Translate this page into:
Technetium-labeled danofloxacin complex as a model for infection imaging
⁎Corresponding author. memoustapha@gmail.com (Moustapha Eid Moustapha) m.moustapha@sau.edu.sa (Moustapha Eid Moustapha)
-
Received: ,
Accepted: ,
This article was originally published by Elsevier and was migrated to Scientific Scholar after the change of Publisher.
Peer review under responsibility of King Saud University.
Abstract
Danofloxacin, a fluoroquinolone derivative antibiotic, was synthesized, successfully labeled with technetium-99m and formulated for the development of a potential diagnostic imaging agent of the bacterial infection and inflammation with higher efficiency than that of the commercially available 99mTc-ciprofloxacin. Factors affecting the labeling yield were optimized. The radiolabeled antibiotic was subjected to preclinical assessments such as purity, stability, and pharmacokinetic investigations in animals. The biodistribution studies indicated that the uptake of 99mTc-danofloxacin was high in the infectious lesion (T/NT = 7.2 ± 0.1) at 2 h post injection. The abscess to normal ratio indicated that the danofloxacin tracer can be used for infection diagnosis. The radiolabeled compound was cleared quickly from most of the body organs. The results displayed that 99mTc-danofloxacin could not differentiate between infection and sterile inflammation.
Keywords
Danofloxacin
Labeling
Technetium
Inflammation
Infection
Diagnosis

1 Introduction
The rate of morbidity and mortality could be reduced if infection and inflammation can be clinically diagnosed and treated with appropriate drugs at early stage (Qaiser et al., 2010). Modern imaging techniques and procedures for diagnosis of infection such as Magnetic Resonance Imaging (MRI), Computer Tomography (CT), X-ray and Ultrasonography (US) are commercially available and have been used for the detection of infection. These tools are not the appropriate methods for the diagnosis and localization of infection at early stages because they cannot reveal the change in the morphology of tissues following the abscess formation (Motaleb, 2007; Lucignani, 2007 and Gemmel et al., 2009). The role of several Nuclear Medicine Scintigraphy (NMS) techniques in early diagnosis or discrimination between infection and inflammation is clinically vital for the appropriate management of patients with microbial infectious or inflammatory diseases (Saha, 2004; Lupetti et al., 2007 and Langer et al., 2005). The introduction of a number of radiopharmaceuticals in Nuclear Medicine used as infection imaging agents was reported including radiolabeled leukocytes and 67Ga-citrate (Palestro, 2007 and Yapar et al., 2001). Due to the inconveniences and limitations of the conventional infection imaging agents such as time-consuming preparation, the lack of specificity, sensitivity and high radiation burden, the development of new radiopharmaceuticals for infection imaging represents a major challenge for nuclear medicine and molecular imaging (Singh et al., 2003; Martin-Comin et al., 2004; Sakr et al., 2013 and Moustapha et al., 2011).
Several radiolabeled antibiotics have been synthesized and are potentially promising kits for the diagnostic imaging of infective lesions (Singh et al., 2010; Esposito et al., 2009; Asikoglu et al., 2000 and Brunton and Parker, 2008). The ideal radiolabeled antibiotic agents should highly localize in the infectious foci, where they are frequently taken up, metabolized and display fast clearance from the body. Quinolones (ceftriaxone, norfloxacin, sitafloxacin, enrofloxacin, ciprofloxacin, lomefloxacin, sparfloxacin, difloxacin, levofloxacin, pefloxacin, moxifloxacin and ceftizoxime) are broad spectrum antibiotics that are widely used as the major treatment of serious bacterial infections (Hazari et al., 2009; Nieto et al., 2005; Starovoitova et al., 2014; Anderson and Kodukula, 2014 and Ziessman et al., 2014). Ciprofloxacin labeled with 99mTc is considered as one of the most important radiopharmaceuticals commercially available for infection imaging (Chattopadhyay et al., 2010; Dumarey et al., 2002; Gemmel et al., 2004 and IAEA, 2008). However, data of biodistribution in experimental animals and in human studies reported that the specificity of 99mTc-ciprofloxacin for infection is contradictory (Yapar et al., 2001; Sarda et al., 2002, 2003; Larikka et al., 2002a,b; Siaens et al., 2004; Pauwels et al., 2001). 99mTc-ciprofloxacin preparation has many disadvantages which are discussed in detail in the literatures (Signore et al., 2008; Siaens et al., 2004; Zolle, 2007 and Britton et al., 1997).
