Translate this page into:
Synthesis and application of a novel cold brand reactive dye to impart colouration, mosquito repellency, and UV protection to nylon
⁎Corresponding author at: TX110C, Dept. of Textile and Fibre Engineering, IIT Delhi, India. jnsheikh@iitd.ac.in (Javed Sheikh)
-
Received: ,
Accepted: ,
This article was originally published by Elsevier and was migrated to Scientific Scholar after the change of Publisher.
Peer review under responsibility of King Saud University.
Abstract
Mosquito-borne diseases are a great concern for human health, and an increment in urbanization causes a rise in the population of mosquitoes. The efficient methods and products for countering mosquito bites are the urgent need of the hour. To protect against mosquitoes, mosquito repellent textiles are an attractive substrate. An impartment of simultaneous dyeing and finishing effects on nylon is an urgent requirement as it can enhance its applicability in various technical applications. The present study reports the preparation of a mosquito repellent-cum-UV protective nylon using a novel reactive dye. The synthesis of dye involves the reaction of cyanuric chloride with H-acid (sodium 4-amino-5-hydroxy-2,7-naphthalene disulfonic acid) to generate cyanuric-H-acid, which was further reacted with diazotised 4-amino-N, N-diethyl-3-methylbenzamide (DEET-NH2) to synthesise a novel cold brand reactive dye. Fourier-transform infrared spectroscopy (FTIR), proton nuclear magnetic resonance (1H NMR), elemental analysis (CHN analyser), UV–vis spectroscopy, and thermogravimetric analysis (TGA) were used to characterise the synthesised dye. The colouration of nylon was done in an infrared lab dyeing machine. The exhaustion and fixation of dye on nylon and the colouration properties (L*, a*, b*, C*, K/S) of dyed nylon were explored. Functional properties (mosquito repellency and UV protection) were evaluated even after laundering treatments. The dyed fabrics were also characterised using TGA and scanning electron microscope (SEM) analysis. An outstanding mosquito repellency (100 %) and good UV protection were achieved.
Keywords
Cold brand reactive dye
Mosquito repellent
UV protective
Nylon
1 Introduction
A variety of textile dyes is available in the market; reactive dyes are the most utilised. Excellent fastness and availability of wide colour ranges make them suitable for apparel, technical textiles, and decoration purposes. A covalent bonding between textile fibres (wool, cotton, nylon) and reactive dyes (Kamel et al., 2003; Lewis, 2014; Tissera et al., 2016) provides a wash-durable colouration property. Sulphatoethylsulphone and triazine-based hetero functional azo reactive dyes were prepared by Siddiqua et al. (Siddiqua et al., 2020). Bis-ethyl-sulphone-based reactive dyes were synthesised, and more than 71 % exhaustion was obtained on cotton fabric (Lewis and Siddique, 2006). The researchers synthesised disperse reactive dyes for the waterless colouration of textiles (Zhang et al., 2016). The reactive dyes containing sulfonate groups were explored in the printing of textiles (Zhang et al., 2020). Tertiary amine-based reactive dyes were prepared, which showed good fixation at neutral pH (Croft et al., 1992). The reactive dyes synthesised from polyether amine segments were utilised for the salt-free dyeing of cellulosic fibre (Wang et al., 2016). H-acid-based monoazo-anthraquinone reactive dyes demonstrated more than 88 % fixation on cotton fabric (Shan et al., 2015). Monochloro-s-triazinyl reactive dyes were prepared, and their hydrolysis kinetics were studied (Huang and Wu, 2021). Mokhtari et al. prepared monochloro-s-triazinyl reactive dyes, and the cotton fabric was dyed with the prepared reactive dyes (Mokhtari et al., 2004). The reactive dyes made from disazo pyrazoloprymidine derivatives and sulfatoethylsulfone indicated good fastness ratings on cotton, wool, and silk (Youssef et al., 2014). The dyeing of wool fibres using reactive dyes in a ternary solvent system was performed (Wang et al., 2021). Electrospun nylon 6 fibres were dyed by the reactive dyes using electron beam irradiation (Park et al., 2016). Quinazolinone-based reactive dyes were prepared and studied for the dyeing of cotton, wool, and silk fibres (Patel and Patel, 2011). Various studies utilised H-acid to prepare reactive dyes (Desai et al., 2022; Mohamed et al., 2020, 2018; Naqvi and Clark, 2011; Zhang et al., 2019). Fluorescent reactive disperse dyes were developed, and their application to polyamide 6 and wool fabrics was reported (Aysha et al., 2019). The authors prepared bisazo reactive dyes based on 4,4′-methylene-bis(2-methyl-5-nitro aniline) (Patel et al., 2016). Saeed & Shabir prepared azo reactive dye based on 4′,4-diamino diphenylamine-2-sulfonic acid to obtain high wash fastness (Saeed and Shabir, 2018). P-sulphophenoxy-s-triazines based reactive dyes were prepared, and ink-jet printing of wool by using reactive dyes was performed (Yang et al., 2019). Faisal et al. developed 2-sulphophenoxy-4-chloro-s-triazine based reactive dye, and the dye was used for ink-jet printing of wool (Faisal et al., 2020).
Technical textiles are mostly non-apparel grade textile materials that have technical applications. Their wide applications include various fields such as agriculture (Bhavani et al., 2017), building construction (Crini et al., 2020), functional protection for human beings (Singh and Sheikh, 2020), and space application (Kuess et al., 2022). The demand for textiles with functional properties has increased in the last decade. The development of functional nylon is a quest for research because it has wide applications in apparel, army uniforms, composites (Wang et al., 2011) and other technical textiles. Nylon was also used for making a composite for dye decolourisation (Saeed et al., 2021).
