Translate this page into:
The application of magnetic nanoparticles based β-cyclodextrin as recoverable catalyst in various organic transformations: An overview
⁎Corresponding author. a.poursattar@urmia.ac.ir (Ahmad Poursattar Marjani)
-
Received: ,
Accepted: ,
This article was originally published by Elsevier and was migrated to Scientific Scholar after the change of Publisher.
Abstract
Abstract
Magnetite nanoparticles (MNPs), owing to their vast surface area, low toxicity, and biocompatibility, present a lot of chemical and biotechnological uses. Over the past decade, extensive attention has been expended on applying Fe3O4 nanoparticles as magnetically reusable solid support for various transition metals. The benefits of magnetic nanocatalytics are their simple separation by an external magnet, extreme reactivity, and a vast surface area of the catalysts. Also, the magnetic features of these catalysts induce their dispersion to be reversibly controlled via the magnetic field. Therefore, MNPs can be dispersed well in the reaction media. β-Cyclodextrin (β-CD), as a cyclic oligomer, has been extensively employed as an economical and eco-friendly alternative catalyst in diverse organic conversions that improved reaction efficiency. The modified β-CD with Fe3O4 led to mainly advantageous catalysts because of their helpful catalytic properties, eco-friendliness, easy separation, and ability to isolate from the reaction medium. In this report, our goal is to present an overview of magnetically reusable nanoparticles-based β-CD. This review’s major focus is the application of magnetic nanomaterials as catalysts in different organic transformations.
Keywords
β-Cyclodextrin (β-CD)
Supramolecular catalysis
Fe3O4 nanoparticles
Organic transformation
- CD
-
Cyclodextrin
- β-CD
-
β-Cyclodextrin
- NPs
-
Nanoparticles
- PhB(OH)2
-
Phenylboronic acid
- MGO
-
Magnetic graphene oxide
- C–C
-
Carbon-carbon
- AM
-
Acrylamide
- MBA
-
Methylenebisacrylamide
- NS
-
Nanosponge
- ArX
-
Aryl halides
- PU
-
Polyurethane
- HA
-
Hydroxyapatite
- HAp
-
Hydroxyapatite (Ca10(PO4)6(OH)2
- KF
-
Kabachnik–Fields
- MCRs
-
Multi-component reactions
- MNPs
-
Magnetite nanoparticles
- DOX
-
Doxorubicin
- MNP-AP
-
3-Aminopropyltriethoxysilane
- NaClO
-
Sodium hypochlorite
- H2O2
-
Hydrogen peroxide
- GPTS
-
3-Glycidyloxypropyltrimethoxysilane
- PGMA
-
Poly(glycidyl methacrylate)
- ATRP
-
Atom transfer radical polymerization
- GO
-
Graphene oxide
- β-CDPU
-
β-Cyclodextrin-polyurethane polymer
- DMF
-
N, N-dimethylformamide
- GA
-
Gluconic acid
- CIT
-
Citric acid
- p-NP
-
p-Nitrophenol
- SPIONs
-
Iron oxide nanoparticles
- ICP-AES
-
Inductively coupled plasma atomic emission spectroscopy
- VSM
-
Vibrating-sample magnetometer
- XRD
-
X-ray diffraction analysis
- FT-IR
-
Fourier Transform Infrared
- SEM
-
Scanning electron microscopy
- TGA
-
Thermogravimetric Analysis
Abbreviations
1 Introduction
Nanotechnology is one of the most crucial events of the revolution in the science and technology industry in the 21st century. Nano-sized compounds present numerous significant advantages due to their particular size and physical features, and they have turned out to be one of the most essential research studies in modern science (Tegart, 2004). Iron oxide NPs are hopeful for nanomaterials among magnetic materials because of their compatibility. Today, magnetite nanoparticles (Fe3O4) have been praised for their non-toxicity, superior superparamagnetic characteristics, easy preparation procedures, and favorable biocompatibility. Ferrite nanoparticles are used as supports for catalysts owing to their high surface area, facile separation, functionalization capability, and chemical stability. Their magnetic nature is a fantastic benefit owing to magnetic recoverability. Fe3O4 NP systems supply the connection between the catalyst and reactants, thus significantly increasing the activity of the catalysts. Day by day, using magnetically recycled nanomaterials to develop more efficient and eco-friendly chemical processes has increased (Nguyen et al., 2021).
They are employed for different applications like nanocarriers for drug delivery (Shabatina et al., 2020), magnetic labeling (Tamanaha et al., 2008), and catalysts for plenty of organic reactions (Govan and Gun’ko, 2014). Iron oxide NPs can recover because the magnetic separation of MNPs from the reaction media by an exterior magnet is easier and more effective than a traditional separation (by filtration and centrifugation). Iron oxide NPs are available from low-cost compounds and can be used as magnetically recoverable solid support for transition metals like palladium (Niu et al., 2013; Gholinejad et al., 2023; Xiao et al., 2022), nickel (Folsom et al., 2021; Moghaddam-Manesh et al., 2020; Prasad et al., 2017), copper (Mahdavi et al., 2016; Bahadorikhalili et al., 2018; Azizi et al., 2017), and gold (Hu et al., 2014; Wang et al., 2017) in organic conversion.
In organic chemistry, the most extensive families of organic compounds belong to the heterocyclic compounds. In our lives, heterocyclic compounds are crucial. It has a vast range of uses in medicinal chemistry and agrochemical products. Synthetic biologically active compounds include mostly five-membered nitrogen-comprising heterocyclic ring framework. The N-possess polycyclic heterostructures have been reported to be connected with a wide range of biological activity. In heterocyclic five-membered ring structures, the imidazole core exhibits various properties. The drugs that have imidazole rings have widened the scope of curing a variety of dispositions in clinical medicines. Recent developments of imidazole-based compounds in a broad spectrum of medicinal chemistry include antifungal, anticancer, antibacterial, antiviral, antiobesity, antiparasitic, and anti-inflammatory. Imidazole derivatives have been recognized as having a special place in the pharmaceutical area. The involvement of the imidazole platform is a key synthetic technique in the drug discovery system. The imidazole ring is a part of many vital naturally occurring products, comprising histidine, purine, histamine, and nucleic acid (Hossain and Nanda, 2018; Siwach and Verma, 2021). The strategy of synthesizing imidazoles is improved by utilizing nanocomposites as catalysts, increasing the reaction's efficacy and performance. Employing nanocomposites to synthesize imidazoles is a significant breakthrough in organic synthesis, furnishing more specific, effective, and eco-friendly procedures for developing these invaluable platforms (Sharma et al., 2024).
Cyclodextrins (CDs) are macrocycles made of glucose units and produced by the enzymatic degradation of starch (Szejtli, 1998). Based on the number of glucose groups, they are categorized as gamma-CD (8), beta-CD (7), and alpha-CD (6), respectively. Β-CDs are the most significant and hopeful hosts due to their low price, readily functionalized, non-toxic, water-soluble, and commercially available (Crini, 2014). They contain a hydrophobic hole, which attaches the substrate selectivity and catalyzes several organic conversions with excellent selectivity. They catalyze the reaction by non-covalent bonding with the reversible construction of host–guest complexes, as noticed in enzymes (Dalal et al., 2018). β-CD, owing to a hydrophilic exterior surface and a hydrophobic internal hollow, has demonstrated outstanding possibility as a green media for catalytic reactions. β-CDs have unique features that make them superb candidates for creating inclusion complexes with multiple organic compounds, leading to stable host–guest relationships. The unique configuration of CD allows them to capture numerous diverse small compounds and make inclusion complexes that are essential in the improvement and stability of the guest molecules. In the cavity of β-CDs, the guest materials can build an inclusion complex through non-covalent bond interaction. β-CDs can be modified with various transition metals to design and develop novel catalysts that catalyze various organic reactions (Komiyama, 2024). CDs have been employed in diverse applications like drug carriers in the pharmaceutical field (Perchyonok and Oberholzer, 2012), textile finishing (Abou-Okeil and El-Shafie, 2011), catalysts (Hapiot et al., 2014; Hapiot et al., 2017), removal of toxic substances from industrial effluent (Azimi et al., 2017), and design of biomimetic systems (Niess et al., 2014). Utilizing β-CD as a catalyst has rapidly received significant attention in organic synthesis. Lately, different kinds of β-CDs catalysts have been synthesized and used for diverse organic processes such as reactions the Suzuki reaction (Payamifar et al., 2024a; Payamifar et al., 2024c), oxidations (Shen and Ji, 2012), nitro reduction (Payamifar and Poursattar Marjani, 2023a), and Heck coupling (Dindulkar et al., 2016).
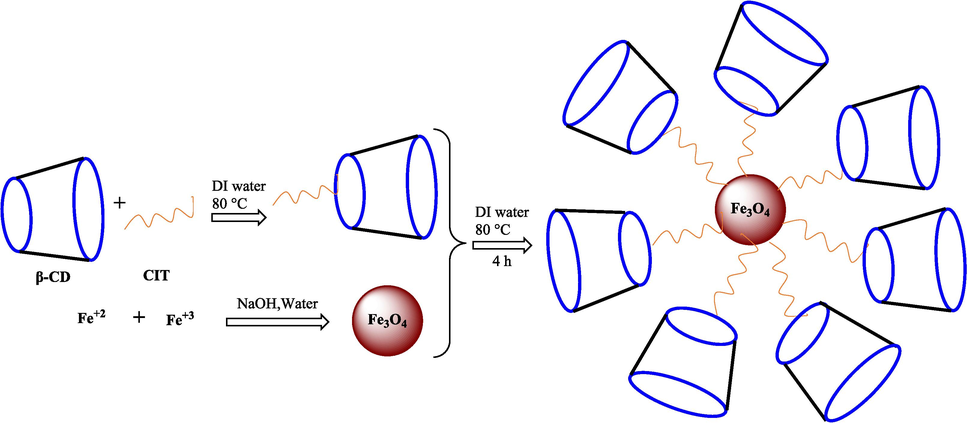
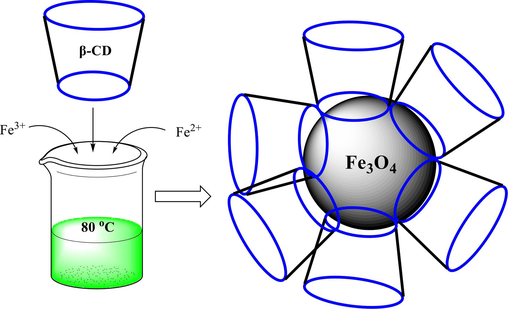
In previous years, researchers have indicated some successful construction procedures for CD-functionalized superparamagnetics (Cao et al., 2009; Banerjee and Chen, 2009; Xia et al., 2007). Magnetic CD nanocomposites (β-CD-Fe3O4) have been utilized in detecting and removing organic pollution to remove heavy metals from wastewater. Owing to the hydrophobic internal cavity, it can hold some organic pollution (Cova et al., 2018; Li et al., 2013). In 2016, Song et al. synthesized magnetic CD to eliminate β-naphthol (common organic pollution) from wastewater (Song et al., 2016). In 2023, Almutairi et al. described that β-CD/Fe3O4NPs could be utilized as a potential material for the degradation of methylene blue dye and also has the highest efficiency antioxidant and antibacterial properties (Almutairi et al., 2023). In 2024, Cai et al. designed and synthesized Fe3O4-GA@β-CD nano-microspheres to eliminate toxic p-nitrophenol compounds (Cai et al., 2024). Hosseinzadeh et al. prepared the amino-functionalized β-CD/Fe3O4@SiO2 in the same year. They used it as a nanocarrier for the controlled release of doxorubicin in drug-delivery systems for cancer therapy (Hosseinzadeh et al., 2024).
Modifying β-CD with MNP can be several applications in various areas such as medical (Sharaf et al., 2024), adsorbents in separation systems (Kakhki, 2015), treatment of dyeing wastewater (Cai et al., 2017), and drug delivery (Sina et al., 2023).
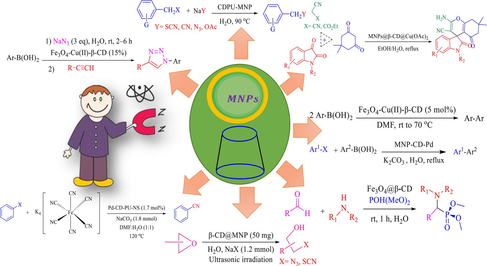
It has been revealed that the immobilization of β-CD on NPs can be utilized as a heterogeneous nanocatalyst in organic reactions. In previous years, many research groups have explored Fe3O4-based β-CD catalysts for vital organic conversions, including carbon–carbon cross-coupling, click reaction, reduction of nitroarenes, oxidation of alcohols, cyanation of aryl halides, ring-opening of epoxides and synthesis heterocyclic compound. The use of the magnetic nanoparticles-based β-CD system as a recoverable catalyst for diverse organic transformation is debated.
2 The use of magnetic nanoparticles based β-CD catalyst in various organic reaction
Over the past decade, magnetic nanocatalysts, as a fantastic alternative to conventional catalysts, have been increasingly developed. Nanocatalysis can design and develop catalysts with superior activity, high stability, and higher selectivity. One of the most valuable properties of magnetic nanocatalysts is their isolation features. These nanocatalysts can be recycled through an exterior magnet from reaction media owing to the paramagnetic characteristic of the support, with no requirement for a filtration stage (Polshettiwar et al., 2011; Hajareh Haghighi et al., 2024; Gebre, 2023; Veisi et al., 2023; Kalantari et al., 2023; Lambat et al., 2023; Kumar et al., 2022). Combining magnetic nanoparticles and β-CD led to an effective and recoverable nanocatalyst that could be employed in different organic reactions. We study the application of these magnetic nanocatalysts β-CD for various organic conversions, like reduction of nitroarenes, click reaction, carbon–carbon coupling, Kabachnik-fields reaction, oxidation of alcohols, ring-opening of epoxides, cyanation of aryl halides, nucleophilic substitution reactions of benzyl halides.
2.1 Click reaction
Click reaction is well-known as one of the most important reactions in organic chemistry, and it is mainly used to fabricate 1,2,3-triazoles (Binder and Kluger, 2006). These invaluable compounds have broad applications in the pharmaceutical and material science fields (Matin et al., 2022). They display various biological traits like antiviral activity (Andreeva et al., 2021), Gram-positive bacteria (Maji and Haldar, 2017), and antitumor (Yamada et al., 2018). Many reports have been published on using magnetic nanocatalysts in Click reactions (Sarreshtehdar Aslaheh et al., 2024; Saadoon et al., 2024; Khaleghi et al., 2024). In previous years, the utilization of magnetic nanoparticle-based β-CD systems as recoverable green nanocatalysts in click reactions has been notable.
A practical, readily recyclable, and highly efficient nanocatalyst Fe3O4-β-CD-Cu2 was prepared and used in the creation of 1,2,3- triazoles reported by Kaboudin et al. (Kaboudin et al., 2013). The preparation of magnetic nanocatalyst is shown in Scheme 1. The 1,2,3-triazole derivatives 3 were obtained from ArB(OH)2 1 and acetylene 2 through a one-pot reaction at ambient temperature in the air with no additives (Scheme 2). The TGA curve of the Fe3O4-β-CD-Cu2 indicates a weight loss of 3.69 % below 160 °C due to the loss of the adsorbed water in the sample. The mass loss of approximately 8.33 % by weight in the 160–340 °C range is attributed to the thermal decomposition of Cu(II)-β-CD. This result showed the nanocatalysts are stable thermally. The average diameter of Fe3O4 is almost 10–20 nm, with a mainly spherical figure, as demonstrated by using TEM images. The frontier between the particles was not detectable in the TEM of the Fe3O4-β-CD-Cu2, which might be owing to interactions between the adjacent particles. No noteworthy Cu(II)-β-CD layer could be noticed on these NPs. The recyclability of this magnetic catalyst was checked for four cycles without a colossal decline in catalytic efficiency. The recovery percentage for this magnetic nanocatalyst at the four runs involving 95 (1st run), 92 (2nd run), 90 (3rd run), and 87 (4th run). The average yield was 91 %. The method's main benefit was utilizing a recoverable catalyst, which is eco-friendly, low-price, and has high catalytic performance.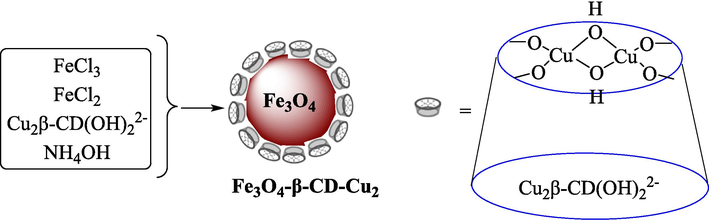
The construction of Fe3O4-β-CD-Cu2.