Danofloxacin is a fluoroquinolone derivative with broad spectrum antibacterial activity toward both Gram positive and Gram negative pathogenic bacteria. The structure of danofloxacin is shown in Fig. 1 (USP/NF, 2008). The purpose of the present study was to establish a simple and efficient method for synthesis and labeling danofloxacin with 99mTc as a potential infection imaging agent to reveal the sites of bacterial infections in vivo. The formulation of the labeled drug will have to be optimized based on preclinical assessments with respect to in vitro and in vivo stability, radiochemical purity and pharmacokinetic studies in animals.
Chemical structure of danofloxacin.
2 Materials and methods
Danofloxacin (C19H20FN3O3; M.wt. = 357.37 g/mol) was purchased from Sigma–Aldrich, USA. Whatman No. 1 paper chromatography (PC) was obtained from Whatman International Ltd., Maidstone, UK. Technetium-99 m (t1/2 = 6 h) was eluted as 99mTcO4− from 99Mo/99mTc generator, Gentech, Turkey. Measurement of radioactivity was performed in a Well-type NaI(Tl) gamma(γ)counter. All reagents and solvents used were of analytical reagent grade and used without further purification. Deionized water was used in all experiments.
2.1 Labeling procedure
99mTc-danofloxacin was synthesized by direct reaction of danofloxacin with technetium-99m. An accurate amount of 1 mg danofloxacin was transferred to an evacuated penicillin vial. A freshly prepared deoxygenated aqueous solution containing exactly 50 μg of SnCl2·2H2O was added and the pH of the preparation was adjusted to 11 using phosphate buffer. One ml of 99mTc eluate containing 400 MBq was introduced to the above reaction mixture, mixed and left to react at room temperature (25 °C) under sterile conditions for 30 min before estimating the yield of the produced complex. The reaction conditions affecting the radiolabeling yield such as stannous chloride amount (25–200 μg), danofloxacin amount (0.5–2.5 mg), pH of the reaction mixture (5–12) and the reaction time (1–480 min) were investigated and optimized in order to maximize the labeling efficiency. Paper chromatography (PC) and high performance liquid chromatography (HPLC) were used to assess the radiochemical purity.
2.2 Analysis
Radiochemical purity of a radiopharmaceutical product is the proportion of the total radioactivity in the desired radiochemical form. Different analytical methods including PC and HPLC are used to assess the radiochemical impurities present in the reaction mixture with the 99mTc-labeled complex. Radiochemical impurities are hydrolyzed 99mTc and free 99mTcO−4. Radiochemical purity of 99mTc-danofloxacin was determined by the ascending paper chromatographic technique using strips of Whatman No. 1 paper chromatography (PC). Two strips (1 cm width, 13 cm length) were marked 2 cm from the bottom edge of the strip and lined into segments 1 cm each up to 13 cm. A spot of 1–2 μl of the filtered reaction mixture containing the labeled complex was applied above the lower edge and allowed to evaporate spontaneously. Then one strip was placed in a closed jar and developed with acetone to determine the percentage of free pertechnetate (99mTcO4−) while the other strip was developed with a mixture of ethanol: water: ammonia (2:5:1) to detect the percentage of reduced hydrolyzed technetium-99m. After complete development the strips were removed, dried and cut into segments of 1 cm each. The radioactivity was counted using a Well-type γ-scintillation detector.
The radiochemical purity was further confirmed by HPLC. Chromatographic analysis was performed by injection of 10 μl of purified reaction mixture containing 99mTc-danofloxacin at the optimum conditions into a reversed-phase column (Lichrosorb RP C-18, 4 mm × 250 mm; 5 μm) coupled to a UV detector (SPD-6A) set at 320 nm and eluted with a mobile phase consisting of a mixture of 10% ethanol in 0.2 M phosphate buffer at pH 7.2. The filtered mobile phase was degassed prior to use and the flow rate was adjusted to 0.5 ml/min. (Moustapha et al., 2013; Al-wabli et al., 2011). Radioactivity measurements in the HPLC eluates were detected by NaI(Tl) scintillation detector coupled to a single channel analyzer.
