Translate this page into:
The preventive effect of secondary metabolites of Dendrobium officinale on acute alcoholic liver injury in mice
⁎Corresponding authors. qinlin1115@163.com (Lin Qin), yqhe.pharm@foxmail.com (Yu-qi He)
-
Received: ,
Accepted: ,
This article was originally published by Elsevier and was migrated to Scientific Scholar after the change of Publisher.
Peer review under responsibility of King Saud University.
Abstract
Dendrobium officinale (D. officinale) is a valuable Chinese herbal medicine and rich in chemical components. The current research on the pharmacological active ingredients of D. officinale mainly focus on polysaccharides, however, there are very few studies on the activity of its secondary metabolites. Our study aimed to investigate the potential pharmacological activity of secondary metabolites of D. officinale. Firstly, we determined the polysaccharide content of D. officinale from four different origins and analyzed their secondary metabolites using UPLC Q-TOF/MS. We found that the average polysaccharide content of D. officinale from Anlong, Guizhou was the lowest, and the secondary metabolites were also significantly different from those of Dendrobium from the other three origins. Then, ten differential compounds with high content in D. officinale from Anlong, Guizhou were identified using Orthogonal Projection to Latent analysis (OPLS-DA), and the potential targets of these secondary metabolites associated with acute alcoholic liver injury through network pharmacology. Finally, pharmacodynamic experiments verified that only D. officinale from Anlong significantly improved liver damage in mice with acute alcoholic liver injury, confirming the pharmacodynamic activity of D. officinale secondary metabolites in the prevention of acute alcoholic liver injury. The present study found that the preventive effect of D. officinale on acute alcoholic liver injury in mice was not directly correlated with the polysaccharide content, indicating that the polysaccharide content alone cannot be used as a quality indicator to evaluate the medicinal activity of D. officinale. Our study provides more references for the study of the substance basis of the pharmacological effect of D. officinale, and also provides a research idea and method for the discovery of the pharmacological active ingredients of Chinese medicine.
Keywords
Dendrobium officinale
Secondary metabolites
Network Pharmacology
Acute alcoholic liver injury
1 Introduction
Dendrobium officinale Kimura et Migo is a perennial epiphytic herb, whose main medicinal part is the stem. According to the records of “Shennong’s Herbal Classic” (Dong Han Dynasty, A.D.25–220) and “Compendium of Herbology” (Ming Dynasty, A.D. 1,552–1,578), D. officinale is mainly used as traditional medicine in China with the effect of “removing paralysis and lowering qi, and tonify five viscera, relieve fatigue” And modern research has found that D. officinale has pharmacological effects such as enhancing immunity, anti-fatigue, antioxidant, hypoglycemia and hypotension (Chang et al., 2019; Committee et al., 2020; Zeng et al., 2020). D. officinale can reduce renal pathological damage and improve renal function in diabetic patients (Chang et al., 2019). Moreover, D. officinale has a protective effect on the liver, which can prevent carbon tetrachloride-induced liver injury in mice (Li et al., 2014), and also reduce hepatic fat storage ameliorating high-fat diet (HFD)-induced non-alcoholic fatty liver injury in mice (Yin et al., 2021).
However, the place of cultivation and cultivation techniques of D. officinale can affect its quality (Zuo et al., 2020). This has led to the uneven quality of D. officinale in the market, which hinders the drug development and clinical application of D. officinale. Moreover, the chemical composition of D. officinale is complex, containing a wide range of compounds such as polysaccharides, bibenzyls, phenanthrenes, alkaloids, amino acids and trace element (Chen et al., (2021);Li et al., 2009; Wang et al., 2021), and the pharmacological activity of these chemical components is still unclear. Although D. officinale polysaccharides have been shown to regulate intestinal homeostasis and protect against carbon tetrachloride-induced liver injury in mice (Wang et al., 2020). The Chinese Pharmacopoeia (Ch.P., 2020 edition), uses polysaccharide content as a criterion for evaluating the quality of D. officinale, however, D. officinale is rich in secondary metabolites in addition to polysaccharides.
Secondary metabolites (SMs) are an important component of the plant's defence systems against pathogenic attacks and environmental stresses. Secondary metabolites of plants have been shown to have significant biological activity and can be used as pharmaceutical ingredients, among which secondary metabolites such as perillylamine and paclitaxel have been found to have anticancer activity and have been successfully developed as clinical anticancer drugs (Newman et al., 2016; Weaver et al., 2014). At the same time, the biosynthesis and accumulation of secondary metabolites are also susceptible to external environmental factors such as light and temperature (Yang et al., 2018). However, it is still unclear whether secondary metabolites in D. officinale have medicinal effects and play a role in the prevention and treatment of diseases. This research aims to explore the association between secondary metabolites of D. officinale and its protection activities against acute alcoholic liver injury. In this study, the polysaccharide contents of different D. officinale samples were detected. Meanwhile, the secondary metabolites were detected and analyzed through profile analysis. Further, potential mechanism of significant secondary metabolites of D. officinale were predicted and screened based on network pharmacology. Further, the prevention activities of D. officinale samples were conducted and validated though mouse model of acute alcoholic liver injury. The study can be a useful supplement to the current quality standard of D. officinale, which is only based on polysaccharides, and provide a new reference for more comprehensive evaluation of D. officinale quality, which is important for guiding the cultivation, quality evaluation and clinical use of D. officinale.
2 Materials and methods
2.1 Chemicals and reagents
D. officinale samples were collected from the cultivation bases of Anlong, Guizhou, Xingyi, Guizhou, Dushan, Guizhou, and Yueqing, Zhejiang, and the collected samples were identified as Dendrobium officinale Kimura et Migo. Concentrated sulphuric acid (AR grade) purchased from Sinopharm Chemical Reagent Co. Phenol (AR grade) and anhydrous ethanol (AR grade) was purchased from Chengdu Kellen Chemical Reagent Factory. Glucose (purity > 99%) was purchased from Sigma-Aldrich (St. Louis, MO, USA). Acetonitrile (LC-MS grade) and methanol (LC-MS grade) were purchased from TEDIA Reagents Co, USA. Formic acid (LC-MS grade) was purchased from Shanghai Ampoule Experimental Technology Co. Silymarin (purity ≥ 98%) was purchased from Shanghai Yuanye Biotechnology Co. Glutathione aminotransferase (ALT/GPT) and glutathione aminotransferase (AST/GOT) kits were purchased from Nanjing Jiancheng Institute of Biological Engineering.
2.2 Extraction and content determination of polysaccharides in D. officinale samples
D. officinale samples were divided into four groups according to the origin of their production (n AL = 171, n DS = 71, n XY = 53, n YQ = 27). Approximately 0.6 g of D. officinale powder (sieved by No.3) was weighed precisely, then 400 mL of distilled water was added for 2 h reflux extraction, let the extract cool down and fix the volume with distilled water to 500 mL, shake it and filter it through, take 2 mL of the renewed filtrate and add 10 mL of anhydrous ethanol, shake it and refrigerate it for 1 h, centrifuge and discard the supernatant, wash the precipitate with 80% ethanol twice, 8 mL each time, centrifuge and discard the supernatant. The precipitate was dissolved by heating water to obtain the aqueous solution of D. officinale polysaccharide, and then the polysaccharide content was determined by phenol–sulfuric acid method using D-glucose as the control (Masuko et al., 2005).
2.3 UPLC-Q/TOF-MS analysis of secondary metabolites of D. officinale
D. officinale samples were divided into four groups according to the origin of their production (n AL = 171, n DS = 71, n XY = 53, n YQ = 27). Approximately 75 mg of D. officinale powder (sieved by No.3) was weighed precisely, then 1.0 mL of 70% methanol was added for 30 min of ultrasonic extraction (power 400 W, frequency 50 kHz), the extracts were then centrifuged at 12,000 rpm for 5 min and the supernatant was aspirated for determination.
