Translate this page into:
Ultrasound-assisted extraction of Cordyceps cicadae polyphenols: Optimization, LC-MS characterization, antioxidant and DNA damage protection activity evaluation
⁎Corresponding authors at: Xinkang road, Ya’an, Sichuan 625014, China. 14126@sicau.edu.cn (Zizhong Tang), chen62hui@163.com (Hui Chen)
-
Received: ,
Accepted: ,
This article was originally published by Elsevier and was migrated to Scientific Scholar after the change of Publisher.
Abstract
In this study, ultrasound-assisted extraction of polyphenols from C. cicadae was optimized by response surface methodology (RSM). The optimized conditions were determined as extraction time of 39 min, liquid-to-solid ratio of 1:29 g/mL, extraction temperature of 69 °C and ethanol concentration of 55% with a yield of 21.9 mg gallic acid equivalent/g dry weight. Four resins were used for polyphenol purification. D101 resin had the highest ratio of adsorption and was further applied in polyphenol purification test. A total of 19 different phenolic compounds were identified by LC-MS, including 12 phenolic acids and 7 organic acids. In addition, C. cicadae polyphenols displayed higher antioxidant activity in vitro and anti-aging activity of C. elegans in vivo. Lastly, C. cicadae polyphenols showed the potential to protect DNA from oxidative damage. Overall, our results suggest that polyphenols from C. cicadae may be considered as novel sources of anti-oxidation, anti-aging and recommended as reagents to protect DNA from oxidative damage in food and pharmaceutical industries.
Keywords
C. cicadae
Polyphenol
Ultrasonic extraction
Antioxidant activity
Oxidative DNA damage
1 Introduction
Cordyceps cicadae is one of the oldest and most famous traditional Chinese medicine. It has a history of more than 1600 years, but it is not the most popular among the Cordyceps family. (Nxumalo et al., 2020). Among nearly 90 species of Cordyceps that have been found in China so far, only C. sinensis has been officially included in Chinese Pharmacopoeia (Wu et al., 2014). Sure, Cordyceps is commonly used in China for prevention and treatment of a variety of diseases and has been utilized in Chinese herbal medicinal prescriptions for thousands of years (Kim et al., 2012). According to Compendium of Materia Medica, C. cicadae can improve eyesight, remove cloudiness of eyes, promote eruption, dispel wind and heat, and relieve convulsion (Nxumalo et al., 2020). Modern pharmacological studies indicate that C. cicadae performs many functions such as anti-tumor, immunomodulatory, anti-oxidation, hypoglycemic, and hypolipidemic activities (Olatunji et al., 2016; Kim et al., 2012). Chemicals isolated from C. cicadae include polysaccharides, galactomannan, cordycepin, adenosine, organic acid, and phenolic compounds (Zhang and Xuan, 2007; Zhang and Xuan, 2008). Phenolic compounds are abundant secondary metabolites that have attracted research attention due to their potential benefits to human health (Limanaqi et al., 2020; Guo et al., 2019). However, current studies on the purification and bioactive components of C. cicadae have mainly focused on the polysaccharides and cordycepin (Olatunji et al., 2016; Liu et al., 2018), and very few studies have been conducted on polyphenols.
Extraction of polyphenols can be accomplished by various extraction procedures. Conventional extraction methods such as heating, boiling, or refluxing, have several disadvantages, including the loss of polyphenols due to oxidation, ionization and hydrolysis during the complex extraction processes and the long extraction times (Dragovíc-Uzelac et al., 2012). Recently, several studies indicate that ultrasound yields more abundant biomolecular extracts with shorter extraction time compared to conventional extraction techniques (Elez Garofulic et al., 2018; Roselló-Soto et al., 2015; Vilkhuet al., 2008; Šic Žlabur et al., 2015). Chmelová et al. (2020) found that the content of phenolic compounds extracted from Picea abies by UAE was 1.1–7.1 times higher than that obtained by SE (solvent extraction). Ultrasonic assisted extraction is affected by many factors. An optimal extraction method is very important for the extraction rate of polyphenols.
Different experimental design methods are used to optimize the extraction procedure, but the two most commonly used methods are single factor experiment and response surface methodology(RSM) (Živković et al., 2018). RSM is a powerful statistical method involving multiple variables. It is more advantageous than the traditional single parameter optimization because it reduces the use of time, space and raw materials (Tang et al., 2021). In this sense, the Box-Behnken design (BBD) is one of the most commonly used RSMs, requiring less experiments and running at least three levels (low, medium, and high: coding −1, 0 and +1). However, according to the literatures, few studies involved the optimization extraction of active substances from C. cicadae. In this study, considering the four independent variables of ethanol concentration, temperature, time and liquid–solid ratio, polyphenols were extracted from C. cicadae by ultrasonic extraction technology and optimized by RSM (BBD).
At present, it has been found that excess reactive oxygen species (ROS) in the body could cause lipid peroxidation, producing lipid peroxidation products that attack biofilms of polyunsaturated fatty acids, DNA, proteins, and enzymes. In addition, the oxidative damage caused by ROS is a major contributor to body aging (Zhu et al., 2020). In this sense, numerous lines of evidence suggest that polyphenols have the capacity to mitigate age-associated cellular damage that is related to oxidative stress, chronic inflammation, and toxin accumulation (Bonomini et al., 2015). Because of the existence of multiple aging-related diseases and disorders, the need for a comprehensive understanding of the possible role of polyphenols in regulating these diseases is critical (Ayuda-Duran et al., 2019). However, the beneficial effects of polyphenolic compounds have been mainly inferred from in vitro studies or studies with short-term dietary supplements. Because of their cost and duration, little is known about whether polyphenols are beneficial for the whole animal, especially in the context of aging (Wilson et al., 2006). In order to address this question, we studied the effects of C. cicadae polyphenols on the C. elegans.
It is already well documented that oxidative stress may lead to DNA damage, which is closely associated with several serious illnesses such as cancer, infertility, stem cell dysfunction, neurodegenerative disorders, cardiovascular diseases, metabolic syndrome and ageing problems (Dong et al., 2021). However, to the best of our knowledge, no studies have addressed the effects of C. cicadae polyphenols on DNA stability and damage so far.
Therefore, the objective of the present study was to apply RSM to optimize the process parameters of UAE including ethanol concentration, extraction time, extraction temperature and liquid-to-solid ratio, and to maximize the content of the extracted polyphenols. Individual polyphenolic compounds purified with D101 were identified by LC-MS, and their antioxidant activities were further evaluated, including 2,2-diphenyl-1-picrylhydrazyl (DPPH) free radical scavenging ability, hydroxyl radical (•OH) scavenging ability, 2,2-azino-bis-(3-ethylbenzothiazoline-6-sulfonic acid) radical (ABTS+) scavenging ability and chelating of ferrous ions ability. Lastly, the effects of polyphenols on longevity and in vivo enzyme activity in C. elegans, as well as protection against oxidative DNA damage were also studied. The current study will provide new knowledge about this fungus and expand the possibility of its application.
2 Materials and methods
2.1 Materials and reagents
Folin-Ciocalteau reagent was purchased from Beijing Bio Lebo Technology Co. Ltd. (Beijing, China). Gallic acid (GA) was purchased from Sinopharm Chemical Reagent Co. Ltd. (Shanghai, China). S-8, D101, AB-8 and NKA-9 resins were procured from Shanghai Yuanye Biotechnology Co. Ltd. (Shanghai, China). ABTS and DPPH were obtained from Sigma-Aldrich Corporation (USA). Unless otherwise stated, all reagents used were of analytical grade.
2.2 Experimental method
2.2.1 Extraction of polyphenols
Fresh C. cicadae were obtained from Dazhu County, China. After washing, the C. cicadae was freeze-dried at −80 °C (Scientz-10N, Ningbo, China) for 48 h and ground into powder. Polyphenols were extracted with ethanol solvent using an ultrasonic bath (410HTD, Jato Co. Guangdong, China) with a fixed frequency of 40 kHz and 240 W power. In all experimental runs, cicadae powder samples (1 g) was mixed with ethanol solvent in 250 mL flask and the extractions were completed at different extraction times and temperatures. The extracts were filtered through filter paper under vacuum and the ethanol solvent was removed using a rotary vacuum evaporator (RE-52, Shanghai, China) under reduced pressure and at a temperature of 45 ± 5 °C.
2.2.2 Single factor extraction experiments
In this study, the effects of four factors (temperature, time, liquid-to-solid ratio, and ethanol addition) on the yield of polyphenols were investigated. The single factor experimental design was shown in Table 1. Single factor experiments were used as the basis for the possible extraction range for RSM.
Single factor
Ethanol concentration
(%)Liquid-solid ratio
(mL/g)Time
(min)Temperature
(°C)
Ethanol concentration
30, 40, 50, 60, 70
40
40
70
Liquid-solid ratio
50
20, 30, 40, 50, 60
40
70
Time
50
30
20, 30, 40, 50, 60
70
Temperature
50
30
40
50, 60, 70, 80, 90
2.2.3 Experimental design of RSM
A reasonable range of parameters was selected for the RSM based on the results of the single-factor experiments. The four process variables were optimized at three levels according to the Box-Behnken experimental design (BBD), which was run 29 times (Table 2).
Level
A
Ethanol concentration(%)B
Liquid-solid ratio(mL/g)C
Time(min)D
Temperature(°C)
−1
40
20
30
60
0
50
30
40
70
1
60
40
50
80
2.3 Determination of polyphenols content
The content of total polyphenols was determined by the Folin-Ciocalteu method described by Vrhovsek et al. (2001), using GA as the standard. Absorbance (Abs) was measured at 750 nm using a microplate detector (Spectra Max M2, Molecular Devices, USA). The total polyphenol content was expressed as mg of GA equivalent (GAE) of dry weigh of C. cicadae powder (mg GAE/g DW).