2.3 Stability of 99mTc-danofloxacin in serum
Stability of the labeled complex was determined by mixing 1.8 ml of the normal serum and 0.2 ml of 99mTc-danofloxacin at 37 °C for 24 h. During the in vitro incubation period, aliquots of 0.2 ml each were withdrawn at different time intervals for up to 24 h and analyzed using instant thin layer chromatography (ITLC) and HPLC to determine the percentage of the formed 99mTc-complex, reduced hydrolyzed technetium and free pertechnetate.
2.4 Induction of inflammation in mice
Induction of infectious foci was evaluated by the method reported by Al-wabli in which a single clinical isolation of Staphylococcus aureus from biological samples was used to produce focal infection (Al-wabli et al., 2011). Individual colonies were diluted in order to obtain turbid suspension. Groups of three mice were intramuscularly injected with 200 μl of the suspension in the left lateral thigh muscle. Then, the mice were left for 24 h to get a gross swelling in the infected thigh. On the other hand, non-infected inflammation (sterile inflammation) was induced by injecting 200 μl of turpentine oil sterilized by autoclaving at 121 °C for 20 min., intramuscularly administered in the left lateral thigh muscle of the mice. Two days later, swelling appeared (Mostafa et al., 2010; Asikoglu et al., 2000). Similarly, induction of heat killed non-infected inflammation was induced by injecting 200 μl of heat killed S. aureus (Larikka et al., 2002a,b; Oyen et al., 2001 and Kaul et al., 2013).
2.5 Biodistribution studies
In vivo experiments were conducted in accordance with the guidelines of the Egyptian Atomic Energy Authority and the animal ethics committee. Prior to the biodistribution experiment, normal Albino mice were housed in groups (n = 5) for different intervals of time under normal conditions. Animals were injected intravenously in the tail vein with 100 μl of 99mTc-danofloxacin (4 MBq). Mice were sacrificed by cervical dislocation at 2, 4 and 24 h after administration of 99mTc-danofloxacin. A blood sample was obtained by heart puncture. Both target and non-target thighs were dissected and counted. After dissection, the organs were removed, washed with saline, collected in plastic tubes and weighed. The radioactivity of each sample and the background was counted in a Well-type NaI(Tl) gamma spectrometer. Results were reported as percent-injected dose per gram organ (% ID/g organ ± SD) in a population of five mice for each time point. Statistics are evaluated with the Student’s t test and all results are given as mean ± SEM.
3 Results and discussion
Radiochemical purity and in vitro stability of 99mTc-danofloxacin complex were assessed by PC and further confirmed by HPLC. Acetone was used as the developing solvent to determine free 99mTcO4−, which moved with the solvent front (Rf = 1), while 99mTc-danofloxacin and reduced hydrolyzed technetium remained at the origin (Rf = 0). Reduced hydrolyzed technetium was detected using a mixture of ethanol: water: ammonium hydroxide (2:5:1 v/v) as the developing solvent, where it remains at the origin (Rf = 0) while other species migrate with the solvent front (Rf = 1). The undesirable radiochemical species such as the free and hydrolyzed fractions must be removed or reduced to a minimum level in order not to change the biodistribution of the labeled compound resulting in a significant interference with the diagnostic image. The radiochemical purity was determined by subtracting the sum of the percent of reduced hydrolyzed technetium and free pertechnetate from 100%. The radiochemical yield is the mean value of three experiments. The radiochemical purity was further confirmed by HPLC analysis, where the retention time of free 99mTcO4− and 99mTc-danofloxacin was 5 and 18.9 min, respectively as illustrated in the radiochromatogram (Fig. 2). In other words, quality control and purification of 99mTc-danofloxacin were performed by HPLC. It was able to separate the labeled compound from the unlabeled compound and the free pertechnetate. Factors affecting the labeling yield will be discussed in detail.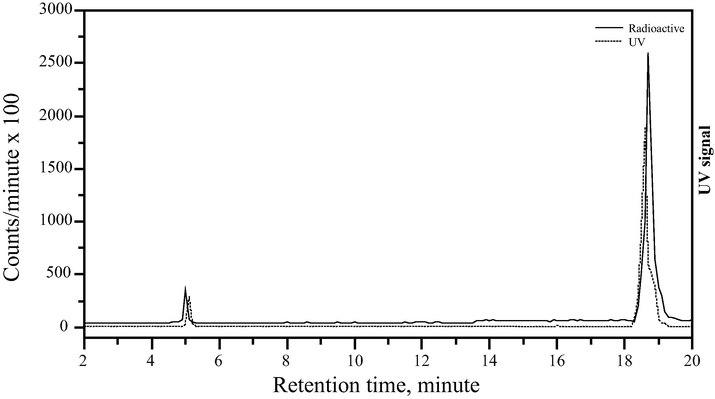
HPLC radiochromatogram of 99mTc danofloxacin.