The gradient elution was carried out on octadecylsilane bonded phase silica gel (Waters CORTECS UPLC C18, 100 mm × 2.1 mm, 1.6 μm) as stationary phase and 0.1 % formic acid water (A) − 0.1 % formic acid acetonitrile (B) as mobile phase for the sample solution to be tested, with the following elution gradients: 0–0.5 min, 5% B; 0.5–4 min, 40% B; 4––5 min, 75% B; 5–5.1 min, 95% B; 5.1–6.5 min, 95% B; 6.5–6.6 min, 5% B; 6.6–10 min, 5% B. The flow rate was 0.4 mL/min, the injection volume was 1 μL, the column temperature was 40 °C and the UV detection wavelengths were set at 280 nm, 254 nm and 210 nm. The TOF/MS system used electrospray ionisation (ESI) mode to acquire the primary mass spectrometry information separately, with a capillary voltage (CV) of 4000 V, an atomisation gas pressure of 45 psi, a cone hole voltage (NV) of 1000 V, a dry gas flow rate of 10 L/min, a sheath gas temperature and ion source temperature of 350 °C, a sheath gas flow rate of 11 L/min and a collision energy (CE) of 10 V. The secondary mass spectral information was acquired using an automated mass spectrometry/mass spectrometry mode scan with an ion scan range of m/z 50 to 1200.
2.4 Predicting the potential disease targets of the differential chemical composition of D. officinale
The PubChem database (https://www.ncbi.nlm.nih.gov/pccompound/), Scifinder database were searched to obtain the 2D structures of the differential chemical components of D. officinale and the molecular descriptors SMILES of the compounds were recorded, the compound structure files were downloaded and saved as “*.mol2″ format. The SEA database (https://sea.bkslab.org/) and Swiss Target Prediction database (https://www.swisstargetprediction.ch/) were then used to simulate the potential targets of action of the differential secondary metabolites: (1) Input the molecular descriptor SMILES of the retrieved compounds into the SEA database, retrieve the disease targets, and set the parameters P < 0.05 and species as Human to filter the retrieved results; (2) the structure files of the compounds were imported into Swiss Target Prediction database and the targets of secondary metabolites of D. officinale were searched, and the parameter probability (probability) > 0 was set to screen the targets of the different chemical components. Finally, the search results of the two databases were combined to obtain the disease targets of the secondary metabolites.
Using “Acute alcoholic liver injury” as the keyword, we searched the Gene Cards database (https://www.genecards.org/) and the DisGeNET database (https://www. disgenet.org/) to search for disease genes related to Acute Alcoholic Liver Injury (AALI), and the data obtained from the two databases were combined and duplicate values were removed to construct a database of disease targets for AALI.
2.5 GO function annotated analysis
The bioinformatics platform WebGestalt (https://www.webgestalt.org/option.php) was used for GO functional annotation of potential targets of acute alcoholic liver injury acting on differential compounds, with the source set as “Homo sapiens” and the statistical method as “ORA”, the confidence interval for the BH statistic was FDR < 0.01, and the minimum number of proteins per GO enrichment was 5 (n ≥ 5).
2.6 Preparation of D. officinale extract
Weigh 63 g of the dried D. officinale, add 30 times the volume of distilled water (m/v) and soak overnight, then boil and reflux the extract for 1 h. Repeat the extraction twice, filter the extracted liquid through gauze and concentrate the filtrate to 70 mL under reduced pressure using a rotary evaporator to obtain D. officinale extract at a concentration of 0.9 g/mL, dispense and store at −80℃ in a refrigerator.
D. officinale extract of 0.9 g/mL was measured and diluted to 50 mL with distilled water, and then shaken well to obtain a solution of D. officinale extract with a concentration of 0.18 g/mL.
2.7 Animal experiments
Male C57BL/6J mice weighing 22 g to 25 g at 8 weeks of age were purchased from Hunan Slaughter Jingda Laboratory Animal Co Ltd (license: SCXK (Xiang) 2019–0004). The mice were fed in an SPF (specific pathogen free) class environment (room temperature 22 ± 1 ℃, humidity 55% ± 5%) with a 12-hour photoperiod, and the mice were free to drink and eat during the feeding period. Mice were randomly divided into 11 groups after one week of acclimatization feeding, with 10 mice in each group as follows: blank control group (BG), alcohol model group (AG), silymarin group (SFJB), Guizhou Anlong D. officinale high and low dose group (AL-H, AL-L), Guizhou Dushan D. officinale high and low dose group (DS-H, DS-L), Guizhou Xinyi D. officinale high and low dose group (XY-H, XY-L), Zhejiang Yueqing D. officinale high and low dose group (YQ-H, YQ-L). The BG and AG groups were fed with distilled water once a day, the SFJB group was fed with silymarin once a day (0.005 g/mL), and each D. officinale group was fed once a day with Dendrobium extract of the corresponding origin at a dose of 10 mL/kg, with the concentration of Dendrobium extract in the high dose group being 0.9 g/mL and that in the low dose group being 0.18 g/mL. The feeding was continuously for 14 days. After 14 days, each group, except the BG group, was given 53% ethanol (10 mL/kg) once, and the mice were fasted without water for 12 h. 1 ∼ 2 mL of blood was removed from the eyes, and the serum was collected for the determination of serum transaminases. About 100 mg of the middle part of the liver lobules of mice were taken and fixed in 10% formaldehyde solution for histopathological examination.
2.8 Histopathological evaluation
HE staining was used to observe the pathological changes in the liver. Liver lobule samples were fixed in 10% formaldehyde, dehydrated in alcohol and xylene in turn. The dehydrated liver lobule samples were embedded in paraffin and cut into 6–––8 μm slices using a microtome, then stained with hematoxylin solution and eosin solution, respectively. After staining, the samples were dehydrated with 80%, 90% and 100% ethanol in that order and finally sealed with coverslips for microscopic observation.
2.9 Serum transaminase assay
The mouse serum alanine aminotransferase (ALT/GPT) and aspartate aminotransferase (AST/GOT) kits purchased from Nanjing Jiancheng Institute of Biological Engineering were used for the determination of mouse serum aminotransferase (Enzymatic assay method) according to the manufacturing instructions of the kits.
2.10 Statistical analysis
Peak matching and alignment of the raw data was performed using Agilent Mass Hunter Profinder software (version 10.0). Principal component analysis (PCA) and Orthogonal partial least squares discriminant analysis (OPLS-DA) were performed in SIMCA-P software (version 14.0). Box line plots, heat maps were plotted in the R program (version 4.1.0) and data were expressed as mean ± SEM and compared between two groups using an independent samples t-test with SPSS Statistics 18.0 (IBM, Chicago, USA) and a one-way ANOVA comparing differences between multiple groups, p < 0.05 was considered statistically significant.
3 Results
3.1 The average polysaccharide content of D. officinale from AL was the lowest.
The average polysaccharide content of D. officinale from the four origins was in the following order: YQ > XY > DS > AL, among which the average polysaccharide content of D. officinale from the YQ group was the highest, 41.62%±0.24% and the average polysaccharide content from the AL group was the lowest, 15.32%±0.05%. There was a significant difference in the polysaccharide content of D. officinale from the four origins (P < 0.05) (Fig. 1).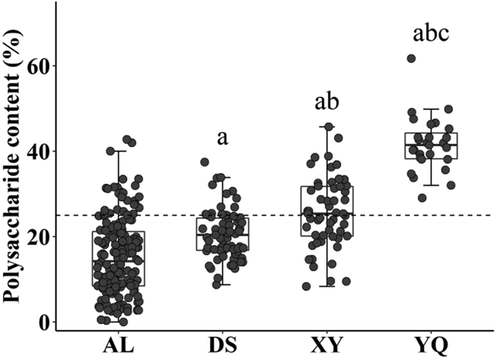
Significant differences in the polysaccharide content of D. officinale from different origins. AL represents D. officinale samples from Anlong, Guizhou, XY represents D. officinale samples from Xingyi, Guizhou, DS represents D. officinale samples from Dushan, Guizhou and YQ represents D. officinale samples from Yueqing, Zhejiang. a P < 0.05, compared with the AL group; b P < 0.05, compared with the DS group; c P < 0.05, compared with the XY group. n AL = 171, n DS = 71, n XY = 53, n YQ = 27.
