Translate this page into:
Surface synthesization of magnesium alloys for improving corrosion resistance and implant applications
⁎Corresponding author. nayem.hossain@iubat.edu (Nayem Hossain),
-
Received: ,
Accepted: ,
This article was originally published by Elsevier and was migrated to Scientific Scholar after the change of Publisher.
Peer review under responsibility of King Saud University.
Abstract
In the field of biodegradable material, a new research area has emerged for magnesium (Mg) and its alloys because of its high biocompatibility and biomechanical compatibility. This review summarizes many important types of research that have been done on degradable coatings on magnesium and its alloys for various implant applications. When magnesium alloys come into contact with other metals, they have a low open circuit potential and are consequently prone to galvanic corrosion. When exposed to air or a humid environment, magnesium may rapidly oxidize and generate a thin layer of loose MgO. Its applications were limited due to these drawbacks. Different types of corrosion have been studied in relation to magnesium and its alloys. Several coating methods are described, split into conversion and deposition coatings based on the individual processing procedures employed. This paper covers the most recent advancements in the development of biodegradable Mg alloy coatings over the last decade, revealing that the corrosion resistance of Mg and its alloys increases in most of circumstances due to coatings. Corrosion rate, coating morphology, adhesion, and surface chemistry were identified and explored as significant elements affecting coating performance. Calcium phosphate coatings made by deposition or conversion processes established for orthopedic purposes are the focus of many investigations according to a review of the literature. More research is needed on organic-based biodegradable coatings to improve corrosion resistance. Improved mechanical qualities are also crucial for coating materials. Developing adequate methodologies for studying the corrosion process in depth and over time is still a hot topic of research.
Keywords
Corrosion Resistance
Conversion Coating
Biomedical Mg Implants
1 Introduction
Before degrading in biological media, Mg alloys can work as a transient structure to generate tissue and that is why Mg alloys are considered biodegradable, bioactive, and bio-tolerant third-generation biomaterials (Heinlein et al., 2003; Shi et al., 2015; Uppal et al., 2022). Ceramics and metallic implants are considered as first generation biomaterials and these are bio-inert while second-generation biomaterials (bio-ceramic and polymer) are bioactive and biodegradable (Damayanti et al., 2020). Mg has a density of 1.74 to 2.00 g/cm3 which is close to the bone has a density of 1.8 to 2.00 g/cm3. Mg has a 158 ken-m/kg tensile strength-to-weight ratio. These are all advantages of Mg alloys over permanent implant materials (Li and Zheng, 2013). Despite the fact that Mg alloys provide a number of advantages over permanent implants, in the bodily fluid solution, however, they exhibit uncontrolled corrosion and deterioration. Clinical trials with Mg alloy implants, such as Mg-Sr (Born pour et al., 2015), Mg-Ca (Zeng et al., 2015), Mg-Zn (Zhang et al., 2010), Mg-rare earth (RE) (Hurt et al., 2010), and Mg-based hybrid implants (Wong et al., 2013; Hade et al., 2013), were investigated as bone implants (Cha et al., 2013) as well as cardiovascular stents (Erbil et al., 2007). Apart from FDA permission, the following are the significant drawbacks of employing magnesium alloys in biomedical applications:(See Table 1).
Coating
Method
Corrosive media
Corrosion rate
Reference
Chitosan
Dip coating
SBF
0.013 ∼ 0.029 ml cm−2h−1
(Gu et al., 2009)
PLA
Dip coating
SBF
0.21 mm/a
(Li et al., 2019)
NanoCap
Dip coating
(Sangeetha et al., 2011)
HA
Spin coating
NaCl
99.28 % inhibition
(Zainab, 2022)
Collagen type-I
Spreading
(Zhao and Zhu, 2014)
PCL
Electro spinning
SBF
∼ five times lower corrosion test
(Keerthi Soujanya et al., 2014)
Gelatin
Electro spinning
Cell culture medium
1.60 ∼ 3.25 mM/3d
(Chan et al., 2013)
Silane
Sol-gel
SBF
(Gaur et al., 2016)
Bioactive glass
AC-EPD
Ringer's solution
0.08 ∼ 0.15 mm/y
(Akram et al., 2020)
nHA
TPA deposition
SBF
90 % corrosion reduced
(Tian et al., 2019)
Silk fibroin
Spin coating
Hank's solution
1.19 ml cm−2/14 d
(Xiong et al., 2019)
a. Corrosion makes the hydrogen gas (H2) released in the physiological environment and increases significantly the pH value of the bodily fluid.
b. Early implant failure because of the mismatch of bone tissue and implant material in the biological environment.
c. Inferior mechanical properties.
Surface treatment, surface modification, improvement of grain size, and microstructure of the main alloy with a coating to manage the deterioration rate are all approaches to overcome the aforesaid restrictions. Various types of coating techniques such as single double or multilayer hydroxyl apatite (HA) coatings, fluoride-based micro-arc oxidation, and polymer and sol–gel coatings are all utilized for corrosion prevention. Combining with organic, inorganic, or hybrid coatings on biodegradable Mg alloys these techniques constitute an intriguing option for improving corrosion and biological characteristics. Bioactive, bio-inert, as well as bio-mimetic hybrid coatings, are the main focus of this review they can improve the corrosion resistance of Mg and Mg alloys by stimulating the bone tissue for biomedical applications.
Besides Mg, other metal such as Zn is used as implant material because of their good biodegradation properties (Yang et al., 2022; Yang et al., 2021). However, Mg shows promising properties in terms of mechanics and degradation. Excellent biocompatibility is shown by Mg and its alloys in physiological conditions (Chen et al., 2014; Pompa et al., 2015). Besides, good mechanical properties for example a good strength-to-weight ratio are obtained (Walker et al., 2014; Riaz et al., 2018). Mg has an elastic modulus of 45 GPa which is close to the elastic modulus of natural bone.
2 Corrosion of Mg alloys
2.1 Galvanic corrosion
Poor design and assembly practices as well as flux contamination or high levels of heavy metals may cause galvanic corrosion in magnesium alloys. For example, when Mg parts are welded or connected with the steels in automotive bodies and lightweight power trains galvanic corrosion occurs. Besides, severe galvanic corrosion occurs in human body fluids when titanium and stainless steel made bone plate is connected with the Mg screws (Zeng et al., 2018). Metals including iron, copper, and nickel can serve as efficient cathodes if their hydrogen overvoltage is low, resulting in severe galvanic corrosion. Metals such as Zn, Sn, Al, and Cd are less damping because they can combine active corrosion potential with high hydrogen over potential. Substrates as well as inner secondary phases that make galvanic corrosion are macroscopically called overall corrosion. Tong et al (Zhen-song et al., 2004). Investigated the galvanic corrosion laws and behavior at atmospheric pressure of AM50, AM60, and AZ91D cast magnesium alloys coupled with LY12 aluminum alloy, H62 brass, A3 steel, and 316L stainless steel. They observed that when magnesium alloys are combined with the four metals mentioned above, they behave as anodes and their corrosion rates increase. The greatest impact of atmospheric galvanic of magnesium alloys was obtained in combination with A3 steel. However, the minimum impact was obtained from LY 12 aluminum alloy. The risk of galvanic corrosion can be considerably decreased by careful material selection and design, insulation materials, and judicious use of coatings. Aluminum 6000 series fasteners for example reduce galvanic corrosion greatly reduce galvanic corrosion in salt spray tests (Skar, 1999). An example of galvanic corrosion can be seen in Fig. 1.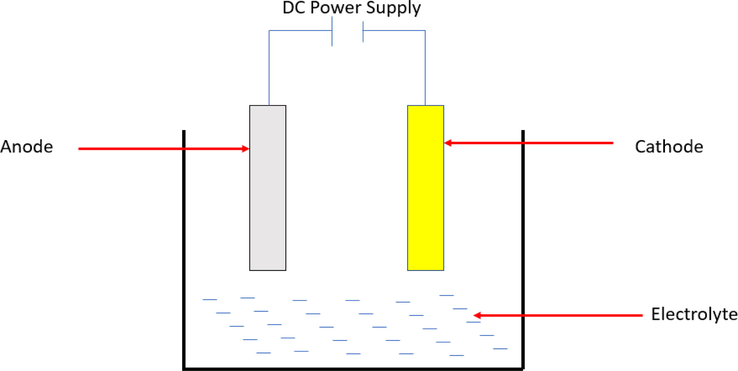
Schematic diagram of galvanic corrosion cell.
