Synthesis and antimicrobial evaluation of 1,4-disubstituted 1,2,3-triazoles with aromatic ester functionality
⁎Corresponding author. Tel.: +91 1662 263152; fax: +91 1662 276240. cpkaushik4@yahoo.co.in (C.P. Kaushik)
-
Received: ,
Accepted: ,
This article was originally published by Elsevier and was migrated to Scientific Scholar after the change of Publisher.
Peer review under responsibility of King Saud University.

Abstract
A series of 1,4-disubstituted 1,2,3-triazoles having p-substituted aromatic ester functionality were synthesized via Cu(I) catalysed click reaction between p-substituted benzoic acid prop-2-ynyl esters and aralkyl azides. The synthesized triazoles were characterized by IR, 1H NMR, 13C NMR and mass spectral techniques. These compounds were evaluated for their antimicrobial activity against Escherichia coli, Staphylococcus aureus, Bacillus subtilis, Pseudomonas aeruginosa, Mycobacterium tuberculosis, Candida albicans, Aspergillus niger and Aspergillus flavus by two fold serial dilution method. Some of the synthesized 1,4-disubstituted 1,2,3-triazoles possess comparable or even better antibacterial, antitubercular and antifungal activities than reference drugs against tested bacterial, mycobacterial and fungal strains, respectively.
Keywords
Click chemistry
1,4-Disubstituted 1,2,3-triazoles
1,3-Dipolar cycloaddition
Antibacterial activity
Antifungal activity
1 Introduction
The synthesis of substituted 1,2,3-triazoles is of key importance due to their large biological spectrum as antibiotic (Aufort et al., 2008), antimicrobial (Lal et al., 2012; Gaur et al., 2012; Demaray et al., 2008), antimalarial (D’hooghe et al., 2011), anticancer (Salmon et al., 2012), antihistaminic (Buckle et al., 1986), anti-HIV (Whiting et al., 2006) and antitubercular agents (Labadie et al., 2011). Good stability and high aqueous solubility of these compounds in biological system boost for appreciable biological activities. Further the 1,4-disubstituted 1,2,3-triazoles have also been used as ligation tool for the synthesis of neoglyco-conjugates (Perez-Balderas et al., 2003), multivalent dendrimeric peptides (Wu et al., 2004), ionic receptors (Kumar and Pandey, 2008), triazolophanes (Haridas et al., 2008), dendrimers (Haridas et al., 2007), cyclic peptides (Turner et al., 2007), peptide nanotubes (Horne et al., 2003), peptidomimetics (Angell and Burgess, 2007) etc. Huisgen cycloaddition, the general method for the synthesis of 1,4-disubstituted 1,2,3-triazoles includes a 1,3-dipolar cycloaddition between azides and alkynes under thermal conditions to afford the equal mixture of 1,4- and 1,5-disubstituted isomers (Huisgen, 1963). A practical solution to avoid the formation of isomeric mixture in products, was given by Sharpless (Rostovtsev et al., 2002) and Meldal (Tornøe et al., 2002) through the catchy term “click chemistry” which refers to facile, efficient, selective and versatile chemical transformation of reactant to a single isomeric product. These reactions are simple to perform, modular, high yielding and lead to excellent selectivity in the product. Among various reactions, Cu(I) catalysed variant of Huisgen 1,3-dipolar cycloaddition of azides and terminal alkynes to give only 1,4-disubstituted 1,2,3-triazoles has been generally pointed as the primary standard of click chemistry. Herein, we report the synthesis of a series of 1,4-disubstituted 1,2,3-triazoles (3a–3p) from various azides and alkynes containing p-substituted aromatic ester functionalities. All the synthesized 1,4-disubstituted 1,2,3-triazoles were characterized by IR, 1H NMR, 13C NMR spectroscopy and mass spectrometry and also screened for their antibacterial, antitubercular and antifungal activities.
2 Experimental
2.1 Measurements
Melting points of synthesized compounds were recorded in °C by applying open capillary method and are uncorrected. The IR spectra were recorded on Shimazdu IR Affinity-I FT-IR spectrophotometer using potassium bromide (KBr) powder and values are given in cm−1. The 1H NMR spectra were recorded on Bruker Avance II 400 MHz/Bruker 300 MHz spectrophotometer and 13C NMR on Bruker Avance II 400 at 100 MHz/Bruker 300 at 75 MHz, in deuterated chloroform using tetramethylsilane (TMS) as an internal standard (chemical shift in δ, ppm). Coupling constant (J) values are given in Hertz (Hz). Mass spectra were recorded on a Waters Micromass Q-Tof Micro (ESI) spectrophotometer. The completion of reactions and the purity of the compounds were analysed by thin layer chromatography (TLC) using readymade silica gel plates (SIL G/UV254, ALUGRAM) and visualized under ultraviolet lamp.
