Translate this page into:
Synthesis, biological activity and assembly of pH-responsive alkyl-substituted naphthalene-type hydrazonotriazole organogelators
⁎Corresponding authors. ffshaaban@uqu.edu.sa (Fathy Shaaban), n_elmetwaly00@yahoo.com (Nashwa M. El-Metwaly) nmmohamed@uqu.edu.sa (Nashwa M. El-Metwaly)
-
Received: ,
Accepted: ,
This article was originally published by Elsevier and was migrated to Scientific Scholar after the change of Publisher.
Peer review under responsibility of King Saud University. Production and hosting by Elsevier.
Abstract
Hydrazones have been a significant group of materials with diverse pharmacological and industrial applications, such as treatment of cardiovascular diseases, parasitic infections, and viral disorders, as well as cosmetic and food additives. In this context, we present the synthesis and characterization of novel hydrazonotriazole analogues with different hydrophobic alkoxy units. The synthesized alkyl-substituted naphthalene-type hydrazonotriazoles were able to act as pH-sensitive and thermoreversible organogelators. They were prepared by a straightforward aldol condensation of 2-naphthalaldehyde with different adducts of 1-(5-methyl-1-(4-alkoxyphenyl)-1H-1,2,3-triazol-4-yl)ethan-1-one at room temperature in ethanolic solution and in the presence of sodium hydroxide as a catalytic agent. The provided compounds were exposed to condensation reaction with (2,4-dinitrophenyl)hydrazine in a refluxing acid ethanolic solution. Different spectroscopic methods were utilized to analyze and prove the chemical structures of the naphthalene-type hydrazonotriazoles, inclusing FT-IR, elemental analysis, and NMR spectra. The photophysical properties of the prepared hydrazonotriazoles were reported. The alkyl-substituted naphthalene-type hydrazonotriazole gelators were able to gelate a variety of solvents, displaying a sol–gel reversible response to pH changes together with colorimetric change from yellow to purple. The optimal gelation was monitored for nonyl-substituted hydrazone in a variety of solvents, demonstrating thermal stability up to 58 °C, and critical gelator concentration (CGC) of ∼ 1–11 mM. Several analytical methods were used to inspect the morphological properties of the hydrazonotriazole-based organogelators, displaying self-assembled nanofibers (7–15 nm). Both cytotoxic and antimicrobial activity of the alkyloxy-containing naphthalene-type hydrazonotriazole gelators was investigated.
Keywords
Naphthalene-type hydrazone
Organogel
Hydrophobic alkyl units
Nanofibers
pH-Responsive
1 Introduction
Low molecular mass organogelators (LMMO) are intriguing materials because of their micro(nano)-structure diversity, chemical sensitivity and thermal reversibility (Khattab et al., 2016; Ren et al., 2020). LMMO have been widely used in many industries, including those related to food, cosmetics, and medicine (Feng et al., 2022; Ru et al., 2020; Zhang et al., 2022). The self-assembly of LMMO has the ability to form non-covalent physical textures with unique properties. It has been widely noted that LMMO has the unique capacity to gelate solvents via non-covalent bonding such as π-stacks, van der Waals forces and hydrogen bonds (Zeng et al., 2021). Intriguingly, current research and development in the area of LMMO have focused on creating smart organogels able to respond to external stimuli such as pH and heat (Zhao et al., 2018). Important implications for the development of functional nanomaterials have been reported to emerge from the self-assembly of LMMO to generate supramolecular nanostructures. Nanorods, nanoparticles, nanoribbons, and nanofibers are only few examples of those supramolecular designs (Katla et al., 2022). In order to create those assemblies, the intermolecular attraction forces have been crucial to rearrange LMMO into higher order structures without switching into the crystalline dense form. Smart LMMO have lately emerged as important materials because of their potential use in many different fields, including tissue engineering, pollutant elimination, drug delivery and pharmaceutics (Ni et al., 2019; Yu et al., 2021; Zhang et al., 2020). As a unique class of soft materials, low molecular mass (LMM) pH-responsive organogels can be identified as materials with sol–gel transitions that are tunable by adjusting the pH of those materials. This is useful for creating new types of soft materials that can encase and release biologically active molecules like vitamins. For these reason, research on pH-sensitive smart organogels has been a priority (Khattab 2018).
Chalcones have been presented as a key structural component in many strongly active biological agents. Chalcones exhibit a variety of pharmaceutical applications, such as anticancer, antidiabetic, antileishmanial, antituberculosis, anti-HIV, antihypertensive, antiviral, antimalarial, antimicrobial, anti-inflammatory, antiprotozoal, antifungal, antioxidants, and antiulcer (Jain et al., 2021; Karaca et al., 2022; Rammohan et al., 2020). Their nonlinear optical and photochemical characteristics have also been determined. Light emitting diodes, chemosensors, and organic fluorescent probes are only few examples of many applications reported for chalcones (Chen et al., 2020; Farooq et al., 2020; Mahapatra et al., 2019; Thapa et al., 2021). They have been used as raw materials for making a wide variety of heterocyclic compounds. As one of several chalcone derivatives, hydrazones are particularly important in the sensing sector because of their hydrazone-containing structure (El-Nagar et al., 2022; Montes-González et al., 2019; Sharmoukh et al., 2022). The commercial potential of chalcone-based hydrazones as dyestuffs, as well as antiviral, antibacterial, and anticancer agents has attracted a lot of interest (Chen et al., 2021; Farooq et al., 2021; Gupta et al., 2020; Liu et al., 2021; Vinaya et al., 2019). Hydrazones have also been put to use as sensors for a variety of analytes, including heavy metals, cyanides, fluorides, and poisonous gases. Recent publications have shown many prototypes of LMM organic gelator structures. However, the development of pH-sensitive LMMO has showed limited studies (Abdelrahman et al., 2021). Moreover, hydrazone-based LMMO have showed limited studies. Many cutting-edge optoelectronics innovations have recently been made possible by hydrazone derivatives of chalcones. Many different products have been shown to rely on hydrazones as electroluminescent and photoluminescent agents, fluorescent sensors, light-emitting devices, logic memories, optics and photorefractives, and lasers (Choi et al., 2016; Elsawy et al., 2022; Snari et al., 2022). Also, many bioactive components and pharmaceutical medicines include 1, 2, 3-triazoles, which are a notable heterocyclic molecule. Anticancer, antibacterial, antifungal, antimalarial, and cytotoxic activities have all been shown in 1, 2, 3-triazole analogues (Bozorov et al., 2019; Qurban et al., 2022). The electron-withdrawing triazole moiety provides a unique opportunity to form push–pull molecular structures in conjugation with electron-donating substituent. Therefore, the molecular assembly of hydrazone-based gelators will be crucial not only for the notion of building innovative pH-sensitive LMMO, but also for extending the area of soft materials toward attractive electronics and photonics functions (Apostolides et al., 2018; Qian et al., 2015). Long-chain alkyl groups were shown to drive the supramolecular assembles of the novel hydrazone analogues. The synthesis of functional organogels required fine-tuning the alkyl chain length (Alqarni et al., 2021). Herein, we detail the creation of pH-responsive low-molecular-weight organogelators based on alkyl-substituted naphthalene-type chalcone-based hydrazonotriazoles. By elongating the terminal alkyl tails, the gelation characteristics were fine-tuned. It was shown that the naphthalene-type chalcone-based hydrazone organogelators could effectively gelate a range of organic solvents. In response to a change in pH, they exhibited reversible responsiveness, changing color from yellow to purple during a sol–gel phase transition. The nonyloxy-containing hydrazone organogelator was shown to have the best gelation performance. Variations in the gel-sol phase transition in response to acid or alkaline vapors suggest a wide range of possible uses, from medication delivery to gas detection. The morphological properties of xerogels made by the air-drying of the respective organogel from n-butanol were explored by transmission electron microscopy (TEM) and scanning electron microscopy (SEM). Cooperating interaction forces of naphthalene-driven π-stacks with alkyl-driven van der Waals introduced gel in mixed and pure organic solvents, which are likely responsible for the self-assembly of those alkyl-substituted naphthalene-type chalcone-based hydrazonotriazole gelators.
