Translate this page into:
Triton X-100 catalyzed synthesis of α-aminophosphonates
⁎Corresponding author. Tel.: +91 9849694958; fax: +91 877 2289555. csrsvu@gmail.com (Cirandur Suresh Reddy)
-
Received: ,
Accepted: ,
This article was originally published by Elsevier and was migrated to Scientific Scholar after the change of Publisher.
Peer review under responsibility of King Saud University.
Abstract
Synthesis of α-aminophosphonates by a three-component condensation of an aldehyde, amines and dialkyl phosphites in the presence of a non-ionic surfactant Triton X-100 catalyst at 70 °C in aqueous medium is accomplished. The advantages are high yield, mild reaction conditions, simple work-up and eco-friendliness. All the newly-synthesized compounds (4a–j) exhibited moderate in vitro antibacterial and antifungal activities.
Keywords
α-Aminophosphonates
Triton X-100
Non-ionic surfactant catalyst
Dialkylphosphites
1 Introduction
The synthesis of α-amino phosphonates has attracted much attention recently due to their structural analogy to α-amino acids (Naydenova et al., 2010) and significant biological activities. They act as peptide mimics (Fields, 1999), enzyme inhibitors (Allen et al., 1989; Giannousis and Bartlett, 1987), antibiotics and pharmacological agents (Atherton et al., 1986). As a result different methods have been developed for the synthesis of α-amino phosphonates (Romanenko and Kukhar, 2006; Ordonez et al., 2009; Thirumurugan et al., 2010). Among them, the Kabachnik–Fields reaction appears to be still one of the simplest and most direct approaches (Tillu et al., 2011; Chandrasekhar et al., 2001). This reaction proceeds via an imine formed by the reaction of carbonyl compounds and amines, where it is converted to the corresponding aminophosphonates by phosphite addition. This one-pot reaction can be promoted by acid or base catalysts, microwave irradiation or by heating (Ranu and Hajra, 2002). Several acid catalysts, such as Lewis acids, examples are BiCl3 (Zhan and Li, 2005), FeCl3 (Rezaei et al., 2009), YbCl3 (Xu et al., 2006), Al(OTf3) (Sobhani and Tashrifi, 2009), CAN (Kasthuraiah et al., 2007), SbCl3/Al2O3 (Ambica et al., 2008) and Brønsted acids, examples are sulfamic acid (Mitragotri et al., 2008), oxalic acid (Vahdat et al., 2008), heteropoly acids (Heydari et al., 2007), solid acids montmorillonite KSF (Yadav et al., 2001), silica sulfuric acid (Yang et al., 2009), and Amberlite-IR 120 (Bhattacharya and Rana, 2008) and base catalysts, such as CaCl2 (Kaboudin and Zahedi, 2008) and PPh3 (Tian et al., 2009), as well as other catalysts, such as ZnO (Kassaee et al., 2009), TiO2 (Hosseini-Sarvari, 2008), tosylchloride (Kaboudin and Jafari, 2008), phenyltrimethylammoniumchloride (Heydari and Arefi, 2007), (bromodimethyl) sulfoniumbromide (Kudrimoti and Rao Bommena, 2005), tetramethyl-tetra-3,4-pyridinoporphyrazinato copper(II) methyl sulfate [Cu(3,4-tmtppa)(MeSO4)4] (Sobhani et al., 2008), tetra-tert-butylphthalocyanine (Matveeva et al., 2003), β-cyclodextrine (β-CD) (Kaboudin and Sorbiun, 2007) and NBS (Wu et al., 2006), have been used to promote this reaction. However, all the reported methods have drawbacks, such as the long reaction time, unsatisfactory yields, difficult operations and environmental pollution caused by toxic reagents and organic solvents. Owing to the importance of α-aminophosphonates from pharmaceutical, industrial and synthetic points of view, there is a great demand for the development of more convenient, practical and efficient method for their synthesis.
In continuation of our work on the synthesis of various biologically important compounds, we report here a highly efficient procedure for the preparation of α-aminophosphonate and its derivatives via one-pot three component Kabachnik–Fields reaction using non-ionic surfactant catalyst Triton X-100 (5 mol%) in aqueous media. Even though Lewis and Bronsted acid surfactant catalyzed reactions are reported for them, there are very few reports involving non-ionic surfactants as catalyst (Bhattacharya et al., 2003). The non-ionic surfactant Triton X-100 (TR) is one of the most commonly used detergents in biochemistry as solubilizer with a wide range of applications to biological systems (Jones, 1999). Solubilization of lipid membranes triggered by Triton X-100 is a well-described phenomenon. It is also used as an emulsifier, and complexing agent in both aqueous and non-aqueous media.
