Translate this page into:
Synthesis, characterization and biological behavior of some Schiff's and Mannich base derivatives of Lamotrigine
⁎Corresponding author. amolkulkarni89@rediffmail.com (A.A. Kulkarni) amolk301@rediffmail.com (A.A. Kulkarni)
-
Received: ,
Accepted: ,
This article was originally published by Elsevier and was migrated to Scientific Scholar after the change of Publisher.
Peer review under responsibility of King Saud University.

Abstract
A series of various Schiff's and Mannich base derivatives (N1–2 & ND1–6) of Lamotrigine with isatin and substituted isatin were synthesized to get more potent anticonvulsant agents. The starting material for the synthesis of various new Schiff's and Mannich base derivatives was isatin (1H-indole- 2, 3-dione) which in turn was prepared from substituted isonitrosoacetanilide using aniline. Lamotrigine reacts with isatin & substituted isatin gave Schiff's bases (N1–2) which on reaction with various secondary amines (dimethylamine, diethylamine, morpholine) produced Mannich bases (ND1–6). The structures of newly synthesized compounds were characterized by using TLC, UV, FT-IR, 1HNMR and studied for their anticonvulsant activity. Anticonvulsant activity of all the derivatives was evaluated by MES method using phenobarbitone sodium & Lamotrigine as standard drugs and % reduction of time spent by animals in extension, flexion, clonus, and stupor phase were noted. Compounds ND-4 and ND-6 showed significant anticonvulsant activity when compared with that of standard drugs. The remaining all compounds show moderate activity. Biological activity data of the synthesized derivatives revealed that, the synthesized derivatives are good anticonvulsant agents as compared to Lamotrigine.
Keywords
Lamotrigine
Isatin
Schiff's base
Mannich base
Anticonvulsant
1 Introduction
Lamotrigine is a member of drug class of phenyltriazine, used for the adjunctive treatment of partial seizures in epilepsy and generalized seizures of Lennox–Gastaut syndrome (Lebre et al., 2003; Qian et al., 2009; Sagud et al., 2008; Calabrese et al., 1999). It is also used for the maintenance treatment of bipolar I disorder and depression. (Barbee and Jamhour, 2002; Botts and Raskind, 1999). Isatin is an endogenous molecule identified in human beings which has anticonvulsant (Bhattacharya and Chakrobarti,1998; Gursoy and Karali, 1996; Popp and Donogn, 1979), antimicrobial, tuberculostatic, analgesic and various other pharmacological activities. Extensive literature review has been made regarding the activities of the isatin, especially for its anticonvulsant, antimicrobial, analgesic and anti-inflammatory activities (Silva et al., 2001; Sridhar et al., 2001). Schiff's bases and Mannich bases of isatin (Pandeya et al., 2000) were reported to possess anticonvulsant activity and various other pharmacological activities (Aboul-fadl and Aboul-wafa, 2010; Agarwal et al., 2006; Chakraborty et al., 2010; Liu et al., 2005; Pandeya et al., 2002; Verma et al.,2004).
Literature review indicates that, Lamotrigine is less potent and shows side effects. In order to increase the potency and reduce the side effects of Lamotrigine, we have considered isatin as one of the conjugate which can be coupled with Lamotrigine due to its structural similarities with already known anticonvulsants such as Mephobarbital, Phenytoin. Herein we report the synthesis, characterization and in vivo anticonvulsant activity of Lamotrigine derivatives (Scheme 1).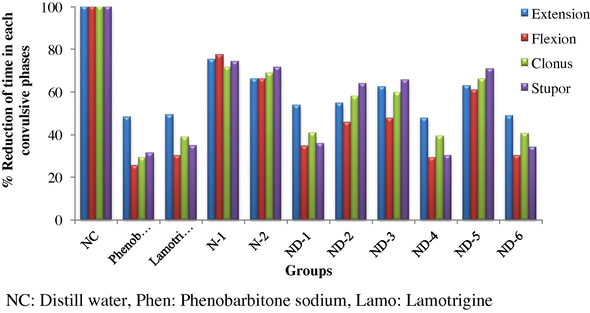
Scheme of synthesis. Step 1: Synthesis of Schiff’s bases (imines) from Lamotrigine and substituted isatin. Step 2: Synthesis of Mannich bases of synthesized Schiff’s bases.
