The removal of COD, TSS and colour of black liquor by coagulation–flocculation process at optimized pH, settling and dosing rate
⁎Corresponding author at: Center for Environmental Protection Studies, Pakistan Council of Scientific and Industrial Research (PCSIR) Laboratories Complex, PCSIR Labs Complex Ferozpur Road, Lahore 54600, Pakistan. Tel.: +92 321 8512451. sahalirfan@gmail.com (Muhammad Irfan)
-
Received: ,
Accepted: ,
This article was originally published by Elsevier and was migrated to Scientific Scholar after the change of Publisher.
Peer review under responsibility of King Saud University.

Abstract
Pulp and Paper mills are generating millions of tons of wastewater and are major source of water pollution. In this research work, pulping wastes have been characterized and found to contain very high COD, TSS and colour. Coagulation–flocculation process was performed to find out the performance of different coagulants and flocculants like alum, ferric chloride, aluminium chloride, ferrous sulphate, poly aluminium chloride (PAC), cationic and anionic polyacrylamide polymers in individual form as well as in different combinations. The effects of dosing rate, settling time and pH were examined for reduction of COD, TSS and colour. Coagulants used in combinations were found to be more effective in reducing COD, TSS and colour instead of using individual form. The initial pH of the effluent for coagulation process was found to have remarkable effect on COD, TSS and colour removal. The most effective results were found using cationic and anionic polyacrylamide combination with ferric chloride and aluminium chloride and reduction of 76% COD, 95% TSS and 95% colour were observed at pH < 3.
Keywords
Paper effluent
Coagulants
Flocculants
COD
TSS
Colour reduction
1 Introduction
At present, in Pakistan there are about 100 units in the organized and unorganized sectors. Collectively, all of these units have a set up capacity of 650 thousand tons per annum (Hassan et al., 2012). These units are producing writing and printing papers, wrapping and packing paper, white duplex coated, un-coated boards and chip boards. There have been number of techniques involved in making pulp and paper. Main types involve mechanical, thermal and chemical (Burger, 2007; Pokhrel and Viraraghavan, 2004). Chemical pulping may be involved in sulphate or Sulphite process whereas mechanical pulping involves grinding of desecrated wooden chips. Kraft process involves boiling of wood chips in the solution of sodium hydroxide and sodium sulphate while in the sulphite process sodium sulphite is used. In thermal pulping, steam is provided to wooden chips for softening (Holmberg and Gustavsson, 2007). The entire process of paper and pulp industry consumes lot of energy and water (Pokhrel and Viraraghavan, 2004). During pulping process water consumption may get as high as 60 m3/ton paper produced (Thompson et al., 2001). This wastewater is treated and recycled to conserve energy and raw materials. Industrial effluent is treated by various wastewater treatment technologies including lagoons systems (Bajpai, 2001), activated sludge process (Chandra, 2001), anaerobic treatment process (Bajpai, 2001) and fungal treatment process (Sakurai et al., 2001) to remove most of the suspended solids and soluble organic materials.
Paper mill effluent contains dissolved inorganic and organic chemicals like dyes, heavy metals, detergents, starch etc. which results in high COD, turbidity, temperature, toxic substances/elements, organic compound, inconsistent pH and intense colour (Monte et al., 2009). The colour of the wastewater generating from pulp and paper mill is mainly due to organic compounds and is comprised of extractives, tannin resins, synthetic dyes, lignin and their degradation products formed by the action of chlorine on lignin during the bleaching process (Sakam, 1987; Bajpai, 2001. Lignin is a complex heterogeneous phenyl propanoid biopolymer containing a diverse array of stable carbon–carbon bonds with aryl/alkyl ether linkages and may be cross-linked to hemicelluloses (Sjostrom, 1993). The dark brown colour of black liquor is formed during the degradation processing of lignocelluloses and is an indirect measurement of the amount of lignin compounds present in the effluent stream. The greater the amount of lignin compounds, the darker the effluents and greater the tendency to produce foam. The poisonous properties of these by-products in the effluents on environment have been studied and reported that pulp and paper industries wastewater have fatal effects on the daphnia, fish, planktons and other bioata in the receiving water bodies (Kovacs et al., 2002). If this wastewater is discharged into water bodies without any particular treatment system it can cause adverse effects on aquatic life even at very low concentrations (Miège et al., 2008).
Wastewater is treated and recycled in pulp and paper mills to conserve energy and raw materials. Excess effluent is treated by wastewater treatment plant which often consists of reception tank, clarifier (primary), biological (secondary) treatment and secondary clarifier to remove most of the suspended solids and soluble organic materials. Several physical and chemical processes including combination of ultra filtration and reverse osmosis techniques, ion-exchange, chromatography, lime precipitation and modified bleaching sequences, activated carbon and allophonic compounds have been used for paper’s effluent treatment. (Koyuncu et al., 1999; Thompson et al., 2001; Chakradhar and Shrivastava, 2004; Herath et al., 2011; Freire et al., 2000; Shawwa et al., 2001). Biological treatment is also widely used for the treatment of industrial effluents (Schnell et al., 2000) and for the degradation of lignin to reduce the load of pulping wastewater (Bajpai, 2001; Oeller et al., 1997; Franta and Wilderer, 1997). Biological treatment method is not much impressive in reducing colour (Cecen et al., 1992) but reduction in BOD and COD has been found to be much satisfactory. However, this treatment involves high energy consumption, the production of large amounts of sludge (Sreekanth et al., 2009) and operational problems including colour, foaming and bulking in secondary clarifiers that are associated with activated sludge plants (Oz et al., 2004). The effect of membrane technologies was also studied by using inorganic MF and organic UF membranes to reduce the COD of black liquor (Liu et al., 2004). Membrane based technologies, including ultra filtration (Jönsson and Wallberg, 2009) electrochemical membrane reactor (Chanworrawoot and Hunsom, 2012) have also been tried with limited success for the separation of valuable constitutes from black liquor. Electro-coagulation (EC) technique for treatment of pulping wastewater has been used with different combinations of aluminium (Al) and iron (Fe) electrodes (Katal and Pahlavanzadeh, 2011).