2.4 Adsorption and desorption capacities of the four resins
The pretreated S-8, D101, AB-8 and NKA-9 resins were added with 60 mL of polyphenol crude extraction solution each. They were shook with 100 rpm at 25 °C for 24 h, and the adsorption rate of polyphenols with the standard curve was calculated. The adsorbed resin was washed with distilled water and then 60 mL of ethanol solution (60%). The mixture was shook under the same conditions, and the desorption rate of polyphenols was calculated by the same method. The adsorption rate and desorption rate were calculated as follows (Guo et al., 2019): where C0 represents the concentration of crude polyphenols, Cr represents the concentration of adsorbed polyphenols, Cd represents the concentration of desorbed polyphenols.
2.5 FT–IR analysis
The FT-IR spectra of the C. cicadae polyphenols were recorded using a Nicolet 6700 spectrophotometer (Thermo Scientific, USA) with the frequency range of 4000–400 cm−1. The polyphenols were mixed with spectral grade potassium bromide powders and then pressed into 1.0 mm pellets.
2.6 Analysis of phenolic acids using LC–MS
The LC-MS (Waters, UPLC; Thermo, Q Exactive) analysis platform was used in this study. Separation was performed on an ACQUITY UPLC HSS T3 (2.1 * 100 mm 1.8 μm). The mobile phase consisted of two solvents: water-formic acid (0.05%) (A) and acetonitrile (B). The constant temperature of the column was controlled at 40 °C. The injection volume was 10 μL and the flow rate was set to 0.3 mL/min. The gradient procedure was used: 0–1 min, 95% A; 1–12 min, 95–5% A; 12–13.5 min, 5% A; 13.5–13.6 min, 5–95% A; 13.6–16 min, 95% A. The electrospray Ionization (ESI) source was operated in negative ion mode and obtained full-scan mass spectrometry data in the range of m/z 70–1050. ESI- parameters were defined as: Heater Temp 300 °C, Sheath Gas Flow rate 45arb; Aux Gas Flow Rate 15arb; Sweep Gas Flow Rate 1arb; spray voltage 3.2KV; Capillary Temp 350 °C; S-Lens RF Level 60%.
2.7 Antioxidant activity of purified polyphenols in vitro
Polyphenols of 0.2, 0.4, 0.6, 0.8, and 1.0 mg/mL were prepared accurately. The scavenging ability for DPPH, ABTS+, hydroxyl radicals and ferrous ion chelating activity were assayed with reference to Patial et al. (2019), Zhao et al. (2020), Cao et al. (2019) and Kuang et al. (2020).
2.8 Anti-aging activity of purified polyphenol in vivo
2.8.1 Strains and culture
C. elegans strains N2 (wild type) and E. coli OP50 were obtained from the Botany Laboratory of Sichuan Agricultural University. The strains were kept at 25 °C according to standard methods, cultured on NGM plates, and seeded with E. coli OP50 (Stiernagle, 1999).
2.8.2 Food clearance assay
The proper polyphenol concentrations were estimated by the consumption rate of food sources. 10 μL smedium liquid medium containing 20–30 pieces of L4 phase N2 was added to 96 well plate, and then added 90 μL E. coli OP50 suspension containing polyphenols (0, 0.25, 0.5, 1.0, 1.5, 2.0, 2.5, 3 mg/mL), cultured at 20 °C. The absorbance at 595 nm was measured every day.
2.8.3 Life span assay
Worms synchronized to the L4 stage were selected and provided with sufficient E. coli OP50 as food on plain NGM plates with different concentrations of polyphenols. During spawning, hermaphrodites were transferred to new NGM (with polyphenols) daily to exclude an influence of their offsprings. Worms were observed every 24 h until they were all dead. Worms were considered dead if they did not move when gently touched by the worm picker. Those worms that disappeared from the dish and those that died prematurely due to internal hatching or vulval orgies were excluded from the analysis.
2.8.4 Reproductive experiments
At least 10 synchronized L4 larvae were randomly transferred to fresh NGM plates treated with polyphenols. Only one nematode was living on each NGM plate. They were transferred to a new NGM plate every 24 h. Offspring was counted at the L2 or L3 stage. The total offspring number within 6 days was denoted as initial reproduction.
2.8.5 Motility Measurement.
The worms were cultured in the same way as in the ordinary lifespan assay. Motility was measured on day 1 (L4 stage), day 5, day 8, and day 12. The motility of worms was classified as A, B or C (Herndon et al., 2002). Worms that were able to perform sinusoidal movements spontaneously were classified in category A. Worms that could not perform sinusoidal movements under stimulation but could still move belonged to category B. Worms that could only move their head or tail under stimulation were classified as Class C.
2.8.6 Effect of polyphenol on the lifespan of C. elegans with heat stress assay
The worms synchronized to L4 stage were picked and provided with sufficient E. coli OP50 as food on plain NGM plates with different concentrations of polyphenols. These NGM plates had 25 μM 5-fluoro-2′-deoxyuridine (FUdR) and were replaced a when worm eggs were found during the experiment.
After 5 days, worms were transferred to new NGM plates. Then, their survival at 37 °C was monitored every 1 h until they were all dead. Worms were considered dead if they did not move when gently touched by a worm picker. Those worms that disappeared from the plate and died prematurely from internal hatching or vulval rapture were excluded from the analysis.
2.8.7 Effect of polyphenols on antioxidant-related enzymes in C. elegans
N2 at L4 stage was cultured in NGM medium containing different concentrations of polyphenols, 50 µg/mL FUdR and E. coli OP50 for 48 h, then treated with heat stress at 37 °C for 12 h. N2 was flushed down with M9 buffer. The cleaned N2 was ultrasonicated in PBS (PH 7.4) (300 W, 6 min, 2 s, 4 s interval), and further centrifuged at 4 °C for 10 min (12,000 r/min). The resulted supernatant was stored at −80 °C.The content of MDA and the activities of SOD, CAT and GSH-Px in C. elegans were determined according to the instructions of the kits.
2.9 Determination of DNA damage protection activity
The inhibition of superhelical xyn11A plasmid DNA strand breaks by ciclopirox polyphenols was estimated using a DNA stapling assay as described by Jin et al. (2016). The reaction mixture contained 10 mM PB (2 μL), plasmid DNA (3 μL, 1462 μg/mL), purified polyphenols (2 μL, 0.1, 0.2 and 0.4 mg/mL), 1 mM FeSO4 (2 μL) and 1 mM H2O2 (2 μL). The mixture was incubated at 37 °C for 40 min. After incubation, 3 μL of a loading buffer was added to stop the reaction, followed by electrophoresis of the reaction mixtures on a 1% agarose gel at 120 V for 30 min. After electrophoresis, the gel was stained with ethidium bromide and then visualized under the UV Transilluminator using the Gel Doc XR system (BioRad, Hercules, CA, USA).
2.10 Statistical analysis
All results were obtained by evaluating the means ± standard deviation (SD) and all experiments were performed in triplicate. Data were assessed by ANOVA (analysis of variance) and the statistical significance of the results was analyzed by applying Duncan’s multiple range test and t-test. Statistical analyses were performed by using IBM SPSS Statistics (Version 25.0, IBM Corp., Armonk, NY, USA).
3 Results
3.1 Single factor experiment
As shown in Fig. 1A, the polyphenol yield increased with increasing ethanol concentration, ultimately reaching a maximum at 50%. These results also indicate that ethanol concentration has a significant positive effect on polyphenol yield when it is below 50%. The effect is not significant when ethanol concentration is above 50%. When the concentration of ethanol continued to increase, the polarity of the solution increased, leading to a decrease in the solubility of polyphenols and a decreasing trend in the extraction rate (Feng et al., 2015). Therefore, 50% was selected as the central point of ethanol concentration in the RSM experiments.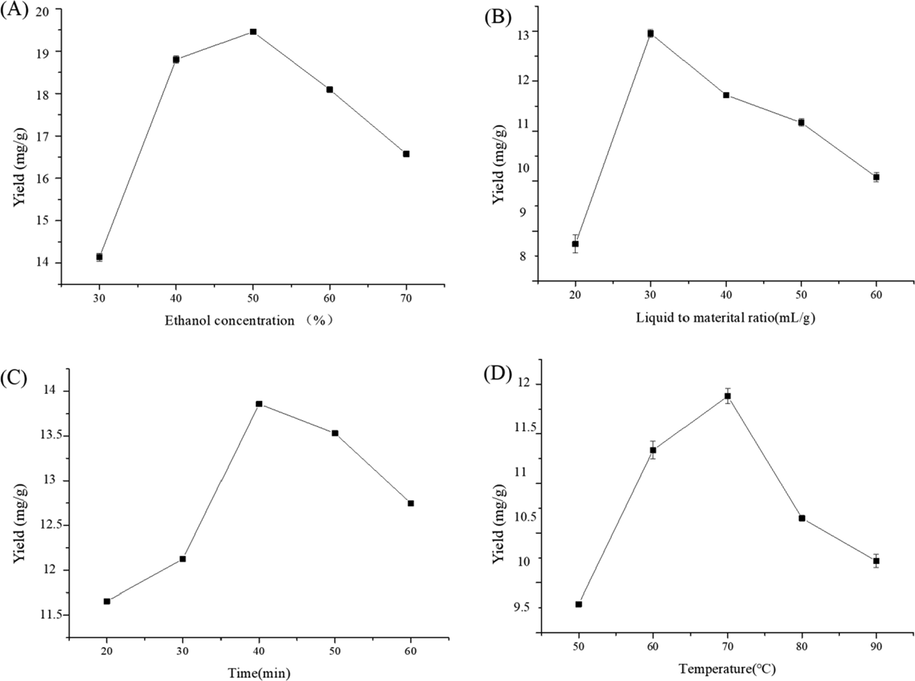
Effect of different ethanol concentration (A), liquid to material ratio (B), time (C), temperature (D) on extraction yield of polyphenol.
