Translate this page into:
Synthesis, spectral studies, antimicrobial and insect antifeedant activities of some substituted styryl 4′-fluorophenyl ketones
*Corresponding author. Tel.: +91 414420015 drgtnarayanan@gmail.com (Ganesamoorthy Thirunarayanan)
-
Received: ,
Accepted: ,
This article was originally published by Elsevier and was migrated to Scientific Scholar after the change of Publisher.
Available online 8 January 2011

Peer review under responsibility of King Saud University.
Abstract
Good yield of some substituted styryl 4′-fluorophenyl ketones were synthesized by solvent free fly-ash:water catalyzed eco-friendly environmentally benign Aldol reaction. These chalcones were characterized by physical constants, micro analysis and spectral data. Antimicrobial and insect antifeedant activities were measured in all chalcones. The group frequencies of all chalcones like carbonyl stretches νCO, C–F and the deformation modes of vinyl part of CH– out of plane, in-plane, CH⚌CH out of plane and >C⚌C< out of plane (cm−1), the vinyl hydrogen and carbons δ(ppm) of Hα, Hβ, Cα, Cβ and CO were assigned and these frequencies were correlated with various kinds of substituent constants. From the results of statistical analysis the influence of electronic effects of substituents on the spectral data of carbonyl group, vinyl proton and carbons of the ketones have been explained.
Keywords
Environmentally benign reaction
4′-Fluorophenyl chalcones
IR and NMR spectra
Antimicrobial and insect antifeedant activities
Hammett correlation
1 Introduction
Green chemistry provides good eco-friendly methods for the synthesis of organic compounds without solvent. This method involving the aqueous phase reaction is of very interesting due to operative simplicity, work up easier, good yield, non-hazardousness and is safer for environment. Chemists and environmental scientists reported various solvent methods for organic synthesis. The use of bases for synthesis of chalcones with solvent or without solvent in chalcone chemistry is important (Thirunarayanan et al., 2010; Guthrie and Wang, 1991; Chaloner et al., 1991; Schmid and Whitesides, 1991; Straub, 1995). Literature survey reveals that there is less number of work reported for synthesis of chalcones using bases in aqueous phase aldol condensation reaction between ketones and aldehydes. Various reagents are used for solvent free synthesis like, metals and metal chelates (Waldemar et al., 2000; Babua and Perumal, 1997), carbonates (Zhang et al., 2003), chiral boronate ester (Richard et al., 2006), phosphate (Pore et al., 2007), organolithium (Daskiewicz et al., 1999), sodium hydroxide (Fringueli et al., 2002), silica-sulphuric acid (Thirunarayanan, 2007a,b; Thirunarayanan and Vanangamudi, 2006a, 2007), alumina (Esmaeili et al., 2005), organic ionic liquids (Ranu and Jana, 2005, 2006; Ranu et al., 2003; Ranu and Banerjee, 2005) metal-nanoparticles (Raveendran et al., 2003) and potassium hydroxide-ethanol (Straub, 1995). Kalluriya and Ray (2003) synthesized more than 60% yield of sydenone chalcones using grinding of aqueous sodium hydroxide-heterogeneous reaction medium with aldehydes. Basaif et al. (2005) reported more than 60% yield of some heteroaryl chalcones obtained by the solvent free reaction with aldehydes in cooling condition in presence of surfactants. Venkat Reddy et al. (2000) reported more than 70% yield of chalcones synthesized using zinc chloride in microwave techniques. Thirunarayanan (2008a) reported aqueous potassium hydroxide used as a reagent for synthesis of some aryl chalcones by grinding aryl aldehydes and ketones. Gopalakrishnan et al. (2006, 2007) have reported that fly-ash is one of the good green catalyst for organic synthesis and they synthesized some heterocyclic compounds by solvent free fly-ash catalyzed reaction. The authors wish to report an efficient and selective method for condensation of 4-fluorophenyl methyl ketone with various m- and p-substituted benzaldehydes under solvent free conditions in presence of fly-ash:water catalyst to yield the respective E-2-propen-1-ones and to study the antibacterial, antifungal, insect antifeedant activities and the quantitative structure property relationship from the group frequency. In this method during the reaction of 4-fluoroacetophenone and aldehydes, they decomposed slowly, forming the products in good yield. The methylene units of chalcones derived from cyclic or acyclic ketones are found in many naturally occurring compounds and they are useful for the synthesis of pyrimidine derivatives (Lévai, 2004). The basic skeleton of chalcones is widely figured in natural products and are known to have multipronged activity (Thirunarayanan, 2003, 2008a; Thirunarayanan et al., 2010). Many of the chalcones are used as agrochemicals and drugs (Mirinda et al., 2000; Monostory et al., 2003; Nowakowska, 2007; Majinda et al., 2001; Sitaram Kumar et al., 2007).
2 Experimental
2.1 Materials and methods
All chemicals used were purchased from Sigma–Aldrich and E-Merck chemical company. Fly-ash is collected from Thermal Power Plant-II, Neyveli Lignite Corporation, Neyveli, Tamil Nadu, India. Melting points of all chalcones were determined in open glass capillaries on Mettler FP51 melting point apparatus and are uncorrected. Infrared spectra (KBr, 4000–400 cm−1) were recorded on AVATAR-300 Fourier transform spectrophotometer. The NMR spectra are recorded in INSTRUM AV300 spectrometer operating at 300 MHz for 1H spectra and 75.46 MHz for 13C spectra in CDCl3 solvent using TMS as internal standard. Electron impact (EI) (70 eV) and chemical ionization mode FAB+ mass spectra were recorded with a JEOL JMS600H spectrometer. Microanalyses of all chalcones were performed in Elementar Model Vario EL III Analyzer.
