Translate this page into:
The evaluation of the interaction of baicalein (5,6,7-trihydroxyflavone) with human IgG and a glioblastoma multiforme (GBM) cell line
⁎Corresponding author. P.Huang_wz@hotmail.com (Ping Huang)
-
Received: ,
Accepted: ,
This article was originally published by Elsevier and was migrated to Scientific Scholar after the change of Publisher.
Abstract
Baicalein, a nutritional phenolic bioactive small molecule present in the roots of Scutellaria baicalensis and Scutellaria lateriflora, shows promising therapeutic effects against a wide range of cancer cells. However, its potential binding with blood carrier proteins and its anticancer mechanisms have not been well investigated. In this study, the interaction between baicalein and immunoglobulin G (IgG) was analyzed by analyzing intrinsic fluorescence, ellipticity changes, as well as molecular docking. The anticancer effects of baicalein against a glioblastoma multiforme (GBM) cell line, U87MG, and one normal immortalized human astrocytes were assessed by MTT, real-time PCR, caspase assay, and western blot. It was shown that the fluorescence quenching mechanism of human IgG by baicalein was based on a static quenching, where one binding site was responsible for the formation of baicalein-IgG complex derived by the formation of hydrogen bonds and van der Waals forces. A molecular docking study indicated that tyrosine residues (Tyr 91 and Tyr 98) were mostly involved in the binding site of baicalein to IgG. The cellular assay demonstrated that baicalein induced U87MG cell line death with an IC50 concentration of 73.19 µM, whereas normal astrocytes depicted over 82.94% portion of viability at 100 µM baicalein. The Bax mRNA overexpression, Bcl-2 mRNA downexpression, as well as the elevation of caspase-9 and caspase-3 activities confirmed that baicalein could effectively trigger the apoptosis of U87MG cells. Moreover, it was explored that baicalein downregulated the expression of the Axl/STAT3 signaling pathway in the U87MG cells, which is responsible for cellular proliferation and growth. In conclusion, these data may provide useful information regarding the pharmaceutical application of baicalein.
Keywords
Baicalein
Human immunoglobulin G
Interaction
Glioblastoma
1 Introduction
Interaction of small molecules showing different therapeutic applications with different blood proteins such as albumin (Bhardwaj et al., 2023), hemoglobin (Salam et al., 2023), and immunoglobulin G (IgG) (Shahlaei et al., 2020) serve as carrier proteins can provide useful information about the therapeutic efficacy of these bioactive compounds.
IgG has received a great deal of attention because of its wide utilization in diagnostics and therapeutics (Li et al., 2021). Human IgG not only serves as a potential macromolecule in the human immune response but also as a promising drug carrier derived from its capability to interact with several metabolites and small molecules as well as antigens (Palma et al., 2005; Wang et al., 2016). The metabolisms and potency of several small molecules are associated with their interaction with IgG (Giragossian et al., 2013). Indeed, the interaction of small molecules with IgG may lead to an alteration in their free concentration and subsequently affect their potency and cytotoxicity. Thus, the investigation of the interaction of small molecules with IgG can be studied as a model for developing potential delivery platforms and small molecules-based therapies.
IgG with a typical β-structure has four polypeptide chains, namely one homodimer γ heavy (H) chain (50 kDa) and one homodimer κ or λ light (L) chain (25 kDa) interacted by inter-chain disulfide bonds (Scott-Taylor et al., 2018; de Taeye et al., 2019).
Brain cancer with very poor survival rates and different categories has been widely increased recently (Tandel et al., 2019). Gliomas are the most frequent type of brain cancer and the most malignant kind of glioma is glioblastoma multiforme (GBM), which is known as one of the most lethal neoplasms of tumors in humans (Huse and Holland, 2010; Grochans et al., 2022). Patients suffering from GBM are usually treated with surgery resection combined with radiation and chemotherapy (Aldoghachi et al., 2022). Also, it has been shown that immunotherapy can be a potential strategy for cancer therapy (Aldoghachi et al., 2022; Li et al., 2022). In the treatment of overexpressed tumor cells, including lung cancer (Lee et al., 2017), colorectal (Schmitz et al., 2016), or GBM (Zhou et al., 2021), TAM receptors, especially Axl, have been a promising target due to their remarkable roles in the formation and proliferation of cancer. Axl is considerably followed by several signaling pathways, which all result in the initiation of tumor cell proliferation mediated by effector biomolecules (Wu et al., 2014). Also, STAT3 typically influences several signaling pathways derived from its regular effect in both tumor cells and the microenvironment. Indeed, STAT3 overexpression triggers tumor cell proliferation and metastasis in different carcinomas (Kim et al., 2021). Also, it has been shown that STAT3 could be a potential target for cancer therapy (Sadrkhanloo et al., 2022). Therefore, STAT3 along with Axl can be considered as potential targets for the treatment of different types of cancers, especially GBM (Kim et al., 2021). On the other hand, different studies have been described to control the Axl-STAT3 signaling pathway in the pathophysiology of tumors.
