Translate this page into:
The DFT study of hydrogen bonding and thermodynamic parameters of (CH3OH)n(H2O)m (n, m = 1–8) clusters at different temperatures
⁎Corresponding author. Tel./fax: +98 851 3339843. mrsameti@malayeru.ac.ir (Mahdi Rezaei Sameti) mrsameti@gmail.com (Mahdi Rezaei Sameti)
-
Received: ,
Accepted: ,
This article was originally published by Elsevier and was migrated to Scientific Scholar after the change of Publisher.
Peer review under responsibility of King Saud University.
Abstract
For the first time, the interaction of one molecule of water with up to 8 molecules of methanol, and one molecule of methanol with up to 8 molecules of water in different temperatures (273.15–403.15 K) is investigated. The intermolecular hydrogen bonding and ΔG and ΔH of formation of (CH3OH)nH2O (n = 1–8) and CH3OH(H2O)m (m = 1–8) clusters is studied. The calculation is performed at the B3LYP/6-31G∗∗ level of theory. Similar to previous studies, herein a cyclic structure was optimized for (CH3OH)nH2O (n = 2–4) clusters. In the case of (CH3OH)nH2O clusters with n >4, a bicyclic structure was optimized, in which the H2O molecule acts as a bridging group. The cyclic structures were also optimized for CH3OH(H2O)m clusters (m = 2 and 3). However, for latter clusters where the number of water molecules was more than 3, a compact structure with maximum number of intermolecular hydrogen bonds was more stable than both the cyclic and bicyclic structures. It was shown that in all cases both the ΔH and ΔG of the formation of each cluster from the free molecules increase with increasing of the number of molecules in the cluster. The ΔH values of the formation of all clusters are negative in all temperatures but the corresponding ΔG values change to a positive number after a defined temperature, depending on the type and the size of the clusters.
Keywords
DFT
Thermodynamic
Cluster methanol–water
Temperature
1 Introduction
The study of hydrogen-bonded mixtures has been the subject of intense interest in the past decade, with water and methanol molecules receiving the greatest amount of attention (Buck and Huisken, 2000). Water is the most thoroughly investigated hydrogen bonded cluster but is quite different from methanol (Lee et al., 1988).Water can form up to four hydrogen bonds, two as proton acceptors (via the lone-pair electrons on oxygen) and two as proton donors. Methanol generally only forms three strong hydrogen bonds, two as proton acceptors (via the lone-pair electrons on oxygen) and one as a proton donor (Lee et al., 1988). The methyl CH bonds may form weak hydrogen-bonding interactions. The bulky methyl group and the dipole it produces give methanol a more complex and asymmetrical cluster compared with water. Much of the stabilization of water-methanol mixtures comes from the very sensitive electronic interaction of the hydrogen bond (Lee et al., 1988). Computational results indicate that the cyclic methanol clusters are the global minima when compared with chain, branched-cyclic, and branched-chain arrangements (Hagemeister et al., 1998; Boyd and Boyd, 2007). Cyclic structures maximize the number of hydrogen bonds and display an increase in cooperatively, thus yielding more favorable interactions among the members of the mixture (Lee et al., 1988). In this work we want to report the thermodynamic properties of (CH3OH)nH2O (n = 1–8) and CH3OH(H2O)m (m = 1–8) clusters in various temperatures. To the best of our knowledge the CH3OH–H2O clusters with more than four molecules (Mandal et al., 2010) have been never studied. The present study is undertaken to gain a better understanding of the interaction of one molecule of methanol with various numbers of water molecules and vice versa.
2 Computational methods
The geometries of all clusters studied here in the gas phase were fully optimized at DFT (B3LYP) (Becke, 1993; Lee et al., 1988) level of theory using the Gaussian 98 set of programs (Frisch and J., 1998). The standard 6-31G∗∗ basis set was used for all atoms. Vibrational frequency analyses, calculated at the same level of theory, at various temperatures (273.15–403.15) indicate that optimized structures are at the stationary points corresponding to local minima without any imaginary frequency. A starting molecular-mechanics structure for the ab initio calculations was obtained using the HyperChem 5.02 program (Hyper Chem, 1997).