Coagulation and flocculation process is one of the best options to treat pulping wastewater using alum, ferric chloride, lime, ferrous sulphate and poly aluminium chloride (PAC) and can be considered as an efficient treatment method to reduce COD, TSS and colour of pulping effluent (Rahbar et al., 2006; Deegan et al., 2011; Wong et al., 2006; Garg et al., 2005). The major advantage of chemical treatment is that most of the COD and TSS are being reduced during this process therefore it can lead to make it more cost effective before secondary treatment as well as removal of colour for effluent.
2 Experimental
2.1 Materials
A sample of wastewater/black liquor was obtained from M/s packages paper mills (A paper mill situated in Lahore, Pakistan). The major chemicals used in this work for the removal of TSS, COD and colour are Alum, Ferric chloride, Ferrous sulphate, Aluminium chloride, Poly aluminium chloride (PAC) and various polyelectrolytes have been studied extensively. Analytical reagent (AR) grade chemicals have been used for the analysis of the studied parameters during coagulation/flocculation studies. Concentration of each coagulant was taken as 2% while polyeltrolytes used in this study is 0.1%. During this present investigation, 200 ml of effluent sample was taken in a glass beaker. Initially pH and temperature of the effluent were measured using digital pH meter (JENCO 6173). pH was adjusted in different trials by using conc. Sulphuric acid and Sodium hydroxide. A known quantity of each coagulant was added to the effluent to check its performance. It was strongly mixed for one minute by a stirrer at 150 rpm and thereafter slowly mixed for two minute at 50 rpm. The effluent sample was left steady for 30 min for sedimentation. The supernatant liquor was taken and analyzed for its COD, TSS and colour values.
2.2 Coagulation–flocculation methods
In this research work, coagulants and flocculants are used in individual form as well as in various combinations. The following Table 2 shows details of all combinations which are used.
| Coagulants/flocculants used |
|---|
| Individuals form – treatment 1 |
| Alum |
| Ferric chloride |
| Aluminum chloride |
| Ferrous sulfates |
| Poly aluminum chloride (PAC) |
| Poly acrylamide (PAM Cationic) |
| Poly acrylamide (PAM Anionic) |
| Coagulants combination used – treatment 2 |
| Alum + ferric chloride |
| Alum + aluminum chloride |
| Alum + PAC |
| Alum + ferrous sulphate |
| Ferric chloride + aluminum chloride |
| Ferric chloride + PAC |
| Ferric chloride + ferrous sulphate |
| Aluminum chloride + PAC |
| Aluminum chloride + ferrous sulphate |
| PAC + ferrous sulphate |
| Coagulants + flocculants combination used – treatment 3 |
| (Ferric chloride + PAC) + cationic PAM |
| (Aluminum chloride) + PAC + cationic PAM |
| (Ferric chloride + PAC) + anionic PAM |
| (Aluminum chloride + PAC) + anionic PAM |
2.3 Analytical methods used during treatment process
The COD of the effluent was determined by dichromate open reflux method as per standard method 5220-B (Standard methods for examination of water and wastewater 21st edition 2005). TSS values were measured by using 45 micron filter paper as per standard method 2540-D. The colour of the initial and final effluent after the treatment was measured by Tinto meter (ASTM Method D-1209). Initial colour of the effluent is highly dark brown that is why it is measured by making its dilution by three times.
2.4. Process lay-out of chemical treatment study
2.5 Objectives
Coagulation–flocculation process was carried out with the following objectives:
-
To find out the optimize pH for maximum reduction of COD, TSS and colour.
-
To find out the optimum dosing rate for each coagulant at selective pH for maximum COD, TSS and colour removal.
-
Working conditions of each coagulants/flocculants in different combinations.
-
Effect of addition of polyelectrolytes.
-
Selectivity of most effective coagulants/flocculants.
3 Results and discussion
The sample collected from packages paper mills was characterized and following results were observed.
Table 1 shows very high values of COD, TSS and colour. Coagulation flocculation process was used to treat this collected sample which involves destabilization of colloidal particles by neutralizing the forces that remain away from each other. Cationic coagulants impart positive electric charges to reduce the negative charge of the colloids. Consequently particles collide to form macro particles called as flocs. Usually coagulants form hydroxide when dissolved in water. These metal hydroxide polymers have amorphous structure as well as large surface area and possess positive charge. These hydroxides are hydrophobic, causing them to adsorb into the organic anionic particle surface and become insoluble (Licsko, 1997). Generally precipitation is enhanced as the pH is lowered in the presence of multivalent cations (See Fig. 1).
| Sr. # | Parameters | Results |
|---|---|---|
| 1 | pH | 6.73 |
| 2 | Chemical oxygen demand (COD) | 28270 mg/l |
| 3 | Total suspended solids (TSS) | 11455 mg/l |
| 4 | Color | Dark brown/1640 pt-co/hazen |
- Steps involved during Chemical treatment by coagulation/flocculation process.