2.2 Pitting corrosion
Magnesium is a naturally passive material that is exposed to chloride ions resulting in pitting corrosion in a non-oxidizing media at the free corrosion potential of magnesium (Fatoba et al., 2016). Corrosion pits are commonly detected by the passivity breakdown at faults (Fatoba et al., 2016; Sakai, 2022). As a result, the formation of an electrolytic cell is observed in which the cathode forms by the second phase particles and the anode forms by the surrounding Mg matrix. Formation of corrosion pits is also observed surrounding the Almon particles shown in Fig. 2 when extruded Mg alloy AM60 was immersed in a natural solution containing 3.5 % NaCl.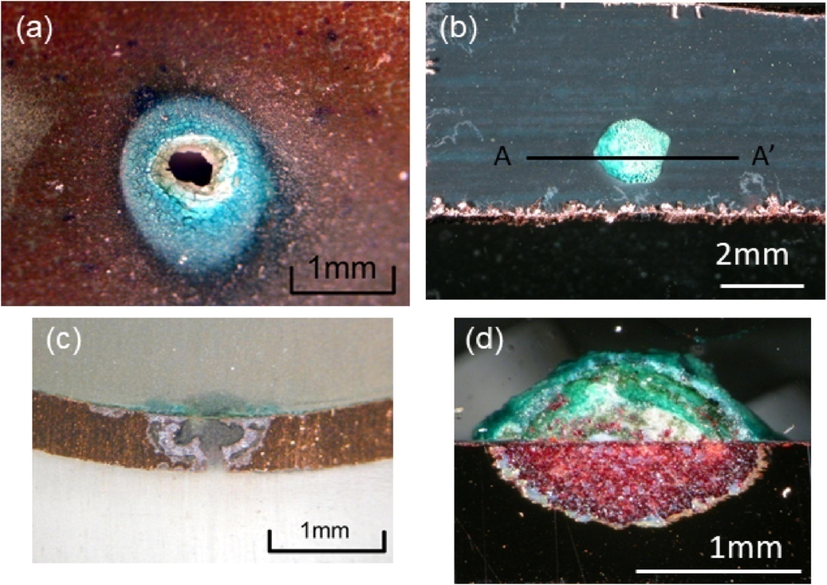
Effect of pitting corrosion on metal (Sakai, 2022).
The following mechanism occurs in pitting corrosion:
1) A protective oxide layer is possessed by the alloy in the air. However, the potentiality of MgO is + 1 V.
2) Upon immersing in a sodium chloride aqueous solution, Cl- ions are absorbed in the surrounding areas of Almon particles.
3) When the oxide film's breakdown potential approaches its free corrosion potential which is 3Com = -1.53 V for AM60.Formation of corrosion nucleus may occur near the Almon particles due to the dissolution of anode compared to Almon particles.
4) Mg (OH)2 is formed and hydrogen is evolved due to corrosion pit developed by nucleus which depends on the following chemical reactions:
Anodic Reaction: Mg = Mg2+ + 2e-.
Cathodic Reaction: 2H20 = H2 + 2OH–.
Overall Reaction: Mg2+ +2OH– =Mg (OH)2.
5) A hemi-spherical corrosion pit or an occlusion cell is developed during the progress of corrosion. Precipitates of Mg hydroxide form on the surface of samples or on the bottom of pits, bringing the pH value to 10.4–10.5 and keeping it there.
2.3 Intergranular corrosion
On the microscale, this sort of corrosion can be seen near grain boundaries in metals. Grain structures will eventually crack if surrounding grain boundaries are attacked quickly. Elements and phases can precipitate and move to grain boundaries because of aging which makes the depletion of intergranular connections (Fig. 3) (Fatoba et al., 2016). There is a mismatch between these migrating particles and the grain boundary composition. Nearby grains in a corrosive medium may lose key elements like precipitates of chromium to the chromium-rich grain boundary, and keep those regions too poor to corrosion by less concentration of chromium.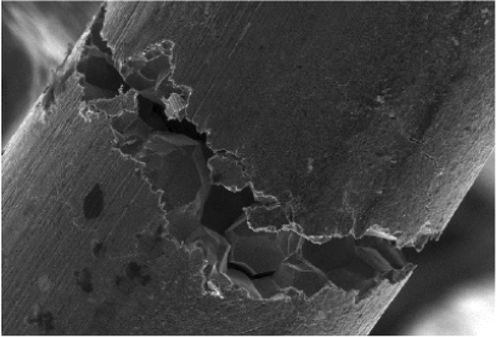
Effect of intergranular corrosion (Gabriel, n.d.).
2.4 Filiform corrosion
On the surface of metals including Mg alloys, Al alloys, and steel, filiform corrosion is frequent (Fig. 4). Active galvanic cells cause it across the metal surface. It has a cathodic tail and an anodic head. It's frequently associated with metal surfaces that have been treated with a protective coating. On pure Mg, this does not occur (Ghali, 2000). The corrosion of AZ91 was studied by LUNDER et al (Lunder et al., 1994), who discovered that the early phases of corrosion for magnesium alloy AZ91 such as filiform corrosion and corrosion features. Pits are created by filiform corrosion. A filiform corrosion model was developed by DEXTER (Dexter, 1987) that could explain the causes of filiform corrosion in magnesium by the difference in the concentration of oxygen in the head and tail.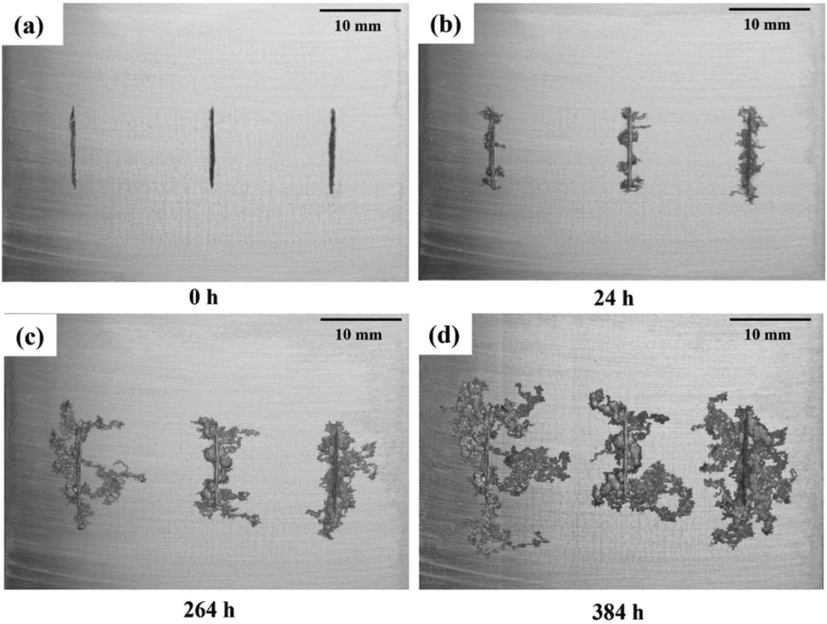
Effect of filiform corrosion (Kousis and Keil, 2022).
2.5 Crevice corrosion
Only a small area is affected by crevice corrosion (Fig. 5). It occurs in metals and alloys with unstable passive coatings when chloride and hydrogen ions are present. Nonferrous metals like zinc, copper, titanium, aluminum, etc are susceptible to this. Under debris deposits in cracks, gaps, riveted sections, flanges, or isolated regions are attacked by this type of corrosion. Deposits of sand and dust as well as products of degradation and scales may work to favorite sites for the difficult reintroduction of corrosive electrolytes (Maeda et al., 2018). For crevice corrosion to occur, the hole must be large enough to accommodate and sustain the electrolyte. Despite the fact that there are no prescribed crevice widths, crevice corrosion can occur when there are gaps of a few micrometers. When there is a high amount of chromium present, crevice corrosion can be minimized (British, n.d.). Tiny gaps in flanges and joints can cause cell or galvanic corrosion whereas a bigger hole allows electrolytes to concentrate and cause crevice corrosion.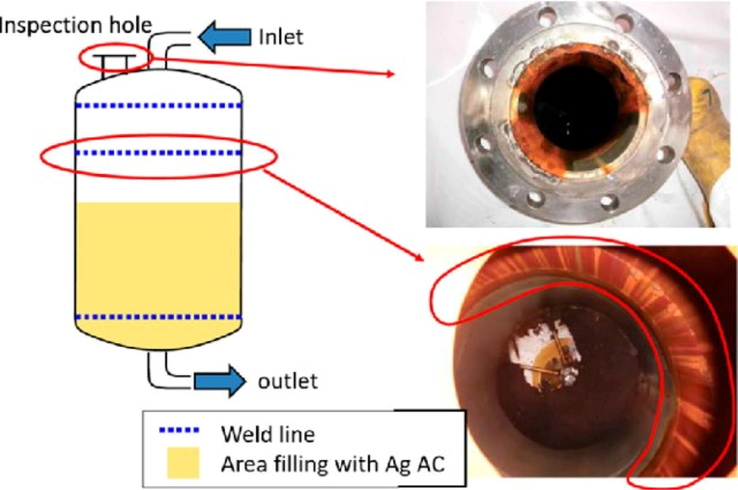
Effect of crevice corrosion (Maeda et al., 2018).
