Translate this page into:
The contribution of mango fruit (Mangifera indica L.) to human nutrition and health
⁎Corresponding author. yahia@uaq.mx (Elhadi M. Yahia),
-
Received: ,
Accepted: ,
This article was originally published by Elsevier and was migrated to Scientific Scholar after the change of Publisher.
Abstract
The mango fruit is of great economic value worldwide and of great nutritional importance. Its functional effects are due to numerous important nutrients and bioactive compounds. In this review, the benefits of mango fruit (pulp, peel, and seed) for human nutrition and health are detailed. The first part of the review presents the nutrient and phytochemical content of the mango fruit, and the second part addresses its health effects. The very diverse phytochemical components in mango fruit can be classified into several groups including macronutrients such as carbohydrates (sugars, pectin, and cellulose), proteins, lipids (Ω-3 and Ω-6 fatty acids); micronutrients such as vitamins A and C, minerals, pigments (chlorophylls, carotenoids, and anthocyanins, depending on the cultivar), phenolic compounds (phenolic acids and flavonoids), and volatile compounds. The beneficial effects of mango fruit and its components have been studied on different non-communicable diseases such as obesity, type 2 diabetes mellitus, hypertension, and cancer. A great diversity of research approaches have been used to study mango fruit health benefits, and those can be grouped into three main categories: a) those that present the associations or direct effects of the edible portion of the fruit on health through observational epidemiological studies and clinical trials; b) in vivo and in vitro experimental approaches that address either the physiological effects or mechanisms of several kinds of extracts or purified components of mango fruit (pulp, peel and seed), and c) preclinical and clinical studies that explore the possible pharmacological uses of mango fruit. Current scientific understanding of mango health benefits suggest that consumption of mango fruit and its byproducts containing bioactive components can be useful as part of a healthy diet in order to reduce the incidence of health problems.
Keywords
Mangifera indica
Phytochemicals
Bioactive compounds
Carotenoids
Phenolic compounds
Bioavailability
1 Introduction
Mango (Mangifera indica L.) is one of the most important fruits worldwide due to its nutritional value and content of diverse phytochemicals, with diverse functions (Brecht and Yahia, 2009; Gupta et al., 2022; Yahia, 2011). Bioactive compounds in mango fruit confer beneficial properties for human health, although their content is variable due to genotypic variation, climatic conditions, preharvest and postharvest treatments (Matheyambath et al., 2016; Yahia, 2011).
Mango is a climacteric fruit, capable of developing characteristic ripening-associated changes such as color, aroma, and taste, before or after harvest (Brecht & Yahia, 2009). Several postharvest handling processes affect mango fruit composition including transport, storage, and processing. In addition, mango fruit are subject to several physiological and pathological disorders that can alter their composition and quality. Chilling injury (CI), a disruption in cell wall membrane structure and functions, changes the flow of cellular fluids in and out of the cell, producing leakage of phytochemicals and disorders such as brown discoloration of the fruit skin, pitting and breakdown of pulp, lenticel spotting, irregular ripening, outflow of electrolytes, and softening of the tissues (Brecht & Yahia, 2009). It is important to understand the effects of these contributing factors on the variability in mango phytochemical composition because of the potential effects that those may have on the potential nutritional and health benefits of mango.
Some of the main bioactive compounds identified in mango fruit include phenolic acids (coumaric acid, ferulic acid and hydroxybenzoic acid), polyphenols (quercetin, mangiferin, catechins, tannins, kaempferol, anthocyanins, gallic acid, ellagic acid), carotenoids, which are the most abundant, and the vitamins ascorbic acid, thiamine, riboflavin, and niacin (Burton-Freeman et al., 2017; Wall-Medrano et al., 2015). These compounds have been reported to exhibit antioxidant activity (Zapata-Londoño et al., 2020), contribute to prevention of cancer (Boateng et al., 2007; Corrales-Bernal et al., 2014a; Ge et al., 2013), diabetes mellitus (DM) (Ediriweera et al., 2017; Gondi et al., 2015; Lucas et al., 2011), and cardiovascular disease and inflammatory processes (Xu et al., 2013). These effects have been attributed to activation by antioxidants of signaling pathways able to regulate the expression of genes and activate molecules leading to antioxidant, immunoregulatory, antiproliferative, and antidiabetic effects. However, many of the mechanisms by which mango bioactive compounds exert their effects are yet to be fully understood (Cervantes-Paz et al., 2016b; Kaulmann and Bohn, 2014; Lauricella et al., 2017; Xavier and Perez-Galvez, 2016; Yahia et al., 2018a). The mechanisms of action explaining the bioactivity of several compounds present in mango fruit are based on in vitro, in vivo and clinical trials, but often at very high concentrations that cannot be achieved by dietary means because these bioactive compounds are found in low levels in plant-based diets, thus unrealistically high intake would be necessary to achieve the levels found to be active using in vivo experiments. However, an active component can be overlooked in an extract or the interaction between several compounds that may be necessary for a strong effect. In addition, it was often observed that the mixture (e.g., an extract) shows a stronger response than adding the single active components together, reason for which the regular consumption of fruits, vegetables and whole grains is associated with reduced risk of developing chronic diseases in spite of the low abundance of some of their bioactive components (Pezzuto and Vang, 2020; Yahia et al., 2019).
Our objective in writing this review is to present an update on the contribution of mango fruit to human health, and to correlate it with fruit composition, as well as describing the factors that impact fruit components and therefore their nutritional and health contributions and effects.
2 Important nutrients and phytochemicals in mango fruit
Mango fruit are rich in nutrients such as carbohydrates, fatty acids, vitamins, and minerals as well as non-nutrient compounds including organic acids, dietary fiber, polyphenols, carotenoids, and other pigments. The energy value of mango fruit pulp varies from 60 to 190 kcal (250 to 795 kJ) per 100 g (Tables 1 to 5). The content of these components changes according to the cultivar and several preharvest and postharvest factors and practices (Yahia et al., 2011). In this review, we focus on the bioactive compounds in mangoes that contribute to human health. a = %, b = µg/ g tissue, n.d. = not detected.
Parameter
Content
(g/100 g DW)
Water
78–83
Ashes
0.34–0.52
Lipids
0.30–0.53
Proteins
0.36–0.40
Carbohydrates
16.20–17.18
Dietary fibers
0.85–1.06
Energy (kcal)
62.1–190
Mineral
mg per 100 g
Calcium
7–16
Iron
0.09–0.41
Magnesium
8–19
Phosphorus
10–18
Potassium
120–211
sodium
0–3
Zinc
0.06–0.15
Copper
0.04–0.32
Manganese
0.03–0.12
Selenium
0–0.6
Amino Acid
Content
(g/100 g DW)
Essential
Histidine
0–0.019
Isoleucine
0–0.029
Leucine
0–0.050
Lysine
0–0.066
Methionine
0–0.008
Phenylalanine
0–0.027
Threonine
0–0.031
Tryptophan
0–0.013
Valine
0–0.042
Nonessential
Alanine
0–0.082
Arginine
0–0.031
Aspartic acid
0–0.068
Glutamic acid
0–0.096
Glycine
0–0.034
Proline
0–0.029
Serine
0–0.035
Tyrosine
0–0.016
Carbon skeleton
Common name
Variety
Content
Part of the Fruit
16:0a
Palmitic acid
Malaysia
Mixed Egypt
Manila México
Kaew Thailand
4 Kenyan varieties6.95–10.93
5.8
9.29
5.4
4.87–10.57Seed
18:0a
Stearic acid
Malaysia
Mixed Egypt
Manila México
Kaew Thailand
4 Kenyan varieties32.8–47.62
38.3
39.07
46.6
24.22–32.80Seed
20:0a
Arachidic acid
Malaysia
Mixed Egypt
Manila México
Kaew Thailand
4 Kenyan varieties1.77–2.43
-
2.48
1.7
0.67–1.64Seed
24:0a
Lignoceric acid
–
–
Seed
18:1 (Δ9)a
Oleic acid
Malaysia
Mixed Egypt
Manila México
Kaew Thailand
4 Kenya varieties37.01–47.28
46.1
40.81
41.1
46.37–58.59Seed
18:2 (Δ9, 12)a
Linoleic acid
Malaysia
Mixed Egypt
Manila México
Kaew Thailand
4 Kenya varieties3.66–6.87
8.2
6.06
3.8
6.73–10.4Seed
18:3 (Δ9, 12,15)a
Α-Linoleic acid
–
–
Seed
14:0b
Myristic acid
Alphonso
Pairi
Kent174.29, 231.21
74.03, 295.16
40.57, 323.9Pulp, Peel
16:0b
Palmitic acid
Alphonso
Pairi
Kent1933.43, 2682.16
896, 3460.13
560.88, 2883.29Pulp, Peel
18:0b
Stearic acid
Alphonso
Pairi
Kent75.63, 123.57
33.36, 238.57
29.76,116.39Pulp, Peel
20:0b
Arachidic acid
Alphonso
Pairi
Kent19.01, 29.21
7.2, 55.24
3.2, 32.56Pulp, Peel
22:0b
Behemic acid
Alphonso
Pairi
Kent24.90, 43.83
8.88, 55.38
3.67, 43.73Pulp, Peel
24:0b
Lignoceric acid
Alphonso
Pairi
Kent35.85, 86.16
27.04117.24
24.88, 71.15Pulp, Peel
16:1, n-7b
Palmitoleic acid
Alphonso
Pairi
Kent2881.90, 1986.59
599.84, 533.59
314.28, 1527.72Pulp, Peel
16:1,n-5b
11-Hexadecenoic acid
Alphonso
Pairi
Kent146.22, 119.07
51.49, 58.01
22.42, 147.05Pulp, Peel
17:1, n-7b
10-Heptadecenoic acid
Alphonso
Pairi
Kent11.82, n.d.
8.76, n.d.
3.76, n.d.Pulp, Peel
18:1, n-9b
Oleic acid
Alphonso
Pairi
Kent856.59, 2376.3
761.792847.25
261.3, 778.48Pulp, Peel
18:1, n-7b
11-Ocatdecenoic acid
Alphonso
Pairi
Kent646.48, 480.59
248.78, 321.16
176.61, 282.14Pulp, Peel
20:1,n-9b
11-Eicosenoic acid
Alphonso
Pairi
Kent6.57, 10.01
2.39, 10.49
n.d., n.d.Pulp, Peel
16:2, n-4
9,12 Hexadecadienoic acid
Alphonso
Pairi
Kent33.86, n.d.
17.71, n.d.
16.09, n.d.Pulp, Peel
18:2, n-6
Linoleic acid
Alphonso
Pairi
Kent83.58, 422.83
139.44, 1956.03
80.05, 1277.41Pulp, Peel
18:2, n-3
9,15 Octadecadienoic acid
Alphonso
Pairi
Kent61.58, n.d
20.24, n.d.
20.93, n.d.Pulp, Peel
7:2, n-3
Hepta-2,4 (E,E)-dienoic acid
Alphonso
Pairi
Kent698.01, 265.93
662.32, 1152.72
835.33, 352.98Pulp, Peel
18:3, n-3
Linolenic acid
Alphonso
Pairi
Kent840.37, 1149.88
522.23, 1991.68
408.42, 1201.18Pulp, Peel
Vitamin
Value per 100 g
Ascorbic acid (Vit C)
13.2–92.8 mg
Thiamine (Vit B1)
0.01–0.04 mg
Riboflavine (Vit B2)
0.02–0.07 mg
Niacin (Vit B3)
0.2–1.31 mg
Panthotenic acid (Vit B5)
0.16–0.24 mg
Pyridoxin (Vit B6)
0.05–0.16 mg
Folate, total
20–69 µg
Vitamin A
54 µg
Vitamin E (α-tocopherol)
0.79–1.02 mg
Vitamin K
4.2 µg
2.1 Carbohydrates
Ripe mango fruit are rich in sugars (glucose, fructose, sucrose), starch, pectins, cellulose and hemicellulose (Belloet al., 2007). Ripe mango fruit pulp is about 15% soluble sugars, which contribute to the sweet flavor and the structure of the fruit. Simple sugars favor obesity and obesity-related diseases, and therefore, the high level intake of particular forms of mango is not advisable in certain age groups such as children because several studies have demonstrated the pro-obesity effects of certain types of fruit attributed to the content of glucose and fructose, the main precursors for inducing fatty acids by hepatic de novo lipogenesis, which may increase the levels of hepatic and circulatory triglycerides, as well as the circulating levels of very-low- and low-density lipoproteins (LDL), thereby resulting in increases in adipose tissue and obesity (Samuel, 2011; Tappy and Le, 2010). These carbohydrates contribute to nutrition and fruit texture, while dietary fiber has some health benefits. Dietary fiber represents 1.6 to 2.6% of the edible portion of Tommy Atkins, Haden, Kent and Keitt mangoes (USDA, 2018). They have nutritional properties based on their ability of hydration and gel formation. Partially soluble fibers have beneficial physiological effects on intestinal motility, weight and volume of the bolus, and intestinal transit time that contribute to the removal of bile acids and reduce cholesterol level in blood; likewise, reduce the risk of cardiovascular diseases and disorders of the colon (Wollowski and Pool-Zobel, 2001).
2.2 Minerals
Mango fruit provide calcium, copper, iron, magnesium, manganese, phosphorus, potassium, selenium, sodium, and zinc to the human diet (Table 2), according to the Recommended Daily Allowance (RDA) (National Research Council (US), 1989). Mango seeds and peels also contain significant amounts, in descending order, of calcium, potassium, magnesium, sodium, iron, manganese, zinc, and copper (USDA, 2018; Yahia et al., 2011).
2.3 Proteins and amino acids
Protein content of mango fruit is low, ranging between 0.5 and 5.5% depending on the cultivar and several other physiological and cultural factors (Saleem-Dar et al., 2016; Yahia et al., 2011). Amino acids (Table 3) content in mango fruit was reported to vary depending on cultivars, maturity, and ripeness stages (Hall et al., 1980; Yahia et al., 2011). Alanine, arginine, glycine, isoleucine, leucine, and serine were the predominant amino acids found in fully ripe mango fruit, with all other amino acids present in only trace amounts (Tharanathan et al., 2016).
2.4 Organic acids
The organic acids in mango fruit are primarily citrate and malate (Matheyambath et al., 2016; Yahia et al., 2011), with citric acid being found at levels ranging from 0.13 to 0.71% FW, as the importnat contributor to acidity (Tharanathan et al., 2016). These and other organic acids are also important for fruit respiratory activity (Vallarino and Osorio, 2019).
2.5 Lipids and fatty acids
Mango fruit pulp contains very low lipid content, but the seeds and the peels contain relatively high quantities of fatty acids (Table 4). Fatty acids are reported to contribute 0.8 to 1.36% of the edible portion of mango fruit, and fruit ripening favors an increase in unsaturated fatty acids (Deshpande et al., 2016).
2.6 Pigments
Mango fruit color is a very important index of maturation, ripeness, and quality (Brecht and Yahia, 2009; Yahia, 2011). The fruit peel of the different mango cultivars is green before maturation, and characterized with different colors on maturation and ripening of different cultivars including green, yellow, orange, and some cultivars also develop a red blush color. The color of the mesocarp (pulp) of all the cultivars is uniform, where it starts as white before maturation and turns into yellow and orange as the maturation and ripening process advances.
2.6.1 Chlorophylls
The peel of developing mango fruit has chloroplasts containing the green pigment chlorophyll (Brecht and Yahia, 2009; Yahia 2011). The potential nutritional and health values of the chlorophyll in mango fruit are still not fully evaluated.
2.6.2 Carotenoids
Carotenoids are lipid-soluble pigments with yellow and orange colors and are present in relatively large amounts in ripe mango mesocarp and peel although some cultivars retain green peel color when ripe (Masibo and Qian, 2008; Yahia, 2011). The red patches that develop on the skin of some cultivars are anthocyanins rather than carotenoids. During mango maturation and ripening, there is a conversion of chlorophyll-containing chloroplasts to carotenoid containing chromoplasts (Ketsa et al., 1999; Yahia, 2011). The presence of some pre-existing carotenoids is masked by chlorophyll during stages of fruit development, but during fruit ripening, the carotenes α-, β-, and γ- and the xanthophylls antheraxanthin, auroxanthin, lutein, neoxanthin, violaxanthin, and zeaxanthin are synthesized (Varakumar et al., 2011).
Carotenoids content in mango fruit varies among different cultivars (Liu et al., 2013; Manthey and Perkins-Veazie, 2009; Saleem-Dar et al., 2016). Carotenoids are 6 to 8 times more abundant during ripening than in unripe fruit (Haque et al., 2015). At the immature stage, lutein is the most abundant carotenoid in mango fruit; other carotenoids include various xanthophylls including all-trans and cis violaxanthin and neoxanthin, neochrome, luteoxanthin, zeaxanthin, antheraxanthin, and auroxanthin (Bramley, 2013; Ediriweera et al., 2017). At the beginning of mango ripening, the most abundant carotenoid is β-cryptoxanthin, while α-, β-, ζ-, and γ-carotenes are the most abundant in later ripening stages (Bramley, 2013; Ediriweera et al., 2017). Mercadante et al. (1997), Ornelas-Paz et al. (2007), and Petry and Mercadante (2016) reported that all-trans-β-carotene, all-trans-violaxanthin, and 9-cis-violaxanthin are common during fruit ripening. According to Manthey and Perkins-Veazie (2009) the main factor influencing the content of β-carotene in mango fruit is the type of cultivar rather than the origin or harvest date of the fruit.
2.7 Phenolic compounds
Phenolic compounds protect plants from different types of stress such as ultraviolet radiation, infections, and reactive oxygen species (ROS), among others (Mathembayath et al., 2016). These secondary metabolites are also essential components of the human diet, with their importance being due both to biological properties and health benefits (Brglez et al., 2016). Flavonoids, stilbenes, and lignans are abundant in plants. Flavonoids comprise about 60% of the dietary phenolics (Ramos, 2007). Current research is focused on the antioxidative, anti-inflammatory, and anti-carcinogenic properties of phenolics. The major phenolics in mango fruit in terms of both quantity and contribution to fruit antioxidative capacity are anthocyanins, the flavonoids catechin, kaempferol, quercetin, and rhamnetin, tannic acid, and the xanthone mangiferin (Masibo and Qian, 2008).
2.7.1 Flavonoids
Mango pulp (100 g) contains (+) - catechin (1.72 mg), and lower quantities of apigenin (10 µg), cyanidin (100 µg), delphinidin (20 µg), pelargonidin (20 µg), luteolin (20 µg), kaempferol (50 µg) and myricetin (60 µg) (Haytowitz et al., 2018). These authors have also reported that Haden, Kent, Keitt and Tommy Atkins mangoes contain isoflavones, proanthocyanidin dimers, trimers, and 4 to 6 dimers. Quercetin and related glycosides are the main flavonoids in mango pulp. The most prevalent is quercetin 3-galactoside, followed by quercetin 3-glucoside, quercetin 3-arabinoside and quercetin aglycone (Berardini et al., 2005; Ediriweera et al., 2017; Matheyambath et al., 2016).