3 Result and discussion
The optimized structures of all 23 clusters studied here are shown in Figs. 1 and 2. Literature review on the structure of clusters of the type (CH3OH)n(H2O)4−n (n = 0–4), shows that the cyclic structures are the most stable structures for this type of compound (Buck and Huisken, 2000; Marcos and Vincent, 2007; Mandal et al., 2010; Gonzalez et al., 1998; Jursic, 1999; Sum and Sandler, 2000; Eudes and Canuto, 2005a,b; Ruckenstein et al., 2005). As it can be seen in the Figs. 1 and 2, in this work we have optimized similar structures for latter clusters. However, in the case of (CH3OH)nH2O clusters with three or four methanol molecules, in addition to the cyclic structure, a bicyclic structure was also optimized in which the H2O molecule acts as bridging group. We found that for (CH3OH)4H2O cluster the cyclic structure (see Fig. 1, n = 4, I) about 2.89 kcal/mol is more stable than bicyclic structure (II). However, for (CH3OH)5H2O cluster the bicyclic structure (Fig. 1, n = 5, II) about 1 kcal/mol is more stable than corresponding cyclic structure (I). We note that one H2O molecule can form up to four intermolecular hydrogen bonds, but it forms only two hydrogen bonds in one cyclic cluster. Thus it can easily act as a bridging group to connect two cyclic clusters (see Fig. 1). Obviously, when the size of the cluster ring is small the cyclic structure is more stable than other possible structures. However, when the numbers of methanol molecules are greater than four then the bicylic structure including two small rings is more stable than a cyclic structure including a single big ring.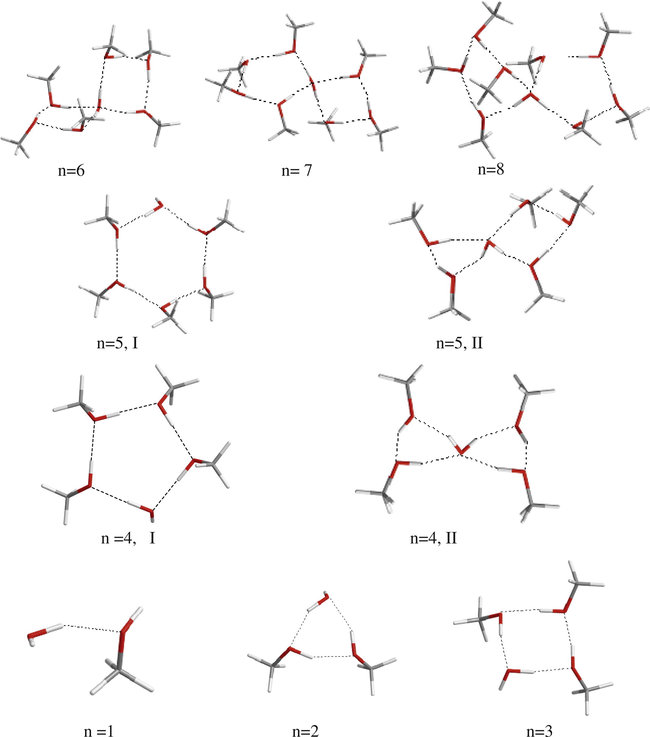
The optimized structures for (CH3OH)nH2O clusters.
