Translate this page into:
Updated review on Indian Ficus species
⁎Corresponding author. bharatsingh217@gmail.com (Bharat Singh)
-
Received: ,
Accepted: ,
This article was originally published by Elsevier and was migrated to Scientific Scholar after the change of Publisher.
Peer review under responsibility of King Saud University. Production and hosting by Elsevier.
Abstract
As per Ayurvedic system of medicine, Indian Ficus (Fam. – Moraceae) plants are used in the treatment of various diseases. The plants are characterized by a specific class of closed inflorescence, named syconia, and are distributed in different states of India. Total 97 species of Ficus genus are naturalized in Indian states. Indian Ficus species possess anti-inflammatory, antimicrobial, antioxidant, antidiabetic, antiarthritic, antistress, anticancer, hepatoprotective, neuroprotective and wound healing properties. The phytochemical analysis reveals the presence of alkaloids, triterpenoids, flavonoids, furanocoumarins, and polyphenolic compounds in different species. Recently, bioavailability of Indian Ficus has been increased due to the presence of antioxidative agents. However, large number of reports have been published on phytochemistry and biological activities of 31 Indian Ficus species but, no reports are available in literature on 66 species. This review summarizes and describes the current knowledge of ethnomedicinal uses, phytochemistry, pharmacological activities, bioavailability, and pharmacokinetic profiles of 31 Indian Ficus species. Moreover, it includes clinical and toxicological studies with an aim to explore their potential in the pharmaceutical industries.
Keywords
Clinical and toxicological studies
Ethnomedicinal properties
Ficus species
Pharmacology
Phytochemistry
- ABTS
-
2,2-Azinobis-(3-ethylbenzothiazoline-6-sulfonate)
- ALT
-
Alanine aminotransferase
- AST
-
Aspartate transaminase
- BSA
-
Bovine serum albumin
- CAT
-
Catalase
- CCl4
-
Carbon tetrachloride
- CFA
-
Complete Freund’s adjuvant
- COX-2
-
cyclooxygenase 2
- DMSO
-
Dimethylsulfoxide
- DPPH
-
2,2-Diphenyl-1-picrylhydrazyl
- FRAP
-
Ferric reducing antioxidant power assay
- HFD-STZ
-
High fat diet fed-streptozotocin- induced
- iNOS
-
Inducible nitric oxide synthase
- LPS
-
Lipopolysaccharide
- MDA
-
malondialdehyde
- MIA
-
Monosodium iodoacetate
- MIC
-
Minimum inhibitory concentration
- MTT assay
-
3-(4,5-Dimethylthiazol-2-yl)-2,5-diphenyl-2H-tetrazolium bromide
- NO
-
Nitric oxide
- ORAC
-
Oxygen radical absorbance capacity
- PGE2
-
Prostaglandin E2
- SGOT
-
Serum glutamic oxaloacetic transaminase
- SGPT
-
Serum glutamic pyruvic transaminase
- SOD
-
Superoxide dismutase
- TNF-α
-
Tumor necrosis factor
- VEGF
-
Vascular endothelial growth factor
Abbreviations
1 Introduction
Medicinal plants play vital roles in primary healthcare system of various developing countries due to lack of modern healthcare infrastructure, traditional acceptance, high cost of pharmaceutical drugs as well as efficacy of medicinal plants against certain disorders that cannot be treated by modern therapeutic drugs (Abdullahi, 2011; Kipkore et al., 2014; Megersa and Tamrat, 2022). Numerous patients in these developing countries combine folklore medicines with standard medicines and use them for the treatment of chronic diseases (Kigen et al., 2013). Ficus genus includes trees, hemi-epiphytes, shrubs, creepers, and climbers and are distributed in the forests, tropical and subtropical areas of Asia, Africa, America, and Australia (Hamed, 2011; Ahmed and Urooj, 2010b). Certain Indian Ficus species do not bear fruits, but they have similar morphological characters that are problematic to be distinguished from their species and variants. Every part of Ficus plants is used in the treatment of peptic ulcers, piles, jaundice, haemorrhage, diabetes, asthma, diarrhoea, dysentery, biliousness, and leprosy (Chopra et al., 1956; Kirtikar and Basu, 1995; Cox and Balick, 1996; Khan and Khatoon, 2007).
Different species of Indian Ficus genus contain sesquiterpenes, monoterpenes, triterpenoids, phenolic compounds, flavonoids, anthocyanins, alkaloids, furanocoumarins, organic acids, volatile components, and phenylpropanoids (Khayam et al., 2019; Shao et al., 2018; Tamta et al., 2021). These metabolites occur in latex, leaves, fruit, stem, and roots of different species (Shahinuzzaman et al., 2021). The Indian Ficus plants possess remarkable analgesic (Mahajan et al., 2012), antimicrobial (Patil and Patil, 2010), antiarthritic (Thite et al., 2014), anticancer (Jamil and Abdul Ghani, 2017), neuroprotective (Ramakrishna et al., 2014) and antidiabetic properties (Anjum and Tripathi, 2019a).
The present review summarizes and discusses the updated knowledge of ethnomedicinal properties, phytochemistry, pharmacological activities of 31 Indian Ficus species. Moreover, toxicological, and clinical studies are also included in this review. Out of 31 species, some species possess potent biological activities, but they have not been evaluated for their clinical research. No information is available in literature on 66 species. This review also provides some critical insights into the current scientific knowledge of bioavailability and pharmacokinetic profiles and its future potential in pharmaceutical research.
2 Methods
The data of identified compounds, studies of pharmacological activities, clinical trials, and toxicological research of 31 species were extracted by using various databases and search-engines e.g., monographs, reference books, MSc/MTech dissertations, PhD theses, PubMed/Medline, Scopus, ScienceDirect, Scifinder, Microsoft Academic, eFloras, Wiley, Google Scholar, DataONE Search, and Research Gate. The meta-analysis of extracted information was also conducted. The authors did not include the pharmacological activities of synthetic and semisynthetic compounds in this review.
3 Results
3.1 Botany and ethnomedicinal properties
Most Indian Ficus species are deciduous and evergreen trees, shrubs, herbs, and climbers. The leaves are reticulate, palmately compound, waxy, and exude white or yellow latex when broken. Many Indian Ficus species have aerial roots, whereas few are epiphytes. The syconium is hollow, enclosing an inflorescence with small male and female flowers lining the inside (Fig. 1). Different parts (bark, fruit, leaves, roots, and latex) of Ficus plants are used in the treatment of leprosy, nose bleeding, cough, paralysis, liver diseases, chest pain, and piles (Kirtikar and Basu, 1995; Khanom et al., 2000; Jaradat, 2005). Leaf infusion of F. carica is recommended as remedy for the treatment of diabetes and hypercholesterolemia (Chaachouay et al., 2019). Powdered roots and leaves of F. deltoidea are taken in the treatment of wounds, rheumatism, and sores (Burkill and Haniff, 1930). Fruits of F. racemosa are given in menorrhea, haemoptysis, visceral obstruction, diarrhoea, and constipation (Chopra et al., 1958; Ahmed et al., 2012b). Mixture of F. religiosa leaf juice and honey is employed for the treatment of asthma, cough, diarrhoea, earache, toothache, migraine, eye troubles, and scabies (Jain et al., 1991; Bhattarai, 1993b; Yadav, 1999). The ethnomedicinal properties of 31 Indian Ficus species are presented in Table 1 and Fig. 2.
Morphological features of Indian Ficus species.

Morphological features of Indian Ficus species.

Morphological features of Indian Ficus species.

Morphological features of Indian Ficus species.

Morphological features of Indian Ficus species.

Morphological features of Indian Ficus species.
Species
State/India
Disease/complaints
Mode/parts of application
References
F. abelii
Arunachal Pradesh, Assam, and Meghalaya
Used in the treatment of diabetes
Leaf decoction
Chaachouay et al. (2019)
F. auriculata (syn.
F. pomifera)Arunachal Pradesh, Assam, Bihar, Jammu &
Kashmir, Jharkhand, Maharashtra, Manipur, Meghalaya, Mizoram, Orissa, Sikkim, Karnataka, and West Bengal
Externally applied for wound healing
Paste of crushed leaves
Kunwar and Bussmann (2006)
Treatment of dysentery
Roasted figs
Zhang et al. (2019)
Used in cholera mumps, and vomiting
Latex of roots
Tamta et al. (2021)
Taken in the treatment of jaundice
Mixture of root powder and bark of Oroxylum indicum
Kunwar and Bussmann (2006)
Useful in diarrhoea
Infusion of stem bark
Cox and Balick (1996)
Employed in the treatment of diabetes
Fruits
Wangkheirakpam and Laitonjam (2012)
F. bengalensis
Uttar Pradesh, Madhya Pradesh, West Bengal, Himachal Pradesh, Rajasthan, Karnataka, Tamil Nadu, and Kerala
Employed in cold, cough and asthma
Boiled stem bark
Shakya (2000)
Used for diarrhoea, dysentery, indigestion, joint pain, dermatitis, gum swelling, gonorrhoea, and snake bite
Milky sap from bark
Cause allergy to children
Leaves
Dangol (2002)
Employed in stopping the menstruation
Aerial root juice
Mishara (1998)
Applied externally for body pain, toothache, diabetes, joint pain, and rheumatism
Kharel and Siwakoti (2002)
Helps in leucorrhoea control
Root bark powder is mixed with Desmostachys bipinnata and is taken with one spoon of sugar
Siwakoti and Siwakoti (2000)
Treatment of boils, wounds and obstinate vomiting
Root latex
Parajuli (2001)
It is used in diarrhoea
Aerial roots decoction and water obtained from rice wash
Chopra et al. (1956)
F. benzamina
Andaman & Nicobar Islands, Arunachal Pradesh, Assam, Bihar, Jharkhand, Madhya
Pradesh, Orissa, Sikkim, and Uttar PradeshApplied on the treatment of boils
Latex
Kunwar and Adhikari (2005)
F. carica
Rajasthan, Uttar Pradesh, Madhya Pradesh, North-east states, Karnataka, and Tamil Nadu
Used in the treatment of diabetes and hypercholesterolemia
Leaf infusion
Chaachouay et al. (2019)
In leprosy and nose bleeding
Fruit powder
Idolo et al. (2010)
Useful in diarrhoea
Decoction of dried fruits and unpeeled almond
Ramazani et al. (2010)
Treatment of abdominal pain
Fruits juice
Khan and Khatoon (2007)
Treatment of leukoderma and ringworm infection
Root’s decoction
Kirtikar and Basu (1995)Dimomfu (1984)Akah et al. (1998)
Used as expectorant, diuretic, and anthelmintic agent
Latex
Bone treatment
Bark poultice
Bronchitis treatment
Aqueous infusion of fresh leaves
Tene et al. (2007)
Taken orally in constipation
Fruit juice
Prajapati et al. (2007)
Taken orally in the treatment of cough
Fruit decoction with honey
Ghazanfar and Al-Abahi (1993)
Expectorant
Fruit
Afzal et al. (2009)
Jaundice
20 mL of leaf juice mixed with a cup of goat milk is taken early in the morning for 3 days
Manjula et al. (2011)
Removal of kidney stone
Bark and leaves
Afzal et al. (2009)
Leukoderma
Roots
Kalaskar et al. (2010)
In menstruation pain
Aqueous infusion of fresh leaf tender is taken orally as a drink
Tene et al. (2007)
Regulates blood stream
Decoction made with dried fruits, lemon peel and Laurus nobilis leaves
Idolo et al. (2010)
To remove weakness
Single dry fruit is soaked in water for a night and is consumed at morning for 15 days
Patil et al. (2011)
Skin disease
Fruits and stem latex
Khan and Khatoon (2007)
F. curtipes
Andaman & Nicobar Islands, Arunachal Pradesh, Assam, Manipur, Meghalaya, Sikkim,
Tripura, and West BengalUsed as immune stimulant
Decoction of stem bark and leaves
Andrade et al. (2019)
F. deltoidea
Assam, and West Bengal
Treatment of wounds, rheumatism, and sores
Powdered roots and leaves
Burkill and Haniff (1930)Khan et al. (2011)
Used as a tonic to contract the uterus and vaginal muscles, and to treat menstrual cycle, and leucorrhoea
Decoction of boiled leaves
Chewed to relieve toothache, cold, and headache
Fruits
Bunawan et al. (2014)
Bouquet (1969)
Taken as an aphrodisiac tonic
Whole plant
F. elastica
Assam, Meghalaya, Sikkim, Assam, and West Bengal
Useful in skin infections and skin allergies
Boiled leaf extract
Kiem et al. (2012)
Employed as a diuretic agent
Cold leaf extract
Teinkela et al. (2018)
Employed as an astringent and styptics for wounds
Stem bark
Rahman and Khanom (2013)
F. erecta
Assam, Sikkim, and Meghalaya
Recommended as medicine in nephritis, and arthritis
Whole plant
Yakushiji et al. (2012)
Treatment of inflammations
Roots, stem bark, and fruits
Kislev et al. (2006)
F. exasperata
Andaman & Nicobar Island, Central and Southern states of India
Used in the treatment of stomach disorders, coughs, epilepsy, high blood pressure, rheumatism, arthritis, intestinal pains, and wounds
Leaf decoction
Dalziel (1948)
Employed as an antipyretic agent
Leaves
Haxaire (1979)
Used in the treatment of malaria
The leaves are macerated in water and the decoction is taken orally
Titanji et al. (2008)
Used in the treatment of haemorrhoids
Aqueous extract of leaves
Focho et al. (2009)
In diarrhoea treatment
Infusion of leaves
Noumi and Yomi (2001)
Treatment of ulcer
Few leaves are chewed and swallowed three times for 4–8 weeks
Berg (1989)
To treat stomach-ache
Infusion of dried leaves
Akah et al. (1997)
Remedy for peptic ulcers
50 Leaves of F. exasperata, 50 leaves of Emilia coccinea and 10 fruits of Capsicum frutescens are boiled in water (1 l), homogenized, and filtered. 150 mL filtrate is taken twice a day for 5 days
Noumi and Dibakto (2000)
Treatment of asthma, bronchitis, tuberculosis, and emphysema
Leaf juice mixed with lemon juice and taken twice a day
Bafor and Igbinuwen (2009)
Used for insomnia
Fresh leaves
Kerharo (1974)
Applied externally to treat eczema
Stem bark crushed with the roots of Croton roxburghii in coconut milk
Harsha et al. (2003)
Eaten to relieve throat pain
Dried flowers
Chhabra et al. (1984)
Used to manage asthma, and venereal diseases
Roots
Chhabra et al. (1990)
Inhaled in case of chest pain
Leaves are boiled in water and the steam
Assi (1990)
Used to arrest bleeding by traditional birth attendants in hastening childbirth
Plant sap
Irene and Iheanacho (2007)
Orally taken and rubbed on the abdomen to stimulate uterus contractions during childbirth
Dried leaf decoction
Hutchinson (1985)
F. fistulosa
Andaman & Nicobar Islands, Arunachal Pradesh, Assam, Bengal, Jharkhand, Meghalaya, Mizoram, and Tripura
Used in the post-natal treatment, and possess diaphoretic property
Whole plant
Mehra et al. (2014)
F. gasparriniana
Bihar, Arunachal Pradesh,
Assam, Meghalaya, Nagaland, and SikkimUsed in the improvement of digestion
Roots
Luo et al. (2019)
F. geniculata
Andaman & Nicobar Islands, Arunachal Pradesh, Assam, Bihar, Jharkhand, Meghalaya, Orissa, Sikkim, Tamil Nadu, and West Bengal
Medicines for haemorrhage, stomach disorder, gastrointestinal, arthritis, headache, and cardiovascular disorder
Stem bark and leaves
Kumari et al. (2019)
F. hirta
Arunachal Pradesh, Assam, Bihar, Meghalaya, Tripura, and West Bengal
Used as child snacks
Ripe female figs
Shi et al. (2014)
F. hispida
Arunachal Pradesh, Assam, West Bengal, Uttarakhand, Uttar Pradesh, and Rajasthan
Is taken for earache
Leaf juice
Basnet (1998)
Used to treat liver complaints
Fumes from twigs
Dangol and Gurung (1995)
Used as emetic and purgative agents
Fruit, seed, and bark
Kharel and Siwakoti (2002)
Remedy to treat diabetes
Infusion of stem bark
Khan et al. (2011)
Used as lactagogue and tonic
Seed infusion
Kirtikar and Basu (1987)
Given to mother as a galactagogue for better milk formation
Boiled green fruits
Behera (2006)
F. lacor
Uttarakhand, West Bengal, and Uttar Pradesh
Used to treat leucorrhoea, ulcers, and boils
Decoction of buds
Manandhar (1985)
Useful in the curing of stomach disorders
Seeds
Bhatt (1977)
Treatment of Harsha
Dried buds
Nakarmi (2001)
Used to treat diabetes
Powder of dried ripened fruits
Khan et al. (2011)
In expelling round worms from stomach
Stem bark
Nadkarni and Nadkarni (1976a)
Used for treatment of various skin problems
Leaves
Gupta and Arora (2013)
F. lamponga
Andaman & Nicobar Islands, Arunachal Pradesh, Assam, Manipur, Meghalaya, and West Bengal
In the treatment of jaundice
Whole plant
Das et al. (2008)
F. lyrata (Syn. F. pandurata Sand)
Andaman & Nicobar Islands
Used as diuretic and antidepressant agent
Leaves
Dhawan et al. (1977)
F. microcarpa
Andaman & Nicobar Islands, Arunachal Pradesh, Assam, Manipur, Meghalaya, Mizoram, Peninsular region, Punjab, Rajasthan, and Sikkim
Used as insecticide to kill housefly
Leaf extract
Kalaskar and Surana (2012)
Taken orally in the treatment of diabetes
Powder of fresh leaves and fruits (equal amounts)
Khan et al. (2011)
F. mollis
Andaman & Nicobar Islands, Bihar, Central and Southern provinces, Jharkhand, Maharashtra, Rajasthan, and Uttar Pradesh
Used to increase lactation after delivery of women
Whole plant
Shahare and Bodele (2020)
Used to treat ulcers and wounds
Leaves
Thapa (2001)Lim (2012)
Applied as a poultice to treat boils
Crushed leaves
Used for stomatitis, and to clean ulcers
Roots
Priya and Abinaya (2018)Ghimire et al. (2000)
To treat skin infections, neck swelling and scabies
Stem bark
F. neriifolia
Arunachal Pradesh, Assam, Meghalaya, Mizoram, Nagaland, Uttar Pradesh, and Western
to Eastern HimalayasGiven in conjunctivitis
Stem bark juice
Manandhar (2001)
Used in the treatment of boils
Milky latex of bark
Khan et al. (2011)
F. palmata
Andhra Pradesh, Bihar, Kerala Madhya Pradesh, Orissa, Rajasthan, and Uttar Pradesh
Employed in ringworm and skin diseases
Fruit paste
Thapa (2001)
Used in dysentery and vomiting
Ripen fruits
Devkota and Karmacharya (2003)
Applied to extract spines deeply lodged in the flesh
Stem latex
Manandhar (1995)
Used to treat digestion complaints
Fruits
Pala et al. (2010)
F. pumila
Rajasthan, Gujarat, Punjab, Uttarakhand, Himachal Pradesh, Assam, Karnataka
Treatment of bleeding, swelling, haemorrhoids, and intestinal disorders
Leaves and fruits
Abraham et al. (2008)
Used to treat diabetes, and high blood pressure
Leaves and fruits
Kaur (2012)
Used for skin infections
Stem latex
Mazid et al. (2012)Sarkar and Devi (2017)Pant and Pant (2004)
Useful in carbuncle, dysentery, haematuria, and piles
Leaves
Used to treat bladder inflammation and dysuria
Roots
Employed for backache, piles, swellings, and tuberculosis of the testicles
Stem or fruit peel
Rahman and Khanom (2013)Vihari (1995)Khare (2007a)
Used for boils, rheumatism, and sore throat
Dried leaves and stems
In the treatment of hernia
Fruit decoction
F. racemosa (syn. F. glomerata)
Assam, Bihar, Chhattisgarh, Jharkhand, Madhya Pradesh, Orissa, Sikkim, Meghalaya, and West Bengal
Used as an astringent, stomachic, carminative given in menorrhea, and constipation
Fruits
Chopra et al. (1958)
Useful in leprosy
A bath made of fruit and bark
Nadkarni et al. (1976)
Raghunatha Iyer (1995)
In the treatment of diabetes
Fruit infusion
Used in bilious infections
Leaf powder mixed with honey
Kirtikar and Basu (1975)
Muller Boker (1999)
Taken in asthma and piles treatment
Bark decoction
Used for boils, blisters, and measles
Leaf latex
Siwakoti and Siwakoti (2000)
Valuable medicine in diabetes
Trunk sap
Paudyal (2000)
Used in burns, swelling, leukorrhea dysentery and diarrhoea
Paste of stem bark
Tiwari (2001)
Used to cure heat stroke, and chronic wounds
Root sap
Thapa (2001)
Taken as aphrodisiac agent
Stem latex
Yadav (1999)
Used to cure stomach-ache, cholera, and mumps
Stem latex
Basnet (1998)
Remedy for cough, asthma, fever, respiratory and liver disorders
Leaf galls
Annon (1976)
Treatment of children’s ear infections, and used to suppress nose bleeding
Leaf galls
Nadkarni (1976)
Used in pulmonary infections, diarrhoea, and vomiting
Leaf galls
Kirthikar and Basu (1935)
F. religiosa
Arunachal Pradesh, Assam, Rajasthan, Uttar Pradesh, Karnataka, Tamil Nadu, Gujarat, Bihar,
Meghalaya, and SikkimEmployed in the treatment of asthma, cough, sexual disorders, diarrhoea, haematuria, earache, toothache, migraine, and gastric complaints
Mixture of leaf juice and honey
Jain et al. (1991)
Used as an analgesic for toothache
Leaf decoction
Bhattarai (1993a)Bhattarai (1993b)
Eaten to facilitate asthma and respiratory system
Fruits
Applied externally to treat scabies
Fruit paste
Siwakoti and Siwakoti (2000)
Taken in scabies
Bark infusion
Chaudhary (1994)
Used in gonorrhoea, wounds, diabetes, diarrhoea, and bone fracture
Stem bark
Shrestha (1997)
Useful in cough, cold and mild fever
Mixture of bark paste and honey (equal amounts)
Dangol (2002)
Employed in the treatment of menstrual complaints
Aerial root juice
Thapa (2001)
F. retusa
Goa, Assam, Meghalaya, and Uttar Pradesh
Used in wounds and bruises
Roots, stem barks, and leaves
Chopra et al. (1956)
(Karki 2001)
Applied to treat decaying or aching tooth
Dried roots are mixed with salt
Used in the treatment of liver diseases
Roots
Semwal et al. (2013)
F. sarmentosa
Arunachal Pradesh, Assam, Himachal Pradesh, Jammu & Kashmir, Meghalaya,
Mizoram, Punjab, Sikkim, Tripura, Uttar Pradesh, and West BengalRecommended as remedy for wounds, fever, swollen joints, inflammations, and ulcers
Whole plant
Ripu et al. (2006)
Joshi and Joshi (2000)Guan et al. (2007)
Taken to cure boils and to increase milk secretion after delivery
Edible bark powder
Used in malaria
Aqueous root extract
Cure for leprosy
A bath made from the fruit and bark
Dimri et al. (2018)Priyanka et al. (2016)Gupta and Acharya (2018)
Lansky (2011)
Curing of fever
Latex
Eaten in diarrhoea
Raw fruits
Applied on forehead to relieve headache
Young fruit juice
F. semicordata
Rajasthan, Uttar Pradesh, Assam, West Bengal, and Karnataka
Applied on forehead to cure headache
Root paste
Rajendra and Prasad (2009)
Taken orally at the time of pregnancy
Fresh decoction of the stem bark and leaves
Ghildiyal et al. (2014)
Curing the fever
Latex
Kunwar and Bussmann (2006)
Taken in typhoid fever
Milky sap of aerial parts diluted once in water
Gopal (2013)
Applied for the growth of hairs on head
Milky latex
Phondani et al. (2010)
Eaten in diarrhoea
Raw fruits
Shashi and Rabinarayan (2018)
Taken orally to get relief from jaundice
Leaf decoction
Gupta and Acharya (2019)
Used in the treatment of constipation
Ripe figs
Kunwar and Bussmann (2006)
Applied to treat wounds and bruises
Juice and powdered stem bark
Khare (2007)
Used to treat boils
Latex
Rajesh et al. (2017)
Applied for curing scabies
Leaf juice
Shubhechchha (2012)
Used in the treatment of menstrual disorders
Juice of stem bark of F. semicordata and M. esculenta
Nikomtat et al. (2011)
F. talbotii
Madhya Pradesh, and Peninsular region
Used for ulcers and venereal diseases
Stem bark
Khare (2007)
Employed as diuretic, spasmolytic, and antidepressant agent
Aerial parts
Shi et al. (2018)
F. tikoua
Assam, Manipur, West Bengal
Used in the treatment of chronic bronchitis, diarrhoea, dysentery, rheumatism, oedema, and impetigo
Rhizomes
Jiangsu New Medical College (1986)
F. tinctoria
Andaman & Nicobar Islands, Bihar, Kerala, Madhya Pradesh, Meghalaya, Orissa, Tamil Nadu, and Uttar Pradesh
Used as a tonic for weakness after the childbirth
Leaf decoction
Smith (1979)
Employed in dressing for broken bones
Plant juice and leaves
Satapathy and Kumar (2017)

Ethnomedicinal properties of some important Indian Ficus species.
3.2 Phytochemistry
The phenylpropanoids, isoflavonoids, flavonoids, phenolic glycosides, monoterpenes, sesquiterpenes, triterpenes, and alkaloids have been isolated and identified from 25 species {F. auriculata (syn. F. pomifera), F. bengalensis, F. benjamina, F. carica, F. curtipes, F. deltoidea, F. elastica, F. erecta, F. exasperata, F. fistulosa, F. geniculata, F. hirta, F. hispida, F. lacor, F. lyrata (Syn. F. pandurata), F. macrocarpa, F. mollis, F. palmata, F. pumila, F. racemosa (syn. F. glomerata), F. religiosa, F. retusa, F. sarmentosa, F. semicordata, and F. tikoua}of Indian Ficus genus while other 72 species {F. abelii Miq, F. filicauda Hand. - Mazz., F. ischnopoda Miq., F. nigrescens King, F. fulva Reinw. ex Blume, F. langkokensis Drake, F. pubigera (Miq. ex Wall.) Brandis, F. laevis Blume, F. diversiformis Miq., F. chartacea (Wall. ex Kurz) Wall. ex-King, F. laevis Blume var. macrocarpa (Miq.) Corner, F. crininervia Miq., F. villosa Blume, F. sagittata Vahl, F. recurva Blume, F. pendens Corner, F. hedaracea Roxb., F. punctata Thunb., F. ampelas Burm. f., F. andamanica Corner, F. assamica Miq., F. copiosa Steud., F. cyrtophylla (Miq.) Miq., F. heterophylla L. f., F. praetermissa Corner, F. montana Burm. f., F. subincisa Buch. -Ham. ex J. E. Sm., F. subincisa Buch. -Ham. ex J. E. Sm. var. paucidentata (Miq.) Corner, F. heteropleura Blume, F. obscura Blume var. borneensis (Miq.) Corner, F. sinuata Thunb., F. subulata Blume, F. guttata (Wight) Kurz ex-King, F. variegata Blume, F. prostrata (Wall. ex Miq.) Miq., F. squamosa Roxb., F. ribes Reinwdt. ex Blume, F. magnoliifolia Blume, F. nervosa B. Heyne ex Roth, F. albipila (Miq.) King, F. callosa Willd., F. capillipes Gagnep., F. alongensis Gagnep., F. amplissima J. E. Sm., F. arnottiana (Miq.) Miq., F. caulocarpa (Miq.) Miq., F. concinna (Miq.) Miq., F. cupulata Haines, F. hookeriana Corner, F. maclellandii King var. rhododendrifolia (Miq.) Corner, F. rigida Jacq., F. rumphii Blume, F. superba (Miq.), F. tsjahela Burm. f., F. virens Aiton, F. altissima Blume, F. beddomei King, F. costata Aiton, F. dalhousiae (Miq.) Miq., F. drupacea Thunb., F. fergusonii (King) Worthington, F. maclellandii King, F. pellucidopunctata Griff., F. stricta (Miq.) Miq., F. sundaica Blume, F. trimenii King, F. gasparriniana Miq., F. macrophylla Desf. ex Pers, F. lamponga Miq., F. neriifolia Sm., F. talbotii King, and F. tinctoria G.Forst} have not been evaluated for the presence of phytoconstituents. The backbone structures of identified compounds are presented in Fig. 3. The state-wise location, types of extracts, parts used, and identified phytoconstituents are described in Table 2.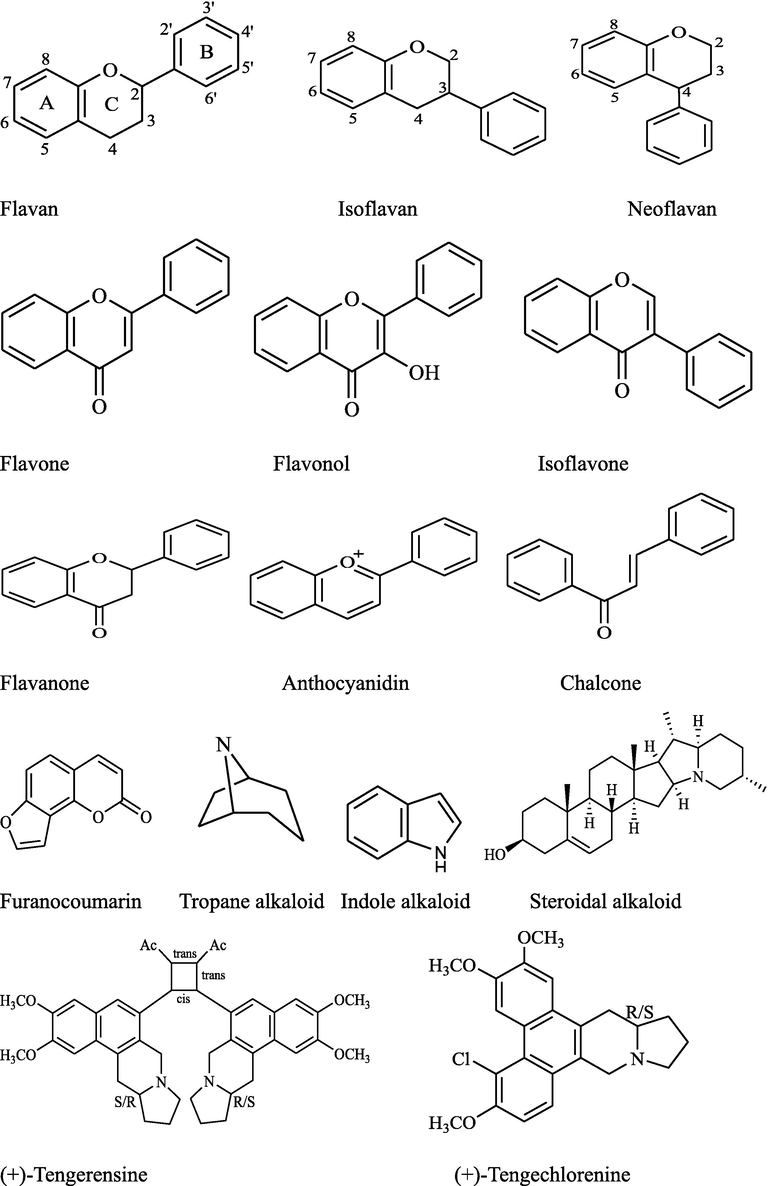
Backbone structures of important isolated and identified compounds of Indian Ficus species.
Backbone structures of important isolated and identified compounds of Indian Ficus species.
