The flavonoid hesperidin methyl chalcone as a potential therapeutic agent for cancer therapy: Molecular docking, in vitro cytotoxicity, and in vivo antitumor activity
⁎Corresponding author at: Department of Pharmaceutics, College of Pharmacy, University of Ha’il, Ha’il 81442, Saudi Arabia. a.abulila@uoh.edu.sa (Amr S. Abu Lila)
-
Received: ,
Accepted: ,
This article was originally published by Elsevier and was migrated to Scientific Scholar after the change of Publisher.
Peer review under responsibility of King Saud University.
Abstract
Hesperidin methyl chalcone (HMC) is a methylation product of the flavanone hesperidin, a flavonoid derived from citrus fruits. Many reports have emphasized the analgesic, anti-inflammatory and antioxidant properties of HMC. However, the anticancer potential of HMC has not been fully elucidated. The objective of this study was to assess the possible anticancer potential of HMC. MTT assay was carried out to assess the in vitro cytotoxicity of HMC against using A549 cancer cell line. In addition, the in vivo antitumor activity of HMC was screened against murine Ehrlich ascites carcinoma (EAC) model, in terms of tumor volume, tumor weight, life span, hematological and biochemical parameters, and compared with that of the anticancer agent, hesperetin. HMC was efficient to suppress the cell viability of A549 cancer cells with an IC50 value of 51.12 µM, which was comparable to that of the anticancer agent hesperetin (IC50 = 49.12 µM). Similarly, in Ehrlich ascites carcinoma model, HMC significantly inhibited the growth of Ehrlich ascites carcinoma with mutual increase in the life span of HMC-treated mice, compared to EAC control. Most importantly, HMC showed a good safety profile as evidenced by restoring hematological profile count of RBCs, WBCs, and hemoglobin to the normal levels. HMC also efficiently reduced the oxidative stress in EAC-bearing mice via increasing the levels of GSH, SOD and Catalase. Collectively, HMC exerted a potent anticancer activity and data presented in this work suggest the usefulness of HMC as a promising candidate in cancer therapy.
Keywords
Cancer
Ehrlich ascites carcinoma
Flavonoid
Hesperidin methyl chalcone
Molecular docking
MTT assay
1 Introduction
Cancer is a fatal disease caused by uncontrolled cell proliferation and stands next to cardiovascular diseases in terms of mortality and morbidity (Nagai and Kim, 2017; Koene et al., 2016; Lila et al., 2014). Various strategies including surgery, chemotherapy, and radiation, employed alone or in combination, are currently adopted to mitigate cancer progression (Moin et al., 2021; Ando et al., 2015; Matlapudi et al., 2015; Abu Lila et al., 2009; Emam et al., 2018). However, multidrug resistance (MDR) and adverse effects are significant barriers to effective cancer therapy (Hussain et al., 2019). Accordingly, the search for safer and more effective chemoprevention and treatment strategies is mandatory for augmenting the overall therapeutic outcomes as well as improving patients’ quality of life.
Recently, there has been a renewed interest for the use of phytochemicals in cancer therapy due to their safety, low toxicity, and readily availability (Hussain et al., 2018). Phytochemicals and their derivatives are viewed as intriguing solutions for enhancing therapy efficacy and alleviating adverse effects in cancer patients (Choudhari et al., 2019; Ranjan et al., 2019). Chalcones, members of the flavonoid family, are simple chemical scaffolds found in a variety of natural plant products such as vegetables, teas, and fruits (Karthikeyan et al., 2015; Singh et al., 2014; Zhou and Xing, 2015). They show structural heterogeneity and can act on a variety of pharmacological targets. Chalcone family members have attracted a lot of attention not only because of their synthetic and biosynthetic capabilities, but also because of their broad spectrum of biological activities, which include antioxidant (Lin et al., 2019), anti-inflammatory (Mahapatra et al., 2017), antimicrobial (Henry et al., 2020); antidiabetic (Rocha et al., 2020), cancer chemopreventive (Xu et al., 2015), and anticancer activities (Gao et al., 2020).
Hesperidin methyl chalcone (HMC) is a product of methylation of hesperidin, a flavonoid present mainly in citrus fruits (Nizamutdinova et al., 2008). Hesperidin has a low water solubility, leading in poor absorption in the small intestine; however, its solubility improves following an alkaline methylation process, which facilitates hesperidin isomerization and the formation of the HMC. Owing to its high-water solubility, HMC grants enhanced absorption, metabolic stability, and systemic bioavailability (Pinho-Ribeiro et al., 2015; Chanal et al., 1981). Many reports have emphasized the analgesic, anti-inflammatory and antioxidant properties of HMC (Pinho-Ribeiro et al., 2015; Guazelli et al., 2021). However, the anticancer potential of HMC has not been fully elucidated.
Since drug discovery and development are both time- and cost-consuming, they provide several hurdles for researchers working on drug design and discovery for diverse diseases such as various forms of cancer. As a result, the adoption of innovative technologies can open the way for the discovery of novel drugs with outstanding therapeutic efficiencies, making a substantial contribution to disease treatment. Molecular docking is a cutting-edge computational drug design technology that predicts the most effective and stable state shape of the ligand-receptor complex (Morris and Lim-Wilby, 2008). Currently, molecular docking is considered one of the commonly adopted therapeutic approaches for anti-cancer drug designing.
In this present work, therefore, we aimed at investigating the possible anticancer potential of the flavonoid conjugated chalcone, hesperidin methyl chalcone (HMC). Molecular docking studies were conducted to target the catalytic site of the cancer specific enzymes, i.e., Polo-like kinase 1 (PLK1) and Epidermal Growth Factor Receptor tyrosine kinase domain (EGFR). Downregulation of both PLK1 and EGFR have been correlated-well with the tumor suppression, and thereby, designing of variety of robust PLK1 and EGFR inhibitors for cancer management is an upcoming strategy against cancer (Gunasekaran et al., 2022; Stratmann and Sebastian, 2019; Su et al., 2022; Alapati et al., 2012). Accordingly, in the present study, the in vitro anticancer activity of HMC was examined against A549 cancer cell line after completing molecular interaction analysis against PLK1 and EGFR. Furthermore, the in vivo antitumor efficacy of HMC was also examined in Ehrlich ascites carcinoma animal model.
