Translate this page into:
The design of natural hybrid biomaterial to promote osteogenic differentiation, collagen i and II expression and relief of musculoskeletal pains: Bone tissue-engineering applications (in-vitro and clinical studies)
⁎Corresponding authors. azadeh.izadyari@gmail.com (Azadeh Izadyari Aghmiuni), saeedhey@gmail.com (Saeed Heidari keshel)
-
Received: ,
Accepted: ,
This article was originally published by Elsevier and was migrated to Scientific Scholar after the change of Publisher.
Abstract
This research aimed to fabricate a natural hybrid biomaterial and assess its physicochemical/biological properties to study the role of the therapeutic biomaterial in the growth of human monocytes, synoviocytes, chondrocytes, expression of type-I/II collagen, and osteogenic differentiation for bone tissue engineering applications, as well as to assess effects of bio-ointment derived from this biomaterial on relief of musculoskeletal pains. Hence, an oily formula [turmeric:ginger:chili pepper:rose oils (1:1:1:1w/w)] was mixed with the base oil [sesame:black seed:olive oils (1:0.4:0.6w/w)] in 3 wt ratios of 1:6(H1), 1.5:5.5(H2), and 2:5(H3). Accordingly, H2 indicated a better performance in cell growth and decrease of inflammatory mediators and led to the highest expression of osteoblastic genes of RUNX2, OCN, and OPN, as well as collagen I and II (COL1A1,COL2A) respectively 1.3-fold, 1.7-fold, and 1.4-fold, as well as %87 % and ∼ 60 % higher than H1, and ∼ 2-fold, 2.1-fold, and 3.4-fold, and ∼ 19 % and ∼ 16 % higher than H3. A clinical study on H2 was also performed on 50 patients with musculoskeletal pains. H2-based bio-ointment could relieve pain in less than 10 min for 40 % of patients and after 30 min from use for 35 %. Moreover, the analgesia period for 60 % and 36 % of patients lasted for longer than 3 h and between 2 h and 3 h, respectively. 76 % also stated that bio-ointment possesses stronger effects than other treatments along with moderate warming functions. Finally, although 6 % of patients reported mild effectiveness in pain relief, 92 % of users stated this therapeutic method has resulted in more effects on their pain relief and damaged organ performance recovery.
Keywords
Natural biomaterials
Osteogenic differentiation
Musculoskeletal pains
Non-steroidal anti-inflammatory drugs (NSAIDs)
Bone regeneration
Bio-ointment
1 Introduction
The disorders, diseases, or injuries of musculoskeletal (such as osteoarthritis, rheumatoid arthritis, psoriatic arthritis, bone, low back, and joint pains, etc.), neuromuscular and neurological problems (such as fasciculations (muscle twitches), myalgia (muscle pain), sciatica, etc.), and trauma, physical activity or exercise (such as muscle cramps or strain, the pains of shoulder and neck, arm, tennis elbow, forearm, and wrist, spinal disc problems and spinal pains, etc.) are one of the main causes of pain and disability in people.
Based on the WHO report (14 July 2022), these injuries cause disability in more than 150 countries resulting in reduced participation in society or early retirement (WHO, 2022). In these injuries or diseases, the most common complaint is related to acute and chronic pains (mild to severe) that non-steroidal anti-inflammatory drugs (NSAIDs), such as diclofenac, ibuprofen, naproxen, etc. in the forms of intravenous, intramuscular, oral, or topical, are widely prescribed by physicians (Ozgoli et al., 2009). The main mechanism of NSAIDs for relieving pain is to inhibit the biosynthesize of cyclooxygenase enzymes 1 and 2 (COX-1 and 2) and convert arachidonic acid into thromboxanes, prostaglandins, and prostacyclins. However, such drugs are mainly used for treating adults with mild to moderate pains and can lead to side effects like indigestion, bleeding gums, stomach upset, bleeding in the gastrointestinal tract, chest pain or tightness, blurred vision, nausea, etc., due to inhibiting both types of COX 1 and 2. Indeed, inhibition of COX-1 leads to a decrease in the secretion of the mucus layers in the stomach and consequently increase in the risk of bleeding the stomach and its perforation (Peddada et al., 2015). Moreover, the excessive use of these relievers increases the doses of the drug and/or the lack of its effectiveness for a long time. Notably, there are no drugs that can be effective on all types of Musculoskeletal and neuromuscular pains, and generally, drugs are different from one pain to another.
Nowadays, the reports indicate, however, that herbs-derived new drugs can be suitable therapeutic alternatives for treating pain and reducing the side effects of chemical drugs (Rondanelli et al., 2020). Based on the studies, several medicinal plants in traditional Iranian and Chinese medicine possess the ability to reduce each type of pain, due to biomolecules or bioactive ingredients such as sesquiterpenes, monoterpenoids, flavonoids, phenols, etc. Zingiber officinale (ginger) is one of these herbs that contains more that more than 400 chemical constituents or bioactive substances (biomacromolecules) and is known as a multi-mechanisms plant with an effective function in reducing pain and inflammation in clinical trials (Ozgoli et al., 2009; Rahnama et al., 2012; Ravindran and Babu, 2016; Therkleson, 2010a, 2010b; Yip and Tam, 2008). Biomolecules of ginger such as 6-gingerol can modulate the types of transient, acute, and chronic pains via various mechanisms such as inhibiting COX (1 and 2) and lipoxygenase (LOX)-pathways and subsequently prostaglandins, suppressing NF-kB activation and TNF-α, increasing antioxidant activities and decreasing inflammatory, and act as a promising natural reliever for reducing the pain of muscles and osteoarthritis, as well as chronic low back pain, after oral or topical administration (Ahn et al., 2009; Daily et al., 2015).
Capsicum annuum (chili pepper) is another plant in this field that contains capsaicinoids, especially capsaicin. The bioactive ingredients of this plant possess a variety of pharmacological effects such as pain-relieving, anti-inflammatory, and antioxidant effects. The studies have demonstrated that chili pepper, as a topical treatment, can effectively reduce peripheral neuropathic pains (Chung and Campbell, 2016; Derry et al., 2017; Giacalone et al., 2015). Indeed, capsaicin is the main factor in the stimulation of peripheral nerve endings which leads to counteraction of nociceptors and consequently, neuropathic pains, due to this natural protoalkaloid possesses a variety of effects on the function of primary sensory neurons (Giacalone et al., 2015; Ilie et al., 2019; Szolcsányi and Pintér, 2013). Accordingly, this biomolecule possesses suitable therapeutic efficacy in reducing pain in patients with Morton’s neuroma such that the establishment of long-term analgesia was an attractive clinical result in this matter (Campbell et al., 2016). Capsaicin also can attenuate hyperalgesia in the knee joint and inhibit genetic ablation of TRPV11 receptors, joint inflammation, and nociception, as well as neural dysfunction (Helyes et al., 2010; Ilie et al., 2019; Lee et al., 2012). However, this bioactive is dose-dependent and its high doses can cause skin irritation or cutaneous reactions, itching, and/or severe allergies (Anand and Bley, 2011; Weisshaar et al., 1998).
Curcuma longa (turmeric) is one of the other plants in this field that is known as a potential natural alternative drug for arthritis pain relief and joint inflammation treatment (Henrotin et al., 2011; Lakhan et al., 2015). Generally, this plant has an activity similar to ginger while it possesses different bioactive ingredients, mainly curcumin. Notably, unlike ginger, NSAIDs, and glucocorticoids, turmeric can selectively modulate COX-2 and NF-κB activities and lead to the modification of pro-inflammatory cytokines like the production of IL-6 and 82 (Appelboom et al., 2014; Goel et al., 2001; Jurenka, 2009; Peddada et al., 2015; Prasad et al., 2014). Moreover, this plant can be the better therapeutic option for patients who are sensitive to pepper and ginger, due to being less hot and spicy. Based on the clinical studies, curcumin can be a suitable alternative to COX-2 inhibitors (such as celecoxib) from which long-term use may lead to cardiovascular toxicity (Lev-Ari et al., 2006).
Another sample in this field is Rosa rubiginosa (rose) which has diverse pharmacological properties and biological activities so that among traditional medicine physicians, this plant is known as a multi-potency drug (Murakkab al quwa) and, according to Ibn Sina, it possesses moderate temperament or Mizaj3 (Mail baEteda) (Analysis, 2012; Ansari et al., 2017). Hence, it seems that rose oil can modulate the side effects of chili pepper such as skin allergies, itching, and irritation. Based on the reports, topical rose oil can relieve low-back pains which are related to pregnancy (Shirazi et al., 2017), and provides joint health in inflammatory musculoskeletal disorders, owing to its antioxidant, anti-inflammatory, and analgesic properties originating from polyphenols (Pekacar et al., 2021). This oil can also lead to control of stem cell differentiation into targeted tissue cells (Kim et al., 2010).
The important point in using these oils is the extraction of their bioactive ingredients by the carrier oil that can lead to transportation and penetration of herbal actives from and into the skin barrier In this field, jojoba, sesame, black seed, and olive oils, etc. are considered as oils with warm Mizaj which possess aromatic and therapeutic properties due to calming and soothing biomolecules such as Sesquiterpenes, thymoquinone, flavonoids, Lignan, squalene, and Vitamins K (Cárdeno et al., 2015; Chin, 2020; Khader and Eckl, 2014; Phitak et al., 2012). The carrier oils can also be used to dilute essential oils and reduce their cytotoxicity, without any antimicrobial antagonism when combined with bioactive ingredients. Hence, here, the combination of 3 carrier oils (sesame, black seed, and olive) is selected due to thermal stability (up to 65 °C), oxidative stability, anti-inflammatory effects, as well as antioxidant and antimicrobial properties (Cárdeno et al., 2015; Michalak, 2018; Phitak et al., 2012; Zhao et al., 2015).
Given the positive effects of herbal active ingredients or biomolecules in regenerating tissue and reducing inflammation pathways for relieving musculoskeletal pains, this study has been firstly focused on the fabrication of a hybrid oily biomaterial and assessment of its physicochemical and biological properties (analysis of oil characterizations, MTT and cytotoxicity assay, IC50, anti-inflammatory effects). Hence, an oily formula containing turmeric, ginger, chili pepper, and rose oils with weight ratios of 1:1:1:1 w/w was mixed with the base oil including sesame, black seed, and olive (1:0.4:0.6 w/w) in 3 wt ratios of 1:6 w/w, 1.5:5.5 w/w, and 2:5 w/w to obtain 3 different oily formulas. Notably, base oils were selected because these oils (i.e. sesame, black seed, and olive oils) are known as natural solvents to extract herbal active ingredients, with higher thermal stability. Moreover, these oils themselves possess therapeutic and warming effects and lead to an increase in cell energy and a decrease in muscular and nervous tensions, improve circulation, and tissue repair or regeneration. The combination of these oils can also provide better stability of the oil, as well as regulation of bone metabolism and bone healing through activating turnover, and reduces a wide range of inflammation and pains (such as rheumatoid arthritis) by increasing the lubrication between the joints.(Griel et al., 2007; Kheiridoost-Langaroodi et al., 2022; Warinhomhoun et al., 2023).
In the following, in-vitro studies were carried out on 3 cells of Human monocytes, human synoviocytes, and human chondrocytes, as well as human adipose-derived stem cells (h-ADSCs) for assessment of cellular behavior in the media containing oily formulas, as well as the evaluation of oil effect on the h-ADSCs differentiation into osteoblast cells (RT-PCR, alkaline phosphatase activities assay, and Alizarin Red Staining assay). After evaluating therapeutic oil characteristics, determining its dosage, and selecting the best formula (as phase I of the study in vitro), a bio-ointment was fabricated by adding beeswax to therapeutic oil, to improve the viscosity of the final product. Afterward, the effects of the herbal bio-ointment were studied on 50 patients with musculoskeletal pains at the clinic of Bone specialty by an orthopedic specialist/ orthopaedist, Tehran province between 2022 and 07-16 and 2022–09-16, as phase II of the study [Female: 35 patients, male: 15 patients, in the age group of 10 to 88 years (10–15 years: 3 patients, 16–30 years: 15 patients, 31–50 years: 11 patients, and 50 years <: 21 patients]. After screening, the patients were defined into two groups: 1- Intervention group: the receiving patients of the herbal anti-pain bio-ointment [no other topical analgesics, and oral NSAIDs and/or COX-2 inhibitors]; 2- Intervention group (Control): the same patients in group (1) who used drugs, analgesia, or other products (a 1-week before using anti-pain bio-ointment) to compare the effectiveness of herbal anti-pain bio-ointment with other drugs and analgesia. Finally, the therapeutic efficacy of this anti-pain bio-ointment containing herbal biomolecules was studied through the Western Ontario and McMaster Universities (WOMAC) index, in 4 items of the speed of pain relief, the time of pain relief or analgesia period (i.e. the time interval between cessation of the pain, after using the bio-ointment, and its resumption), the warming function of the damaged area, and the effectiveness of the formulated bio-ointment than other treatments.
