Translate this page into:
Sustainable adsorptive removal of antibiotic residues by chitosan composites: An insight into current developments and future recommendations
⁎Corresponding authors. emanabdelmonaem5925@yahoo.com (Eman M. Abd El-Monaem), abdelazeemeltaweil@alexu.edu.eg (Abdelazeem S. Eltaweil)
-
Received: ,
Accepted: ,
This article was originally published by Elsevier and was migrated to Scientific Scholar after the change of Publisher.
Peer review under responsibility of King Saud University.
Abstract
Abstract
During COVID-19 crisis, water pollution caused by pharmaceutical residuals have enormously aggravated since millions of patients worldwide are consuming tons of drugs daily. Antibiotics are the preponderance pharmaceutical pollutants in water bodies that surely cause a real threat to human life and ecosystems. The excellent characteristics of chitosan such as nontoxicity, easy functionality, biodegradability, availability in nature and the abundant hydroxyl and amine groups onto its backbone make it a promising adsorbent. Herein, we aimed to provide a comprehensive overview of recent published research papers regarding the removal of antibiotics by chitosan composite-based adsorbents. The structure, ionic form, optimum removal pH and λmax of the most common antibiotics including Tetracycline, Ciprofloxacin, Amoxicillin, Levofloxacin, Ceftriaxone, Erythromycin, Norfloxacin, Ofloxacin, Doxycycline, Cefotaxime and Sulfamethoxazole were summarized. The development of chitosan composite-based adsorbents in order to enhance their adsorption capacity, reusability and validity were presented. Moreover, the adsorption mechanisms of these antibiotics were explored to provide more information about adsorbate-adsorbent interactions. Besides the dominant factors on the adsorption process including pH, dosage, coexisting ions, etc. were discussed. Moreover, conclusions and future recommendations are provided to inspire for further researches.
Keywords
Chitosan
Adsorption
Pharmaceutical residue
Ionic form
Mechanism
1 Introduction
Indeed, drug residues pollutants represent a critical issue due to their harmful impact on the environment particularly in the recent critical time. Notably, pharmaceutical residuals are tenacious contaminants, highly toxic and low biodegradable compounds, so their existence in the drinking water even with the lower acceptable concentration has gigantic jeopardy on human health (Rizzi et al., 2019). Even more dangerous situation is the presence of these detrimental contaminants in the vegetables and the tissues of fishes (Xiong et al., 2018). Moreover, it was reported that the pharmaceutical residuals widely spread in whole water sources; groundwater, drinking water, surface water and wastewater (Alatalo et al., 2019). A plethora of antibiotics comprising of tetracycline, ciprofloxacin, amoxicillin, metronidazole, etc. have been classified among the most used pharmaceuticals, owing to their great activity against bacterial infections and other diseases (Fig. 1) (Leng et al., 2020). Consequently, a high quantity of antibiotics accumulate in wastewater which may cause a threat to ecosystem by increasing the chance of emerging of antibiotic resistance genes (Zhao et al., 2020). Besides, their stability and resistance to biodegradation render them noxious pollutants in wastewater, so the removal of antibiotics from wastewater has become a case of great interest (Yang et al., 2020). Accordingly, diversified water treatment techniques have been sustainably evolved to vanquish these persistent contaminants such as membrane separation (Shin et al., 2020), electrochemical oxidation (Dao et al., 2020), catalytic degradation (El-Maghrabi et al., 2021), photo catalytic degradation (El-Borady et al., 2021, Hosny et al., 2021a) flocculation (Kooijman et al., 2020) and adsorption (Omer et al., 2021a). Great number of research have endorsed that adsorption is the most apposite technique owing to its straightforward nature, low energy consumption, low costs, etc. (Shi et al., 2020, Abdelfatah et al., 2021, Zhang et al., 2021).
Most common types of antibiotic wastes.
The following Table 1 summarized information about a wide scale of antibiotics including the chemical structures, the ionic forms, the maximum wavelength of each antibiotic and the optimum pH range to adsorb these antibiotics according to the previous research.
Antibiotics
Structure
Ionic form
Opt. pH
λmax (n)
Tetracycline
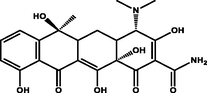
Polyprotic
4–8
354
Ciprofloxacin
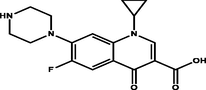
Polyprotic
4–12
275
Amoxicillin
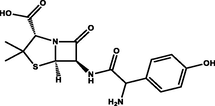
Polyprotic
4–7
228–280
Levofloxacin
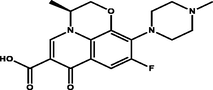
Polyprotic
4–9
288
Ceftriaxone
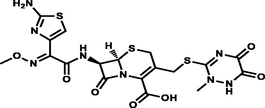
Neutral/Anionic
3–5
242
Erythromycin
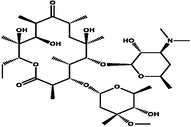
Neutral
3–9
482
Norfloxacin
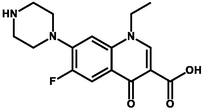
Polyprotic
7–8
271
Ofloxacin
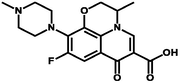
Polyprotic
∼7
293 & 342
Doxycycline
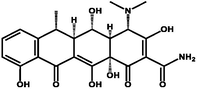
Polyprotic
5–9
351
Cefotaxime
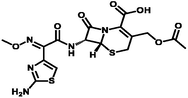
Polyprotic
4–7
235
Sulfamethoxazole
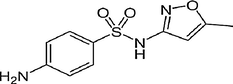
Polyprotic
4–6
268
Natural polymers are commonly supposed to be one of the best options for the removal of pharmaceuticals pollutants from water because they are reckoned to be eco-friendly, biodegradable, easily modified and regenerated and inexpensive in terms of their production (Younas et al., 2019). Natural polymers gained inordinate attention as a result of their structural resemblance with biological macromolecules that made them be easily recognized by the environment and so become easily metabolized to residues that are environmentally-safe and naturally eradicated (Thomas et al., 2012, Bakshi et al., 2020, Omer et al., 2021b). Consequently, these polymers piqued researchers’ interest as a potential candidate in wastewater treatment. Moreover, these polymers were utilized in biomedical applications such as tissue engineering, drug delivery, and permeable membranes (Liu et al., 2020a, Soares et al., 2020, Omer et al., 2021b, Omer et al., 2021c). Plant materials including starch, gum, locust bean gum and pectin are usually utilized for the production of natural polymers. In addition, other sources which are of non-plant origin (bacteria, algae and fungi) are used to prepare alginates, chitin and chitosan (Das and Adholeya 2015, Elieh-Ali-Komi and Hamblin 2016, Eltaweil et al., 2021a). Unfortunately, natural polymers are often unable to remove pharmaceuticals from extremely complex wastewaters, containing various types of contaminants such as heavy metals and dyes that have a detrimental influence on the environment and human health. Thus, to improve their effectiveness in removing pharmaceuticals from complicated wastewaters, these polymers should be modified using chemical or physical techniques (Thakur et al., 2012, Ishak et al., 2020). It's quintessential to adapt the polymers to the intended application's specifications that can be carried out by blending, curing, or grafting. Various materials were used in the modification of natural polymers including Metal Organic Frameworks (MOFs), biochar materials, hydrogels, and lots of different nanomaterials such as Graphene Oxide (GO), reduced Graphene Oxide (rGO), Carbon Nanotubes (CNTs), iron oxide nanoparticles (eg: Fe2O3 and Fe3O4), silica (SiO2), titania (TiO2), Cobalt nanoparticles CoNPs, Zinc oxide nanoparticles (ZnONPs), Gold nanoparticles (AuNPs), Silver nanoparticles (AgNPs), and platinum nanoparticles (PtNPs) (Delfi et al., 2020, Khine and Stenzel 2020, Muqeet et al., 2020, Wang et al., 2020, Eltaweil et al., 2021b, Hosny and Fawzy 2021, Hosny et al., 2021b, Tamer et al., 2018, Jiang et al., 2021, Eltaweil et al., 2022, Hosny et al., 2022, Lv et al., 2022). These new composite materials have gained numerous positive aspects including increased stability, enhanced surface area, improved adsorption capacities, better recyclability, improved applicability under different reaction conditions, boosted charge separation, visible light absorption, and photocatalytic activities (Ge et al., 2021, Kunwar et al., 2021, Wang et al., 2022). Consequently, such modifications particularly with chitosan will be thoroughly discussed in the current review.
2 Chitosan-based adsorbents
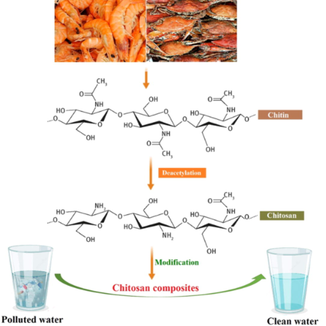
Chitosan (Cs) is the second most abundant biopolymer after cellulose, produced from chitin in basic medium, derived from biological sources such as insects, crustaceans (eg: lobsters, shrimps, and crabs), and fungi (de Farias et al., 2019, Nasrollahzadeh et al., 2020, Kostag and El Seoud 2021, Omer et al., 2022). Chitosan is a linear biopolymer that is made via the deacetylation of chitin, as depicted in Fig. 2 (Duan et al., 2019, Eltaweil et al., 2021c). Because of its distinctive properties, including low cost of processing, biodegradability, hydrophobicity, non-toxicity, and outstanding biocompatibility, it is becoming increasingly popular (Yaqoob et al., 2021). During the last few decades, many researchers have focused on chitosan as a source of potentially bioactive material owing to its distinctive structures, multidimensional characteristics, complex functionality and a wide variety of biological and industrial uses (Dash et al., 2011, Ramya et al., 2012, Kouser et al., 2020). However, its low solubility is a key limiting factor in usage in many applications, so enhancing the solubility of chitosan is critical for issue (Snyman et al., 2002, Alves et al., 2020). Chitosan modification can also be used to stimulate new biological activities and improve its mechanical characteristics (Zhu et al., 2016, Liu et al., 2020b). Recently, chemical derivatives of chitosan have drawn gigantic interest due to their enhanced chemical and biological properties over unmodified chitosan in terms of solubility, gelling properties, the design of hydrophobic derivatives with amphiphilic properties and the ability to harness self-assembling nanostructures and chemical conjugates with a variety of functionalities (Bakshi et al., 2020, Li et al., 2020, Negm et al., 2020). Grafting, cross-linking, composites, substituent insertion, and other physical and chemical techniques can be used to modify chitosan (Saheed et al., 2020, Sanchez-Salvador et al., 2021, Mohy Eldin et al., 2015). Chitosan is a versatile polymer that may be used for a variety of products including biocatalysts, antioxidant, antibacterial, and anticancer agents, wound healing dressing, and food preservatives (Abd El-Hack et al., 2020, Chaiwong et al., 2020, Inanli et al., 2020). Moreover, chitosan has shown to be a safe excipient in medical compositions over the years (Baldrick 2010, Kurakula and Naveen 2020).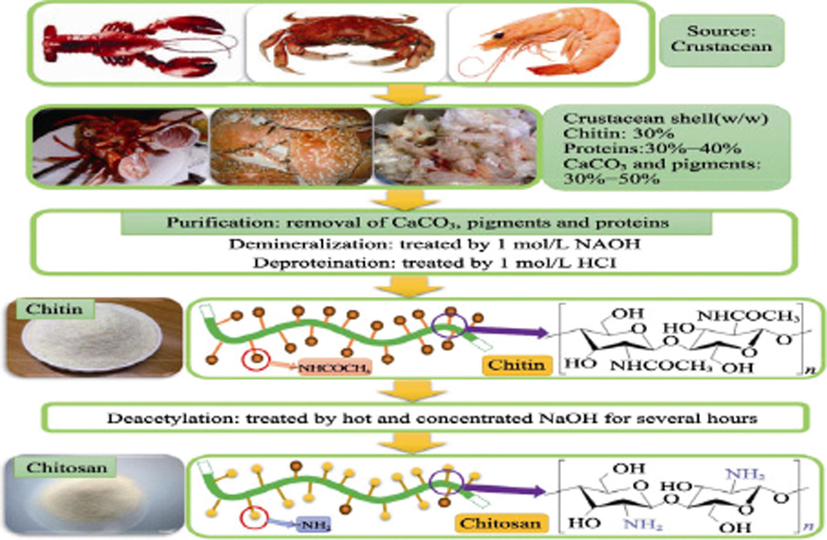
Production of chitosan from crustacean (Duan et al., 2019). Copyright Elsevier 2019.
