Translate this page into:
Ultrasonic assisted extraction of coumarins from Angelicae Pubescentis Radix by betaine-based natural deep eutectic solvents
⁎Corresponding author. liqian1984@gsau.edu.cn (Qian Li)
-
Received: ,
Accepted: ,
This article was originally published by Elsevier and was migrated to Scientific Scholar after the change of Publisher.
Peer review under responsibility of King Saud University.
Abstract
This study focused on extracting coumarins from Angelicae Pubescentis Radix (APR) using deep eutectic solvents (DES). The process parameters were optimized using response surface methodology and characterized by projected scanning electron microscopy analysis. Methods: Eighteen types of DESs based on Choline chloride, Betaine, Sodium acetate, and Ammonium acetate were synthesized. The ultrasonic-assisted effect of these DESs on the yield of coumarins was investigated using high-performance liquid chromatography (HPLC). We studied and optimized the technological conditions for extracting coumarins from APR using a one-factor-at-a-time method and response surface methodology. Additionally, scanning electron microscopy was employed to observe the degree of fragmentation of APR powder before and after extraction with methanol solvent and DESs. The biological activity of DESs extract was evaluated using the free radical scavenging model of 1,1-diphenyl-2-picrylhydrazide (DPPH), while the molecular docking approach was used to explore the binding potential between the coumarins and superoxide dismutase (SOD) enzymes. The results indicated that the natural deep eutectic system with a molar ratio of betaine to ethylene glycol of 1:4 exhibited the most effective extraction. The optimum extraction conditions included a water content of 16.11 % in the DESs, a temperature of 43.52 °C, an extraction time of 59.61 min, and an extraction yield of 3.37 %. Furthermore, scanning electron microscopy revealed that the cells of APR powder obtained through ultrasonically assisted treatment with DESs exhibited the most severe breakage. The experiment demonstrated that the DESs extract exhibited a high scavenging capacity for DPPH free radicals. Conclusion: DESs present as an environmentally friendly solvent option for effectively extracting coumarins from APR, indicating promising potential for substitution of organic solvents.
Keywords
Ultrasonic assisted extraction
Angelicae Pubescentis Radix
Deep eutectic solvents
Coumarins
Antioxidant activity
1 Introduction
Angelicae Pubescentis Radix refers to the dried root of Angelica publicen Maxin f.biserrata Shan et yuan in the Umbelliferae plant family (National Pharmacopoeia Commission, 2020). Described in the Shennong herbal classic as a top-grade drug, APR has been historically significant in treating rheumatism and arthralgia (Gang and Baohua, 2012). Its primary production centers are in Sichuan, Hubei, Anhui, and other regions of China, but it is also distributed in Japan, North Korea, South Korea, and various Southeast Asian countries. APR is characterized by its pungent, bitter taste and is associated with the liver, kidney, and bladder channels. Its primary medicinal functions include dispelling wind and dampnefss, dispersing cold, and relieving pain, making it effective in the treatment of rheumatism, dampness, waist and knee pain, Shaoyin subdued wind headache, headache, and toothache (Wen and Chongchao, 2018).
According to research reports, a total of 69 coumarin compounds and 25 other compounds have been identified and isolated from APR (Janine et al., 2010; Liu et al., 1998; Awale et al., 2006; T et al., 2002). APR contains coumarins and volatile oil components with documented antioxidant, anti-inflammatory, anti-tumor, anti-Alzheimer's, and other pharmacological activities (Luli and Jianguo, 2019; Yao et al., 2018; Markus et al., 2017; Jiahua et al., 2019). Coumarin compounds are the principal active components of APR, and their content serves as a crucial indicator for assessing medicinal value and quality. Its conjugated-double bond structure contributes to a range of pharmacological activities, including anti-inflammatory, anticoagulant, anti-cancer, sedative, anxiolytic, and anti-epileptic effects (Mottaghipisheh et al., 2018; Hou et al., 2019; Chen et al., 2014; Hyun-Ja et al., 2009; Kozioł and Skalicka-Woźniak, 2016). Additionally, APR contains sterols, organic acids, sugars, and various other components (Xiaoliang et al., 2014; Yang et al., 2009). Therefore, the efficient extraction of coumarins from APR holds significant importance for the development and utilization of this resource.
Professor Andrew P. Abbott proposed the concept of deep eutectic solvents (DESs) in 2003 (Abbott et al., 2004). DESs are homogeneous and stable systems formed through hydrogen bonding between hydrogen bond acceptors (HBA) and hydrogen bond donors (HBD). They offer numerous advantages, including being basically non-toxic, simple to synthesize, easy to store, having a high raw material utilization rate, being derived from rich sources, low cost, and biodegradability, among others (Tang et al., 2015; Smith et al., 2014; Jin et al., 2016; Egorova et al., 2017; Hayyan et al., 2015; van Osch et al., 2015; Ying-Jie et al., 2018). This concept has garnered significant attention in the fields of electrochemistry, nanomaterial preparation, catalytic reactions, functional material preparation, and the extraction and separation of bioactive components from medicinal plants (Najmedin and Masoumeh, 2015; Mohammad et al., 2017; Wagle et al., 2014). Previous studies have widely utilized the quaternary ammonium salt choline chloride (ChCl) as a hydrogen bond acceptor (HBA) in the preparation of DESs. However, betaine surpasses ChCl in attractiveness and environmental friendliness, owing to its high biodegradability and low toxicity. Recently, betaine-based DESs have been successful in extracting natural compounds from plant sources, suggesting significant potential for extensive applications (Olivares et al., 2022; Chen et al., 2022; Mohd and Mohd, 2022; Teslić et al., 2022).
Ultrasonic assisted extraction (UAE) utilizes various effects, including strong cavitation, disturbance, high acceleration, crushing, and agitation generated by ultrasonic radiation pressure. This serves to increase the motion frequency and speed of material molecules and enhance solvent penetration, thereby accelerating the entry of target components into the solvent and promoting extraction (Chaoting et al., 2018; Guohui et al., 2001). Furthermore, combining DESs with UAE could significantly enhance the extraction efficiency of phytochemicals from plants.
In this study, eighteen DESs based on Choline chloride, Betaine, Sodium acetate, and Ammonium acetate were synthesized. The ultrasonic-assisted extraction using DESs was applied in the process of extracting coumarins from APR. The results indicated that the DESs with a molar ratio of betaine to ethylene glycol at 1:4 exhibited the most effective solvent performance. The process conditions for extracting coumarins from APR were optimized using the One-factor-at-a-time method, including liquid material ratio, water content, extraction temperature, extraction power, and extraction time, through response surface analysis. This study established an efficient and environmentally friendly extraction method for coumarin compounds in APR. Additionally, it offered a theoretical basis for the development and utilization of coumarin resources in APR and their production.
