Translate this page into:
Tilianin alleviates airway inflammation in ovalbumin-induced allergic asthma in mice through the regulation of Th2 cytokines and TGF-β1/Smad markers
⁎Corresponding author at: Department of Special Need, Xi’an Children’s Hospital, Xi’an 710000, China. qingyou2021@sina.com (Ling Ou)
-
Received: ,
Accepted: ,
This article was originally published by Elsevier and was migrated to Scientific Scholar after the change of Publisher.
Abstract
Background
Allergic asthma is a inflammatory disease defined as a condition of chronic airway inflammation. Asthma can be provoked by various stimuli like allergens inhalation like dust particles, pollen, and pollutants in the air.
Objective
This exploration was dedicated to investigate the anti-asthmatic properties of tilianin against the ovalbumin (OVA)-initiated asthma in mice.
Methodology
The asthma was provoked to the mice via administering 100 μl of aluminum hydroxide containing 20 μg of OVA and treated with the 10 and 20 mg/kg of tilianin, respectively. The levels of Th2 cytokines, OVA-specific IgE, eotaxin, pro-inflammatory mediators, antioxidants, and other markers were inspected by marker specific assay kits. The mRNA expressions of TGF-β1, Smad, iNOS, and COX-2 was assessed using RT-PCR analysis. The lung histology was analyzed microscopically to detect the histological changes.
Results
Tilianin treatment remarkably suppressed the IL-4, IL-5, and IL-13, IFN-γ, eotaxin, and IgE levels. The NO, MPO, and inflammatory makers TNF-α, IL-6, IL-12, and TXB2 was substantially diminished by the tilianin treatment. The TGF-β1, iNOS, and COX-2 expressions were appreciably suppressed by the tilianin. The histological findings proved that the tilianin treatment alleviated the OVA-provoked histopathological changes in the lung tissues.
Conclusion
Our findings proved that tilianin effectively alleviated the OVA-provoked asthma in animals and it could be a talented anti-asthmatic candidate.
Keywords
Allergic asthma
Ovalbumin
Tilianin
Inflammation
Data Availability Statement
The data used to support the findings of this study are available from the corresponding author upon request.
1 Introduction
Allergic asthma is a common inflammatory lung disease distinguished by airway inflammation, obstruction and airway hyper-responsiveness (AHR). The cough, wheezing, breathing difficulty, and chest tightness are the primary signs of asthma (Kim et al., 2010). Generally, asthma is provoked by various stimuli for instance, allergens inhalation like dust particles, pollen, and pollutants in the air (Shin et al., 2013). The chronic exposure to various allergens triggers the Th1 and Th2 response imbalances, further leading to asthmatic reactions of airway. Many inflammatory cells especially eosinophils, generate the chemokines, cytokines, and growth factors, which causes increased inflammatory reactions and over generation of mucus in the airway (Lee et al., 2011).
Chronic airway inflammation along with the eosinophil’s penetration, irregularly elevated the IgE status and Th2 cytokines productions, obstruction of airway, remodeling and structural alterations of airway, and mucus over generation. These are the allergic signs of the asthma, a severe respiratory disorder, which is affects the millions of peoples around the world, with an gradually elevating occurrence in Asian countries (Ellwood et al., 2017). As projected earlier, nearly 241 million peoples affected from asthma with a thousands of mortalities per day worldwide (GBD 2013 MCDC, 2015). The pathogenesis allergic asthma was contributed by various kinds of inflammatory cells, in which airway remodeling is a well recognized feature triggered by frequent damage and repair mechanisms provoked by continuing inflammation (Zhu et al., 2018). The asthmatic condition was initially be reversible as it is a chronic inflammatory ailment, but in the advanced stage may be irreversible because of the airway remodeling (Jo et al., 2018).
Airway inflammation is categorized by the eosinophil penetrations and goblet cells’ hyperplasia (Hoffmann et al., 2016). Th2 cells predominantly activated in an allergic asthma, which plays imperative role in the asthma progression (Gaurav and Agrawal, 2014). Th2 cells generates the more amounts of cytokines, which further enhance the inflammation and airway remodeling. Furthermore, the occurrence of IL-4/IL-13 triggers the B cells to generate the IgE. The recruitment of eosinophils regulated by IL-13 and histamine plays a imperative roles in the asthma progression. Eosinophils generates the numerous chemical mediators that enhance the inflammatory reactions. Mast cells produce the histamine, prostaglandins, leukotrines that further improves the allergic inflammatory reactions (Fulkerson and Rothenberg, 2013).
Allergic asthma is closely related with the Th2-influenced immune reactions that further directs to the more production of IgE, mucus, and eosinophilia in the airway. Th2 cytokines, primarily IL-4 and IL-13, are critical players of the allergic asthma phenotype. IL-4 is a most imperative type of Th2 cytokines that triggers the generation of asthma-specific IgE. Furthermore, IL-5 promotes the eosinophills penetration into the lung tissues with the importance of IL-13 on AHR in a progression of asthma. Hence, the appropriate control of these inflammatory mediators are the hopeful approach to manage the asthma (Zhu et al., 2016).
The TGF-β1 actively participates in the cell differentiation and immunological reactions (Massague, 2012). The stimulated TGF-β1 complexes could bind to the respective receptors that further activates the receptors to trigger signaling to regulate the various target gene expressions participated in the cell differentiation and immunological reactions (Halwani et al., 2011). Earlier investigations has highlighted that the targeting TGF-β1/Smad cascade may provide new therapeutic strategies for asthma mediated airway remodeling (Liu et al., 2019). TGF-β1 is said to be a critical regulator of airway remodeling and responsively disturbs the collagen deposition in the airway layer. The Smad is a imperative protein of the TGF-β1 signaling system and it is plays we key role in the signal transduction (Ojiaku et al., 2017).
Corticosteroids inhalation and bronchodilators are the only known effective approaches to treat the asthma. Though, long-lasting administration of corticosteroids and bronchodilators administration is tightly related with some severe side effects and also the asthma chronicity is remarkable financial challenge for patients (Klimek et al., 2019). In recent times, a massive attention was paid towards the identification of plant-mediated bioactive compounds to treat the respiratory ailments including allergic asthma (Kuo et al., 2012).
