Translate this page into:
The synthesis of N2O2-Schiff base ligand and bulk liquid membrane transport of Cu2+
⁎Corresponding author. Tel.: +963 967637990. wailzoubi@yahoo.com (Wail Al Zoubi) wwailalzoubi@yahoo.com (Wail Al Zoubi)
-
Received: ,
Accepted: ,
This article was originally published by Elsevier and was migrated to Scientific Scholar after the change of Publisher.
Peer review under responsibility of King Saud University.
Abstract
New Schiff base (N2O2) containing nitrogen–oxygen atoms was designed and synthesized by the reaction of 5-amino-2-methoxy-phenol with terephthaldialdehyde. Transport of copper ion across bulk chloroform membrane containing (N2O2) as carrier has been studied. The parameters influencing the transport efficiency such as composition of receiving phase, pH of the feed phase, carrier concentration in the membrane, EDTA concentration in the receiving phase, effect of stirring speed, effect of temperature and time dependency of the process were studied and discussed. The selectivity of the processes toward copper ions were tested by performing the competitive transport experiments on the mixture containing Pb2+, Ni2+, Cd2+, Zn2+ and Co2+ ions. The amount of copper transport through the liquid membrane after 90 min was 99% at 25 °C.
Keywords
N2O2-type Schiff base ligands
Bulk liquid membrane
Copper(II) ions separation
1 Introduction
Usually in many analytical methods the performing of a separation step is necessary during the analysis. Among various separation methods (ion exchange, solvent extraction, selective chemical precipitation, etc.) one interesting approach is based on the recognition, binding and release of specific solutes carried out by facilitated transport membranes, i.e. organic liquid in contact with two separated aqueous phases working under chemical gradient as the driving force. This technique has been widely used for carrier metal ion separations (Jonsson and Matiasson, 1992; Safavi and Shams, 1999; Chaudry et al., 1996; Sirlin et al., 1990) and, to a lesser degree, for organic compounds separation (Kuo and Gergor, 1983; Audunsson, 1986).
Copper is an essential trace element for fundamental biochemical processes, but it can also be very toxic. Copper-based drugs are believed to cause cell death by various mechanisms. Excess Cu ions generate reactive oxygen species that have the potential to damage many components of the cellular machinery (Franke et al., 2003; Himelblau et al., 1998; Harrison and Dameron, 1999). Furthermore, the recovery of copper from industrial waste streams prior to discharge (Parthasarathy and Buffle, 1994) and the prevention of metal accumulation in biomaterials has recently become of interest (Drake and Rayson, 1996). Increased attention has, therefore, been focused on the Cu carrier. Copper ion transport has been examined for different lipophilic carriers, including hydroxyoximes (Szpakowska et al., 2004; Lazarova and Boyadzhiev, 1993; Ilias et al., 1999) crown ethers (Dadfarnia and Shamsipur, 1992; Lee et al., 2001; Omar et al., 2004; Akhond and Shamsipur, 1995; Guyan et al., 1999), Schiff bases (Ameerunisha et al., 1994), β-diketones (Bermejo et al., 2000; Cox, 1992) polyamines (Brinchi et al., 2002; 2004) and other chelating agents (Lmela et al., 2004; Cholivand and Khorsandipoor, 2000; Valenzuela et al., 2003; Gherrou and Kerdjoudj, 2003; Kubo et al., 1998) but most of them have slow rates of transport and lack high selectivity.
A variety of Schiff base ligands of types N4 (Oshima et al., 2001, 2002, 2003), N2O2 (Hirayama et al., 1997; Kara and Alkan, 2002) and N2O3 (Saberyan et al., 2008) were used in liquid–liquid extraction studies. Surprisingly, the mobile–carrier properties of these types of ionophores have been rarely studied. Recently, Rouhollahi et al. (2007) reported the uphill transport of Cu2+ ions using a Schiff base, called N,N′-bis(salicylidene)-naphthylene-1,8-diamine.
We report here the transport of copper ions from picrate solution into EDTA solution through a bulk liquid membrane containing N2O2 as carrier.
