Translate this page into:
Synthesis of UiO-66 with addition of HKUST-1 for enhanced adsorption of RBBR dye
⁎Corresponding author. rediati@chem.its.ac.id (Ratna Ediati)
-
Received: ,
Accepted: ,
This article was originally published by Elsevier and was migrated to Scientific Scholar after the change of Publisher.
Peer review under responsibility of King Saud University.
Abstract
Toxic dye removal, one of the most serious and common industrial pollutants released into natural water, is a critical issue for modern civilization. In this study, a series of UiO-66 composites was synthesized with addition of HKUST-1 using solvothermal method, which was used to remove RBBR dye. The structure, morphology and surface area of the composites were studied by several analyses. HK(5)/UiO-66 possessed a specific surface area of 557.63 m2/g and showed an adsorption capacity of 400 mg/g, higher than that of UiO-66 (261.92 mg/g) with a contact time of 50 min. Several adsorption parameters that influenced RBBR removal efficiency were investigated, such as pH, initial dye concentrations, and temperature. All the composites followed pseudo-first order kinetics and Langmuir isotherm adsorption. Moreover, the adsorption process occurred exothermically and spontaneously, indicating that the adsorption process was advantageous in terms of energy. The possible adsorption mechanism and cost analysis of the adsorbent were also studied in detail.
Keywords
UiO-66
HKUST-1
MOF composite
Adsorption
Remazol Brilliant Blue R
Wastewater treatment
1 Introduction
Synthetic dyes are organic compounds that are widely used in various industries such as textile, cosmetics, paper, and plastics. The use of dye in industries could cause environmental problems due to the wastewater produced. The presence of dyes in wastewater can disrupt the stability of the surrounding ecosystem due to the toxic and insoluble nature of these dyes (He et al., 2014). The textile industry is an example of an industry that uses dyes in a massive amounts. One of the reactive dyes that is mostly used for colouring textile products is the Remazol Brilliant Blue R (RBBR) or Reactive Blue 19 (RB19) dye, which is a heterocyclic compound with anthraquinone as a constituent compound (Arya et al., 2020).
In recent decades, several methods have been reported to remove pollutants in wastewater, among which include ozonation (Ghuge and Saroha, 2018), fenton (Lima et al., 2020), electrochemistry (Devarayapalli et al., 2020), biodegradation (Vedula et al., 2013) and adsorption (Abdollahi et al., 2021). Adsorption is a beneficial method in eliminating the pollutants in wastewater due to the process being quick, very effective and high adsorption capacity, selective, low operational cost, and does not cause side effects such as producing toxic substances (Yoon et al., 2019; Zhao et al., 2020). Porous materials such as active carbon, zeolite, and resin have been widely applied as dye adsorbents (Bayramoglu et al., 2017; Patra et al., 2020; Russo et al., 2021). However, these adsorbents have some disadvantages such as low selectivity, difficult to prepare and low adsorption capacity (Yagub et al., 2014). Metal organic framework (MOF) is a new porous material consisting of hybrid organic and inorganic compounds formed from clusters of metal ions with a variation of organic linkers. MOF has a high and organized pore structure in addition to having a larger specific surface area (Schoedel, 2020). MOF has been widely reported as a selective adsorbent to eliminate organic waste due to the large surface area and pore size, and unsaturated sides which can create bonds with organic wastes (Li et al., 2020; Nanthamathee and Dechatiwongse, 2021; Santoso et al., 2021; Yao et al., 2021).
UiO-66 is a type of MOF that consists of polyhedra Zr6O4(OH)4 which bonded with a carboxylate on the sides, forming a Zr6O4(OH)4(CO2)12 cluster, which are then generally used as selective adsorbents (Ediati et al., 2021; Hidayat et al., 2021; Nanthamathee and Dechatiwongse, 2021). However, the abundance linkers in UiO-66 could obstruct the contact side of the active UiO-66 with foreign molecules, in addition to the micro size contained in UiO-66 could hinder the diffusion process (Chen et al., 2019; Dissegna et al., 2018; Ediati et al., 2021). In order to overcome these problems, an additional compound is needed to increase the selectivity and pore size. Ediati et al. (2021) have reported that adding chitosan could increase the UiO-66 performance in adsorbing methyl orange dye from 256.41 mg/g into 370.37 mg/g (R. Ediati et al., 2021). The addition of MOF on MOF has been reported to have excellent performance as an adsorbent due to MOF producing modifications on the structure and texture (able to increase the intrinsic characteristics of the adsorbent), creating a synergistic effect, and hence increasing the interactions between the adsorbent and adsorbate. Zhang et al. (2020) have reported that the addition of ZIF-8 could increase the performance of UiO-66 in adsorbing inorganic wastes (M. Zhang et al., 2020).
HKUST-1 is a type of MOF which are widely used as an adsorbent due to itsr high affinity characteristics towards water-correlated substances with open metal sites, creating a possibility of an access towards empty orbitals on the copper. Creating a hydrophilic character which is beneficial when used as an adsorbent in water (Burtch et al., 2014). Thus, to enhance the pore channel structure in the composite, HKUST-1 was selected as the dopant in this research in order to enhance the adsorption capacity of dye by increasing the pore channel of the composite. In this research, HKUST-1/UiO-66 composites were synthesized using the solvothermal method with the direct addition of HKUST-1 into the reaction mixture of zirconium chloride and 1,4-benzenedicarboxylic acid in N,N-dimethylformamide solvent. The RBBR adsorption mechanism and model in the HKUST-1/UiO-66 composite have been evaluated, which include kinetic and isothermal adsorptions. In addition, the effects of pH of the solution, the initial concentration of RBBR and the adsorbent dose on the adsorption capacity are also reported.
2 Experimental
2.1 Materials
The materials used in this research were zirconium tetrachloride (ZrCl4) (Sigma-Aldrich, 99 %), copper nitrate trihydrate (Cu(NO3)2·3H2O, 99 %), benzene-1,4-dicarboxylic acid (H2BDC) (Sigma-Aldrich, 99 %), benzene-1,3,5-tricarboxylic acid (H3BTC) (Sigma-Aldrich, 99 %), N,N-dimethylformamide (DMF) (Sigma-Aldrich, 99 %), chloroform (CH3Cl, Merck, 99.9 %), methanol (Merck, 99.9 %), demineralised water, and Remazol Brilliant Blue R (C22H16N2Na2O11S3).
2.2 Synthesis of UiO-66 and HKUST-1/UiO-66
UiO-66 was synthesized using the methods that have been previously reported (Ediati et al., 2019). The synthesis was started by dissolving 0.0045 mol of ZrCl4 into 45 mL DMF. In the other container, 0.0045 mol of H2BDC was dissolved in 45 mL DMF. Both solutions were then mixed together in a sealed reaction bottle, stirred for 30 min, and placed in an oven at 120 °C for 24 h. After the solvothermal process, the reaction mixture was cooled at room temperature for 24 h. The solids formed were washed a couple of times using DMF and chloroform, before being dried at 90 °C for 3 h. The solids obtained were then notated as UiO-66.
The HKUST-1/UiO-66 composites were synthesized using the solvothermal method, where the HKUST-1 was synthesized using the method which have been previously reported (Ediati et al., 2019). HKUST-1 was added into the reaction mixture of ZrCl4 and H2BDC in DMF, prior to performing solvothermal process. The next step was washing the solids using the same step as with the UiO-66 synthesis. The addition of HKUST-1 was varied by 0.008, 0.167 and 0.333 g, and the solids formed were notated as HK(5)/UiO-66, HK(10)/UiO-66 and HK(20)/UiO-66, respectively.