In this communication, we report three component condensation of piperanal, amine/substituted amines and diethylphosphite/dimethylphosphite in water in the presence of Triton X-100 (5 mol%). This reaction led to the formation of diethyl/dimethyl benzo[d][1,3]dioxol-5-yl (phenylamino)methylphosphonate derivatives in good yields (Scheme 1).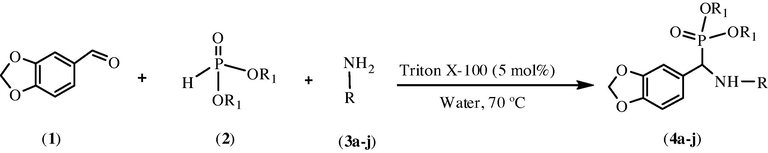
Synthesis of α-aminophosphonates catalyzed by Triton X-100.
2 Experimental
2.1 General
Melting points were recorded on Buchi R-535 apparatus and are uncorrected. IR spectra were recorded on a Perkin–Elmer 683 spectrophotometer using KBr optics. 1H, 13C and 31P NMR spectra were recorded on Bruker avance 500 MHz NMR spectrometer operating at 500 MHz for 1H NMR, 125 MHz for 13C and 202 MHz for 31P NMR. NMR data recorded in CDCl3 were referenced to TMS (1H and 13C) and 85% H3PO4 (31P). Mass spectra were recorded on a JEOL GCMATE II GC–MS spectrometer at SAIF, IIT, Chennai. Elemental analyses were performed using a Perkin–Elmer 2400 instrument at the Central Drug Research Institute (CDRI), Lucknow, India. All chemicals were purchased from Sigma–Aldrich and were used with out further purification. Double distilled water was used as solvent.
2.2 Chemistry
2.2.1 General procedure for the synthesis of diethyl/dimethyl benzo[d][1,3]dioxol-5-yl(phenylamino) methylphosphonate derivatives (4a–j)
In a typical experiment piperanal (1.0 mmol), the respective aniline (1.0 mmol) and the respective phosphite (1.0 mmol) were taken in a mixture of Triton X-100 (5 mol%) and water (2 mL) in a round bottomed flask. The resulting mixture was vigorously stirred at 70 °C until completion of the reaction as monitored by thin-layer chromatography (TLC). After completion of reaction the mixture was extracted with ethyl acetate, the aqueous phase was back extracted with ethyl acetate (3 × 15 mL). The combined organic layers were dried over anhydrous Na2SO4, filtered. The filtrate was evaporated under reduced pressure. The resulting product was purified by column chromatography on silica gel (60–120 mesh, ethylacetate/hexane, 1:2) to afford pure products. Structures of the all the products were confirmed by analytical and spectral data.
2.2.1.1 Diethyl benzo[d][1,3]dioxol-5-yl(phenylamino)methylphosphonate (4a)
Solid, yield 82%, mp 128–129 °C; IR(KBr): νmax = 3230 (N–H), 1232 (P⚌O), 1015 (P–O–C), 750 (P–C) cm−1; 1H NMR (500 MHz, CDCl3): δ = 7.34–6.50 (m, 8Ar-H), 6.92 (s, 1H, NH), 5.80 (s, 2H, OCH2O), 4.59 (d, J = 25.0 Hz, 1H, P–CH), 4.15–3.92 (m, 4H, 2 × P(O)CH2), 1.31–1.26 (t, J = 7 Hz, 6H, 2 × P(O)CH2CH3); 13C NMR (125 MHz, CDCl3): 148.2 (C-5), 147.2 (C-11), 145.2 (C-9), 129.3 (C-3), 126.5 (C-31 & C-51), 114.6 (C-21 & C-61), 126.5 (C-31 & C-51), 124.2 (C-41), 121.3 (C-11), 115.5 (C-10), 110.2 (C-4), 101.2 (C-7), 63.4 (d, J = 7.0 Hz, P(O)CH2), 63.3 (d, J = 6.6 Hz, P(O)CH2), 55.7 (d, J = 152.4 Hz, C-2), 16.4 (d, J = 5.9 Hz, P(O)CH2CH3), 16.2 (d, J = 5.5 Hz, P(O)CH2CH3); 31P NMR(CDCl3): δ = 22.20; GC–MS m/z (%): 363 (M•, 100). Anal. Calc. for C18H22NO5P: C,59.50; H, 6.10; N, 3.85. Found: C, 59.51; H, 6.09; N, 3.83.
