Translate this page into:
Synthesis, characterization, and 3D-molecular modeling and analysis of some copper(II) chelates in O, N-donor coordination pattern involving Schiff bases derived from 4-butyryl-3-methyl-1-phenyl-2-pyrazolin-5-one and some sulfa drugs
*Corresponding author. Tel.: +91 761 2601303; fax: +91 761 2603752 rcmaurya1@gmail.com (R.C. Maurya)
-
Received: ,
Accepted: ,
This article was originally published by Elsevier and was migrated to Scientific Scholar after the change of Publisher.
Peer review under responsibility of King Saud University.

Available online 21 January 2011
Abstract
The synthesis of five new chelates of copper(II) of the general formula[Cu(LH)2(Cl)2], where LH = N-(4′-butyrylidine-3′-methyl-1′-phenyl-2′-pyrazolin-5′-one)sulfamethoxazole (bumphp-smzH, I), N-(4′-butyrylidine-3′-methyl-1′-phenyl-2′-pyrazolin-5′-one)sulfadimidine (bumphp-sdmH, II), N-(4′-butyrylidine-3′-methyl-1′-phenyl-2′-pyrazolin-5′-one)sulfanilamide (bumphp-snmH, III), N-(4′-butyrylidine-3′-methyl-1′-phenyl-2′-pyrazolin-5′-one)sulfamoxole (bumphp-smlH, IV) or N-(4′-butyrylidine-3′-methyl-1’-phenyl-2′-pyrazolin-5′-one)sulfaguanidine (bumphp-sgdH, V) has been carried out. The complexes have been characterized by elemental analyses, copper determination, molar conductance, magnetic and decomposition temperature measurements, electron spin resonance, thermogravimetry, infrared, and electronic spectral studies. A trans octahedral structure has been proposed for these complexes. The 3D molecular modeling and analysis for bond lengths and bond angles have also been carried out for one of the representative compound,[Cu(bumphp-snmH)2(Cl)2] (3) to substantiate the proposed structure.
Keywords
Copper(II) chelates
Sulfa drug based ligands
Medicinal relevance
3D Molecular modeling
1 Introduction
4-Acyl-3-methyl-1-phenyl-2-pyrazolin-5-ones belong to a family of heterocyclic β-diketones, and are comparable to β-diketones because in both classes keto-enol tautomerism is possible. Such ligands have played and continue to play a part in the development of coordination compounds that have found a wide application in several fields (Marchetti et al., 2005), from new materials to catalysts, as precursors for CVD in the microelectronic industry and as potential antitumorals. The 5-pyrazolone derivatives have been extensively investigated due to their wide range of pharmacological activities (Alaudeen et al., 2003).
Sulfonamide derivatives exhibit a range of bioactivities, including anti-angiogenic (Funahashi et al., 2002; Semba et al., 2004), anti-tumor (Semba et al., 2004; Sławínski and Gdaniec, 2005), anti-inflammatory and anti-analgesic (Chen et al., 2005), anti-tubercular (Gadad et al., 2004), anti-glaucoma (Agrawal et al., 2004), anti-HIV (Yeung et al., 2005), cytotoxic (Enćıo et al., 2005), anti-microbial (Nieta et al., 2005) and anti-malarial (Doḿınguez et al., 2005) agents. Sulfonamide derivatives are also known to exhibit a wide variety of pharmacological activities (Yoshino et al., 1992; Toth et al., 1997; Medina et al., 1999) through exchanges of different functional groups without modification of the structural –S(O)2N(H)– feature. The synthesis of metal sulfonamide compounds had received much attention due to the fact that sulfanilamides were the first effective chemotherapeutic agents to be employed for the prevention and cure of bacterial infections in humans (Mohamed and Gad-Elkareem, 2007). The pharmacological activity of these types of molecules is often enhanced by complexation with metal ions (Bult and Sigel, 1983; Casanova et al., 1983). Moreover, some metal complexes of these ligands have been found to promote rapid healing of burns in humans and animals (De Oliveira et al., 2008). The effectiveness of burn treatment seems to depend not only on the presence of the metal ion but also crucially on the nature of the material to which the metal ion is bound (Baenziger et al., 1983).
Within the last decade there has been an upsurge of interest in metal ion therapeutics for both diagnosis and treatment (Schwietert and McCue, 1999; Deschamps et al., 2005). For example Cu(II)-l-histidine (Sarkar, 1999) has been used in the treatment of Menkes disease (Danks et al., 1995; Weder et al., 2002). Such interest has been due to the biochemical and pharmacological properties of the metal-ligand system, with extensive research carried out to determine the role of the ligand in copper uptake into cells. On the other hand many research papers (Dillion et al., 2003; Zvimba and Jackson, 2007) that have indicated the effective role of various copper chelating agents in the alleviation of inflammation associated with rheumatoid arthritis (RA) have also indicated the physiological importance of these agents as well as their therapeutic applications.
The number of people suffering from diabetes mellitus (DM) has been showing an annual increase. The patients with type I DM require daily insulin injections, which is both a physical and mental burden. Furthermore, DM leads to serious life-threatening complications causing severe damage to several organs such as, heart, eyes, kidneys, blood vessels, nerves, gums, teeth, feet, and legs (Marshall, 2004; Ritz and Haxsen, 2005; Moore et al., 1999). Thus, there is an urgent need to establish a treatment regime that can replace painful insulin injections. Since 1980 many researchers have attempted to identify alternative anti-diabetic compounds and have reported that metal ions such as vanadium (Shechter and Karlish, 1980; Sakurai et al., 2002), zinc (Coulston and Dandona, 1980), manganese (Fonteles et al., 2000), copper (Sorenson and Prog, 1989), chromium (Anderson et al., 1997), and tungsten (Munoz et al., 2001) exhibit in vitro insulinomimetic activity and in vivo anti-diabetic ability in experimental animals. In a recent report, a copper(II) chelate in O, N-donor coordination environment involving picolinic acid has been reported as a potent diabetic agent (Yasumatsu et al., 2007).
Previous reports from our laboratory describe the synthesis and characterization of mononuclear ruthenium(II) chelates with some Schiff base ligands derived from sulfa drugs and 4-benzoyl-3-methyl-1-phenyl-2-pyrazolin-5-one (Maurya et al., 1994). Synthesis, magnetic, and spectral studies of some novel binuclear dioxomolybdenum(VI) chelates involving Schiff bases derived from sulfa drugs and 4-benzoyl-3-methyl-1-phenyl-2-pyrazolin-5-one have been recently reported by Maurya et al. (2004). Four new oxovanadium(IV) complexes, formed by the interaction of vanadyl sulfate pentahydrate and the Schiff bases derived from 3-methyl-1-phenyl-4-valeryl-2-pyrazolin-5-one and sulfa drugs viz., N-(3′-methyl-1′-phenyl-4′-valerylidene-2′-pyrazolin-5′-one)sulfadiazine (L1H), N-(3′-methyl-1′-phenyl-4′-valerylidene-2′-pyrazolin-5′-one (L2H), N-(3′-methyl-1′-phenyl-4′-valerylidene-2′-pyrazolin-5′-one)sulfanilamide (L3H), and N-(3′-methyl-1′-phenyl-4′-valerylidene-2′-pyrazolin-5′-one)sulfamethoxazole (L4H) in aqueous ethanol are described by Maurya and Rajput (2006). Some new mixed-ligand ternary complexes of Cu(II), Ni(II), Co(II), Zn(II), Sm(III), Th(IV), and U(VI)O2 with the Schiff base derived from salicylaldehyde and the sulfa drug sulfabenzamide,[N-(salicylidene)-sulfabenzamide] (LH) and the heterocyclic base 1,10-phenanthroline (phen) have been reported by Maurya et al. (2007). Four new dioxomolybdenum(VI) chelates of the general composition,[MoO2(L)2(H2O)3], where LH = N-(o-vanillidene)sulfadiazine (vsdzH), N-(o-vanillidene)sulfanilamide (vsnmH), N-(o-vanillidene)sulfaguanidine (vsgnH), N-(o-vanillidene)sulfamerazine (vsmrH) were recently reported by Maurya et al. (in press, 2015).