1.1 Reagents and apparatus
All the used chemicals were purchased from Aldrich or Merck unless otherwise cited. The C, H and N were analyzed on a Carlo-Erba 1106 elemental analyzer. The IR spectra of the ligand were recorded with a Midac 1700 instrument in KBr pellets. The UV–vis measurements were recorded on a Perkin–Elmer λ 20 UV–vis Spectrometer. A pH meter (Metrohm 691 pH Meter) was also used. Transport experiments were conducted using a thermostated (Grants Instruments, model W14, Cambridge, England) apparatus at 298 K and transport experiments were carried out in a U-type cell (Fig. 1).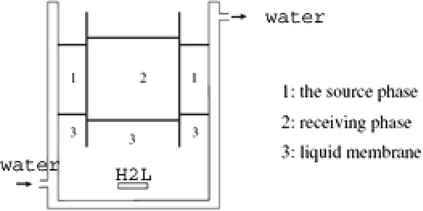
Liquid membrane cell.
1.2 Ligand preparation
In a typical procedure, to a suspension of terephthaldialdehyde (1.34 g, 0.01 mol) in 15 ml of aqueous methanol solution was added 15 ml solution of 5-amino-2-methoxy-phenol (2.78 g, 2 mol) with stirring at 60 °C. The reaction mixture was continuously stirred for 30 min to separate the solid from the reaction mixture and cooled. The solid was filtered and washed with diethyl ether. The corresponding product was recrystallized from methanol and found to be TLC-pure in chloroform and methanol mixture and dried under vacuum over P4O10. Yield%97, m.p. 236–238 °C. Elemental analysis found% (found atomic mass 376 amu), C 70.22%; H 5.4% and N 7.43%. Calculated for C22H20N2O4 (calculated atomic mass 372 amu), C 70.21%; H 5.319% and N 7.44%. IR nmax (film)/cm−1: 2350, 1509, 1582, 1272; ESI m/z calculated for C22H20N2O4 found 376. The series of peaks in the range i.e.39, 52, 65, 79, 96, 100, 124, 145, 159, 173, 195, 209, 237, 253, 289, 315, 333, 348, 361 and 376 amu, etc. may be assigned to various fragments. Their intensity gives an idea of stability of fragments (Fig. 2a).![(a) Electron impact mass spectrum of Schiff base[H2L] (b) IR spectra of Schiff base[H2L].](/content/184/2016/9/5/img/10.1016_j.arabjc.2011.05.006-fig2.png)
(a) Electron impact mass spectrum of Schiff base[H2L] (b) IR spectra of Schiff base[H2L].
The advantages of synthetic procedure are: (1) the process is a simple one step reaction involving inexpensive starting materials: (2) the process is shorter: (3) using water methanol (1:1) as solvent allows the formation of imine without the need for catalysis and (4) yield is high and reaction is fast, and product can be isolated by filtration.
1.3 Transport experiments and analysis
The experimental set-up (Turbeville and Dutta, 1990; Yuzawa et al., 1993) was a double jacket cylindrical glass cell (4.5 cm diameter) holding a glass tube (2.25 cm diameter) for separating the two aqueous phases, which is shown schematically in Fig 1. Temperature of the solution was kept constant using thermostated water circulating through the jacket of the cell. The source phase (10 mL) contained copper picrates [Copper picrates was prepared by successive addition of a 1 × 10−2 mol L−1 metal nitrate solution to 2 × 10−5 mol L−1 aqueous picric acid solution and shaken at 25 °C for 1 h]. This metal picrate was measured by UV–vis using maximum wavelength 352 nm. The receiving phase (20 mL) included EDTA solution (0.1 mol L−1). The pH of the source was adjusted using NaOH or HCl solutions. The aqueous phases were bridged by a chloroform solution (50 mL) of the investigated Schiff bases (N2O2), which is placed below them. The experiment was started by stirring the organic phase (500 rpm) and the concentration of the metals in the aqueous phases was determined by UV–vis using maximum wavelength 352 nm.
