Translate this page into:
Synthesis, characterization, and anticancer activity of some metal complexes with a new Schiff base ligand
⁎Corresponding author. thamer.ayo@gmail.com (Thamer A. Alorini)
-
Received: ,
Accepted: ,
This article was originally published by Elsevier and was migrated to Scientific Scholar after the change of Publisher.
Peer review under responsibility of King Saud University.
Abstract
A new Schiff base ligand, 2-((E)-((4-(((E)-benzylidene)amino)phenyl)imino)methyl)-naphthalene-1-ol, was prepared by the reflux condensation of p-phenylenediamine with 2-hydroxy-1-naphthaldehyde and benzaldehyde. Metal complexes were prepared by reacting the ligand with metal salts: VCl3, CrCl3·6H2O, MnCl2·3H2O, FeCl3·6H2O, CoCl3·6H2O, NiCl2·6H2O, CuCl2·2H2O, and ZnCl2. The ligand and its metallic complexes were characterized by various techniques such as elemental analysis, AAS, NMR, IR, UV–Vis, TGA, DTA, XRD and TEM. The data confirmed that the ligand coordinated with the metal ions in a bidentate nature, bonding through its azomethine nitrogen atom and phenolic oxygen atom; this gave an octahedral geometry. The XRD patterns of the complexes indicated that they were of various structures: the Mn(II), Co(III), and Cu(II) complexes were triclinic, the ligand and Ni(II) complex were orthorhombic, the V(III) and Zn(II) complexes were hexagonal, the Cu(II) complex was monoclinic, and the Fe(II) complex was cubic. TEM analysis confirmed that the complexes were nanoscale in nature. The antibacterial and antifungal activities of the ligand and its complexes against Salmonella enterica serovar typhi and Candida albicans were investigated by the hole plate diffusion method. It was observed that the Co(II) and Zn(II) complexes had intermediate antibacterial activities, while the V(III) complex had the highest activity against C. albicans fungi. The in vitro anticancer activities of the ligand and its metal complexes were tested towards PC-3, SKOV3, and HeLa tumour cell lines, where they exhibited higher antitumour activities against these selected human cell lines than clinically used drugs such as cisplatin, estramustine, and etoposide.
Keywords
Schiff base complex
Antibacterial activity
Antifungal activity
Anticancer activity
Nanoparticle
- AAS
-
Atomic Absorption Spectroscopy
- NMR
-
Nuclear Magnetic Resonance
- IR
-
Infrared Spectroscopy
- UV–Vis
-
Ultraviolet–visible Spectroscopy
- TGA
-
Thermogravimetric analysis
- DTA
-
Differential Thermal Analysis
- XRD
-
X-ray powder Diffraction
- TEM
-
Transmission Electron Microscopy
- DMSO
-
Dimethyl sulfoxide
- SRB
-
Sulforhodamine B assay
- HL
-
Ligand
Abbreviations
1 Introduction
Schiff bases are important chemical compounds in various fields such as inorganic, analytical, and medicinal chemistry due to their versatility; they can form numerous, diverse, stable complexes when they are coordinated with different transition-metal ions. Thus, in recent years, such metal complexes containing Schiff bases have been studied extensively due to their various applications and chemical activities. Schiff bases are formed via the interaction between the carbonyl group of an aldehyde or beta diketone and an amine moiety, and their active group (—N⚌CH—) contains active electrons, making them ideal candidates for developing new drugs (Al-Hakimi et al., 2020; Maurya et al., 2016). In medicinal chemistry, the development of powerful and effective medicinal drugs has been extensively explored. The derivatives of Schiff bases represent a significant category of compounds that have found multiple applications in therapeutic chemistry because of their wide range of pharmacokinetic properties and their prominence in drug discovery programs (El-Saied et al., 2018). Schiff base derivatives and their complexes have reportedly demonstrated a variety of biological properties, such as anti-inflammatory (Azam et al., 2020; Manimohan et al., 2020), antibacterial (El-saied et al., 2020; Shakdofa et al., 2018), antifungal (Al-Hakimi et al., 2020; Bhaskar et al., 2020), antiviral (Buldurun et al., 2020; Slassi et al., 2020), anticancer (Kavitha and Laxma Reddy, 2016; Mbugua et al., 2020), and antioxidant (Keypour et al., 2020; Radha et al., 2020) activities. Schiff bases also have industrial uses (Betiha et al., 2020; Chauhan et al., 2020) and have been used as highly effective sensors (Hosseinzadeh Sanatkar et al., 2020; Mondal et al., 2020) and catalysts (Bocian et al., 2020; Nagalakshmi et al., 2020), as well as beneficial substances for preserving the environment (Kobisy et al., 2020; Zaman Brohi et al., 2020).
Several published studies have demonstrated the extent to which Schiff bases and their transition-metal complexes exhibit biological activities effective against a range of bacterial and fungal species and tumours. For example, Schiff base complexes have shown antitumor and antioxidative activities, as well as lipid peroxidation inhibition (Yang et al., 2000). This is important as microbial infections have threatened human civilization since ancient times, with large proportions of people dying in various parts of the world due to such diseases. According to a report from the Infectious Diseases Society of America, there are several microbial species that pose serious pathogenic risk; these include Klebsiella pneumonia and Pseudomonas aeruginosa, as well as species belonging to the Staphylococcus, Enterococcus, Enterobacter, and Acinetobacter genuses (Malik et al., 2018). Cancer is another category of deadly diseases, and because they lack proper treatment options they threaten humanity in throughout the developing and developed worlds (Mbugua et al., 2020).
Herein, we report the preparation, characterization, and determination of the crystal structure of a new Schiff base constructed from p-phenylenediamine, 2-hydroxy-1-naphthaldehyde, and benzaldehyde as a ligand. Eight complexes derived from this novel base were also prepared. This study assessed the biological activity of the prepared compounds. Namely, all compounds were tested against PC-3, SKOV3, and HeLa tumour cell lines; meanwhile, their antibacterial activities were assayed against Salmonella enterica serovar typhi (S. enterica ser. typhi) and their antifungal activities were evaluated against Fungus Candida albicans.
2 Materials and methods
2.1 Materials and measurements
p-Phenylenediamine, 2-hydroxy-1-naphthaldehyde, and benzaldehyde were purchased from Acros all compounds were of high purity of 98%, while metal salts were obtained from Sigma–Aldrich, ethanol, methanol, dichloromethane, dimethyl sulfoxide (DMSO), and petroleum ether were of analytical grade and purchased from Alfa Aesar and used without purification. 1H and 13C nuclear magnetic resonance (NMR) spectroscopies were performed using a Bruker spectrometer at 850 MHz and 213 MHz, respectively; CDCl3 was used as the solvent, TMS was the standard reference, and the probe temperature was 25 °C. The Fourier-transform infrared (FTIR) spectra of the ligand and its metallic complexes in the solid-state were measured using an Agilent spectrometer (Cary 600 FTIR, USA), which was operated in the wavenumber range of 4000–400 cm−1. UV–Vis spectra of DMSO solutions (1 × 10−4 M) of the ligand and their metal complexes were measured using a Shimadzu spectrophotometer (UV-1650PC, Japan); a 1 cm quartz cell and wavelength range of 250–650 nm were employed. The carbon, hydrogen, and nitrogen contents were determined for the prepared ligand and its metallic complexes using a Eurovector CHN (EA3000, Italy) analyser. The metal elements were determined using AAS (model 200 series AAS spectrometer, Agilent-technologies). TGA and DTA analyses were performed under nitrogen using a Shimadzu simultaneous DTA–TG apparatus (DTG-60AH, Japan); the heating rate was 10 °C/min, the temperature range was 27–525 °C, and Al2O3 served as the reference material for the DTA measurements. The XRD patterns of the compounds were collected with a Rigaku XRD diffractometer (Ultima IV, USA). The anode material was Cu Kα (λ = 1.54180 Å) which operated with a current of 30 mA and a voltage of 40 kV. TEM analysis was conducted using a JEOL-100S (Japan) microscope. The molar conductivity (Λm) values of the prepared metal complexes dissolved in DMSO (1 × 10−3 M) at room temperature were determined using an Oakton Conductivity/DTS meter. The magnetic sensitivities of the prepared solid metal complexes were measured at room temperature with the Magnetic Susceptibility Balance – Auto from Sherwood Scientific (United Kingdom). S. enterica ser. typhi was obtained from the Yemen Standardization, Metrology and Quality Control Organization, while C. albicans was obtained from Althubhani Specialist Medical Laboratory (Sana'a, Yemen).
2.2 Preparation of ligand (HL) (1)
The ligand, 2-((E)-((4-(((E)-benzylidene)amino)phenyl)imino)methyl)-naphthalene-1-ol, was prepared following a literature method (Al-Hakimi et al., 2020), where 1.72 g (0.01 mol) of 2-hydroxy-1-naphthaldehyde and 1.06 g (0.01 mol) of benzaldehyde were dissolved in 20 mL of absolute ethanol and the mixture was stirred until complete dissolution. This solution was then slowly added dropwise to a spherical flask containing 1.08 g (0.01 mol) of p-phenylenediamine dissolved in 15 mL of ethanol. The mixture was left to stir under reflux for 3 h, during which an orange precipitate was formed. The precipitate was then filtered and washed several times with distilled water followed by absolute ethanol. Yield: (84%). M.p.: 230 °C. Colour: Orange. IR, ν(cm−1): 3350(m), 1620(s), 1605(s), 1540(s), 1310(m). 1H NMR (850 MHz, CDCl3) (δ, ppm): 6.65–8.10 (m, 15H, Ar–H), 10.05 (s, 1H, N⚌CH), 10.88 (s, 1H, N⚌CH), 13.20 (s, 1H, ArOH). 13C NMR (213 MHz, CDCl3) (δ, ppm): 149.9, 149.36, 148.77, 140.35, 133.85, 131.64, 129.63, 128.21, 126.85, 124.85, 123.71, 122.36, 118.07, 117.0, 113.26. UV–Vis (DMSO), λmax (nm): 259, 318. Elemental analysis (%): Calculated: C: 82.27, H: 5.19, N: 7.99; Found: C: 82.03, H: 4.93, N: 7.86.