2 Materials and methods
2.1 Materials
All the reagents used for synthesis were obtained from Sigma Aldrich and Loba Chem Ltd. (India). All the solvents used in these studies were dried and distilled before use. Melting points were determined by using Veego digital melting point apparatus (VMP-PM, 32/1104) and are uncorrected. All the reactions were monitored by thin layer chromatography (TLC) using Silica gel G (60 mesh). The solvent system used includes Acetone: Toluene: Ammonia (7:3:1). The IR spectra (KBr) were recorded on a FTIR spectrophotometer with Diffuse Reflectance attachment (Shimadzu 8400S). UV studies were carried out on a UV Visible spectrophotometer (Shimadzu 1700) and the λmax of the respective synthesized compounds was determined using ethanol as the solvent. 1H NMR spectra were recorded on NMR spectrometer (Varian Mercury YH 300) with DMSO as a solvent with TMS as an internal standard.
2.2 Methods
2.2.1 Synthesis of 3,3′{[6-(2,3-dichlorophenyl)-1,2,4-triazine-3,5 diyl]dinitrolo}bis-(1,3-dihydro-2H-indol-2-one) N-1
To 20 ml of hot ethanol, 0.735 gm (0.005 mol) of isatin and 0.640 gm (0.0025 mol) of Lamotrigine were dissolved. To this mixture 1.0 ml of glacial acetic acid was added. The reaction mixture was then refluxed on a water bath in a 250 ml round bottomed flask for 8 h. Completion of the reaction was monitored by TLC. The mixture was allowed to stand for 24 h at room temperature. The product was collected and recrystallized with ethanol-chloroform mixture.
N-1: Yield: 76%; m.p.: 145–148 °C. IR (KBr) (cm−1): 3444 (NH), 3088 (Ar-CH), 1731 (C⚌O), 1616(C⚌N), 771 (C-Cl); 1H NMR (DMSO) δ (ppm): 6.6–7.7 (m, Ar-H), 10.29 (s, 2H, NH); Anal. Calcd for C25H13Cl2N7O: C, 58.38; H, 2.55; N, 19.06; Found: C, 58.31; H, 2.48; N, 19.01%.
2.2.2 Synthesis of 3,3′{[6-(2,3-dichlorophenyl)-1,2,4-triazine-3,5-diyl]dinitrolo}bis-(5-chloro-1,3-dihydro-2H-indol-2-one) N-2
N-2 obtained from 5-choroisatin (0.90 gm, 0.005 mol) under the same conditions as describe above for N-1.
N-2: Yield: 73%; m.p.: 162–165 °C. IR (KBr) (cm−1): 3309 (NH), 3070 (Ar-CH), 1741 (C⚌O), 1612 (C⚌N), 798 (C-Cl); 1H NMR (DMSO) δ (ppm): 6.9–7.9 (m, 9H, Ar-H), 10.29 (s, 2H, NH); Anal. Calcd for C25H11Cl4N7O: C, 51.49; H, 1.90; N, 16.81; Found: C, 51.35; H, 1.78; N, 16.75%.
2.2.3 Synthesis of 3,3′{[6-(2,3-dichlorophenyl)-1,2,4-triazine-3,5-diyl]dinitrolo}bis-{1-[(dimethylamino)methyl]1,3-dihydro-2H-indol-2-one} ND-1
A slurry consisting of the N-1 (0.0025 mol), THF (5 ml) and 37% formalin (2 ml) was made. To this dimethylamine (0.005 mol) was added drop wise with cooling and shaking. The reaction mixture was allowed to stand at room temperature for 1 h with occasional shaking after which it was warmed on a steam bath for 15 min. At the end of the period the contents were cooled and the product was obtained, which was further recrystallized from ethanol.
