Translate this page into:
The biological response of Carica papaya leaves extract to saponin reduction (O/W) emulsion on human bronchial epithelium cell (BEAS-2B)
⁎Corresponding authors. saiful-z@ukm.edu.my (Saiful Irwan Zubairi), zainun@ukm.edu.my (Zainun Nurzahim)
-
Received: ,
Accepted: ,
This article was originally published by Elsevier and was migrated to Scientific Scholar after the change of Publisher.
Peer review under responsibility of King Saud University.
Abstract
Carica papaya leaf has a potentially well-known therapeutic effect in accelerating human blood platelet counts against dengue fever and dengue haemorrhagic fever. However, consuming the extract was considered troublesome due to its bitter taste. The fresh papaya leaves were extracted into two types of preparation: a) Fresh Papaya Leaves Extract (FPL) and b) Papaya Leaves with Saponin Reduction Extract (PLSR). This was followed by the determination of the best edible O/W emulsion formulation of both different extracts with virgin coconut oil (VCO) and whey protein (WP) as surfactant. Through Ternary Phase Diagram (TPD), the optimum ratio (w/w) of FPL/PLSR: VCO: WP were 63: 16: 21 and 65: 16: 19 respectively. Both formulas were examined for their physicochemical properties including pH, creaming index (CI), contact angle and droplet size measurement. The human bronchial epithelium cell (BEAS-2B) was treated using both emulsions for 72 hrs of cell growth response (EC50). The result shows that both FPL and PLSR formulations were slightly acidic and exhibited stable emulsion with no creaming formation (CI) up to 24 hrs of storage (25 ℃). Next, FPL emulsion shows 3 times higher wettability and 4 times bigger nanoparticle size than PLSR. These properties can affect the emulsion absorptivity in the targeted cell microenvironment. Remarkably, the BEAS-2B cell viability (%) for each emulsion was relatively elevated within 24 hrs and increased to more than 100 % at 48 and 72 hrs of exposure. This might hugely represent its potential in repairing damaged blood vessels due to dengue haemorrhagic fever. Besides, the EC50 value also indicated low levels of concentration needed to exponentially increase cell growth and safe for dengue fever treatment. For that reason, the recommended effective dosage by the Ministry of Health (Malaysia) (MOH) for both FPL and PLSR emulsions is two tablespoons twice a day for three consecutive days of treatment (equally to the effective dosage of 102 g extract).
Keywords
Papaya leaves
Ternary phase diagram
Emulsion
Cell response
Effective dosage
Dengue fever treatment
1 Introduction
In Malaysia, the spread of the dengue virus has been widely reported, with 80,014 cases of dengue with 142 deaths in 2017 (Ministry of Health, Malaysia, 2015). The spread of the virus carried by the Aedes aegypti mosquito is rapidly spreading as if urging more studies to be carried out to overcome it (Subenthiran et al., 2013). However, to date, no vaccine nor any specific therapy has been prescribed to prevent the spread of this dengue virus (Bhatt et al., 2013). Hence, Carica papaya leaf or known as papaya leaf is one of the most suitable candidates and popular medicinal herbs used in traditional and modern medicine to treat dengue fever. Typically, mature papaya leaves are readily grown in the fourth month of their growth with mature leaves lasting for three to six months under tropical weather conditions (Saeed et al., 2014). In addition, the papaya leaf extract is rich in phytonutrients (mainly polyphenolic compounds) and important trace minerals such as high carbohydrate, calcium, magnesium, sodium, potassium and manganese (Ali et al., 2011). Worth noting is that papaya leaves extract exhibits larval and pupinid characteristics to fight against chikungunya caused by Aedes aegypti (Subenthiran et al., 2013; Subramaniam et al., 2012).
Pharmacological studies have discovered that the leaves display numerous biological properties (therapeutic agents) such as antioxidant, anti-inflammatory, antiviral, antitumor and antibacterial activities (Khor et al., 2021). The leaves contain numerous bioactive constituents that mainly contribute to the defence against oxidative stress such as manghaslin, clitorin, rutin, nicotiflorin, papain, chymopapain, cystatin, α-tocopherol, σ-coumaric acid and caffeic acid (Subenthiran et al., 2013; Khor et al., 2021). In addition, the efficacy of those bioactive components has shown an incrementing number of white blood cells within 24 hrs after the papaya leaf extract (containing three standardized main markers: manghaslin, clitorin and rutin) was ingested by the 111-intervention group patients who were diagnosed with dengue fever (Subenthiran et al., 2013). In fact, it was effective in increasing the platelet count of dengue fever patients during the intake period of three consecutive days. Not to mention, the efficiency of papaya leaves extract in treating dengue patients was also supported by various extensive studies (Yunita et al., 2012; Siddique et al., 2014).
However, the presence of bitter taste in papaya leaves is often a major challenge for consumers to take it as an additional supplement. The presence of saponins contributes to the bitterness of papaya leaves (Aldin, 2006). Saponins are classified based on their chemical structure into three main groups, namely, triterpenoids (e.g., aescin), spirostanol, and furostanol saponins. However, there are other classifications based on the structure of the saponin genin (aglycone), namely, triterpenes glycosides, steroid glycosides, and steroid alkaloid glycosides (Vincken et al., 2007). The adsorption process is a process of separation of a material removed from a single phase through its method of accumulation on the surface of the adsorbent (Gökmen & Serpen, 2002). The ion conversion process can occur under two conditions, namely, through the separation of all types of ionic charges (positive or negative charge) of the mixture or the separation of molecular types that carry the same charge (Nielsen & Prather, 2009). The adsorption method of the low-cost ion exchange resin Diaion® WA30 can successfully reduce the bitter saponin component of papaya leaves without affecting human health (Syed Amran et al., 2018; Gu et al., 2014). It is a type of ion exchange resin that is porous but has a high resistance to osmotic pressure.
The mixing of these two insoluble liquids makes the emulsion thermodynamically unstable because the emulsion is a metastable dispersion that requires shear forces from the outside to break up large liquid droplets into small droplets in the emulsification process (Khor et al., 2014). Tertiary Phase Diagram (TPD) method is widely used in emulsion production technology, which involves the presence of emulsifiers, for example, in the manufacture of ice cream and cosmetic products. It is one of the methods adopted in obtaining the best formulation and stability of a mixture of several chemical compounds thermodynamically (Siti Salwa & Siti Zulaika, 2015). Toxicity testing is the basis for the extent to which a substance or product is safe to consume in the body. It explains the mechanism of toxin response that may occur in body cells while giving a good picture of the pathogen activity for some diseases. BEAS-2B cells are normal epithelial cells of the human lung obtained from autopsies of non-cancerous individuals (Nguyen et al., 2016). Bronchial epithelial cells are isolated from the epithelial lining of the airways above the bifurcation of the lungs. They are considered part of the lower respiratory tract that includes the larynx, lungs, bronchioles and trachea. This fibroblast type cell mimics the formation and growth of blood vessel connective tissue via endothelial cells signal. For that reason, the innovative approach of this study was to modulate the potential of papaya leaves with saponin reduction extract (PLSR) nanoemulsion that reduces bitterness before consumption and without the saponin reduction of fresh papaya leaves extract (FPL) nanoemulsion in repairing damaged blood vessels due to dengue haemorrhagic fever through the usage of normal fibroblast phenotype. Hence, the objective of this study was to determine the optimum emulsion formulations for both PLSR and FPL through a ternary phase diagram with regards to its effective concentration (EC50) that could potentially enhance cell responses/growth against normal fibroblast human lung epithelial BEAS-2B cells.
2 Materials and methods
2.1 Materials
2.1.1 Carica papaya leaves
The papaya (Carica papaya L. cv. Eksotika) leaves samples were taken from a papaya orchard located in Bukit Changgang, Dengkil, Selangor. Only a mature green papaya leaf with no insect damage and insecticide-free was used to ensure consistency of the emulsion preparation and healthier cell culture response.
2.1.2 Chemicals
The chemicals used in this study are as follows: Premium virgin coconut oil (VCO) brand of Bio-Asli from Desaku Maju Marketing; Filtered Prime Whey Isolate from Lush Protein Armor ASIA, Singapore; Diaion® WA30 ion exchange resin (Sigma-AldrichTM, Germany) and BEAS-2B of the human bronchial epithelial cell line was taken from Advanced Medical and Dentistry Institute (AMDI), Universiti Sains Malaysia (USM). In addition, xanthan gum (Sigma-AldrichTM, Malaysia; Catalogue No.: G1253); deionized water (DIW); methylene blue (Sigma-AldrichTM, Malaysia; Catalogue No.: 03978); PrestoBlueTM MTS (ThermoFisher Scientific, InvitrogenTM; Catalogue No.: A13261); Dulbecco's Modified Eagle's Medium (DMEM) (Sigma-AldrichTM, Malaysia; Catalogue No.: D8437); Trypsin-EDTA solution (Sigma-AldrichTM, Malaysia; Catalogue No.: T4049); Phosphate Buffer Saline (PBS) (Sigma-AldrichTM, Malaysia; Catalogue No.: P5493); 1 % (v/v) streptomycin (Sigma-AldrichTM, Malaysia; Catalogue No.: S6501); 100 μg/mL penicillin (Sigma-AldrichTM, Malaysia; Catalogue No.: P3032) and Trypan Blue Solution, 0.4 % (Gibco®, ThermoFisher Scientific; Catalogue No.: 15250061) were used for TPD emulsion preparation, physicochemical and cytotoxicity analysis.