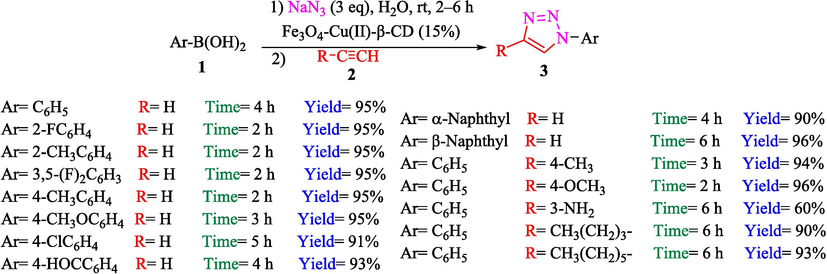
Preparation of 1,2,3-triazoles 3 by Fe3O4-β-CD-Cu2.
In 2016, the Cu@β-CD@SPIONs as a novel reusable magnetic catalyst was synthesized and employed for the assembly of diverse 1-benzyl-1H-1,2,3-triazol dibenzodiazepinones 8 through click cycloaddition (Scheme 3) by Mahdavi et al. (Mahdavi et al., 2016). The construction stages of this catalyst are displayed in Scheme 4. FT-IR, VSM, TGA, and TEM methods characterized the recoverable catalyst. TGA diagrams of nanocatalysts demonstrate that the quantity of loaded β-CD moiety is 43 % w/w. The TEM photos of Fe3O4 NPs appeared as dark spots within bright regions that the size of the nanocatalyst is about 100 nm. This catalyst was separated by utilizing a magnet from the reaction media and reused five times with no considerable degradation in its performance. The amount of leached Cu determined by ICP-AES was less than 1 ppm.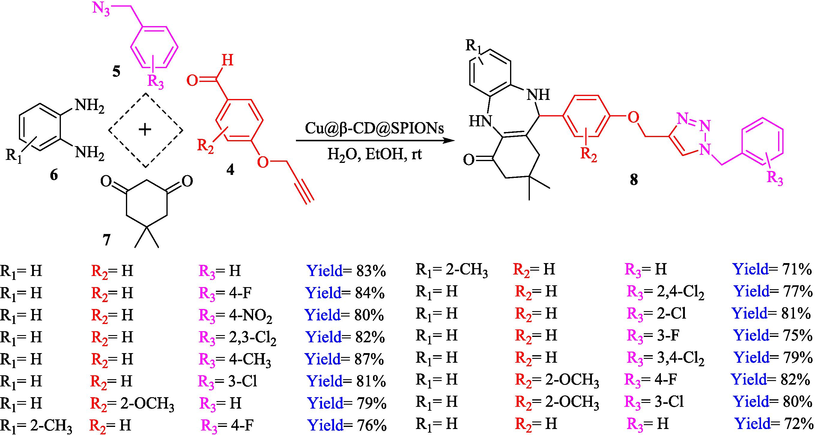
Click reaction catalyzed by Cu@β-CD@SPIONs.
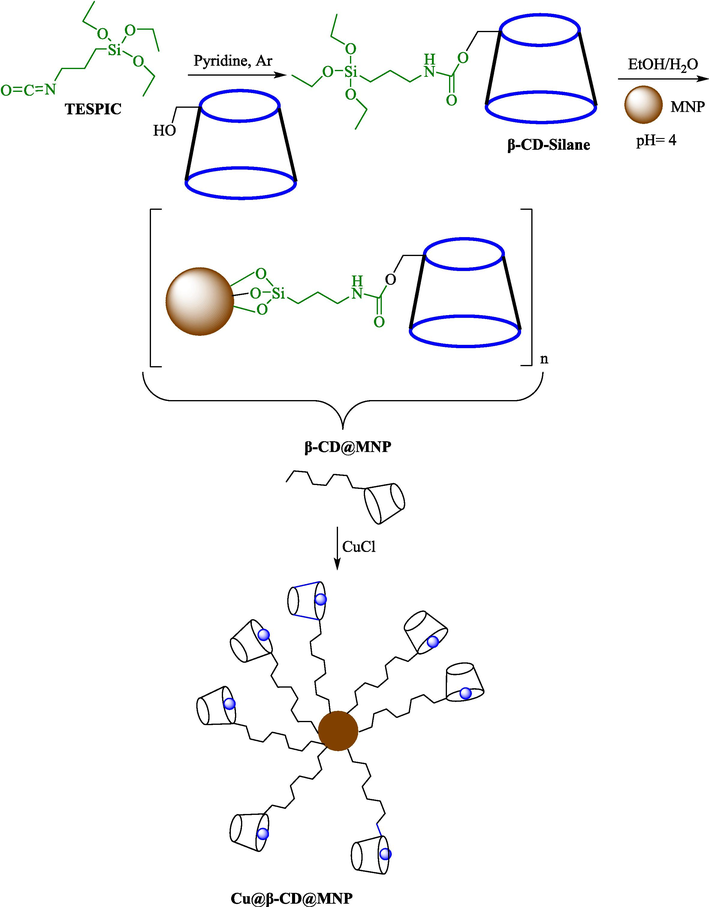
The creation pathway of this nanocatalyst.
Cu@β-CD@SiO2@SPION, as a recycle nanocatalyst, was prepared and used in the click reactions by Bahadorikhalili et al. (Bahadorikhalili et al., 2018). The stepwise preparation pathway of Cu@β-CD@SiO2@SPION is shown in Scheme 5. Diverse analytical methods like VSM, TEM, XRD, SEM, and FT-IR were utilized to analyze nanocatalysts. SEM and TEM images demonstrated that the nanosphere's diameter was approximately 15–20 nm. A weight loss could be noticed in TGA curves below 100 °C, which could be associated with water evaporation. TGA indicates the amount of loaded β-CD moiety to be 39 wt %. The formation of a various scope of 1,2,3‐triazolylquinazolinones 10 from benzyl azide 5, 2‐aminobenzamide (9), and 4‐(prop‐2‐yn‐1‐yloxy)benzaldehydes 4 via click reaction using 1.5 mol % of nanocatalyst in water at 25 °C within 24 h (Scheme 6). Cu@β-CD@SiO2@SPION could be recoverable by magnetic separation for ten successive cycles without any minute reduction in performance. This could be owing to the high stability of the nanocatalyst and the low leaching of copper, which makes the structure of the magnetic nanocatalyst stable during the reaction. The amount of leached Cu is less than 1 ppm.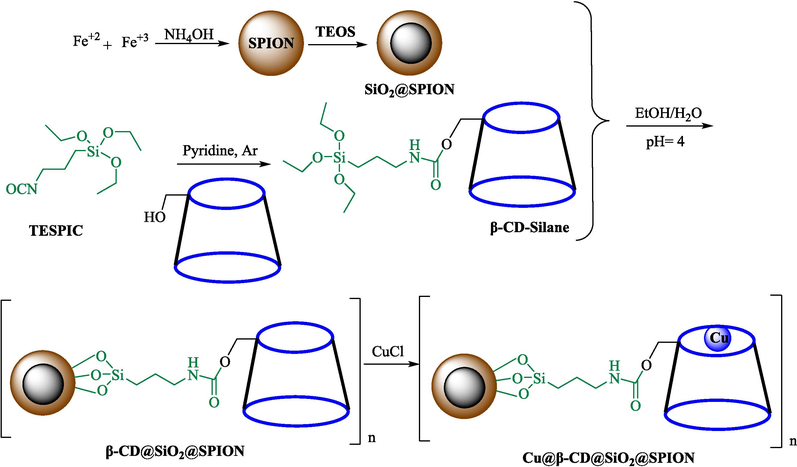
The synthesis pathway of Cu@β-CD@SiO2@SPION.
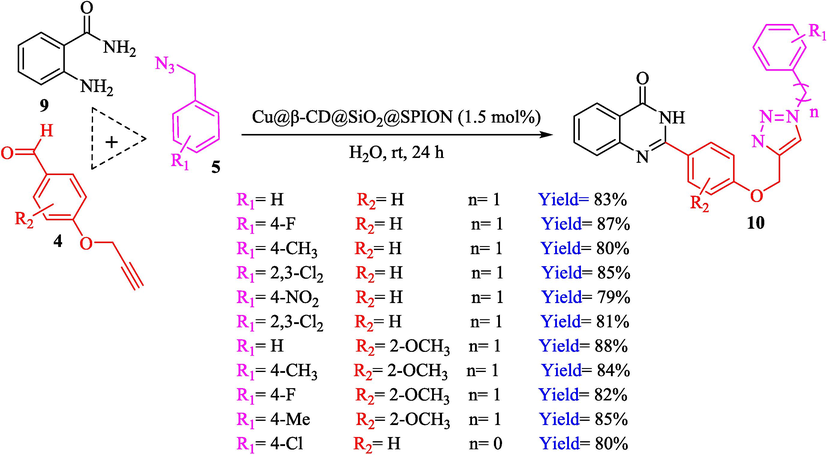
Synthesizing of compounds 10 via click reaction using Cu@β-CD@SiO2@SPION.
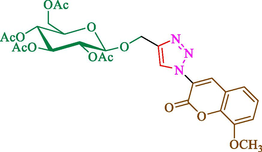
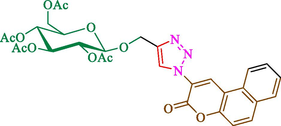
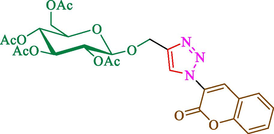
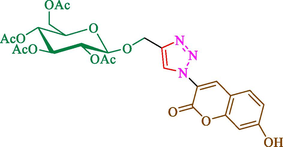
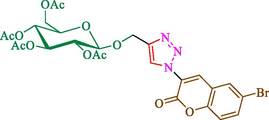
Jain et al. (Jain et al., 2019) explained a green way to the construction of 1,4-disubstituted 1,2,3-triazoles 13 from coumarin azides 12, and terminal alkyl 11 through click cyclization in the existence of 2.5 mol % of Fe3O4@CD-CIT as a nanocatalyst in water at 40 °C under ultrasonic irradiation conditions (Table 1). The pathway of nanocatalyst preparation is indicated in Scheme 7. By applying different techniques, like TEM, XRD, FTIR, 1H NMR, and VSM, Fe3O4@CD-CIT characterized. The TGA thermogram shows a weight loss in two stages: firstly, 2 % weight loss was noticed below 110 °C, which may be owing to the loss of residual water attaching to the sample surface. The second weight loss of 12 % between 165 and 390 °C is due to the thermal decomposition of the CD@CIT moieties. This result exhibits the nanocatalyst is stable thermally. TEM images revealed that nanoparticles are almost spherical with a 5–10 nm range. The 1,4-disubstituted 1,2,3-triazoles were obtained superior outcomes (91–97 %). The catalyst was magnetically recovered 7 times with a slight decrease in activity. TEM photos of this nanocatalyst after the 7th run indicated the preservation of the nanocatalyst structure with a bit of aggregation of nanoparticles. The decline in yield might be due to the separation of the CD-CIT complex from the surface of the Fe3O4.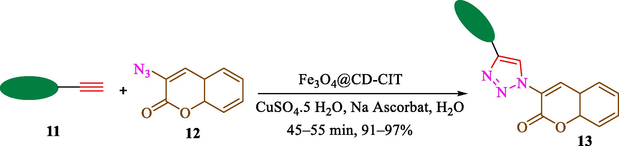
Entry
Product
Time (min)
Yield ( %)
1
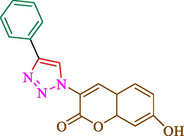
45
95
2
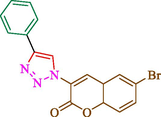
50
94
3
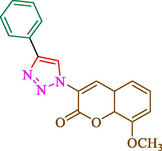
53
96
4
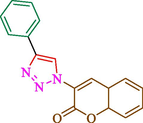
40
97
5
50
97
6
55
91
7
45
97
8
50
92
9
55
94
The preparation of Fe3O4@CD-CIT.
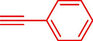
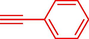
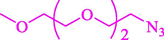
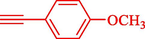
Liu et al. (Liu et al., 2023) prepared nanocomposite hydrogels based on β-CD (CE-β‐CD-Cu) and employed it as a reusable catalyst for click reactions. Click reaction of different azides 14 and alkynes 2 was conducted in the presence of a catalyst (0.12 mol % copper) in H2O at 40 °C within 24h under nitrogen (N2) (Table 2). By using various techniques, such as TEM, FTIR, and UV–vis spectroscopy characterized. In the TEM images, Cu nanoparticles' mean particle sizes were 4.0 nm. The 1,2,3-triazolyl products 15 were obtained with high yields (83–94 %). The recycling of the CE-β‐CD-Cu was tested, and the results displayed that catalysts were recycled well in seven runs with a slight reduction. It was observed that the yield of this reaction still reached up to 92 % after 4 cycles. Even after 6 runs, a satisfied yield of 83 % was also acquired.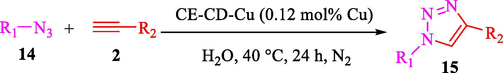
Entry
Azide
Alkyne
Yield ( %)
1
96
2
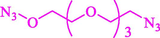
91
3
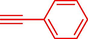
93
4
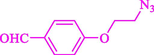
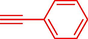
86
5
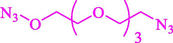

89
6
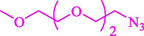
92
7
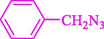

93
8
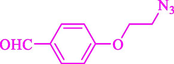

94
9
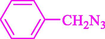
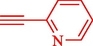
85
10
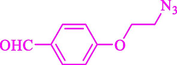
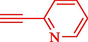
83
2.2 Cyanation of aryl halides
Aryl nitriles (ArCN) are well-known as essential components of many biological, pharmaceuticals, natural products, and agrochemicals (Larock, 1989; Kleemann and Engel, 1999). ArCN are commonly made through the Rosenmund-von Braun procedure. This approach produced metal wastes due to the utility of stoichiometric quantities of copper cyanide (Yan et al., 2017). The cyanation of ArX in the existence of cyanide reagents and metal catalysts is well-known as the most straightforward and robust synthesis process in the generation of ArCN (Yan et al., 2017; Shee et al., 2020; Evano et al., 2018). Various cyanide sources, like Zn(CN)2, KCN, and NaCN, have been utilized to form ArCN employing different metal catalysts. They have several disadvantages, including high prices, long-time reactions, low yields, and harsh conditions. K4Fe(CN)6 as a cyanide source is the most vastly utilized owing to its non-toxicity, excellent efficiency, environmental friendliness, and low price (Yan et al., 2017). Diverse metal catalysts have lately been developed for constructing ArX in the existence of K4Fe(CN)6 (Nikitin et al., 2016; Baran, 2020; Veisi, 2019). Among them, palladium NPs perform a prominent role due to their excellent catalytic efficiency and broad functional group toleration in catalytic conversion (Baran and Sargin, 2020; Çalışkan and Baran, 2022; Turunç et al., 2021; Çalışkan et al., 2021).
In 2020, Baran et al. (Baran and Nasrollahzadeh, 2020) reported the formation of palladium nanoparticles on Schiff base modified β-CD-Sch under the named Pd NPs@β-CD-Sch. They operated it as a heterogeneous nanocatalyst in the cyanation reaction to synthesize benzonitriles. The synthesis steps of Pd NPs@β-CD-Sch nanocatalyst are indicated in Scheme 7. Various methods like TEM, TGA, FE-SEM, FT-IR, XRD, and EDS characterized this heterogeneous nanocatalyst. The result of the TGA analysis illustrated that the thermal durability of the catalyst is considerably high and appropriate for diverse high-temperature catalytic reactions. The most significant degradation recorded was 318 °C for β-CD. The size of palladium NPs was obtained from TEM analysis at almost 20 nm. This catalyst synthesized various benzonitriles with various substrates with high yields (77–98 %) (Table 3). This catalyst recovered eight times. A leaching test was performed after the eighth recycling test, and deficient levels of Pd leaching (0.2 %) were observed. This test demonstrated that Pd leaching from β-CD-Sch is minor during the cyanation reactions. A probable pathway in the cyanation of ArX is shown in Scheme 8.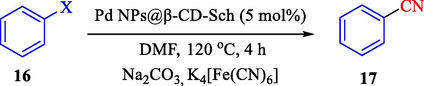
Entry
ArX
Product
Yield ( %)
1
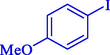
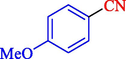
95
2
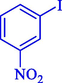
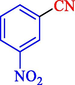
97
3
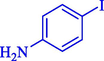
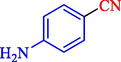
89
4
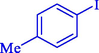
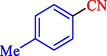
94
5
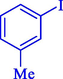
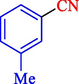
92
6
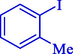
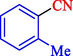
88
7
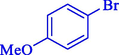
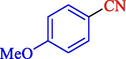
92
8
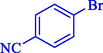
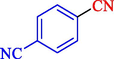
98
9
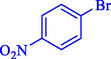
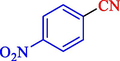
96
10
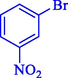
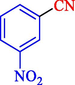
93
11
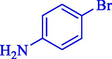
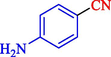
85
12
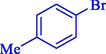
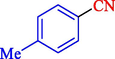
90
13
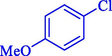
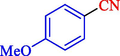
77
14
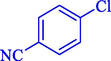
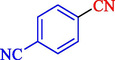
81
15
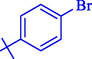
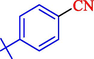
83
16
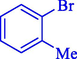
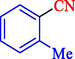
86
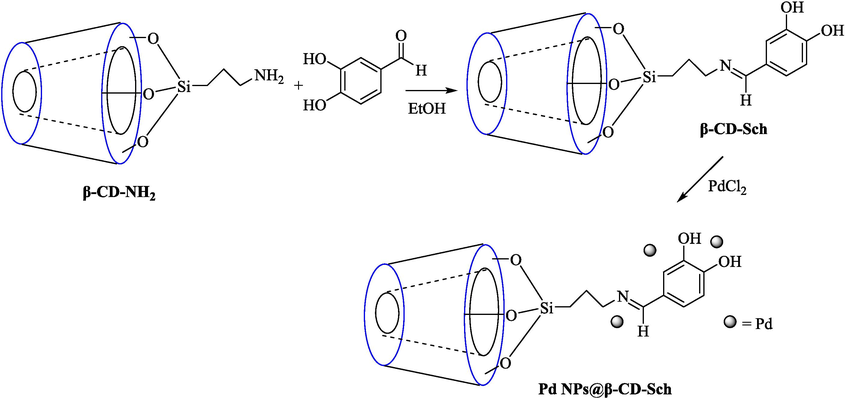
The step synthesized of Pd NPs@ β-CD-Sch.
