Translate this page into:
Synthesis of ruthenium complexes and their catalytic applications: A review
⁎Corresponding authors. nasirrasool@gcuf.edu.pk (Nasir Rasool), zaz@ums.edu.my (Zainul Amiruddin Zakaria)
-
Received: ,
Accepted: ,
This article was originally published by Elsevier and was migrated to Scientific Scholar after the change of Publisher.
Peer review under responsibility of King Saud University.
Abstract
Abstract
Ruthenium complexes are an important class that has multiple catalytic and biological applications. Ruthenium has a remarkably broad and diversified chemical spectrum. In the emerging fields of catalysis, such as homogeneous, heterogeneous, and photo catalysis, ruthenium complexes are being studied. Moreover, Ruthenium complexes can be used in the development of efficient chemotherapeutic drugs for cancer, even though some of them are in the final stage of clinical trials. This review covers the synthesis of the pincer, Shiff base, phosphine, N-heterocyclic carbenes, cycloruthenated, and half sandwiched ruthenium complexes and their potential catalytic and biological applications reported in the last 15 years.
Keywords
Ruthenium
Complexes
Synthesis
Catalysis
Bioactivity
Pincer
Shiff-base
NHC
1 Introduction
Ruthenium metal was discovered by Russian chemist Karl Klaus (1796–1864) in the 18th century (Sahu et al., 2018). Ruthenium serves as a unique catalyst in oxidative reactions due to its variable oxidation state which ranges from +2 to +8 (Ablialimov et al., 2014). Ru exists in two stable oxidation states (II and III), that can be linked with auxiliary ligands of various geometries to form a variety of Ru(II/III) complexes with varied electronic and steric properties (Jabłońska-Wawrzycka et al., 2013; Lee et al., 2020).
Ruthenium metal occupies a significant position in both excited-state chemistry and catalysis due to their well-studied organometallic and coordination chemistries. Various ruthenium compounds have promising applications in catalysis and their low toxicity tends to make them an ideal option for catalytic drug synthesis (Clarke, 2002; Crochet and Cadierno, 2014). These characteristics, together with the fact that ruthenium compounds are less expensive than other platinum-group metal compounds (Pd, Pt, Rh, and Ir), have made them the favored choice for many catalytic reactions. Ruthenium compounds are increasingly being investigated for use as pharmaceutics because of their better biocompatibility compared to many other metallodrugs (Lawrence et al., 2018).
It is worth noting that the medicinal properties of ruthenium are now being discovered, and anticancer agents of ruthenium have recently entered clinical trials, with promising results on resistant tumors. Most investigations of organometallic reagents have targeted their anti-tumor activity throughout the last two decades. While cisplatin is considered the “golden standard” in this respect, it is faced with a vast array of drug resistance and adverse effects (Hartinger et al., 2008; Wheate et al., 2010; Biot et al., 2011; Gasser, 2015). Complex compounds having ruthenium ions are the type of metal complex which has been extensively studied for anticancer action; for example, NAMI-A and KP1019 are now in phase 2 clinical trials. Ruthenium complexes have a wide range of biological features, although their anticancer potential is probably the most well-known. The ruthenium complexes (Ru-1 and Ru-2) exhibit excellent activity against cancer (Fig. 1). The (Ru-2) particularly exhibits excellent results in MCF-7 breast cancer cells (Pastuszko et al., 2016; Skoczynska et al., 2017b; Skoczynska et al., 2017a; Namiecińska et al., 2019; Małecka et al., 2021).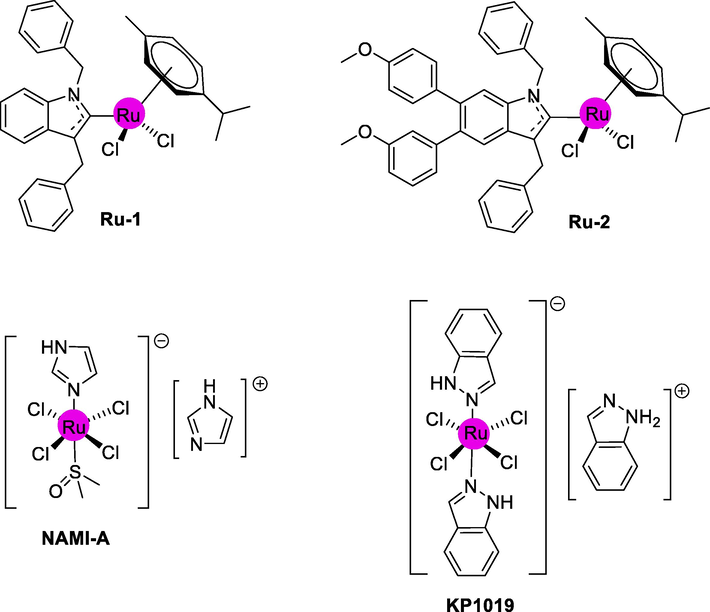
Some important bioactive complexes.
The current research proposed by Lu et al. based on the potential dual-targeting Ru(IV) candidate with antimetastatic and anticancer properties of ruthenium complexes which is under in vitro and in vivo investigation via the PARP/ATM route (Lu et al., 2021). Ruthenium complexes can be used in the development of efficient chemotherapeutic drugs for human lung cancer (Sun et al., 2021). Ruthenium complexes have overall low toxicity and cumulate in cancer cells because these complexes have a range of accessible oxidation states, a fast rate of ligand exchange, and the potential to simulate strong metal interaction with certain biomolecules (Zeng et al., 2022).
The most likely first findings on biological properties triggered by metal N-heterocyclic carbene (NHC) complexes seem between 1996 and 1999 when antifungal and antibacterial activities were described for various rhodium(I) and ruthenium(II) complexes (Çetinkaya et al., 1996; Durmaz et al., 1997; Cetinkaya et al., 1999). Ruthenium compounds have recently been explored as potential antibacterial agents (Namiecińska et al., 2022). Jian et al. reported the antibacterial properties of ruthenium (II) polypyridyl complex [RuCl(MePhtpy)(dpp)]+. These complexes show antibacterial properties in the absence of light which is further enhanced by photoirradiation (Jain et al., 2022).
The chemistry of ruthenium is extremely diverse and vast, and new fields in catalysis are continuously emerging (homogeneous, heterogeneous, photo catalysis, etc.). Ruthenium catalysis has now become a significant catalytic tool in synthetic chemistry (Kaur, 2018; Udvardy et al., 2021). In general, ruthenium metal is far less costly than iridium metal (around ten times cheaper) (Siek et al., 2017).
The ruthenium-catalyzed functionalization and C–H bond activation are widely employed in modern synthetic approaches because of significant aspect of catalysis which based on the pioneering work of Kakiuchi, Ackermann, Bruneau Inoue, and Dixneuf groups (Oi et al., 2001; Oi et al., 2002; Kakiuchi et al., 2003; Ackermann et al., 2006; Ueno et al., 2006; Ackermann et al., 2008; Ferrer Flegeau et al., 2011). Murai, Kakiuchi, and Chatani were the first to describe Ru(0)-complex-mediated coupling of olefins with aromatic ketones in 1993 followed by the pioneering work of Lewis in the synthesis of C–C bonds accelerated by Ru-complexes having ortho-metallated triphenyl phosphite ligand (Murai et al., 1993; Özdemir et al., 2008). Ruthenium complexes also capable of catalyzing the oxidative reactions known as “borrowing hydrogen transformations” (Huq et al., 2009; Sahu et al., 2018). The simple synthetic approaches of cyclo-metallated species through C–H bond breakage, their stability in both water and air, and their stability with currently utilized oxidants are likely reasons for the success of Ru(II) catalysis (Dupont et al., 2005; Boutadla et al., 2009; Djukic et al., 2009). Recently, ruthenium complexes have been utilized as attractive and outstanding catalysts due to good selectivity, versatility, variable oxidation states, high reactivity, and are less expensive than Rh, Pd, Pt, or Ir catalysts (Li et al., 2015; Paterson et al., 2015; Ruiz et al., 2016; Nareddy et al., 2017; Shan et al., 2018).
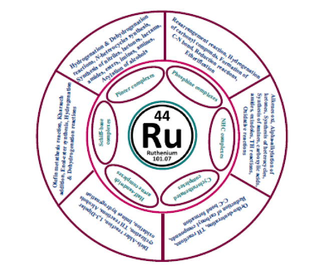
Ruthenium complexes have been classified based on auxiliary ligands that can coordinate with the ruthenium metal such as Ru-pincer complexes, Ru-phosphine complexes, Ru-Schiff base complexes, Ru-half sandwich arene complexes, Ru-NHC complexes, etc. These ruthenium complexes have promising catalytic potential for a vast range of synthetic applications. Dehydrogenation, oxidation, hydrogenation, amination of alcohols, N-alkylation of amines, olefin metathesis reaction, and synthesis of N-heterocycles are some promising catalytic applications of ruthenium complexes. One of the most significant catalytic applications of ruthenium complexes is the formation of α-alkylated ketones which are an important area of interest due to their biological activity and are used as intermediate in organic synthesis. Transfer hydrogenation (TH) is an important alternative method for obtaining a wide range of medicinal and industrial compounds. This is a notably green synthetic method due to features which are simple mechanism, high atom-economy, and inexpensive hydrogen donors. Ruthenium complexes containing various auxiliary ligands have been successfully used in this synthetic approach (Çalık et al., 2016; Türkmen and Kavukcu, 2019; Pakyapan et al., 2020). This review gives a brief account of the synthesis of ruthenium complexes and various catalytic applications which can be exploited for applications in the medical field (Fig. 2).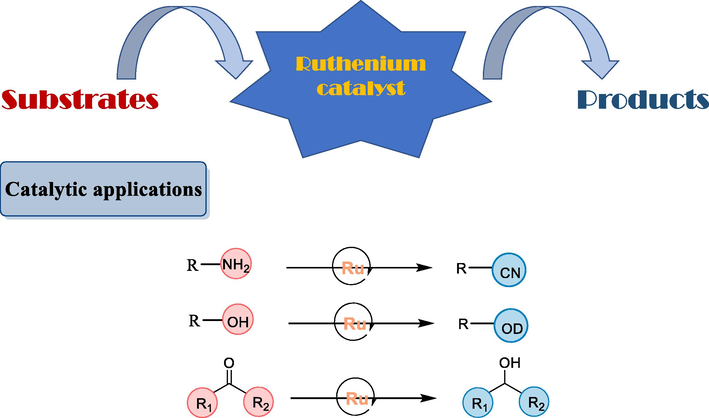
Generalized scheme of catalytic applications of Ru-complexes.
2 Ru-Pincer Complex (RPCs)-Synthesis and applications
Pincer complexes are made up of “tridentate pincer ligands”. These tridentate ligands compel meridional symmetry at the central atom when complexed with metals. Several pincer complexes and their intermediates are become growing more common in chemical synthesis and commercially available because of their wider application and use in chemical synthesis. These unsaturated and saturated ruthenium pincer complexes having aliphatic and hetero-aromatic frameworks exhibit efficient reactivity and serve as effective catalysts in a variety of synthetic methodologies (Gunanathan and Milstein, 2014). Pincer-based catalytic metal complexes have extraordinary stability to reactivity ratios. RPCs have superior functional group tolerance, selectivity, and efficiency than typical ruthenium catalysts. RPCs have a broad range of uses in catalysis. RPCs possess a significant catalytic capability, which is crucial for turning chemicals into useful products. Dehydrogenative oxidation reactions, hydrogenation reactions, terminal alkynes hydroboration, amination of alcohols, cyclopropanation of olefins, hydrolysis of amines, carbonyl compound olefin metathesis, alkynylation by using terminal alkynes, and racemization of alcohols are few examples of RCPs catalyzed reactions (Younus et al., 2015; Zhang et al., 2020).
2.1 Synthesis of Ru(II)-CNN complexes having pyrazoyl-indolyl pyridine ligand and their applications
The N-heteroarenes and carbonyl compounds are very important in medicinal chemistry and organic transformations. Allen and their coworkers reported (2013) that the metal-catalyzed oxidation of organic compounds using air as an oxidant. Eftekhari-Sis and their coworkers reported (2015) the formation of heterocyclic compounds by using α-oxoesters as starting material. Guo and their colleagues (2015) reported the synthesis of a range of significant structural moieties including N,O-heterocycles, N-heterocycles, N,S-heterocycles, and O-heterocycles by using Cu-mediated C−H functionalization. Giustra and their coworkers (2016) reported the homogeneous catalysts for the hydrogenation of heteroarenes and arenes and dehydrogenation of saturated heterocycles, and cycloalkanes respectively (Allen et al., 2013; Eftekhari-Sis and Zirak, 2015; Guo et al., 2015; Giustra et al., 2016). Dehydrogenative catalysis is a cost-effective and green synthetic method (Gunanathan and Milstein, 2013; Peña-López et al., 2015; Daw et al., 2016). This strategy also avoids the use of stoichiometric oxidants and this method is highly desirable in organic synthesis (Suzuki, 2011; Song et al., 2014; Kopylovich et al., 2015; Gülcemal et al., 2016; Crabtree, 2017).
The ruthenium (II) hydride complexes (C3-C4) containing pyrazolyl-(2-indolyl)-pyridine ligand effectively catalyzed the alcohols and N-heterocycles dehydrogenation with a wide substrate scope. Ligands (3a-3b) were obtained in fair to good yields (68-83%) by the reactions of indole or 5-substituted indoles (2a-2b) with pyrazolyl pyridine-based compound (1) via C-N cross-coupling in the presence of a catalytic amount of copper iodide. The ligands (3a-3b) on reaction with RuCl2(PPh3)3 having equimolar concentration with 2-propanol upon refluxing by using NEt3 as a base afforded the Ruthenium (II) complexes (C1-C2) in 86-89% yields. Ru-hydride complexes C3 and C4 were formed in moderate yields by reacting the mixture of complexes (C1-C2) with K2CO3 in 2-propanol upon refluxing (Scheme 1).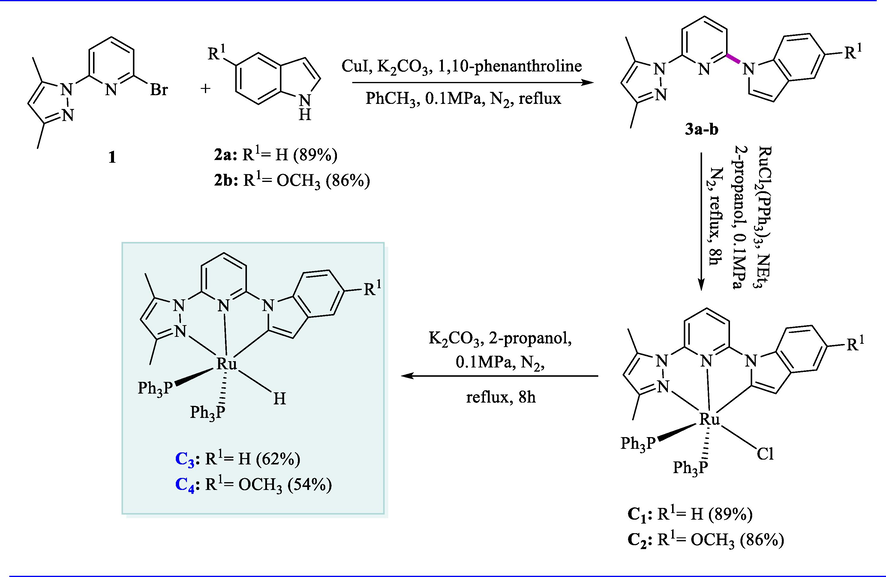
Synthesis of pyrazolyl-(2-indolyl)-pyridine-based Ru(II) complexes.
It was found that complex (C4) is very efficient in catalyzing the dehydrogenation of alcohols and N-heterocycles. The derivatives of N-heterocycles tetrahydroquinoline (4) with complex (C4) were transformed to derivatives of quinoline (5) at 140°C for 48h in the presence of o-xylene (Scheme 2). The complex (C4) exhibit highest catalytic activity then Ru (II) complex (C3), producing the quinoline via dehydrogenation product in good yield. Lepidine (7) possesses anti-bacterial properties obtained in a 75% yield via reaction of 4-methyltetrahydroquinoline (6) (Scheme 3). The tetrahydroquinolines modified at the 3- or 2-position by a phenyl or methyl group yield the target products (72-89%). The substitution on the substrates having aryl moiety was varied to produce products in good yields (83-93%). The reaction efficiency was lowered when the temperature was decreased up to 120°C. The mild conditions such as reduced the loading of ruthenium catalyst C4 (2 mol%) at 140°C, higher TONs or TOFs were achieved. These findings show that the current catalysts have excellent catalytic activity and tolerate a wide range of substrates. Under base-free circumstances, the ruthenium (II) hydride complex (C4) could perform the dehydrogenation process more efficiently.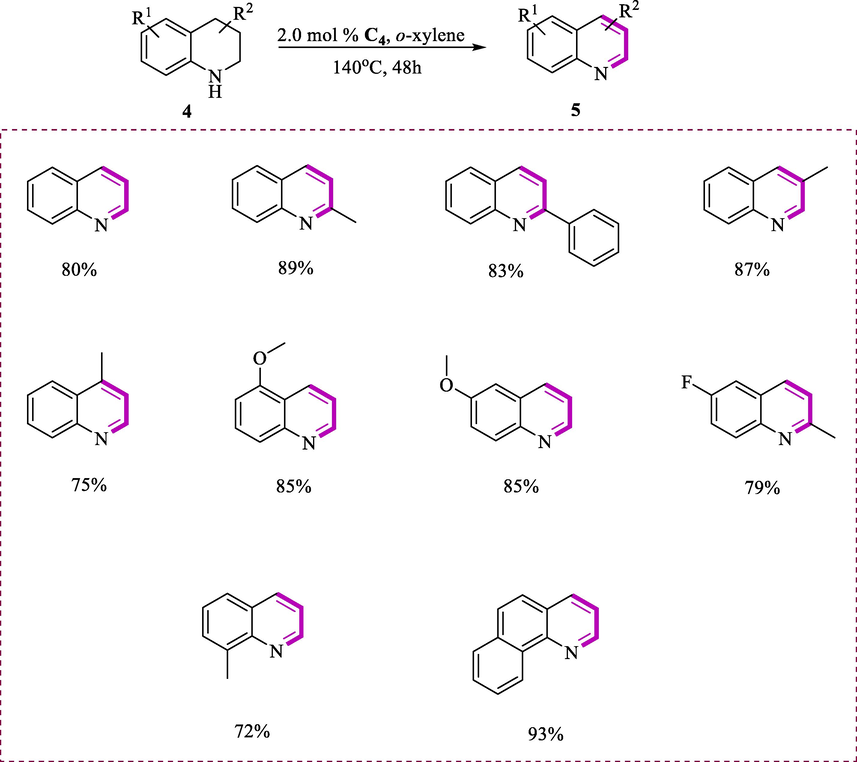
Dehydrogenation of tetrahydroquinolines.
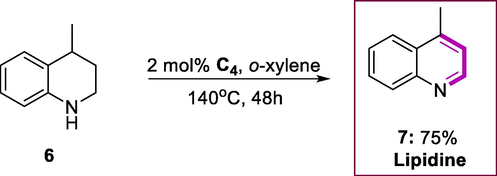
Lepidine synthesis.
A variety of synthetic methods have been devised to produce derivatives of indoles due to their importance in pharmaceutical and chemical fields. Using the ruthenium complex (C4), derivatives of indoline (8) could be effectively dehydrogenated to derivatives of indoles (9) in the presence of o-xylene at 140°C for 48h (Scheme 4). Indole obtains an 82% yield by a reaction of indoline. Dehydrogenation reactions were also successful on indoline substrates containing 2-Ph, 2-Me, or 3-Me substituent, giving substituted indoles in fair to good yields (78-91%). Indolines with electron-donating substituents dehydrogenated to give (76-88%), and the negative steric effect on the reaction effectiveness is due to the 7-methyl substituent on the substrate. On the phenyl ring of indoline substrates, chloro, fluoro, nitro, and bromo substituents lowered reaction outcomes, yielding remarkably low yields (54-75%). These findings show that the fused benzo moiety, as well as NH-functionality in the N-heterocycles, are required for catalytic dehydrogenative reactions to be carried out under the specified conditions.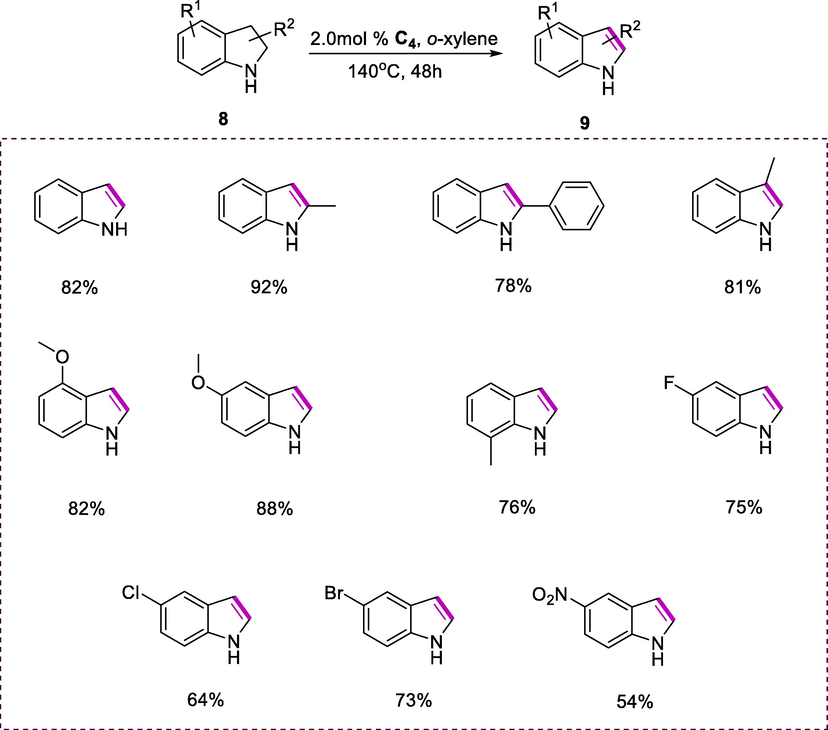
Dehydrogenation of indolines.
The β-carboline motif has long been known to have a significant role in numerous medications. As a result of the Pictet-Spengler reaction of tryptamine (A) and aldehyde (B), 1,2,3,4-tetrahydro-carbolines (10a-10b) were produced in the presence of TFA under reflux. Compounds (10a-10b) underwent comparable dehydrogenative reactions in the presence of complex (C4), yielding the desired β-carboline alkaloids (11a-11b) in high yields (75–80%). The current process is a simple and green synthetic way to obtain derivatives of β-carboline (Scheme 5).
Synthesis of β-Carbolines.
The catalytic dehydrogenation of secondary alcohols (12) into corresponding ketones (13) was achieved by using toluene as a solvent in the presence of complex (C4) at 110°C. The phenylethanol was converted into acetophenone in excellent yield (92%) in 24h using complex C4 (0.5mol%) as the catalyst under reflux in toluene (Scheme 6). The alcohols containing aryl moieties acted as a steric barrier but does not affect the reaction efficacy. Dehydrogenation of 2-methyl-1-phenylethanol, 1-phenylpropanol, and analogs resulted in 94-98% yields of the target ketone products, irrespective of the electron-withdrawing and electron-donating substituents. The dehydrogenation of diphenylmethanol and naphthalene-2-yl-ethanol resulted in the formation of the respective ketones (98%) and (92%). By increasing the catalyst loading up to 1.0 mol %, ketones were also obtained in excellent yields (87-99 %) as a product of efficient catalytic dehydrogenation of corresponding aliphatic alcohols. Despite comparatively less reactivity of cyclohexanol, a good yield (82%) of cyclohexanone was observed.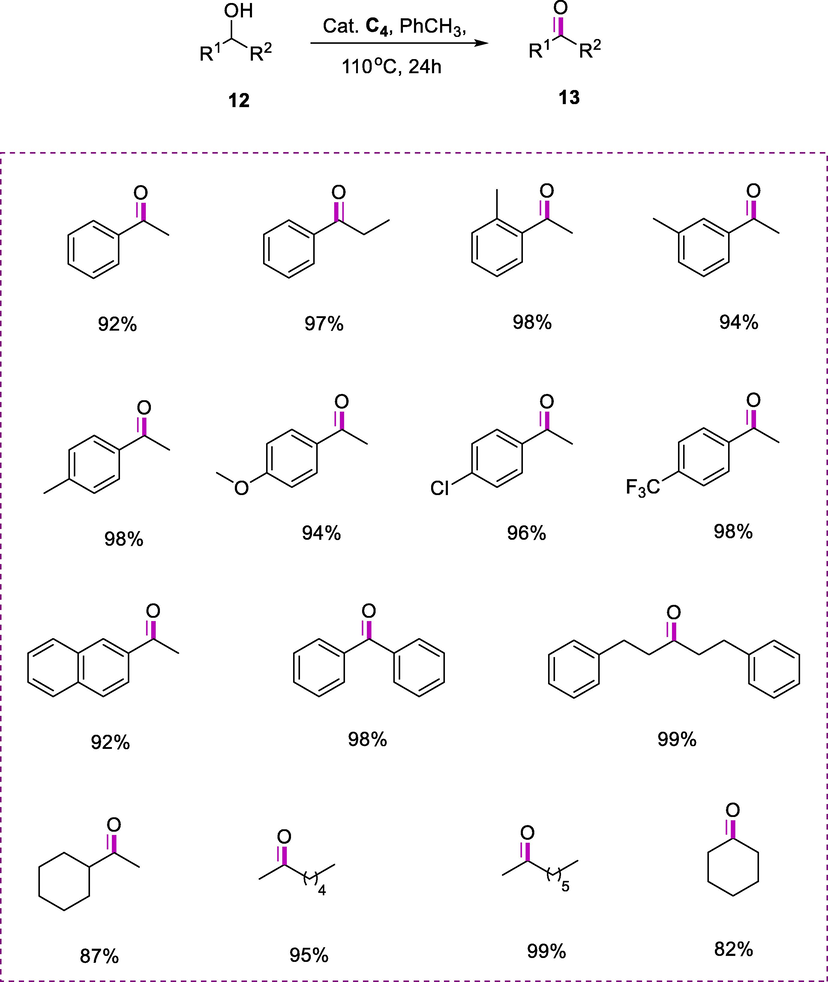
Dehydrogenation of secondary alcohols.
Cholest-5-en-3-ol was also dehydrogenated under the same conditions as α,β-unsaturated ketone cholest-4-en-3-one (14) in moderate yield (62%). methyl glycyrrhetinate and 5-Cholestan-3-ol and were also dehydrogenated to the respective ketones (15 and 16) in (76%) and (52%) yields indicating that the synthesis procedure might be used in specialized pharmaceutical synthesis (Wang et al., 2018).
2.2 Synthesis of PNP-type Ru(II) hydrido borohydride complex and its application
The borohydride transition metal complexes have good reactivities towards organic substrates, thus they are served as good building blocks for transition metal borides and hydrides (White et al., 1991; Dick et al., 1993). These complexes are useful in the catalytic dehydrogenation of diols into cyclic esters (Palard et al., 2004). The diols dehydrogenation to cyclic esters is catalyzed by the bulky, electron-rich tBu-PNP-Ru-complexes (Zhang et al., 2004). The reaction catalyzed by these complexes does not need the base to accelerate the reaction, proceeds very efficiently in neutral or mild conditions (Zhang et al., 2005a).
The RuCl2(PPh3)3 complex reacts with the tBu-PNN pincer ligand (one eq.) yielding the N2-linked binuclear Ru(II) complex [(RuCl2-(PNN))2](-N2) (C5). The ruthenium(II) hydrido borohydride complex (C6) was formed in excellent yield by the reaction of complex (C5) with excess (5 eq.) of reducing agent NaBH4 in the presence of 2-propanol for 12h (Scheme 7).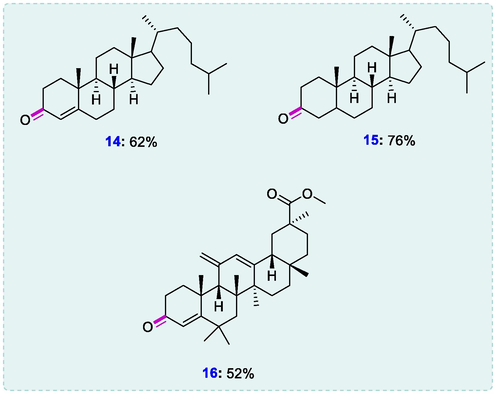
Pharmaceutically active compounds having ketone functionality formed via dehydrogenation.
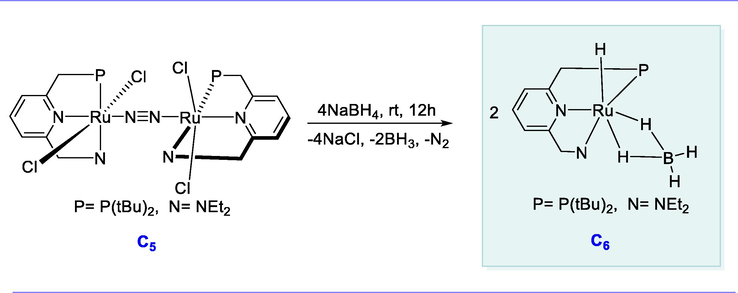
Synthesis of PNP-type Ru(II) hydrido borohydride complex.
The Ru-PNN complex (C6) is the most efficient in catalyzing the dehydrogenative cyclization of diols (17) into lactones (18) under very mild reaction conditions with hydrogen gas evolution (Scheme 8). Thus, a toluene solution containing diol (containing both primary and secondary alcohol groups) and a catalytic quantity of complex (C6) (0.01 mmol) was refluxed for 48h under an argon environment, afforded good yields of lactones (cyclic esters). Several diols were dehydrogenated to the respective lactones in this generic reaction. These dehydrogenative reactions of alcohols are interesting for the storage of hydrogen as well as the synthesis of various organic compounds (Zhang et al., 2011a).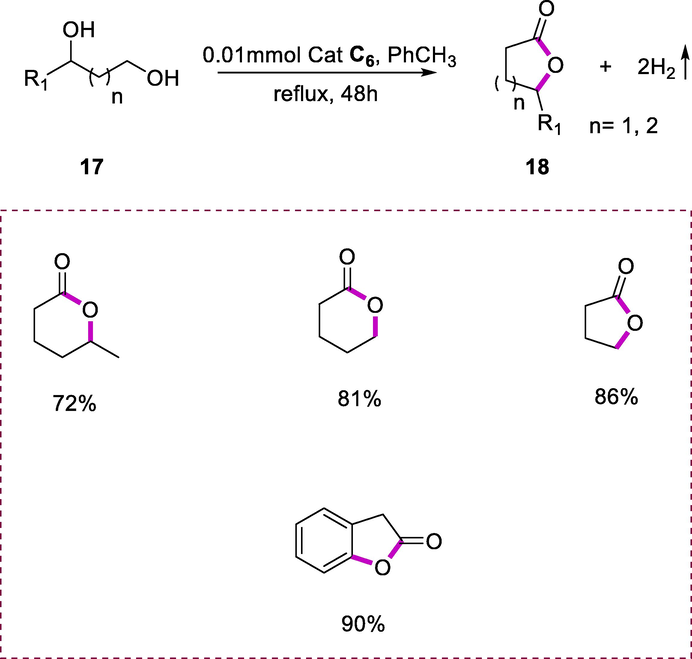
Dehydrogenation of diols into lactones.
2.3 Synthesis of pincer Ru(II) hydrido olefin complexes and their application
Catalytic dehydrogenation of alkene is a significant synthetic reaction because it transforms saturated hydrocarbon having low value into alkenes that are extremely versatile intermediates in synthesis. Choi and their coworkers (2011) reported the dehydrogenation of alkenes and heteroarenes by using pincer complexes. Zhang and their coworkers (2015) reported the synthesis of homogeneous transition-metal catalysts for the dehydrogenation of alkanes (Choi et al., 2011; Pérez, 2012; Fang and Huang, 2015). Alkenes are generally produced in the industry by dehydrogenating alkanes over heterogeneous catalysts (Burk et al., 1984; Felkin et al., 1985; Burk and Crabtree, 1987). In the dehydrogenation of cyclic and linear alkanes, the hydrido Ru(II) olefin complexes are thermally labile at high temperatures. In the absence of a hydrogen acceptor, they are also capable of dehydrogenating the cyclooctane (COA) (Six et al., 1999). The efficacy of these Ru-pincer complexes is based on their thermal lability at high temperatures. The catalyst could be used to modify natural products in a late-stage process by dehydrogenating the alkyl chain with polar functional groups and heterocycles.
The ligand precursors iPrPOCOP-H (a), iPrPCP-H (b), p-C6F5-iPrPOCOP-H (c), and iPrPSCOP-H (d) reacts with RuCl2(PPh3)3 complex in the presence of THF at 80°C for 8h resulted in the production of novel isopropyl-substituted pincer Ru(II) complexes (iPrPOCOP)RuCl(PPh3) (C7), (iPrPCP)RuCl(PPh3) (C8), (p-C6F5-iPrPOCOP)RuCl(PPh3) (C9), and (iPrPSCOP)RuCl(PPh3) (C10). We wanted to replace the coordinated PPh3 in complexes (C7-C10) with a more stable olefin ligand since the presence of PPh3 could hinder the activity of dehydrogenation. The reactions of complexes (C7-C10) with CuCl (used as a PPh3 scavenger) (5 eq. each) and norbornadiene (NBD) resulted in the formation of the respective Ru(II) chloro NBD complexes (C11-C14) in moderate yields (58-72%) as orange solid. The hydrido Ru(II) NBD complexes (C15-C18) were obtained in excellent yields by reacting complexes (C11-C14) with excess NaBH4 (10 eq.) in THF. These complexes are found to be efficient for the catalytic dehydrogenation of alkane and are thermally stable (Scheme 9).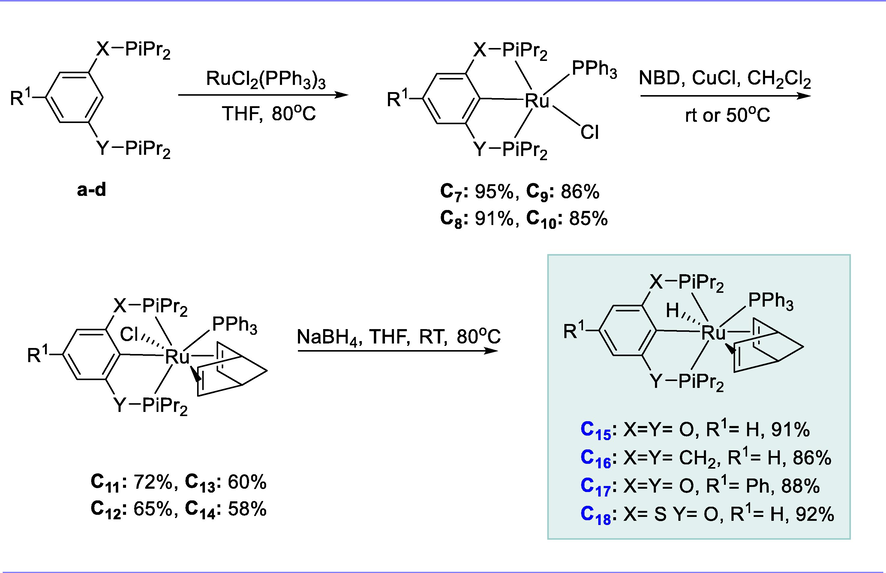
Synthesis of pincer Ru(II) hydrido olefins complexes.
The novel Ru complexes were initially put to the test for cyclooctane 19 (COA) transfer dehydrogenation by using tert-butylethene 20 (TBE) as the H2 acceptor. The 0.35M TBE was found to be effective to carry out transfer dehydrogenation of COA/TBE with several Ru-complexes (C15-C18). The activity of the POCOP Ru-complexes (C15 and C17) and the PCP complex (C16) is good. The PSCOP analog Ru-complex (C18) was found to be inactive for catalytic dehydrogenation. Despite the moderate beginning rate, complexes (C15 and C17) have the higher productivity for the transfer dehydrogenation of COA/TBE among all Ru-complexes. These dehydrogenation reactions, which tolerate ethers, esters, ketones, water, and other impurities in the starting materials, are a significant step forward in the formation of a practical catalyst for dehydrogenation (Scheme 10) (Zhang et al., 2016c).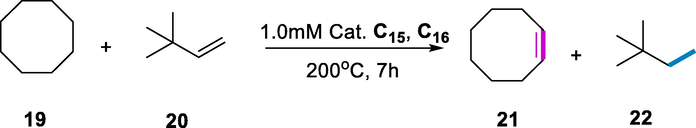
Dehydrogenation of alkene.
2.4 Synthesis of pyridine-based Ru-CNN pincer complexes and their application
The conversion of esters to alcohols via reduction is a principal step in organic synthesis. Traditional methods that utilize stoichiometric reagents like LiAlH4 for laboratory-scale ester reduction are reliable and efficient, but they have a low atom economy (Seyden-Penne, 1997). The formation of effective homogeneous catalysts for the ester hydrogenation that perform under mild conditions is of considerable significance for environmental and economic reasons. The homogenous catalysts that are used for the hydrogenation of esters are based primarily on ruthenium (Turek et al., 1994; Rieke et al., 1997; Teunissen, 1998; Pouilloux et al., 2000; Nomura et al., 2001). Various ruthenium pincer ligand-based complexes have been recently introduced as bifunctional catalysts for coupling primary alcohols to esters via dehydrogenation or ester hydrogenation in the reverse reaction (Del Pozo et al., 2011; Fogler et al., 2011; Sun et al., 2011; Filonenko et al., 2015).
Compounds (25 and 26) were obtained by reaction of suitable secondary amines (24) with pyridine-2-carboxaldehyde (23) via reductive amination. The palladium-catalyzed coupling of mesityl bromide (27) and 1,2-diaminobenzene (28) yielded the known diamine (29), which had previously been produced in two steps. The ligand precursors (30 and 31) were obtained by coupling compounds (25 and 26) with diamine (29). Finally, the ligands (30 and 31) were cyclized using triethylorthoformate and HCl to produce trihydrochloride salts (32 and 33). The ruthenium-pincer complexes (C19 and C20) are formed through transmetalation of the ligands (32 and 33) with Ru-complex RuHCl(CO)(PPh3)3. These Ru-pincer complexes (C19 and C20) are moderately air-stable in solution and so use air-free column chromatography to separate these complexes (Scheme 11).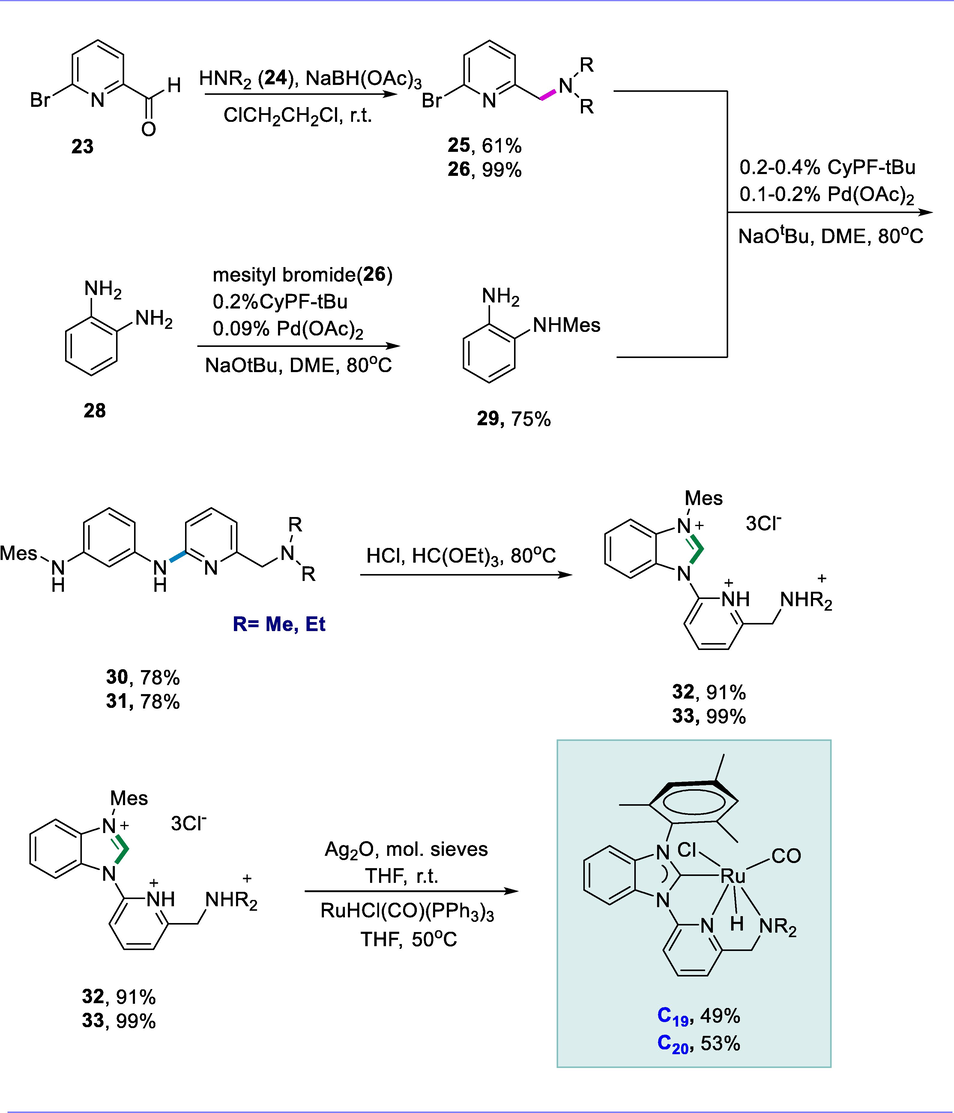
Synthesis of pyridine-based Ru-CNN pincer complexes.
The complexes (C19 and C20) were evaluated as catalysts for the ethyl benzoate hydrogenation by using a catalytic amount of NaOtBu under mild conditions (105°C, 6 bar H2). Remarkably, a subtle change is linked with the improvement of catalytic activity when move from complex C19 to C20. The complex (C20) consider as promise catalyst for this transformation and more active than complex (C19). In comparison to ruthenium complexes, yields of reactions were more consistent when more equivalents of the base were utilized. The Ru-complex (C20) is also much more active than the Ru-complex (C19). Catalyst C20 has been evaluated on a variety of esters (34) hydrogenation. The variety of catalyst loadings was investigated for different ester substrates. The lowest catalyst loading resulted in approximately full conversion. This catalyst tolerates a wide range of aliphatic and aromatic esters involving hexyl, ethyl, and benzyl esters. The complete hydrogenation of phthalide required H2 pressure (30 bar), but only 36% conversion was recorded at 6 bar. While complex C20 is a very effective catalyst but when it comes to hydrogenating methyl esters then this complex becomes remarkably inactive such as ethyl cyclohexane carboxylate and ethyl benzoate hydrogenate very effectively, while this complex produces very low yields of the respective methyl esters. The main reason for the low reactivity of methyl esters in the current catalytic system is because methanol that is formed as a byproduct is a very potent catalyst toxin, reducing catalyst activity (Scheme 12) (Kim et al., 2016).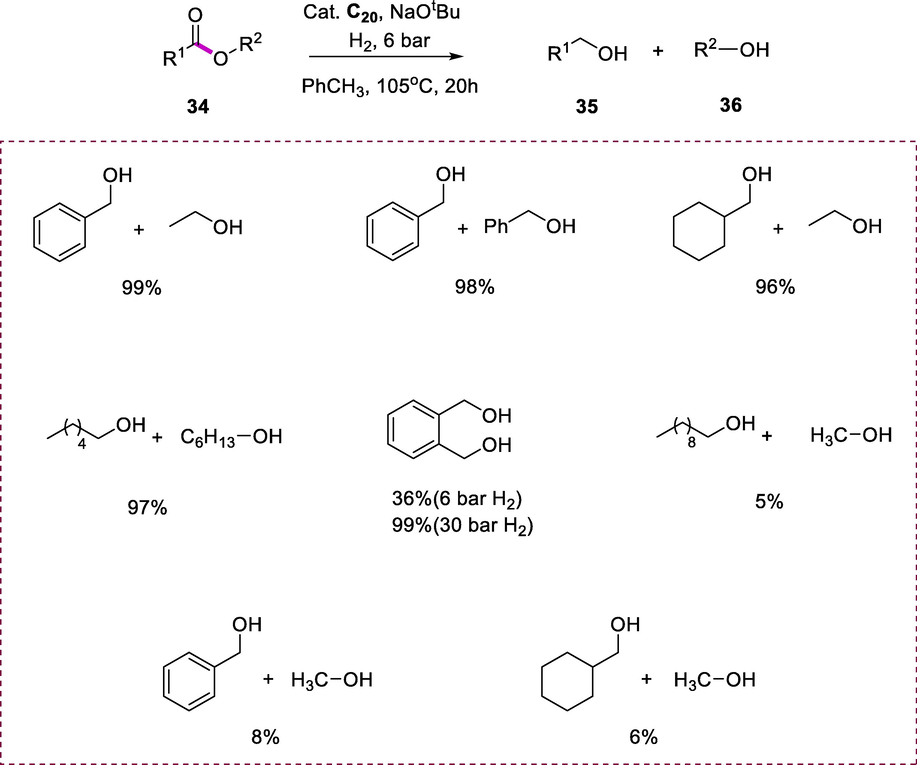
Ester hydrogenation.
2.5 Synthesis of novel dearomatized Ru-PNN complex and its applications
One of the most elementary reactions in organic chemistry is esterification. It has various applications in various fields such as used to make biologically active natural compounds, synthetic intermediate fats and oils esters, polymers, and medicines (Otera and Nishikido, 2009). Among other esterification processes, transesterification is a particularly helpful process for the synthesis of the ester from other alcohols and esters through exchanging alkoxy groups, which avoids the utilization of moisture-sensitive and air-sensitive substrates such as acid halides (Hoydonckx et al., 2004; Otera, 2004; Enders et al., 2007). The Ru-PNN catalyst was used to accelerate the ester transesterification with different secondary alcohols. This reaction is efficient, and non-harmful to the environment. It takes place in neutral conditions without the use of bases or acids, activators, or molecular sieves. The only co-product of this reaction is molecular hydrogen when secondary alcohols and symmetrical ester (such as ethyl acetate) are used as substrate.
The reaction of Ru-complex RuHCl(CO)(PPh3)3 with the novel pyridine-based PNN ligand produced Complex (C21) in 90% yield. Complex (C21) was treated with 1eq. of KOtBu resulting in the benzylic phosphine “arm,” deprotonation occur rather than hydride ligand affording an 89% yield of Ru-complex (C22) at -32°C in the form of brown-red solid. Complex C14 is an effective acylation catalyst that uses ester as the acylation agent (Scheme 13) (Zhang et al., 2005b).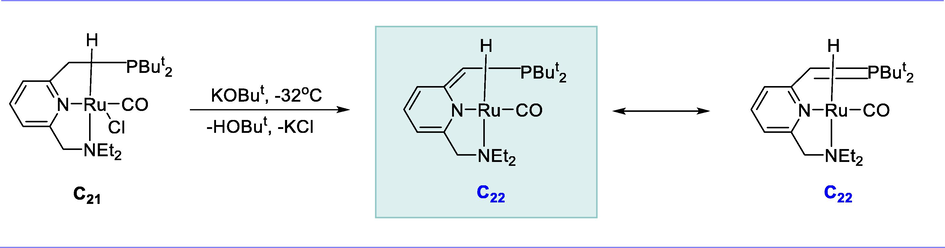
Synthesis of novel dearomatized Ru-PNN complex.
The secondary alcohols (37) acylation by using esters as the acylating reagent (38) was effectively accelerated by complex C22 (0.05 mmol) as a catalyst by refluxing the reaction mixture in toluene under neutral conditions. The symmetrical esters such as ethyl acetate when used in the reaction, both the alkoxy and acyl and portions of the esters as substrate are integrated into the final ester product resulting in hydrogen liberation as a side product. This process of ester to ester conversion is environmentally friendly. Unsymmetrical esters were also used to study these reactions. Unlike the classic transesterification strategy, this method cannot afford alcohol as a byproduct. In this reaction, both the alkoxy and acyl portions of the ester substrate are irreversibly integrated into the final ester (Scheme 14) (Gnanaprakasam et al., 2010a).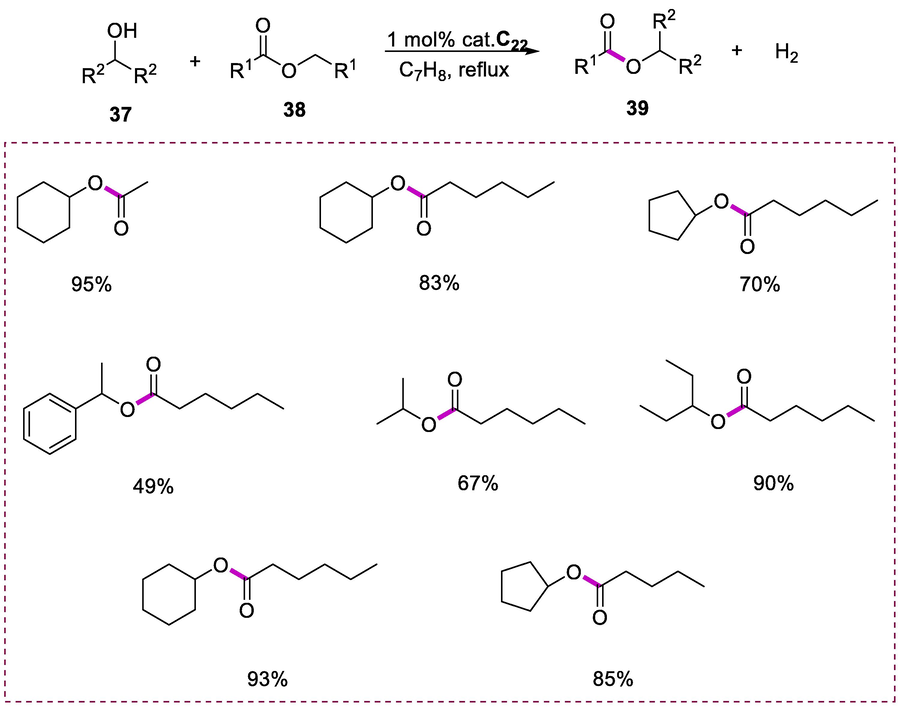
Acylation of alcohols from esters.
The carboxamides are an extremely important functional group in both chemistry and biology. The amide formation from esters is an interesting synthetic approach; however, a catalytic amount of metal mediator or promoters is usually required (Shimizu et al., 1997; Kurosawa et al., 2003; Constable et al., 2007; Ferroud et al., 2008). Complex C21 and its analogous Ru-PNP complex (C22) accelerate the primary alcohols dehydrogenation to produce esters, the formation of alcohols from ester hydrogenation, the secondary alcohol dehydrogenation to ketones, and most recently, the secondary alcohol acylation by using esters (Zhang et al., 2004, 2005b, 2006, 2007; Gnanaprakasam et al., 2010a). The coupling of esters with amines is a new catalytic amide synthesis method. This reaction of amide synthesis is an efficient, broad, and green synthetic method. Neutral conditions are required to proceed with this reaction. The remarkable property of this reaction is that it produces H2 rather than alcohol as a byproduct.