2.2 General procedure for the synthesis of 1,4-disubstituted 1,2,3-triazoles
The starting reactants p-substituted benzoic acid prop-2-ynyl esters (2) were prepared by reacting p-substituted benzoyl chlorides (1) and propargyl alcohol in the presence of N,N-dimethylaminopyridine (DMAP) in dry dichloromethane at 0–10 °C. The 1,4-disubstituted 1,2,3-triazoles (3a–3p) were synthesized by stirring p-substituted benzoic acid prop-2-ynyl esters (1 mmol) with different aralkyl bromides (1 mmol) in the presence of sodium azide (3 mmol), copper sulphate pentahydrate (0.10 mmol) and sodium ascorbate (0.20 mmol) using N,N-dimethylformamide:water (8:2) mixture as solvent at room temperature for 6–12 h (Scheme 1).
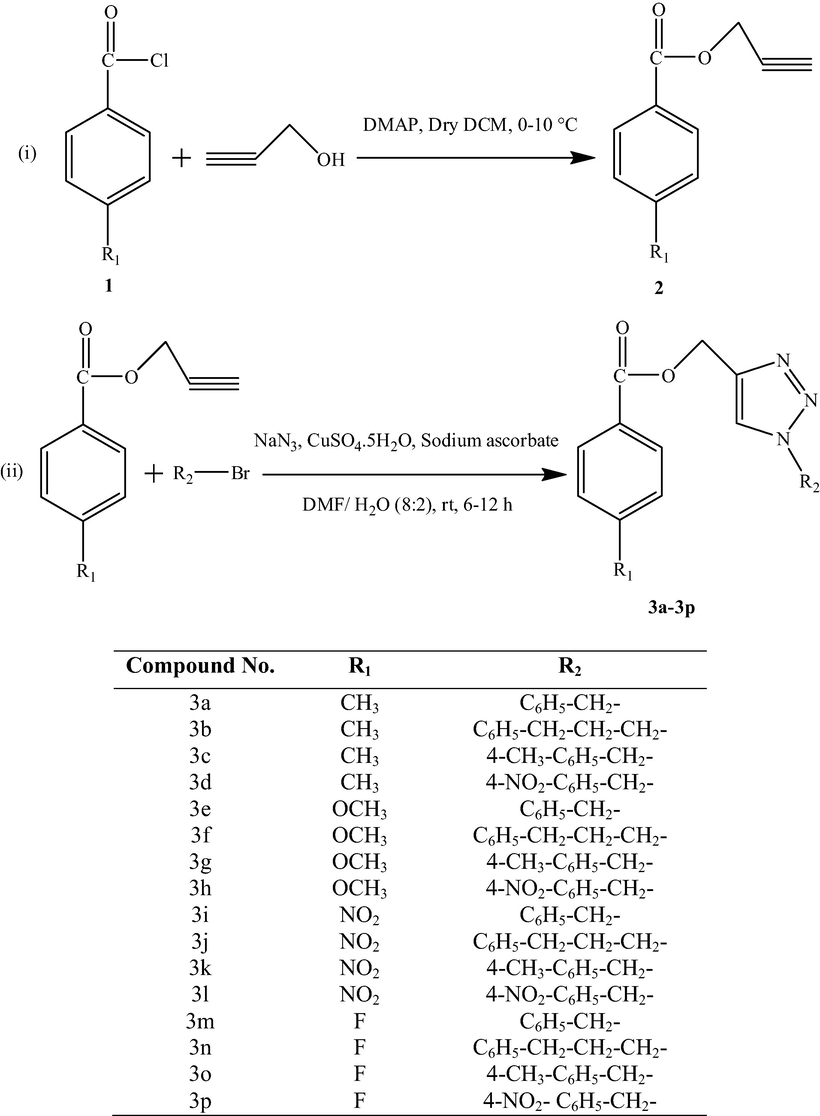
- Synthesis of 1,4-disubstituted 1,2,3-triazoles(3a–3p).
The reaction workup was carried out with aqueous ammonia-ammonium chloride solution and the compound was extracted three times with ethyl acetate. The organic layer was dried over anhydrous sodium sulphate, filtered and concentrated under vacuum to yield 1,4-disubstituted 1,2,3-triazoles.