Baicalein (5,6,7-trihydroxyflavone) is known as a bioactive flavonoid with potential anticancer activities (Gao et al., 2016). Baicalein triggers proapoptotic characteristics mediated by interaction with several receptors involved in carcinogenesis (Wang et al., 2022). It has been reported that baicalein can regulate PI3K/Akt and STAT3-associated signaling pathways (Rahmani et al., 2022; Wang et al., 2022), and therefore it may play a key role in the control of GBM. However, the role of Axl-mediated signaling pathways in the beneficial impact of baicalein has not been well-studied so far.
We therefore performed an investigation to reveal the mechanistic and conformational changes of IgG following interaction with baicalein by different spectroscopic and docking studies. The binding constants as well as thermodynamic parameters in different temperatures were determined. Meanwhile, alterations in IgG secondary structure triggered by the interaction of baicalein were further discovered by circular dichroism spectroscopy. Also, the effect of baicalein on GBM mortality with a focus on the involvement of the Axl-STAT3 signaling pathway was investigated by cellular and molecular studies. For this part, we used one typically used GBM cell line, U87MG, and one normal immortalized human astrocytes.
2 Materials and methods
2.1 Materials
Baicalein (purity > 99 %) and IgG (purity > 99 %) were obtained from Sigma Chemical Company (USA). IgG was solubilized in Tris − HCl buffer solution with pH 7.40 supplemented with NaCl (0.1 mol·L−1). The stock solution (3.0 mM) of baicalein was prepared in DMSO. Cell culture medium, fetal bovine serum (FBS 10 %), MTT (3-(4,5-Dimethylthiazol-2-yl)-2,5-Diphenyltetrazolium Bromide), and antibiotics were purchased from Gibco company (UK).
2.2 Intrinsic fluorescence spectroscopy studies
A quantitative assay of the probable interaction between baicalein and IgG was performed through fluorimetric titration using a F97Pro spectrofluorophotometer (Leng Guang Industrial Co., China): 500 µL IgG solution containing 3 µM IgG was titrated by successive additions of different concentrations of baicalein ranging from 0.1 to 8 µM and incubated for 2 min at room temperature to equilibrate. The fluorescence intensity of IgG at around 340 nm was then recorded at three different temperatures of 298, 310 and 315 K after exciting samples at 280 nm. Furthermore, the fluorescence intensity of IgG was subtracted from inner-filter, dilution and blanks intensities (El-Shamy et al., 2020).
2.3 Circular dichroism (CD) study
CD analysis was carried out at room temperature over wavelengths ranging from 260–190 nm to detect the secondary structural changes of IgG using an Olis DSM 1000CD. For this assay, IgG solution containing 5 µM IgG was titrated by successive additions of increasing concentrations of baicalein ranging from 0.1 to 8 µM and incubated for 2 min at room temperature before reading the CD spectrum.
2.4 Molecular docking study
The crystal structure of human IgG was downloaded from the Protein Data Bank (PDB Nr: 1AJ7). The 3D structure of IgG was optimized with the Amber force field using Kollman-all-atom charges. The 3D crystal structure of baicalein was taken from PubChem and its structure was optimized with the Tripos force field by Gasteiger–Marsili charges. The docking box was fixed at 80*100*70 and docking was done using Vina autodocking software.
2.5 Cell culture
Human glioblastoma cell line, U87MG, and human astrocytes were obtained from the American Type Culture Collection (ATCC) (USA) and Applied Biological Materials (Canada), respectively. Both cells were cultured in DMEM medium at 37 °C with 5 % CO2. The medium contained 10 % FBS and 1 % antibiotics (penicillin–streptomycin).
2.6 MTT assay
U87MG and astrocyte cells (1 × 104 cells/ well) were cultured in a 96-well plate and incubated overnight. Then, the cells were added by increasing concentrations of baicalein ranging from 10 to 100 µM for 24 h, and an MTT assay was carried out based on the previous study (Oliva et al., 2022).
2.7 Caspase-3 and -9 activity assay
The activity of caspase-3 and -9 was assessed using the Sigma colorimetric kits according to the manufacturer’s instructions based on a previous study (Abbasi et al., 2020). Briefly, after seeding and incubating cells for 24 h with IC50 concentration of baicalein, cells were trypsinized, centrifuged, and lysed with lysis buffer (50 μL), incubated (10 min) on ice, and centrifuged (18000–20000 rpm, 4 °C, 5 min). The substrates (1 mM) were then added to the supernatant, incubated for 40 min at 37 °C and finally the absorbance was read at 405 nm using a plate reader (BioTek, USA).
2.8 Real-time PCR assay
After treating cells with baicalein for 24 h, total RNA was extracted with a Total RNA Extraction Kit (Invitrogen, China). The concentration of extracted RNA was then determined using a Nanodrop 1000 (Thermo Fisher Scientific, USA). Afterwards, 1 µg of total RNA was used to synthesize cDNA with a cDNA synthesis kit (Takara, Japan). RT-PCR assay was done by using 1 µl of synthesized cDNA. The primer sequence for Axl and GAPDH was used based on the previous study (Das et al., 2010).