In view of the multiple importance of copper(II) complexes mentioned above, it was, therefore, thought worthwhile to synthesize and characterize some copper(II) complexes with Schiff bases derived from 4-butyryl-3-methyl-1-phenyl-2-pyrazolin-5-one and sulfa drugs, viz., N-(4′-butyrylidine-3′-methyl-1′-phenyl-2′-pyrazolin-5′-one)sulfamethoxazole (bumphp-smzH, I), N-(4′-butyrylidine-3′-methyl-1′-phenyl-2′-pyrazolin-5′-one)sulfadimidine (bumphp-sdmH, II), N-(4′-butyrylidine-3′-methyl-1′-phenyl-2′-pyrazolin-5′-one)sulfanilamide (bumphp-snmH, III), N-(4′-butyrylidine-3′-methyl-1′-phenyl-2′-pyrazolin-5′-one)sulfamoxole (bumphp-sml, IV) or N-(4′-butyrylidine-3′-methyl-1′-phenyl-2′-pyrazolin-5′-one)sulfaguanidine (bumphp-sgd, V) (Fig. 1), not reported hitherto. The results of our studies in this investigation are presented in this paper.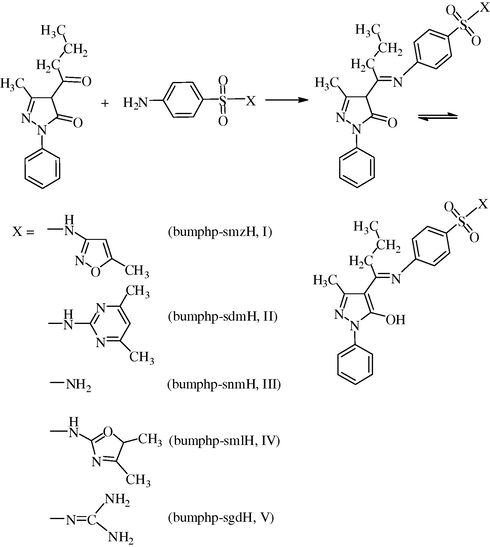
Reaction showing synthesis of Schiff base ligands.
2 Experimental
2.1 Materials
3-Methyl-1-phenyl-2-pyrazolin-5-one was the product of Johnson Chemical Co., Mumbai. The sulfa drugs, viz., sulfamethoxazole, sulfadimidine, sulfanilamide, sulfamoxole and sulfaguanidine were products of Sigma Chemical Co., USA. Copper(II) chloride dihydrate was the product of B.D.H. Chemicals, Mumbai, while butyryl chloride was purchased from Aldrich Chemical Company, USA. All other chemicals used were of analytical reagent grade. 4-Butyryl-3-methyl-1-phenyl-2-pyrazolin-5-one (bumphp) was prepared by the method reported elsewhere (Jensen, 1959).
2.2 Synthesis of sulfa drug Schiff bases
The Schiff bases with sulfa drugs were prepared as follows: an ethanolic solution (15 mL) of bumphp (2.44 g, 0.01 mol) was added to the solution of sulphamethoxazol (2.53 g, 0.01 mol) in ethanol, or sulfadimidine (2.78 g, 0.01 mol) in ethanol, or sulfanilamide (1.72 g, 0.01 mol) in acetone or sulfamoxole (2.67 g, 0.01 mol) in ethanol or sulfaguanidine (2.14 g, 0.01 mol) in ethanol along with two drops of HCl. The resulting solution was refluxed with stirring for 5–6 h and then filtered to remove the insoluble sulfa drug, if any. The filtrate so obtained was concentrated on a water bath and left overnight at room temperature when yellow crystals of Schiff bases separated out from their respective solutions. The crystals thus obtained were washed with ethanol and dried in vacuo.
2.3 Synthesis of complexes
All the complexes were prepared by the general method, namely, the salt cupric chloride dihydrate (0.001 mol, 0.170 g) was dissolved in ethanol (10 mL) by heating and the resulting solution was added to a warmed, stirred solution of the corresponding Schiff base, bumphp-smzH (0.002 mol, 0.958 g), bumphp-sdmH (0.002 mol, 1.8 g), bumphp-snmH (0.002 mol, 0.796 g), bumphp-smxH (0.002 mol, 0.986 g), or bumphp-sgdH (0.002 mol, 0.880 g) in 15 mL of ethanol. The resulting solution was refluxed for 4–5 h when the desired compound separated out as a fine precipitate keeping the reaction mixture overnight. It was filtered by suction, washed several times with ethanol and then dried in vacuo over anhydrous calcium chloride.
2.4 Analysis
Microanalysis of carbon, hydrogen, and nitrogen of the complexes was performed on a Carlo Erba 1108 Heracus elemental analyser at CDRI, Lucknow. Copper was estimated as copper salicyladoximate (Vogel, 1962).
2.5 Physical methods
The solid-state infrared spectra were recorded in KBr pellets with a Perkin Elmer FT-IR spectrophotometer at Central Drug Research Institute, Lucknow. Magnetic measurements were carried out at room temperature on vibrating sample magnetometer at RSIC, Indian Institute of Technology, Chennai. Electronic spectra of the complexes were recorded on an ATI Unicam, UV-2-100 UV–Visible Spectrophotometer in our Department. Conductance measurements were performed at room temperature in dimethylformamide using a Toshniwal conductivity bridge and a dip type cell with a smooth platinum electrode of cell constant 1.02. The decomposition temperatures of the complexes were recorded by an electrically operated melting point apparatus, Kumar Industries, Mumbai, with a heating capacity up to 360 °C. The X-Band EPR spectra of the complexes were measured on a Bruker ESP X-band EPR spectrometer using powdered samples at the Regional Sophisticated Instrumentation Centre, Indian institute of Technology, Chennai at the microwave frequency of 9.45 GHz.
2.6 Molecular modeling studies
The 3D molecular modeling of a representative compound was carried out on ChemBio3D Ultra Molecular Modeling and Analysis Program (www.cambridgesoft.com). It is an interactive graphics program that allows rapid structure building, geometry optimization with minimum energy and molecular display. It has the ability to handle transition metal compounds.
3 Results and discussion
The Copper(II) complexes of sulpha drug Schiff bases were prepared according to the following reaction. where LH = bumphp-smzH (1), bumphp-sdmH, (2), bumphp-snmH (3), bumphp-smxH (4) or bumphp-sgdH (5).
The synthesized complexes are colored (Table 2), non-hygroscopic and air stable solids. They are soluble in dimethylformamide and insoluble in ethanol and methanol. The resulting complexes were characterized using the following physical studies.
3.1 Infrared spectral studies
The sulfa drug based Schiff base ligands used in the present investigation were synthesized by the scheme as shown in Fig. 1. The formation of the Schiff base ligands is consistent with the microanalytical data of the ligands. The C, H, and N data, percentage yields, and important infrared spectral bands are given in Table 1. The formation of the Schiff base ligands is supported by the appearance of a strong band at 1625–1629 cm−1 due to ν(C⚌N) (azomethine) in the IR spectra of these ligands. All of the Schiff base ligands in the present investigation exhibit a broad band centered at 3328–3440 cm−1. This suggests the involvement of the 5-OH group in the intramolecular hydrogen (Maurya et al., 1997) bonding with the lone pair of azomethine nitrogen. It also suggests that the ligands exist in enol form in the solid state (Maurya et al., 1996).