2.2 Papaya leaves extraction
2.2.1 Fresh papaya leaves extract (FPL)
The fresh papaya leaves were extracted according to the Ministry of Health (2015), Nguyen et al. (2016) and Halim et al. (2011) recommendations. Freshly cut papaya leaves were soaked in water for 30 mins, rinsed, air-dried (30 mins) and crushed using mortar. It was then centrifuged using a Gyrozen centrifuge model GZ-406 (BioAstrum Corporation, Maryland, USA) for 5 mins at RCF: 1,006 × g (10 cm of rotor radius). The upper supernatant was collected and stored at −18 °C for further use.
2.3 Papaya leaves with saponin reduction extract (PLSR)
Prior to the extraction process, the fresh papaya leaves were freeze-dried for 48 hrs using a Labconco FreeZone 2.5 Liter (-50 °C) benchtop freeze dryer. It was then ground into a fine powder using an MX-895 M blender prior to the water extraction method (slight modification through response surface optimization) at 80 ˚C with a solvent-to-solid ratio of 20: 1 (mL/g) for 25 mins (Vuong et al., 2015). Then, the PLSR extract was prepared using an ion-exchange resin method (Syed Amran et al., 2018). The extract was shaken for 5 hrs at room temperature with the immersion of 10 % (w/v) WA30 resin. The final extracts were finally filtered via filter paper and kept at −18 ˚C.
2.4 Detection of saponin compound (Aescin) using high-performance liquid chromatography (HPLC)
The detection and quantification of saponin compound before and after treatment of WA30 Low-Ionized Ion Exchange Resin extract were performed using the Reverse Phase (RP)-HPLC (WatersTM) method for comparison of fresh extract samples (without weak ion exchange resin treatment). The detection was carried out based on Sharifah Nurjannah et al. (2018), Ahn & Kim (2005) and Ahmed & Wang (2015) methods with slight modifications. The specifications and conditions of saponin detection in the extracts using high-performance liquid chromatography are as follows: a) Column: Chromolith performance RP-18e; b) detector: UV–vis; c) wavelength 210 nm; operational temperature: 23 ± 1 °C; d) mobile phase (pump A): Deionized water + 0.1 % (v/v) acetic acid; e) mobile phase (pump B): Methanol (HPLC grade); f) flow rate 1.0 mL/min; g) elution mode: Binary gradient and h) injection volume: 20 μL. The analytical standard of aescin was used as a comparison between the treated extract of WA30 ion converter and fresh papaya leaf extract without resin treatment through the external standard method.
2.5 Constructing ternary phase diagram (TPD)
2.5.1 Designing a pseudo-ternary phase diagram
Construction of TPD was performed based on the combined protocols of David & Mary (2019), Wahgiman et al. (2020) and Fadzillah et al. (2020). The first batch of TPD was made with a combination of virgin coconut oil (VCO) and whey protein (WP) in the range of 0: 100 to 100: 0 (VCO: WP (w/w)) is shown in Table 1. These mixtures were then vortexed using Velp Scientifica Vortex Mixture, F202A0173 ZX Classic (Usmate Velate (MB), Italy) for 10 mins for the homogenization process. The theoretical weight is the amount of weight that should be applied to the formulation. Whereas the actual weight is the total weight of the emulsion compound successfully added by approaching the theoretical weight value. Meanwhile, the ‘c’ value was used for the next additional step of deionized water into each test tube. The amount of deionized water to be added (g) into each of these test tubes is calculated using Equation (1) below:
Test tube
Ratio (w/w, %)
Theoretical weight (g)
Actual weight (g)
C = Ce + Cf (g)
VCO
WP
VCO
WP
VCO (Ce)
WP (Cf)
1
100
0
0.5000
0.0000
Ce1
Cf1
C1
2
90
10
0.4500
0.0500
Ce2
Cf2
C2
3
80
20
0.4000
0.1000
Ce3
Cf3
C3
4
70
30
0.3500
0.1500
Ce4
Cf4
C4
5
60
40
0.3000
0.2000
Ce5
Cf5
C5
6
50
50
0.2500
0.2500
Ce6
Cf6
C6
7
40
60
0.2000
0.3000
Ce7
Cf7
C7
8
30
70
0.1500
0.3500
Ce8
Cf8
C8
9
20
80
0.1000
0.4000
Ce9
Cf9
C9
10
10
90
0.0500
0.4500
Ce10
Cf10
C10
Where,
a = Total weight of deionized water to be added (g).
d = Deionized water added for each round (%, w/w).
c = Actual weight of VCO (Ce) (g) + actual weight of WP (Cf) (g).
The deionized water was added drop by drop into the test tube for each round until it reaches the total weight of water. Then, each test tube was vortexed for 5 mins and centrifuged for 20 mins at RCF: 1,006 × g (10 cm of rotor radius). The layer formation was monitored and only homogeneous emulsions were accounted for in the next step. Each edible O/W emulsion formulation that has successfully formed a homogeneous phase was recorded into CHEMIX School 3.51 (Bergen, Norway) software to generate a pseudo-TPD profile to get an initial depiction of the emulsion behaviour in the formulation mixture.
2.5.2 Ternary phase diagram generation
The above-mentioned steps in Section 2.5.1 were repeated by substituting the deionized water (DIW) component with the FPL and PSLR extracts following to the previous emulsion mixture ratios that have reached a homogeneous phase. The emulsion ratio was obtained from the previous pseudo-TPD data distribution as a working baseline (working template).
2.5.3 Extensive examination of the ternary phase diagram
Further tests were carried out by dropping 10 drops of homogenous TPD formulation with surfactants containing 30 % (w/w) or less at random. A total of 20 g of the selected emulsion was added for each formulation and homogenized using vortex for 15 mins, followed by a homogenizer (T10 Basic Ultra-Turrax homogenizer) for 10 mins at RCF: 1,006 × g (10 cm of rotor radius). After 30 mins of storage, the emulsion stability results were observed, and the best FPL and PLSR extract emulsion formulation were added with 0.2 % (w/w) xanthan gum to increase their stability and shelf-life.
2.6 Physicochemical analysis
Once the best FPL and PLSR extract emulsion formulation was developed and identified through the TPD system, the physicochemical properties and storage stability (creaming index) characteristics were carried out prior to cell toxicity studies.
2.6.1 pH analysis
The pH value of the emulsion was determined at 25 °C using a pH meter, PHM 210 analytical radiometer (MeterLab®: LabX, Midland, ON, Canada). The pH meter was calibrated at pH values of 7.01, 4.01, and 10.1 buffer before reading. The reading was taken in triplicate (n = 3).
2.6.2 Creaming index
The creaming index was determined based on the method used by Tangsuphoom & Coupland (2008). Approximately 10 g of the emulsion sample were added into a universal bottle and sealed tightly. It was stored at 25 °C for 24 hrs. The overall height of the emulsion and the height of the bottom layer of droplets formed were calculated based on Equation (2) in triplicate (n = 3):
HL: The height of the droplet layer formed at the bottom.
HE: Total height of emulsion.
2.6.3 Contact angle
The contact angle was measured using a Rame-hart model goniometer (Model 190, USA). Approximately 100 μL of the emulsion was sessile dropped onto a borosilicate glass slide and analysed using a height-width method to measure its angle. The reading was measured in quintuplicate (n = 5) in several different places for consistency.
2.6.4 Particle size measurements
Emulsion particle size was measured based on the method used by Wahgiman et al. (2020) with slight modification using a light scattering laser device (Malvern Zetasizer Ver. 6.00) at 25 °C. and a dynamic light scattering technology. The emulsion extract was first filtered by a 0.45 µm membrane filter. Then, 0.75 mL of the emulsion was inserted into a zeta-sizer device. The intensity distribution was used for mean average (z-average) particle size measurement. The measurement was repeated in triplicate (n = 3).