Plant species
Extract type
Plant parts
Compounds type
Isolated compounds
References
F. auriculata (syn.
F. pomifera)
Ethanolic (70%)
Dried fruits
Sesquiterpene
Aristolone
Ambarwati et al. (2021)
Ethanolic
Roots
Isoflavones
5,7,4′-Trihydroxy-3′-hydroxymethylisoflavone, 3′-formyl-5,4′-dihydroxy-7-methoxyisoflavone, ficuisoflavone and alpinumisoflavone
Qi et al. (2018)
Aerial parts
Flavonoids
Quercetin, epigallocatechin, kaempferol, quercetin, and myricetin
El-Fishawy et al. (2011); Khayam et al. (2019)
Leaves
Volatile oils
4-Phenylmethyl-pyridine, dibutyl phthalate, phytol, 3β-lup-20(29)-en-3-ol-acetate and indol, 4-phenylmethyl-pyridine
Shao et al. (2013)
Stem
Triterpenoids
Betulinic acid, lupeol, stigmasterol, scopoletin, and β-sitosteril-3-O-β-D- glucopyranoside,
El-Fishawy et al. (2011)
Lactone
Ficusine D
Shao et al. (2018); Tamta et al. (2021)
Fruit
Phenolic acids
Galloylquinic acid, 3-O-cafeoylquinic acid, 4-O-cafeoylquinic acid, 5-O-cafeoylquinic acid, procyanidin trimer (B-type), 4-O-feruloylquinic acid derivatives, methoxyl-epicatchin dimer, epicatchin-trimer (A-type), trihydroxy-octadecadienoic acid, trihydroxy octadecanoic acid, gallocatchin-O-hexoside, hydroxy-octadecatrienoic acid, xanthone derivatives, and 3-methyl epigallocatechin gallate
Shahinuzzaman et al. (2021)
Ethyl acetate
Stem
12-Membered and benzoic acid lactones
(3R,4R)-4-Hydroxy-de-O-methyllasiodiplodin, 6-oxolasiodiplodin and ficusines A-C, (R)-(+)-lasiodiplodin, (+)-(R)-de-O-methyllasiodiplodin and (3R,6S)-6-hydroxylasiodiplodin
Shao et al. (2014)
Aqueous
Dried bark
Furanocoumarins
Bergapten
Tiwari et al. (2017)
Flavonoid
Myricetin and querecetin-3-O-β-D-glucopyranoside
Petroleum ether
Leaves
Triterpenoids
3β-Acetoxyurs-12-ene, 3β-hydroxyurs-12-ene, 3β-hydroxyurs-12-en-27-oic acid, 3β-hydroxyolean-12-en-27-oic acid and 3α-acetoxyolean-12-en-27-oic acid
Wangkheirakpam et al. (2015)
F. benghalensis
Methanolic
Leaves
Essential oils
α-Cadinol, germacrene D-4-ol, γ-cadinene, α-muurolene, β-caryophyllene epoxide, cyclosativene, cubenol, τ-cadinol, (E)-β-ionone, δ-cadinene, β-geranylacetone, toluene, γ-muurolene, ethylbenzene, α-copaene, α-phellandrene, linalool, β-caryophyllene, maaliene, n-nonanal, n-hexanal, β-cyclocitral, (E)-α-ionone and limonene
Adebayo et al. (2015)
Flavonoids
Naringenin, and quercetin
Rao et al. (2014)
Stem bark
Triterpenoids
Lanostadienylglucosyl cetoleate, bengalensisteroic acid ester, heneicosanyl oleate, α-amyrin acetate, and lupeol
Naquvi et al. (2015)
Terpenoid
3, 7, 11, 15-Tetramethyl-2-hexadecen-1-ol (phytol)
Kanjikar and Londonkar (2020)
Aerial roots
Flavones and coumarins
Bengalensinone, benganoic acid, apocarotenoid, alpinumisoflavone, 4-hydroxyacetophenone, 4-hydroxybenzoic acid, 4-hydroxymellein, and p-coumeric acid
Riaz et al. (2012)
Lupane triterpenoids
Stigmasterol, lupanyl acetate, and 3-acetoxy-9(11),12-ursandiene
Ethanolic
Aerial roots
Phenolics
Cyanidin 3-glucoside equivalent, cyanidin 3-glucoside, chlorogenic acid, caffeic acid, quercetin, naringenin, and kaempferol
Afzal et al. (2020)
Fruits
Phenolics
Cyanidin 3-glucoside equivalent, cyanidin 3-glucoside, chlorogenic acid, and caffeic acid
Aerial parts
Triterpenoids
Lanosterol, lupeol, amyrin acetate, lupenyl acetate, friedelanol, cyclolaudenol, epifriedelanol, dihydrobrassicasterol, stigmasterol, sitosterol, ergosterol acetate, furostano, 4,22-stigmastadiene-3-one, 1-heptatriacotanol, and protodioscin
Verma et al. (2015)
Stigmasterol
Fegade Sachin and Siddaiah (2019)
Stem bark
Flavonoids
5,7-Dimethyl ether of leucopelargonidin 3-O-α-L rhamnoside and 5,3′-dimethyl ether of leucocyanidin 3-O-α-D galactosyl cellobioside, and quercetin
Daniel et al. (1998)
Fruits
Phenolic acid
Caffeic acid
Gopukumar and Praseetha (2015)
Aqueous- acetone
Stem bark
Anthocyanin
Pelargonidin
Kundap et al. (2017)
Ethyl acetate
Leaves
Flavonoids
5,6,7,3′,5′-Pentamethoxy-4′-prenyloxyflavone, rutin, quercetin and 3-acetyl ursa-14:15-en-16-one
Elgindi (2004)
Chloroform
Leaves
Phenolics
Cinnamic acid, gallic acid, theaflavin-3,3́-digallate, rutin, quercetin-3-galactoside, leucodelphinidin, gallocatechin, kaempferol, leucocydin and apigenin
Almahy et al. (2003)
Stem bark and leaves
Triterpenoids
Stigmasterol, friedelin, lupeol, β-amyrin, 3-friedelanol, betulinic acid, 20-traxasten-3-ol, taraxosterol, and β-sitosterol
Murugesu et al. (2021)
F. benjamina
3% formic acid in 70% methanol/dH2O
Leaves and stem bark
Indole-type
Calycanthidine, akuammidine, ergoline, dasycarpidan, ibogamine, ajamalicine, and dasycarpidol
Novelli et al. (2014)
Indolyzidine-type
Obscurinervinediol, and crinamidine
Isoquinoline-type
Columbamine, laudanosoline, methylcoridaldine, salsoline, reticuline, hydroxymorphine, and isoclaurine
Quinolizidine-type
Sophocarpine, matridine, scoulerine, and lycocernuine
Pyridine-type
Anabasine, nicodicodine, adenocarpine, and lutidine
Carbazol-type
Neblinine, harmine, ellipticine, and aspidospermidin
Pyrrolizidine-type
Indicine N-oxide, and retronecine
Steroidal-type
Tomatidine and solasodine
Quinoline-type
Cinchophen
Pyrrolidine-type
Clemastine
Tropane-type
p-Bromo atropine
Acridine-type
Acridine derivative
Petroleum ether
Leaves
Pentacyclic triterpenoids
Ursolic acid and lupeol
Singh et al. (2020)
Ethyl acetate
Fruits
Isoflavonoids
5,7,4′-Trihydroxy-6-(3,7-dimethyl-2,6-octadienyl)isoflavone, 5,7,2′,4′-tetrahydroxy-8-(3,7-dimethyl-2,6-octadienyl)isoflavone, 6-[(1R*,6R*)-3-methyl-6-(1-methylethenyl)-2-cyclohexen-1-yl]-5,7,4′- trihydroxyisoflavone, 3,5,7-trihydroxy-4′-methoxycoumarano-chroman-4-one, 6-(3-methyl-2-buten-1-yl)-3,5,7-trihydroxy-4′-methoxycoumarano-chroman-4- one, 5,4′-dihydroxy- 2″-hydroxyisopropyldihydrofurano[4,5:7,8]-isoflavone, 5,4′-dihydroxy-2″-(-2-hydroxy-6-methylhept-5-en-2-yl)dihydrofurano[4,5:7,8]- isoflavone, 5,4′-dihydroxy-2″-(-2-hydroxy-6-methylhept-5-en-2-yl)dihydrofurano[4,5:6,7]- isoflavone, lespedezol E1, ficusin A, gancaonin N, lupiwighteone and erythrinin C
Dai et al. (2012); dos Anjos Cruz et al. (2022)
Aqueous methanol (95%)
Root bark
Triterpenoids and ceramide
β-Amyrin acetate, β-amyrin, psoralen, betulinic acid, lupeol, platanic acid, β-sitosterol glucoside, and benjaminamide
Simo et al. (2008)
F. carica
Ethanolic (70%)
Leaves
Isoflavones
Quercetin, luteolin, biochanin A, kaempferol, and rutin
Vaya and Mahmood (2006); Trifunschi et al. (2015)
Polyphenolics
3-O-(Rhamnopyranosyl-glucopyranosyl)-7-O-(glucopyranosyl)-quercetin, 2-carboxyl-1, 4-naphthohydroquinone-4-O-glucopyranoside, luteolin 6-C-glucopyranoside, 8-C-arabinopyranoside, schaftoside, isoorientin, isoschaftoside, rutin, 2″-O-rhamnosylvitexin, isovitexin, isoquercetin, kaempferol-3- O-rutinoside
Li et al. (2021b)
Ethanolic
Root bark
Triterpenoids
α-Amyrin, β-sitosterol, and β-sitosterol-β-D-glucoside
Jain et al. (2007)
Coumarins
Psoralen, bergapten, xanthotoxin, and 6-(2-methoxyvinyl)-7-methylcoumarin
Fruits
Prenylated isoflavone derivatives
Ficucaricones A–D
Liu et al. (2019)
Anthocyanin
Cyanidin-3-O-rutinoside
Solomon et al. (2006)
Leaves
Isoflavonoids
Rutin, isoschaftoside, isoquercetin, chlorogenic acid, caffeoyl malic acid and rutin
Takahashi et al. (2017)
Phenolic acids and flavonoids
Chlorogenic acid, rutin, and psoralen
Teixeira et al. (2006)
Leaves, pulps, and peels
Phenolic acids and flavonoids
3-O- and 5-O-Caffeoylquinic acids, ferulic acid, quercetin-3-O-glucoside, quercetin-3-O-rutinoside, psoralen and bergapten
Oliveira et al. (2009)
Methanolic
Leaves
Triterpenoids
Bauerenol, lupeol acetate, methyl maslinate, calotropenyl acetate and oleanolic acid
Saeed and Sabir (2002)
Furanocoumarin
Psoralen and bergapten
Takahashi et al. (2014); Li et al. (2021a)
Polyphenols and furanocoumarins
Caffeoyl malic acid, psoralic acid-glucoside, rutin, psoralen and bergapten
Yu et al. (2020); Ladhari et al. (2020)
Phenolics
Caffeoylmalic acid, psoralic acid-glucoside, rutin, psoralen and bergapten
Wang et al. (2017)
Fruits
Pentacyclic triterpenoids
Betulinic acid, and oleanolic acid
Wojdyło et al. (2016)
Methanol: water
(80:20%)Leaves
Phenolics
Chlorogenic acid, caffeoylmalic acid, p-coumaroyl derivative, p-coumaroylquinic acid, p-coumaroylmalic acid, caffeic acid, isoschaftoside, schaftoside, rutin, psolaric acid glucoside, quercetin 3-O- glucoside, quercetin 3-O-malonylglucoside, kaempferol 3-O-glucoside, psolaren, and bergapten
Petruccelli et al. (2018)
Water - methanol (40:60)
Leaves
PolyphenolQuercetin-3-glucoside, caftaric acid, quercetin-3, 7-diglucoside, and coumaroyl-hexose, kaempferol-3-O-sophorotrioside, cichoric acid and sinapic acid glucoside
Nadeem and Zeb (2018)
Ammonium sulphate-Ethanol
Leaves
Flavonoids
3-O-(Rhamnopyranosyl-glucopyranosyl)-7-O-(glucopyrnosyl)-quercetin, 2-carboxyl-1,4-naphthohydroquinone-4-O-hexoside, luteolin 6-C-hexoside, 8-C-pentoside, kaempferol 6-C- hexoside −8-C-hexoside, quercetin 6-C-hexobioside, kaempferol 6-C- hexoside −8-C-hexoside, quercetin 3-O-hexobioside, apigenin 2″-O-pentoside, apigenin 6-C-hexoside, quercetin 3-O-hexoside, and kaempferol-3-O-hexobioside
Zhao et al. (2021)
F. curtipes
Methanolic
Stem bark
Phenolics
3-O-Caffeoylquinic acid, catechin, chlorogenic acid isomer, 5-O-caffeoylquinic acid, procyanidin type B, catechin/epicatechin derivative, epicatechin, vicenin-2, procyanidin type C, apigenin-7-O-hex-6/8-C-hex, apigenin-6-C-pt-8-C-hex, cinchonain type II, apigenin-6-C-hex-8-C-pent, cinchonain type I, vitexin, procyanidin type B, isovitexin, and aviculin
Andrade et al. (2019)
F. deltoidea
Methanolic
Leaves
Acyclic monoterpenes
6-Methyl-5-hepten-2-one, myrcene, (Z)-β-ocimene, (E)-β-ocimene, cis-furanoid linalool oxide, trans-furanoid linalool oxide, linalool, cis-pyranoid linalool oxide, trans-pyranoid linalool oxide, hotrienol, perillene
Grison-Pige et al. (2002a)
Cyclic monoterpenes
Limonene
Fruits
Sesquiterpenes
Dendrolasine, α-cubebene, cyclosativene, Α-ylangene, α-copaene, β-bourbonene, 1,5-diepi-β-bourbonene, β-cubebene, β-elemene, α-gurjunene, α-cis-bergamotene, β-caryophyllene, α-santalene, selina-3–6-diene, α-trans-bergamotene, α-humulene, alloaromadendrene, aciphyllene, germacrene D, β-selinene, αd-selinene, α-selinene, bicyclogermacrene, α-muurolene, germacrene A, δ-amorphene, (E,E) α-farnesene, 2-epi-α-selinene, δ-cadinene, cadina-1,4-diene, germacrene B, and caryophyllene oxide
Grison-Pige et al. (2002b)
Ethanolic
Leaves
Flavonoids
Gallocatechin, epigallocatechin, catechin, (epi)afzelechin-(epi)catechin, (epi)afzelechin-(epi)afzelechin, (epi)catechin, epicatechin, lucenin-2, vicenin-2, luteolin-6-C-hexosyl-8-C-pentoside, luteolin-6-C-glucosyl-8-C-arabinoside, isoschaftoside, luteolin-6-C-arabinosyl-8-C-glucoside, schaftoside, orientin, apigenin-6-C-pentosyl-8-C-glucoside, vitexin, apigenin-6-C-glucosyl-8-C-pentoside, apigenin-6,8-C-dipentoside isomer, isovitexin, 4-p-coumarolyquinic acid, rutin, quercetin, and naringenin
Ong et al. (2011); Omar et al. (2011); Choo et al. (2012)
Triterpenoid
Lupeol
Suryati et al. (2011)
Aqueous
Leaves and fruits
Flavonoids
Epicatechin, rutin, quercetin 5,4′-di-O-β-D-glucopyranoside, myricetin and naringenin
Dzolin et al. (2015)
Ethyl acetate
Leaves
Alkaloids
1,1′-(1,1-Ethenediyl) bis(3- methylpiperazine), and nigeglanine
Triadisti et al. (2021)
Terpenoid
1,1,2,3,3-Pentamethylindane
F. elastica
n-Hexane
Leaves
Phenolics
Macluraxanthone, rutin, chlorogenic acid, and psoralen
Teixeira et al. (2009)
Dichloromethane
Leaves
Triterpenoids
Campesterol, stigmasterol, β-sitosterol, α-amyrin, and friedelin
El-Hawary et al. (2012)
Ethanolic (70%)
Leaves
Flavonoids
Quercitrin, morin, myricitrin, and eleutheroside B
Ginting et al. (2020)
Ethanolic
Leaves
Pentacyclic triterpenoids
1-O-Caffaeoyl-D-mannitol, moretenone, glutinol, moretenol, lupeol, β-sitosterol, sakuranin, and kaempferol-3-O-rutinoside
EI-Domiaty et al. (2002)
Methanolic
Aerial root bark
Ceramide, cerebroside and triterpenoid saponin
Ficusamide, ficusoside and elasticoside
Mbosso et al. (2012)
F. erecta
Aqueous ethanol (50:50%)
Leaves and branches
Flavonoids
Chlorogenic acid, rutin, and keampferol-3-O-rutinoside
Sohn et al. (2021)
Ethanolic (70%)
Branches
Organic acids
p-Hydroxybenzoic acid, methyl p-hydroxybenzoate, vanillic acid, methyl vanillate, syringic acid, and ethyl linoleate
Park et al. (2012)
Triterpenoids
β-Sitosterol, and α-amyrin acetate
F. exasperata
Methanolic
Leaves
Isoflavone glycosides
Quercetin-3,7-di-hexoside, quercetin-3-(6-rhamnoside) glucoside, quercetin-3-glucoside, kaempferol-3–92- rhamnoside)hexoside, quercetin-3-(6-malonyl)hexoside, quercetin-3-hexoside-7-ketorhamnoside, kaempferol-3 hexoside, apigenin-7-(6-rhamnoside)hexoside, luteolin 6,8-di-C-hexoside, apigenin-6-C-pentoside-8-C-hexoside pigenin-6-C-hexoside-8-C-pentoside, apigenin-6-C rhamnoside-8-C-hexoside, apigenin-6-C-pentoside-8-C- (3/4-ketorhamnoside) hexoside, apigenin-8-C-glucoside, luteolin-8-C-(3/4-ketorhamnoside)hexoside, apigenin 7-O-ketorhamnoside-8-C-hexoside, and apigenin-8-C-(3/4 ketorhamnoside) hexoside
Mouho et al. (2018)
Ethanolic
Leaves
Phenolics
Quercitrin, chlorogenic acid and caffeic acid
Oboh et al. (2014)
Aqueous- ethanol (50:50%)
Leaves
Isoflavone glycosides
Apigenin C-8 glucoside, isoquercitrin-6-O-4-hydroxybenzoate and quercetin-3-O-β-rhamnoside
Taiwo and Igbeneghu (2014)
F. fistulosa
CH2Cl2/MeOH
Leaves
Bis-benzopyrroloisoquinoline and chlorinated phenanthroindolizidine enantiomers
(±)-Tengerensine, (+)-Tengechlorenine, (±)-fistulosine, (+)-antofine, and (−)-seco-antofine
Al-Khdhairawi et al. (2017); Putra et al. (2020)
Methanolic
Stem bark
Triterpenoids
3β-Acetyl ursa-14:15-en-16-one, lanosterol-11-one acetate, 3β-acetyl-22,23,24,25,26,27-hexanordamaran-20-one, 24-methylenecycloartenol, sorghumol (isoarborinol), 11α,12α-oxidotaraxeryl acetate, and ursa-9(11):12-dien-3β-ol acetate
Tuyen et al. (1998)
Ethanolic
Stem bark
Benzopyrroloisoquinoline alkaloids
Fistulosine
Subramaniam et al. (2009)
Phenanthroindolizidine alkaloids
(−)-13aα-Antofine, (−)-14β-hydroxyantofine and (−)-13aα-secoantofine
Stem bark and leaves
Septicine-type alkaloids
Fistulopsines A and B
Yap et al. (2016)
Phenanthroindolizidine alkaloids
(+)-Septicine, (+)-tylophorine, (+)-tylocrebrine, (–)-3,6-didemethylisotylocrebrine and (+) -(6S,9S)-vomifoliol
F. geniculata
Methanolic
Pulps and peels
Phenolics
3-O- and 5-O-Caffeoylquinic acids, ferulic acid, psoralen, and bergapten
Gupta et al. (2017)
F. hirta
Methanolic
Fruits
Flavonoids
Naringenin-7-O-β-D-glucoside, pinocembrin-7-O-β-D- glucoside, eriodictyol-7-O-β-D-glucoside, luteolin, apigenin, eriodictyol-7-O-β-D-glucoside, methyl-1,2,3,4-tetrahydro-β-carboline-3-carboxylic acid, 2-methyl-1-methyl-1,2,3,4-tetrahydro-β-carboline-3-carboxylate, dihydrophaseic acid, vomifoliol, dehydrovomifoliol, pubinernoid A, 2-phenylethyl-O-β D-glucoside, 1-O-trans-cinnamoyl-β-D-glucopyranosyl-(1 → 6)-β-D-glucopyranoside, 4-O-benzoyl-quinic acid, 3-O-benzoyl-quinic acid, benzyl-β-D-glucopyranoside, (2S) 2-O-benzoyl-butanedioic acid-4-methyl ester, pinocembrin-7-O-β-D-glucoside, naringenin-7-O-β-D-glucoside, eriodictyol-7-O-β-D-glucoside, luteolin, apigenin, and umbelliferone
Wan et al. (2017); Chen et al. (2020)
Methanolic (80%)
Roots
Phenolics
Luteolin, apigenin, psoralen, and bergapten
Yi et al. (2013)
Ethyl acetate
Roots
Phenylpropanoids
(1′S)-Methoxy-4-(1-propionyloxy-5-methoxycarboxyl-pentyloxy)-
(E)-formylvinyl, (8R)-4,5′-dihydroxy-8-hydroxymehtyl-3′-methoxydeoxybenzoin, (2′S)-3-[2,3-dihydro-6-hydroxy-2-(1-hydroxy-1-methylethyl)-5- benzofuranyl] methyl propionate, 3-[6-(5-O-β-D-glucopyranosyl) benzofuranyl] methyl propionate, methylcnidioside A, (E)-3-[5-(6-hydroxy) benzofuranyl] propenoic acid, ficuscarpanoside A, syringaresinol, (7R,8S)-ficusal, trans-p-hydroxycinnamic acid, 1′-O-β-D-glucopyranosyl (2R,3S)-3-hydroxynodakenetin, p-hydroxybenzoic acid, syringic acid, and ficuglucosideCheng et al. (2017a)
Ethyl acetate and n-butanol
Roots
Furanocoumarin glycoside
5-O-[β-D-Apiofuranosyl-(1 → 2)-β-D-glucopyranosyl]- bergaptol
Dai et al. (2018)
Phenolics
Bergapten, umbelliferone, 6-carboxy-umbelliferone, 6,7-furano-hydrocoumaric acid methyl ester, picraquassioside A, and rutin
Ethanolic
Roots
Triterpenoids
Stigmasterol, 22,23-dihydro-stigmasterol, α-amyrin, and β-sitosterol
Li et al. (2006)
Phenolics
Psoralene, 3β-hydroxy-stigmast-5-en-7-one, 5-hydroxy-4′, 6, 7, 8-tetramethoxy flavone, 4′, 5, 6, 7, 8-pentamethoxy flavone, 4′, 5, 7-trihydroxy-flavone, 3β-acetoxy-β-amyrin, 3β-acetoxy-α-amyrin and hesperidin
Epicatechin, chlorogenic acid, (-) (2R,3R)epiafzelechin, psoralenoside, methoxypsoralenoside, hydrasine, 4,5-dihydrogenpsoralenoside, pelargonidin 7-glucoside, aloin A, isoliquiritigenin, vitexin, picraquassioside A, isoeugenitol, kaempferol, psoralen, (±)-naringenin, apigenin, bergapten, resveratrol, pinolenic acid, 2-ethylhexyl ester-2-propenoic acid, n-hexadecanoic acid, ethyl iso-allocholate, bergapten, and 2-methyl-Z, Z-3,13-octadecadienol
Tang et al. (2020)
Lignan
Undescribed lignan
Ye et al. (2021)
Leaves
Ursane triterpenoids
α-Amyrin acetate, 3β-acetoxy-11α-hydroxy- 12-ursenes, and 1β,3β,11α-trihydroxy-urs-12-enyl-3-stearate
Thien et al. (2021)
Phenolics
p-Coumaric acid and isovitexin
Dai et al. (2020)
Ethanolic (95%)
Roots
Phenolics
Cyclomorusin, 3-O-[(6-O-E-sinapoyl)-β-D-glucopyranosyl]-(1 → 2)-β-D-glucopyranoside, 3,5,4′-trihydroxy-6,7,3′-trimethoxyflavone, quercetin, tricin, acacetin, luteolin, apigenin, (E)-suberenol, meranzin hydrate, methyl eugenol(11),3-methoxy-4-hydroxybenzoic acid, p-hydroxybenzoic acid, methyl chlorogenate, and emodin
Zheng et al. (2013)
Fruits
Isoflavonoids
1-Methyl-1,2,3,4-tetrahydro-β-carboline-3-carboxylic acid, methyl 1-methyl-1,2,3,4-tetrahydro-β-carboline-3-carboxylate, vomifoliol, dehydrovomifoliol, icariside B2, dihydrophaseic acid, pubinernoid A, pinocembrin-7-O-β-D-glucoside, naringenin-7-O-β-D-glucoside, eriodictyol-7-O-β-D-glucoside, and 1-phenylpropane-1,2-diol
Wan et al. (2017)
Ethanolic (60%)
Roots
Phenolic glycosides
Ficusides A-G, 3,4-dimethoxyphenyl-1-O-β-D-apiofuranosyl (1 → 2)-β-D-glucopyranoside, khaephuoside A, 2-methoxyphenol-O-β-D-apiofuranosyl-(1 → 2)-β-D-glucopyranoside, methyl 2-hydroxybenzoate 2-O-β-D-apiofuranosyl-(1 → 2)-O-β-D-glucopyranoside, markhamioside F, benzyl-O-β-D-apiofuranosyl-(1 → 2)-β-D-glucopyranoside, 3,4,5-trimethoxyphenyl 1-O-β-apiofuranosyl (1′'→6′)-β-glucopyranoside, di-O-methylcrenatin, 3,4-dimethoxyphenyl-β-D-glucopyranoside, phenyl β-D-glucopyranoside, 2,4,6-trimethoxy-1-O-β-D-glycoside, 2,6-dimethoxy-4-hydroxyphenol-1-O-β-D-glucopyranoside, uralenneoside, glucosyringic acid, 4-(β-D-glucopyranosyloxy) benzoic acid and vanillic acid 4-O-β-D-glucopyranoside, (1′R)-1′-(4-hydroxy-3,5-dimethoxyphenyl) propan-1′-ol 4-O-β-D-glucopyranoside, 3,4,5-Trimethoxybenzaldehyde, 4-(3′-hydroxypropyl)-2,6-dimethoxyphnol-3′-O-β-D-glucoside, and gentisic acid 5-O-β-D-xyloside
Ye et al. (2020)
Ethanolic (75%)
Roots
Phenolics
(Z)-3-[5-(6-O-β-D-Glucopyranosyl) benzofuranyl] methyl propenoate, (Z)-3-[5-(6-methoxy) benzofuranyl] propenoic acid, (10S)-6-(2ʹ-hydroxy-10-O-β-D-glucopyranoside)-7-hydroxycoumarin, (2S)-1-O-β-D-glucopyranosyl-2-O-(2-methoxy-4- phenylaldehyde) propane-3-ol, psoralen, umbelliferon, 7-(2ʹ,3ʹ-dihydroxy-3́-methylbutoxy)-coumarin, nodakenetin, (1R, 2R, 5R, 6S)-6-(4-hydroxy-3, 5-dimethoxyphenyl)-3, 7-dioxabicyclo [3, 3, 0] octan-2-ol, (+)-(7R, 8R)-4-hydroxy-3,3ʹ,5ʹ-trimethoxy-8ʹ,9ʹ-dinor-8,4ʹ-oxyneoligna-7,9-diol-7ʹ-aldehyde, (-)-(7S,8R)-4-hydroxy-3,3ʹ,5ʹ-trimethoxy-8ʹ,9ʹ-dinor-8,4ʹ-oxyneo-ligna-7,9-diol-7ʹ-aldehyde, (1R, 2R, 5R, 6S)-6-(4-hydroxy-3-methoxyphenyl)-3,7-dioxabicyclo [3,3,0] octan-2-ol, (-)-pinoresinol, 2-[4-(3-hydroxy
propyl)-2-methoxyphenoxy] propane-1, 3-diol, vanillin, 3ʹ-hydroxy-4ʹ-metoxy-trans- cinnamaldehyde, vanillin acid, β-hydroxypropiovanillone, 7-O-ethylguaiacylglycerol, evofolin-B, (E)-3-[5-(6-methoxy) benzofuranyl] propenoic acid, (E)-isopsoralic acid 1 → 6-O-β-D-glucopyranoside, (Z)-isopsoralic acid1 → 6-O-β-D-glucopyranoside, phenyl β-D-glucopyranoside, 2, 3-dihydroxy-1-(4-hydroxy-3-methoxyphenyl)-propan-1-one, 3,4,5-trimethoxybenzyl β-D-glucopyranoside, 3,4-dimethoxyphenyl-1-O-β-D-glucopyranoside, 3,4,5-trimethoxy phenoltetraacetyl-β-D- glucopyranoside, and 1,3,5-trimethoxybenzeneCheng et al. (2017b)
n-Hexane
Leaves
Oleanane
triterpenoids3β-Hydroxy-11-oxo-olean-12-enyl-3-stearate, taraxerol, 3β-acetoxy-11α-methoxy-12-ursene and 3β-acetoxy-11α-hydroxy-12-ursene
Thien et al. (2019)
F. hispida
Ethanolic
Stem bark and leaves
Unusual 8,4′-oxyneolignan-alkaloid
Hispidacine
Yap et al. (2015)
Phenanthroindolizidine alkaloids
Hispiloscine and (+)-deoxypergularinine
Twigs and leaves
Amine alkaloids with a rhamnosyl moiety
Ficuhismines A–D, ficushispimine C, magnosprengerine, and ficushispimine A
Jia et al. (2020)
Twigs
Pyrrolidine alkaloids
Ficushispimine A and ficushispimine B
Shi et al. (2016)
Pyrrolidine alkaloids
Ficushispimine A and ficushispimine B
ω-(Dimethylamino)caprophenone alkaloid
Ficushispimine C
Indolizidine alkaloid
Ficushispidine
Isoflavones
Isoderrone, 3′-(3-methylbut-2-en-1-yl)biochanin A, myrsininone A, ficusin A, and 4′,5,7-trihydroxy-6-[(1R*,6R*)-3-methyl-6-(1-methylethenyl)cyclohex-2-en-1-yl]isoflavone
Chloroform
Leaves and twigs
Phenanthroindolizidine alkaloid
O-Methyltylophorinidine
Peraza-Sánchez et al. (2002)
Norisoprenoid
Ficustriol
Hexane
Fruits
Isoflavonoids
Isowigtheone hydrate, 3′-Formyl-5,7-dihydroxy-4′-methoxyisoflavone, 5,7-dihydroxy-4′-methoxy-3′-(3-methyl-2-hydroxybuten-3-yl) isoflavone, and alpinumisoflavone
Zhang et al. (2018)
Coumarin
7-Hydroxycoumarin, 7-hydroxy-6-[2-(R)-hydroxy-3-methyl-but-3-enyl] coumarin, psoralen, and (–)-marmesin
Phenolics
Chlorogenic acid, chlorogenic acid methyl ester, chlorogenine glycoside, protocatechuic acid, gallic acid, benzyl β-D-glucopyranoside, 2-(4-hydroxy-3-methoxy phenyl) ethyl β-D-glucopyranoside
Indole alkaloid
Murrayaculatine
Triterpenoids
Betulinic acid, and sitosterol 3-O-β-D-glucopyranoside
Methanolic
Fruits
Isoflavone
5,7-Dihydroxy-4′-methoxy-3′-(3-methyl-2-hydroxybuten-3-yl) isoflavone
Cheng et al. (2021)
F. lacor
Ethanolic
Aerial roots
Triterpenoids
β-Sitosterol, lupeol, α-amyrin, β-amyrin, stigmasterol, and campesterol
Sindhu and Arora (2013a, b); Ghimire et al. (2020)
Phenolics
Scutellarein glucoside, scutellarein, infectorin, sorbifolin, bergaptol, and bergapten
F. lyrata (Syn. F. pandurata Sand)
Methanolic
Leaves
Flavonoids
(Epi)-catechin digalloyl rhamnoside, (epi)afzelechin-(epi) gallocatechin, epicatechin, (epi)afzelechin-(epi)catechin, (epi)afzelechin(epi)afzelechin-epigallocatechin, benzyl rutinoside, lucenin-2, vicenin-2, rutin, orientin, 3-O-p-coumaroyl epigallocatechin, isoquercetin, luteolin, quercetin, apigenin, and ficuisoflavone
Farag et al. (2014)
F. microcarpa
Ethanolic
Leaves
Triterpenoids
29(20 → 19)Abeolupane-3,20-dione, 19,20-secoursane-3,19,20-trione, (3β)-3-hydroxy-29(20 → 19)abeolupan-20-one, lupenone, and α-amyrone
Hsiung and You (2004)
Aerial roots
Triterpenoids
3β-Acetoxy-11α-hydroxy-11(12 → 13)abeooleanan-12-al, 3β-hydroxy-20-oxo-29(20 → 19)abeolupane, and 29,30-dinor-3β-acetoxy-18,19-dioxo-18,19-secolupane
Chiang and Kuo (2002)
3β-Acetoxy-12,19-dioxo-13(18)-oleanene, 3β-acetoxy-19(29)-taraxasten-20α-ol, 3β-acetoxy-21α,22α-epoxytaraxastan-20α-ol, 3,22-dioxo-20-taraxastene, 3β-acetoxy-11α,12α-epoxy-16-oxo-14-taraxerene, 3β-acetoxy-25-methoxylanosta-8,23-diene, 3β-acetoxy-11α,12α-epoxy-14-taraxerene, 3β-acetoxy-25-hydroxylanosta-8,23-diene, oleanonic acid, acetylbetulinic acid, betulonic acid, acetylursolic acid, ursonic acid, ursolic acid, and 3-oxofriedelan-28-oic acid
Chiang et al. (2005)
Peroxy triterpenoids
3β-Acetoxy-12β,13β-epoxy-11α-hydroperoxyursane, 3β-acetoxy-11α-hydroperoxy-13αH-ursan-12-one, 3β-acetoxy-1β,11α-epidioxy-12-ursene, (20S)-3β-acetoxylupan-29-oic acid, (20S)-3β-acetoxy-20-hydroperoxy-30-norlupane, and 3β-acetoxy-18α-hydroperoxy-12-oleanen-11-one, and 3β-acetoxy-12-oleanen-11-one
Chiang and Kuo (2001)
Methanolic
Syconia
Terpenes
α-Cubebene, 1,2,4-metheno-1H-indene, 3,7-dimethyl-1,3,6-octatriene, (E,E)-2,4-hexadiene, caryophyllene, copaene, and D-limonene
Zhang et al. (2017)
Stem bark
Phenolics
Protocatechuic acid, chlorogenic acid, methyl chlorogenate, catechin, epicatechin, procyanidin B1, and procyanidin B3
Ao et al. (2010)
F. mollis
Methanolic
Stem
Monoterpenes
Sulfurous acid, octadecyl 2-propyl ester, tetratetracontane, hexatriacontyl pentafluoropropionate, octatriacontyl pentafluoropropionate, octacosyl trifluoroacetate, hexadecanoic acid, methyl ester, heptacosanoic acid, and 25-methyl-, methyl ester
Priya and Abinaya (2018)
F. palmata
Methanolic
Fruit
Phenolics
Rutin, isoquercitin, quercitin, kaempferol, luteolin, and cinnamic acid
Sharma et al. (2021)
F. pumila
Chloroform
Stem
Flavonoid glycosides
Astragalin, isoquercitrin, apigenin 6-C-α-L-rhamnopyranosyl-(1 → 2)-β-D-glucopyranoside, kaempferol 3-O-α-L-rhamnopyranosyl- (1 → 6)-β-D-glucopyranoside and kaempferol 3-O-α-L-rhamnopyranosyl-(1 → 6)-β-D-galactopyranoside, rutin and isorhamnetin-3-O-glucoside, apigenin, taxifolin, tricetin, luteolin, hesperitin, and chrysin
Pistelli et al. (2000)
n-Hexane
Stem
Triterpenoids
β-Sitosterol, α-amyrin, taraxasterol and 11α-hydroxy-β-amyrin
Chloroform
Leaves
Furanocoumarin derivatives
Bergapten and oxypeucedanin hydrate
Juan et al. (1997)
Aqueous Pb(OAc)2 solution
Leaves
Triterpenoids
Neohopane
Ragasa et al. (1999)
Ethanolic
Leaves
Norisoprenoids
3,9-Dihydroxy dihydro actinidiolide, 3α-hydroxy-5,6-epoxy-7-megastimen-9-one, dehydrovomifoliol, 3,9-dihydroxy-5,7-megastigmadien-4-one, 9,10-dihydrox-y-4,7-megastigmadien-3-one, 8,9-dihydro-8,9-dihydroxymegastigmatrienone, (6R,9S)-3- oxo-α-ionol, blumenol A, (E)-3-oxo-retro-α-ionol, (6R,9R)-3-oxo-α-ionol-β-d-glucopyranoside, roseoside, and (E)-4-[3′-(β-D-glucopyranosyloxy)butylidene]-3,5,5-trimethyl-2-cyclohexen-l-one
Bai et al. (2019)
Benzofuran derivative
Pumiloside
Trinh et al. (2018)
Flavonoid glycosides
Afzelin, astragalin, quercitrin, isoquercitrin, kaempferol 3-O-rutinoside, rutin and kaempferol 3-O-sophoroside
Flavonoids and phenolic acids
Rutin, kaempferol 3-O-α-L-rhamnopyranosyl (1 → 6)-β-D-glucopyranoside, isoquercitrin, quercitrin, dihydrokaempferol 5-O-β-D-glucopyranoside, dihydro-kaempferol 7-O-β-D-glucopyranoside, maesopsin 6-O-β-D-glucopyranoside, secoisolariciresinol 9-O-β-D-glucopyranoside, chlorogenic acid, protocatechuic acid, caffeic acid, 5-O-caffeoyl quinic acid methyl ester(12), p-hytroxybenzoic acid, vanillic acid, and 5-O-caffeoyl quinic acid butyl ester
Wei et al. (2014)
Ethyl acetate
Roots
Dinorsesquiterpenoids
(6S,9R)-Vomifoliol and (6S)-dehydrovomifoliol
Nguyen and Nguyen (2021)
Stems and roots
Sesquiterpenoids
Phaseic acid, and methyl (2α,3β)-2,3-dihydroxy-olean-12-en-28-oate
Aqueous- ethanol (50:50)
Leaves
Flavonoid glycosides
Rutin, apigenin 6-neohesperidose, kaempferol 3-robinobioside and kaempferol 3-rutinoside
Leong et al. (2008)
Methanolic
Fresh fruits
Acetylated dammarane- triterpenoids
3β-Acetoxy-22, 23, 24, 25, 26, 27-hexanordammaran-20-one, 3β-acetoxy-20, 21, 22, 23, 24, 25, 26, 27-octanordammaran-17β-ol, 3β-acetoxy-(20R, 22E, 24RS)-20, 24-dimethoxydammaran-22-en-25-ol and 3β-acetoxy-(20S, 22E, 24RS)-20, 24-dimethoxydammaran-22-en-25-ol
Kitajima et al. (1999, 2000)
Sesquiterpenoid glucosides
Pumilasides A, B and C
F. racemosa (syn. F. glomerata)
Ethanolic
Stem bark
Flavonoids
Kaempherol, Quercetin, Naringenin, and Baicalein
Keshari et al. (2016)
Triterpenoid
Lupeol
Joshi et al. (2016)
Anthocyanins
Gluanol acetate, leucocyanidin-3-O-β-D-glucopyranc oside, leucopelargonidin-3-O-β-D-glucopyranoside, leucopelargonidin-3-O-α-L-rhamnopyranoside, ceryl behenate
Joy et al. (2001)
Triterpenoids
Lupeol acetate, and α-amyrin acetate, lupeol, friedelin, behenate, stigmasterol, β-sitosterol, β-sitosterol-D-glucoside, bergenin, racemosic acid, friedelin β-sitosterol, β-amyrin, and lupeol acetate
Nguyen et al. (2001); Malairajan et al. (2006); Veerapur et al. (2007)
Fruits
Triterpenoids
β-Sitosterol, gluanol acetate, hentriacontane, tiglic acid, taraxasterol, lupeol acetate, and α-amyrin acetate
Singhal and Saharia (1980);Narender et al. (2009)
Methanolic
Stem bark
Tetracyclic triterpenoids
Gluanol acetate
Rahuman et al. (2008)
Leaves
Tetraterpenoids
Tetra triterpene, glauanolacetate, and racemosic acid
Patil et al. (2010)
n-Hexane
Stem bark
Triterpenoids
Lupeol, lupeol acetate, and β-sitosterol
Bopage et al. (2018)
Root
Triterpenoids
Cycloartenol, euphorbol, taraxerone, and tinyatoxin
Varma et al. (2009)
Latex
Triterpenoids
α-Amyrin, β-sitosterol, cycloartenol, cycloeuphordenol, 4-deoxyphorbol and its esters, euphorbinol, isoeuphorbol, taraxerol, tinyatoxin, and trimethylellagic acid
Paarakh (2009)
F. religiosa
Methanolic
Stem bark
Naphthyl substituted phytosterol
β-Sitosteryl naphthadiolyl linoleinate
Ali et al. (2017, 2020)
Lanostane type-triterpenic
Lanostanoic acid oleate
Naphthyl esters
Lanostanoic acid linolenate, Lanostanoic acid and naphthadiolyl linoleiate
Ali et al. (2014)
Steroids
β-Sitosterol glucoside and β-sitosteryl oleate
Leaves
Phenolics
Eugenol, and tannic acid
Poudel et al. (2015); Rathod et al. (2018)
Monoterpenes
Phytol, linalool, α-cadinol, α-eudesmol, β-eudesmol, epi-α-cadinol, γ-eudesmol, and epi-γ-eudesmol
Triterpenoids
Lupeol α-amyrin, campestrol, and stigmasterol
Murugesu et al. (2021)
Ethanolic
Stem bark
Phenolics
Leucopelargonidin-3-O-β-D-glucopyranoside, leucopelargonidin-3-O-α-L-rhamnopyranoside, leucoanthocyanidin, leucoanthocyanin, bergapten, and bergaptol
Sirisha et al. (2010); Wilson et al. (2016)
Triterpenoids and their derivatives
Lanosterol, lupen-3-one, β-sitosterol, stigmasterol, β-sitosterol-D-glucoside, lupeol acetate, and α-amyrin acetate
Stem
Phenolics
2,6-Dimethoxyphenol, n-hexadecanoic acid, octadecanoic acid, 4H-pyran-4-one,2,3-dihydro-3,5-dihydroxy-6-methyl, and 2,4-bis(1,1-dimethylethyl)
Manorenjitha et al. (2013)
n-Hexane
Stem
Triterpenoids
Stigmasterol, lanosta-8,24-dien-3-ol, acetate(3β), and ergost-5-en-3-ol(3β)
Ethanolic
Roots
Phenolics
Ceryl behenate, leucocyanidin-3-O-β-D-glucopyrancoside, leucopelargonidin-3-O-β-D-glucopyranoside, leucoanthocyanidin, and leucoanthocyanin
Goyal (2014)
Triterpenoids
Lupeol acetate, α-amyrin acetate, lupeol, and β-sitosterol
Fruits
Monoterpenes
β-Caryophyllene, α-terpinene, dendrolasine, α-trans bergamotene, (e)-β-ocimene, α-pinene, limonene, dendrolasine, α-ylangene, α- thujene, α-copaene, β-bourbonene, aromadendrene, δ-cadinene, α-humulene, β-pinene, alloaromadendrene, germacrene, γ-cadinene, bicyclogermacrene [undecane, tridecane, and tetradecane
Rathee et al. (2015); Verma and Gupta (2015)
Triterpenoids
Stigmasterol and lupeol
F. retusa
Ethanolic (96%)
Aerial parts
Polyphenolic
retusaphenol [2-hydroxy-4-methoxy-1,3-phenylene-bis- (4-hydroxy-benzoate)], (+)-retusa afzelechin [afzelechin - (4α → 8) - afzelechin - (4α → 8) - afzelechin], luteolin, (+) - afzelechin, (+)-catechin, and vitexin
Sarg et al. (2011)
Triterpenoids
β-Sitosterol acetate, β-amyrin acetate, moretenone, friedelenol, β-amyrin and β-sitosterol
F. sarmentosa
Methanolic
Stem and leaves
Flavonoids
Eriodictyol, homoeriodictyol, dihydroquercetin, luteolin, quercetin, dihydroquercetin, kaempferol, dihydrokaempferol, naringenin, luteolin, apigenin, chrysoeriol, and 3′,5′,5,7-tetrahydroxylfavanone
Wang et al. (2010a, b)
7-Hydroxycoumarin, apigenin, eriodictyol, and quercetin
Wang et al. (2016)
F. semicordata
Ethanol (70%): acetic acid: formalin (90:5:5%)
Leaves and fruits
Aflatoxins
Aflatoxin B1, aflatoxin B2, aflatoxin G1, and aflatoxin G2
Gupta and Acharya (2019)
Ethanolic
Leaves
Phenolics
Gallic acid and quercetin
Kaur et al. (2017)
Stem
Phenolics
Gallocatechin, epigallocatechin, catechin, rutin, quercetin, quercetrin, (+)-catechins, quercetin and quercitrin
Nguyen (2002); Gupta et al. (2019); Al-Snafi (2020)
Methanolic
Fruits
Monoterpenes
α-Thujene, α-Pinene, sabinene, β-pinene, β-myrcene, limonene,1,8-cineole, (Z)-β-ocimene, (E)-β-ocimene,γ-terpinene, terpinolene, linalool, and perillene
Chen et al. (2009)
Sesquiterpenes
Ylangene, α-copaene, β-panasinsene, β-cubebene, β-elemene, α-gurjunene, β-caryophyllene, α-humulene, alloaromadendrene, γ-muurolene, germacrene D, β-Selinene, α-selinene, α-muurolene, (E,E)-α-farnesene, and δ-cadinene
F. tikoua
Ethanolic (95%)
Whole plant
Isoprenylated flavonoids
Ficustikousins A and B, derrone, alpinumisoflavone, (S)- 5,7,3′,4′-tetrahydroxy-2′-(3-methylbut-2-enyl)flavanone, (S)-paratocarpin K, 3′-(3-methylbut-2-enyl)biochanin A, and genistein
Wu et al. (2015)
Coumarin
Bergapten
Benzofuran glucoside
6-Carboxyethyl-5-hydroxybenzofuran 5-O-β-d-glucopyranoside, and 6-carboxyethyl-7-methoxyl-5-hydroxy-benzofuran 5-O-β-D-glucopyranoside
Wei et al. (2011b)
Rhizome
Isoflavonoids
Ficusin C, 6-[(1R*,6R*)-3-methyl-6-(1-methylethenyl)-2-cyclohexen-1-yl]-5,7,4′- trihydroxyisoflavone, ficusin A, alpinumisoflavone, 4′-O-methylalpinumisoflavone, and quercetin
Fu et al. (2018)
Methanolic
Stem
Pyranoisoflavone
5,3′,4′-trihydroxy-2″,2″-dimethylpyrano (5″,6″:7,8) isoflavone
Wei et al. (2012)
Isoflavones
Wighteone and lupiwighteone
Isoflavanone
Ficustikounone A
Zhou et al. (2018)
Ethanolic (90%)
Stem
Phenolic glycosides
2-Ethylene-3,5,6-trimethyl-4-phenol-1-O-β-D-xylopyranosyl-(1 → 6)-β-D-glucopyranoside, 3-methoxy-4-O-β-D-apiofuranosyl-(1 → 2)-β-D-glucopyranosylpropiophenone, 3-hydroxy-1-(4-O-β-D-glucopyranosyl-3-methoxyphenyl)propan-1-one, 4-hydroxy-3,5-bis(3′-methyl-2-butenyl)benzoic acid-O-β-D-glucopyranoside, 3,4,5-trimethoxyphenol-1-O-β-D-apiofuranosyl-(1 → 6)-β-D-glucopyranoside, 3,4,5-trimethoxyphenol-1-O-β-D-glucopyranoside, 3-methoxy-4-O-β-D-apiofuranosyl-(1 → 6)-β-D-glucopyranosylpropiophenone, baihuaqianhuoside, 3,5-dimethoxy-4-hydroxybenzoic acid-O-β-D-glucopyranoside and 2-methoxy-4-allylphenyl-1-O-β-D-apiofuranosyl-(1 → 6)-β-D-glucopyranoside
Jiang et al. (2013)
3.3 Pharmacological attributes
Indian Ficus species possess analgesic (Marasini et al., 2020), antioxidative (Etratkhah et al., 2019), antidiabetic (Anjum and Tripathi, 2019b), anti-inflammatory (Sabi et al., 2022), antiarthritic (Mathavi and Nethaj, 2019), anti-stress (Murugesu et al., 2021), anticancer (Jain and Jegan, 2019), hepatoprotective (El-hawary et al., 2019), neuroprotective (Hassan et al., 2020), antimicrobial (Raja et al., 2021), radioprotective (Vinutha et al., 2015), and wound healing (Ansari et al., 2021) properties. The summary and related mechanisms of various pharmacological activities are presented in Table 3 and Fig. 4.