Effect of pH on the COD, TSS and colour reduction from wastewater was found to be remarkable. The dosing rate of all coagulants was kept uniform as 0.8 g/L (40 ml stock solution/litre of sample). All trials were taken at different initial pH values, i.e., 1, 2, 3, 4, 5, 7, 9, 11. Coagulants were added and rapidly mixed for one minute by a stirrer at 150 rpm and thereafter slowly mixed for two minutes at 50 rpm and sample was left over for 30mintutes. The supernatant was decanted off and its value for COD, TSS and colour was measured. For Alum and ferrous sulphate, COD reduction was reduced up to 11% and 12%, respectively at pH 5. Aluminium chloride and ferric chloride showed more COD reduction of 13% and 16%, respectively at pH ⩽ 3. Treatment by PAC provides COD reduction of 14% at pH 4 as shown by Fig. 2.
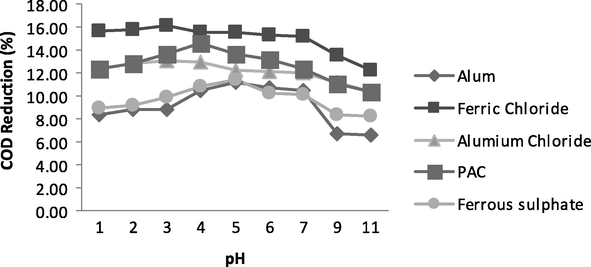
- Variation in COD at various pH values.
The variation in TSS was also studied and it was found to be 46%, 50%, 48%, 47% and 42% for Alum, Ferric Chloride, Aluminium Chloride, PAC and Ferrous sulphate, respectively at optimum pH 4 for Alum and Ferrous Sulpahte, pH 3 for Aluminium Chloride and PAC and pH 2 for Ferric Chloride as shown in Fig. 3. In case of TSS the optimum value of pH is slightly lower than optimum value of pH for COD which might be due to strong precipitation at acidic pH. As the precipitation increased, solution will more clear give very low value of TSS.
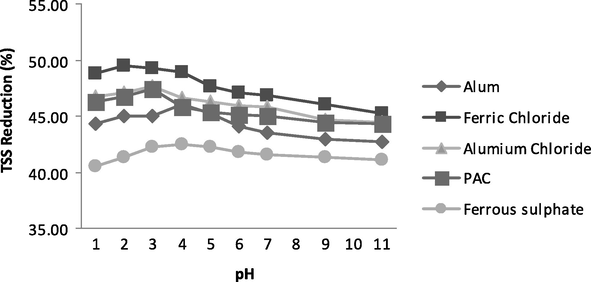
- Variation in TSS at various pH values.
In case of colour reduction, the pH behaviour was very similar as in TSS reduction for all of these coagulants. The optimum colour reduction is found to be 38%, 46%, 52%, 52% and 37% for Alum, Ferric Chloride, Aluminium Chloride, PAC and Ferrous Sulphate, respectively as shown in Fig. 4. Ferric chloride was found to be much better in COD as well as TSS reduction but good in colour reduction at very low concentration. At higher concentration it imparts its own brownish colour. Aluminium chloride and PAC were excellent in colour reduction. Sludge formed by Aluminium chloride was whitish and brownish with PAC. These observations showed that treatment of paper effluents is highly dependent on initial pH value of the effluent sample. Various groups present in wastewater interact with metal cations at alternated pH. Carboxylic and phenolic groups bring together metal cations at low pH while hydroxyl and aliphatic hydroxyl groups interact at elevated value of pH. The removal of dissolved organics during coagulation process at different pH values follows two distinct mechanisms. At low pH, anionic organic molecules interacted with metal cations and form insoluble metal complexes while high pH and elevated coagulant doses lead the organics adsorb onto pre-formed flocs of metal hydroxides and get precipitated (Kumar et al., 2011).
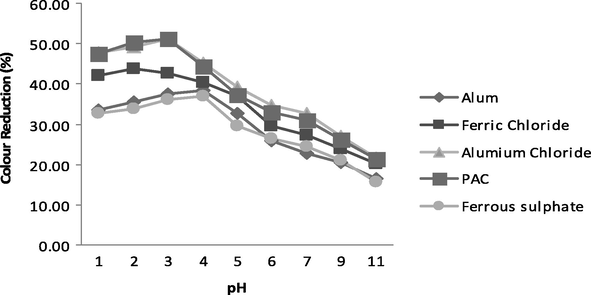
- Variation in TSS at various pH values.
Each coagulant showed a different behaviour from other coagulants; ferric chloride and PAC were good in COD reduction while aluminium chloride and PAC were excellent in colour reduction. Therefore combinations of different coagulants were tried for selectivity of one of the best pairs which can be used for effluent treatment of paper industries. All trials were taken at an initial pH value of 3. Total dosing rate remained constant as 0.8 g/L (40 ml/litre) but it includes 50% conc. of each coagulant. The results shown by each of the pair are shown in Fig. 5. A combination of Ferric chloride and PAC was found to be the best for COD and TSS reduction and it reduced 19% COD and 49% TSS followed by the combination of ferric chloride and aluminium chloride which reduced 18% COD along with attractive 49% reduction in TSS and 52% reduction in colour in comparison to prior combination which reduced 48% colour. It is interesting that although the combination of Alum and Ferric chloride reduced COD up to 16% and TSS 48% it can be a good option for effluent treatment due to low price of Alum in Pakistan.