2.2.1.2 Diethylbenzo[d][1,3]dioxol-5-yl(4-chlorophenylamino)methylphosphonate (4b)
Solid, yield 84%, mp 115–116 °C; IR(KBr): νmax = 3292 (N–H), 1234 (P⚌O), 1015 (P–O–C), 752 (P–C) cm−1; 1H NMR (500 MHz, CDCl3): δ = 7.05–6.50 (m, 7Ar-H), 6.76 (s, 1H, NH), 5.83 (s, 2H, OCH2O), 4.59 (d, J = 25.0 Hz, 1H, P–CH), 4.17–3.94 (m, 4H, 2 × P(O)CH2), 1.31–1.28 (t, J = 7 Hz, 6H, 2 × P(O)CH2CH3); 13C NMR(125 MHz, CDCl3): δ = 148.0 (C-5), 145.3 (C-9), 145.2 (C-11), 131.8 (C-21 & C-61), 129.1 (C-3), 126.5 (C-31 & C-51), 124.2 (C-41), 121.3 (C-11), 115.5 (C-10), 110.2 (C-4), 101.2 (C-7), 63.4 (d, J = 7.0 Hz, P(O)CH2), 63.3 (d, J = 7.0 Hz, P(O)CH2), 55.8 (d, J = 152.4 Hz, C-2), 16.4 (d, J = 5.6 Hz, P(O)CH2CH3), 16.2 (d, J = 5.7 Hz, P(O)CH2CH3); 31P NMR(CDCl3): δ = 22.35; GC–MS m/z (%): 397 (M•, 100). Anal. Calc. for C18H21ClNO5P: C, 54.35; H, 5.32; N, 3.52. Found: C, 54.32; H, 5.30; N, 3.50.
2.2.1.3 Diethyl benzo[d][1,3]dioxol-5-yl(4-bromophenylamino)methylphosphonate (4c)
Solid, yield 83%, mp 135–136 °C; IR(KBr): νmax = 3296 (N–H), 1235 (P⚌O), 1017 (P–O–C), 751 (P–C) cm−1; 1H NMR(500 MHz, CDCl3): δ = 7.19–6.46 (m, 7Ar-H), 6.12 (s, 1H, NH), 5.93 (s, 2H, OCH2O), 4.59 (d, J = 24.0 Hz, 1H, P–CH), 4.16–3.94 (m, 4H, 2 × P(O)CH2), 1.31–1.15 (t, J = 7 Hz, 6H, 2 × P(O)CH2CH3); 13C NMR(125 MHz, CDCl3): δ = 148.2 (C-11), 148.0 (C-5), 147.4 (C-9), 144.7 (C-31 & C-51), 129.2 (C-3), 123.1 (C-21 & C-61), 121.3 (C-10), 121.2 (C-11), 115.3 (C-4), 115.0 (C-41), 101.2 (C-7), 63.4 (d, J = 6.9 Hz, P(O)CH2), 63.2 (d, J = 7.0 Hz, P(O)CH2), 56.4 (d, J = 150.0 Hz, C-2), 16.3 (d, J = 5.7 Hz, P(O)CH2CH3), 16.2 (d, J = 5.4 Hz, P(O)CH2CH3); 31P NMR(CDCl3): δ = 22.25; GC–MS m/z (%): 442 (M•, 100). Anal. Calc. for C18H21BrNO5P: C, 48.89; H, 4.79; N, 3.17. Found: C, 48.85; H, 4.76; N, 3.15.