The synthesis of corresponding amides (42) was achieved by refluxing a reaction mixture of esters (40) and amines (41) with Complex C22 (0.01 mmol) in the presence of toluene/benzene at 135°C in an oil bath. This reaction can tolerate a broad range of esters and amines. This reaction had also great tolerance toward symmetrical esters. The amines acylation with esters as the acylating agent is effectively accelerated by the dearomatized Ru-complex (C22) under mild reaction conditions with no production of waste in this transformation. When symmetrical esters are used as a substrate both the alkoxy and acyl part of the ester substrate are incorporated into the final amide product resulting in the liberation of hydrogen gas. The high turnover numbers (TON) were attained in this reaction which is greater in magnitude up to two orders. The use of primary and secondary amines in this approach was also tolerated (Scheme 15) (Gnanaprakasam and Milstein, 2011).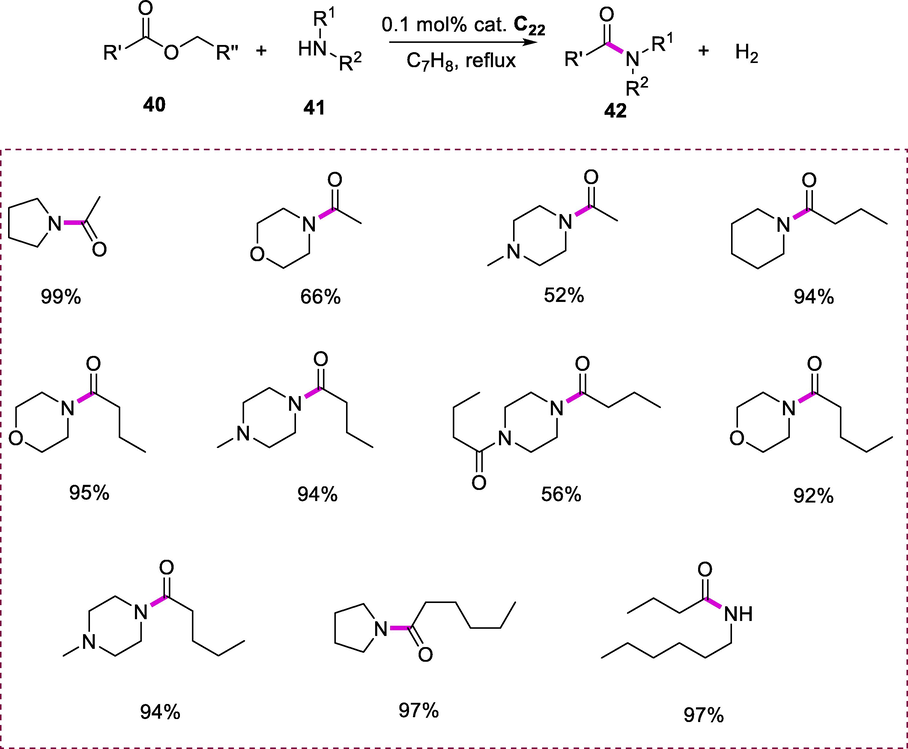
Synthesis of amides from esters and amines.
Imines are a versatile moiety that is used as a synthetic building block for the formation of organic compounds containing nitrogen atoms indicating their significance in synthetic chemistry. Moreover, these imines serve as functional reagents and building blocks in the formation of pharmaceutics and natural products (Bloch, 1998; Kobayashi and Ishitani, 1999; Córdova, 2004). The Ru-pincer complexes have higher reactivities for direct amine coupling to produce an imine via the introduction of imine functionality to the pincer ligand. These complexes provide the simple, direct synthetic route for imine synthesis under a base-free system.
The reaction mixture containing an appropriate amount of Ru-complex C22 (1.0 mol %) and amine (43) in toluene or aniline was heated under an argon environment at the given temperature. The appropriate imines (44) were synthesized in excellent yields. Aniline derivatives were used as the substrate and solvent for benzyl amines selective oxidation to imines. The product imines were obtained in low yields when electron-rich substituents were present on the benzene ring. Aniline with electron-withdrawing substituents like halogens and electron-donating substituents like MeO- results in significant conversion and yields of respective imines. Under oxidant and base-free conditions, this approach affords a systematic strategy for the benzylamines selective oxidation to the respective imines (Scheme 16) (He et al., 2012).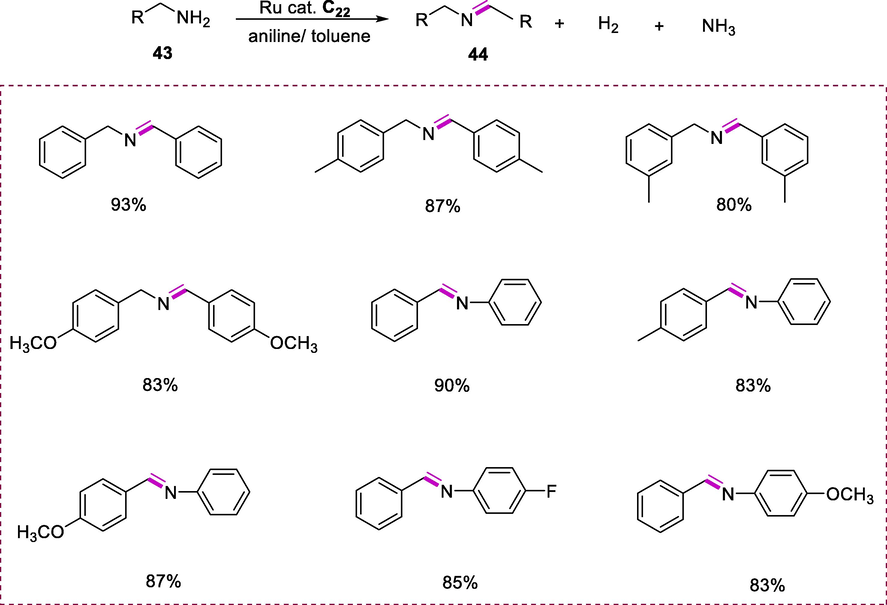
Synthesis of imines via coupling of amines.
Imines are essential compounds due to their broad range of reactivities that lead to their broad applications in laboratory and commercial synthetic methods (Adams, 2000; Gawronski et al., 2008). The formation of a generic and effective process for the imine synthesis from amines and alcohols is highly beneficial due to their wide scope and potential versatility (Blackburn and Taylor, 2001). The coupling of amines with alcohols accelerated by ruthenium pincer complexes leads to the liberation of H2 gas and water. This method does not produce any waste products, making it an effective and green synthetic approach for direct imine synthesis. Furthermore, this strategy was carried out in neutral conditions with no hydrogen acceptor required.
The complex (C22) is also capable of the formation of imines (47) via the reaction of amines (46) and alcohols (45). This reaction proceeds by heating the reaction mixture with complex C27 (0.2 mol%) as a catalyst in PhCH3 (toluene) under refluxing in the argon environment. The water that forms as a byproduct during the reaction does not affect the outcomes of the reaction. The scope of this strategy was examined using a range of amines and alcohols. The amines having either electron-rich or electron-poor substituents undergo effective dehydrogenative coupling with various substituted benzyl alcohol substrates. The yields of the imines were not affected by substituting the amines at the alpha-position such as the reaction of 2-heptylamine with 4-methoxybenzyl alcohol produced the respective imine in high yield. Remarkably, imines were produced as a major product when aromatic primary alcohols were used as substrates. Secondary alcohols can also be used in the reaction. However, it takes more time to completion of the reaction. The reaction of cyclohexanol with benzylamine by using complex (C22) as a catalyst after 22h at reflux resulted in just 20.5% conversion, with the corresponding ketimine produced in a 20% yield. The instability and difficulty in isolation of aliphatic imines make them more difficult to synthesize (Scheme 17) (Gnanaprakasam et al., 2010b).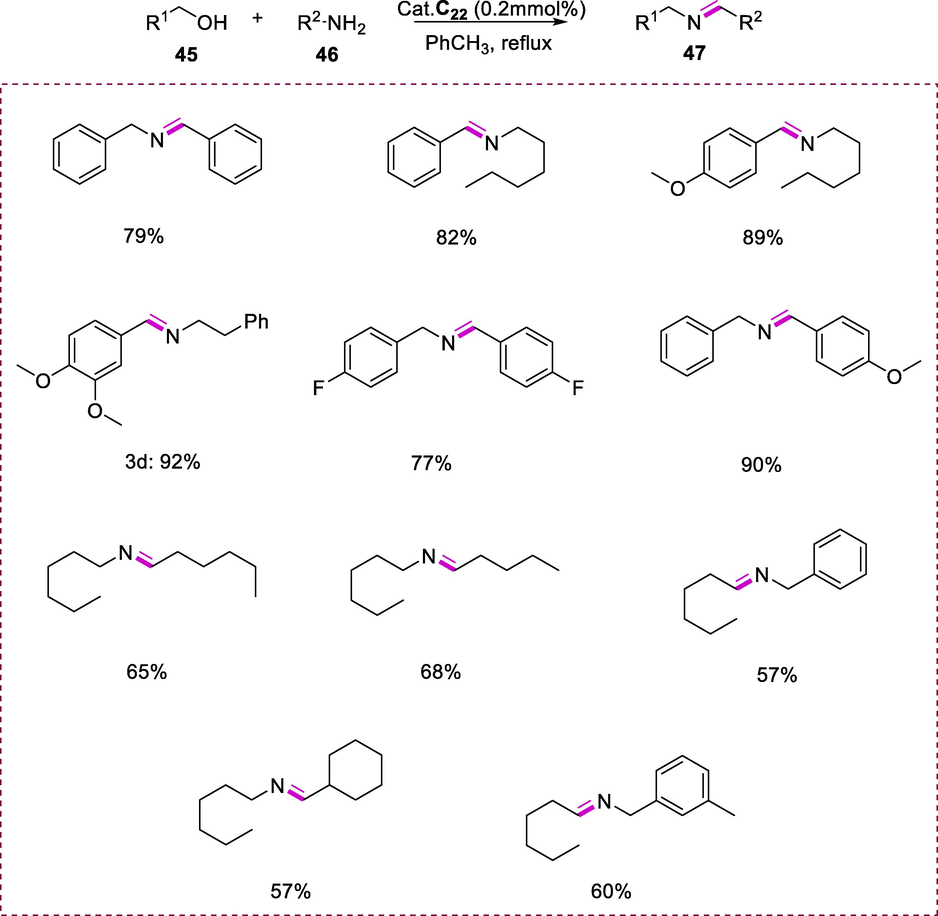
Imines synthesis from amines and alcohols.
2.6 Synthesis of pyridine-based Ru-PNNH complexes and their applications
The amide group reduction can be used in a variety of synthetic transformations such as alcohols and amide synthesis, total synthesis, CO2 recycling, peptide synthesis, and many other organic transformations (Hudlicky, 1996; Andersson and Munslow, 2008). Traditional amide reduction methods are non-catalytic and rely on stoichiometric concentrations of hydride reducing agents, resulting in the production of stoichiometric waste (Seyden-Penne, 1997). It is desirable in this context to develop an efficient catalytic system for amide hydrogenation. The low electrophilicity of the carbonyl group of amide makes amide hydrogenation more challenging than the hydrogenation of many other carboxylic acid derivatives (Smith and Whyman, 2014; Pritchard et al., 2015; Chardon et al., 2018; Zhou et al., 2019). C-N bond cleavage or C-O bond cleavage can both be used to hydrogenate amides. The formation of amines and alcohols via hydrogenation of amides at room temperature is accelerated by low catalyst loading of Ru-PNNH complexes (0.5mol%) under low hydrogen pressures (Fogler et al., 2014; Kumar et al., 2017; Kumar et al., 2018a; Kumar et al., 2018b; Kumar et al., 2019).
The reaction of compound (48) with respective amines (49a-49c), three PNNH ligands with various substituents [R=tertbutyl (50), isopropyl (51), and benzyl (52)] were produced. In the reaction of ligands (50-52) with [Ru(H)Cl(CO)(PPh3)3] complex, the respective Ru-complexes (C23-C25) were produced in excellent yields (85–90%) (Scheme 18). It's worth noting that acidic character of the N-H hydrogen of PNNH ligand helps to reduce the energy demand of rate determining step, enabling hydrogenation to occur even at room temperature via the amido route. This can also illustrate why Ru-C25 has better catalytic activity than Ru-C23 and Ru-C24 because the benzyl substitution of a donor atom makes the PNNH ligand of Ru-C25 less basic than Ru-1 having tBu substitution in the donor atom. Alternatively, the decreased steric hindrance of Bn- group could help to explain the observed higher rate of hydrogenation with Ru-C25 over Ru-C23 and Ru-C24.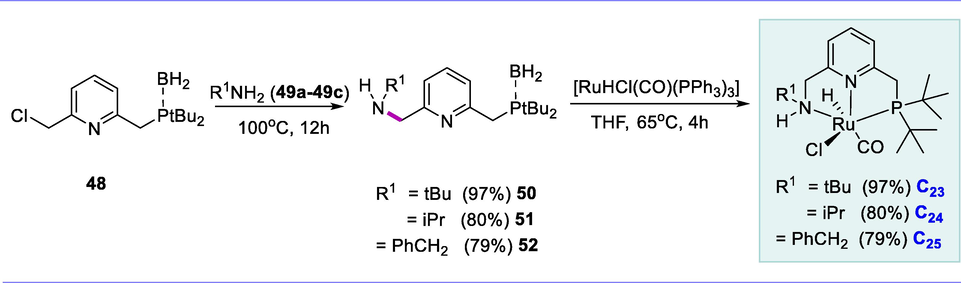
Synthesis of pyridine-based Ru-PNNH complexes.
The amide (53) hydrogenation was accelerated by Ru-PNNH complexes with catalyst loading (0.5mol %) in the presence of tBuOK as a base and THF as a solvent at low Hydrogen pressures (5-10 bars) at ambient temperature resulting in the formation of amines (55) and alcohols (54). The hydrogenation of benzanilide at 45°C with Ru-PNNtBuH complex (Ru-C23; 0.5 mol %) as the catalyst under hydrogen pressure (10 bars) in the presence of tBuOK base (2 mol %) results in the formation of moderate amide conversion to aniline and benzyl alcohol after 20h, indicating that cleavage of C-N bond is the desired route under these conditions. The catalytic activity improved when the N-substitution of the catalyst was replaced from ter-butyl (tBu-) to benzyl (Bn-) group (Ru-C25) resulting in the complete transformation of amide to alcohol and amine was observed after 20h. This transformation can undergo completion even at low temperature (35°C) within 20h by using complex (Ru-C25). Furthermore, complete conversion of benzanilide to aniline and benzyl alcohol can be obtained even at room temperature but a longer reaction time (68h) is required to complete the reaction. Toluene is not an effective solvent for this transformation as THF, presumably because of lack of solubility of benzanilide in the toluene. This catalytic hydrogenation of amides tolerates a wide range of substrates under mild conditions. With catalyst Ru-C25, various substituted benzanilides were transformed into the respective alcohols and aniline at room temperature under H2 pressure (10 bar). The substrates such as diphenylbenzamide, phenyloctanamide, acetylmorpholine, hexylbenzamide, acetylaniline, heptyloctanamide, and formylabenzylamine, were hydrogenated at room temperature or slightly high temperature (35-45°C) that is significantly lesser than the hydrogenation temp. observed with other catalysts previously (Scheme 19) (Kar et al., 2020).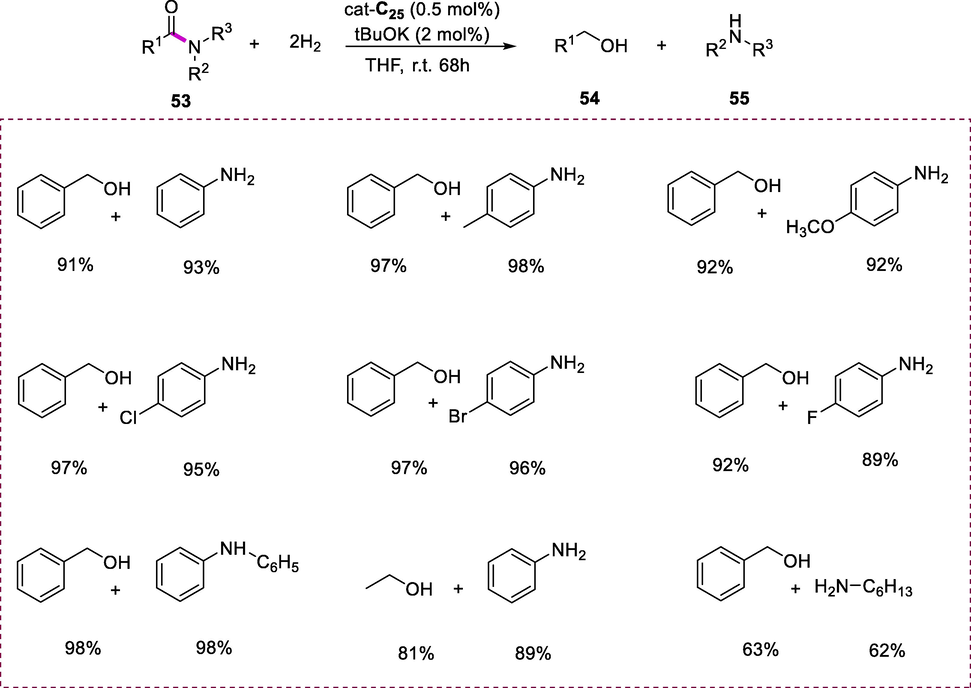
Hydrogenation of amides.
The amide functional group has prime importance in synthetic chemistry. The synthesis of amide bond is a significant step in the formation of medicinally important bioactive compounds, natural compounds, peptides synthesis, polymers like nylons, and industrial chemicals as well as in the formation of carrier system for organic liquid hydrogen (Cupido et al., 2007; Hu et al., 2015; Hu et al., 2016a; Xie et al., 2019). The coupling of amines with acid derivatives such as anhydrides or acid halides has been the classical synthetic approach for amide bond formation, but this method requires high temperature to proceed, generate waste, and other nucleophilic functional groups are poorly tolerated by the classical method (Han and Kim, 2004; Valeur and Bradley, 2009; de Figueiredo et al., 2016; Sabatini et al., 2019). The new environmentally friendly method for amide bond formation is carried out via dehydrogenative coupling of amines and alcohols with hydrogen as the sole byproduct (Gunanathan et al., 2007). The coupling of amine with alcohol in the presence of RuPNNH catalyst under reflux in toluene begins the reaction. The capacity of RuPNNH complexes to activate hemiaminals and alcohols at room temperature with the help of terminal NH-proton accounts for the low-temperature action.
The formation of an amide bond is one of the most significant organic transformations in synthetic chemistry avoiding the poor atom economy. When amines (57) and alcohols (56) were coupled in the presence of complex C25 (0.005mmol), t-BuOK as a base and Et2O as a solvent under reflux resulted in good yields of corresponding amides (58) were obtained. Even at this low temperature, the RuPNNH complexes C23 and C25, which include a terminal NH moiety, showed catalytic activity toward amide production. In comparison to the tert-Bu-substituted complex C23, the N-benzyl substituted PNNH complex C25 showed higher activity. The amides such as N-heptylbenzamide, N-heptylhexanamide, N-benzylbenzamide, N-benzylhexanamide, and N-(furfuryl)hexanamide were also produced in good yields under reflux in diethyl ether. In addition to secondary and primary amines, 1-hexanol reacts with morpholine by using complex C25 as a catalyst at low temperatures resulting in the formation of N-hexanoylmorpholine which is a tertiary amide. Under these conditions, various halogen substituents like F- and Br- were also well tolerated on the aromatic ring (Scheme 20) (Kar et al., 2021).Scheme 20a.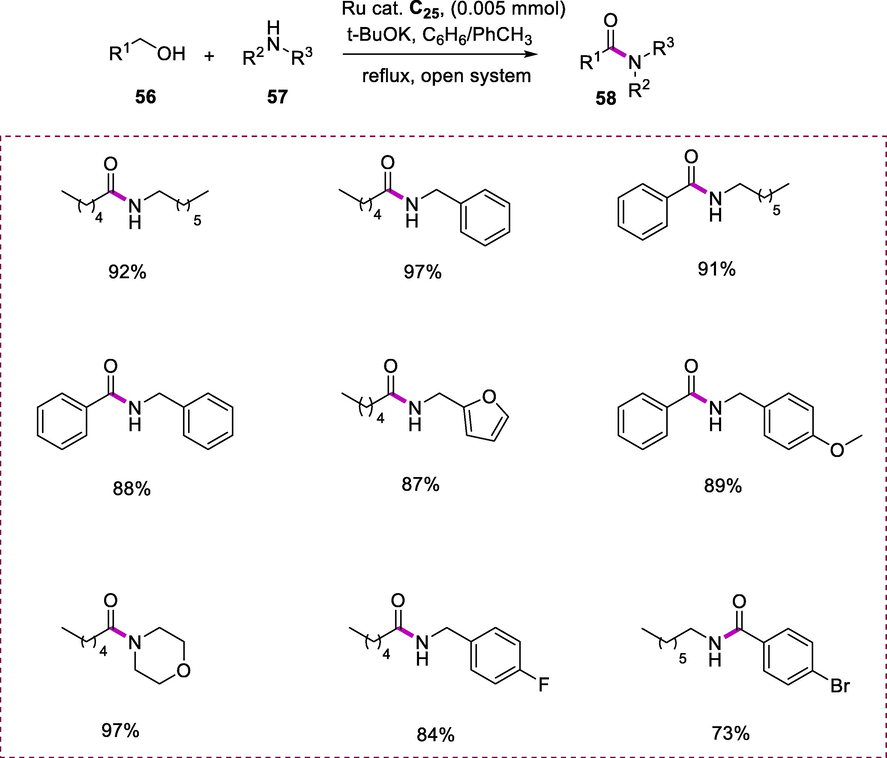
Amides synthesis via dehydrogenation.
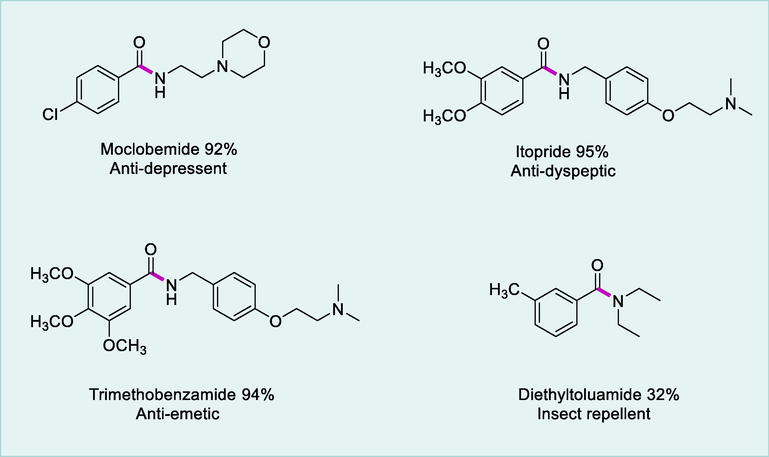
Pharmaceutics containing amide functionality.
This dehydrogenative coupling approach is very useful to synthesize the variety of commercially accessible pharmaceutics containing amide functionality from the respective alcohols and amines.
The metal-ligand coordination (MLC) in catalysis involves both the ligand and metal bond breaking and making with different substrates is very significant (Noyori and Ohkuma, 2001; Noyori et al., 2004; Ikariya and Blacker, 2007; Friedrich and Schneider, 2009; Crabtree, 2011a; van der Vlugt, 2012). Based on dearomatization/aromatization methodology, various new green synthetic transformations catalyzed by PNN or PNP Ru-pincer complexes have been investigated. The alcohols coupling to esters via dehydrogenation, coupling of primary amines with alcohols to synthesize amides with H2 as a sole byproduct, formation of alcohols via hydrogenation of esters, formation of imine by catalytic coupling of amines and nitriles, direct synthesis of imines by coupling of amines and alcohols with H2 as a byproduct, and a variety of other synthetic transformations are among them (Milstein, 2010; Gunanathan and Milstein, 2011b; Gunanathan and Milstein, 2011a; Gunanathan and Milstein, 2013).
The synthesis of corresponding esters (61) results from the primary alcohols (59 and 60) coupling through dehydrogenation with complex C25 (0.1mol %) by using tBuOK as a base and diethyl ether as a solvent under reflux at 35°C (Scheme 21). The reactivity order of the pre-catalysts was C25>C24>C23. PNNH-type Ru pincer complexes were synthesized, which were capable of metal-ligand cooperation (MLC) via ligand amide-amine as well as dearomatization/aromatization pathways. The alcohols coupling via dehydrogenation catalyzed by complex C25 by using base (2.2 eq.) (respective to Ru) under extremely mild conditions. This reaction tolerates a wide range of substituted primary alcohol substrates (Scheme 18) (Fogler et al., 2014).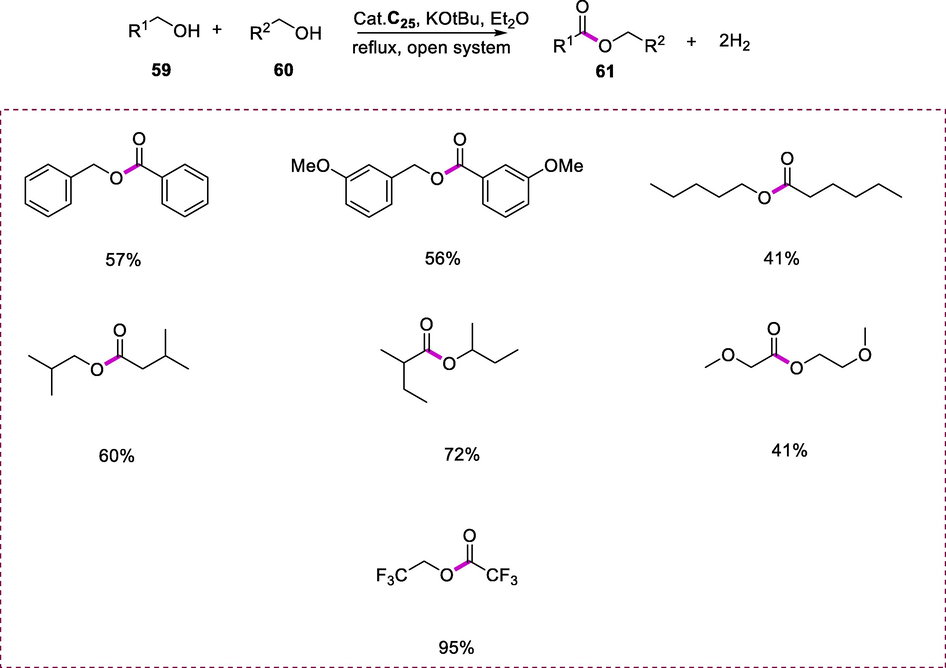
Synthesis of esters from primary alcohols.
2.7 Synthesis of acridine-based PNP ruthenium pincer complex and its applications
Amines are a very important class of compounds due to their significance in pharmaceuticals, dyestuffs, pigments, agrochemicals, fine chemicals, polymers, emulsifiers, and plasticizing agents (Lawrence, 2004). The primary amines are the most valuable amines but their selective formation is more challenging owing to their high reactivity. Traditional primary amine preparation methods typically use stoichiometric concentrations of hazardous chemicals, resulting in poor atom economy and selectivity (White and Elliger, 1965; Miriyala et al., 2004; Bartoli et al., 2008). The new pyridine-based pincer complex is capable of accelerating the highly desirable reaction of alcohols with ammonia to produce water and primary amines preferentially. This reaction can be carried out at low pressures and temperatures, without the use of any solvents, and in water.
The new tridentate electron-rich PNP pincer ligand (63) obtained from compound (62) reacts with Ru-complex [RuHCl-PPh3)3(CO)] in toluene at 65°C for 2h resulting in the formation of novel acridine-based ruthenium pincer complex (C26) considerably in good yield. Complex (C26) is air-stable for up to several months, making it useful in practical applications (Scheme 22).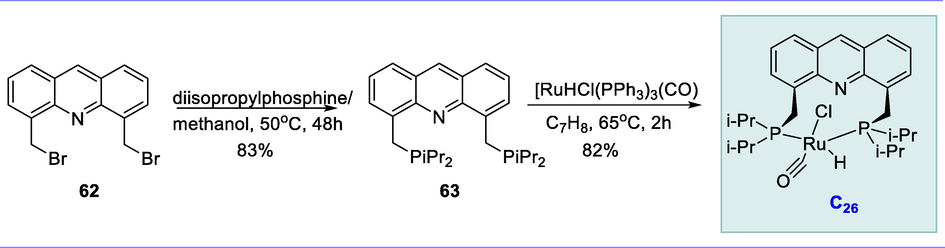
Synthesis of acridine-based PNP ruthenium pincer complex.
Primary alcohols (64) and ammonia (65) were coupled with complex C26 (0.1 mol %) as a catalyst under reflux in toluene resulting in the selective formation of primary amines (66). The scope of direct amination of ammonia with alcohol was examined. Benzyl alcohols undergo a facile reaction to give good yields of benzylamines. Benzyl alcohols having an electron-rich substituent on the phenyl ring reacted faster than those with electron-poor substituents on the phenyl ring. The heteroaryl methanols also showed excellent selectivity for the formation of primary amines. 2-Furylmethanol and pyridinyl-methanol were transformed to the respective primary amines in excellent yields (94.8% and 96%) respectively. 2-methoxyethanol had excellent selectivity for the primary amine, afforded the 94.5% yield of 2-methoxyethylamine. Although linear aliphatic alcohols, aryl, and heteroaryl alcohols were very selective for the formation of primary. The higher steric crowd at the beta-position of alkyl alcohols reduced the rate of secondary amines and imines synthesis, resulting in enhanced yields and selectivity of primary amines. It seems to be worth noting that the oxetane alcohol which is a four-member ring remains intact, resulting in primary amines as a product in good yield. This reaction also proceeded efficiently with simple alcohols and require no additional solvent in this strategy (Scheme 23) (Gunanathan and Milstein, 2008).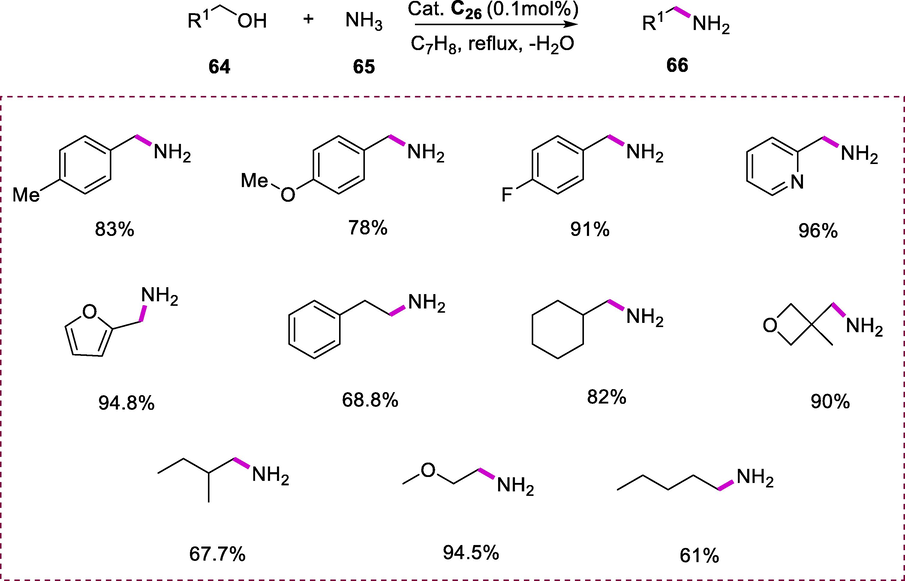
Primary amines synthesis from alcohols and ammonia.
The amine functionality is used as a chemical precursor in different transformations. Chemical reactions including primary amines are extremely important in chemistry and biology. The classical approach for selective deamination includes oxidizing the amines by using HNO2 to produce diazonium ions (unstable) that can then be treated with a variety of nucleophiles while the reaction is carried out by the removal of N2. Such reactions often have limited selectivity due to the formation of carbocation as intermediate and high reactivity of diazonium salts (Baumgarten, 1966; Doyle et al., 1978; Shin et al., 2000; Deechongkit et al., 2004; Cupido et al., 2005). Complex (C26) catalyzes the aliphatic amines deamination into alcohols in the reverse reaction. The synthesis of a hemiaminal intermediate is proposed as a key component that leads to the removal of ammonia as a byproduct in this reaction, which occurs via a reversible dehydrogenation/hydrogenation process. The acridine-based pincer complex (C26) has a distinctive reversible reactivity for deamination of amines and amination of alcohols due to its capacity to facilitate both dehydrogenation of alcohols and amines as well as hydrogenation of both aldehydes and imines within a same complex.
When the solution of amine (67) in water or a dioxane/water mixture is heated by using catalytic amounts of complex (C26) results in the synthesis of alcohol (68) as the main product. The reaction was carried out in a water/dioxane mixture due to the limited solubility of substrates in water and the catalyst which results in minimal conversion. The controlled experiment showed that no reaction proceeded without the use of a catalyst. Overall, deamination that occurs under a hydrogen environment increases the reaction rate by increasing the yield of the required alcohol product and selectivity, even though H2 is not required for this strategy. Under this reaction environment, substituted benzylamines having electron-withdrawing and electron-donating substituents were rigorously tolerated. Substrates such as ethylenediamine, diethylenetriamine, and 2-(aminomethyl)pyridine act as chelating ligands were stable towards deamination due to their strong coordination with the metal center, whereas furfuryl amine goes through non-selective reaction, yielding a mixture of products (Scheme 24) (Khusnutdinova et al., 2013).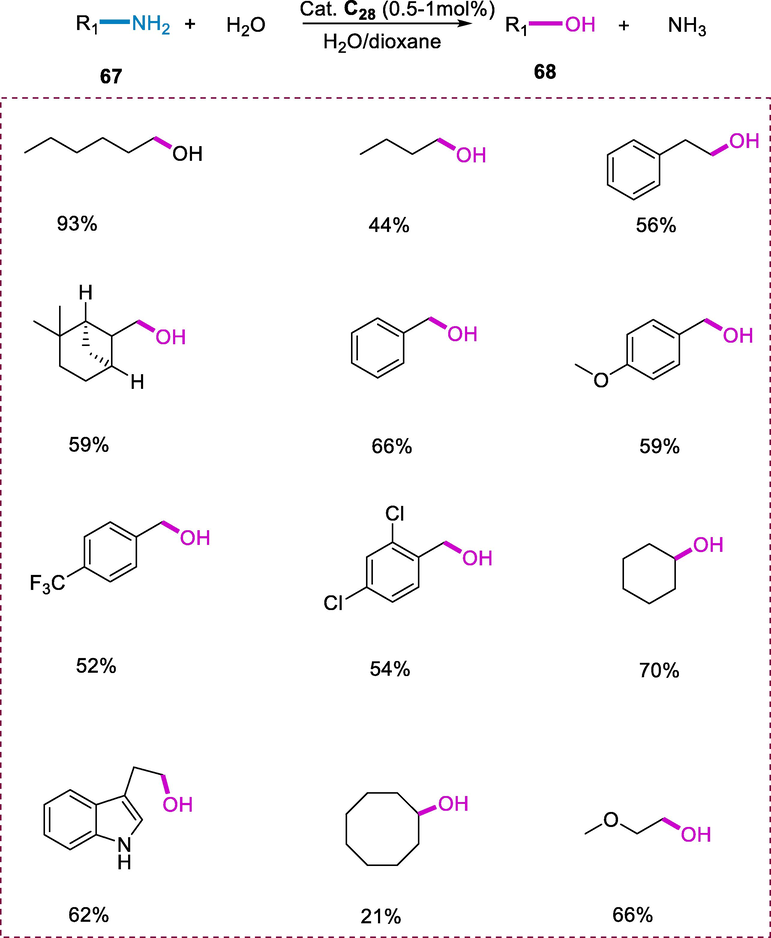
Direct deamination of primary amines.
Lactams are widely utilized as building blocks for the formation of physiologically active chemicals, medicines, and polymers, and their production is one of the most significant conversions in organic synthesis (Moody, 1995). Acridine-based ruthenium pincer complex [Ru(AcrPNP)H(CO)Cl] by using the catalytic quantity of base catalyzes the cyclic amines dehydrogenation in water to form lactams as a product (Gunanathan and Milstein, 2008; Gunanathan et al., 2009; Gunanathan et al., 2010). Water acts as an oxygen source in the amide functionality, and this transformation is accompanied by the release of H2.
Cyclic amines entropically stabilized the cyclic hemiaminal intermediate contrary to deamination. The solution of pyrrolidine (69) in a degassed dioxane-water mixture (1:1 v/v) upon heating for 48h under nitrogen in a closed system at 150°C by using complex C26 (1mol%) results in the formation of a 24% yield of 2-pyrrolidone (70) as well as H2 gas. The production of pyrrolidone in 59% yield was reported when the solution of pyrrolidine was heated in dioxane/water by using complex C26 (1 mol%) in the presence of NaOH (1.5mol%). The significant outcome of the base could be due to the generation of Ru(0) catalyst through deprotonation of complex C26 or the hemiaminal intermediate stability against the elimination of amine. The controlled experiment displayed no conversion would take place without the use of catalyst under similar conditions. Increasing the base concentration to 6mol% did not enhance the lactam yield and resulted in slightly low selectivity. With higher catalyst loadings (5mol% of complex and 5mol% of NaOH), the yield and selectivity could be enhanced, affording the 83% yield and 90% selectivity of 2-pyrrolidone. In addition to water, toluene and lutidine can be used as solvents in this reaction. The substituted pyrrolidines such as 2-methoxymethylpyrrolidine and 2-methylpyrrolidine react similarly and generate the respective lactams in 64.5% and 43.5% yields respectively with the catalyst loading of 1mol%. When 5mol% of the complex was utilized, the 2-methoxymethylpyrrolidine reacts to produce the lactam as a product up to 85% yield, whereas the reaction was less selective with 2-methylpyrrolidine at higher catalyst loadings. Using 5mol% of catalyst and NaOH base, the cyclic sec. amines having six-membered rings reacted, in the same way, to form the respective lactam products in 54-75% yields. Lactam yields for six-membered cyclic amines are often lower than for substrates having five-membered, possibly due to the steric factors or decreased hemiaminal intermediate stability (Scheme 25) (Khusnutdinova et al., 2014).
Lactam synthesis from cyclic amines.
Ammonia is the most basic and important nitrogen source in synthesis, having a relatively good atom economy (Roundhill, 1992). It is used to synthesize amines, amides, amino acids, isocyanates, urea, carbamates, heterocyclic, and N-heteroaromatic, compounds, among other commercially valuable products (Paquette et al., 2009). The coupling of different carbonyl derivatives with ammonia produces a wide range of biologically active natural compounds and pharmaceutically significant N-heteroaromatic molecules (Kharchenko et al., 2003). These techniques have several shortcomings, including a lack of beginning materials, multi-step synthetic operations, and a lot of waste production. For the synthesis of N-heterocycles, an alternative ecologically friendly reaction involving the coupling of amines and alcohols via dehydrogenation, with water and H2 as the only byproducts, is catalyzed by Ru-pincer complexes based on acridine and pyridine backbones (Gunanathan et al., 2007; Gnanaprakasam et al., 2011; Srimani et al., 2013b; Khusnutdinova et al., 2014). Acceptorless dehydrogenative coupling routes for the sustainable development of N-heteroaromatic molecules using amines and alcohols (Michlik and Kempe, 2013; Zhang et al., 2013; Deibl et al., 2015; Daw et al., 2016; Daw et al., 2018).
The pyrazine derivatives (72) were formed by the coupling of gaseous ammonia and 1,2-diols (71). The acridine-based ruthenium pincer complex is used as a catalyst and ammonia is used as a nitrogen source that requires no further additions like a base or an oxidant. The amination of alcohols with ammonia by using a catalyst (C26) results in the synthesis of pyrazine. Heating a solution of toluene with 1,2- hexanediol (1 mmol) in a Fischer-Porter tube with complex C26 (1mol percent) at 150°C (bath temperature) for 36h under 7bar pressures of ammonia results in the quantitative consumption of diols yielding the mixture of 2,5- and 2,6- dibutylpyrazine. In drug development, pyrazine derivatives are important scaffolds in potential bioactive compounds. Long-chain linear alcohols, such as 1,2-tetradecanediol, and 1,2-decanediol resulted in the formation of a 1:1 mixture of both isomers after conversion. The reaction with 1,2-ethanediol-1-phenyl gave quantitative yields of derivatives of diphenylpyrazine with a 68:32 ratio. Reaction with 1,2-butanediol yielded 72% of the desired derivatives of diethylpyrazine, whereas 1,2-propanediol yielded only 42% of the target product, as well as several side products. 2,3-butanediol gave 85% tetramethylpyrazine as the main product, whereas 1,2-cyclohexanediol gave 95% octahydrophenazine with a minor amount of hydrogenated byproducts by using similar conditions (Scheme 26).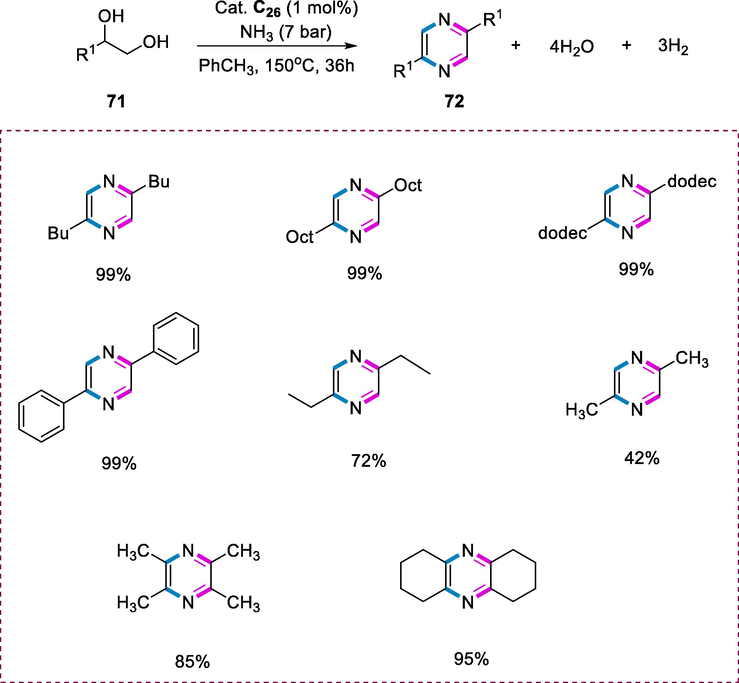
Synthesis of pyrazines from 1.2-diols and ammonia.
Pyrroles are a significant class of compounds because of their anticancer, anti-inflammatory, antibacterial, antioxidant, and antifungal potential in pharmaceutics (Amishiro et al., 2000; Suckling, 2008; Wang et al., 2008b; Wang et al., 2011). They can also be used in conducting polymers and molecular optics (Gimenez and Alves, 1999; de Lacy Costello et al., 2000; Chou and Yeh, 2001). Pyrroles are formed by the coupling of 1,4-diols with amines via dehydrogenation. The acridine-based pincer complex (C26) has a distinctive reversible reactivity for deamination of amines and amination of alcohols due to its capacity to facilitate both dehydrogenation of alcohols and amines as well as hydrogenation of both aldehydes and imines within a same complex.
The synthesis of pyrroles (75) was carried out by reacting derivatives of 1,4-butanediol (73) with primary alcohols (74) and ammonia in the presence of acridine-based pincer complex (C26). The multicomponent dehydrogenative coupling reaction occurred when a 2,5-hexanediol was added to primary alcohols, producing N-substituted pyrroles as a product. The Paal–Knorr reactions are used in traditional strategies for the formation of N-substituted pyrrole as a product. The coupling of secondary alcohols with derivatives of 2-amino alcohol via dehydrogenation also produced derivatives of pyrrole. Treatment of 1-hexanol and 2,5-hexanediol in 0.5 ml toluene with complex C26 (1mol%) under ammonia (7 bar) at 150°C for 24h yielded 2,5-dimethyl-1-hexylpyrrole as the coupling product of dehydrogenation. Various primary alcohols were tolerated for the dehydrogenative coupling with ammonia to generate pyrroles. 1-octanol, 1-butanol, and 1-pentanol generated 83%, 77%, and 76% of the respective N-substituted pyrroles as products respectively, under the optimal reaction conditions. The N,N-dimethylamino-1-propanol yielded the appropriate 1,2,5-substituted pyrrole derivative (74%) as the major product, whereas 3-phenyl-1-propanol and 2-phenyl-1-ethanol yielded the required products in 74% and 76% yields respectively. When using primary alcohols having low boiling point like ethanol, methanol, or 1-propanol, the alcohol (4equiv) were used in comparison to 2,5-hexanediol, producing moderate to good yields of the appropriate 1,2,5-substituted pyrroles respectively. Under the same conditions, replacing straight-chain primary alcohols with benzyl alcohols results in decreased reactivity and a modest yield of 1,2,5- substituted pyrroles were obtained. The corresponding pyrrole derivatives were formed in 48% and 57% by the reaction of 4-methyl benzyl alcohol and benzyl alcohol reactions, respectively, whereas the sterically hindered 3,4-dimethoxybenzyl alcohol yielded the product in just 13% yield. Heteroatom substituted primary alcohols were also well tolerated under the same conditions of the reaction. The corresponding 2,5-dimethyl-N-substituted pyrrole was obtained in 69% and 84% yields from nicotinyl alcohol and furfuryl alcohol respectively (Scheme 27) (Daw et al., 2018).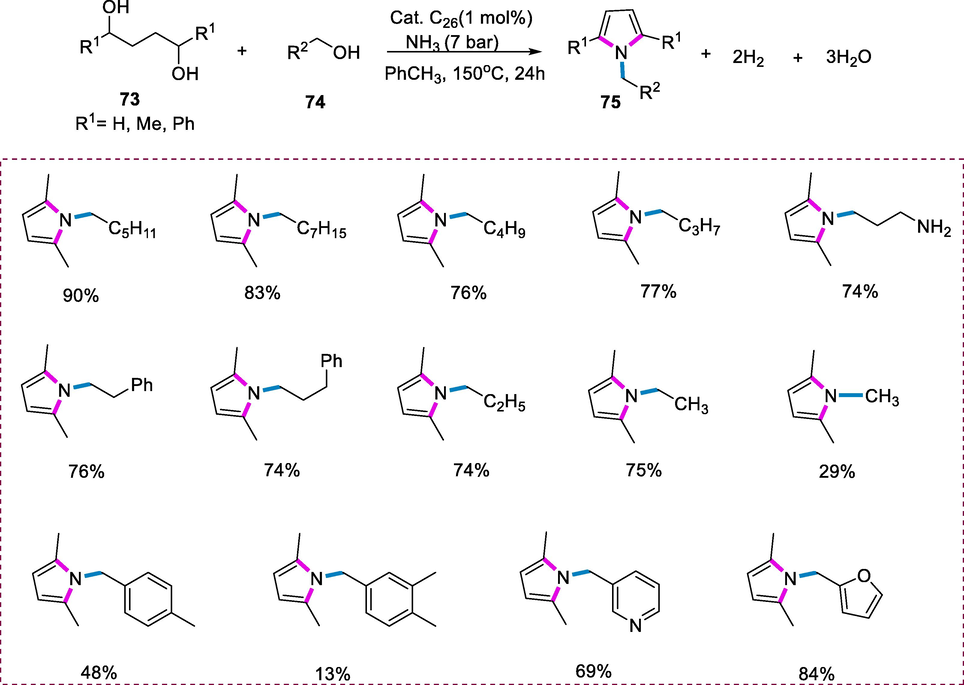
Synthesis of pyrroles from diols.
2.8 Synthesis of amide-derived Ru-NNN hydride complex and its application
Nitriles are the well-known category of organic compounds found in a broad range of biologically active agents, industrial processes (for the synthesis of agrochemicals, polymers, and pigments/dyes), and natural products synthesis as well as being utilized as synthons for further synthesis (Fleming, 1999; Pollak et al., 2000; Fleming et al., 2010; Gunanathan et al., 2011; Srimani et al., 2012a). Conventional nitrile preparation routes are inefficient in terms of atom economy, use of harmful reagents, and low selectivity. The Ru-NNN hydride complex derived from amines (RuH (PPh3)2(bmpi); bmpi =1,3-bis(6-methyl-2-aminopyridinyl)isoindoline) is used as a catalyst to effectively promote the secondary and primary amines dehydrogenation to nitriles and imines respectively. This reaction proceeds without the use of a hydrogen acceptor or exogenous oxidant.
The amide-derived Ru-NNN pincer complexes with the bmpi ligand (1,3-bis(6-methyl-2-aminopyridinyl)isoindoline) promote the fast dehydrogenative reactivity. The deprotonated Hbmpi ligand (76) was added to RuCl2(PPh3)3 in tetrahydrofuran (THF) as solvent resulting in the synthesis of ruthenium complex (C27) RuCl(bmpi)(PPh3) in excellent yield (92%). The complex C27 react with NaHEt3B (1.05 eq) and triphenylphosphine (PPh3) in tetrahydrofuran solution for 2h at ambient temperature, and the Ru-complex RuH(bmpi)(PPh3)2 (C28) was obtained in 89% yield (Scheme 28) (Tseng et al., 2013b).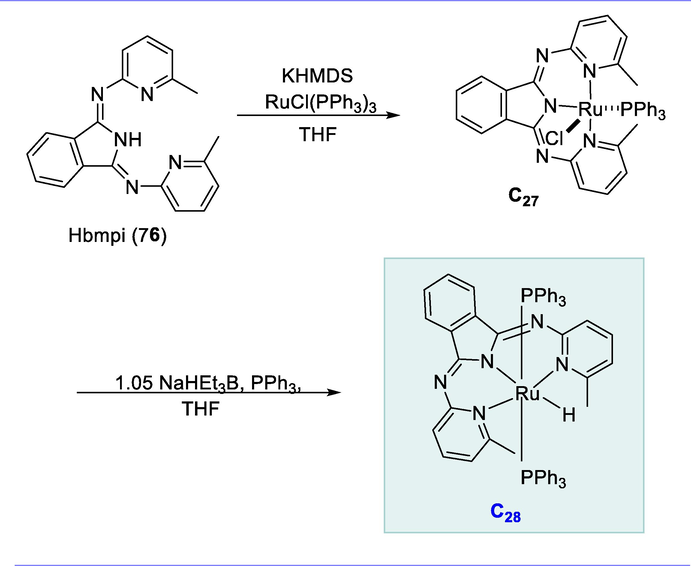
Synthesis of amide-derived Ru-NNN hydride complex.
Amines (77) were dehydrogenated with Ru-pincer complex (C28) (1mol %) in the presence of toluene at 110°C for 24h, resulting in moderate yields of corresponding nitriles (78). The dehydrogenation of aliphatic amines was often used to obtain the respective nitriles as the sole product. The 1-cyclohexylmethanamine as substrate was dehydrogenated to give cyclohexanecarbonitrile a product in good yield (74%). Moreover, the 2-phenethylamine undergoes dehydrogenate to give 2-phenylacetonitrile in good yield (76%) yield which is a key precursor to several pharmaceuticals. The transformation of the respective phenylacetonitriles was dependent on the electron-withdrawing and electron-donating substituent on the phenyl ring when phenethylamines with para- and ortho-substituent were utilized as substrates. The ortho-substituted chlorophenethylamine yielded 33%, but when the chloro group was replaced with an electron-rich substituent such as the methoxy group increased the yield up to 53%. The ortho- and para-substituted methoxy-phenethylamines produced similar yields, and the proximity of the –OCH3 group to the CH2NH2 group had minimal effect on the efficiency of conversion. Benzylic substrates were also used to explore the dehydrogenative process of activated amines which were effectively transformed to the corresponding benzonitriles. A series of functionalized benzylamines were used to investigate substituent effects. Electron-withdrawing groups reduced the yields while in the case of electron-withdrawing substituent on the aromatic ring enhanced the yield of the reaction. Deactivating the chloro group in the ortho-, meta-, or para-positions, for example, resulted in decreased conversions (Scheme 29) (Tseng et al., 2013a).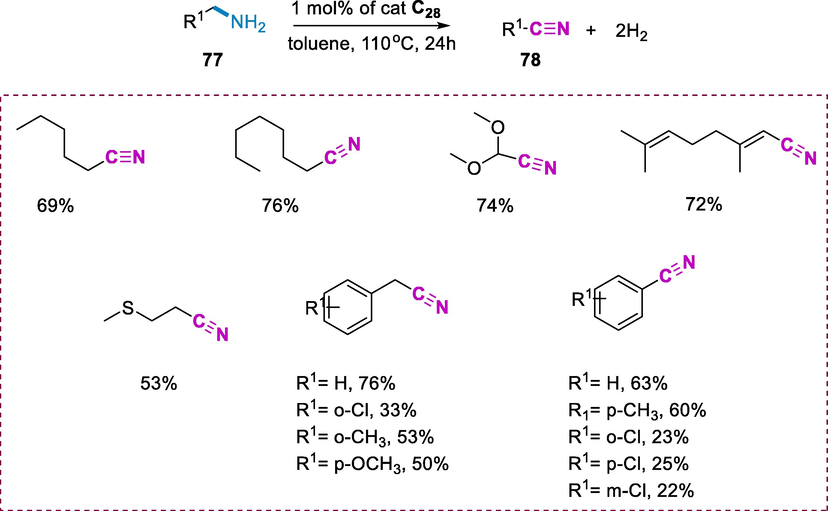
Dehydrogenation of amines to nitriles.
2.9 Synthesis of PNN- and PNP-lutidine-based Ru-complexes and their application
Chemical bond catalysis and activation rely heavily on metal-ligand cooperative bifunctional complexes (Khusnutdinova and Milstein, 2015; Younus et al., 2015; Chen et al., 2016; Gorgas et al., 2017). PNN- and PNP-lutidine-based complexes have methylene protons that are slightly acidic and show fascinating ligand metal coordination based on a dearomatization/aromatization process that has been used in a variety of chemical reactions (Gunanathan and Milstein, 2011a; Jia and Huang, 2016; Xie et al., 2016; Espinosa-Jalapa et al., 2017; Hou et al., 2017). These bifunctional catalysts are effective for β-alkylation of a primary alcohol with secondary alcohols.