2.7 Determination of cell toxicity
Prior to the toxicity analysis, three types of samples which were fresh papaya leaf extract (FPL: positive control) emulsion, papaya leaves saponin reduction extract (PLSR) emulsion and whey protein solution (positive control) was filtered using a sterile 0.22 µm polyvinylidene fluoride (PVDF) filter (Merck Millipore, Germany). The samples were then diluted into five concentrations series of 10, 50, 100, 500, and 1000 ppm or equivalent to log(concentration) of 1.00, 1.699, 2.00, 2.699 and 3.00 ppm respectively with a complete serum-free growth media of Dulbecco’s Modified Eagle’s Media (DMEM) (Sigma-AldrichTM, Malaysia; Catalogue No.: D8437) with the inclusive of 1 % (v/v) streptomycin and 100 μg/mL penicillin. The human lung epithelial cells (BEAS-2B) were used to observe the effect of both emulsions on the normal cell response as either inducing cell proliferation or broad cell death (necrosis). The BEAS-2B cell growth was performed by injecting 1 × 104 cells into the 96-wells of the microplate along with a complete growth media. Then, the plate was placed in an incubator for 24 hrs at a temperature of 37 ⁰C, and 5 % CO2.
2.7.1 Cell viability using diphenyltetrazolium bromide (MTT) assay
For the cell toxicity analysis, about 100 µL of the emulsion was added and swirled evenly onto the entire space of BEAS-2B cells. The microplates were then incubated for 24 hrs at 37 °C and 5 % CO2. Approximately 100 µL of DMEM growth media was set as negative control and a mixture of cells and growth media without extract was set as the positive control. The cell viability (%) was carried out based on the method used by Nguyen et al. (2016). Approximately 10 µL of PrestoBlueTM solution was added to the microplates that had been treated with emulsion solution and incubated for 24 h at 37 °C and 5 % CO2. The absorbance for emulsion solubility was measured at 570 nm every 24 hrs using the FluoStar Absorbance Microplate Reader (BMG Labtech, Germany) in triplicate (n = 3) and measured using Equation (3) below. The data was then used to calculate EC50 values.
The cell morphology was then observed using a BD-S2 Inverted Biological Microscope (Boshida® optical instrument, Shenzhen, China) to analyse any changes throughout 72 hrs of treatment. Methylene blue was used to stain adherent cells, cultured in 96 well plates to distinguish any dead cells prior to cell morphology analysis.
2.7.2 EC50 value measurement
EC50 is an effective concentration of a chemical compound that is tested on cell culture. The EC50 value of both emulsions and WP was determined based on the cell viability (%) absorbance readings taken at 24, 48, and 72 hrs. This value is determined based on the calculation of the equations obtained from the cell viability (%) versus concentration (log; µg/mL), where the y-value of y = 50 % cell viability.
2.8 Statistical analysis
Ternary Phase Diagram (TPD) System was generated using CHEMIX School 3.51: Ternary System Diagram software version 7.0 (Arne Standness, Bergen, Norway). The Minitab® software version 17.0 (Minitab® Inc., Sydney, Australia) was used to determine the significant difference between all samples (p < 0.05) (average ± standard deviation) through t-test (physicochemical analysis), ANOVA (cell viability kinetic profiles) and Fisher test to determine the significant difference between all formulated samples.
3 Results and discussion
3.1 Detection of saponin compound (Aescin) using high-performance liquid chromatography (HPLC)
The detection of saponin compound in papaya leaf extract using HPLC was a confirmatory test to determine the adsorption efficacy of WA30 weak ion resin treatment. Chromatogram profiles of aescin analytical standard were compared with fresh papaya extract (without resin treatment) and resin-treated extract via the external standard method. Based on the prior work, the standard tea extract containing saponin showed two visible peaks at 19 and 21 mins of its retention point (Ahmed & Wang 2015). In the present work (slight modifications), the presence of saponin (aescin) in fresh papaya extract was confirmed to peak at 25.91 ± 1.03 mins (Fig. 1(b)) which was almost the same as standard aescin (Fig. 1(a)). While Fig. 1(c) shows the absence of saponin peak after the treatment with WA30 ion exchange resin. The initial selection of a good resin material of reducing the extract’s bitter taste was primarily introduced using Amberlite RA-67 resin (Noraziani et al., 2016). However, the treatment has resulted in a significant decrease in antioxidant activity (p < 0.05) because a lot of polyphenolic compounds containing (e.g., three standardized main markers: kaempferol (manghaslin) and quercetin (clitorin and rutin)) (Norahmad et al., 2019) in the extract might have been adsorbed throughout the Amberlite RA-67 resin treatment. Therefore, an extensive comparative study of numerous ion exchange resins was carried out between the Amberlite IRA-67, Diaion WA30 and Diaion WA21J with regards to its adsorbability. The highest and lowest saponin adsorption efficiency was recorded for Diaion WA30 and Amberlite IRA-67 ion exchange resin with 97.50 ± 0.25 % (w/w) and 75.71 ± 0.16 % (w/w) respectively (Abidin et al., 2016). Hence, the absence of saponin peak in the present work indeed corresponds to the prior work that produces a satisfactory resin adsorbability of more than 95 %.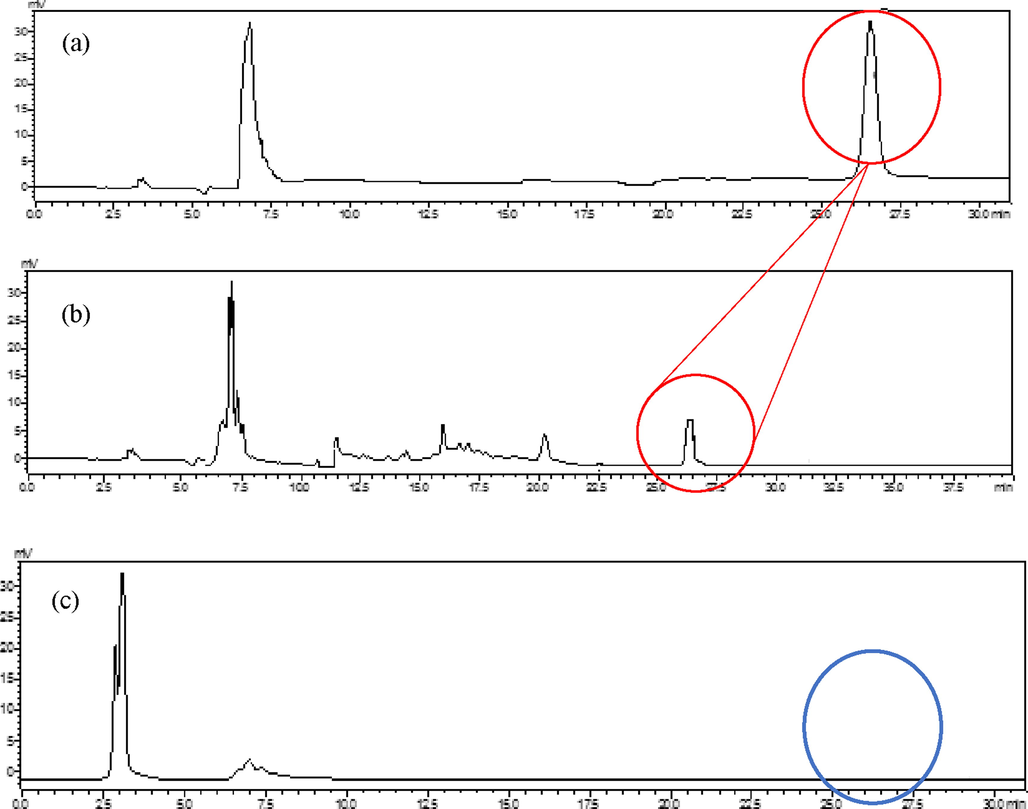
HPLC Chromatogram at 210 nm UV detector of (a) Analytical standard aescin (red circle) at 100 mg/mL concentration; (b) fresh papaya leaves extract without free ion exchange resin treatment and visible aescin detection; (c) fresh papaya leaves extract through 10 % (w/v) Diaion® WA30 ion exchange resin treatment with undetected aescin (blue cycle).
