Translate this page into:
Two-dimensional catena–polymer [[[bis (µ-aqua)(bis (µ-cpiap-K2O1:O2)sodium(I))] (µ-chlorido) bis(µ-cpiap-K5N1:O1:O2:O3,O3′)dicopper(II)]bis(µ-aqua)(aqua)bis(cpiap-K2O1,O2)copper(II)] hexa hydrate
⁎Corresponding author. Elmahdawi@yahoo.com (Ramadan M. El-mehdawi)
-
Received: ,
Accepted: ,
This article was originally published by Elsevier and was migrated to Scientific Scholar after the change of Publisher.
Peer review under responsibility of King Saud University.
Abstract
The new title two-dimensional hetero-tetra nuclear Cu3–Na coordination polymer {[NaCu3Cl(cpiap)2(H2O)3]n·6nH2O} (1) consists of crystallographically two-independent copper(II) centers, each bridged by a sodium cation through carboxylate-oxygen of the deprotonated H3cpiap ligand (H3cpiap = 2-(carboxyphenyl)iminoaceticpropanoic acid) to CuII (2) and CuII (2−) cations, and through water molecules to CuII (1) cation. CuII (2) and CuII (1) cations are bridged by carboxylate-oxygen atoms of the ligand in a syn-anti mode which, alternate regularly within the chain being bridged by a tetra coordinated sodium cation. Each CuII (2) and CuII (2−) cation in (1) is in an octahedral environment formed by four carboxylate-oxygens from two cpiap3− ligands, one nitrogen atom and a bridging chloride atom. CuII (1) cation is in a square pyramidal environment formed by three water molecules and two carboxylate-oxygens from two cpiap3− ligands. The ligand acts simultaneously as monodentate and tridentate toward CuII (1) and CuII (2) cations respectively. The lattice water molecules involved in O—H···O hydrogen bonding are situated in the void spaces between layers. The zigzag chains, which run along the b-axes further construct three-dimensional metal-organic framework via hydrogen bonding and weak face-to-face π-π interactions. Weak C—H···O interactions are also present.
Keywords
Crystal structure
Two-dimension
Lattice water
Hydrogen bonding
Copper–sodium complex
1 Introduction
Poly-carboxylate ligands present very rich coordination chemistry, because of their diverse coordination modes and bridging ability to give rise to a variety of poly-nuclear complexes ranging from discrete entities to three-dimensional systems (Wang et al., 2018; Zhao et al., 2018). In this type of complexes, a carboxylate can adopt diverse bonding modes such as terminal monodentate, chelating to one metal center, bridging bidentate in a syn-syn, syn-anti, and anti-anti configuration to two metal centers and extended architectures with dimensionalities ranging from 1 to 3D arrangements. It is known that one-dimensional system are important in understanding the properties of chains, but will not display long range order, while two and three dimensional system may show long range order and this could find application as new molecular based magnetic materials (Powell et al., 2003; Y. Lu et al., 2019; Fang et al., 2019; Hongwei et al., 2015).
Moreover, recently, intense research activities have been directed toward the development of porous frameworks including assembly of coordination complexes and organic molecules into extended motif, held together by strong metal- ligand bonding or very weak forces (Keeff et al., 2001; Seo et al., 2001), and their useful properties applicable to various fields such as size-selection sorption, gas storage, host- guest recognition and catalysis (Wei-Yin et al., 2011; Wang et al., 2014; Zhao et al., 2011). During the past decade, there have been many reports of the synthesis of coordination polymer compounds, using aromatic carboxylic acids combined with aromatic N-containing chelating ligands (Wei-Yin et al., 2011; Wang et al., 2014; Zhao et al., 2011). Herein, we describe the synthesis and crystal structure of a two-dimensional metal-organic polymer {[NaCu3Cl(cpiap)2(H2O)3]n·6nH2O}(1).
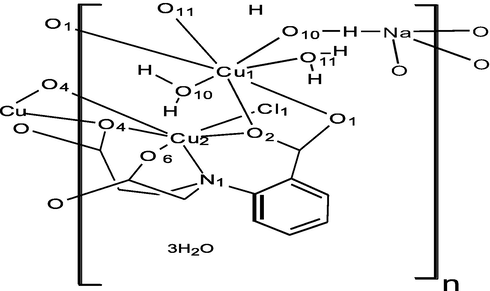
2 Experimental section
2.1 Materials and methods
All chemicals and solvents were used as received without any further purification. All manipulations were carried out under atmospheric pressure.