2 Materials and methods
2.1 Materials
Hesperetin, hesperidin methyl chalcone and 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyl tetrazolium bromide (MTT) was purchased from Sigma Aldrich (St. Louis, MO, USA). Roswell Park Memorial Institute (RPMI) 1640 medium, fetal bovine serum (FBS) and antibiotic–antimycotic solution were procured from Gibco (Gaithersburg, MD, USA).
2.2 Cell lines
Human non-small cell lung cancer A549 cells and murine alveolar macrophages J774A.1 were obtained from National Centre for Cell Science (Pune, India). Ehrlich ascites carcinoma (EAC) cells were provided by the Amala Cancer Research Centre (Thrissur, Kerala). A549 cells were cultured in RPMI-1640 medium while J774A.1 were cultured in Dulbecco’s modified Eagle medium (DMEM). Both media were supplemented with heat-inactivated 10% fetal bovine serum (FBS) and 1% penicillin/streptomycin incubated in a humid atmosphere (5% CO2/95% humidified air).
2.3 Experimental animals
Swiss albino mice (20–25 g) were obtained from Central Animal Facilities, Acharya & BM Reddy College of Pharmacy, Bengaluru, India. Animals were housed under standard temperature- and humidity conditions, and they had free access to mouse chow and water. The Institutional Animal Ethics Committee at Acharya & BM Reddy College of Pharmacy authorized the study protocol (IAEC/ABMRCP/2017–2018/17).
2.4 In silico molecular docking
2.4.1 Target protein and ligand preparation
Protein data bank was used to obtain the 3D structures of target proteins PLK1 (PDB ID: 2OWB) and EGFR (PDB ID: 1 M17) in pdb format. However, each protein was analyzed by Discovery Studio Visualizer tool and heteroatoms were removed. Further, both the target proteins were converted from.pdb to.pdbqt format with the help of AutoDock 4.2 tool by applying the protocol of Rizvi et al. (Rizvi et al., 2013). Kollman united atom charges, solvation parameters and polar hydrogen were also added to the target protein via AutoDock 4.2 before converting them into.pdbqt format.
Three-dimensional structures of PHA-680626 (native ligand for PLK1), Erlotinib (native ligand for EGFR) and Hesperetin (control) were retrieved from the ChemSpider database by using ID 10137725, 154,044 and 65234, respectively. On the other hand, HMC (ID 6436550) 3D structure was obtained from PubChem database. Moreover, OpenBabel tool was used to convert all the ligand structures into.pdbqt format prior to docking.
2.4.2 Docking experiment
AutoDock Vina tool [https://www.ncbi.nlm.nih.gov/pmc/articles/PMC3041641/] was used to dock each ligand to the target proteins by using a protocol described by Moin et al. (Moin et al., 2022). Pre-defined grid coordinates were used to target both the proteins active sites, i.e., x: 0.069; y: 23.58; z: 66.741 for PLK1 enzyme and x: 24.379; y: 0.093; z: 56.141 for EGFR, respectively. However, grid box size was kept as 40 × 40 × 40 Å for both target proteins. Affinity in kcal/mol was obtained for each ligand-target protein interaction, and the algorithm presented results in descending order (10 modes). Further, complex of best docking confirmation was obtained by using PyMol tool, and detailed interaction analysis was done on discovery studio visualizer. Moreover, LigPlot analysis was also performed for each complex to get deeper insight of the interaction. It is noteworthy to mention that both the target proteins were re-docked with native ligand to validate the protocol before performing the docking experiments.
2.5 In vitro cytotoxicity assay
The MTT assay was adopted to estimate the number of viable cells. Briefly, 1 × 104 A549 cells were seeded per well in 96-well microtiter plates. The cells were treated with different concentrations (6.25, 12.5, 25, 50, and 100 µM) of either HMC or the anticancer agent, hesperetin and the plates were incubated at 37 ˚C. After 24 h of incubation, 10 μL of MTT solution (5 mg/mL) was added to each well and the plates were further incubated at 37 °C for 4 h. The formed formazan crystals were then dissolved in 100 μL of acid-isopropanol (0.04 N HCl in isopropanol). The absorbance was measured at a wavelength of 570 nm using an ELISA plate reader.
2.6 In vivo studies
2.6.1 Development of Erlich ascites carcinoma model
Erlich ascitic carcinoma (EAC) cells (1 × 106 cells) were transplanted into the peritoneal cavity of a Swiss albino mouse to propagate. After 16 days, the donor mouse received an intraperitoneal injection of 0.1 mL of normal saline (0.9% w/v). and 1 mL of ascites fluid was collected immediately from the peritoneal cavity and diluted to 10 mL with normal saline. 10 µL of ascites fluid were then placed in Neubauer’s chamber and counted by a hemocytometer.
2.6.2 Experimental design
On day zero, 1 × 106 EAC cells were intraperitoneally injected in Swiss albino mice. 24 h later, the inoculated mice with Ehrlich cells were randomly divided into three groups (n = 12). The first group received phosphate buffer saline and served as EAC-bearing control group. The second group was treated with hesperetin (30 mg/kg) (Aranganathan et al., 2009). The third group was treated with HMC (30 mg/kg). HMC dose was chosen based on previous work (Guazelli et al., 2021). Treatments were given i.p. at 24 h post tumor inoculation and continued once daily for 15 days. On day 16, six mice from each group were euthanized for the estimation of tumor growth, hematological, and hepatic anti-oxidative parameters. The remaining mice in each group were kept alive and monitored for survival parameters.
2.6.2.1 Estimation of tumor volume
The ascitic fluid was collected from peritoneal cavity of each mouse and the tumor volume was estimated by comparing the volume of ascetic fluid present in the peritoneal cavity of treated groups to those in the control group as on day 16 of the experiment.