Sr. No.
Schiff base (empirical formula) (formula weight)
Found (Calcd), %
Yield (%)
ν(C⚌O)
ν(C⚌N) (azomethine)
ν(N–H)
νas(S⚌O2)
νs(S⚌O2)
C
H
N
(I)
Bumphp-smzH
60.25
5.14
14.38
55
1660 s
1608 s
3420 br
1382 m
1158 m
(C24H25N5O4S) (479)
(60.13)
(5.22)
(14.61)
(II)
Bumphp-sdmH
61.68
5.68
16.36
50
1665 s
1605 s
3430 br
1382 m
1160 m
(C26H28N6O3S) (504)
(61.90)
(5.56)
(16.67)
3335
(III)
Bumphp-snmH
60.15
5.48
4.19
50
1670 s
1609 s
3425 br
1381 m
1187 m
(C20H22N4O3S (398)
(60.30)
(5.53)
(14.07)
(IV)
Bumphp-smxH
60.63
5.40
14.08
55
1652 s
1607 s
3410 br
1380 m
1183 m
(C25H27N5O4S) (493)
(60.85)
(5.48)
(14.20)
(V)
Bumphp-sgdH
57.42
5.27
19.22
52
1670 s
1610 s
3430 br
1369 m
1179 m
(C21H24N6O3S) (440)
(57.27)
(5.45)
(19.09)
3342
Sr. No.
Compound (empirical formula) (formula weight)
Found (Calcd), %
Color
Yield (%)
Decom. Temp (°C)
C
H
N
Cu
(1)
[Cu(bumphp-smzH)2(Cl)2]
52.45
4.22
12.53
5.43
Pastel green
53
275
(C48H50Cl2N10O8S2Cu) (1092.54)
(52.72)
(4.58)
(12.81)
(5.82)
(2)
[Cu(bumphp-sdmH)2(Cl)2]
54.43
4.90
14.49
5.83
Venetian green
55
270
(C52H56Cl2N12O6S2Cu) (1142.54)
(54.62)
(4.90)
(14.70)
(5.56)
(3)
[Cu(bumphp-snmH)2(Cl)2]
51.35
4.62
12.23
6.53
Lime
52
285
(C40H44Cl2N8O6S2Cu) (930.54)
(51.58)
(4.73)
(12.04)
(6.83)
(4)
[Cu(bumphp-smxH)2(Cl)2]
53.45
4.42
12.73
5.39
Lime
50
295
(C50H52Cl2N10O8S2Cu) (1120.54)
(53.55)
(4.82)
(12.49)
(5.67)
(5)
[Cu(bumphp-sgd)2(Cl)2]
49.45
4.62
16.33
6.73
Lime
54
280
(C42H48Cl2N12O6S2Cu) (1012.54)
(49.68)
(4.73)
(16.56)
(6.26)
The important infrared spectral bands of the complexes along with their plausible assignments are given in Table 3. All the Schiff base ligands used in this investigation exist in enol form as discussed above. Hence, they possess six potential donor sites: (i) the enolic oxygen, (ii) the cyclic nitrogen N1 pyrazolone moiety, (iii) the cyclic Nitrogen N2 pyrazolone moiety, (iv) the azomethine nitrogen, (v) sulfonamide (SO2NH) oxygen or nitrogen and (vi) the ring nitrogen of the sulfa drug.
Sr. No.
Compound
ν(C⚌O)
ν(C⚌N)
ν(Cu–O)
ν(Cu–N)
Λm (Ohm−1 cm2 mol−1)
μeff (BM)
(1)
[Cu(bumphp-smzH)2(Cl)2]
1607 vs
1577 s
510 w
427 w
7.2
1.81
(2)
[Cu(bumphp-sdmH)2(Cl)2]
1607 vs
1580 s
510 w
416 w
10.2
1.84
(3)
[Cu(bumphp-snmH)2(Cl)2]
1607 vs
1582 s
512 w
489 w
8.6
1.82
(4)
[Cu(bumphp-smxH)2(Cl)2]
1609 vs
1578 s
510 w
419 w
9.5
1.85
(5)
[Cu(bumphp-sgd)2(Cl)2]
1623 vs
1582 s
557 w
490 w
10.8
1.86
The appearance of a band at 1385–1390 cm−1 in the ligands is assigned (Maurya et al., 2007) to νas(O⚌S⚌O). This band remains almost at the same position in the complexes and hence suggests that the sulfonamide oxygen is not taking part in coordination with the metal centre.
The ν(NH) mode(s) of the sulfonamide group/amino group of the free Schiff bases, which is/are observed at 3310–3362 and 3216 cm−1, remain(s) unperturbed in the spectra of their complexes. This suggests that the sulfonamide/amino nitrogen is not taking part in coordination.
All the ligands display a sharp and strong band due to ν(C⚌N) of the azomethine group at 1625–1629 cm−1. The observed low energy shift of this band appearing in the range 1607–1613 cm−1 in the infra red spectra of the complexes suggests the coordination of azomethine nitrogen (Maurya et al., 2007) to the metal centre. The coordination of enolic oxygen of the pyrazolone moiety of the ligands after deprotonation is most likely for the formation of six membered chelate ring including azomethine nitrogen and the metal centre in question, and this should be reflected by the disappearance of the ν(OH) mode of the ligands centered at 3328–3440 cm−1. However, the appearance of ν(OH) mode in all the complexes in the same region suggests the coordination of the enolic oxygen without deprotonation. One of the of the possibilities of the presence of ν(OH) mode in the complexes may be due to lattice or coordinated water in them, but this is ruled out by TG analysis of the complexes wherein no weight loss was observed up to ∼200 °C. The coordination of enolic oxygen without deprotonation has already been observed by us (Maurya et al., 2003a) in a mixed-ligand cyanonitrosyl complexes of molybdenum(II) of the composition,[MoII(NO)(CN)3(BMPHP)], where BMPHP = 4-benzoyl-3-methyl-1-phenyl-2-pyrazolin-5-one, a carbonyl compound similar to 4-butyryl-3-methyl-1-phenyl-2-pyrazolin-5-one, which is one of the component of the Schiff base ligands used in the present studies. The overall IR data conclude that the sulfa Schiff bases behave as neutral bidentate (N, O) ligands.
The ν(C⚌N2) (cyclic) mode arising from the pyrazolone moiety of the ligands appears at 1580–1582 cm−1, and it does not show any appreciable change in its position in the complexes (see Table 3). Similarly, the ν(C⚌N) (cyclic) mode due to the sulpha drug skeleton of all the ligands appearing at ∼1600 cm−1 remains unchanged after complexation, and seems to be merged with ν(C⚌N) (azomethine). These observations indicate the non-participation of the ring nitrogen N2 of the pyrazolone moiety and the cyclic nitrogen of the sulfa drug skeleton of the ligands in bonding. Considering the planarity of the ligands, the coordination of the ring nitrogen N2 is also unlikely due to being in the backside of the effective donor sites, (i) and (iv) discussed above forming six membered chelate ring including the metal. Such a result has already been reported (Maurya and Rajput, 2006) by us. Likewise, the non-participation of the cyclic nitrogen of the sulfa drug skeleton in bonding may be attributed due to being too far from the suitable donor sites, (i) and (iv) and the central metal. The coordination of the ring nitrogen N1 is also unlikely due to a steric demand of the bulky phenyl group attached with it.