2.2 Synthesis of substituted styryl 4-fluorophenyl ketones (Thirunarayanan, 2009, 2010)
Appropriate mixture of 4-fluoroacetophenone (0.01 mol) and m- and p- substituted benzaldehydes (0.01 mol), fly-ash (1 g) and 15 ml of water were refluxed for 4 h (Scheme 1). The completion of reaction was confirmed by TLC. The reaction mixture was transferred into a 100 ml corning glass beaker and extracted with 10 ml of dichloromethane. After evaporation of solvent the pure product were obtained more than 65% by recrystallization with ethanol–dioxane mixture and dried in vacuum desiccator. The purity of known compounds was compared with authentic samples of physical constants and spectral data. The characterization data of all chalcones are presented in Table 1 and the spectral data are shown in Tables 2–4.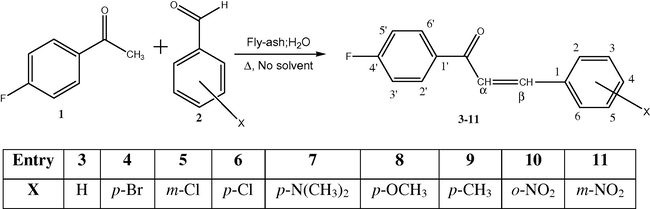
Fly-ash:water catalyzed aldol reaction between 4-fluoroacetophenone and various substituted benzaldehydes.
Entry
X
Mol. formula
Mol. weight
Yield (%)
M.p. (°C)
Found (Calcd.)
Mass m/z
C
H
N
3
H
C15H11FO
226
92
132–133
132a
–
–
–
226(M+), 207, 149, 136, 131, 123, 95, 90, 77, 19
4
p-Br
C15H10BrFO
305
88
117–118
58.93 (59.05)
3.24 (3.27)
–
305(M+), 307(M2+), 306, 304, 226, 240, 166, 125, 107, 95
5
m-Cl
C15H10ClFO
260
90
85–86
69.08 (69.11)
3.76 (3.87)
–
260(M+), 262(M2+), 241, 165, 149, 137, 136, 124, 111, 95, 29, 19
6
p-Cl
C15H10ClFO
260
87
125–126
69.06 (69.11)
3.74 (3.87)
–
260(M+), 262(M2+), 241, 149, 137, 136, 111, 95, 29
7
p-N(CH3)2
C17H16FNO
269
89
113–114
75.76 (75.82)
5.92 (5.99)
5.16 (5.20)
269(M+), 250, 225, 174, 149, 136, 133, 120, 95, 29, 19, 15
8
p-OCH3
C16H13FO2
256
90
97–98
98b
–
–
–
256(M+), 241, 237, 225, 161, 149, 136, 133, 123, 107, 95, 95, 31, 19, 15
9
p-CH3
C16H13FO
240
91
123–124
79.93 (79.98)
5.39 (5.45)
–
240(M+), 225, 221, 145, 140, 136, 95, 91, 29, 19, 15
10
o-NO2
C15H10FNO3
271
86
118–119
66.36 (66.42)
3.66 (3.72)
5.09 (5.16)
271(M+), 255, 252, 176, 149, 136, 135, 95, 29, 19, 16
11
p-NO2
C15H10FNO3
271
91
92–93
66.39 (66.42)
3.68 (3.72)
5.12 (5.16)
271(M+), 255, 252, 149, 136, 95, 29, 16
Entry
X
Ar–F
Ar–H
CO (s-cis)
CO (s-trans)
CHop vinyl
CHip vinyl
CH⚌CHop vinyl
Substituent in styryl part
3
H
1098.35
3028.27
1662.88
1608.98
764.86
1153.30
1032.81
–
4
p-Br
1094.36
2993.26
1665.36
1619.34
772.98
1153.27
1028.31
–
5
m-Cl
1082.64
2923.82
1667.04
1603.99
780.65
1156.87
1020.67
–
6
p-Cl
1088.29
3065.51
1661.53
1607.00
784.22
1157.63
1021.08
–
7
p-N(CH3)2
1073.75
2923.82
1662.14
1601.03
732.65
1157.46
1010.70
3332.55 (–N(CH3)2)
8
p-OCH3
981.06
2988.03
1659.91
1604.54
742.54
1149.49
1021.75
1231.78 (C–O–C)
9
p-CH3
1030.05
3031.04
1659.16
1606.34
739.86
1152.36
1096.61
2852.81 (CH3)
10
o-NO2
1014.95
3054.93
1667.87
1626.15
750.06
1127.94
1072.50
1510.32 (–NO2)
11
m-NO2
1004.85
2998.24
1669.83
1625.25
744.92
1159.75
1020.09
1525.25 (–NO2)
Entry
X
4′-F Ph ring (m, 4H)
Hα (d, 1H)
Hβ (d, 1H)
Styryl Ph ring (m, 4H)
Substituent in styryl part
3
H
8.022–8.079
7.493 (J = 15.6 Hz)
7.829 (J = 15.6 Hz)
7.125–7.414
–
4
p-Br
7.960–8.231
7.563 (J = 16.4 Hz)
7.823 (J = 16.4 Hz)
7.201–7.528
–
5
m-Cl
8.025–7.076
7.513 (J = 16.5 Hz)
7.746 (J = 16.5 Hz)
7.138–7.393
–
6
p-Cl
8.026–8.072
7.498 (J = 15.6 Hz)
7.780 (J = 15.6 Hz)
7.146–7.258
–
7
p-N(CH3)2
8.032–8.049
7.356 (J = 15.8 Hz)
7.765 (J = 15.8 Hz)
6.867–7.247
2.023 (s, 6H, N(CH3)2)
8
p-OCH3
8.036–8.054
7.394 (J = 15.3 Hz)
7.801 (J = 15.3 Hz)
7.119–7.246
3.873 (s, 3H, (OCH3))
9
p-CH3
8.023–8.075
7.490 (J = 15.6 Hz)
7.825 (J = 15.6 Hz)
7.137–7.259
2.390 (s, 3H, (CH3))
10
o-NO2
8.080–8.164
7.590 (J = 15.7 Hz)
7.731 (J = 15.7 Hz)
7.268–7.318
–
11
p-NO2
8.063–8.500
7.646 (J = 15.9 Hz)
7.853 (J = 15.9 Hz)
7.248–7.617
–
Entry
X
CO
Cα
Cβ
C1
C2
C3
C4
C5
3
H
188.73
121.46
144.99
134.68
128.74
130.62
128.44
130.62
4
p-Br
188.76
121.54
144.74
134.62
128.53
131.64
122.65
131.64
5
m-Cl
188.24
122.46
143.35
136.65
126.57
134.45
127.16
130.71
6
p-Cl
188.45
121.85
143.48
133.02
129.24
129.57
134.30
129.57
7
p-N(CH3)2
188.85
119.32
145.30
123.11
127.58
115.46
148.51
115.46
8
p-OCH3
188.80
119.12
144.87
130.24
129.41
114.38
161.70
114.38
9
p-CH3
188.85
120.45
145.11
131.96
128.48
129.69
141.20
129.48
10
o-NO2
188.92
125.03
148.47
130.41
140.43
125.03
129.24
133.55
11
m-NO2
187.89
122.25
141.84
136.45
124.03
148.66
122.23
130.05
C6
C1′
C2′
C3′
C4′
C5′
C6′
Substituent in styryl part
3
H
128.74
134.47
134.43
115.85
167.23
115.85
134.43
–
4
p-Br
128.74
133.68
131.49
116.92
168.93
119.92
131.49
–
5
m-Cl
122.71
134.73
131.56
115.75
167.32
115.85
131.56
–
6
p-Cl
129.24
136.52
131.18
115.92
167.32
115.92
131.18
–
7
p-N(CH3)2
127.58
134.74
131.00
115.75
167.35
115.75
131.00
45.63 (N(CH3)2)
8
p-OCH3
129.41
134.71
130.98
115.75
167.09
115.75
130.98
55.37 (OCH3)
9
p-CH3
128.48
134.67
131.06
115.79
167.17
115.79
131.06
21.51 (CH3)
10
o-NO2
126.95
133.59
131.37
116.03
168.02
116.03
131.37
–
11
p-NO2
131.28
133.84
131.15
116.08
167.53
116.08
131.15
–
3 Results and discussion
