Translate this page into:
Thymus musilii Velen. as a promising source of potent bioactive compounds with its pharmacological properties: In vitro and in silico analysis
⁎Corresponding author at: Department of Biology, University of Hail, College of Science, P.O. Box 2440, 81451 Ha’il, Saudi Arabia. snmejdi@yahoo.fr (Mejdi Snoussi)
-
Received: ,
Accepted: ,
This article was originally published by Elsevier and was migrated to Scientific Scholar after the change of Publisher.
Peer review under responsibility of King Saud University.
Abstract
For the first time, we reported the phytochemical composition of the volatile oil from Thymus musilii Velen (T. musilii). The antioxidant and antimicrobial activities against various food-borne and clinical pathogenic microorganisms were also tested. The thyme oil was particularly rich in thymol (67.697 ± 0.938%), and thymyl acetate (12.993 ± 0.221%). The strongest antioxidant activity of the essential oil was registered with the tests: ABTS (IC50 = 5.6 × 10−4 mg/mL) and β-carotene/linoleic acid (IC50 = 3.2 × 10−3 mg/mL). This thymol-chemotype oil was active against all microorganisms tested with an inhibition growth zone ranging from 21.33 ± 1.52 mm for Proteus mirabilis (P. mirabilis) to 37.33 ± 1.15 mm for Candida vaginalis (C. vaginalis) strain. Overall, the tested oil exhibited bactericidal and fungicidal activities and only a small quantity of the tested essential oil was found to be sufficient for inhibiting the growth of the tested microorganisms. Furthermore, molecular docking results implies that, among the bioactive compounds, β-caryophyllene interacted strongly with the active site residues of TyrRS, GLMS and Gyrase enzymes and consequently support our in vitro results with the highest inhibition potential of this essential oil against tested pathogens, especially Staphylococcus aureus (S. aureus) and Escherichia coli (E. coli). Our results suggested that essential oil of T. musiliii exhibited strong biological activities with a promising source of various natural compounds.
Keywords
Thymus musilii Velen.
GC–MS
Antioxidant
Antibacterial
Antifungal
Molecular docking
1 Introduction
The use of medicinal plants as a source of therapy against various disorders have been practiced in Saudi Arabia since ages and many practices reported in the Prophetic Medicine are currently used in folk medicine in the Arabian Peninsula (Al-Essa et al., 1998). Among this category of plants, there are cultivated plants and others are spontaneous ones. These aromatic plants are grown as needed for their aerial parts (flowers, seeds, leaves, stems, bark) or their underground parts (bulbs, roots). Studies in the past have reported the presence of valuable medicinal plants from the different regions of Saudi Arabia (El-Tawil, 1983). However, the information of the indigenous medicinal plants of Saudi Arabia is scattered in a disorganized manner (Al-Asmari et al., 2014). Scientific studies have proven that these plants, including garlic, pomegranate, black seeds, costus, miswak, henna, ferns, Eucalyptus, ginger, and fenugreek are effective for treating human diseases (Noumi et al., 2017; Adnan, 2019; Reddy et al., 2020; Adnan et al., 2020). These species are exploited in human food, traditional medicine as well as for industrial purposes (agro-food, perfumery, cosmetics, pharmaceutical, etc.).
The mint family (Lamiaceae) is one of the largest and most distinctive families of flowering plants, with about 220 genera and almost 4000 species worldwide (Pirbalouti et al., 2015). This family has an almost cosmopolitan distribution. These plants are frequently aromatic in all parts and include many widely used culinary herbs, such as thyme. The genus Thymus L. belongs to the Nepetoideae subfamily of Lamiaceae family is a well-known aromatic herb and consists of about 330 species of herbaceous perennials and small shrubs in the world (Nickavar et al., 2005; Salehi et al., 2019).
The Mediterranean region can be described as the center of the genus (Cronquist, 1988; Morales, 2002; Jamzad, 2010). Thymus plants also includes many aromatic perennial and herbaceous plant that are cultivated in frequency due to their wide use in the food, cosmetic, and pharmaceutical industries (Nabavi et al., 2015). The genus Thymus is a taxonomically complex group of aromatic plants, traditionally used for medicinal purposes because of their antiseptic, antispasmodic and antitussive properties (Pina-Vaz et al., 2004, Nabavi et al., 2015). Previous chemical investigation on Thymus species have shown the presence of aromatic terpenes and terpenoids, flavonoids, and phenolic acid (Miri et al., 2002; Miguel et al., 2004; Ebrahimi et al., 2008; Tohidi et al., 2017). Thymol and carvacrol are the main phenolic compound of thyme oil. The major non-phenolic compounds were linalool and p-cymene (Piccaglia and Marotti, 1991).
Recent studies have shown that Thymus species have antibacterial, antifungal, and antioxidant activities (Bassam et al., 2004; Rahimmalek et al., 2009; Jordan et al., 2009). Gedikoğlu, et al. (2019) reported that the essential oil of thyme showed antimicrobial activity against Bacillus cereus NRRL (B3711), Staphylococcus aureus (ATCC 9144), Staphylococcus epidermidis (ATCC 12228), Escherichia coli ATCC (25922), Salmonella enteritidis (ATCC 13076) and Salmonella typhimurium (ATCC 14028). The anti-bacterial characteristic of Thymus spp. is due to the occurrence of thymol in this genus. This substance can be used as a disinfectant.
In Saudi Arabia, at least three species of Thymus (endemic and introduced) were identified: T. bovei Benth., T. decussatus Benth. and T. musilii Velen. In addition, T. vulgaris was largely cultivated in many regions of the kingdom. This species, T. musilii Velen. belongs to division: Tracheophyta, subdivision: Spermatophytina, Class: Magnoliopsida, Superorder: Asteranae, Order: Lamiales, Family: Lamiaceae Lindl., and Genus: Thymus L. It is distributed mainly in Iraq, Palestine, and Saudi Arabia (World Checklist of Selected Plant Families, 2010).
Growing to 30–70 cm tall by 40–60 cm wide, it is a bushy, woody-based evergreen subshrub with highly aromatic, green leaves and clusters of white flowers in early summer. Preferred the dry slopes, rocks and maquis, it was always found on clay or limestone soils. It has sessile leaves varying from elliptic to linear or diamond-shaped towards the apex. The flowers have a tube-like calyx and tubular corolla with a three lobed lower lip, and are united in spikes at the top of the branches (Fig. 1). The roots are robust, and the fruit consists of a smooth, dark colored nutlet. In Bedouin population of Saudi Arabia, leaves and flowering tops of T. musilii were used as a garnish or added as a flavoring in cooking variety of foods, as well as in preparing infusion tea. An aromatic tea is made from the fresh or dried leaves. The leaves can be used either fresh or dried. If the leaves are to be dried, the plants should be harvested in early and late summer just before the flowers open and the leaves should be dried quickly.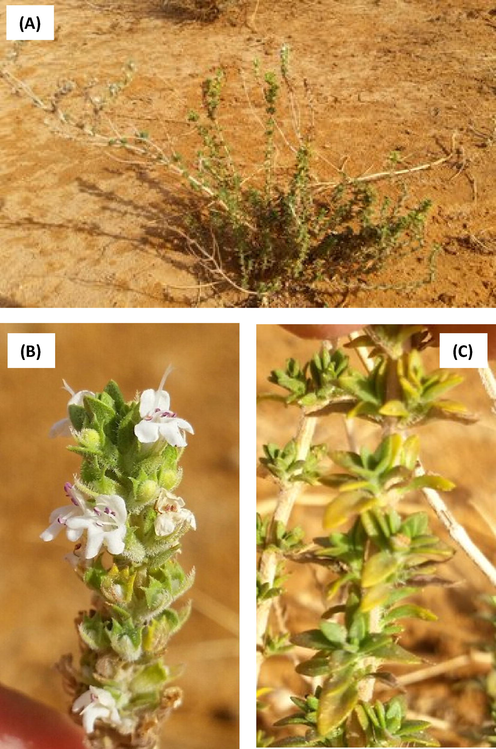
T. musilii Velen specimen. (A): whole plant at flowering stage, (B): clusters of white flowers, (C): green leaves.
The in vitro antimicrobial and antioxidant activities of the essential oil and extract of T. vulgaris have recently been reported. Al-Asmari et al. (2017) have studied the essential oil composition, whereas, Alharbi (2017) reported that the whole plant was used in traditional medicine to treat abdominal pain, and as anti-helminthic and carminative effects. Belonging this genus, T. musilii is a very interesting medicinal plant closely distributed on Arabian Peninsula, Iraq and Jordan landscapes (Batanouny and Sheikh, 1972; Govaerts, 2003). In the north of Saudi Arabia, it is locally used as an antiseptic traditional drug. This species has also been used for curing many bacterial and fungal diseases in traditional medicine in Saudi Arabia (survey, data not shown). In fact, it used by local Saudi population to cure many ailments. Leaves are used in treating respiratory diseases and the flowering tops are used as anti-helminthic, antiseptic and antispasmodic drug. However, antimicrobial and antioxidant properties of T. musilii Velen seem not to have been reported before.
To the best of our knowledge, this study is the first report on the biological properties of T. musilii Velen. The aim of this work was to investigate the chemical composition of the volatile oil obtained from the aerial parts of T. musilii cultivated under greenhouse conditions in Al-Gaad, Hail (Saudi Arabia) by using GC–MS technique. Additionally, the antioxidant and antimicrobial activities of the oil were assessed. To reach this objective, molecular docking studies of the bioactive compounds were also performed against tyrosyl-tRNA synthetase TyrRS from S. aureus, glucosamine 6-phosphate synthase (GLMS) from E. coli and Gyrase from S. aureus enzymes to better understand their mechanism of action.
2 Material and methods
2.1 Plant material sampling and essential oil extraction
The plant used in this study were collected in October 2019 from a nursery belonging to the Ministry of Agriculture in the region of Hail (Al-Gaad, Ha'il, Saudi Arabia). Dr. Ahmed Alghamdi, from the Department of Biology, Faculty of Science, University of Hail, Saudi Arabia identified the plant at the species level. A voucher specimen (AN 001) was deposited in the Department of Biology, University of Hail, Saudi Arabia. The volatile oil was collected using a clevenger-type apparatus after 3 h of hydro-distillation using 100 g from the aerial air-dried organs (flowering stage). The obtained oil was dried using anhydrous sodium sulfate and stored until use at −20 °C. The yield of extraction was calculated after three running cycle and expressed according to the dry weight.
2.2 Characterization of the volatile oil
A Hewlett–Packard 6890 chromatograph equipped with a flame ionization detector (FID) and an electronic pressure control injector was used to study the chemical composition of the obtained volatile oil from T. musilii aerial parts. A gas chromatography apparatus coupled to mass spectrometry (GC–MS) on a gas chromatograph HP 7890 (II) and HP 5975 mass spectrometer (Agilent Technologies, Palo Alto, CA, USA) with an electron impact ionization of 70 eV was used. An HP-5MS capillary column (Agilent Technologies, Hewlett-Packard, CA, USA; 30 m × 0.25 mm), with 0.25 m film thickness was used. Temperature was fixed to rise from 40 °C to 280 °C at a rate of 5 °C/min. The carrier gas was helium with a flow rate of 1.2 mL/min, a split ratio of 60:1, scan time and mass range of 1 s and 40–300 m/z, respectively. The identification of the bioactive components in T. musilii volatile oil was based on the calculated retention index (RI) relative to (C8–C22) n-alkanes and in comparison, with authentic compounds. Further identification of compounds was made by matching their recorded mass spectra with those stored in the Wiley/NBS mass spectral library of the GC–MS data system and other published mass spectra (Adams, 2007) and data expressed as relative percentage of the total peak area as previously described by Essid et al. (2015) and Salem et al. (2018).
2.3 Antioxidant assays
2.3.1 DPPH radical–scavenging activity
The free radical-scavenging activity of the tested essential oil was measured using the protocol described by Chakraborty and Paulraj (2010) and Adnan et al. 2018. The ability to scavenge the DPPH radical was calculated using the following equation (Eq. (1)):
A0 is the absorbance of the control and A1 is the absorbance of the sample.
The antioxidant activity was expressed as IC50 (mg/mL) which represented the extract concentrations scavenging 50% of DPPH radicals (Nishaa et al., 2012).