2.3 Characterization of synthesized compounds
2.3.1 4-Methylbenzoicacid-1-benzyl-1H-[1,2,3]triazol-4-ylmethylester (3a)
Appearance: white, crystalline solid; Yield: 82%; m.p. 118–120 °C; FT-IR (KBr): 3105 (C–H str., triazole ring), 3051, 2962, 1712, 1610, 1446, 1394 cm−1; 1H NMR (400 MHz, CDCl3, δ): 2.41 (s, 3H), 5.45 (s, 2H), 5.55 (s, 2H), 7.23 (d, 2H, J = 8 Hz), 7.28–7.31 (m, 3H), 7.38 (d, 2H, J = 12 Hz), 7.62 (s, 1H), 7.93 (d, 2H, J = 8 Hz); 13C NMR (100 MHz, CDCl3, δ): 21.7, 54.3, 57.9, 123.8, 127, 128.2, 128.9, 129.2, 129.8, 134.4, 143.4, 144, 166.5; MS m/z: 308.0 [M+], 309.0 [M+ + 1].
2.3.2 4-Methylbenzoicacid-1-(phenylpropyl)-1H-[1,2,3]triazol-4-ylmethylester (3b)
Appearance: off-white solid; Yield: 65%; m.p. 90–92 °C; FT-IR (KBr): 3116 (C–H str., triazole ring), 3068, 2943, 1712, 1610, 1448, 1400 cm−1; 1H NMR (300 MHz, CDCl3): 2.17–2.24 (m, 2H), 2.39 (s, 3H), 2.64 (t, 2H), 4.32 (t, 2H), 5.43 (s, 2H), 7.12–7.30 (m, 7H), 7.64 (s, 1H), 7.92 (d, 2H, J = 8 Hz); 13C NMR (75 MHz, CDCI3): 21.7, 36.8, 51.7, 57.9, 124.3, 127, 127.1, 128.7, 128.8, 129.1, 129.8, 136.9, 142.8, 143.9, 166.5; MS m/z: 336.0 [M+], 337.0 [M+ + 1].
2.3.3 4-Methylbenzoicacid-1-(4-methylbenzyl)-1H-[1,2,3]triazol-4-ylmethylester (3c)
Appearance: creamy-white, crystalline solid; Yield: 68.5%. m.p. 104–106 °C; FT-IR (KBr): 3132 (C–H str., triazole ring), 3032, 2954, 2920, 1714, 1610, 1512, 1444 cm−1. 1H NMR (400 MHz, CDCl3): 2.35 (s, 3H), 2.40 (s, 3H), 5.43 (s, 2H), 5.50 (s, 2H), 7.00–7.28 (m, 6H), 7.60 (s, 1H), 7.92 (d, 2H, J = 8 Hz); 13C NMR (100 MHz, CDCI3): 21.2, 21.7, 53.9, 54.1, 57.9, 123.7, 127, 127.2, 128.2, 129.1, 129.2, 129.8, 131.4, 138.8, 143.4, 143.9, 166.5; MS m/z: 322.0 [M+], 323.0 [M+ + 1].
2.3.4 4-Methylbenzoicacid-1-(4-nitrobenzyl)-1H-[1,2,3]triazol–4-ylmethylester (3d)
Appearance: white, crystalline solid; Yield: 62.4%; m.p. 170–172 °C; FT-IR (KBr): 3130 (C–H str., triazole ring), 3082, 2962, 1699, 1606, 1525, 1435, 1348 cm−1; 1H NMR (400 MHz, CDCl3): 2.40 (s, 3H), 5.46 (s, 2H), 5.65 (s, 2H), 7.23 (d, 2H), 7.43 (d, 2H, J = 8.4 Hz), 7.73 (s, 1H), 7.91 (d, 2H, J = 8.0 Hz), 8.23 (d, 2H, J = 8.4 Hz); 13C NMR (100 MHz, CDCI3): 21.7, 53.2, 57.8, 124.1, 126.8, 129.2, 129.5,129.8, 141.4, 142.7, 144.1, 145.4, 148.1, 166.5; MS m/z: 353.0 [M+], 354.0 [M+ + 1].
2.3.5 4-Methoxybenzoicacid-1-benzyl-1H-[1,2,3]triazol-4-ylmethylester (3e)
Appearance: off-white solid; Yield: 79%; m.p. 118–120 °C; FT-IR (KBr): 3136 (C–H str., triazole ring), 3064, 2951, 1712, 1604, 1508, 1452, 1323 cm−1; 1H NMR (300 MHz, CDCl3): 3.82 (s, 3H), 5.39 (s, 2H), 5.50 (s, 2H), 6.86 (d, 2H, J = 8 Hz), 7.28–7.33 (m, 5H), 7.60 (s, 1H), 7.95 (d, 2H, J = 8 Hz); 13C NMR (75 MHz, CDCI3): 54.10, 55.28, 57.71, 113.54, 122.07, 130.73, 128.07, 129.09, 131.81, 134.44, 143.48, 163.45, 165.96; MS m/z: 324.0 [M+], 325.0 [M+ + 1].