Various m- and p-substituted benzaldehydes containing either electron-releasing or withdrawing groups, 4-fluorophenyl methyl ketone, and fly-ash:water were taken in a round bottomed flask and subjected to aldol condensation by refluxation. During the heating the reactants yield the product by decomposition of reactants. The completion of reaction was monitored by TLC. The driving force of the reaction is the heat of formation while the refluxing reactants and fly-ash during which they decompose slowly into the respective E-2-propen-1-ones. The yield of the product is more than 65%. The advantages of this method include mild reaction condition, good yield, slow decomposition of the reactants, environmentally benign reaction, eco-friendly nature since no huge hazardous catalyst or solvent is required.
3.1 Spectral study
Spectroscopic technique is a versatile tool for providing information about the structural diagnosis of most of the organic substrates. It also finds its frequent use in the conformational analysis and in understanding the influence of electronic and conformational effects on chemical shifts and coupling constants. 1H and 13C NMR techniques have been extensively applied in deriving stereo chemical information about a wide variety of systems. Vicinal coupling values have been used in conformational analysis as it can give clue about the orientation of the substituent. Quantitative structure activity relationship study and quantitative property relationships study deal the prediction of ground state molecular equilibration (Wang et al., 2005; Mulliken, 1939) of organic substrates such as s-cis and s-trans isomers α,β-unsaturated ketones (Thirunarayanan et al., 2007) from spectral data. Their use in structure parameter correlations has become popular for studying biological activities (Rajabi et al., 2005), normal co-ordinate (Sharma et al., 2002; Krishnakumar and Ramasamy, 2002) analysis and transition states of reaction mechanisms (Dass, 2001). Infrared spectroscopy is a powerful tool technique for the qualitative and quantitative study of natural and synthetic molecules (Griffiths and Chalmers, 2002). In the importance of material sciences, IR spectroscopy can provide the information about the nature, concentration and structure of samples at the molecular levels (Pellerin and Pelletier, 2005). Numerous works have been devoted to the reactivity of α,β-unsaturated carbonyl compounds particularly, the theoretical aspects of substituent effects were studied on long range interactions in the β-sheet structure (Horváth et al., 2005) of oligopeptides. QSAR study of substituted benzo[α] phenazines (Chen et al., 2005) cancer agents, Diels–Alder reactions (Dumont and Chaquin, 2006), density functional theory (Senthilkumar et al., 2006), gas phase reactivity of alkyl allyl sulfides (Izadar and Gholami, 2006), rotational barriers in selenomides (Kaur et al., 2006). Moraleda et al. (2006) has been studied the quantitative structural relationships in α,β-unsaturated carbonyl compounds between the half wave reduction potential, the frontier orbital energy and the Hammett σp values. Dhami and Stothers (1963a,b) have extensively studied the 1H NMR spectra of a large number of methylketones and styrenes with a view to establish the validity of the additivity of substituent effect in aromatic shielding first observed by Lauterber (1961). Savin et al. (1975) studied the NMR data of unsaturated ketones of the type RC6H4–CH⚌CH–COMe3 and sought Hammett correlations for the ethylenic protons. Solcaniova and Toma (1980) have measured 1H and 13C NMR spectra of substituted styrenes, styryl phenyls and they obtained good Hammett correlations for the olefinic protons and carbons. Now a day’s scientists (Sung and Ananthakrishna Nadar, 2000; Thirunarayanan and Ananthakrishna Nadar, 2006a,b; Shanthi and Kabilan, 2007) have paid more interest to correlate the group frequencies of spectral data with Hammett substituent constants to explain the substituent effects of organic compounds. Recently Thirunarayanan (2008b) investigated elaborately the single and multi-substituent effects on alpha and beta hydrogen and carbons of some naphthyl chalcones. Within the above view there is no information available for the study of structure parameter correlation with the help of infrared and NMR spectral data in literature in the past with substituted styryl 4′-fluorophenyl ketones. Hence the authors have obtained the above ketones by fly-ash:water catalyzed crossed Aldol reaction between 4-fluorophenyl methyl ketone and various m- and p-substituted benzaldehydes and characterized by physical constants, microanalyses and spectral data. In the present study of this section the author evaluates the substituent effects of assigned group frequencies of all chalcones like carbonyl stretches νCO, C–F and the deformation modes of vinyl part CH out of plane, in-plane, CH⚌CH and >C⚌C< out of planes (cm−1), the vinyl hydrogen and carbons δ(ppm) of Hα, Hβ, Cα, Cβ, CO are assigned and these frequencies are correlated with various kinds of substituent constants.
