Translate this page into:
Theoretical study of the reactivity of (CH3)2AlCH2I promoted cyclopropanation reactions
*Corresponding author lichaohui@gmail.com (Zhao-Hui Li)
-
Received: ,
Accepted: ,
This article was originally published by Elsevier and was migrated to Scientific Scholar after the change of Publisher.
Peer-review under responsibility of King Saud University.

Available online 10 July 2010
Abstract
Density functional theory calculations are reported for the cyclopropanation reactions of (CH3)2AlCH2I with ethylene for two reaction channels: methylene transfer and carbometalation. These computational results suggest that the methylene transfer process is favored and the competition from the carbometalation pathway is negligible.
Keywords
Density functional theory
Aluminum carbenoid
Methylene transfer
Carbometalation
1 Introduction
Cyclopropane moieties have been found in wide-range of natural and artificial compounds that exhibit important biological activities and in an array of substances used as starting materials and intermediates in organic synthesis (Rappoport, 1987; Fritschi et al., 1986; Evans et al., 1991; Rodriguez et al., 1993; Zhao et al., 1994; Nishiyama et al., 1994, 1995a,b; Doyle, 1995; Doyle et al., 1998; Boger et al., 1999; Salaun, 2000; Che et al., 2001; Rodriguze-Garcia et al., 2001). This has motivated a large number of research groups to develop new and wide-range methods to produce cyclo-propanated products. A reaction between iodomethylzinc iodide and an olefin that produces a cyclopropane compound was first reported by Simmons and Smith (1959) and Simmons et al. (1973) and is now called the Simmons–Smith (SS) reaction, which is the method for synthesizing cyclo-propanated products from olefins using a metallic carbenoid (SS) reagent. Following this a great deal of work have been done to improve and develop alternative methods to produce Simmons–Smith-type reagents. Many researchers have been trying to find more efficient and highly diastereoselective cyclo-propanating reagents. Metallic carbenoid is an important intermediate in organic synthesis for cyclopropanation reaction. In 1985, there were experimental reports that Al carbenoid is an efficient S–S reagent (Rubottom et al., 1985). The Al/CH2I2 carbenoid is believed to be one of the most efficient and highly diastereoselective cyclo-propanating reagents. The cyclopropanation reactions are usually performed at −40 °C and high yields of cyclo-propanated products can be achieved.
It has been proposed that the carbenoid-promoted cyclopropanation reactions of interest proceed through two likely reaction pathways: methylene transfer and carbometalation (see Scheme 1). The reaction mechanism is system-dependent. For zinc carbenoids, it is thought that the methylene transfer mechanism represents the reaction reality (Rubottom et al., 1985; Charette and Marcoux, 1995; Charette and Beauchemin, 2001; Furukawa et al., 1966; Wittig and Schwarzenbach, 1959; Denmark and Edwards, 1991; Closs and Moss, 1964; Molander et al., 1987; Molander and Harring, 1989; Maruoka et al., 1985, 1989; Bernardi et al., 1997; Dargel and Koch, 1996; Nakamura et al., 1998, 2003; Hermann et al., 2000; Boche and Lohrenz, 2001; Fang et al., 2002; Wang et al., 2002; Zhao et al., 2002). Samarium carbenoid cyclopropanation reactions are believed to have some competition between the methylene transfer mechanism and the carbometalation mechanism (Stiasny and Hoffman, 1995; Zhao et al., 2003; Wang et al., 2004). As for lithium carbenoids, Hoffmann (Stiasny and Hoffman, 1995) reported a possible alternative carbometalation/methylene transfer pathway. In this paper, aluminum carbenoid-promoted cyclopropanation reactions are investigated using theoretical methods. We found that the aluminum carbenoids have a “metal carbene complex” character similar to the classical Simmons–Smith carbenoids previously investigated using density functional theory (DFT) calculations. Our result for the aluminum carbenoids shows that the methylene transfer pathway is favored and competition from the carbometalation pathway is very small.
