Translate this page into:
Tuning of graphitic carbon nitride (g-C3N4) for photocatalysis: A critical review
⁎Corresponding author. yswudil@yahoo.com (Y.S. Wudil)
-
Received: ,
Accepted: ,
This article was originally published by Elsevier and was migrated to Scientific Scholar after the change of Publisher.
Peer review under responsibility of King Saud University.
Abstract
Graphitic carbon nitride (g-C3N4) is a remarkable semiconductor catalyst that has attracted widespread attention as a visible light photo-responsive, metal-free, low-cost photocatalytic material. Pristine g-C3N4 suffers fast recombination of photogenerated electron-hole pairs, low surface area, and insufficient visible light absorption, resulting in low photocatalytic efficiency. This review presents the recent progress, perspectives, and persistent challenges in the development of g-C3N4-based photocatalytic materials. Several approaches employed to improve the visible light absorption of the materials including metal and non-metal doping, co-doping, and heterojunction engineering have been extensively discussed. These approaches, in general, were found to decrease the material’s bandgap, increase the surface area, reduce charge carrier recombination, and promote visible light absorption, thereby enhancing the overall photocatalytic performance. The material has been widely used for different applications such as photocatalytic hydrogen production, water splitting, CO2 conversion, and water purification. The work has also identified various limitations and weaknesses associated with the material that hinders its maximum utilization under visible illumination and presented state-of-the-art solutions that have been reported recently. The summary presented in this review would add an invaluable contribution to photocatalysis research and facilitate the development of efficient visible light-responsive semiconducting materials.
Keywords
Clean energy
Clean water
CO2 conversion
Graphitic carbon nitride
Hydrogen production
Photocatalysis
- g-C3N4
-
Graphitic carbon nitride
- HOMO
-
Highest occupied molecular orbital
- LUMO
-
Lowest unoccupied molecular orbital
- AOPs
-
Advanced oxidation processes
- CB
-
Conduction band
- VB
-
Valence band
- CDs
-
Carbon dots
- NIR
-
near infra-red
- DFT
-
Density functional theory
- UV
-
Ultraviolet
- UCPL
-
up-conversation photoluminescence
Abbreviations
1 Introduction
The ever-increasing demand for clean and sustainable energy for domestic and industrial applications has been a source of concern for both policymakers and researchers as witnessed in deliberations at the international energy summits (Xie et al., 2022; Sharma et al., 2022; Li et al., 2022). This is especially important considering the rising global warming and climate change impacts on our environment (Kanimba et al., 2019; Wudil et al., 2021; Hrahsheh et al., 2017). Sunlight has been considered an incredible and inexhaustible energy source that does not pose any threat to the environment (Zhang et al., 2022). It is considered one of the solutions to the lingering energy scarcity and environmental pollution.
Semiconductor photocatalysts have been employed for various applications including selective synthesis of organic compounds, water splitting, bacteria disinfection, removal of organic pollutants, and CO2 reduction (Zhang et al., 2022; Al-Ahmed, 2022; Boumeriame et al., 2022; Zhao et al., 2022). They are considered clean, safe, economic, and renewable technology. However, the main impediment to the practical applications of these compounds is their wide bandgap which results in low solar energy utilization. For instance, TiO2 has been regarded as an excellent photocatalyst since the discovery of Fujishima et al. (Fujishima and Honda, 1972), who found that by using TiO2 as a photoanode, water can be split into hydrogen. However, the relatively large bandgap of the compound (∼3.2 eV) has become a barrier to its deployment, especially under visible light irradiation. Multiple works have been conducted to overcome the drawbacks of the wide bandgap of TiO2 to enable it to harness the visible portion of solar energy (Su et al., 2017; Ahmed et al., 2010).
Hitherto, it is remarkably challenging to develop a novel photocatalytic material that is efficient, abundant, stable, and facile in synthesis (Zhang et al., 2022; Cheng et al., 2022; Wang et al., 2022; Long et al., 2022). Recently, some 2D materials with outstanding properties such as hexagonal boron nitrides, transition-metal dichalcogenides, graphitic carbon nitrides (g-C3N4), and graphene have been extensively used for different applications including energy storage and generation, chemical sensors, electronic and optical devices, and environmental remediation (Liu et al., 2022; Lu et al., 2022; Zhang et al., 2022; Li et al., 2022; Wan et al., 2022). Specifically, g-C3N4 (Fig. 1) has generated vast interest for its exceptional photocatalytic applications as a metal-free polymer.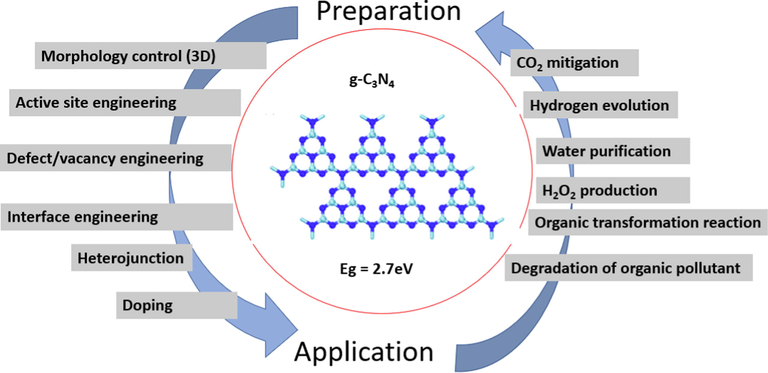
Polymerized g-C3N4 structure (nitrogen: gray, carbon: blue). ().
Adapted from Sun et al., 2018
Basically, g-C3N4 falls in the range of medium bandgap semiconducting compounds that respond to visible light (up to 460 nm) (Kong et al., 2022; Shi et al., 2022). This optimum energy gap together with high chemical stability, facile synthesis, low cost, and environmental friendliness makes it one of the excellent candidates for CO2 reduction and organic synthesis, water splitting, and degradation of organic pollutants under visible light radiation (Altan et al., 2022; Choong et al., 2022; Dong et al., 2022; Rana et al., 2022; Kumar et al., 2022). However, the intrinsic g-C3N4 generally exhibits low photocatalytic performance due to the fast recombination of electron-hole pairs, low surface area, and inadequate light absorption (Mo et al., 2022). The structural lattice architecture and the calculated electronic band structure of the g-C3N4 photocatalyst are presented in Fig. 2. g-C3N4 at varying dimensions has been used for different applications as depicted in Fig. 3. It is evident that g-C3N4 possesses a tunable bandgap with the ability to control the highest occupied molecular orbital (HOMO) and lowest unoccupied molecular orbital (LUMO). This makes it easier to enhance its photoelectronic efficiency. The tunable bandgap allows modifying the material’s property through heterostructure design or by elemental doping (Wudil et al., 2022; Wudil et al., 2020; Al-Najjar et al., 2022). The latter has been reported as an effective means of tuning the material’s energy gap and facilitating visible light absorption, thereby accelerating charge carrier separation (Cheng et al., 2022). Elsewhere (Fronczak et al., 2022), the electronic structure and texture of g-C3N4 have been modified by substitutional and interstitial doping with B, I, C, S, or P, and enhanced photocatalytic efficiency was achieved. Similarly, transition metals and Alkali metals have been doped on g-C3N4 to improve its photo-response.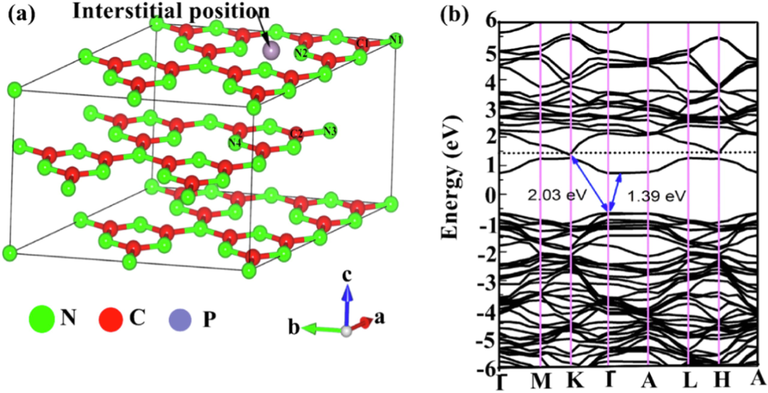
(a)Lattice structure of g-C3N4 with 2:2:1 supercell, (b) calculated band structure of g-C3N4. ().
Adapted from Liu, 2016
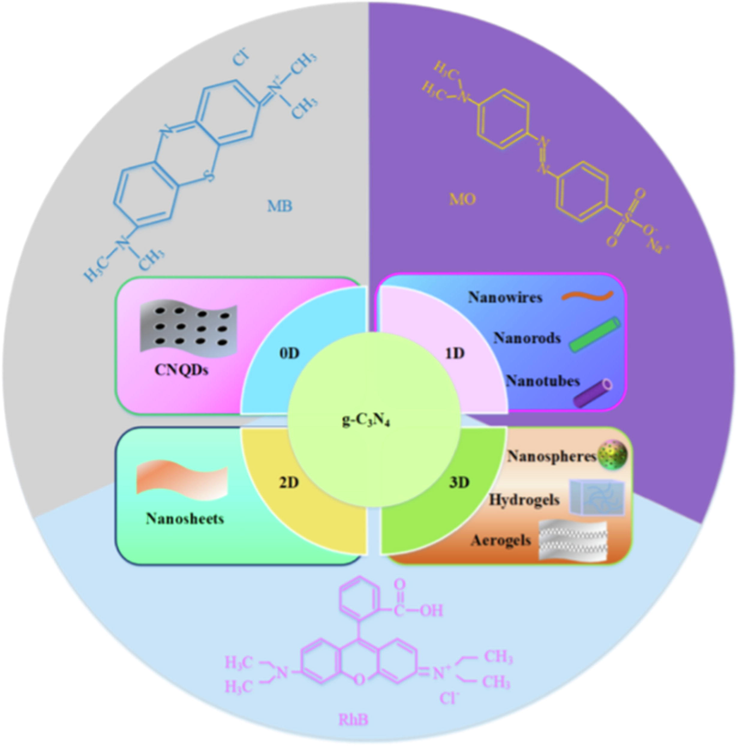
The applications of g-C3N4 with varying dimensions. ().
Adapted from Xie et al., 2022
Herein, we report the recent progress and persistent challenges in the development of g-C3N4 photocatalytic systems to enhance the utilization of solar radiation in the visible range for different applications such as water splitting, hydrogen evolution, water purification, and CO2 reduction. We also present the current challenges that need to be addressed in future works.
2 Fundamentals of heterogeneous photocatalysis mediated by semiconductors
The basics of photocatalysis has been explained in numerous articles (Liang et al., 2022; Wang et al., 2020; Fattahimoghaddam et al., 2022; Meng et al., 2022), therefore, our focus in this work is to give a brief insight into the subject to enable the reader to digest the important aspects of the photocatalytic process (Wang et al., 2020; Wang et al., 2020; Wang et al., 2022). Fig. 4 summarizes the fundamental principles of photocatalysis starting from light harvesting, excitation of charge carriers and separation, charge transfer, recombination of charges, and surface reactions (Koutsouroubi et al., 2022; Rajan and Neppolian, 2022; Shahsavandi et al., 2022). Briefly, the mechanism of photocatalysis involves three main steps: photon absorption, electron-hole generation, and surface activity (Lin et al., 2022; Gao et al., 2022; You et al., 2022; Song et al., 2022). Thus, multiple strategies have been adopted to improve the photocatalytic efficiency of g-C3N4 such as dye sensitization, nanocomposite structure formation, using conductive materials, and exfoliation to 2D nanosheets, fabricating mesoporous g-C3N4, molecular and elemental doping (Yu et al., 2022; Tao et al., 2022; Azami et al., 2022; Zehtab Salmasi et al., 2022; Zhao et al., 2022; Van Thuan et al., 2022; Wang et al., 2022; Niu et al., 2022; Tipplook et al., 2022; Mandari et al., 2022).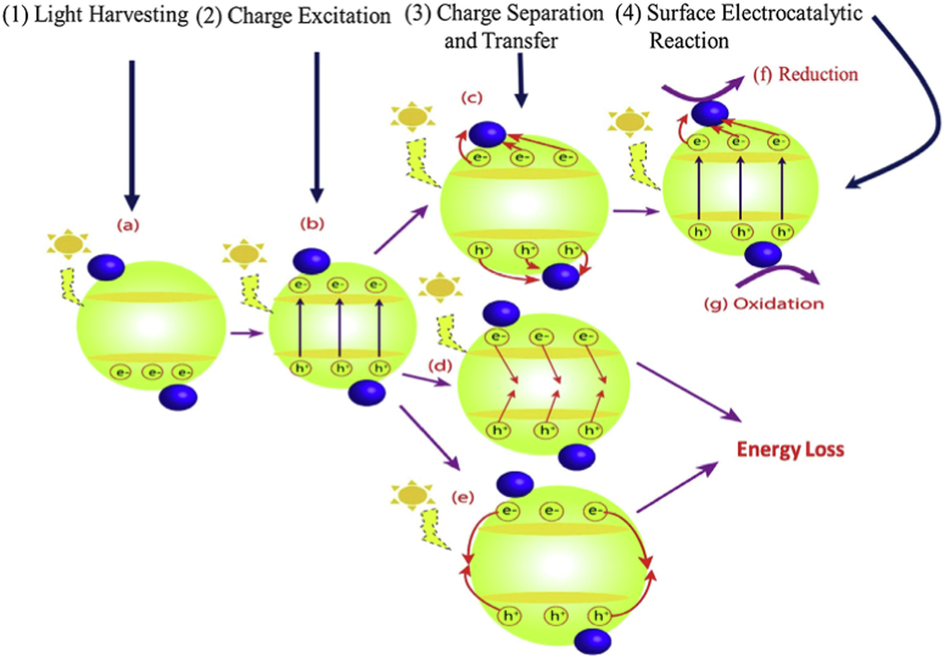
Fundamental principles of photocatalysis depicting (a) Harvesting of light (b) Charge carrier excitation (c) separation of charges and transfer (d) recombination of charges (e) surface charge recombination, (f) surface reduction reactions, and (g) surface oxidation reactions. ().
Adapted from Khan and Tahir, 2019
Several industries (leather, energy, paper, textiles, etc.) employ the application of dyes or synthetic pigments which makes colored wastewater a significant problem to deal with related to these industries, especially as they release large quantity of heat to the environment which often complicate the issue (Wudil et al., 2020; Almessiere et al., 2021; Salhi et al., 2018; Alrebdi et al., 2022). These pollutants are made up of extensive organic compounds and hazardous substances that are poisonous to organisms that live below water. In addition, the increased use of pharmaceuticals in healthcare because of the growth in the world’s population has contributed to the release of these unutilized or dissolved compounds into the environment. For that reason, it is required to mineralize, eliminate, transform and reduce these molecules of pollutants from the aquatic habitat. Apart from that, fossil fuels like coal, gas, and petroleum have serious effects on climate change. Many conventional techniques have been developed in this regard for the purification of water. These techniques involve biological, chemical, physical, coagulation, adsorption, sedimentation, flotation, filtration methods, gravitational separation, and reverse osmosis. However, due to the movement of contaminants from one stage to another stage or partial removal, the performance of these methods is insignificant to exhaustively clean contaminated water. This section presents an advanced technique for dealing with environmental concerns (Asadzadeh-Khaneghah and Habibi-Yangjeh, 2020).
Advanced Oxidation Processes (AOPs) are a category of chemical processes examined to be among the most effective and desirable solutions for the above-mentioned problems. In the past few decades, numerous groundbreaking examinations have been conducted to explore the application of these processes in areas of environmental and wastewater treatment (Asadzadeh-Khaneghah and Habibi-Yangjeh, 2020). These processes do not transfer contaminants from one stage to another, but rather manipulate environmental crises and prevent the production of toxic sludge. This ability made them superior to sedimentation, filtration, physical, and biological processes. These processes are particularly useful as an efficient purification method with high mineralization ability for the non-reactive and rapid degradation of a wide range of highly stable and nonbiodegradable organic chemicals into harmless and environmentally friendly compounds like water, SO4-, PO43-, and carbon dioxide via reactive species such as SO4, O2, and h+.
Various AOPs are stated in Fig. 5. The most cost-effective provides the ideal solution to remediating energy crisis and environmental contamination among the various AOPs is the heterogeneous photocatalysis mediated by semiconductors (Tseng et al., 2022; Lin et al., 2022). The following indicated the characteristics that made heterogeneous photocatalysis appealing: i) The accomplishment of the process in ambient situations. ii) The feasibility of the process to support the photocatalyst on various forms of inert matrices for example, graphene oxides, carbon nanotubes, glasses, and polymers and in general, the utilized catalysts are non-toxic, affordable, and can be recycled. Furthermore, iii) It is the cheapest method that is eco-friendly when it comes to air and water treatment.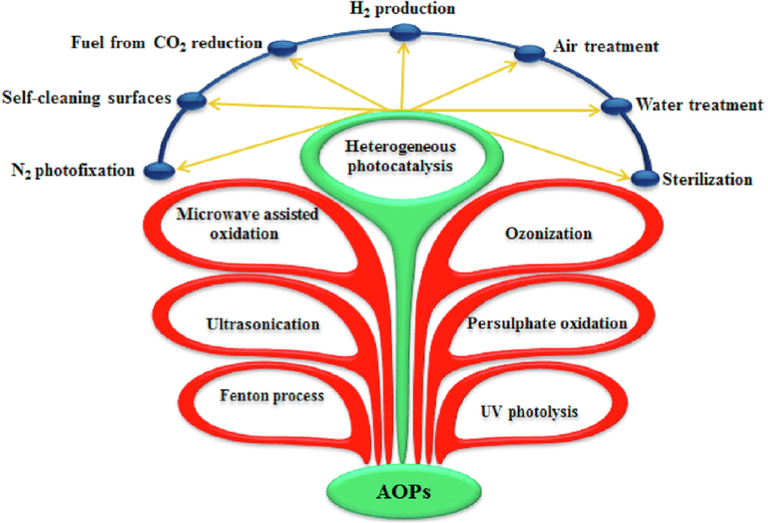
A general representation of the advanced oxidation processes and the potential applications of semiconductor-mediated photocatalysis. ().
Adapted from Li et al., 2019
Moreover, heterogeneous photocatalysis mediated by semiconductors can be employed for various applications among which are the production of hydrogen via splitting of water, degradation of water pollutants, reduction of carbon dioxide for fuel production, disinfection of different microorganisms, and nitrogen photofixation, as shown in Fig. 5.
A photocatalyst under light illumination for activation is schematically displayed in Fig. 6. Four fundamental steps explained the process of heterogeneous photocatalysis. Generally, photocatalysis mediated by semiconductors starts off to absorb proper photon energy that is the same or greater than that of the energy gap (Eg) of the desired photocatalyst to produce e-/h+ pairs which then produce few reactive species and thereby leads to various redox reactions on the surface of the heterogeneous photocatalyst. However, the reverse process of this phase is that absorbed energy is released in form of heat at the time scale of nanoseconds as a result of the recombination of a large portion of the photoinduced charges.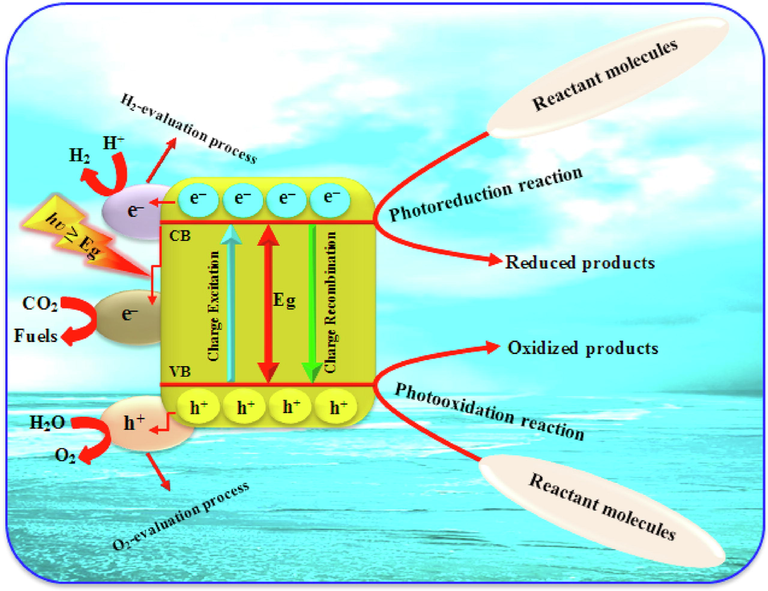
A schematic diagram demonstrating semiconductor-mediated photocatalytic processes. ().
