Translate this page into:
Synthesis of NaKCO3@(Mn-Na2 WO4/SiO2) core-shell catalyst for oxidative coupling of methane
⁎Corresponding author. m_masoumi@iau-tnb.ac.ir (Mir Esmaeil Masoumi)
-
Received: ,
Accepted: ,
This article was originally published by Elsevier and was migrated to Scientific Scholar after the change of Publisher.
Peer review under responsibility of King Saud University.
Abstract
Oxidative Coupling of Methane (OCM) is a process to produce ethylene as one of the most widely used petrochemicals from methane natural gas. The core–shell nano-catalyst of @( ) was prepared with different ratios of , and its activity in the OCM process was investigated and its performance was compared with the conventional nano-catalyst. The effect of temperature and methane-to-oxygen ratio on nano-catalyst performance was investigated. The performance criteria included methane conversion, ethylene selectivity, and yield. The formation of the core–shell structure has been deliberated using XRD and FE-SEM characterization tests. The results of characterization and reactor experiments indicated that the best performance was related to the nano-catalyst prepared with a ratio of = 2/2.4, operating conditions: 850 °C, atmospheric pressure, and ratio = 2 in the reaction feed. The formation of the core–shell structure does not reduce the nano-catalyst activity, but rather increases the selectivity of the main product, ethylene, by improving the nano-catalyst performance even at 850 °C. Stability tests were performed to examine the catalytic activity under high temperatures.
Keywords
Oxidative Coupling of Methane
Ethylene
Nano-catalyst
Core-shell
Shell
1 Introduction
Ethylene is an important chemical compound and is used in the synthesis of the chain products in the petrochemical industry which include raw materials as polyethylene, polypropylene, and polystyrene, which are used in the production of a wide range of products from car antifreeze to plastics (Zhang et al., 2021a). Therefore, ethylene is categorized as a first-class hydrocarbon in the chemical and petrochemical industry. Over the past 30 years, studies have been made in ethylene production by OCM. The reaction mechanism of the catalysts in the OCM process is as follows (Raza et al., 2022):
The OCM process shows promise in conversion to and hence, accounting for a succinct approach to use natural gas for ethylene production as the OCM technique applies direct conversion making it possible to avoid the synthesis gas step (Lee et al., 2022).
The catalyst that is used in the OCM process is vital as it affects the ethylene selectivity and methane conversion percentage. The oxide catalysts are the foremost catalysts used in the OCM reactions. These catalysts can be either, the modified oxides of the transition metals or the promoted or the assorted ones of group IA and IIA elements (Wang et al., 2016).
It has been identified that the catalyst has performed more efficiently, amid all the catalysts synthesized for the OCM process as its stability is high for long duration on the reaction stream. The emerging wetness impregnation technique is the most appropriate synthesis method among the existing methods (Jafarzadeh et al., 2022) to synthesize the catalyst.
The active components (Mn, Na, and W) on or close to the surface of the catalyst are enriched by this process. This could cause a damage of dynamic constituent through lengthy periods on stream which eventually brings about improved catalyst enactment. A distinction of the composition was exactly premeditated (J. Zhang et al., 2021) and it was observed that on protecting the content of Mn stable at 2 % wt. and changing the content of , the supreme activity of OCM was achieved at 5 % wt. of . It has been suggested that and Mn serve as active component and promoters, consistently (Ortiz-Bravo et al., 2021) although the reaction mechanism is still controversial. Researchers have added supporters and changed the support to improve the catalyst (Zhang et al., 2022). Since the OCM reaction is active at very high temperatures, one of the ways to protect the catalyst, the active phase, and the product at the same time is to form a core–shell structure for the catalyst; so, the active phase is selected as the core (Alli et al., 2022).
In the last two decades, OCM research has mainly focused on the synthesis of suitable catalysts and the evaluation of reaction mechanisms to achieve formulations with acceptable outcomes (X. Gao et al., 2022). The main goal of all research in this field is to attain a high ethylene selectivity and acceptable methane conversion percentage, in one step through the catalytic system. Since the 1990 s, major research has focused on optimizing catalysts and examining the types of OCM reactors, in addition to catalyst formulation, selectivity and deactivation of a catalyst in due course is very important (Lyu et al., 2022).
Jaroenpanon et al. (Jaroenpanon et al., 2022) studied the performance of on or catalysts in powder and fiber form for OCM reaction. Their results showed that fiber catalysts increase conversion and selectivity due to improved heat and mass diffusion in the catalyst bed. The activation of at low temperature is strongly associated with the presence of oxygen species at a relatively lower binding energy, which allows the oxygen species to be readily allocated during the reaction. Nevertheless, the efficiency of catalysts, especially for , was reported to be higher than that of under the same conditions, due to the synergistic catalytic effect of the active phases, including , , and .
Tiyatha et al. (Tiyatha et al., 2022) for and catalysts, the effect of loading or , respectively, on two different supports sol–gel (SG) and commercial carbonated (CS) studied. Their results showed that the catalyst with the highest efficiency (21.6 % with 60.8 % selectivity and 35.6 % conversion) was 10 wt% /SG with an olefin/paraffin ratio of 2.2. Also, stability tests for more than 24 h showed that SG-supported catalysts were more durable than those on CS, because the active phase (especially ) was more stable on SG than on CS.
Reportedly, a perfect connection amid the enactment and under the conditions of OCM, the conductivity was detected, and the highest electrical conductivity was scored by (Yentekakis et al., 2021). Although α-cristobalite has been detected in these catalysts composed of other dissimilar elements, W presented the greatest selectivity and greatest conversion over the rest and phase transfer from amorphous (Orooji et al., 2022). A study of different support materials was carried out and concluded that was the best support for the (Song et al., 2022).
Gärtner et al. (Gärtner et al., 2014) used core–shell catalysts to produce ethylene in the oxidative dehydrogenation of ethane. The support of the catalyst was solid and coated by a shell; during the shell process, the active oxygen species diffuse through the molten chloride shell and convert to and, react with ethane on the surface of the shell, the final product is ethylene and water. In similar research, they succeeded to achieve 95 % selectivity with a 25 % conversion percentage by oxidation in the molten shell of this catalyst with an overlayer of 40 % molar; the higher molar percentage of its overlayer corresponds to a lower conversion percentage and higher ethylene selectivity. The best overlayer percentage was 40 % (Liu et al., 2022). In this study, we scrutinize the activity and stability of @( ) as core–shell catalyst and compare it with conventional active/base phase catalyst in the OCM process. The temperature and methane to oxygen ratio have been selected as parameters to investigate the behavior of both catalysts. For core–shell catalyst, in addition to the above two variables, the effect of ratio and for base/active phase catalyst, the effect of ratio have also been investigated.