2.3.2 ABTS radical scavenging activity assay
The radical scavenging activity against ABTS radical cations was measured using the method of Chakraborty and Paulraj (2010). The inhibition percentage of ABTS radical was calculated using the following equation (Eq. (2)):
where
A0 is the absorbance of the control and A1 is the absorbance of the sample.
The antiradical activity was expressed as IC50 (mg/mL) which represented the extract concentrations scavenging 50% of ABTS radicals (Nishaa et al., 2012). A lower IC50 value represents a stronger ABTS scavenging capacity.
2.3.3 Reducing power capability assay
The reducing power was determined using the method of Bi et al. (2013). The extract concentration providing 0.5 of absorbance (IC50) was calculated from the graph of absorbance at 700 nm against sample concentration (Barros et al., 2008). Ascorbic acid was used as a standard.
2.3.4 β-carotene/linoleic acid method
The β-carotene method was carried out according to Ikram et al. (2009). Antioxidant activity (inhibition percentage, PI %) was evaluated using the following equation (Eq. (3), Miraliakbari and Shahidi, 2008):
where
Aβ-carotene t0 and Aβ-carotene T120 refer to the corresponding absorbance values of the test sample, standard and control measured before and after incubation for 2 h, respectively. All tests were performed in triplicate and ascorbic acid (standard) was used for comparison.
2.4 Screening of antimicrobial activities
The antimicrobial activity of the obtained essential oil was tested against four type strains namely E. coli ATCC 35218, P. aeruginosa ATCC 27853, P. mirabilis ATCC 29245, and K. pneumoniae ATCC 27736. Two clinical strains, S. aureus MDR (multidrug resistant bacteria), and Enterobacter cloacae (E. cloacae) were used. The antifungal activity was performed using Candida albicans (C. albicans) ATCC 10231, Cryptococcus neoformans (C. neoformans) ATCC 14116, C. vaginalis (clinical strain), and Candida sp. (clinical strain). Two fungal strains (Aspergillus spp.) were also tested: A. fumigatus ATCC 204305 and A. niger.
Two techniques were used to screen the antimicrobial effect of the obtained essential oil and its main component thymol purchased from Sigma Aldrich®, Germany. The disc diffusion assay was performed on Mueller-Hinton agar plates for all bacteria, Sabouraud chloramphenicol agar for yeasts, and Potato Dextrose agar for the Aspergillus strains. 10 mg of essential oil and thymol/6 mm-disc were tested in triplicate. Ampicillin and Amphotericin B were used as control. The minimal inhibitory concentration (MIC) and minimal bactericidal/fungicidal concentration (MBC/MFC) values were determined by using the microdilution assay as previously described by Snoussi et al. (2018). MBC/MIC ratio and MFC/MIC ratio were used to interpret the activity of the essential oil as described by Gatsing et al. (2009).
2.5 Molecular docking analysis of TyrRS, GLMS and Gyrase with phytochemicals of T. musilii
Crystal structures of tyrosyl-tRNA synthetase TyrRS from S. aureus (PDB: 1JIJ.pdb) (Qiu et al., 2001), glucosamine 6-phosphate synthase (GLMS) from E. coli (PDB: 1XFF.pdb) (Isupov et al., 1996), and Gyrase from S. aureus (PDB: 2XCT.pdb) (Bax et al., 2010) were fetched from Protein Data Bank (RCSBPDB). Following to the retrieval of crystal structures, LCMS identified phytochemicals 3-dimensional structures such as α-thujene, α-pinene, β-myrcene, α-terpinene, p-cymene, (1,8)-cineole, γ-terpinene, α-terpinolene, Borneol, Terpinen-4-ol, α-terpineol, 2-Isopropyl-5-methylanisole, Thymol, Carvacrol, Thymyl acetate, Carvacryl acetate, and β-caryophyllene were acquired from eminent database PubChem and converted to PDB format using Open Babel (O'Boyle et al., 2011). These seventeen compounds were then docked separately against the receptor structure (1JIJ, 1XFF and 2XCT) using molecular docking software Autodock 4.2.6 (Morris et al., 2009). Docking protocol was performed in a similar manner, which can be related to previous analyses (Sonawane and Barage, 2015; Parulekar and Sonawane, 2018). Apart from the grid centre and grid size, all other parameters used for docking with these seventeen compounds were kept same. For the preparation of the grid map using a grid box, Auto Grid (Morris et al., 2009) was used. The grid size was set to 126 × 126 × 126xyz points for TyrRS and gyrase receptors. For GLMS, grid size was set to 96 × 122 × 126 xyz points. Grid spacing was kept to 0.375 Å for all the receptors. The grid centre for TyrRS was designated at dimensions (x, y and z): −11.897, 17.862 and 91.741, for GLMS at (x, y and z): 1.979, 37.952 and 20.512, and for gyrase at (x, y and z): 7.841, 39.224 and 118.021. The grid box is cantered in such a way that it encloses the entire binding site of both the receptors and provides enough space for translation and rotation of ligands. The generated docked conformation was ranked by predicted binding energy and topmost binding energy docked conformation was analyzed using UCSF Chimera (Pettersen et al., 2004) for intermolecular hydrogen bonding of active site amino acid residues from the receptors with docked ligands.
2.6 Statistical analysis
The laboratory biological assays were conducted in triplicates for each sample. The IC50 of DPPH, ABTS, and β-carotene bleaching methods values were calculated by linear regression analysis. ANOVA and Duncan tests were performed with SPSS 16.0. The means of the test’s values were also evaluated with the Least Significant Differences test at 0.05 significance level.
3 Results and discussion
3.1 Chemical composition of T. musilii Velen. essential oil
The air-dried aerial-parts of T. musilii yielded 2.736 ± 0.015% (v/w) essential oil on hydro-distillation. Seventeen components were identified in the obtained oil, belonging mainly to oxygenated monoterpenes (87.010 ± 0.279%) followed by monoterpenes hydrocarbons (11.013 ± 0.039%) and sesquiterpenes hydrocarbons (1.953 ± 0.005%). These data are summarized in Table 1. The chemical structure of the seventeen compounds identified in T. musilii essential oil were depicted in Fig. 2. RI: Retention index on a HP-5MS column. The data are expressed as mean ± SD (n = 3); SD: Standard Deviation.
Peak #
RI* on HP-5MS column
Compounds
Chemical formula
Percentage (Mean ± SD)
1
931
α-Thujene
C10H16
0.437 ± 0.015
2
939
α-Pinene
C10H16
0.303 ± 0.015
3
992
β-Myrcene
C10H16
0.710 ± 0.034
4
1018
α-Terpinene
C10H16
0.853 ± 0.028
5
1026
p-Cymene
C10H14
4.617 ± 0.119
6
1033
1,8-Cineole
C10H18O
0.397 ± 0.005
7
1062
γ-Terpinene
C10H16
2.633 ± 0.072
8
1087
α-Terpinolene
C10H16
1.460 ± 0.081
9
1165
Borneol
C10H18O
0.763 ± 0.030
10
1174
Terpinen-4-ol
C10H18O
0.390 ± 0.017
11
1189
α-Terpineol
C10H18O
0.890 ± 0.036
12
1227
2-Isopropyl-5-methylanisole
C11H16O
0.080 ± 0.138
13
1290
Thymol
C10H14O
67.697 ± 0.938
14
1292
Carvacrol
C10H14O
3.417 ± 0.105
15
1356
Thymyl acetate
C12H16O2
12.993 ± 0.221
16
1367
Carvacryl acetate
C12H16O2
0.383 ± 0.015
17
1404
β-caryophyllene
C15H24
1.953 ± 0.102
Chemical classes
Monoterpene hydrocarbons
11.013 ± 0.039
Oxygenated monoterpenes
87.010 ± 0.279
Sesquiterpenes hydrocarbons
1.953 ± 0.005
Total compounds Identified (%)
100
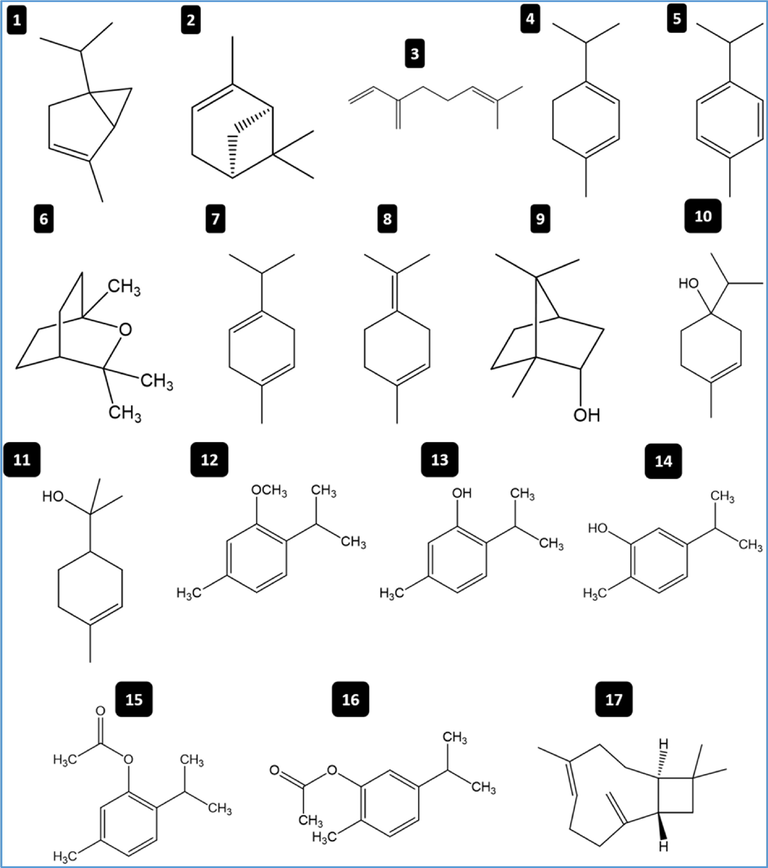
Chemical structures of 17 bioactive molecules identified in T. musilii essential oil using GC–MS technique. Numbers in the figure correspond to the codes in Table 1.
This essential oil can be defined as thymol/thymyl acetate chemotype (67.697/12.993%) as shown in the chromatogram (Fig. 3). Thymol (67.697 ± 0.938%), thymyl acetate (12.993 ± 0.221%), o-cymene (4.617 ± 0.119%), carvacrol (3.417 ± 0.105%), and γ-terpinene (2.633 ± 0.072).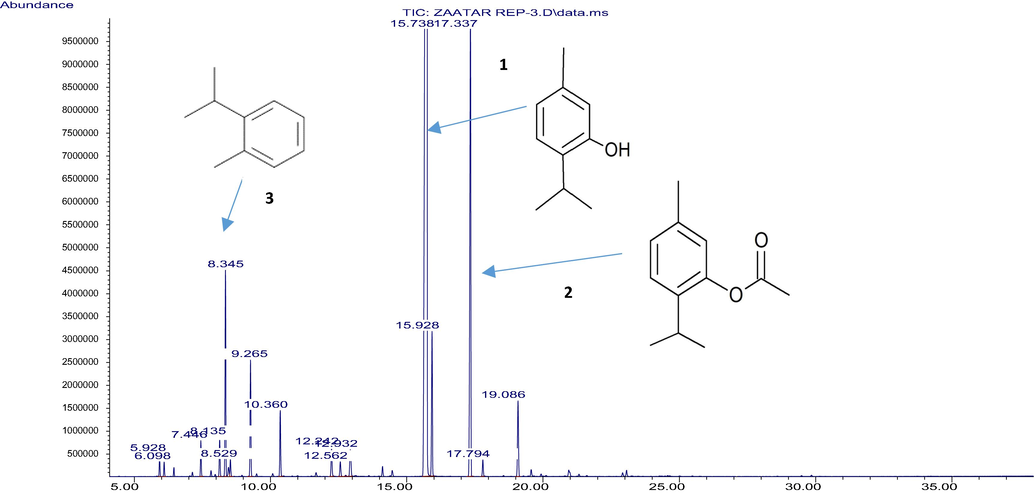
Chromatogram obtained for T. musilii Velen essential oil. The main components identified are: 1 (Thymol), 2 (Thymol Acetate), and 3 (o-cymene).