3.2 Differences in secondary metabolites of D. officinale from different origins
The secondary metabolites of D. officinale from four different origins were analysed separately using UPLC-Q/TOF-MS technique. The chromatograms in positive and negative mode (BPC) showed that the retention times of the peaks were mainly concentrated in the range of 1 ∼ 8 min (Fig. 2A, C), and the chromatogram profiles of the D. officinale in the AL group differed from those of the other three origins (Fig. 2B, D). Then PCA analysis was performed on the the D. officinale from the four origins with the peak area of each ion indicating the content of the corresponding component (Fig. 2E). In the PCA plots, there was a large overlap between the D. officinale samples from the four different origins, but the D. officinale samples from the AL group were the most discrete and could be distinguished from the D. officinale samples from the other three regions (YQ, XY, DS) in terms of their overall chemical profile. It indicates that the secondary metabolites of D. officinale from the AL group as a whole differed from the samples from the other three regions.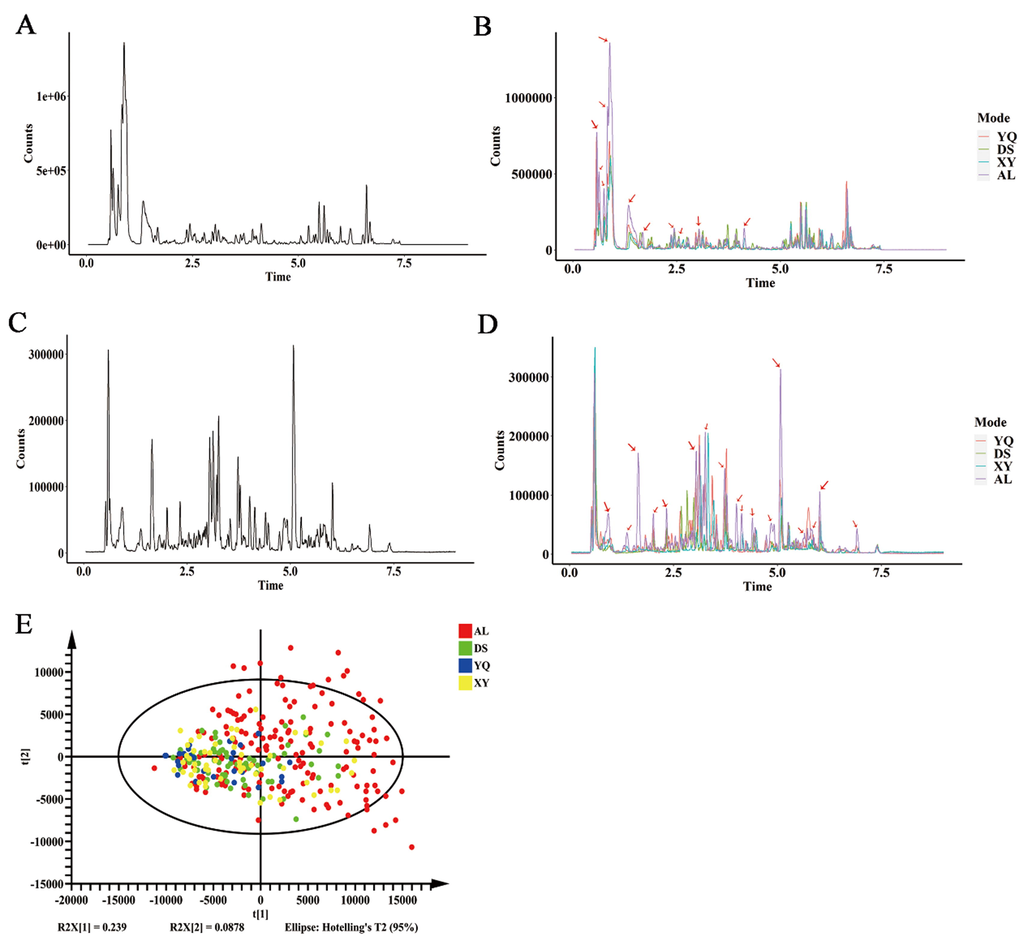
The overall profile of the secondary metabolites of D. officinale in the Anlongu differs markedly from that of the other three regions. (A) Positive ion BPC model total ion flow diagram of D. officinale. (B) Positive ion BPC model total ion flow map of four origins of D. officinale. (C) Total ion flow diagram of D. officinale in negative ion BPC mode. (D) Plot of total ion flow in D. officinale from four origins in the negative ion BPC model. (E) Principal component analysis of the differences in the overall profiles of secondary metabolites of D. officinale from different origins. AL represents D. officinale samples from Anlong, Guizhou, XY represents D. officinale samples from Xingyi, Guizhou, DS represents D. officinale samples from Dushan, Guizhou and YQ represents D. officinale samples from Yueqing, Zhejiang. n AL = 171, n DS = 71, n XY = 53, n YQ = 27.
3.3 67 secondary metabolites differed between D. officinale AL origin and the other three regions
Supervised partial least squares discriminant analysis (OPLS-DA) was used to compare the overall profile of secondary metabolites of the AL D. officinale samples (n = 171) with other three regions D. officinale samples (n = 151) (Fig. 3A). In the OPLS-DA plots, the points representing the AL group of D. officinale samples clustered together and the points representing all other non-AL group of D. officinale. In addition, with R2X = 0.453, Q2 = 0.814 and Q2 > 0.4 at the 95% confidence interval, the OPLS-DA has good confidence, indicating that the AL D. officinale samples differ from the other origins in the overall profile of secondary metabolites.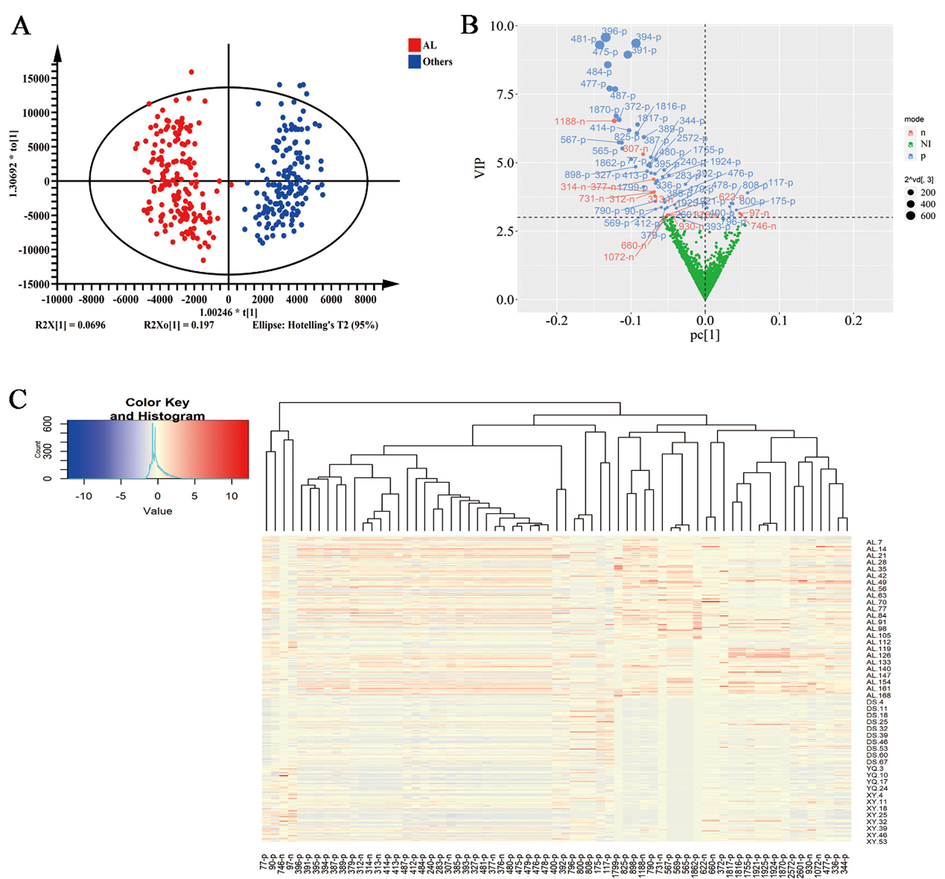
Screening the differential chemical components of AL D. officinale by OPLS-DA. (A) OPLS-DA analysis. (B) VIP map analysis of secondary metabolites. (C) The differences in the content of 67 different chemical constituents in D. officinale from different productions. AL represents D. officinale samples from Anlong, Guizhou, XY represents D. officinale samples from Xingyi, Guizhou, DS represents D. officinale samples from Dushan, Guizhou and YQ represents D. officinale samples from Yueqing, Zhejiang, the others group represents all samples of D. officinale from the three production areas of Xingyi, Guizhou, Dushan, Guizhou and Yueqing, Zhejiang. n AL = 171, n DS = 71, n XY = 53, n YQ = 27.