2.7.2 Xanthones
Xanthones, found primarily in nature as glycosides, are characterized by having a C6-C3-C6 (ABC) ring structure. Rings A and B have hydroxyl, methoxyl and isoprene groups attached (Negi et al., 2013). One of the main xanthones present in mango fruit (peel and pulp) is mangiferin (Matheyambath et al., 2016). Mangiferin, C 2-bD-glucopyranosyl-1,3,6,7-tetrahydroxyxanthone, also called C-glucosyl xanthone, is widely distributed in higher plants, possessing a great diversity of pharmacological effects, notably antioxidant properties. In addition to mangiferin, other xanthones include dimethyl mangiferin, homomangiferin, isomangiferin, and isomangiferin gallate, and mangiferin gallate, with diverse pharmacological effects, including analgesic, anti-allergic, anti-arteriosclerotic, anti-cancer, anti-inflammatory, anti-microbial, and immunomodulatory effects, among others (Berardini et al., 2005; Ediriweera et al., 2017; Imran et al., 2017; Ribeiro et al., 2008; Saleem-Dar et al., 2016). Mangiferin and its derivatives are present in very low quantities in mango fruit pulp with larger amounts present in peels and seeds, ranging from undetectable to a few mg 100 g−1 FW among different cultivars (Luo et al., 2012; Ramirez et al., 2013; Ribeiro et al., 2008). Some mangiferin derivatives of mango include iriflophenone di-O-galloyl-glucose, maclurin-di-O-galloyl-glucose, maclurin-mono-O-galloyl-glucose, and mangiferin gallate (Berardini et al., 2005; Schieber et al., 2003).
2.7.3 Phenolic acids
The phenolic compounds in mango fruit include both hydroxybenzoic acid and hydroxycinnamic acid derivatives (Mattila and Kumpulainen, 2002). The hydroxybenzoic acid derivatives in mango fruit include gallic acid, p-hydroxybenzoic acid protocatechuic acid, vanillic acid, and syringic acid; the derivatives of hydroxycinnamic acid include chlorogenic acid, p-coumaric acid, caffeic acid, and ferulic acid (Ediriweera et al., 2017; Masibo and Qian, 2008). Free and conjugated forms of phenolic acids are present in mango fruit, probably as simple esters of quinic acid or glucose (Burton-Freeman et al., 2017). Phenolic acid types and content vary among different cultivars, geographical locations, and maturity and ripeness stages (Burton-Freeman et al., 2017; Corrales-Bernal et al., 2014b).
Ferulic acid has been reported to be the most abundant phenolic compound in mango fruit, followed by protocatechuic acid, chlorogenic acid, gallic acid, vanillic acid, and caffeic acid (Palafox-Carlos et al., 2012a), although Kim et al. (2009) reported that gallic acid is the major phenolic acid in Azúcar and Tommy Atkins mango pulp. Hu et al. (2018) identified 34 compounds as derivatives of phenolic acids, including maclurin (formed by a ring-opening reaction), gallotannins, quercetin derivates, and rosmarinic acid, both in mango peel and pulp.
Among other phenolic compounds, Berardini et al. (2005) reported 18 gallotannins including hexa-O-galloy glucose, iso-hexa-O-galloy-glucose, iso-penta-O-galloyl-glucose, penta-O-galloyl-glucose, and tetra-O-galloyl-glucose, and tentatively identified five benzophenone derivatives in the pulp of Tommy Atkins mangoes that include galloylated maclurin and iriflophenone glucosides, as well as eight gallotannins. Examples of derivatives of quercetin identified in mango include quercetin-3-O-arabinofuranoside, quercetin-3-O-arabinopyranoside, quercetin-3-O-galactoside, quercetin-3-O-glucoside, and quercetin-3-O-xyloside (Ramirez et al., 2013; Schieber et al., 2003). Ramirez et al. (2013) detected 18 phenolic compounds in Pica and Tommy Atkins mango pulp including procyanidin dimers, acid derivatives, mangiferin, mangiferin gallate, and homomangiferin, and dimethyl mangiferin.
2.8 Vitamins
Water-soluble and fat-soluble vitamins have been reported in Haden, Keitt, Kent, and Tommy Atkins mango fruit, with vitamins C and A being the predominant vitamins (Table 5). Mango consumption can satisfy the daily requirements of these vitamins in people of all age groups (WHO/FAO, 2003).
2.8.1 Vitamin C
Vitamin C content varies from 9.8 to 186 mg 100 g−1 in the edible portion of mango fruit (Manthey and Perkins-Veazie, 2009; Matheyambath et al., 2016; USDA, 2018; Valente et al., 2011). This degree of variability is probably a consequence of variation in preharvest and postharvest factors that affect the biosynthesis and metabolism of this vitamin. Changes in vitamin C content during mango ripening include higher levels in partially ripe mango fruit than in fully ripe mangoes (Hu et al., 2018; Matheyambath et al., 2016). The vitamin C content of ripe mangoes declines rapidly during storage at room temperature (Ibarra-Garza et al., 2015; Yahia, 2011). Several biological processes, such as the ethylene synthesis and metabolism and oxalate and tartrate synthesis, influence vitamin C content where vitamin C acts as a coenzyme (Singh et al., 2011).
2.8.2 Vitamin A
Mango fruit contain provitamin A carotenoids such as α- and β-carotenes, and it was estimated that mango could provide between 300 μg and 1,800 μg of retinol equivalents (RE) (Matheyambath et al., 2016). Recommended safe intake of vitamin A is 450 μg of RE per day for children of 4 to 6 years old, 500 μg of RE per day for children of 7 to 9 years old, and 500 and 600 μg of RE per day for adult females and males between 19 and 65 years old, respectively (WHO/FAO, 2004). Therefore, mango can be an important dietary source of vitamin A, especially in regions with vitamin A deficiency (Muoki et al., 2009). Mouki et al. (2009) considered that 12 μg of β-carotene equal one RE, and estimated that the intake of one fresh ripe mango fruit (approximately 300 g), 3 times per day, could meet and exceed 50% of the daily RE requirements depending on the cultivar and the growing area, and also estimated that the same amount of fresh ripe mango (cv. Apple) would exceed the daily RE requirements for children and adults.
2.8.3 Vitamins D, E and K
The contents of vitamins E and K in mango fruit are low or moderate, while vitamin D is absent in this fruit (Saleem-Dar et al., 2016; USDA, 2018). The natural forms of vitamin E include four tocopherols (α-, β-, λ-, and γ-) and four corresponding tocotrienols (α-, β-, λ-, and γ-), with the α-tocopherol being the most biologically active. Fresh-cut Ataulfo mango from Mexico was found to contain 1.33 mg of α-tocopherol per 100 g FW (Robles-Sanchez et al., 2009), which is higher than that reported by the USDA Nutrient Database (USDA, 2018) for other mango cultivars. The vitamin E content can increase during ripening of some mango cultivars (e.g., Tommy Atkins and Kent) and decreases in other cultivars (e.g., Dasherai) (Barbosa et al., 2017).
Vitamin C contributes to the regeneration of vitamin E because it can reduce the tocopheroxyl radical, which is the oxidized form of vitamin E, but the contrary is also possible. Decreased vitamin C and the consequent reduction of α-tocopherol content vary among cultivars because of the edapho-climatic conditions and genotypic differences (Joas et al., 2009).
2.8.4 Vitamin B complex
The vitamin B complex in mango fruit includes water-soluble enzyme cofactors and their derivatives. These are essential contributors to diverse metabolic processes in both plants and humans. The vitamins that form this complex include B1 (thiamin), B2 (riboflavin), B3 (niacin), B5 (pantothenic acid), B6 (pyridoxine, pyridoxal, and pyridoxamine), B8 (biotin), and B9 (folate or folic acid). Except for biotin, these have all been identified in mango fruit (Saleem-Dar et al., 2016). Vitamin B complex are required in the human diet because humans do not synthesize them. Cell differentiation in plant tissues affects the content of vitamin B.
2.9 Volatile compounds
Volatile compounds are low molecular weight (<400 Da) compounds present in small quantities of 50 ppm or less, very diverse with a wide range of functional groups, occurring as free forms and as glycosidic compounds, have high vapor pressures, and several of them are key components of the characteristic fruit aroma (Li et al., 2017). They can be alcohols, aldehydes, esters, volatile fatty acids, ketones, lactones, and degradation products of carotenoids and phenols (Lebrun et al., 2008; Li et al., 2017; Pino et al., 2005). Mango fruit aroma volatiles in different cultivars and tissues at different stages of maturity are distributed heterogeneously, quantitatively, and qualitatively (Andrade et al., 2000; Lalel et al., 2003b). The importance of most volatile secondary compounds to human health has not been fully investigated.
3 Preharvest and postharvest compositional changes
Fruit biochemistry and physiology affect the composition of mango fruit and its beneficial effects on human nutrition and health. During fruit maturation and ripening, several physiological and metabolic and structural biochemical modifications occur, and these changes determine the attributes of fruit quality, including fruit nutritional quality (Yahia, 2011; Yahia et al., 2011).
Mango fruit maturation and ripening occur around 49 to 77 days after anthesis followed by senescence (Tharanathan et al., 2016). Fruit maturation includes increased structural carbohydrates for cell walls and alcohol-insoluble solids due to starch accumulation, while ripening involves changes in structural polysaccharides related to fruit softening, production of soluble sugars from starch degradation, biosynthesis of volatile compounds, chloroplast degradation and chromoplast development and carotenoids biosynthesis (Joas et al., 2012; Matheyambath et al., 2016; Saleem-Dar et al., 2016; Wongmetha et al., 2015). Activity of the enzyme amylase, responsible for starch breakdown, increases with the onset of mango fruit ripening then decreases towards the full ripe stage (Li et al., 2020). All these changes lead to the ripening of fruit and softening to acceptable eating quality (Gill et al., 2017).
In some mango cultivars (e.g., Alphonso), the ripening process progresses from the skin to the seed, while the opposite occurs in most other cultivars. The development, maturation, and ripening processes involve genetic changes at the transcriptional level. In Alphonso mango flowers, developing fruit at 30 and 60 days postanthesis, mature fruit, and pulp from green, mid-ripe, and ripe fruit contained 4,611 transcripts, which were assigned to hydrolase, isomerase, ligase, lyase, oxidoreductase, and transferase classes, with transferases being most prevalent, followed by hydrolases (Desphande et al., 2017). Genes were identified that encode enzymes involved in 142 biochemical pathways. It was shown for Ataulfo mangoes that novel transcripts are produced during fruit development that are involved in the biosynthesis of monoterpenes, diterpenes, sesquiterpenes, furanones, and lactones that are involved in flavor formation. A large number (79) of novel transcripts were also identified that correspond to inhibitors of cell wall modifying enzymes (Desphande et al., 2017).
Color development in the pulp of mango fruit is due to synthesis of carotenoid pigments, which proceeds from the mesocarp tissue nearest the seed toward the outermost tissues (Tharanathan et al., 2016). The initial content of chlorophyll in the pulp is simultaneously degraded although some cultivars do not change their green color during ripening. To some extent, chlorophyll degradation during mango fruit ripening unmasks previously present carotenoid pigments along with the synthesis of new carotenoids (Yahia, 2011). Carotenoids in mango fruit increase by as much as 400% during maturation and ripening, and this increase could be used as a ripeness and harvest, as well as quality index (Ornelas-Paz et al., 2008b).
Pectins, a component of dietary fiber, play an important role in determining mango fruit texture. Pectin content increases starting at about 5 weeks after fruit set and continues until stone formation. During ripening, pectin chains in mango fruit cell walls are de-esterified, resulting in loss of calcium ions that are integral to bridges linking adjacent pectin polymers (Tharanatan et al., 2016). The insoluble pectin in the middle lamella is converted to soluble pectin as the cell wall is degraded in the process of fruit softening (Li et al., 2022). A feature of degradation of mango cell walls during ripening is the release of monosaccharides derived from the pectin complex, including arabinose and galactose, which leaves galacturonan-rich cell wall polysaccharides (Prasanna et al., 2004).
Regarding mango lipids, some studies have reported that the content of triglycerides increases in the pulp during ripening and that the fatty acid composition also changes (Yahia, 2011). Linoleic acid content also decreases as the fruit ripen, while linolenic acid content increases; a similar reciprocal distribution of palmitic and palmitoleic acids also occurs. The ratio of palmitic to palmitoleic acid influences mango aroma and flavor characteristics (Yahia, 2011). Palmitic acid is transformed to the hydroxy fatty acids that are the precursors of lactone aroma compounds (Prasanna et al., 2004). During the respiratory climacteric stage, the mitochondria metabolize fatty acids like stearic and oleic acids, which produces precursors for the biosynthesis of carotenoids and terpenoid volatiles (Yahia, 2011).
The soluble protein content in mango decreases for about 44 days after fruit set, increasing thereafter until ripeness is achieved (Yahia, 2011). Alanine, arginine, glycine, leucine-isoleucine, and serine are the predominant amino acids in mango fruit, but with the exception of alanine, they decrease during ripening. Studies on Kent and Tommy Atkins mangoes have indicated that the protein content in immature fruit (37.02–40.45 g kg−1 FW) declines through the maturation stage (12.10–13.87 g kg−1 FW; Barbosa et al., 2017). This difference has been associated to the ripening-mediated biosynthesis of enzymes and a decrease in protein content caused by senescence (Andrade et al., 2012; Barbosa et al., 2017).
Most B vitamin group in mango fruit significantly increases from the immature to mature stages (Barbosa et al., 2017). The forms of B vitamin (niacin, pyridine, riboflavin, and thiamine) increased by 1.75- to 3.4-fold during ripening of Kent and Tommy Atkins mangoes (Barbosa et al., 2017).
Vitamin C in mango fruit decreases during ripening, although that does not prevent ripe mangoes from being considered an excellent source of the vitamin (Matheyambath et al., 2016). Vitamin C content in the pulp of different mango cultivars decreases from about 5 weeks after fruit set to maturity (Manthey and Perkins-Veazie, 2009) although Keitt fruit had significantly more vitamin C than Kent and Tommy Atkins at the ripe stage, likely providing better postharvest color and flavor retention due to its direct inhibition of polyphenol oxidase (PPO; Palma-Orozco et al., 2014). The decline in vitamin C during fruit development may be related to its role as an antioxidant in various metabolic pathways and as a coenzyme of ACC-oxidase in ethylene biosynthesis, as well as being a substrate for oxalate and tartrate biosynthesis (Mazid et al., 2011). Nevertheless, only 300 g of ripe Kent and Tommy Atkins mango pulp provide 645 and 1410 mg vitamin C kg−1 FW, respectively; an amount that exceeds the dietary recommendations that are typical in the range of 75–90 mg d-1 (Barbosa et al., 2017; Ibarra-Garza et al., 2015; WHO/FAO, 2003).
Mango fruit vitamin E content also changes during maturation and ripening. Immature Tommy Atkins mango has the highest content (91 mg kg−1 FW), which decreases until the ripe stage (65.81 mg kg−1 FW), however, the opposite has been observed for immature and mature Tommy Atkins and Kent mangoes (51.96–77.50 mg kg−1) (Barbosa et al., 2017).
The content of polyphenols and phenolic acids typically changes little during mango development, ripening, and senescence, although they have the capacity to neutralize free radicals naturally generated during these processes (Palafox-Carlos et al., 2012b). Total polyphenolic contents at the immature and ripe stages of Kent and Tommy Atkins mangoes were 4.81 and 5.24 g kg−1 and 4.42 and 4.18 g kg−1, respectively (Barbosa et al., 2017). Low expression of flavonol synthase in Ataulfo mango may be sufficient to keep the flavonoid levels low (Palafox-Carlos et al., 2012b). The total polyphenolic content also decreased in Keitt (from 97.59 to 59.43 mg GAE 100 g−1 FW) and Xiangya (from 96.15 to 45.15 mg GAE 100 g−1 FW) fruit pulp as a consequence of ripening (Hu et al., 2018). Their flavonoid content was 45% higher at the green stage than when ripe (Hu et al., 2018). Palafox-Carlos et al. (2012b) reported that several phenolic compounds, including chlorogenic, gallic, protocatechuic, and vanillic acids were found in the peel of Ataulfo mango as the fruit progressed through ripeness stages based on green to yellow color development.
The content of chlorogenic acid in Ataulfo mango at ripeness stage one (0 to 10% yellow surface) was 28 mg 100 g−1 DW and rose to 301 mg 100 g−1 DW at ripeness stage four (71 to 100% yellow surface). The contents of gallic, protocatechuic, and vanillic acids changed from 94.60 to 98.70, 16.90 to 24.40, and 0.48 to 1.10 mg 100 g−1 DW, respectively, during the transition between these two stages (Palafox-Carlos et al., 2012b).
Organic acids content and titratable acidity decrease during mango ripening as a consequence of changes in the activity of the Krebs cycle (Yahia, 2011). The activity of some enzymes (i.e., isocitrate dehydrogenase and succinate dehydrogenase) increases during mango ripening, while the opposite occurs for other enzymes (e.g., citrate synthase) (Yahia, 2011).
4 Postharvest factors affecting bioactive compounds in mango fruit
Diverse postharvest treatments and practices are commonly carried out on fresh mango fruit during and after harvest, including in the field, in packinghouses, during transport and storage, or in the market (Yahia et al., 2011). Examples of mango postharvest practices include trimming, latex removal, quarantine treatment, sorting and grading, sanitation and fungicide application, treatments to extend fruit shelf life, packing and packaging, storage, and transport, among others. These practices can affect the nutritional and phytochemical contents of the fruit. Preservation of mango nutritional value is important due to the interest of consumers in health components and food safety in addition to visual quality. However, the information available on the effects of postharvest handling practices on bioactive phytochemicals in mango fruit is limited.
Prasad and Sharma (2018) observed that mechanical and manual harvesting of mango fruit (cvs. Amrapali, Chausa, Dushehari, and Langra) did not influence the total carotenoid content (5.3 and 5.4 mg 100 g−1 FW) and antioxidant capacity (2.98 and 3.00 μ mol Trolox eq g−1 FW). Hot water treatment and fumigation are two methods used to control postharvest mango diseases (Yahia, 2011). Control measures utilized against anthracnose decay can influence the phytochemical content of mango fruit (Kamle et al., 2013). Anthracnose decay is caused by the fungus Colletotrichum gloeosporioides (Penz), that produces the enzyme polygalacturonase that degrades mango fruit cell walls (Kamle et al., 2013). The fungus infection results in oxidation of phenolic compounds by the enzyme PPO, producing quinones, which are polymerized to form brown pigments, resulting in characteristic brown to black spots on the fruit peel. In healthy mango tissue, PPO is located in chloroplasts or chromoplasts and the phenolic substrates are in the cell vacuole, separated from each other, thus preventing the oxidation reaction (Yahia, 2011).