Adapted from Asadzadeh-Khaneghah and Habibi-Yangjeh, 2020
3 Selection of appropriate semiconductors
Numerous semiconductors are applied as photocatalysts to produce energy and facilitate environmental purification (Fronczak et al., 2022; Meng et al., 2022; Zhang et al., 2022; Schirmer et al., 2022; Tan et al., 2022; Nguyen et al., 2022; Liu et al., 2022). Among these photocatalysts used are Fe2O3, NaTaO3, WO3, BiPO4, TiO2, ZnS, SrTiO3, ZnO, and SnO2. Despite several pieces of literature, poor harvesting of visible light by the chosen photocatalysts has made the conventional utilization of this process a great challenge. The UV light spectrum of sunlight radiations can only be harvested by photocatalysts that have a wide energy gap and UV light requires protective support for its application, and UV sources are not cheap (Xu et al., 2022; Pattappan et al., 2022; Shang et al., 2022). Therefore, photocatalysts that are visible-light-induced can be considered appropriate materials for the utilization of the visible fraction of solar energy. Furthermore, an ideal photocatalyst should possess certain characteristics such as slow recombination of charges, appropriate bandgap potential, suitable bandgap, and high surface area (Wang et al., 2022; Xue et al., 2022; Ding et al., 2021). These are the fundamental reasons that made the designation and fabrication of visible-light-induced photocatalysts a hot area of research.
In summary, Graphitic carbon nitride (g-C3N4) has been largely employed in different photocatalytic applications (Li et al., 2022; Li et al., 2019). This is due to its great advantages like cheap precursors, high stability, and preparation methods that are easy. This is the reason why it is among the most favorable materials that can be applied as an important semiconductor in heterogeneous photocatalysis. Nevertheless, characteristics such as low specific surface area, fast recombination of charges, and inefficient absorption of visible light have hindered the extensive and practical deployment of g-C3N4 (Rajan and Neppolian, 2022; Wan et al., 2022; Shi et al., 2022; Guo et al., 2022; Zhang et al., 2022; Cheng et al., 2022). For that reason, the engineering of g-C3N4 has been performed by numerous research efforts to increase the migration of charges, improve the specific surface area, and boost its absorption efficiency. Fig. 7 presents a SWOT (Strengths, Weaknesses, Opportunities, and Threats) analysis of using graphitic carbon nitride as a photocatalytic material in terms of socioeconomic, technical, and environmental sustainability perspectives.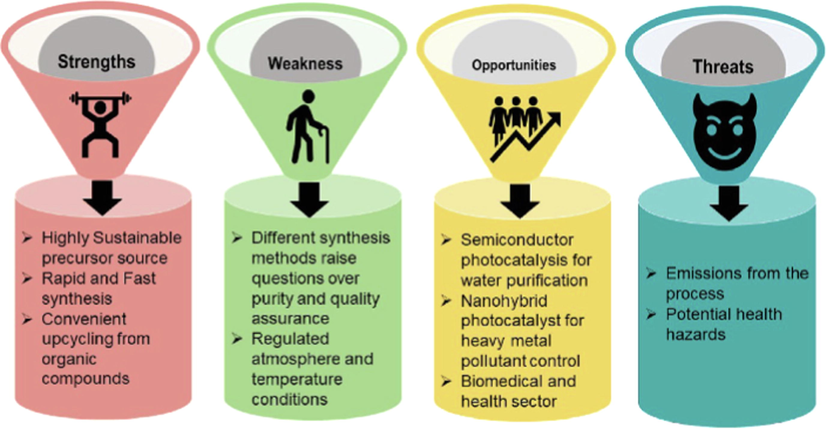
Strengths, weaknesses, opportunities, and threats of using g-C3N4 as an efficient photocatalyst. ().
Adapted from Sharma et al., 2022
4 g-C3N4 preparation and structure-dependent performance
4.1 Design of g-C3N4-based photocatalyst
The preparation of g-C3N4 can be simply carried out by the thermal condensation of several inexpensive nitrogen-rich precursors like melamine, cyanamide, dicyandiamide, urea, thiourea, or combinations thereof (Wang et al., 2022). The typical method for figuring out the phase of carbon nitrides is to employ X-ray powder diffraction (XRD) patterns. There are two distinct diffraction peaks in the g-C3N4 XRD patterns, located at approximately 27.4° and 13.0° (Cao et al., 2015). The former can be indexed for graphitic materials as the 002-peak indicative of interlayer stacking of aromatic systems, and the latter as the 100-peak corresponding to the interplanar separation. Measurements are made using X-ray photoelectron spectroscopy (XPS) to determine the status of nitrogen and carbon elements in g-C3N4. The molecular structure of the polymeric g-C3N4 derived from nitrogen-rich precursors enables the electronic structure to be adjusted by a modest modification of the molecular structure in the copolymerization process of the precursors, along with structure-matching organic additives.
There are several reports on bandgaps and unique surface areas of typical g-C3N4 samples made under various reaction conditions and precursors. These studies imply that an effective method for maximizing the unique surface area and electronic structure of g-C3N4 depends on the choice of various precursors in conjunction with appropriate control over the reaction parameters, such as the time and temperature of the thermal treatment (Vaya et al., 2022). Urea is one of the several precursors that can be utilized to generate thin-layer g-C3N4 with a lot of specific surface area. However, a variety of precursors and experimental circumstances should be investigated, such as the recently described exfoliation procedures, to streamline the synthesis of g-C3N4 materials and enhance their properties.
4.2 Nanostructure design of g-C3N4
g-C3N4 is a polymer with a flexible structure which makes it suitable for forming various morphologies with the aid of various templates. Several common g-C3N4 nanostructures have been made, including 1D nanostructures, hollow spheres, and porous g-C3N4; a brief description of each of these structures is given below.
The unique optical, chemical, and electrical properties that may be obtained by adjusting the length, diameter, and aspect ratio of 1D nanostructured photocatalysts like nanorods, nanowires, nanobelts, and nanotubes are advantageous for enhancing their photocatalytic activity. By thermally condensing cyanamide in the presence of anodic aluminum oxide (AAO) template, Li et al. (Li et al., 2011) created condensed g-C nanorods with an average diameter of 260 nm. The AAO template's confinement effect was essential for enhancing the crystallinity and orientation of the g-C to increase charge-carrier mobility. Additionally, the resulting g-C3N4 nanorods had a more positive VB state, which is necessary for a greater oxidation power.
Hollow sphere photocatalysts are also appealing because they can generate more photoinduced charge carriers and can harvest more incident light through subsequent reflections inside the hollow structure (Chen et al., 2022). Due to the polymeric g-C3N4 layered structure, which is prone to collapsing during processing, it is difficult to manufacture hollow g-C3N4 spheres. However, several attempts to produce hollow g-C3N4 spheres have been successful. Monodisperse silica nanoparticles were covered with thin mesoporous silica shells by Sun et al. (Sun et al., 2012). The preparation of g-C3N4 hollow nanospheres was subsequently carried out using these core–shell structures as hard templates. Recently, it was shown that the supramolecular chemistry of triazine molecules makes g-C3N4 hollow structures easier to manufacture. A precursor hydrogen-bonded supramolecular network, like the cyanuric acid-melamine complex, was created in this instance because of the molecular cooperative assembly of the triazine molecules (Cao et al., 2015). Depending on the solvent used, this complex can be synthesized in a variety of morphological forms. For instance, 3D macro assemblies, flower-like layered spherical aggregates, and an organized pancake-like structure have all been produced in dimethyl sulfoxide and ethanol, respectively.
Porous photocatalysts are incredibly exciting due to their porous structure, which can offer a significant surface area and a huge number of channels to support mass diffusion, charge migration, and separation (Al-Ahmed, 2022). Because they enable customization of the porosity structure of g-C3N4 by selecting different templates, soft and hard templating methods are frequently utilized. Mesoporous g-C3N4 has been produced successfully using a variety of precursors, including ammonium thiocyanate, cyanamide, urea, and thiourea in the presence of silica nanoparticles used as a hard template, the removal of which produced a 3D interconnected structure of g-C3N4 with a large surface area of up to 373 m2 g−1. The pore size that was produced matched the dimensions of the silica nanoparticles utilized as the template.
These studies show that distinct g-C3N4 nanostructures can be created utilizing various templating or non-templating techniques. These nanostructures have several benefits and could make a great foundation for the creation of unique photocatalytic systems. Large surface areas are typically attained for g-C3N4 using porous materials, which might result in an abundance of reactive sites. Porous structures can act as efficient channels to enhance interactions between g-C3N4 and the target reactants in the meantime. Additionally, the requisite specific surface area and charge carrier mobility may both be present in 1D nanostructures of g-C3N4.
5 g-C3N4 as a promising photocatalyst
The C3N4 materials traced back to the time when Berzelius and Liebig produced and reported in 1834 a melon embryonic shape (Scopus - Document details - null | Signed in, (n.d.). https://www.scopus.com/record/display.urieid=2-s2.0-7995330, 2022). This is introduced as the first synthetic polymer in the form of interconnected tri-s-triazine through secondary nitrogen which is a linear polymer. For over 150 years, research on this polymer stopped because of its inability to dissolve in most solvents and its chemical inertness.
Eventually, in the nineties, Liu and Cohen (Cohen, 1993) predicted a diamond-like structure from the fabrication of really hard carbon nitride. Subsequently, bulk hardness and great modulus values were achieved from β- C3N4 and then followed by the discovery of various allotropes of C3N4 that have several properties, for example, g-C3N4, α- C3N4, cubic C3N4, and pseudocubic C3N4. The most resistant among the stated carbon nitrides (CxNy) under surrounding conditions is g-C3N4 and this is because it has a graphene structured layer. The allotropes g-C3N4 have two fundamental tectonic units which are triazene C3N3 known as ‘melam’ and heptazine /tri-s-triazane C6N7 ‘melem’. At ambient conditions, the latter is the one with the most resistant phase.
The field of heterogeneous photocatalysis witnessed the first application of g-C3N4 about a decade ago. It was reported that Wang and his coworkers (Wang et al., 2009) have employed g-C3N4 as a conjugated stable to efficiently produce H2 or O2 from water splitting, as shown in Fig. 8. This was carried out upon visible light illumination because of its supreme optical features, high stability in basic/acidic systems, good physical–chemical stability, and environmentally friendly nature. In the next session, we discussed in more detail the excellent physicochemical characteristics of g-C3N4 which is a result of the great number of publications on the Web of Science that guarantees the material’s popularity. The elemental composition of g-C3N4 as a semiconductor is carbon and nitrogen which are very abundant in the earth (Huang et al., 2022; Pham et al., 2022; Zhong et al., 2022; Che et al., 2022). Fabrication of this organic semiconductor is primarily done with nitrogen-organic precursors which include melamine, urea, cyanamide, dicyanamide, and thiourea among others. Even though, several publications have reported various methods to synthesize g-C3N4. The surface chemical features, electronic band structure, and morphology of the as-synthesized g-C3N4 are strikingly affected by the choice of precursor, reaction temperature, and reaction environment (Chen et al., 2022; Wu et al., 2022; Chu et al., 2022; Altan and Kalay, 2022; Tang et al., 2022; Zhong et al., 2022; Cheng et al., 2022). Many reviews have also thoroughly reported the comprehensive procedures for the preparation of g-C3N4 taking into consideration of the reaction condition and choice of precursor. A thermal polymerization process is a strategy employed on various precursors with nitrogen units (Fig. 9) to fully fabricate g-C3N4. It is described as a ‘bottom-up method’. The bottom-up method is the formation of fairly larger and more complex systems from the self-assembly of any smaller units (for example, atoms, molecules, and nanoparticles). This is achieved by the interactions between the building units utilizing hydrogen bonding, Van der Waals force, and/or electrostatic adsorption.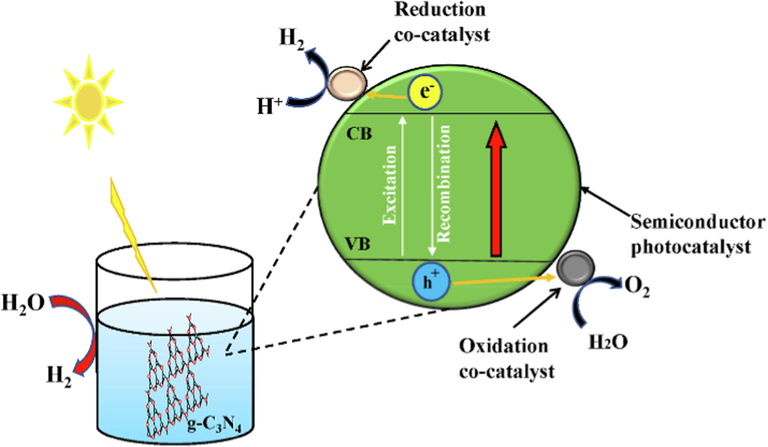
A schematic representation of hydrogen production via water splitting. ().
Adapted from Vaya et al., 2022

The g-C3N4 synthesis route via thermal polymerization of different precursors. ().
Adapted from Li et al., 2019
In short, the carbonization and pyrolysis of some organic precursors (containing in general and ) have made the bottom-up method an efficient process to prepare g-C3N4 on a large scale.
6 Limitations of g-C3N4 and remedy to the obstacles
In the past few years, g-C3N4 has been preferred over other semiconductors in different photocatalytic processes. This is due to its superior advantages such as abundance, nontoxicity, inexpensive, high thermal and photochemical stability, bio-compatibility, and moderation of activity in visible light (due to low bandgap) (Mao et al., 2022; Li et al., 2022; Ye et al., 2022; Zhu et al., 2022; Liu et al., 2022). In addition, the CB of g-C3N4 is severely more negative than the potentials needed for the generation of H2 and O2, CO2 photoreduction processes, and other semiconductors (Swedha et al., 2022). Furthermore, the CB of g-C3N4 can reduce different compounds such as H2O and O2 since the photogenerated electrons have high thermodynamic energy. However, g-C3N4 has some intrinsic drawbacks which include electron-hole rapid recombination rate, low quantum yield, inefficient visible-light absorption (below 460 nm), low charge conductivity, negligible electrical mobility, and inefficient specific surface area (about 10 m2g−1) that result in poor photocatalytic efficiency receiving visible light (Cao et al., 2022; Zeng et al., 2022). In the subsequent paragraphs, we, therefore, discussed the numerous modifications that have been suggested to reduce these problems and enhance the photocatalytic potential of g-C3N4 by utilizing several strategies.
The design of perfect-activity g-C3N4 has been established to largely depend on the surface area, plentiful reactive sites, morphology size, and predominantly extended light-harvesting capability (Wang et al., 2022; Xu et al., 2022; Gholipour et al., 2022; Gao et al., 2022). As it is known, g-C3N4 has the characteristics of a 2D polymer and also possesses a unique layered structure. The delocalized π-conjugated system in the g-C3N4 is made up of C and N with sp2 hybridization. The rapid recombination of the charges is a result of the random movement of the charge carriers in the plane caused by the inherent graphite π-conjugated structure that leads to chemical sluggishness. To modify and construct g-C3N4-based systems and defects engineering method is adopted among the various methods of surface modification. The defect generally occurs from the breakage and disturbance of the ideal periodic arrangement of the fundamental unit in g-C3N4 and that affects its photoactivity. Recombination of chargers is prevented by broadening its visible-light absorption area as a result of surface defects engineered on g-C3N4 containing C and N defects. In general, the electronic structure and features of photocatalytic materials are affected by these defects. Several studies have been carried out to increase g-C3N4 visible-light activity through defects engineering. Usually, the defects could create molecules’ reaction sites of the reactants and transform the features of photocatalysis. Recently, there have been several suggestions to improve the defect-induced photoactivity in g-C3N4. For instance, the N-defected g-C3Nx was constructed by Yu et al. (Yu et al., 2017) with an adjustable band structure that is controlled via surface cyano groups and N vacancies. The N-defective g-C3Nx harvests visible light more impressively than the pristine g-C3N4 due to its ability to separate photoexcited charges. Boosting the activity of g-C3N4 can also be carried out by another procedure and that is to morphologically and texturally fabricate the controlled g-C3N4. Consequently, structural engineering (such as size controlling, shapes, and dimensions) is a great approach to increasing the performance of g-C3N4. This in turn enlarges the surface area with different morphologies such as nanobelts, nanorods, nanowires tubes, porous/mesoporous microspheres, and nanosheets via several methods. The construction of nano-scale structures is interestingly governed by two-dimensional (2D) structures labeled as well-defined building blocks. Thus, g-C3N4 with various morphologies has leading photocatalytic capability when compared to a conventional bulk structure, because of its efficiency, and simplicity, and has more reactive parts as well as a higher specific surface area. Excellent photocatalytic proficiency and boosted optical characteristics can be achieved by open mesoporous morphology (Fig. 10) with tabular nanostructures to strikingly transmit photoinduced charges along the 2D route. This consist of fast electronic immigration and direct route. Based on numerous established literature, the second strategy to improve photocatalytic performance is the preparation of nanocomposites/heterojunction with various semiconductors that contain suitable band energies. This makes the procedure outstanding in impressively slowing down the electron-hole pairs recombination rate. The third strategy is to dope g-C3N4 with metal and non-metal elements. Recently, energy gap engineering of g-C3N4 via doping with different elements yielded excellent results that leads to tuning the redox band potentials, distinctly modulation of the light-receiving ability, as well as changing luminescent and electronic features of g-C3N4 (Yu et al., 2022; Xia et al., 2022; Wang et al., 2022; Alejandra Quintana et al., 298 (2022)). Finally, the simplistic procedure adopted to enhance the photocatalytic performance of g-C3N4 is to couple it with carbon nanomaterials. Carbon materials that have unique properties such as excellent chemical stability, appropriate electrical conductivity, and large surface area are nowadays employed for various applications. Fabricating heterojunctions with various carbon-rich materials not only compensates for the drawbacks of single semiconductors by increasing visible light utilization, improving charge separation, and increasing photo durability, but also induces synergetic effects that lead to faster electron transition from the surface of a nanocomposite due to carbon’s electron transport capability. The photocatalytic ability of the hybrids is successfully enhanced when the carbonaceous materials are coupled with g-C3N4. Carbon dots (CDs) among different types of carbonaceous materials (for example activated carbon, graphene, fullerene, and carbon nanotubes) have attracted substantial attention calling for the enhancement of their photocatalytic performance. This is because CDs have properties such as good physicochemical durability, water-solubility, nontoxicity, and ultrasound small dimension. In addition, CDs are excellent material when it comes to utilization with regards to application in photocatalysis because they have emissive traps and excellent electron reservoir/transfer. The former is because of the quantum size effect with conjugated π structure. Moreover, multi-photon activation brings about the famous feature of CDs which is known as the up-conversation photoluminescence (UCPL) feature. Considering the drawbacks highlighted for g-C3N4, the photoactivity of the system can be enhanced by employing CDs for bandgap engineering. Also, CDs give an avenue for excess orbits for e- by sp2 that which slows down the recombination of charges. The utilization of metal-free CDs as a magnificent co-catalyst produces a fundamental basis for interface engineering g-C3N4 semiconductors for various applications.
SEM images of hollow mesoporous carbon nitride. ().
Adapted from Al-Ahmed, 2022
Hence, given the substantial state of this review, we focused our study on the global survey of utilizing g-C3N4/CDs-based nanocomposite for energy production technology and treatment of photocatalytic pollutants. Around November 2019, the world witnessed almost 100 published research articles that explored the photocatalytic capability of g-C3N4/CDs-based nanocomposite with enormous research interest and broad significance. The increase in popularity of g-C3N4/CDs nanocomposite is confirmed by the rapid increase in relevant research papers that have been published in the field of g-C3N4/CDs nanocomposite within the last 5 years. Gao et al. (Gao et al., 2015) presented for the first time in 2015; the use of the first principal calculation in the photocatalytic term to explore the interaction between the electronic and optical properties of g-C3N4 and CDs. They illustrated that through the process of water splitting, hybrid g-C3N4/CDs favored the production of hydrogen gas. Consequently, the development of g-C3N4/CDs-based materials and diverse synthesis techniques, as well as the application of these photocatalysts to the degradation of various pollutants and antibiotics, water splitting, CO2 photocatalytic reduction, and other applications, has been significant.