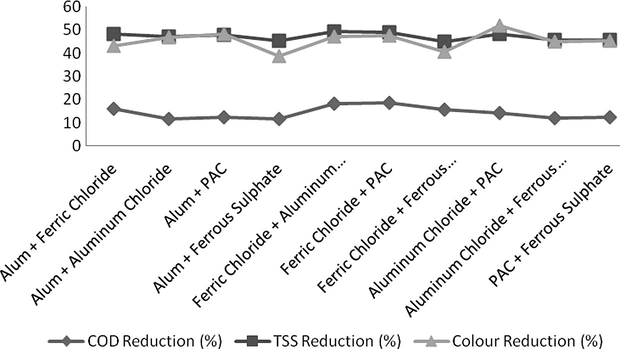
- COD, TSS and Colour Reduction by different combinations of coagulants.
In addition to these coagulants, workings of flocculants such as cationic as well as anionic polyacrylamide (PAM) were also investigated. In flocculation process, usually destabilized particles produced by various coagulants actually stiff into heavy particles which can be easily separated from the wastewater. Therefore these flocculants can be used individually as well as used after dosing of any coagulant. Dosing rate for these flocculants stock solution was kept as 0.004 g/L (4 ml of stock solution/litre of sample). These flocculants were found to be outstanding in reduction of COD, TSS and colour. The effect of pH on working of these flocculants was very noticeable. The workings of these flocculants were found to be very limited in alkaline conditions as well as in neutral pH. When the value of pH was moved below to 7, reduction of the COD, TSS and colour was remarkable and it was found to be the best below pH < 3 and was maximum around pH 2. The reduction in COD, TSS and Colour by PAM cationic and PAM anionic was measured in order of 74%, 93%, 77% and 72%, 93%, 78%, respectively as shown by Figs. 6–8. It was very interesting that pH adjustment by sulphuric acid was excellent and it provides more clear solution and compact sludge as compared to hydrochloric acid. It might be due to strong oxidizing behaviour of sulphuric acid.
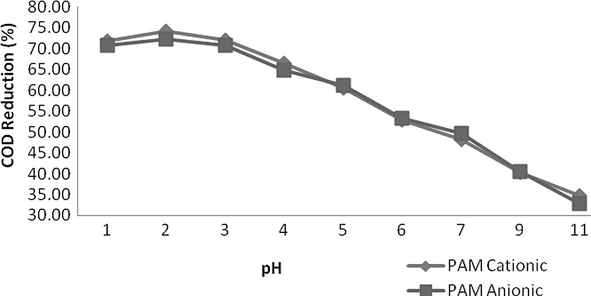
- Variation in COD at various pH values.

- Variation in TSS at various pH values.

- Variation in colour at various pH values.
Flocculants were also used after coagulant addition and results are observed to be more effective. In this case an excellent reduction was observed in all of the parameters i.e., COD, TSS and colour. Naturally, suspended particles are not easily sedimented by conventional settling therefore addition of coagulants combine these particles into colossal particles while flocculants further enhance the destabilization of dispersed particles making more health flocs improving the settling capability. As in this study, combinations of Ferric chloride and PAC and Aluminium chloride and PAC were found to be excellent for reduction of COD, TSS and colour therefore only these combinations were used along with these polyelectrolytes. The dosing rate of each coagulant was 0.2 g/L and 0.004 g/L for flocculants. The results were excellent and a tremendous reduction was observed in all aforementioned parameters as shown in Figs. 9–11. Combination of Ferric chloride, PAC and cationic polymer was excellent in COD reduction and reduced more than 81% of COD as well as 95% TSS. This combination was not found to be good in case of colour reduction which might be due to the presence of ferric chloride that induces its own colour at little higher dosing rate. For significant colour reduction, combination of Aluminium chloride, PAC and Anionic PAM was good and it reduced more than 88% colour as well as 96% TSS and 78% COD.
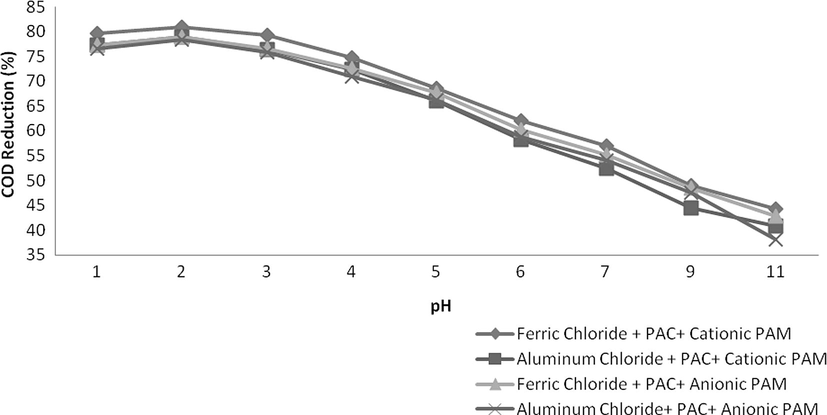
- Variation in COD at various pH values.
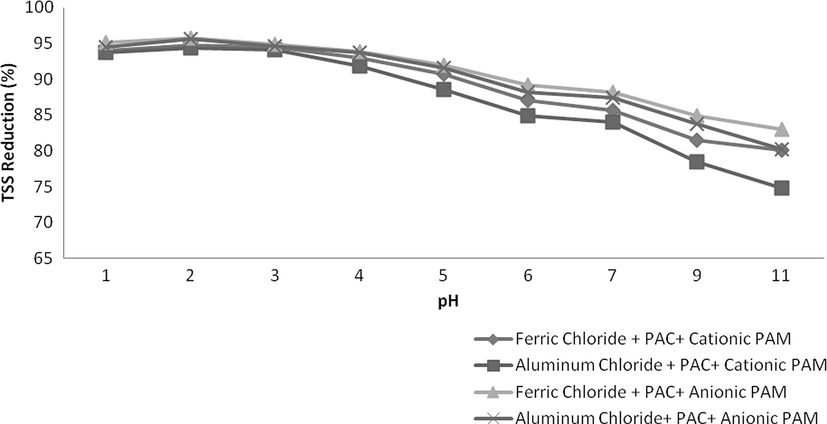
- Variation in TSS at various pH values.