SEM images of before and after corrosion analysis to compare the effect of corrosion is shown in Figs. 6 and 7 (Istrate et al., 2022; Istrate et al., 2021; Istrate et al., 2020; Istrate et al., 2020).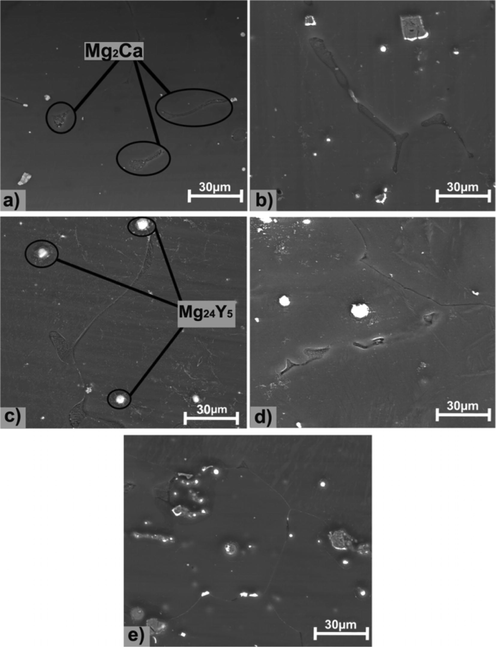
SEM images of Mg alloy before corrosion test (a) Mg-0.5Ca-0.5Y, (b) Mg-0.5Ca-1.0Y, (C) Mg-0.5Ca-1.5Y, (d) Mg-0.5Ca-2.0Y, (e) Mg-0.5Ca-3.0Y.
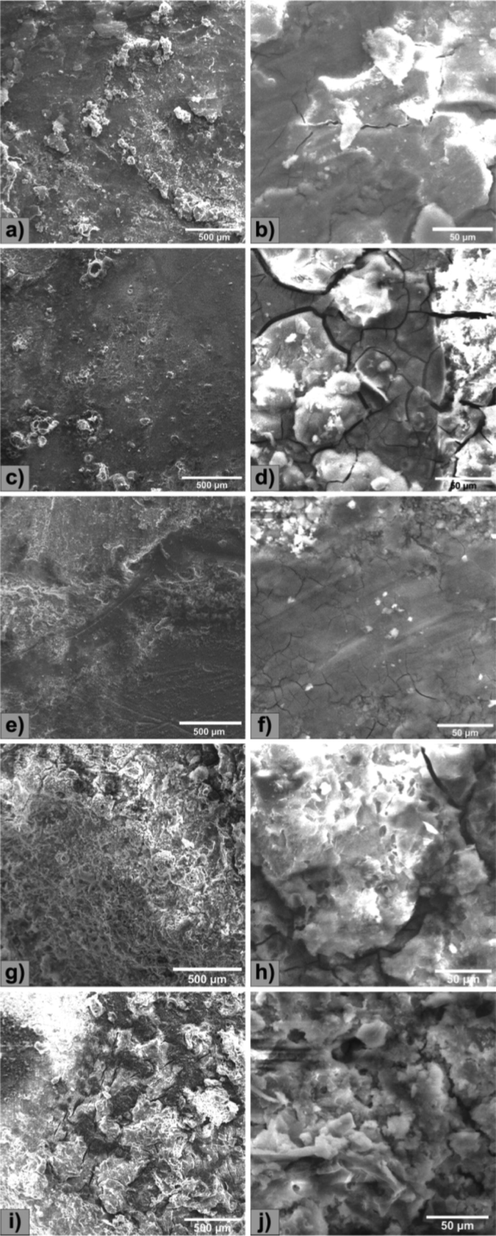
SEM images of Mg alloy after corrosion test (a,b) Mg-0.5Ca-0.5Y, (c,d) Mg-0.5Ca-1.0Y, (e,f) Mg-0.5Ca-1.5Y, (g,h) Mg-0.5Ca-2.0Y, (i,j) Mg-0.5Ca-3.0Y.
3 Coating on Mg alloys for anticorrosion
3.1 Chemical conversion coatings
Surface films of phosphates (Sadio et al., 1999; Tang and Jaworski, n.d.), oxides (Yukio and Toshihiko, 1980; Kataoka, 1980; Furta et al., 1988) chromates (Yoshinori, n.d.), and other compounds (Ger et al., 2003; Kozo et al., 2007; Takenaga et al., 2008) formed by the electrochemical or chemical treatment of a metal surface are known as conversion coatings. The metal surfaces are chemically linked to the superficial coatings. The films give corrosion protection as well as good paint-base qualities to magnesium alloys. Conversion films come in a variety of forms, including chromate (Hebert and De, 1947; Osamu et al., 1980; Leuzinger, 1966; Hagans, 1986; Heller, 1960), phosphate, and anodizing films. The most effective and mature process is chromate conversion, which is described in detail by B. Luan (Wang et al., 2019). It is widely used in the industry as it is highly stuck and corrosion-resistant. Neither uniform iridescent brassy color nor gray powder material was achieved after the immersion in chromate solution. Moreover, no sparking was observed during the wire brushing grinding operation of the workpiece. On the other hand, Cr6+ in chromate baths is an extremely dangerous carcinogen that is rapidly becoming illegal. The creation of an environment-friendly process is in high demand as strict legislation is already in place or proposed to protect the environment. Investigations are going on phosphate treatment to replace chromate conversion films (Sadio et al., 1999). Phosphate films are produced by the reaction of Mg substrate and dehydrogenate phosphates M(H2PO4)2. The process can be expressed by the following reaction: Mg + 2e- = Mg2+ (x + y + 3z)Mg2+ + (2x + 2y + 6z)H2PO4-= xMg(H2PO4)2 + yMgHPO4+ ZMg3 (PO4)2- +(y + 4z)H3PO4 Meanwhile,M(H2PO4)2 = MHPO4 + H3PO4 3MHPO4 = M3(PO4)2 + H3PO4
Though parts of Mg alloy such as 3C products are treated in the industry by phosphate, it is not without its drawbacks. To begin with, the phosphating film grains are coarse, and because of the higher activity of magnesium alloys, cracks develop in the grains. To get fine grains, the phosphate solution's composition should be adjusted. Second, heavy metal ions in phosphate solution might pollute the environment, resulting in higher waste treatment costs. Another frequently used approach in the industry is anodizing technology (Ger et al., 2006; Katsuhiko, 2001; Jiang et al., 2002; Zhang et al., 2009), which produces stable as well as thick oxide film on metals for being an electrolytic process. Paint adherence to metals can be increased by using anodic oxide coating (Okuda et al., 2008; Okuda et al., 2008), as a coloring key, or as a passivity treatment (Atsushi et al., 1983; Takeshita and Kobayashi, 1985). In general, there are two primary anodizing processes: oxygen precipitation and film formation. The following equation depicts the reaction at the anode and cathode.
