Translate this page into:
Synthesis, computational studies and preliminary pharmacological evaluation of 2-[4-(aryl substituted) piperazin-1-yl]-N-benzylacetamides as potential antipsychotics
⁎Corresponding author. Tel.: +91 591 2360817; fax: +91 591 2360818. sushilmpharm@rediffmail.com (Sushil Kumar)
-
Received: ,
Accepted: ,
This article was originally published by Elsevier and was migrated to Scientific Scholar after the change of Publisher.
Peer review under responsibility of King Saud University.
Abstract
A series of 2-[4-(aryl substituted) piperazin-1-yl]-N-benzylacetamides were synthesized and the target compounds (3a–j) were evaluated for atypical antipsychotic activity by studying apomorphine induced climbing behavior, 5-HTP induced head twitches behavior and catalepsy in mice. The physicochemical similarity of the target compounds with respect to standard drugs clozapine, ketanserin and risperidone was assessed by calculating from a set of physicochemical properties using software programs. The test compounds (3a–j) demonstrated good similarity values with respect to the standard drugs. Among them, compound 3b has emerged as an important lead compound showing potential atypical antipsychotic-like profile.
Keywords
N-benzylacetamide
Arylpiperazines
Antipsychotic activity
Dopamine
Serotonin
Antagonists
1 Introduction
Dopaminergic and serotonergic neurotransmission modulates the activity of the CNS and its deregulation is associated with the onset of schizophrenia (Baldessarini and Tarazi, 1996). Schizophrenia is a complex psychological disorder affecting about 1% of the population worldwide (Reynolds, 1992). The use of classical neuroleptics such as chlorpromazine and haloperidol are associated with severe mechanism related side effects including extrapyramidal symptoms (EPS) (Altar et al., 2003; Stahl, 2000). The adverse effects presented by typical antipsychotic drugs along with their ineffectiveness in the treatment of negative symptoms of schizophrenia have led, through the years, to the arduous search for new molecules (Gonzalez-Gomez et al., 2003). Several dopaminergic, serotonergic and glutamergic approaches have been used for the development of atypical antipsychotic drugs. To date, however, the mixed 5-HT2/D2 receptor antagonism strategy is an important approach for the development of atypical antipsychotic drugs (Carro et al., 2009; Meltzer et al., 1989). Long chain arylpiperazines have been (LCAPs) recognized as the largest and most diverse classes of compounds exerting actions on the central nervous system in particular serotonin (5-HT) and dopamine affinity (Obniska et al., 2003; Gonzalez-Gomez et al., 2003; Kumar et al., 2011a). Their general chemical structure consists of the arylpiperazine moiety connected by an alkyl chain with the terminal amide or imide fragment (Gonzalez-Gomez et al., 2003; Perrone et al., 1999; Kumar et al., 2011b). In view of these observations, we herein report the synthesis, computational studies of some new amide arylpiperazines and evaluated them for a possible atypical antipsychotic potential.
2 Materials and methods
2.1 General
Melting points of the synthesized compounds were determined by open capillary method and are uncorrected. The IR spectra of synthesized compounds were recorded in potassium bromide discs on Perkin Elmer Spectrum RX1. The 1H and 13C NMR spectra were recorded on a Bruker DRX-300 spectrophotometer (1H at 300 MHz and 13C at 75 MHz) in CDCl3 containing TMS as an internal standard. Elemental analyses were performed on Elementar Vario EL III analyzer. The electrospray mass spectra were recorded on a Thermo Finnigan LCQ Advantage Max ion trap mass spectrometer. All reagents were of commercial quality and were used without further purification. The reactions progress was monitored by thin-layer chromatography (TLC) using silica gel G and spots were visualized in an iodine chamber.
