Translate this page into:
UPLC-MS/MS for simultaneous quantification of vortioxetine and its metabolite Lu AA34443 in rat plasma and its application to drug interactions
⁎Corresponding authors. xra@wmu.edu.cn (Ren-ai Xu)
-
Received: ,
Accepted: ,
This article was originally published by Elsevier and was migrated to Scientific Scholar after the change of Publisher.
Abstract
Vortioxetine is currently used as the first-line therapy drug for major depressive disorder (MDD). In the present study, we aimed to develop and fully validate an ultra performance liquid chromatography tandem mass spectrometry (UPLC-MS/MS) method for the simultaneous quantification of vortioxetine and its major metabolite Lu AA34443 in plasma and to investigate the effects of dronedarone and amiodarone on vortioxetine metabolism in rats. After protein precipitation with acetonitrile, the separation of vortioxetine, Lu AA34443 and duloxetine (internal standard, IS) were finished on an Acquity BEH C18 (2.1 mm × 50 mm, 1.7 μm) column and their detections were conducted by a Xevo TQ-S triple quadrupole tandem mass spectrometer in the positive ion mode. The assay displayed excellent linearity in the range of 0.5–50 ng/mL for vortioxetine, and 5–1000 ng/mL for Lu AA34443. The results of this method exhibited that the precision, accuracy, matrix effect, recovery, and stability of vortioxetine and Lu AA34443 met all requirements for the quantitation in plasma samples. The validated assay was further successfully employed to study the effects of dronedarone (80 mg/kg) and amiodarone (60 mg/kg) on vortioxetine metabolism in rats. The results showed that dronedarone and amiodarone could increase the concentration of vortioxetine and have inhibitory effect on vortioxetine metabolism. Thus, vortioxetine dose interruption or reduction may be considered.
Keywords
Vortioxetine
Lu AA34443
Pharmacokinetic interaction
UPLC-MS/MS
Inhibitory effect
1 Introduction
Vortioxetine (Fig. 1A), a new antidepressant with multimodal activity, extensively studied for use in major depressive disorder (MDD) (Cipriani et al., 2018; Tran et al., 2019), a global prevalence of 4.7% (Ferrari et al., 2013). Vortioxetine received its approval in the treatment of MDD in the United States of America (USA) and in the European Union (EU), and is currently used as the first-line therapy agent (Gibb & Deeks, 2014). The unique mechanism of vortioxetine is considered to be two different pharmacological targets: transporters and serotonin receptors (Connolly & Thase, 2016; Salagre, Grande, Sole, Sanchez-Moreno, & Vieta, 2018; Sowa-Kucma et al., 2017).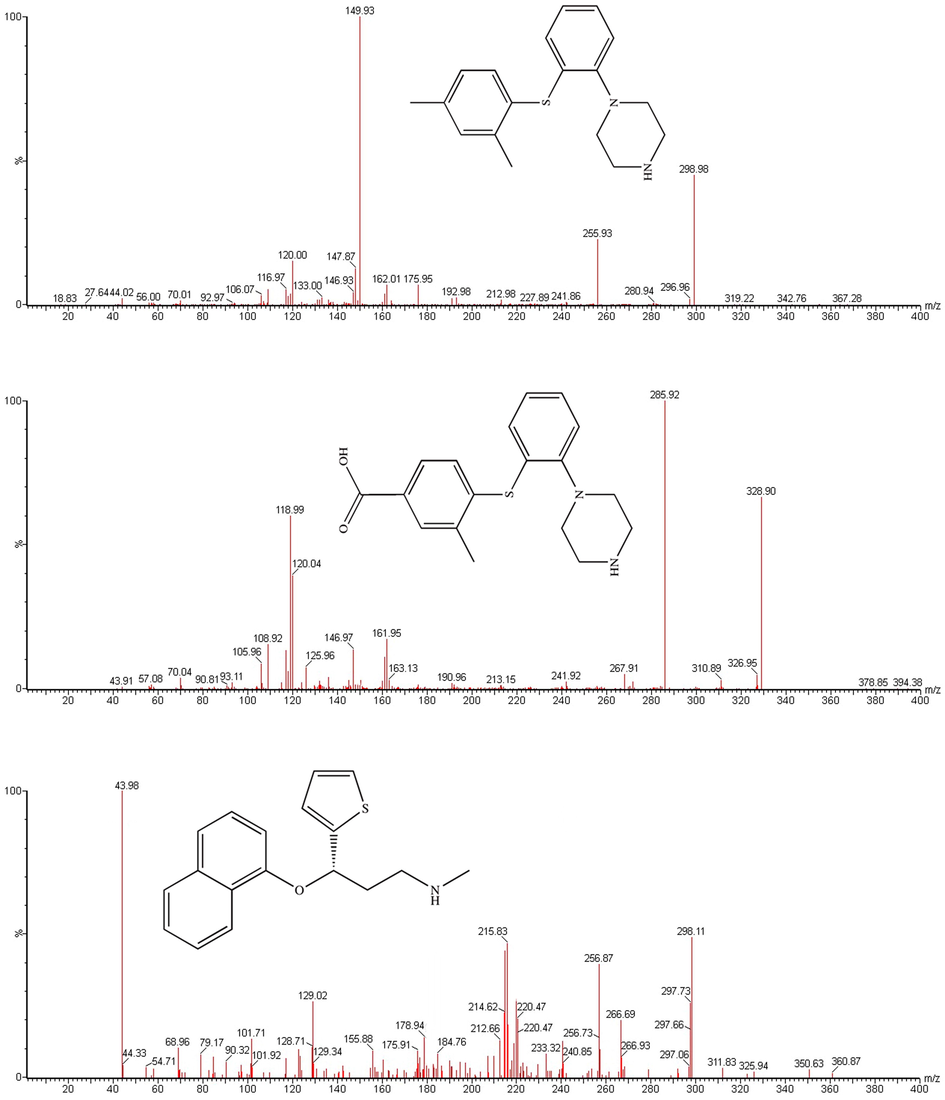