2 Materials and methods
2.1 Materials
Phosphate-buffered saline (PBS), dimethyl sulfoxide (DMSO), and Tetrazolium salt 3-(4, 5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT) were obtained from Sigma (Sigma-Aldrich Inc., St. Louis, MO, USA). Zingiber officinale, Capsicum annuum, Curcuma longa, and Rosa rubiginosa were also purchased from the Nikan-Star Company (Tehran, Iran). Moreover, 3 carrier oils of sesame, black seed, and olive were purchased from ZIMA Company (Tehran, Iran). Pen-Strep [Penicillin-streptomycin, 1/10 U/ml], dulbecco’s modified eagle’s medium–low glucose (DMEM-LG), Ham's F12 medium, and fetal bovine serum (FBS) were purchased from Gibco (Massachusetts, USA).
2.2 Methods
2.2.1 Preparation of herbal oils
The oily extract of medical plants of turmeric, ginger, chili pepper, and rose is obtained in the process of herb/plant powder maceration into oil to extract herbal bioactive ingredients and their biomacromolecules. The extraction process was carried out at 50 °C for 4 h by a bubble column for better mass transfer. Notably, sesame oil was used as the extracting oil of the active ingredient for the mentioned plants due to higher thermal stability (Espitia Cubillos et al., 2019).
The composition of the extracted oils, with weight ratios of 1:1:1:1 w/w, was mixed with the base oil including sesame, black seed, and olive (1:0.4:0.6 w/w) in 3 wt ratios of 1:6 w/w, 1.5:5.5 w/w, and 2:5 w/w, at 50 °C for 1 h (Table 1).
No.
The weight ratio for the composition of turmeric, ginger, chili pepper, and rose oils
The weight ratio for the composition of sesame, black seed, and olive oils
Code of combined therapeutic oils (w/w)
1
1
6
H1 (1:6 w/w)
2
1.5
5.5
H2 (1.5:5.5 w/w)
3
2
5
H3 (2:5 w/w)
Notably, a preclinical study was performed on healthy subjects to check the anti-allergic properties of the formulas. Based on the results, therapeutic oils are non-allergenic when combined in equal proportions. Moreover, base oil was prepared by mixing sesame, black seed, and olive with a ratio of 1, 0.4, and 0.6w/w, respectively. Sesame oil possesses a greater weight ratio than two other oils because this oil has good thermal stability for the extraction process.
2.2.2 Characterizations of combined therapeutic oils
Gas Chromatography-Mass (GC–MS) study: In the following, the phytochemical study was carried out for bio-macromolecules determination in the structure of 3 therapeutic oil groups, by GC–MS Spectrometry (N-5973 Model, Agilent). Notably, the GC–MS consisted of a column with a height of 10 m and diameter of 0.25 mm (injection volume: 2 µl, solvent: chloroform, detector temperature: 290 °C, retention time: 10 min); a 5 °C/min ramp was determined for oven temperature. The process was run in the range of 40–500 m/z and the data were compared with NIST-library and Willey-library (Amiri et al., 2023; Smeriglio et al., 2017).
Fourier Transform Infrared Spectroscopy (FTIR) analysis: To assess the interactions of active ingredients in the 3 groups of H1, H2, and H3, FTIR spectra analysis was carried out with FTIR spectrometer (ALPHA, Bruker, Germany), at 400–4000 cm−1 (Izadyari Aghmiuni et al., 2019).
1,1-Diphenyl-2-picrylhydrazyl (DPPH) assay: The study of radical scavenging activities was also determined for the formula of combined therapeutic oils (i.e. H1, H2, and H3), by a spectrophotometric method based on a methanol solution of DPPH. To this end, 1 ml of various concentrations of oils (1.56, 3.12, 6.25, 12.5, 25, 50, 100, 150, 200 µg/ml) was separately added to 2 ml of 0.004 % DPPH in methanol solution. The mixtures were vigorously shaken and kept in darkness for 30 min (25 °C). Afterward, the colorimetric change of each sample, from violet to light yellow, was measured at 510 nm by a spectrophotometer (Shimadzu UV-1601, Japan). Notably, absolute methanol and DPPH: Methanol (1:1 v/v) solutions were respectively used to zero the spectrophotometer and apply as the control group. Radical scavenging activities were determined by equation (1), as inhibition (%) of DPPH radical (Kumar et al., 2019; Warinhomhoun et al., 2023). Moreover, the tests were performed in triplicate (n = 3) and evaluated for freshly prepared samples and samples preserved for 6 and 12 months.
The antioxidant activity of all 3 groups -for freshly prepared oils- was also assessed as IC50 (µmolar concentration required for inhibiting DPPH radicals formation by 50 %), by plotting inhibition percentage vs. oil concentrations.
2.2.3 Biological analyses (In vitro study on combined therapeutic oils)
To assess the cellular behavior of designed oily formulas in vitro studies were carried out on the cells of Human monocytes, human synoviocytes, and human chondrocytes, as well as human adipose-derived stem cells for evaluation of oil effect on the cell differentiation into osteoblast cells.
Cell seeding and culture: To this end, human monocytes [NCBI Code: C563, Iranian Pasture Institute cell bank, Tehran, Iran] were seeded in 24-well plates (1 × 106 cells/well) and allowed to attach for 1 h. Afterward, cells were cultured with low-endotoxin RPMI1640 medium (Gibco) containing the human serum (1 %) and then incubated humidified incubator at 37 °C (24 h).
Moreover, a human synoviocyte line from a healthy donor [NCBI Code: C616, Iranian Pasture Institute cell bank, Tehran, Iran] was seeded into 24-well plates (1 × 106 cells-well) and then cultured by DMEM-low glucose (DMEM-LG), containing 10 % FBS, at 37 °C for 24 h.
Likewise, human chondrocytes [NCBI Code: C620, Iranian Pasture Institute cell bank, Tehran, Iran] were seeded into 24-well plates (1 × 106 cells-well) and then cultured by DMEM and Ham's F12 (1:1) medium and 10 %FBS, at 37 °C (24 h).
Moreover, the 3rd passage of h-ADSCs isolated in our previous study was applied in this study (Aghmiuni et al., 2022). The cells were then seeded into 24-well plates (1 × 106 cells/well), and cultured by DMEM-LG and FBS (15 %), pen/strep (1 %) (24 h, 37 °C).
MTT Assay: In the following, to study cell viability (%) by MTT assay, the mentioned cells (1 × 104 cells/well) were seeded in a 96-well plate and treated with 25, 50, 100, 150, 200 μg/ml concentrations of therapeutic oils which were combined with weight ratios of H1, H2, and H3, for 24 and 48 h; by the protocol performed in our previous study (Izadyari Aghmiuni et al., 2020). The cell viability (%) was obtained by equation (2).
Cytotoxicity assay: Moreover, to assess the cytotoxicity of oily samples (H1, H2, and H3), the level of the lactate dehydrogenase (LDH) released in the culture media was evaluated using the LDH Assay kit (KLDH96, KiaZist Company, Hamadan, Iran) after 24 h and 48 h. Based on the manufacturer's protocol, Permisolution was applied to generate the maximum LDH release (as positive control). After 1 h incubation with Permisolution, the plates were centrifuged at 400 × g for 5 min; then 50 μl of obtained supernatants were transferred to a 96‑well plate included in the kit and 50 μl of the working Buffer (LDH Assay Buffer 2X; KLDH96, KiaZist Company, Hamadan, Iran) were added to each well. The plates were then incubated at 37C in darkness, for 30 min. Finally, the absorbance was read at 540 nm using a microplate spectrophotometer (Detector Zenyth 3100). The results were calculated according to the equation (3) (Kumar et al., 2018).
Real-time polymerase chain reaction (RT-PCR): The 3rd passage of h-ADSCs isolated in our previous study was applied in the assessment of the osteogenic differentiation of these cells induced by the media containing herbal oils (Aghmiuni et al., 2022). To this end, the h-ADSCs were cultivated on 24-well plates (1 × 106 cells/well) supplemented DMEM-LG-Han’s/F2 along with 10 % FBS, and pen-strep (1 %). After 24 h, herbal oils of H1, H2, and H3 were added to culture media, according to selected concentrations in the cytotoxicity test (level of the LDH). Notably, the oil-free culture medium and the osteogenic differentiation medium [DMEM-F12 containing FBS (10 %), L-ascorbic acid (50 µg/ml), dexamethasone (100 nanomolar), and β-glycerol phosphate disodium salt (10 µM), 10 mM L-glutamine, 1 % pen-strep] were respectively applied as negative and positive control groups. After 7, 14, and 21 days, the osteogenic differentiation potential of h-ADSCs was determined via the RT-PCR, based on the modified protocol reported in our previous study (Aghmiuni et al., 2020; Izadyari Aghmiuni et al., 2021). Briefly, mRNA isolation (from cultured cells on the oil-based media) and reverse transcription (RT) were respectively carried out by RNA extraction kit and the Revert Aid-first strand cDNA synthesis kit (TAKARA, Japan). Finally, the PCR reaction was carried out by the following program:
The denaturation step includes 60 sec at 95 °C; 40 cycles (10 sec, 95 °C), then 15 sec at 60 °C, and 15 sec at 72 °C. The final stage also includes the extension step (300 sec, 40 °C). Notably, the expression level of the genes was normalized and quantified by β-actin and the 2-ΔΔCt method, respectively. Statistical analysis was performed using ANOVA (p < 0.05).
The primer sequences (5′->3′) for runt-related transcription factor 2 (RUNX2), osteopontin (OPN), and osteocalcin (OCN) were used as below:
RUNX2 (Accession No.: Hs_00231692) forward: CACTGGCGCTGCAACAAGA, reverse: CATTCCGGAGCTCAGCAGAATAA (Tm = 60 °C); OPN (Accession No.: NM001040060) forward: GATGAATCTGATGAACTGGTCACT, reverse: GGTGATGTCCTCGTCTGTAGCA (Tm = 60 °C); OCN (Accession No.: NM199173) forward: GACGAGTTGGCTGACCACA, reverse: CAAGGGGAAGAGGAAAGAAGG (Tm = 60 °C), β-actin (Accession No.: NM001101.4) forward: GGCGCCCTATAAAACCCAGC, reverse: GCTCGATGGGGTACTTCAGG (Tm = 60 °C).
Likewise, the expression of COL1A1 and COL2A was assessed during the human chondrocyte culture, after 14 and 21 days. To this end, total RNAs were isolated from the cultivated cells by the RNEasy Micro Kit (74004; Qiagen), then diluted (12.0 μl), and reverse transcribed by the cDNAs synthesis Kit (iScript, Bio-Rad). Finally, the PCR reaction was carried out by the following program: 95 °C 60 sec, 25 cycles (95 °C 30 sec, 65 °C 180 sec), then 65 °C 180 sec.
The primer sequences (5′->3′) for collagens 1 and 2 (COL1A1 and COL2A1) were used as below: COL1A1 (Accession No.: NC_000017.11) forward: CGAAGGTTCCCCTGGACGAGACG, reverse: GGCACAAGGGATGACACGCGTTC; COL2A1 (Accession No.: NC_000012.12) forward: GACGGGTGAACCGGGTATTGC; reverse: ACTTCTCCCTTCTCGCCGTTAG.
Alkaline Phosphatase (ALP) Activity Assay: the cultured cells were assessed on days 7, 14, and 21, to measure alkaline phosphatase activity (ALP) (Li et al., 2016). To this end, the cells were lysed by 0.01 % Triton X-100 (Sigma-Aldrich) and the ALP activity was obtained using an ALP assay kit (P-Nitrophenylphosphate hydrolysis method, VANDIDAZ company, Tehran, Iran). Finally, the absorbance at 420 nm was measured and amounts of alkaline phosphatase in cells were determined.
Alizarin Red Staining Assay: At day 21, the cells were also washed with PBS (two times), fixed in 96 % ethanol (25 °C, 10 min), and stained by Alizarin red S (0.1 %, 37 °C) for 30 min to determine the cell mineralization level (Li et al., 2020). Finally, the calcium nodules were observed under an optical microscope.
In Vitro anti-inflammatory effect of composed oils on LPS-treated cells: To assess whether the oil samples can suppress inflammation in LPS-treated cells, the expression of COX-2, IL-6, and TNF-α4 as pro-inflammatory cytokines, with a modified protocol, were analyzed by RT-PCR.