Numerous studies have reported that oxygenated monoterpenes were the dominant family of compounds found in the Thymus genus essential oil (De Martino et al., 2009; Zarshenas and Krenn, 2015). The diversity of the composition of the volatile oil obtained from different species and subspecies belonging to the genus thymus can be explicated by endogenous (plant varieties, vegetative state, organ tested) and exogenous factors like climatic features, soil characteristics, and seasons (Tzakou et al., 1998; Cosentino et al., 1999; Pirbalouti et al., 2013a,b). It has also been reported that the frequency of irrigation and salicylic acid concentration can affect the yield and the content of essential oil obtained from T. daenensis Celak. and T. vulgaris L. (Khazaie et al., 2008, Pirbalouti et al., 2013c; Alavi-Samani et al., 2013). In addition, application of fertilizers increases the vegetative biomass, oil yield and diversity, and antioxidant activities of T. daenensis Celak. (Bistgani et al., 2018).
Thymol and carvacrol are the main phenolic compound of thyme oil. The major nonphenolic compounds were linalool and p-cymene (Piccaglia and Marotti, 1991). Thymol was the dominant phenolic compound detected in several Thymus species with different percentage as reported by Tohidi et al. (2019) including: T. carmanicus (40.8%), T. daenensis (20–80.4%), T. eriocalyx (5.3–66.34%), T. fallax (19.88–65.9%), T. fedtschenkoi (31.8%), T. kotschyanus (6.8–66.15%), T. migricus (55.6–79.74%), T. pubescens (37.9–63.5%), T. serpyllum (52.45%), T. transcaucasicus (35.83–62.92%), and T. trauveterri (24.43–63.33%).
It has also been reported that thymol is the main phenolic compound in the essential oil of T. cappadocicus Boiss. (Albayrak and Aksoy, 2012), T. pulegioides (Pinto et al., 2006), T. fontanesii (Dob et al., 2006), T. hyemalis (Rota et al., 2008), T. ciliatus Desf. Benth. (Kabouche et al., 2009), T. marschallianus Willd (Cavar Zeljkovic et al., 2015), T. pannonicus (Pluhár et al., 2010), T. vulgaris (Asbaghian et al., 2011), T. zygis (Ballester-Costa et al., 2013), T. numidicus Poiret (Mina et al., 2014), T. quinquecostatus Celak. (Kim et al., 2014) and T. lanceolatus (Khadir et al., 2016a,b). More recently, Jan et al. (2020) reported that T. afghanicus harvested from the Himalayan-Afghanistan area was a thymol chemotype (27.7%).
In this study, thymyl acetate, which is formed after acetylation of thymol produced directly by terpene synthases (Keszei et al. 2008), was found to be the second phenolic compound in T. musilii oil (12.993%). This molecule has been reported in the essential oil of some Thymus species with different percentage including T. longicaulis (0–12.8%) and T. pulegioides L. (0.4–0.7%) from Italy (De Martino et al., 2009), T. caespititius Brot. from Portugal (11–15%, Mendes et al., 2013), T. serpyllum L. from Serbia (38.5%, Cancarevic et al., 2013), and T. lanceolatus from Algeria (0.006%; Khadir et al., 2016a,b).
3.2 Antioxidant activities of T. musilii essential oil
Because of the complex chemical compounds effect of the plants volatile oil, the antioxidant capacity of T. musilii essential oil is studied by four methods, DPPH, ABTS, FRAP and β-carotene bleaching methods in order to estimate the effectiveness of these compound diversity. Table 4 summarizes the free radicals scavenging activities of T. musilii essential oil and the commercialized standards, ascorbic acid and butylated hydroxyl-toluene (BHT). The IC50 of the essential oil and the standards, which is the concentration required for scavenging half (50%) of the tested radicals, showed that ABTS and peroxyl radicals were strongly significantly inhibited by T. musilii (Table 4). Interestingly, T. musilii oil possess high antioxidant activities using ABTS (IC50 = 5.6 × 10−4 ± 2 × 10−5 mg/mL) and β-carotene bleaching (IC50 = 3.2 × 10−3 ± 5 × 10−4 mg/mL) methods, followed by DPPH test (IC50 = 0.049 ± 1 × 10−4 mg/mL). This essential oil is significantly active on peroxyl radicals than the both tested standards (Table 4).
Literature review showed that no previous work was countered on T. musilii essential oil antioxidant capacity. However, several studies were conducted on the genus Thymus essential oils and on its antioxidant capacity (El-Bakkal et al., 2020; Goudjil et al., 2020). For instance, the anti-radicalar essential oils from the cultivated T. carmanicus, T. kotschyanus, T. migricus, and T. vulgaris collected, under various conditions, from Iran were studied by DPPH method (Tohidi et al., 2020). Under red, red-blue, blue, white and greenhouse light treatments, T. carmanicus (IC50 = 278; 259.2; 281; 467.4; 198.2 µg/mL), T. kotschyanus (IC50 = 621.8; 421.1; 304.6; 557.4; 384.7 µg/mL), T. migricus (IC50 = 358; 911.6; 176.8; 1274; 631.8 µg/mL), and T. vulgaris (IC50 = 560; 766; 400.6; 227.6; 314.3 µg/mL) inhibited DPPH radicals (Tohidi et al., 2020). Thymus longicaulis C. Presl subsp. longicaulis var. longicaulis essential oil collected from Turkey had strong radical inhibition percentage (IP = 87.69% at 0.4 mg/mL; 93.28% at 1 mg/mL; 94.15% at 2 mg/mL) using b-carotene–linoleic acid method. The same plant species possess moderate effect (IP = 28.17% at 0.1 mg/mL; 46.32% at 0.2 mg/mL, 63.26% at 0.5 mg/mL) using DPPH method, moderate effect using reducing power protocol (Absorbance = 0.128 at 0.2 mg/mL, 0.241 at 0.4 mg/mL, 0.550 at 1 mg/mL), and it had no chelating activity till 1 mg/mL (Sarikurkcu et al., 2010).
Compared to the previous studies, T. musilii essential oil in the present study exhibited a strong antioxidant effect. This activity can be explained by the chemical composition classes, monoterpene hydrocarbons (11.01%) and oxygenated monoterpenes (87.01%), of the volatile oil. Most researchers revealed the antiradical effect of monoterpenes (Badawy et al., 2019; Wojtunik‐Kulesza et al., 2019). The antioxidant capacity of thymol (IC50 = 31.426 mg/mL), β-cymene (IC50 = 916.89 mg/mL), α-terpineol (IC50 = 480.56 mg/mL), myrcene (IC50 = 22.136 mg/mL), α-pinene (IC50 = 880.74 mg/mL) were evaluated using N,N-dimethyl-1,4-phenylenediamine (DMPD) reagent (Badawy et al., 2019).
Other study focused on the antioxidant of α-terpinene (IC50 = 0.6 and 7.5 mM) and γ-terpinene (IC50 = 2.8 and 30.0 mM) using ABTS and DPPH methods, respectively (Li and Liu, 2009). Previous work demonstrated that γ-terpinene (IC50 = 15.5 mg/mL) inhibited DPPH radicals (Sonboli et al., 2005). This antioxidant assay may be related to a high area of thymol (67.7%). Several studies confirmed the strong in vitro and in vivo biological effect of thymol (Abd El-Naby et al., 2020; Arafa et al., 2020; Jafari et al., 2020). The registered effect may referred to the major compound, thymol (67.7%), and/or to the synergism between main and minor compounds of the essential oil (Ciesla et al., 2016). The antioxidant activities were studied, in literature, towards the whole essential oils, to single compounds and as well as to combination (Graßmann, 2005; Tohidi et al., 2020).
3.3 Antimicrobial activities of T. musilii essential oil
The antibacterial activity of T. musilii essential oil was tested against six bacteria, four yeasts and two fungal strains using both disc diffusion (Fig. 4) and microdilution assays. Obtained results showed that, the tested bacteria were resistant to ampicillin with a mean diameter of growth inhibition zone ranging from 6.33 ± 0.57 mm to 7.33 ± 0.57 mm. In addition, the mean diameter of growth inhibition zones ranged from 21.33 ± 1.52 mm for P. mirabilis to 36.33 ± 1.15 mm for K. pneumoniae. The clinical strain S. aureus MDR, resistant to ampicillin, was susceptible to the oil tested (25.33 ± 1.15 mm). Small quantities of oil (12.5 mg/mL) can inhibit the growth of all tested bacteria, except for E. cloacae (MIC value = 3.125 mg/mL). MBCs values were ranging from 6.25 mg/mL (E. cloacae) to 100 mg/mL for P. aeruginosa. As compared to the single bioactive molecule, thymol, T. musilii essential oil exhibited bactericidal activity for all tested bacteria with MBC/MIC ratio inferior to 4 except for P. aeruginosa (MBC/MIC ratio = 8). All these data are summarized in Table 2. Using the literature review, high antimicrobial activity of Thymus species (chemotype thymol) was recorded against a large collection of bacterial and fungal species (Table 5). *Inhibition zone around the discs impregnated with the essential oil (10 mg/disk) expressed as mean of three replicates (mm ± SD). SD: standard deviation. a: Minimal Inhibitory Concentration (mg/ml). b: Minimal Bactericidal Concentration (mg/ml). c: MBC/MIC ratio interpreted using the scheme of antimicrobial substances are considered as bacteriostatic agents when the ratio MBC/MIC>4 and bactericidal agents when the ratio MBC/MIC≤4 (Gatsing et al., 2009). The letters (a–c) indicate a significant difference between the inhibition zones of essential oil, thymol and ampicillin against the tested bacteria according to the Duncan test (p < 0.05). BHT: Butylated hydroxytoluene. The letters (a–c) indicate a significant difference between the different antioxidant methods according to the Duncan test (p < 0.05).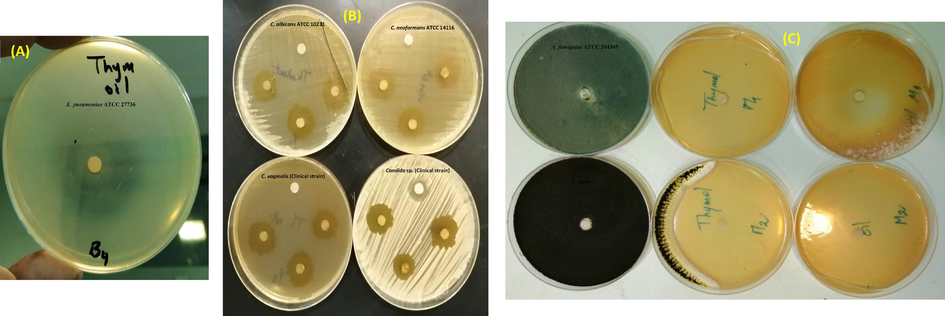
Selected photos showing the antibacterial (A), anti-Candida spp. (B), anti-Aspergillus spp. (C) activity of the tested essential oil and its main component thymol.