The ligand (79) undergoes a reaction with ruthenium complex RuHCl(CO)(PPh3)3 in methanol under reflux resulting in the formation of Ru-complex (C29). Then ligands (80) and (81) were reacted with RuHCl(CO)(PPh3)3 resulting in the synthesis of Ru-complexes (C30) and (C31) in good yields respectively. These ruthenium complexes demonstrated strong catalytic activity for beta-alkylation of primary alcohols with secondary alcohols, which is considered an ecologically sustainable approach to β-alkylation (Scheme 30).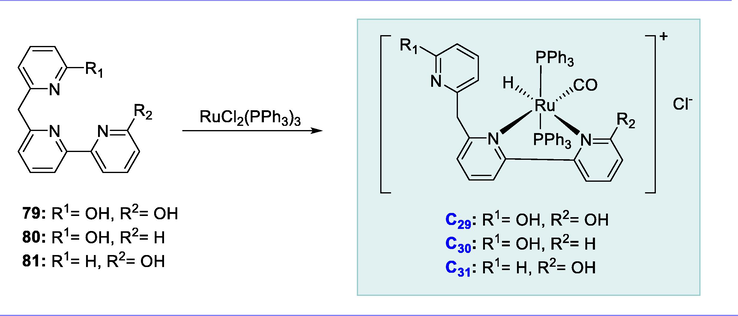
Synthesis of PNN- and PNP-lutidine-based Ru-complexes.
The coupling of benzyl alcohol (82) and 1-phenylethanol (83) by using Ru-complexes (C29-C31) was used as a model transformation. This reaction was proceeded by using Ru-complexes (0.5 mol%) as catalysts and tBuOK (0.5 eq) used as a base in PhCH3 solvent for 60 minutes under a nitrogen environment at 110°C. Complex (C31) exhibits excellent catalytic efficiency resulting in 94% of conversion in 1h. The conversion rate was greatly enhanced by using strong bases like KOH, t-BuOK, and NaOH in comparison to weak bases. Surprisingly, the conversion rate did not decrease when the reaction proceeded under the air atmosphere. This was optimistic because such reactions usually necessitate the presence of N2. Derivatives of phenylethanol were β-alkylated with a wide range of primary alcohols. The majority of substrates show high selectivity and conversion. Because of a steric hindrance effect, benzyl alcohols with chloro-substituent in para- and meta-position with 1-phenylethanol converted into the corresponding secondary alcohols with better selectivity and conversion than those substituted at ortho-position. Furthermore, when an electron-donating or electron-withdrawing substituents were added to the benzyl alcohol at para-position, no noticeable substituents effect was observed. The electron-poor substituent on the 1-phenylethanol substrate at the para-position had no discernible effect on the reaction, whereas an electron-rich substituent drastically reduces the selectivity and reactivity (Scheme 31) (Shi et al., 2018).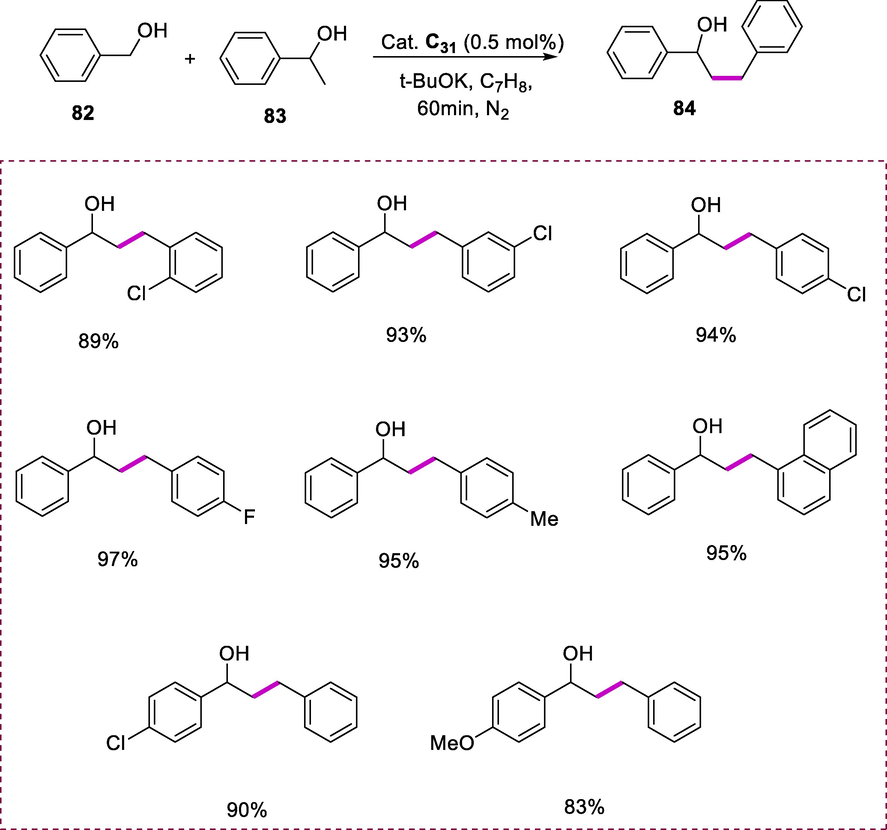
β-alkylation of alcohols by using alcohols.
2.10 Synthesis of bipyridine-based electron-rich Ru(II)-PNN pincer complex and its applications
Hydrogenation of polar organic carbonyl groups has attracted a lot of attention because of its environmentally friendly nature and synthetically useful way to make elementary synthetic intermediates like amines and alcohols (Clapham et al., 2004; Ito and Ikariya, 2007; Wylie et al., 2010). Carbamate hydrogenation is of great importance both theoretically and practically, because carbamates may be easily obtained from CO or CO2, and their mild hydrogenation would result in the conversion into methanol. Pyridine and acridine-based Ru-pincer complexes accelerate the hydrogenation of organic carbamates into alcohols and amines. This reaction is very selective and proceeds effectively under neutral or mild conditions in the absence of solvent has a high turnover rate, and produces no waste. Furthermore, this novel reaction offers intriguing alternatives for mild CO and CO2 hydrogenation into methanol.
The tridentate ligand BPy-tBuPNN (85) was reacted with [RuHCl(PPh3)3(CO)] (86) in tetrahydrofuran at 65°C to produce the bipyridine-based electron-rich Ru(II)-PNN pincer complex (C32). Complex (C32) was deprotonated with KOtBu at -32°C, yielding the dearomatized, coordinatively unsaturated complex (C33) (Scheme 32) (Balaraman and Milstein, 2014).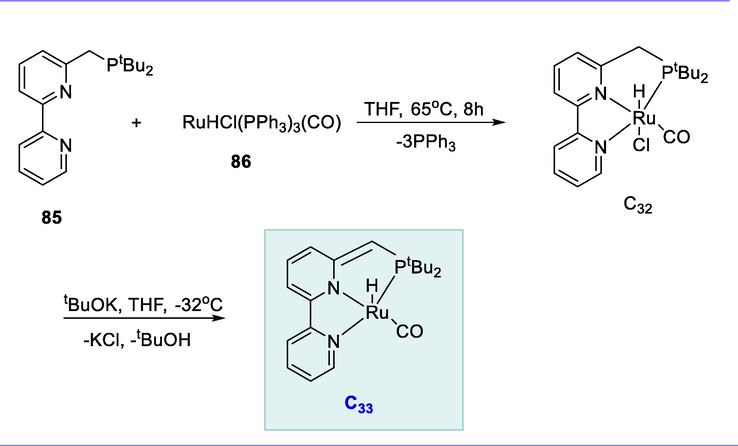
Synthesis of bipyridine-based electron-rich Ru(II)-PNN pincer complex.
The methyl carbamates (87) hydrogenation to corresponding amines (88) and methanol (89) was effectively catalyzed by Complex (C33). The solution of dihydrogen and methyl N-benzyl carbamate was heated in THF for 48h at 110°C with a catalytic quantity of complex C33 (0.01 mmol) afforded the methanol and benzylamine in quantitative yield. The benzyl carbamates were hydrogenated selectively without breakage of the benzyl O-bond to give methanol, respective benzyl alcohols, and amines. The benzyl morpholine-4-carboxylate reacts with hydrogen (10atm) at 110°C in dry tetrahydrofuran for 52h with the catalytic quantity of C33 (1mol percent) resulting in the formation of benzyl alcohol (88%), morpholine (87%), and methanol (81%). It is worth noting that in the hydrogenolysis of benzyl carbamates, the carbamate-N functional groups are deprotected (Scheme 33) (Balaraman et al., 2011).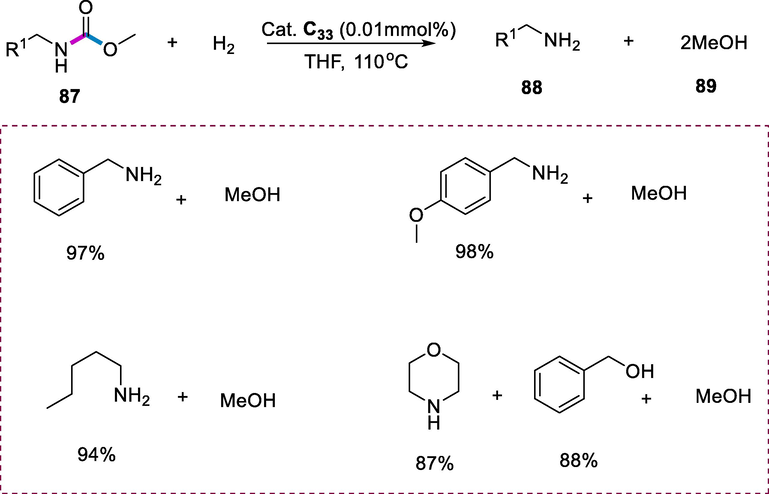
Hydrogenation of carbamates.
The catalytic hydrogenation of derivatives of carbonyl (C=O) compound using molecular H2 is a significant transformation in synthetic chemistry. The hydrophilicity of the carbonyl carbon is inversely proportional to its liability to nucleophilic attack through hydride (Carey and Sundberg, 2000). Hydrogenation of derivatives of urea is the most difficult among other carbonyl compounds because of less electrophilicity of the carbonyl group due to the resonance effect. Alkylated urea derivatives have also been employed as solvents in the hydrogenation transformations of other chemicals as well as as “green” solvents in metal-assisted organic reactions (Imperato et al., 2006).
The urea derivatives (90) hydrogenation was carried out under milder reaction conditions specifically 110°C and H2 pressure (13.6atm) in the presence of complex (C33) resulting in the cleavage of strong C–N bonds occurring selectively yielding the corresponding amines (91) and methanol (92) in high yields. The alkyl- and aryl-urea derivatives may be easily made from amines and CO2, and their hydrogenation provides a mild, eco-friendly, and efficient method of converting CO2 to methanol (Scheme 34) (Balaraman and Milstein, 2014).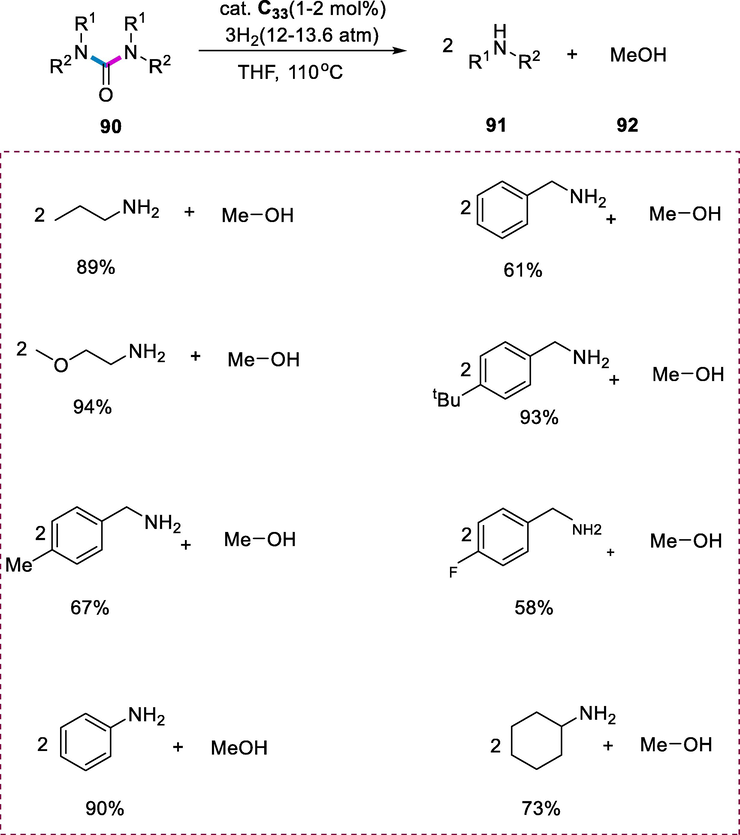
Hydrogenation of urea derivatives.
The selective catalytic alcohol oxidation is a very significant transformation in the formation of industrial building blocks and chemicals (Yamaguchi and Mizuno, 2010; Sheldon, 2012; Simon and Li, 2012). The bipyridine-based ruthenium complex (C33) catalyzed the coupling of different alcohols at basic pH with water, resulting in high yield and large turnovers of the required salts of carboxylic acid and hydrogen gas development. Water might serve as both oxygen donor and reaction medium in a catalytic coupling between alcohols and water without the use of any other oxidant under neutral conditions.
The catalytic transformation was proceeding rapidly by refluxing the mixture of alcohol (93), water, and complex C33 (0.2 mol%) under an argon atmosphere. The appropriate salts of carboxylic acid were produced in high yields after 18h of reaction time and converted to corresponding carboxylic acids (94) via the treatment of acid. Both aromatic and aliphatic alcohols demonstrate significant turnover and selectivity in this catalytic system (Scheme 35) (Balaraman et al., 2013).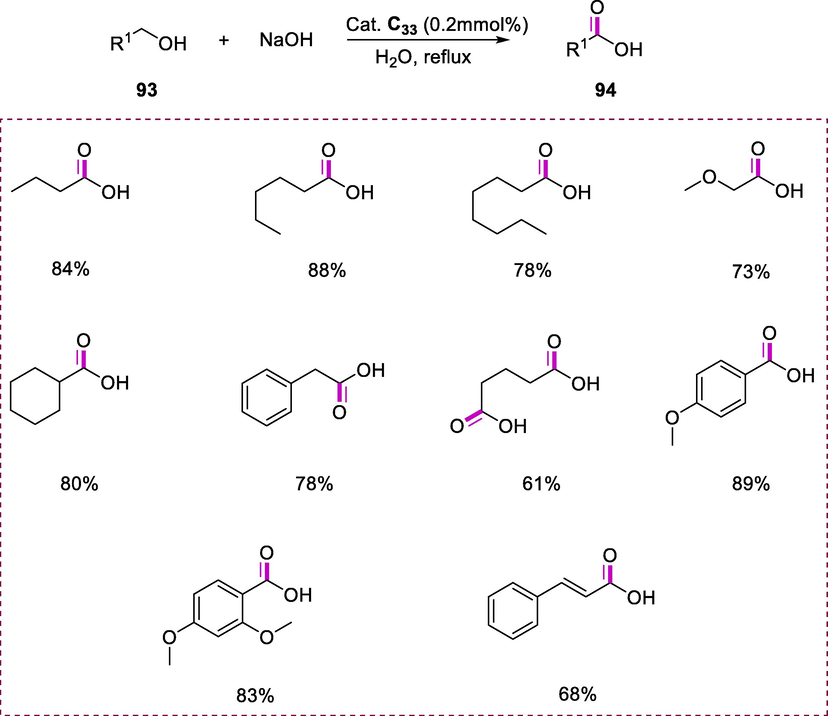
Synthesis of carboxylic acid derivatives.
N-Heterocycles are a significant class of compounds because of their abundance in pharmaceutics and natural products (Joule et al., 2020). Substituted pyridines are used as important building blocks in bioactive compounds among the N-heterocycles, and they are widely applied in medicines, functional materials, and agrochemicals (Henry, 2004; Michael, 2005). The Ru–bipyridine-based pincer complexes catalyze a variety of environmentally friendly reactions. Direct pyrrole synthesis proceeds through the dehydrogenative coupling of secondary alcohols with β-aminoalcohols, as well as efficient, one-step pyridine synthesis via dehydrogenative coupling of secondary alcohols with γ-amino-alcohols was efficiently accelerated by these complexes.
The reaction of 3-aminoalcohol (95) with secondary alcohol (96) was initially selected as model substrates for the synthesis of pyridines (97) via dehydrogenative cross-coupling. Refluxing an equimolar quantity of 1-phenylethanol (2 mmol), 3-aminopropanol, with KOtBu as a base in toluene for 24h in the presence of 0.5mol% complex (C32) yielded 45% of 2-phenylpyridine. The yield of the product was not improved by increasing the duration of reaction up to 40h or increasing the loading of catalyst up to 1mol%. The solvent influence on the reaction rate was also investigated. The 2-phenylpyridine was obtained in higher yields (68%) when the mixture of tetrahydrofuran and toluene was used as a solvent than in THF (25%) or toluene (62%) alone. The reaction of various 3-aminoalcohols with secondary alcohols (cyclic and acyclic) substrates was favored by these reaction conditions and resulted in the synthesis of substituted pyridines in moderate to good yields (Scheme 36).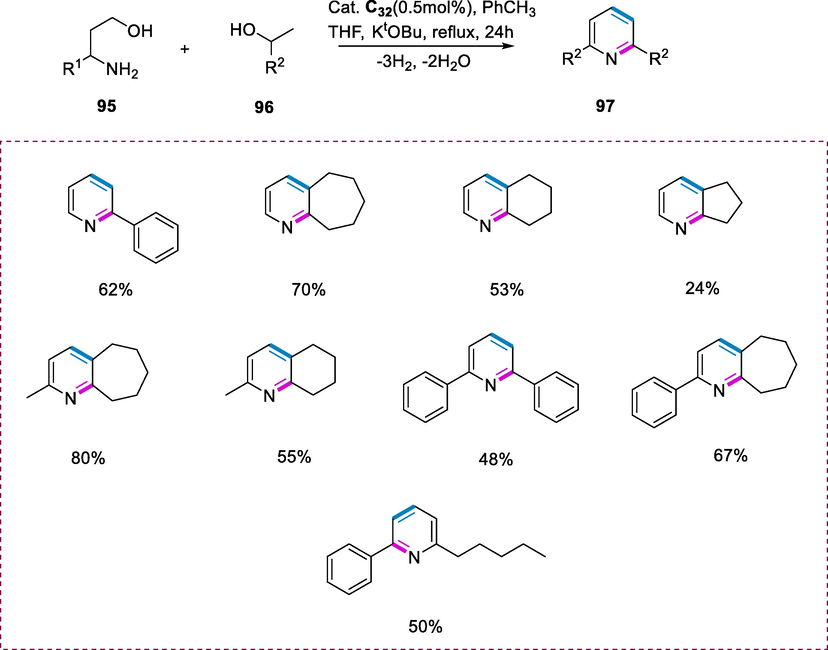
Synthesis of substituted pyridines.
Many bioactive natural compounds including the quinoline molecule and diverse quinoline derivatives have a wide range of pharmaceutical activities which allow them to employ anti-HIV, anticancer, antihypertensive, anti-Alzheimer, and anti-tuberculosis medicines. Exploring the potential of quinoline synthesis, we investigated the reaction of various secondary alcohols, including cyclohexanol, 1-phenylethanol, cyclododecyl alcohol, and cycloheptanol with 2-aminobenzyl alcohol to obtain tetrahydroacridine, 2-phenylquinoline, decacyclododeca[b]quinoline, and tetrahydro-6H-cyclohepta[b]quinoline in modest to good yields (Scheme 37) (Srimani et al., 2013a).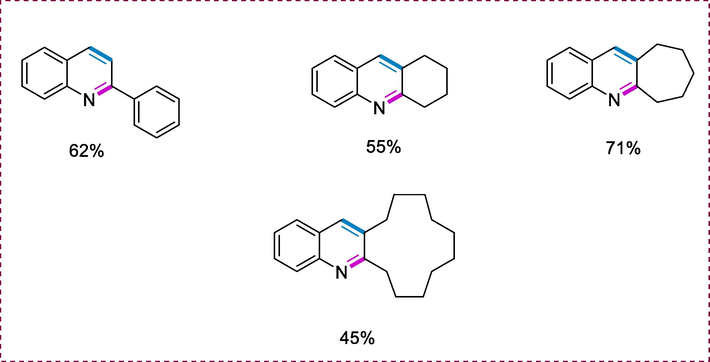
Synthesis of substituted quinolines.
2.11 Synthesis of lutidine-based Ru-CNC complexes and their application
Pincer complexes derived from lutidine have become a popular class of organometallic derivatives (van der Vlugt and Reek, 2009; Gunanathan and Milstein, 2011a). Hydrogenation of C=N bonds of different imines catalyzed by Ru complexes containing dearomatized lutidine-derived pincer ligands (Andersson and Munslow, 2008).
The corresponding 1-substituted 1H-imidazole and 2,6-bis(halomethyl)pyridine were refluxed (1: 2 ratio) in acetonitrile or THF solutions to synthesize the new bis-imidazolium salts (98a-98e). The bis-imidazolium salts were then treated with 1 eq. of Ag2O at room temperature in CH2Cl2 resulting in the synthesis of bimetallic silver reagents (99a-99e). Complexes C34(Cl) and C36(Cl) were easily prepared in THF at 55°C using RuHCl(CO)(PPh3)3 and a suitable silver reagent. Complexes C35(BF4) and C37(Br) were similarly prepared by reacting the corresponding silver reagents with RuHCl(CO)(PPh3)3 respectively. Finally, the formation of 3,5-xilyl-substituted C38(Cl) was carried out more efficiently with RuHCl(CO)(PPh3)3 at room temperature. These complexes are specifically deprotonated by potassium tertiary butoxide at one of the methylene arms in the pincer complex, resulting in catalytically active species in the imines hydrogenation. (Scheme 38).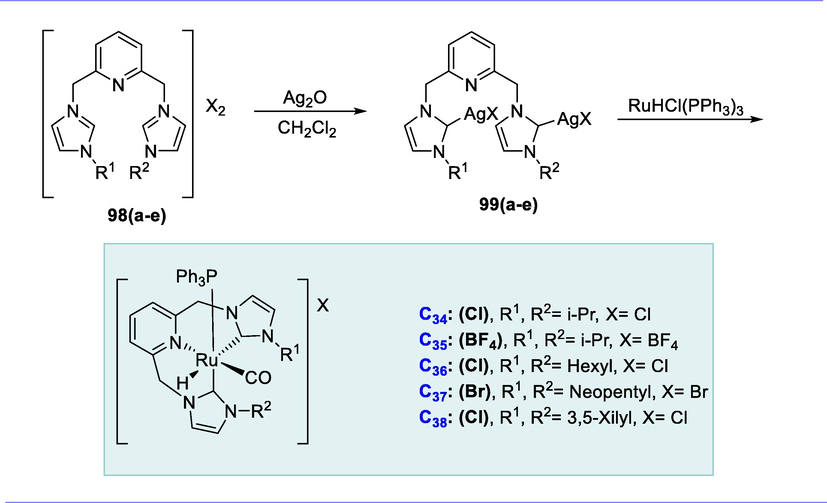
Synthesis of lutidine-based Ru-CNC complexes.
The Ru-complexes (C34-C38) exhibit outstanding catalytic activity for the imine (100) hydrogenation. Complexes (C34-C38) catalyze the N-benzylideneaniline hydrogenation in 2-methyltetrahydrofuran under H2 (5 bar) at 70°C by using tBuOK as a base. Complex C36(Cl) is the more active catalyst in the series. This reaction tolerated a variety of N-alkyl and N-aryl aldimines. Substrates having electron-donating substituents enhanced the reactivity, but the substrates having highly electron-poor substituents in both of the aryl groups greatly minimize the reactivity. The N-phenyl imine was also hydrogenated very efficiently than the analogous N-benzyl aldimine. Complex C36(Cl) was also found to be effective in the catalytic hydrogenation of a variety of N-arylated ketimines with excellent turnover (Scheme 39) (Hernández-Juárez et al., 2013).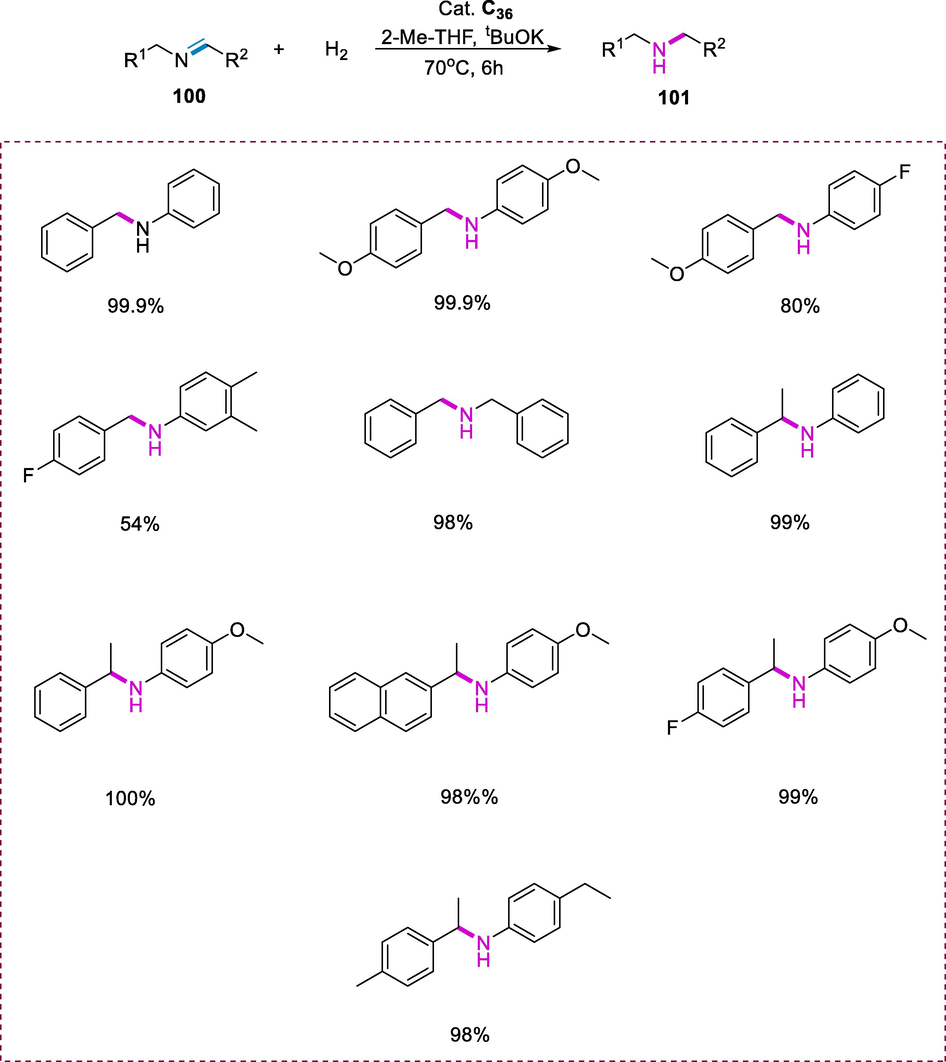
Hydrogenation of imines.
2.12 Synthesis of PNP-Ru(II) and Ru-MACHO-BH Ru(II) complexes and their applications
One of the most significant challenges in modern chemistry is the development of sustainable strategies for converting biomass into valuable compounds (Caetano and Fernandes, 2018). The addition of nitrogen to biomass-derived chemicals increases their value and broadens their industrial application (Dunbabin et al., 2017). Furfurylamines (amines generated from furfurals) are used to make a variety of medicines in the pharmaceutical industry, including Furtrethonium, Furesomide, which are used against the hepatitis-B drug, and Barmastine used as antiseptics as well as used in polymers, agrochemicals, synthetic resins, and insecticides (He et al., 2020). Various reducing agents and catalysts have been used to study the formation of furfurylamines through reductive amination by using furfurals. This conversion as the synthetic tool is non-toxic, environmentally benign, does not use combustible gases, and uses an easy-to-handle, stable, and low-cost hydrogen supply (Yao, 2001; Wang et al., 2008a; Wang and Astruc, 2015; Werkmeister et al., 2015; Farrar-Tobar et al., 2020; Piccirilli et al., 2020). The Ru-MACHO (carbonylhydrido-(tetrahydroborato)[bis(2-diphenylphosphinoethyl)amino] Ru(II) and RuMACHO-BH complexes are appropriate catalysts for hydrogenation via reductive amination due to their excellent selectivity and activity.
Ru-MACHO-BH ruthenium(II) complex (C40) was produced by reducing ruthenium complex (C39) with NaBH4. The PNP-Ru(II) complex (C39) was prepared from commercially available bis-(2-diphenylphosphinoethyl)ammonium chloride (102) and carbonylchlorohydridotris-(triphenylphosphine)-Ru(II). These complexes are miscible in protic solvents like tetrahydrofuran, ethanol, and methanol as well as in toluene (Scheme 40) (Yao, 2001).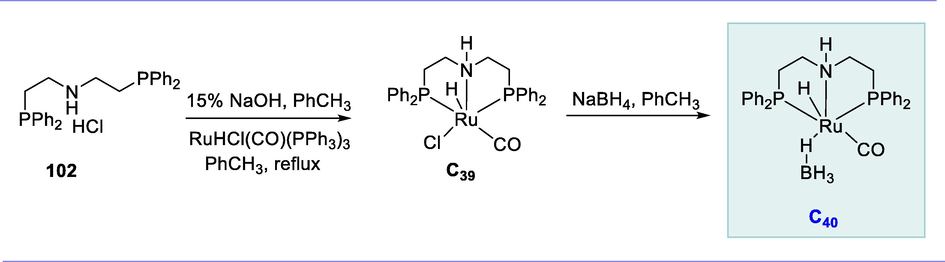
Synthesis of PNP-Ru(II) and Ru-MACHO-BH Ru(II) complexes.
Ru-MACHO-BH (C44) catalyzed the one-pot formation of furfurylamines (105) by using anilines (103) and furfurals (104) in the presence of MgSO4 and iPrOH at 90°C. Furfurylamines were obtained in modest to excellent yields in most cases. The furfurylamine was obtained in excellent yield (93%) from the parent aniline compound. When anilines with electron-withdrawing or electron-donating substituents were compared, the substrates carrying electron-withdrawing groups afforded excellent yields than others. The substituted anilines such as 4-CF3-aniline, 4-F-aniline, and 4-aminopyridine have produced the highest yields of different substituted furfurylamines (74 to 95%). The anti-hepatitis-B molecule is analogous to a compound (106) which demonstrates the direct utilization of this strategy for the development of pharmacologically active molecules. The furfurylamine was obtained at a low yield (61%) when aniline having an electron-donating group such as 4-CH3-aniline was used in the reaction. Various halogens containing aniline substrates were also well-tolerated given the yields ranged from modest to excellent. Compounds like 3-Cl-aniline and 2-F-aniline exhibit good tolerance, giving good yields (60% and 56%) respectively. This strategy was also examined with N-methylaniline (secondary amine) which resulted in a high yield of the tertiary amine (89%) (Scheme 41), (Scheme 41a).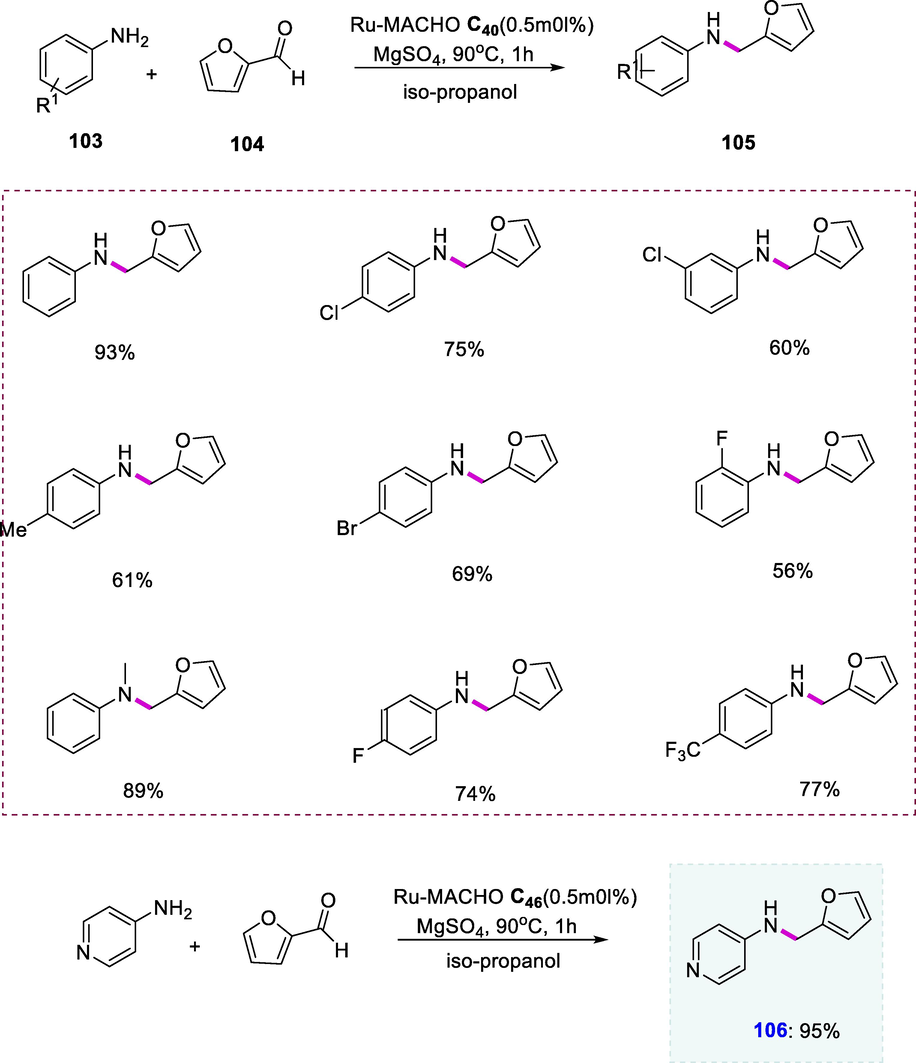
Synthesis of furfurylamines.
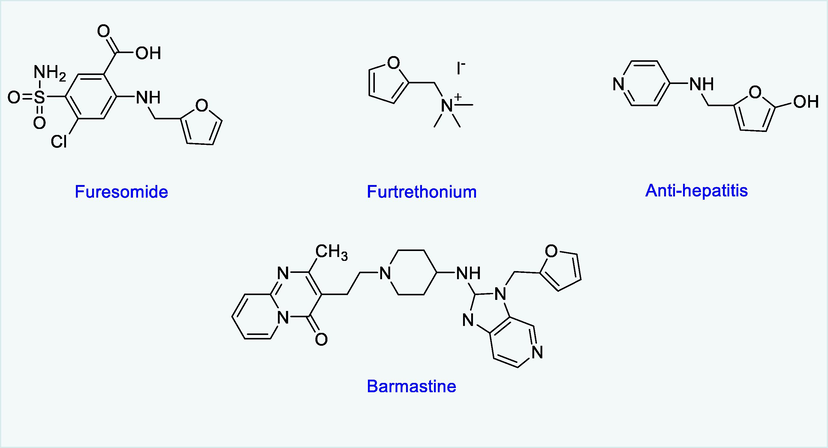
Pharmaceutically active compounds derived from furfurylamines.
Several furfurylamines synthesized from this method are used to make biopolymers (polyamides) and pharmaceuticals like Furtrethonium (the drug that is used against hepatitis-B), Barmastine, and Furesomide. The N-(5-methylfurfuryl)aniline is a key ingredient in the production of epoxyisonindoles and bioactive molecules such as anticancer, antibacterial, antituberculosis, and anti-inflammatory agents (Pinheiro and Nielsen, 2021).
CO2 is an appealing building block in the formation of organic compounds, carbohydrates, and materials because it is a cost-effective, safe, and renewable carbon resource (Aresta, 2010). Milstein and coworkers introduced an indirect route for the formation of methanol from CO2 by homogeneous carbonates hydrogenation under mild conditions employing pincer-type ruthenium (II) catalysts having a common structure [(PNP)Ru-(CO)(H)]. Furthermore, this approach is cost-effective. The utilization of an easily available (PNP)Ru(II) complex for the homogenous cyclic carbonates hydrogenation from readily available epoxides and CO2 to produce corresponding diols and methanol with high efficiency of the catalyst.
The pincer-type ruthenium (II) complex is used as a catalyst to accelerate the hydrogenation of cyclic carbonates (107) effectively. The reaction was carried out in THF at 140°C in the presence of the catalytic Ru(II) complex (C39) and the base tBuOK under a pressure of 50 atm of H2. Ru(II) complex was shown to be active for hydrogenation at a catalyst loading of 0.1 mol %, yielding methanol and ethylene glycol 108 (EG) at varied yields. When the loading of catalyst was reduced to 0.01 mol%, the process could be accomplished in 48h, producing >99% methanol and EG. The reaction was carried out smoothly under an H2 environment (60atm) with an 89% transformation of ethylene carbonate within 72h, affording the ethylene glycol (87%) and methanol (84%) yields respectively by reducing a load of catalyst up to 0.001 mol%. This result indicates the good stability and excellent efficiency of the catalyst. Various cyclic carbonates such as 5-membered as well as six-membered 1,3-dioxolan-2-ones having one, two, or even four substituents can be hydrogenated selectively and efficiently into their corresponding diols and methanol under similar conditions. The sterically hindered substituents on the substrates of cyclic carbonates have a major impact on the activity of reactions, as it requires a greater loading of catalyst and more reaction time to complete the transformation of more sterically demanding substrates (Scheme 42) (Han et al., 2012).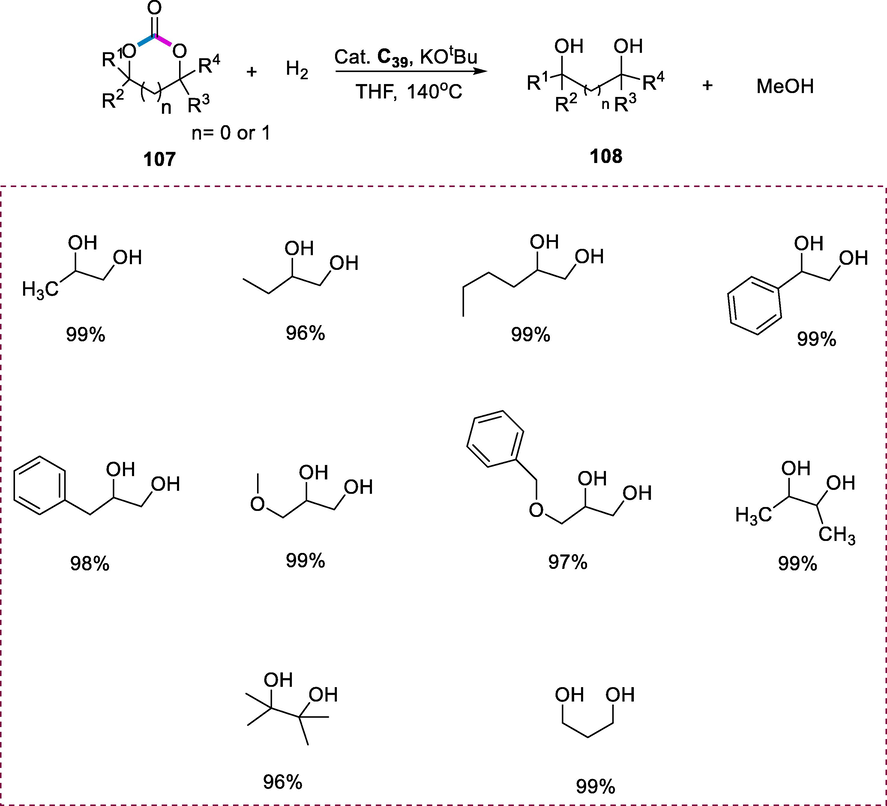
Hydrogenation of cyclic carbonates.
The chemical synthesis relies heavily on the synthesis of the C=C bond (Maryanoff and Reitz, 1989). Only ruthenium-catalyzed alkene metathesis is available as a catalytic technique for intermolecular olefin synthesis (Hoveyda and Zhugralin, 2007; Vougioukalakis and Grubbs, 2010). Vinyl nitriles are common in pharmaceuticals, and natural products and are essential intermediates in organic transformations. They are important intermediate and building blocks in a variety of chemical reactions. In traditional vinyl-nitrile synthesis, a stoichiometric quantity of base is used to mediate the reaction between nitriles and carbonyl compounds (Knoevenagel condensation) (Guillot et al., 2005). Base-promoted condensation transformations with cross-reactions like the aldol condensation, self-condensation of nitriles, and the Cannizzaro reactions are limited to base-sensitive substrates (Arseniyadis et al., 1984). The Ru-catalyzed α-alkylation of aryl methyl nitriles with alcohols as an alkylating agent was recently reported (Thiyagarajan and Gunanathan, 2017). This environmentally friendly and effective alkylation reaction was facilitated by ligand-metal coordination in ruthenium-pincer complex, and the reactions were carried out by borrowing hydrogen pathway. Remarkably, water and liberated H2 are the byproducts obtained from this green synthetic method.
The dehydrogenative coupling of nitriles (109) and alcohols (110) takes place through amide-amine ligand-metal coordination in the activated complex (C39). The phenyl acetonitrile and cyclohexanol were reacted in the presence of Ru-pincer complex (mol%, Ru-MACHO) in toluene resulting in the synthesis of the desired olefin in moderate yield. Although full nitrile conversion was observed in 16h. However, no alkylated product was obtained by using the primary alcohol as a substrate. Additionally, reducing the catalyst loading (1 to 0.5mol%) and decreasing the temperature from 135 to 120°C increase the yield of the reaction. The olefination reaction requires a catalyst and base to proceed with the transformation. The range of substrates including various nitriles was tolerated concerning cyclohexanol in catalytic olefination reaction. The reaction of cyclohexanol with 4-methyl phenyl-acetonitrile afforded the respective olefin product in good yield (78%). When 2-methoxy phenyl-acetonitrile was used in the reaction under controlled conditions result in a lower yield (72%), showing that the olefination reaction is sensitive to steric hindrance in the substrates in proximity. However, excellent conversions were achieved with meta-and para-methoxyphenylacetonitrile and the resultant products were extracted in excellent yields. This catalytic transformation was particularly effective on heteroarylmethyl nitriles, aryl methyl nitriles, and aliphatic nitriles. In this reaction, unsymmetrical and symmetrical secondary alcohols, as well as acyclic and cyclic secondary alcohols, were also used resulting in a variety of α-vinyl nitrile derivatives. The electron-poor substituent on the substrates was also permitted (Scheme 43) (Thiyagarajan and Gunanathan, 2018).
Synthesis of Nitriles α‑olefination via secondary alcohols.
Deuteration of alcohols is selectively important due to a variety of applications, including the production of deuterated medicines and other biologically active molecules (Pleiss and Voges, 2001). Although the interaction of simple hydrocarbons with D2O is a well-known catalytic deuteration reaction. The direct and selective catalytic deuteration of alcohols is less common.
The efficient selective alpha-deuteration was observed when benzyl alcohol was subjected to selective deuteration in the presence of ruthenium complex Ru-C39 (0.2mol%) catalyst and KOtBu in D2O (0.4 mL) at 60°C. The deuteration occurred at the alpha-position within only 3h to provide benzyl alcohol-d3 as a product in 96% yield, while there was no detectable H/D interchange with aromatic protons. The catalytic deuteration of a variety of heteroaryl methanols and aryl methanol were observed using similar catalytic conditions. This methodology was also used to selectively deuterate linear aliphatic alcohols. The variety of aliphatic alcohols involving heteroaryl-appended straight chain aliphatic alcohols demonstrated strong deuterium integration selectively at the alpha position when they are exposed to the catalytic deuterated condition. However, several of these linear alcohols have a minor amount of beta-deuteration (9-20%) (Scheme 44) (Chatterjee and Chidambaram, 2018).
Selective alpha-deuteration of alcohols.
2.13 Synthesis of lutidine-based Ru-CNN(H) complexes and their applications
Metal complexes derived from lutidine have gotten a lot of attention because they can be easily deprotonated at the methylene arms in the pincer complex. Pincer complexes containing lutidine and N-H have proven to be highly selective and active catalysts for a wide range of dehydrogenation and hydrogenation transformations. The reduction of cyclic N-heteroaromatic to their respective saturated derivatives and the saturated N-heterocycles to their parent N-heteroaromatic compounds via oxidation, respectively, were green synthetic approaches involving hydrogenation and dehydrogenation (Milstein, 2015).
The imidazolium salts (114a-114c) were mixed with Ag2O in CH2Cl2 and the silver complexes (115a–115c) were formed. The Ru-CNN(H) complexes (C41-C43) were isolated in good to moderate yields (33–85%) by subsequent reactions of compound (115a–115c) with Ru-complex [RuHCl(CO)(PPh3)3] in tetrahydrofuran, followed by reaction with NaBF4 and PPh3 in CH3CN. Ru complexes based on lutidine-based ruthenium pincer complexes (C41-C43) having CNN(H) ligands with side donor of secondary amine are used as precatalysts for the dehydrogenation and hydrogenation of N-heterocycle. The Ru-CNN(H) complex reacts with an excess of base leads to ruthenium (0) complex observed under catalytic conditions. The Ru-CNN(H) complex (C41) was found to be more effective among other complexes to catalyze both the dehydrogenation and hydrogenation of a series of N-heterocyclic compounds (Scheme 45).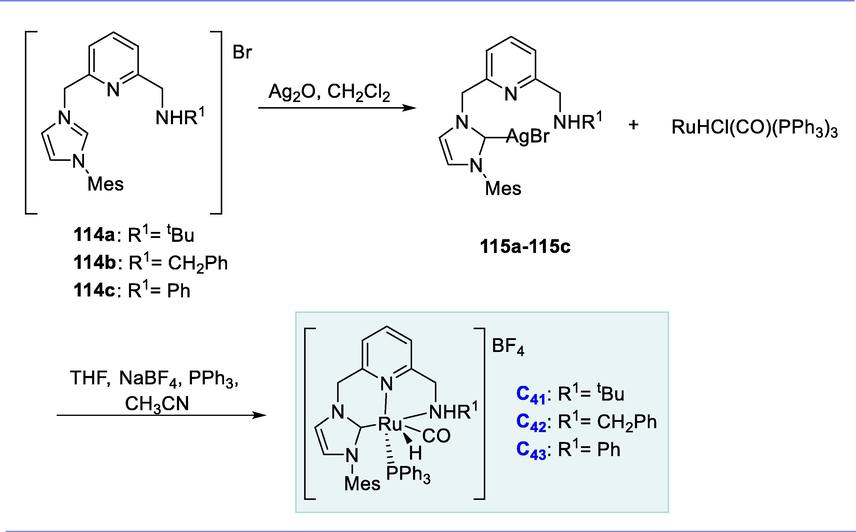
Synthesis of lutidine-based Ru-CNN(H) complexes.
The catalytic reactivity of complex (C41-C43) was studied in the N-heterocycles hydrogenation. Complex C41 (0.5 mol%) catalyzed the N-heterocycles (116) hydrogenation under H2 (4 bar) at 80°C in the presence of KOtBu, resulting in complete conversion in 6h. Complexes (C42 and C43) were shown to be less active than complex (C41) under the same conditions. The 2-methylquinoxaline was reduced with excellent conversions in 24h under similar conditions. Similarly, phenanthridine and acridine were hydrogenated with good conversions in 19h using complex C41 (0.5 mol%). However, to achieve greater than 95% conversion, the hydrogenation of quinoline and quinaldine need harsh reaction conditions (1.0mol% C41, 48h, 95°C). (Scheme 46)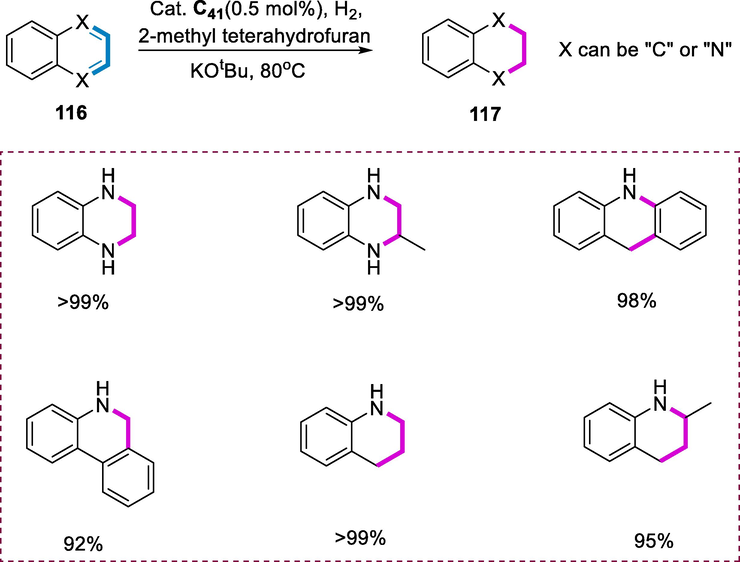
Hydrogenation of N-heterocycles.
It was observed that complex (C41) is also efficient for catalytic N-heterocycles (118) dehydrogenation. Initially, 1,2,3,4-tetrahydroquinoxaline dehydrogenation was investigated in 2-methyltetrahydrofuran at 85°C with complex C41 (4.0mol%) and KOtBu, yielding complete production of quinoxaline after 24h. However, the reaction of 2-methylquinoxaline (160°C, o-xylene) required a greater temperature. Other N-heterocyclic substrates were also investigated for dehydrogenation. With moderate catalytic activity, 9,10-dihydroacridine was also dehydrogenated under reflux in o-xylene. A variety of N-heterocyclic substrates were tolerated for this transformation. The quinoline and 2-methylquinoline were formed via dehydrogenation after 48h with relatively low yields (Scheme 47) (Sánchez et al., 2020).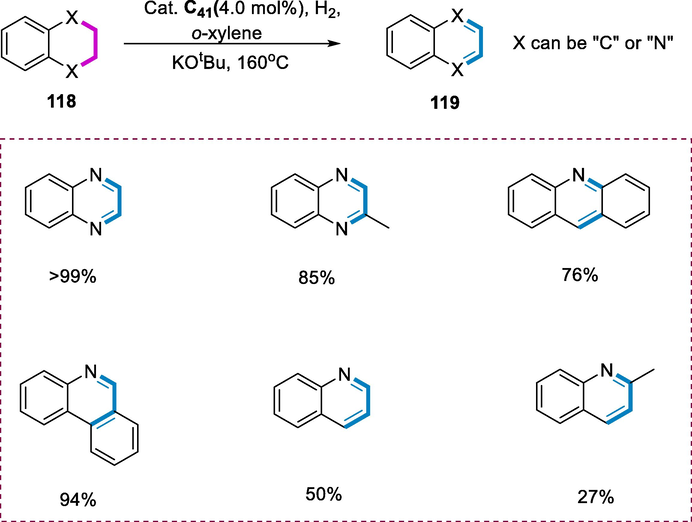
Dehydrogenation of N-heterocycles.
3 Cycloruthenated complexes-synthesis and applications
The cyclometallated compounds are used as mesogenic, photoluminescent chemicals and as chiral auxiliaries as well as their potential use as biologically active molecules against cancer (Dupont and Pfeffer, 2008). The use of cycloruthenated compounds as catalysts demonstrates that they are particularly effective for hydrogenation transformation, either by hydride transfer or H2, C-C bond formation, and ortho-deuteration (Djukic et al., 2009). The cycloruthenated compounds are also used in dye-sensitized solar cells as molecular sensitizers (Wadman et al., 2007).
3.1 Synthesis of mono-nuclear ortho-metallated cyclic ruthenium complexes and their applications
The C-H bonds at the ortho-position of phenyl are the active sites of ligand for deuteration catalyzed by cycloruthenated triphenyl phosphite and -phosphine complexes.