2.2 Synthesis of compounds
2.2.1 Synthesis of 2-chloro-N-benzylacetamide (2)
Benzylamine 1 (4.37 ml, 0.04 mol) in 2N aqueous sodium hydroxide (150 ml) was treated with chloroacetylchloride (3.18 ml, 0.04 mol) as a solution in dichloromethane (100 ml) at 0 °C. After1 h, the layers were separated and the aqueous phases extracted with an additional portion of dichloromethane. The organic phases were combined, washed with an aqueous solution of 1N HCl, saturated NaHCO3, dried (Na2SO4), and concentrated to afford 2. Yield: 78%; m.p. 94–96 °C; IR (KBr, cm−1): 3268, 3017, 2945, 1669, 1220, 1066, 769, 689. 1H NMR (300 MHz; CDCl3 δ): 4.11 (s, 2H); 4.49–4.51 (d, J = 6, 2H); 6.88 (br s, NH); 7.26–7.39 (m, 5H, Ar-H); Anal. Calcd. for C9H10ClNO: C, 58.86; H, 5.49; N, 7.63; Found: C, 58.81; H, 5.43; N, 7.58.
2.2.2 General procedure for the synthesis of 3a–j
2-Chloro-N-benzylacetamide 2 (0.91 g, 0.005 mol) was dissolved in 100 ml of acetonitrile in a 250 ml round bottomed flask. Anhydrous K2CO3 (0.69 g, 0.005 mol) and catalytic amount of potassium iodide and 0.005 mol of appropriate arylpiperazine were added to the above solution. The above mixture was allowed to reflux with continuous stirring on a magnetic stirrer for 12 h. After completion of reaction the solvent was removed by vacuum distillation and residue was dissolved in chloroform and water. The organic layer was washed with brine and dried over MgSO4, removal of the solvent afforded target compounds 3a–j.
2.2.2.1 2-[4-(Phenyl) piperazin-1-yl]-N-benzylacetamide (3a)
Yield: 53%; m.p. 97–100 °C; IR (KBr, cm−1): 3301, 3061, 2827, 1657, 1213, 1017. 1H NMR (300 MHz; CDCl3 δ): 2.67–3.12 (m, 4H, piperazine); 3.13–3.36 (m, 4H, piperazine); 4.39 (s, 2H, COCH2); 4.47 (d, J = 6, 2H, CH2); 6.86–6.91 (m, 5H, Ar-H); 7.25–7.33 (m, 5H, Ar-H); 7.51 (br s, 1H, NH). 13C NMR (75 MHz, CDCl3 δ): 42.6, 49.0, 53.0, 76.5, 115.3, 118.6, 124.0, 127.3, 128.5, 128.7, 137.9, 150.7, 170.1. MS (EI) m/z: 310 (M+1). Anal. Calcd. for C19H23N3O: C, 73.76; H, 7.49, N, 13.58. Found: C, 73.61; H, 7.34; N, 13.51.
2.2.2.2 2-[4-(2-Methoxyphenyl) piperazin-1-yl]-N-benzylacetamide (3b)
Yield: 48%; m.p. 85–88 °C; IR (KBr, cm−1): 3307, 3057, 2829, 1664, 1219, 1016. 1H NMR (300 MHz; CDCl3 δ): 2.71–3.06 (m, 4H, piperazine); 3.14–3.36 (m, 4H, piperazine); 3.81 (s, 3H, OCH3); 4.39 (s, 2H, COCH2); 4.49 (d, J = 6, 2H, CH2); 6.84–7.07 (m, 4H, Ar-H); 7.25–7.34 (m, 5H, Ar-H); 7.53 (br s, 1H, NH). MS (EI) m/z: 340 (M+1). Anal. Calcd. for C20H25N3O2: C, 70.77; H, 7.42; N, 12.38. Found: C, 70.67; H, 7.31; N, 12.21.
2.2.2.3 2-[4-(3-Methoxyphenyl) piperazin-1-yl]-N-benzylacetamide (3c)
Yield: 44%; m.p. 82-85 °C; IR (KBr, cm−1): 3307, 3035, 2831, 1661, 1219, 1016. 1H NMR (300 MHz; CDCl3 δ): 2.72–3.06 (m, 4H, piperazine); 3.14–3.36 (m, 4H, piperazine); 3.78 (s, 3H, OCH3); 4.39 (s, 2H, COCH2); 4.49 (d, J = 6, 2H, CH2); 6.84–7.04 (m, 4H, Ar-H); 7.25–7.32 (m, 5H, Ar-H); 7.53 (br s, 1H, NH). MS (EI) m/z: 340 (M+1). Anal. Calcd. for C20H25N3O2: C, 70.77; H, 7.42; N, 12.38. Found: C, 70.63; H, 7.34; N, 12.30.