2.3.6 4-Methoxybenzoicacid-1-(phenylpropyl)-1H-[1,2,3]triazol-4-ylmethylester (3f)
Appearance: creamy-white solid; Yield: 64%; m.p. 78–80 °C; FT-IR (KBr): 3109 (C–H str., triazole ring), 3070, 2947, 1712, 1600, 1502, 1448, 1377 cm−1; 1H NMR (300 MHz, CDCl3): 2.13–2.29 (m, 2H, J = 7.2 Hz), 2.65 (t, 2H, J = 7.2 Hz), 3.83 (s, 3H), 4.34 (t, 2H J = 7.2 Hz), 5.43 (s, 2H), 6.89 (d, 2H, J = 8.7 Hz), 7.14–7.30 (m, 5H), 7.65 (s, 1H), 7.99 (d, 2H, J = 8.7 Hz); 13C NMR (75 MHz, CDCI3): 31.6, 32.5, 49.6, 55.5, 57.9, 113.7, 122.2, 126.4, 128.3, 128.4, 128.6, 131.8, 140.0, 143.2, 163.6, 166.3; MS m/z: 352.0 [M+], 353.0 [M+ + 1].
2.3.7 4-Methoxybenzoicacid-1-(4-methylbenzyl)-1H-[1,2,3]triazol-4-ylmethylester (3g)
Appearance: dull-white solid; Yield: 69.4%; m.p. 108–110 °C; FT-IR (KBr): 3188 (C–H str., triazole ring), 3022, 2947, 2920, 1705, 1606, 1512, 1448 cm−1; 1H NMR (300 MHz, CDCl3): 2.34 (s, 3H, CH3), 3.84 (s, 3H, OCH3), 5.40 (s, 2H, CH2), 5.47 (s, 2H, CH2), 6.88 (d, 2H, J = 8.7 Hz), 7.17–7.26 (m, 4H), 7.64 (s, 1H), 7.97 (d, 2H, J = 8.7 Hz); 13C NMR (75 MHz, CDCI3): 21.2, 54.1, 55.4, 57.8, 113.6, 122.1, 128.2, 129.8, 131.4, 131.8, 138.8, 143.5, 163.5, 166.2; MS m/z: 338.0 [M+], 339.0 [M+ + 1].
2.3.8 4-Methoxybenzoicacid-1-(4-nitrobenzyl)-1H-[1,2,3]triazol-4-ylmethylester (3h)
Appearance: shiny creamy-white solid; Yield: 72.8%; m.p. 172–174 °C; FT-IR (KBr): 3124 (C–H str., triazole ring), 3080, 2848, 1701, 1606, 1523, 1431, 1340 cm−1; 1H NMR (300 MHz, CDCl3): 3.84 (s, 3H), 5.43 (s, 2H), 5.63 (s, 2H), 6.88 (d, 2H, J = 8.7 Hz), 7.41 (d, 2H, J = 8.4 Hz), 7.70 (s, 1H), 7.96 (d, 2H, J = 8.7 Hz), 8.21 (d, 2H, J = 8.4 Hz); 13C NMR (75 MHz, CDCI3): 51.52, 53.17, 55.46, 56.96, 57.66, 113.67, 121.89, 124.33, 128.69, 131.79, 141.41, 144.15, 148.10, 163.63, 166.19; MS m/z: 369.0 [M+], 370.0 [M+ + 1].
2.3.9 4-Nitrobenzoicacid-1-benzyl-1H-[1,2,3]triazol-4-ylmethylester (3i)
Appearance: creamy-white solid; Yield: 81%; m.p. 134–136 °C; FT-IR (KBr): 3124 (C–H str., triazole ring), 3076, 1726, 1604, 1521, 1435, 1350 cm−1; 1H NMR (400 MHz, CDCl3): 5.50 (s, 2H), 5.56 (s, 2H), 7.28–7.33 (m, 2H), 7.40–7.41 (m, 3H),7.63 (s,1H), 8.21 (d, 2H J = 6.8 Hz), 8.29 (d, 2H J = 6.8 Hz); 13C NMR (100 MHz, CDCI3): 54.3, 58.8, 123.5, 124, 128.2, 129, 129.2, 130.9, 134.2, 135.1, 142.5, 164.6; MS m/z: 339.0 [M+], 340.0 [M++1].
