Translate this page into:
The electrochemical coupling reactions of organic halides compound in a valuable and practical manner for C—C and C–heteroatom formation: An overview
⁎Corresponding author. a.poursattar@urmia.ac.ir (Ahmad Poursattar Marjani) a.poursattar@gmail.com (Ahmad Poursattar Marjani)
-
Received: ,
Accepted: ,
This article was originally published by Elsevier and was migrated to Scientific Scholar after the change of Publisher.
Abstract
Owing to the environmental and energy problems nowadays, one of the enormous challenges is expanding clean, renewable energy to reduce the destructive ecological effects of chemical industries. Compared with other traditional processes, the electrochemical method has some benefits, such as being selective, causing minor waste, and working under mild conditions with no external oxidizing or reducing reagents, making this approach more economical and eco-friendly. Electrochemistry represents a powerful tool within synthetic organic chemistry. The development part of chemistry intends the performance of organic reactions employing electrons, including adding or removing electrons. Using electric current as a reagent has some advantages, such as simplicity of reactions, accessibility of materials, replacing toxic oxidizing or reducing reagents, and applying an eco-friendly environment. During the last decades, there has been much effort to decrease the usage of fossil fuels, and electricity has emerged as a common energy source. The conversion of electricity energy into chemical energy leads to new synthetic paths, and this energy is naturally safe and environmentally friendly. Developing electrochemistry approaches for performing high-performance organic reactions is essential for chemical industries. The electrochemical reaction is a green strategy because of the utilization of electric current instead of stoichiometric oxidants or reductants. Due to its helpful and environmentally friendly ability to produce reactive intermediates, organic electrochemistry has advanced considerably in oxidative hydrogen evolution coupling and sacrificial anode electroreduction. New developments in organic electrochemistry focus on developing new electrolytic catalysts,photoelectrocatalysis, bioelectrosynthesis, optimizing electrode materials, paired electrolysis, and artificial intelligence-assisted electrosynthesis. Thus, organic electrochemistry is expanding daily because of the significant number of construction projects and the possibilities of applying organic electrochemistry. Electrochemical methods for constructing organic compounds have tolerated renewal for the past decades. Organic electrochemistry has a wealthy background in organic synthesis. We hope this review helps to attract more attention to organic electrochemistry from researchers by using electrochemical processes in the laboratory and the industrial centers, which decrease pollution and create an eco-friendly. In this review, the electrochemical coupling reaction of organohalides for synthesizing various coupling products has been summarized and discussed since 2000.
Keywords
Electrochemical methods
Coupling reaction
C=C bond and C-hetero atom bond formation
Electrosynthesis
Halides
- ERCC
-
Cyclization–carboxylation sequence
- [OctV2+][Tf2N]2
-
1,10-Dioctyl-4,40-bipyridinium bis(trifluimide)
- Zn
-
Zinc
- SmI2
-
Samarium(II) iodide
- Bu3SnH
-
Tributyltin hydride
- Mg
-
Magnesium
- Co
-
Cobalt
- NiBr2bpy
-
(2,2′-Bipyridine)nickel(II) dibromide
- dmbp
-
4,4′-Dimethoxybenzophenone
- DMF
-
Dimethylformamide
- RTILs
-
Room temperature ionic liquids
- Al
-
Aluminium
- [octyl-mim][BF4]
-
Octyl-methylimidazoliniumtetrafluoroborate
- Pd-PDR
-
Palladium-pyridyl aldehyde complex
- TBABF4
-
Tetrabutylammonium tetrafluoroborate
- Fe
-
Iron
- NaI
-
Sodium iodide
- NiBr2
-
Nickel(II) bromide
- Pd
-
Palladium
- THF
-
Tetrahydrofuran
- PdCl2(PPh3)2
-
Bis(triphenylphosphine)palladium chloride
- Pt
-
Platinum
- Viologen
-
(C5H4NR)2n+
- RT
-
room temperature
- CH3CN
-
Acetonitrile
- CoCl2
-
Cobalt(II) chloride
- Py
-
Pyridine
- CoBr2
-
Cobalt(II) bromide
- MET
-
Mediated electron transfer
- PDIs
-
Perylene diimides
- DMSO
-
Dimethyl sulfoxide
- SET
-
Single electron transfer
- NatOBu
-
Sodium tert-butoxide
- Pd(OAc)2
-
Palladium(II) acetate
- NH4Br
-
Ammonium bromide
- Cat
-
Cathode
- Cu(OAc)2
-
Copper(II) acetate
- RVC
-
Reticulated vitreous carbon
- DMA
-
Dimethylacetamide
- NiCl2 dme
-
Nickel(II) chloride ethylene glycol dimethyl ether complex
- dtbbpy
-
4,4′-Di-tert-butyl-2,2′-dipyridyl
- mA
-
Milliampere
- MgBr2
-
Magnesium bromide
- bpy
-
2,2′-Bipyridine
- Olefin
-
Alkene
- NEt3
-
Triethylamine
- Fe(CO)5
-
Iron pentacarbonyl
- H2SO4
-
Sulfuric acid
- TEAP
-
Tetraethylammonium perchlorate
- C6H5Cl
-
Chlorobenzene
- C6H5CO2H
-
Benzoic acid
- TEACl
-
Tetraethylammonium chloride
- BMIMBF4
-
1-Butyl-3-methylimidazolium tetrafluoroborate
- NSAIDs
-
Nonsteroidal anti-inflammatory drugs
- CV
-
Cyclic voltammetry
- CH3I
-
Iodomethane
- TBAI
-
Tetra-n-butylammonium iodide
- LiBr
-
Lithium bromide
- HBpin
-
Pinacolborane
- TBAB
-
Tetrabutylammonium bromide
- (CF3SO2)2NLi
-
Lithium bis(trifluoromethanesulfonyl)-amide
- [DEME][TFSA]
-
N,N-Diethyl-N-methyl-N-(2-methoxyethyl)ammonium bis-(tri-fluoromethanesulfonyl)amide
- sCO2
-
Supercritical CO2
- IL
-
Ionic liquid
- [DEME][TFSI]
-
Diethylmethyl(2-methoxyethyl)ammonium bis(trifluoromethylsulfonyl)imide
- B2Cat2
-
Bis(catecholato)diborane
- NLO
-
Nonlinear optics
- Cyclam
-
1,4,8,11-Tetraazacyclotetradecane
Abbreviations
Data availability
All data have been given in the article.
1 Introduction
During the past few decades, efforts to design greener synthetic strategies in chemical procedures have significantly increased. Developing chemical approaches that remove the usage of catalysts is a critical and more attractive method that is proper for all chemistry facets. Organic compounds' electrosynthesis is highly selective compared to the typical chemical reaction. In recent years, electrosynthesis has been a vital research hotspot in organic synthesis transformation. For the electrosynthesis reaction, intermediates exist evenly scattered in the reaction media. The service of electrochemistry persists these days and constructs tons of invaluable chemicals. Electrochemistry is part of chemistry that investigates the interconnection of chemical and electrical impacts. Studying chemical changes that influence the passing electric current is the most crucial section of this field. The principal forces in chemistry-electrochemistry are the electrostatic attractions between electrons and nuclei. Overcoming the kinetic limitations even at low temperatures by utilization of potential and their chemical and stereochemical specific properties make electrochemical reactions beneficial in chemical synthesis (Francke and Little, 2014; Suprun et al., 2014; Suprun et al., 2020; Muster et al., 2011; Rapino et al., 2015; Guan et al., 2023).
Recently, electroorganic conversions have become known as a robust and efficient tool for cross-coupling reactions. Electrochemistry supplies a new area in manufacturing carbon-heteroatom and carbon–carbon bonds and describes probable mechanistic. Organic electrochemistry has a wealthy background in organic synthesis. The construction of new bonds is very crucial in chemical synthesis. Chemistry scientists have developed cross-coupling reactions as an efficient method for carbon–carbon bond generation. The carbon-heteroatom bond formation leads to products that draw considerable interest owing to their role as structural units in enormous synthetic methods for bioactive and natural compounds. Various helpful pharmaceutical agents and materials with various functionalities have been synthesized by constructing carbon–carbon or carbon-heteroatom bonds. Biaryl is a potential structural unit with many applications in natural products, synthetic bioactive compounds, and pharmaceuticals. The compounds in which carbon–nitrogen, carbon–oxygen, or carbon–sulfur bonds exist in the ring structure are seen in many chemistry and biology applications (Hartwig, 2008; Bringmann et al., 1990; Bhunia et al., 2012; Kerru et al., 2019).
Organic electrochemistry explains the oxidation and reduction of organic molecules at the electrodes. Electrochemical reactions are a kind of electron transition that occurs on the surface of electrodes. The reduction occurs on the cathode electrode, and the oxidation happens on the anode electrode; the reductant is the electron receptor, and the oxidant is the electron donor species. The electron transition process is performed between the electrode surface's and the electro-activated species' energy levels (Alvarado et al., 2022; Rein et al., 2022; Park et al., 2021; Zhu et al., 2021; Liu et al., 2023).
Studies were performed in the nineteenth century led to the emergence of electrochemistry of organic compounds in the twentieth century. In 1800, Volta Pile invented the electric battery for the first time (Volta and Nat, 1800). In 1807, Davy started to survey the chemical effects of electricity on electrolyzing the alkalis, which led to the discovery of potassium and sodium through the electrolysis of metal hydroxides (Davy, 1808). Faraday was the first one to introduce the terms electrolysis, anode, cathode, and ion; he also expressed in his two laws of electrolysis in 1830 (Faraday, 1834). Electrolysis of carboxylic acids to access alkyl radicals was carried on by Kolbe in 1847 (Kolbe, 1847). Hydrocarbon preparation, reached from the cathodic reduction in 1907, is well-known as Tafel rearrangement (Tafel et al., 1907). Each electrochemical reaction includes the composition of two half-reactions.
Modern electrochemical setups are divided into two parts: three- and two-electrode setups. Two electrode setups contain working electrodes, which are reactive intermediates generated by the electron transfer to the substrate molecules on this electrode, and another electrode is needed to continue the current in this circuit, that is, a counter electrode. While precise measuring of the potential in the working electrode is essential, (generally Ag/Ag+ or saturated calomel electrode as a standard electrode) is vital. This new setup is known as a three-electrode type setup. If working and the counter electrode are put in one chamber, it is called an undivided cell (Fig. 1A).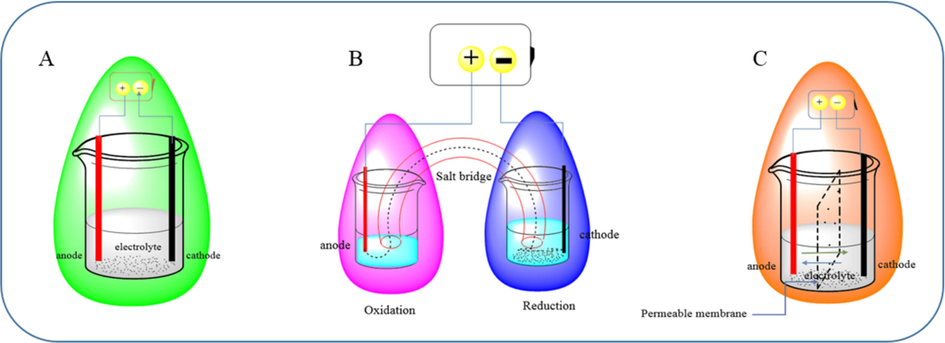
Undivided cell (A), divided cell, cathode, anode, and electrodes are connected with a salt bridge (B), and split cell, anode, and cathode electrodes are associated with a penetrable membrane (C).
The drawback of this setup is that high energy intermediates produced on the working electrode rapidly reduced in the counter electrode (vice versa), so scientists suggested a divided cell. In this cell, the cathode and anode are placed in independent cells and joined with a salt bridge or in a chamber segregated with a permeable membrane (Fig. 1B and 1C, respectively).
Heyrovsky's pioneering effort in polarography, using dropping mercury electrodes, led to today's cyclic voltammetry, where the current and voltage are associated (Heyrovsky, 1922; Heyrovsky, 1923). In 1942, Hickling opened a new approach to electrochemical synthesis. Before his invention, all electrosynthesis was carried out under a galvanostatic condition, where the current was constant and potential increased over time. Hickling designed potentiostat conditions that are currently reduced in constant potential (Hickling, 1942; Lingane et al., 1943). Undivided galvanostatic setups are operationally simple, making them practical despite all the benefits of divided cells and potentiostat setups.
In electrochemical reactions, there are two types of interactions for the transition of electrons between electrode and substrate. In type I, electrons transform from the surface of the electrode to substrate molecules directly. The disadvantage of this type is the high kinetic barrier that interacts between the ions/molecules in the solution and the electrode surface, producing high-energy species like radical anions and cations accumulated in the area close to the electrode superficial (double layer) (Perry and Wright, 2006). Created reactive species cause some problems in the system, and to avoid this, a mediator (or catalyst) is needed to facilitate the transition of electrons (type II).
Nitroxyl radicals (Semmelhack et al., 1983), triarylamines (Seo et al., 1966), transition metal complexes, and ionic halides are important category mediators with many applications in indirect oxidation and reduction Table 1. From the green chemistry point of view, organic electrochemical processes are precious because of their high selectivity for functional groups, scalability, and the requirement to moderate reactivity (low temperature, applied accurate of potential, adopted economical solvents), low waste and environment-friendly (Horn et al., 2016) so displays a remarkable role in enormous research, practical field, and industrial applications (Surucu and Abaci, 2017) Organic electrochemistry is a stepping stone to replace with general greenness chemical reaction. The building of carbon–carbon and carbon-heteroatom bonds is a critical key for assembling a compound for connection to material science, agriculture, and medical chemistry Fig. 2 (Roughley and Jordan, 2011; Alvarez-Builla et al., 2011; Boström et al., 2018).
Entry
Discovery
Explorer
Time
References
1
Built the first electric battery
Volta Pile
1800
Volta and Nat, 1800
2
Survey the chemical effects of electricity on electrolyzing the alkalis
Davy
1807
Davy, 1808
3
Introduce the terms electrolysis, anode, cathode, and ion
Faraday
1830
Faraday, 1834
4
Electrolysis of carboxylic acids to access alkyl radicals
Kolbe
1847
Kolbe, 1847
5
Hydrocarbon preparation, achieved from the cathodic reduction
Tafel
1907
Tafel et al., 1907
6
Designed potentiostat conditions
Hickling
1942
Hickling, 1942
7
Anodic oxidation pathways of aromatic amines studies
Eddie T. Seo et al.
1966
Seo et al., 1966
8
Nitroxyl-mediated electrooxidation of alcohols to aldehydes and ketones
M. F. Semmelhack et al.
1983
Semmelhack et al., 1983
Examples of biological structure applications by electrochemical approach.
Recently, several groups have given special attention to this field and published several reviews. Lei and colleagues illustrated the recent progress of electrochemical oxidative cross-coupling with iodine-mediated in 2018 (Liu et al., 2018). Waldvogel and coworkers explained electrochemical arylation reactions and recent developments in electrochemical synthesis (Wiebe et al., 2018; Waldvogel et al., 2018).
Recently, different groups studied the electrosynthesis of organic compounds and published many reports (Huang et al., 2022; Liang et al., 2018; Minteer and Baran, 2020; Möhle et al., 2018; Novaes et al., 2021; Röckl et al., 2022; Rodrigues et al., 2022; Sauermann et al., 2018; Shi et al., 2020; Wirtanen et al., 2020). Organic electrochemistry possesses a great past in the synthetic organic field. It is a hopeful substitute for traditional chemical methods because it prevents using stoichiometrically dangerous and venomous reactants. This review highlights the electrochemical methods for carbon-heteroatom and carbon–carbon formation from organohalides since 2000. Herein, we provide a profound summary of the recent advances in electrochemical cross-coupling reactions of organohalides.
2 Electrochemical C—C bond establishment
2.1 Homo-coupling of organic halides
In 2000, Cassol et al. demonstrated the synthesis of 6,6′-dimethyl-2,2′-bipyridine (2) using homocoupling of 6-bromopicoline (Scheme 1) (Cassol et al., 2000). They introduced two appropriate and high-yielding methods for the 6,6′-dimethyl-2,2′-bipyridine synthesis (74–67 %). The first procedure was electroconductive homocoupling of 6-bromopicoline (1) by Zinc rod anode, nickel foam cathode was used for electrolysis, and another one was palladium(II) acetate catalyzed coupling of 6-bromopicoline. Either method was straightforward and described a significant advance over the recognized syntheses of disubstituted bipyridine.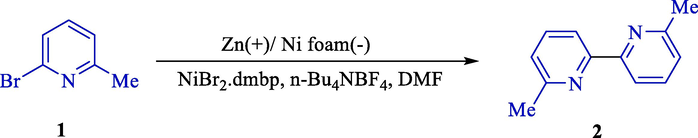
Electroreductive homocoupling of 6-bromopicoline.
Electrochemical reactions were accomplished in an undivided cell by Zn (anode) and NiBr2bpy in the role of the catalyst. Various products were obtained in mild status with isolated yields between 58 and 98 %. This report surveyed the effects of methyl groups' position on the ring, temperature, and solvent. The nature of the influence of the methyl and its place on the aromatic ring influenced the dimerization effectiveness. These effects were presumably associated with one or more stage (s) of the catalytic cycle. At ambient temperature, the electric resistance of the media became excessively elevated, and the solubility of the nickel complex was low; also, at 50 °C, no development was reached; therefore, the ambient temperature appeared to be the most suitable.
In 2002, França et al. indicated electrochemical homocoupling of 2-bromomethylpyridines and 2-bromopyridine by nickel complexes (De França et al., 2002). Electrochemical reactions were accomplished with Zinc as (anode) and NiBr2bpy as the catalyst. With mild conditions, divers of products were provided with isolated yields (58–98 %). This report surveyed the effects of the methyl group's position on the ring, temperature, and solvent. The nature of the methyl (Me) group effect and its position on the ring influenced the dimerization efficiency. These effects were presumably associated with one or more stag (s) of the catalytic cycle. Under room temperature, the electric resistance of the medium became too high, and the solubility of the nickel complex was low, also at 50 °C; no development was reached. Therefore, the ambient temperature appeared to be the most suitable. DMF was also better than acetonitrile because the nickel complex solubility in acetonitrile was low.
In 2003, Barhdadi et al. illustrated the direct or nickel-mediated electroconductive homocoupling of organic halides and coupling of them by activated alkenes at room temperature in ionic liquids (RTILs) as a solvent-electrolyte media (Scheme 2) (Barhdadi et al., 2003). They accomplished the electroreductive homocoupling reaction of several alkyl/benzyl halides 3. The electrolysis was performed by arranging a steady current severity between the Mg or Al bar anode and the nickel grid cathode. Bibenzyl 4 (78–48 %) was achieved from benzyl chloride/bromide in good yields.![Direct electroreductive coupling of organic halides in [octyl mim][BF4].](/content/184/2024/17/8/img/10.1016_j.arabjc.2024.105822-fig4.png)
Direct electroreductive coupling of organic halides in [octyl mim][BF4].
Two years later, Navarro et al. studied the supporting electrolyte Zn anode's mixed impact on the homocoupling of 2-bromopyridines (1, Scheme 3) (De França et al., 2005). A spectrum of reactions was conducted with different sorts and concentrations of supporting electrolytes, and it was shown that an essential stage for this procedure was the production of aryl zinc via a nickel-zinc transmetalation. Dimerization with ammonium salts was inefficient. In contrast, using sodium iodide (supporting electrolyte) did not inhibit the dimerization, although the organozinc was prepared, and their yields of product 5 were excellent.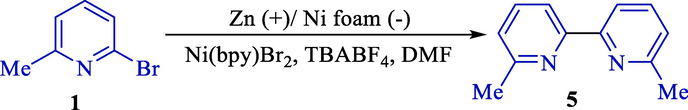
The electrochemical 2-bromopyridine homocoupling.
Then, the group Navarro reported mono and dihalopyridines (6 and 7) electrochemical coupling for the formation of unsymmetrical 2,2′-bipyridines 8 catalyzed using nickel complex (Scheme 4) (Oliveira et al., 2012). Reactions were applied to an undivided cell enclosed by surrounded by a nickel foam (cathode) and a zinc rod (anode). 2,2′-Bipyridine derivatives 8 were attained in good yields (58–98 %).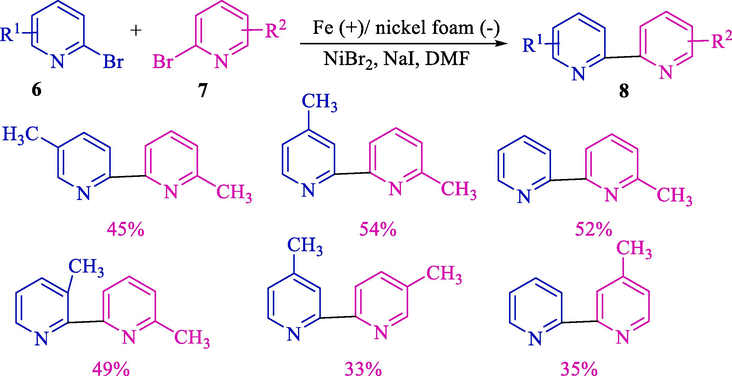
Heterocoupling of 2-bromopyridines with 2-bromomethylpyridines.
In 2013, the group Tanaka described the electroreductive homocoupling of aryl bromides 9 successfully to prepare the corresponding biaryls 4 (6–73 %) (Scheme 5) (Tanaka et al., 2013). By using a double mediatory system including N-alkyl-4-alkoxycarbonylpyridinium salts and [(C6H5)3P]2PdCl2, the electro-reductive coupling was conducted in an undivided cell with a platinum (cathode) and a zinc (anode). The mechanism proposal (Scheme 6) showed that in the cathode, OctV2+ reduced to quinoid OctV0, which reduces Pd(II) to provide Pd(0) active species together with OctV0+. Oxidative addition of aryl bromides on the Pd(0) generated Br—Pd(II)—Ar, then this compound was reduced by OctV0 and afforded [Ar—Pd(0)]−. In the next step, aryl bromide reacted with [Ar—Pd(0)]− and produced Ar—Pd(II)—Ar, which finally underwent reductive elimination to have biaryls.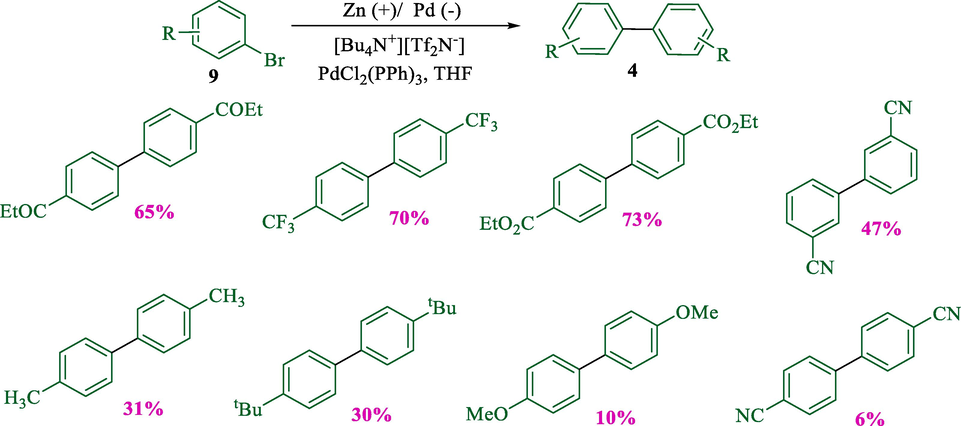
Electroreductive homocoupling of various aryl halides with Pt (cathode) and Zn (anode).
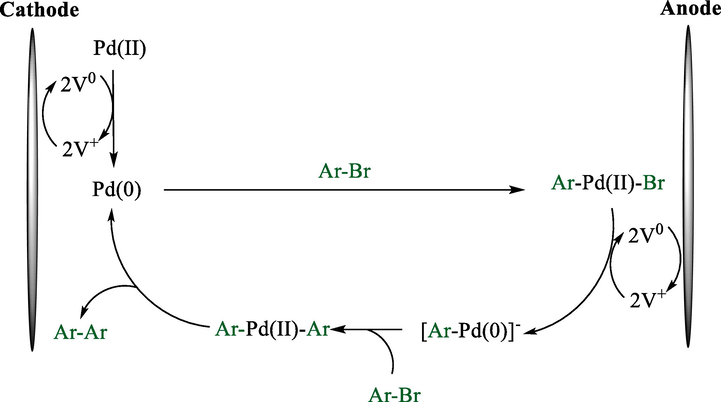
Mechanistic pathway representing the reductive coupling.