2 Experimental
2.1 Materials
The amorphous (35/60 mesh size, Davisil grade 646), manganese (II) nitrate hexahydrate (Mn(NO3)2 6H2O), sodium tungstate (VI) dehydrate (Na2WO4 2H2O) , potassium carbonate ( ) and sodium carbonate ( ) were purchased from Sigma-Aldrich.
2.2 Preparation of
The amorphous
was used as support for all samples. An aqueous solution comprising an 50 ml of
(20 M) was prepared by impregnating a 20 g of silica gel (
) particles. The granules were air-dried at a temperature of 130 °C for 5 h. Then 50 ml of 10 M
solution was added to the granules. Next, the samples were dehydrated for 8 h at 130 °C. The end, all samples was calcined at 800 °C (Ortiz-Bravo et al., 2021), for 8 h with the heating rate set at 10 °C min(-1) (Table 1).
Name
Sample
AS-1
AS-2
AS-3
@(
)
CS-1
@(
)
CS-2
@(
)
CS-3
2.3 Preparation of @( ) core-shell structures
The core–shell catalyst @( ) was synthesized by combining different percentages of K and Na using wet impregnation method. was used as the core for this catalyst with the new method. Certain amount of was slowly added to a 250 ml deionized water containing 50 ml of solution while being stirred at 500 rpm at 80 °C. After complete dissolution of the material, it was placed in an oven at 100 °C for 12 h until water evaporated and, a porous solid remained. The dried solid was separated and powdered. Then, it was added to a 250 ml solution containing the second layer including an aqueous solution of , similar to the previous step. The mixture was placed in an oven at 100 °C for 12 h and after complete drying, for the calcination step and removal of excess material from the catalyst structure, the resulting powder was placed in a furnace and exposed to a flow of synthetic air gas at a temperature of 800 °C (Ortiz-Bravo et al., 2021) with a ramp of 10 °C min(-1) for 8 h (Table 1).
2.4 Characterization of catalyst
The crystal constructions of the arranged catalysts were established through measuring the powder X-ray diffraction (XRD) through an X’pert-Pro diffractometer (Philips, Model: PW1730, Netherlands) by radiation (Step size = 0.05 deg, Time Per Step = 1 s, Wavelength of copper X-ray lamp = 1.54056 Ã…, Voltage = 40 kV, Current = 30 mA, Software XPert HighScore Plus v3.0e). The BET test was carried out with Quanta chrome Chem BET 3000 instruments, for measuring the surface area (The temperature of liquid nitrogen 77 K, Dehydration temperature of 120 °C for 2 h). A scanning electron microscope with an energy dispersive X-ray spectrometer (FE-SEM/EDS, Tescan, Czech) was used to image the surface morphology of the catalysts. Transmission electron microscope (TEM, HT7800 Hitachi, Japan) was used to investigate the core–shell structure of catalysts. Thermal Gravimetric Analysis test was performed using PL-TGA device for measuring the amount of carbon deposition on the catalysts.
2.5 Catalyst activity and stability
In order to perform the activity test, the reactor temperature and pressure were fixed at 850 °C and 1 atm, respectively. A quartz tube reactor with an inner diameter of 5 mm at atmospheric pressure was applied to measure the catalytic activity in the OCM reaction. (1) g of synthesized catalysts were diluted by 3 g sieved quartz powder (30/60 mesh size). The bed of the catalyst was placed at the midpoint of the reactor. To avoid the catalyst from spilling to the base of the reactor, the mixture of catalyst and quartz was poured into the quartz reactor. To mix and preheat the gases, some quartz powder was present on top of the catalyst. For the next step, the reactor was placed inside a furnace and gas transmission lines were joined. was applied to dilute the and with different ratio ( / = 2, 4 and 6) of reactants. This mixture of preheated gases was then poured onto the catalyst bed.
A mass flow controller (MFC, Brooks Instrument) controlled each gas flow. The mass flow controller contributed to set the nitrogen gas flow rate to a definite amount. To flow oxygen, methane and nitrogen gases in certain proportions, the reactor furnace was turned on and temperature was increased from 775 °C with a gradient of 10 °C min(-1). To analyze output gases from the reactor, the flame ionization detector (FID) and thermal conductivity detector (TCD) were equipped with online gas chromatography (RGA, Agilent 7890B, PerkinElmer). The entire schematic design of the OCM process is presented in Fig. 1.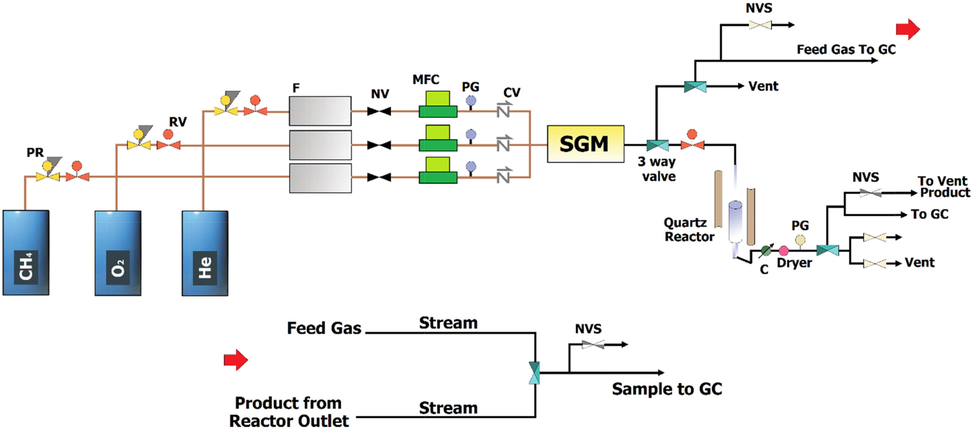
Schematic Design of the OCM Process.
To study the stability of the catalyst, the test temperature was adjusted at 850 °C and the ratio of / in the feed 2; the stability tests were performed in 50 h.