The appearance of the picrate ion in the receiving and source phase was followed by UV spectrophotometry at regular time intervals. Experiments with no carrier present were performed, indicating no transport of metal picrates. The experimental procedure has already been described elsewhere (Marcos et al., 1999) as well.
2 Results and discussion
The preparation of ligand containing nitrogen and oxygen donor atoms are shown in Scheme 1. The structure of the new compound was characterized by a combination of IR, elemental analysis, NMR and MS spectral data. Compound was synthesized according to reported procedure (Lazarova and Boyadzhiev, 1993).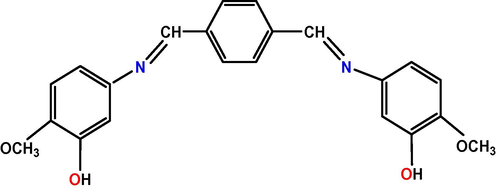
Synthesis of Schiff base.
The IR spectra (Fig. 2b) of the free ligand exhibit various bands in the 400–4000 cm−1 region. A full assignment of the IR spectra of Schiff bases is very difficult due to the extensive vibrational coupling in the molecules (Turbeville and Dutta, 1990; Yuzawa et al., 1993; Majerz et al., 2000; Ognyanova et al., 1999; Ambroziak et al., 2002). The observed bands in the “fingerprint” region are the result of strongly mixed vibrations. The O–H stretching frequency of the free ligand is expected in 3200–3800 cm−1 region, however this frequency was at 3350 cm−1, for the Schiff base. Very strong band near 1272 cm−1 have been assigned to in-plane bending (O–H) vibrations of the ligand (Zolezzi et al., 1999; Percy et al., 1973). The C⚌N stretching frequencies are in the 1509–1582 cm−1 region as reported for similar ligands (Turbeville and Dutta, 1990; Yuzawa et al., 1993; Majerz et al., 2000; Downing and Urbach, 1969).
The liquid membrane used in this study is shown schematically in Fig. 1. The Cu2+ ion is transported from the source phase to the receiving phase via a chloroform membrane. After complexation of the carrier with Cu2+ ion on the left side of the membrane, the complex diffuses down its concentration gradient. On the right side of the membrane, the metal ion would be released into the receiving phase via formation of a ternary complex (carrier-metal ion-EDTA). At this stage, the free carrier diffuses back across the liquid membrane. The net result is the transport of Cu2+ ion from the aqueous source phase to the aqueous receiving phase across the bulk of organic phase.
In the preliminary experiments, it was found that N2O2 had the desired ability for the extraction of the copper ion from the source phase into the organic membrane. Regarding the fact that this transport is proton-driven, it is expected that the transport of copper to the receiving phase be completed by the time the receiving phase acidified. Despite adding different concentrations of various acid solutions, the transport of copper into the receiving phase was quite low. In the next step, the different complexing agents that are well known to form very stable complexes with copper ion were added to the receiving phase at various concentrations and measurements. But in the best state, the maximum percentage of copper transported into the receiving phase was about 15% (Fig. 3). In all the experiments, the concentration of copper in the source phase decreased to about 20–30% after 2 h. It reveals that the problem is in the back extraction stage, where the exchange of Cu2+ and H+ in the membrane/receiving phase interface had some difficulties. We think this problem might have arisen from low surface area of the liquid membrane/aqueous receiving phase and/or low interaction of the complexed copper ion in the liquid membrane and the receiving phase. This causes high activation energy for the transfer of copper ion from chloroform to the aqueous receiving phase and a phase transfer catalyst is needed to improve the transfer process kinetics by lowering its activation energy.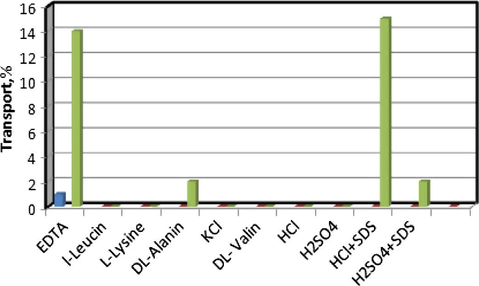
Effect of composition of receiving phase on the transport of copper ions. Conditions, source phase, 20 ml of 2.0 × 10−5 mol L−1 Cu(II) ion; liquid membrane phase, 50 ml of 4.0 × 10−4 mol L−1 N2O2 in chloroform; receiving phase, 20 ml solution of 0.1 mol L−1 time of transport, 2 h.
