Synthesis of iron-based magnetic nanocomposites: A review
-
Received: ,
Accepted: ,
This article was originally published by Elsevier and was migrated to Scientific Scholar after the change of Publisher.
Abstract
Iron oxide-based magnetic nanocomposites have been interesting materials for a wide range of applications in various fields of environment, energy, industries, and medicals. The formation of iron oxide-based magnetic nanocomposites is varied and tuneable referred to their purposes. This paper presents the reviews of the development of magnetic iron oxide nanocomposites by several synthesis method and materials. The highlight is focused on various method for controlling size, magnetization, and the nanoparticles distribution within solid support such as mesoporous silica, clay, graphene, carbon, and zeolite. The role of synthesis method on the magnetic properties and other characteristics as the function of various parameters such as iron content, synthesis condition such as pH, dispersion method, and the presence of intensification are summarized. Furthermore, future perspective for development is also highlighted.
Keywords
Magnetic
Magnetic nanocomposite
Iron oxide nanoparticles
1 Introduction
Iron oxide magnetic nanoparticles and nanocomposites are one of the promising materials in many fields’ application due to its unique properties both chemical and physical. As naturally, the magnetic material can be classified into three major types, that are maghemite (γ-Fe2O3), magnetite (Fe3O4) and hematite (α-Fe2O3). These classifications are useful to describe the disadvantages and advantages of the materials when they were applied in specific applications. For example, magnetite material has been commonly used in medical application for drug delivery because of the highest magnetic saturation properties than the others (almost > 250 times) (Chandra et al., 2010). Therefore, determining the basic type of magnetic material is one of the important factors when the material employed in different type application. In addition, besides magnetite types, hematite magnetic nanomaterials have been widely reported in therapy, cancer treatment, hypothermia treatment, and drug delivery systems. In other hands, the material properties such superparamagnetic, which corelated with thermal energy and the ferromagnetic nanoparticle is one of important character for sensor and biomedical purposes (Cardoso et al., 2017). The magnetic properties of iron oxide material depend on the type of the individual and synthesis pathways of magnetic material as shown in Table 1. The basic idea of magnetic nanocomposite development was that magnetic materials are a multimagnetic domain structure, and as their size is reduced to nanoscale, they have a single magnetic domain structure, and their magnetism turns to paramagnetic (Ladj et al., 2013). The change in particle size into the critical size influences the magnetic spin of the magnetic nanomaterials into the disordered and superparamagnetic (Medeiros et al., 2011; Schleich et al., 2014). The effect of particle size affected to magnetic properties already reported. The magnetic properties (Ms, Mr, and Hc) is directly influence by reducing or increasing the particle size. However, the particle size and magnetic properties have a linear correlation, for example increasing particle size will also increase magnetic intrinsic properties, including Ms, Mr and Hc (Li et al., 2017). These phenomena are useful for tumor thermotherapy. In different mechanism, magnetic nanoparticles can be served as vectors to bind biomolecules and then be separated from the biomolecules at the targeted area under the action of the magnetic field and thus used for targeted therapy or diagnosis. In the environmental applications, the nanocomposites were superior in adsorption, photocatalysis and catalysis, as well as in sensor and biosensor applications. Particularly, the easy in separation due to magnetically recoverable nano catalytic, adsorption and chemical bonding interaction are the potencies for adsorption and catalysis area. The combination of magnetic properties and such specific characters for specific purposes is basic idea for magnetic nanocomposite development (Cao et al., 2013). Moreover, the development of the high stability of iron oxide nanocomposite is still a challenging issue, especially in biomedicine applications. Although iron salts are relatively more stable like Mohr's salts; however, in the liquid phase, the stability becomes decreasing. Therefore, increasing stability with a polymer already reported could increase the stability of iron ions. This technique has been widely developed for the synthesis process because it can produce a relatively more significant 3-fold percentage of iron ions composition (Spiridonov et al., 2018). The magnetic polymer core–shell was developed as a protein adsorbent material due to the material's hydrophobic properties (Elaïssari and Bourrel, 2001). Thermosensitive properties are the main characteristics of polymer-coated materials for biomedical applications. Furthermore, surface modification with polymer cation (polycation) is also widely developed and reported as absorbing and detecting viruses because it can increase the adsorption power of the material through electrostatic mechanisms (Veyret et al., 2005).
| Iron oxide | Chemical formula | Color | Magnetic saturation A m2 kg−1 | Grain size (nm) | Curie transition |
|---|---|---|---|---|---|
| Maghemite | γ-Fe2O3 | Grey, grown, red | 60–80 | ± 10 | ∼820–980 |
| Magnetite | Fe3O4 | Black | 92–100 | ± 6 | ∼850 |
| Hematite | α-Fe2O3 | Grey, grown, red | 0.3 | – | ∼1000 |
The properties and structure of combining material influence the procedure and route and synthesis method. This review highlights the synthesis of magnetic nanocomposite. Considering that the specific surface area, particle size and magnetic saturation are the important physicochemical characters, the studies focused on these parameters. In fact, the relationship among parameters affects to the properties of nanocomposite. For example, from the synthesis of magnetic iron oxide nanocomposite using clay materials, following factors significantly influence the properties of magnetization (Szabó et al., 2007):
-
MNP content and phase
-
Average particle size of MNPs, size distribution, and aggregation state
-
Specific surface area and the porous structure of clay
-
Homogeneity dispersion of iron oxide crystals in the clay matrix.
Surface characteristics consist of hydrophobicity, specific surface area, pore distribution, and nanoparticle distribution are critical parameters of the nanocomposites. For adsorption and catalytic purposes for example, higher specific surface areas provide more space for adsorption. Synthesis method and route are important factors for designing the properties.
2 Synthesis of magnetic nanocomposites
2.1 Co-precipitation method
The synthesis of iron-based nanocomposites by co-precipitation method is the most efficient and straightforward wet chemical route which is generally synthesized from its salt species like Fe2+ and/or Fe3+ in alkali solution as shown in Table 2. the precipitation process of Fe3+/Fe2+ salt depends on the pH of the solution which generally occurs at a pH range between 8 and 14 with the Fe3+/2+ ratio at 2:1 under non-oxidizing conditions. The reaction of the synthesis of wet chemical route of magnetite nanoparticles is shown in Eq. (1)–(4).
| Magnetic source | Modifier | Type of iron oxide | Reaction temperature (oC) | Reaction period | Solvent | Particle size (nm) | Magnetic saturation value (emu. g−1) | Application | Ref. |
|---|---|---|---|---|---|---|---|---|---|
| FeCl3, FeSO4·7H2O | Zn-Mn | Magnetite | 80 | 20 min | Water | 13 | 74 | Hyperthermia | (de Mello et al., 2019) |
| FeCl3, FeSO4·7H2O | Egg shell | Magnetite | 40 | 2 h | Water | 24 | 14.46 | Cr removal | (Sundararaman, 2020) |
| Fe(NO3)3·9H2O | Ho(NO3)3·5H2O | Hematite | 78.3 | 60 min | Ethanol | 25–30 | 0.71 | Catalyst | (Nguyen et al., 2020) |
| FeCl3 | SnCl2·2H2O | Magnetite | 100 | 30 min | Ethylene glycol | 4.85 | 49.7 | NA | (Radoń et al., 2020) |
| FeCl3·6H2O | NiCl2·6H2O/ZnCl2 | Ferrite | NA | 15 min | Water | NA | NA | NA | (Yi et al., 2019) |
| FeCl3·6H2O | ZnCl2 | Ferrite | 80 | 40–60 min | Water | 19 | 12 | Hyperthermia | (Ait Kerroum et al., 478 (2019)) |
| Fe2(SO4)3·5H2O/FeSO4·7H2O | Ce4+, Co2+, Mn2+, Ni2+ | Magnetite | 70 | 30 min | Water | 78.42 | 10.39 | Photocatalyst | (Andrade Neto et al., 242 (2020)) |
| FeCl3, FeCl2 | Sucrose | Magnetite | NA | NA | Water | 4 | 17 | NA | (Jesus et al., 2020) |
| FeSO4·7H2O, FeCl3·6H2O | Malic acid | Magnetite | RT | 15 min | Water | 10–50 | NA | NA | (Klencsár et al., 2019) |
| FeCl2·4H2O, FeCl3·6H2O | Cellulose | Hematite | 30 | 2.5 h | Water | 5–100 | 13.2 | As removal | (Yu et al., 2013) |
| FeCl3·6H2O | NiCl2·6H2O/ZnCl2 | Ferrite | NA | 15 min | Water | NA | 148.1 | NA | (Peng et al., 2017) |
| FeSO4, FeCl3·6H2O | Biopolymer | Magnetite | 70 | 25 min | Water | 498 | ∼30 | Adsorbent | (Lassalle et al., 2011) |
| FeCl3 | CoCl2, porous carbon | Ferrite | RT | 6 h | Water | NA | 9.875 | Phosphate removal | (Karthikeyan et al., 2020) |
| Fe(NO3)3·9H2O | Zn(NO3)2·6H2O, TiO2 | Ferrite | 90 | 2 h | Water | 11 | NA | Photocatalyst | (Chandrika et al., 2019) |
| FeCl3·6H2O, FeCl2·4H2O | Polydextrose sorbitol carboxyl methyl ether | Magnetite | 70 | 40 min | Water | 12.5 | 69.2 | NA | (Li et al., 2017) |
Mello et al. (2019) synthesized the Zn-Mn-doped magnetite nanoparticles by co-precipitation method with the FeCl3 and FeSO4·7H2O as the precursors with the average of particle size of 10–15 nm (de Mello et al., 2019). The authors reported that the type of precursors is a mainly factor in the synthesis of magnetite nanoparticles by co-precipitation method. In addition, the several factors that have affected the particle size of the prepared nanoparticles by this method are operating temperature, the absence or presence of the stabilizing agent, reaction time, and the pH (Mascolo et al., 2013). The doping with other metals like Zn2+ and Mn2+ can improve the magnetization properties compared to pure magnetite. In their research, the addition of Zn2+ and Mn2+ can change the crystal structure which affects the electron migration in the structure. The doping system has been widely reported that it can improve intrinsic properties, especially its permanent magnetic properties. For example, Li et al. (2019) reported that intrinsic properties of the microstructure of nanocomposites (La, Pr)3Fe14B is dependent on La content. The doping system with rare earth metal could improve the permanent magnetic properties (Li et al., 2019). Rare earth metal has an electron in orbital 4f that close with atomic core and show spin–orbit solid interaction. Therefore, the metal has been widely exploited as doping for producing permanent magnetic materials (Skomski et al., 2006).
Yu et al. (2013) prepared the magnetic composites of cellulose/iron oxide nanoparticles in the NaOH-thiourea-urea-H2O at operating temperature of 30 °C (Yu et al., 2013). The solution is used to increase the dissolution of cellulose chains in the preparing nanocomposites. The presence of magnetite nanoparticles can increase the specific of surface area until 100-fold with the average particle size of 61 nm in the cellulose matrix. Although, the cellulose is non-conductive polymer, however the nanocomposite shows the sensitive-magnetic induced with the magnetization value of 13.2 emu g−1. In their research, the synthesized nanocomposite has been applied for removing the heavy metal ions. With the presence of cellulose in the composite structure, the material exhibited a high adsorption capacity for As3+ due to the presence of functional group like hydroxyl (OH−). In other words, beside the addition of organic material for arranging the size, several studies also have reported that the shape and particle size of iron-based nanoparticles in the matrix composites can be controlled by adjusting ionic strength, pH, temperature, and type of salts (nitrates, acetates, chlorides, and sulfates) (Gnanaprakash et al., 2007; Yang et al., 2012). However, the ratio of precursors salts is a major factor to produce the high percentage of yields and desirable size of nanoparticles (Ramimoghadam et al., 2014). In fact, the previous studies have revealed that the increasing ratio of Fe3+/2+ can produce the larger particle size of nanoparticles. In other words, based on the previous research, the smaller particle size will be obtained under high pH conditions and ionic strength (Tartaj et al., 2004). Besides that, another study also reported and revealed that the temperature significantly increased the particle size of iron oxide nanoparticle due to the formation of goethite phase (Gnanaprakash et al., 2007). The co-precipitation method offers a high quantity of products; however, the produced material has not uniform size particle distribution (Liu et al., 2019).
