Translate this page into:
Total phenolic and flavonoid contents, antioxidant, antidiabetic and antiplasmodial activities of Garcinia forbesii King: A correlation study
⁎Corresponding author at: Taslim Ersam, Department of Chemistry, Institut Teknologi Sepuluh Nopember, Sukolilo-Surabaya 60111, Indonesia. paktichem@gmail.com (Taslim Ersam)
-
Received: ,
Accepted: ,
This article was originally published by Elsevier and was migrated to Scientific Scholar after the change of Publisher.
Peer review under responsibility of King Saud University.
Abstract
Garcinia forbesii King belongs to Clusiaceae is a source of secondary metabolites especially xanthones with various biological activities. G. forbesii King is also known for its empirical use for malaria and diabetes. This study investigated the total phenolic and flavonoid contents, in vitro antioxidant, antidiabetic and antiplasmodial activities of four extracts attained from the stem bark of G. forbesii King. The total phenolic and flavonoid contents were determined by spectrophotometric methods and antioxidant activity was evaluated by DPPH, ABTS, FRAP assays. In vitro antidiabetic activity was assessed by α-glucosidase and α-amylase assays and antiplasmodial activity was studied against chloroquine sensitive Plasmodium falciparum strain 3D7. The highest value of total phenolic (187.37 ± 0.06 mg GAE/g) and flavonoid (35.97 ± 0.02 mg QE/g) contents were recorded in n-hexane and methanolic extracts. n-Hexane extract showed the highest DPPH activity with IC50 of 8.12 ± 0.02 μg/mL. Ethyl acetate extract exhibited better scavenging ability for ABTS with IC50 of 3.88 ± 0.04 μg/mL. The FRAP assay showed better activity in methanol extract with an inhibition value of 73.68 ± 3.66 µM Fe2+/g. The strong inhibition against α-glucosidase and α-amylase were displayed by dichloromethane extract with IC50 of 35.13 ± 2.01 μg/mL and 4.83 ± 0.20 μg/mL. n-Hexane and methanol extracts showed significant antiplasmodial activity with IC50 of 0.23 ± 0.01 μg/mL and 0.73 ± 0.01 μg/mL, respectively. The correlation analysis indicated a positive relationship of total phenolic and flavonoid contents with antiplasmodial activity. The results revealed that n-hexane and methanol extracts could be used as a potential natural antiplasmodial, while dichloromethane extract is a promising natural antidiabetic.
Keywords
Garcinia forbesii King
Antidiabetic
Antioxidant
Flavonoids
Phenolics
1 Introduction
Garcinia forbesii King is a small to medium-sized perennial tree belongs to the family Clusiaceae and is native to Indonesia (Sumatera, Kalimantan) and Malaysia (Perry and Metzger, 1980; Aravind et al., 2016, Lim, 2012). G. forbesii King is known as Red Mangosteen, Forest Mangosteen, or Rose Kandis and locally referred as Kandis, Mundar (Sumatera), Bunoh, Itan, Bua’ Tetoh Telato, Aiti Kitung (Kalimantan) in Indonesia and as Kandis, Assam Roi, Babata in Malaysia (Lim, 2012; Sutomo et al., 2020). It is an evergreen tropical plant adapted to a wet, hot, humid environment mainly found in tropical rain forests (Lim, 2012). The white and sour–sweet fruit of this plant is edible, and the rind has been used as flavouring for various types of cuisines (Lim, 2012; Noor and Senin, 2017).
Phytochemical studies indicate that Garcinia species including G. forbesii King are rich of secondary metabolite especially xanthones. Xanthones are well known for their various bioactivities such as anti-inflammatory (Zhang et al., 2014), antivirus, antimalarial (Elfita et al., 2009), antidiabetic (Phukhatmuen et al., 2020), antifungal (Gopalakrishnan et al., 1997) and anticancer (Sukandar et al., 2020). Furthermore, xanthones are effective against free radicals due to the presence of hydroxy groups in its skeleton (Taher et al., 2012). Harrison and co-worker reported the isolation of a chromenoxanthone namely forbexanthone along with pyranojacareubin and 1,3,7-trihydroxy-2-(3-methylbut-2-enyl)-xanthone from the branches of G. forbesii King (Harrison et al., 1993). Forbesione, a caged xanthone, was also obtained from G. forbesii King (Leong et al., 1996). Ethnobotanically G. forbesii King has been empirically used by local people in East Part of Indonesia as a folk medicine to treat malaria and diabetes.
