Translate this page into:
Synthesis of CoMnFe2O4 hollow microstructure decorated GO for photocatalytic degradation of organic dyes
⁎Corresponding author. omar.s@coeng.uobaghdad.edu.iq (Omar S. Dahham)
-
Received: ,
Accepted: ,
This article was originally published by Elsevier and was migrated to Scientific Scholar after the change of Publisher.
Abstract
Over the past decade, safeguarding marine life and aquatic ecosystems against deleterious dye pollutants has emerged as a paramount concern. Methylene blue dye stands out as one such pollutant capable of inflicting irreversible damage to marine ecosystems even at minute concentrations. Addressing this pressing issue, we synthesized a novel CoMnFe2O4/graphene oxide nanocomposite employing a microwave-ultrasonic method. This composite, comprising soft superparamagnetic CoMnFe2O4 hollow microstructures integrated onto graphene oxide surfaces, revealed a mesoporous structure with a notably high surface area, which was about 96.4654 m2.g−1. Various analytical techniques were employed to scrutinize the crystal structure, functional groups, surface chemical composition, and morphologies of the synthesized CoMnFe2O4/graphene oxide nanocomposite (X-ray diffraction, Fourier-Transform Infrared Spectroscopy, energy-dispersive X-ray spectroscopy, field emission scanning electron microscopy, and high-resolution transmission electron microscopy). The CoMnFe2O4 crystal phase appears to be cubic in the X-ray diffraction with a 28.91 nm Avg. crystallite size. The measured band gap energies for the CoMnFe2O4, graphene oxide, and CoMnFe2O4/graphene oxide nanocomposite are 2.23 eV, 2.90 eV, and 1.89 eV, respectively. Remarkably, under visible light irradiation, the nanocomposite exhibited an impressive degradation efficiency of 97.54 % within just fifty minutes (at pH = 7, Methylene blue conc. = 15 mg/L, and catalyst dose = 0.05 g.), attributed to a photo degradation rate constant (k value) reaching 0.07330 min−1. Notably, this efficiency nearly doubled with the introduction of H2O2 peroxide. The outstanding recyclability of the CoMnFe2O4/graphene oxide nanocomposite, sustaining optimal performance over four cycles without significant degradation, underscores its potential for long-term environmental remediation efforts. Moreover, its magnetic extractability from contaminated solutions enhances its suitability for advanced environmental applications.
Keywords
Photocatalysis
Hollow ferrite
Photocatalytic efficiency
Organic dyes
1 Introduction
Pollutants derived from industries such as textiles, particularly those associated with dyes, pose significant environmental risks owing to their enduring and harmful characteristics. These substances have the potential to taint water reservoirs, leading to adverse effects on ecosystems and human well-being (Weldekirstos et al., 2024; Katubi et al., 2024; Othman et al., 2023; Kir et al., 2023). Their carcinogenic and hazardous properties make them detrimental to both human and marine organisms, even at minimal concentrations. The presence of toxic dye molecules can result in various health issues, including allergic dermatitis, skin allergies, and dysfunction of vital organs such as the sex organs, kidneys, brain, and liver (Chand et al., 2023; Ren et al., 2018; Haounati et al., 2021). Currently, numerous researchers are dedicated to mitigating these risks by designing catalysts to reduce these pollutants (Abdallah et al., 2020; Othman et al., 2021; Xu et al., 2020; Bagi et al., 2024; Amor et al., 2023; Mohammed et al., 2023). Advanced oxidation technologies (AOTs) have gained importance in removing dye molecules from water (Kalaycıoğlu et al., 2023). Among these technologies is semiconductor-based photocatalysis, which has consistently emerged as an efficient, cost-effective, reusable, capable of fully degrading dyes even at low concentrations, and environmentally friendly process (Borthakur and Saikia, 2019; Sambaza et al., 2019; Bhuyan and Ahmaruzzaman, 2024; Samarasinghe et al., 2024). The photodegradation mechanism of dyes relies on the generation of electrons (e−) and holes (h+) in semiconductor photocatalysts under appropriate light irradiation. Effectively preventing the recombination of photogenerated e−/h+ pairs is essential to maximize the availability of charge carriers for photocatalytic reactions, ensuring superior photodegradation performance in semiconductors (Cai et al., 2020; Yan et al., 2020). Numerous semiconductor-based photocatalysts have undergone studies; nevertheless, they encounter significant challenges, such as issues with charge separation and transfer, elevated bandgap energy, agglomerations, and the difficulty of separating the catalyst from the aqueous solution post the completion of the photochemical reaction (Li et al., 2022; Ansari and Cho, 2016; Visan et al., 2019). In response to the challenges faced, numerous researchers have undertaken the design of novel materials to overcome these obstacles. Photocatalysis using visible light is a sustainable technology that has gained immense popularity among researchers and industries due to its wide range of applications (Mishra et al., 2024; Mishra et al., 2023; Sahoo and Acharya, 2021; Hasan et al., 2023; Salmi et al., 2024). By utilizing sunlight to trigger chemical reactions, photocatalysis offers an eco-friendly solution to dealing with toxic organic pollution caused by industrial processes. (Muzammil et al., 2023; Lin et al., 2024; Zidane et al., 2022). Among these materials, spinel ferrites (MFe2O4; where M: Cu, Ni, Mg, Mn, Co, Zn, etc.) have garnered significant attention due to their distinctive properties, including a narrow band gap energy (∼1–2.5 eV), utilization of visible light, high electromagnetism performance, cost-effectiveness, ease of preparation, elevated chemical stability, notable adsorption properties, and non-toxicity (Mishra et al., 2023; Mohamed et al., 2023; Harikrishnan and Rajaram, 2024; Meneceur et al., 2024). The ferrite crystal lattice incorporates one or more bivalent oxides with Fe+3 at the tetrahedral and octahedral sites. respectively. Recent studies have focused on the substitution of elements in ferrite to enhance photocatalytic properties, including improvements in particle size, large surface area, thermal magnetic properties, etc. (Singh et al., 2023; Helmy et al., 2023; Keshu and Rani, 2023; Aldhalmi et al., 2023). Despite the many advantages of spinel ferrite, there is a major drawback that adversely affects its photocatalytic efficiency, which is the rapid recombination of electron-hole pairs under visible light radiation (Ying et al., 2020; Das et al., 2021). Furthermore, the agglomeration that occurs due to the preparation method of spinel ferrite reduces the surface area and results in a decline in its degradation performance.
Semiconductor hollow structures have garnered significant interest from researchers due to their distinct morphology and multifaceted characteristics (Cadenbach et al., 2023; Cao et al., 2018; Yin et al., 2022). The void enclosed within a thin shell of these structures offers a broad range of opportunities for applications across various domains (Chen et al., 2024; Sakti et al., 2021; Liu et al., 2019). The morphology, surface chemistry, precisely controlled size, and localized voids of these hollow structures provide them with unique features that are well-suited for applications such as catalysis, drug delivery, energy storage, adsorption, and separation. In particular, hollow structures have proven to be highly effective in the field of photocatalysis by offering efficient light absorption and faster reaction kinetics. Additionally, the increased surface area and optimized crystal facets of hollow structures provide a higher density of active sites for catalytic reactions, which facilitates a more efficient overall process. The photocatalytic reaction of nanomaterials is mainly influenced by their band gap. In comparison to single nanomaterials, nanocomposites have a higher response rate for removing contaminants (Mahdi et al., 2023). Presently, many scientists are investigating the potential of carbon-based materials doped with spinel ferrite for enhancing the photocatalytic activity involved in removing pollutants from wastewater (Acharya et al., 2022; Mishra and Acharya, 2023), H. Subramanian et al. have studied the synthesis of CuFe2O4/GO (Subramanian et al., 2023), while S. Fakhri-Mirzanagh et al. have undergone the preparation of ZnCdS/MnFe2O4/GO (Fakhri-Mirzanagh et al., 2024), both studies have shown excellent photocatalytic efficiency in removing dye pollutants from wastewater.
The use of ferrite hollow microstructures, particularly in conjunction with GO, to enhance photocatalytic efficiency represents a novel approach. There are only limited reports available on this specific application, highlighting the uniqueness of this work. The present study deals with the synthesis of a novel photocatalyst that can effectively overcome the challenges encountered in the elimination of contaminants. The proposed photocatalyst is designed using a ferrite hollow microstructure by substituting Co2+ on the MnFe2O4, leading to the development of CoMnFe2O4 hollow microstructures (CMFO) that exhibit a remarkable ferromagnetic performance. However, Adding Mn2+ ions to CoFe2O4 can significantly improve the material's structure and magnetic properties, as studies have shown (Phumying et al., 2014). By combining Fe, Co, and Mn ions, we can customize their characteristics for specific applications, particularly in optimizing their performance in photocatalyst applications. This is crucial in the study of CMFO as a photocatalyst, and there are few articles available that explore its potential (Arba and Utari, 2020; Srinivas, 2021). The CMFO hollow microstructures are then mixed with graphene oxide nanosheets (GO). Although there have been several studies conducted on CoFe2O4 and MnFe2O4 ferrites, none have been focused on CMFO hollow microstructures with photocatalytic applications. GO is a derivative of graphene that exhibits unique properties owing to its oxygen-containing functional groups. These features include exceptional mechanical strength, high thermal and electrical conductivity, and a large surface area. Incorporating GO into photocatalyst materials is driven by its role as a support matrix, favourable electron transfer properties, and its ability to enhance light absorption and separation of charge carriers. These advantages collectively enhance the efficiency of the photocatalytic process. The novel magnetic CMFO/GO photocatalyst exhibits impressive efficiency in degrading MB molecules under visible light irradiation. This is due to the presence of two-dimensional GO nanosheets in the photocatalyst. The addition of hydrogen peroxide further enhances the Fenton reaction, and significantly improves the photocatalytic performance. We synthesized our hollow structure using simple adsorption–calcination method with colloidal carbon spheres as templates. To combine CMFO with GO, we employed the microwave-ultrasonic method, which creates rapid and intimate reactions in nanoscale and colloidal systems. This method produces magnificent agitation and transient local heating, which is supported by the microwave energy input. Microwave heating is highly efficient because it directly heats the reaction mixture, minimizing energy loss compared to traditional methods. This efficiency supports the sustainability of the synthesis process.
2 Experimental and methods part
2.1 Chemicals
All chemicals used in this study were of high analytical purity (99 % purity) and were employed without further purification. Milli-Q water, with a resistivity greater than 18.2 MΩ cm, was utilized in the synthesis of the photocatalyst, the resistivity greater than 18.2 MΩ cm indicates its extremely low conductivity, making it suitable for various applications where any impurities could interfere, such as in the synthesis of photocatalysts. Graphite powder, sodium nitrate (NaNO3) and sulfuric acid (H2SO4) were supplied by Sigma-Aldrich. Hydrogen peroxide (H2O2) and potassium permanganate (KMnO4) were supplied by Fisher Scientific. Hydrochloric acid (37 wt%) and aqueous ammonia (25 wt%) were supplied by Merck. Ferric chloride hexahydrate FeCl3·6H2O, cobalt chloride hexahydrate CoCl2·6H2O, and manganese chloride tetrahydrate MnCl2·4H2O were supplied by Glentham Life Sciences Ltd., England. Glucose and Polyvinylpyrrolidone (PVP) were supplied by Shanghai Macklin Biochemical Technology Co., Ltd.
2.2 Synthesis of graphene oxide (GO)
Graphene oxide (GO) was synthesized through a modified Hummers' method (Hegazy et al., 2024) by oxidizing natural graphite powder. The process involved the dispersion of 5 g of graphite in 230 mL of 98 % sulfuric acid (H2SO4) in a glass beaker placed in an ice bath. The mixture was then stirred continuously with the progressive addition of 5 g of sodium nitrate (NaNO3) and 30 g of potassium permanganate (KMnO4). Following a two-hour incubation period, the temperature was raised to 98 °C, and 300 mL of deionized water were added. The mixture was then cooled to room temperature and treated with 175 mL hydrogen peroxide (35 % H2O2). The resulting product was separated through centrifugation, washed multiple times with 5 % hydrochloric acid (HCl) solution and deionized water, and finally dried in a vacuum oven at 50 °C for 24 hr.