2.2 Chemical vapor deposition (CVD)
Chemical vapor deposition (CVD) has proved that the method can produce the iron oxide nanocomposites with high performance as a solid material, produced one-dimensional nanomaterials, and high purity. This method is commonly used in a chamber with a reactive gas which can deposit on a substrate to produce a coating material. Moreover, this method offers high flexibility in producing material because of many databases reported (Majidi et al., 2016). Atchudan et al. (2019) reported a simple method for synthesis of iron oxide nanoparticles filled multi wall carbon nanotubes (IONP/MWCNTs) by CVD method (Atchudan et al., 2019). The results confirmed that the IONPs inside in the MWCNTs which have the narrow distribution of diameter at 9 nm. The authors reported that the purified materials could be done by washing the produced material with a simple acid etching method, while the other methods that have been reported the purified materials commonly used the mixture of nitric acid and hydrochloric acid. Chen et al. (2020) studied the Fe3C/N-doped carbon nanofibers nanocomposites by CVD as lithium storage anode. In this method, the nanocomposites was prepared at low annealing temperature with Fe3O4 as iron source and acetylene as carbon source (Chen et al., 2020). The microstructure and morphology of the prepared material showed that the material has ununiform diameter at 50–60 nm. Their studies proved that the diameter of nanomaterials can be controlled by the addition of some organic compounds like surfactant or hydrophilic/hydrophobic molecules when the synthesis process. The presence of organic molecules like surfactants plays an important role in the controlling of particle size on the dispersion and stability of solution (Ordóñez et al., 2020). However, the high modifier on the composites also can decrease the pore volume of the prepared materials due to the agglomeration process. Fig. 1 shows the illustration of the synthesis of iron-based nanocomposite materials.
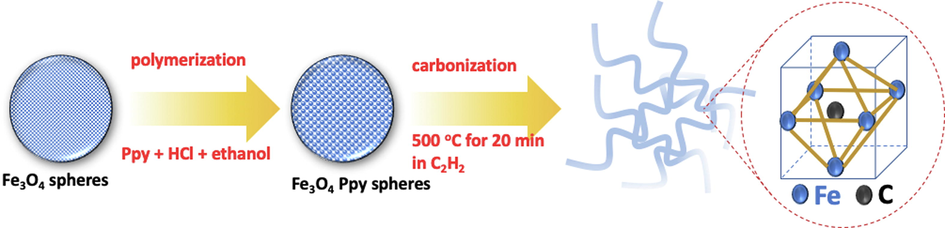
- The representative of the synthesis of iron-based nanocomposite materials using the organic molecules as modifier (Chen et al., 2020).
Ren et al. (2018) reported the synthesis of amorphous iron (III) phosphate/carbon nanotubes (FePO4/CNTs) at low processing temperature (∼300 °C) and low vacuum conditions (4–8 Torr) (Ren et al., 2018). The authors proved that the temperature is an essential factor for the decomposition of precursors on the substrate. At temperature less than 250 °C, the precursors did not decompose and slowly deposition on the substrate. Besides the temperatures, the other factors that have affected the synthesis process by CVD such as the processing time, the gas flow rate, the catalyst, and also the precursors (Lopez et al., 2015). However, recently, the CVD is probably the most versatile synthesis method for producing iron-based nanocomposites because this method offers several advantages such as cheap and simple method for mass production of nanomaterials, and it can be used to grow the different forms of nanostructure. Table 3 shows the list of some commonly used magnetic source for producing iron-based nanocomposites by CVD methods (Shah and Tali, 2016).
| Magnetic source | Modifier | Type of iron oxide | Reaction temperature (oC) | Reaction period | General Remarks | Particle size (nm) | Magnetic saturation value (emu. g−1) | Application | Ref. |
|---|---|---|---|---|---|---|---|---|---|
| Fe(CO)5 | Alloy (Na, Ga, B) | NA | 120 | 30 min | Ar atmosphere | 50–100 | NA | NA | (Jun et al., 2013) |
| Fe(NO3)3·9H2O | CNTs | Goethite | 700 | 30 min | Ar and CH4 atmosphere | 40–60 | 13.79 | Pb removal | (Alijani et al., 2014) |
| Fe(acac)3 | MWCNTs | Magnetite | 800 | 30 min | Ar and C2H2 atmosphere | NA | NA | Capacitors | (Atchudan et al., 2019) |
| Fe pure | 3-triethoxysilypropylamine, paracyclophane dimer | NA | 650 | NA | Vacuum atmosphere | 65 | 125.8 | Coating layer | (Zhang et al., 2020) |
| Fe3(CO)12 | Fe/Fe oxides/metal oxide | Magnetite | 450 | 40 min | Preassure at 10−3/−4 Pa without using any carrier gas | 3 | NA | NA | (Vangelista et al., 2012) |
| Ni0.5Zn0.5Fe2O4 | MWCNTs | Hematite | 750 | 30 min | Ar atmosphere | ∼50 | NA | MW absorber | (Mustaffa et al., 2019) |
| Fe(hfa)2 TMEDA (hfa = 1,1,1,5,5,5-hexafluoro-2,4-pentanedionate; TMEDA = N,N,N’,N’–tetramethylethylenediamine) | Au metal | Ferrite | 400 | 60 min | Dry O2 (P = 3.0 mbar)end | 60 | NA | NA | (Maccato et al., 2018) |
| FeCl3·6H2O | CNFs/Polypyrole | Magnetite | 500 | 20 min | C2H2 atmosphere | 50–60 | NA | Energy storage | (Chen et al., 2020) |
| FeCl3 | Carbon | Magnetite | 450 | 10–30 min | C2H2 atmosphere and Ar carry gas | <100 nm | NA | Energy storage | (Wang et al., 2015) |
| Fe(NO3)3·9H2O | Co2+ | Ferrite | 700 | 30 min | C2H2 atmosphere and N2 carry gas | 500 | 2.61 | Catalyst | (Dhand et al., 2017) |
2.3 Electrochemical synthesis
Electrochemical method has been widely used for generating synthesis of iron-based nanocomposites with different phases of iron oxide like magnetite and maghemite as shown in Table 4. In general, electrochemical synthesis can be done by passing an electric current between two or more electrodes called anodes and cathodes on the electrolyte solution medium. In this technique, the anode metal will be oxidized into its ion species and then the ion will be reduced by the cathode to metal with the presence of stabilizers. In fact, electrochemical synthesis occurs at the interface of electrolyte and electrode and form electric double layers. This method can highly produce a large percent yield of products compared to the chemical synthesis routes. Ansari et al. (2020) studied the synthesis of iron/graphene composites with electrochemical exfoliation using a surfactant as stabilizers to produce nanoparticles (Ansari et al., 2020). The authors presented that the surfactant is a major component in the synthesis of iron oxide nanocomposites because it can control the stabilization of the iron species in the electrolyte solution and can reduce their oxidization by the dissolved oxygen. However, the increasing concentration of surfactants can reduce the magnetization properties of the composites by forming a layer on the surface of the nanoparticles which causes the agglomeration process (Haramagatti et al., 2018). Besides, the presence of surfactants as stabilizer, several factors affect the process of electrosynthesis of iron-based nanocomposites such as type of both electrodes (anode and cathode), the type and concentration of electrolyte solution, temperature, pH, and also electrolysis type (galvanostatic or potentiostatic) (Ramimoghadam et al., 2014). However, two key factors that influence the reaction process that occurs are the cell potential and the current applied which can be changed during the reaction as a function of time.
| Magnetic source | Modifier | Type of iron oxide | Reaction temperature (oC) | Reaction period | General Remarks | Particle size (nm) | Magnetic saturation value (emu. g−1) | Application | Ref. |
|---|---|---|---|---|---|---|---|---|---|
| Fe pure | NA | Magnetite | RT | 4 h | LiCl-C2H5OH solution, Potential at 0,4 V | 40 | 35.5 | NA | (Starowicz et al., 2011) |
| Fe metals | NA | Magnetite | RT | 10 min | ((CH3)4NCl) and NaCl in ethanol:water (1:1) | 8–13 | 86.85 | NA | (Marín et al., 2016) |
| (NH4)2Fe(SO4)2(H2O)6 | Graphene | Magnetite, maghemite | RT | 3 h | HCl-H2O solvent, Potential at 10 V, SDSs as surfactant | 20–30 | 57.3 | NA | (Ansari et al., 2020) |
| FeCl3·6H2O | Triethanolamine | Maghemite | RT | 2 h | NaOH solution | 450 | 84.40 | Supercapacitor | (Elrouby et al., 2017) |
| Sponge Iron | NA | NA | 30–50 | >10 h | Working electrode at 1 cm2, OH− solution | NA | NA | NA | (Sun et al., 2018) |
| Iron rods | Ethylene glycol | Magnetite, hematite | RT | 15 min | Ammonium fluoride solution, Potential at 50 V | NA | NA | Water splitting | (Lucas-Granados et al., 2018) |
| Iron metals | 1, 3, 5- benzenetricarboxylic acid (BTC) | NA | NA | NA | Potential at 12 V | 100–200 | NA | As removal | (Zhang et al., 2018) |
| FeSO4 | Polyvinyl alcohol, CNTs | Magnetite | RT | 3 h | The current density at 348 mA cm−2 | 33 | NA | Antibacterial | (Sadeghfar et al., 2018) |
| FeSO4·7H2O | MWCNTs | NA | NA | NA | Na2SO4 solution | 50 | NA | Catalyst | (Torabi and Sadrnezhaad, 2010) |
| Iron plate | Hexagonal mesoporous spheres (HMS) | NA | RT | 3 h | Na2S2O3 solution | >200 | 2.9 | NA | (Wang et al., 2007) |
Starowicz et al. (2011) revealed that the particle size of iron-based nanoparticles can be controlled by the type of electrolyte solution (Starowicz et al., 2011). In the water medium, the particle size of iron nanoparticles has an average size of nanoparticles between 20 and 40 nm, while at the presence of another solvent like ethanol, the particle size can be obtained less than 20 nm. The electrosynthesis of iron-based nanoparticles can be conducted in constant current (galvanostatic), at this condition the iron will be oxidized in anode electrode while in the cathode electrode will be produced the hydroxyl ion (OH−). Then, the iron ion produced will lead to form Fe(OH)2 which will be converted to iron oxide through the Schikorr reaction 3Fe(OH)2 → Fe3O4 + 2H2O + H2. Based on the principle, the water medium plays an important role to generate the production of iron hydroxyl species. However, in the pure water medium, the process will occur at a high rate so that the presence of ethanol in the water can regulate the water concentration and reduce the formation rates which can produce smaller particle sizes (Karimzadeh et al., 2016). The electrochemical method offers some advantages in iron oxide synthesis such as easily controlling size distribution and high production rate; however, this method is difficult to control the high crystallinity of the produced material (Setyawan and Widiyastuti, 2019). Fig. 2 shows the illustration of electrochemical cell for synthesis of iron-based nanoparticles.
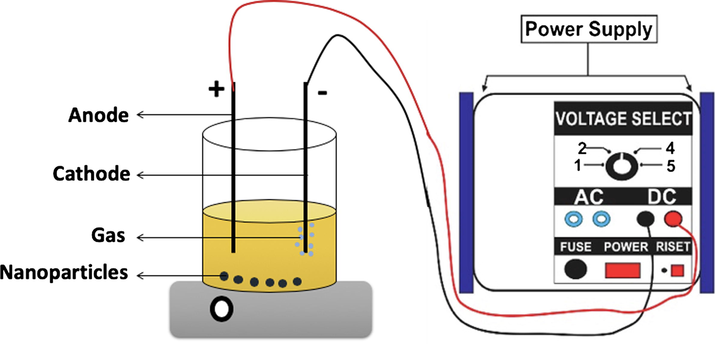
- The illustration of electrochemical synthesis of iron-based nanoparticles.