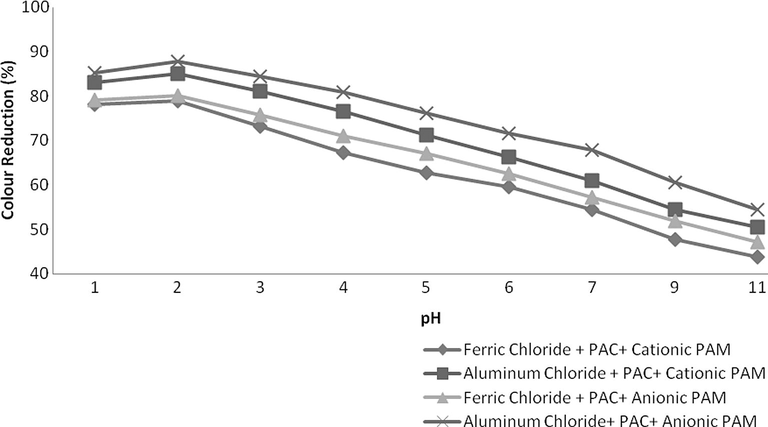
- Variation in Colour at various pH values.
Effect of dosing rates for all these coagulants and flocculants were also studied for maximum reduction of COD, TSS and colour for cost feasibility of the treatment process. Dosing of various coagulants in the effluent along with rapid mixing initiates appropriate coagulation conditions. Moderate mixing thereafter starts floc development and adsorption of the organic particles ensured the precipitation results by settling of the insoluble solids.
The effect of dosing rate on the COD reduction was examined at their optimum pH as shown by Fig. 12. The dosing rates of all coagulants were varied from 0.2–1.2 g/L. In case of Alum and ferrous sulpahte it was observed that as dosing quantity is increased up to 0.8 g/L, reduction in COD is very quantifiable and then slope of graph is very minute. Therefore the optimum dose for both Alum and Ferrous sulphate is taken as 0.8 g/L resulting in 11% reduction in COD. For Aluminium chloride and ferric chloride, a rapid reduction in COD was observed till 0.6 g/L providing 13% and 16% COD reduction, respectively and hereafter, increase in dosing quantity does not reduced COD marginably. For PAC, COD reduction is increased if dosing rate is increased up to 1 g/L coagulant dose, and then reduction was almost unchanged. Therefore dosing rate of 1 g/L is considered as optimum dose of PAC and providing 17% COD reduction. Ferric chloride was found to be excellent at very low dosing rate with remarkable reduction in COD therefore it could be beneficial with minimum chemical cost at industrial scale.
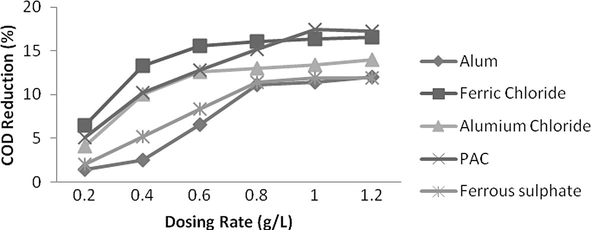
- Effect of dosing rate for various coagulants on COD reduction.
The effect of dosing rate on TSS removal was also observed at their optimum pH (pH = 3 for aluminium and PAC, pH = 4 for ferrous sulphate and alum, pH = 2 for ferric chloride). Reduction in TSS at their corresponding optimum pH for various coagulants is illustrated in Fig. 13. Ferric Chloride showed maximum TSS reduction of 57% at dosing rate of 1.2 g/L. Aluminium chloride and PAC showed reduction of 52% and 50%, respectively at a dosing rate of 1 g/L. Hereafter increase in dosing rate was not much effective. Hence 1 g/L was the optimum dosing rate for both of these coagulants. Ferrous sulphate remarkably reduced TSS up to 0.8 g/L but was no more effective at a higher dosing rate.
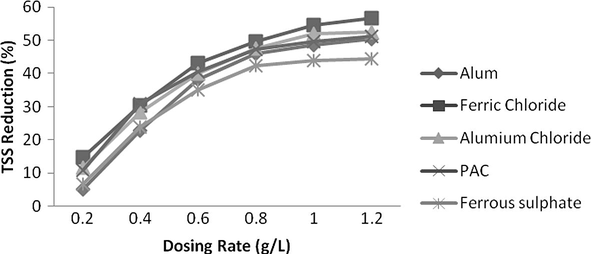
- Effect of dosing rate for various coagulants on TSS reduction.
Effect of dosing rate for colour reduction was observed at same pH level as was in TSS measurement. Aluminium chloride and PAC were both excellent in colour reduction and these showed up to 60% colour reduction with dosing rate of 1.2 g/L. Ferric chloride provides 44% colour reduction at 0.8 g/L and after an increase in dosing quantity colour reduction was not more and decrease up to 28% at a dosing rate of 1.2 g/L as shown by Fig. 14.