Pharmacological activity
Plant species
Used plant parts
Tested extract/ compound
Tested concentration/dose
Tested model/mode of administration
Study outcomes
References
Analgesic activity
F. benghalensis
Stem bark
Aqueous
400 mg/kg b.w./p.o.
Swiss albino mice/ tail-flick and formalin-induced pain assay/i.p.
Tail-flick model - extract (P < 0.001) increased mean tail-flick latency when compared to the control group/formalin-induced pain – extract significantly reduced pain response when compared with control group animals (P < 0.001)
Rajdev et al. (2018)
Methanol
400 mg/kg b.w./p.o.
Swiss albino mice/ acetic acid-induced writhing/i.p.
Extract prevented acetic acid induced writhing movements significantly in mice (P < 0.01) when compared to control
Thakare et al. (2010)
Leaves
Methanol
100 mg/kg b.w./p.o.
Swiss albino Wistar rats/ acetic acid-induced writhing and hot plate assays/i.p.
Extract showed maximum inhibition to writhing responses (43%) when compared to aspirin (20 mg/kg; P < 0.05)/maximum nociception inhibition of stimulus displayed by extract at 15 min (P < 0.05) in hot plate model
Mahajan et al. (2012)
F. carica
Fruits
Aqueous (boiled)
2000 mg/kg b.w./i.p.
Wistar male rats/ formalin-induced paw licking/i.p.
Extract showed no significant difference between control and extract treated animals (P > 0.05)
Mirghazanfari et al. (2019)
F. deltoidea
Leaves
Aqueous
100 mg/kg b.w./i.p.
Male ICR mice/acetic acid-induced abdominal writhing, formalin-induced pain, and hot plate assays/i.p.
Extract produced significant antinociceptive effect in tested assays when compared with control (P < 0.001)
Sulaiman et al. (2008)
Leaves and roots
Aqueous
200 mg/kg b.w./i.p.
Male ICR mice/ acetic acid-induced abdominal writhing (i.p.)/formalin (s.c.)-induced pain and hot plate assays
Extract produced significant antinociceptive effect in acetic acid-induced abdominal writhing (P < 0.001 compared to control)/extract showed significant inhibition in the early phase of the formalin-induced pain assay (P < 0.001 compared to control)/increased latency time significantly in the hot plate assay (P < 0.0001)
Salihan et al. (2015)
F. elastica
Stem bark
Methanol (98%)
10 mg/kg b.w./i.p.
Wistar albino rats/ acetic acid-induced writhing/i.p.
The inhibitory effect of extract on squirming count was significant (P < 0.05; compared to control)
Aziba and Sokan (2009)
F. exasperata
Leaves
Methanol
250 mg/kg b.w./i.p.
Swiss albino mice/ acetic acid-induced writhing/i.p.
Extract reduced the numbers of writhes (36.87%) when compared with control (69.7%; P < 0.001)
Zubair et al. (2014)
F. pumila
Stem and leaves
Methanol
1 g/kg b.w./p.o.
Male ICR mice/acetic acid-induced writhing and formalin-induced paw licking/i.p.
Extract significantly decreased writhing responses in the acetic acid assay (P < 0.01) and licking time in the formalin-induced pain (P < 0.001)
Liao et al. (2012)
F. racemosa (syn. F. glomerata)
Leaves
Ethanol
400 mg/kg b.w./i.p.
Male Swiss albino mice/ acetic acid-induced writhing and formalin-induced paw licking/i.p.
Extract displayed significant activity (P < 0.01) in acetic-induced writhing/extract showed significant reduction in paw biting and licking response (P < 0.01)
Ghawate et al. (2012)
F. religiosa
Leaves
Methanol
40 mg/kg b.w./p.o
Wistar albino rats/ tail flick latency period/ acetic acid- induced writhing in mice/i.p.
Extract found more effective (P < 0.01) in preventing acetic acid induced writhing and in increasing latency period in tail flick method (P < 0.01)
Gulecha et al. (2011)
Leaves and bark
Ethanol
400 mg/kg b.w./p.o.
Swiss albino mice/ Eddy’s hot plate and acetic acid-induced writhing/p.o.
Hot plate – both extracts increased latency time 70.81% (8.54 min) and 70.78% (8.53 min), respectively (P < 0.05)/ both extracts inhibited the number of writhings induced by acetic acid (P < 0.05)
Marasini et al. (2020)
Anti-inflammatory activity
F. benghalensis
Stem bark
Ethanol
600 mg/kg/day/b.w./p.o.
Wistar albino rats/ carrageenan-induced rat paw oedema and cotton pellet granuloma models/i.p.
Extract showed significant inhibition (69.04%; P < 0.05) after 3 h on carrageenan-induced paw edema/extract displayed significant (39.03%; P < 0.05) inhibition after 3 h on cotton pellet granuloma model
Patil and Patil (2010)
200 mg/kg b.w./p.o.
Sprague Dawley rats/ carrageenan-induced paw edema/i.p.
Extract (69.86%) showed significant (P < 0.0001) inhibition at 3 h on the carrageenan-induced inflammation
Wanjari et al. (2011)
Methanol
400 mg/kg b.w./p.o./given for 6 h
Wistar albino rats/carrageenan-induced paw edema/s.c.
Extract (3.8 ± 0.1 mL) and diclofenac sodium (2.9 ± 0.1 mL) elicited significant inhibition of edema formation at 3 h (P < 0.01)
Thakare et al. (2010)
300 mg/kg b.w./i.p. in case of FCA-induced edema arthritis, formalin –induced arthritis while p.o. in case of agar- induced
Swiss albino rats/FCA-induced edema arthritis/s.c./ formalin –induced arthritis/ agar- induced edema/i.p.
FCA-induced edema arthritis - extract exhibited significant inhibition (40.48%) when compared with acetyl salicylic acid (26.98%; P < 0.05)/ formalin-induced paw edema-extract displayed significant inhibition (69.9%) when compared with acetyl salicylic acid (67.72%; P < 0.05)/agar- induced edema-extract exhibited maximum inhibition which was as good as indomethacin (P < 0.01)
Manocha et al. (2011)
Methanol (70%)
60 µg/mL
RAW 246.7 cells/in vitro induced using LPS (500 ng/mL)
Significant decrease in the amount of uric acid, nitric oxide, lipid peroxidation and xanthine oxidase activity reported in treated cell (in vitro)
Sabi et al. (2022)
Leaves
Methanol
200 mg/kg b.w./p.o.
Wistar albino rats/formalin-induced inflammation/i.p.
Extract showed a significant (P < 0.001) decrease in paw volume at 3 h (inhibition 65.21% when compared to 62.31% of diclofenac)
Kothapalli et al. (2014)
F. benjamina
Leaves
Aqueous
264 mg/kg b.w./p.o.
Wistar male albino rats/ carrageenan-induced paw edema/i.p.
Extract showed significant anti-inflammatory (inhibition 39.715%) effects when compared to the negative control (inhibition 70.12%; P < 0.05)
Bunga and Fernandez (2021)
F. carica
Leaves
Ethanol
600 mg/kg b.w/p.o.
Wistar albino rats/ carrageenan-induced paw edema/i.p.
Extract showed (75.90%; P < 0.05) significant inhibition after 3 h when compared with indomethacin (79.72%)
Patil and Patil (2011)
Wistar albino rats/carrageenin, serotonin, histamine, dextran-induced rat paw oedema/i.p.
Extract exhibited maximum anti-inflammatory effect (inhibition 33.73%) at the end of 3 h on carrageenin, serotonin, histamine, and dextran-induced rat paw oedema when compared to indomethacin (P < 0.001)
Patil et al. (2013)
Extract gel (carbopol 940 base swelling + fig leaves)
Rats were topically treated for 3 days
BALB/c rats/croton oil-induced inflammation on the back of rats
The gel displayed significant differences in the treatment group (P = 0.688 negative control; gel P = 0.470)
Kurniawan et al. (2021)
Methanol (50%)
500 mg/kg b.w./p.o.
Wistar albino rats/ formalin-induced rat paw oedema/ i.p.
Extract showed significant inhibition (66.43%) after 4 h (P < 0.001) of administration
Ali et al. (2009, 2012)
Methanol
50 mg/pouch
Male Wistar albino rats/ carrageenan-induced pouches/ i.p.
Extract significantly reduced the formation of TNFα, PGE2, and VEGF, while angiogenesis was significantly suppressed when compared to diclofenac (
< 0.001)
Eteraf-Oskouei et al. (2015)
Branches
Ethyl acetate
–
RAW264.7 cells/in vitro assay
Extract suppressed nitric oxide formation in RAW264.7 cells. The level of tumor necrosis factor-α was significantly decreased (P < 0.01)
Park et al. (2013)
Fruits
Ficucaricones A–D
0.89 ± 0.05 to 8.49 ± 0.18 μM
RAW264.7 cells/in vitro assay
Compounds showed significant anti-inflammatory effects (IC50 0.89 ± 0.05 to 8.49 ± 0.18 μM)
Liu et al. (2019)
F. deltoidea
Leaves
Aqueous
300 mg/kg b.w./p.o.
Wistar albino rats/carrageenan-induced paw edema, cotton pellet-induced granuloma/i.p.
Extract showed significant (P < 0.05) anti-inflammatory activity in all tested assays
Zakaria et al. (2012)
Methanol
–
Wistar albino rats/lipoxygenase inhibitory activity/ hyaluronidase inhibition assay and 12-otetradecanoylphorbol 13-acetate (TPA)-induced ear oedema
Extract exhibited 10.35 ± 0.04% inhibition in case of lipoxygenase activity/ extract showed 51.0% inhibition in case of hyaluronidase inhibition assay/extract showed strong decrease of oedema (85.46 ± 8%; P < 0.05) in (TPA)-induced ear oedema
Abdullah et al. (2009); Ashraf et al. (2021)
F. elastica
Root bark
Aqueous
10 mg/kg b.w./p.o.
Male Wistar albino rats/ carrageenin-induced paw oedema/i.p.
Extract and indomethacin produced potent inhibition (68.92% and 69.26%) to paw edema
Sackeyfio and Lugeleka (1986)
Stem bark
Methanol (98%)
10 mg/kg b.w/p.o.
Wistar albino rats/carrageenan-induced paw oedema/i.p.
Extract significantly inhibited carrageenan induced inflammation when compared with control (P < 0.05)
Aziba and Sokan (2009)
F. erecta
Leaves
Dichloromethane
1 μg/mL
Raw 264.7 murine macrophage cell lines /LPS-induced in vitro assay
Extract inhibited the production of pro-inflammatory factors (NO, iNOS, COX-2, and PGE2)
Jung et al. (2018)
F. exasperata
Stem bark
Ethanol (70%)
300 mg/kg b.w./p.o
Male Wistar rats/carrageenan- induced foot edema/i.p.
Extract significantly suppressed carrageenan induced foot edema (68.57 ± 3.342%) when compared to diclofenac (71.56 ± 3.43%) and dexamethasone (74.53 ± 5.21%)
Amponsah et al. (2013)
F. hirta
Roots
(1′S)-methoxy-4-(1-propionyloxy-5-methoxycarboxyl-pentyloxy)- (E)-formylviny, (8R)-4,5′-dihydroxy-8-hydroxymehtyl-3′-methoxydeoxybenzoin, (2′S)-3-[2,3-dihydro-6-hydroxy-2-(1-hydroxy-1-methylethyl)-5- benzofuranyl] methyl propionate, 3-[6-(5-O-β-D-glucopyranosyl) benzofuranyl] methyl propionate
–
Murine macrophage RAW 264.7 cells/in vitro assay
Isolated compounds (1, IC50 > 100 μM; 2, IC50 68.42 ± 4.96 μM; 3, IC50 46.32 ± 3.67 μM; 4, IC50 > 100 μM) showed pronounced inhibitory effects on the LPS induced NO production in murine macrophage RAW 264.7 cells when compared to indomethacin (IC50 48.3 ± 2.8 μM)
Cheng et al. (2017)
F. hispida
Twigs and leaves
Ficuhismines A–D
–
HEK293/NF-κB cells/in vitro luciferase assay
Ficuhismine B exhibited significant inhibitory effects in NF-κB pathway luciferase assay (IC50 0.52 ± 0.11 μM) when compared with bortezomib (IC50 0.12 ± 0.04 μM)
Jia et al. (2020)
Fruits
Methanol
200 mg/kg b.w./p.o.
Male Wistar albino rats/ carrageenan and histamine-induced inflammation/i.p./ RAW 264.7 cells/LPS in vitro assay
Extract showed significant activity in a dose dependent manner on carrageenan and histamine-induced inflammation/MTT assay – extract (IC50 125 μg/mL) and indomethacin on RAW 264.7 (IC50 50 μg/mL)
Choudhury et al. (2021b)
Leaves
Ethanol
800 mg/kg b.w./p.o.
Female Wistar albino rats/ acetic acid-induced colitis/intra-colonic administration/disease activity index/colon mucosal damage index were determined
Acetic acid group-mucosal disease index 4.33 ± 0.51 (control)/sulfasalazine score 2.00 ± 0.63/extract score 2.50 ± 0.54 (P < 0.001) which was significantly less than control/disease activity index - acetic acid group score (5.50 ± 0.54)/ sulfasalazine score (1.83 ± 0.75)/extract score (2.50 ± 0.54) which was significantly less than control (P < 0.001)
Gunaseelan et al. (2015)
Methanol
400 mg/kg b.w./ p.o.
Swiss albino mice/xylene-induced ear edema test/i.p.
Extract exhibited significant (P < 0.05, vs. control) ear weight differences and inhibition of ear edema
Mushiur Rahman et al. (2018)
Hexane
300 mg/kg b.w./p.o.
Sprague Dawley rats/carrageenan-induced paw edema/i.p.
Extract showed significant inhibition within 90 min when compared with prednisolone (control; P < 0.05) and dichlofenac sodium (control; P < 0.01)
Anasane and Chaturvedi (2017)
Stem bark
Ethanol
400 mg/kg b.w./p.o.
Wistar albino rats/histamine and carrageenan-induced paw oedema/i.p.
Extract (59.49%) and indomethacin (67.72%) inhibited the inflammation significantly in carrageenan-induced paw edema/extract (60.12%) and indomethacin (69.64%) inhibited inflammation significantly (P < 0.01) in histamine-induced paw edema
Howlader et al. (2017)
F. lacor
Aerial roots
Ethanol
100 mg/kg b.w./p.o.
Wistar albino rats/carrageenan-induced paw edema/i.p.
Extract showed (75.40%) inhibition on carrageenan-induced paw edema when compared with indomethacin (80.80%; P < 0.001)
Sindhu and Arora (2014)
100 mg/kg b.w./p.o.
Wistar albino rats/ carrageenan and histamine-induced paw edema/i.p.
Extract showed maximum inhibition (75.11%) on carrageenan induced edema (P < 0.001) when compared with indomethacin (81.83%)/ extract exhibited significant inhibition on histamine-induced edema (35.66%; P < 0.05)
Pandey et al. (2019)
F. Microcarpa
Leaves
Methanol
200 mg/kg b.w./p.o.
Male Wistar albino rats/carrageenan- induced edema, histamine, and serotonin- induced paw edema/i.p.
Extract significantly reduced the formation of oedema induced by carrageenan, histamine and serotonin when compared with control (P < 0.05)
Bairagi et al. (2012)
F. palmata
Fruits
Methanol
150 mg/kg b.w./p.o.given for 4 h
Male Wistar albino rats/ carrageenan-induced paw edema/i.p.
Paw volume was significantly reduced (P < 0.01) in treated animals as compared to control group
Chandra and Saklani (2016)
Ethanol
100 μg/mL concentration
RAW264.7 cells/in vitro assay/ lipopolysaccharide-induced inflammation assay
NO and PGE2 production was significantly inhibited by the extracts in a dose-dependent manner (P < 0.05)
Khajuria et al. (2018)
F. pumila
Fruits
Methanol (75%)
250 mg/kg b.w./p.o.
Clean Kunming mice/carrageenan-induced toe swelling/ear swelling in mice with bilateral adrenalectomy induced by xylene
Extract reduced the swelling significantly in carrageenan-induced model (P < 0.001)/extract also inhibited xylene-induced ear swelling (P < 0.05) when compared with control
Zeng et al. (2020)
Stems and leaves
Methanol
1.0 g/kg b.w./p.o.
Male ICR mice/carrageenan-induced mouse paw edema/i.p.
Extract showed about equal amount of inhibition as exhibited by indomethacin (P < 0.01 and P < 0.001) when compared to control
Liao et al. (2012)
F. racemosa (syn. F. glomerata)
Stem bark
Ethanol
400 mg/kg b.w./p.o.
Wistar albino rats/ carrageenan-induced paw edema(i.p.)/ formalin induced peritonitis (i.p.)/ cotton pellet induced granuloma (s.c.)
Extract (P < 0.001) produced 61.37% inhibition to carrageenan-induced paw edema when compared with diclofenac (62.95%) after 3 h/extract decreased formalin-induced peritonitis when compared with diclofenac significantly (P < 0.001)/in cotton pellet induced granuloma, extract and diclofenac decreased the formation of granuloma significantly (P < 0.001)
Priya Mohan et al. (2021)
Aqueous (hot)
0.1 µg/mL
Albumin denaturation assay/ inhibition of protein denaturation (%)
Extract showed higher inhibition (0.1 μg/mL) than the reference drugs (P < 0.05)
Dharmadeva et al. (2018)
Fruits
Ethanol
500 mg/kg b.w./p.o.
Swiss albino mice/ carrageenan-induced rat paw edema/i.p.
Extract showed significant inhibition (P < 0.001) when compared with the vehicle control (distilled water) and indomethacin (standard) after 3 h
Rahman et al. (2016)
Methanol
500 mg/kg b.w./p.o.
Male albino Wistar rats/ carrageenan-induced paw oedema/i.p.
Extract (37.8%) caused significant inhibition on carrageenan-induced rat paw oedema when compared to control (P < 0.001)
Saneja et al. (2008)
F. religiosa
Leaves
Kaempferol
–
In silico approach using molecular docking/cyclooxygenase-2 receptor
Kaempferol strongly was tied to TYR385 and SER530 of the receptor
Utami et al. (2020); Biju et al. (2020)
F. retusa
Fruits
Methanol
400 mg/kg b.w./p.o.
Wistar albino rats/ carrageenan-induced paw edema/i.p.
Extract produced significant reduction on paw oedema when compared with diclofenac sodium (50 mg/kg; P < 0.05)
Raju and Sreekanth (2011)
Antimicrobial activity
F. auriculata (syn. F. pomifera)
Fruits
Methanol: water (80:20)
60 µg concentration/disc
P. vulgaris, S. epidermidis, E. coli, K. pneumoniae, N. gonorrhoeae, M. genitalium and P. aeruginosa/disc diffusion assay
Potent activity displayed against M. genitalium and S. epidermidis
Raja et al. (2021)
Ethanol
50 mg/mL/ disc
E. coli, K. pneumoniae, E. gergoviae, S. enterica, S. flexneri, S. aureus, S. epidermidis, S. pyogenes, and B. cereus/ disc diffusion assay
Extract showed strong activity (inhibition zone 14 ± 1 mm, 13 ± 1 mm and 12 ± 1 mm) against S. flexneri, E. coli and S. epidermidis
Saklani and Chandra (2012)
(Z)-5,7,4′-trihydroxy-3′-[3-hydroxy-3-methyl-1-butenyl] isoflavone
1.25 to 20 μg/mL
E. coli, S. aureus, S. epidermidis, S. pyogenes/ MIC determination
Compound exhibited significant antibacterial effects against selected pathogenic bacterial strains (MIC 1.25 to 20 μg/mL)
Shao et al. (2022)
Roots
5,7,4′-Trihydroxy-3′-hydroxymethylisoflavone, 3′-formyl-5,4′-dihydroxy-7-methoxyisoflavone, ficuisoflavone and alpinumisoflavone
1.30 to 39.93 μM concentration
B. cereus, S. epidermidis, S. albus, E. coli, P. solanacearum and P. aeruginosa /MIC determination
Isolated compounds showed strong antibacterial activity against selected pathogenic bacteria (MIC 1.30 to 39.93 μM)
Qi et al. (2018)
F. benghalensis
Roots
Ethanol
75 mg/mL/disc
K. pneumoniae, S. aureus and E. coli /disc diffusion assay
Extract showed potent antibacterial activity against S. aureus (IZ 30 mm) when compared with ampicillin (IZ 30 mm)
Murti and Kumar (2011)
Leaves
Ethanol
100, 50, 25, 12.5, 6.25 mg/mL
S. mutans, Lactobacillus species, K. pneumoniae, and C. albicans
Extract showed maximum antimicrobial activity against K. pneumoniae (IZ 23 mm; MIC 6.25 mg/mL)
Samuel et al. (2022)
Fruit latex
Latex
100 μL concentration
S. aureus, S. pyogenes, E. coli, P. aeruginosa, P. mirabilis, K. pneumoniae, Salmonella spp., Seratia spp., C. albican, C. tropicalis, C. cruzii, C. kefyr, C. sojae
Candida species were found more susceptible to latex than selected bacterial pathogens
Faisal (2017)
F. benjamina
Leaves
Methanol
40 mg/ mL/disc
S. typhimurium, P. aeruginosa, S. typhimurium and E. coli
S. typhimurium [3.0 mm] > P. aeruginosa [2.0 mm] > S. typhimurium (TA100) [1.0 mm] = E. coli [2.5 mm]
Bhawana et al. (2018)
Ethanol (96%)
50 μL/disc
K. pneumoniae, P. aeruginosa, E. coli, S. aureus, S. aureus and S. pneumoniae/ disc diffusion assay
Extract showed varied levels of inhibitory effects against all the test organisms
Truchan et al. (2017)
F. deltoidea
Leaves
Methanol
50 mg/mL/disc
S. aureus (IMR S-277), B. subtilis (IMR K-1), E. coli (IMR E-940), P. aeroginosa (IMR P-84)} and C. albicans (IMR C-44)
Extract showed greater activity to S. aureus (IZ 15.67 ± 0.58 mm; MIC 3.125 mg/mL) than other bacterial strains
Abdsamah et al. (2012)
Lupeol
150, 220 and 130 μg/mL concentration
E. coli, B. subtilis and S. aureus/MIC determination
Potent activity displayed against E. coli, B. subtilis and S. aureus with different concentrations (MIC 150, 220 and 130 μg/mL)
Suryati et al. (2011)
F. exasperata
Leaves
Ethanol
100 mg/mL/disc
A. flavus, A. niger, B. theobromae, F. oxysporum, F. solani, P. chrysogenum, P. oxalicum, R. stolonifera, Pseudomonas spp. and Klebsiella spp.
Extract exerted significant antimicrobial effects against the test organisms
Oyelana et al. (2011)
F. microcarpa
Stem bark
Methanol
10 mg/mL/disc
B. brevis, B. cereus, B. subtilis, E. coli and A. polymorph/ disc diffusion assay
Maximum activity displayed against E. coli (inhibition zone 17.8 mm) when compared with ampicillin (IZ 34.5 mm) while mild activity against other microbes
Ao et al. (2008)
Whole plant
Aqueous
37.5 mg/mL/disc
S. aureus, P. aeruginosa and E. coli/disc diffusion assay/MIC determination
Extract showed moderate inhibitory effect against P. aeruginosa (18 mm) followed by S. aureus and E. coli
Nair and Mahesh (2016)
F. palmata
Leaves
Catechin, genistein, β-sitosterol, stigmasterol
100 µg /mL/disc
B. cereus, S. enterica, S. aureus, S. epidermidis and S. pyogenes/disc diffusion assay
Genistein demonstrated potent antibacterial activity against B. cereus (IZ 23 mm) when compared to erythromycin (35 mm)
Chandra and Saklani (2017)
Fruits
Ethanol
20 mg/10 mL/disc
E. coli and S. aureus/disc diffusion assay
Extract showed maximum activity against E. coli (IZ 8 mm)
Kumar et al. (2012)
F. pumila
Leaves
Ethanol
200 μL/disc
A. hydrophila, C. freundii, P. fluorescens, Y. ruckeri/disc diffusion assay
Extract showed maximum activity against Y. ruckeri (IZ 14 mm)
Halyna et al. (2018)
F. racemosa (syn. F. glomerata)
Roots
Ethanol
75 mg/mL/disc
K. pneumoniae, S. aureus and E. coli/disc diffusion assay
Extract showed maximum activity against S. aureus (IZ 35 mm) when compared to ampicillin (10 μg/disc; IZ 40 mm)
Murti and Kumar (2011)
Leaves
Ethanol
100 µL/disc
E. coli, P. aeruginosa, and S. aureus, A. flavus, Rhizopus species and R. solani/ disc diffusion assay
Extract showed moderate activity against E. coli (IZ 10 mm), S. aureus (IZ 14 mm) and Rhizopus (6 mm)
Thilagavathi and Kathiravan (2017)
100 μL/well
E. coli, B. subtilis, P. aeruginosa, K. pneumonia, S. aureus and S. mutans/ well-diffusion assay
Extract manifested strong zone of inhibition against E. coli (13. 1 mm), B. subtilis (10.5 mm), P. aeruginosa (10.4 mm), S. aureus (11.0 mm) and S. mutans (12.4 mm)
Danie Kingsley et al. (2014)
Fruits
Ethanol
100 µg/mL/disc
Staphylococcus spp, Pseudomonas spp, Klebsiella spp and E. coli/MIC determination/disc diffusion assay
Extract showed strong antibacterial activity against Staphylococcus spp. and Klebsiella spp. (IZ 26 ± 0.10 and 24 ± 0.13 mm)/ Staphylococcus spp. showed lowest MIC (0.07 mg/mL)
Bagyalakshmi et al. (2019)
Ethyl acetate
1 mg/mL
E. coli, K. pneumoniae, S. aureus, S. typhi, C. albicans and A. niger/agar well diffusion assay
In well diffusion method- ethyl acetate extract showed significant bactericidal activity against S. aureus (0.98 mg/mL) and fungistatic against A. niger (1.39 mg/mL)
Pingale et al. (2019)
Methanol
200 µg/disc for bacteria and 150 µg/disc for fungal organisms
S. aureus, B. subtilis, V. cholera, B. cereus, S. typhi, S. dysenteriae, P. aeruginosa, Klebsiella spp., Proteus spp., Alternaria spp., Colletotrichum spp., Curvularia spp. and Fusarium spp/disc diffusion assay
Maximum activity displayed against S. aureus (IZ 18 mm) and Fusarium spp. (IZ 12 mm)
Hossain et al. (2014)
Stem bark
Methanol
–
S. aureus, B. cereus, P. aeruginosa, E. coli and B. subtilis/ disc diffusion assays
Disc diffusion assay - extract showed antibacterial effect against S. aureus (IZ 12.1 mm) B. cereus (10.3 mm), B. subtilis (8.14 mm)
Faiyaz et al. (2010)
F. religiosa
Leaves
Methanol
50 µL/disc
E. coli/disc diffusion assay
Methanolic extract showed activity to E. coli (12 mm)
Parasharami et al. (2014)
50 μL/well
S. aureus, E. coli, P. aeruginosa, S. typhi, A. niger and Penicillium notatum/well diffusion method
Methanolic extract exhibited greater activity against E. coli than other tested microbes
Pathania et al. (2021)
F. retusa
Aerial parts
Ethanol
–
S. aureus, P. aeruginosa, E. coli and P. mirabilis/disc diffusion assay
Extract showed moderate activity against S. aureus (7.00 ± 0.32 mm), E. coli (7.04 ± 0.32 mm) and P. aeruginosa (7.05 ± 0.32 mm)
Subramani et al. (2014)
F. sarmentosa
Fruits
Eriodictyol, homoeriodictyol, dihydroquercetin, and luteolin
–
F. graminearum, P. fusarium, C. lunata, S. zeicola, B. cinerea, and R. solani/MIC determination
Luteolin showed the strongest inhibitory activity (IC50 56.38 and 81.48 mg/L) against F. graminearum and S. zeicola
Wang et al. (2010)
F. tikoua
Whole plant
Essential oil
0.20–6.25 mg/mL
S. aureus, B. subtilis, E. faecalis, E. coli, P. aeruginosa and P. vulgaris/MIC determination
Essential oil showed strong antibacterial activity (IZ 7.89–10.59 mm), MIC (0.20–6.25 mg/mL) and MBC (0.20–12.50 mg/mL) against S. aureus, B. subtilis, E. faecalis, E. coli, P. aeruginosa and P. vulgaris
Tian et al. (2020)
Antioxidant activity
F. auriculata (syn. F. pomifera)
Stem bark
Methanol
0.1 mg/mL
DPPH free radical scavenging assay/ in vitro assay
DPPH - IC50 0.042 mg/mL; inhibition − 84.088%
Gaire et al., (2011)
Branches
Ethanol: water (50:50)
50 μL
DPPH radical scavenging assay/in vitro assay
DPPH – IC50 190.57 ± 4.25 µg/mL
Bertoletti et al. (2020)
F. benghalensis
Roots
Ethyl acetate fraction
250 μg/mL
DPPH free radical scavenging and FRAP reducing power assays/in vitro assays
DPPH – IC50 315.5 ± 7.98 μg/mL/FRAP − 173.5 ± 4.32 µmol Fe2+mg dry weight
Etratkhah et al. (2019)
Fruit
Methanol
–
DPPH radical scavenging, and total reducing power assays/in vitro assays
DPPH - IC50 3.18 µg/mL/ total reducing power − 243.89 ± 1.6 µg ascorbic acid equivalents/mg extract
Ahmed et al. (2017)
Leaves
Hydroalcoholic
1,000 μg/mL
DPPH, and ABTS radical scavenging assays/in vitro assays
DPPH – IC50 32.3 ± 1.320 μg/mL/ABTS – IC50 52 ± 0.722 μg/mL
Bhanwase and Alagawadi (2016)
F. benjamina
Leaves
Methanol
200 µg/mL
DPPH, iron chelating, and FRAP assays/in vitro assays
DPPH - IC50 59.07 µg/mL/ iron chelating – 131.12 μg/mL/g ascorbic acid equivalents/FRAP – 433.32 μg/mL/g ascorbic acid equivalents
Jain et al. (2013)
F. carica
Latex
Ethanol (75%)
500 µg/mL
DPPH and ABTS radical scavenging and FRAP reducing power assays/in vitro assays
Significant activity to DPPH − 65.91 ± 1.73% inhibition/ABTS − 98.96 ± 1.06% inhibition/ FRAP − 27.08 ± 0.34 mg Trolox equivalents/g
Shahinuzzaman et al. (2020)
Fruit
Peel juice
–
DPPH scavenging assay/in vitro assays
DPPH - TPC of juices extracts 74.16 mg gallic acid equivalents/g fresh weight
Harzallah et al. (2016)
Methanol (80%)
21 μL extract (for DPPH) and 10 μL extract (for ABTS)
DPPH and ABTS assays/in vitro assays
DPPH − 418.51 mg Trolox equivalent antioxidant capacity/100 g dry matter/ABTS − 207.43 mg Trolox equivalent antioxidant capacity/100 g dry matter (significant difference at P < 0.05)
Khadhraoui et al. (2019)
1 mL
DPPH radical and ABTS scavenging assays/in vitro assays
DDPH − 41.63% inhibition/ABTS − 676.13 equivalent vitamin C mg/100 g fresh weight/ difference P < 0.05
Aljane et al. (2020)
Leaves and twigs
Aqueous- methanol (80:20)
0.25 mg/mL
DPPH, ABTS and FRAP assays/in vitro assays
DPPH - IC50 346.2 μg/mL (leaf extract) with significant difference (P < 0.05)/ABTS - IC50 288.3 μg/mL (leaf extract) with significant difference (P < 0.05)/ FRAP - IC50 50.8 μg/mL (leaf extract) with significant difference (P < 0.05)
Farid et al. (2018)
Leaves
Methanol
250 µg/mL
DPPH radical scavenging assay/in vitro assay
DPPH − 10.222% scavenging inhibition
Ahmad et al. (2013)
F. deltoidea
Leaves
Methanol
1 mg/mL
DPPH radical scavenging and reducing power assays/in vitro assays
DPPH - IC50 288.04 μg/mL/reducing power IC50 0.02–0.24 μg/mL/DPPH (P < 0.001 compared to quercetin – DPPH)/reducing power assay (P < 0.001 compared to ascorbic acid – reducing power)
Mohd Dom et al. (2020)
F. elastica
Leaves
Ethanol (50%)
2 mg/mL
DPPH, ABTS, and ferric ion reducing power assays/in vitro assays
DPPH - EC50 6.4166 ± 0.3329 mg/mL/ ABTS EC50 0.0768 ± 0.0020 mg/mL/ ferric reducing power EC50 0.4027 ± 0.0016 mg/mL
Flayyh et al. (2019)
Methanol
500 µg/mL
DPPH, iron chelating, and reducing power assay/in vitro assays
DPPH - IC50 20.17 µg/mL (P < 0.05)/ iron chelating - IC50 300 µg/mL (P < 0.05)/ reducing power OD 1.25 ± 0.047 at 1 mg/mL (P < 0.05)
Preeti et al. (2015)
F. erecta
Leaves
Ethyl acetate fraction of Ethanol (70%) extract
500 μg/mL
DPPH, xanthine oxidase, nitric oxide, and superoxide dismutase assays/in vitro assay
DPPH - IC50 75.02 ± 0.02 μg/mL/xanthine oxidase - IC50 422.69 ± 10.09 μg/mL/SOD - IC50 85.72 ± 1.23 μg/mL
Jung et al. (2018)
Fruits
Petroleum ether
500 μg/mL
DPPH radical scavenging assay/in vitro assay
DPPH - IC50 7.35 ± 0.08 μg/mL, whereas standard ascorbic acid - IC50 5.80 ± 0.22 μg/mL
Al Faysal et al. (2018)
F. fistulosa
Leaves
Methanol
250 µg/mL
DPPH free radical scavenging assay/in vitro assay
Extract displayed significant radical scavenging activity (IC50 16.66 μg/mL) when compared with ascorbic acid (IC50 11.93 μg/mL)
Raka et al. (2019)
Fruits
Acetone
–
DPPH, FRAP, and ORAC assays/in vitro assays
High content of FRAP (321.75 mg/g dry weight; P < 0.05)/DPPH − 90.54% (P < 0.05)/ORAC 158.36 ± 0.65 Trolox equivalents/100 g (P < 0.05)
Hlail et al. (2014)
F. hirta
Fruits
Ethanol (90%)
0.2 mg/mL (DPPH and other radicals)/ reducing power assays (10 mg/mL)
DPPH, FRAP, ABST, hydrogen peroxide, hydroxyl radicals, chelating and reducing power assays/in vitro assays
Extract showed significant antioxidant activity {DPPH- EC50 − 345.9 ± 2.71 μg/mL/FRAP - EC50 327.5 ± 3.71 μg/mL/ABST radicals – EC50 325.4 ± 3.15 μg/mL/ hydrogen peroxide - EC50 486.5 ± 2.96 μg/mL/ hydroxyl radical - EC50 551.1 ± 3.88 μg/mL/ chelating power - EC50 380.6 ± 2.25 μg/mL/ reducing power assay − 646.6 ± 3.50 μg/mL}
Tamuly et al. (2015)
Ethyl acetate
–
DPPH, ABTS, and FRAP assays/in vitro assays
DPPH - IC50 2.52 mg/mL/ABTS IC50 3.06 mg/mL/extract showed maximum antioxidant potential to reduce ferric ions (P < 0.05)
Chen et al. (2020)
F. lyrata
Leaves
Methanol
–
DPPH radical scavenging assay/ in vitro assay
DPPH - SC₅₀ =8.27 μg/mL
El-SayedSaleh (2009)
F. microcarpa
Stem bark
Ethyl acetate
4.83, 1.62 and 63.2 μg/mL
DPPH, ABTS radical dot+, superoxide radicals scavenging assays/in vitro assays
DPPH – EC50 4.83 μg/mL/ABTS radical dot + EC50 1.62 μg/mL/superoxide radicals scavenging – EC50 63.2 μg/mL
Ao et al. (2008)
F. palmata
Leaves
Ethyl acetate
500 μg/mL
DPPH radical scavenging assay/in vitro assay
Extract showed strong DPPH scavenging effects (97.0%) when compared with ascorbic acid (98.1%)
Alqasoumi et al. (2014)
Fruits
Aqueous
2 mg/mL
DPPH and ABTS radical scavenging assays/in vitro assays
DPPH radical scavenging 175.08 mg TE/g with maximum potency/ABTS radical scavenging activity was 265 mg TE/g
Tewari et al. (2021)
F. racemosa (syn. F. glomerata)
Stem bark
Methanol
200 µg/mL
DPPH scavenging assay/in vitro assay
DPPH scavenging activity - IC50 73.46154%
Sultana et al. (2013)
Leaf gall
Aqueous and methanol
125, 250 and 500 µg/mL
DPPH, NO scavenging, hydroxyl scavenging and FRAP reducing power assays/in vitro assays
DPPH - Inhibition 59% (250 µg/mL aqueous)/NO - IC50 172.37 ± 02 µg/mL (methanol)/hydroxyl radical scavenging activity − 42.31% inhibition (125 µg/mL methanol)/FRAP – methanol extract showed maximum reducing ability at 500 µg/mL
Eshwarappa et al. (2015)
Fruits
Ethanol (90%)
0.2 mg/mL (DPPH and other radicals); reducing power assays (10 mg/ml)
DPPH, FRAP, ABST, hydrogen peroxide, hydroxyl radicals, chelating and reducing power assays/in vitro assays
Extract showed significant antioxidant activity {DPPH- EC50 256.1 ± 5.03 μg/mL; FRAP - EC50 260.7 ± 2.55 μg/mL; ABST radicals – EC50 262.7 ± 2.68 μg/mL; hydrogen peroxide - EC50 402.6 ± 2.47 μg/mL; hydroxyl radical - EC50 433.3 ± 4.33 μg/mL; chelating power - EC50 172.1 ± 1.28 μg/mL; reducing power assay − 470.7 ± 4.17 μg/mL}
Tamuly et al. (2015)
F. religiosa
Leaves
Chloroform and aqueous
80 µg/mL
DPPH, and ABTS assays/in vitro assays
DPPH – IC50 − 4.76 ± 0.15 µg/mL (chloroform)/ ABTS – IC50 − 0.14 ± 0.04 µg/mL (aqueous)
Sharma et al. (2020)
F. semicordata
Fruits
Ethanol (90%)
0.2 mg/mL (DPPH and other radicals)/ reducing power assays (10 mg/mL)
DPPH, FRAP, ABST, hydrogen peroxide, hydroxyl radicals, chelating and reducing power assays/in vitro assays
DPPH- EC50 − 301.0 ± 1.23 μg/mL/FRAP - EC50 305.3 ± 3.73 μg/mL/ABST radicals – EC50 316.9 ± 3.07 μg/mL/hydrogen peroxide - EC50 430.2 ± 4.01 μg/mL/hydroxyl radical - EC50 517.2 ± 3.06 μg/mL/chelating power - EC50 251.7 ± 2.70 μg/mL/reducing power assays − 579.1 ± 4.50 μg/mL
Tamuly et al. (2015)
Anti-diabetic activity
F. auriculata (syn. F. pomifera)
Stem bark
Aqueous
100 µg/mL
α-Amylase inhibitory assay/in vitro assay
Extract showed significant inhibition (31.3%)
Tiwari et al. (2017)
Fruits
Methanol
500 μg/mL
α-Amylase and α-glucosidase inhibitory activity/in vitro assay
Methanol showed maximum α-amylase and α-glucosidase inhibitory activity (IC50 161.73 ± 0.43 and 103.43 ± 0.67 μg/mL) when compared to acarbose (IC50 155.08 ± 1.75 and 95.63 ± 1.71 μg/mL)
Anjum and Tripathi (2019a)
F. benghalensis
Aerial roots
Aqueous
300 mg/kg b.w./p.o.