2 Materials and methods
2.1 Experimental materials
2.1.1 Experimental raw materials
Angelicae Pubescentis Radix from Zhaozhuang village, Maxia Town, Huating City, Gansu Province, was collected and identified by Professor Yuan Chen from the Department of Chinese Herbal Medicine Cultivation and Identification at Gansu Agricultural University. The high-quality Angelicae Pubescentis Radix was selected, washed, dried in the oven, and crushed for further use.
2.1.2 Main reagents
The analytical pure grade of choline chloride, lactic acid, malic acid, glucose, fructose, betaine, ethylene glycol, xylitol, urea, citric acid, glycerol, acetic acid, 1,3-butanediol, 1,4-butanediol, 1,2-propanediol, ammonium acetate, sodium acetate and the chromatographic pure grade of methanol and acetonitrile were purchased from Shanghai Sinopharm Chemical Reagent Co., Ltd. Osthole (batch No. 18032005, purity ≥ 99 %), Columbianadin (batch No. 19091001, purity ≥ 99 %), Imperatorin (batch No.18051502, purity ≥ 99 %), Isoimperatorin (batch No. 18062202, purity ≥ 99 %), Xanthotoxin (batch No. 21070206, purity ≥ 99 %) and Decursin (batch No.21071602, purity ≥ 99 %) were purchased from Chengdu PFID Biotechnology Co., Ltd.
2.2 Experimental method
2.2.1 Chromatographic conditions
The mobile phase consisted of a methanol to water ratio of 73:27, with the detection wavelength set at 325 nm. The flow rate was 1 mL/min, and the column temperature was maintained at 30 °C, with an injection volume of 5 μL.
Fig. 1 illustrates the high-performance liquid chromatography (HPLC) chromatogram of the mixed standard solution and the herbal extract. In addition, Fig. 2 displays the chemical structural formulas of six coumarin compounds.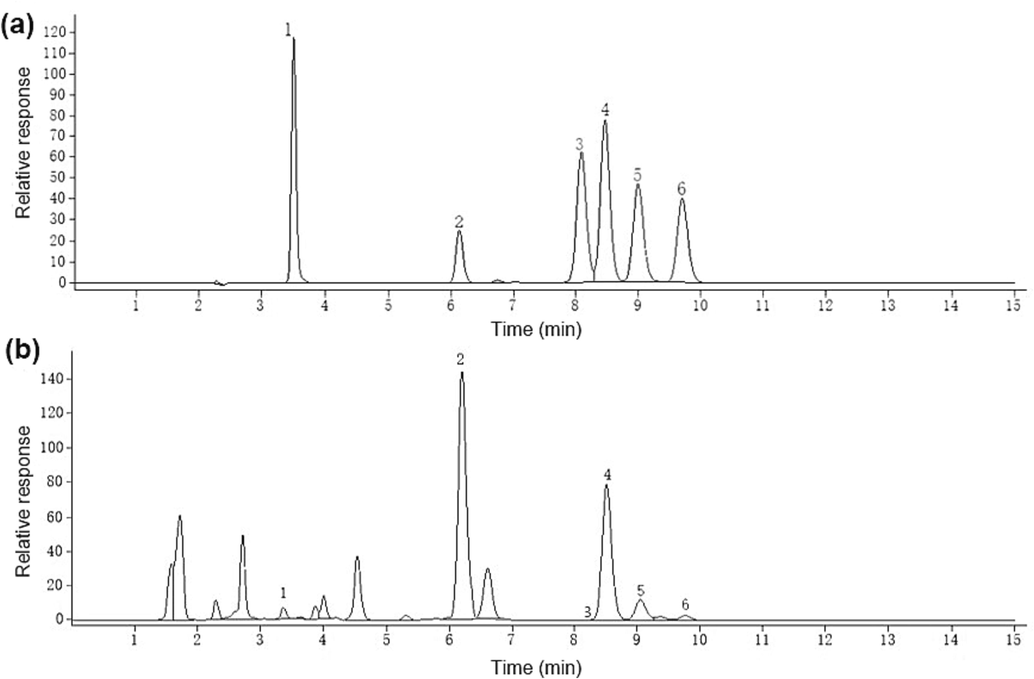
High performance liquid chromatogram of mixed standard solution (a) and herbal extract (b). Notes: 1 — Xanthotoxin, 2 — Imperatorin, 3 — Decursin, 4 — Osthole, 5 — Columbianadin, 6 — Isoimperatorin.
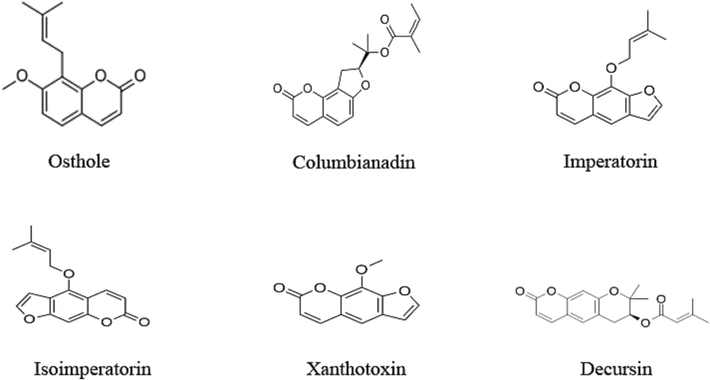
Structural formula of 6 coumarin compounds.
2.2.2 Methodological validation
2.2.2.1 Drawing of standard curve
The standard solutions of Osthole, Columbianadin, Imperatorin, Isoimperatorin, Xanthotoxin, and Decursin, each with a concentration of 1 mg/mL, were accurately prepared using methanol as the solvent. Various concentrations of mixed standard solutions were prepared by combining equal amounts of each standard solution and diluting them with methanol. The known concentration mixed standard solution was injected into the high-performance liquid chromatograph, and the resulting peak area was recorded. Subsequently, linear regression analysis confirmed a strong linear relationship for each component, as evidenced by the values of the regression equation and the correlation coefficient (R2) in Table 1.
No.
Standard
Linear curve
R2
Linear range (μg/mL)
1
Osthole
y = 14.436x-4.2653
0.9992
0.8333 ∼ 100.00
2
Columbianadin
y = 11.543x + 7.2943
0.9993
0.8333 ∼ 100.00
3
Imperatorin
y = 4.7298x-1.3991
0.9994
0.8333 ∼ 100.00
4
Isoimperatorin
y = 10.424x-6.9469
0.9991
0.8333 ∼ 100.00
5
Xanthotoxin
y = 11.587x + 16.109
0.9991
0.8333 ∼ 100.00
6
Decursin
y = 11.145x + 2.7497
0.9991
0.8333 ∼ 100.00
2.2.2.2 Precision test, repeatability test and stability test
The precision of the instrument was assessed through intra-day and inter-day precision tests. A standard sample of a specific concentration was repeatedly tested six times over three consecutive days to establish intra-day and inter-day precision. The relative standard deviations for intra-day and inter-day precision were below 1.20 % and 2.04 %, respectively. Additionally, the test solution was injected six times consecutively to assess repeatability. The stability test involved storing the test solution at room temperature and analyzing it at 0, 2, 4, 6, 8, 10, 12, and 24 h. The relative standard deviation for repeatability test results was less than 1.34 %, and for stability test results, it was less than 1.12 %. These results indicated that the established method was accurate, sensitive, and suitable for the quantitative analysis of 6 components in APR. All the aforementioned data are presented in Table 2.