2.2.1.4 Diethylbenzo[d][1,3]dioxol-5-yl(3-chloro-4-fluorophenylamino) methylphosphonate (4d)
Solid, yield 79%, mp 130–132 °C; IR(KBr): νmax = 3319 (N–H), 1232 (P⚌O), 1023 (P–O–C), 754 (P–C)cm−1; 1H NMR(500 MHz, CDCl3): δ = 7.20–6.95 (m, 6Ar-H), 6.56 (s, 1H, NH), 5.82 (s, 2H, OCH2O), 4.60 (d, J = 25.0 Hz, 1H, P–CH), 4.16–3.89 (m, 4H, 2 × P(O)CH2), 1.31–1.19 (t, J = 7 Hz, 6H, 2 × P(O)CH2CH3); 13C NMR(125 MHz, CDCl3): 147.5 (C-5), 145.6 (C-11), 144.9 (C-9), 128.3 (C-3), 124.2 (C-41), 120.4 (C-11), 119.8 (C-31), 116.0 (C-61), 114.9 (C-21), 113.9 (C-51), 112.8 (C-10), 112.6 (C-4), 101.5 (C-7), 63.2 (d, J = 6.8 Hz, P(O)CH2), 63.1 (d, J = 7.0 Hz, P(O)CH2), 60.5 (d, J = 151.2 Hz, C-2), 16.4 (d, J = 5.5 Hz, P(O)CH2CH3), 16.2 (d, J = 5.7 Hz, P(O)CH2CH3); 31P NMR(CDCl3): δ = 22.32; GC–MS m/z (%): 415 (M•, 100). Anal. Calc. for C18H21ClFNO5P: C, 52.00; H, 4.85; N, 3.37. Found: C, 51.09; H, 4.84; N, 3.35.
2.2.1.5 Diethyl benzo[d][1,3]dioxol-5-yl(4-methoxyphenylamino)methylphosphonate (4e)
Solid, yield 86%, mp 126–127 °C; IR(KBr): νmax = 3320 (N–H), 1238 (P⚌O), 1017 (P–O–C), 753 (P–C) cm−1; 1H NMR(500 MHz, CDCl3): δ = 7.20–6.58 (m, 7Ar-H), 6.64 (s, 1H, NH), 5.80 (s, 2H, OCH2O), 4.58 (d, J = 25.2 Hz, 1H, P–CH), 4.15–3.84 (m, 4H, 2 × P(O)CH2), 2.54 (s, 3H, OCH3), 1.30–1.26 (t, J = 7 Hz, 6H, 2 × P(O)CH2CH3); 13C NMR(125 MHz, CDCl3): 150.6 (C-41), 148.9 (C-5), 146.8 (C-9), 140.1 (C-11),129.7 (C-3), 120.5 (C-11), 117.2 (C-31 & C-51), 115.6 (C-21 & C-61), 112.8 (C-10), 112.5 (C-4), 101.4 (C-7), 64.5 (d, J = 7.0 Hz, P(O)CH2), 64.3 (d, J = 7.0 Hz, P(O)CH2), 60.5 (d, J = 152.0 Hz, C-2), 56.8 (Ar-OCH3), 16.3 (d, J = 5.5 Hz, P(O)CH2CH3), 16.2 (d, J = 5.4 Hz, P(O)CH2CH3); 31P NMR(CDCl3): δ = 22.30; GC–MS m/z (%): 381 (M•, 100). Anal. Calc. for C19H24NO6P: C, 58.01; H, 6.15; N, 3.56. Found: C, 58.00; H, 6.13; N, 3.5.
2.2.1.6 Dimethyl benzo[d][1,3]dioxol-5-yl(phenylamino)methylphosphonate (4f)
Solid, yield 82%, mp 142–143 °C; IR(KBr): νmax = 3290 (N–H), 1236 (P⚌O), 1067 (P–O–C), 750 (P–C) cm−1; 1H NMR (500 MHz, CDCl3): δ = 7.19–6.50 (m, 8Ar-H), 6.54 (s, 1H, NH), 5.82 (s, 2H, OCH2O), 4.59 (d, J = 24.9 Hz, 1H, P–CH), 4.15 (d, J = 9.2 Hz, 3H, P(O)CH3), 4.12 (d, J = 9.0 Hz, 3H, P(O)CH3); 13C NMR(125 MHz, CDCl3): δ = 148.5 (C-5), 146.9 (C-11), 145.1 (C-9), 129.2 (C-3), 128.4 (C-31 & C-51), 120.5 (C-41), 120.0 (C-11), 112.8 (C-10), 112.4 (C-21 & C-61), 112.2 (C-4), 102.3 (C-7), 64.3 (d, J = 152.4 Hz,C-2), 53.3 (d, J = 5.6 Hz, P(O)CH3), 53.2 (d, J = 5.5 Hz, P(O)CH3); 31P NMR(CDCl3): δ = 22.24; GC–MS m/z (%): 335 (M•, 100). Anal. Calc. for C16H18NO5P: C, 57.31; H, 5.41; N, 4.18. Found: C, 57.29; H, 5.40; N, 4.17.