2.3 Characterization
X-ray characterisation (PANanalytical X’pert Pro) was performed to investigate the crystal structure. The ray source used for measurement was Cu Kα radiation (λ = 1,5406 Å), with a voltage of 40 kV and current of 30 mA. The analysis was performed at low angles at a range of 2θ of 5 to 50°. The functional group identification was carried out using FTIR characterization (SHIMADZU 96500). FTIR spectra were detected at wavenumbers of 400 to 4000 cm−1. SEM-EDX characterization was performed to investigate the structure of the crystal morphology, particle size, and the element spread of the synthesized material (SEM, JEOL 6360 LA). The N2 adsorption desorption was carried out to identify the surface area of the material, and pore volume and diameter of the synthesized materials using Nova 1200 e Quantachrome instrument. Prior to analyzing, the samples were evacuated at 130 °C for 24 h.
2.4 Adsorption performance of UiO-66 and composites
Before proceeding to adsorption tests, all the materials were activated at 60 °C for 24 h. The adsorption experiments were performed using batch method at varied contact time of 10 to 70 min with 10 min intervals and varied RBBR concentrations of 100, 150, 200, 250, 300 dan 350 mg/L. The adsorbent doses used were 10 mg in 30 mL solution of RBBR. In order to determine the remaining RBBR in the solution after the process of adsorption, UV–vis instrument was used at wavelength of 591 nm. The data obtained were plotted between Qe (equilibrium adsorption capacity) with time (minutes). The adsorption capacity was calculated using Eq. (1).
Where Co and Ce are the initial and final concentrations of the adsorbate (mg/L), respectively, when at equilibrium condition, W is the adsorbent mass (g) and V is the adsorbate volume (L).
2.5 Adsorption kinetic
The adsorption results towards the synthesized materials were analyzed using the pseudo first and second order equations to determine the kinetic adsorption and the Weber Morris plot in order to obtain the rate constants. The pseudo first order equation is shown in Eq. (2).
The results from the equations were plotted in a graph of time t in minutes as x-axis and ln (Qe – Qt) as y-axis. Thus the K1 values (the pseudo first order adsorption rate constant) were obtained from the slope of the linear equations and from the calculated Qe (Qecal) based on the 2.303 log intercept. The pseudo second order equation is shown in Eq. (3).
By plotting the data on a graph of time t (in minutes) as the x-axis and t/Qt as the y-axis, the value of calculated Qe (Qecal) can be obtained from 1/gradient of the slope of the linear equation. Simultaneously, K2 (pseudo second order adsorption rate constant) can also be obtained from slope2/intercept. The correlation coefficient value (R2) acquired can be used to determine the suitability of the kinetic adsorption. The Weber Moris equation (intraparticle diffusion) is stated in Eq. (4).
By plotting the graph of t1/2 (in minutes) as x-axis and Qt as y-axis, the rate constant value can be found from the slope and the intercept acquired was used to identify the thickness of the boundary layer.
2.6 Adsorption isotherm
The results of adsorption at different concentrations of RBBR were analyzed using the isothermal adsorption equation of Langmuir and Freundlich. The Langmuir isothermal equation is based on Eq. (5).
Subsequently a graph plot was made where 1/Ce was used as the x-axis and 1/Qe was sued as the y-axis. Thus the value of Qm (maximum adsorption capacity) was gathered from 1/intercept, whereas the value of KL (Langmuir constant) was obtained from the intercept/slope. The Freundlich isothermal equation is shown in Eq. (6).
The data were plotted on a graph of ln Ce as the x-axis and ln Qe as the y-axis, thus the value of KF (Freundlich constant) was obtained from 2.303 log intercept. The coefficient correlation value of R2 obtained was used to determine the suitability of kinetic adsorption.
2.7 Adsorption thermodynamic
The adsorption process can be understood from the thermodynamic aspect by determining the Gibbs free energy, enthalpy, and entropy of a system. The temperatures used in this research were varied at 30, 40, and 50 °C with an RBBR concentration of 300 mg/L for 50 min with an adsorbent dose of 10 mg. The value of Gibbs free energy (
G), which is calculated using Eqs. (7) (Dimian et al., 2014).
Where
G,
H dan
S are the change in Gibbs free energy (kJ/mol), the change in enthalpy (kJ/ mol) and the change in entropy (J/mol K), respectively. Based onVan’t Hoff Equation, the value of
G could be calculated using Eq. (8) (Santoso et al., 2021):
Where T is the absolute temperature (K) and R is the universal gas constant (8.314 J/mol K), Kd is Qe/Ce × 1000.
By substituting and merging Eqs. (7) and (8), this results in Eq. (9).
The values of S dan H were obtained from the intercept and the slope of the regression equation, respectively.
3 Results and discussion
3.1 Synthesis and characterization
Characterization using X-ray Diffraction (XRD) was performed to investigate the crystal structure and crystallinity of the synthesized materials and the obtained diffraction patterns are shown in Fig. 1. The UiO-66 diffractograms of the synthesized materials show the main peaks at 2θ of 7.31° with a high intensity and 8.40° with moderate intensity which similiar with previous reported (Ediati et al., 2019). As a comparison, Fig. 1 also shows HKUST-1 diffractograms which also have main characteristic peaks at 2θ of 11.65° with a high intensity, while the next characteristic peaks were identified at 2θ of 6.7, 9.5 and 13.5° (Ediati et al., 2019). The characteritic peak intensity of UiO-66 in composites HKUST-1/UiO-66 decreased with increasing HKUST-1 contents. Meanwhile, the XRD patterns of composites HKUST-1/UiO-66 possesses similar XRD patterns with the pristine UiO-66, which points out that the addition of HKUST-1 did not affect the crystal structure of UiO-66 and it is in accordance with the previous result that was reported by Ediati, et al (Ediati et al., 2021). Furthermore, even when the content of HKUST-1 increased up to 20 %, the characteristic peaks of HKUST-1 could not be found in the XRD pattern of HK(20)/UiO-66. It suggests that the presence of well dispersed of HKUST-1 (Yang et al., 2018).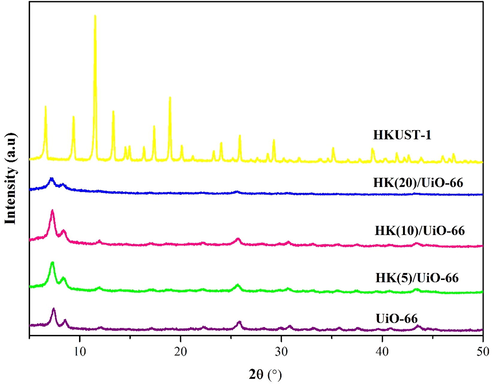
Diffractogram of the synthesized materials.
The FTIR spectra of the synthesized solids are displayed in Fig. 2. The aromatic C⚌C vibration from the benzene structure on the organic ligands was observed from the absorption band of UiO-66 on the wavenumber of approximately 1577–1581 cm−1. The absorption band on the wavenumber of 1400 cm−1 is a stretching vibration of C—O from C-OH carboxylate acid. The absorption band in the wavenumbers of 3421–3446 cm−1 is a region of a stretching vibration of O—H of the carboxylate. The region of approximately 1630–1660 cm−1 shows a vibration of C⚌O from the carboxylate. The absorption band of O—H appeared simultaneously with the adsorption band of C⚌O at approximately 1630–1660 cm−1. The absorption band that appeared at approximately 663–673 cm−1 is a stretching vibration of Zr-O (Hidayat et al., 2021). The successful synthesis of HKUST-1 is signified by the appearance of the characteristic absorption peak of HKUST-1, which is Cu–O on the wavenumber of 731.05 cm−1 (Ediati et al., 2021). This indicates that a bond was formed between the Cu2+ with the oxygen atom which originated from the ligands. In reference to the FTIR spectra, it was discovered that the addition of HKUST-1 did not cause any new absorption band. However, this caused the peaks to shift insignificantly, as shown in Table 1. The addition of HKUST-1 also did not change the crystal structure UiO-66, as shown by the XRD patterns (Ediati et al., 2019).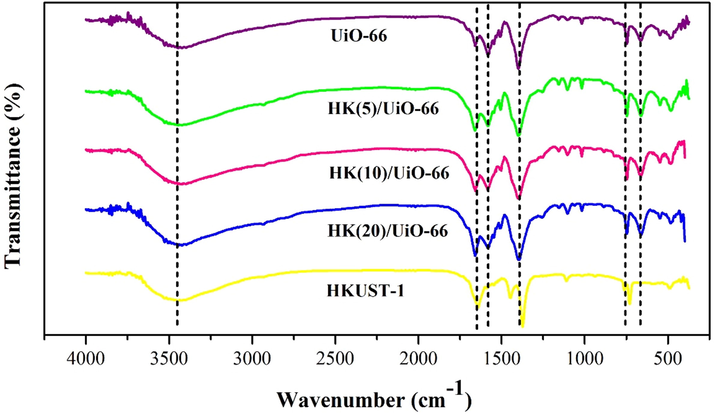
FTIR spectra of the synthesized materials.
