Untargeted GC-MS Metabolomics applied to wild leaves and callus produced by plant tissue culture of Hibiscus sabdariffa L.
⁎Corresponding author at: Federal University of Para. Laboratory of Systematic Investigation In Biotechnology and Molecular Biodiversity. CEP. 66075-110. R. Augusto Corrrea, 01. Guamá, Belém, Pará, Brazil. alberdan@ufpa.br (Alberdan Silva Santos)
-
Received: ,
Accepted: ,
This article was originally published by Elsevier and was migrated to Scientific Scholar after the change of Publisher.
Peer review under responsibility of King Saud University.
Abstract
Hibiscus sabdariffa L. is a naturalized medicinal species in Brazil commonly called a “vinagreira” and is a member of the Malvaceae Juss. family, which has a rich potential of bioactive compounds presenting extracts with antioxidant, antibacterial, anti-inflammatory, hepatoprotective, antiviral, antidiabetic, and antiobesity, among others. The production of secondary metabolites of medicinal plants using biotechnological tools such as the culture of callus of plant tissues is increasingly being used to produce high-quality compounds under in vitro conditions. From this perspective, the objective of this work was to analyze the chemical compounds of the leaves and callus culture of H. sabdariffa using techniques of Gas Chromatography Coupled to the Mass Spectrum (GC-MS),. The analysis methodology used consisted of removal of liposoluble compounds, acid hydrolysis, and derivatization, all stages were submitted to ultrasonic-assisted agitation, using a reduced amount of biomass. Based on the results obtained in the study, a total of 38 metabolites identified by GC-MS analysis can be observed. Among the identified substances, protocatechuic acid (26A) stands out as the main constituent, with a relative abundance of 26.86% and 16.68% for leaves and callus of H. sabdariffa, respectively. The principal component analysis (PCA) allowed the discrimination of the chemical composition of each sample, being useful for the observation and detection of the compounds trends patterns. The analysis of the hierarchical group combined with the heat map represented the visual relationship between the samples of the data set indicating the values of higher and lower concentrations of chemical compounds respectively, confirming that protocatechuic acid is the most abundant, for the leaves and callus of H. sabdariffa, followed by eicosanoid and isocitric acid, produced only in callus. It was concluded that the GC-MS technique combined with chemometric tools, helped identify the diversity of the compounds present in the leaves and callus of H. sabdariffa and that callus culture enables the production of bioactive compounds continuously and uniformly in a controlled environment and free of contamination.
Keywords
Vinagreira
Medicinal compounds
Biotechnology
Metabolomics
Hibiscus
1 Introduction
Existing plant biodiversity provides an essential support structure for the maintenance and survival of living beings, contributing to basic human needs such as food and medicines (Biavatti et al., 2007). Thus, it is of strategic importance for developing countries to use this biodiversity mainly for countries that have forests and have a tradition in the use of plants, and plant species with medicinal potential. Medicinal plants continue to be the main reference sources for various types of medicines currently used, especially in tropical countries and economically underdeveloped countries, in addition, medicinal plants naturally provide highly complex biosynthesized molecules, presenting reduced costs when compared with synthetic molecules, allowing the obtaining of drugs with lower side effects (Cardoso et al., 2019).
In this scenario, the medicinal species naturalized in Brazil, Hibiscus sabdariffa L. known as “vinagreira” belongs to the Malvaceae Juss. the family has several pharmacologically active metabolites that are responsible for its biological activity, including organic acid, anthocyanin, flavonoid, and polyphenol (Mayasari, et al., 2018; Mohammed et al., 2020). These molecules are known as secondary metabolites that are usually of complex structure, low molecular weight, have remarkable biological activities and, unlike the primary metabolites, present in low concentrations and certain groups of plants, in turn, require the use of refined analytical techniques capable of separating and identifying it (Queiroz and Hostettmann, 2006).
In recent decades, plant biotechnology through callus culture has gained prominence for presenting as an adequate system for the in vitro production of secondary and large-scale metabolites when compared with other techniques, mainly because in vitro callus induction is a direct and rapid system of cell multiplication (Cardoso et al., 2019). In this system, almost any part of the plant can be used to generate callus crops, callus crops can be maintained indefinitely in vitro, within the optimal cultivation conditions, presenting advantages over conventional cultivation, such as plant compounds can be generated independently of external factors; cultured cells are not threatened by the attack of microorganisms or insects; the cells of any plant can be easily maintained to produce their secondary metabolites, ensuring sustainable production of secondary metabolites under controlled conditions, and can be extracted directly from callus without sacrificing the entire plant (Efferth, 2019). In addition to the advantages mentioned above, in vitro culture allows the production of new compounds that are not normally found in the natura plant (Castro et al., 2016).
Analyses for in vitro plant culture, when integrated, into metabolomics studies provide a huge advantage for the study of secondary metabolites. Gas chromatography combined with a mass spectrometer (GC-MS) is a relevant method for the study of secondary metabolites, presenting excellent separation capacity, selectivity, sensitivity, and reproducibility (Mastrangelo et al. 2015), in addition to the considerable data contribution, which allows differentiating both species and individuals of the same species (Lo et al., 2021).
Considering the complexities of some samples the use of multivariate techniques has proven effective in dealing with complex data sets, through techniques such as principal component analysis (PCA) which is a powerful tool to identify patterns in high dimensional data and to emphasize differences and similarities between the samples studied, condensing the main sources of data variability, producing new linear combinations of the original variables, called main components (PCs). The PCA provides useful data set information and contributes to better visualization (Brereton, 2003; Ren et al., 2015). The PCA is one of the most important statistical techniques used to characterize the interrelationships between variables and visualization of data patterns (Ghelichkhani et al. 2019, Samiee et al. 2020), and the heat map is used to represent the values of similar or very different expression status characteristics, (Arabameri et al., 2019, Heydarieh et al. 2020).
2 Material and methods
2.1 Reagents and materials
All organic solvents used in extractions and chromatographic processes were of analytical quality: hexane and ethyl acetate, supplied by TEDIA® COMPANY (Fairfield, USA). Ultrapure water with a resistivity of 18.3 MΩ /cm3 produced in a water purification system model Scholar UV UP 900 (Biohuman, Curitiba, Brazil) was used for the preparation of the acid solution. For the analysis of analytical thin-layer chromatography (TLC), SiliaPlate TLC chromatographic plates were used, with aluminum, silica, 250 μm, 20 × 20 cm, F 254 (SiliCycle Inc., Quebec, Canada). The plates were cut in dimensions of 10 x 2 cm, and aliquots of the samples were applied in the form of bands using a capillary tube.