2.2.1.7 Dimethyl benzo[d][1,3]dioxol-5-yl(4-chlorophenylamino)methylphosphonate (4g)
Solid, yield 78%, mp 126–127 °C; IR(KBR): νmax = 3340 (N–H), 1234 (P⚌O), 1020 (P–O–C), 754 (P–C) cm−1; 1H NMR (500 MHz, CDCl3): δ = 7.61–6.40 (m, 7Ar-H), 6.42 (s, 1H, NH), 5.79 (s, 2H, OCH2O), 4.54 (d, J = 24.8 Hz, 1H, P–CH), 4.05 (d, J = 9.8 Hz, 3H, P(O)CH3), 3.08 (d, J = 9.2 Hz, 3H, P(O)CH3), 13C NMR(125 MHz, CDCl3): δ = 148.2 (C-5), 145.9 (C-11), 145.5 (C-9), 128.5 (C-3), 128.9 (C-31 & C-51), 124.5 (C-41), 120.5 (C-11), 112.6 (C-10), 115.5 (C-21 & C-61), 110.2 (C-4), 101.2 (C-7), 65.5 (d, J = 152.2 Hz, C-2), 52.4 (d, J = 6.8 Hz, P(O)CH3), 52.3 (d, J = 6.2 Hz, P(O)CH3); 31P NMR(CDCl3): δ = 22.22; GC–MS m/z (%): 369 (M•, 100). Anal. Calc. for C16H17ClNO5P: C, 51.98; H, 4.63; N, 3.79. Found: C, 51.96; H, 4.61; N, 3.78.
2.2.1.8 Dimethyl benzo[d][1,3]dioxol-5-yl(4-bromophenylamino)methylphosphonate (4h)
Solid, yield 76%, mp 132–133 °C; IR(KBr): νmax = 3294 (N–H), 1236 (P⚌O), 1024 (P–O–C), 758 (P–C) cm−1; 1H NMR (500 MHz, CDCl3): δ = 7.57–6.39 (m, 7Ar-H), 5.80 (s, 2H, OCH2O), 4.50 (d, J = 25.8 Hz, 1H, P–CH), 4.20 (d, J = 10.2 Hz, 3H, P(O)CH3), 4.10 (d, J = 9.8 Hz, 3H, P(O)CH3); 13C NMR(125 MHz, CDCl3): δ = 148.4 (C-5), 145.8 (C-9), 145.5 (C-11), 132.2 (C-31 & C-51), 128.4 (C-3), 120.5 (C-11), 114.9 (C-41), 113.8 (C-21 & C-61), 113.0 (C-4), 111.9 (C-10), 102.4 (C-7), 65.3 (d, J = 150.8 Hz, C-2), 53.4 (d, J = 7.2 Hz, P(O)CH3), 53.2 (d, J = 6.8 Hz, P(O)CH3); 31P NMR(CDCl3): δ = 22.29; GC–MS m/z (%): 414 (M+, 100). Anal. Calc. for C16H17BrNO5P: C, 46.40; H, 4.14; N, 3.38. Found: C, 46.39; H, 4.12; N, 3.36.
2.2.1.9 Dimethylbenzo[d][1,3]dioxol-5-yl(3-chloro-4-fluorophenylamino) methylphosphonate (4i)
Solid, yield 76%, mp 122–123 °C; IR(KBr): νmax = 3324 (N–H), 1240 (P⚌O), 1020 (P–O–C), 758 (P–C) cm−1; 1H NMR(500 MHz, CDCl3): δ = 7.81–6.20 (m, 6Ar-H), 6.18 (s, 1H, NH), 5.84 (s, 2H, OCH2O), 4.58 (d, J = 25.5 Hz, 1H, P–CH), 4.15 (d, J = 9.2 Hz, 3H, P(O)CH3), 4.10 (d, J = 9.0 Hz, 3H, P(O)CH3); 13C NMR(125 MHz, CDCl3): δ = 148.3 (C-5), 145.9 (C-9), 145.2 (C-41), 143.9 (C-11), 130.1 (C-3), 121.2 (C-31), 120.3 (C-11), 115.0 (C-61), 114.5 (C-21), 113.9 (C-51), 112.5 (C-10), 111.9 (C-4), 101.3 (C-7), 68.2 (d, J = 152.0 Hz, C-2), 54.3 (d, J = 6.0 Hz, P(O)CH3), 54.2 (d, J = 6.4 Hz, P(O)CH3); 31P NMR(CDCl3): δ = 22.19; GC–MS m/z (%): 387 (M•, 100). Anal. Calc. for C16H16ClFNO5P: C, 49.56; H, 4.16; N, 3.61. Found: C, 49.54; H, 4.14; N, 3.60.