As an eco-friendly biopolymers class, chitosan-based composites have recently shown great potential for the efficient adsorption of several pollutants particularly pharmaceuticals including antibiotics, analgesics, antipyretics and anti-inflammatories. Chitosan composites provide wide range of possible adsorption mechanisms as a result of their introduced adsorbent functional groups. As such, the main aim of this review is to provide a detailed survey on the most recent and updated chitosan composites utilized in the removal of pharmaceutical wastes, indicating their different modifications, the postulated adsorption mechanisms of the bountiful antibiotic pollutants. Parameters affecting the adsorptive removal of numerous antibiotic residues, especially the dominion of pH onto the adsorption process were well-discussed. Due to its appealing properties, chitosan based adsorbents have been developed for numerous pollutants, not only pharmaceutical residues. I would recommend the authors to briefly mention the recent applications of chitosan based adsorbents on other common pollutants like heavy metal ions (Yang et al., 2021), dyes (Vakili et al., 2014), heavy oil (Lozano-Navarro et al., 2020) and toxic gases (Liao and Pan 2021).
3 Antibiotics removal by chitosan composites-based adsorbents
3.1 Removal of tetracycline
Owing to the extremely high antimicrobial activity of tetracycline (TC) against multifarious types of bacterial infections, it assorted as the second most used antibiotic (Cao et al., 2020). Furthermore, TC is excessively utilized as a food additive to enhance the animals growing rate since it is a cheap medicine and widely produced (Xiong et al., 2018). Nonetheless, it was found that humans or animals could not metabolize TC, so around 50–80% of the absorbed dose is excreted through the urine (Alatalo et al., 2019). Moreover, several recent research has recommended that TC may be an effective medicine for treating COVID-19 infection owing to its anti-inflammatory and anti-apoptotic activities (Mosquera-Sulbaran and Hernández-Fonseca 2020, Sodhi and Etminan 2020, Abdelfatah et al., 2021, Thomson 2021). Accordingly, escalating quantities of TC have accumulated in the water bodies (Ranjbari et al., 2020). So, various attempts have been executed using Cs composite-based adsorbents for the adsorptive removal of tetracycline as summarized in Table 2.
Adsorbent
Drug
qmax (mg/g)
Opt. pH
Ref.
(MSCsG)
TC
183.47
6
(Huang et al., 2017)
MMICs-Fe3+
TC
516.29
7–8
(Chen et al., 2017)
CNTs–C@Fe–Cs
TC
104
–
(Yin et al., 2015)
Cs-TCMA
TC
20.85
7
(Ahamad et al., 2019)
CDF@MF resin nanocomposite
TC
168.24
6
(Ma et al., 2019)
Na-Mt-CMCs
TC
178.57
4
(Liu et al., 2019)
BCs-Fe/S
TC
183.01
5
(Zhao et al., 2020)
MOF-Cs
TC
495.04
8–9
(Rizzi et al., 2019)
Cs-olive pomace
TC
1.6
8
(Lu et al., 2018)
Cs-g-AMPS
TC
806.60
3.3
(Ahamad et al., 2020)
CsTM@Fe3O4
TC
215.31
7
(Raeiatbina and Açıkelb 2017)
Cs-Fe3O4
TC
78.11
5
(Ahamad et al., 2019)
Ahamad et al. prepared another mesoporous nanocomposite possesses superparamagnetic property, high surface area of (376 m2 g−1) and high pore volume (0.3828 cm3 g−1) using Fe3O4, chitosan, thiobarbituric acid, malondialdehyde (CTM@Fe3O4) (Fig. 3a). CTM@Fe3O4 showed a maximum removal of TC at pH 7 with qm of 215.31 mg/g. FESEM of CTM@Fe3O4 nanocomposite (Fig. 3b) showed rough surface with many bright spots as a result from the aggregation Fe3O4 nanoparticles as confirmed by TEM results (Fig. 3c). HRTEM image of CTM@Fe3O4 nanocomposite (Fig. 3d) demonstrated that the polymers are amorphous in nature while, Fe3O4 nanoparticles are crystalline with two lattice fringe spacing were observed about to 0.296 nm and 0.263 nm, due to the inter-planar distance of the (2 2 0) and (3 1 1) plane which was further confirmed by SAED measurements (Fig. 3e).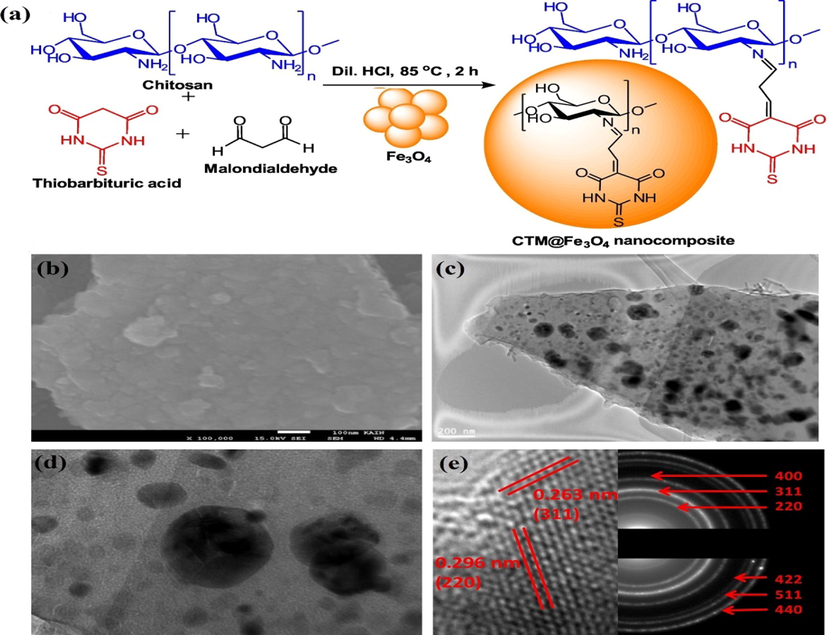
(a) Scheme of synthesis of CTM@Fe3O4, (b) SEM image, (c) TEM image, (d) HRTEM image and (e) d-spacing and SAED of CTM@Fe3O4. Copyright Elsevier.
In this vein, Huang et al. scrutinized the adsorptive performance of Fe3O4@SiO2-Cs/GO (MSCsG) nanocomposite towards TC. In fact, pH is the most predominant among the key parameters that control any adsorption process, especially in such multiform TC (Fig. 4a) that exist as a cation (TCH3+) at pH < 3.3, zwitterion (TCH20) at 3.3 < pH < 7.7 and anion (TCH- or TC2-) at pH > 7.7 (Fig. 4b). It was recorded that the adsorption capacity of TC onto MSCsG increased with the rising in pH from 3 to 6 reached the highest adsorption capacity (67.57 mmol/kg) at pH 6. This behavior reflected the lower electrostatic repulsion between TCH20 and the negatively charged surface of MSCsG where the PZC of MSCsG was vaulted to be 3–4. Furthermore, the dwindling in the adsorption capacity at pH higher than 7.7 could be ascribed to the electrostatic repulsion between TCH-, TC2- and MSCsG. This finding strongly suggested the dominion of pH onto the TC adsorption mechanism onto MSCsG, agreeing with Murat et al. (Topal and Topal 2020), Tansir Ahamad et al. (Ahamad et al., 2020), and Jianzhe Ma et al. (Ma et al., 2019). Besides, π-π conjugation between the benzene rings in GO and TC also had a rule in the adsorption capacity of TC. Moreover, the adsorption of TC was investigated in the presence of interfering cations such as Na+, K+, Ca2+ and Mg2+, clarifying that the four interfering cations had a low effect on the TC adsorption which Compatible with Juanli et al. (Liu et al., 2019). While it was deduced that the presence of Cu2+ increased the adsorption capacity of TC onto MSCsG at pH range 3–7. This most likely due to the increase in the positive charge of TC and the formation of a complex between TC and Cu2+ which led to the increase in the attraction between MSCsG and TC species. Moreover, the adsorption of TC onto MSCsG was studied using XPS spectra before and after the adsorption process. In detail, by comparing the N1s spectra of MSCsG before and after the TC adsorption there were an increase in the molar ratio of N+ from 16.8% to 40.6%, -N= incremented from 9.2% to10.2% and a diminution in –NH– from 74.0% to 49.2%. This finding suggested the interaction between TC and amino groups in MSCsG. While, O1s spectra revealed that there was no any change in the molar ratio of oxygen function groups after the adsorption process. Thus the positive N-containing groups might be one of the most significant adsorption driving forces for TC, evincing the presence of electrostatic interaction between TC and the positive N-containing groups on the MSCsG surface (Huang et al., 2017).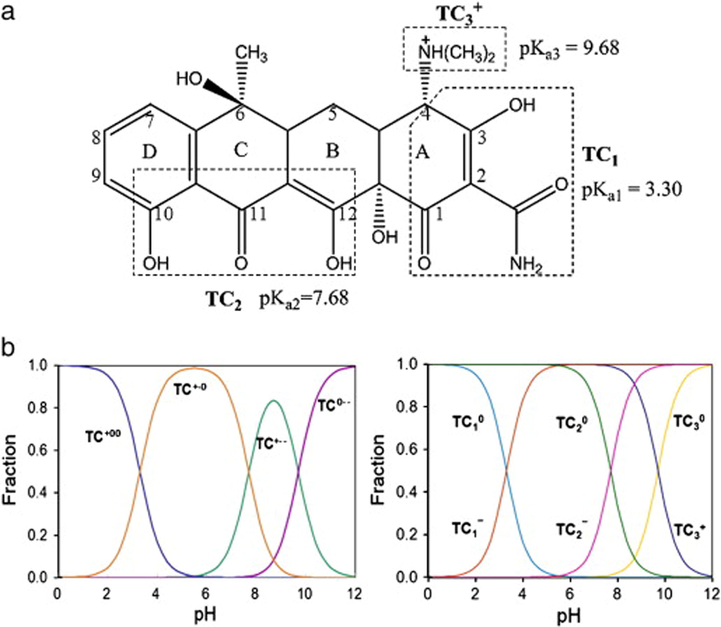
Multiform of (a) TC and (b) pH-dependent speciation of TC (Zhao et al., 2011). Copyright 2021, Springer.
In yet another study, Anwei Chen et al. studied the adsorbability of carbondisulfide-modified magnetic ion-imprinted Cs-Fe3+ composite (MMICs-Fe3+) towards the removal of TC. The addition of carbon disulfide modified the surface of magnetic Cs- Fe3+ beads by additional holes and contact area, resulting in an enhanced qmax reached 516.29 mg/g comparing with the qmax of TC onto pristine Cs derived-mussel shells (28.07 mg/g) (Ranjbari et al., 2020). Moreover, it was noticed that the presence of TC and Cd2+ in the same solution boosted the adsorption capacity of TC which may be expounded by the interaction between Cd2+-MMICs-Fe3+, and Cd2+-TC participated in a bridging interaction between MMICs-Fe3+ and TC. Furthermore, the adsorption capacity of TC incremented with the rising in the concentration of Cd2+ in TC solution until reached 80 mg/L, occurring a competition between TC and Cd2+ for the binding sites of MMICs-Fe3+, so the adsorption capacity declined (Chen et al., 2017).
In this regard, Jie Ma et al. inspected the as-fabricated magnetic CNTs–C@Fe–Cs composite as an adsorbent for the removal of TC from an aqueous solution. It was elucidated that the presence of CNTs provides long-term stability to the composite in water by forming a barrier against oxidation. In addition to the magnetic property facilitates the separation of the composite after the adsorption process, attaining an easy and safe wastewater treatment process. Furthermore, the oxygen functional groups in Cs boost the chemical adsorption of TC via the formation of hydrogen bonds. On the other hand, BET analysis inferred the physical adsorption contribution on the adsorption of TC at which the specific surface area of CNTs–C@Fe–Cs composite diminished from 6.8 to 5.1 m2/g after the adsorption process. Moreover, qmax increased from 103 to 117 mg/g with the augmentation of the system temperature from 15 to 40 °C, reflecting the endothermic nature of the TC adsorption process. It was observed that the adsorption of TC on CNTs–C@Fe–Cs composite enhanced by increasing the concentration of Cu2+ which may be due to the formation of TC-Cu2+ complex. Humic acid also enhanced TC adsorption via the formation of a complex between the anionic sites in humic acid and cationic sites in TC which means that humic acid used as a bridge to facilitate the interaction between TC and CNTs–C@Fe–chitosan composite (Ma et al., 2015).