2.2 Obtaining and processing samples
2.2.1 Leaves of H. sabdariffa
The leaves of H. sabdariffa collected in the village of Pau D'Arco municipality of Santa Bárbara-Pará / Brazil, with georeferenced coordinates S: 1° 15' 3.88“ were used; W: 48° 16' 36.84”, with the registration of activities of access to genetic heritage in the National System of Management of Genetic Heritage and Associated Traditional Knowledge (SisGen) with access number AF05B99. After collection, the leaves were stored in paper bags and transported to the Laboratory of Systematic Research in Biotechnology and Molecular Biodiversity (LabISisBio) of the Federal University of Pará. The first step consisted of cleaning the leaves in running water, the fresh vegetable material was dehydrated on a stove of air circulation at a constant temperature of 50 °C, for 48 hours.
2.2.2 Callus of H. sabdariffa
For the callus induction process, Murashige and Skoog, (1962) (MS) culture medium was used with mineral and vitamin formulations of the basic medium. The MS culture medium was supplemented with 30 g. L-1 sucrose, solidified with Phytagel 0.2 % in the concentrations of growth regulators of 0.1 mg. L-1 of 2,4 D (2,4-dichlorophenoxyacetic acid) and 0.1 mg L−1 BAP (6-benzylaminopurine), with the pH of the medium adjusted to 5.8, the system has sterilized an autoclave at 121 °C for 20 minutes.
Explants of hypocotyls of H. sabdariffa from axenic cultivation were used. In a laminar flow chamber the explants were fragmented (1 cm) and their segments inoculated, using four segments per culture vial (5 cm x 10 cm), containing 40 ml of the MS culture medium with a total of two replications and kept an incubator type B.O.D at 30 °C with a photoperiod of 16 h of light and intensity of 90 μmol m−2 s−1 for the induction of callus (Figure 1) for 120 days.
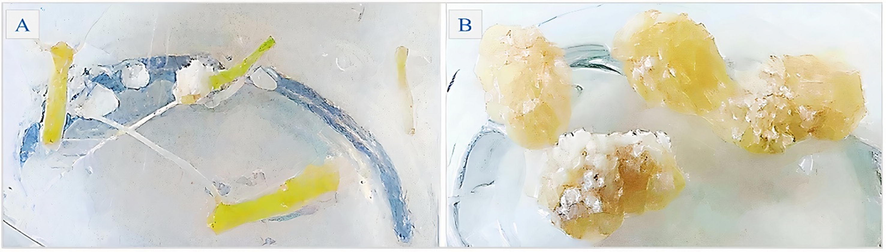
- Hypocotyl segments of H. sabdariffa inoculated in MS culture medium (A) for induction of hypocotyledonary callus in vitro (B).
The experimental assay was derived from the study of biomass accumulation. After 120 days of cultivation, the in vitro hypocotiletiledonary callus of H. sabdariffa were dehydrated in an air circulation stove at a constant temperature of 50 °C for 48 hours.
2.3 Treatment of samples
The samples of dehydrated leaves and callus were sprayed with the aid of a mortar and pestle, then submitted to a solid-liquid extraction process. 200 mg of each plant material was weighed separately in cylindrical glass vials (5 cm x 10 cm), and 10 ml of hexane was added for each sample, this system was carried to ultrasonic machine-assisted agitation (UNIQUE, model USC 5000 A, frequency of 40 kHz, 155 Watts RMS-USA) for 30 minutes. At the end of the extractions, the solutions were filtered with the aid of a filter paper, the filtrate was reserved, and the residual biomass of each sample started a new extraction, this procedure was performed in triplicate at the end of the extractions the hexagonal filtrates of each plant material were gathered, and the solvent evaporated in an automated system at 45 °C, 8psi, for 2 hours and stored until chromatographic analyses. The defatted residual biomasses of each sample were stored in cylindrical glass vials with dimensions of 5 cm x 10 cm, at room temperature of 25 °C until the preparation of ethyl acetate extracts.
2.4 Preparation of ethyl acetate extract
Approximately 100 mg of each defatted biomass of leaves and callus (after treatment, item 2.3), their masses were measured in conical polypropylene vials of 2mL. Then 500 μl of HCl 4M were added to each conical vial and the system was shaken at full speed in a vortex mixer (Vision Bionex KMC-1300 V, Indonesia) for 30 seconds and submitted to an ultrasonic machine (UNIQUE, model USC 5000 A, frequency of 40 kHz, 155 Watts RMS-USA) for 60 minutes at 70 °C and cooled for 10 minutes. Subsequently, 600 μL of ethyl acetate was added, stirred in a vortex mixer for 30 seconds, and centrifuged for 2 minutes at 10,000 rpm. (Microhemato® centrifuge, mod. 2410, Fanen®, São Paulo, Brazil). The supernatant was transferred to another 2 mL polypropylene conical all. This procedure was performed in sextuplicate, and the spare solutions were collected, and the solvents formed evaporated in an automated system at 45 °C, 8psi, for 2 hours and stored until chromatographic analyses were performed.
2.5 Analysis in analytical thin layer chromatography (A-TLC)
For the analysis, silica chromatographic plates were used, cut in dimensions of 10 x 2cm, and activated by heating the heating plate at 100 °C for 2 minutes. The concentrated extracts of the leaves and callus of H. sabdariffa (items 2.3 and 2.4) were solubilized in hexane and ethyl acetate at a concentration of 1mg/mL, and the aliquots were applied to the chromate plates in the form of bands through a capillary tube. The development of A-TLC of hexane extract of leaves and callus of H. sabdariffa was performed using the mobile phase composed of hexane; ether; and acetone drops (8:2:2 drops v /v /v) and for the ethyl acetate extract elution the combination of dichloromethane; methanol with 1% formic acid and drops of ether (9:1:10 drops) was used. Each mobile phase was taken to glass vats for the development of the plates. After the development of the plaques was revealed, using reagents such as vanillin acidified with sulfuric acid (VAS) for hexane extracts, being heated to 110 °C for 5 minutes for optimal staining development and with the developer diphenylboryloxiethylamine / polyethylene glycol (NP-PEG) for ethyl acetate extracts, this developer reacts especially with flavonoids and phenolic acids through its phenolic hydroxyl groups that under the incidence of UV radiation in 365nm, reveals the constituents in the form of fluorescent bands.
