Translate this page into:
Synthesis, antifungal activity and in vitro mechanism of novel 1-substituted-5-trifluoromethyl-1H-pyrazole-4-carboxamide derivatives
⁎Corresponding authors. zbwu@gzu.edu.cn (Zhibing Wu), wxue@gzu.edu.cn (Wei Xue)
-
Received: ,
Accepted: ,
This article was originally published by Elsevier and was migrated to Scientific Scholar after the change of Publisher.
Peer review under responsibility of King Saud University.
Abstract
Inspired by the wide application of amides in plant pathogens, a series of novel 1-substituted-5-trifluoromethyl‑1H‑pyrazole-4-carboxamide derivatives were designed and synthesized. Bioassay results indicated that some target compounds exhibited excellent and broad-spectrum in vitro and certain in vivo antifungal activities. Among them, the in vitro EC50 values of Y13 against G. zeae, B. dothidea, F. prolifeatum and F. oxysporum were 13.1, 14.4, 13.3 and 21.4 mg/L, respectively. The in vivo protective activity of Y13 against G. zeae at 100 mg/L was 50.65%. SAR analysis revealed that the phenyl on the 1-position of the pyrazole ring was important for this activity. An antifungal mechanism study of Y13 against G. zeae demonstrated that this compound may disrupt the cell membrane of mycelium, thus inhibiting the growth of fungi. These mechanistic study results were inconsistent with those for traditional amides and may provide a novel view for deep study of this series of pyrazole carboxamide derivatives.
Keywords
Pyrazole carboxamides
Molecular design
Fungicidal activity
Molecular docking
SAR analysis
Antifungal mechanism
- 1H NMR
-
1H nuclear magnetic resonance
- 13C NMR
-
13C nuclear magnetic resonance
- 19F NMR
-
19F nuclear magnetic resonance
- HRMS
-
high-resolution mass spectrometry
- THF
-
tetrahydrofuran
- PDA
-
potato dextrose agar
- EC50 value
-
median effective concentration
- SD
-
standard deviation
- CK
-
blank control
- PDB
-
potato dextrose broth
- FM
-
fluorescence microscopy
- PI
-
propidium iodide
- MDA
-
malondialdehyde
- SAR
-
structure-activity relationship
- PBS
-
phosphate buffer saline
- SDH
-
succinate dehydrogenase
- SEM
-
scanning electron microscope
Abbreviations
1 Introduction
Plant diseases reduce the global production of major food and economic crops by 20% per year (Xiong et al., 2013). Phytopathogenic fungi cause a drastic threat to agricultural development and human health (McColl et al., 2018). The consequences of plant diseases caused by pathogenic fungi can be catastrophic (Wang et al., 2014). Some fungi can produce mycotoxins, which pose a significant threat for human and animal health (Ninomiya et al., 2020). Currently, chemical means are still the main method to prevent and control fungal diseases (Al-Otibi et al., 2021). Due to the frequent use of commercialized fungicides, pathogenic fungi have evolved severe resistance (Oporto et al., 2019). Therefore, it is necessary to develop novel structural skeletons with clear targets or molecules with novel mechanisms (Wang et al., 2021).
Amides are basic molecular structures that have been widely studied and applied and from which a series of commercial fungicides have been derived. They exhibit wide biological activities, including insecticidal (Luo et al., 2020), herbicidal (Wang et al., 2019), antifungal (Yang et al., 2019) and anticancer activities (Chen et al., 2020). Analysis of the commercially available amide fungicides revealed that many of them use a pyrazole amide as the active skeleton. Among the structures of pyrazole-4-carboxamides, there are two typical types of substitutions on the pyrazole ring: 1,3-disubstitution and 1,3,5-trisubstitution (Fig. 1) (Abrigach et al., 2016; Hua et al., 2020; Xiong et al., 2017; Zhang et al., 2017; Liu et al., 2019; Zhang et al., 2019; Du et al., 2018; Yan et al., 2019; Liu et al., 2018; Gonzalez-Lopez et al., 2020).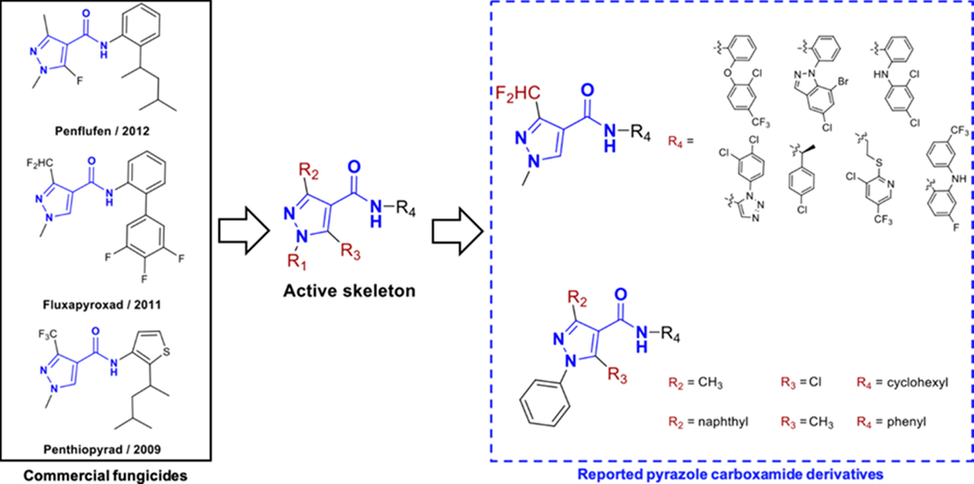
Representative fungicides containing pyrazole carboxamide moieties.
In previous work, we found an active skeleton of 5-trifluoromethyl-4-pyrazole carboxamide, and its derivatives exhibited excellent inhibitory activity against Fusarium oxysporum as potential succinate dehydrogenase inhibitors (Wu et al., 2021). In the present study, the 5-trifluoromethyl on the pyrazole ring of the active skeleton was preserved, the phenyl on the 1-position of the pyrazole ring was substituted by a series of alkyl and cyclohexyl groups, and a series of novel 1-substituted-5-trifluoromethyl-4-pyrazole carboxamide derivatives were designed and synthesized. Bioassay evaluation and docking analysis were used to determine the necessity of the phenyl on the pyrazole ring. The antifungal mechanism of active compounds against Gibberella zeae (Schwein.) Petch was also investigated.