Code
Strain
T. musilii Velen essential oil
Main Compound (Thymol)
Ampicillin
Mean ± SD*
(mm)MICa
MBCb
MBC/MIC
ratioMean ± SD
(mm)MIC
MBC
MBC/MIC
ratioMean ± SD
(mm)
B1
E. coli ATCC 35218
35.33 ± 1.15c
12.5
50
4
12.66 ± 0.57b
3.125
6.25
2
7 ± 0a
B2
P. aeruginosa ATCC 27853
35.33 ± 1.15b
12.5
100
>4
7 ± 0a
12.5
50
4
7.33 ± 0.57a
B3
Proteus mirabilis ATCC 29245
21.33 ± 1.52b
12.5
25
2
6 ± 0a
3.125
6.25
2
6.33 ± 0.57a
B4
K. pneumoniae ATCC 27736
36.33 ± 1.15c
12.5
25
2
9 ± 1b
3.125
6.25
2
6.66 ± 0.57a
B9
S. aureus MDR (Clinical strain)
25.33 ± 1.15b
12.5
25
2
6 ± 0a
0.78
1.56
2
7.33 ± 0.57a
B10
E. cloacae (Clinical strain)
31.00 ± 1.00c
3.125
6.25
2
8.66 ± 1.15b
0.39
0.78
2
6.66 ± 0.57a
Code
Strain
T. musilii Velen essential oil
Main Compound (Thymol)
Amphotericin B (10 mg/ml)
Mean ± SD* (mm)
MIC
MFC
MFC/MIC ratio
Mean ± SD* (mm)
MIC
MFC
MFC/MIC ratio
Mean ± SD* (mm)
Y1
C. albicans ATCC 10231
34.00 ± 1.00c
6.25
25
4
13.66 ± 0.57a
12.5
100
8
22.66 ± 1.15b
Y2
C. neoformans ATCC 14116
36.66 ± 1.15c
3.125
6.25
2
12 ± 1a
50
100
2
15.33 ± 0.57b
Y3
C. vaginalis (Clinical strain)
37.33 ± 1.15c
6.25
12.5
2
12.66 ± 0.57b
25
100
4
6.66 ± 0.57a
Y4
Candida sp. (Clinical strain)
37.33 ± 1.15b
6.25
12.5
2
11.66 ± 0.57a
25
100
4
12.33 ± 0.57a
M1
A. fumigatus ATCC 204305
88.66 ± 1.15c
–
–
–
82.66 ± 2.31b
–
–
–
15.00 ± 1.00a
M2
A. niger
87.33 ± 1.15c
–
–
–
74.33 ± 0.57b
–
–
–
6.00 ± 0.00a
Essential oil and standards tested
Test System
DPPH
IC50 (mg/mL)ABTS
IC50 (mg/mL)β- carotene
IC50 (mg/mL)FRAP
IC50 (mg/mL)
T. musilii Velen
0.049 ± 1 × 10−4b
5.6 × 10−4 ± 2 × 10−5 a
3.2 × 10−3 ± 5 × 10−4 a
>1c
BHT
0.023 ± 3 × 10−4a
0.018 ± 4 × 10−4b
0.042 ± 3.5 × 10−3c
0.05 ± 3 × 10−3 a
Ascorbic Acid
0.022 ± 5 × 10−4 a
0.021 ± 1 × 10−3b
0.017 ± 1 × 10−3b
0.09 ± 7 × 10−3b
Thymus species
Origin
Main Components
Bacteria and Fungi tested
Reference
T. vulgaris L.
Yemen
Thymol (51.34%), p-cymene (18.35%), ß-caryophyllene (4.26%).
B. subtilis, S. aureus, S. epidermidis, P. aeruginosa, E. coli, Mycobacterium smegmatis, C. albicans and C. vaginalis.
Al Maqtari et al., 2011
Romania
Thymol (47.59%), γ-terpinene (30.90%) and p-cymene (8.41%).
S. aureus ATCC 25923, P. aeruginosa ATCC 27853, S. Typhimurium ATCC 14028, E. coli ATCC 25922, K. pneumoniae ATCC 13882, E. faecalis ATCC 29212 and C. albicans ATCC 10231
Borugă et al., 2014
Balkan Peninsula
Thymol (49.1%), p-Cymene (20%), carvacrol (3.5%), α-thujene (1.9%), α-pinene (1.2%), ß-mycrene (1.3%), trans-ß-ocimene (1.4%), γ-Terpinene (4.2%), borneol (1.7%), terpinene-4-ol (2%), ß-caryophyllene (3.7%), δ-cadinene (2.3%).
C. albicans ATCC 10234, C. glabrata, C. krusei, C. tropicalis ATCC 750, P. aeruginosa, E. faecalis, S. sanguinis, S. salivarius, S. mutans, L. acidophilus, S. aureus.
Nikolic et al., 2014
Italy
Thymol (46.2–67.5%), caryophyllene oxide (2.2–7.3%), geranyl propanoate (0–2.2%), linalool (0.3–2.7%), trans-myrtanol (0–2.3%), citronellyl formate (0–2.5%), ethyl-2-octynoate (0–1.8%).
S. aureus ATCC 25923, E. faecalis ATTC 29212, B. cereus ATCC 1177, B. subtilis ATCC 6633, E. coli ATCC 25922, P. aeruginosa ATCC 27853, S. epidermidis ATCC 12228, K. pneumoniae ATCC 10031, S. typhi Ty2 ATCC 19430 and P. vulgaris ATCC 13315.
Mancini et al., 2015
France
Thymol (47.06%), p-cymene (20.07%), γ-terpinene (9.03%), linalool (5.00%), carvacrol (3.24%).
C. albicans ATCC 18804, Cryptococcus neoformans 24067 (serotype D or var. neoformans), Aspergillus niger ATCC 16888.
Satyal et al., 2016
Republic of Moldova
Thymol (55.44 ± 0.62%), m-Cymene (11.88 ± 0.32%), γ-Terpinene (5.74 ± 0.20%), o-Cymen-5-ol (5.14 ± 0.19%), ß-caryophyllene (1.53 ± 0.07%), Terpinen-4-ol (1.04 ± 0.04%), 2-Carene (1.04 ± 0.04%).
A. flavus MUCL 19006
Aprotosoaie et al., 2019
T. longicaulis C. Presl
Italy
Thymyl acetate (0–12.8%), t-Cadinol (0.3–9.2%), p-cymene (0.4–9.0%), ß-caryophyllene (2.2–5.7%), γ-terpinene (0.9–5.5%), Germacrene D (5.3%), thymol (6.4–9.3%), thymol methyl ether (0.8–5.5%), carvacrol (0–12.8%), Carvacryl acetate (0–13.6%).
S. aureus ATTC 25923, S. faecalis ATTC 29212, B. subtilis ATCC 6633, B. cereus PCI 213, P. mirabilis ATCC 12453, E. coli ATCC 25922, S. typhi Ty2 ATCC 19430, P. aeruginosa (ATCC 27853).
De Martino et al., 2009
Balkan Peninsula
Thymol (46.3%), δ-3-Carene (1.6%), p-Cymene (9.4%), γ-terpinene (16.2%), linalool (1.4%), borneol (2.2%), thymyl methyl ether (11.4%), β-Caryophyllene (2.1%), carvacrol (1.4%).
H. influenzae, N. meningitidis, S. aureus, S. pneumoniae, S. pyogenes, C. albicans
Vladimir-Knežević et al., 2012
T. pulegioides L.
Italy
Thymol (21.8–26.3%), p-cymene (17.6–19.9%), linalool (4.7–5.6%), ß-caryophyllene (5.9–7.5%), thymol methyl ether (6.0–10.8%), carvacrol (3.1–4.7%).
S. aureus ATTC 25923, S. faecalis ATTC 29212, B. subtilis ATCC 6633, B. cereus PCI 213, P. mirabilis ATCC 12453, E. coli ATCC 25922, S. typhi Ty2 ATCC 19,430 and P. aeruginosa ATCC 27853.
De Martino et al., 2009
T. daenensis Celak.
Iran
Thymol (3.8–78.3%), ρ-cymene (2.7–11.6%), caryophyllene (2.1–5.6%), methyl carvacrol (2.9–4.9%), g-terpinene (2.5–12.9%), geraniol (0–3.4%), α-humulene (0–3.2%), carvacrol (2–15.2%), γ-terpinene (3.9–12.9%), aromadendrene (0–3.9%), carvacrol methyl ether (3.4–4.27%), δ-terpinene (0–4.3%).
L. monocytogenes, S. aureus, S. iniae, E. coli, P. aeruginosa, K. pneumonia, H. pylori, A. niger, A. fumigatus, C. albicans and S. cerevisiae.
Zarshenas and Krenn, 2015
Iran
α-pinene (0.51%), 1,8-cineole (0.58%), γ-terpinene (5.74%), linalool (0.52%), thymol (74.32%), carvacrol (4.31%), trans-caryophyllene (3.56%), caryophyllene oxide (0.42%).
C. albicans vaginal, E. coli O157:H7; B. cereus, L. monocytogenes and S. aureus.
Pirbalouti et al., 2009 Pirbalouti et al., 2010 Pirbalouti et al., 2014
T. capitatus L.
Algeria
Thymol (51.22%), carvacrol (12.59%), γ-terpinene (10.3%), trans-13-octadecenoic acid (9.04%), linalool (2.29%), caryophyllene (2.01%), pentadecanoic acid (1.92%), α-terpinene (1.78%), ß-myrcene (1.49%), caryophyllene oxide (1.21%).
E. coli, S. typhi, S. aureus, S. pneumoniae, Cladosporium herbarum, Alternaria infectoria, A. ochraceus, and Trichophyton sp.
Goudjil et al., 2020
Tunisia
Thymol (69.95–81.49%), α-cubebene (0–3.44%), β-ocimene (3.09–3.16%), carvacrol (0–2.56%), α-terpinene (2.25–3.83%).
E. coli ATCC 8739, S. typhimurium NCTC 6017, S. aureus ATCC 29213, P. aeruginosa ATCC 27853, A. hydrophila, L. monocytogenes ATCC 7644, B. cereus, A. flavus, A. niger and C. albicans.
Aouadhi et al., 2013
Thymol (89.06%), p-cimene (5.04%), γ-terpinene (3.19%).
S. aureus CIP7625, L. monocytogenes Scott A 724, E. coli ATCC 10536, K. pneumoniae CIP8291, S. cerevisiae ATCC 4226, C. albicans IPA 200, M. ramamnianus ATCC 9314, A. westerdijkiae NRRL 3174.
Mkaddem et al., 2010
T. cappadocicus Boiss.
Turkey
Thymol (70.82%), cymene (9.52%), g-terpinene (9.27%).
A. hydrophila, E. coli, M. morganii, K. pneumoniae, P. mirabilis, P. aeruginosa, S. typhimurium, Y. enterocolitica, B. brevis, B. cereus, B. subtilis, L. monocytogenes, S. aureus, C. albicans and S. cerevisiae.
Albayrak and Aksoy, 2012
T. striatus
Balkan Peninsula
Thymol (59.5%), γ-terpinene (11.6%), p-cymene (6.4%), carvacrol-methyl ether (5.9%), carvacrol (4.9%), α-terpinene (3.3%), E-caryophyllene (2.3%).
A. alternata, A. niger, A. ochraceus, A. versicolor, A. flavus, A. terreus, C. cladosporioides, P. funiculosum, P. helianthi, T. viride, T. mentagrophytes, M. canis, and E. floccosum
Couladis et al., 2004
T. algeriensis Boiss. and Reut
Balkan Peninsula
Thymol (36%), carvacrol (14%), α-pinene (1.1%), ß-mycrene (2.3%), p-cymene (6.3%), ß-bisabolene (4%0, α-terpinene (1.6%), γ-terpinene (4.8%), linalool (1.3%), camphor (1.1%), caryophyllene oxide (1%).
C. albicans ATCC 10234, C. glabrata, C. krusei, C. tropicalis ATCC 750, P. aeruginosa, E. faecalis, S. sanguinis, S. salivarus, S. mutans, L. acidophilus, S. aureus.
Nikolic et al., 2014
T. numidicus Poiret
Algeria
Thymol (40.40%), carvacrol (13.37%), thymol methyl ether (8.30%), β-myrcene (2.37%), p-cymene (7.18%), γ-terpinene (6.41%), linalool (4.06%), β-caryophyllene (2.48%), β-bisabolene (3.26%).
S. aureus ATCC 25923, E. coli, P. aeruginosa ATCC 27853, C. albicans.
Messara et al., 2016
T. zygis
Spain
α-pinene (36.8 ± 1.7–93.9 ± 4.8 mM), myrcene (32.7 ± 0.6–145.6 ± 6.4 mM), α-terpinene (14.6 ± 0.5–102.1 ± 5.5 mM), p-cymene (705.7 ± 22.9–1212.8 ± 13.0 mM), γ-terpinene (448.5 ± 22.4–1462.8 ± 38.2 mM), linalool (223.6 ± 2.8–386.8 ± 13.6 mM), terpinen-4-ol (8.9 ± 0.3–45.7 ± 0.3 mM), thymol (1923.2 ± 27.5–3636.2 ± 15.2 mM), carvacrol (34.3 ± 1.3–112.9 ± 2.5 mM), E-β-caryophyllene (24.1 ± 0.3–50.4 ± 1.0 mM)
S. aureus ATCC 6538, E. coli ATCC 8739, P. aeruginosa ATCC 9027, C. albicans ATCC 10231.
Cutillas et al., 2018
T. serpillum L.
Balkan Peninsula
Thymol (38.5%), carvacrol (4.7%), α-pinene (2%), camphene (2.4%), γ-terpinene (7.2%), linalool (2.4%), borneol (6%), thymol methyl ether (3.8%), thymol acetate (2.8%).