2.3 Synthesis of CMFO hollow microstructures
The magnetic material CMFO hollow microstructures were synthesized by the adsorption–calcination method (Zhou et al., 2020). Initially, carbon spheres (CS) were employed as a template for the synthesis of CMFO. To prepare the carbon spheres, 8 g of anhydrous glucose was dissolved in 60 mL of deionized water, followed by the addition of 0.04 g of surfactant (polyvinylpyrrolidone (PVP)), by incorporating PVP, we aimed to enhance the stability of the dispersion and prevent aggregation of glucose. The solution was continuously stirred for 10 min until the surfactant was completely dissolved. The resulting solution was then sealed in a 100 mL Teflon-lined autoclave and maintained at 180 °C at a rate of 5 °C\min for 10 hr. After completion of the reaction, the autoclave was cooled down to room temperature. The resultant products were centrifuged, washed, and redispersed in water, and this cycle was repeated five times. Subsequently, the products were centrifuged, washed, and redispersed in ethanol, and this cycle was again repeated five times. The obtained carbon spheres were then subjected to oven-drying at 50 °C for 24 hr. After the synthesis of the CS template, the CMFO precursors (FeCl3·6H2O (5 mmol), CoCl2·6H2O (1.25 mmol), and MnCl2·4H2O (1.25 mmol)) were added to 20 mL of deionized water and mixed with 5g of colloidal CS solution. The solution was then magnetically stirred for 24 hr to allow for efficient adsorption of metal ions on the surface of CS. The mixture was centrifuged at 4000 rpm for 15 min, and the resulting colloidal CS solution was dried in a vacuum oven at 50℃ for 24 hr. The dried product was then calcined in a muffle furnace at 700℃ for 5 hr to remove the CS templates and formation of CMFO crystal structure (heating rate, 5℃ /min). The end product was hollow microstructures of CMFO.
2.4 Synthesis of CMFO/GO NCs
CMFO/GO NCs photocatalyst was successfully prepared using a microwave-ultrasonic procedure (Waheed et al., 2022), as shown in Fig. 1. To prepare this photocatalyst, 0.5 g of CMFO hollow microstructures were dispersed in 50 mL of deionized water. Then, they were added to another dispersion of 0.3 g GO and 50 mL of deionized water. The product mixture was sonicated for 2 hr and then microwaved for 10 min. At the end of the process, the CMFO/GO photocatalyst was separated using an external magnetic field, washed several times with DI water and ethanol, and dried for 6 hr at 60 °C.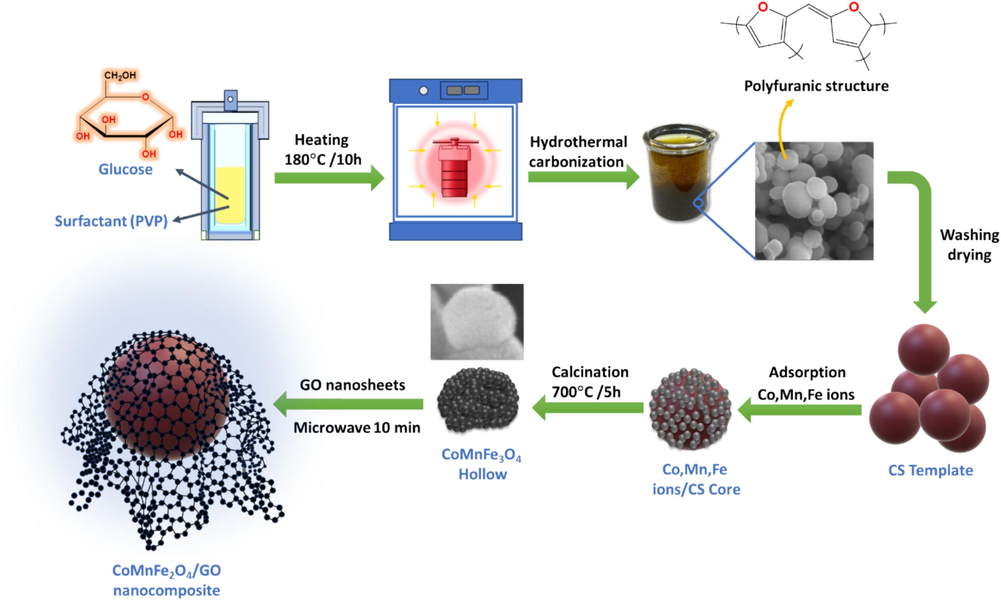
A schematic diagram depicting the synthesis of CMFO/GO NCs.
2.5 Characterization techniques
The synthesized CMFO hollow microstructures, GO nanosheets and novel CMFO/GO photocatalyst were thoroughly characterized using various techniques. Fourier-Transform Infrared Spectroscopy (FT-IR) (Shimadzu FT-IR 8400 spectrophotometer using KBr pellets) elucidated the chemical composition and bonding characteristics, while Raman spectroscopy (WItec Alpha 300 Raman spectrometer) offered valuable information on the structural integrity of GO and CMFO/GO nanocomposite. The study of photocatalysts' crystalline structures was carried out using a powder X-ray diffraction device (Shimadzu XRD-6000), which employed nickel filtered Cu-Kα radiation (λ = 1.54060 Å). The average size (D) of the crystals in the synthesized samples was estimated using Debye-Scherrer's equation, based on the full-width half maximum (FWHM) of the most intense peak, as shown in Eq. (1) (Ye et al., 2019).
2.6 Study of photocatalytic activity
For our study, methylene blue dye (MB) was used as a model to assess the photocatalytic performance of our prepared photocatalyst samples under visible light. To conduct the experiment, we dispersed 0.05 g of photocatalyst in a 50 mL solution of 15 mg/L dye in a 250 mL beaker. The solution was then left in the dark for an hour to allow for adsorption and desorption equilibrium between the MB and photocatalyst. Then placed the beaker and its contents under a halogen lamp (300 W, ∼891,082 lx) for 50 min. During irradiation, around 5 mL of the suspension was withdrawn at five-minute intervals using a syringe and transferred to a vial. After removing any floating residues using an external magnetic field and a centrifuge (4500 rpm), this protocol is similar to a previously published work (Waheed et al., 2022). The photocatalyst efficiency of our samples was investigated using a UV–visible spectroscope with a maximum wavelength of 664 nm. The dye degradation efficiency was calculated over time using Eq. (3).
3 Results and discussion
3.1 Structural characterization
The crystalline structures of pure CMFO hollow microstructure, GO nanosheets, and CMFO/GO NCs were examined via XRD-powder as shown in Fig. 2a. The XRD analysis of the pristine CMFO hollow microstructure revealed distinct diffraction peaks positioned at 18.3°, 30.4°, 35.7°, 37.3°, 43.2°, 49.8°, 54.4°, 57.0°, 62.6°, 72.3°, and 73.9°, these peaks are associated with the crystal planes (1 1 1), (2 2 0), (3 1 1), (2 2 2), (4 0 0), (3 3 1), (4 2 2), (5 1 1), (4 4 0), (6 2 0), and (5 3 3) of the cubic spinel structure (space group Fm3m) respectively, which is in a good agreement with a previously published paper and with the standard data (JCPDS: 22–1086 and JCPDS: 74–2403) (Ansari et al., 2020). However, a weak intensity peak was found at 2θ = 33.4 corresponding to a hematite phase, Fe2O3 (JCPDS card no. 33–0664), and there is no presence of any other phases at all. When observing the XRD pattern of GO nanosheets (JCPDS No. 41–1487), two distinct peaks become apparent. One of these peaks is a sharp and strong peak at 2θ = 11.3° ((0 0 1), d-spacing = 0.776 nm), which indicates the presence of its ordered layered structure and introduction of oxygen-containing functional groups (C–O, C–OOH, and C–OH) in the structure of GO, resulting in a higher d-value of GO compared to original graphite (Zhu et al., 2020). The second peak displays a broad and low-intensity peak centered at 2θ = 26.2° ((0 0 2) d-spacing = 0.339 nm) which may be due to incomplete oxidation (Anjum et al., 2023). In XRD pattern of CMFO/GO NCs, the disappearance of the characteristic diffraction peak at 2θ = 11.3° signifies the full exfoliation of the organized layered arrangement of GO by CMFO hollow microstructure. Furthermore, the broader and reduced intensity peak of interplane (0 0 2), confirms the less stress in the crystal structure of GO and more exfoliation. These hollow microstructures are affixed to the surface of GO. Additionally, the same diffraction crystallographic planes of CMFO hollow microstructures were observed in CMFO/GO nanocomposite, with a reduction in the degree of crystallinity. This change could potentially be ascribed to the presence of amorphous structures within the CMFO/GO nanocomposite. Despite this, the degree of crystallinity of CMFO/GO remained relatively elevated (as shown in Table 1). This elevated crystallinity is of great significance as it plays a crucial role in enhancing the efficiency of the photocatalyst (Waheed et al., 2023). The average crystallite size (D) of the synthesized photocatalysts was determined using Debye-Scherrer's equation. For the CMFO photocatalyst, the crystallite size was computed as 29 nm, while for the CMFO/GO nanohybrid, it measured 39 nm. The incorporation of CMFO hollow microstructure onto GO nanosheets through the microwave-assisted method led to a notable change. Specifically, due to this decoration, the d-spacing expanded from 2.51183 to 2.51802 Angstrom (for the (3 1 1) plane), which in turn caused an enlargement in the lattice volume. As a result of this alteration, the crystallite size experienced an increment. As shown in Table 1, we see that the average size of the CMFO hollow microstructures is 28.91 nm, which is relatively small. However, when we look at the average diameter calculated by TEM images, we get a much larger size, ranging from 2–5 µm. This discrepancy can be explained by the fact that XRD analysis typically provides information about the crystallite size, which represents the size of the ordered domains within the crystal lattice. However, when it comes to hollow structures, there can be a distinction between the crystallite size and the overall size of the microstructure. If the XRD analysis interprets the shell of the hollow microstructure as the crystallite, it could lead to a smaller crystallite size being reported. The hollow interior may not contribute significantly to the XRD signal, especially if it lacks ordered structure or is less dense compared to the shell. In such cases, it's important to consider the interpretation of the XRD results and acknowledge that the reported crystallite size may pertain specifically to the shell of the hollow microstructure. The overall size of the microstructure, which includes both the shell and the hollow interior, could be larger and may be determined by other characterization techniques or imaging methods which in our case are TEM and FE-SEM.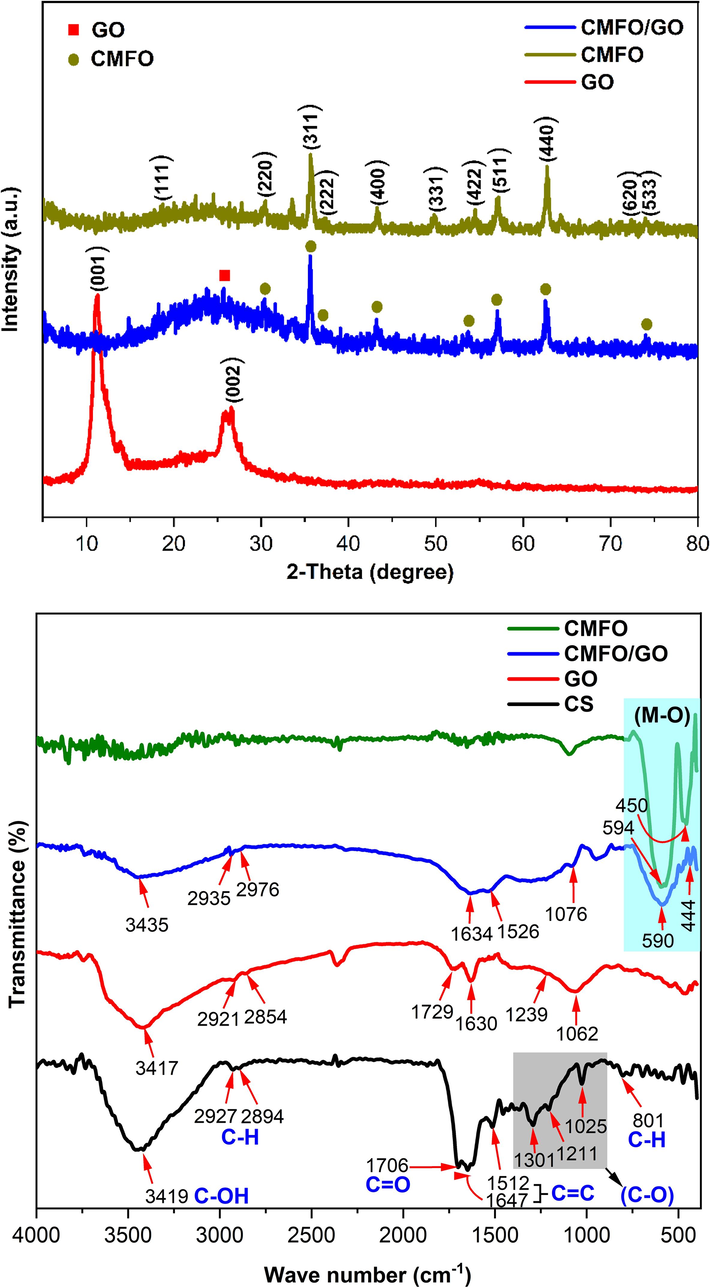
(a) XRD patterns for GO nanosheets, pure CMFO, and CMFO/GO NCs, and (b) FTIR for the same compounds in addition to CS.