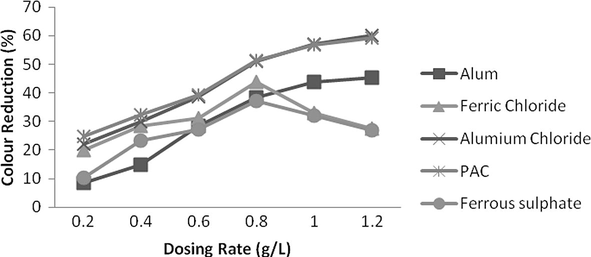
- Effect of dosing rate for various coagulants on colour reduction.
The addition of flocculants was used and an excellent reduction of 93% reduction was measured at a dosing rate of 0.004 g/L at pH 2. Both of anionic and cationic polymer reduced COD up to a dosing rate of 0.004 g/L and then COD increased. This might be due to organic nature of these polymers which increase COD level at higher quantity. In case of TSS, reduction was instantly reduced up to 0.003 g/L and curve becomes less sloppy and almost straight at a dosing rate of 0.005 g/L or above. Anionic PAM was more effective in colour reduction as compared to cationic PAM but was less effective in case of COD reduction. In case of colour, both of these flocculants reduced colour with increasing a dosing rate of polymers but reduction was very remarkable up to 0.005 g/L and after then this change was insignificant and curve becomes almost horizontal to x-axis. Results are shown in Figs. 15–17.
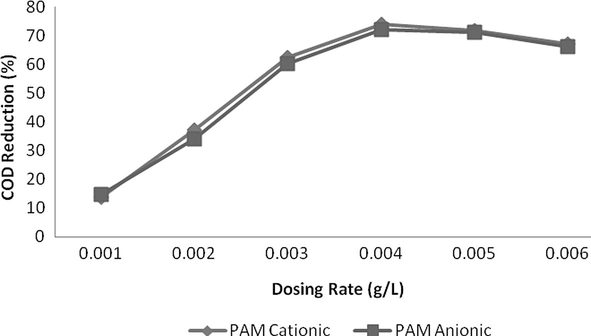
- Effect of dosing rate for Flocculants on COD reduction.
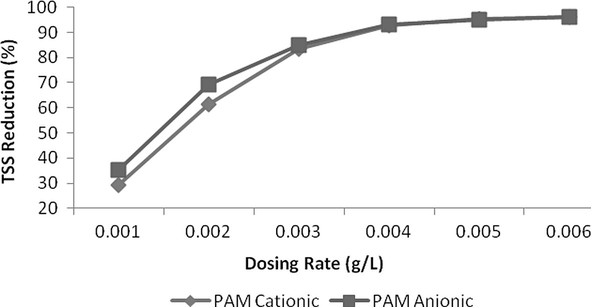
- Effect of dosing rate for Flocculants on TSS reduction.
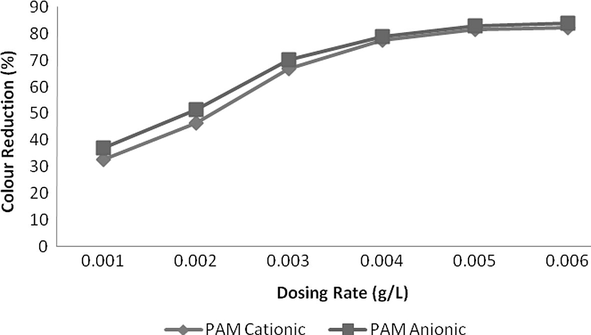
- Effect of dosing rate for Flocculants on Colour reduction.
The effect of change in concentration of both flocculent and coagulant is studied. If concentration of flocculent is kept constant and rate of coagulant is increased consistently than it was observed not a huge reduction in COD, TSS and colour was found. For keeping dosing rate of flocculent as 0.004 g/L but changing the coagulant concentration from 0.2 g/L up to 1 g/L the increase in COD, TSS and colour reduction was only 2%, 3% and 6%, respectively. On contrary, if concentration of flocculent was changed from 0.001 g/L up to 0.005 g/L with constant concentration of coagulant as 0.4 g/L, the change in reduction in COD, TSS and colour was increased from 57%, 67% and 49% as shown in Figs. 18–23. It means that change in concentration of flocculants has played more vital role as compared to coagulant.
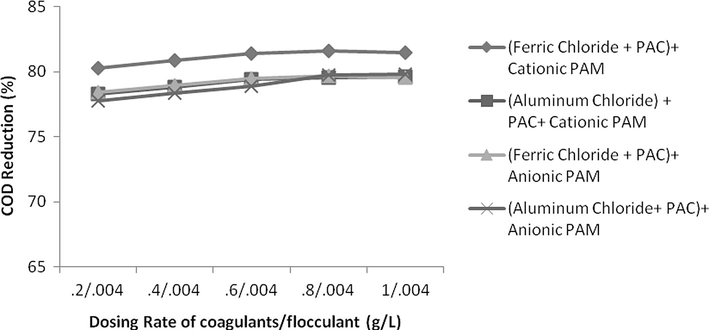
- Effect of dosing rate by keeping constant flocculants conc. and variable coagulant conc. on COD reduction.
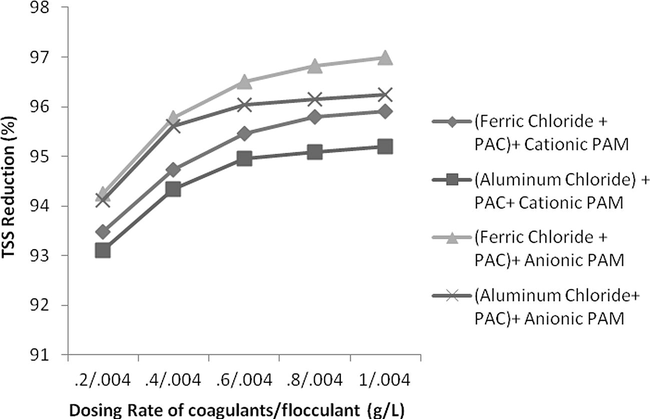
- Effect of dosing rate by keeping constant flocculants conc. and variable coagulant conc. on TSS reduction.