3.1 Effect of danofloxacin amount
Danofloxacin was labeled with technetium-99m using the direct technique in which the reduced technetium-99m reacted with danofloxacin to form the labeled chelate. The influence of labeling yield on the amount of danofloxacin actually present in the reaction mixture is illustrated in Fig. 3. The reaction was performed at different danofloxacin amounts (0.5–2.5 mg). Exactly 1 mg was the optimum ligand amount required to obtain maximum radiochemical yield, 90 ± 2%. Below this value, the ligand amount was insufficient to complex all the reduced technetium-99m toward the formation of 99mTc-danofloxacin, as a result the reduced hydrolyzed technetium was 53% at 0.5 mg of danofloxacin. At ligand amount above 1 mg, the yield slightly decreased but did not change substantially and remained stable.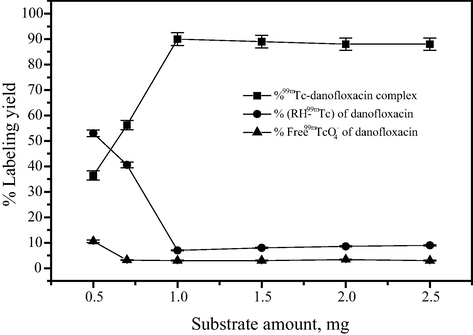
Effect of substrate content on the percent labeling yield of 99mTc-danofloxacin complex.
3.2 Effect of stannous chloride amount
Stannous chloride (SnCl2·2H2O) is the most commonly used reducing agent for the reduction of the pertechnetate ion (99mTcO−4), having the oxidation state (VII) to lower oxidation state, which favors its chelation by danofloxacin. The labeling process was affected by the amount of stannous chloride present in the labeling reaction as demonstrated in Fig. 4. The labeling yield of 99mTc-danofloxacin was 73% after adding 25 μg SnCl2·2H2O to the reaction mixture. This may be attributed to incomplete reduction of 99mTcO−4 and hence unreliable yield of the complex due to the presence of free 99mTcO−4 (13%). The labeling yield was maximized significantly (90%) by increasing the amount of SnCl2·2H2O from 25 to 50 μg. Increasing the amount of SnCl2·2H2O to 200 μg leads to a substantial decrease in the labeling yield (48%) due to the formation of tin colloids (52%). Increasing the amount of SnCl2·2H2O above the optimum value (50 μg) leads to competition of danofloxacin for the reduced 99mTc. The formation of the undesired tin colloid may be explained according to the fact that most of the ligand molecules were consumed in the formation of complex, hence the reduced pertechnetate may form 99mTc–Sn-colloid and other Sn-complexes in the absence of the ligand. On the other hand, the raise of stannous chloride amount leads to the formation of stannous hydroxide colloid Sn(OH)3− impurities in alkaline medium, which in turn compete with the chelation process of danofloxacin and thus reduces the yield of the 99mTc chelate (Jurisson and Lydon, 1999; Saha, 2004).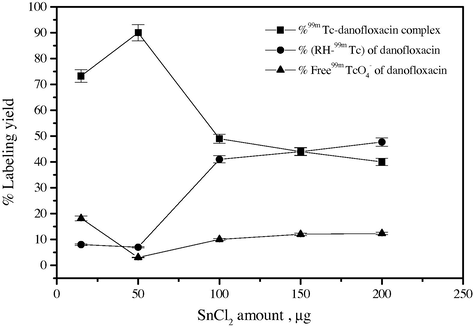
Effect of SnCl2.2H2O content on the percent labeling yield of 99mTc-danofloxacin complex.