Parameters
GO
CMFO
CMFO/GO
Avg. diameter (DSEM) nm
–
258.80
–
Diameter (DTEM) nm
–
75–350
75–350*
Avg. crystallite size (DXRD) nm
4.1
28.91
39.59
Crystallinity (%)
35.83
64.94
52.73
Specific surface area (SBET) m2.g−1
91.8
6.5063
96.4654
Pore volume (Vp) cm3.g−1
0.015339
0.033964
0.100602
Pore diameter (Dp) nm
7.5704
5.4305
4.6930
Saturation magnetization (MS) emu. g−1
–
24.11
17.32
Remanence (MR) emu. g−1
–
7.26
4.36
Coercive field (HC) kOe
–
0.227
0.131
Bandgap energy (eV)
2.90
2.23
1.89
Fig. 2b demonstrates the results of FT-IR spectra of carbon spheres (CS), pure CMFO hollow microstructure calcinated at 700 °C for 5 h, GO nanosheets, and CMFO/GO heterostructure nanocomposite. Prior to the formation of CNFO ferrite, the FT-IR of the CS surface exhibited several bands, as follows, 3419 cm−1 corresponding to O–H stretching, 2927 and 2894 cm−1 representing asymmetric and symmetric C–H aliphatic alkyl stretching, 1706 cm−1 indicating C=O stretching, 1647 and 1512 cm−1 associated with the furan skeleton C=C, 1301,1211, and 1025 cm−1 related to C–O stretching vibrations arising from hydroxyl, carboxyl, and furan rings. Additionally, the band at 801 cm−1 was attributed to C–H in-plane bending vibrations (Zhou et al., 2020). In the spectrum of pure CMFO hollow microstructure, most of the bands of CS disappeared after calcination, with a reduction in intensity for the other bands, on the other hand, two new stretching frequencies formed at 594 and 446 cm−1, which are related to the metal–oxygen (Mn, Co and Fe) bonds at A (tetrahedral) and B (octahedral) sites in spinel structure, respectively, indicating the structure of CMFO, these results are similar to what R. Jabbar et al. has studied previously (Jabbar et al., 2020). GO nanosheets spectrum exhibited characteristic peaks, at 3417, (2921, 2854), 1729, 1630, 1239, and 1062 cm−1 which are attributed to bands C-OH stretching, asymmetric and symmetric C–H stretching, C=O stretching, C=C stretching, C–H bending, and C-O stretching respectively (Pooresmaeil et al., 2021). The characteristic peaks of GO nanosheets and CMFO hollow ferrite remain in the spectrum of the new CMFO/GO nanocomposite, with an obvious reduction in bands intensity as well as a slight shift in the peaks, this indicates the occurrence of an interaction between the starting materials.
Raman spectroscopy is one of the most important techniques to examine the structure changes in carbon networks (Liang et al., 2015). Consequently, this technique was employed to characterize the prepared GO nanosheets and CMFO/GO nanocomposite, as presented in Fig. 3. The Raman spectrum of GO exhibits two prominent peaks, one at 1359 cm−1 (D band), signifying breathing mode of A1g κ point phonons of disordered sp3 carbon framework of graphite, and the other at 1600 cm−1 (G band), indicative of the tangential second-order stretching of the E2g vibrational mode of in-plane sp2 carbon atoms and represents the degree of graphite formation (Al-Janabi et al., 2023). In addition to the G and D bands, two weak peaks at ∼2600 cm−1 (2D band) and ∼3000 cm−1 (G+D band) were observed in the GO Raman spectrum. In the case of GO decorated by CMFO NPs Raman spectrum, peaks positions experienced a shift, the D band peak presented a red shift (1359 to 1349 cm−1), while the G band exhibited a blue shift (1600 to 1605 cm−1). These shifts arise due to charge transfer from GO towards CMFO, indicating a strengthened electron–phonon coupling (Majumder et al., 2018). Additionally, in the Raman spectrum of the CMFO/GO nanocomposite, a few additional bands in the spectral range of 207–749 cm−1 are also observed (T2g(1) (207 cm−1), Eg (329 cm−1), T2g(2) (471 cm−1), T2g(3) (579 cm−1), A1g(2) (632 cm−1), and A1g(1) (749 cm−1)). These bands are characteristic of the spinel type CMFO structure which is in good agreement with a previously published work (Doumeng et al., 2021; Shi et al., 2023). Moreover, the ID/IG intensity ratio, which is indicative of disorder and defects in graphene sheets, exhibited a slight increase from 0.97 (in GO nanosheets) to 1.03 (in CMFO/GO nanocomposites). This shift suggests a greater reduction of GO into rGO and an increase in sheet defects due to the doping effect of CMFO NPs (Zhao et al., 2021).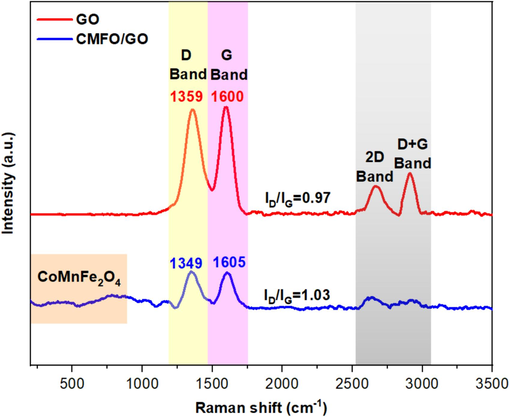
Raman spectroscopy for GO nanosheets and CMFO/GO NCs.
3.2 Morphology studies
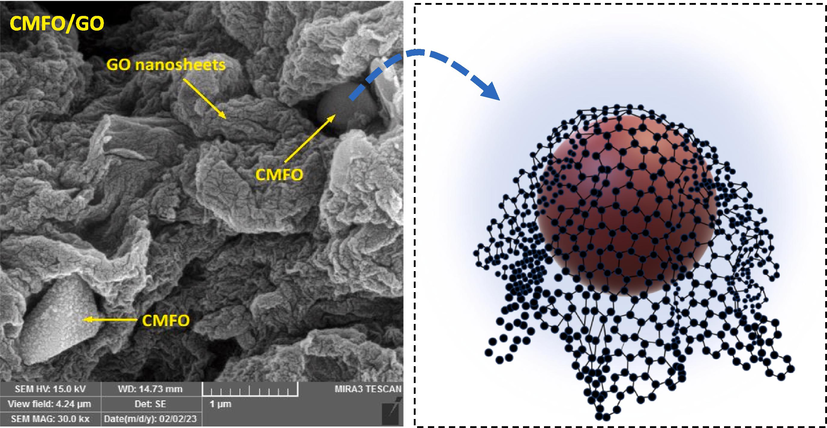
We used various imaging techniques like FE-SEM, EDX analysis, TEM, HR-TEM, and SAED to characterize the surface morphology and constituent elements of CS, pure CMFO hollow microstructure, GO nanosheets, and CMFO/GO nanocomposite. Fig. 4 displays the FE-SEM image of the synthesized micro-spherical carbon with an average diameter of 716.97 nm. The surface of these microspheres became distorted after ion adsorption (Mn, Co, and Fe) and the calcination process due to the release of organic compounds, the volume of the particle shrunk, making the structure more compact. As a result, CMFO hollow microstructure, with a mean diameter of 258.80 nm, adopted hollow polyhedron and spherical shapes. The morphological nature of GO nanosheets, appearing as nanosheets. When hollow CMFO and GO nanosheets are combined, the surface exhibits two types of anchoring. One type involves CMFO agglomerations on the surface of GO, while the other type features CMFO capped by GO nanosheets. This anchoring enhances light absorption and facilitates the separation of the catalyst from the solution using an external magnetic field (Zhao et al., 2023). Fig. 5 showcases the FE-SEM mapping diagrams and the combined composition diagrams with EDX analysis, for each of the starting materials, CMFO hollow microstructure and GO nanosheets. The resulting CMFO/GO NCs was also analyzed. The CS mapping diagram illustrated a uniform distribution of oxygen on the carbon surface, which allowed for the uniform and easy adsorption of metal ions on the surface of CS. The weight percentage of elements appeared as expected for CMFO hollow microstructure (Co0.5Mn0.5Fe2O4), with a small amount of carbon due to traces of the used CS. In GO, the atomic percentage of oxygen reached around 26.66 %, reflecting the introduction of various oxygen functionalities. The CMFO/GO NCs was analyzed and found to contain C, O, Co, Mn, and Fe elements with uniform dispersion, confirming the successful grafting of CMFO hollow microstructure onto the GO surface.
FE-SEM images depicting CS, and the pristine hollow microstructures of CMFO with their histograms. Additionally, images of GO nanosheets. In addition to two distinct CMFO/GO images, with the second image providing an illustrative representation.
FE-SEM images depicting CS, and the pristine hollow microstructures of CMFO with their histograms. Additionally, images of GO nanosheets. In addition to two distinct CMFO/GO images, with the second image providing an illustrative representation.
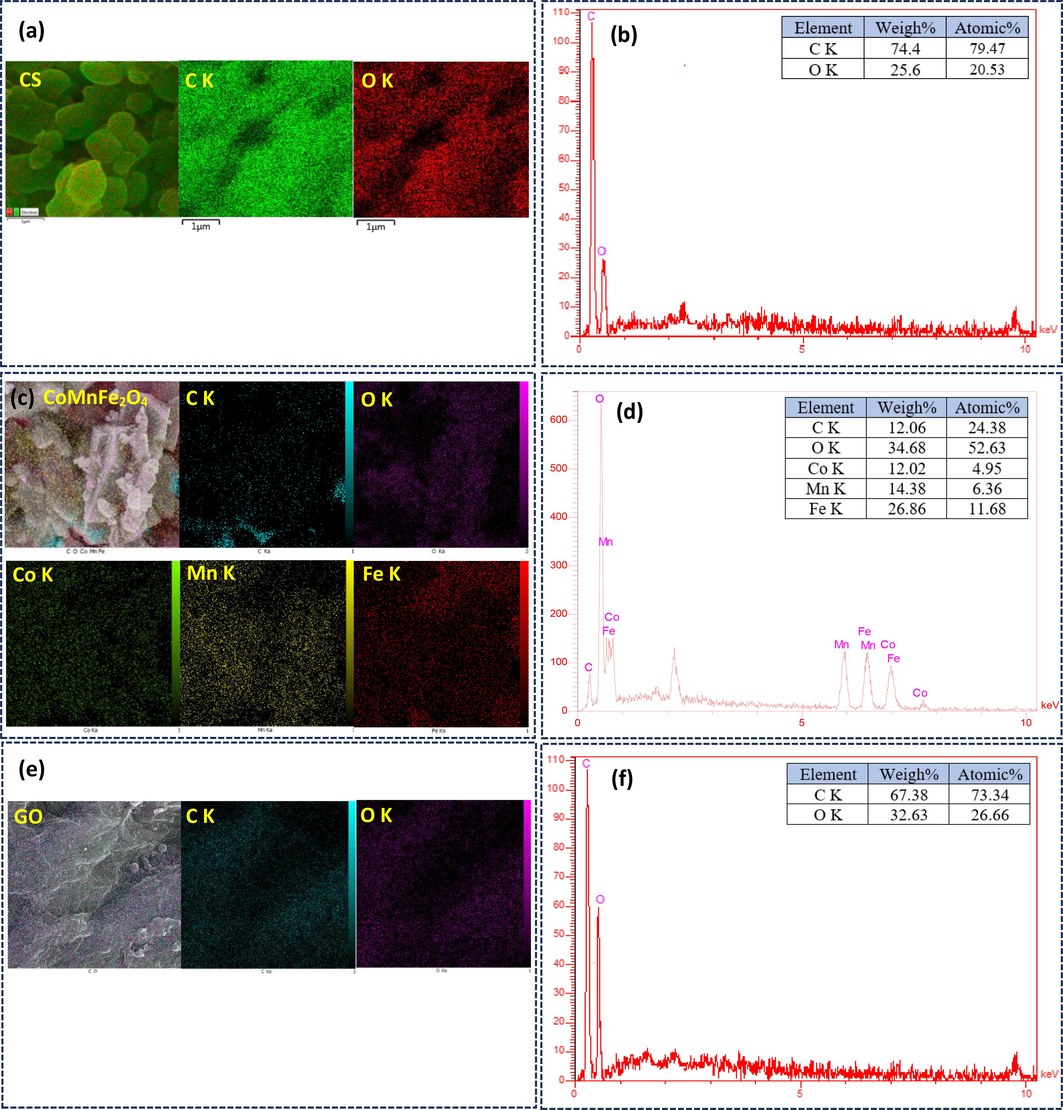
Elemental mapping micrographs for (a, c, e, and g) CS, pure CMFO hollow microstructures, GO nanosheets, and CMFO/GO NCs, along with their EDX spectra (b, d, f, and h).