Conversion (
), selectivity (
) and yield (
) of each catalyst were calculated according to Eq. 5–7:
3 Result and discussion
3.1 Catalyst characterization
The XRD test results for
samples show in Fig. 2, In the
samples, α-cristobalite
(JCPDS 00–003-0271) was identified to improve the catalyst activity (Almana et al., 2016). In both phase samples,
(JCPDS 12–772) and
(JCPDS 01–073-2509) were also observed (Kaviani et al., 2022).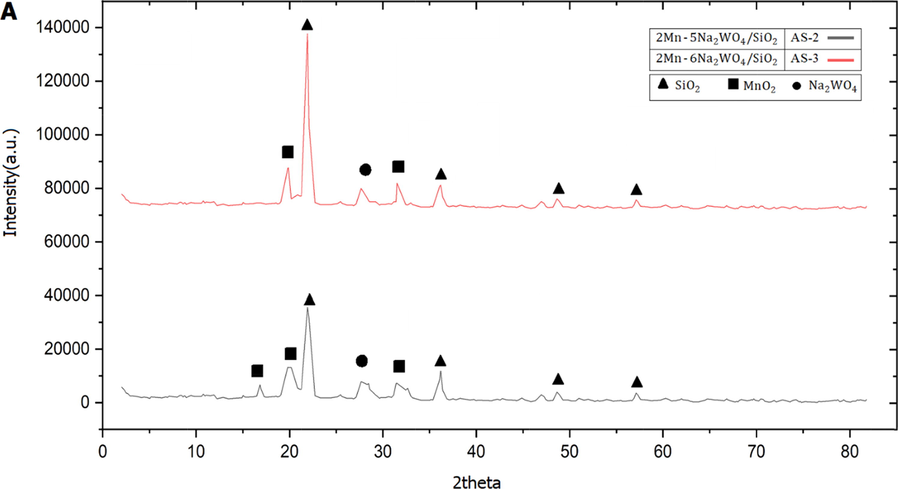
XRD patterns of catalysts samples: a) AS-2 and AS-3, b) CS-1 and CS-2, and c) CS-3 and AS-1.
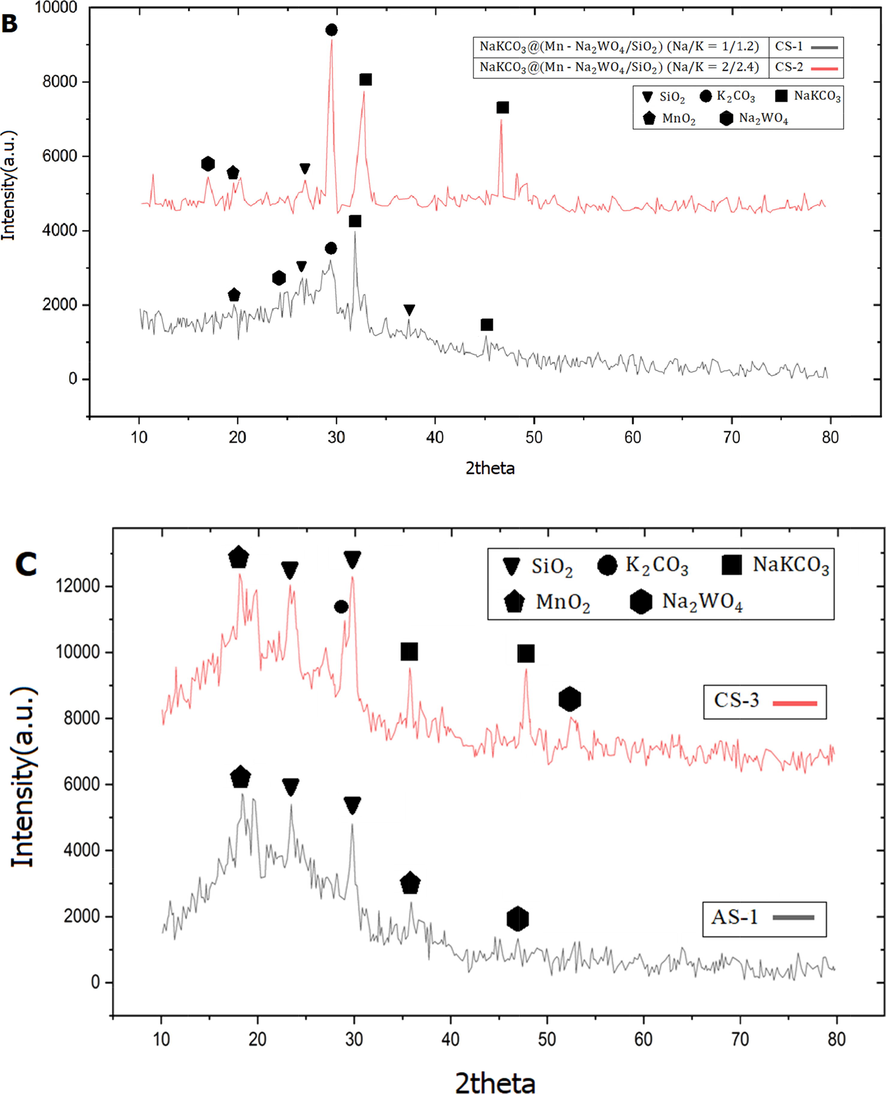
XRD patterns of catalysts samples: a) AS-2 and AS-3, b) CS-1 and CS-2, and c) CS-3 and AS-1.
Using Scherer equation and based on the maximum peak, the crystalline size of AS-2 and AS-3 catalyst was calculated, 28 and 48 nm, respectively. Therefore, as the ratio decreased from 3 to 2.5, the particle size decreased. In @( ) samples, it was found that by increasing the amount of and from 0.010 and 0.012 to 0.02 and 0.024, respectively, the catalyst got a more regular structure; Also, the side phase of (JCPDS 00–16-820) (Lee et al., 2022) was removed from the catalyst structure. The reason for the presence of this phase in the catalyst structure of CS-1 is related to the fact that some initial amount of did not enter the shell structure, and presented in the free phase, which will reduce the efficiency and activity of the catalyst. About the CS-2 sample, no additional peaks related to the side phases of (JCPDS 00–16-820) or (JCPDS 08–0448) are observed (Ziyadi et al., 2021). In the all of samples, the peak of α-cristobalite was observed. Using the Scherer equation (Y. Gao et al., 2022), the crystalline size calculated for the catalyst samples CS-1 and CS-2 is 60 nm and 51 nm, respectively.