7 Multiple strategies to improve the photocatalytic efficiency of g-C3N4
Here, the different strategies for improving the photocatalytic efficiency of g-C3N4 is discussed.
7.1 Metal doping
Essentially, the injection of metallic impurity creates more binding functions which provide the semiconducting material with enhanced photocatalytic activity by minimizing the bandgap and improving visible light absorption (Adegoke and Maxakato, 2022; Hoang et al., 2022; Lin et al., 2023). To incorporate metallic ions into the matrix of the material, the precursor of g-C3N4 is uniformly mixed with the corresponding soluble salt of the metal, thereby paving way for doping the metallic impurity into the framework of g-C3N4 during the precursor’s thermal condensation.
7.1.1 Transition metal
Transition metals such as Zr, W, Cu, Fe, and Pd have been widely used to tune the electronic and optical behavior of g-C3N4. Specifically, they prolong carrier lifetime, accelerate carrier mobility, reduce bandgap, and increased light absorption which is critical for enhanced photocatalytic performance. Elsewhere, Pan et al. (Pan et al., 2011) employed first-principles simulations to design novel g-C3N4-based nanotubes. Their investigations revealed that the optical and electronic properties of the compound can easily improve by functionalization using metallic elements such as palladium and platinum. These results indicate that metal-functionalization could enhance absorption in the visible region, narrow the bandgap and increase carrier mobility. Despite the remarkable potential of noble metals in enhancing the photocatalytic activity of g-C3N4, their practical applications are limited by their high price.
Several reports suggested that introducing a transition metal such as Zr, Mo, Zn, W, Cu, and Fe as a dopant into the matrix of g-C3N4 improves its photocatalytic efficiency (Le et al., 2016). For example, Tonda and his team (Tonda et al., 2014) used a simple, cost-effective synthesis technique to fabricate Fe-doped g-C3N4 nanosheets. They observed that the Fe-doped g-C3N4 nanosheets exhibited redshifts and the Fe-doping has tremendously increased the light absorption in the visible region and improved the photocatalytic activity. The developed Fe-species serve as photo-generated electrons as they were found to exist in a + 3-oxidation state, also making them a potential hole-trapping site. The holes existing in the valence band can oxidize OH– while O2 can be reduced to O2– by the trapped electrons. Furthermore, Wang et al. (Wang et al., 2016) employed a simple pyrolysis technique using common precursors to fabricate molybdenum-doped g-C3N4. Their results suggested that Mo-doping narrows the bandgap, extends the visible light response, enlarges surface area, and reduces the rate of the photogenerated charge carriers. Hence, Mo-doped g-C3N4 demonstrated high performance in the reduction of CO2. Similarly, Li et al. (Wang et al., 2016) used a facile pyrolysis method to introduce the rare earth element yttrium on g-C3N4 using urea as a precursor. They reported an enhanced degradation of Rhb.
7.1.2 Alkali metal
Apart from the transition metals, Alkali metals like sodium and potassium were also introduced into the framework of g-C3N4 to enhance their photocatalytic activity. Recently, Xiong and his colleagues employed density functional theory to investigate the role of the atoms (Na and K) in the increased photocatalytic performance of the doped g-C3N4 (Xiong et al., 2016). They observed that while both dopants narrow the bandgap of the material, however, they exhibit varying impacts on the photocatalytic performance and electronic structure of g-C3N4. They observed that while Na atoms were doped into the conjugated planes, K atoms existed in the interlayers of g-C3N4. Interestingly, K atoms can form a bond with the neighboring two layers thus they could bridge the layers and form the channel of charge delivery, hence facilitating charge transfer and reducing the chances of carrier recombination as depicted in Fig. 11. Moreover, Hu et al. (Hu et al., 2014) used potassium hydrate and dicyandiamide as precursors to prepare K-doped g-C3N4. They observed that the valence band and conduction band potentials of the material could be controlled by altering the concentration of the dopant. Therefore, both O2 and OH could be generated, thus yielding a greater photodegradation rate. In a similar work by Zhang et al. (Zhang et al., 2014), where they fabricated Na-doped g-C3N4, using sodium hydrate and dicyandiamide as precursors, they observed that both VB and CB potentials could be controlled by changing the dopant’s concentration. In general, as reported in the work of Hu et al. (Hu et al., 2014), although both Na and K are suitable for photocatalytic performance enhancement of g-C3N4, the degradation rate is higher with K-doped compound, thus making it more suitable for photocatalytic activity improvement.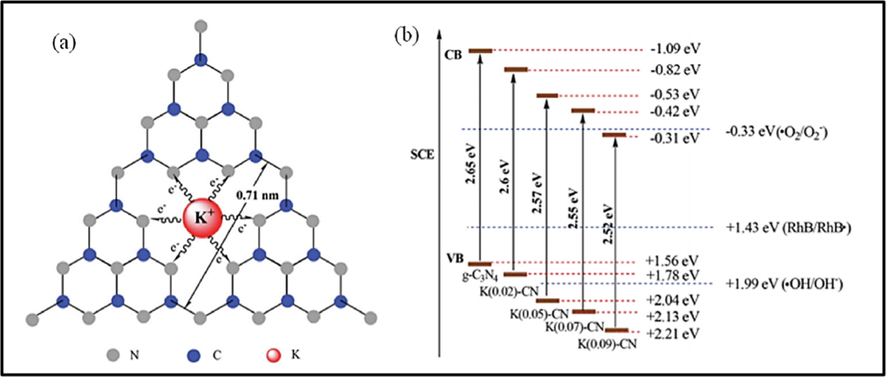
(a) Possible doping sites for potassium ions in K-doped g-C3N4 (b) Band gap structures of as-prepared g-C3N4 and K-doped g-C3N4. ().
Adapted from Asadzadeh-Khaneghah and Habibi-Yangjeh, 2020
In summary, metals have been extensively used as dopants on g-C3N4 for photocatalytic applications. Mostly, their incorporation can suppress the charge carrier recombination rate, extend the visible light response, and create a new energy level in the bandgap of the parent compound. Despite the successful experimentations of metal doping on g-C3N4, there exist a few drawbacks halting the practical applications of the developed material such as poor thermal stability. Also, their newly created energy levels within the bandgap might serve recombination centers, thus resulting in low quantum efficiency.
7.2 Non-metal doping
The doping of non-metallic species on g-C3N4 has also attracted widespread attention in the photocatalysis community. Several non-metals such as halogens, boron, oxygen, nitrogen, carbon, sulfur, and phosphorus have been reported as dopants on g-C3N4 material (Lin et al., 2023; Alcantara et al., 2022; Wang et al., 2022). Apart from conserving the metal-free characteristic, non-metals often possess higher electronegativity and ionization energies. They easily form covalent bonds with other materials by gaining electrons. Furthermore, non-metal doping can serve to reduce the impact of thermal variation of chemical states.
7.2.1 Phosphorus doping
The first phosphorus-doped g-C3N4 was fabricated by Zhang and his team (Zhang et al., 2010) using a simple polycondensation of a mixture containing phosphorus-containing ionic liquid and dicyandiamide as the g-C3N4 precursor. The PF6 reacts with the amine group with increasing temperature resulting in joining the C N matrix. NMR, XPS, and FTIR investigations demonstrated that the P heteratoms substitute the bay or corner C in the framework to create a P—N bond in the C3N4 structure. Their findings revealed that doping with a controlled amount of phosphorus heteratoms could significantly increase the electrical conductivity, lower the optical bandgap and change the electronic structure of g-C3N4. They concluded that P-doped g-C3N4 possesses a remarkable potential for photovoltaic applications. On the other hand, Zhang et al. (Zhang et al., 2013) used the same precursors to prepare P-doped g-C3N4. They observed a significant enhancement of the photocatalytic activity with MO and RhB as pollutants. The phosphorus source influences the P doping site and this may affect the photocatalytic behavior of the developed material. Consequently, different phosphorus sources such as phosphorous acid, ammonium hexafluorophosphate, hexachloro-cyclotriphosphazene (HCCP), (hydroxy ethylidene) diphosphonic acid, 2-aminoethylphosphonic acid, diammonium hydrogen phosphate, etc. have been employed to prepare P-doped g-C3N4.
Zhou and his team (Zhou et al., 2015) used a thermal polymerization route to fabricate phosphorus-doped g-C3N4 using guanidinium hydrochloride as a precursor and HCCP as a phosphorus source. Their results revealed that phosphorus atoms preferentially occupy the bay-carbon and corner carbon sites, and their incorporation into the lattice of g-C3N4 becomes seamless, thereby modulating the electronic structure and consequently reducing the rate of recombination of charge carries. Finally, they recorded an excellent increase in the photocatalytic activity both in RhB degradation and generation of H2. Elsewhere, Hu et al. (Hu et al., 2014) utilized diammonium hydrogen phosphate as the P-source and dicyandiamide as the precursor to synthesize g-C3N4. They observed that phosphorus incorporation improves the separation efficiency of charge carriers, narrows the bandgap, and inhibits the crystal growth of g-C3N4. Their work also suggested that P atoms were interstitially doped into the lattice of g-C3N4 to create the P—N bond. This contradicts the work of Zhou et al. (Zhou et al., 2015) which states that phosphorus atoms would preferentially occupy substitutional bay-carbon and corner carbon sites. In summary, it can be inferred that the phosphorus precursor used can strongly influence the P-doping site either substitutional or interstitial.
On the other hand, nanostructured materials with certain morphology have been used as active ingredients to provide active sites for photocatalytic activity and accelerate mass transfer (Wang et al., 2022; Ou et al., 2022). They also facilitate the mobility of the photogenerated carriers and help in their separation. Recently several forms of nanostructured g-C3N4 with improved photocatalytic performance such as nanofibers, nanorods, mesostructures, nanotubes, and nanosheets have been employed. Moreover, some researchers combined the structural engineering of g-C3N4 with phosphorus doping to achieve higher degradation efficiency. For example, Zhu et al. (Zhu et al., 2015) used the co-condensation of melamine to prepare g-C3N4 nanoflowers. They noted that phosphorus atoms could bond chemically with neighboring carbon and nitrogen and exhibit planar coordination in the C3N4 structure. The designed flower-shaped morphology together with phosphorus inclusion yielded higher charge separation and transfer, and superior trapping of light for enhanced hydrogen production under the visible region. The specific surface area was also found to increase due to increased mass transfer of molecules as well as high porosity.
For an insightful understanding of the phosphorus doping on the photocatalytic performance and the bandgap, Qiao and his team (Ran et al., 2015) used a combination of thermal exfoliation and p-doping to prepare porous p-doped g-C3N4 nanosheet using 2-aminoethylphosphonic acid as the P-source. X-ray photoelectron spectroscopy investigations revealed that phosphorus preferential substituted carbon to create a P—N bond in the g-C3N4 framework. Although past reports indicated a preference for the exfoliated g-C3N4 over the bulk one, there remains the challenge of a large bandgap in the thermally exfoliated nanosheets, which impedes its full utilization of the wide solar spectrum. However, with the presence of strong tail absorption, the bandgap of P-doped g-C3N4 can be decreased, and this leads to the creation of midgap states within the bandgap.
7.2.2 Sulfur doping
The electronic structure of g-C3N4 has also been modified by doping Sulfur atoms to improve carrier mobility, redox potential, light absorbance, and ultimately, photocatalytic activity (He et al., 2014; Wang et al., 2015; Lu et al., 2017). Liu and his colleagues (Liu et al., 2010) first fabricated sulfur-doped g-C3N4 by heating the powdered g-C3N4 in a gaseous H2S atmosphere. The S-doped system demonstrated an elevation in the conduction band minimum in combination with an increase in the valence band width and a slight decrease in absorbance. The concomitant quantum confinement effect plus the homogeneous substitution of Sulfur for Nitrogen led to the evolution of a unique electronic structure which made the S-doped system promising for the photooxidation of phenol and photoreduction of hydrogen evolution.
In a study by Feng and coworkers (Feng et al., 2014), thermal condensation was utilized to synthesize microrods of S-doped g-C3N4. Their investigations revealed that the S-doped system exhibited improved visible light absorption, satisfactory stability, and a larger surface area. They also observed an activity more than 9 times higher than the pristine system for H2 production. Similarly, Fan and his team (Fan et al., 2016) fabricated porous rods of S-doped g-C3N4. They also observed faster degradation of RhB under visible radiation. The synergistic roles of S-doping and the creation of unique structures led to an S-doped g-C3N4 system with narrower bandgaps, a broader light absorption range, and larger surface areas than the pristine system, thus yielding enhanced photoreactivity.
The photocatalytic efficiency of the S-doped g-C3N4 system was found to improve with both the in situ and ex situ sulfur doping with a small percentage of the dopant (less than1.0 wt%). The use of thiourea (TU) as an efficient precursor for the construction of an S-doped g-C3N4 system has recently been reported (Hong et al., 2012; Cao et al., 2015). Hong and his colleagues (Hong et al., 2012) first fabricated in situ S-doped g-C3N4 systems using a unit of TU. X-ray photoelectron spectroscopy analysis revealed the possibility of the dopant to substitute C with a likely downshift of 0.25 eV in the CB. Remarkably, the prepared S-doped g-C3N4 system performed 30 times more than the pristine system for H2 production. The excellent photo reactivity was attributed to extended and stronger light absorbance by S-doping and increased charge and mass transfer in the mesoporous system.
Some research works proposed sulfur doping to replace the lattice nitrogen atoms instead of the carbon atoms in the g-C3N4 framework to form an S—C bond. Considering the similarity of the electronegativities of nitrogen and sulfur, the substitution seems to be favorable. This notion has been substantiated by first-principles density functional theory calculations in the work of Ma et al (Ma et al., 2012). The DFT works demonstrated the presence of impurity states as a consequence of sulfur doping. However, the bandgap was not significantly affected. Therefore, this makes it easy for the photogenerated electrons to jump from the valence band to the impurity states and then to the conduction band. In summary, both theoretical and experimental works revealed that sulfur incorporation into the framework of g-C3N4 modified the electronic properties of the materials leading to enhanced carrier mobility, efficient charge separation, and narrowed bandgap.
7.2.3 Oxygen doping
Li and coworkers first fabricated O-doped g-C3N4 via a simple H2O2 hydrothermal route (Li et al., 2012). X-ray photoelectron spectroscopy studies revealed that O was transmitted directly into the lattice forming N—C—O, thus indicating that oxygen atoms bonded with Sp2 hybridized C. Interestingly, while the oxygen doping does not alter the valence band maximum, the conduction band minimum exhibited a downshift by 0.21 eV. Hence, the oxygen-doping on g-C3N4 could trigger band and electronic structure modifications, which in turn lead to improved separation efficiency of the photogenerated carriers, extended visible light response, and improved surface area.
Elsewhere, Huang et al. (Huang et al., 2015) fabricated oxygen-doped g-C3N4 with a porous network (MCN). XPS investigations revealed a new peak at 531.4 binding energy which has been attributed to the presence of N—C—O and C—O species within the lattice. First principles simulations showed that the bandgap reduced slightly due to oxygen incorporation, in line with the absorption band edge redshift. Similarly, differential charge density calculations showed a dramatic reduction in the electron density at the surrounding carbon atoms of oxygen dopant as well as a remarkable increase at the surrounding nitrogen atoms. Hence, based on DFT and experimental observations, they reported that oxygen-doping preferentially takes place on 2-coordinated nitrogen positions, and together with a porous network, oxygen-doping promotes charge separation and extended light trapping.
In another study, She and coworkers employed a facile calcination process to prepare two-dimensional porous ultra-thin nanosheets of oxygen-doped g-C3N4 (She et al., 2016). They simultaneously modulated the morphology, band positions of the bulk material, and intrinsic electronic structure. The synthesized ultra-thin O-doped g-C3N4 system demonstrated 5.2 times photocatalytic activity for hydrogen evolution and 71 times higher for MO degradation than that of the bulk system. The photocatalytic performance enhancement is attributed to the synergistic effects of increased bandgaps, the introduction of the electrophilic groups, and the 2D porous ultra-thin framework. Guo et al. (Guo et al., 2016) employed a photo-Fenton reaction to design holey-structured g-C3N4 sheets with O-doping. The prepared oxygen-doped holey nanosheet structure yielded efficient photocatalytic performance for RhB degradation and H2 generation under visible light irradiation due to increased surface area and reduced bandgap. Fig. 12 represents the fabrication route of the 3D holey g-C3N4 using the mixture of dicyandiamide and water heat-treated to get the melamine-cyanaurate complex.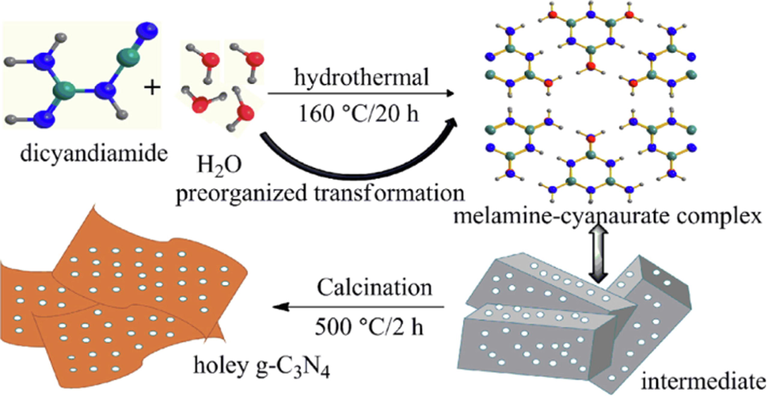
Representation of synthesis of 3D holey g-C3N4 nanosheets. ().
Adapted from Liu et al., 2018
7.2.4 Carbon, nitrogen, or boron doping
Through the density functional theory investigations g-C3N4 system, Dong and coworkers (Dong et al., 2012) observed that the substitution of bridging nitrogen atoms with carbon atoms leads to the creation of delocalized π-bonds among the substituted C atoms and the hexatomic ring, thereby enhancing the electrical conductivity of the material. Moreover, the bandgap of the material would be reduced by the carbon self-doping thereby enhancing light absorption in the visible spectrum. In agreement with the theoretical findings, the fabricated C-doped g-C3N4 exhibited improved absorption in the visible region and demonstrated higher surface area and electrical conductivity, thereby improving the photocatalytic activity. In a similar study by Zhao and colleagues (Zhao et al., 2015), C self-doped g-C3N4 material was prepared for the purification of NO in air. Their study revealed that the developed photocatalyst showed enhanced carrier separation, extended light absorption, and more surface area. Zhang and his team (Zhang et al., 2015) used glucose and melamine as precursors to prepare nanosheets of C-doped g-C3N4 using a facile hydrothermal approach. The introduction of C into the CN framework led to improved absorption of light due to reduced bandgap, and also enhance the electrical conductivity because electron transfer was favored by the delocalized big π -bonds.
Elsewhere (Fang et al., 2015), nitrogen self-doped g-C3N4 was fabricated from melamine and hydrazine via the thermal condensation route. X-ray photoelectron spectroscopy investigations demonstrated that nitrogen substituted the sp2 carbon atom. The UV–vis spectroscopic studies revealed that nitrogen doping decreases the bandgap of the material and weakens the red-shift of the light adsorption band edge of the photocatalyst. The EPR results confirmed that nitrogen self-doping has successfully modified the electronic structure of g-C3N4. Interestingly, nitrogen incorporation significantly improves charge carrier mobility and promoted the separation of photogenerated electrons and holes. Consequently, nitrogen-doped g-C3N4 demonstrated more capacity in photocatalytic hydrogen production under visible light than pure g-C3N4 material.