Mass spectra of vortioxetine (A), Lu AA34443 (B) and duloxetine (IS, C) in the present study.
After oral administration, vortioxetine is absorbed slowly and mainly metabolized by cytochrome P450 2D6 (CYP2D6) to yield its major pharmacologically inactive metabolite (Lu AA34443, Fig. 1B) (Hvenegaard et al., 2012). In previous study, a lot of drug-drug interaction studies have been assessed, and the exposure of vortioxetine could be influenced by some drugs, including bupropion, rifampin, ketoconazole and fluconazole (Chen et al., 2013). In order to support the upcoming pharmacokinetic interaction for vortioxetine, it is important to develop an efficient bioanalytical method to detect the concentrations of vortioxetine and its metabolite Lu AA34443 in plasma simultaneously.
Until now, several liquid chromatography tandem mass spectrometry (LC-MS/MS) have been characterized for the quantification of vortioxetine in biological fluids (Gu et al., 2015; Guan et al., 2018; Huang et al., 2016). All the above methods determined vortioxetine without its metabolite Lu AA34443. Recently, only one published paper reported an isocratic cation exchange HPLC-MS/MS method utilizing time-consuming C8-SPE sample extracts and a reversed-phase UPLC-MS/MS method with gradient elution of protein precipitated sample extracts, for the simultaneous determination of the concentrations of vortioxetine and Lu AA34443 in biological samples (Kall, Rohde, & Jorgensen, 2015). However, both methods had used limited availability of isotope-labeled internal standards and lacked the practical application in pharmacokinetic interaction study.
Dronedarone is a novel antiarrhythmic drug, and has been shown to inhibit CYP2D6 (Patel, Yan, & Kowey, 2009). Amiodarone, the most commonly used antiarrhythmic agent, is also found to be an inhibitor of CYP2D6 (Fukumoto et al., 2006; Jaruratanasirikul & Hortiwakul, 1994; Ohyama et al., 2000). In clinic, patients with MDD may develop arrhythmias. Thus, co-administrated dronedarone or amiodarone with vortioxetine as one treatment protocol in those patients is likely to occur. However, the effects of dronedarone and amiodarone on the pharmacokinetics of vortioxetine have not been assessed. Therefore, the purpose of the present study was to establish an ultra performance liquid chromatography tandem mass spectrometry (UPLC-MS/MS) method for the simultaneous quantification of vortioxetine and Lu AA34443 in rat plasma, and also to investigate whether dronedarone and amiodarone could change the pharmacokinetic profiles of vortioxetine and Lu AA34443 in rats.