Usually, a larger liquid-to-solid ratio can dissolve constituents more effectively and thus increase the extraction yield (Jiang et al., 2020). However, on the one hand, large liquid-to-solid ratio causes solvent wastage. On the other hand, a small liquid-to-solid ratio will result in lower extraction yield. Therefore, it may be important to choose a proper liquid-to-solid ratio (Kaderideset al., 2019). As shown in Fig. 1B, the polyphenol yield increased slowly with increasing liquid-to-solid ratio, ultimately reaching a maximum at 1:30 g/mL. These results also indicate that the liquid-to-solid ratio has a significant positive effect on polyphenol yield when it is below 1:30 g/mL. Therefore, from an economic perspective, 1:30 g/mL was selected as the central point of liquid-to-solid ratio in the RSM experiments.
As shown in Fig. 1C, polyphenol yields increased slowly with increasing extraction time, eventually reaching a maximum at 40 min, indicating that extraction time has a significant positive effect on polyphenol yield when it is below 40 min. The effect was not significant when extraction time was longer than 40 min. There was a gradual decrease in polyphenol yield with increasing time, probably because the molecular structure of polyphenols was disrupted for a long time and therefore the extraction rate decreased (Liu et al., 2020; Oreopoulou et al., 2019). Therefore, 40 min was selected as the central point of extraction time in the RSM experiments.
As shown in Fig. 1D, the polyphenol yield increased with the increase of extraction temperature, and finally reached the maximum at 70 °C. The increase in temperature increased the movement of solvent molecules and solute molecules and promoted diffusion, so the extraction rate increased. When the temperature continued to rise, the extraction rate showed a downward trend, which was mainly due to the fact that polyphenols are heat-sensitive substances that are easily oxidized by heat, resulting in a decline in extraction rate (Al-Dhabi and Ponmurugan, 2020). These results also indicate that extraction temperature has a significant positive effect on polyphenol yield when it is below 70 °C. The effect is not significant when extraction temperature is higher than 70 °C. Therefore, 70 °C was selected as the central point of extraction time in the RSM experiments.
3.2 Optimization of UAE conditions
3.2.1 Model fitting
Table 3 shows the results of the experimentally measured responses based on the experimental design for the 29 runs.
Coded (Independent variables)
Yield (mg/g)
Runa order
A
B
C
D
1
50
30
40
70
22.2
2
50
30
40
70
23.2
3
50
40
40
80
17.8
4
50
40
30
70
18.0
5
50
20
50
70
17.0
6
50
30
40
70
22.5
7
50
20
40
60
19.8
8
40
40
40
70
21.1
9
40
30
30
70
19.7
10
50
30
40
70
21.5
11
50
30
40
70
23.2
12
40
20
40
70
19.1
13
50
30
30
80
17.1
14
60
30
40
60
16.7
15
50
40
40
60
16.3
16
60
30
50
70
17.0
17
50
30
50
60
18.6
18
50
30
50
80
18.8
19
40
30
50
70
19.5
20
50
20
30
70
19.5
21
40
30
40
80
18.5
22
40
30
40
60
21.5
23
50
20
40
80
16.8
24
60
30
30
70
16.5
25
60
20
40
70
16.3
26
60
40
40
70
15.6
27
60
30
40
80
16.5
28
50
30
30
60
19.6
29
50
40
50
70
19.0
Based on experimental data, a second-order polynomial model was constructed by BBD, which can be described as follows: Y=22.2+1.733A−0.058B−0.042C−0.583D−0.675AB+0.175AC+0.700AD+0.875BC+1.125BD+0.675CD−2.019A2−2.232B2−1.732C2−2.019D2 where Y is the predicted response, A, B, C and D are ethanol concentration, liquid-to-solid ratio, extraction time, and extraction temperature, respectively.
The results of the RSM experiments were analyzed using Design Expert software version 12.0.The impact and significance of each term (linear terms, squared terms and interactions) in the regression equation were evaluated by ANOVA analysis of the quadratic model, as shown in Table 4. The ANOVA showed that the results of the model were significant. The significant contribution of each coefficient was determined by the p-value of F-test (P < 0.05). As the p-value becomes smaller, the corresponding variables become more effective. In addition, the p-value can be employed to check the strength of interaction between independent factors (Barman and Badwaik, 2017; Asfaram et al., 2017). Low values of the coefficient of variance clearly indicated that the model was reproducible and reliable (Jiang et al., 2014). The goodness-of-fit of the model was also evaluated by the coefficient of determination (R2 = 0.9490) and the adjusted coefficient of determination (Adj-R2 = 0.8980), which indicated that 89.80% of the variation could be accounted for by the fitted model and 94.90% of the total variation was explained by the model. Furthermore, it could be seen that the linear coefficients (A and D), cross coefficients (BC and BD) and quadratic term coefficients (A2, B2, C2, and D2) closely affect the extraction rate of polyphenols (P < 0.05), whereas the other term coefficients are not significant. Note: in the table, * represents significant difference (P < 0.05), and ** represents extremely significant difference (P < 0.01).
Source
Sum of squares
dfa
Mean square
F-value
p-value
Significant level
Model
126.23
14
9.02
18.61
<0.0001
**
A
34.34
1
34.34
70.88
<0.0001
**
B
0.0408
1
0.0408
0.0843
0.7758
C
0.03
1
0.03
0.0619
0.8071
D
3.41
1
3.41
7.05
0.0189
*
AB
1.82
1
1.82
3.76
0.0729
AC
0.01
1
0.01
0.0206
0.8878
AD
1.96
1
1.96
4.05
0.064
BC
3.06
1
3.06
6.32
0.0248
*
BD
5.06
1
5.06
10.45
0.006
**
CD
2.72
1
2.72
5.62
0.0327
*
A2
29.04
1
29.04
59.94
<0.0001
**
B2
33.3
1
33.3
68.74
<0.0001
**
C2
23.5
1
23.5
48.5
<0.0001
**
D2
29.38
1
29.38
60.65
<0.0001
**
Residual
6.78
14
0.4845
Lack of Fit
5.29
10
0.5291
1.42
0.3936
Pure Error
1.49
4
0.373
Cor Total
133.01
28
R2 = 0.9490
R2Adj = 0.8980
3.2.2 Analysis of response surface
Three-dimensional (3D) response surface of polyphenol yield and influence factors were constructed using BBD based on the regression equation to illustrate the interactions between the dependent variables, the experimental levels of each independent variable, and the two test variables while keeping the other independent variables at zero level (Fig. 2). Predictably, polyphenol yield is sensitive to changes in the variables. The steeper the slope of the three-dimensional response surface, the more sensitive the response value is to the change of experimental factors (Sady et al., 2019). According to the model analysis, the interaction between extraction temperature and liquid-to-solid ratio had the most prominent effect on polyphenol yield (Fig. 2E). According to the result of obvious analysis, ethanol concentration (A) and extraction temperature(D) had a greater effect (P < 0.05) on polyphenol yield, whereas liquid-to-solid ratio (B) and extraction time (C) were less correlation (P > 0.05). The order of the effects of the four independent variables on polyphenol yield was A > D > B > C.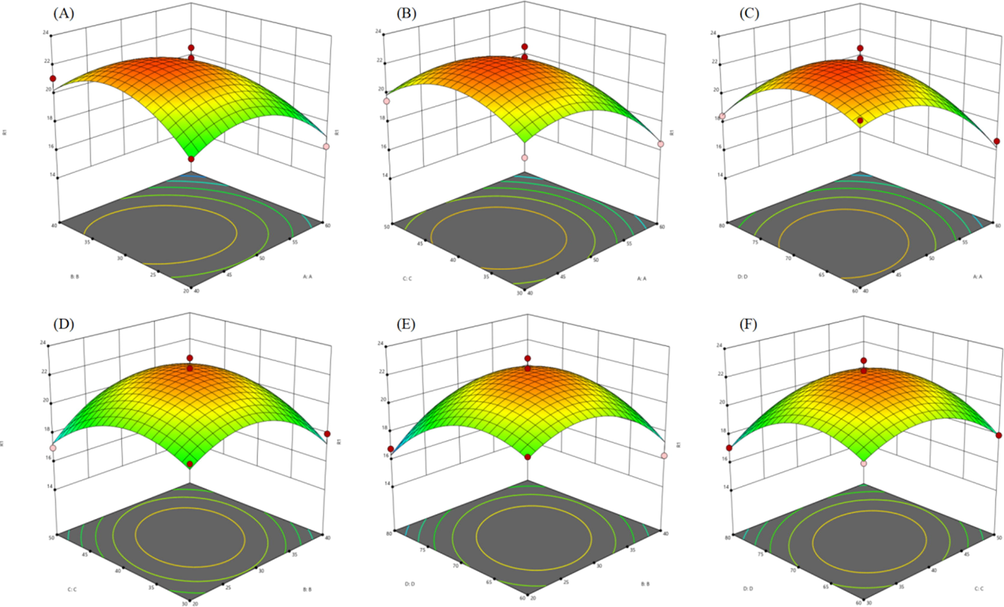
Response surface plots representing the effect of extraction parameters on extraction yield. (A) Ethanol concentration and liquid–solid ratio; (B) Ethanol concentration and time; (C) Ethanol concentration and temperature; (D) Liquid-solid ratio and time; (E) Liquid-solid ratio and temperature; (F) Time and temperature.
3.2.3 Validation of the model
Using Design-Expert, the optimum values of the tested variables for the extraction of polyphenols were: ethanol concentration of 55%, solid–liquid ratio of 29 g/mL, extraction time of 39 min and extraction temperature of 69 °C. The maximum extraction rate of polyphenols was 20.9 mg/g, which was in good agreement with the actual yield (21.9 ± 0.28 mg/g, n = 3). These results suggested that the model designed in this study was valid.