2 Methods and materials
2.1 Instruments and chemicals
1H NMR, 13C NMR, and 19F NMR spectra were obtained on a JEOL-500 (JEOL CO., Ltd., Japan) or a Bruker 400 NMR spectrometer (Bruker Corporation, Germany) with tetramethylsilane as an internal standard and CDCl3 or DMSO‑d6 as the solvent. HRMS data were acquired on a Thermo Scientific Q Exactive mass spectrometer (Thermo Scientific, USA). The morphology of the fungus was observed using an Olympus BX53 fluorescence microscope (Olympus Ltd, Japan) and a Nova Nano SEM 450 instrument (FEI Company, USA). The MDA contents were recorded on a Cytation 5 imaging reader (BioTek Instruments, USA). The MDA assay kit was purchased from Beijing Solarbio Science & Technology Co., and all reagents and solvents were of analytical grade.
2.2 Fungi
Seven plant pathogenic fungi were used for antifungal evaluation. Thanatephorus cucumeris, Diaporthe phaseolorum var. phaseolorum (Cooke & Ellis) Sacc, Gibberella zeae (Schwein.) Petch, Fusarium oxysporum, and Fusarium oxysporum f. sp. Cucumerinum were purchased from Beijing Beina Chuanglian Biotechnology Institute, China. Fusarium prolifeatum was isolated and identified in our lab from Guizhou University, and Botryosphaeria dothidea was provided by Guiyang University. These fungi were grown on PDA plates at 25 ± 1 °C and maintained at 4 °C.
2.3 Synthesis
2.3.1 General procedure for the synthesis of 3a-3e
In a round-bottom flask, ethyl trifluoroacetoacetate (0.1 mol), triethyl orthoformate (0.2 mol) and acetic anhydride (0.3 mol) were mixed, stirred at 130 °C for 4 h, and then subjected to vacuum distillation to obtain 2. Cyclohexyl hydrazine hydrochloride (3.45 g, 22.90 mmol) was dissolved in ethanol (50 mL); the pH value was adjusted to 7 with sodium hydroxide solution; and then, 2 was added and reacted at 100 °C for 2 h. The solvent was removed by vacuum distillation. The mixture was extracted with ethyl acetate, washed with brine, dried over anhydrous sodium sulfate and concentrated to obtain the crude product. The crude product was further purified by column chromatography (PE/EA = 50/1) to obtain 3c. The physical and spectral data of 3a-3e are provided in the Supporting Information.
2.3.2 General procedure for the synthesis of 4a-4e
Intermediate 3c (6.0 g, 20.7 mmol), THF (30 mL), and water (30 mL) were added in turn. After the solution was uniformly stirred, lithium hydroxide (2.0 g, 82.7 mmol) was added. The reaction mixture was stirred for 2 h at 80 °C. The solution was concentrated in vacuo. Hydrochloric acid (2 M) was used to adjust the pH value to approximately 4; some solid precipitates formed, and the mixture was filtered. The filtrate was washed with water and dried to obtain 4c. The physical and spectral data of 4a-4e are provided in the supporting information.
2.3.3 General procedure for the synthesis of 5a-5e
According to a method reported in the literature (Komane et al., 2020), using SOCl2 as the solvent, 5a-5e were synthesized. The solvent was removed under vacuum after the reaction was finished, and the crude product was directly used for the next reaction.
2.3.4 General procedure for the synthesis of Y1-Y20
Taking Y9 as an example, a solution of 4-aminopyridine (3.2 mmol), intermediates 5c (3.5 mmol) and NaH (6.4 mmol) in anhydrous THF (5 mL) was stirred at room temperature overnight. The residue was extracted with ethyl acetate and water, dried over anhydrous sodium sulfate, and purified by silica gel column chromatography to obtain Y9. The physical and spectral data of target compounds Y1-Y20 are provided in the supporting information.
2.4 Bioassays
2.4.1 In vitro antifungal activity
According to a previously reported method (Jian et al., 2016), the fungicidal activities of target compounds Y1-Y20 were tested against seven pathogenic fungi. The preliminary activity screening concentration of the compounds was 100 mg/L. DMSO (1%) in sterile distilled water served as a blank control, whereas the commercial fungicides boscalid, penthiopyrad, fluopyram and phenamacril served as the positive controls. Compounds with high inhibition rates were measured at the EC50 value. Inhibitory effects were calculated as follows: inhibition efficiency (%) = [(L0 - L)/(L0 − 0.5)] × 100, where L0 represents the diameter of fungal growth in the blank control and L represents the diameter of the fungi treated with compound. Each condition was performed in triplicate. SD values were calculated based on the inhibition data for triplicates of each inhibition rate. The regression equations and R2 values are provided in the Supporting Information.
2.4.2 In vivo protective activity bioassay
Maize seeds (Zhengdan 958) were planted in moist soil and cultured in a constant temperature incubator at 25 °C until germination. Seedlings with the same growth and health were transplanted into pots and cultivated for 4 d. A solution of Y13 (100 mg/L) and phenamacril (100 mg/L) was sprayed on the leaves. DMSO (1%) was used as a blank control. After two days in the incubator, the corn leaves were polished with sandpaper, and then, mycelia were placed on the wounds and moisturized with wet cotton. Lesion length was measured after 10 d. The control efficacies were calculated as follows: control efficacy (%) = (A0 - A1)/A0 × 100, where A0 represents the length of the lesion in the blank control group and A1 represents the length of the lesion treated with compound. SD values were calculated based on the inhibition data for triplicates of each inhibition rate (Wang et al., 2021).
2.5 Molecular docking
The SDH crystal structures were downloaded from the RCSB PDB database (PDB codes: 2FBW, 3AEG and 7D6V) and used for the docking study. All bound water and key ligands were eliminated from SDH. Six compounds (Y1, Y5, Y9, Y13, fluopyram and penthipyrad) were selected as the docking ligands, and energies of ligands were minimized using MM2 energy minimizations in ChemBio 3D (Fu et al., 2021). Software of Sybyl X 2.1 was used for molecular docking, and the ligands were embedded well in the same protein activity pocket (established by the extraction carboxin or boscalid from SDH or established automatically) (Kim et al., 2009; Kim et al., 2009). Surflex-Dock method was used to simulate and evaluate the interactions between the ligands and the target protein using an empirical scoring function in Surflex-Dock Geom (SFXC) mode (Kaddouri et al., 2020; Wang et al., 2020; Kaddouri et al., 2021).
2.6 Antifungal mechanism study
2.6.1 Mycelial quantity after treatment of G. Zeae with Y13
Mycelia of G. zeae were cultured on PDB medium on a rotary shaker at 25 °C and 180 rpm. Then, solutions of Y13 at different concentrations (0 mg/L, 6.25 mg/L, 12.5 mg/L, 25 mg/L, 50 mg/L, 100 mg/L and 200 mg/L) were added and incubated with the mycelia for 24 h at 25 °C. Mycelial samples were filtered out of the medium and dried by vacuum freezing. The weighing was repeated three times, each reading difference did not exceed 0.01 g, and each dry mycelium weight is reported as the average dry mycelium weight (Zhu et al., 2020).