2.2 Synthesis
2.2.1 2-(carboxyphenyl)iminoaceticpropanoic acid (H3cpiap)
The ligand was prepared using previously described method (Powell et al., 2009).
2.2.2 {[NaCu3Cl(cpiap)2(H2O)3]n·6nH2O} (1)
The title complex (1) was prepared by refluxing (0.14 g, 0.52 mmol) H3cpiap and (0.08 g, 2.0 mmol) NaOH with (0.30 g, 1.25 mmol) Cu (NO3)2·.3H2O in 50% ethanolic solution for one hour. Filtration and slow evaporation at ambient temperature of the resulting blue solution, gave blue block crystals of (1) suitable for x-ray analysis within two days.
2.2.3 Details of the crystal structure determination and refinement of 1
Suitable blue single crystals of the complex were obtained by slow evaporation of the filtrate of the reaction solution at ambient temperature. A blue block shaped crystal of a suitable size was selected for data collection. Reflection data for the X-ray analysis of (1) were collected on a Bruker APEX-II diffractometer equipped with CCD area detector and Cu K/a radiation (1.54178 Å) at 150 K.
Data collection, reduction and absorption correction (SCALE3 ABSPACK routine) were carried out through the suite CrysAlysPro. The structure of the complex was solved by direct methods of the SHELXD program and then refined by full-matrix least squares against F2 using all data (Sheldrick, 2008). The non-hydrogen atoms were refined anisotropically. H atoms were positioned geometrically at distances 0.95 Å (aromatic CH), 0.99 Å (CH2) and 0.98 Å (CH3). The Uiso (H) values were constrained to be 1.2 Ueq (carrier atom) except for CH3 where it was 1.5Ueq (C). The details of the crystallographic data, data collection and structure refinement (Sheldrick, 2014) of (1) are summarized in (Table 1). CIF file is available from the Cambridge Crystallographic Data Center (CCDC number 1495820).
Empirical formula
C24 H38 Cl Cu3 N2 Na O21
Formula weight
939.62
Temperature
150(2) K
Wavelength
1.54178 إ
Crystal system
Orthorhombic
Space group
P21212
Unit cell dimensions
a = 22.7156(6) β = 90°
b = 7.4159(2)
c = 10.1829(3)
Volume
1715.38(8) 3
Z
2
Density (calculated)
1.819 Mg/m3
Absorption coefficient
3.813 mm−1
F (0 0 0)
958
Crystal size
0.220 × 0.150 × 0.060 mm3
Theta range for data collection
4.342–72.379
Index ranges
−27≤h≤23, −9≤k≤8, −10≤l≤12
Reflections collected
14,040
Independent reflections
3392 [R(int) = 0.0410]
Completeness to theta = 67.679°
99.9%
Absorption correction
Semi-empirical from equivalents
Max. and min. transmission
0.804 and 0.727505
Refinement method
Full-matrix least-squares on F2
Data/restraints/parameters
3392 / 0 / 254
Goodness-of-fit on F2
1.102
Final R indices [I > 2sigma (I)]
R1 = 0.0427, wR2 = 0.1189
R indices (all data)
R1 = 0.0446, wR2 = 0.1207
Absolute structure parameter
0.041(17)
Extinction coefficient
n/a
Largest diff. peak and hole
0.874 and −1.861 e.3−
3 Results and discussion
3.1 Crystal structure of the coordination polymer (1)
The structure of the new two dimensional hetero-tetranuclear CuII-NaI coordination polymer {[NaCu3Cl(cpiap)2(H2O)3]n·6nH2O} (1), consists of asymmetric unit contains two crystallographically independent copper(II) centers bridged by carboxylate oxygen atoms via a syn(eq)-anti(eq) mode. The two copper (II) ions Cu1 and (Cu2, Cu2i) are coordinated to Na+1 ion through O- atoms of the carboxylate groups of deprotonated cpiap3− ligand (Cu2 and Cu2i) and through water molecules (Cu1). The two CuII(2) (Cu2 and Cu2i) cations, which are bridged by a chloride atom, (source of the chloride may due to the formation of (RR’R”) NH+ Cl− as part of the ligand during the acidification process) and through the