No
Type of binding
Wavenumber (cm−1)
UiO-66
HKUST-1/UiO-66
Ref
(5)
(10)
(20)
1.
OH stretching vibration
3437
3421
3412
3433
(Hidayat et al., 2021)
2.
C⚌O stretching vibration
1658
1660
1658
1658
(Hidayat et al., 2021)
3.
Aromatic vibration C⚌C
1581
1581
1581
1581
(Hidayat et al., 2021)
4.
C—O stretching vibration
1400
1400
1400
1396
(Hidayat et al., 2021)
5.
Zr-O stretching vibration
663
661
663
661
(Hidayat et al., 2021)
6.
Cu–O stretching vibration
–
746
746
746
(Ratna Ediati et al., 2021)
The surface morphology and distribution of elements in UiO-66 and the composites have been characterized using SEM-EDX, which was also used to investigate the effects of adding HKUST-1 on the surface morphology of the composites (Fig. 3). The obtained UiO-66 has surface morphology in the form of clustered circles, similar to the previous research reported (Fig. 3a) (Ediati et al., 2018; Ediati et al., 2021). The addition of HKUST-1 to the synthesis of UiO-66 resulted in an irregular morphology of HK(5)/UiO-66 and HK(10)/UiO-66. The surprising result of 5 % HKUST-1 on the synthesis of UiO-66 appeared (Fig. 3b). Two morphologies appeared, one is clustered circles referring to UiO-66 and the second is irregular octahedral refers to HKUST-1, it is confirmed by EDX results. The morphology of HK(10)/UiO-66 (Fig. 3c) is drastically different from pristine UiO-66 (Fig. 3a). It shows a dominant irregular octahedral morphology than clustered circles morphology which is designed by increasing the amount of HKUST-1 up to 10 %. As can be clearly shown, the more HKUST-1 amount in composites caused reducing of clustered circles from pristine UiO-66 but enhancing the irregular octahedral morphology from HKUST-1 (Butova et al., 2020; Ediati et al., 2020). Fig. 4 and Table 2 show the distribution and quantity of the elements contained in UiO-66 and the composites. The EDX results show that UiO-66 comprises carbon (C), oxygen (O), and zirconium (Zr) elements which originated from the precursor used. In addition, no other elements were found which proved that UiO-66 was freed from the impurities. The addition of HKUST-1 led to the occurrence of the addition of copper (Cu) contents in the composites developed. Furthermore, the presence of Cu element on the composites obtained proves that the impregnation process of HKUST-1 on UiO-66 has been successfully carried out in addition to proving the success of Cu element to enter into the frameworks of HKUST-1/UiO-66 composite.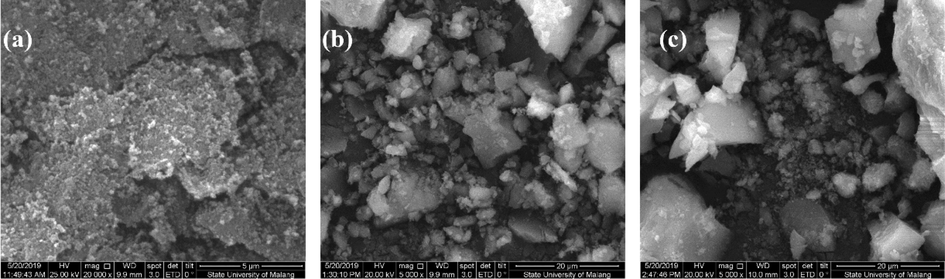
SEM images of (a) UiO-66 (b) HK(5)/UiO-66 (c) HK(10)/UiO-66.
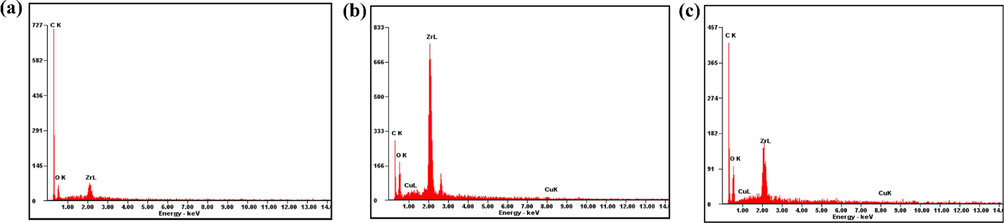
EDX spectra of (a) UiO-66 (b) HK(5)/UiO-66 (c) HK(10)/UiO-66.
Materials
Element
Weight (%)
UiO-66
C
88.2
O
11.0
Zr
0.8
HK(5)/UiO-66
C
67.1
O
24.5
Zr
08,25
Cu
0.17
HK(10)/UiO-66
C
77.2
O
20.1
Zr
02.5
Cu
0.21
Fig. 5 signifies that all the synthesized materials followed the type I adsorption–desorption pattern with a type H1 and H3 loop hysteresis, as stated by IUPAC (Sing, 1982; J. Zhang et al., 2020). The type I isotherm indicates that the material produced was a microporous material, with type I isotherm indicated by the presence of a fast rate of nitrogen gas adsorption at a low P/P0 (Ediati et al., 2021). UiO-66 has a type H3 hysteresis loop which shows that there was a presence of mesoporous structure. However, during the addition of HKUST-1, the hysteresis loop changed into type H1 which expressed that there was a late occurrence of nitrogen capillary condensation in the mesopores. The change in loop hysteresis from H3 into H1 was possible due to the addition of HKUST-1 which covered the mesopores of UiO-66 (Janus et al., 2020; Vo et al., 2019). As listed in Table 3, it can be seen that the addition of 5 % HKUST-1 during the synthesis of UiO-66 produced a composite with a larger surface area, mesopore volume and pore diameter than that of the ordinary UiO-66. However, the addition of HKUST-1 by more than 5 % resulted in a decrease in the surface area, mesopore volume and diameter of the obtained composites. It is believed that the addition of HKUST-1 for greater than 5 % may cause the plugging of the active site, reducing surface area, mesoporous volume and pore diameter (Subagyo et al., 2022).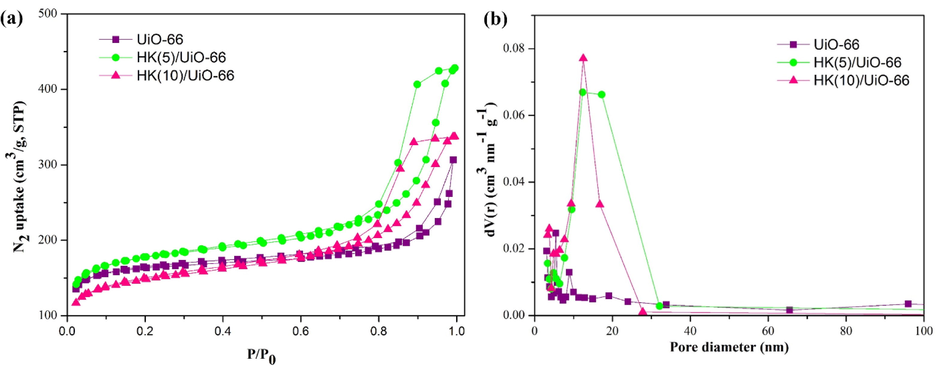
(a) N2 adsorption–desorption isotherm (b) Pore size distribution.