At the same time, this group developed the electrochemical treatment of N-alkylpyridinium salts and electro-reductive coupling of aryl bromides 10 in a double mediatory system, including PdCl2(PPh3)2 (catalytic amounts) and N-alkylpyridinium salts (Scheme 7) (Kuroboshi et al., 2013). Viologen served as a recyclable organic reductant. Compared with viologen compounds, pyridinium derivatives have complex structures, and their redox potential could be adjusted by introducing substituents. Zinc (anode) and platinum (cathode) were used for electrolysis. The variety of the desired biaryls 11 were obtained in good to moderate (65–93 %). They proposed a mechanism like the previous work.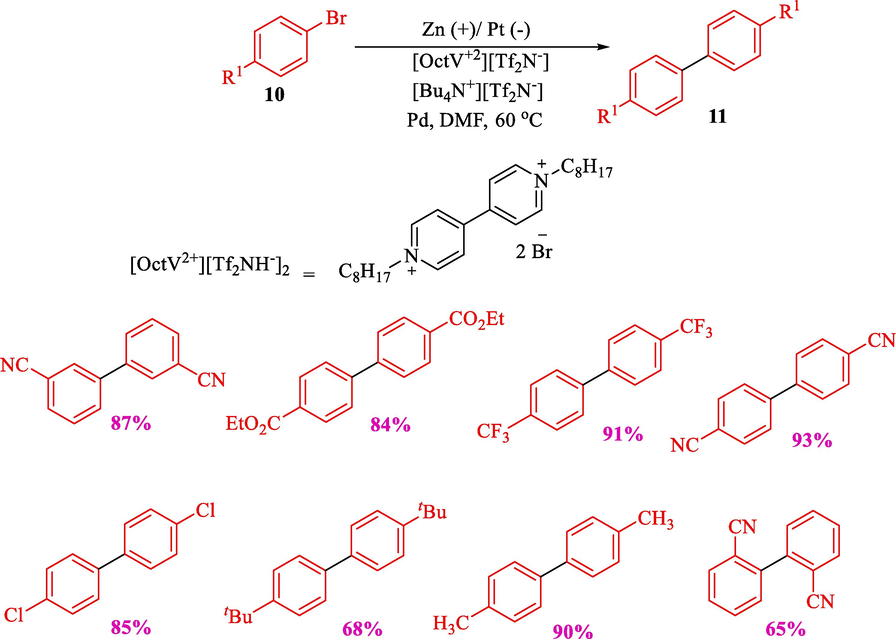
Electroreductive homocoupling of aryl halides.
A new electrochemical procedure for synthesizing symmetrical 2,2′-bipyridines 13 (31–89 %) from the transition metal-catalyzed homocoupling of 2-halopyridine derivatives 12 was reported by Oliveira et al. in 2015 (Scheme 8) (Oliveira et al., 2015). The graphite/nanotube powder cavity cell was employed for the electrocatalytic homocoupling of 2,6-dihydropyridines and 2-bromopyridines. A graphite powder/carbon nanotube (9:1 ratio) was used for the optimum cathode material. The homocoupling product obtained excellent yield. The cathode material could be reusable with three cycles with no tremendous drop.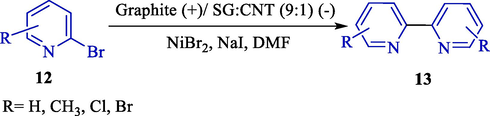
Electrocatalytic homocoupling of 2-halopyridines.
Electrochemical homocoupling of aryl/hetero halides 9 for synthesizing symmetrical biaryls 4 (54–90 %) was developed by Léonel and colleagues in 2018 (Scheme 9) (Rahil et al., 2018). The electrochemical reaction was done in an undivided electrochemical cell surrounded by a nickel foam (cathode) and a nickel/iron (36/64) (anode). The former reports comprised homo couplings of aryl halides using nickel catalysis and faced obstacles like long reaction times, mandatory thermal activation, and scope limitations (Semmelhack et al., 1971; Hashim and Kappe, 2007).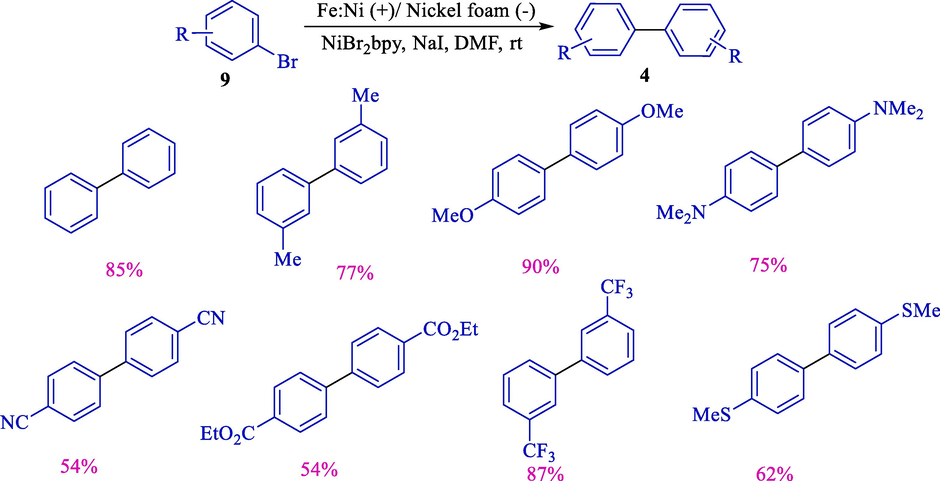
Electrochemical homocoupling of aryl halides.
The proposal mechanism demonstrated that NiBr2bp by cathodic dual electron transfer reduced to [Ni]0 (Scheme 10). Oxidative addition of Ni(0) to the aryl halide carbon-halide bond makes Ar[Ni]IIX complex an intermediate. Monoelectronic reduction of the mediator, pursued by a second oxidative addition to the aryl halide, led to the Ar[Ni]III(X)Ar complex. Finally, a reductive elimination furnished the related coupling compounds.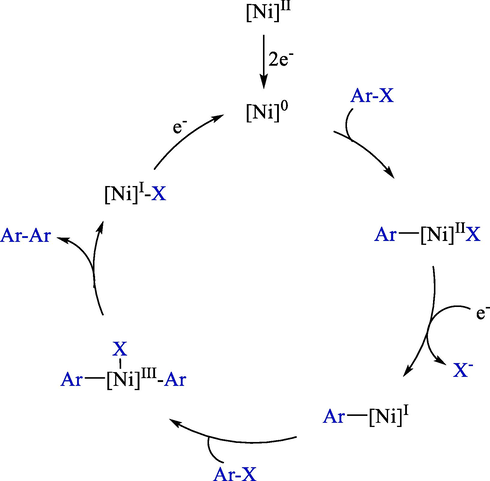
Probable reaction mechanism.
2.2 Cross-coupling of organic halides
Electrochemical synthesis is a very particular type of organic transformation and has been an ongoing research area in recent years. The improvement of the electrochemical cross-coupling method was significantly considered, and various research groups have entered this field. In 2000, Gosmini et al. reported electrochemical coupling of 2-chloropyrazine/2-chloropyrimidine 14 and functionalized aryl halides 3 catalyzed by 2,2′-bipyridine nickel complex (Scheme 11) (Gosmini et al., 2000a). This reaction was conducted with an iron rod (anode) and a nickel foam (cathode). 2-Aryl pyrimidine/pyrazine derivatives 15 were afforded good yields (31–79 %).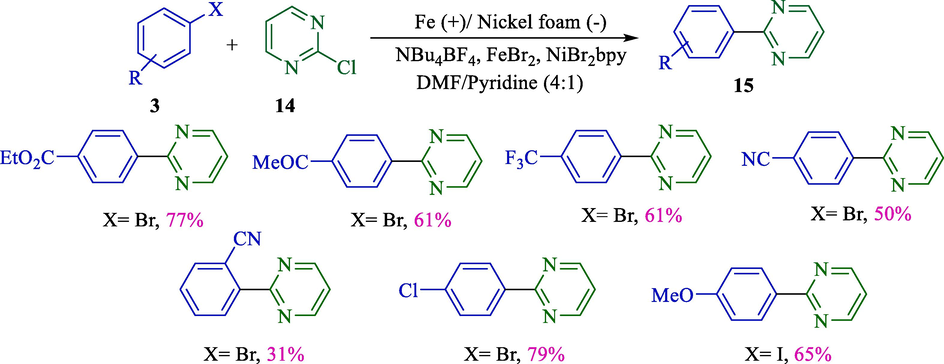
Electrochemical coupling 2-chloropyrimidine and aryl halides.
In the same years, this group explained the electrosynthesis of 2-arylpyridines 17 from the reaction between aryl halides 3 and 2-halo pyridine 16 (Scheme 12) (Gosmini et al., 2000b). Nickel foam cathode and iron anode were used. Under mild condition derivations, 2-arylpyridines achieved good to high yields (40–76 %).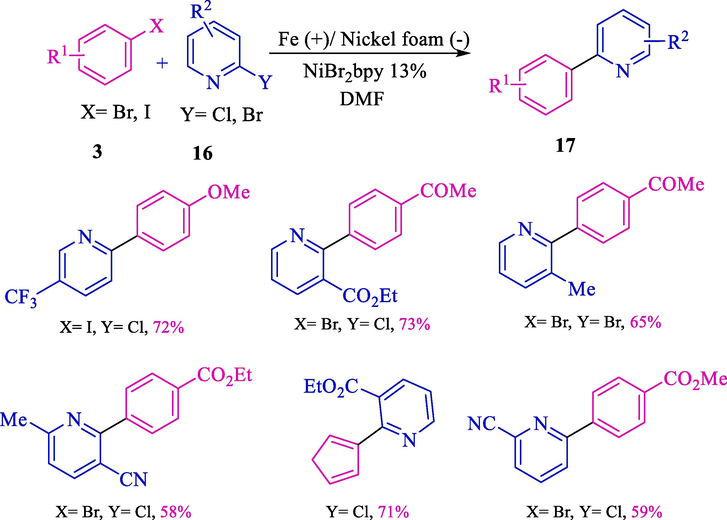
Electrosynthesis of functionalized 2-arylpyridines.
The electrochemical coupling reaction of 4-chloroquinoline 18 and aryl halides 3 by cobalt-catalyzed described by Gosmini's group (Scheme 13) (Le Gall et al., 2001a). By using an iron rod (anode) and a stainless steel grid (cathode), scarcely 4-phenylquinoline derivatives 19 obtained 48–81 % yield.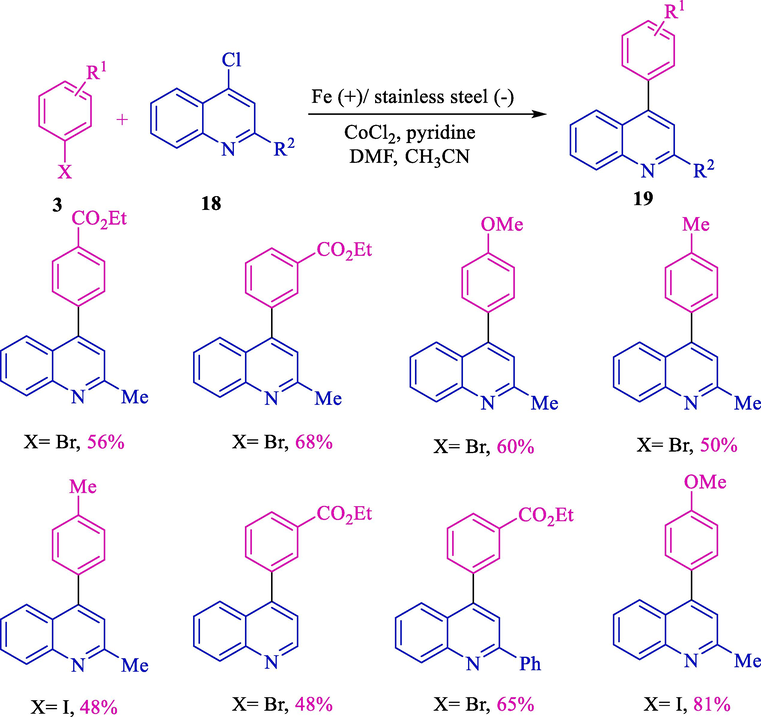
Electrochemical coupling of 4-chloroquinoline and aryl halides.
Both withdrawing and electron-donating groups coupling products achieved acceptable yields. The selection of iron rods was essential for the effectiveness of the electrochemical reaction.
In 2002, this group showed the electrochemical coupling of diverse aryl halides (chlorides, bromides, and iodides) 3 and 20 for synthesizing unsymmetrical biaryls 4 (Scheme 14) (Gomes et al., 2002a). A stainless steel grid (cathode) and an iron rod (anode) were used. The coupling reaction was suitable with diverse withdrawing or electron-donating substituents and efficient with o-substituted aryl halides.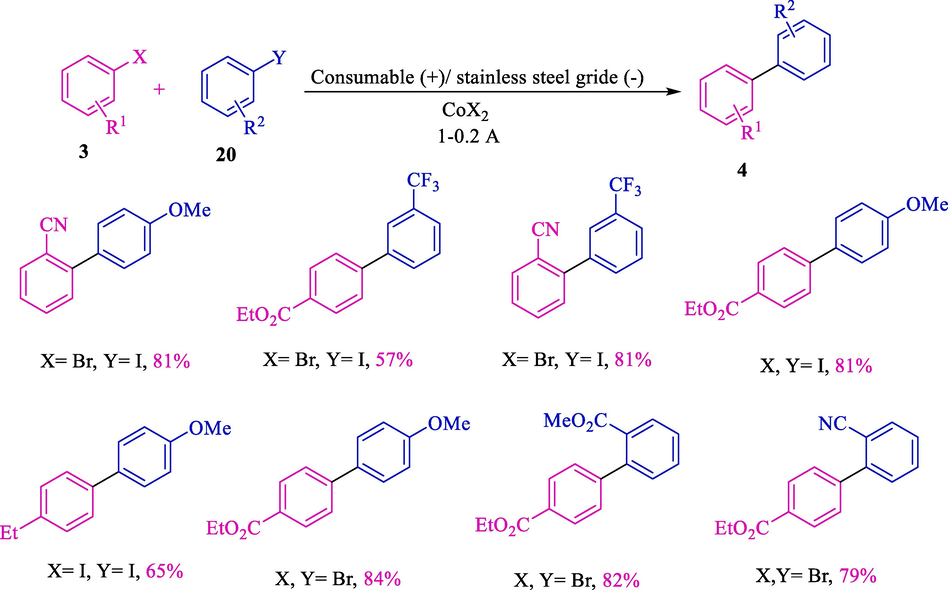
Electrochemical coupling of two various aryl halides.
Electrochemical coupling of aryl/hetero halides 3 and various allylic substrates such as carbonates or acetates 21 using the cobalt-pyridine complex was reported by the group of Gosmini in 2003 (Scheme 15) (Gomes et al., 2003a). A stainless steel cathode and an iron anode were used for electrolysis. Under mild conditions, the corresponding arylallyls 22 were achieved in high yields (30–74 %). This procedure appeared beneficial for synthesizing aryl-allyl compounds.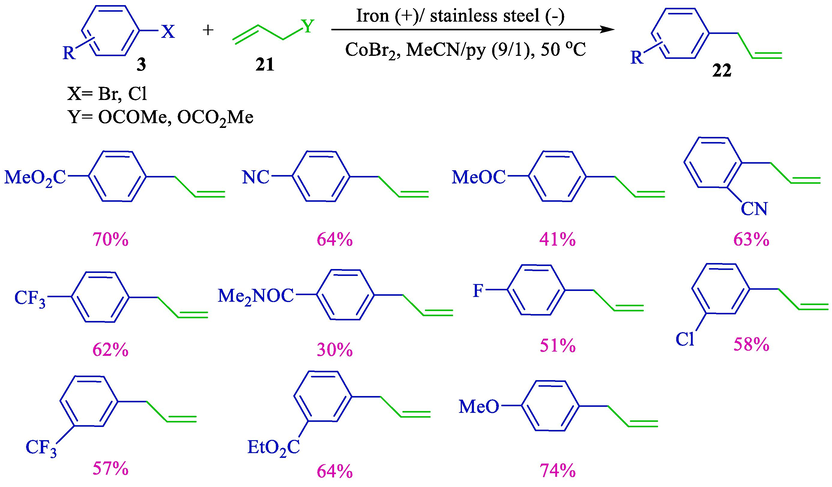
Coupling substituted aryl halides and allyl acetate.
In 2003, the group Gosmini represented a novel coupling reaction of aryl halides 3 with vinylic acetates 23 using cobalt as a catalyst in an undivided cell with a stainless steel grid (cathode) and a consumable iron (anode) (Scheme 16) (Gomes et al., 2003b) This simple electrochemical procedure is a mild and helpful method for synthesizing different vinyl-aryl compounds. Styrene derivatives 24 were obtained with good yields (20–92 %). Cobalt associated with bi-pyridine appeared more efficient for this reaction than palladium complexes.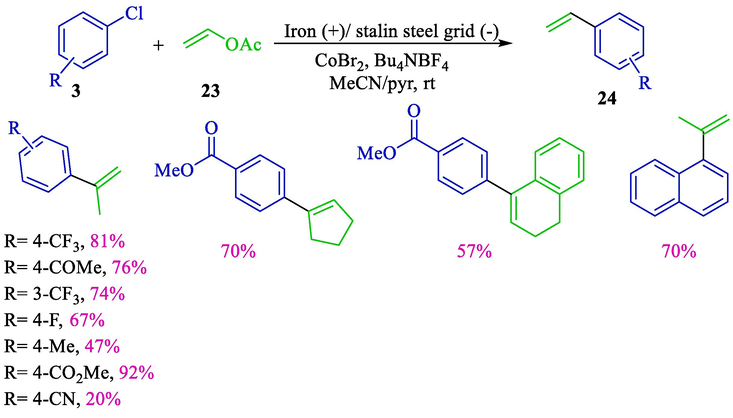
Electrochemical coupling vinylic acetates and aryl halides.
In 2007, Leonel et al. exhibited a proper electrochemical procedure for the syntheses of aryl/hetero aryl pyridazine 26 from the reaction of 3-chloro-6-aryl pyridazine 25 and phenyl halides 3 using Ni-catalyzed (Scheme 17) (Sengmany et al., 2007). An iron rod (anode) covered by a nickel foam (cathode) was applied. Under the standard condition, the differences substituted aryl/hetero arylpyridazines obtained moderate to good yields (22–83 %).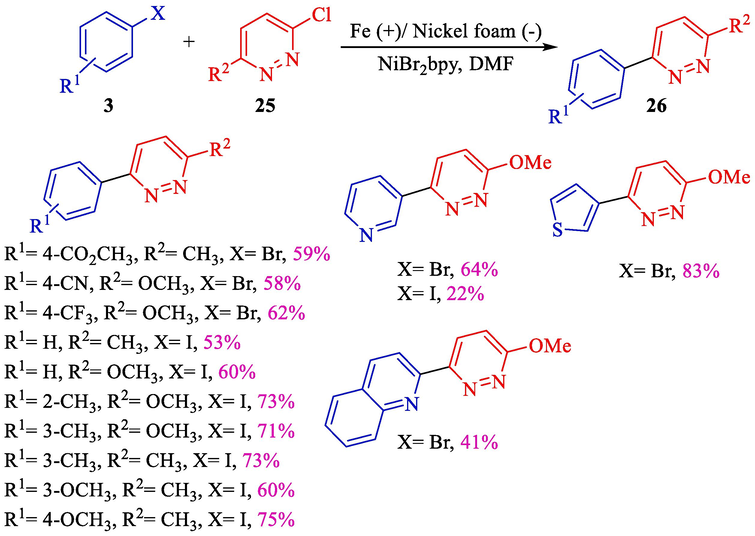
Electrochemical synthesis of substituted aryl/hetero arylpyridazines.
In 2011, Léonel et al.displayed an electrochemical reductive coupling aryl halides 3 and 4-amino-6-chloropyrimidines 27 for the synthesis of scope of 4-amino-6-arylpyrimidines 28 (Scheme 18) (Sengmany et al., 2011). Using an iron rod (anode) and a nickel foam (cathode) several 4-amino-6-arylpyrimidines were provided with moderate to excellent yields (34–99 %). The putative mechanism showed that, firstly, Ni(II) was redacted into Ni(0) (Scheme 19). In this step, the oxidative addition of Ni(0) into the carbon-halide bond of either aryl halide or chloropyrimidine occurred, leading to the generation of HetArNi(II)Cl. The monoelectronic reduction of the complex provided a HetArNiI that was affected in another oxidative addition onto the aryl halide, providing a HetArNi(III)(Ar)X complex. Reductive elimination in the final step produced the desired coupling product.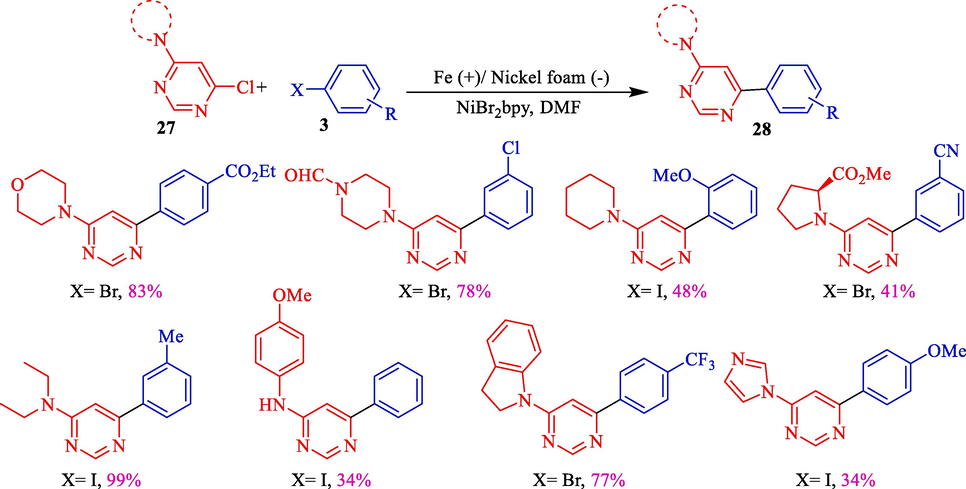
The coupling of aryl halides with 4-amino-6-chloropyrimidines.
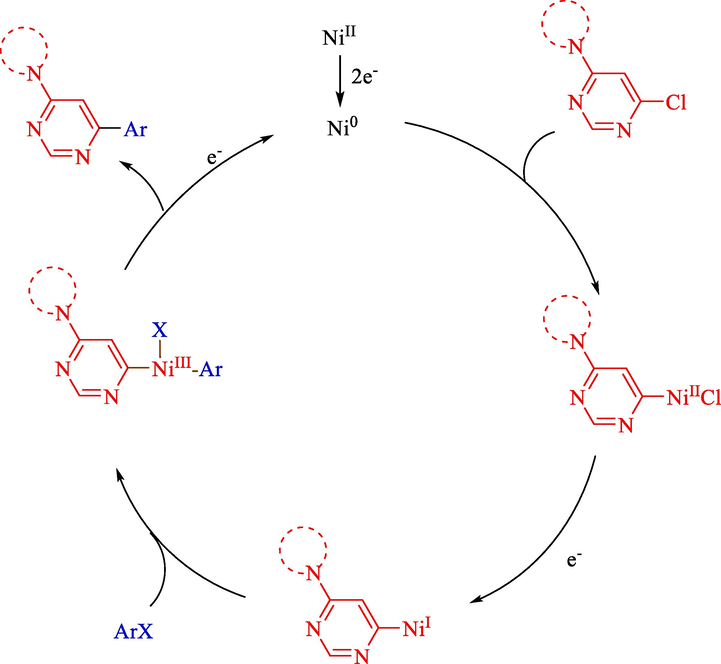
Proposed reaction mechanism.
The indirect electroreductive coupling of pyrroles 29 and aryl halides 3 employing perylene bisimide (as an electron-transfer mediator) was offered (Scheme 20) (Sun et al., 2016). For the first time, this group introduced perylene bisimides as an indirect electroreductive mediator. The extraordinary electron transfer capabilities and outstanding stabilities of perylene bisimides, with their flexibility for structural modification, make them encouraging nominees as indirect electroconductive mediators. The electrochemical reaction was performed with no utilization of a metal catalyst and the addition of a base using a sacrificial zinc anode. Under mild status, pyrrole compounds 30 were provided with an average to good yield (50–71 %). A proposal mechanism depicted those radical anions deriving from the cathodic reduction reduced aryl halides to aryl radicals, which underwent other reactions by pyrroles generating coupling products. (Scheme 21).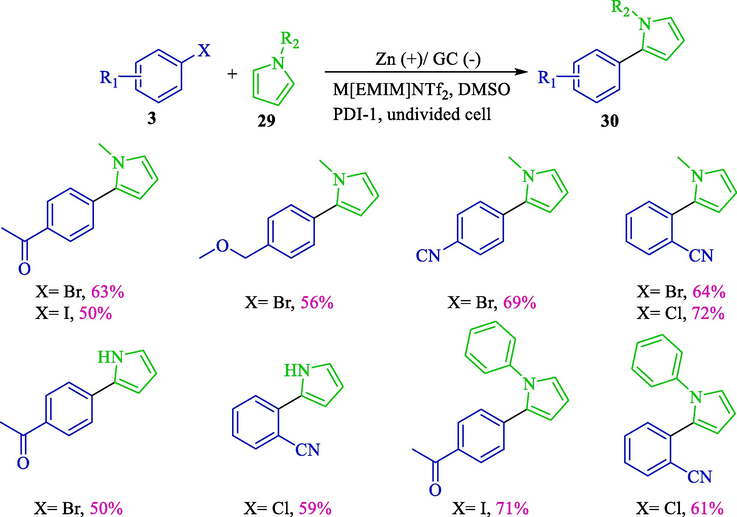
The electroreductive coupling of pyrroles and aryl halides.
