Translate this page into:
Synthesis and antimicrobial evaluation of novel 1,3,4-thiadiazole derivatives of 2-(4-formyl-2-methoxyphenoxy) acetic acid
⁎Corresponding author. Tel.: +91 9417563874; fax: +91 1881263655. mnoolvi@yahoo.co.uk (Malleshappa N. Noolvi)
-
Received: ,
Accepted: ,
This article was originally published by Elsevier and was migrated to Scientific Scholar after the change of Publisher.
Peer review under responsibility of King Saud University.
Abstract
A series of 1,3,4-thiadiazole derivatives of 2-(4-formyl-2-methoxyphenoxy) acetic acid (6a–s) were synthesized by cyclization of carboxylic acid group of 2-(2-methoxy-4-(3-oxo-3-substituted phenylprop-1-enyl)phenoxy) acetic acid (4a–s) with thiosemicarbazide in the presence of POCl3 or PPA. The structures of the compounds were confirmed by IR, 1H NMR and mass analysis. All the compounds have been evaluated in vitro for their antimicrobial activities against several strains of microbes and show significant activity.
Keywords
Phenoxyacetic acid
1,3,4-Thiadiazole
Antimicrobial
1 Introduction
The treatment of infectious disease caused by bacteria, fungi and viruses still remains an important and challenging problem because of a combination factors including newly emerging infectious diseases and increasing number of multi-drug resistant gram-positive pathogens (Tenover and McDonald, 2005; Pfeltz and Wilkinson, 2004; Roberts, 2004; Dessen et al., 2001), such as methicillin-resistant Staphylococcus aureus (MRSA), penicillin resistant Streptococcus pneumoniae (PRSP), and vancomycin-resistant Enterrococci (VRE), compounded problems in the therapeutics (Babu et al., 2008; Dalhoff, 1994). Thus it is still necessary to search for new antimicrobial agents.
Five membered aromatic systems having three hetero atoms at symmetrical position have interesting physiological properties (Hetzhein and Mockel, 1996; Sandstrom, 1968). During recent years there has been intense investigation of different classes of thiadiazole compounds, many of which known to possess interesting biological properties such as antimicrobial (Demirbas et al., 2009; Kadi et al., 2007; Bekhit and Abdel-Aziem, 2004), anti-inflammatory (Mullican et al., 1993; Song et al., 1999; Mathew et al., 2007), anticonvulsants (Chapleo et al., 1986, 1988), antioxidant (Cressier et al., 2009), anticancer (Matysiak et al., 2006; Chou et al., 2003; Radi et al., 2008) and antifungal (Swamy et al., 2006) activities. The activity of 1,3,4-thiadiazoles is possibly due to the presence of the ⚌N–C–S moiety (Bauer et al., 1966). In view of these facts, we have synthesized several new 1,3,4-thiadiazole derivatives of 2-(4-formyl-2-methoxyphenoxy) acetic acid moiety in order to study their biodynamic behavior. The present study reports the synthesis of 3-(4-((5-amino-1,3,4-thiadiazol-2-yl)methoxy)-3-methoxyphenyl)-5-(substituted)-4,5-dihydro-1H-pyrazole-1 carbothioamide (6a–s) by appropriate methods and their evaluation for antibacterial and antifungal potentials.
2 Chemistry
The synthetic route of compounds (6a–s) is shown in Scheme 1. 2-(4-Formyl-2-methoxyphenoxy) acetic acid (2) was prepared by reacting vanillin with chloroacetic acid in the presence of sodium hydroxide. Various chalcone derivatives (4a–s) were synthesized by treating (2) with different derivatives of acetophenone (3a–s). Compounds (5a–s) were obtained by refluxing (4a–k) and thiosemicarbazide in the presence of glacial acetic acid and ethanol. 1,3,4-Thiadiazole derivatives (6a–s) were obtained by cyclization of (5a–s) by treating with thiosemicarbazide and POCl3 or PPA. The physical data of all the synthesized compounds is shown in Table 1.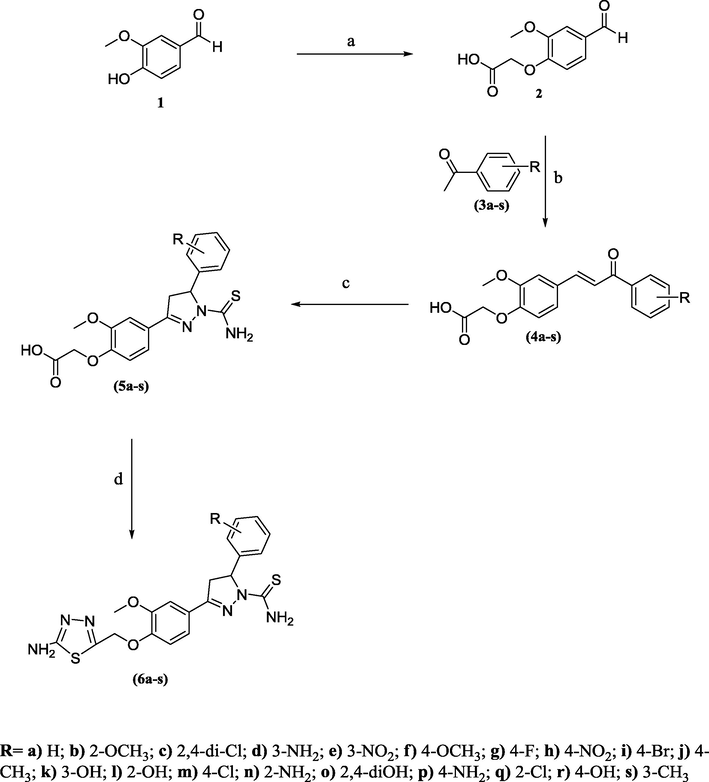
Reagents: (a) chloroacetic acid, NaOH, HCl; (b) EtOH, KOH, petroleum ether; (c) thiosemicarbazide, glacial acetic acid; (d) thiosemicarbazide, PPA or POCl3.