Tilianin is a flavonoid compound occurs in the various parts of Agastache rugosa and Dracocephalum moldavica. Additionally, these plants were highly nutritious and extensively utilized in the form of healthy drinks. Tilianin demonstrated the remarkable antidiabetic, anti-inflammatory, and antihyperlipidemic properties (Garcia-Diaz et al., 2016). Tilianin was reported to exhibit the anti-inflammatory, cardioprotective, antidiabetic, and antioxidant properties (Akanda et al., 2019; Zielinska and Matkowski, 2014; Guo et al., 2015; Zeng et al., 2018). Earlier study disclosed that the tilianin has exhibited the remarkable anti-asthmatic property in an in vitro dendritic cell model (Park et al., 2021). Though, the protective role of tilianin against the asthma provoked animal models were not investigated yet. Consequently, this exploration was designed to explore the protective properties of tilianin against the ovalbumin (OVA)-stimulated allergic asthma in mice via TGF-β1 mediated Smad signaling inhibition.
2 Materials and methods
2.1 Chemicals
Tilianin (Fig. 1), OVA, aluminum hydroxide, and other chemicals were purchased from Sigma-Chemicals, USA. All the marker specific assay kits were attained from Solarbio, China, Abcam, UK, Thermofisher, USA, and Mybiosource, USA, respectively.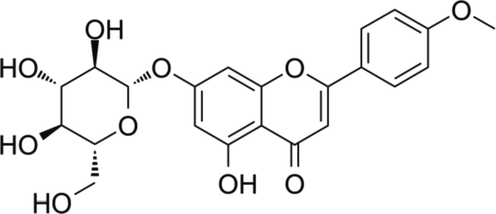
Chemical structure of tilianin.
2.2 Experimental animals
3–4 weeks old BALB/c mice weighing approximately 24 ± 3 g was acquired from institutional animal facility and the same was maintained in a sanitized cabins. All the experiments were carried out in accordance with the guidelines of institutional animal ethics committee. Animals were maintained on a systematic laboratory conditions with temperature 26 ± 1˚C, 60 to 70% air moisture, and 12 h dark/light series. All mice were adapted to laboratory situations for 7-days prior to experiments initiation.
2.3 Experimental groups
Animals were allocated into five groups with six mice in each. Group-I comprises control mice. Group-II are asthma triggered animals by OVA-sensitization. Animals were challenged by injecting (i.p.) a solution of 100 μl of aluminum hydroxide containing 20 μg of OVA on 1st and 14th day. Successively, animals were exposed to the intranasal spray of OVA solution (1%) from 21st to 23rd day. The animals from Group III and IV received 10 and 20 mg/kg of tilianin, respectively by oral gavage in 1 h before the OVA-challenge. Group V animals administered with the 3 mg/kg of standard drug dexamethasone (DEX). On the 24th day, all animals were sacrificed after CO2 asphyxiation euthanasia and then lungs were removed and wet weight (W) was noted (Fig. 2). Afterwards, the lung tissues were dehydrated in a oven at 80 °C and then dry weigh were weighed (D). The W/D ratio was then determined to detect the lung weight index.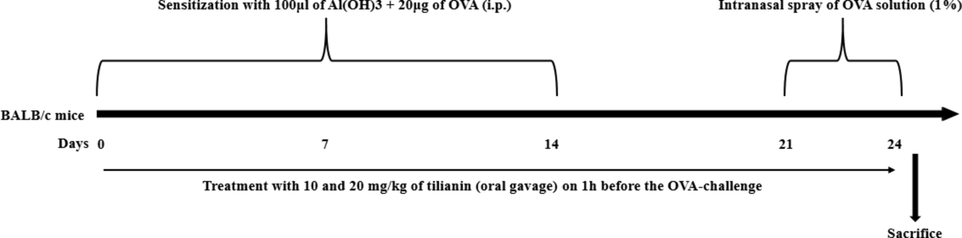
Experimental design and timeline.
2.4 Separation of BALF and enumeration of inflammatory cells
The BALF was gathered from the euthanized animals. The tracheas were detached and lavaged thrice with the 1 ml of chilled buffered saline. Then it was centrifuged at for 10 min at 3000 rpm at 4 °C. The inflammatory cells for instance, eosinophils, neutrophils, macrophages, and lymphocytes from the BALF was stained by respective kits (Solarbio, China) then cells were enumerated using microscope.
2.5 Quantification of Th2 cytokines, eotaxin, IFN-γ, and OVA-specific IgE levels
The status of Th2 cytokines (IL-4, IL-5, and IL-13), IFN-γ, eotaxin, and OVA-specific IgE in the BALF of control and experimental mice were evaluated using respective kits as per the industrial guidelines provided by manufacturer (Abcam, UK).
2.6 Measurement of and MPO and nitric oxide (NO) level
Lung tissues were excised from the animals and homogenized using chilled saline and centrifuged at 10000 rpm for 15 min at 4 °C. The resultant supernatant were gathered to examine the MPO activity and NO status using assay kits (Thermofisher, USA). The absorbance was taken at 550 nm by microplate reader.
2.7 Measurement of oxidative and antioxidant biomarkers
The oxidative stress in the OVA-challenged mice were inspected by examining the malondialdehyde (MDA) and antioxidants superoxide dismutase (SOD) and GSH status with the aid of commercial assay kits using instructions described by the manufacturer (Mybiosource, USA).
2.8 Quantification of pro-inflammatory cytokine levels
The TNF-α, IL-6, IL-12, and Thromboxane B2 (TXB2) status in the BALF of control and experimental mice were assessed using marker specific assay kits as suggested by the manufacturer instructions (Thermofisher, USA).
2.9 RT-PCR analysis
The RT-PCR analysis were executed to determine the inhibitory properties of tilianin on the expressions of TGF-β1, iNOS, COX-2, Smad2, and Smad3 genes in the OVA-challenged animals. For this, total RNA was seperated from lung tissues using Trizol reagent by applying the guidelines of kit’s manufacturer (Thermofisher, USA). The concentration and purity of the isolated RNA was detected by measuring absorbance at 260 nm. Then purified RNA was utilized for cDNA construction using PCR kit (Abcam, UK). The primers for TGF-β1- sense 5′-ATGTCGTCCATCTTGCCATTC-3′, antisense- 5′-AACCGTCCTGTTTTCTTTAGCTT-3′; Smad2- sense 5′-ATGTCGTCCATCTTGCCATTC-3′, antisense 5′-AACCGTCCTGTTTTCTTTAGCTT-3′; Smad3- sense 5′-CCCCCACTGGATGACTACAG-3′, antisense 5′-TCCATCTTCACTCAGGTAGCC-3′; COX-2- sense 5′-ACCAGCAGTTCCAGTATCAGA-3′, antisense 5′-CAGGAGGATGGAGTTGTTGTAG-3′; iNOS- sense 5′-TTCCACAACCACCTCAAGCA-3′ antisense 5′-TTAAGGCATCACAGTCCGAGTC-3′. The GAPDH were employed as a internal control to standardize the marker gene expressions.