In another study, Sara Ranjbari et al. synthesized ionic liquid-impregnated Cs hydrogel beads (Cs-TCMA) via the addition of tricaprylmethylammonium chloride (TCMA) in a volume of 0.1 mL to Cs bead, resulting in an enhancement in the removal efficiency of TC from 9.8% to 66%. This recorded multiplied increase in the removal efficiency of TC may be attributed to the good distribution TCMA into Cs beads, getting a smooth surface with a small pore diameter. Furthermore, it was found that the removal efficiency of TC was highly affected by the amount of Cs-TCMA where it was obviously increased with the increase in Cs-TCMA dosage reached 90% using 3 mg/L of Cs-TCMA. Besides, the superb affinity of Cs-TCMA towards TC may be explained by the different adsorption mechanisms between TC and Cs-TCMA such as electrostatic attraction, hydrogen bonding and XH/π interactions (Ranjbari et al., 2020). Also, Ahamad et al. reported that this interaction mechanism provides a strong adsorbent-adsorbate interaction at which the maximum adsorption capacity (qmax) of TC onto MnFe2O4 nanoparticles embedded Cs-diphenylureaformaldehyde CDF@MF) resin nanocomposite was 168.42 mg/g at pH 6 (Ahamad et al., 2019). One more developed chitosan-based adsorbent that was utilized for the removal of TC from aqueous media was Na-montmorillonite intercalated carboxymethyl chitosan (Na-Mt-CMCs) composite (Fig. 5). XRD spectra confirmed the successful intercalation of CMCs into the layers of Na-Mt at which the diffraction peak of Na-Mt shifted from 7.15° to 5.45°. It was found that from studying of the apt condition to adsorb TC onto Na-Mt-CMCs that the adsorption capacity was lower at a highly acidic medium due to the protonation of amino groups of CMCs, leading to a strong repulsion with TCH3+. The adsorption capacity increased clearly at pH 4 reached 48.10 mg/g due to the electrostatic attraction and cation exchange capacity (Ma et al., 2019).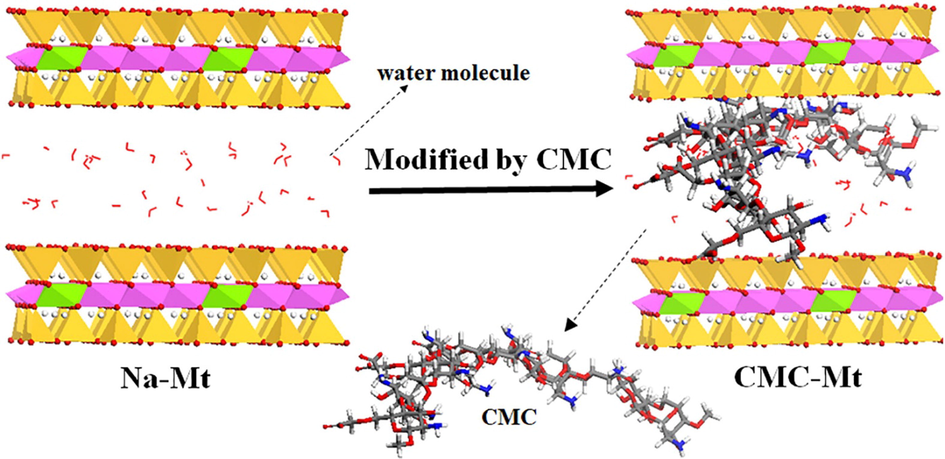
Schematic diagram of Na-Mt-CMCs) composite. Color legend for the atoms in the picture: Mg (purple), Al (green), Si (blue), C (gray), O (red), N (yellow), and H (white) (Ma et al., 2019). Copyright, Elsevier, 2021.
Juanli et al. investigated the adsorption performance of Cs modified-biochar/FeSO4 (BCs-Fe/S) composite towards TC. A series of composites with different mass ratios between biochar, Cs and FeSO4 were prepared as follow 1:1:1, 1:3:5, 5:3:1, 3:1:5, 3:5:1, 5:1:3 and 1:5:3 nominated as BCs-Fe/S-1, BCs-Fe/S-2, BCs-Fe/S-3, BCs-Fe/S-4, BCs-Fe/S-5, BCs-Fe/S-6, and BCs-Fe/S-7, respectively. It was observed that the amount of adsorbed TC increased by increasing the FeSO4 proportion where BCs-Fe/S-4 represented the higher adsorption capacity (150.97 mg/g) than the composites that contained lower proportions of FeSO4. Furthermore, the presence of biochar in the composite also increased the adsorption amount at which BCs-Fe/S-6 revealed more adsorption capacity (140.76 mg/g) than BCs-Fe/S-7 (86.85 mg/g). The diffusion mechanism was studied by applying two different models, intra-particle diffusion and film diffusion model. From the results of two diffusion mechanism, it was concluded that intra-particle diffusion had the higher contribution than film diffusion. Besides, electrostatic attraction, the pore filling, silicate bonding, ion exchange and hydrogen bonding could be considered also in the adsorption mechanism as shown in Fig. 6. The impact of the presence of coexisting cations such as Na+, K+, NH4+ and Ca2+ on the aptitude of the TC adsorption process. The results clarified that the adsorption of TC wasn’t highly affected by the existence of Ca2+ due to the higher ability of Fe2+ to chelate TC than Ca2+. Whereas, NH4+ had extremely effect on TC uptake where NH4+ has a small size and a high ability to chelate with binding sites of the adsorbent (Liu et al., 2019).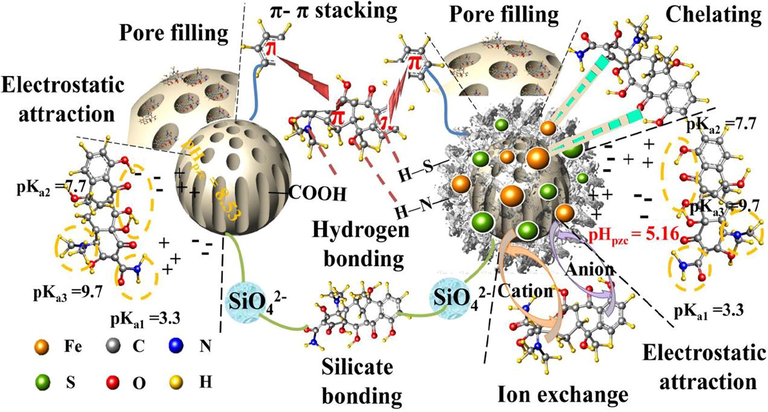
The proposed adsorption mechanisms of TC onto BCs-Fe/S composite (Liu et al., 2019). Copyright, Elsevier, 2021.
3.2 Removal of Ciprofloxacin
Ciprofloxacin (CIP) is a fluoroquinolone antibiotic that excessively used for treatment a wide range of bacterial infections. Although it used in tiny concentrations, its accumulation in wastewater can cause bacterial resistance to antibiotic because it’s incomplete degradation in water (Avcı et al., 2020), in addition to its violence impact on the human health; vomiting, diarrhea, skin disease and headache. Furthermore, CIP can inhibit the plant growth by its effect on the photosynthesis process (Lima et al., 2020). Accordingly, the presence of CIP without treatment has prejudicial effects on human health, plants and entire living beings. It was found that the removal of CIP by conventional techniques is difficult, so applying the adsorption by using adsorbent with high adsorption capacity has a great attention (Wang et al., 2016). Table 3 listed the recent adsorption studies of CIP onto chitosan composite from wastewater.
Adsorbent
Drug
qmax (mg/g)
Opt. pH
Ref.
MCsGO
CIP
282.9
4–9
(Wang et al., 2016)
MACCs
CIP
90.01
–
(Danalıoğlu et al., 2017)
MgO/Cs/GO
CIP
1111
7
(Nazraz et al., 2019)
Fe-CS NCs
CIP
142.85
6
(Rasoulzadeh et al., 2019)
Cs/BC
CIP
>76
3
(Afzal et al., 2018)
CoFe2O4/AC@Cs
CIP
188.68
5
(Malakootian et al., 2018)
Cs/kaolin/Fe3O4
CIP
47.85
6
(Ma et al., 2014)
Cs-grafted SiO2/Fe3O4
CIP
100.74
12
(Danalıoğlu et al., 2018)
Zn(II)–Impregnated Cs/GO
CIP
210.96
6.5
(Rahman and Varshney 2021)
Cs-EDTA-β-CD
CIP
49.37
4–6
(Zhao et al., 2017)
Humic acid/BC@Cs
CIP
153.00
8
(Afzal et al., 2019)
In this perspective, Wang et al. prepared a novel magnetic chitosan grafted graphene oxide (MCsGO) nanocomposite for removing CIP from aqueous media. Magnetization measurements showed that MCsGO has a high magnetic property with a saturation magnetization 14.76 emu/g, providing easy and perfect separation to the composite. The other indication on its magnetic performance had been noticed in XRD results where the characteristic diffraction peaks of pristine Fe3O4 clearly appeared without change in XRD pattern of MCsGO composite. Actually, CIP has multi-forms at different pH where CIP dwell as a cation at pH lower than 6.1, neutral ion at pH range 6.1–8.7 and anion at pH higher than 8.7. This is due to presence of carboxylic acid group and amine group on CIP, so the different pH has a different effect on the adsorption of CIP. It was deduced that the adsorption of CIP onto MCsGO diminished beyond pH 5, this due to the decrease in the positive charged on the composite surface as well as the dimension of protonated –NH2 numbers at pH > 6.5, resulting in strong repulsion forces between the anionic CIP and MCsGO composite. Moreover, the adsorption of CIP was also examined at a solution containing NaCl and CaCl2. The results showed that Ca2+ had more inhibition role for the adsorption ability of MCsGO than Na+ due to its ability to form a complex with MCsGO, resulting in strong vie between CIP and Ca2+ for the active sites of MCsGO composite (Wang et al., 2016). In another case in point, Selen et al. focused on examining the adsorbability of magnetic Cs/carbon material composite towards CIP. Magnetic activated carbon/chitosan (MACCs) composite was synthesized by using co-precipitation method. The characterization clarified that MACCs synthesized without change in the structure of chitosan and Fe3O4 concurring with Wang et al. (Wang et al., 2016). Furthermore, there were some changes in the physical properties like decreasing in the surface area of both AC and Fe3O4 and increasing in that of pure chitosan. In addition to a decline in the saturation magnetization of Fe3O4 when chitosan and AC were added, owing to their non-magnetic behavior. The adsorption efficiency of MACCs was scrutinized in different antibiotics where the CIP showed the highest adsorption efficiency reached 82%, while the adsorption efficiency of both erythromycin and amoxicillin were 54% and 72% respectively, reflecting the high selectivity of MACCs towards CIP. It was recorded that desorbed CIP was detected after 7 h was 15.4% which indicated the strong grasp of CIP molecules by MACCs (Danalıoğlu et al., 2017). Furthermore, Mahsa et al. studied the adsorption of CIP by using a composite consisted of magnesium oxide, chitosan and graphene oxide (MgO/Cs/GO). The three compounds were selected depend on some criteria. Cs has a high affinity toward biomacromolecules, hydrophilicity and high adsorption capacity. MgO has a high surface area and it isn’t toxic which make it an eco-friendly adsorbent to treat wastewater. GO contains large amount of oxygen functional groups that can be dispersed on the surface of chitosan to provide more adsorption sites. This perfect combination produced a propitious adsorbent with supreme adsorption capacity towards CIP since the reckoned qmax under Langmuir was 1111 mg/g. The removal efficiency of CIP by GO, MgO/Cs and MgO/Cs/GO was assessed to clarify the role of the matrix components in the adsorption process. The concert results revealed that the removal efficiency of GO < MgO/Cs/GO which most likely due to the strong interplanar interaction of GO that strengthens its tendency to aggregate, dwindling the available surface area for the CIP adsorption. On the other hand, MgO/Cs showed enhanced removal percentage which may be attributed to the immobilized Cs on MgO surface that boosts the number of the available binding sites. Accordingly, the high removal efficiency MgO/Cs/GO that approximately reached 100 % owing to the synergistic effect between GO and Cs as well as the abundant binding sites of MgO/Cs. Moreover, it was recorded that the highest adsorption capacity was at pH 7 were CIP is mostly zwitterionic and PZC of MgO/Cs/GO was 5.5, suggesting strong electrostatic interaction (Nazraz et al., 2019). Also, Rasoulzadeh et al. (Rasoulzadeh et al., 2019) reported a similar result at which the optimum pH to adsorb CIP onto magnetite imprinted chitosan (Fe-CS NCs) nanocomposites was pH 6. It was found that the decrease in pH led to decreasing the adsorption capacity of CIP onto Fe-CS NCs which may be due to the existence of CIP as a cation at pH lower than 6, leading to a competition between the cationic CIP and the protons in the solution that have a smaller size than CIP for the adsorption sites of the composite. In addition, the electrostatic repulsion between positively charged surface of Fe-CS NCs composite and the cationic CIP took place. Whereas, at higher pH range from 5.9 to 8.9, the hydrophobicity of CIP increases, resulting in an increase the hydrophobic interaction between CIP and Fe-CS NCs as clarified in Fig. 7.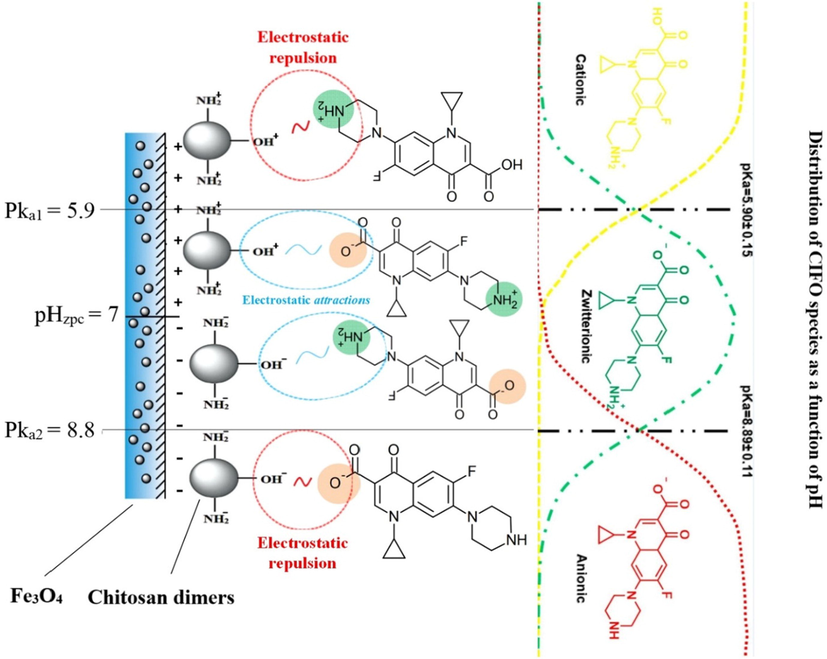
Schematic illustration of CIF adsorption mechanism onto Fe-CS NCs at different pH (Rasoulzadeh et al., 2019). Copyright, Elsevier, 2021.