Anode: 2OH = H2O + O + 2e O + Mg = MgO Mg = Mg2+ + 2e 2OH + Mg2+ =Mg (OH)2
Cathode: 2H+ + 2e = H2
As illustrated in Fig. 8, compact layer formation, porous layer formation, and porous layer growth are the stages used to separate the growth of the anodizing coating. A number of factors influence the properties of the coating such as electrolyte content, voltage, and time. Dow Chemical's (Co, 1959) chemical treatment Dow 17 and the HAE procedure can be used on any magnesium alloy. The therapy procedure is as follows: Degrease -Pickle with Acid - Electro polishing or brightness - Anodizing - Waterproofing.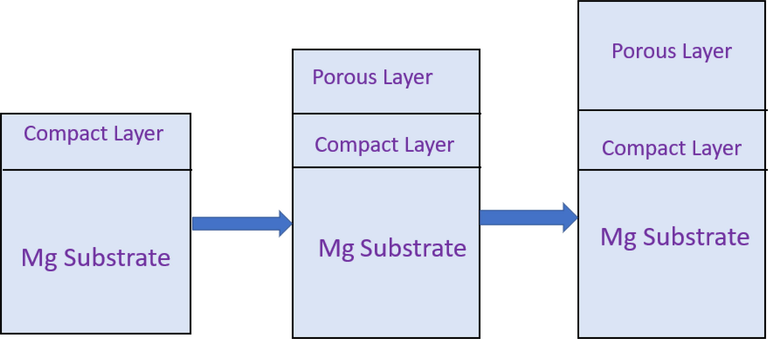
Schematic diagram of the growth of anodizing coating.
3.2 Plating
Electroplating and electroless plating are the two types of plating processes. A metal salt in solution is converted to its metallic state in both circumstances and deposited on the workpiece's surface. Electroplating uses an external power source to supply electrons. A chemical reducing agent supplies electrons in the solution in electroless plating. The plating process has three major phases, as depicted in Fig. 9. Concentration diffusion collects the cations at the cathode surface first. Second, during the displacement reaction, the cations are consumed at the cathode. An example of generic formula can be Men+ + ne Me. A layer is formed at the end due to metal crystal deposition at the surface of the substrate. The use of plating on Mg and alloy surfaces has been demonstrated in applications. However, because of magnesium's higher chemical reactivity, a good number of hydrogen evolved in the platting process with a huge replacement reaction. Because of this, poor adherence is observed between the plating layer and Mg alloys. Usually, it is difficult for plating to perform pretreatment on Mg alloys. Consequently, ASTM B 480–1988 (ASTM B 480. 1988) standard was established to manufacture Mg alloys in the electroplating process. Improved approaches were proposed to reduce production cost and simplify the process.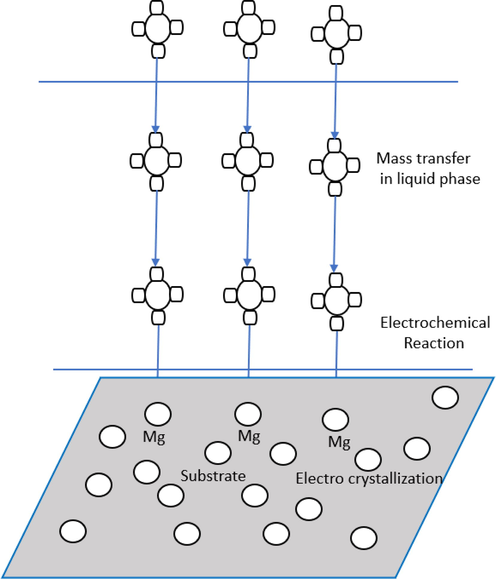
Schematic diagram of electroplating process.
3.3 Sol-gel, dip and spin coatings
A sol–gel deposition is a common method for manufacturing ceramic ultra structures at practically room temperature by reacting inorganic precursors or hydrolyzing organometallic compounds (alkoxides), resulting in a gel of hydrous oxides that will sinter into a dense and homogeneous ceramic body. Chemical solution deposition is a wet chemical technology frequently used in ceramic engineering and material science as also known as the sol–gel method. These technologies are generally utilized to synthesize materials for an integrated network where a chemical solution serves as a precursor (Heimann and Lehmann, 2015). Dip coating ad sol–gel techniques are similar but in dip coating volatile suspension media evaporates quickly as the substrate is withdrawn from the liquid coating medium at a precise rate. Compared to the traditional sol–gel process, dip coating is faster because different solvents take only a few seconds to transfer to solid from liquid. Spin speed, as well as viscosity, are independent parameters principally responsible for spin coating. The spin velocity is indirectly proportional to the coatings or deposited film thickness. The layer thickness fluctuates with spin velocity and time in the absence of solvent evaporation. Sol-gel derived coatings containing primarily biocompatible polymers such as glycidoxypropyl-trimethoxysilane or methyltriethoxysilane, silane/Mg (OH)2 composites (Gaur et al., 2016), and ndecyltriethoxysilane and tetramethoxysilane co-polymers (Ding et al., 2005) were developed for corrosion reduction of Mg alloys in vivo. Some of the used materials are nanostructured magnesium phosphate/poly (lactic acid) coatings (Ren et al., 2018), hybrid TiO2/silane and collagen/chitosan coatings (Córdoba et al., 2018), and silica-based sol–gel coatings doped with quinaldic acid, betaine, dopamine hydrochloride, and Diazolidinyl urea (Upadhyay et al., 2017). Nano-sized amorphous magnesium phosphate composite coatings were created using spin coating on AZ31 magnesium alloy. Development of massive bone-like apatite precipitates was observed on the coated samples surface that showed significant biomineralization capabilities during the immersion time in SBF. Because of this quality, the composite coating can be used on biodegradable Mg alloys as a bioactive and protective covering for orthopedic applications. The degradation of Mg-based substrates can be prevented and bone-implant integration can be promoted by the composite coatings of Nanostructured hydroxylapatite (nHAp)/poly (lactic-co-glycolic acid) (PLGA). Composites of nHAp/PLGA were spin-coated on the substrate of Mg and its alloys. The findings showed that the coatings had nHAp spread nanoscale features throughout the matrix of PLGA. Corrosion potential was increased and corrosion current was lowered by coated Mg compared to the uncoated Mg in SBF. Mg substituted calcium phosphate (CaP) and increased CaP was observed after 24 h in rSBF at the surface of nHAp/PLGA-coated Mg which is an indication of higher potential bioactivity. No deposition of substantial levels of CaP was observed when the control was only PLGA-coated Mg. Polypyrrole-coated WE43 Mg alloy’s corrosion behavior was examined by P.M. Ascencio et al (Ascencio, 2016). with daily electrolyte replenishment in SBF. During the study period, hydrogen gas formed from polypyrrole (PPy) decreased the rate of corrosion of WE43 Mg alloy. The properties of PPy coating and dissolved CO2 species adsorption on the PPy coating attribute the PPy’s corrosion resistance decreases the conductivity of PPy and decoupling of coating/substrate because of coatings uncompensated positive charge as well as Fermi levels misalignment of coating and substrate. Copolymers of poly commonly known as l-lactic acid and poly also were frequently used for Mg alloys coating material (Shi et al., 2017; Li et al., 2015).
3.4 Organic coatings
Organic (polymer) coatings are commonly used as a final or top layer for magnesium alloy protection (Song and Atrens, 1999). Pleasing appearance and corrosion resistance both are provided by the organic coating. Enhanced adhesion, superhydrophobic features, wear abrasion, and self-healing properties may also be displayed by the organic coating after suitable pre and/or post-treatment (Yao et al., 2020). Vehicle layer or binder, pigments, and different additives including stabilizing agents, dispersions agents, dryers, surface activating chemicals, and hardening agents make up an organic coating. Corrosion-inhibiting compounds may also be incorporated in polymer coatings, boosting the level of protection.
Before applying the coating, the surface of the substrate should be made clean, free from oxides, moisture, contaminants, foreign particles, and surface pores (Gray and Luan, 2002). Cleaning has two main effects: (1) eliminating all pollutants from the surface, and (2) roughening the surface to promote coating adhesion. Cleaning procedures include mechanical cleaning, solvent cleaning, alkaline cleaning, and pickling or etching.
Painting, powder coating, cathodic electrocoating (E-coating), sol–gel coating, polymer plating, plasma polymerization, and the application of lacquers, enamels, and varnishes are some of the methods used to apply organic coatings. It should be noted that the benefits and drawbacks of each of the processes discussed vary based on the solvent used. Several regularly used solvent bases once constituted significant harm to the environment and humanity. Alternative approaches have become more widely available in recent years, such as various powder coatings that do not contain any solvent or compliance solvents such as waterborne solvents.
In the vehicle industry, Mg components are protected from corrosion by E-coating and powder coating according to Wang et al (Song and Atrens, 1999). E-coating is good for covering complex geometries and comparatively cheap as it is not a coating method based on line of sight whereas better galvanic corrosion protection is obtained from powder coating. E-coatings are available from PPG, BASF, and DuPont, while powder coatings are available from Pro-Tech, AkzoNobel, and DuPont (Song and Atrens, 1999).