Mosquito repellent, N, N-diethyl-3-methylbenzamide (DEET), is widely used to give mosquito-repellent protection to human beings. The microcapsules prepared from citronella oil were used to provide mosquito-repellent protection to cotton (Specos et al., 2010). Singh & Sheikh prepared multifunctional mosquito repellent cotton fabric by azoic dyeing method with the help of Terminalia Chebula and ethyl anthranilate (Singh and Sheikh, 2021a). Two mosquito repellent disperse dyes have been prepared using 4-amino-N, N-diethyl-3-methylbenzamide (DEET-NH2), ethyl anthranilate, and 4-hydroxy coumarin (Singh and Sheikh, 2021b). To develop wash-durable mosquito repellent cotton fabric, Teli & Chavan reacted DEET-NH2 with dimethylol dihydroxyethylene urea on cotton fabric (Teli and Chavan, 2017a). The capsules synthesised from thyme oil, cypress oil, and grapefruit oil were used to provide mosquito repellent protection to bamboo/Tencel fabric (Geethadevi and Maheshwari, 2015). Microcapsules prepared from Justicia Adhatoda extract as a core and gum acacia as a shell imparted mosquito repellent action to cotton fabric (Karthigeyan and Premalatha, 2019). Teli & Chavan prepared azoic dyes for the development of multifunctional mosquito repellent cotton fabric (Teli and Chavan, 2018). Mosquito repellent nylon was developed using reactive dye synthesised from DEET-NH2 (Teli and Chavan, 2017b). Microcapsules prepared from cinnamon oil as a core and chitosan–gelatin as a shell were used to develop the multifunctional linen fabric (Singh and Sheikh, 2021c). The reactive dyes to impart mosquito repellency and antibacterial properties were prepared by Mokhtari et al. (Mokhtari et al., 2014). Mosquito repellent (4-hydroxy coumarin)-containing azo dye was prepared and applied to polyester for obtaining multifunctional mosquito repellency (Singh and Sheikh, 2022).
Various methods were reported for developing UV-protective (Alebeid and Zhao, 2017) and antibacterial textiles (Gulati et al., 2022). UV protection to textiles was imparted by using nanoparticles (NPs) such as TiO2 and ZnO (Khan et al., 2020). Nosheen et al. developed multifunctional cotton using a synthesised fibre-reactive antibacterial bio-agent based on chloroxylenol (Nosheen et al., 2022b). Acid black 1 dye was modified to impart antibacterial and UV protection to textiles (Nosheen et al., 2022a). Rehman et al. developed dyed cotton fabric with antibacterial properties by using l-cysteine (Rehman et al., 2018). Multifunctional properties (antibacterial activity, self-cleaning, and UV protection) were imparted to fabric using trimethyl[3-trimethoxysilyl)propyl]ammonium chloride and TiO2 NPs (Riaz et al., 2022). Safdar et al. used TiO2 nanoparticles to develop antibacterial and UV-protective cotton fabric (Safdar et al., 2022).
Finishing and dyeing are two distinct operations in textile chemical processing, and both involve the utilisation of a large amount of chemicals and other utilities. The method and manner to achieve the combined dyeing-finishing goal is an interesting area to explore, and dyes that generate combined dyeing and finishing effects are termed as functional dyes. Aesthetically demandable clothes are coloured; however, the synthesis of novel functional dyes to generate functional textiles remains a quest for research. Mosquito repellent hot brand reactive dyes, mosquito repellent disperse dyes, and mosquito repellent azoic dyes were explored in the literature. Few studies were available on mosquito repellent reactive dye, and mosquito repellent cold-brand reactive dye has not been explored. Cold-brand reactive dyes are less energy-intensive than hot-brand reactive dyes because they require lower fixation temperatures. Moreover, exploring new dyes to impart mosquito repellent protection to textiles will give a suitable solution for making multifunctional mosquito repellent textiles. Hence, there is a need to explore multifunctional cold brand reactive dyes.
The present work reports the synthesis of a novel cold brand reactive dye. The reactive group-containing intermediate (cyanuric chloride) was reacted with H-acid (sodium 4-amino-5-hydroxy-2,7-naphthalene disulfonic acid) to synthesise cyanuric H-acid. Cyanuric H-acid was reacted with the diazotised 4-amino-N, N-diethyl-3-methylbenzamide (DEET-NH2) to synthesise a novel cold brand reactive dye. The characterisation of the dye, colouration, and functional (mosquito repellent and UV protection) properties of the dyed substrates were studied in detail.
2 Materials
H-acid (Sodium 4-Amino-5-hydroxy-2,7-naphthalene disulfonate hydrate), cyanuric chloride, N, N-diethyl-3-methylbenzamide (DEET) were obtained from Tokyo Chemical Industry (TCI chemicals). Hydrochloric acid (36 %), sodium nitrite, and solvents (ethanol, dimethylformamide, dimethyl sulfoxide, acetone, ethyl acetate, and 2-propanol) were obtained from Sigma chemicals. Ready-for-dyeing (RFD) nylon-6 fabric (Ends per inch = 80, Picks per inch = 64, Grams per square meter = 81.3) was utilised for dyeing experiments.
3 Methods
3.1 Synthesis of novel cold brand reactive dye
Cyanuric H-acid was prepared by modifying the reported method (Naqvi and Clark, 2011). Cyanuric chloride (0.0165 mol, 3.02 g, 98 %) was dissolved in 25 mL of acetone. The solution of cyanuric chloride was stirred under ice (0–5 °C). This was followed by the addition of H-acid hydrate (0.015 mol, 6.40 g, 80 %) to the solution of cyanuric chloride. The condensation was performed with a maintained pH (3–4) and temperature (0–5 °C). The condensation was proceeded until no colour was developed with the Ehrlich reagent (p-dimethyl amino benzaldehyde).
The reported procedure was used to prepare the DEET-NH2 (modified DEET) (Singh and Sheikh, 2021b). Modified DEET (0.015 mol) was dissolved in 40 mL of water using 3.3 mL of hydrochloric acid (36 %). Sodium nitrite (1.04 g) was reacted with modified DEET/HCl at 0–5 °C. The diazotisation was performed for 3 h, and the solution was neutralised to a pH of 4–5 using sodium acetate.