The conclusion from these studies that pH has a key role in the adsorption mechanism of CIP at which the function groups in chitosan like amino and hydroxyl groups interact with CIP. In addition to ion exchange and π-π interaction could also contribute in adsorption mechanism (Nazraz et al., 2019, Rasoulzadeh et al., 2019).
In another study, Afzal et al. scrutinized the adsorptive performance of chitosan/biochar (Cs/BC) hydrogel beads towards CIP. It was found that qmax of CIP onto Cs/BC reached 76 mg/g at pH 3, reflecting strong electrostatic interaction between CIP and Cs/BC. Moreover, three solvents such as ethanol, methanol and water were chosen to investigate the effect of the solvent on the adsorption efficacy where water showed the highest adsorption due to the contribution of –OH groups in hydrogen binding with CIP. The effect of the ion strength was investigated by using different electrolytes like NaCl, NaNO3, Na2SO4 and Na3PO4 where the results showed that Na3PO4 had the highest effect on the adsorption of CIP. This may be ascribed to the high increase in solution pH in the presence of Na3PO4. Furthermore, the effect of NaCl was investigated at different concentrations, revealing that the increase in the concentration of NaCl led to a decrease in the adsorption of CIP which may be due to the pores filling of the Cs/BC surface by NaCl. The adsorption mechanism was explained by FTIR and XPS of Cs/BC before and after adsorption. FTIR pointed out the formation of hydrogen bond between –COOH in CIP and –NH in Cs/BC. In addition to other oxygen functional groups in CIP like –OH and –CO that could be contributed in the hydrogen bonding. Besides, π -π electron donor–acceptor interaction and hydrophobic interaction between the Cs/BC and CIP that contribute to the adsorption mechanism. While XPS spectra clarified a significant change in the peak intensity of C-NH2 at 400.2 eV after CIP adsorption, suggesting the interaction between C-NH2 on the Cs/BC surface and CIP. Furthermore, there were a slight increase in the peak intensity of NH and N-C=O at 398.78 and 399.48 eV, respectively. Meanwhile, C1s spectrum after the CIP adsorption pointed out a considerable increment in the peak intensity. This finding indicated that the oxygen containing functional groups may increase the adsorption capacity of Cs/BC towards CIP by H-bond, hydrophobic and π -π interactions, agreeing with FTIR results (Afzal et al., 2018).
One more study highlighted fabricating an environmentally-friendly adsorbent (Cs-grafted SiO2/Fe3O4) for removing CIP from wastewater. This composite showed higher saturation magnetization (25.260 emu/g) compared with other magnetic Cs composites such as MACCs, MCsGO, m-Cs/c-Fe2O3/MWCNTs, Cs/kaolin/Fe3O4 and CoFe2O4/AC@Cs with saturation magnetization values 5.78, 14.76, 4.81, 2.38 and 22.03 emu/g, respectively (Zhu et al., 2013, Ma et al., 2014, Wang et al., 2016, Danalıoğlu et al., 2017, Malakootian et al., 2018). Moreover, the addition of Cs to SiO2/Fe3O4 boosted the surface area from 104.34 to 126.16 m2/g and the pore volume from 0.148 to 0.179 cm3/g (Fig. 8a). It was found from the optimization study that the system temperature had a reverse effect on the CIP adsorption onto Cs-grafted SiO2/Fe3O4, suggesting the exothermic adsorption process. In addition, the higher adsorption capacity (52.14 mg/g) was recorded at pH 12 which might be due to the electrostatic interaction (Fig. 8b) (Danalıoğlu et al., 2018).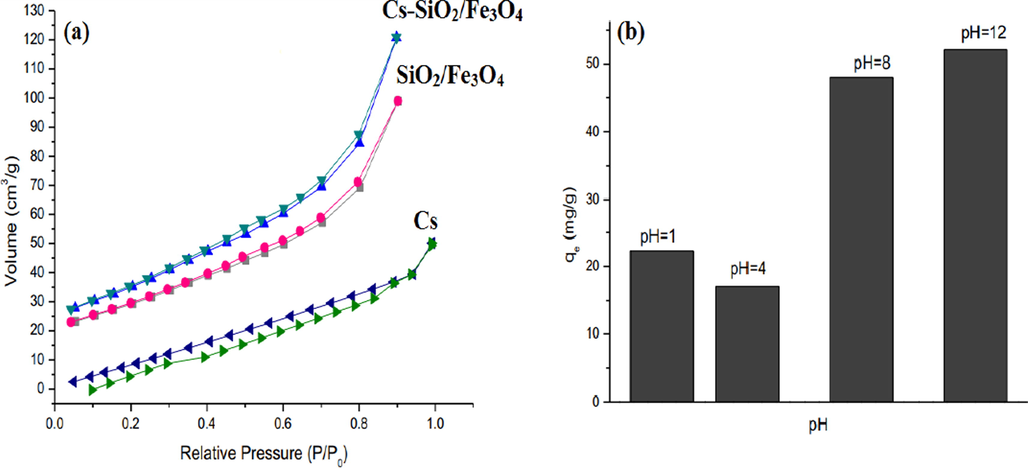
BET of (a) Cs, SiO2/Fe3O4 and Cs-grafted SiO2/Fe3O4 and (b) effect of pH on the adsorption of CIP onto Cs-grafted SiO2/Fe3O4 (Danalıoğlu et al., 2018). Copyright, Elsevier, 2021.
3.3 Removal of Amoxicillin
Amoxicillin (AMX) is a semi-synthetic and broad-spectrum β-lactam antibiotic that has high stability in water. AMX is Excessively applied in medicine as an effective bacteriostatic as well as in veterinary medicine as a growth stimulant (Shakak et al., 2020). Thence, massive accumulated amounts of AMX in are existence in water bodies, threatening human health and the biotic environment, rendering the removal of AMX from wastewater is a big challenge (Ren et al., 2020).
On this consideration, Xiulan et al. studied the removal of AMX in presence of other contaminate like Cd2+ at which wastewater contains enormous types of pollutants. Chitosan was used as a stabilizer to form bimetallic Fe/Ni (Cs-Fe/Ni) nanoparticles. It was observed that the removal efficiency of AMX and Cd2+ in individual solutions is much higher than a mixed solution since the removal efficiency of AMX in single and binary solutions was 93% to 68.9%, respectively. This finding is most likely due to the competition between Cd2+ and AMX towards the adsorption sites of Cs-Fe/Ni. The removal of AMX was explained by occurring iron corrosion that produces electrons, resulting in the formation of hydrogen radicals. Then, and when the AMX was adsorbed on the surface of CS–Fe/Ni, the reductively degraded H radical (Ni - H) degraded AMX via cleave its b-lactam bond as depicted in the following equations (Weng et al., 2013).
3.4 Removal of Levofloxacin and Ceftriaxone
Levofloxacin (LEVO) is the most third produced among the fluoroquinolone antibiotics since it treats bountiful bacterial infections such as skin, gynecological, the upper and lower respiratory tract, soft tissue and genitourinary (Yu et al., 2017). Due to this wide usage and incomplete absorption of LEVO by humans or livestock, tremendous quantities of this detrimental pollutant are accumulated in water bodies (Wu et al., 2020).
In one attempt to remove LEVO from wastewater, Geaneth et al. demonstrated using mesoporous carbon/cyclodextrin–Cs (MMPC/Cyc-Cs) nanocomposite as an adsorbent. Because of the high porosity of the composite with a surface contains 60 % mesopores and a high specific surface area (1264 m2/g), the pore filling mechanism had great contribution in the adsorption mechanism of LEVO. Also, the electrostatic interaction led to increase in the adsorption owing to the ionization of MMPC/Cyc-Cs highly affected by pH where LEVO could be adsorbed via cation exchange in case of the positively charged surface or by electrostatic attraction in case of the negatively charged surface. The as-fabricated composite was applied in real wastewater and the results showed that the removal efficiency in range 90–99% which make MMPC/Cyc-Cs applicable adsorbent in wastewater treatment (Mashile et al., 2020).
Another study on the removal of LEVO was achieved by Mahmoud et al. in this study, nanotitanium oxide-Cs and nanotitanium oxide-bentonite were companied to prepare the composite (NBent-NTiO2-Cs). The highest removal efficiency of LEVO by NBent-NTiO2-Cs was 90.2% at pH 4, due to the electrostatic attraction between LEVO and the composite. While, the lower removal efficiency of LEVO at pH lower than 4 is most likely due to the strong electrostatic repulsion forces between the protonated amino groups of LEVO and the positively charged surface of NBent-NTiO2-Cs. This finding indicated that the adsorption of LEVO was mainly depended on the electrostatic interaction. Besides, the effect of different ions like Na2SO4, K2SO4, MgSO4, NaCl, KCl and CaCl2 on the removal efficiency was investigated. It was noticed that the removal efficiency was highly affected by these ions where both Na and K interact with the carboxylic groups in LEVO to form their stable form while Mg and Ca bind with the active sites on the composite surface (Mahmoud et al., 2020).
Ceftriaxone (CFT) is a new ‘third generation’ semisynthetic cephalosporin with a long half-life owing to its aromatic-ring structure (Acuna et al., 1981). CFT has effectiveness against Gram-negative bacteria stronger than that of cephalosporins of the 'first' and 'second' generations. While its effectiveness against Gram-positive bacteria is less than cephalosporins of the previous generations (Angehrn and Probst 1981, Richards et al., 1984). Besides, CFT is commonly used in the treatment of different infections including those affecting the respiratory tract, urinary system and pelvic cavity. However, it has to be noticed the discharge of CFT into aquatic ecosystems over a long period of time poses a deleterious risk to the aquatic ecology and human health (Li et al., 2018a).
In this regard, Mahmoud et al. (Mahmoud et al., 2020) used formaldehyde to crosslink two distinct nanocomposites, nano titanium oxide/chitosan/nano-bentonite (NBent-NTiO2-Cs), to obtain an enhanced nanocomposite for the sake of CFT removal from wastewater. BET results indicated that NBent-NTiO2-Cs has a good surface area (16.385 m2/g) which was regarded to play a quintessential role in improving CFT. It was reported that the higher removal efficiency of CIF (93.5%) was obtained at pH 5 as a result of the unsteadiness of cephalosporins in an alkaline medium. Furthermore, a noticeable dwindle in the uptake aptitude of CFT onto NBent-NTiO2-Cs was observed in the presence of co-existing ions such as Na+, K+, Mg2+ and Ca2+. This decline is a result of the probable interaction of Na+ and K+ with CFT to form their sodium or potassium salts which are commonly recognized to favor the solubilized forms in aqueous solution than being adsorbed onto the surface of NBent-NTiO2-Cs nanocomposite. On the other hand, Mg2+ and Ca2+ ions could bind with the active surface functional groups and blocking some of the nanocomposite free sites.