Compound
R
Molecular formula
Yield (%)
Melting point (°C)
6a
H
C20H20N6O2S2
42
262–267
6b
2-OCH3
C21H22N6O3S2
44
274–278
6c
2,4-di-Cl
C20H18Cl2N6O2S2
36
232–238
6d
3-NH2
C20H21N7O2S2
26
252–256
6e
3-NO2
C20H19N7O4S2
36
298–303
6f
4-OCH3
C21H22N6O3S2
42
268–272
6g
4-F
C20H19FN6O2S2
20
244–247
6h
4-NO2
C20H19N7O4S2
44
302–306
6i
4-Br
C20H19BrN6O2S2
66
274–279
6j
4-CH3
C21H22N6O2S2
62
282–286
6k
3-OH
C20H20N6O3S2
66
312–317
6l
2-OH
C20H20N6O3S2
68
258–262
6m
4-Cl
C20H19ClN6O2S2
65
228–232
6n
2-NH2
C20H21N7O2S2
59
264–268
6o
2,4-diOH
C20H20N6O4S2
67
306–310
6p
4-NH2
C20H21N7O2S2
70
222–226
6q
2-Cl
C20H19ClN6O2S2
65
254–258
6r
4-OH
C20H20N6O3S2
69
288–292
6s
3-CH3
C21H22N6O2S2
64
272–276
3 Biological activity
All the synthesized 1,3,4-thiadiazole derivatives (6a–s) have been screened for both antibacterial and antifungal activities using cup-plate agar diffusion method by measuring zone of inhibition in mm. Eight different bacterial cultures S. aureus, Salmonella enterica, Vibrio cholera, Bacillus subtilis, Proteus mirabili, Escherichia coli V517, Mycobacterium smegmatics, Pseudomonas aeruginosa in nutrient agar medium, and one fungal culture Candida albicans in sabouraud’s dextrose agar medium (Holla et al., 2002) were used. The results were compared with positive control, the standard drug ampicillin (50 μg/ml) for bacteria and amphotericin B (50 μg/ml) for fungi and negative control, the DMSO poured disk. These sterilized agar media were poured into petri-dishes and allowed to solidify. On the surface of the media microbial suspensions were spread with the help of sterilized triangular loop. A stainless steel cylinder of 8 mm diameter (pre-sterilized) was used to bore cavities. All the synthesized compounds (50 μg/ml) were placed serially in the cavities with the help of micropipette and allowed to diffuse for 1.0 h. DMSO was used as a solvent for all the compounds, and as a control. These plates were incubated at 37 °C for 24 h and 28 °C for 48 h, for the antibacterial and antifungal activities, respectively. The zone of inhibition was observed around the cup after respective incubation and was measured and percent inhibition of the compounds was calculated.
4 Results and discussion
The structures of synthesized compounds were established on the basis of their spectral data. Spectral data of compounds were in full agreement with proposed structures. The formation of 1,3,4-thiadiazoles (6a–s) was supported by the presence of N–H band in the IR spectra and absence of carbonyl stretching band of the carboxylic acid function. In general, infra red spectra (IR) revealed a bilobe of NH2 stretch at ∼3100, 3300, and C⚌N, C–N and C⚌S peak at ∼1530, 1328, and 1126 cm−1, respectively. In the nuclear magnetic resonance spectra (1H NMR) the signals of the respective protons of the prepared titled compounds were verified on the basis of their chemical shifts, multiplicities, and coupling constants. The spectra showed a singlet at δ ∼5.2 ppm corresponding to OCH2 group; doublet at δ ∼4.0 ppm corresponding to C4 methylene group; singlet at δ ∼3.8 ppm corresponding to methoxy group; singlet at δ ∼3.2 ppm corresponding to C5 CH group; singlet at δ ∼5.8 ppm corresponding to NH2 and multiplet at δ ∼6.5–8.2 ppm for aromatic proton; singlet at δ ∼8.5 ppm corresponding to another NH2 group.