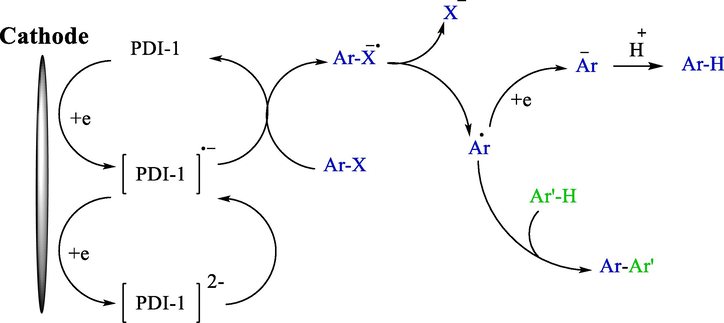
Proposal catalytic cycle.
During the same time, Khrizanforov et al. presented a novel approach to single-step preparation of perfluoro alkylated arenes 33 through cross-coupling of bromo/chloro arenes or hetero arenes (aryl, furan, and pyridine) 31 and organic perfluoro alkyl halides 32 (Scheme 22) (Khrizanforov et al., 2016). Perfluoroalkylated compounds were achieved in good yields. They chose several complexes, including nitrogen ligands that stabilize the low-oxidation-state metals, for instance, α-di-imine compound (dtbpy, tpy, bpy, and dmphen). These reactions used a complex of cobalt and nickel in various ligand environments. The more negative the reduction potential is, the more efficient the catalyst is.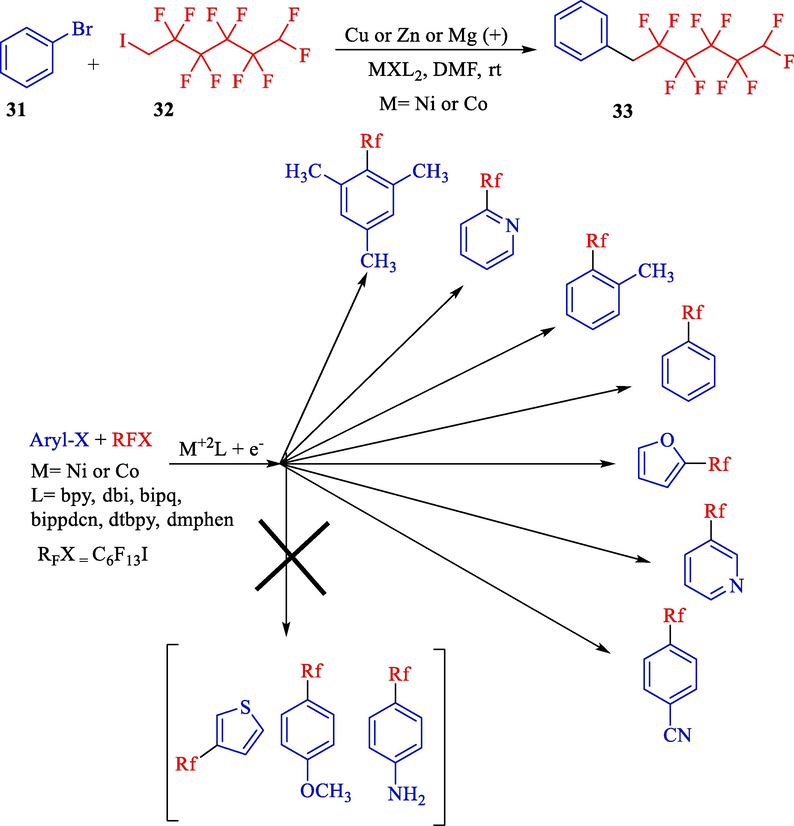
The electrocatalytic cross-coupling of organic halides by nickel and cobalt complexes catalyst.
Also in 2016, Leonel’s group demonstrated the synthesis of the 4-aryl/hetero-6-pyrrolyl pyrimidines 36 (34–70 %) by electroreductive nickel catalyzed from cross-coupling chloro pyrimidines 34 and aryl halides 35 (Scheme 23) (Sengmany et al., 2016). This reaction was a catalyst in an undivided cell wrapped by nickel foam (cathode), iron (anode), and NiBr2bpy. They revealed that the scarcely described aryl/hetero aryl pyrrolyl pyrimidines, potentially attractive novel structures for the transition-metal ligands or pharmaceutical industry, could be easily provided by reductive and electrochemical cross-coupling.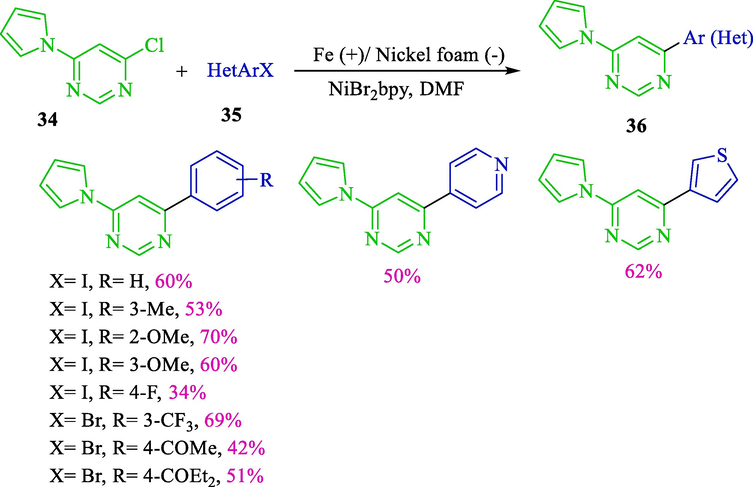
Electrochemical cross-couplings aryl/hetero halides and 4-chloro-6-pyrrolylpyrimidine.
In 2017, Atobe et al. designed a new cathodic coupling reaction of arenes 37 with aryl halides 3 (Scheme 24) (Qu et al., 2017). Using the cathodic SET mechanism for the activation of aryl halides allows the coupling reaction (31–76 %) to continue with no the requirement for any transition metal catalysts and single electron donors in a moderate status. Mechanism studies reveal that the SET from a cathode starts a radical chain by producing an anion radical of the aryl halide (Scheme 25). Platinum plates were utilized both for the cathode and anode.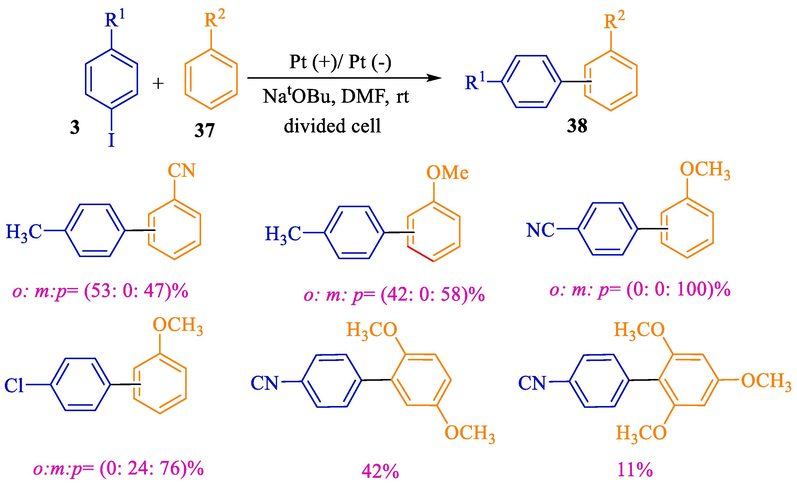
The cathodic coupling reaction of arenes with aryl halides.
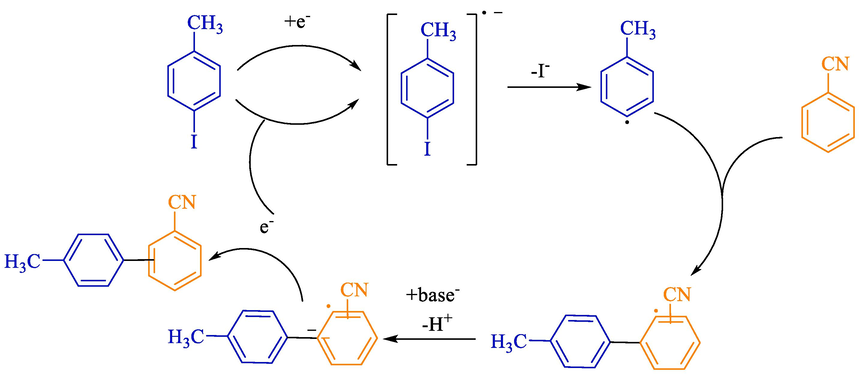
The suitable mechanism for the cathodic reaction.
The Huang group created a novel pathway for directly coupling allylic/alkyl halides utilizing an electrochemical approach (Scheme 26) (Lai and Huang, 2017). This reaction was performed in an undivided cell with zinc foil (cathode) and a platinum wire (anode) in an aqueous medium by catalyzed palladium(II) acetate. This method was helpful for the coupling reaction (76–91 %) of alkyl halides 39 with allylic halides 40, such as tertiary, secondary, and inactivated primary halides, as well as activated halides.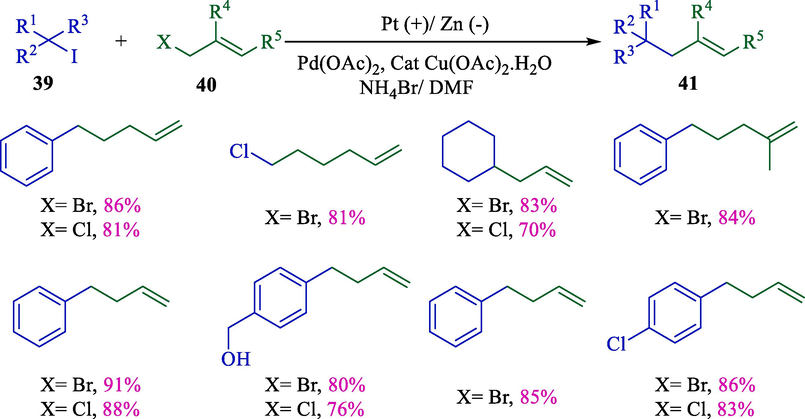
Electrochemical allylic alkylation between allylic and alkyl halides.
This year, Hansen et al. declared the electrochemical procedure for nickel-catalyzed reductive sp3-sp2 couplings alkyl 42 and aryl bromides 3 (Scheme 27) (Perkins et al., 2017). A Reticulated Vitreous Carbon (RVC) foam (cathode) and zinc rod (anode) were used for electrolysis. RVC was selected as the cathode substance for its chemical inertness and high surface area. The yields of product 43 were attained at 51–86 %. The mechanism offered by Weix et al. for metal powder reductive coupling (Biswas and Weix, 2013), where Ni(0) enters the catalytic cycle through the reduction of Ni(II) at the cathode (Scheme 28).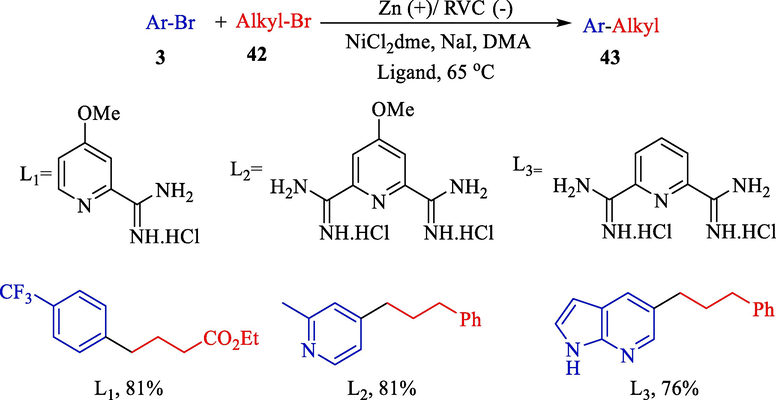
Electrochemical for sp3–sp2 cross-electrophile coupling of unactivated alkyl halides.
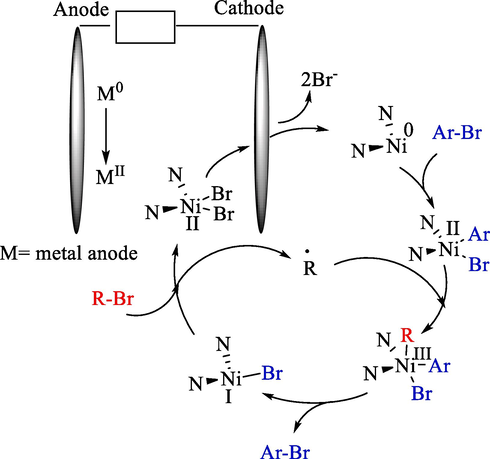
The mechanism that explains the electrochemical cross-electrophile coupling.
In 2018, Li et al. indicated nickel-catalyzed reductive coupling of N-hydroxy phthalimide esters 44 and aryl halide derivatives 45 (Scheme 29) (Li et al., 2018).This report provides a new and practical approach to using carboxylic acids as precursors to carbon–carbon bond-making. The reaction proceeded with mild status in a divided cell and employed triethylamine (as the reductant). This decarboxylative C(sp2)-C(sp3) bond- making showed great substrate functional group adaptability 46 (39–76 %). They proposed to utilize electron-rich amines as sacrificial reductants, which readily underwent anodic oxidation and donated electrons to the system.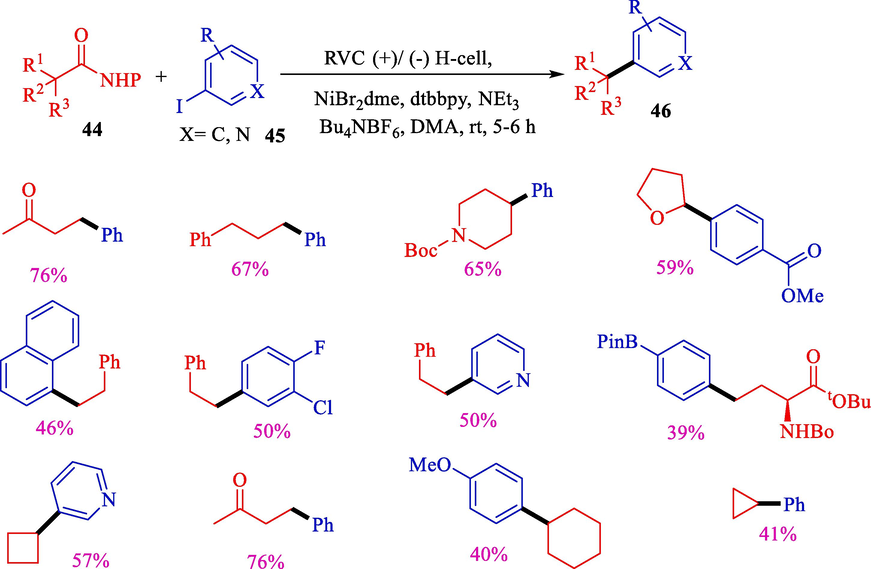
Electrochemical decarboxylative arylations.
Mechanism determination revealed that cathodic reduction of the redox-active NHP ester 44 appeared in a decarboxylative fragmentation, resulting in the production of the C(sp3) radical (a) (Scheme 30). Afterward, alkyl radical (a) is intercepted by a homogeneous nickel catalyst, which could be either Ni(II) or Ni(0) created from the oxidative addition of aryl halides. Upon production of Ni(III) species (b), the aryl/alkyl groups were expected to undergo reductive elimination to afford the C(sp2)-C(sp3) coupling product.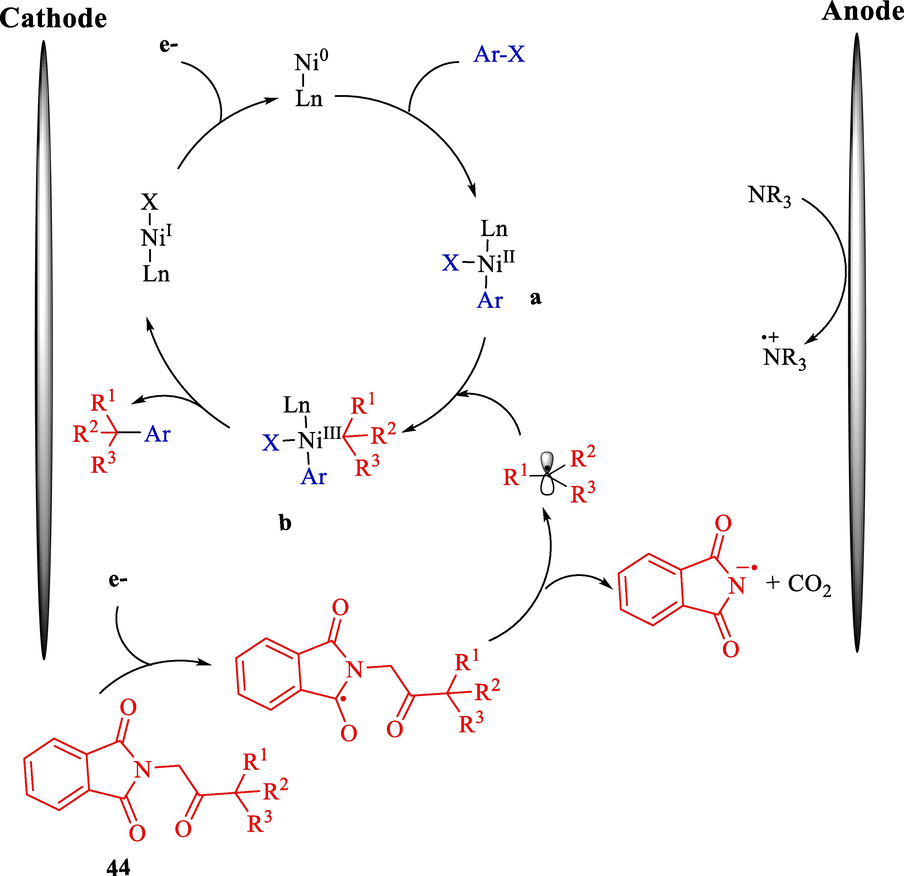
Proposed mechanism of electrochemical-driven decarboxylative arylation by nickel-catalyzed.
In 2021, Jiao et al. reported a simple synthesis of 1,1-dialkyl ketones 49 via a nickel catalyst electrochemical reductive cross-coupling of alkyl bromides 48 with alkyl acids 47 in an undivided cell. Alkyl bromides having various functional groups like ether, fluoro, chloro trifluoromethoxy, trifluoromethyl, and OTIPS were used and obtained products in good yields in contrary alkyl bromide with polysubstituted aryl rings did not went well and provided low results (Scheme 31) (Jiao et al., 2021).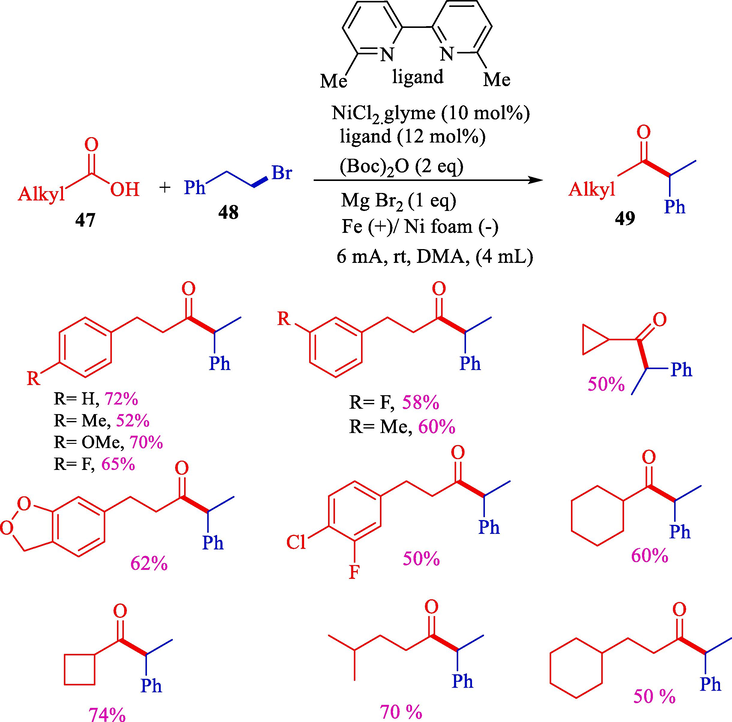
Electrochemical nickel-catalyzed coupling of alkyl halides with alkyl carboxylic acids .
The chemical method for reductive acylation between alkyl bromides and alkyl carboxylic acids by nickel-catalyzed has been reported Wang et al. This approach suffers from a long reaction time (24h) (He et al., 2019). A common limitation is employing stoichiometric amounts of manganese or zinc as reductants. The electrochemical method can be an excellent choice for solving this matter. The mechanism is offered in Scheme 32.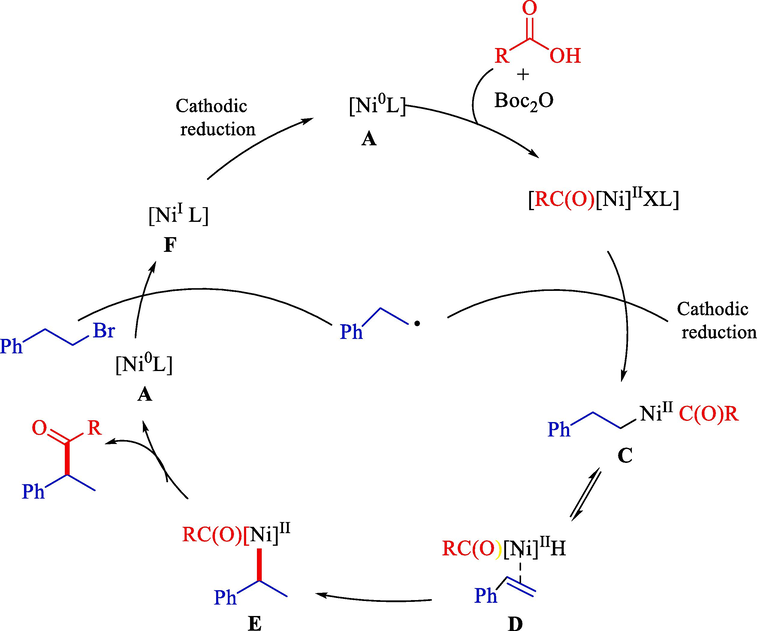
Plausible catalytic process.
In 2023, Cantillo et al. described the electrochemical coupling of alkyl bromides 42 and alkyl tosylates 50 by nickel catalyzed (Scheme 33) (Ibrahim et al., 2023). The results illustrated that the reaction proceeds high selectivity under a constant current (4 mA) using NaBr as the electrolyte in DMSO. The alkyl–alkyl bond-making reactions (42–78 %) are essential in medicinal chemistry, so electroorganic synthesis of this compound is growing. The mechanistic pathway representing electrochemical cross-coupling was depicted in Scheme 34.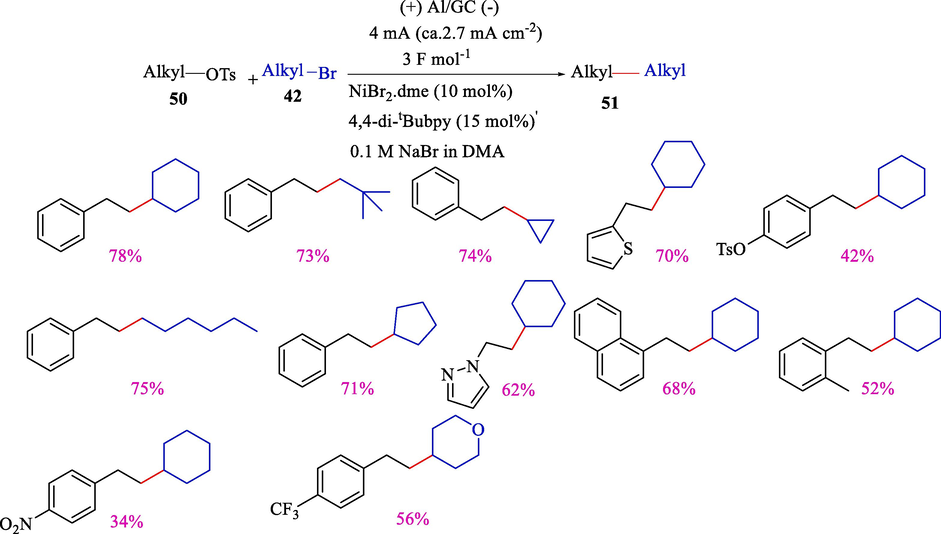
Electrochemical cross-coupling of alkyl tosylates with alkyl bromides.