2.6.2 Cell membrane integrity study
Morphological observation of hyphae via FM: Mycelia of G. zeae were cultured on PDB medium on a rotary shaker at 25 °C and 180 rpm. Then, solutions of Y13, penthiopyrad and phenamacril at different concentrations (0 mg/L, 50 mg/L, 100 mg/L and 200 mg/L, respectively) were added and incubated with the mycelia for 24 h at 25 °C. The PDB medium was removed by centrifugation at 4 °C and 3381 g for 5 min, and the hyphae were stained with 10 μL of a PI solution (20 mg/L). The hyphae were incubated at 37 °C for 15 min in the dark and then washed with PBS three times. A coverslip was placed on the hyphae, and the samples were observed and photographed using an Olympus-BX53 fluorescence microscope (Hou et al., 2021).
Morphological observation of hyphae via SEM: Mycelia of G. zeae were cultured on PDB medium on a rotary shaker (180 rpm) at 25 °C and 180 rpm. Then, prepared solutions of Y13, penthiopyrad and phenamacril at different concentrations (0 mg/L and 200 mg/L, respectively) were added and incubated with the mycelia for 24 h at 25 °C. The mycelia were then washed three times with PBS solution, and 2.5% glutaraldehyde solution was added for fixation for 24 h. The mycelia were washed with 30%, 50%, 70%, 90%, 100% ethanol and 100% tert-butanol, dried in a freeze-dryer, sprayed with gold, made into samples and observed (Ke et al., 2022).
2.6.3 Determination of MDA contents
Mycelia of G. zeae were cultured on PDB medium on a rotary shaker (180 rpm) at 25 °C and 180 rpm. Then, prepared solutions of compound Y13 at different concentrations (0 mg/L, 25 mg/L, 50 mg/L, 100 mg/L, and 200 mg/L) were added and incubated with the mycelia for 24 h at 25 °C. The medium was filtered out, and the hyphae were collected in a vacuum freeze dryer. Mycelia (0.1 g) were homogenized in an ice bath with 1 mL of MDA extract (produced by Solarbio Technology Co., Ltd., Beijing, China) and centrifuged at 8000 g and 4 °C for 15 min. The supernatant was removed, added to each reagent according to the kit instructions, held in a 100 °C water bath for 60 min, cooled in an ice bath, and centrifuged at 10,000 g for 10 min at room temperature. The supernatant was aspirated, and then, the absorbance of each sample was measured at 450 nm, 532 nm and 600 nm. Each group was repeated three times. MDA content in mycelia was determined using an MDA assay kit (Beijing Solarbio Science & Technology Co.) (Mo et al., 2021).
3 Results and discussion
3.1 Chemistry
3.1.1 Synthesis
The synthetic route of 1-substituted-5-trifluoromethyl-1H-pyrazole-4-carboxamide derivatives is shown in Fig. 2. Intermediate 2 was obtained by condensing 1 and triethyl orthoformate and then cyclized with different hydrazines to obtain intermediates 3a-3e. Intermediates 4a-4e were synthesized through a hydroxide reaction of 3a-3e in the presence of lithium hydroxide. The acylation products 5a-5e were obtained by electrophilic substitution reaction of 4a-4e with SOCl2 and reacted with substituted 4-pyridine amines to yield the target compounds Y1-Y20. All the target compounds were confirmed by 1H NMR, 13C NMR, and 19F NMR spectroscopy and HRMS, and their physical and spectral data are provided in the Supporting Information.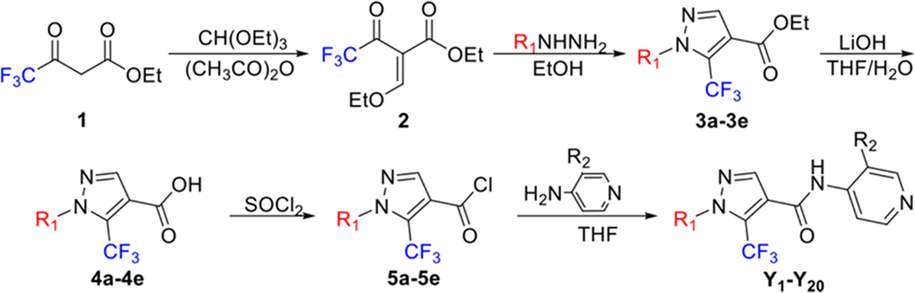
Synthesis routes of the target compounds Y1-Y20.
3.1.2 Crystal structure of Y11
As shown in Fig. 3, the crystal structure of Y11 was determined by single-crystal X-ray difference traction analysis. The skeleton of Y11 was composed of a pyrazole ring and a pyridine ring connected by amide bonds C (9) and C (12). The benzene ring and the trifluoromethyl on the pyrazole ring were attached to N (1) and C (8), respectively, indicating that 2-Cl-benzyl was attached at the 1-position of the pyrazole and trifluoromethyl was attached at the 5-position of the pyrazole. The crystallographic data of Y11 have been deposited in the Cambridge Crystallographic Data Centre under deposition number 2,116,345 and can also be downloaded in the Supporting Information.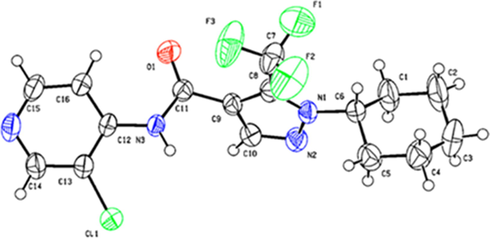
Crystal structure of Y11.
