Translate this page into:
Substituent, structural and positional isomerisation alter anti-oxidant activity of organochalcogen compounds in rats’ brain preparations
⁎Corresponding authors at: Departamento de Química, Centro de Ciências Naturais e Exatas, Universidade Federal de Santa Maria, 97105-900 Santa Maria, RS, Brazil. Tel.: +55 55 3220 8140; fax: +55 55 3220 8978. waseem_anw@yahoo.com (Waseem Hassan), jbtrocha@yahoo.com.br (Joao Batista Teixeira da Rocha)
-
Received: ,
Accepted: ,
This article was originally published by Elsevier and was migrated to Scientific Scholar after the change of Publisher.
Peer review under responsibility of King Saud University.
Abstract
The objective of the present study was to elucidate the biochemical potencies of eighteen structurally related organoselenium and organosulfur compounds against Fe(II) induced thiobarbituric acid reactive species (TBARS) formation in rat’s brain homogenate. The efficacies of these compounds (only organosulfur) were further confirmed by radical scavenging and thiol peroxidase-like (TPx) activities. Our data revealed that electron-donating groups significantly improve, while an electron-withdrawing group decreases antioxidant activities. The effect of structural isomerisation proved that electron-donating groups attached to the benzyl moiety at ortho-, meta- or para-positions decreased antioxidant potential. The compound benzyl-p-tolyl selenide (C-6) showed the highest in vitro activity and was selected for the in vivo experiments. Treatment with C-6 at 0, 10, 25 or 50 mg/kg was not associated with mortality, body weight loss or oxidative stress as measured by TBARS production. Similarly it did not inhibit delta-aminolevulinate dehydratase (α-ALA-D) enzyme, in fact treatment with C-6 increased the non-protein thiol content. Exposure to the same compound did not affect plasma transaminase activities or levels of urea and creatinine, indicating negligible toxicity to hepatic and renal tissues. The present study gives useful information for the synthesis of organochalcogens with desired biological and pharmacological potential.
Keywords
Organochalcogens
Structure activity relationship
Oxidative stress

1 Introduction
Reactive oxygen species (ROS) are an inevitable by-product of cellular respiration which causes oxidation of lipids, nucleic acids and proteins. ROS induced damage is the underlying cause of various neurodegenerative diseases (Valko et al., 2006; Angel et al., 2005). Cells have sophisticated antioxidant regulatory systems to maintain proper balance of ROS. However, disruption in homeostasis can result in oxidative stress and tissue injury (Perry et al., 2002).
Literature have demonstrated that organoselenium compounds possess very interesting biological activities like, glutathione peroxidase-mimic, lipid peroxidation, radical-scavenging, antiinflammatory, antinociceptive, hepatoprotective, nephroprotective and neuroprotective activity (Nogueira et al., 2004). In this regard our group has shown that selenium-containing molecules are better nucleophiles (and therefore better antioxidants) than classical antioxidants and this has led to the a significant development in synthetic organoselenium compounds with pharmacological potential (Arteel and Sies, 2001; Hassan et al., 2009a,b,c). Moreover, clinical trials in humans revealed beneficial effects of organoselenium compounds such as ebselen in pathological situations (Parnham and Sies, 2000). Similarly, organoselenium compounds are good glutathione peroxidase (GPx) mimics and have shown interesting biological activities in different models of oxidative stress induced damages (Meotti et al., 2004; Hassan et al., 2009a,b). Sulfur compounds are also studied for their antioxidant properties and a recent study indicates that aqueous garlic extract protects against arsenic toxicity (Chowdhury et al., 2008). Biological sulfur-containing compounds, including cysteine, methionine, taurine, glutathione (GSH), N-acetylcysteine (NAC), and other sulfur compounds have been extensively studied for their antioxidant properties (Parcell, 2002; Atmaca, 2004).