2.6 Derivatization method of ethyl acetate extract
According to the literature, the amounts of derivatizing agents for the silylation of plant extracts vary between 30-125 μL, organic acids and sugars need relatively low volumes of the derivatizing agent (Roessner et al., 2000). The derivatization stage consists of promoting a silylation reaction of phenolic groups with trimethylsilyl, which converts the compounds mentioned into more volatile substances. In this sense, after evaporation of the ethyl acetate extract of leaves and callus (item 2.4), approximately 10 mg of each sample was weighed for conical polypropylene tubes. Next, 100 μL of the derivatizing agent N, O-Bis(trimethylsilyl) trifluoroacetamide (BSTFA) + 1% trimethylchlorosilane (TMCS) was added, stirred in a vortex mixer (Vision Bionex KMC-1300V, Indonesia) for 2 minutes and submitted to incubation in an ultrasonic machine (UNIQUE, model USC 5000 A, frequency of 40 kHz, 155 Watts RMS-USA) for 1 hour at a temperature of 60 °C to modify some chemical and physical characteristics of the sample constituents that would enable its detection and identification by GC-MS. The derivatized samples were extracted with 500μL of ethyl acetate under agitation in a vortex mixer for 30 seconds and centrifuged for 2 minutes at 10,000 rpm (Microhemato® centrifuge, mod. 2410, Fanen®, São Paulo, Brazil). The supernatant of each sample was transferred to a 2 ml glass vial with a lid and septum for the analysis in the gas chromatography coupled to the mass spectrum (GC-MS).
2.7 Analysis and identification by CG-MS
Gas chromatography analyses were performed using a Thermo Scientific Trace 1300 Gas Chromatograph (GC) coupled to a Thermo Scientific MS-ISQ Single Quadrupole mass spectrometer (EM), with self-sampler AI 1310, equipped with capillary column ZB-5HT INFERNO (30m x 0.25mm x 0.10μm), Helium gas as a carrier at a flow of 1mL / min. 1.0 μL sample injection in Splitless mode. The injector with operation at 250 °C and the oven temperature programming starting at 100 °C, for 5 min., rising to 375 °C (10 °C/min.), for 5 min. The MS-ISQ with an interface at 275 °C, ionization source at 250 °C, using a solvent delay of 3 minutes, with mass range (50-1100 Da). Electronic ionization at 70 eV. The Automated Mass Spectral Deconvolution and Identification System (AMDIS) software was used for the deconvolution of the spectra, each research produced a list of spectra in the library that were classified by similarity to the target spectrum. After deconvolution, the cleaned mass spectrum of each trimethylsililated metabolites was identified by matching the mass spectra with those of reference compounds, using the commercial libraries such as NIST2011, WILEY2009, FAMES2011, and Golm Metabolome Database (GMD).
2.8 Statistical analysis
First, a spreadsheet was elaborated with data from the percentage areas of the chemical compounds (encoded from 1A to 38A) of the leaves and callus of H. sabdariffa in Microsoft Excel 2013®. The percentage areas of the identified compounds were used because it is widely accepted that the peak area of an analyte in a chromatograph is directly proportional to its concentration (Pinkard et al., 2020). The resulting data matrix based on the relative abundance of metabolites was exported in the format. CSV (separated by comma) for a web server called ClustVis: a web tool for visualizing clustering of multivariate data (v. BETA), which is available for free, and no need for login in http://biit.cs.ut.ee/clustvis/. ClustVis was used for multivariate statistical analyses, including principal component analysis (PCA) and heat map (Metsalu, Vilo, 2015). The PCA was performed using self-staggered data and under the NIPALS algorithm and the dendrogram of the heat map was generated using Euclidean distance for the samples.
3 Results and discussion
3.1 Chemical characterization by A-TLC
The analytical thin layer chromatography (A-TLC) technique, using silica as a stationary phase was used to verify the complexity of each extract. The results of A-TLC analyses of hexane extracts and ethyl acetate of leaves and callus are shown in Figure 2.
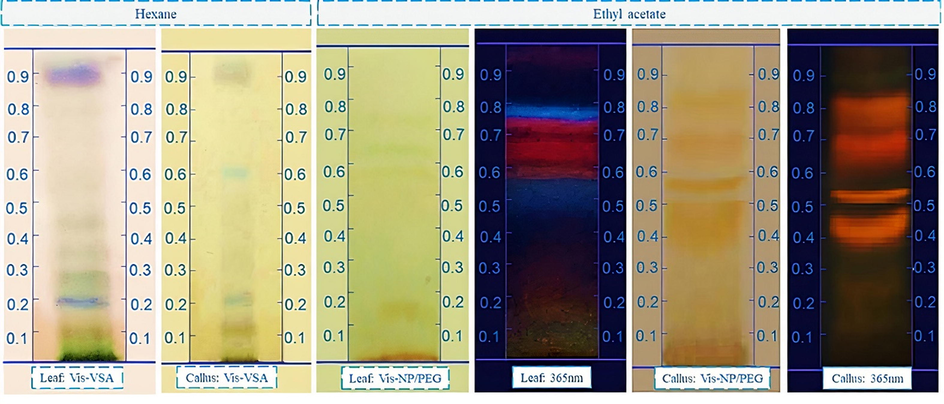
- Analytical thin layer chromatography (A-TLC) performed using hexane extract of leaves and callus of H. sabdariffa eluded in hexane; ether; and acetone drops (8:2: 2 drops v /v/v) and sprayed with VAS and ethyl acetate extract eluided in dichloro : methanol with 1% formic acid and drops of ether (9:1:10 drops v/v/v) and sprayed with chromogenic agent NP/PEG visualized at 365 nm.
The chemical profile in TLC of hexane extracts and acetate of leaves and callus allowed the evaluation of the metabolic complexity of the compounds. The use of the VSA solution as a chemical developer allows the detection of fatty acids (blue), in addition to terpenes, limonoids, and steroids (purple) (Karthika et al., 2014; Salazar et al., 2018), thus, it was possible to observe in Figure 2 that the hexane extract (left) of the leaves and calluses presented few bands with blue and/or purple tones after the revelation of the plate with VSA, being possible to suggest the low occurrence of substances of these classes.
In Figure 2, the ethyl acetate extract (right) of leaves and callus presents fluorescent bands observed at 365nm after revelation with NP-PEG, presenting blue bands, under ultraviolet light, at 365 nm for leaf extract, and may be indicative of the presence of phenolic compounds (phenolic acids or cinnamic derivatives) existing in the extract (Cretu et al., 2013). Similarly, red bands were observed, indicating the presence of chlorophyll in the ethyl acetate extract of the leaves. While for the ethyl acetate extract of the callus it was possible to observe yellow-orange spots endorsed with fluorescence after the revelation with NP-PEG, suggesting the presence of flavonoid derivatives. The results obtained in these analyses indicate that despite the low separation capacity of the most advanced analytical chromatographic techniques, TLC has advantages because it is easy, fast, economical, and allows the screening of a wide range of phytochemicals. In addition, it provides the first approach for the investigation of the chemical composition of plants (Skalicka et al., 2008).