Though G. forbesii King shows beneficial potency in traditional medication, there is a void in the relationship of phytochemical profile and it’s in vitro biological activities. Considering these aspects, here we report a correlation study on total phenolic and flavonoid contents, antioxidant activity using DPPH, ABTS, and FRAP assays, α-glucosidase and α-amylase inhibitory and antiplasmodial activities of G. forbesii King using four solvents to verify their medication potency. This study, to the best of our knowledge, is the first report focusing on the correlation of phenolic and flavonoid contents with antioxidant, antidiabetic and antiplasmodial activities of G. forbesii King stem bark.
2 Material and methods
2.1 Plant material
The stem bark of G. forbesii King was collected from the Somaetek forest in North Halmahera, Indonesia (1°25′22 “LU 127°46′59″ E). This plant was identified and the specimen (VII.G.237a) was stored in the Bogor Botanical Gardens.
2.2 Preparation of extracts
The bark of G. forbesii King was dried at 50 °C and crushed into powder (60–100 mesh) using a milling machine. A total of 15 g of the powder was weighed and extracted by maceration (150 mL) with four solvents: n-hexane, dichloromethane, ethyl acetate and methanol. The obtained extracts were then filtered and evaporated under reduced pressure (40–60 °C) to give dry extracts.
2.3 Total phenolic and flavonoid contents
The Folin-Ciocalteu method was employed for total phenolic content determination following previously reported paper (Badrulhisham et al., 2020) with slight modifications using gallic acid as standard (0–200 mg/L). Briefly, 0.5 mL of the extracts in methanol (1000 ppm) was mixed with 2.5 mL of 10% aqueous Folin-Ciocalteu solution, stirred and left for 5 min. A 2.0 mL of 5% aqueous Na2CO3 solution was then added. The mixture was further incubated at 40 °C for one hour and the absorbance was measured at a wavelength of 765 nm by using a UV–Vis spectrophotometer (Genesys UV–Vis Spectrophotometer, Thermo Fisher Scientific, Madison, WI, USA) with methanol as the blank.
The aluminum chloride method was used for total flavonoid content (Armania et al., 2013) evaluation using quercetin as standard (0–50 mg/L) in methanol. A total of 0.5 mL of each extract (100–1000 ppm) was mixed with 0.5 mL of 2% AlCl3 (in methanol) followed by incubation for one hour and the absorbance was measured at 415 nm using UV–Vis spectrophotometer (Genesys UV–Vis Spectrophotometer, Thermo Fisher Scientific, Madison, WI, USA) with methanol as the blank.
2.4 DPPH radical scavenging assay
The DPPH antioxidant activity of extracts was determined following the reported papers (Brand-Williams et al., 1995; Dudonné et al., 2009). A 3.0 mL of 6 × 10−5 M DPPH was added to 100 µL of extracts (maximum dissolved concentration) in methanol. After 20 min of incubation at 37 °C, the absorbance of the mixture was assessed at a wavelength of 517 nm (As). The absorbance of the blank was also measured at the same wavelength (Ab). This experiment was carried out in triplo using quercetin and gallic acid as controls. The radical inhibitory activity was calculated using the equation [(Ab-As) / Ab] × 100%.
2.5 ABTS radical scavenging assay
The ABTS radical scavenging activity was evaluated based on the previous papers (Armania et al., 2013; Arteaga-Crespo et al., 2020). The ABTS solution was prepared from 5.0 mL of 7 mM ABTS and 88 mL of potassium peroxydisulfate (K2S2O6). The mixture was left to stand for 12–16 h at room temperature and a dark blue solution was formed. A 250 mL of 99.5% ethanol was then added to the solution, which provided an absorbance of 0.7 ± 0.02 at 734 nm. This was referred as working solution. A total of 1.0 mL of working solution and 10 μL of the extracts (maximum dissolved concentration) in methanol was shaken for 10 s and incubated at 30 °C for 4 min. The absorbance of the mixture (As) was measured at a wavelength of 734 nm with ethanol as a blank (Ab). Quercetin and gallic acid were used as controls. The ABTS inhibition was calculated by [(Ab-As) / Ab] x100.
2.6 Ferric reducing-antioxidant power (FRAP) assay
The iron reduction power of G. forbesii King extracts was evaluated by a method of Dudonné et al. (2009) with a slight modification. This assay was conducted based on the reduction of a colorless iron complex of Fe3+-tripyridyltriazine to a blue complex of Fe2+-tripyridyltriazine. The FRAP reagent was mixed with freshly prepared 300 mM acetate buffer, 40 mM HCl, 10 mM TPTZ and 20 mM FeCl3·6H2O at a ratio of 10:1:1 (acetate buffer:TPTZ:FeCl3·6H2O). The standard curves were made using various concentrations of FeSO4·.7H2O and all solutions were prepared in a fresh condition. About 100 μL of sample solution, 900 μL of distilled water, and 2 mL of FRAP reagent were mixed and incubated at 37 °C for 30 min and the absorbance was measured at 593 nm. The blank sample measurement was carried out using acetate buffer. The difference between the absorbance of the samples and the absorbance of the blank was calculated and utilized to obtain the FRAP value and extracts’ reduction capacity. The FRAP value was expressed as µM Fe2+/g ((FRAP Value of sample (µM)) = abs (sample) × FRAP value of std (µM) / abs (std)). Ascorbic acid was used as control and all measurements were carried out in triplicate.