Female albino Wistar rats/p.o./ streptozotocin-induced diabetic rats/glucose tolerance test
Extract significantly reduced the fasting blood glucose level (43.8%) when compared with control after 6 h (P < 0.01)/extract showed significant reduction (40.7%) in glucose tolerance test after 3 h (P < 0.01)
Singh et al. (2009)
Leaf
Petroleum ether
200 mg/kg b.w./p.o.
Alloxan monohydrate- induced diabetic male Wistar albino rats/i.p./blood glucose test
Extract reduced the blood glucose level to 537 ± 31.9, 451 ± 43.8 and 310 ± 12.6 mg/dl on 7, 14 and 21 days, respectively (P ≤ 0·05 compared to diabetic control)
Sidhu and Sharma (2014)
Stem bark
Hexane extract, cycloartenol and 24-methylenecycloartanol
100 mg/kg b.w./p.o. and 1 mg/kg (for isolated compounds)/given for 25 days
Male Wistar albino rats and male Swiss albino diabetic mice/glucose administered/p.o./ high fat diet-streptozotocin- induced type II diabetic rats/oral glucose tolerance test
Extract reduced the levels of glucose [test/control values are 100.2 ± 3.2 mg/dl and 124 ± 4.1 mg/dl)] at 150 min after glucose administration [P < 0.001 when compared to control)/Both compounds showed significant activity in high fat diet-streptozotocin-induced diabetic rats and normalized the derailed blood glucose levels {from 348.4 ± 6.8 mg/dl to 153.7 ± 2.5 mg/dl; P < 0.001}
Nair et al. (2020)
Lcucodelphinidin derivative
250 mg/kg b.w./p.o.
Alloxan monohydrate- induced diabetic Wistar albino rats/ i.p./glucose tolerance test
In glucose tolerant test - blood glucose (%) in leucodelphinidin and glibenclamide treated groups were 48% and 33% when compared with control (71%)
Geetha and Mathew (1994)
Fruits
Ethanol
120 mg/kg b.w./p.o./once a daily for 15 days
Alloxan-induced diabetic male Wistar albino rats/i.v./GOD-POD kit method
Extract reduced the levels of blood glucose in treated animals (31.72% when compared with glibenclamide positive control 34.43%; P < 0.01)
Sharma et al. (2007)
Stem bark and leaves
Aqueous
300 mg/kg b.w./p.o./given for 28 days
Alloxan monohydrate- induced male Wistar albino diabetic rats/i.p.
Extract showed significant decrease in the blood glucose levels when compared with control (P < 0.0001)
Chikaraddy and Maniyar (2017)
F. benjamina
Leaves
Ethanol (80%)
30 µ/mL
α-Glucosidase and α-amylase inhibitory assays/in vitro assays
Extract exhibits strong α-glucosidase and α-amylase inhibitory effects (IC50 9.65 ± 1.04 µg/mL and IC50 13.08 ± 1.06 µg/ mL, respectively)
Mumtaz et al. (2018)
F. carica
Leaves
Basic and chloroform fraction of aqueous extract
0.5 mL olive oil per animal/i.p.
Streptozotocin-induced diabetic Wistar albino rats/p.o./erythrocyte assays/CAT and MDA assays
Both fractions tended to normalize the values of fatty acids and plasma vitamin E values (P < 0.01 versus normal rats)/diabetic group injected with the basic fraction (1.00 ± 0.19 µg/mg; P < 0.01)/diabetic group injected with the chloroform fraction (0.99 ± 0.15 µg/mg; P < 0.05)
Pèrez et al. (2003)
Methanol (70%)
2 mg/mL
α-Amylase inhibitory activity/in vitro assay
Extract showed significant inhibition (IC50 0.896 µg/mL; P < 0.001) when compared with control
Ara et al. (2020)
Aqueous
500 mg/kg b.w./p.o./given twice daily for 9 days
Streptozotocin- induced diabetic Wistar albino (male and female) rats/ i.p./glucose tolerance test
Extract showed significant reduction in the blood glucose level when compared to diabetic control (P < 0.001)/ maximum glucose tolerance was reported (106.5 ± 2.1) in 90 min
Roy et al. (2021)
Ethyl acetate
500 mg/kg b.w./p.o./given for 28 days
High fat diet-fed streptozotocin -induced type 2 diabetic Wistar rats/i.p./ biochemical and immunohistochemical parameters were analyzed
Extract treatment significantly (P < 0.005) reduced the increase of blood glucose levels (129.14 ± 8.23 mg/dl) at 120 min
Irudayaraj et al. (2017)
Chloroform
Streptozotocin-induced hyperglycaemic and hypertrigliceridaemic female Wistar albino diabetic rats/i.p./glucose oxidase method, triglycerides, total cholesterol methods
Plasma glucose levels in treated animals: 38.22 ± 3.63 mM, basal; 33.55 ± 4.12 mM, 60 min (P < 0.001 compared to the basal value); 36.08 ± 3.85 mM, at 24 h (P < 0.05 compared to the 60 min value)
Canal et al. (2000)
F. deltoidea
Leaves
Vitexin or isovitexin
1 mg/kg b.w./ 30 min
Sucrose loaded normoglycemic mice and induced diabetic rats/p.o./ α-glucosidase inhibition assay
Vitexin or isovitexin significantly (P < 0.05) decreased the levels of postprandial blood glucose in sucrose loaded normoglycemic mice
Choo et al. (2012)
Vitexin, and isovitexin
–
α-Amylase inhibition assay/in vitro assay
α-Amylase inhibition – vitexin IC50 0.02 μg/mL/isovitexin IC50 0.06 μg/mL (P < 0.05)
Abu Bakar et al. (2018)
F. exasperata
Leaves
Aqueous
100 mg/kg/day/ p.o./given for 30 days
Streptozotocin-induced spontaneously hypertensive Wistar albino rats/i.p./ serum cholesterol, lipoproteins and triglyceride levels were determined
Extract significantly reduced (P < 0.05) blood glucose concentrations/ lipid parameters were significantly improved (P < 0.05) towards normal values
Adewole et al. (2011)
E. hirta
Leaves
Ethanol (90%)
400 mg/kg b.w./p.o./given for 28 days
Streptozotocin-induced male Wistar albino rats/i.p./ triglycerides, cholesterol, HDL-cholesterol, and LDL-cholesterol were estimated
Extract significantly reduced the levels of blood glucose (113.23 ± 9.22 mg/dl) on 28th day when compared with diabetic control (278.92 ± 11.90 mg/dl)/showed significant reduction (P < 0.05) in total cholesterol, LDL, VLDL, triglycerides and significant increase (P < 0.05) in HDL level
Maurya et al. (2012)
F. hispida
Stem bark
Aqueous suspension (water soluble portion of alcoholic extract)
1.25 g/kg b.w./p.o.
Alloxan monohydrate-induced diabetic rats/ i.v.
Extract reduced the levels of fasting blood sugar (14.75% of extract and 25.37% of glibenclamide P < 0.01)/extract displayed significant decrease in the blood glucose levels both in the normal (P < 0.01) and diabetic (P < 0.001) rats
Ghosh et al. (2004)
F. lacor
Fruits
Ethanol
400 mg/kg b.w./p.o./given daily for 21 days
Streptozotocin-induced diabetic female Wistar albino rats/i.p./ oral glucose tolerance test
Extract reduced the elevated levels of blood glucose when compared to diabetic control group (P < 0.001)
Mule and Naikwade (2022)
F. lyrata
Leaves
Ethanol (80%)
400 mg/kg /given for 30 days
Alloxan-induced diabetic Sprague–Dawely rats/i.p./ plasma total cholesterol, triglycerides, and LDL-cholesterol levels estimated
Extract reduced the blood glucose levels significantly (127.7 ± 6.889 mg/dl when compared with gliclazide 110.8 ± 7.240 mg/dl; P < 0.05)/LDL 80.51 ± 8.235 mg/dl/cholesterol 163.0 ± 7.922 mg/dl/ triglycerides 81.80 ± 1.686 mg/dl
El-Kashoury et al. (2013)
F. microcarpa
Leaves
Ethanol (60%)
200 mg/ kg b.w./ p.o./given for 15 days
Alloxan-induced Wistar albino rats/i.p./ total cholesterol, triglycerides, HDL, LDL and VLDL levels estimated
Extract showed significant decrease in the levels of blood glucose (101.5 ± 8.58 mg/dl) better than the glibenclamide (5 mg/kg: 101. 26 ± 8.16 mg/dl)
Kumar et al. (2007)
F. mollis
Leaves
Ethyl acetate
400 mg/kg b.w./p.o./given for 30 days
Dexamethasone-induced hyperglycemia and hyperlipidemia Wistar albino rats/ s.c./biochemical parameters studied
Extract significantly (P < 0.01 and P < 0.05) reduced the level of glucose (123.5 ± 7.5 mg/dl), cholesterol (88 ± 8 mmol/L), LDL (37.5 ± 2.5 mmol/L), triacyl glycerides (63.5 ± 2.5 mmol/L), SGOT (305 ± 6.5 IU/L), SGPT (77.5 ± 0.5 IU/L) but, significantly (P < 0.01 and P < 0.05) increased the level of HDL (22.5 ± 0.5 mmol/L)
Munna and Saleem (2013)
F. palmata
Fruits
Methanol
500 μg/mL
α-Amylase and α-glucosidase inhibitory assays/in vitro
Extract showed strong α-amylase and α-glucosidase inhibitory effects (IC50 166.91 ± 2.73 and 118.73 ± 0.67 μg/mL) when compared with acarbose (IC50 154.87 ± 2.33 and 105.63 ± 1.71 μg/mL)
Anjum and Tripathi (2019b)
F. racemosa (syn. F. glomerata)
Leaves
Petroleum ether
300 mg/kg b.w./p.o./given twice a day for 7 days
Streptozotocin- induced diabetic Wistar albino rats/p.o./high density lipoprotein, total triglycerides, and total cholesterol, GSH, SOD, CAT and MDA levels were estimated
Extract exhibited maximum hypoglycemic effect/reduced glucose concentration (from 290 to 205 mg/dl; P < 0.001) when compared with control /reduced cholesterol level (157.5 ± 6.45 and 150.33 ± 2.43 mg/dl)/ extract reduced thiobarbituric acid reactive substances and protein carbonyl levels in liver of diabetic rats
Yadav et al. (2015)
Ethanol (70%)
500 mg/kg b.w./p.o./given for 10 days
Alloxan monohydrate induced male Wistar albino rats/p.o./ levels of serum urea, serum creatinine, serum cholesterol, and serum protein were estimated
Extract significantly reduced the levels of fasting blood glucose (189.83 ± 3.31 mg/dl when compared with control; P < 0.001 vs diabetic control)/also reduced the levels of biochemical parameters (P < 0.001)
Sharma et al. (2010)
Benzene
–
α-Amylase and α-glucosidase inhibitory assay/in vitro
Benzene extract showed significant inhibition to α-amylase and α-glucosidase activities (P < 0.05)
Abusufyan et al. (2018)
Fruits
α-Amyrin acetate
100 mg/kg b.w./p.o.
Streptozotocin- induced diabetic Wistar albino rats/p.o.
Isolated compound reduced the levels of blood glucose (18.4% and 17.0%) at 24 h (P < 0.05)
Narender et al. (2009)
Petroleum ether
300 mg/kg b.w./ p.o./given once a daily for 14 days
Alloxan-induced diabetic male Wistar rats/i.p./ the levels of blood glucose level, serum urea level, serum cholesterol level and serum triglycerides were estimated
Extract reduced the levels of serum glucose significantly (P < 0.01 vs. control)/levels of the serum urea, serum cholesterol and serum triglyceride were reduced in treated diabetic animals significantly (P < 0.01 vs. control)
Patil et al. (2006)
Stem bark
Baicalein, kaempferol, naringenin, and quercetin
100 mg/kg b.w./p.o. given once a day for 7 days
Streptozotocin-induced diabetic Wistar albino rats/p.o./lipid profile parameters and high-density lipoproteins estimated
Baicalein reduced the blood glucose level and restored biochemical parameters significantly (P < 0.001)
Keshari et al. (2016)
Methanol
400 mg/kg b.w./p.o.
Alloxan-induced diabetic Wistar albino rats/i.p.
Extract exhibited significant hypoglycemic effects by reducing the levels of glucose (P < 0.001)
Rao et al. (2002)
Ethanol
400 mg/kg/p.o.
High-fat diet and streptozotocin-induced diabetic Wistar albino rats/i.p./glucose tolerance assay
Extract altered the biochemical parameters and significantly improved glucose tolerance and HDL-c levels/extract showed inhibition of PTP-1B (IC50 12.1 μg/mL) and DPP-IV (42.5%)
Veerapur et al. (2012)
400 mg/kg b.w./p.o.
Streptozotocin-induced diabetic male Wistar albino rats/i.v./levels of the serum TG, T-C and LDL-C1 were estimated
Extract restored the levels of blood glucose and lipids (P < 0.001), also decreased creatinine kinase (P < 0.001), lactate dehydrogenase (P < 0.001), C-reactive protein (P < 0.001), creatinine (P < 0.001), blood urea nitrogen (P < 0.001), collagen (P < 0.05) and albumin (P < 0.001) levels/ reduced the level of sodium (P < 0.001), creatinine (P < 0.001), albumin (P < 0.001) and malondialdehyde (P < 0.01) in heart and kidney tissue along with enhanced activities of superoxide dismutase (P < 0.001) and reduced glutathione (P < 0.001)
Joshi et al. (2016)
Ethanol (90%)
400 mg/kg b.w./i.p./given for 1–3 h
Alloxan-induced diabetic Sprague-Dawley rats/i.v./ blood glucose levels measured
Extract significantly lowered the blood sugar levels (28.66 %)/also reduced blood glucose level (45.03%)
Sachan et al. (2009)
Roots
Ethanol
200 mg/kg b.w./p.o./given for 11 days
Alloxan-induced male Wistar albino diabetic rats/i.p./ levels of glucose, cholesterol, triglycerides, and high-density lipoprotein were estimated
Extract significantly reduced the levels of glucose, cholesterol, triglycerides, but increased high-density lipoprotein in diabetic rats (P < 0.05)
Upadhye et al. (2020)
F. religiosa
Leaves
Ethanol
500 mg/kg b.w./p.o./ given for 9 weeks
High-fat-diet-induced hypercholesterolemic rats/p.o.
Extract exerted significant hypolipidaemic and antioxidant effects (P < 0.001)
Hamed (2011)
Aqueous
250 mg/kg b.w./i.p./given once a daily for 21 days
Alloxan-induced diabetic male Wistar albino rats/plasma glucose, total cholesterol, triglyceride, phospholipids, HDL-cholesterol, lipoprotein lipase, HMG CoA reductase activity
Extract showed moderate decrease in the blood glucose, serum cholesterol, triglyceride and increase phospholipids levels (P < 0.001)
Pochhi and Muddeshwar (2017)
Fruits
Ethanol
250 mg/kg b.w./p.o./ administered once a daily for 30 days
Alloxan-induced male Wistar albino rats/i.p./ biochemical parameters (blood glucose, total cholesterol, and triglyceride) were evaluated
Extract and glibenclamide significantly reduced (P < 0.001) the levels of blood sugar and other parameters
Choudhary et al. (2011)
Stem bark
Aqueous
100 mg/kg b.w./p.o./ given a daily for 3 weeks
Glucose-loaded hyperglycemic and streptozotocin-induced diabetic Wistar albino rats/p.o./ serum insulin, body weight and glycogen estimation assays
Extract reduced the blood glucose levels (26.2%); P < 0.05)/showed significant reduction in the levels of serum triglyceride and total cholesterol (P < 0.05 and P < 0.01)
Pandit et al. (2010)
F. sarmentosa
Leaves
Aqueous
500 mg/kg/p.o./given a daily for 9 days
Streptozotocin-induced diabetic Wistar albino rats/i..p./oral glucose tolerance test
Extract significantly reduced the levels of blood glucose (201.8 ± 22.1 mg/100 mL) when compared to diabetic control (339.8 ± 1.81 mg/100 mL)/extract showed maximum glucose tolerance (106.5 ± 2.1 mg/100 mL) when compared with normal control (135.7 ± 2.917 mg/100 mL)
Negi et al. (2021)
F. semicordata
Leaves
Ethanol
50 mg/kg b.w./p.o./given a daily for 21 days
α-Amylase and α-glycosidase inhibitory assays/in vitro assays/ streptozotocin-induced Wistar albino rats/i.p./in vivo assay/blood glucose levels estimated
Extract showed significant α-amylase (IC50 3.352 µg/mL) and α-glycosidase inhibitory effects (IC50 3.448 µg/mL) when compared to standard acarbose (IC50 3.175 µg/mL)/extract also significantly reduced the levels of blood glucose (P < 0.001)
Kaur et al. (2017)
F. tinctoria
Leaves and stem bark
Aqueous- alcohol (50:50%)
250 mg/kg/p.o./ administered a daily for 35 days
Streptozotocin-induced diabetic male Sprague Dawley rats/i.p./ glucose tolerance test
Leaf extract showed significant (P < 0.05) decrease in the elevated blood glucose levels at 120 min/similarly stem bark extract significantly (P < 0.01) reduced the elevated glucose levels at 120 min
Kumar et al. (2020)
Anti-arthritic activity
F. benghalensis
Stem bark
Methanol
400 mg/kg b.w./p.o./given daily for 21 days
Formalin and CFA- induced arthritis in Wistar albino rats (i.p./formalin/s.c./ CFA/ AST, ALT and LDH were assayed
Extract significantly inhibited on 8th day (P < 0.05) and the order of edema inhibition was diclofenac sodium > extract > dexamethasone > methotrexate reduced the arthritic score significantly on the 10th day (P < 0.05)/ maximum edema inhibition reported on the 21st day (methotrexate > dexamethasone ≥ diclofenac sodium > extract/extract, methotrexate, dexamethasone, and diclofenac sodium treatment to arthritic rats prevented the increase in serum AST, ALT and LDH levels significantly (P < 0.001)
Thite et al. (2014)
300 mg/kg/i.p./given for 16 days
CFA and formalin, agar induced-induced arthritis in Swiss albino rats/s.c.
Extract showed significant anti-inflammatory effects, especially on the secondary immunological arthritis (P < 0.05)/extract also showed dose dependent inhibition in early (69.9%) and late phases (78.42%) of licking responses (P < 0.01)/extract significantly (P < 0.05) suppressed the development of acute edema of rat paw (P < 0.05)
Manocha et al. (2011)
Methanol-water (50:50)
400 μg/mL
Egg albumin denaturation assay/in vitro assay
Extract showed maximum inhibition of denaturation (83.80 ± 5.16%) when compared with diclofenac sodium (91.45 ± 6.84%; P < 0.01)
Mathavi and Nethaj (2019)
Aerial roots
Emulgel
Emugel (ethanol extract – 0.01 g + glycerin −2.1 mL + triethanolamine-2.0%+ethanol − 0.1 mL
Drug release analysis/in vitro assay/ franz diffusion cell assay
Emugel activity compared to diclofenac/emulgel confirms (spread ability, and viscosity) the anti-arthritic activity (P < 0.001)
Sonali et al. (2021)
Ethanol (95%)
300 mg/kg b.w./p.o./given for 28 days
CFA-induced arthritis Wistar albino rats/i.p./ hemoglobin content, total WBC, RBC, and erythrocyte sedimentation rate were estimated
Extract (63.64%) and indomethacin (62.34%) showed significant decreases in paw swelling on 28th day as compared to control group (P < 0.01)
Bhardwaj et al. (2016)
Leaves
Ethanol (95%)
300 mg/kg b.w./p.o./given daily for 21 days
CFA-induced arthritic male Wistar albino rats/s.c./hemoglobin content, total WBC, RBC, and erythrocyte sedimentation rate were estimated
Extract significantly (P < 0.01) inhibited the development of swelling/extract showed maximum inhibition (66.88%) of paw edema compared to indomethacin (75.42%)/extract presented significant increase in the WBC count, a decrease in RBC count, and hemoglobin content
Bhardwaj et al. (2010)
F. carica
Leaves
Aqueous
500 µg/mL
Bovine serum denaturation/egg albumin denaturation assays/in vitro assays
BSA method - denaturation of protein was increased in dose dependent manner (inhibition 76.9% extract and 86.44% diclofenac sodium)/egg albumin method - denaturation of protein was increased (inhibition 64.64% extract and 76.16% diclofenac sodium; P < 0.01) significantly
Rajesh et al. (2020)
F. erecta
Leaves
Ethanol (70%)
500 mg/kg/p.o./given for 21 days
Monosodium iodoacetate (MIA)-induced osteoarthritis/i.p.
Behavioral score began to reduce from week 1st when compared with MIA-treated arthritis-induced model. The decrease was lower than that of the positive control indomethacin (P < 0.05)
South Korean Patent (2018)
F. exasperata
Leaves
Ethanol (70%)
300 mg/kg b.w./ p.o./ given for 28 days
CFA-induced arthritis/Sprague Dawley rats/i.p.
Extract significantly reduced the total ipsilateral paw edema (inhibition 34.46 ± 11.42%)/extract reduced the maximum arthritic index (67.35%) than dexamethasone (95.91%) and methotrexate (85.71%; P < 0.001), respectively
Abotsi et al. (2010)
F. lacor
Aerial roots
Petroleum ether and ethanol
150 mg/kg b.w./p.o./given for given 21 days
CFA-induced arthritis/Wistar albino rats/i.p.
Both extracts showed statistically significant inhibition of arthritic lesions (
< 0.05) from day 16, (
< 0.01) from day, 20 and (
< 0.001) from day 21 onwards/both extracts showed significant increase in body weight (
< 0.001) as compared to arthritic control group and increase in liver weight (
< 0.01), decrease in liver weight (
< 0.001), and increase in spleen weight (
< 0.001) in arthritis control
Sindhu and Arora (2013)
F. religiosa
Stem bark
Methanol
400 mg/kg b.w./p.o./given for 28 days
CFA-induced arthritic Wistar albino rats/i.p./serum parameter (glutamic oxaloacetic transaminase, serum glutamate-pyruvate transaminase, urea, and creatinine) were estimated
Extract reduced paw volume significantly (0.99 ± 0.12 mL) on day 28 when compared to Piroxicam (0.96 ± 0.32 mL)/higher arthritic score (control 3 + 2 + 1), and the individuals treated with extract have the least arthritic score/ the levels of SGOT (102.8 ± 1.50 U/L), SGPT (42.4 ± 1.24 U/L) were decreased with piroxicam and extract (SGOT 107.5 ± 1.08U/L, SGPT 43.6 ± 1.23 U/L)
Garg et al. (2018)
Leaves
Ethanol
400 mg/kg/p.o./given for 3 weeks
CFA-induced arthritic male Wistar albino rats/ i.d./body weight, arthritic score, paw volume and ankle diameter were estimated
The weight loss was significantly (
< 0.001) reduced by extract in adjuvant induced arthritis/extract attenuated mean arthritic severity score significantly (
< 0.001)/extract significantly (
< 0.001) reduced ankle diameter (
< 0.001)
Rathod et al. (2018)
Anti-stress activity
F. benghalensis
Fruits
Methanol
500 mg/kg/ p.o/given for 21 days
Anoxia stress tolerance test, swimming endurance test/ Wistar albino rats
Anoxia stress tolerance time – extract (51.1 ± 1.4 min) showed antistress activity closer to that of the standard drug (64.5 ± 2.0 min)/swimming endurance test in mice – extract displayed increase in swimming performance time (402.80 ± 6.14 min) when compared with (Withania somnifera) standard (418.56 ± 5.71 min; P < 0.001)
Jahagirdar et al. (2020)
Stem bark
Methanol
228.3 µg/mL concentration
Acetylcholinesterase inhibitory effect against SHSY5Y cell lines/in vitro assay
Showed significant stress-relieving effects (IC50 228.3 µg/mL; P < 0.05)
Vignesh et al. (2019); Murugesu et al. (2021)
Ethyl acetate
50 mg/kg each/p.o.
Milk-induced leucocytosis and Milk-induced eosinophilia/in vivo/ Male Swiss albino mice/s.c.
Extract showed significant protective effect against milk-induced leucocytosis (P < 0.05) compared with control group/extract displayed significant decrease in eosinophil count when (P < 0.05) compared to the control group
Taur et al. (2007)
F. carica
Leaf-derived callus
Aqueous
–
Stress-hormone-induced damage in skin
Extract reduced the trans-epidermal water loss, the sebum production, the desquamation, and facial skin turning to a pale color from acute stress
Dini et al. (2021)
Anticancer/antitumor activity
F. auriculata (syn. F. pomifera)
Leaves
Methanol and chloroform
100 µg/mL
Lung carcinoma A549 cells/in vitro/ cytotoxicity MTT assay
Both extracts did not show any significant cancer cell killing efficacy against P1C, P1M, P2C and P2M
Kumari et al. (2018)
3β-Acetoxyurs-12-ene and 3β-hydroxyurs-12-ene
3 and 15 μg/mL concentration
Human lung cancer cell line A549/in vitro MTT assay
Both compounds displayed significant cytotoxicity against selected cell lines (IC50 15 µg/mL and 3 µg/mL)
Wangkheirakpam et al. (2015)
Fruits
Ethanol (70%)
50 μg/mL
Human lung adenocarcinoma cell line (A549)/in vitro assay/cell cycle phase distribution and cell death analysis
Extract induced significant decrease in G0/G1 phase (P < 0.05) and accumulation of A549 cells in G2/M phase/extract triggered A549 cells to undergo late apoptosis (Annexin V + PI + ) after 48 h of treatment (14.7 ± 3.4%; P < 0.05)
Jamil and Abdul Ghani (2017)
F. benghalensis
Aerial roots
Ethyl acetate
100, 50, 25, 12.5, 6.25, and 3.125 µg/mL
A549 cell line, MDA-MB-231 cell line, Hela cell lines/in vitro MTT assay
Extract showed significant activity against A549 cell line (IC50 17.817 μg/mL)/MDA-MB-231 cell line (IC50 97.8992 μg/mL)/Hela cell line (IC50 49.27276 μg/mL)
Jain and Jegan (2019)
Leaf
Methanol
100 µg/mL concentration
Human cervical cancer cell line/in vitro mitochondrial reduction assay
Extract showed increased cell toxicity/increase in concentration of extract displayed decrease in the rate of cell proliferation
Kumaresan et al. (2018)
Roots
Aqueous
10 mg/mL
Antimitotic activity by mitotic index determination
Extract showed significant mitotic index (22 ± 1.15%) when compared with methotrexate (30 ± 1.35%)
Ahirrao et al. (2020)
F. carica
Leaves
Leaf latex
0.1% concentration
MDA-MB-231 cells/in vitro MTT assay/ genotoxicity and cytotoxicity analysis
Latex showed stress and apoptosis after 24 h/latex showed significant (P < 0.05) decrease in cell viability when compared to control/treated cells demonstrate a range of unhealthy morphological changes like nucleus blebbing, shrinkage, crescent shape
AlGhalban et al. (2021)
Methanol and aqueous
1.0 mg/mL concentration
Human breast adenocarcinoma (MDA-MB-231) cells and mouse fibroblast (L929) cells/ in vitro cell viability assay
Methanol extract significantly suppressed MDA-MB-231 cell proliferation (P < 0.05) in a dose dependent manner (IC50 0.081 mg/mL)/ aqueous extract decreased the cell viability (IC50 > 1 mg/mL; P < 0.05) significantly
Ergül et al. (2019)
Leaves
Methanol
2000 µL/well
Huh7it cells/in vitro/ cancer cell proliferation test/apoptosis and necrosis test
Extract showed significant inhibition (82.78%) of cell growth/extract displayed IC50 > 653 μg/mL/about 0.44% cells experienced total apoptosis at the 2000 μL/well of extract
Purnamasari et al. (2019)
Fruits
Ethanol
1000 μg/mL
Breast cancer (MCF-7) cell lines/in vitro MTT assay
Extract showed significant inhibition (90.5%) at 72 h/also reduced cell viability in time and dose dependent manner
Jasmine et al. (2015)
F. deltoidea
Aerial parts
Chloroform and isovitexin
–
PC-3 cell line, LNCaP clone FGC cell, and human dermal fibroblasts/ cell viability/ apoptosis and cell migration and invasion assays
Extract induced cell death (P < 0.05) via apoptosis as evidenced by nuclear DNA fragmentation accompanied by an increase in MMP depolarization (P < 0.05), activation of caspases 3 and 7 (P < 0.05) in both PC3 and LNCaP cell lines/inhibited both migration and invasion of PC3 cells (P < 0.05)/isovitexin showed an antiproliferative effect (IC50 = 43 µg/mL) against PC-3 cells
Hanafi et al. (2017)
Leaves
Aqueous
500 mg/kg b.w./p.o./ given for 10 weeks
Male Sprague-Dawley rats/in vivo/4-nitroquinoline-1-oxide-induced tongue neoplastic and preneoplastic lesions/p.o./chemo preventive and chemotherapeutic study
Extract significantly decreased the incidence of oral squamous cell carcinoma (100%)/extract reduced the expression of the key tumor marker cyclin D1 but, significantly enhanced the expression of the β-catenin and e-cadherin antibodies which are associated with enhanced cellular adhesion
Al-koshab et al. (2020)
Ethanol (20%)
100 μg/mL concentration
Prostate cancer cell line (DU145)/in vitro MTT assay/ Acridine orange and propidium iodode staining assay/ Annexin V-FITC/PI by flow cytometry assay
Extract showed 54.55 ± 0.36% cell viability when compared to vitexin (90.29 ± 0.35%) and curcumin (89.63 ± 0.09%)/early-stage apoptotic cells-induced by extract statistically higher than the control, paclitaxel was statistically higher than control (P < 0.05)
Soib et al. (2019)
Ethanol
125 μg/mL
Human breast adenocarcinoma (MCF-7) cells/in vitro MTT assay
Extract did not show any anticancer activity against MCF-7 cell
Wei et al. (2011a)
F. elastica
Aerial roots
Methanol
62.5 μg/mL
HeLa cancer cell line/in vitro assay
Extract showed potent anticancer activity (IC50 20 μg/mL) when compared with standard drug emetine (IC50 0.04 μM)
Teinkela et al. (2018)
F. exasperata
Leaves
Hexane
100 μg/mL concentration
A2780 human ovarian carcinoma cell lines/in vitro MTT assay
Extract showed potent inhibitory effects (97.2%) when compared to control (P < 0.05)
Bafor et al. (2017)
Roots and stem barks
Methanol
1200 µg/mL concentration
PC-3 human prostate cancer cell line/ in vitro MTT assay
Both extracts significantly reduced the cell viability (P < 0.05 to P < 0.001)/both extracts suppressed the growth of PC-3 cells by modulating the [Ca2+]i and stimulating apoptosis through Bax/Cytochrome C/Caspase 3–9 signaling pathway
Deeh et al. (2022)
F. hirta
Fruits
Ethyl acetate
–
HeLa cells cell viability assay
Extract showed significant decrease in G1 population of cancerous cells (P < 0.01)
Zeng et al. (2012)
F. hispida
Fruits
Isowigtheone hydrate, 3′-formyl-5,7-dihydroxy-4′-methoxyisoflavone, 5,7-dihydroxy-4′-methoxy-3′-(3-methyl-2-hydroxybuten-3-yl) isoflavone, chlorogenic, and sitosterol 3-O-β-D-glucopyranoside
400 µg/mL concentrations
Human cancer cell lines (HL60, A549, SKBR3, KB, Hela, HT29, and HepG2) and a normal cell (LO2)/in vitro MTT assay
Isowigtheone hydrate, 3′-formyl-5,7-dihydroxy-4′-methoxyisoflavone, 5,7-dihydroxy-4′-methoxy-3′-(3-methyl-2-hydroxybuten-3-yl) isoflavone, chlorogenic acid, and sitosterol 3-O-β-D-glucopyranoside showed potent inhibitory activities on EBV-EA induction (IC50 271 to 340 M ratio 32 pmol−1 TPA; P < 0.05)
Zhang et al. (2018)
F. microcarpa
Leaves
Ethyl acetate and plectranthoic acid
100 μg/mL concentration
Melanoma (A375) and prostate (DU145, PC3, CWRV1 and NB26) cancer cells /in vitro MTT assay
Ethyl acetate extract showed potent antiproliferative effect against tested cells in dose dependent manner/ plectranthoic acid significantly suppressed the viability of DU145, PC3, CWRV1, NB26 and A375 cells (IC50 25.4, 32.2, 41, 53.1 to 77 μM; P < 0.001)
Akhtar et al. (2015)
F. palmata
Stem
Ethanol
100 μg/mL concentration
RAW264.7 cells/ in vitro assay
MTT assay confirmed that there were no significant changes in cell viabilities reported with extract treatments (P < 0.05)
Khajuria et al. (2018)
F. pumila
Leaves
Aqueous- alcoholic (50:50%)
–
CEM, Jurkat, HL-60 and PNT2 cell line/ in vitro MTT assay
Extract showed strong inhibitory effect against the cells (Jurkat cells, IC50 130.97 µg/mL)/extract displayed strong inhibitory activity (HL-60, IC50 56.31 µg/mL)
Larbie et al. (2015)
F. racemosa (syn. F. glomerata)
Fruits
Chloroform
–
Human hepatocellular carcinoma cell line (HepG-2)/in vitro apoptosis assay/ DAPI method
Extract showed greater fluorescence glow in ethidium bromide, acridine orange and DAPI when compared to the IC50 concentration and control of HepG-2 cells (P < 0.05)
Sivakumar et al. (2019)
Ethanol (70%)
80 µg/mL concentration
MCF7 human breast cancer cell line/ in vitro SRB assay
Extract showed significant reduction against cell lines (LC50, TGI and GI50 ≥ 80 µg/mL)
Gavhane et al. (2016)
Leaves
Ethanol
200 μg/mL concentration
Dalton lymphoma ascites cell line/in vitro MTT assay
Extract showed potent cell death (57.37% of cell death; IC50 175 µg/mL)
Khan et al. (2017a)
Methanol
0.005 – 100 μg/mL
Cancer cell lines (HL-60, HepG2, NCI-H23 and HEK-293 T)/in vitro MTT assay
Extract showed cytotoxic activity against HL-60 and HepG2 cell lines (IC50 276.85 and 362.95 μM) but did not show any activity against HEK-293 T and NCI-H23 cell lines
Sukhramani et al. (2013)
Stem bark
Methanol
100 μg/mL concentration
HEK-293 T, NCI-H23, HepG2 and HL-60/in vitro XTT assay
Extract displayed significant cytotoxicity against HepG2 and HL-60 (IC50 321.742 and 287.126 μM) but did not show any activity against HEK-293 T, and NCI-H23 cell lines
Sukhramani and Patel (2013)
F. religiosa
Leaves
Chloroform
1000 µg/mL concentration
Human breast cancer cell (MDA-MB-231) lines/in vitro cytotoxicity SRB assay
Extract showed maximum dead cell percentage (25%)/extract found mild cytotoxic at 1000 µg/mL (CC50 4944.772 µg/mL)
Shaikh et al. (2020)
Hepatoprotective activity
F. auriculata (syn. F. pomifera)
Leaves
Ethanol
100 mg/kg/day/p.o./given for 14 days
Male Wistar albino rats/intrahepatic cholestasis-induced with 17α-ethinyl estradiol/serum liver function test/ 5′-nucleotidase, total bile acids, total cholesterol and phospholipids were assayed
Extract preserved liver functions, total bile acids, total cholesterol, and phospholipids/suppressed the pro-inflammatory cytokines but, increased hepatic regeneration and antioxidant defense system
El-hawary et al. (2019)
Ethanol (70%)
800 mg/kg b.w./p.o.
Adult mice/CCl4-induced hepatotoxicity/ levels of serum aspartate aminotransferase and alanine aminotransferase were estimated
Extract restored the increased levels of serum aspartate aminotransferase and alanine aminotransferase to the normal levels in treated animals (P < 0.01)
El-Fishawy et al. (2011)
Fruit
Methanol
400 mg/kg b.w./given for 28 days/p.o.
Wistar albino male rats/CCl4-induced hepatotoxicity/ SGPT, SGOT, ALP and bilirubin levels estimated
Extract caused significant reduction in SGPT, SGOT, ALP and bilirubin levels/also showed moderate improvement with mild vacuolization of hepatocytes (P < 0.05)
Tamta et al. (2021)
F. benghalensis
Stem bark
Methanol
250 mg/kg b.w./p.o./ given a daily for seven days
Wistar albino rats/CCl4-induced hepatotoxicity/SGPT/SGOT, alkaline phosphatase, ALP were estimated
Extract significantly (P < 0.001) decreased the levels of enzymes (SGOT, SGPT, ALP and bilirubin total and direct levels)/when compared to silymarin (P < 0.05)
Baheti and Goyal (2011)
Fruits
Ethanol
500 mg/kg b.w./p.o./given for 7 days
Wistar albino rats/CCl4-induced hepatotoxicity
Extract showed strong activity in terms of the restoration of reduced enzyme level as compared to the standard drug silymarin, which also restored the altered level of catalase enzyme (P < 0.01)
Karmakar et al. (2020)
Leaves
Ethanol
400 mg/kg/p.o./given for 7 days
Male Wistar albino rats/CCl4 and ethanol-induced hepatotoxicity/ AST, ALT, total protein, and total albumin were determined
Extract ameliorated the effects of hepatotoxins and significantly (P < 0.05) reduced the elevated levels of the biochemical marker enzymes in treated animals
Shinde et al. (2012)
F. benjamina
Leaves
Aqueous
500 mg/ml b.w./p.o./given daily for 7 days
Male Balb/c mice/ethanol-induced hepatotoxicity/p.o./ alanine aminotransferase levels estimated/ distortion of the liver architecture, presence of foci of necrosis and presence of mild to moderate steatosis was also studied
Alanine aminotransferase showed significant hepatic damage to the negative control group (mean = 196.88 U/L) as compared to the positive control group (mean = 42.58 U/L) and the treated group (mean = 52.40 U/L) (P < 0.05)/A mild distortion of liver parenchymal architecture was observed in extract-treated mice while a moderate distortion of liver parenchymal architecture was observed in untreated mice
Pilapil et al. (2017)
Ethanol
500 mg/kg b.w./p.o.
Wistar albino rats/CCl4-induced hepatotoxicity/i.p./SGOT, SGPT, and serum alkaline phosphatase were estimated
Extract showed significant decrease in the increased levels of SGPT, SGOT, ALP, TBL and comparable with the standard silymarin hepatoprotective drug/extract restored the altered level of enzymes significantly (P < 0.01).
Kanaujia et al. (2011)
F. carica
Leaves
Petroleum ether
200 mg/100 g/p.o./given for 10 days
Wistar albino rats with rifampicin-induced hepatic damage/SGOT, SGPT determined by the Reitman and Frankel method/ bilirubin by the Malloy and Evelyn method
A significant decrease was observed in SGPT, SGOT levels in the animals of treated group/a significant decrease in liver weight was also observed (P < 0.05)
Gond and Khadabadi (2008)
Methanol
500 mg/kg b.w./p.o.