sodium ion (Fig. 1a) are located at the apices of an isosceles triangle, and are bridged by four carboxylate oxygen atoms of two cpiap3− ligands, exhibiting syn-anti mode to form Cu2NaO4 core. The CuII cation in the title complex (1) exhibit two types of coordination spheres, five-coordinate (Cu1) and six-coordinate (Cu2 and Cu2i). Cu(1) has a square pyramidal geometry, and is chelated by two carboxylate-O atoms O1 and O1ii of two cpiap3− ligands in a trans-arrangement, and by three water molecules and two carboxylate-O atoms belonging to two different ligands O1 and O1ii, while the apical position is occupied by a water molecule O11 (w), giving an overall [4 + 1] coordination polyhedron, which can be described as a highly distorted square pyramid. Cu (2) and Cu (2i) atoms are bridged by a chloride atom and each CuII cation is coordinated by four carboxylate-O atoms of two cpiap3− ligands, and through nitrogen atom of one chelating ligand and a chloride atom. The equatorial positions are occupied by carboxylate oxygen atoms O2, O6 and N1 and O4 from another neighboring cpiap3− ligand. The axial positions are occupied by a Cl atom and carboxylate oxygen atom O4ii from the former cpiap3− ligand, giving an overall [4 + 1 + 1] coordination polyhedron, which can be described as a distorted octahedron (Fig. 1a).![(a) Part of the polymeric chain of (1), showing the atom-numbering scheme. Displacement ellipsoids are drawn at 50% probability level. C-bound H atoms omitted for clarity. [Symmetry codes: (1) 1-x, 1-y, z; (ii) 1-x,2-y, z; (iii) 1-x,1-y,1 + z; (iv) x, y, −1 + z; (v) x, y, −1 + z]. (b) Part of asymmetric unit of (1) showing the coordination environment of the Cu1.](/content/184/2020/13/5/img/10.1016_j.arabjc.2020.03.006-fig1.png)
(a) Part of the polymeric chain of (1), showing the atom-numbering scheme. Displacement ellipsoids are drawn at 50% probability level. C-bound H atoms omitted for clarity. [Symmetry codes: (1) 1-x, 1-y, z; (ii) 1-x,2-y, z; (iii) 1-x,1-y,1 + z; (iv) x, y, −1 + z; (v) x, y, −1 + z]. (b) Part of asymmetric unit of (1) showing the coordination environment of the Cu1.
The tripodal moiety of cpiap3− ligand, which has unequal carboxylate arms, has potential coordination sites involving both the nitrogen atom and all of the carboxylate oxygens, coordinate to CuII (2) and CuII(2i) cations as monodentate and tridentate and to CuII (1) cation as a monodentate. The near equalities of the C—O bond distance in the carboxylate groups of the ligand cpiap3−, C10/O3/O4 (1.240, 1.280 Å), C1/O1/O2 (1.261, 1.259 Å) and C12/O5/O6 (1.235, 1.270 Å) indicate delocalized bonding arrangement, rather than delocalized single and double bonds. The bond distances between CuII (2) cation and O2, O6, O4 and O4ii are within the usual range for complexes where monodentate carboxylate groups are present. Selected bond lengths and angles for 1 are given in (Table 2). The Cu—O bond lengths are in the range 1.935(4) – 2.441(4) Å and the two independent Cu···Cu distances within the trinuclear units are 4.626 Å for {Cu2···Cu2i} and 4.533 Å for {Cu2···Cu1}. These units are linked by (cpiap)2Nai(H2O)2 through the oxygen atoms (O5, O6) and (O5i,O6i) of two different cpiap3− ligands via a syn-anti acetate bridge to CuII (2) and CuII(2i) cations respectively and through O10(w) and O10i(w) of two water molecules to CuII1 and CuII1i cations respectively. These distances are in agreement with those reported for similar trinuclear Copper (II) complexes (Christou et al., 2004; Bondi, 1964). Symmetry codes: (i) –x, +1, -y + 1, z, (ii) –x + 1, -y + 2, z.