2.3 Ring-opening of epoxides
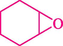
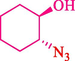
Epoxides are well-known as the most valuable versatile building blocks in the organic synthesis field, and their chemistry is exceptionally varied (Mamedova et al., 2022) Several biologically important molecules include these three-membered rings within their constructions (Yudin, 2006) The epoxide ring-opening reactions by diverse nucleophilic addition have been vastly investigated and draw considerable interest for the synthesis of 1,2-disubstituted products (Lidskog et al., 2020).
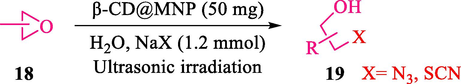
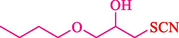
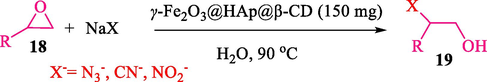
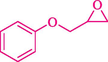
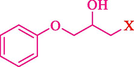
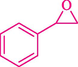
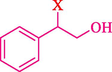
In 2016, the reaction of ring opening of the epoxide 18 was investigated by Sayyahi et al. (Sayyahi et al., 2016) employing β-CD@MNP nanocatalyst. The nanocatalyst was synthesized based on the processes shown in Scheme 9. This nanocatalyst has been examined using SEM, TGA, and VSM. The size of a magnetic nanoparticle is about 67 × 86 nm, which is evaluated from SEM data. According to the TGA analyst, there are two weight loss steps in the region from room temperature to 600 °C. The slight mass loss (∼5 % w/w) below 150 °C is related to the removal of the water; the main mass loss (∼30 % w/w) in the range of 250–325 and 325–450 °C might be associated with the thermal decomposition of β-CD and linkers, respectively. This new magnetic nanocatalyst could be applied well in the ring-opening of epoxides (with azide and thiocyanate as nucleophiles), and the expected compounds 19 were acquired in 84–96 % yields (Table 4). The catalyst recycling was performed for four consecutive cycles with no massive reduction in catalytic performance. The recovery percentage for this magnetic nanocatalyst at the four runs involved 86 (1st run), 84 (2nd run), 80 (3rd run), and 80 (4th run). The average yield was 82.5 %. The benefits of β-CD@MNP are an eco-friendly approach, using green solvents, facile work-up, excellent yield, and short reaction times.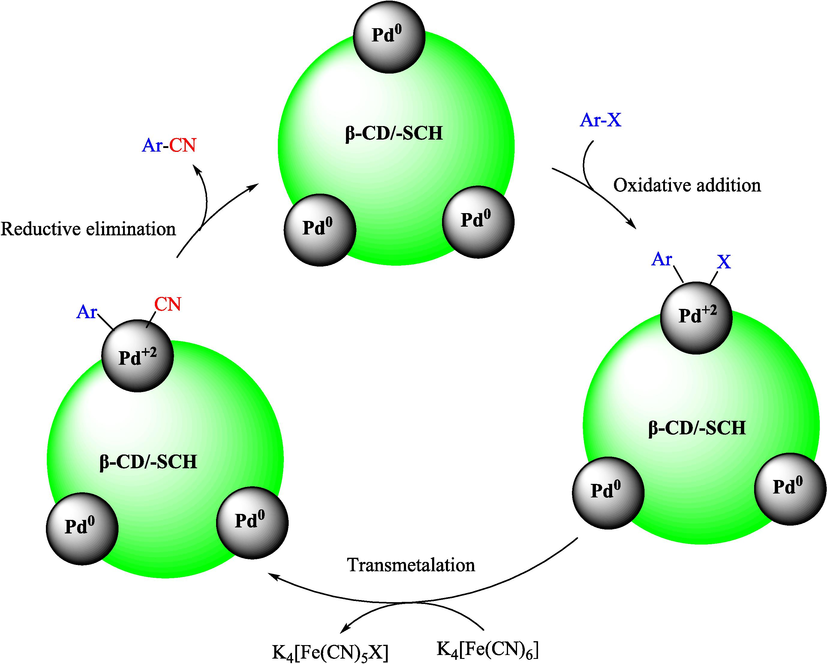
The probable mechanism for the cyanation of ArX.
Entry
Epoxide
Product
Time (min)
Yield ( %)
1
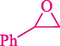
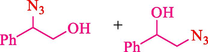
5
84
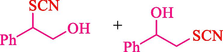
15
85
2
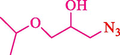
5
92
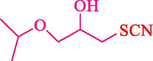
10
91
3
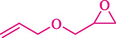
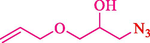
25
96
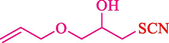
10
87
4

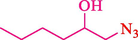
10
91
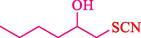
20
88
5
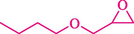
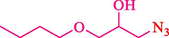
5
92
10
91
6
10
85
20
86
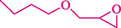
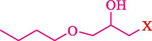
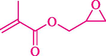
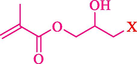
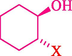
In 2019, Khosravinia et al. (Khosravinia et al., 2019) designed and prepared the γ-Fe2O3@HAp@β-CD catalyst from β-CD supported, hydroxyapatite encapsulated γ-Fe2O3 (Scheme 10). It is used as a phase transfer catalyst in the nucleophilic ring opening of epoxides to synthesize β-CDs, β-nitro alcohols, and β-azido alcohols in water media. The catalytic feature is owing to the inclusion of the complex creation of epoxides through the hydrogen bonding of the epoxide oxygen to the external OH groups of the β-CD (Scheme 11). This nanocatalyst was analyzed using different methods such as TGA, SEM, FTIR, and XRD. TGA curve indicated the first weight loss of 5 % below 120 °C, possibly due to the loss of absorbed water sticking to the sample surface and adsorbing in the β-CD cavities. The second weight loss step of almost 35 % in the 190–380 °C region was due to the decomposition of β-CD moieties. Therefore, this nanocatalyst is stable thermally. The SEM images of the nanoparticles in all the samples had spherical shapes, displaying that γ-Fe2O3@HAp@β-CD had a large surface area. This reaction was conducted at 90 °C in H2O as a solvent, supplying products with good to high yields (66–94 %) (Table 5). This nanocatalyst is recovered in four cycles for the ring-opening reactions. The results showed that the nanocatalyst did not drop-in activity even after four runs. This approach presents some advantages comprising easy work-up, recoverable catalyst, excellent yields, short-time reaction, and superb regioselectivity.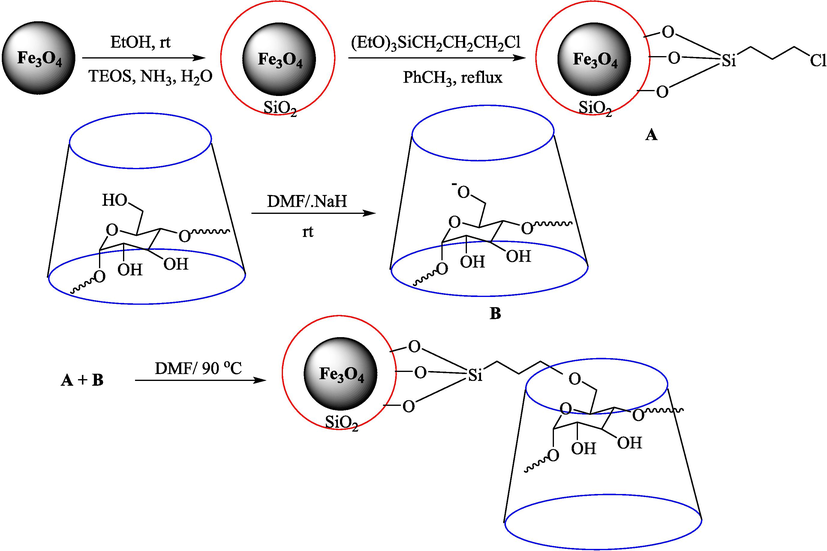
The preparation of β-CD@MNP.
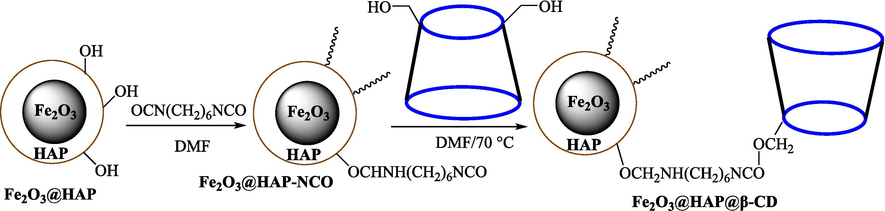
Synthesis of γ-Fe2O3@HAp@β-CD.
Entry
Reactant
Product
X
Time (min)
Yield ( %)
1
CN
N3
NO2
75
55
8066
89
66
2
CN
N3
NO2
65
35
585
89
83
3
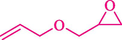
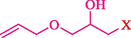
CN
N3
NO2
25
60
577
88
66
4
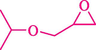
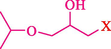
CN
N3
NO2
18
20
991
88
93
5
CN
N3
NO2
22
30
875
90
71
6
CN
N3
NO2
5
20
1871
91
84
7

CN
N3
NO2
21
25
1094
87
75
2.4 Kabachnik–Fields reaction
The Kabachnik–Fields (KF) reaction is well known as a three-component reaction including a hydrophosphoryl, an amine, and a carbonyl compound (aldehyde or ketone) that leads to the synthesis of α-aminophosphonates. The KF reaction has been verified to have a vast worth and range in organic and pharmaceutical chemistry. α-Aminophosphonates have drawn significant attention due to their biological activities (Zefirov and Matveeva, 2008).
Rostamnia and Doustkhah (Rostamnia and Doustkhah, 2015) reported the preparation of α-amino phosphonates from aldehyde 20, amine 21, and dimethyl phosphonate 22 using Fe3O4@β-CD as a highly water-dispersed nanocatalyst via the Kabachnik–Fields reaction. The pathway for the preparation of Fe3O4@CD is displayed in Scheme 12. Various techniques identified this new magnetic catalyst, containing FT-IR, SEM, XRD, and VSM. SEM data indicated the average particle of magnetic nanoparticles was 23 nm. The products were acquired with excellent yields (85–96 %) (Scheme 13). This catalyst was recycled for ten cycles with no remarkable decline in catalytic efficiency. After the ten runs, a considerable reduction in catalyst performance was observed.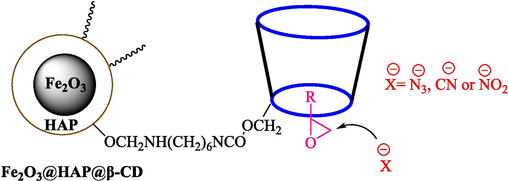
Proposed inclusion complex construction of the nanocatalyst with epoxides in the nucleophilic ring opening.
The path for the synthesis of Fe3O4@CD.
2.5 Multi-component reactions (MCRs)
Multi-component reactions (MCRs) are the most valuable means in modern organic chemistry to synthesize functionalized organic material. Also, they have been utilized to prepare a vast spectrum of diverse categories of heterocyclic material showing varied biological and pharmaceutical uses. This procedure has benefits like eco-friendliness, efficiency, simplicity, and economics compared to traditional chemical reactions (Cioc et al., 2014; Ugi, 2001; Younus et al., 2021; Payamifar et al., 2024b). Using the nanocatalyst in the synthesis of heterocycle compounds attracted massive attention from various research groups (Arabmarkadeh et al., 2021; Fajer et al., 2023a; Fajer et al., 2023b; Hou and Kazemi, 2024a; Hou and Kazemi, 2024b; Kazemi and Mohammadi, 2020; Ghobadi, 2022). β-CDs and their derivatives as catalysts compared to the previously published works (using nanocatalysts) for the synthesis of heterocycles have some advantages such as availability, non-toxicity, low cost, renewability, eco-friendly and high activity in organic transformations, which makes this type of catalysts more proper and valuable. Lately, β-CDs and their derivatives as helpful catalysts have attracted notable for synthesizing heterocyclic compounds due to making this reaction green and environmentally friendly (Abbasi, 2017; Mishra, et al., 2018).
A green approach for the formation of imidazo[2,1-b][1,3,4]thiadiazol-5-amines 26 and imidazo[1,2-a]pyridines 27 from the aldehyde 20, semicarbazide 23, and isocyanides 24 applying βCD-IL@MStarch in ethanol as a solvent at 50 °C were explained by Bahadorikhalili et al. (Bahadorikhalili et al., 2019). The construction of β-CD-IL@M starch is exhibited in Scheme 14. This new nanocatalyst was analyzed using several methods, including TGA, VSM, TEM, and FTIR. According to the TGA curve, this nanocatalyst is stable below 450 °C. A weight loss of approximately 100 °C, which could be observed in the TGA curve, could be related to the evaporation of water from the nanocatalyst. Significant weight loss could be observed in temperatures above 450 °C, in which starch and other organic linkers start decomposition. In the TEM image of the nanocatalyst, the dark point represents the existence of Fe3O4 NPs. The brighter parts were associated with the organic groups, including starch, ionic liquid β-CD, and the polymer linker. Starch and organic groups are observed in the TEM photo as brighter sites, while the Fe3O4 NPs are noticed as dark spots. This catalyst was recycled ten times. The results of the recovery tests display that the catalyst has been active during the 10 sequential reactions. No substantial decrease was noticed in the activity of the βCD-IL@M−Starch catalyst after 10 cycles. The imidazo-thiadiazolamine derivatives were achieved in high isolated yields (80–91 %) (Scheme 15).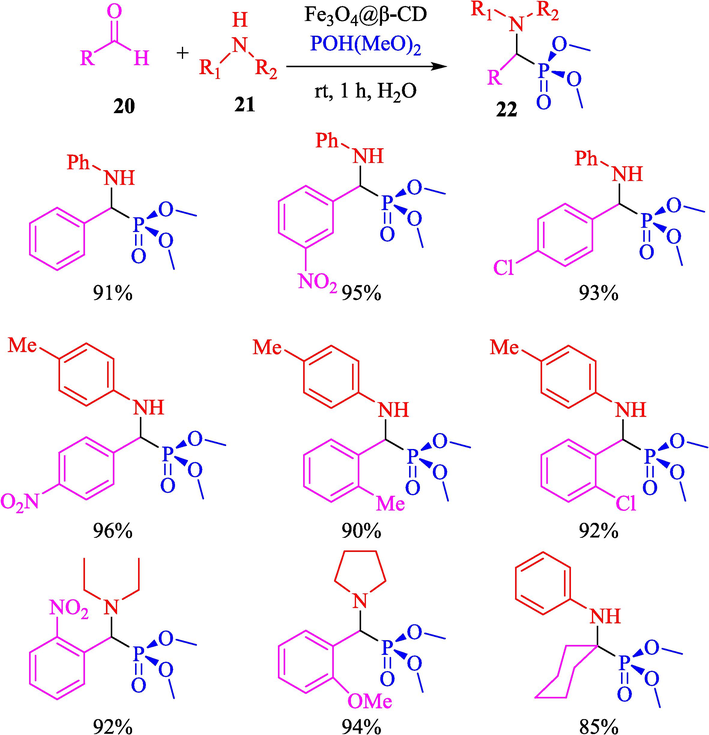
Preparation of α-minophosphonate products.