2.10 Histopathological analysis
The lung tissues were processed with 10% of formalin then fixed on paraffin and blocks were sliced at 5 μm using microtome. Then tissues stained using hematoxylin and eosin (H&E) and the histological alterations were inspected using microscope at 200× magnification.
2.11 Statistical analysis
Outcomes were scrutinized using SPSS software (ver.17.0) and data was given as mean ± SD of triplicates. The one-way ANOVA and Student’s t-test were adapted to assess the changes and significance were fixed at p < 0.05.
3 Results
3.1 Effect of tilianin on the Th2 cytokines, eotaxin, IFN-γ, and OVA-specific IgE in the BALF of OVA-provoked mice
Fig. 3 demonstrates the influence of tilianin on the Th2 cytokines, eotaxin, IFN-γ, and IgE status in both control and treated animals. The OVA-triggered animals revealed a drastic augmentation in the status of IL-4, IL-5, and IL-13, IFN-γ, eotaxin, and OVA-specific IgE in the BALF. Conversely, the treatment with the 10 and 20 mg/kg of tilianin appreciably suppressed the Th2 cytokines, eotaxin, IFN-γ, and IgE status in the BALF of OVA-triggered animals (Fig. 3). The DEX administration also diminished the status of Th2 cytokines, eotaxin, IFN-γ, and IgE status in the BALF of OVA-triggered animals. The DEX and 20 mg/kg of tilianin treatment revealed a similar pattern of results.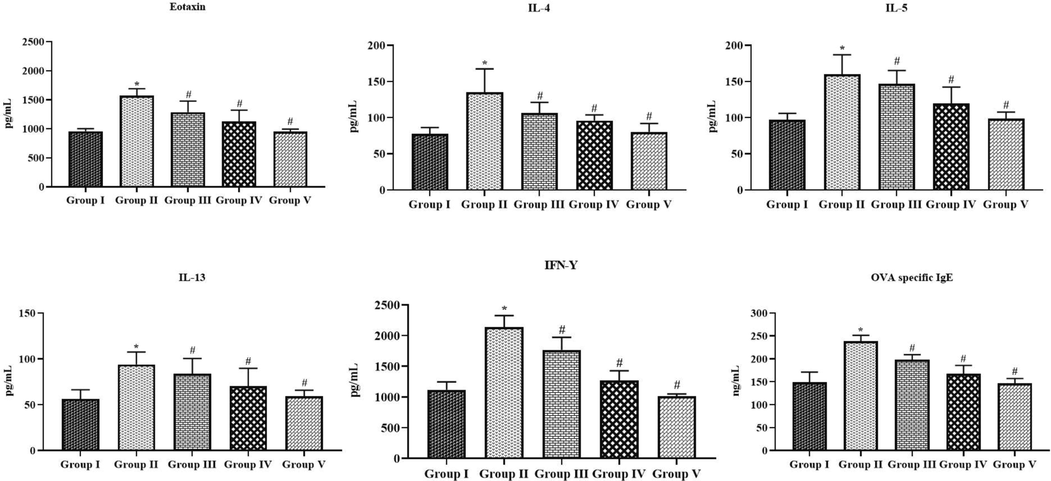
Effect of tilianin on the Th2 cytokines, eotaxin, IFN-γ, and OVA-specific IgE levels in the BALF of OVA-provoked mice. The levels of Th2 cytokines, eotaxin, IFN-γ, and IgE were effectively reduced by the treatment with 10 and 20 mg/kg of tilianin in the OVA-sensitized mice. Values were given as a mean ± SD of triplicates. Data were assessed statistically by one-way ANOVA and Student’s t-test. Note: ‘*’ represents that value differs at p < 0.05 from control and ‘#’ represents that value differs at p < 0.01 from OVA-provoked animals. Group I: Control mice; Group-II: OVA-sensitized asthma mice; Group III and IV: OVA-sensitized and 10 and 20 mg/kg of tilianin treated mice, respectively; Group V: OVA-sensitized and 3 mg/kg of standard drug dexamethasone treated mice.
3.2 Effect of tilianin on the inflammatory cell counts in the BALF of OVA-provoked mice
As represented in the Fig. 4, the total cell count and neutrophils, lymphocytes, eosinophils, and macrophages were drastically augmented in the BALF of OVA-provoked animals, when compared with control. The increases in these cell numbers were effectively suppressed by the tilianin. The 10 and 20 mg/kg of tilianin administration to the OVA-challenged mice revealed the remarkable diminution in the eosinophils, neutrophils, macrophages, and lymphocytes cell numbers in the BALF (Fig. 4).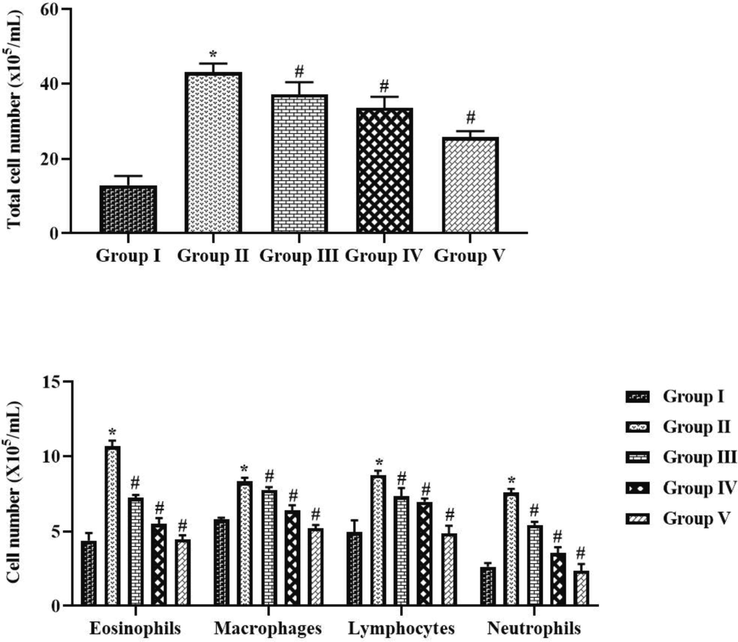
Effect of tilianin on the inflammatory cell counts in the BALF of OVA-provoked mice. The increased inflammatory cell counts were noted in the OVA-sensitized mice and the treatment with the 10 and 20 mg/kg of tilianin was appreciably reduced the level of inflammatory cells. Values were given as a mean ± SD of triplicates. Data were assessed statistically by one-way ANOVA and Student’s t-test. Note: ‘*’ represents that value differs at p < 0.05 from control and ‘#’ represents significantly differs at p < 0.01 from OVA-provoked animals. Group I: Control mice; Group-II: OVA-sensitized asthma mice; Group III and IV: OVA-sensitized and 10 and 20 mg/kg of tilianin treated mice, respectively; Group V: OVA-sensitized and 3 mg/kg of standard drug dexamethasone treated mice.