2.9 Western blot assay
The western blot analysis was done based on the previous study (Kim et al., 2021). Briefly, after loading of samples (20 µg) and electrophoresis, transferring to a nitrocellulose membrane was done (80 V for 2 h). Then, the samples were blocked with skimmed milk (5 %) for 2 h. The membrane was incubated with different primary antibodies overnight: Axl and STAT3 obtained from Cell Signaling Technology (USA). Accordingly, the membranes were incubated with secondary antibodies for 1 h at room temperature, and immunoblots were detected by enhanced chemiluminescence solution.
2.10 Statistical analysis
Data were expressed as the mean ± standard deviation of three assays. Statistical analyses were then carried out with SPSS by one-way ANOVA followed by a Tukey test. p < 0.05 was determined as statistical significance.
3 Results and discussion
3.1 Molecular docking study
The interaction of baicalein with human IgG was assessed using molecular docking analysis and the best-docked outcome is depicted in Fig. 1. It was found that baicalein can interact with both light and heavy chains through different forces. It was displayed that Tyr 91, Arg 96, and Lue 89 in the light chain and His 35, Try 98, and Ala 96 in the heavy chain are the main amino acid residues involved in the interaction of human IgG with baicalein. Indeed, it was displayed that there was one H-bond between baicalein and one amino acid residue His35. Also, a π-cation interaction was observed between the baicalein and Arg96, and π−π interaction between the benzene ring and Tyr98 as well as Tyr 91.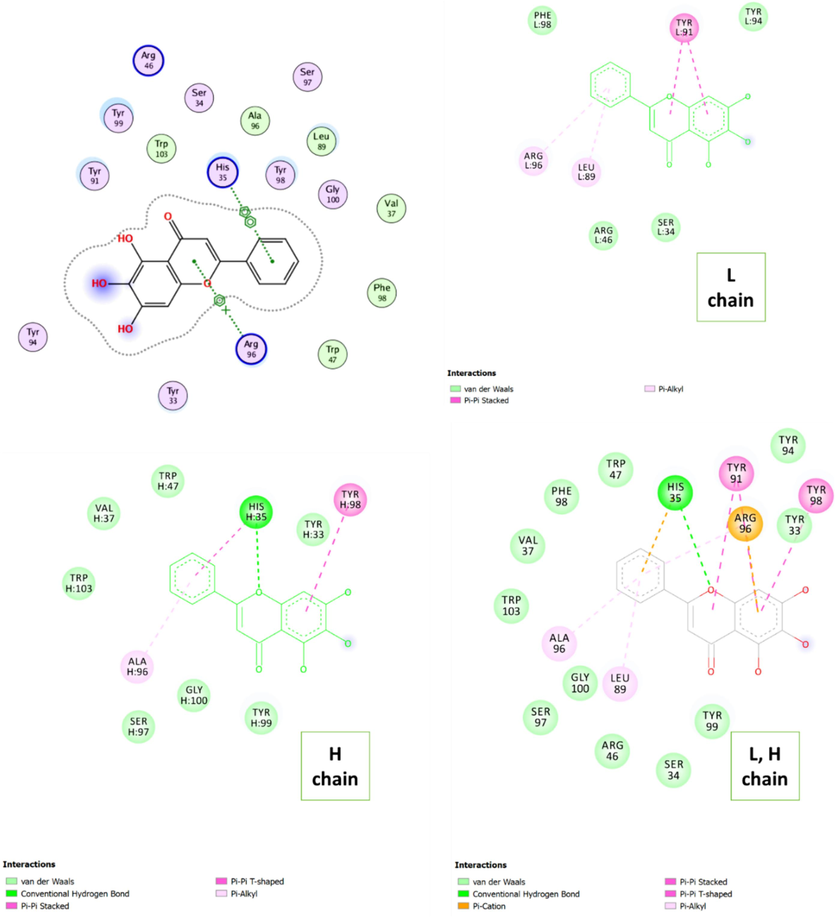
Molecular docking study for the interaction between baicalein and human IgG.
The calculated ΔG was found to be in the range of −4.5 to −6.7 kcal·mol−1 (Table 1), suggesting a strong spontaneous interaction between baicalein and human IgG (Jia et al., 2023). These outcomes of molecular docking furnished a theoretical basis for experimental analyses.