Drop-wise addition of diazotised modified DEET into the cyanuric H-acid solution was done at 0–5 °C. The coupling was done for 2 h (confirmation of consumption of diazotised modified DEET) with a maintained temperature (0–5 °C). A dry powder of sodium chloride (NaCl) was added to the reaction mixture, and the salted-out dye was filtered, washed with salt-containing water (brine solution), and dried at 30 °C. The dye was purified to obtain the salt and impurity-free product. The dye was dissolved in DMF (dimethylformamide) and filtered to remove the salt. Diethyl ether solvent was added to the solution, and the precipitated dye was washed several times with ethyl acetate, diethyl ether, and acetone. The dye was dried and further used for the dyeing of nylon fabric. Fig. 1 shows the reaction schemes for the preparation of the reactive dye.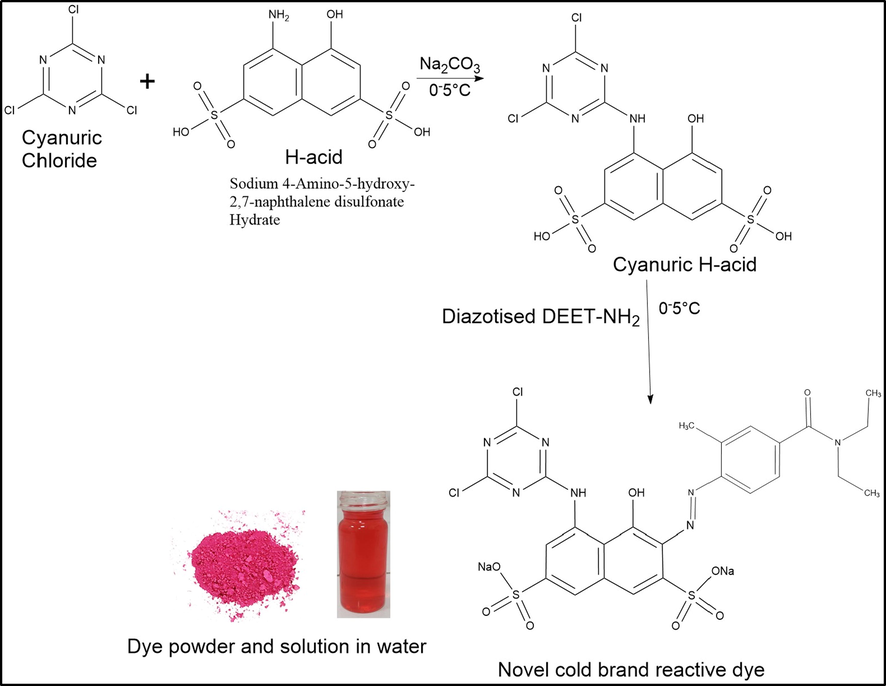
Preparation of novel cold brand reactive dye.
3.2 Characterisation of the dye and dyed fabrics
Functional groups present in synthesised reactive dye were confirmed through FTIR (Fourier transform infrared) spectroscopy (Nicolet 6700(Thermo)). 1H NMR spectra of synthesised reactive dye were obtained using AV spectrophotometer (Burker, 500MHZ) in Dimethyl sulfoxide-d6 (DMSO‑d6). UV–visible spectrophotometer (UV-1900i, Shimadzu) was used to record the UV–visible spectrum and extinction coefficient of the dye. The elemental composition (C, H, N) of the dye was analysed using an elemental analyser (PerkinElmer). Thermal stability of dye and dyed fabrics were studied using thermogravimetric analysis (PerkinElmer) in the temperature range of 50–800 °C at a heating rate of 20 °C/min under the nitrogen atmosphere. The morphology of dyed fabric was also analysed using scanning electron microscope (SEM Zeiss EVO 18, Germany).
3.3 Dyeing of nylon fabric with the reactive dye
The nylon fabric was dyed with the synthesised reactive dye in different shades (1 %, 2 %, 3 %, 4 %, and 5 %) using an infrared dyeing machine. The required quantities of dye and water were calculated based on fabric weight, maintaining material to liquor ratio of 1:10. The dyeing was continued for 1 h at 40 °C. At the end of dyeing cycle, the fabrics were taken out from the machine, and post-dyeing treatments were performed. The dyed fabrics were washed with cold water (at room temperature) for 5 min followed by soaping (1 g/L) at 60 °C for 10 min in a shaker bath. Samples were also given a hot and cold wash and further dried in the air to obtain the final dyed fabric.
3.4 Evaluation of exhaustion, fixation, and colouration property of the reactive dye
Exhaustion of the dye to nylon was measured using the UV–vis spectrophotometer (UV-1900i, Shimadzu). After dyeing, dye solutions were taken from the dyebath. The exhaustion value was calculated using the following formula: where, Ab = Absorbance of dye bath before dyeing, Aa = Absorbance of dye bath after dyeing.
Before and after soaping, the dyed samples were compared to evaluate the fixation (%) using the below-given formula:
A spectrophotometer (Premier Colorscan, SS5100H) was used to obtain colour values (L*, a*, b*, C*) and colour strength (K/S) of dyed fabrics.
3.5 Measurement of fastness and functional properties of dyed samples
Washing fastness, rubbing fastness, and light fastness of dyed samples were determined using ISO 105- C06, ISO 105-X12, and ISO 105 BO2 (ISO manual 2006), respectively. Mosquito repellent efficiency and UV protection activity of dyed samples were evaluated using the “Arm in cage method” (Singh and Sheikh, 2021b) and AS / NZS 4399 (1996), respectively.
The arm-in-cage test was done using a cage with dimensions of 200 mm × 180 mm × 150 mm. Anopheles female mosquitoes were used for the experiment, and no blood feeding was provided to the mosquitoes prior to the two days of the testing. The dyed and undyed fabrics were wrapped on the arm of the volunteer. The volunteer put the arms in the cage containing mosquitoes, and observations for the landing of mosquitoes were recorded to obtain the mosquito repellency by utilising the below-given formula. M = Quantity of mosquitoes landing on undyed fabric, N = Quantity of mosquitoes landing on dyed fabric.
The durability of functional properties of dyed fabrics against repeated washings was evaluated using the AATCC-61 A method (AATCC Technical manual, 2007).
4 Results and discussion
4.1 Thin layer chromatography and 1H NMR of DEET-NH2
The solution of DEET-NH2 in ethanol was spotted on a TLC plate, and the plate was put in an eluent containing n-hexane, water, and ethyl acetate in the ratio 2:1:3 (V/V). The organic purity of DEET-NH2 was suggested through a single spot on the plate (Fig. 2).
TLC image of DEET-NH2.