2.7 Rat intestinal α-glucosidase inhibitory activity
The inhibitory activity of rat intestinal α-glucosidase was determined according to a modified method (Worawalai et al., 2012). This technique was carried out by grouping four mixtures into four different categories: (1) blank enzyme reaction, (2) enzyme reaction, (3) blank samples reaction, (4) samples reaction. The mixture of (1) contained DMSO (10 µL), sodium phosphate buffer (50 µL), glucose kit (80 µL). The enzyme reaction group (2) consisted of mixture (1) with the addition of maltose or sucrose substrates (20 µL). The positive control of acarbose was similarly prepared as (2). The group (3) was loaded with identical mixture of (1) with samples (10 µL) replacing the DMSO. The last mixture of (4) composed of (3) and substrates maltose or sucrose (20 µL). The well plates were then incubated for 10 min (maltose) or 40 min (sucrose). A microplate reader was used to measure the absorbance at 520 nm (BioTek ELx800TM, BioTek Instruments, Inc., Winooski, VT, USA).
2.8 α-Amylase inhibitory activity
The α-amylase activity was evaluated using a method modified by Unuofin et al., 2018. A 5.0 mg of α-amylase (Porcine pancreatic α-amylase) in 1.0 mL 0,1 M phosphate buffer pH 6,9 was added to a solution of 10 mg samples in 1.0 mL DMSO. The samples (20 µL) were mixed with freshly prepared potato substrate (50 µL) and 30 µL of phosphate buffer (pH 6.9). The substrate was obtained by heating potato starch (100 mg) in 5.0 mL 0.1 M phosphate buffer for 5 min and cooled at room temperature. After a 5-minute pre-incubation period at room temperature, 20 µL of α-amylase was then added. Following that, the solution was incubated at 37 °C for 15 min and a color change was noticed by adding 50 µL of 1 M HCl and 50 µL of 10% iodine solution. A UV–Vis spectrophotometer (Genesys UV–Vis Spectrophotometer, Thermo Fisher Scientific, Madison, WI, USA) was used for absorbance measurement at a wavelength of 650 nm.
2.9 Antiplasmodial activity
The antiplasmodial activity of the extracts was judged by a method of Budimulja et al. (1997) using chloroquine sensitive Plasmodium falciparum strain 3D7. Samples were made at concentrations of 1000, 100, 10, 1 and 0.1 µg/mL. The parasites used in this test were synchronous (Ring stage) with ± 1% parasitemia. A 2.0 µL of the test solution with various concentrations was pipetted to 96 wells followed by 198 µL of parasites. The well was further placed in a chamber with O2 5%, CO2 5%, and N2 90% atmosphere. The chamber was then incubated at 37 °C for 48 h and subsequently the culture was harvested, and a thin blood layer was made with 20% Giemsa staining. Data analysis was carried on the blood smear that has been made by counting the number of infected erythrocytes in every 1000 normal erythrocytes under a microscope. The data was further used to determine the percent growth and inhibition.
2.10 Statistical analysis
The statistical analysis was carried out with IBM 23 statistical software to determine total phenolic and flavonoid contents, antioxidant activities and antidiabetic activities. This study was carried out in thrice to determine the mean value (mean SD). A linear regression equation was used to determine the percentage of α-glucosidase and α-amylase inhibition of each extract's concentration. Meanwhile, probity analysis was used to determine the proportion of growth inhibition on P. falciparum.