Surfactant systems have been recognized as very useful agents for improving surface properties and development of new concepts in analytical chemistry. Surfactants, well known as wetting agents, lower the surface tension of a liquid, allow easier spreading, and reduce the interfacial tension between the two liquids (Emadi et al., 2007; Yaftian et al., 2005). Surfactants are usually organic compounds that are amphipathic, meaning they contain both hydrophobic groups (their “tails”) and hydrophilic groups (their “heads”). Therefore, they are typically sparingly soluble in both organic solvents and water. Surfactants reduce the interfacial tension between organic solvents and water by being adsorbed at the liquid–liquid interface. They are coupling agents normally functioning at the interface between bulk materials (for example; organic solvents and water) such that what would not normally mix does so in the form of an emulsion or colloid. The hydrophobic non-polar end attaches to organic solvents while the hydrophilic end attaches to water (Yilmaz et al., 2008; Asharf, 2004; Pretsch et al., 2000; Hinze, 1979; Cline Love et al., 1984). In fact, they serve as a bridge between two immiscible solvents in liquid–liquid interface through which the transport and exchange of molecules and ions take place easily. Although the maximum percentage of copper that transports to the receiving phase that contains 0.1 M hydrochloride acid after 2 h does not exceed 100%, the percentage does exceed 16% only after 2 h when sub-millimolar quantities of the anionic surfactant, sodium dodecyl sulfate (SDS), is added to the receiving phase. It seems that the surfactants lying at the membrane/receiving phase interface catalyze the exchange process of Cu2+ and H+ to the carrier.
2.1 Effect of pH of the source phase
The effect of pH of the source phase on the efficiency of copper transport was studied (Fig. 4). The results revealed that the maximum copper transport occurs at pH 5. At lower pH values there was a decrease in the percentage of copper transport, probably due to a decrease in the hydroxide concentration for exchanging protons with Cu2+ at the interface of the source/membrane phase or slow partition coefficients of Cu2+ at interface between the source/membrane phases. The efficiency of copper transport decreases at higher values, probably due to the complex formation of copper ion with hydroxide. From the results, a pH of 4.8 was selected for further studies.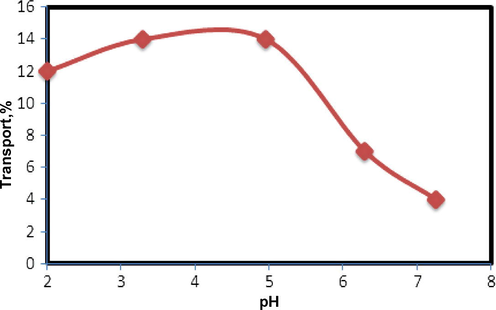
Effect of pH of the source phase on the transport of copper ions. Conditions; source phase, 20 ml of 2.0 × 10−5 mol L−1 Cu(II) ion at various pH; liquid membrane phase, 50 ml of 4.0 × 10−4 mol L−1 N2O2 in chloroform; (
 ) receiving phase, 20 ml solution of 0.1 M EDTA, time of transport, 2 h.
) receiving phase, 20 ml solution of 0.1 M EDTA, time of transport, 2 h.
According to the couple-proton mechanism that we suggested for the Cu2+ transport, we should adjust a basic media for the source phases. It was observed that the carrier is soluble in basic media, therefore, we had to work in pH of distilled water.