A single peak for the ν(Cu–Cl) should appear at ∼300 cm−1 in the infrared spectra of all the complexes. Unfortunately, this peak could not be assigned due to recording of the spectra up to 500 cm−1. The two non-ligand bands occurring at 510–557 and 416–490 have been assigned (Maurya et al., 2008) to ν(Cu–O) and ν(Cu–N), respectively. The IR spectrum of a Schiff base ligand, bumphp-snmH (III) and its complex, (3), are given in Figs. 2 and 3, respectively.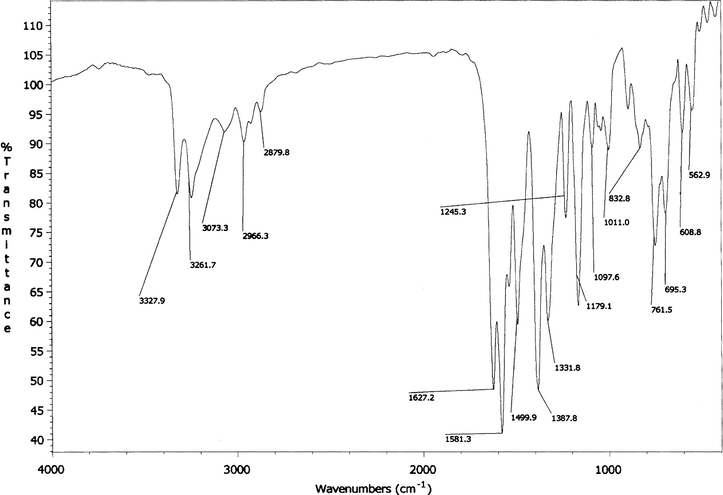
IR spectrum of bumphp-snmH (III).
![IR spectrum of[Cu(bumphp-snm)2(Cl)2] (3).](/content/184/2015/8/2/img/10.1016_j.arabjc.2011.01.015-fig3.png)
IR spectrum of[Cu(bumphp-snm)2(Cl)2] (3).
3.2 Electron spin resonance spectra
The ESR spectra of polycrystalline compounds, namely,[Cu(bumphp-smzH)2(Cl)2] (1) and[Cu(bumphp-sdmH)2(Cl)2] (2) were recorded at room temperature at the microwave frequency of 9.45 GHz. Both parallel and perpendicular features of 63Cu are resolved in both the spectra, which are characteristic of axial symmetry. Nuclear hyperfine coupling is observed in the
region with one component clearly resolved. Thus, it appears that the second, third, and fourth components are obscured by the broad
component (Garcio-Lozano et al., 1994). The
,
, and gav values for these complexes were calculated as 2.074/2.079, 2.174/2.190, and 2.107/2.116, respectively. The trend
(2.174/2.190) >
(2.074/2.079) > ge (2.0036, free ion value) observed in these two complexes shows that the unpaired electron is in the
orbital of Cu(II). The
and
values deviating considerably from the free ion value are close to those reported for a number of distorted copper(II) complexes (Maurya et al., 2003b). Moreover, the observed g values less than 2.3, suggest the covalent (Neiman and Kivelson, 1961) nature of metal-ligand bonds in these complexes. The ESR spectra of compound (1) and (2) are given in Fig. 4a and b, respectively.![ESR spectrum of ESR spectrum of[Cu(bumphp-smzH)2(Cl)2] (1) (a)[Cu(bumphp-sdmH)2(Cl)2] (2) (b).](/content/184/2015/8/2/img/10.1016_j.arabjc.2011.01.015-fig4.png)
ESR spectrum of ESR spectrum of[Cu(bumphp-smzH)2(Cl)2] (1) (a)[Cu(bumphp-sdmH)2(Cl)2] (2) (b).
3.3 Electronic spectra
The electronic spectra of all these compounds were recorded in 10−3 molar dimethylformamide solutions in the range 200–800 nm. The λmax of electronic spectral peaks and respective molar extinction coefficients along with their tentative assignments are given in Table 4. The high intensity peak(s) in all the complexes at 288–326 nm is/are assigned as intra-ligand transition(s). Normally, octahedral copper(II) complexes exhibit a broad band around 714–740 nm described by the transition 2T2g ← 2Eg. However, due to distortion caused by weak axial ligands, the broad bands split distinctly into two or three bands. Three spin-allowed transitions are theoretically possible and are attributed to 2A1g ← 2B1g, 2B2g ← 2B1g, and 2Eg ← 2B1g. But when the difference between 2A1g and 2B1g is small, the transition 2A1g ← 2B1g could remain unobserved. Based on the energy level diagram (Sathyanarayana, 2001) reported elsewhere, and also considering the low intensity of the remaining one/two d–d transition(s) in the visible region at 400–441 and 680–710 nm is/are assigned to the transitions 2Eg ← 2B1g and 2B2g ← 2B1g, respectively. However, the band at 400–441 nm in these complexes may also be considered as the metal-ligand charge transfer transition as reported elsewhere in case of the axially distorted octahedral (Jaskova et al., 2007) copper(II) complexes. The electronic spectrum of compound (1) is given in Fig. 5.
Compound No.
Compound
λmax (nm)
ε (L cm−1 mol−1)
Peak assignment
(1)
[Cu(bumphp-smzH)2(Cl)2]
291
3116
Charge transfer transition
441
109
2Eg ← 2B1g
(2)
[Cu(bumphp-sdmH)2(Cl)2]
288
3173
Charge transfer transition
296
3176
Charge transfer transition
420
142
2Eg ← 2B1g
700
71
2B2g ← 2B1g
(3)
[Cu(bumphp-snmH)2(Cl)2]
288
3215
Charge transfer transition
307
3230
Charge transfer transition
325
3120
Charge transfer transition
410
135
2Eg ← 2B1g
700
65
2B2g ← 2B1g
(4)
[Cu(bumphp-smxH)2(Cl)2]
288
3161
Charge transfer transition
400
150
2Eg ← 2B1g
680
62
2B2g ← 2B1g
(5)
[Cu(bumphp-sgd)2(Cl)2]
291
3310
Charge transfer transition
326
3595
Charge transfer transition
430
180
2Eg ← 2B1g
710
70
2B2g ← 2B1g
![Electronic spectrum of[Cu(bumphp-smzH)2(Cl)2] (1).](/content/184/2015/8/2/img/10.1016_j.arabjc.2011.01.015-fig5.png)
Electronic spectrum of[Cu(bumphp-smzH)2(Cl)2] (1).
3.4 Thermogravimetric studies
Thermogravimetric curves of two compounds[Cu(bumphp-smzH)2(Cl)2](1) (Fig. 6) and[Cu(bumphp-smxH)2(Cl)2] (4) (Fig. 7) were recorded in the temperature range from room temperature to 1300 °C. Both compounds are stable up to ∼200 °C. This excludes the possibility of lattice as well as coordinated water molecule(s) in these two complexes. The compound (4) shows a first weight loss of 43.03% at around 300 °C and this corresponds to the elimination of one molecule of sulfa Schiff base ligand (calcd 43.99%). The second weight loss of 86.5% observed around 600 °C in this compound corresponds to the elimination of second molecule of the sulfa Schiff base ligand (calcd 87.9%). Compound (1) shows weight losses in two steps and the total observed weight loss of 84% around 750 °C roughly corresponds to the elimination of two molecules of the sulfa Schiff base ligand of this complex (calcd 87.68%). Thus, the overall the thermogravimetric results are consistent with the formulation of these complexes along with the conclusion derived from infrared spectral studies (vide supra).![TG curve of[Cu(bumphp-smzH)2(Cl)2] (1).](/content/184/2015/8/2/img/10.1016_j.arabjc.2011.01.015-fig6.png)
TG curve of[Cu(bumphp-smzH)2(Cl)2] (1).