3 RESULTS AND discussion
3.1 Total phenolics and total flavonoids contents
In this study, the total phenolic and flavonoid contents of n-hexane, dichloromethane, ethyl acetate and methanol extracts from the bark of G. forbesii King were tested. The results of total phenolic content were expressed as gallic acid equivalents using the equation of a standard curve y = 0.0042x + 0.0234, R2 = 0.9901, as shown in Fig. 1a. The n-hexane extract showed the highest phenolic content (187.37 ± 0.06 mg GAE/g dry extract). Ramirez and co-workers (2019) found the same profile of n-hexane extract with high phenolics from the epicarp and leaves of G. madruno. In contrast, methanol extract, as shown in Table 1, had the lowest phenolic contents (73.40 ± 0.11 mg GAE/g dry extract). This result indicated that phenolic compounds of G. forbesii King were accumulated in nonpolar and semipolar extracts, and less found in polar extract. This finding was supported by the isolation of five xanthones from dichloromethane extract, including lichexanthone, subelliptenone H, 12b-hydroxy-des-D-garcigerrin A, garciniaxanthone B and garcigerin A (Wairata et al., 2021). The flavonoid contents were expressed as quercetin equivalent using the equation y = 0.0337x: R2 = 0.9973, as shown in Fig. 1b. The methanol extract displayed the highest flavonoids (35.97 ± 0.02 mg EQ/g dry extract) while the n-hexane extract was the lowest (6.60 ± 0.14 mg EQ/g dry extract). It was noticed that flavonoid compounds were accumulated in polar solvents and lower in nonpolar solvents (Arteaga-Crespo et al., 2020; Barbouchi et al., 2020).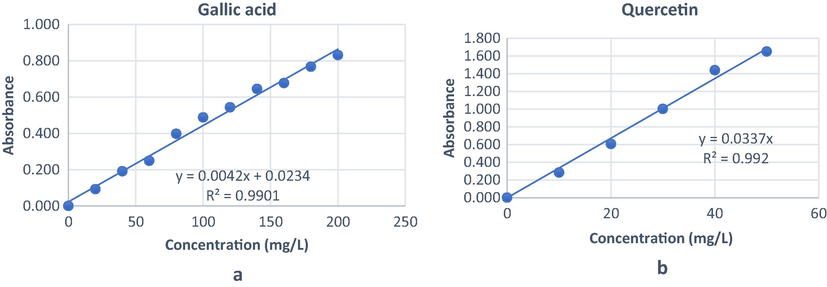
Standard curves for gallic acid (a) and quercetin (b).
G. forbesii Extracts
TPC (mg GAE/g dry extract)a
TFC (mg QE/g dry extract)b
n-hexane
187.37 ± 0.06
6.60 ± 0.14
Dichloromethane
127.84 ± 0.05
24.25 ± 0.12
Ethyl Acetate
116.65 ± 0.06
34.21 ± 0.01
Methanol
73.40 ± 0.11
35.97 ± 0.02
3.2 Antioxidant activity
3.2.1 Radical-scavenging activity (DPPH and ABTS assays)
The antioxidant assay was carried out by in vitro DPPH method. This method has been employed for the evaluation of free radical scavenging activity of several natural product extracts (Terpinc et al., 2012; Mbaoji and Nweze, 2020). Table 2 showed the free radical scavenger activity based on DPPH method at a concentration of 159.7 μg/mL with quercetin and gallic acid as controls. The n-hexane extract showed stronger DPPH radical-scavenging activity with IC50 of 8.12 ± 0.02 µg/mL. This result was positively correlated with phenolic contents of n-hexane extract. Nt = Not tested.
G. forbesii Extract
Antioxidant activity
FRAP
DPPH
ABTS
µM Fe2
% Inhibitiona
IC50 (μg/mL)
% Inhibitionb
IC50 (μg/mL)
n-hexane
24.79 ± 1.16
90.05 ± 0.02
8.12 ± 0.02
83.93 ± 0.01
11.38 ± 0.06
Dichloromethane
68.63 ± 2.01
93.95 ± 0.05
14.40 ± 0.04
90.64 ± 0.04
10.28 ± 0.03
Ethyl Acetate
69.14 ± 1.53
91.82 ± 0.08
10.80 ± 0.01
97.53 ± 0.01
3.88 ± 0.04
Methanol
73.68 ± 3.66
88.71 ± 0.05
10.80 ± 0.02
90.73 ± 0.03
3.97 ± 0.02
Ascorbic acid
30.62 ± 0.27
Nt
Nt
Nt
Nt
Quercetin (C)
Nt
96.34 ± 0.01
1.24 ± 0.01
97.58 ± 0.01
0.05 ± 0.01
Gallic acid (C)
Nt
97.21 ± 0.01
0.51 ± 0.01
96.30 ± 0.01
0.12 ± 0.01
Furthermore, the ABTS which stabilizes the free radicals through proton donors and provides lipophilic and hydrophilic compounds (Chohra et al., 2020; Aguirre-Becerra et al., 2020) was also utilized for the evaluation of free radical scavenging activity of extracts using quercetin and gallic acid as standards. The results, as shown in Table 2, indicated that the ethyl acetate extract exhibited better inhibitory activity with IC50 of 3.88 ± 0.04 µg/mL. The ABTS activity of ethyl acetate and methanol extracts was higher compared to dichloromethane and n-hexane extracts. This trend was also reported by Floegel and co-workers (2011).