3.5 Removal of erythromycin and Norfloxacin
Macrolide antibiotic erythromycin (ERM) is a broad-spectrum antibacterial agent that is commonly utilized to treat a vast array of pathogenic bacteria (Schafhauser et al., 2018). In addition to the pharmaceutical industry widely uses ERM in livestock and poultry breeding (Hua et al., 2019). Several studies have confirmed the enormous presence of ERM in wastewater effluents and surface waters. As a result, the spread of antibiotic resistance genes by pathogenic bacteria through horizontal gene transfer is another concern that has perilous human health effects (Liu et al., 2018). So, the adoption of apposite and cost-effective technologies to remove ERM from wastewater is urgently needed.
For this purpose, Ou et al. (Ou et al., 2015) scrutinized the aptitude of chitosan-stabilized Pickering emulsion (MIPs-Cs) prepared via an oil-in-water Pickering emulsion polymerization process from aqueous solution towards the adsorptive removal of ERM. The optimization study of the ERM adsorption process revealed that apt pH was 9. In detail, it was determined that the adsorption capacity of ERM augmented inconsiderably with the rising of pH from 2 to 4 owing to the slight dehydration of ERM functional groups (Viz., carbonyl and hydroxyl) under acidic conditions. While at a pH range of 5–9 a dramatic boost in the ERM adsorption capacity as the dehydration of ERM improves and its competitiveness for binding sites becomes better until the adsorption capacity being stabilized at approximately pH 9. However, the adsorption capacity of MIPs-Cs towards ERM started to slightly diminish when pH exceeded 9 that could be attributed to the crystallization of ERM. Moreover, equilibrium and kinetic investigations revealed that the adsorption was fitted to Freundlich and pseudo-second order with a maximum adsorption capacity of 52.32 μmol/g at 15 °C. In addition, it has to be mentioned that the MIPs-CS demonstrated superb regeneration efficiency with just 5.04% loss in the adsorption efficacy after three consecutive cycles because of its magnetic nature and subsequent facile separation.
Norfloxacin (NOR), a fluoroquinolone antibiotic, possesses high ability to interfere with DNA helicase function during DNA replication and inhibit cell division, killing bacteria. Fluoroquinolones may alter bacterial composition and diversity (Cui et al., 2014, Wang et al., 2021). NOR concentrations have been reported to be around 9.8 mg/kg in contaminated soil, and 6.8 g/L in urban sewage and surface water (Zou et al., 2011). NOR is also one of the most commonly detected antibiotics in sediment, with levels ranging from 6.5 to 2616.0 mg/kg because of its complex unbiodegradable structure (Li et al., 2018b).
Wu et al. (Wu et al., 2016) targeted the removal of NOR by magnetic molecular imprinted chitosan/γ-Fe2O3 composite (MICs). The BET specific surface area of MICs was 11.04 m2/g larger than the non-imprinted composite (8.23 m2/g). Despite the decline in the saturation magnetization of γ-Fe2O3 (32.98 emu/g) compared with MICs (5.87 emu/g), MICS could possess the ability to magnetically separate by an external magnet. In fact, NOR is a polyprotic molecule since at pH < 6.22, NOR exists in a cationic state, while it dwells as an anion at pH > 8.51. In addition to NOR exists in zwitterionic state at 6.22 < pH < 8.51 (Fang et al., 2020). Accordingly, the optimum pH to adsorb NOR onto MICS was proceeded at a wide pH scale and the results clarified that the optimum adsorption pH was 7 with a higher qe value of 6.29 mg/g. Furthermore, it was concluded that the adsorption mechanism of NOR is affected by hydrogen bonds and van der Waals forces. This finding agree with Nazraz et al. (Nazraz et al., 2019) at which it was determined that the apt pH to adsorb NOR onto magnesium oxide-chitosan-graphene oxide nanocomposite (MgO/Cs/GO) was 7 as well. Moreover, the maximum adsorption capacity of NOR under Langmuir was 1000 mg/g. This quite high adsorption capacity of NOR may be assigned to the high surface area of MgO/Cs/GO which was 294 m2/g. On the contrary, Zhou et al. (Zhou et al., 2018) deduced that the apt pH of the NOR adsorption onto Core-brushes shaped chitosan/Fe3O4 composite particles (Cs-MCPs) was between 3 and 4, suggesting that the adsorption was mainly derived by the electrostatic attraction between Cs-MCPs and NOR. Furthermore, it was proved that lower or higher pH is unfavorable for the adsorption process of NOR because of the competing adsorption between NOR and the large number of H+ ions in the strongly acidic conditions. Whereas at higher pH levels, the electrostatic attraction between Cs-MCPs and NOR declined as a result of NOR transformation from cationic species to neutral or anionic ones.
3.6 Removal of Ofloxacin and Doxycycline
Ofloxacin (OFL) is one of the most widely used antibiotics owing to its finite side effects. However, Humans and animals could not able to utterly absorbed OFL (Yu and Wu 2020). In addition to the complex structure of OFL that increases its persistence in the environment and renders its removal is quite complicated. Consequently, great amounts of OFL have been found in municipal solid waste leachates, surface water, soil and sediments (Li et al., 2017).
In this study, Zhu et al. (Zhu et al., 2018) inspected the removal of OFL by a chitosan/biochar composite (CsBC). It was concluded that the adsorption was enhanced in neutral pH conditions (Fig. 9a). This may be expounded by the alter in OFL ionization form according to the solution pH; OFL is mostly in a cationic form (OFL+) at pH < 6.1, anionic form (OFL−) is the dominant species at pH > 8.28. While, at the range of pH 6.1–8.28, part of OFL exists as the zwitterion (OFL±) and the rest exists as the neutral molecule (OFL0) as shown in Fig. 9b. Meanwhile, zeta potential measurement demonstrated that pHpzc of CsBC was 6.6. So, the surface charge of CsBC was positive at pH < 6.6, while it charged with a negative charge at pH > 6.6. Consequently, strong electrostatic repulsion between OFL and CsBC occurred whether under acidic or alkaline medium, hindering the OFL adsorption onto CsBC. The high adsorption capacity was attributed to the hydrophobic interaction between OFL and CsBC under neutral pH conditions and also to the large surface area of CsBC at which SBET was 141 m2/g.
(a) Effect of solution pH on OFL adsorption (b) Distribution of OFL species as a function of pH (Zhu et al., 2018). Copyright, Taylor & Francis, 2021.
Doxycycline (DC) that has the chemical formula of C22H24N2O8 is commonly utilized for the treatment of infectious diseases caused by various types of bacteria and protozoans including pneumonia, chlamydia, and cholera. Under natural environmental conditions DC is considered to be persistent because of having benzene rings, –NH2 and –CH3 groups (Olusegun and Mohallem, 2020). Several environmental problems have recently stemmed from the excessive use of DC including the detrimental impacts on human health and marine photosynthetic organisms (Azanu et al., 2018). In addition, lots of resistant-bacterial strains have lately evolved from DC uncontrolled use. Therefore, searching for an appropriate material that could be utilized for the removal of DC from water in order to lessen its perilous effects has become a quintessential issue (Okoli and Ofomaja, 2019).
In this respect, Tang et al. (Tang et al., 2021) explored the possibility of using magnetic Fe3O4/alkaline Ca-bentonite (MACB) and chitosan modified magnetic Fe3O4/alkaline Ca-bentonite (MACB-Cs) the removal of DC from an aqueous medium. MACB-Cs was successfully synthesized by modifying the MACB with Cs via Schiff base reaction. Fascinating adsorption efficiency for DC reached 96% accompanied with the advantageous magnetic separation capability was demonstrated by the modified MACB-Cs compared to the efficacy of the un modified MACB which was found to be 84%. According to this study, two main factors were scrutinized including the pH level effect and the contact time between MACB-Cs and MACB with DC. Regarding the first factor, it was found that the surface functionalities of both adsorbents were substantially influenced by the pH value. Therefore, the pH effect on the adsorption process of DC onto MACB and MACB-Cs was investigated. In detail, at a pH range from 2 to 3, DC was found in the form of DC+ so a strong competitive adsorption between DC and the large number of H+ ions for the active sites of both adsorbents, resulting in a diminished adsorption efficacy. Similarly, reduced adsorption efficiency was observed at a pH range of 8–11 since the deprotonated anionic form of DC are the predominant species that caused an electrostatic repulsion with the deprotonated functional groups of MACB and MACB-Cs. On the other hand, high adsorption capacity was obtained in the pH range of 5–6 at which DC is neutral and the surfaces of both MACB and MACB-Cs were positively charged (pHpzc of MACB = 6.55 and pHpzc of MACB-Cs = 5.93). Consequently, strong electrostatic forces between DC and both MACB and MACB-Cs is the main dominant mechanism in the adsorption process. This results agreed with the removal of DC using CuO@poly(AMPA)/Cs) composite and MCCs that evinced that the higher adsorption efficiency was recorded at pH 7 (Bai et al., 2018, Rahman and Varshney, 2021a,b). Also, complexation, electrostatic attraction and deposition mechanisms have a significant role in the DC adsorption onto MACB and MACB-Cs. Moreover, it was determined that the adsorption efficiency increased significantly with increasing the time till reaching an equilibrium after two hours for both adsorbents. The enhanced performance of MACB-Cs compared to MACB can be anticipated by the high surface area of MACB-Cs (957 m2/g) and also the synergistic modification of MACB-Cs.
3.7 Removal of Cefotaxime and Sulfamethoxazole
Cefotaxime (CTX) is a third-generation semisynthetic cephalosporin with a broad spectrum of activity against Gram-negative and Gram-positive bacterial infections (Zhang et al., 2006, Gothwal and Shashidhar, 2015, Hayasi and Saadatjoo, 2018). CTX was found to be persistent in various aquatic environments since it is commonly degraded with just 20% or even less even after three months. In spite of the low eco-toxicity of CTX in different environments, it usually results in the evolvement of multi-resistant bacterial strains that cause severe global health issues (Moreira et al., 2016, Wang et al., 2018). Therefore, it is pivotal to endeavor for finding a suitable material that could be applied in CTX removal.
