Translate this page into:
Synthesis, characterization and antimicrobial studies on 13-membered-N6-macrocyclic transition metal complexes containing trimethoprim
⁎Corresponding author. Tel.: +91 0431 2407082; fax: +91 431 2407045. drgm@bdu.ac.in (G. Muralitharan)
-
Received: ,
Accepted: ,
This article was originally published by Elsevier and was migrated to Scientific Scholar after the change of Publisher.
Peer review under responsibility of King Saud University.
Abstract
Asymmetrical macrocyclic complexes of MnII, CoII, NiII, CuII and ZnII have been synthesized by the template process using bis(benzil)ethylenediamine as precursor. Bis(benzil)ethylenediamine reacts with transition metal chlorides and trimethoprim in a 1:1:1 molar ratio in methanol to give several solid metal complexes of the general composition [M(L)X2] (M = MnII, CoII, NiII, CuII and ZnII, L = ligand and X = Cl−). They were characterized by physicochemical and spectroscopic techniques. Based on analytical, spectral and magnetic moments, all the complexes are identified as distorted octahedral structures. All the complexes are of the [M(L)X2] type. The shifts of the ν(C⚌N) (azomethine) stretches have been monitored. To find out the donor sites of the ligands, the activity data show that the metal complexes are more potent than the parent ligand. The [M(L)X2] complexes showed a broad spectrum of antimicrobial activity in vitro against both gram-positive and gram-negative human pathogenic bacterial isolates and the antimicrobial spectrum enhanced only with a combination of metal chlorides and trimethoprim complex. From the results it is imperative that the synthesized macrocyclic [M(L)X2] complexes exhibit potent broad spectrum antibacterial activity.
Keywords
Trimethoprim
Bis(benzil)ethylenediamine
Antimicrobial activity
Schiff bases
1 Introduction
The intense interest in synthetic macrocycles and their metal complexes depends on the fact that they mimic naturally occurring macrocyclic molecules in their structural and functional features due to rich chemical properties (Lindoy, 1989; Dietrich et al., 1993; Izatt et al., 1991). One of these properties is the enhanced thermochemical and kinetic stability of the complexes with regard to their dissociation which is due tolesser liability and larger association constants than the complexes with homologous open-chain chelating ligands. The application of polyazamacrocycle precursors in the synthesis of transition metal macrocyclic complexes stems mainly from their use as models for protein-metal binding sites in biological systems (Kimura et al., 1985; Fenton and Okawa, 1993; Kimura, 1993; Karlin and Tyeklar, 1991) and as selective complexing agents for metal ions (Muller et al., 1983; Tsubuke et al., 1991; Beklemishev et al., 1994; Mohite et al., 1994).
In this paper, we report on the synthesis and characterization of new bivalent transition metal complexes from the reaction of bis(benzil)ethylenediamine with trimethoprim which show a marked effect of any build-up of ligand strain on the coordination geometry of the central metal. Due to emerging multidrug resistance to antibiotics like ampicillin (A), chloramphenicol (C), trimethoprim–sulfamethoxazole (Tm-Smz), ciprofloxacin (Cp), fluoroquinolones, (Fq) etc., treatment of infection becomes a problem during outbreaks of disease (Brusch, 2009; Dutta et al., 2008; Saha et al., 2006). Currently, the broad spectrum antibiotics were the preferred drugs of choice in many Asian countries, due to increased indiscriminate use of antibiotics for the treatment of humans and animals diseases, thereby developing multidrug resistance organisms (Mijovic et al., 2012), which pose serious threats in developing countries like India. With this view, the synthesized macrocyclic [M(L)X2] complexes were tested against human pathogenic bacterial isolates for evaluating their antimicrobial properties through disc diffusion and minimal inhibitory concentration (MIC) assay.
2 Experimental
2.1 Materials and methods
All the chemicals used for the preparation of the ligands were of BDH quality, AR grade. Molar conductance of the complexes was measured using a Toshniwals conductivity bridge using dip type platinized platinum electrode. Molecular weights were determined by the Rast camphor method. The magnetic moments were measured out by using the gouy balance. Proton NMR spectra were recorded on a EM 300–30 MHz NMR spectrometer in DMSO. IR spectra (KBr) of the samples were recorded on a Shimadzu FTIR-8400s spectro-photometer. The electronic spectra (chloroform) were recorded on the Labomed UVD-3200 spectrophotometer (U.S.A.). The X-ray powder diffraction patterns were obtained with a SEIFERT model XRD 3000p powder X-ray diffractometer using Cu-Kα radiation filtered by a nickel foil over the range of diffraction angle, 2θ = 5–80 in the complexes studied, where θ is the Bragg angle.
2.2 Synthesis of the complexes
To a methanolic solution of the precursor complex (Radhakrishna et al., 1996), bis(benzil)ethylenediamine, Trimethoprim (4.3 mM) and metal chlorides (4.3 mM) in methanol (25 ml) were added and the resulting mixture was heated under reflux for 5–6 h. The solution was cooled and transferred to an evaporating dish and set aside overnight at room temperature. The solid product that separated out was collected, washed with hot water, then with cold methanol and dried.