Further screening of the differential secondary metabolites of D. officinale origin using VIP plots (Fig. 3B) revealed 67 differential secondary metabolites with VIP scores>3, and most of which were detected in positive ion mode. Moreover, the thermogram (Fig. 3C) analysis revealed that the abundance of these 67 secondary metabolites differed between D. officinale AL origin and other non-AL regions, and most of them were in higher abundance in D. officinale AL origin and may be important marker components of D. officinale AL.
3.4 These 10 high content of secondary metabolites may be specific chemical components of D. officinale from AL group
The structural identification of 67 differential secondary metabolites in D. officinale AL were performed, and a total of 37 differential secondary metabolites were identified by removing false positive results as well as fragment ions. Due to the lack of corresponding reference compounds, the chemical structures of the 11 differential secondary metabolites were finally determined and the other components could only be identified as isomers of their corresponding predicted structures (See Table 1, Fig. 4), with five secondary metabolites detected in the cationic mode and six in the anionic mode. The chemical types of the differential secondary metabolites were mainly fatty acids (Pinellic acid, Hydroxy-α-linolenic acid), sugars (Sucrose, Raffinose), flavonoids (Vicenin 3, Apigenin-7-rutinoside), alkaloids (2-Propen-1-yl2-(acetylamino)-2-deoxy-3-O-β-D-galactopy-ranosyl-6-O-methyl-α-D-galactopyranoside, Mycosporine, 6O-L-isoleucyl-sucrose), amino acids (L-tert-Leucine).
No
ID
tR
Adduct ions
m/z(Theoretical)
m/zMeasured
VIP
Error (ppm)
Molecular formula
Chinese name
Identification
1
77-p
0.57
[M + K]+
381.0816
381.0785
4.9094
−2.17
C12H22O11
蔗糖
Sucrose
2
413-p
0.936
[M + H]+
438.1970
438.1972
4.6199
0.21
C18H31NO11
NA
2-Propen-1-yl2-(acetylamino)-2-deoxy-3-O-β-D-galactopyranosyl-6-O-methyl-α-D-galactopyranoside
3
414-p
0.936
[M + H]+
456.20755
456.2074
6.1807
−0.46
C18H33NO12
NA
6-O-L-isoleucyl-sucrose
4
97-n
0.58
[M−H]-
503.1618
503.1613
3.1509
−0.5
C18H32O16
棉子糖
Raffinose
5
376-n
1.310
[M−H]-
147.0452
147.0451
3.1025
−0.29
C9H6O2
香豆素
Coumarin
6
731-n
3.1
[M−H]-
577.1563
577.1553
3.9127
−2.62
C27H30O14
芹菜素-7-O-芸香糖苷
Apigenin-7-rutinoside
7
283-p
0.755
[M + H]+
262.1285
262.1291
4.3448
2.24
C11H19NO6
NA
Mycosporine
8
307-n
0.884
[M−H]-
130.0874
130.0875
5.3095
0.80
C6H13NO2
L-叔亮氨酸
L-tert-Leucine
9
660-n
2.951
[M−H]-
563.14063
563.1398
3.0751
−1.57
C26H28O14
维采宁-3(芹菜素-6-C-β-D-葡萄糖-8-C-β-D-木糖苷)
Vicenin 3
10
1188-n
5.052
[M−H]-
329.23335
329.2329
6.5286
−1.49
C18H34O5
NA
Pinellic acid
11
1816-p
5.61
[M + H]+
295.2274
295.2274
6.0715
0.6
C18H30O3
羟基亚麻酸
Hydroxy-α-linolenic acid
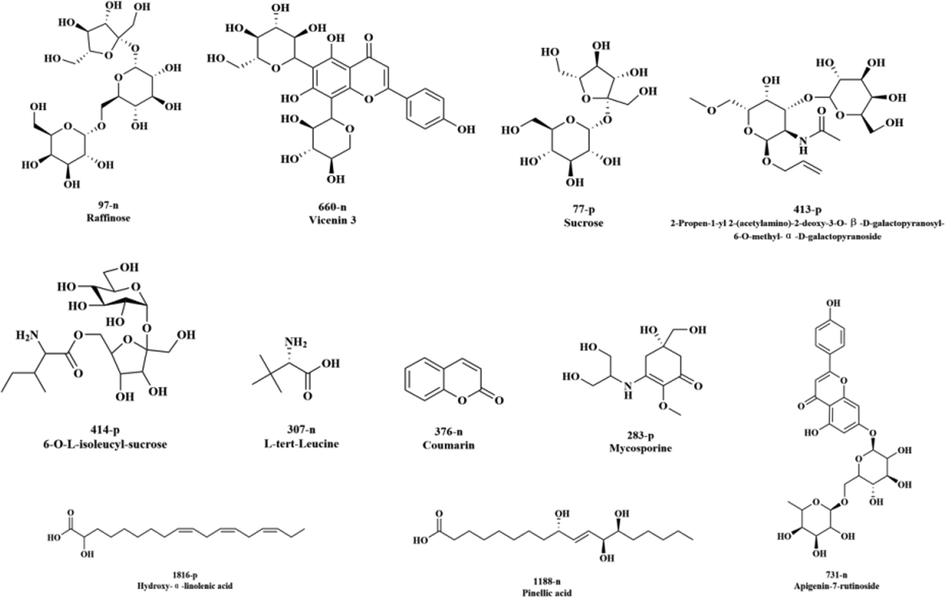
Structure of the differential chemical composition of D. officinale.
The 11 differential secondary metabolites with identified structures were analyzed for their contents differences in D. officinale samples from four different origins (Fig. 5). The content of Raffinose was lowest in D. officinale AL and significantly lower than in D. officinale samples from YQ and XY (P < 0.05). Except for Raffinose, the content of the other 10 secondary metabolites was the highest in D. officinale AL, which were all significantly higher than those from the other three regional sources (P < 0.05), indicating that these 10 high levels of secondary metabolites may be the main components that distinguish it from other regions and may be specific chemical components of D. officinale AL.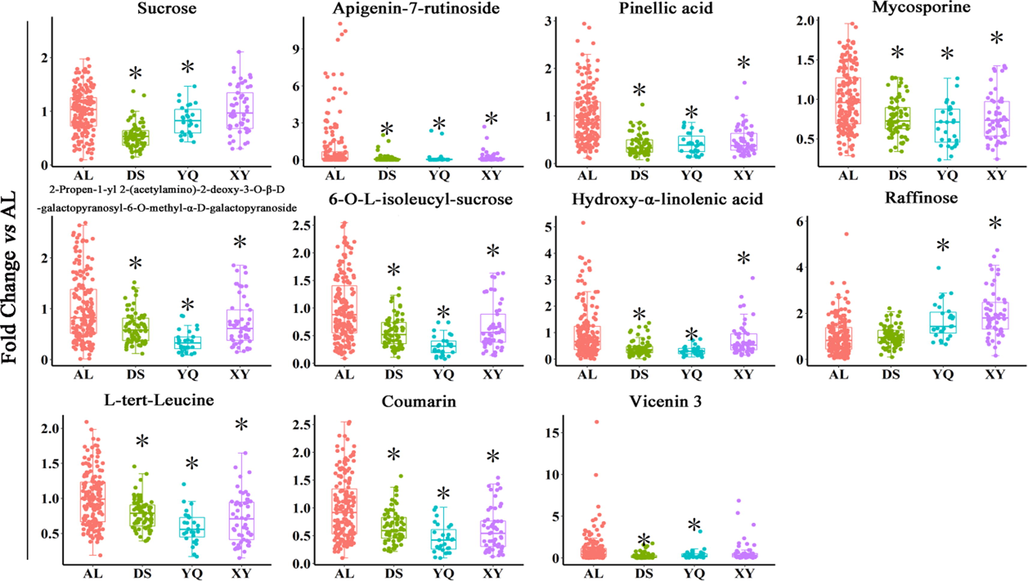
The content of the differential chemical constituents in D. officinale AL was significantly different from that of the other three origins of D. officinale. AL represents D. officinale samples from Anlong, Guizhou, XY represents D. officinale samples from Xingyi, Guizhou, DS represents D. officinale samples from Dushan, Guizhou and YQ represents D. officinale samples from Yueqing, Zhejiang. *P < 0.05, compared with the AL group. n AL = 171, n DS = 71, n XY = 53, n YQ = 27.