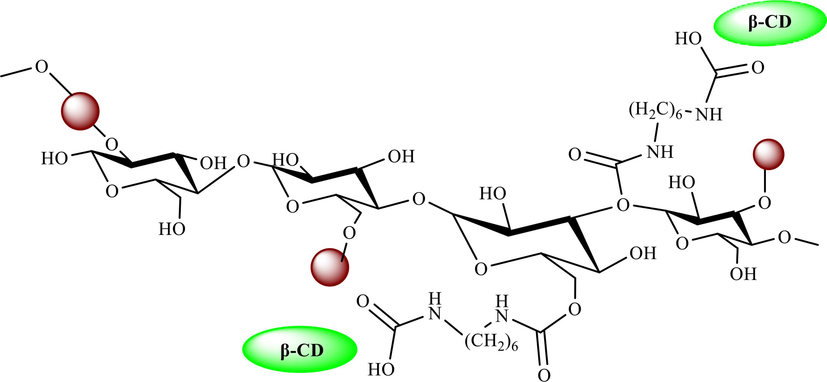
The construction of β-CD-IL@M starch.
Ahadi et al. (Ahadi et al., 2019) designed and prepared MNPs@β‐CD@Cu(OAc)2 as a valuable nanocatalyst for the synthesized of spiropyrans 31 from isatins 28, dimedone (29), and malononitrile (30) (Scheme 16). The construction stage of MNPs@β‐CD@Cu(OAc)2 is demonstrated in Scheme 17. XRD, FT-IR, VSM, and SEM analyses identified the nanocatalyst. The result from the SEM data exhibited the average diameter of the nanocatalyst was almost 33 nm. The recovery of the nanocatalyst was studied, and it was revealed that the catalyst recovered six times with no notable loss in activity and a slight decline in product yield.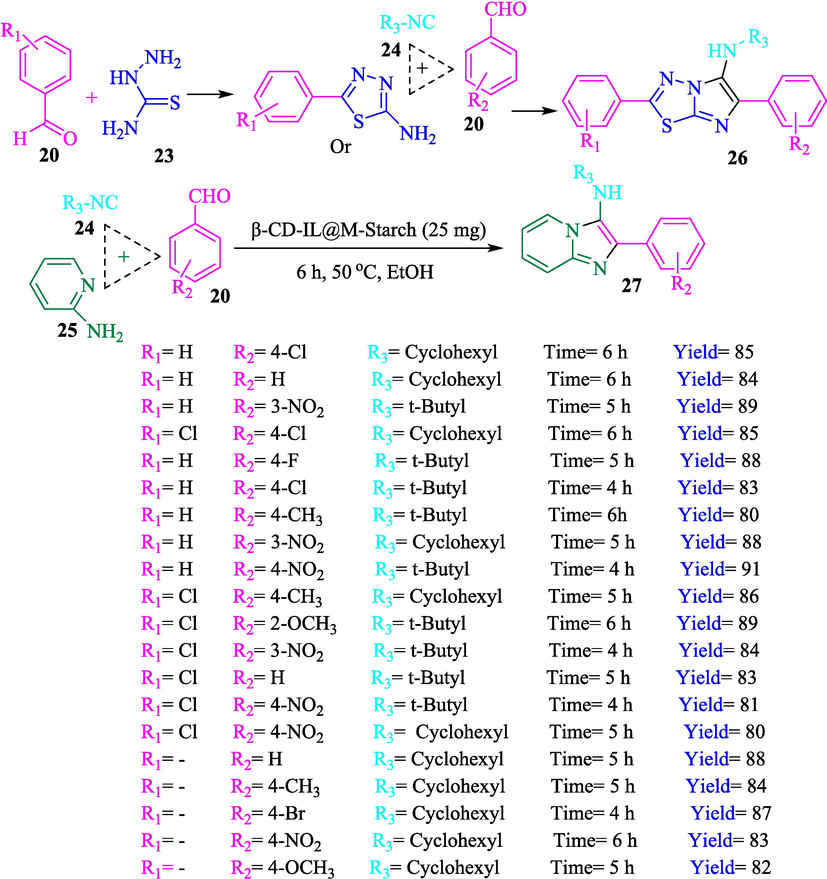
The preparation of derivatives 26 and 27.
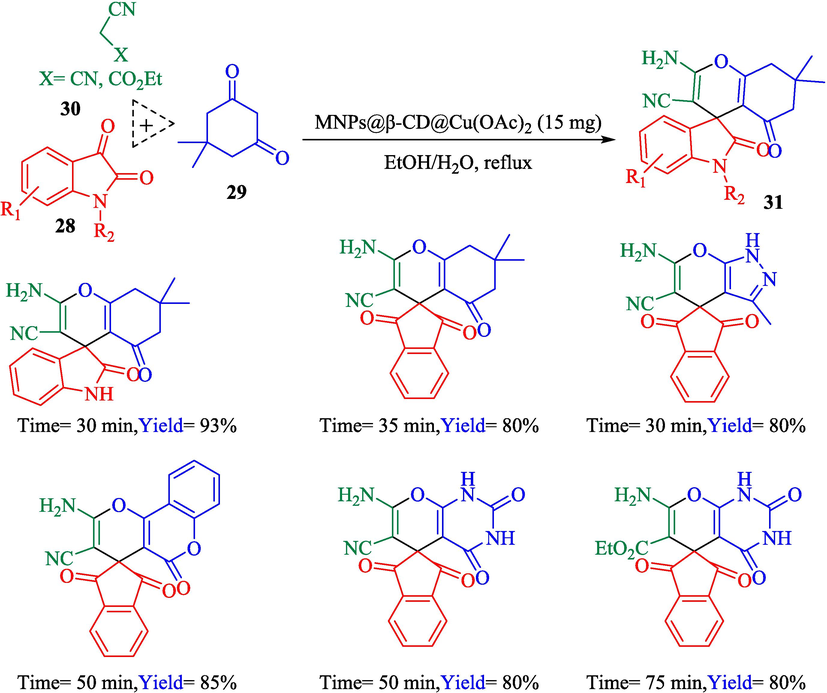
The synthesis of the 31 derivatives by MNPs@β‐CD@Cu(OAc)2.
Mohammadian et al. (Mohammadian and Akhlaghinia, 2019) presented Fe3O4/COS@β-CD-SO3H NPs as recyclable nanocatalysts for the creation of spirooxindoles 32 through MCR from dimedone/cyclohexane-1,3-dione (29), malononitrile (30), and isatins 28 in water at 50 °C. The structure of this new catalyst is displayed in Scheme 18. Magnetic nanocatalysts were characterized through FT-IR, XRD, EDX, TGA, VSM, SEM, and TEM analyses. The TGA thermogram of this nanocatalyst displayed about 2.0 % weight loss at nearly 100 °C, which is owing to the loss of absorbed water from the sample. Additionally, the subsequent weight loss of almost 20 % from 150 to 630 °C is attributed to the decomposition of the β-CD-SO3H part on the surface of Fe3O4/COS NPs. This high temperature for β-CD-SO3H elimination demonstrates the high thermal stability of this nanocatalyst. The SEM images of this nanocatalyst indicated spherical morphology. The TEM photo revealed that most nanoparticles demonstrated uniform spherical morphology with a mean particle size of almost 20–50 nm. Spiro-oxindole derivatives were obtained by reacting different isatins, ethyl cyanoacetate or malononitrile, and 1,3-dicarbonyl compounds (Scheme 19). The recyclability of the nanocatalyst was performed in eight runs without a noticeable decrease in activity. The recovery percentage for this magnetic nanocatalyst at the eight runs involving 98 (1st run), 95 (2nd run), 94 (3rd run), 94 (4th run), 90 (5th run),89 (6th run), 85 (7th run), and 85 (8th run). The average yield was 91.25 %. The procedure's notable advantages are the easy work-up, short-time reaction, catalyst recyclability, and high yields (see Scheme 20).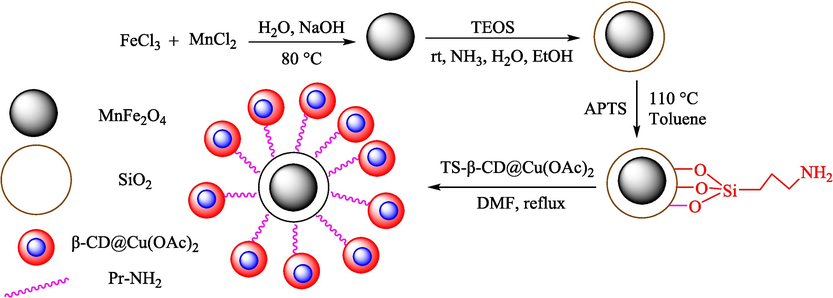
The preparation of this nanocatalyst.
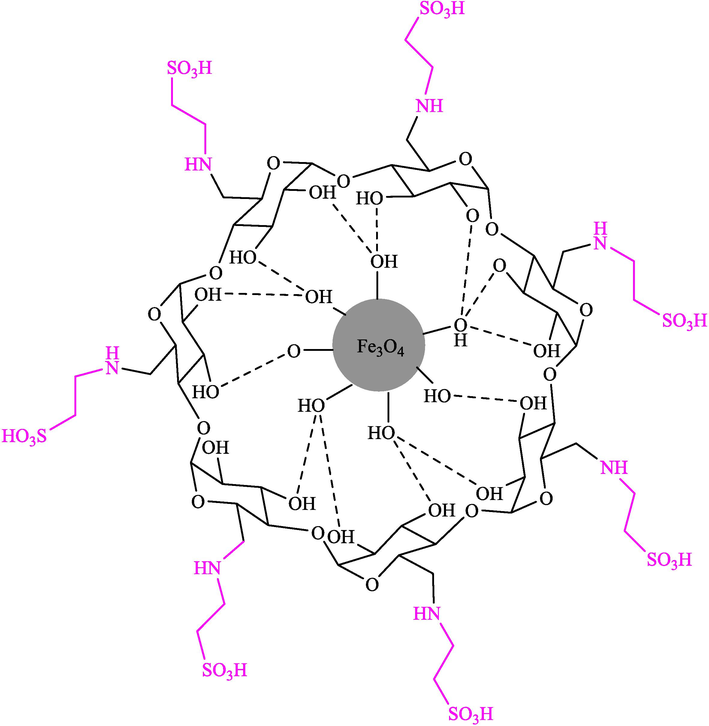
Construction of Fe3O4/COS@β-CD-SO3H NPs.
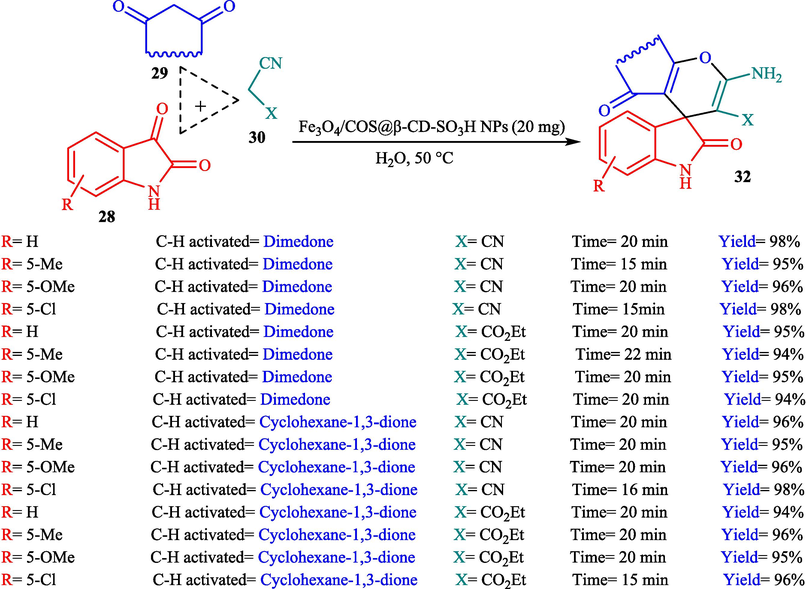
The synthesis of diverse compounds 32.
The preparation of Cu@β-CD@MGO as a recyclable nanocatalyst for the synthesis of N-(alkyl)-2-phenylimidazo[1,2-a]pyridin-3-amines 27 through an efficient method was introduced by Bahadorikhalili et al. (Bahadorikhalili et al., 2020). This novel nanocatalyst was characterized by methods such as TGA, XRD, FT-IR, TEM, SEM, ICP, and VSM. The TGA curve observed that a weight loss occurred at 100 °C, which could be related to the evaporation of the adsorbed water. The weight loss in higher temperatures was owing to the decomposition of the organic moieties in the structure of this nanocatalyst. SEM and TEM data exhibited that Fe3O4 (20–25 nm) were noticed as dark sites within the GO nanosheets. The TCR obtained aldehydes (from 33), pyridine-2-amine (25), and isocyanides 24 were conceived, and N-(alkyl)-2-phenylimidazo[1,2-a]pyridine-3-amine provided in good-yield (65–75 %) (Scheme 21). This nanocatalyst was reused for ten cycles without a noticeable reduction in catalyst efficiency. The authors presented the probable pathway mechanism indicated in Scheme 22 (Bahadorikhalili et al., 2020).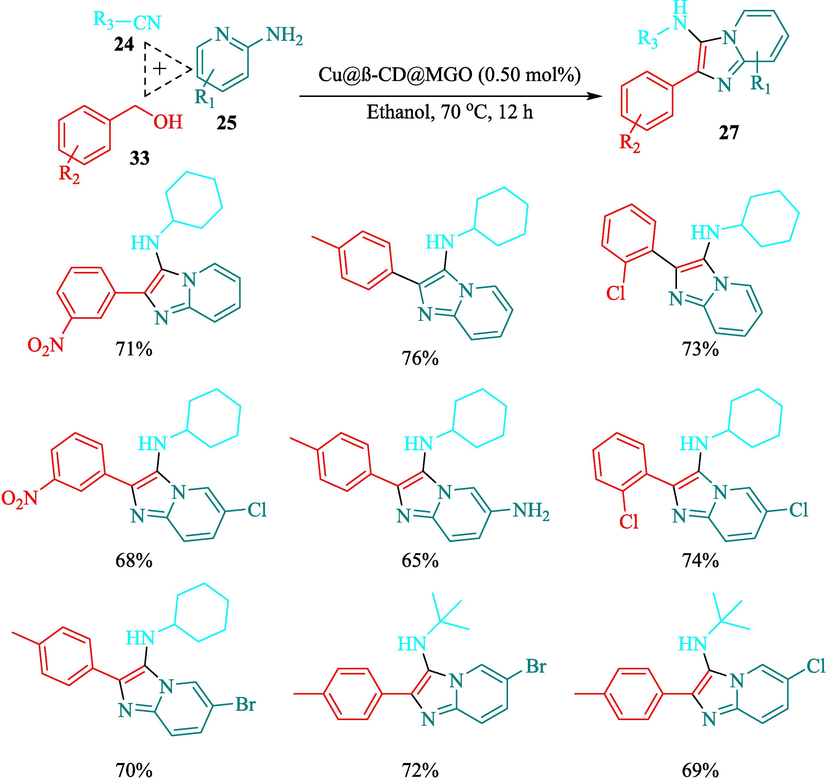
The preparation of various derivatives 27.
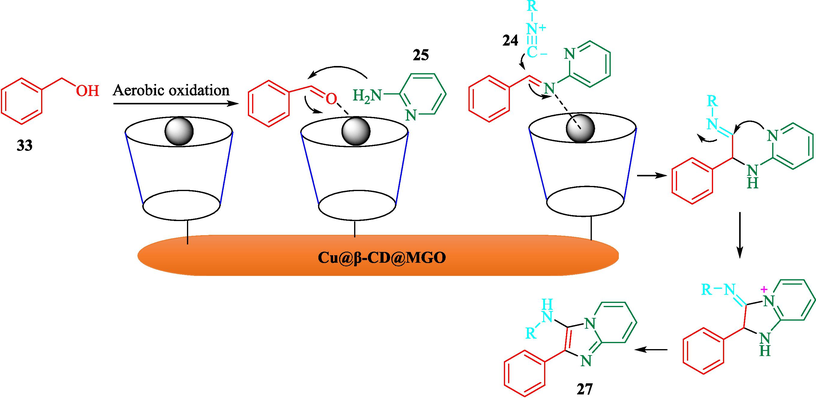
The proposed path for this reaction utilizes Cu@β-CD@MGO.
