Translate this page into:
Synthesis of a reusable magnetic photocatalyst based on BiOCl / MnxZn1-xFe2O4 composites and its application on RhB degradation
⁎Corresponding author. cqwhl@sicnu.edu.cn (Hailong Wang)
-
Received: ,
Accepted: ,
This article was originally published by Elsevier and was migrated to Scientific Scholar after the change of Publisher.
Peer review under responsibility of King Saud University.
Abstract
Water pollution is becoming an increasingly serious issue, which threatens human survival. However, existing technology for addressing this issue has not developed adequately, and the recovery of polluted water remains difficult. Therefore, it is critical to develop innovative wastewater treatment technology, which is highly effective, uses little energy, and produces no secondary pollution. In this study, a Z-type heterojunction BiOCl/MnxZn1-xFe2O4 magnetic composite photocatalyst was created by the hydrothermal method. Using RhB as the target dye, the photocatalytic performances of the composite samples were assessed. The structure of composite samples was studied by SEM, X-ray diffraction, and UV–vis diffuse reflectance spectroscopy.The findings demonstrate that the heterojunction created by the addition of MnxZn1-xFe2O4 and BiOCl lowers the rate at which photogenerated electrons and holes recombine. Meanwhile, the prepared BiOCl/MnxZn1-xFe2O4 magnetic composite photocatalyst exhibited a notable catalytic degradation effect on RhB. Among them, the degradation rate of BiMn-5 was as high as 94.6%, showing better photocatalytic performance than other composite samples with different mass ratios. In addition, the product can be recycled. After five repeated tests, the average degradation rate remained as high as 88.7%. Therefore, the BiOCl/MnxZn1-xFe2O4 magnetic composite photocatalyst developed herein can be a new technology for efficient and environmentally friendly sewage treatment in line with contemporary needs.
Keywords
Z-type heterojunction
BiOCl/MnxZn1-xFe2O4 magnetic composite photocatalyst
RhB
Hydrothermal method
1 Introduction
Rapid industrial development has led to several environmental safety issues. Currently, people are becoming increasingly aware of environmental degradation, particularly water contamination. Organic pollutants in water upset the equilibrium of the natural environment and seriously endanger people’s health. (Gao et al., 2020; Hu et al., 2020). Several technologies have been developed to address water pollution, including adsorption (Shao et al., 2020), membrane separation (Qu et al., 2017), chemical oxidation, and photocatalysis (Zeng et al., 2020; Chu et al., 2019). Over the years, considerable progress has been made in the field of water environment management and protection through the continuous efforts of the government. However, the problem of aquatic environmental safety has not yet been adequately addressed.
Dye wastewater is produced from several sources and is characterized by high toxicity and difficult degradation. Several organic dyes are carcinogens that directly affect human health. The photosynthesis of aquatic plants is also inhibited by dark dyes, which affect the self-purification of water. The most important of these is the continuous inflow and persistence of organic micropollutants in water bodies, which seriously affects the sustainable health of global organisms (Chawla et al., 2021; Shnada et al., 2017; Hu et al., 2021). Therefore, the creation of a revolutionary wastewater treatment technology that is highly effective, energy-efficient, and produces no secondary pollution, is critical. As a green technology that can solve the issues of continuous deterioration of the environment and energy shortage, semiconductor photocatalysis has attracted widespread attention (Xu et al., 2020; Zhu et al., 2021).
Semiconductor photocatalytic water pollution treatment technology involves the use of semiconductor photocatalysts to convert low-density light energy into chemical energy under light irradiation, thereby initiating a series of chemical reactions and eventually achieving the decomposition of organic pollutants. This method is inexpensive and highly efficient. The reduction products are nontoxic and harmless, such as water and carbon dioxide, and the process is free from the risk of secondary pollution. Semiconductor photocatalysts such as TiO2 (Zahra et al., 2017); ZnO (Chin et al., 2018), Bi2O3 (Chen et al., 2019), g-C3N4 (Masih et al., 2017); SnIn4S8 (Zhang et al., 2023) and BiOCl (Yao et al., 2020) can effectively degrade wastewater containing organic dyes such as rhodamine B (RhB) (Wang et al., 2018), methylene blue (MB) (Wu et al., 2019), and methyl orange (MO) (Reddy et al., 2020). Photocatalytic technology also exhibits considerable application potential for the treatment of heavy metal ions in water (Sang et al., 2020) and toxic organic pollutants (Zhang et al., 2015), reduction of chemical oxygen demand (COD) in wastewater (El-Mekkawi et al., 2016), digestion and treatment of toxic and harmful gases (Choi and Park, 2021), and photolysis of water to produce hydrogen (Huogen et al., 2019).
In recent years, BiOCl has been considered a promising photocatalyst for the photodegradation of dyes because of its distinct layering, simple oxygen vacancy production, wide band gap, low toxicity, and affordable cost (Lee et al., 2017; Siao et al., 2019; Liu et al., 2018). Moreover, the structure of BiOCl is easily controlled, making it an important material for photocatalytic research (Wei et al., n.d.). BiOCl has attracted considerable interest as a new type of semiconductor photocatalyst because of its distinct stratified tetragonal structure. The [Bi2O2]2+ layer and the alternating double Cl atomic layer have an inner electric field that efficiently separates the photogenerated electron–hole pairs. (He and Zhou, 2014; Ye et al., 2011). BiOCl can be prepared by simple hydrothermal, calcination, or precipitation methods. It exhibits good chemical stability and a unique structure. However, BiOCl suffers from the common drawbacks of traditional photocatalysts such as complex recovery, difficult reuse, and secondary pollution. To address the problem of recycling, the magnetic ferrite of the photocatalyst is combined to prepare a magnetic photocatalyst that can be quickly recovered under an external magnetic field. MnxZn1-xFe2O4 has garnered the most interest among bulk magnetic oxide materials because of its excellent chemical stability, the lack of toxicity, and simplicity of magnetic recovery (Thakur et al., 2020). and has established itself as an ideal method for producing magnetic composite photocatalytic materials. MnxZn1-xFe2O4-based heterojunctions such as BiVO4 (Xie et al., 2018); BiOI (Feng et al., 2019); BiFeO3 (Zhang et al., 2023) and BiOBr0.5Cl0.5 (Kaewmanee et al., 2020) have been constructed to achieve efficient photodegradation of organic pollutants. In this study, BiOCl photocatalysts were combined with MnxZn1-xFe2O4 with strong magnetism, by a simple hydrothermal method to prepare magnetic BiOCl, and the ability of the product to degrade RhB dye wastewater was tested. Characterization was performed to explore the photocatalytic mechanism and provide a reference for the further development and application of semiconductor photocatalytic technology.