2.2 Effect of N2O2 concentration in the organic phase
The influence of N2O2 concentration in the organic phase on the transport efficiency of copper was also studied. The Fig. 5 shows the percentage of copper transport increases with an increase in N2O2 concentration in the organic phase. Maximum transport occurs at a concentration of about 8.0 × 10−4 mol L−1 N2O2. Further excess of the carrier had no considerable effect on the transport efficiency.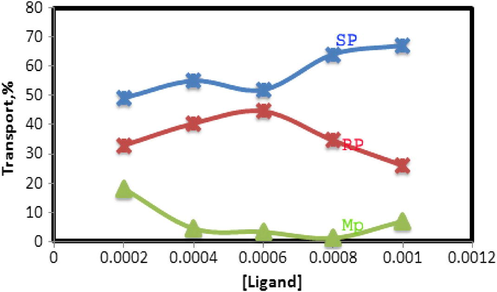
Effect of N2O2 concentration in the membrane phase on the transport of copper ions. Conditions; source phase, 20 ml of 2 × 10−5 mol L−1 Cu(II) ion and pH 4.8; liquid membrane phase, 50 ml of N2O2 in chloroform at different concentrations; receiving phase, 20 ml solution of 0.1 mol L−1 EDTA; transport time, 2 h.
2.3 Effect of time on transport
Fig. 6 shows the time dependence of copper transport through the liquid membrane under experimental conditions. It is obvious that the extraction of copper ion from the source phase into the organic membrane occurs almost completely after 100 min. However, the plot shows the transport rate decreases gradually with time and a steady state situation is reached in which the amount of Cu2+ ions in MP hardly changes, whereas the decrease in SP and increase in RP are constant and equal. After 2 h, the concentrations of Cu2+ ions were independent of time and transport was completed.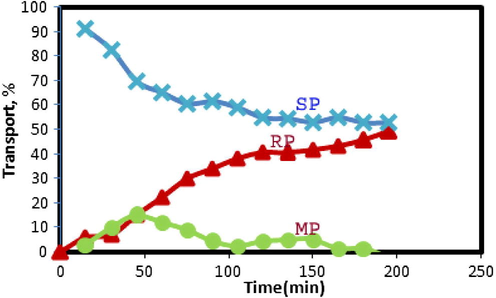
Percentage of the transported copper ions from source phase into the receiving phase through the bulk liquid membranes containing N2O2 ligand as a function of time. Conditions, source phase, 20 ml of 2 × 10−5 mol L−1 Cu(II) ion and pH 4.8; liquid membrane phase, 50 ml of N2O2 in chloroform at concentration (4 × 10−4 mol L−1); receiving phase, 20 ml solution of 0.1 mol L−1 EDTA; time transport, 2 h.
It was found that, under the optimum conditions, the transport of copper ion from the aqueous source phase into the receiving phase after 2.5 h is almost quantitative.
2.4 Effect of EDTA concentration in the receiving phase
The effect of EDTA concentration in the receiving phase on efficiency of copper transport was also investigated (Fig. 7), and it was found that maximum copper transport occurs at EDTA 0.09–0.1 M.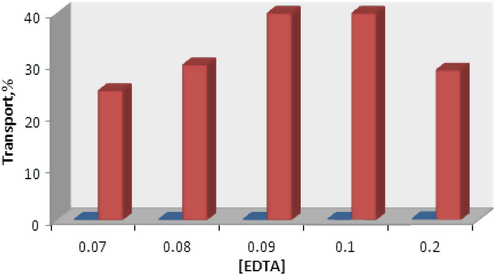
Effect of EDTA concentration in the receiving phase in the transport of copper ions. Conditions, source phase, 20 ml of 2.0 × 10−5 mol L−1Cu(II) ion and pH 4.8; liquid membrane phase, 50 ml of N2O2 in chloroform at concentration(4 × 10−4 mol L−1); receiving phase, 20 ml solution of EDTA different concentrations, time of transport, 2 h.