3.2 Antifungal activity
3.2.1 In vitro antifungal activity
The preliminary in vitro antifungal activity results for the target compounds against seven types of pathogenic fungi are depicted in Table 1. Some compounds exhibited excellent and broad-spectrum antifungal activities at a concentration of 100 mg/L, such as Y13, Y14 and Y17. As shown by the EC50 values in Table 2, Y13 showed good antifungal activities against G. zeae (13.1 mg/L), B. dothidea (14.4 mg/L), F. prolifeatum (13.3 mg/L) and F. oxysporum (21.4 mg/L); Y14 exhibited good antifungal activities against B. dothidea (18.1 mg/L); and Y17 exhibited good antifungal activities against G. zeae (16.8 mg/L) and B. dothidea (13.9 mg/L). Analysis of the in vitro antifungal activities revealed that when the substituents in the 1-position of pyrazole were ortho-substituted phenyls, target compounds exhibited good antifungal activities, such as Y13, Y14 and Y17; but lower activities were observed when methyl, isopropyl and cyclohexyl were introduced to the 1-position of the pyrazole ring, such as Y1 to Y12. However, when 4-pyridinyl was introduced into the amine part of the carboxamide group, such as in Y13 and Y17, the antifungal activities were obviously better than those of other compounds. a Values are the means ± SD of three replicates. ‘--’, not tested. GZ: Gibberella zeae (Schwein.) Petch; TC: Thanatephorus cucumeris; BD: Botryosphaeria dothidea; DP: Diaporthe phaseolorum var. phaseolorum (Cooke & Ellis) Sacc; FP: Fusarium prolifeatum; FOC: Fusarium oxysporum f. sp. Cucumerineum; FO: Fusarium oxysporum. a Values are the means ± SD of three replicates. ‘--’, not tested. GZ: Gibberella zeae (Schwein.) Petch; TC: Thanatephorus cucumeris; BD: Botryosphaeria dothidea; DP: Diaporthe phaseolorum var. phaseolorum (Cooke & Ellis) Sacc; FP: Fusarium prolifeatum; FOC: Fusarium oxysporum f. sp. Cucumerineum; FO: Fusarium oxysporum.
Compound no.
R1
R2
Inhibition rate ± SD (%)
GZ
TC
BD
DP
FP
FOC
FO
Y1
methyl
H
0
11.0 ± 3.0
0
31.2 ± 1.2
0
–
8.6 ± 0.6
Y2
methyl
CH3
0
0
0
0
0
–
9.4 ± 2.3
Y3
methyl
Cl
12.8 ± 1.6
29.4 ± 1.5
0
21.8 ± 0.6
10.5 ± 2.2
–
15.7 ± 1.1
Y4
methyl
F
0
0
17.2 ± 2.1
17.5 ± 0.9
0
–
13.5 ± 0.0
Y5
isopropyl
H
40.9 ± 0.5
0
9.6 ± 0.7
34.0 ± 1.8
32.8 ± 1.1
–
23.9 ± 0.6
Y6
isopropyl
CH3
15.0 ± 1.4
13.7 ± 2.5
36.8 ± 2.5
0
63.2 ± 1.0
–
49.5 ± 1.1
Y7
isopropyl
Cl
64.7 ± 1.3
40.8 ± 1.0
68.9 ± 2.1
32.0 ± 0.6
81.8 ± 2.3
66.8 ± 1.8
60.0 ± 2.0
Y8
isopropyl
F
41.9 ± 1.6
53.1 ± 0.8
47.4 ± 0.4
78.9 ± 2.0
76.2 ± 0.6
47.8 ± 0.2
55.4 ± 1.1
Y9
cyclohexyl
H
77.2 ± 1.1
75.7 ± 0.4
69.2 ± 1.7
20.3 ± 9.6
65.6 ± 0.6
55.7 ± 1.2
63.6 ± 3.0
Y10
cyclohexyl
CH3
23.4 ± 1.1
51.4 ± 0.3
31.8 ± 1.2
0
23.5 ± 0.6
–
22.6 ± 2.3
Y11
cyclohexyl
Cl
0
20.2 ± 1.6
15.9 ± 1.4
0
0
–
0
Y12
cyclohexyl
F
27.8 ± 3.2
37.7 ± 3.0
28.5 ± 1.3
0
30.5 ± 1.7
–
31.8 ± 0.1
Y13
o-methylphenyl
H
86.6 ± 0.2
68.2 ± 1.4
86.1 ± 0.6
100
61.8 ± 0.7
92.3 ± 0.3
70.8 ± 0.9
Y14
o-methylphenyl
CH3
67.6 ± 0.4
84.3 ± 0.0
79.7 ± 0.4
100
55.0 ± 0.7
67.2 ± 0.6
63.3 ± 0.6
Y15
o-methylphenyl
Cl
45.4 ± 3.2
62.0 ± 1.4
33.6 ± 3.1
52.2 ± 3.2
42.0 ± 0.0
21.8 ± 1.3
50.6 ± 1.1
Y16
o-methylphenyl
F
68.0 ± 1.1
77.6 ± 3.1
73.9 ± 0.3
83.7 ± 0.7
39.1 ± 0.7
59.0 ± 4.0
53.2 ± 1.7
Y17
o-trifluorophenyl
H
83.7 ± 1.5
54.1 ± 1.3
76.4 ± 0.4
89.8 ± 0.9
81.5 ± 1.5
81.2 ± 0.8
79.8 ± 0.0
Y18
o-trifluorophenyl
CH3
62.1 ± 1.5
64.3 ± 3.0
74.8 ± 1.9
94.3 ± 1.4
41.2 ± 1.9
59.0 ± 0.5
45.3 ± 0.6
Y19
o-trifluorophenyl
Cl
0
24.7 ± 0.7
19.7 ± 5.2
40.0 ± 0.7
0
–
0
Y20
o-trifluorophenyl
F
37.3 ± 2.0
29.4 ± 0.6
57.3 ± 0.2
51.4 ± 0.7
24.4 ± 1.3
–
46.8 ± 1.7
boscalid
16.3 ± 0.6
100
28.5 ± 1.5
39.6 ± 1.4
0
–
12.4 ± 1.1
penthiopyrad
0
100
–
–
–
–
–
fluopyram
72.5 ± 0.6
–
–
–
–
–
–
phenamacril
100
–
–
–
–
–
–
Compound no.
EC50 (mg/L)
GZ
TC
BD
DP
FP
FOC
FO
Y13
13.1 ± 2.7
49.8 ± 5.7
14.4 ± 0.6
36.4 ± 0.4
13.3 ± 1.4
38.9 ± 1.1
21.4 ± 0.6
Y14
50.7 ± 1.7
37.5 ± 2.4
18.1 ± 3.9
26.6 ± 3.9
–
–
26.0 ± 1.1
Y16
56.0 ± 0.8
44.3 ± 1.4
20.6 ± 0.3
21.8 ± 3.0
34.3 ± 1.7
112.3 ± 1.7
–
Y17
16.8 ± 0.4
56.8 ± 0.9
13.9 ± 0.6
–
–
–
37.8 ± 2.0
Y18
–
94.6 ± 16.7
–
–
–
–
–
fluopyram
12.3 ± 0.3
–
–
–
–
–
–
penthiopyrad
–
0.1 ± 0.0
–
–
–
–
–
boscalid
–
3.5 ± 0.1
–
–
–
–
–
phenamacril
0.5 ± 0.0
–
–
–
–
–
–
3.2.2 In vivo antifungal activity of Y13
Target compound Y13 was selected for further in vivo antifungal activity evaluation against G. zeae on the leaves of maize. As shown in Table 3., Y13 displayed obvious protective activity (50.65%) at 100 mg/L, which was lower than that of the commercialized fungicide phenamacril (79.83%). Further optimization of this series of target compounds containing ortho-substituted phenyl at the 1-position of pyrazole may lead to identification of compounds with higher activity that can be developed as potential fungicides against G. zeae. a b c The significant difference analysis adopts Duncan's test method, and IBM SPSS Statistics 20 data processing software was used. Different letters in the same column in the table indicate significant differences, p < 0.05.