3.3 Analysis of static adsorption and desorption tests
The adsorption and desorption rates of different macroporous resins for polyphenols were shown in Fig. 3A. There were significant (P < 0.05) differences in the adsorption and desorption rates of the four resins (S-8, D101, AB-8 and NKA-9). Among the four resins, the adsorption rate of macroporous resin D101 was the highest, which was 62.20%. The desorption rate of NKA-9 was the highest at 88.28%, but the desorption rate was only 31.58%, while the desorption rate of D101 was 75.24%. Therefore, D101 macroporous resin was selected to purify the polyphenols of C. cicadae. The purified polyphenol content (9.66%) was 2.3 times higher than that of the crude polyphenol (4.13%).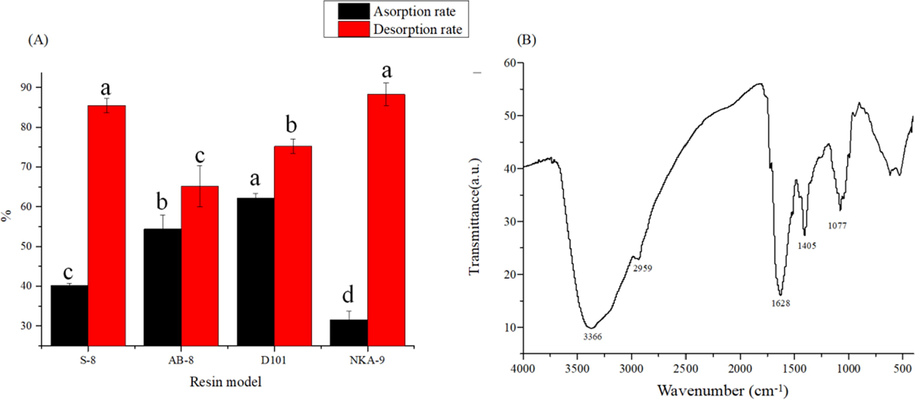
(A) Adsorption and desorption of polyphenols from wine residue by 4 resins; (B) FT-IR spectra of D101 purification sample. Different letters represent the significant difference (P < 0.05).
3.4 FT-IR analysis
The structural feature of the polyphenol samples were further analyzed by FT-IR spectra (Fig. 3B). A strong absorption peak appears at 3366 cm−1 (3500–3100 cm−1), which is caused by the stretching vibration of —OH. This is an important feature of phenolics. An absorption peak near 2959 cm−1 is the stretching vibration of methyl or methylene C—H side chain; peaks in the range 2000–1400 cm−1 indicate that the structure has an aromatic character. At 1628 cm−1 is the C⚌C stretching vibration absorption peak, 1405 cm−1 is the —CH2 deformation vibration absorption peak, and the in-plane bending vibration of aromatic —CH is 1077 cm−1. The stretching vibration of aromatic —CH is mainly detected in the wavelength range less than 900 cm−1(Zheng et al., 2019). Therefore, the FT-IR results can be deduced that the C. cicadae polyphenols have the characteristic structure of phenolic compound.
3.5 Identification of compound by LC-MS analysis
To characterize the functional antioxidant components of the optimized ultrasonic C. cicadae extract, the optimized extracts were analyzed using LC-MS.
3.5.1 Phenolic acid and its derivatives
In total, 12 compounds (Table 5) were compared with the LC-MS database for retention time (RT), molecular weight, and m/z of molecules. Based on matches with an LC-MS library, three of them were characterized as flavanols (Zhuang et al., 2018; Jang et al., 2018; Kumar et al., 2017), compound 4 (RT 0.94 min, m/z 338.98837), compound 14 (RT 5.204 min, m/z 463.0813), compound 11 (RT 4.256 min, m/z 463.09106), compound 17 (RT 12.795 min, m/z 609.50897) and compound 16 (RT 9.211 min, m/z 269.04517) were identified as quercetin, rutin, isoquercitrin, quercetin 3-galactoside and apigenin, respectively. One compound were characterized as isoflavones, and compound 9 (RT 1.418 min, m/z 227.06668) was identified as resveratrol. Two compounds were identified as phenolic acids, compound 10 (RT 3.093 min, m/z 153.01817), compound 13 (RT 4.753 min, m/z 153.01817) were identified as gentian acid and protocatechuic acid, respectively. Two compounds were characterized as flavanones, compound 6 (RT 1.263 min, m/z 271.05698) and compound 15 (RT 6.78 min, m/z Hesperetin) were identified as naringenin and hesperetin, respectively. Two compounds were presumed to be flavanols (Luo et al., 2020; Elez Garofulic et al., 2018), compound 12 (RT 4.54 min, m/z 289.07172), compound 3 (RT 0.871 min, m/z 305.06232) were identified as epicatechin and (-)-epicallocatechin.
Number
RT.[min]
Formula
Molecular.
Weight[M−H]−
(m/z)Name
1
0.777
C6 H12 O7
196.05723
195.04996
Gluconic acid
2
0.851
C4 H6 O5
134.02019
133.01291
Malic acid
3
0.871
C15H14O7
306.06937
305.06232
(-)-Epigallocatechin
4
0.94
C15H10O7
302.03977
338.98837
Quercetin
5
1.171
C6 H8 O7
192.02601
191.01874
Citric acid
6
1.263
C15H12O5
272.06428
271.05698
Naringenin
7
1.299
C6 H6 O6
174.01535
173.00807
trans-Aconitic acid
8
1.405
C4H6O4
118.02517
117.01789
Succinic acid
9
1.418
C14H12O3
228.0742
227.06668
Resveratrol
10
3.093
C7H6O4
154.02545
153.01817
Gentisic acid
11
4.256
C21H20O12
464.09834
463.09106
Quercetin 3-galactoside
12
4.54
C15H14O6
290.07899
289.07172
Epicatechin
13
4.753
C7H6O4
154.02545
153.01817
Protocatechuic acid
14
5.204
C21H20O12
464.08858
463.0813
Isoquercitrin
15
6.78
C15H10O7
302.07857
301.07129
Hesperetin
16
9.211
C15H10O5
270.05244
269.04517
Apigenin
17
12.795
C27H30O16
610.51625
609.50897
Rutin
18
12.804
C18 H32 O2
280.23966
279.23239
Linoleic acid
19
13.502
C18 H34 O2
282.25524
281.24796
Oleic acid
3.5.2 Organic acid and its derivatives
Seven compounds were identified as organic acids and their derivatives. Among them, Compound 1 (RT 0.777 min, m/z 195.04996) was tentatively characterized as gluconic acid. Compound 7 (RT 1.299 min, m/z 173.00807) was identified as trans-aconitic acid. Compound 5 (RT 1.171 min, m/z 191.01874) was roughly speculated as citric acid. Compound 8 (RT 1.405 min, m/z 117.01789) was presumed to be succinic acid. Compound 2 (RT 0.851 min, m/z 133.01291) was identified as malic acid. Compound 18 and 19 (RT 12.804 and 13.502 min, m/z 279.23239 and 281.24796) were identified as linoleic acid and oleic acid, respectively.
3.6 In vitro antioxidant activity
Four antioxidant modes including DPPH radical scavenging activity, ABTS+ radical scavenging activity, hydroxyl radical scavenging ability and chelating rate of ferrous ion were chosen to evaluate the antioxidant capacity of C. cicadae polyphenol extracts.
The purified polyphenols showed much higher DPPH radical scavenging activity than the crude extracts, as revealed in Fig. 4A. The DPPH radical scavenging activity of the purified samples reached 45.36 ± 1.530% when the concentration of the polyphenol extract was 1 mg/mL. However, the activity of the crude sample was only 21.97 ± 1.610% at the same concentration. The DPPH radical scavenging activity of the purified sample (IC50 = 1.05 ± 0.015 mg/mL) was significantly higher than that of the crude sample (IC50 = 1.35 ± 0.028 mg/mL) (P < 0.05, Table 6). IC50 value is half maximal inhibitory concentration. Data were presented as the mean value (n = 3). Mean with different letters in a row are significantly different (P < 0.05).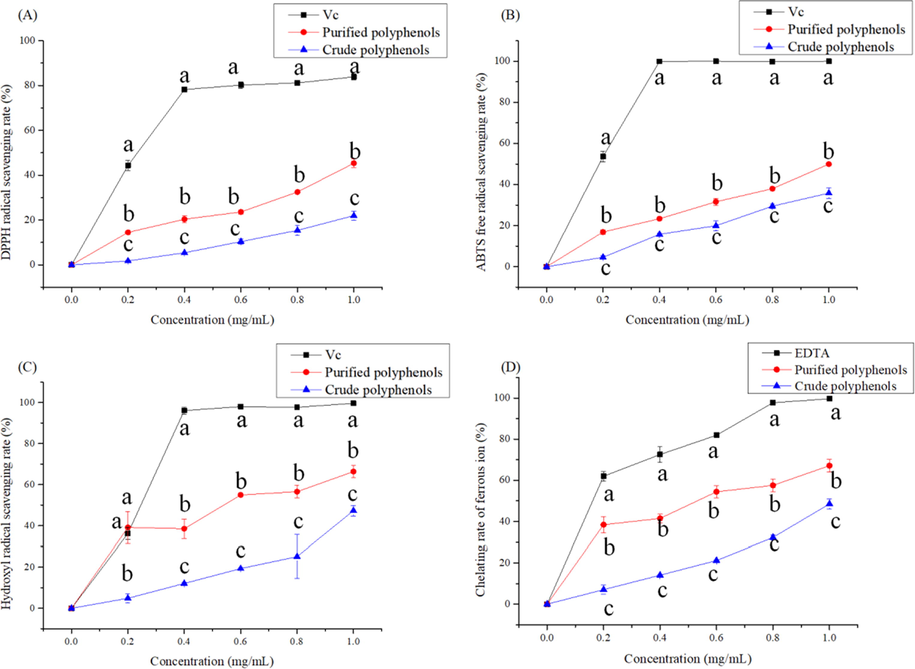
Antioxidant activity of polyphenols samples from C. cicadae. (A) Scavenging activity to DPPH, (B) scavenging activity to ABTS+, (C) scavenging activity to •OH and (D) Chelating rate of ferrous ion. Different letters represent the significant difference (P < 0.05) at the same concentration for different samples. The letter ‘a’ represents the highest value.