The ortho-bonded complex (C44) is formed when triphenyl phosphite reacts with chlorohydridotris(triphenyphosphine)-ruthenium. When complex (C44) reacts with hydrogen, it forms ruthenium-(chlorohydrido tetrakis-triphenyl phosphite), which rapidly returns to complex (C44) when heated. The reversibility of triaryl phosphites is a distinguishing property of this reaction. Lewis developed this catalytic system that allowed for effective ortho-deuteration (Scheme 48) (Parshall et al., 1969).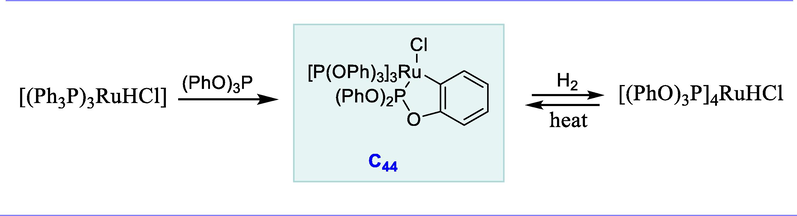
Synthesis of ortho-bonded ruthenium complex.
The ortho-deuteration of phenol (120) by using deuterium (D2), in the presence of cycloruthenated complex (C44) as a catalyst and potassium phenoxide (KOPh) as co-catalyst, and toluene as a solvent at 25°C for 66h results in the synthesis of 2,6-dideutrophenol (121). Only the addition of a transesterification co-catalyst dramatically enhanced the catalytic deuteration of phenol. Complex (C44) is a selective catalyst for the H–D exchange at the ortho-position of phenols by Lewis and coworkers. This was the first example of catalysis carried out by an ortho-metallated complex by the use of a complexing atom (e.g., phosphorus) to improve catalytic activity (Scheme 49) (Lewis, 1985).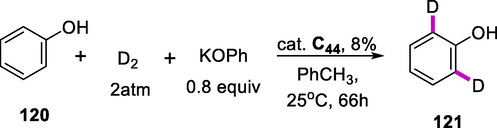
Ortho-deuteration of the substrate.
The Ru(CO)2Cl core part in (N,C)-chelates promotes C–C coupling processes. Metal-mediated “dimerization” of terminal alkynes via C-C bond formation produces conjugated eneynes, which have antimicrobial activity (Yamaguchi et al., 1995; Liu et al., 2006; Ogata and Toyota, 2007; Ciclosi et al., 2008).
The complex [Ru(CO)2Cl2]n reacts with 2-[tricarbonyl(6-phenyl)chromium]pyridine (122) in the presence of degassed and dry 1,2-dimethoxyethane, Na2CO3, and the subsequent suspension is refluxed for 15h, complex (C45) formed as dense yellow and deep red precipitate in 83% yields (Hijazi et al., 2006). The reaction of ruthenium complex [Ru(CO)2Cl2]n with 2-phenyl-4,5-pinenopyridine (123) in the presence of 2-methoxyethanol and the resultant solution was refluxed overnight producing complex (C46) in 80% yield. Complex (C45), which is only moderately soluble in dimethylformamide and slightly soluble in warm tetrahydrofuran while complex (C46) shows greater solubility in most polar protic and aprotic solvents (Scheme 50).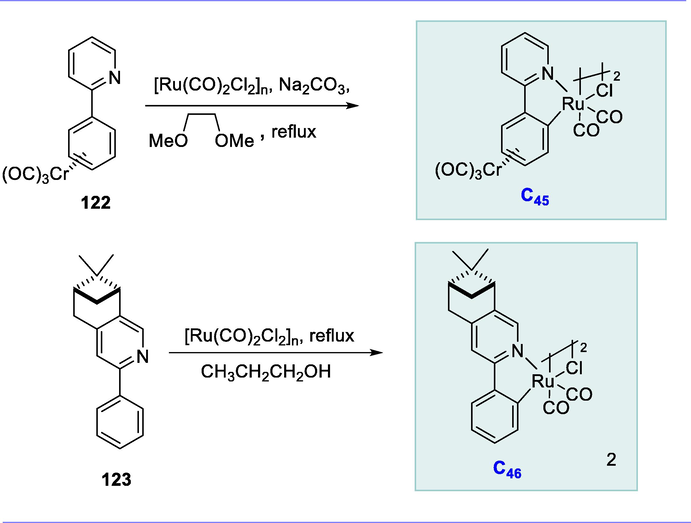
Synthesis of cyclo ruthenium complexes.
Homo-coupling of arylethynes (124) with catalytic quantities of dimeric ruthenacycles (C45 and C46) having the chloro-(dicarbonyl)-ruthenium motif [Ru(CO)2Cl] selectively and efficiently produces (E)-1,4-diaryl-but-1-en-3-ynes (125) in excellent yields. This reaction proceeds in the presence of sodium carbonate (Na2CO3) (excess), i-PrOH/THF (2:1) at 80°C for 15–18h (reflux) resulting in the synthesis of conjugated 1,3-enynes in excellent to good yield (54–100%) (Scheme 51) (Hijazi et al., 2008).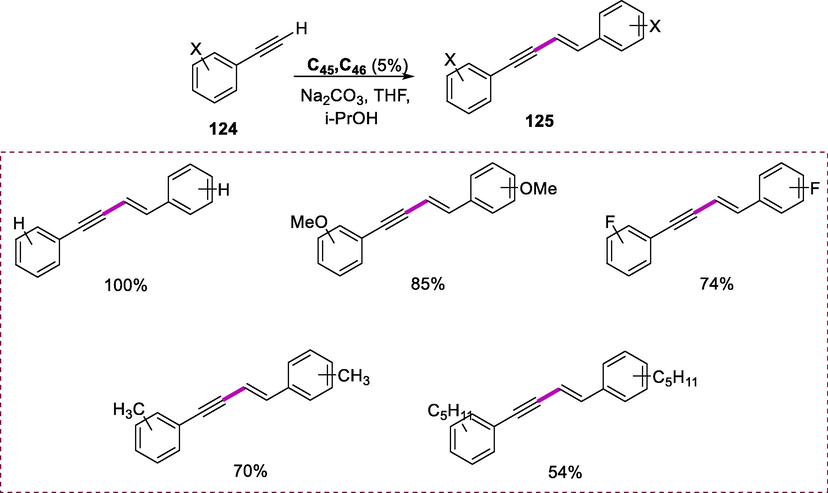
Synthesis conjugated 1,3-enynes.
Transfer hydrogenation is a chemical reaction in which addition of hydrogen into organic molecules from the source pother than gaseous hydrogen. Noyori pioneered transfer hydrogenation and awarded with noble prize (Hashiguchi et al., 1995; Ohkuma et al., 1995; Fujii et al., 1996; Gao et al., 1996; Haack et al., 1997; Matsumura et al., 1997; Noyori and Hashiguchi, 1997; Yamakawa et al., 2000; Noyori and Ohkuma, 2001; Noyori, 2002; Sandoval et al., 2003; Ikariya et al., 2006). The catalytic systems which are Ru-based are successful in the reduction of ketones by transfer hydrogenation (HT) among the many metal-catalyzed reactions (Naota et al., 1998). In the presence of isopropanol, the ruthenacyclic complex [RuBr(AsPh3)2(azo–OMe)] in the presence of isopropanol was found to be a good catalyst for ketone reduction with excellent yields.
The ruthenium (III) complex [RuBr3(AsPh3)3] (C47) as a catalyst was reacted with 2-(arylazo)-phenol (126) under reflux in the presence of dry benzene to produce a series of mono-nuclear ortho-metallated Ru-complexes (C48[a-e]) [RuX(AsPh3)2(L)] (X = Br or Cl; L = CNO donor of the ligands). Because of their versatility as ligands, these compounds have biological and catalytic significance. Among other complexes, the complex [RuBr(AsPh3)2(azo–OMe)] was found to be effective catalyst for the ketones hydrogenation with excellent yields under suitable reaction conditions (Scheme 52).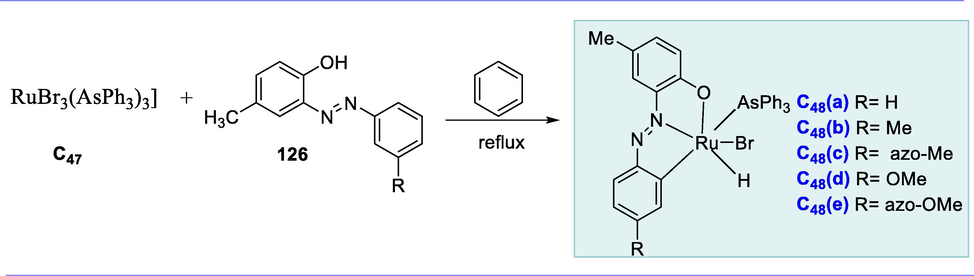
Synthesis of mono-nuclear ortho-metallated Ru-complexes.
Ru complex [RuBr(AsPh3)2(azo–OMe)] C48(c) was used for catalytic hydrogenation of a variety of aromatic and aliphatic ketones (127). When the ketone, ruthenium catalyst C48(c), and KOH were heated under reflux in i-PrOH at 80°C for a suitable period in an inert environment resulted in the formation of alcohols (128) in good yield. There is no hydrogenation of ketones without the use of the base. The base promotes the catalytic hydrogenation of ketones. The catalyst works well for both aliphatic and aromatic ketones with excellent yields and turnover. The reduction of p-chloro and p-methoxyacetophenone was and had lower reaction yields as compared to the reduction of phenylethanone. It was noticed that hydrogenation of aliphatic ketones is challenging and takes a long reaction time to achieve higher conversion(Scheme 53) (Venkatachalam and Ramesh, 2005).
Reduction of ketones.
3.2 Synthesis of amino-pyridine based cycloruthenated complexes and their applications
The secondary alcohols are useful chiral auxiliaries and intermediate in the fine chemical industry (Sheldon, 1993). Hydrolytic enzymes such as esterases and lipases are commonly used to attain a variety of enantioenriched alcohols from racemic sec. alcohol by kinetic resolution (KR) (Bakker et al., 2000). The ruthenium-amino structural core in the ruthenacycles and amino-pyridine Ru-complexes is very effective and is also used in the kinetic resolution of sec. alcohols via hydrogen transfer.
The thermally stable ortho-metallated Ru(II) complex (C49) is obtained in high yield by treating [RuCl2(PPh3)(dppb)] (129) an equimolar quantity of 6-(4-methylphenyl)-2- pyridylmethylamine (130) in the presence of 2-propanol under reflux by using NEt3 (Baratta et al., 2005). Ruthenacycles (C50) were produced by cyclo-metalating an enantiopure aromatic primary amine (131) with [(p-cymene)RuCl2]2 (132) by using NaOH, KPF6 for 72h at room temperature (Scheme 54) (Sortais et al., 2005).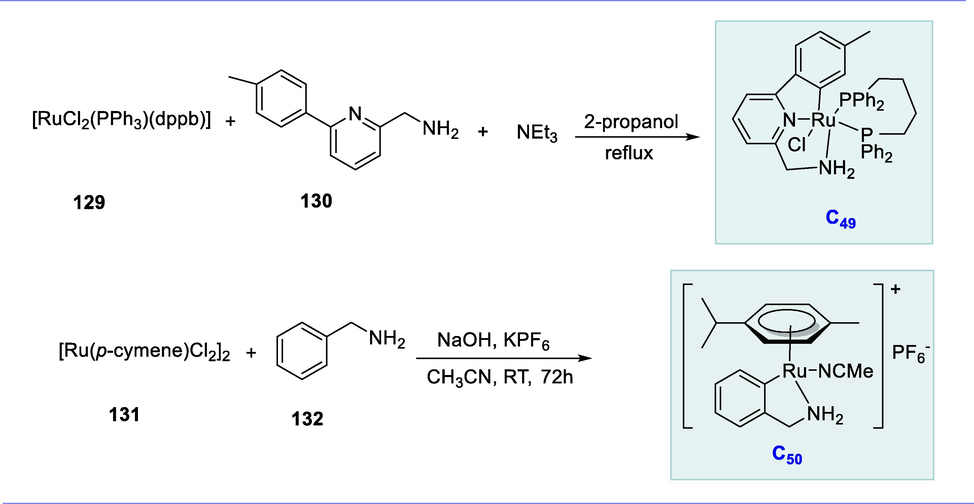
Synthesis of amino-pyridine based cycloruthenated complexes.
Amino-ruthenium complexes (C49) and (C50) were subjected to dynamic kinetic resolution in toluene (PhCH3) at 70°C by using potassium tert-butoxide and CAL-B enzyme. The isopropyl is used as an acyl donor. The racemization of (S)-1-phenylethanol is catalyzed very efficiently by complexes (C49) and (C50). These ruthenacycles resolve rac-1-phenylethanol (133) into enantiopure (R)-1-phenyl ethylacetate (135) in excellent yields and enantioselectivity (86% and 99% ee) after 48 hours at 70°C by using isopropyl acetate (134) as an acyl donor and Candida Antarctica lipase B as an enzyme. In comparison to complex C49, the ruthenacycle C50 gives good results (Scheme 55) (Eckert et al., 2007).
Dynamic kinetic resolution of the secondary alcohols.
The synthesis of a broad range of alcohols relies heavily on transition metal-catalyzed catalytic hydrogenation of carbonyl compounds (Gladiali and Alberico, 2006; Samec et al., 2006). The tridentate RuCl(CNN)(dppb) catalyst quantitatively and chemo-selectively converted the aromatic, aliphatic, and, α,β-unsaturated aldehydes into primary alcohols in the presence of 2-propanol as hydrogen donor. The Ru-complex [RuX(CNN)(dppb)] (dppb = Ph2P(CH2)4PPh2; X = Cl, H), which is a very active catalyst for transfer hydrogenation (Yang et al., 1997; Braunstein et al., 1999; Clark et al., 1999; Toner et al., 2000).
The aldehydes (137) solution was refluxed in isopropanol with complex (C49) and K2CO3 at 82°C, quantitative formation of alcohols (138) occurs in 1-5 minutes with excellent yields. The reaction takes 30 and 20 seconds to complete when the base concentration is increased to 5 and 10mol percent. The effectiveness of applying complex (C49) in synthesis is due to the reaction taking place at a lower complex (C49) loading. Because of the excellent catalytic activity of this complex, the wide range of aldehyde substrates was reduced. The short reaction time (minutes) necessary for full reduction and minimum chances of side reactions making this approach a highly chemoselective for hydrogenation of aldehydes (Scheme 56) (Baratta et al., 2007b).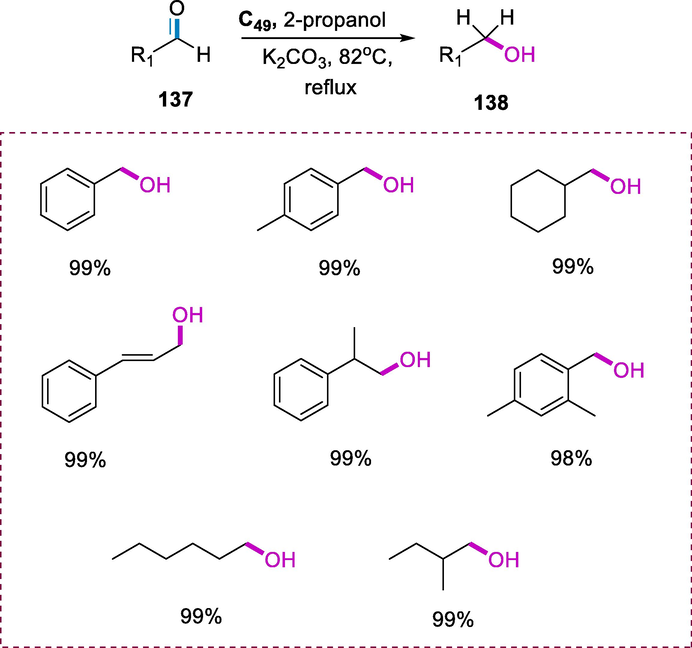
Reduction of aldehydes.
4 Half-sandwich arene Ru-complexes-synthesis and application:
Half-sandwich arene Ru-complexes have a broad range of applications in synthetic chemistry, including hydrogenation, cycloaddition, esterification, Diels–Alder cycloadditions, olefin-metathesis, and 1,3-dipolar (Moriarty et al., 1988; Le Bozec et al., 1989; Noyori et al., 1997). These complexes have proven to be effective precursors in organometallic chemistry due to the mild reaction conditions mandatory for synthesis, good yields, and a vast range of solubility and stability under an aqueous environment (Canivet and Süss-Fink, 2007; Singh et al., 2007). The chemical and biological activity of these complexes is substantially influenced by the type of the arenes, chelating ligands, and leaving group, which display structure-activity relationships (Habtemariam et al., 2006). Furthermore, these complexes are biologically active compounds that could be used to treat cancer (Kurzwernhart, 2013).
4.1 Synthesis of unsaturated cationic arene β-diketiminato ruthenium complexes and their application
The Diels–Alder (DA) reactions due to their high efficiency and selectivity catalyzed by optically active Lewis acids are one of the most valuable tools in the formation of complex compounds. The enantioselective Diels–Alder reactions between dienes and α,β-unsaturated aldehydes catalyzed by half-sandwich cationic complexes having chiral [(arene)-M(L1L2)] motif with only one vacant site (Carmona et al., 1997; Davies et al., 1997; Faller et al., 2001).
The coordinatively unsaturated cationic complexes (C52) and (C53) are formed by the abstraction of chloride from complex (C51) using the weakly coordinating anion sodium tetrakis(3,5-bis(trifluoromethylphenyl)borate) ([Na]BArF), (BArF = B(3,5-(CF3)2C6H3)4) results in the formation of arene Ru(II) complexes having fluorinated β-diketiminate ligands. In comparison to complex (C53), the complex (C52) has a higher endo selectivity in cyclopentadiene and acrolein synthesis. The beta-diketiminate ligand in complex (C52) improves the Diels–Alder processes' selectivity. The respective unsaturated cationic complexes (C52) and (C53) are attained by abstraction of chlorine atom in the presence of poorly coordinated anion BArF. Remarkably, these ruthenium complexes were discovered to be strong lewis acid catalysts for Diels-Alder reaction of dienes (2,3-dimethyl-1,3-butadiene, cyclopenatdiene) and α,β-unsaturated aldehydes (acrolein, methacrolein). The structurally robust ligand (β-diketiminate ligand) not only gives the sufficient Lewis acidity at the central metal center but also improves the selectivity of the Diels-Alder reactions. Significantly, complexes (C52) and (C53) allow the use of mild condition of reaction, lowering the possibility of side products (Scheme 57).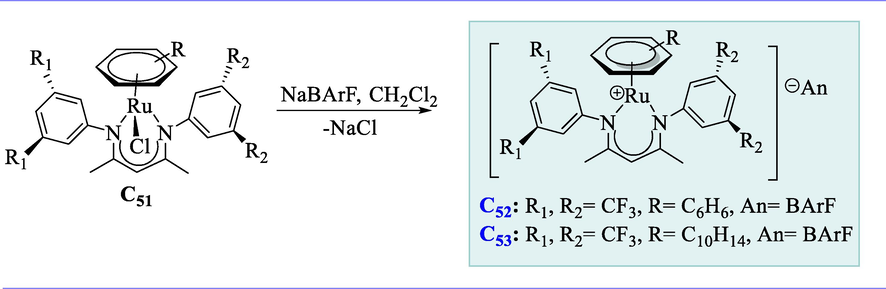
Synthesis of coordinatively unsaturated cationic ruthenium complexes.
The bicyclic endo- or exo-2-methyl-bicyclo[2.2.1]hept-5-ene-2-carboxaldehyde (141a or 141b) and endo- or exo-bicyclo[2.2.1]hept-5-ene-2-carboxaldehyde (142a or 142b) are efficiently formed in excellent yields by Diels-Alder cycloaddition between acrolein or methacrolein (139) and cyclopentadiene (140) catalyze by either complex (C52) or (C53) in the presence of dichloromethane at 20°C. Complexes (C52) and (C53) facilitate the use of milder reaction conditions, which reduces the chances of side products in the reaction (Scheme 58) (Schreiber et al., 2011).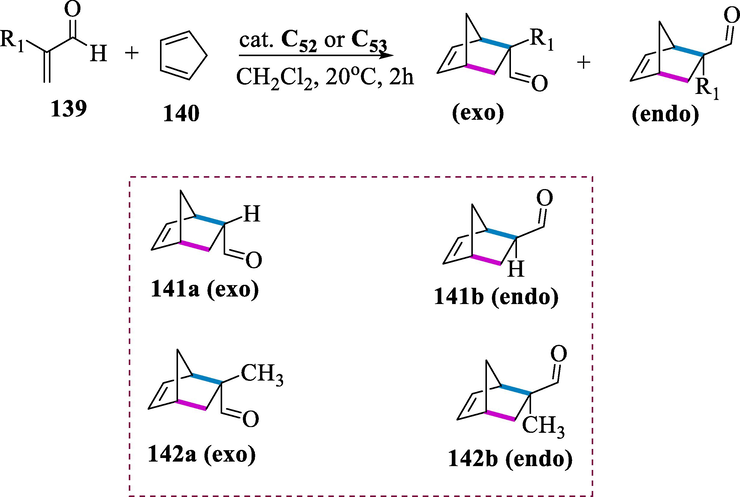
Diels-Alder reaction.
4.2 Synthesis of cationic cyclopentadienyl Ru-complex and its application
Isoxazolidines are enantiopure which are used as precursors for the formation of chiral 1,3-amino alcohols that are useful synthons for building physiologically active compounds (Frederickson, 1997). The 1,3-dipolar cycloaddition process between alkenes and nitrones mediated by chiral lewis acids is the versatile and direct method for these molecules (Gothelf and Jørgensen, 1998; Gothelf and Jørgensen, 2000). The transition metal lewis acid catalyst [Ru (acetone)-(R,R)-BIPHOP-F)Cp][SbF6] catalyzes the 1,3-dipolar cycloaddition reaction between α,β-unsaturated aldehydes, and aryl nitrile oxides.
The reaction of hdrobenzoin (143) with NEt3 and BrP(C6F5)2 in the presence of THF at room temperature for 24 hours yields the chiral ligand [(R,R)-BIPHOP-F] (144), which is then further reacted with Ru3(CO)12, CpH, and CHI3 to form complex (C54). Then complex (C54) react with AgSbF6 and acetone at room temperature, and a cationic cyclopentadienyl Ru-complex (C55) [Ru (acetone)(R,R)-BIPHOP-F)Cp][SbF6] is formed after 1.5hours. The enantioselective 1,3-dipolar cycloaddition of methacrolein with nitrones was efficiently mediated by Ru complex (C55) as catalyst (Scheme 59) (Brinkmann et al., 2007).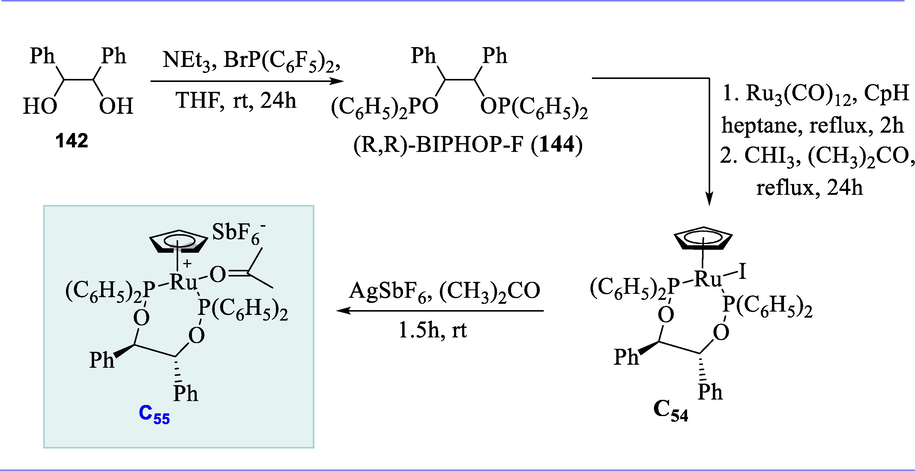
Synthesis of cationic cyclopentadienyl Ru-complex.
The 1,3-dipolar cycloaddition reaction of methacrolein (145) and C,N-diaryl nitrones (146) gave endo and exo-isoxazolidines (147a and 147b) by using complex (C55). Through coordination, the catalyst activates the substrate aldehyde, making it more susceptible to nitrone attack. Catalytically active cationic complex (C55) were found to be active in catalyzing the 1,3-dipolar cycloaddition reaction of a broad range of substrates with good to excellent yields (79% to 92%) and moderate to excellent enantiomeric excess (66% to 94%) (Scheme 60) (Viton et al., 2002).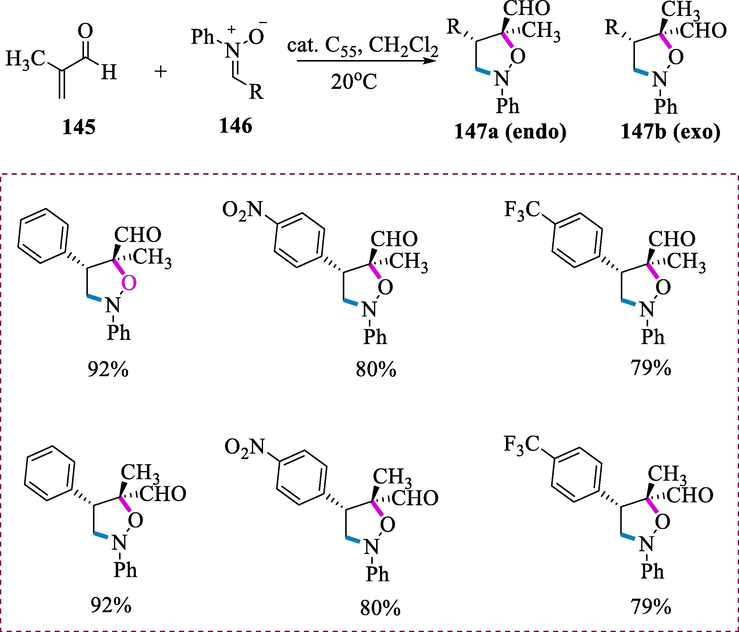
1,3-dipolar cycloaddition reaction.
4.3 Synthesis of Dichloro(p-cymene)[(R)-(isopropyl)(1-naphthyl)phenylphosphine] Ru(II) complex and its application
One of the most effective approaches for the selective production of optically active cyclopropane chemicals is catalytic asymmetric cyclopropanation of alkenes (Lebel et al., 2003). Ru-complexes having mono-dentate phosphorus ligands are efficient for cyclopropanation-competent systems (Lasa et al., 2005).
The synthesis of Dichloro(p-cymene)[(R)-(isopropyl)(1-naphthyl)phenylphosphine] Ru(II) complex (C56) results from the reaction of ruthenium p-cymene dimer (148) with phosphine ligand (149) by using dichloromethane at ambient temperature. Ruthenium complex (C56) was utilized as an efficient catalyst in the asymmetric cyclopropanation reaction between ethyl diazoacetate and styrene with high diastereoselectivity and enantioselectivity (up to 74%) (Scheme 61).![Synthesis of Dichloro(p-cymene)[(R)-(isopropyl)(1-naphthyl)phenylphosphine] Ru(II) complex.](/content/184/2022/15/11/img/10.1016_j.arabjc.2022.104165-fig67.png)
Synthesis of Dichloro(p-cymene)[(R)-(isopropyl)(1-naphthyl)phenylphosphine] Ru(II) complex.
The formation of chiral cyclopropanic compounds (152a and 152b) is achieved via cyclopropanation of styrene (150) through ethyl diazoacetate (151) with complex (C56) by using dichloromethane for 6h. The more electron-rich nature of α-methylstyrene gives better results via cyclopropanation than unsubstituted styrene. The presence of a bulky alkyl group along with complex (C56) containing phosphines with an isopropyl group gives the best results in the cyclopropanation reaction (Scheme 62) (Grabulosa et al., 2012).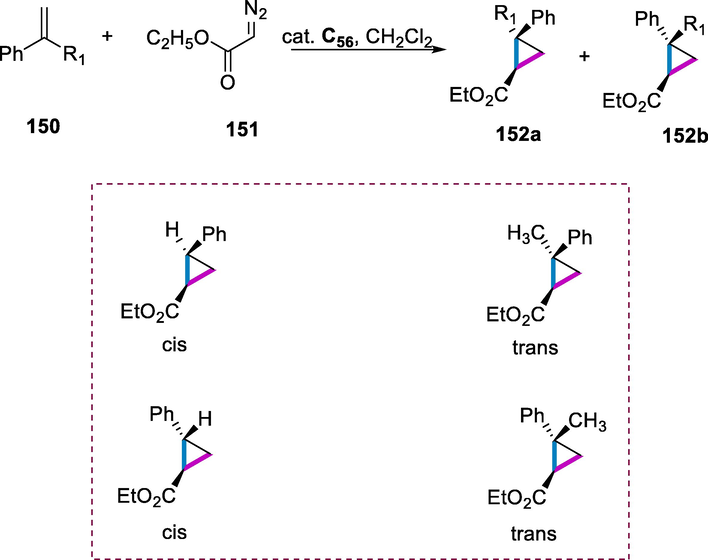
Cyclopropanation reaction.
4.4 Synthesis of dinuclear Shvo type Ru-complex and its application
The 1,3-cycloalkanediols are used as building blocks in many pharmaceutics. They can be synthesized from corresponding diones via reduction (Fransson et al., 2006). The Shvo complex serves as an effective catalyst for the transfer of hydrogenation of ketones in the presence of 2-propanol (Blum et al., 1985).
The ligand tetraarylcyclopentadienone (155) was prepared by the reaction of diaryl-α-diketones (153) with diphenylacetone (154) in the presence of NaOH under reflux in EtOH for 2 to 16 hours. The dinuclear Ru-complex (C57) was obtained by reacting tetraphenylcyclopentadienone (155) with Ru3(CO)12 (156) in methanol under microwave irradiation. It was discovered to be one of the most active catalysts for the transfer hydrogenation of carbonyl compounds and related reactions (Scheme 63) (Cesari et al., 2014).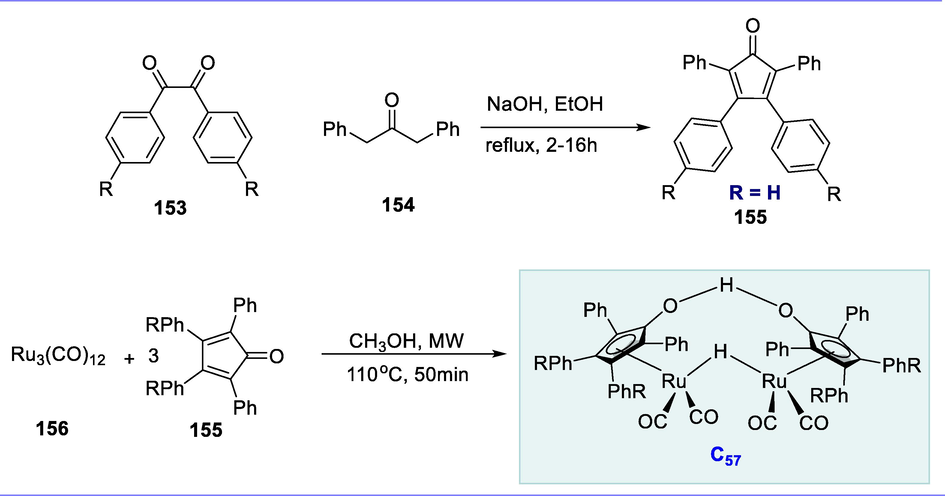
Synthesis of dinuclear Shvo type Ru-complex.
The 1,3-cycloalkanediones (157) react in the presence of ruthenium catalyst (C57) and 2-propanol by using toluene as solvent at 120°C resulting in the effective synthesis of corresponding diols (158) through the reduction in good yield. The loading of the catalyst could be reduced by increasing the amount of 2-propanol, and the reaction was completed within 60 minutes. Six-membered cyclic 1,3-diones undergo transfer hydrogenation by using ruthenium catalyst (C57) in the presence of isopropanol as an H2 donor under microwave heating resulting in the formation of corresponding 1,3-diols. The diols were obtained with a yield of up to 93 percent. The reduction of seven-membered and five-membered cyclic 1,3-diones to the respective diols was also feasible, but a longer reaction duration and hydrogen gas were required. In dynamic kinetic asymmetric transformations, diols are significant building blocks (Scheme 64) (Leijondahl et al., 2006).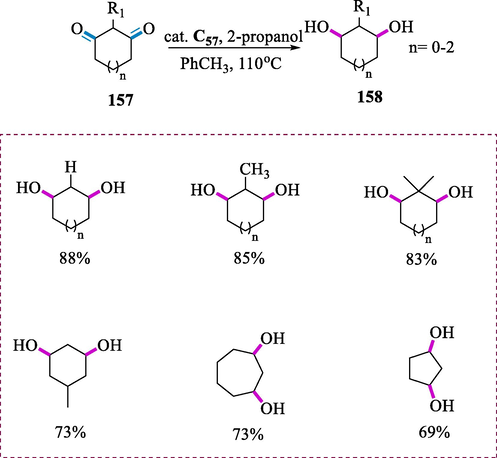
Transfer of hydrogenation of ketones.
4.5 Synthesis of arene Ru(II) complexes carrying pyridine-2-thiocarboxamide ligands and their application
The oxidation of secondary and primary alcohols into their corresponding ketones and aldehydes without further oxidation into carboxylic acids by using N-methylmorpholine N-oxide (NMO) oxidant is a significant transformation in synthetic chemistry.
The substituted pyridine-2-thiocarboxamide (162a-f) ligands were formed by reacting an aromatic amine (161) with sulfur (160) and 2-methylpyridine (159) for 48 hours at 150°C. The reaction of bidentate thiocarboxamide ligands (162a-f) with ruthenium complex [Ru(p-cymene)Cl2]2 (163) and AsPh3 in methanol yielded arene Ru(II) complexes C58(a-f) carrying pyridine-2-thiocarboxamide ligands of the type [Ru(p-cymene)(AsPh3)(L)]. These Ru(II) complexes are colored, light and air-stable, and soluble in dichloromethane, chloroform, DMSO, and DMF. These complexes are diamagnetic in nature. The complex (C58a) found to be more effective among other complexes for this transformation (Scheme 65).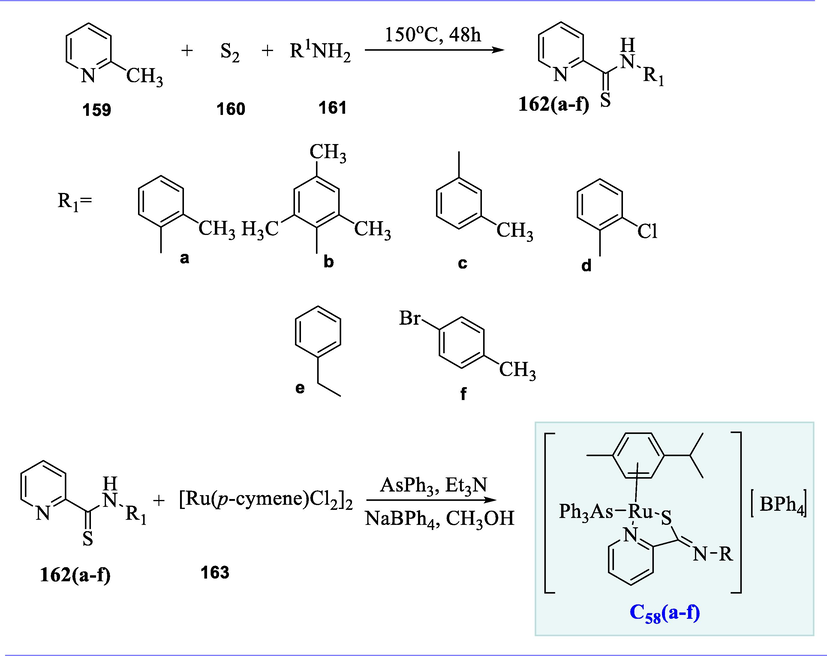
Synthesis of arene Ru(II) complexes carrying pyridine-2-thiocarboxamide ligands.
Ruthenium (II) complexes C58(a-f) catalyze the oxidation of secondary or primary alcohols (164) to the corresponding ketones or aldehydes (165) in the presence of NMO as a co-oxidant. The corresponding ketones and aldehydes were obtained in good yield by using the complex (C58a) as a catalyst and secondary or primary alcohols as substrates in the presence of NMO (N-methylmorpholine N-oxide) as oxidant and dichloromethane by refluxing for three hours (Scheme 66) (Raja and Ramesh, 2012).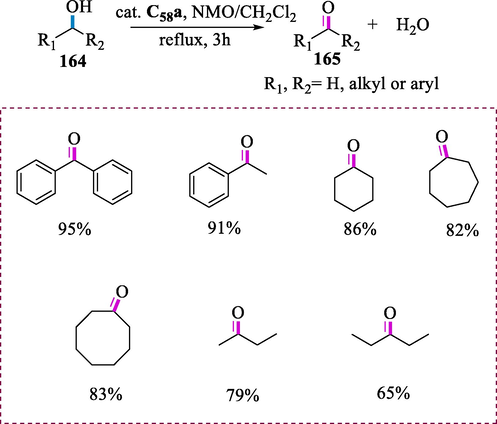
Oxidation of alcohol.
4.6 Synthesis of arene/N-tosylethylenediamine ruthenium (II) complex and its application
The asymmetric hydrogenation of C=N double bonds effectively proceeds by half-sandwich Ru-complexes due to their potential applicability. Chiral amines are essential synthetic intermediate in the pharmaceutical, chemical industry, and agriculture (Kumar et al., 2014). Catalytic systems for the heterocycles hydrogenation such as quinolines, indoles, and pyrroles based on the combination of Ru-complexes. Ruthenium complexes with diamines as a chiral ligand are very effective catalysts for the quinolines asymmetric hydrogenation in ionic liquids. This transformation under extremely concentrated and solvent-free conditions produced excellent outcomes (Kuwano and Kashiwabara, 2006; Zhou et al., 2008a).
Noyori and Ikariya's sulfonyl/arenediamine ruthenium (II) complex (C59) has been demonstrated to have outstanding catalytic activity in the vast range of asymmetric hydrogenations of imines, including quinolone hydrogenation. The synthesis of the arene/sulfonyldiamine-Ru(II) complex (C59) arises from the complexation of the (R,R)-TsDPEN ligand (166) with ruthenium complex [RuCl2(p-cymene)]2 (167) by using Et3N, 2-propanol at 80°C for 1h (Scheme 67) (Touge and Arai, 2016).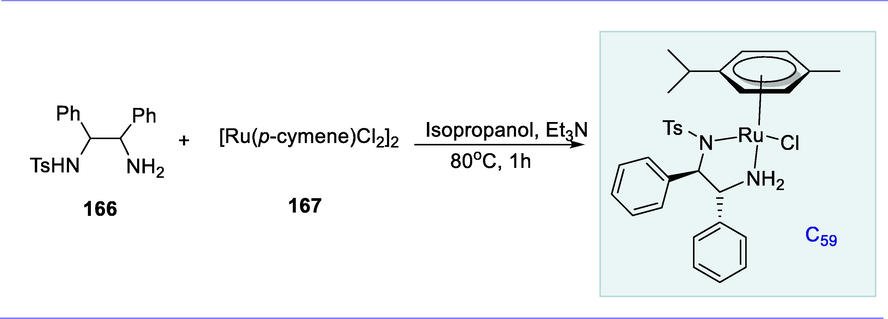
Synthesis of arene/N-tosylethylenediamine ruthenium (II) complex.
Hydrogenation of quinoline substrates (168) yielded 1,2,3,4-tetrahydroquinolines (169) with good enantioselectivity (up to 99% ee). Complex (C59) catalyzes the asymmetric hydrogenation of quinoline derivatives in [BMIM]PF6 in the presence of hydrogen at 25°C for 15-24h, yielding 1,2,3,4-tetrahydroquinolines with good enantioselectivities (up to 99% ee). All 2-alkyl-substituted quinolines were hydrogenated with high yields and enantioselectivities in general (up to 99 percent ee). In an ionic liquid, hydrogenation with complex 1 was selective for C=N bond (quinoline) over C=O bonds (Scheme 68) (Zhou et al., 2008a).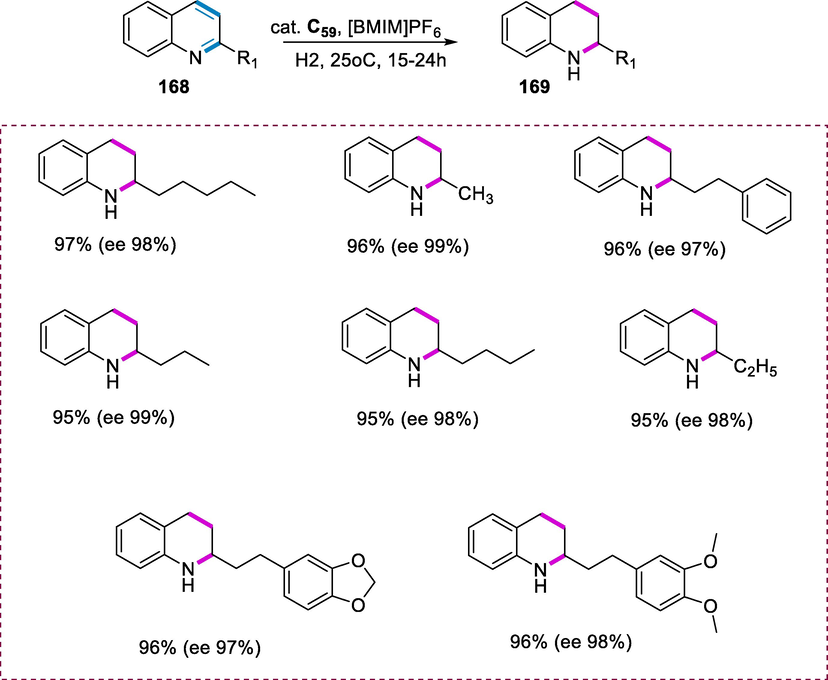
Hydrogenation of imines.
5 Ruthenium phosphine complexes
5.1 Synthesis of Ru-phosphine complexes having 2-aminopiperidine ligand and its application
Baratta and colleagues examined the excellent ability of ruthenium (II) complexes having the 2-aminomethylpiperidine (ampi) ligand to catalyze the reduction of ketones (Baratta et al., 2007a; Del Zotto et al., 2007; Baratta et al., 2010). The transfer hydrogenation (TH) of polar substrates like ketones from hydrogen donors such as formic acid and iso-propanol is rapidly catalyzed by the ruthenium (II) phosphine complex. However, they are shown to be active in the presence of hydrogen donors (Gladiali et al., 2004; Ikariya and Blacker, 2007; Soltani et al., 2010).
Ru(II) complexes containing 2-aminomethylpiperidine ligand and different phosphine moieties were found to be effective in TH. The complexes C60(a-d) were formed by reacting RuCl2(PPh3)3 (171) with 2-aminomethylpyridine (ampi) 170 and the diphosphine ligands in dichloromethane at room temperature. The diphosphine ligands (PP) are well-known due to their excellent chelating properties with transition metals, enabling them to adjust the reactivity, stability, and catalytic performance of their complexes. The ampi is a suitable neutral bidentate ligand of Ru(II) complexes for transfer hydrogenation catalysis. The electron-rich NH and NH2 groups stimulate the TH activity. This ligand provides more hydrogen bonds at the metal atom. The remarkable catalytic activity of these [RuCl2(phosphine)(ampi)] complexes can be attributed to the Ru-NH2 linkage and flexible ampi system, which are both involved in the H-bonding interaction with the alcohol and ketone and are important for substrate access to the ruthenium core. (Scheme 69).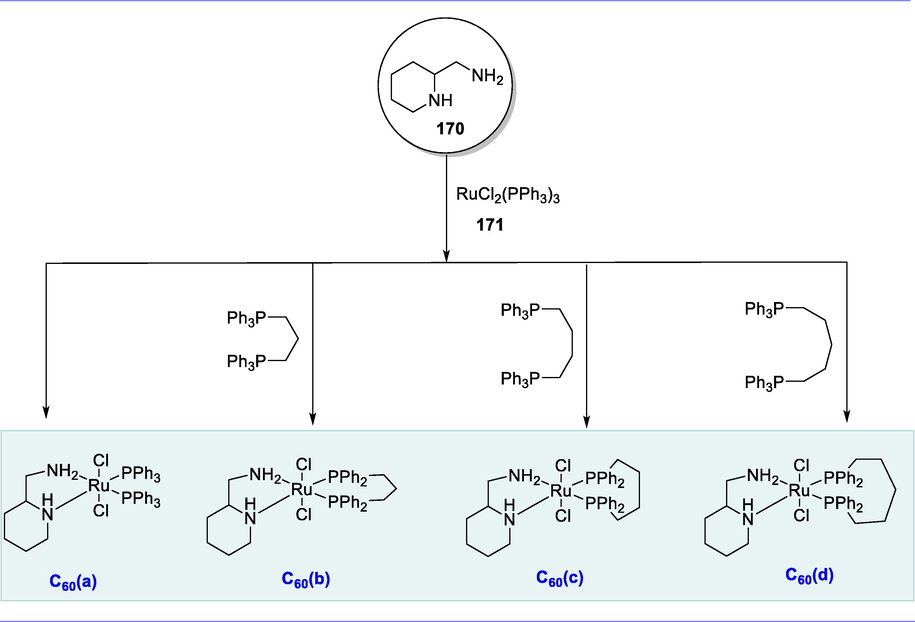
Synthesis of Ru-phosphine complexes having 2-aminopiperidine ligand.
Transfer hydrogenation (TH) of acetophenone (172) by using 2-propanol at 80°C in the presence of complex 5 and using KOH as a base to yield phenylethanol (173). Complexes C60(b-d), which include the diphosphine ligand [(PPh2(CH2)nPPh2, n = 3, 4, and 5] have much more activity than complex C60(a), which has two PPh3 ligands. Complex C60(d) with the diphosphine ligand [PPh2(CH2)5PPh2] and ampi exhibits the maximum catalytic activity, resulting in 99 percent ketone conversion in 5 minutes. Potassium hydroxide was found to be the most efficient than other bases such as NaOH, K3PO4, Et3N, K2CO3, and pyridine being slightly less effective and requiring longer reaction times. Ampi ligand, which is commercially available and has a particularly strong acceleration effect on transfer hydrogenation catalyzed by ruthenium (II) complexes in the presence of 2-propanol (Scheme 70) (Türkmen, 2012).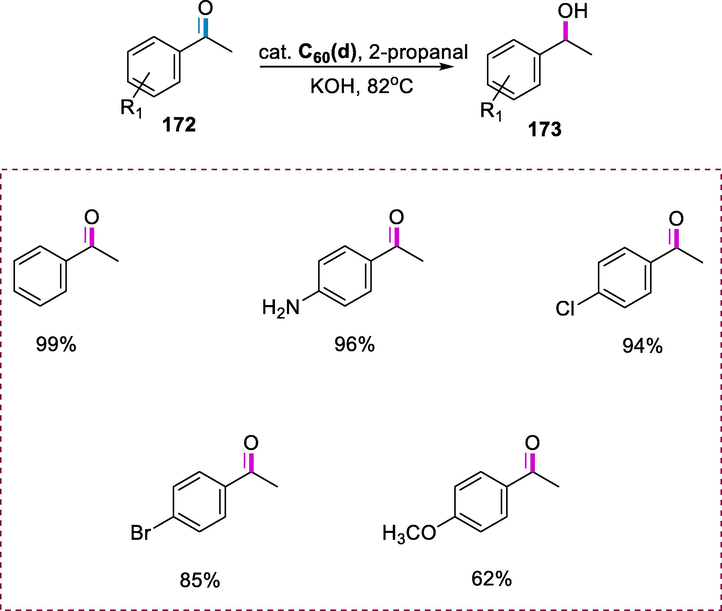
Hydrogenation of arylated ketones.
5.2 Synthesis of ruthenium (II) complexes carrying the 2-(2-(diphenylphosphino) benzylidene)-N-ethylthiosemicarbazone ligand and its application
N-alkylation of amides/amines by using alcohols could be a relatively eco-friendly and green synthetic alternative due to the formation of water being the only byproduct. Furthermore, alcohols are generally available, less toxic, very stable, easily handled and stored, cost-effective, and relatively high in atom efficiency enabling them to use as an alkylating agent which is very simple and easy (Chang et al., 2009; Dobereiner and Crabtree, 2010; Bähn et al., 2011; Crabtree, 2011b; Zhang et al., 2011b). The electronic effects (basicity) of ligand and the steric (bulkiness) enable the complexes having phosphine coordinating arms usually to show excellent catalytic activity (Prakash et al., 2014; Prakash and Viswanathamurthi, 2014; Ramachandran et al., 2014).
The 4-ethyl-3-thiosemicarbazide (174) reacts with the 2-(diphenylphosphino)-benzaldehyde (175) in the presence of acetic acid resulting in the synthesis of PNS-Et (176) in 96 percent yield. The complexes (C61-C63) were formed by reacting isolated PNS-Et (176) with an equimolar quantity of [RuH2(CO)(PPh3)3], [RuHCl(CO)(PPh3)3, and [RuCl2(PPh3)3]. These new complexes were miscible in common organic solvents like chloroform, benzene, dichloromethane, acetonitrile, dimethylsulfoxide, ethanol, methanol, and dimethylformamide. The complexes (C61-C63) have been shown to be effective catalysts for the N-alkylation reaction under moderate conditions. Additionally, these complexes have demonstrated significant tolerance for the functional groups found in hetero(aromatic) amine and benzyl alcohol moieties. Futhermore, these complexes exhibit excellent selectivity for the mono-alkylation of hetero(aromatic) amines. (Scheme 71).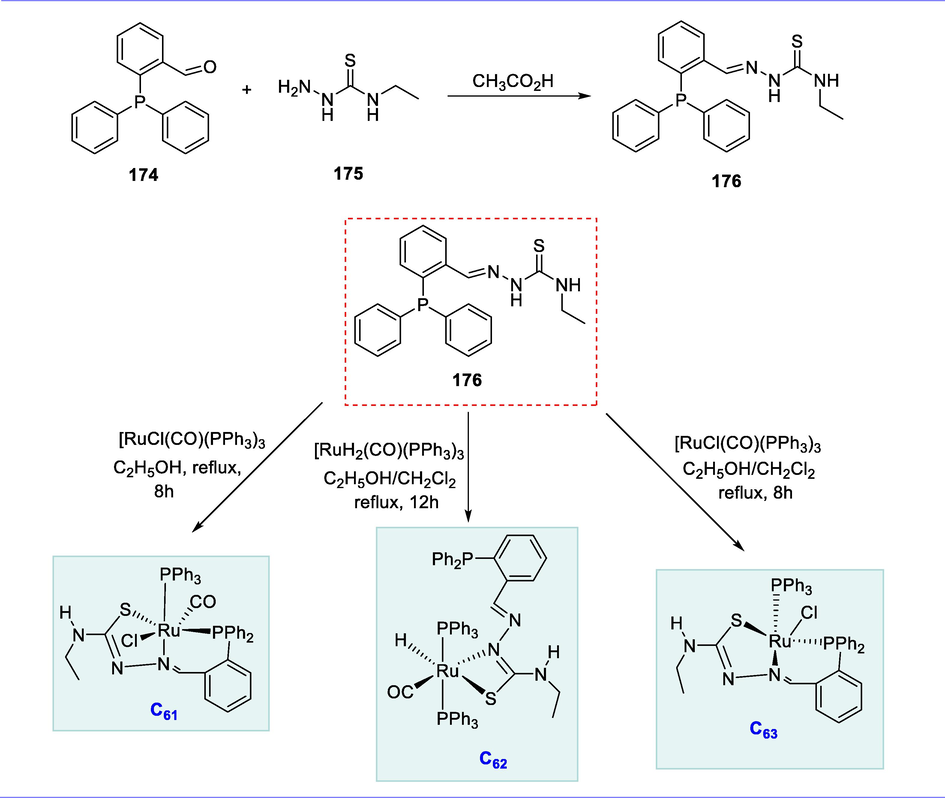
Synthesis of ruthenium (II) complexes carrying the 2-((diphenylphosphino) benzylidene)-N-ethylthiosemicarbazone ligand.
