Translate this page into:
Synthesis of new azetidinonyl/thiazolidinonyl quinazolinone derivatives as antiparkinsonian agents
*Corresponding author. Tel.: +91 0121 2764084 rajputak@gmail.com (Ashok Kumar)
-
Received: ,
Accepted: ,
This article was originally published by Elsevier and was migrated to Scientific Scholar after the change of Publisher.
Abstract
Several 3-amantadinyl-2-[(2-substituted benzylidenehydrazinyl)methyl]-quinazolin-4(3H)-ones (5a–5l) were prepared by the reaction of 3-amantadinyl-2-hydrazinylmethyl substituted quinazolin-4(3H)-ones (4a–4b) with various substituted aromatic aldehydes. Cycloaddition of compounds (5a–5l) with thioglycolic acid in the presence of anhydrous zinc chloride yielded 3-amantadinyl-2-[((substitutedphenyl)-4-oxo-thiazolidin-3-yl)methylamino]-quinazolin-4(3H)-ones (6a–6l). Compounds 5a–5l on further reaction with chloro acetyl chloride in the presence of triethylamine gave 3-amantadinyl-2-[((substitutedphenyl)-3-chloro-2-oxo-azetidin-1-yl)methylamino]-quinazolin-4(3H)-ones (7a–7l). The compounds 5a–5l, 6a–6l and 7a–7l were screened for their antiparkinsonian activity. The most active compound was 6g i.e. 3-amantadinyl-6-bromo-2-[((3,4-dimethoxyphenyl)-4-oxo-thiazolidin-3-yl)methylamino]-quinazolin-4(3H)-ones. Structures of the newly synthesized compounds were established on the basis of elemental and spectral (IR, 1H NMR and mass) analysis.
Keywords
Amantadinylbenzylidinylquinazolinones
Amantadinylazetidinonyl/thiazolidinonyl quinazolinones
Antiparkinsonian activity
Acute toxicity
1 Introduction
The deficiency of dopamine in the basal ganglia of parkinsonian patients has been established as a biochemical lesion in all forms of Parkinsonism. The Levodopa (L-DOPA), a precursor of dopamine, acts on the biochemical defects of parkinsonism and is the most effective drug available for treatment of disease. Furthermore, amantadine is another adjustment drug (dopamine facility drug) for the treatment of Parkinsonism (Singer et al., 2006; Luginger et al., 2000; Severy, 1977). Recently various heterocyclic derivatives of quinazolinone have been reported to possess CNS and antiparkinsonian activity, at substitution IInd and IIIrd positions of quinazolinones play a pivotal role in modulating the antiparkinsonian properties. However, five membered thiazolidinone and four membered azetidinones do not appear to have been linked with quinazolinone, so for our study has shown that the substitution of amantadinyl at IIIrd position and azetidinone and thiozolidinone moieties at IInd position of quinazolinone yielded better antiparkinsonian agent (Panday et al., 2005; Nathani et al., 1989; Srivastava et al., 1987). These compounds were evaluated for their biological activity. However, abundance of dopa decarboxylase in peripheral tissue has necessitated the use of L-dopa for its entry into brain to liberate dopamine by dopa decarboxylase and thereby maintaining optimal dopamine concentration for desired beneficial effects. In addition, the various side effects associated with L-dopa therapy prompted the synthesis of substituted quinazolinones containing 3,4-dimethoxydopamine moiety in their structure in an attempt to provide preferential transport of these compounds to brain and their possible biotransformation to liberate dopamine and/or dopamine like substances by the action of the drug metabolizing enzyme systems and thus being independent of the use of brain dopa decarboxylase. Furthermore, thiazolidinones and azetidinones of various heterocycles have also been reported to possess antiparkinsonian activity (Kumar et al., 1982; Goel et al., 2005; Srivastava et al., 1990). In the light of the above observations we have synthesized new substituted quinazolinone derivatives by incorporating azetidinones and thiazolidinones moieties with the hope to get better antiparkinsonian agents.
2 Chemistry
The synthetic route of compounds is outlined in Scheme 1. Compounds 1a and 1b were synthesized by the reaction of anthranilic acid and 5-bromo anthranilic acid with acidic anhydride, respectively. Compounds 1a–1b on reaction with amantadine yielded substituted 2-methyl benzo [1,3]oxazin-4-ones i.e. compounds 2a–2b. Compounds 2a–2b were reacted with bromine in glacial acetic acid to give 3-amantadinyl-2-bromomethylsubstitutedquinazolinon-4(3H)-ones 3a–3b which on reaction with hydrazine hydrate in methanol yielded 3-amantadinyl-2-(hydrazinylmethyl)substitutedquinazolin-4(3H)-ones (4a–4b). Compounds 4a–4b were reacted with various substituted aromatic aldehydes in the presence of 2% NaOH to give 3-amantadinyl-2-[(substitutedbenzylidinehyrazinyl)methyl]substitutedquinazolin-4(3H)-ones i.e. compounds 5a–5l, which on reaction with thioglycolic acid in the presence of anhydrous zinc chloride yielded 3-amantadinyl-2-[((substitutedphenyl)-4-oxo-thiazolidin-3-yl)methylamino]-quinazolin-4(3H)-ones 6a–6l. Compounds 5a–5l on further reaction with chloroacetyl chloride in the presence of triethylamine yielded 3-amantadinyl-2-[((substitutedphenyl)-3-chloro-2-oxo-azetidin-1-yl)methylamino]-quinazolin-4(3H)-ones (7a–7l).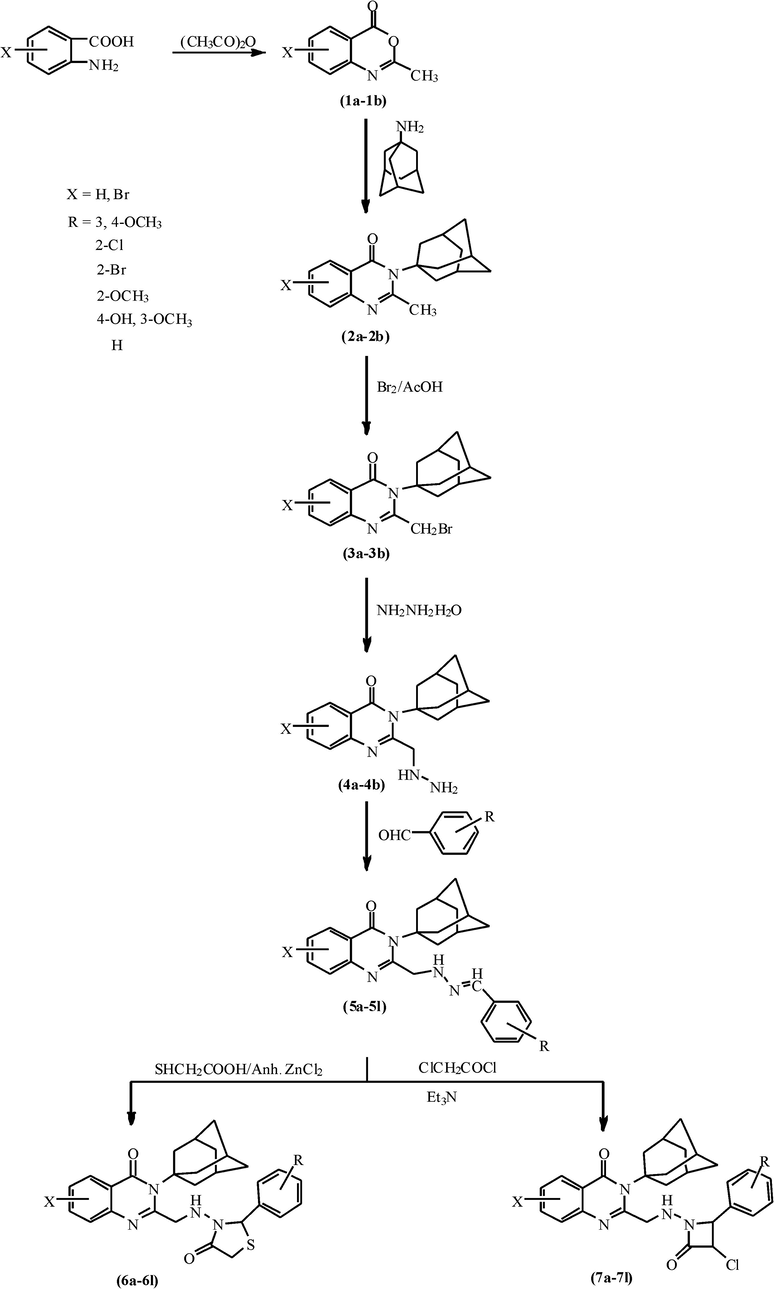
3 Pharmacology
All the newly synthesized compounds were evaluated for their antiparkinsonian activity.
The study was carried out on albino rats weighing 150–200 g and mice 25–30 g of either sex. The animals were fed and allowed water ad libidum. The number of animals in each group was 5. All the compounds were administered in a dose of 100 mg/kg i.p.
3.1 Tremor
This activity was done by the method of Coward and Doggett (1977). Tremors were induced by oxotremorine (OT) (0.5 mg/kg i.p) in mice 45 min after pretreatment with the test compounds. After 5 min of OT injection tremors were assessed visually and scored as: 0 = no tremor; 1 = occasional tremor; 2 = intermittent tremors; 3 = continuous tremors. Each animal of a group was scored and tremor index (mean score for each group) was determined.
3.2 Rigidity
Reserpine (5 mg/kg i.p.) was administered in rats to produce rigidity and after 15 min test compounds were injected. Rigidity was measured 1 h after reserpine administration. To measure rigidity, rats were grasped immediately below forelimbs and slight pressure was applied upward against the hind limbs. The degree of resistance was scored according to(Goldstein et al., 1975): 0 = no resistance; 1 = normal resistance; 2 = complete resistance. A score of 2 was selected as criterion for rigidity and expressed as percentage of animals showing rigidity (score 2) in a group.
3.3 Hypokinesia
This was performed according to the method of Morpugo (1962). It was produced by reserpine (5 mg/kg i.p.) in rats. Locomotor activity was measured after 2 h by placing each group of rats in a photoactometer for 15 min and total counts were recorded. The test compounds were administered 15 min after reserpine administration. The percent increase or decrease in counts was calculated on the basis of counts of untreated groups.
3.3.1 Catatonia
Reserpine (5 mg/kg i.p.) was administered in rats and after 15 min test compounds were administered. Catatonia was observed after 4 h and was scored according to the method of Dews (1953).
3.3.2 Acute toxicity study
The compounds which showed significant antiparkinsonian activity were investigated for their acute toxicity study in mice (25–30 g) of either sex. The compounds were given orally at graded doses to separated group of six animals. After 24 h of administration, percent mortality in each group was observed from the data obtained. LD50 values were calculated by the method Smith (1960).
4 Results and discussion
Compounds 5a–5l, 6a–6l and 7a–7l were screened for their antiparkinsonian activity. Pharmacological data of all the synthesized compounds of this series have been reported in Tables 1–3.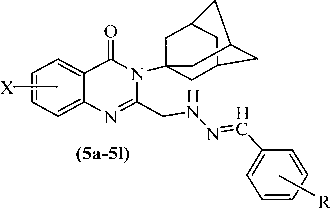
Comp No.
X
R
Oxotremorine induced tremors in mice (0.5 mg/kg)
Reserpine (5 mg/kg induced)
LD50 mg/kg i.p.
Rigidity (%)
Hypokinesia (% counts)
Catatonia (mean scores)
Control
3.0 ± 0
100
14.06
3.0 ± 0
L-dopa
2.60 ± 0.24
80
30.39
2.80 ± 0.08
5a
H
3,4-OCH3
2.65 ± 0.17
80
25.20
2.24 ± 0.16
>1000
5b
H
2-Cl
2.67 ± 0.28
100
22.10
2.25 ± 0.18
>1000
5c
H
2-Br
2.71 ± 0.13
70
21.08
2.60 ± 0.24
>1000
5d
H
2-OCH3
2.73 ± 0.17
80
15.20
2.82 ± 0.22
>1000
5e
H
4-OH, 3-OCH3
2.76 ± 0.21
90
14.70
2.71 ± 0.23
>1000
5f
H
H
2.50 ± 0.26
100
13.50
2.90 ± 0.26
>1000
5g
Br
3,4-OCH3
2.55 ± 0.25
40
41.70
1.90 ± 0.19
>1000
5h
Br
2-Cl
2.57 ± 0.29
50
39.40
2.43 ± 0.22
>1000
5i
Br
2-Br
2.59 ± 0.25
60
36.50
2.67 ± 0.25
>1000
5j
Br
2-OCH3
2.61 ± 0.32
80
34.60
2.70 ± 0.23
>1000
5k
Br
4-OH, 3-OCH3
2.52 ± 0.17
70
32.10
2.90 ± 0.25
>1000
5l
Br
H
2.76 ± 0.30
90
30.20
2.92 ± 0.28
>1000
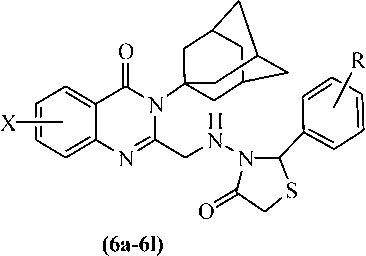
Comp No.