The 1,3,4-thiadiazole derivative 6h showed activity against all the strains. It showed maximum activity (97%) against S. enterica (95%), against V. cholera and (87.9%) inhibition of E. coli V517 when compared with standard drug ampicillin. Compound 6e showed (93.2% and 89.5% inhibition) against S. enterica and V. cholera, respectively. Compound 6k showed maximum inhibition (87.1%) against S. aureus. Compound 6o was found to be active (96.5% inhibition) against E. coli V517 and 6p showed (90.2% inhibition) against P. aeruginosa. Rest of all the 1,3,4-thiadiazole derivatives showed moderate to good antibacterial activity. The 1,3,4-thiadiazole derivative 6h showed maximum inhibition (87.8%) whereas, compound 6e showed (83.3%) inhibition against fungal strain C. albicans. Rest of all the 1,3,4-thiadiazole derivatives showed moderate to low antifungal activity. The results are presented in Table 2. (–): No zone of inhibition. Std.: Standard (ampicillin for bacteria and amphotericin B for fungi).
Compound
% Inhibition
S. aureus
S. enterica
V. cholera
B. subtilis
P. mirabilis
E. coli V517
M. smegmatics
P. aeruginosa
C. albicans
6a
–
–
–
49.6
–
62.9
33.7
44.7
–
6b
–
76.8
38.2
54.7
66.6
77.0
63.9
69.4
–
6c
75
85.0
78.3
–
66.6
82.7
–
–
70.5
6d
–
68.6
–
59.8
–
–
58.1
62.6
48.0
6e
78.5
93.2
89.5
77.3
80.0
84.4
75.5
74.6
83.3
6f
–
78.3
44.4
59.8
70.0
80.1
67.4
74.6
–
6g
75.7
76.8
83.3
80.2
77.3
87.9
72.0
83.5
77.5
6h
80.7
97.0
95.0
81.7
87.3
8.9
81.3
82.8
87.8
6i
–
69.4
52.4
49.6
46.6
62.9
–
–
53.8
6j
–
73.1
46.9
59.1
52.0
79.3
58.1
58.2
–
6k
87.1
79.8
–
–
–
64.6
–
–
55.1
6l
77.1
73.8
–
–
59.3
75.8
–
–
62.8
6m
80.0
79.1
71.4
–
58
85.3
80.2
73.1
72.4
6n
–
70.8
–
67.8
–
88.7
70.3
75.3
62.8
6o
84.2
76.8
–
78.8
70.6
96.5
79.6
86.5
66
6p
–
86.5
–
82.4
–
83.6
83.1
90.2
51.2
6q
81.4
85.8
79
70
66
73.2
74.4
60.4
69.8
6r
76.4
81.3
–
85.4
–
–
65.6
55.9
63.4
6s
–
70.8
54.9
70.8
52
75
73.2
81.3
–
Stand.
100
100
100
100
100
100
100
100
100
5 Experimental
Chemicals were purchased from Merck India, Spectrochem and Sigma–Aldrich etc. Most of the solvents and chemicals used were of LR grade. The purity of the compounds was confirmed by thin layer chromatography using precoated TLC plates and solvent systems of benzene–acetone (9:1), (8:2); T–E–F (5:4:1), and chloroform–methanol (9:1), (9.5:0.5). The spots were visualized under ultraviolet lamp. Melting points were determined in one end open capillary tubes on a liquid paraffin bath and are uncorrected. Infrared (IR) and 1H nuclear magnetic resonance (1H NMR) spectra were recorded for the compounds on Perkin Elmer IR (νmax in cm−1) spectrophotometer in KBr pellets and Bruker model avance II (400 MHz, 1H NMR) instrument, respectively. Chemical shifts are reported in parts per million (ppm) using tetramethylsilane (TMS) as an internal standard.
5.1 Synthesis of 2-(4-formyl-2-methoxyphenoxy) acetic acid (2)
Compound (2) was prepared by the procedure given in the literature (Zubrys and Stebenmann, 1954).
5.2 Synthesis of 2-{4-[3-(substituted)-3-oxo-1-propenyl]-2-methoxyphenoxy} acetic acid (4a–k) and 2-{4-[1-amino (thioxo) methyl-5-(substituted phenyl)-4,5-dihydro-1H-3-pyrazolyl]-2-methoxyphenoxy} acetic acid (5a–k)
Compounds (4a–k) and (5a–k) were prepared by the procedure given in the literature (Mohammad and Mohammad, 2007).