2 Experimental
2.1 Chemicals materials
Vortioxetine, dronedarone, amiodarone (all purity > 98%) and duloxetine (IS, purity > 98%, Fig. 1C) were obtained from Beijing sunflower and technology development CO., LTD (Beijing, China). Lu AA34443 was gifted from Jiangsu Giebell Pharmaceuticals CO., LTD (Jiangsu, Chian). LC-grade methanol and acetonitrile were purchased from Merck Company (Darmstadt, Germany). Analytical grade of formic acid was also bought from Beijing sunflower and technology development CO., LTD (Beijing, China). A Milli-Q Reagent System (Millipore, Bedford, USA) was employed to prepare the distilled water.
2.2 Animal experiments
Eighteen male Sprague-Dawley (SD) rats (200 ± 20 g) were offered from Animal Experiment Center of Wenzhou Medical University (Zhejiang, China). Before experiments, the rats were provided with water freely except for fasting 12 h. The animal experiment was approved by the Animal Care and Use Committee of Wenzhou Medical University. Vortioxetine, dronedarone, and amiodarone were all suspended in 0.5% carboxymethyl cellulose sodium (CMC-Na). 18 rats were randomly divided into three groups (n = 6): the control group (0.5% CMC-Na, group A), dronedarone group (80 mg/kg, group B), and amiodarone group (60 mg/kg, group C). Thirty minutes later, 4 mg/kg vortioxetine was orally administered to each group. Approximately 0.15 mL of blood samples were harvested at the different time points of 0.333, 0.667, 1, 2, 4, 8, 12, 24, and 36 h from the tail vein into 1.5 mL tubes containing heparin. Subsequently, 50 µL plasma was harvested by centrifuging the blood sample at 4000 g for 10 min at room temperature and then stored at −80 °C until analysis.
2.3 Instrumentations and analytical conditions
A Waters Acquity ultra performance liquid chromatography (UPLC) system (Milford, MA, USA), including an I-CLASS binary solvent delivery manager, a column oven (set at 40 °C) and a sample manager (FTN, set at 10 °C), was used for the liquid chromatography. The chromatographic separation was carried out on an Acquity BEH C18 column (2.1 mm × 50 mm, 1.7 μm). Meanwhile, the flow rate of the mobile phase which consisted of acetonitrile (solvent A) and 0.1% formic acid in water (solvent B), was set 0.30 mL/min, and a procedure for the gradient elution was optimized as follows: 10–70% A at 0–0.3 min, 70% A at 0.3–1.5 min, 70–10% A at 1.5–1.6 min, and finally the column was equilibrated with 10% A for 1.4 min. The entire run time was 3.0 min with a 2.0 µL injection volume.
Quantification was conducted on a Waters Xevo TQ-S triple quadrupole tandem mass spectrometer (Milford, MA, USA) through an electro-spray ionization (ESI) interface operated in positive ion mode. The selected multiple reaction monitoring (MRM) mode was employed for the determination of each transition: m/z 298.98 → 149.93 for vortioxetine, m/z 328.90 → 285.92 for Lu AA34443 and m/z 298.11 → 43.98 for IS, respectively. Table 1 demonstrated the parameters of the analytes and IS for the MS. Instrument control and data acquisition was used by the Masslynx 4.1 software (Milford, MA, USA).
Analyte
Precursor ion
Product ion
CV (V)
CE (eV)
RT (min)
Vortioxetine
298.98
149.93
10
25
0.92
Lu AA34443
328.90
285.92
10
20
0.83
IS
298.11
43.98
30
10
0.88
2.4 Standard solutions, calibration standards and quality control (QC) samples
1.00 mg/mL stock solution of each analyte was separately obtained by dissolving the corresponding standard compound in methanol. A serial dilution from the stock solution of each analyte was operated with methanol to gain the quality control (QC) working solution and calibration standard. Likewise, IS working solution was obtained by the dilution of the stock solution (1.00 mg/mL) with acetonitrile to 50 ng/mL. The final concentration levels of the calibration standard obtained from mixing blank plasma (90 µL) with the corresponding standard working solution (10 µL) were 0.5, 1, 2, 5, 10, 20, and 50 ng/mL for vortioxetine, 5, 10, 50, 100, 200, 500 and 1000 for Lu AA34443. Similary, the concentrations of QC samples were operated at 1.5, 15, and 40 ng/mL for vortioxetine, and 15, 80, and 800 ng/mL for Lu AA34443. All the working and stock solutions were stored at −80 °C before analysis.
