Translate this page into:
Synergistic improvement of foam stability with SiO2 nanoparticles (SiO2-NPs) and different surfactants
⁎Corresponding authors. at: Yangtze University WuHan Jingzhou, Hubei, CN 434023, China(P. Xu, M. Xu); Hubei Cooperative Innovation Center of Unconventional Oil and Gas, Wuhan 430100, China(P. Xu, M. Xu). cdxupeng@yangtzeu.edu.cn (Xu Peng), cjdxxmb@126.com (Xu Mingbiao),
-
Received: ,
Accepted: ,
This article was originally published by Elsevier and was migrated to Scientific Scholar after the change of Publisher.
Peer review under responsibility of King Saud University.
Abstract
Foam fluids are widely used in petroleum engineering, but long-standing foam stability problems have limited the effectiveness of their use. The study explores the synergistic effects and influencing factors of SiO2 nanoparticles (SiO2-NPs) with different wettability properties and three different surfactants. The paper investigates the foaming performance of different types of surfactants and analyzes and compares the stability of foam after adding hydrophilic and hydrophobic SiO2-NPs from macroscopic as well as microscopic perspectives, and the effects of temperature and inorganic salts on the stability of mixed solutions. The experimental results show that: 1) hydrophilic nanoparticles can significantly enhance the foam stability of amphoteric surfactants, with a small increase in the foam stability of anionic and cationic surfactants; 2) The concentration of nanoparticles did not have a significant effect on the stability of the cationic surfactants and this conclusion was verified in the experimental results of the surface tension measured below;3) The cationic surfactants showed better temperature resistance at temperatures of 50–90 °C. Both amphoteric surfactant solutions with the addition of hydrophilic SiO2-NPs or hydrophobic SiO2-NPs significantly improved the temperature resistance of the foam at high temperatures. The anionic surfactant solution with hydrophobic SiO2-NPs did not enhance the solution temperature resistance; 4) The surface tension of the surfactant solution gradually increases with increasing concentration of hydrophilic or hydrophobic SiO2-NPs and then levels off; 5) the hydrophilic SiO2-NPs had a significant effect on the salt tolerance of the anionic and amphoteric surfactant solutions. The salt tolerance of cationic surfactant solutions with hydrophobic SiO2-NPs was better than that of surfactants with hydrophilic SiO2-NPs.
Keywords
Foam stability
Silica nanoparticles
SiO2-NPs
Surfactant
Hydrophilic
Hydrophobic
1 Introduction
Foam has been used as an important material in many fields (Karthick et al.,2019; Karthick et al.,2016;), and due to its simple preparation and low cost with little damage to the formation, air foam flooding technology has a greater potential for application as technology in oil recovery for increasing oil and gas production and other work (Guo et al.,2016; Liu et al.,2019). It also shows good prospects in the application of low permeability oil field conditioning because of the advantages of foam systems such as strong injectability and multiple injection methods (Peng et al.,2017). However, the application is limited by the disadvantages such as poor foam stability and easy rupture. Researchers have studied various aspects of improving foam stability (Karthick et al.,2019)and proposed that adding solid nanoparticles can solve this problem (Qiang et al.,2007; Hunter et al.,2009; Horozov et al.,2008; Kumar et al.,2017; Vatanparast et al.,2018; Singh et al.,2015; Karthick et al.,2017). Nowadays, nanoparticle-stabilized foams are used in various industries, such as in various technical fields like food and cosmetics and in oil recovery applications where nanoparticles are used as stabilizers (Bouwmeester et al.,2009; Li et al.,2019; MD et al.,2015; Karthick et al., 2019; Abhishek S et al., 2022; Behbahani et al., 2015). Therefore, it is important to research the synergistic interaction between surfactants and nanoparticles.
The addition of nanoparticles to surfactants is a novel system used to stabilize foam stability (Karthick et al.,2019; Chattopadhyay et al.,2017; Karthick et al.,2021). Most of the current studies have been conducted for silica on its own or stability analysis of surfactants on its own. Milad et al. studied the interfacial properties of the anionic surfactant SDBS in the presence of SiO2-NPs of different scales and concentrations (Eftekhari et al.,2020). Hamid et al. investigated the interfacial behavior of anionic surfactants in the presence of hydrophilic SiO2-NPs and proposed that in the presence of surfactants, nanoparticles retain their non-surface activity, and the surface activity of surfactants increases directly with increasing SiO2-NPs concentration, and this fact is attributed to the electrostatic repulsion between negatively charged nanoparticles and anionic surfactant molecules effect (Vatanparast et al.,2018).
In addition to studying the addition of hydrophilic SiO2-NPs to anionic surfactants, Santini et al. also studied the formation of different structures of hydrophilic SiO2-NPs with cationic surfactants at the water/air interface and it was shown that the adsorption of CTAB on the surface of solid particles changed their hydrophilic/oleophilic state becoming more hydrophobic (Santini et al.,2011). According to the experimental results, CTAB molecules from unsaturated monolayers envelop SiO2-NPs at relatively high concentrations, making hydrophilic SiO2-NPs hydrophobic. Due to the hydrophobic nature of particles, systems consisting of SiO2-NPs and CTAB can form irregular solid structures at the water/air interface.
At the same time, researchers have started to investigate the addition of hydrophobic SiO2-NPs to surfactants to probe the stability. Hunter et al. investigated the effect of the nonionic surfactant TX100 on the foaming properties between hydrophobic SiO2-NPs can improve foam stability in a specific low to medium concentration range, suggesting that the main process of foam stability generated by the surfactant-silica mixture solution is through the interfacial “flocculation” of the particles, forming large molecular structures and maintaining the spatial integrity of the interface (Hunter et al.,2009). Sun et al. evaluated the effect of adding partially hydrophobically modified SiO2-NPs to sodium dodecyl sulfate SDS solution to improve foam stability and enhance recovery using the micro-model drive and sand-filled drive, and the experiments showed that the foam stability gradually increased and the foam volume decreased after the addition of SiO2-NPs, in the effect of hydrophilic and hydrophobic SiO2-NPs on surfactant properties (Sun et al.,2014), Mohammad et al. used both types of SiO2 in combination with anionic surfactants and showed that the addition of both types of nanoparticles to low and high concentrations of surfactants resulted in contrasting interfacial behavior. The addition of a small amount of anionic surfactant (SDS) results in a fairly stable suspension of nanoparticles (Nasim, 2014 . Shoja ei et al. investigated the effect of different charge (anionic, cationic, and nonionic) surfactants on foam stability in the presence of charge-stabilized SiO2-NPs and explored foam coarsening by gas diffusion or bubble coalescence. The experimental results showed that the foam prepared by adding anionic surfactants with nanoparticles increased the original apparent viscosity of the foam and the stability of gravity drainage; For the foam prepared by adding cationic surfactants with nanoparticles, the apparent viscosity measured within the Hele Shaw cell indicated that the foam stability was poor. This conclusion is consistent with our experimental conclusions on the stability of foam obtained from solutions prepared with cationic surfactants and hydrophobic SiO2-NPs surfactants by foam foaming volume and half-life experiments (Shojaei et al.,2021).
This study takes the foam drilling fluid required for low-pressure leak-prone formations as the research background and the stability problem of surfactants added to the foam drilling fluid as the starting point to stabilize the foam using SiO2-NPs. The foaming performance and mechanism of action of anionic surfactant (SDS), cationic surfactant (CTAB), and amphoteric surfactant (CAB-35) were investigated, and then the foaming performance of added hydrophilic and hydrophobic nanoparticle-surfactant solutions was studied, as well as the interaction principle of three different surfactants with pro/hydrophobic SiO2-NPs, and the surface tension and temperature and salt resistance of mixed solutions of different surfactants and hydrophobic SiO2-NPs were further analyzed.
2 Material and methods
2.1 Experimental materials
Three different ionic types of surfactants (anionic surfactants, cationic surfactants, and amphoteric surfactants) were used in this study. Sodium dodecyl sulfate (SDS) (Sinopharm Reagent), cetyltrimethyl ammonium bromide (CTAB) (China National Drug Reagent), and Cocamidopropyl betaine35 (CAB-35) (China National Drug Reagent). The properties of these surfactants are summarized in Table 1.