To this end, the cells differentiated into osteogenic differentiation medium (at a density of 1 × 104 cells/well) were cultured into 24-well plates and then treated by LPS (1 µg/ml, for 1 h), as an inflammation-stimulating agent, for mimicking the inflammatory environment in-vitro. Afterward, the cells were incubated with selected concentrations of herbal oils (H1, H2, and H3) based on the cytotoxicity tests, for 5 and 24 h. Notably, the cells treated with LPS were considered as the negative control groups. Likewise, the highest concentration of the most nontoxic oily sample in the 25–200 μg/ml (i.e. 200 μg/ml concentrations) was selected as the positive control group. In the following, cells were harvested for total RNA isolation on the 5 and 24 days. Then the cDNA first strand was formed with total RNA (2 μg) via Super-Script II (Invitrogen, Carlsbad, CA, USA). Finally, the gene expressions were studied by the real-time PCR and with the reaction program of 95 °C (20 sec), 60 °C (15 sec), 72 °C (20 sec), and the amplification of 50 cycles. The levels of gene expression were also normalized by β-actin and quantified by the 2-ΔΔCt method.
COX-2 (Accession No.: NM000963.3) forward: CCAAATCCTTGCTGTTCCCACCCAT, reverse: GTGCACTGTGTTTGGAGTGGGTTT (Tm = 60 °C); IL-6 (Accession No.: 3569, NC000007.14) forward: ATGAACTCCTTCTCCACAAG, reverse: GTGCCTGCAGCTTCGTCAGCA (Tm = 60 °C); TNF-α (Accession No.: NM_000594.3) forward: AAGGACGAACATCCAACCTTCCCAA, reverse: TTTGAGCCAGAAGAGGTTGAGGGT (Tm = 60 °C), and β-actin (Accession No.: NM001101.4) forward: GGCGCCCTATAAAACCCAGC, reverse: GCTCGATGGGGTACTTCAGG (Tm = 60 °C).
2.2.4 Clinical study
Study design: The 2-month, double-blind, randomized controlled, parallel-group trial study (N = 50, as phase II) was conducted among patients with types of musculoskeletal pains, at the clinic of Bone specialty by orthopedic specialist/ orthopaedist, Tehran province between 2022 and 07-16 and 2022–09-16. The protocol of this study followed the 1975 Declaration of Helsinki, as revised in 2000. Notably, the rand function of Excel software was used for the randomization of patients, as well as, an informed consent was signed by the participants, before the start of the study. The patients, study assessors, and data analysts were also blinded to perform analysis without prejudice and treatment assignment. While, the researcher, clinical caregiver (doctor and physiotherapist), and Data Safety and Monitoring Committee were aware of the herbal structure and nature of the bio-ointment. Moreover, it was approved by the Institutional Research Ethics Committee of the School of Advanced Technologies in Medicine, Vice-Chancellor in Research Affairs- Shahid Beheshti University of Medical Sciences with ethics code IR.SBMU.RETECH.REC.1399.758 and Iranian Registry of Clinical Trials No. (Trial registration): IRCT20211231053573N1.
Patient selection and the study protocol: The patients with transient/acute/chronic musculoskeletal pains (with International Classification of Diseases-10 [ICD-10], diseases of the musculoskeletal system and connective tissue including R29.8 Other and unspecified symptoms and signs involving the nervous and musculoskeletal systems, M54.5 Low back pain, M05 rheumatoid arthritis, M54.3 Sciatica, M41.4 Neuromuscular scoliosis, and M13 arthritis) diagnosed by orthopaedist were selected for this study.
Inclusion criteria were (1) the existence of one of the types of musculoskeletal pains (transient/acute/chronic), and (2) age between 10 and 88 years. Likewise, the exclusion criteria were (1) the allergy to the fabricated herbal bio-ointment used during the study, and (2) body mass index (BMI) of 30 kg/m2 and above.
After screening, the patients eligible for this study were randomly selected. Accordingly, the two groups were defined:
-
Intervention group: the receiving patients (N = 50) of the herbal anti-pain bio-ointment, used it every day (1–2 times) for 2 months, topically [no other topical analgesics, and oral drugs NSAIDs and/or COX-2 inhibitors].
-
Intervention group (Control): the same patients in group (1) who used drugs, analgesia, or other products (a 1-week before using anti-pain bio-ointment). This group was selected to compare the effectiveness of herbal anti-pain bio-ointment with other drugs and analgesia.
Notably, the most common analgesia and drugs used by patients include naproxen, ibuprofen, diclofenac, topical ibuprofen gel, 1 % diclofenac gel, piroxicam 0.5 % gel which were received every 6 to 8 h based on the intensity of the pain.
Treatment method: the use of therapeutic oil called H2 containing active oils of turmeric, ginger, chili pepper, and rose (1:1:1:1 w/w) and base oil containing sesame, black seed, and olive oils (1:0.4:0.6 w/w) which combined with an active oil: base oil weight ratios of 1.5:5.5 w/w. Notably, 10 %wt beeswax was added to this therapeutic oil to form bio-ointment and improve viscosity. In the following, to study the rheology of ointment, the viscosity of the sample was also determined using a Brookfield Synchro-Lectric Viscometer (Model RVT). Briefly, 40 g of the H2 formula was equilibrated in a beaker at 3, 6, 10, 15, 20, 30, 40, 50, 60, 70, 80, 90, and 100 rpm, for 5 min by a T-D spindle. At each speed, viscosity was noted on the viscometer in centipoises (CPS). The pH of the sample was also measured by a pH meter (pH Tutor, Eutech Instruments). To this end, 1g of H2 bio-ointment was added to 50 ml of distilled water and heated in the water bath at 50–60 °C. Finally, the pH of the bio-ointment was determined using the pH meter in triplicate. Moreover, this bio-ointment was assessed to characterize the parameters of appearance, odor, color, texture or homogeneity, and viscosity measurements.
Assessment: the therapeutic efficacy of herbal anti-pain bio-ointment was studied through the Western Ontario and McMaster Universities (WOMAC) index which consists of the subscales of pain, stiffness, and physical function (based on scores of pain [0–20 points], stiffness [0 to 10 points], and physical function [0 to 70 points]) (Amorndoljai et al., 2017). All the studied patients had complete medical workups, including history taking, physical examinations, and laboratory studies. Such that, the assessments included the visual analog scale (VAS), tender (TJC), swollen (SJC) and joint counts, the disease activity score (DAS) (Sami et al., 2023), and the modified health assessment questionnaire (MHAQ) before and after the use of the bio-ointment. Accordingly, the Patient’s Global Assessment (PGA) was used in a 5-point Likert scale (1 = very good or so much effectiveness, 2 = good or so much effectiveness, 3 = average or moderate, 4 = poor or mild effectiveness, 5 = very poor or very mild effectiveness) (Kuptniratsaikul and Rattanachaiyanont, 2007; Pham et al., 2004). The responder criteria were also defined as per the Outcome Measures in Rheumatology (OMERACT) Committee and Osteoarthritis Research Society International (OARSI) Committee (Amorndoljai et al., 2017) that cover domains of pain, function, and PGA, as well as, as a participant with > 50 % improvement in pain or function. Finally, efficacy assessments of herbal anti-pain bio-ointment were carried out at baseline and after 2 months of treatment.
Notably, we used the CONSORT reporting guidelines; Schulz KF, Altman DG, Moher D, for the CONSORT Group. CONSORT 2010 Statement: updated guidelines for reporting parallel group randomized trials.
2.2.5 Statistical analysis
All tests in the parts of characterizations analysis of oil and biological studies were performed 3 times and the data were determined as mean ± standard deviation (SD). For the clinical study, there was no missing data, and no adverse events were observed. Generally, continuous variables were analyzed by repeated ANOVA and statistical tests of two-sided in IBM SPSS Statistics 22 software, (p < 0.05, 0.01, and 0.001).
3 Results and discussion
3.1 Characterizations analysis of the designed therapeutic oils
The bioactive ingredients or biomacromolecules of oily samples (H1, H2, and H3) were analyzed by GC–MS. The data illustrated the identification of 23 bio-actives including protein derivatives (N-Acetyl-L-proline, Phenylpropanoid), sesquiterpenes (beta-caryophyllene, alpha-longipinene, beta-bisabolene), beta-hydroxy ketone (6-gingerol), sesquiterpenoid (Ar-turmerone), monoterpene (thymoquinone), phenol (shogaol), monoterpenoids (citronellol, nerol, geraniol), flavonoid (heptadecane), terpene (limonene), phenylpropanoid (estragole), capsaicinoid (capsaicin), lignan (sesamin), terpene glycoside (harpagoside), triterpenoid (squalene), naphthoquinones (vitamin K1), and other compounds (short-chain saturated fatty acid, vitamin C, Retinol (vitamin A)) that have been listed in Table 2. Based on the results, sesamin (in the group of lignans) was known as the main compound of all three oil samples that can induce inflammatory effects via inhibition of interleukin-1β (IL-1β) release as well as D5-desaturase in the biosynthesis pathway of polyunsaturated fatty acids (Phitak et al., 2012). Moreover, the existence of a high percentage of triterpenoids, terpenes, terpene glycoside, and sesquiterpenes in these oils can increase the anti-bacterial, antioxidant, and anti-inflammatory properties of formulas (Dugasani et al., 2010; Katsukawa et al., 2011; Mohd Yusof, 2016; Prasad and Tyagi, 2015). Indeed, these active ingredients can specifically inhibit and suppress the effects of COX-2 and oxidative stress (Al Wafai, 2013; Huang et al., 2006). Moreover, they lead to a significant reduction in the level of pro-inflammatory mediators such as interleukin-6, TNFα, IFNγ, and PGE-2, as well as suppression of NF-κB and MAPKs signaling pathways, and subsequently inhibition of interleukin-1β-induced inflammation in osteoarthritis chondrocytes (Wang et al., 2015). Notably, N-Acetyl-L-proline (acetylated form of L-proline) known as a protein derivative, can play an important role in regenerating collagen-containing organs. Based on the reports, this protein derivative is the main structure of collagen fibers in the body, especially in the knee joint (Likhitthummagun et al., n.d.; Wu et al., 2011). Such that the use of collagen-synthesizing agents can help regenerate cartilage in patients with osteoarthritis patients (de Paz-Lugo et al., 2018; Wu et al., 2011).
NO.