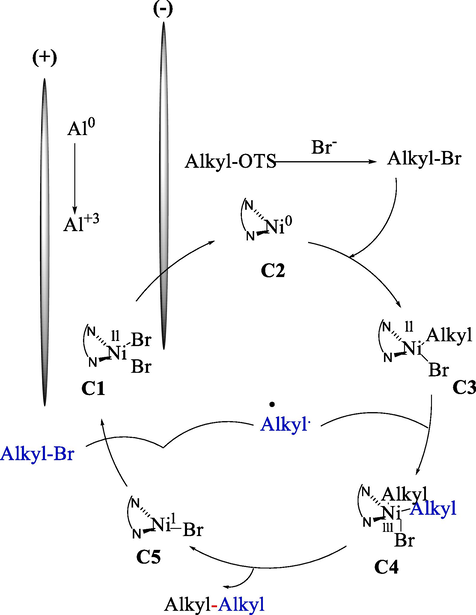
Mechanistic pathway for electrochemical cross-coupling.
In the same year, Qiu et al. reported NiH-catalyzed electrochemical coupling of alkyl alkenes/halides in an undivided cell with 4.0 mA (constant current) for 12h (Scheme 35) (Li et al., 2023). Alkyl halides perform double roles as coupling substrates and hydrogen sources to cause NiH species to be at electrochemical status. The approach displays a broad precursor range and generates good to high yields with up to seventy examples.
Electrochemical coupling of unactivated alkenes and alkyl bromide.
2.3 Addition of organic halides to unsaturated groups
Conjugate additions onto activated alkenes are valuable for carbon–carbon bond creation in organic synthesis (Perlmutter, 1992). In 2000, Gosmini and colleagues demonstrated that CoBr2 was an impressive catalyst for the electrochemical addition of aryl bromides 3 onto activated olefins 54 (Scheme 36) (Gomes et al., 2000). No additional product was acquired for other metals, e.g., Mg, Zn, or Al; nickel foam (cathode) and iron (anode) were used. Electrolysis was conducted at 0.2 A (constant current) in an undivided cell with good yield products 55 (20–70 %). The solvent acetonitrile /pyridine offered advantages over pyridine/DMF, affording slightly better results and avoiding using 2,2′-bipyridine as a ligand.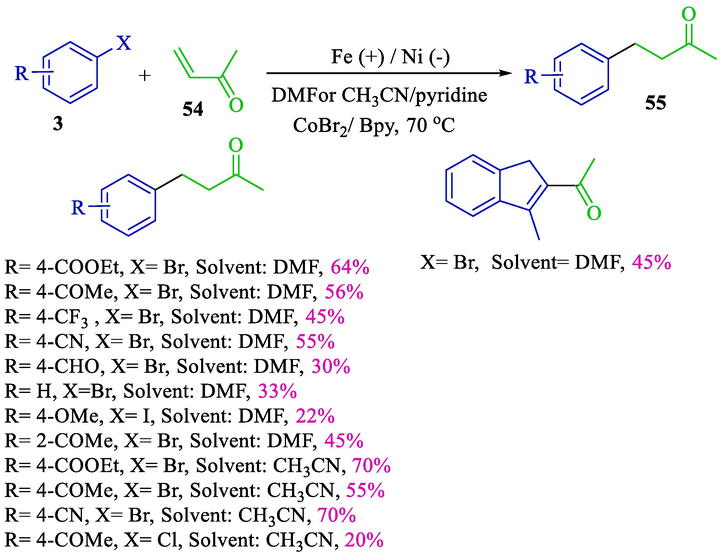
Electrochemical addition of aryl halides onto activated alkenes.
In the same year, Condon et al. demonstrated a direct procedure of activation of alkenyl halides, enabling the fabrication of substantial target compounds, including ketones, γ,δ- or β,γ- unsaturated esters, nitriles, or conjugated dienes as well as alkenylated aryl products (Scheme 37) (Cannes et al., 2000). Homo and cross-coupling, including alkenyl halides 56, were carried out efficiently using electroassisted nickel complex catalysis with (50–77 %) yield. They also exhibited that activating alkenyl chlorides, bromides, or iodides was possible. However, the reaction was born at 50 °C or higher. In cross-coupling reactions, using alkenyl iodides is not beneficial because of the dimerization; this is also observed with bromo styrene.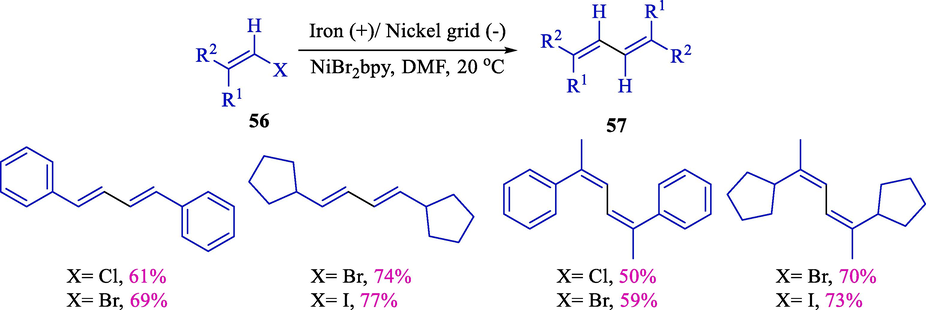
Electrochemical couplings of vinyl halides.
In 2001, Durandetti et al. indicated the electrochemical coupling of aldehydes 58 with organic halide 3 to synthesize arenecarboxaldehydes 59 (the Nozaki-Hiyama-Kishi reaction) (Scheme 38) (Durandetti et al., 2001). The electrochemical procedure includes a one-compartment cell fitted with a nickel sponge (cathode) and a stainless steel rod (anode) using chromium and nickel catalyst. Secondary alcohols were achieved in the mild to good yields (50–70 %). Different benzhydrol derivatives could be obtained to satisfy outcomes in one stage by a highly facile electrochemical procedure. The reaction was catalytic in chromium salt (7 %/C6H5CHO). Also, the process was used to add α-chloroester, allyl acetate, or vinyl halide to aryl aldehydes.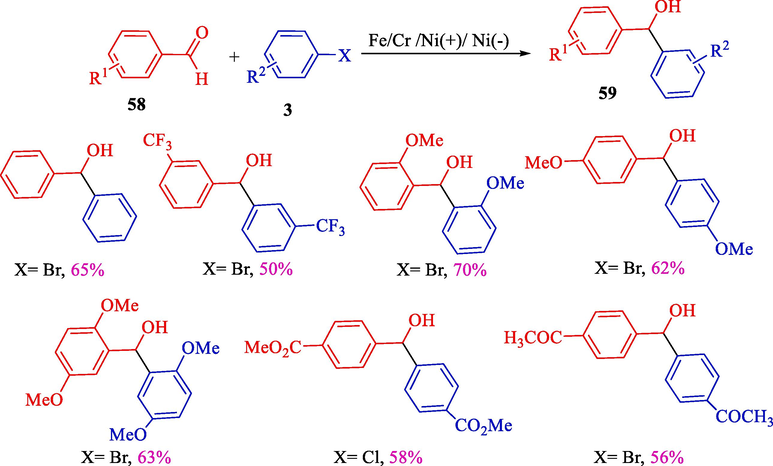
Electroreductive cross-coupling between aryl halides and benzaldehydes.
One year later, Condon et al. described electrochemical heteroarylation of activated olefins 61 by nickel catalyst (Scheme 39) (Condon et al., 2002). The conjugate addition reaction of hetero/aryl halides 60 to functionalized olefins had been favorably attained with nickel catalysis combined with the consumable anode method and prepared available to diverse functionalized hetero aryl products 62 (5–76 %). The structures of hetero aromatic are critical building blocks for synthesizing biologically active fragments as agrochemicals or pharmaceuticals. The reactions were conducted in an undivided cell flushed with a concentric nickel foam (cathode) and a stainless steel rod or iron (anode) in an argon atmosphere.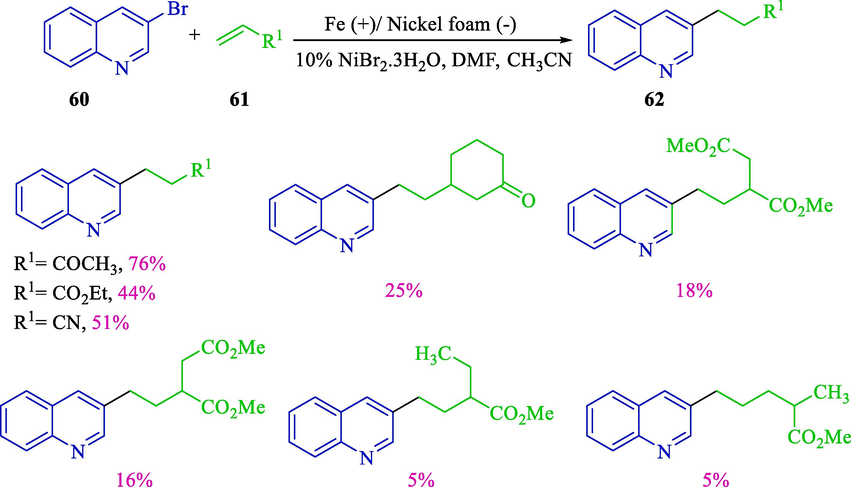
Reaction of activated olefins with 3-bromoquinoline.
The same year, Gosmini et al. demonstrated electrochemical vinylation of vinyl/aryl halides 3 with acrylate esters 63 using CoBr2 (Scheme 40) (Gomes et al., 2002b). A stainless steel grid (cathode) and an iron (anode) were performed. Pyridine/triethylamine/acetonitrile was used as a solvent, and bipyridine was used as a ligand. The CoBr2, 2,2′-bipyridine (specifically substituted by an electron-donating group), and acrylate esters with vinyl chlorides were used. This stereoselective reaction generated only E-olefins 64 (45–61 %) in all the investigated cases. Aryl bromides (substituted by an electron-donating group) in the para status gave the best results.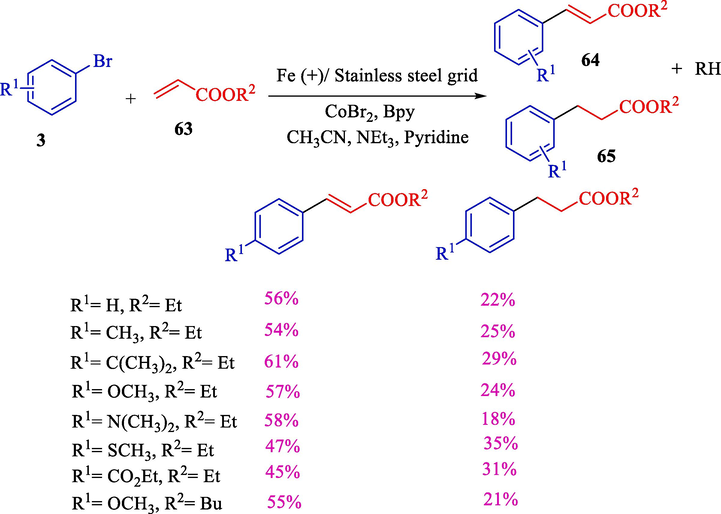
Coupling of acrylate esters with vinyl/aryl halides using CoBr2.
In 2002, Nédélec et al. revealed two pathways to obtain medium-sized lactones based on the electrochemical arylation of activated olefins 63 catalyzed by a nickel complex (Scheme 41) (De Mendonça Cavalcanti et al., 2002). Preparation of 6-, 7-, and 8- membered lactones 68 was applied through electrochemical arylation of electron-deficient olefins. The electrochemical reaction was done in the undivided cell with an iron rod (anode) and a nickel foam (cathode). This group found an exciting alternative based on the coupling between an α-β-unsaturated ester 63 and an o-aryl halide 66 (60–85 %), followed by lactonization.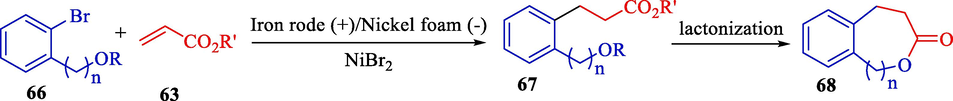
Bimolecular coupling between esters of acrylic acid and o-aryl halides.
Direct electrochemical cross-coupling between a mixture of hetero/aryl chlorides or bromides 3 and carbonates or allylic acetates 22 was offered by Gomes et al. (Scheme 42) (Gomes et al., 2003a). The electrochemical cell has a stainless steel grid (cathode) and a sacrificial iron (anode). This catalytic reaction is a suitable path for the SN2 with aryl halides. Various aryl allyl compounds 22 were achieved in good yields (30–81 %).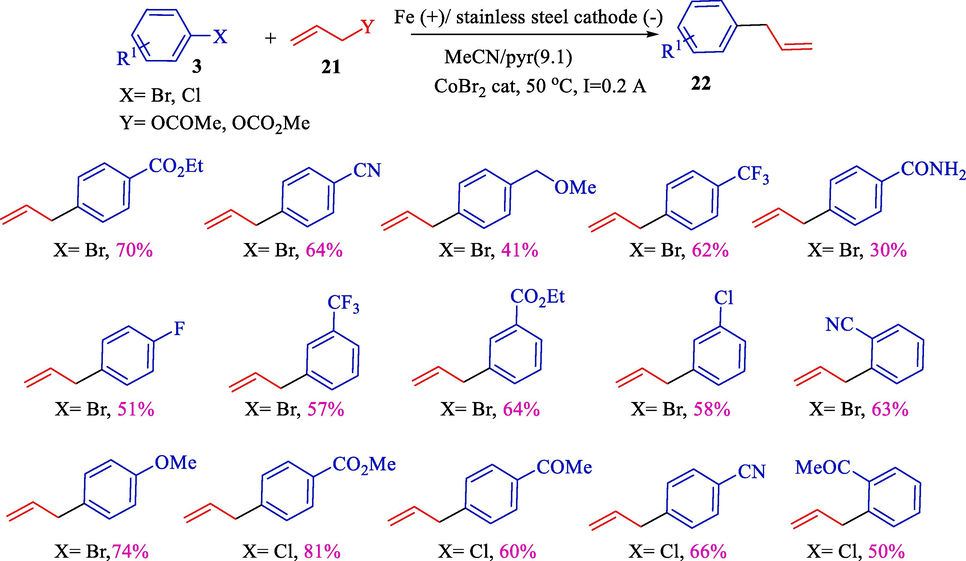
Coupling reaction between allyl acetate and aryl halides.
In 2003, Condon et al. illustrated the arylation of organic halides 3 with acrolein diethyl acetal 69 by nickel(II) bromide as a catalyst precursor (Scheme 43) (Condon et al., 2003). (E)- and (Z)-enol ethers 70 (28–58 %) were obtained by allylic displacement of an alkoxy group. The reactions were carried out using an iron or stainless steel (Fe/Cr/Ni, 72/18/10) anode in steady current intensity at 70 °C, and SN2 substitution derivatives were not attained. Furthermore, the Z-isomer is barely preferred; the isomer ratio was constant in the reaction.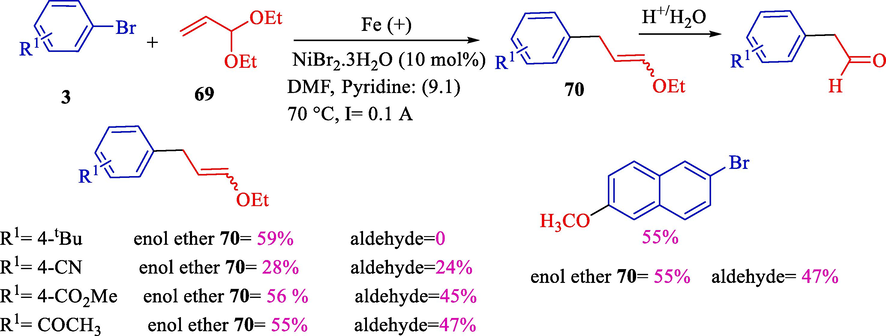
Arylation of acrolein diethyl acetal using nickel catalyzed.
In 2005, Moeller and Tian published the status for electrochemically assisted Heck reactions (Scheme 44) (Tian and Moeller, 2005). This reaction not only notably accelerates the room-temperature Heck reactions without adding ligands but also leads to high product yields 71 (62–89 %). Coupling iodobenzene 3 to electron-rich, electron-poor, and conjugated olefins series 61 was performed. The reactions were accomplished using a straightforward design utilizing a battery as the energy supply.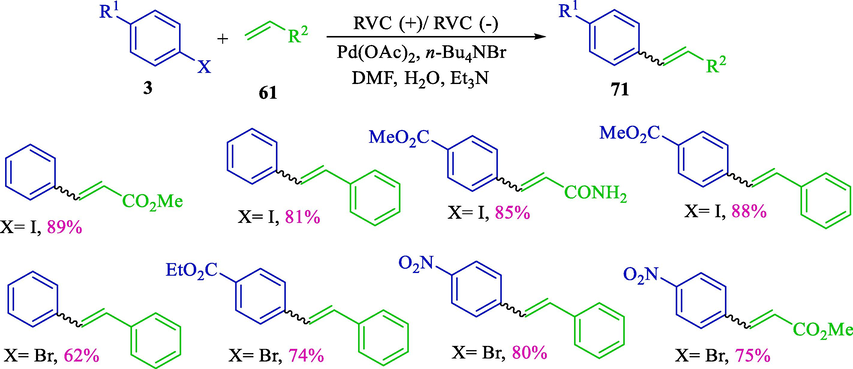
Coupling bromo/iodobenzene to different olefin series.
2.4 Carboxylation and carbonylation of organic halides
Electrochemical fixation of CO2 to organic chemicals is a beneficial procedure for synthesizing different carboxylic acids under neutral and moderate status. These electrochemical carboxylations occur efficiently even in the atmospheric pressure of CO2; meanwhile, an active metal, including Al or Mg, is utilized as a sacrificial anode in the electrolysis (Wang et al., 2022; Zhao et al., 2023). CO2 is a plentiful, cheap, non-toxic, and renewable carbon source; the kinetic and thermodynamic stability of the molecule limits its utilization in the chemical industry for the transformation to helpful chemicals (Costentin et al., 2012; Benson et al., 2009; Kumar et al., 2012).
In 2001, Troupel et al. reported the fabrication of unsymmetrical carbonyls 73 from aryl iodide 3 and benzylic chlorides 72 employing iron pentacarbonyl like the origin of CO (carbon monoxide) (Scheme 45) (Dolhem et al., 2001). The electrochemical reaction was done in an undivided cell supplied by a sacrificial stainless steel rod (anode) encircled by a cylindrical nickel grid (cathode). Applying carbon monoxide gas in mild status, this procedure provided symmetrical ketones derived with good yields (76–88 %).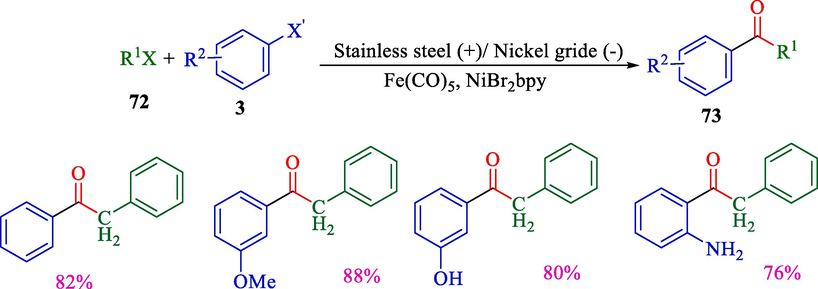
Electrosynthesis of various ketones from different aryl halides.
Reddy et al. declared electrochemical carboxylation of benzyl chlorides 74 catalyzed using Pd(II) complex for synthesizing 2-aryl propionic acids (Scheme 46) (Damodar et al., 2001). This reaction was performed in a cell with an Mg (anode) and Pt (cathode) under atmospheric pressure of carbon dioxide at constant current, producing 2-aryl propionic acids 75 in good yields (74–84 %). Organometallic catalysts did not play any chemical role in the stage of carboxylation but aided in activating the C—X bond, allowing the two-electron reduction of organic halide. Mechanistic research illustrated the reduced Palladium species' cooperative function for carbon dioxide activation.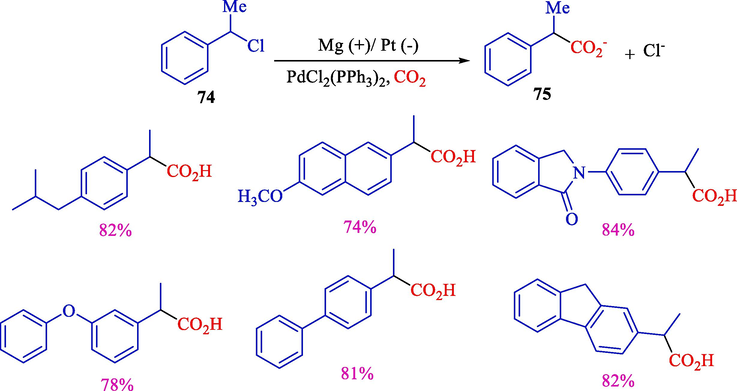
Electrochemical preparation of 2-aryl propionic acids.
In 2002, Gennaro and Isse explained that electrocarboxylation of benzyl chlorides was performed with no use to homogeneous catalysts (Isse and Gennaro, 2002). This work's most significant advantage is that the silver cathode process occurred at a more optimistic potential than other published catalysts. This method gave carboxylic acids excellent yields. Silver has displayed satisfactory catalytic attributes in the electroreduction of various organic halides.
One year later, Raju and colleagues described the preparation of 6-amino nicotinic acid 78 from 2-amino-5-chloropyridine 76 using H2SO4/CO2 in a cathode surface (Scheme 47) (Raju et al., 2003). Electrochemical carboxylation was executed at an undivided cell by platinum (cathode) and magnesium (anode).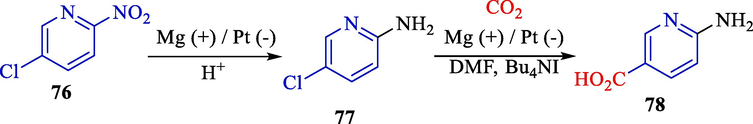
The carboxylation of 2-amino-5-chloropyridine by electrochemical reaction.
This new procedure involved the combination of electrochemical carboxylation and hydrogenation for the synthesis of 6-amino nicotinic acid. Scheme 48 offers a proposed mechanistic rationalization for reductive carboxylation. The two-electron reduction of 2-amino-5-chloropyridine generates an intermediate carbanion, which attacks CO2 to generate CO2−. The catch of the anion by metal ions afforded by the dissolution of the anodic metal provided a metal carboxylate. Lastly, treating the metal carboxylate with acid furnished the desired product (60–84 %).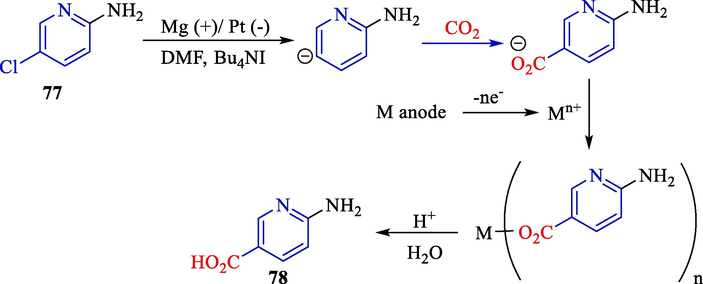
Proposed mechanism of reductive-carboxylation.
In 2004, Gennaro et al. demonstrated a novel method for preparing 6-amino nicotinic acid with the electrocatalytic reduction of 2-amino-5-bromopyridine at silver cathodes (Gennaro et al., 2004). They discovered that the procedure required just the stoichiometric charge at Ag cathodes. They gave good yields of 6-amino nicotinic acid, while in DMF/CH3CN, reduction of the halide compounds in the typically employed electrodes, including Pt, Hg, and GC, required negative potentials, including Pt, Hg, and GC, required very negative possibilities, which were only slightly more positive than that of carbon dioxide.