The BET surface area and pore volume of all catalysts as determined by
adsorption are presented in Table 2. CS-2 had the highest BET (89.25
). According to the FE-SEM images for AS-2 and AS-3 as shown in Fig. 3a-b, the particle size increased with increase the
ratio from 2.5 to 3, the homogeneity and uniformity of the particles decreased, and the particles size of AS-3 sample was reported to be larger and more heterogeneous, the particle size of the AS-2 sample was observed from 29 to 48 nm and with increasing the
ratio from 2.5 to 3, the particle size in AS-3 catalyst varied between 30 and 56 nm. According to Fig. 3c-d, the core–shell samples CS-1 and CS-2, it is found that the catalyst structure becomes more regular, and the growth of the catalyst core–shell structure is increased with increasing Na and K. According to the FE-SEM analysis, the catalysts are amorphous, but according to the TEM images, it can be concluded that the catalysts are almost like spheres. Also, the size of the molecules is reduced due to the growth of the molecular network because of the improvement in the core–shell structure. The first sample, CS-1, contains 0.012 of K and 0.01 of Na had a particle with a size in the range of 54 to 96 nm and the second sample, CS-2, contains 0.024 of K and 0.02 of Na had a particle in the range of 41 to 71 nm, which is the reason for this decrease in size and is related to the growth of the core–shell structure network using increasing values of K and Na. The images related to the EDX analysis of the samples also reveal the absence of impurities in the structure of the catalysts. According to Fig. 4, the TEM image results show that the core–shell structure is formed.
Catalysts
BET Surface area (
)
Pore-volume (
)
AS-1
68.26
0.75
AS-2
50.25
0.8
AS-3
43.12
0.9
CS-1
48.56
0.02
CS-2
89.25
0.91
CS-3
78.36
0.74
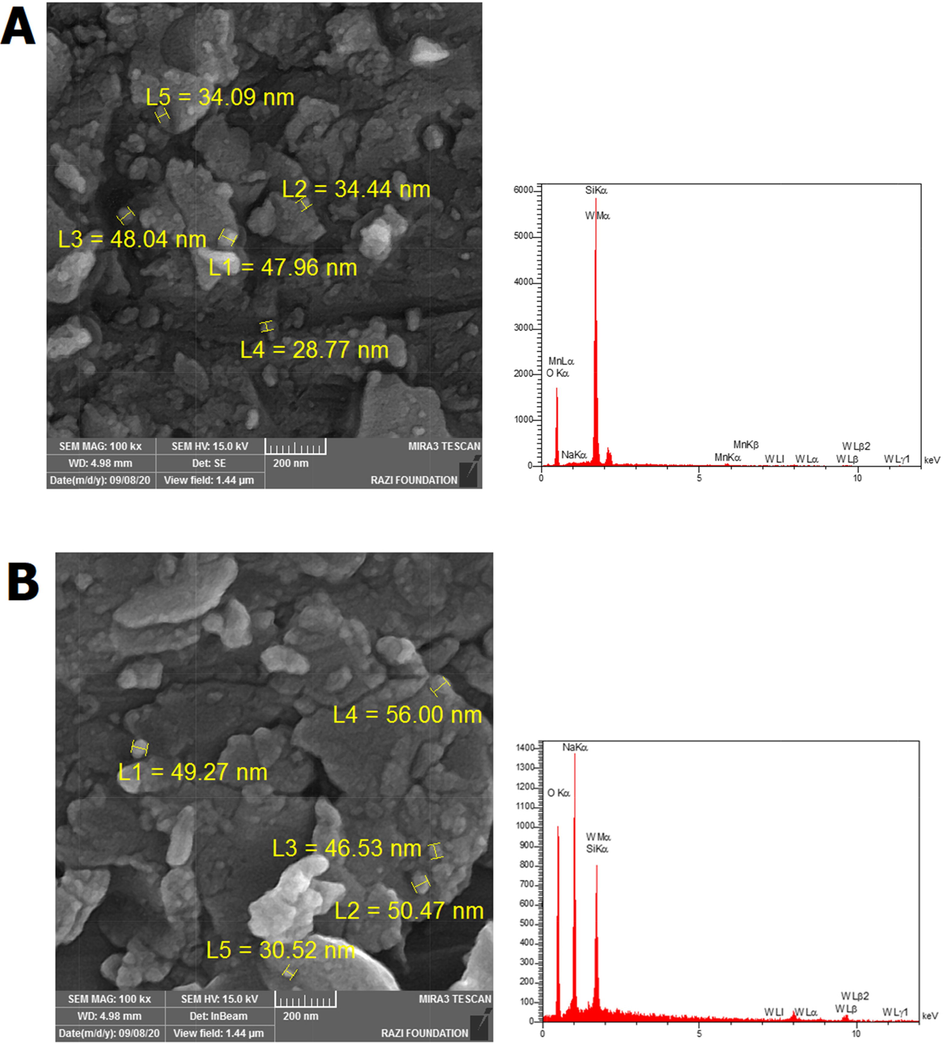
FE-SEM images and EDX graphs for all samples AS-2 (a), AS-3 (b), CS-1 (c), and CS-2 (d).
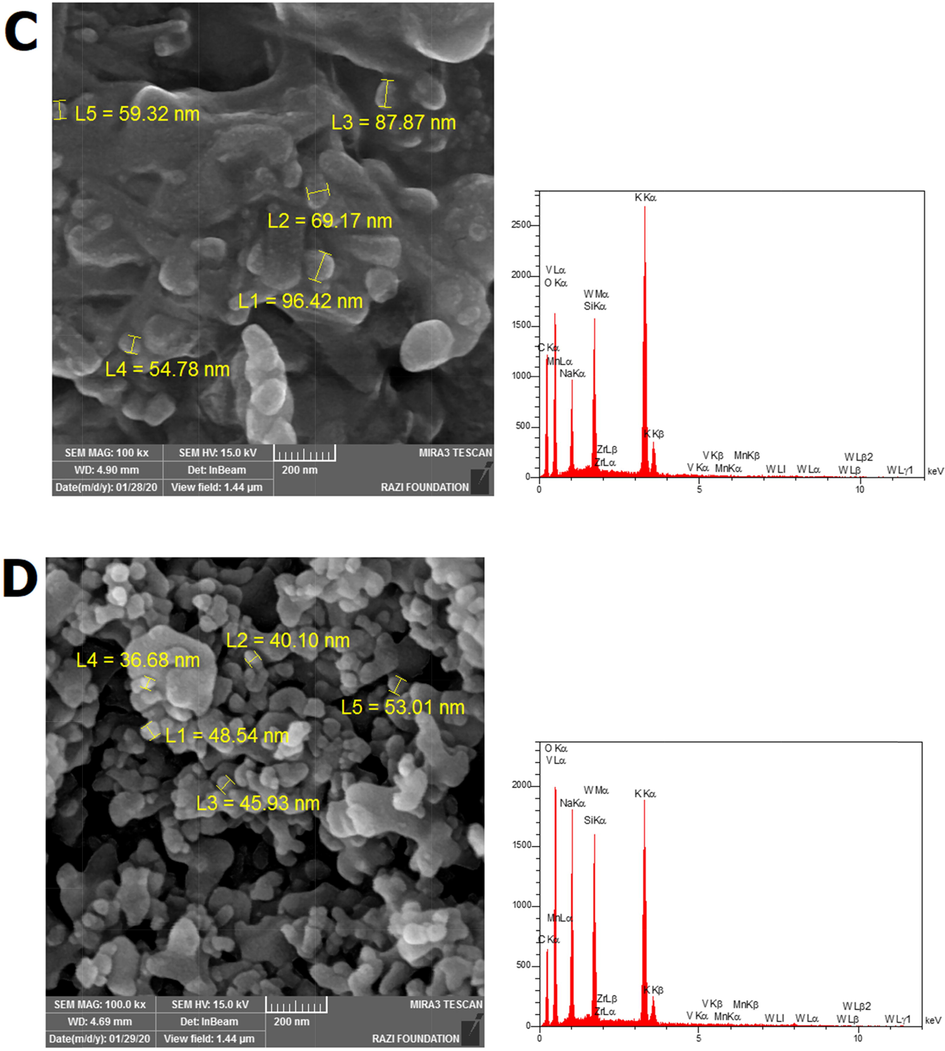
FE-SEM images and EDX graphs for all samples AS-2 (a), AS-3 (b), CS-1 (c), and CS-2 (d).