2.3.10 4-Nitrobenzoicacid-1-(phenylpropyl)-1H-[1,2,3]triazol-4-ylmethylester (3j)
Appearance: off-white solid; Yield: 70.15%; m.p. 106–108 °C; FT-IR (KBr): 3124 (C–H str., triazole ring), 3076, 1722, 1606, 1516, 1454, 1348 cm−1; 1H NMR (300 MHz, CDCl3): 2.27–2.31 (m, 2H), 2.67 (t, 2H J = 6.9 Hz), 4.40 (t, 2H J = 6.9 Hz), 5.52 (s, 2H), 7.15–7.32 (m, 5H), 7.9 (s, 1H), 8.26 (m, 4H); 13C NMR (75 MHz, CDCI3): 31.6, 32.5, 49.7, 58.8, 123.6, 126.5, 128.4, 128.7, 130.9, 135.1, 139.9, 142.1, 150.7, 164.6; MS m/z: 367.0 [M+], 368.0 [M+ + 1].
2.3.11 4-Nitrobenzoicacid-1-(4-methyl-benzyl)-1H-[1,2,3]triazol-4-ylmethylester (3k)
Appearance: off-white solid; Yield: 77.3%; m.p. 136–138 °C; FT-IR (KBr): 3116 (C–H str., triazole ring), 3066, 2951, 1722, 1606, 1521, 1442, 1350 cm−1; 1H NMR (400 MHz, CDCl3): 2.37 (s, 3H), 5.49 (s, 2H), 5.51 (s, 2H), 7.21–7.28 (m, 4H), 7.61 (s, 1H), 8.21 (d, 2H, J = 8.8 Hz), 8.28 (d, 2H, J = 8.8 Hz); 13C NMR (100 MHz, CDCI3): 21.2, 54.2, 58.8, 123.5, 123.8, 128.3, 129.9, 130.9, 142.4, 167.0; MS m/z: 353.0 [M+], 354.0 [M+ + 1].
2.3.12 4-Nitrobenzoicacid-1-(4-nitrobenzyl)-1H-[1,2,3]triazol-4-ylmethylester (3l)
Appearance: very light yellow coloured solid; Yield: 79.9%; m.p. 142–144 °C; FT-IR (KBr): 3151 (C–H str., triazole ring), 3078, 1724, 1606, 1541, 1436, 1352 cm−1. 1H NMR (300 MHz, CDCl3): 5.0 (s, 2H), 5.17 (s, 2H), 6.94 (d, 2H, J = 10), 7.66–7.78 (m, 6H), 7.7 (s, 1H). 13C NMR (75 MHz, CDCI3): 53.14, 58.54, 123.51, 124.31, 128.69, 130.84, 134.90, 141.27, 142.98, 148.12, 150.65, 164.40; MS m/z: 384.0 [M+], 385.0 [M+ + 1].
2.3.13 4-Flourobenzoicacid-1-benzyl-1H-[1,2,3]triazol-4-ylmethylester (3m)
Appearance: white puffy solid; Yield: 85.3%; m.p. 98–100 °C; FT-IR (KBr): 3120 (C–H str., triazole ring), 3068, 1720, 1602, 1508, 1450, 1242 cm−1; 1H NMR (400 MHz, CDCl3): 5.45 (s, 2H), 5.51 (s, 2H), 7.10 (t, 2H, J = 8.8 Hz), 7.28–7.32 (m, 3H), 7.38–7.40 (d, 2H), 7.62 (s, 1H), 8.04–8.07 (d, 2H); 13C NMR (100 MHz, CDCI3): 54.3, 58.8. 115.6, 123.8, 126, 128.2, 128.9, 129.2, 132.3, 134.3, 143.2, 164.6, 165.5; MS m/z: 312.0 [M+], 313.0 [M+ + 1].
2.3.14 4-Flourobenzoicacid-1-(phenyl-propyl)-1H-[1,2,3]triazol-4-ylmethylester (3n)
Appearance: white solid; Yield: 72.1%; m.p. 80–82 °C; FT-IR (KBr): 3111 (C–H, str., triazole ring), 3066, 1716, 1598, 1502, 1450, 1236 cm−1; 1H NMR (400 MHz, CDCl3): 2.24–2.32 (m, 2H), 2.68 (t, 2H J = 7.2 Hz), 4.37 (t, 2H, J = 7.2 Hz), 5.50 (s, 2H), 7.12 (d, 2H, J = 8.4 Hz), 7.17–7.33 (m, 5H) 7.67 (s, 1H), 8.07 (d, 2H, J = 8.4 Hz); 13C NMR (100 MHz, CDCI3): 31.6, 32.5, 49.6, 58.2, 115.5, 115.7, 124.0, 126.0, 126.4, 128.4, 128.6, 132.3, 132.4, 140.0, 142.8, 164.6, 165.5, 167.2; MS m/z: 340.0 [M+], 341.0 [M+ + 1].