X
R
Oxotremorine induced tremors in mice (0.5 mg/kg)
Reserpine (5 mg/kg induced)
LD50 mg/kg i.p.
Rigidity (%)
Hypokinesia (% counts)
Catatonia (mean scores)
Control
–
–
3.0 ± 0
100
14.06
3.0 ± 0
L-dopa
–
–
2.6 ± 0.24
80
30.39
2.8 ± 0.08
6a
H
3,4-OCH3
2.32 ± 0.19
40
57.10
1.82 ± 0.17
>1000
6b
H
2-Cl
2.33 ± 0.22
30
56.40
1.86 ± 0.21
>1000
6c
H
2-Br
2.37 ± 0.25
40
54.70
1.95 ± 0.24
>1000
6d
H
2-OCH3
2.40 ± 0.27
30
52.90
2.10 ± 0.25
>1000
6e
H
4-OH, 3-OCH3
2.42 ± 0.26
50
51.30
2.60 ± 0.23
>1000
6f
H
H
2.45 ± 0.29
60
49.20
2.80 ± 0.26
>1000
6g
Br
3,4-OCH3
2.25 ± 0.15
10
82.70
1.30 ± 0.13
>2000
6h
Br
2-Cl
2.27 ± 0.18
20
81.10
1.40 ± 0.17
>1000
6i
Br
2-Br
2.28 ± 0.22
30
77.50
1.90 ± 0.19
>1000
6j
Br
2-OCH3
2.27 ± 0.26
60
75.30
2.05 ± 0.23
>1000
6k
Br
4-OH, 3-OCH3
2.29 ± 0.21
100
74.60
2.10 ± 0.26
>1000
6l
Br
H
2.30 ± 0.24
100
73.70
2.21 ± 0.29
>1000
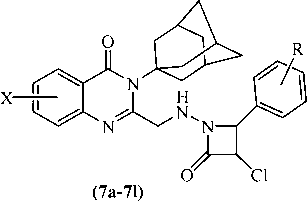
Comp No.
X
R
Oxotremorine induced tremors in mice (0.5 mg/kg)
Reserpine (5 mg/kg induced)
LD50 mg/kg i.p.
Rigidity (%)
Hypokinesia (% counts)
Catatonia (mean scores)
Control
–
–
3.0 ± 0
100
14.06
3.0 ± 0
L-dopa
–
–
2.60 ± 0.26
80
30.39
2.80 ± 0.08
7a
H
3,4-OCH3
2.52 ± 0.18
30
60.20
1.82 ± 0.23
>1000
7b
H
2-Cl
2.54 ± 0.22
40
59.10
1.86 ± 0.24
>1000
7c
H
2-Br
2.53 ± 0.23
50
58.30
1.95 ± 0.27
>1000
7d
H
2-OCH3
2.56 ± 0.25
60
56.60
2.10 ± 0.24
>1000
7e
H
4-OH, 3-OCH3
2.58 ± 0.26
40
55.40
2.60 ± 0.27
>1000
7f
H
H
2.59 ± 0.29
80
55.20
2.80 ± 0.29
>1000
7g
Br
3,4-OCH3
2.42 ± 0.24
20
64.60
1.30 ± 0.12
>1000
7h
Br
2-Cl
2.43 ± 0.26
30
63.70
1.40 ± 0.15
>1000
7i
Br
2-Br
2.46 ± 0.27
40
62.40
1.90 ± 0.16
>1000
7j
Br
2-OCH3
2.48 ± 0.29
40
61.30
2.05 ± 0.19
>1000
7k
Br
4-OH, 3-OCH3
2.51 ± 0.31
60
63.20
2.10 ± 0.23
>1000
7l
Br
H
2.49 ± 0.32
60
73.70
2.21 ± 0.26
>1000
4.1 Antitremor activity
The 6a–6l showed better antitremor activity than L-dopa and 6g exhibited the most potent activity. Compounds 7g–7j in general showed lesser antitremor activity than thiazolidinones. However, these compounds exhibited almost equipotent activity as L-dopa.
4.2 Antirigidity activity
Compounds 6g, 6h and 7g decreased rigidity by 90%, 80% and 80%, respectively, while L-dopa decreased rigidity up to only 20% at the same dose. Compounds 5a, 5d, 5j, and 7f were equipotent to L-dopa. Compounds 5c, 5g–5i, 5k, 6a–6f, 6i–6j, 7a–7e, 7g–7i, 7k and 7l, of this series have shown good antirigidity activity. It can be concluded that thiazolidinone derivatives elicited better antirigidity activity than benzylidenes and azetidinones derivatives.
4.3 Antihypokinetic activity
Compounds 5g–5k showed almost approximately equal antihypokinetic activity as L-dopa, whereas compounds 6a–6l elicited better activity than standard drug. The most active antihypokinetic compound of this series is 6g, which exhibited 82.70% antihypokinetic activity.
4.4 Anticatatonic activity
Catatonia was significantly decreased by compounds 6g, 6h, 7g, and 7h. Compounds 5g, 6i, and 7i exhibited good anticatatonic activity.
4.5 Acute toxicity
The newly synthesized compounds were also tested for approximate lethal dose LD50 and were found to exhibit a higher value of LD50 i.e. more than 1000 mg/kg i.p. except compound 6g, which exhibited LD50 of more than 2000 mg/kg i.p. (maximum dose tested), thus indicating the safer nature of the compounds.
5 Conclusion
Thiazolidinone derivatives showed more potent antiparkinsonian activity than azetidinone derivatives. Moreover, the substitution with 3,4-dimethoxyphenyl group was found to beneficial for antiparkinsonian activity.
6 Experimental protocols
6.1 Chemistry
All reagents and solvents were generally used as received from the commercial supplier. Reactions were routinely performed in an oven-dried glassware. The melting points of compounds were determined in open capillaries with the help of thermonic melting point apparatus and were uncorrected. The purity of the compounds was checked by thin layer chromatography (TLC) performed on silica gel G coated plate of 0.5 mm thickness. The eluent was a mixture of different polar and nonpolar solvents in different proportions and spots were visualized under iodine chamber. Elemental analysis (C, H, N) of all the compounds was determined through the Perkin–Elmer 2400 elemental analyzer and results were found within ±0.4% of theoretical values. Infra red (IR) spectra were recorded in KBr on the Perkin–Elmer-spectrum RX-I instrument and υmax was recorded in cm−1. 1H NMR spectra were recorded by the Bruker DR-X-400 FT-NMR instrument using CDCl3 and DMSO-d6 as solvents and tetramethysilane (TMS) as an internal reference as δ (ppm).
6.1.1 General procedure for synthesis of 2-methyl-6-substitutedbenzo(1,3)oxazin-4-ones (1a–1b)
These compounds were prepared according to the method of Bogert and Soil (1907). A mixture of unsubstituted/6-bromoanthranilic acid (1.0 mol) and acetic anhydride (0.02 mol) was refluxed for 2–3 h with occasional stirring. The excess of acetic anhydride was distilled off. On cooling, a solid was separated out which was filtered and washed with appropriate solvent and dried 1a–1b.
6.1.1.1 2-Methyl-4H-benzo(1,3)oxazin-4-one (1a)
Yield 86% (Methanol): m.p. 79–81 °C; IR (KBr) υmax in cm−1 1701 (C⚌O), 1610 (C C of aromatic ring), 1570 (C⚌N), 1302 (C–N); 1H NMR (CDCl3) δ in ppm: 6.65–7.73 (m, 4H, Ar-H), 1.31 (s, 3H, CH3). MS: [M]+ at m/z 161.16. Anal. Calc. for C9H7NO2: C, 67.07; H, 4.38; N, 8.69. Found: C, 67.15; H, 4.35; N, 8.65%.
C of aromatic ring), 1570 (C⚌N), 1302 (C–N); 1H NMR (CDCl3) δ in ppm: 6.65–7.73 (m, 4H, Ar-H), 1.31 (s, 3H, CH3). MS: [M]+ at m/z 161.16. Anal. Calc. for C9H7NO2: C, 67.07; H, 4.38; N, 8.69. Found: C, 67.15; H, 4.35; N, 8.65%.
6.1.1.2 6-Bromo-2-methyl-4H-benzo(1,3)oxazin-4-one (1b)
Yield 83% (Petroleum ether): m.p. 80 °C; IR (KBr) υmax in cm−1 1704 (C⚌O), 1612 (C C of aromatic ring), 1575 (C⚌N), 1304 (C–N), 610 (C–Br); 1H NMR (CDCl3) δ in ppm: 6.71–7.65 (m, 3H, Ar-H), 1.30 (s, 3H, CH3). MS: [M]+ at m/z 240.5. Anal. Calc. for C9H6BrNO2: C, 45.03; H, 2.52; N, 5.83. Found: C, 45.15; H, 2.55; N, 5.83%.
C of aromatic ring), 1575 (C⚌N), 1304 (C–N), 610 (C–Br); 1H NMR (CDCl3) δ in ppm: 6.71–7.65 (m, 3H, Ar-H), 1.30 (s, 3H, CH3). MS: [M]+ at m/z 240.5. Anal. Calc. for C9H6BrNO2: C, 45.03; H, 2.52; N, 5.83. Found: C, 45.15; H, 2.55; N, 5.83%.
6.1.2 General procedure for the synthesis of 3-amantadinyl-2-methyl-6-monosubstitutedquinazolin-4(3H)-ones 2a–2b
To a solution of compound 1a–1b amantadine (0.02 mol) was added, the mixture was heated on a free flame for 10–20 min in a conical flask. After the disappearance of water droplets in a conical flask it was kept at room temperature. On cooling a jelly like mass was obtained which was dissolved in ethanol, was refluxed and poured into water. The solid thus obtained was filtered, dried and finally recrystallized from the appropriate solvent to obtain compounds 2a–2b.
6.1.2.1 3-Amantadinyl-2-methylquinazolin-4(3H)–one (2a)
Yield 82% (Methanol): m.p. 205 °C; IR (KBr) υmax in cm−1: 1706 (C⚌O), 1615 (C C of aromatic ring), 1571 (C⚌N), 1300 (C–N); 1H NMR (DMSO-d6) δ in ppm: 6.73–7.70 (m, 4H, Ar-H), 1.27 (s, 3H, CH3), 1.30 (m, 15H, amantadinyl ring). MS: [M]+ at m/z 294.39. Anal. Calc. for C19H22N2O: C, 77.52; H, 7.53; N, 9.52. Found: C, 77.63; H, 7.67; N, 9.44%.
C of aromatic ring), 1571 (C⚌N), 1300 (C–N); 1H NMR (DMSO-d6) δ in ppm: 6.73–7.70 (m, 4H, Ar-H), 1.27 (s, 3H, CH3), 1.30 (m, 15H, amantadinyl ring). MS: [M]+ at m/z 294.39. Anal. Calc. for C19H22N2O: C, 77.52; H, 7.53; N, 9.52. Found: C, 77.63; H, 7.67; N, 9.44%.
6.1.2.2 3-Amantadinyl-6-bromo-2-methylquinazolin-4(3H)-one (2b)
Yield 78% (Ethanol): m.p. 201 °C; IR (KBr) υmax in cm−1: 1708 (C⚌O), 1616 (C C of aromatic ring), 1571 (C⚌N), 1302 (C–N), 613 (C–Br); 1H NMR (CDCl3) δ in ppm: 1.29 (m, 15H, amantadinyl ring), 6.78–7.37 (m, 3H, Ar-H), 1.32 (s, 3H, CH3). MS: [M]+ at m/z 373.29. Anal. Calc. for C19H21BrN2O: C, 61.13; H, 5.67; N, 7.50. Found: C, 61.22; H, 5.65; N, 7.53%.