2 Experimental
2.1 Chemicals
Sodium chloride (NaCl AR), Bismuth nitrate (Bi(NO3)3·5H2O AR), Zinc sulfate heptahydrate (ZnSO4·7H2O AR), Manganese Sulfate Monohydrate (MnSO4·H2O AR), Ferric sulfate (Fe2(SO4)3 AR), Ethylene glycol (C2H6O2 AR) and Sodium hydroxide (NaOH AR) were purchased from the Chengdu Kelong Chemical Co. And Rhodamine B (RhB AR) was purchased from theTianjin Guangfu Fine Chemical Research Institute.
2.2 Preparation of photocatalyst
In this study, using NaCl and Bi(NO3)3·5H2O as the starting materials, a hydrothermal calcination process was used to synthesize a combined photocatalyst of BiOCl/MnxZn1-xFe2O4. The MnxZn1-xFe2O4 ferrite production procedure is shown in Fig. 1. First, MnxZn1-xFe2O4 was prepared as an additive. A mixture of 0.96 g ZnSO4·7H2O, 1.38 g MnSO4·H2O, and 5.39 g Fe2(SO4)3 was diluted in 50 mL of distilled water. Then, with 10 min of ultrasonic stirring, 2 mol/L sodium hydroxide solution was added to the original liquor until the pH raised to 13.The resultant solution was stirred for 10 min and transferred to a high-pressure reactor. BiOCl/MnxZn1-xFe2O4 was created through a 5 h, 200 ℃ reaction.The microemulsion method was used to effectively synthesize the BiOCl/MnxZn1-xFe2O4 nanocomposite. First, 20 mL of ethylene glycol was added to the beaker, and 0.97 g of Bi(NO3)3·5H2O was added to it to obtain suspension A. Subsequently, 30 mL of 0.0661 mol/L NaCl solution was added to the suspension A, and the suspension B was obtained by stirring for 10 min.The previously synthesized MnxZn1-xFe2O4 was added to suspension B, agitated and then put into the reactor, where it reacted for 12 h at 160 °C. After filtering, it was repeatedly rinsed with anhydrous ethanol and distilled water.Then it was dried in an oven at 80 ℃ for 6 h. The dry sample was crushed, put in the ceramic crucible,and put it into the muffle furnace heating to 500 °C for 2 h. After cooling and grinding the resultant, BiOCl/MnxZn1-xFe2O4 was obtained as a solid powder. BiOCl/MnxZn1-xFe2O4 with MnxZn1-xFe2O4 weight fractions of 5 %, 7.5 %, 10 %, 12.5 % and 15 % were prepared and expressed as BiMn-5, BiMn-7.5, BiMn-10, BiMn-12.5 and BiMn-15, respectively.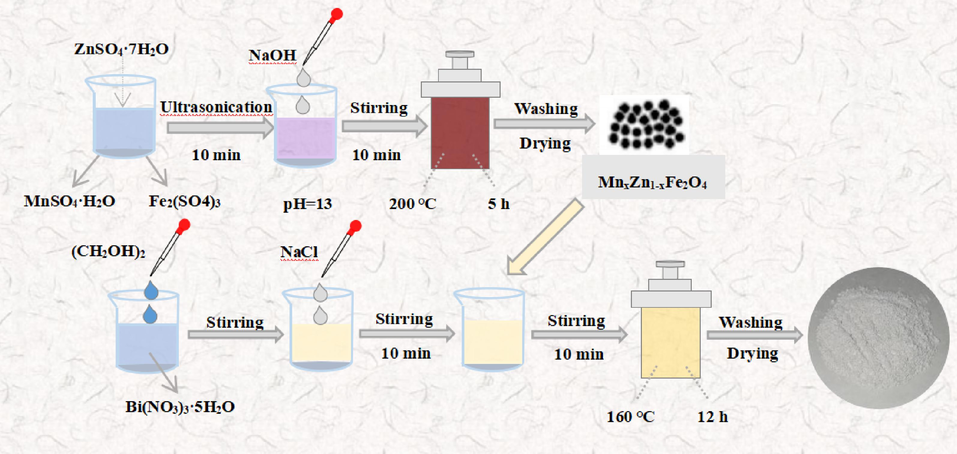
Flowchart describing the process of BiOCl/MnxZn1-xFe2O4 synthesis.
2.3 Characterization
X-ray diffraction (XRD; Shimadzu XRD 6000, Japan) was performed to study the phases of the synthesized photocatalyst. Based on the XRD results, the sizes of samples and the cell parameters were computed to preliminarily determine the sample structure. Transmission electron microscopy (TEM; Tecnai G2 F20, USA) and scanning electron microscopy (SEM; S4800, Japan) were performed to study the microstructures of the materials and identify their precise structures. Furthermore, X-ray photoelectron spectroscopy (XPS) was applied to determined the elements present in the photocatalyst and their valence states. The samples' particular area of surface and distribution of pore sizes were computed using the Brunauer-Emmett-Teller (BET, Quadrasorb 2 MP) analysis, and the adsorption type and mechanism of BiOCl/MZF were studied based on the nitrogen adsorption–desorption curve. UV–vis absorption spectroscopy (UV–vis, TU-1901, China) was used to analyze the degraded RhB solution, and the concentration of RhB was determined by its spectral peak to determine the photocatalytic degradation effect. The saturation magnetization of BiOCl/MZF was measured using a vibrating sample magnetometer (VSM) to determine the magnetism of the sample. The magnet type and stability of the sample were determined according to the shape of the hysteresis loop and residual magnetization. Photoluminescence (PL) spectroscopy (F-4700, Japan) was used to determine the lifetime the pairs of photogenerated electron-holes in the sample to evaluate its photocatalytic effect.