Yan et al. (Yan et al., 2010) prepared the first boron-doped g-C3N4 by heat-treating the mixture of boron oxide and melamine. Typically, they heat-treated the precursors in a furnace to 500 °C temperature for 2 h and further heated the mixture at increased temperature for another 2 h. The pristine g-C3N4 powder was synthesized using simple pyrolysis. Several other starting materials were used to synthesize B-doped g-C3N4 for effective B incorporation. For example, an enhancement in CO2 reduction was achieved by Sagara and coworkers (Sagara et al., 2016) when they used BH3NH3 as the boron source. Lu and coworkers (Lu et al., 2016) employed co-polycondensation H3BO3 and thiourea to fabricate boron-doped g-C3N4 photocatalytic material. They observed a significant enhancement in visible light absorption and a simultaneous reduction in the bandgap. Therefore, carbon, nitrogen, and boron are essential elements that can be used to improve the photocatalytic performance of graphitic carbon nitride-based compounds.
7.2.5 Halogen doping
The photocatalytic performance of g-C3N4 was enhanced by incorporating several halogen species as dopants. For example, Wang and colleagues (Wang et al., 2010), fabricated fluorine-doped g-C3N4 using ammonium fluoride as the fluorine source. Owing to the difference in electronegativity of fluorine and nitrogen, the incorporated F binds easily with C instead of N thereby causing C-sp2 to sp3. They observed a decrease in the energy gap from 2.69 to 2.63 eV. On the other hand, first principles investigations revealed that the introduction of fluorine at the bay C site shifts both the conduction band and valence band to upper energy levels. The fluorine-doped g-C3N4 photocatalyst exhibit 2.7 times more performance than the pristine material for hydrogen evolution application.
In another work, Zhang and his team (Zhang et al., 2014) used ammonium iodide and dicyandiamide to fabricate iodine-doped g-C3N4 via the co-condensation route. Their results indicated that iodine incorporation enhances the separation of photogenerated electrons and holes, increased optical absorption, broadens the surface area, and consequently stimulated hydrogen evolution. First principles investigations revealed that iodine preferentially substitutes the sp2-bonded nitrogen in the C—N framework. The π-conjugated system was extended by the interaction between carbon nitride and iodine, this could be helpful for the transfer of generated charge electrons and holes. In another fascinating work by Han and colleagues (Han et al., 2015), a simple ball milling of bulk g-C3N4 catalyst with iodine yields nanosheets of I-doped g-C3N4. They observed an upshift of both the conduction band minimum and valence band maximum depicting a reduction in bandgap and alignment of energy levels. The I-modified g-C3N4 demonstrated a significant increase in photocatalytic H2 evolution under visible light due to the enhanced charge separation, increased light absorption, enlarged specific surface area, and better-aligned energy levels.
Elsewhere, Lan et al. (Lan et al., 2016) prepared a bromine-doped g-C3N4 photocatalyst using ammonium bromine and urea as precursors via a co-condensation route. Their work showed that bromine incorporation into the framework of g-C3N4 has indeed modulated the carrier separation efficiency, electron conductivity, optical absorption, and texture. They also achieved high stability under visible light and recorded twice the H2 production rate with the optimal sample compared to the unmodified catalyst.
7.3 Codoping
The method of cooping combines the advantages of individual dopants to yield a better photocatalyst. The technique has recently generated widespread interest among researchers working on the g-C3N4 photocatalyst because of the positive impacts it showed on the optical and structural properties. For example, Hu and coworkers (Hu et al., 2014) conducted a metal/non-metal doping on g-C3N4 material using diammonium hydrogen phosphate, ferric nitrate, and dicyandiamide as precursors to yield phosphorus and iron co-doped g-C3N4. It was observed that iron atoms coordinated with the nitrogen atoms at the interstitial sites of the N pots of the material. Meanwhile, P atoms formed a P—N bond at the interstitial position of the g-C3N4. The enhanced photocatalytic performance was ascribed to the combined effects of P and Fe doping which halted the crystal growth of g-C3N4, improved carrier separation, narrowed the bandgap, and enhance the surface area of the photocatalyst.
Furthermore, Zeng and coworkers (Zhang et al., 2014) prepared a g-C3N4 co-doped with Fe and C to reduce the bandgap and extend visible light absorption with a more positive valence band. Their results showed that the developed photocatalyst displayed improved photocatalytic activity under visible light irradiation for the degradation of RhB compared with the pristine and single-specie-doped g-C3N4. The significant improvement could be ascribed to an increase in electrical conductivity and a more positive valence band, increased surface area and charge separation, and reduction in bandgap which ultimately facilitated visible light absorption.
Apart from the metal/non-metal co-dopants, some works also reported non-metal/non-metal co-dopants as a substitute. For instance, Ma and colleagues (Ma et al., 2015) fabricated oxygen and phosphorus co-doped g-C3N4 for improved photocatalytic performance under anoxic conditions. Their performance enhancement was also attributed to features similar to the work of Zeng et al. (Zhang et al., 2014). In another paper by Wang and Lin (Lin and Wang, 2014), boron/fluorine codoped g-C3N4 was prepared by polymerizing urea with an ionic liquid. XPS analysis showed that both F and B heteroatoms have been incorporated into the material’s matrix by the formation of B-F and B-N bonds. They attributed the enhanced hydrogen production rate to higher electron-hole separation and enhanced optical harvesting.
Recently, some works reported the doping of g-C3N4 with three different heteroatoms. For example, Ma and coworkers (Ma et al., 2015) developed a novel S-Co-O tri-doped g-C3N4 through a hydrothermal approach in the absence of H2O2. They first synthesized S and Co codoped g-C3N4 by annealing the mixture of Co(NO3)2·6H2O and thiourea and then conducted hydrothermal treatment to acquire S-Co-O tri-doped photocatalyst. They observed a modification of the bandgap, separation efficiency of charge carriers, and the surface area of the photocatalyst.
Noteworthy, apart from improving the adsorption ability of g-C3N4, oxygen doping creates photogenerated holes for the degradation of RhB by capturing the generated electrons. In summary, while cooping and tridoping improve the photocatalytic activity of the developed catalyst, it is remarkable to note that excessive doping of these materials on the g-C3N4 may degrade the photocatalytic performance due to the creation of more defects for charge carrier recombination.
7.4 Heterojunction based on doped g-C3N4
In general, elemental doping of g-C3N4 has been considered a remarkable way to enhance its photocatalytic activity by modifying its surface area and modulating its electronic properties (Koutsouroubi et al., 2022; Chen et al., 2022; Mo et al., 2022; Xu et al., 2022; Ma et al., 2022; Saeed et al., 2022; Choudhury et al., 2022; Che et al., 2022; Verma et al., 2022). The heterojunction development has always been adopted to facilitate the separation of charge carriers to prevent carrier recombination. The simultaneous effect of employing doping and heterojunction engineering is expected to yield excellent photocatalytic performance by reducing the bandgap, improving carrier separation, and widening the surface area for effective visible light harvesting.
Many research works have reported on the heterojunction engineering of g-C3N4 (Kong et al., 2022; Altan et al., 2022; Ma et al., 2022). Wide bandgap inorganic semiconducting oxides have been useful in the heterojunction engineering of g-C3N4 catalysts based on their band energy levels. They would promote the separation of photogenerated electrons and holes. Titanium dioxide is one of the deeply studied photocatalysts owing to its low-cost, chemical stability, and suitable conduction and valence band positions for redox reactions. However, its employability is halted by its relatively large bandgap that does not utilize the visible portion of the light. Bu et al. (Bu and Chen, 2014) synthesized nanostructured-oxygen-doped C3N4@TiO2 composites. They found that a significant reduction in carrier recombination can be achieved due to the formation of interfacial chemical bonds between TiO2 and O-C3N4 which serves as a medium for the transfer of photogenerated electrons.
Elsewhere, Raziq and colleagues (Raziq et al., 2016) fabricated nanosheets of B-doped g-C3N4 and its composite with nanostructured TiO2. The resulting nanocomposite exhibited superior photocatalytic performance compared to the pristine and B-doped g-C3N4. They ascribed this to the improved separation of electrons and holes following B incorporation and subsequent TiO2 coupling. The boron-induced states close to the top of the valence band trapped holes, and they created heterojunctions to channel electrons from boron to CN to TiO2. Al2O3 is yet another semiconducting material that has been used as catalyst support owing to its broad bandgap, good thermal stability, high specific surface area, and chemical stability. Wang and his team (Wang et al., 2016) combined H2O2-treated g-C3N4 (O-g-C3N4) using the hydrothermal technique. Their investigations revealed that the defects sites in Al2O3 led to a dramatic enhancement in charge separation which consequently increased the photocatalytic performance for water splitting. In addition, Luo and colleagues (Luo et al., 2015) developed CeO2/P- C3N4 photocatalyst by incorporating phosphorus and then coupling it with cerium dioxide. Their study found that the developed catalyst performed 7.9 times higher than pristine g-C3N4 and 12.2 times higher than pure cerium dioxide. They ascribed the improvement to the extended absorption of light from the visible range and increased separation efficiency of the photogenerated electrons and holes.
Li and coworkers (Li et al., 2016) fabricated DyVO4/g-C3N4I composite semiconducting material using a facile hating process. They utilized the advantage of the narrow bandgap of DyVO4 (2.3 eV) and the fact that it exhibits strong absorption in the visible region. The evolution of hydrogen was 1.7, 4.7, and 10.6 times higher than that of g-C3N4I, g-C3N4, and DyVO4 respectively. The notable photocatalytic activity enhancement was attributed to enhanced separation efficiency, improved visible light absorption, reduced bandgap, and increased specific surface area.
Apart from wide bandgap semiconductors, several semiconducting materials have been utilized to modify g-C3N4 for improved photocatalytic activity. These materials include zinc phthalocyanine (Liang et al., 2016); Zn0.8Cd0.2S (Tian et al., 2016); BiPO4 (Yuan et al., 2014), BiVO4 (Kong et al., 2016), ZnIn2S4 (Chen et al., 2016), and many others.
7.5 Carbon dots modified g-C3N4
Firstly, we introduced the various functions carbon dots (CDs) can adhere to on g-C3N4 surface in the field of photocatalysis applications which contain spectra converter, electron acceptor and mediator, and photosensitizer. Fast generation of charge carriers is one of the key subjects in photocatalysis after the absorption of suitable photons over photocatalysts. For this reason, overpowering the recombination of these charges is among the significant parameters to enhance photocatalytic capability. An increase in photocatalytic ability in g-C3N4/CDs-based nanocomposite is achieved using CDs to slow down the recombination of electron-hole pairs. Therefore, the applications of CDs as spectral converters are on the principle of their extraordinary feature of multi-photon irradiation. The UCPL is a feature that the applied excitation wavelength is greater than the PL emission wavelength. That is to say, the lights with high wavelengths can be transformed into lights with low wavelengths to generate charge carriers, increase photocatalytic ability, and enable the utilization of CDs for various purposes. Moreover, the CDs can serve as an electron mediator when the arrangement of electrons between the semiconductors is a Z-scheme photocatalytic system or a type-II heterojunction. CDs function as suitable electron conduction mediation in such mechanisms to impressively enhance the photocatalytic ability and this is due to their best electron acceptor/donor feature.
As a result of the foregoing, g-C3N4/CDs-based nanocomposites can contribute significantly to photocatalytic activities in a variety of applications. Linked CDs with g-C3N4 play a key role in the degradation of different pollutants, generation of H2, and reduction of CO2, as will be explored in greater depth in the following sections.
7.6 Degradation of contaminant by g-C3N4/CDs-based photocatalysts
Rapid population increase and major industrialization have resulted in the introduction of hazardous, toxic, and limitless contaminants into the environment that not only exacerbates environmental issues but poses a risk to human life and health (Singh et al., 2022; Guo et al., 2022; Ni et al., 2022; Praus, 2022). Therefore, photocatalytic degradation of contaminants is globally recognized as a popular research topic for environmental preservation and societal sustainable growth. In this respect, the use of g-C3N4/CDs-based nanocomposites used as novel hybrid photocatalysis has in recent times attracted a lot of attention because of their potential for removing a wide range of contaminants (Li et al., 2018; Xiang et al., 2020). Considering this, Zhang et al. (Zhang et al., 2016) fabricated a binary g-C3N4/CDs-based nanocomposites using a simple impregnation thermal technique via coupling of various fractions of CDs mole with g-C3N4. The photocatalyst that contains 0.5 % of CDs resulted has displayed the most superior photodegradation ability of phenol compared to other photocatalysts. The phenol elimination in this photocatalyst had a 3.7-folds premier rate in comparison with g-C3N4. The activity of photocatalyst can be finally improved through excitation of the up-converted light in g-C3N4 since CDs has the UCPL feature that could convert long wavelengths into short wavelengths of light that is less than 460 nm. As a result, electrons migrate from the CB of g-C3N4 to CDs, facilitating charge separation. The stability of the g-C3N4/CDs photocatalyst was also significantly improved, with the nanocomposite's photocatalytic ability remaining great following five consecutive photocatalytic recoveries under visible light. In another work, Fang et al. (Fang et al., 2016) studied the photoactivity of g-C3N4 reformed with CDs that were produced using dicyandiamide and CDs as precursors in a new process and used to eliminate RhB and create H2, respectively, in the presence of UV and visible light. The findings of this study showed the performance of CDs (0.25 wt%)/g-C3N4 was approximately 3-folds larger than the single g-C3N4. Also, Zhang et al. (Zhang et al., 2017) integrated carbon quantum dots (CQDs) with g-C3N4 nanosheets with the exceptional ability for evaluating the photoelectrocatalytic characteristics of the nanocomposites stated in the removal of MB under visible light. The results showed that the photo-electrocatalytic performance is significantly affected by the surface hybrid heterojunction structures between g-C3N4 nanosheets and CQDs.
In other work, urea and sugarcane juice were used as precursors by Sim et al. (Sim et al., 2018) to fabricate g-C3N4/CDs nanocomposite via the hydrothermal method. Bisphenol A (BPA) degradation showed enhanced photocatalytic performance in comparison to g-C3N4 under irradiation from natural sunlight. Furthermore, a superior BPA elimination rate was displayed by this binary photocatalyst, and this was 3.87-fold premier more than that of g-C3N4. Eventually, a carbon precursor specifically human fingernails serving as low-cost organic waste was used by Tai et al 2018 to prepare CQDs/ g-C3N4 through the hydrothermal method. High performance for 2, 4-dichlorophenol (2, 4-DCP) degradation which is relative to the sample of immaculate g-C3N4 was displayed by the fabricated CQDs/g-C3N4. Therefore, it can be deduced that the stated outstanding efficiency obtained by research workers was because of CDs' ability to serve as electron sinks, also slowing down the recombination rate of e-/h+ pairs and improving their separation rate. CDs also operate as a photosensitizer to sharpen g-C3N4, as evidenced by UV–vis DRS results, resulting in a broad absorption spectrum from the light source. More electrons are produced because of this broad spectral absorption, which boosts photocatalytic activity.
A research group of Habibi-Yangajeh (Asadzadeh-Khaneghah et al., 2018) conducted a study that proved the degradation of pollutants like MB, MO fuchsine, RhB, and unexpectedly Cr (VI) photoreduction, as a typical heavy metal ion pollutant, applying g-C3N4 nanosheet/CDs (CNNs/CDs) linked to BiOX (X: Br, I), were superior to that of g-C3N4 under visible light. The presence of the formation of an interface between the components of BiOX, CDs, and CNNs was confirmed. The improved activity relative to the sample of g-C3N4 is about 129 times from the photodegradation of RhB by the best composite that contains CNNs/CDs/BiOBr (20 %). Electrons are excited to the CBS of CNNs and BiOBr by the utilized light in the sample of CNNs/CDs/BiOBr (20 %). Subsequently, by satisfying the Z-scheme mechanism, the electrons from CB are transmitted to CDs, thereafter to the VB of BiOBr. Hence, a dramatic reduction in the recombination rate is caused by electrons that gather in the holes in the VB of BiOBr and CB of CNNs as presented (Fig. 13).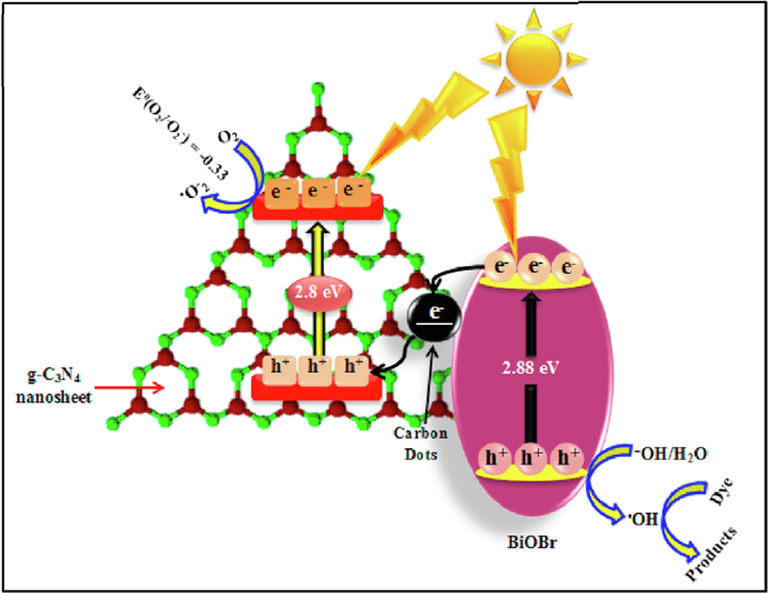
Proposed mechanism in the form of Z-scheme for the CNNS/CDs/BiOBr photocatalysts. ().
Adapted from Asadzadeh-Khaneghah et al., 2018
The preparation of graphene oxide/g-C3N4/CDs was also carried out by Prakash et al. (Prakash et al., 2019) to study its effectiveness via hydrothermal technique on the photodegradation of crystal violet (CV) and RhB. Maximum photoactivity was shown by the GO/g-C3N4/CDs nanocomposite under the illumination of visible light. Apart from that, a Z-scheme photocatalyst was formed from the addition of CDs that is doped with nitrogen over the g-C3N4/Ag3PO4 nanocomposite. When compared with binary g-C3N4/Ag3PO4 under visible light, the ternary-prepared heterojunction photocatalyst showed an enhanced photoelectrocatalytic performance for phenol, RhB, and MB degradations. The rise in light-harvesting capacity, acceleration of electron transfer, and activation of molecular oxygen is more significantly achieved by the presence of NCDs and the impressive amelioration in the ability of g-C3N4/Ag3PO4/NCDs photocatalyst was given to the Z-scheme. The rate of charge recombination and the transfer of interfacial charges of the stated nanocomposites has exhibited a more powerful photocurrent response than those of the binary and pure ones. A superior photoactivity by the ternary photocatalyst over the binary ones was displayed due to the subsequent addition of NCDs that improve the performance of visible-light absorption.
In a study by Dadigala et al. (Dadigala et al., 2017), a photochemical reduction technique was used to integrate g-C3N4 nanosheet/CDs nanocomposite with Ag nanoparticles (Fig. 14). The stated photocatalyst displayed superior ability when the visible light efficiency of the optimized sample of AgNPs/CDs/CNNs that has 2 wt% of CDs was evaluated for p-nitrophenol (PNP) and MO degradation when compared to that of the single counterpart. This was 5.8 and 11.5 -folds premier than that of the degradation of PNP and MO respectively for CNNs. Broad visible-light absorption was a result of the sensitization of CDs and also the recombination of e-/h+ pairs, which was caused by the movement of electrons in the AgNPs/CDs/CNNs nanocomposite from CNNs approaching AgNPs and CDs.
Schematic diagram of the photocatalytic process over the AgNPs/CDs/CNNs. ().
Adapted from Dadigala et al., 2017
Jian et al. (Jian et al., 2016) used an electrostatic adsorption approach with positively charged HpCN and negatively charged CQDs to remove MB under visible-light irradiation using CQDs and proton functionalized g-C3N4 (CQDs/HpCN). The electrostatic self-assembly approach, according to this study group, is a good way to make a well-dispersed HpCN/CQDs heterojunction. The heterostructure was used to accelerate the decomposition of MB in this study, which took 90 min. The enhancement was attributed to a strong absorption peak in the visible region between 400 and 800 nm, indicating that CQDs/HpCN can absorb more energy from visible light, resulting in increased photocatalytic activity.