3.2 Chemical characterization by GC-MS
When it comes to highly complex matrices such as organic acids, it is advisable to use a derivatization process to improve separation parameters in gas chromatography, such as volatility, thermal stability, resolution, as well as detection parameters. During this process, the derivatizing reagent plays an important role in the separation and resolution of analytes. Among the different derivatization reagents for organic acids, silylants are the most popular, such as BSTFA (Jarukas et al., 2020). In silylation reactions, it occurs by nucleophilic substitution (SN2) and the products are generally more volatile and thermally stable (Schummer et al., 2006), labile hydrogen of acids, alcohols, thiols, amines, starches, or enolizable ketones and aldehydes is replaced by a trimethylsilyl group (TMS).
Table 1 presents the secondary metabolites present in ethyl acetate extracts of leaves and callus of H. sabdariffa analyzed by GC-MS. The results show 38 substances, with their respective retention times (RT), relative abundances described by the percentage of peak area, both for leaves and callus (%), molecular ion (M+), and the m/z ratio of greater abundance (m/z (abundance)).
| Code | RT | Compounds | Leaf (%) | Callus (%) | M+ | m/z (abundance) |
|---|---|---|---|---|---|---|
| 1A | 3.15 | Butanoic acid, 2-hydroxy-, ethyl ester | 2.32 | - | 132 | 59 (100); 31 (25); 29 (13) |
| 2A | 3.80 | Furan-2-carboxilic acid-(1TMS) | 0.05 | 0.02 | 184 | 125 (100); 169 (49); 95 (35) |
| 3A | 4.19 | Benzoic acid-(1TMS) | 0.37 | 1.09 | 194 | 179 (100); 105 (90); 135 (75) |
| 4A | 4.29 | Phosphoric acid-(3TMS) | 0.10 | 8.25 | 314 | 299 (100); 133 (30); 283 (23) |
| 5A | 4.89 | Succinic acid-(2TMS) | 13.36 | 9.6 | 262 | 147 (100); 247 (92); 73 (50) |
| 6A | 4.92 | Valeric acid, 5-amino-(3TMS) | 0.42 | - | 333 | 173 (100); 73 (46); 147 (23) |
| 7A | 5.30 | Threonic acid-1,4 lactone-(2TMS) | 0.06 | - | 262 | 101 (100); 247 (80); 116 (76) |
| 8A | 5.35 | Malonic acid, methyl-(2TMS) | 11.92 | - | 262 | 247 (100); 218 (76); 133 (72) |
| 9A | 5.44 | Glyceric acid-(3TMS) | 0.22 | - | 322 | 189 (100); 103 (62); 292 (50) |
| 10A | 5.73 | Fumaric acid-(2TMS) | 0.05 | 2.75 | 259 | 245 (100); 143 (26); 83 (22) |
| 11A | 7.12 | Hydroquinose-(2TMS) | 0.66 | - | 254 | 239 (100); 117 (25); 129 (11) |
| 12A | 7.22 | Lactic acid dimer-(2TMS) | 1.39 | - | 306 | 117 (100); 73 (80); 147 (30) |
| 13A | 7.73 | Tridecan-1-ol, n-(1TMS) | - | 1.21 | 272 | 257 (100); 103 (30); 83 (25) |
| 14A | 7.79 | 1-Dodecanol-(TMS) | 0.30 | 0.76 | 259 | 243 (100); 103 (25); 90 (20) |
| 15A | 8.59 | Mallic acid 2 methyl-(3TMS) | 4.89 | 2.43 | 349 | 247 (100); 115 (68); 85 (24) |
| 16A | 8.62 | Mallic acid-(3TMS) | 1.39 | 1.91 | 350 | 233 (100); 133 (93); 245 (70) |
| 17A | 8.66 | Alanine, Beta-(1TMS) | 0.30 | - | 161 | 117 (100); 143 (48); 75 (23) |
| 18A | 8.69 | Butanoic acid, 4-hydroxy-(2TMS) | 0.14 | - | 249 | 117 (100); 233 (54); 143 (48) |
| 19A | 10.06 | Glutaric acid 2-hydroxy-(3TMS) | 0.11 | 0.33 | 364 | 129 (100); 247 (65); 203 (34) |
| 20A | 10.48 | Benzoic acid, 4-hydroxy-(2TMS) | 0.61 | 0.98 | 282 | 223 (100); 267 (90); 193 (77) |
| 21A | 10.71 | alfa-Rhamnose, (TMS) | 1.44 | 0.82 | 453 | 204 (100); 191 (25); 217 (15) |
| 22A | 11.28 | Eicosan-1-ol, n-(1TMS) | - | 14.09 | 370 | 355 (100); 103 (28); 83 (21) |
| 23A | 11.3 | Hexanedioic acid, hydroxy-(3TMS) | 6.92 | 2.14 | 378 | 129 (100); 171 (53); 261 (47) |
| 24A | 11.53 | Epinephrine-(3TMS) | 2.24 | - | 355 | 75 (100); 149 (38); 245 (30) |
| 25A | 12.12 | Vanillic acid-(2TMS) | 0.81 | 1.02 | 312 | 297 (100); 267 (94); 223 (89) |
| 26A | 12.73 | Protocatechuic acid-(3TMS) | 26.86 | 16.68 | 370 | 193 (100); 77 (25); 311 (14) |
| 27A | 12.84 | Isocitric acid-(4TMS) | - | 12.56 | 480 | 273 (100); 245 (81); 465 (50) |
| 28A | 13.38 | Quinic acid-(5TMS) | 2.90 | 1.12 | 435 | 345 (100); 255 (79); 191 (28) |
| 29A | 14.05 | Cinnamic acid 4-Hydroxy-(2TMS) | 0.87 | 1.63 | 308 | 219 (100); 293 (76); 249 (65) |
| 30A | 14.08 | Hydrocaffeic acid-(3TMS) | 2.33 | - | 398 | 179 (100); 73 (91); 267 (25) |
| 31A | 14.19 | Gallic acid-(4TMS) | 1.77 | 4.78 | 458 | 281 (100); 443 (25); 179 (17) |
| 32A | 14.66 | Glucopyranose, D-(5TMS) | 0.28 | - | 540 | 204 (100); 191 (64); 129 (28) |
| 33A | 15.19 | Hexadecanoic acid-(1TMS) | 12.45 | 7.29 | 328 | 117 (100); 129 (47); 313 (32) |
| 34A | 15.71 | Tricosane | 0.21 | - | 324 | 71 (100); 85 (70); 99 (31) |
| 35A | 15.85 | Caffeic acid, cis-(3TMS) | 0.95 | - | 396 | 219 (100); 73 (88); 191 (27) |
| 36A | 15.87 | Caffeic acid trans-(3TMS) | 1.31 | 2.09 | 396 | 219 (100); 191 (28); 381 (18) |
| 37A | 24.25 | Stigmasterol-(1TMS) | - | 5.10 | 484 | 83 (100); 129 (71); 394 (15) |
| 38A | 24.68 | Sitosterol, Beta-(1TMS) | - | 1.35 | 486 | 129 (100); 327 (39); 396 (30) |
RT = Retention times. M+ = Molecular ion.