5.3 General procedure for the synthesis of 3-(4-((5-amino-1,3,4-thiadiazol-2-yl)methoxy)-3-methoxyphenyl)-5-(substituted)-4,5-dihydro-1H-pyrazole-1-carbothioamide (6a–k)
A mixture of (5a–k) (0.05 mol), thiosemicarbazide (0.05 mol) and POCl3 (13 ml) was heated at 75 °C for 0.75 h. After cooling down to room temperature, water was added. The reaction mixture was refluxed for 4 h. After cooling, the mixture was basified to pH 8 by the drop-wise addition of 50% NaOH solution under stirring. The precipitate was filtered and recrystallized from ethanol.
5.3.1 3-(4-((5-Amino-1,3,4-thiadiazol-2-yl)methoxy)-3-methoxyphenyl)-5-phenyl-4,5-dihydro-1H-pyrazole-1-carbothioamide (6a)
Yield 42%; mp: 262–267 °C; IR (KBr, cm−1): 3327.40, 3290.63 (N–H stretch), 3010.29 (Ar C–H stretch), 2919.01 (C–H stretch), 1570.29 (Ar C⚌C stretch); 1H NMR (DMSO-d6): 9.19 (s, 2H, NH2), 6.72–7.94 (m, 8H, Ar-H), 5.48 (s, 2H, NH2), 5.12 (s, 2H, OCH2), 4.00 (d, 2H, CH2), 3.84 (s, 3H, OCH3), 3.41 (s, 1H, CH), MS (m/z %): 441.08 [M+1]+.
5.3.2 3-(4-((5-Amino-1,3,4-thiadiazol-2-yl)methoxy)-3-methoxyphenyl)-5-(2-methoxyphenyl)-4,5-dihydro-1H-pyrazole-1-carbothioamide (6b)
Yield 44%; mp: 274–278 °C; IR (KBr, cm−1): 3157.02, 3111.20 (N–H stretch), 3041.76 (Ar C–H stretch), 2986.18 (C–H stretch), 1570.41 (Ar C⚌C stretch); 1H NMR (DMSO-d6): 9.26 (s, 2H, NH2), 7.26–8.26 (m, 7H, Ar-H), 5.78 (s, 2H, NH2), 5.20 (s, 2H, OCH2), 4.23 (d, 2H, CH2), 3.82 (s, 6H, OCH3), 3.53 (s, 1H, CH); MS (m/z %): 471.18 [M+1]+.
5.3.3 3-(4-((5-Amino-1,3,4-thiadiazol-2-yl)methoxy)-3-methoxyphenyl)-5-(2,4-dichlorophenyl)-4,5-dihydro-1H-pyrazole-1-carbothioamide (6c)
Yield 36%; mp: 232–238 °C; IR (KBr, cm−1): 3349.34, 3329.20 (N–H stretch), 3086.49 (Ar C–H stretch), 2998.26 (C–H stretch), 1559.31 (Ar C⚌C stretch), 1H NMR (DMSO-d6): 8.92 (s, 2H, NH2), 6.75–7.98 (m, 6H, Ar-H), 5.89 (s, 2H, NH2), 5.29 (s, 2H, OCH2), 4.24 (d, 2H, CH2), 3.83 (s, 3H, OCH3), 3.63 (s, 1H, CH); MS (m/z %): 508. 09 [M+], 509.11 [M+1]+.
5.3.4 3-(4-((5-Amino-1,3,4-thiadiazol-2-yl)methoxy)-3-methoxyphenyl)-5-(3-aminophenyl)-4,5-dihydro-1H-pyrazole-1-carbothioamide (6d)
Yield 26%; mp: 252–256 °C; IR (KBr, cm−1): 3263.24, 3230.38 (N–H stretch), 3018.76 (Ar C–H stretch), 2986.18 (C–H stretch), 1598.83 (Ar C⚌C stretch); 1H NMR (DMSO-d6): 12.6 (s, 2H, NH2), 10.1 (s, 2H, NH2), 6.72–8.44 (m, 7H, Ar-H), 5.38 (s, 2H, NH2), 5.15 (s, 2H, OCH2), 4.15 (d, 2H, CH2), 3.80 (s, 3H, OCH3), 3.26 (s, 1H, CH); MS (m/z %): 455.15 [M+].
5.3.5 3-(4-((5-Amino-1,3,4-thiadiazol-2-yl)methoxy)-3-methoxyphenyl)-5-(3-nitrophenyl)-4,5-dihydro-1H-pyrazole-1-carbothioamide (6e)
Yield 36%; mp: 298–303 °C; IR (KBr, cm−1): 3129.46, 3116.16 (N–H stretch), 3014.18 (Ar C–H stretch), 2912.64 (C–H stretch), 1545.45 (Ar C⚌C stretch); 1H NMR (DMSO-d6): 8.59 (s, 2H, NH2), 6.70–7.51 (m, 7H, Ar-H), 5.42 (s, 2H, NH2), 5.07 (s, 2H, OCH2), 3.95 (d, 2H, CH2), 3.84 (s, 3H, OCH3), 3.50 (s, 1H, CH); MS (m/z %): 485.10 [M+].