C. albicans ATCC 10234, C. glabrata, C. krusei, C. tropicalis ATCC 750, P. aeruginosa, E. faecalis, S. sanguinis, S. salivarius, S. mutans, L. acidophilus, S. aureus.
Nikolic et al., 2014
T. lanceolatus
Algeria
Thymol (69.61%), γ-terpinene (8.38%), p-cymene (5.07%), carvacrol (3.57%), α-terpinene (1.31%), linalool (1.01%), β-mycrene (1.72%), α-thujene (1.07%), α-pinene (0.73%), d-limonene (0.62%), β-pinene (0.43%).
S. aureus ATCC 29213, S. epidermidis ATCC 14990, S. capitis ATCC 35661, S. pyogenes ATCC 12344, S. agalactiae ATCC 27956, Bacillus subtilis ATCC 6051, P. fluorescens ATCC 13525, S. typhimurium ATCC 14028, S. flexneri ATCC 700930, E. coli ATCC 25922, A. fumigatus ATCC 1022, Geotrichum candidum ATCC 12784, S. racemosum ATCC 14831, C. albicans (ATCC 90028).
Khadir et al., 2016a,b
T. linearis Benth.
India
Thymol (54.9%), γ-terpinene (16.6%), p-cymene (5.2%), α-thymol methyl ether (3.2%), terpinene (2.6%), thymyl acetate (2.8%), β-bisabolene (2.3%), (E)-caryophyllene (2.0%), myrcene (1.8%), α-thujene (1.6%), carvacrol (1.5%), borneol (1.1%).
S. aureus MRSA 33591, S. epidermidis MRSE 51625, S. aureus MRSA (BAA-44), S. aureus MTCC-96, S. epidermidis MTCC-435, E. faecalis MTCC-439, C. albicans ATCC 14053, C. tropicalis ATCC 2013180, C. glabrata ATCC-15126.
Kumar et al., 2020
T. kotschyanus
Iran
α-pinene (5.49–12.72%), β-Myrcene (0.80–1.51%), α-terpinene (1.62–1.80%), p-cymene (0–21.35%), m-cymene (0–8.87%), 1,8-cineole (4.57–4.79%), γ-terpinene (4.00–8.01%), 4-terpineol (0–2.19%), α-terpineol (0.92–1.08%), thymol methyl ether (2.10–2.44%), carvacrol methyl ether (0–4.14%), thymol (29.96–47.48%), carvacrol (0.62–3.79%), β-bourbonene (0.15–3.30%), caryophyllene (1.27–2.92%).
E. faecalis ATCC 29212, S. aureus ATCC 25952, S. aureus ATCC 33591, S. aureus ATCC 29213, S. sanguis PTCC 1449, E. aerogenes ATCC 13048, K. pneumoniae ATCC 700603, P. mirabilis ATCC 43071, E. coli O157:H7
Mobaiyen et al., 2017
T. eigii
Turkey
Thymol (24.77%), carvacrol (14.00%), p-cymene (10.91%), γ-terpinene (6.53%), borneol (6.48%), caryophyllene (3.92%), α-pinene (2.03%), α-thujene (2.34%), β-myrcene (2.68%), α-terpinene (2.28%), 1-octen-3-ol (2.94%), 17 trans-sabinene hydrate (2.19%), 4-terpineol (2.55%), (-)-caryophyllene oxide (2.01%).
E. faecalis ATCC 29212, E. casseliflavus ATCC 700327, S. aureus ATCC 29213, S. aureus ATCC BAA 977, E. hormaechei ATCC 700323, K. pneumoniae ATCC 700603, P. aeruginosa ATCC 27853, E. coli ATCC 25922, C. parapsilosis ATCC 22019, C. albicans ATCC 14053.
Ulukanli et al., 2018.
T. willdenowii Boiss & Reut
Morocco
Thymol (35.5–47.3%), p-cymene (13.9–23.8%), γ-terpinene (8.9–20.3%), carvacrol (3–5.6%), linalool (3–3.5%), camphor (0.9–3.7%), borneol (0.7–4.7%).
E. coli ATCC 25922, P. mirabilis ATCC 35659, B. cereus ATCC 10876, C. albicans ATCC 10231, A. brasilliensis ATCC 16404.
Ouknin et al., 2019
T. musilii Velen
Saudi Arabia
Thymol (67.69 ± 0.93%), thymyl acetate (12.99 ± 0.22%), p-cymene (4.61 ± 0.11%), Carvacrol (3.41 ± 0.10%), γ-terpinene (2.63 ± 0.07%).
E. coli ATCC 35218, P. aeruginosa ATCC 27853, P. mirabilis ATCC 29245, K. pneumoniae ATCC 27736, S. aureus MDR, E. cloacae, C. albicans ATCC 10231, Cryptococcus neoformans ATCC 14116, C. vaginalis, Candida sp., A. fumigatus ATCC 204305 and A. niger.
This study
Similar results were obtained with the yeast and fungi strains tested. Interestingly, high diameter of inhibition zone was recorded for the two clinical yeast strains: Candida sp. (37.33 ± 1.15 mm), and C. vaginalis (37.33 ± 1.15 mm). The MIC and MFC values were 6.25 mg/mL and 12.5 mg/mL, respectively for both strains. Using the MFC/MIC ratio scheme proposed by Gatsing et al. (2009), T. musilii seems to be more effective than thymol on the four tested yeast strains as they have the lowest ratio ranging from 2 to 4. It is important to highlight also that the tested (thymol/thymyl acetate) chemotype oil was very active on the two Aspergillus strains with mean inhibition zone about 88.66 ± 1.15 mm for A. fumigatus to 87.33 ± 1.15 mm for A. niger. All these data are summarized in Table 3.
Using the disc diffusion test, Vladimir-Knežević and colleagues (2012) reported similar results with T. longicaulis species (Chemotype thymol, 46.3%) tested against Haemophilus influenzae (IZ = 42 mm), Neisseria meningitidis (IZ = 53 mm), S. aureus (IZ = 35 mm), S. pneumoniae (IZ = 43 mm), and S. pyogens (IZ = 41 mm). Additionally, Bozin et al. (2006) reported that T. vulgaris essential oil (chemotype thymol) was active against a wide range of Gram-positive and Gram-negative bacteria, including the same species tested in our study. In fact, the highest growth inhibition zones were recorded for Micrococcus flavus (IZ = 48.2 mm), S. epidermidis (IZ = 48 mm), S. aureus (IZ = 26.2 mm), B. subtilis (IZ = 40.6 mm), E. coli (IZ = 29.4 mm), and P. aeruginosa (IZ = 12 mm).
Previous reports have noticed the anti-C. albicans activity of different species belonging to the Thymus genus. In fact, Pinto et al. (2006) reported a significant activity of T. pulegioides oil (thymol 26%/carvacrol 21% chemotype) against Candida, Aspergillus and dermatophyte species explained by the alteration in the cytoplasmic membrane and ergosterol content.
In addition, Pirbalouti et al. (2009) founded that T. daenensis Celak. essential oil effectively inhibits the growth of vaginal C. albicans strains at high concentration (50–55 µl). The same oil was active against E. coli O157:H7, B. cereus, L. monocytogenes, and C. albicans with a diameter of growth inhibition zone and MIC values about (7 mm/>10 mg·mL−1, 25 mm/0.625 mg·mL−1, 16 mm/2.5 mg·mL−1, and 19 mm/<0.039 mg·mL−1 respectively (Pirbalouti et al., 2010). Thymol-rich chemotype of T. daenensis Celak essential oil can inhibit the growth of S. aureus isolated from milk with MIC and MBC values about 62 µg/mL and 630 µg/mL, respectively (Pirbalouti et al., 2014). Couladis et al. (2004) reported the high activity of T. striatus (Chemotype thymol, 59.5%) against a large collection of Aspegillus, Cladosporium, Penicillium, Trichoderma, Tricophyton, Microsporum, and Epidermophyton strains with MICs values ranging from 0.5 to 2 µl. In 2014, Nikolic and colleagues reported that T. serpyllum (Thymol, 38.5%) was active against four Candida species (C. albicans, C. tropicalis, C. glabrata, and C. krusei) with MICs values ranging from 01. to 0.2 µl. More recently, Satyal et al. (2016) demonstrated that T. vulgaris essential oils inhibit the growth of C. neoformans var. neoformans, and C. albicans with MICs values about (313/156) µg·mL−1, and (1250/625) µg·mL−1, respectively for linalool and geraniol chemotypes.
A brief literature review summarized the antimicrobial activity of thymol against a large collection of bacteria, yeast and fungi (Table 6). High activity of the Thymus plant species can be associated to the dominance of thymol with different percentage. In fact, this molecule is known to exhibit antimicrobial, antioxidant, immunological, anti-inflammatory, anticancer, and cardiovascular protection properties (Nagoor et al., 2017; D’agostino et al., 2019). This terpenoid molecule inhibits the hyphal production in Fusarium graminearum (Gao et al., 2016), decreases the membrane permeability leading to the loss of cytoplasmic membrane integrity and loss of electrolytes in C. albicans species by binding to ergosterol (De Castro et al., 2015), and inhibits the telomerase activity in S. cerevisiae species (Darvishi et al., 2013). It has been demonstrated that thymol can kill Methicillin-resistant S. aureus strain by increasing the formation of reactive oxygen species (Li et al., 2014).
Strains Tested
MIC
MBC/MFC
Reference
Bacillus cereus
327.581 ppm
–
Falcone et al. 2005
Bacillus subtilis
422.332 ppm
–
Bacillus licheniformis
422.811 ppm
–
Lactobacillus curvatus
723.45 ppm
–
Lactobacillus plantarum
941.01 ppm
–
Candida lusitaniae
307.901 ppm
–
Pichia subpelliculosa
422.781 ppm
–
Saccharomyces cerevisiae
337.761 ppm
–
Staphylococcus aureus ATCC 68380
0.31 mg/mL
–
Tombetta et al., 2005
Escherichia coli ATCC 15221
5.00 mg/mL
–
Candida albicans ATCC 10231
0.16 µl/mL
0.32 µl/mL
Pinto et al., 2006
Candida guilliermondii MAT23
0.16 µl/mL
0.16 µl/mL
Candida parapsilosis ATCC 90018
0.32 µl/mL
0.32 µl/mL
Candida krusei ATCC 6258
0.16 µl/mL
0.32 µl/mL
Candida tropicalis ATCC 13803
0.16 µl/mL
0.32 µl/mL
Candida albicans
0.16 µl/mL
0.32 µl/mL
Candida tropicalis
0.16 µl/mL
0.32 µl/mL
Candida glabrata
(0.16–0.32) µl/mL
0.32 µl/mL
Candida krusei
0.16 µl/mL
0.32 µl/mL
Trichophyton rubrum
0.16 µl/mL
0.16 µl/mL
Trichophyton mentagrophyte
0.16 µl/mL
0.32 µl/mL
Epidermophyton floccosum
0.16 µl/mL
0.16 µl/mL
Microsporum gypseum
0.16 µl/mL
0.32 µl/mL
Microsporum canis
0.08 µl/mL
0.16 µl/mL
Aspergillus niger ATCC 16404
0.16 µl/mL
0.64 µl/mL
Aspergillus niger CECT 2574
0.16 µl/mL
0.64 µl/mL
Aspergillus fumigatus CECT 2071
0.16 µl/mL
0.64 µl/mL
Aspergillus fumigatus ATCC 46645
0.16 µl/mL
0.64 µl/mL
Aspergillus flavus
0.32 µl/mL
0.64 µl/mL
Aspergillus niger
0.16 µl/mL
0.64 µl/mL
Aspergillus fumigatus
0.16 µl/mL
0.64 µl/mL
Salmonella typhimurium SGI1
2.5 mM
–
Palaniappan and Holley, 2010
Escherichia coli N00-666
2.5 mM
–
Staphylococcus aureus blaZ+
2.5 mM
–
Streptococcus pyogenes ermB+
0.31 mM
–
Escherichia coli O157:H7
500–1000 µg/mL
1000–2000 µg/mL
Rivas et al., 2010
Escherichia coli O26
1000 µg/mL
1000 µg/mL
Escherichia coli O111
1000 µg/mL
2000 µg/mL
Escherichia coli O103
1000 µg/mL
1000 µg/mL
Escherichia coli O145
1000 µg/mL
>2000 µg/mL
Salmonella Typhimurium
2000 µg/mL
2000 µg/mL
Listeria monocytogenes
1000 µg/mL
1000 µg/mL
Hafnia alvei
500 µg/mL
500 µg/mL
Staphylococcus aureus
500 µg/mL
500 µg/mL
Lactobacillus sakei
1000 µg/mL
2000 µg/mL
Pseudomonas putida
1000 µg/mL
2000 µg/mL
Bacillus thermosphacta
–
250 µg/mL
Streptococcus mutans MTCC 890
125 µg/mL
–
Mathela et al., 2010
Staphylococcus aureus MTCC 96
62.5 µg/mL
–
Bacillus subtilis MTCC 121
125 µg/mL
–
Staphylococcus epidermidis MTCC 435
125 µg/mL
–
Escherichia coli MTCC 723
250 µg/mL
–
Escherichia coli
< 0.019–0.039 mg/mL
–
Pirbalouti et al., 2011
Pseudomonas aeruginosa
<0.019–0.039 mg/mL
–
Staphylococcus aureus
< 0.019–156 mg/mL
Bacillus cereus
< 0.019–0.156 mg/mL
Micrococcus luteus
1250 µg/mL
Hernández-Hernández et al., 2014
Phytophthora infestans
400.26 µl/l
–
Ben and Hamada, 2014
Phytophthora ultimum
263 µl/l
–
Botrytis cinerea
>600 µl/l
–
Rhizoctonia solani
64.56 µl/l
–
Aspergillus niger
100 mg/mL
–
Abbaszadeh et al., 2014
Aspergillus fumigatus
150 mg/mL
–
Aspergillus flavus
100 mg/mL
–
Aspergillus ochraceus
100 mg/mL
–
Alternaria alternata
100 mg/mL
–
Botrytis cinerea
100 mg/mL
–
Cladosporium spp.