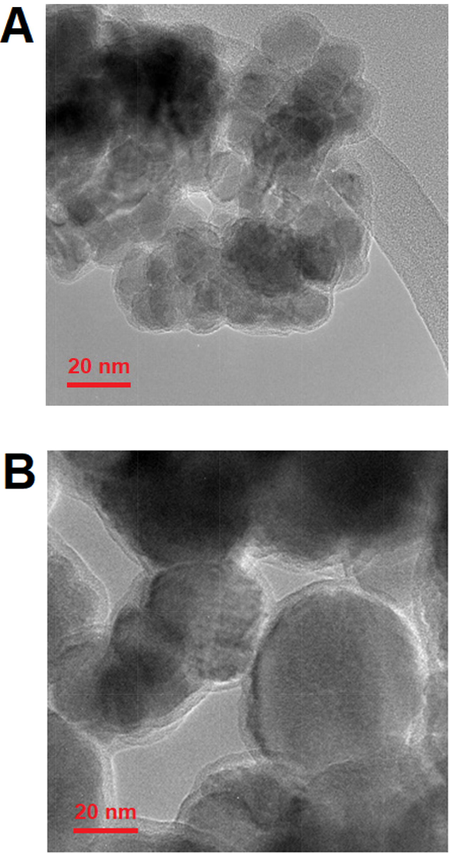
TEM images for samples AS-2 (a) and CS-2 (d).
According to Fig. 7, for the two catalysts CS-2 and AS-2, it is determined that the amount of carbon deposition is 0.71 % and 0.81 %, respectively, which shows the high resistance of these two catalysts against carbon sediment. The catalyst has lesser carbon sediment on its structure, which can be attributed to the presence of oxygen on its surface structure. The oxygen prevents the formation and deposition of carbon on it in the sense that by separating methane, the carbon reacts immediately with oxygen and reduces the amount of carbon deposition on the catalyst. However, it increases the amounts of CO and as by-products. Less carbon deposition on the core–shell catalyst than a conventional AS-2 catalyst indicates better resistance of the core–shell structure because of the higher oxygen content in the CS-2 which thus increases catalyst activity and makes it more stable.
3.2 Catalyst activity
The results of the
and
@(
) catalyst activity test with different ratios of Mn and Na2WO4 are presented in Table 3. The effect of the methane/oxygen ratio on conversion, selectivity, and yield are shown in Table 2, these experiments were performed inside the reactor. According to Table 2, as the ratio of methane to oxygen increases, the percentage of conversion decreases; The highest conversion percentage related to the ratio of methane/oxygen = 2 was reported. However, the selectivity of ethylene alone had an increase–decrease trend and the highest percentage of selectivity was recorded in the ratio of methane/oxygen equal to 4. Since achieving the highest conversion with the highest yield of
was the goal of optimization, the ratio of methane/oxygen = 2 was chosen (Nematollahi and Neyts, 2020).
T °C
Catalyst
21.79
27.99
11.02
17.07
66.78
1.82
23.68
43.10
25.56
775
2
AS-2
20.05
27.05
11.00
16.70
68.12
1.93
23.25
44.87
24.51
775
4
18.74
29.03
10.25
15.37
66.12
2.00
22.04
44.08
23.25
775
6
24.82
31.47
15.22
22.79
74.71
2.01
24.82
49.89
30.50
850
2
20.58
29.70
15.10
21.72
72.74
2.28
22.18
50.56
29.86
850
4
19.87
33.70
13.06
18.52
68.23
2.39
20.13
48.10
27.15
850
6
4.86
6.58
15.15
21.39
66.35
2.43
19.34
47.01
32.24
775
2
CS-2
5.91
8.31
15.17
21.20
66.63
2.52
18.95
47.68
31.82
775
4
7.67
12.58
14.07
19.59
64.10
2.55
18.06
46.05
30.55
775
6
8.82
12.42
18.54
25.42
69.59
2.69
18.84
50.75
36.52
850
2
11.02
14.39
18.40
24.79
70.32
2.88
18.13
52.19
35.25
850
4
14.35
18.05
17.50
23.43
68.62
2.95
17.37
51.25
34.15
850
6
Oxygen concentration is considered one of the main variables controlling the progress of reactions. The results showed that in the presence of oxygen, the formed
hydrocarbons can be easily converted into
. By increasing the oxygen concentration in the feed gas and producing
, methane was consumed at a higher rate through oxidation reactions (Fig. 5a). The yield of
is the product of methane conversion percentage and the selectivity of