5.3.6 3-(4-((5-Amino-1,3,4-thiadiazol-2-yl)methoxy)-3-methoxyphenyl)-5-(4-methoxyphenyl)-4,5-dihydro-1H-pyrazole-1-carbothioamide (6f)
Yield 42%; mp: 268–272 °C; IR (KBr, cm−1): 3187.53, 3157.32 (N–H stretch), 3027.35 (Ar C–H stretch), 2995.45 (C–H stretch), 1562.80 (Ar C⚌C stretch); 1H NMR (DMSO-d6): 9.24 (s, 2H, NH2), 7.24–8.22 (m, 7H, Ar-H), 5.89 (s, 2H, NH2), 5.29 (s, 2H, OCH2), 4.14 (d, 2H, CH2), 3.82 (s, 6H, OCH3), 3.68 (s, 1H, CH); MS (m/z %): 471.08 [M+1]+.
5.3.7 3-(4-((5-Amino-1,3,4-thiadiazol-2-yl)methoxy)-3-methoxyphenyl)-5-(4-fluorophenyl)-4,5-dihydro-1H-pyrazole-1-carbothioamide (6g)
Yield 20%; mp: 244–247 °C; IR (KBr, cm−1): 3363.09, 3352.25 (N–H stretch), 3130.92 (Ar C–H stretch), 2969.22 (C–H stretch), 1593.69 (Ar C⚌C stretch); 1H NMR (DMSO-d6): 9.48 (s, 2H, NH2), 6.60–7.79 (m, 7H, Ar-H), 5.83 (s, 2H, NH2), 5.27 (s, 2H, OCH2), 4.30 (d, 2H, CH2), 3.84 (s, 3H, OCH3), 3.56 (s, 1H, CH); MS (m/z %): 458.12 [M+].
5.3.8 3-(4-((5-Amino-1,3,4-thiadiazol-2-yl)methoxy)-3-methoxyphenyl)-5-(4-nitrophenyl)-4,5-dihydro-1H-pyrazole-1-carbothioamide (6h)
Yield 44%; mp: 302–306 °C; IR (KBr, cm−1): 3299.55, 3257.54 (N–H stretch), 3051.18 (Ar C–H stretch), 2992.21 (C–H stretch), 1590.21 (Ar C⚌C stretch); 1H NMR (DMSO-d6): 8.28 (s, 2H, NH2), 6.69–7.94 (m, 7H, Ar-H), 5.83 (s, 2H, NH2). 5.27 (s, 2H, OCH2), 4.24 (d, 2H, CH2), 3.84 (s, 3H, OCH3), 3.67 (s, 1H, CH); MS (m/z %): 485.04 [M+].
5.3.9 3-(4-((5-Amino-1,3,4-thiadiazol-2-yl)methoxy)-3-methoxyphenyl)-5-(4-bromophenyl)-4,5-dihydro-1H-pyrazole-1-carbothioamide (6i)
Yield 66%; mp: 274–279 °C; IR (KBr, cm−1): 3186.70, 3157.40 (N–H stretch), 3025.65 (Ar C–H stretch), 2963.90 (C–H stretch), 1570.29 (Ar C = C stretch); 1H NMR (DMSO-d6): 9.26 (s, 2H, NH2), 6.69–8.32 (m, 7H, Ar-H), 5.67 (s, 2H, NH2), 5.27 (s, 2H, OCH2), 4.27 (d, 2H, CH2), 3.86 (s, 3H, OCH3), 3.56 (s, 1H, CH); MS (m/z %): 518.08 [M+], 519.01 [M+1]+.
5.3.10 3-(4-((5-Amino-1,3,4-thiadiazol-2-yl)methoxy)-3-methoxyphenyl)-5-p-tolyl-4,5-dihydro-1H-pyrazole-1-carbothioamide (6j)
Yield 62%; mp: 282–286 °C; IR (KBr, cm−1): 3195.20, 3169.97 (N–H stretch), 3063.47 (Ar C–H stretch), 2956.65 (CH3 stretch), 1585.97 (Ar C⚌C stretch); 1H NMR (DMSO-d6): 8.59 (s, 2H, NH2), 6.72–7.94 (m, 7H, Ar-H), 5.48 (s, 2H, NH2), 5.12 (s, 2H, OCH2), 4.08 (d, 2H, CH2), 3.84 (s, 3H, OCH3), 3.41 (s, 1H, CH), 2.55 (s, 3H, CH3); MS (m/z %): 454.21 [M+].