Oil Code
RT1 (min)
MW2 g/mol
Molecular formula
Common name
Group
Content (%)
H3
H2
H1
1
H1
10.74
157.17
C7H11NO3
N-Acetyl-L-proline
protein derivatives
0.38
0.38
0.24
H2
10.89
H3
10.94
2
H1
10.87
74.08
C3H6O2
Propionic acid
short-chain saturated fatty acid
0.20
0.19
0.11
H2
11.54
H3
11.92
3
H1
11.05
176.12
C6H8O6
Ascorbic acid
Naturally in citrus fruits and many vegetables
0.11
0.09
0.04
H2
11.95
H3
12.10
4
H1
13.27
368.4
C21H20O6
Curcumin
Curcuminoids
3.13
3.12
2.54
H2
13.97
H3
14.01
5
H1
14.72
204.35
C15H24
beta-caryophyllene
Sesquiterpene
4.02
3.51
3.28
H2
14.28
H3
14.31
6
H1
16.31
294.4
C17H26O4
6-Gingerol
beta-hydroxy ketone
4.90
4.87
3.10
H2
16.92
H3
17.41
7
H1
20.99
276.4
C17H24O3
Shogaol
Phenols
3.04
2.99
2.34
H2
21.19
H3
21.50
8
H1
22.92
156.26
C10H20O
Citronellol
Monoterpenoid
6.93
6.69
5.21
H2
23.11
H3
23.21
9
H1
25.00
240.50
C17H36
Heptadecane
Flavonoid
2.77
2.73
1.59
H2
25.72
H3
26.10
10
H1
25.65
204.35
C15H24
alpha-Longipinene
Sesquiterpene
1.11
1.09
0.86
H2
26.10
H3
26.20
11
H1
26.00
136.23
C10H16
Limonene
Terpenes
2.51
2.48
2.13
H2
26.81
H3
26.98
12
H1
26.31
148.20
C10H12O
Estragole
Phenylpropanoid (natural product in herbs)
2.93
2.86
2.31
H2
27.26
H3
26.99
13
H1
26.99
154.25
C10H18O
Nerol
Monoterpenoid
1.80
1.76
1.12
H2
27.90
H3
27.21
14
H1
27.49
154.25
C10H18O
Geraniol
Monoterpenoid
2.27
2.25
1.18
H2
28.23
H3
28.52
15
H1
28.21
164.20
C10H12O2
Thymoquinone
Monoterpene
1.88
1.84
1.30
H2
29.20
H3
29.36
16
H1
29.10
216.32
C15H20O
Ar-turmerone
Sesquiterpenoid
2.24
2.26
2.00
H2
29.89
H3
30.11
17
H1
30.00
204.35
C15H24
beta-Bisabolene
Sesquiterpene (cyclohexene)
2.23
2.17
1.01
H2
30.81
H3
31.00
18
H1
31.01
305.4
C18H27NO3
Capsaicin
Capsaicinoid
3.69
3.65
3.47
H2
31.88
H3
32.01
19
H1
31.93
354.4
C20H18O6
Sesamin
Lignan
27.01
27.54
27.39
H2
32.78
H3
32.98
20
H1
32.97
286.5
C20H30O
Retinol
Naturally in citrus fruit and vegetable oils
1.45
1.41
1.15
H2
33.41
H3
33.98
21
H1
33.98
494.4
C24H30O11
Harpagoside
Terpene glycoside
2.12
2.09
1.75
H2
34.75
H3
34.97
22
H1
35.04
410.7
C30H50
Squalene
Triterpenoid
19.21
19.36
18.05
H2
35.96
H3
36.38
23
H1
38.98
450.7
C31H46O2
Vitamin K1 (phytonadione)
Naphthoquinones
2.17
2.16
1.45
H2
39.61
H3
39.99
Main compounds
Lignan
27.01
27.54
27.39
Triterpenoid, terpene, and terpene glycoside
23.84
23.93
21.93
Monoterpenoids
11.00
10.7
7.51
Sesquiterpenes
7.36
6.77
5.15
Vitamins (C, K1 and A)
3.73
3.66
2.64
Protein derivatives
3.31
3.24
2.55
Phenol
3.04
2.99
2.34
Flavonoid
2.77
2.73
1.59
Other
16.41
16.19
12.72
Our results also indicated that the H3 formula possessed higher biomolecules and bioactive ingredients than H2 and H1 which can be due to the percentage composition of oils (Table 2, Fig. 1(a)). However, it seems that the use of these 3 samples can possess potential therapeutic effects, especially for treating inflammatory diseases and consequently reducing pain (Almatroodi et al., 2021; Ballester et al., 2022; Wang et al., 2015). In this matter, the reports demonstrated that the use of natural materials such as capsaicin, curcumin, and shogaol play an important role in relieving pain especially neuropathic, osteoarthritis, rheumatoid arthritis, and muscular low back pains, so that, the frequent use of these biomaterials as the NSAIDs not only can desensitize secreting fibers of substance-P and relieve pain via reducing this substance secretion (Ballester et al., 2022; Derry et al., 2017; Ebrahimzadeh et al., 2021; Fayazi et al., 2015; Kou et al., 2023; Kurien et al., 2015; Morgan and Jeffrey, 2022; van Breemen et al., 2011) but also possess therapeutic properties due to an increase in the synthesis of collagen- containing matrices (de Paz-Lugo et al., 2018; Likhitthummagun et al., n.d.; Wu et al., 2011).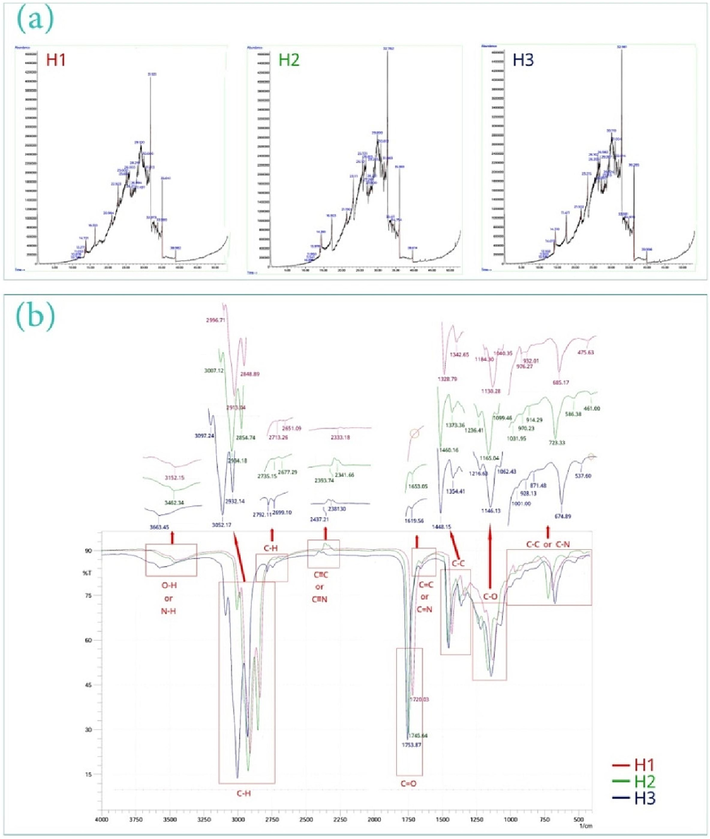
GC–MS chromatogram for therapeutic oils (a), FTIR spectra for the studied oils (b).
In the following, the study of FTIR spectra for all 3 samples indicated when the ratio of extracted oils to base oil is less than 1.5 % v/v (i.e. H1), the intensity of peaks is decreased or in some cases, the peak is removed that can be due to lower active ingredients in H1 formula (such as C≡C and C≡N, as well as C–H stretching vibrations, C = C, and C = N) (Fig. 1(b)). Moreover, shifting left in the position of peaks of the H3 sample for –OH/N–H stretching vibration, C–H stretching, and triple bands, and then shifting right for C–C bending, C-O stretching, and C-N bending of this sample than H2 demonstrated that the increase of bioactive ingredients plays an important role in how functional groups of O–H, N–H, C-O, C–C, and C-N interact. This is related to the extracted compounds of medicinal plants such as proteins, sesquiterpenes, monoterpenoids, and flavonoids which have led to inter-chain interactions during processing. The GC–MS analyses can confirm this case.
In the following, DPPH inhibiting activity of the oily samples was studied for freshly prepared samples and samples preserved for 6 and 12 months (Fig. 2(a)). Based on the results, the H1 sample possessed more changes in free radical inhibition trend, especially after 12 months. Indeed, the lower content of active ingredients in this oily sample especially in phenols, sesquiterpenes, flavonoids, and vitamin C (Ascorbic Acid) content led to a decrease in its antioxidant activity than the two other samples. Moreover, based on the reports, when phenol content increases, lipid oxidation is reduced due to scavenging free radicals at the air-oil interface (Bao and Pignitter, 2023). Hence, the H1 formula has been oxidized more than H2 and H3, after 12 months. Hence, it seems that the shelf-life of this formulation is lower than 12 months (between 6 and 12 months). Our results also indicated that although H2 and H3 possess the same antioxidant activity (almost equal amount of Ascorbic Acid), the H3 formula in concentrations less than 100 µg/ml illustrated a lower antioxidant activity that can be due to decreased content of lignans, triterpenoids, terpenes, and terpene glycosides compared to H2. Accordingly, H2 (in all concentrations) and H3 (only for concentrations more than 100 µg/ml) can provide a shelf-life of 12 months or more. Therefore, oxidative stability in lipid formulation is dependent on weight ratios of the composition of the extracted oils with base oil. The results of IC50 can confirm these results (Fig. 2(b)). Accordingly, IC50 values for freshly prepared samples of H1, H2, and H3 were respectively obtained at 44.75 µg/ml, 42.01 µg/ml, and 42.98 µg/ml (Fig. 2(b)) that showed H2 sample can lead to 50 % inhibition of free radicals at a lower concentration than H1 and H3 (although insignificant); subsequently, more concentrations than that can significantly increase antioxidant activity.![DPPH free radical inhibiting activity [% inhibition vs concentration parameter (µg/ml) for therapeutic oils] (a), IC50 values of therapeutic oils based on the normalized inhibition (%) vs log[concentration of oil] (b).](/content/184/2024/17/6/img/10.1016_j.arabjc.2024.105766-fig2.png)
DPPH free radical inhibiting activity [% inhibition vs concentration parameter (µg/ml) for therapeutic oils] (a), IC50 values of therapeutic oils based on the normalized inhibition (%) vs log[concentration of oil] (b).
3.2 Biological analyses
Cell viability (%): The biocompatibility study of herbal oil samples on human monocytes, human synoviocytes, human chondrocytes, and human adipose-derived stem cells illustrated a general trend in cell proliferation, after 48 h (>80 %) (Fig. 3).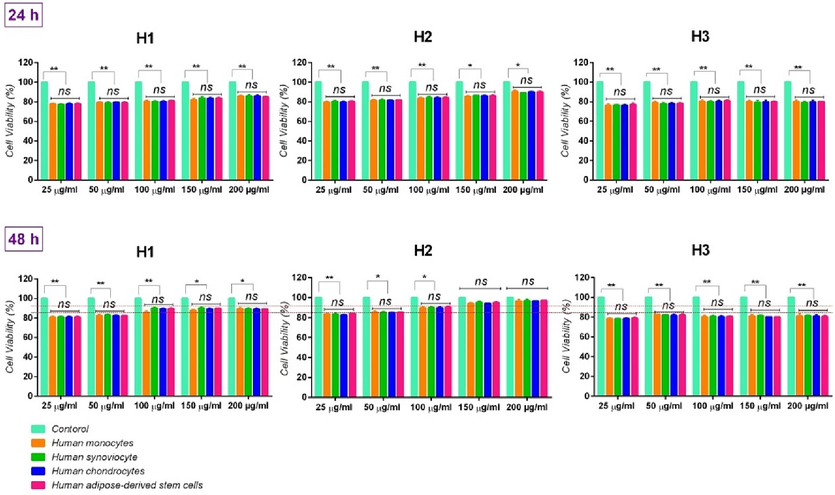
Cell viability (%) of cells on media containing therapeutic oils, with different concentrations (during 24 and 48 h). ns: no significant difference (p > 0.001), *: p < 0.1, and **: p < 0.05.
Although no significant difference was observed between every concentration of H1 and H3, after 48 h, when the concentration of over 100 μg/ml (in H1) and 50 μg/ml (in H3) increased, it was observed that H1 and H3 at these concentrations were led to a minor decrease in cell proliferation (red lines along with the statistical analysis of internal groups in Fig. 3); it can be related to their bioactive ingredient in the formula (for H1, it is lower; and for H3, it is higher than the H2 formula). Notably, red lines are helpful lines for better distinguishing and comparing the concentration difference of each formula with another formula. Namely, the statistical analysis of the internal group in each formula and its comparison with two other formulas occurs more easily through these red lines.
Hence, a dose-dependent behavior of the concentration of herbal oils on the proliferation of all studied cells was observed. Such that, after 48 h, higher cell proliferation with H1, H2, and H3 formulas was respectively obtained in the concentrations of 100 μg/ml, 200 μg/ml, and 50 μg/ml, especially in human chondrocytes. It can be due to protein derivatives such as protein and phenylpropanoid can possess a positive effect on the synthesis of proteoglycans by chondrocytes. In this field, Paz-Lugo et al. stated that such protein derivatives can lead to the synthesis of collagen II in particular chondrocytes, and the regeneration of the cartilage matrix (de Paz-Lugo et al., 2018).
Based on the results, no significant differences were also observed between the control group and the treatment group of H2, at the concentrations of 150 μg/ml and 200 μg/ml, compared to the other two. It demonstrates that this formula can be suitable in high concentrations. Notably, at 50 μg/ml and 100 μg/ml concentrations, the H2 formula possessed more biocompatibility compared to H1 and H3 formulas containing the same concentrations (red lines in Fig. 3). Hence, it is expected that the H2 formula will lead to an increase in osteogenic differentiation due to the existence of optimal active ingredients in its formula compared to the H1 and H3 formulas, even at the 50 μg/ml concentration.
Cytotoxicity study: To study the possible cytotoxic effect induced by the herbal oils on the studied cells, the cytotoxicity assay was performed at 25–200 concentrations of the therapeutic oils, at 24, and 48 h, by measuring the levels of LDH released in the culture media [as a factor for assessing membrane integrity of cells (Chan et al., 2013) (Fig. 4).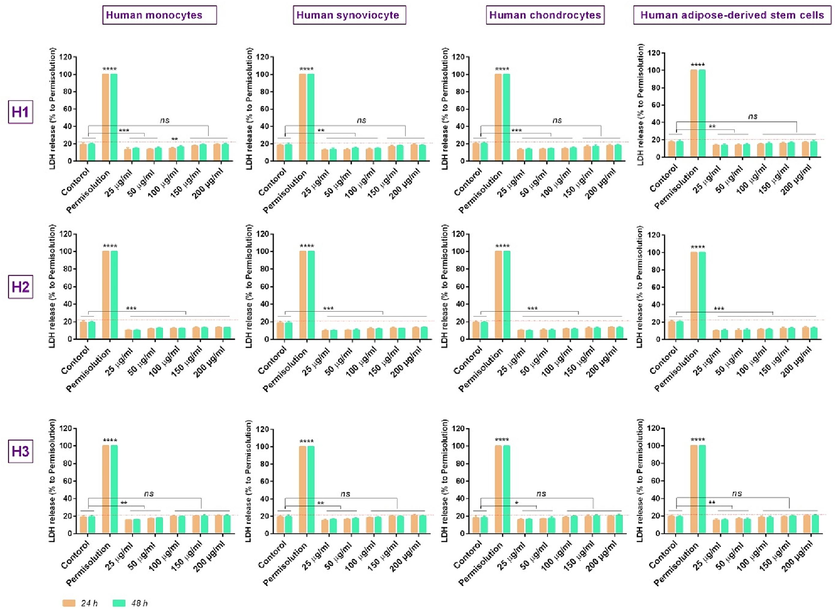
In vitro study of lactate dehydrogenase (LDH) release, in the different concentrations of oil (25, 50, 100, 150, and 200 μg/ml), ****: p < 0.001; ***: p < 0.01, **: p < 0.05, and ns: p > 0.01.