The one-pot reaction of various aldehyde 20, methyl acetoacetate (34), and ammonium acetate (35) has been reported for the synthesis of 1,4-dihydropyridines 36 via the symmetric Hantzsch reaction by using the Cell-β-CD hydrogel/GO/Cu2O/Fe3O4 nanocatalyst by Almajidi et al. (Almajidi et al., 2024) (Table 6). The TGA curve displayed three distinct weight loss ranges at various temperature intervals. The first range was noticed between 50 and 110 °C, pursued by a second scope between 190 and 250 °C, and ultimately a third range between 250 and 600 °C. Generally, the TGA study illustrated that the first weight loss could be associated with removing adsorbed water. The second weight loss indicates the removal of water molecules and the thermal decomposition of the oxygen-containing groups, like carboxyl, hydroxyl, and epoxy groups, in the GO structure. The third weight loss observed was related to the degradation of glucose units, which constitute the β-CD and cell molecules. A range of products was acquired utilizing this nanocatalyst with a high yield (77–93 %). The result demonstrated that the nanocatalyst was recovered for five cycles and could be reused several times with no notable reduction in its catalytic efficiency. This new nanocatalyst showed the potential for broad uses in different organic conversions owing to its good catalytic activity, green character, and ease of recycling.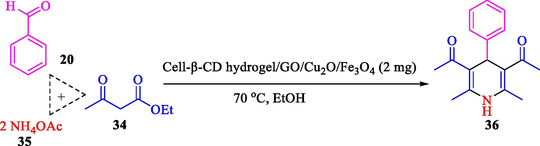
Entry
Aldehyde
Product
Yield ( %)
1
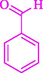
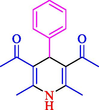
92
2
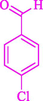
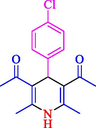
92
3
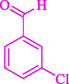
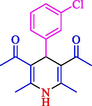
87
4
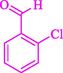
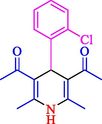
85
5
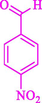
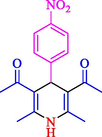
93
6
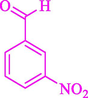
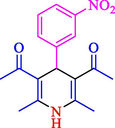
90
7
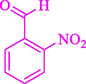
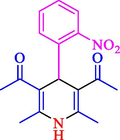
88
8
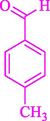
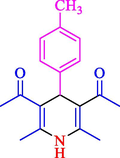
85
9
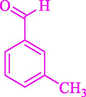
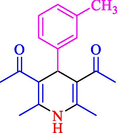
84
10
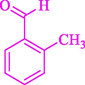
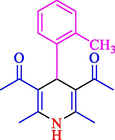
80
11
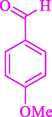
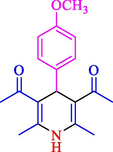
81
12
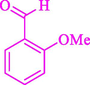
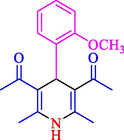
77
13
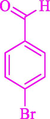
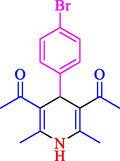
86
14
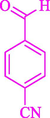
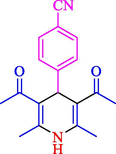
81
15
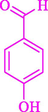
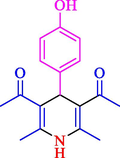
90
16
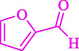
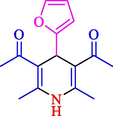
86
17

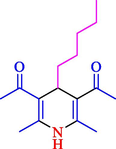
85
We very recently published a review regarding recent advances in β-CD-based catalyst systems for the synthesis of heterocyclic compounds via MCRs that discussed the advantages of using β-CD as green catalysts for synthesizing heterocyclic compounds that lead to the method of milder, easier, and less toxic, approaching an environmental replacement compared to the formerly reported procedures (Payamifar et al., 2024b). A broad spectrum of the reactions catalyzed by β-CD nanocatalysts compared to previously published works for the synthesis of heterocycles using nanocatalysts (Sonawane et al., 2022) can be performed in the H2O media, making those methods green and eco-friendly. One of the most significant benefits of β-CD catalyst is that it can be recycled and reused many runs without reducing efficiency.
2.6 Carbon-carbon coupling
To create carbon–carbon bonds, several common and robust approaches involve the use of transition metal-mediated; among them, the Suzuki coupling is quite an essential and clean procedure. The Suzuki coupling is an appropriate way to construct various kinds of compounds, mainly biaryls (Hooshmand et al., 2019; Chatterjee and Ward, 2016; Payamifar et al., 2024a; Payamifar et al., 2021; Payamifar and Poursattar Marjani, 2023b; Bibak et al., 2024). Biaryls have diverse uses in synthesizing pharmaceuticals, biologically active compounds, and natural products (Bringmann et al., 1990; Adlington et al., 2019). Using the nanocatalyst in coupling reactions has attracted considerable attention recently (Lakshman, 2023; Chetia et al., 2024; Mondal et al., 2024).
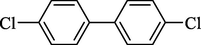
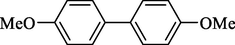
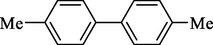
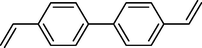
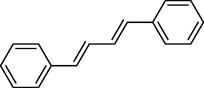
In 2013, Kaboudin et al. (Kaboudin et al., 2013) employed iron oxide-supported Cu2-β-CD as a recoverable magnetic catalyst to prepare symmetrical biaryls derivates 37 from homocoupling 1 (Table 7). These reactions were conducted at room temperature to 70 °C in dimethylformamide as a solvent, obtaining various symmetrical biaryl compounds with good to excellent yields (40–90 %). The mean diameter of Fe3O4 is nearly 10–20 nm, with a mostly spherical shape, as shown by a TEM photo. This nanocatalyst reused four runs with a slight drop in its activity. The recovery percentage for this magnetic nanocatalyst at the four runs involved 90 (1st run), 89 (2nd run), 87 (3rd run), and 83 (4th run). The average yield was 87 %. According to a previous report, the mechanism for the homocoupling ArB(OH)2 is shown in Scheme 23.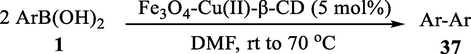
Entry
T (oC)
Product
Time (h)
Yield ( %)
1
rt
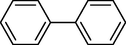
3
90
2
rt
2
89
3
rt
14
74
4
rt
7
66
5
10
82
6
80
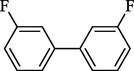
2
85
7
rt
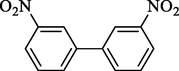
4
40
8
rt
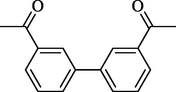
10
48
9
rt
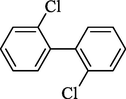
2
80
10
rt
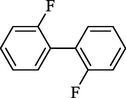
1
82
11
70
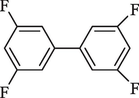
6
75
12
rt
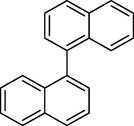
15
50
13
rt
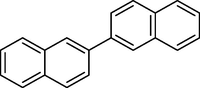
7
90
14
rt
2
50
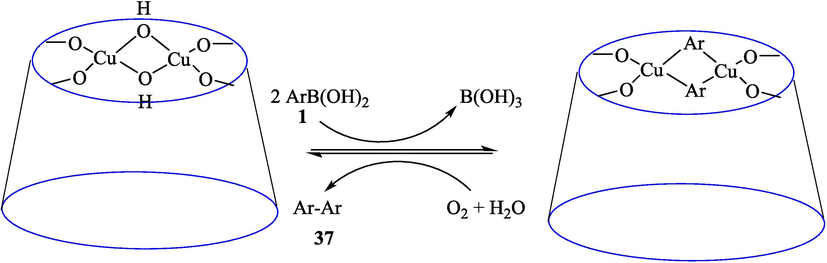
The suggested mechanism for homocoupling reaction.
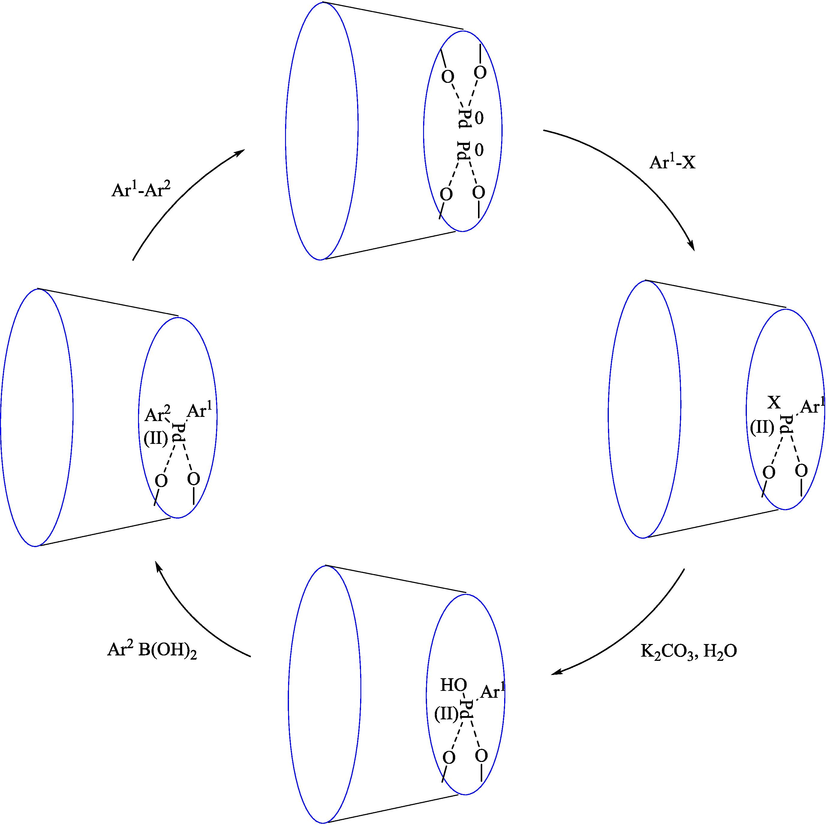
Salemi et al. (Salemi et al., 2016) introduced the MNP-CD-Pd as a recoverable magnetic catalyst in the Suzuki coupling to synthesize diverse biaryls 38. The various steps for preparing this catalyst are exhibited in Scheme 24. The catalyst was analyzed by XRD, TEM, VSM, CHN, TGA, EDS, and FT-IR. For this nanocatalyst, the TGA curve displayed two significant weight loss steps; the second step was 400–520 °C associated with β-CD decomposition. Thus, this nanocatalyst is stable thermally. The TEM image exhibited the palladium nanoparticles, which are nearly spherical and have an average diameter of approximately 25 nm. The obtained nanocatalyst indicated superior performance for a broad scope of substrates in aqueous media under mild reaction. The MNP-CD-Pd is an efficient nanocatalyst applied in the Suzuki coupling reaction of Ar–X (X = I, Br, and Cl) 16 with ArB(OH)2 1 in water/DMF solvent that biaryls products obtained in high yield (62–100 %) (Table 8). The result demonstrated that this nanocatalyst was recovered five times with minor loss of catalytic activity. The recovery percentage for this magnetic nanocatalyst at the five runs involved 96 (1st run), 95 (2nd run), 97 (3rd run), 90 (4th run) and 80 (5th run). The average yield was 91 %. The offered mechanism for the Suzuki reaction by the MNP-CD-Pd catalyst is indicated in Scheme 25.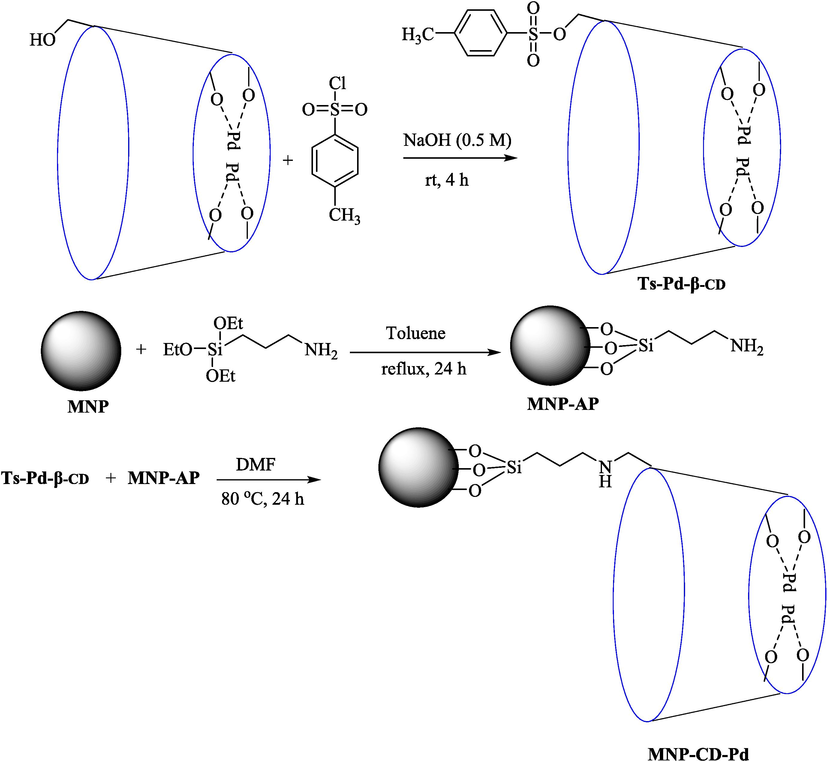
Schematic path for the creation of MNP–CD–Pd.
Entry
Ar1
Ar2
X
Cat (mol %)
Time (h)
Yield ( %)
1
C6H5
C6H5
I
0.03
4
96
2
C6H5
4-MeC6H4
I
0.03
4
100
3
C6H5
4-MeOC6H4
I
0.03
4
100
4
C6H5
3-O2NC6H4
I
0.075
8
98
5
C6H5
3,5-Difluoro-C6H3
I
0.03
4
99
6
C6H5
2-Benzofuranyl
I
0.03
6
100
7
C6H5
β-Naphtyl
I
0.03
6
100
8
C6H5
α-Naphtyl
I
0.075
8
99
9
4-ClC6H4
C6H5
I
0.03
6
98
10
2-MeC6H4
C6H5
I
0.075
6
99
11
4-O2NC6H4
C6H5
I
0.03
4
98
12
4-CHO-C6H4
C6H5
Br
0.03
4
98
13
C6H5
C6H5
Br
0.03
4
71
14
4-CHO-C6H4
4-MeC6H4
Br
0.03
4
100
15
4-CHO-C6H4
4-MeOC6H4
Br
0.03
4
100
16
C6H5
4-MeC6H4
Br
0.06
6
100
17
C6H5
4-MeOC6H4
Br
0.06
6
84
18
4-MeOC6H4
C6H5
Br
0.015
6
96
19
4-MeOC6H4
3,5-Difluoro-C6H3
Br
0.075
8
98
20
4-MeOC6H4
4-MeC6H4
Br
0.015
8
84
21
4-O2NC6H4
C6H5
Cl
0.3
24
65
22
4-CHO-C6H4
C6H5
Cl
0.3
24
62
23
5-Pyrimidine
C6H5
Br
0.03
8
91
The suggested mechanism for the Suzuki reaction.
In 2019, Heidari et al. (Heidari et al., 2019) designed and introduced a novel magnetic catalyst under the name M‐GO/AM‐MBA‐β‐CD@Pd for Suzuki coupling Ar–X (X = I, Br, and Cl) 16 and ArB(OH)2 1 in water media. The different steps of creating this nanocatalyst are illustrated in Scheme 26. Characterization of magnetic catalysts was examined employing FT-IR, SEM, XPS, TEM, XRD, and VSM measurements. Based on the TEM photo, round‐shaped structures of the Pd NPs are noticed, showing that Pd NPs were successfully loaded onto the surface of M‐GO/(AMMBA‐β‐CD). This magnetic catalyst was checked in Suzuki coupling of diverse Ar–X and PhB(OH)2 in water at 40–80 °C within 2–5 h, and different biaryl products 38 obtained in good to excellent yields (45–100 %) (Table 9). This catalytic was reused six times with no significant loss in activity. A leaching test was executed after the sixth run, and low levels of Pd leaching (6 %) were observed.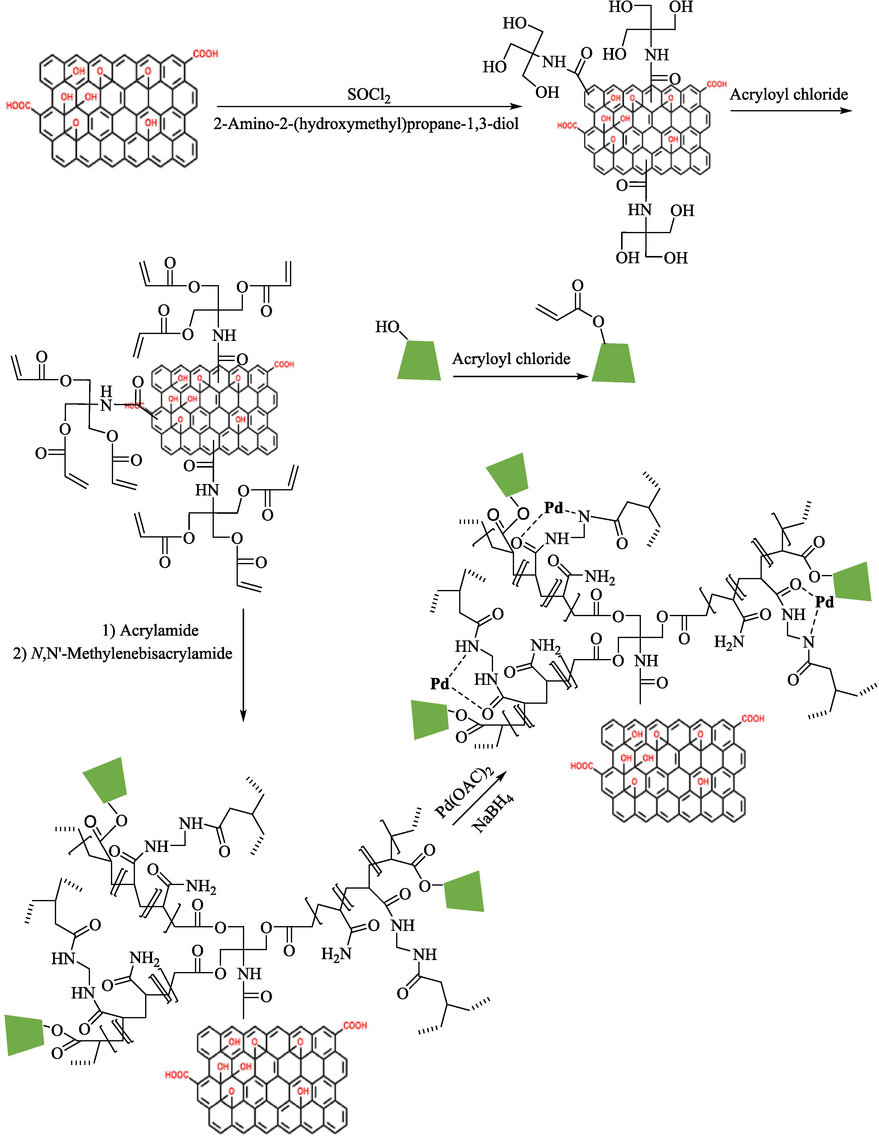
The creation of M‐GO/AM‐MBA‐β‐CD@Pd.