Compound no.
Protective activity
diameter of lesions (cm)
control efficacy (%)
CK
3.0 ± 1.0a
Y13
1.4 ± 0.4b
50.65 ± 3.3
phenamacril
0.6 ± 0.1c
79.83 ± 4.8
3.3 SAR analysis
To further explore the SARs of the target compounds, reported SDH proteins (PDB: 2FBW, 3AEG and 7D6V) were used for docking studies. As shown by the docking results presented in Table 4, the total scores of ligands Y3 and fluopyram docking to SDH protein (PDB: 2FBW) were 6.3 and 7.1, respectively, indicating that fluopyram exhibited a stronger interaction with 2FBW than Y13. This is consistent with the activity results depicted in Tables 1 and 2. Through comparison of the interactions between Y13 and the different SDH proteins (2FBW, 3AEG and 7D6V), this binding model can be effectively evaluated for SAR analysis of this series of target compounds (Yan et al., 2021).
PDB code
Ligand
Structure
Total score
2FBW
Y1
4.3
Y5
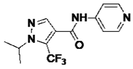
3.5
Y9
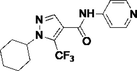
5.4
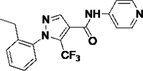
Y13
6.3
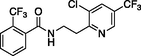
fluopyram
7.1
penthipyrad
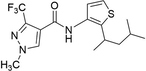
7.3
3AEG
Y13
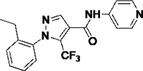
6.2
7D6V
Y13
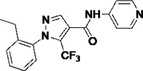
6.2
The docking results in Fig. 4A revealed that the trifluoromethyl group of Y13 formed a hydrogen bond with D/TYR58 (2.2 Å) of 2FBW. As shown in Fig. 4B, the oxygen atom of carbonyl in penthiopyrad formed hydrogen bonds with B/TRP173 (1.9 Å) and D/TYR58 (2.0 Å). Compared the binding modes of Y13 and penthiopyrad found that they bound tightly with 2FBW in the same pocket through roughly the similar conformation. As shown in Fig. 4C, the binding conformations of Y13 and fluopyram were similar, and the key group trifluoromethyl of two ligands both formed the hydrogen bonds with D/TYR58. The docking results demonstrated that the trifluoromethyl may be the key component of this series of molecules, contributing to the enhanced antifungal activity.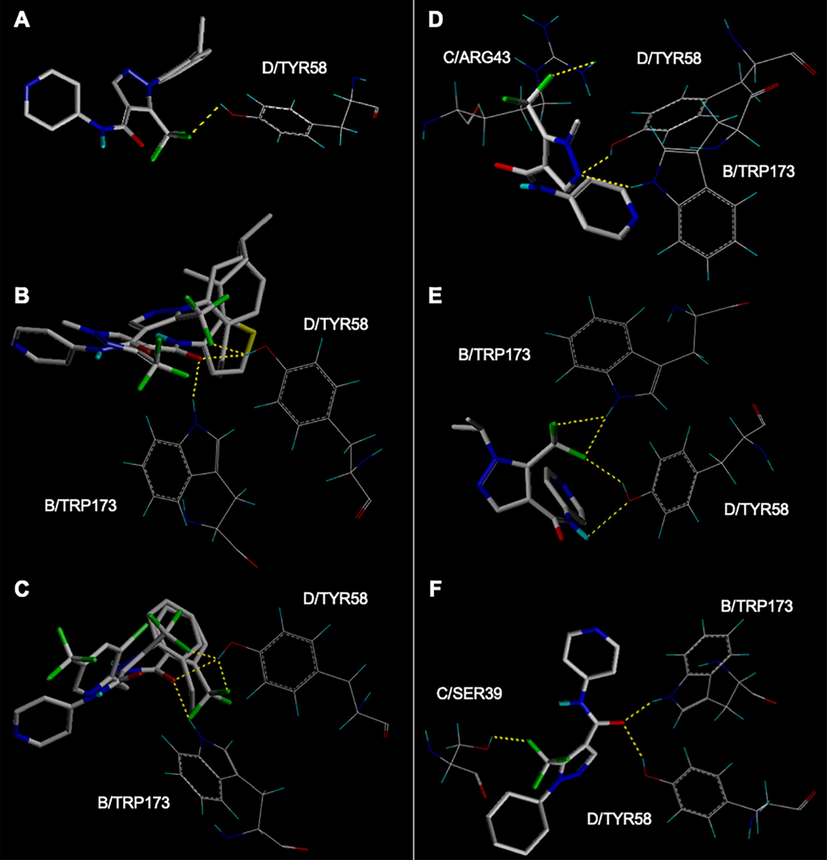
Binding modes between ligands and SDH protein. (A) Y13; (B) Y13 and penthiopyrad; (C) Y13 and fluopyram; (D) Y1; (E) Y5; (F) Y9.
However, the docking poses and the docking scores were slightly changed when the substituent at the 1-position of the pyrazole ring was substituted. Compared the docking scores, binding modes (Fig. 4D, 4E and 4F) and antifungal activities of ligands Y1, Y5, and Y9 found that when methyl, isopropyl and cyclohexyl were introduced at the 1-position of the pyrazole ring to replace the phenyl, the antifungal activities of the target compounds were decreased. Detailed analysis of the docking results for this series of target compounds revealed that Y13 exhibited better antifungal activity when phenyl was introduced to the 1-position of the pyrazole ring. These results were in agreement with the results of the preliminary SAR analysis. Thus, this molecular docking study may provide a benchmark for understanding the antifungal activity against plant phytopathogenic fungi G. zeae.