Wistar albino rats/CCl4-induced hepatotoxicity/ serum ASP, ALT, total serum bilirubin, and malondialdehyde equivalent, and an index of lipid peroxidation were estimated
Extract significantly (P < 0.001) lowered the serum enzyme levels. ALT, AST, total bilirubin serum enzyme levels in treated animals were like the normal control values/ Malondialdehyde levels in the extract treated animals were significantly lower (P < 0.001) than those of toxic control values
Krishna Mohan et al. (2007)
Ethyl acetate
400 mg/kg b.w./p.o./given for 7 days
Male albino mice/CCl4-induced hepatotoxicity/ alanine transaminase, aspartate aminotransferase, alkaline phosphatase and total bilirubin were estimated
Extract showed significant decrease (P < 0.05) in the serum ALT, ASP, alkaline phosphatase, and total bilirubin levels almost like those in the silymarin treated animals
Hira et al. (2021)
Ethanol (80%)
200 mg/kg b.w./p.o./given for 5 days
Male albino mice/CCl4-induced hepatotoxicity/ ALT and ASP levels were estimated
Extract increased significant protection against CCl4- induced hepatic damage (P < 0.05) in treated animals
Aghel et al. (2011)
Ethanol
200 mg/kg b.w./p.o./given daily for 4 days
Male albino rats/ CCl4-induced hepatotoxicity/ SGOT, SGPT, and total bilirubin were estimated
Extract showed significant hepatoprotection in carbon tetrachloride intoxicated rats (P < 0.05)
Mujeeb et al. (2011)
Stem
Aqueous
–
Male Wistar albino rats/methanol-induced hepatotoxicity/i.p./administered for 30 days/ ALT, AST, ALP, and LDH and hepatic lipid peroxidation were estimated
Extract reverts the enzyme activities near to normal status in the treated animals (P < 0.001)
Saoudi and El Feki (2012)
F. hirta
Leaves
Aqueous
300 mg/kg b.w./p.o./given for 5 days
C57BL/6 mice and ICR mice/N, N-dimethylformamide induced acute liver injury/i.p./ ALT, AST and LDH and the pathological changes were determined
Extract significantly reduced the elevated levels of ALT, AST and LDH liver injury (P < 0.05)
Lv et al. (2008)
Roots
Aqueous
300 mg/kg b.w./p.o./given for 5 days
Male ICR mice/cocaine-induced hepatotoxicity/serum ALT, AST activity and the activity of CAT in liver homogenate were estimated
The serum transferase and catalase levels in liver homogenate were reduced significantly (P < 0.01)
Cai et al. (2007)
F. hispida
Leaves
Methanol
400 mg/kg b.w./p.o./given daily for 7 days
Male albino mice/paracetamol-induced hepatotoxicity/p.o./ serum levels of transaminase, bilirubin and alkaline phosphatase were estimated
Extract exhibited a significant protective effect by reducing the serum levels of transaminase (SGOT and SGPT), bilirubin and alkaline phosphatase (P < 0.01)
Mandel et al. (2000)
F. lacor
Stem bark
Ethanol (90%)
50 mg/kg b.w./p.o./given for 5 days
Adult albino rats/CCl4-induced hepatotoxicity/SGOT, SGPT and total bilirubin were determined
Extract showed significant levels of protection against hepatotoxicity (P < 0.05) and reduced the levels of enzyme parameters
Tripathi and Patel (2007)
F. lyrata
Leaves
Butanol (95%)
100 mg/kg b.w./p.o./given for 14 days
Male Wistar albino rats/CCl4-induced hepatotoxicity/ AST, ALT, ALP, and GGT levels were estimated
Extract significantly decreased the elevated levels of AST, ALT, ALP, GGT, and total protein levels when compared to the CCl4 group (P ≤ 0.05)
Awad et al. (2019)
F. microcarpa
Stem bark
Ethyl acetate
200 mg/kg/p.o./given for 21 days
Male Wistar albino rats/CCl4- and paracetamol-induced hepatotoxicities/ AST, ALT, and ALP levels were estimated
Extract reduced the elevated levels of AST, ALT and ALP enzymes and showed their protective effects in treated animals (P < 0.01)
Kalaskar and Surana (2011)
F. mollis
Leaves
Petroleum ether
250 mg/kg b.w./p.o./given for 14 days
Wistar albino rats/ CCl4-induced hepatic damage/ ALT, AST, ALP, bilirubin, and total protein levels were measured
Extract showed significant liver protection against the toxicant as evident by the presence of normal hepatic cords, absence of necrosis and lesser fatty infiltration (P < 0.01)
Rama Devi et al. (2010)
F. palmata
Leaves
Ethanol (95%)
400 mg/kg b.w./p.o./given for 7 days
Wistar albino rats/CCl4-induced hepatotoxicity/ ASP, ALT, γ- glutamyl transpeptidase, alkaline phosphatase and total bilirubin were estimated
Extract showed a significant (P < 0.001) reduction in the levels of AST, ALT, GGT, ALP and bilirubin indicating good protection against liver damage
Alqasoumi et al. (2014)
F. pumila
Leaves
Aqueous-ethanol (50:50)
100 mg/kg b.w./p.o./given for 7 days
Sprague-Dawley rats/CCl4-induced hepatotoxicity/ ALT, ALP, GGT and total bilirubin were estimated
CCl4 produced a 2-fold increase in the levels of ALT, ALP, GGT and total bilirubin and a 1.5-fold increase in AST and direct bilirubin levels/extract restored these increases to near normal levels (P < 0.05)
Larbie et al. (2016)
F. racemosa (syn. F. glomerata)
Stem bark
Methanol
500 mg/kg b.w./p.o./given for 7 days
Male Wistar rats/CCl4-induced hepatotoxicity/ ALT, ASP and alkaline phosphatase activities were determined
Extract attenuated the increase of AST, ALT and ALP activities and showed their protective effect against CCl4-induced hepatotoxicity (P < 0.05)/extract exhibited maximum suppression of AST, ALT, and ALP activities nearer to the normal levels (P < 0.001)
Ahmed and Urooj (2010b)
500 mg/kg b.w./p.o./given daily for 7 days
Wistar albino rats/ CCl4-induced hepatotoxicity/ serum levels of transaminase, bilirubin and alkaline phosphatase were estimated
Extract showed a significant reversal of the serum enzyme changes towards the normal when compared to control values (P < 0.05)
Channabasavaraj et al. (2008)
F. religiosa
Leaves
Methanol
300 mg/kg b.w./ p.o./given for once daily for 7 days
Male Wistar albino rats/isoniazid and rifampicin-induced hepatotoxicity/i.p./ ALP, ALT, AST, total protein, and bilirubin levels were estimated
Extract significantly stopped isoniazid-rifampicin and paracetamol-induced increase in the levels of serum diagnostic liver marker enzymes and TBARS level/total protein and reduced glutathione levels were significantly (P < 0.001) increased
Parameswari et al. (2013)
Ethanol
200 mg/kg b.w./p.o./given for 7 days
Male Wistar rats/CCl4 and paracetamol- induced hepatotoxicity/ ASP, and ALT were estimated
Extract prevented the paracetamol-induced rise in serum enzymes. The hepatotoxic dose of carbon tetrachloride (1.5 mL/kg; p.o.) also raised the serum AST and ALT levels/ extract also prevented the CCl4-induced rise in serum enzymes (P < 0.05)
Selvan and Chourasia (2017)
Stem bark
Methanol
200 mg/kg/p.o./given for 10 days
Male albino Wistar rats/paracetamol- induced hepatotoxicity/ ALT, ASP, alkaline phosphatase, and total bilirubin were estimated
Extract showed significant hepatoprotective activity by reducing the elevated levels of SGOT, SGPT, ALP, and total bilirubin (P < 0.01)
Suryawanshi et al. (2011)
Latex
Methanol
300 mg/kg b.w./p.o./given for 10 days
Male Wistar albino rat/cisplatin induced liver injury/ ALT, ALP and AST and lipid peroxidation, GSH, and SOD were estimated
Extract normalized the levels of serum ALT, ALP and AST and lipid peroxidation, GSH, SOD in the liver of treated animals (P < 0.05)
Yadav (2015)
F. retusa
Leaves
Ethyl acetate
400 mg/kg b.w./p.o./given for 7 days
Wistar albino rats/ CCl4-induced hepatotoxicity/ SGOT, SGPT, alkaline phosphatase and total bilirubin were estimated
Extract showed significant decrease in SGOT, SGPT, SALKP and total bilirubin levels when compared to control (P < 0.01)
Jaya Raju and Sreekanth (2011)
F. semicordata
Leaves
Aqueous
200 µg/mL/in vitro/
HepG2 cell line/ D-galactosamine induced toxicity
Extract showed significant protection against toxicity as compared to D-galactosamine control (P < 0.05)
Gupta et al. (2020)
Neuroprotective and neuroregenerative activity
F. benghalensis
Stem bark
Aqueous
1.0 mg/mL media for 24 h
SK-N-SH cell line/ hydrogen peroxide- induced DNA damage/in vitro neutral comet assay
Extract showed a significant reduction in the intensity of DNA damage in terms of comet tail length and brought to control level (P < 0.05)
Ramakrishna et al. (2014)
Leaves
Methanol
10 μL concentration
Acetylcholinesterase inhibition in vitro assay/ 50% enzyme inhibition was determined
Extract was found most potent acetylcholine esterase inhibitor (IC50 = 194.6 ± 7.961 μg/mL) that is close to that of donepezil (IC50 = 186.1 ± 7.1 μg/mL)
Hassan et al. (2020)
F. deltoidea
Fruits
Aqueous
1 mg/mL concentration
Human neuroblastoma SH-SY5Y cell lines/ hydrogen peroxide toxicity test (IC50 concentration) of cells was determined
Extract showed strong neuroprotective effect (P < 0.05; 80–160% of cell viability)
Dzolin et al. (2012)
F. erecta
Leaves and branches
Aqueous-ethanol
100 mg/kg b.w./p.o./given for 20 days
Male C57BL6 mice/amyloid-β aggregates were injected at intracerebroventricular area/passive avoidance task was performed
Extract significantly suppressed the inflammatory cytokines such as interleukin 1β and tumor necrosis factor-α, and expression of ionized calcium-binding adaptor molecule 1, a marker of microglial activation, in brain tissues of Aβ-injected mice, suggesting anti-neuroinflammatory effects (P < 0.001)
Sohn et al. (2021)
F. racemosa (syn. F. glomerata)
Stem bark
Aqueous
500 mg/kg b.w./p.o./given for 28 days
Male Wistar albino rats/anticholinesterase levels were determined
Extract significantly increased (P ≤ 0.05) Ach levels in hippocampi of rats (38%)
Ahmed et al. (2011)
F. religiosa
Leaves
Petroleum ether
400 mg/kg b.w./p.o./given for 7 days
Male Wistar albino rats/ 3-nitropropionic acid-induced Huntington’s disease/i.p.
Extract significantly improved motor and cognitive performance and significantly attenuated oxidative damage (P < 0.001)
Bhangale et al. (2015)
Radioprotective activity
F. racemosa (syn. F. glomerata)
Stem bark
Ethanol
200 µg b.w./p.o./given once a daily for 15 days
Swiss albino mice/radiation dose rate of 72 Gy/min/p.o./comet assay
Extract significantly reduced the radiation effects induced DNA damage (P < 0.001) in treated animals
Vinutha et al. (2015)
150 mg/mL
V79 cells exposed to γ-irradiation at a dose of 1.0 Gy/min/in vitro assay with cytokinesis-block and proliferation index
Extract resulted in a significant (P < 0.05 and P < 0.001) decrease in the percentage of micronucleated binucleate cells
Veerapur et al. (2009)
Wound healing
F. benghalensis
Stem bark
Aqueous
200 mg/kg/ day /p.o./given for 10 days
Female Wistar albino rats/excision and incision wound models
Excision wound model - extract showed a significant reduction in the wound area (P < 0.001) and epithelialization period/ extract showed healing in 18.33 days as compared with 21.50 days of control/ incision wound model - a significant increase in the wound-breaking strength recorded (P < 0.001)
Garg and Paliwal (2011)
Leaves
Ethanol
200 mg/kg b.w./p.o.
Wistar albino rats/ excision wound and inclusion models
Extract showed significant wound-healing activity by decreasing the period of epithelialization and increasing in the rate of wound contraction (P < 0.05)
Imran et al. (2021)
Aqueous
Extract ointment 100 mg/g concentration
Wistar albino rats/ excision wound model/topically applied
Extract increased wound closure and completed in 12 days (activity 96%; P < 0.05)
Mothilal et al. (2020)
Roots
Aqueous and ethanol
–
Wistar albino rats/excision, incision wound, and dead space wound models/ topically applied
Incision wounds model – breaking strength was increased significantly (502.30 ± 2.26%) when compared with control (305.20 ± 5.15%; P < 0.05)/ extract showed significant wound contraction and achieved 100% with the wound closure time of 14.17 days (P < 0.05)
Murti et al. (2011)
F. carica
Leaves
Methanol
Ointment (5% extract)
Wistar albino rats/ excision wound model/applied topically once a day
The wounds were completely healed in treated group (epithelization period − 14 ± 2 days in treated whereas 24 ± 2 days in the control animals (P < 0.001)
Begum et al. (2013)
F. deltoidea
Leaves
Aqueous
50 μg/mL
Human skin fibroblast (HSF 1184) cell/scratch in vitro assay
Extract reduced the wound area significantly (6 h treatment; 5.96%; P < 0.05)
Mustaffa et al. (2015)
Whole plant
Aqueous
Ointment (10%; extract)
Male Sprague Dawley rats/ experimentally wounded in the posterior neck area/ topically applied
Extract showed lesser scar width at the wound enclosure and more fibroblast proliferation in the granulation tissue greater than blank placebo-treated wounds (P < 0.05) and intrasite gel (positive control)
Abdulla et al. (2010)
F. exasperata
Leaves
Aqueous
Ointment (base of the extract)
Adult albino rats/excision wounds model/ topically applied
Significant wound contraction was observed in ointment treated animals (25%; P < 0.05) /contraction like that of cicatrin powder (standard) on the11th day
Umeh et al. (2014)
F. hispida
Leaves
Methanol
150 mg/kg/b.w.
Albino Wistar rats/excision wound model/ topical application
Significant increase in wound healing reported on 20th day successively in treated animals (P < 0.05)
Singh et al. (2014)
Roots
Ethanol (95%)
150 mg/kg/b.w./p.o./given for 10 days
Wistar albino rats/excision, incision, and dead space wound models
Extract showed maximum breaking strength compared to control group. The rate of epithelialization and wound contraction in excision model was better as compared to control groups (P < 0.05).
Murti et al. (2011a, b)
F. racemosa (syn. F. glomerata)
Roots
Aqueous
Extract ointment (10%)/ applied topically
Wistar albino rats/ incision and excision wound models/wound closure, epithelization time and scar area on complete epithelization were measured
Incision model - extract increased breaking strength (394.70 ± 6.61) when compared to povidone iodine (352.00 ± 2.83; P < 0.05)/ extract showed 100% contraction which was almost better than that of the povidone iodine (17 days)
Murti and Kumar (2012)
Stem bark
Lupeol and β-sitosterol
35 μM concentration
BHK 21 (ATCC, CCL-10) and MDCK (ATCC, PTA 6502) cell lines/in vitro scratch wound assay
Both compounds showed significant wound healing by cell migration enhancement activity on BHK 21 and MDCK cell lines (>80%) in par with the asiaticoside (25 μM; P < 0.001)
Bopage et al. (2018)
Leaves
Methanol
Extract ointment (10%)
Wistar albino rats/ excision and incision wound models/topically applied
Excision model - extract showed significant (P < 0.01) increase in wound contraction (5%age)/incision wound model – extract displayed significant (P < 0.01) increase in breaking strength
Londhe et al. (2013)
F. religiosa
Stem bark
Ethanol
Topical gel (10%)
In vitro wound healing model /albino Wistar rats/excision wound model
Extract increased the RBC membrane stabilization activity in in vivo and in vitro wound healing models (P < 0.001)
Raisagar et al. (2019)
Leaves
Ethanol (70%)
Extract ointment
Wistar albino rats/excision and incision wound models
Excision model - extract showed faster epithelialization of wound (18 ± 0.60%) when compared with povidine iodine ointment treated animals (P < 0.001 vs control)/incision model - showed increase in breaking strength (562.2 ± 6.93 g)
Roy et al. (2009); Verma and Kumar (2021)
Aqueous
Ointment (50 mg of simple ointment base)
Male Sprague Dawley rats/ incision wound, excision wound and dead space wound models/ topical application
Incision model - extract showed significant tensile strength (76.31%) when compared with povidone iodine (94.36%; P < 0.05)/rapid wound closure in standard and extract treated groups was observed between 4 and 12 days/maximum percentage inhibition (%) of wet and dry granuloma were reported
Chowdhary et al. (2014)
Methanol (70%)
10% emulsifying ointment
Sprague Dawley rats/excision wound, incision wound and burn wound models/ topically applied once a day
Excision wound and the burn wound models-extract showed significant decrease in the period of epithelization and in wound contraction (50%; P < 0.001)/a significant increase in the breaking strength was observed in the incision wound model (P < 0.001)
Nayeem et al. (2008); Putra et al. (2020)
F. retusa
Aerial parts
Ethanol
Extract ointment (5%)
Wistar albino rats/ excision wound, and incision wound model/topically applied
Excision wound model - extract showed significant decrease in wound area on day 21 (5%) when compared with framycetin (20%; P < 0.05)/incision wound model – increase in tensile strength reveals better wound healing induced by the applied ointment
Asija and Pareek (2014)
F. sarmentosa
Stem bark
Ethanol
200 mg/kg b.w/p.o./given daily for 10 days
Wistar albino rats/ excision and incision wound models/base ointment applied topically
Excision wound model - showed a significant reduction in the wound area (P < 0.001) and epithelialization period (17.16 days)/incision wound model – extract showed significant increase in the wound-breaking strength (P < 0.001)
Dimri et al. (2018)
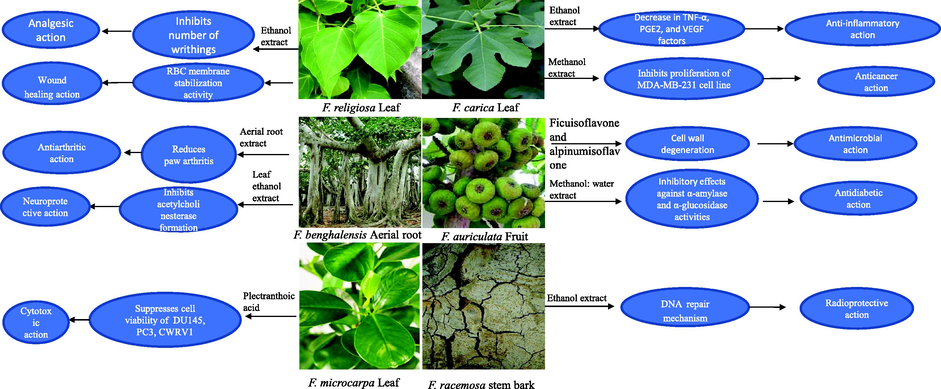
Different pharmacological actions of different parts of some important Indian Ficus species.
3.3.1 Analgesic activity
Pain is a nonspecific expression of various diseases in humans. The non-steroidal anti-inflammatory molecules and opiates have been used traditionally in these conditions, but several adverse effects arise with these drugs such as gastrointestinal disorders, renal injury, and respiratory problems (Domaj et al., 1999; Farshchi et al., 2009). Nowadays, the researchers are showing their interests in searching of novel analgesic compounds from medicinal plants with possibly fewer adverse effects. Aqueous extract (400 mg/kg, p.o.) of F. bengalensis, in the early (0–5 min) and late phases (25–30 min) of pain, showed significant reduction in the duration of licking responses in formalin-induced pain model. The responses were compared to morphine-treated animals (P < 0.001 as compared to the control; Rajdev et al., 2018). The hot aqueous extract (500, 1000 and 2000 mg/kg) of F. carica fruits did not show any significant difference between control and treated animals (P greater than 0.05), but a significant variability reported in between the petroleum ether extract (1000 mg/kg) and the dimethyl sulfoxide treated animals (P < 0.05; Mirghazanfari et al., 2019). Ethanol extract of F. religiosa leaves (400 mg/kg b.w.) showed significant increase in latency time (70.81 %; P < 0.05) in Eddy’s hot plate model when compared to control. Leaf extract (400 mg/kg b.w.) suppresses the number of writhings (68.47 %), induced by acetic acid, when compared to diclofenac (68.47 %; P < 0.05; Marasini et al., 2020). Ethanol extract of F. iteophylla leaves (200 mg/kg) decreases the number of acetic acid-induced abdominal constriction (3.0 ± 0.82) when compared to ketopfofen (reference drug; 10 mg/kg; 4.30 ± 1.28; P < 0.05; Abdulmalik et al., 2011).
3.3.2 Anti-inflammatory activity
Ethanol extract of F. carica leaves (600 mg/kg b.w.) demonstrated potent anti-inflammatory activity in acute (75.90%) and chronic (71.66%) inflammations when compared to indomethacin (P < 0.001; Patil and Patil, 2011). Aqueous extract of F. benjamina leaves (264 mg/kg b.w.) exhibits higher anti-inflammatory effect (39.71%) than the negative control in experimental animals (70.12%; P < 0.05 Bunga and Fernandez, 2021). F. carica leaf extract decreases the formation of TNFα, PGE2, and VEGF in treated models ( < 0.001; Eteraf-Oskouei et al., 2015). Ethanol extract of F. hispida stem bark (400 mg/kg) showed significant inhibition (60.12%) to histamine-induced paw oedema when compared to indomethacin (69.64%; P < 0.01; Howlader et al., 2017).
3.3.3 Antimicrobial activity
The microbial drug resistance to widely used antimicrobial drugs has increased the universality of microbial infections and their related problems (Ginovyan et al., 2017). Methanol extract (60 µg/disc) of F. auriculata fruits demonstrates strong antimicrobial effect against S. epidermidies (28 mm), and M. genetalium (MTCC 2288; 28 mm; Raja et al., 2021). Two compounds (ficuisoflavone and alpinumisoflavone) from F. auriculata fruits exhibit potent antibacterial effect against pathogenic bacteria (S. aureus, K. pneumoniae, B. cereus, N. gonorrhoeae, and P. aeruginosa; MIC 1.25 to 20 μg/ml; Shao et al., 2022). Four isoflavones (5,7,4′-trihydroxy-3′-hydroxymethylisoflavone, 3′-formyl-5,4′-dihydroxy-7-methoxyisoflavone, ficuisoflavone and alpinumisoflavone) from F. auriculata roots displays strong antibacterial activity against S. pneumoniae, S. pyogenes, S. typhi, S. dysenteriae, E. coli and V. cholerae (MIC from 1.30 to 39.93 μM; Qi et al., 2018). Methanol extract F. religiosa leaves (50 μL/well concentration) exhibited greater activity than aqueous extract against the tested microorganisms (S. aureus, E. coli, P. aeruginosa, S. typhi, A. niger and Penicillium notatum; Pathania et al., 2021). The n-hexane extract of F. vogelii leaves demonstrates potent antibacterial effect against E. coli and S. typhimurium (MIC 12.5 μg/mL; Uche Stephen, 2020).
3.3.4 Antioxidant activity
Oxidative stress is known as a main reason for the occurrence and continuance of various diseases (Singh et al., 2021). Plants are considered as a rich source of exogenous antioxidants (Sies, 1997; Singh and Sharma, 2020). Ethanol extract of F. racemosa fruits showed strong antioxidant activity on ABTS (EC50 226.0 ± 1.77 µg/mL), FRAP (EC50 234.8 ± 1.72 µg/mL), DPPH (EC50 28.4 ± 0.50 µg/mL) radical scavenging, hydrogen peroxide radical scavenging (EC50 376.7 ± 2.05 µg/mL), hydroxyl radical scavenging (EC50 427.2 ± 3.06 µg/mL), chelating power (EC50 176.6 ± 3.00 µg/mL), and reducing power (EC50 356.3 ± 4.75 µg/mL) assays (Tamuly et al., 2015). The aqueous-ethanol (50:50) extract of F. auriculata branches demonstrates inhibition to DPPH radical (IC50 190.57 ± 4.25 μg/ml) when compared to gallic acid (standard, 21.66 ± 0.19 μg/mL; Bertoletti et al., 2020). Methanol extract of F. deltoidea leaves displayed diverse levels of potential to DPPH (IC50 288.04 μg/mL; P < 0.001; compared to quercetin) radical and reducing power (IC50 0.02–0.24 μg/mL; compared to ascorbic acid P < 0.001) assays (Mohd Dom et al., 2020). The aqueous extract (5.0 mg/mL) of F. asperifolia leaves possesses strong DPPH scavenging effects (78.65 ± 1.15%; P < 0.05; Ojo and Akintayo, 2014).
3.3.5 Anti-diabetic activity
Diabetes mellitus, a metabolic disorder of carbohydrate, has caused the large number morbidity and mortality in people (Patel et al., 2011). Methanol fraction (500 μg/mL) of methanol: water (4:1) extract of F. auriculata fruits demonstrated strong suppressive effects against α-amylase (91.45%; IC50 161.73 ± 0.43 μg/mL) and α-glucosidase (97.75%; IC50 103.43 ± 0.67 μg/mL) activities when compared with acarbose (IC50 155.08 ± 1.75 and 95.63 ± 1.71 μg/mL; Anjum and Tripathi, 2019). Cycloartenol + 24-methylenecycloartanol (1 mg/kg), from F. auriculata fruits, displayed significant antidiabetic activity in the high fat diet-streptozotocin stimulated type II diabetic rats. Cycloartenol + 24-methylenecycloartanol increased cell viability in the RIN-5F cells (in vitro) and showed a significant protection to β cells from glucose toxicity. Cycloartenol + 24-methylenecycloartanol presented a significant increase of insulin release from the β cells in both in vivo and in vitro studies (Nair et al., 2020).
3.3.6 Anti-arthritic activity
Arthritis and its associated disorders are characterized by swelling, pain, and stiffness of the synovial joints (Tiwari et al., 2021). The exact reasons of arthritis and its associated disorders are not known, but these are strongly associated to the autoimmune responses generated by many genetic and external factors (Alamgeer, 2017). Significant levels of terpenoids (28 mg/g), saponin (26 mg/g), flavonoids (97 mg/g) and phenol (110 mg/g) have been reported in ethanol extract of F. benghalensis stem bark. The ethanolic extract (400 μg/mL) showed significant inhibition (83.80 ± 5.16%) to egg albumin denaturation assay when compared to diclofenac sodium (91.45 ± 6.84%; Mathavi and Nethaj, 2019). Ethanolic (0.01 g) and petroleum ether (0.01 g) extracts of F. benghalensis aerial roots and Carbopol 934 (1%, w/w), glycerine (2.1 mL), Carbopol 940 (1%, w/w), and liquid paraffin (4.5 mL) were mixed to prepare Emulgel. The formulated Emulgel showed a significant homogeneity, lower levels of skin irritation and great stability. The formulated Emulgel, when compared to diclofenac, confirms the anti-arthritic activity through in vitro release assay (Sonali et al., 2021).
3.3.7 Anti-stress activity
Stress is a general physiological response of the body focussed on available resources and minimizing the influence on the body of pessimistic aspects (Seyle, 1973; Doreddula et al., 2014). Stress is linked to pathological processes of hypertension, peptic ulcer, immunosuppression, and reproductive complications (Piato et al., 2008; Ahmed et al., 2011b). Methanol extract of F. benghalensis fruits showed acetylcholinesterase inhibitory effect in SHSY5Y cells lines (IC50 228.3 μg/mL; Vignesh et al., 2019). The methanol extract (500 mg/kg) also displayed dose and duration dependent significant delay in clonic convulsions (51.1 ± 1.4) on anoxia stress tolerance time in mice when compared to positive control (Withania somnifera, 100 mg/kg, p.o.; 64.5 ± 2.0; P < 0.001; Jahagirdar et al., 2020).
3.3.8 Anticancer/antitumor activity
Cancer is a one of the important causes of morbidity and mortality in humans. The proliferation of this disease is growing rapidly in the population of Central and South America, Africa, and Asia (Nguyen et al., 2020). The ethyl acetate extract of F. benghalensis aerial roots exhibited strong anticancer effects against A549 cell line (IC50 13.027 μg/mL). The values were compared to that of doxorubicin (IC50 13.0463 μg/mL). Similarly, the extract was also found effective against MDA-MB-231 cell line (IC50 70.089 μg/mL) and results were compared to the doxorubicin (IC50 59.2523 μg/mL; Jain and Jegan, 2019). Methanol extract F. carica leaves significantly suppressed the proliferation of MDA-MB-231 cell line (P < 0.05) in dose dependent manner (IC50 0.081 mg/mL; Ergül et al., 2019). Chloroform extracts of F. deltoidea var. angustifolia and F. deltoidea var. deltoidea leaves showed cytotoxic effects against the prostate cancer cell lines (IC50 23 and 29 µg/mL for PC3 and IC50 19 and 23 µg/mL for LNCaP; Hanafi et al. 2017). The plectranthoic acid, from ethyl acetate extract of F. macrocarpa leaves, significantly suppressed the viability of DU145, PC3, CWRV1, NB26 and A375 cell lines (IC50 25.4, 32.2, 41, 53.1 to 77 μM; Akhtar et al., 2015).
3.3.9 Neuroprotective activity
Neurodegenerative disorders cause slow neuronal death that led to the loss of cognitive functions and sensory dysfunctions (Mattson et al., 2004). Nowadays, these disorders are linked to various multifactorial pathologies, social, and financial issues (Adewusi et al., 2010; Saxena and Caroni, 2011). Methanolic extract of F. benghalensis leaves showed potent inhibitory effect to acetylcholine esterase activity (IC50 = 194.6 ± 7.961 μg/mL) when compared to donepezil (IC50 = 186.1 ± 7.1 μg/mL; Hassan et al., 2020). Ethanol extract of F. erecta leaves significantly reduced neuronal loss and neuronal nuclei expression in the brain tissues of Aβ injected mice. Extract significantly changed the Aβ-induced inhibition of cAMP response element-binding protein phosphorylation and the expression of brain-derived neurotrophic factor, showing mechanism of neuroprotection. Extract significantly suppressed the formation of interleukin-1β and tumour necrosis factor-α, and the ionized calcium-binding adaptor molecule 1 expression in brain tissues of Aβ-injected mice, proposing anti-neuroinflammatory actions (Sohn et al., 2021).
3.3.10 Radioprotective activity
Radiations can cause mutagenic alterations, and lead to the formation of cancers. Plants that could defend the body from radiation effects would be of great interest (Mamedov et al., 2011). The radioprotective effect of ethanol extract of F. racemosa stem bark was tested on electron beam radiation induced-DNA damage. The extract (50 µg) displayed significant inhibition on radiation induced-DNA damage when compared to control (P < 0.001; Vinutha et al., 2015). Ethanol extract of F. racemosa (20 μg/mL) showed a significant radioprotection (P < 0.01) to 4 Gy γ-irradiation when compared to the radiation controls. The cytokinesis-block proliferative index revealed that extract does not change radiation stimulated cell cycle delay (Veerapur et al., 2009).
3.3.11 Wound healing activity
Wounds are defined as physical, chemical, or thermal damages that result in an opening or breaking of skin integrity or the damage of anatomical and functional integrity of living tissues (Meenakshi et al., 2006). Ethanolic extract of F. benghalensis leaves (200 mg/kg, p.o.) showed significant wound-healing effects by reducing the period of epithelialization as well as enhancing the rate of wound contraction (P < 0.05; Imran et al., 2021). Methanolic extract of F. carica leaves demonstrated significant wound healing activity in the excision wound model. Extract significantly reduced the wound closure time but increased wound contraction percentage (10%, w/w) in treated animals. The wound was totally healed in treated animals within 14 ± 2 days. Healing was compared to negative (24 ± 2 days) as well as positive (12 ± 2 days) controls (Begum et al., 2013). Ethanolic extract (2.0 mg/mL) of F. religiosa displayed strong RBC membrane stabilization activity (90.84%, in vitro assay). Ethanol extract presented the significant decrease in wound size on day 20th when compared to the control (Raisagar et al., 2019).
4 Discussion
Plant-derived medicines are used in the treatment and/or management of various diseases. The large population of developing countries (70–90%) is still relies on plants and plant-derived medicines (Benzie and Watchel-Galor, 2011; Rahman et al., 2022). Ficus bark, root, leaves, fruits, and latex are frequently used in the treatment of various illnesses (Sirisha et al., 2010). Ethanol extract of F. religiosa leaves significantly inhibits the number of writhings, when compared to diclofenac (Marasini et al., 2020). Ethanol extract of F. iteophylla leaves reduces the number of acetic acid-induced abdominal constriction greater than ketopfofen (Abdulmalik et al. 2011). F. carica leaf extract significantly decreases the formation of TNFα, PGE2, and VEGF in rat air pouch model (Eteraf-Oskouei et al., 2015), which shows its strong anti-inflammatory effects. The ethanol extract of F. hispida stem bark exhibits suppression in histamine-induced paw oedema which also displays their anti-inflammatory potential (Howlader et al. 2017). Procyanidin B2, isolated from F. tikoua leaves, up-regulates the expression of p62 and exert a positive loop between Nrf2 and p62 for the treatment of inflammation (Lu et al., 2018; Ma et al., 2021; Chen et al., 2022). Flavanonols {(2R, 3R)-(+)-dihydroquercetin, and aromadendrin} have been reported from F. tikoua leaves (Zhou et al., 2022). Aromadendrin regulates the formation of IL-2 and IFNγ in Jurkat T cells (in vitro). It inhibits the expression of surface molecules (CD69, CD25, and CD40L) as well as the ATP hydrolysis of nuclear factor of activated T cells (Lee and Jeong, 2020). Methanol extract of F. auriculata fruits displays strong antimicrobial effect against M. genetalium and S. epidermidies (60 µg/disc concentration; Raja et al., 2021). The ficuisoflavone and alpinumisoflavone, from F. auriculata fruits, demonstrate strong antibacterial effects against pathogenic bacterial species (S. aureus, K. pneumoniae, B. cereus, N. gonorrhoeae, and P. aeruginosa; MIC 1.25 to 20 μg/ml; Shao et al., 2022). The n-hexane extract of F. vogelii leaves exhibits potent antibacterial activity against E. coli and S. typhimurium (MIC 12.5 μg/mL; Uche Stephen, 2020). Ethanol extract of F. racemosa fruits demonstrates strong antioxidant effects on ABTS, FRAP, DPPH radical scavenging, hydrogen peroxide radical scavenging, hydroxyl radical scavenging, chelating power, and reducing power assays (Tamuly et al., 2015). The aqueous extract of F. asperifolia leaves possesses strong DPPH scavenging effects (P < 0.05; Ojo and Akintayo, 2014).
Methanol fraction of methanol: water (4:1) extract of F. auriculata fruits demonstrates strong inhibitory effects against α-amylase and α-glucosidase activities which compared to acarbose (Anjum and Tripathi, 2019). Ethanol extract of F. asperifolia leaves reduces blood glucose levels in streptozotocin-induced diabetes when compared to diabetic control (Pwaniyibo et al. 2020). The ethyl acetate fraction of acetone extract of F. lutea leaves exhibits significant increase in insulin secretion in RIN-m5F pancreatic β-cells when compared to glibenclamide (Olaokun et al. 2016). The formulated Emulgel (ethanol extract of F. benghalensis), when compared to diclofenac, validates the anti-arthritic activity through in vitro release assay (Sonali et al., 2021). Methanol extract of F. vogelii leaves significantly (P ≤ 0.05) inhibits adjuvant-induced paw arthritis when compared to indomethacin (reference drug; 10 mg/kg; Nwaehujor, 2021). The methanol extract F. carica leaves significantly suppresses the proliferation of MDA-MB-231 cell line (P < 0.05) in dose dependent manner (Ergül et al., 2019). The petroleum ether fraction of ethanol extract of F. glumosa stem bark demonstrates significant cytotoxicity on HT-29 (90.26%), and on A549 (88.38%) cell lines, respectively. Similarly, ethanol extract also displays potent cytotoxic effect against HFL-1 cells (IC50 232.66 µg/mL; Mutungi et al. 2021).
5 Bioavailability and pharmacokinetic profile
Indian Ficus species are used in the Ayurvedic system of medicine for treatment of various diseases in diverse geographical areas (Trivedi et al. 1969). Different species of Ficus genus (F. racemosa, F. glomerata, F. glumosa, F. carica, F. religiosa and F. benghalensis) are used to treat diabetic disorders such as regulating enzymatic activities, rate of carbohydrates assimilation, enhancing insulin sensitivity, release of insulin, formation of hepatic glycogen, and the uptake of peripheral glucose of body (You and Nicklas, 2006; Veberic et al., 2008). The figs of F. carica serve as excellent source of dietary carotenoids, anthocyanins, polyphenols, tocopherols, and vitamin C, therefore the consumption of F. carica fruits must be stimulated (Idolo et al., 2010; Manjula et al., 2011; Sirajo, 2018). Fruits have various health benefits due to the presence of phenolic constituents. The fig latex is used in the treatment of warts, toothache, haemorrhoids, cough, and cancers (Caxito et al. 2017; Abdel-Aty et al. 2019). The aqueous extract of F. sycomorus is useful in the treatment of sickle cell disease (Ramde-Tiendrebeogo et al. 2012).
5.1 Ascorbic acid (vitamin C)
F. carica figs contain rich amount of ascorbic acid, a potent antioxidant molecule, useful in checking of nonenzymatic browning of fruits and vegetables. The molecule is used as an alternative to synthetic antioxidants (Fasakin et al., 2011). Ascorbic acid plays essential role in strengthening of immune system, wound healing, orthogenesis, absorption of iron, biosynthesis of collagen, metabolite detoxification, and stopping the blood vessels from clotting (Tomita et al. 2005). The accumulation of ascorbic acid relies on environmental factors, species diversity, time of harvest, and stages of development and storage. Various isolation and characterization techniques also influence the reliability, and stability of ascorbic acid (Carvalho et al. 2015).
5.2 Carotenoids and anthocyanins
Carotenoids are bioactive compounds, occur in plants, responsible for the colour of Ficus species. Indian Ficus species are an excellent source of carotenoids. They act as an antioxidant agent and reduce the speed of aging process by utilizing reactive oxygen species [Campos et al. 2013]. Ficus fruit consists of diversity of health-advantageous bioactive compounds such as polyphenols (Del Caro and Piga, 2008), alkaloids, sterols (Su et al. 2002), anthocyanins (Dueñas et al. 2008), minerals, and carotenes (Adams and Richardson, 1977). The colour of fruits, and vegetables is associated to the presence of anthocyanins. Normally yellow and orange shades of many foods are developed due to the presence of carotenoids and anthocyanins. Carotenoids and anthocyanins play defensive roles against cancer and cardiovascular diseases (Stintzing and Carle, 2004).
5.3 Phenolic compounds
Phenolic constituents play essential role in protection of cells from hydrogen peroxide injury, as well as absorption of free radicals. Ficus plants contain high quantity of phenolic compounds (Juániz et al. 2016). Due to high profiles of phenolic compounds in Ficus plants, they have explored for their health-beneficial properties such as anti-inflammatory, antitumor, and cardioprotective properties (Dias et al. 2016).
5.4 Pharmacokinetic profile
The oral bioavailability complications can emerge, F. hispida stem bark, leaves, and roots, due to the presence of lipophilic constituents. These lipophilic compounds can influence various metabolic activities. The lipophilic molecule delivery, through oral route, has weak bioavailability because of their poor dissolution. Therefore, it is required that assimilation of F. hispida into dosage form may enhance its bioavailability (Touitou and Rubinstein, 1986; Arunkumar et al. 2009). Different strategies such as solid dispersion, microemulsion, liposome, lipid emulsion, solid lipid nanoparticle, nanosuspension, cyclodextrin complex or phospholipids are used with bioactive molecule to increase dissolution as well bioavailability (Shah et al. 2010; Ali and Chaudhary, 2011). Lanosterol, isolated from F. religiosa, examined for drug similarity using Five parameters of the Lipinski Rule. The reported values were found as 10 (hydrogen bond acceptor), 5 (hydrogen bond donor), 500 Dalton (molecular weight), 5 (H2O partition coefficient, logP), and 40–130 (molar refractivity). These parameters support its strong pharmacokinetics and bioavailability. Therefore, this molecule may be used as an anti-inflammatory agent and could be taken by oral route (Lipinski et al. 2001; Yueniwati et al. 2021).
6 Clinical studies
Since prehistoric times, the plant-derived medicines have been used in drug and cosmetic industries. As per WHO records, plant-derived medicines are used by millions of people of the developing countries (Al Rashid et al., 2019). Panchavalkal, prepared as per the literature available in Ras shastra, showed significant wound healing effects in 60 patients suffering from Dushta Vrana (infected wound). Dressing of patients was done with Panchavalkal ointment for 21 days and 36.7% wound healing recorded in the treated patients (Kulkarni and Dwivedi, 2019). Three F. carica paste packs (300 g/day) improve the colon transit time in 109 subjects of functional constipation when compared with the placebo group (P = 0.045). No serious adverse effects were recorded in the patients during the treatment period. The treatment was continued for 56 days (Baek et al., 2016). Consumption of fruits (90 g/day) produces a significant improvement in the irritable bowel syndrome symptoms such as pain frequency, distention, frequency of defecation and hard stool in patients. The treatment is given for 120 days (Pourmasoumi et al., 2019). Melfi cream (F. carica aqueous extract of sun-dried fruit and base cream; topical use) significantly increases efficacy in terms of reducing the SCORAD index, pruritus, and intensity scores when compared to hydrocortisone (1.0%; P < 0.05; Abbasi et al., 2017). Aqueous extract of F. racemosa stem bark, given two times before each meal, presents significant increase (P < 0.05) in insulin levels in the patients of type 2 diabetes (Ahmed et al., 2011). The details of clinical studies of Ficus species are mentioned in Table 4.