2.3 Preparation of metal complexes
The ratio of ligand to metal (L:M) was calculated from the molar ratios of several solutions that contained fixed proportions of the metal salt solution with variable quantities of the ligand solution. Mn(II) and Cu(II) metallic complexes (4 and 8, respectively) were prepared using MnCl2·3H2O and CuCl2·2H2O, respectively, in L:M molar ratios of 1:1. V(III), Cr(III), Fe(III), Co(III), Ni(II), and Zn(II) metallic complexes (2–9, except 4 and 8, respectively) were prepared using VCl3, CrCl3·6H2O, FeCl3·6H2O, CoCl3·6H2O, NiCl2·6H2O, and ZnCl2, respectively, in L:M molar ratios of 2:1. All the metal complexes were prepared using the following, general method:
An ethanolic solution of the metal salt was mixed with a suitable amount of an ethanolic solution of the ligand in the molar ratios previously mentioned for 3 h on an electric stirrer under reflux at 70 °C. A precipitate was formed, which was then filtered and washed several times with distilled water and then with absolute ethanol to remove the non-reacting organic materials. The solid was then dried in an oven at 50 °C for 2 h.
2.3.1 V(III) Complex (2)
Dark Green precipitate. Yield: (64%). M.p.: 265 °C. IR, ν(cm−1): 3345(m), 1610(s), 1542(s), 1518(s), 1300(m), 620(m), 520(m), 450(s). UV–Vis (DMSO), λmax (nm): 233, 322, 486. Elemental analysis (%): Calculated: C: 70.17, H: 4.29, N: 6.82; Found: C: 70.29, H: 4.12, N: 6.57.
2.3.2 Cr(III) complex (3)
Red precipitate. Yield: (73%). M.p.: 260 °C. IR, ν(cm−1): 3342(m), 1608(s), 1580(s), 1495(s), 1305(m), 615(s), 535(w), 405(s). UV–Vis (DMSO), λmax (nm): 227, 317, 344, 459, 484. Elemental analysis (%): Calculated: C: 70.08, H: 4.31, N: 6.81; Found: C: 69.94, H: 4.19, N: 6.94.
2.3.3 Mn(II) complex (4)
Burgundy precipitate. Yield: (78%). M.p.: 290 °C. IR, ν(cm−1): 3520–3300(br), 3440(m), 1617(s), 1595(s), 1530(s), 1300(s), 620(w), 570(m), 410(s). UV–Vis (DMSO), λmax (nm): 231, 320, 464, 484, 612. Elemental analysis (%): Calculated: C: 56.27, H: 4.33, N: 5.47; Found: C: 56.70, H: 4.42, N: 5.70.
2.3.4 Fe(III) complex (5)
Dark brown precipitate. Yield: (75%). M.p.: >300 °C. IR, ν(cm−1): 3420–3130(br), 1615(s), 1592(s), 1520(s), 1305(s), 652(m), 567(m), 425(m). UV–Vis (DMSO), λmax (nm): 261, 318, 379, 463, 487. Elemental analysis (%): Calculated: C: 71.34, H: 4.49, N: 6.93; Found: C: 71.57, H: 4.25, N: 6.67.
2.3.5 Co(III) complex (6)
Brown precipitate. Yield: (81%). M.p.: >300 °C. IR, ν(cm−1): 3330–3030(br), 1615(s), 1594(s), 1515(s), 1305(s), 653(m), 535(s), 412(m). UV–Vis (DMSO), λmax (nm): 262, 317, 433, 458. Elemental analysis (%): Calculated: C: 71.07, H: 4.47, N: 6.91; Found: C: 70.82, H: 4.38, N: 6.98.
2.3.6 Ni(II) complex (7)
Green precipitate. Yield: (74%). M.p.: >300 °C. IR, ν(cm−1): 3430–3120(br), 3320(m), 1617(s), 1600(s), 1536(s), 1250(m), 619(s), 520(m), 418(m). UV–Vis (DMSO), λmax (nm): 264, 316, 363, 455. Elemental analysis (%): Calculated: C: 71.00, H: 4.59, N: 6.90; Found: C: 70.52, H: 4.51, N: 6.77.
2.3.7 Cu(II) complex (8)
Dark burgundy precipitate. Yield: (75%). M.p.: 270 °C. IR, ν(cm−1): 3520–3230(br), 3443(m), 1612(s), 1544(s), 1511(s), 1298(s), 634(s), 520(s), 416(m). UV–Vis (DMSO), λmax (nm): 258, 332, 460, 490. Elemental analysis (%): Calculated: C: 55.34, H: 4.26, N: 5.38; Found: C: 55.17, H: 4.06, N: 5.31.
2.3.8 Zn(II) complex (9)
Orange precipitate. Yield: (79%). M.p.: 283 °C. IR, ν(cm−1): 3410(m), 1615(s), 1594(s), 1515(s), 1305(s), 688(m), 534(s), 430(m). UV–Vis (DMSO), λmax (nm): 260, 330. Elemental analysis (%): Calculated: C: 68.87, H: 4.33, N: 6.70; Found: C: 68.64, H: 4.27, N: 6.89.
2.4 Cell culture
The American Type Culture Collection (ATCC) provided the following human cell lines: prostate adenocarcinoma (PC-3), ovarian adenocarcinoma (SKOV3), and cervical cancer (HeLa). Under humid, 5% (v/v) CO2 conditions, cells were incubated in RPMI-1640 (100 g/mL) enriched with penicillin (100 units/L) and heat-inactivated foetal bovine serum (10% v/v) at 37 °C (Ghfar et al., 2021).
2.5 Cytotoxicity assay
Using the sulforhodamine B (SRB) assay, the cytotoxicities of the prepared chemical compounds were assessed against human tumour cells (PC-3, SKOV3, and HeLa). Before being treated with the chemical compounds, 80% of the confluent proliferating cells were trypsinised and cultivated in a 96-well tissue culture plate for 24 h. Untreated cells (control) were also added to cells that were exposed to the six concentrations of each compound (0.01, 0.1, 1, 10, 100, and 1000 µg/mL). The cells were exposed to the doses for 72 h before being fixed with TCA (10% w/v) for 1 h at 4 °C. After repeated washes, the cells were stained for 10 min in the dark with a 0.4% (w/v) SRB solution. Glacial acetic acid, 1% (v/v), was used to remove any remaining discoloration. The SRB-stained cells were dissolved in Tris-HCl after drying for 12 h, and their colour intensities were quantified using a microplate reader at 540 nm. Using SigmaPlot 12.00 software, the correlation between the viability percentage of each tumour cell line and the chemical concentrations was analysed to determine the IC50 values (i.e., drug dose that reduces survival to 50%) (Alam et al., 2021).
2.6 In vitro antibacterial and antifungal activity
The ligand and its metallic complexes were diluted with DMSO to concentrations of 100 parts per thousand (ppt). All antimicrobial and antifungal activities tests were conducted in the Department of Botany, Laboratory of Microbiology at Sana'a University (Yemen). The antibacterial and antifungal activity assays of the ligand and its metallic complexes were carried out by the hole plate diffusion method (Magwa et al., 2006). Petri dishes were pre-inoculated with the appropriate bacteria and fungi in the following manner:
One hundred µL of the bacteria and fungi suspension was spread over plates containing Mueller–Hinton agar using a sterile cotton swab (Gachkar et al., 2007; Hanbali et al., 2005). Three wide holes (with diameters of 5 mm) were then made in the agar using a cork borer. Different amounts of the compounds (30 µL, 20 µL, and 10 µL) were introduced into each of the holes in appropriately labelled petri dishes using a sterile micropipette. Gentamicin and clotrimazole (10 µg/ mL) were used as positive controls for bacteria and fungi, respectively (El Malti et al., 2007). The bacteria dishes were then incubated at 37 °C for 24 h, while the yeast, mould, and fungi plates were incubated at 28 °C for 24 h, 3 d, and 24 h, respectively. After incubation, the zones of inhibition were measured and recorded. The zone of inhibition was taken to be the diameter of the zone that visibly shows an absence of growth, including the 5 mm well. Additionally, when no inhibition occurred, the value of 0 mm was assigned to the test sample (Magwa et al., 2006).
3 Results and discussion
3.1 Chemistry
Our approach began with the preparation of the ligand (Scheme 1), which was achieved by reacting one equivalent of 2-hydroxy-1-naphthaldehyde with one equivalent of benzaldehyde in absolute ethanol, and then slowly adding the completely dissolved mixture dropwise to one equivalent of p-phenylenediamine in 15 mL of ethanol. The metallic complexes were constructed in L:M ratios of 1:1 (Scheme 2) or 2:1 (Scheme 3) by reacting ethanolic solutions of the ligand and various metal chloride salts. All obtained complexes were characterized by FT-IR spectroscopy, UV–Vis electronic absorption, elemental analysis, thermal analysis, X-ray powder diffraction, and TEM, as well as molar conductivity and magnetic susceptibility studies.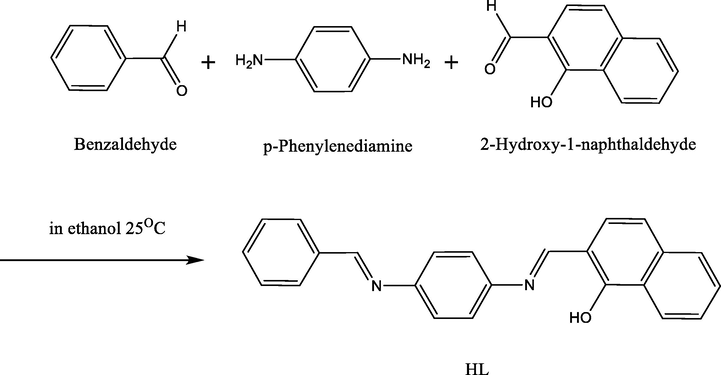
Preparation of the Ligand.