3.2.2 Ferric reducing-antioxidant power (FRAP) assay
The antioxidant potential of all extracts was also evaluated by using the FRAP assay (Tai et al., 2011). The iron reduction properties are frequently used as an indicator of electron activity which is an important part of antioxidant activity (Prior et al., 2005). Table 2 displayed that the reduction power of methanol extract was the highest (73.68 ± 3.66 µM Fe2+/g) compared to other extracts and ascorbic acid as standard (30.62 ± 0.27 µM Fe2+/g). The reduction properties of all extracts indicated that increasing polarity may lead to an increase in the reduction power. The antioxidant potential of G. forbesii King extracts was verified by FRAP assay.
3.3 α-Glucosidase and α-amylase inhibitory activities
The enzyme inhibitory activity for all extracts was assessed using α-glucosidase and α-amylase. The α-glucosidase and α-amylase are key enzymes involved in carbohydrate metabolism. α-Amylase degrades complex carbohydrates into oligosaccharides and disaccharides which further degrade into monosaccharides by α-glucosidase. The liberated glucose is then absorbed by the intestine and causes postprandial hyperglycemia. The inhibition of this process reduces the postprandial glucose levels by slowing the hydrolysis and absorption of carbohydrates in the blood which is useful in the treatment of type 2 diabetes (Shinde et al., 2008).
The α-glucosidase inhibitory activity of extracts from G. forbesii King was observed using rat intestinal enzymes with sucrose and maltose substrates. α-Glucosidase breaks sucrose into glucose and fructose while maltose becomes two glucoses (Kadouh et al., 2016; Unuofin et al., 2018). Table 3 showed that the inhibition percentage and IC50 value of dichloromethane extract using sucrose substrate were 86.68 ± 0.05% and 35.13 ± 2.01 µg/mL at a concentration of 312.5 µg/mL. This value was the highest compared to other extracts. The n-hexane extract produced lowest inhibition in sucrose with 63.14 ± 0.02% of inhibition and IC50 of 60.87 ± 1.06 µg/mL. In contrast, the n-hexane extract displayed the highest activity using maltose with a percent inhibition and IC50 value of 86.32 ± 0.02% and 38.94 ± 1.45 µg/mL. The methanol extract was the least active extract in maltose with 64.14 ± 0.01% of inhibition and IC50 of 29.89 ± 1.54 µg/mL. These data indicated that nonpolar extracts were very active for α-glucosidase inhibition compared to others.
G. forbesii Extract
α-glucosidase
α-amylase
Rat intestinal
% Inhibition
Sucrose IC50 (μg/mL)
% Inhibition
Maltose IC50 (μg/mL)
% Inhibition
Starch IC50 (μg/mL)
n-hexane
63.14 ± 0.02
60.87 ± 1.06
86.32 ± 0.02
38.94 ± 1.45
69.41 ± 0.05
8.12 ± 0.50
Dichloromethane
86.68 ± 0.05
35.13 ± 2.01
94.89 ± 0.05
34.24 ± 1.60
84.27 ± 0.01
4.83 ± 0.20
Ethyl Acetate
68.26 ± 0.02
55.47 ± 1.50
95.50 ± 0.01
24.88 ± 1.27
74.62 ± 0.04
7.91 ± 0.35
Methanol
70.33 ± 0.01
54.27 ± 1.20
91.60 ± 0.01
29.89 ± 1.54
65.96 ± 0.02
16.47 ± 0.51
Acarbose
96.76 ± 0.01
2.97 ± 0.02
97.60 ± 0.01
2.21 ± 0.27
94.11 ± 0.02
2.61 ± 0.32
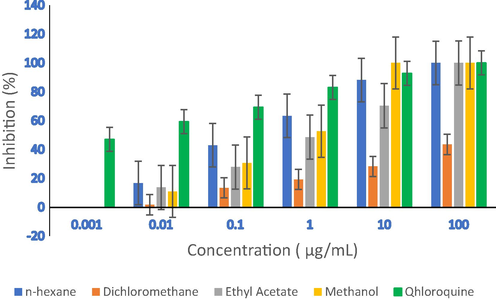
Percentage of antiplasmodial inhibition activities of G. forbesii extracts.