Materials
SBET (m2/g)
Volume mesopore (cm3/g)
Volume micropore (cm3/g)
Average pore size diameter (nm)
UiO-66
502.77
0.229
0.2453
3.774
HK(5)/UiO-66
557.67
0.423
0.2396
4.752
HK(10)/UiO-66
470.50
0.320
0.2012
4.43
3.2 Adsorption, kinetic and thermodinamic behavior on RBBR adsorption
3.2.1 Effect of pH
The pH is an important parameter in the adsorption process of RBBR, because the pH solution determines the degree of ionization, adsorbate speciation, and the charges on the surface of the adsorbent (Guo et al., 2015). To investigate the effect of the solution's initial pH on the adsorbent's ability to absorb RBBR, the pH of the solution was adjusted to a range of values between 3 and 11. The adsorption results at various pH of RBBR solutions are presented in Fig. 6. As shown in Fig. 6, the maximum adsorption capacity of RBBR was achieved at pH 3 for all adsorbents. In the acidic solution, the protonation of adsorbent occurs which causes an increase in the surface positive charge. The increase in electrostatic attraction between the surface of the adsorbent and the SO3− functional group on RBBR increases the adsorption capacity of RBBR (Al-Degs et al., 2008; Embaby et al., 2018). In the alkaline solution, the deprotonation of the adsorbent occurs, which causes a decrease in the surface positive charge. The behavior of adsorption due to the repulsive of electrostatic between SO3- on RBBR with adsorbent decreased the adsorption capacity of RBBR. In addition, the decrease in adsorption capacity is also caused by the number of OH– ions that are dominant and compete with the SO3− functional group on RBBR to bind to the surface of the adsorbent (Hidayat et al., 2021). Hence, electrostatic attraction forces have a substantial role in the adsorption of RBBR onto adsorbent in addition to hydrogen bonding and π-π interaction.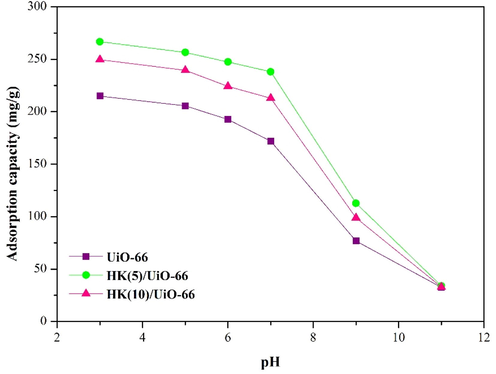
Effect of initial pH (C0 = 100 mg/L; t = 50 min; dosage = 10 mg).
3.2.2 Effect of contact time
As exhibited in Fig. 7, it is seen that all the materials adsorbed the RBBR dye, where the rate of adsorption increased during the initial stage of the process. At the initial stage of the adsorption, the adsorption sites of the material surface are provided by the active functional groups of the adsorbents that have not yet interacted optimally with the RBBR. After passing the 50 min contact time, the adsorption rate reached a constant stage, which indicated that the adsorbents were in a saturated condition (Ediati et al., 2021). The contact time observed for all the materials was 50 min. HK(5)/UiO-66 demonstrated the best adsorption performance with an adsorption capacity of 269.8 mg/g, which was higher than that of a pure UiO-66 with an adsorption capacity of 228.87 mg/g. The addition of HKUST-1 by larger than 5 % resulted in a decrease of adsorption capacity due to the pores being closed and a decrease in surface area, which have been confirmed by the results of N2 adsorption–desorption analysis (R. Ediati et al., 2021; Subagyo et al., 2022). The adsorption capacity at different contact times was then used to determine the adsorption kinetics using pseudo-first order and pseudo-second order models and intraparticle diffusion to identify the adsorption process and rate-determining step. The kinetic parameters are listed in Table 4. Based on the first and second pseudo-order graph plots, both exhibits that the RBBR adsorption by each of the adsorbents followed the second pseudo order kinetic adsorption since the correlation coefficient (R2) is approaching 1. The pseudo second order kinetics indicated that the adsorption process chemically went through the electrostatic interaction mechanisms (Subagyo et al., 2022). The pseudo second order kinetic adsorption also demonstrated that the adsorption was determined by two variables, which are the RBBR concentration and the concentration of the active sites on the surface of the RBBR (Santoso et al., 2021).
(a) Effect of contact time (C0 = 100 mg/L; pH = 3; dosage = 10 mg) (b) Plot pseudo-first order (c) Plot pseudo second order (D) Plot intraparticle diffusion.
Adsorbent
Qe(exp) (mg/g)
PFO
PSO
Qe(cal) (mg/g)
k1
R2
Qe(cal) (mg/g)
k2 × 10-3
R2
UiO-66
228.87
95.12
0.060
0.8126
238.09
0.93
0.9894
HK(5)/UiO-66
269.80
224.64
0.081
0.8154
277.78
1.1
0.9907
HK(10)/UiO-66
249.62
27.78
0.043
0.2682
263.16
0.84
0.9895
HK(20)/UiO-66
171.59
37.89
0.069
0.7614
181.81
1.77
0.9941
Adsorption is a process of removal through a step-by-step mechanism from the interaction of the adsorbate in solution with the adsorbent to the penetration of the adsorbate into the pores of the adsorbent. Therefore, it is important to understand the diffusion process that takes place at the surface or pores through the Weber Moris model (intra-particle diffusion) and to determine the rate-determining step. As displayed in Fig. 7(d) and listed in Table 5, adsorption takes place in two phases namely mass diffusion and intraparticle diffusion. In the beginning, there were many active sites on the surface of the adsorbent, so that the adsorbate could be easily adsorbed on the surface of the adsorbent, which was characterized by a high value of Kp1. Further, the adsorbate adsorbed on the surface of the adsorbent penetrated the pores of the adsorbent, followed by binding of the active site in the pores of the adsorbent. However, at this stage, it caused diffusion resistance resulting in a decrease in the amount of adsorbate in the solution which is characterized by a low Kp2 value (Cui et al., 2019; Subagyo et al., 2022). The decrease of the constant value in the second stage correlated with the C value which specified the boundary thickness. C1 has a smaller value on all the adsorbents compared to the values of C2. The intercept value (C) increased with the decrease in the HKUST-1 addition, which implied that the composite with the least addition of HKUST-1 had the largest adsorption capacity (Wu et al., 2009).