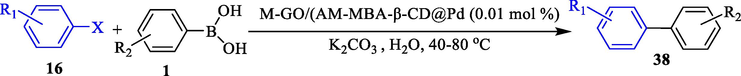
Entry
R1
X
R2
Time (h)
Yield ( %)
1
H
I
H
2
100
2
4-CH3
I
H
2
100
3
4-OCH3
I
H
2
100
4
H
Br
H
3
97
5
4-NO2
Br
H
3
98
6
4-CH3
Br
H
3
95
7
H
Cl
H
5
67
8
4-CH3
Cl
H
5
62
9
4-CH3
Cl
H
5
70
10
H
Br
CH
3
95
11
4-CH3
Br
CH3
3
94
12
4-CH3
I
CH3
2
97
13
4-OCH3
I
CH3
2
96
14
H
I
CH3
2
98
15
4-OCH3
Cl
CH3
5
45
The nanocomposite hydrogels are based on β-CD prepared and developed as a reusable nanocatalyst in Suzuki coupling as reported by Liu et al. (Liu et al., 2023) Cellulose/β-CD hydrogel was created (according to a previous report from cellulose, β-CD, and epichlorohydrin reaction. CE-CD-Cu, CE-CD-Pd, CE-CD-Au, and CE-CD-Ag were recyclable nanocomposite hydrogels. This procedure was a safe and eco-friendly process with nanocomposite hydrogels bearing excellent catalytic performance and satisfactory reusability. Various biaryls 38 acquired excellent yields (93–97 %) (Table 10). The nanocomposite hydrogels recovered eight cycles with no massive reduction in their performance.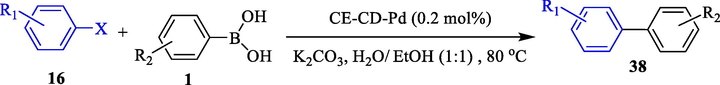
Entry
R1
X
R2
Yield ( %)
1
H
Br
H
97
2
H
I
H
95
3
4-CH3
Br
H
94
4
4-COH3
Br
H
93
5
4-NO2
Br
H
96
6
H
Br
4-COH3
94
7
H
I
4-COH3
92
8
4-CH3
Br
4-COH3
93
9
4-COH3
Br
4-COH3
97
10
4-NO2
Br
4-COH3
96
11
H
Br
4-Me
97
12
H
I
4-Me
95
13
4-Me
Br
4-Me
93
14
4-COH3
Br
4-Me
95
15
4-NO2
Br
4-Me
93
2.7 Oxidation of alcohols
Selective oxidation of alcohols is one of the most fascinating research subjects. The transformation of alcohols to aldehydes, ketones, and carboxylic acids is essential and valuable because they are broadly employed in preparing pharmaceuticals and chemical materials (Najafishirtari et al., 2021).
In 2011, Kang et al. (Kang et al., 2011) prepared and characterized the Fe3O4@SiO2-PGMACD and employed it as a magnetic catalyst in the oxidation of alcohols and adsorption. This novel catalyst was prepared using a series of procedures comprising thermal decomposition, sol–gel method, ATRP, and the ring-opening reaction of the epoxy group. The steps of the synthesis of this catalyst are indicated in Scheme 27. Characterization of Fe3O4@SiO2-PGMA catalysts was analyzed utilizing FT-IR, TEM, XPS, TGA, VSM, and UV–Vis measurements. The TEM image of Fe3O4 indicated the diameter of Fe3O4 was almost 9 nm, and the Fe3O4@SiO2 NPs with a diameter of 60 nm. Oxidation of benzyl alcohol and 1-octanol was studied by using Fe3O4@SiO2-PGMA (32 mg) in H2O at 50 °C, and the mean transformation of oxidation of benzyl alcohol was 84 %. In addition, this catalyst was reused five times without a noticeable reduction in its catalytic activity. After five cycles, the nanocatalyst is still active and helpful.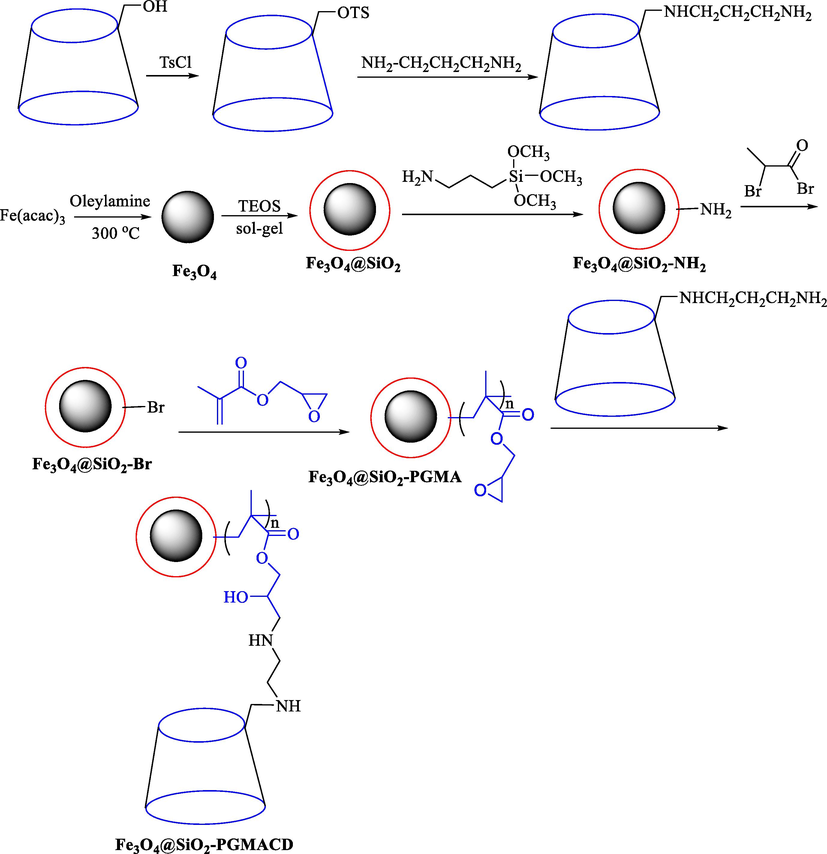
Synthetic pathway for preparation of β-CD-modified MNPs.
In 2017, Zhou et al. (Zhou et al., 2017) designed and constructed Fe3O4@SiO2-GPTSCD (Scheme 28), which was used to catalyze the oxidation of benzyl alcohol. The new catalyst was characterized by diverse procedures such as IR, TGA, TEM, and VSM. Compared with the DTA curves of Fe3-O4@SiO2 and Fe3O4@SiO2-GPTS, a new mighty endothermic peak in the Fe3O4@SiO2-GPTSCD curve might be found at about 300 °C, which was related to the loss of β-CD. TEM images of the catalyst demonstrated that the diameter of the nanoparticles is approximately 10 nm. The oxidation reaction was carried out in the presence of Fe3O4@SiO2-GPTSCD (50 mg) as a catalyst, 10 % oxidant reagent (H2O2 and NaClO) in H2O at 50 °C. This catalyst had good catalytic performance for the oxidation of benzyl alcohol (Table 11). Sodium hypochlorite and hydrogen peroxide are the oxidants used in the oxidation reaction of benzyl alcohol. The oxidation activity of hydrogen peroxide is lower than that of sodium hypochlorite.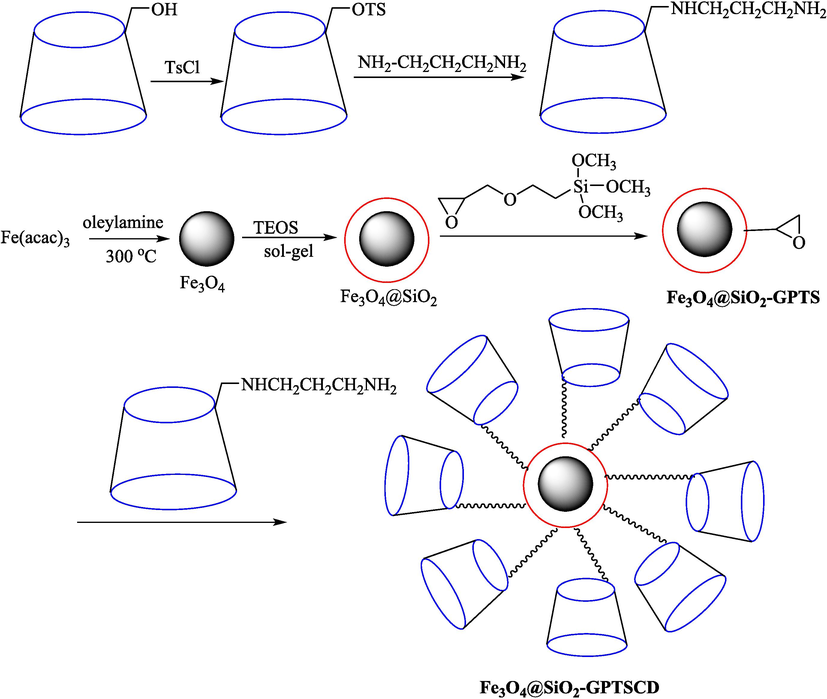
Synthetic way for Fe3O4@SiO2-GPTS.
Entry
Oxidant
Catalyst
Solvent
Product
Conversion ( %)
1
NaClO
No CD
H2O
Benzaldehyde
Trace
2
NaClO
CD
H2O
Benzaldehyde
41.9
3
NaClO
GPTS-CD
H2O
Benzaldehyde
5.4
Benzoic acid
94.5
4
NaClO
CD
H2O/EA
Benzaldehyde
40.9
Benzoic acid
20.6
5
NaClO
GPTS-CD
H2O/EA
Benzaldehyde
80.1
6
H2O2
CD
H2O
Benzaldehyde
6
7
H2O2
GPTS-CD
H2O
Benzaldehyde
76.5
Benzoic acid
17.6
8
H2O2
CD
H2O/EA
Benzaldehyde
8.1
9
H2O2
GPTS-CD
H2O/EA
Benzaldehyde
5.1
Benzoic acid
10.4
10
NaClO
GPTS
H2O
Benzaldehyde
Trace
Benzoic acid
15.2
11
H2O2
GPTS
H2O
Benzaldehyde
4.3
Benzoic acid
Trace
2.8 Nucleophilic substitution reactions of benzyl halides
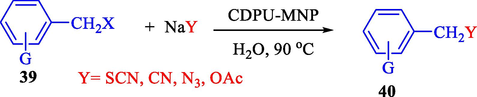
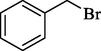
In 2012, Kiasat and Nazari (Kiasat and Nazari, 2012) synthesized and introduced the β-CDPU-MNPs as a phase-transfer catalyst and used it in nucleophilic substitution reactions of benzyl halides 39. The β-CD-polyurethane polymer was grafted onto Fe3O4 NPs to prepare this catalyst, displayed in Scheme 29. Different methods, such as IR, XRD, TGA, SEM, TEM, and VSM identified this new magnetic nanocatalyst. Based on TGA, the first weight loss of 10 % below 300 °C may be due to the loss of the adsorbed water and dehydration of the surface OH groups. The second weight-loss stage of around 58 % in the region of 305–370 °C was owing to the cleavage of the urethane linkage, which is entirely high compared to conventional polyurethanes, which generally start to decompose around 200–220 °C. The enhanced thermal stability of β-CDPUMNPs was related to the crosslinked nature. The final stage of degradation in the region of 370–500 °C, presumably, is owing to the thermal decomposition of β-CD. TEM and VSM analysis revealed that the synthesized magnetic nanocatalysts are superparamagnetic, with an average size of 59 nm. This research presented a safe, low-cost, and straightforward route for forming benzyl thiocyanates, acetates 40, cyanides, and azides via nucleophilic substitution reaction in water (Table 12). This catalyst readily separated and reused ten cycles without massive degradation in its efficiency. The recovery percentage for this magnetic nanocatalyst at the ten runs involved 85 (1st run), 82 (2nd run), 83 (3rd run), 83 (4th run), 82 (5th run), 79 (6th run),78 (7th run),78 (8th run), 76 (9th run) and 73 (10th run). The average yield was 79.9 %.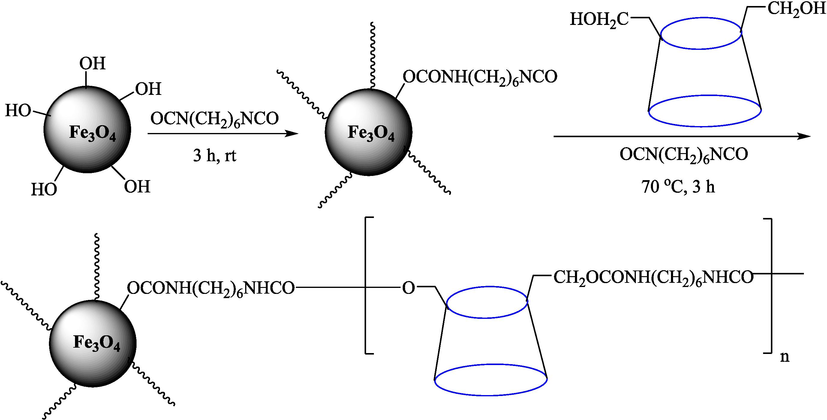
Preparation of β-CDPU-MNPs.
Entry
Benzyl halide
Nucleophile
Time (min)
Yield ( %)
1
SCN
N3
CN
OAc5
10
25
4089
82
79
80
2
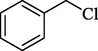
SCN
N3
CN
OAc
SCN10
15
35
40
4085
78
82
80
80
3
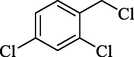
N3
CN
OAc55
75
No reaction84
82
–
4
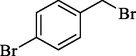
SCN
N3
CN
OAc70
80
75
11580
81
81
79
5
SCN
N3
CN
OAc15
15
25
3583
88
81
75
6
SCN
N3
CN
OAc35
40
50
8084
81
87
83
In 2013, the same group (Kiasat and Nazari, 2013) explained the using MNPs-β-CD as the host system in the nucleophilic substitution reaction of various benzyl halides 39. The products were acquired in pure form without by-products and with more purification. The schematic method for the preparation of MNPs-β-CD is shown in Scheme 30. The TGA analysis of the nanocatalyst indicated the first weight loss of 7 % below 220 °C, which could be due to the loss of residual water attaching to the sample surface and adsorbed in the β-CD cavities. The second weight-loss stage of almost 60 % of the 290–390 °C region was due to the decomposition of β-CD moieties. Therefore, this nanocatalyst is stable thermally. MNPs-β-CD performed nucleophilic substitution conversion of benzyl halides with (SCN and N3) in H2O and corresponding products 40 provided a good yield (78–86 %) (Table 13). This method's principal benefits were a green, clean reaction, short reaction time, high yields, catalyst reusability, and cost-effective procedure. This nanocatalyst readily reused three runs without massive degradation in its performance and the recovery percentage in 86, 81 and 83 % yield, respectively.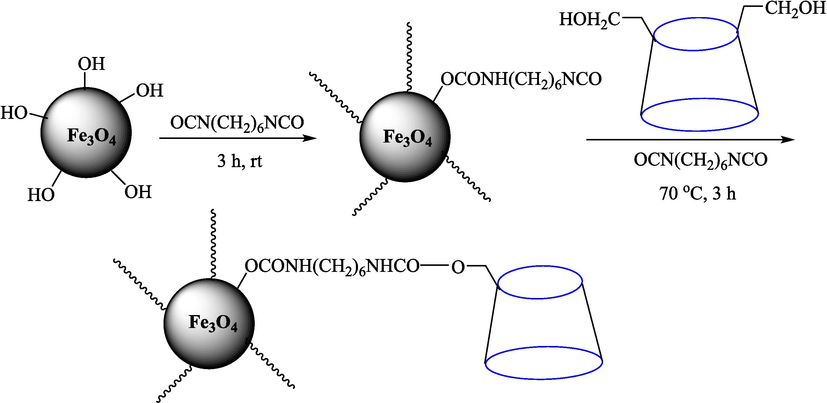
Construction of MNPs-β-CD.