Docking number
Binding energy (kcal·mol−1)
1
−6.7
2
−5.6
3
−5.6
4
−5.4
5
−5.1
6
−5.1
7
−4.8
8
−4.5
9
−4.5
3.2 Quenching study
The intrinsic emission of proteins is mostly driven by aromatic amino acids, especially tryptophan and tyrosine residues due to high quantum yield (Zeinabad et al., 2016). Therefore, the main intrinsic emission of IgG is mainly attributed to tryptophan and tyrosine residues (Liu et al., 2008). When ligands interact with IgG, the microenvironment surrounding these aromatic residues can be changed. This microenvironmental change in the vicinity of tryptophan and tyrosine residues lead to a decrease in the quantum yield of emission from IgG serves as a fluorophore upon interaction with a ligand as a quencher, causing fluorescence quenching of IgG (Zhang et al., 2016). Following excitation at 280 nm, IgG displays maximum fluorescence around 340 nm, mainly because of tryptophan and tyrosine residues (Sun et al., 2021). Titration of baicalein with a fixed concentration of IgG led to an apparent decrease in the emission intensity of IgG (Fig. 2).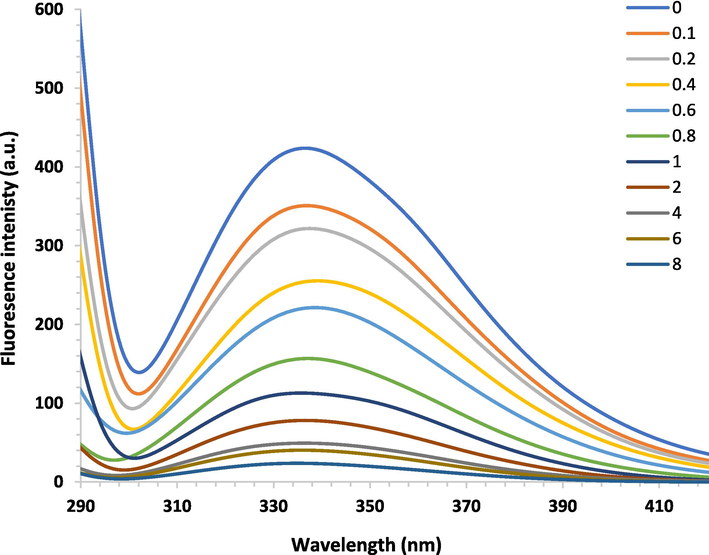
Fluorescence quenching spectra of human IgG (3 µM) with increased concentrations of baicalein (0.1–8 µM) at 298 K.
Fluorescence quenching occurs through two different mechanisms categorized under two classes; static (complex formation in the ground state) and dynamic (complex formation in the excited state) (Zeinabad et al., 2016). To further investigate the class of quenching mechanism, intrinsic fluorescence quenching analysis was performed at three different temperatures of 298, 310, and 315 K based on the well-known Stern-Volmer equation (Esfandfar et al., 2016):
Temperature (K)
KSV (M−1) × 106
kq (M−1 S-1) × 1015
298
1.9
1.9
310
1.06
1.06
315
0.68
0.68
A reducing mode in the Ksv and kq values was detected upon increasing the temperature of the medium. In dynamic or diffusion quenching, enhanced temperatures lead to a larger diffusion coefficient, causing enhanced values of Ksv and kq constants. However, in static quenching, enhanced temperatures reduce the resultant stability of formed complexes, leading to lower values of Ksv and kq constants (Zeinabad et al., 2016). The data calculated values for the IgG-baicalein system indicated that the Ksv and kq constants were inversely correlated to temperature (Fig. 3), proposing that IgG-baicalein complex formation is derived from a static quenching.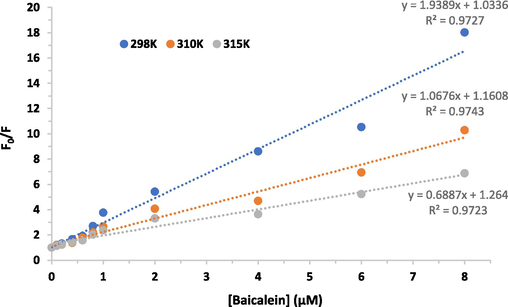
Stern-Volmer plots for the emission quenching of IgG by enhanced concentrations of baicalein at three different temperatures.
To further determine the mechanism of fluorescence quenching, kq values were also calculated by using the above equation. The kq values for baicalein at all studied temperatures were found to be greater than 2 × 1010 M−1s−1, stating the involvement of static quenching (Negrea et al., 2023).
3.3 Binding parameters
Binding parameters, including binding constant (Kb) and number of the binding site (n) were also estimated by using the following equation (Zeinabad et al., 2016):
By plotting a linear graph between log [F0-F]/F versus log [Q] the values of n and Kb can be calculated by using the slope and Y-intercept, respectively. The resultant double logarithmic plots at three different temperatures of 298 K, 310 K and 315 K are depicted in Fig. 4, and the obtaining binding parameters are summarized in Table 3. The Kb values were reduced with an elevation in the corresponding temperature, revealing a reduction in the stability of the formed complex with an enhancement in temperature (Bhardwaj et al., 2023). The n values were determined and realized to be around 1, revealing that only one binding site is responsible for the formation of the IgG- baicalein complex.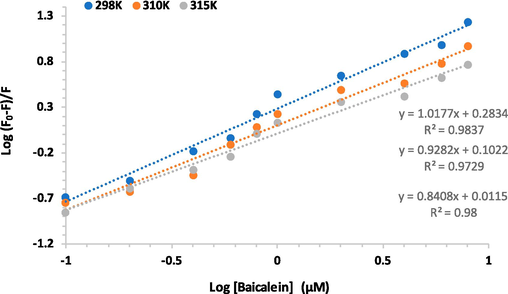
Double logarithm plots of the IgG-baicalein complex at three different temperatures.
