Translate this page into:
Ultrasonic promoted catalyst-free N-formylation of amines in neutral ionic liquid 1-butyl-3-methylimidazolium tetrafluoroborate
⁎Corresponding author. Tel.: +91 02462 229215; fax: +91 02462 229220. vtkd@rediffmail.com (Vinod T. Kamble)
-
Received: ,
Accepted: ,
This article was originally published by Elsevier and was migrated to Scientific Scholar after the change of Publisher.
Peer review under responsibility of King Saud University.

Abstract
A catalyst-free, simple and efficient protocol for N-formylation of alkyl, aryl, and heteroaryl amines with formic acid under ultrasonic irradiation at room temperature using 1-butyl-3-methylimidazolium tetrafluoroborate [Bmim]BF4 as a neutral medium is described.
Keywords
1-Butyl-3-methylimidazolium tetrafluoroborate
N-formylation of amines
Catalyst-free
Room temperature
1 Introduction
In recent years, nonclassical methods, particularly, microwave-assisted synthesis, ultrasonic irradiation, and supercritical fluids serve as appealing methods for the efficient high-throughput synthesis of organic compounds (Nuchter et al., 2000). The development of synthetic protocols employing ultrasound irradiation has led to an epoch-making change in organic reactions and has enabled the activation of poorly reactive substrates (Cravotto and Cintas, 2006; Xu et al., 2007). The notable features of the ultrasound approach are enhanced reaction rates, formation of purer products in high yields, easier manipulation and improved energy conservation and waste minimization compared with traditional methods (Wang et al., 2003). Furthermore, one of the most important aspects in green chemistry is the use of ionic liquids (ILs) as greener solvents in organic reactions that is in combination with some advantages such as control of product distribution (Earle et al., 2004), enhanced rate (Earle et al., 1999; Vijayaraghavan and MacFarlane, 2004; Rosa et al., 2001), and/or reactivity (Chauvin et al., 1995), ease of product recovery (Klingshirn et al., 2005; Mizushima et al., 2001), catalyst immobilization (Yadav et al., 2005; Johansson et al., 2005; Serbanovic et al., 2005), and recycling (Picquet et al., 2003; Forsyth et al., 2005; Reetz et al., 2002). Since ILs are neither completely nonvolatile nor non-flammable, the use of ILs omits the risk of combustion by replacement of volatile organic compounds widely used as solvents in organic reactions.
The protection of reactive amino group by formyl (–CHO) group leading to the formation of amide is a commonly required process in organic synthesis. Formamides have been widely used in organic synthesis of biologically important compounds (Jackson and Meth-Cohn, 1995; Chen et al., 2000; Kobayashi et al., 1995; Kakehi et al., 1995). Due to their Lewis basicity, they are also used to catalyze the reactions such as allylation (Kobayashi and Nishio, 1994), hydrosilylation (Kobayashi et al., 1996) of carbonyl compounds, and asymmetric allylation of aldehydes (Iseki et al., 1999). In addition, they have been used in the synthesis of formamidines (Han and Cai, 1997) and isocyanides (Effenberger and Eichhorn, 1977; Schollkopf, 1977; Humer et al., 1971). Recently enormous progress has been made in expanding the scope of N-formylation of amines by employing a wide array of catalysts and formylating agents (Chen and Benoiton, 1979; Sheehan and Yang, 1958; Strazzolini et al., 1990; Giesemann and Ugi, 1983; Waki and Meienhofer, 1977; Yale, 1971; Camps et al., 1987; Duezek et al., 1996; Reddy et al., 2000; Desai et al., 2005; Hosseini-Sarvari and Sharghi, 2006; Akbari et al., 2009; Jung et al., 2002; Zhang et al., 2006; Blick and Lu, 1952; Panella et al., 2006; Hill et al., 2002; Staab et al., 1962; Ma’mani et al., 2010; Das et al., 2008; Krishnakumar and Swaminathan, 2011; Kim and Jang, 2010; Baharami et al., 2013; Majumdar et al. 2013). However, many of these methods suffer from various drawbacks such as use of toxic or somewhat unstable reagents, tedious purification, requirement of inert atmosphere, long reaction time, application of expensive formylating agents and catalysts. Therefore, the development of new methods with good efficiency, convenient, and delivery of better yields is of great interest.