(Fig. 5c). Therefore, the efficacy of
decreases as the molar ratio of methane to oxygen increases. If the amount of oxygen in the feed gas is reduced, the
production rate will always be lower than the
production rate. Therefore, as the methane to oxygen ratio increases from 2 to 6, methane conversion reduces, while the selectivity of
enhances (Fig. 5b). Therefore, higher methane conversion and
yield was obtained with a higher oxygen ratio (lower
ratio), and the hydrocarbons in the process are oxidized less to carbon dioxide for a
feed ratio (less than 2) (Liu et al., 2022).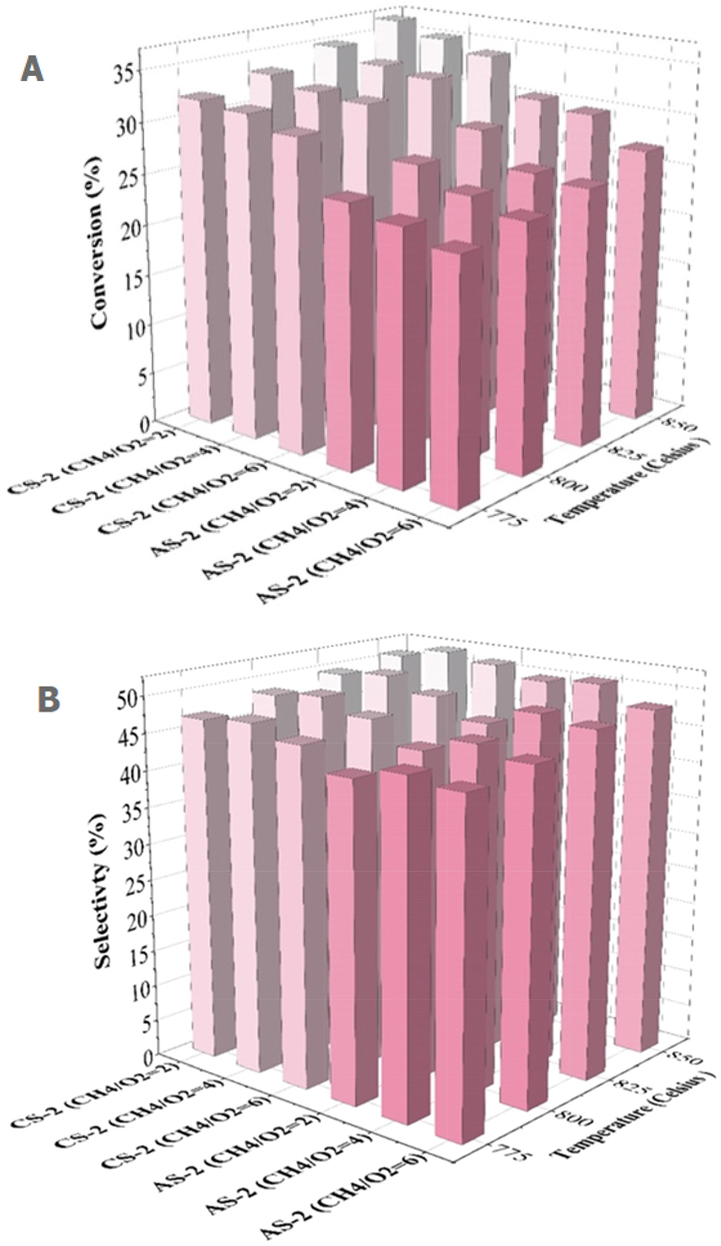
Methane conversion (a), selectivity (b) and
yield (c) curves in performance of AS-2 and CS-2 catalyst.
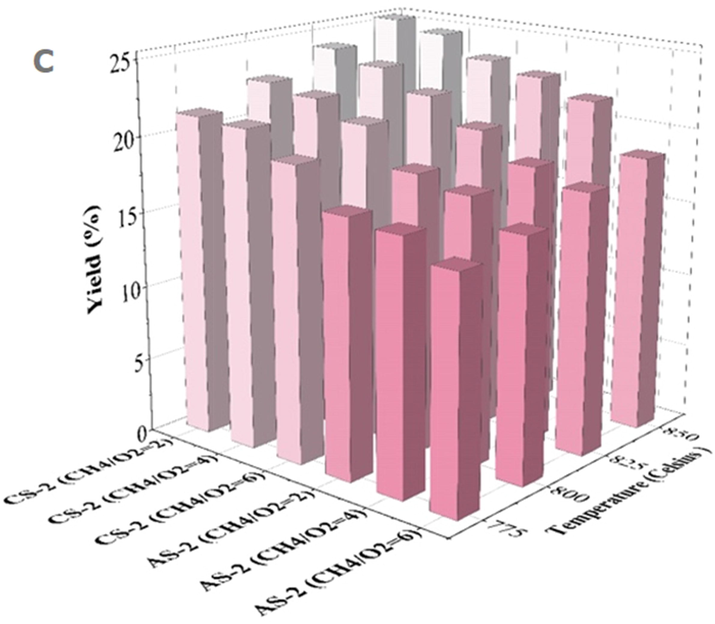
Methane conversion (a), selectivity (b) and
yield (c) curves in performance of AS-2 and CS-2 catalyst.
According to Fig. 5, the reactor tests for nano-catalytic activity suggest the reactor temperature range of 775–850 °C, the methane conversion, selectivity, and yield of compounds have presented the highest results. The optimum temperature was 850 °C. Methane conversion was insignificantly affected at high feed ratios for temperatures above 850 °C. The selectivity and the yield of compounds at the reactor output were enhanced by increasing the reaction temperature to 850 °C.
At both temperatures, below 775 °C and above 850 °C, the gas phase reaction controls the OCM reactions. Selectivity and performance both benefit from successive reactions resulting from the gas phase reaction and reach their highest values. Above 850 °C the oxidation of compounds was the main reaction causing low ethylene selectivity. As a result, product performance was diminished. Maximum performance for the catalytic activity was achieved when the whole oxygen in the reactor was consumed (Rosli et al., 2022). The best performance for these two families of catalysts in similar operating conditions is mentioned below:
I) AS-2 catalyst in the feed ratio = 2 and temperature of 850 °C resulting in methane conversion percentage of 30.5 % and yield of 22.79 %, selectivity of 74.71 % for and selectivity of 49.89 % for ethylene.
II) CS-2 which in the same operating conditions results in, the percentage of methane conversion of 36.52 %, yield of 25.42 %, selectivity of 69.59 % for , and selectivity of 50.75 % for ethylene.
Another distinguishing result was the difference in the amount of ethane produced as one of the by-products, which for the core–shell catalyst was lesser than the conventional AS-2 catalyst. Ethane reduces the selectivity and yield of the product for the core–shell catalyst.
The ethylene produced by the core–shell catalyst was about 21 % more in comparison with the amount of ethylene produced by the conventional catalyst. And the ethane produced as a by-product was approximately 10 % lower for the core–shell catalyst resulting in a higher ratio of ethylene to ethane that was produced (Yang et al., 2022).