Resin, which comes in a variety of forms, including epoxy, polyvinyl butyral, vinyl, acrylic polyurethane, and baked phenolic with zinc chromate or strontium chromate (Hu et al., 2012), is the major component of an organic coating.
The coatings layer that will be used to protect Mg alloys should be uniform, well adhering to the substrate, self-healing, and pore free. The use of pigments of corrosion inhibition or other additives in the coating material can make the coating self-healing. Coatings of more than one layer made of primer, topcoat, and numerous mid-coats may be required in such an environment that is demanding and harsh. Different types of decorative effects can be obtained on Mg alloys by different coating compositions and treatment processes (Gray and Luan, 2002).
3.5 Hydrothermal coating
Under high temperature and pressure, the precursor is placed into an autoclave by a liquidus synthetic process to manufacture hydrothermal coating. It has the advantage of promoting crystallization, being simple to use, and being affordable. Hydrothermal coatings include double-layered hydroxide (LDH) (Fig. 10). It is anionic clay that has a highly changeable brucite structure (Tedim et al., 2012). LDH stands for M2 + 1-xMg3 + x(OH)2Anx/n·mH2O, where M2 + is the divalent metal coating and M3 is the trivalent metal coating, x stands for the molar ratio of M3+/(M2 + M3 + ) ranging from 0.2 to 0.33; An is the interlayer anions including PO43, CO32, MO42, NO3 and Cl (Guo et al., 2018; Zhao et al., 2012). Fig. 8 shows the typical structure of LDH (Bi et al., 2014).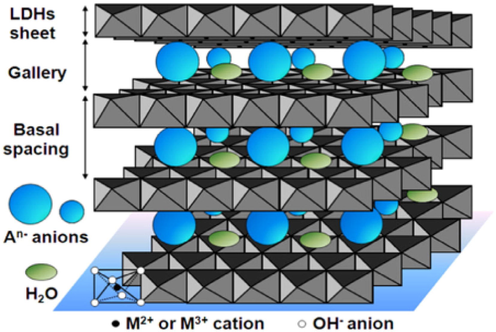
Schematic representation of the LDH (Bi et al., 2014).
Size, the orientation of anions, the ratio of metal cations, and charge were used to calculate the crystal structure properties, anion exchange capacity, and bond strength of LDH. The octahedral holes present in the brucite are occupied by the anions during the synthesis process and release gradually from this intercalated structure (Dong et al., 2017). Anions present in the double layer structure are replaced by the anions that are present in the environment which later forms precipitation on the coated surface interacting with the metal ions. The ion is exchanged in the coating which decreases the aggressive anions concentration for example ions of chlorides that makes the substrate more protective (Zeng et al., 2014). Different researches show that covering which is like an interlocking plate can exchange ions and are self-healing. This covering can be used in different applications. It works as a “smart” coating responding to ambient stimuli. A Zn-Al-LDH coating was synthesized by Zeng et al. (Zhang et al., 2014) which was made of hydrothermally treated and co-precipitated on Mg alloy AZ31 compact and uniform nano-plates and then sealed by a vacuum freeze-dried porous poly coating. The ion exchangeability, barrier function, and self-healing ability made the composite coating corrosion-resistant. Zhang et al (Zhang et al., 2018; Zhang et al., 2018; Zhang et al., 2018). manufactured different types of LDH coatings using various techniques. The demonstration was done to evaluate the self-healing ability of a PA/LDH/Mg (OH)2 coating. An LDH/PDA composite coating was manufactured by Li et al (Li et al., 2018). on AZ31 Mg alloy with surface heparinization and the composite was tested.
3.6 Water–repellent antistatic coatings
Refining polymer foils as well as antistatic qualities in completing textiles are offered by a surface-treated hydrophobic sol–gel system that results in hydrophobic sol–gel coatings. Sol-gel formulations are mixed with hydrophilic as well as hydrophilic components at the same time (Fayna et al., 2005; Tiwari and Hihara, 2015; Brusatin et al., 2004). Alkyl chains and alkoxysilanes are used to modify hydrophobic sections but amino-functionalized alkoxysilanes are used to modify hydrophobic parts (Koc et al., 2008; Conde et al., 2003). The surface of the formed coatings is hydrophobic which is the strongest when comes to contact with solid or air whereas the deeper region of the coatings is hydrophilic. Textiles can have enough humidity absorbing ability, and water repellency, and can provide antistatic qualities treated with these coatings (Liu et al., 2009; Guo et al., 2011). Researchers are going on to develop SiO2 sols coating for textile finishing having antistatic characteristics and hydrophobic properties for simultaneous applications. Bulk coating or surface could be directed by active substances using hydrophobic or hydrophilic groups instead of sol–gel-based coating (Kasanen et al., 2009; Liu et al., 2013; Darmanin et al., 2013).
3.7 Super hydrophobic surfaces
Silica/fluoropolymers nano and micro scale binary structured composite particles were synthesized by sol–gel method mediated by emulsion technique. Lotus leaves surface microstructure was then replicated by applying those composite particles to different substrates (Santana et al., 2015; Cai et al., 2014; Bessede, 1990). Potential uses can be obtained when the contact angle between water contact and ultra-hydrophilic surfaces will be more than 150° for anti-fog, rust-resistant, and self-cleaning applications (Jonsson et al., 2006; Pengyao et al., 2019). The formed Si–O–Si chemical bonds between Si-OH of the glass substrate and Si-OH groups from TMSM and TMOS made the silica-based coating more adhesive which is the main beneficial thing about the hybrid coating. The coating shows more plastic behavior due to the presence of organic chains compared to the TEOS or TMOS-made inorganic coating (Tanem and Svenningsen, 2005) (Fig. 11).
Schematic diagram for super hydrophobic surfaces (Pengyao et al., 2019).
3.8 Self‑cleaning coatings
Reduction in cost and unique applications made self-cleaning coatings more usable in the field of window glasses, paints, cement, and textiles (Fig. 12). Woven goods life is improved and cleaning money is saved by the self-cleaning fabrics. Hydrophilic and hydrophobic are the two types of self-cleaning coatings that can clean themselves when they are in contact with water (Metroke et al., 2002). The high hydrophobicity of the surface repels water and keeps the surface free from the direct touch of filth. Photocatalysis is the basis of the hydrophilic self-cleaning coatings which allows them to break down pollutants in the presence of light. This coating material has already been used to make self-cleaning windows (Fayna et al., 2005; Davo et al., 2004).
Self-cleaning coating (Davo et al., 2004).
4 Implant applications
4.1 Orthopedic application of FLU-coated Mg against bare Mg
4.1.1 Bone repair
-
New bone formation
In eight studies (Sun et al., 2016; Jiang et al., 2017; Li et al., 2017; Iglesias et al., 2015; Sun et al., 2013; Barbeck et al., 2020; Naujokat et al., 2020; Bodelón et al., 2015), new bone production was described. Better new bone was developed than BM by FLU-coated Mg in four trials. Mg coated with FLU had higher bone density with smaller holes whereas the bone density of BM is low. The screws of Mg coated with FLU had higher tissue mineral content (TMC) and tissue mineral density (TMD) than BM which implies that the osteo inductivity is improved by the FLU coating. The new bone made of Mg coated with FLU was more compact compared to BM. No significant statistical difference between BM and Mg coated with FLU were observed in the studies, and there was no evidence that the FLU-coated Mg group was superior to the BM group in bone repair. Both the FLU-coated Mg and the BM groups showed new bone growth, but no comparison was made. The degradation rate is obtained at 0.31 ± 0.13 mm/year. Besides, compared to the current Mg alloys, coated Mg alloys have higher anti-corrosion properties (Yang et al., 2020; Yang et al., 2021; Yang et al., 2021).
-
Bone-implant contact
The bone-implant contact has been described in two studies. At three months, the study found closer contact and consistent implants. No statistical significant difference was observed between the two groups.
4.1.2 Material properties
-
Degradation
The deterioration of magnesium alloys has been studied in eight types of research (Keerthi Soujanya et al., 2014; Chan et al., 2013; Gaur et al., 2016; Akram et al., 2020; Tian et al., 2019; Xiong et al., 2019; Sun et al., 2016; Jiang et al., 2017). The degrading performance of FLU-coated Mg was found to be superior to that of BM in four experiments. There was apparent corrosion and fracture in the BM group, substantially slower degradation is shown by the Mg coated with FLU, suggesting that it might provide enough support for the bone. Between the two groups, there was a statistically minor difference in re-absorption. Implant remnants were found in both groups, according to the study, although no comparison was done.