2.2.1.10 Dimethylbenzo[d][1,3]dioxol-5-yl(4-methoxyphenylamino)methylphosphonate (4j)
Solid, yield 80%, mp 138–139 °C; IR(KBr): νmax = 3321 (N–H), 1248 (P⚌O), 1028 (P–O–C), 753 (P–C)cm−1; 1H NMR(500 MHz, CDCl3): δ = 7.56–6.38 (m, 7Ar-H), 6.14 (s, 1H, NH), 5.80 (s, 2H, OCH2O), 4.60 (d, J = 25.5 Hz, 1H, P–CH), 4.06 (d, J = 10.3 Hz, 3H, P(O)CH3), 3.93 (d, J = 9.1 Hz, 3H, P(O)CH3), 2.46 (s, 1H, OCH3); 13C NMR(125 MHz, CDCl3): δ = 150.5 (C-41), 148.2 (C-5), 145.9 (C-9), 135.5 (C-11), 128.5 (C-3), 120.1 (C-11), 115.0 (C-31 & C-51), 114.9 (C-21 & C-61), 112.9 (C-4), 112.0 (C-10), 101.8 (C-7), 68.5 (d, J = 150.8 Hz, C-2), 55.4 (Ar-OCH3) 53.4 (d, J = 6.2 Hz, P(O)CH3), 53.2 (d, J = 6.0 Hz, P(O)CH3); 31P NMR(CDCl3): δ = 22.28; GC–MS m/z (%): 365 (M•, 100). Anal. Calc. for C17H20NO6P: C, 55.89; H, 5.52; N, 3.83. Found: C, 55.86; H, 5.50; N, 3.81.
2.3 Biological evaluation
2.3.1 Antibacterial activity assay
Antibacterial activity of (4a–j) was assayed against the growth of Staphylococcus aureus and Escherichia coli following the disc-diffusion assay at three concentrations (100, 50, 25 ppm). The inhibition zone was measured from the border of the disc to the edge of the clear zone. The majority of the compounds exhibited moderate to good activity against both the bacteria (Table 1).
Product
Zone of inhibition (%)
Escherichia coli
Staphylococcus aureus
100 ppm
50 ppm
25 ppm
100 ppm
50 ppm
25 ppm
4a
08
05
02
09
05
01
4b
07
05
03
07
04
02
4c
08
04
–
08
04
–
4d
05
03
01
07
04
–
4e
07
04
–
06
05
–
4f
08
05
03
10
06
02
4g
08
04
–
09
04
01
4h
07
05
–
08
05
02
4i
06
03
–
09
05
–
4j
05
02
–
07
03
–
Penicillina
12
07
–
11
08
–
2.3.2 Antifungal activity assay
The compounds (4a–j) were screened for their antifungal activity against Aspergillus niger and Helminthosporium oryzae species along with the standard fungicide Griseofulvin by the disc diffusion method (Benson et al., 1990) at three different concentrations (100, 50, 25 ppm). The majority of the compounds exhibited moderate activity against both the bacteria (Table 2).
Product
Zone of inhibition (%)
Aspergillus niger
Helmenthosphorium oryzae
100 ppm
50 ppm
25 ppm
100 ppm
50 ppm
25 ppm
4a
07
05
03
09
06
03
4b
08
05
03
08
05
02
4c
08
06
02
09
05
03
4d
05
03
01
07
04
–
4e
09
04
–
06
04
–
4f
08
06
03
09
06
02
4g
08
04
–
09
04
01
4h
08
04
–
08
04
–
4i
09
05
02
09
05
01
4j
07
03
–
08
04
01
Griseofulvina
11
08
06
13
08
06
3 Results and discussion
In our study, the simplest and most typical starting materials were used in different combinations. In most of the cases, piperanal (1), primary amines (3a–j) such as aniline and its derivatives, para-chloroaniline, para-bromoaniline, 4-floro3-choroaniline, para-methoxyaniline and diethyl phosphite/ dimethyl phosphite (2) were used.