In related work, Jiang et al. (Jiang et al., 2018) suggested a technique to raise the effectiveness of the visible-light harvesting performance of a fabricated hybrid photocatalyst hybrid (i.e., utilizing Cds quantum dots (Cds QDs), g-C3N4, and CQDs). This was achieved by integrating the CDs with CdS/g-C3N4 and the occurrence of a red shift due to the light absorption trait of CdS QDs and CDs. The CDs conductivity, in addition to the diversity in CB energy levels of the introduced nanomaterials, is the most important factor that enhances the performance of the ternary photocatalysts. The conductivity of the CDs serves as an electron-transfer mediator for the electron that moves from g-C3N4 to the surface of the components. Here, an appropriate passway was promoted and produced by the systematic movement between the components using CDs and the separation of charges. Due to the cohabitation of CDs and CdS QDs on the g-C3N4, these major improvements also resulted in rectified visible-light harvesting and, as a result, premier photoactivity of ternary nanocomposites for organic pollutants destruction.
In another example, Liu et al. (Liu et al., 2017) applied a simple impregnation technique to fabricate a Fe(III)/CDQs/Fe doped with g-C3N4 (Fe(III)/CDQs/Fe- g-C3N4) which has been used for several environmental purification purposes. These developed nanocomposites were created using a simple impregnation process. Over the Fe-g-C3N4, the presence of CQDs with a size of 2–5 nm was well-recognized. The phenol and MO removal ability by the Fe(III)/CQDs/Fe-CN photocatalyst showed good photoactivity when compared to g-C3N4 Fe-doped g-C3N4, CQDs/Fe-g-C3N4, and Fe(III)/Fe-g-C3N4. This increase was attributed to improved light absorption, shorter e-/h+ pair movement distances, and OH being confirmed as a main active component.
7.7 g-C3N4/CDs-based photocatalysts in degradation of different antibiotics
Human health and the environment are at risk due to the presence of antibiotics and other surfacing contaminants in drinking and surface waters. This is a result of their non-biodegradable nature and their ability to remain in the environment for a long time. Thus, several research works have been published that resulted in several growing pieces of literature with regard to the removal of antibiotics. Wang et al. (Wang et al., 2017) investigated the removal of antibiotics by the utilization of g-C3N4/CDs-based nanocomposite. This was achieved via the fabrication of N-doped CDS(NCDs)/ g-C3N4 nanocomposite. A small amount of NCDs (1.0 wt%) was introduced to raise the degradation ability of indomethacin (IDM) under visible light illumination. The ability of the photocatalyst to respond to visible light is enhanced as a result of the effect that NCDs have, which has been established extraordinarily by UV–vis DRS. A decrease in the energy gap was remarkably illustrated by the NCDs/g-C3N4 (i.e., after the addition of NCDs) when compared with g-C3N4. Furthermore, this substantial progress is a result of the premier duty of the nanocomposite that has been formed in the migration of charges and boosted absorption in the visible area. A diagram of the simplified energy band of the NCDs /g-C3N4 photocatalyst is demonstrated in Fig. 15. The probability to recombine charge carriers was slowed down and the photocatalytic ability in the degradation of IDM remarkably improved according to the described mechanism.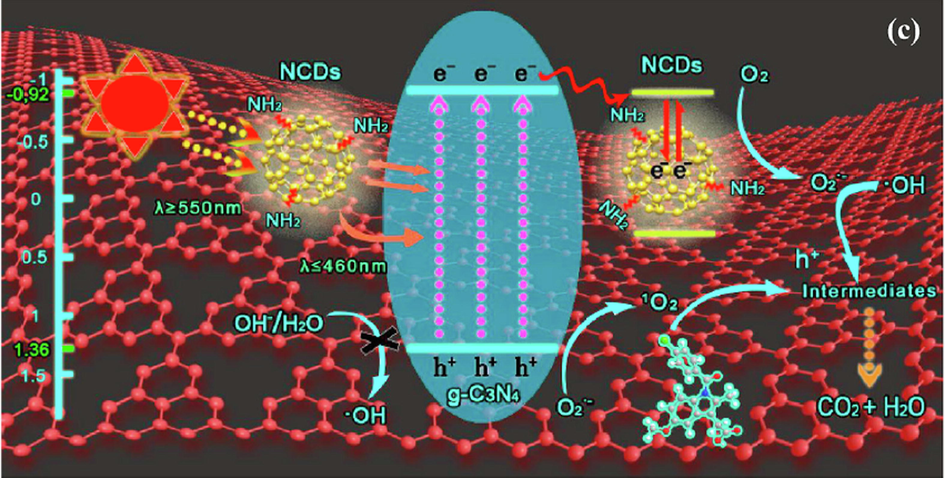
The proposed photocatalytic mechanism in the Nitrogen-doped CDs/g-C3N4. ().
Adapted from Wang et al., 2017
To increase the degradation ability for tetracycline (TC) treatment, the fabrication of hybrid CDs/g-C3N4/ZnO was carried out by Guo and coworkers (Guo et al., 2017) via an easy thermal impregnation process. The preparation of the CDs/g-C3N4/ZnO photocatalyst was done and compared with other species. As the ideal photocatalyst, superior ability in the removal of this pollutant was particularly shown by CDs/g-C3N4/ZnO with the solution of CDs. After 30 min of visible light illumination, the ideal nanocomposite can make TC fully decomposed. The CDs on the g-C3N4/ZnO photocatalyst are the major driver of excellent ability. It also not only boosts the ability to respond to visible light but also makes the e-/h+ pair separation ability efficient and this is due to its feature of electron migrations. Likewise, Xie et al. (Xie et al., 2018) reported that the TC photodegradation rate was improved over the CDs/g-C3N4/ZnO nanocomposites. The Z scheme heterojunction structure, the high separation capacity of e-/h+ pairs, and the superior ability of UCPL are the synergistic effect behind this improvement. The literature demonstrated an excellent photocatalytic efficiency for TC degradation by the CDs/g-C3N4/MoO3 photocatalyst when compared with MoO3/g-C3N4 nanocomposite and the original g-C3N4 under UV–vis irradiation. The Proposed mechanisms for the photocatalytic elimination of TC under visible light over the CDs/g-C3N4/MoO3 composites are presented in Fig. 16.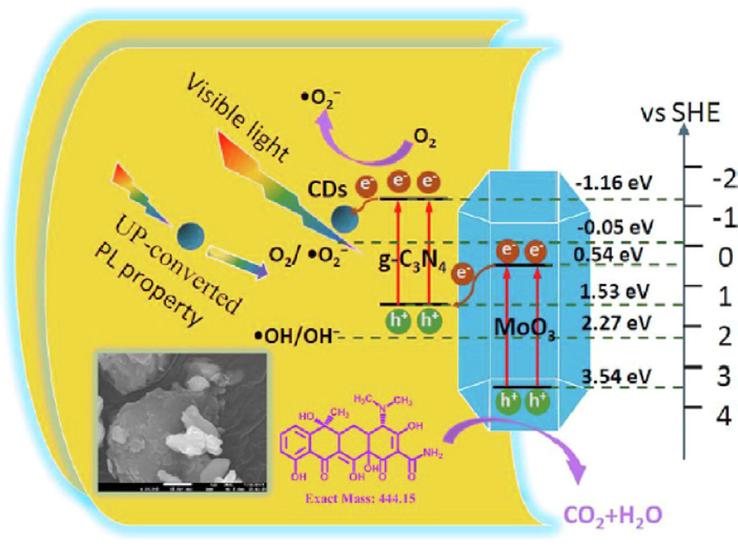
Proposed mechanisms for the photocatalytic elimination of TC under visible light over the CDs/g-C3N4/MoO3 composites. ().
Adapted from Xie et al., 2018
Similarly, the Z-scheme system is responsible for the enhanced photocatalytic process after the irradiation of UV–vis. Because of the UCPL properties of CDs, long-wavelength lights can be converted to short-wavelength lights, allowing MoO3 and g-C3N4 semiconductors to be effectively stimulated by the absorption of the up-converted lights. The separation of charges is afterward improved due to the Z-scheme heterojunction produced as a result of the migration of generated electrons from CB of MoO3 to VB of g-C3N4. Furthermore, the photocatalytic ability is raised by the transfer of electrons gathered in g-C3N4 following the slowing down of the charges from recombination.
In another study, the extraordinary photocatalytic ability was witnessed in the company of 1.0 wt% CDs by Su and colleagues (Su et al., 2017) via the fabrication of CDs/TiO2/g-C3N4 nanocomposites. This photocatalyst is substantially better at degrading enrofloxacin (ENF) because of its efficiency in separating the CDs charges, efficiency in migration of electrons, and distinctive UCPL feature. The up-converted emissions were strikingly seen within the 350 nm to 600 nm region and CDs are excited from 600 nm to 900 nm are the premier UCPL feature. These occurrences show that CDs can step up the photocatalytic activity by converting wavelengths of light that are near infra-red (NIR) to wavelengths that are of visible light, and this remarkably improves the nanocomposite’s light utilization. The stated UCPL feature of CDs can also be used by TiO2/g-C3N4 photocatalyst nanocomposite to convert lights with NIR wavelengths to lights in the visible-light region following the direct introduction of the Z-scheme mechanism. In this place, CDs exist to facilitate the mechanism of Z-scheme movement. The active components in the common mechanism that is heterostructured can convert ENX into some ultimate materials (like CO2 and H2O) or significantly get rid of it to form inorganic compounds. In other research work, combined calcination of silica colloid, CQDs, and cyanamide was performed by Wang and coworkers (Wang et al., 2018) to prepare g-C3N4/CQDs composite that is mesoporous. For photodegradation of fluoroquinolone antibiotics, the stated composite in this literature presented a degradation efficiency of about 1.7 and 2.7- folds superior to the mpg-C3N4 under visible light with a wavelength greater than 420 nm and simulated sunlight with a wavelength greater than 290 nm illuminations, respectively and this is the most superior photocatalytic performance. The unique UCPL features of the CDs, the effective charge separation, and the high surface area of mpg-C3N4 are the major reasons behind the enhancement of this photoactivity. Similarly, Zhang et al. (Zhang and De Zhang, 2019) showed that the removal of CIP by g-C3N4 photocatalyst which has a deficiency of CDs@nitrogen (CDs@ND-g-C3N4) has a photocatalytic ability that is superior to that of fresh g-C3N4. They associated the addition of nitrogen defects and CDs reduce the recombination of the e-/h+ pairs as well as incense the nanocomposite’s absorption capacity to visible light which are responsible for boosting the photocatalytic ability of CDs@nitrogen-g-C3N4 photocatalyst.
7.8 g-C3N4/CDs-based nanocomposite in H2 generation
As a favorable energy source, the use of a photocatalyst and solar energy is introduced to generate H2 as another fuel from H2O. This will also reduce the over-dependence on fossil fuel reserves (Fattahimoghaddam et al., 2022; Shahsavandi et al., 2022; Zhao et al., 2022; Zhang et al., 2022; Cao et al., 2022; Martinez et al., 2022; Alaghmandfard and Ghandi, 2022; Huang et al., 2022; Hao et al., 2022). The amount of energy density of H2 is significantly higher in comparison to that of petrol and diesel (hydrocarbons). In the past few years, there has been a large number of increased expansions in the performance of g-C3N4-based photocatalysts for water-splitting applications. The electron-hole pairs are transferred towards the surface reaction area on the CDs or g-C3N4 in photocatalytic water splitting to generate H2 and O2 under the illumination of light over the g-C3N4/CDs photocatalyst without recombination. This can change the adsorbed H2O molecules to gaseous O2 and H2. By introducing 7 mL of CQDs ethanol solution to g-C3N4 nanosheets (CNNS), Li et al. (Li et al., 2016) found that the rate of H2 production was 116.1 μmol h−1, which was three times higher than that of plain CNNS (37.8 μmol h−1). The increased photocatalytic ability is because of the discovered CQDs PL spectra to appropriately quench electrons which leads to promoting charge separation of the e-/h+ pairs in CNNs. The construction of heterojunctions was demonstrated in a research work by Qin et al. (Qin et al., 2017) to produce Ag NPs/CQDs/g-C3N4 heterostructures of CQDs/g-C3N4 and Ag NPs. The fabricated photocatalyst was analyzed using SEM and it confirmed the attachment of Ag NPs and CQDs nanoparticles over the g-C3N4 a. The UV–vis DRS of the 6 mL CQDs/g-C3N4 (6CCN) and 3 wt% Ag NPs/6 mL CQDs/g-C3N4 (3S6CCN) nanocomposite showed a redshift to visible light, indicating excellent coupling of CQDs with g-C3N4 and Ag NPs, as well as the presence of surface plasmon resonance from silver nanoparticles. Furthermore, in the interface regions, the synergistic action of CQDs, Ag NPs, and g-C3N4 can ease electron production, separation, and transfer, hence increasing photoactivity.
In comparison to the binary photocatalyst g-C3N4/TiO2, the ternary CDs/g-C3N4/TiO2 (CGT) nanocomposite likewise was used to generate H2 and the investigators reported that the positive effects of CDs, as well as the formation of heterojunction between TiO2 nanosheets and g-C3N4, are the fundamental reasons behind the improved photoactivity. Thereby, face-to-face orientation and strong integration of TiO2 and g-C3N4 nanosheets can be provided by the well-defined heterojunction and application CDs as electron traps (electron reservoirs). This improves the photocatalytic ability to generate H2 because of the slowing down of the recombination of charges. One more interesting ternary photocatalyst that demonstrated an impact on H2 generation is the g-C3N4 which is doped with CDs/MoS2 quantum dots (g-C3N4/NCDs/MoS2QDs). This is because of the formation of the Z-scheme mechanism among g-C3N4 and NCDs, which remarkably facilitate the separation and migration of charge carriers.
7.9 H2O2 production using g-C3N4 photocatalyst
Hydrogen peroxide has been extensively employed in the paper industry, chemical synthesis, disinfection, textile bleaching, and production of rocket fuel (Pattappan et al., 2022). More than 95 % of the H2O2 produced industrially is produced via the anthraquinone method, namely the Riedl-Pfleiderer process. Utilizing catalysts made of precious metals, this procedure is carried out in organic solvents. Nevertheless, it has a lot of shortcomings: (i) The hazardous nature of the organic solvents utilized in preparation. (ii) The price of precious metal catalysts is high. (iii) Both hydrogenation and oxidation require a significant amount of energy. (iv) The industrial process produces a large amount of waste, which causes environmental damage (Yang et al., 2019). Thus, it is necessary to look for alternative effective, clean, and secure H2O2 preparation techniques·H2O2 was said to be produced directly from oxygen and hydrogen in literature. The explosive risk associated with this technique, which involves the direct interaction of hydrogen and oxygen, makes it challenging to use in large-scale industrial manufacturing. Another method is to use ultrasonic chemistry to prepare H2O2 by separating oxygen and water. However, the long sonochemical exposure duration could result in H2O2 decay and lower the yield. Recently, solar-powered photocatalysis for the creation of H2O2 has garnered a lot of interest. This method uses plentiful water and oxygen as raw materials to create H2O2 using semiconductor photocatalysts, as opposed to employing an explosive mixture of hydrogen and oxygen. Throughout the entire production process, no pollutants are produced. Consequently, it is thought that photocatalytic H2O2 production is a potential technique.
With water and oxygen as the raw materials, photocatalytic H2O2 synthesis is an upward process, and the associated Gibbs free energy is 117 kJ mol-1 (Wang et al., 2016). One-step two-electron reduction and two-step single-electron reduction are the two primary processes used in the synthesis of H2O2 via photocatalysis. Numerous studies demonstrated the potential of g-C3N4 for photocatalytic H2O2 generation, but their activity is severely constrained by the unfavorable recombination of photoinduced excitons. To improve the separation and transfer of photoinduced carriers, numerous efforts have been made. For instance, Yang et al. (Yang et al., 2019) used a chemical etching technique to create Ti3C2 and g-C3N4 nanosheets by protonating bulk g-C3N4 with hydrochloric acid and supplementing it with ultrasound exfoliation. Finally, they used electrostatic self-assembly to create a mixture of Ti3C2 and g-C3N4. The combination of g-C3N4and of Ti3C2 is seen in the TEM image. The Ti3C2/ g-C3N4 composite's ability to absorb light was greatly enhanced by Ti3C2. Additionally, the Schottky connection between Ti3C2 and g-C3N4 considerably aided in exciton dissociation and molecular oxygen activation, which was advantageous for the production of H2O2. Therefore, the most active sample's H2O2 generation rate reached 2.20 μmol/L min−1 or about 2.1 times that of g-C3N4.
8 CO2 mitigation using g-C3N4-based nanocomposite
Among the assured byproducts resulting from the combustion of fossil fuels is carbon dioxide (CO2), which is one of the major greenhouse gases present in the atmosphere due to its constant increase in concentration. It is one of the most fundamental problems that lead to climate change and global warming (Liang et al., 2022; Tseng et al., 2022; Mahdavi et al., 2022; Zhou et al., 2022; Sudhaik et al., 2022; Al-Hajji et al., 2022; Li et al., 2022; Sakuna et al., 2022). Because of the stated environmental problems, numerous research works have been focused to improve societal and industrial standards via the reduction of inorganic compounds, particularly CO2. Its photoreduction to organic fuels and value-added chemicals has been proposed as a means of not only reducing greenhouse gas emissions but also increasing demand for renewable fuels. The developing interest in the photocatalytic conversion of CO2 is notably due to the schematic and feasible nature of the process, and in addition, only light, H2O, CO2, and the right photocatalyst are needed in the process. Therefore, the photoreduction of CO2 in the generation of chemical fuels has been ideally chosen to solve energy and environmental crises. The emergence of a range of products can be possible from the photoreduction of CO2 under light irradiation employed on various photocatalysts, like CO, CH4, CH3OH, and HCOOH which incorporate multi-electron processes. Thus, various semiconductors have been investigated for the photocatalytic reduction of CO2 by several researchers (Liu et al., 2022; Zhang et al., 2022; Li et al., 2022; Liu et al., 2022; Gorai and Kundu, 2022). Because of the specific properties of CDs, such as photosensitization, increased electrical conductivity, and electron-withdrawing effects, g-C3N4 sensitized by CDs has been used to simplify the photocatalytic CO2 transformation in the last six years. Zhao et al. (Zhao et al., 2018) used a photochemical one-step approach to synthesize Au/CDs/g-C3N4 electrocatalysts for CO2 photoreduction. At 0.5 V, the improved electrocatalyst had a significant CO faradic efficiency of 79.8 %. The synergetic effect between Au, CDs and g-C3N4 nanoparticles is the major reason behind the improved photocatalytic ability. Also, the simplified transformation of CO from CO2 can be achieved through the coupling of CDs with g-C3N4, which is a result of its superior ability to adsorb H+ and CO2 and the impressive conductivity of the CDs. The photocatalyst was very active in the photoreduction of CO from CO2 because it has a particularly high surface area (SBET) of 117 m2 g−1. Protonated g-C3N4 and CDs obtained respectively from glucose and urea precursors were hybridized by Ong et al. (Ong et al., 2016) to derive a better performance. The fabrication of the photocatalyst was carried out for 8 h and in the presence of water, vapors produced CH4 and CO with respectively 29.23 and 58.82 μmol∙g-1catalyst as the total amount. Also, the outstanding ability of the photocatalyst ability to separate e-/h+ pairs is confirmed by the considerable trapping intensity presented by the PL spectra, and this was found to have a significant effect on the photocatalyst’s performance. Feng et al. (Scopus - Document details - null | Signed in, (n.d.). https://www.scopus.com/record/display.urieid=2-s2.0-8506987, 2022) investigated the reactivity of CO2 reduction products using a CQDs/g-C3N4 nanocomposite with CQDs with an average size of 2.5 nm. Under the same conditions as the single g-C3N4 nanocomposite, which produced H2, CO, and O2, the CQDs/g-C3N4 nanocomposite produced 6 times more O2, CO and comparable CH4 in the absence of detectable H2. The Nyquist plots depicted effective e-/h+ pairs transfer among the improved photocatalyst components. As a result, electron migration significantly reduced the recombination of charge carriers. The most effective photocatalyst with 2 wt% CQDs had elevated rates of O2, CH4, and CO evolution, as well as a yield greater than g-C3N4. Thus, g-C3N4/CDs systems displayed extremely interesting methods that can be employed for photocatalytic CO2 reduction and provided critical feedback for future advancements.