The hydrogen auto-transfer reaction with KOH as the promoter and in the presence of solvent catalyzed the heteroaromatic amines (177) N-alkylation by using substituted benzyl alcohols (178) into the respective N-alkylated products (179) by the ruthenium (II) complexes (C61-C63). The 2-aminobenzothiazole is N-alkylated with 4-methoxybenzyl alcohol by using different bases. The results demonstrate that bases were screened as catalytic reaction initiators. The use of toluene as a solvent and a strong base KOH resulted in excellent yields of the desired product. Catalysts (C61-C63) were found to be the most effective complexes for the 2-aminobenzothiazole N-alkylation in terms of selectivity and yield. The catalyst loading of 0.5 mol% was chosen due to the good yields and short reaction durations required. These reactions have a lot of appealing features such as cost-effectiveness, water as a by-product, use of less toxic organic materials, and excellent selectivity to respective products (Scheme 72) (Ramachandran et al., 2015).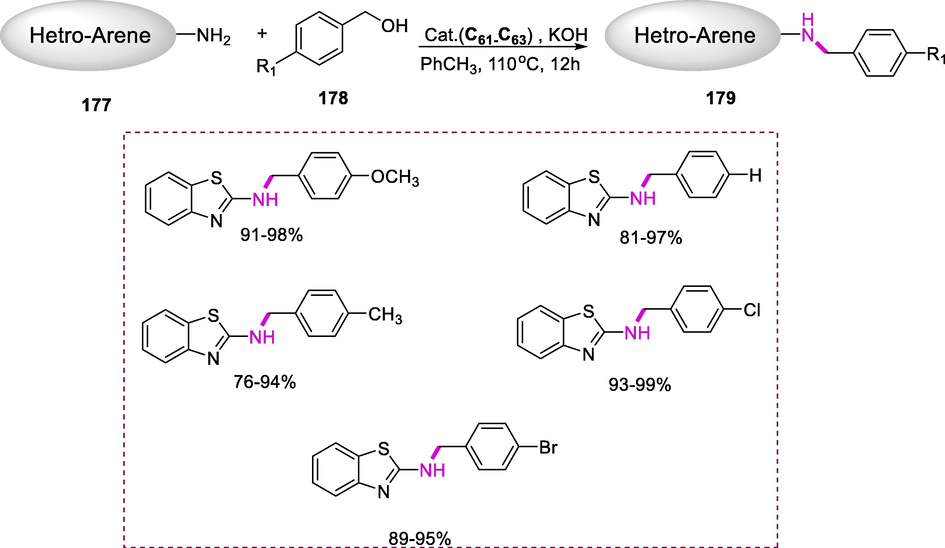
N-alkylation of amines.
5.3 Synthesis of tris-(N-pyrrolylphosphine) ruthenium complex and its application
The new ruthenium phosphine complex is catalytically active for the propargylic alcohol etherification and the synthesis of new and known xanthones through propargylic alcohols. The P(pyr)3 ligand is easily assessable and exhibits improved pi-acidity and electronic properties similar to CO in the [RuCl(ind)(PPh3)P(pyr)3] complex (Moloy and Petersen, 1995; Angurell, 2004; Menye-Biyogo et al., 2007). In the production and application of ruthenium complexes, phosphonates are the most extensively used ligand class (Mezzetti, 2010; Delaude and Demonceau, 2012).
The ruthenium complex [RuCl(ind)(PPh3)2] has been utilized as a precursor for ligand-substitution reactions to synthesize ruthenium complexes. The mono(pyrrolylphosphine) ruthenium complex [RuCl(ind)(PPh3)-P(pyr)3] C64 was isolated in moderate yield (73%) as a red solid after chromatographic workup when [RuCl(ind)(PPh3)2] (180) was treated with P(pyr)3 (181) ligand upon heating in THF under reflux for 4h. The synthesis of P(pyr)3 analogs [RuCl(ind)(PPh3)2] to attain Ru-complexes with higher Lewis acidity to enhance the catalytic activity for the transformation of propargylic alcohol (Scheme 73).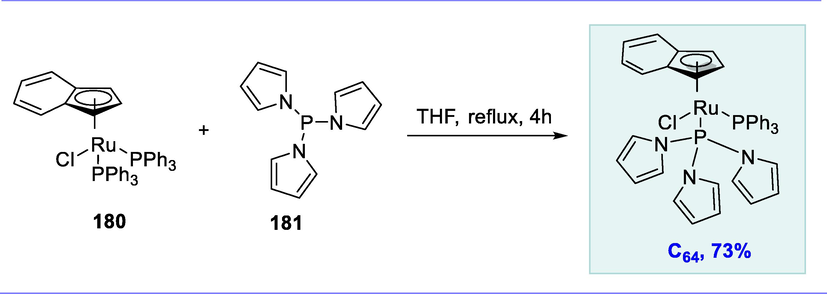
Synthesis of tris-(N-pyrrolylphosphine) ruthenium complex.
The activated ruthenium complex [RuCl(ind)(PPh3)P(pyr)3] (C64) catalyzed the etherification of various propargylic alcohols (182) into respective propargyl ethers (183) in isolated yields of 42% to 27% in the presence toluene at 70 to 95°C for 16–72h. In the etherification of propargylic alcohols, these new complexes are catalytically active (Scheme 74) (Stark et al., 2016).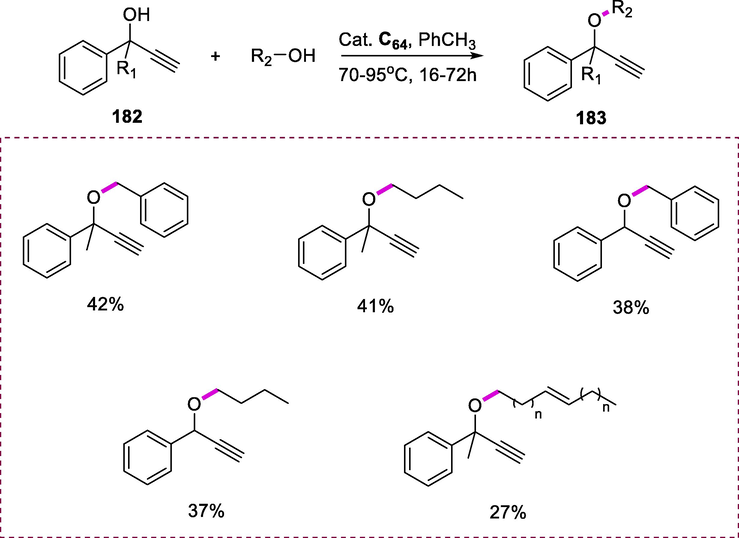
Etherification of propargylic alcohols.
5.4 Synthesis of phosphino-oxime containing Ru(II) complex and its applications
The hybrid ligands with soft phosphorus donor sites and hard nitrogen are extremely useful in coordination chemistry and homogeneous catalysis (Kostas, 2008; Zhang et al., 2011c; Noël and Van der Eycken, 2013; García-Álvarez et al., 2014). The phosphino-oxime 2-Ph2PC6H4CH=NOH containing ruthenium complexes have enormous synthetic potential and are the efficient catalyst in the aldoximes rearrangement into amides (Crochet and Cadierno, 2015).
The cis-[RuCl2(DMSO)4] (184) was treated with phosphino-oxime ligand (186) under reflux in THF, to produce the octahedral Ru(II) derivative [RuCl2((P,N)-2-Ph2PC6H4CH=NOH)2] (C65) in 87% yield. The reaction of [RuCl2(p-cymene)]2 (185) with phosphino-oxime ligand (186) in the presence of toluene was heated under reflux overnight as an alternative approach for preparing Ru complex (C65). The resulting complex (C65) was isolated with a 75% yield. Other aromatic, heteroaromatic, aliphatic, and, unsaturated aldoximes were used to test the flexibility of the most active catalyst (C65). The Ru complex (C65) is found to be very effective in catalyzing the conversion of aldoximes to amides in high yields (Scheme 75).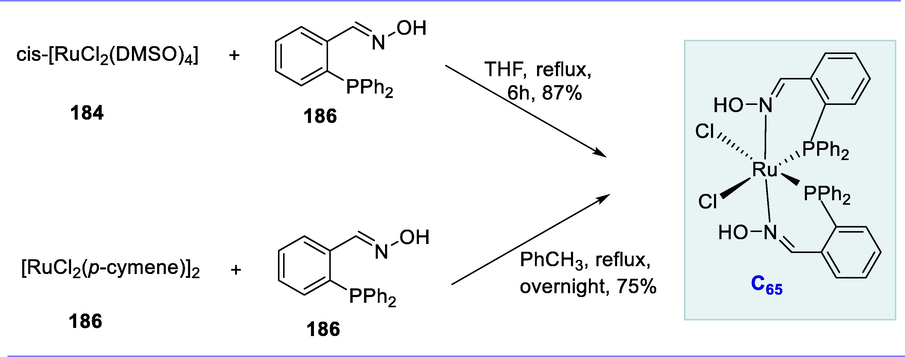
Synthesis of phosphino-oxime containing Ru(II) complex.
The aldoxime (187) and water mixture were added to a Teflon-capped sealed tube containing complex (C65), and the reaction mixture was stirred at 100°C for 5-10 minutes, producing excellent yields of corresponding amides (188). The electronic properties of the phenyl rings were shown to influence the activity of complex in the family of substituted benzaldoximes, with those having electron-withdrawing groups reacting faster. Ortho-substituted substrates showed lower reactivity than their para- and meta-substituted substrates due to steric grounds (Scheme 76) (Francos et al., 2016).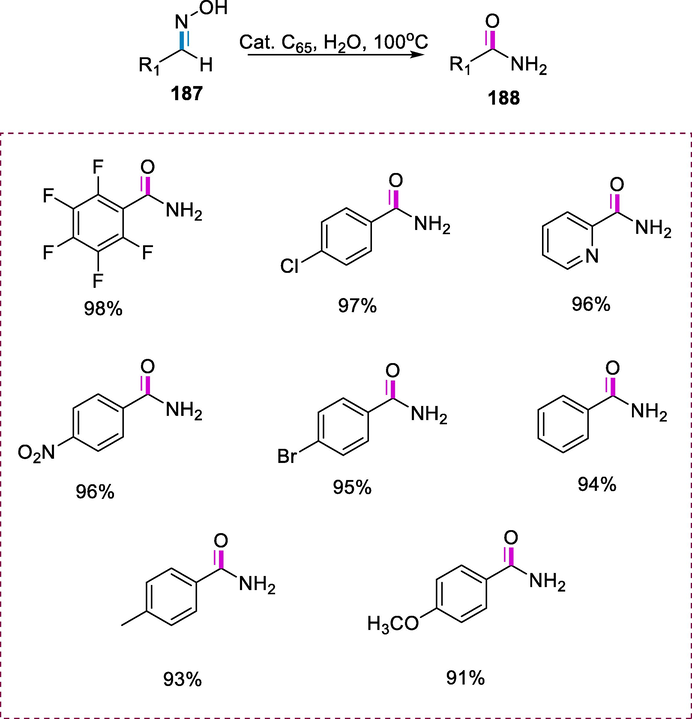
Aldoximes rearrangement into primary amides.
The Ru complex with the phosphino-oxime 2-Ph2PC6H4CH=NOH (C65) ligand catalyzes the reduction/α-alkylation of acetophenones in the presence of primary alcohols very efficiently (Lundberg and Adolfsson, 2016). Alcohols have been used as alkylating agents in the alpha-alkylation of methyl ketones, and both homogeneous and heterogeneous catalysts have been used (Guillena et al., 2007; Td, 2009; Obora and Ishii, 2011; Obora and Catal, 2016).
The acetophenones (189) undergo reaction with alcohols (190) in the presence of KOH, complex (C65) as a catalyst, and toluene as a solvent at 120°C for 3h resulting in the synthesis of secondary aryl-carbinols (191) in 77-91 percent yields. The variety of substituted ketones and alcohols was well tolerated by this reaction (Scheme 77) (Francos et al., 2016).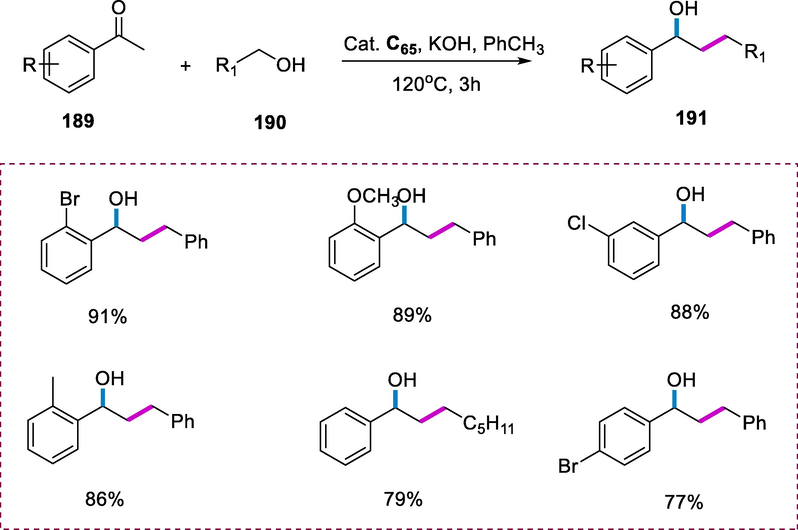
Reduction of acetophenone.
6 Schiff Base-Ruthenium Complexes
In the targeted chemical transformations, the Schiff base ruthenium complexes have attractive catalytic properties, including enhanced activity and selectivity (Dragutan et al., 2000; Dragutan et al., 2001; Katayama and Ozawa, 2004; Rigaut et al., 2004; Winter and Záliš, 2004). The use of Schiff bases ligands to develop new selective and active sites in ruthenium catalytic species has proven to be very attractive (De Clercq and Verpoort, 2001; Vigato and Tamburini, 2004). Since they are excellent catalysts in heterogeneous and homogeneous catalysis activity (Deshmukh et al., 2017). A large number of Schiff bases and their ruthenium (II) complexes have been broadly investigated. Schiff bases are the most extensively used as ligands in inorganic chemistry due to their excellent sigma-donor and pi-acceptor properties. The electron acceptor and electron donor features of the ligand affect the coordination compounds' reactivity and have a significant influence on this activity owing to the relationship between reactivity and structure (Tümer, 2011; Çalık et al., 2016; Jia et al., 2017). These novel ruthenium complexes can be easily prepared from very stable and readily available Ru-compounds, have high chemoselectivity and activity in chemical transformations, and have excellent tolerance to organic functionalities, air, and moisture (Nguyen et al., 1992; Schwab et al., 1996).
6.1 Synthesis of Schiff-base substituted ruthenium benzylidene complexes and their applications
Metathesis reaction is a chemical reaction in which two hydrocarbons such as alkane, alkynes, alkenes are transformed into new hydrocarbons by the exchange of C-C bond. It is widely explored by Grubbs (Nguyen et al., 1992; Fu et al., 1993; Scholl et al., 1999b; Scholl et al., 1999a; Louie et al., 2001; Sanford et al., 2001; Trnka and Grubbs, 2001; Love et al., 2002; Michrowska et al., 2004; Lippstreu and Straub, 2005; Colacino et al., 2007; Hong et al., 2007; Alcaide et al., 2009; Vorfalt et al., 2010). The ring-closing metathesis (RCM) reaction has broad scope and reliability and effectively simplified the total synthesis of a broad range of structurally complicated artificial and natural products (Fürstner and Langemann, 1996; Scholl and Grubbs, 1999; Dixon et al., 2000; Paquette et al., 2000). Schiff base ligands containing Ru complexes have very high excellent stability with activity. Furthermore, the electronic and steric environment of the Schiff base has a significant impact on the catalytic activity of these catalysts.
The well-established two-step synthesis of arene Ru-complexes (C66-C68) having the bidentate Schiff base N,O bonding system and linked with the p-cymene motif was successful. The Schiff bases were produced in the first stage by condensation of aliphatic amines (193) with salicylaldehydes (192) in THF under reflux for 2h. The crude yellow, viscous oily product was separated by column chromatography resulting in good yields of salicylaldimines 194 (Schiff bases) (90-99%). Then these Schiff bases (194) were transformed into the respective thallium salts (195) by treating them with thallium ethoxide in the presence of THF at room temperature for 2h under nitrogen to make the arene Ru-complexes (C66-C68). The salts were then employed in the second reaction step, which was carried out at room temperature for 6h with [RuCl2(p-cymene)]2 (196) in the presence of THF resulting in the synthesis of Schiff base ruthenium arene complexes (C66-C68). The complexes are highly multifunctional catalysts to promote ring-closing metathesis reaction and Kharasch addition. (Scheme 78) (Drozdzak et al., 2005c).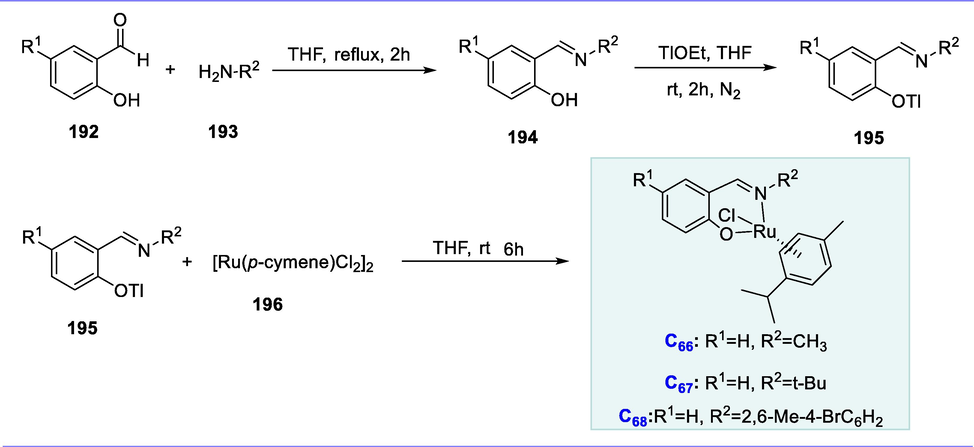
Synthesis of Schiff-base substituted ruthenium benzylidene complexes.
The tri- and tetrasubstituted cycloalkenes (198) are synthesized from the respective dienes (197) using a 5 mol% catalyst (C66-C68) in toluene and trimethylsilyldiazomethane (added to generate the metal-carbene) at 70°C. The activity of these complexes is sufficiently high at 70°C. The three catalysts were formed in moderate to good yields even under more harsh conditions. The strongest, non-transferable chelated Schiff base ligand is present along with the coordinatively stable p-cymene group in complexes (C66-C68) which is favorable for their catalytic activity. The capacity of catalysts in RCM is clearly influenced by substituents linked to the N-atom in the Schiff base complex (C66-C68) (Scheme 79) (De Clercq and Verpoort, 2001).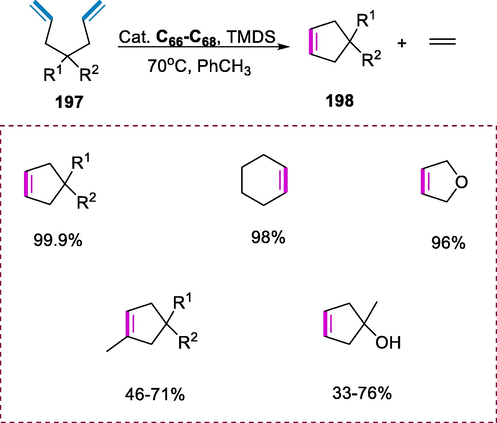
Ring-closing metathesis.
There are several Schiff base-ruthenium that are used as pre-catalysts to perform the Kharasch addition reaction and also known as atom-transfer radical addition (ATRA) of carbon tetrachloride to a variety of olefinic substrates (De Clercq and Verpoort, 2002c; De Clercq and Verpoort, 2002d). The Kharasch reaction of CCl4 across olefins was mediated by Ru-Schiff base complexes (C66-C68) with high yields that were very dependent on the catalyst and substrate utilized (De Clercq and Verpoort, 2002c). Standard conditions were used for the Kharasch addition reactions. The catalytic system and the olefin used appeared to have a significant effect on the reaction's outcome.
Complexes (C66-C68) have been shown to successfully catalyze the Kharash addition of haloalkanes (200) into olefins (199) resulting in the formation of polyhalogenated alkanes (201). The styrene and methylmethacrylate undergo clean mono-addition of carbon tetrachloride by using the best catalytic complex (C68) for 17h at 65°C affording up to 88 and 73 percent product respectively. When the reactions were carried out with diethylallylmalonate, the various catalytic complexes were distinguished. Methacrylates convert more smoothly than acrylates with all three catalytic systems. This reaction was also proceeded at 85°C instead of 65°C to achieve better results in the presence of the best catalytic system (C68) for 17 h. In this instance, all substrates were transformed at a higher rate. The best substrate for these catalysts is styrene, which was almost quantitatively transformed, while methylmethacrylate was also converted to an extent of up to 84 percent. The complexes (C66-C68) are multifunctional catalysts that promote Kharasch addition reactions with great efficiency. It is worth noting that complexes (C66-C68) can easily catalyze the Kharasch addition (ATRA) reaction without the use of a co-catalyst (Scheme 80) (De Clercq and Verpoort, 2002a; Drozdzak et al., 2005b).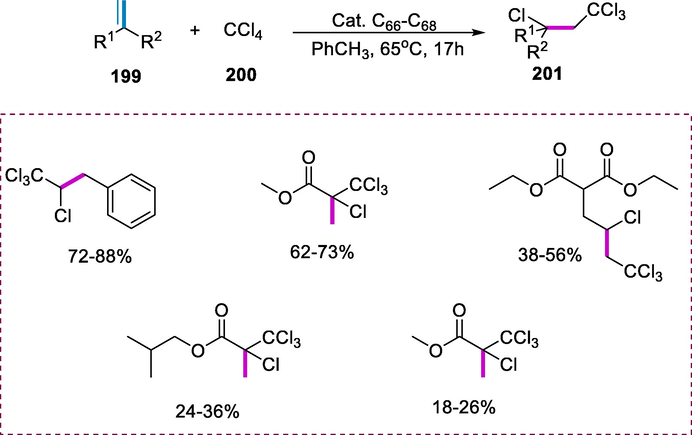
Kharasch addition reaction.
6.2 Synthesis of ruthenium complexes containing Schiff base and N-heterocyclic carbene (NHC) ligand and its applications
The Ru-complexes containing Schiff base and N-heterocyclic carbene (NHC) ligand is effective in catalyzing enol ester synthesis reactions that were highly dependent on the catalyst and substrate used (Opstal and Verpoort, 2002).
The synthesis of complexes C70(a-c) was accomplished by replacing the phosphane in the known complexes C69(a-c) with a bulky 1,3-dimesityl-4,5-dihydroimidazol-2-ylidene group (203) which resulted in excellent yields of compounds C70(a-c) as brown solids. The number of imidazolylidene compounds is stable as their free carbene. The free imidazolylidene carbene was formed in situ using the tetrafluoroborate salt and KOtBu as a base. The suspension of tetrafluoroborate salt (202) reacts with potassium tert-butoxide in the presence of THF at room temperature to produce an intermediate (203). A syringe was used to add one equivalent solution of complexes C69(a-c) in dry PhCH3 after 5 minutes. Complexes C70(a-c) were obtained as brown microcrystalline solids in good yields upon heating the mixture to 70-80°C for 1 hour (Scheme 81) (De Clercq and Verpoort, 2003).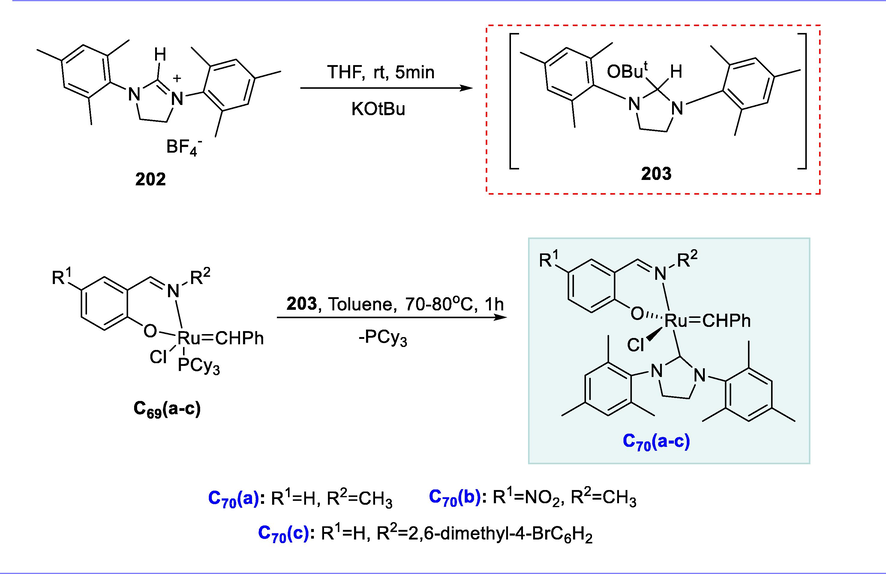
Synthesis of ruthenium complexes containing Schiff base and N-heterocyclic carbene (NHC) ligand.
The regioselective addition of carboxylates (205) into terminal alkynes (206) by using toluene at 100°C provides direct access to enol esters (207) of anti-Markovnikov and Markovnikov types respectively. When octadiyn was utilized as a substrate then the addition of carboxylic acids results in the selective synthesis of (E)-alk-1-en-yl esters respective to a stereo- and regioselective anti-Markovnikov type addition of the acid along with the triple bond. The (E)-alk-1-en-yl ester was obtained in good yield varied between 74 and 83 percent for both acids and all six catalytic systems. The total yield is highly reliant on the acid and catalyst utilized. A small amount of the (Z)-alk-1-en-yl ester is formed in addition to the (E)-alk-1-en-yl ester (Scheme 82) (De Clercq and Verpoort, 2003).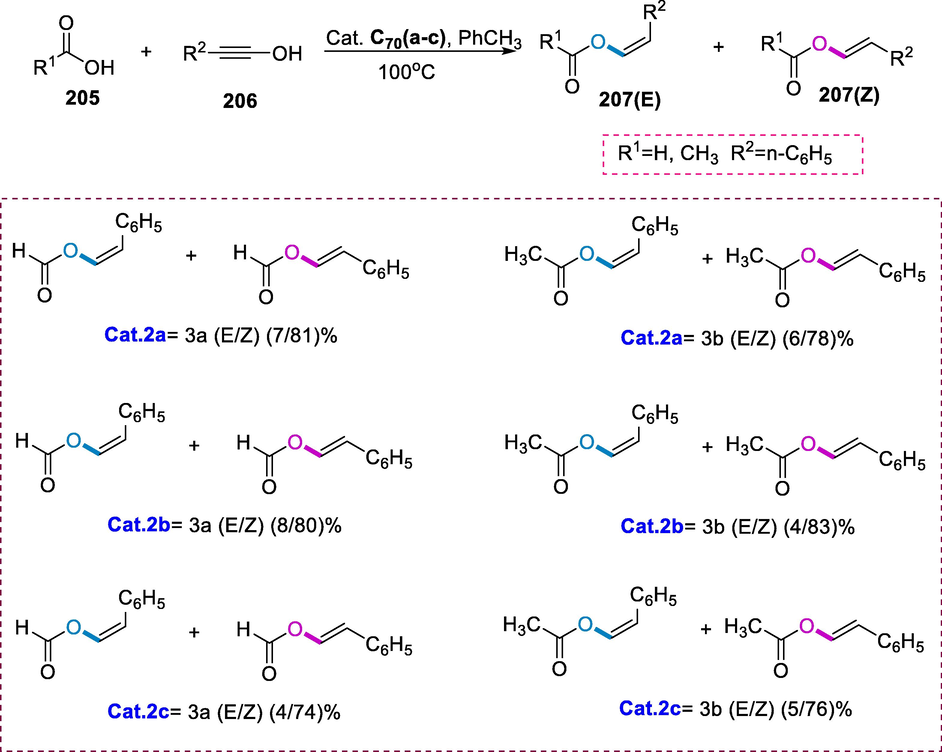
Enol-ester synthesis.
Ring-opening metathesis polymerization (ROMP) has paved the way for novel synthetic methods for a wide range of polymeric materials, resulting in polymers with attractive mechanical and electrical properties (Brintzinger et al., 1995; Ivin and Mol, 1997; Buchmeiser, 2000). The ruthenium-based catalysts proved to be effective in catalyzing ROMP reactions when combined with Schiff base ligand and 1,3-dimesityl-4,5-dihydroimidazol-2-ylidene (De Clercq and Verpoort, 2002b).
The catalytic systems C70(a-c) are successful in ring-opening metathesis polymerization reactions with 5-substituted norbornenes (208) to give ring-opening products (209). In these complexes, the electron-poor properties of the Schiff base substituents and the bulkiness of the Schiff base have a significant impact on the ROMP activity. In ROMP reactions, increasing the steric hindrance of the Schiff base has a detrimental effect on the activity of the catalyst. For example, cyclohexenylnorbornene is converted with yields of 100 and 83 percent respectively when treated with complexes C70(a and c). The substrates having electron-poor nitro substituents undergo systematically lower conversions due to the influence of the electronic environment of Schiff bases in complexes C70(a and c). Furthermore, the steric hindrance of the Schiff base has a greater influence on ROMP catalytic activity than the influence of Schiff base substituents electronic environment (Scheme 83) (De Clercq and Verpoort, 2002b).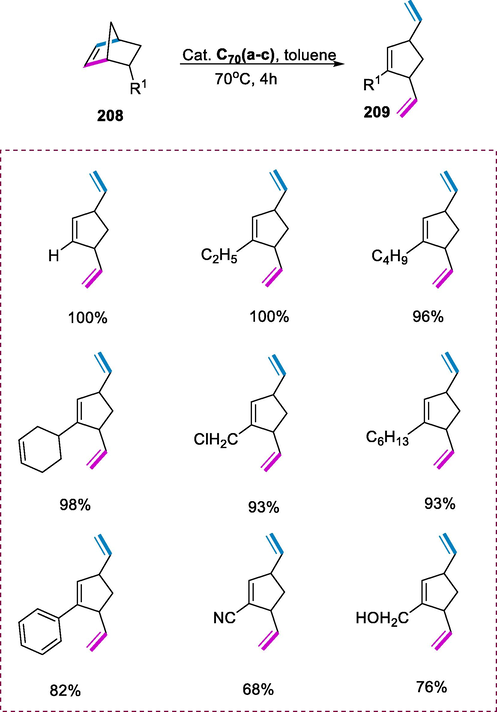
Ring-opening metathesis polymerization reaction.
6.3 Synthesis of Ru(II) Schiff-base complexes containing pyridine ligand and its application
The ruthenium (II) complexes were used as catalysts for the hydrogenation of a variety of ketones in a 2-propanol solution. The catalytic reactions showed that Ru(II) complexes comprising Schiff base ligands containing pyridine were effective for transfer hydrogenation as catalysts in the presence of various bases (Gichumbi et al., 2016; Ramos et al., 2019).
Condensation of 6-tertbutyl 3-ethyl 2-amino-4,5-dihydrothieno[2,3-c]pyridine-3,6(7H)-dicarboxylate with various substituted benzaldehydes yielded Schiff base ligands (210). Ruthenium (II) complexes C71(a-d) were formed by reacting the Schiff base (210) with the metal salt [RuCl2(p-cymene)]2 (211) in methanol at 70°C for 4-5 hours. The transfer hydrogenations of acetophenone derivatives were catalyzed by a series of new Ru(II) complexes containing pyridine group-based Schiff bases. These complexes having both electron poor and electron rich groups coordinating to the aryl ring displayed extremely high catalytic activity. Instead of the electrical effect of groups contained in the structure of these Ru(II) complexes employed, this is likely depending on the structure of the ketone that was used as the substrate. (Scheme 84).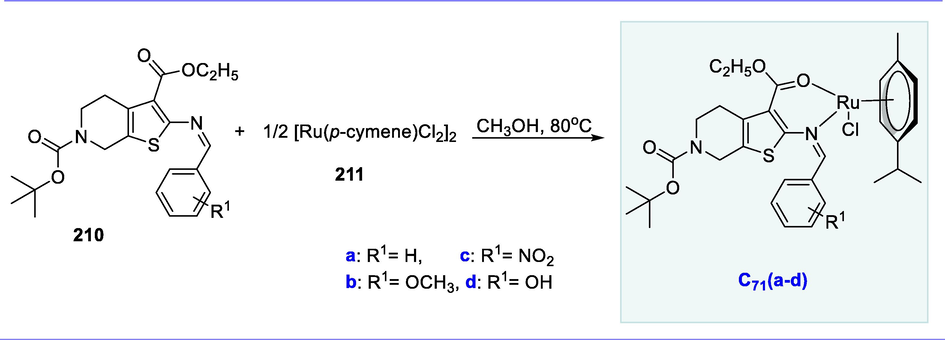
Synthesis of Ru(II) Schiff-base complexes containing pyridine ligand.
Catalytic transfer hydrogenation of various ketones (212) (m-methoxyacetophenone, acetophenone, p-bromoacetophenone, benzophenone, and p-methoxyacetophenone) using ruthenium (II) Schiff base complexes C71(a-d) as a catalyst by using KOH base and iso-propanol as H2 donor by refluxing at 80°C for 8h results in the synthesis of respective secondary alcohols (213). The base is used as a promoter to proceed with this transformation. The transfer hydrogenation reactions, on the other hand, required only 8h with an additional base. The strong inorganic bases (such as KOH and NaOH) give better conversion results in the corresponding alcohol products in excellent yields. The weak inorganic bases (such as Cs2CO3, Na2CO3, KOBut, and K2CO3), on the other hand, were shown to be less effective than strong bases. KOH is thought to be a better base. The electronic effects of the groups in the ligand structure and the structures of the ketones used are the factors responsible for the high conversion (Scheme 85) (Buldurun and Özdemir, 2020).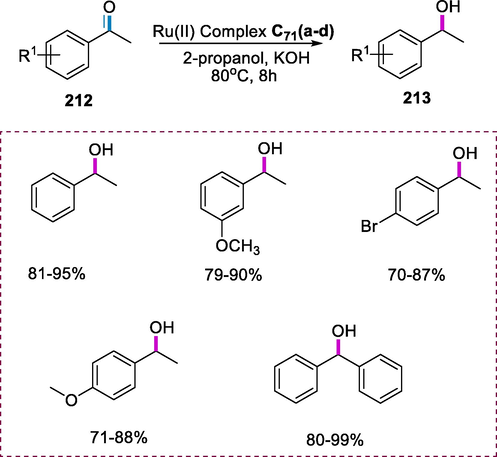
Hydrogenation of ketones.
6.4 Synthesis of bidentate Schiff-base ruthenium complexes and their applications
The effective oxidation of alcohols into corresponding ketones or aldehydes is significant in synthetic chemistry (Yamaguchi and Mizuno, 2010; Parmeggiani and Cardona, 2012; Steves and Stahl, 2013; Ballester et al., 2014; Cardona and Parmeggiani, 2014). Carbonyl compounds and their derivatives are important and versatile building blocks in the formation of a variety of medicinal and fine chemicals (Spasyuk and Gusev, 2012; Koh et al., 2015; Hu et al., 2016b). Acceptorless alcohol dehydrogenation (AAD) reactions are important in the synthesis of carbonyl compounds without the use of oxidants (Gunanathan and Milstein, 2013; Chelucci, 2017; Crabtree, 2017; Li et al., 2019).
The Schiff base bidentate ligands RN=CH-(2,4-(tBu)2C6H2OH (R= C6H5, L1; R = 4-MeC6H4, L2; R = 4-ClC6H4, L3; R = 4-BrC6H4, L4) were produced via condensation reactions of 3,5-di-tert-butyl-2-hydroxybenzaldehyde with the respective primary amine. The RuHClCO(PPh3)3 (214) reacts with Schiff bases (L1-L4) under reflux in THF in the presence of a base (trimethylamine) giving the Ru(II) complexes C72(a-d) in isolated yields ranging from 42 to 57 percent. The complex C72(b) was then prepared by reaction of Schiff base bidentate ligand with ruthenium complex in which one PPh3 was replaced with one Cl in the initial ruthenium compound. These complexes were shown to be air and moisture stable. These complexes were shown to be efficient catalytic activity in the acceptorless dehydrogenation of secondary alcohols and good compatibility of functional groups yielding the respective ketones in 82-94 percent yields. This type of catalytic system can work in mild reaction conditions and tolerates a broad variety of functional groups. It offers an environmentally friendly approach to synthesizing ketones (Scheme 86).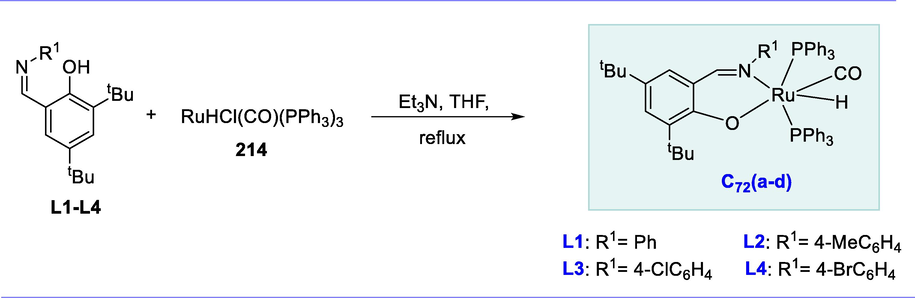
Synthesis of bidentate Schiff base ruthenium complexes.
Secondary alcohols (215) are reacted with Ru Schiff base catalysts C72(a-d) utilizing t-BuOK as a base in the presence of toluene as a solvent at 110°C for 12 hours under an N2 environment to produce excellent yields of respective ketones (216). The variety of substituted 1-phenylethanols with electron-donating and electron-withdrawing groups on the aryl ring, such as 1-(naphthalen-2-yl)ethan-1-ol can be transformed into corresponding ketones. It is worth mentioning that this catalytic system also dehydrogenated aliphatic alcohols very well providing the aliphatic ketones in good yields (>80%). The complexes C72(c and d) having electron-poor substituents on the aromatic ring of the Schiff base ligand are slightly more effective than complexes C72(a and b) with electron-donating substituents. The yield increased dramatically when organic bases (such as DBU, t-BuOK, and DABCO) were used, and t-BuOK was shown to be far more efficient than the other organic bases. The limited solubility of inorganic bases in toluene affects their performance less than that of organic bases. The PhCH3 was found to be very effective for this transformation. Xylene even at a higher temperature under reflux gives very low yields in other solvents such as THF, CH2Cl2, 1,4-dioxane, or acetone as compared to toluene (Scheme 87) (Hao et al., 2021).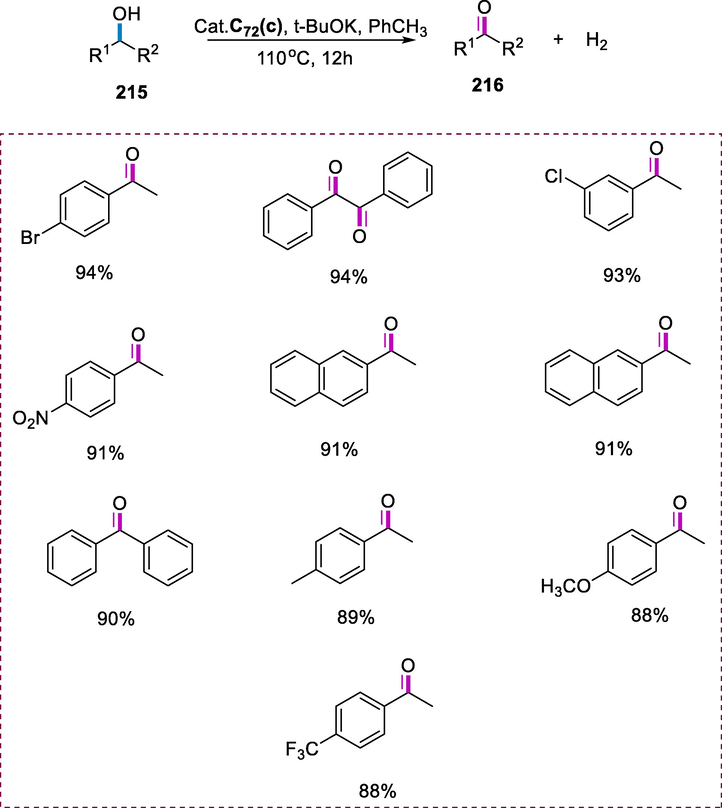
Dehydrogenation by secondary alcohol.
Ruthenium complexes are very effective at catalyzing the nitroarenes hydrogenation into aromatic anilines by using a reducing agent such as sodium borohydride in an ethanol solvent (Indra et al., 2011; Tomkins et al., 2015). Aromatic anilines are used in the synthesis of medicines, pigments, agrochemicals, and dyes as intermediates and precursors (Tafesh and Weiguny, 1996; Vaidya et al., 2003). Complex C72(c), in particular, was found to be a very effective catalyst for the hydrogenation of nitroarene compounds with a broad substrate scope functional group compatibility.
The reduction of 4-nitroanisole by using a reducing agent (NaBH4) under homogenous conditions proceeds in the presence of Schiff-based Ru-complexes. The Ru-complex C72(c) was discovered to be extremely effective for reducing nitro compounds. The nitroarene (217) underwent reaction with 0.5mol% of complex C72(c) at 30°C in the presence of four eq. of NaBH4 in ethanol as solvent resulting in the formation of respective amine (218) in good yield. A vast range of aryl functional groups involving electron-withdrawing and electron-donating groups were well tolerated by the reaction. The steric bulkiness of the methyl group on nitroarene appeared to have no impact on the catalytic efficiency of the nitroarene to toluidine conversion. The very sterically crowded 2,6-dimethyl nitrobenzene may be reduced by 95% yield under optimal conditions. The reaction offers broad substrate scope, vast compatibility of functional group, and provides arylated anilines in good to excellent yields (Scheme 88) (Jia et al., 2016).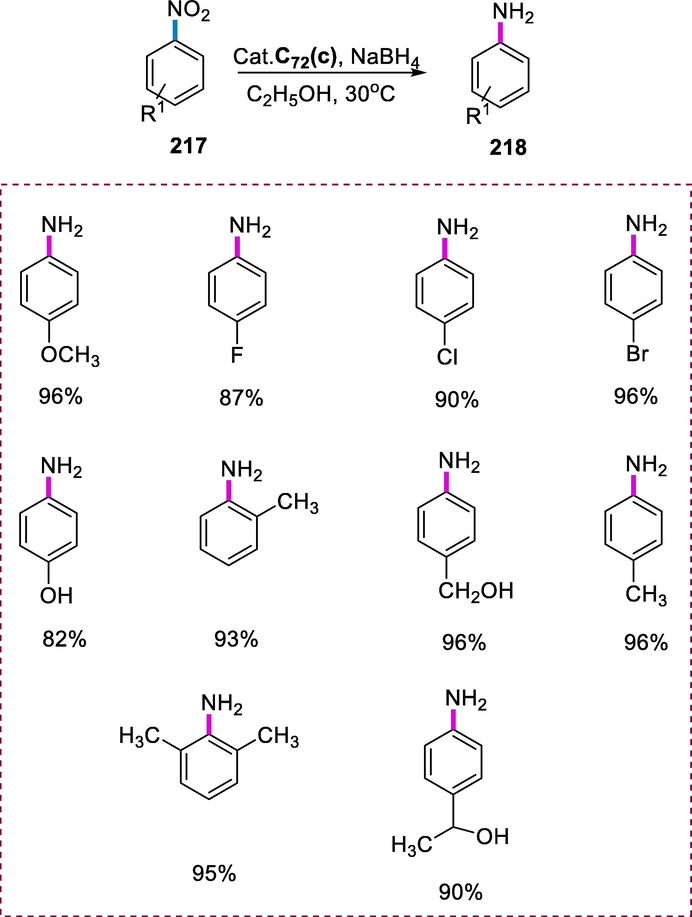
Hydrogenation of nitroarenes.
6.5 Synthesis of Ru(III) Schiff base bis-bidentate complexes and their application
The Schiff base bidentate ligands of the O, N bonding system were used to make a series of new stable Ru(III) complexes [RuCl(PPh3)(L)2] (where L = O, N donor of Schiff bases) that are excellent catalysts in the imine hydrogenation into amines with high to moderate conversions. In terms of selectivity and reactivity, ruthenium Schiff base complexes outperform catalysts with amine/diamine or oxazoline ligands in transfer hydrogenation reactions (Gao et al., 2000; Lindner et al., 2003; Hannedouche et al., 2004).
These Ru complexes C73(a-d) were formed in benzene by reacting Schiff base bidentate ligands (L5-L8) with Ru(III) precursors [RuCl3(PPh3)3] (219) in a 2:1 molar ratio under reflux for 5 hours. The complexes were then purified using CH2Cl2 (60–80°C) to yield a new class of Ru(III) Schiff base bis-bidentate complexes [RuCl(PPh3)(L)2] (L = bidentate Schiff base ligands). These compounds are extremely effective at catalyzing imine transfer hydrogenation (Scheme 89).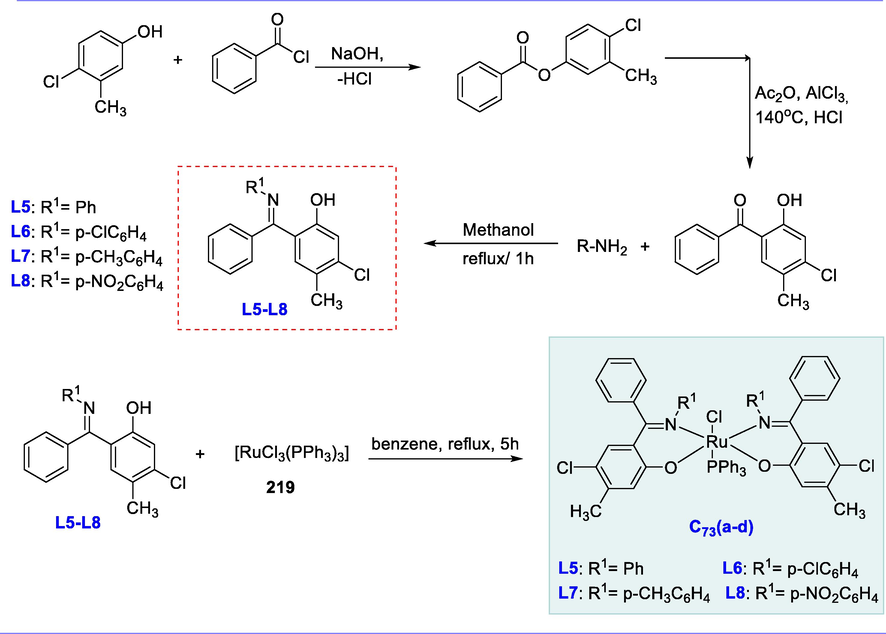
Synthesis of Ru(III) Schiff base bis-bidentate complexes.
The synthesis of corresponding amines (220) arises from the catalytic imine (221) hydrogenation by Ru(III) Schiff-based bis-bidentate complexes [RuCl(PPh3)(L1)2] C73(a-d) by using KOH which is catalyzed by heating to reflux for 3h in 2-PrOH under inert atmosphere. Both aldimines and ketimines were converted to respective amines with high conversion by using these complexes. The arylated aldimine having a p-methoxy group was converted into the corresponding amine after 3h in 78.5% yield and after 10h, it was 92.6 percent. Ketimines are more difficult to convert to amines than aldimines. When aromatic ketamine with electron-donating groups is reduced, better yields are obtained than when aromatic ketamine with electron-withdrawing groups is reduced. The transfer hydrogenation of imines requires the presence of a catalytic quantity of a base (Scheme 90) (Venkatachalam and Ramesh, 2006).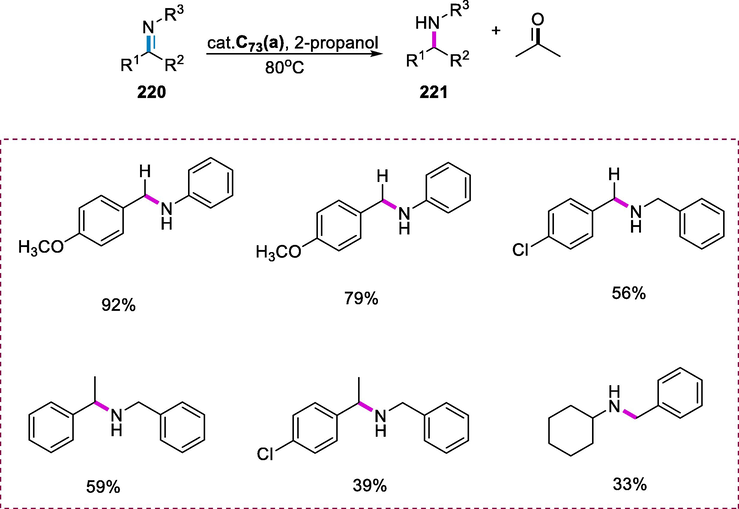
Hydrogenation of imines.
6.6 Synthesis of Schiff base Ru-complexes having p-cymene ligand and its application
The Ru(II) Schiff base complexes showed efficient catalytic activity for the one-pot conversion of aldehydes to amides in the presence of NH2OH.HCl/NaHCO3. Amides are a significant class of organic compounds that have long been used as chemical building blocks in organic synthesis and as functional groups, as well as biologically active compounds and pharmaceuticals (Drozdzak et al., 2005a). Ruthenium complexes have been widely used because of their catalytic importance in a range of organic conversions, such as the imine synthesis from alcohols and amines.
The dimeric [(p–cymene)RuCl2]2 complex (223) reacts with the suitable Schiff base ligands 222(a-d) in CH2Cl2 at room temperature, and the Ru(II) Schiff base complexes C74(a-d) were readily produced in good yields. The complexes C74(a-d) are solid and were stable in air. They are highly miscible in polar solvents but insoluble in non-polar solvents. These Schiff base complexes of ruthenium (II) were produced as reddish-brown solids in excellent yields of up to 90% (Scheme 91).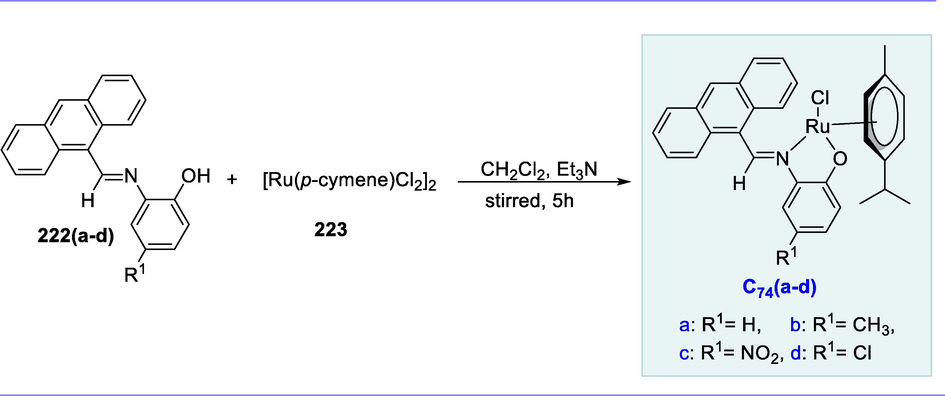
Synthesis of Schiff base Ru-complexes having a p-cymene ligand.
The catalytic conversion of aldehydes (224) into amides (225) is catalyzed by Ru(II) Schiff base complexes C74(a-d) in toluene in the presence of NaHCO3/NH2OHHCl. These complexes are very effective for all Aromatic, heterocyclic aromatic, and conjugated aldehydes. These Schiff-based complexes of Ru(II) effectively catalyze the transformation of aldehydes to their respective amides in a one-pot reaction. In the absence of a catalyst or base, no considerable reaction was seen. Toluene as a solvent and NaHCO3 as a base was found to be the most effective for the catalytic transformation of aldehydes to amines with good yields. The presence of electron-donating groups in the substrates, such as –OH and –OCH3 varies the reactions and the appropriate amides were produced in good yield (87-79%). When compared to substrates having electron-withdrawing groups and electron-donating groups such as the -NO2 and -Cl substituents offer higher yields (98-82%). Among all the complexes, catalyst C74(a) is thought to be particularly effective of transforming aldehydes to amides in high yields (Scheme 92) (Premkumar et al., 2019).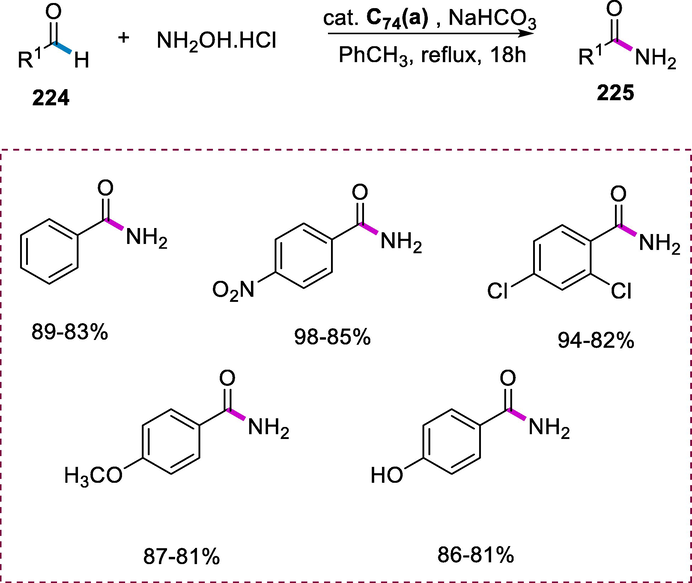
Synthesis of amides.