Temperature (K)
logKb (M−1)
n
298
0.28
1.01
310
0.10
0.92
315
0.01
0.84
3.4 Thermodynamic study
The interaction between ligands and proteins is mediated by several forces: van der Waals, H-bonds, ionic, and hydrophobic interactions, which can be determined by thermodynamic parameters. The van't Hoff equation was used to determine the thermodynamic parameters (Bhardwaj et al., 2023):
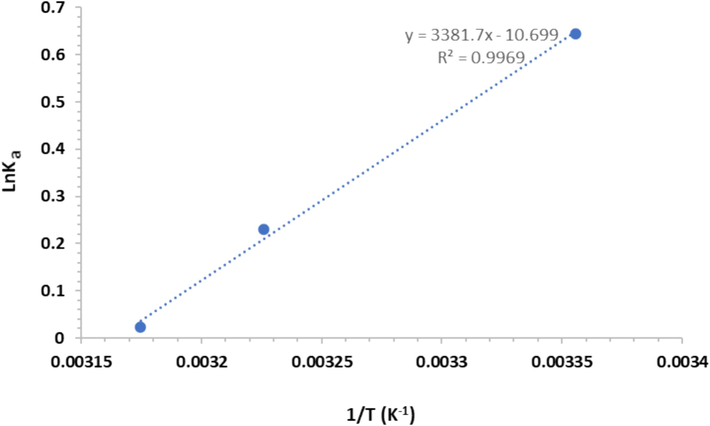
Van't hoff plot for the igg-baicalein at three different temperatures.
Temperature (K)
ΔH° (kJ/mole)
TΔS° (kJ/mole)
ΔG° (kJ/mole)
298
−28.06
−26.44
−1.62
310
−28.06
−27.50
−0.56
315
−28.06
−27.94
−0.11
3.5 Far UV-CD
Circular dichroism as a sensitive technique is commonly used for recording the structural changes in proteins triggered by small molecules (Pishkar et al., 2017). In the case of IgG, the recorded CD spectrum is derived from the asymmetric signal of the protein backbone (Sun et al., 2021). The data of far-UV CD is used to receive information about the protein’s secondary conformers (Zeinabad et al., 2016). Therefore, CD spectroscopy was used to determine the impact of baicalein on the IgG structure. The CD spectra of IgG without and with baicalein are displayed in Fig. 6. The one minimum attained at around 218 nm (π-π*) for free IgG determines the IgG’s majorly β-sheet structure (Sun et al., 2021) Apparently, there was not an evident variation in the ellipticity changes for the minimum of IgG after addition of increasing concentrations of baicalein even at 8 µM.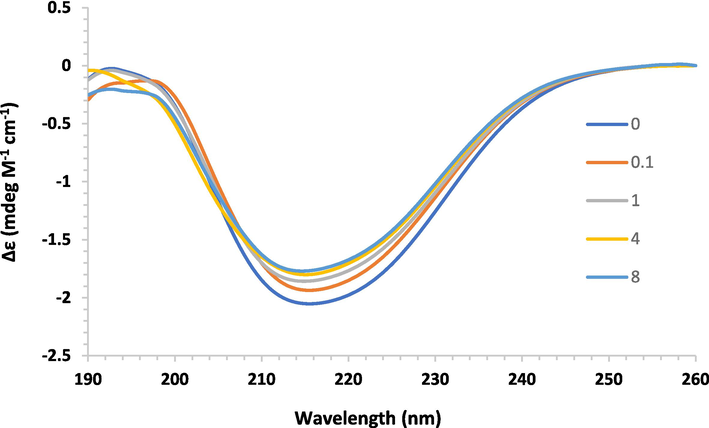
Circular dichroism study of human IgG with increased concentrations of baicalein (0.1–8 µM) at 298 K.
This observation indicated that the non-covalent interaction between baicalein and IgG is causing a partial secondary structural change even at high concentrations (Zeinabad et al., 2016). In fact, the hydroxyl groups possibly increase the hydrophilic interaction between baicalein and IgG. Most significantly, all secondary structural changes in IgG following interaction with baicalein are not significant and therefore favor the promising application of this small molecule pharmaceuticals areas.
3.6 Viability assay
Baicalein is depicted to induce anticancer effects in GBM through different mechanisms and regulation of several signaling pathways (Zhang et al., 2014; Liu et al., 2019; Yang et al., 2021). Astrocytes normal cells and U87MG cancer cells were incubated with different concentrations of baicalein up to 100 µM and the percentage of cell viability was detected by MTT assay. It was depicted that baicalein even up to 100 µM did not trigger the viability of astrocytes normal cells, as these cells depicted over 82.94 % portion of viability, but this small molecule led to highly reduced viability in the U87MG cells (Fig. 7).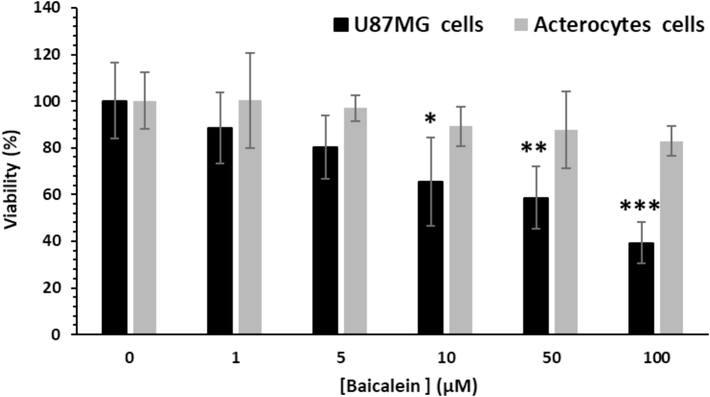
Effect of baicalein on cell viability of U87MG and astrocytes normal cells after 24 h. MTT data were displayed as mean ± SD of three sets of experiments. *p < 0.05, **p < 0.01, ***p < 0.001 vs. untreated cells.