In this prospect, Li et al. (Kentsa et al., 2020) examined the CTX removal by the novel NiFe2O4-COF-chitosan-terephthalaldehyde nanocomposites film (NCCsT) (Fig. 10a). It was determined by utilizing the salt addition approach that pHpzc of NCCsT was around 8.6. Besides, CTX ionization form changes with the change of pH; when pH < 3.4, CTXH3+ and CTXH2 are the predominant species. While as pH increases in the range of 3.4–6 the CTX became negatively charged CTXH−. Thence, the higher removal of CTX was achieved at pH 4 which was 54%, resulting from the strong electrostatic interactions between CTXH− and the positively charged NCCsT. Nevertheless, when the solution pH ranged from 6 to 8.6, the positively charged NCCsT slightly impact the adsorption of CTXH− and CTX2−, denoting that there was another factor controlling the adsorption process and it works only in an acidic condition such as condensation reaction as well as other controlling forces such as hydrogen bonding and π–π interaction. Regarding the influence of other interfering ions on the CTX removal including Na+, K+, Mg2+, and Ca2+, it was concluded that there was a noticeable reduction in the CTX removal in the existence of the four coexisting cations. This may be assigned to the adsorption of these cations onto the negatively charged CTX and resulting in the diminution of the electrostatic attraction between CTX and NCCsT. Also, it has to be mentioned that the removal efficiency of CTX on NCCsT still achieved above 90% of the initial percentage after six consecutive adsorption-desorption cycles, indicating that NCCsT possesses a well potential to utilize as a reusable adsorbent.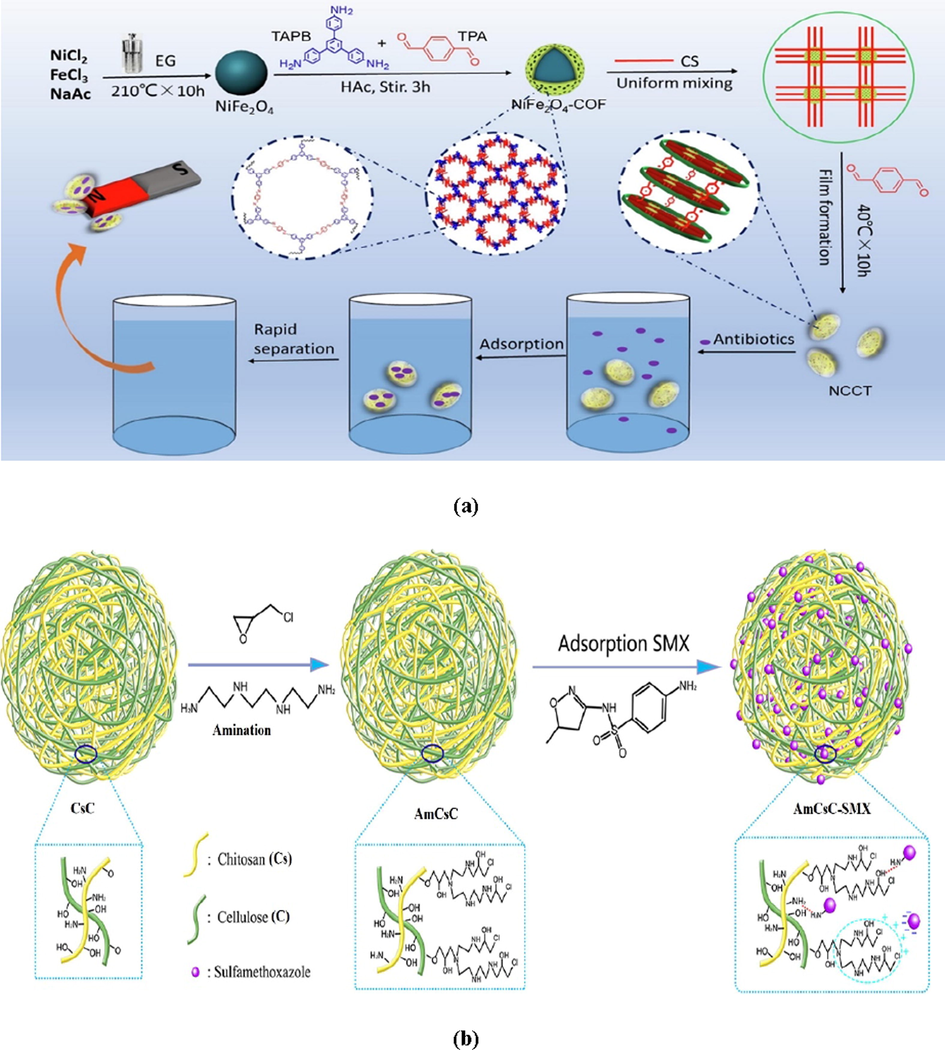
(a) Schematic illustration of preparation of NiFe2O4/COF/CS/TPA nanocomposites film (NCCT) and adsorption of CTX on NCCT (Kentsa et al., 2020). Copyright, Elsevier, 2021. (b) Schematic representation for the fabrication of AmCsC and the adsorption mechanism of SMX on AmCsC.
In another study, Li et al. (Li et al., 2021) compared the adsorption aptitude of chitosan particles (CsP) and chitosan film (CsF) toward the adsorptive removal of CTX from wastewater. It was found that the computed maximum adsorption capacity of CTX onto CsP and CsF were 648.05 and 1003.64 mg/g, respectively at pH 4. This superb adsorption capacity of CTX was explained by the strong interaction between both CsP and CsF and CTX via plane strengthening effect, electrostatic attraction, nucleophilic addition reaction and hydrogen bonding. Besides, the effects of common cations including NaCl, KCl, MgCl2 and CaCl2 on the CTX adsorption process were investigated. It was found that the removal efficiency of CTX diminished upon increasing the concentrations of these interfering cations. Such a result was attributed to the competitive adsorption between the cations and CsF because of the easy combination of these inorganic salts with CTX molecules.
Soares et al. (Soares et al., 2019) fabricated two magnetic chitosan-based adsorbent consist of Fe3O4 core coated with chitosan siloxane hybrid shells (Fe3O4@SiO2/SiCs) and Fe3O4 core coated with trimethyl chitosan/siloxane hybrid shells (Fe3O4@SiO2/SiTMCs) using a simple one-step coating procedure. The fabricated composites were tested for the removal of the antibiotic sulfamethoxazole (SMX) at optimum pH 5. It was found that TMC-based adsorbent has a higher SMX adsorption capacity (598.0 mg/g) than the that of pristine chitosan (24.1 mg/g). This high capacity of Fe3O4@SiO2/SiTMCs to remove SMX could be attributed to the quaternary amine groups of trimethyl chitosan where at pH 5 about 20% of SMX molecules are in the anionic form and thus could interact electrostatically with trimethylammonium groups of the Fe3O4@SiO2/SiTMCs rather than of pristine chitosan. In this context, aminated chitosan/cellulose nanocomposite was fabricated by in-situ solid phase grafting methods for the removal of low SMX concentration (Fig. 10b) (Gong et al., 2021). The removal of SMX onto AmCsC nanocomposite fitted with Langmuir isothermal model and pseudo 2nd order kinetic model with maximum adsorption capacity of 45.98 mg/g at 298 K and pH 6. Interestingly, the adsorption capacity of SMX onto AmCsC to was not large changed in the pH range 5–9 which could be explained by the fact that SMX was affected by the aromatic amine and sulfonamide groups on its surface (Gong et al., 2021). In another study by Zhou et al (Zhou et al., 2020), activated carbon embedded chitosan-polyvinyl alcohol (AC/CS/PVA) composite was prepared for the adsorptive removal of SMX from water. Adsorption followed pseudo 2nd order kinetic model and fitted Langmuir isotherm model with maximum adsorption capacity of 9.10 mg/g at optimum pH of 4. The adsorption of SMX on AC/CS/PVA was slightly affected by both temperature and ionic strength.
Overall, Table 4 concluded the optimum adsorption pH values as it considered yhe main affecting parameter on the removal process. In addition, the gained maximum adsorption capacities of the reported chitosan composites for the removal of various antibiotics were also summarized.
Adsorbent
Drug
qmax mg/g
Opt. pH
Ref.
MACCs
AMX
526.31
–
(Danalıoğlu et al., 2017)
Leached C black waste-chitosan composite
AMX
12.00
5.5
(Yaqubi et al., 2021)
MMPC/Cyc-Cs
LEVO
165.00
–
(Mashile et al., 2020)
NBent-TiO2-Cs
LEVO
90.91
4
(Mahmoud et al., 2020)
MMPC/Cyc-Cs
LEVO
165.00
–
(Mashile et al., 2020)
Chitosan film
LEVO
1003.64
4
(Li et al., 2021)
NBent-TiO2-Cs
CFT
90.91
5
(Mahmoud et al., 2020)
NBent-NTiO2-Cs
CFT
90.91
5
(Mahmoud et al., 2020)
MIPs-Cs
ERM
52.32
9
(Ou et al., 2015)
Cs/ MCs
ERM
–
3
(Ghodrat and Asrari 2018)
MICs
OFL
–
5–9
(Wu et al., 2016)
(MgO/Cs/GO)
OFL
1000
7
(Nazraz et al., 2019)
Cs-MCP
OFL
165
3
(Zhou et al., 2018)
CsBC
OFL
6.64
6.6
(Zhu et al., 2018)
MACB-Cs
DC
599
5–6
(Tang et al., 2021)
CuO@poly(AMPA)/Cs
DC
203.94
7
(Rahman and Varshney 2021)
(MCM)
DC
4.816
7–9
(Bai et al., 2018)
NCCsT
CTX
309.26
4
(Kentsa et al., 2020)
CsP
CTX
648.05
4
(Li et al., 2021)
CsF
CTX
1003.64
4
(Li et al., 2021)
Fe3O4@SiO2/SiCs
SMX
598.00
5
(Soares et al., 2019)
AmCsC
SMX
45.98
6
(Gong et al., 2021)
AC/Cs/PVA
SMX
9.10
4
(Zhou et al., 2020)
4 Conclusions and future recommendations
Removal of antibiotics from wastewater has been recently investigated numerous studies due to their harmful effects for aquatic life and human being (Välitalo et al., 2017). This review article concerns with the adsorption of the most common antibiotics using chitosan-based materials. The most common types of chitosan modifications reported in this review were the incorporation of metals, metal oxides, GO, biochar, carbonaceous materials and magnetic nanoparticles. The optimum pH and adsorption mechanism for the adsorption of each antibiotic has been reported and discussed briefly. It was deduced that the main adsorption mechanisms of antibiotics onto chitosan-based are electrostatic attraction, π-π, n-π and hydrogen bonding. The main advantages of chitosan-based composites for the removal of antibiotics are their high biocompatibility, recyclability and considerable high adsorption capacity. However, there are many challenges to be addressed including:
-
Future studies can be oriented toward the development of sustainable adsorbents via simple, low-cost and operative modification procedures. It is crucial to evidence the application viability of such chitosan composites-based adsorbents, in addition to broaden their adsorption performance and lessen their operation time.
-
The need to develop new large-scale and low-cost chitosan composites -based for real water treatment environments that contains multiple pollutants instead of single antibiotic pollutant. More researches regarding the selectivity of chitosan composites-based adsorbents are significant
-
The most important challenging aspects are the regeneration of the chitosan based-adsorbents which has direct inferences in terms of the manufacture costs. New regeneration techniques are needed to offer more cost-effective and reusable adsorbents with superior mechanical characteristics.
-
Comprehensive economic and market investigations are obligatory; since the foremost future aim is to interchange the adsorption process of pharmaceutical residues from lab scale to the industrial scale.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
References
- Antimicrobial and antioxidant properties of chitosan and its derivatives and their applications: a review. Int. J. Biol. Macromol.. 2020;164:2726-2744.
- [Google Scholar]
- Efficient adsorptive removal of tetracycline from aqueous solution using phytosynthesized nano-zero valent iron. J. Saudi Chem. Soc.. 2021;25:101365.
- [Google Scholar]
- In-vitro studies with ceftazidime against aerobic Gram-negative bacilli and Bacteroides fragilis group. J. Antimicrob. Chemother.. 1981;8:83-89.
- [Google Scholar]
- Enhancement of ciprofloxacin sorption on chitosan/biochar hydrogel beads. Sci. Total Environ.. 2018;639:560-569.
- [Google Scholar]
- Enhanced removal of ciprofloxacin using humic acid modified hydrogel beads. J. Colloid Interface Sci.. 2019;543:76-83.
- [Google Scholar]
- Fabrication of MnFe2O4 nanoparticles embedded chitosan-diphenylureaformaldehyde resin for the removal of tetracycline from aqueous solution. Int. J. Biol. Macromol.. 2019;134:180-188.
- [Google Scholar]
- Preparation of chitosan based magnetic nanocomposite for tetracycline adsorption: kinetic and thermodynamic studies. Int. J. Biol. Macromol.. 2020;147:258-267.
- [Google Scholar]
- Mechanistic insight into efficient removal of tetracycline from water by Fe/graphene. Chem. Eng. J.. 2019;373:821-830.
- [Google Scholar]
- Development of Spirulina/chitosan foam adsorbent for phenol adsorption. J. Mol. Liq.. 2020;309:113256.
- [Google Scholar]
- Antibacterial properties of Ro 13–9904, a long-acting new cephalosporin. Chemotherapy. 1981;27:9-14.
- [Google Scholar]
- Adsorption of ciprofloxacin hydrochloride on multiwall carbon nanotube. J. Mol. Struct.. 2020;1206:127711.
- [Google Scholar]
- Occurrence and risk assessment of antibiotics in water and lettuce in Ghana. Sci. Total Environ.. 2018;622:293-305.
- [Google Scholar]
- Bai, B., Xu, X., Li, C., Xing, J., Wang, H., Suo, Y., 2018. Magnetic Fe3O4@ chitosan carbon microbeads: removal of doxycycline from aqueous solutions through a fixed bed via sequential adsorption and heterogeneous Fenton-like regeneration. Journal of Nanomaterials. 2018.
- Chitosan as an environment friendly biomaterial–a review on recent modifications and applications. Int. J. Biol. Macromol.. 2020;150:1072-1083.
- [Google Scholar]
- The safety of chitosan as a pharmaceutical excipient. Regul. Toxicol. Pharm.. 2010;56:290-299.
- [Google Scholar]
- Efficient charge transfer in aluminum-cobalt layered double hydroxide derived from Co-ZIF for enhanced catalytic degradation of tetracycline through peroxymonosulfate activation. Chem. Eng. J.. 2020;382:122802.
- [Google Scholar]
- Antioxidant and moisturizing properties of carboxymethyl chitosan with different molecular weights. Polymers.. 2020;12:1445.
- [Google Scholar]
- Carbon disulfide-modified magnetic ion-imprinted chitosan-Fe (III): a novel adsorbent for simultaneous removal of tetracycline and cadmium. Carbohydr. Polym.. 2017;155:19-27.
- [Google Scholar]
- Influence of ciprofloxacin on microbial community structure and function in soils. Biol. Fertil. Soils. 2014;50:939-947.