3.4 Effect of Y13 on mycelial growth of G. Zeae
As shown in Fig. 5, the dry weight of G. zeae hyphae treated with Y13 at different concentrations (0, 6.25, 12.5, 25, 50, 100, and 200 mg/L) for 24 h decreased significantly. Furthermore, the weight of hyphae decreased sharply as the concentration of Y13 increased, indicating that the target compound can inhibit the growth of G. zeae (Guo et al., 2020).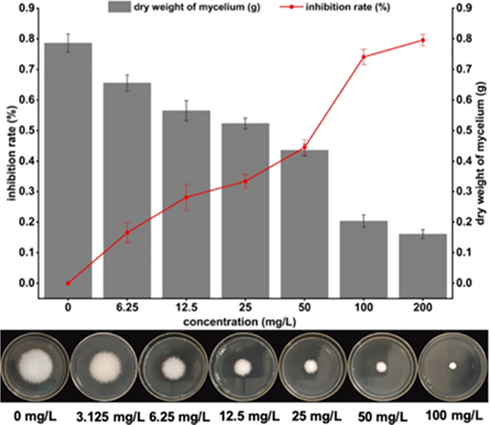
Effects of Y13 treatment on the growth of G. zeae.
3.5 Effect of Y13 on the cell membrane integrity of G. Zeae mycelia
3.5.1 Morphological analysis via FM
PI, a nucleic acid dye that can enter through a disrupted cell membrane, stains upon binding to double-stranded nucleic acids (Li et al., 2020). The more and brighter the red fluorescence appears, the worse the cell membrane damage. As shown in Fig. 6, no fluorescence was observed in the CK groups (0 mg/L). As concentration increased, the fluorescence of hyphae treated with phenamacril (Fig. 6B) and Y13 (Fig. 6C) became stronger, which indicated that these compounds can destroy the integrity of the cell membrane and that the effects are concentration-dependent (Hou et al., 2021). However, the hyphae treated with penthiopyrad did not show obvious fluorescence, even at a concentration of 200 mg/L (Fig. 6A).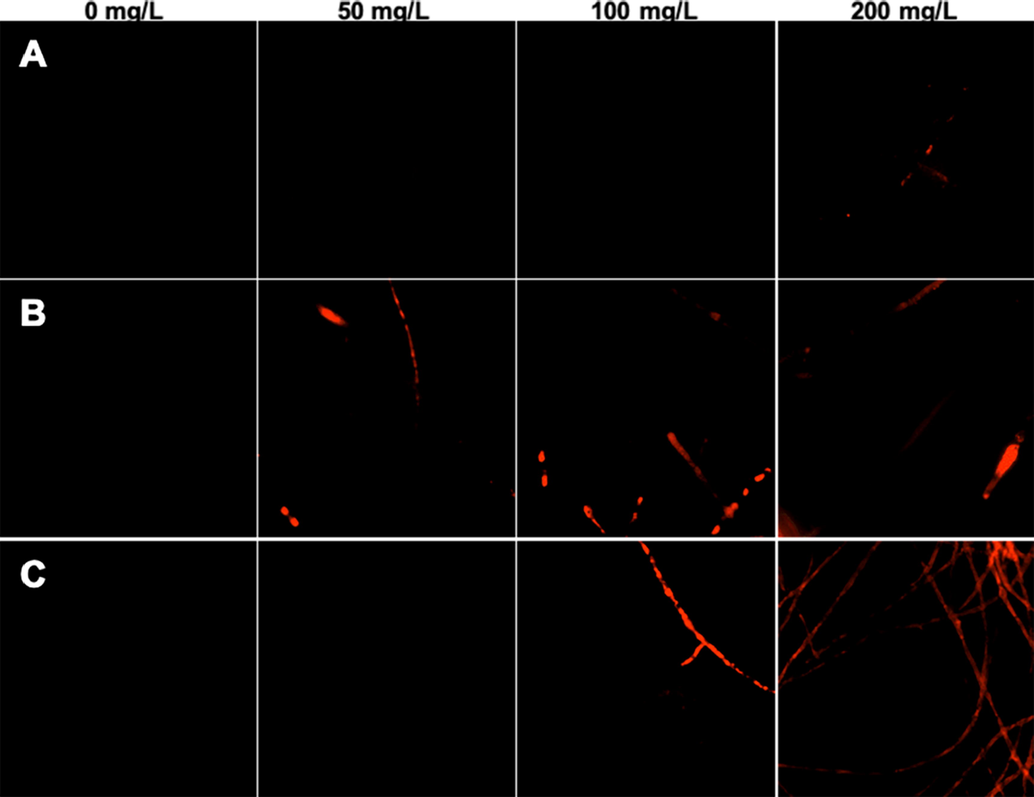
Fluorescence observation of G. zeae treated with different compounds. A: Treatment with penthiopyrad at 0, 50, 100, and 200 mg/L; B: treatment with phenamacril at 0, 50, 100, and 200 mg/L; C: treatment with Y13 at 0, 50, 100, and 200 mg/L.
3.5.2 Morphological analysis via SEM
Morphological observation results for the hyphae of G. zeae treated with penthiopyrad, phenamacril, or Y13 at 200 mg/L are shown in Fig. 7. Compared with the hyphae treated with the different compounds, the normal hyphae presented regular, smooth and uniform mycelia (blank control groups), and the hyphae treated with penthiopyrad exhibited a degree of shrinking (red arrows in Fig. 7C) but no breakage. However, the hyphae treated with phenamacril and Y13 showed obvious breakage, indicated by the red circles in Fig. 7C, which revealed that Y13 and phenamacril may disrupt the cell membrane of the mycelium, thus inhibiting the growth of the fungi (Zhao et al., 2020). This result was consistent with the findings of the FM morphological analysis described above.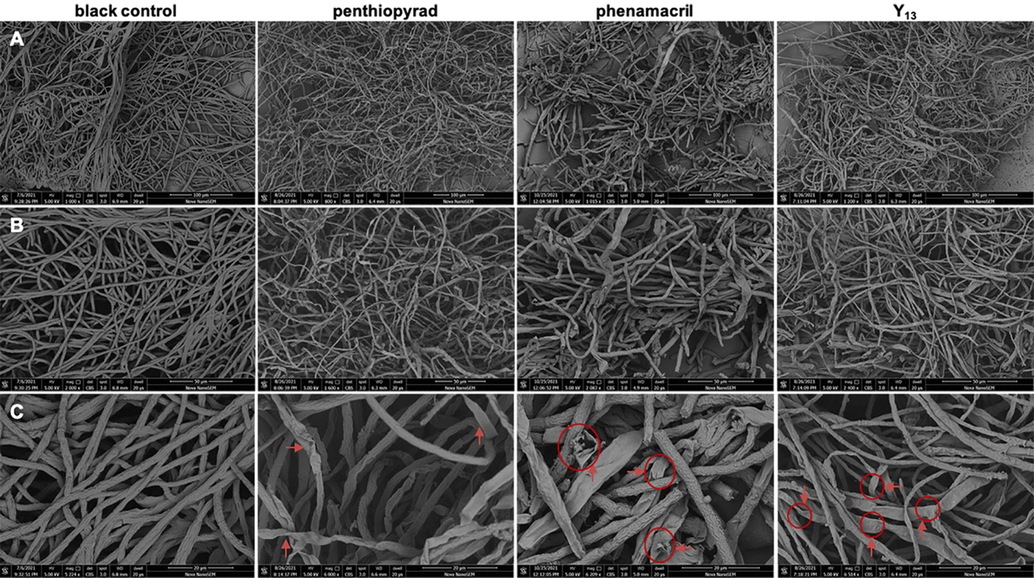
SEM analysis: scanning electron micrographs of hyphae exposed to penthiopyrad phenamacril and Y13 at a concentration of 200 mg/L. A: 100 μm. B: 50 μm. C: 20 μm. Arrows and arrowheads indicate hyphal shrinkage and partial distortion, respectively.