3.1.1 Correlation of IR spectral data
The effect of substituents on the infrared carbonyl frequencies has been reported previously in several studies (Thirunarayanan and Ananthakrishna Nadar, 2006a,b; Jones et al., 1957; Krueger, 1973; Stewart and Yates, 1958, 1960; Thirunarayanan and Vanangamudi, 2006b; Thirunarayanan and Jaishankar, 2003; Iida et al., 2007; Arul Kumaran et al., 2010). The carbonyl group stretching frequency can be assumed to be “mass insensitive”. The carbonyl group frequency has been successfully correlated with Hammett σ constants in acetophenones (Brown and Okamoto, 1957), benzophenones (Fuson et al., 1954) and benzoyl chlorides (Flett, 1948).
While seeking Hammett correlation involving group frequencies, the form of the Hammett equation employed is
The series of ketones chosen in the present study possess α,β-unsaturated carbonyl system. They are expected to exist in s-cis and s-trans conformations are shown in Fig. 1. The carbonyl stretching frequencies (cm−1) of s-cis and s-trans isomers of present study are presented in Table 2. The stretching frequencies for carbonyl absorption are assigned based on the assignments made by Hays and Timmons (1968). The lowest carbonyl frequency is observed in both the conformers when strongest electron withdrawing groups are present in phenyl ring while the highest frequency is noted when the strongest electron attracting group present in phenyl ring. The same trend was followed for assigned carbonyl frequencies of s-cis and s-trans conformers. These frequencies are separately analyzed through various Hammett sigma constants using single and multi-regression analysis. The results of the statistical analysis are presented in Table 5. In all the correlation positive ρ values are obtained. This shows that the normal substituent effects operate in all the compounds. In the case of the s-cis conformers the correlation of νCO with Hammett σ, σ+ and σI values seems satisfactory when H substituent is excluded. The correlation of σR is poor. This is due to the conjugative effect on the carbonyl group from the substituent shown in Fig. 2. The multi-regression analysis (Swain and Lupton, 1968) answers satisfactorily with σI and σR or F and R parameters. The correlation equations (2) and (3) are:
r = Correlation coefficient; ρ = slope; I = intercept; s = standard deviation; n = number of substituents.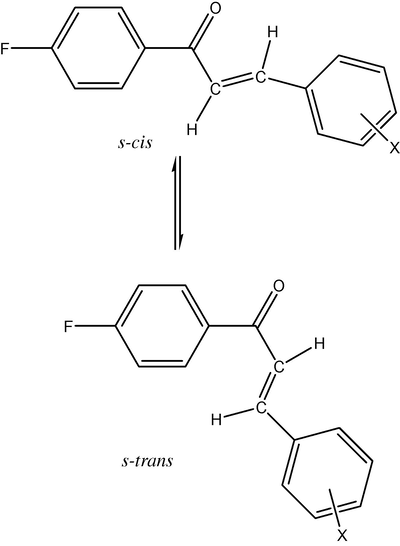
The s-cis and s-trans conformers of substituted styryl 4′-fluorophenyl ketone.
Frequency
Constants
r
ρ
I
s
n
Correlated derivatives
νC–F (cm−1)
σ
0.910
3.840
1016.57
0.15
8
p-Br, m-Cl, p-Cl, p-N(CH3)2, p-OCH3, p-CH3, o-NO2, m-NO2
σ+
0.992
6.245
1021.65
0.11
8
p-Br, m-Cl, p-Cl, p-N(CH3)2, p-OCH3, p-CH3, o-NO2, m-NO2
σI
0.683
3.867
1048.41
0.12
9
H, p-Br, m-Cl, p-Cl, p-N(CH3)2, p-OCH3, p-CH3, o-NO2, m-NO2
σR
0.900
6.434
1628.73
1.60
8
H, m-Cl, p-Cl, p-N(CH3)2, p-OCH3, p-CH3, o-NO2, m-NO2
νCO(s-cis) (cm−1)
σ
0.900
8.940
1657.70
0.15
7
m-Cl, p-Cl, p-N(CH3)2, p-OCH3, p-CH3, o-NO2, m-NO2
σ+
0.900
12.465
1659.04
0.16
7
m-Cl, p-Cl, p-N(CH3)2, p-OCH3, p-CH3, o-NO2, m-NO2
σI
0.900
32.817
1648.41
0.12
7
m-Cl, p-Cl, p-N(CH3)2, p-OCH3, p-CH3, o-NO2, m-NO2
σR
0.713
14.444
1658.73
1.60
8
H, m-Cl, p-Cl, p-N(CH3)2, p-OCH3, p-CH3, o-NO2, m-NO2
νCO(s-trans) (cm−1)
σ
0.769
11.723
1607.37
5.41
9
H, m-Br, m-Cl, p-Cl, p-N(CH3)2, p-OCH3, p-CH3, o-NO2, m-NO2
σ+
0.684
6.456
1609.46
6.13
9
H, m-Br, m-Cl, p-Cl, p-N(CH3)2, p-OCH3, p-CH3, o-NO2, m-NO2
σI
0.817
16.462
1603.60
6.71
9
H, m-Br, m-Cl, p-Cl, p-N(CH3)2, p-OCH3, p-CH3, o-NO2, m-NO2
σR
0.846
10.887
1609.29
7.17
9
H, m-Br, m-Cl, p-Cl, p-N(CH3)2, p-OCH3, p-CH3, o-NO2, m-NO2
(ppm)
σ
0.930
0.171
7.479
0.03
8
H, m-Cl, p-Cl, p-N(CH3)2, p-OCH3, p-CH3, o-NO2, m-NO2
σ+
0.936
0.106
7.511
0.03
8
H, m-Cl, p-Cl, p-N(CH3)2, p-OCH3, p-CH3, o-NO2, m-NO2
σI
0.900
0.211
7.433
0.71
8
H, m-Cl, p-Cl, p-N(CH3)2, p-OCH3, p-CH3, o-NO2, m-NO2
σR
0.983
0.113
7.505
0.09
7
H, m-Cl, p-Cl, p-N(CH3)2, p-CH3, o-NO2, m-NO2
(ppm)
σ
0.900
0.692
7.792
0.35
7
H, m-Cl, p-Cl, p-N(CH3)2, p-OCH3, p-CH3, m-NO2
σ+
0.673
0.015
7.701
0.46
9
H, m-Br, m-Cl, p-Cl, p-N(CH3)2, p-OCH3, p-CH3, o-NO2, m-NO2
σI
0.910
0.458
7.805
0.31
7
H, m-Cl, p-Cl, p-N(CH3)2, p-OCH3, p-CH3, o-NO2
σR
0.726
0.490
7.790
0.48
9
H, m-Br, m-Cl, p-Cl, p-N(CH3)2, p-OCH3, p-CH3, o-NO2, m-NO2
δCO (ppm)
σ
0.903
−0.381
188.64
0.33
7
H, m-Cl, p-Cl, p-N(CH3)2, p-OCH3, p-CH3, m-NO2
σ+
0.900
−0.230
188.57
0.33
6
H, m-Cl, p-Cl, p-OCH3, p-CH3, m-NO2
σI
0.902
−0.700
188.81
0.32
7
H, m-Cl, p-Cl, p-N(CH3)2, p-OCH3, p-CH3, m-NO2
σR
0.781
−0.132
188.58
1.43
9
H, p-Br, m-Cl, p-Cl, p-N(CH3)2, p-OCH3, p-CH3, o-NO2, m-NO2