Surfactant type
Name
Purity
Molecular weight
Anionic surfactants
Sodium dodecyl sulfonate(SDS)
≥ 99.0 %
288.38 g/mol
Amphoteric ionic surfactants
Cocoamidopropyl Betaine (CAB-35)
99.0 %
348.48 g/mol
Cationic surfactants
cetyltrimethyl ammonium bromide (CTAB)
≥ 99.0 %
364.45 g/mol
Hydrophobic gas-phase SiO2-NPs (Shanghai Macklin Biochemical Technology) with a specific surface area of 230 m2/g and a particle size of 7–40 nm or hydrophilic gas-phase SiO2-NPs (Shanghai Macklin Biochemical Technology) with a specific surface area of 200 m2/g and a particle size of 7–40 nm were added to the three different surfactant solutions, respectively.
2.2 Experimental apparatus
An electronic balance (METTLER-TOLEDO ME104E) was used to determine the weight of the material. Firstly, the foam was prepared by a high-speed stirrer (Qingdao Tongchun Petroleum Instrument Co., ltd., China), and the prepared foam was poured into a measuring cylinder (Changzheng Chemical Glass Instrument) to measure the foaming volume, and a stopwatch was used to record the foam half-life, and this experiment was used to compare the foaming performance and foam stability of three surfactants. Then the hydrophilic SiO2-NPs and hydrophobic SiO2-NPs were dispersed in an ultrasonic pulverizer (LAWSON SCIENTIFIC), and then a water bath heater (Changzhou Guoyu Youqi Manufacturing Co., ltd.) was used to evaporate the alcohol-water mixture to disperse the hydrophobic SiO2-NPs. The surface tension of the solution was measured by using QBZY series automatic surface tension meter (Shanghai Fangrui Instrument Co., ltd.); the contact angle of hydrophilic SiO2-NPs and hydrophobic SiO2-NPs was measured by using YIKE-360B contact angle measuring instrument (Chengde Yi Ke Test Equipment Factory).
2.3 Experimental methods
2.3.1 Dispersion preparation of SiO2-NPs
Because the hydrophobic SiO2-NPs used in the experiment are extremely hydrophobic, they cannot be dissolved in the surfactant solution in the experiments, but float on the water surface. Therefore, it is necessary to pretreat the SiO2-NPs when configuring the hydrophobic SiO2-NPs solution, to disperse the hydrophobic SiO2-NPs. Firstly, the hydrophobic SiO2-NPs were wetted using anhydrous ethanol (Sun et al.,2015), anhydrous ethanol was mixed with hydrophobic SiO2-NPs in a 4:1 ratio, stirred to a certain paste, and deionized water was added to a certain amount and then sonicated for 30 min for uniform dispersion. After fixing the volume to 200 ml and transferring to a water bath heated pot for evaporation to a liquid volume of 50 ml, continue to add deionized water and evaporate again, repeating the above steps several times to ensure that only trace amounts of ethanol remain. After the dispersed solution of hydrophobic SiO2-NPs, the solution was stirred for 30 min using a high-speed stirrer to ensure uniform dispersion (Sun et al.,2014). The treated SiO2-NPs can be uniformly dispersed in water.
2.3.2 Foam evaluation experiments
The surfactant aqueous solution was configured and the surfactant was added to the well-dispersed SiO2-NPs solution to form the SiO2-NPs and surfactant dispersion. The effect of SiO2-NPs on the foaming and stabilization performance of SDS, CTAB, and CAB-35 foam systems was studied.
(1) Foam foaming performance.
There are many experiments to evaluate foam performance, and in this paper, the Waring-Blender method (Kitty et al.,1946) is used to determine the foaming capacity and foam stability. Take 100 ml of dispersion in a high stirring cup, stir at high speed (8000r/min) for 2 min, then quickly pour the foam into the measuring cylinder, record the initial volume of foam (i.e. foaming volume) and start timing, the time required when the foam discharge volume reaches half of the original dispersion volume (i.e., the discharge volume reaches 50 ml) the time required is the foam half-life, each concentration ratio is measured at least three times, take The average value is taken as the final result.
(2) Microstructure of foam.
The microstructure of foams needs to be carried out under wet-phase conditions(Manas et al.,2012), and cryoelectron microscopy and optical microscopy observation are the main ways to observe them. In this study, the microstructure of the foam was observed by optical microscopy, and a piece of foam solution was taken after mixing the surfactant-water mixture with a high-speed stirrer at 8000 r/min and immediately placed on a clean slide. In this study, the foam morphology was photographed at 1 min, 10 min, and 20 min to observe the microstructural changes of surfactant SDS under the conditions with and without the addition of SiO2-NPs.
(3) Surface tension experiment.
The QBZY series automatic tensiometer is used to measure the surface tension of surfactants and different concentrations of hydrophobic SiO2-NPs mixtures at room temperature. The specific experimental method is: firstly, connect the power supply and turn on the machine to warm up for 30 min, clean the platinum sheet with anhydrous ethanol, leave it to burn red in the external flame of the alcohol lamp, and then gently hang it on the measuring hook of the instrument after cooling; place the petri dish for weighing and placing the sample in the measuring module on the sample table, wait for the liquid level and the platinum sheet to stabilize, tare to zero and then measure the surface tension according to the measuring steps. Each sample should be measured three times to take the average value to ensure the accuracy of the experimental data. At the end of the experiment, the platinum sheet is cleaned and stored.(See Fig. 1).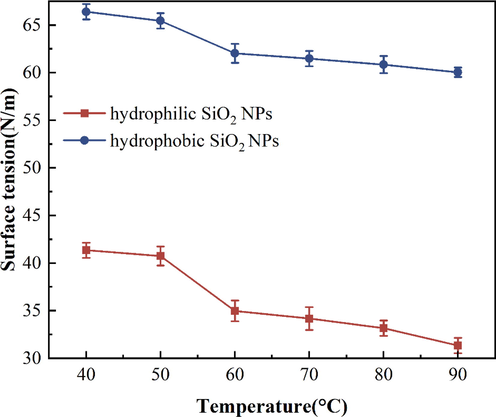
Surface tension isotherms of hydrophilic and hydrophobic SiO2-NPs.
(4) Surfactant temperature resistance experiment.
The surfactant was added to the dispersed hydrophilic or hydrophobic SiO2-NPs solution, and then the solution was placed in a water bath heating pot with heating temperatures of 50, 60, 70, 80, and 90 °C, respectively. After heating to a certain temperature, the foam was stirred for 2 min with a high-speedstirrer, and the foam was quickly poured into a 500 ml measuring cylinder and timed with a stopwatch to record the volume and half-life of the foam.
(5) Surfactant salt tolerance experiment.
Prepare 5 cups of 100 ml of hydrophilic or hydrophobic SiO2-NPs dispersion solution adding surfactant and sodium chloride at 0.2 %, 0.4 %, 0.6 %, 0.8 %, 1 % of the solution mass. The prepared mixed solution was stirred for 2 min under a high-speed stirrer, and then the foam was quickly poured into a 500 ml measuring cylinder and timed by a stopwatch to record the foam volume and half-life.
(6) Contact angle measurement.
The contact angles of aqueous droplets of hydrophilic and hydrophobic SiO2-NPs on slides were measured using the YIKE-360B contact angle measuring instrument from the domestic Chengde Yike Test Equipment Factory. The contact angle was measured by the static drop method. The configured silica particle solution was loaded into a syringe equipped with a needle and measured using contact angle measurement software. Firstly, the syringe was mounted and fixed, the assay software was opened to turn on the video, the syringe was advanced slowly until the droplet fell on the slide, the video was stopped immediately, and the contact angle of the droplet at this moment was drawn on the picture, which is given in Figure 2 for hydrophobic SiO2-NPs and hydrophilic SiO2-NPs.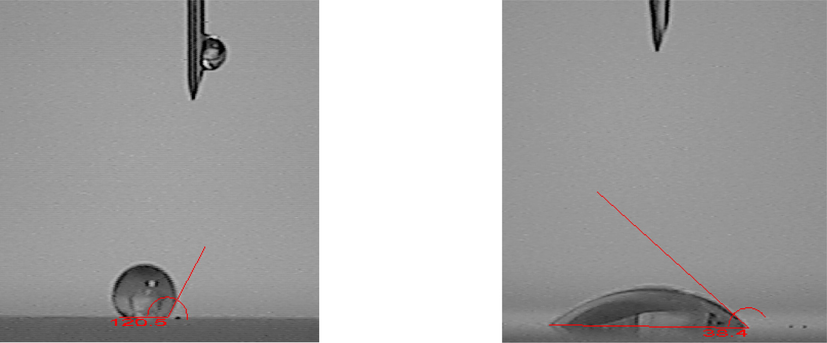
Nano-silica contact angle (hydrophobic nano-silica on the left, contact angle of 120.6°; hydrophilic nano-silica on the right, contact angle of 38.4°).