Table 1 was constructed by determining the area of each chromatographic peak, being related to the total area of all peaks identified for the leaves and calluses of H. sabdariffa, thus obtaining a total percentage ratio of 100% for the samples, according to the number of chromatographic peaks detected, with the retention time, expressed in minutes, and the relative percentage area for leaves and callus expressed in percentage (%). All compounds were identified by comparing the experimental mass spectrum and the electronic reference databases of mass spectral data (NIST2011-WILEY2009-FAMES2011-GMD) and the depth and increase in the identification of these compounds were due to the processing of files by mass spectrometry with the AMDIS software that aided in the adjacent deconvolution of the peak, subtraction, and increased detection limits (Halket et al., 1999).
A total of 38 different bioactive metabolites were identified by GC-MS analysis (Table 1). Among the identified substances, protocatechuic acid (26A) stands out as the main constituent, with a relative abundance of 26.86% and 16.68% for leaves and callus of H. sabdariffa, respectively. Protocatechuic acid is a derivative of benzoic acid (Marchiosi et al., 2020) widely distributed and occurs naturally in most edible plants used in folk medicine, it is a phenolic acid with a chemical structure like that of gallic acid, caffeic acid, and vanillic acid, which are well-known antioxidant compounds (Sahil, Souravh, 2014).
3.3 Exploratory data analysis
All data were normalized to calculate the covariance matrix and its auto values and autovectors, which were derived from the peak area of the 38 chemical compounds presented in Table 1, being exported to the ClustVis tool for principal component analysis (PCA), calculation of the corresponding contribution rates (PC1 and PC2) (Figure 3).
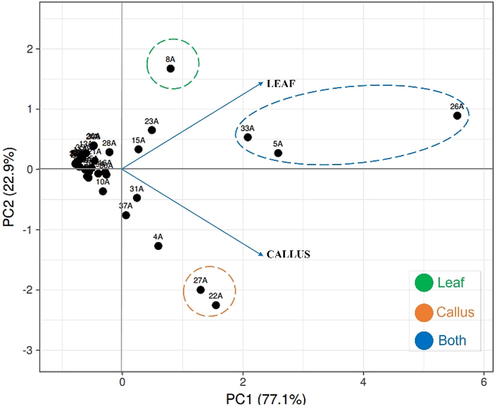
- Principal component analysis (PCA) biplot, based on the variability of chemical profiles of leaf and callus samples of H. sabdariffa. Source: Authors. Created using the ClustVis.
The result shows in Figure 3 the representation of the PCs in a two-dimensional graph, providing an overview of the data, pointing out patterns of similarity or differentiability in the data set, and it is possible to identify correlations between the variables. Each point on the graph represents a chemical compound in the samples, which show similar clustered variations. Thus, the PCA shows a tendency to separate the different samples into two principal components, a scatter in the axes corresponding to the PC1 and PC2 that explain 77.1%, and 22.9% respectively. To the components were assigned the most informative variability, explaining 100% of the total variation in the data. PCA is one of the most important statistical techniques used to characterize the interrelationships between variables and the visualization of data patterns (Ghelichkhani et al. 2019, Samiee et al. 2020), and is useful in discriminating the chemical composition of each sample, highlighting the compounds present in the leaves and callus of H. sabdariffa.
The PCA graph indicated the variable distribution of components of higher concentration present only in the leaves (green circle - compound 8A - methylmalonic acid), exclusive of callus (orange circle – compounds 22A and 27A – eicosanol and isocitric acid) and in both samples (blue circle – compounds 5A, 33A, and 26A – succinic acid, hexadecanoic acid and protocatechuic acid respectively).
Figure 4 shows the chemical structures silylated from these higher concentration organic compounds identified by GC-MS.
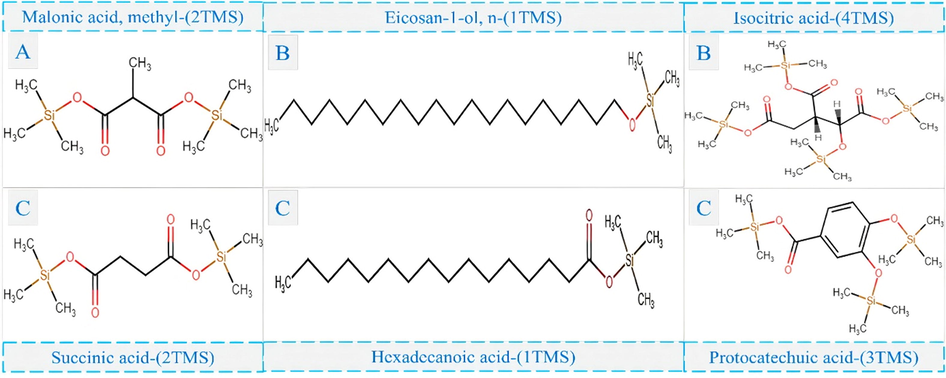
- Sililated structures of the major compounds present in the acetate extract of leaves and callus of H. sabdariffa. A: Leaves; B: Callus; C: Both.