In 2005, Isse et al. surveyed the electrochemical reduction of some aryl ethyl chlorides by silver and glassy carbon electrodes in dimethylformamide /acetonitrile (Isse et al., 2005). In these two solvents, silver showed a significant catalytic influence. Better results were obtained at lower temperatures. The acid product yields were 70–81 % and 61–73 % at 273.15 K and 298.15, respectively.
In 2007, Aishah et al. explained the electrosynthesis of benzoic acid 80 from chlorobenzene 79 by CO2 fixation procedure (Scheme 49) (Aishah et al., 2007). The electrolysis was conducted at a one-compartment cell provided by palladium (cathode) and aluminium (anode) under the optimum status of electrocarboxylation, which showed a 72 % yield of C6H5CO2H from C6H5Cl. In addition, these statuses were employed in 1,2- and 1,3- dichlorobenzene to transform them into their desired benzoic acids.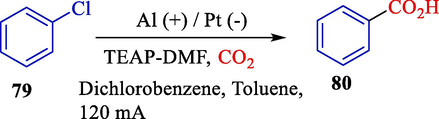
Electrosynthesis of C6H5CO2H from C6H5Cl.
At the same time, Yamauchi et al. indicated the electrochemical carboxylation of α,α-difluorotoluene derivatives 81, and 84 by the electrochemical reduction using carbon dioxide in an undivided cell with a magnesium rod (anode) and platinum plate (cathode). (Scheme 50) (Yamauchi et al., 2008). They illustrated that this procedure is highly effective for synthesizing α-fluorinated nonsteroidal and 2-fluoro-2-arylpropanoic acids 82, 83, and 85. In an efficient carbon dioxide fixation, the related α-fluorophenylacetic acids were achieved in high yields (40–74 %).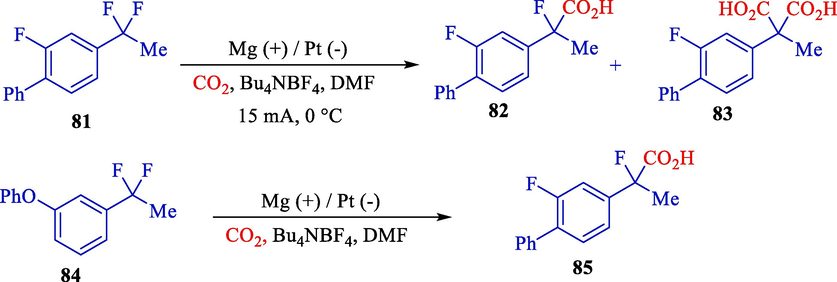
The carboxylation of α,α-difluorotoluene compound by electrochemical method.
In 2008, Niu et al. demonstrated an efficient and easy electrocarboxylation reaction of aliphatic halides 86 (Scheme 51) (Niu et al., 2008). They showed that diverse status, including the supporting electrolyte, temperature, and the nature of the electrode, could affect C6H5CO2H yield. Under the standard reaction status, medium to high yields (22–89 %) were achieved to form corresponding carboxylic acids 87.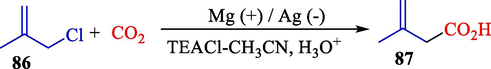
Electrochemical carboxylation of 3-chloro-2-methylpropene.
For the first time, the electrocarboxylation of benzyl chloride was surveyed at the silver cathode in CO2-saturated ambient temperature ionic liquid (BMIMBF4) solution by Jiaxing et al. in 2009 (Niu et al., 2009). The electrochemical treatment was investigated at various electrodes by cyclic voltammetry, which exhibited the remarkable electrocatalytic impact of the silver electrode in the reduction of benzyl chloride. The most elevated yield of phenylacetic acid (45 %) was acquired under optimization status. The ionic liquid was recovered after four cycles of minimal efficiency loss.
In 2010, Yamauchi et al. illustrated the electrochemical reduction of 1-bromo-2,2,2-trifluoroethyl benzene (88) to synthesize 2-aryl-3,3,3-trifluoropropanoic acid (89) using CO2 in an undivided cell fitted by a zinc plate (anode) and a Pt plate (cathode) (Scheme 52) (Yamauchi et al., 2010). The desired products with good yields (23–70 %). Also, this current reaction was effectively employed to synthesize β,β,β-trifluorinated non-steroidal anti-inflammatory medicines (NSAIDs).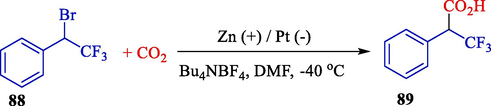
Electrochemical carboxylation of 1-bromo-2,2,2-trifluoroethyl benzene.
In 2010, Hiejima et al. studied the electrochemical carboxylation of α-chloroethyl benzene 90 with CO2 in ionic liquids compressed (Scheme 53) (Hiejima et al., 2010). The current efficiency increased with pressure and temperature. The influence of carbon dioxide pressure on the present efficiency of the carboxylation reaction was surveyed. The sacrificial magnesium is satisfactorily utilized in TFSA-DEME compressed with carbon dioxide, allowing it to handle the auxiliary oxidation in the counter electrode and enhance the existing carboxylation efficiency (32–60 %).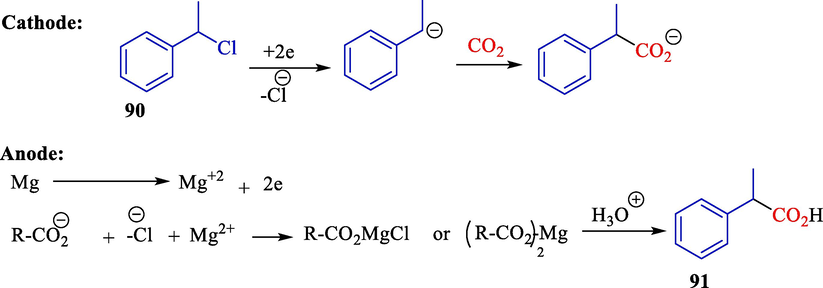
Electrochemical carboxylation of α-chloroethylbenzene.
A novel electrochemical method for the electrocatalytic carboxylation of 2-amino-5-bromopyridine and CO2 in BMIMBF4 (ionic liquid) to 6-amino nicotinic acid was surveyed in 2010 by Feng et al (Feng et al., 2010). The reactions were completed in three electrodes undivided cells without catalysts or toxic and volatile solvents under mild status. Cyclic voltammetry shows that BMIMBF4 could serve as a medium for 2-amino-5-bromopyridine reduction and the effect of working potential, temperature, passed charge, and concentrations. Under optimized conditions, 6-amino nicotinic acid can be obtained in 75 % yield with excellent selectivity. In addition, the ionic liquid was recycled.
In 2010, Yuan et al. indicated that the electrochemical pathway with nickel cathode was efficient for the electrocarboxylation of polycyclic aryl hydrocarbons 92 with carbon dioxide without additional catalysts under mild status (Scheme 54) (Yuan et al., 2010). Nickel cathode catalyzed in reducing polycyclic aryl hydrocarbons or carbon dioxide. The work provided an efficient and convenient method for synthesizing dicarboxylic acids from CO2 and polycyclic aryl hydrocarbons. The formation of trans-dicarboxylic acids during electrolyzes is due to electrostatic repel and the steric hindrance effect between two CO2 groups in the products. The related trans-dicarboxylic acids 93 were found in 62–90 % yields.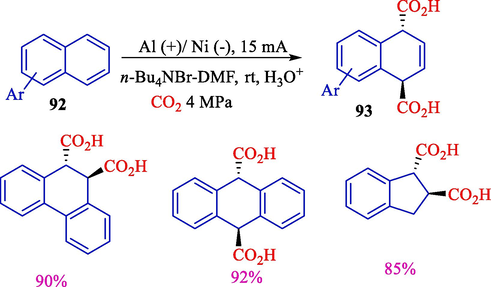
Electrochemical dicarboxylation of naphthalene with CO2.
An efficient and straightforward electrocatalytic carboxylation of arylic bromides 3 was extended by Lu et al. in 2011. Magnesium as an anode and silver as a cathode were used (Scheme 55) (Zhang et al., 2011). The influences of fundamental factors (including temperature, the current density, and the nature of the cathode material) on this reaction were examined. The desired carboxylic acids 94 were achieved in 30–78 % yields. The electrochemical treatment was checked in various electrodes (Ni, Ti, Cu, and Ag) by cyclic voltammetry, which exhibited the notable electrocatalytic effect of the silver electrode in the reductive carboxylation of aryl bromides.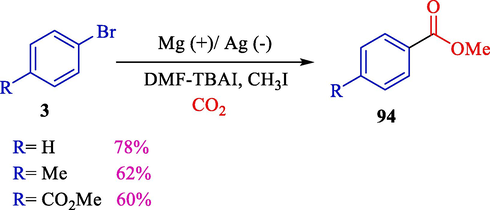
Electrocatalytic carboxylation of arylic bromides.
The same group presented the electrochemical reduction of di-bromobenzenes 95 using carbon dioxide (Scheme 56) (Lan et al., 2012). Diverse selectivity of individual isomers determined by cyclo voltammetry. Several cathode materials were surveyed, including titanium, copper, silver, and nickel. The most satisfactory results were achieved with silver as a cathode material owing to the less harmful reduction potential. By using o-di-bromobenzene, a transformation of just 49 % could be observed.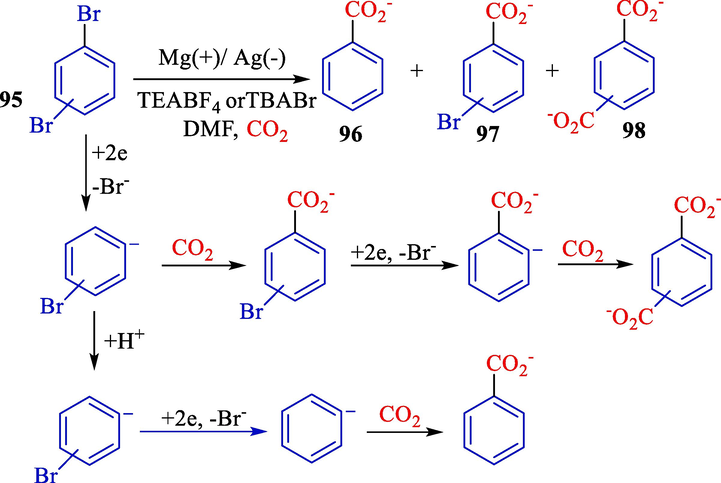
The electrochemical carboxylation of di-bromobenzenes compounds.
Conversely, an average conversion of 71 and 68 % was found in p- and m- di-bromobenzene, respectively. These conversions were not selective. O-di-bromobenzene was mainly monocarboxylate, which could be explained by a significantly more significant reduction potential of both reduction events in 1.61 V.
In 2013, Senboku et al. surveyed the electrochemical carboxylation of hexafluorobenzene 99 (Scheme 57) (Senboku et al., 2013). The electrolysis was stereo- and regio- selective, and polyfluorobenzoic acids 100 were attained in medium to superb yields (44–84 %).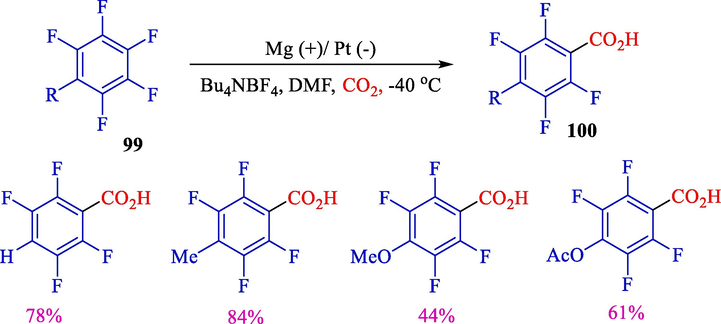
Electrochemical carboxylation of polyfluroarenes.
In 2014, Chen et al. reported the asymmetric electrocarboxylation of 1-phenylethylchloride 90 with carbon oxide using a chiral [CoI(salen)]-complex catalyst (Scheme 58) (Chen et al., 2014). This process was accomplished in an undivided glass cell by GC (cathode) and magnesium (anode). Although the yield (13–66 %) and the enantiomeric excess values are moderate, this elegant study rendered an alternative method for synthesizing optically active carboxylic acids 101.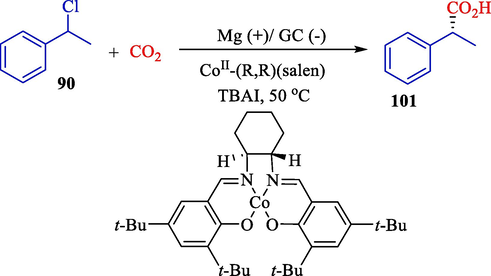
The asymmetric carboxylation of achiral 1-phenylethyl chloride.
The electrochemical virtues of ionic liquid and CO2 systems were surveyed as new reaction media by Tateno et al. in 2015 (Scheme 59) (Tateno et al., 2015). The Electrochemical reaction in ionic liquid and supercritical CO2 to diverse organohalide compounds 90 illustrated appropriate yields (22–59 %). The obtained consequences exhibit that this system is a valuable medium for electrochemical carboxylation reactions. The chemical fixation of carbon dioxide as a raw material is usually challenging owing to its high stability. Therefore, Grignard and organolithium reagents generally react directly with carbon dioxide (Cai and Xie, 2013). The drawbacks of these approaches are the problem of handling and using costly and toxic reagents and the production of enormous amounts of reagent waste. The electrochemical fixation of carbon dioxide to organic compounds is an attractive alternative to traditional chemical procedures (Senboku, 2021).![Electrochemical carboxylation of diverse organohalide compounds using [DEME][TFSI].](/content/184/2024/17/8/img/10.1016_j.arabjc.2024.105822-fig61.png)
Electrochemical carboxylation of diverse organohalide compounds using [DEME][TFSI].
3 Electrochemical Hetero-Coupling of organic halides
3.1 C—N bond formation reaction
Carbon-nitrogen cross-coupling is one of the most widespread and valuable transforms in organic synthesis fields. C—N cross-coupling reactions are vital for providing N-containing compounds with numerous applications in synthetic, biological, pharmaceutical, and materials science (Kafi-Ahmadi et al., 2021; Parsa Habashi and Poursattar Marjani, 2022; Payamifar et al., 2024a; Payamifar and Poursattar Marjani, 2023a, 2023b, 2024b, Poursattar Marjani et al., 2017, 2019b, 2019a; Schlummer and Scholz, 2004).
In 2017, Baran et al. introduced an electrochemical procedure to attain the cross-coupling between aliphatic amines 103 and aryl halides 3 and 102 (Scheme 60) (Li et al., 2017). The range of the electrolytic protocol comprises aryl chlorides, bromides, iodides, and triflates. Additionally, amides and alcohols could be used as nucleophiles. Electrochemical reactions were performed in an undivided cell by RVC (anode) and nickel (cathode) without an external base. Under milder conditions, various aryl donors (Ar—OTf, Ar—I, Ar—Br, and Ar—Cl) and secondary and primary amines with functional group tolerance are viable electrophiles in this reaction. This work displayed using an inexpensive nickel catalyst to enable carbon–nitrogen bond formation and exhibited a rare instance of anodic and cathodic processes adapted to synergistic produce reactive catalyst species in various oxidation states; therefore, a sacrificial electrode was not included. There are many previous reports on the formation of aryl carbon–nitrogen bonds between aryl halides and amines (Lavoie et al., 2016; Park et al., 2014; Ge et al., 2014; Fine Nathel et al., 2014; Manolikakes et al., 2008). The harsh reaction conditions prevented them from being extended. In this electrochemical method, cross-coupling between aryl halides and alkyl amines was performed without an external base at room temperature.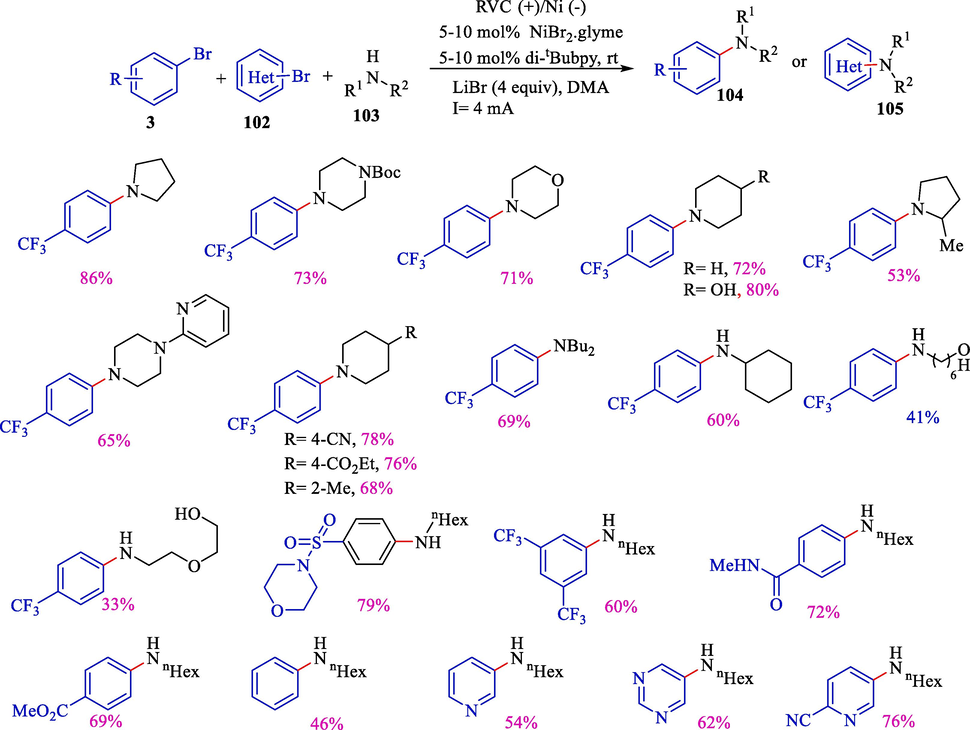
Electrochemical amination of aryl halides by nickel-catalyzed.
3.2 C—S bond formation reaction
The C—S bond generation reactions are used as powerful means in synthetic organic chemistry for the synthesis of different pharmaceutical, biological, and molecule materials (Feng et al., 2016; Boyd, 2016; Evano et al., 2013).
Recently, Wang et al. revealed electrochemical thiolation of aryl iodides 106 for carbon–sulfur bond formation by nickel-catalyzed (Scheme 61) (Wang et al., 2019). The reaction was attained in an undivided cell by graphene/nickel foam electrodes, which were chosen to enhance the charge exchange. The electrochemical reaction was performed under moderate electrochemical status and provided alkyl and aryl sulfides 108 in superb yields (54–99 %). In an undivided cell unit, radical-mediated nickel species of various oxidation states, the cathodic and anodic processes synergistically harness. Manuel reported that the palladium-catalyzed coupling of aryl halides with thiols was used using the chemical method (Fernández-Rodríguez et al., 2006). This reaction was performed in DME as the solvent and DME as the base at 110 °C. This reaction needs a high mole percent palladium catalyst at high temperatures to afford sulfide derivatives.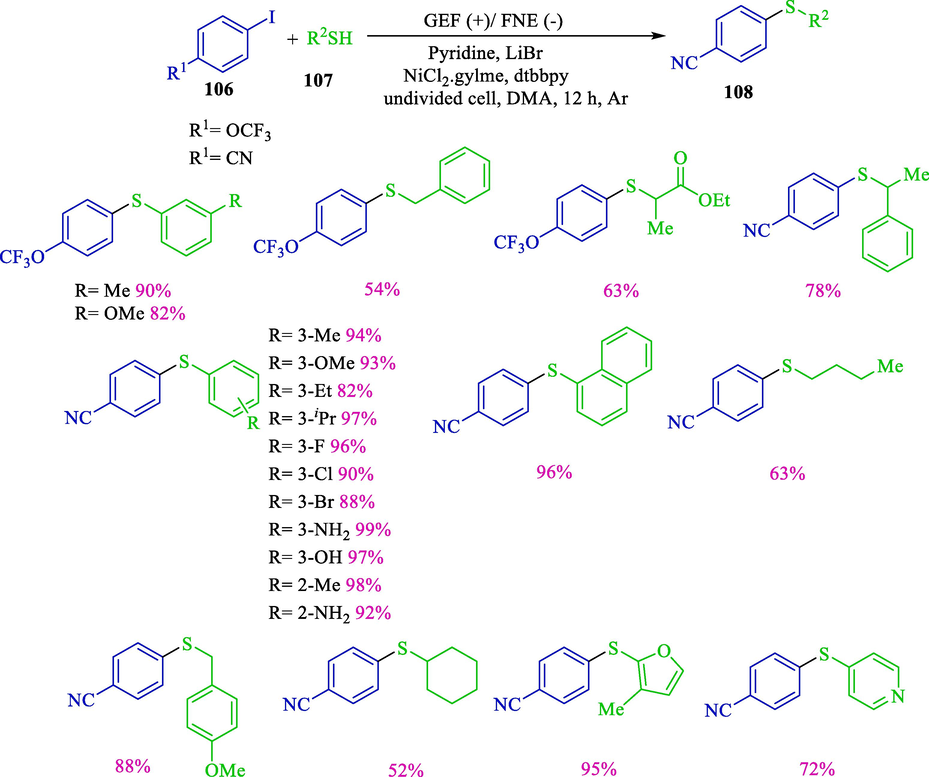
Electrochemical cross-coupling thiols and aryl halides.
The proposal mechanism (Scheme 62) showed that the anodic oxidation of thiol 107 caused cation radical F. Hydrogen abstraction from cation radical by pyridine generated the thiol radical G with aryl sulfide H. Meanwhile, Ni(0)—X B was produced from a cathodic reduction of NiCl2·dtbbpy A. Oxidative addition B with aryl halide 106 generated X—Ni(II)—Ar species C, which trapped the thiol radical G to deliver a Ni(III)—complex D. Lastly, reductive elimination of D furnished the coupling product 108 and Ni(I)—X complex E observed with the cathodic reduction to regenerated Ni(0)—X B.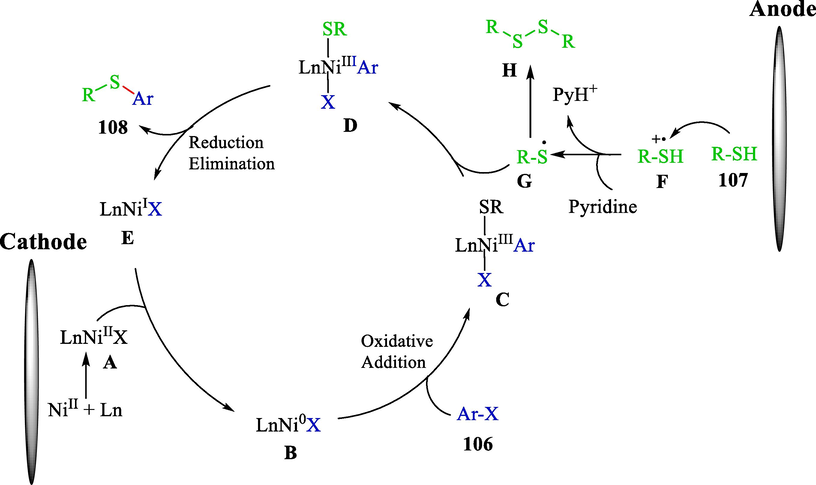
The proposed mechanism reaction.
The cyclic voltammetry studies were performed, as shown in Fig. 3. According to this data, aryl iodide and nickel (II) catalysts exhibited relatively high oxidative potentials (2.17 and 1.03 V vs SCE, respectively). Thiol indicated multiple irreversible oxidative waves from 0.88 V vs SCE. By adding pyridine to thiol, only one oxidative wave showed at 1.04 V, demonstrating that pyridine could stabilize the oxidation process of thiols. These anodic events substantiated that the initiation of the catalytic cycle was more likely to be the oxidation of thiol to its radical R—S radical.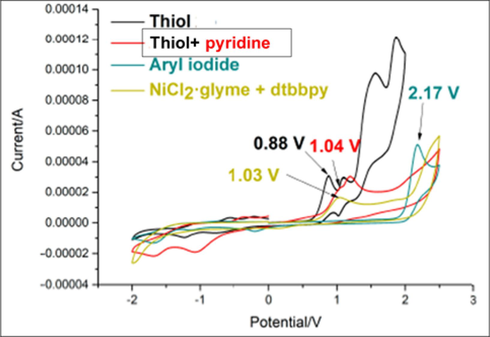
The cyclic voltammetry study Wang et al., 2019.