2.3 General properties
Mn(II), Co(II), Ni(II) and Cu(II) complexes are coloured but the zinc complexes are colourless (Table 1). All the complexes are soluble in dimethyl formamide (DMF), acetonitrile, DMSO and chloroform.
Complex
Empirical formula
Colour
Mol. Wt.
M.P. (°C)
Yield (%)
[Mn(L)Cl2]
C44H38MnN6O3Cl2
Red
824
220
60
[Co(L)Cl2]
C44H38CoN6O3Cl2
Pink
828
242
55
[Ni(L)Cl2]
C44H38NiN6O3Cl2
Green
828
216
58
[Cu(L)Cl2]
C44H38CuN6O3Cl2
Pale blue
833
232
61
[Zn(L)Cl2]
C44H38ZnN6O3Cl2
Colourless
835
256
52
2.4 Bacterial strains
Five different bacterial strains used in this study were originally isolated from patient’s samples. The bacterial isolates tested include gram positive species of Staphylococcus aureus and gram-negative species like Escherichia coli, Shigella flexneri, Pseudomonas aeruginosa and Vibrio cholerae. The bacterial strains were identified based on standard phenotypic, biochemical tests and grown overnight at 37 °C in nutrient broth. Cultures were maintained in nutrient agar (Hi-media, M002) slants at 4 °C. Subcultures were being made freshly before assaying.
2.5 Determination of antibacterial activity by disc diffusion assay
Antimicrobial activity of the synthesized macrocyclic [M(L)X2] complexes was carried out by the disc diffusion method. Two different concentrations of synthesized compounds (400–300 μg/ml) were prepared and their efficacy was assessed on Muller-Hinton agar (MHA) plates. Bacterial strains grown on nutrient broth medium at 37 °C for 24 h were adjusted to a final turbidity of 0.5 Mc Farland standard (108 CFU/ml) (Saad et al., 2011). A lawn culture was made with this bacterial load on the surface of MHA (Merck, U.S.A.). Aseptically, the synthesized [M(L)X2] complex was aseptically impregnated on a sterile 6 mm filter paper disc with 25 μl of each concentration and placed on bacterial seeded solid medium surface. All the plates were incubated at 37 °C for 24 h and tested in duplicate. The clear zone of inhibition around each disc was measured after incubation and recorded in millimetre. The standard antibiotics like, ampicillin (30 μg), nalidixic acid (30 μg), norfloxacin (10 μg), ciprofloxacin (5 μg), methicillin (5 μg), were included as control antibiotics and the zone of inhibition measured for these antibiotics were interpreted in accordance to manufacturers’ standard (Hi-Media). The CLSI (NCCLS) standard method was employed to determine antimicrobial susceptibility to reflect the resistance rate of test pathogens for the tested compounds (Skov et al., 2000).
2.6 Determination of minimal inhibitory concentration (MIC) assay
The microdilution method was used to determine the lowest or minimum complex test material required for inhibiting the growth of the tested microorganism. A serial dilution of each macrocyclic [M(L)X2] complex was prepared with dilution starting at 256–0.05 μg/ml using DMSO as solvent system (Irith et al., 2008). The MIC was performed in a 96 well assay plate filled with Muller-Hinton broth medium with different concentrations of the synthesized macrocyclic [M(L)X2] complex with standard antibiotic as positive and DMSO solvent as negative controls. An equal volume of 100 μl of fresh bacterial broth suspension (108 CFU/ml) was added to the wells without altering the dilution factor. The entire assay was prepared in triplicate in 96 well MIC plates and incubated at 37 °C for 24 h. The minimum inhibitory concentration (MIC) was determined by comparing the various concentrations of the synthesized macrocyclic [M(L)X2] complex showing different inhibitory effect and selecting the lowest concentration that inhibits the growth of the tested microorganism (Prasannabalaji et al., 2012).
2.7 Determination of minimum bactericidal concentration (MBC)
To determine the Minimum Bactericidal Concentration (MBC), the lowest concentration of the synthesized macrocyclic [M(L)X2] complex or standard antibiotic which destroys the tested bacterial strains in the MIC assay was cultured on freshly prepared MHA plates and incubated at 37 °C for 24 h. The highest diluted compound showing no growth on agar plates after incubation was considered as the MBC value.
3 Results and discussion
The elemental analysis is shown in Table 2. The molar conductance values obtained for these complexes at the concentration of 10−3 M are in the range of 10–50 Ω−1 cm2 mol−1. These values are too low to account for any dissociation of the complexes in acetonitrile. Hence these complexes can be regarded as non-electrolytes. The ligand (L) is soluble in common organic solvents such as THF, DMSO and methanol. The octahedral metal complexes are highly soluble in DMSO, DMF and chloroform.