2.4 Photocatalytic assessment
The concentration of a 3 mL mixture was measured in each test. A constant 0.1 g of photocatalyst and 0.1 L of RhB-simulated wastewater (10 mg/L) was used. To ensure that the photocatalyst was in a saturated adsorption state, a beaker containing the photocatalyst solution was first mechanically agitated for 30 min in the dark. The samples were then exposed to light and periodically tested. The entire reaction was performed under a 300 W xenon lamp, acting as a substitute for sunlight.
3 Results and discussion
3.1 Photocatalytic activity
The photocatalytic activity of BiOCl/MnxZn1-xFe2O4 was investigated,by simulating the degradation of RhB under simulated sunlight. As shown in Fig. 2(a), after an 80-min illumination, the degradation rates of RhB by BiMn-5, BiMn-7.5, BiMn-10, BiMn-12.5, and BiMn-15 were 94.06 %, 80.86 %, 85.57 %, 92.18 %, and 69.06 %, respectively. In this degradation process, BiMn-5 exhibited the fastest degradation rate and the best degradation effect.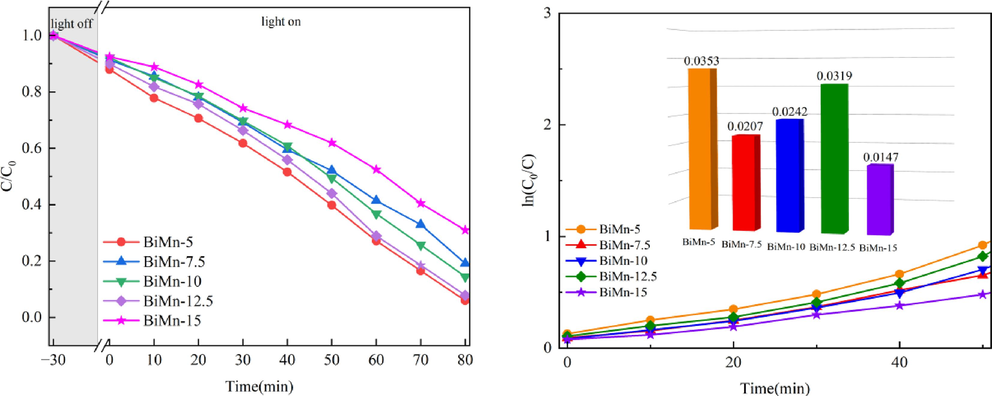
(a) RhB degradation rate; (b) Kinetic linear fitting curves (the illustration is the chemical reaction's rate constants).
A first-order kinetic model represented by ln(C0/C) = kt (Sakkas and Konstantinou, 2004) was used to quantitatively investigate the photocatalytic degradation of the RhB solution. Studying the kinetic model and the results of the calculations based on the aforementioned formula suggested that the degradation of RhB by the BiOCl/MnxZn1-xFe2O4 composite photocatalyst corresponded to the kinetic model for first-order reactions, as illustrated in Fig. 2(b). BiOCl, BiMn-5, BiMn-7.5, BiMn-10, BiMn-12.5, and BiMn-15 exhibited the constants for first-order reaction rate of 0.0673, 0.0353, 0.0207, 0.0319, and 0.0147 min−1, respectively. Because BiMn-5 exhibited better photocatalytic performance than other composite photocatalysts, BiMn-5 was studied in depth.
3.2 Magnetic stability and recovery capability
In this experiment, the magnetism of the composite photocatalyst was crucial to the recyclability of the photocatalyst, and a VSM was employed to study the magnetism of MZF and BiMn-5. Fig. 3 shows a loop that causes hysteresis. The saturation magnetization (Ms) of the MZF was 69.7 emu·g−1. The residual magnetization (Mr) of MZF was low (1.53 emu·g−1) indicating that the hysteresis loss of MZF was small under the magnetic field. The Ms of BiMn-5 was 1.80 emu·g−1, and the decrease of saturation magnetization was owing to the low content of MZF in the hybrid magnetic photocatalyst. BiMn-5 exhibited a remanent magnetization of 0.14 emu/g with a tight hysteresis loop, similar to MZF, suggesting that it also had a low hysteresis loss. Despite having a lower Ms than that of MZF, the magnetic photocatalyst rapidly recovered when subjected to the outside magnetic field.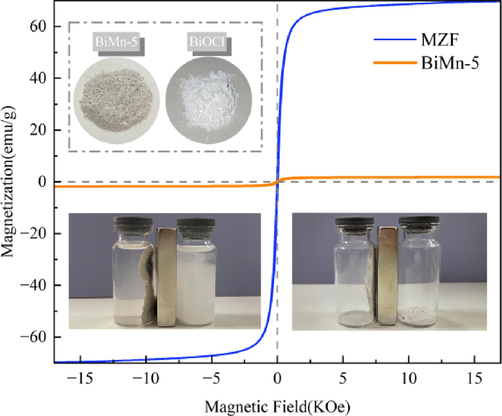
Loops of magnetic hysteresis (Illustration shows BiOCl and BiMn-5 attracted by a magnet).
The cycling stability of photocatalytic materials is crucial for their sustained applicability. Therefore, the cycling stability of the composite magnetic photocatalytic materials was also tested. The catalyst was magnetically separated, washed several times, dried, and repeatedly used to degrade RhB. The experimental results are shown in Fig. 4. After five cycles of use, the average degradation rate of BiMn-5 composite photocatalytic material reached 88.7 %, and the adsorption was not significantly weakened. The degradation remained excellent after five cycles. This indicates that BiMn-5 is highly stable and recyclable. This also shows that the RhB adsorbed by the composite photocatalytic material was effectively degraded.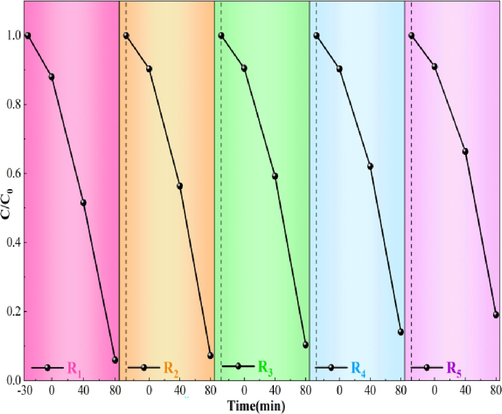
Degradation rate of RhB by BiMn-5 after being recycled.