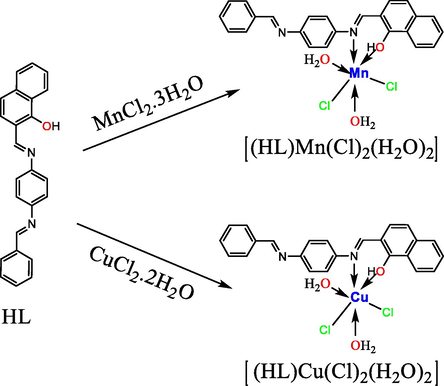
Preparation of the metallic complexes in ligand-to-metal(L. M) ratios of 1:1.
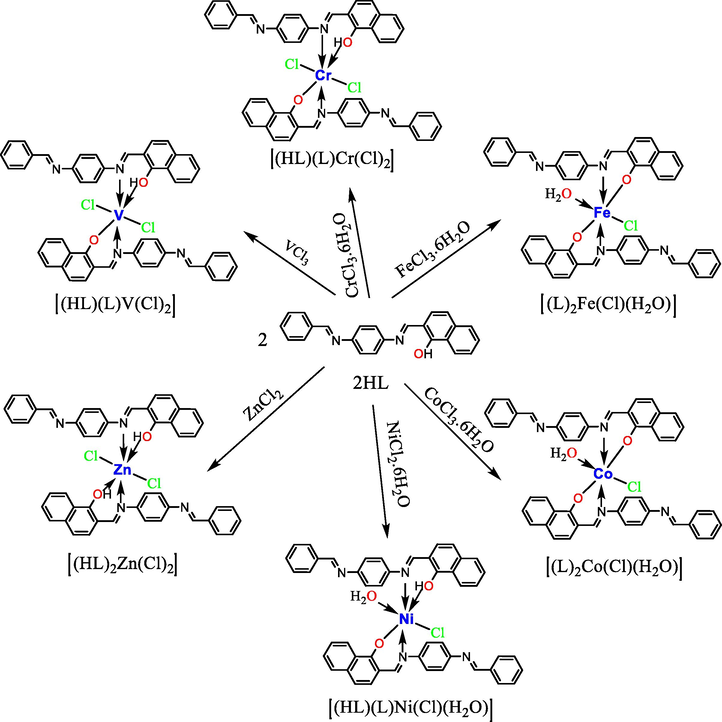
Preparation of the metallic complexes in L. M ratios of 2:1.
3.1.1 1H NMR spectra
The 1H NMR spectrum of the ligand in CDCl3 (Fig. 1) showed signals consistent with the expected values. Importantly, the spectrum lacked the signal of the amino group (—NH2) characteristic of the starting material. The spectrum showed one peak as a singlet at 13.20 ppm, which was assigned to the hydroxyl (—OH) moiety (Ren et al., 2020), along with two peaks at 10.88 ppm and 10.05 ppm, which were assigned to the —N⚌CH— groups; the difference in the shifts of these two protons is due to the polarity of the 2-hydroxy-1-naphthaldehyde moiety, which is in closer proximity to the group whose proton appears at 10.88 ppm (Al-Hakimi et al., 2020). Another signal appeared as a multiplet within the range of 6.65–8.10 ppm, which is attributed to aromatic protons (Al-Hakimi, 2020; Al-Hakimi et al., 2020).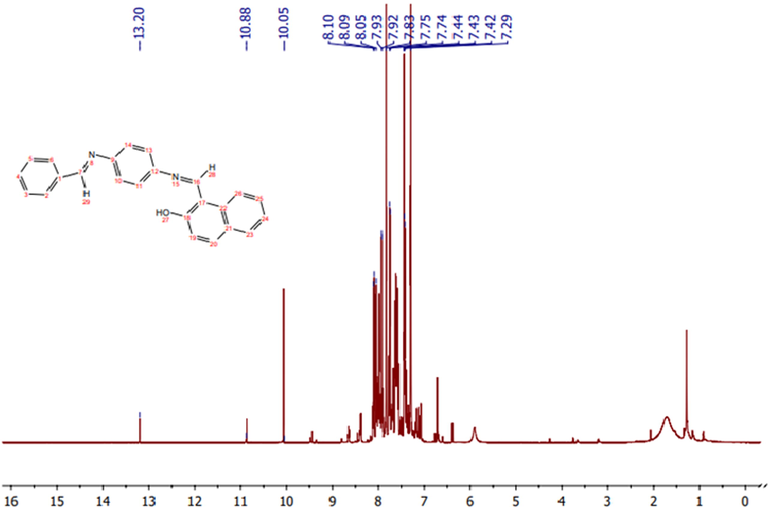
1H NMR spectrum of the ligand.
3.1.2 13C NMR spectra
The 13C NMR spectrum of the ligand (Fig. 2) showed peaks appearing at 149.9, 149.36 and 148.77 ppm. These peaks can be attributed to the C—OH, HC⚌N, and HC⚌N groups, respectively. The peaks within the 113.26–140.35 ppm range were assigned to the aromatic carbons (Al-Hakimi, 2020; Al-Hakimi et al., 2020).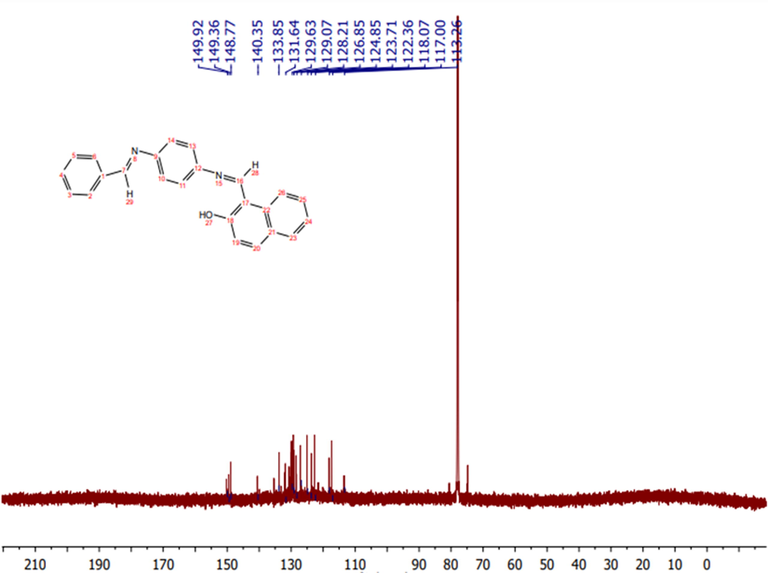
13C NMR spectrum of the ligand.
3.1.3 Elemental analysis and physical properties
Elemental analysis and physical data (Table 1) supported the proposed structures of the ligand and its metal complexes, with the V(III), Cr(III), Fe(III), Co(III), Ni(II), and Zn(II) complexes showing L:M molar ratios of 2:1 and the Mn(II) and Cu(II) complexes showing L:M molar ratios of 1:1. Furthermore, the molar conductivity values of the complexes indicate that the chloride anions are located within their inner-spheres.
Comp.
No.Compound
(m.w)Colour
Yield
(%)M.p.
(°C)Ω−1 mol−1 cm2
μeff
(μB)Found (Calc.) (%)
C
H
N
M
1
[HL][C24H18N2O]
(350.41)Orange
84
230
–
–
82.03
(82.27)4.93
(5.19)7.86
(7.99)–
2
[(HL)(L)V(Cl)2]
(821.66)Dark
Green64
265
3.8
2.85
70.29
(70.17)4.12
(4.29)6.57
(6.82)6.05
(6.20)
3
[(HL)(L)Cr(Cl)2]
(822.72)Red
73
260
7.4
3.9
69.94
(70.08)4.19
(4.31)6.94
(6.81)6.47
(6.32)
4
[(HL)Mn(Cl)2(H2O)2]
(512.29)Burgundy
78
290
21.1
5.96
56.70
(56.27)4.42
(4.33)5.70
(5.47)10.78
(10.72)
5
[(L)2Fe(Cl)(H2O)]
(808.12)Dark
Brown75
>300
26.9
5.94
71.57
(71.34)4.25
(4.49)6.67
(6.93)6.72
(6.91)
6
[(L)2Co(Cl)(H2O)]
(811.21)Brown
81
>300
25.6
4.8
70.82
(71.07)4.38
(4.47)6.98
(6.91)7.14
(7.26)
7
[(HL)(L)Ni(Cl)(H2O)]
(811.98)Green
74
>300
8.4
2.85
70.52
(71.00)4.51
(4.59)6.77
(6.90)7.03
(7.23)
8
[(HL)Cu(Cl)2(H2O)2]
(520.89)Dark
Burgundy75
270
27.3
1.7
55.17
(55.34)4.06
(4.26)5.31
(5.38)12.06
(12.20)
9
[(HL)2Zn(Cl)2]
(837.12)Orange
79
283
6.0
Dia.
68.64
(68.87)4.27
(4.33)6.89
(6.70)7.51
(7.81)
3.1.4 Molar conductivity
To verify the ionic formulas of the prepared metal complexes in solution, the molar conductivities of DMSO solutions of the complexes (1 × 10−3 M) were determined (Table 1). A range of 6.0–27.3 Ω−1 mol−1 cm−2 was obtained; such low values indicate that the complexes are non-electrolytic in nature (Alhakimi et al., 2021; El-tabl et al., 2008). This confirms that the anions are coordinated with the metal ions in all complexes. To further ensure this absence of an electrolytic nature, a AgNO3 solution was added to the metal complex solutions; as expected, there was no formation of a white precipitate or any turbidity, which confirmed that the chlorine ions are not located outside the coordination sphere as accompanying ions. This supports the proposed shapes of the complexes.