(ppm)
σ
0.905
0.053
120.37
0.23
8
H, m-Cl, p-Cl, p-N(CH3)2, p-OCH3, p-CH3, o-NO2, m-NO2
σ+
0.900
0.400
120.42
0.23
9
H, p-Br, m-Cl, p-Cl, p-N(CH3)2, p-OCH3, p-CH3, o-NO2, m-NO2
σI
0.805
−0.736
120.61
0.71
9
H, p-Br, m-Cl, p-Cl, p-N(CH3)2, p-OCH3, p-CH3, o-NO2, m-NO2
σR
0.175
−1.330
120.32
1.25
8
H, m-Cl, p-Cl, p-N(CH3)2, p-OCH3, p-CH3, o-NO2, m-NO2
(ppm)
σ
0.911
−2.589
143.47
1.40
7
H, m-Cl, p-Cl, p-N(CH3)2, p-OCH3, o-NO2, m-NO2
σ+
0.906
−1.540
143.00
1.45
7
H, m-Cl, p-Cl, p-N(CH3)2, p-OCH3, o-NO2, m-NO2
σI
0.900
−3.280
144.19
1.73
7
H, m-Cl, p-Cl, p-N(CH3)2, p-OCH3, o-NO2, m-NO2
σR
0.613
−0.878
143.36
2.35
9
H, p-Br, m-Cl, p-Cl, p-N(CH3)2, p-OCH3, p-CH3, o-NO2, m-NO2
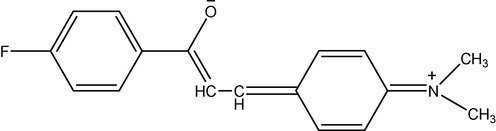
Resonance structure of chalcone.
The correlations observed between νCO s-trans (cm−1) and Hammett sigma constants are presented in Table 5 and all correlations fail including F and R parameters. This may mean that the substituents are incapable of predicting substituent effects independently on carbonyl frequencies as per the reason stated earlier. It is well known that polar and inductive effects (Thirunarayanan and Ananthakrishna Nadar, 2002) less pronounced considerably with distance. The substituents are separated from the carbonyl group by four or more carbon atoms in the compounds.
A comparison of ρ values for two isomers in relation to a constant reveals that their relative abilities to transmit electronic effects are different. The ratio of ρ cis/ρ trans is 0.762. This result is in accordance with the hypothesis that, as the two conformers have different approaches of degree of coplanarity and their abilities to transmit electronic effects become different.
The assigned νC–F (cm−1) stretches (Kemp, 1987; Silverstein et al., 1963) are presented in Table 2. These frequencies are correlated with various Hammett substituent constants. The results of statistical analysis are presented in Table 5. The correlation of C–F vibrations with σ, σ+ and σR constants gave satisfactory and good result and that with other constants σI, F and R fails. Similarly the multiparameter correlations also fail with the above stretches.
The measured deformation modes of C–H in-plane and out of plane and CH⚌CH and >C⚌C< out of plane modes in substituted styryl 4′-fluorophenyl ketones are presented in Table 2. The statistical analysis of all observed deformation modes with various Hammett substituent constants are presented in Table 5. In Table 2, the observed deformation modes predict that the reactions occur normally with substituent constants by the absorption trend. In the styryl moiety, with electron withdrawing groups, absorption is higher and with electron donating groups, absorption is lower. This trend fails in present ketones. Because ketones with nitro-group absorb at some what less values. Therefore the correlation is reduced or reversed. The polar effects, inductive and resonance effects of substituents do not affect the above group absorptions. Therefore these substituents reversed their substituent effect and unable to predict the reactivity on the deformation modes.
The correlations of all deformation modes of styryl 4′-fluorophenyl ketones have been done with all kinds Hammett substituents constants. The results of statistical analysis of these ketones are shown in Table 5. In this series of ketones all correlations are very poor including multi-regression analysis. In this series of ketones some of the correlations produce negative ρ values. This shows that the def. modes of vibrations are unable to predict the reactivity through their substituent effects. This is due to the reversal of substituent effect and the conjugative structure affects the effect of substituents on the absorptions in all ketones shown in Fig. 2. The ratio of ρ between –CH in-plane def. and –CH out of plane def. is −0.60. This value shows that the correlations of all kinds of –CH absorptions with substituent constants are not obeyed.
3.2 NMR spectral correlation
3.2.1 1H NMR spectra
The 1H NMR spectra of nine chalcones under investigation are recorded in deuterochloroform solutions employing tetramethylsilane (TMS) as internal standard. The signals of the ethylenic protons were assigned. They were calculated as AB or AA′ BB′ systems, respectively. The chemical shifts of Hα are at higher field than those of Hβ in this series of ketones. The ethylenic protons give an AB pattern and the β-proton doublet in most cases is well separated from the signals of the aromatic protons. The assigned chemical shifts of the ethylenic protons are presented in Table 3.