2 Experimental
2.1 Materials
Commercially available materials and reagents from Sigma-Aldrich (Egypt) were used with no additional purification. Ammonium hydroxide (NH4OH; 30%), dimethylformamide (DMF; 99.99%), absolute ethanol (99.8%) and hydrochloric acid (ACS reagent; 37%) were provided from Frontier-Scientific and Merck (Egypt). Fluka and Aldrich supplied the spectroscopic grade solvents used in the present investigation. Reactions were watched in real time using a Merck Al-TLC plate covered with silica gel (PF-254). In order to use the plates, we put them under a UV light source (254/365 nm). Re-crystallization and flash column chromatography were used in the purification processes. The alkoxy-substituted triazole derivates 1a-e and 2a-e were prepared using previously established procedures (Mohamed et al., 2022; Qurban et al., 2022), and used as starting intermediates for the synthesis of the corresponding alkyl-substituted naphthalene-type hydrazonotriazole derivatives.
2.2 Synthetic methods
2.2.1 General synthesis of alkyl-substituted naphthalene-type hydrazonotriazole 3a-e
The corresponding compound 2a-e (6 mmol) was dissolved in absolute ethanol (50 mL). Both of 2,4-dinitrophenylhydrazine (6 mmol) and hydrochloric acid (1 mL) were added to the above prepared solution. The admixture was exposed to reflux for 4 h. After cooling, the generated precipitate was filtered under vacuum, washed with absolute ethanol and recrystallized from dimethylformamide.
2.2.2 Synthesis of methyl-substituted hydrazonotriazole 3a
Compound 3a was synthesized from compound 2a as yellow solid (87%); mp 226–228 °C; 1H NMR (400 MHz, DMSO‑d6): 2.65 (s, 3H), 7.26 (s, 1 H), 7.53 (d, 1 H, J = 8.0 Hz), 7.64 (d, 1 H, J = 8.0 Hz), 7.65–8.02 (m, 13H), 9.18 (s, 1 H), 11.71 and 12.57 (s, 1 H); 13C NMR (100 MHz, DMSO‑d6): 10.83, 116.95, 117.38, 117.50, 123.47, 124.26, 126.66, 127.29, 127.77, 128.25, 128.47, 128.54, 128.74, 128.79, 128.97, 129.30, 129.78, 132.45, 133.79, 135.78, 138.12, 138.41, 144.45, 145.34, 162.22, 164.19. IR (cm−1): 3345 (N–H), 3064 (aromatic C–H), 2938 (aliphatic C–H); Elemental Analysis: Calculated for C29H23N7O5 (549.54): C, 63.38; H, 4.22; N, 17.84; Found: C, 63.29; H, 4.15; N, 17.96.
2.2.3 Synthesis of propyl-substituted hydrazonotriazole 3b
Compound 3b was synthesized from compound 2b as yellow solid (85%); mp 236–238 °C; 1H NMR (400 MHz, DMSO‑d6): 2.53 (s, 3H), 7.48 (d, 1H, J = 8.0 Hz), 7.52 (s, 1H), 7.83 (d, 1H, J = 8.0 Hz), 7.93–8.16 (m, 12H), 8.84 (s, 1H), 11.78 and 12.57 (s, 1H); 13C NMR (100 MHz, DMSO‑d6): 10.86, 116.87, 116.99, 117.18, 117.34, 117.52, 124.28, 126.68, 127.31, 127.79, 128.27, 128.51, 128.71, 128.76, 128.99, 129.06, 129.32, 133.70, 133.81, 135.80, 136.43, 138.43, 144.47, 145.36, 162.24, 164.21. IR (cm−1): 3347 (N–H), 3060 (aromatic C–H), 2941 (aliphatic C–H); Elemental Analysis: Calculated for C31H27N7O5 (577.59): C, 64.46; H, 4.71; N, 16.98; Found: C, 64.40; H, 4.65; N, 17.04.
2.2.4 Synthesis of hexyl-substituted hydrazonotriazole 3c
Compound 3c was synthesized from compound 2c as yellow solid (83%); mp 244–246 °C; 1H NMR (400 MHz, DMSO‑d6): 2.49 (s, 3H), 7.44 (d, 1H, J = 8.7 Hz), 7.48 (s, 1H), 7.79 (d, 1H, J = 8.7 Hz), 7.89–8.12 (m, 12H), 8.80 (s, 1H), 11.74 and 12.53 (s, 1H); 13C NMR (100 MHz, DMSO‑d6): 10.80, 116.81, 116.90, 117.22, 117.57, 117.53, 124.20, 126.57, 127.39, 127.83, 128.25, 128.42, 128.70, 128.57, 128.07, 129.13, 129.47, 133.78, 133.86, 135.86, 136.72, 138.60, 144.76, 145.25, 162.35, 164.50. IR (cm−1): 3355 (N–H), 3069 (aromatic C–H), 2931 (aliphatic C–H); Elemental Analysis: Calculated for C34H33N7O5 (619.67): C, 65.90; H, 5.37; N, 15.82; Found: C, 65.79; H, 5.42; N, 15.68.