5.3.11 3-(4-((5-Amino-1,3,4-thiadiazol-2-yl)methoxy)-3-methoxyphenyl)-5-(3-hydroxyphenyl)-4,5-dihydro-1H-pyrazole-1-carbothioamide (6k)
Yield 66%; mp: 312–317 °C; IR (KBr, cm−1): 3612.26 (O–H stretch), 3166.10, 3152.56 (N–H stretch), 3020.71 (Ar C–H stretch), 2998.16 (C–H stretch), 1594.25(Ar C⚌C stretch); 1H NMR (DMSO-d6): 10.71 (s, 1H, OH), 9.97 (s, 2H, NH2), 7.61–8.41 (m, 7H, Ar-H), 5.48 (s, 2H, NH2), 4.02 (s, 2H, OCH2), 4.05 (d, 2H, CH2), 3.80 (s, 3H, OCH3), 3.50 (s, 1H, CH); MS (m/z %): 456.14 [M+].
5.3.12 3-(4-((5-Amino-1,3,4-thiadiazol-2-yl)methoxy)-3-methoxyphenyl)-5-(2-hydroxyphenyl)-4,5-dihydro-1H-pyrazole-1-carbothioamide (6l)
Yield 68%; mp: 258–262 °C; IR (KBr, cm−1): 3626.26 (O–H stretch), 3157.20, 3145.56 (N–H stretch), 3059.21 (Ar C–H stretch), 2967.66 (C–H stretch), 1568.35 (Ar C⚌C stretch); 1H NMR (DMSO-d6): 10.24 (s, 1H, OH), 9.53 (s, 2H, NH2), 7.82–8.66 (m, 7H, Ar-H), 5.50 (s, 2H, NH2), 4.46 (s, 2H, OCH2), 4.35 (d, 2H, CH2), 3.84 (s, 3H, OCH3), 3.32 (s, 1H, CH); MS (m/z %): 456.09 [M+].
5.3.13 3-(4-((5-Amino-1,3,4-thiadiazol-2-yl)methoxy)-3-methoxyphenyl)-5-(4-chlorophenyl)-4,5-dihydro-1H-pyrazole-1-carbothioamide (6m)
Yield 65%; mp: 228–232 °C; IR (KBr, cm−1): 3134.40, 3167.96 (N–H stretch), 3040.85 (Ar C–H stretch), 2967.07 (C–H stretch), 1559.59 (Ar C⚌C stretch); 1H NMR (DMSO-d6): 9.32 (s, 2H, NH2), 7.34–8.24 (m, 7H, Ar-H), 5.26 (s, 2H, NH2), 4.35 (s, 2H, OCH2), 4.03 (d, 2H, CH2), 3.86 (s, 3H, OCH3), 3.20 (s, 1H, CH); MS (m/z %): 475.02 [M+1]+.
5.3.14 3-(4-((5-Amino-1,3,4-thiadiazol-2-yl)methoxy)-3-methoxyphenyl)-5-(2-aminophenyl)-4,5-dihydro-1H-pyrazole-1-carbothioamide (6n)
Yield 59%; mp: 264–268 °C; IR (KBr, cm−1): IR (KBr, cm−1): 3283.34, 3270.08 (N–H stretch), 3027.16 (Ar C–H stretch), 2976.08 (C–H stretch), 1578.93 (Ar C⚌C stretch); 1H NMR (DMSO-d6): 11.3 (s, 2H, NH2), 10.8 (s, 2H, NH2), 6.74–8.41 (m, 7H, Ar-H), 5.76 (s, 2H, NH2), 5.36 (s, 2H, OCH2), 4.20 (d, 2H, CH2), 3.87 (s, 3H, OCH3), 3.24 (s, 1H, CH); MS (m/z %): 455.19 [M+].
5.3.15 3-(4-((5-Amino-1,3,4-thiadiazol-2-yl)methoxy)-3-methoxyphenyl)-5-(2,4-dihydroxyphenyl)-4,5-dihydro-1H-pyrazole-1-carbothioamide (6o)
Yield 67%; mp: 306–310 °C; IR (KBr, cm−1): 3662.26 (O–H stretch), 3156.50, 3146.76 (N–H stretch), 3020.71 (Ar C–H stretch), 2978.76 (C–H stretch), 1598.95 (Ar C⚌C stretch); 1H NMR (DMSO-d6): 11.01 (s, 1H, OH), 10.35 (s, 1H, OH), 9.78 (s, 2H, NH2), 7.78–8.45 (m, 7H, Ar-H), 5.67 (s, 2H, NH2), 4.37 (s, 2H, OCH2), 4.10 (d, 2H, CH2), 3.87 (s, 3H, OCH3), 3.08 (s, 1H, CH); MS (m/z %): 472.22 [M+].