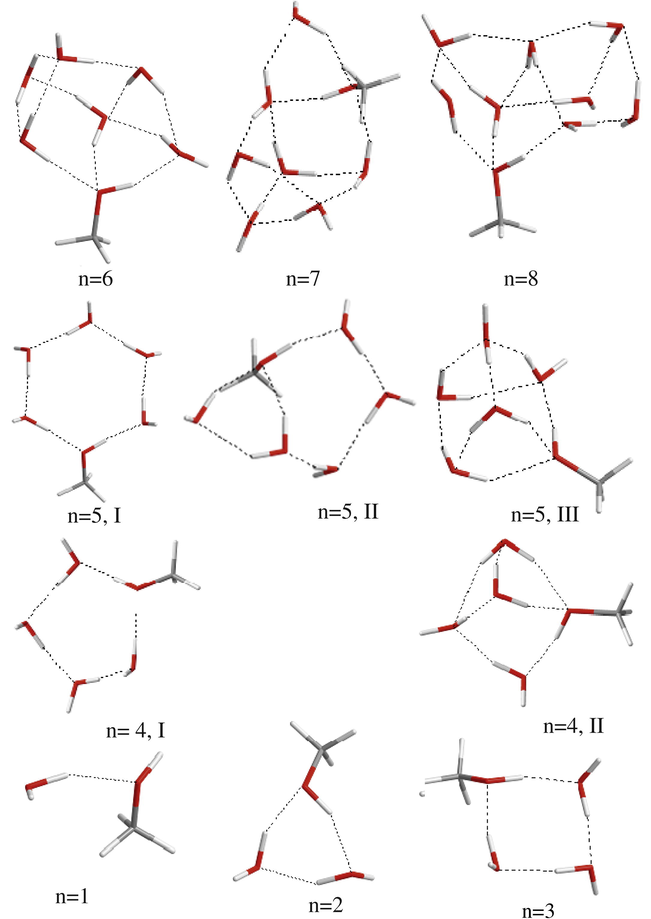
The optimized structures for CH3OH(H2O)m clusters.
On the other hand, we found that the most stable structure for CH3OH(H2O) clusters with more than three H2O molecules, is a structure with the maximum number of intermolecular hydrogen bonds (see Fig. 2). For CH3OH(H2O)4 cluster the cyclic structure (Fig. 2, n = 4, I) is about 3.1 kcal/mol less stable than the corresponding compact structure (II) in which the maximum number of hydrogen bonds are formed. As can be seen in Fig. 2, three different structures were optimized for CH3OH(H2O)5 cluster. We found that the compact structure, III, is about 5.5 and 6.8 kcal/mol more stable than the corresponding cyclic and bicyclic structures. Obviously, for bigger clusters the compact structure with maximum number of intermolecular hydrogen bonds will be more stable than other possible structures.
The ΔG and ΔH of formation of (CH3OH)nH2O and CH3OH(H2O)m clusters in various temperatures were calculated when considering the following reactions, respectively (see Figs. 3 and 4):
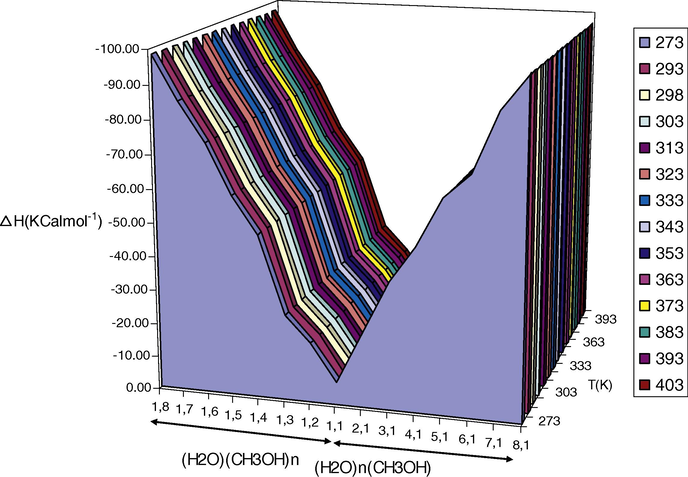
Variations of calculated ΔH values for (CH3OH)nH2O and CH3OH(H2O)m clusters at different temperatures.