Study
Age (years)
Treatment
Dose
Recommended time
Useful outcomes
References
F. benghalensis
Randomized comparative clinical study
60 Patients (males and females) suffering from Dushta Vrana (infected wound)/ 16 to 60 years
Wound cleaning was done with normal saline. In group A, dressing was done with Panchavalkal ointment and in group B with framycetin sulfate, using sterile gauze
Panchavalkal (stem bark of F. Benghalensis, F. glomerata, F. religiosa, F. lacor and T. populnea, tila tail and bees wax) was prepared as per mentioned in Ras shastra
21 days
Group A − 36.7% healed, 26.7 regenerated and 6.7% improved. Group B − 30.0% healed, 43.3% regenerated and 13.3% improved. Panchvalkal ointment was found effective cream in the management of infected wound
Kulkarni and Dwivedi (2019)
F. carica
A randomized, double-blind, placebo-controlled study
109 subjects (both sexes) with functional constipation/19 to 39 years
Three F. carica paste packs given to subjects with functional constipation/ given three times a daily before meals
Three paste per day (300 g/day)
56 days
Colon transit time was significantly improved in the paste treated group compared with the placebo group (P = 0.045)/no serious adverse effects were reported during treatment period/ no significant differences in dietary intake (calorie, carbohydrate, protein, fat, and fiber) were observed between the groups during the intervention period
Baek et al. (2016)
A single-blind randomized clinical trial
150 patients with irritable bowel syndrome with predominant constipation/18 to 70 years
45 g fruits before breakfast and lunch were taken with a glass of water every day
90 g/day
120 days
Consumption of fruits caused a significant improvement in irritable bowel syndrome symptoms such as frequency of pain, distention, frequency of defecation and hard stool. The consumption also showed a significant increase in the quality of life, as well as satisfaction with overall bowel habits
Pourmasoumi et al. (2019)
A single center, randomized, double-blind crossover study
10 healthy adults (7 women and 3 men; 7 White/Caucasian, 1 Hispanic, and 2 Asian) acute postprandial glucose and insulin homeostasis /18 to 45 years
Glucodin™ powder (abscisic acid ≥ 300 ppm, drug extract ratio of native extract is 50–60:1) and extract-50× (abscisic acid ≥ 50 ppm with drug extract ration of native extract is 7–1:1)
51.4 g Glucodin™ powder (Valeant Pharmaceuticals, Australia) dissolved in 250 mL water/taken daily
120 min
Extract supplementation is a promising nutritional intervention for the management of acute postprandial glucose and insulin homeostasis, and it is a possible adjunctive treatment for glycemic management of chronic metabolic disorders such as prediabetes and type 2 diabetes mellitus
Atkinson et al. (2019)
A double-blind cross-over clinical trial
28 patients with type 2 diabetic mellitus/40 to 60 years
Aqueous decoction once a day
13 g of leaf powder boiled in 500 mL of distilled water (aqueous decoction)
21 days
The postprandial blood sugar was significantly altered after treatment with aqueous decoction {decreased from 230 ± 64.67 mg/dL at baseline to 193 ± 61.70 mg/dL in the intervention group, while it was 229 ± 70.13 mg/dL in the control group (P < 0.001)}
Mazhin et al. (2016)
A double blind randomized clinical trial
40 patients with multiple sclerosis and constipation/ 25 to 50 years
Carica paste three times a day
10 g
90 days
Mean reductions in the frequency of hard stool in intervention group showed no significant difference (P = 0.518) with the placebo group. One patient in fig paste group reported nausea after taking the supplement
Sardari et al. (2015)
A double-blinded, randomized, and placebo-controlled trial
45 children with mild to moderate atopic dermatitis /4 months to 14 years
Melfi cream (aqueous extract of sundried fruit and base cream)/ cream applied twice a day for two weeks
30 g for topical use
14 days
The treatment had significantly increased efficacy in terms of reducing the SCORAD index, pruritus, and intensity scores in comparison with hydrocortisone (1.0%; P < 0.05) and the placebo failed to ameliorate the symptoms
Abbasi et al. (2017)
A randomized controlled parallel group clinical trial
56 patients with rheumatoid arthritis/over 18 years
Herbal supplement (combination of olive oil, olive fruit and F. carica fruit/2:5:1 w/w)/supplement given with meals and were asked not to change the usual dietary intake
15 g (equal to 1 tablespoonful)
10 days
The supplement treatment did not show any adverse effects on the concentration of plasma lipids and fasting blood sugar. No other significant adverse effects contributed to the herbal supplement was seen in the study groups except one case with severe unclassified hiccup in the intervention group that lead to his withdrawal from the study
Bahadori et al. (2016)
A randomized, open, single-blinded, placebo-controlled, observer-blinded study
31 female subjects with facial wrinkle, especially the crow’s feet region of eyes/45–65 years
2% Combined fruit extracts (Punica granatum, Ginkgo biloba, Ficus carica, and Morus alba) were mixed with a formulation containing water, carbomer, glycerine, disodium EDTA, methyl paraben, triethanolamine, tocopheryl acetate, polysorbate 60, stearyl alcohol, PEG-100 stearate, sorbitan stearate, caprylic/capric triglyceride, dimethicone, mineral oil, propylparaben, butylene glycol, beeswax, and fragrance/ formulated fruit extract topically applied on one side of the face (crow’s feet) twice a day
2% topical formulated fruit cream
56 days
Treatment significantly reduced the percentage of wrinkle depth, length, and area by 11.5%, 10.07%, and 29.55%, respectively, when compared to the placebo. The dermatological scores of the treated sides decreased significantly (P = 0.05) with 1.5-fold lower than that of the placebo
Ghimeray et al. (2015)
Single blinded and comparison study
11 Asian healthy males with skin irritation/ between 20 and 35 years
Cream containing F. carica fruits/ formulation were applied to the cheeks of human volunteers
Cream (4% concentrated extract of fruits)
56 days
Cream reduced the skin melanin, trans–epidermal water loss and skin sebum significantly but enhanced the skin hydration significantly
Khan et al. (2014)
Open right/left comparative trial
25 patients with common warts/5 to 20 years
Patients were asked the self-application of fig tree latex to warts on one side of the body
Latex
180 days
Fig tree latex therapy of warts offers several beneficial effects including short-duration therapy, no reports of any side-effects, ease-of-use, patient compliance, and a low recurrence rate
Bohlooli et al. (2007)
A randomized, placebo-controlled study
10 Patients (6 men and 4 women) with insulin-dependent diabetes mellitus/ 22–38 years
Patients were given a decoction as a non-sweet commercial tea
Aqueous decoction of fig leaves
30 days
Post-prandial glycemia was significantly lowered during treatment (156.6 ± 75.9 mg/dl versus control 293.7 ± 45.0 mg/dl; P < 0.001). Medium average capillary profiles were also lowered in the treated patients (166.7 ± 23.6 mg/dl, versus control 245.8 ± 14.2 mg/dl; P < 0.05)
Serraclara et al. (1998)
F. fistulosa
–
10 healthy volunteers with oily skin/20 to 25 years old
Aqueous extract tested for safety and in-vivo sebum controlled efficacy
Hydrogel containing extract
28 days
After 28 days of application, the sebum levels of volunteers were significantly decreased (P < 0.05)/mean percentage of sebum content was also reduced in hydrogel treated patients (54.36%±13.7%) on day 28
Ditthawutthikul et al. (2021)
F. hispida
–
30 Patients of vitiligo/20 to 30 years
Fine powder of the fruits given orally twice a day with equal quantity of jaggery/bark powder was also applied to the lesion of vitiligo in the form of an ointment with Til Tail
24 g/day
42 days
More than 50% of patients attained rapid pigmentation. Lesions of the neck, leg, hand, chest responded rapidly while the lesion of the lips and hips were slow to respond
Morale (2021)
F. lacor
–
A male patient having non healing ulcer on right leg medially above the ankle joint, associated pain and foul-smelling discharge from the wound/55 years old
Ointment (malahar) was applied locally for thirty days followed by alternate day for next fifteen days. Supportive treatment of Vitamin B and C along with zinc was also given orally
Aqueous extract of stem bark was used for the preparation of ointment/ ointment prepared as per the formulation described in ‘Ras tarangini’, an ancient compendium
45 days
After 45 days, the wound was healed with healthy granulation tissue, purulent discharge and foul smell was totally absent. Bark extract rich in caffeic acid, have regulatory mechanism on glucose metabolism in diabetes
Changade et al. (2021)
F. pumila
–
3814 Japanese patients with upper borderline high BP and dyslipidaemia/20 to 50 years
Ishimaki tea, prepared from dried stems and leaves, followed by extraction of the major components with water. Patients were asked to drink Ishimaki tea a day
200–300 mL
90
Ishimaki tea significantly reduced mean body mass index/systolic BP/diastolic BP/total cholesterol/low density lipoproteins/γ-glutamyl trans peptidase /uric acid/ triglyceride/ and total glycerides/ high-density lipoprotein cholesterol-C, whereas high-density lipoprotein cholesterol was significantly increased
Suzuki et al. (2021)
–
28 Human T-cell leukemia virus type 1/19 were female (58 to 97 years old) and 9 were male (82 to 94 years old)
Ishimaki tea administered to the patients
2.441 mg/200–300 g leaves/day of rutin and approximately 1.411 mg/200–300 g leaves/day of apigenin
–
Those who were administered extracts had no human T-cell leukemia virus type 1-related symptoms, while those who were not administered extracts had human T-cell leukemia virus type 1-related diseases
Gonda et al. (2021)
F. racemosa
–
Volunteers were healthy, non-smokers, had not taken any medications, including aspirin
Two concentrations (50 and 100 µg/mL) dissolved in 25 µL phosphate buffered saline to 450 µL aliquots of platelet-rich plasma were given to volunteers
Hot and cold aqueous bark extracts (100 μg/mL)
14 days
Both the extracts induced aggregation of platelets to an extent of 3–51% compared to control. However, the extent of aggregation induced by both extracts were significantly lower (P ≤ 0.05) than those induced by collagen (2 µg/mL), adenosine diphosphate (10 µM) and epinephrine (10 µM), respectively
Ahmed et al. (2012b)
A double-blinded, randomized, placebo-controlled trial
30 (18 men and 12 women) patients with type 2 diabetes/35 to 50 years
Aqueous extract of stem bark given two times before each meal
1.2 g⁄day (400 mg × 3 hard gelatin capsules)
360 days
A significant increase (P < 0.05) in insulin levels was observed in the extract-supplemented group, but no significant (P > 0.05) changes were observed in the control group
Ahmed et al. (2011a)
A single/double blinded, randomized placebo-controlled trial
50 Patients with type 2 diabetes/35 to 50 years
Aqueous extract (1 gelatin capsule) of leaves taken thrice a day before each meal
1 capsule (400 mg)
56 days
A significant reduction in fasting and postprandial blood glucose levels were achieved in the patients. Moreover, a significant increase in the serum insulin level was recorded in treated patients. Regenerated pancreatic β-cells resulting in increased synthesis and secretion of insulin into the blood stream
Urooj and Ahmed (2013)
F. religiosa
A randomized clinical trial
30 Patients with type II diabetes mellitus/30 to 70 years
Aqueous extract of stem bark taken twice a day before meals with water
500 mg
30 days
Extract showed beneficial effects on fasting & post meal blood sugar levels. The drug was well tolerated by all patients throughout the treatment period. There was no evidence of adverse side-effects reported in the patients
Vaishali et al. (2014)
A multicentric double blind homoeopathic pathogenetic trial
24 healthy volunteers (19 males and 5 females)/18 to 50 years/ elicit the pharmacodynamic response
4–6 globules given four times a day, dry on tongue/ two stages of two different potencies viz. 30C and 200C
56 doses
14 days
Heaviness with coryza and pain in throat (2, 30C)/headache with malaise and pain in left hip and left foot (1, 30C)/constipation with headache (1, 200C)/bursting pain in eyeballs, walking (1, 200C)/pain in joints of lower extremities, sensation as if broken down (1, 30C) observed in volunteers
Dey et al. (2008)
44 patients with diabetes mellitus and erectile dysfunction/21 to 60 years
Ashvattha powder made from its root and stem barks, fruit and tender leaf buds/powder dissolved in 1 glass of milk and taken twice a day (morning – before meal and evening – after dinner)
10 g
45 days
In both the diabetic and the nondiabetic subjects, Ashvattha provided encouraging results on erectile dysfunction as well as on seminal parameters in comparison to the placebo
Virani et al. (2010)
–
18 normal healthy subjects/post prandial glycemic response and glycemic index/24 to 32 years
The food products: dal samose (10% leaf powder) and bati (5% bark powder) were given for two days
The dal samose and bati were supplemented with 5 and 10% powder of leaves and stem bark
2
The dal samose (10% leaves) and Bati (5% bark) were insignificant at P ≤ 0.05 level which was comparable with standard. Glycemic index and glycemic load values were found to be lowest for 10% leaves incorporated dal samose (35 and 13) when compared to 5% bark incorporated Bati (53 and 20)
Chaturvedi et al. (2014)
7 Toxicological effects
The large number of people of developing countries (approximately 80%) use medicinal plants regularly, without prescription, for the treatment of various diseases (WHO, 2002). Usage of medicinal plants for longer duration, in customary medicine, may cause low levels of toxicity in the people (Yuet Ping et al., 2013). Many recent studies indicate that the medicinal plants, used in traditional system of medicine, show various adverse effects (Ertekin et al. 2005; Ukwuani et al., 2012). Therefore, traditional medicines are getting substantial support in health debates world-wide (Tilburt and Kaptchuk, 2008). The rats showed negative behavioral changes at 5000, 5500, 5750 and 6000 mg/kg dosages of aqueous extract of F. carica leaves. The LD50 obtained was higher than 6000 mg/kg (Odo et al., 2016). No signs of symptoms of acute toxicity or behavioral changes and mortality were reported within 72 h to 14 days. Ethanol extract of F. deltoidea leaves (2000 mg/kg dose) did not affect the body weight of mice (Nugroho et al., 2020). Some symptoms of toxicity recorded were unease, sluggishness, and dizziness three hours after the administration of methanol extract of F. exasperata leaves (Shemishere et al., 2020). F. religiosa ethanol extract (2000 mg/kg/p.o.) decreased the levels of water intake in Wistar rats when compared to the control (Elavarasi et al., 2018). The results of toxicity studies of Indian Ficus species are described in Table 5.
Plant species
Plant parts
Extract/compound
Dose
Type of toxicity
Model/symptoms
References
F. benghalensis
Root
Ethanol and aqueous
3000 mg/kg b.w. of each extract
Behavioral, neurological, autonomic profiles and lethality were observed for 28 days during antiarthritic study
Extracts were found safe/no behavioral changes and mortality recorded up to 28 days in male Wistar rats
Bhardwaj et al. (2016)
F. carica
Leaf
Aqueous
Rats fasted for 12 h/first phase animals received 2000, 3000, 4000 and 5000 mg/kg b.w. for 24 h/in second phase animals received 5500, 5750 and 6000 mg/kg b.w. of extract
Hematological and some biochemical parameters
There was no mortality recorded when all the doses of the extract were administered orally to the rats. The rats showed negative behavioral changes at 5000, 5500, 57,250 and 6000 mg/kg dosages. The LD50 obtained was higher than 6000 mg/kg by implication
Odo et al. (2016)
F. deltoidea
Leaf
Ethanol
2000 mg/kg b.w. given for 14 days in case of acute toxicity while 1000 mg/kg b.w. given for 28 days in case of sub-chronic toxicity
Acute and subchronic toxicities were studied in mice
Acute toxicity - no signs of symptoms of toxicity or behavioral changes and mortality reported within 72 h–14 days. Extract at this dose did not affect the body weight of mice.
Sub-chronic toxicity - extract did not produce any symptoms of toxicity, changes in behavior, and mortality in miceNugroho et al. (2020)
2000 mg/kg b.w./p.o./monitored for 14 days in case of acute and 1000 mg/kg/p.o. monitored for 28 days in case of subchronic toxicity
Symptoms of physical
and behavioral changes, mortality rate were determined (LD50)Acute toxicity - Extract did not show any symptoms of toxicity and mortality, LD50 was above 2000 mg/kg b.w.
Subchronic toxicity - Extract did not produce any symptoms of toxicity, changes in behavior, and mortality in animalsNugroho et al. (2020)
Methanol
5000 mg/kg given for 14 days in case of genotoxicity and acute and subchronic toxicities recorded at 2500 mg/kg dose (monitored for 28 days)
Mortality, clinical signs, body weight changes, hematological and biochemical parameters, gross findings, organ weights, and histological parameters were studied
Acute toxicity - there were no significant changes in behavior, such as apathy, hyperactivity, or morbidity reported in any of the animals.
Subchronic toxicity - did not show any mortality, body weight gains, behavioral changes or food and water consumption between the animals of the treated and control groupFarsi et al. (2013)
F. exasperata
Leaf
Aqueous
2.5, 5, 10 and 20 g/kg administered daily for 14 days
Hematological parameters, body weight and body temperature in mice/ acute toxicity over 24 h and 14-day periods
Extract caused neither mortality nor changes in behavior, body weight and body temperatures. No significant differences in hematological parameters (WBC count, platelet, and hemoglobin estimation) in control or treated animals were recorded. However, the daily dose up to 14 days showed significant increase in body temperature (P < 0.05) but, a significant decrease in the red blood cells count, hemoglobin count and hematocrit values (P < 0.05) were recorded
Bafor and Igbinuwen (2009)
Methanol
1500, 3000, and 5000 mg/kg/p.o./ acute toxicity observed for 14 days
Changes in behavior, and body weight parameters were measured
Symptoms of toxicity recorded were as unease, sluggishness, and dizziness three hours after administration of extract in male Wistar rats
Shemishere et al. (2020)
F. hispida
Leaf
Methanol
4000 mg/kg b.w./p.o./acute toxicity observed for 14 days
Rate of mortality, behavioral pattern changes such as weakness, aggressiveness, food or water refusal, diarrhea, salivation, discharge from eyes and ears, noisy breathing, changes in locomotor activity, convulsion, coma, injury, pain, or any sign of toxicity in each group of animals were recorded
No mortality and behavioral changes or symptoms of toxicity observed in treated animals during this period of study
Mushiur Rahman et al. (2018)
F. racemosa
Stem bark
Aqueous
2000 mg/kg b.w./p.o./acute toxicity observed for 7 days
Acute toxicity/the observation in tremors, convulsion, salivation, diarrhea, lethargy, or unusual behaviors such as self-mutilation, walking backward, and difference in body weights were studied
No lethal effect or mortality was observed in animals throughout the test period following single oral administration. Animals did not show any tremors, convulsion, salivation, diarrhea, lethargy, or unusual behaviors such as self-mutilation, walking backward, and difference in body weights before and after the study period
Solanki and Bhavsar (2014)
F. religiosa
Stem bark
Ethanol
2000 mg/kg b.w./p.o./acute toxicity monitored for 14 days
Acute toxicity/changes in body weight, food intake, water intake, relative organ weight, hematological parameters and histoarchitecture of vital organs were studied
There were no remarkable alterations in the general behavior, toxicity signs and mortality recorded in rats treated with extracts (p.o.). In extract treated rats, a significant decrease in the levels of water intake recorded when compared to the control
Elavarasi et al. (2018)
Acetone
2000 mg/kg b.w.
Acute toxicity studied in Wistar albino rats (p.o.)/ animals were observed for general behavioral, neurological, autonomic profiles, any toxicity and mortality during antiulcer study
Extract did not show any mortality, changes related to behavior, autonomic, neurologic, and physical disorder within the first 24 h and during the 14 days follow-up. The plant extracts were found to be safe at the tested dose.
Panchawat and Pradhan (2020)
8 Conclusions and perspectives
Ficus is the largest genus of Moraceae family and found in Neotropical America, Madagascar, Indian Ocean islands, Malaysia, the Arabian Peninsula, India–Asia, New Guinea, Pacific islands, and Australia. The leaves, stem, aerial roots, and stem bark of Ficus plants have been used in alleviating fever and relieving pain and to treat flu, malaria, acute enteritis, tonsillitis, bronchitis, and rheumatism. Ficus plants contain various classes of compounds including monoterpenes, diterpenes, sesquiterpene, triterpenes, alkaloids, and flavonoids. The isolated compounds from Indian Ficus species possess antioxidant, antimicrobial, anticancer, anti-inflammatory, radioprotective, neuroprotective, and wound healing properties. The clinical study of F. carica capsules reveals its applications in the treatment of constipation. Panchavalkal formulation (ethanol extract of F. benghalensis) improves the healing of Dushta Vrana (infected wound) in the patients. Many studies on ethnomedicinal properties, characterization of phytoconstituents, pharmacological activities of 31 Ficus species have been conducted but there is still a need to perform further experimental studies on the exploration of chemical characterizations and pharmacological evaluations of 66 Indian Ficus species. Very few clinical studies (only 8 Ficus species) have been performed on the Indian Ficus species whereas no clinical studies have attempted on 23 Indian Ficus species. Therefore, intensive research is to be conducted by the researchers on the examination of therapeutic and toxicological effects of Indian Ficus species that will help in the development of new pharmaceutical drugs in the future.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
References
- A new topical treatment of atopic dermatitis in pediatric patients based on Ficus carica L. (Fig): A randomized, placebo-controlled clinical trial. Complement. Ther. Med.. 2017;35:85-91.
- [Google Scholar]
- Abdel-Aty, A.M., Hamed, M.B., Salama, W.H., Ali, M.M., Fahmy, A.S., Mohamed S.A., 2019. Ficus carica, Ficus sycomorus and Euphorbia tirucalli latex extracts: phytochemical screening, antioxidant and cytotoxic properties. Biocatal Agric Biotechnol 20, Article ID 101199.
- Antimicrobial activity of Ficus deltoidea Jack (Mas Cotek) Pak. J. Pharm. Sci.. 2012;25:675-678.
- [Google Scholar]
- Role of Ficus deltoidea extract in the enhancement of wound healing in experimental rats. Biomed. Res.. 2010;21:241-245.
- [Google Scholar]
- Anti-inflammatory activity of standardised extracts of leaves of three varieties of Ficus deltoidea. Int. J. Pharmacol. Clin. Res.. 2009;1:100-105.
- [Google Scholar]
- Trends and challenges of traditional medicine in Africa. African J. Trad. Complement. Altern. Med.. 2011;8:115-123.
- [Google Scholar]
- Evaluation of analgesic and anti-inflammatory effects of ethanol extract of Ficus iteophylla leaves in rodents. African J. Tradit. Complement. Altern. Med.. 2011;8:462-466.
- [Google Scholar]
- Antiarthritic and antioxidant effects of the leaf extract of Ficus exasperata P. Beauv. (Moraceae) Pharmacog. Res.. 2010;2:89-97.
- [Google Scholar]
- Antioxidant flavonoid glycosides from the leaves of Ficus pumila L. Food Chem.. 2008;109:415-420.
- [Google Scholar]
- Experimental and computational approaches to reveal the potential of Ficus deltoidea leaves extract as α-amylase inhibitor. Nat. Prod. Res.. 2018;32:473-476.
- [Google Scholar]
- Comparative in vitro antidiabetic and antioxidant activity of various extracts of Ficus species. Pharmacog. J.. 2018;10:349-354.
- [Google Scholar]
- Nutritive Value of Foods. Deptt Agric Agricultural Res Serv Consum Food Econ Inst. 1977;No. 72
- [Google Scholar]
- Constituent of essential oil from Ficus benghalensis L. European J. Med. Plants. 2015;9:1-6.
- [Google Scholar]
- Hypoglycaemic and hypotensive effects of Ficus exasperata Vahl. (Moraceae) leaf aqueous extract in rats. African J. Trad. Complement. Altern. Med.. 2011;8:275-283.
- [Google Scholar]
- Medicinal plants with cholinesterase inhibitory activity: A review. African J. Biotechnol.. 2010;9:8257-8276.
- [Google Scholar]
- Ethnobotanical studies from Northern Pakistan. J. Ayub. Medical College, Abbottabad. 2009;21:52-57.
- [Google Scholar]
- Phenolic compounds proliferation by HPLC: to find out antibacterial activities in Ficus benghalensis plant extract. Int. J. Bot. Studies. 2020;5:140-144.
- [Google Scholar]
- Hepatoprotective effect of Ficus carica leaf extract on mice intoxicated with carbon tetrachloride. Iranian J. Pharm. Res.. 2011;10:63-68.
- [Google Scholar]
- Effect of aqueous extract on total phenolic content and antimitotic activity of Ficus benghalensis root on Allium cepa root meristamatic cells. Archiv. Biomed. Eng. Biotechnol.. 2020;4:1-5.
- [Google Scholar]
- Evaluation of antioxidant and antimicrobial activity of Ficus carica leaves: an in vitro approach. J. Plant Pathol. Microb.. 2013;4:1.
- [Google Scholar]
- Ahmed, F., Narendra Sharath Chandra, J.N., Manjunath, S., 2011b. Acetylcholine and memory-enhancing activity of Ficus racemosa bark. Pharmacog. Res. 3, 246 – 249.
- Antihyperglycemic activity of Ficus racemosa bark extract in type 2 diabetic individuals. J. Diabetes. 2011;3:318-319.
- [Google Scholar]
- Phytochemical analysis and antioxidant potential of Ficus benghalensis L. J. Bioresource Manage. 2017;4:17-26.
- [Google Scholar]
- Traditional uses and pharmacological potential of Ficus exasperata Vahl. Syst. Rev. Pharm.. 2012;3:15-23.
- [Google Scholar]
- Hepatoprotective effects of Ficus racemosa stem bark against carbon tetrachloride-induced hepatic damage in albino rats. Pharm. Biol.. 2010;48:210-216.
- [Google Scholar]
- Studies on the gastrointestinal properties of Ficus exasperata. Fitoterapia. 1997;68:17-20.
- [Google Scholar]
- Evaluation of Nigerian Traditional medicines on peptic ulcer. J. Ethnopharmacol.. 1998;62:123-127.
- [Google Scholar]
- The pentacyclic triterpenoid, plectranthoic acid, a novel activator of AMPK induces apoptotic death in prostate cancer cells. Oncotarget. 2015;7:3819-3831.
- [Google Scholar]
- In vitro thrombolytic activity, antioxidant, and cytotoxic properties of fruit extracts of Ficus erecta (Thunb.) J. Med. Plants Res.. 2018;12:50-54.
- [Google Scholar]
- Preclinical and clinical trials of Indian medicinal plants in disease control. In: Sen S., Chakraborty R., eds. Herbal Medicine in India: Indigenous Knowledge, Practice, Innovation and its Value. Singapore: Springer Singapore; 2019. p. :119-142.
- [Google Scholar]
- Comparative anticancer activities of Ficus carica and Ficus salicifolia latex in MDA-MB-231 cells. Saudi J. Biol. Sci.. 2021;32:3225-3234.
- [Google Scholar]
- Ficus hispida Linn.: A review of its pharmacognostic and ethnomedicinal properties. Pharmacog. Rev.. 2011;5:96-102.
- [Google Scholar]
- New naphthyl esters from the bark of Ficus religiosa Linn. Nat. Prod. J.. 2014;4:248-253.
- [Google Scholar]
- A new naphthyl substituted β-sitosterol and fatty acids from the bark of Ficus religiosa L. Indian Drugs. 2017;54:18-22.
- [Google Scholar]
- New naphthyl substituted phytosterol and lanostane type-triterpenic esters from the stem bark of Ficus religiosa L. Indian J. Pharm. Education Res.. 2020;54:750-754.
- [Google Scholar]
- Anti-inflammatory effects of hydro-methanolic extract of Ficus carica. Biomed. Pharmacol. J.. 2009;2:129-132.
- [Google Scholar]
- Phytochemical characteristics and antioxidant activity of several fig (Ficus carica L.) ecotypes. Ital. J. Food Sci.. 2020;32:755-768.
- [Google Scholar]
- A bis-benzopyrroloisoquinoline alkaloid incorporating a cyclobutane core and a chlorophenanthroindolizidine alkaloid with cytotoxic activity from Ficus fistulosa var. tengerensis. J. Nat. Prod.. 2017;80:2734-2740.
- [Google Scholar]
- Al-koshab, M., Alabsi, A.M., Bakri, M.M., Ali-Saeed, R., Naicker, M.S., 2020. Antitumor activity of Ficus deltoidea extract on oral cancer: An in vivo study. J. Oncol. 2020, Article ID 5490468.
- The chemical constituents of Ficus benjamina Linn. and their biological activities. Pertanika J. Sci. Technol.. 2003;11:73-81.
- [Google Scholar]
- Phytochemical and pharmacological study of Ficus palmata growing in Saudi Arabia. Saudi Pharm. J.. 2014;22:460-471.
- [Google Scholar]
- Phenolics and flavonoids contents of medicinal plants, as natural ingredients for many therapeutic purposes- A review. IOSR J. Pharm.. 2020;10:42-81.
- [Google Scholar]
- Aristolone content analysis in Ficus auriculata as predicted apoptotic activity in Hela cells. J. Health Technol. Assessment Midwifery. 2021;4:15-20.
- [Google Scholar]
- Anti-inflammatory, antioxidant and antimicrobial activity of the stem bark extract and fractions of Ficus exasperata Vahl. (Moraceae) J. Pharmacog. Phytochem.. 2013;2:38-44.
- [Google Scholar]
- Evaluation of anti-inflammatory effects if Ficus hispida L. leaves extract against carrageenan induced paw edema in rats. J. Pharm. Sci. Res.. 2017;9:364-367.
- [Google Scholar]
- Phenolic profiling and biological potential of Ficus curtipes Corner leaves and stem bark: 5-Lipoxygenase inhibition and interference with NO levels in LPS-stimulated RAW 264.7 macrophages. Biomolecules. 2019;9:400.
- [Google Scholar]
- In vitro alpha-amylase and alpha-glucosidase inhibitory activities of fruits of Ficus auriculata Lour. Int. J. Pharma. Bio. Sci.. 2019;10:134-141.
- [Google Scholar]
- Phytochemical screening and in vitro evaluation of antidiabetic activity of Ficus palmata fruits. European J. Pharm. Med. Res.. 2019;6:251-258.
- [Google Scholar]
- Annon., 1976. The Wealth of India: A Dictionary of Indian Raw Materials, and Industrial products. Publ Information Directorate, CSIR, New Delhi.
- Isolation and identification of antioxidant and hyaluronidase inhibitory compounds from Ficus microcarpa L. fil. Bark. J. Enzyme Inhib. Med. Chem.. 2010;25:406-413.
- [Google Scholar]
- Comparative antioxidative and antidiabetic activities of Ficus carica pulp, peel and leaf and their correlation with phytochemical contents. J. Pharm. Res.. 2020;4:000197
- [Google Scholar]
- Nanosuspension technology and its applications in drug delivery. Asian J. Pharm.. 2009;3:168-173.
- [Google Scholar]
- An overview of phytochemical and biological activities: Ficus deltoidea Jack and other Ficus spp. J. Pharm. Bioallied Sci.. 2021;13:11-25.
- [Google Scholar]
- Utilisation de diversesespèces de Ficus (Moraceae) dans la pharmacopéetraditionnelleafricaine de Côte d’Ivoire. MitteilungenausdemInstitutfürallgemeine Botanik Hamburg. 1990;23:1039-1046.
- Abscisic acid standardized fig (Ficus carica) extracts ameliorate postprandial glycemic and insulinemic responses in healthy adults. Nutrients. 2019;11:1757.
- [Google Scholar]
- Quantitation and Characterization of phenolic compounds in butanol fraction of Ficus lyrata leaves and in vivo assessment of hepatoprotective and hypocholesterolemic activities in rats. J. Herbs Spices Med. Plants. 2019;25:330-346.
- [Google Scholar]
- Anti-inflammatory and analgesic activities of methanolic extracts of the stem bark of Alstonia boneei, Ficus elastica and Xylopia aethiopica in rodents. African J. Biomed. Res.. 2009;12:249-251.
- [Google Scholar]
- Baek, H.I., Ha, K.C., Kim, H.M., Choi, E.-K., Park, E.-O., Park, B-.H., Yang, H.J., Kim, M.J., Kang, H.J., Chae, S.-W., 2016. Randomized, double-blind, placebo-controlled trial of Ficus carica paste for the management of functional constipation. Asia Pac. J. Clin. Nutr. 25, 487 – 496.
- Acute toxicity studies of the leaf extract of Ficus exasperata on haematological parameters, body weight and body temperature. J. Ethnopharmacol.. 2009;123:302-307.
- [Google Scholar]
- Characterisation of the antiproliferative constituents and activity of Ficus exasperata (Vahl) on ovarian cancer cells - a preliminary investigation. Nat. Prod. Res.. 2017;31:2164-2168.
- [Google Scholar]
- Does regular use of a complementary medicine of Olea europe and Ficus carica have adverse effects on lipid profile and fasting blood glucose of rheumatoid arthritis (RA) patients under treatment with DMARD regimens containing methotrexate? Iranian. J. Pharm. Res.. 2016;15:933-940.
- [Google Scholar]
- Evaluation of hepatoprotective activity of Ficus bengalensis. Int. J. Res. Pharm. Sci.. 2011;2:522-525.
- [Google Scholar]
- A new norisoprenoid from the leaves of Ficus pumila. Nat. Prod. Res.. 2019;33:1292-1297.
- [Google Scholar]
- Analgesic and anti-inflammatory evaluation of Ficus microcarpa L. leaves extract. Asian J. Pharm. Clin. Res.. 2012;5:258-261.
- [Google Scholar]
- Basnet, B.K., 1998. Medicinal plants in Sindhuli district: utilization, trade, and management practice. Central Deptt Soc Sci, Tribhuvan Univ, Kathmandu, Nepal.
- Begum, F., Shivakrishna, P., Savya, B., Lalitha, U., Ashok kumar, K., Hazeera, Sunil, R., 2013. Wound healing activity of methanolic leaf extract of Ficus carica in albino rats. Int. J. Curr. Res. 5, 2631 – 2635.
- Ethnomedicinal plants used by the tribals of Similipal Bioreserve, Orissa, India: A pilot study. Ethnobot. Leaflets. 2006;10:149-173.
- [Google Scholar]
- Herbal Medicine: Biomolecular and Clinical Aspects. Boca Raton, USA: CRC Press; 2011.
- Extracts of branches and fruits of Ficus auriculata Lour: antioxidant, antimicrobial, and phytotoxic activities. Agric. Conspec. Sci.. 2020;85:303-310.
- [Google Scholar]
- Neuroprotective effect of pet ether extract of Ficus religiosa (L.) leaves in 3-nitropropionic acid induced Huntington disease. Int. J. PharmTech. Res.. 2015;8:57-69.
- [Google Scholar]
- Antioxidant and immunomodulatory activity of hydroalcoholic extract and its fractions of leaves of Ficus benghalensis Linn. Pharmacog. Res.. 2016;8:50-55.
- [Google Scholar]
- Study on efficacy of treatment with Ficus benghalensis leaf extracts on Freundís adjuvant induced arthritis in rats. Int. J. Drug Dev. Res.. 2010;2:744-749.
- [Google Scholar]
- Evaluation of anti-arthritic activity of Ficus benghalensis Linn. root extracts on Freund’s adjuvant induced arthritis in rats. J. Phytopharmacol.. 2016;5:10-14.
- [Google Scholar]
- Natural history and economic botany of Nepal. India: Orient Longman Pvt Ltd Hyderabad; 1977.
- Folk medicinal use of plants for respiratory complaints in central Nepal. Fitoterapia. 1993;64:163-171.
- [Google Scholar]
- Folk herbal remedies for diarrhoea and dysentery in central Nepal. Fitoterapia. 1993;64:243-250.
- [Google Scholar]
- Evaluation of antibacterial potential of Ficus species. J. Pharm. Sci. Res.. 2018;10:1251-1255.
- [Google Scholar]
- A comparative evaluation of Ficus religiosa with Ficus species for its anti-inflammatory activity: A review. J. Appl. Pharm. Res.. 2020;8:13-16.
- [Google Scholar]
- Comparative study of fig tree efficacy in the treatment of common warts (Verruca vulgaris) vs. cryotherapy. Int. J. Dermatol.. 2007;46:524-526.
- [Google Scholar]
- Bopage, N.S., Kamal Bandara Gunaherath, G.M., Jayawardena, K.H., Wijeyaratne, S.C., Abeysekera, A.M., Somaratne, S., 2018. Dual function of active constituents from bark of Ficus racemosa L in wound healing. BMC Complement. Altern. Med. 18, 29.
- Féticheursetmé decinestraditionnelles du Congo (Brazzaville) Mem ORSTOM. 1969;36:282.
- [Google Scholar]
- Anti-inflammatory activities of aqueous extract of beringin (Ficus benjamina L.) and kersen (Muntingia calabura L.) leaves in albino rats. J. Katalisator. 2021;6:254-260.
- [Google Scholar]
- Effect of roots of Ficus hirta on cocaine-induced hepatotoxicity and active components. Zhongguo Zhong Yao Za Zhi. 2007;32:1190-1193.
- [Google Scholar]
- Polyphenols, ascorbic acid and carotenoids contents and antioxidant properties of habanero pepper (Capsicum chinense) fruit. Food Nutr. Sci.. 2013;04:47-54.
- [Google Scholar]
- A chloroform extract obtained from a decoction of Ficus carica leaves improves the cholesterolaemic status of rats with streptozotocin-induced diabetes. Acta Physiol. Hung. 2000;87:71-76.
- [Google Scholar]
- Bioactive compounds and antioxidant activity of pepper (Capsicum sp.) genotypes. J. Food Sci. Technol.. 2015;52:7457-7464.
- [Google Scholar]
- Antiproliferative activity of extracts of Euphorbia tirucalli L (Euphorbiaceae) from three regions of Brazil. Trop. J. Pharm. Res.. 2017;16:1013-1020.
- [Google Scholar]
- Ethnobotanical and ethnopharmacological studies of medicinal and aromatic plants used in the treatment of metabolic diseases in the Moroccan Rif. Heliyon. 2019;5:e02191.
- [Google Scholar]
- Evaluation of the anti-inflammatory activity of the methanolic extract of the fruits of Ficus palmata. British J. Pharm. Res.. 2016;12:1-5.
- [Google Scholar]
- Isolation and identification of Ficus palmata leaves and their antimicrobial activities. J. Sci. Res.. 2017;9:193-200.
- [Google Scholar]
- Formulation and efficacy of Plaksha (Ficus lacor) ointment in the management of non-healing ulcer. Res. J. Pharm. Technol.. 2021;14:5680-5682.
- [Google Scholar]
- Hepatoprotective and antioxidant activity of methanol extract of Ficus glomerata. J. Nat. Med.. 2008;62:379-383.
- [Google Scholar]
- Glycemic index of Ficus religiosa based bakery products for healthy normal subjects. Food Sci.. 2014;4:210-212.
- [Google Scholar]
- Plants used in the treatment of domestic cattle in Narayani zone (central Nepal). Royal Nepal Acad Sci Technol, Kathmandu, Nepal: In Proc III Nat Conf Sci Technol; 1994.
- Private channel: a single unusual compound assures specific pollinator attraction in Ficus semicordata. Funct. Ecol.. 2009;23:941-950.
- [Google Scholar]
- Antioxidant, antifungal activities of ethnobotanical Ficus hirta Vahl. and analysis of main constituents by HPLC-MS. Biomedicines. 2020;8:15.
- [Google Scholar]
- Procyanidins and their therapeutic potential against oral diseases. Molecules. 2022;27:2932.
- [Google Scholar]
- Phenolic compounds from Ficus hispida L.f. as tyrosinase and melanin inhibitors: Biological evaluation, molecular docking, and molecular dynamics. J. Mol Structure. 2021;1244:130951
- [Google Scholar]
- Anti-inflammatory phenylpropanoids and phenolics from Ficus hirta Vahl. Fitoterapia. 2017;121:229-234.
- [Google Scholar]
- Phenolics from the roots of hairy fig (Ficus hirta Vahl.) exert prominent anti-inflammatory activity. J. Funct. Foods. 2017;31:79-88.
- [Google Scholar]
- Phytochemical screening of Tanzanian medicinal plants. I. J. Ethnopharmacol.. 1984;11:157-179.