3.3 Structure characteristics
3.3.1 XRD analysis
The crystalline structures of BiOCl, MZF, and BiMn-5 were investigated using XRD. The cubic form of MnxZn1-xFe2O4 is suggested by diffraction peaks that match with those in the PDF standard diagram 74–2399. As shown in Fig. 5, BiOCl exhibits significant peaks in diffraction at 2θ = 11.98°, 25.86°, 32.50°, 33.45°, 40.89°, 46.64°, 49.70°, 54.09°, and 58.60°, which matches with the crystal planes of BiOCl (PDF # 06–0249) at (0 0 1), (1 0 1), (1 1 0), (1 0 2), (1 1 2), (2 0 0), (1 1 3), (2 1 1), and (2 1 2), respectively, indicating that the prepared BiOCl is a lamellar tetragonal crystal. MnxZn1-xFe2O4 exhibits strong diffraction peaks at 35.18° and 61.83°. The typical peak shapes of the BiMn-5 composites were nearly identical to those of BiOCl, indicating that MnxZn1-xFe2O4 was successfully combined with BiOCl. By using the Scheller formula, the median size of the grains of BiMn-5 was determined to be 52.11 nm, and there were no more impurity peaks observed in the XRD pattern of BiMn-5. This indicated that the purity of the prepared BiMn-10 composite photocatalyst was relatively high.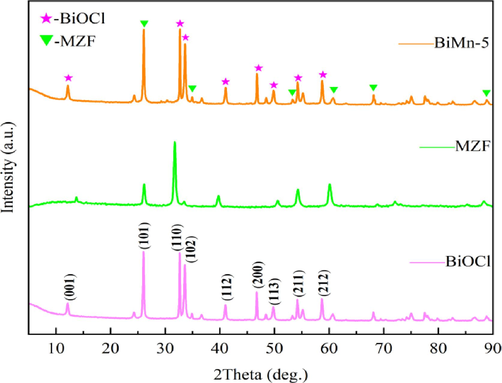
XRD patterns of BiOCl, MnxZn1-xFe2O4, and BiMn-5.
3.3.2 SEM analysis
The surface structures of the specimens were investigated by SEM. BiOCl and MnxZn1-xFe2O4 consisted of unevenly formed nanosheets and sphere-shaped particles, respectively. As shown in Fig. 6(c), BiOCl flakes adhered to the surface of MnxZn1-xFe2O4. XRD results show that the two materials are successfully combined, indicating that the combination of BiOCl and MnxZn1-xFe2O4 did not destroy the original semiconductor structure. In addition, the TEM images indicated that BiOCl was successfully compounded with MnxZn1-xFe2O4, which verified the SEM results. Fig. 6(g–i) shows the full EDS spectra of BiOCl/MnxZn1-xFe2O4. The composite samples contained Cl, Bi, O, Mn, Zn, Fe, and other elements. The elemental compositions of the samples were analyzed and characterized. It is clearly observed that each element was evenly distributed in the sample, and the distribution of the elements was also more concentrated. Moreover, the Mn, Zn, and Fe elements were more active on the surface of the BiMn-5 composite sample.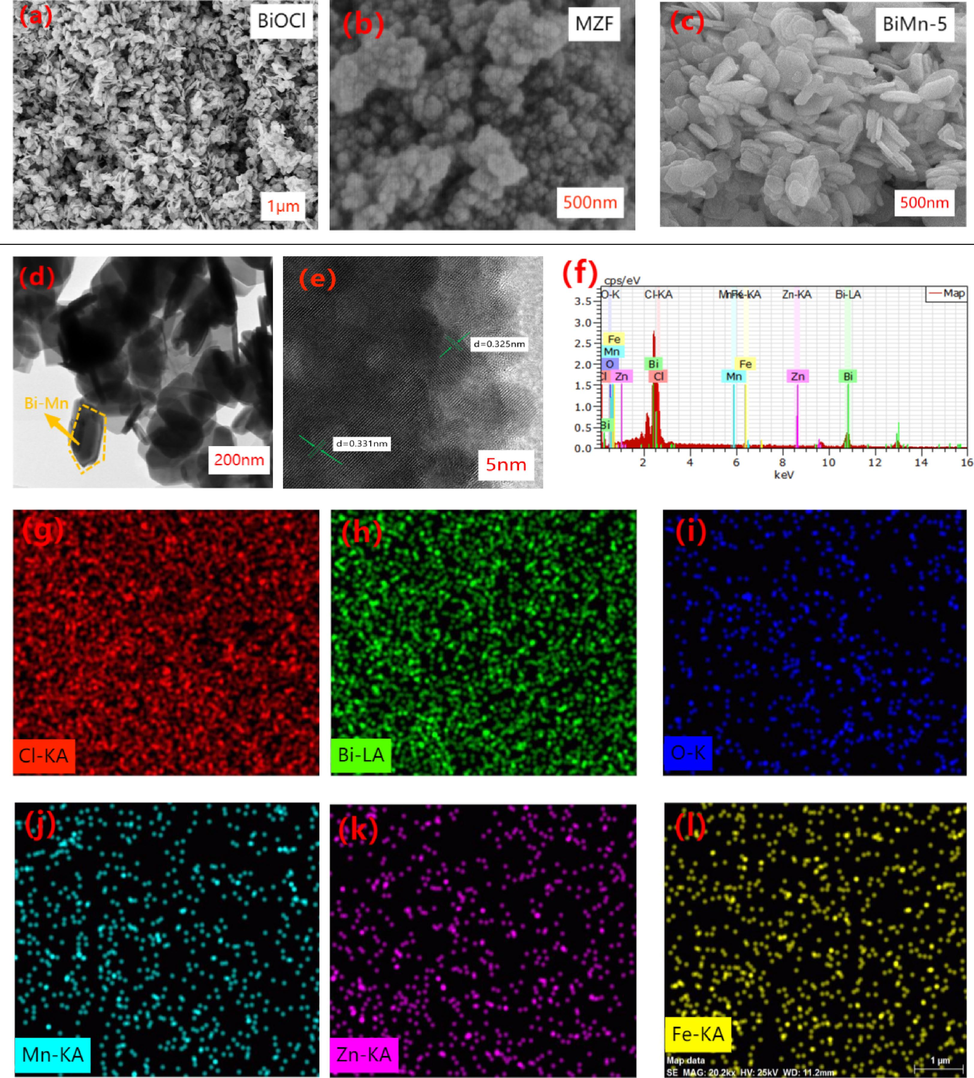
(a)–(c) SEM images of BiOCl, MZF, and BiMn-5; (d) TEM image of BiMn-5; (e) HRTEM image of BiMn-5; (f) EDS spectrum of BiMn-5; (g)–(i) EDS elemental mapping of Cl, Bi, O, Mn, Zn, and Fe.