In nuclear magnetic resonance spectra, the proton or the 13C chemical shifts (δ) depend on the electronic environment of the nuclei concerned. These shifts can be correlated with reactivity parameters. Thus the Hammett equation may be used in the form as
The assigned Hα and Hβ proton chemical shifts (ppm) are correlated with various Hammett sigma constants. The results of statistical analysis are presented in Table 5. All the attempted correlation involving substituent parameters gave only positive ρ value. This shows that the normal substituent effect operates in all chalcones. The Hα proton chemical shifts satisfactorily correlate with Hammett substituent constants. The σI constants produce the correlation excluding the methoxy substituent in styryl part. The correlations of Hβ proton chemical shifts with Hammett σ and σI constants are satisfactory excluding nitro substituents in styryl part and with the other constants fail.
From Table 5 the r values show that the substituents predicting the reactivity on Hα and Hβ are not equal in all ketones. This is in contrast to the findings of Solcaniova and Toma (1980). In fact the extent of transmission of electrical effect is almost same from the substituents to Hα and Hβ in the present investigation. It has been the observation of Solcaniova et al. (1976) that the chemical shifts of the β-protons do not correlate with any type of substituent parameters. But in the present investigation the chemical shifts of both protons correlate satisfactorily with Hammett sigma constants.
Application of Swain–Lupton (Swain and Lupton, 1968) treatment to the relative chemical shifts of Hα and Hβ with F and R values is successful with either resonance, inductive or F and R parameter generates the multi-regression equations (5)–(8):
3.2.2 13C NMR spectra
Physical organic chemists and scientists (Annapoorna et al., 2002; Dhami and Stothers, 1963a,b; Thirunarayanan, 2007a,b) have made an extensive study of 13C NMR spectra for a large number of different ketones and styrenes. They found a linear correlation of the chemical shifts of Cβ carbons with Hammett σ constants in styrenes. Attempts to correlate the Cα chemical shifts with any kind of σ constants fail in their systems. An attempt is made in the present investigation to determine substituent effects on vinyl Cα, Cβ and carbonyl carbon chemical shifts of the 4′-fluorophenyl chalcones to what extent. The 13C chemical shifts of vinyl Cα, Cβ and carbonyl carbons of all ketones are given in Table 4.
The chemical shifts (ppm) observed for the carbonyl carbons are correlated with Hammett constants and the results of statistical analysis are presented in Table 5. All correlations gave negative slopes with fair degree of r values. This negative slope shows that the substituent effect reverses on the carbonyl carbon absorptions in all ketones. The correlation of σ, σ+ and σI constants with δC⚌O (ppm) are satisfactory excluding some substituents such as nitro and methoxy groups and when they are included, the correlation fails. Poor correlation was obtained with σR constants. This is due to the reason stated earlier that the conjugation exists between the substituent and the carbonyl group shown in Fig. 2.
In view of the inability of some of the σ constants to produce individually satisfactory correlations, it was thought worthwhile to seek multiple correlations involving either σI and σR constants or Swain–Lupton (Swain and Lupton, 1968) F and R parameters produce Eqs. (9) and (10):
The 13C chemical shift (ppm) values of vinyl carbons of all ketones are correlated with various Hammett substituent constants. The results of statistical analysis of substituent effects on carbonyl carbons are shown in Table 5. Satisfactory correlations obtained with Cα carbon chemical shifts and produce positive ρ values with σ and σ+ constants. This implies that the normal substituent effect operates in all constants with Cα carbons in all ketones. The negative ρ values in σI and σR constants imply that the substituent effects are reversed in these constants and give poor correlation. This is due to the fact that the resonance and inductive effects are not operating considerably for prediction of reactivity through the conjugative structure of all chalcones in Fig. 2. The Swain–Lupton (Swain and Lupton, 1968) parameters correlation also fails within these carbon chemical shifts. The individual correlations of Cβ carbon chemical shifts with Hammett σ, σ+ and σI constants produce satisfactory correlation excluding p-CH3 substituent and fail with σR constants. All correlations gave negative ρ values. The negative ρ values imply that the substituent effect is reversed within these constants. The degree of transmission of substituent effect is found to be high in Cα chemical shifts than Cβ carbon chemical shifts. Uniformly σI and σR parameters or F and R values adequately explain substituent effects in all cases as evidenced from the correlation equations (11) and (12) are
3.3 Microbial activities
Chalcones possess a wide range of biological activities such as antibacterial (Sivakumar et al., 2007), antifungal (Lahtcher et al., 2008), antiviral (Trivedi et al., 2007), antifeedant (Thirunarayanan, 2008a; Thirunarayanan et al., 2010) anticancer (Modzelewska et al., 2006), antimalarial (Dominguez et al., 2005), anti-tuberculosis (Lin et al., 2002), antiAIDS (Deng et al., 2007) and antioxidant (Werber et al., 2005) activities. These multipronged activities present in different chalcones are intended to examine their above activities against respective microbes-bacteria’s, fungi and insect antifeedant activities against caster semilooper.
3.3.1 Antibacterial activity
The antibacterial activities of all prepared chalcones were evaluated against two gram positive pathogenic strains Staphylococcus aureus, Entrocccus faecalis while Escherichia coli, Klebsiella species, Psuedomonas and Proteus vulgaris were the gram negative strains. The disc diffusion technique was followed using the Kirby–Bauer (Bauer et al., 1996) method, at a concentration of 250 μg/ml with ampicillin and streptomycin taken as the standard drugs. The measured antibacterial activities of all chalcones are presented in Table 6. Against E. coli, two compounds 4 and 7 show maximum zone inhibition with greater than 20 mm while 3, 5, 6, 8, 9, 10 and 11. The chalcones 4 and 7 were active against Staphylococcus, showing maximum inhibition. The other chalcones show less effectiveness against S. aureus. Three chalcone derivatives 3, 7 and 8 are more active against Pseudomona at greater than 20 mm zone inhibition and the other derivatives inhibit the growth of bacterial between 12 and 19 mm zone inhibitions. The chalcones 4, 7 and 8 are effective against Klebsiella in 20–24 mm zone inhibition while the other ketones show a moderate activity. The chalcones 3, 4, 7 and 9 are active when they are screened against P. vulgaris and the other compounds are less effective. The chalcones 4 and 8 shows moderate activities against E. faecalis when then are screened with 13–19 mm zone inhibition. Disc size: 6.35 mm; duration: 24–45 h; standard: ampicillin (30–33 mm) and streptomycin (20–25 mm); control: methanol; —: no activities; ±: active (8–12 mm); +: moderately active (13–19 mm); ++: active (20–24 mm).