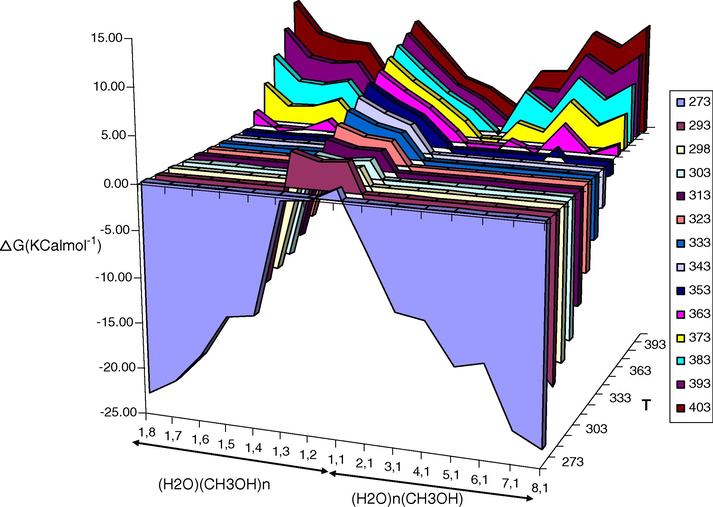
Variations of calculated ΔG values for (CH3OH)nH2O and CH3OH(H2O)m clusters at different temperatures.
ΔH (k cal mol−1) (H2O)n(CH3OH)m
273
293
298
303
313
323
333
343
353
363
373
383
393
403
1.8
−98.41
−98.23
−98.18
−98.13
−98.03
−97.92
−97.81
−97.70
−97.58
−97.45
−97.33
−97.19
−97.06
−96.92
1.7
−85.45
−85.30
−85.26
−85.22
−85.12
−85.04
−84.95
−84.85
−84.75
−84.65
−84.54
−84.43
-84.31
−84.19
1.6
−73.81
−73.69
−73.66
−73.63
−73.56
−73.49
−73.41
−73.34
−73.25
−73.17
−73.08
−72.99
−72.90
−72.80
1.5
−59.06
−58.97
−58.95
−58.92
−58.87
−58.81
−58.75
−58.69
−58.62
−58.55
−58.48
−58.40
−58.33
−58.24
1.4
−48.39
−48.31
−48.29
−48.26
−48.22
−48.16
−48.11
−48.05
−48.00
−47.94
−47.87
−47.81
−47.74
−47.67
1.3
−25.34
−25.25
−25.22
−25.20
−25.15
−25.09
−25.04
−24.98
−24.92
−24.86
−24.80
−24.74
−24.67
−24.61
1.2
−17.58
−17.52
−17.51
−17.49
−17.46
−17.43
−17.40
−17.36
−17.32
−17.29
−17.25
−17.21
−17.17
−17.13
1.1
−6.62
−6.59
−6.58
−6.57
−6.56
−6.54
−6.52
−6.50
−6.48
−6.46
−6.43
−6.41
−6.39
−6.36
2.1
−22.43
−22.42
−22.41
−22.41
−22.40
−22.38
−22.37
−22.35
−22.33
−22.31
−22.28
−22.26
−22.23
−22.20
3.1
−37.64
−37.62
−37.62
−37.61
−37.60
−37.57
−37.55
−37.53
−37.50
−37.46
−37.43
−37.39
−37.35
−37.31
4.1
−48.35
−48.33
−48.33
−48.32
−48.30
−48.27
−48.24
−48.21
−48.17
−48.13
−48.09
−48.04
−47.99
−47.93
5.1
−62.72
−62.71
−62.70
−62.70
−62.68
−62.64
−62.61
−62.57
−62.53
−62.47
−62.42
−62.36
−62.29
−62.23
6.1
−70.01
−69.97
−69.95
−69.93
-69.90
−69.84
−69.79
−69.73
−69.66
−69.59
−69.51
−69.42
−69.33
−69.24
7.1
−87.69
−87.68
−87.67
−87.66
−87.63
−87.59
−87.55
−87.49
−87.44
-87.36
−87.29
−87.21
−87.12
−87.03
8.1
−97.79
−97.74
−97.73
−97.71
−97.66
−97.60
−97.53
−97.45
−97.37
−97.27
-97.17
−−97.06
−96.95
−96.83
ΔG (k cal mol−1 (H2O)n(CH3OH)m
273
293
298
303
313
323
333
343
353
363
373
383
393
403
1.8
−23.09
−17.58
−16.20
−14.83
−12.08
−9.34
−6.60
−6.42
−1.13
1.60
4.32
7.05
9.76
12.48
1.7
−21.36
−16.67
−15.50
−14.33
−4.42
−9.66
−7.33
−7.36
−2.67
−0.35
1.97
4.29
6.60
8.91
1.6
−18.19
−14.12
−13.11
−12.09
−10.06
−8.03
−6.01
−6.06
−1.97
0.05
2.06
4.08
6.08
8.10
1.5
−13.54
−10.21
−9.38
−8.55
−6.89
−5.23
−3.58
−3.75
−0.27
1.38
3.03
4.68
6.32
7.97
1.4
−13.03
−10.44
−9.80
−9.15
−7.86
−6.57
−5.29