The compounds presented in Figure 4 present several biological activities. Methylmalonic acid can stabilize polyphenols to maintain antioxidant and anticancer activities (Wenfeng et al., 2019), eicosanol exhibits antibacterial activity (Krichen et al., 2020), isocitric acid fights oxidative stress (Kamzolova, et al., 2021), while succinic acid has a variety of pharmacological effects, including cardioprotective, antithrombotic, anti-inflammatory, and antibacterial (Hao et al., 2020). In addition, hexadecanoic acid exhibits anti-inflammatory, antioxidant, pesticide, and larvicidal activity (Parveen et al., 2021), and finally, protocatechuic acid presents antioxidant action (Li et al., 2011), antibacterial (Chao, Yin, 2009), anticancer (Tanaka et al., 2011), antiulcer (Kore et al., 2011), antidiabetic (Scazzocchio et al., 2011; El-Sonbaty et al., 2019), antifibrotic (Li et al., 2012), antiviral (Zhou et al., 2007), anti-inflammatory, analgesic (Lende et al., 2011), antihyperlipidemic (Borate et al., 2011), cardioprotective (Sahil, Souravh, 2014), hepatoprotective (Liu et al, 2002; El-Sonbaty et al., 2019), neuroprotector (Guan et al., 2006) and nefro protector (Lee et al., 2009).
For the perception of the different metabolites present in the leaves and callus of H. sabdariffa, a heat map was also generated (Figure 5) based on the data in Table 1, and the samples of the leaves and callus of H. sabdariffa were arranged in columns according to their similarity and thus forming clusters between the samples. While, the metabolites stayed on the lines, following the same criteria mentioned above. Using normalized similarity distances, which are based on the Euclidean distance, it was possible to illustrate the general similarity between the samples and the profiles of the metabolites.
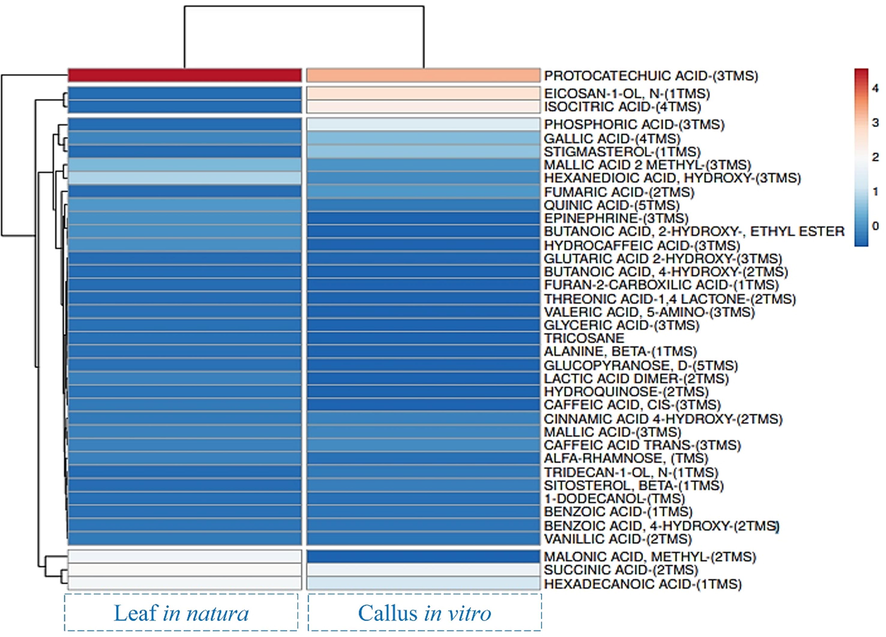
- Heat map with gradient of color from red to blue, which represents in this order the levels of higher and lower concentration of chemical compounds of in natura leaves and callus in vitro of H. sabdariffa. Source: Authors, built using the ClustVis.
Figure 5 shows the hierarchical grouping combined with a heat map, which represents the values of similar or very different expression status characteristics and is an unsupervised technique often used to determine the visual relationship between the samples of the dataset (Arabameri et al., 2019), Heydarieh et al. 2020). It was observed that the color gradient varied between the extreme distance values from 4 (intense red) to 0 (dark blue) indicating the values of higher and lower concentrations of chemical compounds, respectively. Therefore, it is observed in the heat map the confirmation that protocatechuic acid is the most abundant, in the leaves and callus of H. sabdariffa, followed by eicosanoid and isocitric acid, present only in the callus.
Protocatechuic acid is the main compound found in the flowers of H. sabdariffa (Semaming et al., 2014). The level of protocatechuic acid in H. sabdariffa has also been reported as the majority in comparison with other flowers, i.e. the Rosa hybrida and Camellia japonica flowers (Hapsari et al, 2021). Eicosanoids are a group of biologically active compounds derived from precursors of polyunsaturated fatty acids, in addition, they are key mediators in the binding between fatty acids, presenting several biological functions (Tsikas, Zoerner, 2014). Isocitric acid and its derivatives are widely used in industries in pharmaceutical, and cosmetic production, presenting the superior activity to ascorbic acid in the model of induced oxidative stress (Kamzolova, et al., 2021). In addition, the study by authors Morgunov et al., (2019) indicated that isocitric acid reduces neurotoxification by a high concentration of lead salts and molybdenum, in addition to restoring memory and accelerating learning.
Given the above, it is possible to conclude that the identified compounds are highly diversified, and their changes depend on chemical interactions between plants and their environment, but the in vitro culture approach of plant tissues is independent of environmental conditions, so there is the possibility of continuously producing these bioactive compounds, providing a source of uniform plant material, avoiding complex interactions between different organs and/or plant tissues. (Karuppusamy, 2009; López-Laredo et al., 2009; Ric-Varas, et al., 2020), enabling the manipulation of the biosynthesis pathways of plant cells to produce secondary metabolite derivatives with improved characteristics (Hussain et al., 2012), expanding the production of metabolites of interest through cell suspension cultures (Morales-Rubio, Espinosa-Leal, Garza-Padrón, 2016).
4 Conclusion
GC-MS analysis, in combination with exploratory data analysis, can effectively classify and identify the compounds present in the leaves and callus of H. sabdariffa. The PCA allowed the separation of the compounds from the samples, evidencing the importance of the technique for classifying and discriminating the substances presented in the samples, allowing the extraction of the relevant information, in addition, the heat map showed that both leaves and callus present protocatechuic acid as a major, which is a compound with antioxidant activity, antibacterial, anticancer, antiulcer, antidiabetic, antifibrotic, antiviral, anti-inflammatory, analgesic, antiatherosclerotic, cardioprotective, hepatoprotective, neuroprotective and nephroprotective. In addition, other compounds identified in the study for the species such as methylmalonic acid that presents antioxidant and anticancer activities, eicosanol with antibacterial activity, isocitric acid agent to combat oxidative stress, succinic acid that is cardioprotective, antithrombotic, anti-inflammatory, and antibacterial, hexadecanoic acid with anti-inflammatory activity, antioxidant, larvicidal pesticide.