3.5 Potential targets for the action of differential chemical components of D. officinale mainly act on acute alcoholic liver injury
After literature research and database search, the 10 differential chemical components in D. officinale AL could potentially act on 373 targets, and most of these targets were associated with acute alcoholic liver injury. A total of 1248 targets related to acute alcoholic liver injury were obtained from the gene database search, and these targets were further compared with the potential targets of the differential chemical components of D. officinale and found that 123 targets out of 373 targets of the potential effects of the 10 differential chemical components were associated with acute alcoholic liver injury.
A “compound-target (C - T)” network was constructed to reveal the relationship between the differential metabolites and potential targets of D. officinale (Fig. 6), with the lines between the nodes representing the potential relationships between compounds and targets. The C-T network shows that one target is linked to multiple chemical components, suggesting that these chemical components may play a synergistic role in the prevention of acute alcoholic liver injury; the same chemical component is linked to multiple target genes, with each compound acting on an average of 57 potential targets, reflecting that the pharmacological activity of the differential metabolites of D. officinale against acute alcoholic liver injury may be related to the synergistic mechanism of “multi-component, multi-target”.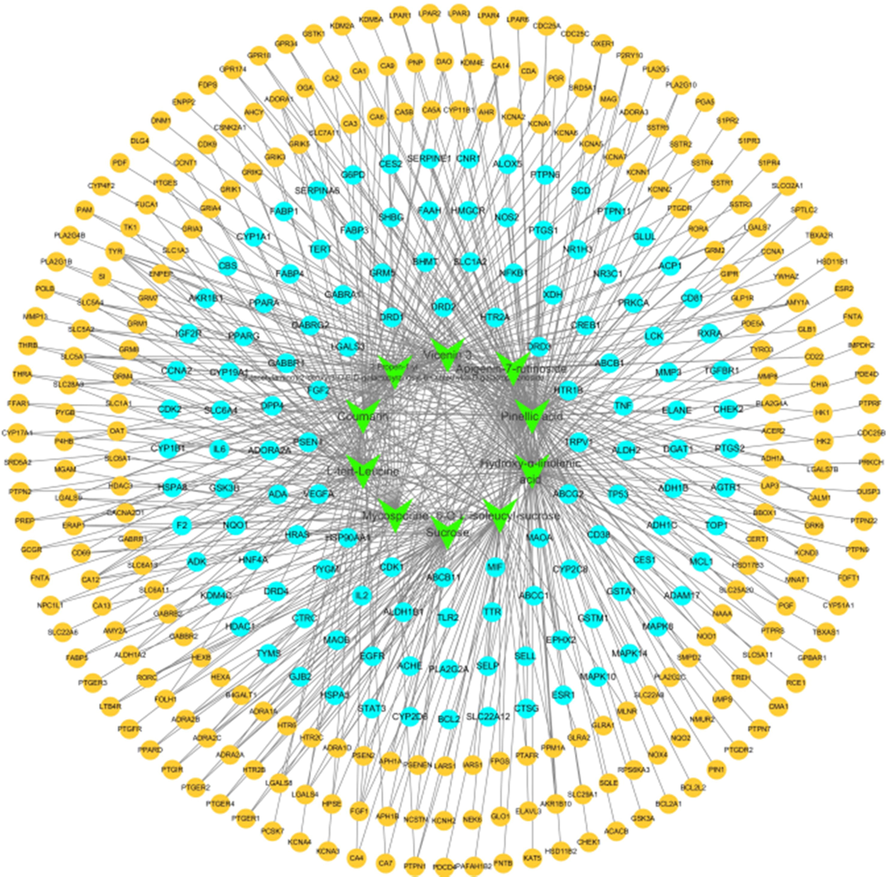
Potential targets of differential chemical action of D. officinale associated with acute alcoholic liver injury. The green diamond nodes represent differential compounds in D. officinale, the blue circular nodes represent disease targets related to acute alcoholic liver injury, the orange circular nodes represent other disease targets, and the lines between the nodes represent potential relationships of compounds to the targets.
The 123 acute alcoholic liver injury targets involved a total of 966 BP entries (Biological Progress) (FDR < 0.01, n ≥ 5) and we show the top 40 GO entries (Fig. 7A). Meanwhile, we constructed a “compound – target - GO function” network to further reveal the interactions between compounds and targets of acute alcoholic liver injury and related biological mechanisms (Fig. 7B). Edges represent the association between the target and GO function, while 179 black edges represent the interaction between the compound and the target. GO functions were ranked according to the size of the CD, and most of the compound targets were found to be enriched in response to oxidation (GO: 1901700), drug response (GO: 0042493), small molecule metabolic processes (GO: 0044281), cell proliferation (GO: 0008283), transport regulation (GO: 0051049), secretion regulation (GO: 0046903) and other GO functions (See Table 2). These biological pathways are closely related to liver health, involving oxidative stress, immune regulation, cellular metabolism and other biological processes related to acute alcoholic liver injury, indicating that the potential targets of D. officinale differential chemical composition acting on acute alcoholic liver injury are related to the acute alcoholic liver injury, and secondary metabolites may be important chemical components of D. officinale to intervene in acute alcoholic liver injury.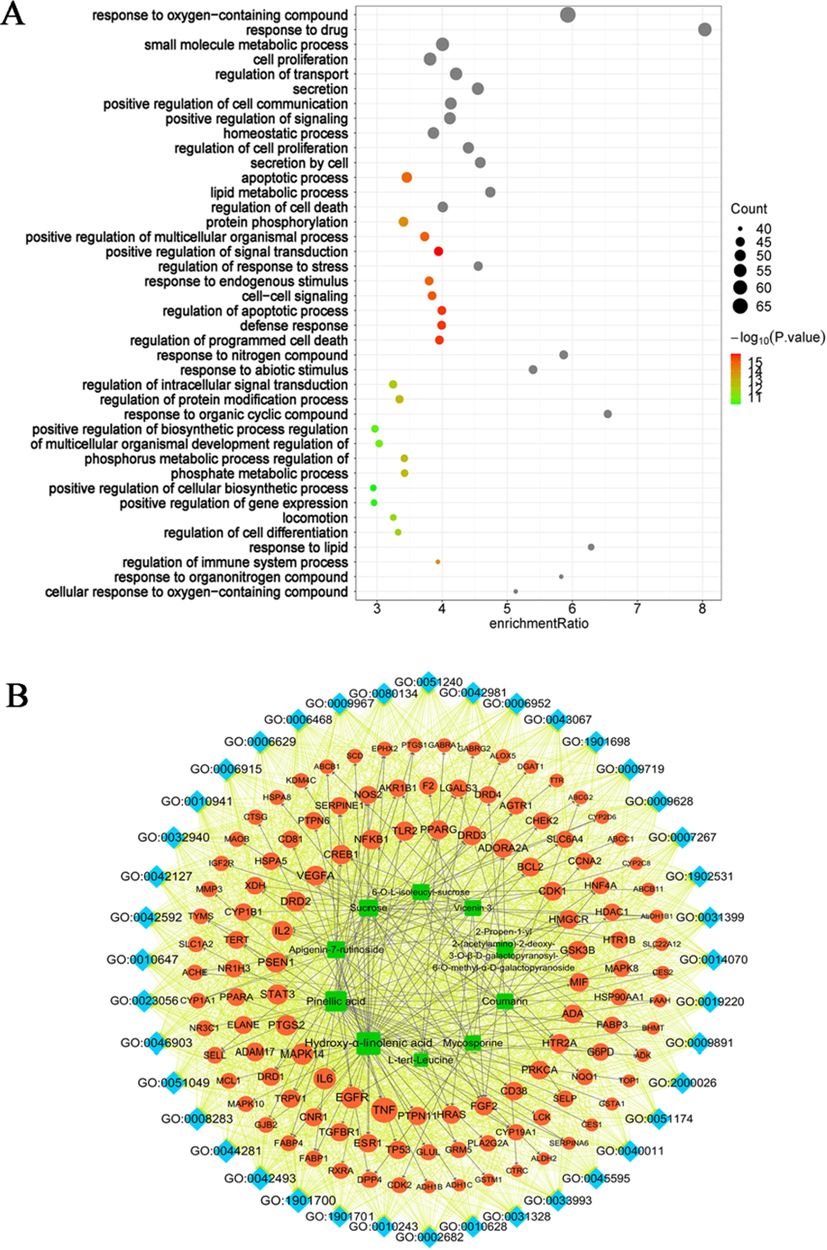
The targets of acute alcoholic liver injury on which the differential chemical composition of D. officinale acts are related to the acute alcoholic liver injury. (A) GO functional annotation analysis of the biological functions of potential acute alcoholic liver injury targets on which the compounds act. (B) “Compound-target-GO function” network analysis of the relationship between compounds, potential targets of acute alcoholic liver injury, and GO biological functions. Blue diamonds represent GO terms, red round nodes represent chemical targets, and green square nodes represent D. officinale differential compounds.