C of aromatic ring), 1571 (C⚌N), 1302 (C–N), 613 (C–Br); 1H NMR (CDCl3) δ in ppm: 1.29 (m, 15H, amantadinyl ring), 6.78–7.37 (m, 3H, Ar-H), 1.32 (s, 3H, CH3). MS: [M]+ at m/z 373.29. Anal. Calc. for C19H21BrN2O: C, 61.13; H, 5.67; N, 7.50. Found: C, 61.22; H, 5.65; N, 7.53%.
6.1.3 General procedure for synthesis of 3-amantadinyl-2-bromomethylsubstitutedquinazolin-4-(3H) ones 3a–3b
Bromine (0.4 mol) in acetic acid was added dropwise to the solution of compound 2a–ab in acetic acid (50 mL). The reaction mixture was poured onto crushed ice, then left overnight at room temperature. The precipitate thus obtained was recrystallized with suitable solvents to furnish compounds 3a–3b.
6.1.3.1 3-Amantadinyl-2-bromomethylquinazolin-4(3H)-one (3a)
Yield 76% (Methanol): m.p. 198 °C; IR (KBr) υmax in cm−1: 1710 (C⚌O), 1619 (C C of aromatic ring), 1579 (C⚌N), 1305 (C–N); 1H NMR (CDCl3) δ in ppm: 6.67–7.72 (m, 4H, Ar-H), 1.32 (m, 15H, amantadinyl ring), 1.25 (s, 2H, CH2). MS: [M]+ at m/z 373.29. Anal. Calc. for C19H21BrN2O: C, 61.13; H, 5.67; N, 7.50. Found: C, 61.24; H, 5.58; N, 7.43%.
C of aromatic ring), 1579 (C⚌N), 1305 (C–N); 1H NMR (CDCl3) δ in ppm: 6.67–7.72 (m, 4H, Ar-H), 1.32 (m, 15H, amantadinyl ring), 1.25 (s, 2H, CH2). MS: [M]+ at m/z 373.29. Anal. Calc. for C19H21BrN2O: C, 61.13; H, 5.67; N, 7.50. Found: C, 61.24; H, 5.58; N, 7.43%.
6.1.3.2 3-Amantadinyl-6-bromo-2-(bromomethyl)quinazolin-4(3H)-one (3b)
Yield 75% (Methanol): m.p. 201 °C; IR (KBr) υmax in cm−1: 1712 (C⚌O), 1621 (C C of aromatic ring), 1574 (C⚌N), 1303 (C–N), 615 (C–Br); 1H NMR (DMSO-d6) δ in ppm: 6.79–7.92 (m, 3H, Ar-H), 1.31 (s, 2H, CH2), 1.25 (m, 15H, amantadinyl ring). MS: [M]+ at m/z 452.18. Anal. Calc. for C19H20Br2N2O: C, 50.47; H, 4.46; N, 6.20. Found: C, 50.43; H, 4.45; N, 6.25%.
C of aromatic ring), 1574 (C⚌N), 1303 (C–N), 615 (C–Br); 1H NMR (DMSO-d6) δ in ppm: 6.79–7.92 (m, 3H, Ar-H), 1.31 (s, 2H, CH2), 1.25 (m, 15H, amantadinyl ring). MS: [M]+ at m/z 452.18. Anal. Calc. for C19H20Br2N2O: C, 50.47; H, 4.46; N, 6.20. Found: C, 50.43; H, 4.45; N, 6.25%.
6.1.4 General procedure for synthesis of 3-amantadinyl-2-(hydrazinylmethyl)substitutedquinazolin-4(3H)-one 4a–4b
A compound 3a–3b (0.01 mol) and hydrazine hydrate (0.02 mol) in methanol were refluxed for 10 h. The excess of solvent was distilled off and the reaction mixture was poured onto ice. The solid thus obtained was filtered washed with water dried and recrystallized from appropriate solvents to yield compounds 4a–4b.
6.1.4.1 3-Amantadinyl-2-hydrazinylmethylquinazolin-4(3H)-one (4a)
Yield 73% (Ethanol): m.p. 195 °C; IR (KBr) υmax in cm−1: 3341 (N–H), 1713 (C⚌O), 1625 (C C of aromatic ring), 1579 (C⚌N), 1306 (C–N), 1260 (N–N), 624 (C–Br); 1H NMR (CDCl3) δ in ppm: 8.34 (s, 1H, NH exchangeable with D2O), 8.15 (s, 2H, NH2), 6.68–7.67 (m, 4H, Ar-H), 3.28 (d, 2H, J = 9.1 Hz, CH2NH), 1.34 (m, 15H, amantadinyl ring). MS: [M]+ at m/z 324.42. Anal. Calc. for C19H24N4O: C, 70.23; H, 7.46; N, 17.27. Found: C, 70.22; H, 7.45; N, 17.23%.
C of aromatic ring), 1579 (C⚌N), 1306 (C–N), 1260 (N–N), 624 (C–Br); 1H NMR (CDCl3) δ in ppm: 8.34 (s, 1H, NH exchangeable with D2O), 8.15 (s, 2H, NH2), 6.68–7.67 (m, 4H, Ar-H), 3.28 (d, 2H, J = 9.1 Hz, CH2NH), 1.34 (m, 15H, amantadinyl ring). MS: [M]+ at m/z 324.42. Anal. Calc. for C19H24N4O: C, 70.23; H, 7.46; N, 17.27. Found: C, 70.22; H, 7.45; N, 17.23%.
6.1.4.2 3-Amantadinyl-6-bromo-2-hydrazinylmethyl)quinazolin-4(3H)-one (4b)
Yield 70% (Ethanol): m.p. 193 °C; IR (KBr) υmax in cm−1: 3345 (N–H), 1710 (C⚌O), 1617 (C C of aromatic ring), 1581 (C⚌N), 1304 (C–N), 614 (C–Br); 1H NMR (CDCl3) δ in ppm: 8.12 (s, 1H, NH exchangeable with D2O), 8.10 (s, 2H, NH2), 6.62–7.58 (m, 3H, Ar-H), 3.28 (d, 2H, J = 9.1 Hz, CH2NH), 1.33 (m, 15H, amantadinyl ring), MS: [M]+ at m/z 403.32. Anal. Calc. for C19H23BrN4O: C, 56.58; H, 5.75; N, 13.89. Found: C, 56.55; H, 5.78; N, 13.85%.
C of aromatic ring), 1581 (C⚌N), 1304 (C–N), 614 (C–Br); 1H NMR (CDCl3) δ in ppm: 8.12 (s, 1H, NH exchangeable with D2O), 8.10 (s, 2H, NH2), 6.62–7.58 (m, 3H, Ar-H), 3.28 (d, 2H, J = 9.1 Hz, CH2NH), 1.33 (m, 15H, amantadinyl ring), MS: [M]+ at m/z 403.32. Anal. Calc. for C19H23BrN4O: C, 56.58; H, 5.75; N, 13.89. Found: C, 56.55; H, 5.78; N, 13.85%.
6.1.5 General procedure for synthesis of 3-amantadinyl-2-[2-(substitutedbenzylidine)hydrazinyl]methylquinazolin-4(3H)-ones 5a–5l
A mixture of compound 4a–4b (0.02 mol) and substituted benzaldehydes (0.01 mol) was dissolved in absolute ethanol and (60 mL) in the presence of few drops of glacial acid. The reaction mixture was refluxed for 12 h and poured onto crushed ice and the resultant solid was recrystallized from suitable solvents to yield compounds 5a–5l.
6.1.5.1 3-Amantadinyl-2-[(2-(3,4-dimethoxybenzylidine)hydrazinyl)methyl]quinazolin-4(3H)-one (5a)
Yield 75% (Methanol): m.p. 182 °C; IR (KBr) υmax in cm−1: 3340 (N–H), 1725 (C⚌O), 1620 (C C of aromatic ring), 1580 (C⚌N), 1570 (N⚌CH), 1309 (C–N), 1290 (N–N), 1224 (OCH3); 1H NMR (DMSO-d6) δ in ppm: 8.29 (s, 1H, CH-Ar), 8.13 (s, 1H, NH exchangeable with D2O), 6.69–7.72 (m, 7H, Ar-H), 3.35 (d, 2H, J = 9.1 Hz, CH2NH), 3.29 (s, 6H, 2 × OCH3), 1.31 (m, 15H, amantadinyl ring). MS: [M]+ at m/z 472.58. Anal. Calc. for C28H32N4O3: C, 71.16; H, 6.83; N, 11.86. Found: C, 71.15; H, 6.85; N, 11.84%.
C of aromatic ring), 1580 (C⚌N), 1570 (N⚌CH), 1309 (C–N), 1290 (N–N), 1224 (OCH3); 1H NMR (DMSO-d6) δ in ppm: 8.29 (s, 1H, CH-Ar), 8.13 (s, 1H, NH exchangeable with D2O), 6.69–7.72 (m, 7H, Ar-H), 3.35 (d, 2H, J = 9.1 Hz, CH2NH), 3.29 (s, 6H, 2 × OCH3), 1.31 (m, 15H, amantadinyl ring). MS: [M]+ at m/z 472.58. Anal. Calc. for C28H32N4O3: C, 71.16; H, 6.83; N, 11.86. Found: C, 71.15; H, 6.85; N, 11.84%.
6.1.5.2 3-Amantadinyl-2-[(2-(2-chlorobenzylidine)hydrazinyl)methyl]quinazolin-4(3H)-one (5b)
Yield 74% (DMF): m.p. 175 °C; IR (KBr) υmax in cm−1: 3343 (N–H), 1719 (C⚌O), 1625 (C C of aromatic ring), 1583 (C⚌N), 1570 (N⚌CH), 1310 (C–N), 1293 (N–N), 726 (C–Cl); 1H NMR (CDCl3) δ in ppm: 8.40 (s, 1H, NH exchangeable with D2O), 8.30 (s, 1H, CH-Ar), 6.59–7.62 (m, 8H, Ar-H), 3.45 (d, 2H, J = 9.1 Hz, CH2NH), 1.35 (m, 15H, amantadinyl ring). MS: [M]+ at m/z 446.97. Anal. Calc. for C26H27ClN4O: C, 69.87; H, 6.09; N, 12.53. Found: C, 69.85; H, 6.13; N, 12.54%.
C of aromatic ring), 1583 (C⚌N), 1570 (N⚌CH), 1310 (C–N), 1293 (N–N), 726 (C–Cl); 1H NMR (CDCl3) δ in ppm: 8.40 (s, 1H, NH exchangeable with D2O), 8.30 (s, 1H, CH-Ar), 6.59–7.62 (m, 8H, Ar-H), 3.45 (d, 2H, J = 9.1 Hz, CH2NH), 1.35 (m, 15H, amantadinyl ring). MS: [M]+ at m/z 446.97. Anal. Calc. for C26H27ClN4O: C, 69.87; H, 6.09; N, 12.53. Found: C, 69.85; H, 6.13; N, 12.54%.
6.1.5.3 3-Amantadinyl-2-[(2-(2-bromobenzylidine)hydrazinyl)methyl]quinazolin-4(3H)-one (5c)
Yield 71% (Ethanol): m.p. 173 °C; IR (KBr) υmax in cm−1: 3346 (N–H), 1716 (C⚌O), 1624 (C C of aromatic ring), 1585 (C⚌N), 1574 (N⚌CH), 1311 (C–N), 1295 (N–N), 612 (C–Br); 1H NMR (DMSO-d6) δ in ppm: 8.32 (s, 1H, CH-Ar), 8.20 (s, 1H, NH exchangeable with D2O), 6.62–7.63 (m, 8H, Ar-H), 3.43 (d, 2H, J = 9.1 Hz, CH2NH), 1.38 (m, 15H, amantadinyl ring). MS: [M]+ at m/z 491.42. Anal. Calc. for C26H27BrN4O: C, 63.55; H, 5.54; N, 11.40. Found: C, 63.52; H, 5.52; N, 11.43%.
C of aromatic ring), 1585 (C⚌N), 1574 (N⚌CH), 1311 (C–N), 1295 (N–N), 612 (C–Br); 1H NMR (DMSO-d6) δ in ppm: 8.32 (s, 1H, CH-Ar), 8.20 (s, 1H, NH exchangeable with D2O), 6.62–7.63 (m, 8H, Ar-H), 3.43 (d, 2H, J = 9.1 Hz, CH2NH), 1.38 (m, 15H, amantadinyl ring). MS: [M]+ at m/z 491.42. Anal. Calc. for C26H27BrN4O: C, 63.55; H, 5.54; N, 11.40. Found: C, 63.52; H, 5.52; N, 11.43%.