This paper describes the synthesis of a series of sulfur and selenium agents (according to the literature methods) and evaluation of their antioxidant potential. In order to understand the biochemical efficacies of these chalcogens, we particularly emphasized on a chemically multidimensional approach towards antioxidant characterization that includes (i) effect of chain length i.e., introduction of CH2 group (ii) different ring substituent effects i.e., both electron donating and electron withdrawing groups and (iii) not only the effect of ortho, meta and para positioning but also structural changes of the same molecule leading to different isomers and its anti-oxidant effect are described in detail. We have tested the efficacy of these compounds against Fe(II) induced TBARS formation in rat’s brain homogenate. The activities of these compounds are also measured and confirmed by their thiol peroxidase like activity followed by DPPH radical scavenging assay. In vivo work followed the in vitro assays to get a better understanding of its possible toxic profile.
2 Material and method
2.1 Synthesis of organoselenium and organosulfur compounds
The unsymmetrical diorganyl selenides and sulfides (Fig. 1) were synthesized using the literature procedure (Narayanaperumal et al., 2009; Brindaban et al., 2004). Diphenyl disulphide (CAS No: 882-33-7) was purchased and used for the experiments.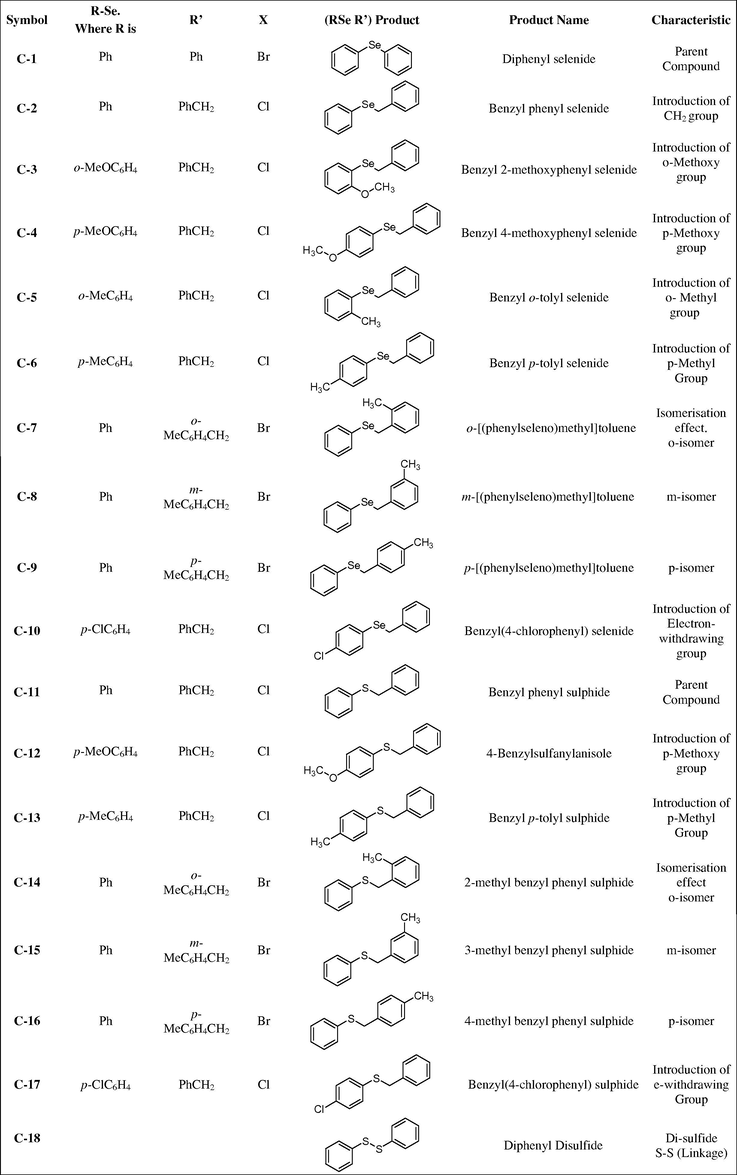
Structures of organochalcogen compounds.
Reaction 1. Synthesis of diorganyl selenides.
Reaction 2. Synthesis of diorganyl sulfides.