2.6.2.2 Hematological parameters
To assess the impact of HMC treatment on hematological parameters in EAC-bearing mice, blood samples was collected from retro-orbital venous plexus into EDTA Eppendorf tubes. Hematological parameters such as hemoglobin (Hb) content, red blood cells (RBCs) and white blood cells (WBCs) counts were estimated.
2.6.2.3 Hepatic antioxidant parameters
The liver of each mouse was dissected, washed with cold saline, and blotted on filter paper. A definite weight of 1 g of liver tissue was then homogenized with 10 mL cold PBS (0.2 M, pH 7) at 4000 rpm. Liver homogenates were then centrifuged at 10,000 rpm at 4 ˚C for 15 min. The collected supernatant was subjected to different biochemical estimations such as Superoxide dismutase (SOD), glutathione synthetase (GSH), catalase (CAT), Nitric oxide (NO) (Sinha, 1972; Habig et al., 1974; Kakkar et al., 1984).
2.6.2.4 Survival parameters
The effect of Hesperetin (30 mg/kg) and HMC (30 mg/kg) on tumor growth was monitored in terms of median survival time (MST) and percentage increase in life span (% ILS). MST was estimated by recording the mortality rate daily until all the animals were dead and % ILS was calculated using the following formula (Saad et al., 2017):
2.6.2.5 Weight variation parameter
To estimate the differences in body weight between the control EAC group and treated groups, animals were weighed every other day from day zero.
2.7 Statistical analysis
All the data are expressed as mean ± SD. GraphPad Prism 7 software was employed to analyze the data using one-way ANOVA followed by Dunnett's t-test for multiple comparisons. *P < 0.05 was considered significant.
3 Results and discussion
3.1 In silico studies
Molecular docking interaction experiments were performed to target the native ligand, HMC and Hesperetin on both the proteins (PLK1 and EGFR) by using AutoDock Vina. Table 1 and Fig. 1 shows the docking interaction results of native ligand (PHA-680626, a selective PLK inhibitor), HMC and Hesperetin (control) on the catalytic site of PLK1. To validate the protocol re-docking experiment with native ligand was performed on PLK1; interestingly, the re-docked native ligand bound to the same active site cavity as the native ligand in the crystal structure (Kothe et al., 2007). The superimposition image (Fig. 1a) confirms the standardization of the protocol. In fact, HMC and hesperetin also bounded to the same catalytic site as native ligand (Fig. 1b). Detailed interaction analysis showed that native ligand once re-docked with PLK1 active site showed binding energy of −9.0 kcal/mol, and eleven amino acids of PLK1 (Arg57, Leu59, Ala80, Val114, Glu131, Leu132, Cys133, Arg134, Arg136, Phe183, Glu186) were involved in this interaction (Table 1; Fig. 1c and d). Among these amino acids, Leu59 and Arg134 showed strong hydrogen bonding interaction with the native ligand. Whereas HMC showed −8.5 kcal/mol binding energy with the involvement of twenty amino acids of PLK1 'active site' in the interaction (Table 1; Fig. 1e and f). Interestingly, HMC showed strong hydrogen bonding with five amino acids (Glu131, Cys133, Arg136, Glu140, Lys178) of PLK1 catalytic site during 'HMC-PLK1′ molecular interaction. On the other hand, 'hesperitin-PLK1′ interaction showed binding energy of −7.8 kcal/mol, and three hydrogen bonding with Glu131, Cys133 and Glu140 amino acid residues of PLK1 (Table 1; Fig. 1g and h). It has been reported by Shakil et al (Shakil et al., 2019) that six-amino acids, namely, Leu59, Cys67, Leu130, Cys133, Arg136 and Phe183 are crucial for binding of inhibitors to the kinase domain of PLK1. They also revealed that Cys67 and Phe183 forms the top and bottom of the ATP-binding pocket of PLK1, respectively; however, Leu132 forms its hinge-region. In the present study, among the important kinase domain amino acids of PLK1, HMC interacted with Cys133 and Arg136, hesperetin interacted with Cys13,3 and native ligand interacted with Leu59 through strong hydrogen bonds. Moreover, HMC has shown interaction with all the crucial amino acids of PLK1 mentioned in the Shakil et al (Shakil et al., 2019) report via hydrogen or hydrophobic interactions. These results confirmed the strong bonding of our targeted molecule (HMC) against a potent anticancer target PLK1. section may be divided by subheadings. It should provide a concise and precise description of the experimental results, their interpretation, as well as the experimental conclusions that can be drawn.
| Ligands | Free Binding Energy | Interacting Amino Acids |
|---|---|---|
| PHA-680626 (Re-docked Native Ligand) | −9.0 kcal/mol | Arg57, Leu59, Ala80, Val114, Glu131, Leu132, Cys133, Arg134, Arg136, Phe183, Glu186 |
| HMC | −8.5 kcal/mol | Leu59, Gly60, Lys61, Gly62, Ala65, Lys66, Cys67, Ala80, Lys82, Val114, Glu131, Leu132, Cys133, Arg136, Ser137, Leu139, Glu140, Lys178, Gly180, Phe183 |
| Hesperetin | −7.8 kcal/mol | Leu59, Cys67, Ala80, Val114, Leu130, Glu131, Leu132, Cys133, Arg136, Ser137, Leu139, Glu140, Gly180, Phe183 |
*Hydrogen bonded amino acids are presented in bold.
![Interaction analysis of different ligands with PLK1. (a) Superimposition image of native ligand (brown) and re-docked native ligand (blue) after binding to PLK1 active site; (b) Superimposition image of all the ligands [native ligand (brown); re-docked native ligand (blue); HMC (magenta) and hesperitin (green)] after binding to PLK1 active site; (c-d) Interaction of re-docked native ligand with PLK1 and its LigPlot analysis; (e-f) Interaction of HMC with PLK1 and its LigPlot analysis; and (g-h) Interaction of hesperitin with PLK1 and its LigPlot analysis.](/content/184/2023/16/6/img/10.1016_j.arabjc.2023.104769-fig1.png)
- Interaction analysis of different ligands with PLK1. (a) Superimposition image of native ligand (brown) and re-docked native ligand (blue) after binding to PLK1 active site; (b) Superimposition image of all the ligands [native ligand (brown); re-docked native ligand (blue); HMC (magenta) and hesperitin (green)] after binding to PLK1 active site; (c-d) Interaction of re-docked native ligand with PLK1 and its LigPlot analysis; (e-f) Interaction of HMC with PLK1 and its LigPlot analysis; and (g-h) Interaction of hesperitin with PLK1 and its LigPlot analysis.