Ligand/complex
Elemental analysis % found (calculated)
μeff. BM
Molar Conductance (Ω−1 cm2 mol−1)
C
H
N
M
C44H38MnN6O3Cl2
64.01
4.55
10.10
6.59
5.94
26.15
(64.08)
(4.61)
(10.19)
(6.66)
C44H38CoN6O3Cl2
63.69
4.52
10.09
7.07
5.01
31.14
(63.77)
(4.59)
(10.14)
(7.11)
C44H38NiN6O3Cl2
63.71
4.56
10.10
7.01
3.01
26.68
(63.77)
(4.59)
(10.14)
(7.09)
C44H38CuN6O3Cl2
63.31
4.52
10.00
7.57
2.01
28.21
(63.39)
(4.56)
(10.08)
(7.62)
C44H38ZnN6O3Cl2
63.19
4.51
10.03
7.82
Dia
30.5
(63.23)
(4.55)
(10.06)
(7.86)
3.1 Conductance
The observed molar conductance values of the complex in acetonitrile are in the range of 10–50 Ω−1 cm2 mol−1 at room temperature. Hence from conductivity measurements, it is concluded that the chloride ions are covalently bonded to metal ions, which indicates that they act as ligands and not as simple ions. Based on the metal–ligand ratio calculated by the analytical data and the nature of the electrolytes given by the conductance measurements, compositions were assigned for the prepared complexes. From the magnetic and conductometric analysis it is predicted that the complexes may have the following structures [Mn(L)Cl2], [Co(L)Cl2], [Ni(L)Cl2], [Cu(L)Cl2] and [Zn(L)Cl2].
3.2 Magnetic moments
The magnetic moment values of Co(II), Ni(II) and Cu(II) complexes are shown in Table 2. The magnetic moment of Co (II) complex is in the range of 5.01 BM indicating that the Co(II) complexes are typically high spin complexes having an octahedral structure. The Ni(II) complexes exhibit the magnetic moment values in the range 3.01 BM, indicating octahedral coordination of the ligands around Ni(II) ion. The Cu(II) complexes exhibit magnetic moment in the range 2.01 BM suggesting a distorted octahedral nature. The absorption bands observed for the electronic spectra of the metal complexes also support the octahedral geometry.
3.3 Infrared spectral analysis
The IR spectra of Ligand (L) with its octahedral complexes have been studied in order to characterize their structures. The IR spectra of the free ligand and its metal complexes were carried out in the 4000–400 cm−1 range. The infrared spectra of the ligand C30H24N2O2 and the metal complexes show the absence of uncondensed functional groups (NH2 and C⚌O), stretching modes of the starting material and the appearance of bands characteristic of the imine group (Shakir et al., 1995). The bands characteristic of the benzil moiety appeared in all the complexes at 1500–1551 cm−1 (νasymC6H5) and 1316–1399 cm−1 (νsymC6H5). The major changes observed in the IR spectra of the macrocyclic complexes are the absence of stretching and deformation vibrations of NH2 groups, indicating their deprotonation and the appearance of strong bands due to coordinated ν(C⚌N) vibrations (Kumari et al., 1993), in the range 1635–1644 cm−1. Strong and sharp bands for C—H stretching and bending vibrations appear in 2835–2837 and 1444–1453 cm−1, respectively (Coltrain and Jackels, 1981). The presence of new bands in the spectra of the metal complexes in the far-IR region at 419–439 cm−1 due to the ν(M—N) vibrations supports the coordination of the imine nitrogen to the metal ion (Singh et al., 1989). Fig. 2 describes the FT-IR spectrum of the Co(C44H38N6O3)Cl2 complex.
Suggested structure of the complex.
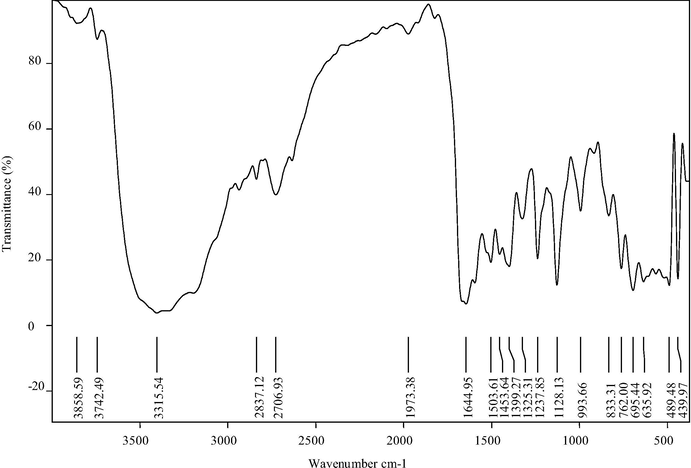
FT-IR spectrum of the Co (C44H38N6O3) Cl2 complex.
3.4 Electronic spectra
The electronic spectra of bis(benzil)ethylenediamine and its derivatives have been recorded on a Lambda 35 spectrometer. The absorption maximum at 375 nm in the case of bis(benzil)ethylenediamine may be assigned to the n → π∗ transition of the azomethine group. The shift of this band (15 nm) in the spectra of the complexes indicated the coordination of the nitrogen to the metal atom (Dixit and Tandon, 1990; Malik et al., 1983). In addition to this band, the spectra of the metal complexes exhibit bands around 270 and 335 nm due to π → π∗ electronic transitions. However, the position of these bands remains almost unchanged on complexation. The UV–visible absorption spectral data of the Schiff base metal complexes are given in Table 3, which includes the absorption regions, band assignments and the proposed geometry of the complexes. These values are comparable with other reported values (Larabi et al., 2003).