3 Results and discussion
3.1 Foaming and foam stabilization of different surfactants
Firstly, to study the foam performance of the three surfactants at different concentrations and to select the best surfactant dosage. Preparation of three surfactant solutions of anionic surfactant (SDS), cationic surfactant (CTAB), and amphoteric surfactant (CAB-35), the added masses are 0.1 %, 0.3 %, 0.5 %, 0.7 %, and 0.9 %, and the foam foaming volume and half-life evaluation experiments were carried out, and the experimental results are shown in Fig. 3 and Fig. 4.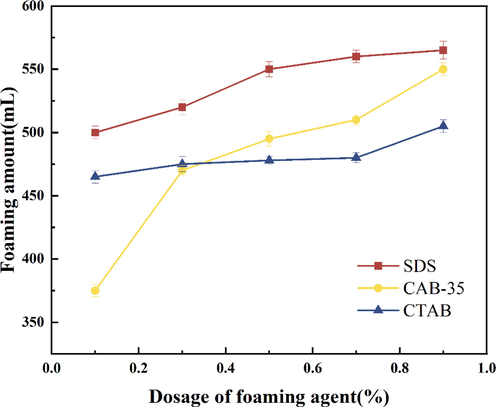
Experimental results of different surfactant foaming amounts.
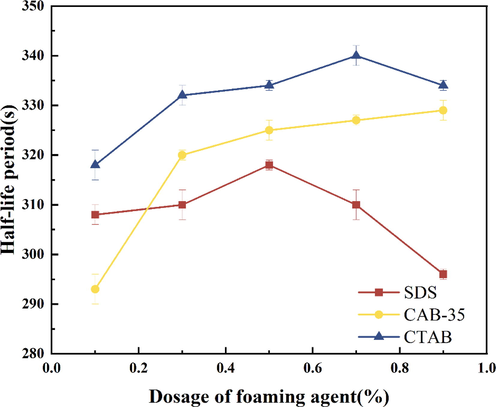
Experimental results of the half-life of different surfactants.
From Figs. 3, 4 three foaming agents on their own foaming volume and half-life: with the increase of foaming agent concentration three foaming agent foaming volumes are gradually rising, but the half-life of anionic surfactants and cationic surfactants show a trend of first rising and then falling. The half-life of the amphoteric surfactants showed a slow increase after the addition of more than 0.3 %. However, the half-life of the amphoteric surfactant increased only by 5 s when the addition amount was increased from 0.3 % to 1 % content. The anionic surfactant foaming amount was the best, and its half-life showed a decreasing trend at the addition of 0.5 %, indicating that the foam stability decreased after 0.5 % addition; the addition of cationic surfactant from 0.1 % to 1 % foam foaming amount only increased by about 35 ml, which indicates that the cationic concentration has less influence on the foaming amount. However, the half-life was influenced by the addition amount, and the stability of the foam showed a decreasing trend at the addition amount greater than 0.7 %. According to the form of foaming performance presented by the three foaming agents, the optimal amount of foaming agent addition was preferred.
3.2 Effect of hydrophilic SiO2-NPs and surfactant on foam stability
With the above hydrophilic SiO2-NPs contact angle measurements, it is clear that the contact angle ranges from 37 to 39°, and the SiO2-NPs carry a strong hydrophilic effect with ionic surfactants. To study the effect of hydrophilic SiO2-NPs on three surfactants, the interaction between hydrophilic nanoparticles and surfactants at different concentrations was investigated using foam foaming volume and stability experiments.
Hydrophilic SiO2-NPs (0.1 %,0.3 %,0.5 %,0.7 %,0.9 %) is uniformly dispersed in anionic surfactant (SDS), amphoteric surfactant (CAB-35), cationic surfactant (CTAB) solution. According to the above analysis, this paper selected SDS solution mass fraction of 0.5 %, CAB-35 mass concentration of 0.3 %, CTAB mass concentration of 0.7 % for foam experiments, the results are shown in Table 2, Fig. 5, and Fig. 6.
Hydrophilic SiO2-NPs mass fraction/%
SDS
CAB −35
CTAB
Foaming volume (mL)
Half-life period(s)
Foaming volume (mL)
Half-life period(s)
Foaming volume (mL)
Half-life period(s)
0
550
318
470
320
480
340
0.1
545
400
475
465
478
332
0.3
540
406
460
602
475
337
0.5
532
410
410
907
468
340
0.7
534
418
400
1057
442
467
0.9
530
450
385
1573
440
805
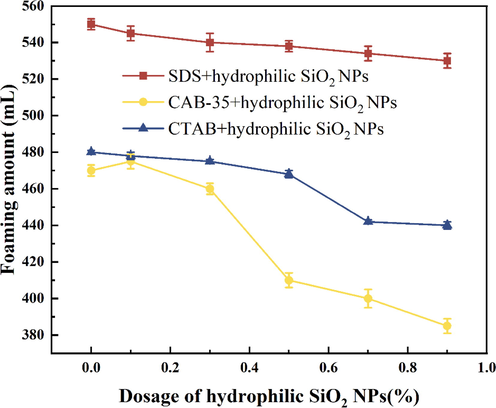
Experimental results of the amount of surfactant foaming by adding different concentrations of hydrophilic SiO2-NPs.
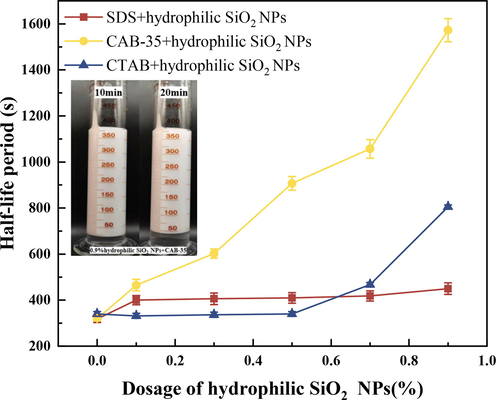
Experimental results of half-life of surfactants with different concentrations of hydrophilic SiO2-NPs.
For the cationic surfactant CTAB mixed with hydrophilic SiO2-NPs solution (Fig. 7), the foaming amount after adding hydrophilic SiO2-NPs is lower than that of the solution without adding hydrophilic SiO2-NPs. The reason is that CTAB is positively charged and hydrophilic SiO2-NPs are negatively charged after dissolving in water, resulting in charge adsorption between hydrophilic SiO2-NPs and CTAB, which transforms hydrophilic nanoparticles into partially hydrophobic SiO2-NPs (Ravera et al.,2006). However, at this time, the SiO2-NPs still have a high hydrophilicity. When the CTAB concentration was below 0.5 % (Fig. 7a), the half-life of CTAB mixed with hydrophilic SiO2-NPs solution did not have a large change. When the hydrophilic SiO2-NPs content is less than 0.5 % (As shown in Fig. 7a), the half-life of the solutions prepared by CTAB and hydrophilic SiO2-NPs did not change significantly. And when the concentration of hydrophilic SiO2-NPs is added above 0.5 %, the electrostatic adsorption of cationic surfactant and hydrophilic SiO2-NPs will gradually increase the foam stabilization ability. With the addition of a lower concentration of hydrophilic SiO2-NPs at a certain concentration of CTAB (Fig. 8a), the cationic surfactant is adsorbed on the surface of SiO2 particles due to the electrostatic interaction between positive and negative charges, and the number of surfactants is reduced and the foaming amount is decreased.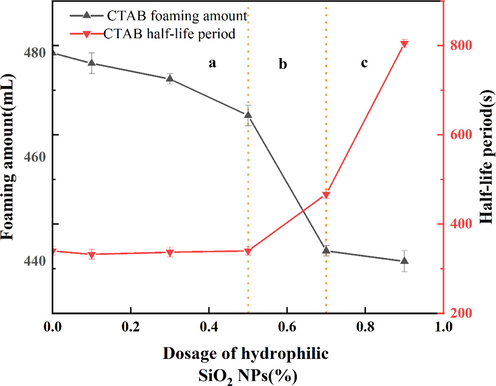
Foaming performance of cationic surfactants with hydrophilic SiO2-NPs.