2.5 Sample preparation
For each 50 µL plasma sample, 150 µL of acetonitrile (50 ng/mL IS in acetonitrile) was spiked for protein precipitation before the mixture was vortexed for 3.0 min and centrifugated at 13,000 g at 4 °C for 10 min. Then, 100 µL supernatant was transferred to the autosampler vial, and only 2.0 µL clear supernatant was used for analysis in the UPLC-MS/MS system.
2.6 Method validation
A complete validation of the bioanalytical assay was conducted in light of the regulatory principles by the US FDA (Center for Drug Evaluation and Research of the U.S. Department of Health and Human Services Food and Drug Administration, Guidance for industry; Bioanalytical method validation, 2018, http://www.fda.gov/Drugs/GuidanceComplianceRegulatoryInformation/Guidances/ucm064964.htm, Accessed: August 2, 2018.), which need to assess the lower limit of quantification (LLOQ), selectivity, calibration curve, precision and accuracy, matrix effect, recovery and stability under various conditions.
2.6.1 Selectivity
The selectivity of this method was investigated by comparing the chromatographic results of six individual rat blank plasma samples, plasma samples spiked with vortioxetine, Lu AA34443 and IS standards, and a real rat sample obtained from the pharmacokinetic interaction study, to ensure no endogenous substance interference.
2.6.2 Linearity and LLOQ
Through the weighted (1/x2) least-squares regression algorithm, the peak area ratio of each analyte to that of IS against the nominal concentration was calculated to be the linearity in this study. LLOQ was considered to be the lowest concentration in the calibration curve, of whose accuracy should be within ± 20%, and precision should not be>20%.
2.6.3 Accuracy and precision
At 3 consecutive analytical days, 6 replicates of QC samples and LLOQ were analyzed for the evaluation of intra- and inter-day accuracy and precision. Accuracy and precision was calculated as relative error (RE) and relative standard deviation (RSD), respectively, and required not to exceed ± 15%, except for the LLOQ should not be more than ± 20%.
2.6.4 Matrix effect and recovery
The matrix effects from the spiked plasma samples were calculated by comparing the peak areas of vortioxetine and Lu AA34443 in post-extracted plasma samples with those of corresponding standard solutions at three QC levels (n = 6). The recoveries of the analytes were determined by comparing the peak areas of the post-extracted QC plasma samples with those of the pre-extracted plasma samples at three different concentratiton levels (n = 6).
2.6.5 Stability
The stability in rat plasma was detected using three QC levels under the following conditions: at room temperature for 3 h as the short-term stability, at −80 °C for 21 days as the long-term stability, and three complete freeze (-80 °C)/thaw (RT) cycles. In addition, the post-preparative stability was assessed through an autosampler at 10 °C for 6 h.
2.7 Statistical analysis
Following the quantification of each analyte concentration in different groups, Origin 8.0 (Originlab Company, Northampton, MA, USA) was used to describe the average plasma concentration vs time profile of vortioxetine and Lu AA34443 in rat plasma, and DAS (Drug and statistics) software (Version 2.0, Shanghai University of Traditional Chinese Medicine, China) was employed to calculate the pharmacokinetic parameters of vortioxetine and Lu AA34443 in non-compartmental mode. In addition, Statistical Package for the Social Sciences (version 17.0; SPSS Inc., Chicago, IL, USA) was employed to compare the main pharmacokinetic parameters within each group by one-way analysis of variance coupled with the Dunnett’s test. In all cases, a p-value below 0.05 was deemed to be of statistical significance.