7 NHC–Ru complexes-Synthesis and applications
Organometallic and inorganic coordination chemistry, N-heterocyclic carbenes are frequently used. They can act as auxiliary ligands in the growing number of transition-metal catalysts, or even as a catalyst and nucleophilic reagents in a variety of organic syntheses have gotten a lot of attention (Crabtree, 2005; Díez-González and Nolan, 2005; Lai et al., 2005; Singh and Nolan, 2005; Crabtree, 2006). The NHC complexes have exhibited extraordinary stability toward moisture, air, and heat because of their great sigma-donating ability (Egbert et al., 2011; Fortman and Nolan, 2011). NHC ligands were produced and demonstrated excellent catalytic activity in C-C bond coupling, hydrosilylation, and hydrogenation reactions.
7.1 Synthesis of pyridazine-based N–heterocyclic carbene diruthenium complex and its application
The bimetallic N-heterocyclic carbene (NHC) complexes have a high catalytic activity for alkene oxidation processes (Zhou et al., 2008b; Liu et al., 2010). The Ru....Ru cooperative effect is considered to be liable for the strong activity of the di-ruthenium complex (C75) towards alkene oxidation. The pyridazine moiety can binucleate, the high donating ability of carbene, and hexadentate characteristic of structure all lead to ensuring the oxidation state stability of complex (C75) which could potentially increase its catalytic efficiency in oxidation transformations.
The imidazolium salt of the functionalized pyridazine bis(NHC) ligand precursor (H2L(PF6)2) 228 was produced by reacting the 2-(chloromethyl)pyridine (227) and 3,6-di(1H-imidazol-1-yl)pyridazine (226) in DMF under heat followed by the exchange of anion in water. The salt of imidazoline (228) was obtained in the form of a white solid with a moderate yield (64%). The colorless solution of [Ag6(L)4]-(PF6) was obtained by reacting the imidazolium salt H2L(PF6)2 (L = 3,6-bis-(N-(pyridylmethyl)imidazolylidenyl)pyridazine) with Ag2O in the presence of CH3CN at 50°C. The diruthenium complex [Ru2Cl(L)(CH3CN)4](PF6)3 (C75) was obtained by adding [Ru(p-cymene)Cl2]2 complex to solution. The dinuclear ruthenium (II) complex has efficient catalytic activity for the oxidation of the alkene into diketones(Scheme 93).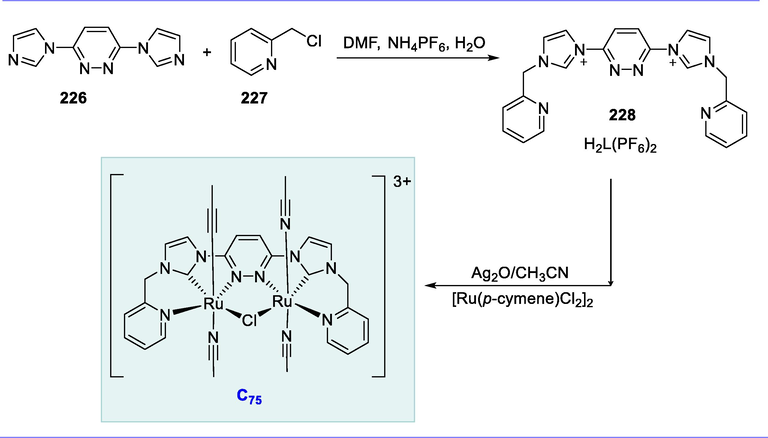
Synthesis of pyridazine-based N-heterocyclic carbene diruthenium complex.
The reaction mixture containing alkene (229), complex (C75), and NaI was stirred in 3.0mL of CH3CN. Then add the TBHP as an oxidant to this solution with the help of a syringe at r.t. The reactions required only 10 minutes to complete at room temperature. The alkenes were transformed into their diketone (230) counterparts. Acetonitrile was found to be a better solvent than toluene for obtaining good yields, and TBHP was utilized as an oxidant. Alkenes with electron-rich groups yielded better yields than those with electron-poor groups. It is worth noting that N-substituted indoles were often used successfully resulting in high yields. Furthermore, heterocyclic alkenes like those containing carbazole and thiophene can be used to give the target product in moderate yields (Scheme 94) (Liu and Chen, 2012).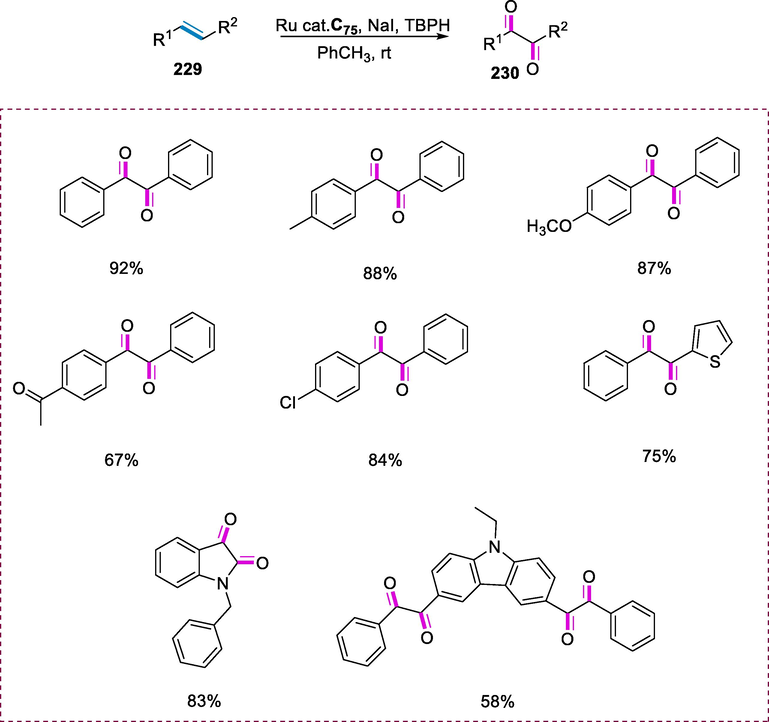
Alkene oxidation.
7.2 Synthesis of benzimidazole-based ruthenium(II)-NHC complexes and their applications
The borrowing hydrogen method was found to be efficient with Ru-complexes that have strong sigma-donor ligands, such as N-heterocyclic carbenes (NHC) (Hamid et al., 2007; Td, 2009; Kerdphon et al., 2015; Corma et al., 2018). Borrowing hydrogen (BH) for the alkylation of ketone enolates with a ruthenium(II)-NHC catalyst has emerged as a potent and environmentally friendly method for forming C-C bonds (Gunanathan and Milstein, 2013; Ketcham et al., 2014; Huang et al., 2016). Primary alcohols were employed as alkylating agents and could be used in a one-to-one ratio with the ketones (Srimani et al., 2012b; Xu et al., 2014; Elangovan et al., 2015). Compared to previous methods, the Ru-NHC complexes catalyzed by the borrowing hydrogen strategy offer several advantages including low cost, high atom efficiency, and H2O as a byproduct (Yan et al., 2014). The pharmaceutically active intermediate core moiety was produced in stages using the hydrogen auto-transfer process.
The reaction of anhydrous K2CO3 with 5,6dimethylbenzimidazole (231) in the presence of acetonitrile proceeds by the addition of alkyl halide in stoichiometric quantity under reflux for 16h producing the appropriate benzimidazolium salt 232(a-f) in good yield (70–90%). The Ru (II)NHC complexes C76(a-f) were made using a silver-NHC intermediate 233(a-f) formed by reacting the silver(I)oxide with ligand precursor to produce Ag-NHC. The Ag-NHC 233(a-f) was then treated with ruthenium(II) arene precursors and stirred at r.t. for 6h. Then, by adding pet-ether into the filtrate, brown ruthenium(II)-NHC complexes C76(a-f) are precipitated in high yield. The complex C76(a) demonstrated excellent catalytic efficiency with 92 percent yield of alkylated product. This complexes have smaller wingtip as compared to other complexes C76(a-f) having bulky wingtips along with arene lability probably effect on hydrogen borrowing reaction in α‐alkylation. Based on findings, it can be seen that the yield of acetophenone's alkylation was slightly reduced when compared to catalysts C76(a-f) with various arene moieties and wingtip, in the following sequence: benzene moiety-ethyl wingtip C76(a) > p–cymene moiety-ethyl wingtip C76(d) > benzene moiety-cyclohexylmethyl wingtip C76(b) > p-cymene moiety-cyclohexylmethyl wingtip C76(e) > benzene moiety-benzyl wingtip C76(c) > p-cymene moiety-benzyl wingtip C76(f) (Scheme 95).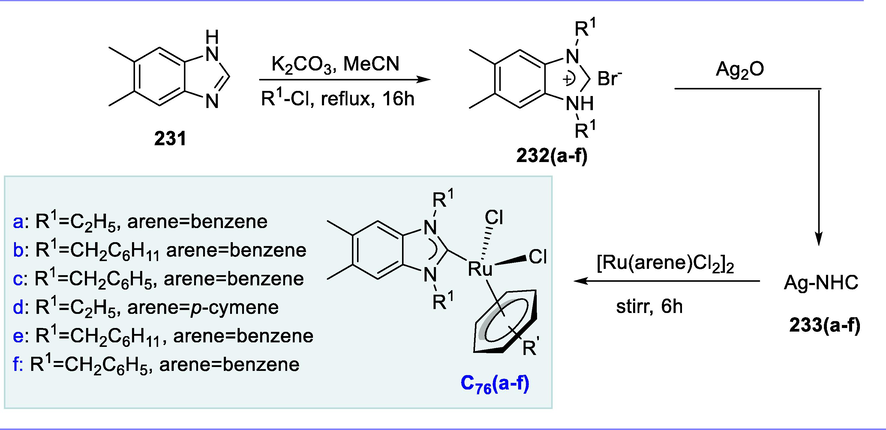
Synthesis of benzimidazole-based ruthenium(II)-NHC complexes.
Ketones (234) react with alcohols (235) in the presence of complex C76(a), NaOH, and toluene at 105°C for 8 hours, resulting in high yields of alkylated ketones (236). Compound C76(a) demonstrated remarkable catalytic activity. The optimal reaction conditions for alpha-methylation of ketones were NaOH/toluene at 105°C. The yield of the product was dramatically reduced when the reaction proceeded at low temperatures. When compared with other bases used in this reaction, the catalyst reactivity was determined to be efficient by using NaOH. The substrate with electron-donating substituents improves the alkylation reaction with benzyl alcohol which results in excellent yields of respective products (86-92%). In the case of the substrate having electron-withdrawing substituents reduce the yields of alkylated products by 79-86%(Scheme 96) (Ortega et al., 2013).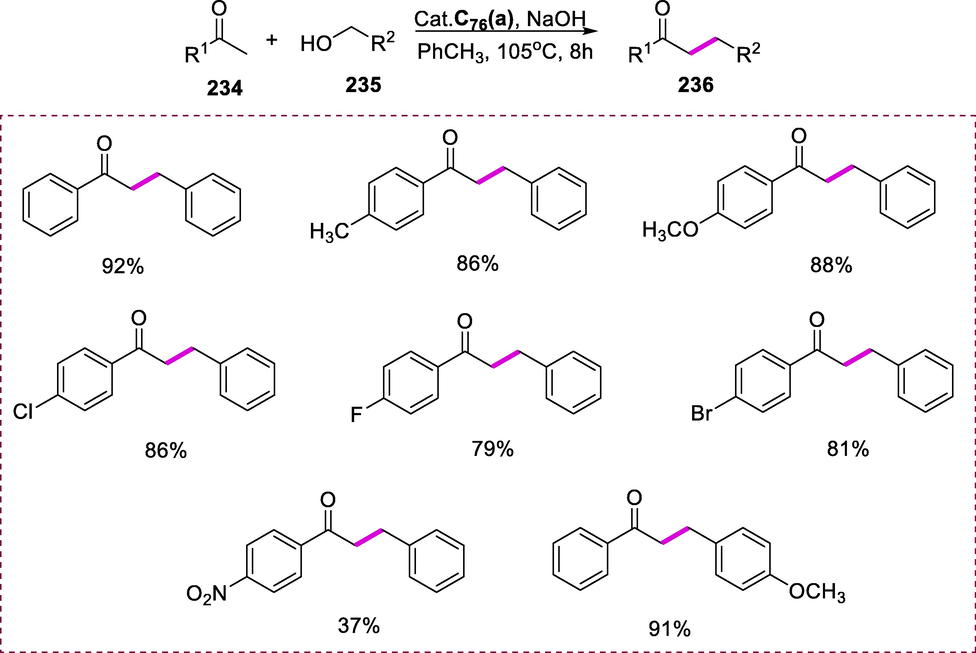
α-Alkylation of methylene ketones.
The pharmaceutically active intermediate core moiety was synthesized successfully using the hydrogen auto-transfer technique (Leonard et al., 2015). The arene ruthenium(II)-NHC complexes were used as efficient catalysts in the acceptorless dehydrogenative coupling of 2aminobenzyl alcohol and ketone to produce quinoline derivatives (Sindhuja et al., 2012; Sindhuja et al., 2014; Manikandan et al., 2016; Manikandan et al., 2018). Quinoline derivatives are generally found to be excellent candidates for heterocyclic compounds. These scaffolds have significant applications in pharmacology (antitubercular, anticancer, antipsychotic, antibacterial, antiviral, and neurodegenerative disease treatment), material chemistry (OLED complexes and chemo sensors), and catalysis (asymmetric synthesis) (Kim et al., 2005; Jin et al., 2011; Solomon and Lee, 2011; Keri and Patil, 2014; Vlahopoulos et al., 2014; He et al., 2015).
Ketones (237) react with 2-aminobenzyl alcohols (238) in the presence of catalyst C76(a), NaOH, and toluene at 105°C for 8h, yielding quinolines and their derivatives (239) in good yields. The optimal reaction conditions for quinolines derivatives synthesis were NaOH/toluene at 105°C. The yield of the product was dramatically reduced when the reaction proceeded at low temperatures. Under ideal reaction conditions, a vast range of aliphatic, aromatic ketones, and their derivatives offer good yields. The cyclization of 2-aminobenzyl alcohol with ketones includes an electron-rich substituent at the para-position affording an excellent yield (90–92%). The ketones with an electron-poor substituent at the para-position afford the appropriate products in 88% yields (Scheme 97) (Balamurugan et al., 2019).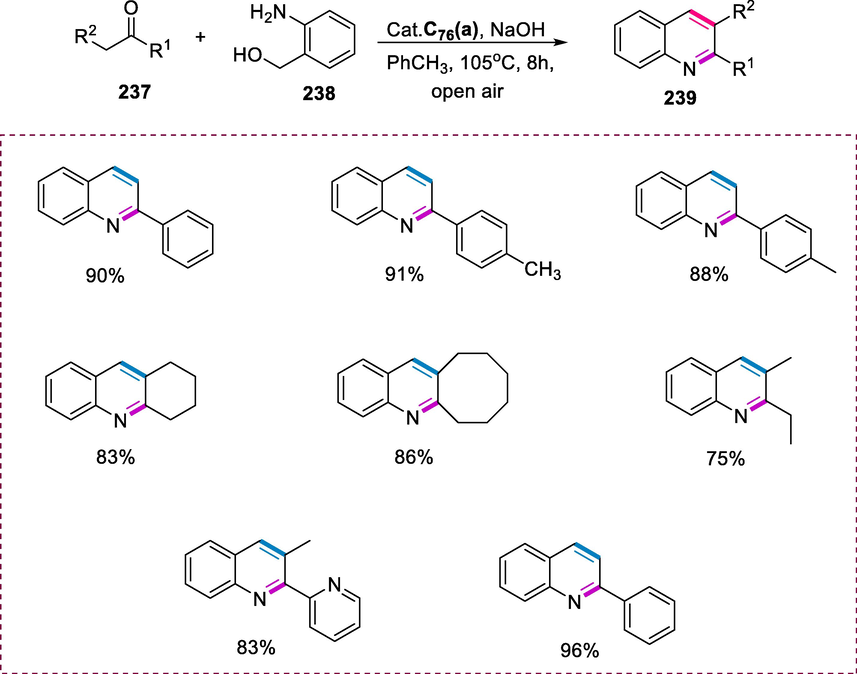
Synthesis of quinolines.
7.3 Synthesis of Ru(II) NHC carbonyl complex and its application
The amide bonds are important functionality in both natural and synthetic systems. Amido linkages can be found in biopolymer proteins and a variety of synthetic polymers (Lectka, 2001; Peptides, 2002; Pattabiraman and Bode, 2011). Madsen introduced the ruthenium N-heterocyclic carbene (NHC) complex for amidation reactions (Nordstrøm et al., 2008; Dam et al., 2010; Makarov et al., 2012).
The Ru(II) NHC carbonyl complex [Ru(py-NHC)(CO)2Br2] (C78) was obtained by treating 3-methyl-1-(pyridine-2-yl)-imidazolium bromide (240) with [Ru2(CO)4(CH3CN)6](BF4)2 complex (241) by using tetrabutylammonium bromide (TBABr) in dichloromethane at r.t. Catalyst (C78) shows a wide substrate range for phosphine-free coupling of amines and alcohols into amides at moderate catalyst loading (Scheme 98).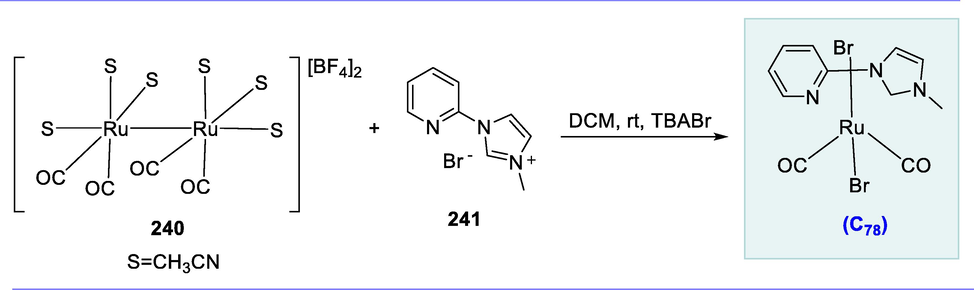
Synthesis of Ru(II) NHC carbonyl complex.
The catalytic activity of complex (C78) for coupling of amines (242) and alcohol (243) via dehydrogenation was studied. The reaction of amine and alcohol in toluene solvent for 24 hours at 110°C by using a catalyst (C78) and NaH gave an amide (244) as the only product in excellent yields. The base is required as a promoter for this transformation although strong bases such as KOH, KOtBu, and NaH were found to be effective. The scope of the substrate was investigated under optimal conditions (1mol% catalyst (C78), alcohol, amine, 5mol% NaH at 110°C in PhCH3 for 24h). The electron-donating p-methoxybenzyl alcohol with benzylamine, p-methylbenzylamine, cyclohexylamine, and hexylamine generated good conversions of the respective amides (90-92%). A variety of amines were tolerated along with the benzyl alcohol and p-methylbenzyl alcohol resulting in moderate to excellent yields of respective amides (69-90%). The yields of electron-withdrawing p-fluorobenzyl alcohol and p-nitrobenzyl alcohol were lower than those of electron-donating alcohols. The straight-chain alcohols such as hexanol and octanol when treated with hexylamine and benzylamine afforded fewer yields than benzyl alcohol (Scheme 99) (Saha et al., 2014).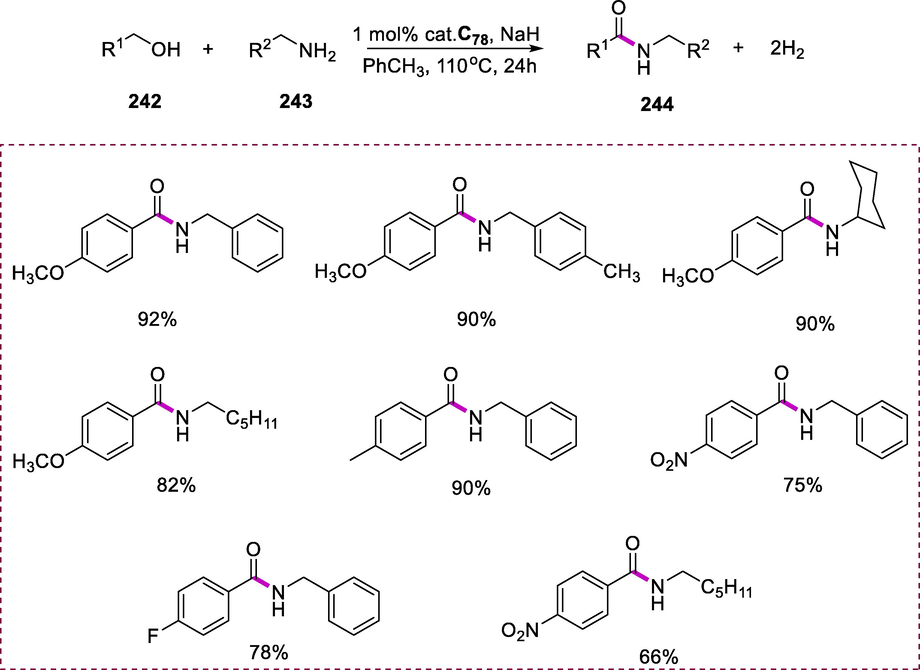
Synthesis of amides.
7.4 Synthesis of ruthenium complexes [RuCl(p-cymene)(iPr-bimy)(PPh3)]PF6 and [RuCl(p-cymene)(Bn-bimy)(PPh3)]PF6 and its application
The dehydrogenation of alcohols catalyzed by transition metals is used to make amides and amines, which are significant in organic chemistry, medicinal chemistry, and biochemistry (Ricci, 2008b; Valeur and Bradley, 2009). Complexes with NHC (N-heterocyclic carbene) ligands are particularly effective in this reaction (Nordstrøm et al., 2008; Ghosh et al., 2009; Zhang et al., 2010; Makarov et al., 2012; Kim et al., 2015). Alcohols are less effective for amines as alkylating agents due to their low electrophilicity. However, utilizing transition metal complexes through borrowing hydrogen strategy makes the N-alkylation with alcohols potentially atom-efficient and safer process (Guillena et al., 2010; Fogler et al., 2011; Fernandez et al., 2012).
The neutral [RuCl2(p-cymene)(bimy)] complexes (C79 and C80) were treated with triphenyl phosphine and KPF6 in acetone to produce ruthenium complexes [RuCl(p-cymene)(iPr-bimy)(PPh3)]PF6 (C81) and [RuCl(p-cymene)(Bn-bimy)(PPh3)]PF6 (C82) . The required complexes (C81 and C82) were obtained in good yields as yellow solids by chlorido displacement (97% for complex C81 and 94% for complex C82). These complexes are soluble in polar aprotic solvents like DMF and DMSO, as well as MeOH, CH3CN, and acetone, but in nonpolar solvents like hexane, diethyl ether, and toluene, they are almost insoluble. Complexes C81 and C82 with the 1,3-dibenzyl benzimidazolin-2-ylidene ligand can catalyze both mono- and di-amination (N-alkylation) via dehydrogenation in the presence of simple amines with alcohols, resulting in a variety of secondary amines. The complex C81 has been shown to be effective and versatile catalyst in the N-alkylation reaction under mild conditions. Additionally, this complex has demonstrated a high level of tolerance for functional groups found in alcohol and amine moieties. (Scheme 100).![Synthesis of ruthenium complexes [RuCl(p-cymene)(iPr-bimy)(PPh3)]PF6 and [RuCl(p-cymene)(Bn-bimy)(PPh3)]PF6.](/content/184/2022/15/11/img/10.1016_j.arabjc.2022.104165-fig106.png)
Synthesis of ruthenium complexes [RuCl(p-cymene)(iPr-bimy)(PPh3)]PF6 and [RuCl(p-cymene)(Bn-bimy)(PPh3)]PF6.
The production of n-alkylated secondary amines (247) arises from the interaction of alcohols (245) with amines (246) by using a catalyst (C81) and NaHCO3 as a base. The required amines were produced in a modest yield after 24h without the use of an additional base at 120°C. The production of amines increased in the presence of NaHCO3 and Na2CO3, respectively. The catalytic performance was also improved by increasing the temperature in steps. Complex (C81) can activate benzylic, and aliphatic alcohols in the presence of NaHCO3 and then couple with benzylamine to produce sec. amines in moderate to good yields. The alkylation of aniline derivatives with a variety of alcohols produced the appropriate amines in reasonable yields. In a polar system, a weaker base is responsible for proton transfer to start the amination pathway (Scheme 101) (Xie and Huynh, 2015).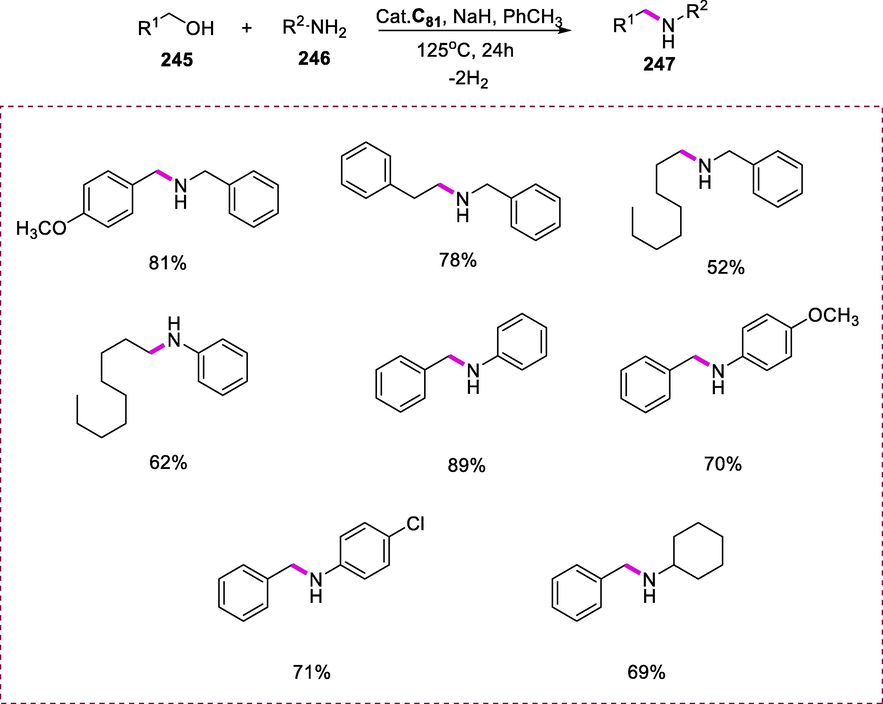
N-alkylation of amines.
7.5 Synthesis of ortho-metallated imidazolylidene-based Ru(II)-NHC complex and its application
Transfer hydrogenation of carbonyl compounds is usually a less hazardous and more convenient process than direct hydrogenation through hydrogen gas and it can reduce a vast range of substrates (such as imines, nitriles, carbonyl groups, C=C bonds, and so on), making it one of the most studied hydrogenation reactions (Nieto et al., 2011; Wang and Astruc, 2015). Ru(II)-NHC complexes are effective catalysts in a variety of important chemical reactions, including the transfer hydrogenation reaction (Daw et al., 2014).
The ortho-metallated Ru(II)-NHC complex (C83) was produced in moderate yield (60.8%) by reacting imidazolium salt (248)with [(p-cymene)RuCl2]2 (249) in the presence of Cs2CO3 as the base. This complex is found to be particularly efficient in the transfer hydrogenation of ketones even at minimal catalyst loading (Scheme 102).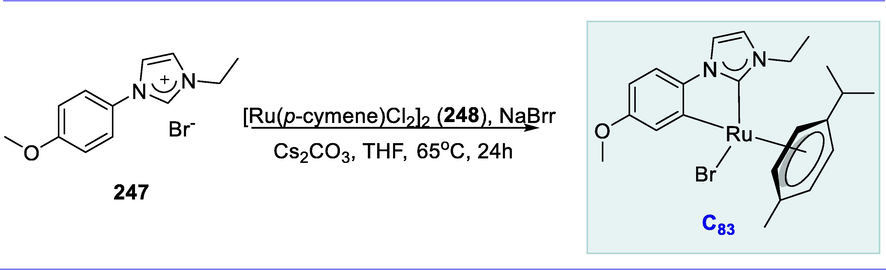
Synthesis of ortho-metallated imidazolylidene-based Ru(II)-NHC complex.
The synthesis of corresponding alcohols (249) arises from the catalytic hydrogenation of ketones (250) and proceeds via Ru-complex (C83) in the presence of KOH as a base and isopropanol as a hydrogen donor. When a para-methoxy substituent is present on the N-heterocyclic carbene ligand backbone, the catalytic activity of complex (C83) is significantly increased while other parameters remain unchanged. This reaction tolerates a variety of substrates including aliphatic (cyclic and acyclic) ketones, aromatic, and hetero-aromatic ketones, and secondary alcohols were obtained in excellent yields (89-98%). Although electron-donating carbonyl substrates provide a lower yield of corresponding products than electron-withdrawing carbonyl substrates. This catalytic system performs well for both electron-withdrawing and electron-donating substituents. This may be related to the electrophilicity of carbonyl groups which is affected by the electron-withdrawing and electron-donating character of substituents (Scheme 103) (Hey et al., 2018).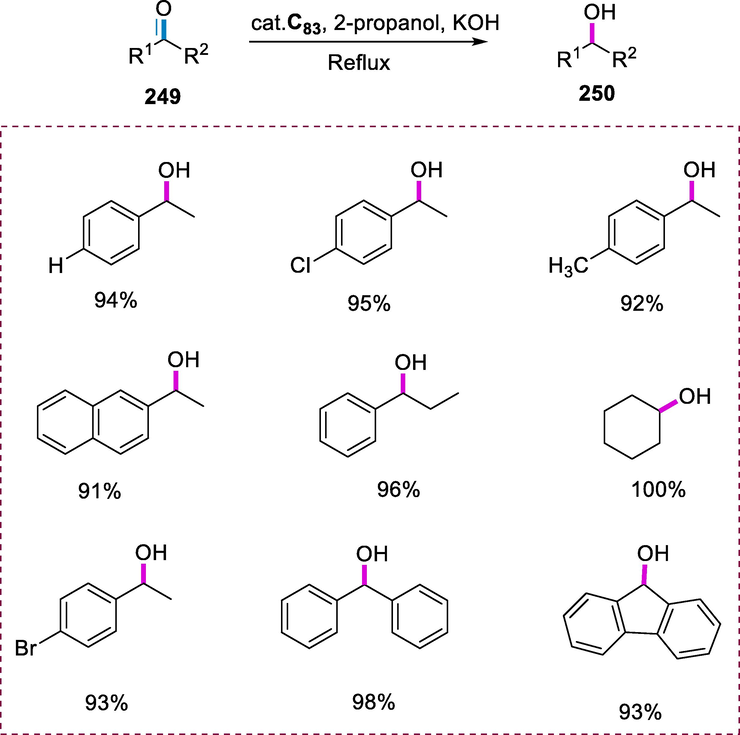
Hydrogenation of ketones.
7.6 Synthesis of ruthenium Schiff-base complexes of the type [Ru(p-cym)(NHC)Cl2] and its application
The catalytic reaction of alcohols with hydrosilanes is a simple way of producing pure molecular hydrogen. Complexes of the type [Ru(p-cym)(NHC)Cl2] are based on a less expensive metal like ruthenium that can effectively catalyze the alcohol-silane coupling reaction at even lower temperatures and catalyst loading (Ventura-Espinosa et al., 2017; Ventura Espinosa et al., 2017).
The silver carbene intermediate (252) is obtained by reacting the imidazolium salt (251) with silver oxide in dichloromethane. This intermediate was not separated and further reacts with the ruthenium dimer in situ to form the catalyst (C84). The reaction should take 4h to complete and overnight stirring gives a higher yield of catalyst (C84) (Scheme 104).![Synthesis of Ru-complexes of the type [Ru(p-cym)(NHC)Cl2] .](/content/184/2022/15/11/img/10.1016_j.arabjc.2022.104165-fig110.png)
Synthesis of Ru-complexes of the type [Ru(p-cym)(NHC)Cl2] .
When Ru-NHC complex (C84) is used as a catalyst, catalytic dehydrogenative coupling between alcohols (254) and hydrosilanes (253) produces silyl ethers (255) as a major product and hydrogen gas as a by-product at high rates. Model substrates include dimethylphenyl silane and methanol. The selective coupling of alcohols and hydrosilanes via dehydrogenation is mediated by Complex (C84). The catalytic alcohol/silane coupling is versatile, allowing different hydrosilanes and alcohols to be used (Scheme 105) (Mollar-Cuni et al., 2021).
Dehydrogenative coupling of alcohols and silanes.
7.7 Synthesis of Ru-CNN hydride NHC complex and its application
The hydrogenation of esters to respective alcohols is one of the most important transformations in organic chemistry because it is used in the synthesis of valuable compounds. The process of catalytic hydrogenation is environmentally friendly (Gribble, 1998; Clarke, 2012). The ester hydrogenation reactions have mostly utilized Ru (II) complexes of NHCs (Sun et al., 2011; Morris, 2013; Filonenko et al., 2014; Le et al., 2018; Le et al., 2019). Because of their high sigma-donor and comparatively weak pi-acceptor properties, these complexes have a significant increase in catalytic activity.
The CNN-NHC ligand with electron-donating diethylamino and imidazolyl NHC groups as side-arms was synthesized in good yields by nucleophilic replacements using suitable imidazole, 2,6-bis-(bromomethyl)pyridine, and diethylamine. Then this ligand (256) on treatment with [RuHCl(CO)(PPh3)3] complex in the presence of lithium bromide yielded ruthenium hydride complex (C85) (Scheme 106).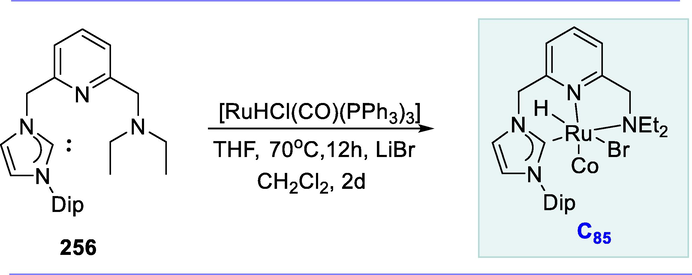
Synthesis of ruthenium hydride NHC complex.
The Ru-CNN NHC complex (C85) catalyzes the hydrogenation of inactivated esters (257) by using base (KHMDS or KOtBu) under mild conditions (5.3 bar H2 and 105°C). The Ru(II) complex (C85) displays excellent activity and high TONs for various aliphatic and aromatic esters hydrogenation (yields up to 92%)(Scheme 107) (Pandey and Choudhury, 2020).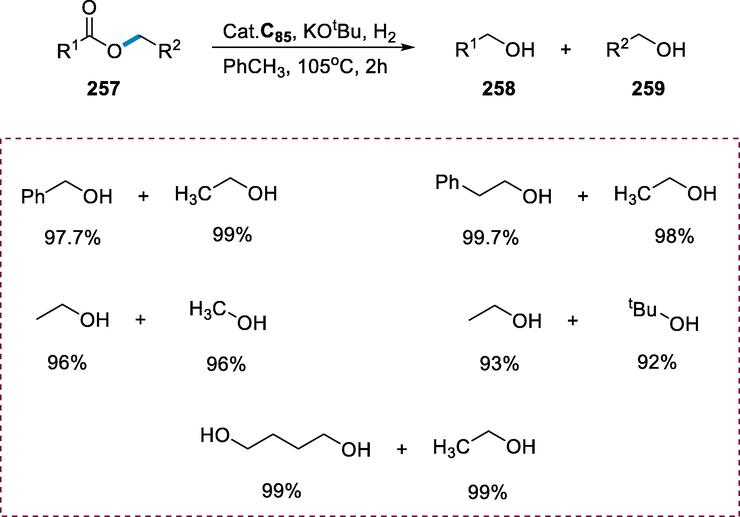
Hydrogenation of esters.
7.8 Synthesis of pyridyl-functionalized and alkenyl-tethered NHC Ru(II) complex and its application
Amines are one of the most significant class of compounds found in a vast range of natural products, medicines, polymers, and pigments, as well as agricultural chemicals (Lawrence, 2004). Metal N-heterocyclic carbene (NHC) complexes which are effective for hydrogenation processes, particularly for nitrile hydrogenation are being developed (Baskakov et al., 2007; Camm et al., 2007; Chen et al., 2007; Addis et al., 2009).
The [L1H]PF6 (260) solution in dichloromethane (DCM) was treated with silver oxide and the resulting suspension was stirred for 4h at room temperature under an N2 atmosphere. The [RuCl2(p-cymene)]2 was then added to the solution after 4h, which was then stirred at room temperature for12h resulting in the formation of [RuL1(p-cymene)Cl]PF6 (C86) in high yield (85%). Complex (C86) was found to be effective for the hydrogenation of nitriles (Scheme 108).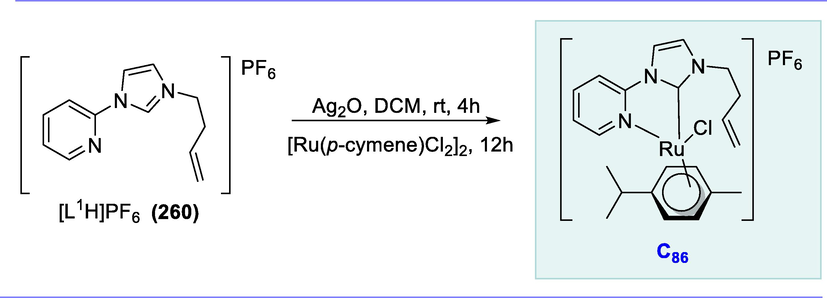
Synthesis of pyridyl-functionalized and alkenyl-tethered NHC Ru(II) complex.
Catalyst (C86) was found to be effective for the hydrogenation of nitriles (261) into secondary amines (262) in 90 percent conversion when used with hydrogen (60 bar) at 80°C in dry 2-propanol for 12h. The yield of the product was reduced at low temperature, H2 pressure, and reaction time, as well as altering the solvent from 2-propanol to 1,4-dioxane, toluene, and THF. The Ru(II) complex (C86) is an effective homogeneous catalyst for the coupling of nitriles via hydrogenation to produce symmetrical amines with high yields and selectivity. Aromatic nitriles having electron-deficient groups afforded higher yields than aromatic nitriles having electron-rich groups under optimal reaction conditions resulting in the formation of symmetrical secondary amines (Scheme 109) (Saha et al., 2016).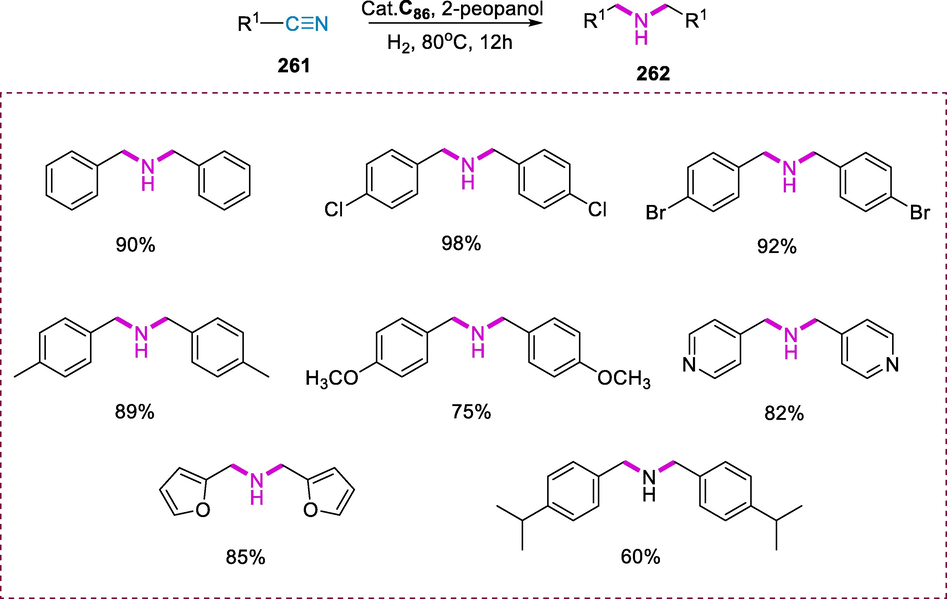
Hydrogenation of nitriles.
7.9 Synthesis of pyridyl-based pyrazolyl-NHC ruthenium complex and its application
The Oppenauer-type oxidation of alcohols in the absence of catalytic amounts of oxidants has been frequently used to synthesize carbonyl compounds. Beyond traditional reduction and oxidation reactions, Oppenauer-type dehydrogenative oxidation has proven to be a reliable synthetic protocol (Addis et al., 2009; Coleman et al., 2010; Moyer and Funk, 2010; Baratta et al., 2011).
The Pd-catalyzed Suzuki reaction of compound (263) with para-tolylboronic acid produced compound (264) in 86 percent yield. Ru(II) complex (C87) was produced in 65 percent yield by the reaction of compound (264) and equimolar quantity of [Ru(PPh3)3Cl2] complex heated under reflux in isopropanol in the presence of trimethylamine. Air-stable red-brown crystals were obtained by recrystallizing complex (C87) from toluene/n-hexane. The unsymmetrical environment around the metal center is thought to be capable of the increase in the catalytic activity of complex (C87), implying that the combined steric and electronic effects due to two different coordinating donor arms at the 2,6 positions of the backbone of the pyridinyl group are essential for the high catalytic efficiency of complexes (Scheme 110).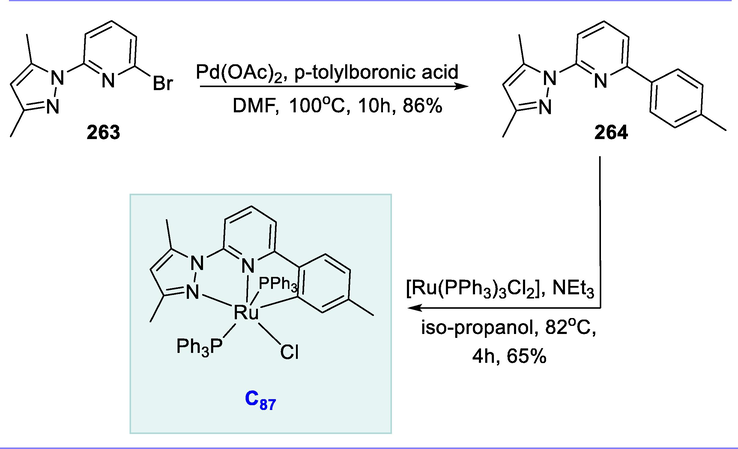
Synthesis of pyridyl-based pyrazolyl-NHC ruthenium complex.
Complex (C87) was used as the catalyst in the presence of tBuOK as a base for the dehydrogenative oxidation of secondary alcohols (265) into ketones (266) in acetone under reflux. Secondary alcohols were selectively oxidized to their respective ketones within 1min to 3h. Most of the alcohol substrates give more than 97% conversion within 1–20 minutes which makes these complexes the best catalytic systems for this sort of reaction. The Ru-complex having an unsymmetrical NNC ligand exhibit excellent activity for the Oppenauer-type oxidation of alcohols. Using a greater catalyst loading and longer reaction duration, aliphatic alcohols were also oxidized to the appropriate ketones. The ortho substituents on the aromatic ring of substrates had a negative influence on the yield of reaction because of the increased steric hindrance (Scheme 111) (Du et al., 2012).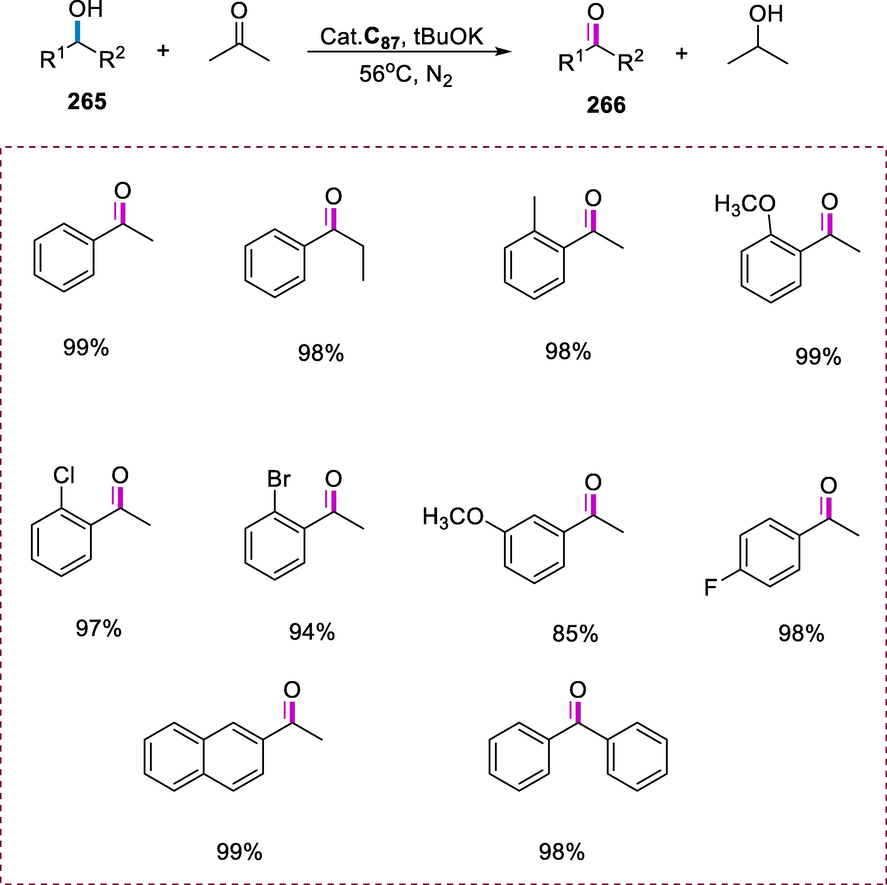
Oxidation of alcohols.
7.10 Synthesis of tri-nuclear Ru-NHC complex and its application
The potential of Ru mono, bi, tri, and multi-dentate NHC complexes in a variety of C-C coupling processes in synthetic chemistry has been studied (Colacino et al., 2007). Besides their industrial significance, group (XI) metal NHC chemistry is primarily concerned with the development of novel anti-cancer and antibiotics drugs (Patil et al., 2015).
The reaction of [Ru3(CO)12] (267) with methyl/1,3-Diisopropyl substituted six-membered NHC free NHC ligand (268) in the presence of THF under a nitrogen environment at ambient temperature results in the formation of corresponding trinuclear Ru complex (C88) in good yields. The catalytic acylation of pyridine by Complex (C88) was investigated (Scheme 112) (Ellul et al., 2018).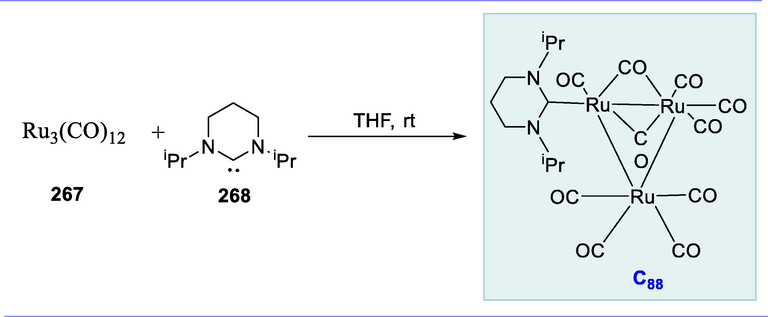
Synthesis of trinuclear Ru-NHC complex.
Complex (C88) catalyzed the insertion of terminal alkene (270) and CO into the ortho C-H bond of pyridine (269) at high pressure (10 atm CO) for 16h at 150°C affording the primarily linear acylation products (271 and 272) in low yield. The activity of Complex (C88) was lower than that of the beginning precursor [Ru3(CO)12] (Scheme 113) (Narayana et al., 2021).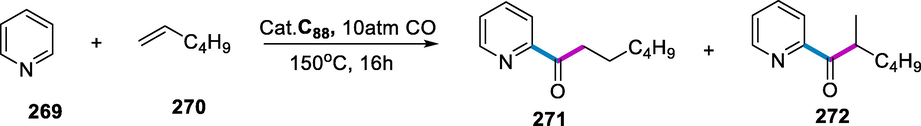
Acylation of pyridine.
7.11 Synthesis of benzimidzole-based Ru-HNC complex and its application
Oxindole is a nitrogen-based heterocycle that can be found in a wide range of pharmaceuticals and naturally occurring compounds. The arylidene-oxindoles, in particular, are oxindole analogs and a preferred scaffold in medicinal chemistry due to their widespread use in a variety of physiologically active compounds as anti-cancer and anti-malarial drugs (Huber et al., 2010; Eissenstat et al., 2012; Lv et al., 2013; Ribeiro et al., 2014; Kaur et al., 2016). Chen et al. reported the direct amidation from amines and alcohols by using bidentate NHC-based Ru-complex and carbonate containing Ru-NHC complex (Wu et al., 2019; Wang et al., 2020). The air-stable Ru N-heterocyclic carbene (Ru-NHC) was used as the catalyst for alpha-olefination of 2-oxindole (amide) by coupling of diaryl methanols via dehydrogenation. As a result, the formation of a C=C bond that yields derivatives of alpha-vinyl 2-oxindole is extremely useful in the synthesis of organic compounds (Dalpozzo et al., 2012; Gicquel et al., 2013; Zhang et al., 2016a, 2016b).
The Ru(II) complexes containing NHC ligands of the general type (arene)(NHC)Ru(II)X2 (where X = halide) were investigated as antibacterial and anticancer agents. The two-step process was used to make the (p-cymene)(NHC)Ru(II)Cl2 complex. The benzimidazole (273) was alkylated by using benzyl bromide in the first stage, yielding benzimidazolium bromide (274). The target compound (C89) was produced in the second stage in a one-pot reaction of compound (274) with silver oxide and then transmetalation with [(p-cym)RuCl2]2. Complex (C89) is often employed for the α-olefination of 2-oxindole because of its high catalytic activity (Scheme 114) (Burmeister et al., 2021).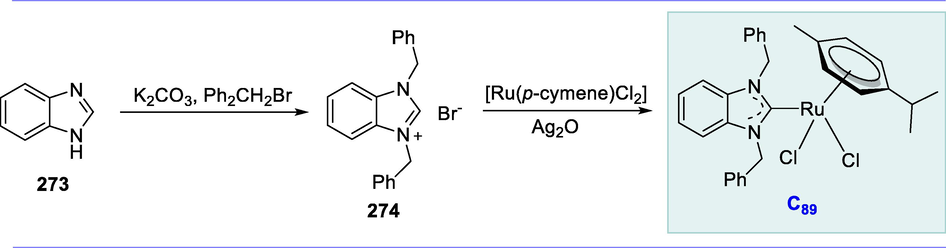
Synthesis of benzimidzole-based Ru-NHC complex.