Furthermore, we concluded that baicalein stimulated dose-dependent toxicity in the U87MG cells but not in astrocytes as normal cells (Fig. 7) as evidenced by the MTT assay. It was then calculated that the IC50 concentration of baicalein against U87MG cells was around 73.19 µM, which was used for incubating cells for further experiments.
3.7 Baicalein triggers apoptotic in the U87MG cells
To examine the probable apoptosis triggered by baicalein in the U87MG cells, we treated U87MG with IC50 concentration of baicalein (73.19 µM) for 24 h and assessed the expression levels of Bax and Bcl-2 mRNA using real-time PCR. The results indicated that baicalein induced the overexpression of the apoptotic pathway in the U87MG cells through the upregulation of Bax (Fig. 8a) and downregulation of Bcl-2 (Fig. 8b). An additional caspase activity assay determined the apoptotic impact of baicalein through the elevation of caspase-3 and caspase-9 activities (Fig. 8c). All these data depicted that baicalein (73.19 µM) induced a significant apoptotic effect in the U87MG cells after 24 h.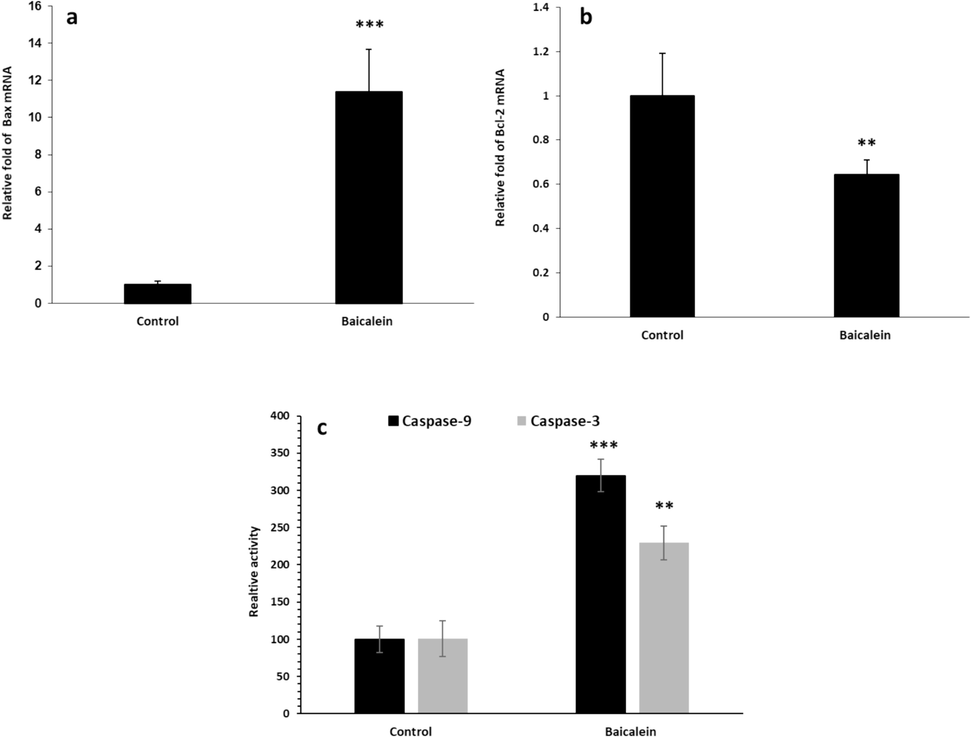
Effect of baicalein (73.19 µM) on mRNA expression of Bax (a) and Bcl-2 (b) in the U87MG cells after 24 h, assessed by real-time PCR. Caspase-3 and −9 activity assays in the U87MG cells after incubation with baicalein (73.19 µM) for 24 h. **p < 0.01, ***p < 0.001 vs. untreated cells.