Elemental mapping micrographs for (a, c, e, and g) CS, pure CMFO hollow microstructures, GO nanosheets, and CMFO/GO NCs, along with their EDX spectra (b, d, f, and h).
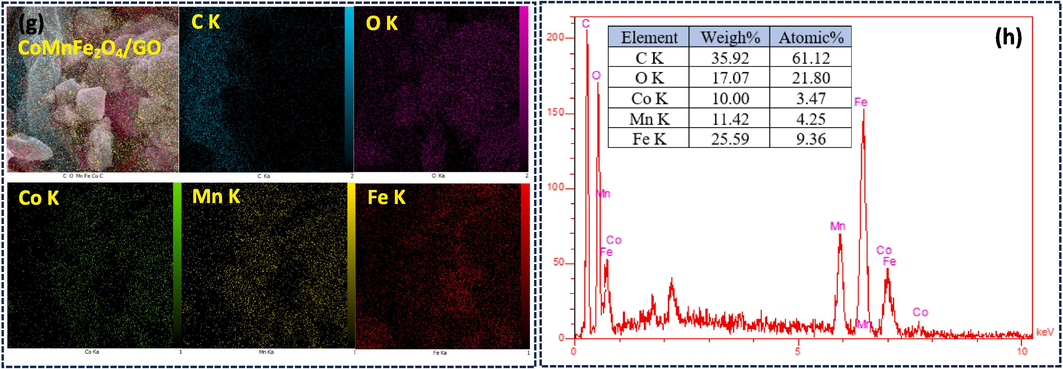
The morphology and microstructure of various samples were examined via transmission electron microscopy (TEM) images, as presented in Fig. 6. The calcination and decomposition process for CS-metal ions was observed to produce CO2 gases inside the spherical shell, resulting in the formation of larger cavities or pores. As a result, the CMFO grains on the surface collapsed without proper connection, leading to three forms of CMFO observed in Fig. 6a. These included complete hollow polyhedrons, hollow semi-spheres with small particle size, and partially cross-linked hollow shapes with large pores on the surface. In Fig. 6b, GO exhibited natural 2D sheets with wrinkled surfaces, while Fig. 6c showed the TEM photograph of the CMFO/GO photocatalyst. It is noteworthy that CMFO microstructures with various shapes have anchored on GO nanosheets, and this non-complete sheet coating is significant as it enhances active site access on the surface, resulting in better photocatalytic efficiency. The morphology properties mentioned above underpin the incorporation of CMFO microstructures upon GO nanosheet, leading to the development of a photocatalyst that may overcome some of the issues associated with other photocatalysts.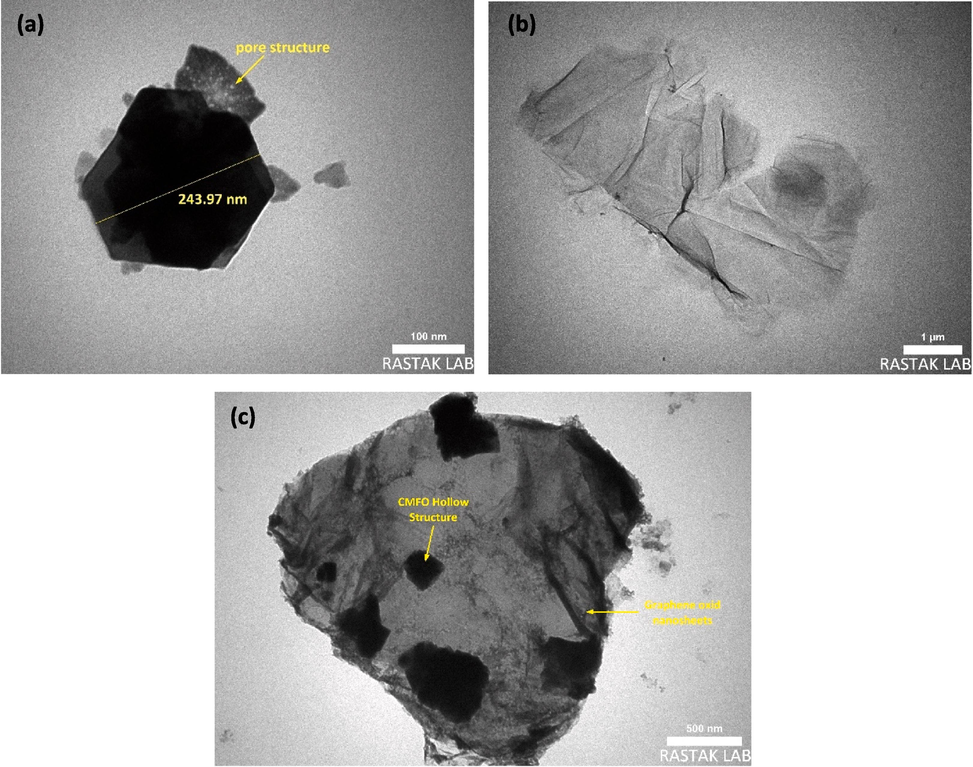
TEM micrographs for (a) pure CMFO hollow microstructures, (b) GO nanosheets, and (c) CMFO/GO NCs.
3.3 Specific surface area and BET analysis
The BET method was employed to investigate the specific surface area (SBET), while the pore volume and pore size distribution were determined using the BJH methods applied to the N2 gas isotherm at 77 K, as illustrated in Fig. 7 and summarized in Table 1. Fig. 7a-c shows a hysteresis loop plot of the isotherm processes of all prepared samples. According to the International Union of Pure and Applied Chemistry (IUPAC) classification (Khan et al., 2020), the graphs are classified as type IV; H3 adsorption isotherm, and the observed hysteresis loops provide evidence of the existence of mesopores (Wang et al., 2022). Calculated surface area (SBET) for a CMFO hollow structure, GO nanosheet, and CMFO/GO NCs were 6.50, 91.8, and 96.46 m2.g−1, respectively. Additionally, the pore dimeters distribution of these compounds were 7.57, 5.43, and 4.69 nm, respectively (as depicted in Fig. 7d-f). The pore size distribution of CMFO was higher than other compounds due to the decomposition of organic compounds that are present in the CoMnFe-carbon precursor during the calcination process. The increase in the surface area and identified porosity in CMFO/GO photocatalyst, resulting from the addition of CMFO onto the GO surface, is noteworthy. This enhancement, evidenced by the significantly greater surface area compared to CMFO's hollow microstructure and GO's nanosheets, is attributed to the presence of defects such as increased voids and cracking. These structural modifications are deemed beneficial for photocatalytic efficiency as they augment reactive sites and facilitate reactant diffusion. However, it's important to note that the relationship between surface area and adsorption volume is complex and influenced by various factors including porosity and pore structure. While the GO's nanosheets may exhibit a higher volume of nitrogen adsorbed, indicating greater gas adsorption per gram of material, it doesn't necessarily correlate with a higher surface area. Surface area measurements encompass both external and internal surface areas within pores, suggesting that the CMFO/GO NCs might have a higher overall surface area despite lower nitrogen adsorption volume. This underscores the importance of considering both external and internal surfaces in assessing the adsorption characteristics of materials. Detailed pore volume values have been inserted in Table 1.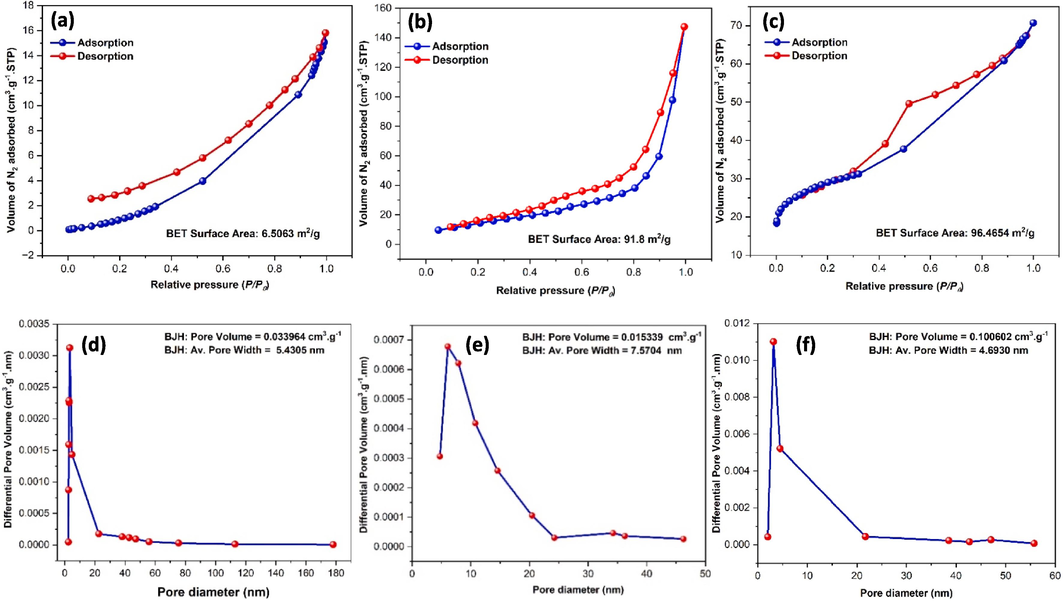
N2 adsorption–desorption isotherm profiles (a-c), and corresponding pore diameters (d-f), of pristine CMFO hollow microstructures, GO nanosheets, and the novel CMFO/GO photocatalyst, respectively.
3.4 Magnetic properties
CMFO hollow microstructures were added to GO nanosheets to enhance the speed of the catalyst separation from the treated water post-reaction. This also helps in the easy recovery of the photocatalyst particles under an external magnetic field. The magnetic properties of the photocatalysts we prepared were evaluated using a vibrating sample magnetometer (VSM) at room temperature. In Fig. 8 we can see the magnetic hysteresis curves of the hollow CMFO and CMFO/GO nanocomposite. The saturation magnetization (Ms) values for CMFO hollow microstructures and CMFO/GO NCs were 24.11 and 17.32 emu.g−1, respectively, as shown in Table 1. The decrease in Ms values for the CMFO/GO NCs is likely due to the diamagnetic shielding effects of GO nanosheets, and the possible presence of a magnetically dead or antiferromagnetic layer on the GO surface. However, this specific magnetic property of the CMFO/GO NCs is still suitable for isolating the photocatalyst from the solution by using an external magnetic field. The coercivity (HC) of magnetic nanoparticles can be determined by identifying the intersection point of the magnetization curve and X-axis. Values of Hc were 0.227 kOe for CMFO hollow microstructures which is similar to the work of S. Phumying et al. (Fakhri-Mirzanagh et al., 2024), in addition to the value of CMFO/GO NCs which was 0.131 kOe, so the M–H hysteresis loops of the CMFO/GO NCs exhibit a soft superparamagnetic that can be magnetically recovered after photocatalytic process. CMFO hollow microstructure exhibits remarkable ferromagnetic properties, while there might not be a direct correlation between the magnetic properties and the photocatalytic performance, there are some instances where magnetic properties affect the photocatalytic performance, especially when the material has both ferromagnetic and photocatalytic properties, which our compound has.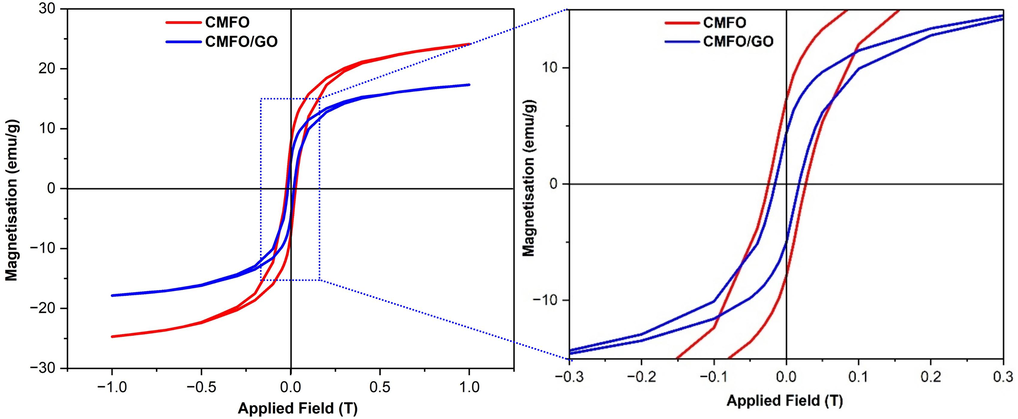
Magnetic hysteresis loops for CMFO hollow microstructures and CMFO/GO NCs with their magnification at room temperature.