Based on the results, although all formulas (H1, H2, and H3) possess lower cytotoxicity than the positive control (the MTT results confirm this), the comparison of released LDH levels in the supernatants of the cells for each of 3 formulas illustrates that the higher levels of released LDH (for all studied cells) were related to 100–200 μg/ml concentrations in H3 formula (the LDH mean of more than 18 %, 19 % and 20 %, respectively). Likewise, the lower LDH release % (<13.5 % for all cells) was observed for H2 formula at 25‑200 μg/ml concentrations. The lowest LDH levels in the H1 formula were also assigned to 25–100 μg/ml concentrations of this herbal oil (>13.5 %, >14 %, and > 15 %, respectively).
Accordingly, the membrane integrity of cells was unaffected at 25‑200 μg/ml concentrations for the H2 formula, 25‑100 μg/ml concentrations for the H1 formula, and 25‑50 μg/ml concentrations for the H3 formula. Indeed, concentrations of 50 μg/ml, 100 μg/ml, and 200 μg/ml, respectively for the H1, H3, and H2 formulas, were the maximum concentration that can maintain the membrane integrity of cells.
Hence, based on the MTT and cytotoxicity results, the 100 μg/ml, 200 μg/ml, and 50 μg/ml concentrations, as effective dosage, can be selected respectively for H1, H2, and H3 formulas. However, we selected the lowest dose i.e. 50 μg/ml concentration, for all three formulas, to provide similar concentration conditions.
Gene expression: RT-PCR analysis was carried out to assess the osteogenic differentiation from h-ADSCs in the media containing 50 μg/ml of herbal oils (H1, H2, and H3), after 7, 14, and 21 days (Fig. 5(a)). It was found that all formulas can lead to osteogenic differentiation; however, the highest expression of osteoblastic genes (RUNX2, OCN, and OPN) was observed for the H2 formula. Such that, after 7 days, the expression of RUNX2, OCN, and OPN genes by H2 formula was respectively recorded 1.3-fold, 1.7-fold, and 1.4-fold the higher than H1, as well as ∼ 2-fold, 2.1-fold, and 3.4-fold the higher than H3 (p < 0.01). It can be due to the presence of optimized bioactive ingredients or biomolecules in this formula (H2) which are considered the main factor in osteogenic differentiation (such as sesquiterpenes (Chircov et al., 2021; Fateh et al., 2022; Kim and Jang, 2017) and phenols (Abdel-Naim et al., 2017; Lambert et al., 2021)). In this matter, the reports have indicated that sesquiterpenes can act as differentiation-inducing/-inhibiting agents and lead to control and timely modulation of differentiation subsequently bone regeneration (Fateh et al., 2022; Jang, 2016; Laid et al., 2008).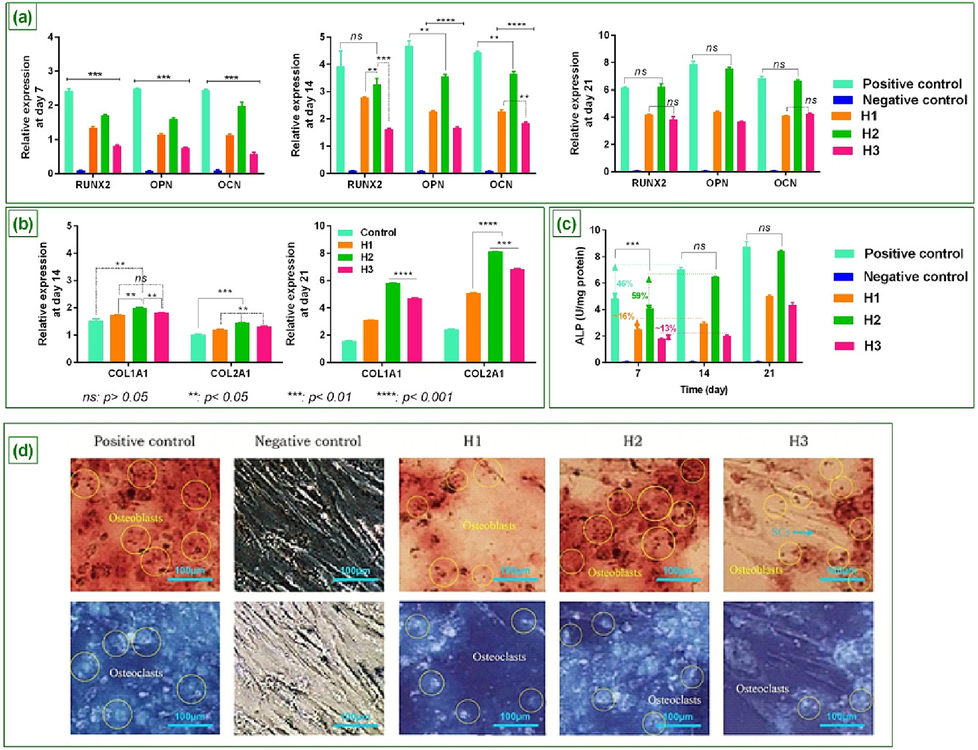
The expression of osteoblastic genes (RUNX2, OPN, and OCN) on media containing 50 μg/ml of therapeutic oils at days 7, 14, and 21 (a), The expression level of COL1A1 and COL2A on media containing 50 μg/ml of therapeutic oils at days 14 and 21 along with p-value (b), ALP activity on day 7, 14, and 21 (c), Alizarin Red S staining of cell cultures (Scale Bars 100 μm) along with mineralized deposits and expression of osteoblast, as well as osteoclasts and un-differentiated h-ADSCs at day 21 (d) ****: p < 0.001; ***: p < 0.01, **: p < 0.05, and ns: p > 0.01.
The results also demonstrated that 50 μg/ml concentration of H2 possesses a better performance than the same concentration for two other formulas to mimic the osteogenic differentiation pattern, and regenerate bone. Although a ∼ 17 % (RUNX2), 25 % (OPN), and 17.5 % (OCN) difference in expression of osteoblastic genes was observed for this formula compared with the positive control group (p < 0.05), after 14 days, that can be owing to the use of lower concentration than effective concentration of H2 (i.e. 200 μg/ml) which is obtained from cell viability and cytotoxicity assay. In the following, the comparison of these two groups indicated that after 21 days there was no significant difference between these groups.
Generally, the reports indicate that herbs in the forms of oil, extract, etc. can promote cellular functions, both cell proliferation and differentiation (de Paz-Lugo et al., 2018; Wu et al., 2011). Therefore, it seems that although H2 can enhance cell proliferation due to better synergy of active ingredients and consequently lead to an increase in differentiation, the presence of optimized bioactive ingredients or biomolecules in H2 formula, such as sesquiterpenes and phenols themselves are the main factor in osteogenic differentiation.
In the following, the results of RT-PCR analysis were also studied to assess the expression of COL1A1 and COL2A (collagen I and II) from human chondrocytes in the media containing the 50 μg/ml of H1, H2, and H3, after 14 and 21 days (Fig. 5(b)). Based on the results, the chondrocyte cells grown on these media indicated an increase in the expression of COL1A1 and COL2A at days 14 and 21 compared to the control group (cells grown on medium without oil) (p < 0.05 and 0.001, respectively). Indeed, all three formulas led to a suitable level of collagen expression that can be related to protein derivatives of N-Acetyl-L-proline and phenylpropanoid in the oily samples. This can provide a positive effect on collagen synthesis and regenerate its fiber structure, especially in the knee joints and patients with osteoarthritis (de Paz-Lugo et al., 2018; Wu et al., 2011).
However, a higher expression of collagen was reported for the H2 formula. Such that, the expression of COL1A1 and COL2A by H2 formula was respectively ∼ 16 % and 20 % (after 14 days) and %87 % and ∼ 60 % (after 21 days) the higher than H1, as well as ∼ 9 % and ∼ 9.5 % (after 14 days) and ∼ 19 % and ∼ 16 % (after 21 days) the higher than H3 (p < 0.01). It can be due to the presence of optimized bioactive ingredients along with proteins in the H2 formula compared with two other groups.
The study of ALP Activity: ALP activity is a well-known factor for reflecting the degree of osteogenic differentiation. Here, measuring the activity of this factor to determine the osteogenesis of all three formulas indicated there was no significant difference between H2 and positive control groups after 14 and 21 days (p > 0.01, 0.001, respectively). Moreover, it was found that the use of the H2 formula as an osteogenic induction medium during 14 and 21 days can respectively lead to a 59 % and 107 % (i.e. 1.07-fold) increase in the ALP activity, compared to the 7th day (Fig. 5(c)). This is in a situation where the ALP activity, in the positive control group, after 14 and 21 days were respectively 45 % and 81 % more than on the 7th day. This can be due to the content of the H2 formula and the existence of herbal bioactive molecules that provide an enriched medium for osteogenic differentiation and consequently increase tissue repair rate. In this matter, H1 and H3 samples illustrated a lower level of ALP activity than H2 which could be related to the smaller amount of lignans, triterpenoids, terpenes, and terpene glycosides in these two samples.
Alizarin red S staining analysis: Osteoblasts and osteoclasts are responsible respectively for the formation of new bones and the re-sorption of aged bones (Chen et al., 2017). The balance between the osteoblasts and osteoclasts' activities ensures that bone is neither over-formatted nor over-degraded, so that, physiologically, the resorption and formation functions will be in stable conditions. Hence, it seems that the mimicking materials of such conditions can be effective in bone regeneration and improvement of musculoskeletal disorders. Accordingly, the results of Alizarin Red S staining indicated that the H2 formula can create these stable conditions as well as the osteogenic differentiation media, after 21 days (Fig. 5(d)). In this field, although H1 and H3 demonstrated mineralized deposits, the higher expression of osteoblast was related to the H2. Moreover, it was found that the H1 possesses the rate of bone resorption more than its formation. It means that differentiation potential has been reduced in the 3rd week. In contrast, the H3 indicated a higher rate of bone formation than its resorption, along with un-differentiated H-ADSCs, after 21 days. Based on the results, H2 can be a more successful and promising formula for clinical applications in the treatment of bone-related diseases/disorders.
The study of in-vitro anti-inflammatory effects: In the following, to determine whether studied herbal oils can suppress LPS-stimulated inflammation in osteoblast cells, the expression levels of COX-2, TNF-α, and IL-6 in the cells differentiated into osteogenic differentiation medium, after culturing on H1, H2, and H3 for 5 h and 24 h, were assessed by RT-PCR (Fig. 6(a)). Given the nontoxic of H2 in the 25–200 μg/ml concentrations, the 200 μg/ml concentration was intentionally selected as the positive control group. Based on the results, LPS-treated cells illustrated the highest expression level of COX-2, TNF-α, and IL-6 compared to other groups at both hours (p < 0.001). It was also found that H2 could significantly down-regulate expression levels of inflammatory mediators than H1 and H3 (p < 0.001 and 0.01).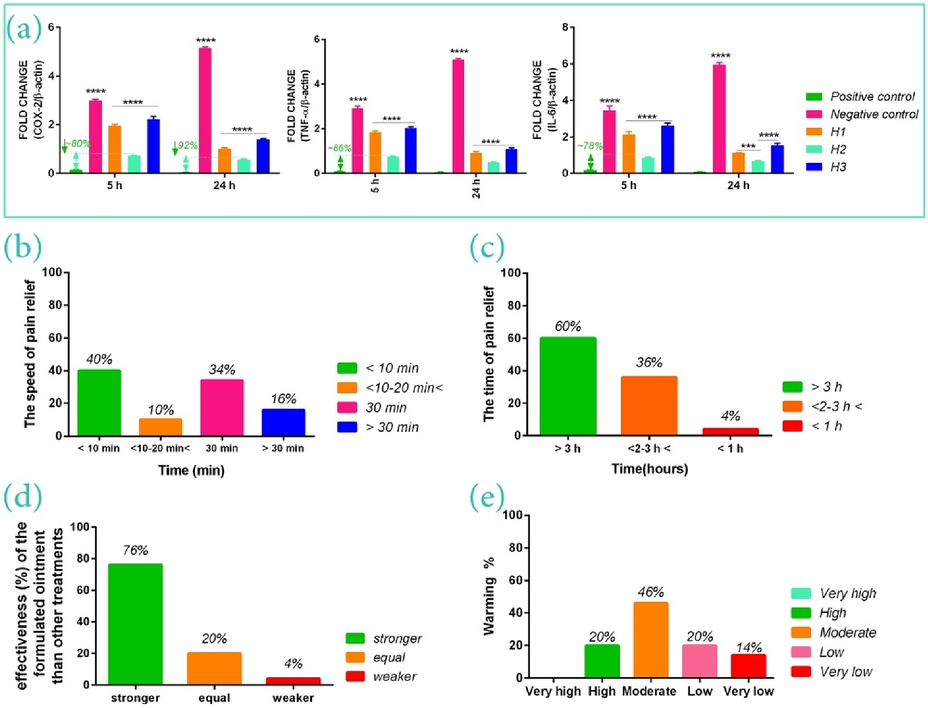
Anti-inflammatory effects of therapeutic oils at 50 μg/ml concentration on the expression of cytokines of COX-2, IL-6, and TNF-α (a), the speed of pain relief (b), the time of pain relief or analgesia period (i.e. the time interval between cessation of the pain, after using the bio-ointment, and its resumption) (c), the effectiveness of the formulated bio-ointment than other treatments (d), and warming function of therapeutic bio-ointment on the damaged area (e).