ND-1: Yield: 80%; m.p.: 94–97 °C. IR (KBr) (cm−1): 2887 (N-CH2-N), 3064 (Ar-CH), 1735 (C⚌O), 1610 (C⚌N), 757 (C-Cl); 1H NMR (CDCl3) δ (ppm): 2.19 (s, 12H, CH3), 4.00 (s, 4H, N-CH2-N), 6.6–8.0 (m, 11H, Ar-H); Anal. Calcd for C31H27Cl2N9O2: C, 59.24; H, 4.33; N, 20.06; Found: C, 59.19; H, 4.28; N, 19.96%.
2.2.4 Synthesis of 3,3′{[6-(2,3-dichlorophenyl)-1,2,4-triazine-3,5-diyl]dinitrolo}bis-{5-chloro-1-[(dimethylamino)methyl]-1,3-dihydro-2H-indol-2-one} ND-2
ND-2 obtained by using N-2 (0.0025 mol) under the same conditions as describe above for ND-1.
ND-2: Yield: 75%; m.p.: 103–106 °C; IR (KBr) (cm−1): 2840 (N- CH2-N), 3072 (Ar-CH), 1742 (C⚌O), 1608 (C⚌N), 725 (C-Cl), 1H NMR (CDCl3) δ (ppm): 2.34 (s, 12H, CH3), 3.97 (s, 4H, N-CH2-N), 6.7–7.6 (m, 9H, Ar-H); Anal. Calcd for C31H25Cl4N9O2: C, 53.39; H, 3.61; N, 18.08; Found: C, 53.25; H, 3.49; N, 18.06%.
2.2.5 Synthesis of 3,3′{[6-(2,3-dichlorophenyl)-1,2,4-triazine-3,5-diyl]dinitrolo}bis-{1-[(diethylamino)methyl]-1,3-dihydro-2H-indol-2-one} ND-3
A slurry consisting of the N-1 (0.0025 mol), THF (5 ml) and 37% formalin (2 ml) was made. To this diethylamine (0.005 mol) was added drop wise with cooling and shaking. The reaction mixture was allowed to stand at room temperature for 1 h with occasional shaking after which it was warmed on a steam bath for 15 min. At the end of the period the contents were cooled and the product was obtained, which was further recrystallized from ethanol.
ND-3: Yield: 66%; m.p.: 68–71 °C; IR (KBr) (cm−1): 2813 (N-CH2- N), 3058 (Ar-CH), 1730 (C⚌O), 1614 (C⚌N), 767 (C-Cl); 1H NMR (CDCl3) δ (ppm): 1.08 (q, 12H, CH3), 2.35 (q, 8H, CH2), 4.06 (s, 4H, N-CH2-N), 6.7–7.9 (m, 11H, Ar-H); Anal. Calcd for C35H35Cl2N9O2: C, 69.40; H, 5.15; N, 18.41; Found: C, 69.19; H, 5.10; N, 18.36%.
2.2.6 Synthesis of 3,3′{[6-(2,3-dichlorophenyl)-1,2,4-triazine-3,5-diyl]dinitrolo}bis-{5-chloro-1-[(diethylamino)methyl]-1,3-dihydro-2H-indol-2-one} ND-4
ND-4 obtained from N-2 (0.0025 mol) under the same conditions as describe above for ND-3.
ND-4: Yield: 70%; m.p.: 84–87 °C; IR (KBr) (cm−1): 2868 (N-CH2-N), 3062 (Ar-CH), 1735 (C⚌O), 1611 (C⚌N), 760 (C-Cl); 1H NMR (CDCl3) δ (ppm): 1.12 (q, 12H, CH3), 2.51 (q, 8H, CH2), 3.92 (s, 4H, N-CH2-N), 6.8–7.9 (m, 9H, Ar-H); Anal. Calcd for C35H33Cl4N9O2: C, 55.79; H, 4.41; N, 18.82; Found: C, 55.58; H, 4.36; N, 18.75%.