-
Gas formation
The gas formation was described in five studies. In three trials, there was less hydrogen, as evidenced by the absence of an evident gas hole, no sign of a gas shadow, and lower gas emission. On the other hand, according to another research, results for the Mg group coated with FLU were not provided, and only the BM group's gas production and absorption were documented. Both groups saw a drop in gas volume, but no comparison was made.
4.1.3 Systemic host responses
-
Influence on the major organs
The effect of transplants on major organs was studied in two investigations. In this study, the outcomes of the Mg groups coated with FLU were not specified. No effect was observed from the BM and Mg coated with FLU on the physiological functions of the liver and kidney at three months. Besides, statistically, no significant difference was observed between the two groups.
-
Ion concentration in serum
Only one study looked at serum ion concentrations. The study found that the degradation of both groups had no effect on serum Ca2+ levels and that the increase in serum Mg2+ had no statistically significant difference.
-
Clinical findings
In two investigations, the FLU-coated Mg group outperformed the BM group in terms of infection resistance. Inflammatory cell infiltration was seen in the BM group at one and two weeks, as well as local hemorrhage. After one week, only limited inflammatory cell infiltration was observed by the Mg group coated with FLU, until two months, bleeding symptoms and inflammatory reaction were not observed, and at three months, no inflammatory response was observed. At three months, neither group showed any visible signs of inflammation. During the degradation process, however, phagocytes consume more Mg particles in the BM group, whereas the Mg group coated with FLU contained only a few microscopic Mg particles. In five investigations, the FLU-coated Mg or BM group revealed no foreign cells or symptoms of inflammation. Other investigations have not made any comparisons.
Besides, the coated Mg alloy provides a better environment for bone-implant integration and can be applied in many clinical orthopedic surgeries, hard tissue engineering, and bone regeneration. It can also act as a drug carrier, drug delivery, and bone tissue engineering (Rezk et al., 2019; Zhu et al., 2021).
Mg metal is converted into implants in various coating processes such as biodegradable polymer coatings, biodegradable silane coatings, biodegradable calcium phosphate coatings, graphene-derivative based coatings which make the implant excellent biocompatible, provide adequate biological response, has good mechanical properties, excellent corrosion resistance, and high resistance to fatigue (Banerjee et al., 2019).
5 Conclusion and future outlook
The most promising green engineering materials are the Mg alloys. Magnesium has exceptional technical and mechanical qualities, particularly in terms of its lightness and density. Magnesium's greatest strength is its capacity for biodegradation, but the greatest drawback is the higher rate of degradation. Magnesium declines rapidly in a physiological milieu. Galvanic corrosion, pitting corrosion, filiform corrosion, intergranular corrosion (IGC), and crevice corrosion are the many kinds of magnesium corrosion. Every year, the number of patents linked to magnesium alloys grows. However, only a small percentage of these patents are actually used in the industry. When developing a coating process for commercial applications, a number of aspects such as capital expenditure, ease of production, coating performance, and environmental issues must be considered. In order to protect Mg from corrosion, Mg alloys impurities and alloying must be controlled according to the limit of endurance. Magnesium alloy corrosion mechanisms are still poorly understood. As a result, it is imperative that this investigation be continued. The creation of a flawless coating on Mg alloys is difficult for corrosion experts. Future R&D focuses on the coating techniques that provide strong adhesion, corrosion and wear resistance, and high impact resistance. However, there are currently no coatings that can meet all of these requirements. Phosphate dialogue films, polymers, and other non-metallic coatings are examples. These films are incredibly thin and fragile, with little mechanical strength and hardness, making them difficult to integrate into an assembly. The development of new designs will open new areas of applications by the use of resorbable implants in the future that will tailor the material, project deterioration, and new methods for manufacturing the implants of these Mg. The Ca-covered Mg alloy had improved biocompatibility, osteoconductive properties, and corrosion resistance, indicating that it might be used in a variety of orthopedic applications. Although the described polymer coatings have improved the performance of Mg alloys, no coating method is yet ready for clinical use. Some essential coating properties must be considered such as permeability, adhesions, and degradation for the successful use of implants. Further improvement should be done on degradation mode. Innovative design should be introduced to improve mechanical strength that fits with natural bone. Besides, bio-safety still remains a concerning question that requires further research.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
References
- ACS Appl. Bio Mater.. 2020;3:7052.
- Investigation of the corrosion behaviour of bare and polypyrrole-coated WE43 magnesium alloy for the development of biodegradable implants. McGill University; 2016. Ph.D. Thesis.
- ASTM B 480. In: Standard Guide for Preparation of Magnesium and Magnesium Alloys for Electroplating 1988.
- Atsushi F, Mitsuru SKI, Toshio I. Method for forming colored protective film on surface of magnesium material. JP patent 56096564, 1983.
- Degradation, bone regeneration and tissue response of an innovative volume stable magnesium-supported GBR/GTR barrier membrane. Int. J. Mol. Sci.. 2020;21(9):3098. View at: Publisher Site | Google Scholar
- [Google Scholar]
- Bessede JL (1990) PhD dissertation, INSA, Lyon, 3233 France,p 112.
- Pharmaceutics. 2014;6:298-332.
- Analysis of metallic traces from the biodegradation of endomedullary AZ31 alloy temporary implants in rat organs after long implantation times. Biomed. Mater.. 2015;10(4):045015. View at: Publisher Site | Google Scholar
- [Google Scholar]
- British Stainless Steel Association. Principles and Prevention of Crevice Corrosion.
- Poled sol–gel materials with heterocycle push–pull chromophores that confer enhanced second-order optical nonlinearity. Adv. Funct. Mater.. 2004;14:1160-1166.
- [Google Scholar]
- Sol-gel preparation of hydrophobic silica antireflective coatings with low refractive index by base/acid two-step catalysis. ACS Appl. Mater. Interf.. 2014;6:11470-11475.
- [Google Scholar]
- Biodegradability engineering of biodegradable Mg alloys: tailoring the electrochemical properties and microstructure of constituent phases. Sci. Rep.. 2013;3:1-6.
- [Google Scholar]
- Mater. Sci. Eng. C. 2013;33:5019.
- Recent advances on the development of magnesium alloys for biodegradable implants. Acta Biomater.. 2014;10:4561-4573.
- [Google Scholar]
- Co DC. Anodizing magnesium. US patent 2901409, 1959.
- Polymeric sol–gel coatings as protective layers of aluminum alloys. Prog. Org. Coat.. 2003;46:288-296.
- [Google Scholar]
- Hybrid coatings with collagen and chitosan for improved bioactivity of Mg alloys. Surf. Coat. Technol.. 2018;341:103-113.
- [Google Scholar]
- Implant surface modification strategies through antibacterial and bioactive components. Biopolymer-Based Formulations: Elsevier, Amsterdam; 2020. p. :647-673.
- Superhydrophobic surfaces by electrochemical processes. Adv. Mater.. 2013;25:1378-1394.
- [Google Scholar]
- Inhibition of stress corrosion cracking of alloy AA8090 T-8171 by addition of rare earth salts. Corros. Sci.. 2004;47:1227-1237.
- [Google Scholar]
- DEXTER S C. Metals Handbook (Vol.l3) [M]. 9th ed. OH: ASM International, 1987. 106. WILLIAM K, MILLER. Stress.
- Ding WB, Jiang HY, Zeng XQ, et al. Method of B4C+Al remelting on magnesium alloy surface. CN patent 200510025065.2, 2005
- J. Magnes. Alloy.. 2017;5:320-325.
- Temporary scaffolding of coronary arteries with bioabsorbable magnesium stents: a prospective, non-randomizedmulticenter trial. Lancet. 2007;369:1869-1875.
- [Google Scholar]
- Computational dynamics of anti-corrosion performance of laser alloyed metallic materials. Fiber Laser. 2016;20:461-469.
- [Google Scholar]
- Mechanicalproperties of hybrid organic–inorganic materials. J. Mater. Chem.. 2005;15:3787-3794.
- [Google Scholar]
- Mechanical properties of hybrid organic–inorganic materials. J. Mater. Chem.. 2005;15:3787-3794.
- [Google Scholar]
- Furta M, Uehara K, Kobayashi W. Anodizing solution for anodic oxidation of magnesium or its alloys. US patent 19870030941, 1988.