6.1.5.4 3-Amantadinyl-3-[(2-(2-methoxybenzylidine)hydrazinyl)methyl]quinazolin-4(3H)-one (5d)
Yield 68% (DMF): m.p. 167 °C; IR (KBr) υmax in cm−1: 3343 (N–H), 1718 (C⚌O), 1626 (C C of aromatic ring), 1589 (C⚌N), 1575 (N⚌CH), 1311 (C–N), 1293 (N–N), 1226 (OCH3); 1H NMR (CDCl3) δ in ppm: 8.45 (s, 1H, NH exchangeable with D2O), 8.29 (s, 1H, CH-Ar), 6.66–7.72 (m, 8H, Ar-H), 3.46 (s, 3H, OCH3), 3.43 (d, 2H, J = 9.1 Hz, CH2NH), 1.30 (m, 15H, amantadinyl ring). MS: [M]+ at m/z 442.24. Anal. Calc. for C27H30N4O2: C, 73.28; H, 6.83; N, 12.66. Found: C, 73.25; H, 6.86; N, 12.63%.
C of aromatic ring), 1589 (C⚌N), 1575 (N⚌CH), 1311 (C–N), 1293 (N–N), 1226 (OCH3); 1H NMR (CDCl3) δ in ppm: 8.45 (s, 1H, NH exchangeable with D2O), 8.29 (s, 1H, CH-Ar), 6.66–7.72 (m, 8H, Ar-H), 3.46 (s, 3H, OCH3), 3.43 (d, 2H, J = 9.1 Hz, CH2NH), 1.30 (m, 15H, amantadinyl ring). MS: [M]+ at m/z 442.24. Anal. Calc. for C27H30N4O2: C, 73.28; H, 6.83; N, 12.66. Found: C, 73.25; H, 6.86; N, 12.63%.
6.1.5.5 3-Amantadinyl-2-[(2-(4-hydroxy-3-methoxybenzylidine)hydrazinyl)methyl] quinazolin-4(3H)-one (5e)
Yield 64% (Methanol), m.p. 176 °C; IR (KBr) υmax in cm−1: 3430 (OH), 3345 (N–H), 1718 (C⚌O), 1622 (C C of aromatic ring), 1588 (C⚌N), 1579 (N⚌CH), 1308 (C–N), 1294 (N–N), 1223 (OCH3); 1H NMR (CDCl3) δ in ppm: 8.33 (s, 1H, CH-Ar), 8.32 (s, 1H, NH exchangeable with D2O), 11.15 (s, 1H, OH exchangeable with D2O), 6.69–7.75 (m, 7H, Ar-H), 3.42 (s, 3H, OCH3), 3.37 (d, 2H, J = 9.1 Hz, CH2NH), 1.34 (m, 15H, amantadinyl ring). MS: [M]+ at m/z 458.55. Anal. Calc. for C27H30N4O3: C, 70.72; H, 6.59; N, 12.22. Found: C, 70.76; H, 6.54; N, 12.24%.
C of aromatic ring), 1588 (C⚌N), 1579 (N⚌CH), 1308 (C–N), 1294 (N–N), 1223 (OCH3); 1H NMR (CDCl3) δ in ppm: 8.33 (s, 1H, CH-Ar), 8.32 (s, 1H, NH exchangeable with D2O), 11.15 (s, 1H, OH exchangeable with D2O), 6.69–7.75 (m, 7H, Ar-H), 3.42 (s, 3H, OCH3), 3.37 (d, 2H, J = 9.1 Hz, CH2NH), 1.34 (m, 15H, amantadinyl ring). MS: [M]+ at m/z 458.55. Anal. Calc. for C27H30N4O3: C, 70.72; H, 6.59; N, 12.22. Found: C, 70.76; H, 6.54; N, 12.24%.
6.1.5.6 3-Amantadinyl-2-(2-benzylidine)hydrazinyl)methyl]quinazolin-4(3H)-one (5f)
Yield 62% (Acetone): m.p. 171 °C; IR (KBr) υmax in cm−1: 3348 (N–H), 1737 (C⚌O), 1623 (C C of aromatic ring), 1586 (N⚌CH), 1582 (C⚌N), 1307 (C–N), 1291 (N–N); 1H NMR (CDCl3) δ in ppm: 8.35 (s, 1H, CH-Ar), 8.25 (s, 1H, NH exchangeable with D2O), 6.65–7.63 (m, 9H, Ar-H), 1.31 (d, 2H, J = 9.2 Hz, CH2NH), 1.23 (m,15H, amantadinyl ring). MS: [M]+ at m/z 412.53. Anal. Calc. for C26H28N4O: C, 75.10; H, 6.84; N, 13.58. Found: C, 75.15; H, 6.91; N, 13.46%.
C of aromatic ring), 1586 (N⚌CH), 1582 (C⚌N), 1307 (C–N), 1291 (N–N); 1H NMR (CDCl3) δ in ppm: 8.35 (s, 1H, CH-Ar), 8.25 (s, 1H, NH exchangeable with D2O), 6.65–7.63 (m, 9H, Ar-H), 1.31 (d, 2H, J = 9.2 Hz, CH2NH), 1.23 (m,15H, amantadinyl ring). MS: [M]+ at m/z 412.53. Anal. Calc. for C26H28N4O: C, 75.10; H, 6.84; N, 13.58. Found: C, 75.15; H, 6.91; N, 13.46%.
6.1.5.7 3-Amantadinyl-6-bromo-2-[(2-(3,4-dimethoxybenzylidine)hydrazinyl)methyl] quinazolin-4(3H)-one (5g)
Yield 59% (Ethanol): m.p. 169 °C; IR (KBr) υmax in cm−1: 3349 (N–H), 1726 (C⚌O), 1626 (C C of aromatic ring), 1588 (C⚌N), 1570 (N⚌CH), 1312 (C–N), 1291 (N–N), 1224 (OCH3), 614 (C–Br); 1H NMR (DMSO-d6) δ in ppm: 8.34 (s, 1H, CH-Ar), 8.46 (s, 1H, NH exchangeable with D2O), 6.69–7.72 (m, 6H, Ar-H), 3.47 (d, 2H, J = 9.1 Hz, CH2NH), 2.29 (s, 6H, 2 × OCH3), 1.31 (m, 15H, amantadinyl ring). MS: [M]+ at m/z 551.47. Anal. Calc. for C28H31BrN4O3: C, 60.98; H, 5.67; N, 10.16. Found: C, 60.95; H, 5.65; N, 10.19%.
C of aromatic ring), 1588 (C⚌N), 1570 (N⚌CH), 1312 (C–N), 1291 (N–N), 1224 (OCH3), 614 (C–Br); 1H NMR (DMSO-d6) δ in ppm: 8.34 (s, 1H, CH-Ar), 8.46 (s, 1H, NH exchangeable with D2O), 6.69–7.72 (m, 6H, Ar-H), 3.47 (d, 2H, J = 9.1 Hz, CH2NH), 2.29 (s, 6H, 2 × OCH3), 1.31 (m, 15H, amantadinyl ring). MS: [M]+ at m/z 551.47. Anal. Calc. for C28H31BrN4O3: C, 60.98; H, 5.67; N, 10.16. Found: C, 60.95; H, 5.65; N, 10.19%.
6.1.5.8 3-Amantadinyl-6-bromo-2-[(2-(2-chlorobenzylidine)hydrazinyl)methyl]quinazolin-4(3H)-one (5h)
Yield 56% (DMF): m.p. 168 °C; IR (KBr) υmax in cm−1: 3345 (N–H), 1720 (C⚌O), 1624 (C C of aromatic ring), 1585 (C⚌N), 1574 (N⚌CH), 1313 (C–N), 1291 (N–N), 623 (C–Cl), 615 (C–Br); 1H NMR (CDCl3) δ in ppm: 8.46 (s, 1H, NH exchangeable with D2O), 8.30 (s, 1H, CH-Ar), 6.59–7.62 (m, 7H, Ar-H), 3.44 (d, 2H, J = 9.1 Hz, CH2NH), 1.35 (m, 15H, amantadinyl ring), MS: [M]+ at m/z 525.87. Anal. Calc. for C26H26BrClN4O: C, 59.38; H, 4.98; N, 10.65. Found: C, 59.35; H, 4.95; N, 10.63%.
C of aromatic ring), 1585 (C⚌N), 1574 (N⚌CH), 1313 (C–N), 1291 (N–N), 623 (C–Cl), 615 (C–Br); 1H NMR (CDCl3) δ in ppm: 8.46 (s, 1H, NH exchangeable with D2O), 8.30 (s, 1H, CH-Ar), 6.59–7.62 (m, 7H, Ar-H), 3.44 (d, 2H, J = 9.1 Hz, CH2NH), 1.35 (m, 15H, amantadinyl ring), MS: [M]+ at m/z 525.87. Anal. Calc. for C26H26BrClN4O: C, 59.38; H, 4.98; N, 10.65. Found: C, 59.35; H, 4.95; N, 10.63%.
6.1.5.9 3-Amantadinyl-6-bromo-2-[(2-(2-bromobenzylidine)hydrazinyl)methyl] quinazolin-4(3H)-one (5i)
Yield 52% (Acetone): m.p. 165 °C; IR (KBr) υmax in cm−1: 3341 (N–H), 1715 (C⚌O), 1627(C C of aromatic ring), 1589 (C⚌N), 1576 (N⚌CH), 1313 (C–N), 612 (C–Br); 1H NMR (DMSO-d6) δ in ppm: 8.32 (s, 1H, CH-Ar), 8.30 (s, 1H, NH exchangeable with D2O), 6.62–7.63 (m, 7H, Ar-H), 3.45 (d, 2H, J = 9.1 Hz, CH2NH), 1.38 (m, 15H, amantadinyl ring), MS: [M]+ at m/z 570.32. Anal. Calc. for C26H26Br2N4O: C, 54.76; H, 4.60; N, 9.82. Found: C, 54.78; H, 4.65; N, 9.66%.
C of aromatic ring), 1589 (C⚌N), 1576 (N⚌CH), 1313 (C–N), 612 (C–Br); 1H NMR (DMSO-d6) δ in ppm: 8.32 (s, 1H, CH-Ar), 8.30 (s, 1H, NH exchangeable with D2O), 6.62–7.63 (m, 7H, Ar-H), 3.45 (d, 2H, J = 9.1 Hz, CH2NH), 1.38 (m, 15H, amantadinyl ring), MS: [M]+ at m/z 570.32. Anal. Calc. for C26H26Br2N4O: C, 54.76; H, 4.60; N, 9.82. Found: C, 54.78; H, 4.65; N, 9.66%.
6.1.5.10 3-Amantadinyl-6-bromo-2-[(2-(2-methoxybenzylidine)hydrazinyl)methyl]quinazolin-4(3H)-one (5j)
Yield 56% (Methanol): m.p. 163 °C; IR (KBr) υmax in cm−1: 3342 (N–H), 1629 (C C of aromatic ring), 1720 (C⚌O), 1590 (C⚌N), 1578 (N⚌CH), 1313 (C–N), 1294 (N–N), 1225 (OCH3), 618 (C–Br); 1H NMR (CDCl3) δ in ppm: 8.45 (s, 1H, NH exchangeable with D2O), 8.34 (s, 1H, CH-Ar), 6.66–7.72 (m, 7H, Ar-H), 3.50 (d, 2H, J = 9.1 Hz, CH2NH), 3.30 (s, 3H, OCH3), 1.30 (m, 15H, amantadinyl ring). MS: [M]+ at m/z 521.45. Anal. Calc. for C27H29BrN4O2: C, 62.19; H, 5.61; N, 10.74. Found: C, 62.15; H, 5.65; N, 10.76%.
C of aromatic ring), 1720 (C⚌O), 1590 (C⚌N), 1578 (N⚌CH), 1313 (C–N), 1294 (N–N), 1225 (OCH3), 618 (C–Br); 1H NMR (CDCl3) δ in ppm: 8.45 (s, 1H, NH exchangeable with D2O), 8.34 (s, 1H, CH-Ar), 6.66–7.72 (m, 7H, Ar-H), 3.50 (d, 2H, J = 9.1 Hz, CH2NH), 3.30 (s, 3H, OCH3), 1.30 (m, 15H, amantadinyl ring). MS: [M]+ at m/z 521.45. Anal. Calc. for C27H29BrN4O2: C, 62.19; H, 5.61; N, 10.74. Found: C, 62.15; H, 5.65; N, 10.76%.