Sample
IC50 value (mg/mL)
DPPH scavenging activity
•OH scavenging activity
ABTS+ scavenging activity
Ferrous ion chelating activity
Crude extract
1.35 ± 0.028a
1.02 ± 0.031a
1.13 ± 0.021a
1.012 ± 0.026a
Purifcation
1.05 ± 0.015b
0.64 ± 0.012b
1.02 ± 0.014b
0.626 ± 0.002b
Vc
0.34 ± 0.008c
0.24 ± 0.011c
0.18 ± 0.019c
0.272 ± 0.003c
ABTS+ radical scavenging activity of the crude sample (IC50 = 1.13 ±0.021 mg/mL) was higher than that of the purified sample (IC50 = 1.02 ± 0.014 mg/mL) (P < 0.05, Table 6). The ABTS+ radical scavenging activity of the purified sample was clearly higher (P < 0.05) than that of crude samples with a concentration dependence (Fig. 4B). In general, the tendency was almost the same as that of the DPPH radical scavenging activity.
The hydroxyl radical scavenging ability of all samples is shown in Fig. 4C. The results indicated that the scavenging ability of the crude samples (47.33 ± 2.101%) was significantly higher than that of the purified samples (66.37 ± 2 0.509%) at a concentration of 1 mg/mL. The IC50 of the crude sample (IC50 = 1.02 ± 0.031 mg/mL) was found to be higher than that of the purified sample (IC50 = 0.64 ± 0.012 mg/mL) or VC (IC50 = 0.24 ± 0.011 mg/mL) (Table 6).
The Fe2+-chelating activity of the crude samples and purified samples were evaluated (Fig. 4D). Both samples exhibited good concentration-dependent ferrous ion-chelating ability. The chelating potential increased with increasing concentration up to 1 mg/mL, and the purified samples were always stronger for than crude ones. At 1 mg/mL, the chelating potential of purified sample, crude sample, and EDTA were 67.13 ± 2.452%, 48.5 ± 2.054%, and 99.67 ± 0.448%, respectively. Among the different samples tested, the purified samples showed higher ferrous ion chelating activities with low IC50 values as 0.626 ± 0.002 mg/mL (P < 0.05, Table 6).
3.7 Anti-aging activity of purified polyphenols in vivo
3.7.1 Concentration range for in vivo screening of polyphenols in C. Elegans
C. elegans has strong fecundity and can quickly consume the supply of E. coli in a limited resource space. Therefore, the appropriate polyphenols concentration can be estimated by the consumption rate of food sources. To efficiently assess the anti-aging and anti-stress capacity of C. cicadae polyphenols, we first determined the appropriate concentrations of polyphenols used to maintain C. elegans. As shown in Fig. 5, C. elegans were exposed to 1.5–3 mg/mL of polyphenols that delayed the food clearance. When the polyphenol concentration was 0–0.5 mg/mL, the values decreased rapidly after polyphenol treatment according to the change in absorbance of the control group, indicating that this concentration was more suitable for the growth of worms. For polyphenols, the optimal concentration for C. elegans was below 0.5 mg/mL and was selected for the following tests.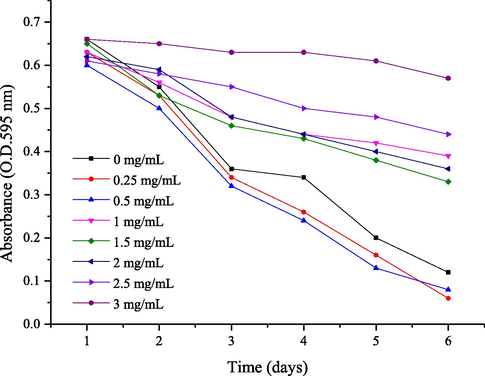
The OD of E. coli is reported daily for each concentration of C. cicadae polyphenols.
3.7.2 Longevity analysis
Age-synchronized adult wild type N2 worms were fed with polyphenols (0.125, 0.25, 0.5 mg/mL) for their lifetime. The results showed that polyphenols significantly prolonged the lifespan of C. elegans, compared with those of the control group (P < 0.05) (Fig. 6A). The high-dose group exhibited a positive effect of delaying aging with a maximum lifespan of 28 days, increasing the mean and median lifespan of C. elegans by 27.66% and 20.12%, respectively. The medium-dose group treatment also increased the mean lifespan of worms by 23.54% and 26.32%, with corresponding value of 19.18% and 21.79% for the low-dose group (Table 7). This result suggests that polyphenols might delay aging of wild type worms under normal cultural condition. Note: 37 °C: hours, 20 °C: days. Date were expressed as means ± standard deviation (n = 3). Mean with different letters in a row are significantly different (P < 0.05).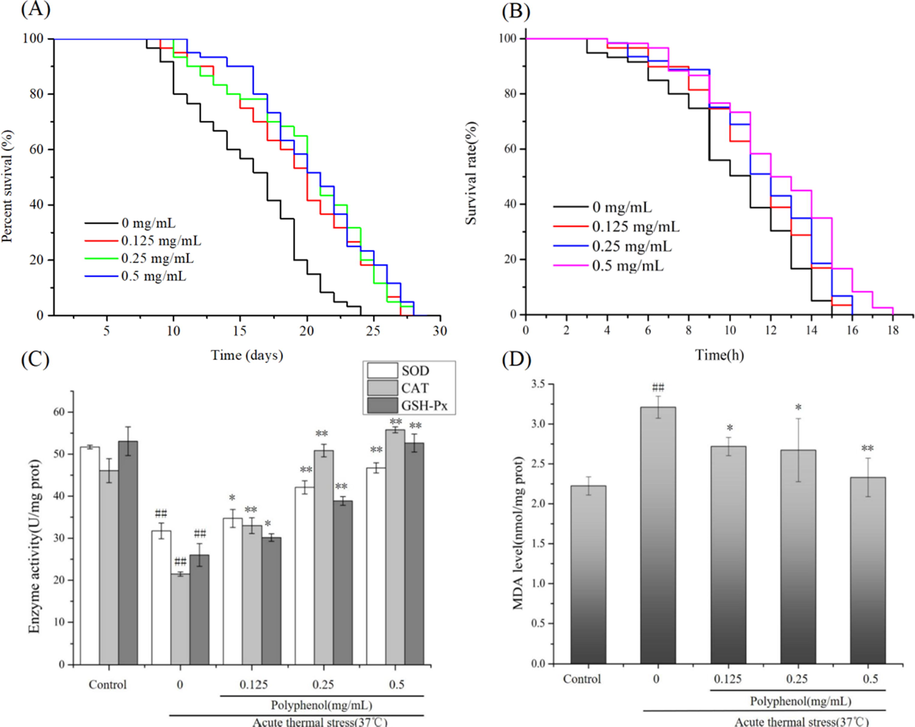
Effects of C. cicadae polyphenols on the lifespan of wild type C. elegans, (A) 20 °C, (B) 37 °C; Effects of polyphenol (C) GSH-Px, SOD, CAT activity and (D) MDA levels acute thermal stress-treated C. elegans. Data were presented as means ± S.D. (n = 3). ## P < 0.01 as compared to control; * P < 0.05 as compared to the acute thermal stress group; **P < 0.01 as compared to the acute thermal stress group.
Group
(mg/mL)Mean lifespan
Maximum lifespan
Median lifespan
37 °C
20 °C
37 °C
20 °C
37 °C
20 °C
Control
8.27 ± 0.62b
15.80 ± 1.15b
13.67 ± 0.47c
23.67 ± 0.47b
9.88 ± 0.16c
16.34 ± 0.91c
0.125
10.31 ± 0.19a
18.83 ± 0.36a
14.67 ± 0.47bc
27.00 ± 0.00a
10.89 ± 0.17b
19.90 ± 0.35b
0.25
11.36 ± 0.71a
19.52 ± 0.75a
15.00 ± 0.00ab
27.67 ± 0.47a
11.11 ± 0.45b
20.64 ± 0.80ab
0.5
11.25 ± 0.16a
20.17 ± 0.59a
16.00 ± 0.82a
28.00 ± 0.00a
11.83 ± 0.21a
21.12 ± 0.46a
3.7.3 Effects of polyphenols on reproduction in C. elegans
Fig. 7A presents the results for the duration of reproduction of the F1 generation. On day 2, the three treatments led to an increase in the total progeny. Moreover, we found that the duration of reproduction of worms in the high dose group was significantly increased on the day 2 compared to the control (P < 0.001). On the day 3 to 6, it also led to an increase in total progeny for all three treatments although there was no significant difference (P > 0.01). Interestingly, the total progeny within 6 days were significantly (0.125 and 0.25 mg/mL) or extremely significantly (0.5 mg/mL) higher than that the control (0 mg/mL). The results suggested that polyphenols could enhance the reproductive capacity of worms.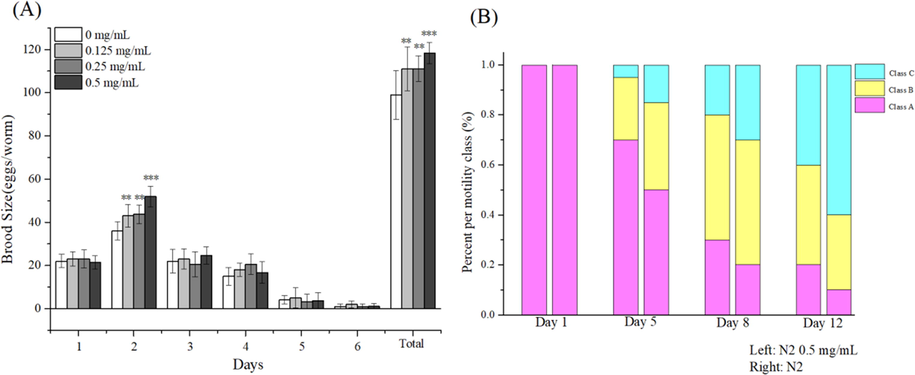
(A) Percent of per motility class of N2 cultured with or without polyphenols on day 1, day 5, day 8, and day 12; (B) Effect of polyphenols on reproduction of N2. **P < 0 01, ***P < 0 001.