- [Google Scholar]
- Efficient removal of antibiotics by a novel magnetic adsorbent: Magnetic activated carbon/chitosan (MACC) nanocomposite. J. Mol. Liq.. 2017;240:589-596.
- [Google Scholar]
- Chitosan grafted SiO 2–Fe 3 O 4 nanoparticles for removal of antibiotics from water. Environ. Sci. Pollut. Res.. 2018;25:36661-36670.
- [Google Scholar]
- Recent trends in removal pharmaceuticals and personal care products by electrochemical oxidation and combined systems. Water. 2020;12:1043.
- [Google Scholar]
- Potential uses of immobilized bacteria, fungi, algae, and their aggregates for treatment of organic and inorganic pollutants in wastewater. Water challenges and solutions on a global scale. ACS Publications; 2015. p. :319-337.
- Chitosan—A versatile semi-synthetic polymer in biomedical applications. Prog. Polym. Sci.. 2011;36:981-1014.
- [Google Scholar]
- Production of low molecular weight chitosan by acid and oxidative pathways: effect on physicochemical properties. Food Res. Int.. 2019;123:88-94.
- [Google Scholar]
- Functionalization of polymers and nanomaterials for biomedical applications: Antimicrobial platforms and drug carriers. Prosthesis.. 2020;2:117-139.
- [Google Scholar]
- Chitosan as a preservative for fruits and vegetables: a review on chemistry and antimicrobial properties. J. Bioresources Bioprod.. 2019;4:11-21.
- [Google Scholar]
- Antioxidant, anticancer and enhanced photocatalytic potentials of gold nanoparticles biosynthesized by common reed leaf extract. Appl. Nanosci. 2021:1-12.
- [Google Scholar]
- Catalytic and Medical Potential of a Phyto-Functionalized Reduced Graphene Oxide-Gold Nanocomposite Using Willow-Leaved Knotgrass. ACS Omega 2021
- [CrossRef] [Google Scholar]
- Chitin and chitosan: production and application of versatile biomedical nanomaterials. Int. J. Adv. Res.. 2016;4:411.
- [Google Scholar]
- Fabrication of attapulgite/magnetic aminated chitosan composite as efficient and reusable adsorbent for Cr (VI) ions. Sci. Rep.. 2021;11:1-15.
- [Google Scholar]
- Green synthesis of platinum nanoparticles using Atriplex halimus leaves for potential antimicrobial, antioxidant, and catalytic applications. Arabian J. Chem.. 2022;15:103517.
- [CrossRef] [Google Scholar]
- Highly Efficient Removal for Methylene Blue and Cu2+ onto UiO-66 Metal-Organic Framework/Carboxylated Graphene Oxide-Incorporated Sodium Alginate Beads. ACS Omega. 2021;6(36):23528-23541.
- [Google Scholar]
- Chitosan based adsorbents for the removal of phosphate and nitrate: a critical review. Carbohydr. Polym.. 2021;274:118671.
- [Google Scholar]
- High-efficiency adsorption of norfloxacin using octahedral UIO-66-NH2 nanomaterials: Dynamics, thermodynamics, and mechanisms. Appl. Surf. Sci.. 2020;518:146226.
- [Google Scholar]
- Z-scheme heterojunction based on NiWO 4/WO 3 microspheres with enhanced photocatalytic performance under visible light. Dalton Trans.. 2021;50:13801-13814.
- [Google Scholar]
- Comparative study of the performance of chitosan and chitosan adsorbents modified with Fe3O4 to eliminate erythromycin from aqueous solutions. Iranian J. Health Environ.. 2018;10:471-482.
- [Google Scholar]
- Aminated chitosan/cellulose nanocomposite microspheres designed for efficient removal of low-concentration sulfamethoxazole from water. J. Mol. Liq.. 2021;339:116407.
- [Google Scholar]
- Antibiotic pollution in the environment: a review. Clean-Soil, Air, Water.. 2015;43:479-489.
- [Google Scholar]
- Preparation of magnetic nanoparticles functionalized with poly (styrene-2-acrylamido-2-methyl propanesulfonic acid) as novel adsorbents for removal of pharmaceuticals from aqueous solutions. Adv. Polym. Tech.. 2018;37:1941-1953.
- [Google Scholar]
- Instantaneous phytosynthesis of gold nanoparticles via Persicaria salicifolia leaf extract, and their medical applications. Adv. Powder Technol. 2021
- [CrossRef] [Google Scholar]
- Comparative study on the potentialities of two halophytic species in the green synthesis of gold nanoparticles and their anticancer, antioxidant and catalytic efficiencies. Adv. Powder Technol.. 2021;32:3220-3233.
- [Google Scholar]
- Comparative study between Phragmites australis root and rhizome extracts for mediating gold nanoparticles synthesis and their medical and environmental applications. Adv. Powder Technol. 2021
- [Google Scholar]
- Biogenic synthesis, characterization, antimicrobial, antioxidant, antidiabetic, and catalytic applications of platinum nanoparticles synthesized from Polygonum salicifolium leaves. J. Environ. Chem. Eng.. 2022;10:106806.
- [CrossRef] [Google Scholar]
- Degradation performance and microbial community analysis of microbial electrolysis cells for erythromycin wastewater treatment. Biochem. Eng. J.. 2019;146:1-9.
- [Google Scholar]
- Effect of Cu (II) ions on the enhancement of tetracycline adsorption by Fe3O4@ SiO2-Chitosan/graphene oxide nanocomposite. Carbohydr. Polym.. 2017;157:576-585.
- [Google Scholar]
- The impact of chitosan on seafood quality and human health: A review. Trends Food Sci. Technol.. 2020;97:404-416.
- [Google Scholar]
- The application of modified natural polymers in toxicant dye compounds wastewater: A review. Water.. 2020;12:2032.
- [Google Scholar]
- Optimizing the metal-support interactions at the Pd-polymer carbon nitride Mott-Schottky heterojunction interface for an enhanced electrocatalytic hydrodechlorination reaction. J. Hazard. Mater.. 2021;411:125119.
- [Google Scholar]
- Characterization of Akilbenza clay from Cameroon and its performance for the removal of copper (II) ions from aqueous solution. Environ. Sci. Pollut. Res.. 2020;27:36487-36497.
- [Google Scholar]
- Surface modified cellulose nanomaterials: a source of non-spherical nanoparticles for drug delivery. Mater. Horiz.. 2020;7:1727-1758.
- [Google Scholar]
- Perspectives of coagulation/flocculation for the removal of pharmaceuticals from domestic wastewater: A critical view at experimental procedures. J. Water Process Eng.. 2020;34:101161.
- [Google Scholar]
- Sustainable biomaterials based on cellulose, chitin and chitosan composites-A review. Carbohydrate Polymer Technol. Appl.. 2021;2:100079.
- [Google Scholar]
- Functionalization of halloysite nanotube with chitosan reinforced poly (vinyl alcohol) nanocomposites for potential biomedical applications. Int. J. Biol. Macromol.. 2020;165:1079-1092.
- [Google Scholar]
- Transformation of Supercapacitive Charge Storage Behaviour in a Multi elemental Spinel CuMn 2 O 4 Nanofibers with Alkaline and Neutral Electrolytes. Adv. Fiber Mater. 2021:1-10.
- [Google Scholar]
- Prospection of recent chitosan biomedical trends: Evidence from patent analysis (2009–2020) Int. J. Biol. Macromol. 2020
- [Google Scholar]
- Use of microalgae based technology for the removal of antibiotics from wastewater: a review. Chemosphere. 2020;238:124680.
- [Google Scholar]
- Preparation of RuO2-TiO2/Nano-graphite composite anode for electrochemical degradation of ceftriaxone sodium. J. Hazard. Mater.. 2018;351:250-259.
- [Google Scholar]
- Migration of two antibiotics during resuspension under simulated wind–wave disturbances in a water–sediment system. Chemosphere. 2018;192:234-243.
- [Google Scholar]
- Sorption behavior of ofloxacin to kaolinite: effects of pH, ionic strength, and Cu (II) Water Air Soil Pollut.. 2017;228:46.
- [Google Scholar]
- Biochar supported CuO composites used as an efficient peroxymonosulfate activator for highly saline organic wastewater treatment. Sci. Total Environ.. 2020;721:137764.
- [Google Scholar]
- A high-efficiency and plane-enhanced chitosan film for cefotaxime adsorption compared with chitosan particles in water. Chem. Eng. J.. 2021;413:127494.
- [Google Scholar]
- Self-indicating and high-capacity mesoporous aerogel-based biosorbent fabricated from cellulose and chitosan via co-dissolution and regeneration for removing formaldehyde from indoor air. Environ. Sci. Nano. 2021;8:1283-1295.
- [Google Scholar]
- Degradation of antibiotic ciprofloxacin by different AOP systems using electrochemically generated hydrogen peroxide. Chemosphere. 2020;247:125807.
- [Google Scholar]
- Photocatalytic hydrogen production coupled with selective benzylamine oxidation over MOF composites. Angew. Chem.. 2018;130:5477-5481.
- [Google Scholar]
- A novel Biochar modified by Chitosan-Fe/S for tetracycline adsorption and studies on site energy distribution. Bioresour. Technol.. 2019;294:122152.
- [Google Scholar]
- Efficient and selective adsorption of dye in aqueous environment employing a functional Zn (Ⅱ)-based metal organic framework. J. Solid State Chem.. 2020;121740
- [Google Scholar]
- Bacterial cellulose-based composite scaffolds for biomedical applications: a review. ACS Sustainable Chem. Eng.. 2020;8:7536-7562.
- [Google Scholar]
- Chitosan–starch films modified with natural extracts to remove heavy oil from water. Water.. 2020;12:17.
- [Google Scholar]
- Magnetic chitosan–based adsorbent prepared via Pickering high internal phase emulsion for high-efficient removal of antibiotics. Int. J. Biol. Macromol.. 2018;106:870-877.
- [Google Scholar]
- Nitrate reduction by nanoscale zero valent iron (nFe0)-based Systems: Mechanism, reaction pathway and strategy for enhanced N2 formation. Chem. Eng. J.. 2022;430:133133.
- [Google Scholar]
- Adsorption properties, kinetics & thermodynamics of tetracycline on carboxymethyl-chitosan reformed montmorillonite. Int. J. Biol. Macromol.. 2019;124:557-567.
- [Google Scholar]
- Facile method for the synthesis of a magnetic CNTs–C@ Fe–chitosan composite and its application in tetracycline removal from aqueous solutions. PCCP. 2015;17:15936-15944.
- [Google Scholar]
- Preparation and characterization of chitosan/kaolin/Fe3O4 magnetic microspheres and their application for the removal of ciprofloxacin. Adsorpt. Sci. Technol.. 2014;32:775-790.
- [Google Scholar]
- Enhanced adsorption of Levofloxacin and Ceftriaxone antibiotics from water by assembled composite of nanotitanium oxide/chitosan/nano-bentonite. Mater. Sci. Eng., C. 2020;108:110199.
- [Google Scholar]
- Preparation of CoFe2O4/activated carbon@ chitosan as a new magnetic nanobiocomposite for adsorption of ciprofloxacin in aqueous solutions. Water Sci. Technol.. 2018;78:2158-2170.
- [Google Scholar]
- A biodegradable magnetic nanocomposite as a superabsorbent for the simultaneous removal of selected fluoroquinolones from environmental water matrices: isotherm, kinetics, thermodynamic studies and cost analysis. Polymers. 2020;12:1102.
- [Google Scholar]
- Mohy Eldin, M.S., Omer, A.M., Wassel, M.A., Tamer, T.M., Abd Elmonem, M.S. and Ibrahim, S.A. Novel smart pH sensitive chitosan grafted alginate hydrogel microcapsules for oral protein delivery: II. evaluation of the swelling behavior, (2015) International Journal of Pharmacy and Pharmaceutical Sciences, 7 (10), 331-337.
- Photocatalytic ozonation of urban wastewater and surface water using immobilized TiO2 with LEDs: Micropollutants, antibiotic resistance genes and estrogenic activity. Water Res.. 2016;94:10-22.
- [Google Scholar]
- Tetracycline and viruses: a possible treatment for COVID-19? Arch. Virol. 2020:1-7.
- [Google Scholar]
- Insight into cellulose-based-nanomaterials-A pursuit of environmental remedies. Int. J. Biol. Macromol. 2020
- [Google Scholar]
- Starch, cellulose, pectin, gum, alginate, chitin and chitosan derived (nano) materials for sustainable water treatment: a review. Carbohydr. Polym.. 2020;116986
- [Google Scholar]
- Chitosan-based sorbent for efficient removal and extraction of ciprofloxacin and norfloxacin from aqueous solutions. Microchim. Acta. 2019;186:1-9.