6.1.5.11 3-Amantadinyl-6-bromo-2-[(2-(4-hydroxy-3-methoxybenzylidine)hydrazinyl)methyl]quinazolin-4(3H)-one (5k)
Yield 53% (Ethanol): m.p. 160 °C; IR (KBr) υmax in cm−1: 3433 (OH), 3342 (N–H), 1721 (C⚌O), 1624 (C C of aromatic ring), 1589 (C⚌N), 1580 (N⚌CH), 1313 (C–N), 1294 (N–N), 1227 (OCH3), 618 (C–Br); 1H NMR (CDCl3) δ in ppm: 8.48 (s, 1H, NH exchangeable with D2O), 8.35 (s, 1H, CH-Ar), 10.95 (s, 1H, OH exchangeable with D2O), 6.69–7.75 (m, 6H, Ar-H), 3.48 (d, 2H, J = 9.0 Hz, CH2NH), 3.46 (s, 3H, OCH3), 1.34 (m, 15H, amantadinyl ring). MS: [M]+ at m/z 537.45. Anal. Calc. for C27H29BrN4O3: C, 60.34; H, 5.44; N, 10.42. Found: C, 60.36; H, 5.46; N, 10.40%.
C of aromatic ring), 1589 (C⚌N), 1580 (N⚌CH), 1313 (C–N), 1294 (N–N), 1227 (OCH3), 618 (C–Br); 1H NMR (CDCl3) δ in ppm: 8.48 (s, 1H, NH exchangeable with D2O), 8.35 (s, 1H, CH-Ar), 10.95 (s, 1H, OH exchangeable with D2O), 6.69–7.75 (m, 6H, Ar-H), 3.48 (d, 2H, J = 9.0 Hz, CH2NH), 3.46 (s, 3H, OCH3), 1.34 (m, 15H, amantadinyl ring). MS: [M]+ at m/z 537.45. Anal. Calc. for C27H29BrN4O3: C, 60.34; H, 5.44; N, 10.42. Found: C, 60.36; H, 5.46; N, 10.40%.
6.1.5.12 3-Amantadinyl-6-bromo-2-[(2-benzylidine)hydrazinyl)methyl]quinazolin-4(3H)-one (5l)
Yield 52% (Ethanol): m.p. 162 °C; IR (KBr) υmax in cm−1: 3350 (N–H), 1722 (C⚌O), 1627 (C C of aromatic ring), 1591 (C⚌N), 1583 (N⚌CH), 1312 (C–N), 1290 (N–N); 1H NMR (DMSO-d6) δ in ppm: 8.47 (s, 1H, NH exchangeable with D2O), 6.65–7.63 (m, 8H, Ar-H), 3.51 (d, 2H, J = 9.1 Hz, CH2NH), 3.36 (s, 1H, CH-Ar), 1.23 (m, 15H, amantadinyl ring). MS: [M]+ at m/z 491.42. Anal. Calc. for C26H27BrN4O: C, 63.55; H, 5.54; N, 11.40. Found: C, 63.54; H, 5.59; N, 11.43%.
C of aromatic ring), 1591 (C⚌N), 1583 (N⚌CH), 1312 (C–N), 1290 (N–N); 1H NMR (DMSO-d6) δ in ppm: 8.47 (s, 1H, NH exchangeable with D2O), 6.65–7.63 (m, 8H, Ar-H), 3.51 (d, 2H, J = 9.1 Hz, CH2NH), 3.36 (s, 1H, CH-Ar), 1.23 (m, 15H, amantadinyl ring). MS: [M]+ at m/z 491.42. Anal. Calc. for C26H27BrN4O: C, 63.55; H, 5.54; N, 11.40. Found: C, 63.54; H, 5.59; N, 11.43%.
6.1.6 General procedure for synthesis of 3-amantadinyl-2-[((substitutedphenyl)-4-oxo-thiazolidin-1-yl)methylamino]quinazolin-4(3H)-ones 6a–6l
To a solution of compound 5a–5l (0.01 mol) and anhydrous ZnCl2 in dry benzene (50 mL), thioglycolic acid (0.02 mol) was added dropwise with stirring at ambient temperature and the reaction mixture was kept for 3 days at room temperature and then refluxed for 14 h. The reaction mixture was filtered. The filtrate was concentrated and poured on crushed ice. The resultant solid was recrystallized from appropriate solvent to yield the desired compounds 6a–6l.
6.1.6.1 3-Amantadinyl-2-[((3,4-dimethoxyphenyl)-4-oxo-thiazolidin-3-yl)methylamino]-quinazolin-4(3H)-one (6a)
Yield 55% (DMF): m.p. 175 °C; IR(KBr) υmax in cm−1: 1768 (C⚌O), 1626 (C C of aromatic ring), 1591 (C⚌N), 1312 (C–N), 1224 (OCH3), 674 (C–S–C); 1H NMR (DMSO-d6) δ in ppm: 8.48 (s, 1H, NH exchangeable with D2O), 6.69–7.72 (m, 7H, Ar-H), 5.93 (s, 1H, CH-Ar), 3.53 (s, 2H, CH2-S), 2.85 (s, 6H, 2 × OCH3), 2.52 (d, 2H, J = 9.1 Hz, CH2NH), 1.17 (m, 15H, amantadinyl ring). MS: [M]+ at m/z 546.68. Anal. Calc. for C30H34N4O4S: C, 65.91; H, 6.27; N, 10.25. Found: C, 65.92; H, 6.25; N, 10.23%.
C of aromatic ring), 1591 (C⚌N), 1312 (C–N), 1224 (OCH3), 674 (C–S–C); 1H NMR (DMSO-d6) δ in ppm: 8.48 (s, 1H, NH exchangeable with D2O), 6.69–7.72 (m, 7H, Ar-H), 5.93 (s, 1H, CH-Ar), 3.53 (s, 2H, CH2-S), 2.85 (s, 6H, 2 × OCH3), 2.52 (d, 2H, J = 9.1 Hz, CH2NH), 1.17 (m, 15H, amantadinyl ring). MS: [M]+ at m/z 546.68. Anal. Calc. for C30H34N4O4S: C, 65.91; H, 6.27; N, 10.25. Found: C, 65.92; H, 6.25; N, 10.23%.
6.1.6.2 3-Amantadinyl-2-[((2-chlorophenyl)-4-oxo-thiazolidin-3-yl)methylamino]-quinazolin-4(3H)-one (6b)
Yield 60% (Ethanol): m.p. 173 °C; IR (KBr) υmax in cm−1: 1772 (C⚌O), 1625 (C C of aromatic ring), 1532 (C⚌N), 1257 (C–N), 728 (C–Cl), 678 (C–S–C); 1H NMR (CDCl3) δ in ppm: 8.34 (s, 1H, NH exchangeable with D2O), 6.65–7.69 (m, 8H, Ar-H), 5.83 (s, 1H, CH-Ar), 3.93 (s, 2H, CH2-S), 2.87 (d, 2H, J = 9.1 Hz, CH2NH), 1.17 (m, 15H, amantadinyl ring). MS: [M]+ at m/z 521.07. Anal. Calc. for C28H29ClN4O2S: C, 64.54; H, 5.61; N, 10.75. Found: C, 64.42; H, 5.32; N, 10.43%.
C of aromatic ring), 1532 (C⚌N), 1257 (C–N), 728 (C–Cl), 678 (C–S–C); 1H NMR (CDCl3) δ in ppm: 8.34 (s, 1H, NH exchangeable with D2O), 6.65–7.69 (m, 8H, Ar-H), 5.83 (s, 1H, CH-Ar), 3.93 (s, 2H, CH2-S), 2.87 (d, 2H, J = 9.1 Hz, CH2NH), 1.17 (m, 15H, amantadinyl ring). MS: [M]+ at m/z 521.07. Anal. Calc. for C28H29ClN4O2S: C, 64.54; H, 5.61; N, 10.75. Found: C, 64.42; H, 5.32; N, 10.43%.
6.1.6.3 3-Amantadinyl-2-[((2-bromophenyl)-4-oxo-thiazolidin-3-yl)methylamino]-quinazolin-4(3H)-one (6c)
Yield 64% (Methanol): m.p. 167 °C; IR (KBr) υmax in cm−1: 1772 (C⚌O), 1627 (C C of aromatic ring), 1531 (C⚌N), 1306 (C–N), 677 (C–S–C), 612 (C–Br); 1H NMR (CDCl3) δ in ppm: 8.23 (s, 1H, NH exchangeable with D2O), 6.69–7.75 (m, 8H, Ar-H), 5.86 (s, 1H, CH-Ar), 3.60 (s, 2H, CH2-S), 2.54 (d, 2H, J = 9.1 Hz, CH2NH), 1.25 (m, 15H amantadinyl ring). MS: [M]+ at m/z 565.52. Anal. Calc. for C28H29BrN4O2S: C, 59.47; H, 5.17; N, 9.91; Found: C, 59.49; H, 5.14; N, 9.93%.
C of aromatic ring), 1531 (C⚌N), 1306 (C–N), 677 (C–S–C), 612 (C–Br); 1H NMR (CDCl3) δ in ppm: 8.23 (s, 1H, NH exchangeable with D2O), 6.69–7.75 (m, 8H, Ar-H), 5.86 (s, 1H, CH-Ar), 3.60 (s, 2H, CH2-S), 2.54 (d, 2H, J = 9.1 Hz, CH2NH), 1.25 (m, 15H amantadinyl ring). MS: [M]+ at m/z 565.52. Anal. Calc. for C28H29BrN4O2S: C, 59.47; H, 5.17; N, 9.91; Found: C, 59.49; H, 5.14; N, 9.93%.
6.1.6.4 3-Amantadinyl-2-[((2-methoxyphenyl)-4-oxothiazolidin-3-yl)methylamino]-quinazolin-4(3H)-one (6d)
Yield 58% (Methanol): m.p. 163 °C; IR (KBr) υmax in cm−1: 1773 (C⚌O), 1626 (C C of aromatic ring), 1529 (C⚌N), 1308 (C–N), 1220 (OCH3), 676 (C–S–C); 1H NMR (DMSO-d6) δ in ppm: 8.35 (s, 1H, NH exchangeable with D2O), 6.68–7.78 (m, 8H, Ar-H), 5.89 (s, 1H, CH-Ar), 3.65 (s, 2H, CH2-S), 2.85 (s, 3H, OCH3), 2.69 (d, 2H, J = 9.2 Hz, CH2NH), 1.18 (m, 15H, amantadinyl ring). MS: [M]+ at m/z 516.65. Anal. Calc. for C29H32N4O3S: C, 67.42; H, 6.24; N, 10.84. Found: C, 67.42; H, 6.22; N, 10.83%.
C of aromatic ring), 1529 (C⚌N), 1308 (C–N), 1220 (OCH3), 676 (C–S–C); 1H NMR (DMSO-d6) δ in ppm: 8.35 (s, 1H, NH exchangeable with D2O), 6.68–7.78 (m, 8H, Ar-H), 5.89 (s, 1H, CH-Ar), 3.65 (s, 2H, CH2-S), 2.85 (s, 3H, OCH3), 2.69 (d, 2H, J = 9.2 Hz, CH2NH), 1.18 (m, 15H, amantadinyl ring). MS: [M]+ at m/z 516.65. Anal. Calc. for C29H32N4O3S: C, 67.42; H, 6.24; N, 10.84. Found: C, 67.42; H, 6.22; N, 10.83%.
6.1.6.5 3-Amantadinyl-2-[((4-hydroxy-3-methoxyphenyl)-4-oxo-thiazolidin-3-yl)methyl amino]-quinazolin-4(3H)-one (6e)
Yield 53% (DMF): m.p. 160 °C; IR (KBr) υmax in cm−1: 3434 (OH), 1776 (C⚌O), 1619 (C C of aromatic ring), 1532 (C⚌N), 1313 (C–N), 1228 (OCH3), 673 (C–S–C); 1H NMR (CDCl3) δ in ppm: 8.38 (s, 1H, NH exchangeable with D2O), 11.12 (s, 1H, OH exchangeable with D2O), 6.75–7.70 (m, 7H, Ar-H), 5.65 (s, 1H, CH-Ar), 3.69 (s, 2H, CH2-S), 2.83 (s, 3H, OCH3), 2.68 (d, 2H, J = 9.1 Hz, CH2NH), 1.18 (m, 15H, amantadinyl ring). MS: [M]+ at m/z 532.65. Anal. Calc. for C29H32N4O4S: C, 65.39; H, 6.06; N, 10.52. Found: C, 65.35; H, 6.08; N, 10.56%.