3.3.3 XPS analysis
The surface chemical characteristics of BiMn-5 were studied by XPS. The findings suggested that bond formation and the transfer of chemicals occurred as a result of the combination of BiOCl and MnxZn1-xFe2O4. As shown in Fig. 7(a), the XPS spectra of BiMn-5 proved the presence of Cl, Bi, O, Mn, Zn, Fe, and C is consistent with the EDS and HRTEM findings. The Cl 2p spectra exhibit a pair of distinct peaks at 199.8 eV (Cl 2p3/2) and 198.3 eV (Cl 2p1/2), indicating the presence of Cl-. In addition, the characteristic peaks of the Bi 4f spectrum at 159.4 eV (Bi 4f7/2) and 164.67 eV (Bi 4f5/2) further confirmed the existence of Bi3+ (Li and Wu, 2018). The distinctive peak at 530.2 eV can be integrated into the O 1 s spectrum, as illustrated in Fig. 7(d). MnxZn1-xFe2O4 is the primary source of the major signals at 637.28, 1023.68, and 702.6 eV, which are the major signals in the Mn 2p, Zn 2p, and Fe 2p spectra in Fig. 7(e–g). In addition, the strong interaction between BiOCl and MnxZn1-xFe2O4 in BiMn-5 slightly shifted the highest points of Cl 2p, Bi 4f, and O 1 s to lower binding energies compared to those in BiOCl. In summary, the heterojunction interface between BiOCl and MnxZn1-xFe2O4 was formed by a chemical combination of chemicals rather than simple physical interactions.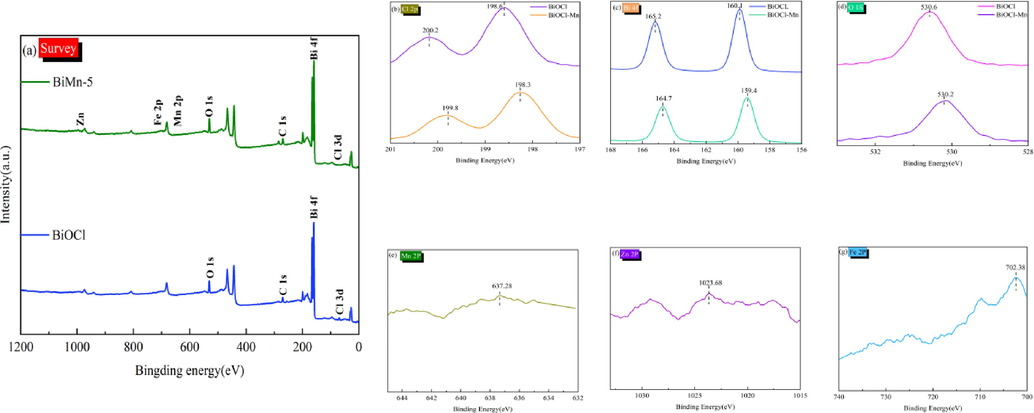
(a) XPS survey spectra; (b–g) XPS spectra corresponding to each element.
3.3.4 BET analysis
According to the BiMn-5 desorption and adsorption rates for nitrogen (Fig. 8), the desorption curve does not coincide with the adsorption line, and the isotherm significantly increases with an increase in P/P0. The most probable pore size distribution of the BiMn-5 composite photocatalyst was 25.2 nm. The overall pore volume of the BiMn-5 was 0.0311 cm3/g and the Brunauer–Emmett–Teller (BET) specific surface area was 14.73 m2/g. RhB can be more easily adsorbed, and the rate of its photocatalytic degradation can be increased by increasing the pore volume and specific area of the surface.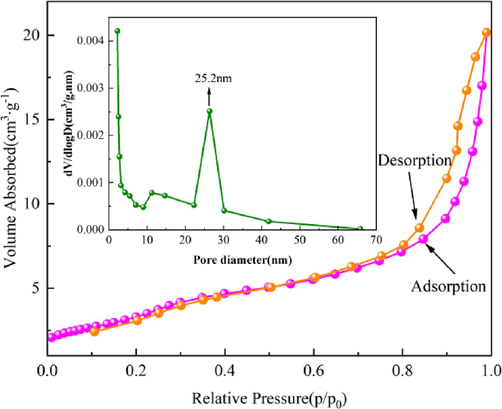
Nitrogen adsorption–desorption isotherms of BiMn-5 (The pore size dispersion curve is shown above).
3.3.5 Band analysis
The photoresponse characteristics of BiMn-5 and BiOCl were studied by UV–Vis diffuse reflectance spectroscopy (DRS),and the results are shown in Fig. 9. The equation ahv = A (hv-Eg)1/2 was used to determine the band gap energy. It is important to note that BiMn-5 exhibited substantial absorption in the visible light range, but BiOCl mostly absorbed UV light, indicating that the addition of MnxZn1-xFe2O4 is beneficial to expand the absorption region of the composite photocatalyst. The bandgap is the key element in determining the occurrence of pairs of electrons and holes in photocatalysts. BiMn-5 and BiOCl exhibit bandgap energies of 3.09 and 3.15 eV, respectively. Compared to BiOCl, the bandgap energy of BiMn-5 is lower, which also suggests the enhanced photocatalytic activity of BiMn-5.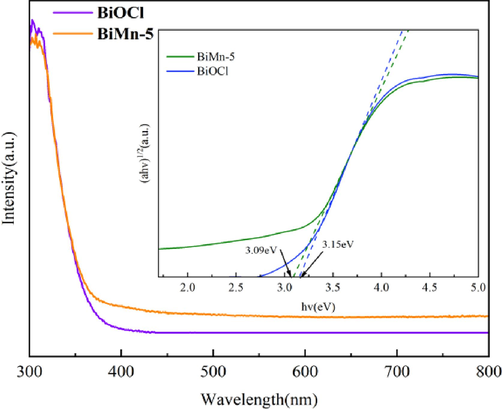
UV–vis diffuse reflectance spectra of BiOCl and BiMn-5 (Inset shows the (ahv) 1/2-hv curve).