3 Results and discussion
3.1 Method development and optimization
Vortioxetine, Lu AA34443 and IS (200 ng/mL in methanol:water (50:50, v/v) for each substance) were directly infused into the mass spectrometer, and the conditions of product ion mass spectra of vortioxetine, Lu AA34443 and IS were optimized. Different settings that produced the highest sensitivity by monitoring the product ion transition were compared. In positive full mass scan (as shown in Fig. 1), vortioxetine, Lu AA34443 and IS generated a protonated molecular ion [M + H]+, and the most abundant fragment ions for MRM were m/z 298.98 → 149.93, m/z 328.90 → 285.92 and m/z 298.11 → 43.98, with collision energy values of 25 eV, 20 eV and 10 eV for vortioxetine, Lu AA34443 and IS, respectively.
Due to the redundant numbers of plasma samples produced in pharmacokinetic interaction study, a simple and quick sample preparation method is always desirable. After many attempts, it was found that acetonitrile yielded a significantly shorter retention time and better symmetrical peak shape than methanol. Therefore, acetonitrile was employed for sample preparation in this study. In addition, in order to improve the sensitivity of ESI positive detection, the addition of formic acid in mobile phase was necessary. Finally, an gradient elution was developed for all the analytes and IS, which was using a mobile phase of acetonitrile and 0.1% formic acid in water, and the total run time was only 3.0 min with 0.30 mL/min as flow rate.
3.2 Method validation
3.2.1 Selectivity
The spectra of blank rat plasma sample, plasma sample containing vortioxetine, Lu AA34443 and IS, and rat plasma sample from the pharmacokinetic interaction study are shown in Fig. 2. The results indicated that no apparent interferences from endogenous compounds were observed at the corresponding retention times of vortioxetine, Lu AA34443 and IS, which were 0.92, 0.83, 0.88 min, respectively.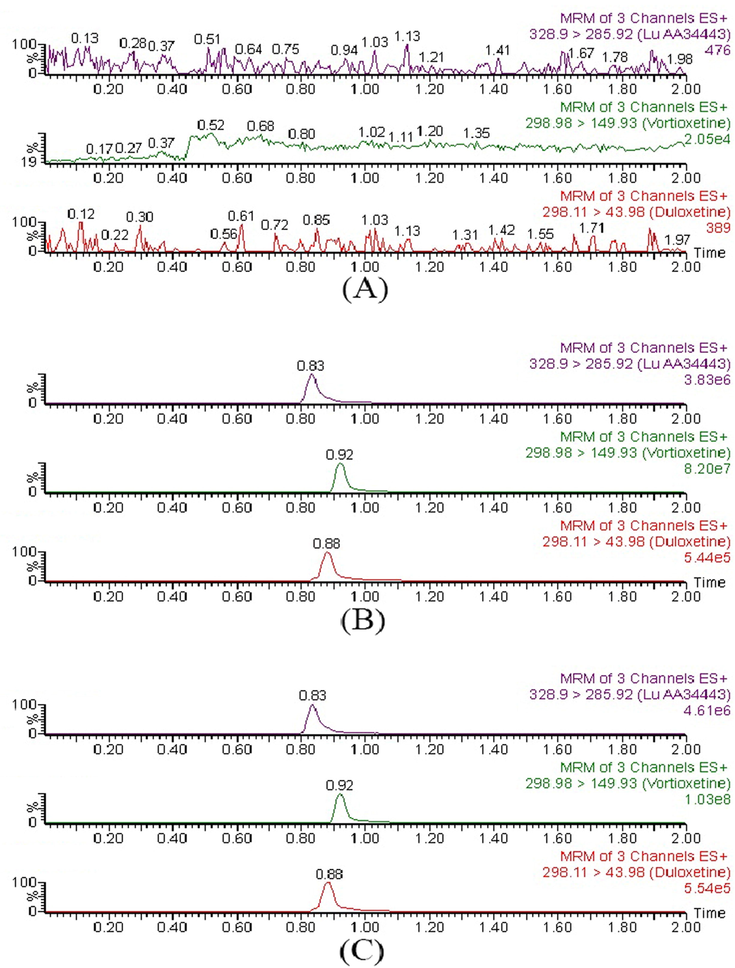
Representative MRM chromatograms of vortioxetine, Lu AA34443 and IS in rat plasma sample: blank plasma (A), blank plasma spiked with standard solutions (B) and real plasma sample collected from a rat after 2.0 h oral administration of 4 mg/kg vortioxetine (C).