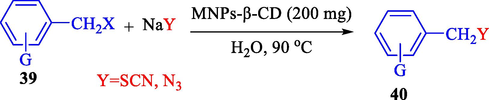
Entry
Benzyl halide
Nucleophile
Time (min)
Yield ( %)
1
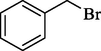
SCN
N3
15
3586
84
2
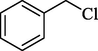
SCN
N3
25
5081
83
3
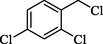
SCN
N3
100
15080
87
4
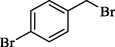
SCN
N3
150
18080
82
5
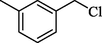
SCN
N3
40
5083
79
6
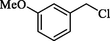
SCN
N3
65
8083
82
2.9 Reduction of nitroarenes
Water pollution has been known as a significant menace factor responsible for numerous diseases in humans and animals. The harmful pollutants, like p-NP and azo dyes that have high stability, lead to water pollution, which induces health risks. Therefore, designing safe systems and green catalysts to decrease toxic pollutants like nitro derivates and dyes is a big issue. One strategy to devastate this concern is the catalytic reduction of nitro materials to valuable amine compounds, which are beneficial and non-toxic. Various procedures have been developed to reduce nitro compounds utilizing a simple economic catalyst. Since this reaction is prolonged, using a proper catalyst for the catalytic transformation of the nitro materials is hugely required (Orlandi et al., 2016; Goksu et al., 2017; Kovacic and Somanathan, 2014). Owing to financial and environmental factors, the hydrogenation of nitro compounds by employing eco-friendly catalysts like β-CD has gained importance. β-CD based on nanomagnetic catalysts, compared to other β-CD catalysts, has some advantages like straightforward isolation and reuse, ample surface area, and low toxicity. We recently reported a review of the most recent developments associated with the utility of β-CD catalysts in the reduction of nitroaromatic. In that review, we thoroughly studied the latest advances in β-CD catalysts, including various catalysts like homogeneous and heterogeneous magnetic nanoparticles. We are employing β-CD catalysts based on MNPs as a recoverable and highly effective catalyst in the nitro compound reduction field has been studied (Payamifar and Poursattar Marjani, 2023a).
A summary of various magnetic nanoparticles of β-CD-catalyst in various organic transformations is exhibited in Table 14. The catalyst used in entry 9 of the report related to Rostamnia and Doustkhah (2015) had better performance. They use Fe3O4@β-CD as a nanocatalyst for the Kabachnik–Fields MCR. The aminophosphonate derivatives were obtained in high yield (85–96 %) at room temperature within 1 h in H2O.
Entry
Catalyst
Temp. (°C)
Time (h)
Solvent
Yield ( %)
Ref.
1
Fe3O4-β-CD-Cu(II)
rt
2–6
H2O
60–96
(Kaboudin et al., 2013)
2
Cu@β-CD@SiO2@SPION
rt
24
H2O
79–88
(Bahadorikhalili et al., 2018)
3
Cu@β-CD@SPIONs
rt
24
H2O-EtOH
71–87
(Mahdavi et al., 2016)
4
Fe3O4@CD-CIT
rt
45–55 min
H2O
91–97
(Jain et al., 2019)
5
CE-β‐CD-Cu
40
24
H2O
83–94
(Liu et al., 2023)
6
Pd NPs@β-CD-Sch
120
4
DMF
77–98
(Baran and Nasrollahzadeh, 2020)
7
β-CD@MNP
rt
5–25 min
H2O
84–96
(Sayyahi et al., 2016)
8
γ-Fe2O3@HAp@β-CD
90
5–80 min
H2O
66v94
(Khosravinia et al., 2019)
9
Fe3O4@β-CD
rt
1
H2O
85–96
(Rostamnia and Doustkhah, 2015)
10
β-CD-IL@M starch
50
6 h
EtOH
80–91
(Bahadorikhalili et al., 2019)
11
MNPs@β‐CD@Cu(OAc)2
reflux
30–75 min
EtOH/H2O
80–93
(Ahadi et al., 2019)
12
Fe3O4/COS@β-CD-SO3H NPs
50
15–22 min
H2O
94–98
(Mohammadian and Akhlaghinia, 2019)
13
Cu@β-CD@MGO
70
12
EtOH
65–75
(Bahadorikhalili et al., 2020)
14
Cell-β-CD hydrogel/GO/Cu2O/Fe3O4
70
15 min
EtOH
77–93
(Almajidi et al., 2024)
15
Fe3O4-β-CD-Cu(II)
rt-70
1–24
DMF
40–90
(Kaboudin et al., 2013)
16
MNP-CD-Pd
reflux
4–24
H2O
62–100
(Salemi et al., 2016)
17
M‐GO/AM‐MBA‐β‐CD@Pd
40–80
2–5
H2O
45–100
(Heidari et al., 2019)
18
CE-CD-Pd nanoparticles
85
10
EtOH/H2O
92–97
(Liu et al., 2023)
19
Fe3O4@SiO2-PGMACD
50
24
H2O
84
(Kang et al., 2011)
20
Fe3O4@SiO2-GPTSCD
50
24
H2O
76.5–94.5
(Zhou et al., 2017)
21
β-CDPU-MNPs
90
5–115 min
H2O
75–89
(Kiasat and Nazari, 2012)
22
MNPs-β-CD
90
15–150
H2O
78–86
(Kiasat and Nazari, 2013)
3 Conclusion
Due to ever-increasing environmental and economic issues, the rising attraction has been paid to expanding superb and reusable catalysts for organic transformation. Along this line, due to their outstanding properties, Fe3O4 NPs have attracted much attention over the past decade. The magnetic nanoparticles improve and enhance the efficiency of organic transformation in simplicity, selectivity, activity, and sustainability. The nano-sized magnetic nanoparticles raise the active spots on the surface of the catalyst system for interaction with the reactants. Also, the excellent dispersal of the Fe3O4 nanocatalysts in various solvents is a further advantage since this makes the active areas of the catalyst more available to the reactants. Significantly, the surfaces of magnetic nanoparticles can be modified easily with diverse organic ligands that produce appropriate regions for the adsorption of catalytically active metal nanoparticles. β-CD is a low-cost, non-toxic, eco-friendly, and commercially available ligand that can be functionalized with Fe3O4 and used as a recoverable catalyst in organic reactions. Remarkably, an expansive scope of the reactions catalyzed by β-CD makes procedures green and eco-friendly. β-CD catalysis can be recycled, which is highly critical from environmental and economic points of view. Their most crucial feature is to form inclusion complexes with reactants, intermediates, and catalysts.
Consequently, their cavities act as sterically restricted and polar reaction fields to promote the efficiency and selectivity of reactions. Furthermore, unstable reagents and intermediates are protected from undesired side reactions. The benefits of magnetic nanoparticles β-CD catalysts include being reused, simple isolation, and cheap and readily accessible starting materials.
Furthermore, the easy separation of the reaction media under mild conditions obtains ideal advantages to green chemistry goals. These days, numerous interest has been prompted in the functionalization of β-CDs as hosts for organic reactions. The combination of magnetic nanoparticles with β-CD has been studied, a reusable nanocatalyst used in many organic reactions. This review outlines the progress in magnetic nanoparticle-based β-CD catalysts in different organic conversions.
CRediT authorship contribution statement
Sara Payamifar: Writing – original draft, Investigation. Majid Abdouss: Supervision. Ahmad Poursattar Marjani: Writing – review & editing, Supervision, Investigation.
Acknowledgments
The authors would like to acknowledge the support from the Research Council of Urmia University and Amirkabir University of Technology.
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
References
- β‐Cyclodextrin as an efficient and recyclable supramolecular catalyst for the synthesis of heterocyclic compounds. J. Chin. Chem. Soc.. 2017;64:896-917.
- [CrossRef] [Google Scholar]
- Urea phosphate/β-cyclodextrin inclusion complex to enhance thermal behavior of cotton fabric. Carbohydr. Polym.. 2011;84:593-598.
- [CrossRef] [Google Scholar]
- Process design and optimization in the pharmaceutical industry: a Suzuki–Miyaura procedure for the synthesis of savolitinib. J. Org. Chem.. 2019;84:4735-4747.
- [CrossRef] [Google Scholar]
- Cu(II)‐β‐cyclodextrin complex stabilized on magnetic nanoparticles: a retrievable hybrid promoter for green synthesis of spiropyrans. Appl. Organomet. Chem.. 2019;33:e4738
- [CrossRef] [Google Scholar]
- A versatile magnetic nanocomposite based on cellulose-cyclodextrin hydrogel embedded with graphene oxide and Cu2O nanoparticles for catalytic application. Int. J. Biol. Macromol.. 2024;260:129367
- [CrossRef] [Google Scholar]
- Green synthesis of magnetic supramolecules β-cyclodextrin/iron oxide nanoparticles for photocatalytic and antibacterial applications. ACS Omega.. 2023;8:32067-32077.
- [CrossRef] [Google Scholar]
- Synthesis of 1,2,3-triazolyl nucleoside analogues and their antiviral activity. Mol. Divers.. 2021;25:473-490.
- [CrossRef] [Google Scholar]
- Nanomaterials: catalysis in the synthesis of highly substituted heterocycles. Synth. Commun.. 2021;51:880-903.
- [CrossRef] [Google Scholar]
- Removal of heavy metals from industrial wastewaters: a review. ChemBioEng Rev.. 2017;4:37-59.
- [CrossRef] [Google Scholar]
- Direct oxidative esterification of toluene with 1,3‐dicarbonyl compounds catalyzed by copper complex supported on magnetic nanoparticles. Appl. Organomet. Chem.. 2017;31:e3658
- [CrossRef] [Google Scholar]
- Copper‐supported β‐cyclodextrin‐functionalized magnetic nanoparticles: efficient multifunctional catalyst for one‐pot ‘green’ synthesis of 1,2,3‐triazolylquinazolinone derivatives. Appl. Organomet. Chem.. 2018;32:e4212
- [CrossRef] [Google Scholar]
- The use of magnetic starch as a support for an ionic liquid-β-cyclodextrin based catalyst for the synthesis of imidazothiadiazolamine derivatives. Int. J. Biol. Macromol.. 2019;135:453-461.
- [CrossRef] [Google Scholar]
- Efficient one-pot synthesis of phenylimidazo[1,2‐a]pyridine derivatives using multifunctional copper catalyst supported on β‐cyclodextrin functionalized magnetic graphene oxide. Appl. Organomet. Chem.. 2020;34:e5913.
- [CrossRef] [Google Scholar]
- Cyclodextrin-conjugated nanocarrier for magnetically guided delivery of hydrophobic drugs. J. Nanoparticle Res.. 2009;11:2071-2078.
- [CrossRef] [Google Scholar]
- Pd NPs@Fe3O4/chitosan/pumice hybrid beads: a highly active, magnetically retrievable, and reusable nanocatalyst for cyanation of aryl halides. Carbohydr. Polym.. 2020;237:116105
- [CrossRef] [Google Scholar]
- Green synthesis of palladium nanocatalyst derived from the β-cyclodextrin used as an effective heterogeneous catalyst for cyanation of aryl halides. Inorg. Chem. Commun.. 2020;119:108117
- [CrossRef] [Google Scholar]
- Green synthesis of a palladium nanocatalyst anchored on magnetic lignin-chitosan beads for synthesis of biaryls and aryl halide cyanation. Int. J. Biol. Macromol.. 2020;155:814-822.
- [CrossRef] [Google Scholar]
- MCM-41 supported 2-aminothiophenol/Cu complex as a sustainable nanocatalyst for Suzuki coupling reaction. Sci. Rep.. 2024;14:18070
- [CrossRef] [Google Scholar]
- Azide/alkyne- “click” reactions: applications in material science and organic synthesis. Curr. Org. Chem.. 2006;10:1791-1815.
- [CrossRef] [Google Scholar]
- The directed synthesis of biaryl compounds: modern concepts and strategies. Angew. Chem. Int. Ed.. 1990;29:977-991.
- [CrossRef] [Google Scholar]
- Supramolecular Fe3O4-gluconic acid@β-cyclodextrin magnetic composite: green synthesis using sucrose and application in removal of p-nitrophenol. J. Environ. Chem. Eng.. 2024;12:111503
- [CrossRef] [Google Scholar]
- Quaternary ammonium β-cyclodextrin-conjugated magnetic nanoparticles as nano-adsorbents for the treatment of dyeing wastewater: synthesis and adsorption studies. J. Environ. Chem. Eng.. 2017;5:2869-2878.
- [CrossRef] [Google Scholar]
- Design of a palladium nanocatalyst produced from Schiff base modified dialdehyde cellulose and its application in aryl halide cyanation and reduction of nitroarenes. Cellulose. 2022;29:4475-4493.
- [CrossRef] [Google Scholar]
- Facile preparation of nanostructured Pd-Sch-δ-FeOOH particles: a highly effective and easily retrievable catalyst for aryl halide cyanation and p-nitrophenol reduction. J. Phys. Chem. Solid. 2021;152:109968
- [CrossRef] [Google Scholar]
- Fabrication of cyclodextrin-functionalized superparamagnetic Fe3O4/amino-silane core-shell nanoparticles via layer-by-layer method. Appl. Surf. Sci.. 2009;255:7974-7980.
- [CrossRef] [Google Scholar]
- Recent advances in the palladium-catalyzed Suzuki–Miyaura cross-coupling reaction in water. Catal. Lett.. 2016;146:820-840.
- [CrossRef] [Google Scholar]
- A Comprehensive review on magnetic NiFe2O4 nanoparticles: synthesis approaches and catalytic proficiency in various coupling reactions. Tetrahedron. 2024;134172
- [CrossRef] [Google Scholar]
- Multicomponent reactions: advanced tools for sustainable organic synthesis. Green Chem.. 2014;16:2958-2975.
- [CrossRef] [Google Scholar]
- Cyclodextrin-based materials for removing micropollutants from wastewater. Curr. Org. Chem.. 2018;22:2150-2181.
- [CrossRef] [Google Scholar]
- β‐Cyclodextrin: a green and efficient supramolecular catalyst for organic transformations. Chem. Rec.. 2018;18:1560-1582.
- [CrossRef] [Google Scholar]
- Functionalized β-cyclodextrin as supramolecular ligand and their Pd(OAc)2 complex: highly efficient and reusable catalyst for Mizoroki–Heck cross-coupling reactions in an aqueous medium. Carbohydr. Res.. 2016;430:85-94.
- [CrossRef] [Google Scholar]
- Metal-mediated halogen exchange in aryl and vinyl halides: A review. Front. Chem.. 2018;6:114
- [CrossRef] [Google Scholar]
- Synthesis of pyrano-pyrimidines: recent advances in catalysis by magnetically recoverable nanocatalysts. Mol. Divers. 2023:1-33.
- [CrossRef] [Google Scholar]
- Recent advances on multicomponent synthesis of pyranopyrazoles using magnetically recoverable nanocatalysts. Polycycl. Aromat. Comp. 2023:1-47.
- [CrossRef] [Google Scholar]
- Nickel-Fe3O4 magnetic nanoparticles supported on multiwalled carbon nanotubes: an effective catalyst in Suzuki cross-coupling reactions. Catalysts. 2021;11:495
- [CrossRef] [Google Scholar]
- Recent developments of supported and magnetic nanocatalysts for organic transformations: an up-to-date review. Appl. Nanosci.. 2023;13:15-63.
- [CrossRef] [Google Scholar]
- Based on copper ferrite nanoparticles (CuFe2O4 NPs): catalysis in the synthesis of heterocycles. J. Synth. Chem.. 2022;1:84-96.
- [CrossRef] [Google Scholar]
- Palladium supported on hydrophilic magnetic nanoparticles as a new efficient catalyst in aqueous media. J. Mol. Struct.. 2023;1288:135804
- [CrossRef] [Google Scholar]
- Recent advances in the reduction of nitro compounds by heterogeneous catalysts. Curr. Org. Chem.. 2017;21:794-820.
- [Google Scholar]
- Recent advances in the application of magnetic nanoparticles as a support for homogeneous catalysts. Nanomaterials. 2014;4:222-241.
- [CrossRef] [Google Scholar]
- Magnetic iron oxide nanomaterials for lipase immobilization: promising industrial catalysts for biodiesel production. Catalysts. 2024;14:336
- [CrossRef] [Google Scholar]
- Recent breakthroughs in aqueous cyclodextrin-assisted supramolecular catalysis. Cat. Sci. Technol.. 2014;4:1899-1908.
- [CrossRef] [Google Scholar]
- Catalysis in cyclodextrin-based unconventional reaction media: recent developments and future opportunities. ACS Sustain. Chem. Eng.. 2017;5:3598-3606.