5.3.16 3-(4-((5-Amino-1,3,4-thiadiazol-2-yl)methoxy)-3-methoxyphenyl)-5-(4-aminophenyl)-4,5-dihydro-1H-pyrazole-1-carbothioamide (6p)
Yield 70%; mp: 222–226 °C; IR (KBr, cm−1): 3157.80, 3146.06 (N–H stretch), 3056.51 (Ar C–H stretch), 2989.61 (C–H stretch), 1549.52 (Ar C⚌C stretch); 1H NMR (DMSO-d6): 10.2 (s, 2H, NH2), 9.87 (s, 2H, NH2), 7.98–8.89 (m, 7H, Ar-H), 5.87 (s, 2H, NH2), 4.67 (s, 2H, OCH2), 4.31 (d, 2H, CH2), 3.83 (s, 3H, OCH3), 3.56 (s, 1H, CH); MS (m/z %): 455.22 [M+].
5.3.17 3-(4-((5-Amino-1,3,4-thiadiazol-2-yl)methoxy)-3-methoxyphenyl)-5-(2-chlorophenyl)-4,5-dihydro-1H-pyrazole-1-carbothioamide (6q)
Yield 65%; mp: 254–258 °C; IR (KBr, cm−1): 3189.20, 3179.36 (N–H stretch), 3067.17 (Ar C–H stretch), 2978.87 (C–H stretch), 1578.52 (Ar C⚌C stretch); 1H NMR (DMSO-d6): 8.98 (s, 2H, NH2), 7.21–8.81 (m, 7H, Ar-H), 5.87 (s, 2H, NH2), 4.85 (s, 2H, OCH2), 4.29 (d, 2H, CH2), 3.83 (s, 3H, OCH3), 3.78 (s, 1H, CH); MS (m/z %): 475.23 [M+1]+.
5.3.18 3-(4-((5-Amino-1,3,4-thiadiazol-2-yl)methoxy)-3-methoxyphenyl)-5-(4-hydroxyphenyl)-4,5-dihydro-1H-pyrazole-1-carbothioamide (6r)
Yield 69%; mp: 288–292 °C; IR (KBr, cm−1): 3645.54 (O–H stretch), 3177.10, 3168.06 (N–H stretch), 3045.91 (Ar C–H stretch), 2930.09 (C–H stretch), 1578.76 (Ar C⚌C stretch); 1H NMR (DMSO-d6): 10.98 (s, 1H, OH), 9.87 (s, 2H, NH2), 7.89–8.78 (m, 7H, Ar-H), 5.89 (s, 2H, NH2), 4.87 (s, 2H, OCH2), 4.60 (d, 2H, CH2), 3.80 (s, 3H, OCH3), 3.64 (s, 1H, CH); MS (m/z %): 456.14 [M+].
5.3.19 3-(4-((5-Amino-1,3,4-thiadiazol-2-yl)methoxy)-3-methoxyphenyl)-5-m-tolyl-4,5-dihydro-1H-pyrazole-1-carbothioamide (6s)
Yield 64%; mp: 272–276 °C; IR (KBr, cm−1): 3167.90, 3187.16 (N–H stretch), 3034.91 (Ar C–H stretch), 2999.16 (C–H stretch), 1580.75 (Ar C⚌C stretch); 1H NMR (DMSO-d6): 8.97 (s, 2H, NH2), 7.91–8.71 (m, 7H, Ar-H), 5.49 (s, 2H, NH2), 4.30 (s, 2H, OCH2), 4.16 (d, 2H, CH2), 3.89 (s, 3H, OCH3), 3.02 (s, 1H, CH), 2.67 (s, 3H, CH3); MS (m/z %): 454.02 [M+].
6 Conclusion
A total of 19 compounds were synthesized and screened for their antibacterial activity against S. aureus, S. enterica, V. cholera, B. subtilis, P. mirabili, E. coli V517, M. smegmatics, P. aeruginosa and antifungal activity against C. albicans. The % inhibition of all the compounds was determined by observing the zones of inhibition formed around the cup after 24 h of incubation for antibacterial and 48 h for antifungal activities. Among the tested compounds 6h, 6e, 6k, 6o, 6p and 6h, 6e possess significant antibacterial and antifungal activities, respectively while rest of all the 1,3,4-thiadiazole derivatives showed moderate antimicrobial activity as compared to standards.
Acknowledgment
The authors would like to thanks Sardar Sangat Singh Longia, Vice-president, ASBASJSM College of Pharmacy, Bela (Ropar) for providing the necessary facilities.
References
- Synthesis of quinoline analogues: search for antimalarial agents. Monatsh. Chem.. 2008;39:179-181.
- [Google Scholar]
- Antibiotics susceptibility testing by a standardized single disk method. Am. J. Clin. Pathol.. 1966;45:493-496.