100 mg/mL
–
Penicillium citrinum
100 mg/mL
–
Penicillium chrysogenum
100 mg/mL
–
Fusarium oxysporum
100 mg/mL
–
Rhizoctonia oryzae
100 mg/mL
–
Escherichia coli
187.5 μg/mL
375 μg/mL
Du et al., 2015
Clostridium perfringens
375 μg/mL
750 μg/mL
Salmonella Typhimurium
375 μg/mL
750 μg/mL
Salmonella Enteritidis
750 μg/mL
1500 μg/mL
Salmonella Pullorum
375 μg/mL
750 μg/mL
Lactobacillus acidophilus
1500 μg/mL
3000 μg/mL
Lactobacillus reuteri
1500 μg/mL
3000 μg/mL
Lactobacillus salivarius
1500 μg/mL
3000 μg/mL
Pythium insidiosum
160–320 µg/mL
Jesus et al., 2015
Helicobacter pylori
0.043 ± 0.024 µl/mL
Falsafi et al., 2015
Mycobacterium tubercolosis
0.75 µg/mL
Andrade-Ochoa et al., 2015
Mycobacterium bovis
2.02 µg/mL
Candida albicans
39 µg/mL
–
De Castro et al., 2015
Candida krusei
39 µg/mL
–
Candida tropicalis
78 µg/mL
–
Aspergillus flavus CGMCC 32890
80 μg/mL
–
Shen et al., 2016
Bacillus cereus
0.007 mg/mL
–
Guimarães et al., 2019
Salmonella Typhimurium
0.003 mg/mL
0.12 mg/mL
Escherichia coli
0.007 mg/mL
0.12 mg/mL
Staphylococcus aureus
0.007 mg/mL
0.12 mg/mL
Cronobacter sakazakii lv27
0.05%
–
Berthold-Pluta et al., 2019
Cronobacter malonaticus lv31
0.05%
–
Cronobacter muytjensii s50
0.05%
–
Cronobacter turicensis lv53
0.05%
–
Cronobacter condimenti s37
0.05%
–
Escherichia coli ATCC 35218
3.125 mg/mL
6.25 mg/mL
This study
Pseudomonas aeruginosa ATCC 27853
12.5 mg/mL
50 mg/mL
Proteus mirabilis ATCC 29245
3.125 mg/mL
6.25 mg/mL
Klebsiella pneumoniae ATCC 27736
3.125 mg/mL
6.25 mg/mL
Staphylococcus aureus MDR
0.78 mg/mL
1.56 mg/mL
Enterobacter cloacae
0.39 mg/mL
0.78 mg/mL
Candida albicans ATCC 10231
12.5 mg/mL
100 mg/mL
Cryptococcus neoformans ATCC 14116
50 mg/mL
100 mg/mL
Candida vaginalis (Clinical strain)
25 mg/mL
100 mg/mL
Candida sp. (Clinical strain)
25 mg/mL
100 mg/mL
3.4 Molecular docking analysis
In order to correlate the binding of isolated Thymus bioactive molecules with its biological activities, the main compounds were docked to the active site of TyrRS, GLMS and Gyrase, respectively to demonstrate their potential inhibition against S. aureus and E. coli pathogens. The binding affinities of top-rated pose of different ligand-receptor complex (Table 4) revealed that among all tested bioactive compounds, the best binding affinity was found with β-caryophyllene-enzymes with values of −5.4 kcal/mol, −6.8 kcal/mol and −6.2 kcal/mol, respectively for β-caryophyllene-TyrRS, β-caryophyllene-GLMS and β-caryophyllene-Gyrase, suggesting its highest binding efficiency and therefore was selected for further investigation.
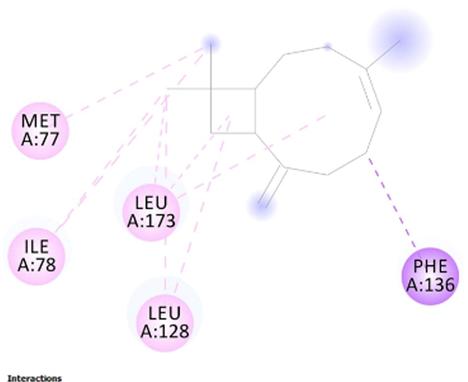
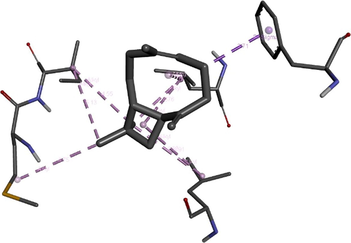
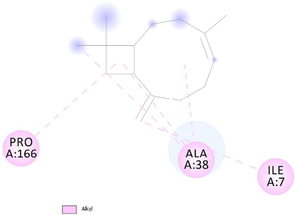
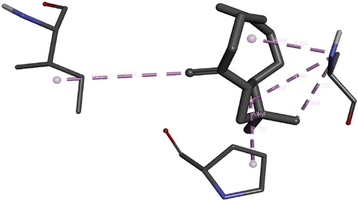
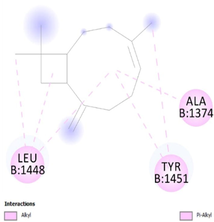
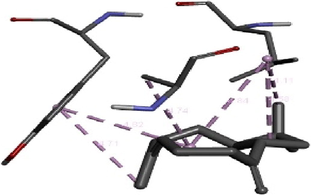
To get insight into the mechanism of TyrRS, GLMS and Gyrase inhibition by β-caryophyllene, we elucidate their molecular interaction mode in the active site residues of receptors. The outcomes compiled in Table 8 showed that β-caryophyllene-TyrRS complex was mainly stabilized by Alkyl interactions with Met77, Ile78 and Leu128, Pi-Alkyl interactions with Leu128 and Leu173 and Pi-sigma interactions with Phe 136 residues. Alkyl and Pi-Alkyl interactions were also formed between β-caryophyllene and GLMS residues of Ile7, Ala38 and Pro166. However, the amino acid residues involved in stabilizing the complex caryophyllene-Gyrase are Ala1374 (Pi-Alkyl), Leu1448 (Pi-Alkyl and Alkyl) and Tyr1451 (Pi-Alkyl). As shown, Phe136 and Leu173 of TyrRS from S. aureus, Ala38 from Gyrase in S. aureus and Leu1448 from GLMS in E. coli formed stronger Pi-Sigma, Alkyl and Pi-Alkyl interactions with the natural bioactive compounds (Tables 7 and 8) and therefore, could possibly inhibit the activity of enzyme resulting in the neutralization of their virulence. 1XFF: glucosamine 6-phosphate synthase (GLMS) from E. coli, 1JIJ: tyrosyl-tRNA synthetase TyrRS from S. aureus, 2XCT: Gyrase from S. aureus.
Compounds
1XFF
1JIJ
2XCT
α-Thujene
−4.5
−5.5
−4.9
α-Pinene
−4.4
−5.6
−4.5
β-Myrcene
−3.3
−5.1
−4
α-Terpinene
−4.5
−6
−4.9
p-Cymene
−4.3
−5.7
−5
1,8-Cineole
−4.8
−5.1
−4.8
γ-Terpinene
−4.5
−6
−4.9
α-Terpinolene
−4.4
−5.9
−5.3
Borneol
−4.9
−5.4
−4.8
Terpinen-4-ol
−4.6
−5.8
−5
α-Terpineol
−4.9
−6.1
−5.1
2-Isopropyl-5-methylanisole
−4.4
−4.9
−5.1
Thymol
−4.5
−5.9
−5.4
Carvacrol
−5.2
−6.3
−5.4
Thymyl acetate
−5.1
−6.1
−4.8
Carvacryl acetate
−5
−6.1
−5.6
β-Caryophyllene
−5.4
−6.8
−6.2
2D interactions, Receptor Ligand Interactions, Distance in Angstroms
3D interaction Receptor–Ligand
Receptor – Ligand: 1JIJ – β-Caryophyllene
Receptor – Ligand: 1JIJ – β-Caryophyllene
(MET77) S---S (Ligand) Alkyl interaction: 4.75 A°; (ILE78) C---C (Ligand) Alkyl interaction: 4.55 A°; (ILE78) C---C (Ligand) Alkyl interaction: 5.13 A°; (LEU128) C---C (Ligand) Alkyl interaction: 4.63 A°; (LEU128) C---C (Ligand) Pi-Alkyl interaction 4.91A°;(PHE136) phenyl ring---C (Ligand) Pi-sigma interaction: 3.71 A°; (LEU173) C---C (Ligand) Alkyl interaction: 3.85 A°; (LEU173) C---C (Ligand) Pi-Alkyl interaction: 4.69 A°; (LEU173) C---C (Ligand) Pi-Alkyl interaction: 4.76 A°.
Receptor – Ligand: 1XFF – β-Caryophyllene
Receptor – Ligand: 1XFF – β-Caryophyllene
(ILE7) CC---CH (Ligand): 5.37 A°; (ALA38) C---Phenyl ring (Ligand) Pi-Alkyl interaction: 3.64 A°; (ALA38) C---Alkyl ring (Ligand) Pi-Alkyl interaction: 4.40 A°; (ALA38) C---C (Ligand) Alkyl interaction: 4.07 A°; (PRO166) phenyl ring---Alkyl ring (Ligand) Pi-Alkyl interaction: 4.76 A°.
Receptor – Ligand: 2XCT – β-Caryophyllene
Receptor – Ligand: 2XCT – β-Caryophyllene
(ALA1374)C---phenyl ring (Ligand) – Pi-alkyl interaction: 4.74 A°; (LEU1448) C---phenyl ring (Ligand)–Pi-alkyl interaction: 4.84 A°; (LEU1448) C---alkyl ring (Ligand)–Alkyl interaction: 4.58 A°; (LEU1448) C---C (Ligand)–Alkyl interaction: 4.11 A°; (TYR1451) phenyl ring---C (Ligand) Pi-alkyl interaction: 4.71 A°; (TYR1451) phenyl ring---phenyl ring (Ligand) Pi-alkyl interaction 4.82 A°.
4 Conclusion
In the present study, the antioxidant and the antimicrobial assays of the essential oil from T. musilii were evaluated. The obtained findings suggest that this cultivated species can constitute a good source of antioxidant, antibacterial and antifungal compounds, namely, thymol. Nevertheless, these biological results deserve further deep in vivo studies in order to use this plant as possible bio-source in food and pharmaceutical industries. Molecular docking results together with the findings of in-vitro antimicrobial potency suggest that T. musilii essential oil is a potent inhibitor of S. aureus and E. coli and subsequently lead to novel discovery of plant-based therapeutic products.