2.4 Solvothermal method
Synthesis of magnetic nanomaterials, classifications, and its applications by solvothermal method have been presented in the literature Table 5. The solvothermal method is one of the well-known techniques in the fabrication of magnetite materials. This method is almost similar to the hydrothermal method in the synthesis procedure, except the use of water as a solvent is replaced by an organic solvent (Feng et al., 2017). The solvothermal method is sometimes referred to the alcohothermal and glycothermal method when the class of alcohol and glycerol are used as a solvent in the reaction (Erdemi and Baykal, 2015). In fact, there have been many experiments using both classes of solvents, where this is an important strategy to control the shape, phase, and size distribution of crystal in magnetite materials. The physical properties of magnetite materials can be adjusted using several parameters such as the type of precursor, solvent, temperature and reaction time (Kefeni et al., 2017). In the one-step supercritical synthesis of magnetic iron nanoparticles, pressure and temperature conditions facilitate the product crystallization process through the dissolution and diffusion of iron salts. The reaction mechanisms are naturally highly system dependent. The aqueous solution of the iron salt is brought to its subcritical temperature, and the low dielectric constant will instantly force the nanoparticles to precipitate so that nucleation occurs instantaneously. Then tiny and homogeneous iron particles with a very narrow size distribution obtained from the simultaneous deposition of numerous iron nuclei (Nunes et al., 2019).
| Magnetic Source | Modifier | Solvent | Reaction Temperature (oC) | Reaction Period | Particle Size (nm) | Magnetic Saturation Value (emu.g−1) | Iron Form | Application | Classification | Ref. |
|---|---|---|---|---|---|---|---|---|---|---|
| Fe(acac)3 | Cu2+ | Triethylene glycol | 260 | 24 h | 19.9 | 53.1 | Magnetite | Magnetic fluid hyperthermia | Metal Oxide-based Material | (Fotukian et al., 2020) |
| FeCl3·6H2O | Mn2+, Carbonyl Iron (CI) | Ethylene glycol, Silicone Oil | 200 | 12 h | 21.4 | 58.8 | Magnetite | MR Fluids (Smart Material) | Metal Oxide-based Material | (Wang et al., 2020) |
| FeCl3·6H2O | Trisodium Citrate (TSC) Dihydrate | Ethylene glycol | 200 | 10 h | 280 | 70.2 | Magnetite | Identification of glycopeptides in human saliva | Metal Oxide-based Material | (Chu et al., 2020) |
| FeSO4·7H2O | Zn2+, Mn2+, Bi3+, TEOS | Ethylene glycol | 180 | 5 h | 60 | 17.72 | Magnetite | Adsorption and photocatalytic degradation of dyes | Metal Oxide-based Material | (Kaewmanee et al., 2020) |
| FeCl3, and FeCl2 | Biomass-based Carbon, and Graphene | Water, Ethanol | 260 | 2 h | 5–20 | 14.28 | Magnetite and Maghemite | NA | Graphene-Mixed Carbon Material | (Siddiqui et al., 2020) |
| FeCl3·6H2O | Mn2+, Graphene Oxide (GO) | Ethylene glycol, PEG-2000 | 200 | 10 h | 30–100 | 25.5 | Magnetite | Photocatalytic degradation of dye | Reduced Graphene Oxide-Based Material | (Huang et al., 2019) |
| FeCl3·6H2O, FeCl3·4H2O | Graphene oxide (GO), chitosan | Ethylene glycol | 180 | 12 h | ∼ 250 | 46.5 | Magnetite and Hematite | Adsorption of aromatic compounds | Graphene-Mixed Carbon Material | (Rebekah et al., 2020) |
| FeCl3·6H2O | Mg2+, Al3+, Graphene oxide and C2H8N2 | Ethylene glycol | 200 | 8 h | 5–10 | ∼ 18 | Magnetite | Adsorption of metal ions | Graphene Oxide-Based Material | (Huang et al., 2018) |
| FeCl3·6H2O, FeSO4·7H2O | Ti4+, Graphene oxide | Isopropanol | 180 | 16 h | 100–500 | 2.74–8.89 | Magnetite | Photocatalytic ozonation | Graphene Oxide-Based Material | (Chávez et al., 2020) |
| Fe(C5H5)2 | N/A | Hydrogen Peroxide, Acetone | 200 | 48 h | 5.6–18.6 | 26.8 | Magnetite | Photocatalytic degradation | Carbon-Based Material | (Zhang et al., 2020) |
| C15H21FeO6, Co(NO3)2·6H2O | Spherical activated carbon (SAC) | Benzyl Alcohol, Methanol, C4H6N2 (Hmim) | 175 | 48 h | 200–400 | 8.2 | Magnetite | Catalyst for Knoevenagel condensation | Activated Carbon-Based Material | (Xiang et al., 2020) |
| FeCl3·6H2O | Mn2+, Glucose, Amine –NH2 | Ethylene glycol, polyethylene glycol | 200 | 12 h | 40 | 26.5 | Magnetite | Heterogeneous Fenton catalyst | Carbon-Based Material | (Qin et al., 2020) |
| Fe(C5H5)2 | PF-127, C10H6(OH)2 | Ethanol, Hydrogen Peroxide | 220 | 24 h | ∼306 | 5.7–26.38 | Magnetite | Adsorbent of organic pollutant | Carbon-Based Material | (Cui et al., 2020) |
| FeCl3·6H2O, FeSO4·7H2O | Biomass-based Carbon | Water | 230 | 24 h | ∼ 10–20 | 9.73 | Magnetite | Adsorption of Tetracycline | Activated Carbon-Based Material | (Rattanachueskul et al., 2017) |
| FeCl3·6H2O, FeSO4·7H2O | Biomass-based carbon | Water | 200 | 24 h | ∼ 300 | 15.58 | Hematite | Adsorption of Mercury | Activated Carbon-Based Material | (Wang et al., 2018) |
| FeCl3·6H2O, FeSO4·7H2O | Starch-based carbon | Water, NH4OH | 200 | 12 h | ∼ 2–10 | ∼ 130 | Magnetite | Biomedical application | Carbon-Based Material | (Lee et al., 2019) |
| FeCl3·6H2O | 3D carbon nanofiber, Dopamine hydrochloride | Ethylene Glycol, PEG-2000 | 200 | 8 h | ∼ 200–250 | 13.4–39.7 | Magnetite | Electromagnetic shielding | Carbon-Based Material | (Zhan et al., 2018) |
| FeCl3 | Biomass-based carbon | Diethylene Glycol (DEG) | 200 | 24 h | ∼ 10–11 | 11.9–22.7 | Magnetite | Adsorption and Fenton degradation of dye | Activated Carbon-Based Material | (Liu et al., 2017) |
| FeCl3·6H2O | MCNTs, SDBS | Ethylene Glycol | 180 | 8 h | 400 | ∼ 50–60 | Magnetite | Microwave adsorption and Supercapacitor | Carbon-Based Material | (Zeng et al., 2020) |
| FeCl3·6H2O | Ni2+, Carbon Paste, Graphite Powder, Paraffin oil | Ethanol, Ethylene Glycol, PEG | 160 | 7 h | 31 | 70–80 | Magnetite | Electrochemical sensor | Carbon-Based Material | (Tajyani and Babaei, 2018) |
| FeCl3·6H2O | Glucose, Ammonia | Ethylene Glycol, PEG | 200 | 6 h | 138–416 | 40 | Magnetite | Laccase immobilization | Carbon-Based Material | (Lin et al., 2017) |
| FeSO4·4H2O | C31H28O12 | Water | 160 | 8 h | ∼ 40–80 | ∼ 25 | Magnetite | Adsorption of heavy metal ions | Natural Polymer-Based Material | (Shi et al., 2020) |
| FeCl3·6H2O | Montmorillonite, Hexandiamine | Ethylene Glycol | 198 | 6 h | 30–50 | 15 | Magnetite | Adsorption of heavy metal ions | Clay-Based Material | (Irawan et al., 2019) |
| FeCl3·6H2O | Bentonite, C2H4(NH2)2 | Ethylene Glycol | 200 | 8 h | 10–50 | 15.1–37.4 | Magnetite | Adsorption of heavy metal ions | Clay-Based Material | (Yan et al., 2016) |
| FeCl3·6H2O, CoCl2·6H2O | Mn2+, Bentonite, APTES | Ethylene Glycol | 200 | 12 h | ∼ 110 | 7 | Magnetite | Adsorption of heavy metal ions | Clay-Based Material | (Zhou et al., 2019) |
| FeCl3·6H2O, FeSO4·7H2O | Halloysite, Carbon paste, Graphite powder, Paraffin oil | Water | 105 | 12 h | ∼ 30–50 | 25.1 | Magnetite | Electrochemical detection of Hg (II) | Clay-Based Material | (Fayazi et al., 2016) |
The solvothermal process of most common magnetic material is carried out through the process of hydrolysis and oxidation of iron salt solutions as a precursor or neutralization process of mixed metal hydroxides in aqueous media which can cause the formation of ferrite. During the solvothermal process, the chemical reaction between the precursor and the solvent takes place in a sealed autoclave reactor at very high temperature (ca. 130–250 °C) and autogenous pressure (ca. 0.3–4 MPa) (Sharifianjazi et al., 2020; Qiao et al., 2019). This condition can increase the solvent ability to dissolve the precursors and accelerate the formation of products.
For example, Fotukian et al. (2020) successfully synthesized of Fe3O4 and monodisperse CuFe2O4 with triethylene glycol as the solvent and stabilizer for the application of magnetic fluid hyperthermia through solvothermal process at 260 °C for 24 h. The obtained Fe3O4 and CuFe2O4 exhibited very small particles size with great superparamagnetic properties. Another experiment was conducted by Huang et al. (2019) to fabricate magnetically separable reduced graphene oxide supported MnFe2O4 hybrids (rGO/MnFe2O4) by one-pot solvothermal synthesis for photocatalytic degradation of dye. The results show that the rGO nanosheets was embedded by monodisperse MnFe2O4 with uniform particles size. In the general procedure, the appropriate proportions of precursors and solvents are inserted into the sealed autoclave reactor and then placed in the oven at a predetermined temperature and time as shown in Figs. 3 and 4. Therefore, the solvothermal method can be used to control the shape and size of nanoparticles, also to improve physical and chemical properties of magnetic materials which can be utilized for industrial and biomedical applications. Some of the significant advantages of hydro/solvothermal synthesis are the use of appropriate eco-friendly solvents for the nucleation of homogeneous particles, the chemical activity of the reactant improve, intermediate and metastable products may be quickly produced, easy and precise control of the size, shape distribution, and crystallinity of the final product. While some of the disadvantages of this method are the need for quite an expensive autoclave, safety issues during the reaction process, and the impossibility of observing the reaction process (Rane et al., 2019).
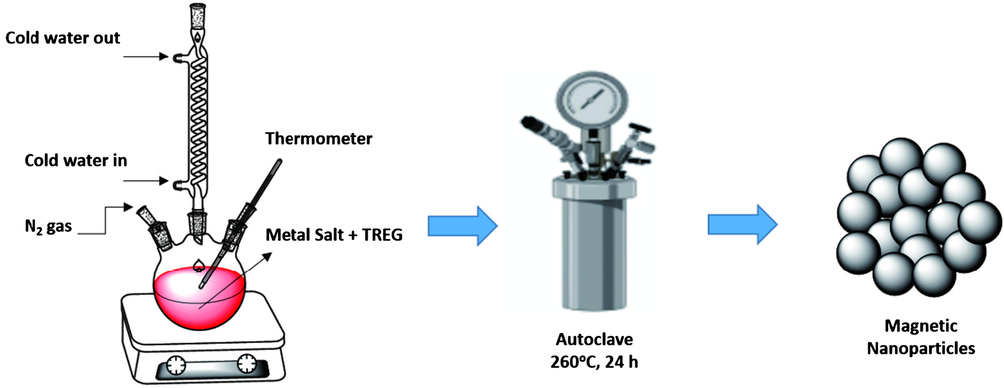
- The synthesis of Fe3O4 and monodisperse CuFe2O4 through solvothermal method by Fotukian et al. (2020).