3.3 Effect of tilianin on the lung weight index, NO level, and MPO activity in the OVA-provoked mice
Fig. 5 demonstrates the influence of tilianin on the lung weight index, NO level, and MPO activity in both control and treated mice. The OVA-triggered mice exhibited the severe augmentation in the provoked mice lung weight index, NO level, and MPO activity when compared with the normal. Conversely, the supplementation of 10 and 20 mg/kg of tilianin to the OVA-challenged mice revealed the remarkable suppression in the lung weight index, NO level, and MPO activity (Fig. 5).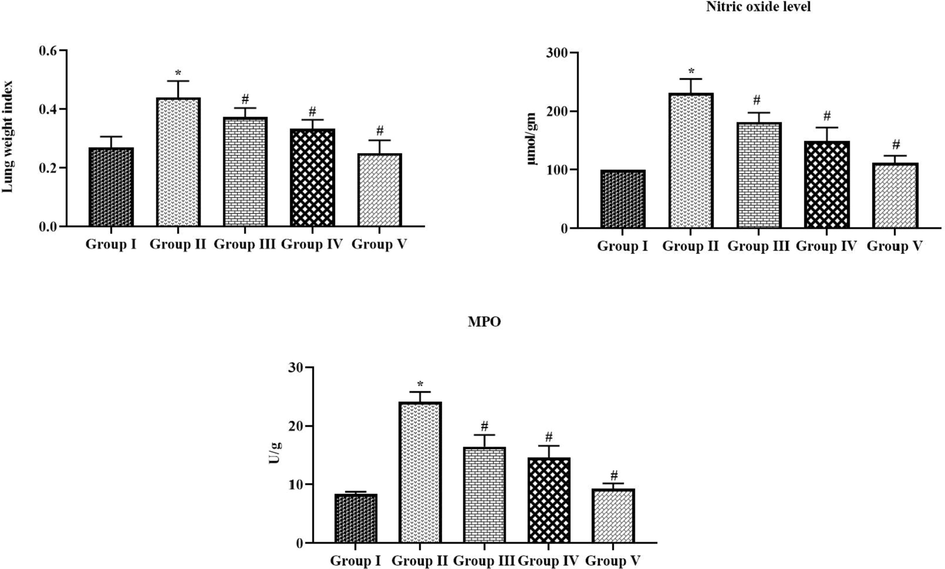
Effect of tilianin on the lung weight index, NO level, and MPO activity in the OVA-provoked mice. The increased lung weight, NO, and MPO was noted in the OVA-sensitized mice, which is effectively reduced by the 10 and 20 mg/kg of tilianin treatment. Values were given as a mean ± SD of triplicates. Data were assessed statistically by one-way ANOVA and Student’s t-test. Note: ‘*’ represents that value differs at p < 0.05 from control and ‘#’ represents significantly differs at p < 0.01 from OVA-provoked animals. Group I: Control mice; Group-II: OVA-sensitized asthma mice; Group III and IV: OVA-sensitized and 10 and 20 mg/kg of tilianin treated mice, respectively; Group V: OVA-sensitized and 3 mg/kg of standard drug dexamethasone treated mice.
3.4 Effect of tilianin on the oxidative stress and antioxidants in the OVA-provoked mice
Fig. 6 represents the MDA, SOD and GSH level in both control and experimental mice. The OVA-triggered mice revealed the appreciable elevation in the MDA as well as suppressed the SOD activity and GSH. The administration of 10 and 20 mg/kg of tilianin to the OVA-challenged asthmatic mice revealed the remarkable reduction in the MDA content and also remarkably augmented the SOD activity and GSH level (Fig. 6). The 20 mg/kg of tilianin possessed the remarkable action.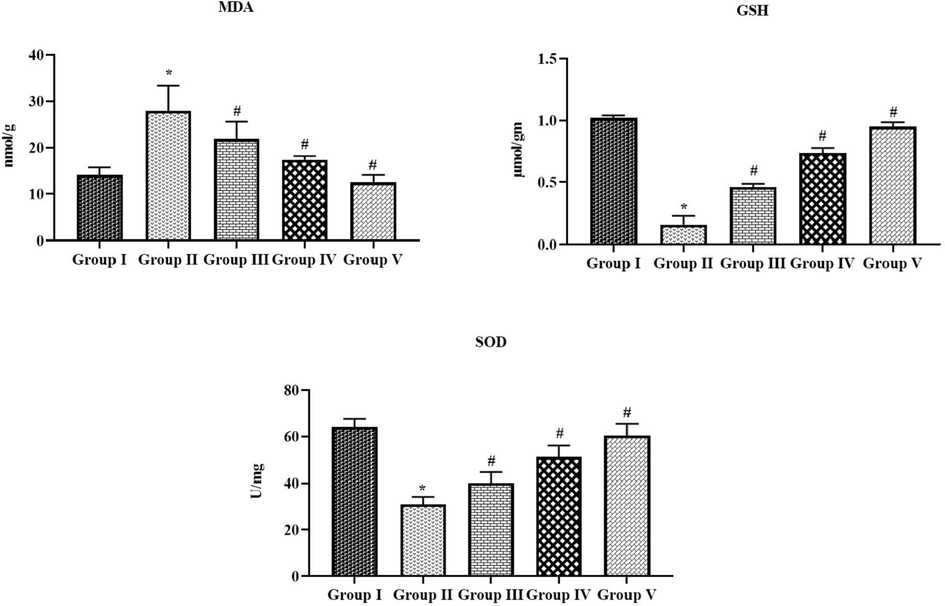
Effect of tilianin on the oxidative stress and antioxidants level in the OVA-stimulated mice. The OVA-sensitized mice revealed a increased MDA, and decreased GSH and SOD activity when compared with control. The treatment with the 10 and 20 mg/kg of tilianin was appreciably reduced the MPO and increased the GSH and SOD in the OVA mice. Values were given as a mean ± SD of triplicates. Data were assessed statistically by one-way ANOVA and Student’s t-test. Note: ‘*’ represents that value differs at p < 0.05 from control and ‘#’ represents significantly differs at p < 0.01 from OVA-provoked animals. Group I: Control mice; Group-II: OVA-sensitized asthma mice; Group III and IV: OVA-sensitized and 10 and 20 mg/kg of tilianin treated mice, respectively; Group V: OVA-sensitized and 3 mg/kg of standard drug dexamethasone treated mice.