Adsorbent
Intraparticle diffusion
C1 (mg/g)
k1 (mg g−1 min0.5)
C1 (mg/g)
k1 (mg g−1 min0.5)
UiO-66
8.31
36.68
195.78
3.88
HK(5)/UiO-66
16.70
44.90
224.88
6.08
HK(10)/UiO-66
11.26
39.78
207.37
5.32
HK(20)/UiO-66
4.60
31.26
146.96
3.85
3.2.3 Effect of initial concentrations of RBBR
The adsorption process with varying concentrations of RBBR aims to determine the concentration of RBBR required to achieve optimal adsorption, as shown in Fig. 8a. It can be seen in Fig. 8a, all the materials experienced a significant increase in adsorption capacity up to an RBBR concentration of 300 mg/L, and remain unchanged at RBBR concentrations of higher than 300 mg/L, which indicated that adsorbents have reached its saturation point. The increase in adsorption capacity along with the increase in the concentration of RBBR indicates that the high concentration of RBBR in the solution causes the active site of the adsorbent to be surrounded by more RBBR. When compared to a pure UiO-66, the HK(5)/UiO-66 composite has an adsorption capacity of 400 mg/L or 1.53 times higher. The increase in adsorption capacity of the HK(5)/UiO-66 composite was due to the addition of the active site of HKUST-1 and the reduction in particle aggregation which could reduce the active site of the material (Subagyo et al., 2022). This result also correlates with the results of the N2 adsorption–desorption analysis which states that HK(5)/UiO-66 has a larger surface area and meso-volume than UiO-66, which is beneficial for the adsorption process. Moreover, the increase in adsorption capacity was also caused by the addition of the intercalation of the pi bond of the HKUST-1 carboxylate ring with RBBR (R. Ediati et al., 2021). The electrostatic attraction and the increased hydrogen bonding due to the addition of HKUST-1 may be the cause of the increase in adsorption capacity (Hidayat et al., 2021). Table 6 shows the high adsorption capacity of the HK(5)/UiO-66 composite compared to other adsorbents. Some beneficial interactions that can occur between HK(5)/UiO-66 and RBBR include: hydrogen bonds interactions, π-π bond interactions between the adsorbent and the RBBR, electrostatic attraction, and acid-base interactions.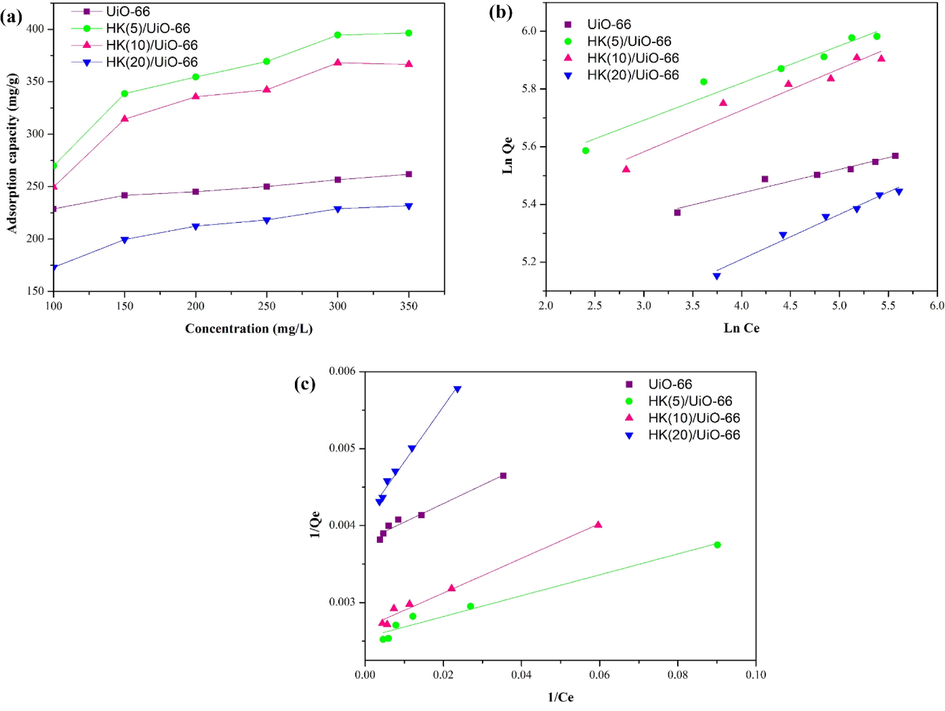
(a) Effect of initial concentration (C0 = 100 mg/L; pH = 3; t = 50 min; dosage = 10 mg) (b) Ploti isotherm Freundlich (c) Plot isotherm Langmuir.
Adsorben
Langmuir
Freundlich
Qmax (mg/g)
KL (L/mg)
R2
1/n
KF (mg/g)
R2
UiO-66
263.16
0.16
0.9676
0.0818
166.05
0.953
HK(5)/UiO-66
400
0.18
0.9687
0.1334
198.40
0.9542
HK(10)/UiO-66
370.37
0.12
0.984
0.1432
172.67
0.9444
HK(20)/UiO-66
243.90
0.06
0.9891
0.1554
98.35
0.9798
The value of the adsorption capacity of the synthesized adsorbents at different initial concentrations of RBBR was then used to determine the isothermal adsorption of Langmuir and Freundlich, as. shown in Fig. 8b and c, respectively. The suitable choice of isotherm was based on three references, which are the isotherm produced must be linear, the maximum concentration must have an adsorption capacity limit and the gradient obtained must be positive on all the varied concentrations (Bachmann et al., 2021). As listed in Table 7, the gradient obtained on all the variations, on both the Langmuir and Freundlich plot, produced positive outcomes, thus the gradient was determined based on the correlation coefficient value. The correlation coefficient value (R2) of the Langmuir plot was found to be higher when compared to the correlation coefficient value (R2) of Freundlich plot, which applied to all the adsorbent types. Therefore it can be deduced that all the adsorbents followed the Langmuir isothermal, which implies that the adsorption occurred in monolayer, chemisorption, and has a uniform energy. The chemisorption process led to the adsorbate to being quickly saturated as although the concentration and contact time were increased, the amount of adsorbate that was adsorbed by the adsorbent stayed constant (R. Ediati et al., 2021; Hidayat et al., 2021). The constant which states the deviation of the linearity and was used to verify the adsorption type is notated by n. A value of 1/n = 1 implies a linear adsorption, 1/n greater than 1 implies the adsorption occurred chemically, and 1/n less than 1 implies that the adsorption process occurred physically (Wen et al., 2020). As stated in Table 8, it was found that the obtained value of 1/n on all of the adsorbents was less than 1, implementing that the Freundlich isothermal plot correlated with Langmuir isothermal, which further confirms that the adsorption process took place chemically and was fitted more to following Langmuir isothermal.
Adsorbent
Temperature (K)
Time (min)
Concentration (mg/L)
pH
Qmax (mg/g)
Ref
HK(5)/UiO-66
303
50
350
3
400
This research
Bone char
298
240
500
6.54
20.6
(Bedin et al., 2017)
Biochar
303
60
100
10
87.03
(Raj et al., 2021)
HSNMIL88(Fe)
303
480
200
4.5
213.2
(Zhang et al., 2021)
Poly(NOPMA)
293
59.91
100
7
60.85
(Torgut et al., 2017)
Activated carbon
303
1440
500
3
204
(Ahmad et al., 2014)
Chitosan Coated Montmorillonite
298
120
400
2
322.58
(Altıntıg et al., 2022)
Chi/Sep/Alg
303
17.5
250
4
292.4
(Zain et al., 2022)
Yarrowia lipolitica
298
180
150
2
119.6
(Aracagök, 2022)
Jhingan hydrogel
298
420
100
6
9.88
(Mate and Mishra, 2020)
Adsorben
H° (kJ/mol)
S° (J/mol K)
G° (kJ/mol)
303 K
313 K
323 K
HK(5)/UiO-66
−92.27
209.22
−29.29
−25.90
−25.16
HK(10)/UiO-66
−65.29
126.94
−27.19
−24.80
−24.70
3.2.4 Effect of adsorption temperature
The effects of temperature towards adsorption is related to the thermodynamic parameters, which are Gibbs free energy (to determine the spontaneity of a system), enthalpy change (to determine the reactions that took place, endothermic or exothermic), and entropy (to determine the randomness of a system and the change in the structure on the adsorbent), all of which are given in Fig. 9 and Table 8. As presented in Fig. 9, it can be seen that as the temperature increased, the adsorption capacity decreased. The decrease in adsorption capacity was a consequence of the equilibrium condition which was reached at high temperatures, thus the adsorption system produced became saturated (Zhang et al., 2012).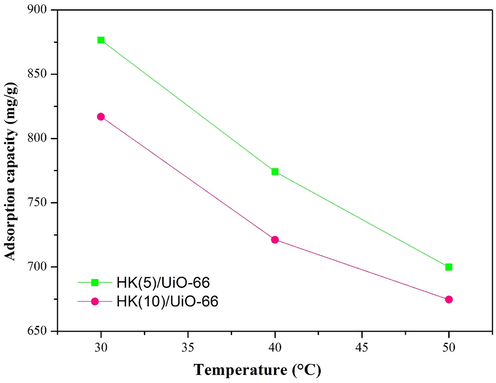
Effect of initial temperature.