2.2.5 Synthesis of nonyl-substituted hydrazonotriazole 3d
Compound 3d was synthesized from compound 2d as yellow solid (81%); mp 251–253 °C; 1H NMR (400 MHz, DMSO‑d6): 2.59 (s, 3H), 7.54 (d, 1H, J = 8.7 Hz), 7.58 (s, 1H), 7.89 (d, 1H, J = 8.7 Hz), 7.99–8.22 (m, 12H), 8.90 (s, 1H), 11.84 and 12.63 (s, 1H); 13C NMR (400 MHz, DMSO‑d6): 10.75, 116.88, 116.79, 117.07, 117.35, 117.50, 124.30, 126.56, 127.35, 127.81, 128.32, 128.09, 128.68, 128.86, 128.90, 129.44, 129.38, 133.69, 133.77, 135.85, 136.39, 138.35, 144.40, 145.32, 162.09, 164.38. IR (cm−1): 3359 (N–H), 3073 (aromatic C–H), 2928 (aliphatic C–H); Elemental Analysis: Calculated for C37H39N7O5 (661.75): C, 67.15; H, 5.37; N, 14.82; Found: C, C, 67.23; H, 5.44; N, 14.89.
2.2.6 Synthesis of dodecyl-substituted hydrazonotriazole 3e
Compound 3e was synthesized from compound 2e as yellow solid (77%); mp 266–268 °C; 1H NMR (400 MHz, DMSO‑d6): 2.62 (s, 3H), 7.55 (d, 1H, J = 8.7 Hz), 7.55 (s, 1H), 7.71 (d, 1H, J = 8.7 Hz), 7.83–8.10 (m, 12H), 8.83 (s, 1H), 11.78 and 12.55 (s, 1H); 13C NMR (100 MHz, DMSO‑d6): 10.82, 116.94, 116.93, 117.50, 117.42, 117.60, 124.71, 126.51, 127.90, 127.77, 128.25, 128.56, 128.72, 128.82, 128.95, 129.07, 129.46, 133.73, 133.90, 135.82, 136.54, 138.32, 144.58, 145.75, 162.45, 164.30. IR (cm−1): 3366 (N–H), 3078 (aromatic C–H), 2925 (aliphatic C–H); Elemental Analysis: Calculated for C40H45N7O5 (619.67): C, 68.26; H, 6.44; N, 13.93; Found: C, 68.12; H, 6.61; N, 14.02.
2.3 Methods
The melting points of the naphthalene-type hydrazonotriazole derivatives and associated intermediates were measured using differential scanning calorimetry (DSC; TA 2920). Perkin-Elmer 2400 (Norwalk, United States) was utilized to explore the elemental analysis results of the synthesized hydrazonotrizole derivatives. FTIR-ATR Bruker Vectra-33 (Bruker, USA) spectrophotometer was utilized to collect FTIR spectra. Absorption spectra in the UV–Vis range were measured using HP-8453 (HEWLETT PACKARD) spectrophotometer. The BRUKER AVANCE 400 (Germany) was used to collect the NMR spectra at 400 MHz, and the chemical shifting (ppm) was reported comparative to tetrametylsilane (TMS) as standard reference at 25 °C. 1H NMR spectra were analyzed by increasing the concentration of compound 3d in DMSO‑d6. The addition of the appropriate amount of hydrazonotriazole 3d to a solution of DMSO‑d6 resulted in a rise in the concentration of compound 3d from 0.15 to 0.63 mM. The pH was measured using pHI340 with a calomel-glass electrode. The Hitachi S-2600 N SEM (Japan) was used to capture the 20 kV SEM micrographs. The hydrazonotrizole derivative 3d was dissolved in n-butanol to create an organogel, which was then put onto carbon tape adhered to a metal stub and dried in a desiccator placed under vacuum to produce the corresponding xerogel suitable for scanning electron microscopy analysis. The samples were dried, and annealed in an oven at 45 °C for 12 h before being coated with a 10 nm of gold layer. Jeol TEM 1200EX (Japan) was used for TEM study, and the voltage used was 80 kV. For TEM examination, Cu-grid (200 mesh) was deposited with the organogel of the hydrazonotrizole derivative 3d in n-butanol and dried for two hours under a vacuum.
2.4 pH sensory study
According to previously presented method (Al-Ahmed et al., 2022), trifluoroacetic acid (TFAA) miscible with methanol (1.5 × 10-2 M) was poured into a DMSO solution of compound 3d (1.25 × 106 M) in order to lower the pH value. The DMSO solution of compound 3d (1.25 × 106 M) was treated with tetrabutylammonium hydroxide (TBAH) miscible with methanol (1 M) to produce the corresponding hydrazone anion. All of the measurements were taken using a pH meter that employed a glass-calomel electrode. The Canon A710-IS camera was used to take photographs of organogel prepared from compound 3d (∼1 mM) dissolved in DMSO at a range of different pH values.
2.5 Gelation method
The gelation procedure was performed by boiling the hydrazonotrizole adduct 3d at 125 °C in n-butanol. It took between 15 and 25 min, depending on the gelator concentration, to produce the corresponding organogel when the hot sample was left to cool down to ambient temperature (25 °C). The melting point of the generated organogel was determined by inverting the glass vial containing the organogel in an oil bath according to the “stable to inversion” technique (Yang et al., 2018). The melting point of an organogel is said to be the temperature at which it breaks down in free fall. By performing the aforementioned steps multiple times and recording the melting point of the organogel after each cycle, we were able to report that the sol–gel phase transition is reversible with no fatigue.
2.6 Sensor examination
Tests were conducted on the produced hydrazonotriazole xerogel 3d generated by drying the respective gel from n-butanol for the detection of ammonia gas. The variations in the absorbance intensity at 594 nm after being exposed to ammonia and air were monitored to probe the reversibility test. High reversibility without fatigue was shown by reporting the absorbance intensity after each cycle of exposure to ammonia and air, and compared it to the first cycle.
2.7 Biological studies
Human skin fibroblast cell line (BJ1) were used in an in vitro proliferation MTT assay to determine the cytotoxic activity of the produced naphthalene-type hydrazonotriazoles (Khalil et al., 2019). Standard methods were used to determine the antimicrobial efficacy of the naphthalene-type hydrazonotriazole derivatives against E. coli (ATCC 25922) and S. aureus (ATCC 25923) (Qiao et al., 2021).