3.5 Effect of tilianin on the pro-inflammatory cytokines in the OVA-provoked mice
Fig. 7 demonstrates the status of inflammatory markers in both control and treated animals. The status of TNF-α, IL-6, IL-12, and TXB2 were drastically augmented in the OVA-challenged mice. Meanwhile, the tilianin treatment effectively modulated these alterations. The administration of 10 and 20 mg/kg of tilianin appreciably suppressed the TNF-α, IL-6, IL-12, and TXB2 in the OVA-triggered animals (Fig. 7). These outcomes were correlated with the outcomes of DEX (3 mg/kg) treated asthma animals.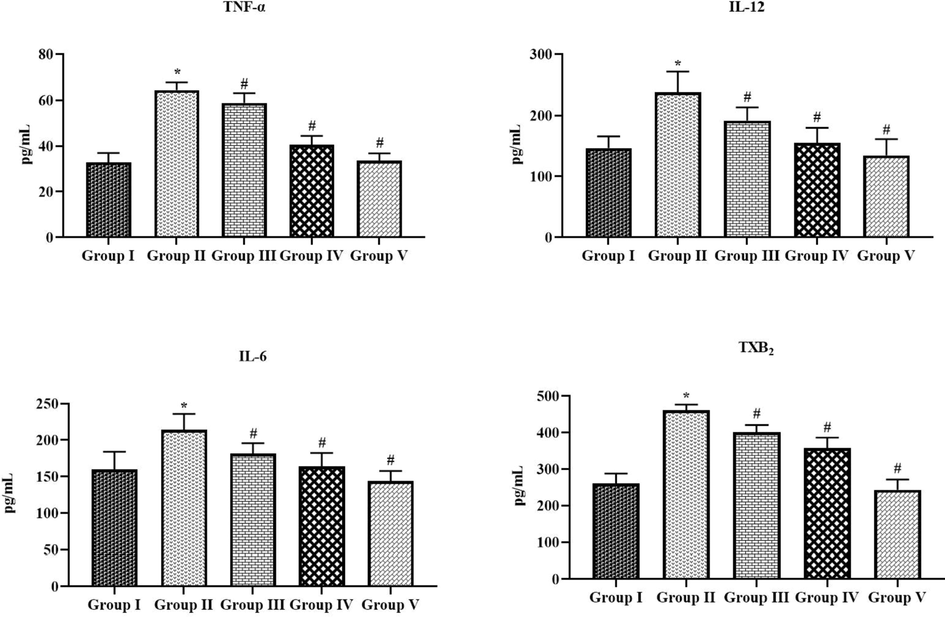
Effect of tilianin on the pro-inflammatory cytokines level in the OVA-provoked mice. The treatment with the 10 and 20 mg/kg of tilianin was appreciably reduced the TNF-α, IL-12, IL-6, and TXB2 levels in the OVA-sensitized mice. Values were given as a mean ± SD of tripicates. Data were assessed statistically by one-way ANOVA and Student’s t-test. Note: ‘*’ represents that value differs at p < 0.05 from control and ‘#’ represents significantly differs at p < 0.01 from OVA-provoked animals. Group I: Control mice; Group-II: OVA-sensitized asthma mice; Group III and IV: OVA-sensitized and 10 and 20 mg/kg of tilianin treated mice, respectively; Group V: OVA-sensitized and 3 mg/kg of standard drug dexamethasone treated mice.
3.6 Effect of tilianin on the TGF-β1/Smad pathway in the OVA-provoked mice
Fig. 8 represents the inhibitory potentials of tilianin on the mRNA expressions of TGF-β1, Smad2/3, iNOS, and COX-2 in the OVA-triggered animals. The expressions of TGF-β1, iNOS, and COX-2 were elevated in the OVA-triggered animals. Surprisingly, the tilianin appreciably regulated the expression of these genes (Fig. 8). The 10 and 20 mg//kg of tilianin appreciably down-regulated the TGF-β1, iNOS, and COX-2 expressions in the OVA-challenged animals. On the contrary the expression levels of Smad2/3 has upregulated by the tilianin treatment 20 mg/kg of tilianin and 3 mg/kg of DEX treatments demonstrated the similar results.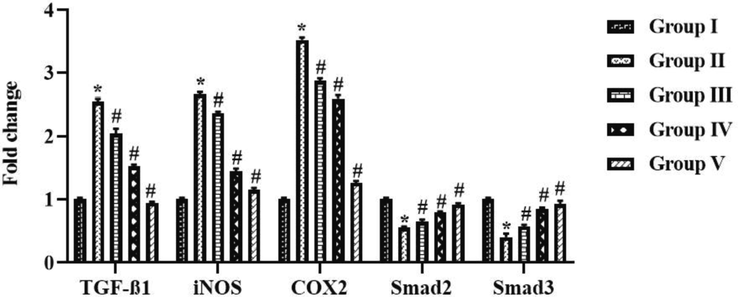
Effect of tilianin on the TGF-β1/Smad signaling pathway in the OVA-provoked mice. The 10 and 20 mg/kg of tilianin effectively regulated the expression of TGF-β1, iNOS, COX-2, Smad2, and Smad3 in the OVA-sensitized mice. Values were given as a mean ± SD of triplicates. Data were assessed statistically by one-way ANOVA and Student’s t-test. Note: ‘*’ represents that value differs at p < 0.05 from control and ‘#’ represents significantly differs at p < 0.01 from OVA-provoked animals. Group I: Control mice; Group-II: OVA-sensitized asthma mice; Group III and IV: OVA-sensitized and 10 and 20 mg/kg of tilianin treated mice, respectively; Group V: OVA-sensitized and 3 mg/kg of standard drug dexamethasone treated mice.