The in vitro callus culture of H. sabdariffa proved to be an attractive technique to produce secondary metabolites, mainly phenolic compounds, besides being an ecological and sustainable alternative to produce this species with high bioactive potential. And even with the complexity of phenolic compounds, in vitro culture enables manipulation to increase the production of certain compounds. This cutting-edge technology is a promising system for studying the production of new or known metabolites in a highly controlled environment.
Acknowledgments
The authors thank the Coordenação de Aperfeiçoamento de Pessoal de Nível Superior Brasil (CAPES) - Financing Code 001 and PROPESP-UFPA, granting of a grant and financial support.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
References
- Oxidative stability of virgin olive oil: evaluation and prediction with an adaptive neuro-fuzzy inference system (ANFIS) J. Sci. Food Agric.. 2019;99(12):5358-5367.
- [CrossRef] [Google Scholar]
- Ethnopharmacognostic survey on botanical compendia for potential cosmeceutic species from Atlantic Forest. Rev. Bras. Farmacogn.. 2007;17(4):640-653.
- [CrossRef] [Google Scholar]
- Antihyperlipidemic effect of protocatechuic acid in fructose-induced hyperlipidemia in rats. Int. J. Pharma. Bio. Sci.. 2011;2(4):456-460.
- [Google Scholar]
- Pattern Recognition. In: Chemometrics: Data Analysis for the Laboratory and Chemical Plant. John Wiley & Sons Ltd; 2003. p. :183-269.
- [Google Scholar]
- Cardoso, J.C., Oliveira, M., Cardoso, Cardoso, F., 2019. Advances and challenges on the in vitro production of secondary metabolites from medicinal plants. Hortic. Bras. 37 (2), 124-132. https://doi.org/10.1590/S0102-053620190201
- Callus induction and bioactive phenolic compounds production from Byrsonima verbascifolia (L.) DC. (Malpighiaceae) Rev. Ciênc. Agron.. 2016;47:143-151.
- [CrossRef] [Google Scholar]
- Antibacterial effects of roselle calyx extracts and protocatechuic acid in ground beef and apple juice. Foodborne Pathog. Dis.. 2009;6(2):201-206.
- [CrossRef] [Google Scholar]
- A high-performance thin-layer chromatographic method for chlorogenic acid and hyperoside determination from berry extracts. Rom. Biotechnol. Lett.. 2013;18(5):8657-8665.
- [Google Scholar]
- Biotechnology applications of plant callus cultures. Engineering. 2019;5(1):50-59.
- [CrossRef] [Google Scholar]
- Protocatechuic acid exhibits hepatoprotective, vasculoprotective, antioxidant, and insulin-like effects in dexamethasone-induced insulin-resistant rats. Biochimie. 2019;167:119-134.
- [CrossRef] [Google Scholar]
- Effect of the spray and freeze dryers on the bioactive compounds of olive leaf aqueous extract by chemometrics of HCA and PCA. J. Food Meas. Charact.. 2019;13:2751-2763.
- [CrossRef] [Google Scholar]
- Protective effect of protocatechuic acid from Alpinia oxyphylla on hydrogen peroxide-induced oxidative PC12 cell death. Eur. J. Pharmacol.. 2006;538:73-79.
- [CrossRef] [Google Scholar]
- Deconvolution gas chromatography/mass spectrometry of urinary organic acids-potential for pattern recognition and automated identification of metabolic disorders. Rapid Commun. Mass Spectrom.. 1999;13:279-284.
- [CrossRef] [Google Scholar]
- Succinic acid inhibits the activity of cytochrome P450 (CYP450) enzymes. Pharm. Biol.. 2020;58(1):1159-1164.
- [CrossRef] [Google Scholar]
- Hapsari, B.W., Manikharda, Setyaningsih, W., 2021. Methodologies in the Analysis of Phenolic Compounds in Roselle (Hibiscus sabdariffa L.): Composition, Biological Activity, and Beneficial Effects on Human Health. Horticulturae. 7 (35). https://doi.org/10.3390/horticulturae7020035
- Heydarieh, A., Arabameri, M., Ebrahimi, A., As’habi, A., Marvdashti, L.M., Yancheshmeh, B.S., Abdolshahi, A., 2020. Determination of magnesium, calcium, and sulphate ion impurities in commercial edible salt. J. Chem. Health Risks. 10, 93-102. https://doi.org/10.22034/jchr.2020.1883343.1067
- Current approaches toward production of secondary plant metabolites. J. Pharm. Bioallied Sci.. 2012;4(1):10-20.
- [CrossRef] [Google Scholar]
- Investigation of organic acids in saffron stigmas (Crocus sativus L.) extract by derivatization method and determination by GC/MS. Molecules. 2020;25(15):3427.
- [CrossRef] [Google Scholar]
- Isocitric acid production from ethanol industry waste by Yarrowia lipolytica. Fermentation. 2021;7:146.
- [CrossRef] [Google Scholar]
- TLC and HPTLC Fingerprint profiles of different bioactive components from the tuber of solena amplexicaulis. J. Pharmacogn. Phytochem.. 2014;3:198-206.
- [Google Scholar]
- A review on trends in production of secondary metabolites from higher plants by in vitro tissue, organ, and cell cultures. J. Med. Plant Res.. 2009;3(13):1222-1239.
- [CrossRef] [Google Scholar]
- Evaluation of anti-ulcer activity of protocatechuic acid ethyl ester in rats. Int. J. Pharm. Life Sci.. 2011;2(7):909.
- [Google Scholar]
- Essential oil from pistachio by-product: potential biological properties and natural preservative effect in ground beef meat storage. J. Food Meas. Charact.. 2020;14:3020-3030.
- [CrossRef] [Google Scholar]
- Rhus verniciflua Stokes prevents cisplatin-induced cytotoxicity and reactive oxygen species production in MDCK-I renal cells and intact mice. Phytomedicine. 2009;16(2):188-197.
- [CrossRef] [Google Scholar]
- Anti-inflammatory and analgesic activity of protocatechuic acid in rats and mice. Inflammopharmacology. 2011;19(5):255-263.
- [CrossRef] [Google Scholar]
- Antifibrotic effects of protocatechuic aldehyde on experimental liver fibrosis. Pharm. Biol.. 2012;50(4):413-419.
- [CrossRef] [Google Scholar]
- Li, X., Wang, X., Chen, D., Chen, S. Antioxidant activity and mechanism of protocatechuic acid in vitro. Funct. Foods Health Dis. 7, 232-244. https://doi.org/10.31989/ffhd.v1i7.127.