3 Results and discussion
3.1 Chemistry and characterization
To serve as thermally reversible pH-sensitive LMMO, a series of new alkyl-substituted naphthalene-type hydrazone analogues with various terminal alkoxy units was synthesized. The simple method utilized to synthesize the hydrazonotriazole chromophores is described in Scheme 1, where R represents different alkoxy chains. The derivatives of 1a-e and 2a-e were produced using previously established procedures (Qurban et al., 2022), and were used as starting materials for the synthesis of 3a-e. An aldol condensation of 2-naphthalaldehyde with different derivatives of 1-(5-methyl-1-(4-alkoxyphenyl)-1H-1,2,3-triazol-4-yl)ethan-1-one 1a-e was performed at room temperature in ethanolic solution and in the presence of sodium hydroxide as a catalytic agent to provide the corresponding 2a-e. Reacting compounds 2a-e with (2,4-dinitrophenyl)hydrazine in dry ethanol (100%) with some drops of HCl as a catalytic agent resulted in high yields of the appropriate alkoxy-substituted hydrazonotriazole chromophore. The synthesized naphthalene-type hydrazone analogues were structurally verified using elemental analysis, nuclear magnetic resonance and infrared spectroscopy. The 1H NMR spectra provided conclusive evidence that the hydrazone N–H group has a distinct signal at 11.35 ppm. FTIR spectra further showed that the hydrazone N–H group exhibited a clear absorption band at about 3318 cm−1.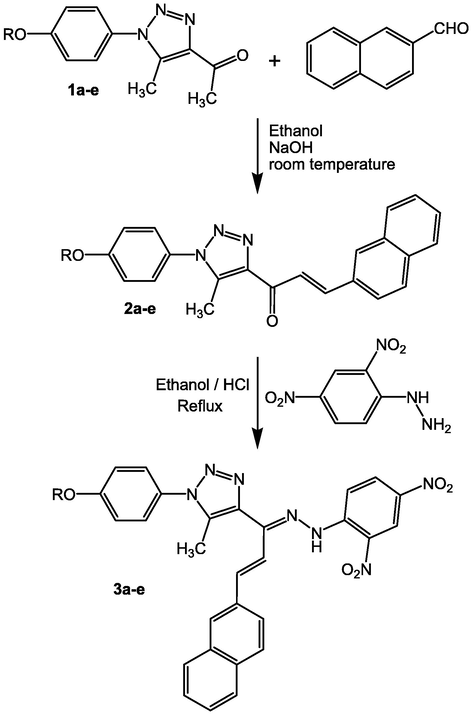
Synthesis of alkyl-substituted naphthalene-type chalcone-based hydrazonotriazoles 3a-e; R = H (3a), C3H7 (3b), C6H13 (3c), C9H19 (3d), C12H25 (3e).
3.2 Photophysical studies
The chromophores 3a-e of the hydrazonotriazole family are soluble in both polar and nonpolar solvents. Due to their similar molecular structures, the hydrazonotriazole derivatives 3a-e exhibited similar absorption characteristics in solutions of various organic solvents. The absorbance spectra of the naphthalene-type chalcone-based hydrazonotriazoles in various solvents are shown in Table 1. In various pure solvents, the hydrazonotriazole chromophores 3a-e display a halochromic switching from yellow to purple. As the polarity of the solvent increases, the principal absorption peak showed a bathochromic activity to indicate positive solvatochromism. This demonstrates that the charge transfer is attributed to the π-π* transitions, which is completely suitable for such positive solvatochromism. This is because the deprotonated hydrazone anion functions as a push–pull molecular system, whereby the negatively charged hydrazone anion isomer donates electrons to the electron-withdrawing triazole fragment (Al-Ahmed et al., 2022). The acidic N–H hydrazone in the dinitrophenyl group allows them to generate anions with a higher electron-donating capacity after the abstraction of a proton.
Solvent
λmax (nm)
3a
3b
3c
3d
3e
Ethanol
n-Butanol DMSO
THF
CH3CN
Ethylacetate447
453
448
452
456
450444
451
441
443
449
442439
441
438
434
440
431433
435
434
430
434
424424
423
429
424
430
420
3.3 pH-sensory study
The capacity to reversibly sense pH-induced molecular switching was established by adding an acid or a base to a solution of compound 3d in DMSO (1.25 × 106 M). Colorimetric changes of the hydrazonotriazole chromophore 3d are shown as reversible absorbance spectra in Fig. 1. Changes in colorimetry were tracked from yellow to reddish-purple and purple when the pH was varied from 5.5 to 7.5. When TBAH was added to a solution of compound 3d in DMSO, the maximum emission wavelength was shifted from 439 to 594 nm, and a new peak appeared at 594 nm. However, when TFAA was added, the absorbance band at 439 nm was once again visible. Reversible shift from 439 to 594 nm was achieved by alternating addition of TFAA and TBAH to a solution of hydrazonotriazole 3d dissolved DMSO.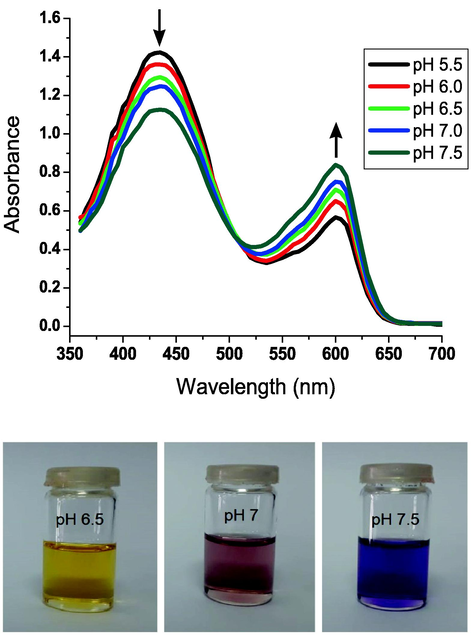
Ph-dependent absorption spectra (top), and colorimetric shifts (bottom) of the naphthalene-type chalcone-based hydrazone 3d in DMSO (1.24 × 10-6 M).
An isosbestic point was detected at 504 nm when TBAH was introduced to a solution of the naphthalene-type hydrazone 3d in dimethyl sulfoxide (Fig. 1). The maximum absorption wavelength moved to a longer wavelength (594 nm), indicating a bathochromic shifting, as a consequence of hydrazone anion formation. Therefore, the hydrazonotriazole anion exhibits a robust interaction of the lone-pair of electrons on the N–H hydrazone-nitrogen with both dinitrophenyl moity and triazole group. Thus, we may infer that the dinitrophenyl moiety interacts strongly with the lone-pair of electrons on the N–H hydrazone-nitrogen, as shown in Scheme 2.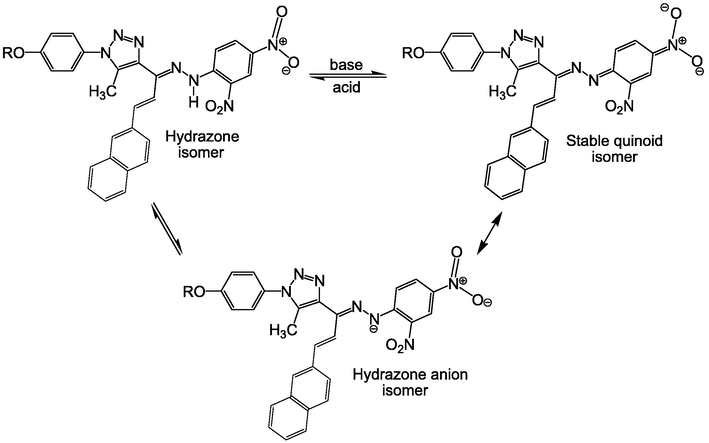
Ph-dependent remarkable effects on the molecular structure of the naphthalene-type hydrazonotriazoles.