Herein, we describe convenient and highly efficient protocol for the synthesis of formamides under ultrasonic irradiation at room temperature using 1-butyl-3-methylimidazolium tetrafluoroborate [Bmim]BF4 as a neutral medium (Scheme 1).
2 Experimental
IR spectra were recorded on a Bruker spectrophotometer using KBr disks, and the absorption bands are expressed in cm−1. 1H NMR spectra were recorded on a Varian as 400 MHz spectrometer in CDCl3/DMSO-d6, chemical shifts (d) are in ppm relative to TMS, and coupling constants (J) are expressed in Hertz (Hz). Mass spectra were taken on a Macro mass spectrometer (Waters) by electro-spray method (ES). Bandelin Sonorex (with a frequency of 35 kHz and a nominal power 200 W) ultrasonic bath was used for ultrasonic irradiation. Built in heating (25–30 °C) is thermostatically adjustable. The reaction flask was placed inside the ultrasonic bath containing water.
2.1 General procedure
A mixture of aniline (1 mmol), formic acid (2 mmol) and [Bmim]BF4 (0.5 mL) was placed in a 25 mL pyrex flask. This mixture was irradiated for the appropriate time (Table 3) at room temperature. The progress of the reaction was monitored by TLC. The ultrasonic apparatus used showed the temperature automatically, so the temperature was controlled and fixed at room temperature by pouring water in the bath in case of elevation of temperature. After completion of the reaction, ethyl acetate (5 mL) was added and washed with water (3 × 5 mL) and dried over anhydrous Na2SO4. The ethyl acetate was evaporated under vacuum and the products were purified by chromatographic column, using deactivated silica and a mixture of ethyl acetate/petroleum ether (1:9) as the eluent. To recover [Bmim]BF4, after the isolation of products, water was evaporated, and the remaining viscous liquid was washed with ethyl acetate (5 mL) and dried under reduced pressure ([Bmim]BF4 was recovered in 96% yield). The structure of the products was confirmed by IR, 1H NMR and comparison with authentic samples obtained commercially or prepared by reported methods.
Entry
Amine (1)
Product (3)
Time (min)
Yielda (%)
a
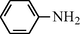
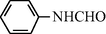
15
99
b
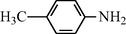
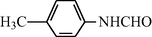
15
92
c
15
93
d
25
89
e
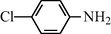

20
90
f
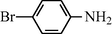
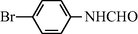
20
88
g
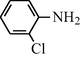
25
87
h
15
90
i
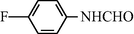
20
89
j
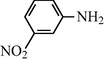

25
87
k
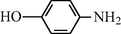
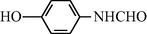
20
87
l
25
85
m
30
85
n
25
88
o
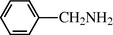
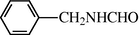
40
90
p
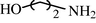
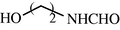
15
92
q
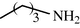
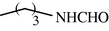
15
90
r
(CH3)2CHNHCH(CH3)2
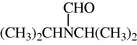
40
85
s

45
82
t
40
85
u
40
85
v


45
85
w
no reaction
–
–
2.2 Spectral data of synthesized compounds
2.2.1 N-Phenyl formamide (3a): IR (KBr)
3245, 3058, 1660, 1571, 1338, 1204 cm−1; 1H NMR (400 MHz, CDCl3): δ 9.25 (br s, 1H, trans), 8.65 (br s, 1H, cis), 8.55 (d, 1H, J = 11.20 Hz, trans), 8.10 (s, 1H, cis), 7.95–7.55 (m, 5H, Ar-H); EI-MS: m/z = 121 (M+).
2.2.2 N-(4-Methylphenyl) formamide (3b)
IR (KBr): 3260, 3050, 1650, 1565, 1340, 1225 cm−1; 1H NMR (400 MHz, CDCl3): δ 9.20 (br s, 1H, trans), 8.75 (br s, 1H, cis), 8.20 (d, 1H, J = 10.20 Hz, trans), 8.10 (s, 1H, cis), 7.90–7.50 (m, 4H, Ar-H), 2.30 (s, 3H); EI-MS: m/z = 135 (M+).