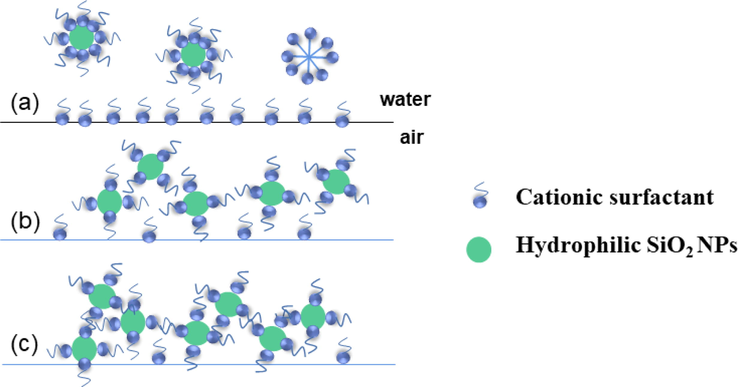
Low concentration of hydrophilic SiO2-NPs (a) in the presence of CTAB solution, when there are still a large number of surfactant molecules on the surface of the gas–liquid interface. When the medium concentration of hydrophilic SiO2-NPs (b) is present with CTAB solution, the surfactant in the solution is partly adsorbed on the surface of nanoparticles and partly at the gas–liquid interface. When a high concentration of hydrophilic SiO2-NPs(c) is present in the CTAB solution, most of the surfactant is adsorbed on the surface of the nanoparticles (JIANG et al.,2016).
As the concentration of hydrophilic SiO2-NPs increases, more surfactants move from the gas–liquid interface to the surface of nanoparticles to form surfactant-coated nanoparticles, which tend to be hydrophobic and enhance the surface activity of the particles, making it easier for the particles to adsorb at the gas–liquid interface to improve foam stability. Jia et al. also demonstrated this by using Zeta potential experiments to illustrate the electrostatic adsorption between the two. (Jia et al.,2020). When the hydrophilic SiO2-NPs concentration is high (Fig. 8c) there are fewer surfactant molecules at the gas–liquid interface at this time, resulting in a lower foaming volume. This indicates that hydrophilic SiO2-NPs can form synergistic foam stabilization with surfactants at a more suitable CTAB concentration (Binks et al.,2008).
Amphoteric surfactants are characterized by high water solubility and good foam stability. As shown in Fig. 5, Fig. 6 as the concentration of hydrophilic SiO2-NPs gradually increases, the solution foaming volume gradually decreases, When the hydrophilic SiO2-NPs content is lower than 0.3 %, the CAB-35 foaming volume is more balanced without much change, which is mainly because the content of hydrophilic SiO2-NPs added at this time is less, and the surfactant present in the solution is the main factor that mainly causes the foaming performance. Continuing to increase the concentration of SiO2-NPs, the foaming volume decreases substantially, at which time the content of hydrophilic SiO2-NPs added is higher and adsorbs the surfactant within the solution, making the surfactant content within the solution decrease. In terms of half-life, the foam stability gradually increased with the increase of hydrophilic SiO2-NPs concentration. The reason for this is the adsorption of the positively charged part of the amphoteric surfactant (Mclachlan et al.,2006) with the hydrophilic surfactant, and from the contact angle in Fig. 9 increasing the amount of silica addition its contact angle gradually increases. Combined with the foam half-life, it can be seen that the foam stability gradually increases in the appropriate contact angle range.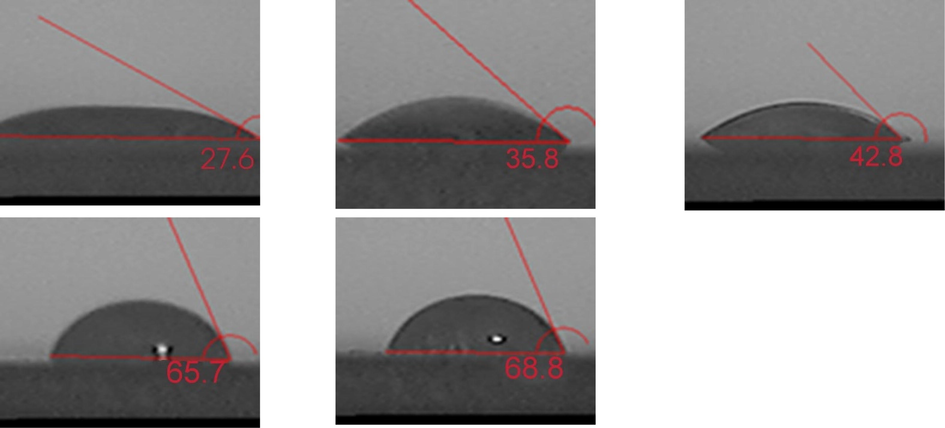
Contact angle between CAB-35 and hydrophilic nano-silica (CAB-35 dosing is 0.3%, from left to right added hydrophilic nano-silica dosing is 0.1%, 0.3%, 0.5%, 0.7%, 0.9% respectively).
3.3 Effect of hydrophobic SiO2-NPs and surfactant on foam stability
The dispersion-treated hydrophobic SiO2-NPs solutions (0.1 %, 0.3 %, 0.5 %, 0.7 %, 0.9 %) were mixed with three different surfactants and the foaming properties were recorded as shown in Figs. 10, 11.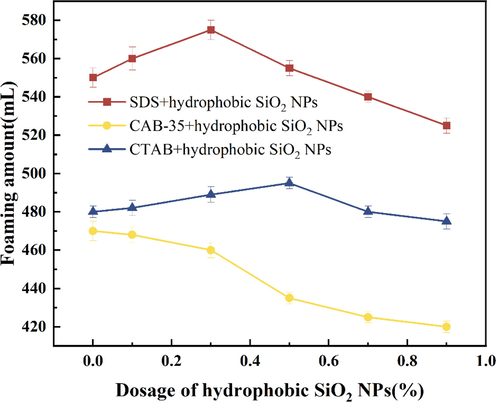
Experiment with the amount of surfactant foaming by adding different concentrations of hydrophobic SiO2-NPs.
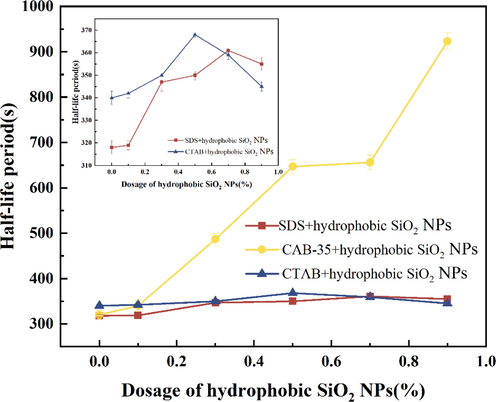
Half-life experiments of surfactants with different concentrations of hydrophobic SiO2-NPs.