Cl1—Cu2i
2.3852(17)
Cu2—O2
1.935(4)
Cu1—O1ii
1.978(4)
Cu2—O6
1.955(4)
Cu1—O10ii
1.986(5)
Cu2—O4ii
1.989(4)
Cu1—O11
2.182(7)
Cu2—N1
2.051(4)
O1—Cu1—O1ii
O10—Cu1—O10ii
167.1(2)
176.4(3)O1—Cu1—O11
96.43(12)
As expected for Cu (II) ion in square-pyramidal geometry, the apical Cu1—O11(w) bond distance {2.182(6) Å} is longer than the remaining four distances in the Cu1 coordination polyhedron {Cu1—O10(w) = 1.986(4) Å}, (Table 1). The weak apical bond reflects a weak axial contact, as expected for Jahn-Teller sensitive copper (II) complexes. A short Cu1····O2 and Cu1····O2ii contacts of 2.719 (4) Å [symmetry code: (ii) 1-2x,2-y,z], which is shorter than the sum of Van der Waals radii 2.92A° (Elmehdawi et al., 2014) and even shorter than those reported by (Elmehdawi et al., 2015, Rogan et al., 2011, Palenik et al., 2004), should also be mentioned since the O11—Cu1—O2 angle is 147.78°. So, we consider the coordination geometry about Cu1 is between a [4 + 1] distorted square pyramidal and [4 + 1 + 2] as a distorted capped trigonal prism polyhedron (Fig. 1b), which is not common for the chemistry of Cu (II) complexes (Ruiz-Perez et al., 2000). The two carboxylate-O atoms O2 and O2ii which are in contact with Cu1 were provided by two cpiap3− ligands through bridging Cu1 and Cu2 atoms using anti-syn-syn diatomic fashion (Scheme 1).
The geometry around CuII (2) and CuII(2i) cations are hexa-coordinate octahedral with a distortion caused by a Jahn-Teller (4 + 1 + 1) elongated octahedron environment. The axial Cu2—O4i and Cu2—Cl1 bond lengths (2.441(4) and 2.385(2) Å) respectively, are longer than the equatorial Cu2—O bond lengths (1.989(4), 1.936(4) and 1.955(4) Å) for O4, O2 and O6) respectively. Large deviation from the ideal value of 180˚ was observed for the bond angle around CuII (2) cation for Cl1—Cu2—O4i (164.52°). The Na+(1) ion which is linked to CuII(2) and CuII(2i) cations through O5iii and O5iv of carboxylate groups in syn - anti mode of two different capip3− ligands and to CuII(1) cation through O10(w) of a water molecule, acts as bridge connecting adjacent trinuclear units, forming an infinite one – dimensional chain (Fig. 2).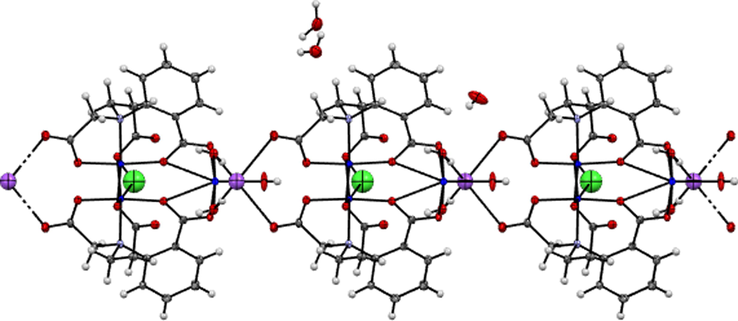
A projection of the 1D chain of 1, formed by trinuclear CuII units through a sodium cationic bridge. Water molecules are omitted for clarity.