3.7.4 Motility measurement
Motility is a measure of muscle integrity and its measurement has a direct impact on quality of life. We found that N2 cultured with 0.5 mg/mL polyphenols were able to maintain better locomotion than the control group (Fig. 7B). At day 12, 40% of N2 incubated with 0.5 mg/mL polyphenols still belonged to Class A, but only 10% of the control group remained in Class A. All these results indicate that polyphenols enhance the ability of worms to maintain a considerably high level of muscle integrity.
3.7.5 Effect of purified polyphenol on the lifespan of C. elegans with thermal stress
From Table 7 and Fig. 6B, it can be seen that the mean lifespan, maximum and median lifespan of C. elegans in all dose groups of purified polyphenols were prolonged after polyphenol treatment. Comparatively, the most significant effect was observed in the high dose treatment group (P < 0.05). Compared with the blank control group, the mean lifespan, maximum lifespan, and median lifespan of the high dose treatment group were prolonged by 36.03%, 17.04% and 19.74%, respectively. From the above results, it can be seen that polyphenols of C. cicadae have a certain therapeutic effect on heat stress in C. elegans, and this therapeutic effect obviously extended the lifespan of C. elegans.
3.7.6 Effect of purified polyphenol on antioxidant-related enzymes of C. elegans under thermal stress
In fact, oxidative damage caused by excessive ROS has an accelerating effect on the body’s aging process. Fortunately, common antioxidative enzymes, such as SOD, CAT and GSH-Px, constitute the body's first line of defense against foreign oxidative damage by scavenging excessive ROS, and thus delay aging processes (Zhu et al., 2020). Herein, we studied the effect of C. cicadae polyphenols on antioxidant-related enzymes in C. elegans under acute thermal stress. As indicated in Fig. 6C, prior treatment of C. elegans with polyphenols dramatically increased their SOD activities under acute thermal stress from 31.74 U/mg protein to 34.71, 42.10, 46.72 U/mg protein (0.125, 0.25 and 0.5 mg/mL of polyphenols, respectively) (P < 0.05). Likewise, the activities of CAT and GSH-Px were significant increased in all pretreated C. elegans groups compared to the group under acute thermal stress (P < 0.05). Furthermore, a significant increase was observed in the MDA levels in C. elegans exposed to 37 °C (144.37%) (P < 0.05). However, pretreatment with polyphenols significantly decreased the up-regulated levels of MDA in a dose-dependent manner (P < 0.05) (Fig. 6D). MDA is a product of free radicals reactions, which directly indicates the degree of oxidation in C. elegans (Tian et al., 2006). This result suggests that C. cicadae polyphenols are able to protect C. elegans from acute thermal stress by reducing lipid peroxidation products and enhancing the activities of antioxidant enzyme.
3.8 Inhibitory effect of polyphenols on oxidative DNA damage
In the present study, the capacity of C. cicadae polyphenols in preventing hydroxyl radical-induced oxidative DNA damage was evaluated. As shown in Fig. 8, in the absence of Fe2+ and H2O2, plasmid DNA was mainly in the form of supercoiled (Fig. 8A, lane 1). After incubation with Fe2+ and H2O2 (Fig. 8A, lane 2), the supercoiled form of DNA was converted into the relaxed cyclic or linear form, suggesting that the hydroxyl radical generated by the Fenton reaction induced DNA breaks. Adding polyphenols at concentrations of 0.1, 0.2 and 0.4 mg/mL, significantly reduced the DNA damage in a dose-dependent manner (Fig. 8A, lanes 3–5). The proportion of superhelical DNA was further analyzed by statistical electrophoresis. The results also visually reflected the significant protective effect of C. cicadae polyphenols against plasmid DNA oxidative damage (P < 0.01) in an obvious dose-dependent manner (Fig. 8B).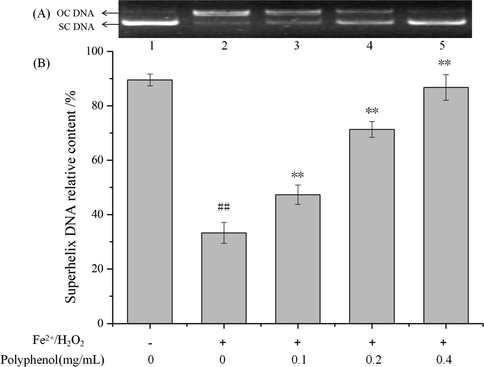
Inhibitory effect of the purified C. cicadae polyphenols on •OH induced DNA damage. (A) DNA electrophoretic analysis. Lanes 1 and 2 were the normal DNA and treated with 1 mM FeSO4 and 1 mM H2O2, respectively. Lanes 3–5 were treated with various concentrations of polyphenols (0.1, 0.2 and 0.4 mg/mL). (B) Statistics of relative content of superhelix DNA. Data were presented as means ± S.D. (n = 3). ## P < 0.01 as compared to control; **P < 0.01 as compared to the oxidative induction group. +induction, −no induction.
4 Discussion
The application of effective extraction procedures should achieve the maximum recovery and minimum degradation of the target bioactive compounds (Živković et al., 2018). Ultrasonic assisted extraction has been widely used in the extraction of phenolic compounds because of its low cost and high efficiency (Bouaoudia-Madi et al., 2019). It is reported that the extraction rate of phenolic compounds was affected by many factors, including solid solution ratio, extraction time and temperature (Gao and Wang, 2021). When multiple variables affect the output, response surface method (RSM) is a suitable technology for the optimization process (Famuwagun et al., 2017). Therefore, RSM method was used in this study to determine the effects of ethanol concentration, extraction time, extraction temperature, and liquid–solid ratio on the content of polyphenols in C. cicadae. The polyphenol content (21.9 mg/g) extracted by the present method was significantly higher than the reported extraction rate (2.46 mg/g), which was extracted from rye bran by UAE (Iftikhar et al., 2020). In polyphenol extraction from C. cicadae, UAE resulted in a 75% increase in polyphenol content (from 12.5 mg/g to 21.9 mg/g) and a significant decrease in extraction time (from 60 min to 49 min) compared to that reported by Yin et al.(2017).
The purification method of macroporous adsorption resin is to separate and purify polyphenols and other substances from samples by the adsorption and desorption properties of resin (Dong et al., 2021). Through adsorption and analytical experiments, we chose D101 to purify polyphenols. The content of purified polyphenol (9.66%) was 2.3 times higher than that of crude polyphenol (4.13%). The results were similar to a study carried out by Guo et al. (2019), in which the concentration of fungus suillus polyphenols purified by D101 was increased from 0.54 ± 0.02 to 4.89 ± 0.11 mg/g, with 3.5 times more polyphenols than that crude extract.
Twelve phenolic acids were identified by LC-MS. Several polyphenolic compounds were also detected in other edible mushrooms. For example, Moghaddam et al. (2019) identified ferulic acid, gallic acid, and myricetin compounds in some edible and medicinal mushrooms from Turkey, and Sulkowska-Ziaja et al. (2012) detected protocatechuic acid, p-hydroxybenzoic acid, and vanillic acid in polyporoid mushrooms from Poland. Our study found that alcohol extract of C. cicadas contain flavonols, flavanols, flavones, and isoflavones, all of which are all flavonoids with different degrees of heteroepoxidation (Scalbert et al., 2005). Phenolic acids and flavonoids are considered to be the major contributor to antioxidant activity (Chang et al., 2017; Li et al., 2012; Yang et al., 2015). In vitro experiments showed that the polyphenol extract of C. cicadae had the ability to scavenge DPPH, ABTS+, •OH free radicals and chelating ferrous ions. These free radicals are primarily inhibited by antioxidant compounds, such as phenolic acids and flavonoids, by donating electrons and hydrogen atoms to these harmful free radicals (Ampofo and Ngadi, 2020). For example, Ioku et al. (2001) found that quercetin 4-O-β-glucoside and quercetin 3, 4-O-β-glucoside from onion polyphenols have strong antioxidant effect. Yan et al. (2002) showed that flavonols in cranberry have strong DPPH scavenging ability and their scavenging effect is superior to that of the antioxidant VE. Therefore, the antioxidant capacity of C. cicadae polyphenols in vitro may be related to the presence of these phenolic acids. In addition, our study found purified polyphenols had stronger antioxidant capacity in vitro. The results were similar to a study carried out by Guo et al. (2019). Furthermore, our results suggest that under certain conditions may promote the release of other compounds of the matrix that are different from the polyphenolic compounds, such as linoleic acid, oleic acid and other organic acids. Their interactions in solution affect the antioxidant ability (Wei et al., 2014).
In fact, oxidative damage caused by excessive ROS can accelerate the aging process of human body. The longevity of nematodes may be caused by two reasons: i) polyphenols of C. cicadae can remove excess environmental reactive oxygen species, which usually leads to rapid aging and death of nematodes (Zhang et al., 2019); ii) Polyphenols are a kind of antioxidant substances which may enhance the activity of antioxidant enzymes in nematodes. In vitro experiments, it has been confirmed that polyphenols have DPPH, ABTS, and ·OH scavenging abilities. To clarify the underlying mechanisms of C. cicadae polyphenols in prolonging the life span of nematodes under heat stress, we studied whether it had effects on the levels of SOD, CAT, and GSH-Px in nematodes. Our results showed that the activity levels of three enzymes were increased in C. elegans, which was consistent with their survival rate during heat stress. The addition of C. cicadae polyphenols can significantly reduce the level of MDA under heat stress, indicating that it may reduce the degree of lipid peroxidation by reducing the level of ROS. Similar studies also showed that blueberry polyphenols significantly improved the antioxidant capacity of C. elegans under heat stress, thus improving the longevity of C. elegans (Wilson et al., 2006). Anthocyanins from purple wheat not only have high DPPH radical scavenging activity and strong antioxidant protective capability in vivo, but also extended the average lifespan of wild type C. elegans by 10.5% (Chen et al., 2003).