2.2.2.4 2-[4-(4-Methoxyphenyl) piperazin-1-yl]-N-benzylacetamide (3d)
Yield: 37%; m.p. 70–75 °C; IR (KBr, cm−1): 3301, 3061, 2827, 1661, 1221, 1028. 1H NMR (300 MHz; CDCl3 δ): 2.72–2.92 (m, 4H, piperazine); 3.14–3.36 (m, 4H, piperazine); 3.76 (s, 3H, OCH3); 4.39 (s, 2H, COCH2); 4.49 (d, J = 6, 2H, CH2); 6.87–7.04 (m, 4H, Ar-H); 7.25 -7.34 (m, 5H, Ar-H); 7.53 (br s, 1H, NH). MS (EI) m/z: 340 (M+1). Anal. Calcd. for C20H25N3O2: C, 70.77; H, 7.42; N, 12.38. Found: C, 70.60; H, 7.28; N, 12.25.
2.2.2.5 2-[4-(3-Methylphenyl) piperazin-1-yl]-N-benzylacetamide (3e)
Yield: 51%; m.p. 126–128 °C; IR (KBr, cm−1): 3309, 3057, 2831, 1659, 1213, 1032. 1H NMR (300 MHz; CDCl3 δ): 2.26 (s, 3H, CH3); 2.73–3.14 (m, 4H, piperazine); 3.19–3.35 (m, 4H, piperazine); 4.41 (s, 2H, COCH2); 4.48 (d, J = 6, 2H, CH2); 6.80–7.07 (m, 4H, Ar-H); 7.25 -7.34 (m, 5H, Ar-H); 7.48 (br s, 1H, NH). MS (EI) m/z: 324 (M+1). Anal. Calcd. for C20H25N3O: C, 74.27; H, 7.79; N, 12.99. Found: C, 74.18; H, 7.67; N, 12.91.
2.2.2.6 2-[4-(4-Methylphenyl) piperazin-1-yl]-N-benzylacetamide (3f)
Yield: 55%; m.p. 89–91 °C; IR (KBr, cm−1): 3307, 3051, 2829, 1661, 1213, 1032. 1H NMR (300 MHz; CDCl3 δ): 2.26 (s, 3H, CH3); 2.73–3.06 (m, 4H, piperazine); 3.10–3.35 (m, 4H, piperazine); 4.41 (s, 2H, COCH2); 4.48 (d, J = 6, 2H, CH2); 6.80–7.05 (m, 4H, Ar-H); 7.26–7.33 (m, 5H, Ar-H); 7.48 (br s, 1H, NH). MS (EI) m/z: 324 (M+1). Anal. Calcd. for C20H25N3O: C, 74.27; H, 7.79; N, 12.99. Found: C, 74.21; H, 7.70; N, 12.93.
2.2.2.7 2-[4-(2-Chlorophenyl) piperazin-1-yl]-N-benzylacetamide (3g)
Yield: 56%; m.p. 153–155 °C; IR (KBr, cm−1): 3311, 3057, 2831, 1664, 1210, 1015. 1H NMR (300 MHz; CDCl3 δ): 2.67–3.11 (m, 4H, piperazine); 3.21–3.35 (m, 4H, piperazine); 4.43 (s, 2H, COCH2); 4.50 (d, J = 6, 2H, CH2); 6.82–7.15 (m, 4H, Ar-H); 7.25–7.34 (m, 5H, Ar-H); 7.51 (br s, 1H, NH). MS (EI) m/z: 344 (M+1). Anal. Calcd. for C19H22ClN3O: C, 66.37; H, 6.45; N, 12.22. Found: C, 66.30; H, 6.38; N, 12.16.
2.2.2.8 2-[4-(3-Chlorophenyl) piperazin-1-yl]-N-benzylacetamide (3h)
Yield: 48%; m.p. 77–80 °C; IR (KBr, cm−1): 3313, 3059, 2829, 1667, 1213, 1012. 1H NMR (300 MHz; CDCl3 δ): 2.67–3.11 (m, 4H, piperazine); 3.21–3.35 (m, 4H, piperazine); 4.43 (s, 2H, COCH2); 4.50 (d, J = 6, 2H, CH2); 6.84–7.03 (m, 4H, Ar-H); 7.15–7.32 (m, 5H, Ar-H); 7.51 (br s, 1H, NH). MS (EI) m/z: 344 (M+1). Anal. Calcd. for C19H22ClN3O: C, 66.37; H, 6.45; N, 12.22. Found: C, 66.28; H, 6.35; N, 12.18.