2 Computational details
The hybrid B3LYP density functional method (Becke, 1993, 1988; Lee et al., 1988) was used to investigate the cyclopropanation reaction mechanisms of the aluminum carbenoids with ethylene. The stationary structures of the potential energy surfaces were fully optimized at the B3LYP level of theory. Analytical frequency calculations at the same level of theory were performed in order to confirm the optimized structures to either a minimum or a first-order saddle-point as well as to obtain the zero-point energy correction. Furthermore, intrinsic reaction coordinate (IRC) calculations Gonzalez and Schlegel, 1989, 1990 were performed to confirm that the optimized transition state correctly connects the relevant reactants and products. Geometry optimization for all of the reactants, intermediates, transition states and products as well as the frequency calculations were carried out with the 6-311G∗∗ basis set for all atoms of the reactions investigated (Glukhovtsev et al., 1995). All the calculations were carried out using the Gaussian 98 and Gaussian 03 program suites (Frisch et al., 1998).
3 Results and discussion
3.1 Cyclopropanation reaction of the (CH3)2AlCH2I with ethylene
The optimized geometry for the Al carbenoid (CH3)2AlCH2I is shown in Fig. 1 along with the optimized geometry of the reactant complex (RC1) and the transition states (TS1, TS2) for cyclopropanations of ethylene through two different pathways to produce cyclopropane (c-C3H6) and (CH3)2AlI. The methylene transfer pathway involves a concerted[2 + 1] addition through a transition state, TS1, in which the pseudotrigonal methylene group of the carbenoid adds to the ethylene π-bond to form new C–C bonds asynchronously. This process is accompanied by a 1,2-migration of the I anion from the carbon atom to the aluminum atom. According to the transition state proposed by Simmons and Smith (1959) and Moser (1969), this “butterfly” transition structure can explain the stereochemical features of this type of reaction. Another pathway named a carbometalation process, involves a[2 + 2] addition of ethylene to the Al–C bond to form an intermediate (IM) through a four-centered transition state (TS2). A subsequent intramolecular substitution reaction of this intermediate produces the final cyclopropane product. In the methylene transfer pathway, the Al carbenoid (CH3)2AlCH2I approaches ethylene from above the molecular plane in an asymmetric manner; while in the carbometalation process, the ethylene molecule simultaneously moves to the Al carbenoid to form a π-complex which can be regarded as the reactant complex for both reaction pathways. In the transition structure TS1, the ethylene molecule has changed its planar structure with a significant pyramidalization of about 7.3° for C2, which indicates that the sp2 → sp3 rehybridization is necessary for cyclopropane formation; whereas the pyramidalization of C3 is only 0.8°. There is another significant piece of evidence for an asynchronous approach of the CH2CH2 molecule in the methylene transfer mechanism. The C1–C2 distance in TS1 is 2.224 Å, which is 0.257 Å shorter than the C1–C3 distance. The interactions of the (CH3)2AlCH2I moiety with the π-olefin orbital are mainly responsible for the slight lengthening of C2⚌C3 bond and C1–Al bond from the reactant complex (RC1) to the transition state (TS1) where the C2⚌C3 bond length is elongated by 0.015 Å and the C1–Al bond length is elongated by 0.046 Å, respectively. Relatively large changes are associated with the ∠I–C1–Al, the ∠I–Al–C1, the C1–I and Al–C1 distances that vary from 102.5°, 41.31°, 2.227 Å, 1.989 Å in RC1, to 65.4°, 71.45°, 2.819 Å, 2.035 Å in TS1, respectively, as shown in Fig. 1. Notably, in the transition state TS1, the C1–I bond becomes nearly broken and the electron-rich I atom is attracted by the metal center to almost result in a complete Al–I bond. These changes in the bond lengths and angles are attributed to partial formation of the (CH3)2AlI byproduct in the transition