2.2 In vitro assays
2.2.1 Preparation of brain homogenate and thiobarbituric acid reactive species (TBARS) assay
Rats were decapitated under mild ether anesthesia and the cerebral tissue (whole brain) was rapidly removed, placed on ice and weighed. Tissue was immediately homogenized in cold 10 mM Tris–HCl, pH 7.4 (1/10, w/v) with 10 up-and-down strokes at approximately 1,200 rev/min in a Teflon-glass homogenizer. The homogenate was centrifuged for 10 min at 4,000 g to yield a pellet that was discarded and a low-speed supernatant (S1) was obtained. An aliquot of 100 μL of S1 was incubated for 1 h at 37 °C in the presence of both organoselenides (final concentrations range of 0–100 μM) with the pro oxidant Iron (Fe(II) at a final concentration of 20 μM. Production of TBARS was determined as described by the method of Ohkawa et al. (1979), except that the buffer of color reaction has a pH of 3.4. The color reaction was developed by adding 200 μL of 8.1% SDS to S1, followed by sequential addition of 500 μL of acetic acid/HCl (pH 3.4) and 500 μL of 0.8% of thiobarbituric acid (TBA). This mixture was incubated at 95 °C for 1 h. TBARS produced were measured at 532 nm and the absorbance was compared to the standard curve obtained using malondialdehyde (MDA).
2.2.2 Thiol peroxidase-like activity
The catalytic activity of organochalcogens as a GPx model enzyme was evaluated according to Iwaoka and Tomoda (1994) using benzenethiol as a glutathione alternative. The reduction of H2O2 was monitored through the UV absorption increase at 305 nm, due to diphenyl disulfide formation.
2.2.3 DPPH• radical scavenging activity assay
The measurement of the organochalcogens scavenging activity against the radical DPPH• was performed in accordance with Choi et al. (2002). Briefly, 85 μM DPPH• was added to a medium containing different organochalcogen concentrations. The medium was incubated for 30 min at room temperature. The decrease in absorbance measured at 518 nm depicted the scavenging activity of the organochalcogens against DPPH•. DPDS was used as a positive control to determine the maximal decrease in DPPH• absorbance. The values are expressed in percentage of inhibition of DPPH• absorbance in relation to the control values.
2.3 In vivo assay
2.3.1 Animal’s treatment
Rats were divided into four groups, daily weighted and treated (orally) for 3 days (at the interval of 24 h) with C-6 at 0 (canola oil) 10, 25 and 50 mg/kg. 24 h after the last injection animals were anesthetized for the collection of blood via heart puncture and then killed by decapitation. All the experiments were conducted at least four times and similar results were obtained.
2.3.2 Determination of TBARS levels
TBARS were determined in the brain homogenate by the method of Ohkawa et al. (1979), in which malondialdehyde (MDA), an end-product of fatty acid peroxidation, reacts with thiobarbituric acid (TBA) to form a colored complex. In brief, samples were incubated at 100 °C for 60 min in acid medium containing 0.45% sodium dodecyl sulfate and 0.6% thiobarbituric acid. After centrifugation the reaction product was determined at 532 nm and the results were expressed as nmol MDA/mg of tissues.
2.3.3 δ-Aminolevulinic dehydratase (δ-ALA-D) activity
δ-ALA-D activity was assayed according to the method of Sassa (1982). The enzyme activity was measured by determining the amount of porphobilinogen formed at 37 °C. The reaction was started by adding the substrate (δ-ALA) and incubated for 90 min at 37 °C. The reaction product (porphobilinogen) was determined using modified Ehrlich’s reagents and measured at 555 nm.
2.3.4 Nonprotein thiol (NPSH) content
Cerebral NPSH levels were determined by the method of Ellman (1959). A sample of supernatant (500 μL) was mixed (1:1) with 10% trichloroacetic acid (500 μL). After centrifugation, the protein pellet was discarded and free –SH groups were determined as nmol/g tissue in a clear supernatant. An aliquot (100 μL) of supernatant was added in a 1 M potassium phosphate buffer (850 μL), pH 7.4, and 10 mM 5,5′-dithio-bis(2-nitrobenzoic acid) (DTNB) (50 μL). The color reaction was measured at 412 nm.