2.5 Effect of the stirring speed on the transport efficiency of copper(II)
It is well known that hydrodynamic conditions play an important role in mass transfer from one phase to another phase at the interface between two liquid phases. In order to examine the effect of hydrodynamics on the copper ion transfer, experiments were carried out with different stirring speeds (80–300 rpm). A plot of %Cu in SP and RP against stirring speed is given in Fig. 8.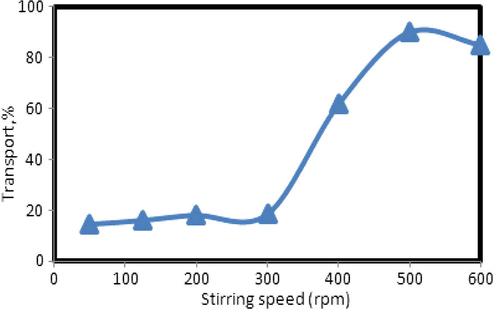
Effect of the stirring speed on the transport efficiency of copper ions. Conditions are given in Fig. 5.
As can be seen, the ion transport at the SP/membrane interface shows a curve in the range 50–500 rpm, as would be expected for diffusion-controlled transport, with a leveling off at values >300 rpm.
2.6 Effect of temperature
The effect of temperature on the transport of the Cu2+ through the liquid membrane containing N2O2 in CHCl3 was examined at 298, 305, 323 and 330 °K, respectively. The experimental results are shown in Fig. 9. It is quite obvious that transport increases with an increase in the temperature.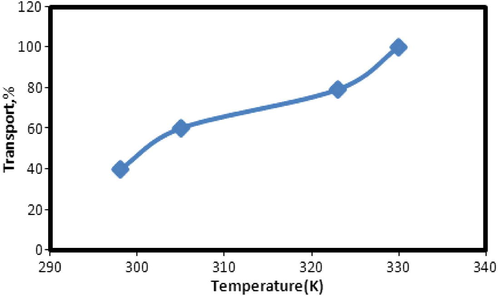
Effect of temperature on the transport efficiency of copper ions. Experimental conditions are given in Fig. 6.
2.7 Selectivity of the processes
Selectivity of the transport experiments using N2O2 carrier under similar experimental conditions toward copper ions over lead, nickel, cobalt, cadmium and zinc ions from binary mixtures was evaluated (Table 1). The results present the suitable selectivity of the studied carriers toward copper ions over the examined ions. It is noteworthy that, the presence of other metal ions in the mixture decreases the percentage of copper transported in comparison with those in the single species experiments. This is probably due to a multi-ion competition or crowding effect (Alguacil et al., 2004; De Gyves and Rodriguez de, 1999; Parinejad and Yaftian, 2007).
Mixture
M2+
Transported value (%)
Mix. 1
Cu2av
88.2
Zn2+
9
Mix. 2
Cu2+
81.7
Co2+
13.6
Mix. 3
Cu2+
88.2
Pb2+
22
Mix. 4
Cu2+
80.1
Ni2+
5.5
Mix. 5
Cu2+
89.6
Cd2+
2
2.8 Suggested mechanism
The copper ions are transported from source phase to the receiving phase through a chloroform membrane with simultaneous counter-transport of protons. The Cu2+ transport can be explained as follows: at source phase/membrane interface, the carrier complex of copper ion forms uncharged complex CuL. At this stage, the carrier splits off protons into the source phase.
The formed complex diffuses across the membrane and at the membrane/receiving phase interface, the release of the Cu2+ ion into the receiving phase occurs via the decomposition of the complex. At this stage, the carrier associates with proton from the receiving phase releasing Cu2+ ion into the receiving phase. The free carrier diffuses back across the membrane to the source phase/membrane interface, where the cycle starts again.
3 Conclusion
In the first stage, substituted Schiff base is obtained from the condensation of terephthaldialdehyde and 5-amino-2-methoxy-phenol in dry ethanol. The present study demonstrates that the ligand N2O2 is an excellent carrier for efficient transport of Cu2+.
Uphill transport of Cu2+ against its concentration gradient is easily performed with the illustrated system (Fig. 10). Also, this study demonstrates the usefulness of the liquid membrane technique for making it possible to combine extraction and stripping operations in a single process and reducing the solvent inventory requirements. In conclusion, this system has the advantages of high precision, efficiency, simplicity and speed. Also the percentage of copper transport is reached at 98% at temperature of 330 °K and stirring speed (500 rpm) after 48 min.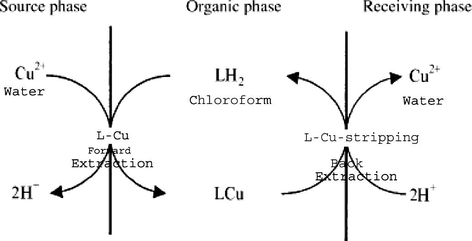
Liquid membrane system for transport of Cu (II) ions.