Moreover, the positive control group after 24 h indicated a 30 %, 51 %, and ∼ 50 % decrease respectively in expression levels of COX-2, TNF-α, and IL-6 than the first 5 h for the same group. Likewise, the expression levels of these mediators after 24 h for H2-treated cells were 35 %, 65.8 %, and 77.35 % less than the first 5 h. It shows that lower concentrations of the H2 formula (50 μg/ml) can also reduce the level of the mentioned inflammatory mediators. However, the higher concentration of this herbal oil (i.e. positive control group, 200 μg/ml concentration,) could significantly decrease inflammatory mediators expression after 5 and 24 h compared to the H2 formula as well as H1 and H, such that the positive control group in the first 5 h indicated ∼ 80 %, 86.32 and 77.81 % decrease in the expression levels of COX-2, TNF-α, and IL-6 respectively, than the 50 μg/ml concentration of this formula (i.e. H2 formula). Accordingly, it seems that H2 can be a suitable option for bone disorders-related pain relief (such as musculoskeletal, arthritis, etc.) in a dose-dependent manner.
Hence, based on the comparison of results, the H2 formula was selected to continue the study and carry out the clinical trial in topical management of musculoskeletal pains and treatment of inflammation involved in these diseases. To this end, the beeswax was added to the H2 formula at the rate of 10 % of the total weight of oil, to form bio-ointment and improve its viscosity.
Before the clinical study, the pH and viscosity of H2 bio-ointment were measured. Accordingly, the pH of the bio-ointment was estimated at 6.78 ± 0.122 which can be considered almost neutral. The viscosity assay also indicated with the increase in spindle speed the viscosity decreases, so that the viscosity values were 5119, 2899, 1884, 1328, 1047, 775.8, 607.4, 493.1, 419.9, 365.9, 328.4, 293.3, and 265.7 CPS, respectively at the speeds of 3, 6,10, 15, 20, 30, 40, 50, 60, 70, 80, 90, and 100 rpm. Therefore, it seems that deep penetration occurs when a particular interaction with the skin, such as an effective massage, creates that influences the spreadability through heat and speed generated by touching the skin. The H2 bio-ointment was also tested for physical appearance, color, and texture. These characteristics were determined by visual observation. Accordingly, the homogeneity of the appearance was assessed by pressing a small quantity of bio-ointment between fingers. The results demonstrated that this bio-ointment possesses the consistency of the formulation without the presence of coarse particles, along with an orange-yellow color and homogeneous soft grease texture in immediate skin feel.
3.3 The study of clinical efficacy
50 participants were randomized to relieve and treat musculoskeletal pains (Female: 35 patients, male: 15 patients, in the age group of 10 to 88 years (10–15 years: 3 patients, 16–30 years: 15 patients, 31–50 years: 11 patients, and 50 years <: 21 patients). All 50 patients completed the protocols and were eligible for Outcomes Assessment (OA). Accordingly, one patient had rheumatoid arthritis, 10 patients had osteoarthritis, 2 patients had muscle cramps or strain, 8 patients had shoulder and neck pains, 7 patients arm, tennis elbow, forearm, and wrist pains, 7 patients had neuromuscular, sciatica and back pains, 6 patients were bone pain or chronic pain that has continued long after the fracture finished healing, 2 patients were spinal disc problems and spinal pains, 1 patient was pain caused by torn meniscus, and 6 patients were pains caused by trauma (damage to the tissue), physical activity or exercise. No one of the patients possessed concomitant medication, during the study period. An herbal anti-pain bio-ointment of 20 g was used for each patient, for 2 months.
In the following, the effectiveness of bio-ointment was assessed in terms of the speed of pain relief [with four answers: (1) less than 10 min, (2) between 10 and –20 min, (3) 30 min, and (4) more than 30 min], the time of pain relief or analgesia period (i.e. the time interval between cessation of the pain, after using the bio-ointment, and its resumption) [with three answers: (1) more than 3 h, (2) between 2 and 3 h, and (3) less than 1 h], warming function of the damaged area [with five answers: (1) very low, (2) low, (3) moderate, (4) high, and (5) very high], and the effectiveness of the formulated bio-ointment than other treatments [with three answers: (1) stronger, (2) equal, and (3) weaker].
Based on the results, 40 % of patients stated that the suggested bio-ointment has led to the reduction of pains, in less than 10 min. Moreover, 35 % of patients also reported that this bio-ointment can reduce pain after 30 min from the use of bio-ointment (Fig. 6(b)). Hence, as indicated in Fig. 6B, 84 % of patients that included all groups i.e. rheumatoid arthritis, osteoarthritis, muscle cramps/strain, shoulder and neck pains, the pains of the arm, tennis elbow, forearm, and wrist, as well as neuromuscular, sciatica, back pains, and bone pain, have found that the mentioned bio-ointment is suitable to relieve both chronic and acute pains, in less than 30 min. It can be due to the existence of active ingredients such as curcumin, shogaol, 6-gingerol, and capsaicin which lead to inhibition of the inflammatory process and consequently pain relief by providing anti-inflammatory effects similar to NSAIDs such as ibuprofen, and diclofenac, especially for treating acute inflammation (Cameron, 2013; Paultre et al., 2021; Shep et al., 2020, 2019). Moreover, anti-inflammatory cytokines in these bio-actives reduce the transmission of pain signals from nerve endings to the brain, by decreasing the synthesis of substance P or inhibition of its release which results in the depletion of sensory nerves of substance P content, and result in the chronic or neuropathic pain relief (Akgol Gur et al., 2023; Persson et al., 2018).
In the following, the analysis of questionnaire data indicated that the pain-relieving effect of this bio-ointment (analgesia period) for 60 % of patients has lasted for longer than 3 h (Fig. 6(c)), while lasted between 2 h and 3 h, for 36 % of patients. Accordingly, 76 % of patients stated that the formulated bio-ointment possesses stronger effects than other treatments (Fig. 6(d)). 20 % of them also reported an equal effect to other pain relief medications/methods. This result is related to vitamins and active compounds in the bio-ointment formula such as Ascorbic Acid, capsaicin, alpha-longipinene, limonene, estragole, geraniol, citronellol, and heptadecane that significantly possess antioxidant and anti-inflammatory properties and other pharmacological activities, as well as help reduce oxidative stress, regulate cell growth, and establish long-term analgesia (Chung and Campbell, 2016; da Costa et al., 2022; Katsukawa et al., 2011; Tobyn et al., 2011; Zahra et al., 2019). In this field, the reports illustrated that the mentioned compounds can act as a strong sedative agent and a possible alternative to treat pains pharmacologically (Canuto et al., 2022; da Costa et al., 2022; Elshafie et al., 2019; Ferreira Farias et al., 2019). Indeed, these bio-actives can help suppress COX-2 activities, and inhibit leukotriene synthesis (Katsukawa et al., 2011).
Regarding the warming property of the bio-ointment, 14 % of patients stated that this bio-ointment possesses few warming functions. Likewise, 20 % of patients also reported this function was to be low. However, according to Fig. 6(e), this formulated bio-ointment possesses an average warming function which is assigned to oils of ginger, chili pepper, sesame, black seed, and olive oils. These oils can create significant pain-relieving properties by warming the damaged area and reducing inflammation-induced pain (Rondanelli et al., 2020). Moreover, the studies of traditional medicine indicate that oils with hot/warm Mizaj (warm temperament) possess better therapeutic potential and are pharmacologically potent (Farsani et al., 2014; Fazmiya et al., 2022; Parvinroo et al., 2014). Indeed, it seems that biomolecules of warm Mizaj in these herbal oils lead to an increase in cell energy and subsequently a decrease in muscular and nervous tension. Such oils can also soothe stiff muscles, improve circulation, warm damaged areas, and lead to tissue repair/regeneration. Hence, these herbal oils not only can act as a reliever but also therapeutic; it means that they heal the cause of pain (such as osteoarthritis, neuromuscular, sciatica, etc.) by supplying energy to the cells and restoring the ability of cells, over time.
Here, it is important that the formulated bio-ointment possesses a moderate warming function and acts as a drug of moderate temperament because it will not be toxic or allergic, and not cause skin irritation. Moreover, given that hot temperamental drugs are suitable for cold temperamental individuals and conversely, paying attention to the moderate temperament in designing medicinal formulas can reduce the risks of inappropriate doses and allergies (Farsani et al., 2014; Fazmiya et al., 2022). In this matter, rose oil used in the formula of this bio-ointment plays an important role in reducing and inhibiting allergies caused by chili pepper and ginger due to its moderate temperament. Moreover, the clinical trials demonstrate that citronellol and geraniol in such herbal oils can reduce the production of inflammatory mediators by affecting COX-2 and inhibiting enzymes involved in the prostaglandin (PGE) production from arachidonic acid (Fazmiya et al., 2022; Jeon et al., 2009; Mahboubi, 2019). Citronellol also can act as an effective bioactive for the removal of allergic agents or the treatment of allergic diseases via inhibiting TNF-α production by mast cells (Kobayashi et al., 2016).
Hence, the final analysis of the mentioned results was performed in the form of two separate questions regarding the effectiveness or success of the bio-ointment in reducing pain and damaged tissue performance recovery, as well as the patient's satisfaction with the bio-ointment function (generally). Based on the results, 34 % of patients stated this bio-ointment possesses so much effectiveness in reducing/relieving pain and damaged tissue performance recovery. Likewise, 36 % of them also reported this therapeutic method has resulted in many effects on their pain relief and the increase in performance of the target tissue (Fig. 7(a)). It is related to the multi-mechanism function of bio-ointment and the stimulation of collagen synthesis in damaged organs via protein derivative in the bio-ointment formula that led to improvement and increase in performance of this organ (Likhitthummagun et al., n.d.; Wu et al., 2011). However, 24 % and 6 % of the studied patients declared moderate and mild effectiveness for bio-ointment, respectively. Accordingly, 40 % and 52 % of users of formulated bio-ointment were respectively satisfied and very satisfied with the final performance of the product (Fig. 7(b)). Only 6 % of patients were dissatisfied with the bio-ointment function. This group was the same people who stated a mild effectiveness in pain relief.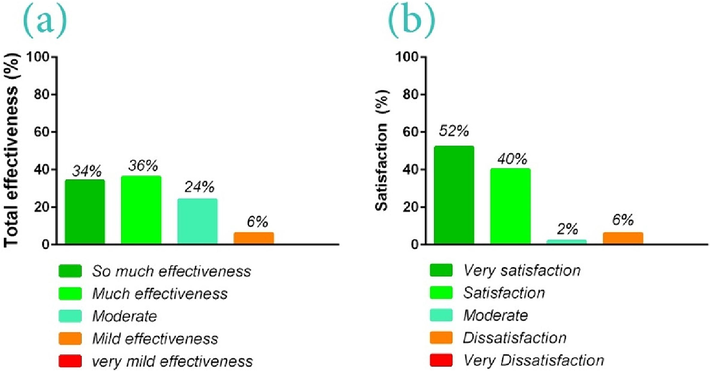
The effectiveness or success of the bio-ointment in reducing pains based on themodified health assessment questionnaire (MHAQ) before and after the use of the bio-ointment and the PGA in a 5-point Likert scale (so much effectiveness (70–100), so much effectiveness (50–70), moderate (30–50), mild effectiveness (10–30), very mild effectiveness (0–10 value)(a); the patient's satisfaction (%) with the bio-ointment function based on the 4 parameters of the speed of pain relief, the time of pain relief or analgesia period, the effectiveness of the formulated bio-ointment than other treatments, and warming function of therapeutic bio-ointment on the damaged area (b).