Pérez-Márquez et al., (2016) observed that phenolic compounds in the skin of 'Super Haden' mango gradually decreased as the severity of anthracnose infection increased, and the amount of soluble phenolic compounds in diseased fruit was lower than in healthy fruit. The combination of technologies to reduce anthracnose infection avoids oxidation and polymerization of phenolic compounds. Hot water treatment (5 min at 53 °C), application of polyethylene wax (10%) containing the fungicide Imazalil (800 mg L-1) and storage in the presence of an ethylene absorber (Conserver 21) reduced the loss of soluble phenolic compounds in Super Haden mango fruit infected with anthracnose, showing a final content of these compounds that was higher than that of the untreated control group (Pérez-Márquez et al., 2016). This beneficial effect was attributed to the elimination of spores or fungal mycelium and to the heat-mediated increased expression of mango polygalacturonases, which are capable of inhibiting the fungal endopolygalacturonase (Li et al., 2013). Kim et al. (2007) reported similar results in mature green Tommy Atkins mangoes. The concentrations of gallic acid and hydrolyzable tannins in the fruit were unaffected by the USDA APHIS quarantine hot water treatment (46.1 °C for 75 min) combined with 2 weeks’ storage at 10 °C in controlled atmospheres of 3 kPa O2 + 97 kPa N2 or 3 kPa O2 + 10 kPa CO2 + 87 kPa N2 followed by ripening in air at 25 °C, a procedure used for inhibiting anthracnose, while the content of total phenols decreased in mangoes treated either with hot water or controlled atmosphere alone.
If improperly applied, heat treatments that inactivate microorganisms and spoilage enzymes may induce chemical changes in the fruit that may not only affect organoleptic properties but also may reduce the content or bioavailability of some phytochemicals. For example, short heat treatments such as 80 °C for 5 min blanching may cause loss of carotenoids in mango fruit, while longer, lower temperature hot-water treatments such as those used for insect quarantine treatments (e.g., 46 °C, 65 to 110 min) may decrease gallic acid, gallotannins, and total soluble phenolics (Kim et al. 2009).
On the contrary, hot water treatment of Keitt mango fruit at 50 °C for 30 min or 46 °C for 75 min maintained vitamin C contents during storage for 3 days; however, when mango fruit were treated by combining the same times and temperatures, vitamin C content rapidly declined (Djioua et al., 2009). These results highlight the need to use proper time–temperature combinations to preserve the phytochemical content of mango. The content of carotenoids in Keitt mango fruit treated with hot water at 46 °C for 75 min, or 50 °C for 30 min, or 50 °C for 75 min increased during storage due to induction of cell membrane disruption and enhancement of carotenoid extractability and stability of total carotenoid contents compared with control fruit (Djioua et al., 2009).
The postharvest life of mangoes can be extended by physical and chemical methods that reduce respiration and ethylene production, among other effects. Some organic compounds, such as methyl jasmonate, can preserve mango fruit quality by enhancing anthocyanin content and thus favoring uniform red color, inducing phenylalanine ammonia lyase (PAL) activity, and promoting synthesis or inhibiting polymerization of phenolic compounds (Lalel et al., 2003a); and may also improve β-carotene and vitamin C contents (Muengkaew et al., 2016).
Other postharvest treatments used include the application of chemicals such as 1-methylcyclopropene (1-MCP) and Ethrel, which delay and accelerate the ripening process, respectively. These compounds caused opposite effects on the biochemistry of mango fruit (cv. Dashehari), with 1-MCP, for example, decreasing lipid peroxidation and increasing the superoxide dismutase (SOD) and catalase activities, while Ethrel causing opposite effects on lipids and enzymes (Singh and Dwivedi, 2008). The treatment of ‘Kensington Pride’ mangoes with 10 or 25 μL L-1 1-MCP affected the aroma volatile production, especially aldehydes, monoterpenes, and esters (Lalel et al., 2003b).
Irradiations may affect the levels of phytochemicals in fruits. Electron-beam ionizing radiation treatments (1.0 to 3.1 Gy) were evaluated for their effect on Tommy Atkins mango phenolic compounds, carotenoids, and vitamin C during storage (Reyes and Cisneros-Zevallos, 2007). After storage for 18 days at 15 °C, the ripening of all irradiated mangoes was delayed compared to the untreated control; the 3.1 Gy irradiated fruit had higher flavanol content with no effect on total phenolics, while ≥ 1.5 kGy treatment resulted in vitamin C content declining by half, but there were no significant changes in carotenoid content.
Molecular biology techniques like antisense RNA have been used to control mango ripening (Xu et al., 2018). Antisense technology involves insertion of a unique DNA transcript with 19 to 23 nucleotides that is complementary to mRNA. The result is that gene expression is downregulated in terms of replication, transcription, and translation. Genetic modification can also be used to either manipulate existing biosynthetic pathways or introduce genes from other species to boost the production of desired bioactive compounds, such as β-carotene (Tang et al., 2009), flavonal (Bovy et al., 2002), stilbenes, and other polyphenols (Giovinazzo et al., 2008) in different commodities.
5 Bioaccessibility and bioavailability of mango bioactive compounds
Mango fruit contain many phytochemicals, but the absorption process of carotenoids and phenolics are the most studied, mostly in vitro. The number of such studies in mango is small, contrasting with much more studies on absorption of phytochemicals from other fruits and vegetables such as carrots, spinach, tomatoes, and berries (Castro et al., 2019). However, the absorption process of phytochemicals from tropical fruits like mangoes has gained some attention lately (Liu et al., 2016; Sáyago-Ayerdi et al., 2019). In vitro approaches have been widely used to determine the release, diffusion, and micellarization of carotenoids and phenolic compounds from mango during digestion (Epriliati et al., 2009a, 2009b). Other studies have been carried out in humans and animals to determine the digestive metabolism and bioavailability of these mango compounds (Barnes et al., 2020; Gouado et al., 2007; Liu et al., 2016; Low et al., 2016; Low et al., 2015; Ornelas-Paz et al., 2010b).
The absorption efficiency of mango phytochemicals has been determined using the terms ‘bioaccessibility’ and ‘bioavailability’. Bioaccessibility refers to the proportion of consumed phytochemicals released from mango during digestion and available in the required form for absorption or incorporation into the epithelial cells. Bioavailability refers to the proportion of consumed phytochemicals reaching target tissues or stored in the human organism. This concept includes the processes of digestion, absorption, metabolism, tissue distribution, and elimination from the body of bioactive compounds or their metabolites due to changes such as degradation or conjugation with functional groups, proteins, or carbohydrates (Burton-Freeman et al., 2017). In some cases, the simple release of mango phytochemicals during digestion was wrongly considered as bioaccessibility or bioavailability.
5.1 General absorption processes for carotenoids and phenolics
The first step for absorption of carotenoids and phenolics is their release from the food matrix. This is achieved by mechanical actions (e.g., chewing, movements of the gastrointestinal tract, etc.), chemical actions (e.g., gastric HCl and Na2CO3 of pancreatic secretions), or enzymatic actions in the gastrointestinal tract (e.g., digestive enzymes secreted by mouth, stomach, pancreas, and intestine (Yahia et al., 2018a).
In order to be absorbed, carotenoids must be solubilized in droplets of dietary fat dispersed in the aqueous contents of the stomach. The size of these droplets is reduced at the duodenum due to the emulsifying effect of the bile (Yahia et al., 2018a). The reduction of the lipid droplet size favors the hydrolysis of triglycerides by pancreatic lipase at the lipid droplet surface and the formation of micelles, which are structured with the products of lipid digestion, bile salts, and cholesterol (Cervantes-Paz et al., 2016b). This lipolysis also favors the transference of carotenoids from the oil droplets to the micelles. Micellarization of carotenoids during digestion is largely recognized as a key step for their subsequent absorption. Enterocytes, or intestinal absorptive cells, are able to absorb the micellarized carotenoids (Cervantes-Paz et al., 2017). Small quantities of carotenoids are incorporated into the enterocytes by passive diffusion; however, this mechanism is quickly saturated under high concentrations of carotenoids and an active mechanism is activated for further absorption. Active absorption of carotenoids is performed by several protein transporters (e.g. SR-BI, CD36, and NPC1L1). In the enterocyte, the carotenoids are packed in chylomicrons, which are secreted into the blood system where they are exposed to the action of lipoprotein lipases leading to the formation of chylomicrons remnants, and these carotenoid-containing structures are taken up by the liver. Carotenoids are exported from the liver to different tissues by lipoproteins, especially by LDL, HDL and VLDL (Yahia et al., 2018a).
Absorption of phenolic compounds is relatively simple compared to carotenoid absorption. Phenolics diffuse from the chyme to the epithelial cells found in the stomach and small intestine, entering passively into the portal blood circulation. Unabsorbed phenolic compounds can also be metabolized by the gut microbiota, leading to the production of human health-related compounds secreted by the microorganisms, and absorbed in the large intestine (Crozier et al., 2010).
5.2 Bioaccesibility and bioavailability of mango carotenoids
The first step in the carotenoid absorption process involves the release of carotenoids from the food matrix. The natural location of carotenoids in mango cells in a lipid-dissolved form makes them more easily releasable during the digestion than the crystalline forms of some carotenoids found in tomato or carrot. Disruption of the mango tissue structure improves carotenoid release during digestion. Low et al. (2015) showed that the release of β-carotene and xanthophylls (mainly violaxanthin isomers) from Kensington Pride mango fruit flesh during in vitro digestion increased with the intensity of mastication (particle sizes of 0.075 to 2.8 mm). The release of β-carotene after gastric versus intestinal digestion was ∼ 8 to 10 and 20 to 32%, respectively, while the release of xanthophylls after these digestive stages varied between 5 and 18 and 35 to 55%, depending on the mastication intensity. Treatment of Ataulfo mango paste by ultrasound significantly increased the release of β-cryptoxanthin, β-carotene, and lutein after in vitro gastric digestion. However, digestion reactions of untreated samples showed a higher release of these carotenoids during the intestinal phase of digestion (Mercado-Mercado et al., 2018). Overall, xanthophylls are more easily released in vitro from mango tissues than from carotenes (Low et al., 2015).
Micellarization of mango carotenoids during digestion is determined by many factors. Veda et al. (2007) observed that the content of β-carotene in six varieties of mango varied between 0.55 and 3.21 mg 100 g−1, with 24.5 to 39.1% of this compound being micellarized during in vitro digestion. Badami and Mallika mangoes showed the highest concentration and micellarization of β-carotene. These differences might be attributed to differences in the concentration of carotenoids or differences in fiber content, because low carotenoids doses are more efficiently absorbed than high doses while fibers compromise lipid emulsification, lipolysis, and micelle formation (Cervantes-Paz et al., 2016a, 2017; Yahia and Ornelas-Paz, 2010). In plant foods with low fiber content, the varietal differences in carotenoid micellarization have been related to carotenoid concentration in the food, but mango pectin seems to also compromise the micellarization of carotenoids (Ornelas-Paz et al., 2008b). Fruit ripeness stage is another factor that determines the micellarization efficiency of mango carotenoids. Ornelas-Paz et al. (2008a) demonstrated that 4.5 to 6.9% of β-carotene in Ataulfo mango is micellarized during in vitro digestion, and increased with fruit ripening. This effect of ripening was attributed to the ripening-mediated softening of mango, indicating a similar positive effect of mango processing on carotenoid micellarization.
Treatment of mango paste with ultrasound improved micellarization by 44–46% (Mercado-Mercado et al., 2018). Pulsed electric fields (35 kV cm−1, 4 ms bipolar pulses, 200 Hz, 1800 ms) increased the micellarization of neoxanthin and cis-violaxanthin by 79% in a fruit juice mixture including mango, as compared with the untreated beverage (Rodríguez-Roque et al., 2016). Increased carotenoid micellarization have been observed for juice mixtures containing mango after treatment with pulsed electric fields (Buniowska et al., 2017).
Carotenoids are non-polar compounds, requiring the presence of dietary fat for their micellarization and subsequent absorption (Victoria-Campos et al., 2013). Studies have been conducted to investigate the impact of dietary fat on bioaccessibility and bioavailability of mango carotenoids. The addition of fat and protein to the digestion reaction increased the micellarization of mango β-carotene from 26 to 231%, as compared to digestions without fat and protein (Ornelas-Paz et al., 2008a). Veda et al. (2007) observed that digestion reactions of mango that included milk had 11 to 12% increased bioaccessibility of β-carotene. The milk protein has an emulsifying effect during digestion, improving lipolysis, which favors carotenoid micellarization, and improves absorption (Yahia and Ornelas-Paz, 2010).
The type of fat also influences the micellization efficiency of mango carotenoids. Liu et al. (2016) observed that the micellarization of carotenoids increased by emulsifying mango pulp with either medium or long chain triacylglycerols before digestion, with the micellarization efficiency of carotenoids without pre-emulsification or emulsified with medium or long chain triacylglycerols being 30, 54, and 85%, respectively. The effect of fat type on carotenoid micellarization has also been related to fat digestibility and alteration of micelle/chylomicron size (Yahia and Ornelas-Paz, 2010). The fat type also determines the particle size in the digestive media of mango, with medium or long chain fatty acids decreasing particle size from a range of 10 μm to greater than 1 μm in the oral phase, to 0.2 μm in the gastric phase, and finally to 0.13 μm in the intestinal phase. This reduction of particle size caused a hydrolysis of 80 to 100% of triglycerides during digestion, as compared with a non-emulsified test meal, where lipolysis did not occur (Liu et al., 2016).
Several studies have demonstrated that the interaction between fat and fibers determine the micellarization of carotenoids under digestive conditions. This interaction depends on the type and quantity of fat and fibers, although little is known on this in mango fruit. Ornelas et al. (2008a) demonstrated that micellarization in vitro of β-carotene from a pill decreased 10 to 14% in the presence of pectin from slightly ripe mango (cv. Ataulfo) and 3 to 28% with pectin from fully ripe mango, depending on pectin concentration in the digestion reaction. Ripening significantly alters the properties of mango pectin.
Only a minor fraction of micellarized carotenoids reaches the enterocytes. In early studies on bioavailability of mango carotenoids it was hypothesized that the consumption of mangoes was implicated in the absence of symptoms of vitamin A deficiency in Senegalese children (Carlier et al., 1992). Yuyama et al. (1991) observed that rats fed 4 IU of retinyl palmitate per g diet or 4 IU of vitamin A from mango fruit per g diet or 4 IU of vitamin A from peach palm fruit per g diet caused similar levels (28 to 32 μg) of vitamin A in plasma, although rats fed peach palm fruit stored more vitamin A in the liver than those fed with mango fruit.
Ornelas-Paz et al. (2010a) demonstrated that feeding Wistar rats with Ataulfo mango along with soybean oil caused vitamin A accumulation in the liver that was 37% higher than that of rats fed with carrots. They also demonstrated that soybean oil improved the bioavailability of mango carotenoids. Mango and carrots provided the same quantity of β-carotene to the rats. This study is very relevant because carrots have high concentrations of two provitamin A carotenoids (α- and β-carotenes) while mangoes only contain significant quantity of one provitamin A carotenoid (β-carotene), and carrots are the most important source of provitamin A carotenoids in the human diet. Ornelas-Paz et al. (2008a) found that 17% of micellarized β-carotene in mango digestions can be incorporated by intestinal cells in culture. Of course, these kinds of measurements do not represent the bioavailability of this compound because carotenoids can be secreted by intestinal cells into the digestive medium or even returned from the blood to the intestinal content again (Yahia & Ornelas-Paz, 2010). Further human studies are needed to understand the potential impact of mango consumption on circulating carotenoids, as has been done for other fruits like tomatoes.
5.3 Bioaccesibility and bioavailability of mango phenolic compounds
The release of phenolics from mango pulp during digestion depends on the ripeness stage of the fruit, because ripening alters the structure of mango tissue. Quirós-Sauceda et al. (2019) observed that although the concentration of phenolic compounds decreased in mango pulp (cv. Ataulfo) during ripening, the release of these compounds increased in the digestive media (in vitro) as ripening progressed, accounting for 26 to 40 and ∼ 30 to 45% of the phenolic content of mango pulp in the gastric and intestinal media, respectively. The release of phenolics from mango pulp during digestion may be differentially impacted by gastric and intestinal phases of digestion with the intestinal phase causing a higher release of phenolic compounds than the gastric phase, as reported for example for phenolic compounds from commercially available mango juices (Attri et al., 2017). Chen et al. (2014) also observed that phenolics in the chyme slightly decreased during the gastric phase of in vitro digestion of mango (cvs. Hainan and Shuixian) pulp while the intestinal phase caused the opposite effect.
The increase of phenolic compounds in the digestion medium has mainly been attributed to Na2CO3 in digestive secretions, which favors the release of phenolic compounds from Na2CO3-soluble pectin rich in phenolic compounds, as demonstrated for other fruits (Epriliati and Ginjom, 2012). These increases, measured commonly as the content of total phenols, not only suggest a release of phenolic compounds, but also their metabolism, as reported for example for anthocyanins after digestion, making it difficult to estimate the absorption efficiency of mango phenols.
The pH of the gastric medium plays important roles in the release of food phenolic compounds in the digestive medium and their subsequent metabolism. This is particularly true in the case of mango fruit because the most abundant phenolics in mango fruit, gallic acid and gallotannins, experience structural modifications during the digestion (Barnes et al., 2016). The gallotannins are polymers of gallic acid that are hydrolyzed to gallic acid and other metabolites during the gastrointestinal digestion. The gastric pH is responsible for the reduction of the molecular weight of gallotannins to species ranging from 4-O-galloylglucose to 8-O-galloylglucose. The enzymatic modification of gallotannins in the gastrointestinal tract seems to have minor relevance (Barnes et al., 2020).
Overall, the structure of phenolic compounds can be modified during the entire digestion process, including the oral phase, and the final metabolites are generally phenolic acids already contained in the food, making it difficult to estimate the bioavailability of the original compounds (Kim et al., 2021). The gallic acid is mainly released from penta-O-galloylglucose or trigalloyl moieties from 11- or 12-O-galloylglucose (Krook and Hagerman, 2012). The depolymerization of gallotannins involves both m-depside and galloyl-glucose bond hydrolysis (Barnes et al., 2016; 2020). This effect of digestive pH makes it difficult to estimate the absorption efficiency of the parent compounds. While there may be other factors that play a role in the release and digestive metabolism of phenolics in plant foods, the impact of such factors has received little attention in the case of mango.
A minor quantity of the phenolic compounds released during digestion is bio-accessible. Quirós-Sauceda et al. (2019) demonstrated that only ∼ 10 to 11.5% of mango phenols released during in vitro digestion were considered to be bio-accessible as determined by permeation into a cellulose dialysis bag (12 to 14 kDa). This absorption process mainly includes passive diffusion, and in some cases, active mechanisms can take place in the stomach and intestine, according to studies on other fruits (Crozier et al., 2010).
Plant tissues contain some water-soluble flavonoids that can be converted into lipid-compatible molecular complexes called phytosomes, which follow the same absorption pathway as lipid-soluble phytochemicals like carotenoids (Kidd and Head, 2005). Liu et al. (2016) observed that some phenolic compounds are micellarized during mango digestion, showing bioaccessibility values 1.5 times higher than those of carotenoids.