In the mixed system after adding hydrophobic SiO2-NPs (Figs. 10, 11), the foaming amount of SiO2/CTAB and SiO2/SDS is higher. The adsorption of SiO2-NPs at the air–water interface is mainly controlled by the interaction between the particle interface and the particles, and some of the hydrophobic SiO2-NPs are positively charged. At constant SDS concentration (Fig. 12a) due to adsorption at the air–water interface resulting in SDS adsorption on lower concentrations of SiO2-NPs. At moderate SDS concentrations (Fig. 12b) particles become more hydrophobic, particle-to-particle and particle-to-interface electrostatic repulsions are smaller, and particle adsorption at the interface is enhanced. SDS molecules are adsorbed on the SiO2 surface by hydrophobic interaction, leaving the negative hydrophilic groups exposed (Vatanparst et al.,2017). The concentration of SiO2-NPs is higher (Fig. 12c) when the positive charge on the particle surface is reduced and more uncharged particles are adsorbed on the bubble surface. This conclusion can also be illustrated according to Jia et al. regarding the absolute value of zeta potential between anionic surfactants and hydrophobic nanoparticles (Jia et al.,2020), by zeta potential it was found that the addition of anionic surfactants to hydrophobic SiO2-NPs greatly increased the absolute value of zeta potential, while the addition of hydrophilic SiO2-NPs slightly increased the absolute value of zeta potential, while the increase in zeta potential indicated that more SDS was adsorbed on the surface of hydrophobic SiO2-NPs. It was demonstrated that the SDS in Fig. 10 showed a trend of increasing and then decreasing foaming after the addition of hydrophobic SiO2-NPs.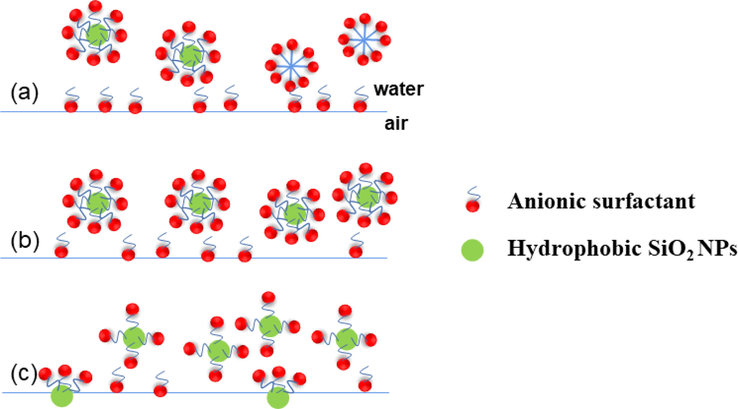
The adsorption process of SDS and hydrophobic SiO2-NPs (Jiang et al., 2016).
As the concentration of hydrophobic SiO2-NPs increased, there was no relatively obvious trend of increasing or decreasing the foaming volume and half-life of the mixed solution of CTAB and hydrophobic SiO2-NPs, indicating the stability of the foam was only stabilized by the surfactant at this time. After adding amphoteric surfactant CAB-35 to the dispersion solution of hydrophobic SiO2-NPs, the foam half-life gradually increased with the increase of hydrophobic SiO2-NPs, and the foam half-life reached 15 min. The half-life of hydrophilic SiO2-NPs was 25 min.
3.4 Microstructure of SiO2-NPs stabilized foam
To observe the microscopic morphology of the foam with SiO2-NPs, we put the prepared foam under an optical microscope to observe its morphology.
The foamed film of anionic surfactant and hydrophobic nano silica particle solution is thicker than that of the anionic surfactant itself (Fig. 13), meanwhile, as the bubble continues to gather and burst (Sun et al., 2016), the water phase flowed between the liquid film, and after 10 min the foam liquid film gradually thinned and the foam became coarse. After 20 min, the foam gradually ruptured and thicker, and finally disappeared. After adding SiO2-NPs to the solution, the nanoparticles are well dispersed between the liquid film and have certain adsorption properties, which makes the foam have better toughness (Xue et al.,2021).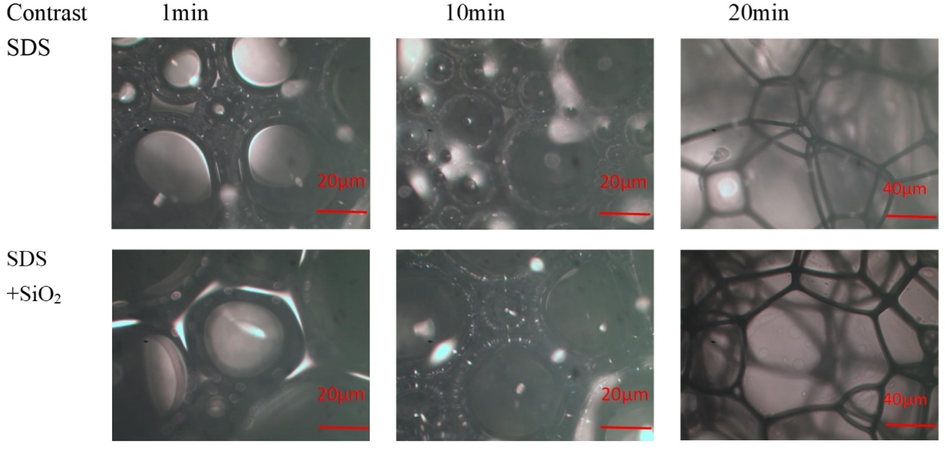
Microstructure of foam with added SiO2-NPs (200x magnification before 10 min, 100x magnification after 10 min).
3.5 Effect of hydrophilic or hydrophobic SiO2-NPs concentration on surface tension
Surface tension is also one of the factors affecting foam stability. In this paper, the surface tension of different surfactants with hydrophilic or hydrophobic SiO2-NPs at different concentrations was determined. The trend of the addition of different concentrations of hydrophilic and hydrophobic SiO2-NPs on the surface tension of the three surfactants was investigated (Fig. 14). The addition amounts of anionic, cationic and amphoteric surfactants are 0.5 %, 0.7 % and 0.3 % respectively.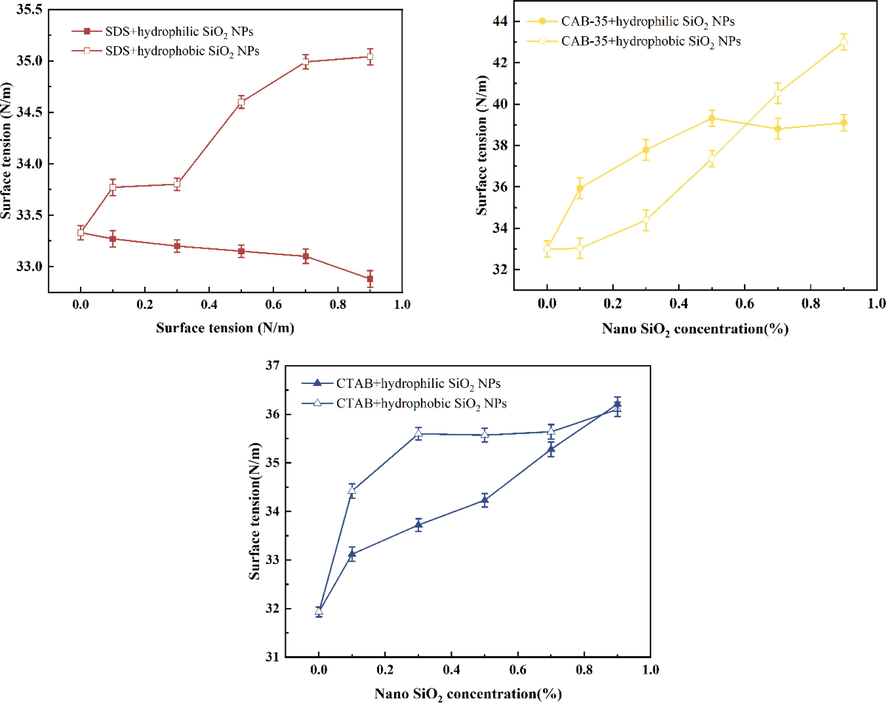
The surface tension of surfactant solutions with the addition of hydrophilic and hydrophobic SiO2-NPs.
With the increase of hydrophilic SiO2-NPs concentration, the surface tension curve of SDS mixed solution showed a smooth trend (Fig. 14), which shows that the number of surfactants at the interface does not decrease after the addition of hydrophilic SiO2-NPs. As the concentration of SiO2-NPs increases raises the viscosity within the solution (Yan et al.,2006), reducing the rate of liquid film drainage thereby increasing the stability of the foam. After the addition of hydrophobic SiO2-NPs, the surface tension increased with the increase of hydrophobic SiO2-NPs particle content, which indicates that the amount of surfactant within the solution decreased at this time, and we believe that this may be due to the adsorption of nanoparticles. The increasing surface tension of CAB-35 and CTAB both in hydrophobic and hydrophilic SiO2-NPs solutions indicates that both surfactants are adsorbed to the SiO2-NPs at this time leading to the adsorption of the surfactant on the liquid surface to the SiO2-NPs and the formation of surfactant-encapsulated nanoparticles. The difference is that the surface tension of CTAB increases first with hydrophobic SiO2-NPs at lower SiO2-NPs solubility and then increases slowly. The surface tension of the solution increases continuously after the addition of hydrophilic SiO2-NPs to the cationic surfactant solution. Because the amphiphilic surfactant is hydrophilic and adsorbs with hydrophobic SiO2-NPs resulting in high surface tension. Surfactant adsorption on the nanoparticles in small amounts of hydrophilic nanoparticle solutions raises the surface tension of the liquid surface. However, the surface tension changes less with the increasing concentration of hydrophilic SiO2-NPs.