α-Amylase is an enzyme responsible for starch processing into glucose (Venkatachalam et al., 2020). Therefore, the inhibitory activity of the extracts from G. forbesii King was also tested against this enzyme. The dichloromethane extract showed a strong inhibition activity against α-amylase with IC50 value of 4.83 ± 0.20 μg/mL at a concentration of 99.01 μg/mL ( Table 3). The lowest activity was shown by methanol extract with IC50 of 16.47 ± 0.51 μg/mL. The standard acarbose had an IC50 value of 2.61 ± 0.32 μg/mL. The α-amylase activity evaluation confirmed the profile of nonpolar and semipolar extracts in G. forbesii King.
3.4 Antiplasmodial activity
The in vitro antiplasmodial assay of G. forbesii King stem bark extracts were performed against P. falciparum strain 3D7. The results, as presented in Table 4 and Fig. 2, revealed that n-hexane, ethyl acetate, and methanol extracts showed promising activity at a 100 μg/mL concentration compared to chloroquine standard. The methanol extract produced the highest activity at a concentration of 10 μg/mL compared to other extracts and chloroquine. Furthermore, at a concentration of 1 μg/mL, the inhibition percentage of n-hexane extract was 63.32%. The IC50 values of the n-hexane and methanol extracts were of 0.23 ± 0.01 μg/mL and 0.73 ± 0.01 μg/mL close to the chloroquine standard (IC50 0.002 ± 0.01 μg/mL). The IC50 values in the range of 10 to ≤ 10 μg/ mL were expressed as very active. Therefore, the extracts of n-hexane and methanol were active (IC50 value ≥ 10 μg/mL) (Kainama et al., 2019). The results indicated that the extracts of G. forbesii King are potential therapeutics against P. falciparum 3D7 strain (Lulan et al., 2020).
G. forbesii Extracts
IC50 (μg/mL)
n-hexane
0.23 ± 0.01
Dichloromethane
>100 ± 0.50
Ethyl Acetate
1.11 ± 0.10
Methanol
0.73 ± 0.01
Chloroquine
0.002 ± 0.01
3.5 Correlation analysis
This study correlates the total phenolic and flavonoid contents, antioxidant, antidiabetics, and antiplasmodial activities of G. forbesii Kings extracts. The relationship was calculated by using Kendall's Tau correlation. Hunter and co-workers (2021) reported the use of Kendall’s Tau calculation in the correlation of Manuka-type honeys with their bioactivities. The total phenolics negatively correlated with total flavonoids (r = -1,000, p < 0.01) means that the higher the total phenolic content, the lower the total flavonoid content. In general, total phenolics and flavonoids play an important role in the antioxidant activity acting as a chain breaker, free radical scavenger and electron donor (Kainama et al., 2020). Table 5 shows a strong positive correlation between DPPH vs total phenolics and DPPH vs total flavonoids with r = 0.718 (p < 0.005). A weak correlation was observed between ABTS vs total phenolics and ABTS vs total flavonoids with r = 0.174 (p < 0.05), but ABTS had a strong correlation with DPPH (r = 0.718, p < 0.05). The total phenolics and FRAP were negatively fierce (r = -1,000, p < 0.01) which shows an inverse relationship between phenolics and Fe3+ reduction activity. The higher the phenolic, the lower the reduction activity. In contrast, a positive correlation was occurred for total flavonoids vs FRAP (r = 1.000, p < 0.01). This indicated the flavonoid compounds contained in the extracts were directly proportional to the reduction power. Furthermore, the ABTS was strongly correlated with α-glucosidase inhibitory activity (sucrose r = 1.000, p 0.05; maltose r = 1.000, p < 0.01). In addition, the α-amylase inhibitory activity was also showed a relationship with ABTS (r = 1.000, p < 0.05). This data concluded that there is a correlation between antidiabetic and antioxidant activities. The lipophilic and hydrophobic compounds are active in ABTS and as antidiabetics (Huang et al., 2020).
TPCa
TFCb
DPPHc
ABTSc
FRAPc
Sucrosed
Maltosed
Strachd
TPC
1
TFC
−1.000**
1
DPPH
0.718*
0.718*
1
ABTS
0.174*
0.174*
0.718*
1
FRAP
−1.000**
1.000**
0.718*
0.174*
1
Sucrose
0.497*
0.497*
0.071*
1.000*
0.497*
1
Maltose
0.174*
0.174*
0.718*
1.000**
0.174*
1.000*
1
Strach
0.497*
0.497*
0.279*
1.000*
0.497*
0.497*
1.000*
1
4 Conclusion
This study reports on correlation of total phenolics and flavonoids, in vitro antioxidant, antidiabetic and antiplasmodial activities of G. forbesii King stem bark using n-hexane, dichloromethane, ethyl acetate and methanol. The use of four different solvents was purposedly for designing the in vitro assays which yielded a various class of phytochemicals from nonpolar, semipolar, and polar. The use of organic solvents in the in vitro assay is acceptable due to their volatility and they have been completely removed from the extract. A comprehensive study revealed that G. forbesii King is a promising resource of potential bioactive metabolites for medicinal and pharmaceutical purposes. The n-hexane and methanolic extracts of G. forbesii King stem bark recorded with higher content of phenolics and flavonoids. These compounds possess strong positive correlation with in vitro antioxidant results. A correlation was also noted between antidiabetic and antioxidant activities. This study noticed that the nonpolar extract correlated with antidiabetic activity while the polar extract showed a relationship with antiplasmodial activity. Investigations are needed to understand the mechanism of action of purified compounds composed the extracts based on the results of this study.