3.3 Effect of pH
The labeling yield was affected by changes in pH of the reaction mixture as illustrated in Fig. 5. At pH 11 the labeling efficiency of 99mTc-danofloxacin was equal to 90 ± 2%. The maximum yield may be due to the deprotonation of the danofloxacin at high pH values, which increased the stability of 99mTcO(V)-danofloxacin complex. At alkaline pH values, the presence of high OH- concentration in the reaction mixture may lead to the partial hydrolysis of the formed complex and incomplete reduction of 99mTc to the desired oxidation state, and hence produce an unreliable yield of the 99mTc-danofloxacin besides the unreacted 99mTcO−4.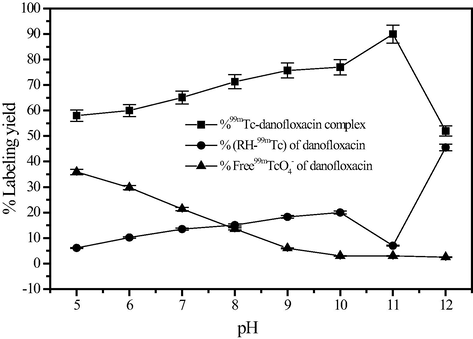
Effect of pH value of the reaction mixture on the percent labeling yield of 99mTc-danofloxacin complex.
3.4 Effect of in vitro stability of 99mTc-danofloxacin and reaction time
Fig. 6 indicates that the rate of formation of 99mTc-danofloxacin complex was strongly dependent on reaction time. The labeling yield increased from 85% to 90% when increasing the reaction time from 1 to 30 min. In vitro stability of 99mTc-danofloxacin was investigated over time. Fig. 7 indicates that the labeled fluoroquinolone derivative was stable for up to 120 min after labeling. The stability was found to be time-dependent with respect to some factors such as temperature, light and radiolysis, which may lead to the decomposition of the compound and limited its availability prior to injection.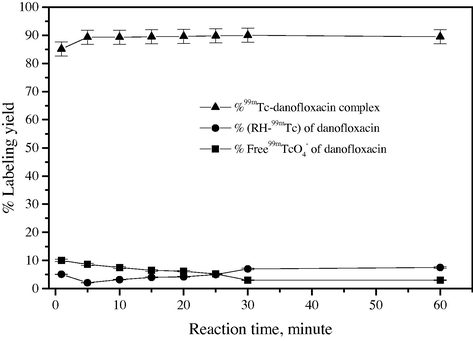
Effect of reaction time on 99mTc-danofloxacin complex.
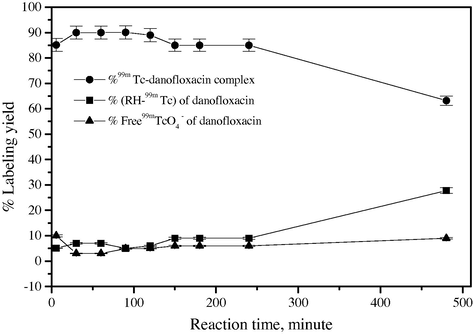
Stability test for 99mTc-danofloxacin.
3.5 Stability test
As shown in Fig. 8, incubation of 99mTc-danofloxacin in normal serum for 24 h at 37 °C resulted in a small release of radioactivity (13 ± 1%, n = 5 experiments) from the 99mTc-complex, as determined by HPLC and ITLC. Therefore, the stability of the labeled complex indicated its suitability for in vivo application over time.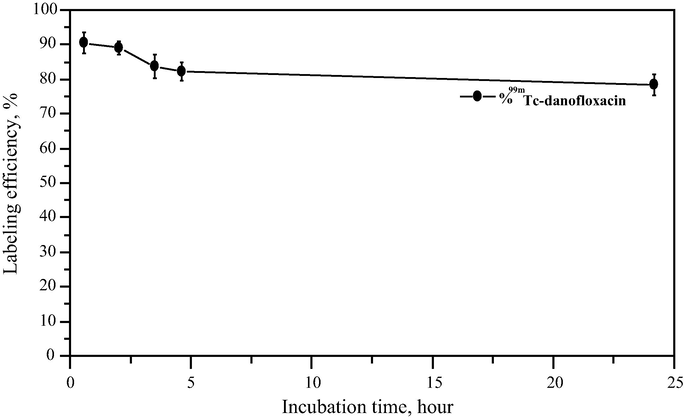
In vitro stability of 99mTc-danofloxacin in normal serum.