Funding
This research has been funded by Scientific Research Deanship at University of Ha'il - Saudi Arabia through project number 160991.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
References
- Antifungal efficacy of thymol, carvacrol, eugenol and menthol as alternative agents to control the growth of food-relevant fungi. J. Med. Mycol.. 2014;242:e51-e56.
- [Google Scholar]
- Dietary combination of chitosan nanoparticle and thymol affects feed utilization, digestive enzymes, antioxidant status, and intestinal morphology of Oreochromis niloticus. Aquaculture. 2020;515:734577.
- [Google Scholar]
- Identification of Essential Oil Components by Gas Chromatography/Quadrupole Mass Spectroscopy. Carol Stream, III, USA: Allured Publishing Corporation; 2007.
- Bioactive potential of essential oil extracted from the leaves of Eucalyptus globulus (Myrtaceae) J. Pharmacogn. Phytochem.. 2019;8(1):213-216.
- [Google Scholar]
- Effect of Adiantum philippense extract on biofilm formation, adhesion with its antibacterial activities against foodborne pathogens and characterization of bioactive metabolites: An in vitro-in silico approach. Front. Microbiol.. 2020;11:823.
- [CrossRef] [Google Scholar]
- Formulation, evaluation and bioactive potential of Xylaria primorskensis terpenoid nanoparticles from its major compound xylaranic acid. Sci. Rep.. 2018;8(1):1740.
- [Google Scholar]
- Chemical composition and antimicrobial activity of essential oil of Thymus vulgaris from Yemen. Turk. J. Biochem.. 2011;36:342-349.
- [Google Scholar]
- Chemical composition of essential oil of Thymus vulgaris collected from Saudi Arabian market. Asian Pac. J. Trop. Biomed.. 2017;7(2):147-150.
- [Google Scholar]
- A review of hepatoprotective plants used in Saudi traditional medicine. Evid. Based Complement. Alternat. Med. 2014:1-22.
- [Google Scholar]
- The influence of reduced irrigation on herbage, essential oil yield and quality of Thymus vulgaris and Thymus daenensis. J. Herbal Drugs. 2013;4(3):109-113.
- [Google Scholar]
- Essential oil composition and in vitro antioxidant and antimicrobial activities of Thymus cappadocicus Boiss. J. Food Process. Preserv. 2012:1-10.
- [Google Scholar]
- Parental awareness of the liver disease among children in Saudi Arabia. Ann Saudi Med.. 1998;18(1):79-81.
- [Google Scholar]
- Survey of plant species of medical importance to treat digestive tract diseases in Tabuk Region, Saudi Arabia. J. King Abdulaziz Univ.-Sci.. 2017;29(1):51-61.
- [Google Scholar]
- Quantitative structure-activity relationship of molecules constituent of different essential oils with antimycobacterial activity against Mycobacterium tuberculosis and Mycobacterium bovis. BMC Complement. Altern. Med.. 2015;15:332.
- [Google Scholar]
- Comparison of chemical composition, antioxidant and antimicrobial activities of Thymus capitatus L. essential oils from two Tunisian localities (Sousse and Bizerte) Int. J. Agron. Plant. Prod.. 2013;4(8):1772-1781.
- [Google Scholar]
- Essential oils of Moldavian Thymus species: Chemical composition, antioxidant, anti-Aspergillus and antigenotoxic activities. Flavour Frag. J.. 2019;34:175-186.
- [Google Scholar]
- Thymol efficacy against coccidiosis in pigeon (Columba livia domestica) Prevent. Veterin. Med.. 2020;176:104914.
- [Google Scholar]
- Comparison of volatile constituents, and antioxidant and antibacterial activities of the essential oils of Thymus caucasicus, T. kotschyanus and T. vulgaris. Natural Prod. Commun.. 2011;6(1):137-140.
- [Google Scholar]
- Antimicrobial and antioxidant activities of hydrocarbon and oxygenated monoterpenes against some foodborne pathogens through in vitro and in silico studies. Pestic. Biochem. Physiol.. 2019;158:185-200.
- [Google Scholar]
- Chemical composition and in vitro antibacterial properties of essential oils of four Thymus species from organic growth. Ind. Crops Prod.. 2013;50:304-311.
- [Google Scholar]
- Antioxidant activity of Agaricus sp. mushrooms by chemical, biochemical and electrochemical assays. Food Chem.. 2008;111(1):61-66.
- [Google Scholar]
- Antibacterial activities of some plant extracts utilized in popular medicine in Palestine. Turk. J. Biol.. 2004;28:99-102.
- [Google Scholar]
- Ecological observations along baghdad-huseiba road. Western Desert, Iraq With 2 figures. Feddes Repertor.. 1972;83(4):245-263.
- [Google Scholar]
- Type IIA topoisomerase inhibition by a new class of antibacterial agents. Nature. 2010;466:935-940.
- [Google Scholar]
- Antibacterial activities of plant-derived compounds and essential oils against Cronobacter strains. Eur. Food Res. Technol.. 2019;245:1137-1147.
- [Google Scholar]
- Antifungal activity of chemically different essential oils from wild Tunisian Thymus spp., Natural Product Research: Formerly. Natural Prod. Lett. 2014:1-5.
- [Google Scholar]
- Structural elucidation and antioxidant activity of a water-soluble polysaccharide from the fruit bodies of bulgaria inquinans (Fries) Food Chem.. 2013;138(2–3):1470-1475.
- [Google Scholar]
- Ind. Crops Prod.. 2018;121(1):434-440.
- Thymus vulgaris essential oil: chemical composition and antimicrobial activity. J. Med. Life. 2014;7(Special Issue 3):56-60.
- [Google Scholar]
- Characterization of the volatile composition of essential oils of some Lamiaceae spices and the antimicrobial and antioxidant activities of the entire oils. J. Agric. Food. Chem.. 2006;54(5):1822-1828.
- [Google Scholar]
- Biological activity and ethnomedicinal use Thymus vulgaris and Thymus serpyllum. Med. Mater.. 2013;33(33):3-17.
- [Google Scholar]
- Chemical composition and bioactivity of essential oil from Thymus species in Balkan Peninsula. Phytochem. Rev.. 2015;14:335-352.
- [Google Scholar]
- Sesquiterpenoids with free-radical-scavenging properties from marine macroalga Ulva fasciata Delile. Food Chem.. 2010;122(1):31-41.
- [Google Scholar]
- Antioxidant synergism and antagonism between selected monoterpenes using the 2, 2-diphenyl-1-picrylhydrazyl method. Flavour Fragrance J.. 2016;31(6):412-419.
- [Google Scholar]
- In-vitro antimicrobial activity and chemical composition of Sardinian Thymus essential oils. Lett. Appl. Microbiol.. 1999;29:130-135.
- [Google Scholar]
- Chemical analysis and antifungal activity of Thymus striatus. Phytother. Res.. 2004;18(1):40-42.
- [Google Scholar]
- The Evolution and Classification of Flowering Plants. New York, USA: The New York Botanical Garden; 1988.
- Thyme essential oils from Spain: Aromatic profile ascertained by GC-MS, and their antioxidant, anti-lipoxygenase and antimicrobial activities. J. Food Drug. Anal.. 2018;26:529-544.
- [Google Scholar]
- Essential oils and their natural active compounds presenting antifungal properties. Molecules. 2019;24:3713-3734.
- [Google Scholar]
- Thymol antifungal mode of action involves telomerase inhibition. Med. Mycol.. 2013;51:826-834.
- [Google Scholar]
- Antifungal activity and mode of action of thymol and its synergism with nystatin against Candida species involved with infections in the oral cavity: An in vitro study. BMC Complement. Altern. Med.. 2015;15:417.
- [Google Scholar]
- Chemical composition and antimicrobial activity of the essential oils from two species of Thymus growing wild in southern Italy. Molecules. 2009;14(11):4614-4624.
- [Google Scholar]
- Composition and antimicrobial activity of the essential oil of Thymus fontanesii. Pharm. Biol.. 2006;44(8):607-612.
- [Google Scholar]
- In vitro antibacterial activity of thymol and carvacrol and their effects on broiler chickens challenged with Clostridium perfringens. J. Anim. Sci. Biotechnol.. 2015;24(6):58-69.
- [Google Scholar]
- Essential oil composition and antibacterial activity of Thymus caramanicus at different phonological stages. Food Chem.. 2008;110:927-931.
- [Google Scholar]
- Comparison of yield chemical composition and biological activities of essential oils obtained from Thymus pallidus and Thymus satureioides Coss. grown in wild and cultivated conditions in Morocco. J. Essential Oil Bear. Plants 2020:1-14.
- [Google Scholar]
- Chemical constituents of indigenous plants used in native medicine of Saudi Arabia. Arab Gulf J. Sci. Res.. 1983;1:395-419.
- [Google Scholar]
- Antileishmanial and cytotoxic potential of essential oils from medicinal plants in northern Tunisia. Ind. Crop. Prod.. 2015;77:795-802.
- [Google Scholar]
- A Study on the Antimicrobial Activity of Thymol Intended as a Natural Preservative. J. Food Prot.. 2005;68(8):1664-1670.
- [Google Scholar]
- Chemical composition and anti-Helicobacter pylori effect of Satureja bachtiarica Bunge essential oil. Phytomedicine. 2015;221:173-177.
- [Google Scholar]
- In vitro antibacterial activity of Crinum purpurascens Herb. leaf extract against the Salmonella species causing typhoid fever and its toxicological evaluation. Iran. J. Med. Sci.. 2009;34:126-136.
- [Google Scholar]
- Evaluation of Thymus vulgaris and Thymbra spicata essential oils and plant extracts for chemical composition, antioxidant, and antimicrobial properties. Food Sci. Nutrit.. 2019;7(5):1704-1714.
- [Google Scholar]
- The Fungicidal activity of thymol against Fusarium graminearum via inducing lipid peroxidation and disrupting ergosterol biosynthesis. Molecules.. 2016;21:770-782.
- [Google Scholar]
- Biological activities of essential oils extracted from Thymus capitatus (Lamiaceae) S. Afr. J. Bot.. 2020;128:274-282.
- [Google Scholar]
- Govaerts, R., 2003. World checklist of selected plant families database in ACCESS: 1-216203. The Board of Trustees of the Royal Botanic Gardens, Kew. (Thymus musilii Velen., Sitzungsber. Königl. Böhm. Ges. Wiss., Math.-Naturwiss. Cl. 11: 5 (1911 publ. 1912). R.Govaerts.
- Terpenoids as Plant Antioxidants. In: Litwack G., ed. Vitamins & Hormones. Academic Press; 2005. p. :505-535.
- [Google Scholar]
- Antibacterial activity of terpenes and terpenoids present in essential oils. Molecules.. 2019;24(13):2471-2481.
- [Google Scholar]
- Hernández-Hernández E., Regalado-González C., Vázquez-Landaverde P., Guerrero-Legarreta I., & García-Almendárez B. E., 2014. Microencapsulation, chemical characterization, and antimicrobial activity of Mexican Lippia graveolens H.B.K. and European Origanum vulgare L. oregano essential oils. Sci. World J., 641814.
- Antioxidant capacity and total phenolic content of Malaysian underutilized fruits. J. Food Compos. Anal.. 2009;22(5):388-393.
- [Google Scholar]
- Substrate binding is required for assembly of the active conformation of the catalytic site in Ntn amidotransferases: evidence from the 1.8 å crystal structure of the glutaminase domain of glucosamine 6-phosphate synthase. Structure. 1996;4(7):801-810.
- [Google Scholar]
- Protective effects of orally administered thymol against titanium dioxide nanoparticle–induced testicular damage. Environ Sci. Pollut. Res. Int.. 2020;27(2):2353-2360.
- [Google Scholar]
- Jamzad, Z., 2010. Thymus and Satureja spp. of Iran, Research instituted of Forests and Rangelands Press, 172 P.
- Composition of the essential oil of Thymus afghanicus. Chem. Nat. Compd.. 2020;56(1):156-157.
- [Google Scholar]
- In vitro activity of carvacrol and thymol combined with antifungals or antibacterials against Pythium insidiosum. Journal de Mycologie Médicale. 2015;252:e89-e93.
- [Google Scholar]
- Polyphenolic extract and essential oil quality of Thymus zygis ssp. gracilis shrubs cultivated under different watering levels. Ind. Crop. Prod.. 2009;29:145-153.