Acknowledgments
This study financially supported by the Ministry of Research, Technology and Higher Education, Indonesia (contract number 3/E1/KP.PTNBH/2021). The authors would like to thank Directorate General of Higher Education Ministry of Education, Culture, Research, and Technology of The Republic of Indonesia for providing Doctoral Scholarship Fund (JW).
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
References
- Jacaranda flower (Jacaranda mimosifolia) as an alternative for antioxidant and antimicrobial use. Heliyon. 2020;6:e05802
- [CrossRef] [Google Scholar]
- Aravind A.P.A, Lekshmi N. Menon and Rameshkumar, K.B., 2016. Phytochemistry and Phytopharmacology Division Jawaharlal Nehru Tropical Botanic Garden and Research Institute Palode, Thiruvananthapuram-695562, Kerala, India. https://doi.org/10.25173/978-81-924674-5-0.
- Dillenia suffruticosa exhibited antioxidant and cytotoxic activity through induction of apoptosis and G2/M cell cycle arrest. J. Ethnopharmacol.. 2013;146:525-535.
- [CrossRef] [Google Scholar]
- Optimisation of ultrasound-assisted extraction of phenolic antioxidants from Ilex guayusa Loes. leaves using response surface methodology. Heliyon. 2020;6:e03043
- [CrossRef] [Google Scholar]
- Harvested locations influence the total phenolic content, antioxidant levels, cytotoxic, and anti-inflammatory activities of stingless bee honey. J. Asia-Pac. Entomol.. 2020;23:950-956.
- [CrossRef] [Google Scholar]
- A comparative study on phytochemical screening, quantification of phenolic contents and antioxidant properties of different solvent extracts from various parts of Pistacia lentiscus L. J. King Saud Univ. - Sci.. 2020;32:302-306.
- [CrossRef] [Google Scholar]
- Use of a free radical method to evaluate antioxidant activity. LWT - Food Sci. Technol.. 1995;28:25-30.
- [CrossRef] [Google Scholar]
- The sensitivity of Plasmodium protein synthesis to prokaryotic ribosomal inhibitors. Mol. Biochem. Parasitol.. 1997;84:137-141.
- [CrossRef] [Google Scholar]
- Phenolic profiles, antioxidant activities and enzyme inhibitory effects of an Algerian medicinal plant (Clematis cirrhosa L.) South Afr. J. Bot.. 2020;132:164-170.
- [CrossRef] [Google Scholar]
- Comparative Study of Antioxidant Properties and Total Phenolic Content of 30 Plant Extracts of Industrial Interest Using DPPH, ABTS, FRAP, SOD, and ORAC Assays. J. Agric. Food Chem.. 2009;57:1768-1774.
- [CrossRef] [Google Scholar]
- Antiplasmodial and other constituents from four Indonesian Garcinia spp. Phytochemistry. 2009;70:907-912.
- [CrossRef] [Google Scholar]
- Comparison of ABTS/DPPH assays to measure antioxidant capacity in popular antioxidant-rich US foods. J. Food Compos. Anal.. 2011;24:1043-1048.
- [CrossRef] [Google Scholar]
- Evaluation of the Antifungal Activity of Natural Xanthones from Garcinia mangostana and Their Synthetic Derivatives. J. Nat. Prod.. 1997;60:519-524.
- [CrossRef] [Google Scholar]
- Antioxidant and pancreatic lipase inhibitory effects of flavonoids from different citrus peel extracts: An in vitro study. Food Chem.. 2020;326:126785
- [CrossRef] [Google Scholar]
- The bioactive, antioxidant, antibacterial, and physicochemical properties of a range of commercially available Australian honeys. Curr. Res. Food Sci.. 2021;4:532-542.
- [CrossRef] [Google Scholar]
- α-Glucosidase inhibiting activity and bioactive compounds of six red wine grape pomace extracts. J. Funct. Foods. 2016;26:577-584.