![TG curve of[Cu(bumphp-smxH)2(Cl)2] (4).](/content/184/2015/8/2/img/10.1016_j.arabjc.2011.01.015-fig7.png)
TG curve of[Cu(bumphp-smxH)2(Cl)2] (4).
3.5 Magnetic susceptibility
The copper(II) has d9 electronic configuration and its complexes contain one unpaired electron in the d-shell. A majority of complexes are formed by the involvement of d-orbital and they are square planar or distorted octahedral. The John Teller distortions contribute a major role in the distortion of the geometry of the complexes. The formation of tetrahedral complexes of copper(II), with no involvement of d-orbital is also reported to be formed. When the Cu(II) complexes display magnetic moment values equivalent to one unpaired electron, the complexes are referred to as magnetically dilute. In such complexes the paramagnetic metal centers are situated apart and the metal ions are surrounded by the ligand molecules in such a way that unpaired spins of the neighboring metal ions remain unaffected. In case of tetrahedral or distorted octahedral Cu(II) complexes the room temperature magnetic moment values are usually observed in the range of 1.8–2.2 BM, which are not affected appreciably by temperature and magnetic field. In case of planar dimeric or polynuclear species, the complexes display subnormal magnetic moments. In practice, compounds whose geometry approaches an octahedral geometry usually exhibit magnetic moments at the lower end, while those approaching a tetrahedral geometry are at the higher end. In the present investigations complexes exhibit magnetic moment 1.81–186 BM. These data indicate the octahedral (Maurya et al., 2003) geometry for these compounds.
3.6 3D Molecular modeling and analysis
In view of the hexa-coordination of the present complexes (vide infra), and also taking into account of the well established hexa-coordinate octahedral structure of[Cu(en)2(H2O)2]2+ cation (Jaskova et al., 2007) ([having neutral bidentate (N, N-donor) “en” ligands similar to neutral (O, N)-donor sulfa drug based Schiff base ligands in the present investigation], wherein four nitrogen atoms of the ethylenediamine (en) form the coordination plane around the copper(II) atom with the oxygen atoms of the two water molecules lying in the axial positions, the molecular modeling of a representative compound,[Cu(bumphp-snmH)2(Cl)2] (3) with two Cl groups at the axial position trans to each other and the two (O, N)-donor sulfa drug based Schiff base ligands at the equatorial positions in cis-arrangement to each other, is based on its octahedral structure. The details of bond lengths and bond angles as per the 3D structure (Fig. 8) are given in Tables 5 and 6, respectively. For convenience of looking over the different bond lengths and bond angles, the various atoms of the compound in question are numbered in Arabic numerals. In all, 323 measurements of the bond lengths (114 in numbers), plus the bond angles (209 in numbers) are listed. Except few cases, optimal values of both the bond lengths and the bond angles are given in the tables along with the actual ones. The actual (calculated) bond lengths/bond angles given in tables are obtained as a result of energy optimization in CHEM 3D Ultra, while the optimal bond length/optimal bond angle values are the most desirable/favorable (standard) bond lengths/bond angles established by the builder unit of the CHEM 3D. The missing of some values of optimal bond lengths/bond angles may be due to the limitations of the software, which we had already noticed in modeling of other systems (Maurya and Rajput, 2007; Maurya et al., 2007, 2008). In most of the cases, the calculated bond lengths and bond angles are close to the optimal values, and thus the proposed structure of the compound (3) as well as of the others are acceptable (Maurya and Rajput, 2007; Maurya et al., 2007, 2008, 2011).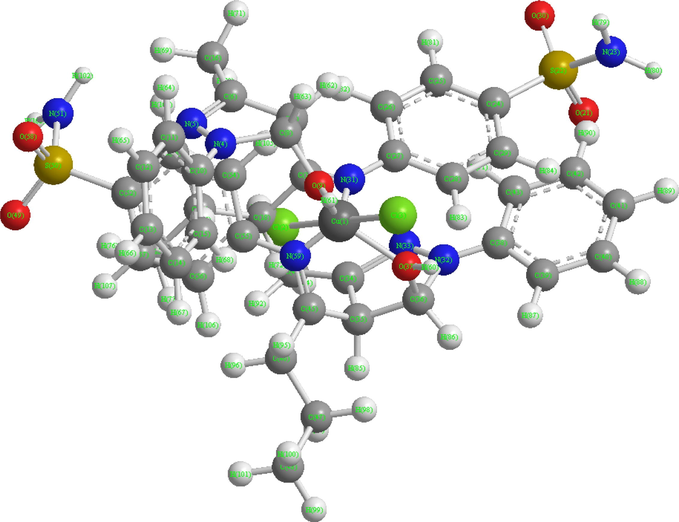
3D Structure of compound (3).
Sr. No.
Atoms
Actual bond length
Optimal bond length
Sr. No.
Atoms
Actual bond length
Optimal bond length
1
C(57)–H(107)
1.1
1.1
58
N(31)–C(27)
1.26
1.456
2
C(56)–H(106)
1.1
1.1
59
C(27)–C(28)
1.3948
1.42
3
C(56)–C(57)
1.3949
1.42
60
C(26)–H(82)
1.1
1.1
4
N(59)–C(55)
1.26
1.456
61
C(26)–C(27)
1.3948
1.42
5
C(55)–C(56)
1.3948
1.42
62
C(25)–H(81)
1.1
1.1
6
C(54)–H(105)
1.1
1.1
63
C(25)–C(26)
1.3949
1.42
7
C(54)–C(55)
1.3948
1.42
64
C(24)–C(29)
1.3948
1.42
8
C(53)–H(104)
1.1
1.1
65
C(24)–C(25)
1.3948
1.42
9
C(53)–C(54)
1.3949
1.42
66
N(23)–H(80)
1.02
1.02
10
C(52)–C(57)
1.3948
1.42
67
N(23)–H(79)
1.02
1.02
11
C(52)–C(53)
1.3948
1.42
68
S(22)–O(30)
1.45
1.45
12
N(51)–H(103)
1.02
1.02
69
S(22)–C(24)
1.79
–
13
N(51)–H(102)
1.02
1.02
70
S(22)–N(23)
1.696
–
14
S(50)–O(58)
1.45
1.45
71
O(21)–S(22)
1.45
1.45