- [CrossRef] [Google Scholar]
- Novel palladium nanoparticles supported on β‐cyclodextrin@graphene oxide as magnetically recyclable catalyst for Suzuki–Miyaura cross‐coupling reaction with two different approaches in bio‐based solvents. Appl. Organomet. Chem.. 2019;33:e4632
- [CrossRef] [Google Scholar]
- Recent advances in the Suzuki–Miyaura cross-coupling reaction using efficient catalysts in eco-friendly media. GreenChem.. 2019;21:381-405.
- [CrossRef] [Google Scholar]
- A review on heterocyclic: synthesis and their application in medicinal chemistry of imidazole moiety. Science. 2018;6:83-94.
- [CrossRef] [Google Scholar]
- Preparation of amino-functionalized β-cyclodextrin/Fe3O4@SiO2 magnetic nanocarrier for controlled release of doxorubicin, an anticancer drug. Arab. J. Sci. Eng.. 2024;49:459-473.
- [CrossRef] [Google Scholar]
- A comprehensive review on synthesis of oxazoles: research on magnetically recoverable catalysts. Res. Chem. Intermed.. 2024;50:1845-1872.
- [CrossRef] [Google Scholar]
- Recent advances on application nanomagnetic recoverable catalysts for synthesis of 1,4-dihydropyridine (DHPs) Res. Chem. Intermed.. 2024;50:1713-1743.
- [CrossRef] [Google Scholar]
- A highly efficient catalyst: in situ growth of Au nanoparticles on graphene oxide–Fe3O4 nanocomposite support. Chem. Eng. J.. 2014;236:1-8.
- [CrossRef] [Google Scholar]
- Robust synthesis of sugar-coumarin based fluorescent 1,4-disubstituted-1,2,3-triazoles using highly efficient recyclable citrate grafted β-cyclodextrin@magnetite nano phase transfer catalyst in aqueous media. Carbohydr. Res.. 2019;482:107736
- [CrossRef] [Google Scholar]
- Fe3O4 nanoparticle-supported Cu(II)-β-cyclodextrin complex as a magnetically recoverable and reusable catalyst for the synthesis of symmetrical biaryls and 1,2,3-triazoles from aryl boronic acids. Green Chem.. 2013;15:2266-2274.
- [CrossRef] [Google Scholar]
- Application of magnetic nanoparticles modified with cyclodextrins as efficient adsorbents in separation systems. J. Incl. Phenom. Macrocycl. Chem.. 2015;82:301-310.
- [CrossRef] [Google Scholar]
- Syntheses and structures of magnetic nanodendrimers and their catalytic application in organic synthesis. Appl. Organomet. Chem.. 2023;37:e7064
- [CrossRef] [Google Scholar]
- β-Cyclodextrin-modified hybrid magnetic nanoparticles for catalysis and adsorption. J. Mater. Chem.. 2011;21:3704-3710.
- [CrossRef] [Google Scholar]
- Magnetically recoverable catalysts: catalysis in the synthesis of polyhydroquinolines. Appl. Organomet. Chem.. 2020;34:e5400
- [CrossRef] [Google Scholar]
- Copper-supported modified magnetic carrageenan as a bio-based catalyst for the synthesis of novel scaffolds bearing the 1,2,3-triazole unit through the click reaction. Nanoscale Adv.. 2024;6:2337-2349.
- [CrossRef] [Google Scholar]
- Synthesis and characterization of γ-Fe2O3@HAp@ β-CD core-shell nanoparticles as a novel magnetic nanoreactor and its application in the one-pot preparation of β-azido alcohols, β-nitro alcohols, and β-cyanohydrins. Iran. J. Chem. Chem. Eng.. 2019;38:61-68.
- [CrossRef] [Google Scholar]
- Magnetic nanoparticles grafted with β–cyclodextrin–polyurethane polymer as a novel nanomagnetic polymer brush catalyst for nucleophilic substitution reactions of benzyl halides in water. J. Mol. Catal. A Chem.. 2012;365:80-86.
- [CrossRef] [Google Scholar]
- β-Cyclodextrin conjugated magnetic nanoparticles as a novel magnetic microvessel and phase transfer catalyst: synthesis and applications in nucleophilic substitution reaction of benzyl halides. J. Incl. Phenom. Macrocycl. Chem.. 2013;76:363-368.
- [CrossRef] [Google Scholar]
- Kleemann, A., Engel, J., 1999. Pharmaceutical Substances: Syntheses, Patents, Applications. https://lccn.loc.gov/00709735.
- Monomeric. oligomeric, polymeric, and supramolecular cyclodextrins as catalysts for green chemistry. Research. 2024;7:0466
- [CrossRef] [Google Scholar]
- Nitroaromatic compounds: environmental toxicity, carcinogenicity, mutagenicity, therapy and mechanism. J. Appl. Toxicol.. 2014;34:810-824.
- [CrossRef] [Google Scholar]
- Magnetically active iron oxide nanoparticles for catalysis of organic transformations: A review. Tetrahedron. 2022;106:132641
- [CrossRef] [Google Scholar]
- Recent developments in coupling reactions catalyzed by copper ferrite nanoparticles (CuFe2O4 NPs) J. Synth. Chem.. 2023;1:148-154.
- [CrossRef] [Google Scholar]
- Recent developments in the organic synthesis using nano-NiFe2O4 as reusable catalyst: a comprehensive synthetic & catalytic reusability protocol. Results Chem. 2023101176
- [CrossRef] [Google Scholar]
- Larock, R.C., 1989. Comprehensive Organic Transformations. Wiley Online Library. doi: 10.1002/9781118662083.
- Synthesis of water-dispersible Fe3O4@β-cyclodextrin by plasma-induced grafting technique for pollutant treatment. J. Chem. Eng.. 2013;229:296-303.
- [CrossRef] [Google Scholar]
- Asymmetric ring-opening of epoxides catalyzed by metal–salen complexes. Catalysts. 2020;10:705
- [CrossRef] [Google Scholar]
- Cellulose/β-cyclodextrin hydrogel supported metal nanoparticles as recyclable catalysts in the 4-nitrophenol reduction, Suzuki–Miyaura coupling, and click reactions. Cellulose. 2023;30:953-971.
- [CrossRef] [Google Scholar]
- Copper-supported β-cyclodextrin grafted magnetic nanoparticles as an efficient recyclable catalyst for one-pot synthesis of 1-benzyl-1H-1,2,3-triazoldibenzodiazepinone derivatives via click reaction. RSC Adv.. 2016;6:28838-28843.
- [CrossRef] [Google Scholar]
- 1-(2-Aminophenyl)-1H-1,2,3-triazole-4-carboxylic acid: activity against Gram-positive and Gram-negative pathogens including Vibrio cholerae. Soc. Open Sci.. 2017;4:170684
- [CrossRef] [Google Scholar]
- Epoxides: methods of synthesis, reactivity, practical significance. Russ. Chem. Rev.. 2022;91:11.
- [CrossRef] [Google Scholar]
- Triazoles and their derivatives: chemistry, synthesis, and therapeutic applications. Front. Mol. Biosci.. 2022;9:864286
- [CrossRef] [Google Scholar]
- One-pot multicomponent approach towards the synthesis of heterocyclic compounds by using β-cyclodextrin as a catalyst. Curr. Org. Chem.. 2018;22:1959-1985.
- [CrossRef] [Google Scholar]
- Synthesis of bioactive magnetic nanoparticles spiro [indoline‐3,4′‐[1,3]dithiine]@Ni(NO3)2 supported on Fe3O4@SiO2@CPS as reusable nanocatalyst for the synthesis of functionalized 3,4‐dihydro‐2H‐pyran. Appl. Organomet. Chem.. 2020;34:e5543
- [CrossRef] [Google Scholar]
- Magnetic calcined oyster shell functionalized with taurine immobilized on β-cyclodextrin (Fe3O4/COS@β-CD-SO3H NPs) as green and magnetically reusable nanocatalyst for efficient and rapid synthesis of spirooxindoles. Res. Chem. Intermed.. 2019;45:4737-4756.
- [CrossRef] [Google Scholar]
- Mondal, M., Ghosh, S., Hajra, A., 2024. Nano Copper-catalyzed Coupling Reactions, Vol. 9. ACS Publications, pp. 231–256. doi: 10.1021/bk-2024-1466.ch009.
- A perspective on heterogeneous catalysts for the selective oxidation of alcohols. Chem. Eur. J.. 2021;27:16809-16833.
- [CrossRef] [Google Scholar]
- Fe3O4 nanoparticles: structures, synthesis, magnetic properties, surface functionalization, and emerging applications. Appl. Sci.. 2021;11:11301
- [CrossRef] [Google Scholar]
- Molecular muscles: from species in solution to materials and devices. Chem. Lett.. 2014;43:964-974.
- [CrossRef] [Google Scholar]
- Polymer biquinolyl-containing complexes of Pd(Ⅱ) as efficient catalysts for cyanation of aryl and vinyl halides with K4Fe(CN)6. New J. Chem.. 2016;40:10465-10473.
- [CrossRef] [Google Scholar]
- Preparation of recoverable Pd catalysts for carbonylative cross-coupling and hydrogenation reactions. ChemCatChem. 2013;5(2013):349-354.
- [CrossRef] [Google Scholar]
- Recent developments in the reduction of aromatic and aliphatic nitro compounds to amines. Org. Process Res. Dev.. 2016;22:430-445.
- [CrossRef] [Google Scholar]
- Nickel/β‐CD‐catalyzed Suzuki–Miyaura cross‐coupling of aryl boronic acids with aryl halides in water. Appl. Organomet. Chem.. 2021;35:e6378
- [CrossRef] [Google Scholar]
- The recent development of β‐cyclodextrin‐based catalysts system in Suzuki coupling reactions. Appl. Organomet. Chem.. 2024;38:e7458
- [CrossRef] [Google Scholar]
- Recent advances in β-cyclodextrin-based catalyst systems for the synthesis of heterocyclic compounds via multicomponent reactions (MCRs) Arab. J. Chem. 2024105967
- [CrossRef] [Google Scholar]
- The electrochemical coupling reactions of organic halides compound in a valuable and practical manner for C–C and C–heteroatom formation: an overview. Arab. J. Chem.. 2024;17:105822
- [CrossRef] [Google Scholar]
- Recent advances in β‐cyclodextrin‐based catalysts for reducing toxic nitroaromatic: an overview. Appl. Organomet. Chem.. 2023;37:e7287
- [CrossRef] [Google Scholar]
- A new β-cyclodextrin-based nickel as green and water-soluble supramolecular catalysts for aqueous Suzuki reaction. Sci. Rep.. 2023;13:21279
- [CrossRef] [Google Scholar]
- Cyclodextrins as oral drug carrier molecular devices: origins, reasons and in-vitro model applications. Curr. Org. Chem.. 2012;16:2365-2378.
- [CrossRef] [Google Scholar]
- Bio inspired green synthesis of Ni/Fe3O4 magnetic nanoparticles using Moringa oleifera leaves extract a magnetically recoverable catalyst for organic dye degradation in aqueous solution. J. Alloys Compd.. 2017;700:252-258.
- [CrossRef] [Google Scholar]
- Synthesis of water-dispersed magnetic nanoparticles (H2O-DMNPs) of β-cyclodextrin modified Fe3O4 and its catalytic application in Kabachnik–Fields multicomponent reaction. J. Magn. Magn. Mater.. 2015;386:111-116.
- [CrossRef] [Google Scholar]
- Construction and characterization of Fe3O4-Bis[Imine-pyridine]-Cu(OAc)2 nanocomposite: a novel and ecofriendly reusable nanocatalyst for Click synthesis of 1-aryl-1,2,3-triazole derivatives. Polycycl. Aromat. Comp.. 2024;44:607-622.
- [CrossRef] [Google Scholar]
- Highly water-dispersible magnetite nanoparticle supported-palladium–β-cyclodextrin as an efficient catalyst for Suzuki–Miyaura and Sonogashira coupling reactions. RSC Adv.. 2016;6:52656-52664.
- [CrossRef] [Google Scholar]
- Review of the use of the nickel catalyst in azide‐alkyne cycloaddition reactions. Appl. Organomet. Chem.. 2024;38:e7692.
- [CrossRef] [Google Scholar]
- Fe3O4 nanoparticle-bonded β-cyclodextrin as an efficient and magnetically retrievable catalyst for the preparation of β-azido alcohols and β-hydroxy thiocyanate. Res. Chem. Intermed.. 2016;42:511-518.
- [CrossRef] [Google Scholar]
- Magnetic nanoparticles for biomedical purposes: modern trends and prospects. Magnetochemistry. 2020;6:30
- [CrossRef] [Google Scholar]
- Adsorption dynamics of uremic toxins to cyclodextrin-coated magnetic nano-adsorbents. Carbohydr. Polym.. 2024;347:122573.
- [CrossRef] [Google Scholar]
- Green synthesis of imidazoles: the catalytic efficacy of magnetic nanoparticles. Tetrahedron. 2024;167:134246.
- [CrossRef] [Google Scholar]
- Organophotoredox assisted cyanation of bromoarenes via silyl-radical-mediated bromine abstraction. Chem. Commun.. 2020;56:4240-4243.
- [CrossRef] [Google Scholar]
- Amino alcohol-modified β-cyclodextrin inducing biomimetic asymmetric oxidation of thioanisole in water. Carbohydr. Res.. 2012;354:49-58.
- [CrossRef] [Google Scholar]
- An arrangement of β-cyclodextrin chitosan supported on magnetic graphene oxide and its application for in-vitro drug delivery. Int. J. Biol. Macromol.. 2023;246:125696
- [CrossRef] [Google Scholar]
- Synthesis and therapeutic potential of imidazole-containing compounds. BMC Chem.. 2021;15:1-69.
- [CrossRef] [Google Scholar]
- Reusable nano catalyzed synthesis of heterocycles: an overview. ChemistrySelect. 2022;7:e202103900
- [CrossRef] [Google Scholar]
- β-Cyclodextrin modified with magnetic nanoparticles noncovalently for β-naphthol removal from wastewater. Syn. React. Inorg. Metaorg. Nanometal Chem.. 2016;46:143-146.
- [CrossRef] [Google Scholar]
- Introduction and general overview of cyclodextrin chemistry. Chem. Rev.. 1998;98:1743-1754.
- [CrossRef] [Google Scholar]
- Magnetic labeling, detection, and system integration. Biosens. Bioelectron.. 2008;24:1-13.
- [CrossRef] [Google Scholar]
- Nanotechnology: the technology for the twenty‐first century. Foresight. 2004;6:364-370.
- [CrossRef] [Google Scholar]
- An easily fabricated palladium nanocatalyst on magnetic biochar for Suzuki–Miyaura and aryl halide cyanation reactions. New J. Chem.. 2021;45:12519-12527.
- [CrossRef] [Google Scholar]
- Recent progress in the chemistry of multicomponent reactions. Pure Appl. Chem.. 2001;73:187-191.
- [CrossRef] [Google Scholar]
- Efficient cyanation of aryl halides with K4[Fe(CN)6] catalyzed by encapsulated palladium nanoparticles in biguanidine–chitosan matrix as core–shell recyclable heterogeneous nanocatalyst. Polyhedron. 2019;159:212-216.
- [CrossRef] [Google Scholar]
- Recent advances in the application of magnetic nanocatalysts in multicomponent reactions. RSC Adv.. 2023;13:20530-20556.
- [CrossRef] [Google Scholar]
- Fabrication of thermo-responsive polymer functionalized reduced graphene oxide@Fe3O4@Au magnetic nanocomposites for enhanced catalytic applications. J. Mater. Chem. A. 2017;5:5088-5097.
- [CrossRef] [Google Scholar]
- Facile fabrication of water-soluble magnetic nanoparticles and their spherical aggregates. Chem. Mater.. 2007;19:4087-4091.
- [CrossRef] [Google Scholar]
- Rapid one-pot synthesis of magnetically separable Fe3O4–Pd nanocatalysts: a highly reusable catalyst for the Suzuki-Miyaura coupling reaction. Dalton Trans.. 2022;51:11485-11490.
- [CrossRef] [Google Scholar]
- Synthesis, antitumor activity, and cytotoxicity of 4-substituted 1-benzyl-5-diphenylstibano-1H-1,2,3-triazoles. Bioorg. Med. Chem. Lett.. 2018;28:152-154.
- [CrossRef] [Google Scholar]
- Recent advances in the synthesis of aryl nitrile compounds. Adv. Synth. Catal.. 2017;359:4068-4105.
- [CrossRef] [Google Scholar]
- Multicomponent reactions (MCR) in medicinal chemistry: a patent review (2010-2020) Expert Opin. Ther.. 2021;31:267-289.
- [CrossRef] [Google Scholar]
- Aziridines and Epoxides in Organic Synthesis. John Wiley & Sons; 2006. doi: 10.1002/3527607862
- Catalytic Kabachnik-Fields reaction: new horizons for old reaction. ARKIVOC. 2008;1:1-17.
- [CrossRef] [Google Scholar]
- High-efficiency and magnetically separable nanocatalyst: β-cyclodextrin modified core-shell hybrid magnetic nanoparticles. J. Incl. Phenom. Macrocycl. Chem.. 2017;87:45-51.
- [CrossRef] [Google Scholar]