3.5 Optical properties analysis
UV–vis-DRS spectroscopy was utilized to analyze the optical absorption of the compounds prepared in the left graph of Fig. 9. This analysis helps to determine the bandgap energy and optical absorption capability, which are important for the effective use of visible light in the photocatalytic degradation approach. Based on the UV–vis-DRS spectra analysis, it is evident that the presence of CMFO on the surface of GO nanosheets significantly modifies the optical properties of GO. This effect can be attributed to the porous structure of the nanocompounds which enhances the reflection of incident light, leading to an increased light-capturing capacity (Cai et al., 2023). Additionally, the loading of CMFO onto GO nanosheets results in a remarkable visible light absorption up to 800 nm, which is in the proximity of the IR region. This suggests that the optical properties of CMFO/GO nanocomposites have improved in the visible light area. To investigate the optical properties of CMFO, GO nanosheets, and CMFO/GO nanocomposite, it is essential to calculate their bandgap energy (Eg). As these samples are classified as direct band gap semiconductors, we can determine the band gap energy (Eg) of these compounds by using Tauc plot Eq. (4) (Wang et al., 2022).
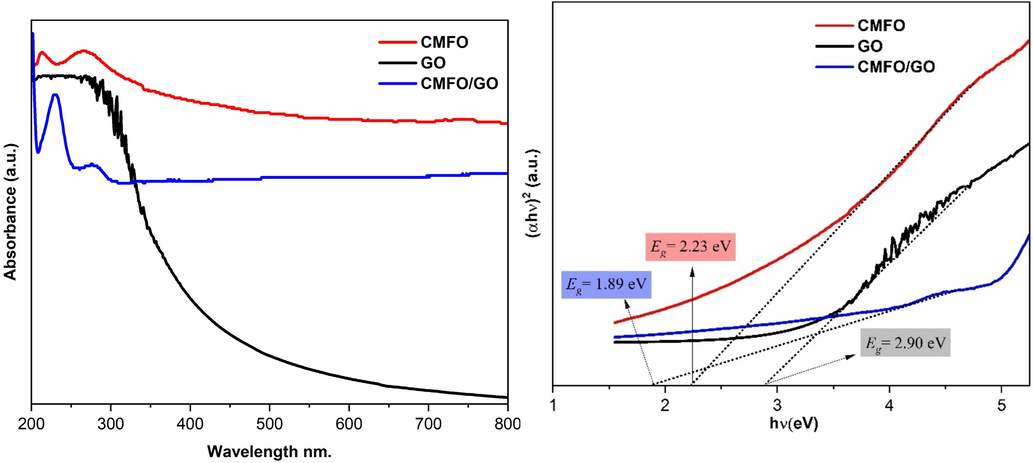
(left) DRS spectra, and (right) Plots of the (αhν) 2 versus photon energy (hν) for of the prepared photocatalysts.
3.6 Photodegradation analysis
In this study, we evaluated the photocatalytic degradation efficiency of as-prepared samples by measuring the degradation of MB in an aqueous solution under visible light within 50 min. During UV–visible absorbance measurement, it was observed that the intensity of the main peak of MB (with a maximum wavelength of 664 nm) decreased over time in the presence of binary CMFO/GO. This indicates a reduction in the concentration of MB in the aqueous solution, as shown in Fig. 10a. The photocatalytic efficiencies of CMFO hollow microstructure and CMFO/GO NCs with the presence of H2O2 were also studied for comparison purposes as Fig. 10b shows. When we compared the degradation of MB using our prepared photocatalyst in the dark and under visible light, it was clear that the process was a result of photocatalytic degradation, rather than adsorption. Before irradiating with visible light, the mixed solution was stirred in the dark for an hour to achieve a physical adsorption–desorption equilibrium on the photocatalyst surface. The weak decolorization indicated a poor adsorption effect during the process. In Fig. 10b, we can observe the pristine CMFO hollow microstructure, a photodegradation efficiency of 39.51 % was noted, which is quite low when compared to the binary CMFO/GO (97.54 %) under visible light after 50 min at pH = 7 and MB concentration = 15 mg/L. However, when 1 ml of H2O2 was added, the CMFO/GO nanocomposite's photocatalytic performance improved significantly, up to 98.62 % compared to the CMFO/GO NCs without H2O2 over 30 min under light exposure. Conversely, when we used H2O2 and visible light irradiation to degrade MB pollutants in an aqueous solution, we found a negligible amount of degradation in the photolysis process. This suggests that the pollutants are more stable in environmental conditions, possibly due to the limited amount of •OH released by the autogenous decomposition of H2O2 (Waimbo et al., 2020). Pure CMFO exhibits poor photocatalytic performance due to agglomerate formation during synthesis, leading to decreased SBET (6.506 m2.g−1) and rapid electron-hole (e-/h+) recombination, making the photocatalytic efficiency low. The enhanced degradation efficiency of the binary CMFO/GO photocatalyst is attributed to several reasons that lie behind the improvement of photocatalytic ability, such as a large surface area and mesoporous structure, these factors could help decrease the agglomerations and provide more active sites that are able to absorb the most amounts of photons. In addition to this discussion, GO nanosheets provide a swift separation capability to the photogenerated e−/h+ pairs. This high efficiency of separation can be attributed to the well-matched energy level between GO and CMFO. The efficiency of CMFO/GO in degrading MB dye was significantly improved when H2O2 was introduced into the dye solution. This was due to the reaction that occurred between charge carriers and H2O2 molecules, which generated reactive radicals that increased the degradation of MB dye performance (Ren et al., 2021).![(a) The reduction in absorption spectra of MB dye after multiple regular intervals of time in the presence of CMFO/GO photocatalyst, (b) the concentration change of MB dye over time with the presence and absence of various catalysts in dark and under visible light conditions, (c) kinetic plots of MB dye degradation through different photocatalysts. [reaction conditions]: pH = 7, MB conc. = 15 mg/L, and catalyst dose = 0.05 g).](/content/184/2024/17/9/img/10.1016_j.arabjc.2024.105934-fig12.png)
(a) The reduction in absorption spectra of MB dye after multiple regular intervals of time in the presence of CMFO/GO photocatalyst, (b) the concentration change of MB dye over time with the presence and absence of various catalysts in dark and under visible light conditions, (c) kinetic plots of MB dye degradation through different photocatalysts. [reaction conditions]: pH = 7, MB conc. = 15 mg/L, and catalyst dose = 0.05 g).
The photodegradation reaction of MB dye by our synthesized compounds was analyzed by plotting ln(Ci/Ct) against irradiation time. The plots showed a linear relationship (with R2 values close to 0.99) as depicted in Fig. 10c, indicating that the reactions followed a pseudo-first-order rate equation as shown in the formula (Wang et al., 2020):
CR = Congo red, CV = Crystal violet.
Photocatalysts
Light source
Reaction conditions
Degradation time (min)
Removal (%)
Ref.
ZnFe2O4/GO
300 W Xe lamp
0.2 g/L of photocatalyst, [CR] 20 mg/L.
180
93 %
(Zhao et al., 2023)
Ni0.65Zn0.35Fe2O4/rGO
300 W Xe lamp
0.1 g/L of photocatalyst, [MB] 5 mg/L.
56
95 %
(Javed et al., 2019)
NixMg0.5Zn0.5-xFe2O4
[x = 0.0 to 0.5]160 W lamp with 535 lx)
1 g/L of photocatalyst, [MB] 10 mg/L.
120
95.3 %
(Bagade et al., 2023)
MnFe2O4/rGO
UV lamp (λ = 365 nm, Intensity = 40 W)
0.03 g/L of photocatalyst, [MB] 10 mg/L.
60
97 %
(Mandal et al., 2020)
NiCe0.05Fe1.95O4-rGO
200 W light bulb with tungsten filament
5 mg/L of photocatalyst, [MB] 5 mg/L.
70
94.67 %
(Rahman et al., 394 (2020))
CuxNi1−xFe2O4/CNTs
sunlight
10 mg/L of photocatalyst, [CV] 5 mg/L.
90
92.9 %
(Jamil, 2021)
CMFO/GO
300 W halogen lamp
0.05 g/L of photocatalyst, [MB] 15 mg/L.
50
97.54 %
Current Study
Photocatalysts
Degradation time (min)
Removal (%)
Ref.
NiFe2O4/GO
60
99.14 %
(Thy et al., 2022)
PBC/CoFe2O4/Mn-Fe-LDH
50
94.5 %
(Zarei et al., 2023)
Fe3O4@rGO@TiO2
120
99 %
(Yang et al., 2015)
MCA-CN/WO3
30
98 %
(Gao et al., 2022)
ZnFe2O4/SiO2
90
90 %
(Huang and Nan, 2021)
CMFO/GO
30
98.62 %
Current Study
3.7 Mechanism and pathways of MB degradation
To understand how MB is removed from a new binary CMFO/GO photocatalyst through photodegradation, it is important to determine the energy band alignment of the condition band (CB) and valence band (VB). This will determine whether or not charge separation can occur. To do this, we need to know more about the CMFO hollow microstructure and GO nanosheets. Mulliken electronegativity equations were used to calculate the CB and VB values. (Truong et al., 2020):
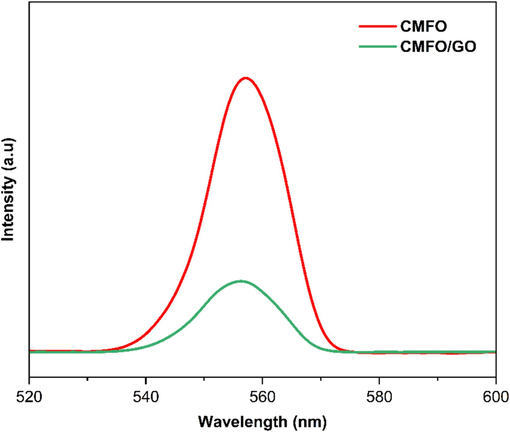
PL spectra of the prepared pure CMFO and CMFO/GO NCs.