1H NMR of DEET-NH2 is recorded in Fig. 3. The multiplet at 7.33–7.01 ppm due to aromatic hydrogens, singlet at 4.38 ppm due to NH2, multiplet at 3.31 – 3.08 ppm due to CH2, singlet at 2.30 ppm due to CH3, and multiplet at 1.18 – 0.99 ppm due to methyl group were obtained.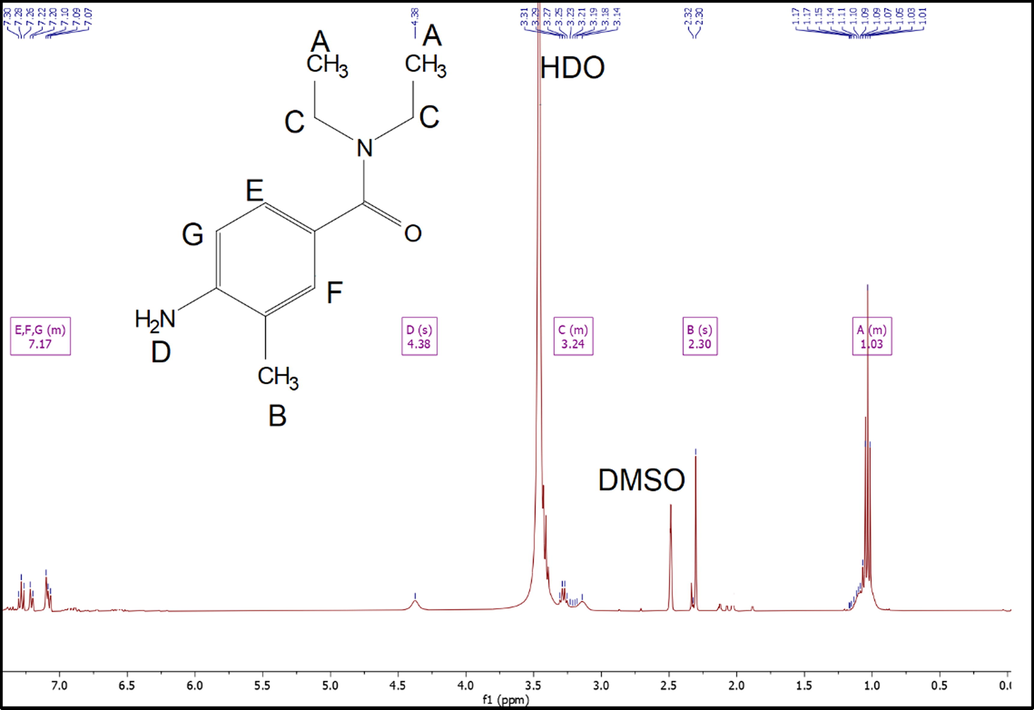
1H NMR of DEET-NH2.
1H NMR (500 MHz, DMSO‑d6): δ = 7.33–7.01(m, 3H,ArH), 4.38 (s, 2H,–NH2), 3.31 – 3.08 (m, 4H,–CH2),2.30(s,1H,Ar-CH3),1.18 – 0.99 (m, 6H,–CH3).
4.2 Characterisation of reactive dye
FTIR spectrum of the reactive dye is recorded in Fig. 4. Various peaks were observed at 3475 cm−1 (N—H stretching), 3419 cm−1(OH stretching), 2974 cm−1(=C—H stretching), 2937 cm−1(C—H stretching), 1639 cm−1(C⚌O stretching), 1622 cm−1(N⚌N stretching), 1560 cm−1(C⚌C stretching), 1411 cm−1(C—H bending), 1136 cm−1(S⚌O stretching), 1049 cm−1(C—Cl stretching), and 626 cm−1(C—H bending due to rocking). Hence, the FTIR spectrum confirmed the presence of azo and other functional groups of the reactive dye.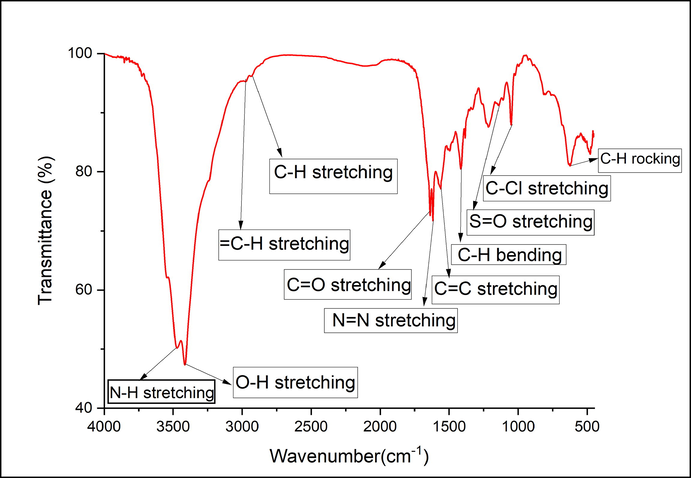
FTIR spectrum of the reactive dye.
Fig. 5 indicates the 1H NMR spectra of the dye. A broad singlet due to methyl group attached to methylene at 1.11 ppm, a singlet at 2.36 ppm due to methyl group attached to the aromatic ring, and two double peaks due to methylene groups at 2.76 and 2.93 ppm were observed. Similar nature of peaks of methyl and methylene groups in DEET was reported (Jensen and Fort, 2001). Moreover, aromatic hydrogens in the region of 6.92–8.14 ppm, singlet due to NH at 9.00 ppm, and singlet due to —OH group at 10.95 ppm were also obtained.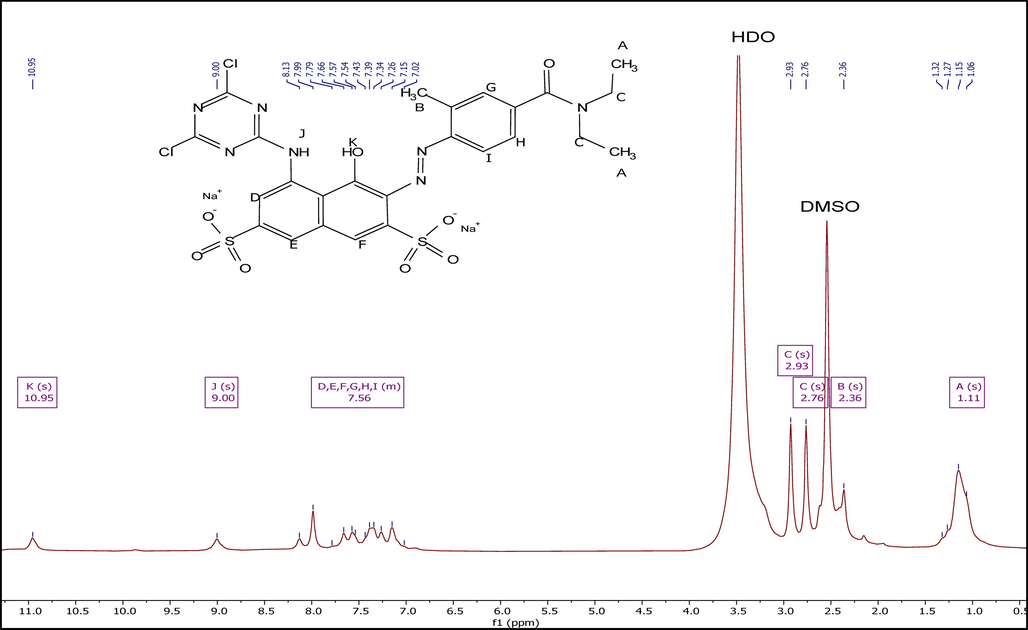
1H NMR spectra of the reactive dye.
1H NMR (500 MHz, DMSO‑d6): δ = 10.95(s,1H,OH), 9.00(s,1H,NH), 8.14–6.92(m,6H,ArH), 2.93(s,2H,CH2), 2.76(s, 2H,CH2), 2.36(s,1H,Ar-CH3), 1.11(s,6H,–CH3).