C of aromatic ring), 1532 (C⚌N), 1313 (C–N), 1228 (OCH3), 673 (C–S–C); 1H NMR (CDCl3) δ in ppm: 8.38 (s, 1H, NH exchangeable with D2O), 11.12 (s, 1H, OH exchangeable with D2O), 6.75–7.70 (m, 7H, Ar-H), 5.65 (s, 1H, CH-Ar), 3.69 (s, 2H, CH2-S), 2.83 (s, 3H, OCH3), 2.68 (d, 2H, J = 9.1 Hz, CH2NH), 1.18 (m, 15H, amantadinyl ring). MS: [M]+ at m/z 532.65. Anal. Calc. for C29H32N4O4S: C, 65.39; H, 6.06; N, 10.52. Found: C, 65.35; H, 6.08; N, 10.56%.
6.1.6.6 3-Amantadinyl-2-[((phenyl)-4-oxo-thiazolidin-3-yl)methylamino]-quinazolin-4(3H)-one (6f)
Yield 51% (Methanol): m.p. 158 °C; IR (KBr) υmax in cm−1: 1777 (C⚌O), 1622 (C C of aromatic ring), 1587 (C⚌N), 1315 (C–N), 679 (C–S–C); 1H NMR (CDCl3) δ in ppm: 8.36 (s, 1H, NH exchangeable with D2O), 6.74–7.72 (m, 9H, Ar-H), 5.62 (s, 1H, CH-Ar), 3.72 (s, 2H, CH2-S), 2.85 (d, 2H, J = 9.1 Hz, CH2NH), 1.24 (m, 15H, amantadinyl ring). MS: [M]+ at m/z 486.63. Anal. Calc. for C28H30N4O2S: C, 69.11; H, 6.21; N, 11.51. Found: C, 69.15; H, 6.26; N, 11.54%.
C of aromatic ring), 1587 (C⚌N), 1315 (C–N), 679 (C–S–C); 1H NMR (CDCl3) δ in ppm: 8.36 (s, 1H, NH exchangeable with D2O), 6.74–7.72 (m, 9H, Ar-H), 5.62 (s, 1H, CH-Ar), 3.72 (s, 2H, CH2-S), 2.85 (d, 2H, J = 9.1 Hz, CH2NH), 1.24 (m, 15H, amantadinyl ring). MS: [M]+ at m/z 486.63. Anal. Calc. for C28H30N4O2S: C, 69.11; H, 6.21; N, 11.51. Found: C, 69.15; H, 6.26; N, 11.54%.
6.1.6.7 3-Amantadinyl-6-bromo-2-[((3,4-dimethoxyphenyl)-4-oxo-thiazolidin-3-yl)methyl amino]-quinazolin-4(3H)-one (6g)
Yield 55% (Ethanol): m.p. 155 °C; IR (KBr) υmax in cm−1: 1780 (C⚌O), 1621 (C C of aromatic ring), 1584 (C⚌N), 1310 (C–N), 1228 (OCH3), 679 (C–S–C), 617 (C–Br); 1H NMR (CDCl3). δ in ppm: 8.32 (s, 1H, NH exchangeable with D2O), 6.65–7.62 (m, 6H, Ar-H), 5.68 (s, 1H, CH-Ar), 3.66 (s, 2H, CH2-S), 3.40 (s, 6H, 2 × OCH3), 2.84 (d, 2H, J = 9.1 Hz, CH2NH), 1.28 (m, 15H, amantadinyl ring). MS: [M]+ at m/z 625.58. Anal. Calc. for C30H33BrN4O4S: C, 57.60; H, 5.32; N, 8.96. Found: C, 57.62; H, 5.35; N, 8.98%.
C of aromatic ring), 1584 (C⚌N), 1310 (C–N), 1228 (OCH3), 679 (C–S–C), 617 (C–Br); 1H NMR (CDCl3). δ in ppm: 8.32 (s, 1H, NH exchangeable with D2O), 6.65–7.62 (m, 6H, Ar-H), 5.68 (s, 1H, CH-Ar), 3.66 (s, 2H, CH2-S), 3.40 (s, 6H, 2 × OCH3), 2.84 (d, 2H, J = 9.1 Hz, CH2NH), 1.28 (m, 15H, amantadinyl ring). MS: [M]+ at m/z 625.58. Anal. Calc. for C30H33BrN4O4S: C, 57.60; H, 5.32; N, 8.96. Found: C, 57.62; H, 5.35; N, 8.98%.
6.1.6.8 3-Amantadinyl-6-bromo-2-[(2-chlorophenyl)-4-oxo-thiazolidin-3-yl)methylamino]-quinazolin-4(3H)-one (6h)
Yield 58% (Acetone): m.p. 150 °C; IR (KBr) υmax in cm−1: 1771 (C⚌O), 1621 (C C of aromatic ring), 1582 (C⚌N), 1306 (C–N), 729 (C–Cl), 682 (C–S–C); 1H NMR (DMSO-d6) δ in ppm: 8.35 (s, 1H, NH exchangeable with D2O), 6.67–7.76 (m, 7H, Ar-H), 5.65 (s, 1H, CH-Ar), 3.68 (s, 2H, CH2-S), 2.87 (d, 2H, J = 9.2 Hz, CH2NH), 1.32 (m, 15H, amantadinyl ring). MS: [M]+ at m/z 599.97. Anal. Calc. for C28H28BrClN4O2S: C, 56.05; H, 4.70; N, 9.34. Found: C, 55.02; H, 4.73; N, 9.35%.
C of aromatic ring), 1582 (C⚌N), 1306 (C–N), 729 (C–Cl), 682 (C–S–C); 1H NMR (DMSO-d6) δ in ppm: 8.35 (s, 1H, NH exchangeable with D2O), 6.67–7.76 (m, 7H, Ar-H), 5.65 (s, 1H, CH-Ar), 3.68 (s, 2H, CH2-S), 2.87 (d, 2H, J = 9.2 Hz, CH2NH), 1.32 (m, 15H, amantadinyl ring). MS: [M]+ at m/z 599.97. Anal. Calc. for C28H28BrClN4O2S: C, 56.05; H, 4.70; N, 9.34. Found: C, 55.02; H, 4.73; N, 9.35%.
6.1.6.9 3-Amantadinyl-6-bromo-2-[((2-bromophenyl)-4-oxo-thiazolidin-3-yl)methylamino]-quinazolin-4(3H)-one (6i)
Yield 64% (Methanol): m.p. 148 °C; IR (KBr) υmax in cm−1: 1776 (C⚌O), 1623 (C C of aromatic ring), 1591 (C⚌N), 1309 (C–N), 678 (C–S–C), 618 (C–Br); 1H NMR (CDCl3) δ in ppm: 8.39 (s, 1H, NH exchangeable with D2O), 6.63–7.75 (m, 7H, Ar-H), 5.66 (s, 1H, CH-Ar), 3.65 (s, 2H, CH2-S), 2.84 (d, 2H, J = 9.1 Hz, CH2NH), 1.36 (m, 15H, amantadinyl ring). MS: [M]+ at m/z 644.42. Anal. Calc. for C28H28Br2N4O2S: C, 52.19; H, 4.38; N, 8.69. Found: C, 52.16; H, 4.37; N, 8.65%.
C of aromatic ring), 1591 (C⚌N), 1309 (C–N), 678 (C–S–C), 618 (C–Br); 1H NMR (CDCl3) δ in ppm: 8.39 (s, 1H, NH exchangeable with D2O), 6.63–7.75 (m, 7H, Ar-H), 5.66 (s, 1H, CH-Ar), 3.65 (s, 2H, CH2-S), 2.84 (d, 2H, J = 9.1 Hz, CH2NH), 1.36 (m, 15H, amantadinyl ring). MS: [M]+ at m/z 644.42. Anal. Calc. for C28H28Br2N4O2S: C, 52.19; H, 4.38; N, 8.69. Found: C, 52.16; H, 4.37; N, 8.65%.
6.1.6.10 3-Amantadinyl-6-bromo-2-[((2-methoxyphenyl)-4-oxo-thiazolidin-3-yl)methyl amino]-quinazolin-4(3H)-one (6j)
Yield 67% (Ethanol): m.p. 145 °C; IR (KBr) υmax in cm−1: 1777 (C⚌O), 1629 (C C of aromatic ring), 1579 (C⚌N), 1305 (C–N), 1226 (OCH3), 675 (C–S–C), 622 (C–Br); 1H NMR (DMSO-d6) δ in ppm: 8.40 (s, 1H, NH exchangeable with D2O), 6.67–7.74 (m, 7H, Ar-H), 5.68 (s, 1H, CH-Ar), 3.68 (s, 2H, CH2-S), 3.55 (s, 3H, OCH3), 2.88 (d, 2H, J = 9.1 Hz, CH2NH), 1.38 (m, 15H, amantadinyl ring). MS: [M]+ at m/z 595.55. Anal. Calc. for C29H31BrN4O3S: C, 58.49; H, 5.25; N, 9.41. Found: C, 58.45; H, 5.17; N, 9.43%.
C of aromatic ring), 1579 (C⚌N), 1305 (C–N), 1226 (OCH3), 675 (C–S–C), 622 (C–Br); 1H NMR (DMSO-d6) δ in ppm: 8.40 (s, 1H, NH exchangeable with D2O), 6.67–7.74 (m, 7H, Ar-H), 5.68 (s, 1H, CH-Ar), 3.68 (s, 2H, CH2-S), 3.55 (s, 3H, OCH3), 2.88 (d, 2H, J = 9.1 Hz, CH2NH), 1.38 (m, 15H, amantadinyl ring). MS: [M]+ at m/z 595.55. Anal. Calc. for C29H31BrN4O3S: C, 58.49; H, 5.25; N, 9.41. Found: C, 58.45; H, 5.17; N, 9.43%.
6.1.6.11 3-Amantadinyl-6-bromo-2-[((4-hydroxy-3-methoxyphenyl)-4-oxo-thiazolidin-3-yl)methylamino]-quinazolin-4(3H)-one (6k)
Yield 53% (Ethanol): m.p. 141 °C; IR (KBr) υmax in cm−1: 3440 (OH), 1773 (C⚌O), 1625 (C C of aromatic ring), 1592 (C⚌N), 1312 (C–N), 1228 (OCH3), 680 (C–S–C), 624 (C–Br); 1H NMR (DMSO-d6) δ in ppm: 11.16 (s, 1H, OH exchangeable with D2O), 8.44 (s, 1H, NH exchangeable with D2O), 6.80–7.79 (m, 6H, Ar-H), 5.68 (s, 1H, CH-Ar), 3.72 (s, 2H, CH2-S), 3.62 (s, 3H, OCH3), 2.92 (d, 2H, J = 9.1 Hz, CH2NH), 1.35 (m, 15H, amantadinyl ring). MS: [M]+ at m/z 611.55. Anal. Calc. for C29H31BrN4O4S: C, 56.96; H, 5.11; N, 9.16. Found: C, 56.94; H, 4.16; N, 9.12%.
C of aromatic ring), 1592 (C⚌N), 1312 (C–N), 1228 (OCH3), 680 (C–S–C), 624 (C–Br); 1H NMR (DMSO-d6) δ in ppm: 11.16 (s, 1H, OH exchangeable with D2O), 8.44 (s, 1H, NH exchangeable with D2O), 6.80–7.79 (m, 6H, Ar-H), 5.68 (s, 1H, CH-Ar), 3.72 (s, 2H, CH2-S), 3.62 (s, 3H, OCH3), 2.92 (d, 2H, J = 9.1 Hz, CH2NH), 1.35 (m, 15H, amantadinyl ring). MS: [M]+ at m/z 611.55. Anal. Calc. for C29H31BrN4O4S: C, 56.96; H, 5.11; N, 9.16. Found: C, 56.94; H, 4.16; N, 9.12%.
6.1.6.12 3-Amantadinyl-6-bromo-2-[((phenyl)-4-oxo-thiazolidin-3-ylmethylamino]-quinazolin-4(3H)-one (6l)
Yield 61% (Methanol): m.p. 145 °C; IR (KBr) υmax in cm−1: 1772 (C⚌O), 1628 (C C of aromatic ring), 1587 (C⚌N), 1305 (C–N), 681 (C–S–C), 615 (C–Br); 1H NMR (CDCl3) δ in ppm: 8.47 (s, 1H, NH exchangeable with D2O), 6.75–7.81 (m, 8H, Ar-H), 5.66 (s, 1H, CH-Ar), 3.70 (s, 2H, CH2-S), 2.90 (d, 2H, J = 9.1 Hz, CH2NH), 1.45 (m, 15H, amantadinyl ring). MS: [M]+ at m/z 565.52. Anal. Calc. for C28H29BrN4O2S: C, 59.47; H, 5.17; N, 9.91. Found: C, 59.46; H, 5.15; N, 9.93%.