3.2.2 Linearity and LLOQ
The calibration curve displayed an significant linearity within the ranges of 0.5–50 for vortioxetine and 5–1000 ng/mL for Lu AA34443, respectively. Representative linear regression equation was Y = 2.29053 × X ± 0.0333131 (r2 = 0.9991) for vortioxetine, and Y = 0.131341 × X ± 0.00786217 (r2 = 0.9996) for Lu AA34443, respectively. The value of LLOQ was established as 0.5 ng/mL for vortioxetine and 5 ng/mL for Lu AA34443, respectively, with sufficient accuracy and precision (Table 2). RSD, relative standard deviation; RE, relative error.
Analyte
Concentration (ng/mL)
Intra-day
Inter-day
RSD%
RE%
RSD%
RE%
Vortioxetine
0.5
12.6
4.6
14.1
4.8
1.5
11.3
−2.8
13.0
2.2
15
8.2
−2.5
8.9
4.9
40
5.2
−0.8
5.4
−1.8
Lu AA34443
5
10.1
5.9
14.6
−9.5
15
8.8
4.2
14.4
−12.2
80
7.0
−3.5
7.2
−2.2
800
2.4
−3.9
4.4
−0.3
3.2.3 Accuracy and precision
The intra-day and inter-day of accuracy and precision of each analyte at four different levels were presented in Table 2. The accuracy was within ± 15%, and the precision was within 15%. The newly established method was judged to be reliable and reproducible.
3.2.4 Recovery and matrix effect
At three QC concentration levels, the recovery of the method ranged from 80.8% to 92.8%. The matrix effect varied from 87.8% to 99.2%, which illustrated no significant plasma matrix effects on the compounds of vortioxetine and Lu AA34443. In addition, the matrix effect and the recovery for the IS were 97.8 ± 6.7% and 89.5 ± 5.9%, respectively.
3.2.5 Stability
Stability was tested for plasma samples in various storage and processing conditions at three QC concentrations. It was proved to be stable when plasma vortioxetine and Lu AA34443 samples were stored at room temperature for at least 3 h and −80 °C for at least 21 days, also in the auto-sampler (10 °C) for at least 6 h and three complete freeze–thaw cycles.
3.3 Animal study
A pharmacokinetic interaction study between vortioxetine and dronedarone/amiodarone in rats was employed to confirm the application of the validated UPLC-MS/MS method. After orally administrated a single dose of 4 mg/kg vortioxetine, the average plasma concentration vs time profiles of vortioxetine and Lu AA34443 in different groups were represented in Fig. 3 and Fig. 4, and the main calculated of pharmacokinetic parameters were demonstrated in Table 3 and Table 4 using non-compartment model analysis. AUC, Area under the concentration–time curve; MRT, mean residence time; t1/2, half-life time; Cmax, maximum plasma concentration; Tmax, time to Cmax; CLz/F, clearance. Compared with Group A, *P < 0.05. AUC, Area under the concentration–time curve; MRT, mean residence time; t1/2, half-life time; Cmax, maximum plasma concentration; Tmax, time to Cmax; CLz/F, clearance. Compared with Group A, *P < 0.05.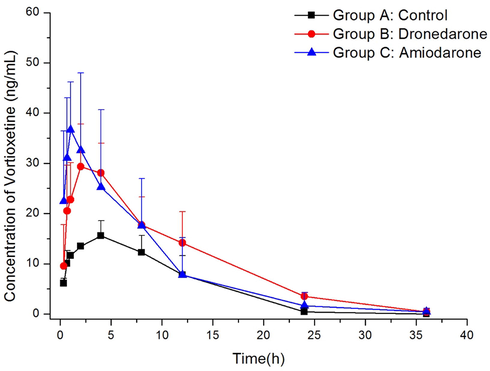
Mean plasma concentration–time curves of vortioxetine in different treatment groups of rats. Group A: the control group, Group B: 80 mg/kg dronedarone, and Group C: 60 mg/kg amiodarone. (n = 6).