- [Google Scholar]
- Advancement on modification of chitosan biopolymer and its potential applications. Int. J. Biol. Macromol.. 2020;152:681-702.
- [Google Scholar]
- Development of sustainable magnetic polyurethane polymer nanocomposite for abatement of tetracycline antibiotics aqueous pollution: response surface methodology and adsorption dynamics. J. Cleaner Prod.. 2019;217:42-55.
- [Google Scholar]
- Comparative adsorption mechanism of doxycycline and Congo red using synthesized kaolinite supported CoFe2O4 nanoparticles. Environ. Pollut.. 2020;260:114019.
- [Google Scholar]
- Omer, A. M., E. M. Abd El-Monaem, G. M. El-Subruiti, M. M. Abd El-Latif and A. S. Eltaweil, 2021. Fabrication of easy separable and reusable MIL-125(Ti)/MIL-53(Fe) binary MOF/CNT/Alginate composite microbeads for tetracycline removal from water bodies. 11, 23818.
- pH-Sensitive Alginate/Carboxymethyl Chitosan/Aminated Chitosan Microcapsules for Efficient Encapsulation and Delivery of Diclofenac Sodium. Pharmaceutics. 2021;13:338.
- [Google Scholar]
- Insights into recent advances of chitosan-based adsorbents for sustainable removal of heavy metals and anions. Arabian J. Chem.. 2022;15:103543.
- [Google Scholar]
- Formulation and Antibacterial Activity Evaluation of Quaternized Aminochitosan Membrane for Wound Dressing Applications. Polymers.. 2021;13:2428.
- [Google Scholar]
- Selective removal of erythromycin by magnetic imprinted polymers synthesized from chitosan-stabilized Pickering emulsion. J. Hazard. Mater.. 2015;289:28-37.
- [Google Scholar]
- Removal of tetracycline by magnetic chitosan nanoparticles from medical wastewaters. Desalination Wter Treat.. 2017;73:380-388.
- [Google Scholar]
- Effective removal of doxycycline from aqueous solution using CuO nanoparticles decorated poly (2-acrylamido-2-methyl-1-propanesulfonic acid)/chitosan. Environ. Sci. Pollut. Res. 2021:1-19.
- [Google Scholar]
- Facile Synthesis and Characterization of Zn (II)-Impregnated Chitosan/Graphene Oxide: Evaluation of Its Efficiency for Removal of Ciprofloxacin from Aqueous Solution. J. Inorg. Organomet. Polym Mater. 2021:1-18.
- [Google Scholar]
- Biomedical applications of chitosan: an overview. J. Biomater. Tissue Eng.. 2012;2:100-111.
- [Google Scholar]
- Efficient tetracycline adsorptive removal using tricaprylmethylammonium chloride conjugated chitosan hydrogel beads: mechanism, kinetic, isotherms and thermodynamic study. Int. J. Biol. Macromol.. 2020;155:421-429.
- [Google Scholar]
- Mechanistic investigation of ciprofloxacin recovery by magnetite–imprinted chitosan nanocomposite: isotherm, kinetic, thermodynamic and reusability studies. Int. J. Biol. Macromol.. 2019;133:712-721.
- [Google Scholar]
- Biomaterial-based flower-like MnO2@ carbon microspheres for rapid adsorption of amoxicillin from wastewater. J. Mol. Liq.. 2020;309:113074.
- [Google Scholar]
- Ceftriaxone. Drugs.. 1984;27:469-527.
- Removal of tetracycline from polluted water by chitosan-olive pomace adsorbing films. Sci. Total Environ.. 2019;693:133620.
- [Google Scholar]
- Saheed, I. O., O. W. Da and F. B. M. Suah, 2020. Chitosan Modifications for Adsorption of Pollutants-A review. J. Hazard. Mater. 124889.
- Chitosan grafted/cross-linked with biodegradable polymers: a review. Int. J. Biol. Macromol. 2021
- [Google Scholar]
- Global review and analysis of erythromycin in the environment: occurrence, bioaccumulation and antibiotic resistance hazards. Environ. Pollut.. 2018;238:440-451.
- [Google Scholar]
- Synthesis and characterization of nanocomposite ultrafiltration membrane (PSF/PVP/SiO2) and performance evaluation for the removal of amoxicillin from aqueous solutions. Environ. Technol. Innovation. 2020;17:100529.
- [Google Scholar]
- High-capacity adsorption of Cr (VI) by lignin-based composite: Characterization, performance and mechanism. Int. J. Biol. Macromol.. 2020;159:839-849.
- [Google Scholar]
- Fluorine-containing polyimide/polysilsesquioxane carbon molecular sieve membranes and techno-economic evaluation thereof for C3H6/C3H8 separation. J. Membr. Sci.. 2020;598:117660.
- [Google Scholar]
- The relationship between the absolute molecular weight and the degree of quaternisation of N-trimethyl chitosan chloride. Carbohydr. Polym.. 2002;50:145-150.
- [Google Scholar]
- Polymer-hybrid nanoparticles: Current advances in biomedical applications. Biomed. Pharmacother.. 2020;131:110695.
- [Google Scholar]
- Trimethyl chitosan/siloxane-hybrid coated Fe3O4 nanoparticles for the uptake of sulfamethoxazole from water. Molecules. 2019;24:1958.
- [Google Scholar]
- Therapeutic Potential for Tetracyclines in the Treatment of COVID-19. Pharmacotherapy.. 2020;40:487.
- [Google Scholar]
- Tamer, T.M., Abou-Taleb, W.M., Roston, G.D., Mohyeldin, M.S., Omer, A.M., Khalifa, R.E. and Hafez, A.M. Formation of zinc oxide nanoparticles using alginate as a template for purification of wastewater,(2018) Environmental Nanotechnology, Monitoring and Management, 10, 112-121.
- Fabrication of carboxymethyl cellulose and chitosan modified Magnetic alkaline Ca-bentonite for the adsorption of hazardous doxycycline. Colloids Surf., A. 2021;610:125730.
- [Google Scholar]
- Surface modification of natural polymers to impart low water absorbency. Int. J. Polym. Anal. Charact.. 2012;17:133-143.
- [Google Scholar]
- Natural polymers, biopolymers, biomaterials, and their composites, blends, and IPNs. CRC Press; 2012.
- Thomson, J. A., 2021. RE: Transparency in the selection of therapeutic treatments: Where are the clinical trials of the tetracycline family (doxycycline) for SARS-CoV-2/COVID-19?.
- Optimization of tetracycline removal with chitosan obtained from mussel shells using RSM. J. Ind. Eng. Chem.. 2020;84:315-321.
- [Google Scholar]
- Application of chitosan and its derivatives as adsorbents for dye removal from water and wastewater: A review. Carbohydr. Polym.. 2014;113:115-130.
- [Google Scholar]
- Toxicological impacts of antibiotics on aquatic micro-organisms: a mini-review. Int. J. Hyg. Environ. Health.. 2017;220:558-569.
- [Google Scholar]
- Facile construction of novel organic–inorganic tetra (4-carboxyphenyl) porphyrin/Bi2MoO6 heterojunction for tetracycline degradation: Performance, degradation pathways, intermediate toxicity analysis and mechanism insight. J. Colloid Interface Sci.. 2022;605:727-740.
- [Google Scholar]
- Photocatalytic degradation of fluoroquinolone antibiotics using ordered mesoporous g-C3N4 under simulated sunlight irradiation: kinetics, mechanism, and antibacterial activity elimination. Appl. Catal. B. 2018;227:114-122.
- [Google Scholar]
- Effects of antibiotic norfloxacin on the degradation and enantioselectivity of the herbicides in aquatic environment. Ecotoxicol. Environ. Saf.. 2021;208:111717.
- [Google Scholar]
- Removal of ciprofloxacin from aqueous solution by a magnetic chitosan grafted graphene oxide composite. J. Mol. Liq.. 2016;222:188-194.
- [Google Scholar]
- Pd-TiO2 Schottky heterojunction catalyst boost the electrocatalytic hydrodechlorination reaction. Chem. Eng. J.. 2020;381:122673.
- [Google Scholar]
- Chitosan stabilized bimetallic Fe/Ni nanoparticles used to remove mixed contaminants-amoxicillin and Cd (II) from aqueous solutions. Chem. Eng. J.. 2013;229:27-34.
- [Google Scholar]
- Visible light photocatalytic degradation of tetracycline over TiO2. Chem. Eng. J.. 2020;382:122842.
- [Google Scholar]
- Recognizing removal of norfloxacin by novel magnetic molecular imprinted chitosan/γ-Fe2O3 composites: selective adsorption mechanisms, practical application and regeneration. Sep. Purif. Technol.. 2016;165:92-100.
- [Google Scholar]
- Adsorption of tetracycline antibiotics from aqueous solutions on nanocomposite multi-walled carbon nanotube functionalized MIL-53 (Fe) as new adsorbent. Sci. Total Environ.. 2018;627:235-244.
- [Google Scholar]
- Mesoporous cellulose-chitosan composite hydrogel fabricated via the co-dissolution-regeneration process as biosorbent of heavy metals. Environ. Pollut.. 2021;286:117324.
- [Google Scholar]
- Enhanced removal of antibiotics in wastewater by membrane bioreactor with addition of rice straw. Int. Biodeterior. Biodegrad.. 2020;148:104868.
- [Google Scholar]
- Chitosan-based nanocomposites for gene delivery: Application and future perspectives. Polysaccharide-Based Nanocomposites for Gene Delivery and Tissue Engineering. Elsevier; 2021. p. :245-262.
- Adsorptive removal of tetracycline and amoxicillin from aqueous solution by leached carbon black waste and chitosan-carbon composite beads. J. Environ. Chem. Eng.. 2021;9:104988.
- [Google Scholar]
- Design, synthesis, and characterization of graphene–nanoparticle hybrid materials for bioapplications. Chem. Rev.. 2015;115:2483-2531.
- [Google Scholar]
- A review on versatile applications of blends and composites of CNC with natural and synthetic polymers with mathematical modeling. Int. J. Biol. Macromol.. 2019;124:591-626.
- [Google Scholar]
- High adsorption for ofloxacin and reusability by the use of ZIF-8 for wastewater treatment. Microporous Mesoporous Mater.. 2020;308:110494.
- [Google Scholar]
- Enhanced levofloxacin removal from water using zirconium (IV) loaded corn bracts. Environ. Sci. Pollut. Res. Int.. 2017;24:10685.
- [Google Scholar]
- Solubiliy of sodium cefotaxime in aqueous 2-propanol mixtures. J. Chem. Eng. Data. 2006;51:2239-2241.
- [Google Scholar]
- Enhanced adsorption of tetracycline by an iron and manganese oxides loaded biochar: Kinetics, mechanism and column adsorption. Bioresour. Technol.. 2021;320:124264.
- [Google Scholar]
- One-pot synthesis of trifunctional chitosan-EDTA-β-cyclodextrin polymer for simultaneous removal of metals and organic micropollutants. Sci. Rep.. 2017;7:1-14.
- [Google Scholar]
- Uniform and stable immobilization of metal-organic frameworks into chitosan matrix for enhanced tetracycline removal from water. Chem. Eng. J.. 2020;382:122893.
- [Google Scholar]
- Tetracycline adsorption on kaolinite: pH, metal cations and humic acid effects. Ecotoxicology. 2011;20:1141-1147.
- [Google Scholar]
- Fabrication of hydrophobic/hydrophilic bifunctional adsorbent for the removal of sulfamethoxazole and bisphenol A in Water. J. Environ. Chem. Eng.. 2020;8:104161.
- [Google Scholar]
- Enhanced adsorption of pharmaceuticals onto core-brush shaped aromatic rings-functionalized chitosan magnetic composite particles: Effects of structural characteristics of both pharmaceuticals and brushes. J. Cleaner Prod.. 2018;172:1025-1034.
- [Google Scholar]
- Ofloxacin adsorption on chitosan/biochar composite: kinetics, isotherms, and effects of solution chemistry. Polycyclic Aromat. Compd. 2018
- [Google Scholar]
- Enhanced water-solubility and antibacterial activity of novel chitosan derivatives modified with quaternary phosphonium salt. Mater. Sci. Eng., C. 2016;61:79-84.
- [Google Scholar]
- Preparation, characterization and adsorption properties of chitosan modified magnetic graphitized multi-walled carbon nanotubes for highly effective removal of a carcinogenic dye from aqueous solution. Appl. Surf. Sci.. 2013;285:865-873.
- [Google Scholar]
- Occurrence and distribution of antibiotics in coastal water of the Bohai Bay, China: impacts of river discharge and aquaculture activities. Environ. Pollut.. 2011;159:2913-2920.
- [Google Scholar]