2.3.5 Hepatic and renal evaluation
Hepatic toxicity was analyzed using serum alanine aminotransferase (ALT) and aspartate aminotransferase (AST) according to Reitman and Frankel (1957), using a commercial Kit (LABTEST, Diagnostica S.A., Minas Gerais, Brazil) and expressed as U/dl. Renal function was analyzed using a commercial Kit (LABTEST, Diagnostica S.A., Minas Gerais, Brazil) by determining plasma urea (Mackay and Mackay, 1927) and creatinine (Jaffe, 1886) and expressed as mg/dL.
2.4 Statistical analysis
The results are expressed as the mean ± standard error (SEM). Data were analyzed statistically by analysis of variance (Two-way ANOVA).
3 Results and discussion
The structures of all tested compounds are shown in Fig. 1. The influence of the substituent on organoselenium compounds can be observed through the potential shift in anti-oxidant activities as shown in Fig. 2A. Addition of Methoxy –OCH3 (mesomerically electron donating group) group at ortho (C-3) and para (C-4) position, significantly improved antioxidant activity. Importantly, the para isomer displayed significantly a higher antioxidant potential than ortho isomer. The results were replicated (C5 and C6) by the addition of another electron donating group (CH3, inductively electron donating group). Hypothetically, the electron donating group increases the electronic density on selenium atom which may increase its anti-oxidant activity. For this purpose we synthesized three isomers (C7–9), where an electron donating group i.e., (CH3) was attached to the benzyl ring (this ring does not have a direct bond with selenium). None of the compound showed better anti-oxidant potential than C-6. However, these isomers (C7–9) have better antioxidant activity than parent C1 and C2. These results prove that isomerisation has a significant effect on the anti-oxidant activity of organoselenium compounds. Contrarily, the presence of an electron withdrawing group might destabilize the selenium moiety by withdrawing electronic density and may lead to a decrease in the antioxidant potential. This phenomenon is observed in our results, where C-10, a compound with an electron withdrawing group (chlorine-Cl) did not show any antioxidant potential. In fact, C-10 at the highest tested concentration showed pro-oxidant behavior as apparent from increased TBARS formation as shown in Fig. 2A.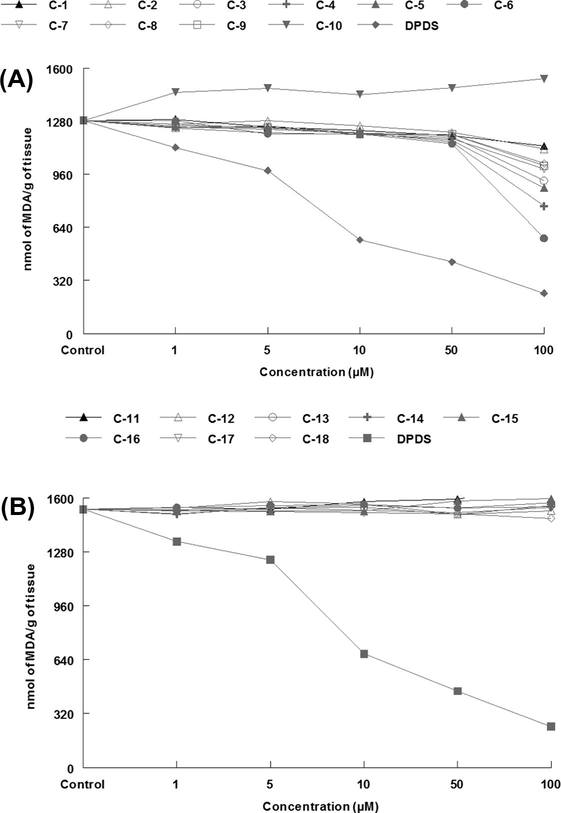
Effect of organoselenium (A) and organosulfur compounds (B) against Fe(II) induced TBARS formation.