- [CrossRef] [Google Scholar]
- In vitro and In vivo Antiplasmodial of Stem Bark Extract of Garcinia husor. HAYATI J. Biosci.. 2019;26:9.
- [Google Scholar]
- The Relationship of Free Radical Scavenging and Total Phenolic and Flavonoid Contents of Garcinia lasoar PAM. Pharm. Chem. J.. 2020;53:1151-1157.
- [CrossRef] [Google Scholar]
- Forbesione, a modified xanthone from Garcinia forbesii. J. Chem. Res.- Part S. 1996;8:392-393.
- [Google Scholar]
- Edible Medicinal And Non-Medicinal Plants. Springer, Netherlands, Dordrecht. 2012
- [CrossRef] [Google Scholar]
- α-VINIFERIN as a potential antidiabetic and antiplasmodial extracted from Dipterocarpus littoralis. Heliyon. 2020;6:e04102
- [CrossRef] [Google Scholar]
- Antioxidant and hepatoprotective potentials of active fractions of Lannea barteri Oliv. (Anarcadiaceae) in rats. Heliyon. 2020;6:e04099
- [CrossRef] [Google Scholar]
- Noor, O.D.A., Senin, T.D., 2017. Mundar (Garcinia forbesii) Si Manggis Merah Sumber Daya Genetik Kalimantan Selatan 10.
- Medicinal Plants of East & Southeast Asia. Attributed Properties & Uses. London: The MIT Press; 1980.
- Bioassay-guided isolation and identification of antidiabetic compounds from Garcinia cowa leaf extract. Heliyon. 2020;6:e03625
- [CrossRef] [Google Scholar]
- Standardized Methods for the Determination of Antioxidant Capacity and Phenolics in Foods and Dietary Supplements. J. Agric. Food Chem.. 2005;53:4290-4302.
- [CrossRef] [Google Scholar]
- Chemical constituents and antioxidant activity of Garcinia madruno (Kunth) Hammel. J. King Saud Univ. – Sci.. 2019;31:1283-1289.
- [CrossRef] [Google Scholar]
- α-Glucosidase inhibitory activity of Syzygium cumini (Linn.) Skeels seed kernel in vitro and in Goto-Kakizaki (GK) rats. Carbohydr. Res.. 2008;343:1278-1281.
- [CrossRef] [Google Scholar]
- Picrorhizones A-H, Polyprenylated Benzoylphloroglucinols from the Stem Bark of Garcinia picrorhiza. J. Nat. Prod.. 2020;83:2102-2111.
- [CrossRef] [Google Scholar]
- Pharmacognostic Study and Antioxidant Activity of Mundar (Garcinia forbesii King.) leaves from Banua Botanical Gardens of South Kalimantan. Borneo J. Pharm.. 2020;3:209-215.
- [CrossRef] [Google Scholar]
- Apoptosis, antimicrobial and antioxidant activities of phytochemicals from Garcinia malaccensis Hk.f. Asian Pac. J. Trop. Med.. 2012;5:136-141.
- [CrossRef] [Google Scholar]
- Antioxidant activity and chemical constituents of edible flower of Sophora viciifolia. Food Chem.. 2011;126:1648-1654.
- [CrossRef] [Google Scholar]
- Studies of the correlation between antioxidant properties and the total phenolic content of different oil cake extracts. Ind. Crops Prod.. 2012;39:210-217.
- [CrossRef] [Google Scholar]
- In vitro α-amylase, α-glucosidase, lipase inhibitory and cytotoxic activities of tuber extracts of Kedrostis africana (L.) Cogn. Heliyon.. 2018;4:e00810
- [CrossRef] [Google Scholar]
- Various solvent effects on phytochemical constituent profiles, analysis of antioxidant and antidiabetic activities of Hopea parviflora. Process Biochem.. 2020;89:227-232.
- [CrossRef] [Google Scholar]
- Evaluation of the Antioxidant, Antidiabetic, and Antiplasmodial Activities of Xanthones Isolated from Garcinia forbesii and Their In Silico Studies. Biomedicines. 2021;9:1380.
- [CrossRef] [Google Scholar]
- Concise synthesis of (+)-conduritol F and inositol analogues from naturally available (+)-proto-quercitol and their glucosidase inhibitory activity. Bioorg. Med. Chem. Lett.. 2012;22:1538-1540.
- [CrossRef] [Google Scholar]
- Cytotoxic and Anti-Inflammatory Prenylated Benzoylphloroglucinols and Xanthones from the Twigs of Garcinia esculenta. J. Nat. Prod.. 2014;77:1700-1707.
- [CrossRef] [Google Scholar]