3.3 C—P bond formation reaction
Organophosphorus is a beneficial reagent in catalysis, organic synthesis, coordination chemistry, and medicinal chemistry (Montchamp, 2014; Tang and Zhang, 2003). An appropriate procedure for their preparation commonly comprises carbon-phosphorus coupling between nucleophilic phosphorus-having compounds and aryl halides (Tappe et al., 2010; Demmer et al., 2011). The electrochemical synthesis of aryl/hetero phosphonates from di-methyl phosphite and aryl/hetero halides for the first time was published by the group's Léonel in 2018 (Scheme 63) (Sengmany et al., 2018). These reactions were accomplished in the galvanostatic mode in an undivided cell with an iron/nickel rod anode enclosed by a Ni foam cathode. NiBr2bpy was applied as the comfortably available pre-catalyst, TBAB (Supporting electrolyte), and trimethylamine as a base. Both aryl iodides and bromides could be utilized 109, providing the related hetero/aryl phosphonates 111 with good yields. Electron-rich and electron-deficient aryl bromides coupling with di-methyl phosphite 110 produce products in excellent yields (40–86 %). The other di-alkyl phosphites were used in this process. In the chemical method, Yamaguchi reported the synthesis of arylphosphinates by zinc-mediated nickel-catalyzed coupling of aryl halides and H-phosphinate (Kinbara et al., 2015). This reaction needs long reaction times at high temperatures to afford phosphorus derivatives. The electrochemical method has some advantages, such as a short-time reaction and high yield at room temperature.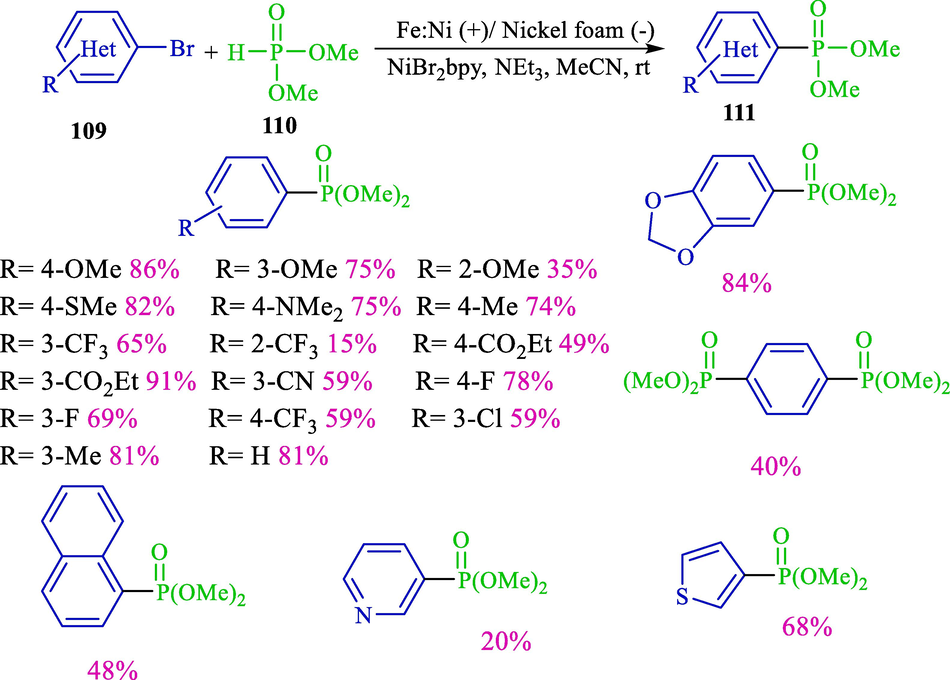
Electrochemical cross-coupling of aryl halides and di-methyl phosphite.
The admissible mechanism showed that an initial cathodic reduction of the nickel(II) pre-catalyst led to the Ni(0) species, pursued by oxidative addition of the halide and reductive electron transfer to produce Ni(I) species (Scheme 64). Di-methyl phosphite was then added to create a Ni-phosphorus ylide. Proton abstraction on the hydroxyl of by Et3N led to the critical complex, which would be oxidized to generate Ar—Ni(II)P(O)(OMe)2. Reductive elimination afforded the target compound 111 and the catalyst of the process Ni(0).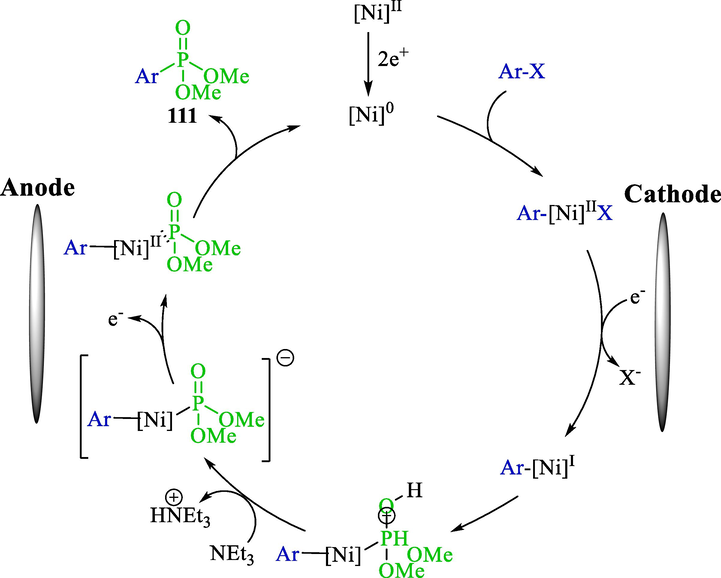
The suggested mechanism of electrochemical carbon-phosphorus bond fabrication.
3.4 C—B bond fabrication reactions
The electrochemical methodology suggests an appealing alternative to the classical preparation of organoboronic derivatives. In unique boronic acids and esters, boronic derivatives constitute highly valuable intermediates for their broad applications in the agrochemical and pharmaceutical areas and polymer and material chemistry (Silva et al., 2020). However, the chief interest of esters and boronic acids is their usage as intermediates in coupling reactions. Coupling reactions permit the creation of new carbon–carbon bonds to form novel and complex molecules exhibiting particular features.
In 2002, Dunach et al. reported electrochemical coupling of aromatic or heteroaromatic halides 3 and a trialkyl borate including B(OiPr)3 or B(OMe)3 112 for the obtaining of aryl/hetero boronic acids 113 (Scheme 65) (Laza et al., 2002). The selectivities of aryl boronic acids were in the scope of 32–73 %. The reactions were employed in a single-compartment cell with a magnesium or aluminum (anode) and stainless steel or nickel foam (cathode).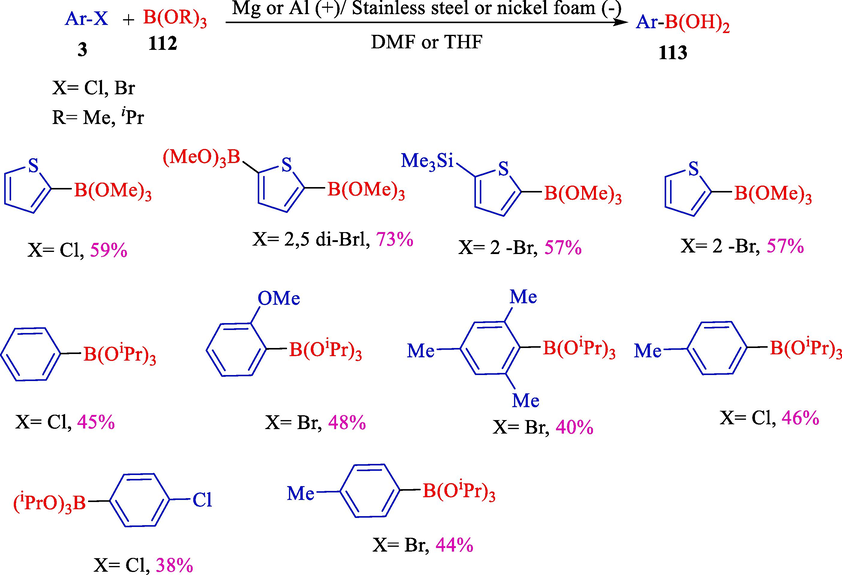
Electrosynthesis of aryl boronic acids with B(Oipr)3 or B(OMe)3.
One year later, the same group represented a novel electrosynthesis of aryl boronic esters 115 from pinacolborane 114 and aryl halides 3 (Scheme 66) (Laza and Dunach, 2003). They used pinacolborane (HBpin) as a suitable substance for the functionalization of aryl halide. In this case, aryl boronic esters yielded up to 95 %. The electrochemical reaction was conducted in a single-compartment cell fitted with a nickel foam (cathode) and a consumable magnesium (anode). The nature of the electrodes ultimately affected the results. A magnesium anode was shown to be more efficient than aluminium or zinc. This electrochemical method exhibited several advantages compared to the classical boration of aryl halides. The boration occurred in one-pot without the requirement to prepare the Grignard reagent or its Li equivalent.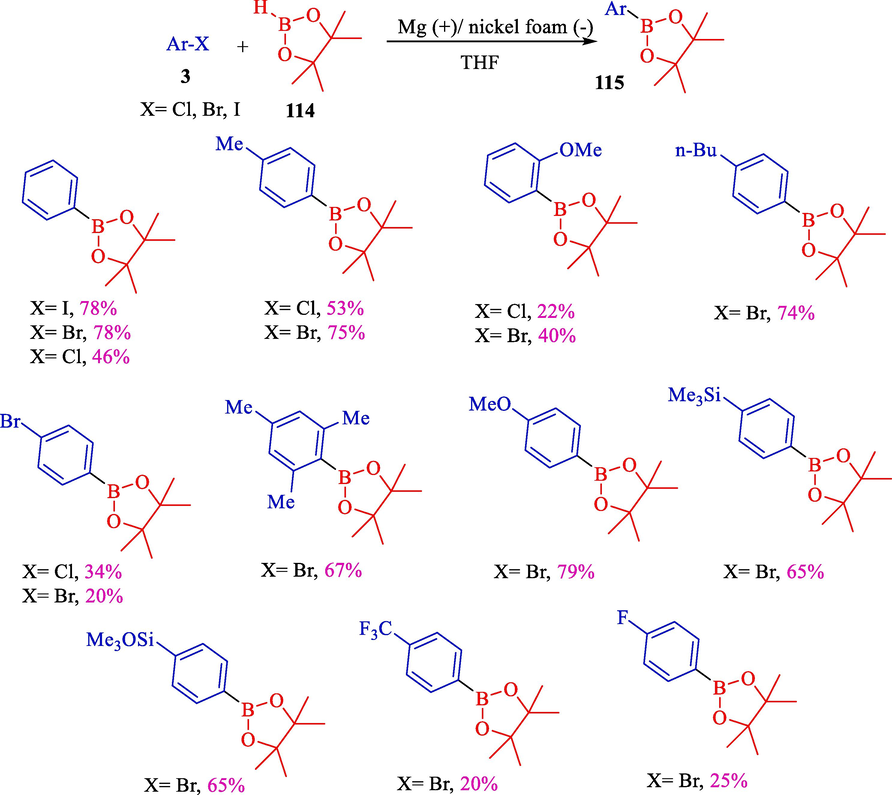
Electrosynthesis of aryl boronic pinacol esters from pinacol borane and aryl halides.
In 2004, the group Dunach demonstrated the electrochemical procedure, including the straightforward coupling of pinacolborane 114 and benzylic halides 116 for synthesizing benzyl boronic pinacol esters 117 (Scheme 67) (Pintaric et al., 2004). Under mild status, the reaction was conducted in a single-compartment cell with a magnesium (anode) and a nickel (cathode). Benzyl boronic pinacol esters were attained in 61–82 % yields.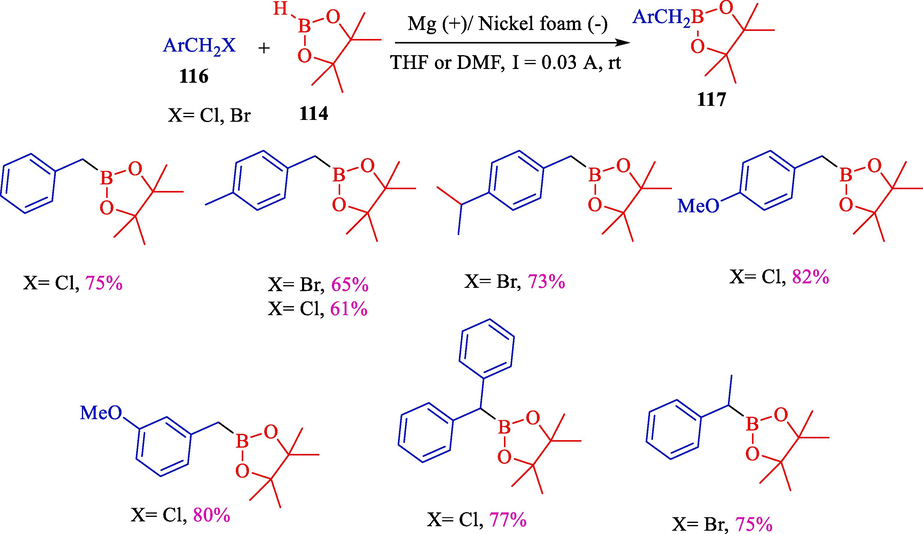
Electrosynthesis of benzylboronic pinacol esters derivatives.
The electrochemical reduction of aryl halide compounds 118 using pinacolborane (119) as Dunach's group had reported the electrophile to obtain aryl boronic acids selectively that still bear halogen substituents for further functionalization (Scheme 68) (Laza et al., 2005). Reactions were acted in a single cell compartment using a magnesium (anode) and a nickel foam (cathode) in tetrahydrofuran. The relative nature and place of the halogen substituents affect the boration outcomes. Some halogenated aryl boronic esters 120–122 achieved satisfactory yields at unfinished transformations.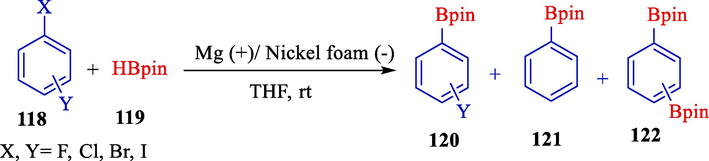
Electrosynthesis of aryl boronic esters.
By control of the electrolysis conditions, p-F, p-Cl, p-Br, and m-Br phenyl pinacol boronic esters were obtained in 45, 75, 67, and 60 % yields, respectively. Also, m-phenyl-di-boronic ester yields 57 % after 4F/mol electrolysis of m-di-bromobenzene.
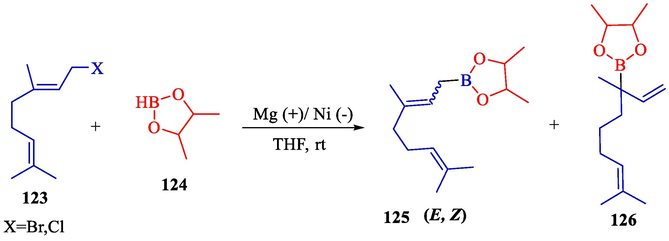
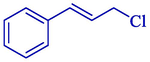
Electrosynthesis of allyl boronic pinacol esters from the electrochemical boration of pinacolborane 124 with various allyl halides 123 illustrated by the group of Dunach in 2009 (Table 2) (Godeau et al., 2009). The electrosynthesis was done in a single-compartment cell with a nickel foam (cathode) and a consumable Al (anode) in a tetrahydrofuran solution comprising (CF3SO2)2NLi. The allyl boronic 125 and 126 derivatives were acquired in 60–86 % yields, generally as mixtures of linear and branched isomers, with the linear isomers usually being the significant compounds. The process is simple and does not require organometallic catalysis, and the reaction takes one step. The terminal borated substance having the less hindered achieved preferential with 68–91 % selectivities. The E-derivative was the principal or particular stereoisomer.
Entry
Substrate
Products ratio
E/Z ratio of 125
Boration yield
1
90:10
70/30
81 %
2
91:9
74/26
86 %
3
84:16
96/4
70 %
4
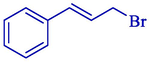
7:90
–
7 %
5
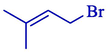
79:21
–
76 %
6
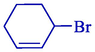
–
65 %
7

83:17
100/-
74 %
8

68:32
100/-
64
In 2023, for the first time, Wang et al. declared electrochemical borylation of alkyl boronic esters to unactivated alkyl halides 127 at high current (Scheme 69) (Wang et al., 2021). The tertiary, secondary, primary, and alkyl boronic esters provide good to superb yields 129 (70 examples). Mechanistic investigations revealed that B2cat2 128 acts as both a cathodic mediator and a reagent, which could electro reduction at a low potential of about alkyl bromides or chlorides difficult-to-reduce. Recently, transition-metal catalyzed borylation of alkyl halides has developed as a valuable strategy for synthesizing alkylboron compounds. Regardless, these methods depend mainly on transition-metal catalysts, long reaction times, and stoichiometric quantities of activators (Yang et al., 2012; Yi et al., 2012; Wang et al., 2021; Ito and Kubota, 2012). The Electrochemical approach is milder, safer, simpler, and environmentally efficient for synthesizing alkyl boron compounds. The cyclic voltammetry (CV) experiments are shown in Fig. 4. The mechanism for the reaction can be explained using cyclic voltammetry data.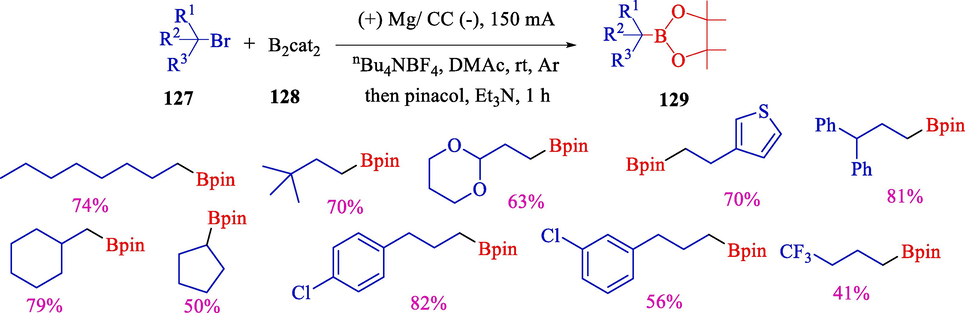
Electrochemical borylation of alkyl halides for synthesizing alkyl boronic esters.
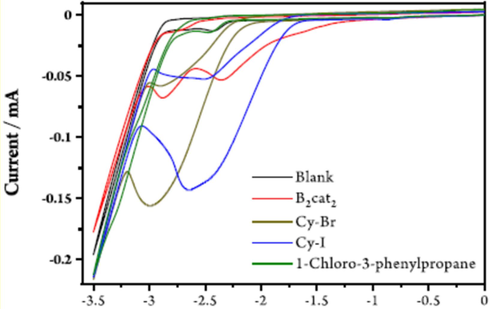
The cyclic voltammetry data Wang et al., 2021.
3.5 C—Si bond formation reaction
In 2001, Moreau et al. illustrated the electrochemical synthesis of functional aryl/hetero arylchlorosilanes 132 (Scheme 70) (Moreau et al., 2001). An efficient and selective procedure for synthesizing functionalized di- and mono-chlorosilanes as an essential intermediate for polarized organosilicons that are fascinating for NLO applications was described. This reaction was done in an undivided cell with an aluminium or magnesium (anode), a concentric stainless steel grid, or carbon (cathode). Due to its versatility and selectivity, the electrosynthesis pathway is especially beneficial for constructing new organosilicon models. Electrochemical reduction of aryl halides, halopyridines, halofurans, and halothiophenes 130 for synthesizing various functional aryl/hetero arylchlorosilanes (43–88 %).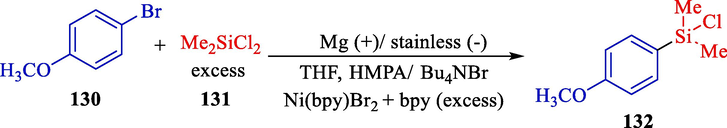
Ni-catalyzed electrochemical synthesis of p-methoxyphenyldimethylchlorosilane.
4 Electrochemical cyclizations of organic halides
Employing the electron as a reagent usually provides inexpensive and actively suitable reactions in organic electrochemistry. In the many synthetic strategies, radical cyclizations include a primary process for forming diverse cyclic and natural products comprising heterocyclic rings (Giese, 1986; Giese et al., 1996; Ezzati et al., 2017; Poursattar Marjani et al., 2018; Khalafy et al., 2014; Poursattar Marjani et al., 2019). Radical cyclization methods show benefits over other techniques, which may need the laborious multi-step alternative synthesis.
The electrochemical nickel catalyst was offered for the impressive fabrication of benzolactones using carbon dioxide for disubstituted epoxides 133 by Dunach and Tascedda in 2000 (Scheme 71) (Tascedda and Duñach, 2000). This reaction suggested that the first reductive carboxylation was pursued with the oxirane ring-opening and cyclization. For terminal epoxides, a cyclic carbonate was established from the straight placing CO2 into the epoxide. Six-membered ring isocoumarines 134 and 135 (48–88 %) were achieved using 2,2′-bipyridine, whereas five-membered ring benzolactones were selectively formed with cyclam as the ligand.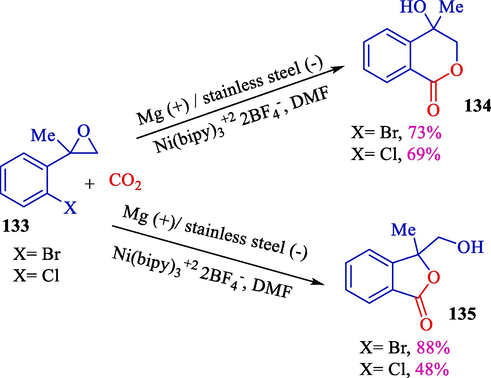
Electrosynthesis of cyclic carbonates and benzolactones from epoxide-aryl halides.
The synthesis of benzothiophenes 137 through the cyclization of o-halo allyl/aryl thioethers 136 had been described using nickel (catalyst) by Dunach et al. in 2004 (Scheme 72) (Pelletier et al., 2004). This electrochemical process had an effortless setup and equipped a carbon fiber cathode and an Mg anode. Dihydrobenzo[b]thiophenes were obtained in superb yields (59–95 %). The procedure was highly reliant on the type of ligand related to the metal center. Ni(II) complexes associated with tetra aza macrocyclic ligands, including cyclam, could catalyze intramolecular cyclization efficiently.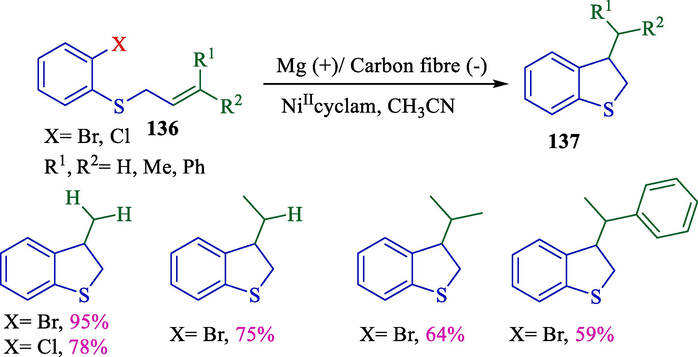
The cyclization of o-halo allyl/aryl thioethers using nickel(II)-catalyst.
Senboku et al. (2011) offered facile preparation of 2,3-dihydrobenzofuran-3-ylacetic acids 139 (33–85 %). Corresponding analogs were attained using a new electrochemical aryl radical cyclization–carboxylation sequence of 2-allyloxy-2-bromobenzenes 138 by applying methyl 4-tert-butylbenzoate (electron-transfer mediator) (Scheme 73) (Senboku et al., 2011). The outcomes indicated that the current became a vital instrument for synthesizing (2,3-dihydrobenzofuran-3-yl)acetic acids and desired analogs utilizing carbon dioxide with no metal substance, including SmI2 and Bu3SnH.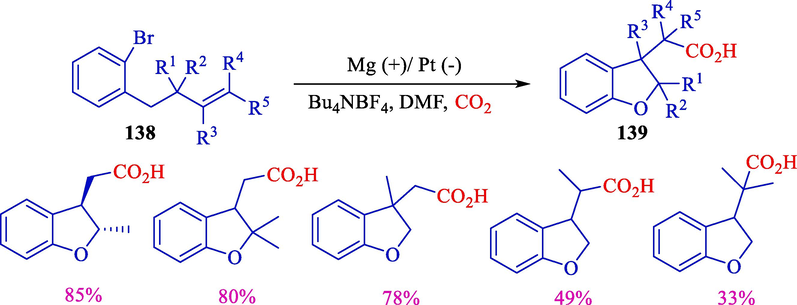
Formation of (2,3-dihydrobenzohuran-3-yl)acetic acids using ERCC.