3.4 Gelation studies
The gelation properties of the synthesized compounds 3a-e were investigated in different solvents using the “stable to inversion” method (Yang et al., 2018). Compounds 3a-b were tested in a variety of pure and mixed organic solvents, and it was found that they are unable to form organogels when subjected to the standard heating–cooling treatment. This is because they are insoluble in common organic solvents. As a result of its high solubility, compound 3e was incapable to immobilize solvents. Compounds 3c-d were very soluble in some solvents, like DMF and THF, reducing their ability to form organogels in those solvents. Because of their poor solubility in hexane, benzene and toluene, compounds 3c-d were unable to form organogels in those solvents. On the other hand, compounds 3b-d performed well as organogelators in pure alcohols. Compounds 3c and 3d were found to form organogels in mixes of solvents such as cyclohexane/DMSO (1/1) and cyclohexane/CHCl3 (3/1). The key results of the gelation activity are described in Table 2. The critical gel concentration (CGC) varied from ∼ 1 mM to ∼ 10 mM depending on the solvent used. Gels produced from 1,3-propanediol and n-butanol utilizing the hydrazonotriazole organogelators 3c and 3d were very stable for many weeks when stored at room temperature. However, the cyclohexane/CHCl3 based gel was only stable for a few days when stored at room temperature. These results highlight the importance of the alkyl unit length for the gelation performance. The hydrazonotriazole gelators 3c and 3d have showed improved solubility in a wide range of solvents due to increasing the alkyl chain length. However, the intermolecular affinity required for self-assembly into higher order supramolecular structures is likely reduced due to the intense solvation induced by the alkyl tail. The optimal tail length of the alkyl group is crucial in the production of a suitable organogelator, as shown by a comparison of the gelation activities of adducts 3a, 3b, and 3e. The gelation ability is indeed lost when the alkyl tail length is greatly increased, as in compound 3e due to the high solubility. In contrast, the gelation ability is lost in compounds where the alkyl chain length is drastically shortened, such as compounds 3a and 3b.
Solvent
3a
3b
3c
3d
3e
Ethanol
n-Butanol
n-Octanol
Benzyl alcohol
1,3-Dihydroxypropane
1,6-Dihydroxyhexane
Cyclohexane
Hexane
CHCl3/cyclohexane (1/3)DMSO/cyclohexane
(1/1)
THF
CHCl3
DMSO
Ethylacetate
CH3CN
DMF
Toluene
BenzeneSol
Sol
Ppt
Ppt
Ppt
Ppt
Ppt
Ppt
Ppt
Ppt
Sol
Ppt
Sol
Ppt
Ppt
Sol
Ppt
PptSol
Sol
Ppt
Ppt
Ppt
Ppt
Ppt
Sol
Sol
Sol
Sol
Ppt
Sol
Ppt
Ppt
Sol
Ppt
PptSol
Gel (2.59)
PGel
PGel
PGel
PGel
PGel
Ppt
Gel (3.56)
Gel (4.46)
Sol
Ppt
Gel (3.82)
PGel
PGel
Sol
Ppt
PptSol
Gel (1.02)
Gel (1.55)
PGel
Gel (3.65)
Gel (2.49)
PGel
Ppt
G (2.81)
G (2.37)
Sol
PGel
Gel (2.87)
Gel (9.99)
Gel (4.71)
Sol
Ppt
PptSol
Sol
Sol
Sol
Sol
Sol
Sol
Ppt
Sol
Sol
Sol
Sol
Sol
Sol
Sol
Sol
Ppt
Ppt
The hydrazone anion isomer was not effective in immobilizing organic solvents, but the neutral hydrazone was capable to create gels. It was found that the hydrazone anion isomeric forms of the hydrazonotriazole gelators 3c and 3d were unable to form gels in a wide range of organic solvents. If used in the hydrazone isomer form, stable organogels can be made in a variety of organic solvents. A simple test was run on compound 3d dissolved in n-butanol to show acid/base-dependent reversible transitions amid gel → sol → gel. An organogel of compound 3d (Fig. 2) was shown to collapse into sol state when exposed to gaseous ammonia, with a corresponding color change from yellow to purple owing to the creation of hydrazone anion. The organogel of compound 3d was irreproducible in the presence of ammonia despite heating and cooling the hydrazone gelator 3d in n-butanol. After being exposed to the vapor of hydrochloric acid and stirring for 5 min, the previously generated purple solution of the hydrazonotriazole anion form turned back to yellow solution of the hydrazonotriazole form. After heating and cooling the hydrazone yellow solution, the organogel can be reproduced again. The hydrazonotriazole 3d was unable to gelate solvents bellow a pH of 6. It is noteworthy that compound 3d forms a yellow solution in n-butanol below a pH of 7 and a purple solution at a pH value higher than 7. Based on these results, we infer that the nonyl-based hydrazone gel is quite stable in a pH range of 6–7. The organogel was found to entirely precipitate out into solid and solution phases for the pH values below 6 and above 7. The hydrazone N–H was found to dissociate to hydrazone anion at a pH greater than 7 to trigger the gel → sol transition. When the pH is between 6 and 7, the N–H remained protonated. This decreases the organogelator solubility, resulting in a higher tendency to form gel. However, reducing the pH value less than 6 results in higher solubility of the gelator, leading to organogel instability.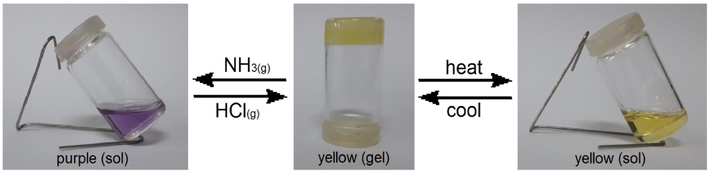
Reversible sol → gel → sol transitions displaying base instabilized and acid stabilized gel formation of compound 3d in n-butanol.