- Liu, C.L., Wang, J.M., Chu, C.Y., Cheng, M.T., Tseng, T.H., 2002. In vivo protective effect of protocatechuic acid on tert-butyl hydroperoxide-induced rat hepatotoxicity. Food Chem. Toxicol. 40 (5), 635-641, 2002. https://doi.org/10.1016/s0278-6915(02)00002-9.
- Extraction and identification of volatile organic compounds emitted by fragrant flowers of three tillandsia species by HS-SPME/GC-MS. Metabolites. 2021;11(9):594.
- [CrossRef] [Google Scholar]
- Comparison of metabolite levels in callus of Tecoma stans (L.) Juss. ex Kunth. cultured in photoperiod and darkness. In Vitro Cell. Dev. Biol. Plant.. 2009;45(5):550-558.
- [CrossRef] [Google Scholar]
- Marchiosi, R., dos Santos, W.D., Constantin, R.P., de Lima, R.B., Soares, A.R., Finger-Teixeira, A., Mota, T.R., de Oliveira, D.M., Foletto-Felipe, M. de P., Abrahão, J., Ferrarese-Filho, O., 2020. Biosynthesis and metabolic actions of simple phenolic acids in plants, Phytochem. Rev. 19, 865-906. https://doi.org/10.1007/s11101-020-09689-2
- From sample treatment to biomarker discovery: A tutorial for untargeted metabolomics based on GC-(EI)-Q-MS. Anal. Chim. Acta. 2015;900(21–35):2015.
- [CrossRef] [Google Scholar]
- Mayasari, N.R., Susetyowati, Wahyuningsih, M.S.H., Probosuseno., 2018. Antidiabetic Effect of Rosella-Stevia Tea on Prediabetic Women in Yogyakarta, Indonesia. J. Am. Coll. Nutr. 37 (5), 373-379. https://doi.org/10.1080/07315724.2017.1400927
- ClustVis: a web tool for visualizing clustering of multivariate data using principal component analysis and heatmap. Nucleic Acids Res.. 2015;43:W566-W570.
- [CrossRef] [Google Scholar]
- The antidiabetic and antilipidemic effects of Hibiscus sabdariffa: a systematic review and meta-analysis of randomized clinical trials. Food Res. Int.. 2020;130
- [CrossRef] [Google Scholar]
- Cultivo de tejidos vegetales y su aplicación en productos naturales. In: Rivas-Morales C., Oranday-Cardenas M.A., Verde-Star M.J., eds. Investigación en plantas de importancia médica (1st edn.). Barcelona: OmniaScience; 2016. p. :351-410.
- [Google Scholar]
- Biosynthesis of isocitric acid in repeated-batch culture and testing of its stress-protective activity. Appl. Microbiol. Biotechnol.. 2019;103:3549-3558.
- [CrossRef] [Google Scholar]
- Revised medium for rapid growth and bioassays with tobacco tissue cultures. Physiol Plant.. 1962;15:473-497.
- [CrossRef] [Google Scholar]
- Parveen, R., Shamsi, T.N., Fatima, S., 2021. Sandalwood, an Indian medicinal plant attenuates the microbial growth and influence up / down regulation of the metabolites. https://doi.org/10.1101/2021.03.01.433331.
- Raman spectroscopic data from formic acid decomposition in subcritical and supercritical water. Data Brief. 2020;29:105312
- [CrossRef] [Google Scholar]
- A Importância das Técnicas Acopladas (CL/UV, CL/EM, CL/RMN) para Procura de Princípios Ativos. Rev. Fitos.. 2006;2(3):39-53.
- [Google Scholar]
- Computational and statistical analysis of metabolomics data. Metabolomics. 2015;11:1492-1513.
- [CrossRef] [Google Scholar]
- Exploring the use of fruit callus culture as a model system to study color development and cell wall remodeling during strawberry fruit ripening. Plants. 2020;9(7):805.
- [CrossRef] [Google Scholar]
- Simultaneous analysis of metabolites in potato tuber by gas chromatography–mass spectrometry. Plant J.. 2000;23:131-142.
- [CrossRef] [Google Scholar]
- A review on protocatechuic acid and its pharmacological potential. Int. Sch. Res. Notices 2014
- [CrossRef] [Google Scholar]
- Salazar, M.A.R., Costa, J.V., Urbina, G. R.O., Cunha, V.M.B., Silva, M.P., Bezerra, P. do N., Pinheiro, W.B.S., Leal, W.G., Lopes, A. S., Carvalho Junior, R.N., 2018. Chemical composition, antioxidant activity, neuroprotective and anti-inflammatory effects of cipó-pucá (Cissus sicyoides L.) extracts obtained from supercritical extraction J. Supercrit. Fluids, 138, 36-45. https://doi.org/10.1016/j.supflu.2018.03.022
- The concentration of polycyclic aromatic hydrocarbons (PAHs) in the processed meat samples collected from Iran’s market: a probabilistic health risk assessment study. Environ. Sci. Pollut. Res.. 2020;27(17):21126-21139.
- [CrossRef] [Google Scholar]
- Cyanidin-3-O-β-glucoside and protocatechuic acid exert insulin-like effects by upregulating PPARγ activity in human omental adipocytes. Diabetes. 2011;60(9):2234-2244.
- [CrossRef] [Google Scholar]
- Analysis of t-butyldimethylsilyl derivatives of chlorophenols in the atmosphere of urban and rural areas in East of France. Chromatographia. 2006;63:189-195.
- [CrossRef] [Google Scholar]
- Protocatechuic acid exerts a cardioprotective effect in type 1 diabetic rats. J. Endocrinol.. 2014;223(1):13-23.
- [CrossRef] [Google Scholar]
- Plant Materials in Modern Pharmacy and Methods of Their Investigations. In: Thin Layer Chromatography in Phytochemistry. CRC Press; 2008. p. :16-35.
- [Google Scholar]
- Potential cancer Chemopreventive activity of protocatechuic acid. J. Exp. Clin. Med.. 2011;3(1):27-33.
- [CrossRef] [Google Scholar]
- Analysis of eicosanoids by LC-MS/MS and GC-MS/MS: a historical retrospect and a discussion. J. Chromatogr. B Anal. Technol. Biomed. Life Sci.. 2014;964:79-88.
- [CrossRef] [Google Scholar]
- Chemical composition, antioxidant activity, and antitumor activity of tumorous stem mustard leaf and stem extracts. CyTA. J. Food Sci.. 2019;17(1):272-279.
- [CrossRef] [Google Scholar]
- Protocatechuic aldehyde inhibits hepatitis B virus replication both in vitro and in vivo. Antivir. Res.. 2007;74(1):59-64.
- [CrossRef] [Google Scholar]