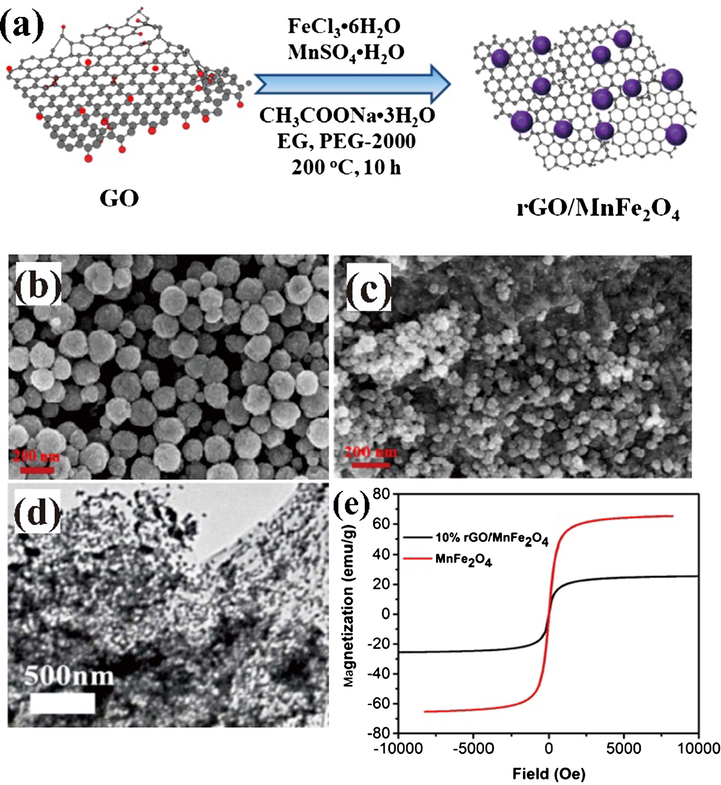
-
(a) A synthesis model of magnetically separable reduced graphene oxide supported MnFe2O4 hybrids (rGO/MnFe2O4) by one-pot solvothermal synthesis by Huang et al. (2019); SEM images: (b) pure MnFe2O4 and (c) 10% rGO/MnFe2O4; TEM image of (d) 10% rGO/MnFe2O4 and (e) Hysteresis loops comparison of pure MnFe2O4 and 10% rGO/MnFe2O4.
2.5 Microemulsion
Synthesis of magnetic nanomaterials, classifications and its applications by microemulsion method have been presented in the literature Table 6. Microemulsion is a stable colloidal suspension in two phases which do not dissolve each other and combine into one phase with the help of surfactants (such as Tween 80, sodium dodecyl sulphate, brij, etc.). The microemulsion solvent can be either the water/oil (w/o) or oil/water (o/w) system (Qiao et al., 2019). While magnetic nanoparticle precursors are dispersed into aqueous phase with the size of 1–100 nm. The microemulsion mechanism begins with the formation of a micelle system as a nanoreactor caused by water droplets containing magnetic nanoparticles encircled by surfactant molecules. Nucleation, growth and agglomeration of magnetic nanoparticles are restricted by micelle layers. After that, the second emulsion is added to the solution to precipitate magnetic nanoparticles (Sharifianjazi et al., 2020).
| Magnetic Source | Modifier | Solvent | Reaction Temperature (oC) | Reaction Period | Particle Size (nm) | Magnetic Saturation Value (emu.g−1) | Iron Form | Application | Classification | Ref. |
|---|---|---|---|---|---|---|---|---|---|---|
| FeCl3, FeCl2 | Mn2+, Zn2+, S2−, SDS and TEOS | Water, Toluene, Isopropanol | 60 | NA | 45 | 31.7 | Magnetite | Drug delivery system | Metal Oxide-based Material | (Dung et al., 2016) |
| K4[Fe(CN)6] | NaOH, Hydrazine | Water, PEG-2000, Triton X-100, Amyl alcohol, Cyclohexane | 180 | 20 h | 200–600 | NA | Magnetite | Lithium-Air battery | Metal Oxide-based Material | (Lv et al., 2015) |
| FeCl3 | TEOS, SDBS, Pyrrole (Py), Trisodium citrate (TSC) Dihydrate | Water, Ethanol, Ethylene Glycol | 4 | 12 h | 110–240 | 0.02–0.48 | Magnetite | Microwave absorber | Polymer-based Material | (Liu et al., 2019) |
| Fe(acac)3 | Oleic acid, Oleylamine, CTAB, Urea | Water, Chloroform, Cyclohexane, Dibenzylether, Butanol | 120 | 12 h | 16.4 | 4.8 | Magnetite | Drug delivery system | Silica-based Material | (Asgari et al., 2019) |
| FeCl3·6H2O | Sr2+, CTAB, TEOS | Water, Butanol, Isooctane | RT and 1050 | 24 h and 4 h | 6.6 | 18.4 | ε-Fe2O3, Magnetite, Hematite | NA | Silica-based Material | (Nikolic et al., 2017) |
| FeCl3, FeCl2·4H2O | Ta5+, PVP, Dipicolinic acid | Water, Hexane | 25 | 15 min | 38 | 6.58 | Magnetite | Lipase immobilization | MOF-based Material | (Sargazi et al., 2018) |
| FeCl3·6H2O | Oleic acid and Sodium oleate | Water, Ethanol, Hexane, Octadecene | 20 and 320 | 30 min and 24 h | ∼ 8–18 | ∼ 6–21 | Magnetite | Magnetic fluid fields | Metal Oxide-based Material | (Yu et al., 2018) |
| FeCl3·6H2O, (NH4)2Fe(SO4)2·6H2O | Fatty Acids (C6-C11) | Water, Acetone | 80 | 30 min | 8–12 | NA | Magnetite | Adsorption of PAHs compound | Metal Oxide-based Material | (Liao et al., 2015) |
| FeCl3·6H2O | Ca2+, Mg2+, Brij-35 | Water, Hexane, Butanol | 500–900 | 180 min | 40–100 | ∼ 6–9.5 | Magnetite | Drug delivery system | Calcium-based Material | (Foroughi et al., 2016) |
| NiCl2·6H2O, FeCl2·4H2O | CTAB, Hydrazine | Water, Hexanol, Octane, Butanol | 70 | 60 min | ∼ 7 | 11.4 | Magnetite | NA | Metal Oxide-based Material | (Beygi and Babakhani, 2017) |
| NH4Fe(SO4)2·12H2O, (NH4)2Fe(SO4)2·6H2O | Ag3+, CTAB, Glucose | Water | 50 | 24 h | ∼ 9–11 | 53 | Magnetite | NA | Metal Oxide-based Material | (Singh and Upadhyay, 2018) |
| FeCl3·6H2O, FeSO4·7H2O | TMAAC, Sodium citrate, Chitosan | Water, Atolin, Span-80, Tween-80 | 60 | 60 min | <10 | 21.57 | Magnetite | Adsorption of dye | Polymer-based Material | (Yu et al., 2016) |
| Fe(acac)3 | TEOS, CTAB, Oleic acid | Water, Benzyl ether, Choloroform, Ethanol | 70 | 30 min | 220–260 | 39.7 | Magnetite | Adsorption of metal ion | Silica-based Material | (Meng et al., 2018) |
| FeCl3·6H2O, FeCl2·4H2O | ω-TA, Benzoyl peroxide, PVA, DTAC, Glutaraldehyde | Water, Butanol, Ethanol | 70 | 7 h | 30–40 | 47.84 | Magnetite | Biocatalyst | Polymer-based Material | (Jia et al., 2016) |
For example, the synthesis process of core–shell Fe3O4@SiO2 nanoparticles using the reverse microemulsion method. In this method, one or two surfactants become stabilizers of the hydrolysis reaction and condensation in water droplets that contain magnetic material precursors that are well dispersed in the organic phase. Therefore, the aggregation process can be prevented when the formation of silica layers on the magnetic surface of nanoparticles happens (Asgari et al., 2019). Lv et al. (2015) revealed the novel synthesis of Fe3O4 particles pagoda-like microstructures using the microemulsion -assisted hydrothermal method for the application of Lithium-air batteries. The experimental results show that the increase in reaction time is very influential on the morphological evolution of samples from pagoda-like to flower- like shapes. In addition, the concentration of NaOH and polyethylene glycol (PEG)-2000 has a major role in the morphology of the final product.
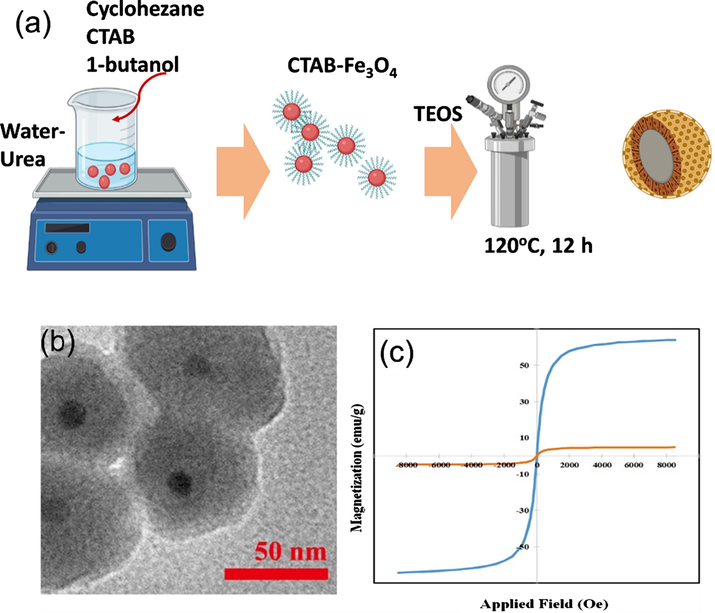
Asgari et al. (2019) synthesized the core–shell structure of monodisperse magnetic mesoporous silica nanoparticles (MMSN) via inverse microemulsion method for anticancer drug carriers. In that study, Fe3O4 in urea solution acts as a water phase, silica precursors in cyclohexane as an oil phase, as well as cetyltrimethylammonium bromide (CTAB) and 1-butanol as surfactants and co-surfactants, respectively. This method is different from the conventional inverse microemulsion method because the magnetic precursors used will be coated by CTAB and oleic acid as surfactants in the core-phase, then silica formation in shell-phase. The experimental results reveal that the increase in reaction temperature from 70 to 120 °C affects the increase in the thickness of the silica layer as the shell-phase from 3 to 17 nm.
Another experiment by Sargazi et al. (2018) exhibited the synthesis of of Ta-MOF@Fe3O4 core/shell nanostructures through novel ultrasound-assisted reverse micelle (UARM) method in optimum conditions for a novel candidate for enzyme immobilization. The obtained product from this experiment has the particle size distribution of 38 nm, thermal stability at 200 °C and a large surface area. The SEM image of Ta-MOF@Fe3O4 core/shell reveals that the targeted enzyme is stably and efficiently loaded on the material substrate. Therefore, the method used and the obtained results can be developed for the application of other compounds in biology.
Some of the microemulsion synthesis methods' advantages are easy preparation, effectively control of nucleation, preventing agglomeration, low viscosity, low energy consumption, thermodynamic stability, and reversible microemulsion formation. While the drawbacks of the microemulsion synthesis are the use of a large number of surfactants, pH and temperature affect the stability of the microemulsion, the limited dissolving capacity for substances with high melting points, and has low crystallinity due to slow nucleation rate at low temperature resulting in low yields (Kefeni et al., 2017; Sharifianjazi et al., 2020; Simonazzi et al., 2018). The synthesis schematic of several magnetic materials via the microemulsion method can be seen in Figs. 5–7.