2.2.2.9 2-[4-(4-Nitrophenyl) piperazin-1-yl]-N-benzylacetamide (3i)
Yield: 62%; m.p. 156–158 °C; IR (KBr, cm−1): 3301, 3030, 2823, 1661, 1251, 1004. 1H NMR (300 MHz; CDCl3 δ): 2.67–3.10 (m, 4H, piperazine); 3.13–3.35 (m, 4H, piperazine); 4.41 (s, 2H, COCH2); 4.48 (d, J = 6, 2H, CH2); 6.62–7.07 (m, 4H, Ar-H); 7.25–7.34 (m, 5H, Ar-H); 7.60 (br s, 1H, NH). MS (EI) m/z: 355 (M+1). Anal. Calcd. for C19H22N4O3: C, 64.39; H, 6.26; N, 15.81. Found: C, 64.31; H, 6.20; N, 15.76.
2.2.2.10 2-[4-(4-Fluorophenyl) piperazin-1-yl]-N- benzylacetamide (3j)
Yield: 58%; m.p. 123–125 °C; IR (KBr, cm−1): 3307, 3041, 2827, 1657, 1213, 1013. 1H NMR (300 MHz; CDCl3 δ): 2.72–3.10 (m, 4H, piperazine); 3.12–3.36 (m, 4H, piperazine); 4.41 (s, 2H, COCH2); 4.48 (d, J = 6, 2H, CH2); 6.84–7.02 (m, 4H, Ar-H); 7.15–7.32 (m, 5H, Ar-H); 7.48 (br s, 1H, NH). MS (EI) m/z: 328 (M+1). Anal. Calcd. for C19H22FN3O: C, 69.70; H, 6.77; N, 12.83. Found: C, 69.68; H, 6.45; N, 12.66.
2.3 Computational studies
2.3.1 Computation of physiochemical properties
A set of molecular parameters were computed for the target compounds as well as three standard drugs clozapine, ketanserin and risperidone using Chem 3D Ultra version, 11.0, and Chem Silico online free software. The important molecular parameters for antipsychotics are blood-brain barrier (BBB), log P and topological polar surface area. The literature review suggests that TPSA is a measure of a molecule's hydrogen bonding capacity and its value should not a exceed certain limit if the compound is intended to be CNS active. Two differing limits have been proposed: van de Waterbeemed et al. (1998) suggested a limit of 90 A2, where, Kelder et al. (1999) suggested 60–70 A2.
2.3.2 Similarity calculations
The physicochemical similarity of the target compounds was calculated with respect to the standard drugs (Bali et al., 2010).
Firstly, the distance di of a particular target compound j to drug molecules e.g., clozapine was calculated by the formula: where Xi,j is the value of molecular parameter ‘i’ for compound ‘j’, Xi,std is the value of the same molecular parameter for the standard drug, e.g., clozapine, ketanserin and risperidone. Then, the similarity of compound ‘j’ to the standard drug was calculated as where R = √d2 is the quadratic mean (root mean square), a measure of central tendency.
2.4 Preliminary pharmacological evaluation for atypical antipsychotic effect
All the target compounds were subjected to preliminary pharmacological evaluation to determine their ability to inhibit apomorphine induced climbing behavior (Chung et al., 2002), inhibit 5-hydroxy tryptophan (5-HTP) induced head twitches behavior (Chung et al., 2002) and catalepsy studies (Kumar et al., 2011a,b; Ferre et al., 1990). Prior permission of the Animal Ethics Committee was obtained and all experiments were conducted according to the approved protocol (837/ac/04/CPCSEA). Clozapine and haloperidol group were employed as standard (positive control). Statistical analysis of the results in the test group was done by comparison with the results in the control group employing non-parametric Kruskal Wallis test or one way ANOVA (Jandel Sigmastat version 2.0). Level of significance was fixed at p < 0.05.