The dye was dissolved in water to perform UV–visible analysis. A UV–visible spectrum of the reactive dye showed a maximum absorbance peak at 236 nm in the UV region for the benzene ring due to the π- π* transition (Fig. 6(A)). The shoulder peak in the region (523 to 548 nm) was also obtained due to the n → π ∗ transition, which confirmed the formation of the azo group. The reactive dye’s extinction coefficient was determined using the absorbance and concentration plot (calibration plot) at 523 nm (Fig. 6(B)). The slope of calibration is related to the extinction coefficient of the dye (Haque and Islam, 2015). The extinction coefficient of the synthesised dye was found to be 3.8 L g−1 cm−1.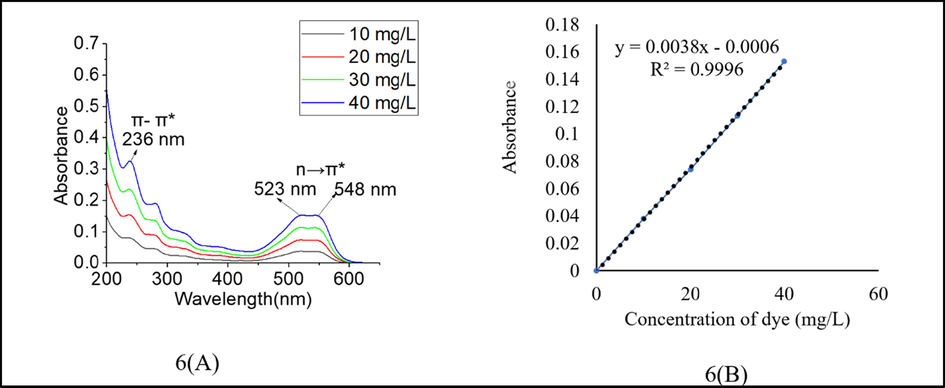
(A) UV–vis analysis of the reactive dye; (B) Calibration plot of the reactive dye.
The obtained elemental compositions (Cob, Hob, and Nob) were very close to the calculated elemental compositions (Ccal, Hcal, and Ncal), as recorded in Table 1. Hence, the molecular formula of the dye was confirmed. The molecular formula and IUPAC name are also recorded in Table 1.
Molecular formula
IUPAC name
Ccal/Cob, (%)
Hcal/Hob, (%)
Ncal/Nob, (%)
C25H21Cl2N7O8 S2Na2
sodium (E)-5-((4,6-dichloro-1,3,5-triazin-2-yl)amino)-3-((4-(diethylcarbamoyl)-2-methylphenyl)diazenyl)-4-hydroxynaphthalene-2,7-disulfonate
43.99/42.90
3.10/3.78
14.37/12.6
Thermal stability is an essential parameter to examine the suitability of parameters for the application of dyes. In order to study the thermal stability of synthesised reactive dye, a thermogram is recorded (Fig. 7). Table 2 indicates the various transition temperatures, such as T2 (2 % weight loss temperature), T5 (5 % weight loss temperature), and T10 (10 % weight loss temperature). The weight loss at different temperatures (50–800 °C) is depicted in Table 3. Significantly less weight loss at 800 °C indicated that the dye was thermally stable. Similar thermal stability of some reactive dyes was also found in the literature (Patel and Patel, 2015).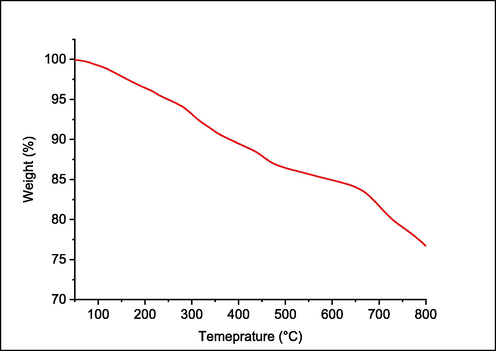
TGA curve of the reactive dye.
T2 (°C)
T5 (°C)
T10 (°C)
145
249
381
Temperature (°C)
150
300
450
600
750
800
Weight loss (%)
2.12
6.82
12.09
15.09
20.95
23.30
4.3 Colour values, colour strength (K/S), exhaustion, fixation, and fastness properties
Dyed fabrics showed L* values of 56 to 67 at 1–5 % shade (Table 4). a* values in the range of 19 to 37 were obtained. b* values were in the range of −8 to −12, and chroma in the range of 21 to 39 was observed. Pink shades were generated on nylon after dyeing, and it is recorded in Table 4. The improvement in colour strength (K/S) was obtained with increasing dye add-on (% shade), suggesting an incrementing amount of the dye molecules on the fabric. ΔE* indicates the colour uniformity of the dyed samples. ΔE* of less than one represents that the variation of coloration properties throughout the dyed substrate is not perceptible by human eyes (Minaker et al., 2021). Thus, the dyed samples showed uniform coluration properties.
Sample no.
Sample name
Colour valuesa
K/Sa
Colour appearance
L*
a*
b*
C*
ΔE*
1
1 % shade nylon
67.903
19.062
−8.981
21.072
0.64
0.785

2
2 % shade nylon
64.206
27.220
−12.575
29.984
0.49
1.188

3
3 % shade nylon
61.443
36.017
−11.609
37.842
0.54
1.705

4
4 % shade nylon
57.601
34.830
−11.740
36.755
0.66
2.170

5
5 % shade nylon
56.824
37.441
−12.541
39.486
0.71
2.437

“Good” to “excellent” wash and rubbing fastness ratings also confirmed strong covalent interaction between the dye and fibre (Table 5). Lightfastness in the range of 5 to 6 was obtained. The dyed fabric was boiled with dimethylformamide and dimethyl sulfoxide solvents. The colourless extracts, in both cases, indicated strong bonding (covalent) between dye and nylon fibre. Fig. 8 shows the interaction between dye and fibre, where one chlorine atom of reactive dye can react with the —NH2 group of nylon through a nucleophilic substitution mechanism. The neutral-pH fixation mechanism of the reactive dye with nylon through covalent interaction was reported by Kamel et al. (2003).