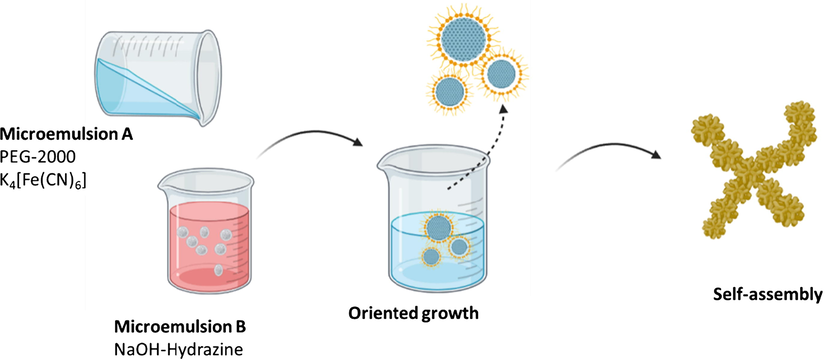
- Schematic representation of the self-assembly in Fe3O4 particles synthesis (adapted from Lv et al., 2015).
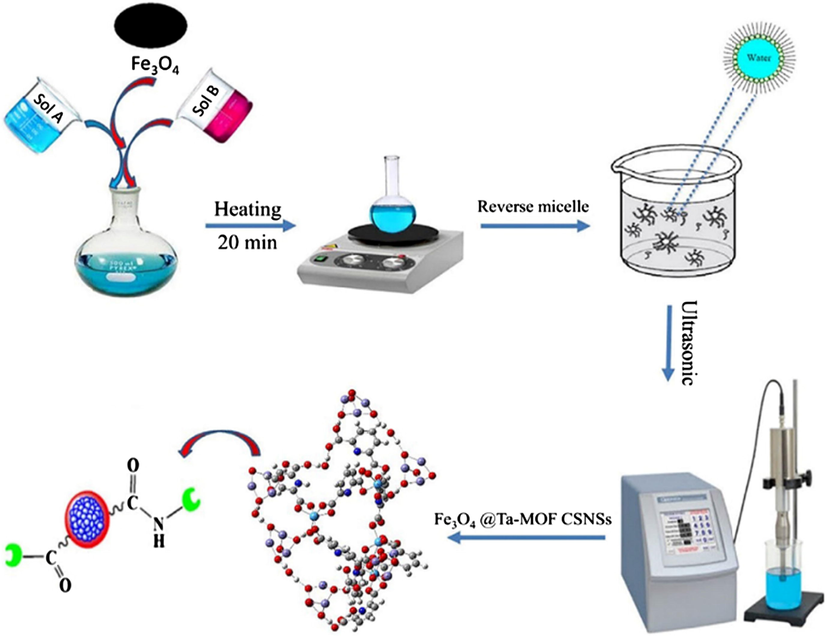
- The synthesis of Ta-MOF@Fe3O4 nanostructures via ultrasound assisted reverse micelle (UARM) by Sargazi et al. (2018).
-
(a) The synthesis route of Fe3O4@mSiO2 nanoparticles via modified inverse microemulsion technique by Asgari et al. (2019); (b) TEM image of naked Fe3O4 nanoparticles; (c) Hysteresis loops of Fe3O4@oleic acid (blue) and Fe3O4@mSiO2 (orange).
2.6 Microwave-assisted synthesis
Synthesis of magnetic nanomaterials, classifications and its applications by microwave-assisted method have been presented in the literature Table 7. At present, synthesis of multipurpose material has widely used microwave-assisted heating methods to increase the reaction rate and good control of the magnetic nanoparticles formation (Kefeni et al., 2017). In the synthesis of magnetic nanoparticles by microwave-assisted method, electromagnetic irradiation occurs through ionic conduction and molecular motion of precursor solvents and reducing agents. Thermal energy will be generated from this process as the conversion form from electromagnetic energy (Bolade et al., 2020). Generally, the sample irradiation process exerts the temperature of 100–200 °C with fast reaction time (Gonzalez-Moragas et al., 2015).
| Magnetic Source | Modifier | Solvent | Reaction Temperature (oC) | Microwave Power (Watt) | Reaction Period | Particle Size (nm) | Magnetic Saturation Value (emu.g−1) | Iron Form | Application | Classification | Ref. |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Fe(acac)3 | Oleic acid | Benzyl alcohol | 205 | 800 | 30 min | 5–7 | 97–98 | Magnetite | Magnetic fluid hyperthermia | Metal Oxide-based Material | (Kostyukhin and Kustov, 2018) |
| FeCl3, FeCl2 | Sm3+ | Ethylene Glycol, PEG | 200 | 300 | 180 min | ∼ 4.5 | 33 | Maghemite | Biomedicine | Metal Oxide-based Material | (Lastovina et al., 2018) |
| FeCl3·6H2O, FeCl2·4H2O | Zn2+, Ag+, Br− | Ethanol, Water | NA | 550 | 10 min | ∼ 200 | 3.31 | Magnetite | Antifungal | Metal Oxide-based Material | (Hoseinzadeh et al., 2016) |
| Fe(acac)3 | Trisodium citrate dihydrate | Benzyl alcohol | 180–210 | 300–500 | ∼ 8 min | ∼ 4–6 | 62 | Hematite and Maghemite | NA | Metal Oxide-based Material | (Gonzalez-Moragas et al., 2015) |
| Fe(NO3)3·9H2O | Citric acid | Water | NA | 750 | 2 min | 25–30 | 28.8–86.5 | Magnetite | NA | Metal Oxide-based Material | (Radpour et al., 2017) |
| Fe(acac)3 | Oleic acid, Oleylamine | Ethylene Glycol, Isooctane | 230–300 | 850 | 15–30 min | 4, 20.50 and 200 | 42–95 | Magnetite | NA | Metal Oxide-based Material | (Liang et al., 2017) |
| Fe2(SO4)3 | Sodium acetate | Ethylene Glycol, PEG | 200 | 300 | 10 min | 52 | 58 | Magnetite | Hyperthermia application | Metal Oxide-based Material | (Sathya et al., 2017) |
| FeCl3·6H2O, FeCl2·4H2O | Zr4+ | Water | RT | 100–900 | 100 min | 35 | ∼ 2 | Magnetite | Antimicrobial in dental filler | Metal Oxide-based Material | (Imran et al., 2019) |
| Fe(acac)3 | Pectin, β-isopropylglutaric acid | Water, Acetic acid, Oleic acid, Oleylamine | 185 | 100 | 30 min | 4.3 | 50.1 | Magnetite | Adsorption of dye | Metal Oxide-based Material | (Rakhshaee and Noorani, 2017) |
| Fe(acac)3, Co(acac)3 | NA | Ethanol | 150 | 800 | 180 min | 10 | NA | Magnetite | Acetone sensor | Metal Oxide-based Material | (Zhang et al., 2019) |
| FeCl3·6H2O | Zn2+, TEOS, Trisodium citrate dihydrate | Water, Ethylene Glycol, Diethylene Glycol (DEG) | 160 | NA | 15–60 min | ∼ 500 | ∼ 18–80 | Magnetite | Photocatalytic degradation | Metal Oxide-based Material | (Liu et al., 2019) |
| Fe(C5H5)2, FeCl3 | NA | Ethanol, Acetonitrile | NA | 800 | 1.5 min | 5–10 | 30.4 | Magnetite | NA | Carbon-based Material | (Kumar et al., 2017) |
| FeCl3·6H2O | Graphene Oxide, Oleic acid | Benzyl alcohol | 176 | 300 | 10 min | 2–8 | ∼ 5–15 | Magnetite and Maghemite | NA | Reduced Graphene Oxide-Based Material | (Bertran et al., 2020) |
| Fe(C5H5)2 | Graphite Oxide Powder | Ethanol | NA | 700 | 1.75 min | ≤ 400 | NA | Magnetite | High performance Lithium batteries | Reduced Graphene Oxide-Based Material | (Kumar et al., 2018) |
| FeCl3·6H2O | NaAc, H3BTC | Water, Ethylene Glycol | 150 | 300 | 30 min | ∼ 200 | 15.1 | Magnetite | Adsorbent and Photocatalyst | MOFs-Based Material | (Li et al., 2019) |
| FeCl3·6H2O, FeCl2·4H2O | SDS, DVB, Acrylic Acid, KPS and PVP40 | Water, Ethylene Glycol, PEG-200 | 200 and 80 | 900 | 30 min | 25–33 | 22.5 | Magnetite | Biomedical, catalysis and Magnetic sensor | Polymer-Based Material | (Jaiswal et al., 2018) |
| FeCl3·6H2O | Mg2+, TEOS, Ammonia chloride | Water, Ethanol, Ethylene Glycol, PEG200 | 160 | NA | 30 min | ∼ 615 | 66.5 | Magnetite | Adsorption of heavy metal ions | Silica-Based Material | (Zhao et al., 2017) |
| FeCl3, FeCl2 | HCl, NaOH, Sodium cilicate, APTES | Water | 80 | 1400 | ∼ 12 min | ∼ 4–9 | NA | Magnetite | Adsorption of heavy metal ions | Silica-Based Material | (Mahmoud et al., 2016) |
Compared to conventional heating methods, microwave-assisted synthesis is based on efficient heating of the sample by dielectric heating effect. Dielectric heating in the sample works by two main mechanisms, namely dipolar polarization and ion conduction which will induce electrons in the sample, and then convert them into kinetic energy which is ultimately converted into heat (Morsali et al., 2020). Furthermore, the major advantages of microwave-assisted synthesis are increased uniform reaction rate, high reproducibility, high yield and purity of product, lower processing cost, small and narrow particle size distribution, and energy saving compared to other conventional approaches. While this method has major limitations, namely the type of vessel/reactor used and in-situ temperature measurement (Gupta et al., 2018). Mahmoud et al. (2016) synthesized a functionalized nanomagnetic iron oxide with 3-aminopropyltriethoxysilane [Nano-Fe3O4–SiO2–NH2] through microwave-assisted heating method for heavy metals ions extraction from aqueous solution. The results of microwave-assisted adsorption revealed that the maximum adsorption capacity of Cu (II), Cd (II) and Hg (II) was 1050, 350 and 350 µmol.g−1 after 25, 15 and 25 s heating periods, respectively. In addition, the microwave-assisted adsorption method exhibited the excellent performance in the process of extracting heavy metal ions in aqueous solution.
Jaiswal et al. (2018) reported the rapid synthesis of highly crystalline superparamagnetic Fe3O4 nanoparticles via microwave-assisted method. The obtained Fe3O4 nanoparticles have the crystallite size of 25 to 33 nm. Furthermore, the synthesis of spherical polymers magnetic composite (PMCs) from PVP-stabilized Fe3O4, is carried out through microwave-irradiated emulsion polymerization using vinyl monomers. The characterization results indicated that the superparamagnetic Fe3O4 nanoparticles were integrated in a cross-linked polymer matrix (styrene–divinylbenzene-acrylic acid). The obtained Fe3O4 nanoparticles revealed superparamagnetic properties at 300 K with the saturation magnetization of 67.5 emu.g−1. Whereas the synthesized responsive magnetic PMC [Fe3O4/P(St-DVB-AA)] showed ferromagnetic properties at temperature intervals of 10–350 K which were different from the synthesized Fe3O4.