3.1.5 Magnetic susceptibility
The room-temperature magnetic moments of the complexes (2–9) are shown in Table 1. The V(III) and Ni(II) complexes (2 and 7, respectively) show magnetic moments of 2.85 μB, indicating they possess two unpaired electrons in their outer valence shells as well as octahedral geometries (Güngör and Gürkan, 2019). However, the Cr(III) complex (3) showed a magnetic moment of 3.9 μB, confirming the presence of three unpaired electrons in its outer valence shell, resulting in an octahedral geometry around the Cr(III) ion (El-Tabl, 2002; El-tabl et al., 2008). The magnetic moment values for the Mn(II), Fe(III), and Co(III) complexes (4, 5, and 6, respectively) are 5.96, 5.94 and 4.8 μB, respectively, indicating high-spin Mn(II), Fe(III), and Co(III) ions, as well as five, five, and four unpaired electrons in their respective outer valence shells; they also showed octahedral geometries (Murukan and Mohanan, 2006). Cu(II) complex 8 had a magnetic moment of 1.70 μB, which corresponds to one unpaired electron and an octahedral geometry (Singh et al., 2017). Finally, the Zn(II) complex (9) showed a diamagnetic value.
3.1.6 FT-IR spectra
The important absorption bands of the ligand and its metal complexes are presented in Table 2. The IR spectrum of the ligand (Fig. 3) shows a sharp, weak band centred at 3350 cm−1, which is attributed to the hydroxyl group (ν(O—H)) (AL-FAKEH et al., 2020). The spectrum also shows strong bands at 1620 and 1605 cm−1, which are assigned to the two azomethine groups (ν(C⚌N)) (Aly et al., 2012; El-tabl et al., 2008). In addition, medium and strong bands appeared at 1540 and 1310 cm−1, corresponding to ν(C⚌C) and ν(C—O), respectively (El-tabl et al., 2008). By comparing the IR spectrum of the ligand with the spectra of the metal complexes, the method of metal–ligand coordination can be elucidated. The ν(O—H) vibrations of complexes 2, 3, 4, 7, 8, and 9 were observed as strong bands at 3345, 3342, 3440, 3320, 3443, and 3410 cm−1, respectively (El-tabl et al., 2008). Except for complexes 2, 3, and 9, these complexes also show broad band in the 3520–3030 cm−1 range, which is assigned to coordinated water molecules (El-Tabl et al., 2008; Mosa’d Jamil et al., 2018). Medium and strong bands are noted at complexes 2–9 in the 1620–1608 cm−1 and 1595–1542 cm−1 ranges and are due to the ν(C⚌N) vibrations involving the benzene group and the C⚌N unit coordinating with a metal ion, respectively (Al-Hakimi et al., 2020; El-tabl et al., 2008). However, in compounds, 1–9 the medium and strong bands that appear in the 1536–1495 cm−1 and 1305–1250 cm−1 ranges are due to ν(C⚌C) and ν(C—O) vibrations, respectively (El-tabl et al., 2008; El-Tabl et al., 2008). Additionally, complexes 2–9 shows medium bands in the ranges of 688–615, 570–520, and 450–405 cm−1, which are due to ν(M—N), ν(M—O), and ν(M—Cl) vibrations, respectively (El-tabl et al., 2008). These results combined with the elemental analysis indicate that the Schiff base coordinated to the metal ions via the hydroxyl oxygen of its naphthaldehyde moiety and the N atom of one of its azomethine groups.
Comp.No.
ν(OH)
ν(H2O)
ν(C⚌N)
ν(C⚌C)
ν(C-O)
ν(M—O)
ν(M—N)
ν(M—Cl)
1
3350
–
1620,1605
1540
1310
–
–
–
2
3345
–
1610,1542
1518
1300
620
520
450
3
3342
–
1608,1580
1495
1305
615
535
405
4
3440
3520–3300(br)
1617,1595
1530
1300
620
570
410
5
–
3420–3130(br)
1615,1592
1520
1305
652
567
425
6
–
3300–3030(br)
1615,1594
1515
1305
653
535
412
7
3320
3430–3120(br)
1617,1600
1536
1250
619
520
418
8
3443
3520–3230(br)
1612,1544
1511
1298
634
520
416
9
3410
–
1615,1594
1515
1305
688
534
430
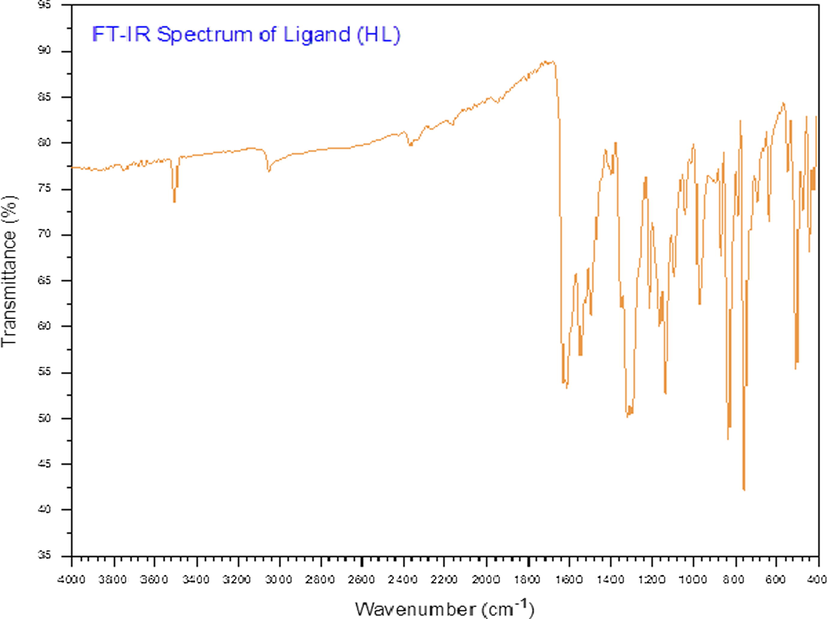
FT-IR spectrum of the ligand.
3.1.7 UV–Visible spectra
The electronic spectral data of the ligand (HL) and its metal complexes in DMSO are presented in Table 3. The ligand UV–Vis spectrum (Fig. 4) showed absorbance bands at 318 nm and 259 nm, which may correspond to the n
and
transitions, respectively, within the aromatic rings in the ligand. On the other hand, these transitions in the complexes appeared as bands that were shifted compared to those of the ligand; specifically, the bands corresponding to the n
and
transitions appeared in the ranges of 310–332 nm and 227–264 nm, respectively (Al-Hakimi et al., 2020). This indicates bonding between the ligand and the metallic elements. Furthermore, the V(III) complex showed a band at 486 nm representing a 3T1g (F)
3T2g (P) transition, further indicating an octahedral geometry for the V(III) ion (Dorn et al., 2020). The Cr (III) complex shows three bands at 484, 459, and 344 nm corresponding to the transitions 4A2g (F)
4T1g (P), 4A2g (F)
4T2g (F), and 4A2g (F)
4T1g (F), respectively; this supports the previously identified octahedral geometry of the Cr(III) ion (Tarafder et al., 2000). The Mn(II) complex showed three bands at 612, 484, and 464 nm that were assigned to 4T1g
6A1g, 4T2g (G)
6A1g, and 4T1g (D)
6A1g transitions, respectively, suggesting an octahedral geometry around the Mn(II) ion (Mahmoud et al., 2020). The Fe(III) complex showed bands at 487, 463, and 379 nm corresponding to 4T1g (D)
6A1g, 4T2g (G)
6A1g, and 4T2g (G)
6A1g transitions, respectively, which are indicative of an octahedral geometry (Mahmoud et al., 2020). The Co(III) complex showed two bands at 458 and 433 nm that were assigned to 4T1g (F)
4T2g (F) and 4T1g (F)
4A2g transitions, suggesting an octahedral geometry for the complex (Mahmoud et al., 2020). The Ni(II) complex showed bands at 455 and 363 nm, which are attributable to 3A2g (F)
3Tg (F) and 3A2g (F)
3T1g (P) transitions, respectively, indicating an octahedral Ni(II) complex (El-tabl et al., 2008). The Cu(II) complex exhibited bands centred at 490 and 460 nm that were assigned to ligand → metal charge transfer, 2B1
2B2, and 2B1
2E transitions, respectively, indicating a distorted octahedral structure (El-tabl et al., 2008). Finally, the Zn(II) complex showed bands at 330 and 260 nm, indicating the presence of intra-ligand transitions and LMCT (Al-Hakimi et al., 2020; El-Tabl et al., 2008).
Comp. No.
compounds
λmax (nm)
λmax (cm−1)
Assignment
1
[HL][C24H18N2O]
318
31,445
n
259
38,610
2
[(HL)(L)V(Cl)2]
486
20,576
3T1g (F)
3T2g (P)
322
31,055
n
233
42,918
3
[(HL)(L)Cr(Cl)2]
484
20,661
4A2g (F)
4T1g (P)
459
21,786
4A2g (F)
4T2g (F)
344
29,070
4A2g (F)
4T1g (F)
317
31,546
n
227
44,052
4
[(HL)Mn(Cl)2(H2O)2]
612
16,339
4T1g
6A1g
484
20,661
4T2g (G)
6A1g
464
12,551
4T1g (D)
6A1g
320
31,250
n
231
43,290
5
[(L)2Fe(Cl)(H2O)]
487
20,533
4T1g (D)
6A1g
463
21,598
4T2g (G)
6A1g
379
26,385
4T2g (G)
6A1g
318
31,447
n
261
38,314
6
[(L)2Co(Cl)(H2O)]
458
21,834
4T1g (F)
4T2g (F)
433
23,094
4T1g (F)
4A2g
317
31,546
n
262
38,168
7
[(HL)(L)Ni(Cl)(H2O)]
455
21,978
3A2g (F)
3Tg (F)
363
27,548
3A2g (F)
3T1g (P)
316
31,646
n
264
37,879
8
[(HL)Cu(Cl)2(H2O)2]
490
20,408
2B1
2B2
460
21,739
2B1
2E
332
30,120
n
258
38,760
9
[(HL)2Zn(Cl)2]
330
30,303
n
260
38,462
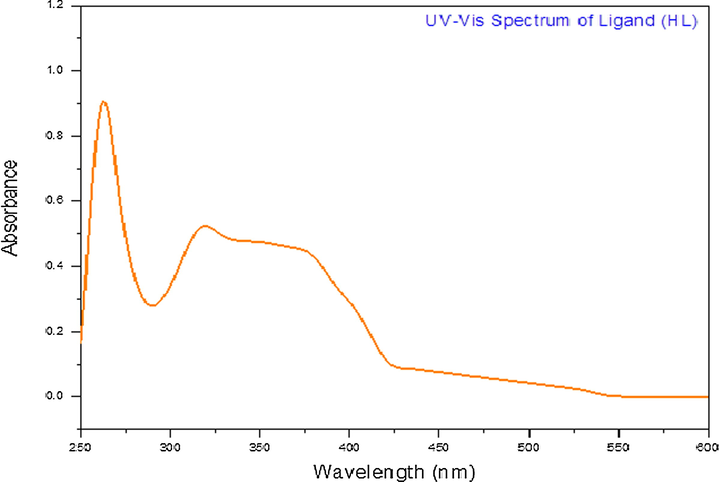
UV–Vis spectrum of the ligand.