15
S(50)–C(52)
1.79
–
72
C(20)–H(78)
1.113
1.113
16
S(50)–N(51)
1.696
–
73
C(20)–H(77)
1.113
1.113
17
O(49)–S(50)
1.45
1.45
74
C(20)–H(76)
1.113
1.113
18
C(48)–H(101)
1.113
1.113
75
C(19)–H(75)
1.113
1.113
19
C(48)–H(100)
1.113
1.113
76
C(19)–H(74)
1.113
1.113
20
C(48)–H(99)
1.113
1.113
77
C(19)–C(20)
1.523
1.523
21
C(47)–H(98)
1.113
1.113
78
C(18)–H(73)
1.113
1.113
22
C(47)–H(97)
1.113
1.113
79
C(18)–H(72)
1.113
1.113
23
C(47)–C(48)
1.523
1.523
80
C(18)–C(19)
1.523
1.523
24
C(46)–H(96)
1.113
1.113
81
C(17)–N(31)
0.961
1.26
25
C(46)–H(95)
1.113
1.113
82
C(17)–C(18)
1.497
1.497
26
C(46)–C(47)
1.523
1.523
83
C(16)–H(71)
1.113
1.113
27
C(45)–N(59)
1.2185
1.26
84
C(16)–H(70)
1.113
1.113
28
C(45)–C(46)
1.497
1.497
85
C(16)–H(69)
1.113
1.113
29
C(44)–H(94)
1.113
1.113
86
C(15)–H(68)
1.1
1.1
30
C(44)–H(93)
1.113
1.113
87
C(14)–H(67)
1.1
1.1
31
C(44)–H(92)
1.113
1.113
88
C(14)–C(15)
1.3949
1.42
32
C(43)–H(91)
1.1
1.1
89
C(13)–H(66)
1.1
1.1
33
C(42)–H(90)
1.1
1.1
90
C(13)–C(14)
1.3948
1.42
34
C(42)–C(43)
1.3949
1.42
91
C(12)–H(65)
1.1
1.1
35
C(41)–H(89)
1.1
1.1
92
C(12)–C(13)
1.3948
1.42
36
C(41)–C(42)
1.3948
1.42
93
C(11)–H(64)
1.1
1.1
37
C(40)–H(88)
1.1
1.1
94
C(11)–C(12)
1.3949
1.42
38
C(40)–C(41)
1.3948
1.42
95
C(10)–C(15)
1.3948
1.42
39
C(39)–H(87)
1.1
1.1
96
C(10)–C(11)
1.3948
1.42
40
C(39)–C(40)
1.3949
1.42
97
O(9)–H(61)
0.942
0.961
41
C(38)–C(43)
1.3948
1.42
98
C(8)–H(63)
1.113
1.111
42
C(38)–C(39)
1.3948
1.42
99
C(8)–O(9)
1.402
1.41
43
O(37)–H(60)
0.942
0.961
100
C(7)–H(62)
1.113
1.113
44
C(36)–H(86)
1.113
1.111
101
C(7)–C(17)
1.497
1.497
45
C(36)–O(37)
1.402
1.41
102
C(7)–C(8)
1.523
1.514
46
C(35)–H(85)
1.113
1.113
103
C(6)–C(16)
1.497
1.497
47
C(35)–C(45)
1.497
1.497
104
C(6)–C(7)
1.497
1.497
48
C(35)–C(36)
1.523
1.514
105
N(5)–C(6)
1.5306
1.26
49
C(34)–C(44)
1.497
1.497
106
N(4)–C(10)
1.266
1.462
50
C(34)–C(35)
1.497
1.497
107
N(4)–C(8)
1.47
1.47
51
N(33)––C(34)
1.4515
1.26
108
N(4)–N(5)
1.23
1.426
52
N(32)–C(38)
1.266
1.462
109
O(37)–Cu(1)
1.81
–
53
N(32)–C(36)
1.47
1.47
110
O(9)–Cu(1)
1.81
–
54
N(32)–N(33)
1.23
1.426
111
N(59)–Cu(1)
1.303
1.303
55
C(29)–H(84)
1.1
1.1
112
N(31)–Cu(1)
1.303
1.303
56
C(28)–H(83)
1.1
1.1
113
Cu(1)–Cl(3)
2.16
–
57
C(28)–C(29)
1.3949
1.42
114
Cu(1)–Cl(2)
2.16
–
Sr. No.
Atoms
Actual bond angles
Optimal bond angles
Sr. No.
Atoms
Actual bond angles
Optimal bond angles
1
H(103)–N(51)–H(102)
119.9999
104.5
106
C(46)–C(45)–C(35)
123.8202
117.2
2
H(103)–N(51)–S(50)
119.9998
–
107
H(84)–C(29)–C(28)
119.9994
120
3
H(102)–N(51)–S(50)
120.0003
–
108
H(84)–C(29)–C(24)
120.0008
120
4
H(101)–C(48)–H(100)
109.5199
109
109
C(28)–C(29)–C(24)
119.9998
–
5
H(101)–C(48)–H(99)
109.4617
109
110
O(30)–S(22)–C(24)
109.5198
–
6
H(101)–C(48)–C(47)
109.4621
110
111
O(30)–S(22)–N(23)
109.4419
–
7
H(100)–C(48)–H(99)
109.4416
109
112
O(30)–S(22)–O(21)
109.442
116.6
8
H(100)–C(48)–C(47)
109.4421
110
113
C(24)–S(22)–N(23)
109.4617
–
9
H(99)–C(48)–C(47)
109.5
110
114
C(24)–S(22)–O(21)
109.462
–
10
H(98)–C(47)–H(97)
109.52
109.4
115
N(23)–S(22)–O(21)
109.4999
–
11
H(98)–C(47)–C(48)
109.4621
109.41
116
C(29)–C(24)–C(25)
120.003
120
12
H(98)–C(47)–C(46)
109.4616
109.41
117
C(29)–C(24)–S(22)
119.9984
–
13
H(97)–C(47)–C(48)
109.4421
109.41
118
C(25)–C(24)–S(22)
119.9986
–
14
H(97)–C(47)–C(46)
109.4417
109.41
119
H(81)–C(25)–C(26)
120.0013
120
15
C(48)–C(47)–C(46)
109.4999
109.5
120
H(81)–C(25)–C(24)
120.0016
120
16
H(80)–N(23)–H(79)
119.9998
104.5
121
C(26)–C(25)–C(24)
119.9971
–
17
H(80)–N(23)–S(22)
120.0004
–
122
H(83)–C(28)–C(29)
120.0016
120
18
H(79)–N(23)–S(22)
119.9997
–
123
H(83)–C(28)–C(27)
120.0016
120
19
H(78)–C(20)–H(77)
109.5202
109
124
C(29)–C(28)–C(27)
119.9968
–
20
H(78)–C(20)–H(76)
109.462
109
125
H(82)–C(26)–C(27)
119.999
120
21
H(78)–C(20)–C(19)
109.4612
110
126
H(82)–C(26)–C(25)
119.9999
120
22
H(77)–C(20)–H(76)
109.4424
109
127
C(27)–C(26)–C(25)
120.0011
–
23
H(77)–C(20)–C(19)
109.4416
110
128
C(55)–N(59)–C(45)
123.1877
–
24
H(76)–C(20)–C(19)
109.4999
110
129
C(55)–N(59)–Cu(1)
123.1876
–
25
H(75)–C(19)–H(74)
109.5197
109.4
130
C(45)–N(59)–Cu(1)
113.6247
–
26
H(75)–C(19)–C(20)
109.4619
109.41
131
H(60)–O(37)–C(36)
125.2505
–
27
H(75)–C(19)–C(18)
109.4616
109.41
132
H(60)–O(37)–Cu(1)
125.2498
–
28
H(74)–C(19)–C(20)
109.4421
109.41
133
C(36)–O(37)–Cu(1)
109.4997
–
29
H(74)–C(19)–C(18)
109.442
109.41
134
N(31)–C(27)–C(28)
119.9987
120
30
C(20)–C(19)–C(18)
109.5002
109.5
135
N(31)–C(27)–C(26)
119.9992
120
31
H(107)–C(57)–C(56)
120.0002
120
136
C(28)–C(27)–C(26)
120.0022
120
32
H(107)–C(57)–C(52)
120.0002
120
137
N(59)–Cu(1)–O(37)
109.3273
–
33
C(56)–C(57)–C(52)
119.9996
–
137
N(59)–Cu(1)–N(31)
109.5001
–
34
O(58)–S(50)–C(52)
109.5198
–
139
N(59)–Cu(1)–O(9)
109.5
–
35
O(58)–S(50)–N(51)
109.4417
–
140
N(59)–Cu(1)–Cl(3)
136.5963
–
36
O(58)–S(50)–O(49)