The metal-metal separation through bridging carboxylate–O5 and water–O10 are 5.652 Å [Cu(2)···Na(1)] and 3.830 Å [Cu(1)···Na(1)], respectively. The Na1—O bond lengths are (2.684(5)Å and 2.695(6)Å for Na(1)—O10(w) and Na (1)—O5 (carboxylate) respectively, are longer than Cu (II)—O bonds and a little bit shorter than that found in [Na2Ni(mal)2 (H2O)6]n 22. The bond angles around Na (1) atom O5—Na (1)—O5ii, O10(w)—Na (1)—O5 and O10(w)—Na(1)—O10ii(w) are 103.91°, 113.89° and 135.21° respectively, describe a highly distorted tetrahedral environment around sodium ion. The crystal packing structure of (1) indicates that the zigzag chains, which run along the b axis, further construct a three-dimensional metal-organic framework via face-to-face π-π interactions and hydrogen bonding as well as C—H···O type involving the H-atoms of the benzene rings of the ligand (Fig. 3).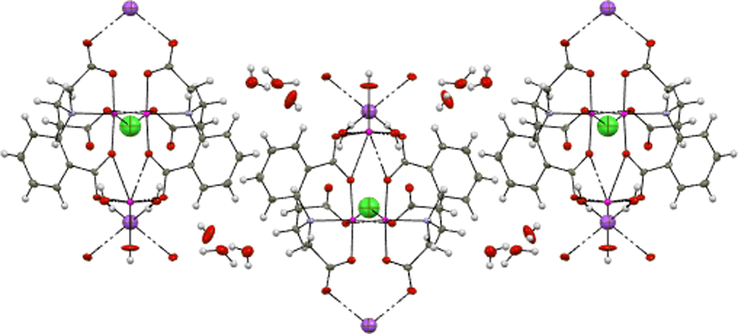
The zigzag chain of (1) running along the b axis.
There is an extensive hydrogen bonding located in the void spaces of the well- defined framework between the three lattice water molecules O7(w), O8(w), O9(w) and the symmetry related O7−(w), O8−(w), O9−(w), within and between layers also, between the carboxylate-O atoms O3vii and, O6viii [symmetry code: (vii) 1-x, 2-y, z, and (viii) x, y, 1 + z], and the coordinated water molecules H2O10(w) and H2O11(w). The hydrogen bond geometry is shown in Table 3. Symmetry codes: (ii) −x + 1, -y + 2, z (iii) x, y + 1, z (iv) −x + 1/2, y + 1/2, −z + 1 (v) –x + 1/2, y−1/2, −z (vi) –x + 1/2, y + 1/2, −z (vii) x, y-1,z (viii) −x + 1/2,y−1/2,−z + 1 (ix) x, y, z + 1.
D—H···A
d(D—H)
d(H···A)
d(D···A)
<(DHA)
C(9)—H(9B)···O(7)iii
0.99
2.59
3.411(8)
140.7
O(7)—H(7A)···O(9)iv
0.90
1.86
2.735(9)
163.0
O(7)—H(7B)···O(8)v
0.77
2.14
2.775(8)
141.0
O(8)—H(8C)···O(5)vi
0.98
1.94
2.874(7)
159.4
O(8)—H(8D)···O(7)iii
0.94
2.53
3.021(9)
113.0
O(8)—H(8D)···O(7)vi
0.94
2.22
2.775(8)
117.1
O(9)—H(9C)···O(1)vii
0.55(14)
2.42(14)
2.963(7)
167(22)
O(9)—H(9D)···O(8)viii
0.90(15)
2.12(15)
2.978(10)
159(12)
O(10)—H(10A)···O (3)ii
0.71(8)
2.05(8)
2.753(7)
166(8)
O(11)—H(11)···O(6)ix
0.86(11)
2.00(11)
2.804(6)
156(13)
The three lattice water molecules O7(w), O8(w), O9(w) and the symmetry related O7−(w) and O8−(w) are arranged in a non-planar cyclic pentamer (Fig. 4a) The water pentamer is similar to the cyclopentane with a one water molecule O8(w) deviate from the plane formed by the other four oxygens O7(w), O9(w), O7−(w) and O8−(w). The O····O distances within the pentamer are in the range 2.735–3.020 Å, which is comparable with the values reported by Powell et al. (2003). The two pentamers are connected through O7 (w) and its symmetry related O8−(w) with an O····O distance of 3.020 Å. The pentamers are clearly shown encapsulated in the void space of the framework of 1 (Fig. 4b).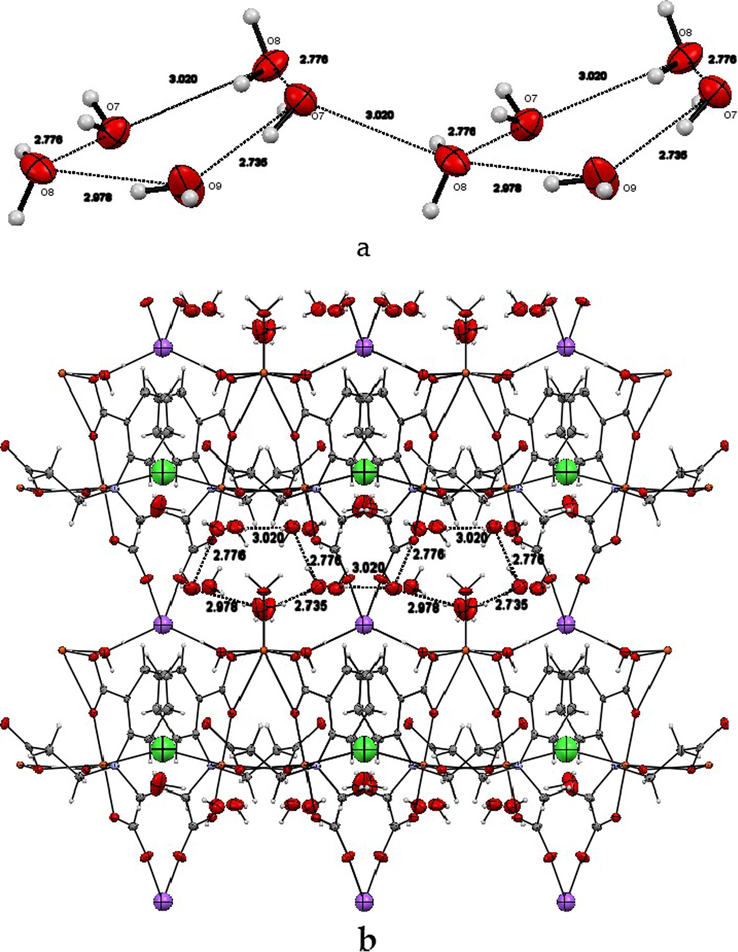