3.5 Morphological properties
It was shown that the nonyloxy unit on compound 3d optimally improved the gelator solubility in various organic solvents, facilitating its self-assembly through van der Waals forces, and ultimately led to formation of gel. The N–H signal in the concentration-reliant 1H NMR spectrum showed a clear downfield shift as the nonyl-substituted gelator concentration increased. These results suggest that H-bonding occurs between the N–H groups during gel formation. The = C–H group exhibited the same behavior, shifting downfield when the gelator concentration was raised. In addition, when the concentration of the gelator was raised, the peak of the aryl groups shifted slightly upfield, indicating that π-π stacks between the aromatic rings contributed to the gel stability. After the xerogel of compound 3d was entirely extracted of n-butanol by drying under vacuum, the morphology was studied by SEM and TEM. Fig. 3 shows SEM images of xerogel generated from 3c, revealing three-dimensional platelet sheets. Fig. 4 shows TEM images of xerogel generated from 3d, revealing three-dimensional nanofibrous entanglements with sizes between 7 and 15 nm. The produced nanofibrous intertwined network becomes swollen in the gelated solvent to form gel.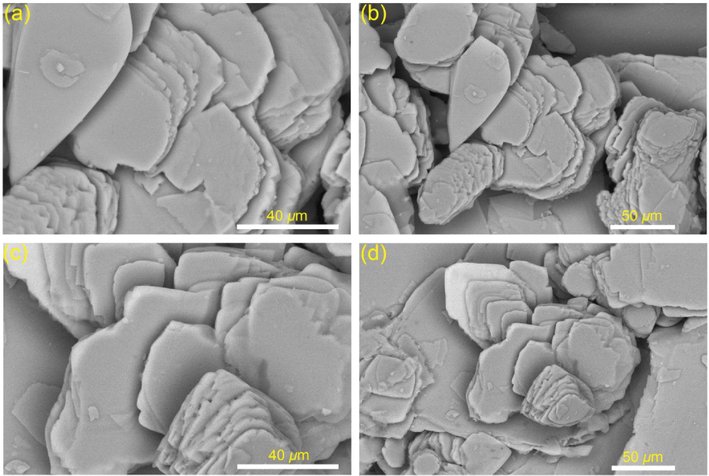
SEM images of xerogel of compound 3c extracted from the corresponding organogel in n-butanol (∼1 mM).
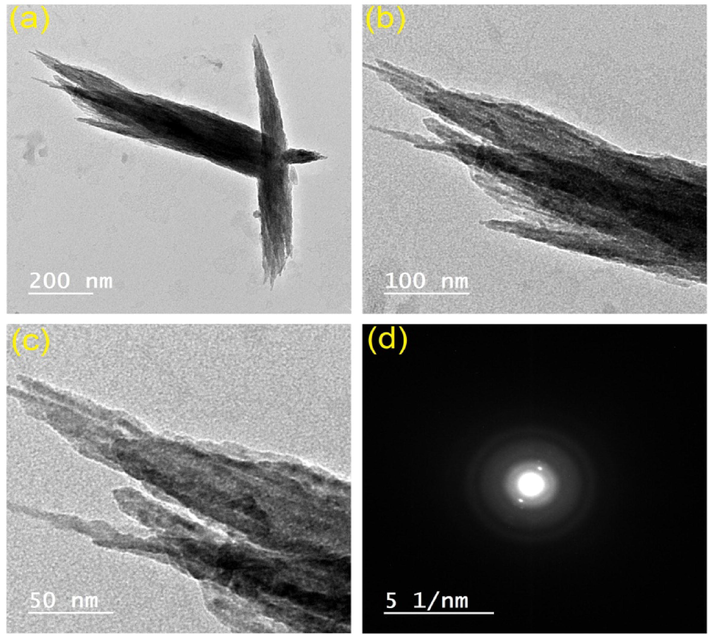
TEM (a–c), and electron diffraction of selected area (d) images of compound 3d extracted from the corresponding organogel in n-butanol (∼1 mM).
The dried xerogel film changed color from yellow to purple when exposed to alkaline vapor in an interestingly reversible manner. Since the large surface area of these pH-responsive nanofibers allows for easier diffusion of the gaseous analyte through the fibrous entanglements, they show increased sensitivity to alkaline analytes. As a result, the xerogel nanofibrous film displays remarkable sensitivity to ammonia gas. Ammonia was reported as a dangerous and colorless industrial gaseous agent with many different uses, such as refrigeration industry. The absorbance spectra of the hydrazone gelator 3d in n-butanol as a function of ammonia are shown in Fig. 5. Fig. 6 shows the excellent reversibility of the hydrazonotriazole xerogel 3d extracted from the corresponding organogel in n-butanol (∼1 mM) when exposed to both atmospheric air and ammonia gas.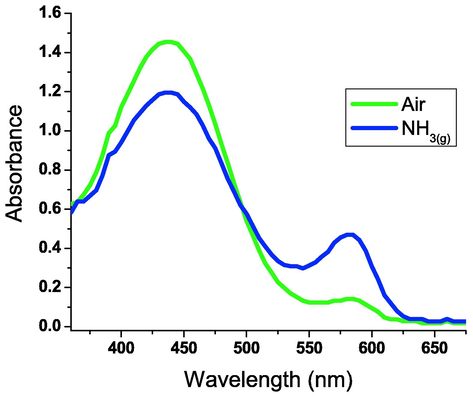
Absorption spectra of hydrazonotriazole xerogel 3d extracted from the corresponding organogel in n-butanol (∼1 mM) under exposure to atmospheric air (439 nm) and (NH3)g (594 nm).
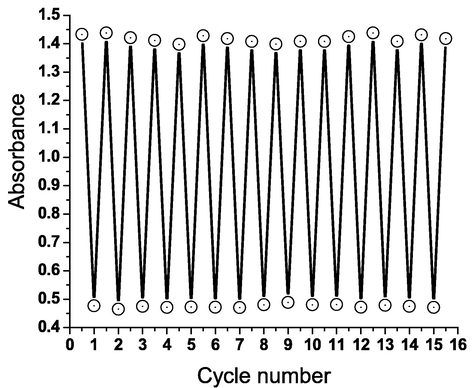
Variations in absorbance intensity of xerogel 3d extracted from the corresponding organogel in n-butanol (∼1 mM) under mutual exposure to atmospheric air (439 nm) and (NH3)g (594 nm).
3.6 Thermal properties
The thermal stability of the nonyl-based hydrazone-based gel in n-butanol was studied by reporting the concentration-reliant gel → sol melting point at a constant pH value (6.5), as shown in Fig. 7. Increasing the concentration of organogelator from ∼ 1 mM to ∼ 10 mM increased the gel → sol melting point from 43 to 58 °C. Nonetheless, increasing the gelator content over 10 mM lowered the organogel melting temperature. The gelator thermal stability decreases above a concentration of 10 mM. A higher number of the gelator molecules being integrated into the fibrous aggregations can account for the enhanced thermal stability. Therefore, the creation of extremely flexible one-dimensional nanofibrous aggregates that generate increased nanofibrous tangling is linked to an increase in the gel → sol melting point.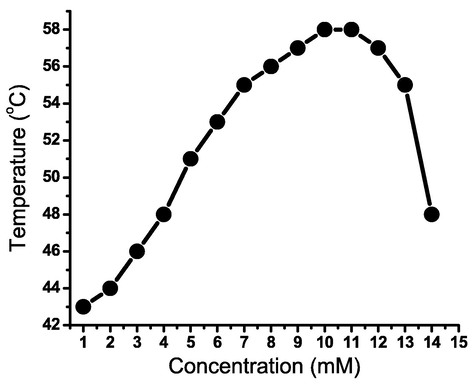
Organogel melting points of organogelator 3d in n-butanol (pH = 6.5).