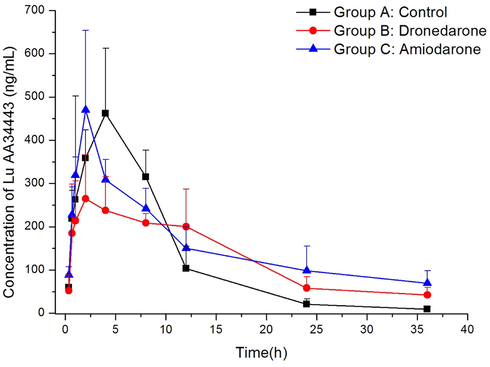
Mean plasma concentration–time curves of Lu AA34443 in different treatment groups of rats. Group A: the control group, Group B: 80 mg/kg dronedarone, and Group C: 60 mg/kg amiodarone. (n = 6).
Parameters
Group A
Group B
Group C
AUC0→t (µg/mL•h)
197.06 ± 42.46
381.52 ± 61.37*
318.07 ± 55.58*
AUC0→∞ (µg/mL•h)
205.08 ± 47.45
387.75 ± 61.69*
321.23 ± 56.73*
MRT0→t (h)
7.37 ± 1.11
8.73 ± 1.11
7.70 ± 1.35
MRT0→∞ (h)
7.56 ± 1.20
9.42 ± 2.00
7.89 ± 1.57
t1/2 (h)
3.98 ± 1.59
5.60 ± 1.75*
3.79 ± 1.68
Tmax (h)
3.20 ± 1.10
1.93 ± 1.03
1.50 ± 0.48
CLz/F (L/h)
20.39 ± 4.79
16.85 ± 4.08*
17.46 ± 4.15*
Cmax (µg/mL)
16.42 ± 2.85
33.92 ± 3.33*
39.75 ± 3.03*
Parameters
Group A
Group B
Group C
AUC0→t (µg/mL•h)
5581.11 ± 804.89
4741.86 ± 741.15
5900.48 ± 897.28
AUC0→∞ (µg/mL•h)
5647.44 ± 841.27
4804.97 ± 776.65
6073.06 ± 974.42
MRT0→t (h)
11.04 ± 1.11
11.77 ± 1.03
11.04 ± 2.01
MRT0→∞ (h)
11.42 ± 1.16
12.05 ± 1.06
11.85 ± 2.04
t1/2 (h)
10.21 ± 1.72
9.96 ± 2.20
10.82 ± 2.07
Tmax (h)
3.40 ± 1.82
3.42 ± 1.42
2.67 ± 1.05
CLz/F (L/h)
0.78 ± 0.15
0.73 ± 0.12
0.63 ± 0.18
Cmax (µg/mL)
474.22 ± 111.52
301.54 ± 102.70
483.18 ± 136.09
From the results of our study, when vortioxetine was combined with dronedarone in group B, the AUC0→t, AUC0→∞, t1/2 and Cmax of vortioxetine as the main pharmacokinetic parameters in this study increased largely (P < 0.05), while CLz/F decreased greatly (P < 0.05). It indicated that dronedarone had an inhibitory effect on vortioxetine metabolism. However, the main pharmacokinetic parameters (AUC0→t, AUC0→∞, t1/2 , CLz/F and Cmax) of Lu AA34443 in group B had no obvious changes when compared with group A. As for amiodarone in group C, the same results were observed, for the values of AUC0→t, AUC0→∞, and Cmax of vortioxetine also increased largely (P < 0.05) when compared with the control group A, while CLz/F reduced obviously (P < 0.05). In addition, no obvious changes were found for Lu AA34443 in group C. These data showed that amiodarone also had inhibitory effect on vortioxetine metabolism. As the study of the effects of dronedarone and amiodarone on vortioxetine metabolism was performed in rats with a few number, further researches should be done.
4 Conclusions
In conclusion, the newly developed method for the UPLC-MS/MS determination of vortioxetine and Lu AA34443 concentrations in rat plasma was simple and reliable. In addition, this developed UPLC-MS/MS method demonstrated great accuracy and precision, excellent sensitivity, and appropriate recovery, and was also successfully used to investigate the effects of dronedarone and amiodarone on the pharmacokinetics of vortioxetine and Lu AA34443 in rats. Interesting, both dronedarone and amiodarone increased the exposure of vortioxetine and had inhibitory effect on vortioxetine metabolism. Thus, caution should be given when vortioxetine with dronedarone or amiodarone are used together in clinic.