There is a dearth of available information regarding the influence of mango consumption on the concentration of phenolics in human fluids. Concentrations of catechol-O-sulfate, 4-O-methylgallic acid, 4-O-methylgallic acid-3-O-sulfate, pyrogallol-O-sulfate, and methylpyrogallol-O-sulfate in human plasma were 9520, 2790, 6030, 5990, and 4020 μg L-1, respectively, following consumption of 400 g of Ataulfo mango pulp (Barnes et al., 2020). Quirós-Sauceda et al. (2017) detected ferulic, gallic, gentisic, and protocatechuic acids in mango pulp and juice and observed that the consumption of these foods caused increases in the concentration of these compounds in plasma, reaching maximum concentrations of 7.9–8.7, 49.7–109, 11.8–12.2, 30.8–34.5 ng mL−1 after 2.3 to 4.4 h, suggesting that the absorption of these compounds took place in the intestine. Quirós-Sauceda et al. (2017) also noted that urinary excretion of chlorogenic, ferulic, gallic, sinapic, vanillic, and p-coumaric acids and pyrogallol increased after 8 to 24 h from mango consumption. Barnes et al. (2016) detected pyrogallol-1-O-glucuronide, 4-O-methyl-gallic acid, 4-O-methylgallic acid-3-O-sulfate, O-methylpyrogallol-O-sulfate, pyrogallol-O-sulfate, deoxypyrogallol-O-sulfate, and O-methylpyrogallol-O-sulfate in human urine after mango consumption. They hypothesized that due to their rapid appearance in urine 6 h after mango consumption the compounds 4-O-methyl-gallic acid and 4-O-methylgallic acid-3-O-sulfate were probably absorbed in the small intestine. The other metabolites were conjugates of pyrogallol, which are absent in mango pulp; however, those compounds can be produced from gallic acid via the action of decarboxylase enzymes in the microbiota in the colon, where gallic acid can be absorbed and then conjugated by hepatic and renal enzymes prior to elimination in urine (Kim et al., 2021). Barnes et al. (2016) demonstrated that gallic acid metabolites are not detectable in the plasma after mango consumption. This might be a consequence of the efficiency of the release of phenolics during digestion (food matrix effect) mediated by ripening or other factors, because several gallic acid metabolites have been observed in plasma after mango consumption (Pimpao et al., 2015).
The absorbed phenolic compounds may be transformed into other compounds as a result of conjugation reactions such as glucuronation, methylation, and sulphation catalyzed by Phase I and II enzymes in the small intestine and liver (Kim et al., 2021). However, some compounds are excreted into the small intestine via bile and reabsorbed by enterohepatic circulation, although the most important clearance pathway is through the kidney and urine excretion (Burton-Freeman et al., 2017).
The phenolic compounds that are not absorbed can be metabolized by microbiota in the large intestine and the resulting metabolites absorbed there (Epriliati and Ginjom, 2012; Kim et al., 2021). In rats, Kim et al. (2018) observed that mango juice altered the composition of gut microbiota, increasing the population of L. plantarum, L. lactis, and Clostridium butyrium, which synthesized tannase and gallic acid decarboxylase that promoted the hydrolysis of gallotannins to gallic acid and then to pyrogallol. These microorganisms can also produce bioavailable short chain fatty acids (SCFA; propionic, butyric, isobutyric, valeric, isovaleric, etc.) from mango phenolic compounds and fiber. Thus, the SCFA might be considered as bioactive compounds from mango. In the same way, Ojo et al. (2016) found that feeding a high fat diet supplemented with freeze-dried ripe Tommy Atkins mango pulp to C57BL/6 mice for 12 weeks resulted in high levels of acetic and butyric acids in the feces along with increases in the beneficial gut bacteria Bifidobacteria and Akkermansia.
Hernández-Maldonado et al. (2019) tested an in vitro gastrointestinal and colonic fermentation model using mango-based fruit bars as a dietary fiber and phenolic compounds source. They reported that the bio-accessibility of phenolic compounds was 53.78%, and during colonic fermentation these compounds were mainly converted into hydroxyphenolic acids and acetic acid followed by propionic and butyric acids as the main SCFA after 48 h. Finally, the unabsorbed phenolic compounds and microbial metabolites other than SCFA may be excreted in feces. Moreover, this in vitro experiment showed that mango-based fruit bars could also modify colonic microbiota through increasing carbohydrate metabolism that also could prevent metabolic dysbiosis (Gutiérrez-Sarmiento et al., 2020).
In a study performed using lean and obese human subjects, Barnes et al. (2019) showed that supplementing the participants’ diet with 400 g d-1 of Ataulfo mango pulp for 6 weeks increased gallotannin-metabolites in urine, however, only lean participants increased fecal SCFA (butyric and valeric acids). Besides, obese participants had increased levels of Lactococcus lactis and decreased populations of Clostridium leptum and Bacteroides thetaiotaomicron, bacterial species that are associated with obesity. Mango intake positively modulates fecal microbial composition by significantly increasing the presence of Lactobacillus spp. and fecal butyric acid (Kim et al., 2020). This is particularly important because inflammatory bowel disease is a major risk factor for gastrointestinal neoplasias such as colorectal cancer, and knowledge of the positive effects of mango consumption on fecal microbial composition may contribute to the development of novel therapies (Kim et al., 2020).
6 Effects of mango fruit bioactive compounds
6.1 Antioxidant activity of carotenoids
Foods containing carotenoids, such as mango, in which β-carotene is the prevalent type of carotenes in the pulp, possess potent antioxidant properties. β-carotene contributes to the antioxidant capacity of mango by direct and indirect mechanisms. It can directly neutralize free radicals such as peroxyl (ROO.), hydroxyl (.OH), singlet oxygen (1O2), and superoxide (O2.-) radicals (Birben et al., 2012), resulting in 5,8-carotene endoperoxides (Choe and Min, 2009). This occurs via the donation of a hydrogen to produce a carotene radical, which at low oxygen concentrations can react with another ROO. leading to non-radical carotene peroxides (Chen et al., 2011). By binding to the Antioxidant Response Element (ARE) the transcription factor Nrf2 activates indirect antioxidant mechanisms. This is necessary for the induction of phase II enzymes including glutathione S-transferases (GSTs), NAD(P)H:quinone oxidoreductase (NQO1) and thioredoxin (Tanaka et a., 2012).
Anilakumar et al. (2003) reported that male Wistar rats that were fed a diet supplemented with 10% mango for 12 weeks presented reduced cytotoxic effects induced by dimethyldrazine via hepatic increase of vitamin A and optimized activities of hepatic catalase and GST activities.
In another study using human dermal fibroblasts (FEK4), it was observed that after UVA exposure, β-carotene specifically exerted its antioxidant effect by suppressing the up-regulation of Haem oxygenase-1 gene expression in a dose-dependent manner (Trekli et al., 2003), which is consistent with the direct antioxidant mechanism described for this carotenoid via singlet oxygen quenching. As explained by Pavan et al. (2006), this Haem oxygenase-1 gene expression can be activated by β-carotene and retinoids via the nuclear retinoic acid receptors, RAR and RXR, after binding to retinoic acid-responsive elements (RARE) in the promoter regions. However, as shown by Obermuller-Jevic et al. (1999), the activation of retinoid signaling via RARs and RXRs might not be involved because the Haem-oxygenase-1 can be expressed at low concentrations of β-carotene (0.2 μM) in response to UVA treatment of human skin fibroblasts.
In contrast, high in vitro concentrations (<10 μM) of β-carotene favor oxidation by promoting production of reactive oxygen species (ROS) and oxidized glutathione (Palozza et al., 2003). Paolini et al. (2001) showed that this effect is due to activation of NF-kB and upregulation of c-myc expression, causing inhibition of cell growth in human leukemia and colon adenocarcinoma cell lines and induction of apoptosis; however, these effects were blocked by treatment with α-tocopherol and N-acetylcysteine. Moreover, α-tocopherol and N-acetylcysteine reduced upregulated expression of Phase I enzymes and ROS overproduction in intestines, kidneys, livers, and lungs of rats fed diets supplemented with β-carotene (Paolini et al., 2001).
If β-carotene induces oxidative stress in cells, it follows that oxidative breakdown products of β-carotene will be produced. One such products are the β-apo-carotenals, which can serve as precursors of vitamin A via the enzyme β-carotene 15,15′-monooxygenase (CMO1) (Tanaka et al., 2012). The β-apo-carotenals comprise a group of oxidized compounds that have been found in smokers. Therefore, β-carotene supplementation is not indicated for chemoprevention of lung cancer because cigarette smoking causes retinoid signaling to be suppressed via reduced RAPβ gene expression and activation of the AP-1 transcription factor (Yu et al, 2015), part of the mitogenic signaling pathway of insulin growth factor type 1 (IGF-1) among others (Angel and Karin 1991).
The AP-1 complex comprises the protein families Jun (c-Jun, JunB and JunD) and Fos (c-Fos, FosB, Fra-1 and Fra-2), which exist as homodimers (Jun/Jun) or heterodimers (Jun/Fos) (Albanese et al., 1995). As described by Palozza et al. (2010), these components of the AP-1 complex can be induced by mitogenic stimuli and tumor-promoting agents, binding to the AP-1 site or TPA response element (TRE). The TRE is found on the promoter of many genes that are related to cell proliferation such as cyclin-D. The AP-1 complex proteins can also be part of the ARE transcription complex, which raises the possibility expressed by Palozza et al. (2010) that retinoic acid reduces growth factor-induced stimulation of AP-1 transcriptional activity by altering the composition of AP-1 complexes that bind to DNA.
Taken together these mechanisms may partially explain why high doses of β-carotene did not protect against lung cancer in several intervention trials (Omenn et al., 1996). Supplementation with high doses of β-carotene along with inhalation of tobacco smoke induce 3- to 4-fold increases in expression of c-Jun and c-Fos genes, leading to proliferation response in lung tissue and squamous metaplasia, along with increased cell proliferation marker and proliferation of cell nuclear antigens. Despite this evidence related to lung cancer, dietary carotenoids have been shown in numerous studies to prevent cancer and the mechanism is thought to involve induction of cell death via the oxidative stress caused by cytochrome c release from mitochondria, which leads to apoptosis, a way to kill other types of cancer cells (Tanaka et al., 2012).
6.2 Antioxidant activity of phenolics
Catechin and epicatechin react directly with H2O2 and prevent the Fenton reaction between Fe2+ and H2O2 (Masibo and Qian, 2008). Another mango flavonoid with significant reported antioxidant activity is quercetin, which has the ability to sequester ROS such as O2.-, NO., and other reactive nitrogen species, a feature that has been attributed by Boots et al. (2008) to the catechol motif in its B-ring, and the hydroxyl group at position 3 of the C-ring.
The antioxidant capacity of mangiferin, present in mango leaves and bark, and to a lesser extent in the fruit pulp, has been widely demonstrated in different studies such as its capacity to inhibit the Fenton reactions and lipidic peroxidation through the formation of a stable complex with Fe3+ (Benard and Chi, 2015). The antioxidant capacity of mangiferin is comparable to the neutralization of ROS, such as ROO., O2.-, H2O2, and.OH by ascorbic acid (Benard and Chi, 2015). Antioxidant activity of mangiferin has also been demonstrated using the 2,2′-azinobis-(3-ethylbenzothiazolin-6-sulfonic acid (ABTS) and DPPH assays (Tang et al., 2004). Mangiferin showed in vitro values of 0.61, 1.67, and 3.69 µmol of Trolox, using DPPH, ABTS, and Oxygen Radical Absorbance Capacity (ORAC) assays, respectively (Malherbe et al., 2014). Mangiferin has also been shown to protect against oxidative stress in Wistar rats and in healthy older adults (Pardo-Andreu et al., 2008).
Among the phenolic acids in mango fruit with reported antioxidant activity are gallic and ellagic acids. Gallic acid is capable of inhibiting lipid peroxidation and neutralizing ROS such as.OH, O2.-, and ROO., as well as other oxidizers like H2O2 and HClO (Badhani et al., 2015), which is partially attributed to the hydroxyl groups in the ortho position. The antioxidant activity of ellagic acid measured by the DPPH assay has been shown to inhibit lipid peroxidation and enhance SOD, catalase, and glutathione peroxidase (GPx) activities in lung fibroblasts (V79-4) of Chinese hamsters (Han et al., 2006).
Zapata-Londoño et al. (2020) reported that mango juice (cv. Azúcar) consumption for 26 days did not significantly affect plasma antioxidant capacity and biomarkers of oxidative stress [lipid peroxidation, total glutathione, and 8-hydroxy-guanosine (8-OHdG)] in healthy adults with dietary habits that favor colorectal cancer risk. In contrast, Pardo-Andreu et al. (2006) reported that 30 days of consumption of 14.67 µmol L-1 of Vimang, an extract from mango bark that is rich in phenolic acids and esters, flavan-3-ols, and mainly mangiferin (10 µM), decreased TBARS levels. In this study, the effect of the extract consumption on total glutathione was also determined, but no differences were observed after taking the extract (Pardo-Andreu et al., 2008). Differences between these studies may be attributed to the quality of dietary habits of the participants, such as the low fruits and vegetables consumption by the participants in the study of Zapata-Londoño et al. (2020), which is associated with increased levels of lipid peroxidation and oxidative damage to DNA (Suwimol et al., 2012), indicating that the ability of these foods to protect against oxidative stress is due, in part, to their phenolic compounds. Plasma antioxidant capacity is directly associated with consumption of foods rich in phenolic compounds, which was evidenced in a study by Wang et al. (2012) reporting that a high consumption of foods rich in antioxidants led to an improvement in plasma antioxidant capacity.
On the other hand, considering that mangiferin’s antioxidant capacity in vitro and in vivo has been shown in different studies (Márquez et al., 2012; Pardo-Andreu et al., 2006), the plasma content of mangiferin following consumption of mango juice was evaluated by Zapata-Londoño et al. (2020) who found higher values of mangiferin in participants (38.64 ± 6.75 ng mL−1), more than those reported by Hou et al. (2012) after the administration of mangiferin (0.9 g) via the oral route to healthy individuals. These differences may be attributed to the accumulation of mangiferin present in mango juice consumed daily and because absorption of mangiferin may be greater when it is in this matrix.
Studies performed in humans and rats have shown that mango consumption increases antioxidant capacity in plasma or serum (García-Solís et al., 2008; Robles-Sánchez et al, 2011). Robles-Sanchez et al. (2011) found that healthy individuals who consumed peeled and sliced Ataulfo mangoes for 30 days had increased plasma antioxidant capacity as measured by ORAC and ABTS assays compared to the controls.
6.3 Immunomodulation activity
One of the observed effects of carotenoids and polyphenols of mango is their anti-inflammatory effect on epithelial cells such as Caco-2 in the gastrointestinal tract (Biehler et al., 2011), HT-29 cells (Ribeiro et al., 2006), human gastric epithelial adenocarcinoma (AGS) (Jang et al., 2012), ulcerative colitis (Kim et al., 2016), breast cancer cells (Arbizu-Berrocal et al., 2019), obesity (Gomes Natal et al., 2016; 2017), and in juvenile upper respiratory tract (Anaya-Loyola et al., 2020). The inflammation is initiated by an irritant agent or infection and involves movement of fluid containing macrophages and leukocytes into extravascular tissue, then tissue regeneration or repair involving cell proliferation. A respiratory burst is characteristic of this process, which is associated with ROS overproduction, oxidative stress, and damage to lipid membranes, producing lipid peroxidation and activation of the arachidonic pathway, further producing ecoisanoids that are able to induce cell proliferation. Oxidative stress also damages DNA and proteins with modification of structure and function (Hussain et al., 2003), a condition that can lead to chronic diseases like cancer. As outlined by Reuter et al. (2010), the affected cells produce proinflammatory cytokines (TNF-α, IL-1β), chemokines, and prostaglandin E2 (PGE2) because of activation of cyclooxygenase 2 (COX-2), inducible nitric oxide synthase (iNOS), and activation of the transcription factors NF-κB and Nrf2 that have been associated with inflammation and oxidative stress responses (Fig. 1). A clinical trial performed with 10 adult patients with inflammatory bowel disease, Crohn disease, and ulcerative colitis, showed that supplementation with 200–400 g d-1 of ripe Keitt mango pulp for 8 weeks improved their clinical condition, including simple Clinical Colitis Activity Index, decreased plasma pro-inflammatory cytokines related to neutrophil-induced inflammation (i.e., Il-8, growth regulated oncogene, ranulocyte macrophage colony-stimulating factor) (Kim et al., 2020).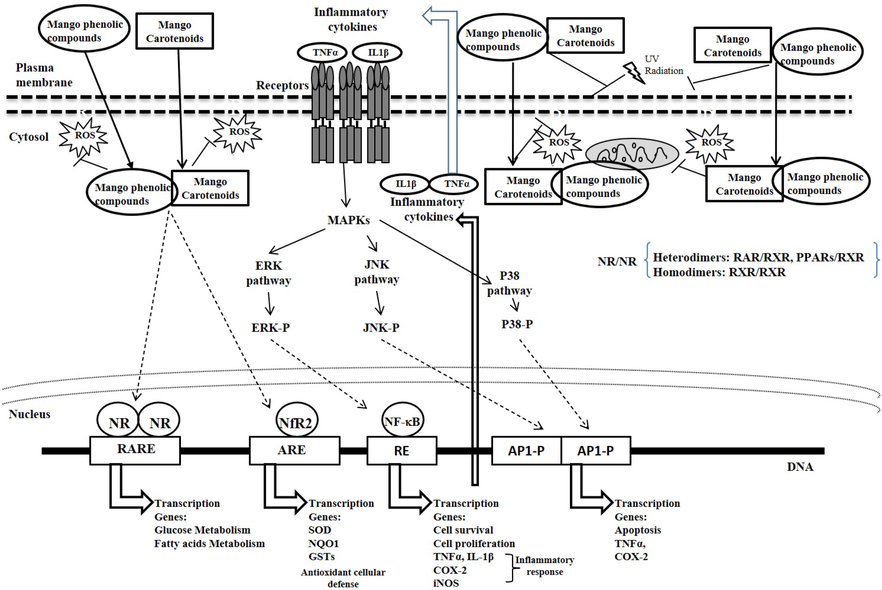
Possible mechanisms of the effects of mango carotenoids and phenolic compounds. ARE: antioxidant response element; AP1-P: activator protein 1 phosphorylated; COX-2: cyclooxygenase-2; DNA: deoxyribonucleic acid; IL1b: interleukin 1-b; iNOS: inducible nitric oxide-synthase; MAPKs: mitogen-activated protein kinases; ERK: extracellular signal-regulated kinase; ERK: extracellular signal-regulated kinase phosphorylated; GSTs: glutathione-S-transferases; JNK: c-Jun N-terminal kinase; JNK-P: c-Jun N-terminal kinase phosphorylated; NR: nuclear receptor; NF-kB: nuclear factor kappa-B; Nrf2: nuclear factor erythroid 2-related factor 2; NQO1: NAD(P)H quinone oxidoreductase 1; P38: mitogen-activated protein kinase p38; P38-P: mitogen-activated protein kinase p38 phosphorylated; RAR: retinoic acid receptor; RARE: retinoic acid response element; RXR: retinoid X receptor; PPARs: peroxisome proliferator-activated receptors; RE: Response element; ROS: reactive oxygen species; SOD: superoxide dismutase; TNFa: tumor necrosis factor-alpha; UV: Ultraviolet.
As described in Fig. 1, carotenoids (including β-carotene) and phenolic compounds (phenolic acids and polyphenols) from mango may act directly as antioxidants from the effects of UV radiation, but can also interact with a wide group of cell molecules such as nuclear transcription factors (NR: nuclear receptors, Nrf2, RAR, RXR, NF-κB, MAPKs, PPARs) and MAPKs enzymes, able to modulate through the RARE, the antioxidant response element (ARE), the transcription of genes involved in cellular defense such as antioxidant enzymes (SOD, GSTs, NQO1), genes involved in the inflammatory system such as the cytokines TNFα and IL1β, the pro-inflammatory enzymes (COX-2, iNOS) for producing compounds such PGE-2 by COX-2, genes involved in apoptosis, glucose and fatty acid metabolism, and genes participating in cell survival and/or proliferation.