The fecundity of C. elegans was also significantly increased when worms were incubated with 0.5 mg/mL of polyphenols. This result seems to be somewhat contradictory to previous studies that suggest a trade-off between survival and fitness in life-extending interventions. It has been found that long-lived C. elegans can reduce egg production, and that ablation of germ-line precursor cells leads to increased lifespan in C. elegans (Hsin and Kenyon, 1999). Resveratrol increases both the mean and maximum lifespan in C. elegans but causes a significant reduction in fertilization rate during the early gravid period (Gruber et al., 2007). However, there may be other trade-offs for longevity, such as between increased lifespan and decreased pharyngeal pumping rates when worms were cultured with blueberry polyphenols (Joseph et al. 1999). However, we also had the opposite result, with C. cicadae polyphenols extending lifespan without reducing motility. This further supports to the findings of Oh et al. (2015) who showed that hat N-acetyl-L-cysteine and extracts from Tenebrio molitor extended the lifespan of C. elegans and simultaneously improved the fertility, and Zhang et al. (2019) who found that lycium barbarum polysaccharide can increase the lifespan of C. elegans while enhancing reproductive potential and maintain muscle integrity. Therefore, it seems reasonable that C. cicadae polyphenols prolong the lifespan of C. elegans without reducing fertility and muscle integrity. There may be other trade-offs that need to be further examined in future studies.
Fenton reaction can induce the production of hydroxyl radicals (Halliwell, 1991). Free radicals damage DNA strands, resulting in DNA base modifications, DNA site mutations, DNA duplex distortion and other forms of DNA oxidative damage, which has been suspected to be a major cause of cancer (Chandrasekara and Shahidi, 2011; Meira et al., 2008). C. cicadae phenolics may protect superhelical plasmid DNA from hydroxyl radicals by suppressing the reaction of Fe2+ with H2O2 or by directly quenching hydroxyl radicals by donating hydrogen atoms or electrons (Wang et al., 2015). The second mechanism is the capacity of phenolic extracts to scavenge hydroxyl radicals generated in the system. In a previous study we showed that C. cicadae phenolic extracts could effectively chelate ferrous ions as well as scavenge hydroxyl radicals in the in vitro system. Therefore, the results obtained in this study could be due to a multifactorial effect of all possible inhibitory activities aforementioned, hence suggesting that the C. cicadae phenolics are effective against DNA cleavage mediated by hydroxyl radicals. This further supports the findings of Wang et al. (2017), who showed that the higher accumulation of polyphenolics was intended to enhance the protection of supercoiled DNA. In the present study, the C. cicadae phenolics showed higher protective effects for supercoiled DNA. It is thought that the C. cicadae phenolics inhibits oxidative DNA damage and prevent the development of cancer.
5 Conclusion
In this study, UAE was successfully applied to extract polyphenols from C. cicadae and BBD was used to optimize the extraction variables. As a result, the following optimal conditions were determined: ethanol concentration 55%, solid-to-liquid ratio 29 g/mL, extraction time of 39 min and extraction temperature of 69 °C. Under these optimal conditions, the maximum extraction rate was 21.9 mg GAE/g DW. The adsorption/desorption capacities of the four resins were examined, and the D101 resin was found to have good adsorption and desorption ratios for the purification of C. cicadae polyphenols. In addition, 19 compounds were identified by LC-MS. Furthermore, their in vitro antioxidant activities and anti-aging properties were evaluated on C. elegans. The results showed that C. cicadae polyphenols exhibited strong antioxidation properties in vitro and anti-aging properties in vivo. Finally, the protective effect of polyphenols against oxidative DNA damage was investigated. This result indicated that C. cicadae polyphenols have a good inhibitory effect on DNA oxidative damage, suggesting C. cicadae are a valuable source of polyphenols.
Acknowledgments
This study was supported by Enzyme Resources Sharing and Service Platform of Sichuan Province (Project No. 2020JDPT0018) and Dazhu Wankang Ecological Agriculture Development Co., Ltd. Cordyceps cicadae polysaccharide beverage research project.
Autho contributions
Zizhong Tang and Wenjie Lin: designed experiment and performed experiment.
Yusheng Chen, Shiling Feng, Yihan Qin, Hong Chen and Yuntao Liu: analyzed the data, drawing diagrams and tables.
Yirong Xiao Jing Yang: drafted the work.
Hui Chen: supervise and administrated the work.
Chunbang Ding, Tongliang Bu, Qinfeng Li, Yi Cai, Huipeng Yao: revised it critically for important content.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
References
- Microwave assisted extraction and characterization of polysaccharide from waste jamun fruit seeds. Int. J. Biol. Macromol.. 2020;152:1157-1163.
- [CrossRef] [Google Scholar]
- Ultrasonic assisted phenolic elicitation and antioxidant potential of common bean (Phaseolus vulgaris) sprouts. Ultrason. Sonochem.. 2020;64:104974
- [CrossRef] [Google Scholar]
- Ultrasound assisted combined molecularly imprinted polymer for selective extraction of nicotinamide in human urine and milk samples: Spectrophotometric determination and optimization study. Ultrason. Sonochem.. 2017;34:640-650.
- [CrossRef] [Google Scholar]
- Antioxidant characterization and biological effects of grape pomace extracts supplementation in Caenorhabditis elegans. Foods. 2019;8(2)
- [CrossRef] [Google Scholar]
- Effect of ultrasound and centrifugal force on carambola (Averrhoa carambola L.) slices during osmotic dehydration. Ultrason. Sonochem.. 2017;34:37-44.
- [CrossRef] [Google Scholar]
- Metabolic syndrome, aging and involvement of oxidative stress. Aging. Dis.. 2015;6(2):109-120.
- [Google Scholar]
- Optimization of ultrasound-assisted extraction of polyphenols from myrtus communis L. pericarp. Antioxidants. 2019;8, 205:1-17.
- [Google Scholar]
- Comparative study on the monosaccharide compositions, antioxidant and hypoglycemic activities in vitro of intracellular and extracellular polysaccharides of liquid fermented Coprinus comatus. Int. J. Biol. Macromol.. 2019;139:543-549.
- [CrossRef] [Google Scholar]
- Antiproliferative potential and DNA scission inhibitory activity of phenolics from whole millet grains. J. Funct. Foods. 2011;3:159-170.
- [Google Scholar]
- Quercetin suppresses the metastatic ability of lung cancer through inhibiting Snail-dependent Akt activation and Snail-independent ADAM9 expression pathways. Biochim. Biophys. Acta. Mol. Cell. Res.. 2017;1864(10):1746-1758.
- [CrossRef] [Google Scholar]
- Anthocyanin-rich purple wheat prolongs the life span of Caenorhabditis elegans probably by activating the DAF-16/FOXO transcription factor. J. Agr. Food. Chem.. 2003;61:3047-3053.
- [Google Scholar]
- Ultrasonic-assisted extraction of polyphenols and antioxidants from picea abies bark. J. Biotechnol. 2020:314-315.
- [Google Scholar]
- Composition of bound polyphenols from carrot dietary fiber and its in vivo and in vitro antioxidant activity. Food. Chem.. 2021;339
- [Google Scholar]
- Fractionation and structural characterization of polysaccharides derived from red grape pomace. Process. Biochem.. 2021;109:37-45.
- [Google Scholar]
- Dragovíc-Uzelac, V., Elez Garofulíc, I., Jukíc, M., Peníc, M., & Dent, M., 2012. The influence of microwave-assisted extraction on the isolation of sage (Salvia offificinalis L.) polyphenols. Food Technol. Biotechnol. 50(3), 77–383.
- UPLC-MS(2) Profiling of Blackthorn Flower Polyphenols Isolated by Ultrasound-Assisted Extraction. J. Food. Sci.. 2018;83(11):2782-2789.
- [CrossRef] [Google Scholar]
- Gao, Y., Wang, S.F., Dang, S.K., Han, S.L., Yun, C.L., Wang, W.J., Wang, H.M., 2021. Optimized ultrasound-assisted extraction of total polyphenols from Empetrum nigrum and its bioactivities. J. Chromatogr. B, 1173.
- Gruber, J., Tang, S.Y., Halliwell, B., 2007. Evidence for a trade-off between survival and fitness caused by resveratrol treatment of caenorhabditis elegans. Annals of the New York Academy of Sciences 1100(Apr), 530–542.
- Extraction optimization and antioxidant properties of african eggplant (solanum macrocarpon) leaf polyphenols. J. Food. Quality. 2017;2017:1-14.
- [Google Scholar]
- Ultrasonic-assisted extraction and purification of phenolic compounds from sugarcane (Saccharum officinarum L.) rinds. Lwt.. 2015;60(2):970-976.
- [CrossRef] [Google Scholar]
- Purification and antioxidant activities of polyphenols from Boletus edulis Bull.: Fr. J. Food. Meas. Charact.. 2019;14(2):649-657.
- [CrossRef] [Google Scholar]
- Stochastic and genetic factors inflfluence tissue-specifific decline in ageing C. elegans. Nature. 2002;419:808-814.
- [Google Scholar]
- Signals from the reproductive system regulate the lifespan of C. elegans. Nature. 1999;399(6734):362-366.
- [Google Scholar]
- Various cooking methods and the flavonoid content in onion. J. Nutr. Sci. Vitaminol.. 2001;47(1):78-83.