3.8 Baicalein probably suppresses STAT3/Axl signaling pathway
In the cancer cell microenvironment, the STAT3/Axl signaling pathway serves as an inducer for cancer cell proliferation and metastasis, and there has been a correlation between STAT3/Axl overexpression with glioma tumor grade and overall proliferation (West et al., 2018; Kim et al., 2021). We then assessed the STAT3 expression in the U87MG cells and found out that baicalein reduced STAT3 phosphorylation (Fig. 9). Also, we analyzed whether baicalein was able to probably influence Axl receptor tyrosine kinase in the U87MG cells. We evaluated U87MG cells to explore the change in protein expression of Axl triggered by baicalein (73.19 µM). As shown in Fig. 9, Axl protein was downregulated in the U87MG cells, depicting an apparent reduction in expression after 24 h.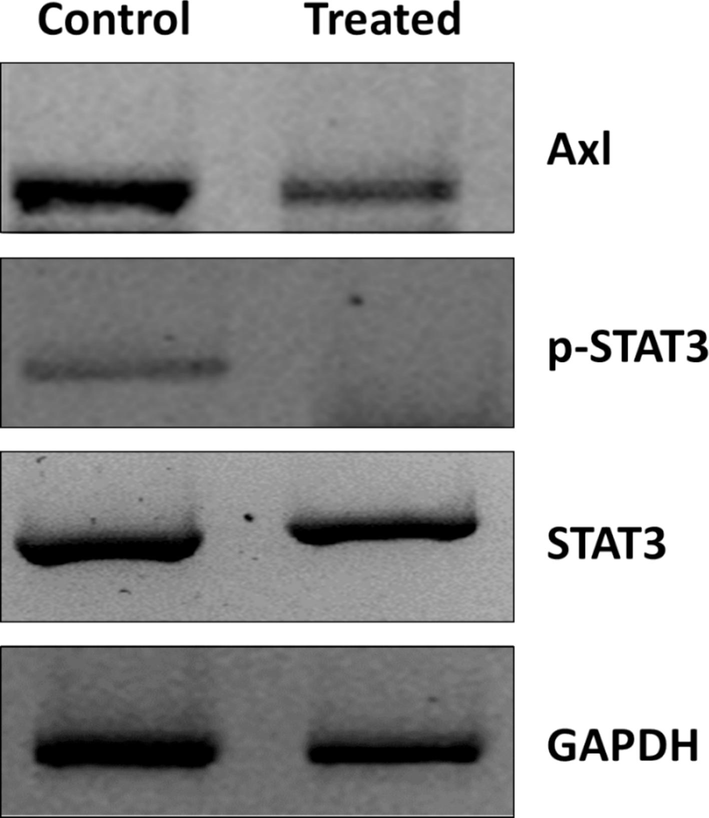
Western blot analysis of U87MG cells after incubation with baicalein (73.19 µM) for 24 h.
4 Conclusion
In conclusion, the interaction of baicalein (5,6,7-trihydroxyflavone) with human IgG and a GBM cancer cell line, U87MG, was assessed by using different spectroscopic studies molecular docking, cellular, and molecular studies. Fluorescence analysis indicated the spontaneous formation of a baicalein-IgG complex through static quenching and involvement of hydrogen bonds and van der Waals forces. Docking analysis displayed that tyrosine residues (Tyr 91 and Tyr 98) were placed in the vicinity of the binding site of baicalein in IgG. CD analysis also showed a partial secondary structural change of IgG induced by interaction with baicalein. The anticancer assays demonstrated that baicalein had a more cytotoxic effect in the U87MG cancer cell line than normal astrocytes, which was assisted with apoptosis induction through the elevation of Bax mRNA expression and caspase-9 and caspase-3 activities. Also, it was shown that baicalein regulated the expression of the Axl/STAT3 signaling pathway in the U87MG cells as a crucial m for cellular mechanism for cancer cell proliferation. Future studies should be planned to assess the anticancer effects of baicalein in vivo.
CRediT authorship contribution statement
Lihe Zhu: Conceptualization, Methodology, Data curation, Writing – original draft, Visualization. Ying Zhang: Methodology, Validation, Writing – original draft, Visualization. Wei Zhu: Writing – review & editing, Visualization. Jianle Ji: Writing – review & editing, Visualization. Xin Bai: Writing – review & editing, Visualization. Ping Huang: Conceptualization, Data curation, Writing – review & editing, Supervision, Project administration, Funding acquisition.
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
References
- Evaluation of the cytotoxic and apoptogenic effects of cinnamaldehyde on U87MG cells alone and in combination with doxorubicin. Res. Pharmaceut. Sci.. 2020;15(1):26.
- [Google Scholar]
- Recent advances in the therapeutic strategies of glioblastoma multiforme. Neuroscience 2022
- [Google Scholar]
- Study on the interaction of the bromodomain inhibitor JQ1 with human serum albumin by spectroscopic and molecular docking studies. J. Mol. Struct.. 2023;5(1273):134374
- [Google Scholar]
- Flavonoids activated caspases for apoptosis in human glioblastoma T98G and U87MG cells but not in human normal astrocytes. Cancer: Interdiscip. Int. J. Am. Cancer Soc.. 2010;116(1):164-176.
- [Google Scholar]
- Probing the interaction of iron complex containing N3S2 macrocyclic ligand with bovine serum albumin using spectroscopic techniques. Spectrochim. Acta A Mol. Biomol. Spectrosc.. 2020;228:117811
- [Google Scholar]
- Spectroscopic studies of interaction between CuO nanoparticles and bovine serum albumin. J. Biomol. Struct. Dyn.. 2016;34(9):1962-1968.
- [Google Scholar]
- Neonatal Fc receptor and its role in the absorption, distribution, metabolism and excretion of immunoglobulin G-based biotherapeutics. Curr. Drug Metab.. 2013;14(7):764-790.
- [Google Scholar]
- Epidemiology of glioblastoma multiforme-literature review. Cancers. 2022 May 13;14(10):2412.
- [Google Scholar]
- Targeting brain cancer: advances in the molecular pathology of malignant glioma and medulloblastoma. Nat. Rev. Cancer. 2010;10(5):319-331.
