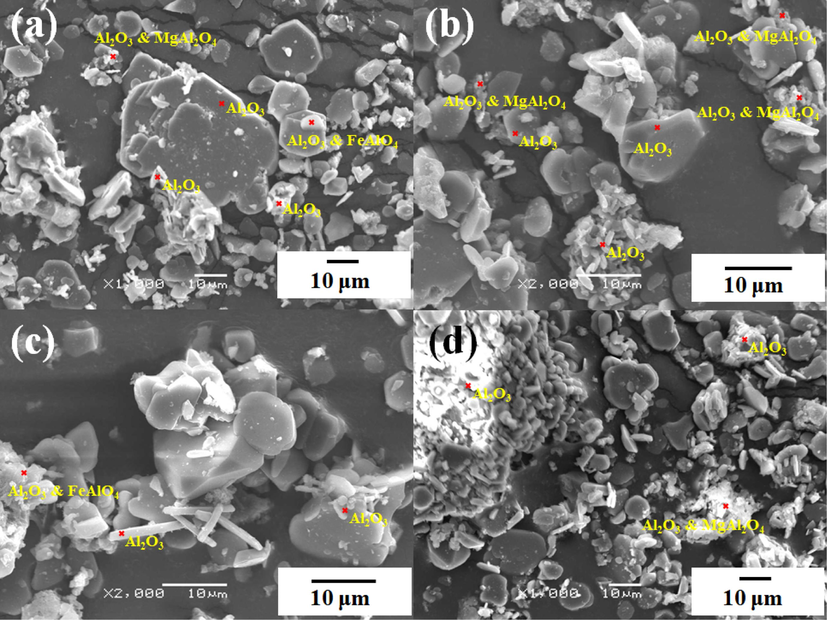
SEM morphology of prepared corundum-based refractories.
4 Conclusion
The hazardous solid waste BAD produced in the aluminum recycling industry is studied in this paper, and a calcination-leaching process is proposed for the preparation of corundum materials. The results of TG-DTG-DSC&MS of BAD at high temperature were discussed in detail. The following conclusions have been drawn:
The quantity of gaseous products can be obtained by TG-DTG-DSC&MS analysis, which can be used to quantitatively analyze the reaction rate of aluminum nitride, metal aluminum, fluoride salt and other substances in BAD.
The reaction between AlN and O2 occurs in the range of 500–1000 °C, and the reaction rate is slow. The gaseous products are mainly N2 and a small amount of NO. The volatilization of salts such as NaCl and KCl mainly occurs above 800 °C, and the volatilization rate is also slow. The average volatilization rate of salts in BAD under Ar atmosphere is 0.065 mg/min. The volatilization rate in Ar + O2 atmosphere is 0.089 mg/min, which is slightly higher than the volatilization rate in pure Ar atmosphere.
The addition of calcium oxide to BAD does not significantly change its reaction behavior in the high-temperature process. The chemical reactions and the volatilization of salt substances that occur at high temperatures are similar, and the main difference lies in the difference in the amount of reaction or volatilization.
After the calcination-leaching process, the BAD mainly contains more than 95 % corundum and magnesia-aluminum spinel, with a particle size of d50 = 37.95 μm and d90 = 242.54 μm. It can be used as a high-quality raw material for the preparation of refractory materials.
Acknowledgments
This study was supported by the National Key Research and Development Program of China (2022YFC2904401)
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
References
- Evaluating the chemical composition and the molar heat capacities of a white aluminum dross. Energy Procedia. 2015;75:2099-12015.
- [Google Scholar]
- The aluminium industry: a review on state-of-the-art technologies, environmental impacts and possibilities for waste heat recovery. Int. J. Thermofluids. 2020;1–2:100007
- [Google Scholar]
- A study of stabilization and recycling for aluminum dross. Appl. Mech. Mater.. 2013;275–277:2237-2240.
- [Google Scholar]
- Acid dissolution of alumina from waste aluminium dross. Hydrometall.. 2008;92:48-53.
- [Google Scholar]
- Hydrolysis of aluminum dross material to achieve zero hazardous waste. J. Hazard. Mater.. 2012;209–210:501-509.
- [Google Scholar]
- Surface composition, charge and texture of active alumina powders recovered from aluminum dross tailings chemical waste. Powder Technol.. 2003;132:137-144.
- [Google Scholar]
- Study on the extraction of aluminum from aluminum dross using alkali roasting and subsequent synthesis of mesoporous γ-Alumina. Metall. Mater. Trans. B. 2018;49:2906-2916.
- [Google Scholar]
- A comparative study of acid and alkaline aluminum extraction valorization procedure for aluminum saline slags. J. Environ. Chem. Eng.. 2022;10:107546
- [Google Scholar]
- The aluminum smelting process and innovative alternative technologies. J. Occup. Environ. Med.. 2014;56:23-32.
- [Google Scholar]
- Evaluation of aluminum dross as raw material for high-alumina refractory. Ceram. Int.. 2014;40:12585-12590.
- [Google Scholar]
- Reduction of secondary aluminum dross by a waste pickling liquor containing ferrous chloride. Sustain Environ. Res.. 2013;23:61-67.
- [Google Scholar]
- Study on calcination catalysis and the desilication mechanism for coal gangue. ACS Sustain. Chem. Eng.. 2021;9:10318-10325.
- [Google Scholar]
- A thermodynamic and kinetic study of catalyzed hydrolysis of aluminum nitride in secondary aluminum dross. J. Mater. Res. Technol.. 2020;9:9735-9745.
- [Google Scholar]
- Hazardous aluminum dross characterization and recycling strategies: a critical review. J. Environ. Manage.. 2018;223:452-468.
- [Google Scholar]
- Recovery of valuable products from hazardous aluminum dross: a review. Resour. Conserv. Recycl.. 2018;130:95-108.
- [Google Scholar]
- Conversion of aluminum dross residue into value-added ceramics. Key Eng. Mater.. 2016;690:71-75.
- [Google Scholar]
- On trending technologies of aluminium dross recycling: a review. Process Saf. Environ. Prot.. 2023;171:38-54.
- [Google Scholar]
- Production of alpha-alumina from black aluminum dross using NaOH leaching followed by calcination. JOM. 2020;72:3358-3366.
- [Google Scholar]
- Preparation and properties of resin concrete using aluminum dross wastes as aggregates. J. Japan. Inst. Metals. 1998;48:276-281.
- [Google Scholar]
- Characteristic analysis of hazardous waste from aluminum reduction industry. Light Met. 2020:1261-1266.
- [Google Scholar]
- Fine Al2O3 powder produced by radio-frequency plasma from aluminum dross. IEEE t. Plasma Sci.. 2014;42:3751-3755.
- [Google Scholar]
- A new approach to recover the valuable elements in black aluminum dross. Resour. Conserv. Recy.. 2021;174:105768
- [Google Scholar]