If the enthalpy value of the system is less than 40 kJ/mol, it indicates that the adsorption mechanism occurred physically. Whereas if the enthalpy value of the system is more than 40 kJ/mol, it indicates that the adsorption mechanism occurred physically (Wang et al., 2022). The enthalpy change value for HK(5)/UiO-66 and HK(10)/UiO-66 composites are −92.27 and −65.29 kJ/mol, which implied that the reaction occurred exothermically (Hidayat et al., 2021). The results correlated with the adsorption capacity values where they decrease with increasing temperature. In an exothermic process, the optimum adsorption performance is obtained at low temperatures (Horsfall and Spiff, 2005). A positive entropy value signified that there was an increase in the randomness that occurred throughout the adsorption process (Hidayat et al., 2021). This circumstance took place due to the RBBR molecules that were being damaged throughout the adsorption process as a result of a variety of interactions between the RBBR and the material (Rehman et al., 2021). A negative Gibbs free energy value hinted that the reaction took place spontaneously which benefited in terms of energy. While a positive Gibbs value specified that the reaction did not occur spontaneously, which did not benefit energetically since the reaction needed more energy from the surroundings in order for the reaction to take place spontaneously (González-López et al., 2021). The negative Gibbs free energy value on both materials of the RBBR adsorption was caused by the spontaneous reaction, which revealed that the reaction was beneficial (Hidayat et al., 2021).
3.3 Reusability of HK(5)/UiO-66
To investigate the regenerability and recyclability of HK(5)/UiO-66, the three successive recycling processes were carried out by adding restored of HK(5)/UiO-66 to fresh RBBR solutions. As shown in Fig. 10, the adsorption capacity of HK(5)/UiO-66 toward RBBR after three successive cycle of adsorption still maintained above 300 mg/g and the ability of HK(5)/UiO-66 to regeneration was reached to 75 %. It can be seen that HK(5)/UiO-66 is a promising adsorbent to removal of RBBR from aqueous solutions.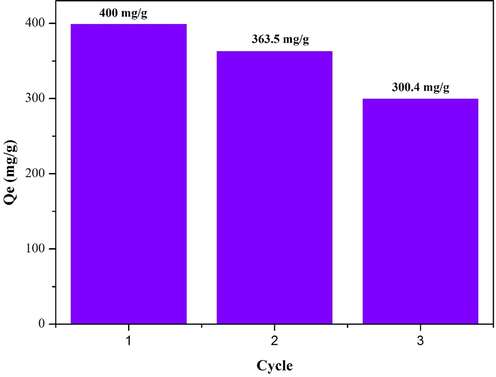
Reusability of HK(5)/UiO-66.
3.4 Adsorption mechanism
To study the interaction mechanism between HK(5)/UiO-66 and RBBR dyes, FTIR spectra of RBBR dye, HK(5)/UiO-66 before and after adsorption were showed in Fig. 11. The intensity of the absorption band at 1579.5 cm−1 is significantly increased, which was corresponded to the strengthening due to the presence of π-π stacking between adsorbent and adsorbate. Besides, a small adsorption band at a wavenumber of 1273 cm−1 was observed, which is a region of -S⚌O- stretching vibration of the sulfonate (-SO3-) at the region of 1200–1365 cm−1. This was possibly due to the new formation of electrostatic force between metal ion with the active -SO3- group that contained within RBBR (Wang et al., 2015). However, simultaneously, The characteristic peaks in the 1105.25 cm−1 (C—O vibration) and 1660.77 cm−1 (C⚌O group) regions were disappeared, indicating that the adsorption process had been occured. The adsorption mechanism was intended to explain the adsorption process that took place. The first stage of the adsorbent was adsorbing the RBBR before interacting through π-π stacking, hydrogen bonds and electrostatic force between the adsorbent with the RBBR, as shown in Fig. 12. The hydrogen bonds were formed when the adsorbent was dissolved and interacted with H2O, which was continued to forming a bond between the H2O with the RBBR molecules (Hasan et al., 2014). The π-π interactions were a consequence of the interactions between π aromatic groups on the MOF with the aromatic groups in RBBR (Azhar et al., 2017; Yoon et al., 2019). The electrostatic force occurred between the SO3- groups in RBBR with the composite metal cluster in the composite, which are Zr4+ and Cu2+. Zr4+ and Cu2+ acted as the Lewis acid which supplied the empty orbitals and the SO3- groups in RBBR acted as the Lewis acid which has a pair of free electrons (Hidayat et al., 2021; Tran Ba Luan, 2020). In this circumstance, the presence of HKUST-1 provided a vital role in increasing the interactions that occurred. From the calculations using Avogrado’s equation, the RBBR size was found to be 1.30 × 1.03 × 1.30 nm (Fig. 13). The RBBR molecules were found to be smaller compared to the resulted pores diameter, which are 3.774, 4.752 and 4.43 nm for UiO-66, HK(5)/UiO-66 and HK(10)/UiO-66 materials, respectively, consequently allowing RBBR to diffuse into the adsorbent pores. This process continued until the active sites of the adsorbent became saturated to interact with the RBBR molecules.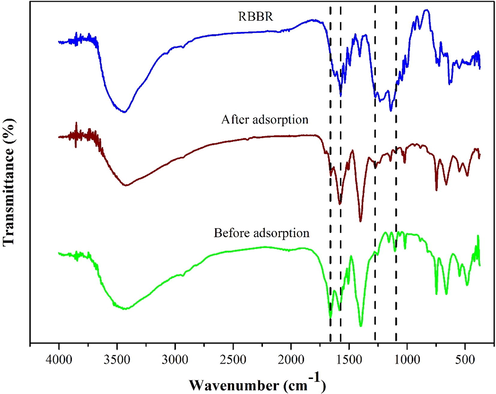
Comparison between after and before adsorption.
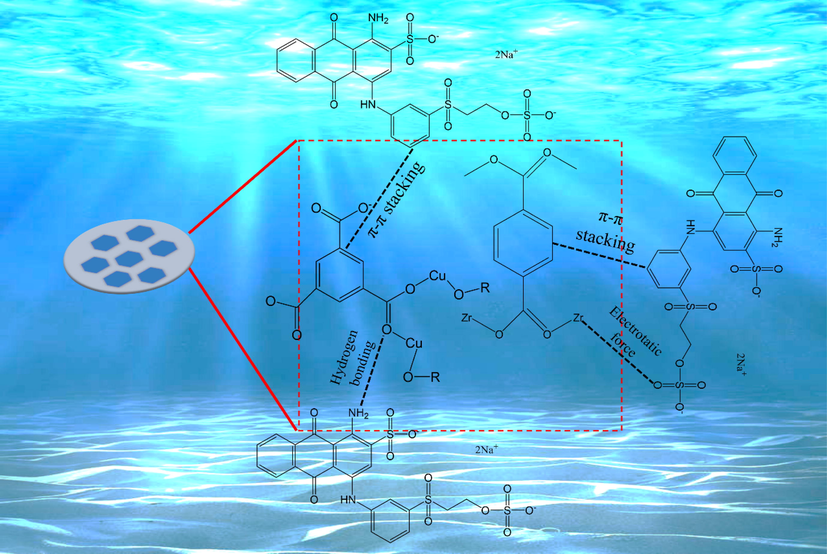
Possible mechanism of adsorption RBBR.
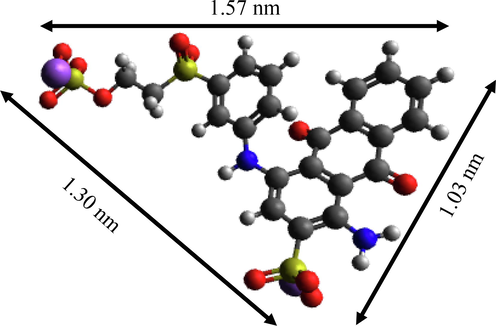
Structure of RBBR using avogadro.