2.4.1 Apomorphine induced mesh climbing behavior
Swiss albino mice (six mice in each group) of either sex (24–26 g) were habituated by individually placing in a circular cage made of wire mesh of diameter 13 cm and height 14 cm. Mice in the test, control and standard groups were injected, respectively, with test compound, normal saline and clozapine intraperitoneally and returned to the home cage. After a gap of 10 min, apomorphine (2.5 mg/kg) was injected intraperitoneally. Mesh climbing behavior was noted at 5 min intervals for up to 20 min, starting 10 min after the apomorphine administration using the following scoring system: 0 – no paws on the cage, 1 – one paw on the cage, 2 – two paws on the cage, 3 – three paws on the cage, 4 – four paws on the cage (Fig. 1). The score recorded for each animal was based on the position of the animal at the moment it was first observed. A score of 20 is the maximum possible. Recording was done by an observer who was unaware of the specific drug treatments.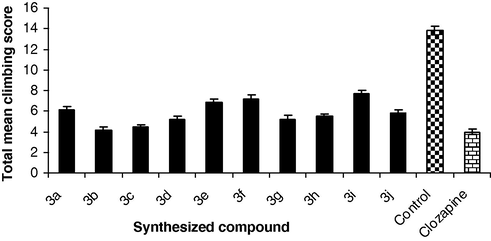
The effect of synthesized compounds (3a–j) on the apomorphine induced climbing behavior. Each column represents the mean ± SEM of total climbing score for group of six mice assessed at 5-min intervals for 20 min, starting 10 min after apomorphine treatment. All values are statistically significant with respect to control at p < 0.05.
2.4.2 5-Hydoxytryptophan (5-HTP) induced head twitches behavior
Swiss albino mice in the control group (n = 6) was injected with pargyline (75 mg/kg, i.p.) in order to prevent the rapid degradation of 5-HTP. Thirty minutes later, the test compound was administered. After a further 30 min, the mice received 5-HTP (50 mg/kg, i.p.). The mice were returned to the test cages and then head twitches were assessed at 10 min intervals for 30 min, starting 20 min after the 5-HTP treatment. Head twitches were monitored using the following scoring system: 0 – absent, 1 – moderate, 2 – marked (Fig. 2). A score of 20 is the maximum possible. An observer made all observations unaware of the specific drug treatments.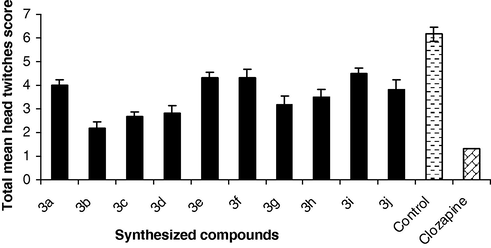
The effect of synthesized compounds (3a–j) on the 5-HTP induced head twitches behavior. Each column represents the mean ± SEM of total head twitches score for group of six mice assessed at 10-min intervals for 30 min, starting 20 after the 5-HTP treatment. All values are statistically significant with respect to control at p < 0.05.
2.4.3 Catalepsy
Catalepsy was induced in albino mice (n = 6) with haloperidol (1.0 mg/kg, i.p.) and was assessed at 30 min intervals until 120 min and at the end of 240 min by means of a standard bar test. Catalepsy was assessed in terms of the time (s) for which the mouse maintained an imposed position with both front limbs extended and resting on a 4 cm high wooden bar (1.0 cm diameter). The endpoint of catalepsy was considered to occur when both front paws were removed from the bar or if the animal moved its head in an exploratory manner. The cataleptic behavior was scored as 1 if the imposed posture was maintained for at least 20 s and every additional 20 s one extra point was given for severity of catalepsy (Fig. 3). A cut-off time of 1100 s was applied during the recording of observations. The animals were returned to their individual home cages in between determinations. All observations were made between 10.00 and 16.00 h in a quiet room at 23–25 °C. The animals in the test group were administered with test drugs instead of haloperidol and the remaining procedure for assessment of catalepsy was same as mentioned above.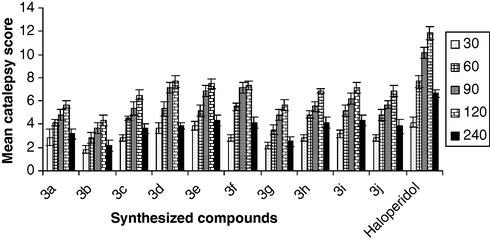
Induction of catalepsy of synthesized compounds (3a–j). Results are expressed as the mean ± SEM. (n = 6). All values are statistically significant with respect to control at p < 0.05.