9 Conclusion and future perspective
The distinguishing properties of graphitic carbon nitride such as suitable bandgap, thermal and chemical stability, visible light absorption, less hazardous, excellent redox ability, polymeric structure, and easy fabrication make it an attractive material for different photocatalytic applications like hydrogen production, CO2 reduction, water splitting and degradation of organic pollutants in wastewater. This review reported the recent advancements and lingering challenges hindering the large-scale utilization of g-C3N4 semiconductor as an efficient visible light-responsive photocatalytic material. Various methodologies used to improve the photocatalytic performance of the compound such as metal and non-metal doping, co-doping, and heterojunction engineering have been discussed in detail. The paper has also highlighted some of the hitherto weaknesses and limitations of the material that requires attention in future works. In summary, metal doping creates a new energy level within the bandgap and enhances the spectral response which can reduce the charge carrier recombination. However, the newly created energy levels serve as recombination centers, thereby decreasing quantum efficiency. The doped ions also exhibit poor thermal stability. On the other hand, non-metal doping serves as an effective technique to modulate the mobility of photogenerated charge carriers and enhance absorbance and redox potentials. Meanwhile, codoping or tridoping combines the advantages of these single dopants and has led to improved photocatalytic activity. Despite the many successes achieved, some challenges need to be addressed in future works. For instance, the mechanism of photocatalytic enhancement by elemental doping, how the defect influences the electronic structure, the nature of the chemical states, and the role and site of the metal ion in heteroatom are not fully understood.
Some aspects in which future research works could be directed include codoping and tridoping as a strategy to integrate the benefits of single dopants. Also, heterojunction and simultaneous doping could improve charge carrier separation for enhanced photocatalytic performance. Furthermore, combining elemental doping with nanostructuring to obtain different morphologies such as nanofibers, nanosheets, nanorods, and nanotubes could be a new direction of research. Finally, innovative doping strategies should be employed to modulate the higher occupied molecular orbital and lower unoccupied molecular orbital with orientation for high oxidation and reduction potential.
Acknowledgment
The authors acknowledge the support received from the Deanship of Research, Oversight and Coordination (DROC), King Fahd University of Petroleum & Minerals (KFUPM), Dhahran, Saudi Arabia under project # INCB2216. We are also thankful to Physics Department and Interdisciplinary Research Center for Construction and Building Materials, KFUPM for supporting this work. M.A. Gondal and Y.S. Wudil are thankful to the DROC, KFUPM for the support received through project # DF201018.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
References
- Efficient strategies for boosting the performance of 2D graphitic carbon nitride nanomaterials during photoreduction of carbon dioxide to energy-rich chemicals. Mater. Today Chem.. 2022;23:100605
- [CrossRef] [Google Scholar]
- Heterogeneous photocatalytic degradation of phenols in wastewater: A review on current status and developments. Desalination. 2010;261:3-18.
- [CrossRef] [Google Scholar]
- A. Alaghmandfard, K. Ghandi, A Comprehensive Review of Graphitic Carbon Nitride (g-C3N4)–Metal Oxide-Based Nanocomposites: Potential for Photocatalysis and Sensing, Nanomater. 2022, Vol. 12, Page 294. 12 (2022) 294. https://doi.org/10.3390/NANO12020294.
- Photocatalytic properties of graphitic carbon nitrides (g-C3N4) for sustainable green hydrogen production: recent advancement. Fuel. 2022;316:123381
- [CrossRef] [Google Scholar]
- R. Alcantara, J. Qian, Y. Liu, W. Zheng, B. Zhou, X. Dong, Covalent Modification of Iron Phthalocyanine into Skeleton of Graphitic Carbon Nitride and Its Visible-Light-Driven Photocatalytic Reduction of Nitroaromatic Compounds, Catal. 2022, Vol. 12, Page 752. 12 (2022) 752. https://doi.org/10.3390/CATAL12070752.
- M. Alejandra Quintana, R.R. Solís, M. Ángeles Martín-Lara, G. Blázquez, F. Mónica Calero, M.J. Muñoz-Batista, Enhanced boron modified graphitic carbon nitride for the selective photocatalytic production of benzaldehyde, Sep. Purif. Technol. 298 (2022) 121613. https://doi.org/10.1016/J.SEPPUR.2022.121613.
- RuO2 nanoparticles-accommodated graphitic carbon nitride for significant enhancement in photocatalytic oxidation of trichloroethylene. Opt. Mater. (Amst).. 2022;125:112086
- [CrossRef] [Google Scholar]
- Impact of Gd substitution on the structure, hyperfine interactions, and magnetic properties of Sr hexaferrites. Ceram. Int.. 2021;47:33853-33864.
- [CrossRef] [Google Scholar]
- O.A. Al-Najjar, Y.S. Wudil, U.F. Ahmad, O.S.B. Al-Amoudi, M.A. Al-Osta, M.A. Gondal, Applications of laser induced breakdown spectroscopy in geotechnical engineering: a critical review of recent developments, perspectives and challenges, Https://Doi.Org/10.1080/05704928.2022.2136192. (2022) 1–37. https://doi.org/10.1080/05704928.2022.2136192.
- Predicting the thermal conductivity of Bi2Te3-based thermoelectric energy materials: a machine learning approach. Int. J. Therm. Sci.. 2022;181:107784
- [CrossRef] [Google Scholar]
- The influence of band bending phenomenon on photocatalytic Suzuki-Miyaura coupling reaction: the case of AgPd alloy nanoparticles supported on graphitic carbon nitride. Appl. Surf. Sci.. 2022;580:152287
- [CrossRef] [Google Scholar]
- The rational design of a graphitic carbon nitride-based dual S-scheme heterojunction with energy storage ability as a day/night photocatalyst for formic acid dehydrogenation. Chem. Eng. J.. 2022;441:136047
- [CrossRef] [Google Scholar]
- Graphitic carbon nitride nanosheets anchored with BiOBr and carbon dots: exceptional visible-light-driven photocatalytic performances for oxidation and reduction reactions. J. Colloid Interface Sci.. 2018;530:642-657.
- [CrossRef] [Google Scholar]
- g-C3N4/carbon dot-based nanocomposites serve as efficacious photocatalysts for environmental purification and energy generation: a review. J. Clean. Prod.. 2020;276:124319
- [CrossRef] [Google Scholar]
- Exploiting the potential of silver oxo-salts with graphitic carbon nitride/fibrous silica-titania in designing a new dual Z-scheme photocatalyst for photodegradation of 2-chlorophenol. Sep. Purif. Technol.. 2022;292:120984
- [CrossRef] [Google Scholar]
- Graphitic carbon nitride/few-layer graphene heterostructures for enhanced visible-LED photocatalytic hydrogen generation. Int. J. Hydrogen Energy. 2022;47:25555-25570.
- [CrossRef] [Google Scholar]
- Effect of oxygen-doped C3N4 on the separation capability of the photoinduced electron-hole pairs generated by O-C3N4@TiO2 with quasi-shell-core nanostructure. Electrochim. Acta. 2014;144:42-49.
- [CrossRef] [Google Scholar]
- Polymeric photocatalysts based on graphitic carbon nitride. Adv. Mater.. 2015;27:2150-2176.
- [CrossRef] [Google Scholar]
- Synthesis and characterization of sulfur self-doped g-C3N4 with efficient visible-light photocatalytic activity. Mater. Lett.. 2015;149:50-53.
- [CrossRef] [Google Scholar]
- Efficient visible light driven degradation of antibiotic pollutants by oxygen-doped graphitic carbon nitride via the homogeneous supramolecular assembly of urea. Environ. Res.. 2022;210:112920
- [CrossRef] [Google Scholar]
- In situ fabrication of NiS2-decorated graphitic carbon nitride/metal-organic framework nanostructures for photocatalytic H2 evolution. ACS Appl. Nano Mater.. 2022;5:5416-5424.
- [CrossRef] [Google Scholar]
- Construction of a 2D layered phosphorus-doped graphitic carbon Nitride/BiOBr heterojunction for highly efficient photocatalytic disinfection. Chem. – An Asian J.. 2022;17:e202200095.
- [CrossRef] [Google Scholar]
- Graphitic carbon nitride-based photocatalysts for biological applications. Adv. Sustain. Syst.. 2022;6:2100294.
- [CrossRef] [Google Scholar]
- Novel mesoporous P-doped graphitic carbon nitride nanosheets coupled with ZnIn2S4 nanosheets as efficient visible light driven heterostructures with remarkably enhanced photo-reduction activity. Nanoscale. 2016;8:3711-3719.
- [CrossRef] [Google Scholar]
- Potassium doped and nitrogen defect modified graphitic carbon nitride for boosted photocatalytic hydrogen production. Int. J. Hydrogen Energy. 2022;47:14044-14052.
- [CrossRef] [Google Scholar]
- Modification of graphite carbon nitride by adding an ultra-micro amount of triaminotriphenylamine for superior photocatalytic hydrogen evolution. New J. Chem.. 2022;46:9057-9063.
- [CrossRef] [Google Scholar]
- NH2-MIL-125(Ti) modified graphitic carbon nitride with carbon vacancy for efficient photocatalytic NO removal. Chemosphere. 2022;307:135660
- [CrossRef] [Google Scholar]
- Regulation on polymerization degree and surface feature in graphitic carbon nitride towards efficient photocatalytic H2 evolution under visible-light irradiation. J. Mater. Sci. Technol.. 2022;98:160-168.
- [CrossRef] [Google Scholar]
- Highly conjugated graphitic carbon nitride nanofoam for photocatalytic hydrogen evolution. Langmuir. 2022;38:1471-1478.
- [CrossRef] [Google Scholar]
- Copper and platinum dual-single-atoms supported on crystalline graphitic carbon nitride for enhanced photocatalytic CO2 reduction. Chinese J. Catal.. 2022;43:451-460.
- [CrossRef] [Google Scholar]
- Interfacial coupling perovskite CeFeO3 on layered graphitic carbon nitride as a multifunctional Z-scheme photocatalyst for boosting nitrogen fixation and organic pollutants demineralization. Chem. Eng. J.. 2022;427:131406
- [CrossRef] [Google Scholar]
- Hematite nanoparticles decorated nitrogen-doped reduced graphene oxide/graphitic carbon nitride multifunctional heterostructure photocatalyst towards environmental applications. New J. Chem.. 2022;46:13100-13116.
- [CrossRef] [Google Scholar]
- Preparation of sodium and boron co-doped graphitic carbon nitride for the enhanced production of H2O2 via two-electron oxygen reduction and the degradation of 2,4-DCP via photocatalytic oxidation coupled with Fenton oxidation. Chem. Eng. J.. 2022;431:134020
- [CrossRef] [Google Scholar]
- M.L. Cohen, Predicting useful materials, Science (80-.). 261 (1993) 307–308. https://doi.org/10.1126/SCIENCE.261.5119.307/ASSET/A3111EC4-4183-4A1D-ADE2-ABFAD03DB4CC/ASSETS/SCIENCE.261.5119.307.FP.PNG.
- Carbon dots and Ag nanoparticles decorated g-C3N4 nanosheets for enhanced organic pollutants degradation under sunlight irradiation. J. Photochem. Photobiol. A Chem.. 2017;342:42-52.
- [CrossRef] [Google Scholar]
- Core-shell covalently linked graphitic carbon nitride-melamine-resorcinol-formaldehyde microsphere polymers for efficient photocatalytic CO2 reduction to methanol. J. Am. Chem. Soc. 2021
- [CrossRef] [Google Scholar]
- In situ fabrication of niobium pentoxide/graphitic carbon nitride type-II heterojunctions for enhanced photocatalytic hydrogen evolution reaction. J. Colloid Interface Sci.. 2022;608:1951-1959.
- [CrossRef] [Google Scholar]
- Carbon self-doping induced high electronic conductivity and photoreactivity of g-C3N4. Chem. Commun.. 2012;48:6178-6180.
- [CrossRef] [Google Scholar]
- W. Fan, B.K. Lok, F.K. Lai, Evaluation of printed heating elements for continuous flow PCR application, Proc. 2016 IEEE 18th Electron. Packag. Technol. Conf. EPTC 2016. (2017) 360–364. https://doi.org/10.1109/EPTC.2016.7861505.
- Nitrogen self-doped graphitic carbon nitride as efficient visible light photocatalyst for hydrogen evolution. J. Mater. Chem. A. 2015;3:13819-13826.
- [CrossRef] [Google Scholar]
- Effect of carbon-dots modification on the structure and photocatalytic activity of g-C3N4. Appl. Catal. B Environ.. 2016;185:225-232.
- [CrossRef] [Google Scholar]
- Coral-like potassium and phosphorous doped graphitic carbon nitride structures with enhanced charge and mass transfer dynamics toward photocatalytic hydrogen peroxide production and microbial disinfection. J. Colloid Interface Sci.. 2022;617:326-340.
- [CrossRef] [Google Scholar]
- Nanoporous sulfur-doped graphitic carbon nitride microrods: a durable catalyst for visible-light-driven H2 evolution. Int. J. Hydrogen Energy. 2014;39:15373-15379.
- [CrossRef] [Google Scholar]
- Photocatalytic performance of alkali metal doped graphitic carbon nitrides and Pd-alkali metal doped graphitic carbon nitride composites. Diam. Relat. Mater.. 2022;125:109006
- [CrossRef] [Google Scholar]
- A. Fujishima, K. Honda, Electrochemical Photolysis of Water at a Semiconductor Electrode, Nat. 1972 2385358. 238 (1972) 37–38. https://doi.org/10.1038/238037a0.
- The stable and elastic graphitic carbon nitride/polyvinyl alcohol photocatalytic composite sponge: simple synthesis and application for wastewater treatment. J. Environ. Chem. Eng.. 2022;10:107814
- [CrossRef] [Google Scholar]
- Carbon nanodot decorated graphitic carbon nitride: New insights into the enhanced photocatalytic water splitting from ab initio studies. Phys. Chem. Chem. Phys.. 2015;17:31140-31144.
- [CrossRef] [Google Scholar]
- Bi-doped graphitic carbon nitride nanotubes boost the photocatalytic degradation of Rhodamine B. New J. Chem.. 2022;46:3588-3594.
- [CrossRef] [Google Scholar]
- B. Gholipour, A. Zonouzi, M. Shokouhimehr, S. Rostamnia, Integration of plasmonic AgPd alloy nanoparticles with single-layer graphitic carbon nitride as Mott-Schottky junction toward photo-promoted H2 evolution, Sci. Reports 2022 121. 12 (2022) 1–13. https://doi.org/10.1038/s41598-022-17238-4.
- Platinum-silicon doped graphitic carbon nitride: a first principle calculation. Phys. B Condens. Matter.. 2022;627:413547
- [CrossRef] [Google Scholar]
- Carbon dots/g-C3N4/ZnO nanocomposite as efficient visible-light driven photocatalyst for tetracycline total degradation. Sep. Purif. Technol.. 2017;173:295-303.
- [CrossRef] [Google Scholar]
- Pomelo biochar as an electron acceptor to modify graphitic carbon nitride for boosting visible-light-driven photocatalytic degradation of tetracycline, Chinese. J Chem. Eng.. 2022;48:1-11.
- [CrossRef] [Google Scholar]
- Constructing benzene ring modified graphitic carbon nitride with narrowed bandgap and enhanced molecular oxygen activation for efficient photocatalytic degradation of oxytetracycline. Sep. Purif. Technol.. 2022;294:121170
- [CrossRef] [Google Scholar]
- Holey structured graphitic carbon nitride thin sheets with edge oxygen doping via photo-Fenton reaction with enhanced photocatalytic activity. Appl. Catal. B Environ.. 2016;185:315-321.
- [CrossRef] [Google Scholar]
- One-step preparation of iodine-doped graphitic carbon nitride nanosheets as efficient photocatalysts for visible light water splitting. J. Mater. Chem. A. 2015;3:4612-4619.
- [CrossRef] [Google Scholar]
- Y. Hao, Y.C. Hou, D. Song, W.M. Yin, X.Y. Chen, C. Wang, Y.R. Guo, L. Li, Q.J. Pan, Interfacial coupling enhanced photocatalytic activity: an experimental/DFT combined study of porous graphitic carbon nitride/carbon composite material, Cellul. 2022 297. 29 (2022) 3759–3772. https://doi.org/10.1007/S10570-022-04529-2.
- The sulfur-bubble template-mediated synthesis of uniform porous g-C3N4 with superior photocatalytic performance. Chem. Commun.. 2014;51:425-427.
- [CrossRef] [Google Scholar]
- T.V.A. Hoang, E.W. Shin, T.K.A. Nguyen, D.Q. Dao, P.A. Nguyen, D.H. Jeong, Solvent Etching Process for Graphitic Carbon Nitride Photocatalysts Containing Platinum Cocatalyst: Effects of Water Hydrolysis on Photocatalytic Properties and Hydrogen Evolution Behaviors, Nanomater. 2022, Vol. 12, Page 1188. 12 (2022) 1188. https://doi.org/10.3390/NANO12071188.
- Mesoporous carbon nitride with in situ sulfur doping for enhanced photocatalytic hydrogen evolution from water under visible light. J. Mater. Chem.. 2012;22:15006-15012.
- [CrossRef] [Google Scholar]
- Confined phase separation of aqueous–organic nanodroplets. Phys. Chem. Chem. Phys.. 2017;19:26839-26845.
- [CrossRef] [Google Scholar]
- A simple and efficient method to prepare a phosphorus modified g-C 3N4 visible light photocatalyst. RSC Adv.. 2014;4:21657-21663.
- [CrossRef] [Google Scholar]
- Enhanced visible light photocatalytic performance of g-C3N4 photocatalysts co-doped with iron and phosphorus. Appl. Surf. Sci.. 2014;311:164-171.
- [CrossRef] [Google Scholar]
- Band gap-tunable potassium doped graphitic carbon nitride with enhanced mineralization ability. Dalt. Trans.. 2014;44:1084-1092.
- [CrossRef] [Google Scholar]
- Cadmium-sulfide/gold/graphitic-carbon-nitride sandwich heterojunction photocatalyst with regulated electron transfer for boosting carbon-dioxide reduction to hydrocarbon. J. Colloid Interface Sci.. 2022;613:575-586.
- [CrossRef] [Google Scholar]
- Precursor-modified strategy to synthesize thin porous amino-rich graphitic carbon nitride with enhanced photocatalytic degradation of RhB and hydrogen evolution performances. Chinese J. Catal.. 2022;43:497-506.
- [CrossRef] [Google Scholar]
- Carbon nitride with simultaneous porous network and O-doping for efficient solar-energy-driven hydrogen evolution. Nano Energy.. 2015;12:646-656.
- [CrossRef] [Google Scholar]
- Construction of carbon quantum dots/proton-functionalized graphitic carbon nitride nanocomposite via electrostatic self-assembly strategy and its application. Appl. Surf. Sci.. 2016;370:514-521.
- [CrossRef] [Google Scholar]
- W. Jiang, H. Wang, X. Zhang, Y. Zhu, Y. Xie, Two-dimensional polymeric carbon nitride: structural engineering for optimizing photocatalysis, Sci. China Chem. 2018 6110. 61 (2018) 1205–1213. https://doi.org/10.1007/S11426-018-9292-7.
- E. Kanimba, Z. Tian, H. Choi, Y.J. Kim, J. Song, C.S. Kim, G.S. Lee, S.S. Kim, J. Park, S.H. Yim, S.H. Park, H.R. Hwang, M. Hong, P. Veluswamy, B.J. Cho, P.M. Rodrigo, A. Valera, E.F. Fernández, F.M. Almonacid, K. Yang, K. Cho, S. Yang, Y. Park, S.S. Kim, D. Kong, W. Zhu, Z. Guo, Y. Deng, Y. Zhou, S.S. Zhang, X. Xu, W. Liu, S.S. Zhang, G. Li, J. He, S.M. Pourkiaei, M.H. Ahmadi, M. Sadeghzadeh, S. Moosavi, F. Pourfayaz, L. Chen, M.A. Pour Yazdi, R. Kumar, K. Karthick, S. Suresh, H. Singh, G.C. Joy, R. Dhanuskodi, H.M. Elmoughni, A.K. Menon, R.M.W. Wolfe, S.K. Yee, A.A. Angeline, L.G. Asirvatham, D.J. Hemanth, J. Jayakumar, S. Wongwises, M. Marefati, M. Mehrpooya, Z. Yuan, X. Tang, Y. Liu, Z. Xu, K. Liu, J. Li, Z. Zhang, H. Wang, K. Karthick, S. Suresh, M.M.M.D. Hussain, H.M. Ali, C.S.S. Kumar, X. Zhao, W. Han, C. Zhao, S. Wang, F. Kong, X. Ji, Z. Li, X. Shen, L.K. Allison, T.L. Andrew, Y.J. Cui, B.L. Wang, K.F. Wang, L. Zheng, E. Houshfar, A. Mohammadnia, B.M. Ziapour, F. Sedaghati, L.A. Rosendahl, A. Rezania, S. Mahmoudinezhad, P.A. Cotfas, D.T. Cotfas, L.A. Rosendahl, A. Rezania, Y.S. Wudil, M.A. Gondal, S.G. Rao, S. Kunwar, Thermal conductivity of PLD-grown thermoelectric Bi2Te2.7Se0.3 films using temperature-dependent Raman spectroscopy technique, Appl. Therm. Eng. 4 (2019) 25–43. https://doi.org/10.1016/j.seta.2019.02.008.