No.
Analyte
Precision
Repeatability RSD (%)
Stability RSD (%)
Intra-Day RSD (%)
Inter-Day RSD (%)
1
Osthole
0.22
1.38
1.03
0.18
2
Columbianadin
0.44
1.54
1.34
1.03
3
Imperatorin
0.50
1.55
0.45
1.12
4
Isoimperatorin
0.78
2.04
0.43
0.72
5
Xanthotoxin
1.20
1.62
0.47
0.38
6
Decursin
0.21
1.65
0.42
0.74
2.2.3 Preparation of DESs
The hydrogen bond acceptor and donor were mixed in specific molar ratios, heated in a water bath at 80 °C, and stirred to form a transparent liquid, resulting in the formation of deep eutectic solvents (DESs). These DESs were then sealed and stored for later use. Table 3 displays the preparation of 18 DESs using the aforementioned method.
No.
HBAs
HBDs
HBA:HBD: (Water)
Appearance at Room Temperature
1
Choline chloride
Lactic acid
1:2
Yellow viscous liquid
2
Choline chloride
DL-malic acid
1:1:(2)
Yellow transparent liquid
3
Choline chloride
D-glucose
1:1:(3)
White transparent liquid
4
Choline chloride
Fructose
1:1:(3)
White transparent liquid
5
Choline chloride
Xylitol
1:1:(1)
White transparent liquid
6
Choline chloride
Urea
1:1:(2)
White transparent liquid
7
Choline chloride
Citric acid
1:1:(2)
White transparent liquid
8
Choline chloride
Glycerol
1:2
White transparent liquid
9
Choline chloride
Ethylene glycol
1:2
White transparent liquid
10
Choline chloride
Acetic acid
1:2
White transparent liquid
11
Choline chloride
1,3-butanediol
1:2
White viscous liquid
12
Choline chloride
1,4-butanediol
1:2
White viscous liquid
13
Choline chloride
1,2-propanediol
1:2
White transparent liquid
14
Betaine
Ethylene glycol
1:4
White viscous liquid
15
Betaine
Glycerol
1:2
White viscous liquid
16
Sodium acetate
Lactic acid
1:3
Yellow transparent liquid
17
Ammonium acetate
Lactic acid
1:3
Yellow transparent liquid
18
Ammonium acetate
Glycerol
1:3
White viscous liquid
2.2.4 Extraction of coumarins from APR
A 1 g sample of APR powder was combined with a specified amount of DESs in a 150 mL conical flask. Subsequently, the conical flask containing the solution was placed in an ultrasonic bath after mixing and agitation. The ultrasonic treatment was conducted at 300 W and 50 °C for 60 min. Following ultrasonication, the solution was transferred to a centrifuge tube and centrifuged at a speed of 8000 r/min for 10 min. Post-centrifugation, 1 mL of the supernatant was transferred to a 5 mL volumetric flask and diluted to volume with methanol. The extract was filtered using a 0.45 μm microporous membrane and subsequently analyzed by high-performance liquid chromatography. Ultimately, the peak area was measured.
2.2.5 Selection of the best DESs
Nineteen parts of APR powder were accurately weighed and placed in a conical flask. Subsequently, a specific amount of 18 DESs at a liquid material ratio of 20:1 was added to the mixture. The remaining portion was treated with an equivalent amount of methanol solution, enabling a comparison of the extraction yield of DESs with traditional solvents. Following treatment as per 2.2.4, the total yield of coumarins in each extraction solvent represented the combined yield of six different coumarin compounds.
2.2.6 One-factor-at-a-time method
2.2.6.1 Selection of liquid material ratio of DESs
Six 1 g portions of APR powder were precisely weighed and placed in individual conical flasks, to which a specific amount of DESs was added. The study investigated the impact of varying liquid material ratios (10:1, 15:1, 20:1, 25:1, 30:1, and 35:1 mL/g) on the total extraction yield of coumarins. This investigation was conducted under specific conditions, including ultrasonic power of 300 W, a duration of 60 min, a temperature of 50 °C, and a DESs water content of 0 %.
2.2.6.2 Selection of water content of DESs
Six 1 g portions of APR powder were precisely weighed and deposited into conical flasks. DESs with water contents ranging from 0 % to 50 % were sequentially added. The liquid-to-material ratio was 20:1. The impact of varying water contents on the overall extraction yield of coumarins was studied at 300 W ultrasonic power, 60 min processing time, and 50 °C temperature.
2.2.6.3 Selection of extraction temperature of ultrasonic assisted DESs
Five 1 g portions of APR powder were precisely weighed and deposited into conical flasks. DESs were added with a liquid material ratio of 20:1. The impact of various ultrasonic temperatures (30, 40, 50, 60, and 70 °C) on the total extraction yield of coumarins was investigated with 300 W ultrasonic power, 60 min processing time, and no added water.
2.2.6.4 Selection of extraction time of ultrasonic assisted DESs
Five 1 g portions of APR powder were precisely weighed and deposited into conical flasks. DESs were added with a liquid material ratio of 20:1. The impact of varying ultrasonic times (30, 40, 50, 60, and 70 min) on the total extraction yield of coumarins was explored at 300 W ultrasonic power, 50 °C temperature, without added water.
2.2.6.5 Selection of ultrasonic power in ultrasonic assisted extraction of DESs
Six 1 g portions of APR powder were precisely weighed and placed into conical flasks. DESs were added with a liquid material ratio of 20:1. The impact of various ultrasonic powers (240, 300, 360, 420, 480, and 540 W) on the total extraction yield of coumarins was examined at a 60 min ultrasonic time, 50 °C temperature, and 0 % water content of DESs.
2.2.7 Experimental design of ultrasonic assisted extraction of Box-Behnken design (BBD) with DESs
The single factor screening experiment identified three influential factors for APR coumarins, and the extraction process parameters were optimized using the BBD method. The experiment utilized the total extraction yield of six coumarins as the response variable. The experimental designs were created and assessed using Design Expert 8.0.6 software.