- [Google Scholar]
- Thymus ciliatus the highest thymol containing essential oil of the genus. Nat. Prod. Commun.. 2009;4(9):1251-1252.
- [Google Scholar]
- A molecular perspective on terpene variation in Australian Myrtaceae. Aust. J. Bot.. 2008;56:197-213.
- [Google Scholar]
- Chemical composition and biological activity of the essential oil from Thymus lanceolatus. Z. Naturforsch.. 2016;71:155-163.
- [Google Scholar]
- Chemical composition and biological activity of the essential oil from Thymus lanceolatus. Z. Naturforsch C. J. Biosci.. 2016;71(5–6):155-163.
- [Google Scholar]
- Effect of irrigation frequency and planting density on herbage biomass and oil production of thyme (Thymus vulgaris) and hyssop (Hyssopus officinalis) Ind. Crop. Prod.. 2008;27(3):315-321.
- [Google Scholar]
- Thymol from Thymus quinquecostatus Celak. protects against tert-butyl hydroperoxide-induced oxidative stress in Chang cells. J. Nat. Med.. 2014;68(1):154-162.
- [Google Scholar]
- Chemical composition, antimicrobial activity, kinetics and mechanism of action of Himalayan-thyme (Thymus linearis Benth.) J. Essent. Oil Res.. 2020;32:64-73.
- [Google Scholar]
- Unusual antioxidant behavior of α-and γ-terpinene in protecting methyl linoleate, DNA, and erythrocyte. J. Agric. Food Chem.. 2009;57(9):3943-3948.
- [Google Scholar]
- Antibacterial activity and mechanism of action of Monarda punctate essential oil and its main components against common bacterial pathogens in respiratory tract. Int. J. Clin. Exp. Pathol.. 2014;7:7389.
- [Google Scholar]
- Studies on chemical composition, antimicrobial and antioxidant activities of five Thymus vulgaris L. essential oils. Molecules. 2015;20:12016-12028.
- [Google Scholar]
- Synthesis and in vitro antibacterial activity of thymol and carvacrol derivatives. Acta Poloniae Pharm.-Drug Res.. 2010;67(4):375-380.
- [Google Scholar]
- Essential oil production in shoot cultures versus field-grown plants of Thymus caespititius. Plant Cell Tiss. Organ Cult.. 2013;113:341-351.
- [Google Scholar]
- Chemical composition, antibacterial, and antifungal activities of the essential oil of Thymus numidicus Poiret from Algeria. Phytothérapie 2016:1-6.
- [Google Scholar]
- Composition and antioxidant activities of the essential oils of Thymus caespititius, Thymus camphoratus and Thymus mastichina. Food Chem.. 2004;86:183-188.
- [Google Scholar]
- Antipseudomonal activity of the essential oil of Thymus numidicus Poiret. Int. J. Pharm. Sci. Rev. Res.. 2014;25(2):149-153.
- [Google Scholar]
- Antioxidant activity of minor components of tree nut oils. Food Chem.. 2008;111(2):421-427.
- [Google Scholar]
- Composition of the volatile oil of Thymus transcaspicus Klokov from Iran. Flavour Frag. J.. 2002;17:245-246.
- [Google Scholar]
- Essential oil of Thymus capitatus Hoff. et Link. from Matmata, Tunisia: gas chromatography-mass spectrometry analysis and antimicrobial and antioxidant activities. J. Med. Food.. 2010;13(6):1500-1504.
- [Google Scholar]
- The Comparison of Composition and biological activities in wild and cultivated of Thymus kotschyanus essential oils and methanolic extracts from East Azarbayjan, Iran. Crescent J. Med. Biol. Sci.. 2017;4:17-22.
- [Google Scholar]
- Morales, R., 2002. The history, botany and taxonomy of the genus Thymus. In: Stahl-Biskup., Saez, F. (Eds.), Thyme: The Genus Thymus. Taylor & Francis, London. pp. 1–43.
- AutoDock4 and AutoDockTools4: Automated docking with selective receptor flexibility. J. Comput. Chem.. 2009;30(16):2785-2791.
- [Google Scholar]
- Plants belonging to the genus Thymus as antibacterial agents: from farm to pharmacy. Food Chem.. 2015;15(173):339-347.
- [Google Scholar]
- Pharmacological properties and molecular mechanisms of Thymol: prospects for its therapeutic potential and pharmaceutical development. Front. Pharmacol.. 2017;26(8):380.
- [Google Scholar]
- Analysis of the essential oils of two Thymus species from Iran. Food Chem.. 2005;90:609-611.
- [Google Scholar]
- Chemical composition, antimicrobial, antioxidant and antitumor activity of Thymus serpyllum L., Thymus algeriensis Boiss. and Reut. and Thymus vulgaris L. essential oils. Ind. Crops Prod.. 2014;52:183-190.
- [Google Scholar]
- Antioxidant activity of ethanolic extract of Maranta Arundinacea L. tuberous rhizomes. Asian J. Pharm. Clin. Res.. 2012;5(4):85-88.
- [Google Scholar]
- Phytochemical composition, anti-biofilm and anti-quorum sensing potential of fruit, stem and leaves of Salvadora persica L. methanolic extracts. Microb. Pathog.. 2017;109:169-176.
- [Google Scholar]
- Comparative study of the chemical profiling, antioxidant and antimicrobial activities of essential oils of different parts of Thymus willdenowii Boiss & Reut. Nat. Prod. Res.. 2019;33:2398-2401.
- [Google Scholar]
- Use of natural antimicrobials to increase antibiotic susceptibility of drug resistant bacteria. Int. J. Food Microbiol.. 2010;1402:164-168.
- [Google Scholar]
- Molecular modeling studies to explore the binding affinity of virtually screened inhibitor toward different aminoglycoside kinases from diverse MDR strains. J. Cell Biochem.. 2018;119(3):2679-2695.
- [Google Scholar]
- UCSF Chimera–a visualization system for exploratory research and analysis. J. Comput. Chem.. 2004;25(13):1605-1612.
- [Google Scholar]
- Composition of the essential oil of an Italian Thymus vulgaris L. ecotype. Flavour Frag. J.. 1991;6:241-244.
- [Google Scholar]
- Antifungal activity of Thymus oils and their major compounds. J. Eur. Acad. Dermatol.. 2004;18:73-78.
- [Google Scholar]
- Antifungal activity of the essential oil of Thymus pulegioides on Candida, Aspergillus and dermatophyte species. J. Med. Microbiol.. 2006;55:1367-1373.
- [Google Scholar]
- Anti- Candida activity of Iranian medicinal plants. Electron. J. Biol.. 2009;5:85-88.
- [Google Scholar]
- Environment effect on diversity in quality and quantity of essential oil of different wild populations of kerman Thyme. Genetika. 2013;45(2):441-450.
- [Google Scholar]
- Essential oil and chemical compositions of wild and cultivated Thymus daenensis Celak and Thymus vulgaris L. Ind. Crop. Prod.. 2013;48:43-48.
- [Google Scholar]
- Antimicrobial activity of some of the Iranian medicinal plants. Arch. Biol. Sci. Belgrade.. 2010;62:633-642.
- [Google Scholar]
- Chemical composition and antibacterial activity of essentials oils of Iranian herbs against Staphylococcus aureus isolated from milk. Int. J. Food Prop.. 2014;17:2063-2071.
- [Google Scholar]
- Salicylic acid affects growth, essential oil and chemical compositions of thyme (Thymus daenensis Celak.) under reduced irrigation. Plant Growth Regul. 2013:1-13.
- [Google Scholar]
- Variation in antibacterial activity, thymol and carvacrol contents of wild populations of Thymus daenensis subsp. daenensis Celak. P.O.J.. 2011;4(4):209-214.
- [Google Scholar]
- Essential oil polymorphism of wild growing Hungarian thyme (Thymus pannonicus) populations in the Carpathian Basin. Nat. Prod. Commun.. 2010;5:1681-1686.
- [Google Scholar]
- Crystal structure of Staphylococcus aureus tyrosyl-tRNA synthetase in complex with a class of potent and specific inhibitors. Protein Sci.. 2001;10:2008-2016.
- [Google Scholar]
- Genetic variability and geographical differentiation in Thymus daenensis subsp. daenensis Cleak, an endangered aromatic and medicinal plant as revealed by Inter Simple Sequence Repeat (ISSR) markers. Biochem. Genet.. 2009;47:831-842.
- [Google Scholar]
- Evaluation of anticancer, antibacterial and antioxidant properties of a medicinally treasured fern tectaria coadunata with its phytoconstituents analysis by HR-LCMS. Anti-Cancer Agents Med. Chem.. 2020;20:1-12.
- [Google Scholar]
- Inhibition of verocytotoxigenic Escherichia coli in model broth and rumen systems by carvacrol and thymol. Int. J. Food Microbiol.. 2010;1391:70-78.
- [Google Scholar]
- Antimicrobial activity and chemical composition of Thymus vulgaris, Thymus zygis and Thymus hyemalis essential oils. Food Control. 2008;19(7):681-687.
- [Google Scholar]
- Thymus spp. Plants-Food applications and phytopharmacy properties. Trends Food Sci. Technol.. 2019;85:287-306.
- [Google Scholar]
- Variation in chemical composition of Eucalyptus globulus essential oil under phenological stages and evidence synergism with antimicrobial standards. Ind. Crop. Prod.. 2018;124:115-125.
- [Google Scholar]
- Essential oil composition and antioxidant activity of Thymus longicaulis C. Presl subsp. longicaulis var. longicaulis. Food Chem Toxicol.. 2010;48(7):1801-1805.
- [Google Scholar]
- Essential Oil characterization of Thymus vulgaris from various geographical locations. Foods. 2016;5:70-81.
- [Google Scholar]
- ROS involves the fungicidal actions of thymol against spores of aspergillus flavus via the induction of nitric oxide. PLoS ONE. 2016;11(5):e0155647.
- [Google Scholar]
- Antioxidant properties and anti-quorum sensing potential of Carum copticum essential oil and phenolics against Chromobacterium violaceum. J. Food Sci. Technol.. 2018;55(8):2824-2832.
- [Google Scholar]
- Structural analysis of membrane-bound hECE-1 dimer using molecular modeling techniques: insights into conformational changes and Abeta1-42 peptide binding. Amino Acids. 2015;47(3):543-559.
- [Google Scholar]
- Antibacterial and antioxidant activity and essential oil composition of Grammosciadium scabridum Boiss. from Iran. Z. Naturforsch C J. Biosci.. 2005;60(7–8):534-538.
- [Google Scholar]
- Essential oil composition, total phenolic, flavonoid contents, and antioxidant activity of Thymus species collected from different regions of Iran. Food Chem.. 2017;220:153-161.
- [Google Scholar]
- Thymol, carvacrol, and antioxidant accumulation in Thymus species in response to different light spectra emitted by light-emitting diodes. Food Chem.. 2020;307:125521.
- [Google Scholar]
- Review on essential oil, extracts composition, molecular and phytochemical properties of Thymus species in Iran. Ind. Crop. Prod.. 2019;134:89-99.
- [Google Scholar]
- Mechanisms of antibacterial action of three monoterpenes. Antimicrob. Agents Chemother.. 2005;496:2474-2478.
- [Google Scholar]
- Chemical composition and antibacterial properties of Thymus longicaulis subsp. chaoubardii oils: three chemotypes in the same population. J. Essent. Oil Res.. 1998;10:97-99.
- [Google Scholar]
- Antimicrobial and Herbicidal Activities of the Essential Oil from the Mediterranean Thymus eigii. J. Essent. Oil Bear. Plants.. 2018;21:214-222.
- [Google Scholar]
- Antimicrobial activity of Thymus longicaulis C. Presl essential oil against respiratory pathogens. Cent. Eur. J. Biol.. 2012;7(6):1109-1115.
- [Google Scholar]
- World Checklist of Selected Plant Families, 2010. copyright © The Board of Trustees of the Royal Botanic Gardens, Kew. Thymus musilii Velen.. Accessed through: Euro+Med PlantBase at http://ww2.bgbm.org/euroPlusMed/PTaxonDetail.asp?UUID=B7F1730F-E243-4A96-9592-12FB15BFDE1A.
- Critical overview on Thymus daenensis Celak: phytochemical and pharmacological investigations. J. Integr. Med.. 2015;13(2):91-98.
- [Google Scholar]