Complex
λmax (cm−1)
Band assignments
Geometry
C44H38MnN6O3Cl2
18,811
6A1g(S) → 4T1g(S)
Octahedral
31,296
6A1g(S) → 4T1g (4P)
C44H38CoN6O3Cl2
9302
4T1g(F) → 4T2g(F)
Octahedral
14,669
4T1g(F) → 4A2g(F)
17,886
4T1g(F) → 4T1g(P)
C44H38NiN6O3Cl2
10,394
3A2g → 3T2g
Octahedral
15,869
3A2g → 3T1g(F)
28,253
3A2g → 3T1g(P)
C44H38CuN6O3Cl2
18,164
2Eg → 2T2g
Tetragonal
3.4.1 Manganese(II) complexes
The Mn(II) complex shows a magnetic moment of 5.94 BM at room temperature corresponding to the five unpaired electrons. The diffuse reflectance spectra of MnII tetraaza macrocyclic complexes exhibit bands in the region 14,280 cm−1 which may be a charge transfer band. Bands in the region 18,811 and 31,296 cm−1 may be assigned to 6A1g(S) → 4T1g(S) and 6A1g(S) → 4T1g(4P) multiplicity forbidden transitions respectively (Satpathy et al., 1992). Thus the electronic spectral findings favour the geometry to be octahedral for MnII complexes.
3.4.2 Cobalt(II) complexes
The electronic spectrum of the cobalt complexes exhibited three bands at 9302, 14,669 and 17,886 cm−1 that are assigned to the transitions 4T1g(F) → 4T2g(F), 4T1g(F) → 4A2g(F) and 4T1g(F) → 4T1g(P) respectively, suggesting octahedral geometry around the Co(II) ion. The magnetic moments of Co(II) complexes were found to be 5.01 BM also indicating octahedral geometry.
3.4.3 Nickel(II) complexes
The absorption spectra of Ni(II) complexes display three d–d transition bands at 10,394, 15,869 and 28,253 cm−1 which correspond to 3A2g → 3T2g, 3A2g → 3T1g(F) and 3A2g → 3T1g(P) (Fig. 4). The magnetic moments of Ni (II) complexes were found to be 3.01 BM supporting the d8 high spin distorted octahedral structure.![UV-visible spectrum of the [Ni(C44H38N6O3)Cl2] complex.](/content/184/2019/12/7/img/10.1016_j.arabjc.2014.11.033-fig3.png)
UV-visible spectrum of the [Ni(C44H38N6O3)Cl2] complex.
3.4.4 Copper(II) complexes
The magnetic moment values of Cu(II) complexes are 2.01 BM that fall within the normal range observed for distorted octahedral complexes. From the results, Cu (II) complexes show a single broad band in the range 18,164 cm−1 due to transition between 2Eg → 2T2g suggesting tetragonal geometry. Tetragonal or square planar Cu (II) complexes are expected to give three bands. However, these three bands usually overlapped in tetragonal complexes, to give one broad absorption band. The electronic spectra and magnetic moment data for all Cu(II) complexes coupled with the analytical, conductance data suggest the tetragonal geometry for all the complexes.
3.5 EPR spectral studies
3.5.1 EPR spectrum of Cu(II) complex
The EPR spectra of the Cu(II) complexes were recorded in a polycrystalline sample as well as in solution at room temperature in different frequencies. The analysis of spectra gives the values of gII = 2.1755 and g⊥ = 2.0568. The observed ‘g’ values for the complexes are less than 2.3 in agreement with the covalent character of the metal ligand bond. The trend gII > g⊥ > 2.0023 observed for the complexes indicates that the unpaired electron is localized in orbital of the Cu(II) ion and spectral features are characteristic of axial symmetry. Tetragonal elongated structure is confirmed for the aforesaid complex.
3.5.2 1H NMR spectra
The bonding pattern in the resulting complexes has been further substantiated by the proton magnetic resonance spectra of the precursor and the metal complexes of the macrocycles. The 1H NMR spectra of the complexes do not show any signal corresponding to primary amino protons. This suggested that the proposed macrocyclic skeleton has been formed. A singlet observed at δ 3.709 ppm in the complex may be assigned to nine methoxy protons in the 3,4,5-trimethoxy benzil group of Trimethoprim. Another singlet is observed at δ 3.608 ppm which corresponds to benzil protons. A Singlet peak is observed at δ 3.533 ppm for the methylene protons. The shift of the signals towards the lower field is an indication of the coordination of the macrocycles. The multiple of aromatic protons was observed at δ 7.3–7.9 ppm in the spectra of the precursor and the metal complexes of the macrocycles (Fig. 3).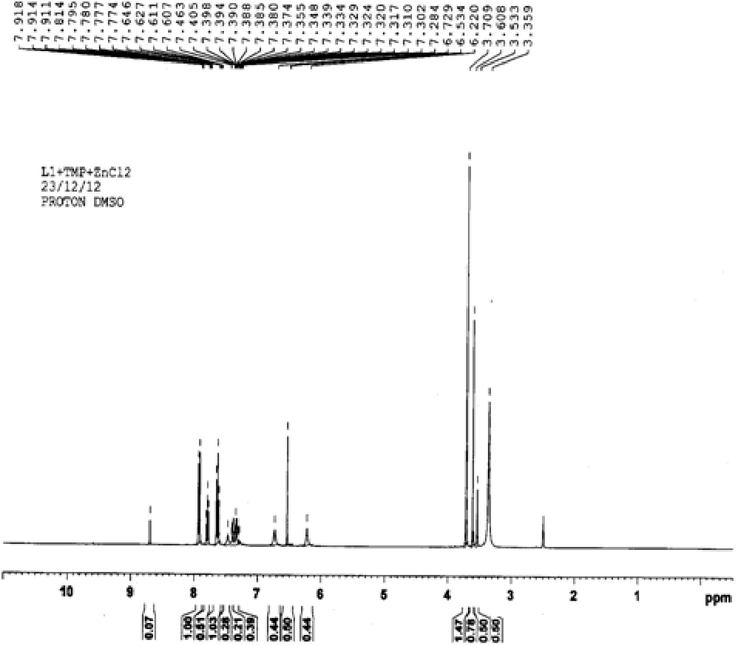
1H NMR spectrum of the Zn (C44H38N6O3) Cl2 complex.