6.4 Anti-inflammatory activity of mango carotenoids
It has been shown in in vitro studies that β-carotene (2 to 20 μM) is able to significantly reduce ROS production and activation of the NF-κB in AGS cells after Helicobacter pylori stimulation (Bouayed and Bohn, 2010; Jang et al., 2009), including down-regulated iNOS and COX-2 expression, and production of PGE2 (Jang et al., 2009; Palozza et al., 2003). PGE2 plays an important role in the process by activating PGE2 receptors, which increase β-catenin nuclear accumulation and transcriptional activity (Castellone et al., 2005). The PGE2 also activates signal transduction pathways that favor growth of malignant tissue by enhancing cellular proliferation, promoting angiogenesis, inhibiting apoptosis, stimulating invasion or migration, and suppressing immune responses (Wang and Dubois, 2006). Corrales-Bernal et al. (2016) showed that consumption of Azúcar mango juice during the 8 weeks following AOM injections to induce colorectal cancer, significantly reduced PGE2 levels compared with the control group, a result correlated with aberrant crypt foci (ACF), indicating mango consumption might contribute to control of carcinogenesis in colonic mucosa.
The effect of carotenoids on Mitogen-activated protein kinases pathway (MAPK) has been investigated in other studies. Oxidative stress stimuli, IL-1β, and TNF-α activate these serine/threonine kinases. The three MAPK-pathways that have been characterized are the p38 kinases, the c-Jun N-terminal kinases (JNK1, JNK2, and JNK3), and the extracellular signal-regulated kinases (ERK1/ERK2) (Johnson and Lapadat, 2002). It has been observed that β-carotene inactivates the p38 and ERK pathways by decreasing phosphorylation, but this effect was not found for JNK in the cytosol of AGS cells (Jang et al., 2009).
Reducing biomarkers of inflammation that has been identified in cancer chemoprevention studies, such as TNFα and IL1β, has been addressed in several animal studies by providing foods containing carotenoids (Bousserouel et al., 2011). Proinflammatory cytokines TNFα and IL1 (α and β) are considered tumor promoting factors that act via stimulation of chemokine expression and promotion of ROS production in epithelial cells (Babbar and Casero, 2006). IL1β has been shown as the cause of inflammation and induction of pro-inflammatory gene expression. Pro-inflammatory cytokine levels in the blood of Balb/c mice injected with azoxymethane (AOM) then provided with 0.3% w/v of mango juice for 8 weeks were only slightly, but not significantly different compared with the control group (AOM + water) (Corrales-Bernal et al., 2016). The TNFα levels in the experimental group were undetectable, and IL6 was not different in treated and control animals. Elevated IL1β levels induced by AOM soon after initiation of colon carcinogenesis and diminished IL1β with mango juice consumption were novel results of this study. Choi et al. (2006) reported similar results when rats with colon carcinogenesis induced using AOM were given 0.5% β-carotene for 33 weeks, with the β-carotene failing to reduce inflammatory markers such as COX-2. Although the reasons for these results are unexplained, the approach of using in vivo models and administering water-soluble preparations of carotenoids (i.e., mango juice) is interesting.
In spite these promising results, when effects of carotenoids are evaluated by intake of carotenoid-rich foods such as mango, there are prevailing confounding factors, including the presence of other components of the food matrix such as dietary fiber, minerals, and vitamins. However, carotenoids and micronutrients provided as supplements may not result in the same synergistic effects as when they are in the food matrix, overdosing is a possibility, and bioavailability may be altered (Kaulmann and Bohn, 2014).
The effect of carotenoid supplementation on factors involved in the NF-κB pathway has been analyzed in a few studies, but foods rich in lycopene (not found in mango) have received the most attention. However, one example using β-carotene was the study of Vaisman et al. (2006) in which the subjects received enteral nutrition enriched with carotenoid (2 µg kcal−1), resulting in reduced expression of lymphocyte NF-κB after 3 months compared with the control. On the other hand, daily consumption for 4 weeks of either 2 or 8 servings of carotenoid-containing fruits and vegetables had no effect on TNF-α, IL-12, or C-reactive protein serum levels, but consumption of greater than 8 daily servings did reduce C-reactive protein serum levels (Watzl et al. 2011).
6.5 Anti-inflammatory activity of mango phenolics
The anti-inflammatory activity of mango phenolic compounds like gallic acid and gallotannins was reported by Kim et al. (2016) to inhibit the nuclear factor kappa-B (NF-κB) in experimental models of ulcerative colitis employing an enriched fraction of Keitt mango polyphenolic extracts. They examined the miR-126/PI3K/AKT/mTOR signaling pathway in in vitro lipopolysaccharide (LPS)-treated CCD-18Co cells and observed that a mango extract with 10 mg GAE L-1 suppressed NF-κB, p-NF-κB, PI3K (p85β), HIF-1α, p70S6K1, and RPS6 protein (Kim et al., 2016). They also observed that a mango beverage protected rats against dextran sodium sulfate (DSS)-induced colonic inflammation (47%) and significantly reduced expression of iNOS, the proinflammatory cytokines TNF-α, IL-1β, as well as PI3K, AKT, and mTOR. Similar results were obtained in TNF-α treated breast non-cancer cells MCF-12A and breast cancer cells MDA-MB231 exposed to a polyphenol-rich extract of Keitt mango pulp (Arbizu-Berrocal et al., 2019). These findings suggest that mango phenols have potential as anti-inflammatory therapeutic agents in the prevention of breast cancer and as cancer-specific cytotoxic treatments of breast cancer without inducing cytotoxicity in non-cancer cells.
Inflammation is not only associated with infectious processes or cancer, but also with obesity modulated by adipokines. When Gomes Natal et al. (2016; 2017) evaluated the effect of consumption of Ubá mango juices by obese Wistar rats (35 mL d-1) for 7 days they noted that the juice prepared from pulp + peel had higher concentrations of phenolic compounds, mangiferin, and ascorbic acid than juice prepared from pulp alone. Nevertheless, both mango juices reduced obesity indicators such as total body weight, body weight gain, visceral fat, and adiposity index (Gomes Natal et al., 2016). In addition, they observed reduced expression of peroxisome proliferator-activated receptor gamma (PPAR-γ), a transcription factor of adipogenic genes, and increased blood levels of HDL-cholesterol while blood glucose and reducing total cholesterol, fatty acid synthase expression, and TNF-α were decreased (Gomes Natal et al., 2016). Moreover, both Ubá mango juice, pulp, and pulp + peel, reduced seric resistin levels and hepatic steatosis in obese rats (Gomes Natal et al., 2017). Thus, taken together, their results indicate that Ubá mango has potential as a functional food able to reduce the metabolic risk for obesity that is associated with adiposity and inflammation.
Anaya-Loyola et al. (2020) proposed that a mango juice-by-product (JBP) containing peel and pulp remanent may reduce symptoms related to gastrointestinal and upper respiratory acute infections in healthy school-age children (6 to 8 years old) via modulation of immune-related proteins due to its high content of dietary fiber (1.1 g) and 278.6 mg extractable polyphenols (mono-to-hepta galloyl hexosides and mangiferin) and 7.8 mg of hydrolyzable polyphenols (ellagic and gallic acid) per portion (2 g d-1) supplemented for 2 months. The JBP showed beneficial effects on gastrointestinal and upper respiratory tract, as most of the children did not present gastrointestinal and respiratory infections at the end of the study. These effects might be attributed to the antimicrobial and antiviral activities associated with the high content of phenols such as gallic acid and gallotannins (Lee et al., 2016b). In addition, these effects in children were associated by Anaya-Loyola et al. (2020) with the increase in serum levels of immune-related proteins, plasminogen activator inhibitor (PAI-I), which confers protection against yersiniosis by promoting the innate immune system; macrophage inflammatory protein (MIP-1α), which promotes the ability of cytotoxic T lymphocytes to protect against a secondary viral challenge; and MIP-1β, which promotes IgA and IgE antibodies. However, in this study, the serum levels of these immunoglobulins were not significantly affected by mango JBP supplementation. On the other hand, decreases in levels of IgG, macrophage migration inhibitory factor (MIF), and osteopontin were the result of a lower number of repeated infections and less production of pro-inflammatory cytokines as a result of inflammatory process in organs such as the gastrointestinal tract (Anaya-Loyola et al., 2020).
6.6 Antiproliferative action of mango phytochemicals
Epidemiological evidence has shown inverse association between intake (food or supplements) of phytochemicals (phenolic compounds and carotenoids) and cancer risk, because the phytochemicals are able to regulate cellular differentiation and growth cell (Yu et al., 2015). Studies on the mechanisms of action of the phytochemicals present in fruits and vegetables in cancer have allowed their classification according to their ability to block the initiation stage of carcinogenesis (cancer-blocking agents) or to suppress (cancer-suppressing agents) the proliferative capacity of preneoplastic lesions in the stages of tumor promotion and progression (Surh, 2003; Yahia, 2009; Yahia et al., 2018b). Phenolic compounds are blocking agents that inhibit carcinogens by protecting DNA from nitrogen reactive intermediates and ROS attack because of their scavenging potentials shown for quercetin (Bub et al., 2003) and gallic acid (Lauricella et al., 2019). Flavonoids also induce carcinogen detoxifying systems, alter metabolism of procarcinogens in favor of conjugation and excretion of reactive metabolites, and can interact with the aryl hydrocarbon receptor as agonists or antagonists depending on structure and cell context, which influences the expression of drug metabolizing enzymes such as cytochromes P450 (Romagnolo and Selmin, 2012) as well as induction of phase II enzymes, and may facilitate the elimination of certain carcinogens or their reactive intermediates (Zhang et al., 2003). On the other hand, the suppressing mechanisms induced by polyphenols include suppression or elimination of tumor cells by interfering with cell cycle regulation, signal transduction pathways, transcriptional regulation, inhibition of cyclooxygenase activity, suppression of oncogenes and tumor formation, and induction of apoptosis of cancer cells (Surh, 2003). Many flavonoids or combinations of flavonoids induce G2/M arrest in CaCo-2 and SW620 human colon carcinoma cells (Dorai and Aggarwal, 2004). Another suppressing mechanism considered is the inhibitory effect on arachidonic acid metabolism leading to production of pro-inflammatory or mitogenic metabolites including prostaglandins and ROS (Corrales-Bernal et al., 2016).
Over the past few years, research has focused on demonstrating that the polyphenol mangiferin presents antiproliferative activity. Mangiferin has been reported by Li et al. (2016) to reduce the proliferation of human prostate cancer PC3 cells in a concentration- and time-dependent manner. In addition, mangiferin promotes apoptosis and induces caspase-3 activity of human prostate cancer PC3 cells. Mangiferin treatment significantly reduces the expression levels of Bcl-2 and improves miR-182 expression in PC3 cells (Li et al., 2016). Mangiferin in CNE2 cells, a nasopharyngeal carcinoma cell line, induced apoptosis, and inhibited cell proliferation via cell cycle arrest in phase G2/M in a dose-dependent manner (Pan et al., 2014). Moreover, mangiferin reduced antiapoptotic protein Bcl-2 and its associated mRNA and increased proapoptotic protein Bax in CNE2 cells (Pan et al., 2014).
In rhabdomyosarcoma (RD) cells, mangiferin caused cell death in a dose-dependent manner (Padma et al., 2015). It was found that the IC50 value was 70 µM and its cytotoxic effect increased depending on the dose and induced the loss of lactate dehydrogenase, the release of nitric oxide, the increase in intracellular production of ROS and intracellular calcium level, and the decrease in the antioxidant enzymes catalase, SOD, glutathione-s-transferase, and glutathione with decreasing the mitochondrial membrane potential in DR cells (Padma et al., 2015).
In in vivo animal models, the chemopreventive activity of mangiferin has been tested in ovarian adenocarcinoma cell line xenograft (OVCAR3) in nude BALB/c female mice. Xenograft tumor volume decreased significantly with increasing mangiferin dose during a 14-d treatment, and xenograft tumor volume decreased in mice treated with mangiferin plus cisplatin, suggesting that mangiferin increased the sensitivity of ovarian cells to cisplatin (Zou et al., 2017). Mangiferin has also been reported to inhibit in vivo growth of fibrosarcoma in Swiss mice (Derese et al., 2017).
The way in which carotenoids are thought to prevent development of certain types of cancers is by modulating the RARβ gene that codes for a potential tumor suppressor (Pavan et al., 2006). Contrary to this anticancer effect of β-carotene, this carotenoid and its oxidative metabolite, β-apo-14′-carotenoic acid, down-regulate RARβ in bronchial epithelial cells in the presence of smoke-borne carcinogens by affecting the transactivation of the RARβ promoter, and consequently the transcriptional activation of anti-proliferative or pro-apoptotic genes necessary for the elimination of neoplastic and preneoplastic cells with irreparable alterations (Prakash et al., 2004).
The anti-carcinogenic action of mango has been studied from in vitro cultures of cancer cells and in vivo animal models in the early stages of carcinogenesis (Boateng et al., 2007; Corrales-Bernal et al., 2014a; 2016). Cell growth of human fibroblasts (Stivala et al., 2000) and human colon cancer cell lines (COLO 320 HSR, LS-174, HT-29 and WiDr) (Palozza et al., 2002), as well as some prostate cancer cell lines (PC-3, DU 145 and LNCaP) (Williams et al., 2000) have been shown to be inhibited by β-carotene independently of p53 and/or p21WAF1 status or gene expression with the arrest of cell cycle in G2/M phase and the induction of apoptosis. These inhibitory effects occur in parallel to the accumulation of carotenoids inside the cells and their conversion into retinol, leading to a reduced expression of cyclin A, a key regulator of G2/M progression without modification of p21 or p27, two cyclin kinase inhibitors. In relation to the apoptosis that was observed in Bcl-2, Bcl-xL, and Bax, a similar effect was observed in SW480 adenocarcinoma cell line after treatment with carotenoid and polyphenol extracts from Ataulfo and Haden mangoes, respectively. This was achieved by ∼ 2-fold increase in levels of the enzyme Caspase-8 and 1.3- and 1.7-fold increases in expression of the pro-apoptotic genes BAX and BIM, respectively; and by increases in the cell cycle regulators P21 and PKMYT1 of ∼ 1.3- and 1.4-fold, respectively, compared to untreated cells. In addition, polyphenols from Ataulfo mango (10 mg GAE L-1) induced cell cycle arrest in the G2/M phase within 24 h and this was associated with increased expression of PKMYT1, a gene that has been shown to negatively regulate the G2/M cell cycle transition (Noratto et al., 2010).
In addition, there is important evidence to support the relationship between treatment with whole mango juices, extracts, and bioactive phytochemicals from mango fruit and the modulation of many molecular pathways to prevent the in vitro development and progression of cancer as well as in murine cancer models. The effect of whole and fractions of mango juice was evaluated using HL-60 cells that showed cell cycle arrest in the G0/G1 phase after treatment (Percival et al., 2006). Furthermore, it has been shown that aqueous extract of ‘Ataulfo’ mango has inhibitory effect on viability of breast cancer cells MCF-7 (García-Solís et al., 2009). In addition, mango inhibited neoplastic transformation of BALB/3T3 cells in a dose-dependent manner (Percival et al., 2006). Similarly, exposure to mango peel ethanol extracts (125–1000 µg mL−1) inhibited proliferation in human cervical cancer HeLa cells, human gastric cancer AGS cells, and human hepatocarcinoma HepG2 cells in a dose-dependent manner (Kim et al., 2010).
In a preclinical model of colorectal cancer induced by AOM using mango juice from Azúcar mango (0.3% to 1.25% w/v) offered daily for 10 weeks to Balb/c mice, Corrales-Bernal et al. (2014a) reported that the formation of ACF in the colon was inhibited by more than 60% in mice receiving 0.3% (p = 0.05) before and after AOM injection when compared with controls receiving water. A similar result (p = 0.013) was obtained when mango juice was given for 8 weeks just after AOM injection. Furthermore, the analysis of crypt multiplicity in ACF by Boateng et al. (2007) indicated that consumption of 5% w/w of mango juice highly inhibited ACF formation (by 83.3%).
6.7 Anti-diabetic effects and mechanisms of mango phytochemicals
Type 2 diabetes mellitus (T2DM) is a complex, multifactorial, systemic chronic disease characterized by hyperglycemia and other metabolic disorders. It is generally accepted that the main pathophysiological contributors to T2DM are insulin resistance (IR) and β-cell dysfunction (Sangeetha et al., 2017). As the available hypoglycemic drugs have adverse side effects, there is an increasing interest in identifying natural sources and/or natural bioactive compounds as therapeutics to treat T2DM and its complications (Shukri et al., 2011). The study of the antidiabetic properties of natural products focuses on the effects involved in carbohydrate digestion, glucose absorption, glucose uptake by adipose tissue and skeletal muscles, synthesis of sorbitol, formation of advanced glycation end products (AGEs), gluconeogenesis/glycogenolysis, and synthesis and secretion of insulin (Alam et al., 2019; Sangeetha et al., 2017). The role of mango on the preventive and adjuvant effects in the treatment of T2DM and its complications have been studied in different ways, by clinical studies, studies in experimentation animals, and in vitro studies.
One of the main concerns about the consumption of mango pulp is that it could have a hyperglycemic effect in patients with T2DM due to its high content of sugars (sucrose, glucose, and fructose). However, several studies have shown that the glycemic response to mango in healthy subjects and patients with T2DM is low with respect to the response to the consumption of glucose or other fruits such as banana, sapote, rambutan, durian, and pineapple (Elizondo-Montemayor et al., 2015; Redfern et al., 2017). A clinical trial carried out in healthy patients shows that fresh Tommy Atkins mango puree has a low glycemic index (GI; Elizondo-Montemayor et al., 2015). The GI is a quantitative measure that determines the increase in blood glucose that occurs following consumption of 50 g of the food carbohydrate equivalents compared with the consumption of the same quantity of glucose. A GI ≤ 55 is considered low, ≥70 is considered high, and the GI described for fresh ripe mango puree is 42.7 ± 19.5. Furthermore, Elizondo-Montemayor et al. (2015) reported that high hydrostatic pressure (HHP) processed mango puree had significantly reduced GI (32.7 ± 13.4). Consumption of low GI foods has been associated with improvements in both body weight and lipid profiles. The mechanism by which HHP processing reduces mango GI is unknown, but it has been postulated that an increase in the viscosity and alcohol insoluble residues solubility could delay the absorption of carbohydrates. The effect of mango fruit powder obtained from unripe Kili-Mooku mangoes, called CarelessTM, on glucose metabolism and microcirculation in healthy subjects was studied in a double-blind, randomized placebo-controlled trial (Buchwald-Werner et al., 2017). Either 100 or 300 mg of fruit powder was administered daily for 4 weeks with no modification observed in glucose tolerance, glycosylated hemoglobin (HbA1c), and microcirculation.