2.2.8 Determination of antioxidant activity of plant extracts and molecular docking studies
The antioxidant activity was assessed using the 1,1-diphenyl-2-pyrrolyl (DPPH) free radical method. This study compared the antioxidant activity of extracts from methanol and DESs solvents. Each sample was prepared at varying solution concentrations ranging from 1 to 50 mg/mL. Additionally, a vitamin C (VC) solution with the corresponding concentration was prepared using deionized water as a control test for antioxidant activity. Absorbance was measured at 517 nm with respect to the blank. The percentage of scavenging activity was calculated using the formula: (1-(A1-A2)/A0) × 100 %, where A0 represents the absorbance of the control, A1 represents the absorbance of the sample, and A2 represents the absorbance of the blank sample without DPPH free radical.
Molecular docking technology was utilized to validate the potential antioxidant active ingredients targeting superoxide dismutase (SOD). Retrieve the 3D structure file of the superoxide dismutase protein with the PDB ID 2C9V from the RCSB database. Obtain the structure files of the three small molecules Osthole, Columbianadin, and Imperatorin from the Pubchem database. The 2C9V protein underwent docking with the three small molecules. PyMOL (version 4.3.0) software is utilized to isolate the original ligand and protein structure, dehydrate, and eliminate organic components. Subsequent to the Vina docking, PyMOL and Discovery Studio software are employed to analyze and visualize the forces in 3D and 2D perspectives.
3 Results and discussion
3.1 Selection of the best DESs for extraction
To comprehend the impact of various DESs on the extraction yield of coumarins and their effectiveness in extracting coumarins from APR, the extraction yields of coumarins from APR using methanol and different DESs were compared, as depicted in Fig. 3.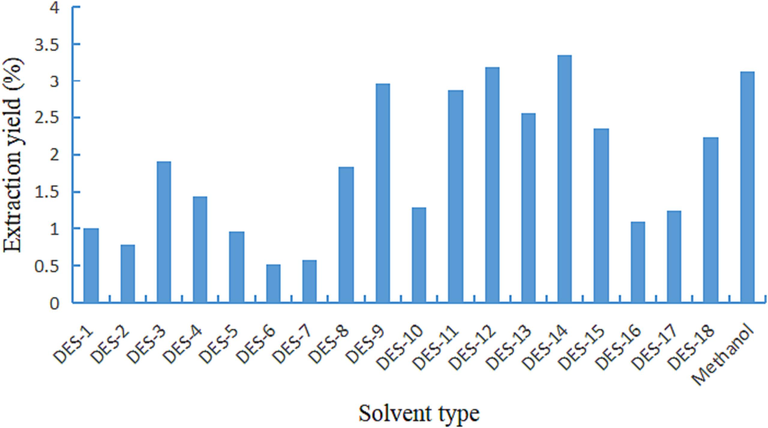
Total extraction yield of six coumarin compounds by different solvent.
Fig. 3 illustrates that the overall extraction yield of six coumarins by certain DESs, such as DESs-6, was low. Conversely, the extraction yields of DESs-9, DESs-11, DESs-12, and DESs-14 were higher. Specifically, the extraction yields of DESs-12 and DESs-14 were 0.07 % and 0.23 % higher, respectively, than that of the traditional solvent methanol (Jie et al., 2020). Moreover, the extraction yield of DESs-14 prepared by betaine: ethylene glycol at the molar ratio of 1:4 was the highest, reaching 3.36 %. This particular DES was selected as the best extraction solvent for coumarin compounds and applied in further experiments.
3.2 One-factor-at-a-time method
3.2.1 Effect of liquid-material ratio on the extraction yield
The extraction yield of six coumarin components at varying liquid-material ratios was assessed under specific conditions: ultrasonic power of 300 W, 60 min duration, temperature of 50 °C, and 0 % water content in the DESs.
Fig. 4 depicts that the extraction yield of coumarins initially increased and then decreased with the varying liquid-material ratio. The yield of coumarins gradually rose when the liquid-material ratio ranged from 10:1 to 20:1 (mL/g). At a 20:1 ratio, the extraction yield peaked. However, when the ratio ranged from 20:1 to 35:1 (mL/g), the coumarin yield declined. This trend indicates that the increase in the liquid-material ratio in the extraction process expanded the contact area between the substance and the solvent, thereby favoring higher coumarin yield (Meng et al., 2012). Hence, 20:1 should be chosen as the optimal extraction liquid-material ratio for coumarins.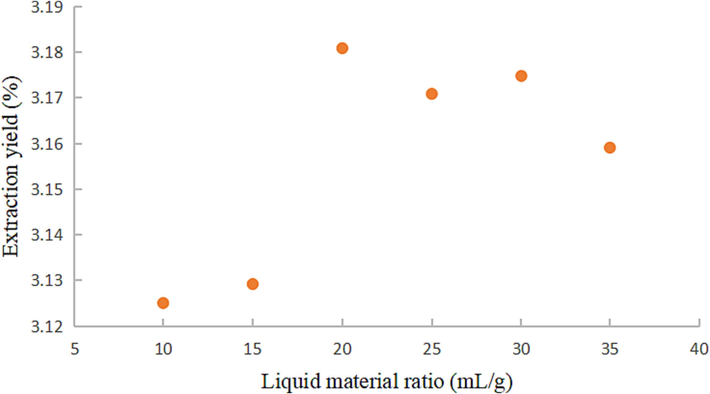
Effect of liquid material ratio on coumarins extraction yield.
3.2.2 Effect of water content of DESs on extraction yield
The extraction yield of coumarin components using DESs with different water content was performed under the following conditions: ultrasonic power of 300 W, extraction time of 60 min, extraction temperature of 50 °C, and a liquid-material ratio of 20:1. As illustrated in Fig. 5, the extraction yield exhibited an increasing trend within the range of 0–20 % water content, and a decreasing trend within the range of 20–50 %. This behavior can be attributed to the elevated polarity of the mixture caused by excessive water, which reduces the interaction between compounds. Consequently, a water content of 20 % was determined to be the most suitable for the DESs in subsequent tests. It is important to note that the screening for low eutectic solvents is not a guarantee for finding an optimal solution, our focus was specifically on water content as an optimization factor for increasing the extraction rate.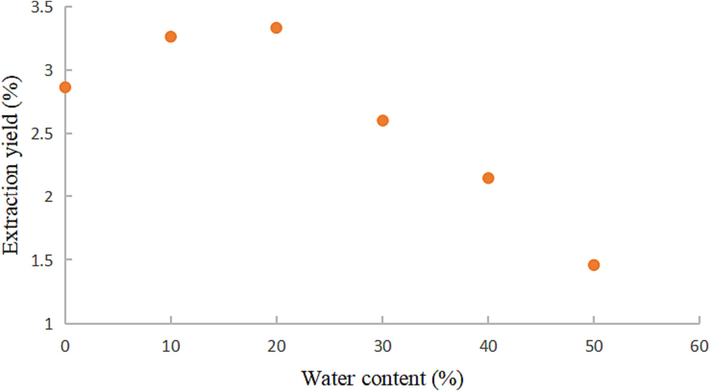
Effect of water content of DESs on extraction yield coumarins.