Like the organoselenium compounds we synthesized structurally related organosulfur compounds and tested their efficacy against Fe(II) Induced TBARS production. The parent compound i.e., diphenyl sulfide (C-11) did not show any activity. It is apparent from Fig. 2B that introduction of the electron donating group did not improve antioxidant potential. Similarly, the electron donating group (CH3) attached to the benzyl moiety of organosulfur compounds at ortho, meta and para position i.e., C-14, C-15 and C-16 did not show any anti-oxidant activity. Similarly the introduction of the electron donating group (Cl) at C-17 showed pro-oxidant behavior.
Our results indicate that organoselenium compounds are better anti-oxidants than its sulfur analogues. Although selenium shares many chemical properties with its neighboring homologue sulfur, selenium differs from sulfur in a number of ways, the most significant being that selenol is a more powerful nucleophile than thiol. In contrast to thiols, selenols exist mostly in anionic form at neutral pH and represent good reducing groups under normal physiological conditions (Burk, 1994). This possibly could be the main reason for the better anti-oxidant activity of organoselenium compounds than organosulfur analogues.
Organoselenium compounds protected against Fe(II) induced TBARS production at highest tested concentration. Ferryl ion (Bors et al., 1979) perferryl ion (Pederson et al., 1973) and Fe+2–O2•–Fe+3 complexes (Bucher et al., 1983) have been proposed to be involved in the initiation of Fe(II)-dependent lipid peroxidation. A plausible mechanism by which organoselenium compounds are conferring protective action against Fe(II)-induced lipid peroxidation in these homogenate is that organoselenium compounds may be interacting with Fe(II) or its oxidized forms.
Organoselenium compounds showed better thiol peroxidase like activities (Fig. 3A) than their sulfur analogues (Fig. 3B). However, it is important to note that in strong contrast to TBARS inhibitory activity (Fig. 2A) the compounds C7, C8 and C9 did not show any improved thiol peroxidase activity than parent C1 or C2 compound (Fig. 3A). The plausible explanation for this contrasting behavior between thiol peroxidase like activity and TBARS inhibitory activities could be that although GPx measurements are typically used to determine antioxidant activity of selenium compounds, these measurements may not accurately reflect cellular conditions. GPx activity measurements are often determined under conditions that are not physiologically relevant, such as using non-aqueous solutions or non-biological thiols (Sarma and Mugesh, 2005; Mareque et al., 2004; Marnett, 2000).
Thiol peroxidase activity data of organoselenium (3A) and organosulfur compounds (3B).
The ability of sulfur compounds to scavenge ROS has been attributed to a possible antioxidant mechanism for these compounds. (Mishra et al., 2008; Kim et al., 2006; Shindo and Brown, 1965; Livingstone and Nolan, 1968; Battin and Brumaghim, 2008). However our results indicated that all sulfur compounds lack anti-oxidant activity. This behavior is apparent from lack of anti-oxidant activity against Fe(II) induced TBARS production (Fig. 2B), thiol peroxidase like activity (Fig. 3B) and inactivity towards DPPH• radical scavenging assay (Fig. 4). The lack of anti-oxidant activity indicates that these organosulfur compounds neither exhibit metal binding capacity nor act as free radical scavenger which may be produced by fenton reactions.
DPPH radical scavenging data of organosulfur compounds.