- [Google Scholar]
- Design, synthesis and biological evaluation of some pyrazole derivatives as anti-inflammatory-antimicrobial agents. Bioorg. Med. Chem.. 2004;12:1935-1945.
- [Google Scholar]
- Substituted 1,3,4-thiadiazoles with anticonvulsant activity. 1. Hydrazines. J. Med. Chem.. 1986;29:2273-2280.
- [Google Scholar]
- Substituted 1,3,4-thiadiazoles with anticonvulsant activity. 4. Amidines. J. Med. Chem.. 1988;31:7-11.
- [Google Scholar]
- Investigation of anticancer mechanism of thiadiazole-based compound in human non small cell lung cancer A549 cells. Biochem. Pharmacol.. 2003;66:115-124.
- [Google Scholar]
- Synthesis, antioxidant properties and radioprotective effects of new benzothiazoles and thiadiazoles. Biol. Med. Chem.. 2009;17:5275-5284.
- [Google Scholar]
- Quinolone resistance in Pseudomonas aeruginosa and Staphylococcus aureus. Development during therapy and clinical significance. Infection. 1994;22:S111-S121.
- [Google Scholar]
- Synthesis of some new 1,3,4-thiadiazol-2-ylmethyl-1,2,4-triazole derivatives and investigation of their antimicrobial activities. Eur. J. Med. Chem.. 2009;44:2896-2903.
- [Google Scholar]
- Molecular mechanisms of antibiotic resistance in gram-positive pathogens. Curr. Drug Targets Infect. Disord.. 2001;1:63-77.
- [Google Scholar]
- New bis-aminomercaptotriazoles and bis-triazolothiadiazoles as possible anticancer agents. Eur. J. Med. Chem.. 2002;37:511-517.
- [Google Scholar]
- Adv. Heterocycl. Chem.. 1996;7:183.
- Synthesis, antimicrobial, and anti-inflammatory activities of novel 2-(1-adamantyl)-5-substituted-1,3,4-oxadiazoles and 2-(1-adamantylamino)-5- substituted-1,3,4-thiadiazoles. Eur. J. Med. Chem.. 2007;42:235-242.
- [Google Scholar]
- Studies on synthesis and pharmacological activities of 3,6-disubstitued-1,2,4-triazole-[3,4-b]-1,3,4-thiadiazoles and their dihydro analogues. Eur. J. Med. Chem.. 2007;42:823-840.
- [Google Scholar]
- Synthesis and antiprolipherative activity of some 5-substituted 2-(2,4-dihydroxyphenyl)-1,3,4-thiadiazoles. Eur. J. Med. Chem.. 2006;41:475-482.
- [Google Scholar]
- Synthesis and evaluation of phenoxy acetic acid derivatives as a anti-mycobacterial agents. Bioorg. Med. Chem.. 2007;15:1896-1902.
- [Google Scholar]
- The escalating challenge of vancomycin resistance in Staphylococcus aureus. Curr. Drug Targets Infect. Disord.. 2004;4:273-294.
- [Google Scholar]
- Design of 5-(3,5-di-tert-butyl-4-hydroxyphenyl)-1,3,4-thiadiazoles, 1,3,4-oxadiazoles, and 1,2,4-triazoles as orally-active, nonulcerogenic, antiinflammatory agent. J. Med. Chem.. 1993;36:1090-1099.
- [Google Scholar]
- Discovery and SAR of 1,3,4-thiadiazole derivatives as potent Abl tyrosine kinase inhibitors and cytodifferentiating agents. Biol. Org. Med. Chem. Lett.. 2008;18:1207-1211.
- [Google Scholar]
- Distribution of macrolide, lincosamide, streptogramin, ketolide and oxazolidinone (MLSKO) resistance genes in gram-negative bacteria. Curr. Drug Targets Infect. Disord.. 2004;4:207-215.
- [Google Scholar]
- Adv. Heterocycl. Chem.. 1968;9:165.
- Synthesis, structure-activity relationships, and in vivo evaluations of substituted di-tert-butylphenols as a novel class of potent, selective, and orally active cyclooxygenase-2 inhibitors. 2. 1,3,4-and 1,2–4-thiadiazole series. J. Med. Chem.. 1999;42:1161-1169.
- [Google Scholar]
- Synthesis of pharmaceutically important condensed heterocyclic 4,6-disubstituted-1,2,4-triazolo-1,3,4-triadiazole derivatives as antimicrobials. Eur. J. Med. Chem.. 2006;41:531-538.
- [Google Scholar]
- Vancomycin-resistant Staphylococci and Enterococci: epidemiology and control. Curr. Opin. Infect. Dis.. 2005;18:300-305.
- [Google Scholar]
- Antituberculous isonicotinyl hydrazones of low toxicity. Can. J. Chem.. 1954;33:11-14.
- [Google Scholar]