The three components (1, 2, 3a–j) were measured in equimolar quantities and the mixtures were allowed to react vigorously in aqueous media at 70 °C for 30–60 min with stirring to afford the corresponding α-aminophosphonates. Several structurally diverse aniline/substituted anilines, diethyl phosphite and dimethyl phosphite were subjected to this novel procedure to give the corresponding α-amino phosphonates in high to excellent yields. The results are summarized in Table 3.
The presence of electron donating groups on aniline gave the corresponding products in good yields. The wide applicability of the present method is evident from the fact that it is tolerant toward various functional groups including alkoxy and halo compounds. Further we studied the role of catalyst concentration on the model reaction 4e. We have varied the catalyst concentration to 5, 10, 15, 20 mol%. The result revealed that, when the reaction was carried out in the presence of 10, 15, 20 mol% of catalyst it gave lower yield of product even after prolonged reaction time. At the same time when the concentration of catalyst was 5 mol% we got excellent yields of product in a short span. Even after increasing the catalyst concentration above 5 mol% the yields of the products did not improve. So it is established that the 5 mol% of catalyst is sufficient to catalyze and bring it to completion. The results are listed in Table 4.
Entry
Catalyst (mol%)
Yield (%)
1
5
86
2
10
50
3
15
40
4
20
30
All the products were purified by column chromatography and were characterized by elemental analysis, 1H NMR, IR, 13C NMR, 31P NMR and mass spectral data.
4 Conclusion
In conclusion, Triton X-100 was found to be an efficient catalyst for the one-pot reaction of aldehyde, amines and diethylphosphite/dimethylphosphite to afford the corresponding α-aminophosphonates in moderate to good yields. The main advantages of the present synthetic protocol are mild reaction, solvent-free conditions, ecofriendly catalyst and easy work-up procedure. The derivatives are characterized by physicochemical and spectral analysis such as 1H NMR, IR, 13C NMR, 31P NMR and mass spectral data. The spectral data obtained were in full agreement with the proposed structures. The majority of the compounds exhibited moderate activity against both bacteria.
Acknowledgments
The authors express their grateful thanks to Prof. C.D. Reddy, Department of Chemistry, Sri Venkateswara University, Tirupati, for his helpful discussions and also thank BRNS, BARC, Mumbai for providing financial assistance (2010/37C/26/BRNS/1424).
References
- Synthesis of transition-state analog inhibitors containing phosphorus acid derivatives at the scissile bond. J. Med. Chem.. 1989;32:1652-1661.
- [Google Scholar]
- One-pot synthesis of α-aminophosphonates catalyzed by antimony trichloride adsorbed on alumina. Tetrahedron Lett.. 2008;49:2208-2212.
- [Google Scholar]
- Synthesis and structure-activity relationships of antibacterial phosphonopeptides incorporating (1-aminoethyl)phosphonic acid and (aminomethyl)phosphonic acid. J. Med. Chem.. 1986;29:29-40.
- [Google Scholar]
- Microbiological Applications (fifth ed.). Boston: W.C. Brown Publications; 1990.
- Amberlite-IR 120 catalyzed three-component synthesis of α-amino phosphonates in one-pot. Tetrahedron Lett.. 2008;49:2598-2601.
- [Google Scholar]
- Environmentally friendly solvent-free processes: novel dual catalyst system in Henry reaction. Org. Process Res. Dev.. 2003;7:254-258.
- [Google Scholar]
- Three component coupling catalyzed by TaCl5–SiO2: synthesis of α-amino phosphonates. Tetrahedron Lett.. 2001;42:5561-5563.
- [Google Scholar]
- Synthesis of natural products containing a C–P bond. Tetrahedron. 1999;55:12237-12273.
- [Google Scholar]
- Phosphorus amino acid analogs as inhibitors of leucine aminopeptidase. J. Med. Chem.. 1987;30:1603-1609.
- [Google Scholar]
- One-pot three-component synthesis of α-amino phosphonate derivatives. Catal. Commun.. 2007;8:1023-1026.
- [Google Scholar]
- A new one-pot synthesis of α-amino phosphonates catalyzed by H3PW12O40. Catal. Commun.. 2007;8:1224-1226.
- [Google Scholar]
- TiO2 as a new and reusable catalyst for one-pot three-component syntheses of α-aminophosphonates in solvent-free conditions. Tetrahedron. 2008;64:5459-5466.