- Gabriel A. Farotade, Patricia A. I. Popoola and Olawale M. Popoola, Computatonal Analysis of System and DesignParameters of Electrodeposition for Marine Applications.
- Swati Gaur, Saumya Nigam, A.S.Khanna, R.K. Singh Raman, Silane-Coated Magnesium Implants with Improved In-Vitro Corrosion Resistance and Biocompatibility, Journal of Materials Science & Surface Engineering Vol. 4 (5), 2016, pp 415-424.
- Silane-coated magnesium implants with improved in vitro corrosion resistance and biocompatibility. J. Mater. Sci. Surf. Eng.. 2016;4(5):415-424.
- [Google Scholar]
- Ger MD, Yang KH, Sung Y, et al. Method for treating magnesium alloy by chemical conversion. US patent 20030230365, 2003.
- Ger MD, Chang CL, Sung Y, et al. Method for treating surface of magnesium or magnesium alloy. US patent 20060390206, 2006.
- Corrosion and protection of magnesium alloys [J] Mater. Sci. Forum. 2000;350–351:261-272.
- [Google Scholar]
- Protective coatings on magnesium and its alloys—a critical review. J. Alloys Compd.. 2002;336:88-113. [Google Scholar] [CrossRef]
- [Google Scholar]
- Biomed. Mater.. 2009;4:44109.
- Superhydrophobic surfaces: from natural to biomimetic to functional. J. Colloid Interface Sci.. 2011;353:335-355.
- [Google Scholar]
- J. Mater. Sci. Technol.. 2018;34:1455-1466.
- Safety and performance of the drug-eluting absorbable metal scaffold (DREAMS) in patients with de-novo coronary lesions: 12-month results of the prospective, multicenter, first-in-man BIOSOLVE-I trial. Lancet. 2013;381:836-844.
- [Google Scholar]
- Hagans PL. Method for providing a corrosion resistant coating for magnesium containing materials. US patent 4569699, 1986.
- Hebert K, De L. Surface treatment of magnesium alloys. US patent 2428749, 1947.
- Bioceramic Coatings for Medical Implants. WileyVCH, Weinheim: Trends and Techniques; 2015.
- B. Heinlein, R. Rohde, V. Kiese, M. Niemeyer, W. Hartung, A. aver ich, Biocorrosion of magnesium alloys: a new principle in cardiovascular plant technology? Heart, 89 (2003) 651-656
- Heller FP. Method of forming a chromate conversion coating on magnesium. CA patent 603580, 1960.
- Recent progress in corrosion protection of magnesium alloys by organic coatings. Prog. Org. Coat.. 2012;73:129-141. [Google Scholar] [CrossRef]
- [Google Scholar]
- N. Hurt, Y. Huang, D. Fechner, M. Stormers, C. Blauert, F. Witte, C. Vogt, H. Drucker, R. Willum it, K. Kaine, Magnesium alloys as implant materials–principles of property design for Mg–RE alloys, Acta Biomaterial, 6 (2010) 1714-1725.54.
- C. Iglesias, O. G. Bodelón, R. Montoya et al., “Fracture bone healing and biodegradation of AZ31 implant in rats,” Biomedical Materials, vol. 10, no. 2, Article ID 025008, 2015.View at: Publisher Site | Google Scholar
- Electrochemical analysis and in vitro assay of Mg-0.5Ca-xY biodegradable alloys. Materials. 2020;13(14):3082.
- [CrossRef] [Google Scholar]
- Bogdan Istrate, Julietta V. Rau, Corneliu Munteanu, Iulian V. Antoniac, Vicentiu Saceleanu, Properties and in vitro assessment of ZrO2-based coatings obtained by atmospheric plasma jet spraying on biodegradable Mg-Ca and Mg-Ca-Zr alloys, Ceramics International, 46, 10, Part B, 2020, 15897-15906
- Microstructural, electrochemical and in vitro analysis of Mg-0.5Ca-xGd biodegradable alloys. Appl. Sci.. 2021;11(3):981.
- [CrossRef] [Google Scholar]
- Current research studies of Mg–Ca–Zn biodegradable alloys used as orthopedic implants—review. Crystals. 2022;12(10):1468.
- [CrossRef] [Google Scholar]
- J. e. Sun, J. Wang, H. Jiang, M. Chen, Y. Bi, and D. Liu, “In vivo comparative property study of the bioactivity of coated Mg-3Zn-0.8Zr alloy,” Materials Science and Engineering: C, Materials for Bilogical Applications, vol. 33, no. 6, pp. 3263–3272, 2013.View at: Publisher Site | Google Scholar
- Biodegradable magnesium alloys for orthopaedic applications: a review on corrosion, biocompatibility and surface modifications. Mater. Sci. Eng. C. 2017;75:1068-1074. View at: Publisher Site | Google Scholar
- [Google Scholar]
- Jiang BL, Zhang SF, Hao JM, et al. Process of surface treatment on magnesium alloy. CN patent 01106741.1, 2002
- Self-cleaning, titanium dioxide based, multilayer coating fabricated on polymer and glass surfaces. J. Appl. Polym. Sci.. 2009;111:2597-2606.
- [Google Scholar]
- Kataoka A. Surface treating method of magnesium or its alloy. JP patent 53149330, 1980.
- Katsuhiko M, Kei taro Y, Takayuki K. Method for surface treatment of magnesium or magnesium alloy. JP patent 11306790, 2001.
- Electrospun nanofibrous polymer coated magnesium alloy for biodegradable implant applications. Procedia Mater. Sci.. 2014;5:817-823.
- [Google Scholar]
- Nano-scale superhydrophobicity: suppression of protein adsorption and promotion of flow-induced detachment. Lab Chip. 2008;8:582-586.
- [Google Scholar]
- Christos Kousis, Patrick Keil, Hamilton, Neil McMurray, Geraint Williams, The kinetics and mechanism of filiform corrosion affecting organic coated Mg alloy surfaces, Corrosion Science, 206, 2022, 110477
- Kozo I, Shuji T, Kinfe T, et al. Surface treatment method for magnesium alloy material and magnesium alloy material treated thereby oral institute of advanced industrial and technology. JP patent 2005350043, 2007.
- Leuzinger JM. Conversion of coating of magnesium alloys surfaces. CA patent 726661, 1966.
- Drug delivery property, antibacterial performance and cytocompatibility of gentamicin loaded poly(lactic-co-glycolic acid) coating on porous magnesium scaffold. Mater. Technol.. 2015;30(sup6):B96-B103.
- [Google Scholar]
- Biodegredable and Bioactive Orthopaedic Magnesium Implants with Multilayered Protective Coating. ACS Applied Bio Materials; 2019.
- Biomater. Sci.. 2018;6:1846-1858.
- In vitro and in vivo corrosion, mechanical properties and biocompatibility evaluation of MgF 2 -coated Mg-Zn-Zr alloy as cancellous screws. Korean J. Couns. Psychother.. 2017;75:1268-1280. View at: Publisher Site | Google Scholar
- [Google Scholar]
- Novel magnesium alloys developed for biomedical application: a review. J. Mater. Sci. Technol.. 2013;29:489-502.
- [Google Scholar]
- Bio-inspired superoleophobic and smart materials: design, fabrication, and application. Prog. Mater. Sci.. 2013;58:503-564.
- [Google Scholar]
- Bioinspired design of a superoleophobic and low adhesive water/ solid interface. Adv. Mater.. 2009;21:665-669.
- [Google Scholar]
- LUNDER 0, LEIN J E, HESJEVIK S M, AWE T KR, NISANCIOGLU. Corrosion morphologies on magnesium alloy A291 [J]. Eristoff und Corrosion, 1994.45: 331-340
- M. Born pour, M. Colicin, M. Peculiarize, Thermal exposure effects on the in vitro degradation and mechanical properties of Mg–Sr and Mg–Ca–Sr biodegradable implant alloys and the role of the microstructure, Materials Science and Engineering: C, 46 (2015) 16-24
- Influenceof activated carbon on crevice corrosion in adsorption tower of advanced liquid processingsystem. Corros. Eng. Sci. Technol.. 2018;53(sup1):39-43.
- [Google Scholar]
- Spectroscopic and corrosion resistance characterization of amine and super acid-cured hybrid organic-inorganic thin films on 2024–T3 aluminiumalloy. Prog. Org. Coat.. 2002;44:185-199.