Hence, this formulated bio-ointment has been effective on 92 % of patients who possessed transient, acute, and chronic musculoskeletal pains (mild to severe), while it possessed mild effectiveness for very severe pains. Accordingly, we believe that this bio-ointment not only can be significantly effective in relieving pain but also possesses a therapeutic potential and regenerative ability of damaged tissue due to its active ingredients. Based on the reports medicinal herbs such as curcumin, black seed, and ginger possess therapeutic effects so that in many cases, they return the functional ability to the damaged tissue and subsequently repair or regenerate it via targeting different pathways (such as bone formation, antioxidant, and anti-inflammatory capacity) (Habibi Ghahfarrokhi and Reisi, 2019; Kheiridoost-Langaroodi et al., 2022).
4 Conclusion
Musculoskeletal pains are one of the most important causes of early retirement or reduced quality of life and participation in society. In this matter, NSAIDs are widely prescribed in various forms, associated with high costs and serious side effects due to inhibiting the biosynthesize of both types of cyclooxygenase 1 and 2 (COX-1 and 2). Traditional medicine research indicates that herbal medicines and their biomolecules can be promising alternatives to inhibit selectively COX-2 and regenerate bone tissue. Here, two in-vitro and clinical studies were performed which were firstly focused on the design of a hybrid oily biomaterial and assessment of its physicochemical and biological properties (in-vitro), and then the fabrication of anti-pain bio-ointment containing this biomaterial for studying effects of the selected formula on 50 patients with musculoskeletal pains, as the phase II of the study. To this end, an oily formula containing turmeric, ginger, chili pepper, and rose oils with weight ratios of 1:1:1:1 w/w was mixed with the base oil including sesame, black seed, and olive (1:0.4:0.6 w/w) in 3 wt ratios of 1:6 w/w (H1), 1.5:5.5 w/w (H2), and 2:5 w/w (H3); the best formula in terms of biological properties and cellular behavior was selected to do a clinical trial. Based on the results, the H2 formula, at 50 μg/ml concentration, due to having an enricher and optimized medium of herbal biomolecules and protein derivatives possessed a better performance than the same concentration for two other formulas and led to the better growth of human monocytes, synoviocytes, and chondrocytes, as well as osteogenic differentiation from h-ADSCs along with higher expression of the types I and II collagen. Such that, the highest expression of osteoblastic genes (RUNX2, OCN, and OPN) and collagen were observed for the H2 formula [respectively 1.3-fold, 1.7-fold, and 1.4-fold the higher than H1, as well as ∼ 2-fold, 2.1-fold, and 3.4-fold the higher than H3 (p < 0.01)]. Moreover, the H2 formula as an osteogenic induction medium indicated led to ∼ 31 % and 32 % increase in ALP activity compared to the positive control group, during 14 and 21 days, respectively. The studies of in-vitro anti-inflammatory and antioxidant illustrated that lower concentrations of the H2 formula also can lead to 35 %, 65.8 %, and 77.35 % decrease in the expression levels of mediators of COX-2, TNF-α, and IL-6, after 24 h, along with better antioxidant property (due to existence of optimized Ascorbic Acid in its formula). In the following, the therapeutic effects of this formula in the form of an herbal bio-ointment, on 50 patients with musculoskeletal pains, indicated that the suggested bio-ointment could relieve pain in less than 10 min for 40 % of patients and after 30 min from the use for 35 % of patients. Moreover, the pain-relieving effect of this bio-ointment (analgesia period) for 60 % of patients has lasted for longer than 3 h and lasted between 2 h and 3 h, for 36 % of patients. Generally, 76 % of patients stated that the formulated bio-ointment possesses stronger effects than other treatments. 46 % of patients also stated that this bio-ointment possesses moderate warming functions. Finally, although 6 % of patients were dissatisfied with the bio-ointment function, 92 % of patients reported this therapeutic method has resulted in many effects on their pain relief and damaged organ performance recovery, so that, 40 % and 52 % of users of formulated bio-ointment were respectively satisfied and very satisfied with the final performance of the product.
Formatting of funding sources
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
CRediT authorship contribution statement
Azadeh Izadyari Aghmiuni: Conceptualization, Data curation, Investigation, Methodology, Project administration, Writing – original draft, Validation. Saeed Heidari keshel: Formal analysis, Supervision, Writing – review & editing. Ali Aghababai: Data curation, Investigation, Resources, Software, Writing – original draft. Mohammad Zahraei: Conceptualization, Formal analysis, Methodology, Writing – original draft. Mostafa Rezaei-tavirani: Data curation, Software, Writing – review & editing.
Acknowledgements
We wish to acknowledge the help provided by the Proteomics research center (No. of Project: 24388) and School of Advanced Technologies in Medicine, Shahid Beheshti University of Medical Sciences to finalize this project with ethics code IR.SBMU.RETECH.REC.1399.758. Drs. Azadeh Izadyari Aghmiuni and Saeed Heidari Keshel contributed equally to this work.
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
References
- Phenolics isolated from Aframomum meleguta enhance proliferation and ossification markers in bone cells. Molecules. 2017;22:1467.
- [Google Scholar]
- Design of novel 3D-scaffold as a potential material to induct epidermal-dermal keratinocytes of human-adipose-derived stem cells and promote fibroblast cells proliferation for skin regeneration. Fibers Polym.. 2020;21:33-44.
- [CrossRef] [Google Scholar]
- Effect of PEG molecular weight and volume ratio of chitosan/PEG and silk fibroin on physicomechanical properties of chitosan/PEG-SF scaffold as a bio-mimetic substrate in skin-tissue engineering applications. Fibers Polym.. 2022;23:3358-3368.
- [CrossRef] [Google Scholar]
- Inhibition of homodimerization of toll-like receptor 4 by 6-shogaol. Mol. Cells. 2009;27:211-215.
- [Google Scholar]
- Topical capsaicin versus topical ibuprofen in acute musculoskeletal injuries: A randomized, double-blind trial. Hong Kong J. Emerg. Med.. 2023;30:210-216.
- [CrossRef] [Google Scholar]
- Nigella sativa and thymoquinone suppress cyclooxygenase-2 and oxidative stress in pancreatic tissue of streptozotocin-induced diabetic rats. Pancreas. 2013;42:841-849.
- [CrossRef] [Google Scholar]
- Thymoquinone, the Most Prominent Constituent of Nigella Sativa, Attenuates Liver Damage in Streptozotocin-Induced Diabetic Rats via Regulation of Oxidative Stress, Inflammation and Cyclooxygenase-2 Protein Expression. Appl. Sci.. 2021;11:3223.
- [CrossRef] [Google Scholar]
- Design of bio-scaffold conjugated with chitosan-PEG nano-carriers containing bio-macromolecules of Verbascum sinuatum L. to differentiate human adipose-derived stem cells into dermal keratinocytes. Int. J. Biol. Macromol.. 2023;127520
- [Google Scholar]
- A comparative of ginger extract in nanostructure lipid carrier (NLC) and 1% diclofenac gel for treatment of knee osteoarthritis (OA) J. Med. Assoc. Thail.. 2017;100:447-456.
- [Google Scholar]
- Topical capsaicin for pain management: therapeutic potential and mechanisms of action of the new high-concentration capsaicin 8% patch. Br. J. Anaesth.. 2011;107:490-502.
- [Google Scholar]
- Therapeutics and pharmacology of Gul-e-Surkh (Rosa damascena Mill): An important Unani drug. Int. J. Adv. Pharm. Med. Bioallied Sci.. 2017;5:195-205.
- [Google Scholar]
- A new curcuma extract (Flexofytol®) in osteoarthritis: results from a Belgian real-life experience. Open Rheumatol. J.. 2014;8:77.
- [Google Scholar]
- Mechanisms of lipid oxidation in water-in-oil emulsions and oxidomics-guided discovery of targeted protective approaches. Compr. Rev. Food Sci. Food Saf.. 2023;22:2678-2705.
- [CrossRef] [Google Scholar]
- Topical herbal therapies for treating osteo-arthritis. J. Evid. Based. Med.. 2013;6:200.
- [CrossRef] [Google Scholar]
- A randomized, double-blind, placebo-controlled trial of injected capsaicin for pain in Morton’s neuroma. Pain. 2016;157:1297-1304.
- [Google Scholar]
- Medicinal plants of the Asteraceae family that contain limonene. Med. PLANT Commun.. 2022;5:1-5.
- [CrossRef] [Google Scholar]
- Squalene targets pro- and anti-inflammatory mediators and pathways to modulate over-activation of neutrophils, monocytes and macrophages. J. Funct. Foods. 2015;14:779-790.
- [CrossRef] [Google Scholar]
- Detection of necrosis by release of lactate dehydrogenase activity. Immune Homeost. Methods Protoc. 2013:65-70.
- [Google Scholar]
- Small molecule therapeutics for inflammation-associated chronic musculoskeletal degenerative diseases: Past, present and future. Exp. Cell Res.. 2017;359:1-9.
- [CrossRef] [Google Scholar]
- Chin, K.Y., 2020. The relationship between vitamin k and osteoarthritis: A review of cur[1] K.Y. Chin, The relationship between vitamin k and osteoarthritis: A review of current evidence, Nutrients. 12 (2020). doi:10.3390/nu12051208.rent evidence. Nutrients 12. doi: 10.3390/nu12051208.
- Essential oils for bone repair and regeneration—mechanisms and applications. Materials (basel).. 2021;14:1867.
- [CrossRef] [Google Scholar]
- Use of capsaicin to treat pain: Mechanistic and therapeutic considerations. Pharmaceuticals. 2016;9:1-20.
- [CrossRef] [Google Scholar]
- Mechanisms of actions involved in the antinociceptive effect of estragole and its β-cyclodextrin inclusion complex in animal models. Plants. 2022;11
- [CrossRef] [Google Scholar]
- Efficacy of ginger for alleviating the symptoms of primary dysmenorrhea: a systematic review and meta-analysis of randomized clinical trials. Pain Med.. 2015;16:2243-2255.
- [Google Scholar]
- High glycine concentration increases collagen synthesis by articular chondrocytes in vitro: acute glycine deficiency could be an important cause of osteoarthritis. Amino Acids. 2018;50:1357-1365.
- [CrossRef] [Google Scholar]
- Topical capsaicin (high concentration) for chronic neuropathic pain in adults. Cochrane Database Syst. Rev.. 2017;2017
- [CrossRef] [Google Scholar]
- Comparative antioxidant and anti-inflammatory effects of [6]-gingerol, [8]-gingerol, [10]-gingerol and [6]-shogaol. J. Ethnopharmacol.. 2010;127:515-520.
- [CrossRef] [Google Scholar]
- Effects of curcumin supplementation on inflammatory biomarkers in patients with Rheumatoid Arthritis and Ulcerative colitis: A systematic review and meta-analysis. Complement. Ther. Med.. 2021;61:102773
- [CrossRef] [Google Scholar]
- Antimicrobial activity and chemical composition of essential oil extracted from Solidago canadensis L. growing wild in Slovakia. Molecules. 2019;24:1206.
- [Google Scholar]
- Comparative study of the thermal properties of Sesame oil and two mineral oils of different viscosity. Inge Cuc. 2019;15:99-109.
- [CrossRef] [Google Scholar]
- Is the iranian traditional medicine warm and cold temperament related to basal metabolic rate and activity of the sympathetic-parasympathetic system? Study Protocol. J. Diabetes Metab. Disord.. 2014;13:1-6.
- [CrossRef] [Google Scholar]
- The effects of sesquiterpene lactones on the differentiation of human or animal cells cultured in-vitro: A critical systematic review. Front. Pharmacol.. 2022;13:1-15.
- [CrossRef] [Google Scholar]
- Effect of capsaicin cream on chronic low back pain in patients with inter-vertebral disc herniation. Jundishapur J. Chronic Dis. Care. 2015;4:3-7.
- [CrossRef] [Google Scholar]
- Current insights on bioactive molecules, antioxidant, anti-inflammatory, and other pharmacological activities of cinnamomum camphora linn. Oxid. Med. Cell. Longev.. 2022;2022:1-23.
- [CrossRef] [Google Scholar]
- Chemical characterization, antioxidant, cytotoxic and microbiological activities of the essential oil of leaf of Tithonia diversifolia (Hemsl) A. Gray (asteraceae). Pharmaceuticals. 2019;12:34.
- [Google Scholar]
- Giacalone, M., Forfori, F., Giunta, F., 2015. Chili Pepper Compounds in the Management of Neuropathic Pain, Bioactive Nutraceuticals and Dietary Supplements in Neurological and Brain Disease: Prevention and Therapy. Elsevier Inc. doi: 10.1016/B978-0-12-411462-3.00020-5.
- Specific inhibition of cyclooxygenase-2 (COX-2) expression by dietary curcumin in HT-29 human colon cancer cells. Cancer Lett.. 2001;172:111-118.
- [Google Scholar]
- An increase in dietary n-3 fatty acids decreases a marker of bone resorption in humans. Nutr. J.. 2007;6:4-11.