3.3.6 Photoelectrochemical properties
PL emission spectra were used to measure the recombination degree of photoinduced electrons and holes. PL spectroscopy can be used to investigate the lifespan of photogenerated pairs of electrons and holes. In the procedure of photocatalysis, the combination of the pairs of electrons and holes generated by photosynthesis can influence the activity of the photocatalyst. In general, the stronger the PL intensity, the greater the recombination probability of photogenerated holes and electrons. By contrast, a lower PL intensity indicates lower recombination efficiency, resulting in a longer lifetime of the photogenerated carriers. As shown in Fig. 10, the BiMn-5 peak is less powerful than that of BiOCl, indicating that the photogenerated electron–hole recombination rate is lower. This is because a heterojunction is formed between MZF and BiOCl, which enhanced the separation of holes and electrons, thereby preventing their recombination. In addition, after the addition of MnxZn1-xFe2O4, the useful life of the carriers produced by photosynthesis increased.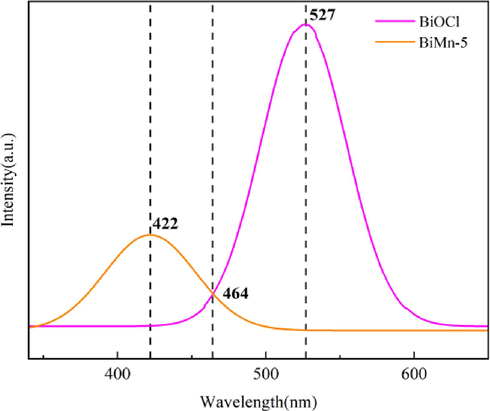
Photoluminescence (PL) emission spectra of BiOCl and BiMn-5.
Electrochemical impedance spectroscopy (EIS) is applied to determine the resistance of photogenerated electrons during transfer. The arc radius of the EIS spectrum was used to characterize the related properties (Yu et al., 2018). If the radius is smaller, the interface resistance during the electron transfer process is also lower, and the recombination rate decreases with decreasing peak strength (Zhang et al., 2021). As shown in Fig. 11, compared to the BiMn-5 composite photocatalyst, BiOCl exhibits a smaller radius of curvature, higher interfacial charge transfer and separation efficiency, and more efficient conductivity.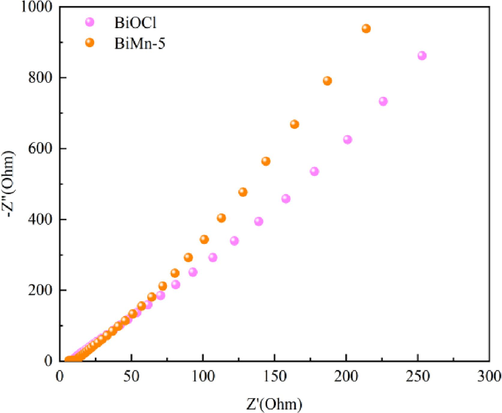
Electrochemical impedance spectra of BiOCl and BiMn-5.
BiOCl and BiMn-5 transient current concentrations were recorded, and the carrier mobility was studied. Within a predetermined duration, the immediate photocurrent reaction intensity fluctuated with the cyclical lighting process. When the BiOCl and BiMn-5 photocatalysts receive light, BiOCl and BiMn-5′s electrons are instantly energized., leading to a rapid increase in the photocurrent density that reaches a peak in an extremely short period. As shown in Fig. 12, under illumination, the photocurrent densities of both materials showed an upward trend. Simultaneously, the current density was relatively low without illumination, and the photocurrent density of BiOCl was higher, indicating that the photocurrent response of BiMn-5 was weaker than that of BiOCl. It can be concluded that the photocatalytic efficiency of BiMn-5 was slightly lower than that of BiOCl.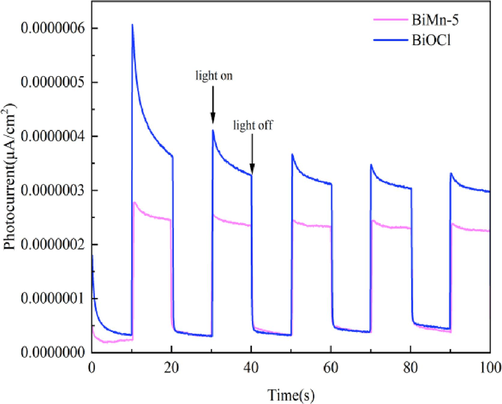
Transient photocurrent response (I–t) of BiOCl and BiMn-5.
3.3.7 Active radical capture experiment
The mechanism of the photocatalytic reaction can be studied by adding a capture agent to the photocatalytic reaction. Fig. 13 shows the experimental results for the BiMn-5 radical capture. After adding O2– capture agent BQ and hole capture agent AO, the degradation rates of RhB by BiMn-5 under simulated sunlight were 12.41 % and 37.29 %, respectively, after 80 min and the photocatalytic activity was significantly reduced. After adding IPA to capture hydroxyl radicals, the degradation rate of RhB was 29.38 %. Therefore, the IPA addition exhibited approximately no impact on the activity of photocatalytic catalysts. This suggests that the principal contributors to the breakdown of RhB by BiMn-5 were the superoxide radicals and photogenerated holes.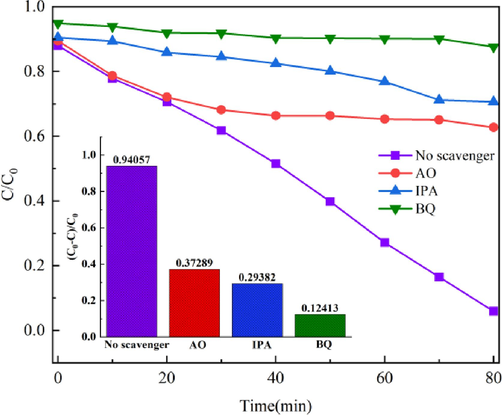
The impact of radical scavengers on the degradation of BiMn-5 by RhB photocatalysis.