2.3.15 4-Flourobenzoicacid-1-(4-methyl-benzyl)-1H-[1,2,3]triazol-4-ylmethylester (3o)
Appearance: white solid; Yield: 76.0%; m.p. 108–110 °C; FT-IR (KBr): 3118 (C–H str., triazole ring), 3066, 2951, 1716, 1598, 1506, 1444, 1224 cm−1; 1H NMR (400 MHz, CDCl3): 2.37 (s, 3H), 5.44 (s, 2H), 5.50 (s, 2H), 7.10 (dd, 2H, J = 8.0), 7.22 (m, 4H), 7.59 (s,1H), 8.05 (dd, 2H, J = 8.0); 13C NMR (100 MHz, CDCI3): 21.2, 54.1, 58.1, 115.4, 115.7, 123.7, 126.0, 128.2, 129.8, 131.3, 132.3, 138.9, 143.1, 164.6, 165.5, 167.2; MS m/z: 326.0 [M+], 327.0 [M+ + 1].
2.3.16 4-Flourobenzoicacid-1-(4-nitro-benzyl)-1H-[1,2,3]triazol-4-ylmethylester (3p)
Appearance: light yellow solid; Yield: 79.3%; m.p. 122–124 °C; FT-IR (KBr): 3130 (C–H str., triazole ring), 3068, 1707, 1602, 1510, 1462, 1226 cm−1; 1H NMR (300 MHz, CDCl3): 5.40 (s, 2H), 5.66 (s, 2H), 7.08 (d, 2H, J = 8.0 Hz), 7.43 (d, 2H, J = 10.0 Hz), 7.74 (s, 1H), 8.03 (d, 2H, J = 8.0 Hz), 8.20 (d, 2H, J = 10.0 Hz); 13C NMR (75 MHz, CDCI3): 53.07, 57.90, 115.78, 124.26, 128.66, 132.39, 141.40, 143.61, 148.06, 163.33, 165.29, 168.40; MS m/z: 357.0 [M+], 358.0 [M+ + 1].
2.4 Determination of antimicrobial activities
2.4.1 Antibacterial/antitubercular activity evaluation
All the synthesized compounds were assessed for their in vitro antibacterial activity against two gram negative bacteria, Escherichia coli (MTCC 1231) and Pseudomonas aeruginosa (MTCC 1036), two gram positive bacteria, Staphylococcus aureus (MTCC 7443) and Bacillus subtilis (MTCC 9023). The in vitro activities of newly synthesized triazoles were tested in nutrient broth (NB, Hi-media, Mumbai) by two fold serial dilution method using a stock solution of 1000 μg/ml concentration. Dimethylsulphoxide was used as solvent control. The stock solutions of test compounds and reference drug were serially diluted to get concentrations of 500, 250, 200, 100, 50, 25 and 12.5 μg/ml. These dilutions were inoculated with 100 μL suspension of respective microorganisms in sterile saline and incubated at 37 °C for 24 h. To check the effect of solvent on bacterial growth, a control test was performed with the test medium supplemented with dimethylsulphoxide at same dilution as used in the experiment. The antibacterial potency of the compounds was compared with a broad spectrum antibiotic ciprofloxacin.
Mycobacterium tuberculosis (ATCC 27294) were assessed in microtiter plates by adding 10 ml aliquots of a culture suspension [whose turbidity was equal to that of a No. 0.5 McFarland standard containing 1.5 × 108 colony forming units (CFU)/ml] to 80 ml of Middlebrook 7H9 medium containing 0.5% glycerol and 10% albumin-dextrose-catalase (ADC) and various concentrations of test compounds. Plates were then incubated for 9 days at 37 °C. At the end of incubation, the number of viable mycobacterium was determined by the MTT method.
2.4.2 Antifungal activity evaluation
All synthesized triazole compounds were evaluated for their in vitro antifungal activity against three fungal strains viz. Candida albicans (MTCC 854), Aspergillus niger (MTCC 282), Aspergillus flavus (MTCC 873) and amphotericin B was used as the standard drug. Sabouraud dextrose broth was employed as culture media and dimethylsulphoxide as solvent control. A spore suspension in sterile saline was prepared from one day old culture of fungus growing on sabourauds dextrose broth (SDB, Hi-Media, Mumbai). The final spore concentration was 100 μL/ml. The stock solutions of 1000 μg/ml of test compounds and standard drug were diluted to get concentrations of 500, 250, 200, 100, 50, 25 and 12.5 μg/ml. These dilutions were inoculated with the suspension of respective microorganism in their culture media and were incubated at 25 °C for 48 h in case of C. albicans and at 25 °C for 120 h in case of A. niger and A. flavus.