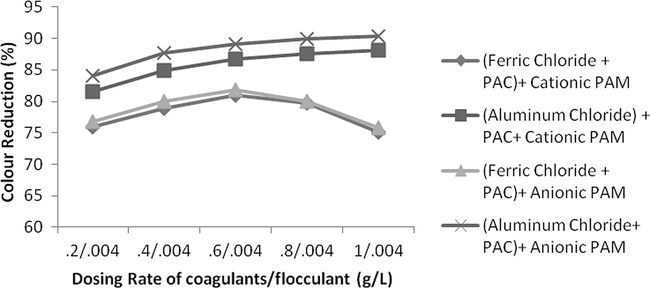
- Effect of dosing rate by keeping constant flocculants conc. and variable coagulant conc. on colour reduction.
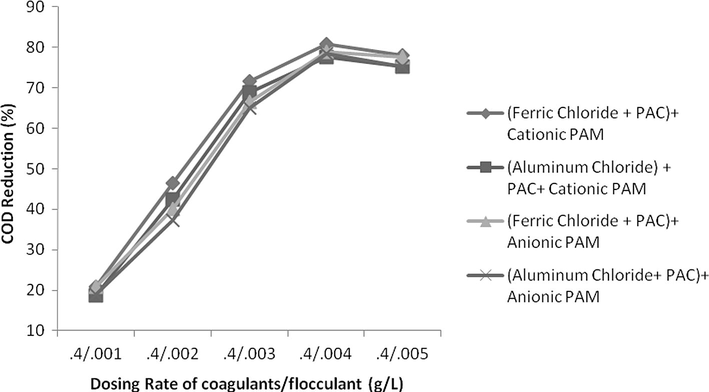
- Effect of dosing rate by keeping constant coagulants conc. and variable flocculants conc. on COD reduction.
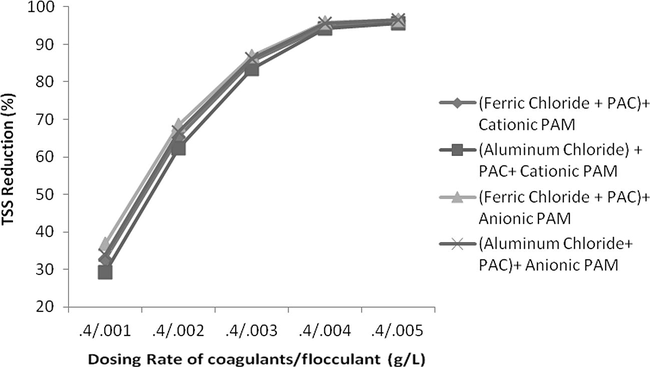
- Effect of dosing rate by keeping constant coagulants conc. and variable flocculants conc. on TSS reduction.
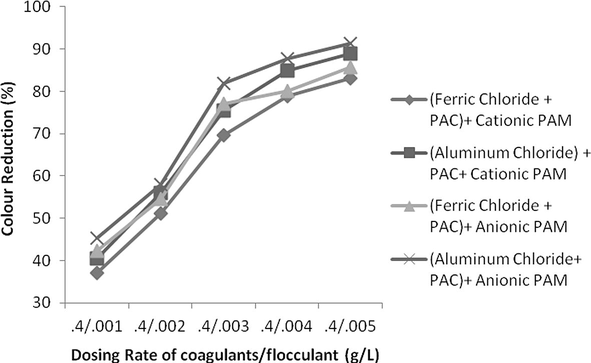
- Effect of dosing rate by keeping constant coagulants conc. and variable flocculants conc. on colour reduction.
4 Conclusion
Present experimental studies of black liquor treatment by various coagulant and flocculants indicate that ferric chloride and aluminium chloride showed good reduction in COD as compared to Alum, PAC and ferrous sulphate. Aluminium chloride was exceptionally good in reducing colour of pulping wastewater. It was also noted that selection of appropriate pH was necessary as it plays a major role in the reduction of all parameters i.e., COD, TSS and colour while dosing of these coagulants. The optimum pH 3, 4, and 5 were optimum values for Aluminium chloride and Ferric chloride, PAC and Alum and ferrous sulphate, respectively. Maximum COD, TSS and colour reduction of 16%, 50%, 46% and 13%, 48%, 52% was achieved by using Ferric chloride and Aluminium chloride at their optimum pH. The addition of cationic PAM and anionic PAM was found to be excellent for reduction of COD, TSS and colour at optimum pH value of 2. The reduction in COD, TSS and Colour by PAM cationic and PAM anionic was measured in order of 74%, 93%, 77% and 72%, 93%, 78%, respectively. The combination of anion and cationic flocculants with ferric chloride, aluminium chloride and PAC was found to be even better in COD, TSS and colour reduction. Combination of Ferric chloride, PAC and cationic polymer was excellent for reduction of 81% COD and 95% TSS while combination of Aluminium chloride, PAC and Anionic PAM was good in 88% colour reduction. On using a combination of flocculants and coagulants, concentration of anionic and cationic PAM plays a more dominant role as compared to concentration of all coagulants.
Reference
- Microbial degradation of pollutants in pulp mill effluents. Adv. Appl. Microbiol.. 2001;48:79-134.