3.5 Adsorbent cost analysis
Cost analysis of HKUST-1/UiO-66 preparation from their raw materials is crucial analysis for economic viability of adsorbent production (Li et al., 2022). During the synthesis, the preparation cost of HKUST-1/UiO-66 comes from the chemical precursor (cost A). Cost B and C could be formed from the solution exchange and electricity. The estimation of cost A, B and C US $0.32/kg, US $26.53/kg and US $0,42/kg, respectively. Taking into account the total cost estimation from each component was about US $27.27/kg. The maximum adsorption capacity for RBBR using HKUST-1/UiO-66 in this research is 400 mg/g. Based on the estimated adsorbent cost per gram of adsorbate removed, The adsorption cost of HKUST-1/UiO-66 is US $0.069/g.
4 Conclusion
HKUST-1/UiO-66 composites have been successfully synthesized using solvothermal method. Such a synthesis produces a different structure and texture and optimum addition of HKUST-1 can enlarge the active specific surface area and pore size, which consequently affects their performance as adsorbent of RBBR in aqueous solution. The addition of HKUST-1 by 5 % produced a HKUST-1/UiO-66 composite with the highest adsorption capacity of 400 mg/g with adsorbent dose of 10 mg. With the increase in dye concentrations, the adsorption capacity also increased and reached equilibrium at an RBBR concentration of 350 mg/L. The adsorption process followed the pseudo-first order kinetic model which revealed that chemisorption and Langmuir isotherm took place during the adsorption, which in turn signifies that the adsorption took place in monolayer with the highest homogeneity. The effect of adsorption temperature was also studied which showed that the adsorption took place exothermically and spontaneously at low temperatures. The π-π stacking interaction, hydrogen bonding and electromagnetic force were considered as the major interactions for the adsorption process. A relatively high adsorption capacity even after three cycles (300.4 mg/g), indicating that the HK(5)/UiO-66 could be a virtuous candidate for removal of RBBR from aquatic environment. Thus, the overall results of this research are expected to give new insights into the prospective applications from a HKUST-1/UiO-66 as an low-cost adsorbent with high adsorption capacity in eliminating dye in wastewater.
Acknowledgements
The authors gratefully acknowledge financial support from the Institut Teknologi Sepuluh Nopember (ITS), Indonesia, for this work, under a project scheme of the Publication Writing and IPR Incentive Program (PPHKI) 2022.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
References
- Fabrication of ZiF-8 metal organic framework (MOFs)-based CuO-ZnO photocatalyst with enhanced solar-light-driven property for degradation of organic dyes. Arab. J. Chem.. 2021;14:103444
- [CrossRef] [Google Scholar]
- Equilibrium, kinetics, and thermodynamics of remazol brilliant blue r dye adsorption onto activated carbon prepared from pinang frond. ISRN Mech. Eng.. 2014;2014
- [CrossRef] [Google Scholar]
- Effect of solution pH, ionic strength, and temperature on adsorption behavior of reactive dyes on activated carbon. Dye. Pigment.. 2008;77:16-23.
- [CrossRef] [Google Scholar]
- Kinetic, equilibrium, adsorption mechanisms of RBBR and MG dyes on chitosan-coated montmorillonite with an ecofriendly approach. Chem. Eng. Res. Des.. 2022;188:287-300.
- [CrossRef] [Google Scholar]
- Biosorption of Remazol Brilliant Blue R dye onto chemically modified and unmodified Yarrowia lipolytica biomass. Arch. Microbiol.. 2022;204:1-8.
- [CrossRef] [Google Scholar]
- Adsorptive removal of Remazol Brilliant Blue R dye from its aqueous solution by activated charcoal of Thuja orientalis leaves: an eco-friendly approach. SN Appl. Sci.. 2020;2:1-10.
- [CrossRef] [Google Scholar]
- One-pot synthesis of binary metal organic frameworks (HKUST-1 and UiO-66) for enhanced adsorptive removal of water contaminants. J. Colloid Interface Sci.. 2017;490:685-694.
- [CrossRef] [Google Scholar]
- Caffeine removal from aqueous media by adsorption: An overview of adsorbents evolution and the kinetic, equilibrium and thermodynamic studies. Sci. Total Environ.. 2021;767:144229
- [CrossRef] [Google Scholar]
- Removal of metal complexed azo dyes from aqueous solution using tris(2-aminoethyl)amine ligand modified magnetic p(GMA-EGDMA) cationic resin: Adsorption, isotherm and kinetic studies. Chem. Eng. Res. Des.. 2017;124:85-97.
- [CrossRef] [Google Scholar]
- Bone char prepared by CO2 atmosphere: Preparation optimization and adsorption studies of Remazol Brilliant Blue R. J. Clean. Prod.. 2017;161:288-298.
- [CrossRef] [Google Scholar]
- Water stability and adsorption in metal-organic frameworks. Chem. Rev.. 2014;114:10575-10612.
- [CrossRef] [Google Scholar]
- Microwave synthesis and phase transition in UiO-66/MIL-140A system. Microporous Mesoporous Mater.. 2020;296:109998
- [CrossRef] [Google Scholar]
- Reticular chemistry in the rational synthesis of functional zirconium cluster-based MOFs. Coord. Chem. Rev.. 2019;386:32-49.
- [CrossRef] [Google Scholar]
- Gel-like ZnO/Zr-MOF(bpy) nanocomposite for highly efficient adsorption of Rhodamine B dye from aqueous solution. J. Phys. Chem. Solids. 2019;134:165-175.
- [CrossRef] [Google Scholar]
- Rapid microwave-assisted construction of ZIF-8 derived ZnO and ZnO@Ta2O5 nanocomposite as an efficient electrode for methanol and urea electro-oxidation. J. Electroanal. Chem.. 2020;878:114634
- [CrossRef] [Google Scholar]
- Generalised Computational Methods in Thermodynamics. Computer Aided Chemical Engineering 2014
- [CrossRef] [Google Scholar]
- Synthesis and catalytic activity of mesoporous Al-MCM-41/UiO-66 for esterification of oleic acid. AIP Conf. Proc.. 2018;2049
- [CrossRef] [Google Scholar]
- One-pot solvothermal synthesis and characterization of UiO-66/HKUST-1 composites. IOP Conf. Ser. Mater. Sci. Eng.. 2019;578
- [CrossRef] [Google Scholar]
- Synthesis of HKUST-1 with addition of Al-MCM-41 as adsorbent for removal of methylene blue from aqueous solution. Mater. Today Proc.. 2020;2020:1-8.
- [CrossRef] [Google Scholar]
- Chitosan/UiO-66 composites as high-performance adsorbents for the removal of methyl orange in aqueous solution. Mater. Today Chem.. 2021;21:100533
- [CrossRef] [Google Scholar]
- Optimization of the use of mother liquor in the synthesis of HKUST-1 and their performance for removal of chromium (VI) in aqueous solutions. J. Water Process Eng.. 2021;39:101670
- [CrossRef] [Google Scholar]
- Chinese Journal of Chemical Engineering The adsorptive properties of UiO-66 towards organic dyes : A record adsorption capacity for the anionic dye Alizarin Red S. Chinese J. Chem. Eng.. 2018;26:731-739.
- [CrossRef] [Google Scholar]
- Ozonation of Reactive Orange 4 dye aqueous solution using mesoporous Cu/SBA-15 catalytic material. J. Water Process Eng.. 2018;23:217-229.
- [CrossRef] [Google Scholar]
- Chemically Modified Polysaccharides for Hexavalent Chromium Adsorption. Sep. Purif. Rev.. 2021;50:333-362.
- [CrossRef] [Google Scholar]
- Adsorption behavior of Cr(vi) from aqueous solution onto magnetic graphene oxide functionalized with 1,2-diaminocyclohexanetetraacetic acid. RSC Adv.. 2015;5:45384-45392.