Based on our in vitro data, C-6 with the highest TBARS inhibition and thiol peroxidase like activity was selected for in vivo work. Our results indicated (Fig. 5A) that C-6 has no effect on TBARS content and confirms the safe position of the tested compound. Organochalcogens can interact directly with low molecular thiols oxidizing them to disulfides (Goeger and Ganther, 1994). Reduced cysteinyl residues from proteins can also react with simple diselenides and ditellurides, which can cause, in the case of enzymes, the loss of catalytic activity. Besides, organoselenium and organotellurium compounds have been reported to inhibit various sulfhydryl group containing enzymes, like 5-lipoxygenase (Björnstedt et al., 1996), protein kinase JNK1 (Park et al., 2000), δ-aminolevulinate dehydratase (Barbosa et al., 1998; Nogueira et al., 2003) and squalene monooxygenase (Gupta and Porter, 2001; Laden and Porter, 2001). However the present study revealed that compound-6 did not inhibit the δ-ALA-D activity (Fig. 5B) rather at the highest tested concentration it increased NPSH content (Fig. 5C). Rats exposed to C-6 presented no hepatic and renal damage apparent from normal enzymatic and biochemical parameters (Fig. 6).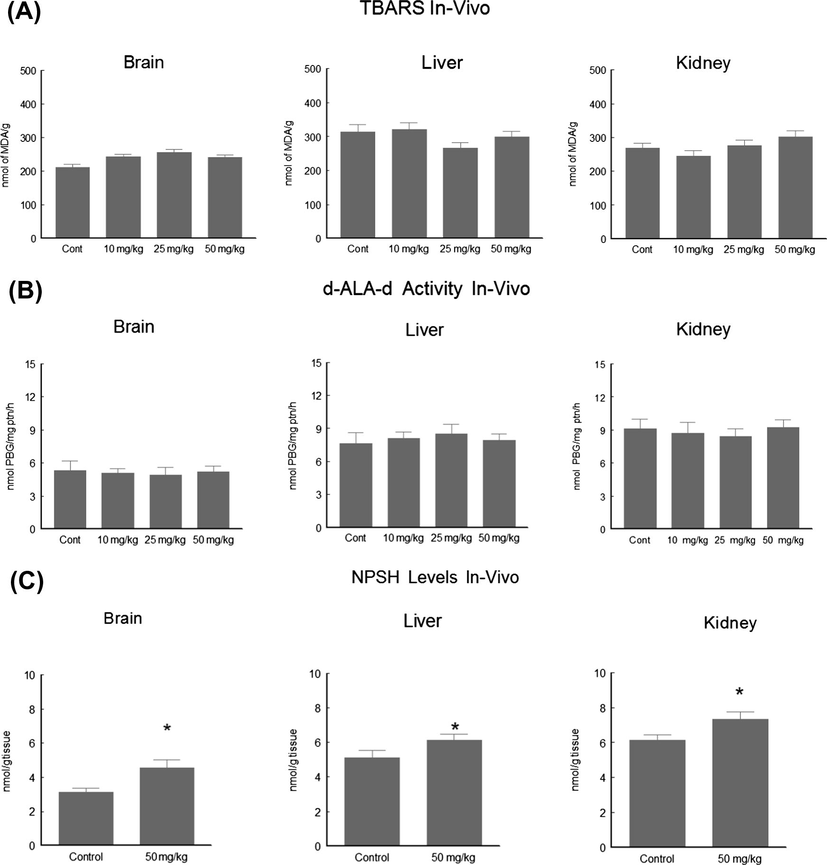
Effect of compound 6 on TBARS formation (A), α-ALA-D activity (B) and NPSH (C) at different doses in rat’s tissue preparation. Data are reported as mean ± SEM for six rats in each group.
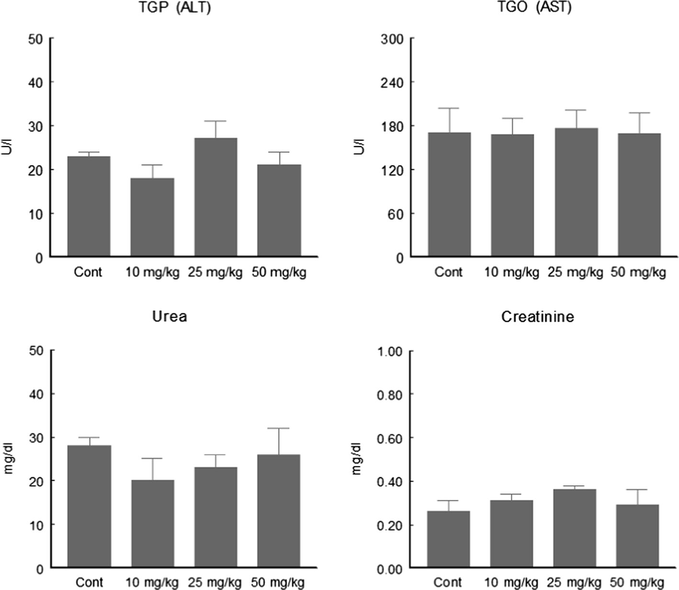
Effect of compound 6 on toxicological parameters after 3-doses of acute treatment. Data are reported as mean ± SEM for six rats in each group.