- Recent advancements in engineering approach towards design of photo-reactors for selective photocatalytic CO2 reduction to renewable fuels. J. CO2 Util.. 2019;29:205-239.
- [CrossRef] [Google Scholar]
- Sulfur-doped g-C3N4/BiVO4 composite photocatalyst for water oxidation under visible light. Chem. Mater.. 2016;28:1318-1324.
- [CrossRef] [Google Scholar]
- Plasmon Ag/Na-doped defective graphite carbon nitride/NiFe layered double hydroxides Z-scheme heterojunctions toward optimized photothermal-photocatalytic-Fenton performance. Appl. Catal. B Environ.. 2022;304:120969
- [CrossRef] [Google Scholar]
- Photochemical deposition of SnS2 on graphitic carbon nitride for photocatalytic aqueous Cr(VI) reduction. Chem. Eng. J. Adv.. 2022;9:100224
- [CrossRef] [Google Scholar]
- Tuning the surface and optical properties of graphitic carbon nitride by incorporation of alkali metals (Na, K, Cs and Rb): effect on photocatalytic removal of organic pollutants. Chemosphere. 2022;287:131988
- [CrossRef] [Google Scholar]
- A facile synthesis of Br-modified g-C3N4 semiconductors for photoredox water splitting. Appl. Catal. B Environ.. 2016;192:116-125.
- [CrossRef] [Google Scholar]
- Cu-doped mesoporous graphitic carbon nitride for enhanced visible-light driven photocatalysis. RSC Adv.. 2016;6:38811-38819.
- [CrossRef] [Google Scholar]
- Ascorbic acid-induced structural defect in photocatalytic graphitic carbon nitride to boost H2O2 fuel cell performance. J. Power Sources. 2022;532:231368
- [CrossRef] [Google Scholar]
- Facile synthesis and enhanced visible-light photoactivity of DyVO4/g-C3N4I composite semiconductors. Appl. Catal. B Environ.. 2016;183:426-432.
- [CrossRef] [Google Scholar]
- Rapid sterilization and accelerated wound healing using Zn2+ and graphene oxide modified g-C3N4 under dual light irradiation. Adv. Funct. Mater.. 2018;28:1800299.
- [CrossRef] [Google Scholar]
- Eradicating multidrug-resistant bacteria rapidly using a multi functional g-C3N4@ Bi2S3 nanorod heterojunction with or without antibiotics. Adv. Funct. Mater.. 2019;29:1900946.
- [CrossRef] [Google Scholar]
- Boosting the extra generation of superoxide radicals on graphitic carbon nitride with carbon vacancies by the modification of pollutant adsorption for high-performance photocatalytic degradation. ACS ES&T Eng. 2022
- [CrossRef] [Google Scholar]
- Two-dimensional antibacterial materials. Prog. Mater. Sci.. 2022;130:100976
- [CrossRef] [Google Scholar]
- Modification of g-C3N4 nanosheets by carbon quantum dots for highly efficient photocatalytic generation of hydrogen. Appl. Surf. Sci.. 2016;375:110-117.
- [CrossRef] [Google Scholar]
- High-efficiency ultrathin porous phosphorus-doped graphitic carbon nitride nanosheet photocatalyst for energy production and environmental remediation. Appl. Catal. B Environ.. 2022;307:121099
- [CrossRef] [Google Scholar]
- Construction of dual transfer channels in graphitic carbon nitride photocatalyst for high-efficiency environmental pollution remediation: Enhanced exciton dissociation and carrier migration. J. Hazard. Mater.. 2022;436:129171
- [CrossRef] [Google Scholar]
- Thiocyanate hydrometallurgy for the recovery of gold. part I: chemical and thermodynamic considerations. Hydrometallurgy. 2012;113–114:1-9.
- [CrossRef] [Google Scholar]
- Application in photocatalytic degradation of zearalenone based on graphitic carbon nitride. Luminescence. 2022;37:190-198.
- [CrossRef] [Google Scholar]
- Recent advances in 3D g-C3N4 composite photocatalysts for photocatalytic water splitting, degradation of pollutants and CO2 reduction. J. Alloys Compd.. 2019;802:196-209.
- [CrossRef] [Google Scholar]
- Condensed graphitic carbon nitride nanorods by nanoconfinement: promotion of crystallinity on photocatalytic conversion. Chem. Mater.. 2011;23:4344-4348.
- [CrossRef] [Google Scholar]
- The incorporation of cocatalyst cobalt sulfide into graphitic carbon nitride: boosted photocatalytic hydrogen evolution performance and mechanism exploration. Nano Mater. Sci. 2022
- [CrossRef] [Google Scholar]
- Sulfur-doped graphitic carbon nitride decorated with zinc phthalocyanines towards highly stable and efficient photocatalysis. Appl. Catal. A Gen.. 2016;519:107-115.
- [CrossRef] [Google Scholar]
- Synthesis of bismuth oxybromochloroiodide/graphitic carbon nitride quaternary composites (BiOxCly/BiOmBrn/BiOpIq/g-C3N4) enhances visible-light-driven photocatalytic activity. Catal. Commun.. 2022;163:106418
- [CrossRef] [Google Scholar]
- Photocatalysts of quaternary composite, bismuth oxyfluoride/bismuth oxyiodide/ graphitic carbon nitride: synthesis, characterization, and photocatalytic activity. Mol. Catal.. 2022;528:112463
- [CrossRef] [Google Scholar]
- Ionic liquid promoted synthesis of conjugated carbon nitride photocatalysts from urea. ChemSusChem.. 2014;7:1547-1550.
- [CrossRef] [Google Scholar]
- Graphitic carbon nitride-based photocatalysts in the applications of environmental catalysis. J. Environ. Sci.. 2023;124:570-590.
- [CrossRef] [Google Scholar]
- Effect of phosphorus doping on electronic structure and photocatalytic performance of g-C3N4: Insights from hybrid density functional calculation. J. Alloys Compd.. 2016;672:271-276.
- [CrossRef] [Google Scholar]
- Grafting Fe(III) species on carbon nanodots/Fe-doped g-C3N4 via interfacial charge transfer effect for highly improved photocatalytic performance. Appl. Catal. B Environ.. 2017;205:173-181.
- [CrossRef] [Google Scholar]
- Construction of Li/K dopants and cyano defects in graphitic carbon nitride for highly efficient peroxymonosulfate activation towards organic contaminants degradation. Chemosphere. 2022;294:133700
- [CrossRef] [Google Scholar]
- Construction of V-doped graphitic carbon nitride with nanotube structure for sustainable photodegradation of tetracycline. Vacuum. 2022;204:111342
- [CrossRef] [Google Scholar]
- Phenanthroline bridging graphitic carbon nitride framework and Fe (II) ions to promote transfer of photogenerated electrons for selective photocatalytic reduction of Nitrophenols. J. Colloid Interface Sci.. 2022;608:2088-2099.
- [CrossRef] [Google Scholar]
- Unique electronic structure induced high photoreactivity of sulfur-doped graphitic C3N4. J. Am. Chem. Soc.. 2010;132:11642-11648.
- [CrossRef] [Google Scholar]
- Boosting photocatalytic nitrogen reduction to ammonia by dual defective -CN and K-doping sites on graphitic carbon nitride nanorod arrays. Appl. Catal. B Environ.. 2022;317:121752
- [CrossRef] [Google Scholar]
- Mesoporous g-C3N4 nanosheets prepared by calcining a novel supramolecular precursor for high-efficiency photocatalytic hydrogen evolution. Appl. Surf. Sci.. 2018;450:46-56.
- [CrossRef] [Google Scholar]
- Construction of chlorine doped graphitic carbon nitride nanodisc for enhanced photocatalytic activity and mechanism insight. Int. J. Hydrogen Energy. 2022;47:16887-16899.
- [CrossRef] [Google Scholar]
- X. Long, C. Feng, S. Yang, D. Ding, J. Feng, M. Liu, Y. Chen, J. Tan, xingjie Peng, J. Shi, R. Chen, Oxygen doped graphitic carbon nitride with regulatable local electron density and band structure for improved photocatalytic degradation of bisphenol A, Chem. Eng. J. 435 (2022) 134835. https://doi.org/10.1016/J.CEJ.2022.134835
- Boron doped g-C3N4 with enhanced photocatalytic UO22+ reduction performance. Appl. Surf. Sci.. 2016;360:1016-1022.
- [CrossRef] [Google Scholar]
- Bamboo-charcoal-loaded graphitic carbon nitride for photocatalytic hydrogen evolution. Int. J. Hydrogen Energy. 2022;47:3733-3740.
- [CrossRef] [Google Scholar]
- Photocatalytic reduction elimination of UO22+ pollutant under visible light with metal-free sulfur doped g-C3N4 photocatalyst. Appl. Catal. B Environ.. 2017;200:378-385.
- [CrossRef] [Google Scholar]
- Enhancing visible-light photocatalytic activity of g-C3N4 by doping phosphorus and coupling with CeO2 for the degradation of methyl orange under visible light irradiation. RSC Adv.. 2015;5:68728-68735.
- [CrossRef] [Google Scholar]
- Novel PO codoped g-C3N4 with large specific surface area: Hydrothermal synthesis assisted by dissolution–precipitation process and their visible light activity under anoxic conditions. Appl. Surf. Sci.. 2015;357:131-138.
- [CrossRef] [Google Scholar]
- A strategy of enhancing the photoactivity of g-C 3N 4 via doping of nonmetal elements: a first-principles study. J. Phys. Chem. C. 2012;116:23485-23493.
- [CrossRef] [Google Scholar]
- GaN/Surface-modified graphitic carbon nitride heterojunction: promising photocatalytic hydrogen evolution materials. Int. J. Hydrogen Energy. 2022;47:7202-7213.
- [CrossRef] [Google Scholar]
- A facile approach to synthesizing S-Co–O tridoped g-C3N4 with enhanced oxygen-free photocatalytic performance via a hydrothermal post-treatment. RSC Adv.. 2015;5:79585-79592.
- [CrossRef] [Google Scholar]
- Visible light photocatalytic degradation and pretreatment of lignin using magnetic graphitic carbon nitride for enhancing methane production in anaerobic digestion. Fuel. 2022;318:123600
- [CrossRef] [Google Scholar]
- Synergistic effects of Tin sulfide Nitrogen-doped titania Nanobelt-Modified graphitic carbon nitride nanosheets with outstanding photocatalytic activity. J. Colloid Interface Sci.. 2022;606:1767-1778.
- [CrossRef] [Google Scholar]
- Rapid high-temperature hydrothermal post treatment on graphitic carbon nitride for enhanced photocatalytic H2 evolution. Catal. Today 2022
- [CrossRef] [Google Scholar]
- Formation of a p-n heterojunction photocatalyst by the interfacing of graphitic carbon nitride and delafossite CuGaO2. J. Chinese Chem. Soc.. 2022;69:1042-1050.
- [CrossRef] [Google Scholar]
- Oxygen-doped porous graphitic carbon nitride in photocatalytic peroxymonosulfate activation for enhanced carbamazepine removal: performance, influence factors and mechanisms. Chem. Eng. J.. 2022;429:130860
- [CrossRef] [Google Scholar]
- Photocatalytic elimination of moxifloxacin by two-dimensional graphitic carbon nitride nanosheets: enhanced activity, degradation mechanism and potential practical application. Sep. Purif. Technol.. 2022;292:121067
- [CrossRef] [Google Scholar]
- Activation of Fe species on graphitic carbon nitride nanotubes for efficient photocatalytic ammonia synthesis. Int. J. Energy Res.. 2022;46:13453-13462.
- [CrossRef] [Google Scholar]
- Fabrication of visible-light-driven tubular F, P-codoped graphitic carbon nitride for enhanced photocatalytic degradation of tetracycline. J. Environ Chem. Eng.. 2022;10:106905
- [CrossRef] [Google Scholar]
- Efficient and reusable photocatalytic river water disinfection by addictive graphitic carbon nitride/magnesium oxide nano-onions with particular “nano-magnifying glass effect”. J. Hazard. Mater.. 2022;439:129533
- [CrossRef] [Google Scholar]
- Recycling spent LiCoO2 battery as a high-efficient lithium-doped graphitic carbon nitride/Co3O4 composite photocatalyst and its synergistic photocatalytic mechanism. Energy Environ. Mater. 2022
- [CrossRef] [Google Scholar]
- W.J. Ong, L.K. Putri, Y.C. Tan, L.L. Tan, N. Li, Y.H. Ng, X. Wen, S.P. Chai, Unravelling charge carrier dynamics in protonated g-C3N4 interfaced with carbon nanodots as co-catalysts toward enhanced photocatalytic CO2 reduction: A combined experimental and first-principles DFT study, Nano Res. 2016 105. 10 (2017) 1673–1696. https://doi.org/10.1007/S12274-016-1391-4.
- Carbon nitride photocatalysts with integrated oxidation and reduction atomic active centers for improved CO2 conversion. Angew. Chem.. 2022;134:e202206579.
- [CrossRef] [Google Scholar]
- Ab initio study on a novel photocatalyst: Functionalized graphitic carbon nitride nanotube. ACS Catal.. 2011;1:99-104.
- [CrossRef] [Google Scholar]
- Graphitic carbon nitride/NH2-MIL-101(Fe) composite for environmental remediation: visible-light-assisted photocatalytic degradation of acetaminophen and reduction of hexavalent chromium. Chemosphere. 2022;286:131875
- [CrossRef] [Google Scholar]
- Visible-light photocatalysis of Ag-doped graphitic carbon nitride for photodegradation of micropollutants in wastewater. Chemosphere. 2022;301:134626
- [CrossRef] [Google Scholar]
- Fruitful fabrication of CDs on GO/g-C3N4 sheets layers: a carbon amalgamation for the remediation of carcinogenic pollutants. J. Photochem. Photobiol. A Chem.. 2019;370:94-104.
- [CrossRef] [Google Scholar]
- P. Praus, A brief review of s-triazine graphitic carbon nitride, Carbon Lett. 2022 323. 32 (2022) 703–712. https://doi.org/10.1007/S42823-022-00319-9.
- Preparation and photocatalytic behavior of carbon-nanodots/graphitic carbon nitride composite photocatalyst. J. Electrochem. Soc.. 2017;164:H211-H214.
- [CrossRef] [Google Scholar]
- Coordinative integration of amorphous nickel-imidazole framework with graphitic carbon nitride for enhanced photocatalytic hydrogen production. Appl. Mater. Today. 2022;28:101524
- [CrossRef] [Google Scholar]
- Porous P-doped graphitic carbon nitride nanosheets for synergistically enhanced visible-light photocatalytic H2 production. Energy Environ. Sci.. 2015;8:3708-3717.
- [CrossRef] [Google Scholar]
- Graphitic carbon nitride based immobilized and non-immobilized floating photocatalysts for environmental remediation. Chemosphere. 2022;297:134229
- [CrossRef] [Google Scholar]
- Enhanced cocatalyst-free visible-light activities for photocatalytic fuel production of g-C3N4 by trapping holes and transferring electrons. J. Phys. Chem. C. 2016;120:98-107.
- [CrossRef] [Google Scholar]
- Reduced graphene oxide-assisted graphitic carbon nitride@ZnO rods for enhanced physical and photocatalytic degradation. Inorg. Chem. Commun.. 2022;142:109623
- [CrossRef] [Google Scholar]
- Photoelectrochemical CO2 reduction by a p-type boron-doped g-C3N4 electrode under visible light. Appl. Catal. B Environ.. 2016;192:193-198.
- [CrossRef] [Google Scholar]
- The influence of metal-doped graphitic carbon nitride on photocatalytic conversion of acetic acid to carbon dioxide. Front. Chem.. 2022;10
- [CrossRef] [Google Scholar]
- Review of recent developments and persistent challenges in stability of perovskite solar cells. Renew. Sustain. Energy Rev.. 2018;90:210-222.
- [CrossRef] [Google Scholar]
- Mesoporous graphitic carbon nitride as a heterogeneous organic photocatalyst in the dual catalytic arylation of Alkyl Bis(catecholato)silicates. Org. Lett.. 2022;24:2483-2487.
- [CrossRef] [Google Scholar]
- Scopus - Document details - null | Signed in, (n.d.). https://www.scopus.com/record/display.uri?eid=2-s2.0-79953302537&origin=inward (accessed August 17, 2022).
- Scopus - Document details - null | Signed in, (n.d.). https://www.scopus.com/record/display.uri?eid=2-s2.0-85069872356&origin=inward&featureToggles=FEATURE_NEW_DOC_DETAILS_EXPORT:1,FEATURE_EXPORT_REDESIGN:0 (accessed August 14, 2022).
- Plasmon-enhanced photocatalytic activity in the visible range using AgNPs/polydopamine/graphitic carbon nitride nanocomposite. Appl. Surf. Sci.. 2022;585:152728
- [CrossRef] [Google Scholar]
- A broom-like tube-in-tube bundle O-doped graphitic carbon nitride nanoreactor that promotes photocatalytic hydrogen evolution. Chem. Eng. J.. 2022;431:133898
- [CrossRef] [Google Scholar]
- Photocatalytic hydrogen production using graphitic carbon nitride (GCN): a precise review. Renew. Sustain. Energy Rev.. 2022;168:112776
- [CrossRef] [Google Scholar]
- Template-free synthesis of 2D porous ultrathin nonmetal-doped g-C3N4 nanosheets with highly efficient photocatalytic H2 evolution from water under visible light. Appl. Catal. B Environ.. 2016;187:144-153.
- [CrossRef] [Google Scholar]
- Enhanced nonsacrificial photocatalytic generation of hydrogen peroxide under visible light using modified graphitic carbon nitride with doped phosphorus and loaded carbon quantum dots: constructing electron transfer channel. J. Colloid Interface Sci.. 2022;628:259-272.
- [CrossRef] [Google Scholar]
- Onion-liked carbon-embedded graphitic carbon nitride for enhanced photocatalytic hydrogen evolution and dye degradation. Appl. Catal. B Environ.. 2022;308:121216
- [CrossRef] [Google Scholar]
- Sugarcane juice derived carbon dot–graphitic carbon nitride composites for bisphenol A degradation under sunlight irradiation. Beilstein J. Nanotechnol.. 2018;935(9):353-363.
- [CrossRef] [Google Scholar]
- Rational design of a graphitic carbon nitride catalytic–biocatalytic system as a photocatalytic platform for solar fine chemical production from CO2. React. Chem. Eng.. 2022;7:1566-1572.
- [CrossRef] [Google Scholar]
- Thickness regulation of graphitic carbon nitride and its influence on the photocatalytic performance towards CO2 reduction. Appl. Surf. Sci.. 2022;577:151810
- [CrossRef] [Google Scholar]
- Decoration of TiO2/g-C3N4 Z-scheme by carbon dots as a novel photocatalyst with improved visible-light photocatalytic performance for the degradation of enrofloxacin. RSC Adv.. 2017;7:34096-34103.
- [CrossRef] [Google Scholar]
- Graphitic carbon nitride-based upconversion photocatalyst for hydrogen production and water purification. Nanofabrication. 2022;7:183-190.