- [Google Scholar]
- Evaluation the binding of chlorogenic acid with bovine serum albumin: spectroscopic methods, electrochemical and molecular docking. Spectrochim. Acta A Mol. Biomol. Spectrosc.. 2023;291:122289
- [Google Scholar]
- Quercetin induces apoptosis in glioblastoma cells by suppressing Axl/IL-6/STAT3 signaling pathway. Am. J. Chin. Med.. 2021;49(03):767-784.
- [Google Scholar]
- Anticancer effect of luteolin is mediated by downregulation of TAM receptor tyrosine kinases, but not interleukin-8, in non-small cell lung cancer cells. Oncol. Rep.. 2017;37(2):1219-1226.
- [Google Scholar]
- Sialylated immunoglobulin G: a promising diagnostic and therapeutic strategy for autoimmune diseases. Theranostics.. 2021;11(11):5430.
- [Google Scholar]
- Recent development in NKT-based immunotherapy of glioblastoma: from bench to bedside. Int. J. Mol. Sci.. 2022;23(3):1311.
- [Google Scholar]
- Baicalein induces autophagy and apoptosis through AMPK pathway in human glioma cells. Am. J. Chin. Med.. 2019;47(06):1405-1418.
- [Google Scholar]
- Interaction of curcumin with intravenous immunoglobulin: a fluorescence quenching and Fourier transformation infrared spectroscopy study. Immunobiology. 2008;213(8):651-661.
- [Google Scholar]
- Spectroscopic studies on binding of ibuprofen and drotaverine with bovine serum albumin. J. Photochem. Photobiol. A Chem.. 2023;438:114512
- [Google Scholar]
- Isoginkgetin—a natural compound to control U87MG glioblastoma cell growth and migration activating apoptosis and autophagy. Molecules. 2022;27(23):8335.
- [Google Scholar]
- Antibodies as drug carriers III: design of oligonucleotides with enhanced binding affinity for immunoglobulin G. Pharm. Res.. 2005;22:122-127.
- [Google Scholar]
- Studies on the interaction between nanodiamond and human hemoglobin by surface tension measurement and spectroscopy methods. J. Biomol. Struct. Dyn.. 2017;35(3):603-615.
- [Google Scholar]
- The multifaceted role of baicalein in cancer management through modulation of cell signalling pathways. Molecules. 2022;27(22):8023.
- [Google Scholar]
- STAT3-EMT axis in tumors: modulation of cancer metastasis, stemness and therapy response. Pharmacol. Res.. 2022;106311
- [Google Scholar]
- Molecular docking and biophysical studies on the interaction between thiram and human hemoglobin. J. Mol. Struct.. 2023;15(1272):134188
- [Google Scholar]
- TAM receptors Tyro3 and Mer as novel targets in colorectal cancer. Oncotarget. 2016;7(35):56355-56370.
- [Google Scholar]
- Immunoglobulin G; structure and functional implications of different subclass modifications in initiation and resolution of allergy. Immun. Inflammation Dis.. 2018;6(1):13-33.
- [Google Scholar]
- Increasing the effectiveness of oxaliplatin using colloidal immunoglobulin G nanoparticles: Synthesis, cytotoxicity, interaction, and release studies. Colloids Surf. B Biointerfaces. 2020;195:111255
- [Google Scholar]
- Elucidating the interaction of propofol as an intravenous anesthetic drug with blood components: IgG and peripheral blood mononuclear cell as targets. Arab. J. Chem.. 2021;14(2):102965
- [Google Scholar]
- A review on a deep learning perspective in brain cancer classification. Cancers. 2019;11(1):111.
- [Google Scholar]
- Latest research progress on anticancer effect of baicalin and its aglycone baicalein. Arch. Pharm. Res.. 2022;45(8):535-557.
- [Google Scholar]
- Molecular modeling and multi-spectroscopic approaches to study the interaction between antibacterial drug and human immunoglobulin G. Luminescence. 2016;31(3):704-711.
- [Google Scholar]
- The role of interleukin-6-STAT3 signaling in glioblastoma. Oncol. Lett.. 2018;16:4095-4104.
- [Google Scholar]
- Baicalein inhibits invasion and promotes apoptosis in glioma cells through the PI3K/Akt pathway. J. BUON.. 2021;1(26):395-401.
- [Google Scholar]
- Thermodynamic and conformational changes of protein toward interaction with nanoparticles: a spectroscopic overview. RSC Adv.. 2016;6(107):105903-105919.
- [Google Scholar]
- Baicalein reduces the invasion of glioma cells via reducing the activity of p38 signaling pathway. PLoS One. 2014;9(2):e90318.
- [Google Scholar]
- Binding analysis of carbon nanoparticles to human immunoglobulin G: Elucidation of the cytotoxicity of CNPs and perturbation of immunoglobulin conformations. Spectrochim. Acta A Mol. Biomol. Spectrosc.. 2016;5(154):33-41.
- [Google Scholar]
- Immuno-oncology: are TAM receptors in glioblastoma friends or foes? Cell Commun. Signal.. 2021;19(1):1-3.
- [Google Scholar]