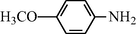
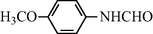
2.2.3 N-(4-Methoxyphenyl) formamide (3c)
IR (KBr): 3345, 3090, 1645, 1570, 1350, 1210 cm−1; 1H NMR (400 MHz, CDCl3): δ 9.10 (br s, 1H, trans), 8.80 (br s, 1H, cis), 8.17 (d, 1H, J = 10.10 Hz, trans), 8.15 (s, 1H, cis), 7.80–7.40 (m, 4H, Ar-H), 3.70 (s, 3H); EI-MS: m/z = 151 (M+).
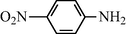
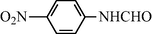
2.2.4 N-(4-Nitrophenyl) formamide (3d)
IR (KBr): 3275, 3060, 1650, 1550, 1310 cm−1; 1H NMR (400 MHz, CDCl3): δ 8.90 (br s, 1H, cis), 8.40 (s, 1H, trans), 8.15 (d, 2H, J = 8.50 Hz, cis), 7.90 (s, 1H, trans), 7.55–7.10 (m, 4H, Ar-H); EI-MS: m/z = 166 (M+).
2.2.5 N-(4-Chloroophenyl) formamide (3e)
IR (KBr): 3265, 3050, 1660, 1540, 1300 cm−1; 1H NMR (400 MHz, CDCl3): δ 9.50 (br s, 1H, trans), 8.41 (s, 1H, cis), 8.30 (d, 1H, J = 8.50 Hz, trans), 8.10 (s, 1H, cis), 7.95 (d, 2H, J = 8.50 Hz, Ar-H), 7.50 (d, 2H, J = 8.50 Hz, Ar-H); EI-MS: m/z = 156 (M+).
2.2.6 N-(4-Bromophenyl) formamide (3f)
IR (KBr): 3275, 3010, 1665, 1550, 1320 cm−1; 1H NMR (400 MHz, CDCl3): δ 9.10 (br s, 1H, trans), 8.50 (d, 1H, J = 10.52 Hz, trans), 8.30 (s, 1H, cis), 8.10 (br s, 1H, cis) 7.85–7.45 (m, 4H, Ar-H); EI-MS: m/z = 200 (M+).
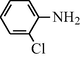
2.2.7 N-(2-Chlorophenyl) formamide (3g)
IR (KBr): 3310, 3040, 1640, 1545, 1320, 1233 cm−1; 1H NMR (400 MHz, CDCl3): δ 8.90 (d, 1H, trans), 8.50 (s, 1H, cis), 8.30 (d, 1H, J = 8.10 Hz, trans), 7.80 (br s, 1H, cis), 7.50–7.30 (m, 4H, Ar-H); EI-MS: m/z = 156 (M+).
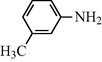
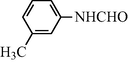
2.2.8 N-(3-Methylphenyl) formamide (3h)
IR (KBr): 3305, 3020, 1645, 1550, 1325, 1215 cm−1; 1H NMR (400 MHz, CDCl3): δ 9.10 (br s, 1H, trans), 8.74 (d, 1H, J = 10.70 Hz, trans), 8.50 (br s, 1H, cis), 8.25 (s, 1H, cis), 7.50–7.35 (m, 4H, Ar-H), 2.34 (s, 3H); EI-MS: m/z = 135 (M+).
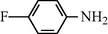
2.2.9 N-(4-Flourophenyl) formamide (3i)
IR (KBr): 3290, 2995, 1665, 1570, 1345, 1210 cm−1; 1H NMR (400 MHz, CDCl3): δ 9.10 (br s, 1H, trans), 8.60 (d, 1H, J = 10.70, trans), 8.25 (s, 1H, cis), 8.10 (br s, 1H, cis), 7.90–7.45 (m, 4H, Ar-H); EI-MS: m/z = 139 (M+).