3.6 Powdered XRD analysis
The diffractogram of the CuII complex consists of fifteen reflections with maxima at 2θ = 24.889° corresponding to the value of d = 3.574 Å (Fig. 5) between 10° and 80°. The unit cell of Cu(II) complex yielded values of lattice constants: a = 9.424 Å, b = 14.680 Å, c = 9.326 Å and a unit cell volume V = 1290.199 Å3 (Table 4). In concurrence with these cell parameters, conditions such as a ≠ b ≠ c and α = γ = 90° ≠ β required for a monoclinic sample are tested and found to be satisfactory. Hence, it can be concluded that the Cu(II) complex belongs to monoclinic crystal system.![X-ray diffraction pattern of the [Cu(C44H38N6O3)Cl2] complex.](/content/184/2019/12/7/img/10.1016_j.arabjc.2014.11.033-fig5.png)
X-ray diffraction pattern of the [Cu(C44H38N6O3)Cl2] complex.
Complexes
2θ values
Unit cell parameters
Density (gcc)
Possible geometry
[Cu(C44H38N6O3)Cl2]
17.392
2.02
Monoclinic
21.742
24.038
24.889
25.872
a = 9.424 Å
26.952
b = 14.680 Å
28.444
c = 9.326 Å
32.16
α = 90.000°
32.758
β = 109.32°
34.731
γ = 90.000°
38.907
45.388
46.429
49.617
53.844
The experimental density of the complex under study is determined by using the specific gravity method, which further enabled to calculate the volume of the unit cell. By using this value of density, molecular weight of the complexes, Avagadro’s number and volume of unit cell, the number of molecules (n) per unit cell is calculated by using the equation, ρ = nM/NV. The experimental density values of the complex are determined using the specific gravity method and found to be 2.02 g cm−3 for this complex (Çakir and Biçer, 2004). By using this value of density, molecular weight of the complex, Avagadro’s number and volume of unit cell, the number of molecules (n) per unit cell is calculated by using the equation, ρ = nM/NV and wasfound to be 2 for this complex (Gale, 1996). With this number, theoretical density is computed and found to be 2.04 g cm−3 for this complex. Comparison of experimental and theoretical density values shows good agreement within the limits of experimental error (Allan et al., 1979).
Thus from the above observations, the following structure is tentatively proposed for the macrocyclic complexes. The proposed structure is shown in Fig. 1.
3.7 Antimicrobial activity of the complex compound
Results of the antimicrobial sensitivity study for the macrocyclic [M(L)X2] complex by the disc diffusion test against all five human pathogenic bacterial strains are depicted in Table 5. Among the five [M(L)X2] complexes, the [Co(L)Cl2] complex exhibits highest bacteriostatic and bactericidal activity towards all pathogenic isolates other than Vibrio sp. Bacteria tested zone of inhibition in mm. AP, ampicillin; NX, norfloxacin; CP, ciprofloxacin; N, nalidixic acid; MT, methicillin; S∗ = sensitive; NA = no activity.