Similar approach was applied on another anti-cancer target i.e., EGFR. Table 2 and Fig. 2 shows interaction of native ligand (erlotinib), HMC and hesperetin with EGFR. Superimposition of native ligand and re-docked native ligand validated the protocol (Fig. 2a). However, superimposition of all the docked structures of EGFR confirmed that HMC and hesperetin have also bounded to the same site as native ligand (Fig. 2b). Native ligand showed binding energy of −7.4 kcal/mol and two hydrogen bonding (Met769 and Cys773) with EGFR (Table 2; Fig. 2c and d). Whereas HMC showed better binding energy of −9.3 kcal/mol with five hydrogen bonding (Lys721, Glu738, Met742, Met769 and Arg817) with EGFR (Table 2; Fig. 2e and f). Similarly, hesperetin also showed five hydrogen bonding (Lys721, Glu738, Leu764, Thr830 and Asp831) with EGFR; however, the binding energy was −8.5 kcal/mol (Table 2; Fig. 2g and h). The result indicated strong interaction of HMC with EGFR better than the native ligand and control hesperetin. In a 2017 study (Puranik and Srivastava, 2017), rugosafavonoid showed strong anticancer activity against breast cancer, and Met769 amino acid of EFGR was common in binding with all the derivatives of rugosafavonoid. In a subsequent investigation, Asn818, Lys721, Leu694, Val702 and Met742 were considered as crucial active site residues for EFGR binding with inhibitor (Yan et al., 2018). Recently, the importance of Glu738, Met742, Asp831 and Arg817 in binding with inhibitors has also been explored (Al-Warhi et al., 2022). Interestingly, HMC showed strong hydrogen bonding with Lys721, Glu738, Met742, Met769 and Arg817 that have been reported as crucial active site amino acids for EGFR.
| Ligands | Free Binding Energy | Interacting Amino Acids |
|---|---|---|
| Erlotinib (Re-docked native ligand) | −7.4 kcal/mol | Leu694, Val702, Ala719, Lys721, Leu764, Thr766, Gln767, Met769, Pro770, Phe771, Gly772, Cys773, Leu820, Thr830, Asp831 |
| HMC | −9.3 kcal/mol | Leu694, Phe699, Val702, Ala719, Lys721, Glu738, Met742, Leu764, Thr766, Leu768, Met769, Gly772, Arg817, Leu820, Thr830, Asp831, Gly833 |
| Hesperetin | −8.5 kcal/mol | Gly695, Phe699, Val702, Ala719, Ile720, Lys721, Glu738, Met742, Leu764, Thr766, Leu820, Thr830, Asp831 |
*Hydrogen bonded amino acids are presented in bold.
![Interaction analysis of different ligands with EGFR. (a) Superimposition image of native ligand (brown) and re-docked native ligand (blue) after binding to EGFR active site; (b) Superimposition image of all the ligands [native ligand (brown); re-docked native ligand (blue); HMC (magenta) and hesperetin (green)] after binding to EGFR active site; (c-d) Interaction of re-docked native ligand with EGFR and its LigPlot analysis; (e-f) Interaction of HMC with EGFR and its LigPlot analysis; and (g-h) Interaction of hesperetin with EGFR and its LigPlot analysis.](/content/184/2023/16/6/img/10.1016_j.arabjc.2023.104769-fig2.png)
- Interaction analysis of different ligands with EGFR. (a) Superimposition image of native ligand (brown) and re-docked native ligand (blue) after binding to EGFR active site; (b) Superimposition image of all the ligands [native ligand (brown); re-docked native ligand (blue); HMC (magenta) and hesperetin (green)] after binding to EGFR active site; (c-d) Interaction of re-docked native ligand with EGFR and its LigPlot analysis; (e-f) Interaction of HMC with EGFR and its LigPlot analysis; and (g-h) Interaction of hesperetin with EGFR and its LigPlot analysis.
Overall, in-silico results indicated that our target molecule 'HMC' could interact strongly with PLK1 as well as EGFR, and has a potential to develop into an anticancer molecule. It has been observed that in-silico findings, correlate-well with the wet lab investigations and provide a preliminary idea about the tested molecule. However, in vitro and in vivo validations are necessary to conclude any in-silico findings. Hence, HMC has been further tested against A549 cells and Ehrlich ascites carcinoma. In fact, there are several reports pertinent to the development of PLK1 and EGFR inhibitors against both these cancer types (Gunasekaran et al., 2022; Stratmann and Sebastian, 2019; Su et al., 2022). Thus, the further in vitro and in vivo investigation has its due clinical relevance.
3.2 In vitro cytotoxicity of HMC against A549 cells
To assess the inhibitory effects of HMC on the cell viability of A549 cells, the cells were exposed to serial dilutions of HMC (0, 6.25, 12.5, 25, 50 and 100 μM) for 24 h and the cell viability were evaluated by MTT assay and compared with that of hesperetin. As shown in Fig. 3A, HMC remarkably inhibited the growth of A549 in a dose-dependent manner. The cell viability of A549 cells was declined from 80.53 ± 3.17% to 12.78 ± 1.82% upon increasing HMC concentration from 6.25 to 100 µM. The calculated IC50 value of HMC against A549 cells was 51.16 ± 1.9 µM, which was comparable to that of a the cytotoxic agent, hesperetin (IC50 49.14 ± 1.4 µM). These results suggest the potent cytotoxic potential of HMC against non-small cell lung cancer A549 cells.