109.4419
116.6
141
N(59)–Cu(1)–Cl(2)
43.1572
–
37
C(52)–S(50)–N(51)
109.462
–
142
O(37)–Cu(1)–N(31)
109.5
–
38
C(52)–S(50)–O(49)
109.4616
–
143
O(37)–Cu(1)–O(9)
109.4998
–
39
N(51)–S(50)–O(49)
109.5003
–
144
O(37)–Cu(1)–Cl(3)
55.6453
–
40
C(57)–C(52)–C(53)
120.0026
120
145
O(37)–Cu(1)–Cl(2)
124.32
–
41
C(57)–C(52)–S(50)
119.9987
–
146
N(31)–Cu(1)–O(9)
109.5
–
42
C(53)–C(52)–S(50)
119.9986
–
147
N(31)–Cu(1)–Cl(3)
113.9036
–
43
H(104)–C(53)–C(54)
120.0016
120
148
N(31)–Cu(1)–Cl(2)
66.3429
–
44
H(104)–C(53)–C(52)
120.0013
120
149
O(9)–Cu(1)–Cl(3)
55.4536
–
45
C(54)–C(53)–C(52)
119.9972
–
150
O(9)–Cu(1)–Cl(2)
124.512
–
45
H(106)–C(56)–C(57)
120.0012
120
151
Cl(3)–Cu(1)–Cl(2)
179.7535
–
47
H(106)–C(56)–C(55)
120.0012
120
152
H(73)–C(18)–H(72)
109.5198
109.4
48
C(57)–C(56)–C(55)
119.9975
–
153
H(73)–C(18)–C(19)
109.4616
109.41
49
H(105)–C(54)–C(55)
119.9997
120
154
H(73)–C(18)–C(17)
109.4619
109.41
50
H(105)–C(54)–C(53)
119.9992
120
155
H(72)–C(18)–C(19)
109.4419
109.41
51
C(55)–C(54)–C(53)
120.0011
–
156
H(72)–C(18)–C(17)
109.4421
109.41
52
H(90)–C(42)–C(43)
120.0015
120
157
C(19)–C(18)–C(17)
109.5001
109.5
53
H(90)–C(42)–C(41)
120.0009
120
158
C(27)–N(31)–C(17)
137.567
–
54
C(43)–C(42)–C(41)
119.9975
–
159
C(27)–N(31)–Cu(1)
137.5671
–
55
H(89)–C(41)–C(42)
119.9991
120
160
C(17)–N(31)–Cu(1)
84.8659
–
56
H(89)–C(41)–C(40)
119.9996
120
161
H(67)–C(14)–C(15)
120.0016
120
57
C(42)–C(41)–C(40)
120.0013
–
162
H(67)–C(14)–C(13)
120.0011
120
58
H(88)–C(40)–C(41)
119.9991
120
163
C(15)–C(14)–C(13)
119.9973
–
59
H(88)–C(40)–C(39)
119.9988
120
164
H(66)–C(13)–C(14)
119.999
120
60
C(41)–C(40)–C(39)
120.0021
–
165
H(66)–C(13)–C(12)
119.9987
120
61
H(91)–C(43)–C(42)
119.9999
120
166
C(14)–C(13)–C(12)
120.0022
–
62
H(91)–C(43)–C(38)
120.0004
120
167
H(65)–C(12)–C(13)
119.9997
120
63
C(42)–C(43)–C(38)
119.9997
–
168
H(65)–C(12)–C(11)
119.9996
120
64
H(87)–C(39)–C(40)
120.0019
120
169
C(13)–C(12)–C(11)
120.0007
–
65
H(87)–C(39)–C(38)
120.002
120
170
H(68)–C(15)–C(14)
120.0001
120
66
C(40)–C(39)–C(38)
119.9962
–
171
H(68)–C(15)–C(10)
120.0002
120
67
C(43)–C(38)–C(39)
120.0033
120
172
C(14)–C(15)–C(10)
119.9997
–
68
C(43)–C(38)–N(32)
119.9985
120
173
H(64)–C(11)–C(12)
120.0015
120
69
C(39)–C(38)–N(32)
119.9983
120
174
H(64)–C(11)–C(10)
120.0015
120
70
H(94)–C(44)–H(93)
109.52
109
175
C(12)–C(11)–C(10)
119.997
–
71
H(94)–C(44)–H(92)
109.4617
109
176
N(31)–C(17)–C(18)
127.0442
115.1
72
H(94)–C(44)–C(34)
109.4618
110
177
N(31)–C(17)–C(7)
105.9115
115.1
73
H(93)–C(44)–H(92)
109.4421
109
178
C(18)–C(17)–C(7)
127.0443
117.2
74
H(93)–C(44)–C(34)
109.4416
110
179
H(61)–O(9)–C(8)
125.2502
–
75
H(92)–C(44)–C(34)
109.5
110
180
H(61)–O(9)–Cu(1)
125.2501
–
76
C(34)–N(33)–N(32)
114.9101
115
181
C(8)–O(9)–Cu(1)
109.4997
–
77
C(38)–N(32)–C(36)
124.5
108
182
H(63)–C(8)–O(9)
108.4684
106.7
78
C(38)–N(32)–N(33)
124.5002
124
183
H(63)–C(8)–C(7)
114.5222
109.39
79
C(36)–N(32)–N(33)
110.9998
–
184
H(63)–C(8)–N(4)
111.7238
107.5
80
H(86)–C(36)–O(37)
108.4686
106.7
185
O(9)–C(8)–C(7)
107.5003
107.7
81
H(86)–C(36)–C(35)
114.5224
109.39
186
O(9)–C(8)–N(4)
110.5
–
82
H(86)–C(36)–N(32)
111.7236
107.5
187
C(7)–C(8)–N(4)
104.0001
–
83
O(37)–C(36)–C(35)
107.5001
107.7
188
C(15)–C(10)–C(11)
120.003
120
84
O(37)–C(36)–N(32)
110.5001
–
189
C(15)–C(10)–N(4)
119.9984
120
85
C(35)–C(36)–N(32)
104.0001
–
190
C(11)–C(10)–N(4)
119.9986
120
86
C(44)–C(34)–C(35)
127.9431
117.2
191
H(71)–C(16)–H(70)
109.5201
109
87
C(44)–C(34)–N(33)
127.9432
115.1
192
H(71)–C(16)–H(69)
109.4621
109
88
C(35)–C(34)–N(33)
104.1137
115.1
193
H(71)–C(16)–C(6)
109.4619
110
89
H(96)–C(46)–H(95)
109.52
109.4
194
H(70)–C(16)–H(69)
109.4416
109
90
H(96)–C(46)–C(47)
109.4615
109.41
195
H(70)–C(16)–C(6)
109.4419
110
91
H(96)–C(46)–C(45)
109.4621
109.41
196
H(69)–C(16)–C(6)
109.4997
110
92
H(95)–C(46)–C(47)
109.4418
109.41
197
H(62)–C(7)–C(17)
107.8496
109.39
93
H(95)–C(46)–C(45)
109.4419
109.41
198
H(62)–C(7)–C(8)
112.9888
109.39
94
C(47)–C(46)–C(45)
109.5
109.5
199
H(62)–C(7)–C(6)
112.9887
109.39
95
H(85)–C(35)–C(45)
107.8499
109.39
200
C(17)–C(7)–C(8)
109.4697
109.51
96
H(85)–C(35)–C(36)
112.9883
109.39
201
C(17)–C(7)–C(6)
109.4699
109.51
97
H(85)–C(35)–C(34)
112.9885
109.39
202
C(8)–C(7)–C(6)
103.9999
109.51
98
C(45)–C(35)–C(36)
109.4701
109.51
203
C(10)–N(4)–C(8)
124.5001
108
99
C(45)–C(35)–C(34)
109.4701
109.51
204
C(10)–N(4)–N(5)
124.4997
124
100
C(36)–C(35)–C(34)
103.9998
109.51
205
C(8)–N(4)–N(5)
111.0002
–
101
N(59)–C(55)–C(56)
119.9991
120
206
C(16)–C(6)–C(7)
129.5073
117.2
102
N(59)–C(55)–C(54)
119.9989
120
207
C(16)–C(6)–N(5)
129.5075
115.1
103
C(56)–C(55)–C(54)
120.002
120
208
C(7)–C(6)–N(5)
100.9853
115.1
104
N(59)–C(45)–C(46)
123.8198
115.1
209
C(6)–N(5)–N(4)
113.9712
115
105
N(59)–C(45)–C(35)
112.36
115.1
4 Conclusions