Scheme 74 offers a suggested mechanistic rationalization using ERCC. A one-electron reduction of aryl halide selectively occurred by a radical anion of the mediator to produce aryl radical. Without a mediator, two-electron reduction of aryl halide competitively occurred to create the related aryl anion furnishing immediately carboxylated benzoic acid. Dissolving the Mg (anode) as magnesium ion prevents any species from oxidizing at the anode.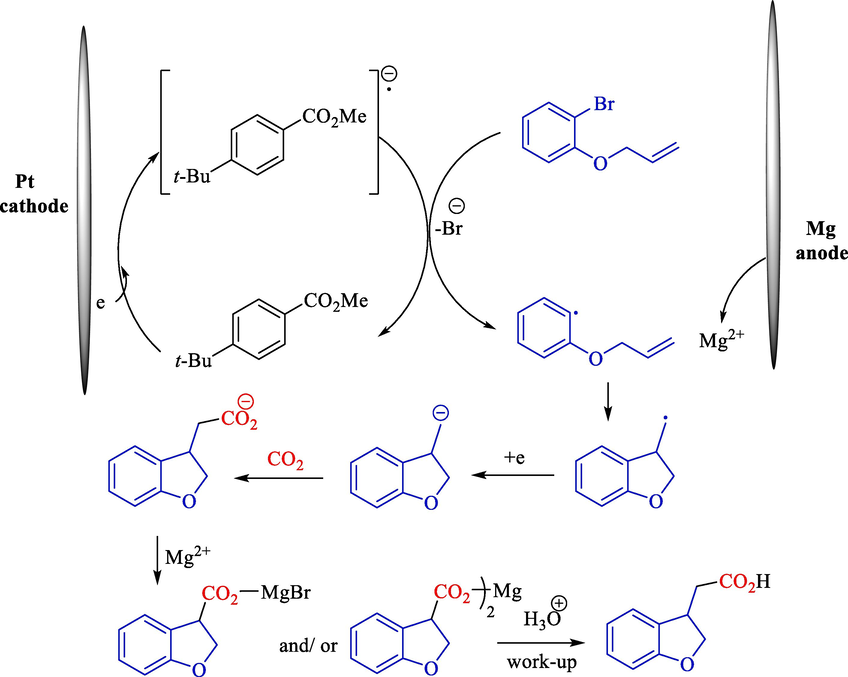
The stepwise reaction of methyl 4-tert-butylbenzoate.
In 2016, Senboku et al. designed a sequential carboxylation aryl radical cyclization with alkyne using electrolysis of 2-(2-propargyloxy)bromobenzenes (Scheme 75) (Katayama et al., 2016). The electrochemical reaction was done in an undivided cell with a platinum (cathode) and magnesium (anode) using CO2 and methyl 4-tert-butyl benzoate as an electron transfer mediator. Aryl radical cyclization, dihydro benzofuran, indoline, indane, and dihydro benzothiophene, also tetrahydropyran framework 140, could be created effectively, and following tandem carboxylation produced the desired derivatives of 2,2-ring-fused succinic acid 141 in excellent yields (38–71 %).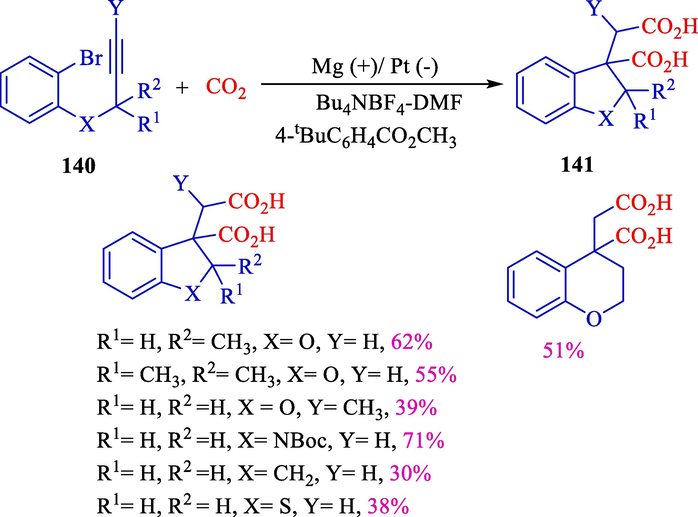
Electrochemical aryl radical cyclization with alkyne.
Mechanism studies revealed that one-electron reduction of methyl p-(tert-butyl)benzoate at the cathode utilized as an electron transfer mediator produced the respective radical anion (Scheme 76). One-electron reduction of aryl bromide 140 using the methyl p-(tert-butyl)benzoate radical anion (II) produced the anion radical (I), resulting in the break of the C—Br bond, yielding the aryl radical A1. Without electron transfer mediator (II), two-electron reduction of aryl bromide 140 at the cathode forms the desired aryl anion species that straight provides carboxylated benzoic acid. The construction of cyclized vinyl radical B1 results from the intramolecular 5-exo cyclization of a C—C triple bond with aryl radical A1. Moreover, one-electron reduction formed the vinyl anion C1, CO2 reacted, creating the α,β-unsaturated carboxylate ion D, which had a cinnamic acid part.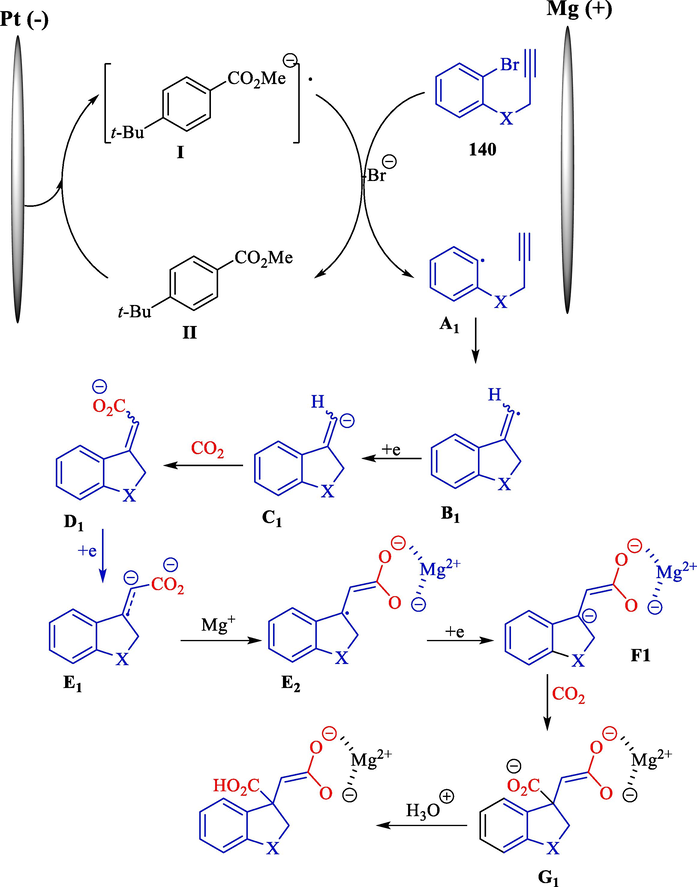
The acceptable mechanism of the radical cyclization.
Consequently, reducing one more electron of α,β-unsaturated carboxylate ion D1 may rapidly produce the radical anion E1. Resonance of the radical anion E2 to stable enol form, followed by the reduction of one-electron, had the anion F1. Selective insertion of CO2 at the benzylic position gave intermediate G1 and created an acid environment, further obtaining the desired product.
In the same year, Senboku and Katayama offered an electrochemical reduction of vinyl bromide 142 using carbon dioxide that produced vinyl radical 143, 144, and 145 utilizing methyl p-(tert-butyl)benzoate as an electron transfer mediator (Scheme 77) (Katayama and Senboku, 2016). Cyclization accompanied by carbon dioxide insertion with a carbon–carbon bond creation produced γ,δ-unsaturated carbo- and heterocycle carboxylic acids in excellent outcomes (39–71 %). Electrochemical reactions were conducted in an undivided cell using a Mg rod (anode) and a platinum plate (cathode). Cyclic voltammetry was used to determine the role of methyl 4-tert-butylbenzoate, as displayed in Fig. 1. A proposal mechanism exhibited that at the cathode, one-electron reduction of methyl p-(tert-butyl)benzoate, utilized as an electron transfer intermediate, took place to afford the related radical anion (Scheme 78).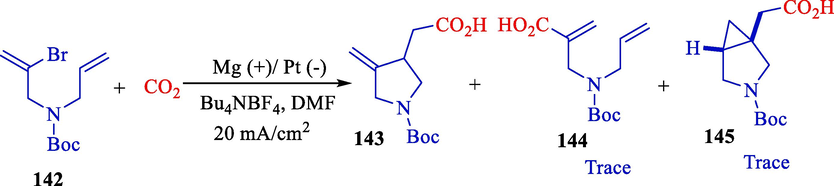
Electrochemical reduction of vinyl radical cyclization-fixation of CO2.
Plausible reaction mechanism.
5 Electrochemical preparation of organo-metal compounds from organic halides
Organozinc materials have earned a remarkably substantial role in advancing novel approaches in the organic synthesis field (Knochel and Singer, 1993).
In 2000, Gosmini et al. explained the synthesis of aryl zinc compounds 146 from the electroreduction of aryl bromides/chlorides 3 using a cobalt catalyst (Scheme 79) (Gosmini et al., 2000c). An electrochemical cell was equipped with a consumable nickel or stainless steel (cathode) and zinc (anode) using cobalt chloride with pyridine as a ligand. Formation of aryl zinc halides achieved excellent yields, and this procedure is especially effective on aryl halides containing an electron-withdrawing group.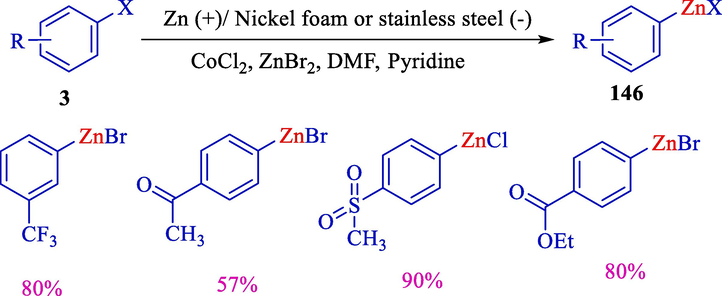
Preparation of aryl zinc compounds from aryl halides using CoCl2.
One year later, this group demonstrated electrosynthesis of diverse aryl organozinc derivatives 147 and their coupling with pyridine using the cobalt catalyzed (Scheme 80) (Le Gall et al., 2001b). Normal protocols, including halogenated compounds and nickel catalysts, were ineffective. This procedure's significant privilege relies on using mild and straightforward reaction status. A stainless steel grid (cathode) and a zinc rod (anode) were used. 4-aryl-pyridines 150 were attained in high yields (54–85 %).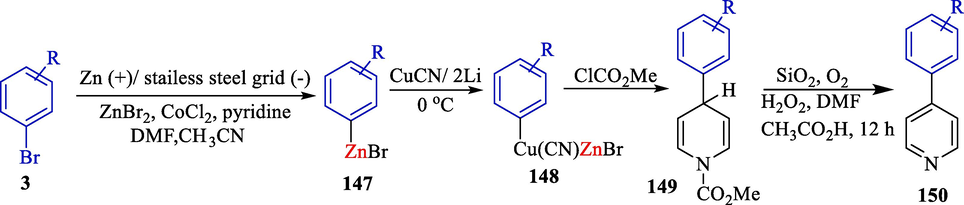
Electrosynthesis of organozinc reagents for functionalized 4-phenyl-pyridines.
The establishment of 3-substituted monothienylzinc species was attained using electrochemical procedures and a selective and original bromination method by Mellah et al., as reported in 2001 (Scheme 81) (Mellah et al., 2001). Electrolysis is arranged into an undivided cell by a sacrificial Zn (anode) utilizing NiBr2bpy (amount catalyst) for the construction of monothienylzinc 152 and 153 archived in moderate to excellent yields (20–100 %).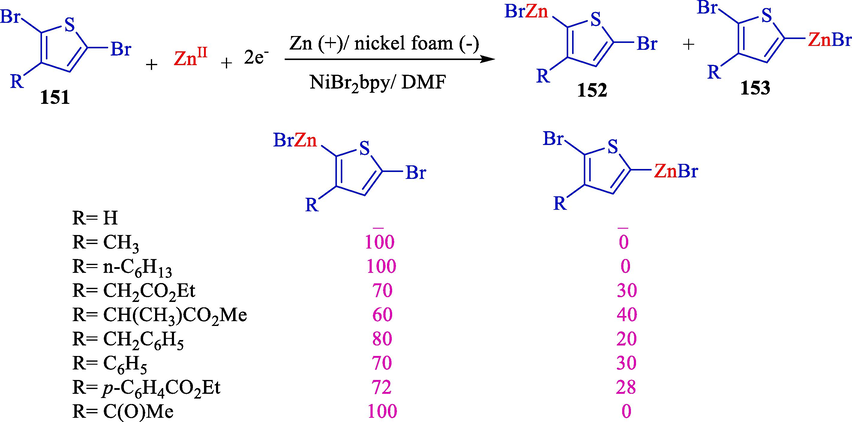
The electrochemical synthesis of thienyl Zn species.
The electrosynthesis of diaryl zinc species using dibromo benzenes or activated dichloro benzene by the group of Gosmini was explained in the same year (Fillon et al., 2001). at a one-compartment cell by a nickel foam (cathode) and a consumable Zn (anode). Diorganozinc compounds were synthesized using cobalt catalysis with good to superior. The di- and mono- Zn products were provided concurrently at 2F/mol, excluding bromochloro benzene, where just the mono-Zn species is noticed. In 4F/mol, the di-Zn species is exclusively provided from di-bromobenzenes. On the contrary, di-chlorobenzene reagents combine di- and mono- Zn materials. In o-dibromobenzene, the yield is decreased.
6 Conclusion
The synthesis of organic compounds requires various methods, and lately, scientists have developed new synthetic methods adaptable to green chemistry aims. One of the most promising ways is electrosynthesis, which has attained notable attraction in the academic community and industrial field. Electrosynthesis is a valuable and practical technique in organic transformation. The application of this powerful method in organic conversion is fascinating and impressive. Photochemistry and electrochemistry are two powerful tools in organic synthesis, and merging both is a highly advantageous approach to fulfilling modern organic synthesis's green and sustainable needs. Also, it can develop a system in which each technique’s defects can be wholly compensated for by their respective benefits, thus creating probable novel reaction paths that are unachievable with individual procedures. Indeed, more developments and applications of this merging will persist and be used in the future in the organic synthesis field (Wu et al., 2022). Organic photoelectrochemistry is a growing field with notable attention due to its potential for renewable energy transformation and storage uses.
Further, reactions in photoelectrochemistry and flow chemistry are two hopeful electrochemistry strategies, and their combination offers excellent potential for use in solar energy and energy conversion technologies. These combination systems can perform more eco-friendly and energy-savingly with high atom and step economies (Murtaza et al., 2023). Photoelectrochemical catalysis can present more efficient, better, cheaper, environmentally safer, and greener replacement reaction methodologies in organic transformations (Hardwick and Ahmed, 2021; Hardwick et al., 2020).
In the current work, we have offered recent expansions for electrochemical coupling reactions of organohalides to create carbon–carbon and carbon-heteroatom bonds. Electrochemistry has some natural benefits involving reaction tenability, recyclable and cheap electrodes, and scalability. Although electro-organic synthesis has been well-known for over 150 years, notable growth has been made in recent decades. Using the electrochemical approach in drug discovery and investigation of physiological activity is considerable for many chemists. The utilization of electrochemical methodologies in organic synthesis is earning notable consideration because transformations can frequently be accomplished more efficiently, and electrochemical methods can reduce contamination and make the chemistry environmentally friendly; hence, more research groups are focusing on these topics. Electrochemical cross-coupling reactions provide a new opportunity for synthesizing green organic carbon–carbon and carbon-heteroatom bonds. Our aim in this study is to exhibit an overall outlook of electrochemical coupling reaction aryl halides as an old tool in the organic reaction. We hope our review will attract enthusiasts and pioneers in the coupling field toward clean, readily available, and inexpensive electrochemical methods. Hence, more attention must be given to organic electrochemistry, and this review will, without a doubt, encourage chemists to develop more future studies in the electrochemistry field and make it a common approach in the modern organic chemistry world shortly.
CRediT authorship contribution statement
Sara Payamifar: Investigation, Writing – original draft. Leila Behrouzi: Investigation, Writing – original draft. Ahmad Poursattar Marjani: Investigation, Supervision, Writing – review & editing.
Acknowledgments
The authors would like to acknowledge the support from the Research Council of Urmia University.
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
References
- Carbon dioxide fixation method for electrosynthesis of benzoic acid from chlorobenzene. J. Nat. Gas Chem.. 2007;16:273-277.
- [CrossRef] [Google Scholar]
- Working at the interfaces of data science and synthetic electrochemistry. Tetrahedron Chem.. 2022;1:100012
- [CrossRef] [Google Scholar]
- Alvarez-Builla, J., Vaquero, J.J., Barluenga, J., 2011. Heterocyclic compounds: an introduction, modern heterocyclic chemistry. 1–9. https://onlinelibrary.wiley.com/doi/book/10.1002/9783527637737#page=20.
- Barhdadi, R., Courtinard, C., Nédélec, J.Y., Troupel, M., 2003. Room-temperature ionic liquids as new solvents for organic electrosynthesis. The first examples of direct or nickel-catalysed electroreductive coupling involving organic halides. ChemComm. 1434–1435. doi: 10.1039/B302944A.
- Electrocatalytic and homogeneous approaches to conversion of CO2 to liquid fuels. Chem. Soc. Rev.. 2009;38:89-99.
- [CrossRef] [Google Scholar]
- Recent advances in transition-metal-free carbon-carbon and carbon-heteroatom bond-forming reactions using arynes. Chem. Soc. Rev.. 2012;41:3140-3152.
- [CrossRef] [Google Scholar]
- Mechanism and selectivity in nickel-catalyzed cross-electrophile coupling of aryl halides with alkyl halides. J. Am. Chem. Soc.. 2013;135:16192-16197.
- [CrossRef] [Google Scholar]
- Expanding the medicinal chemistry synthetic toolbox. Nat. Rev. Drug Discov.. 2018;17:709-727.
- [CrossRef] [Google Scholar]
- Sulfur and its role in modern materials science. Angew. Chem. Int. Ed.. 2016;55:15486-15502.
- [CrossRef] [Google Scholar]
- The directed synthesis of biaryl compounds: modern concepts and strategies. Angew. Chem. Int. Ed. Engl.. 1990;29:977-991.
- [CrossRef] [Google Scholar]
- Direct carboxylative reactions for the transformation of carbon dioxide into carboxylic acids and derivatives. Synthesis. 2013;45:3305-3324.
- [CrossRef] [Google Scholar]
- Nickel-catalyzed electrochemical couplings of vinyl halides: synthetic and stereochemical aspects. J. Org. Chem.. 2000;65:4575-4583.
- [Google Scholar]
- Two convenient and high-yielding preparations of 6,6′-dimethyl-2,2′-bipyridine by homocoupling of 6-bromopicoline. Tetrahedron Lett.. 2000;41:8203-8206.
- [CrossRef] [Google Scholar]
- Asymmetric electrocarboxylation of 1-phenylethyl chloride catalyzed by electrogenerated chiral [CoI (salen)] complex. Electrochem. Commun.. 2014;42:55-59.
- [CrossRef] [Google Scholar]
- Nickel catalyzed electrochemical heteroarylation of activated olefins. Synthesis. 2002;2002:1752-1758.
- [CrossRef] [Google Scholar]
- Nickel-catalyzed arylation of acrolein diethyl acetal: a substitute to the 1,4-addition of aryl halides to acrolein. Org. Lett.. 2003;5:4701-4703.
- [CrossRef] [Google Scholar]
- A local proton source enhances CO2 electroreduction to CO by a molecular Fe catalyst. Science. 2012;338:90-94.
- [CrossRef] [Google Scholar]
- Synthesis of 2-arylpropionic acids by electrocarboxylation of benzylchlorides catalyzed by PdCl2 (PPh3)2. Electrochem. Commun.. 2001;3:762-766.
- [CrossRef] [Google Scholar]
- Electro-chemical research, on the decomposition of the earths; with observations on the metals obtained from the alkaline earths, and on the amalgam procured from ammonia. Philos. Trans. R. Soc. Lond. 1808:333-370.
- [Google Scholar]
- Electrochemical homocoupling of 2-bromomethylpyridines catalyzed by nickel complexes. J. Org. Chem.. 2002;67:1838-1842.
- [CrossRef] [Google Scholar]
- Mixed effect of the supporting electrolyte and the zinc anode in the electrochemical homocoupling of 2-bromopyridines catalyzed by nickel complexes in an undivided cell. J. Org. Chem.. 2005;70:10778-10781.
- [CrossRef] [Google Scholar]
- Synthesis of 6-,7-, and 8- membered lactones via the nickel-catalyzed electrochemical arylation of electron-deficient olefins. Tetrahedron Lett.. 2002;43:6343-6345.
- [CrossRef] [Google Scholar]
- Review on modern advances of chemical methods for the introduction of a phosphonic acid group. Chem. Soc. Rev.. 2011;111:7981-8006.
- [CrossRef] [Google Scholar]
- Nickel catalysed electrosynthesis of ketones from organic halides and iron pentacarbonyl. Part 2: unsymmetrical ketones. Tetrahedron. 2001;57:525-529.
- [CrossRef] [Google Scholar]
- An electrochemical coupling of organic halide with aldehydes, catalytic in chromium and nickel salts. The Nozaki-Hiyama-Kishi reaction. Org. Lett.. 2001;3:2073-2076.
- [CrossRef] [Google Scholar]
- Impact of copper-catalyzed cross-coupling reactions in natural product synthesis: the emergence of new retrosynthetic paradigms. Nat. Prod. Rep.. 2013;30:1467-1489.
- [CrossRef] [Google Scholar]
- The catalyst-free syntheses of pyrazolo[3,4-b]quinolin-5-one and pyrazolo[4′,3′:5,6]pyrido[2,3-d] pyrimidin-5,7-dione derivatives by one-pot, three-component reactions. Tetrahedron. 2017;73:6587-6596.
- [CrossRef] [Google Scholar]
- Ann. Phys. Leipzig.. 1834;47:438.
- Electrocatalytic carboxylation of 2-amino-5-bromopyridine with CO2 in ionic liquid 1-butyl-3-methyllimidazoliumtetrafluoborate to 6-aminonicotinic acid. Electrochim. Acta. 2010;55:5741-5745.
- [CrossRef] [Google Scholar]
- Sulfur-containing scaffolds in drugs: synthesis and application in medicinal chemistry. Curr. Top. Med. Chem.. 2016;16:1200-1216.
- [Google Scholar]
- A general and long-lived catalyst for the palladium-catalyzed coupling of aryl halides with thiols. J. Am. Chem. Soc.. 2006;128:2180-2181.
- [CrossRef] [Google Scholar]
- Electrosynthesis of functionalized organodizinc compounds from aromatic dihalides via a cobalt catalysis in acetonitrile/pyridine as a solvent. Tetrahedron Lett.. 2001;42:3843-3846.
- [CrossRef] [Google Scholar]
- Nickel-catalyzed amination of aryl chlorides and sulfamates in 2-methyl-THF. ACS Catal.. 2014;4:3289-3293.
- [CrossRef] [Google Scholar]
- Redox catalysis in organic electrosynthesis: basic principles and recent developments. Chem. Soc. Rev.. 2014;43:2492-2521.
- [CrossRef] [Google Scholar]
- Controlling first-row catalysts: amination of aryl and heteroaryl chlorides and bromides with primary aliphatic amines catalyzed by a BINAP-ligated single-component Ni (0) complex. J. Am. Chem. Soc.. 2014;136:1617-1627.
- [CrossRef] [Google Scholar]
- Electrocatalytic synthesis of 6-aminonicotinic acid at silver cathodes under mild conditions. Electrochem. Commun.. 2004;6:627-631.
- [CrossRef] [Google Scholar]
- Radicals in Organic Synthesis: Formation of Carbon-Carbon Bonds. Oxford: Pergamon Press; 1986.
- Radical Cyclization Reactions. Organic Reactions. John Wiley & Sons; 1996. p. :301-856.
- Electrochemical preparation of pinacol allyl boronic esters. Electrochim. Acta. 2009;54:5116-5119.
- [CrossRef] [Google Scholar]
- Cobalt bromide as a catalyst in electrochemical addition of aryl halides onto activated olefins. Tetrahedron Lett.. 2000;41:3385-3388.
- [CrossRef] [Google Scholar]
- Synthesis of unsymmetrical biaryls by electroreductive cobalt-catalyzed cross-coupling of aryl halides. Tetrahedron. 2002;58:8417-8424.