2.2.7 Synthesis of 3,3′{[6-(2,3-dichlorophenyl)-1,2,4-triazine-3,5-diyl]dinitrolo}bis-[1-(morpholinomethyl)1,3-dihydro-2H-indol-2-one] ND-5
A slurry consisting of the N-1 (0.0025 mol), THF (5 ml) and 37% formalin (2 ml) was made. To this morpholine (0.005 mol) was added drop wise with cooling and shaking. The reaction mixture was allowed to stand at room temperature for 1 h with occasional shaking after which it was warmed on a steam bath for 15 min. At the end of the period the contents were cooled and the product was obtained, which was further recrystallized from ethanol.
ND-5: Yield: 86%; m.p.: 101–104 °C; IR (KBr) (cm−1): 2854 (N-CH2-N), 3039 (Ar-CH), 1735 (C⚌O), 1609 (C = N), 765 (C-Cl); 1H NMR (CDCl3) δ (ppm): 2.61–2.64 (q, 8H, CH2), 3.5–3.7 (q, 8H, CH2), 4.45 (s, 4H, N-CH2-N), 7.0–7.6 (m, 11H, Ar-H); Anal. Calcd for C35H31Cl2N9O4: C, 58.99; H, 4.38; N, 17.69; Found: C, 58.86; H, 4.36; N, 17.65%.
2.2.8 Synthesis of 3,3′{[6-(2,3-dichlorophenyl)-1,2,4-triazine-3,5-diyl]dinitrolo}bis-[5-chloro-1-(morpholinomethyl)1,3-dihydro-2H-indol-2-one] ND-6
ND-6 obtained from N-2 (0.0025 mol) under the same conditions as describe above for ND-5.
ND-6: Yield: 83%; m.p.: 108–111 °C; IR (KBr) (cm−1): 2785 (N-CH2-N), 3097(Ar-CH), 1743(C⚌O), 1650 (C⚌N), 790 (C-Cl); 1H ]NMR (CDCl3) δ (ppm): 2.54–2.58 (q, 8H, CH2), 3.48–3.60 (q, 8H, CH2), 4.23 (s, 4H, N-CH2-N), 6.8–7.5 (m, 9H, Ar-H); Anal. Calcd for C35H29Cl4N9O4: C, 53.79; H, 3.74; N, 16.13; Found: C, 53.58; H, 3.66; N, 16.09%.
3 Anticonvulsant activity
3.1 General
Anticonvulsant activity of Lamotrigine derivatives was tested by Maximal the Electroshock Method (MES) (DYIPSR/IAEC/10–11/P-23). Epilepsy resembling human epileptic types can be induced in small laboratory animals to screen/evaluate anticonvulsant activity of newly synthesized compounds (Kaminski et al., 2008; Oja et al., 1983). Present study has been carried out by maximal electroshock (MES) convulsions model, which clinically resembles human grand-mal epilepsy. When a stimulus (electric) of specified strength is applied using appropriate electrodes to the test animals, the animals exhibit different phases of convulsions, Such as extension, flexion, clonus, and stupor. The animals may survive/die during or after the shock. During the experiment, time spent by animals in various phases of the convulsion was recorded (see Table 1).
Compound Code
R1
R2
N-1
H
–
N-2
Cl
–
ND-1
H
–N(CH3)2
ND-2
Cl
–N(CH3)2
ND-3
H
–N(C2H5)2
ND-4
Cl
–N(C2H5)2
ND-5
H
–NC4H8O
ND-6
Cl
–NC4H8O
3.2 Procedure
Either sex, 3–4 months, healthy (250–300 gm) albino rats (wistar), overnight fasted were randomly assigned to 10 groups (n = 6), weighed and marked. Group-I animals received a solution in which the sample prepared was given by oral route (Blank/controlled group). Group-II animals received Phenobarbitone sodium (30 mg/kg p.o.) and Lamotrigine (10 mg/kg i.p.). (Reference/standard group). Group III–Group X received the sample in the dose of 10 mg/kg by i.p. route. After 30 min of drug treatment a stimulus of 130 mA for 0.2 s was delivered through electrodes (pinna) using electro convulsometer (INCO India) to each animal. Immediately, time spent by the animal in extension, flexion, clonus and stupor was tabulated Table 2 (mean ± SEM). Recovery/death following the various phases of convulsions was also tabulated. n = mean of six determinations.