- [Google Scholar]
- Study on optimization of ultrasonic assisted extraction of phenolic compounds from rye bran. LWT-. Food. Science. and. Technology. 2020;134(4):110243
- [Google Scholar]
- Characterization and quantification of flavonoid glycosides in the Prunus genus by UPLC-DAD-QTOF/MS. Saudi. J. Biol. Sci. 2018;25(8):1622-1631.
- [CrossRef] [Google Scholar]
- Optimization for ultrasound-assisted extraction of polysaccharides with antioxidant activity in vitro from the aerial root of Ficus microcarpa. Carbohydr. Polym.. 2014;110:10-17.
- [CrossRef] [Google Scholar]
- Two water-soluble polysaccharides from mung bean skin: Physicochemical characterization, antioxidant and antibacterial activities. Food. Hydrocoll.. 2020;100
- [CrossRef] [Google Scholar]
- Antioxidant and DNA damage protecting potentials of polysaccharide extracted from phellinus baumii using a delignification method. Carbohyd. polym. 2016;152:575-582.
- [Google Scholar]
- Reversals of age-related declines in neuronal signal transduction, cognitive, and motor behavioral deficits with blueberry, spinach, or strawberry dietary supplementation. J. Neurosci.. 1999;19(18):8114-8121.
- [Google Scholar]
- Microwave-assisted extraction of phenolics from pomegranate peels: Optimization, kinetics, and comparison with ultrasounds extraction. Chem. Eng. Process.. 2019;137:1-11.
- [CrossRef] [Google Scholar]
- Activation of macrophages by polysaccharide isolated from paecilomyces cicadae through toll-like receptor 4. Food. Chem. Toxicol.. 2012;50(9):3190-3197.
- [Google Scholar]
- Characterization and antioxidant activities of intracellular polysaccharides from Agaricus bitorquis (QueL.) Sacc. Chaidam ZJU-CDMA-12. Int. J. Biol. Macromol.. 2020;156:1112-1125.
- [CrossRef] [Google Scholar]
- Identification and characterization of phenolics and terpenoids from ethanolic extracts of Phyllanthus species by HPLC-ESI-QTOF-MS/MS. J. Pharm. Anal.. 2017;7(4):214-222.
- [CrossRef] [Google Scholar]
- Optimisation of infrared-assisted extraction of rutin from crude Flos Sophorae Immaturus using response surface methodology and HPLC analysis. Phytochem. Anal.. 2012;23(4):292-298.
- [CrossRef] [Google Scholar]
- Merging the multi-Target effects of phytochemicals in neurodegeneration: From oxidative stress to protein aggregation and inflammation. Antioxidants. (Basel). 2020;9(10)
- [CrossRef] [Google Scholar]
- Identification of cordycepin biosynthesis-related genes through de novo transcriptome assembly and analysis in Cordyceps cicadae. R. Soc. Open. Sci.. 2018;5(12):181247
- [CrossRef] [Google Scholar]
- Structural characterization and antioxidant activity of polysaccharides extracted from jujube using subcritical water. Lwt.. 2020;117
- [CrossRef] [Google Scholar]
- Green extraction of antioxidant polyphenols from green tea (Camellia sinensis) Antioxidants. (Basel). 2020;9(9)
- [CrossRef] [Google Scholar]
- Meira, L., B., Bugni, J., M., Green, S., L., 2008. DNA damage induced by chronic inflammation contributes to colon carcinogenesis in mice. J. Clin. Invest., 118, 2516–2525.
- Investigation of polyphenol composition, biological activities and detoxification properties of some medicinal mushrooms from turkey. Turkish. J. Pharm. Sci.. 2019;16(2):155-160.
- [Google Scholar]
- Can Cordyceps cicadae be used as an alternative to Cordyceps militaris and Cordyceps sinensis? - A review. J. Ethnopharmacol.. 2020;257:112879
- [CrossRef] [Google Scholar]
- Lifespan extension and increased resistance to environmental stressors by n-acetyl-l-cysteine in caenorhabditis elegans. Clinics. (São. Paulo,. Brazil). 2015;70(5):380-386.
- [Google Scholar]
- Polysaccharides purified from Cordyceps cicadae protects PC12 cells against glutamate-induced oxidative damage. Carbohydr. Polym.. 2016;153:187-195.
- [CrossRef] [Google Scholar]
- Extraction of polyphenols from aromatic and medicinal plants: an overview of the methods and the effect of extraction earameters. Polyphenols. in. Plants 2019:243-259.
- [Google Scholar]
- Correlation study among the extraction techniques, phytochemicals, and antioxidant activity of Nepeta spicata aerial part. Biocatal. Agric. Biotechnol.. 2019;20
- [CrossRef] [Google Scholar]
- Clean recovery of antioxidant compounds from plant foods, by-products and algae assisted by ultrasounds processing. Modeling approaches to optimize processing conditions. Trends. Food. Sci. Technol.. 2015;42(2):134-149.
- [CrossRef] [Google Scholar]
- Optimisation of ultrasonic-assisted extraction of bioactive compounds from chokeberry pomace using response surface methodology. Acta Scientiarum Polonorum Technologia Alimentaria. 2019;18(3):249-256.
- [Google Scholar]
- Dietary polyphenols and the prevention of diseases. Crit. Rev. Food. Sci. Nutr.. 2005;45(4):287-306.
- [CrossRef] [Google Scholar]
- Optimization of ultrasound assisted extraction of functional ingredients from Stevia Rebaudiana bertoni leaves. Int. Agrophys.. 2015;29(2):231-237.
- [CrossRef] [Google Scholar]
- Ultrasound-assisted extraction of four groups of osmanthus fragrans fruit: optimization, UPLC-Orbitrap-MS/MS characterization and anti-inflammatory activity evaluation. Arab. J. Chem.. 2021;14(4):103086
- [Google Scholar]
- Antioxidation activity of pleurotus nebrodensis mycelium polysaccharides in mice and its effect on senescence-resistance of fruit flies. Food Sci.. 2006;27(4):223-226.
- [Google Scholar]
- Phenolic compounds and antioxidant activity in some species of polyporoid mushrooms from Poland. Int. J. Med. Mushrooms. 2012;14(4):385-393.
- [Google Scholar]
- Applications and opportunities for ultrasound assisted extraction in the food industry — a review. Innov. Food. Sci. Emerg. Technol.. 2008;9(2):161-169.
- [CrossRef] [Google Scholar]
- Analysis of red wine phenolics: comparison of HPLC and spectrophotometric methods. Vitis. 2001;40(2):87-91.
- [Google Scholar]
- Characterization of soluble and insoluble-bound polyphenols from psidium guajava l. leaves co-fermented with monascus anka and bacillus sp. and their bio-activities. J. Funct. Foods. 2017;32:149-159.
- [Google Scholar]
- Reviews on mechanisms of in vitro antioxidant activity of polysaccharides. Oxid. Med. Cell. Longev 2015:1-13.
- [Google Scholar]
- Identification of antioxidant components and fatty acid profiles of the leaves and fruits from averrhoa carambola. LWT – Food Sci. Technol.. 2014;55(1):278-285.
- [Google Scholar]
- Blueberry polyphenols increase lifespan and thermotolerance in Caenorhabditis elegans. Aging. Cell. 2006;5:59-68.
- [Google Scholar]
- Characterization and discrimination of polysaccharides from different species of cordyceps using saccharide mapping based on pace and hptlc. Carbohydr. Polym.. 2014;103:100-109.
- [Google Scholar]
- Antioxidant activities and antitumor screening of extracts from cranberry fruit (Vaccinium macrocarpon) J. Agric. Food. Chem.. 2002;50(21):5844-5849.
- [Google Scholar]
- Three pathways assess anti-inflammatory response of epicatechin with lipopolysaccharide-mediated macrophage RAW264.7 cells. J. Food. Biochem.. 2015;39(3):334-343.
- [CrossRef] [Google Scholar]
- Study on Polyphenol Extraction of Cordyceps sinensis Mycelium and Their Inhibitory Effect on Three Kinds of Cancer Cells. Edible Fungi China. 2017;36(4):48-52.
- [Google Scholar]
- Cyclopentenone and furan derivative from the mycelia of cordyceps cicadae. J. Antibiotics. 2008;61(1):43.
- [Google Scholar]
- Five aromatics bearing a 4-o-methylglucose unit from cordyceps cicadae. Helvetica Chimica Acta. 2007;90(2):404-410.
- [Google Scholar]
- An aqueous polyphenol extract from rosa rugosa tea has antiaging effects on caenorhabditis elegans. J. Food. Biochem. 2019;43:4.
- [Google Scholar]
- Effects of lycium barbarum polysaccharides on health and aging of C. elegans depend on daf-12/daf-16. Oxidative medicine and cellular longevity 2019:1-14.
- [CrossRef] [Google Scholar]
- Structural characterization and antioxidant activity of oligosaccharides from Panax ginseng C. A. Meyer. Int. J. Biol. Macromol. 2020;150:737-745.
- [CrossRef] [Google Scholar]
- Optimization of ultrasound-assisted extraction of polyphenolic compounds from pomegranate peel using response surface methodology. Sep. Purif. Technol.. 2018;194:40-47.
- [Google Scholar]
- Extraction and Antioxidant Activities of Magnolia kwangsiensis Figlar & Noot. Leaf. Polyphenols. Chem. Biodivers.. 2019;16(2):e1800409
- [CrossRef] [Google Scholar]
- Antioxidant and anti-aging activities of polysaccharides from Cordyceps cicadae. Int. J. Biol. Macromol.. 2020;157:394-400.
- [CrossRef] [Google Scholar]
- Chemical profiling and quantitation of bioactive compounds in Platycladi Cacumen by UPLC-Q-TOF-MS/MS and UPLC-DAD. J. Pharm. Biomed. Anal. 2018;154:207-215.
- [CrossRef] [Google Scholar]