Regarding the AS-2 catalyst as well as its core–shell catalyst, it can be noted that the higher activity of these two catalysts in comparison with other catalysts can be related to the proper distribution of on the catalyst structure. The active phase of these catalysts consists of quadrilateral . This structure was changed during the catalytic cycle between and structures during the chemical separation of methane and subsequent methane oxidation. The main role of Na in the catalyst was to stabilize the quadrilateral structure versus the octahedral structure. The Mn in was a mediator that contributes to the use of the oxygen produced in the recovery of . The cooperation of two cycles of reduction of W and Mn leads to the development of OCM. The second role of sodium in the structure of this catalyst was to facilitate the formation of the cristobalite phase at low temperatures such as 800 °C, which was lower than the calcination temperature of . The presence of the cristobalite phase in stabilizes , sodium plays a dual role as a chemical and structural enhancer (Park et al., 2020).
One of the concerns is that at high temperatures of the OCM process, the core–shell structure breaks down to form a composite mixture composed of , , , and . In this research, a solution was found to solve this problem; the presence of excess sodium in the nano-catalytic structure of caused a very good distribution of on containing Mn. This proper distribution makes it easy to form . This prevents from being converted to due to the high amount of sodium and oxygen in the system, and improves the performance of the core–shell catalyst in comparison with a conventional catalyst. Therefore, the core–shell structure and the proper distribution of the phase in its structure improve the performance of the catalyst in the production of compounds (Zhou et al., 2021).
Based on the results of the tests and the comparison of the data obtained from the conversion percentage and the yield of ethylene production, the best performance is related to the two catalysts AS-2 and CS-2. So, a stability test at 850 °C for 50 h with a feed-to-oxygen ratio of 2 was performed on these two catalysts to test their stability in addition to being superior in yield and selectivity. The results of the stability test indicated that both catalysts have high stability in ethylene production (Fig. 6).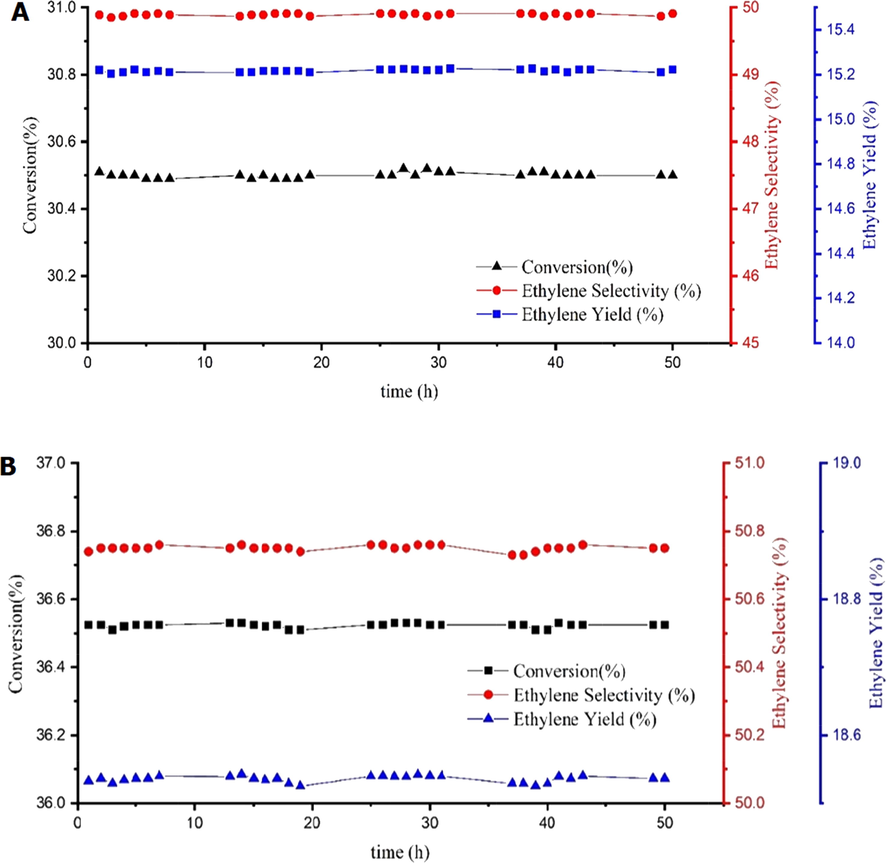
Conversion, ethylene selectivity, and ethylene yield for AS-2 (a) and CS-2 (b) catalyst over 50 h with feed containing methane, oxygen, and nitrogen at a ratio of 2:1:7 at 850 °C.
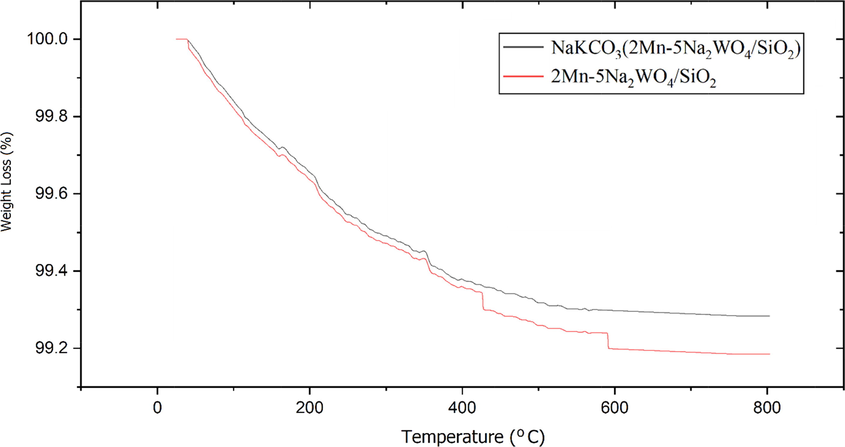
TGA test results for AS-2 and CS-2 catalysts.
4 Conclusion
The purpose of the core–shell catalyst synthesis is to maintain the stability of the catalyst as the core is coated by the shell to withstand the high heat of reaction. Shells protected at high reaction temperatures for longer periods improve catalytic activity. In the current research, core–shell structures composed of were prepared as precursors of OCM catalysts. The aim of this paper was to investigate the proper conditions for higher performance of the catalyst with and without shell, in terms of the selectivity and yield of and in terms of catalyst stability for a longer period. The advantage of the shell is its high thermal resistance since it has no decomposition, no interference in the process, and less carbon deposition. The core–shell catalyst is more stable, and its activity loss and degradation occur later. The core–shell catalyst limits ethylene reabsorption and does not allow it to react with unsaturated cations. In addition to increasing the yield and selectivity of ethylene as the main product, it reduces ethane production as one of the by-products. The advantage of the core–shell catalyst is that it is cheap and readily available. The production of other by-products in the core–shell catalyst is much lower compared to other catalysts, and this reduces the cost of separation of by-products thereby improving the performance of the catalyst in the production of the main ethylene product. In conclusion, the CS-2 core–shell catalyst has a stable methane conversion of about 36.52 % and an ethylene yield of about 18.54 %, and a yield of about 25.42 % at 850 °C with a ratio of 2, which for other catalysts are around 30.50 %, 15.22 %, and 22.79 %, respectively.