3.2.3 Effect of extraction temperature on the extraction yield
The extraction yields of coumarin components at various ultrasonic temperatures were conducted with an ultrasonic power of 300 W, an extraction duration of 60 min, a 0 % water content of DESs, and a liquid-material ratio of 20:1.
Fig. 6 depicts the trend of coumarins' extraction yield, revealing an initial increase followed by a decrease with the rise in extraction temperature. Within the range of 30 to 40 °C, the coumarin yield exhibited a gradual ascent, reaching its peak at 40 °C. However, as the extraction temperature entered the range of 40 to 70 °C, the yield showed a declining pattern. The elevation of the extraction medium's temperature is known to augment solvent diffusion and compound solubility, thus favoring component dissolution. Nonetheless, at higher temperatures, some heat-sensitive coumarins may undergo structural changes, leading to a reduction in extraction yield (Leron and Li, 2013). Consequently, 40 °C was identified as the optimal extraction temperature for coumarins.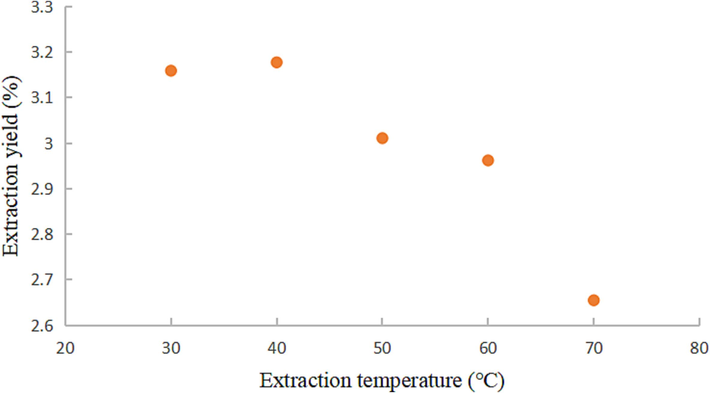
Effect of extraction temperature on extraction yield of coumarins.
3.2.4 Effect of extraction time on the extraction yield
The extraction yields of coumarin components at various ultrasonic durations were conducted under the following conditions: ultrasonic power of 300 W, temperature of 50 °C, 0 % water content of DESs, and a liquid-material ratio of 20:1.
Fig. 7 illustrates an increasing trend in extraction yield within the 30 to 60 min range, reaching its peak at 60 min, and a subsequent decline within the 60 to 70 min range. Prolonged ultrasonic exposure generates a stronger cavitation effect, intensifying the crushing force on the cell wall of medicinal materials and enhancing extraction yield (Milad et al., 2016). However, as the ultrasonic action time is extended, the total extraction yield of the six coumarins decreases, likely attributed to the dual degradation of the system under prolonged ultrasonic radiation and thermal exposure at 50 °C (Jelena et al., 2018). Based on these findings, an extraction time of 60 min is recognized as optimal.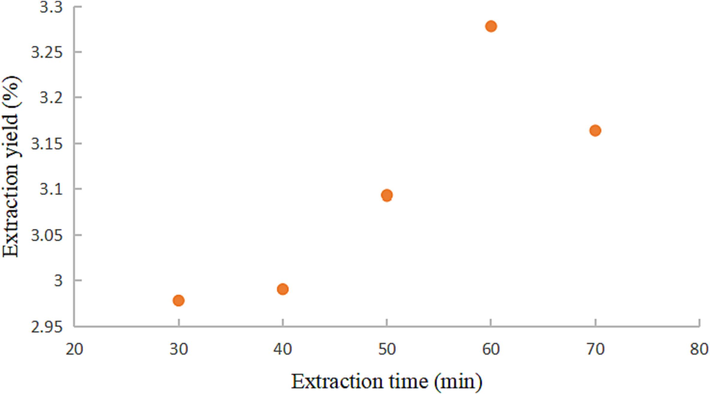
Effect of extraction time on extraction yield of coumarins.
3.2.5 Effect of ultrasonic power on the extraction yield
The extraction yields of coumarin components using various ultrasonic powers were conducted under the following conditions: 60 min of ultrasonic time, a temperature of 50 °C, 0 % water content of DESs, and a liquid-material ratio of 20:1.
As depicted in Fig. 8, the extraction yield of coumarins exhibited an initial increase followed by a decrease in response to the extraction power. Within the range of 240 to 300 W, the coumarin extraction yield gradually rose, reaching its peak at 300 W. However, as the extraction power extended to the range of 300 to 540 W, a declining trend in coumarin extraction yield was observed, likely due to excessive ultrasonic power causing thermal effects that could lead to the degradation of target components. Consequently, an ultrasonic power of 300 W was deemed most suitable for this test.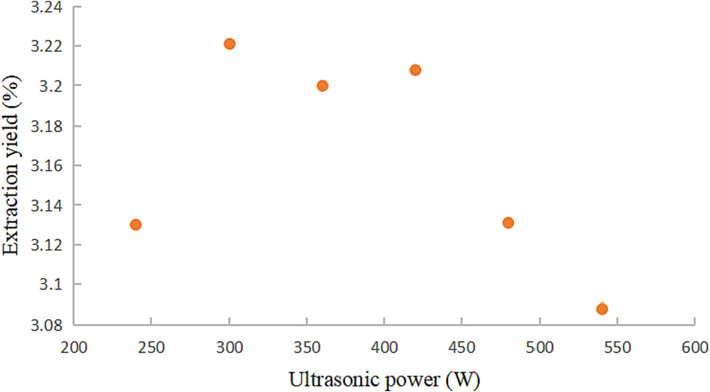
Effect of ultrasonic power on coumarin extraction yield.
3.3 Response surface method
Based on the results of the one-factor-at-a-time method, three significant factors affecting the yield of coumarin components of APR were identified: the water content of DESs, extraction temperature, and extraction time. The response surface method design with three factors and three levels was conducted and analyzed using Design Expert 8.0.6 software, as detailed in Table 4. The response surface design scheme and results along with the analysis of variance are presented in Tables 5 and 6, respectively. Note: **indicates a very significant level (P < 0.01), * indicates significant level (P < 0.05), # It means not significant.
Factor
Level
−1
0
1
A(Water content of DESs /%)
10
20
30
B(Extraction temperature /°C)
30
40
50
C(Extraction time /min)
30
40
50
No.