3.2 Pseudo-ternary phase diagram profiles
The pseudo-ternary phase diagram shows that the formation of the isotropic liquid region depends on the hydrophilicity and lipophilicity of the surfactant. Fig. 2 shows the best pseudo-ternary diagram with a shaded grey area that represents a homogenous region for the emulsion mixture of virgin coconut oil (VCO), surfactant (WP) and deionized water (DIW). It was observed that the combination of WP and VCO gives a wide ratio of solution strength. Besides, the active ingredient contained in WP successfully coats the oil molecule and produces an effective dispersion. The addition of DIW also helps the distribution of surfactant molecules to form a homogeneous solution through external physical shear support such as vortex or sonication. The homogeneous phase distribution is most noticeable in the middle of the diagram that represents a uniform formulation ratio between surfactant, oil and deionized water. The emulsion phase change was observed as it approaches the corner for each emulsion component. Currently, the formulation ratio tends to increase in some components only and this area, most formulations begin to produce a thin white layer on the top of the emulsion after 30 mins of storage. Meanwhile, a stable emulsion was selected based on the formulation’s visual observation of its homogeneous phase. The selected homogeneous area distribution was used as a guideline for the ultimate TPD emulsion profiles by replacing the DIW with FPL and PLSR extract.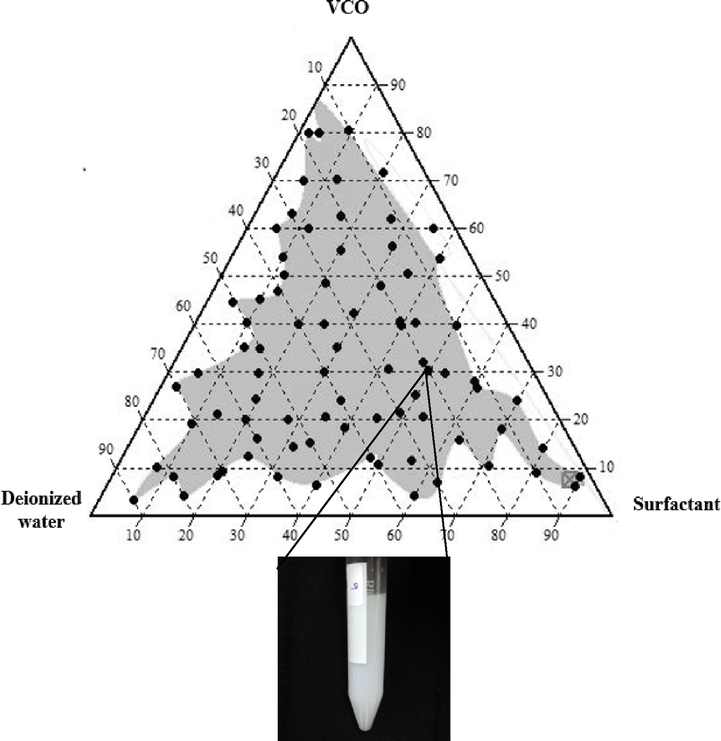
Pseudo-ternary phase diagram of oil and surfactant mixtures with addition of deionized water (DIW) with a wide range of homogenized mixture formation (grey area). The inset picture shows the visual formation of a randomly selected homogenized mixture after 30 mins of storage.
3.2.1 Fresh papaya leaves extract (FPL) emulsion formulation
In this study, the formulation of a mixed VCO, WP and papaya leaf extract was produced based on the ternary phase diagram (TPD) method. The FPL formulation serves as a control formulation (positive) and this formulation is considered the standard extract preparation based on the Ministry of Health (MOH), Malaysia guidelines. Table 2 shows ten emulsion formulation points which were randomly selected from the TPD homogenized region. Fig. 3 shows the TPD profiles generated from the mixture of FPL emulsion formulations. The grey region shows an emulsion formulation reaching a homogeneous phase after being homogenized and stored for 30 mins. The homogenized emulsion formation was found to be concentrated in the range of 30 % to 90 % (w/w) of the extract concentrations. The VCO and WP concentration was set to be<40 % (w/w) and 30 % (w/w) respectively to produce emulsions with moderate viscosity (Sanjeewani & Sakeena, 2013). The emulsions that produce several phases of emulsion were excluded from this observation. Based on those ten formulations tested, formulation #4 (Fig. 3: H point) showed the best emulsion stability characteristics as compared to the other formulations. At this formulation ratio of 21: 16: 63 % (w/w) (VCO: WP: FPL), it has produced a well-blended and homogenized emulsion that did not produce any immiscible layers after more than 30 mins of storage.
Formulation
VCO (g)
WP (g)
FPL (g)
VCO: WP: PLSR (%, w/w)
1
3.50
2.00
4.50
27: 15: 58
2
3.50
3.00
3.50
25: 15: 60
3
2.50
3.00
4.50
23: 16: 61
4
2.40
2.70
4.90
21: 16: 63
5
2.00
2.00
6.00
20: 18: 62
6
2.00
2.50
5.50
19: 24: 57
7
1.50
3.00
5.50
17: 24: 59
8
1.50
2.50
6.00
15: 25: 60
9
1.00
3.00
6.00
13: 27: 60
10
1.00
2.50
6.50
9: 30: 61
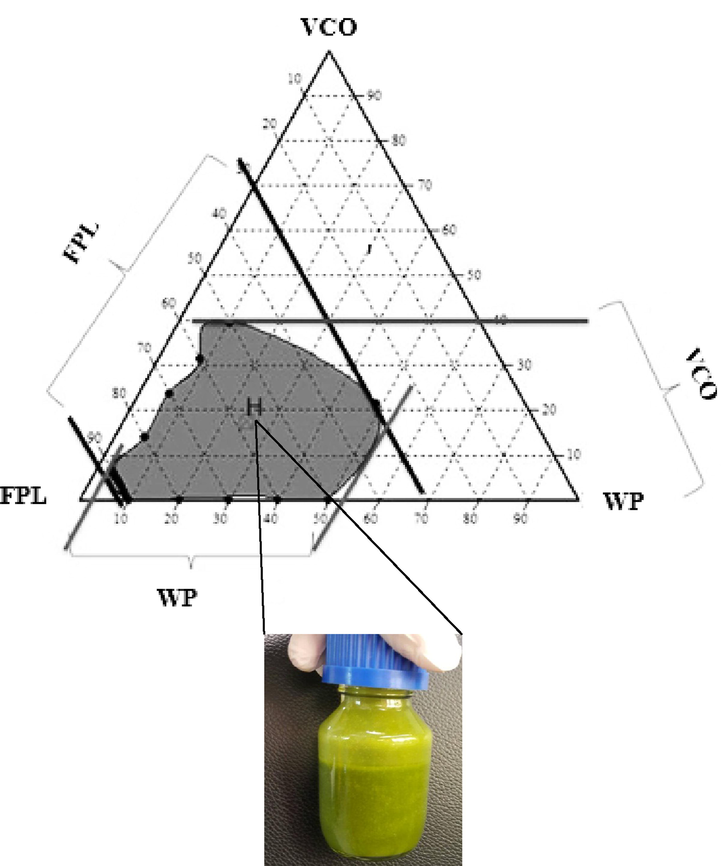
Ternary phase diagram for fresh papaya leaf (FPL) of ten emulsion formulation points which was randomly selected from the homogenized region of TPD (grey region). Formulation #4 (H point) was selected prior to physicochemical and cell response analysis.
3.2.2 Papaya leaves with saponin reduction extract (PLSR) emulsion formulation
At the beginning of the phase formation, the WP was limited to 30 % (w/w) and below. However, the emulsion formulations were randomly assigned to a few points of the increment (WP) to see a full spectrum of formulation behaviour. It was observed that this formulation mixture produces better and broader emulsion stability. Initially, the VCO maximum amount was used (100 %, w/w) with only 60 % (w/w) of WP without the presence of PLSR extract. Next, the highest PSLR extract was used (90 %, w/w) with 9 % (w/w) of WP without the presence of VCO. Finally, the highest WP was utilized (90 %, w/w) with 10 % (w/w) of VCO without the presence of PLSR extract. Ultimately, the construction of the TPD profiles was completed with the generation of a homogenized grey region (stabilized emulsion after 30 mins of storage) that covered almost the entire diagram (Fig. 4). Further analysis was carried out from the homogenized grey region to obtain the best formulation. Table 3 shows ten randomly selected emulsion formulation points and the WP was set below 30 % (w/w) to produce emulsions with moderate viscosity (Sanjeewani & Sakeena 2013). The surfactant (WP) is limited to<30 % (w/w) based on the production cost assessment (economic feasibility study) and health risks factor. High surfactant content (e.g., WP) in any edible food-based formulation can irritate the gastrointestinal tract and serious health issues if taken in a high dosage (Ahmad et al., 2013). Hence, it was observed that formulation #10 (Fig. 4: G point) with a ratio of 19: 16: 65 % (w/w) (VCO: WP: PLSR) shows the best stabilized and well-blended emulsion without any immiscible layer’s formation for more than 30 mins of storage. It was then added with 0.2 % (w/w) xanthan gum to increase its long-term stability and shelf-life.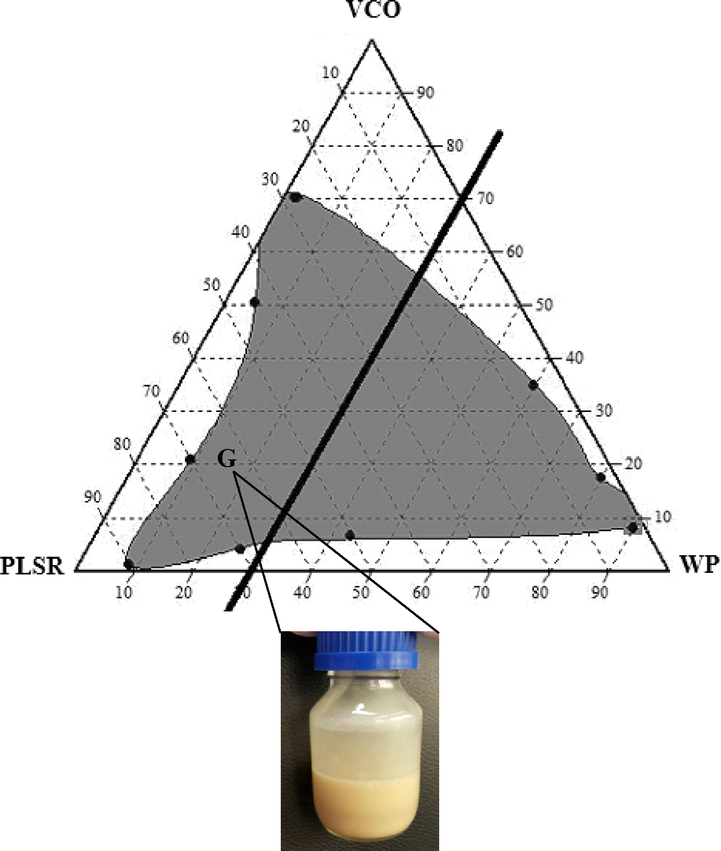
Ternary phase diagram for fresh papaya leaf with saponin reduction (PLSR) of ten emulsion formulation points which was randomly selected from the homogenized region of TPD (grey region). Formulation #10 (G point) was selected prior to physicochemical and cell response analysis.