a. The interconnected pentamer water molecules. b. The encapsulated pentamers in the void spaces of the framework of 1.
Further analysis of the packing structure reveals that two of cpiap3− ligands bridges [NaCu3] units forming a two-dimensional network growing perpendicularly to the a-axis (Fig. 5). This network can be described as a honeycomb structure. The orientation of the benzene rings of the ligand cpiap3− belonging to superposed trinuclear units, symmetry related interacts weakly via face-to-face π-π stacking, were the distances are in the range 3.839–3.954 Å.![A portion of the crystal packing showing two-dimensional undulated layer parallel to the [10–1] plane.](/content/184/2020/13/5/img/10.1016_j.arabjc.2020.03.006-fig6.png)
A portion of the crystal packing showing two-dimensional undulated layer parallel to the [10–1] plane.
4 Conclusion
We have synthesized and characterized a two-dimensional coordination polymer of CuII cations with cpiap3− ligands. The two-dimensional networks are extended into three-dimensional supramolecular structure via H-bonding between uncoordinated water molecules and the carboxylate-oxygen atoms of the ligands. The lattice water molecules which are arranged in a cyclic structure via H-bonding in the void spaces between layers also, forms hydrogen bonding with coordinated water molecules. The presence of an alkali metal as a structure element in the present polymer allowed us to prepare copper (II) complex exhibiting a new two-dimensional structure, in which by replacing the alkali metal by 4f elements, we can construct 3d-4f heterometallic frameworks with interesting magnetic properties.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
References
- J. Phys. Chem.. 1964;68:441-451.
- Angew. Chem. Int. Ed.. 2004;43:2117-2121.
- C. Acta Cryst.. 2014;E70:m270-m271.
- Global J. Res. Anal.. 2015;4(6):400-403. 1SSN No2777-8160
- Inorganic Chem. Acta. 2004;357(1):321-324.
- Inorg. Chem. 2003;42:3492-3500.
- Cryst Eng Comm. 2009;11:1089-1096.
- Acta Cryst.. 2011;C67:m230-m233.
- Inorganica Chem. Acta. 2000;298:245-250.
- Tetrahedron Lett.. 2001;42:6353-6355.
- Acta Cryst.. 2008;A64:112-122.
- Sheldrick, G.M., 2014. SHELXL.
- Cryst. Growth Des.. 2018;18:7114-7121.