While Kumar et al. (2017) exhibited the large-scale synthesis of octahedral iron oxide nanocrystals (Fe3O4-ONCs) embedded on the reduced graphene oxide nanosheets (rGO NSs), (Fe3O4-ONCs@rGO hybrids) via microwave-assisted method. During the synthesis process, rGO NSs provide a structural board to implant positively charged Fe3O4-ONCs using the electrostatic assembly followed by the microwave reduction of rGO NSs. Experimental results indicated that Fe3O4-ONCs@rGO hybrids shows superior electrochemical performance better than Fe3O4-NPs@rGO, including better cycling stability and performance value, which may be affected by the embedded nano-size Fe3O4-ONCs in rGO NSs. Furthermore, this low-cost and fast synthesis method can be used in other structured hybrids for high-performance lithium batteries. The synthesis schematic of several magnetic materials via the microwave-assisted synthesis method can be seen in Figs. 8–10.
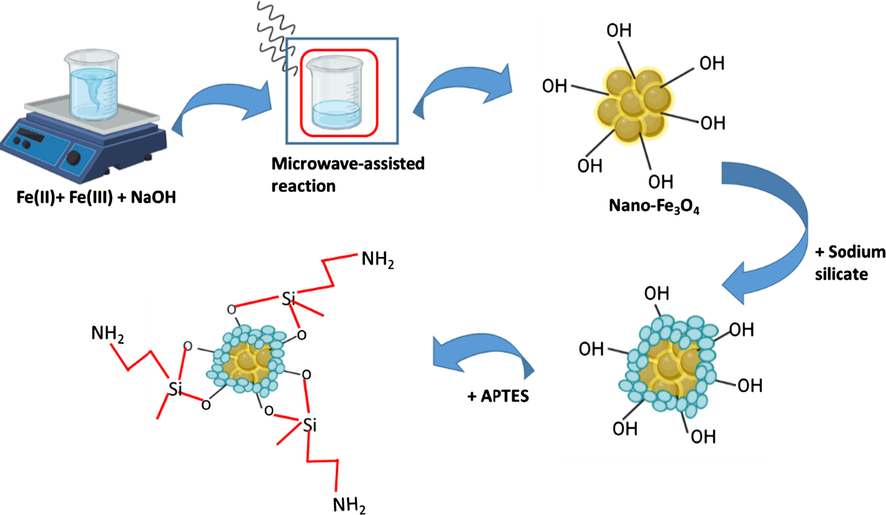
- Illustration of microwave-assisted synthesis of Nano-Fe3O4-SiO2-NH2 adsorbent (Singh and Upadhyay, 2018).
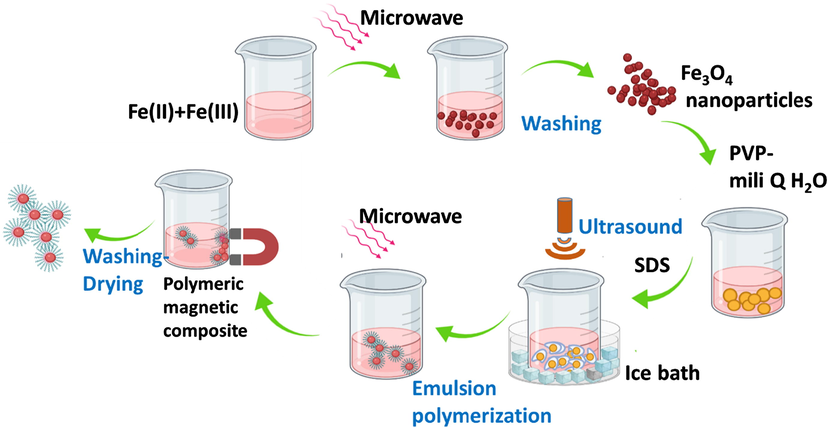
- Schematic of the microwave-assisted synthesis of Fe3O4 nanoparticles and Fe3O4/poly(styrene–divinylbenzene-acrylic acid) composites by Jaiswal et al. (2018).
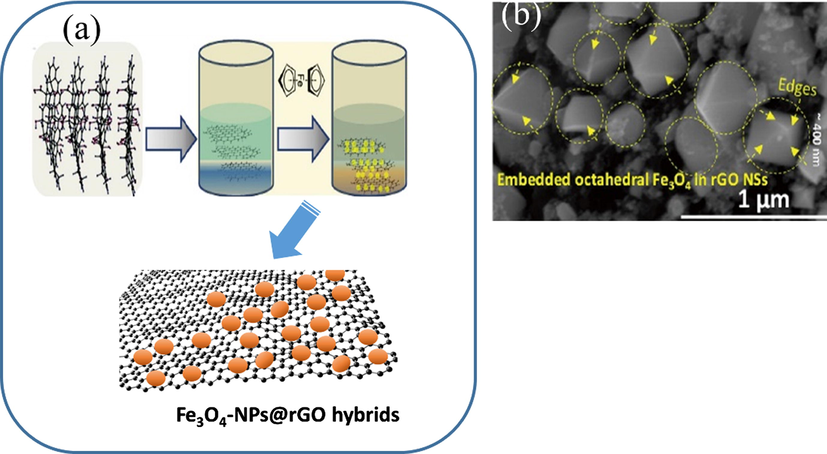
-
(a) The synthesis schematic of Fe3O4-NPs@rGO hybrids by Kumar et al. (2018); (b) SEM Fe3O4-ONCs@rGO hybrids.
3 Future perspective
This review has summarized and highlighted several methods for synthesizing iron oxide nanocomposites, such as co-precipitation, chemical vapor deposition (CVD), electrochemical method, solvothermal, microwave, and micro-emulsion. Generally, the stability of the precursor material plays an essential role in the quantity of nanoparticles produced. In addition, several factors such as pH, temperature, supporting electrolyte, salts, etc., directly influence. The magnetic characteristics of the iron nanocomposite material are determined by the natural type of iron oxide itself. However, monitoring temperature during a process is crucial because the iron oxide can change the phase during the synthesis. Several routes of the synthesis of magnetic iron oxide nanocomposites have been well studied. Review presents the incorporating functional magnetic nanoparticles into the nanocomposite structures by different method with various nanoparticle size and saturation magnetization for such applications. The intensification of the synthesis for fast and greener method consist of microwave-assisted method was also addressed. Thus, we have recommended that researcher’s study large scale production and implementation, including the following:
-
Modeling of synthesis procedure by simulating several factors influence on the performance of nanocomposite for applicability in industrial scale.
-
Life cycle assessment on the synthesis method should be given to minimize chemical waste during synthesis.
Acknowledgement
The research has been funded by Chemistry Department, Universitas Islam Indonesia by Research Excellencies Support 2021.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
References
- The effect of basic pH on the elaboration of ZnFe2O4 nanoparticles by co-precipitation method: Structural, magnetic and hyperthermia characterization. J. Magn. Magn. Mater.. 2019;478:239-246.
- [CrossRef] [Google Scholar]
- A new approach for one step synthesis of magnetic carbon nanotubes/diatomite earth composite by chemical vapor deposition method: Application for removal of lead ions. Chem. Eng. J.. 2014;253:456-463.
- [CrossRef] [Google Scholar]
- Characterization and photocatalytic application of Ce4+, Co2+, Mn2+ and Ni2+ doped Fe3O4 magnetic nanoparticles obtained by the co-precipitation method. Mater. Chem. Phys.. 2020;242:122489
- [CrossRef] [Google Scholar]
- Synthesis of iron/graphene composites with controlled magnetization by electrochemical exfoliation/deposition using sodium dodecyl sulfate as surfactant. J. Magn. Magn. Mater.. 2020;500:166398
- [CrossRef] [Google Scholar]
- A robust method for fabrication of monodisperse magnetic mesoporous silica nanoparticles with core-shell structure as anticancer drug carriers. J. Mol. Liq.. 2019;292:111367
- [CrossRef] [Google Scholar]
- Direct growth of iron oxide nanoparticles filled multi-walled carbon nanotube via chemical vapour deposition method as high-performance supercapacitors. Int. J. Hydrogen Energy. 2019;44:2349-2360.
- [CrossRef] [Google Scholar]
- Particle size determination from magnetization curves in reduced graphene oxide decorated with monodispersed superparamagnetic iron oxide nanoparticles. J. Colloid Interface Sci.. 2020;566:107-119.
- [CrossRef] [Google Scholar]
- Microemulsion synthesis and magnetic properties of FexNi(1–x) alloy nanoparticles. J. Magn. Magn. Mater.. 2017;421:177-183.
- [CrossRef] [Google Scholar]
- Green synthesis of iron-based nanomaterials for environmental remediation: A review. Environ. Nanotechnol. Monit. Manage.. 2020;13:100279
- [CrossRef] [Google Scholar]
- Synthesis and characterization of magnetic ZSM-5 zeolite. Trans. Tianjin Univ.. 2013;19:326-331.
- [CrossRef] [Google Scholar]
- Advances in Magnetic Nanoparticles for Biomedical Applications. Adv. Healthcare Mater.. 2017;7:1700845.
- [CrossRef] [Google Scholar]
- Dendritic magnetite nanocarriers for drug delivery applications. New J. Chem.. 2010;34:648-655.
- [CrossRef] [Google Scholar]
- Studies on structural and optical properties of nano ZnFe2O4 and ZnFe2O4-TiO2 composite synthesized by co-precipitation route. Mater. Chem. Phys.. 2019;230:107-113.
- [CrossRef] [Google Scholar]
- Magnetic graphene TiO2-based photocatalyst for the removal of pollutants of emerging concern in water by simulated sunlight aided photocatalytic ozonation. Appl. Catal. B. 2020;262:118275
- [CrossRef] [Google Scholar]
- A facile self-catalyzed CVD method to synthesize Fe3C/N-doped carbon nanofibers as lithium storage anode with improved rate capability and cyclability. J. Mater. Sci. Technol.. 2020;44:229-236.
- [CrossRef] [Google Scholar]
- One-pot preparation of hydrophilic citric acid-magnetic nanoparticles for identification of glycopeptides in human saliva. Talanta. 2020;206:120178
- [CrossRef] [Google Scholar]
- Magnetic carbon nanospheres: Synthesis, characterization, and adsorbability towards quinoline from coking wastewater. Chem. Eng. J.. 2020;382:122995
- [CrossRef] [Google Scholar]
- Co-precipitation synthesis of (Zn-Mn)-co-doped magnetite nanoparticles and their application in magnetic hyperthermia. J. Alloy. Compd.. 2019;779:698-705.
- [CrossRef] [Google Scholar]
- Synthesis and comparison of different spinel ferrites and their catalytic activity during chemical vapor deposition of polymorphic nanocarbons. Int. J. Precis. Eng. Manuf.-Green Technol.. 2017;4:441-451.
- [CrossRef] [Google Scholar]
- Synthesis of ZnS:Mn–Fe3O4 bifunctional nanoparticles by inverse microemulsion method. J. Sci.: Adv. Mater. Devices. 2016;1:200-203.
- [CrossRef] [Google Scholar]
- Thermosensitive magnetic latex particles for controlling protein adsorption and desorption. J. Magn. Magn. Mater.. 2001;225:151-155.
- [CrossRef] [Google Scholar]
- Synthesis of iron oxides nanoparticles with very high saturation magnetization form TEA-Fe(III) complex via electrochemical deposition for supercapacitor applications. J. Mol. Struct.. 2017;1147:84-95.
- [CrossRef] [Google Scholar]
- Dielectric properties of triethylene glycol-stabilized Mn1−xZnxFe2O4 nanoparticles. Mater. Chem. Phys.. 2015;165:156-167.
- [CrossRef] [Google Scholar]
- Fe3O4 and MnO2 assembled on halloysite nanotubes: A highly efficient solid-phase extractant for electrochemical detection of mercury(II) ions. Sens. Actuators, B. 2016;228:1-9.
- [CrossRef] [Google Scholar]
- Chapter 4 - Hydrothermal and Solvothermal Syntheses. In: Xu R., Xu Y., eds. Modern Inorganic Synthetic Chemistry (second ed.). Amsterdam: Elsevier; 2017. p. :73-104.
- [Google Scholar]
- In situ microemulsion synthesis of hydroxyapatite-MgFe2O4 nanocomposite as a magnetic drug delivery system. Mater. Sci. Eng., C. 2016;68:774-779.