Sample no
Washing fastness
Rubbing fastness
Light fastness
Exhaustion (%)
Fixation (%)
Dry
Wet
1
5
5
5
5
88.23
83.74
2
5
5
5
5–6
85.76
78.36
3
4–5
4–5
4–5
5–6
77.95
75.39
4
4–5
4–5
4–5
5–6
73.09
72.77
5
4
4
4
6
68.61
70.39
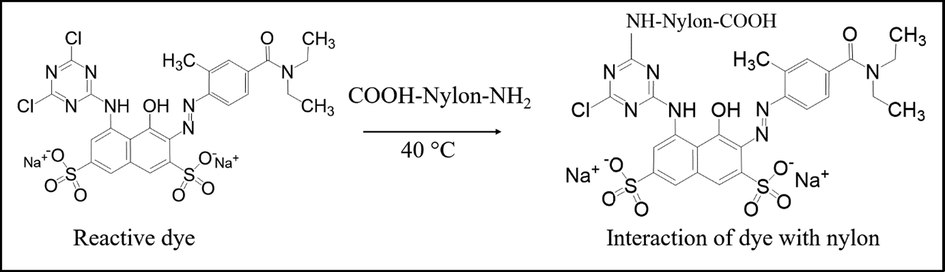
Interaction of reactive with the nylon.
Exhaustion and fixation values are also recorded in Table 5. It can be seen from the outcomes that more than 68 % exhaustion and 70 % fixation were obtained. The higher amount of dye in the dye bath has promoted a lesser amount of exhaustion. Hence, with increasing % shade, the absolute amount of dye exhausted on fibre increased; however, relative quantity decreased (Alam et al., 2008). The reduction in fixation with increasing shade (%) was due to the less availability of the fibre reactive sites.
4.4 Functional properties of dyed nylon fabrics
The mosquito repellent action of dyed nylon is recorded in Table 6. More than 87.8 % repellency was obtained, and the increase in mosquito repellency was also confirmed with the increasing shade (%). The samples dyed at higher than 2 % shade indicated mosquito repellency of 100 %. The wash-durable mosquito repellent action by the dyed nylon was confirmed through outstanding (100 %) repellency of the dyed samples after 20 washes. This verifies the synthesised reactive dye as a mosquito repellent molecule. The reactive dye contains a DEET-based modified part, making it a mosquito-repellent molecule.
The dyed fabric contains the repellent dye, which imparts the action through contact mode. Gustatory receptors, also known as contact chemoreceptors, are present in the labella of mosquitoes (shown in Fig. 9) (Dickens and Bohbot, 2013). The dye must be acting as feeding deterrents and gustatory receptors are targeted by the dye. The neuron’s action potential depends on the concentration of the repellent (Dickens and Bohbot, 2013). The presence of more dye on fabric (as increasing shade %) showed more repellent action to the mosquitoes; Thus, an increase in repellency was found.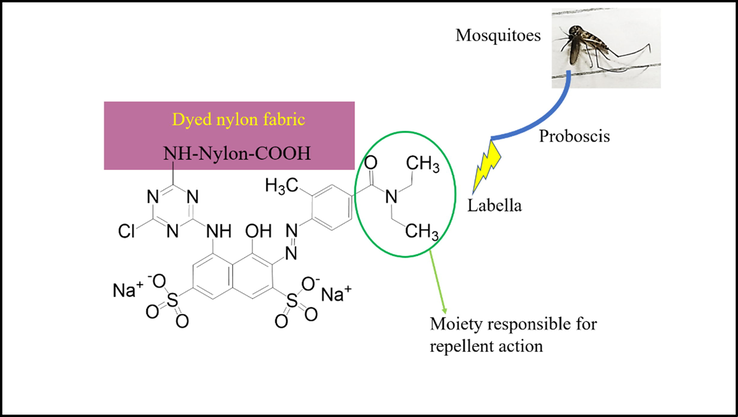
Mechanism of the dyed fabric for showing mosquito repellent action.
Moreover, the repellent action is strongly related to the charges on the atoms available in the amide group of (N—C⚌O) of the repellent (Fig. 9) (Ma et al., 1999). The receptors have positive and negative charges. When repellent dye interacts with the receptors, net repulsion force is generated and imparts the repellent action.
Undyed nylon demonstrated a poor UPF (UV protection factor) rating (Table 6). The nylon dyed with 1 to 5 % shades showed good UPF ratings. This suggests the UV protective action imparted by the synthesised reactive dye. The mechanism of the dye for offering UV protection is based on the UV-absorbing ability of the dye (Singh and Sheikh, 2021b). The UV–vis spectrum of the dye prior confirmed the UV absorption potential of the dye.
Even after 20 washes, the dyed fabric retained good UV protection. As established earlier, the reactive dye reacts with nylon through a covalent bond (Son et al., 2005), due to which the dyed fabrics imparted wash-durable functional effects.
Fig. 10 records the Transmittance curve for dyed and undyed nylon fabrics. A reduction in transmission values after dyeing was obtained. Dyed fabrics, even after washing, showed less transmission than undyed fabric. Thus, the transmission curve also suggested the UV protection action provided by dye to the nylon.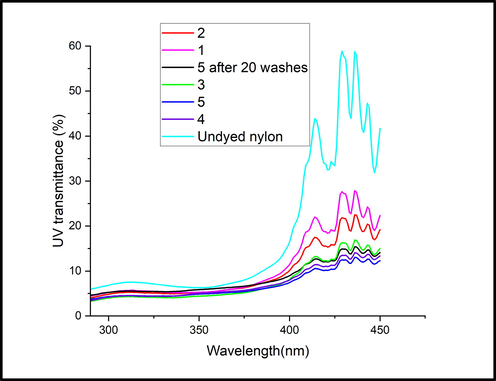
Transmittance curve for dyed and undyed nylon fabrics.
4.5 TGA and SEM analysis of nylon fabrics
Fig. 11 indicates the thermogravimetric analysis of undyed and dyed nylon. Similar nature of curves for undyed and dyed nylon was obtained. A significant weight loss was observed between 400 and 500 °C. Dyed nylon showed slightly higher thermal stability than undyed nylon. Various temperatures (T10, T25, T50, and T75) are also recorded in Table 7, where T10 expresses the temperature at 10 % weight loss. The transition temperature of dyed nylon was higher than that of undyed nylon.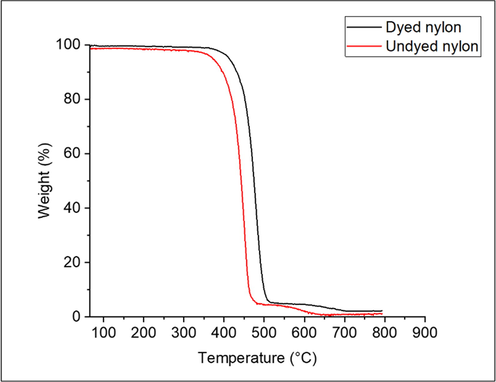
TGA analysis of nylon fabric.