The Ru-NHC catalyst (C89) is often utilized in the borrowing hydrogen method to synthesize bis-arylidene oxindole by alpha-olefination of 2-oxindole. Ru-NHC complex (C89) is air-stable and used as a catalyst in the formation of oxindoles (amide) by dehydrogenative coupling of diaryl methanols (276) and 2-oxindole (275) generates various substituted derivatives of 3-(diphenylmethylene)indolin-2-one (277) in good yield and produces eco-friendly by-products like H2 and H2O. Furthermore, a controlled experiment was carried out with toluene and KOtBu, under heat at 140°C for 48 hours. This transformation was tolerate the number of functional groups involving the -CF3 group, which resulted in the synthesis of 3-(phenyl(3- (trifluoromethyl)phenyl)indolin-2-one in 38 percent yield. Unfortunately, bis(4-fluorophenyl)methanol, bis(4-methoxyphenyl)methanol, and di-substituted diphenyl methanol derivatives were failed to give the expected products. This might be due to the strong nucleophilicity of the base, which causes them to substitute the fluorine in the aryl ring. Intriguingly, the addition of Cs2CO3 (mild base) resulted in the formation of respective products in low yields respectively. In addition, 7-chloro-2-oxindole was reacted with a variety of diaryl methanols to give the corresponding compounds in a reasonable yield (Scheme 115) (Bisht et al., 2018).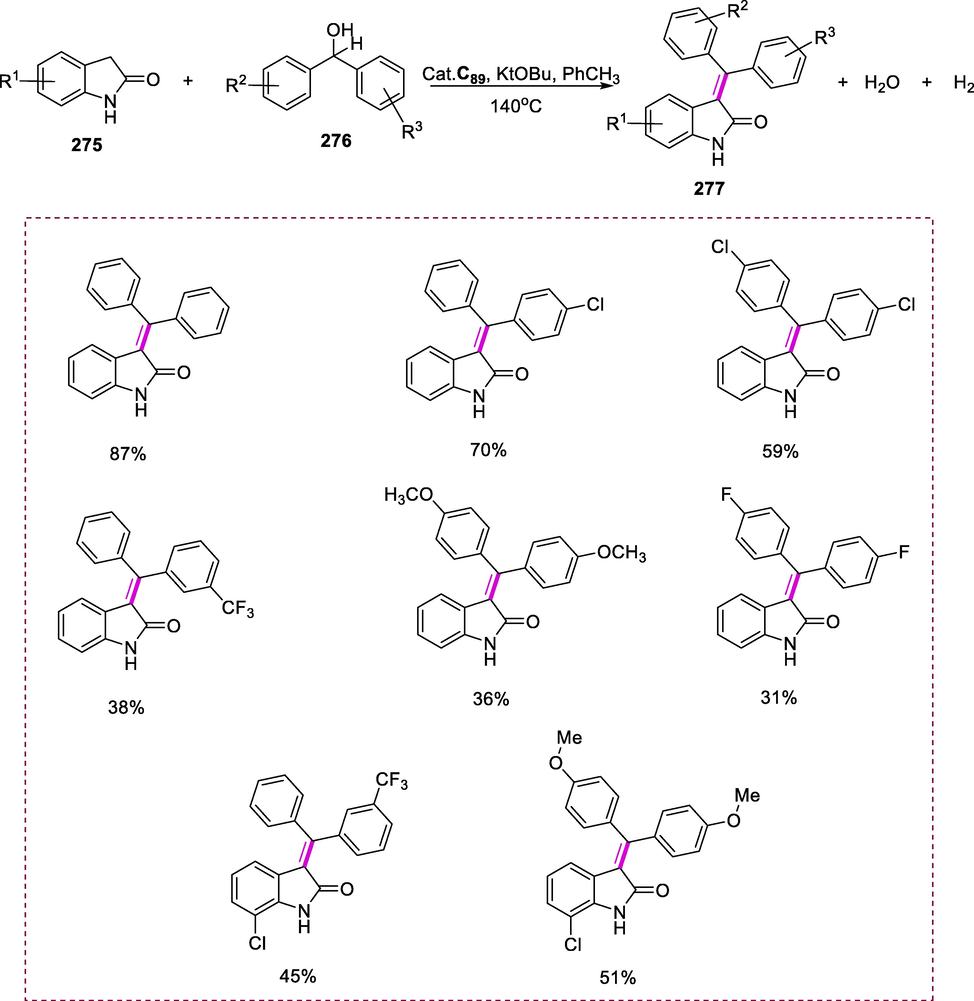
Synthesis of arylidene oxindoles.
7.12 Synthesis of imidazole-based Ru-NHC complex and its applications
One of the most fundamental transformations in synthetic chemistry is the formation of carboxylic acid from primary alcohol (Tojo and Fernández, 2007; Gunanathan and Milstein, 2013). Several ruthenium complexes are used as a catalyst to dehydrogenate the primary alcohol into the appropriate aldehyde. Chen et al. reported the bidentate Ru-NHC complexes for the coupling of alcohols via dehydrogenation into carboxylic acids (Wang et al., 2019; Wu et al., 2022). The dehydrogenation of primary alcohol can result in the formation of a carboxylic acid in the presence of water as a nucleophile (Gianetti et al., 2016).
The imidazole-based complexes have traditionally been made by transferring the free N-heterocyclic carbene ligand into [RuCl2(p-cymene)]2. Carbene transfer has recently been made achievable through the reaction of 1,3-dialkylimidazolium chlorides (277) with Ag2O in the presence of dichloromethane (DCM). The respective silver carbene is produced by this approach and then transmetallated in situ with [RuCl2(p-cymene)]2 complex. Complex (C90) was produced in a high-yields by this method (Scheme 116) (Dam et al., 2010).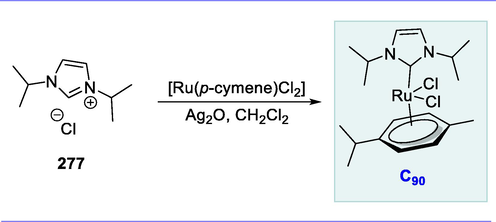
Synthesis of imidazole-based Ru-NHC complex.
The corresponding carboxylic acid (279) was produced from alcohols (278) under optimal conditions, which required the complex (C90), PCy3, in the presence of a stoichiometric quantity of KOH upon refluxing in toluene under an argon flow. However, when using a higher quantity of KOH or lower catalyst loading, the yield of the reaction was reduced. The carboxylic acids are isolated easily using an organic solvent after being precipitated as salts of potassium and then transformed into corresponding acids with HCl. The number of para-substituted benzyl alcohols was transformed into benzoic acids. The oxidation of p-chloro and p-methylbenzyl alcohol went smoothly, producing high yields of carboxylic acids. The same reaction with p-iodo and p-bromobenzyl alcohol yielded slightly lower yields due to the competitive dehalogenation reaction of benzoic acid. The carboxylic acids were also obtained from p-trifluoromethyl and p-phenylbenzyl alcohol, though in a lower yield than the former (Scheme 117) (Santilli et al., 2016).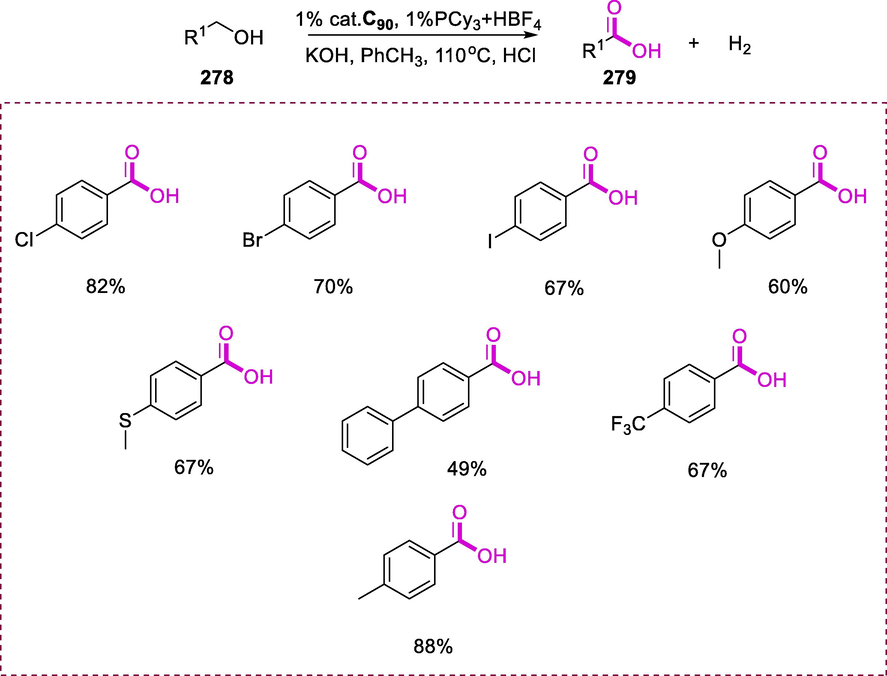
Synthesis of carboxylic acid from aromatic alcohols.
The number of aliphatic primary alcohols (280) was also transformed into their corresponding carboxylic acids (281) by using the Ru-complex (C90), and because these aliphatic alcohols are more stable than benzylic substrates, an 18h reaction period was required to ensure complete conversion. Linear alcohols produced excellent yields, as were substituents at the third position. The yields of alcohols with substituents in the 2-position were slightly lower (Scheme 118).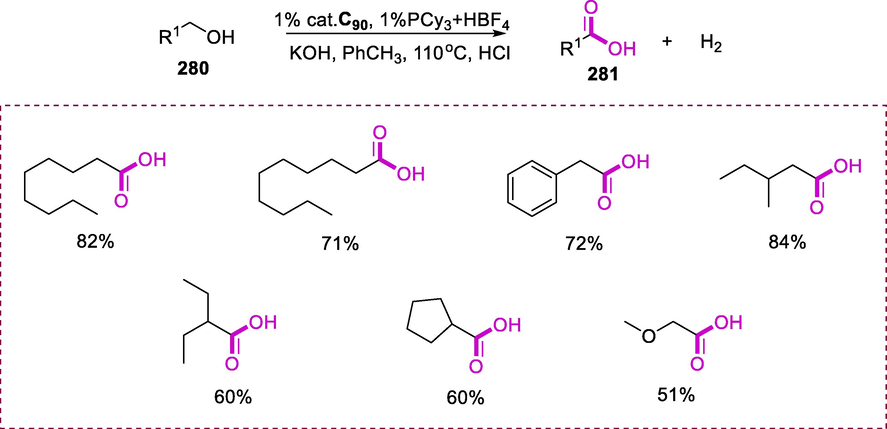
Synthesis of carboxylic acid from aliphatic alcohols.
7.13 Synthesis of imidazolylidene-based untethered Ru-NHC complex and its applications
Amines are undeniably one of the most main classes of organic compounds, particularly because many of them are biologically active. A wide range of natural goods, agrochemicals, medicines, dyes, and pigments contain them (Larock, 1999; Lawrence, 2004; Ricci, 2008a; Ricci, 2008b). For reduction chemistry, Ru-based catalysts have been widely explored, particularly in asymmetric hydrogenation or transfer hydrogenation of imines. [206] Many ruthenium NHC complexes are catalytically active in the reduction of ketimines and aldimines. In the hydrogenation of imines, hydrosilanes are used as a reducing agent along with the Ru-NHC complexes. Aldimines might be converted to the appropriate secondary amine much faster than ketimines due to the steric crowd between the aryl or alkyl group on the ketimine, rather than the hydrogen on the aldimine substrate.
The Ru-NHC complex (C91) was made by transferring the NHC ligand (282) from Ag-NHC produced in situ in the dark from the NHC ligand and silver oxide to Ru in the dimer of dichloro(p-cymene)ruthenium (II). The orange to red-colored crystalline solid of the Ru-NHC complex (C91) was separated in good to excellent yield and these complexes are air-stable (Scheme 119) (Kathuria et al., 2020).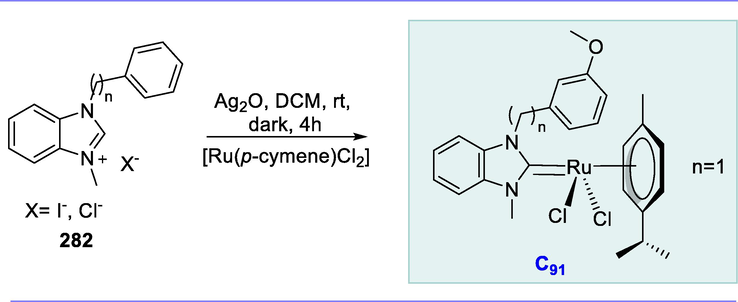
Synthesis of imidazolylidene-based untethered Ru-NHC complex.
Aldimine (283) with PhSiH3 in the presence of Ru-NHC catalyst (C91) and ethanol at room temperature provides the secondary amines (284) in good yields. The complex N-meta-methoxy was tested for the hydrogenation of a variety of aldimines with diverse electronic and steric properties. This transformation tolerates the presence of different functional groups on the substrate, such as halogens, -NO2, -CN, -OH, -NMe2, -CONH2, and –COOMe. So, the vast range of aldimines may be efficiently reduced. These functionalities remained unaffected in the reaction, and the catalyst was not poisoned. Surprisingly, in addition to functional group tolerance, electron-deficient groups such as halide and nitro groups had no adverse effect on reaction rate. The reaction rate was unchanged even by ortho substitution with CH3 on the N-aryl group. The substrate with various electron-rich groups could also be hydrogenated even in a short reaction time. In an ester-containing imine substrate, a complete reduction of imine could be accomplished in 5 minutes without affecting the ester functionality. When the loading of catalyst was increased to 5mol%, full conversion was seen in 2h (Scheme 120).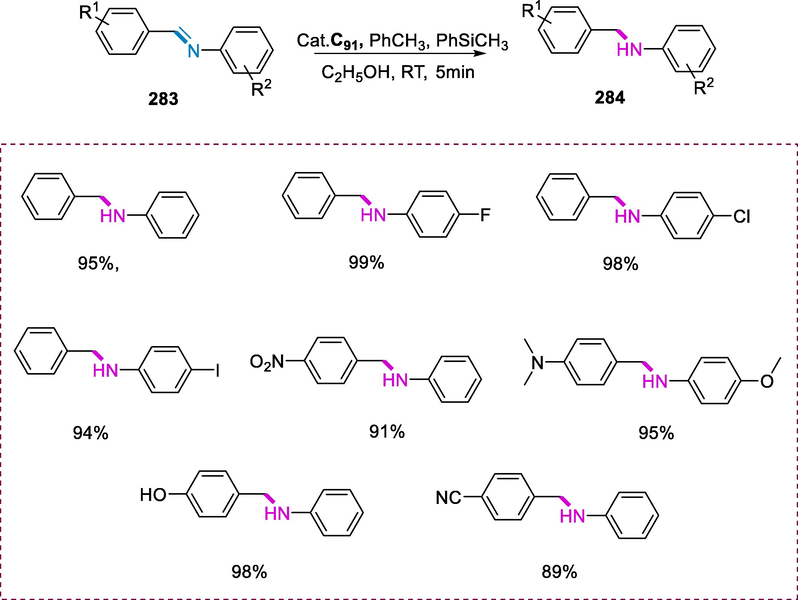
Reduction of aldimines.
Ketimines were reduced by using Ru-NHC complex (C91) (N-mOMe). The reduction of the range of ketimines with various steric and electronic factors was investigated. Ketimines (285) generate high yields of secondary amines (286) when reacting with PhSiH3 in the presence of a Ru-NHC catalyst and ethanol at room temperature. The catalyst was able to efficiently reduce all of the ketimines tested, giving nearly quantitative yields. Unlike aldimines, ketimines acquired a longer reaction time due to steric and electronic effects. The reaction of N-1-diphenylethanimine with an ortho-substituent, for example, took 120 minutes to complete. It was also difficult to reduce a ketimine produced from a cyclic amine. The reaction occurred at a slower rate when a nitro substituent was present on the aryl ring of the imine, and full conversion was detected after 120 minutes. The desirable selectivity was not lost despite the lower reactivity, and the nitro group was unaffected (Scheme 121) (Kathuria and Samuelson, 2020).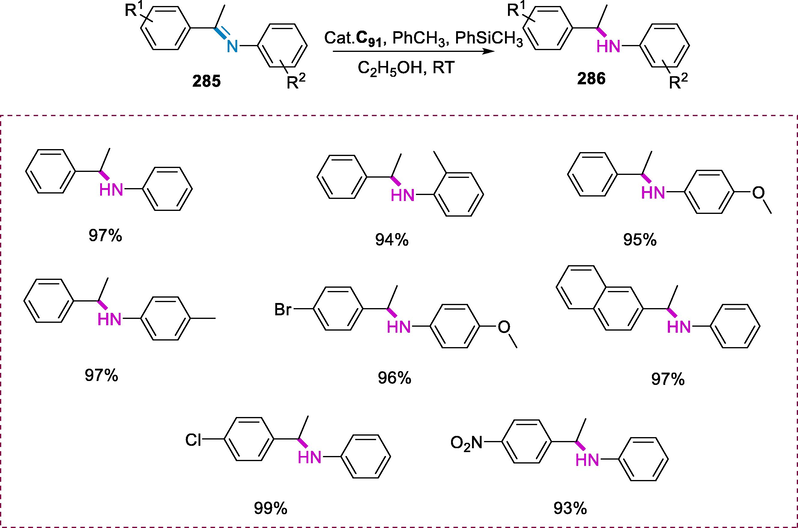
Reduction of kitimines.
8 Future perspective and conclusion
In the case of ruthenium, researchers can still find lots of opportunities in the field of Carbon-heteroatom coupling reactions, which appear to be in their infancy. Many advances in Carbon-Carbon couplings are still feasible, particularly in the less known activation of Csp3–H bonds and couplings with unsaturated small molecules, allenes, or allyl groups. Furthermore, the methods by which these reactions occur are poorly understood: astonishingly, very identical reactions can occur via diverse mechanisms, as demonstrated in cases where intermediates could be identified.
Similarly, molecular modeling of processes has largely been neglected. The diverse photocatalytic activity of ruthenium is not covered here, although it is unquestionably an intriguing field. As a result, this is an exciting field with lots of fresh discoveries and discoveries to come. Ruthenium quickly forms coordination complexes, which have uses in medicine, catalysis, biology, nanoscience, redox, and photoactive materials, among other domains. Ru is utilized in biomedical disciplines for diagnosis and treatment. In comparison to current Pt-based anticancer medications, a variety of Ru complexes have demonstrated enhanced anticancer characteristics, including increased selectivity for cancer cells and reduced toxicity against normal cells. The synthesis of ruthenium complexes, as well as their catalytic and biological uses, are briefly discussed in this article. Further study is likely to lead to even more practical applications of this miracle element in the future years.
Acknowledgement
We strongly acknowledge the synthetic organic chemistry laboratory, Department of Chemistry, Government College University Faisalabad, Punjab, Pakistan for their continuous support.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
References
- Synthesis, structure, and catalytic activity of new ruthenium (II) indenylidene complexes bearing unsymmetrical N-heterocyclic carbenes. Organometallics.. 2014;33(9):2160-2171.
- [Google Scholar]
- Catalytic arylation reactions by C H bond activation with aryl tosylates. Angewandte Chemie International Edition.. 2006;45(16):2619-2622.
- [Google Scholar]
- Assisted ruthenium-catalyzed C− H bond activation: carboxylic acids as cocatalysts for generally applicable direct arylations in apolar solvents. Organic Letters.. 2008;10(11):2299-2302.
- [Google Scholar]
- Imines, enamines and oximes. Journal of the Chemical Society, Perkin. Transactions. 2000;1. (2):125-139.
- [Google Scholar]
- Ruthenium N-heterocyclic carbene catalysts for selective reduction of nitriles to primary amines. Tetrahedron Letters.. 2009;50(26):3654-3656.
- [Google Scholar]
- Grubbs’ ruthenium-carbenes beyond the metathesis reaction: less conventional non-metathetic utility. Chemical reviews.. 2009;109(8):3817-3858.
- [Google Scholar]
- Aerobic copper-catalyzed organic reactions. Chemical reviews.. 2013;113(8):6234-6458.
- [Google Scholar]
- Synthesis and antitumor activity of duocarmycin derivatives: A-ring pyrrole compounds bearing β-(5′, 6′, 7′-trimethoxy-2′-indolyl) acryloyl group. Bioorg. Med. Chem.. 2000;8(7):1637-1643.
- [Google Scholar]
- Modern reduction methods. John Wiley & Sons; 2008.
- Angurell, I. 2004. I, Martínez-Ruiz, O. Rossell, M. Seco, P. Gómez-Salb, A. Martín. Chem. Commun. 1712-1713.
- Carbon dioxide as chemical feedstock. John Wiley & Sons; 2010.
- Arseniyadis, S., Kyler, K.S., Watt, D.S. 1984. Addition and Substitution Reactions of Nitrile‐Stabilized Carbanions Wiley Online Library.
- Synthesis of primary amines from secondary and tertiary amines: ruthenium-catalyzed amination using ammonia. Chemistry–A. European Journal.. 2011;17(17):4705-4708.
- [Google Scholar]
- Highly enantioselective aminoacylase-catalyzed transesterification of secondary alcohols. Tetrahedron: Asymmetry.. 2000;11(8):1801-1808.
- [Google Scholar]
- Synthesis and Structures of Arene Ruthenium (II)–NHC Complexes: Efficient Catalytic α-alkylation of ketones via Hydrogen Auto Transfer Reaction. Appl. Organomet. Chem.. 2019;33(1):e4696.
- [Google Scholar]
- Hydrogenation of polar bonds catalysed by ruthenium-pincer complexes. Ruthenium in Catalysis, Springer. 2014:19-43.
- [Google Scholar]
- Efficient hydrogenation of organic carbonates, carbamates and formates indicates alternative routes to methanol based on CO 2 and CO. Nat. Chem.. 2011;3(8):609-614.
- [Google Scholar]
- Catalytic transformation of alcohols to carboxylic acid salts and H 2 using water as the oxygen atom source. Nature Chemistry.. 2013;5(2):122-125.
- [Google Scholar]
- Efficient and eco-compatible transition metal-free Oppenauer-type oxidation of alcohols. Catalysis Communications.. 2014;47:58-62.
- [Google Scholar]
- Ruthenium (ii) Terdentate CNN Complexes: Superlative Catalysts for the Hydrogen-Transfer Reduction of Ketones by Reversible Insertion of a Carbonyl Group into the Ru H Bond. Angew. Chem. Int. Ed.. 2005;44(38):6214-6219.
- [Google Scholar]
- Catalytic transfer hydrogenation with terdentate CNN ruthenium complexes: the influence of the base. Chem. Eur. J.. 2007;13(26):7479-7486.
- [Google Scholar]
- Fast and chemoselective transfer hydrogenation of aldehydes catalyzed by a terdentate CNN ruthenium complex [RuCl (CNN)(dppb)] Adv. Synth. Catal.. 2007;349(10):1633-1636.
- [Google Scholar]
- Chiral pincer ruthenium and osmium complexes for the fast and efficient hydrogen transfer reduction of ketones. Organometallics.. 2010;29(16):3563-3570.
- [Google Scholar]
- Pincer and diamine Ru and Os diphosphane complexes as efficient catalysts for the dehydrogenation of alcohols to ketones. Chemistry–A. European Journal.. 2011;17(12):3474-3481.
- [Google Scholar]
- Efficient Transformation of Azides to Primary Amines Using the Mild and Easily Accessible CeCl3⊙ 7H2O/NaI System. The Journal of organic chemistry.. 2008;73(5):1919-1924.
- [Google Scholar]
- Chiral N-heterocyclic carbenes with restricted flexibility in asymmetric catalysis. Organometallics.. 2007;26(3):626-632.
- [Google Scholar]
- Aliphatic deaminations in organic synthesis. Journal of Chemical Education.. 1966;43(8):398.
- [Google Scholar]
- The antimalarial ferroquine: from bench to clinic. Parasite: journal de la Société Française de Parasitologie.. 2011;18(3):207.
- [Google Scholar]
- Ru-Catalyzed dehydrogenative synthesis of antimalarial arylidene oxindoles. Organic & biomolecular chemistry.. 2018;16(39):7223-7229.
- [Google Scholar]
- In situ oxidation− imine formation− reduction routes from alcohols to amines. Organic letters.. 2001;3(11):1637-1639.
- [Google Scholar]
- Additions of organometallic reagents to C= N bonds: Reactivity and selectivity. Chemical reviews.. 1998;98(4):1407-1438.
- [Google Scholar]
- (Cyclopentadienone) ruthenium carbonyl complexes-a new class of homogeneous hydrogenation catalysts. Organometallics.. 1985;4(8):1459-1461.
- [Google Scholar]
- Mechanistic Study of Acetate-Assisted C− H Activation of 2-Substituted Pyridines with [MCl2Cp*] 2 (M= Rh, Ir) and [RuCl2 (p-cymene)] 2. Organometallics.. 2009;28(2):433-440.
- [Google Scholar]
- Catalytic transfer hydrogenation of ketones by the use of ruthenium complexes incorporating the new tridentate ligand, bis (2-oxazolin-2-ylmethyl) phenylphosphine. Journal of the Chemical Society, Dalton Transactions.. 1999;4:589-594.
- [Google Scholar]
- Chiral ruthenium Lewis acid-catalyzed nitrile oxide cycloadditions. Tetrahedron.. 2007;63(35):8413-8419.
- [Google Scholar]
- Stereospecific olefin polymerization with chiral metallocene catalysts. Angewandte Chemie International Edition in English.. 1995;34(11):1143-1170.
- [Google Scholar]
- Homogeneous metathesis polymerization by well-defined group VI and group VIII transition-metal alkylidenes: fundamentals and applications in the preparation of advanced materials. Chemical reviews.. 2000;100(4):1565-1604.
- [Google Scholar]
- Ruthenium (II) complexes with pyridine-based Schiff base ligands: Synthesis, structural characterization and catalytic hydrogenation of ketones. Journal of Molecular Structure.. 2020;1202:127266
- [Google Scholar]
- Selective catalytic dehydrogenation of alkanes to alkenes. Journal of the American Chemical Society.. 1987;109(26):8025-8032.
- [Google Scholar]
- Selective stoichiometric and catalytic carbon-hydrogen bond cleavage reactions in hydrocarbons by iridium complexes. Organometallics.. 1984;3(5):816-817.
- [Google Scholar]
- Evaluation of Ruthenium (II) N-Heterocyclic Carbene Complexes as Antibacterial Agents and Inhibitors of Bacterial Thioredoxin Reductase. Molecules.. 2021;26(14):4282.
- [Google Scholar]
- One-pot synthesis of amines from biomass resources catalyzed by HReO 4. Green Chemistry.. 2018;20(11):2494-2498.
- [Google Scholar]
- Ruthenium (II) complexes of NO ligands: Synthesis, characterization and application in transfer hydrogenation of carbonyl compounds. J. Organomet. Chem.. 2016;801:122-129.
- [Google Scholar]
- Tandem ROMP− hydrogenation with a third-generation grubbs catalyst. Journal of the American Chemical Society.. 2007;129(14):4168-4169.
- [Google Scholar]
- Water-soluble arene ruthenium catalysts containing sulfonated diamine ligands for asymmetric transfer hydrogenation of α-aryl ketones and imines in aqueous solution. Green Chemistry.. 2007;9(4):391-397.
- [Google Scholar]
- Cardona, F., Parmeggiani, C. 2014. Transition metal catalysis in aerobic alcohol oxidation Royal Society of Chemistry.
- Isotope effects. Advanced organic chemistry. 2000;4:222-224.
- pez-Ram de VÏu, LA Oro, C. F. Viguri. Chem. Commun.: Vega; 1997. p. :2351.
- Microwave-Assisted Synthesis of Functionalized Shvo-Type Complexes. Organometallics.. 2014;33(11):2814-2819.
- [Google Scholar]
- Antimicrobial activity of carbene complexes of rhodium (I) and ruthenium (II) Arzneimittel-forschung.. 1996;46(8):821-823.
- [Google Scholar]
- Antibacterial and antifungal activities of complexes of ruthenium (II) Arzneimittelforschung.. 1999;49(06):538-540.
- [Google Scholar]
- Synthesis, characterization and catalytic activity of saturated and unsaturated N-heterocyclic carbene iridium (I) complexes. Dalton Transactions.. 2009;5:861-867.
- [Google Scholar]
- Selective Deuteration of Organic Compounds Catalyzed by Ruthenium Pincer Complexes. Pincer Compounds: Elsevier; 2018. p. :519-538.
- Ruthenium and osmium complexes in CC bond-forming reactions by borrowing hydrogen catalysis. Coordination Chemistry Reviews.. 2017;331:1-36.
- [Google Scholar]
- New optically active N-heterocyclic carbene complexes for hydrogenation: A tale with an atropisomeric twist. Organometallics.. 2007;26(4):855-859.
- [Google Scholar]
- Unexpected direct hydride transfer mechanism for the hydrogenation of ethyl acetate to ethanol catalyzed by SNS pincer ruthenium complexes. Chemistry–A. European Journal.. 2016;22(6):1950-1957.
- [Google Scholar]
- Dehydrogenation and related reactions catalyzed by iridium pincer complexes. Chemical reviews.. 2011;111(3):1761-1779.
- [Google Scholar]
- Synthesis of novel sulfonyl-substituted pyrrole chromophores for second-order nonlinear optics. Tetrahedron Letters.. 2001;42(7):1309-1311.
- [Google Scholar]
- An unprecedented iridium (III) catalyst for stereoselective dimerisation of terminal alkynes. Advanced Synthesis & Catalysis.. 2008;350(2):234-236.
- [Google Scholar]
- Mechanisms of the H2-hydrogenation and transfer hydrogenation of polar bonds catalyzed by ruthenium hydride complexes. Coordination Chemistry Reviews.. 2004;248(21–24):2201-2237.
- [Google Scholar]
- Electrophilic substitution reactions at the phenyl ring of the chelated 2-(2 ‘-Pyridyl) phenyl ligand bound to Ruthenium (II) or Osmium (II) Organometallics.. 1999;18(15):2813-2820.
- [Google Scholar]
- Ruthenium metallopharmaceuticals. Coordination Chemistry Reviews.. 2002;232(1–2):69-93.
- [Google Scholar]
- Recent developments in the homogeneous hydrogenation of carboxylic acid esters. Catalysis Science & Technology.. 2012;2(12):2418-2423.
- [Google Scholar]
- Preparation of NHC–ruthenium complexes and their catalytic activity in metathesis reaction. Coordination Chemistry Reviews.. 2007;251(5–6):726-764.
- [Google Scholar]
- Iron-catalyzed Oppenauer-type oxidation of alcohols. Advanced Synthesis & Catalysis.. 2010;352(6):967-970.
- [Google Scholar]
- Key green chemistry research areas—a perspective from pharmaceutical manufacturers. Green Chemistry.. 2007;9(5):411-420.
- [Google Scholar]
- The direct catalytic asymmetric Mannich reaction. Accounts of chemical research.. 2004;37(2):102-112.
- [Google Scholar]
- Advances in one-pot synthesis through borrowing hydrogen catalysis. Chemical reviews.. 2018;118(4):1410-1459.
- [Google Scholar]
- NHC ligands versus cyclopentadienyls and phosphines as spectator ligands in organometallic catalysis. J. Organomet. Chem.. 2005;690(24–25):5451-5457.
- [Google Scholar]
- Some chelating C-donor ligands in hydrogen transfer and related catalysis. Journal of organometallic chemistry.. 2006;691(14):3146-3150.
- [Google Scholar]
- Multifunctional ligands in transition metal catalysis. New Journal of Chemistry.. 2011;35(1):18-23.
- [Google Scholar]
- An organometallic future in green and energy chemistry? Organometallics.. 2011;30(1):17-19.
- [Google Scholar]
- Homogeneous transition metal catalysis of acceptorless dehydrogenative alcohol oxidation: applications in hydrogen storage and to heterocycle synthesis. Chem. Rev.. 2017;117(13):9228-9246.
- [Google Scholar]
- Arene-ruthenium (II) complexes with hydrophilic P-donor ligands: versatile catalysts in aqueous media. Dalton Transactions.. 2014;43(33):12447-12462.
- [Google Scholar]
- Catalytic synthesis of amides via aldoximes rearrangement. Chemical Communications.. 2015;51(13):2495-2505.
- [Google Scholar]
- NO as temporary guanidino-protecting group provides efficient access to Pbf-protected argininic acid. Tetrahedron letters.. 2005;46(39):6733-6735.
- [Google Scholar]
- The synthesis of naturally occurring peptides and their analogs. Current opinion in drug discovery & development.. 2007;10(6):768-783.
- [Google Scholar]
- Recent advances in organocatalytic methods for the synthesis of disubstituted 2-and 3-indolinones. Chemical Society Reviews.. 2012;41(21):7247-7290.
- [Google Scholar]
- Amide synthesis from alcohols and amines catalyzed by ruthenium N-heterocyclic carbene complexes. Chemistry–A. European Journal.. 2010;16(23):6820-6827.
- [Google Scholar]
- Chiral arene ruthenium complexes as asymmetric Diels-Alder catalysts. Chemical Communications.. 1997;15:1351-1352.
- [Google Scholar]
- A highly efficient catalyst for selective oxidative scission of olefins to aldehydes: abnormal-NHC–Ru (II) complex in oxidation chemistry. Journal of the American Chemical Society.. 2014;136(40):13987-13990.
- [Google Scholar]
- Direct synthesis of pyrroles by dehydrogenative coupling of diols and amines catalyzed by cobalt pincer complexes. Angew. Chem.. 2016;128(46):14585-14589.
- [Google Scholar]
- Synthesis of pyrazines and quinoxalines via acceptorless dehydrogenative coupling routes catalyzed by manganese pincer complexes. ACS catalysis.. 2018;8(9):7734-7741.
- [Google Scholar]
- Ring-closing metathesis, Kharasch addition and enol ester synthesis catalysed by a novel class of ruthenium (II) complexes. Tetrahedron Letters.. 2001;42(51):8959-8963.
- [Google Scholar]
- Atom transfer radical polymerization of vinyl monomers mediated by schiff base ruthenium− alkylidene catalysts and the adventitious effect of water in polymerizations with the analogous cationic complexes. Macromolecules.. 2002;35(24):8943-8947.
- [Google Scholar]
- Activity of a new class of ruthenium based ring-closing metathesis and ring-opening metathesis polymerization catalysts coordinated with a 1, 3-dimesityl-4, 5-dihydroimidazol-2-ylidene and a Schiff base ligand. Tetrahedron letters.. 2002;43(50):9101-9104.
- [Google Scholar]
- Radical reactions catalysed by homobimetallic ruthenium (II) complexes bearing Schiff base ligands: atom transfer radical addition and controlled polymerisation. Tetrahedron letters.. 2002;43(26):4687-4690.
- [Google Scholar]
- Kharasch addition and vinylation reactions mediated by ruthenium (II) complexes bearing Schiff base ligands. Catalysis letters.. 2002;83(1):9-13.
- [Google Scholar]
- Synthesis and evaluation of a new class of ruthenium-based catalytic systems for atom transfer radical addition and enol ester synthesis. J. Organomet. Chem.. 2003;672(1–2):11-16.
- [Google Scholar]
- Nonclassical routes for amide bond formation. Chemical reviews.. 2016;116(19):12029-12122.
- [Google Scholar]
- The synthesis of a number of 3-alkyl and 3-carboxy substituted pyrroles; their chemical polymerisation onto poly (vinylidene fluoride) membranes, and their use as gas sensitive resistors. Synthetic Metals.. 2000;114(2):181-188.
- [Google Scholar]
- Synthesis of all nineteen appropriately protected chiral α-hydroxy acid equivalents of the α-amino acids for Boc solid-phase depsi-peptide synthesis. Organic letters.. 2004;6(4):497-500.
- [Google Scholar]
- A sustainable multicomponent pyrimidine synthesis. Journal of the American Chemical Society.. 2015;137(40):12804-12807.
- [Google Scholar]
- Pincer-type pyridine-based N-heterocyclic carbene amine Ru (II) complexes as efficient catalysts for hydrogen transfer reactions. Organometallics.. 2011;30(8):2180-2188.
- [Google Scholar]
- Del Zotto, A., Greco, C., Baratta, W., Siega, K., Rigo, P. (2007) Transfer Hydrogenation Reactions Catalyzed by Free or Silica‐Immobilized [RuCl2 (ampy){RN (CH2PPh2) 2}] Complexes, Wiley Online Library.
- Retracing the evolution of monometallic ruthenium–arene catalysts for C-C bond formation. Dalton transactions.. 2012;41(31):9257-9268.
- [Google Scholar]
- Study of catalytic activity of silica supported Schiff base complexes. Asian Journal of Chemistry.. 2017;29(7):1455-1458.
- [Google Scholar]
- Dick, D.G., Edema, J.J., Duchateau, R., Gambarotta, S. 1993. Novel bis ((trimethylsilyl) benzamidinato) titanium (III) complexes. Preparation and crystal structures of {PhC [(Me3Si) N] 2} 2Ti (. mu.-Cl) 2Li (TMEDA),{PhC [(Me3Si) N] 2} 2Ti (BH4), and {PhC [(Me3Si) N] 2} 2Ti (. eta. 3-allyl). Inorganic Chemistry. 32(10): 1959-1962.
- 8 Carbene and transition metal-mediated transformations. Annual Reports Section“ B”(Organic Chemistry). 2005;101:171-191.
- [Google Scholar]
- A short and efficient stereoselective synthesis of the polyhydroxylated macrolactone (+)-aspicilin. Organic letters.. 2000;2(2):123-125.
- [Google Scholar]
- Cycloruthenated compounds–synthesis and applications. Eur. J. Inorg. Chem.. 2009;2009(7):817-853.
- [Google Scholar]
- Dehydrogenation as a substrate-activating strategy in homogeneous transition-metal catalysis. Chem. Rev.. 2010;110(2):681-703.
- [Google Scholar]
- Alkyl nitrite-metal halide deamination reactions. 5. In situ generation of nitrosyl halides. Effective product control from nitrosyl chloride diazotization of primary aliphatic amines in N, N-dimethylformamide. The Journal of Organic Chemistry.. 1978;43(21):4120-4125.
- [Google Scholar]
- Metathesis catalysed by the platinum group metals. Platinum Metals Review(UK). 2000;44(2):58-66.
- [Google Scholar]
- Single-site ruthenium metathesis catalysts. Platinum Metals Review.. 2001;45(4):155-162.
- [Google Scholar]
- Synthesis of Schiff Base-Ruthenium Complexes and Their Applications in Catalytic Processes. Advanced Synthesis & Catalysis.. 2005;347(14):1721-1743.
- [Google Scholar]
- Ruthenium complexes bearing bidentate Schiff base ligands as efficient catalysts for organic and polymer syntheses. Coordination Chemistry Reviews.. 2005;249(24):3055-3074.
- [Google Scholar]
- Drozdzak, R., Allaert, B., Ledoux, N. 2005a. 1. Dragutan, V. Dargutan, F. Verpoort. Coord. Chem. Rev. 249: 3055.
- A Versatile Ruthenium (II)–NNC Complex Catalyst for Transfer Hydrogenation of Ketones and Oppenauer-Type Oxidation of Alcohols. Chemistry–A. European Journal.. 2012;18(37):11550-11554.
- [Google Scholar]
- Furfurylamines from biomass: transaminase catalysed upgrading of furfurals. Green Chemistry.. 2017;19(2):397-404.
- [Google Scholar]
- The potential of palladacycles: more than just precatalysts. Chemical Reviews.. 2005;105(6):2527-2572.
- [Google Scholar]
- Palladacycles: synthesis. characterization and applications John Wiley & Sons; 2008.
- Antimicrobial activity of rhodium (I) and ruthenium (II) carbene complexes derived from benzimidazole against Staphylococcus aureus isolates. Turkish Journal of Medical Sciences.. 1997;27(1):59-61.
- [Google Scholar]
- Study of the Efficiency of Amino-Functionalized Ruthenium and Ruthenacycle Complexes as Racemization Catalysts in the Dynamic Kinetic Resolution of 1-Phenylethanol. Adv. Synth. Catal.. 2007;349(17–18):2603-2609.
- [Google Scholar]
- Chemistry of α-oxoesters: a powerful tool for the synthesis of heterocycles. Chemical reviews.. 2015;115(1):151-264.
- [Google Scholar]
- Synthesis, Characterization, and Reactivity of N-Heterocyclic Carbene Palladium (II) Hydroxide Dimers. Organometallics.. 2011;30(17):4494-4496.
- [Google Scholar]
- Enamino-oxindole HIV protease inhibitors. Bioorganic & medicinal chemistry letters.. 2012;22(15):5078-5083.
- [Google Scholar]
- Iron-Catalyzed α-Alkylation of Ketones with Alcohols. Angewandte Chemie International Edition.. 2015;54(48):14483-14486.
- [Google Scholar]
- [Ru 3 (6-NHC)(CO) 10]: synthesis, characterisation and reactivity of rare 46-electron tri-ruthenium clusters. Dalton Transactions.. 2018;47(13):4518-4523.
- [Google Scholar]
- Organocatalysis by N-heterocyclic carbenes. Chemical Reviews.. 2007;107(12):5606-5655.
- [Google Scholar]
- Synthesis of cyclic imides by acceptorless dehydrogenative coupling of diols and amines catalyzed by a manganese pincer complex. Journal of the American Chemical Society.. 2017;139(34):11722-11725.
- [Google Scholar]
- An Application of Electronic Asymmetry to Highly Enantioselective Catalytic Diels− Alder Reactions. Journal of the American Chemical Society.. 2001;123(11):2525-2529.
- [Google Scholar]
- Metal-catalysed selective transfer hydrogenation of α, β-unsaturated carbonyl compounds to allylic alcohols. Green Chemistry.. 2020;22(11):3323-3357.
- [Google Scholar]
- Activation of CH bonds in saturated hydrocarbons. The selective, catalytic functionalisation of methyl groups by means of a soluble iridium polyhydride system. Tetrahedron letters.. 1985;26(16):1999-2000.
- [Google Scholar]
- Ruthenium (II) picolyl-NHC complexes: synthesis, characterization, and catalytic activity in amine N-alkylation and transfer hydrogenation reactions. Organometallics.. 2012;31(19):6868-6879.
- [Google Scholar]
- Autocatalysis for C-H bond activation by ruthenium (II) complexes in catalytic arylation of functional arenes. Journal of the American Chemical Society.. 2011;133(26):10161-10170.
- [Google Scholar]
- Microwaves-assisted solvent-free synthesis of N-acetamides by amidation or aminolysis. Tetrahedron Letters.. 2008;49(18):3004-3008.
- [Google Scholar]
- Lutidine-derived Ru-CNC hydrogenation pincer catalysts with versatile coordination properties. ACS Catalysis.. 2014;4(8):2667-2671.
- [Google Scholar]
- Bis-N-heterocyclic carbene aminopincer ligands enable high activity in Ru-catalyzed ester hydrogenation. Journal of the American Chemical Society.. 2015;137(24):7620-7623.
- [Google Scholar]
- Nitrile-containing pharmaceuticals: efficacious roles of the nitrile pharmacophore. Journal of medicinal chemistry.. 2010;53(22):7902-7917.
- [Google Scholar]
- New CNN-type ruthenium pincer NHC complexes. Mild, efficient catalytic hydrogenation of esters. Organometallics.. 2011;30(14):3826-3833.
- [Google Scholar]
- System with potential dual modes of metal-ligand cooperation: highly catalytically active pyridine-based PNNH-Ru pincer complexes. Eur. J. Chem.. 2014;20(48):15727-15731.
- [Google Scholar]
- N-Heterocyclic carbene (NHC) ligands and palladium in homogeneous cross-coupling catalysis: a perfect union. Chem. Soc. Rev.. 2011;40(10):5151-5169.
- [Google Scholar]
- Synthesis and catalytic applications of ruthenium (ii)–phosphino-oxime complexes. RSC advances.. 2016;6(45):39044-39052.
- [Google Scholar]
- Enzymatic resolution, desymmetrization, and dynamic kinetic asymmetric transformation of 1, 3-cycloalkanediols. J. Org. Chem.. 2006;71(17):6309-6316.
- [Google Scholar]
- Optically active isoxazolidines via asymmetric cycloaddition reactions of nitrones with alkenes: applications in organic synthesis. Tetrahedron.. 1997;53(2):403-425.
- [Google Scholar]
- Acceptorless dehydrogenation of alcohols: perspectives for synthesis and H2 storage. ChemCatChem.. 2009;1(1):72-73.
- [Google Scholar]
- Catalytic ring-closing metathesis of functionalized dienes by a ruthenium carbene complex. Journal of the American Chemical Society.. 1993;115(21):9856-9857.
- [Google Scholar]
- Ruthenium (II)-catalyzed asymmetric transfer hydrogenation of ketones using a formic acid− triethylamine mixture. Journal of the American Chemical Society.. 1996;118(10):2521-2522.
- [Google Scholar]
- A concise total synthesis of dactylol via ring closing metathesis. The Journal of organic chemistry.. 1996;61(25):8746-8749.
- [Google Scholar]
- A ruthenium (II) complex with a C 2-symmetric diphosphine/diamine tetradentate ligand for asymmetric transfer hydrogenation of aromatic ketones. Organometallics.. 1996;15(4):1087-1089.
- [Google Scholar]
- New chiral catalysts for reduction of ketones. Chirality: The Pharmacological, Biological, and Chemical Consequences of Molecular Asymmetry.. 2000;12(5–6):383-388.
- [Google Scholar]
- Iminophosphorane–phosphines: Versatile ligands for homogeneous catalysis. Journal of Organometallic Chemistry.. 2014;751:792-808.
- [Google Scholar]
- Recent progress in Lewis base activation and control of stereoselectivity in the additions of trimethylsilyl nucleophiles. Chemical reviews.. 2008;108(12):5227-5252.
- [Google Scholar]
- Direct amide synthesis from alcohols and amines by phosphine-free ruthenium catalyst systems. Advanced Synthesis & Catalysis.. 2009;351(16):2643-2649.
- [Google Scholar]
- Nitrous oxide as a hydrogen acceptor for the dehydrogenative coupling of alcohols. Angew. Chem. Int. Ed.. 2016;55(5):1854-1858.
- [Google Scholar]
- Application of arene ruthenium (II) complexes with pyridine-2-carboxaldimine ligands in the transfer hydrogenation of ketones. Journal of Molecular Catalysis A: Chemical.. 2016;416:29-38.
- [Google Scholar]
- Synthesis of 3, 3′-spirocyclic oxindoles via phosphine catalyzed [4+ 2] cyclizations. Organic letters.. 2013;15(15):4002-4005.
- [Google Scholar]
- Formation of a novel polypyrrole/porous phosphate glass ceramic nanocomposite. Journal of the Brazilian Chemical Society.. 1999;10:167-168.
- [Google Scholar]
- Homogeneous metal catalysis for conversion between aromatic and saturated compounds. Coordination Chemistry Reviews.. 2016;314:134-181.
- [Google Scholar]
- Asymmetric transfer hydrogenation: chiral ligands and applications. Chemical Society Reviews.. 2006;35(3):226-236.
- [Google Scholar]
- Synthesis of Peptides and Pyrazines from β-Amino Alcohols through Extrusion of H2 Catalyzed by Ruthenium Pincer Complexes: Ligand-Controlled Selectivity. Angewandte Chemie.. 2011;123(51):12448-12452.
- [Google Scholar]
- Ruthenium Pincer-Catalyzed Acylation of Alcohols Using Esters with Liberation of Hydrogen under Neutral Conditions. Adv. Synth. Catal.. 2010;352(18):3169-3173.
- [Google Scholar]
- Synthesis of amides from esters and amines with liberation of H2 under neutral conditions. J. Am. Chem. Soc.. 2011;133(6):1682-1685.
- [Google Scholar]
- Direct synthesis of imines from alcohols and amines with liberation of H2. Angewandte Chemie International Edition.. 2010;49(8):1468-1471.
- [Google Scholar]
- Stable, yet highly reactive nonclassical iron (II) polyhydride pincer complexes: Z-selective dimerization and hydroboration of terminal alkynes. Journal of the American Chemical Society.. 2017;139(24):8130-8133.
- [Google Scholar]
- Asymmetric 1, 3-dipolar cycloaddition reactions. Chemical Reviews.. 1998;98(2):863-910.
- [Google Scholar]
- Catalytic enantioselective 1, 3-dipolar cycloaddition reactions of nitrones. Chemical Communications.. 2000;16:1449-1458.
- [Google Scholar]
- Neutral p-cymene ruthenium complexes with P-stereogenic monophosphines. New catalytic precursors in enantioselective transfer hydrogenation and cyclopropanation. J. Organomet. Chem.. 2012;696(26):4221-4228.
- [Google Scholar]
- Sodium borohydride in carboxylic acid media: a phenomenal reduction system. Chemical Society Reviews.. 1998;27(6):395-404.
- [Google Scholar]
- Alcohols as Electrophiles in C C Bond-Forming Reactions: The Hydrogen Autotransfer Process. Angewandte Chemie International Edition.. 2007;46(14):2358-2364.
- [Google Scholar]
- Hydrogen autotransfer in the N-alkylation of amines and related compounds using alcohols and amines as electrophiles. Chemical reviews.. 2010;110(3):1611-1641.
- [Google Scholar]
- Solvent-free condensation of arylacetonitrile with aldehydes. Tetrahedron.. 2005;61(42):10129-10137.
- [Google Scholar]
- Acceptorless Dehydrogenative Oxidation of Secondary Alcohols Catalysed by Cp* IrIII–NHC Complexes. Chem. Eur. J.. 2016;22(30):10513-10522.
- [Google Scholar]
- Selective synthesis of primary amines directly from alcohols and ammonia. Angew. Chem.. 2008;120(45):8789-8792.
- [Google Scholar]
- Metal–ligand cooperation by aromatization–dearomatization: a new paradigm in bond activation and “Green” catalysis. Acc. Chem. Res.. 2011;44(8):588-602.
- [Google Scholar]
- Direct synthesis of amides from alcohols and amines with liberation of H2. Science.. 2007;317(5839):790-792.
- [Google Scholar]
- Gunanathan, C., Hölscher, M., Leitner, W. (2011) Reduction of Nitriles to Amines with H2 Catalyzed by Nonclassical Ruthenium Hydrides–Water‐Promoted Selectivity for Primary Amines and Mechanistic Investigations, Wiley Online Library.
- “Long-Range” Metal− Ligand Cooperation in H2 Activation and Ammonia-Promoted Hydride Transfer with a Ruthenium− Acridine Pincer Complex. Journal of the American Chemical Society.. 2010;132(42):14763-14765.
- [Google Scholar]
- Bond activation by metal-ligand cooperation: Design of “green” catalytic reactions based on aromatization-dearomatization of pincer complexes. Bifunctional molecular catalysis: Springer; 2011. p. :55-84.
- Applications of acceptorless dehydrogenation and related transformations in chemical synthesis. Science.. 2013;341(6143)
- [Google Scholar]
- Bond activation and catalysis by ruthenium pincer complexes. Chem. Rev.. 2014;114(24):12024-12087.
- [Google Scholar]
- Direct conversion of alcohols to acetals and H2 catalyzed by an acridine-based ruthenium pincer complex. Journal of the American Chemical Society.. 2009;131(9):3146-3147.
- [Google Scholar]
- Copper-catalyzed C-H functionalization reactions: efficient synthesis of heterocycles. Chemical reviews.. 2015;115(3):1622-1651.
- [Google Scholar]
- The Catalyst Precursor, Catalyst, and Intermediate in the RuII-Promoted Asymmetric Hydrogen Transfer between Alcohols and Ketones. Angewandte Chemie International Edition in English.. 1997;36(3):285-288.
- [Google Scholar]
- Structure− activity relationships for cytotoxic ruthenium (II) arene complexes containing N, N-, N, O-, and O. O-chelating ligands. Journal of medicinal chemistry.. 2006;49(23):6858-6868.
- [Google Scholar]
- Borrowing hydrogen in the activation of alcohols. Advanced synthesis & catalysis.. 2007;349(10):1555-1575.
- [Google Scholar]
- Recent development of peptide coupling reagents in organic synthesis. Tetrahedron.. 2004;60(11):2447-2467.
- [Google Scholar]
- Catalytic hydrogenation of cyclic carbonates: a practical approach from CO2 and epoxides to methanol and diols. Angewandte Chemie.. 2012;124(52):13218-13222.
- [Google Scholar]
- A new class of “tethered” ruthenium (II) catalyst for asymmetric transfer hydrogenation reactions. Journal of the American Chemical Society.. 2004;126(4):986-987.
- [Google Scholar]
- Ruthenium (II) Complexes Bearing Schiff Base Ligands for Efficient Acceptorless Dehydrogenation of Secondary Alcohols. Chinese Journal of Chemistry.. 2021;39(1):121-128.
- [Google Scholar]
- KP1019, a new redox-active anticancer agent–Preclinical development and results of a clinical phase I study in tumor patients. Chemistry & biodiversity.. 2008;5(10):2140-2155.
- [Google Scholar]
- Asymmetric transfer hydrogenation of aromatic ketones catalyzed by chiral ruthenium (II) complexes. Journal of the American Chemical Society.. 1995;117(28):7562-7563.
- [Google Scholar]
- Enhanced reactivities toward amines by introducing an imine arm to the pincer ligand: Direct coupling of two amines to form an imine without oxidant. Organometallics.. 2012;31(14):5208-5211.
- [Google Scholar]
- Ligand-enabled cross-coupling of C (sp3)–H bonds with arylsilanes. Journal of the American Chemical Society.. 2015;137(14):4618-4621.
- [Google Scholar]
- Sustainable access to renewable N-containing chemicals from reductive amination of biomass-derived platform compounds. Green Chemistry.. 2020;22(20):6714-6747.
- [Google Scholar]
- Hydrogenation of imines catalysed by ruthenium (II) complexes based on lutidine-derived CNC pincer ligands. Dalton Trans.. 2013;42(2):351-354.
- [Google Scholar]
- Current advances on ruthenium (II) N-heterocyclic carbenes in hydrogenation reactions. Coordination Chemistry Reviews.. 2018;374:114-132.
- [Google Scholar]
- Direct Orthoruthenation of Planar Prochiral Pyridine Derivatives by C− H Bond Activation with [Ru (CO) 2Cl2] n and Its Unexpected Stereoselectivity. Inorganic chemistry.. 2006;45(12):4589-4591.
- [Google Scholar]
- Head-to-Head Homo-Coupling of Arylethynes Catalysed by (Dicarbonyl) ruthenium Chloride Metallacycles: Selective Synthesis of (E)-1, 4-Diarylbut-1-en-3-ynes. Adv. Synth. Catal.. 2008;350(10):1493-1496.