- [CrossRef] [Google Scholar]
- Solvothermal synthesis of CuFe2O4 and Fe3O4 nanoparticles with high heating efficiency for magnetic hyperthermia application. J. Alloy. Compd.. 2020;816:152548
- [CrossRef] [Google Scholar]
- Effect of initial pH and temperature of iron salt solutions on formation of magnetite nanoparticles. Mater. Chem. Phys. 2007:168-175.
- [CrossRef] [Google Scholar]
- Scale-up synthesis of iron oxide nanoparticles by microwave-assisted thermal decomposition. Chem. Eng. J.. 2015;281:87-95.
- [CrossRef] [Google Scholar]
- 26 - Microwave synthesized nanocomposites for enhancing oral bioavailability of drugs. In: Inamuddin, Asiri A.M., Mohammad A., eds. Applications of Nanocomposite Materials in Drug Delivery. Woodhead Publishing; 2018. p. :619-632.
- [Google Scholar]
- Role of surfactants on stability of iron oxide yellow pigment dispersions. Prog. Org. Coat.. 2018;120:260-265.
- [CrossRef] [Google Scholar]
- Antifungal activity of magnetically separable Fe3O4/ZnO/AgBr nanocomposites prepared by a facile microwave-assisted method. Progr. Nat. Sci. Mater. Int.. 2016;26:334-340.
- [CrossRef] [Google Scholar]
- Magnetic graphene oxide/MgAl-layered double hydroxide nanocomposite: One-pot solvothermal synthesis, adsorption performance and mechanisms for Pb2+, Cd2+, and Cu2+. Chem. Eng. J.. 2018;341:1-9.
- [CrossRef] [Google Scholar]
- One-pot solvothermal synthesis of magnetically separable rGO/MnFe2O4 hybrids as efficient photocatalysts for degradation of MB under visible light. Mater. Chem. Phys.. 2019;231:68-74.
- [CrossRef] [Google Scholar]
- Microwave assisted synthesis and antimicrobial activity of Fe3O4-doped ZrO2 nanoparticles. Ceram. Int.. 2019;45:10106-10113.
- [CrossRef] [Google Scholar]
- Removal of Pb(II) and As(V) using magnetic nanoparticles coated montmorillonite via one-pot solvothermal reaction as adsorbent. J. Environ. Chem. Eng.. 2019;7:103000
- [CrossRef] [Google Scholar]
- Microwave-assisted rapid synthesis of Fe3O4/poly(styrene-divinylbenzene-acrylic acid) polymeric magnetic composites and investigation of their structural and magnetic properties. Eur. Polym. J.. 2018;98:177-190.
- [CrossRef] [Google Scholar]
- Synthesis and magnetic interaction on concentrated Fe3O4 nanoparticles obtained by the co-precipitation and hydrothermal chemical methods. Ceram. Int.. 2020;46:11149-11153.
- [CrossRef] [Google Scholar]
- Immobilization of ω-transaminase by magnetic PVA-Fe3O4 nanoparticles. Biotechnol. Rep,. 2016;10:49-55.
- [CrossRef] [Google Scholar]
- Microstructure and magnetic properties of bulk Nd2Fe14B/α-Fe nano-composite prepared by chemical vapor deposition. J. Magn. Magn. Mater.. 2013;328:1-6.
- [CrossRef] [Google Scholar]
- Solvothermal synthesis of Mn–Zn Ferrite(core)@SiO2(shell)/BiOBr 0.5Cl0.5 nanocomposites used for adsorption and photocatalysis combination. Ceram. Int.. 2020;46:3655-3662.
- [CrossRef] [Google Scholar]
- Development of a facile and effective electrochemical strategy for preparation of iron oxides (Fe3O4 and γ-Fe2O3) nanoparticles from aqueous and ethanol mediums and in situ PVC coating of Fe3O4 superparamagnetic nanoparticles for biomedical applications. J. Magn. Magn. Mater.. 2016;416:81-88.
- [CrossRef] [Google Scholar]
- Preparation of novel cobalt ferrite coated-porous carbon composite by simple chemical co-precipitation method and their mechanistic performance. Diam. Relat. Mater.. 2020;108:107922
- [CrossRef] [Google Scholar]
- Ferrite nanoparticles: Synthesis, characterisation and applications in electronic device. Mater. Sci. Eng., B. 2017;215:37-55.
- [CrossRef] [Google Scholar]
- The effect of preparation conditions on magnetite nanoparticles obtained via chemical co-precipitation. Mater. Chem. Phys.. 2019;223:122-132.
- [CrossRef] [Google Scholar]
- Microwave-assisted synthesis of magnetite nanoparticles possessing superior magnetic properties. Mendeleev Commun.. 2018;28:559-561.
- [CrossRef] [Google Scholar]
- Enhanced magnetic performance of iron oxide nanoparticles anchored pristine/ N-doped multi-walled carbon nanotubes by microwave-assisted approach. J. Alloy. Compd.. 2017;695:1793-1801.
- [CrossRef] [Google Scholar]
- Rapid and controllable synthesis of Fe3O4 octahedral nanocrystals embedded-reduced graphene oxide using microwave irradiation for high performance lithium-ion batteries. Electrochim. Acta. 2018;281:78-87.
- [CrossRef] [Google Scholar]
- Individual inorganic nanoparticles: preparation, functionalization and in vitro biomedical diagnostic applications. J. Mater. Chem. B. 2013;1:1381-1396.
- [CrossRef] [Google Scholar]
- Novel and facile synthesis of magnetic composites by a modified co-precipitation method. Mater. Chem. Phys.. 2011;130:624-634.
- [CrossRef] [Google Scholar]
- Microwave-assisted synthesis of ultra-small iron oxide nanoparticles for biomedicine. Mendeleev Commun.. 2018;28:167-169.
- [CrossRef] [Google Scholar]
- Magnetic enhancement of carbon-encapsulated magnetite nanoparticles. J. Alloy. Compd.. 2019;790:716-722.
- [CrossRef] [Google Scholar]
- Rapid in situ microwave synthesis of Fe3O4@MIL-100(Fe) for aqueous diclofenac sodium removal through integrated adsorption and photodegradation. J. Hazard. Mater.. 2019;373:408-416.
- [CrossRef] [Google Scholar]
- Fe3O4@PSC nanoparticle clusters with enhanced magnetic properties prepared by alternating-current magnetic field assisted co-precipitation. Colloids Surf., A. 2017;520:348-354.
- [CrossRef] [Google Scholar]
- Correlation between particle size/domain structure and magnetic properties of highly crystalline Fe3O4 nanoparticles. Sci. Rep.. 2017;7:9894.
- [CrossRef] [Google Scholar]
- Magnetic properties and microstructure of nanocomposite (La, Pr)3Fe14B ribbons by doping La element. AIP Adv.. 2019;9:035111
- [CrossRef] [Google Scholar]
- Size-dependent electromagnetic properties and the related simulations of Fe3O4 nanoparticles made by microwave-assisted thermal decomposition. Colloids Surf., A. 2017;530:191-199.
- [CrossRef] [Google Scholar]
- Preparation of fatty acids coated Fe3O4 nanoparticles for adsorption and determination of benzo(a)pyrene in environmental water samples. Chem. Eng. J.. 2015;271:232-239.
- [CrossRef] [Google Scholar]
- Synthesis of amine-functionalized Fe3O4@C nanoparticles for laccase immobilization. Int. J. Biol. Macromol.. 2017;96:377-383.
- [CrossRef] [Google Scholar]
- Tailor-made core/shell/shell-like Fe3O4@SiO2@PPy composites with prominent microwave absorption performance. J. Alloy. Compd.. 2019;779:831-843.
- [CrossRef] [Google Scholar]
- 12 - Magnetic Nanocomposite Adsorbents. In: Kyzas G.Z., Mitropoulos A.C., eds. Composite Nanoadsorbents. Elsevier; 2019. p. :295-316.
- [Google Scholar]
- Effect of Fe3O4 content and microwave reaction time on the properties of Fe3O4/ZnO magnetic nanoparticles. J. Alloy. Compd.. 2019;781:790-799.
- [CrossRef] [Google Scholar]
- Biomass activated carbon supported with high crystallinity and dispersion Fe3O4 nanoparticle for preconcentration and effective degradation of methylene blue. J. Taiwan Inst. Chem. Eng.. 2017;81:265-274.
- [CrossRef] [Google Scholar]
- Temperature effect on the synthesis of carbon nanotubes and core–shell Ni nanoparticle by thermal CVD. Diam. Relat. Mater.. 2015;52:59-65.
- [CrossRef] [Google Scholar]
- Influence of electrolyte temperature on the synthesis of iron oxide nanostructures by electrochemical anodization for water splitting. Int. J. Hydrogen Energy. 2018;43:7923-7937.
- [CrossRef] [Google Scholar]
- Microemulsion-mediated hydrothermal growth of pagoda-like Fe3O4 microstructures and their application in a lithium–air battery. Ceram. Int.. 2015;41:8843-8848.
- [CrossRef] [Google Scholar]
- Magnetic properties of ε iron(III) oxide nanorod arrays functionalized with gold and copper(II) oxide. Appl. Surf. Sci.. 2018;427:890-896.
- [CrossRef] [Google Scholar]
- Microwave-enforced sorption of heavy metals from aqueous solutions on the surface of magnetic iron oxide-functionalized-3-aminopropyltriethoxysilane. Chem. Eng. J.. 2016;293:200-206.
- [CrossRef] [Google Scholar]
- Current methods for synthesis of magnetic nanoparticles. Artif. Cells Nanomed. Biotechnol.. 2016;44:722-734.
- [CrossRef] [Google Scholar]
- Influence of Surface Treatment on Magnetic Properties of Fe3O4 Nanoparticles Synthesized by Electrochemical Method. J. Phys. Chem. B. 2016;120:6634-6645.
- [CrossRef] [Google Scholar]
- Room Temperature Co-Precipitation Synthesis of Magnetite Nanoparticles in a Large pH Window with Different Bases. Materials (Basel). 2013;6:5549-5567.
- [CrossRef] [Google Scholar]
- Stimuli-responsive magnetic particles for biomedical applications. Int. J. Pharm.. 2011;403:139-161.
- [CrossRef] [Google Scholar]
- Preparation of amino-functionalized Fe3O4@mSiO2 core-shell magnetic nanoparticles and their application for aqueous Fe3+ removal. J. Hazard. Mater.. 2018;341:198-206.
- [CrossRef] [Google Scholar]
- Chapter Two - Nanoscale coordination polymers: Preparation, function and application. In: Ruiz-Molina D., van Eldik R., eds. Advances in Inorganic Chemistry. Academic Press; 2020. p. :33-72.
- [Google Scholar]
- An investigation of microstructural, magnetic and microwave absorption properties of multi-walled carbon nanotubes/Ni0.5Zn0.5Fe2O4. Sci. Rep.. 2019;9
- [Google Scholar]
- Optical and magnetic properties of HoFeO3 nanocrystals prepared by a simple co-precipitation method using ethanol. J. Alloy. Compd.. 2020;834:155098
- [CrossRef] [Google Scholar]
- Re-formation of metastable ε-Fe2O3 in post-annealing of Fe2O3/SiO2 nanostructure: Synthesis, computational particle shape analysis in micrographs and magnetic properties. Ceram. Int.. 2017;43:7497-7507.
- [CrossRef] [Google Scholar]
- 2 - Synthesis, design, and morphology of metal oxide nanostructures. In: Nunes D., Pimentel A., Santos L., Barquinha P., Pereira L., Fortunato E., Martins R., eds. Metal Oxide Nanostructures. NY: Elsevier; 2019. p. :21-57.
- [Google Scholar]
- Synthesis of ZrO2 nanoparticles and effect of surfactant on dispersion and stability. Ceram. Int.. 2020;46:11970-11977.
- [CrossRef] [Google Scholar]
- Fe-based soft magnetic composites coated with NiZn ferrite prepared by a co-precipitation method. J. Magn. Magn. Mater.. 2017;428:148-153.