Formulation
VCO (g)
WP (g)
PLSR (g)
VCO: WP: PLSR (%, w/w)
1
1.00
2.50
6.50
10: 30: 60
2
1.00
3.00
6.00
12: 30: 58
3
1.50
2.50
6.00
13: 28: 59
4
1.50
3.00
5.50
14: 27: 59
5
2.00
2.50
5.50
15: 26: 59
6
2.00
2.00
6.00
16: 24: 60
7
2.40
2.70
4.90
17: 22: 61
8
2.50
3.00
4.50
17: 20: 63
9
3.50
3.00
3.50
18: 18: 64
10
3.50
2.00
4.50
19: 16: 65
Furthermore, based on both FPL and FPSR emulsion formulation profiles, the stability of a protein mixture emulsion depends heavily on the interaction between the protein layers absorbed by the oil molecules. The main thermodynamic absorption force is when the removal of excess hydrophobic molecules occurs by replacing them with water molecules in the aqueous phase (Andrews et al., 2005; Asenjo & Andrew, 2011; Loi et al., 2019). During the homogenization process, the molecular droplets are disturbed due to strong shear forces and form a new surface around the molecules. Currently, WP is required in large amounts to cover the surface of the molecules (McClements, 2004). Realistically, as the usage of WP is<10 % (w/w), the emulsion system shows the formation of heterogeneous emulsion formulations. At this point, the presence of WP is quite insufficient to cover the entire surface of the droplet, but in fact, the droplet is likely to clump and produce larger-sized droplets. Nevertheless, as the WP increased to 15 % (w/w) and above, the emulsion began to show a thinner separation layer, until it reaches a desired homogeneous phase. The presence of WP at this point has successfully covered almost all droplets on the new surface and stabilized the oil droplets in the emulsion system (Qian et al., 2011).
In the presence of high emulsifier content (%, w/w), WP can form several layers covering the emulsion droplets. The formation of several layers can alter the interface rheology of emulsion droplets due to the formation of a stable interface layer that is resistant to the occurrence of re-agglomeration (McClements, 2004). Besides, there is an improvement in droplet molecular composition and the reduction of collision interactions between droplets occurring due to the mobilization of each emulsion droplet in the new emulsion system, causing the emulsion concentration to increase in the continuous phase (Tesch & Schubert, 2002).
3.3 Physicochemical properties and stability of the emulsion
Based on the TPD profiles, the two best emulsion formulations have been successfully developed and selected prior to the BEAS-2B human bronchial epithelial cell bioassay. For the FPL emulsions, the best selected formulation was at #4 (Table 2) with a ratio of 21: 16: 63 % (w/w) (VCO: WP: FPL). Whereas, for the PLSR emulsion, the best-selected formulation was at #10 (Table 3) with a ratio of 19: 16: 65 % (w/w) (VCO: WP: PLSR). Throughout this study, the differences between these two emulsions were studied in terms of their physicochemical properties (e.g., pH value, creaming index, contact angle and particle size measurement) and storage stability (e.g., creaming index) which was summarized in Table 4. a-bDifferent letters in the same column represent significant differences (p < 0.05) between formulations (n = 3).
Sample
pH
Creaming Index (CI)
Contact angle
Z-average Emulsion Droplet Size (nm)
Fresh Papaya Leaves Extract Formulation (FPL) - Positive control
6.85 ± 0.07a
0.00 ± 0.00a
33.7° ± 2.90b
406.07 ± 23.53b
Papaya Leaves with Saponin Removal Extraction Formulation (PLSR)
6.76 ± 0.06a
0.00 ± 0.00a
17.1 ± 0.18a
197.30 ± 56a
3.3.1 The pH profiles
The pH value in Table 4 indicates that both FPL and PLSR extract emulsion was slightly acidic and very near to neutral pH (p greater than 0.05). In fact, the removal of saponin (PLSR) did not attribute to any significant difference as compared to FPL extract emulsion (p greater than 0.05). As these emulsions serve as therapeutic supplements for dengue fever patients, neutral pH is seen to be suitable for promoting cell tissue growth (e.g., fibroblast: blood vessels) and at the same time helping other emulsion components (e.g., VCO and WP) to respond well to the human body microenvironment systems. Besides, excessively acidic or alkaline emulsification conditions can cause emulsion instability and might affect the gastrointestinal tract. However, the VCO pH value shows high acidic when they reach a value of as low as 5.40 due to the VCO's fatty acids content (predominantly containing 48.40 % to 52.80 % of lauric acid) which acts as antiviral, anti-inflammatory and wound healing precursors (Carandang, 2008; Nevin & Rajamohan, 2010; Subermaniam et al., 2014). However, the acidity of the VCO does not affect the pH reading of the homogenized emulsion as it has been stabilized by the presence of the WP.
Furthermore, the phase separation (immiscible layers) of the WP mixture emulsion system can occur at pH 5.0 and below due to the formation of flocculation between proteins (Laplante et al., 2005). Adjusting the emulsion pH to 5.5 and above might have resulted in the formation of stable and smooth lipid droplets. The optimal stability of both emulsions was finally obtained ranging from pH 6.0 to 7.0, where WP synergistic effect might have been the main contributor to the ultimate stabilized emulsion formation. However, protein oxidation (WP) can still happen at pH 4.5 in this VCO-based emulsion and produce separate protein deposits in the emulsion system (Chen & Diosady, 2003). This is because the protein contained in VCO-based emulsion can easily turn into sediment and clots at pH 4.0 (Tangsuphoom & Coupland, 2008). Surprisingly, under this nearly neutral pH emulsion condition, the decomposition of casein (WP) can be prevented from occurring in the protein mixtures emulsion and simultaneously prolong its shelf-life for food-based safety purposes (Bos & Van Vliet, 2001).
3.3.2 Creaming index (CI)
The creaming index was commonly used to predict the emulsion formulation stability. Both nano emulsion mixtures of FPL and PLSR extract formulation produced a creaming index of 0 indicating a very stable emulsion has been successfully developed (p greater than 0.05). In fact, both formulations did not show any apparent separation phases (immiscible layers) which indicates that flocculation and creaming conditions were not produced in this emulsion. The mixture remained homogenous which indicates its high stability at 25 °C for 24 hrs of storage. Thus, the stability of both emulsion formulations was high because the mechanical structure of the emulsion system can withstand the occurrence of gravity deposition and creaming precipitation. However, other emulsion formulations (Table 2 and Table 3) began to show separate oil layer formation in<24 hrs of storage. Cream precipitation can occur when the density of oil droplets is heavier or lighter than the density of the aqueous phase, decomposition of the emulsion composition, gravitational or centrifugal forces and lipid oxidation. Moreover, flocculation can also occur when oil droplet molecules overshared some of the protein molecules in the emulsification process due to the number of protein molecules available is quite insufficient to cover the entire oil–water interface layer (Chanamai & McClements, 2000; McClements, 2004; Thevene, 2006; Tadros, 2010).