3.1.8 Thermal analyses (DTA and TGA)
TGA and DTA curves in the range of 27–525 °C were collected for the complexes, which confirmed that they were thermally stable up to 100 °C. The thermal data for the complexes are summarized in Table 4 (El-Saied et al., 2018a; Shakdofa et al., 2021).
Comp. No.
Temp. range (oC)
DTA (peak)
TGA (Wt. loss %)
Assignment
Endo.
Exo.
Calc.
Found
2
201–320
304
8.63
8.11
Loss of two chloride atoms
321–415
393
–
42.65
42.27
Loss of one ligand (HL)
416–466
–
428
30.48
31.63
Decomposition with the formation of V2O3
3
189–286
–
215
8.62
8.26
Loss of two chloride atoms
287–416
–
299
42.60
41.93
Loss of one ligand (HL)
417–493
747
–
30.31
31.72
Decomposition with the formation of Cr2O3
4
133–265
–
160
7.03
6.81
Loss of two molecules of coordination water
266–344
–
–
13.84
14.27
Loss of two chloride atoms
345–496
–
381
65.28
64.30
Decomposition with the formation of MnO
5
160–264
–
256
2.23
2.13
Loss of molecule of coordination water
265–378
374
–
4.39
4.22
Loss of chloride atom
379–498
458
–
73.62
74.20
Decomposition with the formation of Fe2O3
6
159–253
–
–
2.22
2.47
Loss of molecule of coordination water
254–358
–
332
4.37
4.80
Loss of chloride atom
459–494
468
–
72.96
72.31
Decomposition with the formation of Co2O3
7
179–243
–
–
2.22
2.52
Loss of molecule of coordination water
244–361
–
341
4.37
4.88
Loss of chloride atom
362–514
438
–
84.21
83.76
Decomposition with the formation of NiO
8
115–235
–
–
6.92
6.47
Loss of two molecules of coordination water
236–304
–
262
13.61
14.83
Loss of two chloride atoms
305–489
341
–
64.20
65.12
Decomposition with the formation of CuO
9
224–316
–
–
8.47
8.62
Loss of two chloride atoms
317–465
–
382
81.82
82.13
Decomposition with the formation of ZnO
3.1.9 XRD analysis
The XRD patterns were recorded for the ligand and its complexes. The crystal structure details for all compounds are listed in Table 5. It was noted that the V(III) and Zn(II) complexes have hexagonal crystal systems. Meanwhile, the ligand and Ni(II) complex have orthorhombic crystal systems, while that of the Cr(III) complex is monoclinic, that of the Fe(III) complex is cubic, and those of the Mn(II), Co(III), and Cu(II) complexes are triclinic. Peak broadening in the XRD patterns signifies that the particles are on a nanometre scale (El-Shafiy et al., 2017). This is supported by the parameters of the crystal structures ranging from 12 nm to 217 nm (Saeed et al., 2014). The XRD patterns of the Co(III), Cu(II), and Zn(II) complexes are shown in Figs. 5, 6, and 7, respectively.
Parameters
compounds
HL
V(III)
Cr(III)
Mn(II)
Fe(III)
Co(III)
Ni(II)
Cu(II)
Zn(II)
a (Å)
13.179
9.878
24.02
9.692
13.62
8.148
10.082
10.66
12.550
b (Å)
11.045
9.878
8.541
10.072
13.62
9.337
20.16
12.46
12.550
c (Å)
19.731
10.20
14.44
10.300
13.62
11.259
18.92
13.93
7.13
Alfa (°)
90.0
90.0
90.0
80.57
90.0
70.13
90.0
117.8
90.0
Beta(°)
90.0
90.0
93.96
68.40
90.0
77.67
90.0
100.1
90.0
gamma(°)
90.0
120.0
90.0
76.21
90.0
86.09
90.0
94.9
120.0
Volume of unit cell(Å3)
2872
862
2956
904.8
2524
787
3844
1582
973
Crystal size (nm)
217
12
55
43
19
43
31
20
24
Crystal type
Orthorhombic
Hexagonal
monoclinic
Triclinic
Cubic
Triclinic
Orthorhombic
Triclinic
Hexagonal
Space group
Pbcm
P-6
C12 / m1
P-1
Pa-3
P-1
P mmm
P-1
P-6
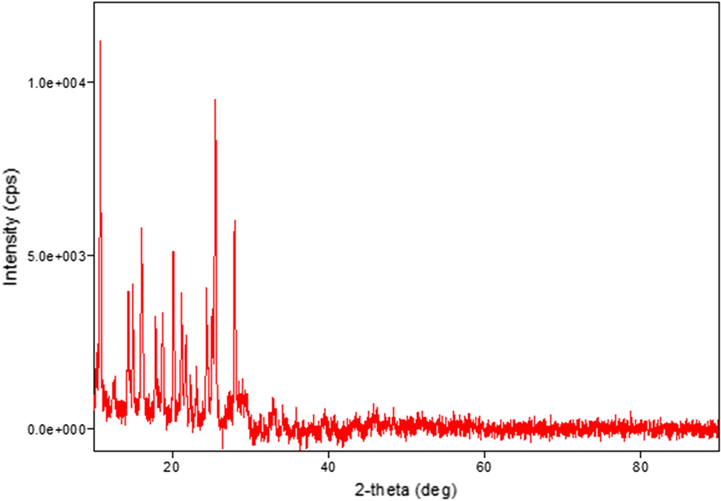
XRD pattern of the Co(III) complex.
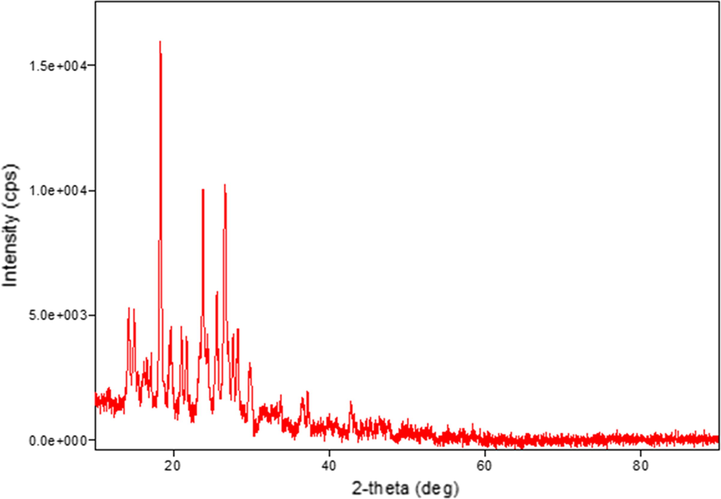
XRD pattern of the Cu(II) complex.
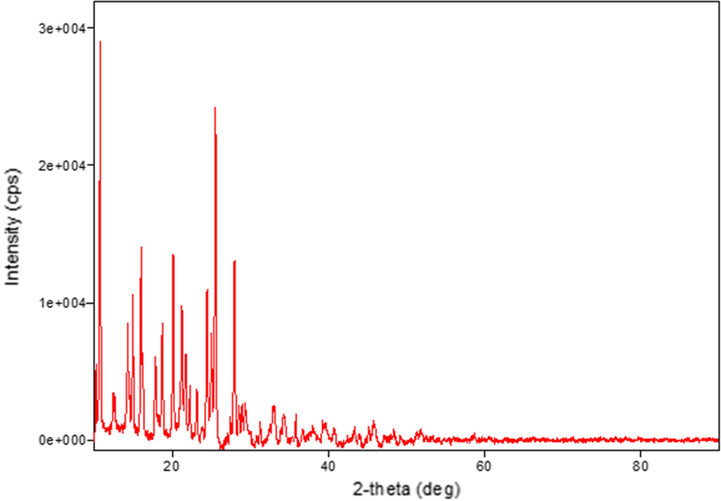
XRD pattern of the Zn(II) complex.
3.1.10 TEM morphological study
TEM was used to further examine the particle sizes and crystallinities of the samples. TEM bright-field images of the ligand and its metal complexes (Figs. 8 and 9) show that the samples comprise small and varying nanoparticles sizes. It can be clearly seen that the metal complex powders show both spherical and rod-shaped nanoparticles, while the ligand powder particles take on rod shapes and are micro-sized. Furthermore, the particles were estimated size of the ligand and metal complexes with a grain size of about 5.0–206.0 nm. These results agree with the XRD data in Table 5.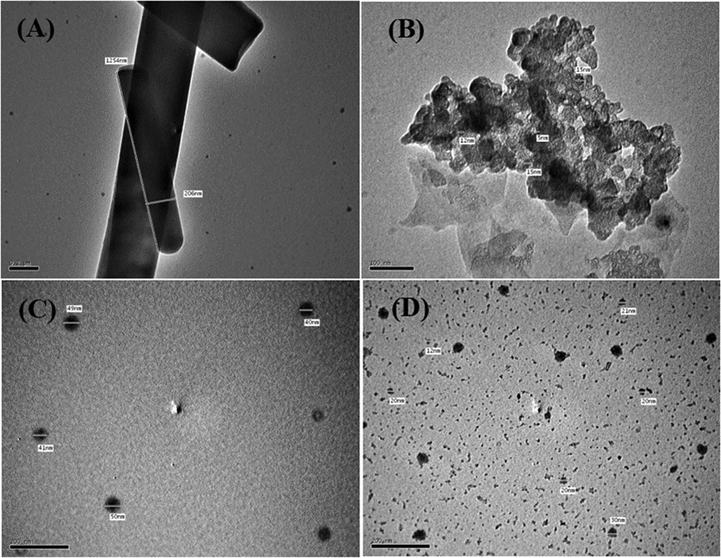
High-resolution TEM images of the (A) ligand, (B)V(III) complex, (C) Mn(II) complex, and (D) Fe(III) complex.