A
B
C
Extraction yield /%
1
10.00
30.00
60.00
3.21169
2
30.00
30.00
60.00
2.51361
3
10.00
50.00
60.00
3.19019
4
30.00
50.00
60.00
3.13384
5
10.00
40.00
50.00
3.18885
6
30.00
40.00
50.00
2.79218
7
10.00
40.00
70.00
3.21383
8
30.00
40.00
70.00
2.5998
9
20.00
30.00
50.00
3.03483
10
20.00
50.00
50.00
3.18528
11
20.00
30.00
70.00
2.92338
12
20.00
50.00
70.00
3.1501
13
20.00
40.00
60.00
3.33555
14
20.00
40.00
60.00
3.21751
15
20.00
40.00
60.00
3.27393
16
20.00
40.00
60.00
3.37497
17
20.00
40.00
60.00
3.32349
Source
Sum of squares
df
Mean square
F value
P value
Significance
Model
0.97
9
0.11
25.91
0.0001
**
A-Water content
0.39
1
0.39
93.39
<0.0001
**
B-Extraction temperature
0.12
1
0.12
28.55
0.0011
**
C-Extraction time
0.012
1
0.012
2.96
0.1292
AB
0.10
1
0.10
24.96
0.0016
**
AC
0.012
1
0.012
2.83
0.1363
BC
1.454 × 10-3
1
1.454 × 10-3
0.35
0.5734
A2
0.18
1
0.18
44.00
0.0003
**
B2
0.030
1
0.030
7.13
0.0320
*
C2
0.092
1
0.092
22.02
0.0022
**
residual
0.029
7
4.170 × 10-3
Lack of Fit
0.014
3
4.800 × 10-3
1.30
0.3902
#
Pure Error
0.015
4
3.698 × 10-3
Cor Total
1.00
16
Table 6 demonstrates that the regression model was highly significant, and the mismatch was insignificant, indicating the appropriateness of using this model for analyzing the influence of various factors on the yield of coumarin extraction. Additionally, the coefficient of determination (R2) of the equation is 0.9709, signifying that the model accounts for 97.09 % of the total variance. This indicates a strong fit of the model and minimal experimental error, establishing the suitability of the best technological conditions for analyzing the extraction of coumarins from APR. Notably, among several factors, the influence on coumarin yield follows the order of DESs water content > extraction temperature > extraction time. It was observed that A, B, AB, A2, and C2 significantly affected the yield of coumarin, while B2 had a significant effect, and C, AC, and BC did not have a significant effect on the yield.
Design Expert 8.0.6 was utilized to fit the data presented in Table 6, resulting in a quadratic multinomial regression model:
Among these, Y represents the extraction yield of coumarin (%), while A, B, and C denote the water content (%), extraction temperature (°C), and extraction time (min) of DESs, respectively. The figure depicted the effects of water content of DESs, extraction temperature, and extraction time on the total yield of coumarins in APR, as well as the interaction among these factors.
The influence of the two variables on the response value is determined by analyzing the inclination of the surface. A steeper slope, higher contour density, and an oval or saddle-shaped contour line indicate a more significant interaction between the variables. Fig. 9 illustrates that the contour lines of the interaction terms AB, AC, and BC are predominantly saddle-shaped or elliptical, signifying a substantial impact of these factors on the content of the six coumarins. These findings from the surface graph align with the analysis of variance results in Table 6.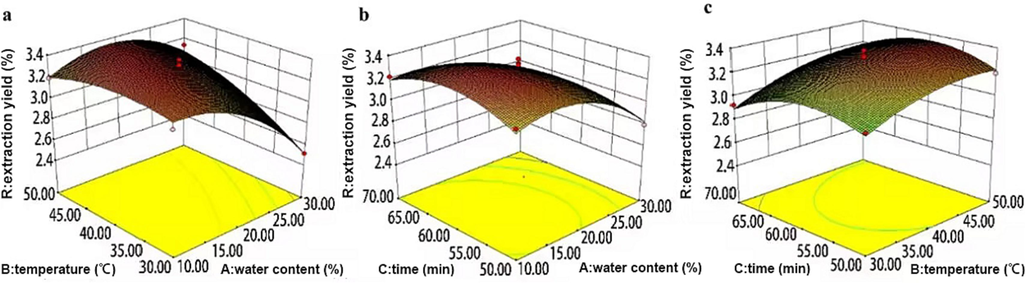
3D response surface diagram of single factor interaction. Note: a represents the interaction between temperature and molar ratio, b represents the interaction between time and molar ratio, and c represents the interaction between time and temperature.
Based on the analysis, the maximum predicted value of the response was 3.37011 %. Under these conditions, the process parameters were as follows: DESs water content at 16.12 %, extraction temperature at 43.51 °C, and extraction time at 59.62 min. For practical purposes, the extraction process conditions were established as DESs water content of 16 %, extraction temperature of 44 °C, and extraction time of 60 min. Three validation tests were conducted under these conditions, resulting in an average coumarin yield of 3.36951 %, closely approximating the predicted value.
3.4 Microstructure of plant materials by scanning electron microscope
The APR powder was fully dried before undergoing ultrasonic-assisted extraction with DESs and methanol. Subsequently, the dried powder was uniformly adhered to the conductive adhesive. Following the coating treatment, the samples were observed using scanning electron microscopy.
Fig. 10 illustrates that the surface of the APR cells before extraction appeared complete, without any evidence of damage. Ultrasonic-assisted extraction using DESs and methanol resulted in a certain degree of damage to the APR cells. The cells were notably disrupted and collapsed, exhibiting a loose structure, with the most significant breakage occurring after ultrasonic-assisted extraction using DESs. Specifically, the extent of microstructural damage in APR showed a positive correlation with the extraction yield of coumarins. Ultrasonic-assisted extraction utilizing DESs led to the destruction of the plant cell wall, resulting in increased compound overflow and higher extraction yields.
Field emission scanning electron microscope scanning image of APR powder before extraction(A), APR powder after ultrasonic assisted extraction by DESs (B), and APR powder after ultrasonic assisted extraction by methanol (C).