Temperature
Undyed nylon
Dyed nylon
T10
397
429
T25
425
457
T50
443
475
T75
454
488
Fig. 12 records the SEM images of undyed and dyed nylon fabrics. Both fabrics showed a very smooth and clear appearance without having traces of any impurities. The nylon fibres were found twisted around each other. An intact appearance of the nylon surface after dyeing was obtained. Thus, the SEM analysis suggested that no fibre surface damage occurred after the dyeing.
(A) SEM image of undyed nylon; (B) SEM image of dyed nylon.
5 Conclusions
The synthesis of novel reactive dye for functional dyeing of nylon was successfully achieved. Different characterisations, FTIR, 1H NMR, CHN analyser, UV–vis spectroscopy, and thermogravimetric analysis (TGA), were explored to characterise and confirm the reactive dye synthesis. The prepared dye was applied to the nylon using the cold brand reactive dyeing method to generate the mosquito repellent-UV protective nylon. After washing treatments, 100 % mosquito repellency and good UV protection were achieved. The exhaustion, fixation, colouration, and fastness properties of the dye on nylon were studied. The dyeing and functional behaviour of the synthesised dye suggested that it is an excellent option for making coloured and multifunctional nylon fabric. Moreover, the paper reports a new reactive dye for generating mosquito repellent textile substrate, which can be directly utilised to make protective textiles.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
References
- AATCC technical manual, 2007. Association of Textile Chemists and Colorists. AATCC technical manual, Research Triangle Park.
- Dyeing of cotton fabrics with reactive dyes and their physico-chemical properties. Indian J. Fibre Text. Res.. 2008;33:58-65.
- [Google Scholar]
- Review on : developing UV protection for cotton fabric. J. Text. Inst.. 2017;5000:1-13.
- [CrossRef] [Google Scholar]
- Synthesis, spectral study and application of solid state fluorescent reactive disperse dyes and their antibacterial activity. Arab. J. Chem.. 2019;12:225-235.
- [CrossRef] [Google Scholar]
- Agro textiles - Their applications in agriculture and scope for utilizing natural fibers in agro tech sector. Int. J. Appl. Home Sci.. 2017;4:653-662.
- [Google Scholar]
- Applications of hemp in textiles, paper industry, insulation and building materials, horticulture, animal nutrition, food and beverages, nutraceuticals, cosmetics and hygiene, medicine, agrochemistry, energy production and environment: a review. Environ. Chem. Lett.. 2020;18:1451-1476.
- [CrossRef] [Google Scholar]
- Neutral-fixing reactive dyes for cotton. Part 1 —synthesis and application of quaternised S-triazinyl reactive dyes. J. Soc. Dye. Colour.. 1992;108:195-199.
- [CrossRef] [Google Scholar]
- Synthesis, application and colorimetric study of some monoazo reactive dyes. Mater. Today Proc.. 2022;51:1099-1106.
- [CrossRef] [Google Scholar]
- Mini review: Mode of action of mosquito repellents. Pestic. Biochem. Physiol.. 2013;106:149-155.
- [CrossRef] [Google Scholar]
- Synthesis, stability and printing properties of a novel 2-sulphophenoxy-4-chloro-s-triazine reactive dye for ink-jet printing of wool. Color. Technol.. 2020;136:153-166.
- [CrossRef] [Google Scholar]
- Long-lasting UV protection and mosquito repellent finish on bamboo/tencel blended fabric with microencapsulated essential oil. Indian J. Fibre Text. Res.. 2015;40:175-179.
- [Google Scholar]
- Antimicrobial textile : recent developments and functional perspective. Polym. Bull.. 2022;79:5747-5771.
- [CrossRef] [Google Scholar]
- The contribution of different vinyl sulphone-reactive dyes to an effluent. J. Taibah Univ. Sci.. 2015;9:594-600.
- [CrossRef] [Google Scholar]
- Synthesis and kinetic study of a series of chloro- and m-carboxypyridium triazinyl reactive dyes. Dye. Pigment.. 2021;188:109147
- [CrossRef] [Google Scholar]
- ISO manual, 2006. ISO Technical Manual. switerzland: Geneva.
- Molecular mechanics and variable-temperature 1H NMR studies on N/N-diethyl-m-toluamide: An undergraduate NMR and molecular modeling experiment. J. Chem. Educ.. 2001;78:538-540.
- [CrossRef] [Google Scholar]
- Ultrasonic-assisted dyeing: I. Nylon dyeability with reactive dyes. Polym. Int.. 2003;52:373-380.
- [CrossRef] [Google Scholar]
- Mosquito Repellent Finish on Cotton Fabric using Justicia Adhatoda Vasica Extract by Micro Encapsulation. Int. J. Res. Eng. Sci. Manag.. 2019;2:520-522.
- [Google Scholar]
- A review of UV radiation protection on humans by textiles and clothing. Int. J. Cloth. Sci. Technol.. 2020;32:869-890.
- [CrossRef] [Google Scholar]
- Characterising potential space suit textiles in proton beams using radiotherapy-based dosimetry. Adv. Sp. Res.. 2022;70:1925-1934.
- [CrossRef] [Google Scholar]
- Developments in the chemistry of reactive dyes and their application processes. Color. Technol.. 2014;130:382-412.
- [CrossRef] [Google Scholar]
- Synthesis of reactive dyes based on the bis-(N-carboxymethylamino)monoquaternary-triazine-bis-ethylsulphone reactive group. Part 1: Application to cotton cellulose. Color. Technol.. 2006;122:217-226.
- [CrossRef] [Google Scholar]
- Predicting mosquito repellent potency of N, N-diethyl-m-toluamide (DEET) analogs from molecular electronic properties. Am. J. Trop. Med. Hyg.. 1999;60:1-6.
- [CrossRef] [Google Scholar]
- Optimizing Color Performance of the Ngenuity 3-Dimensional Visualization System. Ophthalmol. Sci.. 2021;1:100054.
- [CrossRef] [Google Scholar]
- Synthesis, application and antibacterial activity of new reactive dyes based on thiazole moiety. Pigment Resin Technol.. 2018;47:246-254.
- [CrossRef] [Google Scholar]
- Synthesis and application of novel reactive dyes based on dimedone moiety. Egypt. J. Chem.. 2020;63:4447-4455.
- [CrossRef] [Google Scholar]
- Synthesis and evaluation of a series of trisazo, monochloro -s- triazinyl (MCT) reactive dyes for cotton. Dyes Pigment.. 2004;63:51-63.
- [CrossRef] [Google Scholar]
- Synthesis and characterization of novel reactive dyes with simultaneous insect-repellent and anti-bacterial properties. Fibers Polym.. 2014;15:1369-1374.