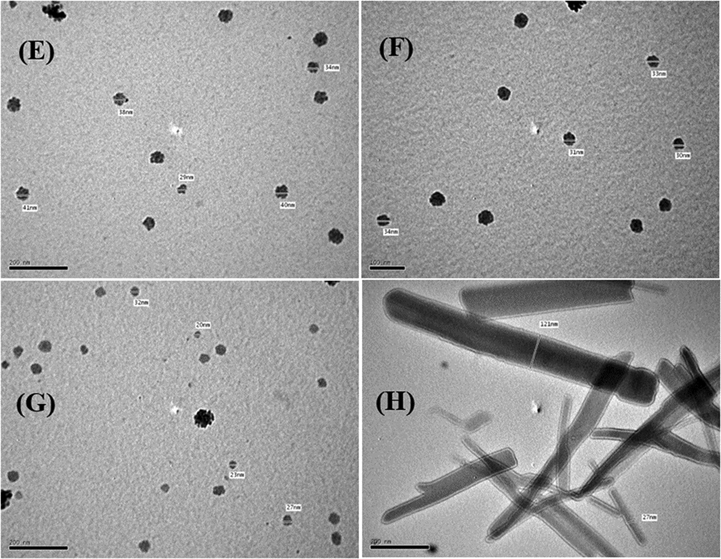
High-resolution TEM images of the (E) Co(III), (F) Ni(II), (G) Cu(II), and (H) Zn(II) complexes.
3.2 Biology
3.2.1 In vitro anticancer activities
The in vitro cytotoxic activities of the ligand and its metal complexes towards PC-3, SKOV3 and HeLa tumour cell lines were determined using SBR assays with six different concentrations of each compound (0.01, 0.1, 1, 10, 100, and 1000 µg/mL). By comparing the results with those of a previous study (Matela, 2020), the tested compounds in the present work exhibited highly cytotoxic activity against the selected human cell lines (Fig. 10). The data in Table 6 show that the Cu(II) complex had the highest activity among the tested compounds against PC-3, SKOV3, and HeLa cells with IC50 values of 0.161, 0.063, and 0.087 µg/mL, respectively. Meanwhile, the Ni(II) complex exhibited the lowest toxicity towards the PC-3, SKOV3 and HeLa cells among the tested compounds, giving IC50 values of 1.8287, 1.2502, and 3.8453 µg/mL, respectively. On the other hand, the range of IC50 values of the ligand and the V(III), Cr(III), Mn(II), Fe(III), Co(III), and Zn(II) complexes for the PC-3, SKOV3, and HeLa cells were 0.1786–1.0607, 0.0615–0.8128, and 0.0719–1.534 µg/mL, respectively. It is worth noting that the prepared compounds (1–9) exhibited higher activities than clinically used drugs such as cisplatin (IC50 ∼ 2.4 µg/mL) (Ray et al., 2007), estramustine (IC50 ∼ 0.35–1.3 µg/mL) (Nicholson et al., 2002), and etoposide (IC50 ∼ 17.4 µg/mL) (Aras and Yerlikaya, 2016). Fig. 11 shows the cytotoxic dose–response curves for compounds 1–9 towards selected human cell lines where cells were exposed to the compounds at different concentrations for 72 h.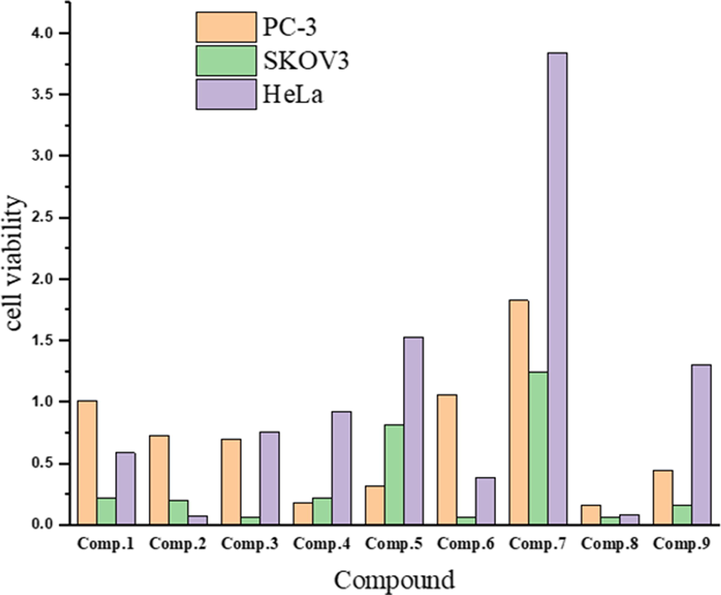
Screening of anticancer activity of the ligand and its metal complexes against selected human cell lines.
No.
Compound
IC50 (µg/mL)
PC-3
SKOV3
HeLa
1
[HL][C24H18N2O]
1.0123 ± 0.05
0.2163 ± 0.005
0.5877 ± 0.14
2
[(HL)(L)V(Cl)2]
0.7262 ± 0.14
0.1997 ± 0.03
0.0719 ± 0.02
3
[(HL)(L)Cr(Cl)2]
0.7027 ± 0.25
0.0678 ± 0.03
0.7606 ± 0.05
4
[(HL)Mn(Cl)2(H2O)2]
0.1786 ± 0.02
0.2189 ± 0.05
0.9254 ± 0.05
5
[(L)2Fe(Cl)(H2O)]
0.3188 ± 0.02
0.8128 ± 0.20
1.5340 ± 0.30
6
[(L)2Co(Cl)(H2O)]
1.0607 ± 0.16
0.0615 ± 0.02
0.3921 ± 0.03
7
[(HL)(L)Ni(Cl)(H2O)]
1.8287 ± 0.10
1.2502 ± 0.20
3.8453 ± 0.32
8
[(HL)Cu(Cl)2(H2O)2]
0.1612 ± 0.005
0.0630 ± 0.003
0.0872 ± 0.01
9
[(HL)2Zn(Cl)2]
0.4436 ± 0.07
0.1646 ± 0.04
1.3064 ± 0.46
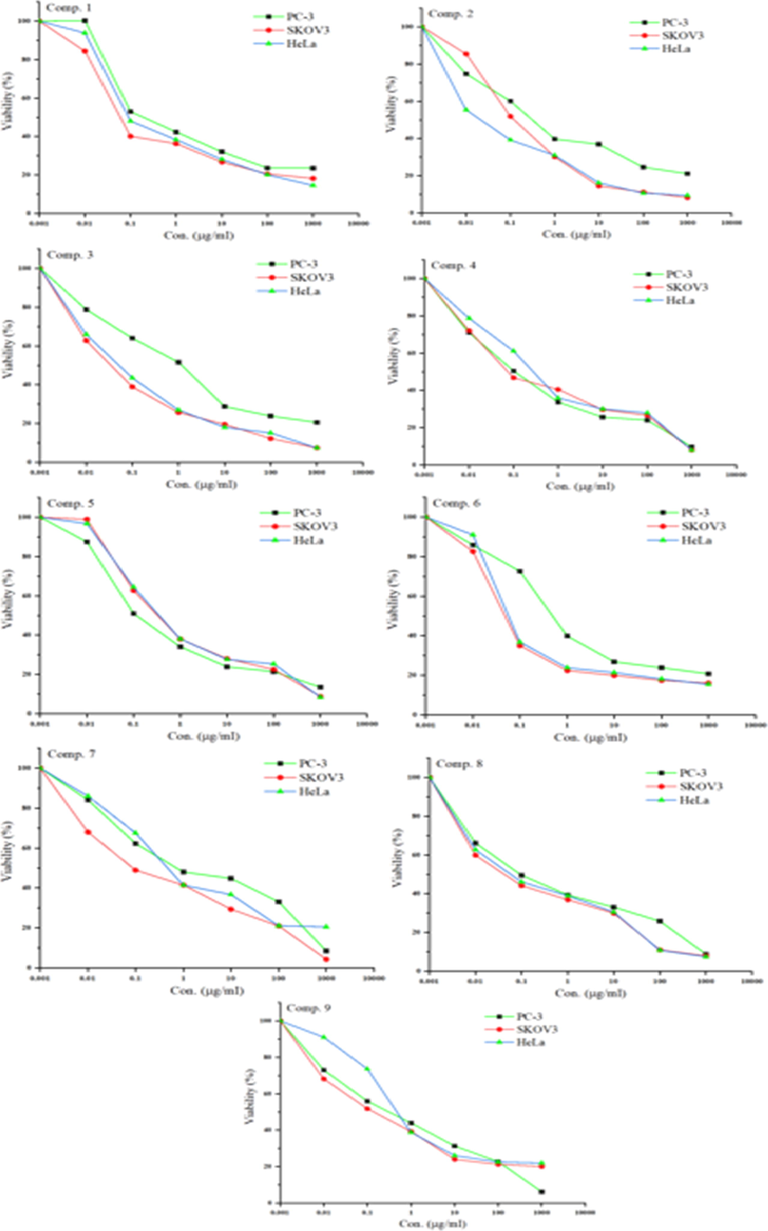
The dose–response curves of the cytotoxicities of compounds 1–9 towards PC-3, SKOV3, and HeLa human cell lines. Cells were exposed to different concentrations of the compounds for 72 h. Cell viability was determined by sulforhodamine B (SRB) staining.