Acknowledgement
This study was supported by grant from the Natural Science Foundation of Zhejiang Province (Grant No. LYQ20H310004).
References
- (Center for Drug Evaluation and Research of the U.S. Department of Health and Human Services Food and Drug Administration, Guidance for industry; Bioanalytical method validation, 2018, http://www.fda.gov/Drugs/GuidanceComplianceRegulatoryInformation/Guidances/ucm064964.htm, Accessed: August 2, 2018.).
- Pharmacokinetic drug interactions involving vortioxetine (Lu AA21004), a multimodal antidepressant. Clin Drug Investig. 2013;33(10):727-736.
- [CrossRef] [Google Scholar]
- Comparative efficacy and acceptability of 21 antidepressant drugs for the acute treatment of adults with major depressive disorder: a systematic review and network meta-analysis. Lancet. 2018;391(10128):1357-1366.
- [CrossRef] [Google Scholar]
- Vortioxetine: a New Treatment for Major Depressive Disorder. Expert Opin Pharmacother. 2016;17(3):421-431.
- [CrossRef] [Google Scholar]
- Burden of depressive disorders by country, sex, age, and year: findings from the global burden of disease study 2010. PLoS Med. 2013;10(11):e1001547
- [CrossRef] [Google Scholar]
- Effect of amiodarone on the serum concentration/dose ratio of metoprolol in patients with cardiac arrhythmia. Drug Metab Pharmacokinet. 2006;21(6):501-505.
- [Google Scholar]
- An UPLC-MS/MS method for the quantitation of vortioxetine in rat plasma: Application to a pharmacokinetic study. J Chromatogr B Analyt Technol Biomed Life Sci. 2015;997:70-74.
- [CrossRef] [Google Scholar]
- Pharmacokinetic and metabolic studies of Vortioxetine in rats using ultra high performance liquid chromatography with tandem mass spectrometry. J Sep Sci. 2018;41(24):4469-4479.
- [CrossRef] [Google Scholar]
- Simultaneous quantification of vortioxetine, carvedilol and its active metabolite 4-hydroxyphenyl carvedilol in rat plasma by UPLC-MS/MS: Application to their pharmacokinetic interaction study. J Pharm Biomed Anal. 2016;128:184-190.
- [CrossRef] [Google Scholar]
- Identification of the cytochrome P450 and other enzymes involved in the in vitro oxidative metabolism of a novel antidepressant, Lu AA21004. Drug Metab Dispos. 2012;40(7):1357-1365.
- [CrossRef] [Google Scholar]
- The inhibitory effect of amiodarone and desethylamiodarone on dextromethorphan O-demethylation in human and rat liver microsomes. J Pharm Pharmacol. 1994;46(11):933-935.
- [Google Scholar]
- Quantitative determination of the antidepressant vortioxetine and its major human metabolite in plasma. Bioanalysis. 2015;7(22):2881-2894.
- [CrossRef] [Google Scholar]
- Inhibitory effects of amiodarone and its N-deethylated metabolite on human cytochrome P450 activities: prediction of in vivo drug interactions. Br J Clin Pharmacol. 2000;49(3):244-253.
- [CrossRef] [Google Scholar]
- Dronedarone. Circulation. 2009;120(7):636-644.
- [CrossRef]
- Vortioxetine: A new alternative for the treatment of major depressive disorder. Rev Psiquiatr Salud Ment. 2018;11(1):48-59.
- [CrossRef] [Google Scholar]
- Vortioxetine: A review of the pharmacology and clinical profile of the novel antidepressant. Pharmacol Rep. 2017;69(4):595-601.
- [CrossRef] [Google Scholar]
- Tran, B. X., Ha, G. H., Vu, G. T., Nguyen, L. H., Latkin, C. A., Nathan, K., . . . Ho, R. C. (2019). Indices of Change, Expectations, and Popularity of Biological Treatments for Major Depressive Disorder between 1988 and 2017: A Scientometric Analysis. Int J Environ Res Public Health, 16(13). doi:10.3390/ijerph16132255