Entry
X
E. coli
S. aureus
Pseudomonas
Klebsiella
P. vulgaris
Entrococcus faecalis
3
H
±
+
±
±
±
—
4
p-Br
++
++
+
++
+
++
5
m-Cl
+
+
+
+
+
—
6
p-Cl
+
+
+
+
+
—
7
p-N(CH3)2
++
++
++
+
+
—
8
p-OCH3
+
+
+
+
+
++
9
p-CH3
±
+
±
±
±
—
10
o-NO2
+
+
+
+
+
—
11
p-NO2
+
+
+
+
+
—
3.3.2 Antifungal activity
Measurement of antifungal activities of all chalcones were done using Candida albicans as the fungal strain and the disc diffusion technique was followed for the antifungal activity while the two other stains Penicillium species and Aspergillus niger, the dilution method was adopted. The drugs dilution was 50 μg/ml. Grisseofulvin is taken as the standard drug. The observed antifungal activities of all chalcones are presented in Table 7. The antifungal activities of all chalcones against C. albicans, the two compounds 4 and 8 are effective at 20 mm as the zone inhibition with 250 μg/disc while chalcones 7 and 8 are active at 13–19 mm zone inhibition and the ketones 3 and 5 are the least active in 8–12 mm zone inhibitions. Against Penicillum species, compound 4 is visible while development of the fungal colony and 2–3 colonies was recorded for the compounds 8. The chalcones inhibition against A. niger was less in two compounds 4 and 7 being highly active followed by 8. Presence of a methoxy, methyl, dimethyl and bromo substituents are responsible for antimicrobial activities of chalcones. Standard: griseofulvin and gentamycin; duration: 72 h; control: methanol; medium: potato dextrose agar; ++: no fungal colony; +: one fungal colony; ±: two–three fungal colonies; —: heavy fungal colony.
Entry
X
Disc diffusion technique (250 μg/ml)
Drug dilution method (50 μg/ml)
Candida albicans
Penicillin
Aspergillus niger
3
H
—
—
—
4
p-Br
++
++
++
5
m-Cl
—
±
+
6
p-Cl
±
—
—
7
p-N(CH3)2
±
++
++
8
p-OCH3
++
++
+
9
p-CH3
+
+
—
10
o-NO2
—
—
—
11
p-NO2
—
—
—
3.3.3 Insect antifeedant activity
The multipronged activities present in different chalcones are intended to examine their insect antifeedant activities against caster semilooper. The larvae’s of Achoea janata L. were reared as described on the leaves of caster Riclmus cammunls in the laboratory at the temperature range of 26 ± 1 °C and a relative humidity of 75–85%. The leaf-disc bioassay method (Thirunarayanan, 2008a) was used against the 4th instar larvae to measure the antifeedant activity. The 4th instar larvae were selected for testing because the larvae at this stage feed very voraciously.
3.3.3.1 Measurement of insect antifeedant activity of chalcones
Leaf discs of a diameter of 1.85 cm were punched from caster leaves with the petioles intact. All ketones were dissolved in acetone at a concentration of 200 ppm dipped for 5 min. The leaf discs were air-dried and placed in 1 l beaker containing little water in order to facilitate translocation of water. Therefore the leaf discs remain fresh throughout the duration of the rest, 4th instar larvae of the test insect, which had been preserved on the leaf discs of all chalcones and allowed to feed on them for 24 h. The area of the leaf disc consumptions was measured by Dethlers (Dethler, 1947) method. The observed antifeedant activity of chalcones was presented in Table 8. Number of leaf discs consumed by the insect (values are mean + SE of five).
Entry
X
4–6 pm
6–8 pm
8–10 pm
10–12 pm
12–6 am
6–8 am
8 am–12 Nn
12 Nn–2 pm
2–4 pm
Total leaf disc consumed in 24 h
3
H
1
1
0.5
0.5
0.5
1
1
1
1
8
4
p-Br
0.5
0.25
0.25
0.5
0.5
0.5
1
1
0.5
0.5
5
m-Cl
0.5
0.5
0.25
1
0.5
0.5
0.25
0.25
0.25
0.4
6
p-Cl
0.25
0.25
0.25
0
0
0.25
0
0
0
0.1
7
p-N(CH3)2
1
2
2
1
0
0
1
1
1
9
8
p-OCH3
1
0.5
0.5
1
1
0
1
1
1
9
9
p-CH3
0.5
0.5
0.5
2
2
1
1
1
1
9
10
o-NO2
2
3
3
1
1
1
0.5
1
0
12
11
p-NO2
1
2
2
2
1
0.5
0.5
1
0
10
The results of the antifeedant activity of 2-propen-1-ones presented in Table 8 reveal that the compounds 4–6 are found to reflect remarkable antifeedant among all other chalcones. This test is performed with the insects which ate only two-leaf disc soaked under the solution of this compound. Compounds 4, 5 also show enough antifeedant activity but lesser than 6. Further compound 6 was subjected to measure the antifeedant activity at different 50, 100, 150 ppm concentrations and the observation reveals that as the concentrations decreased, and the activity also decreased. It is observed from the results in Table 9 and that the ketone 6 (2E)-1-(4′-fluorephenyl)-3-(3-chlorophenyl)-2-propen-1-one shows an appreciable antifeedant activity at 200 ppm concentration.
ppm
4–6 pm
6–8 pm
8–10 pm
10–12 pm
12 am–6 am
6–8 am
8 am–12 Nn
12 Nn–2 pm
2–4 pm
Total leaf disc consumed in 24 h
50
0.5
0.5
0
0
0
0
0
0
0
0.1
100
0
0.25
0.25
0
0
0
0
0
0
0.05
150
0
0
0
0
0
0
0
0
0
0
Acknowledgement
The authors are thankful to The Head, Instrumentation Laboratory, Department of Chemistry, Madurai Kamaraj University, Madurai for recording NMR spectra of all compounds.