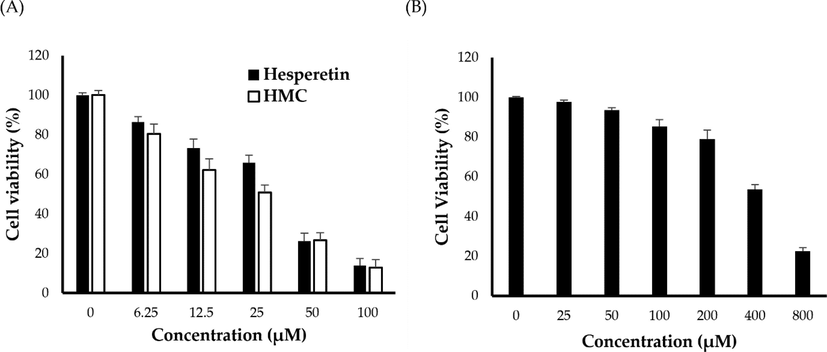
- (A) In vitro cytotoxicty of HMC and hesperetin against A549 cancer cells; (B) In vitro cytotoxicty of HMC against noncancerous murine alveolar macrophages J774A.1 cells.
Finding cytotoxic drugs with great selectivity for cancer cells is critical for improving the survival rates of cancer patients. Consequently, the cytotoxicity of HMC was screened against noncancerous murine alveolar macrophages J774A.1 cells, and the selectivity index was calculated. As shown in Fig. 3B, insignificant decrease in the viability of J774A.1 cells was observed upon treatment with lower HMC concentration (up to 200 μM). Nevertheless, at relatively higher concentrations (400 and 800 μM), HMC could elicit a pronounced cytotoxic effect against J774A.1 cells. The IC50 value of HMC against J774A.1 cells was 649.7 ± 23.4 μM. Most importantly, the calculated selectivity index (S.I.), obtained by dividing the average of the IC50 value in the noncancerous cell line (J774A.1) by the IC50 value in the cancer cell line (A549), was found 12.07, indicating the great selectivity of HMC towards cancerous cell line rather than normal noncancerous cells.
3.3 In vivo antitumor activity of HMC in Erlich ascites carcinoma (EAC) model
Ehrlich ascites carcinoma (EAC), a murine mammary adenocarcinoma, is a rapidly developing undifferentiated malignancy with aggressive behavior (Jaganathan et al., 2010), capable of growing in almost all mice strains (Mishra et al., 2018), and is widely employed in cancer research. In this study, in order to address the anti-tumor potential of HMC against EAC cells, changes in ascitic fluid volume of treated mice were assessed at the end of day 16. Generally, ascitic fluid is a crucial nutritional requirement for the growth of EAC cells, consequently, a rapid rise in ascitic fluid is considered a key indicator for tumor progression (Haldar et al., 2010). As depicted in Fig. 4A, treatment of EAC-bearing mice with HMC for 15 consecutive days significantly decreased ascitic fluid volume, compared to EAC untreated control mice. Furthermore, the antitumor effect of HMC, in terms of ascitic fluid volume, was similar to that of hesperetin.
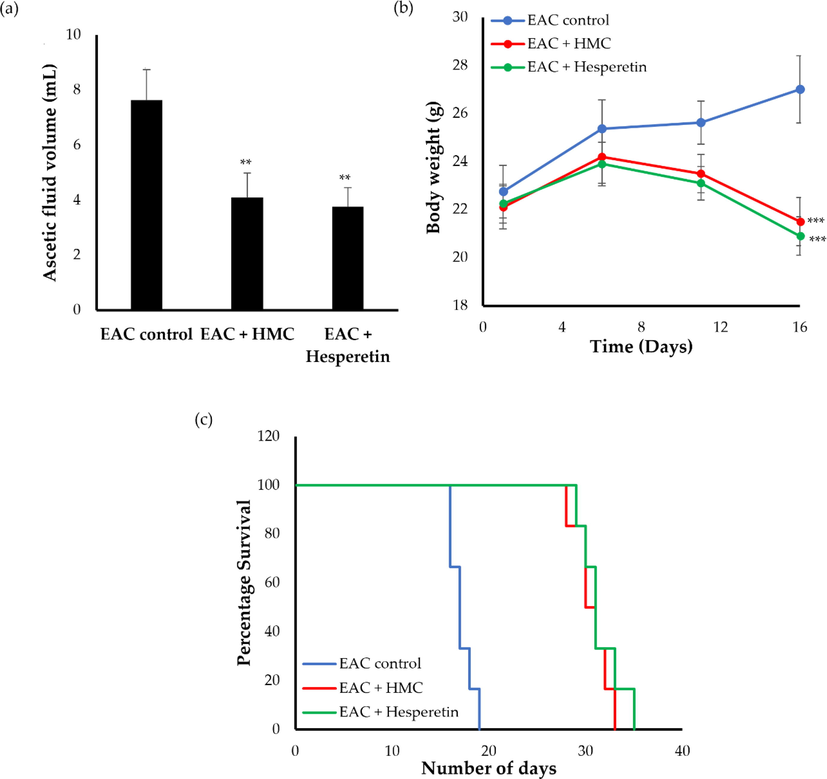
- Effect of HMC and hesperetin on (a) ascetic fluid volumes; (b) body weight and (c) median survival time in EAC-bearing mice. Data were expressed as mean ± SD (n = 6). **P < 0.01, ***P < 0.001 vs. EAC control mice.
Body weight of EAC-bearing mice is another important parameter for estimating the anticancer potential of MHC. Animal body weights were measured on a regular basis on predetermined days. As shown in Figure 4B, a remarkable rise in body weight of the animals was detected in positive control mice, presumably, due to the rapid and progressive accumulation of ascites tumor cells. On the other hand, a significant drop in in body weights was observed in animals treated with either HMC or hesperetin, compared to EAC control mice. The percent decrease in body weight observed upon treatment with either HMC or hesperetin were 20.37% and 22.59%, respectively.