3.3.3 Contact angle profiles
The contact angle describes the wetting value of where the liquid–vapour interface meets on an untreated/treated solid flat surface. There was an almost 2-fold decrease in PLSR contact angle values than the FPL extract emulsion (p < 0.05) (Table 4). In fact, both emulsions contact angles were<55.70° (water contact angle on an uncoated micro slide) which indicates that the emulsion particles were mostly hydrophilic-based liquid on the surface of the micro slide glass (Gao et al., 2006). Moreover, a low contact angle indicates that the surface is high in wetting, meaning that the water droplet spreads out more on the surface (Aydar, 2020; Ramlan et al., 2022). Thus, PLSR demonstrated two times higher wetting capability than FPL extract emulsion to indicate that the removal of saponin bioactive compound (aescin) from the extract has changed its hydrophobicity/hydrophilicity profoundly (p < 0.05). Furthermore, FPL was prepared from fresh papaya leaves without the addition of any water or solvent. Unlike PLSR, the presence of high-water content in the extract produces extra intermolecular interactions and repulsive forces between the particles producing lower surface tension between the particles (Tadros et al., 2004; Snoeijer & Andreotti, 2008). Surfaces with high energy interactions will produce better absorption for later mixing and formulating (Muster & Prestidge, 2002; Ramlan et al., 2018).
3.3.4 Particle size measurement
Particle size measurement is used to measure the particle size distribution for each component in an emulsion system. It was observed that the particle size distribution of PLSR was four times smaller than the FPL emulsion formulation (p < 0.05) (Table 4). In fact, both formulations were also categorized as nanoemulsions since the size range is between 100 and 500 nm (Kabri et al., 2011). Nanoemulsion has good suspension stability at −10 to 55 °C, can withstand the physical instability of the emulsion caused by gravity, flocculation and/or coalescence, is easily prepared at low surfactant content of as low as 20 % or less and has a better wettability and absorbability (Tadros et al., 2004; Anton et al., 2008; Khor et al., 2014). The production of nanoemulsions has always been a major selection in any medicinal product development. Those special features are considered an added value to the developed emulsion by enhancing the drug absorbability into the body’s cellular functions. It helps by increasing the efficiency of the drug delivery system to patients which can be taken orally or by injection (Sanjeewani & Sakeena, 2013). For that reason, good characteristics of papaya leaf extract nanoemulsion will eventually help its absorption into targeted cells and biological systems so that any immune responses that help the body to fight those viruses (e.g., dengue virus) can be activated efficiently.
3.4 The EC50 values
The EC50 value is the treatment concentration value that affects the maximum half-reaction. This value is used to observe the effectiveness of a drug/chemical effect at a rapid rate and low dose (Singh et al., 2020). In this study, the determination of EC50 values of both FPL (control), PLSR emulsions and WP (control) were determined based on the cell viability (%) obtained from 72-hr absorbance readings taken every 24 hrs.
The BEAS-2B cells treated with 100 μL of papaya leaf extract emulsion showed different response patterns to five different concentrations (10 to 1000 ppm) of the solution from 24 hrs up to 48 hrs of exposure (p < 0.05) (Fig. 5). The 24-hr absorbance readings showed a consistent upward trend and responses of cell viability for all three samples, of which PLSR and WP were seeming to exhibit viability of more than 50 % at most of the concentration exposure. Meanwhile, all samples started to exponentially increase towards 100 % cell viability (p < 0.05) over the treatment period between 24 hrs and 48 hrs except FPL which was still adapting to its new microenvironment cocktails. This vindicates a good healing property of these emulsion components (with or without saponin in the extract) in which cells proliferated rapidly with no apparent triggered necrosis. Subsequently, the toxicity level was calculated based on the EC50 values obtained from the cell viability (%) kinetic profiles. This value is determined based on the calculation of the equations obtained from the cell viability (%) versus concentration (log; µg/mL), where the y-value of y = 50 % cell viability is shown in Table 5. a-cDifferent alphabet shows significant differences (p < 0.05) (n = 3). *NA: Not Available.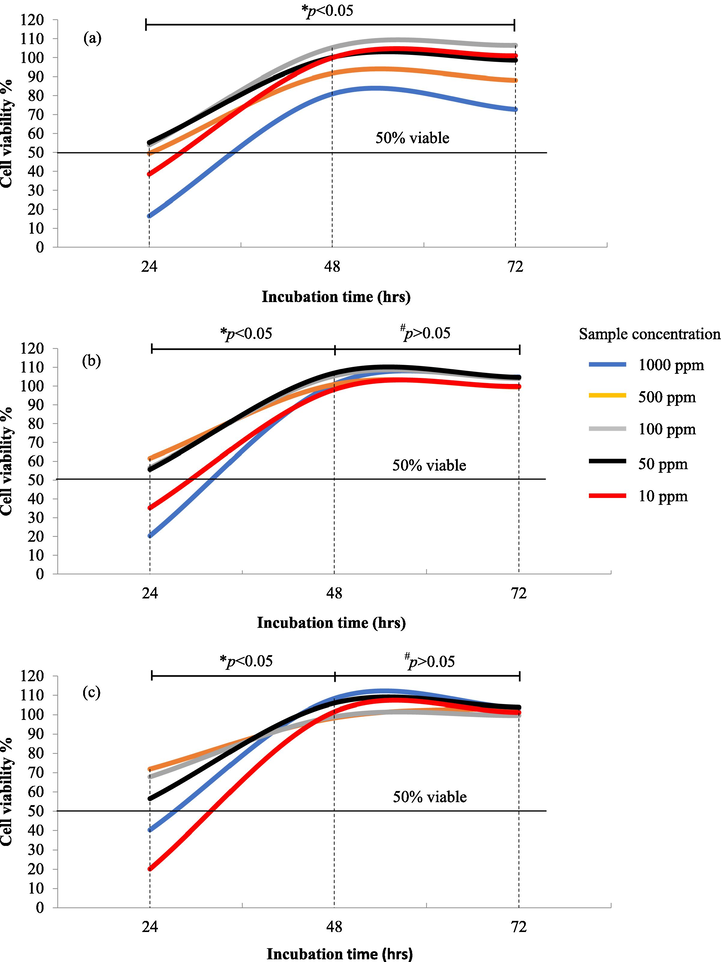
Viability kinetic profiles of (a) Fresh Papaya Leaves (FPL) extract emulsion formulation (positive control); (b) Papaya Leaves with Saponin Removal (PLSR) extract formulation; (c) whey protein (WP) (positive control) in 5 different concentrations (1000, 500, 100, 50, 10 ppm) which ostensibly boost up normal cell growth after 24 hrs of treatment. (*) p < 0.05: Cell viability (%) increased significantly from 24 hrs to 48/72 hrs of treatment in all prepared concentrations (n = 3). (#) p < 0.05: Cell viability (%) remained constant from 48 hrs to 72 hrs of treatment in all prepared concentrations (n = 3).