3.4 Photocatalytic mechanism
BiOCl exhibits a wide energy band, cannot effectively excite photogenerated pairs of electrons and holes under simulated sunlight. The difference in the energy levels between BiOCl and MnxZn1-xFe2O4 leads to the movement of photoinduced holes and electrons into the Z-type heterojunction, as exhibited in Fig.14. Owing to the influence of the energy density disparity, e- on the conduction band of BiOCl tends to transfer and combine with the hole transferred from the valence band of MnxZn1-xFe2O4, which inhibits recombination of carriers produced by photons. The EVB (3.42 eV) of BiOCl is higher than the energy level of·OH/H2O (2.27 eV), resulting in the oxidation of hydroxide to hydroxide radical. Conversely, the ECB (-0.78 eV) of MnxZn1-xFe2O4 possesses a sufficient negative charge so that photoinduced electrons can convert O2 to superoxide radicals. MnxZn1-xFe2O4-doping inhibited the growth of the BiMn-5 crystal plane; therefore, it was easier to adsorb dissolved oxygen in the water to produce superoxide radicals. Inside the BiOCl/MnxZn1-xFe2O4 heterojunction, a field of electromagnetic radiation develops because of the constant motion of holes and electrons. The addition of MnxZn1-xFe2O4 decreased the migration resistance of the photogenerated electron–hole pairs by preventing their recombination.
Schematics of the photocatalytic mechanism.
4 Conclusions
To solve the issues of the nonresponsivity of BiOCl to simulated sunlight and difficulty in its recovery, we prepared a BiOCl/MnxZn1-xFe2O4 magnetic photocatalyst that could be rapidly recovered under an external magnetic field. The BiOCl and MnxZn1-xFe2O4 substrates were prepared using a hydrothermal method, and the new magnetic composite photocatalysts BiOCl/MnxZn1-xFe2O4 were prepared with different mass ratios. Through five repeated experiments, it was concluded that the composite material exhibited strong stability and recyclability. The RhB degradation ability was analyzed using simulated sunlight, and the results and properties were characterized using various characterization methods. The results of the degradation experiments showed that BiMn-5 exhibited better photocatalytic efficiency than other samples, and demonstrated a RhB degradation rate of 94.06 % within 80 min. MnxZn1-xFe2O4 forms a heterojunction with BiOCl, which lowers the rate at which the photogenerated electron–hole recombination occurs. The findings from the current study reveal that the preparation method of the magnetic composite photocatalyst BiOCl/MnxZn1-xFe2O4 is simple, raw material cost for preparing the photocatalyst is low, stability of the photocatalyst in repeated reactions is outstanding, and the recyclability of the photocatalyst is notable, thereby demonstrating the considerable potential for practical uses.
Acknowledgements
This research was supported by the National Natural Science Foundation of China (NSFC, 51374259)
Author Statement
All authors contributed to the study conception and design. Material preparation, data collection and analysis were performed by Hailong Wang, Huasen Guo, Chaodi Zhang,Dan Yang, Yifan Wang, Haiyi Wan, Lei Feng. The first draft of the manuscript was written by Huasen Guo and all authors commented on previous versions of the manuscript. All authors read and approved the final manuscript.
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
References
- Recent advancements in enhancement of photocatalytic activity using bismuth-based metal oxides Bi2MO6 (M=W, Mo, Cr) for environmental remediation and clean energy production[J] J. Ind. Eng. Chem.. 2021;95(1):1-15.
- [Google Scholar]
- Exploration and crystal phase engineering from bismuthinite ore to visible-light responsive photocatalyst of Bi2O3[J] J. Environ. Chem. Eng.. 2019;7(5):103375
- [Google Scholar]
- A review of ZnO nanoparticles as solar photocatalysts: Synthesis, mechanisms, and applications[J] Renew. Sustain. Energy Rev.. 2018;81(1):536-551.
- [Google Scholar]
- Benefits of TiO2 photocatalyst on mechanical properties and nitrogen oxide removal of ultra-high-performance concrete[J] Constr. Build. Mater.. 2021;285:122921
- [Google Scholar]
- Synthesis of g-C3N4-based photocatalysts with recyclable feature for efficient 2,4-dichlorophenol degradation and mechanisms. Appl. Catal. B Environ.. 2019;243:57-65.
- [Google Scholar]
- Photocatalytic activity evaluation of TiO2 nanoparticles based on COD analyses for water treatment applications: a standardization attempt[J] Int. J. Environ. Sci. Technol.. 2016;13(4):1077-1088.
- [Google Scholar]
- Preparation andphotocatalytic activity of BiOI/MnxZn1-xFe2O4 magneticphotocatalyst[J] Ceram. Int.. 2019;45(8):10468-10474.
- [Google Scholar]
- Rational design 2D/2D BiOCl/H+Ti2NbO7- heterojunctions for enhanced photocatalytic degradation activity. Appl. Surf. Sci.. 2020;521:146334
- [Google Scholar]
- He R, Cao S, Zhou P, et al. Recent advances in visiblelight bi-basedphotocatalysts [J].Chinese Journal of Catalysis, 2014, 35 (7): 989–1007.
- Microplastics remediation in aqueous systems: Strategies and technologies[J] Water Res.. 2021;198:117144
- [Google Scholar]
- Step-scheme NiO/BiOI heterojunction photocatalyst for rhodamine photodegradation. Appl. Surf. Sci.. 2020;511:145499
- [Google Scholar]
- Huogen Y, Ying H Yoo D, et al. Improved H2-generation performance of Pt/CdS photocatalyst by a dual-function TiO2 mediator for effective electron transfer and hole blocking[J]. Ceramics International, 2019, 285:122921.