Previously we have shown that acute exposure to high doses of DPDS did not affect plasma transaminase activities or urea and creatinine levels in rodents (Meotti et al., 2003). Furthermore, rabbits chronically exposed to DPDS supplemented diet have hepatic and renal functions within the normal range (de Bem et al., 2007). Taken together, these data suggest that acute exposure to this mono-selenide (C-6) did not induce oxidative stress in rats. Further studies are needed to understand the potential therapeutic and toxicological efficacies of this interesting class of compounds.
Acknowledgments
Waseem Hassan is a beneficiary of the TWAS_CNPq doctoral fellowship program (Brazil). The financial support of Brazilian agencies and foundations like CAPES, SAUX, VITAE and FAPERGS is gratefully acknowledged.
References
- Metal ion chelation in neurodegenerative disorders. Drug Dev. Res.. 2005;56:300-309.
- [Google Scholar]
- The biochemistry of selenium and the glutathione system. Environ. Toxicol. Pharmacol.. 2001;10:153-158.
- [Google Scholar]
- Antioxidant effects of sulfur-containing amino acids. Yonsei Med. J.. 2004;45:776-788.
- [Google Scholar]
- Effect of inorganic forms of selenium on delta-aminolevulinate dehydratase from liver, kidney and brain of adults rats. Toxicol. Appl. Pharmacol.. 1998;149:243-253.
- [Google Scholar]
- Metal specificity in DNA damage prevention by sulfur antioxidants. J. Inorg. Biochem.. 2008;102:3036-3042.
- [Google Scholar]
- Selenite incubated with NADPH and mammalian thioredoxin reductase yields selenide, which inhibits lipoxygenase and changes the electron spin resonance spectrum of the active site iron. Biochemistry. 1996;35:8511-8516.
- [Google Scholar]
- On the nature of biochemically generated hydroxyl radicals. Studies using the bleaching of p-nitrosodimethylaniline as a direct assay method. Eur. J. Biochem.. 1979;95:621-627.
- [Google Scholar]
- Remarkably selective reduction of the α,β-Carbon–Carbon double bond in highly activated α,β,γ,δ-unsaturated alkenes by the InCl3−NaBH4 reagent system. J. Org. Chem. 2004;69:5793-5795.
- [Google Scholar]
- The requirement for ferric in the initiation of lipid peroxidation by chelated ferrous iron. Biochem. Biophys. Res. Commun.. 1983;111:777-784.
- [Google Scholar]
- Burk R.F., ed. Selenium in Biology and Human Health. New York: Springer-Verlag; 1994.
- Antioxidant activity and free radical scavenging capacity between Korean medicinal plants and flavonoids by assay-guided comparison. Plant Sci.. 2002;153:1161-1168.
- [Google Scholar]
- In vitro and in vivo reduction of sodium arsenite induced toxicity by aqueous garlic extract. Food Chem. Toxicol.. 2008;46:740-751.
- [Google Scholar]
- Low toxicity of diphenyl diselenide in rabbits: a long-term study. Basic Clin. Pharmacol. Toxicol.. 2007;101:47S-55S.
- [Google Scholar]
- Oxidation of dimethyl selenide to dimethyl selenoxide by microsomes from rat liver and flavin-containing monooxygenase from pig liver. Arch. Biochem. Biophys.. 1994;310:448-451.
- [Google Scholar]
- Inhibition of human squalene monooxygenase by selenium compounds. J. Biochem. Mol. Toxicol.. 2001;16:18-23.
- [Google Scholar]
- Enhancement of iron-catalyzed lipid peroxidation by acidosis in brain homogenate: comparative effect of diphenyl diselenide and ebselen. Brain Res.. 2009;1258:71-77.
- [Google Scholar]
- Towards the mechanism and comparative effect of diphenyl diselenide, diphenyl ditelluride and ebselen under various pathophysiological conditions in rat’s kidney preparation. Chem. Biol. Interact.. 2009;182:52-58.