The solid-like hydrazonotriazole organogel 3d was heated to its boiling temperature (125 °C for n-butanol) by placing the vial holding it upside down in a hot bath. Temperature was recorded when the organogel, which behaves like a solid, is collapsed. The “stable to inversion” technique verified that the solid-like gel could be reproduced by allowing the resulting solution to settle down at room temperature. Numerous cycles of the above procedures showed no change in the temperature of the gel → sol transition, proving strong reversibility (Fig. 8).
Temperature reversibility of gel → sol transition of organogel 3d.
3.7 Biological screening
The cytotoxicity test and antibacterial activities were studied to investigate the possible use of the synthesized alkoxy-containing naphthalene-type hydrazonotriazole gelators in biomedical applications (Althagafi et al., 2019; Ayoub et al., 2021; Haseeb et al., 2016; Haseeb et al., 2021; Khalil et al., 2019; Mohyuddin et al., 2022; Owayss et al., 2020; Qiao et al., 2021). By tracking BJ1 proliferation in the presence of the alkoxylated hydrazones, we conducted an in vitro cytotoxicity experiment. Hydrazonotriazole derivatives 3d and 3e with the longest alkoxy tails had no effect on the proliferation of BJ1 cells. However, hydrazonotriazole 3c showed a reduction in viability, with a viable cells value of 92.35%. The hydrazonotriazole analogues 3a and 3b with the shortest alkoxy units were found to have a viability of 88.07% and 84.37%, respectively. This relative drop in cell quantity is unrelated to cell death but may be attributable to the propensity of hydrazone precipitation leading to reduced spaces for cell development (Qurban et al., 2022). As a result, the current alkoxylated hydrazonotriazole derivatives can be reported as safe chemicals. The results of testing hydrazonotriazoles gelators 3a-e for antibacterial activity are shown in Table 3. Plate agar counting methods were used to examine the antibacterial activities of hydrazonotriazole gelators against E. coli and S. aureus, and the results showed that the gelators antimicrobial activity improved along with increasing the length of the alkyl unit. This can be attributed to increasing the hydrazone solubility with increasing the length of the alkyl unit. Thus, those alkoxylated hydrazonotriazole-based gelators have shown promise as noncytotoxic compounds for use in antibacterial and bioimaging applications.
Gelator
Reduction %
E. coli
S. aureus
3a
20 ± 1.4
17 ± 1.3
3b
25 ± 1.0
22 ± 1.2
3c
29 ± 1.7
25 ± 1.0
3d
35 ± 1.1
31 ± 1.0
3e
36 ± 1.0
33 ± 1.5
4 Conclusion
In summary, we describe the preparation of novel pH-responsive alkyl-substituted naphthalene-type hydrazonotriazole-based LMMO that can immobilize a wide range of solvents. The pH-sensitive LMMO dyes with an extended π-conjugation and a hydrazonotriazole moiety were synthesized and characterized. The hydrazone analogues were prepared in absolute ethanol utilizing HCl as a catalytic agent by a straightforward condensation between (2,4-dinitrophenyl)hydrazine and different adducts of alkyl-substituted triazoles. Both of photophysical and gelation properties of the prepared pH-sensitive hydrazone gelators were explored. The examination of the absorbance spectra revealed evidence of solvatochromism. The maximum absorption wavelength displayed a bathochromic shifted from 439 to 594 nm. Intriguingly, the hydrazonotriazole gelators were shown to undergo a protonation/deprotonation mechanism in response to changes in pH. It was found that the synthesized hydrazonotriazole gelators were acid stable (hydrazone form) and base unstable (hydrazone anion form). The resulting gels showed a very good thermal stability. Nanofibrous structures with diameters of 7 to 15 nm were monitored in the morphological investigations of the alkyl-substituted naphthalene-type hydrazonotriazole organogels. The current LMMO gelated solvents, indicating a novel category of gelators that can tuned depending on variations in pH. The nonyloxy-substituted hydrazonotriazole (CGC = ∼1–10 mM) gelled well in a wide range of solvents, and had desirable biological properties. It demonstrated remarkable self-assembly efficiency, forming stable nanofiber-like supramolecular structures by the aryl-assisted stacking molecules and alkoxy-supported van der Waals forces. The prepared organogels proved to be very stable in a wide range of alcohols, with the capability to amend the gelation behavior by altering the medium pH.
Acknowledgments
The authors extend their appreciation to the Deputyship for research & innovation, Ministry of Education in Saudi Arabia for funding this research work through the project number: IFP22UQU4361180DSR014.
Data availability
All relevant data are within the manuscript and available from the corresponding author upon request.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
References
- Hydrazone-Based Supramolecular Organogel for Selective Chromogenic Detection of Organophosphorus Nerve Agent Mimic. ChemistrySelect. 2021;6:2002-2009.
- [Google Scholar]
- Preparation of thermochromic and vapochromic cotton fibers finished with poly (N-vinylcaprolactam-co-hydrazone) Cellul.. 2022;29:8457-8472.
- [Google Scholar]
- Development of a Fluorescent Nanofibrous Template by In Situ SNAr Polymerization of Fluorine-Containing Terphenyls with Aliphatic Diols: Self-Assembly and Optical and Liquid Crystal Properties. ACS Omega. 2021;6:35030-35038.
- [Google Scholar]
- Novel Nano-sized bis-indoline Derivatives as Antitumor Agents. J. Heterocycl. Chem.. 2019;56:391-399.
- [Google Scholar]
- Dynamic covalent polymer hydrogels and organogels crosslinked through acylhydrazone bonds: Synthesis, characterization and applications. Polym. Int.. 2018;67:627-649.
- [Google Scholar]
- GC/MS Profiling and Ex Vivo Antibacterial Activity of Salvadora persica (Siwak) against Enterococcus faecalis as Intracanal Medicament. Evid.-based Complement. Altern. Med. 2021
- [Google Scholar]
- 1, 2, 3-Triazole-containing hybrids as leads in medicinal chemistry: A recent overview. Bioorg. Medic. Chem.. 2019;27:3511-3531.
- [Google Scholar]
- Novel D–π-A and A–π-D–π-A three-component photoinitiating systems based on carbazole/triphenylamino based chalcones and application in 3D and 4D printing. Polym. Chem.. 2020;11:6512-6528.