3 Results and discussion
3.1 Synthesis of compounds
The target compounds 2-[4-(aryl substituted) piperazin-1-yl]-N-benzylacetamides (3a–j) were synthesized by benzylamine (1) in 2N sodium hydroxide treated with chloroacetylchloride in dichloromethane to afford 2-chloro-N-benzylacetamide (2). The IR spectrum of compound (2) showed characteristic carbonyl group absorption peak at 1669 cm−1 and 1H NMR spectrum exhibited a broad singlet due to NH group at 6.88 ppm. Compound (2) on treatment with appropriate phenylpiperazine in acetonitrile in the presence of K2CO3 and KI yielded the target compounds (3a–j). All the target compounds (3a–j) were obtained in good yield (37–62%) and characterized by analytical and spectroscopic methods. Mass spectra of target compounds showed characteristic M+1 peak. The reactions are outlined in Scheme 1 and the nature of the substituent is given in Table 1.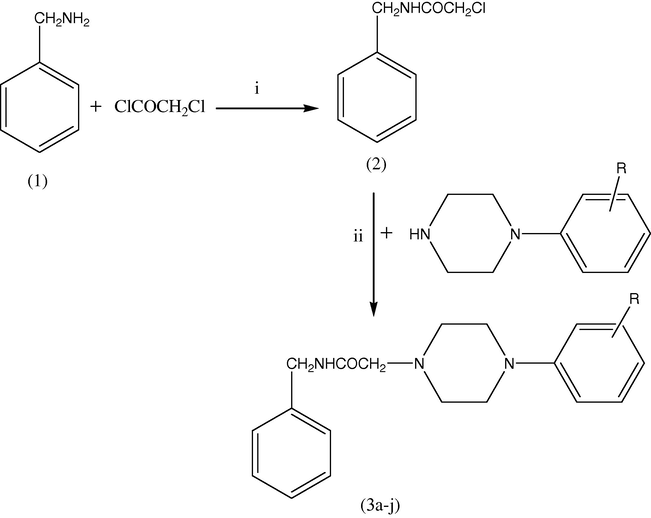
Synthesis of the target compounds. Reagents and conditions: (i) NaOH, dichloromethane (ii) acetonitrile, K2CO3, KI.
Compd. no.
Compd code.
R
1
3a
H
2
3b
2-OCH3
3
3c
3-OCH3
4
3d
4-OCH3
5
3e
3-CH3
6
3f
4-CH3
7
3g
2-Cl
8
3h
3-Cl
9
3i
4-NO2
10
3j
4-F
3.2 Computational studies
A set of molecular parameters were computed for the target compounds as well as three standard drugs clozapine, ketanserin and risperidone using Chem 3D Ultra version, 11.0, and Chem Silico online free software are shown in Table 2. The important molecular parameters for antipsychotics are blood-brain barrier (BBB), log P and topological polar surface area (TPSA). Literature review suggested that TPSA is a measure of a molecule’s hydrogen bonding capacity and its value should not exceed a certain limit if the compound is intended to be CNS active. Two differing limits have been proposed: van de Waterbeemed et al. suggested a limit of 90 A2, where, Kelder et al. suggested 60–70 A2. The TPSA value for test compounds (3a–j) were well within these limits (35.58–87.39) which shows that these compounds have a potential to effectively cross the blood-brain barrier. The log BB values (−0.20 to −0.40) and log P values (1.28–3.22) for the test compounds were noted and suggesting that these have an excellent potential for CNS activity. The physicochemical similarity of the test compounds (3a–j) was calculated with respect to the standard drugs and shown in Table 3. Most of the compounds showed good similarity with respect to standard drugs. Among them, compounds 3b showed good structural similarity with respect to clozapine, ketanserine and risperidone, respectively (57.60%, 78.35% and 78.82%).