- [CrossRef] [Google Scholar]
- Effects of medicinal herbs on osteoporosis: a systematic review based on clinical trials. J. Shahrekord Univ. Med. Sci.. 2019;21:229-236.
- [CrossRef] [Google Scholar]
- Involvement of transient receptor potential vanilloid 1 receptors in protease-activated receptor-2-induced joint inflammation and nociception. Eur. J. Pain. 2010;14:351-358.
- [Google Scholar]
- Nutraceuticals: do they represent a new era in the management of osteoarthritis?–a narrative review from the lessons taken with five products. Osteoarthr. Cartil.. 2011;19:1-21.
- [Google Scholar]
- Harpagoside suppresses lipopolysaccharide-induced iNOS and COX-2 expression through inhibition of NF-κB activation. J. Ethnopharmacol.. 2006;104:149-155.
- [CrossRef] [Google Scholar]
- Capsaicin: Physicochemical properties, cutaneous reactions and potential applications in painful and inflammatory conditions (Review) Exp. Ther. Med.. 2019;916–925
- [CrossRef] [Google Scholar]
- Quince seed mucilage-based scaffold as a smart biological substrate to mimic mechanobiological behavior of skin and promote fibroblasts proliferation and h-ASCs differentiation into keratinocytes. Int. J. Biol. Macromol. 2019
- [CrossRef] [Google Scholar]
- Quince seed mucilage-based scaffold as a smart biological substrate to mimic mechanobiological behavior of skin and promote fibroblasts proliferation and h-ASCs differentiation into keratinocytes. Int. J. Biol. Macromol.. 2020;142:668-679.
- [CrossRef] [Google Scholar]
- Fabrication of 3D hybrid scaffold by combination technique of electrospinning-like and freeze-drying to create mechanotransduction signals and mimic extracellular matrix function of skin. Mater. Sci. Eng. C. 2021;120:111752
- [CrossRef] [Google Scholar]
- Artesunate inhibits adipogeneis in 3T3-L1 preadipocytes by reducing the expression and/or phosphorylation levels of C/EBP-α, PPAR-γ, FAS, perilipin A, and STAT-3. Biochem. Biophys. Res. Commun.. 2016;474:220-225.
- [CrossRef] [Google Scholar]
- Anti-allergic effects of white rose petal extract and anti-atopic properties of its hexane fraction. Arch. Pharm. Res.. 2009;32:823-830.
- [CrossRef] [Google Scholar]
- Anti-inflammatory properties of curcumin, a major constituent of Curcuma longa: A review of preclinical and clinical research. Altern. Med. Rev.. 2009;14:141-153.
- [Google Scholar]
- Citronellol and geraniol, components of rose oil, activate peroxisome proliferator-activated receptor α and γ and suppress cyclooxygenase-2 expression. Biosci. Biotechnol. Biochem.. 2011;75:1010-1012.
- [CrossRef] [Google Scholar]
- Thymoquinone: An emerging natural drug with a wide range of medical applications. Iran. J. Basic Med. Sci.. 2014;17:950-957.
- [Google Scholar]
- The effect of selected herbal medicines on bone turnover markers: A systematic review and meta-analysis. J. Fam. Reprod. Heal.. 2022;16
- [CrossRef] [Google Scholar]
- Enhancement of keratinocyte differentiation by rose absolute oil. Ann. Dermatol.. 2010;22:255-261.
- [CrossRef] [Google Scholar]
- Zaluzanin C (ZC) induces osteoblast differentiation through regulating of osteogenic genes expressions in early stage of differentiation. Bioorg. Med. Chem. Lett.. 2017;27:4789-4793.
- [CrossRef] [Google Scholar]
- Inhibitory effects of geranium essential oil and its major component, citronellol, on degranulation and cytokine production by mast cells. Biosci. Biotechnol. Biochem.. 2016;80:1172-1178.
- [CrossRef] [Google Scholar]
- Effect of curcumin on rheumatoid arthritis: a systematic review and meta-analysis. Front. Immunol.. 2023;14
- [CrossRef] [Google Scholar]
- Antioxidant potential of essential oils from some Himalayan Asteraceae and Lamiaceae species. Med. Drug Discov.. 2019;1:100004
- [CrossRef] [Google Scholar]
- Analysis of cell viability by the lactate dehydrogenase assay. Cold Spring Harb. Protoc.. 2018;2018:465-468.
- [CrossRef] [Google Scholar]
- Validation of a modified Thai version of the Western Ontario and McMaster (WOMAC) osteoarthritis index for knee osteoarthritis. Clin. Rheumatol.. 2007;26:1641-1645.
- [Google Scholar]
- Therapeutic potential of curcumin and curcumin analogues in rheumatology. Int. J. Rheum. Dis.. 2015;18:591-593.
- [CrossRef] [Google Scholar]
- Sesquiterpene lactones from Algerian Artemisia herba-alba. Phytochem. Lett.. 2008;1:85-88.
- [CrossRef] [Google Scholar]
- Zingiberaceae extracts for pain: a systematic review and meta-analysis. Nutr. J.. 2015;14:1-10.
- [Google Scholar]
- The damage-associated molecular patterns (DAMPs) as potential targets to treat osteoarthritis: perspectives from a review of the literature. Front. Med.. 2021;7:607186
- [Google Scholar]
- Functional interactions between NMDA receptors and TRPV1 in trigeminal sensory neurons mediate mechanical hyperalgesia in the rat masseter muscle. PAIN®. 2012;153:1514-1524.
- [Google Scholar]
- Curcumin synergistically potentiates the growth-inhibitory and pro-apoptotic effects of celecoxib in osteoarthritis synovial adherent cells. Rheumatology. 2006;45:171-177.
- [CrossRef] [Google Scholar]
- Low-intensity pulsed ultrasound upregulates osteogenesis under inflammatory conditions in periodontal ligament stem cells through unfolded protein response. Stem Cell Res. Ther.. 2020;11:1-15.
- [CrossRef] [Google Scholar]
- Comparison of alkaline phosphatase activity of MC3T3-E1 cells cultured on different Ti surfaces: Modified sandblasted with large grit and acid-etched (MSLA), laser-treated, and laser and acid-treated Ti surfaces. J. Adv. Prosthodont.. 2016;8:235-240.
- [CrossRef] [Google Scholar]
- Likhitthummagun, P., Koonngam, P., Seeremaspun, A., n.d. Anti-aging effect of oral very high proline complex collagen placebo-controlled clinical study.
- Traditional and integrative medicine Therapeutic and Health Benefits of Rose Fixed Oil (Rowghan-E-Gol). Med: Tradit. Integr; 2019. p. :4.
- The use of carrier oils in aromatherapy massage and their effect on skin. Arch Physiother Glob Res. 2018;22:23-31.
- [Google Scholar]
- Gingerol and Its Role in Chronic Diseases. In: Gupta S.C., Prasad S., Aggarwal B.B., eds. Advances in Experimental Medicine and Biology. Cham: Springer International Publishing; 2016. p. :177-207.
- [Google Scholar]
- In patients with osteoarthritis, is curcumin, compared to placebo, effective in reducing pain? J Okla State Med Assoc. 2022;115:28-30.
- [CrossRef] [Google Scholar]
- Comparison of effects of ginger, mefenamic acid, and ibuprofen on pain in women with primary dysmenorrhea. J. Altern. Complement. Med.. 2009;15:129-132.
- [CrossRef] [Google Scholar]
- The effects of selected hot and cold temperament herbs based on Iranian traditional medicine on some metabolic parameters in normal rats. Iran. J. Pharm. Res.. 2014;13:177-184.
- [Google Scholar]
- Therapeutic effects of turmeric or curcumin extract on pain and function for individuals with knee osteoarthritis: A systematic review. BMJ Open Sport Exerc. Med.. 2021;7
- [CrossRef] [Google Scholar]
- Role of Curcumin in Common Musculoskeletal Disorders: A Review of Current Laboratory, Translational, and Clinical Data. Orthop. Surg.. 2015;7:222-231.
- [CrossRef] [Google Scholar]
- Anti-Inflammatory and Analgesic Effects of Rosehip in Inflammatory Musculoskeletal Disorders and Its Active Molecules. Curr. Mol. Pharmacol.. 2021;14:731-745.
- [CrossRef] [Google Scholar]
- The relative efficacy of topical non-steroidal anti-inflammatory drugs and capsaicin in osteoarthritis: a network meta-analysis of randomised controlled trials. Osteoarthr. Cartil.. 2018;26:1575-1582.
- [CrossRef] [Google Scholar]
- OMERACT-OARSI initiative: Osteoarthritis Research Society International set of responder criteria for osteoarthritis clinical trials revisited. Osteoarthr. Cartil.. 2004;12:389-399.
- [Google Scholar]
- Chondroprotective and anti-inflammatory effects of sesamin. Phytochemistry. 2012;80:77-88.
- [CrossRef] [Google Scholar]
- Curcumin, a component of golden spice: from bedside to bench and back. Biotechnol. Adv.. 2014;32:1053-1064.
- [Google Scholar]
- Ginger and its constituents: Role in prevention and treatment of gastrointestinal cancer. Gastroenterol. Res. Pract.. 2015;2015
- [CrossRef] [Google Scholar]
- Effect of Zingiber officinale R. Rhizomes (ginger) on pain relief in primary dysmenorrhea: A placebo randomized trial. BMC Complement. Altern. Med.. 2012;12
- [CrossRef] [Google Scholar]
- Ginger: the genus Zingiber. CRC Press; 2016.
- Clinical trials on pain lowering effect of ginger: A narrative review. Phyther. Res.. 2020;34:2843-2856.
- [CrossRef] [Google Scholar]
- The role of musculoskeletal ultrasound in assessment of disease activity in rheumatoid arthritis patients with fibromyalgia. Egypt. Rheumatol.. 2023;45:7-11.
- [CrossRef] [Google Scholar]
- Safety and efficacy of curcumin versus diclofenac in knee osteoarthritis: A randomized open-label parallel-arm study. Trials. 2019;20:1-11.
- [CrossRef] [Google Scholar]
- Efficacy and safety of combination of curcuminoid complex and diclofenac versus diclofenac in knee osteoarthritis: A randomized trial. Med. (united States). 2020;99:E19723.
- [CrossRef] [Google Scholar]
- The effect of topical Rosa damascena (rose) oil on pregnancy-related low back pain: a randomized controlled clinical trial. J. Evid. Based. Complementary Altern. Med.. 2017;22:120-126.
- [Google Scholar]
- In vitro evaluation of the antioxidant, cytoprotective, and antimicrobial properties of essential oil from Pistacia vera L. Variety Bronte Hull. Int. J. Mol. Sci.. 2017;18
- [CrossRef] [Google Scholar]
- Transient receptor potential vanilloid 1 as a therapeutic target in analgesia. Expert Opin. Ther. Targets. 2013;17:641-657.
- [Google Scholar]
- A Phenomenological Study of Ginger Compress Therapy for People with Osteoarthritis. Indo-Pacific J. Phenomenol.. 2010;10:1-10.
- [CrossRef] [Google Scholar]
- Ginger compress therapy for adults with osteoarthritis. J. Adv. Nurs.. 2010;66:2225-2233.
- [CrossRef] [Google Scholar]
- Cyclooxygenase-2 inhibitors in ginger (Zingiber officinale) Fitoterapia. 2011;82:38-43.
- [CrossRef] [Google Scholar]
- Thymoquinone Inhibits IL-1β-Induced Inflammation in Human Osteoarthritis Chondrocytes by Suppressing NF-κB and MAPKs Signaling Pathway. Inflammation. 2015;38:2235-2241.
- [CrossRef] [Google Scholar]
- Effects of black seed oil combined with olive oil or honey on antioxidant activities, phenolic content, and identification and quantification of thymoquinone, a key bioactive compound. J. Agric. Food Res.. 2023;14:100891
- [CrossRef] [Google Scholar]
- Effect of topical capsaicin on the cutaneous reactions and itching to histamine in atopic eczema compared to healthy skin. Arch. Dermatol. Res.. 1998;290:306-311.
- [Google Scholar]
- WHO, 2022. Musculoskeletal health [WWW Document]. 14 July 2022. URL https://www.who.int/news-room/fact-sheets/detail/musculoskeletal-conditions.
- Proline and hydroxyproline metabolism: implications for animal and human nutrition. Amino Acids. 2011;40:1053-1063.
- [Google Scholar]
- An experimental study on the effectiveness of massage with aromatic ginger and orange essential oil for moderate-to-severe knee pain among the elderly in Hong Kong. Complement. Ther. Med.. 2008;16:131-138.
- [Google Scholar]
- Essential oil composition of two Scutellaria species from Iran. J. Tradit. Chinese Med. Sci.. 2019;6:244-253.
- [CrossRef] [Google Scholar]
- Effect of carrier oils on the physicochemical properties of orange oil beverage emulsions. Food Res. Int.. 2015;74:260-268.
- [CrossRef] [Google Scholar]