3 Results and discussion
3.1 Chemistry
The triazole compounds were characterized by IR, 1H NMR, 13C NMR and mass spectral techniques. The formation of triazole was confirmed by the presence of an absorption band in the region 3188–3105 cm−1 in IR spectra due to ⚌C–H stretching of triazole ring. The presence of characteristic singlet in 1H NMR due to triazolyl protons in the region of δ 7.59–7.9 and δ 129.8–132.4 in 13C NMR due to C-5 of the triazole ring confirmed the formation of the triazole ring. The results obtained from mass spectral analysis were found to be in accordance to their molecular weights.
3.2 Antibacterial/antitubercular activity
All compounds were assessed for their in vitro antibacterial/antitubercular activity against E. coli, S. aureus, B. subtilis, P. aeruginosa and M. tuberculosis. The antibacterial/ antitubercular potency of the compounds was compared with standard drugs ciprofloxacin and isoniazid. The minimum inhibitory concentration (MIC) values were calculated as summarized in Table 1.
| Compound no. | E. coli (MTCC 1231) | S. aureus (MTCC 7443) | B. subtilis (MTCC 9023) | P. aeruginosa (MTCC 1036) | M. tuberculosis (ATCC 27294) |
|---|---|---|---|---|---|
| 3a | 100 | 500 | 100 | 250 | 50 |
| 3b | 25 | 12.5 | 12.5 | 12.5 | 12.5 |
| 3c | 100 | 100 | 200 | 50 | 50 |
| 3d | 100 | 250 | 200 | 250 | 12.5 |
| 3e | 100 | 250 | 500 | 500 | 50 |
| 3f | 50 | 200 | 100 | 200 | 25 |
| 3g | 200 | 1000 | 500 | 500 | 25 |
| 3h | 100 | 250 | 200 | 200 | 50 |
| 3i | 100 | 200 | 250 | 100 | 25 |
| 3j | 50 | 100 | 50 | 100 | 6.25 |
| 3k | 100 | 500 | 50 | 200 | 50 |
| 3l | 200 | 500 | 250 | 500 | 50 |
| 3m | 500 | 500 | 100 | 250 | 12.5 |
| 3n | 100 | 50 | 50 | 50 | 3.75 |
| 3o | 100 | 100 | 100 | 100 | 25 |
| 3p | 100 | 500 | 500 | 500 | 50 |
| Ciprofloxacin | 25 | 50 | 12.5 | 12.5 | – |
| Isoniazid | – | – | – | – | 3.75 |
The results obtained from their antibacterial studies revealed that compounds 3a to 3p showed moderate to excellent activities against tested bacterial strains. Compound 3b showed an excellent activity against both gram positive and gram negative bacterial strains with MIC values ranging from 12.5–25 μg/ml. Compound 3j exhibited good to moderate activity against E. coli, B. subtilis, M. tuberculosis and compound 3n has better inhibition effect against S. aureus, B. subtilis, P. aeruginosa and M. tuberculosis. Compounds 3c and 3f were found to possess apparent activity against P. aeruginosa and E. coli, respectively.
3.3 Antifungal activity
The in vitro antifungal activity of the synthesized compounds was examined against three fungal strains viz. C. albicans, A. niger, A. flavus and Amphotericin B was used as the standard drug. The MIC values of the test compounds and the standard are furnished in Table 2.
| Compound no. | C. albicans (MTCC 854) | A. niger (MTCC 282) | A. flavus (MTCC 873) |
|---|---|---|---|
| 3a | 500 | 500 | 1000 |
| 3b | 25 | 100 | 100 |
| 3c | 50 | 25 | 50 |
| 3d | 500 | 100 | 100 |
| 3e | 500 | 250 | 125 |
| 3f | 100 | 50 | 100 |
| 3g | 200 | 100 | 100 |
| 3h | 100 | 200 | 500 |
| 3i | 500 | 200 | 250 |
| 3j | 500 | 500 | 500 |
| 3k | 250 | 200 | 100 |
| 3l | 500 | 1000 | 500 |
| 3m | 50 | 100 | 50 |
| 3n | 12.5 | 12.5 | 25 |
| 3o | 25 | 25 | 50 |
| 3p | 100 | 100 | 200 |
| Amphotericin B | 25 | 25 | 50 |
Antifungal activity results indicated that most of the synthesized compounds exhibited good to moderate activity against the tested fungal strains. The compounds 3c, 3n and 3o showed excellent antifungal activity against all tested fungal strains with MIC values ranging from 12.5–50 μg/ml. Compounds 3b, 3f and 3m exhibited good antifungal activity against C. albicans, A. niger and A. flavus, respectively.