Treatment
Mean of time spent by 6 animals in
Extension
Flexion
Clonus
Stupor
Negative control
17.63 ± 1.47
16.68 ± 1.13
23.50 ± 1.81
24.03 ± 1.32
Phenobarbitone sodium
8.51 ± 1.61
4.26 ± 0.61
6.87 ± 1.03
7.55 ± 1.45
Lamotrigine
8.69 ± 0.76
5.03 ± 0.53
9.17 ± 1.07
8.38 ± 0.75
N-1
13.28 ± 1.68
12.92 ± 1.62
16.83 ± 2.46
17.84 ± 1.89
N-2
11.65 ± 2.36
11.04 ± 1.81
16.21 ± 3.13
17.19 ± 1.54
ND-1
9.47 ± 1.19
5.80 ± 0.80
9.64 ± 1.35
8.63 ± 1.12
ND-2
9.64 ± 1.38
7.64 ± 0.94
13.66 ± 2.13
15.35 ± 1.40
ND-3
11.00 ± 0.67
7.97 ± 0.76
14.04 ± 1.35
15.79 ± 1.68
ND-4
8.44 ± 0.83
4.89 ± 0.67
9.26 ± 1.20
7.26 ± 1.44
ND-5
11.12 ± 1.25
10.19 ± 1.82
15.57 ± 3.24
17.02 ± 1.98
ND-6
8.63 ± 1.38
5.07 ± 0.57
9.53 ± 1.14
8.21 ± 1.17
4 Results and discussion
The main aim of the present work was to synthesize some new series of Schiff's and Mannich base derivatives. The structures of all the synthesized compounds were confirmed on the basis of TLC, UV, FT-IR and 1H NMR spectra. All the compounds were screened for anticonvulsant activity using MES method. The anticonvulsant evaluations of synthesized compounds were done in rat at 10 mg kg−1 by i.p. route. Phenobarbitone sodium and Lamotrigine were employed as standard drugs and % reduction of time spent by animals in extension, flexion, clonus, stupor phase was noted. Results are shown in Tables 2 and 3 and Graphs 1 and 2. Compounds ND-4 and ND-6 showed more potent anticonvulsant activity when compared with that of the standard drug. Remaining all compounds show moderate activity.
Treatment
% reduction of time spent by animals in
Extension
Flexion
Clonus
Stupor
Negative control
100
100
100
100
Phenobarbitone sodium
48.26
25.53
29.23
31.41
Lamotrigine
49.29
30.15
39.02
34.87
N-1
75.32
77.45
71.61
74.24
N-2
66.08
66.18
68.97
71.53
ND-1
53.71
34.77
41.02
35.91
ND-2
54.67
45.80
58.12
63.87
ND-3
62.39
47.78
59.74
65.70
ND-4
47.87
29.31
39.40
30.21
ND-5
63.07
61.09
66.25
70.82
ND-6
48.95
30.39
40.55
34.16
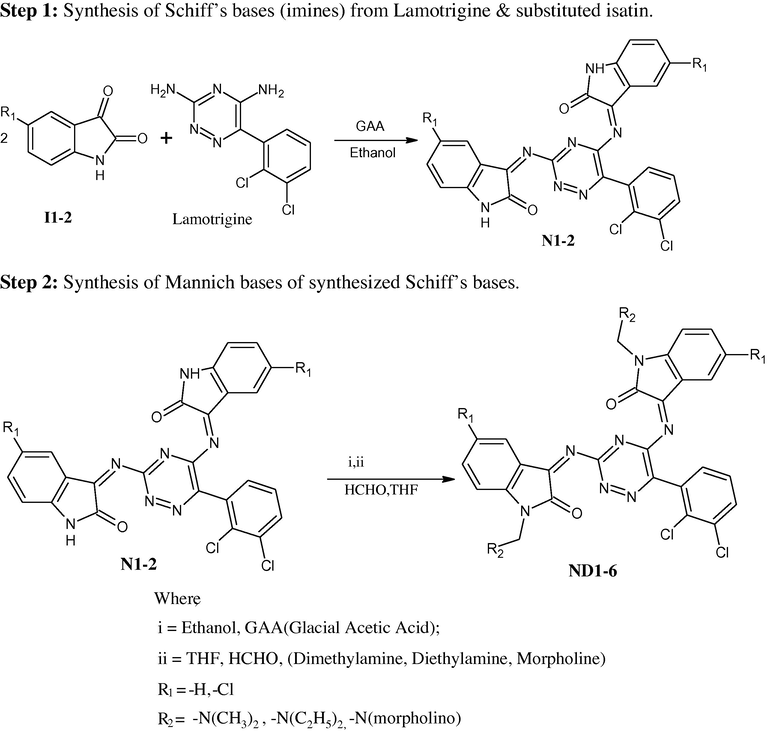
Mean of time spent by animals in extension, flexion, clonus, stupor phase.