3.7 Effect of tilianin on the lung histopathology in the OVA-provoked mice
Fig. 9 shows the histological analysis of lung tissues of control and treated animals. The OVA-challenged animals elicited the inflammatory cell penetrations in the pulmonary tissues, which could leads to the airway epithelium and mucous membranes thickening. The administration of 10 and 20 mg/kg was substantially ameliorated the inflammatory cell penetrations, which is triggered by OVA-challenge. Tilianin treatment also normalized the airway epithelial arrangements and pulmonary tissue constructions in the OVA-challenged asthma mice (Fig. 9). The 3 mg/kg of DEX treatment also prevented the pulmonary tissues from the OVA stimulated histological alterations.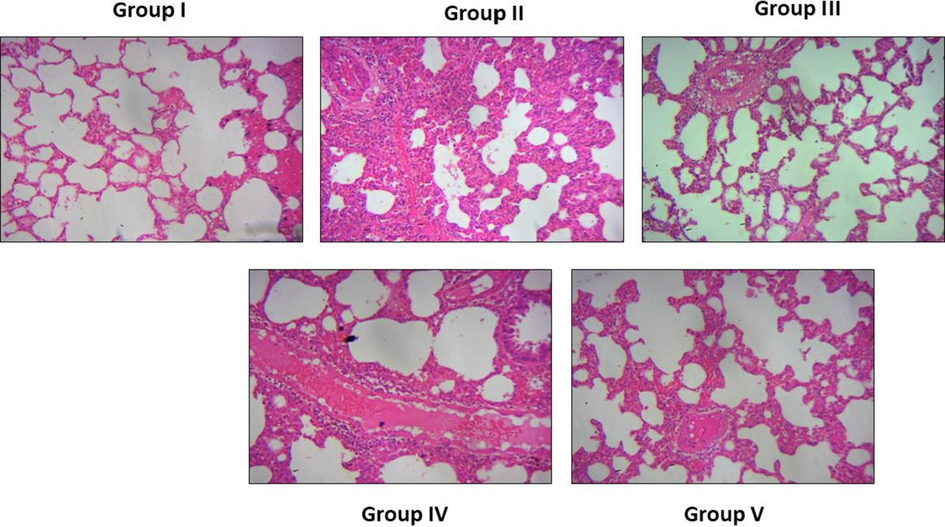
Effect of tilianin on the lung histopathology in the OVA-provoked mice. Control animals demonstrated the normal lung histology (Group I). OVA-challenged animals elicited the inflammatory cell penetrations, thickening of epithelium and mucous membranes (Group II). The 10 and 20 mg/kg of tilianin administered animals ameliorated the inflammatory cell infiltration (Group III and IV). The DEX treatment also prevented the pulmonary tissues from the OVA-stimulated changes (Group V).
4 Discussion
Allergic asthma is a inflammatory ailment with various phenotypes provoked by numerous stimuli. Asthma is defined as a condition of chronic airway inflammation. It is distinguished by AHR, hyperplasia of goblet cells, and eosinophilia as a result of exposures to allergens and other stimuli (Gong et al., 2012). The administration of steroids are the only effective option to treat the ailment and also the long-term administration of these steroidal drugs could cause various adverse for instance, mood change, muscle weakness, high blood pressure, and steroid tolerance. In recent times, the new approaches and natural herbal compounds are being explored continuously for effective asthma management as substitute for steroids with less adverse effects (Lin et al., 2019). This exploration was aimed to disclose the beneficial properties of tilianin against OVA-triggered asthma animals.
The IL-4, IL-5, and IL-13 are the vital players of the inflammation and regulating the eosinophilia in airway, over production of mucus and other inflammatory cytokines. Accordingly, the over-activation of Th2 cytokines is actively participates in the asthma progression. Hence, the inhibition of Th2 cytokines over production could be hopeful strategy to treat the allergic asthma (Woodruff et al., 2009). In response to the serious inflammatory condition of lungs, the Th1 and Th2 cell imbalance stimulates the clinical expression of asthma. The improved status of IL-4, IL-5, and IL-13 trigger the penetration of inflammatory cells into the lungs many studies (Walsh, 2017). These elevated cytokines performs a imperative function in triggering the accumulation of asthma-specific IgE, which is noted in OVA-activated asthma mice. IL-4, a major type of Th2 cytokines, enhances the B-cell multiplication, and stimulation that also improves the penetration of inflammatory cells into lung tissues.
The over stimulation of IL-4 is also participates in the IgE accumulation during asthma progression. IL-5 can control the eosinophil development, IgE accumulation in asthma. IL-6 improves the IL-4 accumulation and regulates the Th1/Th2 equilibrium to Th2, therefore improving the Th2 differentiation and suppressing the Th1 cell differentiation (Iftikhar et al., 2018). Additionally, IL-13 facilitates to the B-cell differentiation and performs a critical function in the triggering of inflammatory responses (Karo-Atar et al, 2018). The over accumulation of IL-13 could directs to the exacerbation of AHR in asthma. Numerous investigations suggested that the inhibition Th2 cytokines accumulation could be hopeful therapeutic option to treat the asthma (Ntontsi et al., 2018). The findings from the current exploration disclosed that the tilianin treatment appreciably suppressed the IL-4, IL-5, and IL-13, IFN-γ, and TNF-α, IL-6, IL-12, and TXB2 in the OVA-challenged animals. These outcomes evidenced the strong anti-inflammatory actions of tilianin against the OVA-provoked inflammatory condition in mice. The anti-inflammatory effects of flavonoid glycoside compounds were already been reported (Hamalainen et al., 2007; Yang and He, 2022). Our findings were also coincides with these earlier literatures. The discharge of numerous cytokines like IL-4, IL-5, and IL-13 was actively participates in the asthma progression (Yuk et al., 2011). The eotaxin liberated from Th2 cells also worsens the eosinophils penetration in the airway (Shi et al., 2009). Tilianin treatment also suppressed the eotaxin level in the OVA-challenged mice.