Organism/[M(L)X2] complex
ZnCl2
Mncl2
CoCl2
NiCl2
CuCl2
AP
NX
CP
N
MT
400 μg
300 μg
400 μg
300 μg
400 μg
300 μg
400 μg
300 μg
400 μg
300 μg
10 μg
10 μg
5 μg
30 μg
10 μg
Staphylococcus aureus
24
13
24
10
32
24
NA
NA
23
15
29
23
30
31
9
E. coli
18
9
15
NA
20
19
10
NA
11
NA
30
32
34
27
–
Shigella flexneri
28
16
18
7
36
27
NA
NA
25
19
29
28
31
12
–
Pseudomonas aeruginosa
27
17
16
NA
29
16
9
NA
10
NA
32
18
34
28
–
Vibrio cholerae
10
NA
13
NA
19
7
NA
NA
12
NA
31
28
30
29
–
Among the tested bacterial strains, E. coli, P. aeruginosa and V. cholerae were susceptible to all tested standard antibiotics, while the gram-positive S. aureus and gram-negative S. flexneri bacterial strains showed resistance to methicillin and nalidixic acid standard antibiotics with a zone of inhibition of 9 and 12 mm respectively. The study of disc diffusion assay indicated that [Co(L)Cl2] is the most active complex with a zone of inhibition of 36 mm (400 μg/ml) against S. flexneri and 32 mm (400 μg/ml) against S. aureus [Zn(L)Cl2] macrocyclic compound showed moderate antibacterial activity (Table 5). The macrocyclic [Mn(L)Cl2] complex did not show much effect against all bacterial isolates tested showing moderate activity only against S. aureus. Similarly [Cu(L)Cl2] showed low to moderate activity against S. aureus and S. flexneri. Of the four complexes tested by disc diffusion assay, [Co(L)Cl2] complex expresses effective bacteriostatic activity against methicillin resistant S. aureus (MRSA) and nalidixic acid resistant S. flexneri pathogenic isolates. Antimicrobial resistance against the first line of drugs like nalidixic acid, trimethoprim and other antibiotics was commonly observed in many countries worldwide during in vivo analysis of pathogens (Rahman et al., 2007; Salam and Bennish, 1988). Similar work done by other researchers with a moderately different macrocyclic ligand and its metal complexes reported antimicrobial activities towards S. aureus, E. coli and P. aeruginosa bacterium which is in accordance with our macrocyclic complexes antibacterial potency (Ahmed et al., 2013). To know the bacteriostatic and bactericidal activities of the synthesized compounds, MIC assay was carried out. All four [M(L)X2] complexes were used at a concentration from 256 to 0.5 μg/ml against the human pathogenic bacterial isolates tested. The MIC results are shown in Table 6. Out of the five complexes tested [Co(L)cl2] showed better activity with an MIC value of 4, 16, 32 and 256 μg/ml against S. flexneri, S. aureus, E. coli and P. aeruginosa respectively, where as [Zn(L)Cl2], [Cu(L)Cl2], and [Mn(L)Cl2] showed an MIC value of 256 μg/ml against the pathogenic bacteria tested. The [Mn(L)Cl2] complex showed activity only against S. aureus, the other bacterial pathogens are resistant to it. None of the synthesized complexes showed activity against V. cholerae. It is interesting to note that [Co(L)Cl2] showed efficient bactericidal activity at a lowest MIC value of 4 and 16 μg/ml against S. flexneri and S. aureus respectively which showed resistance to the standard antibiotic tested (Table 6). Standard antibiotics: AP – ampicillin; NX – norfloxacin; CP – ciprofloxacin; N – nalidixic acid; MT – methicillin; S∗ = sensitive; NA = no activity.
Organism/[M(L)X2] complex
MIC (μg/ml)
ZnCl2
Mncl2
CoCl2
CuCl2
Ni Cl2
AP
NX
CP
N
MT
Staphylococcus aureus
256
256
16
256
NA
s
s
s
s
64
E. coli
NA
NA
32
NA
NA
s
s
s
s
–
Shigella flexneri
256
NA
4
256
NA
s
s
s
32
–
Pseudomonas aeruginosa
256
NA
256
NA
NA
s
s
s
s
–
Vibrio cholerae
NA
NA
NA
NA
NA
s
s
s
s
–
The trimethoprim antibiotic antimicrobial activity did not show much active bacteriostatic or bactericidal activity against the tested pathogenic bacteria, while the activity was highly influenced by forming the macrocyclic Schiff-Base complex. The trimethoprim is a bacteriostatic antibiotic compound mainly used against the treatment of urinary tract infection and inhibits the reduction of dihydrofolic acid (Rang et al., 1999). Thymine utilized in DNA formation was generated by folate metabolism. The trimethoprim prohibits the formation of tetrahydrofolate from dihydrofolate and therefore halts folate synthesis (Heaslet et al., 2009). The [Co(L)Cl2] complex showed MIC activity at 4 μg/ml against the antibiotic resistant S. flexneri, which among other isolates showed a high rate of resistance to standard nalidixic acid antibiotic at 32 μg/ml concentration (NCCL Breakpoint ⩽ 8 μg/ml). Similarly, methicillin resistance was demonstrated by tested Staphylococcal aureus isolate with an MIC valve of 16 μg/ml (NCCL Breakpoint ⩽ 8 μg/ml).
The drug resistant bacterial strains possess resistance by the mechanism of enzymatic inactivation or target site modification with decreased intracellular drug accumulation (Schwart and Nobel, 1999). The rate of resistance to clinically isolated MRSA shows a lower level (⩽20%) of resistance to trimethoprim–sulfamethoxazole than other antibiotics (Kim et al., 2004). The inhibition of nalidixic acid resistant S. flexneri and MRSA growth at lower concentrations indicated the higher antibacterial potency of our synthesized [Co(L)Cl2] and [Zn(L)Cl2] complex than known standard commercial antibiotics.
The [Ni(L)Cl2] complex did respond less significantly to growth inhibition of E. coli and P. aeruginosa with a zone of inhibition of 10 mm (400 μg/ml) and 9 mm (400 μg/ml), while Fujiwara et al. (1990) express the neutral complexes of Ni(II) with Schiff bases exhibits the antimicrobial property for fungal species. The metal complexes possess greater antimicrobial properties against gram negative microorganisms compared to free ligands, which can be correlated to metal theory (Singh et al., 2004).