A mounting body of evidences have revealed that prolongation of life span of tumor bearing animals, along with a decrease in tumor volume are considered reliable criteria for depicting the potential of any anticancer agent (Haldar et al., 2010; Dolai et al., 2012; Ghosh et al., 2011). Herein, treatment with HMC for 15 days significantly prolonged the survival of EAC-bearing mice, compared to EAC-bearing untreated mice (Fig. 4C). The median survival time of mice treated with either HMC or hesperetin significantly increased to 30.5 ± 1.1 days (ILS = 73.78%), and 32 ± 1.9 days (ILS = 78.41%), respectively, when compared to EAC control group (MST 17.5 ± 0.5 days). Collectively, HMC efficiently exerted a potent antitumor activity in EAC-bearing mice as evidenced by a significant decrease in tumor volume, tumor weight, along with a remarkable increase in life span, compared to EAC-bearing control mice.
3.4 Effects of HMC on hematological parameters
The most common complications associated with cancer treatment are myeloid suppression and anemia caused by a decrease in red blood cells (RBCs) or hemoglobin (Hb) content (Opare Kennedy et al., 2001). As summarized in Table 3, EAC-bearing mice had lower RBCs count (2.16 ± 0.34 million/mm3) and Hb content (4.66 ± 0.73 g/dL), which might be attributed, on the one hand, to the suppressive effect of EAC on bone marrow erythropoiesis (DeGowin and Gibson, 1978), and on the other hand, to iron deficiency in a myelopathic or hemolytic condition (Saad et al., 2017). On interest, EAC-bearing animals treated with HMC or the anticancer agent, hesperetin, showed significant increases (p < 0.001) in RBCs count and Hb content toward normal. RBCs counts in HMC- and hesperetin-treated mice were 8.56 ± 0.59 million/mm3 and 7.43 ± 0.62 million/mm3, respectively, and Hb contents in HMC- and hesperetin-treated mice were 10.66 ± 0.54 g/dL and 11.7 ± 0.57 g/dL, respectively. These enhancements clearly show that HMC has a protective effect on the haemopoietic system.
| Groups | Hb (g/dL) | RBC (1x106 /mm3) | WBC (1x103/mm3) |
|---|---|---|---|
| Normal control | 10.98 ± 0.89 | 7.61 ± 0.95 | 16.7 ± 0.61 |
| Cancer Control | 4.66 ± 0.73 | 2.16 ± 0.34 | 53.33 ± 1.77 |
| HMC | 10.66 ± 0.54*** | 8.56 ± 0.59*** | 15.03 ± 1.89*** |
| Hesperetin | 11.7 ± 0.57*** | 87.43 ± 0.62*** | 14.37 ± 1.31*** |
Data represent mean ± SD (n = 6). ***p < 0.001 vs EAC-bearing control mice.
One of the most significant criteria for assessing the therapeutic effectiveness of anticancer medications is their ability to lower white blood cells (WBCs) count in tumor-bearing mice. Generally, chemotherapy not only destroys rapidly divided cancer cells but could also kills some rapidly dividing normal cells in the body, such as those in bone marrow that make WBCs. Accordingly, a transient drop in WBC levels is generally observed with chemotherapy treatments. Herein, as depicted in Table 3, the level of WBCs was increased in EAC control mice (53330 ± 1770 cells/mm3). On the other hand, treatment with either HMC or hesperetin effectively (p < 0.001) restored WBCs count to the normal level, when compared to EAC control group. Collectively, these results evidently emphasized HMC had hematopoietic protective function without causing myelotoxicity, the most prevalent adverse effect of cancer treatment.
3.5 Effects of HMC on antioxidant parameters
Glutathione (GSH) and antioxidant enzymes, catalase (CAT) and superoxide dismutase (SOD), play vital roles in the antioxidant defense mechanisms, via mitigating the harmful effects of free radicals, during carcinogenesis (Rajkapoor et al., 2007; Singh and Ahluwalia, 2002). Imbalance in endogenous antioxidant enzymes along with increased free radical production are considered hallmarks in carcinogenesis (Szatrowski and Nathan, 1991). In this study, a significant decrease in hepatic antioxidants enzymes levels was detected in EAC-bearing control mice, suggesting the existence of an oxidative stress state, which might predispose hepatocytes injury (Table 4). Interestingly, treatment with either HMC or hesperetin significantly elevated GSH content (p < 0.01), CAT levels (p < 0.01), and SOD levels (p < 0.01) in liver tissues of the treated animals compared to EAC control mice (Table 4). The elevated levels of these enzymes can protect liver tissues from oxidative stress by scavenging superoxide radicals and the resulting H2O2; which constitute the substrates of SOD and CAT enzymes, respectively. Accordingly, these results emphasized the potential of HMC to reduce oxidative stress in EAC-bearing mice by regulating antioxidant levels. Similar findings were reported by Samudrala et al. who attributed the antitumor efficacy of Alternanthera brasiliana extract against Ehrlich ascites carcinoma to the potent antioxidant and hemoprotective nature of the extract.
| Groups | SOD (IU/mg protein) | GSH (mM/g) | Catalase (µM/mg protein) | Nitric oxide (mM/mL) |
|---|---|---|---|---|
| Normal Control | 1.85 ± 0.16 | 802.15 ± 24.1 | 0.043 ± 0.010 | 50.01 ± 3.8 |
| Cancer Control | 1.04 ± 0.19 | 153.6 ± 18.9 | 0.012 ± 0.005 | 21.19 ± 1.6 |
| HMC | 1.95 ± 0.11** | 754.75 ± 32.3** | 0.028 ± 0.007** | 43.00 ± 4.4** |
| Hesperetin | 1.99 ± 0.15** | 869.57 ± 48.8** | 0.035 ± 0.009** | 46.23 ± 2.5** |
All the values were expressed as mean ± SD (n = 6). **P < 0.01 vs. EAC-bearing control mice.