- [CrossRef] [Google Scholar]
- Adsorption of pyridine over amino-functionalized metal-organic frameworks: Attraction via hydrogen bonding versus base-base repulsion. J. Phys. Chem. C. 2014;118:21049-21056.
- [CrossRef] [Google Scholar]
- Adsorption behavior of rhodamine B on UiO-66. Chinese J. Chem. Eng.. 2014;22:1285-1290.
- [CrossRef] [Google Scholar]
- Linear and nonlinear isotherm, kinetic and thermodynamic behavior of methyl orange adsorption using modulated Al2O3@UiO-66 via acetic acid. J. Environ. Chem. Eng.. 2021;9:106675
- [CrossRef] [Google Scholar]
- Effects of temperature on the sorption of Pb2+ and Cd 2+ from aqueous solution by Caladium bicolor (Wild Cocoyam) biomass. Electron. J. Biotechnol.. 2005;8:162-169.
- [CrossRef] [Google Scholar]
- On mechanism of formation of SBA-15/furfuryl alcohol-derived mesoporous carbon replicas and its relationship with catalytic activity in oxidative dehydrogenation of ethylbenzene. Microporous Mesoporous Mater.. 2020;299
- [CrossRef] [Google Scholar]
- In-situ growing of metal-organic frameworks on three-dimensional iron network as an efficient adsorbent for antibiotics removal. Chem. Eng. J.. 2020;392:124844
- [CrossRef] [Google Scholar]
- Adsorption performance and optimization by response surface methodology on tetracycline using Fe-doped ZIF-8-loaded multi-walled carbon nanotubes. Environ. Sci. Pollut. Res. 2022
- [CrossRef] [Google Scholar]
- Degradation of antibiotic ciprofloxacin by different AOP systems using electrochemically generated hydrogen peroxide. Chemosphere. 2020;247:125807
- [CrossRef] [Google Scholar]
- Synthesis of borax cross-linked Jhingan gum hydrogel for remediation of Remazol Brilliant Blue R (RBBR) dye from water: Adsorption isotherm, kinetic, thermodynamic and biodegradation studies. Int. J. Biol. Macromol.. 2020;151:677-690.
- [CrossRef] [Google Scholar]
- Kinetic and thermodynamic studies of neutral dye removal from water using zirconium metal-organic framework analogues. Mater. Chem. Phys.. 2021;258:123924
- [CrossRef] [Google Scholar]
- Surface treated acid-activated carbon for adsorption of anionic azo dyes from single and binary adsorptive systems: A detail insight. Environ. Pollut.. 2020;266:115102
- [CrossRef] [Google Scholar]
- Kinetic and thermodynamic investigations of sewage sludge biochar in removal of Remazol Brilliant Blue R dye from aqueous solution and evaluation of residual dyes cytotoxicity. Environ. Technol. Innov.. 2021;23:101556
- [CrossRef] [Google Scholar]
- Physicochemical characterization of Pakistani clay for adsorption of methylene blue: Kinetic, isotherm and thermodynamic study. Mater. Chem. Phys.. 2021;269:124722
- [CrossRef] [Google Scholar]
- Adsorption and catalytic degradation of Tartrazine in aqueous medium by a Fe-modified zeolite. Clean. Eng. Technol.. 2021;4:100211
- [CrossRef] [Google Scholar]
- Facile synthesis of ZIF-8 nanoparticles using polar acetic acid solvent for enhanced adsorption of methylene blue. Microporous Mesoporous Mater.. 2021;310:110620
- [CrossRef] [Google Scholar]
- Schoedel, A., 2020. Secondary building units of MOFs, Metal-Organic Frameworks for Biomedical Applications. Elsevier Inc. https://doi.org/10.1016/b978-0-12-816984-1.00003-2.
- Reporting physisorption data for gas/solid systems with special reference to the determination of surface area and porosity (Provisional) Pure Appl. Chem.. 1982;54:2201-2218.
- [CrossRef] [Google Scholar]
- Converting red mud wastes into mesoporous ZSM-5 decorated with TiO2 as an eco-friendly and efficient adsorbent-photocatalyst for dyes removal. Arab. J. Chem.. 2022;15:103754
- [CrossRef] [Google Scholar]
- Application of response surface methodology for optimization of Remazol Brilliant Blue R removal onto a novel polymeric adsorbent. J. Taiwan Inst. Chem. Eng.. 2017;80:406-414.
- [CrossRef] [Google Scholar]
- Dye Adsorption on UiO-66: the Importance of Electrostatic Attraction Mechanism. J. Water Chem. Technol.. 2020;42:441-449.
- [CrossRef] [Google Scholar]
- Bioremoval of cyanide and phenol from industrial wastewater: An update. Bioremediat. J.. 2013;17:278-293.
- [CrossRef] [Google Scholar]
- Facile Synthesis of UiO-66(Zr) Using a Microwave-Assisted Continuous Tubular Reactor and Its Application for Toluene Adsorption. Cryst. Growth Des.. 2019;19:4949-4956.
- [CrossRef] [Google Scholar]
- Superior removal of arsenic from water with zirconium metal-organic framework UiO-66. Sci. Rep.. 2015;5:1-10.
- [CrossRef] [Google Scholar]
- Behavior and mechanism of atrazine adsorption on pristine and aged microplastics in the aquatic environment: Kinetic and thermodynamic studies. Chemosphere. 2022;292:133425
- [CrossRef] [Google Scholar]
- Ice-templated porous polymer/UiO-66 monolith for Congo Red adsorptive removal. Arab. J. Chem.. 2020;13:5669-5678.
- [CrossRef] [Google Scholar]
- Initial behavior of intraparticle diffusion model used in the description of adsorption kinetics. Chem. Eng. J.. 2009;153:1-8.
- [CrossRef] [Google Scholar]
- Dye and its removal from aqueous solution by adsorption: A review. Adv. Colloid Interface Sci.. 2014;209:172-184.
- [CrossRef] [Google Scholar]
- Modification effects of B2O3 on the structure and catalytic activity of WO3-UiO-66 catalyst. Nanomaterials. 2018;8
- [CrossRef] [Google Scholar]
- Rapid ultrasound-assisted synthesis of controllable Zn/Co-based zeolitic imidazolate framework nanoparticles for heterogeneous catalysis. Microporous Mesoporous Mater.. 2021;314:110777
- [CrossRef] [Google Scholar]
- Removal of acid orange 7 from aqueous solution by metal-organic frameworks. Crystals. 2019;9
- [CrossRef] [Google Scholar]
- A pH-Sensitive Surface of Chitosan/Sepiolite Clay/Algae Biocomposite for the Removal of Malachite Green and Remazol Brilliant Blue R Dyes: Optimization and Adsorption Mechanism Study. J. Polym. Environ. 2022
- [CrossRef] [Google Scholar]
- TiO2-UiO-66-NH2 nanocomposites as efficient photocatalysts for the oxidation of VOCs. Chem. Eng. J.. 2020;385:123814
- [CrossRef] [Google Scholar]
- High-density immobilization of laccase on hollow nano-sphere NH2-MIL88(Fe) host with interfacial defects to improve enzyme activity and stability for remazol brilliant blue R decolorization. Chem. Eng. J.. 2021;405:127003
- [CrossRef] [Google Scholar]
- Adsorption behavior of metal-organic frameworks for thiophenic sulfur from diesel oil. Ind. Eng. Chem. Res.. 2012;51:12449-12455.
- [CrossRef] [Google Scholar]
- 3D-agaric like core-shell architecture UiO-66-NH2@ZIF-8 with robust stability for highly efficient REEs recovery. Chem. Eng. J.. 2020;386:124023
- [CrossRef] [Google Scholar]
- Uniform and stable immobilization of metal-organic frameworks into chitosan matrix for enhanced tetracycline removal from water. Chem. Eng. J.. 2020;382:122893
- [CrossRef] [Google Scholar]