−4.00
−2.72
−1.44
−0.16
1.12
2.39
3.67
1.3
−0.64
3.75
1.62
2.07
2.97
3.87
4.76
5.65
6.55
7.44
8.32
9.21
10.10
10.98
1.2
−0.30
2.94
1.28
1.60
2.23
2.85
3.48
4.11
4.73
5.36
5.98
6.60
7.22
7.84
1.1
1.12
3.19
1.82
1.96
2.25
2.53
2.81
3.09
3.37
3.64
3.92
4.20
4.47
4.75
2.1
−4.72
−3.43
−3.10
−2.78
−2.13
−1.48
−0.84
−1.10
0.45
1.10
1.74
2.39
3.03
3.67
3.1
−10.95
−7.04
−8.51
−8.02
−7.05
−6.07
−5.10
−4.13
−3.15
−2.18
−1.21
−0.24
0.73
1.70
4.1
−11.44
−8.74
−8.06
−7.39
−6.04
−4.69
−3.34
−2.00
−0.65
0.70
2.04
5.00
4.72
6.06
5.1
−15.91
−12.48
−11.62
−10.76
−9.05
−7.34
−5.63
−3.92
−2.21
−0.50
1.20
2.91
4.61
6.31
6.1
−15.08
−11.06
−10.05
−9.04
−7.04
−5.03
−3.03
−1.03
0.98
2.98
4.97
6.97
8.96
10.95
7.1
−21.78
−14.18
−15.75
−14.54
−12.13
−9.72
−7.31
−4.90
−2.49
−0.09
2.31
4.71
7.11
9.50
8.1
−23.32
−17.87
−16.50
−15.14
−12.42
−9.70
−6.98
−4.26
−1.55
1.16
3.87
6.58
9.28
11.98
The calculated ΔG values are given in Table 2. As it can be seen, the ΔG values of the clusters are negative only below a critical temperature (see Fig. 4). Note that for the 1 + 1 cluster of the methanol–water in all temperatures the ΔG value is positive, 1.12 kcal/mol, indicating that the energy of an intermolecular hydrogen bond between these molecules is not enough to compensate the decreasing of the entropy of the system. Thus it seems that in all cases the ΔG values of the clusters are negative below a defined temperature, if the number of intermolecular hydrogen bonds in the cluster is enough. Furthermore we found that in both series of (CH3OH)nH2O and CH3OH(H2O)m clusters, when we move from smaller cluster to the bigger one, the ΔG value increases (becomes more negative), only if there are the maximum numbers of intermolecular hydrogen bonds. The ΔG values of (CH3OH)nH2O clusters at 273.15 K varies form −0.30 kcal/mol for n = 2 to −23.09 for n = 8, indicating that the number of intermolecular hydrogen bonds increases continuously with increasing of the number of methanol molecules. Similarly, the ΔG values of CH3OH(H2O)n clusters at 273.15 K varies form −4.72 kcal/mol for n = 2 to −23.32 for n = 8, indicating that the number of intermolecular hydrogen bonds increases continuously with increasing of the number of water molecules.