The simplicity, excellent efficiency and high selectivity for Cu2+ ion transport shown by the membrane system demonstrate its potential applicability to the selective separation, concentration, or purification of copper from mixtures.
References
- Sep. Sci. Technol.. 1995;30:3061-3072.
- Chemosphere. 2004;57:813-819.
- J. Mol. Struct.. 2002;615:109.
- Bull. Chem. Soc. Jpn. 1994;67:263.
- Tetrahedron. 2004;60:1541-1548.
- Anal. Chem.. 1986;58:2714-2723.
- J. Chem. Res., Synop.. 2000;587:479-481.
- Eur. J. Org. Chem.. 2002;5:930.
- Eur. J. Org. Chem.. 2004;5:1330-1335.
- Sep. Sci. Technol.. 1996;31:1309-1326.
- J. Membr. Sci.. 2000;180:115-120.
- Anal. Chem.. 1984;56:1132A-1148A.
- Principles and Practice of Solvent Extraction. New York: Marcel Dekker; 1992.
- J. Membr. Sci.. 1992;75:61-68.
- Ind. Eng. Chem. Res.. 1999;38:2182.
- J. Am. Chem. Soc.. 1969;91:5977-5983.
- Anal. Chem.. 1996;68:22A-27A.
- Turk. J. Chem.. 2007;31:423-433.
- J. Bacteriol.. 2003;185:3804-3814.
- Desalination. 2003;158:195-200.
- Anal. Chem.. 1999;1:819-826.
- J. Biochem. Mol. Toxicol.. 1999;13:93-106.
- Plant Physiol.. 1998;117:1227-1234.
- Solution Chemistry of Surfactants. New York: Plenum; 1979. p. 79
- Anal. Chem.. 1997;69:4814-4818.
- Sep. Sci. Technol.. 1999;34:1007-1019.
- TRAC. 1992;11:106-114.
- Microchim. J.. 2002;7:129.
- Talanta. 1998;45:963-968.
- Sep. Sci. Technol.. 1983;18:421-440.
- J. Membr. Sci.. 1993;78:239-245.
- Inorg. Chim. Acta. 2001;317:174-180.
- J. Chem. Technol. Biotechnol.. 2004;79:229-305.
- J. Mol. Struct.. 2000;552:243-247.
- J. Phys. Org. Chem.. 1999;12:695.
- J. Mol. Struct.. 1999;513:139.
- Sep. Sci. Technol.. 2004;39:1681-1693.
- Anal. Chim. Acta. 2001;441:157-164.
- Anal. Sci.. 2002;18:1351-1357.
- Talanta. 2003;59:867-874.
- Iran. J. Chem. Chem. Eng.. 2007;26:19-27.
- Nucl. Chem.. 1973;35:2319-2327.
- Pretsch, E., Buhlmann, P., Affolter, C., 2000. Structure Determination of Organic Compounds, Springer-Verlag, Berlin, Heidelberg, New York.
- Sep. Purif. Technol.. 2007;54:28-33.
- Bull. Korean Chem. Soc.. 2008;29:94-98.
- Talanta. 1999;48(5):1167-1172.
- J. Membr. Sci.. 1990;54:299-305.
- Sep. Sci. Technol.. 2004;39:699-707.
- J. Phys. Chem.. 1990;94:4060-4066.
- J. Chil. Chem. Soc.. 2003;48:79-84.
- Sep. Sci. Technol.. 2005;40:2709-2719.
- Polyhedron. 2008;27:125-132.
- Chem. Phys. Lett.. 1993;202:221-226.
- Polyhedron. 1999;18:897-904.