3.6 Biodistribution
Biodistribution data of the mice infected with living, heat killed S. aureus and turpentine oil are shown in Table 1. The uptake of 99mTc-danofloxacin was significantly low in heat killed S. aureus and turpentine oil infected animals (aseptic inflammation) as compared to infected animals with living bacteria (abscess). These data indicated high uptake in the inflamed areas within 2 h after intravenous administration of the labeled compound via the tail vein. A rapid distribution of radioactivity was observed throughout the body. The uptake of 99mTc-danofloxacin in different organs demonstrated that the injected tracer was cleared from the blood stream 24 h post injection. The major remaining activity of the tracer was found in the kidneys (6.6 ± 0.4% ID) and urine (30 ± 3% ID). In contrast, a significant level of radioactivity was retained in the liver (5.4 ± 0.3% ID). Elimination of 99mTc-danofloxacin appeared to be by both renal and hepatic routes.
Organs and body fluids
% Injected dose/organs at different time intervals (h)
S. aureus
Heat killed S. aureus
Turpentine oil
2
4
24
2
4
24
2
4
24
Inflamed muscle
1.8 ± 0.2
1.3 ± 0.1
0.8 ± 0.1
0.97 ± 0.1
0.75 ± 0.2
0.25 ± 0.1
1.1 ± 0.0
0.69 ± 0.2
0.21 ± 0.1
Control muscle
0.25 ± 0.1
0.21 ± 0.2
0.19 ± 0.0
0.29 ± 0.0
0.26 ± 0.1
0.15 ± 0.0
0.30 ± 0.0
0.25 ± 0.0
0.13 ± 0.0
Liver
17.1 ± 3.2
12.9 ± 2.0
5.4 ± 0.3
17.6 ± 2.4
12.2 ± 2.2
4.9 ± 0.5
17.5 ± 3.0
13.2 ± 2.2
6.1 ± 0.6
Urine
18.5 ± 3.5
24.9 ± 4.2
29.9 ± 3.1
17.1 ± 1.3
24.3 ± 1.1
29.4 ± 2.7
18.0 ± 0.9
25.4 ± 1.8
28.8 ± 2.4
Kidneys
11.2 ± 1.2
10.7 ± 1.3
6.6 ± 0.4
10.9 ± 1.3
10.2 ± 2.3
5.5 ± 0.7
11.3 ± 2.5
10.2 ± 1.2
6.9 ± 0.3
Blood
5.3 ± 0.4
3.1 ± 0.2
1.00 ± 0.0
6.10 ± 0.2
4.0 ± 0.2
1.0 ± 0.1
5.5 ± 0.2
4.3 ± 0.2
1.0 ± 0.0
Heart
0.3 ± 0.1
0.1 ± 0.0
0.09 ± 0.0
0.3 ± 0.09
0.1 ± 0.0
0.1 ± 0.0
0.4 ± 0.08
0.2 ± 0.0
0.1 ± 0.0
Lung
1.2 ± 0.2
0.2 ± 0.0
0.1 ± 0.0
1.1 ± 0.09
0.2 ± 0.0
0.1 ± 0.0
1.3 ± 0.09
0.4 ± 0.1
0.2 ± 0.0
Intestine and stomach
19.9 ± 2.5
3.90 ± 0.5
2.10 ± 0.3
19.1 ± 3.4
4.7 ± 0.4
2.2 ± 0.7
18.9 ± 1.9
4.7 ± 0.8
2.4 ± 0.4
Spleen
2.10 ± 0.1
1.00 ± 0.2
0.3 ± 0.1
2.1 ± 0.3
1.1 ± 0.0
0.5 ± 0.1
2.0 ± 0.1
1.3 ± 0.0
0.3 ± 0.0
Bone
0.90 ± 0.0
0.40 ± 0.1
0.10 ± 0.0
1.0 ± 0.0
0.49 ± 0.1
0.1 ± 0.0
1.1 ± 0.2
0.5 ± 0.1
0.1 ± 0.0
Mice with infectious lesions injected with 99mTc-fluoroquinolone revealed a mean abscess-to-muscle (target to non-target, T/NT) ratio equal to 7.2 ± 0.1 after 2 h post injection, which express higher uptake in infected tissue than the commercially available 99mTc-ciprofloxacin (T/NT = 3.8 ± 0.8) (Rien et al., 2004) as shown in Fig 9.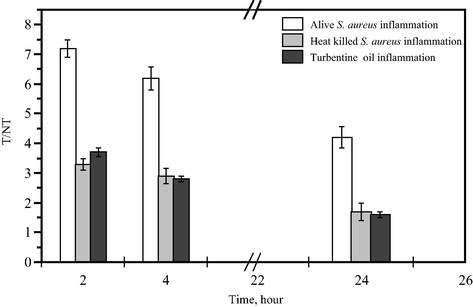
The ratio of target muscle (T) to non-target muscle (NT) of 99mTc-danofloxacin in different inflammation models at different post injection times.