3.5 Determination of antioxidant activity of plant extracts and molecular docking studies
The DPPH free radical scavenging activity of solutions extracted with different solvents is shown in Fig. 11. The free radical scavenging activity of coumarin extracts on DPPH was assessed, with VC used as a control. The findings revealed a gradual increase in the scavenging ability of antioxidants in the various solutions as their concentrations increased, stabilizing at a certain concentration. The decolorization of VC to DPPH remained stable at 30 µg/mL, reaching its maximum at 250 µg/mL with a 99.39 % decolorization. Similarly, the decolorization of DESs extract reached stability at 10 mg/mL, with a maximum decolorization of 98.31 %. Notably, the removal rate of the methanol extraction solution at the same concentration was lower than that of DESs solution, with a maximum removal rate of 88.87 %. In conclusion, the VC control solution exhibited the strongest DPPH free radical scavenging ability, followed by the DESs extract, which outperformed the methanol extract. Although the antioxidant capacity of angelica coumarin in the methanol extraction solution was inferior to that of the DESs solution, it still exhibited a significant effect.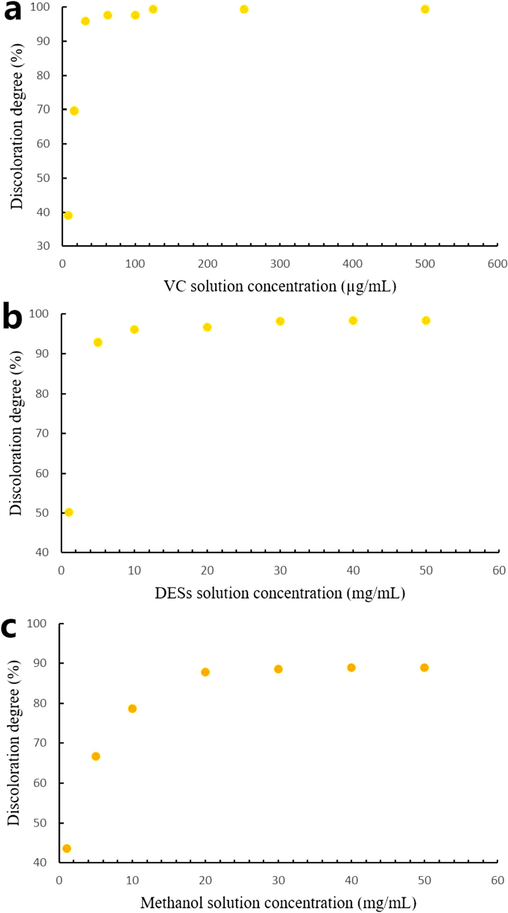
Comparison of DPPH radical scavenging activity in different solutions. (a) VC reference solution; (b) DESs solution; (c) Methanol solution.
The binding mode of SOD with Osthole, Columbianadin and Imperatorin is shown in Fig. 12.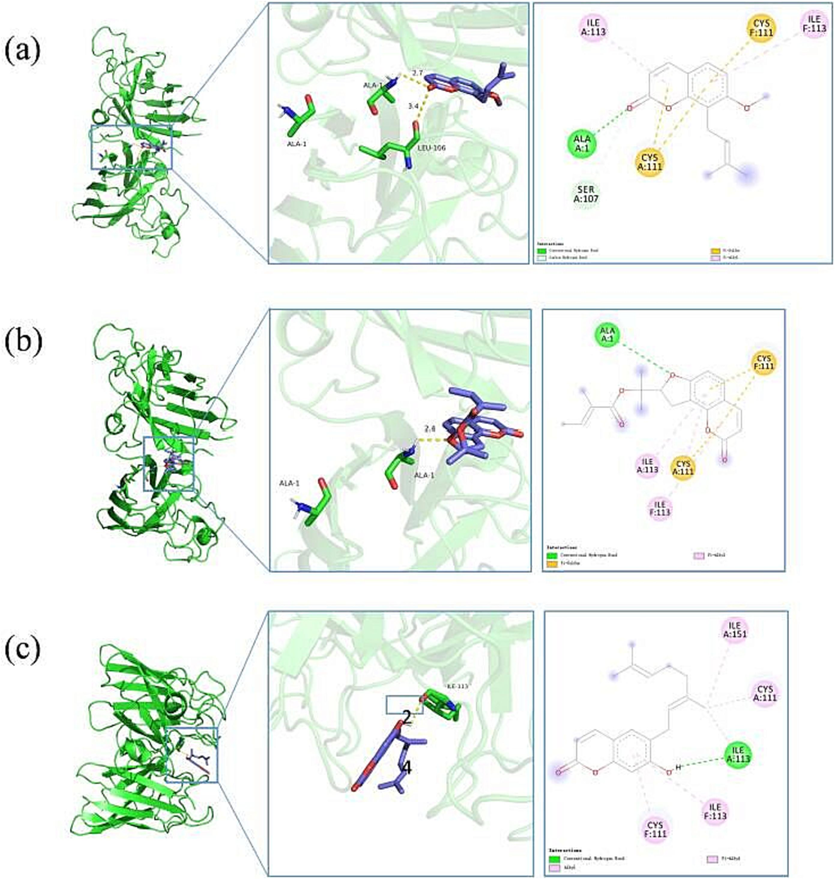
Three-dimensional docking model of Osthole(a), Columbianadin(b) and Imperatorin(c) with SOD.
The binding energy between the protein 2C9V (SOD) and the small molecules Osthole, Columbianadin, and Imperatorin was −7.162 kcal/mol, −7.669 kcal/mol, and −8.151 kcal/mol, respectively. Typically, a binding energy below −5 indicates stable binding between the protein and the small molecule. Therefore, Osthole, Columbianadin, and Imperatorin exhibited strong affinity with the protein 2C9V, suggesting high antioxidant activity of these ligands.
4 Conclusion
In this study, the effects of different combinations of deep eutectic solvents (DESs) on the extraction of coumarins from Angelicae Pubescentis Radix (APR) were screened and compared using the extraction rate of APR as the primary indicator. The extraction process was optimized using the one-factor-at-a-time method and Box-Behnken design to obtain the optimal extraction conditions. These conditions were determined as follows: DESs with a water content of 16.11 %, a temperature of 43.52 °C, an extraction time of 59.61 min, and an extraction yield of 3.37 %. Furthermore, scanning electron microscopy results revealed that the cells of APR treated with ultrasonically assisted DESs suffered the most significant damage, confirming the completeness of the extraction without resource wastage and further validating the superiority of the ultrasonically assisted DESs extraction method. Simultaneously, the antioxidant activity of coumarins from Angelicae Pubescentis Radix (APR) was examined. The results revealed a direct quantitative relationship between the mass concentration and the antioxidant activity of APR. Furthermore, molecular docking results indicated a strong affinity between the ligand small molecules Osthole, Columbianadin, and Imperatorin with protein 2C9V, establishing these components as possessing high antioxidant activity.
In conclusion, the extraction of coumarins using deep eutectic solvents (DESs) exhibits environmentally friendly, cost-effective, efficient, and uncomplicated characteristics. This study established a novel method for extracting coumarins from Angelicae Pubescentis Radix (APR) using DESs synthesized from betaine and ethylene glycol. These findings offer valuable insights into the potential application of DESs in phytochemical extraction and present a new approach to coumarin extraction.
Funding
This work was supported by Longyuan youth innovation and entrepreneurship talent project (GSRC-2023-1-4), Gansu Province university teachers innovation fund project (2023A-53), the National Natural Science Foundation of China (31860102), Youth Tutor Fund project of Gansu Agricultural University (GAU-QDFC-2020-2), Young Talents Introduction Projects of Gansu Agricultural University (GSAU-RCZX201704).
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
References
- Deep eutectic solvents formed between choline chloride and carboxylic acids: versatile alternatives to ionic liquids. J. Am. Chem. Soc.. 2004;126(29):9142-9147.