Oxidative stress is accompanied by disproportion of the production of free radicals and its removal by the antioxidants. Oxidative stress drastically deplete the ability of anti-oxidants, provoke the damaging effects to the numerous tissues. Many studies highlighted that the oxidative stress actively participates to a pathological development of asthma (Jiang et al., 2016). Oxidative stress is known to cause the oxidative damage that facilitates to the asthma development (Nesi et al., 2017). GSH and SOD are some of the essential antioxidants that has a remarkable therapeutic assets in the treatment of numerous diseases like asthma (Younus, 2018). The current findings evidenced that the MDA level was drastically improved and antioxidants SOD and GSH was depleted in the OVA-challenged asthma mice. Interestingly, the tilianin appreciably decreased the MDA and improved the GSH and SOD in the OVA-mice, which demonstrate its strong antioxidant action.
The penetration of inflammatory cells into the pulmonary tissues are the common event of OVA-challenged asthma. These excessive gathered inflammatory cells generate the inflammatory regulators like cytokines and chemokines and enhance the inflammatory responses of lungs (McGregor et al., 2019). The excessive gathering of eosinophils in the pulmonary tissues is a imperative event of pathological progression of allergic asthma (Patel and Sur, 2017). Many inflammatory cells actively participates in the allergic reactions, particularly two main cells, which accomplish this complex response are mast cells and eosinophils (Ghorani et al., 2018). Mast cells are broadly dispersed on the connective and mucosal tissues, infiltrating into the inflammatory areas related with chronic allergic ailments (Vuolo et al., 2015). We also identified that the inflammatory cell counts were appreciably supressed in the OVA-challenged animals by tilianin treatment. The histological findings also proved that the tilianin treatment effectively reduced the inflammatory cell penetrations into the pulmonary tissues.
Accumulating evidences has suggested that the TGF-β1/Smad pathway is a most critical molecular event that participates in the airway remodeling during asthma progression (Wang et al., 2019; Lee et al., 2017). TGF-β1 is a imperative molecule, which performs a essential function in the asthma (Jeon et al., 2016). The Smad proteins is a well known molecule, which is regulate the regulators for TGF-β1 pathway (Feng et al., 2019). Additionally, many investigations has highlighted that the TGF-β1 may have numerous pharmacological properties through the stimulation of down-stream regulators for instance, Smad2 and Smad3 (Chen et al., 2018; Hu et al., 2018). In this investigation, we discovered that the tilianin treatment appreciably down-regulated the TGF-β1/Smad signaling in OVA-provoked asthma animals.
5 Conclusion
Our findings demonstrated that tilianin potentially alleviated the OVA-provoked allergic asthma in mice. Tilianin effectively suppressed the Th2 cytokines, pro-inflammatory cytokines, inflammatory cell counts, TBX2, MPO, and MDA levels and improved the antioxidants level. The expressions of TGF-β1, iNOS, and COX-2 was appreciably inhibited by the tilianin treatment. Hence, it was clear that tilianin could be a talented anti-asthmatic agent in the future.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper. Liyuan Zhang and Xinpeng Han make equal contribution to this research.
References
- The biological and pharmacological roles of polyphenol flavonoid tilianin. Eur. J. Pharmacol.. 2019;842:291-297.
- [Google Scholar]
- Central role of dysregulation of TGF-β/Smad in CKD progression and potential targets of its treatment. Biomed. Pharmacother.. 2018;101:670-681.
- [Google Scholar]
- The global asthma network rationale and methods for phase I global surveillance: Prevalence, severity, management and risk factors. Eur. Respirat. J.. 2017;49(1) pii: 1601605
- [Google Scholar]
- Effect of dexamethasone on TGF-β1/Smad3 signalling pathway in airway remodelling model of asthmatic rats. J. Coll. Physicians Surg. Pak.. 2019;29:537-540.
- [Google Scholar]
- Targeting eosinophils in allergy, inflammation and beyond. Nat. Rev. Drug Discovery. 2013;12(2):117-129.
- [Google Scholar]
- Antidiabetic, antihyperlipidemic and anti-inflammatory effects of tilianin in streptozotocin-nicotinamide diabetic rats. Biomed. Pharmacother.. 2016;83:667-675.
- [Google Scholar]
- Clinical view on the importance of dendritic cells in asthma. Expert Rev. Clin. Immunol.. 2014;9(10):899-919.
- [Google Scholar]
- Global, regional, and national age-sex specific all-cause and cause-specific mortality for 240 causes of death, 1990-2013: A systematic analysis for the Global Burden of Disease Study 2013. Lancet. 2015;385(9963):117-171.
- [Google Scholar]
- The Effects of Allium Cepa Extract on Tracheal Responsiveness, Lung Inflammatory Cells and Phospholipase A2 Level in Asthmatic Rats. Iran. J. Allergy. Asthma. Immunol.. 2018;17:221-231.
- [Google Scholar]
- Kaempferol suppresses eosionphil infiltration and airway inflammation in airway epithelial cells and in mice with allergic asthma. J. Nutrit.. 2012;142(1):47-56.
- [Google Scholar]
- Cardioprotective effects of tilianin in rat myocardial ischemia-reperfusion injury. Mol. Med. Rep.. 2015;11(3):2227-2233.
- [Google Scholar]
- Role of transforming growth factor-β in airway remodeling in asthma. Am. J. Respir. Cell Mol. Biol.. 2011;44:127-133.
- [Google Scholar]
- Anti-Inflammatory Effects of Flavonoids: Genistein, Kaempferol, Quercetin, and Daidzein Inhibit STAT-1 and NF-κB Activations, Whereas Flavone, Isorhamnetin, Naringenin, and Pelargonidin Inhibit only NF-κB Activation along with Their Inhibitory Effect on iNOS Expression and NO Production in Activated Macrophages. Mediators Inflamm.. 2007;2007:45673.