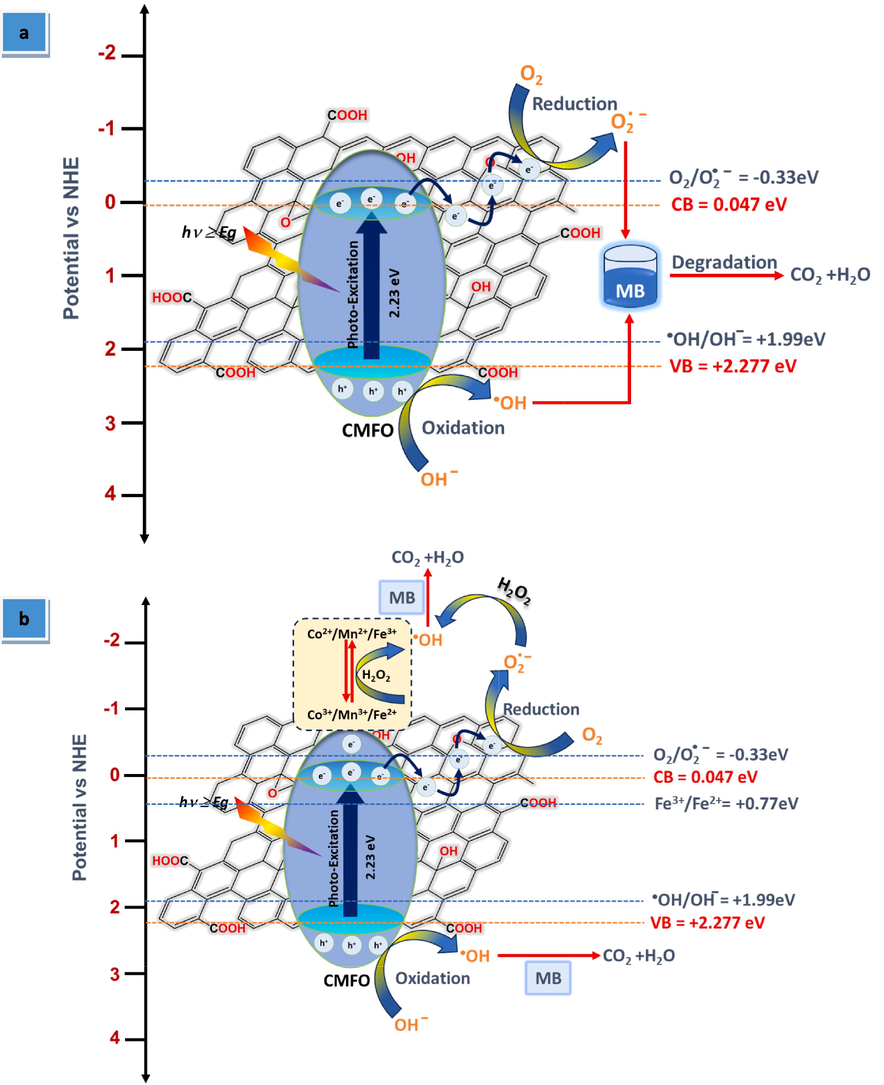
A schematic illustration of the proposed photocatalytic degradation mechanism of MB over CMFO/GO photocatalyst under visible light with the (a) absence and (b) presence of H2O2.
According to the above results and discussion, the collaboration between the photo-Fenton and photocatalyst reactions significantly enhanced the photodegradation efficiency of MB. In addition to the effective separation of e−/h+ pairs.
3.8 Studying the impact of pH, catalyst dosage, and MB concentration on effective photocatalytic degradation of MB
Extensive testing has been conducted on the new CMFO/GO photocatalyst to identify the optimal conditions needed for efficient photodegradation of MB. Several factors such as the quantity of photocatalyst used, the pH of the reaction solution, the concentration of MB, and light source were studied to establish the most suitable conditions for achieving maximum degradation. The effect of solution pH on the degradation efficiency of the MB pollutant over CMFO/GO catalyst is presented in Fig. 12a. The pH value of the solution plays a major role in MB degradation. A series of pH was used between 2 and 10, and the dilution of HCl and NaOH solutions was adjusted under the same conditions as previously studied. The graphical representation of the data indicates that the photocatalyst exhibits suboptimal photocatalytic performance under acidic conditions, specifically within the pH range of 2–4. Conversely, significant improvement in photocatalytic performance is observed when the system is alkaline, with a pH value of 10 yielding a photocatalytic efficiency of 99.94 %. The poor performance of the photocatalyst in acidic conditions can be attributed to the positive charge distribution on the catalyst's surface, resulting in an excess of H+ on the surface, which impedes the adsorption of cationic MB dye. This deficiency, in turn, leads to slower photodegradation. Conversely, the great photocatalytic degradation performance in alkaline conditions can be justified to negative charges gained on the surface of the catalyst due to the adsorption of OH–, leading to an electrostatic attraction of cationic MB dye molecules to the catalyst's surface, thereby increasing the adsorption of MB dye. Furthermore, hydroxyl radicals can be produced by OH– ions in an alkaline pH condition, which increases the degradation rate of MB dye. Therefore, the results indicate that alkaline conditions significantly enhance the photocatalytic performance of the CMFO/GO catalyst for MB dye degradation (Palanivel et al. (2021)). Fig. 12b. presents the effect of different doses of catalyst on the photodegradation of MB dye performance. Based on the data graph, the optimal catalyst dosage was 0.05 g/L, delivering a remarkable photodegradation efficiency of 97.54 %. As expected, increasing the catalyst dosage results in more photons absorbed by the catalyst, leading to higher concentrations of h+/e− pairs. Nevertheless, we chose to use the 0.05 g/L dosage because of its outstanding performance of 97.54 % photodegradation with a small amount of catalyst (0.05 g/L), compared to the 0.08 g/L dosage. The initial dye concentration in wastewater plays a major role in the photocatalytic efficiency, so, four different MB initial concentrations were chosen, which are 5, 10, 15, and 20 mg/L, then were irradiated under visible light for 50 min each, with a catalyst dose of 0.05 g/L and pH of 7, as depicted in Fig. 12c.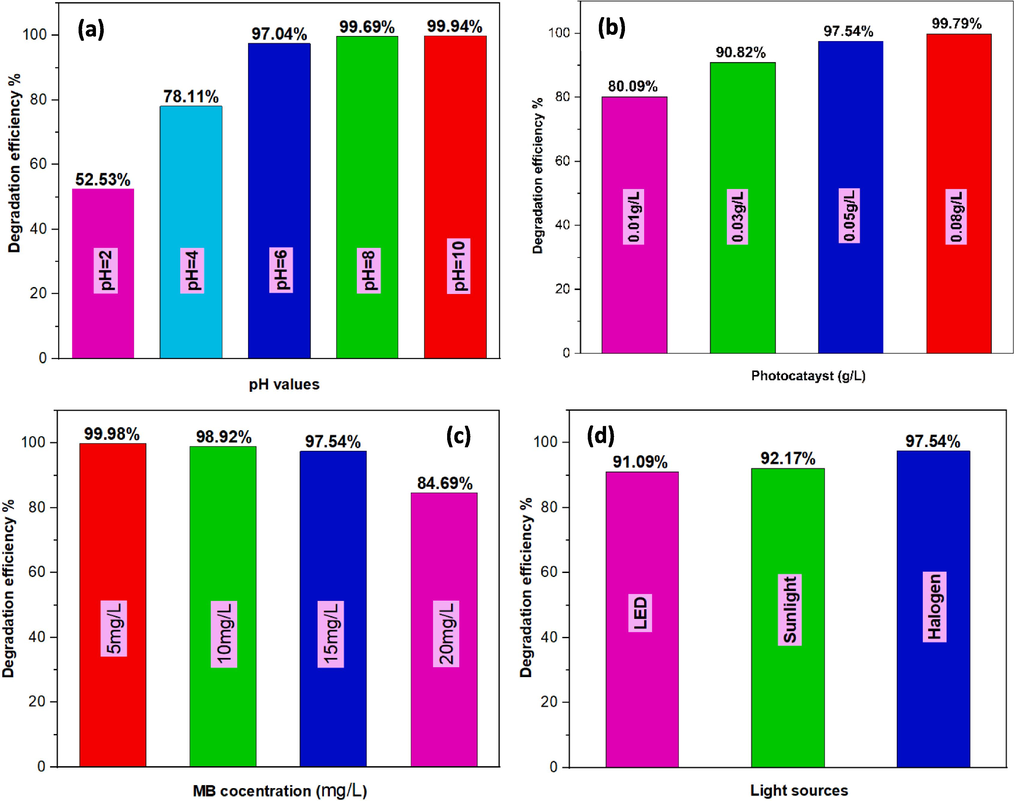
MB degradation efficiency graphs over a 50 min period affected by (a) pH (MB conc. = 15 mg/L, and catalyst dose = 0.05 g), (b) catalyst dose (g/L) (pH = 7, and MB conc. = 15 mg/L), (c) MB conc. (mg/L) (Catalyst dose = 0.05 g, and pH = 7), and (d) light sources (pH = 7, MB conc. = 15 mg/L, and catalyst dose = 0.05 g).
At the MB concentration 20 mg/L, the photocatalyst degradation decreased notably, which may be attributed to the scattering or absorption of light, resulting in a less photons reaching to the catalyst surface, thus, less e-/h+ pairs generation. The data presented in Fig. 12d indicates that MB degradation efficiency was significantly improved when exposed to sunlight/halogen light irradiation compared to LED. This noteworthy outcome could be attributed to the relatively higher intensity of the former (Palanivel et al., 2019).
3.9 Reusability of CMFO/GO catalyst study
The exceptional performance of photocatalysts warrants their application outside laboratory settings. Along with their efficacy, the reusability and stability of these catalysts are noteworthy aspects. Consequently, this study explores the reusability of CMFO/GO photocatalysts under visible light, using identical reaction conditions (Waheed et al., 2023). The results presented in Fig. 13 depict four sequential reusability tests on CMFO/GO photocatalysts, with promising outcomes. Notably, the photocatalytic efficiency may be reduced due to the adsorption of degradation products on the catalyst surface, resulting in compromised reaction sites and a subsequent decline in efficiency. Another factor that could lead to efficiency reduction is catalyst waste generated during washing. Nevertheless, the used CMFO/GO photocatalysts demonstrate an efficiency of 86.76 % even after the fourth cycle, indicating the catalyst's superior reusability and stability.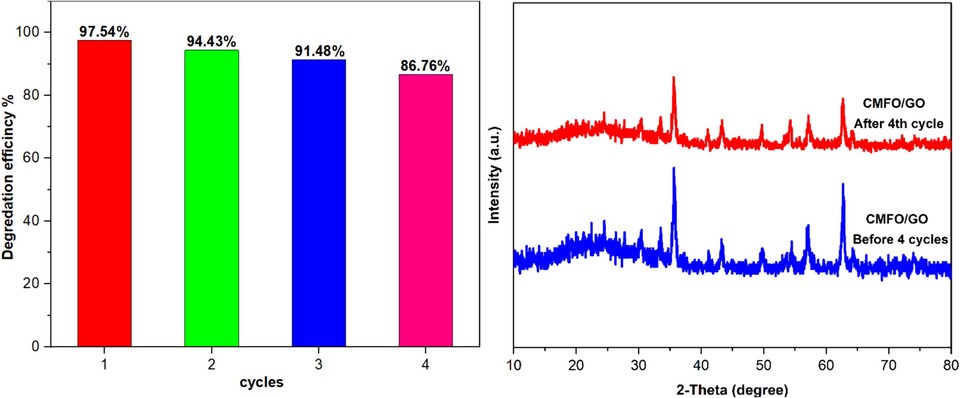
(left) Reusability test data graphs of CMFO/GO photocatalyst for the degradation of MB dye under visible light at pH = 7, MB conc. = 15 mg/L, and catalyst dose = 0.05 g. (right) XRD of recovered CMFO/GO NCs before and after photodegradation cycles.
After the photocatalysis process, the particles that were reused underwent an XRD analysis. The results showed that the particles maintained their structural stability, as indicated by the similar peak positions observed. Additionally, the particles were found to be free from any damage caused by photocorrosion. Hence, CMFO/GO photocatalysts are a suitable candidate for treating wastewater.
4 Conclusions
In this study, we successfully developed a novel heterojunction superparamagnetic nano-photocatalyst, CMFO/GO, utilizing a microwave-ultrasonic synthesis method. The confirmation of CMFO hollow microstructure decoration onto GO nanosheets was meticulously verified through a comprehensive array of analytical techniques including FTIR, XRD, FE-SEM, Raman spectroscopy, and TEM. Notably, our fabricated photocatalyst exhibited a remarkable enhancement in performance compared to the initial materials. This enhancement can be attributed to several factors including the exceptionally high surface area (96.4654 m2.g−1) coupled with a mesoporous hollow microstructure, the synergistic effect of the heterojunction configuration, and the presence of CMFO and GO components which effectively hindered the recombination of h+/e- pairs. Our results unequivocally demonstrate the exceptional efficiency of the CMFO/GO photocatalyst under visible light irradiation, achieving an outstanding MB degradation efficiency of 98.62 % within just half an hour in the presence of H2O2. Even without the use of peroxide, the photodegradation efficiency remained impressively high at 97.54 %. Furthermore, we identified the optimal conditions for maximizing degradation efficiency, including pH (6–10), catalyst dose (0.05 g/L), and MB concentration (15 mg/L). Of particular interest is the magnetic retrievability of the photocatalyst from contaminated solutions, offering added convenience and efficiency in environmental remediation processes. Moreover, our findings underscore the robust stability of the nanocomposite, as evidenced by its ability to be reused for four consecutive cycles without any notable loss in efficiency, thus affirming its practical viability for sustainable environmental applications, such as the removal of colorless and colored pollutions. However, this novel catalyst as any other catalyst has its drawbacks or challenges, which is mainly the low yield of CS template, in addition to its large size, which leads to a longer period of preparation and harder control of size, this also could somewhat make the process costlier.