4 Conclusion
A series of 1,4-disubstituted 1,2,3-triazole compounds were synthesized through an easy, convenient, Cu(I) catalysed click reaction and evaluated for their in vitro antimicrobial activity against E. coli, P. aeruginosa, S. aureus, B. subtilis, M. tuberculosis, C. albicans, A. niger and A. flavus. Compound 3b exhibited significant antibacterial activity against all tested bacterial strains, whereas, compounds 3n, 3o possess excellent antifungal activity among the used fungal strains. Rest of synthesized molecules have moderate to good antimicrobial activity. The significant antimicrobial activity of some of the synthesized compounds highlights them as promising molecules for further synthetic and biological exploration.
Acknowledgements
Authors wishes to thank Sh. Kashmiri Lal, Assistant Professor, Department of Chemistry, GJUS&T, Hisar for his valuable support and University Grants Commission, New Delhi for financial assistance to carry out research work through Major Research Project (CPK).
References
- Peptidomimetics via copper-catalyzed azide-alkyne cycloadditions. Chem. Soc. Rev.. 2007;36:1674-1689.
- [Google Scholar]
- Synthesis and antibiotic activity of a small molecules library of 1,2,3-triazole derivatives. Bioorg. Med. Chem. Lett.. 2008;18:1195-1198.
- [Google Scholar]
- Studies on 1,2,3-triazoles. (Piperazinylalkoxy)[l]benzopyrano[2,3-d]-1,2,3-triazol-9(1H)-ones with combined H, antihistamine and mast cell stabilizing properties. J. Med. Chem.. 1986;29:2262-2267.
- [Google Scholar]
- Synthesis of triazole-oxazolidinones via a one-port reaction and evaluation of their antimicrobial activity. Bioorg. Med. Chem. Lett.. 2008;18:4868-4871.
- [Google Scholar]
- Synthesis of 2-amino-3-arylpropan-1-ols and 1-(2,3-diaminopropyl)-1,2,3-triazoles and evaluation of their antimalarial activity. Beilstein J. Org. Chem.. 2011;7:1745-1752.
- [Google Scholar]
- Synthesis, characterization and antifungal activity of biaryl-based bis(1,2,3-triazoles) using click chemistry. Monatsh. Chem.. 2012;143:283-288.
- [Google Scholar]
- Design and synthesis of triazole-based peptide dendrimers. Tetrahedron Lett.. 2007;48:4719-4722.
- [Google Scholar]
- Design, synthesis and self-assembling properties of novel triazolophanes. Org. Lett.. 2008;10:1645-1647.
- [Google Scholar]
- 1,3-Dipolare cycloadditionen: Ruckschau und ausblick. Angew. Chem. Int. Ed.. 1963;75:604-637.
- [Google Scholar]
- Anion recognition by 1,2,3-triazolium receptors: application of click chemistry in anion recognition. Org. Lett.. 2008;10:165-168.
- [Google Scholar]
- Targetting tuberculosis through a small focused library of 1,2,3-triazoles. Mol. Divers.. 2011;15:1017-1024.
- [Google Scholar]
- Regioselective synthesis and antimicrobial studies of ester linked 1,4-disubstituted-1,2,3-bistriazoles. Bioorg. Med. Chem. Lett.. 2012;22:4353-4357.
- [Google Scholar]
- Multivalent neoglycoconjugates by regiospecific cycloaddition of alkynes and azides using organic-soluble copper catalysts. Org. Lett.. 2003;5:1951-1954.
- [Google Scholar]
- A stepwise Huisgen cycloaddition process: Copper(I) catalyzed regioselective “ligation” of azides and terminal alkynes. Angew. Chem. Int. Ed.. 2002;41:2596-2599.
- [Google Scholar]
- Metallocene-based inhibitors of cancer- associated carbonic anhydrase enzymes IX and XII. J. Med. Chem.. 2012;55:5506-5517.
- [Google Scholar]
- Peptidotriazoles on solid phase: [1,2,3]-triazoles by regiospecific copper(I)-catalyzed 1,3-dipolar cycloadditions of terminal alkynes to azides. J. Org. Chem.. 2002;67:3057-3064.
- [Google Scholar]
- Click chemistry as a macrocyclization tool in the solid-phase synthesis of small cyclic peptides. Org. Lett.. 2007;9:5011-5014.
- [Google Scholar]
- Inhibitors of HIV-1 protease by using in situ click chemistry. Angew. Chem. Int. Ed.. 2006;45:1435-1439.
- [Google Scholar]
- Efficiency and fidelity in a click chemistry route to triazole dendrimers by the copper(I) catalyzed ligation of azides and alkynes. Angew. Chem. Int. Ed.. 2004;43:3928-3932.
- [Google Scholar]