3.6 Effect of temperature on the foam stability of surfactants with added hydrophilic or hydrophobic SiO2-NPs
3.6.1 Effect of temperature stability of hydrophilic nano-surfactant foam
To compare the foam stability of hydrophilic SiO2-NPs surfactants at different temperatures, 50 °C, 60 °C, 70 °C, 80 °C, and 90 °C were chosen to record the foaming volume and half-life of different surfactants to evaluate the temperature resistance of the foam.
Comparing the foaming performance of the two systems of surfactants at different temperatures, in terms of foaming volume (Fig. 15), the foaming volume of the solution with the addition of hydrophilic SiO2-NPs is lower than the foaming volume of the foaming agent solution on its own. From the graph, we can see that the foaming curves of SDS, SDS/SiO2, and CAB-35 solutions show a trend of rising and then falling with the increase in temperature. This is because the increase in temperature decreases the viscosity of the liquid phase and increases the dissolution rate of surfactants, which makes foaming easier (Chen, 2019; Dandigunta et al., 2021). However, the increase in temperature also promotes the coarsening of the foam, which is not conducive to its stability of the foam. SDS exhibits high foaming performance in the temperature range of 50–90 °C with a foaming agent solution on its own as well as with the addition of hydrophilic SiO2-NPs. The foaming amount of CTAB with the addition of hydrophilic SiO2-NPs is higher than the foaming agent solution on its foaming amount.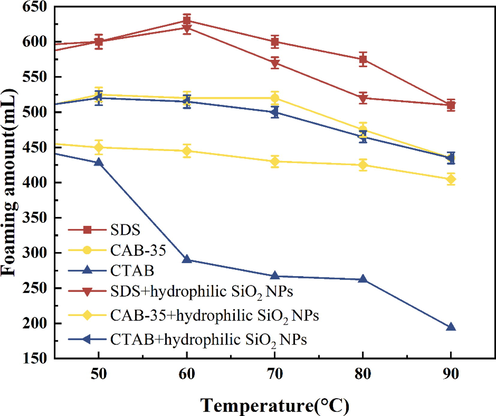
Comparison of temperature resistance of surfactants with the addition of hydrophilic SiO2-NPs (foaming volume).
The results of temperature resistance experiments comparing two different systems of three different surfactants at different temperatures in terms of half-life show (Fig. 16) that the half-life of the foam has been decreasing with the increase in temperature and the stability has become worse. This is mainly caused by two aspects, on the one hand, when the temperature rises, the evaporation of water from the foam film intensifies so that the film becomes thinner, the rate of liquid discharge increases, and the foam is easy to burst (Li et al., 2015); On the other hand, as the temperature increases, the solution viscosity decreases, the rate of liquid discharge from the liquid film increases, and the foam stability becomes less stable (Chen, 2019). In addition, the increase in temperature and the strengthening of gas diffusion also made the foam more unstable comparing the foam stability of different three surfactants, it can be seen that CTAB has better temperature resistance, whereas a CTAB solution on its shows a longer half-life and better foam stability at high temperatures. Comparing the other two surfactants, the foam stabilization effect of SDS with CAB-35 after the addition of hydrophilic SiO2-NPs was better than that of the foaming agent solution on its own. It indicates that the addition of hydrophilic SiO2-NPs can improve the temperature resistance of surfactants.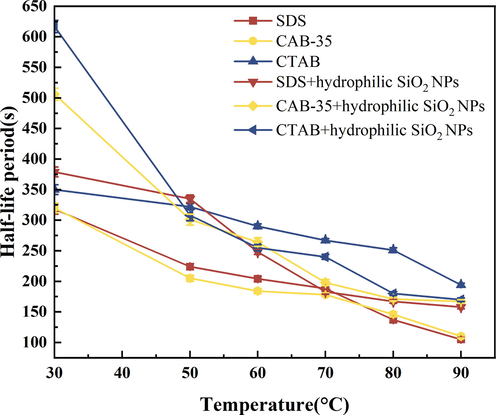
Comparison of temperature resistance of surfactants with hydrophilic SiO2-NPs (Half-life).
3.6.2 Effect of temperature stability of hydrophobic nano-surfactant foam
The surfactant and hydrophobic SiO2-NPs mixed solutions at different temperatures were configured to record the foaming performance of different systems and to study their temperature resistance.
According to Figs. 17, 18 experimental results are shown, anionic surfactants have a higher foaming capacity. The foaming capacity at 50–90 °C for both foaming agent solution on its own and surfactants with added hydrophobic SiO2-NPs is higher than that of cationic and amphoteric surfactants. The half-life curve of the amphoteric surfactant clearly shows that hydrophobic SiO2-NPs play a greater role in temperature resistance, and CAB-35 with hydrophobic SiO2-NPs particles has an increased ability to stabilize foam at 50–90 °C. The foaming volume of cationic surfactants decreases rapidly after the temperature is higher than 50 °C, and only about 180 ml of foaming volume remains at 90 °C, but the half-life can last up to 220 s. Since the low foaming volume of cationic surfactants causes the foam rupture to be less affected by the gravity factor, the half-life at 90 °C does not indicate the temperature resistance of cations. The addition of cationic surfactants with hydrophobic SiO2-NPs improves the foaming ability of the foam. The foaming volume of the foam is much higher than that of the foaming agent solution on its own at a temperature above 50 °C.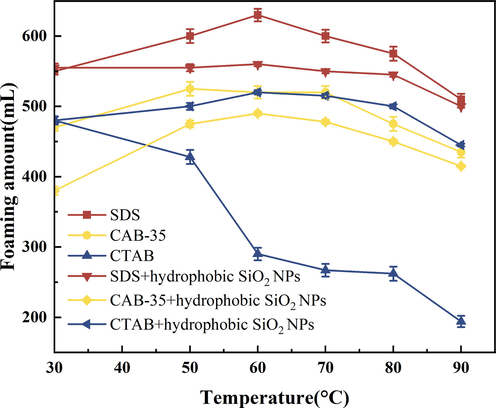
Comparison of temperature resistance of surfactants with added hydrophobic SiO2-NPs (foaming volume).
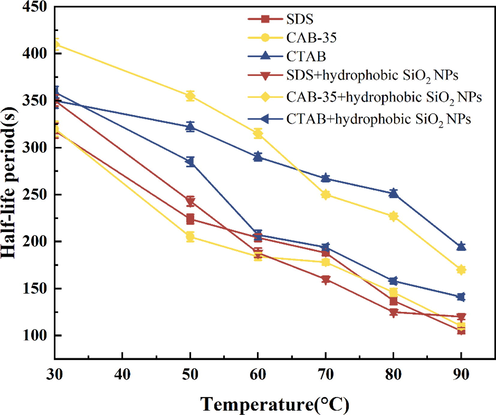
Comparison of temperature resistance of surfactants with hydrophobic SiO2-NPs (Half-life).
3.7 Effect of inorganic salts on the foam stability of surfactants with hydrophilic or hydrophobic SiO2-NPs
In most of the oil fields in China, the mineralization of the formation water is high. Inorganic salt ions interact with surfactant ions, which in turn affects foam stability. The addition of salt to ionic surfactants increases the hydrophobicity of the surfactant, and the increase in electrolyte concentration leads to an increase in the micelle number and aggregation number of the surfactant solution (Cheng et al.,2006). Sammalkorpi et al. investigated the effect of NaCl on the aggregation properties of sodium dodecyl sulfate SDS solutions using a molecular dynamics approach (Sammalkorpi et al.,2009). It was found that Na+ as a head group linking surfactant molecules, plays an important role in stabilizing the structure of micelles as well as increasing the size of micelles, while it can promote the coalescence of counterions around the aggregates, leading to tighter arrangement of surfactant molecules in the aggregates.