4 Conclusions
This study characterized the in vitro and in vivo conditions necessary for designing 99mTc-danofloxacin complex as a potentially useful radiopharmaceutical for diagnosing bacterial infection. Danofloxacin was successfully labeled with technetium-99m by the direct labeling method at room temperature with a labeling yield of 90% using stannous chloride as a reducing agent. The excellent localization of the tracer in the induced foci of inflammation expressed the effectiveness of this complex for targeting infectious lesions, which was higher than the commercially available 99mTc-ciprofloxacin.
Acknowledgement
This project was supported by the Deanship of Scientific Research at Prince Sattam Bin Abdulaziz University under the research project #.2015/01/5126.
References
- Labeling and biodistribution of 99mTc-7-bromo-1,4-dihydro-4-oxo-quinolin-3-carboxylic acid complex. J. Radioanal. Nucl. Chem.. 2011;290:507-513.
- [Google Scholar]
- Biomarkers in pharmacology and drug discovery. Biochem. Pharmcol.. 2014;87:172-188.
- [Google Scholar]
- Detecting inflammation with 131I-labeled ornidazole. Appl. Radiat. Isot.. 2000;53:411-413.
- [Google Scholar]
- Clinical evaluation of technetium-99m infection for the localization of bacterial infection. Eur. J. Nucl. Med.. 1997;24:553-556.
- [Google Scholar]
- Goodman & Gilman’s Manual of Pharmacology and Therapeutics. New York: McGraw-Hill; 2008.
- Synthesis and evaluation of 99mTc-moxifloxacin, a potential infection specific imaging agent. Appl. Radiat. Isot.. 2010;68:314-316.
- [Google Scholar]
- Infection is not specific for bacterial osteo-articular infective pathology. Eur. J. Nucl. Med.. 2002;29:530-535.
- [Google Scholar]
- Italian guidelines for the diagnosis and infectious disease management of osteomyelitis and prosthetic joint infections in adults. Infection. 2009;37:478-496.
- [Google Scholar]
- 99mTc ciprofloxacin imaging for the diagnosis of infection in the postoperative spine. Nucl. Med. Commun.. 2004;25:277-283.
- [Google Scholar]
- Synthesis and biological evaluation of isonicotinic acid hydrazide conjugated with diethylenetriamine penta acetic acid for infection imaging. Open Nucl. Med. J.. 2009;1:33-42.
- [Google Scholar]
- Technical Reports Series No. 466. Technetium-99m Radiopharmaceuticals: Manufacture of Kits. Vienna: International Atomic Energy Agency; 2008.
- Potential technetium small molecule radiopharmaceuticals. Chem. Rev.. 1999;99:2205-2218.
- [Google Scholar]
- Preliminary evaluation of technetium-99m-labeled ceftriaxone: infection imaging agent for the clinical diagnosis of orthopedic infection. Int. J. Infect. Dis.. 2013;17:263-270.
- [Google Scholar]
- In vitro and in vivo evaluation of 18F-ciprofloxacin for the imaging of bacterial infections with PET. Eur. J. Nucl. Med. Mol. Imaging. 2005;32:143-150.
- [Google Scholar]
- 99mTc-ciprofloxacin (infection) imaging in the diagnosis of knee prosthesis infections. Nucl. Med. Commun.. 2002;23:167-170.
- [Google Scholar]
- Comparison of 99mTc ciprofloxacin, 99mTc white blood cell and three-phase bone imaging in the diagnosis of hip prosthesis infections: improved diagnostic accuracy with extended imaging time. Nucl. Med. Commun.. 2002;23:655-661.