- [Google Scholar]
- Photopolymerization and 3D/4D applications using newly developed dyes: Search around the natural chalcone scaffold in photoinitiating systems. Dyes Pigm.. 2021;188:109213
- [Google Scholar]
- Unusual fluorescence and sol–gel transition properties of a pyridine-based polymeric gel formed via the hydrazone reaction. J. Mater. Chem. C. 2016;4:9646-9650.
- [Google Scholar]
- Preparation of novel reversible thermochromic polyethylenimine dendrimer and tricyanofuran hydrazone chromophore. Eur. Polym. J.. 2022;111344
- [Google Scholar]
- One pot and two pot synthetic strategies and biological applications of epoxy-chalcones. Chem. Africa. 2020;3:291-302.
- [Google Scholar]
- One-pot and two-pot methods for chalcone derived pyrimidines synthesis and applications. J. Heterocycl. Chem.. 2021;58:1209-1224.
- [Google Scholar]
- Photo-crosslinkable and ultrastable poly (1, 4-butadiene) based organogel with record-high reversible elongation upon cooling and contraction upon heating. Polymer. 2022;262:125477
- [Google Scholar]
- Development of chalcone-based derivatives for sensing applications. Anal. Methods. 2020;12:5022-5045.
- [Google Scholar]
- Antimicrobial resistance among pilgrims: a retrospective study from two hospitals in Makkah, Saudi Arabia. Int. J. Infect. Dis.. 2016;47(2016):92-94.
- [Google Scholar]
- Antimicrobial Usage and Resistance in Makkah Region Hospitals: A Regional Point Prevalence Survey of Public Hospitals. Int. J. Environ. Res. Public Health. 2021;19:254.
- [Google Scholar]
- Nanocatalysts: Applications in synthesis of chalcones–a review. Synth. Commun.. 2021;51:1-12.
- [Google Scholar]
- The metal sensing applications of chalcones: The synthesis, characterization and theoretical calculations. J. Mol. Struct.. 2022;1248:131454
- [Google Scholar]
- Small Molecule Organogels from AIE Active α-Cyanostilbenes. Handbook of Aggregation-Induced Emission 2022:255-276.
- [Google Scholar]
- Biocompatibility enhancement of graphene oxide-silver nanocomposite by functionalisation with polyvinylpyrrolidone. IET Nanobiotechnol.. 2019;13:816-823.
- [Google Scholar]
- Synthesis and Self-assembly of Novel s-Tetrazine-based Gelator. Helv. Chim. Acta. 2018;101:e1800009.
- [Google Scholar]
- pH triggered smart organogel from DCDHF-Hydrazone molecular switch. Dyes Pigm.. 2016;130:327-336.
- [Google Scholar]
- Design of photoinitiating systems based on the chalcone-anthracene scaffold for LED cationic photopolymerization and application in 3D printing. Eur. Polym. J.. 2021;147:110300
- [Google Scholar]
- Perspectives of medicinally privileged chalcone based metal coordination compounds for biomedical applications. Eur. J. Medic. Chem.. 2019;174:142-158.
- [Google Scholar]
- Novel allyl-hydrazones including 2, 4-dinitrophenyl and 1, 2, 3-triazole moieties as optical sensor for ammonia and chromium ions in water. BMC Chem.. 2022;16:26.
- [Google Scholar]
- Comparative Insights into the Antimicrobial, Antioxidant, and Nutritional Potential of the Solanum nigrum Complex. Processes. 2022;10:1455.
- [Google Scholar]
- Perspectives of ferrocenyl chalcones: synthetic scaffolds toward biomedical and materials science applications. Pure Appl. Chem.. 2019;91:653-669.
- [Google Scholar]
- Supramolecular liquid-crystalline polymer organogel: Fabrication, multiresponsiveness, and holographic switching properties. Chem. Mater.. 2019;31:3388-3394.
- [Google Scholar]
- In vitro antimicrobial activities of Saudi honeys originating from Ziziphus spina-christi L. and Acacia gerrardii Benth. trees. Food Sci. Nutr.. 2020;8:390-401.
- [Google Scholar]
- An emissive and pH switchable hydrazone-based hydrogel. Chem. Commun.. 2015;51:11158-11161.
- [Google Scholar]
- Purification, characterization, and mode of action of a novel bacteriocin BM173 from Lactobacillus crustorum MN047 and its effect on biofilm formation of Escherichia coli and Staphylococcus aureus. J. Dairy Sci.. 2021;104:1474-1483.
- [Google Scholar]
- Synthesis and self-assembly of novel pH triggered smart organogelators from novel alkyl-substituted chalcone-based hydrazonotriazole derivatives. J. Mol. Liq.. 2022;364:120005
- [Google Scholar]
- Chalcone synthesis, properties and medicinal applications: a review. Environ. Chem. Lett.. 2020;18:433-458.
- [Google Scholar]
- A tri-responsive and fast self-healing organogel with stretchability based on multiple dynamic covalent bonds. New J. Chem.. 2020;44:1609-1614.
- [Google Scholar]
- Reversibly thermosecreting organogels with switchable lubrication and anti-icing performance. Angew. Chem.. 2020;132:11974-11978.
- [Google Scholar]
- Development of novel reversible thermometer from N-isopropylacrylamide and dicyanodihydrofuran hydrazone probe. Polym. Eng. Sci.. 2022;62:3820-3830.
- [Google Scholar]
- Chalcone and its analogs: Therapeutic and diagnostic applications in Alzheimer’s disease. Bioorg. Chem.. 2021;108:104681
- [Google Scholar]
- Synthesis, growth and characterization of a long-chain π-conjugation based methoxy chalcone derivative single crystal; a third order nonlinear optical material for optical limiting applications. Opt. Mater.. 2019;89:419-429.
- [Google Scholar]
- A novel aggregation-induced-emission-active supramolecular organogel for the detection of volatile acid vapors. New J. Chem.. 2018;42:17524-17532.
- [Google Scholar]
- Preparation and Sensing Application of Fluorescent Organogels and Hydrogels. Supramolecular Gels: Materials and Emerging Applications; 2021. p. :21-50.
- Recent advances of organogels: From fabrications and functions to applications. Prog. Org. Coat.. 2021;159:106417
- [Google Scholar]
- A Pillar [5] arene-Based Smart Organogel with Effective Iodine Adsorption. Macromol. Chem. Phys. 2022
- [Google Scholar]
- A redox-responsive organogel based on a selenium-containing low molecular mass gelator. New J. Chem.. 2020;44:24-28.
- [Google Scholar]
- Macroscopic layered organogel–hydrogel hybrids with controllable wetting and swelling performance. Adv. Funct. Mater.. 2018;28:1800793.
- [Google Scholar]