- [Google Scholar]
- Plants used in traditional medicine in Eastern Tanzania. IV. Angiosperms (Mimosaceae to Papilionaceae) J. Ethnopharmacol.. 1990;29:295-323.
- [Google Scholar]
- Novel triterpenoids from the aerial roots of Ficus macrocarpa. J. Org. Chem.. 2002;67:7656-7661.
- [Google Scholar]
- Cytotoxic triterpenes from the aerial roots of Ficus macrocarpa. Phytochem.. 2005;66:495-501.
- [Google Scholar]
- New peroxy triterpenes from the aerial roots of Ficus macrocarpa. J. Nat. Prod.. 2001;64:436-439.
- [Google Scholar]
- Evaluation and comparison of hypoglycemic activity of bark and leaf of Ficus bengalensis Linn. in alloxan induced diabetes in albino rats. Indian J. Pharm. Pharmacol.. 2017;4:138-142.
- [Google Scholar]
- Vitexin and isovitexin from the Leaves of Ficus deltoidea with in-vivo α-glucosidase inhibition. J. Ethnopharmacol.. 2012;142:776-781.
- [Google Scholar]
- Indigenous Drugs of India (Second Ed). Calcutta: Academic Publ; 1958.
- Ficus Glossary of Indian Medicinal Plants. New Delhi: CSIR; 1956.
- Evaluation of antidiabetic activity of leaves and fruits of Ficus religiosa Linn. Int. J. Pharm. Life Sci.. 2011;2:1325-1327.
- [Google Scholar]
- Anti-inflammatory activity of methanolic extract of Ficus hispida dried fruit. Int J Basic Clin Pharmacol. 2021;10:997-1004.
- [Google Scholar]
- Wound healing activity of aqueous extracts of Ficus religiosa and Ficus benghalensis leaves in rats. Indian J Res Pharm Biotechnol. 2014;2:1071-1081.
- [Google Scholar]
- Dictionary of Chinese Materia Medica. Shanghai: Shanghai Sci Technical Publ; 1986.
- Ethnobotanical research and traditional health care in developing countries, plants, people, and culture. Freeman Comp: New York, W.H; 1996.
- Isoflavonoids from Ficus benjamina and their inhibitory activity on BACE1. Planta Med. 2012;78:1357-1362.
- [Google Scholar]
- A new furanocoumarin glycoside from the roots of Ficus hirta. Lett Org Chem. 2018;15:1007-1011.
- [Google Scholar]
- Triterpenoids and phenolics from the leaves of Ficus hirta Vahl. in Tuyen Quang province. Vietnam. Int J Food Sci Agric. 2020;4:138-142.
- [Google Scholar]
- Documentation of the ethnobotanical knowledge of the Kumal community of Chitwan district. Central Nepal: Central Department of Botany, Tribhuvan University, Kathmandu, Nepal; 2002.
- Ethnobotanical studies of Darai tribe in Chitwan district Nepal. Kathmandu, Nepal: Research report NEMP/IUCN and NAHSON; 1995.
- Evaluation of antimicrobial activity of Ficus racemosa Linn leaves extract. Int J Pharm Technol. 2014;6:6310-6317.
- [Google Scholar]
- Antioxidant effect of two flavonoids from the bark of Ficus bengalensis Linn in hyperlipidemic rats. Indian J Exp Biol. 1998;36:902-906.
- [Google Scholar]
- Medicinal plants used by different tribes of Cachar district, Assam. Indian Journal of Traditional Knowledge. 2008;7:446-454.
- [Google Scholar]
- Phyllanthus muellerianus and Ficus exasperata exhibit anti-proliferative and pro-apoptotic activities in human prostate cancer PC-3 cells by modulating calcium influx and activating caspases. Biologia. 2022;77:1981-1994.
- [Google Scholar]
- Polyphenol composition of peel and pulp of two Italian fresh fig fruits cultivars (Ficus carica L.) European Food Res Technol. 2008;226:715-719.
- [Google Scholar]
- Documentation of indigenous knowledge of medicinal plants in Gwalek, Baitadi. Nepal. Botanica Orient. 2003;3:135-143.
- [Google Scholar]
- Ficus religiosa: A multicentric double blind homoeopathic pathogenetic trial. Indian J Res Homoeop. 2008;2:10-14.
- [Google Scholar]
- In vitro anti-inflammatory activity of Ficus racemosa L. bark using albumin denaturation method. AYU. 2018;39:239-242.
- [Google Scholar]
- Screening of Indian plants for biological activity: part VI. Indian J Exp Biol. 1977;15:208-219.
- [Google Scholar]
- Effect of ultrasound on the supercritical CO2 extraction of bioactive compounds from dedo de moça pepper (Capsicum baccatum L. var. pendulum) Ultrasonics Sonochem. 2016;31:284-294.
- [Google Scholar]
- L0 art deguerirchezles Kongodu Zaıre, discourmagiqueou Science me dicale? Lescahiersdu CEDA F. Anthropol. 1984;1:71.
- [Google Scholar]
- Wound-healing activity of ethanolic and aqueous extracts of Ficus sarmentosa bark. Int. J. Pharm. Res.. 2018;10:38-42.
- [Google Scholar]
- An extract from Ficus carica cell cultures works as an anti-stress ingredient for the skin. Antioxidants. 2021;10:515.
- [Google Scholar]
- Management of seborrhea and enlarged pore size with a hydrogel containing Ficus fistulosa extract. J Clin Aesthet Dermatol. 2021;14:42-45.
- [Google Scholar]
- Antinociceptive and pharmacological effects of metanicotina, a selective nicotine agonist. J Pharmacol Exp Ther. 1999;291:390-398.
- [Google Scholar]
- Doreddula SK, Bonam SR, Gaddam DP, Desu BS. 2014. Phytochemical analysis, antioxidant, antistress, and nootropic activities of aqueous and methanolic seed extracts of ladies finger (Abelmoschus esculentus L.) in mice. Sci World J 2014, 519848.
- Anthocyanin composition in fig (Ficus carica L.) J. Food Compos Anal.. 2008;21:107-115.
- [Google Scholar]
- Inhibition of free radical and neuroprotective effect of four varieties of Ficus deltoidea. Adv. Materials Res.. 2012;554–556:1371-1380.
- [Google Scholar]
- Flavonoid distribution in four varieties of Ficus deltoidea (Jack) J Med Plant Herb Ther Res. 2015;3:1-9.
- [Google Scholar]
- EI-Domiaty, M., Abdel-Aal, M., Abou-Hashem, M., Abd-Alla, R., 2002. Phytochemical and biological study of Ficus elastica Roxb. var. Decora growing in Egypt. Zagazig J. Pharm. Sci. 5, 34 – 45.
- Acute toxicity evaluation of Ficus religiosa bark extract on albino rats. Int. J. Creative Res. Thoughts. 2018;6:11-20.
- [Google Scholar]
- Phytochemical and pharmacological studies of Ficus auriculata Lour. J Nat Prod. 2011;4:184-195.
- [Google Scholar]
- Antitumor and antioxidant activity of Ficus elastica Roxb and Ficus bengalensis Linn family Moraceae. World Appl. Sci. J.. 2012;19:1532-1539.
- [Google Scholar]
- Hepatoprotective potential of standardized Ficus species in intrahepatic cholestasis rat model: Involvement of nuclear factor-κB, and Farnesoid X receptor signalling pathways. J Ethnopharmacol. 2019;231:262-274.
- [Google Scholar]
- Comparative DNA profiling, phytochemical investigation, and biological evaluation of two Ficus species growing in Egypt. Pharmacog. Res.. 2013;5:291-299.
- [Google Scholar]
- Total phenolic contents and free radical scavenging activity of certain Egyptian Ficus species leaf samples. Food Chem.. 2009;114:1271-1277.
- [Google Scholar]
- In vitro evaluation of the chemical composition and various biological activities of Ficus carica leaf extracts. Turk. J. Pharm. Sci.. 2019;16:401-409.
- [Google Scholar]
- A combination of unusual presentations of Datura stramonium intoxication in a child: Rhabdomyolysis and fulminant hepatitius. J. Emerg. Med.. 2005;28:227-228.
- [Google Scholar]
- Antioxidant activities of Ficus glomerata (moraceae) leaf gall extracts. Pharmacog. Res.. 2015;7:114-120.
- [Google Scholar]
- Eteraf-Oskouei, T., Allahyari, S., Akbarzadeh-Atashkhosrow, A., Delazar, A., Pashaii, M., Gan, S.H., Najafi, M., 2015. Methanolic extract of Ficus carica Linn. leaves exerts antiangiogenesis effects based on the rat air pouch model of inflammation. Evi-Based Complement. Alter. Med. 2015, Article ID 760405.
- Antioxidant activity and phytochemical screening of Ficus benghalensis aerial roots fractions. J. Rep. Pharma. Sci.. 2019;8:24-27.
- [Google Scholar]
- Antimicrobial activity of Ficus bengalensis and Ficus elastica fruit latex against selected bacteria and fungi. Int J Sci: Basic Appl Res. 2017;31:21-26.
- [Google Scholar]
- Antibacterial activities of various sequential extracts of Ficus racemosa stem bark. Pharmacog J. 2010;2:203-206.
- [Google Scholar]
- Profiling the chemical content of Ficus lyrata extracts via UPLC-PDA-qTOF-MS and chemometrics. Nat Prod Res. 2014;28:1549-1555.
- [Google Scholar]
- Antinociceptive effect of promethazine in mice. Iran J Basic Med Sci. 2009;12:140-145.
- [Google Scholar]
- Genotoxicity and acute and subchronic toxicity studies of a standardized methanolic extract of Ficus deltoidea leaves. Clinics. 2013;68:865-875.
- [Google Scholar]
- Antioxidant properties of chlorophyll-enriched and chlorophyll-depleted polyphenolic fractions from leaves of Vernonia amygdalina and Gongronema latifolium. Food Res Int. 2011;44:2435-2441.
- [Google Scholar]
- Isolation and characterization of Ficus bengalensis Linn. J Drug Delivery Ther. 2019;9:207-211.
- [Google Scholar]
- Chemical composition and antioxidant activity of Ficus elastica Roxb. Ex Hornem and Raphanus sativus L. selective dry extracts with potential antidiabetic activity. Farmacia. 2019;67:764-771.
- [Google Scholar]
- Medicinal plants of Aguambu Bamumbu in the Lebialem highlands, southwest province of Cameroon. African J Pharm Pharmacol. 2009;3:1-13.
- [Google Scholar]
- Antioxidant and alpha-glucosidase inhibitory activities of isoflavonoids from the rhizomes of Ficus tikoua Bur. Nat Prod Res. 2018;32:399-405.
- [Google Scholar]
- Phytochemical screening and analysis of antibacterial and antioxidant activity of Ficus auriculata (Lour.) stem bark. Pharmacog J. 2011;3:49-55.
- [Google Scholar]
- Wound-healing activity of ethanolic and aqueous extracts of Ficus benghalensis. J Adv Pharm Technol Res. 2011;2:110-114.
- [Google Scholar]
- Antiarthritic activity of different plant extracts of Ficus religiosa stem bark in complete Freund’s adjuvant-induced arthritis in rat. Asian Pacific J Health Sci. 2018;5:183-188.
- [Google Scholar]
- Cytotoxic and anticancer activity of F. racemosa fruit extract on MCF7 human breast cancer cell line by SRB method. J Animal Res. 2016;6:43-47.
- [Google Scholar]
- Hypoglycemic effects of leucodelphinidin derivative isolated from Ficus bengalensis (Linn.) Indian J Rhysiol Pharmacol. 1994;38:220-222.
- [Google Scholar]
- Evaluation of comparative analgesic activity of two Ficus species. Int J Res Pharm Chem. 2012;2:969-973.
- [Google Scholar]
- Indigenous uses of plants in different women ailments in Garhwal region. Indian J Pharm Biol Res. 2014;2:39-44.
- [Google Scholar]
- In vitro antioxidant, collagenase inhibition, and in vivo anti-wrinkle effects of combined formulation containing Punica granatum, Ginkgo biloba, Ficus carica, and Morus alba fruits extract. Clin Cosmet Investig Dermatol. 2015;8:389-396.
- [Google Scholar]
- A comprehensive review on phytochemical, pharmacognostical properties and pharmacological activities of Ficus lacor L. (Moraceae) Int J Herbal Med. 2020;8:96-102.
- [Google Scholar]
- Plant resource use and human impact around RBNP. Nepal. J Nat History Museum. 2000;19:3-26.
- [Google Scholar]
- Hypoglycemic activity of Ficus hispida (bark) in normal and diabetic albino rats. Indian J Pharmacol. 2004;36:222-225.
- [Google Scholar]
- Antimicrobial activity of some plant materials used in Armenian traditional medicine. BMC Complement Alter Med. 2017;17:50.
- [Google Scholar]
- Antioxidant activities of Ficus elastica leaves ethanol extract and its compounds. Mol. Cell Biomed. Sci.. 2020;4:27-33.
- [Google Scholar]
- Hepatoprotective activity of Ficus carica leaf extract on rifampicin-induced hepatic damage in rats. Indian J. Pharm. Sci.. 2008;70:364-366.
- [Google Scholar]
- Ficus pumila L. improves the prognosis of patients infected with HTLV-1, an RNA virus. Nutrition J.. 2021;20:16.
- [Google Scholar]
- Medicinal Plants as a source of antipyretic agent in Terai region of Western Nepal. Int. J. Appl. Sci. Biotechnol.. 2013;1:118-126.
- [Google Scholar]
- Ficus benghalensis Linn—The sacred indian medicinal tree with potent pharmacological remedies. Int. J. Pharm. Sci. Rev. Res.. 2015;32:223-227.
- [Google Scholar]
- Phytochemistry and in vitro studies on anti-fertility effect of Ficus religiosa fruits extract on uterine morphology of goat (Capra hircus) Int. J. Drug Dev. Res.. 2014;6:141-158.
- [Google Scholar]
- Specific attraction of fig-pollinating wasps: role of volatile compounds released by tropical figs. J. Chem. Ecol.. 2002;28:283-295.
- [Google Scholar]
- Chemical constituents in Ficus tikoua of Miao nationality. Chin Tradit Herb Drugs. 2007;38:342-344.
- [Google Scholar]
- Screening of Ficus religiosa leaves fractions for analgesic and anti-inflammatory activities. Indian J. Pharmacol.. 2011;43:662-666.
- [Google Scholar]
- Effect of Ficus hispida in inflammatory bowel disease in experimental animals. Int. J. Pharm. Sci. Res.. 2015;6:4391-4397.
- [Google Scholar]
- Ethnomedicinal claims of Ficus semicordata Buch.-Ham. ex Sm.: A review. Int J Green Pharm. 2018;12:S206-S213.
- [Google Scholar]
- Antioxidant and nutritional evaluation of Bhu Udumbara (Ficus semicordata Buch.-Ham. ex Sm.) leaves and fruits: An extra pharmacopoeial drug of Ayurveda. Ayu. 2019;40:120-126.
- [Google Scholar]
- Food and nutritional security through wild edible vegetables or weeds in two districts of Jharkhand, India. J Pharmacog Phytochem. 2017;6:1402-1409.
- [Google Scholar]
- Detailed pharmacognostical and phytochemical screening of stem and stem bark of Ficus semicordata Buch.-Ham. Ex sm. - an extra pharmacopoeial drug of Ayurveda. Pharmacogn J. 2019;11:1303-1311.
- [Google Scholar]
- Hepatoprotective activity of Ficus semicordata Buch.-Ham. ex Sm. leaves aqueous extract on D-galactosamine induced toxicity in HepG2 cell line. Indian J Nat Prod Resour. 2020;11:239-243.
- [Google Scholar]
- Antibacterial activity of ethanolic leaf extracts obtained from various Ficus species (Moraceae) against the fish pathogen, Citrobacter freundii. J Ecol Protect Coastline. 2018;20:117-136.
- [Google Scholar]
- Beneficial effect of Ficus religiosa Linn. on high-fat-diet-induced hypercholesterolemia in rats. Food Chem. 2011;129:162-170.
- [Google Scholar]
- In vitro pro-apoptotic and anti-migratory effects of Ficus deltoidea L. plant extracts on the human prostate cancer cell lines PC3. Front Pharmacol. 2017;8:895.
- [Google Scholar]
- Ethnomedicobotany of Uttara Kannada District in Karnataka, India/plants in treatment of skin diseases. J Ethnopharmacol. 2003;84:37-40.
- [Google Scholar]
- Phytochemical content and antioxidant activity of different fruit parts juices of three figs (Ficus carica L.) varieties grown in Tunisia. Ind Crops Prod. 2016;83:255-267.
- [Google Scholar]
- Hassan, H.A., Allam, A.E., Abu-Baih, D.H., Mohamed, M.F.A., Abdelmohsen, U.R., Shimizu, K., Desoukey, S.Y., Hayallah, A.M., Mahmoud, A., Elrehany, M.A., Mohamed, K.M., Kamel, M.S. 2020. Isolation and characterization of novel acetylcholinesterase inhibitors from Ficus benghalensis L. leaves. RSC Adv 10, 36920 – 36929.
- Haxaire C. 1979. Phytotherapieet Medecine Familiale Chezles Gbaya-Kara (Republique Centrafricaine). These de Doctorat, Fac Pharmacie, Universite de Paris, Paris.
- Protective effect of leaf extract of Ficus carica L. against carbon tetrachloride-induced hepatic toxicity in mice and HepG2 cell line. Tropical J Pharm Res. 2021;20:113-119.
- [Google Scholar]
- Variations in antioxidant content in leaves and fruits of Ficus fistulosa. AIP Conf Proc. 2014;1614:619.
- [Google Scholar]
- In-vitro antimicrobial activity of methanolic extract of Ficus racemosa Linn. fruits. J Sci Innovative Res. 2014;3:446-449.
- [Google Scholar]
- Howlader, M.S.I., Siraj, M.A., Dey, S.K., Hira, A., Ahmed, A., Hossain, M.H. 2017. Ficus hispida bark extract prevents nociception, inflammation, and CNS stimulation in experimental animal model. Evidence-Based Complement Altern Med 2017, Article ID 7390359.
- Howlader, M.S.I., Siraj, M.A., Dey, S.K., Hira, A., Ahmed, A., Hossain, M.H. 2017. Ficus hispida bark extract prevents nociception, inflammation, and CNS stimulation in experimental animal model. Evi-Based Complement Altern Med 2017, Article ID 7390359.
- Two novel triterpenes from the leaves of Ficus macrocarpa. Helv Chim Acta. 2004;87:1071-1076.
- [Google Scholar]
- Ethnobotanical and phytomedicinal knowledge in a long history protected area, the Abruzzo, Lazio and Molise National Park (Italian Apennines) J Ethnopharmacol. 2010;127:379-395.
- [Google Scholar]
- Standardization and wound-healing activity of petroleum, ethanolic and aqueous extracts of Ficus benghalensis leaves. Pharm Chem J. 2021;54:1057-1062.
- [Google Scholar]
- Acute effect of administration of ethanol extracts of Ficus exasperata vahl on kidney function in albino rats. J Med Plants Res. 2007;1:27-29.
- [Google Scholar]
- Screening of antistress activity of Ficus benghalensis fruit extract. Res J Pharm Technol. 2020;13:191-196.
- [Google Scholar]
- Secondary metabolites from Ficus carica roots. Proc Nat Acad Sci India - Section A. 2007;77A:99-100.
- [Google Scholar]
- Evaluation of antioxidant and anticancer potential of flavonoids from aerial roots of Ficus benghalensis Linn. Int J Pharm Res. 2019;11:12-20.
- [Google Scholar]
- Notable Plants in Ethnomedicine of India. New Delhi, India: Deep Publ; 1991.
- Medical plants utilized in Palestinian folk medicine for treatment of diabetes mellitus and cardiac diseases. J Al-Aqsa Unv. 2005;9:1-27.
- [Google Scholar]
- Evaluating the antioxidant and anticancer property of Ficus carica fruits. African J Biotechnol. 2015;14:634-641.
- [Google Scholar]
- Alkaloid constituents of Ficus hispida and their anti-inflammatory activity. Nat Prod Bioprospect. 2020;10:45-49.
- [Google Scholar]
- Phenolic glycosides from Ficus tikoua and their cytotoxic activities. Carbohydrate Res. 2013;382:19-24.
- [Google Scholar]
- Indigenous knowledge and uses of medicinal plants by local communities of the Kali-Gandaki Watershed Area. Nepal. J Ethnopharmacol. 2000;73:175-183.
- [Google Scholar]
- Ficus recemosa bark extract attenuates diabetic complications and oxidative stress in STZ-induced diabetic rats. Pharm Biol. 2016;54:1586-1595.
- [Google Scholar]
- Bioactive furanocoumarin derivatives from Ficus pumila (Moraceae) Philip J Sci. 1997;126:143-153.
- [Google Scholar]
- Influence of heat treatment on antioxidant capacity and (poly)phenolic compounds of selected vegetables. Food Chem. 2016;197:466-473.
- [Google Scholar]
- Antioxidant and anti-inflammatory effects of Ficus erecta var. sieboldii leaf extract in murine macrophage RAW 264.7 cells. Korean J Plant Res. 2018;31:303-311.
- [Google Scholar]
- Pharmacognostic and phytochemical investigation of Ficus carica Linn. Ethnobot Leaflets. 2010;14:599-609.
- [Google Scholar]
- Free radical scavenging and hepatoprotective potential of Ficus microcarpa L. fil. bark extracts. J Nat Med 2011 65, Article number 633
- [Google Scholar]
- Pharmacognostic and phytochemical studies on Ficus microcarpa L. fil. Anc Sci Life. 2012;32:107-111.
- [Google Scholar]
- Evaluation of hepatoprotective activity on the leaves of Ficus benjamina Linn. J Nat Prod Plant Resour. 2011;1:59-69.
- [Google Scholar]
- Documentation of indigenous knowledge on the utilization of plant resource by the Chepang community in Dhading district. Nepal: Central Deptt Bot, Tribhuvan Univ, Kathmandu, Nepal; 2001.
- A pharmacological audit and advancement on the recent trend of research on Ficus benghalensis L. including its in vitro hepatoprotective activity. Clinical Phytosci. 2020;6:84.
- [Google Scholar]
- Pharmacognostical and preliminary phytochemical studies on the leaf extract of Ficus pumila Linn. J Pharmacog Phytochem. 2012;1:105-111.
- [Google Scholar]
- Bioassay-guided evaluation of Ficus semicordata for antidiabetic activity. Int J Pharm Pharm Sci. 2017;9:71-77.
- [Google Scholar]
- Historic and ethnopharmacognosic review on the belief and traditional practices in the treatment of sleeping sickness in West Africa. Bull Soc Med African Noire Lang Fr. 1974;19:400-410.
- [Google Scholar]
- Isolated flavonoids from Ficus racemosa stem bark possess antidiabetic, hypolipidemic and protective effects in albino Wistar rats. J Ethnopharmacol. 2016;181:252-262.
- [Google Scholar]
- Phytochemical content, antioxidant potential, and fatty acid composition of dried Tunisian fig (Ficus carica L.) cultivars. J Appl Bot Food Quality. 2019;92:143-150.
- [Google Scholar]
- In-vitro assessment of cytotoxicity, antioxidant and anti-inflammatory activities of Ficus palmata. J Herbal Med. 2018;13:71-75.
- [Google Scholar]
- Effects of cream containing Ficus carica L. fruit extract on skin parameters: In vivo evaluation. Indian J Pharm Sci. 2014;76:560-564.
- [Google Scholar]
- a. In vitro antioxidant and cytotoxicity analysis of leaves of Ficus racemosa. Free Rad Antioxidants. 2017;7:8-12.
- [Google Scholar]
- Ethno-medicinal species of genus Ficus L. used to treat diabetes in Pakistan. J Appl Pharm Sci. 2011;01:209-211.
- [Google Scholar]
- Ethnobotanical studies on useful trees and shrubs of Haramosh and Bugrote valleys, in Gilgit Northern areas of Pakistan. Pak J Bot. 2007;39:699-710.
- [Google Scholar]
- Superoxide-scavenging and prolyl endopeptidase inhibitory activities of Bangladeshi indigenous medicinal plants. Biosci Biotech Biochem. 2000;64:837-840.
- [Google Scholar]
- Indian Medicinal Plants. New Delhi, India: Springer Private Limited; 2007.
- Angiospermic plants in Chakchaki village of Jhapa district, east Lyonia. Nepal. J Nat History Museum. 2002;21:187-198.
- [Google Scholar]
- Biological and phytochemical evaluation of Cotoneaster microphyllus, Ficus auriculata and Calotropis procera. Lat. Americ J Pharm. 2019;38:945-953.
- [Google Scholar]
- Chemical constituents of the Ficus elastica leaves and their antioxidant activities. Bull Korean Chem Soc. 2012;33:3461-3464.
- [Google Scholar]
- Current trends of traditional herbal medicine practice in Kenya: A review. African J Pharmacol Ther. 2013;2:32-37.
- [Google Scholar]
- A study of the medicinal plants used by the Marakwet Community in Kenya. J Ethnobiol Ethnomed. 2014;10 Article 24
- [Google Scholar]
- Indian Medicinal Plants. Vol Vol 3. Allahabad, Allahabad: Lalit Mohan Publ; 1935.
- Kirtikar, K.R., Basu, B.D. 1987. Indian Medicinal Plants. Vol 1-4, Lalit Mohan Basu, Allahabad, India.
- Indian Medicinal Plants (Second edition). Dehra Dun: Bishen Singh & Mahendra Pal Singh; 1975.
- Indian Medicinal Plants. Vol Vols I-IV. India: International Book Distributors, Dehra Dun; 1995.
- Response to comment on Early domesticated fig in the Jordan valley. Science. 2006;314:1683.
- [Google Scholar]
- New dammarane-type acetylated triterpenoids and their related compounds of Ficus pumila fruit. Chem Pharm Bull. 1999;47:1138-1140.
- [Google Scholar]
- Three new sesquiterpenoid glucosides of Ficus pumila fruit. Chem Pharm Bull. 2000;48:77-80.
- [Google Scholar]
- In-vivo anti-inflammatory and analgesic screening of Ficus bengalensis leaf extract in rats. Asian J Res Pharm Sci. 2014;4:174-178.
- [Google Scholar]
- Hepatoprotective activity of Ficus carica Linn. leaf extract against carbon tetrachloride-induced hepatotoxicity in rats. DARU. 2007;15:162-166.
- [Google Scholar]
- Comparative clinical study of ‘Panchavalkal ointment’ & framycetin sulfate cream (local application) in the management of infected wound wrt Dushta Vrana. J Adv Res Ayurveda Yoga Unani Sidhha Homeopat. 2019;6:17-22.
- [Google Scholar]
- Hypoglycemic effect of Ficus microcarpa leaves (Chinese Banyan) on alloxan-induced diabetic rats. J Biol Sci. 2007;7:321-326.
- [Google Scholar]
- Antibacterial activity of methanolic and ethanolic extracts of leaves and fruits of Ficus palmata Forssk. Res J Pharmacog Phytochem. 2012;4:310-313.
- [Google Scholar]
- Antidiabetic activity of Ficus gibbosa Blume extracts in streptozotocin–nicotinamide-induced diabetes in Sprague Dawley rats. J Drug Res Ayurvedic Sci. 2020;5:57-62.
- [Google Scholar]
- Antioxidant and cytotoxic activity of combined extracts prepared using Ficus religiosa and Ficus benghalensis leaves against cervical cancer cell line (Hela) Asian J Pharm Clin Res. 2018;11:407-410.
- [Google Scholar]
- Evaluation of phytochemical, antioxidant, antibacterial and anticancerous activity of Ficus auriculata Lour. and Osyris wightiana Wall. ex Wight. Bull Env Pharmacol Life Sci. 2018;7:64-70.
- [Google Scholar]
- Effect of Pelargonidin isolated from Ficus benghalensis L. on phenotypic changes in zebrafish (Danio rerio) embryos. Saudi Pharm J. 2017;25:249-257.
- [Google Scholar]
- Ficus (Fig) species in Nepal: A review of diversity and indigenous uses. Lyonia. 2006;11:85-97.
- [Google Scholar]
- The anti-inflammatory effect of ethanol extract gel of fig leaves (Ficus carica Linn.) and sidr leaves (Ziziphus mauritiana Linn.) Indian J Pharm Educ Res. 2021;55:S518-S527.
- [Google Scholar]
- Allelopathic potential and phenolic allelochemicals discrepancies in Ficus carica L. cultivars. South African J Bot. 2020;130:30-44.
- [Google Scholar]
- Anti-proliferative effect of Ficus pumila Linn. on human leukemic cell lines. Int J Basic Clin Pharmacol. 2015;4:330-336.
- [Google Scholar]
- Evaluation of the hepatoprotective potential of hydroethanolic extract of Ficus pumila L. on CCl4 induced liver damage in rats. Global J Res Med Plants Indigenous Med Koppa. 2016;5:217-225.
- [Google Scholar]
- Aromadendrin inhibits T cell activation via regulation of calcium influx and NFAT activity. Molecules. 2020;25:4590.
- [Google Scholar]
- Antioxidant flavonoid glycosides from the leaves of Ficus pumila L. Food Chem. 2008;109:415-420.
- [Google Scholar]
- Chemical constituents from roots of Ficus hirta. Zhongguo Zhong Yao Za Zhi. 2006;31:131-133.
- [Google Scholar]
- A comprehensive review on phytochemistry, bioactivities, toxicity studies, and clinical studies on Ficus carica Linn. leaves. Biomed Pharmacother. 2021;137:111393
- [Google Scholar]
- Valorization of Fig (Ficus carica L.) waste leaves: HPLC-QTOF-MS/MS-DPPH system for online screening and identification of antioxidant compounds. Plants. 2021;10:2532.
- [Google Scholar]
- Liao, C.-R., Kao, C.-P., Peng, W.-H., Chang, Y.-S., Lai, S.-C., Ho, Y.-L. 2012. Analgesic and anti-inflammatory activities of methanol extract of Ficus pumila L. in mice. Evi-Based Complement Alter Med 2012, Article ID 340141.
- Edible Medicinal and Non-Medicinal Plants. Switzerland: Springer Nature; 2012.
- Experimental and computational approaches to estimate solubility and permeability in drug discovery and development settings. Adv Drug Deliv Rev. 2001;46:3-26.
- [Google Scholar]
- Anti-inflammatory and antiproliferative prenylated isoflavone derivatives from the fruits of Ficus carica. J Agric Food Chem. 2019;67:4817-4823.
- [Google Scholar]
- Wound healing activity of ethanolic extract of Ficus racemosa leaves. Indian Drugs. 2013;50:15-24.
- [Google Scholar]
- Grape seed procyanidin extract protects against Pb-induced lung toxicity by activating the AMPK/Nrf2/p62 signaling axis. Food Chem Toxicol. 2018;116:59-69.
- [Google Scholar]
- Diversity and use of medicinal plants for soup making in traditional diets of the Hakka in West Fujian. China. J Ethnobiol Ethnomed. 2019;15:60.
- [Google Scholar]
- The hepatoprotective effect of aqueous extracts from Ficus hirta on N, N-dimethylformamide induced acute liver injury in mice. Zhong Yao Cai. 2008;31:1364-1368.
- [Google Scholar]
- Soy isoflavones alleviate polycystic ovary syndrome in rats by regulating NF-κB signaling pathway. Bioengineered. 2021;12:7215-7223.
- [Google Scholar]
- Anti-edematogenic and analgesic activities of Ficus benghalensis. Int J Nutr Pharmacol Neurol Dis. 2012;2:100-104.
- [Google Scholar]
- Analgesic activity of some Indian medicinal plants. J Ethnopharmacol. 2006;106:425-428.
- [Google Scholar]
- Radioprotective activity of some medicinal plant extracts. Acta Hortic. 2011;925:315-320.
- [Google Scholar]
- Ethnobotanical notes on certain medicinal plants used by Tharus of Dang Deokhuri district. Nepal. Int J Crude Drug Res. 1985;23:153-159.
- [Google Scholar]
- A survey of medicinal plants of Jajarkot district. Nepal. J Ethnopharmacol. 1995;48:1-6.
- [Google Scholar]
- Protective effect of leaf extract of Ficus hispida Linn. against paracetamol-induced hepatotoxicity in rats. Phytother Res. 2000;14:457-459.
- [Google Scholar]
- Ethnomedicinal plants used to cure jaundice in Kammam district of Andhra Pradesh, India. J Phytol. 2011;3:33-35.
- [Google Scholar]
- Ethnomedicinal plants used to cure jaundice in Kammam district of Andhra Pradesh. India J Phytol. 2011;3:33-35.
- [Google Scholar]
- Anti-rheumatic and antioxidant activity of extract of stem bark of Ficus bengalensis. Res J Chem Sci. 2011;1:1-8.
- [Google Scholar]
- GC-MS analysis of bioactive components of Ficus religiosa (Linn.) stem. Int J Pharm Bio Sci. 2013;4:99-103.
- [Google Scholar]
- Analgesic activity of bark and leaves of Ficus religiosa L. from Nepal. Int J Pharm Pharm Sci. 2020;12:32-35.
- [Google Scholar]
- A study on anti-arthritic activity of Ficus benghalensis bark extract. J Pharmacog Phytochem. 2019;8:1825-1828.
- [Google Scholar]
- Metal-catalysed disruption of membrane protein and lipid signalling in the pathogenesis of neurodegenerative disorders. Ann N Y Acad Sci. 2004;1012:37-50.
- [Google Scholar]
- Antidiabetic and antihyperlipidemic effect of Euphorbia hirta in streptozotocin induced diabetic rats. Der Pharmacia Lett. 2012;4:703-707.
- [Google Scholar]
- Ficus carica leaves decoction on glycemic factors of patients with type 2 diabetes mellitus: A double-blind clinical trial. Jundishapur J Nat Pharm Prod. 2016;11:e25814.
- [Google Scholar]
- Medicinal plants of rural India: A Review of use of medicinal plant by Indian folks. Indo Global J Pharm Sci. 2012;2:286-304.
- [Google Scholar]
- Ceramide, cerebroside and triterpenoid saponin from the bark of aerial roots of Ficus elastica (Moraceae) Phytochem. 2012;83:95-103.
- [Google Scholar]
- Antimicrobial, wound healing and antioxidant activity of Plagiochasma appendiculatum Lehm. et Lind. J Ethnopharmacol. 2006;107:67-72.
- [Google Scholar]
- Megersa, M., Tamrat, N. 2022. Medicinal plants used to treat human and livestock ailments in Basona Werana district, North Shewa Zone, Amhara Region, Ethiopia. Evi-Based Complement Alter Med 2022, Article ID 5242033.
- Diversity, utilization, and sacred values of ethno-medicinal plants of Kumaun Himalaya. Trop Plant Res. 2014;1:80-86.
- [Google Scholar]
- Evaluation of Ficus carica Linn fruit extracts on formalin induced pain in the male rat paw. Ann Mil Health Sci Res. 2019;17:e97383.
- [Google Scholar]
- A study of traditional medicinal plant and its awareness among people of Dhading district. Nepal: Central Deptt Social Sci, Tribhuvan University, Kathmandu, Nepal; 1998.
- Mohd Dom, N.S., Yahaya, N., Adam, Z., Rahman, N.M.A.N.A., Hamid, M. 2020. Antiglycation and antioxidant properties of Ficus deltoidea varieties. Evi-Based Complement Altern Med 2020, Article ID 6374632.
- Clinical study to evaluate the effect of kakodumbar (Ficus hispida Linn) in shwitra with special reference to vitiligo. Aayushi Int Interdiscipl Res J VIII 2021:66-68.
- [Google Scholar]
- Comparison of the Ficus for the wound healing activity in various species. Int J Res Phytochem Pharmacol. 2020;10:67-70.
- [Google Scholar]
- Chemical findings and in vitro biological studies to uphold the use of Ficus exasperata Vahl leaf and stem bark. Food Chem Toxicol. 2018;112:134-144.
- [Google Scholar]
- Hepatoprotective activity of the ethanolic extract of Ficus carica Linn. leaves in carbon tetrachloride-induced hepatotoxicity in rats. Iranian J Pharm Res. 2011;10:301-306.
- [Google Scholar]
- Antidiabetic effect of fruit and cork extracts of Ficus lacor Buch. Ham. in streptozotocin-induced diabetic rats. J Appl Pharm Sci. 2022;12:131-139.
- [Google Scholar]
- Metabolite profiling and inhibitory properties of leaf extracts of Ficus benjamina towards α-glucosidase and α-amylase. Int J Food Properties. 2018;21:1560-1574.
- [Google Scholar]
- Hypoglycemic and hypolipidemic activity of Ficus mollis leaves. Brazilian J Pharmacog. 2013;23:687-691.
- [Google Scholar]
- Antimicrobial activity of Ficus benghalensis and Ficus racemosa roots L. American J Microbiol. 2011;2:21-24.
- [Google Scholar]
- Enhancement of wound healing with roots of Ficus racemosa L. in albino rats. Asian Pac J Trop Biomed. 2012;2:276-280.
- [Google Scholar]
- Healing promoting potentials of roots of Ficus benghalensis L. in albino rats. Asian Pac J Trop Med. 2011;2011:921-924.
- [Google Scholar]
- Phytochemistry, pharmacological properties, and recent applications of Ficus benghalensis and Ficus religiosa. Plants. 2021;10:2749.
- [Google Scholar]
- An in vitro study of wound healing activity of Ficus deltoidea leaf extract. J Teknol. 2015;3:67-72.
- [Google Scholar]
- Antioxidant and antiproliferative potentials of Ficus glumosa and its bioactive polyphenol metabolites. Pharmaceuticals. 2021;14:266.
- [Google Scholar]
- Impact of maturity on phenolic composition and antioxidant activity of medicinally important leaves of Ficus carica L. Physiol Mol Biol Plants. 2018;24:881-887.
- [Google Scholar]
- Indian Materia Medica. Vol Vol 1. Bombay: Popular Prakashan; 1976.
- Nadkarni, K.M., Nadkarni, A.K. 1976a. Dr. K.M. Nadkarni's Indian Materia Medica: with Ayurvedic, Unani-Tibbi, Siddha, Allopathic, Homeopathic, Naturopathic & Home Remedies, Appendices & Indexes. Popular Prakashan Private Ltd, Bombay, India.
- Indian Materia Medica. Vol Vol 6. Mumbai, India: Popular Prakashan Pvt LTD; 1976.
- Indian Materia Medica. Bombay: Popular Prakashan; 1976.
- Antibacterial activity of Ficus microcarpa L. extract on gram positive and gram-negative bacteria. J Pharmacog Phytochem. 2016;5:102-104.
- [Google Scholar]
- Antidiabetes constituents, cycloartenol and 24- methylenecycloartanol, from Ficus krishnae. PLoS ONE. 2020;15:e0235221.
- [Google Scholar]
- Medicinal plant used by Lama community of Ichhangu village in Kathmandu. Central Nepal: Central Deptt Bot, Tribhuvan Univ, Kathmandu, Nepal; 2001.
- Two new phytosterols from the stem bark of Ficus bengalensis L. J Saudi Chem Soc. 2015;19:650-654.
- [Google Scholar]
- Synthesis of α-amyrin derivatives and their in vivo antihyperglycemic activity. Eur J Med Chem. 2009;44:1215-1222.
- [Google Scholar]
- Wound healing activity of the hydro alcoholic extract of Ficus religiosa leaves in rats. Internet J Altern Med. 2008;6:1-5.
- [Google Scholar]
- Study on the chemical constituents of Ficus semicordata. Tap Chi Hoa Hoc. 2002;40:69-71.
- [Google Scholar]
- Some compounds isolated from Momordica chchinchinensis seed and Ficus glomerata bark. Ly Va Sinh Hoc. 2001;6:66-69.
- [Google Scholar]
- Anticancer activity of novel plant extracts and compounds from Adenosma bracteosum (Bonati) in human lung and liver cancer cells. Molecules. 2020;25:2912.
- [Google Scholar]
- Inhibition of Inulacappa (Ham. Ex D. Don) DC. Extracts on herpes simplex virus infection in vitro. Afr J Microbiol Res. 2011;5:4049-4058.
- [Google Scholar]
- Medicinal plants used for peptic ulcer in the Bangangte region, western Cameroon. Fitoterapia. 2000;71:406-412.
- [Google Scholar]
- Medicinal plants used for intestinal diseases in Mbalmayo Region, Central Province, Cameroon. Fitoterapia. 2001;72:246-254.