Time (hrs)
EC50 values (µg/ml)
Fresh Papaya Leaves Extract Formulation (FPL) - Positive control
Papaya Leaves with Saponin Removal Extract Formulation (PLSR)
Whey protein (WP) - Positive control
24
336.28 ± 5.55a
34.07 ± 3.28b
52.10 ± 2.60b
48
271.33 ± 3.25b
NA
NA
72
266.13 ± 3.49c
NA
NA
From Table 5, it was observed that there was a significant difference (p < 0.05) between the FPL-treated cells with the cells treated with PLSR and WP 24 hrs after treatment. The absence of saponin in the PLSR emulsion intensely propelled cell proliferation at low concentrations (<40 ppm) up to 72 hrs of exposure. Likewise, the WP was considered ‘inert’ as it did not affect the cell’s normal growth kinetics. In fact, the EC50 values for PLSR and WP at 48 hrs and 72 hrs were not determined even though cell viability was beyond the 100 % mark which indicates the cells were too overcrowded. At this point, cell proliferation occurs rapidly and permeates every well of the plate as shown in Fig. 6. The overcrowded cells were seeming to stabilize as it reaches 72 hrs of exposure. Prior to treatment, it was observed that the cell loading distribution was noticeable low and minimum cell-to-cell interaction with visible space unoccupied by the cells. However, apparent cell death (black spots: suspended cell fragments) was considered inevitable (Fig. 6 (b and c): yellow circle) after being treated with FPL, PLSR and WP for up to 72 hrs of treatment due to sacred nutrients, overcrowded cells, and lack of spaces to adhere which eventually led to necrosis. In addition, the effectiveness of both raw papaya leaves saponin reduction and normal leaves extracts have been proven to be safe based on prior work (Noraziani et al., 2016), in which the cell viability (IMR90) treated with saponin reduction extract was found to produce more active cell as compared to the normal extract (p < 0.05). This finding supports that the removal of saponin from the extract positively affects the growth of non-cancerous cells. Whereas other available components in the emulsion such as VCO have been proven not to increase cell growth (present work) but good at killing cancerous cells. A study on two human lung cancer cells (NCI-H1299 and A549) has shown a significant cell death after 72 hrs of VCO treatment (Nurul’ain et al., 2015). Hence, this shows that VCO, WP and papaya leaves extract are safe for consumption, possess no adverse effect on normal cell growth, have the potential to treat cancer and essentially can help in repairing damaged blood vessels due to dengue haemorrhagic fever.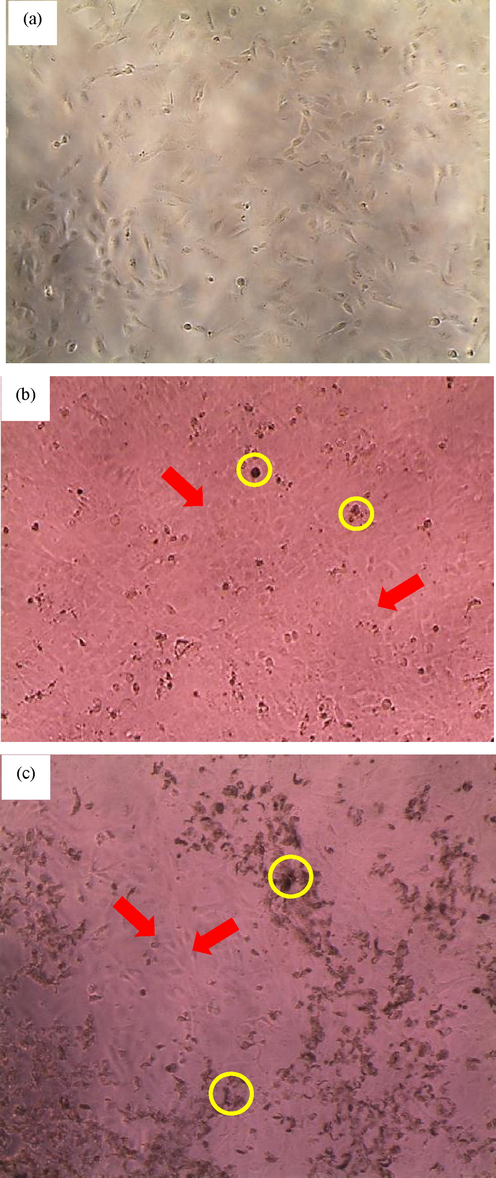
Morphological changes of BEAS-2B cell images at 100x magnification scale for a) before any treatment (negative control); b) cells treated with Papaya Leaves with Saponin Reduction (PLSR) extract emulsion formulation at 48 hrs at 500 ppm; c) cells treated with PLSR emulsion at 72 hrs at 500 ppm. Red arrows: The healthy over crowded fibroblast cells adhered onto glass surfaces. Yellow circles: Suspended dead cells visibly seen in black spots. Lack of nutrients in the growth media and overcrowded spaces could have led to cell death (necrosis).
3.5 Effective dosage of FPL and PLSR emulsion formulation
Based on the Ministry of Health (Malaysia) (MOH) (2015) and Subenthiran et al. (2013), dengue patients were given 2 tablespoons of papaya leaf juice daily for three consecutive days. The weight volume of 2 tablespoons of the extract was estimated to be equal to 34 g, while the weight of one tablespoon of the extract is equal to 17 g. If given on three consecutive days, it will be entirely around 102 g of papaya leaf extract. Hence, the calculation was made based on both emulsion formulations (the best formulation: FPL formulation #4 and PLSR formulation #10) by choosing the composition of the extract ratio in the formulation. As for the FPL extract emulsion, the extract consists of 63 % (w/w), which is equivalent to 10.71 g per tablespoon. Thus, to obtain the quantity of 102 g of papaya leaf extract according to the MOH recommendation, this emulsion should be taken as much as two tablespoons twice a day for 3 consecutive days. Meanwhile, for the PLSR extract emulsion, the extract composition was 65 % (w/w), which is equivalent to 11.05 g per tablespoon. Likewise, this emulsion should also be taken as much as two tablespoons twice a day for three consecutive days, as recommended by the MOH.
4 Conclusion
The optimal edible O/W emulsion formulations of FPL (control) and PLSR were successfully developed based on the ternary phase diagram (TPD) method. The best FPL and PLSR edible emulsion formulation (%, w/w) was generated at 63: 16: 21 (Formulation #4: FPL: VCO: WP) and 19: 16: 65 (Formulation #10: PLSR: VCO: WP) respectively. The physicochemical properties of both FPL and PLSR O/W emulsion formulations produced a slightly acidic condition with stable nanoemulsion textures with non-existence creaming formation at 25 ℃ for 24-hr storage. Next, the wettability of both emulsions was adequately high with the PLSR emulsion containing a much smaller particle size than FPL (p < 0.05), showing a better spreading potential in the cell microenvironment cocktail and superior absorbability of its nano-size emulsion. The biological response of both emulsions through BEAS-2B cell viability (%) was beyond 100 % for 48 hrs of treatment onwards with no apparent ‘knock-off’ effect from the lowest to the highest concentrations used (10 to 1000 ppm) apart from typical cell death due to sacred nutrients and overcrowded cells which led to necrosis. The effective concentration (EC50) to expedite cell growth requires only a low concentration of emulsion ranging from 35 to 350 ppm at a crucial treatment time of within 24 hrs. Hence, the recommended effective dosage for both FPL and PLSR as recommended by the Ministry of Health (MOH), Malaysia effective dosage to reduce dengue haemorrhagic fever (e.g., blood vessel ruptures) is two tablespoons twice a day (ranging from 10 to 11 g/tablespoon with the extract concentrations of 60 to 65 % (w/w)) for three consecutive days.
Acknowledgements
We are grateful to Universiti Kebangsaan Malaysia (UKM) for the financial support (ST-2022-016; ST-2022-21; GUP-2018-057; GUP-2018-080), the Ministry of Higher Education (Malaysia) (FRGS/1/2020/WAB04/UKM/02/4) and the Department of Food Sciences, Faculty of Science and Technology, UKM Bangi for allowing this study to be carried out at the Functional Food and Nutritional laboratory.
Declaration of Competing Interest
The authors declare the following financial interests/personal relationships which may be considered as potential competing interests: Saiful Irwan Zubairi reports financial support, administrative support, article publishing charges, and statistical analysis were provided by National University of Malaysia.
References
- Adsorption of Saponin Compound in Carica papaya Leaves Extract Using Weakly Basic Ion Exchanger Resin. AIP Conf. Proc.. 2016;1784:030039
- [CrossRef] [Google Scholar]
- Construction of pseudo ternary phase diagram and its evaluation: development of self-dispersible oral formulation. Int. J. Drug Develop. Res.. 2013;5(2):84-90.
- [Google Scholar]
- Ahmed, H. O. A. & Wang. C. 2015. Determination of tea saponin in Camellia seed oil with UV and HPLC analysis. World Journal of Engineering and Technology 3(4): 30-37.
- Identification and quantification of steroidal saponins in Polyganatum species by HPLC/ESI/MS. Arch. Pharma. Res.. 2005;28(5):592-597.
- [Google Scholar]
- Bitterness of soy extracts containing isoflavones and saponins. J. Food Sci.. 2006;71(3):S211-S215.
- [Google Scholar]
- Natural products and their active compounds on disease prevention in nutritional and medicinal values of papaya. (Carica Papaya L.): Nova Science Publishers Inc.; 2011. p. :307-324.
- Correlation for the partition behavior of proteins in aqueous two-phase systems: effect of surface hydrophobicity and charge. Biotechnol. Bioeng.. 2005;90(3):380-390.
- [Google Scholar]
- Design and production of nanoparticles formulated from nano-emulsion templates-a review. J. Control. Release. 2008;128(3):185-199.
- [Google Scholar]
- Aqueous two-phase systems for protein separation: a perspective. J. Chromatogr. A. 2011;1218(49):8826-8835.
- [Google Scholar]
- The relationship between the contact angle and some quality parameters of frying oils. La Rivista Italiana Delle Sostanze Grasse J.. 2020;97:17-23.
- [Google Scholar]
- Interfacial rheological properties of adsorbed protein layers and surfactants: a review. Adv. Colloid Interface Sci.. 2001;91(3):437-471.
- [Google Scholar]
- Impact of weighting agents and sucrose on gravitational separation of beverage emulsions. J. Agric. Food. Chem.. 2000;48(11):5561-5565.