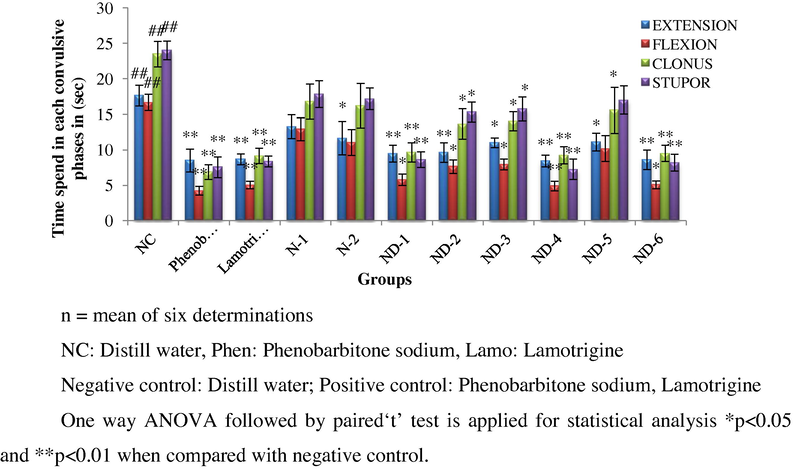
Showing (%) reduction of time spent in various phases of convulsions by treated animals.
5 Conclusion
A series of novel Schiff's and Mannich base derivatives were synthesized, characterized and evaluated for their anticonvulsant activity. The test results showed that compounds ND-4 and ND-6 exhibited significant anticonvulsant activity while remaining compounds showed moderate activity.
Acknowledgements
We are thankful to the SAIF Punjab University, Chandigarh, India for spectral analysis of newly synthesized compounds. One of the authors, Dr. Amol A. Kulkarni is also thankful to the Board of College and University Development (BCUD), the University of Pune for providing the financial support to carry out this research work.
References
- Eur. J. Med. Chem.. 2010;45:4578.
- Eu. J. Med. Chem.. 2006;41:1223.
- J. Clin. Psychiatry.. 2002;63:737.
- Ind. J. Exp. Bio.. 1998;36:118.
- Am. J. Health Sys. Pharm.. 1999;56:1939.
- Am. J. Psychiatry.. 1999;156:1019.
- Eur. J. Pharmacol.. 2010;647:1.
- Farmaco.. 1996;51:437.
- Eur. J. Med. Chem.. 2008;43:53.
- Eur. J. Pharmacol.. 2003;482:163.
- Biorg. Medi. Chem. Lett.. 2005;15:3058.
- Eur. J. Pharmacol.. 1983;87:191.
- Eur. J. Med. Chem.. 2000;35:249.
- Arch. Pharm. Med. Chem.. 2002;4:129.
- J. Pharm. Sci.. 1979;68:519.
- J. Chem. Sci.. 2009;121:463.
- Bipolar Res. Today. 2008;32:1195.
- J. Braz. Chem. Soc.. 2001;12:273.
- Eur. J. Med. Chem.. 2001;36:615.
- Acta Pharm.. 2004;54:49.