- [CrossRef] [Google Scholar]
- Synthesis of an amino coumarin-based fluorescent reactive dye and its application to wool fibres. Color. Technol.. 2011;127:62-68.
- [CrossRef] [Google Scholar]
- A novel approach to modify and functionalize acid black 1 dye for antimicrobial and UV protective textiles. Dye. Pigment.. 2022;205:110486.
- [CrossRef] [Google Scholar]
- Development of protective cotton textiles against biohazards and harmful UV radiation using eco-friendly novel fiber-reactive bioactive agent. Process Saf. Environ. Prot.. 2022;165:431-444.
- [CrossRef] [Google Scholar]
- Dyeing of electrospun nylon 6 nanofibers with reactive dyes using electron beam irradiation. J. Ind. Eng. Chem.. 2016;39:16-20.
- [CrossRef] [Google Scholar]
- Synthesis and characterization of reactive dyes based on 2-phenyl-3-[4’-(4″-aminophenylsulphonamido)]phenyl-4(3H)-quinazolinone-6-sulphonic acid. Arab. J. Chem.. 2011;4:279-285.
- [CrossRef] [Google Scholar]
- Synthesis, characterization and in vitro antimicrobial screening of some new MCT reactive dyes bearing nitro quinazolinone moiety. J. Saudi Chem. Soc.. 2015;19:347-359.
- [CrossRef] [Google Scholar]
- Colorimetric studies of some newly synthesized bisazo reactive dyes. Arab. J. Chem.. 2016;9:S161-S169.
- [CrossRef] [Google Scholar]
- Simultaneous dyeing and anti-bacterial finishing on 100% cotton fabric: process establishment and characterization. Cellulose. 2018;25:5405-5414.
- [CrossRef] [Google Scholar]
- Cationization of TiO2 nanoparticles to develop highly durable multifunctional cotton fabric. Mater. Chem. Phys.. 2022;278:125573.
- [CrossRef] [Google Scholar]
- Preparation of ZnO/Nylon 6/6 nanocomposites, their characterization and application in dye decolorization. Appl. Water Sci.. 2021;11:1-10.
- [CrossRef] [Google Scholar]
- Synthesis and characterization of high wash fastness novel azo reactive dyes incorporating aromatic bridged diamines. Arab. J. Chem.. 2018;11:111-119.
- [CrossRef] [Google Scholar]
- Single step synthesis and functionalization of nano titania for development of multifunctional cotton fabrics. Materials (Basel). 2022;15:38.
- [CrossRef] [Google Scholar]
- A new kind of H-acid monoazo-anthraquinone reactive dyes with surprising colour. Dyes Pigment.. 2015;123:44-54.
- [CrossRef] [Google Scholar]
- Computational and experimental study of heterofunctional azo reactive dyes synthesized for cellulosic fabric. J. Mol. Struct.. 2020;1221:128753.
- [CrossRef] [Google Scholar]
- Microencapsulation and its application in production of functional textiles. Indian J. Fibre Text. Res.. 2020;45:495-509.
- [Google Scholar]
- Development of Mosquito Repellent, Antibacterial, Antioxidant and UV Protective Cotton Using a Novel Method of Azoic Dyeing with Terminalia Chebula. J. Nat. Fibers. 2021;1–14
- [CrossRef] [Google Scholar]
- Development of multifunctional polyester using disperse dyes based through a combination of mosquito repellents. J. Mol. Struct.. 2021;1232:129988.
- [CrossRef] [Google Scholar]
- Multifunctional Linen Fabric Obtained through Finishing with Chitosan-gelatin Microcapsules Loaded with Cinnamon Oil. J. Nat. Fibers. 2021;1–11
- [CrossRef] [Google Scholar]
- Synthesis of novel, coumarin-based, mosquito repellent-cum-multifunctional azo disperse dye for functional dyeing of polyester. J. Text. Inst.. 2022;1–11
- [CrossRef] [Google Scholar]
- A study of heterobifunctional reactive dyes on nylon fibers: Dyeing properties, dye moiety analysis and wash fastness. Dyes Pigment.. 2005;66:231-239.
- [CrossRef] [Google Scholar]
- Microencapsulated citronella oil for mosquito repellent finishing of cotton textiles. Trans. R. Soc. Trop. Med. Hyg.. 2010;104:653-658.
- [CrossRef] [Google Scholar]
- Modified application process on cotton fabric for improved mosquito repellency. J. Text. Inst.. 2017;108:915-921.
- [CrossRef] [Google Scholar]
- Synthesis of reactive dye to impart mosquito repellency to nylon. J. Text. Inst.. 2017;108:226-232.
- [CrossRef] [Google Scholar]
- Dyeing of cotton fabric for improved mosquito repellency. J. Text. Inst.. 2018;109:427-434.
- [CrossRef] [Google Scholar]
- Ultrasound energy to accelerate dye uptake and dye-fiber interaction of reactive dye on knitted cotton fabric at low temperatures. Ultrason. Sonochem.. 2016;29:270-278.
- [CrossRef] [Google Scholar]
- Mechanical and electrical property improvement in CNT/Nylon composites through drawing and stretching. Compos. Sci. Technol.. 2011;71:1677-1683.
- [CrossRef] [Google Scholar]
- Solvent assisted dyeing of wool fibers with reactive dyes in a ternary solvent system for protecting fibers against damage. J. Ind. Eng. Chem.. 2021;103:329-339.
- [CrossRef] [Google Scholar]
- Synthesis and salt-free dyeing characteristics of cationic reactive dyes containing polyetheramine segments. Color. Technol.. 2016;132:344-349.
- [CrossRef] [Google Scholar]
- Synthesis of a di-(p-sulphophenoxy)-s-triazine reactive dye and its application in wool fabric ink-jet printing. Color. Technol.. 2019;135:202-212.
- [CrossRef] [Google Scholar]
- Synthesis and application of disazo reactive dyes derived from sulfatoethylsulfone pyrazolo[1,5-a]pyrimidine derivatives. J. Saudi Chem. Soc.. 2014;18:220-226.
- [CrossRef] [Google Scholar]
- Synthesis of novel green reactive dyes and relationship between their structures and printing properties. Dyes Pigment.. 2020;174:108079.
- [CrossRef] [Google Scholar]
- Ecofriendly synthesis and application of special disperse reactive dyes in waterless coloration of wool with supercritical carbon dioxide. J. Clean. Prod.. 2016;133:746-756.
- [CrossRef] [Google Scholar]
- Synthesis and application of KM-type reactive dyes containing 2-ethoxy-4-chloro-s-triazine. Color. Technol.. 2019;135:335-348.
- [CrossRef] [Google Scholar]