- Solvothermal synthesis of Mn-Zn Ferrite(core)@SiO2(shell)/BiOBr 0.5Cl0.5 nanocomposites used for adsorptionand photocatalysis combination[J] Ceram. Int.. 2020;46(3):3655-3662.
- [Google Scholar]
- A series of bismuth-oxychloride/bismuth- oxyiodide/graphene-oxide nanocomposites: Synthesis, characterization, and photcatalytic activity and mechanism. Mol. Catal.. 2017;432:196-209.
- [Google Scholar]
- Vacancy-rich monolayer BiO2-x as a highly efficient UV, visible, and near-infrared responsive photocatalyst. Angew. Chem. Int. Ed.. 2018;57:491-495.
- [Google Scholar]
- Novel synthesis of PbBiO2Cl/BiOCl nanocomposite with enhanced visible-driven-light photocatalytic activity. Catal. Today. 2018;300:112-123.
- [Google Scholar]
- Graphitic C3N4 based noble-metal-free photocatalyst systems: A review[J] Appl. Catal. B Environ. Int. J. Dev. Catal. Sci. Appl.. 2017;206:556-588.
- [Google Scholar]
- Gold nanoparticles and g-C3N4- intercalated graphene oxide membrane for recyclable surface enhanced Raman scattering. Adv. Funct. Mater.. 2017;27:1701714.
- [Google Scholar]
- Excellent visible-light driven photocatalyst of (Al, Ni) co-doped ZnO structures for organic dye degradation - ScienceDirect[J] Catal. Today. 2020;340:277-285.
- [Google Scholar]
- Metolachlor photocatalytic degradation using TiO2 photocatalysts[J] Appl. Catal. B-Environ.. 2004;49:195-205.
- [Google Scholar]
- Facile one-pot synthesis of novel hierarchical Bi2O3/Bi2S3 nanoflower photocatalyst with intrinsic p-n junction for efficient photocatalytic removals of RhB and Cr (VI)[J] J. Hazard. Mater.. 2020;351:120942
- [Google Scholar]
- An all-in-one strategy for the adsorption of heavy metal ions and photodegradation of organic pollutants using steel slag-derived calcium silicate hydrate. J. Hazard. Mater.. 2020;382:121120
- [Google Scholar]
- Distribution and importance of microplastics in the marine environment: A review of the sources, fate, effects, and potential solutions[J] Environ. Int.. 2017;102:165-176.
- [Google Scholar]
- BiOxCly/BiOmBrn/BiOpIq/GO quaternary composites: Syntheses and application of visible-light-driven photocatalytic activities. J. Colloid Interface Sci.. 2019;544:25-36.
- [Google Scholar]
- A review on MnZ nferrites:synthesis, characterization and applications[J] Ceram. Int.. 2020;46(10):15740-15763.
- [Google Scholar]
- ZnO nanoparticles implanted in TiO2 microchannels as an effective direct Z-scheme heterojunction photocatalyst for degradation of RhB[J] Appl. Surf. Sci.. 2018;456:666-675.
- [Google Scholar]
- Hao Wei, Wang Jie, Xu Shengyuan, Gao Wensheng, Xie Kefeng.A review on the preparation and application of BiOCl photocatalyst [ J/ OL ]:1-17.
- Synthesis of hollow core-shell CdS@TiO2/Ni2P photocatalyst for enhancing hydrogen evolution and degradation of MB[J] Chem. Eng. J.. 2019;360:221-230.
- [Google Scholar]
- Xie T P, Li H, Liu C L, et al. Facile synthesis of magneticphotocatalyst Ag/BiVO4/Mn1-xZnxFe2O4 and its highly visible-light-driven photocatalytic activity[J].Materials, 2018, 11(5):810.
- Fabri-cation of Z-scheme BiVO4/GO/g-C3N4photocatalyst with efficientvisible-light photocatalytic performance. J. Inorg. Mater.. 2020;35(7):839-846.
- [Google Scholar]
- Review Bismuth oxychloride-based materials for the removal of organic pollutants in wastewater[J] Chemosphere. 2020;273:128576
- [Google Scholar]
- The{001}facets-dependent high photo-activity of BiOCl nanosheets [J] Chem. Commun.. 2011;47(24):6951-6953.
- [Google Scholar]
- Synthesis of carbon-doped KNbO3 photocatalyst with excellent performance for photocatalytic hydrogen production. Sol. Energy Mater. Sol. Cell.. 2018;179:45-56.
- [Google Scholar]
- TiO2 photocatalyst for removal of volatile organic compounds in gas phase–A review[J] Chem. Eng. J.. 2017;334(15):2408-2439.
- [Google Scholar]
- Oxidation of 2,4-dichlorophenol in saline water by unactivated peroxymonosulfate: mechanism, kinetics and implication for in situ chemical oxidation. Sci. Total Environ.. 2020;728:138826
- [Google Scholar]
- Dafeng Zhang, Xuhui Liu, Shoucheng Wang, Bingjie Fan, Zhuwang Shao, Changhua Su, Xipeng Pu, Enhanced charges separation to improve hydrogen production efficiency by organic piezoelectric film polarization, Journal of Alloys and Compounds, Volume 869, 2021, 159390, ISSN 0925-8388, https://doi.org/10.1016/j.jallcom. 2021.159390.
- Heterojunctions between amorphous and crystalline niobium oxide with enhanced photoactivity for selective aerobic oxidation of benzylamine to imine under visible light[J] J. Shandong Univ. Fin. Econ.. 2015;3(35):18045-18052.
- [Google Scholar]
- A novel SnIn4S8/ZnFe2O4 S-scheme heterojunction with excellent magnetic properties and photocatalytic degradation activity for tetracycline [J] Dalton Trans.. 2023;52(41):14956-14966.
- [CrossRef] [Google Scholar]
- 3D/2D ZnIn2S4/BiFeO3 as S-scheme heterojunction photocatalyst for boosted visible-light hydrogen evolution. J. Am. Ceram. Soc.. 2023;106:4785-4793.
- [CrossRef] [Google Scholar]
- Application informaldehyde purification in air of flower spherical Bi2S3/BiOI com-posite photocatalyst.Chinese. J. Inorg. Chem.. 2021;37(3):437-442.
- [Google Scholar]