GO num
GO term
Degree
GO:1901700
response to oxygen-containing compound
67
GO:0042493
response to drug
58
GO:0044281
small molecule metabolic process
57
GO:0008283
cell proliferation
55
GO:0051049
regulation of transport
54
GO:0046903
secretion
53
GO:0023056
positive regulation of signaling
52
GO:0010647
positive regulation of cell communication
52
GO:0042592
homeostatic process
51
GO:0042127
regulation of cell proliferation
50
3.6 Validation of the pharmacological activity of secondary metabolites of D. officinale in the prevention of acute alcoholic liver injury in mice
HE staining of liver tissues to observe the effects of D. officinale extracts of different origins on the liver of mice with acute alcoholic liver injury (Fig. 8). The mouse liver cells in the BG group were neatly arranged, with intact cell structure and well-defined nuclei and cell pulp shapes. Compared with the BG group, the liver cells in the AG group were blurred, with swollen cells, incomplete cell membranes, and obvious intracellular vacuoles, and the nuclei were wrinkled or even disappeared, indicating obvious pathological changes in the liver tissue of the AG group. Compared with the AG group, the hepatocyte injury of mice in the AL high-dose group was improved, with gradually well-defined cell outlines, clear cell structures and fewer intracellular vacuoles, while the liver cells of the remaining D. officinale extract administration group were obviously swollen, with incomplete cell membranes, intracellular vacuoles and crumpled or even disappeared nuclei. The results showed that AL D. officinale extract had alleviating effects on acute alcoholic liver injury in mice.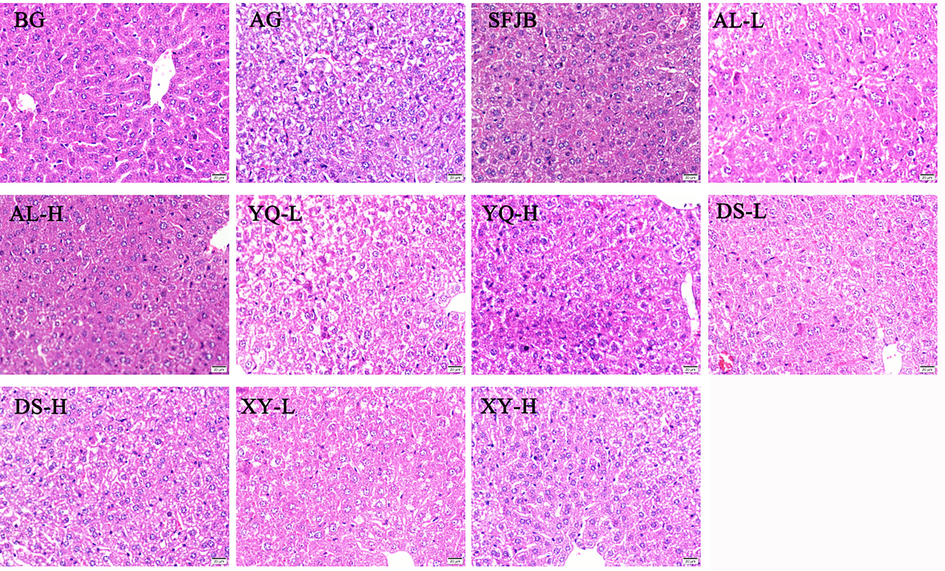
High dose of AL D. officinale extract ameliorates liver pathological damage in mice. HE stain, ×400. BG represents the blank group, AG represents the model group, SFJB represents the positive control group, AL represents the D. officinale extract group in Anlong, Guizhou, YQ represents the D. officinale extract group in Yueqing, Zhejiang, DS represents the D. officinale extract group in Dushan, Guizhou, and XY represents the D. officinale extract group in Xingyi, Guizhou, where -L indicates the D. officinale extract low dose group and -H indicates the D. officinale extract high dose group.
Serum ALT and AST were measured in each group of mice (Fig. 9). Compared with the BG group, the serum ALT levels and AST levels in the AG group were significantly higher (P < 0.05), indicating that a single dose of 53% ethanol (10 mL/kg) by gavage in mice can cause liver damage and the method can be used to establish a mouse model of acute alcoholic liver injury. Compared with the AG group, the serum ALT and AST of mice in the AL high-dose group were significantly reduced (P < 0.05); the serum AST levels of mice in the DS low-dose group and XY low-dose group were significantly reduced (P < 0.05), but the changes in ALT were not significant (P > 0.05). The results indicated that the high dose of AL D. officinale extract could significantly reduce the serum transaminase level in mice with acute alcoholic liver injury. Taken together, the results suggest that AL D. officinale extract has a preventive effect on acute alcoholic liver injury in mice.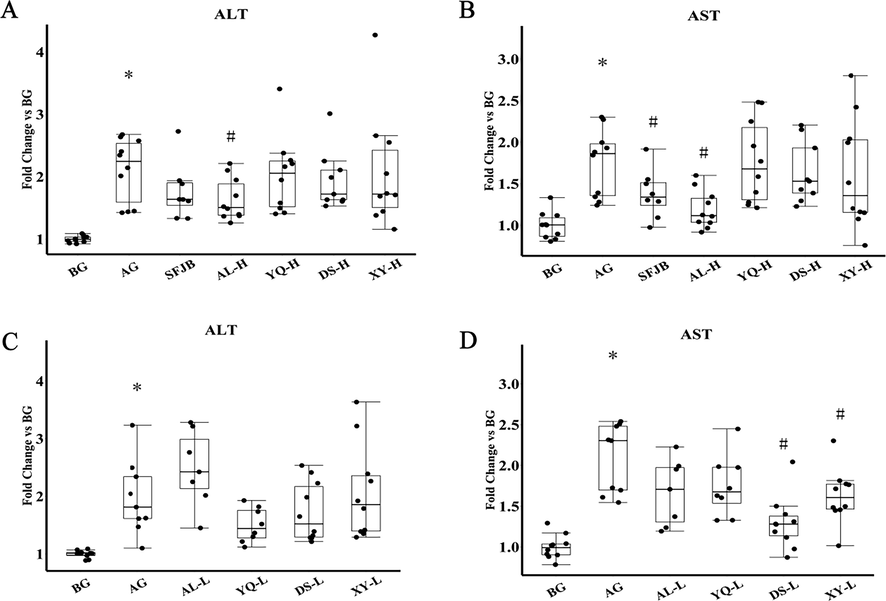
High dose of AL D. officinale extract significantly reduces serum ALT and AST levels in mice with acute alcoholic liver injury. (A) and (C) Serum ALT level. (B) and (D) Serum AST level. BG represents the blank group, AG represents the model group, SFJB represents the positive control group, AL represents the D. officinale extract group in Anlong, Guizhou, YQ represents the D. officinale extract group in Yueqing, Zhejiang, DS represents the D. officinale extract group in Dushan, Guizhou, and XY represents the D. officinale extract group in Xingyi, Guizhou, where -L indicates the D. officinale extract low dose group and -H indicates the D. officinale extract high dose group. * P < 0.05, compared with the BG group, # P < 0.05, compared with the AG group. ¯x ± SEM, n = 10.