3.2.2 Antimicrobial activity
The data in Table 7 show the antibacterial and antifungal activities of ligand and its metal complexes in different volumes against the growth of S. enterica ser. typhi, (Fig. 12) and C. albicans (Fig. 13). It was observed that the Co(III) and Zn(II) complexes had intermediate antibacterial activities against S. enterica ser. typhi when used in volumes of 30, 20, and 10 µL (Fig. 14). On other hand, in the same volumes, the V(III), Cr(III), Mn(II), Fe(III), Ni(II), and Cu(II) complexes and the ligand do not exhibit antibacterial activities against S. enterica ser. typhi. However, the V(III) complex had the highest activity against C. albicans when used in volumes of 30, 20, and 10 µL (Fig. 14). The Fe(III) and Cr(III) complexes also had high antifungal activities against C. albicans in volumes of 30 µL and 20 µL (Fig. 14). The Co(III) and Cu(II) complexes in volumes of 30, 20, and 10 µL had intermediate antifungal activities against C. albicans, while the Mn(II), Ni(II), and Zn(II) complexes and the ligand showed weak antifungal activities against C. albicans when used in the same volumes. Note: Zone of inhibition in mm.
No.
Compound
Salmonella typhi
Candida albicans
30 µL
20 µL
10 µL
30 µL
20 µL
10 µL
–
DMSO
0
0
0
0
0
0
1
[HL][C24H18N2O]
0
0
0
4
3
2
2
[(HL)(L)V(Cl)2]
0
0
0
13
12
9
3
[(HL)(L)Cr(Cl)2]
0
0
0
11
10
8
4
[(HL)Mn(Cl)2(H2O)2]
0
0
0
5
3
0
5
[(L)2Fe(Cl)(H2O)]
0
0
0
12
11
9
6
[(L)2Co(Cl)(H2O)]
9
8
7
10
9
7
7
[(HL)(L)Ni(Cl)(H2O)]
0
0
0
4
3
0
8
[(HL)Cu(Cl)2(H2O)2]
0
0
0
9
8
6
9
[(HL)2Zn(Cl)2]
7
6
5
5
4
2
–
Gentamycin
–
–
2
–
–
–
–
Clotrimazole
–
–
–
–
–
3
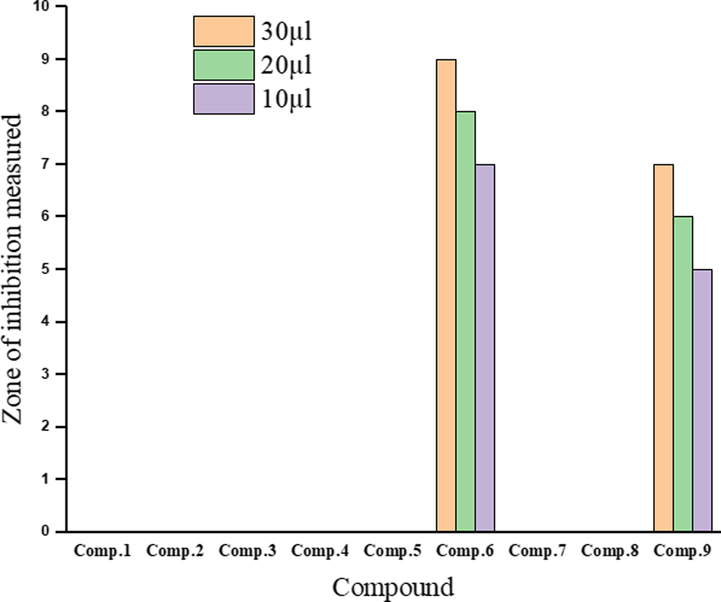
Antibacterial activities of the ligand and its metal complexes.
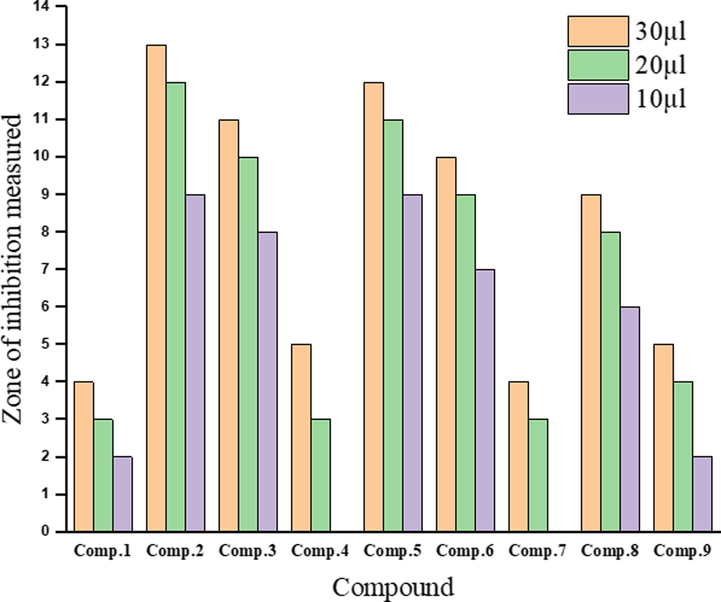
Antifungal activities of the ligand and its metal complexes.
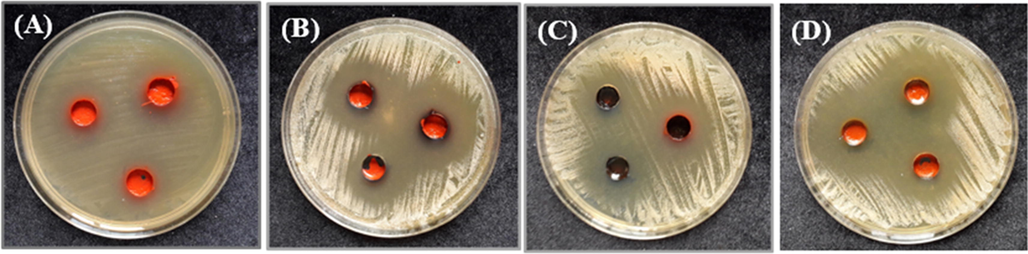
Inhibition zone of the (A) Co(III) complex against S. enterica ser. Typhi growth, and those of the (B) V(III), (C) Fe(III), and (D) Cr(III) complexes against C. albicans growth.
4 Conclusion
In this work, we have synthesized a new Schiff base and employed it as a ligand in various metal complexes. Spectral, elemental, and thermal analyses, as well as conductivity measurements, confirmed that the ligand adopted bidentate behaviour and bonded with the metals through its azomethine nitrogen atom and its phenolic oxygen atom, thus exhibiting octahedral geometries. XRD analysis of the complexes indicated that they were of various structures: triclinic, orthorhombic, hexagonal, monoclinic, and cubic. TEM analysis confirmed that the metal complex powders contain both spherical and rod-shaped nanoparticles, while the ligand powder shows rod-shaped, micro-size particles. The antibacterial and antifungal activities of the ligand and its complexes against S. enterica ser. typhi and C. albicans were investigated by the hole plate diffusion method. It was observed that the Co(III) and Zn(II) complexes had intermediate antibacterial activities, while the V(III) complex demonstrated the highest activity against C. albicans fungi among the tested complexes. Furthermore, the in vitro antitumor results showed that the Cu(II) complex had the highest activity among the tested compounds against PC-3, SKOV3, and HeLa cells. Remarkably, the ligand and all its metal complexes showed higher antitumour activities against these selected human cell lines than clinically used drugs such as cisplatin, estramustine, and etoposide.
Funding
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors
References
- Asian J. Chem.. 2020;32:2502-2506.
- Synthesis, characterization and microbicides activities of N(hydroxy-4-((4-nitrophenyl)diazenyl) benzylidene)-2-(phenylamino) acetohydrazide metal complexes. Egypt. J. Chem.. 2020;63:1509-1525.
- [Google Scholar]
- Al-Hakimi, A.N., Alminderej, F., Aroua, L., Alhag, S.K., Alfaifi, M.Y., M, S.O., Mahyoub, J.A., Eldin I. Elbehairi, S., Alnafisah, A.S., 2020. Design, synthesis, characterization of zirconium (IV), cadmium (II) and iron (III) complexes derived from Schiff base 2-aminomethylbenzimidazole, 2-hydroxynaphtadehyde and evaluation of their biological activity. Arab. J. Chem. 13, 7378–7389. https://doi.org/10.1016/j.arabjc.2020.08.014.
- Naproxen based 1, 3, 4-oxadiazole derivatives as EGFR inhibitors: design, synthesis, anticancer, and computational studies. Pharmaceuticals. 2021;14:870.
- [Google Scholar]
- Transition metal complexes derived from 2-hydroxy-4-(p-tolyldiazenyl) benzylidene)-2-(p-tolylamino) acetohydrazide synthesis, structural characterization, and biological activities. J. Korean Chem. Soc.. 2021;65:93-105.
- [Google Scholar]
- Synthesis and characterization of transition metal coordination polymers derived from 1,4-benzenedicarboxylate and certain azoles. Turkish J. Chem.. 2012;36:69-79.
- [CrossRef] [Google Scholar]
- Bortezomib and etoposide combinations exert synergistic effects on the human prostate cancer cell line PC-3. Oncol. Lett.. 2016;11:3179-3184.
- [Google Scholar]
- Zn (II) complex derived from bidentate Schiff base ligand: synthesis, characterization, DFT studies and evaluation of anti-in flammatory activity. J. Mol. Struct.. 2020;1201:127177.
- [CrossRef] [Google Scholar]
- Experimental evaluation of cationic-Schiff base surfactants based on 5-chloromethyl salicylaldehyde for improving crude oil recovery and bactericide. J. Mol. Liq.. 2020;316:113862.