2.2.10 N-(3-Nitrophenyl) formamide (3j): IR (KBr)
3310, 3015, 1650, 1560, 1350, 1225 cm−1; 1H NMR (400 MHz, CDCl3): δ 8.90 (br s, 1H, trans), 8.50 (d, 1H, J = 8.4 Hz, trans), 8.10 (s, 1H, cis), 7.90 (s, 1H, cis), 7.80–7.20 (m, 4H, Ar-H); EI-MS: m/z = 166 (M+).
2.2.11 N-(4-Hydroxyphenyl) formamide (3k)
IR (KBr): 3320, 3210, 2990, 1645, 1575, 1340, 1210 cm−1; 1H NMR (400 MHz, CDCl3): δ 9.40 (br s, 1H, trans), 9.20 (br s, 1H, cis), 8.45 (d, 1H, J = 10.5 Hz, trans), 8.20 (s, 1H, cis), 7.90–7.40 (m, 4H, Ar-H); EI-MS: m/z = 137 (M+).
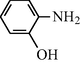
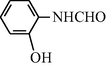
2.2.12 N-(2-Hydroxyphenyl) formamide (3l)
IR (KBr): 3340, 3215, 2995, 1655, 1550, 1360, 1230 cm−1; 1H NMR (400 MHz, CDCl3): δ 9.91 (br s, 1H, trans), 9.50 (s, 1H, cis), 8.90 (br s, 1H, trans), 8.30 (s, 1H, cis), 7.90–7.60 (m, 4H, Ar-H); EI-MS: m/z = 137 (M+).
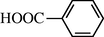
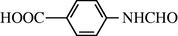
2.2.13 4-Formylamino benzoic acid (3m)
IR (KBr): 3320, 3215, 2995, 1695, 1660, 1545, 1360, 1210 cm−1; 1H NMR (400 MHz, DMSO): δ 11.20 (br s, 1H), 10.25 (s, 1H), 9.52 (s, 1H), 7.90 (d, 2H, Ar-H), 7.60 (d, 2H, Ar-H)); EI-MS: m/z = 165 (M+).
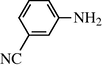
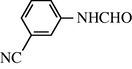
2.2.14 N-(3-cyanophenyl) formamide (3n)
IR (KBr): 3290, 2990, 2145, 1665, 1550, 1360 cm−1; 1H NMR (400 MHz, DMSO): δ 10.50 (br s, 1H, trans), 8.90 (d, 1H, J = 8.80 Hz, trans), 8.50 (s, 1H, cis), 8.32 (s, 1H, cis), 7.80–7.30 (m, 4H, Ar-H); EI-MS: m/z = 146 (M+).
2.2.15 N-benzyl formamide (3o)
IR (KBr): 3260, 2995, 1655, 1545, 1350 cm−1; 1H NMR (400 MHz, CDCl3): δ 8.90 (br s, 1H, cis), 8.60 (d, 1H, J = 11.0 Hz, trans), 8.50 (s, 1H, J = 11.0 Hz, trans), 8.30 (s, 1H, cis), 7.90 (d, 2H, J = 8.80 Hz, Ar-H), 7.65 (d, 2H, J = 8.80, Ar-H), 5.90 (s, 1H, cis), 5.50 (d, 1H, J = 6.0 Hz, cis), 5.10 (d, 2H, J = 6.5 Hz, trans); EI-MS: m/z = 135 (M+).
2.2.16 N-(2-Hydroxyethyl) formamide (3p)
IR (KBr): 3340, 3210, 1645, 1555, 1320 cm−1; 1H NMR (400 MHz, CDCl3): δ 8.21(s, 1H, trans), 8.10 (s, 1H, cis), 6.85(br s, 1H, trans) 6.60 (br s, 1H, cis), 4.75 (br s, 1H, OH), 4.20–3.20 (m, 4H); EI-MS: m/z = 89 (M+).
2.2.17 N-Butyl formamide (3q)
IR (KBr): 3290, 1640, 1530, 1310 cm−1; 1H NMR (400 MHz, CDCl3): δ 8.64 (s, 1H), 5.65 (br s, 1H), 3.40–3.15 (m, 2H), 1.60–1.42 (m, 4H), 1.05 (t, 3H, J = 8.20 Hz); EI-MS: m/z = 101 (M+).