4 Discussion
Secondary metabolites are a group of small organic compounds produced by secondary metabolism that are not essential for the normal functioning of cellular life activities and plant growth and development. They not only play an important role in the life activities of plants, but some of these secondary metabolites also have pharmacological activities. However, due to the many kinds of secondary metabolites, complex composition and highly susceptible to the plant growth environment, little research has been done on them so far, so it is very important to find a suitable method to analyze secondary metabolites, and D. officinale contains rich chemical components, including polysaccharides, dendrobine, lignans, amino acids, flavonoids and many other chemical components, but D. officinale polysaccharide has been used as its quality evaluation standard, whether the amount of D. officinale polysaccharide content can represent its quality, our research answered this question. We analyzed the secondary metabolites and polysaccharide contents of D. officinale with enough samples from four different producing areas and found that the differences in production sources affected the secondary metabolites and polysaccharide contents. This is also consistent with the conclusion that the poor cultivation conditions and cultivation environment of D. officinale affect the planting and growth of D. officinale reported in previous studies (Yuan et al., 2020). On the other hand, D. officinale with low polysaccharide content is rich in secondary metabolites, and the content trend of secondary metabolites in D. officinale is not consistent with that of polysaccharide, which once again proves that it is unreasonable to use polysaccharide content as a single quality evaluation standard for D. officinale.
At the same time, we screened and identified 11 chemical components from D. officinale group, except Raffinose, the contents of the other 10 components in D. officinale samples were significantly higher than those in the other three groups (P < 0.05), and among these different secondary metabolites, some studies have shown that these secondary metabolites are pharmacologically active. For example, α-linolenic acid has cardiovascular protective, anti-inflammatory and antioxidant effects (Kim et al., 2014). α-linolenic acid inhibits the generation of reactive oxygen species (ROS) and suppresses cadmium-induced oxidative stress, neuroinflammation and neurodegeneration in the mouse brain when administered orally (Alam et al., 2021). Pinellic acid is an unsaturated fatty acid with immunomodulatory, immune response enhancing and anti-inflammatory properties, it can enhance the nasal influenza HA vaccine IgA response and produce adjuvant activity when administered via the GI tract (Nagai et al.,5 2002; Choi et al., 2013) Coumarin belongs to the phenylpropanoid compound with various pharmacological effects such as anticoagulant, antibacterial, anti-inflammatory and neuroprotective, and has significant immunomodulatory activity for the treatment of immunosuppressive diseases (Annunziata et al., 2020). However, the pharmacological activity of these chemical components in D. officinale has not been reported, but this does not mean that the secondary metabolites of D. officinale are not important. Such as Apigenin-7-rutinoside is a flavonoid that has been identified and isolated from Dendrobium and has biological activities of oxidative stress inhibition, antioxidant, and hepatocyte protection (Wang et al., 2003). Bibenzylates in D. officinale have been demonstrated to have pharmacological effects as anti-inflammatory, antioxidant, and improve intestinal homeostasis (Li et al., 2020). However, studies on the medicinal active ingredients of D. officinale have been focused on Dendrobium polysaccharides, which are also considered to be the main active ingredients of D. officinale (Chen et al., 2021). Therefore, it is very important to find a suitable method to study the pharmacodynamic activity of the secondary metabolites of D. officinale. And in our study, the pharmacological effects of 11 different chemical components of D. officinale were studied by network pharmacology, and it was found that the secondary metabolites of D. officinale had the effect of preventing acute alcoholic liver injury in mice. In previous studies, chemical components in plants were usually isolated and then the pharmacological effects or pharmacodynamic activities of chemical components were studied one by one. However, the chemical components in traditional Chinese medicine are complex, with the characteristics of multi-component, multi-target and low side effects, and the pharmacodynamic mechanism of traditional Chinese medicine is characterized by multi-level, multi-target and overall regulation. The pharmacodynamic study of a single chemical component is not sufficient to discover the pharmacodynamic effect caused by the interaction of multiple chemical components. While network pharmacology can reveal the interactions between multiple targets in D. officinale from the perspective of complex biological networks, further helping us to discover their pharmacological effects, our network pharmacology results help us to discover the underlying biological activity mechanisms of these differential secondary metabolites involved in several biological processes closely related to acute alcoholic liver injury, such as cellular immunity, inflammation and oxidative stress. Moreover, acute alcoholic liver injury is a type of liver damage associated with excessive alcohol consumption (Torruellas et al., 2014). Excessive alcohol consumption leads to the accumulation of ROS, which in turn leads to oxidative stress, ER stress and steatosis, while excessive alcohol consumption also leads to innate immune activation, which stimulates the production of pro-inflammatory factors by Kupffer cells and promotes the development of liver damage (Shao et al., 2020). These small molecules may play an important role in the prevention of acute alcoholic liver injury of D. officinale by participating in the comprehensive regulation of biological pathways such as oxidative stress, cellular immunity and inflammation.
On the other hand, our pharmacodynamic experiments showed that the prediction results of our network pharmacology were correct, and the AL group with low polysaccharide content but significant differences in secondary metabolites could significantly reduce the elevation of serum ALT and AST induced by alcohol in mice. Alcohol is one of the carcinogens released by the World Health Organization. Excessive daily intake of alcohol is easy to cause damage to the digestive system and nervous system, and induce acute alcoholic gastritis, digestive tract ulcers, peripheral neuritis and other diseases (Akiyama et al., 2008; Zhang et al., 2018). As the largest metabolic organ of the body, liver is also the main place of ethanol metabolism. During liver metabolism, ethanol is metabolized to acetaldehyde mainly by ethanol dehydrogenase and produces a large number of reactive oxygen species (ROS). Liver damage caused by excessive alcohol consumption is one of the important risk factors for liver fibrosis, cirrhosis, liver cancer and other diseases, as well as one of the major preventable causes of high morbidity and mortality in the world (Trimble et al., 2013; Blachier et al., 2013). Moreover, ALT mainly exists in the plasma of liver cells. When liver cells are damaged, the intracellular aminotransferase can enter the blood and cause the level of ALT in serum to increase. AST also exists in the plasma of hepatocytes and is distributed in mitochondria. When hepatocytes are severely damaged, AST releases human blood from mitochondria and increases the serum AST level. Therefore, our pharmacodynamic results once again demonstrate the potential pharmacological activity of D. officinale secondary metabolites in the prevention of acute alcoholic liver injury.
Our study showed that the quality of D. officinale should not be judged by polysaccharide content, but should also consider the content of secondary metabolites. Our study provided a new reference for the quality evaluation standard of Dendrobium officinale in the cultivation process, and also provided a new idea for the pharmacodynamics study of secondary metabolites of D. officinale. On the other hand, most of the current drugs for the treatment of alcoholic liver injury are only effective under certain conditions and have certain side effects, making it difficult to achieve multi-target and multi-level coverage. However, the treatment of most diseases is related to multiple targets, and it is difficult to achieve appropriate therapeutic effects against a single target (Kendrick et al., 2010; Louvet et al., 2015). Our study found that the secondary metabolites of D. officinale are important active components in the prevention of acute alcoholic liver injury of D. officinale. These active components can act on multiple targets, and our study also provides a new idea for the development of therapeutic drugs for acute alcoholic liver injury.