- [Google Scholar]
- Angelmarin, a novel anti-cancer agent able to eliminate the tolerance of cancer cells to nutrient starvation. Bioorg. Med. Chem. Lett.. 2006;16(3):581-583.
- [Google Scholar]
- Advances in ultrasound assisted extraction of bioactive compounds from cash crops – A review. Ultrasonics Sonochemistry. 2018;48:538-549.
- [Google Scholar]
- Furanocoumarins are a novel class of modulators for the transient receptor potential vanilloid type 1 (TRPV1) channel. J. Biol. Chem.. 2014;289(14):9600-9610.
- [Google Scholar]
- Physical properties of betaine-1,2-propanediol-based deep eutectic solvents. Polymers. 2022;14(9):1783.
- [Google Scholar]
- Biological activity of ionic liquids and their application in pharmaceutics and medicine. Chem. Rev.. 2017;117(10):7132-7189.
- [Google Scholar]
- In Vitro and In Vivo toxicity profiling of ammonium-based deep eutectic solvents. PLoS One. 2015;10(2):e117934.
- [Google Scholar]
- Design, synthesis, and evaluation of new series of Imperatorin analogs with potential vasodilatory activity. J. Asian Nat. Prod. Res.. 2019;21(1):43-50.
- [Google Scholar]
- Anti-inflammatory effect of Columbianetin on activated human mast cells. Biol. Pharm. Bull.. 2009;32(6):1027-1031.
- [Google Scholar]
- HPLC-based activity profiling of Angelica pubescens roots for new positive GABA A receptor modulators in Xenopus oocytes. Fitoterapia. 2010;82(3):434-440.
- [Google Scholar]
- Optimization of ultrasound-assisted extraction of polyphenolic compounds from pomegranate peel using response surface methodology. Sep. Purif. Technol.. 2018;194:40-47.
- [Google Scholar]
- The ethnopharmacology, phytochemistry and pharmacology of Angelica biserrata – A review. J. Ethnopharmacol.. 2019;231:152-169.
- [Google Scholar]
- Study on the extraction of Flavonoids from Fructus aurantii Immaturus with low eutectic solvent. Appl. Chem. Indus.. 2020;49(12):3078-3082.
- [Google Scholar]
- Enhanced solubilization and extraction of hydrophobic bioactive compounds using water/ionic liquid mixtures. Green Chem.. 2016;18(12):3549-3557.
- [Google Scholar]
- Imperatorin-pharmacological meaning and analytical clues: profound investigation. Phytochem. Rev. : Proc. Phytochem. Soc. Europe. 2016;15:627-649.
- [Google Scholar]
- Solubility of carbon dioxide in a choline chloride-ethylene glycol based deep eutectic solvent. Thermochim. Acta: Int. J. Concerned Broader Asp. Thermochem. Appl. Chem. Prob.. 2013;551:14-19.
- [Google Scholar]
- Inhibitory effects of Angelica pubescens f. biserrata on 5-lipoxygenase and cyclooxygenase. Planta Med.. 1998;64(6):525-529.
- [Google Scholar]
- Research Progress on chemical constituents and pharmacological activities of Angelica pubescens. Mod. Chinese Trad. Med.. 2019;21(12):1739-1748.
- [Google Scholar]
- Biosynthesis, characterization, and bioactivities evaluation of silver and gold nanoparticles mediated by the roots of chinese herbal Angelica pubescens Maxim. Nanoscale Res. Lett.. 2017;12(1):46.
- [Google Scholar]
- Application of ionic liquid in lycopene extraction. Chem. Res. Appl.. 2012;24(11):1771-1776.
- [Google Scholar]
- Optimization of pulsed ultrasound-assisted technique for extraction of phenolics from pomegranate peel of Malas variety: Punicalagin and hydroxybenzoic acids. Food Chem.. 2016;206:156-166.
- [Google Scholar]
- One-step and low-temperature synthesis of monetite nanoparticles in an all-in-one system (reactant, solvent, and template) based on calcium chloride-choline chloride deep eutectic medium. Ceram. Int.. 2017;43(2):2046-2050.
- [Google Scholar]
- The formulation and physicochemical properties of betaine-based natural deep eutectic solvent. J. Mol. Liq.. 2022;360
- [Google Scholar]
- Antiproliferative and cytotoxic activities of furocoumarins of Ducrosia anethifolia. Pharm. Biol.. 2018;56(1):658-664.
- [Google Scholar]
- Eco-efficiency and scalable synthesis of bisamides in deep eutectic solvent. J. Mol. Liq.. 2015;206:268-271.
- [Google Scholar]
- National Pharmacopoeia Commission, Pharmacopoeia of the People's Republic of China, China Medical Science and Technology Press, 2020: P. 274.
- Betaine-urea deep eutectic solvent improves imipenem antibiotic activity. J. Mol. Liq.. 2022;350
- [Google Scholar]
- Deep eutectic solvents (DESs) and their applications. Chem. Rev.. 2014;114(21):11060-11082.
- [Google Scholar]
- T, I., V. B and K. I, Escuside, a new coumarin-secoiridoid from Fraxinus ornus bark. Fitoterapia, 2002. 73(5): p. 386-389.
- Application of deep eutectic solvents in the extraction and separation of target compounds from various samples. J. Sep. Sci.. 2015;38(6):1053-1064.
- [Google Scholar]
- Simultaneous hydrolysis of ellagitannins and extraction of ellagic acid from defatted raspberry seeds using Natural Deep Eutectic Solvents (NADES) Antioxidants. 2022;11(2):254.
- [Google Scholar]
- Hydrophobic deep eutectic solvents as water-immiscible extractants. Green Chem.. 2015;17(9):4518-4521.
- [Google Scholar]
- Deep eutectic olvents: Sustainable media for nanoscale and functional materials. Acc. Chem. Res.. 2014;47:2299-2308.
- [Google Scholar]
- Textual research on Radix Angelicae pubescens and common drug pairs. J. Shaanxi Univ. Trad. Chinese Med.. 2018;41(01):127-130.
- [Google Scholar]
- Xiaoliang, G., et al., Current situation and Prospect of Angelica pubescens research. Anhui Agricultural Science, 2014. 42(33): p. 11673-11674+11722.
- Novel coumarin and furan from the roots of Angelica pubescens f. biserrata. J. Asian Nat. Prod. Res.. 2009;11(8):698-703.
- [Google Scholar]
- Global proteomics deciphered novel-function of osthole against pulmonary arterial hypertension. Sci. Rep.. 2018;8(1):5556.
- [Google Scholar]
- Purification of anthocyanins from saskatoon berries and their microencapsulation in deep eutectic solvents. LWT. 2018;95:316-325.
- [Google Scholar]