2.2.18 N,N-Diisopropyl formamide (3r)
IR (KBr): 3280, 1665, 1525, 1320 cm−1; 1H NMR (400 MHz, CDCl3): δ 6.95–6.82 (m, 1H), 4.25–4.10 (m, 1H), 3.75 (s, 1H), 1.40–1.18 (m, 12H); EI-MS: m/z = 129 (M+).
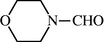
2.2.19 4-Morpholine carbaldehyde (3s)
IR (KBr): 3290, 1660, 1545, 1330, 1120 cm−1; 1H NMR (400 MHz, CDCl3): δ 8.10 (s, 1H), 3.70–3.35 (m, 8H); EI-MS: m/z = 115 (M+).
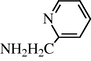
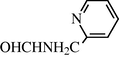
2.2.20 N-(2-Pyridylmethylphenyl) formamide (3t)
IR (KBr): 3285, 1675, 1560, 1360 cm−1; 1H NMR δ (400 MHz, CDCl3): δ 9.06 (s, 1H), 8.50 (s, 1H), 7.8–7.35 (m, 1H), 7.30–7.15 (m, 2H), 6.90 (br s, 1H), 4.66 (s, 2H)); m/z = 136 (M+).
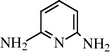
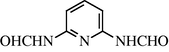
2.2.21 N-(6-Formylamino-2-Pyridyl) formamide (3u)
IR (KBr): 3260, 1670, 1565 cm−1; 1H NMR (400 MHz, CDCl3): δ 9.00 (s, 2H), 8.15 (s, 2H), 7.65 (d, 2H, J = 18.20 Hz), 7.25 (t, 1H, J = 8.20 Hz); m/z = 165 (M+).
2.2.22 N,N-Diphenyl formamide (3v)
IR (KBr): 3270, 1665, 1555, 1320 cm-1; 1H NMR (400 MHz, CDCl3): δ 8.70 (s, 1H), 7.45–7.25 (m, 10H); m/z = 198 (M+).
3 Results and discussion
Initially, we investigated the reaction of aniline 1a with formic acid 2 as a model reaction in order to find the best condition to synthesize formamide 3a. We have varied the amounts of formic acid (1 mmol, 2 mmol, 3 mmol, 4 mmol and excess) and [Bmim]BF4 (0.5 mL, 1 mL, 2 mL, 3 mL and 4 mL) respectively for 1 mmol of aniline. It was observed (Table 1) that the reactants in the amount 1 mmol (aniline): 2 mmol (formic acid): 0.5 mL ([Bmim]BF4) afforded the best results (99% yield of formamide 3a). Next, in order to establish the effect of reaction medium on the yield of product 3a, we investigated various solvents such as ethanol, water, chloroform, PEG-400, acetonitrile and [Bmim]BF4 (0.5 mL each) for the reaction of aniline 1a (1 mmol) with formic acid 2 (2 mmol) in the presence of ultrasonic irradiation at room temperature. The results are summarized in Table 2. [Bmim]BF4 brought the reaction to completion efficiently to furnish the product 3a in excellent 99% yield (Table 2, entry 6). Whereas, reaction in ethanol, water, chloroform, PEG-400 and acetonitrile resulted in low yields (25–40%) and requires long reaction time (Table 2, entries 1–5).
Entry
Aniline 1a (mmol)
Formic acid (mmol)
IL (mL)
Time (min)
Yielda (%)
1
1
1
1
45
90
2
1
2
1
15
94
3
1
2
0.5
15
99
4
1
2
0.3
15
95
5
1
3
1
15
90
6
1
4
1
15
76
7
1
Excess
1
15
65
8
1
1
2
15
94
9
1
2
3
15
90
10
1
3
4
15
89
To explore the generality and scope of this reaction protocol, structurally diverse amines were subjected for N-formylation using 1-butyl-3-methylimidazolium tetrafluoroborate [Bmim]BF4 as a neutral medium. The reactions proceeded smoothly under ultrasonic irradiation at room temperature and were completed within 15–45 min of reaction time. The tolerance of various functional groups under the present reaction conditions has been examined by reacting the substrates with methyl, methoxy, nitro chloro, bromo, fluoro, hydroxy, carboxyl, and cyano groups (Table 3, entries 3b–n), and the reaction conditions are compatible with these functional groups. The reactions proceeded quite cleanly without any side reactions and N-formyl products were obtained in 82–95% yields. Heterocyclic (Table 3, entries 3s–u), and diamine (Table 3, entry u) are also formylated by this procedure without any difficulty. The open chain aliphatic amines reacted smoothly under such reaction conditions (Table 3, entries 3p–r). It is worth noting that primary amines are easily formylated to provide formamide in short reaction time and high yields as compared to secondary amines.