- [Google Scholar]
- Identification of alkaloid’s profile in Ficus benjamina L. extracts with higher antioxidant power. Americ J Plant Sci. 2014;5:4029-4039.
- [Google Scholar]
- Acute and subchronic toxicity study of the ethanol extracts from Ficus deltoidea leaves in male mice. Open Access Macedonian. J Med Sci. 2020;8(A):76-83.
- [Google Scholar]
- Ficus vogelii methanol leaf extract ameliorates inflammation and arthritis by modulating osmotic fragility in C57BL/6J mice. Niger J Physiol Sci. 2021;36:221-226.
- [Google Scholar]
- Phenolic compounds from sandpaper (Ficus exasperata) leaf inhibits angiotensin 1 converting enzyme in high cholesterol diet fed rats. J Ethnopharmacol. 2014;157:119-125.
- [Google Scholar]
- Toxicity and effects of fig (Ficus carica) leaf aqueous extract on haematology and some biochemical indices of Wistar albino rats (Rattus norvegicus) J Med Plants Res. 2016;10:298-305.
- [Google Scholar]
- Assessment of antioxidant activity of Ficus asperifolia Miq aqueous extract - In vitro studies. J Phytopharmacol. 2014;3:16-21.
- [Google Scholar]
- Antidiabetic activity of the ethyl acetate fraction of Ficus lutea (Moraceae) leaf extract: comparison of an in vitro assay with an in vivo obese mouse model. BMC Complement Altern Med. 2016;16:110.
- [Google Scholar]
- Ficus carica L.: Metabolic and biological screening. Food Chem Toxicol. 2009;47:2841-2846.
- [Google Scholar]
- Identification of proanthocyanidin dimers and trimers, flavone C-glycosides, and antioxidants in Ficus deltoidea, a malaysian herbal tea. J Agric Food Chem. 2011;59:1363-1369.
- [Google Scholar]
- Production of Flavonoid compounds in cell cultures of Ficus deltoidea as influenced by medium composition. Int J Med Aromatic Plants. 2011;1:62-74.
- [Google Scholar]
- Antimicrobial activity of Ficus leaf extracts on some fungal and bacterial pathogens of Dioscorea rotundata from Southwest Nigeria. J Biol Sci. 2011;11:359-366.
- [Google Scholar]
- Traditional uses of medicinal plants of Pauri Garhwal, Uttrakhand. Nat Sci. 2010;8:57-61.
- [Google Scholar]
- Comparative evaluation of antiulcer activity of Ficus religiosa (stem bark) extracts prepared by different methods of extraction. Curr Trad Med. 2020;6:350-358.
- [Google Scholar]
- Phytochemical Screening and Anti-inflammatory activity of Murraya Koenigii & Ficus Lacor roots in carrageenan, histamine and serotonin induced paw edema in albino Wistar rats. Int J Rec Adv Sci Technol. 2019;6:1-12.
- [Google Scholar]
- Antidiabetic effect of Ficus religiosa extract in streptozotocin-induced diabetic rats. J Ethnopharmacol. 2010;128:462-466.
- [Google Scholar]
- Indigenous knowledge on medicinal plants in Bhagawati village, Darchula. Nepal. Botanica Orient. 2004;4:79-83.
- [Google Scholar]
- Medicinal plants used in cuts and wounds in Kaski district West Nepal and their antibacterial activities. Central Deptt Bot: Tribhuvan University, Kathmandu, Nepal; 2001.
- Hepatoprotective activity of Ficus religiosa leaves against isoniazid+rifampicin and paracetamol induced hepatotoxicity. Pharmacog Research. 2013;5:271-276.
- [Google Scholar]
- Recent antimicrobial and pharmacological studies in Ficus religiosa Linn. Int J Curr Microbiol Appl Sci. 2014;3:461-475.
- [Google Scholar]
- Antioxidative and anti-inflammatory activities of an ethanol extract from fig (Ficus carica) branches. Food Sci Biotechnol. 2013;22:1071-1075.
- [Google Scholar]
- Constituents with tyrosinase inhibitory activities from branches of Ficus erecta var. sieboldii King. J Enzyme Inhibit Med Chem. 2012;27:390-394.
- [Google Scholar]
- Antidiabetic and in vitro antioxidant potential of Hybanthus enneaspermus (Linn) F. Muell in streptozotocin-induced diabetic rats. Asian Pac J Trop Biomed. 2011;1:316-322.
- [Google Scholar]
- Antimicrobial activity of Ficus religiosa against some pathogenic microorganisms. Int J Progressive Res Sci Eng. 2021;2:759-763.
- [Google Scholar]
- Evaluation of anti-inflammatory activity of Ficus carica Linn. leaves. Indian J Nat Prod Resour. 2011;2:151-155.
- [Google Scholar]
- Anti-inflammatory evaluation of Ficus carica Linn. leaf extract on rats. Biochem Pharmacol. 2013;2:4.
- [Google Scholar]
- A comparative evaluation of anti-inflammatory activity of the bark of Ficus bengalensis in plants of different age. J Basic Clin Pharm. 2010;1:107-113.
- [Google Scholar]
- Indigenous home remedies as applied in Shirpur Taluka of Dhule district (Maharashtra) India. Curr Bot. 2011;2:34-35.
- [Google Scholar]
- Antihyperglycemic and hypoglycemic Effect of Ficus racemosa Leaves. J Nat Remedies. 2010;10:1-16.
- [Google Scholar]
- Hypoglycemic properties of Ficus glomerata fruits in alloxan-induced diabetic rats. J Nat Remedies. 2006;6:120-123.
- [Google Scholar]
- Ethnobotanical Study of the Tharus Living in Central Part of Dang. Midwest Nepal: Central Department of Botany, Tribhuvan University, Kathmandu, Nepal; 2000.
- Constituents of the leaves and twigs of Ficus hispida. Planta Med. 2002;68:186-188.
- [Google Scholar]
- Experimental diabetes treated with Ficus carica extract: Effect on oxidative stress parameters. Acta Diabetol. 2003;40:3-8.
- [Google Scholar]
- Polyphenolic profiling and chemometric analysis of leaves from Italian Ficus carica L. varieties. Polyphenol compounds in common fig. European J Horticult Sci. 2018;83:94-103.
- [Google Scholar]
- Ethnobotanical uses of plants among the bhotiya tribal communities of Niti valley in Central Himalaya, India. Ethnobot Res Appl. 2010;8:233-244.
- [Google Scholar]
- Effects of Marapuama in the chronic mild stress model: Further indication of antidepressant properties. J Ethnopharmacol. 2008;118:300-304.
- [Google Scholar]
- Hepatoprotective effect of crude aqueous leaf extract of fig tree, Ficus benjamina, on ethanol-induced liver damage in mice. Int J Sci Technol Res. 2017;6:118-121.
- [Google Scholar]
- Antibacterial and antifungal approaches of Ficus racemosa. Pharmacog J. 2019;11:355-357.
- [Google Scholar]
- Hypoglycemic and antihyperlipidemic effect of aqueous leaves extract of Ficus religiosa in alloxan induced diabetic rats. Asian J Med Sci. 2017;8:50-55.
- [Google Scholar]
- Composition and bioactivities of the leaf essential oil of Ficus religiosa Linn. Americ J Essen Oil. 2015;2:16-17.
- [Google Scholar]
- Comparison and assessment of flixweed and fig effects on irritable bowel syndrome with predominant constipation: A single-blind randomized clinical trial. Explore (NY). 2019;15:198-205.
- [Google Scholar]
- A handbook of medicinal plants: Section II. Jodhpur, India: Agrobios Publishers; 2007.
- Phytochemical composition and antioxidant activity of Ficus elastica Roxb. (Moraceae) leaves. Res J Pharm Tech. 2015;8:259-264.
- [Google Scholar]
- GC-MS analysis of bioactive components of Ficus mollis, vahl leaves. J Med Plants Studies. 2018;6:98-100.
- [Google Scholar]
- Study of anti-inflammatory activity of Ficus racemosa Linn stem bark extract in albino rats. Int J Basic Clin Pharmacol. 2021;10:231-237.
- [Google Scholar]
- Ethnobotanically important plants of Kishanpur Wildlife Sanctuary, Uttar Pradesh, India, East Himalayan society for spermatophyte taxonomy. Pleione. 2016;10:32-42.
- [Google Scholar]
- Anticancer activity of methanol extract of Ficus carica leaves and fruits against proliferation, apoptosis, and necrosis in Huh7it cells. Cancer Informatics. 2019;18:1-7.
- [Google Scholar]
- Phytochemical activities of Ficus (Moraceae) in Java Island, Indonesia. Bonorowo Wetlands. 2020;10:98-125.
- [Google Scholar]
- Anti-diabetic effects of Ficus asperifolia in streptozotocin-induced diabetic rats. J Diabetes Metab Disor. 2020;19:605-616.
- [Google Scholar]
- Preliminary phytochemical screening, analgesic and anti-inflammatory activity of Ficus glomerata fruit extract. European J Med Plants. 2016;14:1-10.
- [Google Scholar]
- A taxonomic and ethno-medicinal study of species from Moraceae (mulberry) family in Bangladesh flora. Res Plant Sci. 2013;1:53-57.
- [Google Scholar]
- Medicinal plant sources and traditional healthcare practices of forest-dependent communities in and around Chunati Wildlife Sanctuary in southeastern Bangladesh. Env Sustainability. 2022;5:207-241.
- [Google Scholar]
- Mosquito larvicidal activity of gluanol acetate, a tetracyclic triterpene derived from Ficus racemosa Linn. Parasitol Res. 2008;103:333.
- [Google Scholar]
- Comparative study of wound-healing effect of bark extracts of Ficus religiosa & Ficus benghalensis by mice model. J Pharmacog Phytochem. 2019;8:1815-1821.
- [Google Scholar]
- Evaluation of phytochemical screening and antibacterial activity of Ficus auriculata Lour. (Moraceae) – An traditional medicinal plant. Kong Res J. 2021;8:22-29.
- [Google Scholar]
- Antinociceptive effect of Ficus bengalensis bark extract in experimental models of pain. Cureus. 2018;10:e2259.
- [Google Scholar]
- Ethnobotanical study of medicinal plants used by Tharu community of parrohavdc, Rupandehi district. Nepal. Sci World. 2009;7:80-84.
- [Google Scholar]
- Ethno-medicinal Plants used by chepang community in Nepal. J Plant Res. 2017;15:21-30.
- [Google Scholar]
- Phytochemical analysis and anti-arthritic activity of Ficus carica leaves. Asian J Res Chem. 2020;13:151-154.
- [Google Scholar]
- Evaluation of anti-inflammatory activity of Ficus retusa (Moraceae) Int J Res Ayur Pharm. 2011;2:5151-5517.
- [Google Scholar]
- Evaluation of antioxidant and antimicrobial activity of methanolic extract of Ficus fistulosa leaves: An unexplored phytomedicine. PharmacologyOnLine. 2019;1:354-360.
- [Google Scholar]
- Hepatoprotective activity of Ficus mollis (Vahl) leaf extract on CCl4 induced hepatic damage in Wistar albino rats. Res J Pharm Biol Chem Sci. 2010;1:338-343.
- [Google Scholar]
- Protective effect of Ficus bengalensis L. extract against H2O2 induced DNA damage and repair in neuroblastoma cells. Free Rad Antioxidants. 2014;4:3-7.
- [Google Scholar]
- In vitro and in vivo anti-malarial activity of Boerhavia elegans and Solanum surattense. Malaria J. 2010;9:124-130.
- [Google Scholar]
- Antioxidative and antibacterial activities of phenolic compounds from Ficus sur Forssk. and Ficus sycomorus L. (Moraceae): potential for sickle cell disease treatment in Burkina Faso. Int J Brain Cogn Sci. 2012;6:328-336.
- [Google Scholar]
- Glucose lowering efficacy of Ficus racemosa bark extract in normal and alloxan diabetic rats. Phytother Res. 2002;16:590-592.
- [Google Scholar]
- Phytochemical composition and antioxidant activity of Ficus benghalensis (Moraceae) leaf extract. J Biol Act Prod Nat. 2014;4:236-248.
- [Google Scholar]
- HPTLC densitometric quantification of stigmasterol and lupeol from Ficus religiosa. Arabian J Chem. 2015;8:366-371.
- [Google Scholar]
- Antiarthritic activity of ethanolic extract of Ficus religiosa leaves in FCA induced arthritis in rats. World J Pharm Res. 2018;7:778-789.
- [Google Scholar]
- Cholinesterase inhibitory constituents from Ficus bengalensis. J Asian Nat Prod Res. 2012;14:1149-1155.
- [Google Scholar]
- Antihyperglycemic activity of Ficus carica leaves extracts on streptozotocin induced diabetic rats. Res J Pharm Technol. 2021;14:4151-4156.
- [Google Scholar]
- Wound healing potential of leaf extracts of Ficus religiosa on Wistar albino strain rats. Int J PharmTech Res. 2009;1:506-508.
- [Google Scholar]
- Anti-inflammatory and anti-hyperuricemic effect of Ficus benghalensis bark extract in Raw 246.7 cell line. Indian J Pharm Educ Res. 2022;56:520-528.
- [Google Scholar]
- Antidiabetic potential of alcoholic and aqueous extracts of Ficus racemosa Linn. bark in normal and alloxan induced diabetic rats. Int J Pharm Sci Drug Res. 2009;1:24-27.
- [Google Scholar]
- The anti-inflammatory effect of a crude aqueous extract of the root bark of “Ficus elastica” in the rat. Arch Int Pharmacodyn Ther. 1986;281:169-176.
- [Google Scholar]
- Irritant potential of triterpenoids from Ficus carica leaves. Fitoterapia. 2002;73:417-420.
- [Google Scholar]
- In vitro antimicrobial activity, nutritional profile and phytochemical screening of wild edible fruit of Garhwal Himalaya (Ficus auriculata) Int J Pharm Sci Rev Res. 2012;12:61-64.
- [Google Scholar]
- Antinociceptive activity of Ficus deltoidea var trengganuensis aqueous extract in mice. J Pharmacol Toxicol Investig. 2015;1:51-53.
- [Google Scholar]
- Antimicrobial activity of leaves of Ficus benghalensis against isolated dental pathogens. Int J Pharm Sci Res. 2022;13:251-256.
- [Google Scholar]
- Evaluation of anti-inflammatory and analgesic activities of Ficus glomerata Linn. fruit extract. Nat Prod Indian J. 2008;4:187-189.
- [Google Scholar]
- Saoudi, M., El Feki, A. 2012. Protective role of Ficus carica stem extract against hepatic oxidative damage induced by methanol in male Wistar rats. Evi-Based Complement Altern Med 2012, Article ID 150458.
- Ficus carica (fig) paste supplementation in patients with multiple sclerosis associated constipation; a double blind randomized clinical trial. Planta Med. 2015;81 - SL2A_04
- [Google Scholar]
- Two new polyphenolic compounds from Ficus retusa L.“variegata” and the biological activity of the different plant extracts. J Pharmacog Phytother. 2011;3:89-100.
- [Google Scholar]
- Analysis of medicinal and economic important plant species of Hollongapar Gibbon wildlife sanctuary, Assam, northeast India. Trop Plant Res. 2017;4:485-496.
- [Google Scholar]
- Medicinal Ficus species of temple city of Odisha. J Biodivers Conservation. 2017;1:S1-S5.
- [Google Scholar]
- Selective neuronal vulnerability in neurodegenerative diseases: from stressor thresholds to degeneration. Neuron. 2011;71:35-48.
- [Google Scholar]
- Hepatoprotective activity of Ficus religiosa leaf extract in rats. Curr Res Pharm Sci. 2017;7:64-68.
- [Google Scholar]
- Pharmacognostical evaluation of medicinally important Ficus retusa (leaves and bark) J Acute Dis. 2013;2013:300-303.
- [Google Scholar]
- Hypoglycemic action of an oral fig-leaf decoction in type-I diabetic patients. Diabetes Res Clin Pract. 1998;39:19-22.
- [Google Scholar]
- GC-MS analysis of phytoconstituents in Ficus mollis Vahl leaves. Int J Modern Pharm Res. 2020;4:213-220.
- [Google Scholar]
- In vitro antioxidant activity of Ficus carica L. latex from 18 different cultivars. Sci Rep. 2020;10:10852.
- [Google Scholar]
- New insights of phenolic compounds from optimized fruit extract of Ficus auriculata. Sci Rep. 2021;11:12503.
- [Google Scholar]
- Anticancer potential of Ficus religiosa (Linn) against human breast cancer cell line MDA-MB231 and its phytoconstituents by HR-LCMS. Res Square 2020
- [CrossRef] [Google Scholar]
- Shakya, P.R. 2000. Intellectual heritage on folk medicine in Nepal. In: Proceeding of Nepal Japan Joint Symposium on Conservation and Utilization of Himalayan Medicinal Resources, Kathmandu, Nepal, November 6–11, 43 - 49.
- Analysis of the chemical constituents of volatile oil from the leaves of Ficus auriculata Lour by GC-MS. Chem Ind Forest Prod. 2013;33:135-137.
- [Google Scholar]
- Lactones from Ficus auriculata and their effects on the proliferation function of primary mouse osteoblasts in vitro. Bioorg Med Chem Lett. 2014;24:3952-3955.
- [Google Scholar]
- A new12-membered lactone from the stems of Ficus auriculata. Nat Prod Res. 2018;32:2268-2273.
- [Google Scholar]
- A new isoflavone from the fruits of Ficus auriculata and its antibacterial activity. Nat Prod Res. 2022;36:1191-1196.
- [Google Scholar]
- Evaluation of the phytochemicals and antidiabetic activity of Ficus bengalensis. Int J Diabetes Dev Ctries. 2007;27:56-59.
- [Google Scholar]
- Hypoglycemic activity of Ficus glomerata in alloxan-induced diabetic rats. Int J Pharm Sci Rev Res. 2010;1:18-22.
- [Google Scholar]
- Profiling of polyphenolic compounds of Ficus palmata fruits via Ultra high-performance liquid chromatography with diode array detector spectrometry. Med Plants - Int J Phytomed Related Ind. 2021;13:524-528.
- [Google Scholar]
- Comparison of extraction yield, phytochemicals, and in vitro antioxidant potential of different extracts of two Ficus species. Plant Archiv. 2020;20:2669-2673.
- [Google Scholar]
- Ethnomedicinal claims of Ficus semicordata Buch-Ham. Ex Sm: A review. Int J Green Pharm. 2018;12:206-213.
- [Google Scholar]
- Acute toxicity study of crude methanol leaf extract of Ficus exasperata Vahl on male Wistar albino rats. Biokemistri. 2020;32:47-53.
- [Google Scholar]
- An ethnobotanical study of the less known wild edible figs (genus Ficus) native to Xishuangbanna. Southwest China. J Ethnobiol Ethnomed. 2014;10:68.
- [Google Scholar]
- New alkaloids and α-glucosidase inhibitory flavonoids from Ficus hispida. Chem Biodivers. 2016;13:445-450.
- [Google Scholar]
- The genus Ficus (Moraceae) used in diet: Its plant diversity, distribution, traditional uses and ethnopharmacological importance. J Ethnopharmacol. 2018;226:185-196.
- [Google Scholar]
- Hepatoprotective activity of Ficus bengalensis Linn leaves. J Curr Pharma Res. 2012;2:503-507.
- [Google Scholar]
- Documentation of indigenous knowledge on the utilization of plant resources by the Tharu communities around Royal Bardia National Park. West Nepal: Central Deptt Bot, Tribhuvan Univ, Kathmandu, Nepal; 1997.
- Medico-ethnobotany of magar community in Salija VDC of Parbat District, Central Nepal. Our Nat. 2012;10:176-190.
- [Google Scholar]
- Antihyperglycemic activity of petroleum ether leaf extract of Ficus krishnae L. on alloxan-induced diabetic rats. Indian J Pharm Sci. 2014;76:323-331.
- [Google Scholar]
- Benjaminamide: A new ceramide and other compounds from the twigs of Ficus benjamina (Moraceae) Biochem Syst Ecol. 2008;36:238-243.
- [Google Scholar]
- Phytochemical and pharmacognostical investigation on aerial roots of Ficus lacor Buch. Ham. Int J Phytomed. 2013;5:267-277.
- [Google Scholar]
- Anti-inflammatory potential of different extracts isolated from the roots of Ficus lacor Buch. Hum and Murraya koenigii L. Spreng. Archiv Biol Sci Belgrade. 2014;66:1261-1270.
- [Google Scholar]
- Antidiabetic effect of Ficus bengalensis aerial roots in experimental animals. J Ethnopharmacol. 2009;123:110-114.
- [Google Scholar]
- Investigation of antiplasmodial efficacy of lupeol and ursolic acid isolated from Ficus benjamina leaves extract. Nat Prod Res. 2020;34:2514-2517.
- [Google Scholar]
- Ethnopharmacological and phytochemical attributes of Indian Tinospora species: A comprehensive review. Arabian J Chem. 2021;14:103381
- [Google Scholar]
- Wound healing activity of leaf methanolic extract of Ficus hispida Linn. African J Pharm Pharmacol. 2014;8:21-23.
- [Google Scholar]
- Ascorbic acid, total polyphenols, and antioxidant activity of Ficus carica fruits. J Food Chem Nutr. 2018;6:21-27.
- [Google Scholar]
- Antioxidant properties of Ficus species – A review. Int J PharmTech Res. 2010;2:2174-2182.
- [Google Scholar]
- Anticancer effect of fig fruit Ficus racemosa extract against human heptocellar carcinoma (HepG-2) cell line. Int J Res Analytical Rev. 2019;6:767-783.
- [Google Scholar]
- Siwakoti, M., Siwakoti, S. 2000. Ethnomedicinal uses of plants among the Satar tribe of Nepal. In: Ethnobotany and Medicinal Plants of Indian Subcontinent Maheswari, JK (Eds). Scientific publisher, Jodhpur, India, pp 98 - 108.
- A comprehensive flora of Fiji. Hawaii: Pacific Tropical Botanical Garden; 1979.
- Ficus erecta Thunb. leaves ameliorate cognitive deficit and neuronal damage in a mouse model of amyloid-β-induced Alzheimer’s disease. Front Pharmacol. 2021;12:607403
- [Google Scholar]
- Fractionation of aqueous extract of Ficus deltoidea VAR. Kunstleri’s leaves using solid phase extraction method for anticancer activity on DU145 cell line. Malaysian J Anal Sci. 2019;23:534-547.
- [Google Scholar]
- Toxicological evaluation of the aqueous fraction of Ficus racemosa and Bauhinia variegata bark in rats. Int J Toxicol Pharmacol Res. 2014;6:80-85.
- [Google Scholar]
- Antioxidant activities and anthocyanin content of fresh fruits of common fig (Ficus carica L.) J Agric Food Chem. 2006;54:7717-7723.
- [Google Scholar]
- Formulation and evaluation of Ficus benghalensis emulgel for its anti- rheumatoid arthritis effect. J Innov Appl Pharm Sci. 2021;6:31-36.
- [Google Scholar]
- South Korean Patent. 2018. Composition for anti-arthritis using a leaf extract of Ficus erecta Thunb. var. sieboldii(Miq.)King. Application KR1020180031634A.
- Functional properties of anthocyanins and betalains in plants, food, and in human nutrition. Trends Food Sci Technol. 2004;15:19-38.
- [Google Scholar]
- Identification and quantitation of major carotenoids in selected components of the Mediterranean diet: Green leafy vegetables, figs and olive oil. European J Clin Nutr. 2002;56:1149-1154.
- [Google Scholar]
- Studies on phytochemical properties and antimicrobial activities of Passiflora foetida L. and Ficus retusa L. J Res Plant Sci. 2014;3:249-254.
- [Google Scholar]
- A benzopyrroloisoquinoline alkaloid from Ficus fistulosa. Phytochem Lett. 2009;2:88-90.
- [Google Scholar]
- Anticancer screening of Ficus racemosa by XTT bioassay. Res J Pharmacol Pharmacodynamics. 2013;5:309-312.
- [Google Scholar]
- In-vitro screening of Ficus racemosa for anticancer activity. Res J Pharmacog Phytochem. 2013;5:119-122.
- [Google Scholar]
- Evaluation of the antinociceptive activity of Ficus deltoidea aqueous extract. Fitoterapia. 2008;79:557-561.
- [Google Scholar]
- Evaluation of the antioxidant activity of Ficus racemosa plant extracts from North-Western district of Bangladesh. J Life Earth Sci. 2013;8:93-99.
- [Google Scholar]
- Structure elucidation of antibacterial compound from Ficus deltoidea Jack leaves. Indonesian J Chem. 2011;11:67-70.
- [Google Scholar]
- Hepato-protective activity of stem bark extracts of Ficus religiosa Linn in rats. Int J Biomed Res. 2011;8:466-475.
- [Google Scholar]
- Potential curing and beneficial effects of Ooitabi (Ficus pumila L.) on hypertension and dyslipidaemia in Okinawa. J Hum Nutr Diet. 2021;34:395-401.
- [Google Scholar]
- Antioxidant and antibacterial activities of flavonoid glycosides from Ficus exasperata Vahl-Holl (Moraceae) leaves. African J Tradit Complement Altern Med. 2014;11:97-101.
- [Google Scholar]
- Identification of phenylpropanoids in fig (Ficus carica L.) leaves. J Agric Food Chem. 2014;62:10076-10083.
- [Google Scholar]
- Phenylpropanoid composition in fig (Ficus carica L.) leaves. J Nat Med. 2017;71:770-775.
- [Google Scholar]
- Traditional uses, phytochemical, and pharmacological properties of Ficus auriculata: A review. J Drug Deliv Ther. 2021;11:163-169.
- [Google Scholar]
- Assessment of antioxidant activity of six Ficus species—underutilized fruits from Arunachal Pradesh in Northeast India. Int J Fruit Sci. 2015;15:85-99.
- [Google Scholar]
- Analysis of chemical constituents in Ficus Hirta Vahl. by LCMS-IT-TOF and GC-MS. IOP Conf. Ser: Materials Sci Eng. 2020;730:012027
- [Google Scholar]
- Antistress and antiallergic effects of Ficus bengalensis bark in asthma. Nat Prod Res. 2007;21:1266-1270.
- [Google Scholar]
- Biological activities of plant extracts from Ficus elastica and Selaginella vogelli: An antimalarial, antitrypanosomal and cytotoxity evaluation. Saudi J Biol Sci. 2018;25:117-122.
- [Google Scholar]
- Teixeira, D.M., Canelas, V.C., do Canto, A.M., Teixeira, J.M.G., Dias, C.B. 2009. HPLC-DAD Quantification of phenolic compounds contributing to the antioxidant activity of Maclura pomifera, Ficus carica and Ficus elastica Extracts. Analytical Lett 42, 2986 - 3003.
- Comparison between sample disruption methods and solid-liquid extraction (SLE) to extract phenolic compounds from Ficus carica leaves. J Chromatogr A. 2006;1103:22-28.
- [Google Scholar]
- An ethnobotanical survey of medicinal plants used in Loja and Zamora-Chinchipe, Ecuador. J Ethnopharmacol. 2007;111:63-81.
- [Google Scholar]
- Phenolic profiling, antioxidants, multivariate, and enzyme inhibitory properties of wild Himalayan fig (Ficus palmata Forssk.): A potential candidate for designing innovative nutraceuticals and related products. Analytical Lett. 2021;54:1439-1456.
- [Google Scholar]
- Stem bark extraction of Ficus bengalensis Linn for anti-inflammatory and analgesic activity in animal models. Indian J Exp Biol. 2010;48:39-45.
- [Google Scholar]
- Documentation of Traditional Uses of Plants by Tharu Community Around Royal Sukla-Phanta Wildlife Reserve. Far West Nepal: Central Department of Botany, Tribhuvan University, Kathmandu, Nepal; 2001.
- A new oleanane triterpene from the leaves of Ficus hirta. Nat Prod Res. 2019;33:3065-3069.
- [Google Scholar]
- Ursane Triterpenoids from the Leaves of Ficus hirta. Chem Nat Comp. 2021;57:695-697.
- [Google Scholar]
- Phytochemical analysis and antimicrobial activity of ethonolic leaf extract of Ficus racemosa Linn. Research J Pharm Technol. 2017;10:537-540.
- [Google Scholar]
- Anti-arthritic activity profile of methanolic extract of Ficus bengalensis: Comparison with some clinically effective drugs. Biomed Aging Pathol. 2014;4:207-217.
- [Google Scholar]
- Chemical composition, antibacterial and cytotoxic activities of the essential oil from Ficus tikoua Bur. Rec Nat Prod. 2020;14:219-224.
- [Google Scholar]
- Herbal medicine research and global health: An ethical analysis. Bull World Health Organ. 2008;86:594-599.
- [Google Scholar]
- The antimalarial potential of medicinal plants used for the treatment of malaria in Cameroonian folk medicine. African J Tradit Complement Altern Med. 2008;5:302-321.
- [Google Scholar]
- Ethno-medicinal plants of Parsa district Nepal. In: Jha P.K., Baral S.R., Karmacharya S.B., Lekhak H.D., Lacoul P., Baniya C.B., eds. Environment and Agriculture: Biodiversity, agriculture and pollution in South Asia. Kathmandu: Ecol Soc Nepal; 2001. p. :238-244.
- [Google Scholar]
- Anti-inflammatory and anti-arthritic potential of standardized extract of Clerodendrum serratum (L.) Moon. Front Pharmacol. 2021;12:629607
- [Google Scholar]
- In vitro evaluation of alpha amylase activity of bark extracts of Ficus auriculata. Int J Innovative Sci Res Technol. 2017;2:88-92.
- [Google Scholar]
- Metabolite profiling of Ficus deltoidea’s most active fraction as anti-Candida albicans using UPLC-QToF-MS/MS. J Young Pharm. 2021;13:58-62.
- [Google Scholar]
- Flavonoids and polyphenols content and antioxidant activity of Ficus carica L. extracts from Romania. J Nat Sci Novi Sad. 2015;128:57-65.
- [Google Scholar]
- A new benzofuran derivative from the leaves of Ficus pumila L. Nat Prod Res. 2018;32:1648-1652.
- [Google Scholar]
- Hepatoprotective activity of Ficus lacor Buch.-Ham. Int J Pharmacol Biol Sci. 2007;1:33-35.
- [Google Scholar]
- Preliminary phytochemical and pharmacological studies on Ficus racemosa (Gular) Indian J Med Res. 1969;57:1070-1074.
- [Google Scholar]
- Antimicrobial potential of extract fractions of Ficus vogelii Miq. Environ Dis. 2020;5:66-71.
- [Google Scholar]
- Toxicological studies of hydromethanolic leaves extract of Grewia crenata. Int J Pharm Sci Drug Res. 2012;4:245-249.
- [Google Scholar]
- Wound–healing activity of the aqueous leaf extract and fractions of Ficus exasperata (Moraceae) and its safety evaluation on albino rats. J Trad Complement Med. 2014;4:246-252.
- [Google Scholar]
- Antidiabetic effects of ethanolic extract of Ficus glomerata (L.) roots. Curr Bioactive Comp. 2020;16:33-41.
- [Google Scholar]
- Ficus racemosa and Morus indica: Emerging alternative antihyperglycemic agents. Open Conf Proc J. 2013;4:59-65.
- [Google Scholar]
- In silico anti-inflammatory activity evaluation of some bioactive compound from Ficus religiosa through molecular docking approach. J Physics: Conf Ser. 2020;1563:012024
- [Google Scholar]
- Randomized clinical trial of Ficus religiosa on level in type II diabetes mellitus. Int Ayurvedic Med J. 2014;2:1019-1024.
- [Google Scholar]
- Hypoglycemic and antihyperglycemic activity of methanolic root extract of Ficus racemosa in normal and streptozotocin induced diabetic rats. Pharmacologyonline. 2009;2:656-666.
- [Google Scholar]
- Flavonoid content in leaf extracts of the fig (Ficus carica L.), carob (Ceratonia siliqua L.) and pistachio (Pistacia lentiscus L.) BioFactors. 2006;28:169-175.
- [Google Scholar]
- Phenolic acids and flavonoids of fig fruit (Ficus carica L.) in the Northern Mediterranean region. Food Chem. 2008;106:153-157.
- [Google Scholar]
- Ficus racemosa stem bark extract: A potent antioxidant and a probable natural radioprotector. Evid Based Complement Altern Med. 2007;119:1-8.
- [Google Scholar]
- Ficus racemosa stem bark extract: A potent antioxidant and a probable natural radioprotector. eCAM. 2009;6:317-324.
- [Google Scholar]
- Antidiabetic effect of Ficus racemosa Linn. stem bark in high-fat diet and low-dose streptozotocin-induced type 2 diabetic rats: a mechanistic study. Food Chem. 2012;132:186-193.
- [Google Scholar]
- Estimation of phytochemical, nutritional, antioxidant, and antibacterial activity of dried fruit of sacred figs (Ficus religiosa) and formulation of value-added product (hard candy) J Pharmacog Phytochem. 2015;4:257-267.
- [Google Scholar]
- Burn wound healing study of Ficus religiosa’s bark ash and extraction. Annals RSCB. 2021;26:19262-19276.
- [Google Scholar]
- Characterization and screening of bioactive compounds in the extract prepared from aerial roots of Ficus benghalensis. Int J Pharm Sci Res. 2015;6:5056.
- [Google Scholar]
- Antistress activity of methanolic extract of Ficus benghalensis. Drug Invent Today. 2019;12:1281-1283.
- [Google Scholar]
- Radioprotective activity of Ficus racemosa ethanol extract against electron beam induced dna damage in vitro, in vivo and in silico. Int J Pharm Pharm Sci. 2015;7:110-119.
- [Google Scholar]
- Clinical study on erectile dysfunction in diabetic and non-diabetic subjects and its management with Ficus religiosa Linn. AYU. 2010;31:272-278.
- [Google Scholar]
- Chemical constituents and antifungal activity of Ficus hirta Vahl. fruits. Plants. 2017;6:44.
- [Google Scholar]
- Enhanced and green extraction polyphenols and furanocoumarins from fig (Ficus carica L.) leaves using deep eutectic solvents. J Pharm Biomed Anal. 2017;145:339-345.
- [Google Scholar]
- Antifungal flavonoids from Ficus sarmentosa var. henryi (King) Corner. Agricult Sci China. 2010;9:690-694.
- [Google Scholar]
- Insecticidal constructure and bioactivities of compounds from Ficus sarmentosa var. henryi. J Integr Agricult. 2016;10:1402-1410.
- [Google Scholar]
- Comparative study of leaves of Ficus pomifera Wall., Ficus hispida Linn. and Ficus religiosa Linn. for the biochemical contents, minerals and trace elements. Indian J Nat Prod Resources. 2012;3:184-188.
- [Google Scholar]
- Cytotoxic triterpenoids from Ficus pomifera Wall. Indian J Chem Section B. 2015;54:676-681.
- [Google Scholar]
- Anti-inflammatory effect of ethanolic extract of Ficus bengalensis Linn. in carrageenan induced paw edema in rats. Pharmacog J. 2011;3:96-99.
- [Google Scholar]
- Chemical constituents from Ficus pumila. Chinese Trad Herbal Drugs. 2014;24:615-621.
- [Google Scholar]
- A new benzofuran glucoside from Ficus tikoua Bur. Int J Mol Sci. 2011;12:4946-4952.
- [Google Scholar]
- Characterization of antioxidant, antimicrobial, anticancer property, and chemical composition of Ficus deltoidea Jack. leaf extract. J Biol Active Prod Nat. 2011;1:1-6.
- [Google Scholar]
- New antifungal pyranoisoflavone from Ficus tikoua Bur. Int J Mol Sci. 2012;13:7375-7382.
- [Google Scholar]
- WHO Traditional Medicine Strategy 2002–2005. Geneva, Switzerland: World Health Organization; 2002.
- Study of synergistic effects on antioxidant activity and antimicrobial activity of polyherbal formulations containing Ficus species. Int J Pharm Pharm Sci. 2016;8:50-53.
- [Google Scholar]
- Phenolic compounds, antioxidant and antidiabetic activity of different cultivars of Ficus carica L. fruits. J Funct Foods. 2016;25:421-432.
- [Google Scholar]
- Isoprenylated flavonoids with PTP1B Inhibition from Ficus tikoua. Nat Prod Commun. 2015;10:2105-2107.
- [Google Scholar]
- Medicinal plants and traditional medicinal practice in the Eastern part of Parsa district, Nepal. In: Proc III Nat Conf Sci Technol. Kathmandu, Nepal: Royal Nepal Acad Sci Technol; 1999. p. :1421-1426.
- [Google Scholar]
- Hepatoprotective effect of Ficus religiosa latex on cisplatin induced liver injury in Wistar rats. Rev Bras Farmacogn. 2015;25:278-283.
- [Google Scholar]
- Interspecific hybridization of fig (Ficus carica L.) and Ficus erecta Thunb., a source of Ceratocystis canker resistance. Euphytica. 2012;183:39-47.
- [Google Scholar]
- Hispidacine, an unusual 8,4'-oxyneolignan-alkaloid with vasorelaxant activity, and hispiloscine, an antiproliferative phenanthroindolizidine alkaloid, from Ficus hispida Linn. Phytochem. 2015;109:96-102.
- [Google Scholar]
- Fistulopsines A and B antiproliferative septicine-type alkaloids from Ficus fistulosa. Phytochem Lett. 2016;15:136-141.
- [Google Scholar]
- Phenolic glycosides from the roots of Ficus hirta Vahl. and 2 their anti-neuroinflammatory activities. J Agric Food Chem. 2020;68:4196-4204.
- [Google Scholar]
- Lignans and phenylpropanoids from the roots of Ficus hirta and their cytotoxic activities. Nat Prod Res. 2021;35:1-10.
- [Google Scholar]
- Chemical quantification and antioxidant assay of four active components in Ficus hirta root using UPLC-PAD-MS fingerprinting combined with cluster analysis. Chem. Central J.. 2013;7:115.
- [Google Scholar]
- Chronic inflammation: Role of adipose tissue and modulation by weight loss. Curr Diabetes Rev. 2006;2:29-37.
- [Google Scholar]
- Sustainable and efficient surfactant-based microwave-assisted extraction of target polyphenols and furanocoumarins from fig (Ficus carica L.) leaves. J. Mol. Liquids. 2020;318:114196
- [Google Scholar]
- Molecular docking analysis of Ficus religiosa active compound with anti-inflammatory activity by targeting tumour necrosis factor alpha and vascular endothelial growth factor receptor in diabetic wound healing. Open Access Macedonian J Med Sci. 2021;9:1031-1036.
- [Google Scholar]
- Yuet Ping, K., Darah, I., Chen, Y., Sreeramanan, S., Sasidharan, S., 2013. Acute and subchronic toxicity study of Euphorbia hirta L. methanol extract in rats. BioMed. Res. Int. 2013, 182064.
- Anti-inflammatory activity of the aqueous extract of Ficus deltoidea. Biol. Res. Nurs.. 2012;14:90-97.
- [Google Scholar]
- Effects of Ficus hirta Vahl. (Wuzhimaotao) extracts on growth inhibition of HeLa cells. Exp Toxicol Pathol. 2012;64:743-749.
- [Google Scholar]
- Experimental study on the anti-inflammatory effect of Ficus pumila L. Medicinal Plant. 2020;11:26-29.
- [Google Scholar]
- Chemical composition of volatiles from the syconia of Ficus microcarpa and host recognition behavior of pollinating fig wasps. Chinese J. Plant Ecol.. 2017;41:549-558.
- [Google Scholar]
- Taxonomic treatment of the Ficus auriculata complex (Moraceae) and typification of some related names. Phytotaxa. 2019;399:203-208.
- [Google Scholar]
- Flavonoids from fig (Ficus carica Linn.) leaves: The development of a new extraction method and identification by UPLC-QTOF-MS/MS. Appl. Sci.. 2021;11:7718.
- [Google Scholar]
- Chemical studies on roots of Ficus hirta. China J. Chinese Materia Med.. 2013;38:3696-3701.
- [Google Scholar]
- A new hemiacetal chromone racemate and α-glucosidase inhibitors from Ficus tikoua bur. Nat. Prod. Res.. 2022;36:1-9.
- [Google Scholar]
- Analgesic and antiemetic activities of Ficus exasperata Vahl., and Cleome ciliata Schmach and Thonn. World J. Pharm. Res.. 2014;3:1945-1954.
- [Google Scholar]