Comp. code
log BBi
log P
M.W.a
MRb
SAS (A2)c
MSA (A2)d
SEV (A3)e
TPSAf
MTIg
WIh
3a
0.32
2.66
309.41
94.66
575.217
307.199
280.758
35.58
11308
1458
3b
0.27
2.53
339.43
101.91
638.833
338.01
301.468
44.81
13673
1802
3c
0.25
2.53
339.43
101.91
650.064
341.557
300.50
44.81
13901
1836
3d
0.25
2.53
339.43
101.91
650.116
340.422
298.864
44.81
14129
1870
3e
0.40
3.15
323.43
100.56
606.47
325.882
297.658
35.58
12691
1635
3f
0.40
3.15
323.43
100.56
635.774
332.971
292.45
35.58
12822
1652
3g
0.35
3.22
343.85
99.26
614.289
322.626
287.173
35.58
12077
1618
3h
0.37
3.22
343.85
99.26
623.463
326.608
287.197
35.58
12157
1635
3i
−0.20
1.28
354.40
98.64
649.164
341.967
300.969
87.39
15135
2090
3j
0.32
2.82
327.40
95.06
610.728
317.941
278.769
35.58
12237
1652
CLZj
0.75
3.71
326.82
94.58
508.991
259.124
215.892
30.87
8127
1082
KETk
−0.48
2.37
395.43
106.67
589.34
298.729
253.338
69.72
18646
2596
RISl
−0.20
2.10
410.48
114.21
690.021
375.09
351.81
57.50
20311
2793
Compd. code
Similaritya, b (%) to
Clozapine
Ketanserin
Risperidone
3a
76.52
69.90
69.14
3b
57.60
78.35
78.82
3c
56.21
78.61
79.38
3d
55.13
79.11
79.79
3e
67.39
73.66
73.24
3f
66.31
73.95
73.66
3g
69.95
73.69
72.71
3h
69.11
73.78
73.03
3i
18.55
83.39
75.81
3j
69.73
73.84
72.18
3.3 Preliminary pharmacological evaluation for antipsychotic effect
The results of pharmacological evaluation of the target compounds are depicted graphically in Figs. 1–3 and in Table 4. All the ligands showed significant interactions with the dopamine and serotonin receptors, which were found to be dependent, fundamentally on the substitution of the N4-aryl group of the piperazine ring. With respect to the dopamine receptor, compounds bearing a methoxy group at ortho, meta and para position of aryl moiety of piperazine ring (3b, 3c and 3d) produced statistically significant reduction in apomorphine induced climbing behavior than chloro analogs (3g and 3h). A significant reduction in activity was observed, when nitro group was present at para position of aryl moiety of piperazine ring (3i). Other compounds (3a, 3e, 3f and 3j) have lesser efficacy for the dopamine receptor. Regarding the serotonin receptor, the methoxy analogs (3b, 3c and 3d) showed higher inhibition of 5-hydroxy tryptophan (5-HTP) induced head twitches behavior than chloro analogs (3g and 3h). Highest reduction in activity was observed when methyl or nitro substituent was present on arylpiperazine ring (3e, 3f and 3i). The other compounds (3a, and 3j) showed lower activity for the serotonin receptor. The catalepsy results showed all the new compounds (3a–j) were less cataleptogenic than haloperidol. Among them, methoxy analogs 3b exhibited lower propensity to produce catalepsy.
Compd. code
Inhibition of apomorphine induced climbing behavior (ED50, mg/kg, i.p.)
Inhibition of 5-HTP induced head twitches behavior (ED50, mg/kg, i.p.)
Induction of catalepsy (ED50, mg/kg, i.p.)
3a
21.3
11.2
35.0
3b
10.0
7.5
40.0
3c
10.5
8.5
45.0
3d
27.1
10.2
40.0
3e
>40
23.4
>50
3f
>40
23.2
>50
3g
22.0
10.5
44.2
3h
22.5
13.2
43.2
3i
>40
>40
>50
3j
29.2
15.2
38.7
Clozapine
8.7
2.8
–
Haloperidol
–
–
nda
4 Conclusion
A new series of arylpiperazines have been synthesized and their preliminary pharmacological evaluation has shown potential atypical antipsychotic effect in behavioral models. However, in vivo results are insufficient for prediction of an atypical antipsychotic profile similar to clozapine. Among the compounds, 3b has shown potent atypical antipsychotic activity probably by inhibition of dopamine D2 and serotonin 5-HT2 receptors and low induction of catalepsy. Moreover, TPSA, log BB, log P values suggested that this compound has the potential to penetrate the blood-brain barrier and showed good similarity with respect to standard drugs. Further studies on this lead including in vitro assays are required for the refinement of the atypical antipsychotic activity.