- [Google Scholar]
- PH dependent Fe(II) pathophysiology and protective effect of an organoselenium compound. FEBS Lett.. 2009;583:1011-1016.
- [Google Scholar]
- A model study on the effect of an amino group on the antioxidant activity of glutathione peroxidase. J. Am. Chem. Soc.. 1994;116:2557.
- [Google Scholar]
- Structure–activity relationship of neuroprotective and reactive oxygen species scavenging activities for allium organosulfur compounds. J. Agric. Food Chem.. 2006;54:6547-6553.
- [Google Scholar]
- Inhibition of human squalene monooxygenase by tellurium compounds: evidence of interaction with vicinal sulfhydryls. J. Lipid Res.. 2001;42:235-240.
- [Google Scholar]
- Metal chelates of biologically important compounds. I. Complexes of dl-methionine and s-methyl-l-cysteine. Inorg. Chem.. 1968;7:1447-1451.
- [Google Scholar]
- In vitro evaluation of glutathione peroxidase (GPx)-like activity and antioxidant properties of some ebselen analogues. Redox Rep.. 2004;9:81-87.
- [Google Scholar]
- Potential renal and hepatic toxicity of diphenyl diselenide, diphenyl ditelluride and ebselen for rats and mice. Toxicol. Lett.. 2003;143:9-16.
- [Google Scholar]
- Protective role of aryl and alkyl diselenides on lipid peroxidation. Environ. Res.. 2004;94:276-282.
- [Google Scholar]
- Antioxidant status of children with acute renal failure. Pediatr. Nephrol.. 2008;23:2047-2051.
- [Google Scholar]
- Ionic liquid: an efficient and recyclable medium for synthesis of unsymmetrical diorganyl selenides promoted by InI. Org. Biomol. Chem.. 2009;7:4647.
- [Google Scholar]
- Anti-inflammatory and antinociceptive activity of diphenyl diselenide. Inflamm. Res.. 2003;52:56-63.
- [Google Scholar]
- Organoselenium and organotellurium compounds: toxicology and pharmacology. Chem. Rev.. 2004;104(12):6255-6285.
- [Google Scholar]
- Assay for lipid peroxides in animal tissues by 399 thiobarbituric acid reaction. Anal. Biochem.. 1979;95:351-358.
- [Google Scholar]
- Sulfur in human nutrition and applications in medicine. Altern. Med. Rev.. 2002;7:22-44.
- [Google Scholar]
- Selenite inhibits the c-Jun N-terminal kinase/stress-activated protein kinase (JNK/SAPK) through a thiol redox mechanism. J. Biol. Chem.. 2000;275:2527-2531.
- [Google Scholar]
- Ebselen, prospective therapy for cerebral ischaemia. Expert Opin. Inv. Drug. 2000;9:607-619.
- [Google Scholar]
- Microsomal electron transport. The role of reduced nicotinamide adenine dinucleotide phosphate-cytochrome c reductase in liver microsomal lipid peroxidation. J. Biol. Chem.. 1973;248:7134-7141.
- [Google Scholar]
- Metals and oxidative homeostasis in Alzheimer’s disease. Drug Dev. Res.. 2002;56:293-299.
- [Google Scholar]
- A colorimetric method for the determination of serum glutamic oxalacetic and glutamic pyruvic transaminases. Am. J. Clin. Pathol.. 1957;28:56-63.
- [Google Scholar]
- Glutathione peroxidase (GPx)-like antioxidant activity of the organoselenium drug ebselen: unexpected complications with thiol exchange reactions. J. Am. Chem. Soc.. 2005;127:11477-11485.
- [Google Scholar]
- Infrared spectra of complexes of L-cysteine and related compounds with zinc(II), cadmium(II), mercury(II), and lead(II) J. Am. Chem. Soc.. 1965;87:1904-1909.
- [Google Scholar]
- Free radicals, metals and antioxidants in oxidative stress-induced cancer. Chem. Biol. Interact.. 2006;160:1-40.
- [Google Scholar]