state. The Al–I interaction is believed to give a sufficient compensation for the weakening of the Al–C1 bond from RCI to TS1. As shown in Fig. 1, the methylene transfer pathway has a barrier of 14.4 kcal/mol in (CH3)2AlCH2I and is exothermic by about 28.7 kcal/mol at the B3LYP level of theory. Vibrational analysis shows that the TS1 structure is a first-order saddle-point with only one imaginary frequency of 289i cm−1 and the IRC calculations confirmed that TS1 connects the corresponding reactant RC1 and products (c-C3H6) and (CH3)2AlI. Thus, it is evident that TS1 is the transition state of the concerted reaction of (CH3)2AlCH2I with ethylene through the methylene transfer pathway.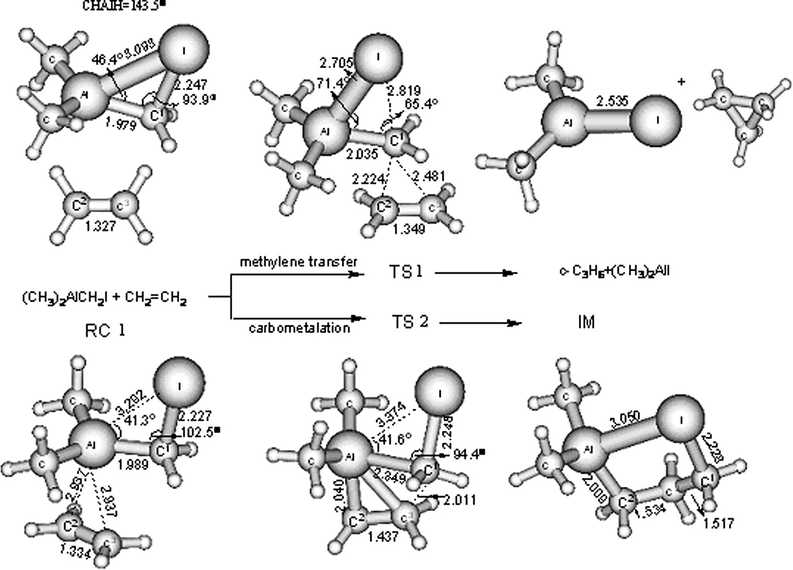
Schematic diagrams of the optimized geometry from the B3LYP/6-311G∗∗ level computations for the aluminum carbenoid (CH3)2AlCH2I, reactant complex RC1, the intermediate IM as well as the transition state for the cyclopropanation with ethylene. TS1 = transition state for the methylene transfer for the reaction of (CH3)2AlCH2I with ethylene. TS2 = transition state for carbometalation for the reaction of (CH3)2AlCH2I with ethylene. Selected structural parameters are shown for each species with the bond lengths in Å and bond angles in degrees.
With regard to the carbometalation pathway, there is an insertion reaction of the ethylene to the Al–C1 bond to produce the intermediate IM through a four-centered TS2 transition state. Compared with the methylene transfer pathway, the carbometalation pathway has larger changes in the geometry from the reaction complex to the transition state. The Al–C2 interaction increases significantly from 2.937 Å in RC1 to 2.04 Å in TS2. The C1–C3 goes from a distance 3.296 Å in RC1 to 2.011 Å in TS2. This is accompanied by the weakening of the C1–Al bonds from 1.989 Å in RC1 to 2.349 Å in TS2. It is interesting that the C1–I bond length and the Al–I interaction only change very slightly during the process from RC1 to TS2. This is different from the methylene transfer pathway and indicates that the Al–I interaction contributes little to the weakening of the C1–Al bonds of TS2 in the carbometalation process. Thus, more energy is needed to overcome the barrier in carbometalation pathway from RC1 to TS2. The reaction barrier height at the B3LYP/6-311G∗∗ level of theory for the reaction system of (CH3)2AlCH2I + CH2CH2, is calculated to be 32.82 kcal/mol. The barrier height of 32.82 kcal/mol predicts that the reaction does not occur easily under room-temperature conditions. Vibrational analysis found that the optimized TS2 structure had one imaginary frequency of 391i cm−1 and was confirmed to connect the corresponding reactants and products by IRC calculations.
4 Conclusions
In this paper we have studied, using a DFT approach, the potential energy surfaces for the reactions between ethylene and (CH3)2AlCH2I carbenoids which represent model systems for aluminum carbenoid-promoted cyclopropanation reactions. Two reaction channels were investigated: methylene transfer and carbometalation. The energy barriers for the methylene transfer pathway (14.4 kcal/mol) are significantly smaller than those of the carbometalation pathway (about 32.82 kcal/mol). The methylene transfer process is favored and the competition from the carbometalation process is likely to be very small and this is consistent with experimental results. We have also demonstrated that the methylene transfer transition state corresponds to a three-centered structure similar to that originally suggested by Simmons and Smith (1959) and Moser (1969). The reactant complexes located on the reaction surface appear to form without any barrier. Our results are consistent with and can help explain the experimental observation that Al carbenoids can undergo efficient cyclopropanation reactions with olefins at −40 °C.
References
- Phys. Rev. A. 1988;38:3098-3100.
- J. Chem. Phys.. 1993;98:5648-5652.
- J. Am. Chem. Soc.. 1997;119:12300-12305.
- Chem. Rev.. 2001;101:697-756.