References
- Indian J. Chem.. 2002;41(A):1341.
- IUP J. Chem.. 2010;3:82.
- Synth. Commun.. 1997;27(21):3677.
- Bull. Korean Chem. Soc.. 2005;26(11):1677.
- Am. J. Clin. Pathol.. 1996;45:493.
- J. Am. Chem. Soc.. 1957;79:1913.
- Tetrahedron Lett.. 1991;32:6037.
- J. Mol. Struct. (Theochem). 2005;756:167.
- Tetrahedron Lett.. 1999;40(39):7095.
- Indian J. Chem.. 2001;40(A):223.
- Bioorg. Med. Chem.. 2007;15:4985.
- Chemical Insect Attractants and Repellents. Philadelphia: Blackistan; 1947. p. 210
- Can. J. Chem.. 1963;43:479.
- Can. J. Chem.. 1963;43:510.
- Il Farmaco. 2005;60:307.
- J. Mol. Struct. (Theochem). 2006;758:161.
- Monatst. Chem.. 2005;136:571.
- Trans. Faraday Soc.. 1948;44:767.
- Tetrahedron. 2002;50(39):11499.
- J. Am. Chem. Soc.. 1954;76:2526.
- Arkivoc. 2006;13:130.
- J. Korean Chem. Soc.. 2007;51(6):520.
- Handbook of Vibrational Spectroscopy. Vol vol. 4. Chichester: John Wiley and Sons Inc.; 2002. p. 2576
- Can. J. Chem.. 1991;69(2):339.
- Acta Cryst. (E) SR. 2006;62:o3251.
- Spectrochim. Acta. 1968;24(A):3239.
- J. Mol. Struct. (Theochem). 2005;755:81.
- Tetrahedron Lett.. 2007;48(11):2037.
- J. Mol. Struct. (Theochem). 2006;759:11.
- Can. J. Chem.. 1957;35:504.
- Indian J. Chem.. 2003;42B:2556.
- J. Mol. Struct. (Theochem). 2006;759:41.
- Organic Spectroscopy (second ed.). ELBS; 1987.
- Indian J. Pure Appl. Phys.. 2002;40:252.
- Can. J. Chem.. 1973;51:1363.
- Eur. J. Med. Chem.. 2008;43(1):1.
- J. Am. Chem. Soc.. 1961;83:1846.
- Arkivoc. 2004;7:14.
- Bioorg. Med. Chem.. 2002;10:2795.
- Pure Appl. Chem.. 2001;73:1197.
- Cancer Lett.. 2000;149(1-2):21-29.
- Bioorg. Med. Chem.. 2006;14:3491.
- Toxicology. 2003;184(2–3):203.
- J. Mol. Struct. (Theochem). 2006;760:113.
- J. Chem. Phys.. 1939;7:121.
- Eur. J. Med. Chem.. 2007;42(2):125.
- Lab Plus Int.. 2005;19:10.
- J. Org. Chem.. 2007;3(7):1088.
- Lett. Appl. Microbiol.. 2005;40:212.
- J. Org. Chem.. 2005;70(11):4517.
- J. Org. Chem.. 2005;70(21):8621.
- Eur. J. Org. Chem.. 2006;71(10):3767.
- Org. Lett.. 2003;5(9):1439.
- J. Am. Chem. Soc.. 2003;125(46):13940.
- Arkivok. 2006;1:95.
- Zh. Org. Khim.. 1975;11:1169.
- J. Am. Chem. Soc.. 1991;113(17):6349.
- J. Mol. Struct. (Theochem). 2006;758:107.
- Spectrochim. Acta. 2007;67(A):479.
- Indian J. Pure Appl. Phys.. 2002;40:246.
- Spectrometric Identification of Organic Compounds (fourth ed.). New York: John Wiley and Sons; 1963.
- Eur. J. Med. Chem.. 2007;42(4):538.
- Bioorg. Med. Chem. Lett.. 2007;17:1965.
- Org. Magn. Reson.. 1980;14:138.
- Org. Mag. Reson.. 1976;8:439.
- J. Am. Chem. Soc.. 1958;80:6355.
- J. Am. Chem. Soc.. 1960;82:4059.
- Tetrahedron Lett.. 1995;36(5):663.
- Indian J. Chem.. 2000;39(A):1066.
- J. Am. Chem. Soc.. 1968;90:4328.
- A. J. Chem.. 2003;15:907.
- Indian J. Chem.. 2007;46B:1551.
- J. Korean Chem. Soc.. 2007;51(2):115.
- J. Indian Chem. Soc.. 2008;84:447.
- J. Korean Chem. Soc.. 2008;52(4):369.
- Thirunarayanan, G., 2009. In: Proceedings of the 46th Annual Convention of Chemists and International Conference on Recent Trends in Chemical Sciences, December 2–6, Abstract No. ORG OP5, p. C13.
- Thirunarayanan, G., 2010. In: Tamil Nadu Science Congress 10th Conference, May 21–23, Abstract No. OP4.18, p. 119.
- A. J. Chem.. 2002;13–14:1518.
- J. Korean Chem. Soc.. 2006;50(3):183.
- J. Indian Chem. Soc.. 2006;83:1107.
- Acta Ciencia Indica. 2003;29:183.
- Arkivoc. 2006;12:58.
- Int. J. Chem. Sci.. 2006;1(1):215.
- E-J. Chem.. 2007;4(1):92.
- Spectrochim. Acta. 2007;67(A):1106.
- Spectrochim. Acta. 2010;75A:152.
- Tetrahedron Lett.. 2007;48(48):8472.
- Synth. Commun.. 2000;13:2884.
- J. Am. Chem. Soc.. 2000;122(23):5654.
- J. Mol. Struct. (Theochem). 2005;755:31.
- Bioorg. Med. Chem.. 2005;13:3811.
- Chem. Lett.. 2003;32(10):966.