Acknowledgements
This research was supported by Bandar Imam Petrochemical Company (0861638903).
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
References
- Tri-reforming of methane for syngas production using Ni catalysts: current status and future outlook. Catal. Today 2022
- [CrossRef] [Google Scholar]
- Design of a core–shell Pt–SiO2 catalyst in a reverse microemulsion system: distinctive kinetics on CO oxidation at low temperature. J. Catal.. 2016;340:368-375.
- [CrossRef] [Google Scholar]
- State-of-art modifications of heterogeneous catalysts for CO2 methanation – active sites, surface basicity and oxygen defects. Catal. Today. 2022;402:88-103.
- [CrossRef] [Google Scholar]
- Insight into deactivation of the carbon-/sintering-resistant Ni@Silicalite-1 for catalytic partial oxidation of methane to syngas. Fuel. 2022;320:123892
- [CrossRef] [Google Scholar]
- Oxidative dehydrogenation of ethane on dynamically rearranging supported chloride catalysts. J. Am. Chem. Soc.. 2014;136:12691-12701.
- [CrossRef] [Google Scholar]
- CS@Cu2O and magnetic Fe3O4@SiO2-pAMBA-CS-Cu2O as heterogeneous catalysts for CuAAC click reaction. Arab. J. Chem.. 2022;15
- [CrossRef] [Google Scholar]
- Synthesis of Na2WO4-MnxOy supported on SiO2 or La2O3 as fiber catalysts by electrospinning for oxidative coupling of methane: synthesis of Na2WO4-MnxOy supported on SiO2 or La2O3 as fiber catalysts. Arab. J. Chem.. 2022;15
- [CrossRef] [Google Scholar]
- High coke resistance Ni-SiO2@SiO2 core-shell catalyst for biogas dry reforming: effects of Ni loading and calcination temperature. Fuel. 2022;330:125609
- [CrossRef] [Google Scholar]
- Development of etched SiO2@Pt@ZrO2 core-shell catalyst for CO and C3H6 oxidation at low temperature. Appl. Surf. Sci.. 2022;575
- [CrossRef] [Google Scholar]
- From fundamentals to chemical engineering on oxidative coupling of methane for ethylene production: a review. Carbon Resour. Convers.. 2022;5:1-14.
- [CrossRef] [Google Scholar]
- Lyu, J.M., Yu, S., Peng, Z., Zhou, J., Liu, Z., Li, X.Y., Yu-Li, Chen, L.H., Su, B.L., 2022. Control of the proximity of bifunctional zeolite@Al2O3 catalysts for efficient methanol conversion into hydrocarbons. Catal. Today. https://doi.org/10.1016/j.cattod.2022.07.017.
- Direct oxidation of methane to methanol on Co embedded N-doped graphene: comparing the role of N2O and O2 as oxidants. Appl. Catal. A Gen.. 2020;602:117716
- [CrossRef] [Google Scholar]
- Valorisation of nuts biowaste: prospects in sustainable bio(nano)catalysts and environmental applications. J. Clean. Prod.. 2022;347
- [CrossRef] [Google Scholar]
- Oxidative coupling of methane (OCM): an overview of the challenges and opportunities for developing new technologies. J. Nat. Gas Sci. Eng.. 2021;96:104254
- [CrossRef] [Google Scholar]
- SiO2@MnO: X@Na2WO4@SiO2core-shell-derived catalyst for oxidative coupling of methane. RSC Adv.. 2020;10:37749-37756.
- [CrossRef] [Google Scholar]
- Methane decomposition for hydrogen production: a comprehensive review on catalyst selection and reactor systems. Renew. Sustain. Energy Rev.. 2022;168:112774
- [CrossRef] [Google Scholar]
- The effect of oxygen mobility/vacancy on carbon gasification in nano catalytic dry reforming of methane: a review. J. CO2 Util.. 2022;63:102109
- [CrossRef] [Google Scholar]
- Insights into mechanism of catalytic ozonation of cinnamyl alcohol over core–shell Fe3O4@SiO2@La2O3 catalyst. Sep. Purif. Technol.. 2022;282:119969
- [CrossRef] [Google Scholar]
- Oxidative coupling of methane—comparisons of MnTiO3–Na2WO4 and MnOx–TiO2–Na2WO4 catalysts on different silica supports. Sci. Rep.. 2022;12:1-16.
- [CrossRef] [Google Scholar]
- NiO@SiO2 core-shell catalyst for low-temperature methanation of syngas in slurry reactor. Ranliao Huaxue Xuebao/J. Fuel Chem. Technol.. 2016;44:548-556.
- [CrossRef] [Google Scholar]
- CO2 conversion via dry reforming of methane on a core-shell Ru@SiO2 catalyst. J. CO2 Util.. 2022;57:101893
- [CrossRef] [Google Scholar]
- A review of recent efforts to promote dry reforming of methane (DRM) to syngas production via bimetallic catalyst formulations. Appl. Catal. B Environ.. 2021;296:120210
- [CrossRef] [Google Scholar]
- Steam reforming of methane: current states of catalyst design and process upgrading. Renew. Sustain. Energy Rev.. 2021;149:111330
- [CrossRef] [Google Scholar]
- A core-shell NiCu@NiCuOOH 3D electrode induced by surface electrochemical reconstruction for the ammonia oxidation reaction. Int. J. Hydrogen Energy. 2022;47:16080-16091.
- [CrossRef] [Google Scholar]
- Facile synthesis of multi-shelled MnO2–Co3O4 hollow spheres with superior catalytic activity for CO oxidation. Ceram. Int.. 2021;47:18411-18416.
- [CrossRef] [Google Scholar]
- Active oxygen center in oxidative coupling of methane on La2O3 catalyst. J. Energy Chem.. 2021;60:649-659.
- [CrossRef] [Google Scholar]
- The synthesis and characterization of Fe2O3@SiO2–SO3H nanofibers as a novel magnetic core-shell catalyst for formamidine and formamide synthesis. Heliyon. 2021;7
- [CrossRef] [Google Scholar]