5 Conclusion
In this study, it was found that the overall profile of secondary metabolites in D. officinale with low polysaccharide content was significantly different from that of other D. officinale. D. officinale had the effect of preventing acute alcoholic liver injury in mice, and the polysaccharide content of D. officinale with obvious pharmacological activity was the lowest, but the difference in the content of secondary metabolites was the most obvious. The contents of secondary metabolites of D. officinale were significantly different, suggesting that the pharmacodynamic activity of D. officinale in preventing acute alcoholic liver injury was related to its secondary metabolites, and polysaccharide was not the only active component of D. officinale in preventing acute alcoholic liver injury. On the other hand, small molecules of differential secondary metabolites, such as hydroxyllinolenic acid, L-tertiary leucine, coumarin, vincristine 3 and apigenin-7-o-rutinoside, which were selected in this study, may be active markers of D. officinale for the prevention of acute alcoholic liver injury, which may be a useful supplement to the current quality standard of D. officinale based only on polysaccharides. It provides a new reference for evaluating the quality of D. officinale more comprehensively.
CRediT authorship contribution statement
Mengting Yang: . Qianru Zhang: . Anjing Lu: . Zhou Yang: Supervision, Validation. Daopeng Tan: . Yanliu Lu: . Lin Qin: . Yu-qi He: .
Acknowledgements
This work was supported by the Department of Science and Technology of Guizhou Province (nos. QKHZC[2019]2953, QKHZC [2020]4Y072), the Science and Technology Innovation Action Plan of Domestic Science and Technology Cooperation Projects in Shanghai (20025800400), and Guizhou Engineering Research Center of Industrial Key-technology for Dendrobium Nobile (QJJ[2022]048).
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
References
- Akiyama, T., Inamori, M., Iida, H., et al, 2008. Hosono K, Yoneda K, Fujita K, Yoneda M, Takahashi H, Goto A, Abe Y, Kobayashi N, Kubota K, Saito S, Nakajima A, Alcohol consumption is associated with an increased risk of erosive esophagitis and Barrett's epithelium in Japanese men. BMC Gastroenterol. 8, 1-6. https://doi.org/10.1186/1471-230x-8-58
- Alpha-linolenic acid impedes cadmium-induced oxidative stress, neuroinflammation, and neurodegeneration in mouse brain. Cells. 2021;10:1-14.
- [CrossRef] [Google Scholar]
- Annunziata, F., Pinna, C., Dallavalle, S., Tamborini, L., 2020. Pinto A, An Overview of Coumarin as a Versatile and Readily Accessible Scaffold with Broad-Ranging Biological Activities. Int J Mol Sci. 21, 1-81. https://doi.org/10.3390/ijms21134618
- The burden of liver disease in Europe: a review of available epidemiological data. J. Hepatol.. 2013;58:593-608.
- [CrossRef] [Google Scholar]
- Chang, J., Zhou, Y., Cong, G., et al, H, 2019. Dendrobium candidum protects against diabetic kidney lesions through regulating vascular endothelial growth factor, Glucose Transporter 1, and connective tissue growth factor expression in rats. J Cell Biochem. 120, 13924-13931. https://doi.org/10.1002/jcb.28666
- Traditional uses, phytochemistry, pharmacology, and quality control of Dendrobium officinale Kimura et. Migo. Front. Pharmacol.. 2021;12:1-23.
- [CrossRef] [Google Scholar]
- Isolation, structural properties, bioactivities of polysaccharides from Dendrobium officinale Kimura et. Migo: A review. Int. J. Biol. Macromol.. 2021;184:1000-1013.
- [CrossRef] [Google Scholar]
- Inhibition of prostaglandin D₂ production by trihydroxy fatty acids isolated from Ulmus davidiana var. Japonica. Phytother. Res.. 2013;27:1376-1380.
- [CrossRef] [Google Scholar]
- Committee for the Pharmacopoeia of PR China(2020), 2020. Pharmacopoeia of the People's Republic of China, Part 1, Beijing, China: China Medical Science Press. (2020) 295.
- Theophylline improves steroid sensitivity in acute alcoholic hepatitis. Hepatology. 2010;52:126-131.
- [CrossRef] [Google Scholar]
- α-Linolenic acid: nutraceutical, pharmacological and toxicological evaluation. Food Chem. Toxicol.. 2014;70:163-178.
- [CrossRef] [Google Scholar]
- Anti-inflammatory bibenzyls from the stems of Dendrobium huoshanense via bioassay guided isolation. Nat. Prod. Res.. 2020;34:563-566.
- [CrossRef] [Google Scholar]
- Dendrobium candidum Wall. ex Lindl. attenuates CCl4-induced hepatic damage in imprinting control region mice. Exp. Ther. Med.. 2014;8:1015-1021.
- [CrossRef] [Google Scholar]
- Four new bibenzyl derivatives from Dendrobium candidum. Chem. Pharm. Bull. (Tokyo). 2009;57:997-999.
- [CrossRef] [Google Scholar]
- Alcoholic liver disease: mechanisms of injury and targeted treatment. Nat. Rev. Gastroenterol. Hepatol.. 2015;12:231-242.
- [CrossRef] [Google Scholar]
- Carbohydrate analysis by a phenol-sulfuric acid method in microplate format. Anal. Biochem.. 2005;339:69-72.
- [CrossRef] [Google Scholar]
- Pinellic acid from the tuber of Pinellia ternata Breitenbach as an effective oral adjuvant for nasal influenza vaccine. Int. Immunopharmacol.. 2002;2:1183-1193.
- [CrossRef] [Google Scholar]
- Natural products as sources of new drugs from 1981 to 2014. J. Nat. Prod.. 2016;79:629-661.
- [CrossRef] [Google Scholar]
- TNF-α-induced p53 activation induces apoptosis in neurological injury. J. Cell Mol. Med.. 2020;24:6796-6803.
- [CrossRef] [Google Scholar]
- Diagnosis of alcoholic liver disease. World J. Gastroenterol.. 2014;20:11684-11699.
- [CrossRef] [Google Scholar]
- Mortality associated with alcohol-related liver disease. Aliment. Pharm. Ther.. 2013;38:596-602.
- [CrossRef] [Google Scholar]
- Traditional uses and pharmacologically active constituents of dendrobium plants for dermatological disorders: A review. Nat. Prod. Bioprospect.. 2021;11:465-487.
- [CrossRef] [Google Scholar]
- Analysis of antioxidative phenolic compounds in artichoke (Cynara scolymus L.) J. Agric. Food Chem.. 2003;51:601-608.
- [CrossRef] [Google Scholar]
- Dendrobium officinale polysaccharide protected CCl4-induced liver fibrosis through intestinal homeostasis and the LPS-TLR4-NF-κB signaling pathway. Front. Pharmacol.. 2020;11:1-14.
- [CrossRef] [Google Scholar]
- How Taxol/paclitaxel kills cancer cells. Mol. Biol. Cell. 2014;25:2677-2681.
- [CrossRef] [Google Scholar]
- Response of plant secondary metabolites to environmental factors. Molecules. 2018;23:1-26.
- [CrossRef] [Google Scholar]
- Protective effects of Dendrobium candidum Wall ex Lindl. on high-fat diet-induced liver damage in mice. J. Food Biochem.. 2021;45:1-10.
- [CrossRef] [Google Scholar]
- The effects of ecological factors on the main medicinal components of Dendrobium officinale under different cultivation modes. Forests. 2020;11:1-16.
- [CrossRef] [Google Scholar]
- Dendrobium officinale attenuates myocardial fibrosis via inhibiting EMT signaling pathway in HFD/STZ-induced diabetic mice. Biol. Pharm. Bull.. 2020;43:864-872.
- [CrossRef] [Google Scholar]
- Role of MCP-1 and CCR2 in ethanol-induced neuroinflammation and neurodegeneration in the developing brain. J. Neuroinflamm.. 2018;15:1-14.
- [CrossRef] [Google Scholar]
- Comparative metabolomic analysis of Dendrobium officinale under different cultivation substrates. Metabolites. 2020;10:1-14.
- [CrossRef] [Google Scholar]