Nitric oxide (NO) plays a crucial role in both the progression and suppression of carcinogenesis depending on its concentration (Khan et al., 2020). Generally, a high concentration of NO may trigger cancer cell apoptosis, and thereby, inhibit tumor growth. On the other hand, at low concentration of NO, cancer growth and proliferation are promoted (Xu et al., 2002). In this study, NO levels were significantly depressed (21.19 ± 1.6 mM/mL) in EAC-bearing control group, which might support cancer growth and proliferation. On the other hand, treatment with HMC efficiently restored the level of NO to normal levels, as compared to the EAC-bearing control group (P < 0.01). The NO levels in both HMC- and hesperetin-treated mice were 43.00 ± 4.4 and 46.23 ± 2.5 mM/mL, respectively. Collectively, the antitumor efficacy of HMC might be attributed, at least in part, to the efficient antioxidant potential observed in EAC model.
4 Conclusions
In this study the anticancer activity of hesperidin methylated chalcone (HMC) was evaluated in silico against PLK1 and EGFR cancer targets, in vitro against A549 cancer cells and in vivo in Ehrlich ascites carcinoma (EAC) animal model. HMC showed strong interaction with the active site of both the cancer targets. HMC exhibited remarkable in vitro cytotoxicity against A549 cancer cell line. In addition, HMC therapy reduced tumor volume, tumor weight, and increased the life span of EAC-bearing mice. The potent antitumor activity detected in this model was correlated to the antioxidant potential of HMC, and was comparable to that of the reference agent, hesperetin. Furthermore, HMC showed a good safety profile as evidenced by restoring hematological profile count of RBCs, WBCs, and hemoglobin to the normal levels. Collectively, HMC might represent a novel candidate in cancer therapy with high antitumor efficiency and reasonable safety profile.
Acknowledgments
This research has been funded by Scientific Research Deanship at University of Ha’il - Saudi Arabia through project number RG-21 061.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
References
- Recent advances in tumor vasculature targeting using liposomal drug delivery systems. Expert. Opin. Drug Deliv.. 2009;6:1297-1309.
- [CrossRef] [Google Scholar]
- In vivo anti-tumour activity of novel Quinazoline derivatives. Eur. Rev. Med. Pharmacol. Sci.. 2012;16:1753-1764.
- [Google Scholar]
- Novel 2-(5-Aryl-4,5-Dihydropyrazol-1-yl)thiazol-4-one as EGFR inhibitors: synthesis, biological assessment and molecular docking insights. Drug Des. Devel. Ther.. 2022;16:1457-1471.
- [CrossRef] [Google Scholar]
- Advanced therapeutic approach for the treatment of malignant pleural mesothelioma via the intrapleural administration of liposomal pemetrexed. J. Control. Release. 2015;220:29-36.
- [CrossRef] [Google Scholar]
- Hesperetin exerts dose dependent chemopreventive effect against 1,2-dimethyl hydrazine induced rat colon carcinogenesis. Invest. New Drugs. 2009;27:203-213.
- [CrossRef] [Google Scholar]
- Absorption and elimination of (14C) hesperidin methylchalcone in the rat. Eur. J. Drug Metab. Pharmacokinet.. 1981;6:171-177.
- [CrossRef] [Google Scholar]
- Phytochemicals in cancer treatment: from preclinical studies to clinical practice. Front. Pharmacol.. 2019;10:1614.
- [CrossRef] [Google Scholar]
- Suppressive effects of an extramedullary tumor on bone marrow erythropoiesis and stroma. Exp. Hematol.. 1978;6:568-575.
- [Google Scholar]
- Evaluation of antitumor activity and in vivo antioxidant status of Anthocephalus cadamba on Ehrlich ascites carcinoma treated mice. J. Ethnopharmacol.. 2012;142:865-870.
- [CrossRef] [Google Scholar]
- Emam, S.E.; Ando, H.; Lila, A.S.A.; Shimizu, T.; Okuhira, K.; Ishima, Y.; Mahdy, M.A.; Ghazy, F.-e.S.; Sagawa, I.; Ishida, T. Liposome co-incubation with cancer cells secreted exosomes (extracellular vesicles) with different proteins expressions and different uptake pathways. Scientific Reports 2018, 8, 14493, doi:10.1038/s41598-018-32861-w
- Chalcone hybrids as potential anticancer agents: current development, mechanism of action, and structure-activity relationship. Med. Res. Rev.. 2020;40:2049-2084.
- [CrossRef] [Google Scholar]
- Evaluation of antitumor activity of stigmasterol, a constituent isolated from Bacopa monnieri Linn aerial parts against Ehrlich Ascites Carcinoma in mice. Orient. Pharm. Experimental Med.. 2011;11:41-49.
- [CrossRef] [Google Scholar]
- Antioxidant and anti-inflammatory effects of hesperidin methyl chalcone in experimental ulcerative colitis. Chem. Biol. Interact.. 2021;333:109315
- [CrossRef] [Google Scholar]
- An investigation of Plk1 PBD inhibitor KBJK557 as a tumor growth suppressor in non-small cell lung cancer. J. Anal. Sci. Technol.. 2022;13:36.
- [CrossRef] [Google Scholar]
- Habig, W.H.; Pabst, M.J.; Jakoby, W.B. Glutathione S-transferases. The first enzymatic step in mercapturic acid formation. J Biol Chem 1974, 249, 7130-7139
- Antitumor activity of Sansevieria roxburghiana rhizome against Ehrlich ascites carcinoma in mice. Pharm. Biol.. 2010;48:1337-1343.
- [CrossRef] [Google Scholar]
- Ferrocenyl chalcone derivatives as possible antimicrobial agents. J. Antibiot.. 2020;73:299-308.
- [CrossRef] [Google Scholar]
- Potentiating effect of ethnomedicinal plants against proliferation on different cancer cell lines. Curr Drug Metab.. 2018;19:584-595.
- [CrossRef] [Google Scholar]
- Cancer drug resistance: a fleet to conquer. J. Cell Biochem.. 2019;120:14213-14225.
- [CrossRef] [Google Scholar]
- Effect of honey and eugenol on Ehrlich ascites and solid carcinoma. J. Biomed. Biotechnol.. 2010;2010:989163
- [CrossRef] [Google Scholar]
- A modified spectrophotometric assay of superoxide dismutase. Indian J. Biochem. Biophys.. 1984;21:130-132.