3.6 MDA content of G. Zeae treated with Y13
MDA, a sustainable biochemical marker, is produced in the process of lipid peroxidation, and the MDA content can be used to evaluate the degree of membrane damage (Zhao et al., 2016). The MDA contents of G. zeae treated with Y13 at different concentrations (0, 25, 50, 100 and 200 mg/L) for 24 h are depicted in Fig. 8. The MDA content of G. zeae mycelia increased when the concentration of Y13 increased (from 25 mg/L to 100 mg/L) and was obviously higher than the content in the control group (0 mg/L). However, when the concentration of Y13 was 200 mg/L, the MDA content decreased, indicating that a high concentration of Y13 can destroy the structure of the cell membrane and result in leakage of cell contents. All the results demonstrated that the target compound Y13 can promote lipid and peroxide reactions in cell membranes, which can lead to breakage of cell membranes (Yang et al., 2020).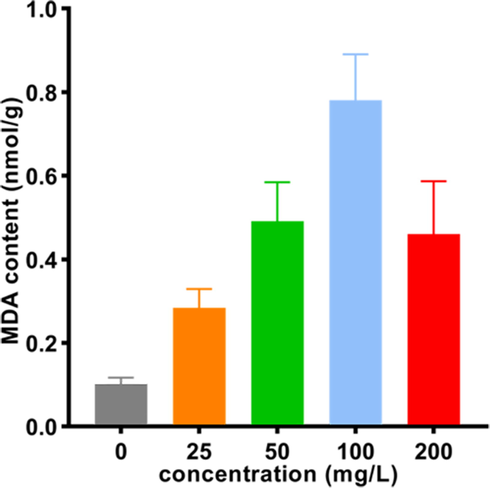
MDA contents of G. zeae treated with Y13.
The cells were treated for 24 h. The bars indicate the mean ± SD (n = 3), and one-way ANOVA was used for statistical analysis among different groups. A significant difference was observed between the different treatment groups (p < 0.05).
4 Conclusion
A series of novel 1-substituted-5-trifluoromethyl-1H-pyrazole-4-carboxamide derivatives were designed and synthesized. In vitro bioassay results indicated that some target compounds exhibited excellent and broad-spectrum antifungal activities. Among them, the EC50 values of Y13 against G. zeae, B. dothidea, F. prolifeatum and F. oxysporum were 13.1, 14.4, 13.3, and 21.4 mg/L, respectively. Y13 also displayed certain protective activity (50.65%) against G. zeae in vivo at 100 mg/L. SAR analysis revealed that the phenyl on the 1-position of the pyrazole ring was important for this activity. An antifungal mechanism study of Y13 against G. zeae demonstrated that this compound may disrupt the cell membrane of mycelium, thus inhibiting the growth of fungi. These findings of the mechanistic studies were inconsistent with the findings for traditional amides and may provide a novel view for deep study of this series of pyrazole carboxamide derivatives.
Acknowledgements
This work was supported by the National Natural Science Foundation of China (No. 32160655); and the Breeding Program of Guizhou University (No. 201931).
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
References
- New N, N, N’, N’-tetradentate pyrazoly agents: synthesis and evaluation of their antifungal and antibacterial activities. J. Med. Chem.. 2016;12(1):83-89.
- [Google Scholar]
- Biosynthesis of silver nanoparticles using Malva parviflora and their antifungal activity. Saudi J. Biol. Sci.. 2021;28(4):2229-2235.
- [Google Scholar]
- Amide derivatives of gallic acid: Design, synthesis and evaluation of inhibitory activities against in vitro α-synuclein aggregation. Bioorg. Med. Chem.. 2020;28(15):115596
- [Google Scholar]
- Synthesis, antifungal activity and QSAR of novel pyrazole amides as succinate dehydrogenase inhibitors. Heterocycles. 2018;96:74-85.
- [Google Scholar]
- A selectivity study of polysubstituted pyridinylimidazoles as dual inhibitors of JNK3 and p38α MAPK based on 3D-QSAR, molecular docking, and molecular dynamics simulation. Struct. Chem.. 2021;32(2):819-834.
- [Google Scholar]
- Synthesis and evaluation of the fungal activity of new pyrazole-carboxamides against Colletotrichum gloeosporioides. J. Braz. Chem. Soc.. 2020;31(9):1917-1925.
- [Google Scholar]
- Cinnamic acid increased the incidence of Fusarium wilt by increasing the pathogenicity of Fusarium oxysporum and reducing the physiological and biochemical resistance of faba bean, which was alleviated by intercropng with wheat. Front. Plant Sci.. 2020;11:608389
- [Google Scholar]
- Studies on the novel pyridine sulfide containing SDH based heterocyclic amide fungicide. Pest Manag. Sci.. 2020;76(7):2368-2378.
- [Google Scholar]
- Design, synthesis and antifungal evaluation of novel mandelic acid derivatives containing a 1,3,4-oxadiazothioether moiety. Chem. Biol. Drug Des.. 2021;98(1):166-174.
- [Google Scholar]
- Synthesis, biological evaluation, and molecular modeling studies of new oxadiazole-stilbene hybrids against phytopathogenic fungi. Sci. Rep.. 2016;6:31045.
- [Google Scholar]
- Synthesis, characterization, reaction mechanism prediction and biological study of mono, bis and tetrakis pyrazole derivatives against Fusarium oxysporum f. sp. Albedinis with conceptual DFT and ligand-protein docking studies. Bioorg. Chem.. 2021;110:104696
- [Google Scholar]
- Kaddouri, Y., Abrigach, F., Ouahhoud, S., Benabbes, R., Kodadi, M. E., Alsalme, A., Al-Zaqri, N., Warad, I., Touzani, R., 2020. Mono-Alkylated ligands based on pyrazole and triazole derivatives tested against Fusarium oxysporum f. sp. albedinis: Synthesis, characterization, DFT, and phytase binding site identification using blind docking/virtual screening for potent fophy inhibitors. Front. Chem. 1073.