The satisfactory analytical data and all the studies presented above indicate that the copper(II) complexes in the present investigation may be formulated as[Cu(LH)2(Cl)2], where LH = N-(4′-butyrylidine-3′-methyl-1′-phenyl-2′-pyrazolin-5′-one)sulfamethoxazole (bumphp-smzH), N-(4′-butyrylidine-3′-methyl-1′-phenyl-2′-pyrazolin-5′-one)sulfadimidine (bumphp-sdmH), N-(4’-butyrylidine-3′-methyl-1′-phenyl-2′-pyrazolin-5′-one)sulfanilamide (bumphp-snmH), N-(4′-butyrylidine-3′-methyl-1′-phenyl-2′-pyrazolin-5′-one)sulfamoxole (bumphp-sml) or N-(4′-butyrylidine-3′-methyl-1′-phenyl-2′-pyrazolin-5′-one)sulfaguanidine (bumphp-sgd). Keeping in view of the non-electrolytic (Geary, 1971) nature of the complexes (Λm = 7.2–10.8 ohm−1 cm2 mol−1), IR, ESR, magnetic, and electronic spectral results, and also considering the well established axially distorted octahedral structure of[Cu(en)2(H2O)2]2+ cation (Jaskova et al., 2007), wherein four nitrogen atoms of the ethylenediamine (en) form the coordination plane around the copper(II) atom with the oxygen atoms of the two water molecules lying in the axial positions, axially distorted octahedral structures (Fig. 9) having two Cl groups at the axial position trans to each other, and the two (O, N)-donor sulfa drug Schiff base ligands at the equatorial positions in cis-arrangement have been proposed for these complexes. X-ray crystallographic studies, which might confirm the proposed structures, could not be carried out, as suitable crystals could not be grown.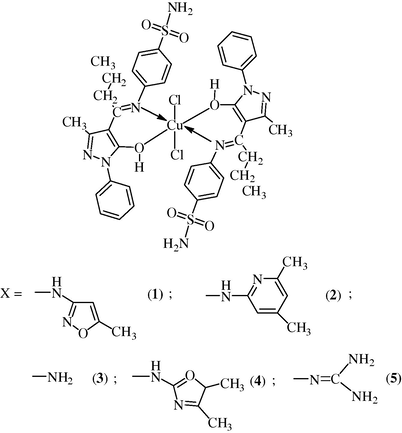
Proposed octahedral structure of complexes.
Acknowledgments
We are thankful to the University Grants Commission and Council of Scientific and Industrial Research, New Delhi, India, for the financial assistance. Analytical facilities provided by the Central Drug Research Institute, Lucknow, and the Regional Sophisticated Instrumentation Centre, Indian Institute of Technology, Chennai, are gratefully acknowledged. Thanks are also due to Professor R.R. Mishra, Vice-Chancellor, Rani Durgavati University, Jabalpur, MP, India, for the encouragement.
References
- Eur. J. Med. Chem.. 2004;39:593.
- Indian J. Chem., Sect. A. 2003;42:1617. and references therein
- Diabetes. 1997;46:1786.
- Acta Crystallogr., Sect. C. 1983;39:1620.
- Metal Ions in Biological Systems. Vol vol. 116. New York: Marcel Dekker; 1983. p. 261
- J. Inorg. Biochem.. 1983;51:689. and references therein
- ChemBio3D Ultra Molecular Modeling and Analysis, Cambridge. <www.cambridgesoft.com>.
- Bioorg. Med. Chem.. 2005;13:2459.
- Diabetes. 1980;29:665.
- Danks D.M., Scriver C.R., Beaudet A.L., Sly W.M., Valle D., eds. Disorders of Copper Transport in “The Metabolic and Molecular Basis of Inherited Disease” (sixth ed.). New York: McGraw-Hill; 1995. p. 2211
- Inorg. Chim. Acta. 2008;361:132.
- Coord. Chem. Rev.. 2005;249:895.
- Chem. Res. Toxicol.. 2003;16:28.
- IL Farmaco. 2005;60:307.
- Br. J. Cancer. 2005;92:690.
- Metab. Res.. 2000;32:129.
- Cancer Res.. 2002;62:6116.
- Bioorg. Med. Chem.. 2004;12:651.
- Synth. React. Inorg. Met.-Org. Chem.. 1994;24:365.
- Coord. Chem. Rev.. 1971;7:81.
- Inorg. Chim. Acta. 2007;360:2711.
- Acta Chem. Scand.. 1959;13:1670.
- Coord. Chem. Rev.. 2005;249:2909. and references therein
- Clin. Med.. 2004;4:277.
- J. Mol. Struct.. 2006;794:24.
- J. Mol. Struct.. 2007;833:133.
- Synth. React. Inorg. Met-Org. Chem.. 1994;26:1013.
- Synth. React. Inorg. Met.-Org. Chem.. 1996;28:1265.
- Indian J. Chem., Sect. A. 1997;36:406.
- Synth. React. Inorg. Met.-Org. Chem.. 2003;33:699.
- Synth. React. Inorg. Met.-Org. Chem.. 2003;33:1063.
- Indian J. Chem., Sect. A. 2004;43:763.
- Indian J. Chem., Sect. A. 2007;46:1594.
- Indian J. Chem., Sect. A. 2008;47:517.
- Maurya, R.C., Martin, M.H., Chourasia, J., Sharma, A.K., in press. Int. J. Curr. Chem.
- Arabian J. Chem.. 2015;8:78.
- Bioorg. Med. Chem. Lett.. 1999;9:1843. and references therein
- Spectrochim. Acta, Part A. 2007;68:1382.
- J. Periodontol.. 1999;70:409.
- J. Diabetes. 2001;50:131.
- J. Chem. Phys.. 1961;35:149.
- Eur. J. Med. Chem.. 2005;40:361.
- Eur. J. Clin. Invest.. 2005;35:66.
- Coord. Chem. Rev.. 2002;226:187.
- Chem. Rev.. 1999;99:2535.
- Electronic Absorption Spectroscopy and Related Techniques (first ed.). University Press (India) Limited; 2001. p. 245
- Coord. Chem. Rev.. 1999;184:67.
- Clin. Cancer Res.. 2004;10:1430.
- Nature. 1980;284:556.
- Eur. J. Med. Chem.. 2005;40:377.
- Med. Chem.. 1989;437:26.
- J. Med. Chem.. 1997;40:1018.
- A Text Book of Qualitative Inorganic Analysis. ELBS, Longman Green and Co. Ltd.; 1962.
- Coord. Chem. Rev.. 2002;232:95.
- Bioorg. Med. Chem.. 2007;15:4917.
- Bioorg. Med. Chem. Lett.. 2005;15:2275.
- J. Med. Chem.. 1992;35:2496.
- Polyhedron. 2007;26:239.