The streptozotocin (STZ) induction model of diabetes mellitus (DM) in rats is widely used to study the antidiabetic effects of natural products. Streptozotocin is a toxic compound that acutely destroys pancreatic β-cells, leading to the development of severe hyperglycemia because of the resulting lack of insulin. This model is particularly useful for studying the hyperglycemic effects and complications of DM such as neuropathy, nephropathy, retinopathy, and cataractogenesis.
Mangiferin has been shown to reduce diabetic nephropathy in STZ-diabetic rats (Li et al., 2010). Mangiferin in STZ-diabetic rats significantly inhibited 24 h albuminuria excretion, glomerular extracellular matrix expansion, and accumulation and transforming growth factor-β1 (TGF-β1) overexpression in glomeruli. Moreover, serum-AGEs, lipoperoxidation, and sorbitol concentration in erythrocytes were significantly decreased by mangiferin (Li et al., 2010). On the other hand, mangiferin also increased both serum SOD and GPx activities and creatinine clearance rate in STZ-diabetic rats (Li et al., 2010). Moreover, in mesangial cells, mangiferin inhibited high-glucose-induced cellular proliferation and also reduced collagen IV overexpression induced by AGEs (Li et al., 2010).
An 8-week high-fat/high fructose diet (HFFD) followed by subdiabetogeneic STZ was used to explore the antidiabetogenic effect of mangiferin. This model is useful to induce IR and β-cell dysfunction and their consequences such as hyperglycemia, depletion in liver glycogen, dyslipidemia, and obesity (Saleh et al., 2014). When 20 mg/Kg i.p. mangiferin was applied for 28 days, insulin sensitivity was improved in HFFD-STZ-diabetic rats (Saleh et al., 2014). Serum fasting glucose and lipid profile were improved and the serum tumor necrosis factor-α (TNF-α) was reduced, whereas serum adiponectin was increased. TNF-α is an adipokine related to development of IR, whereas adiponectin is an adipokine that increases insulin sensitivity. The effects of mangiferin in HFFD-STZ-diabetic rats were similar to those of the standard insulin sensitizer rosiglitazone (Saleh et al., 2014).
Supplementing the high-fat (HF) diet of mice with 1% freeze-dried mango pulp for 8 weeks improved glucose tolerance, with reduced adiposity and lowered insulin resistance in the HF group compared to the hypolipidemic drug fenofibrate and the hypoglycemic drug rosiglitazone (Lucas et al., 2011).
The preclinical model of Gestational Diabetes Mellitus (GDM) in mice has been used to show that the protective effect of mangiferin at 50 mg kg -1 d-1 for 18 gestational days is due to suppression of inflammation, oxidative stress, and endoplasmic reticulum (ER) stress (Sha et al., 2019) because glucose intolerance, insulin resistance, and β-cell dysfunction that contribute to the processes associated with GDM are favored by oxidative stress. The ER regulates protein synthesis and post-translational protein modifications; thus, ER perturbation affects the biological function of proteins resulting in misfolded proteins (Roca-Rodríguez et al., 2014). Therapeutic application of mangiferin was demonstrated to reduce GDM symptoms by rescuing insulin level, decreasing blood glucose, total cholesterol, triacylglycerides and low-density lipoproteins, increasing antioxidant factors (SOD, GPx, and catalase; Roca-Rodríguez et al., 2014). Mangiferin decreased, but did not totally prevent, inflammation [IL-6, monocyte-chemoattractant-protein-1 (MCP-1) e IL-8] and ER stress (GRP78, p-IRE1α, p-eIF2α, and p-PERK) in GDM mouse placenta; the latter may be attributed to poor absorption and low bioavailability of mangiferin.
The mechanisms of the effects of mango fruit (pulp, peel, and seed) on DM are at different physiopathological levels because of very diverse compounds present in different preparations or extracts of mango fruit. Some of these hypoglycemic and hypolipidemic effects observed with mango fruit and the vegetative plant tissues are similar to those observed using pharmacological options, explained from the action of antagonists for peroxisome proliferator-activated receptors (PPARs), which are able to control glucose and lipid metabolism (Wang, 2010). The PPARs comprise a family of three members: α, β/δ, and γ. The PPAR-α activation by ligands regulates the genes involved in the catabolism of fatty acids (β-oxidation, fatty acid synthesis, ketogenesis, and lipoprotein metabolism) (Wang, 2010; Zhang et al., 2015). Upregulation of glucose uptake explains how PPAR-γ improves insulin sensitivity in adipose tissue and skeletal muscle (Wang, 2010). Finally, the PPAR β/δ modulates fatty acid metabolism and regulates endogenous antioxidant levels in the heart as well as regulating mitochondrial biogenesis, cardiomyocyte apoptosis, glucose metabolism and the insulin signaling pathway, and myocardial inflammatory responses induced by lipids (Palomer et al., 2016).
This information suggests that bioactive mango compounds could induce control of glucose and lipid metabolism by acting as PPARs agonists, but the mechanism by which mango fruit and the plant bioactive compounds lower blood glucose levels is unknown. Aderibigbe et al. (1999) reported that an aqueous extract of mango leaf demonstrating hypoglycemic activity did so by stimulating pancreatic β-cells to secrete insulin and reduce intestinal absorption of glucose.
Thus, whether mango compounds could act similarly to other PPAR agonists is not clear at present, and future studies are necessary to examine how mango consumption leads to these effects. Further research is needed to investigate if supplementing the diet with fresh mango containing these bioactive component(s) will have these same benefits.
6.8 Anti-hypertensive effects and mechanisms of mango phytonutrients
Globally, hypertension is one of the most important non-transmissible diseases and it is the most preventable risk factor for all-cause morbidity and mortality (Saiz et al., 2020). Hypertension is also the major risk factor of cardiovascular disease (heart failure, chronic kidney disease, myocardial infarction, peripheral vascular disease, stroke) and it is associated with persistently hypertension in systemic arteries (Saiz et al., 2020). Around 90 to 95% of the patients with hypertension have essential or primary hypertension that has a multifactorial gene-environment etiology (Oparil et al., 2018).
One of the factors involved in the development of hypertension is hyperuricemia (Oparil et al., 2018; Yang et al., 2018). Hyperuricemia involves impairment of nitric oxide (NO) synthesis and subsequent endothelial dysfunction (Yang et al., 2018). The liposoluble free radical NO is involved in several biological processes such as vasodilatation, angiogenesis, immune function, leukocyte-endothelial interactions, platelet aggregation, long-term synaptic transmission, memory, and reproduction (Becerril et al., 2019; Lee et al., 2016a). The three NO synthase (NOS) isoforms, neuronal (nNOS), endothelial (eNOS), and inducible (iNOS), are responsible for NO synthesis. In normal physiological conditions, nNOS and eNOS are both constitutively expressed, whereas iNOS is stimulated by inflammatory cytokines. iNOS promotes excessive NO synthesis that causes oxidative stress and cellular damage via accumulation of peroxynitrites (ONOO–) (Becerril et al., 2019; Lee et al., 2016a). Elevated blood pressure is generally related to reduced eNOS activity and excessive abnormal iNOS expression seems to be related to progression of vascular dysfunction (Lee et al., 2016a).
There are some studies related to NO synthesis and hypertension in which the antihypertensive effect of mangiferin was explored. Yang et al. (2018) showed in rats with hypertension induced by hyperuricemia that mangiferin administered intragastrically (120 mg kg−1) significantly decreased both serum uric acid (UA) and systolic blood pressure (SBP) between 8 and 12 weeks. The reduction of SBP in hyperuricemic rats by mangiferin was associated with increased serum NO and decreased serum C-reactive protein (CRP). Besides, mangiferin increased eNOS, reduced CRP and intercellular adhesion molecule-1 (ICAM-1), and decreased formation of ONOO– in aortic segments. Moreover, in vitro experiments performed in cultured human umbilical vein endothelial cells (HUVECs) treated with UA yielded results consistent with the observed results in vivo. Mangiferin in HUVECs treated with UA reduced protein and mRNA expression of CRP, reduced mRNA expression of ICAM-1, increased mRNA for eNOS, increased formation of NO and reduced the formation of ONOO– (Yang et al., 2018). Moreover, an in vitro study performed with digested mango fruit powder obtained from unripe CarelessTM mangoes in HUEVCs showed 23, 42, and 60% activation of eNOS for doses of 300, 1,500, and 3,000 µg mL−1, respectively (Gerstgrasser et al., 2016).
On the other hand, the antihypertensive effects of mangiferin alone were studied using vascular smooth muscle cells and mesenteric resistance arteries from normotensive (NT) and spontaneously hypertensive (HT) rats (Beltrán et al., 2004). The effect of 1 ng mL−1 interleukin-1β on COX-2 and iNOS protein expression in NT and HT rats was inhibited by 0.025 mg mL−1 mangiferin, but noradrenaline-induced contraction was not affected. Taken together, the results suggest that mangiferin may have an important role to play in decreasing hypertension through regulation of NO synthesis and improvement of endothelial function.
7 Conclusions
Mango fruit are highly nutritious, providing fiber, minerals, vitamins, and macronutrients. In addition, mango fruit are an important source of many bioactive phytochemicals, including polyphenols and carotenoids, which are biologically important as well as serving as fruit quality indicators, in addition to being medically useful. Many of these compounds exist in mango in very low abundance, although their contribution to human health, especially in a combined synergistic action with others might be significant. With better understanding of the chemical composition and changes that occur in mango fruit during development, and the interactions among the bioactive compounds, producers and consumers will be able to benefit from the diverse mango cultivars for fruit phytochemical characteristics that provide added value.
Enhanced fruit color, prolonged ripening, increased antioxidant content, and improved fruit characteristics for fresh consumption, processing or other agro-industrial uses can all contribute to increasing the value of mangoes. It is important to enhance various biotechnological strategies that might contribute to controlling the biochemical changes during mango fruit development before or after harvest in order to obtain fruit with excellent combinations of organoleptic and nutritional qualities, and for health benefits, for the final consumer.
The different signaling pathways that can be activated for antioxidant activity, immunomodulation, antiproliferation, and metabolic control of carotenoids, including β-carotene, and phenolic compounds (phenolic acids and polyphenols) from mango fruit are summarized in Fig. 1. However, the specific molecular mechanisms that underlie these complex biological functions of the carotenoids and phenolics present in mango fruit need to be further understood before mango and mango byproducts rich in carotenoids and phenolics can be fully exploited to aid in the prevention of carcinogenesis, DM, or other non-communicable diseases associated with chronic ROS production and inflammation. At present, no evidence suggests any dangers associated with consuming large amounts of carotenoids and phenolics from natural dietary sources like mango. However, people with alcohol consumption and smoking habits are recommended to avoid high β-carotene supplement doses. This review was limited in terms of us being able to categorically attest that consuming fresh or processed mangoes or dietary supplements derived from mango will provide specific benefits for human health. Much further work is required before such specific recommendations can be made, including does-response relationships and determination of possible synergistic interactions among various mango constituents.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
References
- Antihyperglycaemic effect of Mangifera indica in rat. Phytother. Res.. 1999;13(6):504-507.
- [CrossRef] [Google Scholar]
- Enzymes inhibitors from natural sources with antidiabetic activity: a review. Phytother. Res.. 2019;33(1):41-54.
- [CrossRef] [Google Scholar]
- Transforming p21ras mutants and c-Ets-2 activate the cyclin D1 promoter through distinguishable regions. J. Biol. Chem.. 1995;270:23589-23597.
- [Google Scholar]
- A mango (Mangifera indica L.) juice by-product reduces gastrointestinal and upper respiratory tract infection symptoms in children. Food Res. Int.. 2020;136:109492
- [Google Scholar]
- Andrade, M. J., Toledo, T. T., Nogueira, S. B., Cordenunsi, B. R., Lajolo, F. M., do Nascimento J. R. O., 2012. 2D-DIGE analysis of mango (Mangifera indica L.) fruit reveals major proteomic changes associated with ripening. J. Proteomics, 75, 3331-3341. doi: 10.1016/j.jprot.2012.03.047
- Aroma volatile constituents of Brazilian varieties of mango fruit. J. Food Comp. Anal.. 2000;13:27-33.
- [CrossRef] [Google Scholar]
- The role of Jun, Fos and the AP-1 complex in cell-proliferation and transformation. Biochim. Biophys. Acta. 1991;1072:129-157.
- [CrossRef] [Google Scholar]
- Reduction of dimethylhydrazine-induced cytotoxicity by mango fruit bar: changes in antioxidant enzymes in rats. Plant Foods Hum. Nutr.. 2003;58:1-11.
- [CrossRef] [Google Scholar]
- Polyphenols from mango (Mangifera indica L.) modulate PI3K/AKT/mTOR-associated micro-RNAs and reduce inflammation in non-cancer and induce cell death in breast cancer cells. J. Funct. Foods.. 2019;55:9-16.
- [Google Scholar]
- Effect of in vitro gastric and pancreatic digestion on antioxidant potential of fruit juices. Food Biosci.. 2017;17:1-6.
- [Google Scholar]
- Tumor necrosis factor-α increases reactive oxygen species by inducing spermine oxidase in human lung epithelial cells: a potential mechanism for inflammation-induced carcinogenesis. Cancer Res.. 2006;66:11125-11130.
- [Google Scholar]
- Gallic acid: a versatile antioxidant with promising therapeutic and industrial applications. RSC Adv.. 2015;5(35):27540-27557.
- [CrossRef] [Google Scholar]
- Changes in the nutritional quality of five Mangifera species harvested at two maturity stages. J. Sci. Food Agric.. 2017;97:4987-4994.
- [CrossRef] [Google Scholar]
- Urinary metabolites from mango (Mangifera indica L. cv. Keitt) galloyl derivatives and in vitro hydrolysis of gallotannins in physiological conditions. Mol. Nutr. Food Res.. 2016;60:542-550.
- [CrossRef] [Google Scholar]
- Body mass index as a determinant of systemic exposure to gallotannin metabolites during 6-week consumption of mango (Mangifera indica L.) and modulation of intestinal microbiota in lean and obese individuals. Mol. Nutr. Food Res.. 2019;63:e1800512.
- [Google Scholar]
- Improved recovery of galloyl metabolites from mango (Mangifera indica L.) in human plasma using protein precipitation with sodium dodecyl sulfate and methanol. Food Res. Int.. 2020;129:108812
- [CrossRef] [Google Scholar]
- Functional relationship between leptin and nitric oxide in metabolism. Nutrients. 2019;11(9):2129.
- [CrossRef] [Google Scholar]
- Vascular effects of the Mangifera indica L. extract (Vimang) Eur. J. Pharmacol.. 2004;499(3):297-305.
- [CrossRef] [Google Scholar]
- Medicinal properties of mangiferin, structural features, derivative synthesis, pharmacokinetics and biological activities. Mini Rev. Med. Chem.. 2015;15(7):582-594.
- [CrossRef] [Google Scholar]
- Screening of Mango (Mangifera indica L.) cultivars for their contents of flavonol o- and canthone c-glycosides, anthocyanins, and pectin. J. Agric. Food Chem.. 2005;53(5):1563-1570.
- [CrossRef] [Google Scholar]
- Divalent minerals decrease micellarization and uptake of carotenoids and digestion products into Caco-2 cells. J. Nutr.. 2011;141:1769-1776.
- [Google Scholar]
- Selected fruits reduce azoxymethane (AOM)-induced aberrant crypt foci (ACF) in Fisher 344 male rats. Food Chem. Toxicol.. 2007;45:725-732.
- [Google Scholar]
- Health effects of quercetin: from antioxidant to nutraceutical. Eur. J. Pharmacol.. 2008;585:325-337.
- [Google Scholar]
- Exogenous antioxidants—double-edged swords in cellular redox state: Health beneficial effects at physiologic doses versus deleterious effects at high doses. Oxid. Med. Cell Longev.. 2010;3:228-237.
- [Google Scholar]
- Early modulation of gene expression used as a biomarker for chemoprevention in a preclinical model of colon carcinogenesis. Pathol. Intl.. 2011;61:80-87.
- [Google Scholar]
- High-flavonol tomatoes resulting from the heterologous expression of the maize transcription factor genes LC and C1. Plant Cell. 2002;14:2509-2526.
- [Google Scholar]
- Carotenoid biosynthesis and chlorophyll degradation. In: Seymour G.B., Poole M., Giovannoni J.J., Tucker G.A., eds. The Molecular Biology and Biochemistry of Fruit Ripening. Iowa, USA: Blackwell Publishing Ltd; 2013. p. :75-116.
- [CrossRef] [Google Scholar]
- Brecht, J., Yahia, E.M., 2009. Postharvest physiology, in: R. Litz (Ed.), The mango. Botany, production and uses. CAB International, Wallingford, UK, Second Edition, pp. 484-528. ISBN 978 1 84593 489 7.
- Polyphenols: extraction methods, antioxidative action, bioavailability and anticarcinogenic effects. Molecules. 2016;21:901-939.
- [CrossRef] [Google Scholar]
- Fruit juice consumption modulates antioxidative status, immune status and DNA damage. J. Nutr. Biochem.. 2003;14:90-98.
- [Google Scholar]
- Effects of Mangifera indica (Careless) on microcirculation and glucose metabolism in healthy volunteers. Planta Med.. 2017;83:824-829.
- [CrossRef] [Google Scholar]
- Bioaccessibility of bioactive compounds after non-thermal processing of an exotic fruit juice blend sweetened with Stevia rebaudiana. Food Chem.. 2017;221:1834-1842.
- [Google Scholar]
- Mangos and their bioactive components: adding variety to the fruit plate for health. Food Funct.. 2017;8:3010-3032.
- [CrossRef] [Google Scholar]
- Efficacy of massive oral doses of retinyl palmitate and mango (Mangifera indica L.) consumption to correct an existing vitamin A deficiency in Senegalese children. Br. J. Nutr.. 1992;68(2):529-540.
- [CrossRef] [Google Scholar]
- Prostaglandin E2 promotes colon cancer cell growth through a Gs-axin-beta-catenin signaling axis. Science. 2005;310:1504-1510.
- [Google Scholar]
- Effects on plasma carotenoids and consumer acceptance of a functional carrot-based product to supplement vegetable intake: a randomized clinical trial. J. Functional Food. 2019;60:103421
- [CrossRef] [Google Scholar]
- Cervantes-Paz, B., Victoria-Campos, C. I., Ornelas-Paz, J. de J., 2016b. Absorption of carotenoids and mechanisms involved in their health-related properties, in: Carotenoids in Nature. Subcellular Biochemistry, vol 79, Stange, C. (Ed.), Springer, Cham., Switzerland, pp. 415-454. https://doi.org/10.1007/978-3-319-39126-7_16.
- Effect of pectin concentration and properties on digestive events involved on micellarization of free and esterified carotenoids. Food Hydrocolloides. 2016;60:580-588.
- [CrossRef] [Google Scholar]
- Effects of pectin on lipid digestion and possible implications for carotenoid bioavailability during pre-absorptive stages: A review. Food Res. Int.. 2017;99(2):917-927.
- [CrossRef] [Google Scholar]
- Total phenolic contents of 33 fruits and their antioxidant capacities before and after in vitro digestion. Ind. Crops Prod.. 2014;57:150-157.
- [Google Scholar]
- Direct observation of the β-carotene reaction with hydroxyl radical. J Phys. Chem. B.. 2011;115:2082-2089.
- [Google Scholar]
- Mechanisms of Antioxidants in the Oxidation of Foods. Compr Rev Food Sci Food Safety. 2009;8:345-358.