- [Google Scholar]
- Influence of surface modifications on the degradation of standard-sized magnesium plates and healing of mandibular osteotomies in miniature pigs. Int. J. Oral Maxillofac. Surg.. 2020;49(2):272-283. View at: Publisher Site | Google Scholar
- [Google Scholar]
- Okuda Y, Sakai K, Hino M, et al. Manufacturing method of magnesium or magnesium alloy product having anodic oxidation coating on surface. JP patent 2008137327, 2008.
- Okuda Y, Sakai K, Hino M, et al. Magnesium or magnesium alloy product having electroconductive anodic oxidation coating film on surface thereof and method for production thereof. JP patent 2008137326, 2008.
- Osamu M, Atari Y, Samburu S, et al. Surface treatment of magnesium or magnesium alloy. JP patent 54057110, 1980.
- A facile method of superhydrophobic coating on rubberwood to improve its anti-mildew performance. BioResources. 2019;14(3):7111-7121.
- [Google Scholar]
- Surface characterization and cytotoxicity response of biodegradable magnesium alloys. Mater. Sci. Eng. C. 2015;49:761-768.
- [Google Scholar]
- Nanostructured amorphous magnesium phosphate/poly(lactic acid) composite coatings for enhanced corrosion resistance and bioactivity of biodegradable AZ31 magnesium alloy. Progr. Org. Coat.. 2018;118:1-8.
- [Google Scholar]
- J. Coat. Technol. Res.. 2019;16:477.
- Riaz U, Shabib I, Haider W. 2018. The current trends of Mg alloys in biomedical applications—A review. J Biomed Mater Res B Part B. 2018:9999B:9999B:1–27.
- Sadio I, Yoichi S, Masahiko N. Composition and process for treating magnesium-containing metals and product therefrom. US patent 19970822444, 1999.
- A new type of silica-induced “moundless” pitting corrosion in copper observed in Japan. Heliyon. 2022;8(8):e10110.
- [Google Scholar]
- Mater. Sci. Eng. B. 2011;176:1703.
- Corrosion protection of carbon steel by silica-based hybrid coatings containing cerium salts: effect of silica nanoparticle content. Surf. Coat. Technol.. 2015;265:106-116.
- [Google Scholar]
- P. Shi, B. Neu, S. E, Y. Chen, Q. Li, Preparation and characterization of PLA coating and PLA/MAO composite coatings on AZ31 magnesium alloy for improvement of corrosion resistance, Surface and Coatings Technology, 262 (2015) 26-32
- Y.J. Shi, J. Pei, J. Zhang and 5 additional authors. Enhanced corrosion resistance and cytocompatibility of biodegradable Mg alloys by introduction of Mg(OH)2 particles into poly(l-lactic acid) coating. Sci. Rep., 7 (2017), 41796
- Corrosion and corrosion prevention of magnesium alloys [J] Mater. Corros.. 1999;50:2-6.
- [Google Scholar]
- Corrosion mechanisms of magnesium alloys. Adv. Eng. Mater.. 1999;1:11-33. [Google Scholar] [CrossRef]
- [Google Scholar]
- The fluoride coated AZ31B magnesium alloy improves corrosion resistance and stimulates bone formation in rabbit model. Mater. Sci. Eng.: C, Mater. Biol. Appl.. 2016;63:506-511. View at: Publisher Site | Google Scholar
- [Google Scholar]
- Takenaga S, Kawakami M, Ono T, et al. Highly corrosion-resistant magnesium alimony and its production method. JP patent 2006217864, 2008.
- Takeshita S, Kobayashi W. Aqueous anodizing solution and process for coloring article of magnesium or magnesium-base alloy. US patent 19840631577, 1985.
- Relations between sample preparation and SKPFM Volta potential maps on an EN AW-6005 aluminium alloy. J. Mardalen Corros. Sci.. 2005;47:1506-1519.
- [Google Scholar]
- Tang X, Jaworski M, Hammer Schmidt K. Corrosion resistant, chromate-free conversion coating for magnesium alloys. US patent 20030601247, 2004-12.
- Corros. Sci.. 2012;55:1-4.
- Nano-to-submicron hydroxyapatite coatings for magnesium-based bioresorbable implants – deposition, characterization, degradation, mechanical properties, and cytocompatibility. Sci. Rep.. 2019;9:810.
- [CrossRef] [Google Scholar]
- Sol-gel route for the development of smart green conversion coatings for corrosion protection of metal alloys. In: Intelligent Coatings for Corrosion Control. Boston, MA: Butterworth-Heinemann; 2015. p. :363-407.
- [Google Scholar]
- Silica-based sol-gel coatings on magnesium alloy with green inhibitors. Coatings. 2017;7(7):86.
- [Google Scholar]
- Magnesium based implants for functional bone tissue regeneration – a review. J. Magn. Alloy.. 2022;10(2):356-386.
- [Google Scholar]
- Magnesium biomaterials for orthopedic application: a review from a biological perspective. J. Biomed. Mater. Res. Part B Appl. Biomater.. 2014;102:1316-1331.
- [Google Scholar]
- Surface protection of Mg alloys in automotive applications: a review. AIMS Mater. Sci.. 2019;6:567-600. [Google Scholar] [CrossRef]
- [Google Scholar]
- Low-modulus Mg/PCL hybrid bone substitute for osteoporotic fracture fixation. Biomaterials. 2013;34:7016-7032.
- [Google Scholar]
- Acta Biomater.. 2019;98:160.
- Laser additive manufacturing of Mg-based composite with improved degradation behaviour. Virt. Phys. Prototyping. 2020;15(3):278-293.
- [CrossRef] [Google Scholar]
- Microstructure evolution and texture tailoring of reduced graphene oxide reinforced Zn scaffold. Bioact. Mater.. 2021;6(5):1230-1241.
- [Google Scholar]
- In situ grown rare earth lanthanum on carbon nanofibre for interfacial reinforcement in Zn implants. Virt. Phys. Prototyping. 2022;17(3):700-717.
- [Google Scholar]
- Laser-sintered Mg-Zn supersaturated solid solution with high corrosion resistance. Micromachines. 2021;12(11):1368.
- [CrossRef] [Google Scholar]
- In-situ deposition of apatite layer to protect Mg-based composite fabricated via laser additive manufacturing. J. Magn. Alloy. 2021
- [Google Scholar]
- Superhydrophobic coatings for corrosion protection of magnesium alloys. J. Mater. Sci. Technol.. 2020;52:100-118. [Google Scholar] [CrossRef]
- [Google Scholar]
- Yoshinori T. Surface treatment of magnesium or magnesium alloy. JP patent 60132460, 1986-12-22.
- Yukio O, Toshihiko S. Surface-treating method for magnesium or magnesium alloy. JP patent 53089901, 1980.
- Zainab F. Al-Sherify, Nawal Mohammed Dawood, Zuheir Talib Khulief, Corrosion Behavior of Magnesium Implants Coated with Biocomposite Polymer, International Journal of Mechanical Engineering, 7 (2022)
- Corrosion Types of Magnesium Alloys. In: Tański T., Borek W., Król M., eds. Magnesium Alloys: Selected Issue. London: IntechOpen; 2018.
- [CrossRef] [Google Scholar]
- J. Mater. Chem. A. 2014;2:13049-13057.
- In vitro corrosion of as-extruded Mg–Ca alloys–the influence of Ca concentration. Corros. Sci.. 2015;96:23-31.
- [Google Scholar]
- Zhang DF, Liu YP, Shen YP. Method of surface treatment on magnesium alloy with aboding electrolyte. CN patent 200910103124.1, 2009
- Surf. Coat. Technol.. 2014;258:1152-1158.
- RSC Adv.. 2018;8:2248-2259.
- Corros. Sci.. 2018;139:370-382.
- Appl. Surf. Sci.. 2018;456:419-429.
- Research on an Mg–Zn alloy as a degradable biomaterial. Acta Biomater.. 2010;6:626-640.
- [Google Scholar]
- Adv. Funct. Mater.. 2012;22:675-694.
- PLoS One. 2014;9:e110420.
- TONG Zhen-song, ZHANG Wei, L1 Jiu-Qing, CHENG Fei. Initial laws of atmospheric galvanic corrosion for magnesium alloys [J]. The Chinese Journal of Nonferrous Metals, 2004, 14(4): 554-561. (In Chinese)
- Polymer coatings on magnesium-based implants for orthopedic applications. J. Polym. Sci. 2021
- [Google Scholar]