Competing interests
The authors declare no conflict of interest.
Acknowledgements
The authors are grateful to Prof. R.M. Dubey, Managing Director, IFTM, Moradabad, for providing the research facilities and also thankful to the Head, sophisticated analytical instrument facility, CDRI, Lucknow, for spectral analysis. This work is a part of work done for Ph.D. degree of the Shobhit University by Sushil Kumar.
References
- Burger’s Medicinal Chemistry and Drug Discovery (sixth ed). New Jersey: John Wiley & Sons; 2003. p. 599
- Brain dopamine receptors: a primer on their current status, basic and clinical. Harv. Rev. Psychiatry. 1996;3:301-325.
- [Google Scholar]
- Synthesis, evaluation and computational studies on a series of acetophenone based 1-(aryloxypropyl)-4-(chloroaryl) piperazines as potential atypical antipsychotics. Eur. J. Med. Chem.. 2010;45:2656-2662.
- [Google Scholar]
- Synthesis and binding affinity of potential atypical antipsychotics with the tetrahydroquinazolinone motif. Bioorg. Med. Chem. Lett.. 2009;19:6059-6062.
- [Google Scholar]
- Behavioural pharmacology of polygalasaponins indicates potential antipsychotic efficacy. Pharmacol. Biochem. Behav.. 2002;71:191-195.
- [Google Scholar]
- Is experimental catalepsy properly measured? Pharmacol. Biochem. Behav.. 1990;35:753-757.
- [Google Scholar]
- New arylpiperazine derivatives with high affinity for 1A, D2 and 5-HT2A receptors. Bioorg. Med. Chem. Lett.. 2003;113:175-178.
- [Google Scholar]
- Polar molecular surface as a dominating determinant for oral absorption and brain penetration of drugs. Pharm. Res.. 1999;16:1514-1519.
- [Google Scholar]
- Synthesis, computational studies and preliminary pharmacological evaluation of 2-[4-(aryl substituted) piperazin-1-yl] N,N-diphenylacetamides as potential antipsychotics. Eur. J. Med. Chem.. 2011;46(9):4753-4759.
- [Google Scholar]
- Synthesis and preliminary pharmacological evaluation of 2-[4-(aryl substituted) piperazin-1-yl]-N-phenylacetamides: as potential antipsychotics. Trop. J. Pharm. Res.. 2011;10(3):265-272.
- [Google Scholar]
- The ratios of serotonin-2 and dopamine-2 affinities differentiate atypical and typical antipsychotic drugs. Psychopharmacol. Bull.. 1989;25:390-392.
- [Google Scholar]
- Synthesis and 5-HT1A/5-HT2A receptor activity of new N-[3-(4-phenylpiperazin-1-yl)-propyl] derivatives of 3-spiro-cyclohexanepyrrolidine-2,5-dione and 3-spiro-β-tetralonepyrrolidine-2,5-dione. Pol. J. Pharmacol.. 2003;55:553-557.
- [Google Scholar]
- 1-Aryl-4-[(5-methoxy-1, 2, 3, 4-tetrahydronaphthalen-1-yl) alkyl] piperazines and their analogues: influence of the stereochemistry of the tetrahydronaphthalen-1-yl nucleus on 5-HT1A receptor affinity and selectivity versus α1 and D2 receptors. J. Med. Chem.. 1999;42:490-496.
- [Google Scholar]
- Developments in the drug treatment of schizophrenia. Trends Pharmacol. Sci.. 1992;13(3):116-121.
- [Google Scholar]
- Essential Psychopharmacology: Neuroscientific Basis and Practical Applications (second ed). New York: Cambridge University Press; 2000. pp. 401–458
- Estimation of blood-brain barrier crossing of drugs using molecular size and shape, and H-bonding descriptors. J. Drugs Target. 1998;6:151-165.
- [Google Scholar]