Our present study corroborates with some earlier reports on biological analysis of other metal complexes (Co(II), Cu(II), Ni(II), Mn(II) and Cr(III)) of macrocyclic Schiff-Base ligand, demonstrating both antibacterial and antifungal activities against E. coli, S. aureus, Klebsiella pneumoniae, Mycobacterium smegmatis, P. aeruginosa, Enterococcus cloacae, Bacillus megaterium and Micrococcus leteu (Prakash and Devjani, 2011). The Zn(II) complex showed a wide range of bactericidal activities against the gram positive and gram negative bacteria, with a potency well above the commercial antibiotics (Lei et al., 2009), Our study also authenticates the bacteriostatic and bactericidal nature of synthesized Co(II) and Zn(II) complexes. The results of the present investigation showed that the effective macrocyclic [M(L)X2] complexes possess potent and broad-spectrum antimicrobial activity which could be utilized in the production of pharmaceutically important therapeutic antibiotics.
4 Conclusion
In this work, newly synthesized five different macrocyclic based metal complexes from the Schiff bases were investigated. All the five complexes, [Mn(L)Cl2], [Co(L)Cl2], [Ni(L)Cl2], [Cu(L)Cl2] and [Zn(L)Cl2] were physicochemically characterized using elemental analysis, IR spectrum, UV, magnetic moments, 1HNMR spectroscopies and powder XRD analysis. The elemental analyses correspond to the metal:ligand stoichiometry of 1:1. The electrical conductance value for the ∼10−3 M solutions of the complexes in acetonitrile, showed that all are non-electrolytic in nature. The infrared spectral data show that the ligand coordinates with the metal ion through the four imine nitrogen atoms of the macrocyclic ring. The magnetic moments of the complexes reveal that they are all of high spin type with paramagnetic (except Zn) nature. It suggests that Mn, Co, Ni, Cu and Zn are in the +2 oxidation state in their complexes. The electronic spectral data support that all the complexes were found to have octahedral/distorted octahedral geometry. The integral intensities of each signal in the 1H NMR spectra of the macrocyclic complexes are found to agree with the number of different types of protons present. The X-ray diffraction analysis of Cu(II) complex showed that this complex belongs to the monoclinic crystal system. This is in concurrence with the cell parameters. The conditions such as a ≠ b ≠ c and α = γ = 90° ≠ β required for a monoclinic sample were tested and found to be satisfactory. The complex contains trimethoprim with two chloride ions and exhibits a distorted octahedral structure. All the macrocyclic complexes were evaluated for antimicrobial property against five human pathogenic bacteria namely E. coli, P. aeruginosa, Vibrio cholerae, methicillin resistant S. aureus and nalidixic resistant S. flexneri. Among the five macrocyclic Schiff base complexes, the [Co(L)Cl2] and [Zn(L)Cl2] complex exhibits potent antibacterial activity against drug resistant S. flexneri and S. aureus on comparing with standard drugs. Our data indicated that these complexes were effective to combat the growth of selective drug resistant pathogenic microorganisms.
Conflict of interest
The authors declare that they have no competing interest.
Acknowledgement
The first author acknowledges the management of St. Joseph College for providing the necessary facilities to carry out this work.
References
- Metal complexes of macrocyclic schiff-base ligand: preparation, characterisation, and biological activity. Sci. World J. 2013:1-7.
- [Google Scholar]
- Some first row transition metal complexes of nicotinamide and nicotinic acid. J. Therm. Anal. Calorim.. 1979;16:79-90.
- [Google Scholar]
- Solvent extraction of radium with crown ether carboxylic acids. Anal. Chem.. 1994;66:3521-3524.
- [Google Scholar]
- Brusch J.L., Garvey T., Corales D.O. 2009. Typhoid fever: E-medicine specialities /infectious diseases/ bacterial infections.
- Redox behaviour of cysteine in the presence of ammonium trioxovanadate (V) Bioelectrochemistry. 2004;64:1-6.
- [Google Scholar]
- Coordination chemistry of a copper(ii) tetramine macrocycle: four-, five- and six-coordinate derivatives and reduction transmetalation to the zinc(ii) complex. Inorg. Chem.. 1981;20:2032-2039.
- [Google Scholar]
- Aspects of Organic and Inorganic Supramolecular Chemistry, Macrocyclic Chemistry (first ed.). New York: VCH; 1993.
- Synthesis and spectroscopic studies of some fungitoxic organolead(iv) complexes of sulfur and nitrogen donor ligands. Phosphorus Sulfur Silicon. 1990;53:389-395.
- [Google Scholar]
- Emergence of highly fluoroquinolone resistant Salmonella enterica serovar Typhi in a community-based fever surveillance from Kolkata, India. Int. J. Antimicrob. Agents. 2008;31:387-389.
- [Google Scholar]
- The emergence of trinuclear constellations at metallobiosites. J. Chem. Soc. Dalton Trans. 1993:1349-1357.
- [Google Scholar]
- Preparation of 14-, 18-, and 22-membered tetraaza macrocycles and their complexing ability for copper(II) and nickel(II) ions. Bull. Chem. Soc. Jpn.. 1990;63:3443-3449.
- [Google Scholar]
- Nicotinamide: potential for the prevention of type 1 diabetes? Horm. Metab. Res.. 1996;28:361.