- [Google Scholar]
- Decomposition of ruthenium olefin metathesis catalysts. Journal of the American Chemical Society.. 2007;129(25):7961-7968.
- [Google Scholar]
- When bifunctional catalyst encounters dual MLC modes: DFT study on the mechanistic preference in Ru-PNNH pincer complex catalyzed dehydrogenative coupling reaction. ACS Catalysis.. 2017;7(1):786-795.
- [Google Scholar]
- The remarkable metal-catalysed olefin metathesis reaction. Nature.. 2007;450(7167):243-251.
- [Google Scholar]
- Esterification and transesterification of renewable chemicals. Topics in Catalysis.. 2004;27(1):83-96.
- [Google Scholar]
- A novel liquid organic hydrogen carrier system based on catalytic peptide formation and hydrogenation. Nature communications.. 2015;6(1):1-7.
- [Google Scholar]
- Rechargeable hydrogen storage system based on the dehydrogenative coupling of ethylenediamine with ethanol. Angewandte Chemie.. 2016;128(3):1073-1076.
- [Google Scholar]
- Effect of ceria crystal plane on the physicochemical and catalytic properties of Pd/ceria for CO and propane oxidation. ACS Catalysis.. 2016;6(4):2265-2279.
- [Google Scholar]
- C-Alkylation of Ketones and Related Compounds by Alcohols: Transition-Metal-Catalyzed Dehydrogenation. Angewandte Chemie International Edition.. 2016;55(3):862-875.
- [Google Scholar]
- Novel 3-arylideneindolin-2-ones as inhibitors of NAD+-dependent histone deacetylases (sirtuins) Journal of medicinal chemistry.. 2010;53(3):1383-1386.
- [Google Scholar]
- Hudlicky, M. 1996. Reductions in organic chemistry American Chemical Society.
- Platinum and other heavy metal compounds in cancer chemotherapy. Humana Press Totowa; 2009.
- Asymmetric transfer hydrogenation of ketones with bifunctional transition metal-based molecular catalysts. Acc. Chem. Res.. 2007;40(12):1300-1308.
- [Google Scholar]
- Bifunctional transition metal-based molecular catalysts for asymmetric syntheses. Organic & Biomolecular Chemistry.. 2006;4(3):393-406.
- [Google Scholar]
- Low melting sugar–urea–salt mixtures as solvents for organic reactions—estimation of polarity and use in catalysis. Green Chemistry.. 2006;8(12):1051-1055.
- [Google Scholar]
- Control of chemoselectivity in hydrogenations of substituted nitro-and cyano-aromatics by cluster-derived ruthenium nanocatalysts. Journal of catalysis.. 2011;284(2):176-183.
- [Google Scholar]
- Catalytic hydrogenation of polar organic functionalities based on Ru-mediated heterolytic dihydrogen cleavage. Chemical communications.. 2007;48:5134-5142.
- [Google Scholar]
- Ivin, K.J., Mol, J.C. 1997. Olefin metathesis and metathesis polymerization Elsevier.
- Ruthenium complexes in different oxidation states: Synthesis, crystal structure, spectra and redox properties. Dalton Transactions.. 2013;42(17):6092-6101.
- [Google Scholar]
- Photodynamic antimicrobial studies on a Ruthenium-based metal complex. Inorganica Chimica Acta.. 2022;538:120996
- [Google Scholar]
- Conversion of alkanes to linear alkylsilanes using an iridium–iron-catalysed tandem dehydrogenation–isomerization–hydrosilylation. Nature chemistry.. 2016;8(2):157-161.
- [Google Scholar]
- Synthesis, structures and catalytic activities of half-sandwich ruthenium complexes based on Schiff Base ligands. Inorganic Chemistry Communications.. 2016;66:15-18.
- [Google Scholar]
- Half-sandwich ruthenium complexes with oxygen–nitrogen mixed ligands as efficient catalysts for nitrile hydration reaction. Polyhedron.. 2017;138:1-6.
- [Google Scholar]
- Diastereo-and Enantioselective Synthesis of Oxazine and Oxazolidine Derivatives with a Chiral Quaternary Carbon Center under Multifunctional Catalysis. Organic letters.. 2011;13(4):564-567.
- [Google Scholar]
- Joule, J.A., Mills, K., Smith, G.F. 2020. Heterocyclic chemistry CRC Press.
- A ruthenium-catalyzed reaction of aromatic ketones with arylboronates: a new method for the arylation of aromatic compounds via C− H bond cleavage. Journal of the American Chemical Society.. 2003;125(7):1698-1699.
- [Google Scholar]
- Selective room-temperature hydrogenation of amides to amines and alcohols catalyzed by a ruthenium pincer complex and mechanistic insight. ACS catalysis.. 2020;10(10):5511-5515.
- [Google Scholar]
- Near-Ambient-Temperature Dehydrogenative Synthesis of the Amide Bond: Mechanistic Insight and Applications. ACS catalysis.. 2021;11:7383-7393.
- [Google Scholar]
- Vinylideneruthenium complexes in catalysis. Coordination chemistry reviews.. 2004;248(15–16):1703-1715.
- [Google Scholar]
- N-Heterocyclic Carbene (NHC)-Stabilized Ru0 Nanoparticles. In Situ Generation of an Efficient Transfer Hydrogenation Catalyst. Chemistry–A. European Journal.. 2020;26(34):7622-7630.
- [Google Scholar]
- Ruthenium N-Heterocyclic Carbene Complexes for Chemoselective Reduction of Imines and Reductive Amination of Aldehydes and Ketones. European Journal of Inorganic Chemistry.. 2020;2020(24):2372-2379.
- [Google Scholar]
- Ruthenium catalysis in six-membered O-heterocycles synthesis. Synthetic Communications.. 2018;48(13):1551-1587.
- [Google Scholar]
- Oxindole: A chemical prism carrying plethora of therapeutic benefits. Eur. J. Med. Chem.. 2016;123:858-894.
- [Google Scholar]
- C-N Coupling of Amides with Alcohols Catalyzed by N-Heterocyclic Carbene-Phosphine Iridium Complexes. The Journal of organic chemistry.. 2015;80(22):11529-11537.
- [Google Scholar]
- Synthesis of hexahdrofuro [3, 2-c] quinoline, a martinelline type analogues and invesigation of its biological activity. Biomed. Pharmacother.. 2014;68:1161-1175.
- [Google Scholar]
- Catalytic Enantioselective C H Functionalization of Alcohols by Redox-Triggered Carbonyl Addition: Borrowing Hydrogen. Returning Carbon. Angewandte Chemie International Edition.. 2014;53(35):9142-9150.
- [Google Scholar]
- Reactions of 1, 5-Diketones with Ammonia and Its Derivatives. Chemistry of Heterocyclic Compounds.. 2003;39(9):1121-1142.
- [Google Scholar]
- Oxidant-free conversion of cyclic amines to lactams and H2 using water as the oxygen atom source. J. Am. Chem. Soc.. 2014;136(8):2998-3001.
- [Google Scholar]
- Metal–ligand cooperation. Angewandte Chemie International Edition.. 2015;54(42):12236-12273.
- [Google Scholar]
- Direct deamination of primary amines by water to produce alcohols. Angewandte Chemie International Edition.. 2013;52(24):6269-6272.
- [Google Scholar]
- N-Heterocyclic carbene-based well-defined ruthenium hydride complexes for direct amide synthesis from alcohols and amines under base-free conditions. Tetrahedron.. 2015;71(26–27):4565-4569.
- [Google Scholar]
- Ester hydrogenation catalyzed by CNN-Pincer complexes of ruthenium. Organometallics.. 2016;35(7):982-989.
- [Google Scholar]
- Efficient electrogenerated chemiluminescence from cyclometalated iridium (III) complexes. J. Am. Chem. Soc.. 2005;127(6):1614-1615.
- [Google Scholar]
- Catalytic enantioselective addition to imines. Chemical Reviews.. 1999;99:1069-1094.
- [Google Scholar]
- High-value alcohols and higher-oxidation-state compounds by catalytic Z-selective cross-metathesis. Nature.. 2015;517(7533):181-186.
- [Google Scholar]
- Catalytic oxidation of alcohols: Recent advances. Advances in organometallic chemistry.. 2015;63:91-174.
- [Google Scholar]
- Recent advances on P, N-containing ligands for transition-metal homogeneous catalysis. Current Organic Synthesis.. 2008;5(3):227-249.
- [Google Scholar]
- Direct synthesis of amides by dehydrogenative coupling of amines with either alcohols or esters: manganese pincer complex as catalyst. Angewandte Chemie International Edition.. 2017;56(47):14992-14996.
- [Google Scholar]
- CO 2 activation by manganese pincer complexes through different modes of metal–ligand cooperation. Dalton Transactions.. 2019;48(39):14580-14584.
- [Google Scholar]
- Half-sandwich arene ruthenium complexes: synthetic strategies and relevance in catalysis. Chem. Soc. Rev.. 2014;43(2):707-733.
- [Google Scholar]
- Selective hydrogenation of cyclic imides to diols and amines and its application in the development of a liquid organic hydrogen carrier. Journal of the American Chemical Society.. 2018;140(24):7453-7457.
- [Google Scholar]
- Manganese catalyzed hydrogenation of organic carbonates to methanol and alcohols. Angewandte Chemie International Edition.. 2018;57(37):12076-12080.
- [Google Scholar]
- Stereocontrolled total synthesis of (−)-ephedradine A (orantine) Journal of the American Chemical Society.. 2003;125(27):8112-8113.
- [Google Scholar]
- Kurzwernhart, A. (2013) Tumor-inhibierende Ru II (aren)-Komplexe mit Flavonoid-Liganden-auf dem Weg zu“ multi-target” Chemotherapeutika, uniwien.
- Ruthenium-catalyzed asymmetric hydrogenation of N-Boc-indoles. Org. Lett.. 2006;8(12):2653-2655.
- [Google Scholar]
- Theoretical study on the mechanism of N-heterocyclic carbene catalyzed transesterification reactions. Tetrahedron letters.. 2005;46(37):6265-6270.
- [Google Scholar]
- Larock, R.C. 1999. Comprenhensive organic transformations: a guide to functional group preparations.
- Chiral-at-metal ruthenium (II) complexes as catalysts in the asymmetric cyclopropanation reaction. J. Mol. Catal. A: Chem.. 2005;234(1–2):129-135.
- [Google Scholar]
- Amines: synthesis, properties and applications. Cambridge University Press; 2004.
- Basic Coordination Chemistry of Ruthenium. Ruthenium Complexes: Photochemical and Biomedical Applications; 2018. p. :25-41.
- Organometallic chemistry of arene ruthenium and osmium complexes. Advances in organometallic chemistry.. 1989;29:163-247.
- [Google Scholar]
- Structure–function relationship in ester hydrogenation catalyzed by ruthenium CNN-pincer complexes. Organometallics.. 2018;37(19):3286-3297.
- [Google Scholar]
- Unexpected CNN-to-CC Ligand Rearrangement in Pincer-Ruthenium Precatalysts Leads to a Base-Free Catalyst for Ester Hydrogenation. Organometallics.. 2019;38(17):3311-3321.
- [Google Scholar]
- Buchbesprechung: The Amide Linkage. Selected Structural Aspects in Chemistry, Biochemistry, and Materials Science. Herausgegeben von. Wiley Online Library; 2001.
- Ruthenium complexes as anticancer agents: A brief history and perspectives. Drug Des. Devel. Ther.. 2020;14:5375.
- [Google Scholar]
- Efficient ruthenium-catalyzed transfer hydrogenation/hydrogenation of 1, 3-cycloalkanediones to 1, 3-cycloalkanediols using microwave heating. J. Org. Chem.. 2006;71(22):8622-8625.
- [Google Scholar]
- A survey of the borrowing hydrogen approach to the synthesis of some pharmaceutically relevant intermediates. Org. Process Res. Dev.. 2015;19(10):1400-1410.
- [Google Scholar]
- Reexamination of the deuteration of phenol catalyzed by an orthometalated ruthenium complex. Inorganic Chemistry.. 1985;24(25):4433-4435.
- [Google Scholar]
- N-acyl amino acid ligands for ruthenium (II)-catalyzed meta-C–H tert-alkylation with removable auxiliaries. Journal of the American Chemical Society.. 2015;137(43):13894-13901.
- [Google Scholar]
- Eichele; K. Synthesis, characterization, and catalytic application of a new family of diamine (diphosphine) ruthenium (II) complexes. J. Organomet. Chem.. 2003;665:176-185.
- [Google Scholar]
- Mechanism of Enyne Metathesis Catalyzed by Grubbs Ruthenium− Carbene Complexes: A DFT Study. Journal of the American Chemical Society.. 2005;127(20):7444-7457.
- [Google Scholar]
- Pyridazine-based N-heterocyclic carbene complexes and ruthenium-catalyzed oxidation reaction of alkenes. Organometallics.. 2012;31(18):6614-6622.
- [Google Scholar]
- Copper (II) hydroxide complexes of N-heterocyclic carbenes and catalytic oxidative amination of arylboronic acids. Organometallics.. 2010;29(6):1457-1464.
- [Google Scholar]
- π-Conjugated aromatic enynes as a single-emitting component for white electroluminescence. Journal of the American Chemical Society.. 2006;128(17):5592-5593.
- [Google Scholar]
- Tandem catalysis: The sequential mediation of olefin metathesis, hydrogenation, and hydrogen transfer with single-component Ru complexes. Journal of the American Chemical Society.. 2001;123(45):11312-11313.
- [Google Scholar]
- A practical and highly active ruthenium-based catalyst that effects the cross metathesis of acrylonitrile. Angewandte Chemie.. 2002;114(21):4207-4209.
- [Google Scholar]
- A dual-targeting ruthenium nanodrug that inhibits primary tumor growth and lung metastasis via the PARP/ATM pathway. Journal of nanobiotechnology.. 2021;19(1):1-13.
- [Google Scholar]
- Catalytic α-Alkylation/Reduction of Ketones with Primary Alcohols To Furnish Secondary Alcohols. Synthesis.. 2016;48(05):644-652.
- [Google Scholar]
- Synthesis and in vitro antitumor activity of 1-(3-dimethylamino) propyl indolin-2-one derivatives. Medicinal Chemistry Research.. 2013;22(4):1723-1729.
- [Google Scholar]
- Mechanistic Investigation of the Ruthenium–N-Heterocyclic-Carbene-Catalyzed Amidation of Amines with Alcohols. Chem. Eur. J.. 2012;18(49):15683-15692.
- [Google Scholar]
- Biological properties of ruthenium (II)/(III) complexes with flavonoids as ligands. Coordination Chemistry Reviews.. 2021;436:213849
- [Google Scholar]
- Synthesis and characterisation of cycloruthenated benzhydrazone complexes: Catalytic application to selective oxidative cleavage of olefins to aldehydes. RSC advances.. 2016;6(99):97107-97115.
- [Google Scholar]
- Direct Aerobic Strategy for Synthesis of Imines via Alcohols and Amines Promoted by Ruthenium (II)(η6-p-cymene) Complexes. ChemistrySelect.. 2018;3(5):1561-1568.
- [Google Scholar]
- The Wittig olefination reaction and modifications involving phosphoryl-stabilized carbanions. Stereochemistry, mechanism, and selected synthetic aspects. Chemical Reviews.. 1989;89(4):863-927.
- [Google Scholar]
- Asymmetric transfer hydrogenation of α, β-acetylenic ketones. Journal of the American Chemical Society.. 1997;119(37):8738-8739.
- [Google Scholar]
- Ruthenium-stabilized low-coordinate phosphorus atoms. p-cymene ligand as reactivity switch. Organometallics.. 2007;26(20):5091-5101.
- [Google Scholar]
- Ruthenium complexes with chiral tetradentate PNNP ligands: Asymmetric catalysis from the viewpoint of inorganic chemistry. Dalton Transactions.. 2010;39(34):7851-7869.
- [Google Scholar]
- Quinoline, quinazoline and acridone alkaloids. Nat. Prod. Rep.. 2005;22(5):627-646.
- [Google Scholar]
- Nitro-substituted Hoveyda− Grubbs ruthenium carbenes: enhancement of catalyst activity through electronic activation. Journal of the American Chemical Society.. 2004;126(30):9318-9325.
- [Google Scholar]
- Discovery of environmentally benign catalytic reactions of alcohols catalyzed by pyridine-based pincer Ru complexes, based on metal–ligand cooperation. Top. Catal.. 2010;53(13):915-923.
- [Google Scholar]
- Metal–ligand cooperation by aromatization–dearomatization as a tool in single bond activation. Philosophical Transactions of the Royal Society A: Mathematical, Physical and Engineering Sciences.. 2015;373(2037):20140189.
- [Google Scholar]
- Chemoselective reductive alkylation of ammonia with carbonyl compounds: synthesis of primary and symmetrical secondary amines. Tetrahedron.. 2004;60(7):1463-1471.
- [Google Scholar]
- Introducing Catalysis to Undergraduate Chemistry Students: Testing a Ru–NHC Complex in the Selective Dehydrogenative Coupling of Hydrosilanes and Alcohols. J. Chem. Educ.. 2021;98(8):2638-2642.
- [Google Scholar]
- N-Pyrrolyl Phosphines: An Unexploited Class of Phosphine Ligands with Exceptional. pi.-Acceptor Character. Journal of the American Chemical Society.. 1995;117(29):7696-7710.
- [Google Scholar]
- Synthesis: carbon with two attached heteroatoms with at least one carbon-toheteroatom multiple link. Elsevier; 1995.
- J. Organomet. Chem.. 1988;350:157-190.
- Morris, R.H. 2013. Ester hydrogenation catalyzed by a ruthenium (II) complex bearing an N-heterocyclic carbene tethered with an “NH2” group and a DFT study of the proposed bifunctional mechanism.
- Air-stable iron catalyst for the Oppenauer-type oxidation of alcohols. Tetrahedron Letters.. 2010;51(41):5430-5433.
- [Google Scholar]
- Efficient catalytic addition of aromatic carbon-hydrogen bonds to olefins. Nature.. 1993;366(6455):529-531.
- [Google Scholar]
- Anticancer and antimicrobial properties of novel η 6-p-cymene ruthenium (ii) complexes containing a N, S-type ligand, their structural and theoretical characterization. RSC Advances.. 2019;9(66):38629-38645.
- [Google Scholar]
- Arene-Ruthenium (II) Complexes with Carbothiamidopyrazoles as a Potential Alternative for Antibiotic Resistance in Human. Molecules.. 2022;27(2):468.
- [Google Scholar]
- Ruthenium-catalyzed reactions for organic synthesis. Chemical reviews.. 1998;98(7):2599-2660.
- [Google Scholar]
- Metal-Metal Interactions in Bi-, Tri-and Multinuclear Fe, Ru and Os N-Heterocyclic Carbene Complexes and their Catalytic Applications. European Journal of Inorganic Chemistry. 2021
- [Google Scholar]
- Recent developments in ruthenium-catalyzed C-H arylation: Array of mechanistic manifolds. ACS Catalysis.. 2017;7(9):5721-5745.
- [Google Scholar]
- Ring-opening metathesis polymerization (ROMP) of norbornene by a group VIII carbene complex in protic media. Journal of the American Chemical Society.. 1992;114(10):3974-3975.
- [Google Scholar]
- Transfer hydrogenation in water via a ruthenium catalyst with OH groups near the metal center on a bipy scaffold. Organometallics.. 2011;30(23):6339-6342.
- [Google Scholar]
- Ferrocene-derived P, N ligands: synthesis and application in enantioselective catalysis. Green Processing and Synthesis.. 2013;2(4):297-309.
- [Google Scholar]
- Direct synthesis of 2-phenylethanol by hydrogenation of methyl phenylacetate using homogeneous ruthenium-phosphine catalysis under low hydrogen pressure. Journal of Molecular Catalysis A: Chemical.. 2001;166(2):345-349.
- [Google Scholar]
- Amide synthesis from alcohols and amines by the extrusion of dihydrogen. J. Am. Chem. Soc.. 2008;130(52):17672-17673.
- [Google Scholar]
- Asymmetric catalysis: Science and opportunities (Nobel lecture) Angewandte Chemie International Edition.. 2002;41(12):2008-2022.
- [Google Scholar]
- Asymmetric transfer hydrogenation catalyzed by chiral ruthenium complexes. Accounts of chemical research.. 1997;30(2):97-102.
- [Google Scholar]
- P roc. Natl. Acad. Sci. USA 2004, 101, 5356–5362; c) R. Noyori. S. Hashiguchi. Acc. Chem. Res.. 1997;30:97-102.
- [Google Scholar]
- Asymmetric catalysis by architectural and functional molecular engineering: practical chemo-and stereoselective hydrogenation of ketones. Angewandte Chemie International Edition.. 2001;40(1):40-73.
- [Google Scholar]
- Toward efficient asymmetric hydrogenation: Architectural and functional engineering of chiral molecular catalysts. Proceedings of the National Academy of Sciences.. 2004;101(15):5356-5362.
- [Google Scholar]
- Iridium-catalyzed reactions involving transfer hydrogenation, addition, n-heterocyclization, and alkylation using alcohols and diols as key substrates. Synlett.. 2011;2011(01):30-51.
- [Google Scholar]
- Iridium (I) complex of chelating pyridine-2-thiolate ligand: Synthesis, reactivity, and application to the catalytic E-selective terminal alkyne dimerization via C-H activation. Journal of Organometallic Chemistry.. 2007;692(19):4139-4146.
- [Google Scholar]
- Practical enantioselective hydrogenation of aromatic ketones. Journal of the American Chemical Society.. 1995;117(9):2675-2676.
- [Google Scholar]
- Ruthenium complex-catalyzed direct ortho arylation and alkenylation of 2-arylpyridines with organic halides. Organic letters.. 2001;3(16):2579-2581.
- [Google Scholar]
- Ruthenium complex catalyzed direct ortho arylation and alkenylation of aromatic imines with organic halides. Organic letters.. 2002;4(10):1783-1785.
- [Google Scholar]
- Ruthenium indenylidene and vinylidene complexes bearing Schiff bases: Potential catalysts in enol-ester synthesis. Synlett.. 2002;2002(06):0935-0941.
- [Google Scholar]
- N-formylation of amines by methanol activation. Organic letters.. 2013;15(7):1776-1779.
- [Google Scholar]
- Toward ideal (trans) esterification by use of fluorous distannoxane catalysts. Accounts of chemical research.. 2004;37(5):288-296.
- [Google Scholar]
- Esterification: methods, reactions. and applications John Wiley & Sons; 2009.
- Direct Arylation of Arene C− H Bonds by Cooperative Action of NHCarbene− Ruthenium (II) Catalyst and Carbonate via Proton Abstraction Mechanism. Journal of the American Chemical Society.. 2008;130(4):1156-1157.
- [Google Scholar]
- Synthesis and catalytic applications of Ru and Ir complexes containing N. O-chelating ligand. Journal of Organometallic Chemistry.. 2020;925:121486
- [Google Scholar]
- Unprecedented Polymerization of ε-Caprolactone Initiated by a Single-Site Lanthanide Borohydride Complex,[Sm (η-C5Me5) 2 (BH4)(thf)]: Mechanistic Insights. Chemistry–A. European Journal.. 2004;10(16):4054-4062.
- [Google Scholar]
- Ester Hydrogenation with Bifunctional Metal–NHC Catalysts: Recent Advances. ACS omega.. 2020;5(48):30775-30786.
- [Google Scholar]
- Enantioselective double Michael addition/cyclization with an oxygen-centered nucleophile as the first step in a concise synthesis of natural (+)-asteriscanolide. Journal of the American Chemical Society.. 2000;122(12):2742-2748.
- [Google Scholar]
- Encyclopedia of reagents for organic synthesis Van Dyke. TF: AR; Jamison; 2009.
- Transition metal based catalysts in the aerobic oxidation of alcohols. Green Chemistry.. 2012;14(3):547-564.
- [Google Scholar]
- Ligand-metal hydrogen-transfer reactions in triphenyl phosphite and triphenylphosphine complexes. Journal of the American Chemical Society.. 1969;91(18):4990-4995.
- [Google Scholar]
- The synthesis, lipophilicity and cytotoxic effects of new ruthenium (II) arene complexes with chromone derivatives. Journal of Inorganic Biochemistry.. 2016;159:133-141.
- [Google Scholar]
- Catalytic meta-selective C-H functionalization to construct quaternary carbon centres. Chemical Communications.. 2015;51(64):12807-12810.
- [Google Scholar]
- N-heterocyclic carbene metal complexes as bio-organometallic antimicrobial and anticancer drugs. Future medicinal chemistry.. 2015;7(10):1305-1333.
- [Google Scholar]
- Iron (II) Pincer-Catalyzed Synthesis of Lactones and Lactams through a Versatile Dehydrogenative Domino Sequence. ChemCatChem.. 2015;7(5):865-871.
- [Google Scholar]
- Peptides, S.N.J.H.-D. (2002) Chemistry and Biology Wiley, VCH Weinheim:.
- Alkane CH activation by single-site metal catalysis. Springer Science & Business Media; 2012.
- Recent progress with pincer transition metal catalysts for sustainability. Catalysts.. 2020;10(7):773.
- [Google Scholar]
- Base-Free Synthesis of Furfurylamines from Biomass Furans Using Ru Pincer Complexes. Catalysts.. 2021;11(5):558.
- [Google Scholar]
- Synthesis and applications of isotopically labelled compounds, International Symposium on the Synthesis and Applications of Isotopes and Isotopically Labelled Compounds 2000: Dresden, Germany). John Wiley; 2001.
- Pollak, P., Romeder, G., Hagedorn, F., Gelbke, H.P. 2000. Nitriles. Ullmann's encyclopedia of industrial chemistry.
- Selective hydrogenation of methyl oleate into unsaturated alcohols: Relationships between catalytic properties and composition of cobalt–tin catalysts. Catalysis Today.. 2000;63(1):87-100.
- [Google Scholar]
- New ruthenium (II) carbonyl complexes bearing disulfide Schiff base ligands and their applications as catalyst for some organic transformations. Spectrochimica Acta Part A: Molecular and Biomolecular Spectroscopy.. 2014;129:352-358.
- [Google Scholar]
- Catalytic role of ruthenium (III) complexes bearing cystamine-based disulfide ligands on the N-alkylation of o-substituted anilines. Monatshefte für Chemie-Chemical Monthly.. 2014;145(12):1903-1912.
- [Google Scholar]
- Half-sandwich Ruthenium (II) Schiff base complexes: Synthesis, characterization and effective catalysts for one-pot conversion of aldehydes to amides. Journal of Organometallic Chemistry.. 2019;902:120964
- [Google Scholar]
- Heterogeneous and homogeneous catalysis for the hydrogenation of carboxylic acid derivatives: history, advances and future directions. Chemical Society Reviews.. 2015;44(11):3808-3833.
- [Google Scholar]
- Cationic arene ruthenium (II) complexes bearing N, S chelating thiocarboxamides: Synthesis, structure, characterization and catalytic oxidation of alcohols. J. Organomet. Chem.. 2012;699:5-11.
- [Google Scholar]
- Efficient and versatile catalysis of N-alkylation of heterocyclic amines with alcohols and one-pot synthesis of 2-aryl substituted benzazoles with newly designed ruthenium (II) complexes of PNS thiosemicarbazones. Dalton Transactions.. 2014;43(21):7889-7902.
- [Google Scholar]
- Ruthenium (II) complexes containing a phosphine-functionalized thiosemicarbazone ligand: synthesis, structures and catalytic C-N bond formation reactions via N-alkylation. RSC Advances.. 2015;5(15):11405-11422.
- [Google Scholar]
- Ruthenium-cymene containing pyridine-derived aldiimine ligands: Synthesis, characterization and application in the transfer hydrogenation of aryl ketones and kinetics studies. Journal of Organometallic Chemistry.. 2019;892:51-65.
- [Google Scholar]
- Synthesis and evaluation of spiroisoxazoline oxindoles as anticancer agents. Bioorganic & medicinal chemistry.. 2014;22(1):577-584.
- [Google Scholar]
- Amino group chemistry: from synthesis to the life sciences. John Wiley & Sons; 2008.
- Ricci, A. 2008a. Modern amination methods John Wiley & Sons.
- Fatty methyl ester hydrogenation to fatty alcohol part II: process issues. Journal of the American Oil Chemists' Society.. 1997;74(4):341-345.
- [Google Scholar]
- Ruthenium-allenylidene complexes and their specific behaviour. Coordination chemistry reviews.. 2004;248(15–16):1585-1601.
- [Google Scholar]
- Transition metal and enzyme catalyzed reactions involving reactions with ammonia and amines. Chemical reviews.. 1992;92(1):1-27.
- [Google Scholar]
- Ru-catalysed C-H functionalisations as a tool for selective organic synthesis. Tetrahedron Letters.. 2016;57(31):3413-3432.
- [Google Scholar]
- A green chemistry perspective on catalytic amide bond formation. Nature Catalysis.. 2019;2(1):10-17.
- [Google Scholar]
- Selective hydrogenation of nitriles to secondary amines catalyzed by a pyridyl-functionalized and alkenyl-tethered NHC–Ru (II) complex. Journal of Organometallic Chemistry.. 2016;812:87-94.
- [Google Scholar]
- Amide synthesis from alcohols and amines catalyzed by a RuII–N-heterocyclic carbene (NHC)–carbonyl complex. Journal of Organometallic Chemistry.. 2014;771:124-130.
- [Google Scholar]
- Properties and applications of ruthenium. Nanoscale Effects and Applications, IntechOpen: Noble and Precious Metals-Properties; 2018.
- Mechanistic aspects of transition metal-catalyzed hydrogen transfer reactions. Chemical Society Reviews.. 2006;35(3):237-248.
- [Google Scholar]
- Hydrogenation/dehydrogenation of N-heterocycles catalyzed by ruthenium complexes based on multimodal proton-responsive CNN (H) pincer ligands. Dalton Transactions.. 2020;49(28):9583-9587.
- [Google Scholar]
- Mechanism of asymmetric hydrogenation of ketones catalyzed by BINAP/1, 2-diamine− ruthenium (II) complexes. Journal of the American Chemical Society.. 2003;125(44):13490-13503.
- [Google Scholar]
- A versatile precursor for the synthesis of new ruthenium olefin metathesis catalysts. Organometallics.. 2001;20(25):5314-5318.
- [Google Scholar]
- Dehydrogenative synthesis of carboxylic acids from primary alcohols and hydroxide catalyzed by a ruthenium N-heterocyclic carbene complex. J. Org. Chem.. 2016;81(20):9931-9938.
- [Google Scholar]
- Total synthesis of (−)-and (±)-frontalin via ring-closing metathesis. Tetrahedron letters.. 1999;40(8):1425-1428.
- [Google Scholar]
- Increased ring closing metathesis activity of ruthenium-based olefin metathesis catalysts coordinated with imidazolin-2-ylidene ligands. Tetrahedron Letters.. 1999;40(12):2247-2250.
- [Google Scholar]
- Synthesis and activity of a new generation of ruthenium-based olefin metathesis catalysts coordinated with 1, 3-dimesityl-4, 5-dihydroimidazol-2-ylidene ligands. Organic letters.. 1999;1(6):953-956.
- [Google Scholar]
- Application of Electron-Withdrawing Coordinatively Unsaturated η6-Arene β-Diketiminato–Ruthenium Complexes in Lewis Acid Catalyzed Diels-Alder Reactions. Organometallics.. 2011;30(20):5381-5395.
- [Google Scholar]
- Synthesis and applications of RuCl2 (CHR ‘)(PR3) 2: the influence of the alkylidene moiety on metathesis activity. Journal of the American Chemical Society.. 1996;118(1):100-110.
- [Google Scholar]
- Reductions by the alumino-and borohydrides in organic synthesis. John Wiley & Sons; 1997.
- Mechanistic view of Ru-catalyzed C-H bond activation and functionalization: computational advances. Chemical Society Reviews.. 2018;47(20):7552-7576.
- [Google Scholar]
- Fundamentals of green chemistry: efficiency in reaction design. Chemical Society Reviews.. 2012;41(4):1437-1451.
- [Google Scholar]
- Sheldon, R.A. 1993. Chirotechnology: industrial synthesis of optically active compounds CRC press.
- Synthesis and Reactivity of Metal-Ligand Cooperative Bifunctional Ruthenium Hydride Complexes: Active Catalysts for β-Alkylation of Secondary Alcohols with Primary Alcohols. Organometallics.. 2018;37(16):2795-2806.
- [Google Scholar]
- Efficient method for preparation of N-methoxy-N-methyl amides by reaction of lactones or esters with Me2AlCl MeONHMe· HCl. Tetrahedron letters.. 1997;38(15):2685-2688.
- [Google Scholar]
- Synthesis of optically active phthaloyl D-aminooxy acids from L-amino acids or L-hydroxy acids as building blocks for the preparation of aminooxy peptides. The Journal of organic chemistry.. 2000;65(22):7667-7675.
- [Google Scholar]
- Iridium and ruthenium complexes of N-heterocyclic carbene-and pyridinol-derived chelates as catalysts for aqueous carbon dioxide hydrogenation and formic acid dehydrogenation: The role of the alkali metal. Organometallics.. 2017;36(6):1091-1106.
- [Google Scholar]
- Green chemistry oriented organic synthesis in water. Chemical Society Reviews.. 2012;41(4):1415-1427.
- [Google Scholar]
- Palladium (ii) thiocarboxamide complexes: synthesis, characterisation and application to catalytic Suzuki coupling reactions. Dalton Transactions.. 2012;41(17):5351-5361.
- [Google Scholar]
- Direct synthesis of amides from coupling of alcohols and amines catalyzed by ruthenium (II) thiocarboxamide complexes under aerobic conditions. Organometallics.. 2014;33(16):4269-4278.
- [Google Scholar]
- DNA binding and topoisomerase II inhibitory activity of water-soluble ruthenium (II) and rhodium (III) complexes. Inorganic chemistry.. 2007;46(25):10869-10876.
- [Google Scholar]
- Synthesis of phosphorus esters by transesterification mediated by N-heterocyclic carbenes (NHCs) Chemical communications.. 2005;43:5456-5458.
- [Google Scholar]
- Inter-and intramolecular thermal activation of sp3 C− H bonds with ruthenium bisallyl complexes. Organometallics.. 1999;18(17):3316-3326.
- [Google Scholar]
- Synthesis, structural analysis, redox properties and in vitro antitumor evaluation of half-sandwich complexes of Ru (II) with aminocoumarins. Polyhedron.. 2017;127:307-314.
- [Google Scholar]
- Spectroscopic and cytotoxic characteristics of (p-cymene) Ru (II) complexes with bidentate coumarins and density functional theory comparison with selected Pd (II) complexes. Inorganica Chimica Acta.. 2017;456:105-112.
- [Google Scholar]
- Review of methods for the catalytic hydrogenation of carboxamides. Chemical reviews.. 2014;114(10):5477-5510.
- [Google Scholar]
- Quinoline as a privileged scaffold in cancer drug discovery. Current medicinal chemistry.. 2011;18(10):1488-1508.
- [Google Scholar]
- Transfer hydrogenation in water: Enantioselective, catalytic reduction of α-cyano and α-nitro substituted acetophenones. Organic letters.. 2010;12(13):2893-2895.
- [Google Scholar]
- ACS Catal. 2014, 4, 2889–2895; b) S. Chakraborty, PO Lagaditis, M. Förster, EA Bielinski, N. Hazari, MC Holthausen, WD Jones, S. Schneider, ACS Catal.. 2014;4:3994-4003.
- [Google Scholar]
- Cycloruthenated primary and secondary amines as efficient catalyst precursors for asymmetric transfer hydrogenation. Org. Lett.. 2005;7(7):1247-1250.
- [Google Scholar]
- Acceptorless dehydrogenative coupling of ethanol and hydrogenation of esters and imines. Organometallics.. 2012;31(15):5239-5242.
- [Google Scholar]
- Catalytic coupling of nitriles with amines to selectively form imines under mild hydrogen pressure. Chemical Communications.. 2012;48(97):11853-11855.
- [Google Scholar]
- Ruthenium Pincer-Catalyzed Cross-Dehydrogenative Coupling of Primary Alcohols with Secondary Alcohols under Neutral Conditions. Advanced Synthesis & Catalysis.. 2012;354(13):2403-2406.
- [Google Scholar]
- Direct synthesis of pyridines and quinolines by coupling of γ-amino-alcohols with secondary alcohols liberating H 2 catalyzed by ruthenium pincer complexes. Chemical Communications.. 2013;49(59):6632-6634.
- [Google Scholar]
- Direct Synthesis of Pyrroles by Dehydrogenative Coupling of β-Aminoalcohols with Secondary Alcohols Catalyzed by Ruthenium Pincer Complexes. Angewandte Chemie.. 2013;125(14):4104-4107.
- [Google Scholar]
- Stark, M.J., Shaw, M.J., Rath, N.P., Bauer, E.B. 2016. Synthesis, Structural Characterization and Catalytic Activity of Indenyl tris-N-Pyrrolyl Phosphine Complexes of Ruthenium.
- Copper (I)/ABNO-catalyzed aerobic alcohol oxidation: alleviating steric and electronic constraints of Cu/TEMPO catalyst systems. Journal of the American Chemical Society.. 2013;135(42):15742-15745.
- [Google Scholar]
- Molecular recognition and physicochemical properties in the discovery of selective antibacterial minor groove binders. Journal of Physical Organic Chemistry.. 2008;21(7–8):575-583.
- [Google Scholar]
- Ester hydrogenation catalyzed by Ru-CNN pincer complexes. ChemComm.. 2011;47(29):8349-8351.
- [Google Scholar]
- Ruthenium Complexes as Promising Candidates against Lung Cancer. Molecules.. 2021;26(15):4389.
- [Google Scholar]
- Organic synthesis involving iridium-catalyzed oxidation. Chemical reviews.. 2011;111(3):1825-1845.
- [Google Scholar]
- A review of the selective catalytic reduction of aromatic nitro compounds into aromatic amines, isocyanates, carbamates, and ureas using CO. Chemical reviews.. 1996;96(6):2035-2052.
- [Google Scholar]
- Td, N. 2009. Whittlesey MK. Williams JMJ. Dalton Trans. 753.
- Homogeneous ruthenium catalyzed hydrogenation of esters to alcohols. Chemical Communications.. 1998;13:1367-1368.
- [Google Scholar]
- Facile ruthenium (II)-catalyzed α-alkylation of arylmethyl nitriles using alcohols enabled by metal–ligand cooperation. Acs Catalysis.. 2017;7(8):5483-5490.
- [Google Scholar]
- Ruthenium-catalyzed α-olefination of nitriles using secondary alcohols. ACS Catalysis.. 2018;8(3):2473-2478.
- [Google Scholar]
- Tojo, G., Fernández, M. 2007. Oxidation of primary alcohols to carboxylic acids. A Guide to Current Common Practice.
- Concurrent hydrogenation of aromatic and nitro groups over carbon-supported ruthenium catalysts. ACS Catalysis.. 2015;5(1):203-209.
- [Google Scholar]
- Ruthenium assisted reversible proton transfer from an aromatic carbon to a hydride. Journal of the American Chemical Society.. 2000;122(28):6777-6778.
- [Google Scholar]
- Asymmetric Hydrogenation of Unprotected Indoles Catalyzed by η6-Arene/N-Me-sulfonyldiamine–Ru (II) Complexes. J. Am. Chem. Soc.. 2016;138(35):11299-11305.
- [Google Scholar]
- The development of L2X2Ru CHR olefin metathesis catalysts: an organometallic success story. Accounts of Chemical Research.. 2001;34(1):18-29.
- [Google Scholar]
- Oxidant-free conversion of primary amines to nitriles. Journal of the American Chemical Society.. 2013;135(44):16352-16355.
- [Google Scholar]
- Base-free, acceptorless, and chemoselective alcohol dehydrogenation catalyzed by an amide-derived NNN-ruthenium (II) hydride complex. Organometallics.. 2013;32(7):2046-2049.
- [Google Scholar]
- Synthesis and characterization of the transition metal complexes: their alcohol oxidation and electrochemical properties. Synthesis and Reactivity in Inorganic, Metal-Organic, and Nano-Metal Chemistry.. 2011;41(2):211-223.
- [Google Scholar]
- The catalytic hydrogenolysis of esters to alcohols. Catalysis Reviews—Science and Engineering.. 1994;36(4):645-683.
- [Google Scholar]
- Synthesis of 2-aminomethylpiperidine ruthenium (II) phosphine complexes and their applications in transfer hydrogenation of aryl ketones. Applied Organometallic Chemistry.. 2012;26(12):731-735.
- [Google Scholar]
- A New and Eco-Friendly Method for Reduction of Ketones in Water. ChemistrySelect.. 2019;4(28):8322-8326.
- [Google Scholar]
- Synthesis and catalytic applications of Ru (II)-phosphaurotropine complexes with the use of simple water-soluble Ru (II)-precursors. Coordination Chemistry Reviews.. 2021;438:213871
- [Google Scholar]
- Direct observation of the oxidative addition of the aryl carbon− oxygen bond to a ruthenium complex and consideration of the relative reactivity between aryl carbon− oxygen and aryl carbon− hydrogen bonds. Journal of the American Chemical Society.. 2006;128(51):16516-16517.
- [Google Scholar]
- Synthesis of p-aminophenol by catalytic hydrogenation of p-nitrophenol. Organic process research & development.. 2003;7(2):202-208.
- [Google Scholar]
- Amide bond formation: beyond the myth of coupling reagents. Chem. Soc. Rev.. 2009;38(2):606-631.
- [Google Scholar]
- Cooperative catalysis with first-row late transition metals. European Journal of Inorganic Chemistry.. 2012;2012(3):363-375.
- [Google Scholar]
- Neutral Tridentate PNP Ligands and Their Hybrid Analogues: Versatile Non-Innocent Scaffolds for Homogeneous Catalysis. Angewandte Chemie International Edition.. 2009;48(47):8832-8846.
- [Google Scholar]
- Catalytic transfer hydrogenation of ketones catalyzed by orthometalated ruthenium (III) 2-(arylazo) phenolate complexes containing triphenylarsine. Tetrahedron letters.. 2005;46(31):5215-5218.
- [Google Scholar]
- Ruthenium (III) bis-bidentate Schiff base complexes mediated transfer hydrogenation of imines. Inorganic Chemistry Communications.. 2006;9(7):703-707.
- [Google Scholar]
- Ventura Espinosa, D., Carretero Cerdán, A., Baya, M., García, H., Mata Martínez, J.A. 2017. Catalytic dehydrogenative coupling of hydrosilanes with alcohols for the production of hydrogen on-demand: application of a silane/alcohol pair as a liquid organic hydrogen carrier.
- Ventura-Espinosa, D., Carretero-Cerdán, A., Baya, M., García, H., Mata, J.A. 2017. Catalytic dehydrogenative coupling of hydrosilanes with alcohols for the production of hydrogen on-demand: Application of the pair silane/alcohol as liquid organic hydrogen carrier.
- The challenge of cyclic and acyclic Schiff bases and related derivatives. Coordination Chemistry Reviews.. 2004;248(17–20):1717-2128.
- [Google Scholar]
- Iron and ruthenium Lewis acid catalyzed asymmetric 1, 3-dipolar cycloaddition reactions between nitrones and enals. Journal of the American Chemical Society.. 2002;124(18):4968-4969.
- [Google Scholar]
- New use for old drugs? Prospective targets of chloroquines in cancer therapy. Current drug targets.. 2014;15(9):843-851.
- [Google Scholar]
- Probing the mechanism of olefin metathesis in Grubbs-Hoveyda and Grela type complexes. Angewandte Chemie.. 2010;122(32):5665-5668.
- [Google Scholar]
- Ruthenium-based heterocyclic carbene-coordinated olefin metathesis catalysts. Chemical reviews.. 2010;110(3):1746-1787.
- [Google Scholar]
- Cyclometalated ruthenium complexes for sensitizing nanocrystalline TiO 2 solar cells. Chemical communications.. 2007;19:1907-1909.
- [Google Scholar]
- Acceptorless Dehydrogenation of N-Heterocycles and Secondary Alcohols by Ru (II)-NNC Complexes Bearing a Pyrazoyl-indolyl-pyridine Ligand. Organometallics.. 2018;37(4):584-591.
- [Google Scholar]
- Effect of the side chain size of 1-alkyl-pyrroles on antioxidant activity and ‘Laba’garlic greening. International journal of food science & technology.. 2008;43(10):1880-1886.
- [Google Scholar]
- Highly active bidentate N-heterocyclic carbene/ruthenium complexes performing dehydrogenative coupling of alcohols and hydroxides in open air. Chemical Communications.. 2019;55(59):8591-8594.
- [Google Scholar]
- Broader, greener, and more efficient: Recent advances in asymmetric transfer hydrogenation. Chemistry–An Asian Journal.. 2008;3(10):1750-1770.
- [Google Scholar]
- Design, synthesis and antifungal activities of novel pyrrole alkaloid analogs. European journal of medicinal chemistry.. 2011;46(5):1463-1472.
- [Google Scholar]
- Well-defined N-heterocyclic carbene/ruthenium complexes for the alcohol amidation with amines: The dual role of cesium carbonate and improved activities applying an added ligand. Applied Organometallic Chemistry.. 2020;34(2):e5323.
- [Google Scholar]
- Pincer-type complexes for catalytic (De) hydrogenation and transfer (De) hydrogenation reactions: recent progress. Chemistry–A. European Journal.. 2015;21(35):12226-12250.
- [Google Scholar]
- The status of platinum anticancer drugs in the clinic and in clinical trials. Dalton transactions.. 2010;39(35):8113-8127.
- [Google Scholar]
- The conversion of alcohols into amines. Journal of the American Chemical Society.. 1965;87(22):5261-5262.
- [Google Scholar]
- White III, J.P., Deng, H., Shore, S.G. 1991. Borohydride complexes of europium (II) and ytterbium (II) and their conversion to metal borides. Structures of (L) 4Yb [BH4] 2 (L= CH3CN, C5H5N). Inorganic chemistry. 30(10): 2337-2342.
- Allenylidene complexes of ruthenium: synthesis, spectroscopy and electron transfer properties. Coordination chemistry reviews.. 2004;248(15–16):1565-1583.
- [Google Scholar]
- Efficient and phosphine-free bidentate N-heterocyclic carbene/ruthenium catalytic systems for the dehydrogenative amidation of alcohols and amines. Organic Chemistry Frontiers.. 2019;6(5):563-570.
- [Google Scholar]
- Gram-scale synthesis of carboxylic acids via catalytic acceptorless dehydrogenative coupling of alcohols and hydroxides at an ultralow Ru loading. Applied Catalysis A: General.. 2022;630:118443
- [Google Scholar]
- The hydrogenation of molecules with polar bonds catalyzed by a ruthenium (II) complex bearing a chelating N-heterocyclic carbene with a primary amine donor. Chemical communications.. 2010;46(43):8240-8242.
- [Google Scholar]
- Highly efficient process for production of biofuel from ethanol catalyzed by ruthenium pincer complexes. Journal of the American Chemical Society.. 2016;138(29):9077-9080.
- [Google Scholar]
- A Reversible Liquid Organic Hydrogen Carrier System Based on Methanol-Ethylenediamine and Ethylene Urea. Angewandte Chemie.. 2019;131(15):5159-5163.
- [Google Scholar]
- Tunable dehydrogenative amidation versus amination using a single ruthenium-NHC catalyst. ACS Catalysis.. 2015;5(7):4143-4151.
- [Google Scholar]
- Direct α-Alkylation of Ketones with Alcohols in Water. ChemSusChem.. 2014;7(1):105-109.
- [Google Scholar]
- Green functional group transformations by supported ruthenium hydroxide catalysts. Synlett.. 2010;2010(16):2365-2382.
- [Google Scholar]
- Chemistry and antimicrobial activity of caryoynencin analogs. J. Med. Chem.. 1995;38(26):5015-5022.
- [Google Scholar]
- The metal− ligand bifunctional catalysis: A theoretical study on the ruthenium (II)-catalyzed hydrogen transfer between alcohols and carbonyl compounds. Journal of the American Chemical Society.. 2000;122(7):1466-1478.
- [Google Scholar]
- Efficient ruthenium-catalyzed α-alkylation of ketones using pyridyl methanols. Tetrahedron.. 2014;70(6):1193-1198.
- [Google Scholar]
- Ruthenium (II) complexes containing optically active hemilabile P, N, O-tridentate ligands. Synthesis and evaluation in catalytic asymmetric transfer hydrogenation of acetophenone by propan-2-ol. Organometallics.. 1997;16(7):1401-1409.
- [Google Scholar]
- Ruthenium, carbonyl [2-(diphenylphosphino-κP)-N-[2-(diphenylphosphino-κP) ethyl] ethanamine-κN][tetrahydroborato (1-)-κH]-hydrido,(OC-6-13)-. Encyclopedia of Reagents for Organic Synthesis. 2001:1-3.
- [Google Scholar]
- Ruthenium pincer complexes: Synthesis and catalytic applications. Adv. Synth. Catal.. 2015;357(2–3):283-330.
- [Google Scholar]
- Arene Ru (II) Complexes with Difluorinated Ligands Act as Potential Inducers of S-Phase Arrest via the Stabilization of c-myc G-Quadruplex DNA. Molecules.. 2022;27(6):1897.
- [Google Scholar]
- Electron-rich PNP-and PNN-type ruthenium (II) hydrido borohydride pincer complexes. Synthesis, structure, and catalytic dehydrogenation of alcohols and hydrogenation of esters. Organometallics.. 2011;30(21):5716-5724.
- [Google Scholar]
- Enantioselective construction of spirocyclic oxindoles via tandem michael/michael reactions catalyzed by multifunctional quaternary phosphonium salt. The Journal of organic chemistry.. 2016;81(21):10558-10568.
- [Google Scholar]
- Recent advances in metal catalysts with hybrid ligands. Coordination Chemistry Reviews.. 2011;255(17–18):1991-2024.
- [Google Scholar]
- Zhang, J., Leitus, G., Ben-David, Y., Milstein, D. 2005a. Facile Conversion of Alcohols into Esters and Dihydrogen Catalyzed by New Ruthenium Complexes [J. Am. Chem. Soc. 2005, 127, 10840− 10841]. Journal of the American Chemical Society. 127(35): 12429-12429.
- Synthesis of pincer hydrido ruthenium olefin complexes for catalytic alkane dehydrogenation. Organometallics.. 2016;35(2):181-188.
- [Google Scholar]
- Electron-rich, bulky ruthenium PNP-type complexes. Acceptorless catalytic alcohol dehydrogenation. Organometallics.. 2004;23(17):4026-4033.
- [Google Scholar]
- Electron-rich, bulky PNN-type ruthenium complexes: synthesis, characterization and catalysis of alcohol dehydrogenation. Dalton Transactions.. 2007;1:107-113.
- [Google Scholar]
- Synthesis of α-Amino Acid Amides: Ruthenium-Catalyzed Amination of α-Hydroxy Amides. Angewandte Chemie.. 2011;123(47):11393-11397.
- [Google Scholar]
- General and regioselective synthesis of pyrroles via ruthenium-catalyzed multicomponent reactions. J. Am. Chem. Soc.. 2013;135(30):11384-11388.
- [Google Scholar]
- Facile conversion of alcohols into esters and dihydrogen catalyzed by new ruthenium complexes. J. Am. Chem. Soc.. 2005;127(31):10840-10841.
- [Google Scholar]
- Efficient homogeneous catalytic hydrogenation of esters to alcohols. Angewandte Chemie.. 2006;118(7):1131-1133.
- [Google Scholar]
- Synthesis of cyclic imides from simple diols. Angewandte Chemie.. 2010;122(36):6535-6539.
- [Google Scholar]
- Thiourea–quaternary ammonium salt catalyzed asymmetric 1, 3-dipolar cycloaddition of imino esters to construct spiro [pyrrolidin-3, 3′-oxindoles] Organic letters.. 2016;18(19):4774-4777.
- [Google Scholar]
- Ruthenium [NNN] and [NCN]-type pincer complexes with phosphine coligands: synthesis, structures and catalytic applications. Transition Metal Chemistry.. 2020;45(2):99-110.
- [Google Scholar]
- Dinickel (II) complexes of bis (N-heterocyclic carbene) ligands containing [Ni2 (μ-OH)] cores as highly efficient catalysts for the coupling of aryl chlorides. Organometallics.. 2008;27(22):5911-5920.
- [Google Scholar]
- Ruthenium-catalyzed selective reduction of carboxylic esters and carboxamides. Synthesis.. 2019;51(12):2491-2505.
- [Google Scholar]
- Hydrogenation of Quinolines Using a Recyclable Phosphine-Free Chiral Cationic Ruthenium Catalyst: Enhancement of Catalyst Stability and Selectivity in an Ionic Liquid. Angew. Chem. Int. Ed.. 2008;47(44):8464-8467.
- [Google Scholar]