The reaction conditions are highly specific for the amino group and alcoholic or phenolic hydroxyl groups of the starting material remain unaltered in the protection process. For example, for 4-hydroxy aniline the aniline group was converted to the corresponding formyl group while the hydroxy functionality remained unaffected (Table 3, entry 3k). This result suggests that the protocol is useful for carrying out similar chemoselective reactions.
4 Conclusion
In conclusion, we have developed a novel and highly efficient protocol for the N-formylation of amines using [Bmim]BF4 as a nonvolatile medium. This method is bestowed with several advantages such as high conversions, simplicity in operation, cost efficiency, simple workup, neutral reaction conditions, and high yields of the products.
Acknowledgment
The authors thank the Department of Science and Technology, New Delhi, India for Financial support (SR/FT/CS-019/2008).
References
- ARKIVOC (xi):123.
- Tetrahedron Lett.. 2013;54:5064.
- J. Am. Chem. Soc.. 1952;74:3933.
- Synthesis 1987:511.
- Angew. Chem. Int. Ed. Engl.. 1995;34:2698.
- Tetrahedron Lett.. 2000;41:5453.
- Synthesis. 1979;8(8):709.
- Chem. Soc. Rev.. 2006;35:17.
- Tetrahedron Lett.. 2008;49:2225.
- Tetrahedron Lett.. 2005;46:955.
- Synthesis 1996:37.
- Org. Lett.. 2004;6:707.
- Green Chem.. 1999;1:23.
- Tetrahedron Asym..
- J. Mol. Catal. A Chem.. 2005;231:61.
- Synthesis 1983:788.
- J. Org. Chem.. 2006;71:6652.
- Org. Lett.. 2002;4:111.
- J. Med. Chem.. 1971;14:982.
- Tetrahedron Lett.. 1997;38:5423.
- Tetrahedron. 1999;55:977.
- J. Chem. Soc. Chem. Commun. 1995:1319.
- Bull. Korean Chem. Soc.. 2002;23:149.
- J. Organomet. Chem.. 2005;690:3614.
- Bull. Chem. Soc. Jpn.. 1995;68:3573.
- Synlett. 2010;8:1231.
- J. Organomet. Chem.. 2005;690:3620.
- J. Org. Chem.. 1994;59:6620.
- Chem. Lett. 1996:407.
- Chem. Lett. 1995:575.
- J. Mol. Cat. Chem.. 2011;334:98.
- Tetrahedron Lett.. 2013;54:262.
- Appl. Cat. A Gen.. 2010;377:64.
- Green Chem.. 2001;3:76.
- J. Phys. Org. Chem.. 2000;13:579.
- J. Org. Chem.. 2006;71:2026.
- Green Chem.. 2003;5:153.
- Tetrahedron Lett.. 2000;41:9149.
- Chem. Commun. 2002:992.
- Tetrahedron. 2001;57:4189.
- Angew. Chem. Int. Ed. Engl.. 1977;16:339.
- J. Organomet. Chem.. 2005;690:3600.
- J. Am. Chem. Soc.. 1958;80:1154.
- Ann. chem.. 1962;655:95.
- Tetrahedron. 1990;40:1081.
- Aust. J. Chem.. 2004;57:129.
- J. Org. Chem.. 1977;42:2019.
- Synlett 2003:2377.
- Ultrason. Sonochem.. 2007;14:779.
- Tetrahedron. 2005;61:9541.
- J. Org. Chem.. 1971;36:3238.
- Biotechnol. Prog.. 2006;22:514.