Funding
The author(s) received no financial support for the research, authorship, and/or publication of this article.
CRediT authorship contribution statement
Ibrahim F. Waheed: Resources, Supervision, Writing – editing, Methodology, Formal analysis, manuscipt final review. Maha M. Awsaj: Investigation, Writing – original draft. Omar S. Dahham: Resources, Writing – editing. Mustafa Qutaiba Jabbar: Data curation, Formal analysis, Validation, Visualization. Faiz M. Al‑Abady: Resources, Validation, Visualization. Mohammed Abbas Fadhil Al-Samarrai: Data curation, Formal analysis, Writing – review & editing.
Acknowledgments
We acknowledge university of Tikrit, college of science department of chemistry, for the support they provided us to be able to complete this research.
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
References
- Design and fabrication of Ni(OH)2/BiVO4 heterostructured nano-photocatalyst for high-efficient removal of organic dyes. J. Alloy. Compd.. 2020;831:154828
- [CrossRef] [Google Scholar]
- A review on visible light driven spinel ferrite-g-C3N4 photocatalytic systems with enhanced solar light utilization. J. Mol. Liq.. 2022;357:119105
- [CrossRef] [Google Scholar]
- A novel fabricate of iron and nickel-introduced bimetallic MOFs for quickly catalytic degradation via the peroxymonosulfate, antibacterial efficiency, and cytotoxicity assay. Inorg. Chem. Commun.. 2023;153:110823
- [CrossRef] [Google Scholar]
- Fe3O4@SiO2 functionalized PEG-PPG-PEG triblock copolymer-grafted graphene oxide as novel magnetic nanodemulsifier for water-in-crude oil emulsion separation. Colloids Surf., A Physicochem. Eng. Asp.. 2023;676:132228
- [CrossRef] [Google Scholar]
- Influence of chitosan source and degree of deacetylation on antibacterial activity and adsorption of AZO dye from water. Biomass Conv. Bioref. 2023
- [CrossRef] [Google Scholar]
- Novel synthesis of CuO/GO nanocomposites and their photocatalytic potential in the degradation of hazardous industrial effluents. ACS Omega. 2023;8:17667-17681.
- [CrossRef] [Google Scholar]
- Highly visible light responsive, narrow band gap TiO2 nanoparticles modified by elemental red phosphorus for photocatalysis and photoelectrochemical applications. Sci. Rep.. 2016;6:25405.
- [CrossRef] [Google Scholar]
- Eco-friendly synthesis, crystal chemistry, and magnetic properties of manganese-substituted CoFe2O4 nanoparticles. ACS Omega. 2020;5:19315-19330.
- [CrossRef] [Google Scholar]
- Photocatalytic property of co-precipitated manganese substituted on cobalt ferrite nanoparticles. AIP Conf. Proc.. 2020;2296
- [CrossRef] [Google Scholar]
- Ni-doped Mg-Zn nano-ferrites: Fabrication, characterization, and visible-light-driven photocatalytic degradation of model textile dyes. Catal. Commun.. 2023;181:106719
- [CrossRef] [Google Scholar]
- A comprehensive parametric study on CO2 removal from natural gas by hollow fiber membrane contactor: a computational fluid dynamics approach. Chem. Eng. Technol.. 2024;47:732-738.
- [CrossRef] [Google Scholar]
- Recent advances in MOF-5-based photocatalysts for efficient degradation of toxic organic dyes in aqueous medium. Next Sustainability. 2024;3:100016
- [CrossRef] [Google Scholar]
- ZnFe2O4@g-C3N4 nanocomposites: An efficient catalyst for Fenton-like photodegradation of environmentally pollutant Rhodamine B. J. Environ. Chem. Eng.. 2019;7:103035
- [CrossRef] [Google Scholar]
- Hydrothermal synthesis of bismuth ferrite hollow spheres with enhanced visible-light photocatalytic activity. Molecules. 2023;28:5079.
- [CrossRef] [Google Scholar]
- Tuning catalytic performance by controlling reconstruction process in operando condition. Appl. Catal. B. 2020;260:118103
- [CrossRef] [Google Scholar]
- Synergy of Light Trapping and Water Management in Interconnected Porous PEDOT: PSS Hydrogels for Efficient Solar-Driven Water Purification. Ind. Eng. Chem. Res.. 2023;62:10175-10183.
- [CrossRef] [Google Scholar]
- Enhanced photocatalytic degradation of tetracycline hydrochloride by novel porous hollow cube ZnFe2O4. J. Photochem. Photobiol. A Chem.. 2018;364:794-800.
- [CrossRef] [Google Scholar]
- Enhancing photocatalytic efficiency of spent tea leaf powder on ZnIn2S4 incorporation: role of surface charge on dye degradation. ACS Omega. 2023;8:17880-17890.
- [CrossRef] [Google Scholar]
- Engineering core-shell hollow-sphere Fe3O4@FeP@nitrogen-doped-carbon as an advanced bi-functional electrocatalyst for highly-efficient water splitting. J. Colloid Interface Sci.. 2024;657:684-694.
- [CrossRef] [Google Scholar]
- ZnFe2O4@WO3–X/Polypyrrole: an efficient ternary photocatalytic system for energy and environmental application. ACS Omega. 2021;6(45):30401-30418.
- [CrossRef] [Google Scholar]
- Doumeng, M., Makhlouf, L., Berthet, F., Marsan, O., Delb́e, K., Denape, J., Chabert, F., 2021. A comparative study of the crystallinity of polyetheretherketone by using density, DSC, XRD, and Raman spectroscopy techniques, Polym. Test. 93, 106878, Doi: 10.1016/j.polymertesting.2020.106878.
- The effect of graphene oxide content on the photocatalytic activity of (ZnCdS/MnFe2O4/GO) nanocomposite. Phys. B Condens. Matter. 2024;685:415974
- [CrossRef] [Google Scholar]
- Degradation of methylene blue in the photo-Fenton-like process with WO3-loaded porous carbon nitride nanosheet catalyst. Water. 2022;14:2569
- [CrossRef] [Google Scholar]
- Photo-Fenton degradation of rhodamine B using Fe2O3–Kaolin as heterogeneous catalyst: Characterization, process optimization and mechanism. J. Colloid Interface Sci.. 2014;433:1-8.
- [CrossRef] [Google Scholar]
- Design of direct Z-scheme superb magnetic nanocomposite photocatalyst Fe3O4/Ag3PO4@Sep for hazardous dye degradation. Sep. Purif. Technol.. 2021;277:119399
- [CrossRef] [Google Scholar]
- Constructive Z-scheme interfacial charge transfer of a spinel ferrite-supported g-C3N4 heterojunction architect for photocatalytic degradation. J. Alloy. Compd.. 2024;976:172987
- [CrossRef] [Google Scholar]
- Synergistic effect of novel biosynthesis SnO2@Fe3O4 nanocomposite: A comprehensive study of its photocatalytic of Dyes & antibiotics, antibacterial, and antimutagenic activities. J. Photochem. Photobiol. A Chem.. 2023;443:114874
- [CrossRef] [Google Scholar]
- Magnetic Fe3O4-grafted cellulose/graphene oxide nanocomposite for methylene blue removal from aqueous solutions: Synthesis and characterization. Next Materials. 2024;3:100064
- [CrossRef] [Google Scholar]
- CuCe-ferrite/TiO2 nanocomposite as an efficient magnetically separable photocatalyst for dye pollutants decolorization. Top. Catal.. 2023;66:53-63.
- [CrossRef] [Google Scholar]
- Synergetic adsorption and photo-Fenton degradation of methylene blue by ZnFe2O4/SiO2 magnetic double-mesoporous-shelled hollow spheres. Environ. Technol.. 2021;42:3218-3230.
- [CrossRef] [Google Scholar]
- Structural, dielectric and magnetic properties of Mn+2 doped cobalt ferrite nanoparticles. J. Magn. Magn. Mater.. 2020;494:165726
- [CrossRef] [Google Scholar]
- Cu2+ doped nickel spinel ferrites (CuxNi1−xFe2O4) nanoparticles loaded on CNTs for degradation of crystal violet dye and antibacterial activity studies. J. Taibah Univ. Sci.. 2021;15:814-825.
- [CrossRef] [Google Scholar]
- Reduced graphene oxide-spinel ferrite nano-hybrids as magnetically separable and recyclable visible light driven photocatalyst. Synth. Met.. 2019;254:1-9.
- [CrossRef] [Google Scholar]
- Efficient photocatalytic degradation of methylene blue dye from aqueous solution with cerium oxide nanoparticles and graphene oxide-doped polyacrylamide. ACS Omega. 2023;8:13004-13015.
- [CrossRef] [Google Scholar]
- Fabrication and characterization of ternary composite MgFe2O4/MoO3@CNTs for the enhanced photocatalytic activity to degrade organic pollutants. J. Alloy. Compd.. 2024;978:173327
- [CrossRef] [Google Scholar]
- Shanker, One pot green synthesis of Al doped zinc ferrite nanoparticle decorated with reduced graphene oxide for photocatalytic remediation of organic pollutants: Green synthesis, kinetics, and photoactivity. Chemosphere. 2023;344:140381
- [CrossRef] [Google Scholar]
- Silver-decorated cobalt ferrite nanoparticles anchored onto the graphene sheets as electrode materials for electrochemical and photocatalytic applications. ACS Omega. 2020;5:31076-31084.
- [CrossRef] [Google Scholar]
- Biosynthesis and characterization of novel nanocomposite ZnO/BaMg2 efficiency for high-speed adsorption of AZO dye. Biomass Conv. Bioref. 2023
- [CrossRef] [Google Scholar]
- Challenges of photocatalysis and their coping strategies. Chem. Catal.. 2022;2:1315-1345.
- [CrossRef] [Google Scholar]
- Direct observation of enhanced plasmon-driven catalytic reaction activity of Au nanoparticles supported on reduced graphene oxides by SERS. Phys. Chem. Chem. Phys.. 2015;17:10176-10181.
- [CrossRef] [Google Scholar]
- Defect evolution on the activity of samarium doped Na2TinO2n+1 with different sodium/titanium ratio for photocatalytic degradation of fluoroquinolones drugs, toxicity assessments and eco-toxicity prediction. Ceram. Int.. 2024;50:11939-11948.
- [CrossRef] [Google Scholar]
- α-Fe2O3 hollow microspheres assembled by ultra-thin nanoflakes exposed with (241) high-index facet: Solvothermal synthesis, lithium storage performance, and superparamagnetic property. Int. J. Hydrogen Energy. 2019;44:1070-1077.
- [CrossRef] [Google Scholar]
- Synthesis of magnetically recyclable g-C3N4/NiFe2O4 S-scheme heterojunction photocatalyst with promoted visible-light-response photo-Fenton degradation of tetracycline. Mater. Res. Bull.. 2023;158:112064
- [CrossRef] [Google Scholar]
- A facile construction of NiV2O6/CeO2 nano-heterojunction for photo-operated process in water remediation reaction, antibacterial studies, and detection of D-Amino acid in peroxidase system. Surf. Interfaces. 2023;40:102970
- [CrossRef] [Google Scholar]
- Multifunctional reduced graphene oxide wrapped circular Au nanoplatelets: Enhanced photoluminescence, excellent surface-enhanced Raman scattering, photocatalytic water splitting, and non-enzymatic biosensor. ACS Appl. Nano Mater.. 2018;1:3945-3955.
- [CrossRef] [Google Scholar]
- MnFe2O4 decorated reduced graphene oxide heterostructures: Nanophotocatalyst for methylene blue dye degradation. Vacuum. 2020;173:109150
- [CrossRef] [Google Scholar]
- photocatalytic activity of iron oxide nanoparticles synthesized by different plant extracts for the degradation of diazo dyes Evans blue and Congo red. Biomass Conv. Bioref.. 2024;14:5357-5372.