- Angew. Chem. Int. Ed.. 1999;38:2424-2426.
- Org. React. (N.Y.). 2001;58:1-9.
- Synlett 1995:1197-1207.
- J. Am. Chem. Soc.. 2001;123:4119-4129.
- J. Am. Chem. Soc.. 1964;86:4042-4053.
- J. Chem. Soc. Perkin Trans.. 1996;2:877-881.
- J. Org. Chem.. 1991;56:6974-6981.
- Hegedus L.S., ed. Comprehensive Organometallic Chemistry II. Vol vol. 12. Oxford, UK: Pergamon; 1995.
- Modern Catalytic Methods for Organic Synthesis with Diazo Compounds. New York: Wiley; 1998.
- J. Am. Chem. Soc.. 1991;113:726-728.
- J. Org. Chem.. 2002;67:154-160.
- Frisch, M.J., Trucks, G.W., Schlegel, H.B., Scuseria, G.E., Robb, M.A., Cheeseman, J.R., Zakrzewfki, V.G., Montgomery, J.A., Stratmann, R.E., Burant, J.C., Dapprich, S., Millam, J.M., Daniels, A.D., Kudin, K.N., Strain, M.C., Farkas, O., Tomasi, J., Barone, V., Cossi, M., Cammi, R., Mennucci, B., Pomelli, C., Adamo, C., Clifford, F., Ochterski, J., Petersson, G.A., Ayala, P.Y., Cui, Q., Morokuma, K., Malick, D.K., Rabuck, A.D., Raghavachari, K., Foresman, J.B., Cioslowski, J., Ortiz, J.V., Stefanov, B.B., Liu, G., Liashenko, A., Piskorz, P., Komaromi, I., Gomperts, R., Martin, R.L., Fox, D.J., Keith, T., Al-Laham, M.A., Peng, C.Y., Nanayakkara, A., Gonzalez, C., Challacombe, M., Gill, P.M.W., Johnson, B.G., Chen, W., Wong, M.W., Andres, J.L., Head-Gordon, M., Replogle, E.S., Pople, J.A., 1998. Gaussian 98, Revision A.11 and Gaussian 03 Revision C.02. Gaussian Inc., Pittsburgh, PA.
- Angew. Chem. Int. Ed. Engl.. 1986;25:1005-1006.
- Tetrahedron Lett. 1966:3353-3354.
- J. Chem. Phys.. 1995;103:1878-1885.
- J. Chem. Phys.. 1989;90:2154-2161.
- J. Phys. Chem.. 1990;94:5523-5527.
- Tetrahedron. 2000;56:4109-4115.
- Phys. Rev. B. 1988;37:785-789.
- Org. Chem.. 1985;50:4412-4414.
- Org. Synth.. 1989;67:176-179.
- J. Org. Chem.. 1989;54:3525-3532.
- J. Am. Chem. Soc.. 1987;109:453-463.
- J. Am. Chem. Soc.. 1969;91:1135-1140.
- J. Am. Chem. Soc.. 1998;120:5844-5845.
- J. Am. Chem. Soc.. 2003;125:2341-2350.
- J. Am. Chem. Soc.. 1994;116:2223-2224.
- Bull. Chem. Soc. Jpn.. 1995;68:1247-1262.
- Chem. Lett.. 1995;25:1071-1072.
- Rappoport, Z. (Ed.), 1987. The Chemistry of the Cyclopropyl Group. Wiley, Chichester, UK.
- Tetrahedron Lett.. 1993;34:6233-6236.
- J. Am. Chem. Soc.. 2001;123:6157-6163.
- J. Am. Chem. Soc.. 1985;107:4230-4233.
- de Meijere A., ed. Small Ring Compounds in Organic Synthesis, VI. Vol vol. 207. Berlin: Springer; 2000. p. :1-67.
- J. Am. Chem. Soc.. 1959;81:4256-4264.
- Org. React. (N.Y.). 1973;20:1.
- Chem. Eur. J.. 1995;1:619-624.
- Organometallics. 2002;21:5901-5910.
- J. Org. Chem.. 2004;69:5512-5515.
- Angew. Chem.. 1959;20:652.
- Tetrahedron Lett.. 1994;35:5405-5408.
- J. Am. Chem. Soc.. 2002;124:12903-12914.
- J. Am. Chem. Soc.. 2003;125:15200-15209.