- [CrossRef] [Google Scholar]
- J. Sun, J. Zhang, M. Zhang, M. Antonietti, X. Fu, X. Wang, Bioinspired hollow semiconductor nanospheres as photosynthetic nanoparticles, Nat. Commun. 2012 31. 3 (2012) 1–7. https://doi.org/10.1038/ncomms2152.
- g-C3N4 based composite photocatalysts for photocatalytic CO2 reduction. Catal. Today. 2018;300:160-172.
- [CrossRef] [Google Scholar]
- Graphitic carbon nitride embedded Ni3(VO4)2/ZnCr2O4 Z-scheme photocatalyst for efficient degradation of p-chlorophenol and 5-fluorouracil, and genotoxic evaluation in Allium cepa. J. Ind. Eng. Chem.. 2022;112:244-257.
- [CrossRef] [Google Scholar]
- Visible-light-assisted peroxymonosulfate activation by metal-free bifunctional oxygen-doped graphitic carbon nitride for enhanced degradation of imidacloprid: role of non-photochemical and photocatalytic activation pathway. J. Hazard. Mater.. 2022;423:127048
- [CrossRef] [Google Scholar]
- Uncovering the multifaceted roles of nitrogen defects in graphitic carbon nitride for selective photocatalytic carbon dioxide reduction: a density functional theory study. Phys. Chem. Chem. Phys.. 2022;24:11124-11130.
- [CrossRef] [Google Scholar]
- Photocatalytic degradation of pharmaceuticals by pore-structured graphitic carbon nitride with carbon vacancy in water: identification of intermediate degradants and effects of active species. Sci. Total Environ.. 2022;824:153845
- [CrossRef] [Google Scholar]
- A novel P-doped g-C3N4/Zn0.8Cd0.2S composite photocatalyst for degradation of methylene blue under simulated sunlight. Appl. Surf. Sci.. 2016;361:251-258.
- [CrossRef] [Google Scholar]
- Graphitic carbon nitride nanoflakes decorated on multielement-doped carbon as photocatalysts for bacterial disinfection under visible and near-infrared light. ACS Appl. Nano Mater.. 2022;5:3422-3433.
- [CrossRef] [Google Scholar]
- Fe-doped and -mediated graphitic carbon nitride nanosheets for enhanced photocatalytic performance under natural sunlight. J. Mater. Chem. A.. 2014;2:6772-6780.
- [CrossRef] [Google Scholar]
- Sugarcane bagasse supported graphitic carbon nitride for photocatalytic conversion of carbon dioxide. Catal. Commun.. 2022;164:106431
- [CrossRef] [Google Scholar]
- Development of Indium vanadate and Silver deposited on graphitic carbon nitride ternary heterojunction for advanced photocatalytic degradation of residual antibiotics in aqueous environment. Opt. Mater. (Amst).. 2022;123:111885
- [CrossRef] [Google Scholar]
- Recent advances in graphitic carbon nitride semiconductor: structure, synthesis and applications. Mater. Sci. Semicond. Process.. 2022;137
- [CrossRef] [Google Scholar]
- Heterogeneous graphitic carbon nitrides in visible-light-initiated organic transformations. Green Chem.. 2022;24:438-479.
- [CrossRef] [Google Scholar]
- Promoting intramolecular charge transfer of graphitic carbon nitride by donor–acceptor modulation for visible-light photocatalytic H2 evolution. Interdiscip. Mater.. 2022;1:294-308.
- [CrossRef] [Google Scholar]
- Defect engineered mesoporous graphitic carbon nitride modified with AgPd nanoparticles for enhanced photocatalytic hydrogen evolution from formic acid. Chem. Eng. J.. 2022;429:132388
- [CrossRef] [Google Scholar]
- Phosphorus-oxygen-codoped graphitic carbon nitride for enhanced hydrogen evolution and photocatalytic degradation under visible light irradiation. ACS Appl. Energy Mater.. 2022;2022:5784.
- [CrossRef] [Google Scholar]
- All-solid-state Z-scheme Pt/ZnS-ZnO heterostructure sheets for photocatalytic simultaneous evolution of H2 and O2. Chem. Eng. J.. 2020;385:123782
- [CrossRef] [Google Scholar]
- Facile synthesis of N-doped carbon dots/g-C3N4 photocatalyst with enhanced visible-light photocatalytic activity for the degradation of indomethacin. Appl. Catal. B Environ.. 2017;207:103-113.
- [CrossRef] [Google Scholar]
- P and Cu dual sites on graphitic carbon nitride for photocatalytic CO2 reduction to hydrocarbon fuels with high C2H6 evolution. Angew. Chemie Int. Ed.. 2022;61:e202210789.
- [CrossRef] [Google Scholar]
- Excellent visible-light photocatalysis of fluorinated polymeric carbon nitride solids. Chem. Mater.. 2010;22:5119-5121.
- [CrossRef] [Google Scholar]
- X. Wang, K. Maeda, A. Thomas, K. Takanabe, G. Xin, J.M. Carlsson, K. Domen, M. Antonietti, A metal-free polymeric photocatalyst for hydrogen production from water under visible light, Nat. Mater. 2009 81. 8 (2008) 76–80. https://doi.org/10.1038/nmat2317.
- Sulfur-doped g-C3N4 with enhanced photocatalytic CO2-reduction performance. Appl. Catal. B Environ.. 2015;176–177:44-52.
- [CrossRef] [Google Scholar]
- 3D macropore carbon-vacancy g-C3N4 constructed using polymethylmethacrylate spheres for enhanced photocatalytic H2 evolution and CO2 reduction. J. Energy Chem.. 2020;53:139-146.
- [CrossRef] [Google Scholar]
- Facile fabrication of the Ag nanoparticles decorated graphitic carbon nitride photocatalyst film for indoor air purification under visible light. Build. Environ.. 2022;222:109402
- [CrossRef] [Google Scholar]
- One-step supramolecular preorganization constructed crinkly graphitic carbon nitride nanosheets with enhanced photocatalytic activity. J. Mater. Sci. Technol.. 2022;104:155-162.
- [CrossRef] [Google Scholar]
- CdS-sensitized 3D ordered macroporous g-C3N4 for enhanced visible-light photocatalytic hydrogen generation. J. Mater. Sci. Technol.. 2022;111:204-210.
- [CrossRef] [Google Scholar]
- Novel ternary photocatalyst of single atom-dispersed silver and carbon quantum dots co-loaded with ultrathin g-C3N4 for broad spectrum photocatalytic degradation of naproxen. Appl. Catal. B Environ.. 2018;221:510-520.
- [CrossRef] [Google Scholar]
- Visible LED photocatalysis combined with ultrafiltration driven by metal-free oxygen-doped graphitic carbon nitride for sulfamethazine degradation. J. Hazard. Mater.. 2022;439:129632
- [CrossRef] [Google Scholar]
- Synthesis of Mo-doped graphitic carbon nitride catalysts and their photocatalytic activity in the reduction of CO2 with H2O. Catal. Commun.. 2016;74:75-79.
- [CrossRef] [Google Scholar]
- Improved polyhydroxybutyrate production by Cupriavidus necator and the photocatalyst graphitic carbon nitride from fructose under low light intensity. Int. J. Biol. Macromol.. 2022;203:526-534.
- [CrossRef] [Google Scholar]
- A facile template synthesis of phosphorus-doped graphitic carbon nitride hollow structures with high photocatalytic hydrogen production activity. Mater. Chem. Phys.. 2022;275:125299
- [CrossRef] [Google Scholar]
- In-situ hydrothermal synthesized γ-Al2O3/O-g-C3N4 heterojunctions with enhanced visible-light photocatalytic activity in water splitting for hydrogen. J. Energy Chem.. 2016;25:594-600.
- [CrossRef] [Google Scholar]
- Facile synthesis of Y-doped graphitic carbon nitride with enhanced photocatalytic performance. Catal. Commun.. 2016;84:179-182.
- [CrossRef] [Google Scholar]
- Q. Wu, D. Lu, B. Zhang, K.K. Kondamareddy, Y. Zeng, Y. Zhang, J. Wang, M. Zhou, N. D, H. Hao, H. Fan, Interfacial optimization of CeO2 nanoparticles loaded two-dimensional graphite carbon nitride toward synergistic enhancement of visible-light-driven photoelectric and photocatalytic hydrogen evolution, Int. J. Hydrogen Energy. 47 (2022) 2313–2326. https://doi.org/10.1016/J.IJHYDENE.2021.10.221
- Improved thermoelectric performance of ternary Cu/Ni/Bi2Te2.7Se0.3 nanocomposite prepared by pulsed laser deposition. Mater. Chem. Phys.. 2020;253:123321
- [CrossRef] [Google Scholar]
- Substrate temperature-dependent thermoelectric figure of merit of nanocrystalline Bi2Te3 and Bi2Te2.7Se0.3 prepared using pulsed laser deposition supported by DFT study. Ceram. Int. 2020
- [CrossRef] [Google Scholar]
- The multi-dimensional approach to synergistically improve the performance of inorganic thermoelectric materials: a critical review. Arab. J. Chem.. 2021;14:103103
- [CrossRef] [Google Scholar]
- Hydrostatic pressure-tuning of thermoelectric properties of CsSnI3 perovskite by first-principles calculations. Comput. Mater. Sci.. 2022;201:110917
- [CrossRef] [Google Scholar]
- Leveraging doping and defect engineering to modulate exciton dissociation in graphitic carbon nitride for photocatalytic elimination of marine oil spill. Chem. Eng. J.. 2022;439:135668
- [CrossRef] [Google Scholar]
- A Z-scheme heterojunction of ZnO/CDots/C3N4 for strengthened photoresponsive bacteria-killing and acceleration of wound healing. J. Mater. Sci. Technol.. 2020;57:1-11.
- [CrossRef] [Google Scholar]
- Progress of graphite carbon nitride with different dimensions in the photocatalytic degradation of dyes: a review. J. Alloys Compd.. 2022;901:163589
- [CrossRef] [Google Scholar]
- Construction of carbon dots modified MoO3/g-C3N4 Z-scheme photocatalyst with enhanced visible-light photocatalytic activity for the degradation of tetracycline. Appl. Catal. B Environ.. 2018;229:96-104.
- [CrossRef] [Google Scholar]
- Bridging the g-C3N4 interlayers for enhanced photocatalysis. ACS Catal.. 2016;6:2462-2472.
- [CrossRef] [Google Scholar]
- Nitrogen-doping coupled with cerium oxide loading co-modified graphitic carbon nitride for highly enhanced photocatalytic degradation of tetracycline under visible light. Chemosphere. 2022;293:133648
- [CrossRef] [Google Scholar]
- Z-scheme Cu2O nanoparticle/graphite carbon nitride nanosheet heterojunctions for photocatalytic hydrogen evolution. ACS Appl. Nano Mater.. 2022;5:8475-8483.
- [CrossRef] [Google Scholar]
- Efficient charge transfer in Co-doped CeO2/graphitic carbon nitride with N vacancies heterojunction for photocatalytic hydrogen evolution. J. Colloid Interface Sci.. 2022;627:261-269.
- [CrossRef] [Google Scholar]
- The fabrication of graphitic carbon nitride hollow nanocages with semi-metal 1T’ phase molybdenum disulfide as co-catalysts for excellent photocatalytic nitrogen fixation. J. Colloid Interface Sci.. 2022;608:1229-1237.
- [CrossRef] [Google Scholar]
- Photodegradation of rhodamine B and methyl orange over boron-doped g-C 3N4 under visible light irradiation. Langmuir. 2010;26:3894-3901.
- [CrossRef] [Google Scholar]
- Ti3C2 Mxene/porous g-C3N4 interfacial Schottky junction for boosting spatial charge separation in photocatalytic H2O2 production. Appl. Catal. B Environ.. 2019;258:117956
- [CrossRef] [Google Scholar]
- Q. Ye, L. Xu, Y. Xia, R. Gang, C. Xie, Zinc oxide quantum dots/graphitic carbon nitride nanosheets based visible-light photocatalyst for efficient tetracycline hydrochloride degradation, J. Porous Mater. 2022 292. 29 (2022) 571–581. https://doi.org/10.1007/S10934-022-01197-2.
- Self-assembled graphitic carbon nitride regulated by carbon quantum dots with optimized electronic band structure for enhanced photocatalytic degradation of diclofenac. Chem. Eng. J.. 2022;431:133927
- [CrossRef] [Google Scholar]
- D. Yu, T. Jia, Z. Deng, Q. Wei, K. Wang, L. Chen, P. Wang, J. Cui, One-Dimensional P-Doped Graphitic Carbon Nitride Tube: Facile Synthesis, Effect of Doping Concentration, and Enhanced Mechanism for Photocatalytic Hydrogen Evolution, Nanomater. 2022, Vol. 12, Page 1759. 12 (2022) 1759. https://doi.org/10.3390/NANO12101759.
- Single-atom Ir and Ru anchored on graphitic carbon nitride for efficient and stable electrocatalytic/photocatalytic hydrogen evolution. Appl. Catal. B Environ.. 2022;310:121318
- [CrossRef] [Google Scholar]
- Alkali-assisted synthesis of nitrogen deficient graphitic carbon nitride with tunable band structures for efficient visible-light-driven hydrogen evolution. Adv. Mater.. 2017;29:1605148.
- [CrossRef] [Google Scholar]
- Novel 3-D nanoporous graphitic-C3N4 nanosheets with heterostructured modification for efficient visible-light photocatalytic hydrogen production. RSC Adv.. 2014;4:52332-52337.
- [CrossRef] [Google Scholar]
- Spinel MgAl2O4 nanospheres coupled with modified graphitic carbon nitride nanosheets as an efficient Z-scheme photocatalyst for photodegradation of organic contaminants. Appl. Surf. Sci.. 2022;585:152615
- [CrossRef] [Google Scholar]
- Sulfate doped graphitic carbon nitride with enhanced photocatalytic activity towards degradation of methylene blue. Mater. Lett.. 2022;309:131310
- [CrossRef] [Google Scholar]
- Facile synthesis of phosphorus doped graphitic carbon nitride polymers with enhanced visible-light photocatalytic activity. Mater. Res. Bull.. 2013;48:3485-3491.
- [CrossRef] [Google Scholar]
- Ternary catalysts based on amino-functionalized carbon quantum dots, graphitic carbon nitride nanosheets and cobalt complex for efficient H2 evolution under visible light irradiation. Carbon N. Y.. 2019;145:488-500.
- [CrossRef] [Google Scholar]
- A convenient method to prepare a novel alkali metal sodium doped carbon nitride photocatalyst with a tunable band structure. RSC Adv.. 2014;4:62912-62919.
- [CrossRef] [Google Scholar]
- Graphitic carbon nitride/antimonene van der Waals heterostructure with enhanced photocatalytic CO2 reduction activity. J. Mater. Sci. Technol.. 2022;116:192-198.
- [CrossRef] [Google Scholar]
- Bandgap engineering and mechanism study of nonmetal and metal ion codoped carbon nitride: C+Fe as an example. Chem. – A Eur. J.. 2014;20:9805-9812.
- [CrossRef] [Google Scholar]
- Hydrothermal synthesis of carbon-rich graphitic carbon nitride nanosheets for photoredox catalysis. J. Mater. Chem. A. 2015;3:3281-3284.
- [CrossRef] [Google Scholar]
- Metal free and efficient photoelectrocatalytic removal of organic contaminants over g-C3N4 nanosheet films decorated with carbon quantum dots. RSC Adv.. 2017;7:56335-56343.
- [CrossRef] [Google Scholar]
- Unraveling the dual defect sites in graphite carbon nitride for ultra-high photocatalytic H2O2 evolution. Energy Environ. Sci.. 2022;15:830-842.
- [CrossRef] [Google Scholar]
- Precise carbon doping regulation of porous graphitic carbon nitride nanosheets enables elevated photocatalytic oxidation performance towards emerging organic pollutants. Chem. Eng. J.. 2022;433:134551
- [CrossRef] [Google Scholar]
- Phosphorus-doped carbon nitride solid: enhanced electrical conductivity and photocurrent generation. J. Am. Chem. Soc.. 2010;132:6294-6295.
- [CrossRef] [Google Scholar]
- Synergistic effect of nitrogen vacancy on ultrathin graphitic carbon nitride porous nanosheets for highly efficient photocatalytic H2 evolution. Chem. Eng. J.. 2022;431:134101
- [CrossRef] [Google Scholar]
- Photocatalytic abstraction of hydrogen atoms from water using hydroxylated graphitic carbon nitride for hydrogenative coupling reactions. Angew. Chemie Int. Ed.. 2022;61:e202204256.
- [CrossRef] [Google Scholar]
- EDTA-dominated hollow tube-like porous graphitic carbon nitride towards enhanced photocatalytic hydrogen evolution. J. Colloid Interface Sci.. 2022;619:289-297.
- [CrossRef] [Google Scholar]
- Iodine modified carbon nitride semiconductors as visible light photocatalysts for hydrogen evolution. Adv. Mater.. 2014;26:805-809.
- [CrossRef] [Google Scholar]
- Precise regulation of ultra-thin platinum decorated Gold/Graphite carbon nitride photocatalysts by atomic layer deposition for efficient degradation of Rhodamine B under simulated sunlight. Arab. J. Chem.. 2022;15:103951
- [CrossRef] [Google Scholar]
- In-situ synthesis of dual Z-scheme heterojunctions of cuprous oxide/layered double hydroxides/nitrogen-rich graphitic carbon nitride for photocatalytic sterilization. J. Colloid Interface Sci.. 2022;620:313-321.
- [CrossRef] [Google Scholar]
- Carbon dots decorated graphitic carbon nitride as an efficient metal-free photocatalyst for phenol degradation. Appl. Catal. B Environ.. 2016;180:656-662.
- [CrossRef] [Google Scholar]
- Cyano group-enriched crystalline graphitic carbon nitride photocatalyst: Ethyl acetate-induced improved ordered structure and efficient hydrogen-evolution activity. J. Colloid Interface Sci.. 2022;608:1268-1277.
- [CrossRef] [Google Scholar]
- Single atom Fe-dispersed graphitic carbon nitride (g-C3N4) as a highly efficient peroxymonosulfate photocatalytic activator for sulfamethoxazole degradation. Chem. Eng. J.. 2022;430:132937
- [CrossRef] [Google Scholar]
- Facile synthesis of cadmium-doped graphite carbon nitride for photocatalytic degradation of tetracycline under visible light irradiation. Environ. Sci. Pollut. Res.. 2022;2022:1-19.
- [CrossRef] [Google Scholar]
- Template synthesis of carbon self-doped g-C3N4 with enhanced visible to near-infrared absorption and photocatalytic performance. RSC Adv.. 2015;5:39549-39556.
- [CrossRef] [Google Scholar]
- Enhanced activity for CO2 electroreduction on a highly active and stable ternary Au-CDots-C3N4 electrocatalyst. ACS Catal.. 2018;8:188-197.
- [CrossRef] [Google Scholar]
- Semi-chemical interaction between graphitic carbon nitride and Pt for boosting photocatalytic hydrogen evolution. Chinese Chem. Lett.. 2022;33:3061-3064.
- [CrossRef] [Google Scholar]
- Directional utilization disorder charge via In-plane driving force of functionalized graphite carbon nitride for the robust photocatalytic degradation of fluoroquinolone. Chem. Eng. J.. 2022;442:135943
- [CrossRef] [Google Scholar]
- Perylene diimide supermolecule (PDI) as a novel and highly efficient cocatalyst for photocatalytic degradation of tetracycline in water: A case study of PDI decorated graphitic carbon nitride/bismuth tungstate composite. J. Colloid Interface Sci.. 2022;615:849-864.
- [CrossRef] [Google Scholar]
- Brand new P-doped g-C3N4: enhanced photocatalytic activity for H2 evolution and Rhodamine B degradation under visible light. J. Mater. Chem. A.. 2015;3:3862-3867.
- [CrossRef] [Google Scholar]
- Mesoporous phosphorus-doped g-C3N4 nanostructured flowers with superior photocatalytic hydrogen evolution performance. ACS Appl. Mater. Interfaces. 2015;7:16850-16856.
- [CrossRef] [Google Scholar]
- NaClO-induced sodium-doped cyano-rich graphitic carbon nitride nanosheets with nitrogen vacancies to boost photocatalytic hydrogen peroxide production. Chem. Eng. J.. 2022;443:136501
- [CrossRef] [Google Scholar]