- [Google Scholar]
- Hydrophosphorylation of imines catalyzed by tosyl chloride for the synthesis of α-aminophosphonates. Synlett 2008:1837.
- [Google Scholar]
- β-Cyclodextrin as an efficient catalyst for the one-pot synthesis of α-aminophosphonic esters in water. Tetrahedron Lett.. 2007;48:9015-9017.
- [Google Scholar]
- Calcium chloride as an efficient lewis base catalyst for the one-pot synthesis of α-aminophosphonic esters. Chem. Lett.. 2008;37:540.
- [Google Scholar]
- ZnO nanoparticles as an efficient catalyst for the one-pot synthesis of α-aminophosphonates. Synlett 2009:1326.
- [Google Scholar]
- Syntheses, spectral property, and antimicrobial activities of 6-α-amino dibenzo[d,f][1,3,2]dioxaphosphepin 6-oxides. Heteroat. Chem.. 2007;18:2-8.
- [Google Scholar]
- (Bromodimethyl) sulfonium bromide: an inexpensive reagent for the solvent-free, one-pot synthesis of α-aminophosphonates. Tetrahedron Lett.. 2005;46:1209-1210.
- [Google Scholar]
- A novel catalytic three-component synthesis (Kabachnik–fields reaction) of α-aminophosphonates from ketones. Synlett 2003:2321.
- [Google Scholar]
- Sulfamic acid: An efficient and cost-effective solid acid catalyst for the synthesis of α-aminophosphonates at ambient temperature. Catal. Commun.. 2008;9:1822-1826.
- [Google Scholar]
- Synthesis and biological activity of novel small peptides with aminophosphonates moiety as NOP receptor ligands. Amino Acids 2010:1537-1543. 39
- [Google Scholar]
- An overview of stereoselective synthesis of α-aminophosphonic acids and derivatives. Tetrahedron. 2009;65:17-49.
- [Google Scholar]
- A simple and green procedure for the synthesis of α-aminophosphonate by a one-pot three-component condensation of carbonyl compound, amine and diethyl phosphite without solvent and catalyst. Green Chem.. 2002;4:551-554.
- [Google Scholar]
- Design and one-pot synthesis of α-aminophosphonates and bis(α-aminophosphonates) by iron(III) chloride and cytotoxic activity. Eur. J. Med. Chem.. 2009;44:4266-4275.
- [Google Scholar]
- One-pot synthesis of primary 1-aminophosphonates: coupling reaction of carbonyl compounds, hexamethyldisilazane, and diethyl phosphite catalyzed by Al(OTf)3. Heteroat. Chem.. 2009;20:109-115.
- [Google Scholar]
- An eco-friendly procedure for the efficient synthesis of dialkyl α-aminophosphonates in aqueous media. J. Organomet. Chem.. 2008;693:3313-3317.
- [Google Scholar]
- KHSO4-mediated synthesis of α-amino phosphonates under a neat condition and their 31P NMR chemical shift assignments. Tetrahedron Lett.. 2010;51:5708-5712.
- [Google Scholar]
- PPh3-catalysed one-pot three-component syntheses of α-aminophosphonates under solvent-free conditions. J. Chem. Res.. 2009;2009:78-80.
- [Google Scholar]
- One-pot three-component Kabachnik–Fields synthesis of α-aminophosphonates using H-β zeolite catalyst. Tetrahedron Lett.. 2011;52:863-866.
- [Google Scholar]
- Organocatalytic synthesis of α-hydroxy and α-aminophosphonates. Tetrahedron Lett.. 2008;49:6501-6504.
- [Google Scholar]
- Expeditious approach to α-amino phosphonates via three-component solvent-free reactions catalyzed by NBS or CBr4. Green Chem.. 2006;8:365-367.
- [Google Scholar]
- Facile one-pot synthesis of α-amino phosphonates using lanthanide chloride as catalyst. Heteroat. Chem.. 2006;17:389-392.
- [Google Scholar]
- Montmorillonite clay-catalyzed one-pot synthesis of α-aminophosphonates. Synlett 2001:1131.
- [Google Scholar]
- Silica sulfuric acid as a recyclable catalyst for a one-pot synthesis of α-aminophosphonates in solvent-free conditions. Lett. Org. Chem.. 2009;6:470-473.
- [Google Scholar]
- Bismuth(III) chloride catalyzed three-component coupling synthesis of α-aminophosphonates. Synth. Commun.. 2005;35:2501-2508.
- [Google Scholar]