- [Google Scholar]
- Enzymatic aqueous processing of coconuts. Int. J. Appl. Sci. Eng.. 2003;1(1):55-61.
- [Google Scholar]
- David J. E & Mary, T. E. 2019. Chemical engineering in the pharmaceutical industry: active pharmaceutical ingredients. 2nd edition 424-426.
- Physico-chemical and sensory acceptance of Carica papaya leaves extract edible O/W emulsion as prospective natural remedies. Arabian J. Chem.. 2020;13:7829-7842.
- [Google Scholar]
- Synthesis and characterization of a novel fluorine-containing polymer emulsion with core/shell structure. J. Fluorine Chem.. 2006;127(2):282-286.
- [Google Scholar]
- Equilibrium and kinetic studies on the adsorption of dark-colored compounds from apple juice using adsorbent resin. J. Food Eng.. 2002;53(3):221-227.
- [Google Scholar]
- Effects of dietary plant meal and soya-saponin supplementation on intestinal and hepatic lipid droplet accumulation and lipoprotein and sterol metabolism in Atlantic salmon (Salmo salar L.) Br. J. Nutr.. 2014;111(3):432-444.
- [Google Scholar]
- Acute toxicity study of Carica papaya leaf extract in Sprague Dawley rats. J. Med. Plants Res.. 2011;5(10):1867-1872.
- [Google Scholar]
- Physico-chemical characterization of nano-emulsions in cosmetic matrix enriched on omega-3. J. Nanobiotechnol.. 2011;9(41):1-8.
- [Google Scholar]
- Chemical composition, antioxidant and cytoprotective potentials of Carica papaya leaf extracts: a comparison of supercritical fluid and conventional extraction methods. Molecules. 2021;2021(26):1489.
- [Google Scholar]
- A comparative study of the physicochemical properties of a virgin coconut oil emulsion and commercial food supplement emulsions. Molecules. 2014;19(7):9187-9202.
- [Google Scholar]
- Effect of pH, ionic strength, and composition on emulsion stabilising properties of chitosan in a model system containing whey protein isolate. Food Hydrocolloids. 2005;19:721-729.
- [Google Scholar]
- Protein-stabilised emulsions. In: Melton L., Shahidi F., Varelis P., eds. Encyclopedia of Food Chemistry. Oxford Academic Press; 2019. p. :404-409.
- [Google Scholar]
- Protein-stabilized emulsions. Curr. Opin. Colloid Interface Sci.. 2004;9(5):305-313.
- [Google Scholar]
- Ministry of Health (MOH). 2015. Management of dengue infection in adults. 3rd Edition. Ministry of Health. Malaysia
- Application of time-dependent sessile drop contact angles on compacts to characterise the surface energetics of sulfathiazole crystals. Int. J. Pharm.. 2002;234(1–2):43-54.
- [Google Scholar]
- Effect of topical application of virgin coconut oil on skin components and antioxidant status during dermal wound healing in young rats. Skin Pharmacol. Physiol.. 2010;23(6):290-297.
- [Google Scholar]
- Traditional aboriginal preparation alters the chemical profile of carica papaya leaves and impacts on cytotoxicity towards human squamous cell carcinoma. PLoS ONE. 2016;11(2)
- [Google Scholar]
- In situ product recovery of n-butanol using polymeric resins. Biotechnol. Bioeng.. 2009;102(3):811-821.
- [Google Scholar]
- Norahmad, N. A., Mohd Abd Razak, M. R., Mohmad Misnan, N., Md Jelas, N. H., Sastu, U. R., Muhammad, A., Ho, T. C. D., Jusoh, B., Zolkifli, N. A., Thayan, R., Mat Ripen, A., Zainol, M. & Syed Mohamed., A. F. 2019. Effect of freeze-dried Carica papaya leaf juice on inflammatory cytokines production during dengue virus infection in AG129 mice. BMC Complementary & Alternative Medicine 19, 44
- Noraziani, Z. A., Hazreen, O., Anathasia, E. & Nurulain ’Atikah, K. 2016. Proliferative activity of saponin-reducing carica papaya leaves extracts on human lung fibroblast cell (IMR90). Jurnal Teknologi 78(11): 41-49
- Nurul’ain, K., Yusop, M., Sulaiman, S. & Yahaya, B. 2015. Apoptosis in lung cancer cells induced by Virgin Coconut Oil. Regenerative Research, 4(6): 30-36
- Comparison of biopolymer emulsifier performance in formation and stabilization of orange oil-in-water emulsions. J. Am. Oil Chem. Soc.. 2011;88(1):47-55.
- [Google Scholar]
- Effect of plasma treatment (He/CH4) on the glass surface during drying to reduce powder flux adhesion. Sains Malaysiana. 2018;47(6):1147-1155.
- [Google Scholar]
- Response surface optimization of polydimethylsiloxane (PDMS) on borosilicate glass and stainless steel (SS316) to increase hydrophobicity. Molecules. 2022;27(11):3388.
- [Google Scholar]
- An overview: nutritional and phyto-therapeutic potential of papaya (Carica papaya Linn.) Int. J. Food Prop.. 2014;17(7):1637-1653.
- [Google Scholar]
- Formulation and characterization of Virgin Coconut Oil (VCO) based. Emulsion. 2013;3(12):1-6.
- [Google Scholar]
- Sharifah Nurjannah, S. A., Noraziani, Z. A., Haslaniza, H. & Saiful, I. Z. 2018. Saponin Bitterness Reduction of Carica papaya Leaf Extracts through Adsorption of Weakly Basic Ion Exchange Resins. Journal of Food Quality 2018. Article ID 5602729.
- Effects of papaya leaves on thrombocyte counts in dengue - a case report. J. Pakistan Med. Assoc.. 2014;64(3):364-366.
- [Google Scholar]
- The reciprocal EC50 value as a convenient measure of the potency of a compound in bioactivity-guided purification of natural products. Fitoterapia. 2020;43:104598
- [Google Scholar]
- Phase behaviour study of swiftlet nest using virgin coconut oil with non-ionic surfactants. Malaysian J. Anal. Sci.. 2015;19(1):184-193.
- [Google Scholar]
- Subenthiran, S., Choon, T. C., Cheong, K. C., Thayan, R., Teck, M. B., Muniandy, P. K. & Afzan, A. 2013. Carica papaya leaves juice significantly accelerates the rate of increase in platelet count among patients with dengue fever and dengue haemorrhagic fever. Evidence-based Complementary and Alternative Medicine 2013. Article ID 616737.
- Virgin Coconut Oil (VCO) decreases the level of malondialdehyde (MDA) in the cardiac tissue of experimental sprague-dawley rats fed with heated palm oil. J. Med. Bioeng.. 2014;3(2)
- [Google Scholar]
- Mosquito larvicidal activity of Aloe vera (Family: Liliaceae) leaf extract and Bacillus sphaericus, against Chikungunya vector, Aedes aegypti. Saudi J. Biol. Sci.. 2012;19(4):503-509.
- [Google Scholar]
- Saponin bitterness reduction of Carica papaya leaf extracts through adsorption of weakly basic ion exchange resins. Hindawi J. Food Qual. 2018:1-12.
- [Google Scholar]
- Rheology of dispersions, principles and applications. Germany: Wiley; 2010. p. :218.
- Formation and stability of nano-emulsions. Adv. Colloid Interface Sci.. 2004;108–109:303-318.
- [Google Scholar]
- Effect of pH and ionic strength on the physicochemical properties of coconut milk emulsions. Food Eng. Phys. Properties. 2008;73(6)
- [Google Scholar]
- Influence of increasing viscosity of the aqueous phase on the short-term stability of protein stabilized emulsions. J. Food Eng.. 2002;52(3):305-312.
- [Google Scholar]
- Thevenet, F. 2006. Acacia gum (gum Arabic) in food stabilisers, thickeners and gelling agents. Blackwell Publishing. USA. 2006. 11-30
- Saponins, classification and occurrence in the plant kingdom. J. Phytochem.. 2007;68(3):275-297.
- [Google Scholar]
- Antioxidant and anticancer capacity of saponin-enriched Carica papaya leaf extracts. Int. J. Food Sci. Technol.. 2015;50(1):169-177.
- [Google Scholar]
- Phase behaviour study on medium-chain triglyceride/surfactant/water systems containing gemcitabine using phase inversion composition technique. Malaysian J. Anal. Sci.. 2020;24(3):363-372.
- [Google Scholar]
- The effect of Carica papaya L. leaves extract capsules on platelets count and hematocrit level in dengue fever patient. Int. J. Med. Aromatic Plants. 2012;2(4):2249-4340.
- [Google Scholar]