- [Google Scholar]
- Origin, localization, and immunoregulatory properties of pulmonary phagocytes in allergic asthma. Front. Immunol.. 2016;7
- [Google Scholar]
- New insights into TGF-β/Smad signaling in tissue fibrosis. Chem. Biol. Interact.. 2018;292:76-83.
- [Google Scholar]
- Comparative efficacy of anti IL-4, IL-5 and IL-13 drugs for treatment of eosinophilic asthma: A network meta-analysis. Lung. 2018;196(5):517-530.
- [Google Scholar]
- Aqueous extract of Gumiganghwal–tang, a traditional herbal medicine, reduces pulmonary fibrosis by transforming growth factor-β1/Smad signaling pathway in murine model of chronic asthma. PLoS ONE. 2016;11:e0164833
- [Google Scholar]
- The protective effect of Trillin LPS-induced acute lung injury by the regulations of inflammation and oxidative state. Chem. -Bioloqical Interact.. 2016;243:127-134.
- [Google Scholar]
- Mast cell–derived plasminogen activator inhibitor type 1 promotes airway inflammation and remodeling in a murine model of asthma. J. Allergy Clin. Immunol.. 2018;142:294-297.e5.
- [Google Scholar]
- Therapeutic targeting of the interleukin-4/interleukin-13 signaling pathway: In allergy and beyond. BioDrugs: Clin. Immunotherapeut. Biopharmaceut. Gene Therapy. 2018;32(3):201-220.
- [Google Scholar]
- The many paths to asthma: phenotype shaped by innate and adaptive immunity. Nat. Immunol.. 2010;11:577-584.
- [Google Scholar]
- Current therapeutically strategies for allergic rhinitis. Expert Opin. Pharmacother.. 2019;20(1):83-89.
- [Google Scholar]
- Effect of the velvet antler of formosan sambar deer (Cervus unicolor swinhoei) on the prevention of an allergic airway response in mice. Evid. Based Complement. Altern. Med.. 2012;2012:10.
- [Google Scholar]
- Inhibitory effects of resveratrol on airway remodeling by transforming growth factor–beta/Smad signaling pathway in chronic asthma model. Allergy Asthma Immunol Res. 2017;9:25-34.
- [Google Scholar]
- Anti-asthmatic effects of Angelica dahurica against ovalbumin-induced airway inflammation via upregulation of heme oxygenase-1. Food Chem. Toxicol.. 2011;49:829-837.
- [Google Scholar]
- Advanced molecular knowledge of therapeutic drugs and natural products focusing on inflammatory cytokines in asthma. Cells. 2019;8
- [Google Scholar]
- Protocatechuic acid inhibits TGF–beta1–induced proliferation and migration of human airway smooth muscle cells. J. Pharmacol. Sci.. 2019;139:9-14.
- [Google Scholar]
- Inflammatory and oxidative stress markers in experimental allergic asthma. Inflammation. 2017;40(4):1166-1176.
- [Google Scholar]
- Targeted anti-IL-13 therapies in asthma: Current data and future perspectives. Expert Opin. Invest. Drugs. 2018;27(2):179-186.
- [Google Scholar]
- Transforming growth factor β1 function in airway remodeling and hyperresponsiveness. The missing link? Am. J. Respir. Cell Mol. Biol.. 2017;56:432-442.
- [Google Scholar]
- Tilianin attenuates HDM-induced allergic asthma by suppressing Th2-immune responses via downregulation of IRF4 in dendritic cells. Phytomed.. 2021;80:153392
- [Google Scholar]
- IgE and eosinophils as therapeutic targets in asthma. Curr. Opin. Allergy Clin. Immunol.. 2017;17(1):42-49.
- [Google Scholar]
- Naringenin inhibits allergen-induced airway inflammation and airway responsiveness and inhibits NF-κB activity in a murine model of asthma. Can. J. Physiol. Pharmacol.. 2009;87:729-735.
- [Google Scholar]
- Diallyl-disulfide, an organosulfur compound of garlic, attenuates airway inflammation via activation of the Nrf-2/HO-1 pathway and NF-kappaB suppression. Food Chem. Toxicol.. 2013;62:506-513.
- [Google Scholar]
- Evaluation of Serum Cytokines Levels and the Role of Cannabidiol Treatment in Animal Model of Asthma. Mediators Inflamm.. 2015;2015:538670
- [Google Scholar]
- Biologics targeting IL-5, IL-4 or IL-13 for the treatment of asthma-an update. Expert Rev. Clin. Immunol.. 2017;13(2):143-149.
- [Google Scholar]
- Cryptotanshinone attenuates airway remodeling by inhibiting crosstalk between tumor necrosis factor–like weak inducer of apoptosis and transforming growth factor beta 1 signaling pathways in asthma. Front. Pharmacol.. 2019;10:1338.
- [Google Scholar]
- T-helper type 2-driven inflammation defines major subphenotypes of asthma. Am. J. Respir. Crit. Care Med.. 2009;180:388-395.
- [Google Scholar]
- Anti-inflammatory effects of flavonoids and phenylethanoid glycosides from Hosta plantaginea flowers in LPS-stimulated RAW 264.7 macrophages through inhibition of the NF-κB signaling pathway. BMC Complement. Med. Ther.. 2022;22:55.
- [Google Scholar]
- Therapeutic potentials of superoxide dismutase. Int. J. Health Sci. (Qassim). 2018;12(3):88-93.
- [Google Scholar]
- Effects of astilbic acid on airway hyperresponsiveness and inflammation in a mouse model of allergic asthma. Int. Immunopharmacol.. 2011;11:266-273.
- [Google Scholar]
- Cardioprotection of tilianin ameliorates myocardial ischemia reperfusion injury: role of the apoptotic signaling pathway. PLoS One. 2018;13(3) article e0193845
- [Google Scholar]
- BMS345541 inhibits airway inflammation and epithelialmesenchymal transition in airway remodeling of asthmatic mice. Int. J. Mol. Med.. 2018;42:1998-2008.
- [Google Scholar]
- Th1/Th2/Th17 cells imbalance in patients with asthma with and without psychological symptoms. Allergy Asthma Proc.. 2016;37(2):148-156.
- [Google Scholar]
- Phytochemistry and bioactivity of aromatic and medicinal plants from the genus Agastache (Lamiaceae) Phytochem. Rev.. 2014;13:391-416.
- [Google Scholar]