- [Google Scholar]
- Effects of quercetin and beta-carotene supplementation on azoxymethane-induced colon carcinogenesis and inflammatory responses in rats fed with high-fat diet rich in omega-6 fatty acids. Biofactors. 2006;27:137-146.
- [Google Scholar]
- In vitro and in vivo effects of mango pulp (Mangifera indica cv. Azucar) in colon carcinogenesis. Arch. Latinoam. Nutr.. 2014;64:16-22.
- [Google Scholar]
- Mango de azúcar (Mangifera indica L.) variedad de Colombia: características antioxidantes, nutricionales y sensoriales. Revista Chilena de Nutrición. 2014;41:312-318.
- [CrossRef] [Google Scholar]
- Mango (Mangifera indica cv. Azúcar) antiinflammatory and chemopreventive role during colorectal carcinogenesis. Emirates J. Food Agric.. 2016;28(10):1-9.
- [CrossRef] [Google Scholar]
- Bioavailability of dietary flavonoids and phenolic compounds. Mol. Aspects Med.. 2010;31(6):446-467.
- [CrossRef] [Google Scholar]
- Derese, S., Guantai, E.M., Souaibou, Y., Kuete, V., 2017. Mangifera indica L (Anacardiacea). In: Medicinal spices and vegetables from Africa, Therapeutic potential against metabolic, inflammatory, infectious and systemic diseases. Elsevier: Switzerland, pp. 451-483. https://doi.org/10.1016/B978-0-12-809286-6.00021-2.
- Data on changes in the fatty acid composition during fruit development and ripening of three mango cultivars (Alphonso, Pairi and Kent) varying in lactone content. Data Brief. 2016;9:480-491.
- [Google Scholar]
- Transcriptional transitions in Alphonso mango (Mangifera indica L.) during fruit development and ripening explain its distinct aroma and shelf life characteristics. Sci. Rep.. 2017;7:8711.
- [CrossRef] [Google Scholar]
- Improving the storage of minimally processed mangoes (Mangifera indica L.) by hot water treatments. Postharvest Biol. Technol.. 2009;52:221-226.
- [CrossRef] [Google Scholar]
- A Review on Ethnophamacological Applications, Pharmacological Activities and Bioactive Compounds of Mango (Mangifera indica L.) Evidence-Based Complement Alt. Med.. 2017;24
- [CrossRef] [Google Scholar]
- High hydrostatic pressure processing reduces the glycemic index of fresh mango puree in healthy subjects. Food Funct.. 2015;6(4):1352-1360.
- [CrossRef] [Google Scholar]
- Epriliati, I., Ginjom, I.R., 2012. Bioavailability of phytochemicals. In: Phytochemicals - A global perspective of their role in nutrition and health, Rao, V. (Ed.), IntechOpen, pp. 401-428.
- Nutriomic analysis of fresh and processed fruit products. 1. During in vitro digestions. J. Agric. Food Chem.. 2009;57(8):3363-3376.
- [CrossRef] [Google Scholar]
- Nutriomic analysis of fresh and processed fruit products. 2. During in vitro simultaneous molecular passages using Caco-2 cell monolayers. J. Agric. Food Chem.. 2009;57(8):3377-3388.
- [CrossRef] [Google Scholar]
- Study of the effect of 'Ataulfo' mango (Mangifera indica L.) intake on mammary carcinogenesis and antioxidant capacity in plasma of N-methyl-N-nitrosourea (MNU)-treated rats. Food Chem.. 2008;111(2):309-315.
- [CrossRef] [Google Scholar]
- Screening of antiproliferative effect of aqueous extracts of plant foods consumed in México on the breast cancer cell line MCF-7. Int. J. Food Sci. Nutr.. 2009;60(Suppl 6):32-46.
- [CrossRef] [Google Scholar]
- Carotenoid intake and esophageal cancer risk: a meta-analysis. Asian Pacific J. Cancer Prevention. 2013;14(3):1911-1918.
- [CrossRef] [Google Scholar]
- In vitro activation of eNOS by Mangifera indica (Careless™) and determination of an effective dosage in a randomized, double-blind, human pilot study on microcirculation. Planta Med.. 2016;82(4):298-304.
- [CrossRef] [Google Scholar]
- Physicochemical changes during progressive ripening of mango (Mangifera indica L.) cv. Dashehari under different temperature regimes. J. Food Sci. Tech. 2017:1-7.
- [CrossRef] [Google Scholar]
- Characterization and content of stilbenes and other polyphenols in tomato plants transformed with the stilbene synthase gene. Acta Hortic.. 2008;789:211-219.
- [Google Scholar]
- Ubá mango juices intake decreases adiposity and inflammation in high-fat diet-induced obese Wistar rats. Nutrition. 2016;32(9):1011-1108.
- [CrossRef] [Google Scholar]
- Bioactive compounds of the Ubá mango juices decrease inflammation and hepatic steatosis in obese Wistar rats. J. Funct. Foods. 2017;32:409-418.
- [CrossRef] [Google Scholar]
- Anti-diabetic effect of dietary mango (Mangifera indica L.) peel in streptozotocin-induced diabetic rats. J. Sci. Food Agric.. 2015;95(5):991-999.
- [CrossRef] [Google Scholar]
- Systemic levels of carotenoids from mangoes and papaya consumed in three forms (juice, fresh and dry slice) Eur. J. Clin. Nutr.. 2007;61:1180-2118.
- [CrossRef] [Google Scholar]
- A review on valorization of different byproducts of mango (Mangifera indica L.) for functional food and human health. Food. Bioscience. 2022;48:101783
- [CrossRef] [Google Scholar]
- Changes in intestinal microbiota and predicted metabolic pathways during colonic fermentation of mango (Mangifera indica L.)—Based bar indigestible fraction. Nutrients. 2020;12(3):683.
- [CrossRef] [Google Scholar]
- Protein and amino acid composition of ten tropical fruits by gas-liquid chromatography. J. Agric. Food Chem.. 1980;28(6):1217-1221.
- [CrossRef] [Google Scholar]
- Antioxidant and apoptosis-inducing activities of ellagic acid. Anticancer Res. 2006;26:3601-4366.
- [Google Scholar]
- Total carotenoid content in some mango (Mangifera Indica) varieties of Bangladesh. Inter. J. Pharm. Sci. Res.. 2015;6:4875-4878.
- [CrossRef] [Google Scholar]
- USDA database for the flavonoid content of selected foods release 3.3. U.S. Department of Agriculture; 2018.
- In vitro gastrointestinal digestion and colonic fermentation of high dietary fiber and antioxidant-rich mango (Mangifera indica L.) “Ataulfo”-Based Fruit Bars. Nutrients. 2019;11(7):1564.
- [CrossRef] [Google Scholar]
- Combination of mangiferin and dipeptidyl peptidase-4 inhibitor sitagliptin improves impaired glucose tolerance in streptozotocin-diabetic rats. Pharmacology. 2012;90:177-182.
- [CrossRef] [Google Scholar]
- Phytochemical profiling of the ripening of Chinese mango (Mangifera indica L.) cultivars by real-time monitoring using UPLC-ESI-QTOF-MS and its potential benefits as prebiotic ingredients. Food Chem.. 2018;256:171-180.
- [CrossRef] [Google Scholar]
- Effects of postharvest ripening on the nutraceutical and physicochemical properties of mango (Mangifera indica L. cv Keitt) Postharvest Biol. Technol.. 2015;103:45-54.
- [CrossRef] [Google Scholar]
- Mangiferin: a natural miracle bioactive compound against lifestyle related disorders. Lipids Health Dis.. 2017;16(1):84.
- [CrossRef] [Google Scholar]
- β-carotene inhibits Helicobacter pylori–induced expression of inducible nitric oxide synthase and cyclooxygenase-2 in human gastric epithelial AGS cells. J. Physiol. Pharmacol.. 2009;60(Suppl 7):131-137.
- [Google Scholar]
- Lycopene inhibits Helicobacter pylori–induced ATM/ATR-dependent DNA damage response in gastric epithelial AGS cells. Free Radic. Biol. Med.. 2012;52(3):607-615.
- [CrossRef] [Google Scholar]
- Comparison of postharvest changes in mango (cv Cogshall) using a Ripening class index (Rci) for different carbon supplies and harvest dates. Postharvest Biol. Technol.. 2009;54:25-31.
- [CrossRef] [Google Scholar]
- Physiological age at harvest regulates the variability in postharvest ripening, sensory and nutritional characteristics of mango (Mangifera indica L.) cv. Coghshall due to growing conditions and Mathieu L. echaudel. J. Sci. Food Agric. 2012:1282-1290.
- [CrossRef] [Google Scholar]
- Mitogen-activated protein kinase pathways mediated by ERK, JNK, and p38 protein kinases. Science. 2002;298:1911-1912.
- [Google Scholar]
- Identification and phylogenetic correlation among Colletotrichum gloeosporioides pathogen of anthracnose for mango. Biocatal. Agric. Biotechnol.. 2013;2:285-287.
- [CrossRef] [Google Scholar]
- Carotenoids, inflammation, and oxidative stress–implications of cellular signaling pathways and relation to chronic disease prevention. Nutr Res. 2014;34:907-929.
- [Google Scholar]
- Peel enzymatic activity and color changes in ripening mango fruit. J. Plant Physiol.. 1999;154:363-366.
- [CrossRef] [Google Scholar]
- A review of the bioavailability and clinical efficacy of milk thistle phytosome: A silybin-phosphatidylcholine complex (Silipos®) Altern. Med. Rev.. 2005;10(3):193-203.
- [Google Scholar]
- Mango polyphenolics reduce inflammation in intestinal colitis-involvement of the miR-126/PI3K/AKT/mTOR axis in vitro and in vivo. Mol. Carcinog.. 2016;56(1):197-207.
- [CrossRef] [Google Scholar]
- Antioxidant phytochemical and fruit quality changes in mango (Mangifera indica L.) following hot water immersion and controlled atmosphere storage. Food Chem.. 2007;105:1327-1334.
- [Google Scholar]
- Polyphenolic derivatives from mango (Mangifera indica L.) modulate fecal microbiome, short-chain fatty acids production and the HDAC1/AMPK/LC3 axis in rats with DSS-induced colitis. J. Funct. Foods. 2018;48:243-251.
- [Google Scholar]
- Mango (Mangifera indica L.) polyphenols: Anti-inflammatory intestinal microbial health benefits, and associated mechanisms of actions. Molecules. 2021;26:2732.
- [CrossRef] [Google Scholar]
- Antioxidant phytochemical and quality changes associated with hot water immersion treatment of mangoes (Mangifera indica L.) Food Chem.. 2009;115:989-993.
- [CrossRef] [Google Scholar]
- Antioxidant and antiproliferative activities of mango (Mangifera indica L.) flesh and peel. Food Chem.. 2010;121:429-436.
- [Google Scholar]
- Mango (Mangifera indica L.) polyphenols reduce IL-8, GRO, and GM-SCF plasma levels and increase Lactobacillus species in a pilot study in patients with inflammatory bowel disease. Nutr. Res.. 2020;75:85-94.
- [CrossRef] [Google Scholar]
- Stability of polyphenols epigallocatechin gallate and pentagalloyl glucose in a simulated digestive system. Food Res Int.. 2012;49:112-116.
- [Google Scholar]
- The role of methyl jasmonate in mango ripening and biosynthesis of aroma volatile compounds. J. Hortic. Sci. Biotechnol.. 2003;78:470-484.
- [CrossRef] [Google Scholar]
- The role of ethylene in mango fruit aroma volatiles biosynthesis. J. Hortic. Sci. Biotech.. 2003;78:485-495.
- [CrossRef] [Google Scholar]
- Multifaceted health benefits of Mangifera indica L. (Mango): the inestimable value of orchards recently planted in Sicilian rural areas. Nutrients. 2017;9(5):525.
- [CrossRef] [Google Scholar]
- The anti-cancer effect of Mangifera indica L. peel extract is associated to γH2AX-mediated apoptosis in colon cancer cells. Antioxidants. 2019;8(10):422.
- [CrossRef] [Google Scholar]
- Discrimination of mango fruit maturity by volatiles using the electronic nose and gas chromatography. Postharvest Biol. Technol.. 2008;48:122-131.
- [Google Scholar]
- Altered nitric oxide system in cardiovascular and renal diseases. Chonnam Med. J.. 2016;52(2):81-90.
- [CrossRef] [Google Scholar]
- Antiviral effects of black raspberry (Rubus coreanus) seed and its gallic acid against influenza virus infection. Viruses. 2016;8:157.
- [Google Scholar]
- Mangiferin prevents diabetic nephropathy progression in streptozotocin-induced diabetic rats. Phytother. Res.. 2010;24(6):893-899.
- [CrossRef] [Google Scholar]
- Ripening induced degradation of pectin and cellulose affects the far infrared drying kinetics of mangoes. Carbohydr. Polym.. 2022;291:119582
- [CrossRef] [Google Scholar]
- Mangiferin inhibition of proliferation and induction of apoptosis in human prostate cancer cells is correlated with downregulation of B-cell lymphoma-2 and upregulation of microRNA. Oncol Lett. 2016;182:817-822.
- [Google Scholar]
- Profiling of volatile fragrant components in a mini-core collection of mango germplasms from seven countries. PLoS One. 2017;12(12)
- [CrossRef] [Google Scholar]
- Transcriptional mechanism of differential sugar accumulation in pulp of two contrasting mango (Mangifera indica L.) cultivars. Genomics. 2020;112(6):4505-4515.
- [CrossRef] [Google Scholar]
- Effects of hot water treatment on anthracnose disease in papaya fruit and its possible mechanism. Postharvest Biol. Technol.. 2013;86:437-446.
- [CrossRef] [Google Scholar]
- Enhancement of nutraceutical bioavailability using excipient nanoemulsions: role of lipid digestion products on bioaccessibility of carotenoids and phenolics from mangoes. J. Food Sci.. 2016;81(3):N754-N761.
- [CrossRef] [Google Scholar]
- Physico-chemical and antioxidant properties of four mango (Mangifera indica L.) cultivars in China. Food Chem.. 2013;138:396-405.
- [CrossRef] [Google Scholar]
- Mastication effects on carotenoid bioaccessibility from mango fruit tissue. Food Res. Int.. 2015;67:238-246.
- [CrossRef] [Google Scholar]
- Microbial biotransformation of polyphenols during in vitro colonic fermentation of masticated mango and banana. Food Chem.. 2016;207:214-222.
- [CrossRef] [Google Scholar]
- Mango modulates body fat and plasma glucose and lipids in mice fed a high-fat diet. Br. J. Nutr.. 2011;106(10):1495-1505.
- [CrossRef] [Google Scholar]
- Quantification and purification of mangiferin from Chinese mango (Mangifera indica L.) cultivars and its protective effect on human umbilical vein endothelial cells under H2O2-induced stress. Inter. J. Mol. Sci.. 2012;13:11260-11274.
- [CrossRef] [Google Scholar]
- Chemical composition of mango (Mangifera indica L.) fruit: nutritional and phytochemical compounds. Frontiers. 2019;10, 1073:1-21.
- [Google Scholar]
- Iriflophenone-3-C-glucoside from Cyclopia genistoides: Isolation and quantitative comparison of antioxidant capacity with mangiferin and isomangiferin using on-line HPLC antioxidant assays. J. Chromatogr. B. Analyt. Technol. Biomed. Life Sci.. 2014;951–952:164-171.
- [Google Scholar]
- Influences of harvest date and location on the levels of β-carotene, ascorbic acid, total phenols, in vitro antioxidant capacity, and phenolic profiles of five commercial varieties of mango (Mangifera indica L.) J. Agric. Food Chem.. 2009;57(22):10825-10830.
- [CrossRef] [Google Scholar]
- Mangiferin decreases inflammation and oxidative damage in rat brain after stress. Eur. J. Nutr.. 2012;51:729-739.
- [Google Scholar]
- Major mango polyphenols and their potential significance to human health. Compr. Rev. Food Sci. Food Safety. 2008;7:309-319.
- [Google Scholar]
- Mangoes. In: Caballero B., Finglas P., Toldrá F., eds. Encyclopedia of Food and Health. Netherlands: Elsevier, Amsterdam; 2016. p. :641-645.
- [Google Scholar]
- Determination of free and total phenolic acids in plant-derived foods by HPLC with diode-array detection. J. Agric. Food Chem.. 2002;50:3660-3667.
- [CrossRef] [Google Scholar]
- Occurrence, biosynthesis and potentialities of ascorbic acid in plants. Int. J. Pl. An. Env. Sci.. 2011;1:167-184.
- [Google Scholar]
- HPLC and Mass Spectrometric Analysis of Carotenoids from Mango. J. Agric. Food Chem.. 1997;45:120-123.
- [CrossRef] [Google Scholar]
- Ultrasound-assisted extraction of carotenoids from mango (Mangifera indica L. ‘Ataulfo’) by-products on in vitro bioaccessibility. Food Biosci.. 2018;21:125-131.
- [Google Scholar]
- Changing of physiochemical properties and color development of mango fruit sprayed methyl Jasmonate. Sci. Hortic.. 2016;198:70-77.
- [CrossRef] [Google Scholar]
- Potential contribution of mangoes to reduction of vitamin A deficiency in Kenya. Ecol. Food Nutr.. 2009;48(6):482-498.
- [CrossRef] [Google Scholar]
- National Research Council (US), (1989). Subcommittee on the Tenth Edition of the Recommended Dietary Allowances. Recommended Dietary Allowances: 10th Edition. Washington (DC): National Academies Press (US); 2, Definition and Applications. Available at: https://www.ncbi.nlm.nih.gov/books/NBK234926/ (accessed 4 June 2022).
- Naturally occurring xanthones: chemistry and biology. J. Appl. Chem.. 2013;9
- [CrossRef] [Google Scholar]
- Anticarcinogenic effects of polyphenolics from mango (Mangifera indica) varieties. J. Agric. Food Chem.. 2010;58:4104-4112.
- [Google Scholar]
- Enhancement of the UVA induction of haem oxygenase-1 expression by beta-carotene in human skin fibroblasts. FEBS Lett.. 1999;460:212-216.
- [Google Scholar]
- Mango supplementation modulates gut microbial dysbiosis and short-chain fatty acid production independent of body weight reduction in C57BL/6 mice fed a high-fat diet. J. Nutr.. 2016;146(8):1483-1491.
- [CrossRef] [Google Scholar]
- Effects of a combination of β-carotene and vitamin A on lung cancer and cardiovascular disease. N. Engl. J. Med.. 1996;334:1150-1155.
- [Google Scholar]
- Hypertension. Nature Reviews Disease Primers. 2018;4:18014.
- [CrossRef]
- Ornelas, J. de J., Yahia, E. M., Failla, M., Gardea, A., 2008a. Impact of stage of ripening and dietary fat on in vitro bioaccessibility of β-carotene in ‘Ataulfo’ mango. Journal of Agricultural and Food Chemistry, 56(4), 1511-1516. https://doi.org/10.1021/jf072751r
- Identification and quantification of xanthophyll esters, carotenes, and tocopherols in the fruit of seven Mexican mango cultivars by liquid chromatography-atmospheric pressure chemical ionization-time-of-flight mass spectrometry. J. Agric. Food Chem.. 2007;55:6628-6635.