- [CrossRef] [Google Scholar]
- Electrochemical vinylation of aryl and vinyl halides with acrylate esters catalyzed by cobalt bromide. Tetrahedron Lett.. 2002;43:5901-5903.
- [CrossRef] [Google Scholar]
- Cobalt-catalyzed direct electrochemical cross-coupling between aryl or heteroaryl halides and allylic acetates or carbonates. J. Org. Chem.. 2003;68:1142-1145.
- [CrossRef] [Google Scholar]
- Cobalt-catalyzed electrochemical vinylation of aryl halides using vinylic acetates. Tetrahedron. 2003;59:2999-3002.
- [CrossRef] [Google Scholar]
- Electrochemical cross-coupling between functionalized aryl halides and 2-chloropyrimidine or 2-chloropyrazine catalyzed by nickel 2,2′-bipyridine complex. Tetrahedron Lett.. 2000;41:201-203.
- [CrossRef] [Google Scholar]
- Electrosynthesis of functionalized 2-arylpyridines from functionalized aryl and pyridine halides catalyzed by nickel bromide 2,2′-bipyridine complex. Tetrahedron Lett.. 2000;41:5039-5042.
- [CrossRef] [Google Scholar]
- New efficient preparation of arylzinc compounds from aryl halides using cobalt catalysis and sacrificial anode process. J. Org. Chem.. 2000;65:6024-6026.
- [CrossRef] [Google Scholar]
- An electrochemical strategy to synthesize disilanes and oligosilanes from chlorosilanes. Angew. Chem. Int. Ed. Engl.. 2023;135:e202303592
- [CrossRef] [Google Scholar]
- C-H functionalization via electrophotocatalysis and photoelectrochemistry: complementary synthetic approach. ACS Sustain. Chem. Eng.. 2021;9:4324-4340.
- [CrossRef] [Google Scholar]
- Interfacial photoelectrochemical catalysis: solar-Induced green synthesis of organic molecules. ChemSusChem. 2020;13:1967-1973.
- [CrossRef] [Google Scholar]
- Carbon-heteroatom bond formation catalysed by organometallic complexes. Nature. 2008;455:314-322.
- [CrossRef] [Google Scholar]
- Synthesis of symmetrical bisquinolones via nickel(0)-catalyzed homocoupling of 4-chloroquinolones. Adv. Synth. Catal.. 2007;349:2353-2360.
- [CrossRef] [Google Scholar]
- Migratory reductive acylation between alkyl halides or alkenes and alkyl carboxylic acids by nickel catalysis. ACS Catal.. 2019;9:3253-3259.
- [CrossRef] [Google Scholar]
- Chem. List.. 1922;16:256
- Electrolysis with a Dropping Mercury Cathode. Part I. Deposition of Alkali and Alkaline Earth Metals. The London, Edinburgh, and Dublin; 1923.
- Studies in electrode polarisation, Part IV-The automatic control of the potential of a working electrode. Trans. Faraday Soc. 1942;38:27-33.
- [CrossRef] [Google Scholar]
- Electrochemical carboxylation of α-chloroethylbenzene in ionic liquids compressed with carbon dioxide. PCCP. 2010;12:1953-1957.
- [CrossRef] [Google Scholar]
- Synthetic organic electrochemistry: an enabling and innately sustainable method. ACS Cent. Sci.. 2016;2:302-308.
- [CrossRef] [Google Scholar]
- Recent progress in cathodic reduction-enabled organic electrosynthesis: trends, challenges, and opportunities. eScience. 2022;2:243-277.
- [CrossRef] [Google Scholar]
- Electrochemical nickel-catalyzed C(sp3)-C(sp3) cross-coupling of alkyl halides with alkyl tosylates. J. Am. Chem. Soc.. 2023;145:17023-17028.
- [CrossRef] [Google Scholar]
- Electrocatalytic carboxylation of benzyl chlorides at silver cathodes in acetonitrile. ChemComm. 2002:2798-2799.
- [CrossRef] [Google Scholar]
- Electrocatalytic reduction of arylethyl chlorides at silver cathodes in the presence of carbon dioxide: synthesis of 2-arylpropanoic acids. J. Electroanal. Chem.. 2005;581:38-45.
- [CrossRef] [Google Scholar]
- Copper(I)-catalyzed boryl substitution of unactivated alkyl halides. Org. Lett.. 2012;14:890-893.
- [CrossRef] [Google Scholar]
- Nickel-catalyzed electrochemical reductive relay cross-coupling of alkyl halides with alkyl carboxylic acids. Org. Chem. Front.. 2021;8:6603-6608.
- [CrossRef] [Google Scholar]
- Ultrasonic assisted preparation of Co3O4 and Eu-doped Co3O4 nanocatalysts and their application for solvent-free synthesis of 2-amino-4H-benzochromenes under microwave irradiation. Appl. Organomet. Chem.. 2021;2021(35):e6271
- [CrossRef] [Google Scholar]
- Sequential vinyl radical cyclization/fixation of carbon dioxide through electrochemical reduction of vinyl bromide in the presence of an electron-transfer mediator. ChemElectroChem. 2016;3:2052-2057.
- [CrossRef] [Google Scholar]
- Aryl radical cyclization with alkyne followed by tandem carboxylation in methyl 4-tert-butylbenzoate-mediated electrochemical reduction of 2-(2-propynyloxy) bromobenzenes in the presence of carbon dioxide. Tetrahedron. 2016;72:4626-4636.
- [CrossRef] [Google Scholar]
- Design of carbon-carbon and carbon-heteroatom bond formation reactions under green conditions. Curr. Org. Chem.. 2019;23:3154-3190.
- [CrossRef] [Google Scholar]
- A convenient and mild synthesis of new 2-aryl-3-hydroxy-6,7-dihydro-1H-indol-4(5H)-ones via a one-pot, three-component reaction in water. Tetrahedron Lett.. 2014;55:3781-3783.
- [CrossRef] [Google Scholar]
- Single-stage synthetic route to perfluoroalkylated arenes via electrocatalytic cross-coupling of organic halides using Co and Ni complexes. J. Organomet. Chem.. 2016;820:82-88.
- [CrossRef] [Google Scholar]
- Nickel-catalyzed C-P cross-coupling reactions of aryl iodides with H-phosphinates. Tetrahedron. 2015;71:7614-7619.
- [CrossRef] [Google Scholar]
- Preparation and reactions of polyfunctional organozinc reagents in organic synthesis. Chem. Soc. Rev.. 1993;93:2117-2188.
- [CrossRef] [Google Scholar]
- Beobachtungen über die oxydirende Wirkung des Sauerstoffs, wenn derselbe mit Hülfe einer elektrischen Säule entwickelt wird. J. Prakt. Chem.. 1847;41:137-139.
- [CrossRef] [Google Scholar]
- Photochemical and photoelectrochemical reduction of CO2. Annu. Rev. Phys. Chem.. 2012;63:541-569.
- [CrossRef] [Google Scholar]
- Viologen as catalytic organic reductant: electro-reductive dimerization of aryl bromides in a Pd/viologen double mediatory system. Tetrahedron Lett.. 2013;54:3666-3668.
- [CrossRef] [Google Scholar]
- Palladium-catalyzed electrochemical allylic alkylation between alkyl and allylic halides in aqueous solution. Organic Lett.. 2017;19:2022-2025.
- [CrossRef] [Google Scholar]
- Electroreduction of dibromobenzenes on silver electrode in the presence of CO2. J. Electroanal. Chem.. 2012;664:33-38.
- [CrossRef] [Google Scholar]
- Challenging nickel-catalyzed amine arylations enabled by tailored ancillary ligand design. Nat. Commun.. 2016;7:11073
- [CrossRef] [Google Scholar]
- New electrosynthesis of arylboronic esters from aromatic halides and pinacolborane. Adv. Synth. Catal.. 2003;345:580-583.
- [CrossRef] [Google Scholar]
- Novel synthesis of arylboronic acids by electroreduction of aromatic halides in the presence of trialkyl borates. New J. Chem.. 2002;26:373-375.
- [CrossRef] [Google Scholar]
- Electrochemical reduction of polyhalogenated aryl derivatives in the presence of pinacolborane: electrosynthesis of functionalised arylboronic esters. Electrochim. Acta. 2005;50:4897-4901.
- [CrossRef] [Google Scholar]
- Cobalt-catalyzed electrochemical cross-coupling of functionalized phenyl halides with 4-chloroquinoline derivatives. Tetrahedron Lett.. 2001;42:267-269.
- [CrossRef] [Google Scholar]
- Synthesis of functionalized 4-phenyl-pyridines via electrochemically prepared organozinc reagents. Tetrahedron. 2001;57:1923-1927.
- [CrossRef] [Google Scholar]
- Ni catalyzed electrochemical decarboxylative C-C couplings in batch and continuous flow. Org. Lett.. 2018;20:1338-1341.
- [CrossRef] [Google Scholar]
- Electrochemically enabled, nickel-catalyzed amination. Angew. Chem. Int. Ed.. 2017;129:13268-13273.
- [CrossRef] [Google Scholar]
- Electrochemical NiH-catalyzed C(sp3)-C(sp3) coupling of alkyl halides and alkyl alkenes. Angew. Chem. Int. Ed.. 2023;62:e202311941
- [CrossRef] [Google Scholar]
- Recent advances in the electrochemical α-C-H bond functionalization of carbonyl compounds. Adv. Synth. Catal.. 2018;360:4266-4292.
- [CrossRef] [Google Scholar]
- Polarographically controlled syntheses, with particular reference to organic chemistry. J. Am. Chem. Soc.. 1943;65:1348-1353.
- [CrossRef] [Google Scholar]
- Electroreductive cross-electrophile coupling (eXEC) reactions. Angew. Chem. Int. Ed. Engl.. 2023;62:e202306679
- [CrossRef] [Google Scholar]
- Recent advances in iodine mediated electrochemical oxidative cross-coupling. Org. Biomol. Chem.. 2018;16:2375-2387.
- [CrossRef] [Google Scholar]
- An efficient silane-promoted nickel-catalyzed amination of aryl and heteroaryl chlorides. J. Org. Chem.. 2008;73:1429-1434.
- [CrossRef] [Google Scholar]
- New and efficient access to 3-substituted 2,5-dibromothiophenes. consecutive nickel-catalyzed electrochemical conversion to thienyl zinc species. New J. Chem.. 2001;25:318-321.
- [CrossRef] [Google Scholar]
- Electrifying synthesis: recent advances in the methods, materials, and techniques for organic electrosynthesis. Acc. Chem. Res.. 2020;53:545-546.
- [CrossRef] [Google Scholar]
- Modern electrochemical aspects for the synthesis of value-added organic products. Angew. Chem. Int. Ed. Engl.. 2018;57:6018-6041.
- [CrossRef] [Google Scholar]
- Phosphinate chemistry in the 21st century: a viable alternative to the use of phosphorus trichloride in organophosphorus synthesis. Acc. Chem. Res.. 2014;47:77-87.
- [CrossRef] [Google Scholar]
- Electrochemical synthesis of functional aryl-and heteroarylchlorosilanes. Application to the Preparation of donor- acceptor or donor-donor organosilicon molecules. Organometallics. 2001;20:1910-1917.
- [CrossRef] [Google Scholar]
- Arenes and heteroarenes C-H functionalization under enabling conditions: Electrochemistry, photoelectrochemistry and flow technology.
- A review of high throughput and combinatorial electrochemistry. Electrochim. Acta. 2011;56:9679-9699.
- [CrossRef] [Google Scholar]
- Electrocatalytic carboxylation of aliphatic halides at the silver cathode in acetonitrile. Tetrahedron. 2008;64:10517-10520.
- [CrossRef] [Google Scholar]
- Electrocatalytic carboxylation of benzyl chloride at silver cathode in ionic liquid BMIMBF4. Chin. J. Chem .. 2009;27:1041-1044.
- [CrossRef] [Google Scholar]
- Electrocatalysis as an enabling technology for organic synthesis. Chem. Soc. Rev.. 2021;50:7941-8002.
- [CrossRef] [Google Scholar]
- Electrochemical coupling of mono and dihalopyridines catalyzed by nickel complex in undivided cell. Tetrahedron. 2012;68:2383-2390.
- [CrossRef] [Google Scholar]
- A graphite powder cavity cell as an efficient tool of sustainable chemistry: electrocatalytic homocoupling of 2-halopyridines. Electrochim. Acta. 2015;173:465-475.
- [CrossRef] [Google Scholar]
- Development of an air-stable nickel precatalyst for the amination of aryl chlorides, sulfamates, mesylates, and triflates. Org. Lett.. 2014;16:220-223.
- [CrossRef] [Google Scholar]
- N-methylpyrrolidine as an effective organocatalyst for the regioselective synthesis of 3-hydroxy-3, 5/6-di-aryl-1H-imidazo[1,2-a] imidazol-2(3H)-ones. Res. Chem. Intermed.. 2022;48:2325-2336.
- [CrossRef] [Google Scholar]
- The recent development of β-cyclodextrin-based catalysts system in Suzuki coupling reactions. Appl. Organomet. Chem. 2024e7458
- [CrossRef] [Google Scholar]
- A new β-cyclodextrin-based nickel as green and water-soluble supramolecular catalysts for aqueous Suzuki reaction. Sci. Rep.. 2023;13:21279
- [CrossRef] [Google Scholar]
- Recent advances in β-cyclodextrin-based catalysts for reducing toxic nitroaromatic: an overview. Appl. Organomet. Chem.. 2023;37:e7287
- [CrossRef] [Google Scholar]
- The recent development of β-cyclodextrin-based catalysts system in click reactions: a review. Appl. Organomet. Chem.. 2024;e7365
- [CrossRef] [Google Scholar]
- Nickel-catalyzed electrochemical synthesis of dihydro-benzo[b]thiophene derivatives. Synth. Commun.. 2004;34:3343-3348.
- [CrossRef] [Google Scholar]
- Electrochemical nickel catalysis for sp2-sp3 cross-electrophile coupling reactions of unactivated alkyl halides. Org. Lett.. 2017;19:3755-3758.
- [CrossRef] [Google Scholar]
- Conjugate Addition Reactions in Organic Synthesis. Oxford: Pergamon Press; 1992.
- The application of cathodic reductions and anodic oxidations in the synthesis of complex molecules. Chem. Soc. Rev.. 2006;35:605-621.
- [CrossRef] [Google Scholar]
- Electrosynthesis of benzylboronic acids and esters. Tetrahedron Lett.. 2004;45:8031-8033.
- [CrossRef] [Google Scholar]
- Co3O4 nanoparticles prepared by oxidative precipitation method: an efficient and reusable heterogeneous catalyst for N-formylation of amines. Res. Chem. Intermed.. 2017;43:413-422.
- [CrossRef] [Google Scholar]
- An efficient synthesis of 4H-chromene derivatives by a one-pot, three-component reaction. Iran. J. Chem. Chem. Eng.. 2018;37:149-157.
- [CrossRef] [Google Scholar]
- Synthesis of ethyl 2-amino-4-benzoyl-5-oxo-5,6-dihydro-4H-pyrano[3,2-c] quinoline-3-carboxylates by a one-pot, three-component reaction in the presence of TPAB. J. Heterocycl. Chem.. 2019;56:268-274.
- [CrossRef] [Google Scholar]
- Synthesis of pyrazolopyridine and pyrazoloquinoline derivatives by one-pot, three-component reactions of arylglyoxals,3-methyl-1-aryl-1H-pyrazol-5-amines and cyclic 1,3-dicarbonyl compounds in the presence of tetrapropylammonium bromide. Green Process Synth.. 2019;8:533-541.
- [CrossRef] [Google Scholar]
- Cathodic aromatic C, C cross-coupling reaction via single electron transfer pathway. Molecules. 2017;22:413
- [CrossRef] [Google Scholar]
- Nickel-catalyzed electrochemical reductive homocouplings of aryl and heteroaryl halides: a useful route to symmetrical biaryls. Synthesis. 2018;50:146-154.
- [CrossRef] [Google Scholar]
- Electroorganic synthesis of 6-aminonicotinic acid from 2-amino-5-chloropyridine. Tetrahedron Lett.. 2003;44:4133-4135.
- [CrossRef] [Google Scholar]
- Scanning electro-chemical microscopy reveals cancer cell redox state. Electrochim. Acta. 2015;179:65-73.
- [CrossRef] [Google Scholar]
- High-throughput experimentation for electrochemistry, the power of high-throughput experimentation: general topics and enabling technologies for synthesis and catalysis. ACS Publ.. 2022;1:167-187.
- [CrossRef] [Google Scholar]
- A decade of electrochemical dehydrogenative C, C-coupling of aryls. Acc. Chem. Res.. 2019;53:45-61.
- [CrossRef] [Google Scholar]
- Electrosynthetic C-F bond cleavage. Org. Biomol. Chem.. 2022;20:6707-6720.
- [CrossRef] [Google Scholar]
- Recent advances in electrochemical synthesis of nitriles: a sustainable approach. ChemistrySelect. 2022;7:e202200081
- [CrossRef] [Google Scholar]
- the medicinal chemist’s toolbox: an analysis of reactions used in the pursuit of drug candidates. J. Med. Chem.. 2011;54:3451-3479.
- [CrossRef] [Google Scholar]
- Palladium-catalyzed C-N and C-O coupling a practical guide from an industrial vantage point. Adv. Synth. Catal.. 2004;346:1599-1626.
- [CrossRef] [Google Scholar]
- Nitroxyl-mediated electrooxidation of alcohols to aldehydes and ketones. J. Am. Chem. Soc.. 1983;105:4492-4494.
- [CrossRef] [Google Scholar]
- Synthesis with zerovalent nickel. coupling of aryl halides with bis(1,5-cyclooctadiene) nickel (0) J. Am. Chem. Soc.. 1971;93:5908-5910.
- [CrossRef] [Google Scholar]
- Electrochemical fixation of carbon dioxide: synthesis of carboxylic acids. Chem. Rec.. 2021;21:2354-2374.
- [CrossRef] [Google Scholar]
- Facile synthesis of 2,3-dihydrobenzofuran-3-ylacetic acids by novel electrochemical sequential aryl radical cyclization-carboxylation of 2-allyloxybromobenzenes using methyl 4-tert-butylbenzoate as an electron-transfer mediator. Synlett. 2011;2011:1567-1572.
- [CrossRef] [Google Scholar]
- Regioselective electrochemical carboxylation of polyfluoroarenes. Electrochemistry. 2013;81:380-382.
- [CrossRef] [Google Scholar]
- Preparation of functionalized aryl and heteroarylpyridazines by nickel-catalyzed electrochemical cross-coupling reactions. J. Org. Chem.. 2007;72:5631-5636.
- [CrossRef] [Google Scholar]
- An electrochemical synthesis of functionalized arylpyrimidines from 4-amino-6-chloropyrimidines and aryl halides. Molecules. 2011;16:5550-5560.
- [CrossRef] [Google Scholar]
- Beneficial effects of electrochemistry in cross-coupling reactions: electroreductive synthesis of 4-aryl-or 4-heteroaryl-6-pyrrolylpyrimidines. Eur. J. Org. Chem.. 2016;2016:4865-4871.
- [CrossRef] [Google Scholar]
- A mild electroassisted synthesis of (hetero) arylphosphonates. Org. Biomol. Chem.. 2018;16:4495-4500.
- [CrossRef] [Google Scholar]
- Anodic oxidation pathways of aromatic amines. Electrochemical and electron paramagnetic resonance studies. J. Am. Chem. Soc.. 1966;88:3498-3503.
- [CrossRef] [Google Scholar]
- Electrochemical oxidation induced selective C-C bond cleavage. Chem. Soc. Rev.. 2020;121:485-505.
- [CrossRef] [Google Scholar]
- Boronic acids and their derivatives in medicinal chemistry: synthesis and biological applications. Molecules. 2020;25:4323
- [CrossRef] [Google Scholar]
- Direct arylation of pyrroles via indirect electroreductive C-H functionalization using perylene bisimide as an electron-transfer mediator. Org. Lett.. 2016;18:544-547.
- [CrossRef] [Google Scholar]
- Protein electrochemistry: application in medicine. A review. Electrochim. Acta. 2014;140:72-82.
- [CrossRef] [Google Scholar]
- Advanced electrochemical detection of amino acids and proteins through flow injection analysis and catalytic oxidation on Prussian Blue. Electrochim. Acta. 2020;331:135289
- [CrossRef] [Google Scholar]
- Organic electrochemistry: basics and applications. Int. J. Biosen. Bioelectron.. 2017;3:270-271.
- [CrossRef] [Google Scholar]
- Chem. Ges.. 1907;40:3312
- Electro-reductive homo-coupling reaction of aryl bromides in PdCl2 (PPh3) 2/pyridinium salt double mediatory systems. Electrochemistry. 2013;81:356-358.
- [CrossRef] [Google Scholar]
- New chiral phosphorus ligands for enantioselective hydrogenation. Chem. Soc. Rev.. 2003;103:3029-3070.
- [CrossRef] [Google Scholar]
- Transition-metal-catalyzed CP cross-coupling reactions. Synthesis. 2010;2010:3037-3062.
- [CrossRef] [Google Scholar]
- Electrosynthesis of benzolactones by nickel-catalyzed carboxylation of epoxide-functionalized aromatic halides. Synlett. 2000;2000:245-247.
- [CrossRef] [Google Scholar]
- Electrochemical fixation of CO2 to organohalides in room temperature ionic liquids under supercritical CO2. Electrochim. Acta. 2015;161:212-218.
- [CrossRef] [Google Scholar]
- Electrochemically assisted Heck reactions. Org. Lett.. 2005;7:5381-5383.
- [CrossRef] [Google Scholar]
- Phil. Chem. Arts.. 1800;4:179
- Electrochemical arylation reaction. Chem. Soc. Rev.. 2018;118:6706-6765.
- [CrossRef] [Google Scholar]
- Catalytic boration of alkyl halides with borane without hydrodehalogenation enabled by titanium catalyst. Angew. Chem. Int. Ed.. 2021;60:12298-12303.
- [CrossRef] [Google Scholar]
- Electrochemically promoted nickel-catalyzed carbon-sulfur bond formation. ACS Catal.. 2019;9:1630-1634.
- [CrossRef] [Google Scholar]
- Recent advances in electrocarboxylation with CO2. Chem. Asian J.. 2022;17:e202200543
- [CrossRef] [Google Scholar]
- Electrochemical borylation of alkyl halides: fast, scalable access to alkyl boronic esters. J. Am. Chem. Soc.. 2021;143:12985-12991.
- [CrossRef] [Google Scholar]
- Electrifying organic synthesis. Angew. Chem. Int. Ed. Engl.. 2018;57:5594-5619.
- [CrossRef] [Google Scholar]
- Recent advances in the electrochemical reduction of substrates involving N-O bonds. Adv. Synth. Catal.. 2020;362:2088-2101.
- [CrossRef] [Google Scholar]
- Synthetic molecular photoelectrochemistry: new frontiers in synthetic applications, mechanistic insights, and scalability. Angew. Chem. Int. Ed. Engl.. 2022;61:e202107811
- [CrossRef] [Google Scholar]
- Electrochemical carboxylation of α, α-difluorotoluene derivatives and its application to the synthesis of α-fluorinated nonsteroidal anti-inflammatory drugs. Synlett. 2008;2008:438-442.
- [CrossRef] [Google Scholar]
- Synthesis of 2-aryl-3,3,3-trifluoropropanoic acids using electrochemical carboxylation of (1-bromo-2,2,2-trifluoroethyl) arenes and its application to the synthesis of β, β, β-trifluorinated non-steroidal anti-inflammatory drugs. Tetrahedron. 2010;66:473-479.
- [CrossRef] [Google Scholar]
- Alkylboronic esters from copper-catalyzed borylation of primary and secondary alkyl halides and pseudohalides. Angew. Chem. Int. Ed.. 2012;124:543-547.
- [CrossRef] [Google Scholar]
- Alkylboronic esters from palladium and nickel-catalyzed borylation of primary and secondary alkyl bromides. Adv. Synth. Catal.. 2012;354:1685-1691.
- [CrossRef] [Google Scholar]
- Electrocarboxylation of carbon dioxide with polycyclic aromatic hydrocarbons using Ni as the cathode. Chin. J. Chem .. 2010;28:1983-1988.
- [CrossRef] [Google Scholar]
- Electrocatalytic carboxylation of arylic bromides at the silver cathode in the presence of carbon dioxide. Synth. Commun.. 2011;41:3720-3727.
- [CrossRef] [Google Scholar]
- Site-selective electrochemical C-H carboxylation of arenes with CO2. Angew. Chem. Int. Ed. Engl.. 2023;135:e202214710
- [CrossRef] [Google Scholar]
- Organic electrochemistry: molecular syntheses with potential. ACS Cent. Sci.. 2021;7:415-431.
- [CrossRef] [Google Scholar]