C of aromatic ring), 1587 (C⚌N), 1305 (C–N), 681 (C–S–C), 615 (C–Br); 1H NMR (CDCl3) δ in ppm: 8.47 (s, 1H, NH exchangeable with D2O), 6.75–7.81 (m, 8H, Ar-H), 5.66 (s, 1H, CH-Ar), 3.70 (s, 2H, CH2-S), 2.90 (d, 2H, J = 9.1 Hz, CH2NH), 1.45 (m, 15H, amantadinyl ring). MS: [M]+ at m/z 565.52. Anal. Calc. for C28H29BrN4O2S: C, 59.47; H, 5.17; N, 9.91. Found: C, 59.46; H, 5.15; N, 9.93%.
6.1.7 General procedure for synthesis of 3-amantadinyl-2-[(substitutedphenyl)-3-chloro-2-oxo-azetidin-1-yl)methylamino]-quinazolin-4(3H)-ones 7a–7l
To a solution of compounds 6a–6l (0.01 mol) and triethylamine (5–6 drops) in dry benzene, mono chloro acetyl chloride (0.15 mol) was added dropwise at 50 °C. The reaction mixture was stirred for 40 min at room temperature and refluxed for 7 h. The reaction mixture was filtered to remove triethylamine hydrogen chloride and the resultant solution was poured onto crushed ice with constant stirring. The solid thus obtained was recrystallized from suitable solvents to yield the desired compounds 7a–7l.
6.1.7.1 3-Amantadinyl-2-[((3,4-dimethoxyphenyl)-3-chloro-2-oxo-azetidin-3-yl)methyl amino]-quinazolin-4(3H)-one (7a)
Yield 52% (Methanol): m.p. 175 °C; IR (KBr) υmax in cm−1: 1755 (C⚌O) 1625 (C C of aromatic ring), 1575 (C⚌N), 1322 (C–N), 727 (C–Cl); 1H NMR (CDCl3) δ in ppm: 8.35 (s, 1H, NH exchangeable with D2O), 6.64–7.75 (m, 7H, Ar-H), 5.80 (s, 1H, CH-Ar), 6.42 (d, 1H, J = 6.5 Hz, CHCl), 3.70 (d, 2H, J = 9.1 Hz, CH2NH), 3.45 (s, 6H, 2 × OCH3), 1.13 (m, 15H, amantadinyl ring). MS: [M]+ at m/z 549.06. Anal. Calc. for C30H33ClN4O4: C, 65.63; H, 6.06; N, 10.20. Found: C, 65.59; H, 6.12; N, 10.27%.
C of aromatic ring), 1575 (C⚌N), 1322 (C–N), 727 (C–Cl); 1H NMR (CDCl3) δ in ppm: 8.35 (s, 1H, NH exchangeable with D2O), 6.64–7.75 (m, 7H, Ar-H), 5.80 (s, 1H, CH-Ar), 6.42 (d, 1H, J = 6.5 Hz, CHCl), 3.70 (d, 2H, J = 9.1 Hz, CH2NH), 3.45 (s, 6H, 2 × OCH3), 1.13 (m, 15H, amantadinyl ring). MS: [M]+ at m/z 549.06. Anal. Calc. for C30H33ClN4O4: C, 65.63; H, 6.06; N, 10.20. Found: C, 65.59; H, 6.12; N, 10.27%.
6.1.7.2 3-Amantadinyl-2-[(2-chlorophenyl)-3-chloro-2-oxo-azetidin-3-yl)methylamino]quinazolin-4(3H)-one (7b)
Yield 60% (Acetone): m.p. 173 °C; IR (KBr) υmax in cm−1: 1759 (C⚌O), 1628 (C C of aromatic ring), 1585 (C⚌N), 1317 (C–N), 727 (C–Cl); 1H NMR (CDCl3) δ in ppm: 8.34 (s, 1H, NH exchangeable with D2O), 6.64–7.75 (m, 8H, Ar-H), 5.75 (s, 1H, CH-Ar), 6.48 (d, 1H, J = 6.4 Hz, CHCl), 3.78 (d, 2H, J = 9.2 Hz, CH2NH), 1.12 (m, 15H, amantadinyl ring). MS: [M]+ at m/z 523.45. Anal. Calc. for C28H28Cl2N4O2: C, 64.25; H, 5.39; N, 10.70. Found: C, 64.22; H, 5.35; N, 10.73%.
C of aromatic ring), 1585 (C⚌N), 1317 (C–N), 727 (C–Cl); 1H NMR (CDCl3) δ in ppm: 8.34 (s, 1H, NH exchangeable with D2O), 6.64–7.75 (m, 8H, Ar-H), 5.75 (s, 1H, CH-Ar), 6.48 (d, 1H, J = 6.4 Hz, CHCl), 3.78 (d, 2H, J = 9.2 Hz, CH2NH), 1.12 (m, 15H, amantadinyl ring). MS: [M]+ at m/z 523.45. Anal. Calc. for C28H28Cl2N4O2: C, 64.25; H, 5.39; N, 10.70. Found: C, 64.22; H, 5.35; N, 10.73%.
6.1.7.3 3-Amantadinyl-2-[(2-bromophenyl)-3-chloro-2-oxo-azetidin-3-yl)methylamino]quinazolin-4(3H)-one (7c)
Yield 61% (Methanol): m.p. 169 °C; IR (KBr) υmax in cm−1: 1762 (C⚌O), 1630 (C C of aromatic ring), 1583 (C⚌N), 1315 (C–N), 721 (C–Cl), 616 (C–Br); 1H NMR (CDCl3) δ in ppm: 8.31 (s, 1H, NH exchangeable with D2O), 6.62–7.72 (m, 8H, Ar-H), 5.78 (s, 1H, CH-Ar), 6.45 (d, 1H, J = 6.5 Hz, CHCl), 3.75 (d, 2H, J = 9.1 Hz, CH2NH), 1.22 (m, 15H, amantadinyl ring). MS: [M]+ at m/z 567.90. Anal. Calc. for C28H28BrClN4O2: C, 59.22; H, 4.97; N, 9.87. Found: C, 51.22; H, 4.95; N, 8.85%.
C of aromatic ring), 1583 (C⚌N), 1315 (C–N), 721 (C–Cl), 616 (C–Br); 1H NMR (CDCl3) δ in ppm: 8.31 (s, 1H, NH exchangeable with D2O), 6.62–7.72 (m, 8H, Ar-H), 5.78 (s, 1H, CH-Ar), 6.45 (d, 1H, J = 6.5 Hz, CHCl), 3.75 (d, 2H, J = 9.1 Hz, CH2NH), 1.22 (m, 15H, amantadinyl ring). MS: [M]+ at m/z 567.90. Anal. Calc. for C28H28BrClN4O2: C, 59.22; H, 4.97; N, 9.87. Found: C, 51.22; H, 4.95; N, 8.85%.
6.1.7.4 3-Amantadinyl-2-[((2-methoxphenyl)-3-chloro-2-oxo-azetidin-3-yl)methylamino]quinazolin-4(3H)-one (7d)
Yield 64% (Methanol): m.p. 165 °C; IR (KBr) υmax in cm−1: 1767 (C⚌O), 1622 (C C of aromatic ring), 1587 (C⚌N), 1305 (C–N), 1233 (OCH3), 728 (C–Cl); 1H NMR (CDCl3) δ in ppm: 8.40 (s, 1H, NH exchangeable with D2O), 6.62–7.73 (m, 8H, Ar-H), 5.98 (s, 1H, CH-Ar), 6.45 (d, 1H, J = 6.5 Hz, CHCl), 3.74 (d, 2H, J = 9.1 Hz, CH2NH), 3.21 (s, 3H, OCH3), 1.13 (m, 15H, amantadinyl ring). MS: [M]+ at m/z 519.03. Anal. Calc. for C29H31ClN4O3: C, 67.11; H, 6.02; N, 10.79. Found: C, 66.15; H, 6.05; N, 10.75%.
C of aromatic ring), 1587 (C⚌N), 1305 (C–N), 1233 (OCH3), 728 (C–Cl); 1H NMR (CDCl3) δ in ppm: 8.40 (s, 1H, NH exchangeable with D2O), 6.62–7.73 (m, 8H, Ar-H), 5.98 (s, 1H, CH-Ar), 6.45 (d, 1H, J = 6.5 Hz, CHCl), 3.74 (d, 2H, J = 9.1 Hz, CH2NH), 3.21 (s, 3H, OCH3), 1.13 (m, 15H, amantadinyl ring). MS: [M]+ at m/z 519.03. Anal. Calc. for C29H31ClN4O3: C, 67.11; H, 6.02; N, 10.79. Found: C, 66.15; H, 6.05; N, 10.75%.
6.1.7.5 3-Amantadinyl-2-[((4-hydroxy3-methoxyphenyl)-3-chloro-2-oxo-azetidin-3-yl)methylamino]quinazolin-4(3H)-one (7e)
Yield 59% (DMF): m.p. 161 °C; IR (KBr) υmax in cm−1: 3422 (OH), 1765 (C⚌O), 1624 (C C of aromatic ring), 1583 (C⚌N), 1315 (C–N), 1225 (OCH3), 728 (C–Cl); 1H NMR (CDCl3) δ in ppm: 11.13 (s, 1H, OH exchangeable with D2O), 8.38 (s, 1H, NH exchangeable with D2O), 6.58–7.61 (m, 7H, Ar-H), 5.79 (s, 1H, CH-Ar), 6.41 (d, 1H, J = 6.4 Hz, CHCl), 3.71 (d, 2H, J = 9.0 Hz, CH2NH), 3.25 (s, 3H, OCH3), 1.16 (m, 15H, amantadinyl ring). MS: [M]+ at m/z 535.03. Anal. Calc. for C29H31ClN4O4: C, 65.10; H, 5.84; N, 10.47. Found: C, 64.13; H, 5.85; N, 10.55%.
C of aromatic ring), 1583 (C⚌N), 1315 (C–N), 1225 (OCH3), 728 (C–Cl); 1H NMR (CDCl3) δ in ppm: 11.13 (s, 1H, OH exchangeable with D2O), 8.38 (s, 1H, NH exchangeable with D2O), 6.58–7.61 (m, 7H, Ar-H), 5.79 (s, 1H, CH-Ar), 6.41 (d, 1H, J = 6.4 Hz, CHCl), 3.71 (d, 2H, J = 9.0 Hz, CH2NH), 3.25 (s, 3H, OCH3), 1.16 (m, 15H, amantadinyl ring). MS: [M]+ at m/z 535.03. Anal. Calc. for C29H31ClN4O4: C, 65.10; H, 5.84; N, 10.47. Found: C, 64.13; H, 5.85; N, 10.55%.
6.1.7.6 3-Amantadinyl-2[((phenyl)-3-chloro-2-oxo-azetidin-3-yl)methylamino]-quinazolin-4(3H)-one (7f)
Yield 57% (Ethanol): m.p. 163 °C; IR (KBr) υmax in cm−1: 1762 (C⚌O), 1625 (C C of aromatic ring), 1584 (C⚌N), 1318 (C–N), 726 (C–Cl); 1H NMR (CDCl3) δ in ppm: 8.35 (s, 1H, NH exchangeable with D2O), 6.62–7.56 (m, 9H, Ar-H), 5.83 (s, 1H, CH-Ar), 6.46 (d, 1H, J = 6.5 Hz, CHCl), 3.69 (d, 2H, J = 9.1 Hz, CH2NH), 1.15 (m, 15H, amantadinyl ring). MS: [M]+ at m/z 489.01. Anal. Calc. for C28H29ClN4O2: C, 78.77; H, 5.98; N, 11.46. Found: C, 78.69; H, 5.86; N, 11.52%.
C of aromatic ring), 1584 (C⚌N), 1318 (C–N), 726 (C–Cl); 1H NMR (CDCl3) δ in ppm: 8.35 (s, 1H, NH exchangeable with D2O), 6.62–7.56 (m, 9H, Ar-H), 5.83 (s, 1H, CH-Ar), 6.46 (d, 1H, J = 6.5 Hz, CHCl), 3.69 (d, 2H, J = 9.1 Hz, CH2NH), 1.15 (m, 15H, amantadinyl ring). MS: [M]+ at m/z 489.01. Anal. Calc. for C28H29ClN4O2: C, 78.77; H, 5.98; N, 11.46. Found: C, 78.69; H, 5.86; N, 11.52%.