- [CrossRef] [Google Scholar]
- Spinel-ferrite-decorated graphene-based nanocomposites for enhanced photocatalytic detoxification of organic dyes in aqueous medium: A review. Water. 2023;15:81.
- [CrossRef] [Google Scholar]
- Recent updates in modification strategies for escalated performance of Graphene/MFe2O4 heterostructured photocatalysts towards energy and environmental applications. J. Alloy. Compd.. 2023;960:170576
- [CrossRef] [Google Scholar]
- Boosted photocatalytic accomplishment of 3D/2D hierarchical structured Bi4O5I2/g-C3N4 p-n type direct Z-scheme heterojunction towards synchronous elimination of Cr(VI) and tetracycline. Diam. Relat. Mater.. 2024;142:110834
- [CrossRef] [Google Scholar]
- Mishra, S., Acharya, R., Parida, K. 2023. Spinel-ferrite-decorated graphene-based nanocomposites for enhanced photocatalytic detoxification of organic dyes in aqueous medium: A review, 15, 81, Doi: 10.3390/w15010081.
- Se-doped magnetic Co–Ni spinel ferrite nanoparticles as electrochemical catalysts for hydrogen evolution. ACS Appl. Nano Mater.. 2023;6:7330-7341.
- [CrossRef] [Google Scholar]
- A novel biosynthesis of MgO/PEG nanocomposite for organic pollutant removal from aqueous solutions under sunlight irradiation. Environ. Sci. Pollut. Res.. 2023;30:57076-57085.
- [CrossRef] [Google Scholar]
- Muzammil, K., Zaid, M., Hussein, U. A-R., Abduljabbar, M.H., Jalal, S.S., Najm, M.A.A., Alshahrani, M.Y., Almulla, A.F., Alsaalamy, A., Amer, R.F., Janani, B.J. 2023. Decoration of MoO3-x on clay mineral matrix with great phosphorescence properties for oxygen activation, photochemical properties, bactericidal and oxidase-like mimics for prompt detection of pesticide, Mater. Sci. Semicond. Process., 168, 107847, Doi: 10.1016/j.mssp.2023.107847.
- Facile preparation of magnetic CuFe2O4 on Sepiolite/GO nanocomposites for efficient removal of Pb(II) and Cd(II) from aqueous solution. ACS Omega. 2023;8:38828-38838.
- [CrossRef] [Google Scholar]
- Efficient photocatalytic degradation of organic dyes by AgNPs/TiO2/Ti3C2Tx MXene composites under UV and solar light. ACS Omega. 2021;6(6):33325-33338.
- [CrossRef] [Google Scholar]
- Rational design of ZnFe2O4/g-C3N4 nanocomposite for enhanced photo-Fenton reaction and supercapacitor performance. Appl. Surf. Sci.. 2019;498:143807
- [Google Scholar]
- Activation of persulfate for improved naproxen degradation using FeCo2O4@g-C3N4 heterojunction photocatalysts. ACS Omega. 2021;6:34563-34571.
- [CrossRef] [Google Scholar]
- Photodegradation of methylene blue and photoproduction of hydrogen peroxide using magnesium ferrite-titanium dioxide/reduced graphene oxide as a photo-catalyst under visible light. Opt. Mater.. 2023;139:113832
- [CrossRef] [Google Scholar]
- Structure and magnetic properties of Mn-doped CoFe2O4 nanoparticles prepared by solvothermal route. J. Supercond. Nov. Magn.. 2014;27:2573-2579.
- [CrossRef] [Google Scholar]
- A new pH-sensitive CS/Zn-MOF@ GO ternary hybrid compound as a biofriendly and implantable platform for prolonged 5-Fluorouracil delivery to human breast cancer cells. J. Alloy. Compd.. 2021;885:160992
- [CrossRef] [Google Scholar]
- Fabrication of Ce3+ substituted nickel ferrite-reduced graphene oxide heterojunction with high photocatalytic activity under visible light irradiation. J. Hazard. Mater.. 2020;394:122593
- [CrossRef] [Google Scholar]
- Recent advances of photocatalytic application in water treatment: A review. Nanomaterials. 2021;11:1804.
- [CrossRef] [Google Scholar]
- Facile preparation of 3D regenerated cellulose/graphene oxide composite aerogel with high-efficiency adsorption towards methylene blue. J. Colloid Interface Sci.. 2018;532:58-67.
- [CrossRef] [Google Scholar]
- Sahoo, S., Acharya, R., 2021. An overview on recent developments in synthesis and molecular level structure of visible-light responsive g-C3N4 photocatalyst towards environmental remediation, Mater. Today: Proc., 35, 150–155. Doi: 10.1016/j.matpr.2020.04.008.
- Magnetic hollow buoyant alginate beads achieving rapid remediation of oil contamination on water. J. Environ. Chem. Eng.. 2021;9:104935
- [CrossRef] [Google Scholar]
- biosynthesis of Mn3O4/PVP nanocomposite for enhanced photocatalytic degradation of organic dyes under sunlight irradiation. J. Clust. Sci.. 2024;35:201-215.
- [CrossRef] [Google Scholar]
- Recent advances in visible light-activated photocatalysts for degradation of dyes: A comprehensive review. Chemosphere. 2024;349:140818
- [CrossRef] [Google Scholar]
- Enhanced degradation of BPA in water by PANI supported Ag/TiO2 nanocomposite under UV and visible light. J. Environ. Chem. Eng.. 2019;7:102880
- [CrossRef] [Google Scholar]
- The magnetic properties of Mn-doped cobalt ferrite films prepared by the spin-coating method. J. Mater. Sci. Mater. Electron.. 2023;34:1635.
- [CrossRef] [Google Scholar]
- Role of different fuels and sintering temperatures in the structural, optical, magnetic, and photocatalytic properties of chromium-containing nickel ferrite: kinetic study of photocatalytic degradation of rhodamine B dye. ACS Omega. 2023;8:6302-6317.
- [CrossRef] [Google Scholar]
- Superior photocatalytic activity of Mn-doped CoFe2O4 under visible light irradiation: Exploration of hopping and polaron formation in the spinel structure. Mater. Sci. Eng. B. 2021;270:115222
- [CrossRef] [Google Scholar]
- Hydrothermal synthesis of spindle structure copper ferrite-graphene oxide nanocomposites for enhanced photocatalytic dye degradation and in-vitro antibacterial activity. Environ. Res.. 2023;231:116095
- [CrossRef] [Google Scholar]
- Nickel ferrite nanoparticles-doped graphene oxide as a heterogeneous Fenton catalyst: Synthesis, characterization, and catalytic application. Vietnam J. Chem.. 2022;60:532-539.
- [CrossRef] [Google Scholar]
- H2O2-assisted photocatalysis for removal of natural organic matter using nanosheet C3N4-WO3 composite under visible light and the hybrid system with ultrafiltration. Chem. Eng. J.. 2020;399:125733
- [CrossRef] [Google Scholar]
- Photocatalytic reactor design: guidelines for kinetic investigation. Ind. Eng. Chem. Res.. 2019;58:5349-5357.
- [CrossRef] [Google Scholar]
- Novel MgFe2O4-CuO/GO heterojunction magnetic nanocomposite: Synthesis, characterization, and batch photocatalytic degradation of methylene blue dye. J. Mol. Liq.. 2022;357:119084
- [CrossRef] [Google Scholar]
- Degradation of methylene blue using a novel magnetic CuNiFe2O4/g-C3N4 nanocomposite as heterojunction photocatalyst. Diam. Relat. Mater.. 2023;133:109716
- [CrossRef] [Google Scholar]
- Improved charge separation through H2O2 assisted copper tungstate for enhanced photocatalytic efficiency for the degradation of organic dyes under simulated sunlight. J. Photochem. Photobiol. B Biol.. 2020;204:111781
- [CrossRef] [Google Scholar]
- Wang, D., Zhao, P., Yang, J., Xu, G., Yang, H., Shi, Z., Hu, Q., Dong, B., Guo, Z. 2020. Photocatalytic degradation of organic dye and phytohormone by a Cu(II) complex powder catalyst with added H2O2, 603, 125147. Doi: 10.1016/j.colsurfa.2020.125147.
- A novel self-activation strategy for designing oxygen vacancies-rich nickel ferrite and cobalt ferrite microspheres with large specific surface area for overall water splitting. Int. J. Hydrogen Energy. 2022;47:24343-24357.
- [CrossRef] [Google Scholar]
- Visible-light-driven photo-Fenton reactions using Zn1-1.5xFexS/g-C3N4 photocatalyst: Degradation kinetics and mechanisms analysis. Appl. Catal. B. 2020;266:118653
- [CrossRef] [Google Scholar]
- Magnetic cobalt ferrite/reduced graphene oxide (CF/rGO) porous balls for efficient photocatalytic degradation of oxytetracycline. J. Environ. Chem. Eng.. 2022;10:108259
- [CrossRef] [Google Scholar]
- Enhanced photocatalytic degradation of methylene blue dye using fascily synthesized g-C3N4/CoFe2O4 composite under sun light irradiation. Results Chem.. 2024;7:101306
- [CrossRef] [Google Scholar]
- Piezo-photocatalytic activity of Bi0.5Na0.5TiO3@TiO2 composite catalyst with heterojunction for degradation of organic dye molecule. J. Phys. Chem. C. 2020;124:24126-24134.
- [CrossRef] [Google Scholar]
- Design of ternary CaTiO3/g-C3N4/AgBr Z-scheme heterostructured photocatalysts and their application for dye photodegradation. Solid State Sci.. 2020;100:106102
- [CrossRef] [Google Scholar]
- Rapid degradation of methylene blue in a novel heterogeneous Fe3O4 @rGO@TiO2-catalyzed photo-Fenton system. Sci. Rep.. 2015;5:10632.
- [CrossRef] [Google Scholar]
- Photocatalytic Simultaneous Removal of Nitrite and Ammonia via a Zinc Ferrite/Activated Carbon Hybrid Catalyst under UV–Visible Irradiation. ACS Omega. 2019;4:6411-6420.
- [CrossRef] [Google Scholar]
- A promising Ag2CrO4/LaFeO3 heterojunction photocatalyst applied to photo-Fenton degradation of RhB. Environ. Technol.. 2018;41:1486-1503.
- [CrossRef] [Google Scholar]
- Mechanism research of catalytic degradation of 1, 2-dichlorobenzene over highly efficient hollow calcium ferrite by in situ FTIR spectra. Mater. Today Energy. 2022;26:100996
- [CrossRef] [Google Scholar]
- An innovative magnetic Ni0.1Co0.9Fe2O4/g-C3N4 nano-micro-spherical heterojunction composite photocatalyst with an extraordinarily prominent visible-light-irradiation degradation performance toward organic pollutants. Dalton Trans.. 2020;49:9849-9862.
- [CrossRef] [Google Scholar]
- Photocatalytic decomposition of methylene blue and methyl orange dyes using pistachio biochar/CoFe2O4/Mn-Fe-LDH composite as H2O2 activator. Surf. Interfaces. 2023;43:103571
- [CrossRef] [Google Scholar]
- MnFe2O4 nanoparticles-decorated graphene nanosheets used as an efficient peroxidase minic enable the electrochemical detection of hydrogen peroxide with a low detection limit. Microchem. J.. 2021;166:106240
- [CrossRef] [Google Scholar]
- Micro-/nanostructured ZnFe2O4 hollow sphere/GO composite for structurally enhanced photocatalysis performance. Rare Met.. 2023;42:813-821.
- [CrossRef] [Google Scholar]
- Preparation of CoFe2O4 hollow spheres with carbon sphere templates and their wave absorption performance. Mater. Chem. Phys.. 2020;244:122697
- [CrossRef] [Google Scholar]
- Fluffy cotton-like GO/Zn–Co–Ni layered double hydroxides form from a sacrificed template GO/ZIF-8 for high performance asymmetric supercapacitors. ACS Sustain. Chem. Eng.. 2020;8:11618-11629.
- [CrossRef] [Google Scholar]
- Green synthesis of multifunctional MgO@AgO/Ag2O nanocomposite for photocatalytic degradation of methylene blue and toluidine blue. Sec. Polym. Chem.. 2022;10
- [CrossRef] [Google Scholar]