- [Google Scholar]
- The many roads to infection imaging. Eur. J. Nucl. Med. Mol. Imaging. 2007;34:1873-1877.
- [Google Scholar]
- Radiolabeled antimicrobial peptides for infection detection. Lancet Infect. Dis.. 2007;3:223-239.
- [Google Scholar]
- Diagnosis of bone infection with 99mTc-ceftizoxime. Rev. Esp. Med. Nucl.. 2004;23:357.
- [Google Scholar]
- Labeling of ceftriaxone for infective inflammation imaging using 99mTc eluted from 99Mo/99mTc generator based on zirconium molybdate. Appl. Radiat. Isot.. 2010;68:1959-1963.
- [Google Scholar]
- Preparation and biodistribution of 99mTc-lomefloxacin and 99mTc-ofloxacin complexes. J. Radioanal. Nucl. Chem.. 2007;272:95-99.
- [Google Scholar]
- Synthesis of 99mTc-oxybutynin for M3-receptor-mediated imaging of urinary bladder. J. Radioanal. Nucl. Chem.. 2011;287:35-40.
- [Google Scholar]
- Oxidative radioiodination of aripiprazole by chloramine-T as a route to a potential brain imaging agent: a mechanistic approach. Radiochemistry. 2013;55:116-122.
- [Google Scholar]
- Benzenesulfonamide analogs of fluoroquinolones. Antibacterial activity and QSAR studies. Eur. J. Med. Chem.. 2005;40:361-369.
- [Google Scholar]
- Concerns about 99mTc labelled ciprofloxacin for infection detection—reply. Eur. J. Nucl. Med.. 2001;28:781.
- [Google Scholar]
- Synthesis, biodistribution and evaluation of 99mTc-sitafloxacin kit: a novel infection imaging agent. J. Radioanal. Nucl. Chem.. 2010;284:189-193.
- [Google Scholar]
- Synthesis and comparison, of 99mTc-enrofloxacin and 99mTc-ciprofloxacin. J. Nucl. Med.. 2004;45:2088-2094.
- [Google Scholar]
- The Fundamentals of Nuclear Pharmacy (fifth ed.). New York: Springer; 2004.
- 99mTc-nebivolol as a novel heart imaging radiopharmaceutical for myocardial infarction assessment. J. Radioanal. Nucl. Chem.. 2013;295:1511-1516.
- [Google Scholar]
- Evaluation of (99m)Tc-ciprofloxacin scintigraphy in a rabbit model of Staphylococcus aureus prosthetic joint infection. J. Nucl. Med.. 2002;43:239-245.
- [Google Scholar]
- Inability of 99mTc-ciprofloxacin scintigraphy to discriminate between septic and sterile osteoarticular diseases. J. Nucl. Med.. 2003;44:920-926.
- [Google Scholar]
- Synthesis and comparison of 99mTc-enrofloxacin and 99mTc-ciprofloxacin. J. Nucl. Med.. 2004;45:2088-2094.
- [Google Scholar]
- Can we produce an image of bacteria with radiopharmaceuticals? Eur. J. Nucl. Med. Mol. Imaging. 2008;35:1051-1055.
- [Google Scholar]
- Tc-99m-labelled sparfloxacin: a specific infection imaging agent. World J. Nucl. Med.. 2003;2:103-109.
- [Google Scholar]
- To evaluate the clinical efficacy of a single vial kit preparation of 99mTc-ceftriaxone (Scintibact) for the diagnosis of orthopedic infections—first results. J. Nucl. Med.. 2010;51(Suppl 2):373.
- [Google Scholar]
- Production of medical radioisotopes with linear accelerators. Appl. Radiat. Isot.. 2014;85:39-44.
- [Google Scholar]
- United States Pharmacopeia/National Formulary. USA: US Pharmacopeia; 2008.
- The efficacy of technetium-99m ciprofloxacin imaging in suspected orthopedic infection: a comparison with sequential bone/gallium imaging. Eur. J. Nucl. Med.. 2001;28:822-830.
- [Google Scholar]
- Nuclear Medicine (fourth ed.). USA: Elsevier; 2014. pp. 322–349
- Technetium-99m Radiopharmaceuticals: Preparation and Quality Control in Nuclear Medicine. Berlin: Springer; 2007.