6.1.7.7 3-Amantadinyl-6-bromo-2-[((3,4-dimethoxyphenyl)-3-chloro-2-oxo–azetidin-3-yl)methylamino]-quinazolin-4(3H)-one (7g)
Yield 55% (Methanol): m.p. 159 °C; IR (KBr) υmax in cm−1: 1726 (C⚌O), 1635 (C C of aromatic ring), 1575 (C⚌N), 1323 (C–N), 1226 (OCH3), 728 (C–Cl), 627 (C–Br); 1H NMR (CDCl3) δ in ppm: 8.44 (s, 1H, NH exchangeable with D2O), 6.42–7.46 (m, 6H, Ar-H), 5.77 (s, 1H, CH-Ar), 6.43 (d, 1H, J = 6.5 Hz, CHCl), 3.73 (d, 2H, J = 9.1 Hz, CH2NH), 3.32 (s, 6H, 2 × OCH3), 1.32 (m, 15H, amantadinyl ring). MS: [M]+ at m/z 627.96. Anal. Calc. for C30H32BrClN4O4: C, 57.38; H, 5.14; N, 8.92. Found: C, 57.35; H, 5.15; N, 8.91%.
C of aromatic ring), 1575 (C⚌N), 1323 (C–N), 1226 (OCH3), 728 (C–Cl), 627 (C–Br); 1H NMR (CDCl3) δ in ppm: 8.44 (s, 1H, NH exchangeable with D2O), 6.42–7.46 (m, 6H, Ar-H), 5.77 (s, 1H, CH-Ar), 6.43 (d, 1H, J = 6.5 Hz, CHCl), 3.73 (d, 2H, J = 9.1 Hz, CH2NH), 3.32 (s, 6H, 2 × OCH3), 1.32 (m, 15H, amantadinyl ring). MS: [M]+ at m/z 627.96. Anal. Calc. for C30H32BrClN4O4: C, 57.38; H, 5.14; N, 8.92. Found: C, 57.35; H, 5.15; N, 8.91%.
6.1.7.8 3-Amantadinyl-6-bromo-2-[((2-chlorophenyl)-3-chloro-2-oxo-azetidin-3-yl)methylamino]-quinazolin-4(3H)-one (7h)
Yield 54% (Acetone): m.p. 155 °C; IR (KBr) υmax in cm−1: 1723 (C⚌O), 1614 (C C of aromatic ring), 1582 (C⚌N), 1316 (C–N), 725 (C–Cl), 623 (C–Br); 1H NMR (CDCl3) δ in ppm: 8.38 (s, 1H, NH exchangeable with D2O), 6.65–7.46 (m, 7H, Ar-H), 5.80 (s, 1H, CH-Ar), 3.72 (d, 2H, J = 9.1 Hz, CH2NH), 6.45 (d, 1H, J = 6.5 Hz, CHCl), 1.19 (m, 15H, amantadinyl ring). MS: [M]+ at m/z 602.35. Anal. Calc. for C28H27BrCl2N4O2: C, 55.83; H, 4.52; N, 9.30. Found: C, 51.82; H, 4.55; N, 8.31%.
C of aromatic ring), 1582 (C⚌N), 1316 (C–N), 725 (C–Cl), 623 (C–Br); 1H NMR (CDCl3) δ in ppm: 8.38 (s, 1H, NH exchangeable with D2O), 6.65–7.46 (m, 7H, Ar-H), 5.80 (s, 1H, CH-Ar), 3.72 (d, 2H, J = 9.1 Hz, CH2NH), 6.45 (d, 1H, J = 6.5 Hz, CHCl), 1.19 (m, 15H, amantadinyl ring). MS: [M]+ at m/z 602.35. Anal. Calc. for C28H27BrCl2N4O2: C, 55.83; H, 4.52; N, 9.30. Found: C, 51.82; H, 4.55; N, 8.31%.
6.1.7.9 3-Amantadinyl-6-bromo-2-[(2-bromophenyl)-3-chloro-2-oxo-azetidin-3-ylmethylamino]-quinazolin-4(3H)-one (7i)
Yield 58% (Ethanol): m.p. 149 °C; IR (KBr) υmax in cm−1: 1766 (C⚌O), 1627 (C C of aromatic ring), 1582 (C⚌N), 1312 (C–N), 728 (C–Cl), 614 (C–Br); 1H NMR (CDCl3) δ in ppm: 8.37 (s, 1H, NH exchangeable with D2O), 6.67–7.69 (m, 7H, Ar-H), 5.79 (s, 1H, CH-Ar), 3.68 (d, 2H, J = 9.2 Hz, CH2NH), 6.48 (d, 1H, J = 6.4 Hz, CHCl), 1.18 (m, 15H, amantadinyl ring). MS: [M]+ at m/z 646.80. Anal. Calc. for C28H27Br2ClN4O2: C, 51.99; H, 4.21; N, 8.66. Found: C, 51.92; H, 4.25; N, 8.65%.
C of aromatic ring), 1582 (C⚌N), 1312 (C–N), 728 (C–Cl), 614 (C–Br); 1H NMR (CDCl3) δ in ppm: 8.37 (s, 1H, NH exchangeable with D2O), 6.67–7.69 (m, 7H, Ar-H), 5.79 (s, 1H, CH-Ar), 3.68 (d, 2H, J = 9.2 Hz, CH2NH), 6.48 (d, 1H, J = 6.4 Hz, CHCl), 1.18 (m, 15H, amantadinyl ring). MS: [M]+ at m/z 646.80. Anal. Calc. for C28H27Br2ClN4O2: C, 51.99; H, 4.21; N, 8.66. Found: C, 51.92; H, 4.25; N, 8.65%.
6.1.7.10 3-Amantadinyl-6-bromo-2-[((2-methoxyphenyl)-3–chloro-2-oxo-azetidin-3-yl)methylamino]-quinazolin-4(3H)-one (7j)
Yield 54% (DMF): m.p. 152 °C; IR (KBr) υmax in cm−1: 1761 (C⚌O), 1626 (C C of aromatic ring), 1583 (C⚌N), 1314 (C–N), 1228 (OCH3), 727 (C–Cl), 618 (C–Br); 1H NMR (CDCl3) δ in ppm: 8.36 (s, 1H, NH exchangeable with D2O), 6.62–7.79 (m, 7H, Ar-H), 5.81 (s, 1H, CH-Ar), 6.52 (d, 1H, J = 6.5 Hz, CHCl), 3.72 (d, 2H, J = 9.1 Hz, CH2NH), 3.39 (s, 3H, OCH3), 1.21 (m, 15H, amantadinyl ring). MS: [M]+ at m/z 597.93. Anal. Calc. for C29H30BrClN4O3: C, 58.25; H, 5.06; N, 9.37. Found: C, 58.22; H, 5.13; N, 9.39%.
C of aromatic ring), 1583 (C⚌N), 1314 (C–N), 1228 (OCH3), 727 (C–Cl), 618 (C–Br); 1H NMR (CDCl3) δ in ppm: 8.36 (s, 1H, NH exchangeable with D2O), 6.62–7.79 (m, 7H, Ar-H), 5.81 (s, 1H, CH-Ar), 6.52 (d, 1H, J = 6.5 Hz, CHCl), 3.72 (d, 2H, J = 9.1 Hz, CH2NH), 3.39 (s, 3H, OCH3), 1.21 (m, 15H, amantadinyl ring). MS: [M]+ at m/z 597.93. Anal. Calc. for C29H30BrClN4O3: C, 58.25; H, 5.06; N, 9.37. Found: C, 58.22; H, 5.13; N, 9.39%.
6.1.7.11 3-Amantadinyl-6-bromo-2-[((4-hydroxy3-methoxyphenyl)-3-chloro-2-oxo-azetidin-3-yl)methylamino]-quinazolin-4(3H)-one (7k)
Yield 51% (Ethanol): m.p. 147 °C; IR (KBr) υmax in cm−1: 3432 (OH), 1765 (C⚌O), 1626 (C C of aromatic ring), 1580 (C⚌N), 1310 (C–N), 1234 (OCH3), 726 (C–Cl), 614 (C–Br); 1H NMR (CDCl3) δ in ppm: 11.20 (s, 1H, OH exchangeable with D2O), 8.37 (s, 1H, NH exchangeable with D2O), 6.71–7.72 (m, 6H, Ar-H), 5.78 (s, 1H, CH-Ar), 6.43 (d, 1H, J = 6.5 Hz, CHCl), 3.73 (d, 2H, J = 9.1 Hz, CH2NH), 3.41 (s, 3H, OCH3), 1.27 (m, 15H, amantadinyl ring). MS: [M]+ at m/z 613.93. Anal. Calc. for C29H30BrClN4O4: C, 56.73; H, 4.93; N, 9.13. Found: C, 56.72; H, 4.95; N, 9.23%.
C of aromatic ring), 1580 (C⚌N), 1310 (C–N), 1234 (OCH3), 726 (C–Cl), 614 (C–Br); 1H NMR (CDCl3) δ in ppm: 11.20 (s, 1H, OH exchangeable with D2O), 8.37 (s, 1H, NH exchangeable with D2O), 6.71–7.72 (m, 6H, Ar-H), 5.78 (s, 1H, CH-Ar), 6.43 (d, 1H, J = 6.5 Hz, CHCl), 3.73 (d, 2H, J = 9.1 Hz, CH2NH), 3.41 (s, 3H, OCH3), 1.27 (m, 15H, amantadinyl ring). MS: [M]+ at m/z 613.93. Anal. Calc. for C29H30BrClN4O4: C, 56.73; H, 4.93; N, 9.13. Found: C, 56.72; H, 4.95; N, 9.23%.
6.1.7.12 3-Amantadinyl-6-bromo-2-[((phenyl)3-chloro-2-oxo-azetidin-3-yl)methylamino]-quinazolin-4(3H)-one (7l)
Yield 56% (Methanol): m.p. 152 °C; IR (KBr) υmax in cm−1: 1765 (C⚌O), 1623 (C C of aromatic ring), 1585 (C⚌N), 1315 (C–N), 725 (C–Cl), 624 (C–Br); 1H NMR (CDCl3) δ in ppm: 8.34 (s, 1H, NH exchangeable with D2O), 6.69–7.73 (m, 8H, Ar-H), 5.81 (s, 1H, CH-Ar), 6.42 (d, 1H, J = 6.5 Hz, CHCl), 3.71 (d, 2H, J = 9.2 Hz, CH2NH), 1.27 (m, 15H, amantadinyl ring). MS: [M]+ at m/z 567.90. Anal. Calc. for C28H28BrClN4O2: C, 59.22; H, 4.97; N, 9.87. Found: C, 59.25; H, 4.95; N, 9.88%.
C of aromatic ring), 1585 (C⚌N), 1315 (C–N), 725 (C–Cl), 624 (C–Br); 1H NMR (CDCl3) δ in ppm: 8.34 (s, 1H, NH exchangeable with D2O), 6.69–7.73 (m, 8H, Ar-H), 5.81 (s, 1H, CH-Ar), 6.42 (d, 1H, J = 6.5 Hz, CHCl), 3.71 (d, 2H, J = 9.2 Hz, CH2NH), 1.27 (m, 15H, amantadinyl ring). MS: [M]+ at m/z 567.90. Anal. Calc. for C28H28BrClN4O2: C, 59.22; H, 4.97; N, 9.87. Found: C, 59.25; H, 4.95; N, 9.88%.
References
- J. Am. Chem. Soc.. 1907;29:517-536.
- Psycho Pharmacol.. 1977;52(2):165-171.
- Br. J. Pharmacol.. 1953;8:46-48.
- J. Pharm. Sci.. 2005;8(2):182-189.
- Eur. J. Pharmacol.. 1975;33:183-188.
- Indian J. Chem.. 1982;21B:1128-1129.
- Mov. Disord.. 2000;15:873-878.
- Arch. Int. Pharmacodyn. Ther.. 1962;8:46-48.
- Indian J. Chem.. 1989;28B:745-750.
- Indian J. Chem.. 2005;44B:1940-1944.
- Dis. Nerv. Syst.. 1977;38:605-608.
- J. Appl. Res.. 2006;6(3):240-245.
- Pharmacological Screening Tests Progress in Medicinal Chemistry. Vol vol. 1. London: Butterworth; 1960. pp. 1–33
- Pharmacol. Res. Commun.. 1987;19(9):617-628.
- Indian J. Chem. Soc.. 1990;67:335-338.