- Structural understanding of quorum-sensing inhibitors by molecular modeling study in Pseudomonas aeruginosa. Appl. Microbiol. Biotechnol.. 2009;83(6):1095-1103.
- [Google Scholar]
- Development of inhibitors against TraR quorum-sensing system in Agrobacterium tumefaciens by molecular modeling of the ligand-receptor interaction. Mol. Cells. 2009;28(5):447-453.
- [Google Scholar]
- Functionalized, vertically super-aligned multiwalled carbon nanotubes for potential biomedical applications. Int. J. Mol. Sci.. 2020;21(7):2276.
- [Google Scholar]
- Study on inhibitory activity and mechanism of chitosan oligosaccharides on Aspergillus Flavus and Aspergillus Fumigatus. Carbohydr. Polym.. 2022;275:118673
- [Google Scholar]
- Regioselective [3 + 2]-annulation of hydrazonyl chlorides with 1,3-dicarbonyl compounds for assembling of polysubstituted pyrazoles. Org. Biomol. Chem.. 2018;16(42):7811-7814.
- [Google Scholar]
- Novel 4-pyrazole carboxamide derivatives containing flexible chain motif: design, synthesis and antifungal activity. Pest Manag. Sci.. 2019;75(11):2892-2900.
- [Google Scholar]
- Novel anthranilic amide derivatives bearing the chiral thioether and trifluoromethylpyridine: Synthesis and bioactivity. Bioorg. Med. Chem. Lett.. 2020;30(3):126902
- [Google Scholar]
- Isolation of antimicrobial genes from Oryza rufipogon griffby using a Bacillus subtilis expression system with potential antimicrobial activities. Int. J. Mol. Sci.. 2020;21(22):8722.
- [Google Scholar]
- Resistance to the plant defensin NaD1 features modifications to the cell wall and osmo-regulation pathways of yeast. Front. Microbiol.. 2018;9:1648.
- [Google Scholar]
- Naturally produced magnolol can significantly damage the plasma membrane of Rhizoctonia solani. Pestic. Biochem. Physiol.. 2021;178:104942
- [Google Scholar]
- Mycovirus-induced tenuazonic acid production in a rice blast fungus Magnaporthe oryzae. Front. Microbiol.. 2020;11:1641.
- [Google Scholar]
- Distinct transcriptional changes in response to patulin underlie toxin biosorption differences in Saccharomyces cerevisiae. Toxins (Basel).. 2019;11(7):400.
- [Google Scholar]
- Synthesis, antifungal activities and qualitative structure activity relationship of carabrone hydrazone derivatives as potential antifungal agents. Int. J. Mol. Sci.. 2014;15(3):4257-4272.
- [Google Scholar]
- Discovery of novel N-isoxazolinylphenyltriazinones as promising protoporphyrinogen IX oxidase inhibitors. J. Agric. Food Chem.. 2019;67(45):12382-12392.
- [Google Scholar]
- Expedient discovery for novel antifungal leads targeting succinate dehydrogenase: Pyrazole-4-formylhydrazide derivatives bearing a diphenyl ether fragment. J. Agric. Food Chem.. 2020;68(49):14426-14437.
- [Google Scholar]
- Novel fluorinated 7-hydroxycoumarin derivatives containing an oxime ether moiety: design, synthesis, crystal structure and biological evaluation. Molecules. 2021;26(2):372.
- [Google Scholar]
- Synthesis, biological evaluation, and 3D-QSAR studies of N-(Substituted pyridine-4-yl)-1-(substituted phenyl)-5-trifluoromethyl-1H-pyrazole-4-carboxamide derivatives as potential succinate dehydrogenase inhibitors. J. Agric. Food Chem.. 2021;69(4):1214-1223.
- [Google Scholar]
- Synthesis and biological activity of novel pyrazol-5-yl-benzamide derivatives as potential succinate dehydrogenase inhibitors. J. Agric. Food Chem.. 2021;69(20):5746-5754.
- [Google Scholar]
- In vitro antifungal activity of antifungalmycin 702, a new polyene macrolide antibiotic, against the rice blast fungus Magnaporthe grisea. Biotechnol. lett.. 2013;35(9):1475-1479.
- [Google Scholar]
- Structure-based discovery of potential fungicides as succinate ubiquinone oxidoreductase inhibitors. J. Agric. Food Chem.. 2017;65(5):1021-1029.
- [Google Scholar]
- Target-directed design, synthesis, antiviral activity, and SARs of 9-substituted phenanthroindolizidine alkaloid derivatives. J. Agric. Food Chem.. 2021;69(27):7565-7571.
- [Google Scholar]
- Design, synthesis, and antifungal activity of carboxamide derivatives possessing 1,2,3-triazole as potential succinate dehydrogenase inhibitors. Pestic. Biochem. Physiol.. 2019;156:160-169.
- [Google Scholar]
- Synthesis and biological activity of novel succinate dehydrogenase inhibitor derivatives as potent fungicide candidates. J. Agric. Food Chem.. 2019;67(47):13185-13194.
- [Google Scholar]
- In vitro and in vivo antifungal activity and preliminary mechanism of cembratrien-diols against Botrytis cinerea. Ind. Crops Prod.. 2020;154:112745
- [Google Scholar]
- Synthesis and biological evaluation of novel pyrazole carboxamide with diarylamine-modified scaffold as potent antifungal agents. Chin. Chem. Lett.. 2017;28(8):1731-1736.
- [Google Scholar]
- Discovery of N-(4-fluoro-2-(phenylamino)phenyl)-pyrazole-4-carboxamides as potential succinate dehydrogenase inhibitors. Pestic. Biochem. Physiol.. 2019;158:175-184.
- [Google Scholar]
- Quantitative proteomic analyses identify ABA-Related proteins and signal pathways in maize leaves under drought conditions. Front. Plant Sci.. 2016;7:1827.
- [Google Scholar]
- Mechanism of action of novel pyrazole carboxamide containing a diarylamine scaffold against Rhizoctonia solani. J. Agric. Food Chem.. 2020;68(40):11068-11076.
- [Google Scholar]
- Improving the fermentable sugar yields of wheat straw by high-temperature pre-hydrolysis with thermophilic enzymes of Malbranchea cinnamomea. Microb. Cell Fact.. 2020;19(1):149.
- [Google Scholar]
Appendix A
Supplementary material
Supplementary data to this article can be found online at https://doi.org/10.1016/j.arabjc.2022.103987.
Appendix A
Supplementary material
The following are the Supplementary data to this article:Supplementary data 1
Supplementary data 1