- [CrossRef] [Google Scholar]
- Application of magnetic adsorbents based on iron oxide nanoparticles for oil spill remediation: A review. J. Taiwan Inst. Chem. Eng.. 2019;97:227-236.
- [CrossRef] [Google Scholar]
- Degradation of ofloxacin, amoxicillin and tetracycline antibiotics using magnetic core–shell MnFe2O4@C-NH2 as a heterogeneous Fenton catalyst. Chem. Eng. J.. 2020;396:125304
- [CrossRef] [Google Scholar]
- Structure and magnetic properties of ultrafine superparamagnetic Sn-doped magnetite nanoparticles synthesized by glycol assisted co-precipitation method. J. Phys. Chem. Solids. 2020;145:109530
- [CrossRef] [Google Scholar]
- Microwave-assisted solution combustion synthesis of Fe3O4 powders. Ceram. Int.. 2017;43:14756-14762.
- [CrossRef] [Google Scholar]
- Comparing three methods of simultaneous synthesis and stabilization of Fe3O4 nanoparticles: Changing physicochemical properties of products to improve kinetic and thermodynamic of dye adsorption. J. Magn. Magn. Mater.. 2017;422:128-140.
- [CrossRef] [Google Scholar]
- Progress in electrochemical synthesis of magnetic iron oxide nanoparticles. J. Magn. Magn. Mater.. 2014;368:207-229.
- [CrossRef] [Google Scholar]
- Chapter 5 - Methods for Synthesis of Nanoparticles and Fabrication of Nanocomposites. In: MohanBhagyaraj S., Oluwafemi O.S., Kalarikkal N., Thomas S., eds. Synthesis of Inorganic Nanomaterials. Woodhead Publishing; 2018. p. :121-139.
- [Google Scholar]
- Magnetic carbon composites with a hierarchical structure for adsorption of tetracycline, prepared from sugarcane bagasse via hydrothermal carbonization coupled with simple heat treatment process. Bioresour. Technol.. 2017;226:164-172.
- [CrossRef] [Google Scholar]
- Magnetic graphene/chitosan nanocomposite: A promising nano-adsorbent for the removal of 2-naphthol from aqueous solution and their kinetic studies. Int. J. Biol. Macromol.. 2020;159:530-538.
- [CrossRef] [Google Scholar]
- Conformal vapor deposition of iron phosphate onto carbon nanotubes for flexible high-rate cathodes. Mater. Today Energy. 2018;8:143-150.
- [CrossRef] [Google Scholar]
- Polyvinyl alcohol/Fe3O4@carbon nanotubes nanocomposite: Electrochemical-assisted synthesis, physicochemical characterization, optical properties, cytotoxicity effects and ultrasound-assisted treatment of aqueous based organic compound. J. Ind. Eng. Chem.. 2018;65:349-362.
- [CrossRef] [Google Scholar]
- Ultrasound assisted reverse micelle efficient synthesis of new Ta-MOF@ Fe3O4 core/shell nanostructures as a novel candidate for lipase immobilization. Mater. Sci. Eng., C. 2018;93:768-775.
- [CrossRef] [Google Scholar]
- One-step microwave-assisted synthesis of water-dispersible Fe3O4 magnetic nanoclusters for hyperthermia applications. J. Magn. Magn. Mater.. 2017;439:107-113.
- [CrossRef] [Google Scholar]
- Iron oxide-loaded nanotheranostics: Major obstacles to in vivo studies and clinical translation. J. Control. Rel. Off. J. Control. Release Soc.. 2014;198
- [CrossRef] [Google Scholar]
- Progress in the Preparation of Magnetite Nanoparticles through the Electrochemical Method. Kona Powder Part. J.. 2019;36:145-155.
- [CrossRef] [Google Scholar]
- Synthesis of carbon nanotubes by catalytic chemical vapour deposition: A review on carbon sources, catalysts and substrates. Mater. Sci. Semicond. Process.. 2016;41:67-82.
- [CrossRef] [Google Scholar]
- Shahedi Asl, Magnetic CoFe2O4 nanoparticles doped with metal ions: A review. Ceram. Int.. 2020;46:18391-18412.
- [CrossRef] [Google Scholar]
- Synthesis of proanthocyanidins-functionalized Fe3O4 magnetic nanoparticles with high solubility for removal of heavy-metal ions. Chem. Phys. Lett.. 2020;753:137600
- [CrossRef] [Google Scholar]
- Synthesis of novel magnetic carbon nano-composite from waste biomass: A comparative study of industrially adoptable hydro/solvothermal co-precipitation route, Journal of Environmental. Chem. Eng.. 2020;8:103519
- [CrossRef] [Google Scholar]
- Chapter 3 - Nanotechnology applications in drug controlled release. In: Grumezescu A.M., ed. Drug Targeting and Stimuli Sensitive Drug Delivery Systems. William Andrew Publishing; 2018. p. :81-116.
- [Google Scholar]
- Role of silver nanoshells on structural and magnetic behavior of Fe3O4 nanoparticles. J. Magn. Magn. Mater.. 2018;458:39-47.
- [CrossRef] [Google Scholar]
- Intrinsic and Extrinsic Properties of Advanced Magnetic Materials. In: Liu Y., Sellmyer D.J., Shindo D., eds. Handbook of Advanced Magnetic Materials. US, Boston, MA: Springer; 2006. p. :1-57.
- [Google Scholar]
- Water-Soluble Magnetic Nanocomposites Based on Carboxymethyl Cellulose and Iron(III) Oxide. Polym. Sci. - Series B. 2018;60:116-121.
- [CrossRef] [Google Scholar]
- Electrochemical synthesis of magnetic iron oxide nanoparticles with controlled size. J. Nanopart. Res. Interdiscip. Forum Nanoscale Sci. Technol.. 2011;13:7167-7176.
- [CrossRef] [Google Scholar]
- Electrochemical synthesis of ferrate(VI) using sponge iron anode and oxidative transformations of antibiotic and pesticide. J. Hazard. Mater.. 2018;344:1155-1164.
- [CrossRef] [Google Scholar]
- Synthesis and characterization of chicken eggshell powder coated magnetic nano adsorbent by an ultrasonic bath assisted co-precipitation for Cr(VI) removal from its aqueous mixture. J. Environ. Chem. Eng. 2020103877
- [CrossRef] [Google Scholar]
- Magnetic iron oxide/clay composites: Effect of the layer silicate support on the microstructure and phase formation of magnetic nanoparticles. Nanotechnology. 2007;18:285602-285609.
- [CrossRef] [Google Scholar]
- A new sensing platform based on magnetic Fe3O4@NiO core/shell nanoparticles modified carbon paste electrode for simultaneous voltammetric determination of Quercetin and Tryptophan. J. Electroanal. Chem.. 2018;808:50-58.
- [CrossRef] [Google Scholar]
- From Hollow to Dense Spheres: Control of Dipolar Interactions by Tailoring the Architecture in Colloidal Aggregates of Superparamagnetic Iron Oxide Nanocrystals. Adv. Mater.. 2004;16:529-533.
- [CrossRef] [Google Scholar]
- Electrochemical synthesis of flake-like Fe/MWCNTs nanocomposite for hydrogen evolution reaction: Effect of the CNTs on dendrite growth of iron and its electrocatalytic activity. Curr. Appl Phys.. 2010;10:72-76.
- [CrossRef] [Google Scholar]
- Chemical vapor deposition growth of Fe3O4 thin films and Fe/Fe3O4 bi-layers for their integration in magnetic tunnel junctions. Thin Solid Films. 2012;520:4617-4621.
- [CrossRef] [Google Scholar]
- Magnetic colloids for the generic capture of viruses. Anal. Biochem.. 2005;346:59-68.
- [CrossRef] [Google Scholar]
- Chemical vapor deposition prepared bi-morphological carbon-coated Fe3O4 composites as anode materials for lithium-ion batteries. J. Power Sources. 2015;282:257-264.
- [CrossRef] [Google Scholar]
- Towards a better understanding on mercury adsorption by magnetic bio-adsorbents with γ-Fe2O3 from pinewood sawdust derived hydrochar: Influence of atmosphere in heat treatment. Bioresour. Technol.. 2018;256:269-276.
- [CrossRef] [Google Scholar]
- One-step solvothermal synthesis of porous MnFe2O4 nanoflakes and their magnetorheological properties. J. Alloy. Compd.. 2020;819:153044
- [CrossRef] [Google Scholar]
- Electrochemical synthesis of magnetic nanoparticles within mesoporous silica microspheres. Colloids Surf., A. 2007;294:287-291.
- [CrossRef] [Google Scholar]
- Millimeter-scale magnetic spherical metal-organic framework core-shell structured composites for recyclable catalytic applications. Micropor. Mesopor. Mater.. 2020;300:110152
- [CrossRef] [Google Scholar]
- Facile solvothermal synthesis of Fe3O4/bentonite for efficient removal of heavy metals from aqueous solution. Powder Technol.. 2016;301:632-640.
- [CrossRef] [Google Scholar]
- Size-controlled synthesis, magnetic property, and photocatalytic property of uniform α-Fe2O3 nanoparticles via a facile additive-free hydrothermal route. CrystEngComm. 2012;14:7915-7921.
- [CrossRef] [Google Scholar]
- Influence of heat treatment on microstructures and magnetic properties of Fe-based soft magnetic composites prepared by co-precipitation method. J. Magn. Magn. Mater.. 2019;476:100-105.
- [CrossRef] [Google Scholar]
- Preparation of the chitosan grafted poly (quaternary ammonium)/Fe3O4 nanoparticles and its adsorption performance for food yellow 3. Carbohydr. Polym.. 2016;152:327-336.
- [CrossRef] [Google Scholar]
- One-step synthesis of magnetic composites of cellulose@iron oxide nanoparticles for arsenic removal. J. Mater. Chem. A. 2013;1:959-965.
- [CrossRef] [Google Scholar]
- Fabrication and characterization of morphology-tuned single-crystal monodisperse Fe3O4 nanocrystals. Appl. Surf. Sci.. 2018;439:298-304.
- [CrossRef] [Google Scholar]
- Necklace-like Fe3O4 nanoparticle beads on carbon nanotube threads for microwave absorption and supercapacitors. Mater. Des.. 2020;189:108517
- [CrossRef] [Google Scholar]
- 3D carbon fiber mats/nano-Fe3O4 hybrid material with high electromagnetic shielding performance. Appl. Surf. Sci.. 2018;444:710-720.
- [CrossRef] [Google Scholar]
- Poly-para-xylylene enhanced Fe-based amorphous powder cores with improved soft magnetic properties via chemical vapor deposition. Mater. Des.. 2020;191:108650
- [CrossRef] [Google Scholar]
- Facile electrochemical synthesis of nano iron porous coordination polymer using scrap iron for simultaneous and cost-effective removal of organic and inorganic arsenic. Chin. Chem. Lett.. 2018;29:456-460.
- [CrossRef] [Google Scholar]
- Microwave-assisted solvothermal synthesis of shape-controlled CoFe2O4 nanoparticles for acetone sensor. J. Alloy. Compd.. 2019;788:1103-1112.
- [CrossRef] [Google Scholar]
- Degradation of ibuprofen in the carbon dots/Fe3O4@carbon sphere pomegranate-like composites activated persulfate system. Sep. Purif. Technol.. 2020;242:116820
- [CrossRef] [Google Scholar]
- Microwave-assisted synthesis of magnetic Fe3O4-mesoporous magnesium silicate core-shell composites for the removal of heavy metal ions. Micropor. Mesopor. Mater.. 2017;242:50-58.
- [CrossRef] [Google Scholar]
- Synthesis of amino-functionalized bentonite/CoFe2O4@MnO2 magnetic recoverable nanoparticles for aqueous Cd2+ removal. Sci. Total Environ.. 2019;682:505-513.
- [CrossRef] [Google Scholar]