- [CrossRef] [Google Scholar]
- Synthesis, characterization and antimicrobial studies of novel ONO donor hydrazone Schiff base complexes with some divalent metal (II) ions. Arab. J. Chem.. 2020;13:6559-6567.
- [CrossRef] [Google Scholar]
- The effect of Schiff base ligands on the structure and catalytic activity of cobalt complexes in hydrosilylation of olefins. Appl. Catal. A Gen.. 2020;602:117665.
- [CrossRef] [Google Scholar]
- Synthesis, spectroscopic properties, crystal structures, antioxidant activities and enzyme inhibition determination of Co(II) and Fe(II) complexes of Schiff base. Res. Chem. Intermed.. 2020;46:283-297.
- [CrossRef] [Google Scholar]
- Chitosan-cinnamaldehyde Schiff base: a bioinspired macromolecule as corrosion inhibitor for oil and gas industry. Int. J. Biol. Macromol.. 2020;158:127-138.
- [CrossRef] [Google Scholar]
- A vanadium(III) complex with blue and NIR-II spin-flip luminescence in solution. J. Am. Chem. Soc.. 2020;142:7947-7955.
- [CrossRef] [Google Scholar]
- Antitumor activity of synthesized and characterized Cu (II), Ni (II) and Co (II) complexes of hydrazone-oxime ligands derived from 3-(hydroxyimino) butan-2-one. Beni-Suef Univ. J. basic Appl. Sci.. 2018;7:420-429.
- [Google Scholar]
- New nano-complexes of Zn (II), Cu (II), Ni (II) and Co (II) ions; spectroscopy, thermal, structural analysis, DFT calculations and antimicrobial activity application. J. Mol. Struct.. 2017;1147:452-461.
- [Google Scholar]
- Synthesis, characterisation and antimicrobial activity of manganese (II), nickel (II), cobalt (II), copper (II) and zinc (II) complexes of a binucleating tetradentate ligand. J. Chem. Res.. 2002;2002:529-531.
- [Google Scholar]
- Spectroscopic characterization and biological activity of metal complexes with an ONO trifunctionalized hydrazone ligand. J. Coord. Chem.. 2008;61:2380-2401.
- [Google Scholar]
- Synthesis, spectroscopic characterization and biological activity of the metal complexes of the Schiff base derived from phenylaminoacetohydrazide and dibenzoylmethane. Spectrochim Acta Part A Mol. Biomol. Spectrosc.. 2008;71:90-99.
- [CrossRef] [Google Scholar]
- Anti-neurotoxic evaluation of synthetic and characterized metal complexes of thiosemicarbazone derivatives. Appl. Organomet. Chem.. 2018;32:e4215
- [Google Scholar]
- Transition metal complexes derived from N′-(4-fluorobenzylidene)-2-(quinolin-2-yloxy) acetohydrazide: Synthesis, structural characterization, and biocidal evaluation. Appl. Organomet. Chem.. 2020;34:e5898
- [Google Scholar]
- Antimicrobial activity of Elettaria cardamomum: toxicity, biochemical and histological studies. Food Chem.. 2007;104:1560-1568.
- [Google Scholar]
- Chemical and biological characteristics of Cuminum cyminum and Rosmarinus officinalis essential oils. Food Chem.. 2007;102:898-904.
- [Google Scholar]
- Production of terretonin N and butyrolactone I by thermophilic aspergillus terreus TM8 promoted apoptosis and cell death in human prostate and ovarian cancer cells. Molecules. 2021;26:2816.
- [Google Scholar]
- Potentiometric and antimicrobial studies on the asymmetric Schiff bases and their binuclear Ni(II) and Fe(III) complexes; synthesis and characterization of the complexes. Arab. J. Chem.. 2019;12:2244-2256.
- [CrossRef] [Google Scholar]
- Chemical composition and antibacterial activity of essential oil of Pulicaria odora L. J. Ethnopharmacol.. 2005;99:399-401.
- [Google Scholar]
- Synthesis, crystal structure, and characterization of two Cu(II) and Ni(II) complexes of a tetradentate N2O2 Schiff base ligand and their application in fabrication of a hydrazine electrochemical sensor. Inorganica Chim. Acta. 2020;506:119537.
- [CrossRef] [Google Scholar]
- Pd(II) complexes bearing chromone based Schiff bases: synthesis, characterisation and biological activity studies. Arab. J. Chem.. 2016;9:640-648.
- [CrossRef] [Google Scholar]
- Synthesis, spectral, theoretical and antioxidant studies of copper (II) and cobalt (III) macroacyclic Schiff-base complexes containing homopiperazine moietiy. Chem. Data Collect.. 2020;26:100354.
- [CrossRef] [Google Scholar]
- Mitigation of eco-unfriendly and costly microbial induced corrosion using novel synthesized Schiff base cationic surfactants. J. Chem. Technol. Biotechnol. 2020
- [Google Scholar]
- Chemical composition and biological activities of essential oil from the leaves of Sesuvium portulacastrum. J. Ethnopharmacol.. 2006;103:85-89.
- [CrossRef] [Google Scholar]
- Transition metal complexes of Schiff base ligand based on 4, 6-diacetyl resorcinol. Appl. Organomet. Chem.. 2020;34:e5528
- [Google Scholar]
- Efficacy of novel Schiff base derivatives as antifungal compounds in combination with approved drugs against candida albicans. Med. Chem. (Los. Angeles).. 2018;15:648-658.
- [CrossRef] [Google Scholar]
- Biologically active water soluble novel biopolymer/hydrazide based O -carboxymethyl chitosan schiff bases: synthesis and characterisation. J. Inorg. Organomet. Polym. Mater.. 2020;30:3658-3676.
- [CrossRef] [Google Scholar]
- Schiff bases and complexes: a review on anti-cancer activity. Anti-Cancer Agents Med Chem. (Formerly Curr. Med. Chem. Agents). 2020;20:1908-1917.
- [Google Scholar]
- Oxovanadium(IV) complexes of bioinorganic and medicinal relevance: synthesis, characterization and 3D molecular modeling of some oxovanadium(IV) complexes involving O, N-donor environment of salicylaldehyde-based sulfa drug Schiff bases. Arab. J. Chem.. 2016;9:S1084-S1100.
- [CrossRef] [Google Scholar]
- New palladium(II) and platinum(II) complexes based on pyrrole Schiff bases: synthesis, characterization, X-ray structure, and anticancer activity. ACS Omega. 2020;5:14942-14954.
- [CrossRef] [Google Scholar]
- Novel dextrin-cysteine Schiff base: a highly efficient sensor for mercury ions in aqueous environment. ChemistrySelect. 2020;5:2082-2093.
- [Google Scholar]
- Synthesis, Characterization and comparative thermal degradation study of Co (II), Ni (ΙΙ) and Cu (II) complexes with Asparagine and Urea as mixed ligands. Eclética Química J.. 2018;43:11-24.
- [Google Scholar]
- Synthesis, characterization, electrochemical properties and antibacterial activity of some transiton metal complexes with [(2-hydroxy-1-naphthaldehyde)-3-isatin]-bishydrazone. Transit. Met. Chem.. 2006;31:441-446.
- [Google Scholar]
- Palladium(II) complexes comprising naphthylamine and biphenylamine based Schiff base ligands: synthesis, structure and catalytic activity in Suzuki coupling reactions. J. Organomet. Chem.. 2020;914:121220.
- [CrossRef] [Google Scholar]
- In vitro and in vivo activity of LS 4477 and LS 4559, novel analogues of the tubulin binder estramustine. Eur. J. Cancer. 2002;38:194-204.
- [CrossRef] [Google Scholar]
- Transition metal complexes of novel binuclear Schiff base derived from 3,3′-diaminobenzidine: synthesis, characterization, thermal behavior, DFT, antimicrobial and molecular docking studies. J. Coord. Chem.. 2020;73:1009-1027.
- [CrossRef] [Google Scholar]
- Anticancer and antimicrobial metallopharmaceutical agents based on palladium, gold, and silver N-heterocyclic carbene complexes. J. Am. Chem. Soc.. 2007;129:15042-15053.
- [CrossRef] [Google Scholar]
- High-performance naphthalenediamine-based polybenzoxazine and its cured epoxy resin. J. Mater. Sci.. 2020;55:806-816.
- [Google Scholar]
- One-step thermolysis synthesis of divalent transition metal ions monodoped and tridoped CdS and ZnS luminescent nanomaterials. J. Nanomater.. 2014;2014
- [Google Scholar]
- Transition metal complexes of a hydrazone–oxime ligand containing the isonicotinoyl moiety: synthesis, characterization and microbicide activities. Appl. Organomet. Chem.. 2018;32:e4376
- [Google Scholar]
- Synthesis, characterization, and density functional theory studies of hydrazone–oxime ligand derived from 2, 4, 6-trichlorophenyl hydrazine and its metal complexes searching for new antimicrobial drugs. Appl. Organomet. Chem.. 2021;35:e6111
- [Google Scholar]
- Antimicrobial, spectral and thermal studies of divalent cobalt, nickel, copper and zinc complexes with triazole Schiff bases. Arab. J. Chem.. 2017;10:S978-S987.
- [CrossRef] [Google Scholar]
- New copper (II) and zinc (II) complexes based on azo Schiff base ligand: Synthesis, crystal structure, photoisomerization study and antibacterial activity. Appl. Organomet. Chem.. 2020;34:e5503
- [Google Scholar]
- Complexes of a tridentate ONS Schiff base. Synthesis and biological properties. Transit. Met. Chem.. 2000;25:456-460.
- [CrossRef] [Google Scholar]
- Crystal structure and antitumor activity of some rare earth metal complexes with Schiff base. Polyhedron. 2000;19:2599-2604.
- [CrossRef] [Google Scholar]
- Graphene oxide functionalized with a Schiff Base for the removal of Pb (II) ions from contaminated water: experimental and modeling approach. J. Chem. Technol. Biotechnol.. 2020;95:1694-1704.
- [Google Scholar]