- [Google Scholar]
- Charles Fenerty and His Paper Invention. Toronto: Peter Burger; 2007. pp. 25–30, ISBN 978-0-9783318-1-8
- Biological and advanced treatment of sulfate pulp bleaching effluents. Water Sci. Technol.. 1992;26(1–2):435-444.
- [Google Scholar]
- Colour removal of pulp and paper effluents. Indian J. Chem. Technol.. 2004;11(5):617-621.
- [Google Scholar]
- Microbial decolourisation of pulp mill effluent in presence of nitrogen and phosphorous by activated sludge process. J. Environ. Biol.. 2001;22(No. 1):23.
- [Google Scholar]
- Treatment of wastewater from pulp and paper mill industry by electrochemical methods in membrane reactor. J. Environ. Manag.. 2012;113:399-406.
- [Google Scholar]
- Treatment options for wastewater effluents from pharmaceutical companies. Int. J. Environ. Sci. Technol.. 2011;8:649-666.
- [Google Scholar]
- Biological treatment of papermill wastewater by sequencing batch reactor technology to reduce residual organics. Water Sci. Technol.. 1997;35(1):129-136.
- [Google Scholar]
- Some chemical and toxicological aspects about paper mill effluent treatment with ozone. Environ. Technol.. 2000;21(6):717-721.
- [Google Scholar]
- Thermochemical precipitation as a pretreatment step for the chemical oxygen demand and color removal from pulp and paper mill effluent. Ind. Eng. Chem. Res.. 2005;44(7):2016-2026.
- [Google Scholar]
- Financial analysis review and performance of paper and board industry in Pakistan economy since 2001 to 2010. Global J. Manag. Business Res.. 2012;12(10)
- [Google Scholar]
- Color and phenolic compounds reduction of Kraft Pulp Mill effluent by ozonation with some pretreatments. Am. J. Sci. Ind. Res.. 2011;2(5):798-806.
- [Google Scholar]
- Biomass use in chemical and mechanical pulping with biomass-based energy supply. Resour. Conserv. Recy.. 2007;52(2):331-350.
- [Google Scholar]
- Cost estimates of kraft lignin recovery by ultrafiltration. Desalination. 2009;237(1):254-267.
- [Google Scholar]
- Influence of different combinations of aluminum and iron electrode on electrocoagulation efficiency: application to the treatment of paper mill wastewater. Desalination. 2011;265(1):199-205.
- [Google Scholar]
- Assessing the biological status of fish in a river receiving pulp and paper mill effluents. Environ. Pollut.. 2002;118(1):123-140.
- [Google Scholar]
- Color removal of high strength paper and fermentation industry effluents with membrane technology. Water Sci. Technol.. 1999;40(11):241-248.
- [Google Scholar]
- Treatment of paper and pulp mill effluent by coagulation. IJTEE. 2011;3(3):222-227.
- [Google Scholar]
- Realistic coagulation mechanisms in the use of aluminium and iron (III) salts. Water Sci. Technol.. 1997;36(4):103-110.
- [Google Scholar]
- Treatability of kraft spent liquor by microfiltration and andultrafiltration. Desalination. 2004;160(2):131-141.
- [Google Scholar]
- Removal efficiency of pharmaceuticals and personal care products with varying wastewater treatment processes and operating conditions–Conception of a database and first results. Water Sci. Technol.. 2008;57(1):49-56.
- [Google Scholar]
- Waste management from pulp and paper production in the European Union. Waste. Manag.. 2009;29(1):293-308.
- [Google Scholar]
- Reduction in residual COD in biologically treated paper mill effluents by means of combined ozone and ozone/UV reactor stages. Water Sci. Technol.. 1997;35(2):269-276.
- [Google Scholar]
- Effect of wastewater composition on methanogenic activity in an anaerobic reactor. J. Environ. Sci. Health A. 2004;39(11–12):2941-2953.
- [Google Scholar]
- Treatment of pulp and paper mill wastewater—a review. Sci. Total Environ.. 2004;333(1):37-58.
- [Google Scholar]
- Color removal from industrial wastewater with a novel coagulant flocculant formulation. Int. J. Environ. Sci. Technol.. 2006;3(1):79-88.
- [Google Scholar]
- Industrial Effluents Origin Characteristics Effects Analysis and Treatment. India: Sakthi Publications; 1987.
- Removal of lignin in a liquid system by an isolated fungus. J. Chem. Technol. Biotechnol.. 2001;77:9-14.
- [Google Scholar]
- Enhanced biological treatment of bleached kraft mill effluents—I. Removal of chlorinated organic compounds and toxicity. Water Res.. 2000;34(2):493-500.
- [Google Scholar]
- Color and chlorinated organics removal from pulp mills wastewater using activated petroleum coke. Water Res.. 2001;35(3):745-749.
- [Google Scholar]
- Sjostrom E., ed. Wood Chemistry, Fundamentals and Applications (secoind ed.). San Diego, CA: Academic Press; 1993.
- Thermophilic treatment of bulk drug pharmaceutical industrial wastewaters by using hybrid up flow anaerobic sludge blanket reactor. Bioresour. Technol.. 2009;100(9):2534-2539.
- [Google Scholar]
- The treatment of pulp and paper mill effluent: a review. Bioresour. Technol.. 2001;77(3):275-286.
- [Google Scholar]
- Treatment of pulp and paper mill wastewater by polyacrylamide (PAM) in polymer induced flocculation. J. Hazard. Mater.. 2006;135(1):378-388.
- [Google Scholar]