The effect of Na+ and Mg2+ on foam stability of a solution mixed with surfactants and nanoparticles will be investigated. In this experiment, SDS addition of 0.5 %, CTAB addition of 0.7 %, CAB-35 addition of 0.3 %, and hydrophilic or hydrophobic SiO2-NPs addition according to the above experiments preferably selected the more effective to add to the surfactant solution. Hydrophilic SiO2-NPs with anionic addition of 0.7 %, with cationic addition of 0.9 %, and with amphoteric surfactant addition of 0.7 %; hydrophobic SiO2-NPs with anionic surfactant addition of 0.5 %, with cationic surfactant addition of 0.7 %, and with amphoteric surfactant addition of 0.5 %. As shown by the experimental data (Fig. 19): the amount of Na+ added to the solution ranged from 0 to 1 %, but the anionic surfactant foam stabilization time with the addition of hydrophilic or hydrophobic SiO2-NPs shown by the data did not change significantly. It indicates that for anionic surfactants, the addition of hydrophilic or hydrophobic SiO2-NPs can provide better salt tolerance.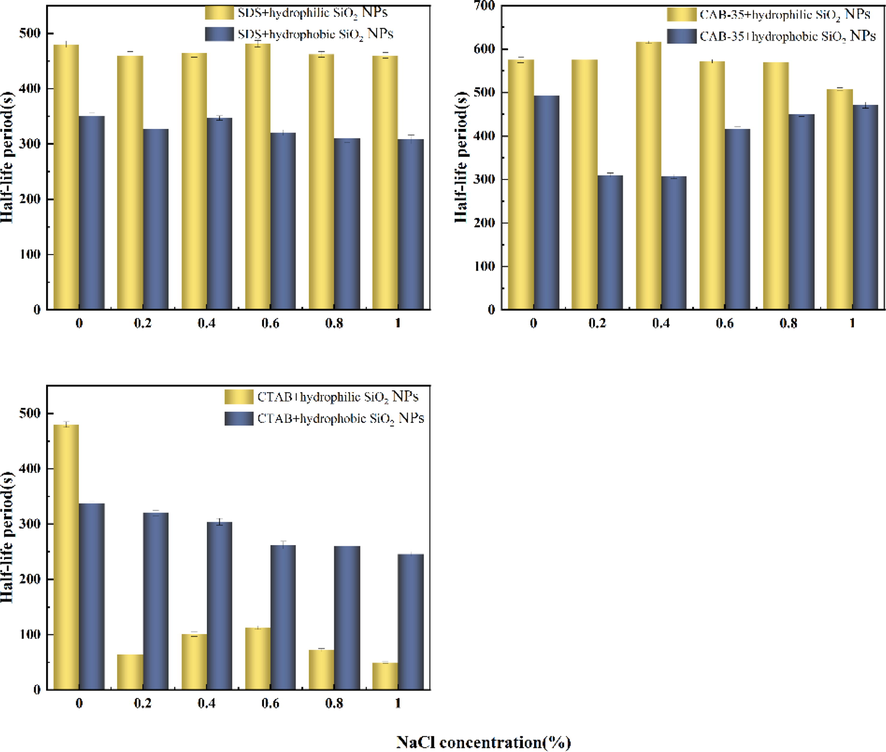
Effect of NaCl on foaming performance of three surfactants mixed with SiO2-NPs system.
The foaming performance was observed by adding NaCl salt solution to the mixed system of CAB-35 and hydrophobic SiO2-NPs (Fig. 19), and the half-life of the mixed solution of CAB-35 with hydrophobic SiO2-NPs showed a trend in the first decreasing and then gradually increasing with the increase of the salt solution concentration. When the concentration of the salt solution is low, the stability of the foam does not change much, and the stability gradually decreases after the increasing salt solution increases. The foaming performance increases and then decreases in the solution mixed with hydrophilic SiO2-NPs. Comparing the two mixed systems, the salt solution added to the CAB-35 hydrophilic SiO2-NPs system solution makes the solution have better foaming performance and better salt tolerance. The foam stability of CTAB and hydrophobic SiO2-NPs mixed with NaCl salt solution remains stable with the increase of salt solution concentration. The half-life of cationic surfactant solution with hydrophilic SiO2-NPs added without NaCl is up to 450 s. Its half-life decreased rapidly after the addition of NaCl, indicates that the cationic surfactant solution with the addition of hydrophilic SiO2-NPs has a weaker salt tolerance.
As shown in Fig. 20, divalent magnesium ions had little effect on the solutions of anionic surfactants mixed with hydrophilic SiO2-NPs and amphoteric surfactants mixed with nanoparticles at the addition amounts between 0.2 % and 1 %. However, from the foam half-life of the mixed solution of anionic surfactant and hydrophobic SiO2-NPs, the low concentration of magnesium ions has the effect of enhancing foam stability, and the foam half-life gradually decreases with the increase of magnesium ion concentration. Magnesium chloride was added to the mixed solution of cationic surfactant and hydrophilic SiO2-NPs, at which time the foam stability decreased substantially, indicating that the magnesium ion has a greater effect on the solution. The foam half-life remains stable within a certain range by adding magnesium chloride to cationic surfactants with hydrophobic SiO2-NPs.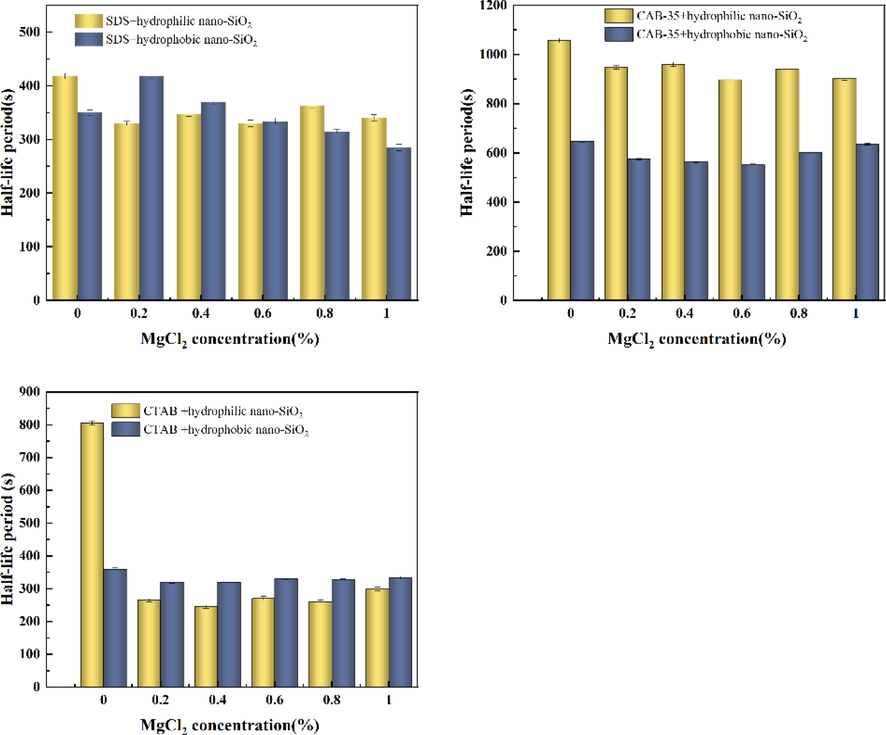
Effect of MgCl2 on foaming performance of three surfactants mixed with SiO2-NPs system.
4 Conclusions
The use of hydrophobic SiO2-NPs with surfactants is now becoming more and more widespread. In this paper, the interaction mechanism between three different types of surfactants including anionic surfactant sodium dodecyl sulfate (SDS), cationic surfactant cetyl dimethyl ammonium bromide (CTAB), amphoteric surfactant Cocamidopropyl betaine (CAB-35) and hydrophilic/hydrophobic SiO2-NPs was investigated. To understand the interaction between ionic surfactants and hydrophilic and hydrophobic SiO2-NPs, the surface tension and temperature, and salt tolerance of surfactants mixed with hydrophilic SiO2-NPs or hydrophobic SiO2-NPs in solution were investigated. The results showed that among the three different ionic surfactants, the half-life of the foam prepared by amphoteric surfactants and hydrophilic SiO2-NPs with 1 % content reached 25 min, which was longer than that of the anionic surfactant and cationic surfactant with SiO2-NPs. However, the foaming volume of the system did not show advantages. From the experimental results of the three surfactants, the anionic surfactant has a better foaming volume, and if the anionic surfactant is mixed with the amphoteric surfactant and then prepared with the hydrophilic SiO2-NPs solution for foam, theoretically there will be better results, and the foam with large foaming volume and good stability can be prepared. These experiments will help in the stability studies of foam fluid systems.