4 Conclusions
The ΔH and ΔG of the formation of (CH3OH)n(H2O)m clusters with up to 8 molecules of methanol or water in different temperatures have been studied at the B3LYP/6-31G∗∗ level of theory. Similar to previous studies, a cyclic structure was optimized for both the above clusters only where the value of n was 2 or 3. In the case of (CH3OH)nH2O clusters with four methanol molecules the cyclic structure was also more stable than other possible structures, but when the methanol molecules was more than four then, a bicyclical structure in which the H2O molecule acts as bridging group was more stable. However, in the case of CH3OH(H2O)m clusters with more than three H2O molecules, a compact structure with the maximum number of intermolecular hydrogen bonds was more stable than cyclic and bicyclic structures. The data show that in both the (CH3OH)nH2O and CH3OH(H2O)m clusters with increasing of the value of n the ΔH value increases. On the other hand, the results show that the ΔG of the formation of one cluster from free molecules has a negative value only below that of a critical temperature, depending on the type and the size of the cluster. The data show that in the both series of the above clusters with increasing of the number of intermolecular hydrogen bonds the ΔG value of system increases.
References
- Chem. Rev.. 2000;100:3863.
- J. Chem. Theory Comput.. 2007;3:54.
- J. Chem. Phys.. 1993;98:5648.
- Int. J. Quantum Chem.. 2005;102:554.
- Int. J. Quantum Chem.. 2005;104:808.
- Frisch, M. J., Trucks, G. W., Schlegel, H. B., Scuseria, G. E., Robb, M. A., Cheeseman, J. R., Zakrzewski, V. G., Montgomery Jr., J. A., Stratmann, R. E., Burant, J. C., Dapprich, S., Millam, J. M., Daniels, A. D., Kudin, K. N., Strain, M. C., Farkas, O., Tomasi, J., Barone, V., Cossi, M., Cammi, R., Mennucci, B., Pomelli, C., Adamo, C., Clifford, S., Ochterski, J., Petersson, G. A., Ayala, P. Y., Cui, K., Morokuma, Q., Salvador, P., Dannenberg, J. J., Malick, D. K., Rabuck, A. D., Raghavachari, K., Foresman, J. B., Cioslowski, J., Ortiz, J. V., Stefanov, B. B., Liu, G., Liashenko, A., Piskorz, P., Komaromi, I., Gomperts, R., Martin, R. L., Fox, D. J., Keith, T., Al-Laham, M. A., Peng, C. Y., Nanayakkara, A., Gonzalez, C., Challacombe, M., Gill, P. M. W., Johnson, B., Chen, W., Wong, M. W., Andres, J. L., Gonzalez, C., Head-Gordon, M., Replogle, E.S., Pople, J. A. GAUSSIAN 98.
- J. Chem. Phys.. 1998;109:139.
- J. Phys. Chem. A. 1998;102:82.
- Hyper Chem, 1997. Released 5.02, Hypercube, INC, Gainesville.
- J. Mol. Struct. (THEOCHEM).. 1999;466:203.
- Phys. Rev. B.. 1988;37:785.
- J. Chem. Theor. Comp.. 2007;3:1073.
- J. Phys. Chem. A. 2010;114:2250.
- J. Phys. Chem. A. 2005;109:807.
- J. Phys. Chem. A. 2000;104:1121.