- [Google Scholar]
- Structural comparison of chromosomal and exogenous dihydrofolate reductase from Staphylococcus aureus in complex with the potent inhibitor trimethoprim. Proteins Struct. Funct. Bioinform.. 2009;76:706-717.
- [Google Scholar]
- Agar and broth dilution methods to determine the minimal inhibitory concentration (MIC) of antimicrobial substances. Nat. Protoc.. 2008;3(2):163-164.
- [Google Scholar]
- Bioinorganic Chemistry of Copper. New York: Chapman and Hall; 1991.
- In vitro activities of 28 antimicrobial agents against Staphylococcus aureus isolates from tertiary-care hospitals in Korea: a nationwide survey. Antimicrob. Agents Chemother.. 2004;48:1124-1127.
- [Google Scholar]
- A proton-driven copper (II) ion pump with a macrocyclic dioxotetra-amine. A new type of carrier for solvent extraction of copper. J. Chem. Soc. Chem. Commun. 1985:1041-1043.
- [Google Scholar]
- Antimicrobial effects of newly synthesized organotin(IV) and organolead(IV) derivatives. Appl. Organomet. Chem.. 1993;7:655-660.
- [Google Scholar]
- Synthesis, structural study and electrochemical properties of copper(II) complexes derived from benzene- and p-toluenesulphonylhydrazone. J. Serb. Chem. Soc.. 2003;68:85.
- [Google Scholar]
- Synthesis, characterization, and biological activity of a Schiff-base Zn(II) complex. J. Coord. Chem.. 2009;62:3471-3477.
- [Google Scholar]
- The Chemistry of Macrocyclic Ligand Complexes. Cambridge, UK: Cambridge University Press; 1989.
- Preparation and characterization of copper, cobalt and nickel complexes of tetradentate N6 macrocyclic ligand. Polyhedron. 1983;2:369-373.
- [Google Scholar]
- Antibiotic susceptibility of Salmonella spp.: a comparison of two surveys with a 5 years interval. J. IMAB Annu. Proc.. 2012;18:216-219. (Scientific Papers)
- [Google Scholar]
- Potassium separation from S-block and other elements using a polymeric crown ether. Anal. Chem.. 1994;66:4097-4099.
- [Google Scholar]
- Lipophilic tetraazamacrocyles: extraction of metal ions by impregnated resin. Helv. Chim. Acta. 1983;66:1525-1531.
- [Google Scholar]
- Application of Schiff bases and their metal complexes – a review. Int. J. ChemTech Res.. 2011;4:1891-1896.
- [Google Scholar]
- Antibacterial activities of some Indian traditional plant extracts. Asian Paci. J. Dis. 2012:291-295.
- [Google Scholar]
- Synthesis, characterization and electrochemistry of unsymmetrical macrocyclic cobalt(II) complexes derived from bis(benzil)ethylenediamine. Indian J. Chem.. 1996;35:677-680.
- [Google Scholar]
- Increasing spectrum in antimicrobial resistance of Shigella isolates in Bangladesh: resistance to azithromycin and ceftriaxone and decreased susceptibility to ciprofloxacin. J. Health Popul. Nutr.. 2007;2:158-167.
- [Google Scholar]
- Pharmacology (fourth ed.). Churchill Livingstone; 1999. 725–731
- Asian Paci. J. Trop. Med.. 2011;4:523-525.
- Molecular basis of resistance displayed by highly ciprofloxacin resistant Salmonella enterica serovar Typhi in Bangladesh. J. Clin. Microbiol.. 2006;44:3811-3813.
- [Google Scholar]
- Therapy of shigellosis: randomized double-blind trial of nalidixic acid in childhood shigellosis. J. Pediatr.. 1988;113:901-907.
- [Google Scholar]
- J. Indian Chem. Soc.. 1992;69:49.
- Aspects of bacterial resistance to antimicrobials used in veterinary dermatological practice. Vet. Dermatol.. 1999;10:163-176.
- [Google Scholar]
- Synthesis and characterization of 5,12-dioxa-7,14-dimethyl-14,8,11-tetraazacyclotetradeca-1,8-diene and its metal complexes with chromium(II), manganese(II), iron(II), copper(II), nickel(II) and zinc(II) metal ions. Indian J. Chem.. 1995;34:72-75.
- [Google Scholar]
- Synthetic, structural and biological studies of semicarbazonato complexes of organotin(IV) Main Group Met. Chem.. 1989;12:155-167.
- [Google Scholar]
- Synthetic, magnetic, spectral, antimicrobial and antifertility studies of dioxomolybdenum (VI) unsymmetrical imine complexes having a N ∩ N donor system. Transition Met. Chem.. 2004;29:70-74.
- [Google Scholar]
- Comparison of different MIC methods and establishment of zone diameter breakpoints for mecillinam, using NCCLS methodology. In: Program and abstracts of the 40th Interscience Conference on Antimicrobial Agents and Chemotherapy (Toronto), Canada, September 17–20. Washington, DC: American Society for Microbiology; 2000.
- [Google Scholar]
- Lipid-bound macrocycles as new immobilized ligand systems for effective separation of metal cations. J. Chem. Soc. Chem. Commun. 1991:1069-1070.
- [Google Scholar]