- [Google Scholar]
- Advances in chalcones with anticancer activities. Recent Pat. Anticancer Drug Discov.. 2015;10:97-115.
- [CrossRef] [Google Scholar]
- The role of nitric oxide in cancer: master regulator or not? Int. J. Mol. Sci.. 2020;21:9393.
- [CrossRef] [Google Scholar]
- Shared risk factors in cardiovascular disease and cancer. Circulation. 2016;133:1104-1114.
- [CrossRef] [Google Scholar]
- Structure of the catalytic domain of human polo-like Kinase 1. Biochemistry. 2007;46:5960-5971.
- [CrossRef] [Google Scholar]
- Selective delivery of oxaliplatin to tumor tissue by nanocarrier system enhances overall therapeutic efficacy of the encapsulated oxaliplatin. Biol. Pharm. Bull.. 2014;37:206-211.
- [CrossRef] [Google Scholar]
- A novel chalcone derivative exerts anti-inflammatory and anti-oxidant effects after acute lung injury. Aging (Albany NY). 2019;11:7805-7816.
- [CrossRef] [Google Scholar]
- Chalcone derivatives: anti-inflammatory potential and molecular targets perspectives. Curr. Top Med. Chem.. 2017;17:3146-3169.
- [CrossRef] [Google Scholar]
- Dual drug conjugate loaded nanoparticles for the treatment of cancer. Curr. Drug Deliv.. 2015;12:782-794.
- [CrossRef] [Google Scholar]
- Subcutaneous Ehrlich Ascites Carcinoma mice model for studying cancer-induced cardiomyopathy. Sci. Rep.. 2018;8:5599.
- [CrossRef] [Google Scholar]
- Formulation, characterization, and cellular toxicity assessment of tamoxifen-loaded silk fibroin nanoparticles in breast cancer. Drug Deliv.. 2021;28:1626-1636.
- [CrossRef] [Google Scholar]
- Dithymoquinone analogues as potential candidate(s) for neurological manifestation associated with COVID-19: a therapeutic strategy for neuro-COVID. Life (Basel). 2022;12:1076.
- [CrossRef] [Google Scholar]
- Cancer prevention from the perspective of global cancer burden patterns. J. Thorac. Dis.. 2017;9:448-451.
- [CrossRef] [Google Scholar]
- Hesperidin, hesperidin methyl chalone and phellopterin from Poncirus trifoliata (Rutaceae) differentially regulate the expression of adhesion molecules in tumor necrosis factor-alpha-stimulated human umbilical vein endothelial cells. Int. Immunopharmacol.. 2008;8:670-678.
- [CrossRef] [Google Scholar]
- Growth inhibitory effect of green tea extract and (−)-epigallocatechin in Ehrlich ascites tumor cells involves a cellular thiol-dependent activation of mitogenic-activated protein kinases. Chem. Biol. Interact.. 2001;134:113-133.
- [CrossRef] [Google Scholar]
- Protective effects of the flavonoid hesperidin methyl chalcone in inflammation and pain in mice: role of TRPV1, oxidative stress, cytokines and NF-κB. Chem. Biol. Interact.. 2015;228:88-99.
- [CrossRef] [Google Scholar]
- First synthesis of rugosaflavonoid and its derivatives and their activity against breast cancer. RSC Adv.. 2017;7:33052-33060.
- [CrossRef] [Google Scholar]
- Antitumor and cytotoxic effects of Phyllanthus polyphyllus on Ehrlich Ascites carcinoma and human cancer cell lines. Biosci. Biotech. Bioch.. 2007;71:2177-2183.
- [CrossRef] [Google Scholar]
- Role of phytochemicals in cancer prevention. Int. J. Mol. Sci.. 2019;20:4981.
- [CrossRef] [Google Scholar]
- A simple click by click protocol to perform docking: AutoDock 4.2 made easy for non-bioinformaticians. EXCLI J.. 2013;12:831-857.
- [Google Scholar]
- A systematic review on anti-diabetic properties of chalcones. Curr. Med. Chem.. 2020;27:2257-2321.
- [CrossRef] [Google Scholar]
- Saad, E.A.; Hassanien, M.M.; El-Mezayen, H.A.; NM, E.L. Regression of murine Ehrlich ascites carcinoma using synthesized cobalt complex. Medchemcomm 2017, 8, 1103-1111, doi:10.1039/c6md00618c
- Molecular and enzoinformatics perspectives of targeting Polo-like kinase 1 in cancer therapy. Semin. Cancer Biol.. 2019;56:47-55.
- [CrossRef] [Google Scholar]
- Studies on the effect of monosodium glutamate (MSG) administration on the activity of xanthine oxidase, superoxide dismutase and catalase in hepatic tissue of adult male mice. Indian J. Clin. Biochem.. 2002;17:29-33.
- [CrossRef] [Google Scholar]
- Recent developments in biological activities of chalcones: a mini review. Eur. J. Med. Chem.. 2014;85:758-777.
- [CrossRef] [Google Scholar]
- Polo-like kinase 1 inhibition in NSCLC: mechanism of action and emerging predictive biomarkers. Lung Cancer (Auckl). 2019;10:67-80.
- [CrossRef] [Google Scholar]
- PLK1 inhibition-based combination therapies for cancer management. Transl. Oncol.. 2022;16:101332
- [CrossRef] [Google Scholar]
- Production of large amounts of hydrogen peroxide by human tumor cells. Cancer Res.. 1991;51:794-798.
- [Google Scholar]
- Chemopreventive effect of chalcone derivative, L2H17, in colon cancer development. BMC Cancer. 2015;15:870.
- [CrossRef] [Google Scholar]
- Anti-breast cancer activity of selected 1,3,5-triazines via modulation of EGFR-TK. Mol. Med. Rep.. 2018;18:4175-4184.
- [CrossRef] [Google Scholar]
- Diverse molecular targets for chalcones with varied bioactivities. Med. Chem. (Los Angeles). 2015;5:388-404.
- [CrossRef] [Google Scholar]