- [CrossRef] [Google Scholar]
- Changes in external and internal color and during postharvest ripening of ‘Manila’ and ‘Ataulfo’ mango fruit and relationship with carotenoid content determined by liquid chromatography-APcI+-time of flight mass spectrometry. Postharvest Biol. Technol.. 2008;50:145-152.
- [Google Scholar]
- Bioconversion efficiency of β-carotene from mango fruit and carrots in vitamin A. Am. J. Agric. Biol. Sci.. 2010;5(3):301-308.
- [CrossRef] [Google Scholar]
- Carotenoid composition in 'Ataulfo' mango and their bioavailability and bioconversion to vitamin A. Acta Hortic.. 2010;877:1245-1252.
- [Google Scholar]
- Mangiferin induces cell death against rhabdomyosarcoma through sustained oxidative stress. Integrative Med. Res.. 2015;4(2):66-75.
- [CrossRef] [Google Scholar]
- Identification and quantification of major phenolic compounds from mango (Mangifera indica, cv. Ataulfo) fruit by HPLC–DAD– MS/MS-ESI and their individual contribution to the antioxidant activity during ripening. Food Chem.. 2012;135:105-111.
- [CrossRef] [Google Scholar]
- Effect of ripeness stage of mango fruit (Mangifera indica L., cv. Ataulfo) on physiological parameters and antioxidant activity. Sci. Hortic.. 2012;135:7-13.
- [CrossRef] [Google Scholar]
- Purification and partial biochemical characterization of polyphenol oxidase from mango (Mangifera indica cv. Manila) J. Agric. Food Chem.. 2014;62(40):9832-9840.
- [CrossRef] [Google Scholar]
- PPARβ/δ and lipid metabolism in the heart. Biochimica et Biophysica Acta (BBA)-Molecular and Cell Biology of. Lipids. 2016;1860(10):1569-1578.
- [CrossRef] [Google Scholar]
- Induction of cell cycle arrest and apoptosis in human colon adenocarcinoma cell lines by beta-carotene through down-regulation of cyclin A and Bcl-2 family proteins. Carcinogenesis. 2002;23:11-18.
- [Google Scholar]
- Beta-carotene regulates NF-kappaB DNA-binding activity by a redox mechanism in human leukemia and colon adenocarcinoma cells. J. Nutr.. 2003;133:381-388.
- [Google Scholar]
- Tomato lycopene and inflammatory cascade: basic interactions and clinical implications. Curr. Med. Chem.. 2010;17(23):2547-2563.
- [Google Scholar]
- Mangiferin induces apoptosis by regulating Bcl-2 and bax expression in the CNE2 nasopharyngeal carcinoma cell line. Asian Pac. J. Cancer Prev.. 2014;15(17):7065-7068.
- [CrossRef] [Google Scholar]
- Induction of cytochrome P450 enzymes and over-generation of oxygen radicals in betacarotene supplemented rats. Carcinogenesis. 2001;22:1483-1495.
- [Google Scholar]
- Mangifera indica L. (Vimang) protection against serum oxidative stress in elderly humans. Arch. Med. Res.. 2006;37:158-164.
- [Google Scholar]
- Protective effects of Mangifera indica L extract (Vimang), and its major component mangiferin, on iron-induced oxidative damage to rat serum and liver. Pharmacol. Res.. 2008;57:79-86.
- [Google Scholar]
- Nuclear retinoic acid receptor β as a tool in chemoprevention trials. Curr. Med. Chem.. 2006;13(29):3553-3563.
- [Google Scholar]
- Neoplastic transformation of BALB/3T3 cells and cell cycle of HL-60 cells are inhibited by mango (Mangifera indica L.) juice and mango juice extracts. J. Nutr.. 2006;136(5):1300-1304.
- [Google Scholar]
- Contenido de fenoles totales en frutos de mango super Haden dañados por antracnosis y tratados en poscosecha. Cultivos Tropicales. 2016;37:71-77.
- [Google Scholar]
- Composition by LC-MS/MS of New Carotenoid Esters in Mango and Citrus. J. Agric. Food Chem.. 2016;64:8207-8224.
- [CrossRef] [Google Scholar]
- Perspective: A positive cocktail effect of the bioactive components in the diet. In: Pezzuto J.M., Vang O., eds. Natural Products for Cancer Chemoprevention. Switzerland: Springer; 2020. p. :613-629.
- [Google Scholar]
- Phenolic sulfates as new and highly abundant metabolites in human plasma after ingestion of a mixed berry fruit puree. Br. J. Nutr.. 2015;113:454-463.
- [Google Scholar]
- Pino, J. A., Mesa, J., Marti, M. P., Muñoz., Marbot, R., 2005. Volatile Components from Mango (Mangifera indica L.) cultivars. Journal of Agricultural and Food Chemistry, 53(6), 2213–2223. doi: 10.1021/jf0402633
- β-Carotene and β-apo-14'-carotenoic acid prevent the reduction of retinoic acid receptor β in benzo[a]pyrene-treated normal human bronchial epithelial cells. J. Nutr.. 2004;134:667-673.
- [Google Scholar]
- Impact of harvesting method on cosmetic appeal, physiological and biochemical attributes of mango fruits (Mangifera indica L.) J. Pharmacogn. Phytochem.. 2018;7:1468-1473.
- [Google Scholar]
- Pectic polysaccharides of mango (Mangifera indica L): structural studies. J. Sci. Food. Agric.. 2004;84:1731-1735.
- [CrossRef] [Google Scholar]
- Processing ‘Ataulfo’ mango into juice preserves the bioavailability and antioxidant capacity of its phenolic compounds. Nutrients. 2017;9:1082.
- [Google Scholar]
- Effects of ripening on the in vitro antioxidant capacity and bioaccessibility of mango cv. ‘Ataulfo’ phenolics. J. Food Sci. Technol.. 2019;56(4):2073-2082.
- [Google Scholar]
- Antioxidant properties and hyphenated HPLC-PDA-MS profiling of Chilean Pica mango fruits (Mangifera indica L. Cv. Piqueno) Mol.. 2013;19:438-458.
- [CrossRef] [Google Scholar]
- Effects of dietary flavonoids on apoptotic pathways related to cancer chemoprevention. J. Nutr. Biochem.. 2007;18:427-442.
- [CrossRef] [Google Scholar]
- Redfern, K. M., Cammack, V. L., Sweet, N., Preston, L. A., SoBHCS Student Team, Jarvis, M. A., Rees, G. A., 2017. Nutrient-extraction blender preparation reduces postprandial glucose responses from fruit juice consumption. Nutrition and Diabetes, 7(10), e288. doi: 10.1038/nutd.2017.36
- Oxidative stress, inflammation, and cancer: how are they linked? Free Radic. Biol. Med.. 2010;49:1603-1616.
- [Google Scholar]
- Electron-beam ionizing radiation stress effects on mango fruit (Mangifera indica L.) antioxidant constituents before and during postharvest storage. J. Agric. Food Chem.. 2007;55:6132-6139.
- [CrossRef] [Google Scholar]
- Phenolic compounds and antioxidant capacity of Brazilian mango (Mangifera indica L.) varieties. Food Chem.. 2008;110:620-626.
- [CrossRef] [Google Scholar]
- Cellular uptake of carotenoid-loaded oil-in-water emulsions in colon carcinoma cells in vitro. J. Agric. Food Chem.. 2006;54:9366-9369.
- [Google Scholar]
- Influence of whole and fresh-cut mango intake on plasma lipids and antioxidant capacity of healthy adults. Food Res. Int.. 2011;44:1386-1391.
- [CrossRef] [Google Scholar]
- Quality index, consumer acceptability, bioactive compounds, and antioxidant activity of fresh-cut “Ataulfo” mangoes (Mangifera indica L.) as affected by low-temperature storage. J. Food Sci.. 2009;74:126-134.
- [CrossRef] [Google Scholar]
- Postpartum development of endothelial dysfunction and oxidative stress markers in women with previous gestational diabetes mellitus. J. Endocrinol. Invest.. 2014;37:503-509.
- [CrossRef] [Google Scholar]
- Food matrix and processing influence on carotenoid bioaccessibility and lipophilic antioxidant activity of fruit juice based beverages. Food Funct.. 2016;7(1):380-389.
- [Google Scholar]
- Flavonoids and cancer prevention: a review of the evidence. J. Nutr. Gerontol. Geriatr.. 2012;31:206-238.
- [Google Scholar]
- Blood pressure targets for the treatment of people with hypertension and cardiovascular disease. Cochrane Database Systematic Rev.. 2020;9(9):CD010315.
- [CrossRef] [Google Scholar]
- Saleem-Dar, M, O. P., Chidley, H., Deshpande, A., Giri, A., Gupta, V., 2016. Nutrient and lavor content of mango (Mangifera indica L.) cultivars: An appurtenance to thel List of staple foods. In: Nutritional composition of fruit cultivars, Simmonds, M. S. J., Preedy, V. R., Switzerland, pp. 445-468. doi: 10.1016/B978-0-12-408117-8.00019-2.
- Modulation of diabetes and dyslipidemia in diabetic insulin-resistant rats by mangiferin: role of adiponectin and TNF-α. An. Acad. Bras. Cienc.. 2014;86(4):1935-1948.
- [CrossRef] [Google Scholar]
- Fructose induced lipogenesis: From sugar to fat to insulin resistance. Trends Endocrinol. Metab.. 2011;22:60-65.
- [Google Scholar]
- Current trends in small molecule discovery targeting key cellular signaling events towards the combined management of diabetes and obesity. Bioinformation. 2017;13(12):394-399.
- [CrossRef] [Google Scholar]
- Prebiotic effect of predigested mango peel on gut microbiota assessed in a dynamic in vitro model of the human colon (TIM-2) Food Res. Int.. 2019;118:89-95.
- [CrossRef] [Google Scholar]
- Identification of flavonol and xanthone glycosides from mango (Mangifera indica L. Cv. “Tommy Atkins”) peels by high-performance liquid chromatography-electrospray ionization mass spectrometry. J. Agric. Food Chem.. 2003;51(17):5006-5011.
- [CrossRef] [Google Scholar]
- Mangiferin ameliorates gestational diabetes mellitus-induced placental oxidative stress, inflammation and endoplasmic reticulum stress and improves fetal outcomes in mice. Eur. J. Pharmacol.. 2019;859:172522
- [CrossRef] [Google Scholar]
- Evaluating the toxic and beneficial effects of jering beans (Archidendron jiringa) in normal and diabetic rats. J. Sci. Food Agric.. 2011;91(14):2697-2706.
- [CrossRef] [Google Scholar]
- Activation of ethylene-responsive phydroxyphenylpyruvate dioxygenase leads to increased tocopherol levels during ripening in mango. J. Exp. Bot.. 2011;44:1254-1263.
- [CrossRef] [Google Scholar]
- Effect of Ethrel and 1-methylcyclopropene (1-MCP) on antioxidants in mango (Mangifera indica var. Dashehari) during fruit ripening. Food Chem.. 2008;111:951-956.
- [CrossRef] [Google Scholar]
- The antiproliferative effect of β-carotene requires p21waf1/cip1 in normal human fibroblasts. Eur. J. Biochem.. 2000;267:2290-2296.
- [Google Scholar]
- Cancer chemoprevention with dietary phytochemicals. Nat. Rev. Cancer. 2003;3:768-780.
- [CrossRef] [Google Scholar]
- Impact of fruit and vegetables on oxidative status and lipid profiles in healthy individuals. Food Public Health. 2012;2:113-118.
- [Google Scholar]
- Golden rice is an effective source of vitamin A. Am. J. Clin. Nutr.. 2009;89:1776-1783.
- [Google Scholar]
- Characterization of antioxidant and antiglycation properties and isolation of active ingredients from traditional chinese medicines. Free Rad. Biol. Med.. 2004;36:1575-1587.
- [Google Scholar]
- Metabolic effects of fructose and the worldwide increase in obesity. Physiol. Rev.. 2010;90:23-46.
- [Google Scholar]
- Mango (Mangifera indica L.), The King of fruits – An Overview. Food Rev. Int.. 2016;22:95-123.
- [CrossRef] [Google Scholar]
- Beta-carotene suppresses UVA-induced HO-1 gene expression in cultured FEK4. Free Radic. Biol. Med.. 2003;34(4):456-464.
- [Google Scholar]
- United States Department of Agriculture (USDA), Agricultural Research Service, 2018. USDA National Nutrient Database for Standard Reference, Release 1 April, Nutrient Data Laboratory Home Page, https://ndb.nal.usda.gov/ndb/ (accessed 6 July 2022).
- Enteral feeding enriched with carotenoids normalizes the carotenoid status and reduces oxidative stress in long-term internally fed patients. Clin. Nutr.. 2006;25:897-905.
- [Google Scholar]
- Ascorbic acid content in exotic fruits: A contribution to produce quality data for food composition databases. Food Res. Int.. 2011;44:2237-2242.
- [CrossRef] [Google Scholar]
- Vallarino, J.G., Osorio, S., 2019. Organic acids. In: Yahia, E.M., Carrillo-López, A., (Eds.), Postharvest Physiology and Biochemistry of Fruits and Vegetables, Elsevier, Switzerland, pp. 207–224).
- Carotenoid composition of mango (Mangifera indica L.) wine and its antioxidant activity. J. Food Chem.. 2011;35:1538-1547.
- [CrossRef] [Google Scholar]
- Varietal differences in the bioaccessibility of β-carotene from mango (Mangifera indica) and papaya (Carica papaya) fruits. J. Agric. Food Chem.. 2007;55(19):7931-7935.
- [Google Scholar]
- Effect of ripening, heat processing, and fat type on the micellarization of pigments from Jalapeño peppers. J. Agric. Food Chem.. 2013;61(41):9938-9949.
- [Google Scholar]
- El mango: aspectos agroindustriales, valor nutricional/funcional y efectos en la salud. Nutr. Hosp.. 2015;31:67-75.
- [Google Scholar]
- PPARs: diverse regulators in energy metabolism and metabolic diseases. Cell Res.. 2010;20:124-137.
- [CrossRef] [Google Scholar]
- Dietary total antioxidant capacity is associated with diet and plasma antioxidant status in healthy young adults. J. Acad. Nutr. Diet.. 2012;112:1626-1635.
- [Google Scholar]
- A 4-wk intervention with high intake of carotenoid-rich vegetables and fruit reduces plasma C-reactive protein in healthy, nonsmoking men. J. Nutr.. 2011;141:1827-1833.
- [Google Scholar]
- WHO/FAO, 2003. Diet, nutrition and prevention of chronic diseases. Report of a join WHO/FAO expert consultation (WHO technical report series 916. World Health Organization. Geneva, Switzerland, 1-149. http://apps.who.int/iris/bitstream/10665/42665/1/WHO_TRS_916.pdf?ua=1. (accessed 3 August 2022).
- WHO/FAO, 2004. Vitamin and mineral requirements in human nutrition, second edn. WHO, Geneva, Switzerland.
- Beta-carotene modulates human prostate cancer cell growth and may undergo intracellular metabolism to retinol. J. Nutr.. 2000;130:728-732.
- [Google Scholar]
- Protective role of probiotics and prebiotics in colon cancer. Am. J Clin. Nutr.. 2001;73:451-455.
- [Google Scholar]
- The changes in physical, biochemical, physiological characteristics and enzyme activities of mango cv. Jinhwang during fruit growth and development. J Life Sci.. 2015;1–6
- [CrossRef] [Google Scholar]
- Carotenoids as a source of antioxidants in the diet. Subcell. Biochem.. 2016;79:359-375.
- [Google Scholar]
- Dietary intake of vitamins A, C, and E and the risk of colorectal adenoma: a meta-analysis of observational studies. Eur. J. Cancer Prev.. 2013;22(6):529-539.
- [CrossRef] [Google Scholar]
- Antisense RNA: the new favorite in genetic research. J. Sci. Biochem.. 2018;19:739-749.
- [CrossRef] [Google Scholar]
- The contribution of fruit and vegetable consumption to human health. In: Benkeblia N., ed. Postharvest Technology for Horticultural Crops. Kerala: Research Signpost Publisher; 2009. p. :139-163.
- [Google Scholar]
- Mango (Mangifera indica L.) In: Yahia E., ed. Postharvest biology and technology of tropical and subtropical fruits. Cocona to mango, Elsevier: Switzerland; 2011. p. :492-567.
- [Google Scholar]
- Chemistry, stability and biological actions of carotenoids. In: González Aguilar G.A., de la Rosa L.A., Alvarez Parrilla E., eds. Fruit and vegetable phytochemicals: Chemistry, nutritional value and stability. USA: Blackwell Publishing; 2010. p. :177-222.
- [Google Scholar]
- Nutritional and health-promoting properties of tropical and subtropical fruits. In: Yahia E.M., ed. Postharvest Biology and Technology of Tropical and Subtropical Fruits. Volume 1. Fundamental Issues. England: Woodhead Publishing; 2011. p. :21-78.
- [Google Scholar]
- Chemistry, stability and biological actions of carotenoids. In: Yahia E.M., ed. Fruit and vegetable phytochemicals. England: Blackwell Publishing; 2018. p. :285-345.
- [Google Scholar]
- The contribution of fruit and vegetable consumption to human health. In: Yahia E.M., ed. Fruit and vegetable phytochemicals: Chemistry and human health. Vol Volume 1. Oxford: John Willey & Sons Ltd; 2018. p. :3-52.
- [Google Scholar]
- Contribution of fruits and vegetables to human nutrition and health. In: Yahia E.M., ed. Postharvest physiology and biochemistry of fruits and vegetables. United Kingdom: Woodhead Publishingp; 2019. p. :19-45.
- [Google Scholar]
- Mangiferin alleviates hypertension induced by hyperuricemia via increasing nitric oxide releases. J. Pharmacol. Sci.. 2018;137(2):154-161.
- [CrossRef] [Google Scholar]
- Association of dietary vitamin A and β-carotene intake with the risk of lung cancer: a meta-analysis of 19 publications. Nutrients. 2015;7(11):9309-9324.
- [CrossRef] [Google Scholar]
- Bioavailability of vitamin A form peach palm (Bactris gaspiaes H.B.K) and from mango Mangifera indica L.) in rats. Nutr. Res.. 1991;11(10):1167-1175.
- [CrossRef] [Google Scholar]
- Effect of mango (Mangifera indica) cv. Azucar juice consumption on plasma and oxidative stress biomarkers. Vitae. 2020;27(1)
- [CrossRef] [Google Scholar]
- Flavonoids as aryl hydrocarbon receptor agonists/antagonists: effects of structure and cell-context. Environ. Health Perspect.. 2003;111(16):1877-1882.
- [CrossRef] [Google Scholar]
- Transcriptional factors mediating retinoic acid signals in the control of energy metabolism. Int. J. Mol. Sci.. 2015;16:14210-14244.
- [CrossRef] [Google Scholar]
- Mangiferin induces apoptosis in human ovarian adenocarcinoma OVCAR3 cells via the regulation of Notch3. Oncol. Rep.. 2017;38(3):1431-1441.
- [CrossRef] [Google Scholar]
- Yahia, E.M., J. J. Ornelas Paz, and R. Ariza. 2006. The Mango (in Spanish). Trillas, México, ISBN 968-24-7380-2, 224 pages.