Acknowledgments
The authors gratefully acknowledge the financial support from, the Open Fund (Grant No. UOG2022-02, UOGBX2022-02, and UOGBX2022-01) of Cooperative Innovation Center of Unconventional Oil and Gas, Yangtze University (Ministry of Education & Hubei Province), and the National Natural Science Foundation of China (Grant No. 51804044).
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
References
- Analyzing milk foam using machine learning for diverse applications. Food Anal. Method 2022
- [CrossRef] [Google Scholar]
- Behbahani, Mohammad, Shahtaheri, Seyed Jamaleddin, & Bojdi, et al. 2015. Solid phase extraction and trace monitoring of cadmium ions in environmental water and food samples based on modified magnetic nanoporous silica. Journal of Magnetism & Magnetic Materials.395,213-220.
- Origin of stabilisation of aqueous foams in nanoparticle–surfactant mixtures. Soft Matter. 2008;4(12):2373-2382.
- [Google Scholar]
- Review of health safety aspects of nanotechnologies in food production. Regul. Toxicol. Pharm.. 2009;53(1):52-62.
- [Google Scholar]
- Remediation of diesel contaminated soil by tween-20 foam stabilized by silica nanoparticles. Int. J. Chem. Eng. Appl.. 2017;8(194–198):2017.
- [Google Scholar]
- Chen,Y.M .,2019.Study on the stability of SiO_2 particles on foam systems. [D]. China University of Petroleum (Beijing). DOI:10.27643/d.cnki.gsybu. 001254
- Interfacial tension between crude oil and decylmethylnaphthalene sulfonate surfactant alkali-free flooding systems. Colloids Surf. A: Physicochem. Eng. Asp.. 2006;276:186-191.
- [Google Scholar]
- Impact of temperature and surfactant addition on milk foams. J. Food Eng.. 2021;299:110509
- [Google Scholar]
- The influence of negatively charged silica nanoparticles on the surface properties of anionic surfactants: electrostatic repulsion or the effect of ionic strength. Phys. Chem. Chem. Phys.. 2020;22(4):2238-2248.
- [Google Scholar]
- An experimental investigation of nanoparticle-stabilized CO2 foam used in enhanced oil recovery. Fuel. dec.15 2016;186:430-442.
- [Google Scholar]
- Foas and foam films stabilised by solid particles. Curr. Opin. Colloid Interface Sci.. 2008;13(3):134-140.
- [Google Scholar]
- Non-ionic surfactant interactions with hydrophobic nanoparticles: impact on foam stability. Colloids Surf. A: Physicochem. Eng. Asp.. 2009;347(1–3):81-89.
- [Google Scholar]
- Systematic investigation on the interaction between SiO2 nanoparticles with different surface affinity and various surfactants. J. Mol. Liq.. 2020;304:112777
- [Google Scholar]
- Jiang, L., Li, S,Y., YU, W,Y., et al.2016. Interfacial study on the interaction between hydrophobic nanoparticles and ionic surfactants[J]. Colloids and Surfaces, A. Physicochemical and Engineering Aspects.48820-27
- Karthick, A, R., Chattopadhyay, P., 2017. Characterization and Application of Surfactant Foams Produced from Ethanol-Sodium Lauryl Sulfate-Silica Nanoparticle Mixture for Soil Remediation. Macromolecular Symposia.376, 1600182, 2017.
- Karthick, A, R., Chattopadhyay, P., 2016.An In-Depth Analysis of Ethanol Based Aqueous Foams for Environmental Applications. International Journal of Chemical Sciences. 14, 1711-1717.
- Optimum conditions of zero-valent iron nanoparticle stabilized foam application for diesel-contaminated soil remediation involving three major soil types. Environ. Monit. Assess.. 2021;193(9):1-15.
- [Google Scholar]
- Karthick,A., Chauhan, M., Krzan,M., et al,2019.Potential of surfactant foam stabilized by Ethylene glycol and Allyl alcohol for the remediation of diesel contaminated soil. Environmental Technology & Innovation.14, 100363.
- Comparison of zero-valent iron and iron oxide nanoparticle stabilized alkyl polyglucoside phosphate foams for remediation of diesel-contaminated soils. J. Environ. Manage.. 2019;240:93-107.
- [Google Scholar]
- A review on the application of chemical surfactant and surfactant foam for remediation of petroleum oil contaminated soil. J. Environ. Manage.. 2019;243:187-205.
- [Google Scholar]
- Investigation on stabilization of co2 foam by ionic and nonionic surfactants in presence of different additives for application in enhanced oil recovery. Appl. Surf. Sci.. 2017;420(Oct. 31):9-20.
- [Google Scholar]
- Li, J, S., Cao, J., Zhou, M., Jia, W, J., et al. 2015. Laboratory study on binary foam system APP-4/CR052111. Drilling & Production Technology.38, 91-94.
- Li, Z, M., Hou, D, W., Lu, T., Huang, S, Z., Zhang, K, M., et al. 2019. Properties of Hydrophobic SiO2 Nanoparticles Stabilized Foam and it’s Temperature Resistance. Oilfield Chemistry. 36(3), 494-500.
- Research progress of nanoparticles-stabilized foam for EOR. Oilfield Chem.. 2019;36:748-754.
- [Google Scholar]
- Manas, R, B, Shailesh,R, V., 2012. Foaming in Micellar Solutions: Effects of Surfactant, Salt, and Oil Concentrations[J]. Ind Eng ChemRes.53(48):18497-18511.
- Interactions between zwitterionic and conventional anionic and cationic surfactants. J. Colloid Interface. 2006;295(1):243-248.
- [Google Scholar]
- Influences of Hydrophilic and Hydrophobic silica Nanoparticles on anionic surfactant Properties: Interfacial and adsorption beha-viors. J. Pet. Sci. Eng.. 2014;119(1):36-43.
- [Google Scholar]
- Research progress of nanoparticle stabilized foam system for profile control in low permeability oilfield. Oilfield Chem.. 2017;34(4):745-748.
- [Google Scholar]
- Synergistic effect of silica nanoparticle and cetyltrimethyl ammonium bromide on the stabilization of o/w emulsions. Colloids Surf. A Physicochem. Eng. Asp.. 2007;302(1–3):126-135.
- [Google Scholar]
- Effect of nanoparticles on the interfacial properties of liquid/liquid and liquid/air surface layers. J. Phys. Chem. B. 2006;110(39):19543-19551.
- [Google Scholar]
- Ionic surfactant aggregates in saline solutions: sodium dodecyl sulfate (SDS) in the presence of excess sodium chloride (NaCl) or calcium chloride (CaCl2) J. Phys. Chem. B. 2009;113(17):5863.
- [Google Scholar]
- Study of the monolayer structure and wettability properties of silica nanoparticles and CTAB using the Langmuir trough technique. Colloids Surf. A: Physicochem. Eng. Asp.. 2011;382(1):186-191.
- [Google Scholar]
- Combined effects of nanoparticles and surfactants upon foam stability. Chem. Eng. Sci.. 2021;238:116601
- [Google Scholar]
- Synergy between nanoparticles and surfactants in stabilizing foams for oil recovery. Energy Fuels. 2015;29(2):467-479.
- [Google Scholar]
- Properties of nanoparticle-stabilized foam and its flow behavior in porous media. China University of Petroleum (East China); 2015.
- Sun, Q., Li, Z,M., Li S,Y., et al. 2016. Surface properties and dissection performance of foam with added nano-sio2 particles. Journal of China University of Petroleum: Natural Science Edition. 40(6), 8.
- Utilization of surfactant-stabilized foam for enhanced oil recovery by adding nanoparticles. Energy Fuels. Mar.-Apr. 2014;28:2384-2394.
- [Google Scholar]
- Surface behavior of hydrophilic silica nanoparticle-SDS surfactant solutions: i. effect of nanoparticle concentration on foamability and foam stability. Colloids Surf. A Physicochem. Eng. Asp.. 2017;513:430-441.
- [Google Scholar]
- The role of electrostatic repulsion on increasing surface activity of anionic surfactants in the presence of hydrophilic silica nanoparticles. Sci. Rep.. 2018;8(1):7251.
- [Google Scholar]
- Xue, polish. 2021. Study of hydrophobic hollow silica to improve foam stability. Chemical Engineer. (4),91-94
- Foam sweep in fractures for enhanced oil recovery. Colloids Surf. A Physicochem. Eng. Asp.. 2006;282:348-359.
- [Google Scholar]







